Abstract
Prostate cancer (PC) is the most commonly diagnosed non-cutaneous cancer and the second leading cause of cancer-related to death in men. The major risk factors for PC are age, family history, and African American ethnicity. Epidemiological studies have reported large geographical variations in PC incidence and mortality, and thus lifestyle and dietary factors influence PC risk. High fat diet, dairy products, alcohol and red meats, are considered as risk factors for PC. This book chapter provides a comprehensive, literature-based review on dietary factors and their molecular mechanisms of prostate carcinogenesis. A large portion of our knowledge is based on epidemiological studies where dietary factors such as cancer promoting agents, including high-fat, dairy products, alcohol, and cancer-initiating genotoxicants formed in cooked meats have been evaluated for PC risk. However, the precise mechanisms in the etiology of PC development remain uncertain. Additional animal and human cell-based studies are required to further our understandings of risk factors involved in PC etiology. Specific biomarkers of chemical exposures and DNA damage in the prostate can provide evidence of cancer-causing agents in the prostate. Collectively, these studies can improve public health research, nutritional education and chemoprevention strategies
Introduction
The World Health Organization (WHO) has reported that prostate cancer (PC) is the second most common cancer in men worldwide, with an estimated 1.1 million incident cases and 0.3 million deaths occurring in 2012 [1]. PC is more commonly diagnosed in economically developed countries, which may be attributed to more extensive PC screening programs. The major risk factors identified for PC include increased incidence with age, family history, and ethnicity, with African-American men having a two-time higher risk compared to Caucasians [2]. The occurrence of PC varies widely worldwide. Many studies of migrant populations show a significant increase in the incidence of PC and mortality rates in migrants from regions of the world with a low prevalence of PC, following their relocation to countries with high risk for PC, suggesting that environmental or dietary factors influence the risk factors for PC development [3]. The frequent consumption of high-fat diets from dairy products and red meats and alcohol are implicated as risk factors for PC [4]. However, the precise role of dietary factors and specific chemicals in the diet and mechanisms involved in the development of this malignancy remain unclear.
The diet as a risk factor for human PC
High-fat diet
Dietary fat and several fatty acids are postulated to play a role in PC etiology and tumor progression, although the findings of epidemiologic studies are inconsistent. Some studies found a strong positive association between fat consumption, PC incidence and mortality [5-10], whereas other investigations have not detected a correlation [11-14].
Several studies conducted in vivo in animal models and in vitro have shown a role for a high-fat diet in the development and progression of PC. Tissue culture medium conditioned with adipose tissue obtained from mice fed with high-fat Western-style foods enhanced cell proliferation, migration, and invasion of human prostate cancer cells in vitro [15, 16]. In the transgenic adenocarcinoma mouse prostate (TRAMP) and xenograft models, circulating adipokine and cytokine alterations and other factors induced by a high-fat diet contributed to PC progression [15-18]. Although strong evidence supports the effects of a high-fat diet on PC development and progression, the exact mechanism(s) by which a high-fat diet underlines PC etiology remain uncertain. Several hypotheses proposed include intake of fatty acids, resulting in inflammation, induction of oxidative stress, and cell signaling alteration.
Fatty acids
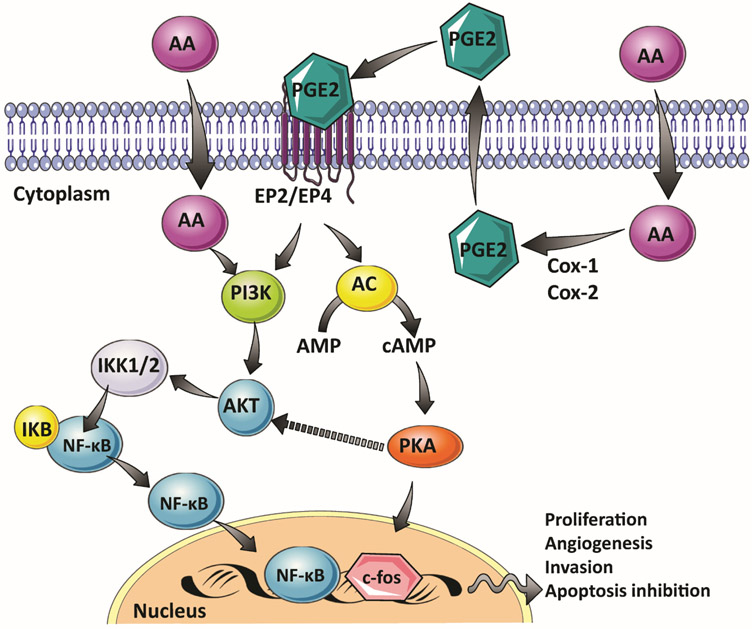
Fatty acids, such as n-3, and n-6 polyunsaturated fatty acids, and their metabolites are involved in numerous pathways that can affect PC development and progression. For example, n-6 fatty acids linoleic acid and arachidonic acid enhance proliferation of human prostate cell lines [19, 20]. Moreover, n-6 fatty acids are precursors of eicosanoids, which are converted to prostaglandins (PGs). The n-6 fatty acid arachidonic acid is metabolized by the enzyme cyclooxygenase (Cox-1 and Cox-2) to form prostaglandin E2 (PGE2) [21]. PGE2 is a short-lived hormone-like molecule involved in cell proliferation, cell differentiation and inflammation [21]. Notably, the growth stimulation of PC cells, by treatment with arachidonic acid, is correlated to the induction of COX2 expression and an increased synthesis of PGE2 [19, 22]. At the molecular level, PGE2 binds to its EP4 and EP2 receptors, resulting in the subsequent activation of the protein kinase A (PKA) pathway, which leads to expression of early growth-related response genes including c-fos [23]. Arachidonic acid also activates the phosphatidylinositol-4,5-bisphosphate 3-kinase signaling pathway (PI3K/Akt) [20]. The PI3K/Akt cascade is involved in the progression and aggressiveness of PC. In fact, after long-term androgen deprivation therapy, there is constitutive activation of the PI3K/Akt pathway, a mechanism that leads to increased resistance of tumor cells to apoptosis [24, 25]. The activation of the PI3K/Akt pathway by arachidonic acid in human prostate cells also results in the activation of the Nuclear Factor Kappa Beta (NF-κB) cascade [20]. The induction of the NF-κB pathway increases cell resistance to chemotherapy and radiation therapy. Moreover, the activation of NF-κB also stimulates tumor cell growth, the inhibition of apoptosis, and enhances tumor invasion, metastasis, and angiogenesis [26] (Figure 1).
Figure 1:
Effect of Arachidonic acid (AA) and its metabolite prostaglandin E2 (PGE2) on cell signaling in human prostate cells
In contrast to the n-6 polyunsaturated fatty acids, n-3 long-chain fatty acids protect against PC development. For example, a significant decrease in the growth of PC xenografts occurs in nude mice fed with a diet containing high levels of eicosapentaenoic and docosahexaenoic acids [27]. These effects have been supported by in vitro studies where both eicosapentaenoic acid and docosahexaenoic acid inhibit the proliferation of human PC cell lines [19, 28]. Moreover, eicosapentaenoic acid and docosahexaenoic acid prevent the progression of human prostate cells toward an aggressive androgen-independent phenotype. At the molecular level, eicosapentaenoic and docosahexaenoic acid treatments inhibit the PI3K/Akt signaling pathway and decrease expression of the androgen receptor (AR), a master regulator of prostate cell proliferation and PC development [29].
Inflammation
Inflammation often occurs in the prostates of aging men, and plays a critical role in the development of benign prostatic hyperplasia and PC incidence [30-32]. Androgen levels, genetic predisposition, obesity, and a high-fat diet are associated with prostatic hyperplasia and PC [33].
The association between a high-fat diet, the induction of inflammation, and PC markers have been reported in several in vivo studies. Prostatic inflammation correlates with cell proliferation and an increase in prostate gland size of mice consuming a high-fat diet [34, 35]. Consumption of a high-fat diet also elevates ataxin levels in the adipose tissue, leading to a significant increase in the production of lysophosphatidic acid, which can act directly on the prostate and induce hyperplasia and cell proliferation [36].
While inflammation is associated with an enhancement of PC development, there is not a clear understanding of the mechanisms involved in this effect. Several studies have reported the involvement of immune cells and the production of pro-inflammatory cytokines. In clinical studies, patients with benign prostatic hyperplasia contain infiltrates of macrophages, T-lymphocytes, and B-lymphocytes that are chronically activated [37]. These infiltrating cells produce cytokines including IL-2, IFN-γ IL-6, IL-8, IL-17, and TGF-β that maintain a chronic immune response and induce persistent intra-prostatic inflammation and fibromuscular growth by an autocrine or paracrine effect [38, 39]. These pro-inflammatory cytokines can modulate prostate growth in mice fed a high-fat diet [40] and correlate with the production of pro-inflammatory cytokines such as IL-1α, IL-1β, IL-6, IL-17 and TNF-α [41, 42]. These cytokines can induce prostate growth through induction of secondary mediators such as Cox-2. For example, IL-17 serves to stabilize and increase the enzymatic activity of Cox-2 [43]. Of note, the induction of Cox-2 expression in the prostate epithelium is associated with increased cell proliferation and apoptotic resistance [44]. Furthermore, the treatment of human prostate cells in vitro with serum obtained from obese mice containing elevated levels of pro-inflammatory cytokines promotes cell proliferation, invasion, migration and, epithelial-mesenchymal transition [45].
Oxidative stress
A disproportionate generation of reactive oxygen species (ROS) causes tissue injury, DNA damage, and post-translational DNA modifications, which can lead to neoplasia in the human prostate [46, 47]. ROS are generated from the mitochondrial respiratory chain, an uncontrolled arachidonic acid cascade, and NADPH oxidase [33]. The expression of NADPH oxidase subunits such as gp91phox, p47phox, and p22phox is increased in the prostates of mice fed a high-fat diet, [34]. Also of note, human PC cells harbor an increased level of ROS compared to normal prostate cells [48]. The ROS activity correlates with dysregulation of the NADPH oxidase system, which is a critical event for the malignant phenotype of human PC cells [47, 49-52].
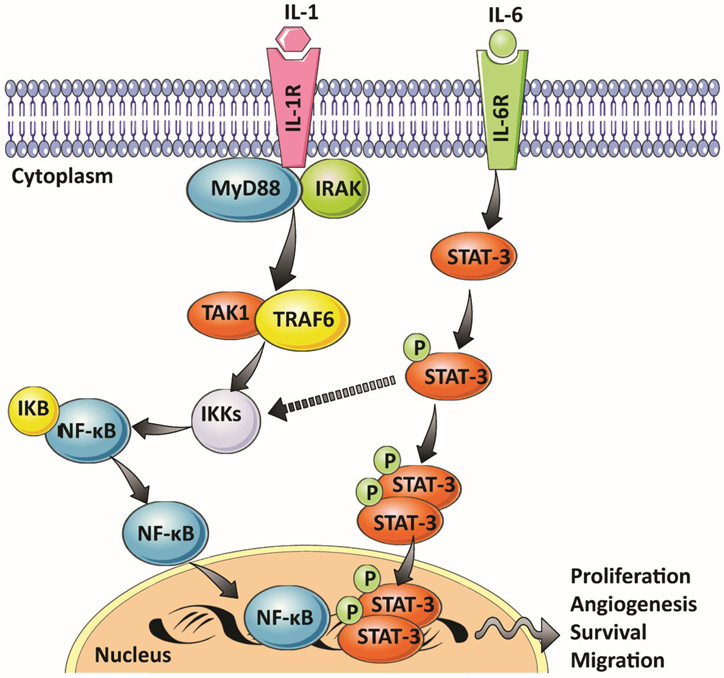
At the molecular level, continual oxidative stress leads to the activation of two critical signaling pathways: the signal transducer and activator of transcription (STAT-3) and NF-κB pathways [33]. The activation of both cascades leads to the expression of transcription factors required for regulating genes involved in proliferation, survival, angiogenesis, invasion, and inflammation [53] (Figure 2).
Figure 2:
Effect of pro-inflammatory cytokines such as IL-6 and IL-1 on the activation and the cross talk of STAT-3 and NF-κB pathways
A high-fat diet also leads to increased activation of NF-κB in many organs of mice, including prostate [54]. In humans, there is constitutive activation of NF-κB in prostate adenocarcinoma [55]. Moreover, this constitutive activation is associated with upregulation of pro-survival molecules including Bcl-2, Bcl-XL, and Mcl-1 [56]. Similarly, increased STAT-3 activation and its DNA binding occur in the prostate of mice fed a high-fat diet [57]. In human PC cells, the inhibition of STAT-3 results in the inhibition of cell proliferation and a significant decrease in cell viability [58].
Dairy products
Several epidemiological studies have reported that frequent intake of high-fat dairy products is associated with an increased risk of developing PC [59-61]; however, other studies failed to observe this association [62, 63]. The role of high-dairy fat intake in PC risk is supported by studies conducted in vitro where milk modulated and promoted the proliferation of the human prostate LNCaP and PC-3 cancer cell lines [64, 65]. Saturated fat intake, high-calcium intake, decreased circulating levels of 1,25-dihydroxy-vitamin D (the active form of vitamin D), and increasing levels of insulin-like growth factor-1 (IGF-1) are several potential mechanisms by which milk and dairy product intake may impact the incidence and the progression of PC.
Saturated fat intake
Saturated fat is another likely factor in dairy products that may influence the development and the aggressiveness of PC. A higher intake of low-fat milk is associated with a greater risk of non-aggressive PC, whereas whole-fat milk intake is frequently associated with a higher incidence of aggressive PC phenotypes [63, 66-69].
High-calcium intake
Intake of calcium above the recommended daily doses (~1000 mg/day) is associated with increased risk of developing PC but also with aggressive and highly malignant PC [70-75]. The underlying mechanisms of high calcium intake and the risk of PC are not yet elucidated. Over-activation of the calcium-sensing receptor and calcium-dependent voltage-gated channel expressed in human prostate cells by the high levels of ionized calcium circulating in the bloodstream are two potential mechanisms involved in PC etiology [76-79]. The stimulation of these receptors by extracellular calcium increases PC cell proliferation, apoptosis resistance, and metastatic potential in vitro and in vivo [80-83].
Vitamin D
Several epidemiological studies have reported an association between low levels of vitamin D and higher risk for PC [84-86]. The modulation of vitamin D metabolism and the decrease of 1,25-dihydroxyvitamin D levels are associated with an increased risk of PC [87]. Once ingested, vitamin D is metabolized to its biologically active form 1,25-dihydroxyvitamin D through a two-step oxidation. The first oxidation reaction catalyzed by CYP2R1 occurs in the liver leading to the formation of 25-hydroxyvitamin D, and the second oxidation catalyzed by CYP27B1 occurs in the kidney, producing the 1,25-dihydroxyvitamin D [88]. 1,25-Dihydroxy vitamin D has anti-proliferative effects that are driven through the nuclear Vitamin D receptor (VDR) pathway, leading to the expression of genes involved in cell cycle arrest, cell apoptosis and differentiation [89]. VDR is expressed in both normal and cancer prostate cells [90-92]. In human prostate cells, 1,25-dihydroxyvitamin D produces anti-proliferative effects [92-96], reduces oxidative stress [97], and up-regulates pro-apoptotic genes [98]. More than 2000 genes are modulated by 1,25-dihydroxy vitamin D, including genes encoding for androgen metabolism [99]. More detailed studies are required to elucidate the critical roles of vitamin D in PC development.
IGF-1
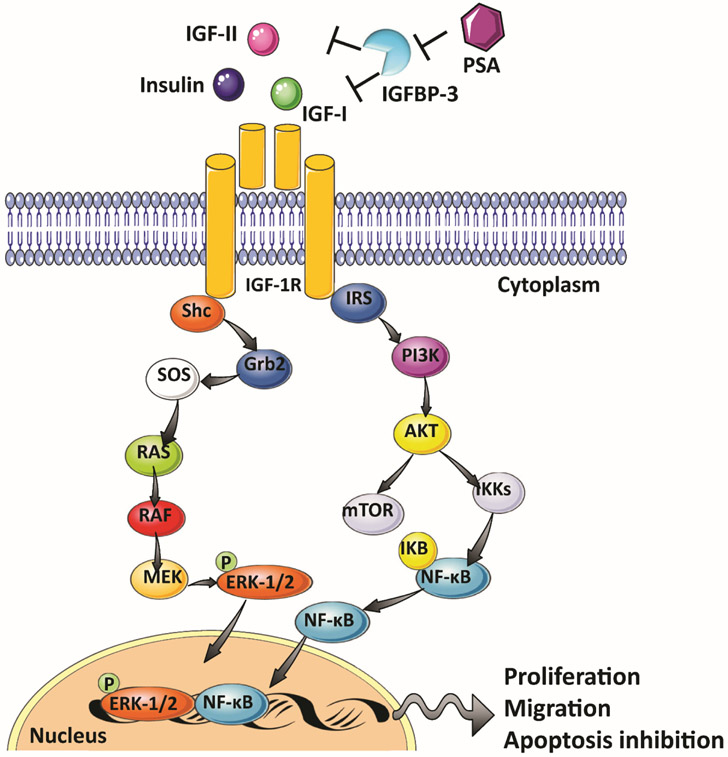
The IGF system includes three ligands (insulin, IGF-1, IGF-2), their receptors (insulin receptor (INSR), IGF-1 receptor (IGF-1R), the mannose 6-phosphate receptor (M6P/IGF-2R), and six circulating IGF-binding proteins (IGFBP1–6) [100]. In the human prostate, every element of this system is expressed in normal, hyperplastic, and neoplastic prostate tissues, as well as in primary and prostate cell lines [101-110]. The IGF system plays a critical role in normal gland growth and development of the prostate [104, 108, 111, 112]. A higher serum IGF-1 concentration is correlated with an increased risk of PC [113-119]. The biological functions of IGF-I are mediated primarily through the IGF-IR, a tyrosine kinase transmembrane receptor that binds IGF-I with higher affinity than IGF-II [120]. Interestingly, inhibition of IGF-1R is associated with decreased androgen-dependent and androgen-independent growth in vitro as well as a suppression of in vivo tumor growth and PC cell invasiveness [108, 121-123]. Conversely, activation of IGF-1R by its ligand IGF-1 leads to the activation of several signaling pathways including mitogen-activated protein kinase (MAPK) and PI3K/Akt [124]. The activation of these signaling pathways induces proliferation and migration, and inhibits apoptosis in the PC cell [125-128] (Figure 3).
Figure 3:
Insulin/IGF signaling in prostate carcinogenesis.
The IGFBPs provide an additional, extracellular mechanism to regulate IGF activity. The IGFBPs bind to IGF-1 and IGF-2 with high affinity and thereby diminish their binding to IGF-R, resulting in the inhibition of the IGF signaling pathway [100]. Altered IGFBP plasma levels are found in PC patients, and a decrease in IGFBP-3 is associated with higher risk and progression of PC [113, 114, 129, 130]. IGFBP-3 is a substrate for the serum protease PSA [104]. Therefore, high levels of PSA in PC patients may result in a decrease in circulating levels of IGFBP-3 by proteolytic cleavage, leading to an increase in IGFs including IGF-1, thus facilitating disease progression (Figure 3).
Alcohol
Alcohol consumption accounts for about 5% of all cancer deaths worldwide [131]. In the USA, 92% of adult males self-report a long-term use of alcohol [132], and up to 3.7% of total cancer deaths are linked to alcohol [133]. Elevated alcohol consumption can contribute to a number of malignancies, including cancer of the oral cavity, pharynx, larynx, esophagus, and liver of both sexes and colorectal cancer in women. However, findings from epidemiological studies on the role of alcohol consumption in PC risk are inconsistent. Several studies found that alcohol consumption is a risk factor for PC [134-136], whereas other studies reported a decreased risk of PC [137]. The compilation of meta-analyses are also inconsistent: several reports found no association between alcohol consumption and PC risk [138-140], whereas others reported a significantly increased risk of PC with alcohol [141-144]. One meta-analysis reported an increased risk for PC for men drinking more than 50 g of alcohol per day, with the risk becoming slightly higher for men who consume more than 100 g per day [141]. Three other meta-analyses also reported a significantly increased risk in PC for light and moderate drinking (one to four drinks per day) [142, 143] or the equivalent of up 24 g of alcohol per day [144]. An association between alcohol intake and the degree of aggressiveness of PC was reported in some studies [145-148], but not other studies [135, 149, 150].
Ethanol is the primary form of alcohol in alcoholic beverages. Ethanol is classified as a human carcinogen [151]. The genotoxic effects and carcinogenicity of ethanol are thought to be driven by its major metabolite, acetaldehyde. Acetaldehyde forms DNA adducts in human cells in vitro and in vitro [152]. In humans, levels of acetaldehyde DNA adducts present in lymphocytes are seven times higher in alcohol users compared non-users [153]. In vivo studies demonstrated that ethanol is efficiently bioactivated into acetaldehyde in rat prostate by different enzymatic pathways involving xanthine oxidoreductase and cytochrome P450 2E1 [154, 155]. Moreover, acetaldehyde formation is linked to an increase in prostate epithelial cell death and ultrastructural alterations in epithelial cells including chromatin condensation around the perinuclear membrane and endoplasmic reticulum dilatation, an ultra-structural marker of endoplasmic reticulum stress [156]. The rat prostate lacks alcohol dehydrogenase and aldehyde dehydrogenase activities, resulting in an accumulation of acetaldehyde and thus, an increase in genomic damage in the prostate of rats exposed to ethanol [156]. Chronic ethanol exposure also leads to oxidative stress and a diminution in the antioxidant defense system in the rat ventral prostate [156, 157].
An association of PC risk with cancer aggressiveness was observed with high intake of beer [145, 146, 148]; while modest protective effects were observed for red wine consumption in some but not all studies [158-160]. The protective effect of wine, especially red wine, is likely attributed to its high contents of polyphenols such as flavonoids and resveratrol [161]. Polyphenolic compounds harbor antioxidant and anti-androgenic activities and therefore are thought to act as anti-carcinogens [162]. In vitro, nanomolar concentrations polyphenols inhibit cell growth in a dose and time-dependent manner in both androgen-dependent LNCaP and androgen-independent DU145 and PC3 PC cell lines. Treatment of LNCaP and PC3 cells with flavonoids, including catechin, epicatechin, and quercetin, inhibites cell proliferation, whereas resveratrol is the most potent inhibitor of DU145 cell growth. The proposed mechanism for the antiproliferative effect of polyphenols is through the modulation of NO production [163].
Red and processed meat
Many epidemiological studies have focused on the role of red and processed meats in PC risk. Some meta-analyses report an elevated risk for PC with frequent consumption of meats, whereas other studies failed to find an overall effect on risk [164, 165]. It is hypothesized that DNA damaging agents, including heme iron, N-nitroso compounds (NOCs) formed in processed meats [166], polycyclic aromatic hydrocarbons (PAHs) formed in smoked meats and meats cooked under flame [167], and heterocyclic aromatic amines (HAAs) formed in well-done grilled meats [168, 169], contribute to PC risk. Of note, the risk of PC for African American men is ~2-fold greater than for Caucasians [2]. One paradigm proposed for the increased risk of PC in African American men is based on their preference for frequent consumption of well-done cooked meats containing the HAA 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). PhIP is a rodent prostate carcinogen and potential human prostate carcinogen [170, 171], and may explain the higher risk of PC for African-American men compared to white men [172].
Heme iron
Once ingested, heme-containing proteins such as myoglobin or hemoglobin are hydrolyzed to peptides, amino acids, and heme iron. Feeding ferriheme to rodents induces cytotoxicity, enhances cell proliferation of colonic mucosa, promotes oxidative stress, and thus, may contribute to colorectal cancer [173]. Heme iron is transported through the bloodstream to all organs of the body and can catalyze oxidative reactions, causing DNA, protein and lipid oxidation in multiple organs including the prostate [174]. The overall level of free radical damage induced by heme-catalyzed oxidation is estimated to be comparable to that resulting from ionizing radiation [174]. Two epidemiological studies have examined the role of heme iron in PC development [175, 176]. One reported a positive association between total heme iron intake and advanced PC risk, whereas another reported no associations of the dietary factors with PC risk irrespective of stage or grade. Thus, additional studies are necessary to evaluate any potential associations between heme iron consumption and PC.
N-Nitroso compounds (NOCs)
Carcinogenic NOCs include two chemical classes, N-nitrosamines and N-nitrosamides, formed by the reaction of nitrosating agents derived from nitrite with amines and amides respectively [177]. Nitrites added to processed meat serve as anti-bacterial agents as well as curing agents, and they produce the characteristic red-pink color of cured meats. However, nitrites also react with amines in processed meats to produce dietary sources of NOCs. Moreover, the consumption of processed meat is a significant dietary source of nitrite, secondary amines, and amides, which can undergo nitrosation to form NOCs within the gastrointestinal tract [177-179]. The ingestion of heme contained in red meat can stimulate the endogenous formation of NOCs in the digestive tract [180-182]. More than 300 NOCs have been detected in 39 different animal species, including six species of nonhuman primates. Of these, 85% of N-nitrosamines and 92% of N-nitrosamides were reported to induce cancer in multiple organs including liver, lung, esophagus, bladder, and pancreas [183]. NOCs or their metabolites alkylate DNA. While N-nitrosamides react spontaneously with DNA, N-nitrosamines require metabolism by cytochromes, such as by cytochrome P450 2E1, which is expressed in the gastrointestinal tract [178]. Among the different types of DNA adducts formed with NOCs, the alkylation of the O6-position of guanine is a primary lesion that induces G to A transitions [184-186]. The majority of epidemiological studies have focused on the role of NOCs in gastric, esophageal, and colorectal cancers [187-192]. Two epidemiology studies studied the etiology of NOCs and PC risk: there was no significant association between dietary NOCs and risk of development of PC in either study [176, 192]. Thus, further studies on the role of processed meats and NOCs in PC risk are warranted.
Polycyclic aromatic hydrocarbons (PAHs)
PAHs constitute a broad class of compounds that have two or more fused aromatic rings. PAHs arise by the incomplete combustion or high-temperature pyrolysis of organic materials [193]. PAHs are ubiquitous environmental pollutants that occur as complex mixtures but never as individual components [194]. Several PAHs are classified as human carcinogens by the World Health Organization [151, 193]. Apart from occupational exposure, such as the case of coke-oven workers [195-197], the general population is exposed primarily to PAHs from dietary sources [167] and cigarette smoke [198]. The preparation of meats, mainly by a direct open flame, results in pyrolysis of the fat drippings, leading to the formation of PAHs, which are deposited through the smoke particulates on the surface of the grilled meats [199, 200]. Various PAHs occur in some charcoal-broiled, grilled, and smoked meats [194, 201-203]. The estimates of the daily dietary intake for the general population are imprecise and range widely: levels of total daily PAH intake range from 3.7 μg up to 17 μg [167], The most well-studied PAH is benzo[a]pyrene (B[a]P). B[a]P occurs in some grilled meats at levels up to ~ 4 ng/g [204, 205].
The carcinogenic properties of PAHs are attributed to their ability to form mutation-prone DNA adducts [193]. PAHs undergo metabolism by cytochrome P450 enzymes to form reactive dihydrodiol epoxides, which react with DNA to form covalent adducts, leading to mutations [206]. B[a]P-DNA adducts are formed in human prostate cells in vitro after exposure to B[a]P [207-209], B[a]P treatment also leads to an increase in DNA double-strand breaks when measured by the comet assay [208, 210]. PAH adducts, including B[a]P adducts, are frequently detected in human prostate tissues by immunohistochemistry (IHC)-[211-215] with an antibody, which was raised against B[a]P-modified DNA, but which also cross-reacted with DNA adducts of at least five other PAHs [216]. Levels of PAH-DNA adducts is higher in adjacent human non-tumor prostate tissue compared with prostate tumor tissue, possibly due a higher cell proliferation rate in the tumor [211, 213, 217]. The occurrence of putative PAH-DNA adducts is associated with a higher risk for PC and cancer recurrence after prostatectomy within one to two years after surgery [211]. This risk was prominent in patients younger than 60 years old, patients with advanced-stage disease, and African Americans patients [211]. However, these data should be interpreted with caution since IHC is not a specific method of DNA adduct detection, even for assays performed with monoclonal antibodies, where possible cross-reactivity of the antibodies with other DNA adducts or endogenous cellular components can lead to false positivity. The occurrence of DNA adducts of B[a]P was not confirmed in one cohort of PC patients when analyzed by liquid chromatography/mass spectrometry (LC/MS), a more specific analytical method than IHC [218]. Thus, there is a critical need to characterize DNA adducts on the same specimens by IHC and LC/MS to determine the validity of the analyses.
Heterocyclic aromatic amines (HAAs) and PC
HAA formation and sources of exposure
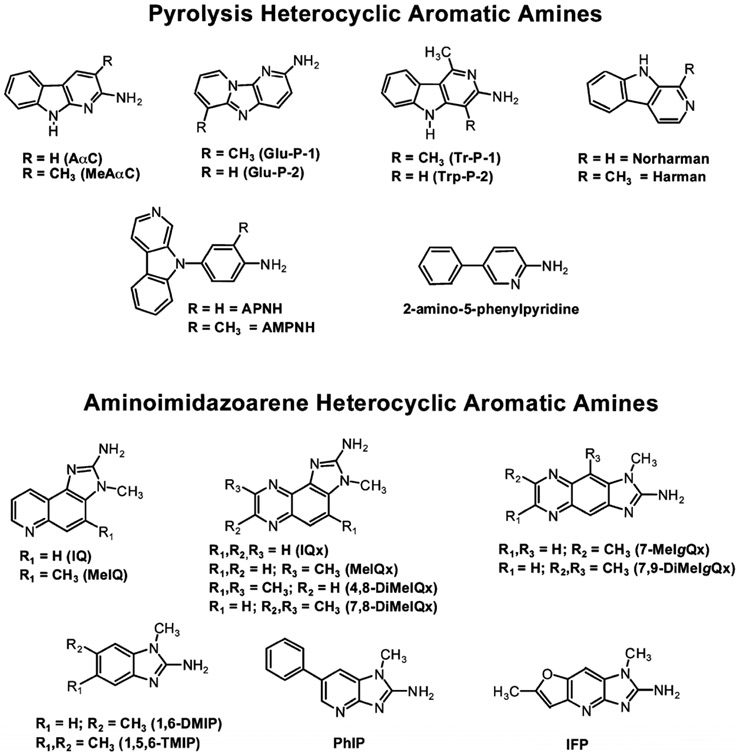
HAAs are a class of more than 25 genotoxic chemicals known to form in cooked meats, fish, poultry, and tobacco smoke [168, 169, 219]. HAAs are sub-classified into the aminoimidazoarenes (AIAs) and the high-temperature pyrolytic HAAs (Figure 4). AIAs contain the N-methyl-2-aminoimidazole moiety derived from creatinine in muscle tissue. The AIAs form in meats, fish, and poultry cooked at temperatures above 150 °C and arise through the reaction of pyridine or pyrazines, derived from Strecker reactions, and condensation with creatine [220, 221]. Pyrolytic HAAs form by high-temperature pyrolysis ( >250 °C) of protein or amino acids, such as glutamic and tryptophan. Pyrolytic HAAs occur when proteinaceous foods are heated at temperatures generally above 250 °C [168, 222, 223]. Several HAAs are also formed in tobacco smoke [223, 224]. 2-Amino-9H-pyrido[2,3-b]indole (AαC), a pyrolysis product of tryptophan, is the major carcinogenic HAA formed in combusted tobacco and occurs in mainstream tobacco smoke at levels up to 258 ng per cigarette [225-227]. Unexpectedly, PhIP, an AIA containing the N-methyl-2-aminoimidazole moiety of creatine, was detected in tobacco smoke [224]; however, the mechanism of PhIP formation in tobacco smoke has not been determined. The principal sources of exposure to most HAAs occur through the consumption of well-done cooked meats and poultry [168, 228]. HAA formation in cooked meats generally occurs at the low parts-per-billion (ppb) range, but the levels of some HAAs can approach several hundred ppb in well-done cooked meats or poultry [168, 219, 229, 230]. PhIP and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) are often the most prevalent HAAs formed in cooked meats and poultry [219, 221, 230-233]. The average dietary HAAs intake ranges from less than 2 to up to 25 ng/kg per day [172, 234].
Figure 4:
Chemical structures of prevalent HAAs in cooked meat.
Bioactivation and Formation of DNA Adducts
HAAs undergo extensive metabolism by hepatic cytochrome P450 1A2 (CYP 1A2)-catalyzed N-oxidation of the exocyclic amine groups, leading to the formation of N-hydroxy-HAAs, as reactive intermediates [228, 235, 236]. CYP 1A1 and CYP 1B1 catalyze this reaction in extrahepatic tissues [228, 235]. The N-hydroxy-HAAs are further bioactivated by acetylation or sulfation catalyzed by N-acetyltransferases (NATs) or sulfotransferases (SULTs), respectively, either in the liver or extrahepatic tissues [228]. These unstable esters react with DNA to form DNA adducts [228, 237]. HAA-DNA adducts are mainly formed at C-8 on deoxyguanosine (dG) through the exocyclic amine of the HAAs, to produce dG-C8-HAA adducts as the major DNA adducts [238, 239].
Carcinogenesis of HAAs
HAAs are multisite carcinogens in rodents and induce cancers of the oral cavity, liver, stomach, colon, pancreas, and the mammary gland in females [168, 170]. Notably, PhIP is the only HAA reported to induce PC in rodents [168, 170] Carcinogenesis studies in rodents used chronic doses of HAAs ranging between 0.1 to 64 mg/kg/day to induce tumors [168, 228]. These doses are more a million-fold higher than the daily intake of HAAs. Thus, one might surmise that the levels of human exposure are too low to contribute to human cancers. However, a linear relationship between HAA dose and HAA-DNA adduct formation occurs in rodent tissues for PhIP, MeIQx, and IQ [240-242], signifying mutation-prone DNA adducts of HAAs can still form in tissues at dosing regimens approaching human exposure levels.
Animal toxicity studies may underestimate the carcinogenic potential of HAAs in humans. For example, the levels of HAA-DNA adducts formed in primary human hepatocytes are significantly higher than those formed in primary rat hepatocytes, under the same doses and times of exposure [243]. Human CYP1A2, which is principally expressed in the liver, is the major CYP involved in the metabolism of many HAAs. Human CYP1A2 is catalytically more efficient than the rat homolog in the bioactivation of PhIP and MeIQx, and perhaps other HAAs [244]. Human CYP1A2 and human liver microsomes preferentially bioactivate HAAs through N-oxidation of the exocyclic amine group. In contrast, rat CYP1A2 and rat liver microsomes preferentially catalyze the detoxication of HAAs by oxidation of the heterocyclic rings [245]. The superior activity (lower Km and higher kcat) and ability of human CYP1A2 to catalyze N-oxidation can explain the higher levels of HAA adducts formed in human compared to rat hepatocytes. Several HAA-DNA adducts have been detected in human tissues by various techniques, indicating that even at low levels of exposure, HAAs can form DNA adducts in humans [246-256].
PhIP DNA Damage, Mutation, and Carcinogenicity in Prostate Rodent Studies
PhIP is the only HAA studied thus far that targets the prostate as a principal site for DNA adduct formation and carcinogenesis in rodents [168]. PhIP undergoes metabolism to form high levels of DNA adducts in the prostate of Wistar and Fischer 344 rats [170, 257-260] and induces high levels of mutations in the prostate of the Big Blue lacI transgenic rat [260, 261]. PhIP is a prostate carcinogen in the Fischer 344 rat [170]. PhIP also induces prostate tumors in CYP1A-humanized (hCYP1A) mice but not in wild-type mice [262]. This finding reinforces the concept that human CYP1A is superior to the rodent orthologue in the bioactivation of PhIP [244, 263].
Extensive inflammation occurs in the dorsolateral prostate lobe marked by CD45+ mononuclear leukocyte and CD8+ T lymphocyte infiltration in PhIP-induced tumors in the prostates of hCYP1A mice [262]. This inflammation is associated with atrophic glands, high-grade prostatic intraepithelial neoplasia, and oxidative stress [262, 264]. In contrast, the prostatic intraepithelial neoplasia lesions are significantly less severe and infrequently associated with inflammation and oxidative stress in the ventral prostate glands [262]. These observations are noteworthy because the dorsolateral prostate is homologous to the human peripheral prostate zone, the most common site of PC development in humans [265]. Similarly, PhIP treatment leads to inflammation with a marked increase in mastocyte and macrophage infiltration and glandular atrophy of the prostate of Fischer 344 rats [261, 266]. These pathologies induced by PhIP in rodent models of PC are significant because inflammation, oxidative stress, and glandular atrophy are common features in the pathology of human PC [267].
Several key features of cancer biology often reported in human PC occur in PhIP-induced prostate tumors in rodents. For example, treatment of hCYP1A mice with PhIP results in a time-dependent increase in expression of AR protein in prostate tumor epithelial cells [262]. An up-regulation of AR leading to a higher rate of cell proliferation occurs in human PC [268]. Furthermore, PhIP-induced tumors in h-CYP1A mice display significant decreases in levels of E-Cadherin and p63 expression [262]. E-Cadherin is an epithelial cell adhesion molecule involved in the maintenance of normal cell architecture, while the p63 transcription factor has multiple functions in cancer cell biology. PhIP-treatment in Fisher 344 and Big Blue rats also results in significant increases in the levels of Ki-67, a well-established marker of cell proliferation, in the intraepithelial neoplasia regions of the prostate [261, 266]. Dysregulated expression and distribution of these proteins are hallmarks of epithelial malignancies and serve as major diagnostic criteria for human PC [269-271].
PhIP-induced tumors in the h-CYP1A mice also exhibit increased levels of oxidative stress markers, including 8-oxo-dG and nitrotyrosine, markers of oxidative DNA damage and reactive nitrogen species. PhIP treatment results in the up-regulation of COX-2 expression, a cyclooxygenase that catalyzes the formation of pro-inflammatory prostaglandins and a loss of Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) a key transcription factor responsible of the expression of many cytoprotective proteins [264]. Oxidative stress is a key contributor to the development of PC in humans [46, 272]. The PTEN/PI3K/Akt signaling pathway is another critical feature of cell proliferation, cell cycle progression, and survival. Activation of AKT in response to oxidative stress and the loss of PTEN are critical events in human PC progression [273-275]. PhIP-induced prostate tumors in h-CYP1A mice display a significant decrease in PTEN expression and an elevation of phospho-AKT, leading to cell proliferation [264].
These mechanistic data in rodent models reinforce the biological plausibility that PhIP plays an important role in dietary-linked human PC. However, the biological events observed in rodents occurred at very high doses of PhIP treatment - up to 200 mg/kg. Humans are exposed to one million-fold or lower daily amounts of PhIP [172], and the capacity of such lower concentrations of PhIP to induce similar biological effects has not been investigated. Therefore, the interpretation of the carcinogenic effects of PhIP in PC of rodents and their extrapolation to human PC should be done with caution.
b. Human studies
PhIP is the only HAA reported thus far to form DNA adducts in the prostate of human PC patients [218, 255, 276-278] (Figure 5). This biomarker data provides support for some of the epidemiological studies that have linked the frequent consumption of well-done cooked red meat containing PhIP with increased risk of PC. [279-281] However, other investigations have failed to find an association between cooked red meat and increased PC risk [175, 282, 283]. The concentrations of PhIP and other HAAs can vary by more than 100-fold in cooked meats [169, 219]. There is a critical need to conduct such epidemiological studies with more precise exposure measurements of HAAs.
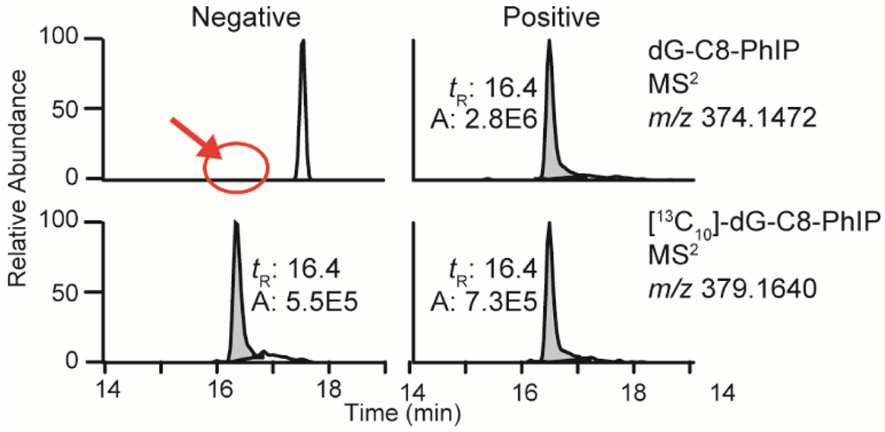
Figure 5:
Representative chromatograms at the MS2 scan stage of human prostate samples that were negative and positive for dG-C8-PhIP.
The frequency of detection and the levels of PhIP-DNA adducts in human prostate range widely between studies, depending on the analytical method of adduct measurement. For example, using a high-resolution LC/MS method, PhIP-DNA adducts were detected in 13 out of 54 PC patients with levels ranging from 2 to 120 adducts per 109 DNA bases [218, 278]. However, PhIP-DNA adducts were detected in a very high percentage of prostate tissues in another cohort, occurring at levels exceeding several adducts per 107 DNA bases, when measured by IHC [255, 276, 277]. These discrepancies in adduct measurements may imply a high level of false positivity obtained by IHC, possibly due to cross-reactivity of the polyclonal antibodies raised against PhIP-modified DNA with other DNA adducts or endogenous cellular components [284].
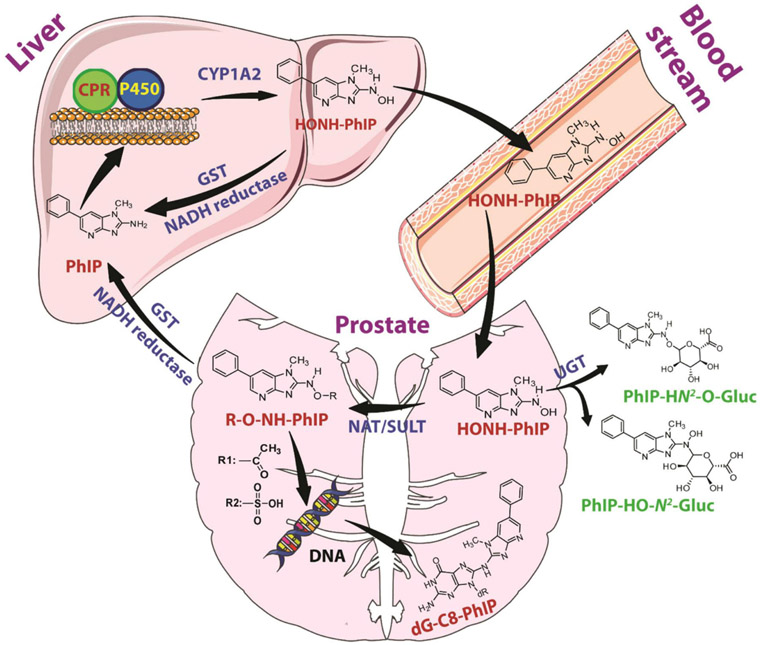
Cytotoxicity and DNA adduct formation induced by PhIP and other HAAs has been studied in primary and PC cell lines. The parent HAAs, PhIP, MeIQx, IQ and AαC were not toxic at doses up to 10 μM and formed low levels of DNA adducts in LNCaP cells [285]. However, HONH-PhIP, the genotoxic metabolite of PhIP, induced a dose-dependent increase in cytotoxicity, whereas HONH-MeIQx, HONH-IQ, and HONH-AαC were not toxic [285, 286]. Moreover, HONH-PhIP forms DNA adducts at levels that are 20-fold higher than other HONH-HAAs in LNCaP cells [285]. These data suggest that the initial bioactivation step of PhIP to form HONH-PhIP occurs in the liver through CYP 1A2-catalyzed N-oxidation, followed by systemic circulation to reach the prostate, where bioactivation is mediated by Phase II enzymes (Figure 6) [285]. Similar data were reported in primary human prostate epithelial cells, where HONH-PhIP formed 50- to 100-fold higher levels of DNA adducts than IQ, MeIQx, and HONH-MeIQx [207, 287].
Figure 6:
Metabolic activation of PhIP and dG-C8-PhIP formation in human prostate.
HONH-PhIP also induces unscheduled DNA synthesis, and DNA single-strand breaks in primary human prostate epithelial cells at 100-time higher levels than HONH-MeIQx [208, 210]. The higher susceptibility of human prostate cells to the DNA-damaging and genotoxic effect of PhIP compared to other prominent HAAs formed in cooked meats recapitulates the DNA adduct biomarker data in prostate tissues of PC patients and provides support for the possible causal role of PhIP in human PC.
PhIP can also act through non-genotoxic mechanisms via androgenic effects to contribute to the development of PC. PhIP binds to the AR and modulates cell proliferation. Using, in silico analysis, binding of PhIP and HONH-PhIP to the AR was found to be comparable to that of the endogenous AR ligand, dihydrotestosterone (DHT), when based on the predicted free energy of binding [288]. Through computational docking studies, both PhIP and HONH-PhIP displayed similar binding modes to DHT and docked with high affinity in the same cavity of the AR ligand binding domain as DHT [288]. Moreover, treatment of the human prostate epithelial cell line LNCaP with PhIP or HONH-PhIP up-regulated AR and PSA expression [288].
PhIP also induces proliferation of human prostate epithelial cells in an AR-independent manner, through the activation of pro-proliferation cell signaling pathways. Low concentrations of PhIP (10−12–10−8 mol/L) increase the proliferation, migration and invasion properties of PC-3, an AR-negative human prostate cell line [289]. Proliferation and migration are mediated through the activation of the ERK signal transduction cascade and a rapid, transient increase in phosphorylation of both MEK1/2 and ERK1/2. Interestingly, mitogenic stimulation with epithelial growth factor (EGF), induces the same pattern of activation [290]. Proliferation, migration, and invasiveness are crucial events in the oncogenic progression of cells [291]. Thus, all these biological phenomena induced by PhIP suggest a carcinogenic potential of PhIP in human prostate. The challenge in risk assessment is to determine if the amounts of PhIP in the diet are sufficient to be a significant risk for PC.
Conclusion
There is growing mechanistic and epidemiological data supporting a role for the diet in the development of PC. Multiple mechanisms and hypotheses have been brought forward for PC risk, ranging from different classes of dietary genotoxicants acting as initiators of PC to different dietary factors involved in tumor promotion. However, the precise roles of specific genotoxicants and nutritional factors in PC remain to be clarified. Prospective epidemiological studies on PC risk with improved assessments of dietary habits, including protective nutrient biomarkers in plasma and urine are needed [292]. The identification of micronutrients that protect against PC [293, 294], and the detection of biomarkers of DNA damage in the prostate, such as DNA adducts, by specific mass spectrometric methods and their linkage to mutations [218, 285], can advance our understanding of the micronutrients and genotoxicants in the diet that impact PC risk.
Acknowledgements
A portion of the research conducted in the Turesky laboratory was supported by R01CA122320.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87–108 [DOI] [PubMed] [Google Scholar]
- 2.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, Morrison H, Sonawane B, Shifflett T, Waters DJ, Timms B (2004) Human prostate cancer risk factors. Cancer 101: 2371–490 [DOI] [PubMed] [Google Scholar]
- 3.Gann PH (2002) Risk factors for prostate cancer. Rev Urol 4 Suppl 5: S3–S10 [PMC free article] [PubMed] [Google Scholar]
- 4.Perdana NR, Mochtar CA, Umbas R, Hamid AR (2016) The Risk Factors of Prostate Cancer and Its Prevention: A Literature Review. Acta Med Indones 48: 228–38 [PubMed] [Google Scholar]
- 5.Pelser C, Mondul AM, Hollenbeck AR, Park Y (2013) Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev 22: 697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CG, Willett WC (1993) A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst 85: 1571–9 [DOI] [PubMed] [Google Scholar]
- 7.West DW, Slattery ML, Robison LM, French TK, Mahoney AW (1991) Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control 2: 85–94 [DOI] [PubMed] [Google Scholar]
- 8.Slattery ML, Schumacher MC, West DW, Robison LM, French TK (1990) Food-consumption trends between adolescent and adult years and subsequent risk of prostate cancer. Am J Clin Nutr 52: 752–7 [DOI] [PubMed] [Google Scholar]
- 9.Lophatananon A, Archer J, Easton D, Pocock R, Dearnaley D, Guy M, Kote-Jarai Z, O'Brien L, Wilkinson RA, Hall AL, Sawyer E, Page E, Liu JF, et al. (2010) Dietary fat and early-onset prostate cancer risk. Br J Nutr 103: 1375–80 [DOI] [PubMed] [Google Scholar]
- 10.Lee MM, Wang RT, Hsing AW, Gu FL, Wang T, Spitz M (1998) Case-control study of diet and prostate cancer in China. Cancer Causes Control 9: 545–52 [DOI] [PubMed] [Google Scholar]
- 11.Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, Jakobsen MU, Johnsen NF, Tjonneland A, Linseisen J, Rohrmann S, Boeing H, Pischon T, Trichopoulou A, et al. (2008) Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 87: 1405–13 [DOI] [PubMed] [Google Scholar]
- 12.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN (2007) Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer 121: 1339–45 [DOI] [PubMed] [Google Scholar]
- 13.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT (1997) A case-control study of diet and prostate cancer. Br J Cancer 76: 678–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghadirian P, Lacroix A, Maisonneuve P, Perret C, Drouin G, Perrault JP, Beland G, Rohan TE, Howe GR (1996) Nutritional factors and prostate cancer: a case-control study of French Canadians in Montreal, Canada. Cancer Causes Control 7: 428–36 [DOI] [PubMed] [Google Scholar]
- 15.Hu MB, Xu H, Zhu WH, Bai PD, Hu JM, Yang T, Jiang HW, Ding Q (2018) High-fat diet-induced adipokine and cytokine alterations promote the progression of prostate cancer in vivo and in vitro. Oncol Lett 15: 1607–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho HJ, Kwon GT, Park H, Song H, Lee KW, Kim JI, Park JH (2015) A high-fat diet containing lard accelerates prostate cancer progression and reduces survival rate in mice: possible contribution of adipose tissue-derived cytokines. Nutrients 7: 2539–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mavropoulos JC, Buschemeyer WC 3rd, Tewari AK, Rokhfeld D, Pollak M, Zhao Y, Febbo PG, Cohen P, Hwang D, Devi G, Demark-Wahnefried W, Westman EC, Peterson BL, et al. (2009) The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila) 2: 557–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llaverias G, Danilo C, Wang Y, Witkiewicz AK, Daumer K, Lisanti MP, Frank PG (2010) A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am J Pathol 177: 3180–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes-Fulford M, Chen Y, Tjandrawinata RR (2001) Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis 22: 701–7 [DOI] [PubMed] [Google Scholar]
- 20.Hughes-Fulford M, Li CF, Boonyaratanakornkit J, Sayyah S (2006) Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res 66: 1427–33 [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Dubois RN (2006) Prostaglandins and cancer. Gut 55: 115–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjandrawinata RR, Dahiya R, Hughes-Fulford M (1997) Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer 75: 1111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Hughes-Fulford M (2000) Prostaglandin E2 and the protein kinase A pathway mediate arachidonic acid induction of c-fos in human prostate cancer cells. Br J Cancer 82: 2000–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeil K, Eder IE, Putz T, Ramoner R, Culig Z, Ueberall F, Bartsch G, Klocker H (2004) Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells. Prostate 58: 259–68 [DOI] [PubMed] [Google Scholar]
- 25.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ (2001) Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology 142: 4795–805 [DOI] [PubMed] [Google Scholar]
- 26.Mendonca MS, Turchan WT, Alpuche ME, Watson CN, Estabrook NC, Chin-Sinex H, Shapiro JB, Imasuen-Williams IE, Rangel G, Gilley DP, Huda N, Crooks PA, Shapiro RH (2017) DMAPT inhibits NF-kappaB activity and increases sensitivity of prostate cancer cells to X-rays in vitro and in tumor xenografts in vivo. Free Radic Biol Med 112: 318–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly JM, Coleman M, Rose DP (1997) Effects of dietary fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutr Cancer 29: 114–9 [DOI] [PubMed] [Google Scholar]
- 28.Rose DP (1997) Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am J Clin Nutr 66: 1513S–22S [DOI] [PubMed] [Google Scholar]
- 29.Friedrichs W, Ruparel SB, Marciniak RA, deGraffenried L (2011) Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr Cancer 63: 771–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciarra A, Mariotti G, Salciccia S, Autran Gomez A, Monti S, Toscano V, Di Silverio F (2008) Prostate growth and inflammation. J Steroid Biochem Mol Biol 108: 254–60 [DOI] [PubMed] [Google Scholar]
- 31.Gurel B, Lucia MS, Thompson IM Jr., Goodman PJ, Tangen CM, Kristal AR, Parnes HL, Hoque A, Lippman SM, Sutcliffe S, Peskoe SB, Drake CG, Nelson WG, et al. (2014) Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 23: 847–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLennan GT, Eisenberg R, Fleshman RL, Taylor JM, Fu P, Resnick MI, Gupta S (2006) The influence of chronic inflammation in prostatic carcinogenesis: a 5-year followup study. J Urol 176: 1012–6 [DOI] [PubMed] [Google Scholar]
- 33.Shankar E, Bhaskaran N, MacLennan GT, Liu G, Daneshgari F, Gupta S (2015) Inflammatory Signaling Involved in High-Fat Diet Induced Prostate Diseases. J Urol Res 2: [PMC free article] [PubMed] [Google Scholar]
- 34.Vykhovanets EV, Shankar E, Vykhovanets OV, Shukla S, Gupta S (2011) High-fat diet increases NF-kappaB signaling in the prostate of reporter mice. Prostate 71: 147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai X, Haleem R, Oram S, Cyriac J, Jiang F, Grayhack JT, Kozlowski JM, Wang Z (2001) High fat diet increases the weight of rat ventral prostate. Prostate 49: 1–8 [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni P, Getzenberg RH (2009) High-fat diet, obesity and prostate disease: the ATX-LPA axis? Nat Clin Pract Urol 6: 128–31 [DOI] [PubMed] [Google Scholar]
- 37.Kramer G, Steiner GE, Handisurya A, Stix U, Haitel A, Knerer B, Gessl A, Lee C, Marberger M (2002) Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate 52: 43–58 [DOI] [PubMed] [Google Scholar]
- 38.Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G, Lee C, Marberger M (2003) Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest 83: 1131–46 [DOI] [PubMed] [Google Scholar]
- 39.Konig JE, Senge T, Allhoff EP, Konig W (2004) Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. Prostate 58: 121–9 [DOI] [PubMed] [Google Scholar]
- 40.St Sauver JL, Jacobsen SJ (2008) Inflammatory Mechanisms Associated with Prostatic Inflammation and Lower Urinary Tract Symptoms. Curr Prostate Rep 6: 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA (2005) Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–9 [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Hu MB, Bai PD, Zhu WH, Liu SH, Hou JY, Xiong ZQ, Ding Q, Jiang HW (2015) Proinflammatory cytokines in prostate cancer development and progression promoted by high-fat diet. Biomed Res Int 2015: 249741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faour WH, Mancini A, He QW, Di Battista JA (2003) T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3'-untranslated region of COX-2 mRNA. J Biol Chem 278: 26897–907 [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Bergh A, Damber JE (2004) Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate 61: 60–72 [DOI] [PubMed] [Google Scholar]
- 45.Price RS, Cavazos DA, De Angel RE, Hursting SD, deGraffenried LA (2012) Obesity-related systemic factors promote an invasive phenotype in prostate cancer cells. Prostate Cancer Prostatic Dis 15: 135–43 [DOI] [PubMed] [Google Scholar]
- 46.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK (2009) Oxidative stress in prostate cancer. Cancer Lett 282: 125–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK (2008) Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68: 1777–85 [DOI] [PubMed] [Google Scholar]
- 48.Szatrowski TP, Nathan CF (1991) Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51: 794–8 [PubMed] [Google Scholar]
- 49.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS (2005) Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 62: 200–7 [DOI] [PubMed] [Google Scholar]
- 50.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, et al. (2003) NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol 285: C353–69 [DOI] [PubMed] [Google Scholar]
- 51.Block K, Ricono JM, Lee DY, Bhandari B, Choudhury GG, Abboud HE, Gorin Y (2006) Arachidonic acid-dependent activation of a p22(phox)-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxid Redox Signal 8: 1497–508 [DOI] [PubMed] [Google Scholar]
- 52.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD (2002) Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A 99: 715–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grivennikov SI, Karin M (2010) Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 21: 11–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, Blomhoff R (2009) Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr 4: 215–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S (2004) Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia 6: 390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catz SD, Johnson JL (2001) Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene 20: 7342–51 [DOI] [PubMed] [Google Scholar]
- 57.Shankar E, Vykhovanets EV, Vykhovanets OV, Maclennan GT, Singh R, Bhaskaran N, Shukla S, Gupta S (2012) High-fat diet activates pro-inflammatory response in the prostate through association of Stat-3 and NF-kappaB. Prostate 72: 233–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gill BS, Kumar S, Navgeet (2016) Evaluating anti-oxidant potential of ganoderic acid A in STAT 3 pathway in prostate cancer. Mol Biol Rep 43: 1411–22 [DOI] [PubMed] [Google Scholar]
- 59.Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, Vatten LJ, Norat T (2015) Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 101: 87–117 [DOI] [PubMed] [Google Scholar]
- 60.Gao X, LaValley MP, Tucker KL (2005) Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 97: 1768–77 [DOI] [PubMed] [Google Scholar]
- 61.Newmark HL, Heaney RP (2010) Dairy products and prostate cancer risk. Nutr Cancer 62: 297–9 [DOI] [PubMed] [Google Scholar]
- 62.Park Y, Mitrou PN, Kipnis V, Hollenbeck A, Schatzkin A, Leitzmann MF (2007) Calcium, dairy foods, and risk of incident and fatal prostate cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 166: 1270–9 [DOI] [PubMed] [Google Scholar]
- 63.Pettersson A, Kasperzyk JL, Kenfield SA, Richman EL, Chan JM, Willett WC, Stampfer MJ, Mucci LA, Giovannucci EL (2012) Milk and dairy consumption among men with prostate cancer and risk of metastases and prostate cancer death. Cancer Epidemiol Biomarkers Prev 21: 428–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SW, Kim JY, Kim YS, Lee SJ, Lee SD, Chung MK (2014) A milk protein, casein, as a proliferation promoting factor in prostate cancer cells. World J Mens Health 32: 76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tate PL, Bibb R, Larcom LL (2011) Milk stimulates growth of prostate cancer cells in culture. Nutr Cancer 63: 1361–6 [DOI] [PubMed] [Google Scholar]
- 66.Song Y, Chavarro JE, Cao Y, Qiu W, Mucci L, Sesso HD, Stampfer MJ, Giovannucci E, Pollak M, Liu S, Ma J (2013) Whole milk intake is associated with prostate cancer-specific mortality among U.S. male physicians. J Nutr 143: 189–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu W, Chen H, Niu Y, Wu H, Xia D, Wu Y (2016) Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr J 15: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tat D, Kenfield SA, Cowan JE, Broering JM, Carroll PR, Van Blarigan EL, Chan JM (2018) Milk and other dairy foods in relation to prostate cancer recurrence: Data from the cancer of the prostate strategic urologic research endeavor (CaPSURE). Prostate 78: 32–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang M, Kenfield SA, Van Blarigan EL, Wilson KM, Batista JL, Sesso HD, Ma J, Stampfer MJ, Chavarro JE (2015) Dairy intake after prostate cancer diagnosis in relation to disease-specific and total mortality. Int J Cancer 137: 2462–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan JM, Giovannucci E, Andersson SO, Yuen J, Adami HO, Wolk A (1998) Dairy products, calcium, phosphorous, vitamin D, and risk of prostate cancer (Sweden). Cancer Causes Control 9: 559–66 [DOI] [PubMed] [Google Scholar]
- 71.Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL (2001) Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. Am J Clin Nutr 74: 549–54 [DOI] [PubMed] [Google Scholar]
- 72.Giovannucci E, Rimm EB, Wolk A, Ascherio A, Stampfer MJ, Colditz GA, Willett WC (1998) Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res 58: 442–7 [PubMed] [Google Scholar]
- 73.Kesse E, Bertrais S, Astorg P, Jaouen A, Arnault N, Galan P, Hercberg S (2006) Dairy products, calcium and phosphorus intake, and the risk of prostate cancer: results of the French prospective SU.VI.MAX (Supplementation en Vitamines et Mineraux Antioxydants) study. Br J Nutr 95: 539–45 [DOI] [PubMed] [Google Scholar]
- 74.Mitrou PN, Albanes D, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Leitzmann MF (2007) A prospective study of dietary calcium, dairy products and prostate cancer risk (Finland). Int J Cancer 120: 2466–73 [DOI] [PubMed] [Google Scholar]
- 75.Wilson KM, Shui IM, Mucci LA, Giovannucci E (2015) Calcium and phosphorus intake and prostate cancer risk: a 24-y follow-up study. Am J Clin Nutr 101: 173–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prevarskaya N, Skryma R, Bidaux G, Flourakis M, Shuba Y (2007) Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ 14: 1295–304 [DOI] [PubMed] [Google Scholar]
- 77.Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Brown EM (2001) Ca(2+)-sensing receptor expression and PTHrP secretion in PC-3 human prostate cancer cells. Am J Physiol Endocrinol Metab 281: E1267–74 [DOI] [PubMed] [Google Scholar]
- 78.Yano S, Macleod RJ, Chattopadhyay N, Tfelt-Hansen J, Kifor O, Butters RR, Brown EM (2004) Calcium-sensing receptor activation stimulates parathyroid hormone-related protein secretion in prostate cancer cells: role of epidermal growth factor receptor transactivation. Bone 35: 664–72 [DOI] [PubMed] [Google Scholar]
- 79.Weaver EM, Zamora FJ, Puplampu-Dove YA, Kiessu E, Hearne JL, Martin-Caraballo M (2015) Regulation of T-type calcium channel expression by sodium butyrate in prostate cancer cells. Eur J Pharmacol 749: 20–31 [DOI] [PubMed] [Google Scholar]
- 80.Liao J, Schneider A, Datta NS, McCauley LK (2006) Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res 66: 9065–73 [DOI] [PubMed] [Google Scholar]
- 81.Lin KI, Chattopadhyay N, Bai M, Alvarez R, Dang CV, Baraban JM, Brown EM, Ratan RR (1998) Elevated extracellular calcium can prevent apoptosis via the calcium-sensing receptor. Biochem Biophys Res Commun 249: 325–31 [DOI] [PubMed] [Google Scholar]
- 82.Vaz CV, Rodrigues DB, Socorro S, Maia CJ (2015) Effect of extracellular calcium on regucalcin expression and cell viability in neoplastic and non-neoplastic human prostate cells. Biochim Biophys Acta 1853: 2621–8 [DOI] [PubMed] [Google Scholar]
- 83.Sun Y, Selvaraj S, Varma A, Derry S, Sahmoun AE, Singh BB (2013) Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels. J Biol Chem 288: 255–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, Lin Z (2014) Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. J Cancer Res Clin Oncol 140: 1465–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, Kristal AR, Peters U, Neuhouser ML (2014) Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 23: 1484–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, Schenk JM, Thompson IM, Meyskens FL Jr., Goodman GE, Minasian LM, Parnes HL, Klein EA (2014) Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 23: 1494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonjour JP, Chevalley T, Fardellone P (2007) Calcium intake and vitamin D metabolism and action, in healthy conditions and in prostate cancer. Br J Nutr 97: 611–6 [DOI] [PubMed] [Google Scholar]
- 88.Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21: 319–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvala H, Vienonen A, Tuohimaa P (2002) Antiproliferative action of vitamin D. Vitam Horm 64: 357–406 [DOI] [PubMed] [Google Scholar]
- 90.Kivineva M, Blauer M, Syvala H, Tammela T, Tuohimaa P (1998) Localization of 1,25-dihydroxyvitamin D3 receptor (VDR) expression in human prostate. J Steroid Biochem Mol Biol 66: 121–7 [DOI] [PubMed] [Google Scholar]
- 91.Miller GJ, Stapleton GE, Ferrara JA, Lucia MS, Pfister S, Hedlund TE, Upadhya P (1992) The human prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1 alpha,25-dihydroxyvitamin D3. Cancer Res 52: 515–20 [PubMed] [Google Scholar]
- 92.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D (1994) Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res 54: 805–10 [PubMed] [Google Scholar]
- 93.Miller GJ, Stapleton GE, Hedlund TE, Moffat KA (1995) Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res 1: 997–1003 [PubMed] [Google Scholar]
- 94.Moreno J, Krishnan AV, Feldman D (2005) Molecular mechanisms mediating the anti-proliferative effects of Vitamin D in prostate cancer. J Steroid Biochem Mol Biol 97: 31–6 [DOI] [PubMed] [Google Scholar]
- 95.Skowronski RJ, Peehl DM, Feldman D (1993) Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 132: 1952–60 [DOI] [PubMed] [Google Scholar]
- 96.Zhao XY, Peehl DM, Navone NM, Feldman D (2000) 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology 141: 2548–56 [DOI] [PubMed] [Google Scholar]
- 97.Bao BY, Ting HJ, Hsu JW, Lee YF (2008) Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int J Cancer 122: 2699–706 [DOI] [PubMed] [Google Scholar]
- 98.Guzey M, Kitada S, Reed JC (2002) Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther 1: 667–77 [PubMed] [Google Scholar]
- 99.Trump DL, Aragon-Ching JB (2018) Vitamin D in prostate cancer. Asian J Androl 20: 244–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8: 915–28 [DOI] [PubMed] [Google Scholar]
- 101.Fiorelli G, De Bellis A, Longo A, Giannini S, Natali A, Costantini A, Vannelli GB, Serio M (1991) Insulin-like growth factor-I receptors in human hyperplastic prostate tissue: characterization, tissue localization, and their modulation by chronic treatment with a gonadotropin-releasing hormone analog. J Clin Endocrinol Metab 72: 740–6 [DOI] [PubMed] [Google Scholar]
- 102.Bonnet P, Reiter E, Bruyninx M, Sente B, Dombrowicz D, de Leval J, Closset J, Hennen G (1993) Benign prostatic hyperplasia and normal prostate aging: differences in types I and II 5 alpha-reductase and steroid hormone receptor messenger ribonucleic acid (mRNA) levels, but not in insulin-like growth factor mRNA levels. J Clin Endocrinol Metab 77: 1203–8 [DOI] [PubMed] [Google Scholar]
- 103.Kaicer E, Blat C, Imbenotte J, Troalen F, Cussenot O, Calvo F, Harel L (1993) IGF binding protein-3 secreted by the prostate adenocarcinoma cells (PC-3): differential effect on PC-3 and normal prostate cell growth. Growth Regul 3: 180–9 [PubMed] [Google Scholar]
- 104.Cohen P, Peehl DM, Baker B, Liu F, Hintz RL, Rosenfeld RG (1994) Insulin-like growth factor axis abnormalities in prostatic stromal cells from patients with benign prostatic hyperplasia. J Clin Endocrinol Metab 79: 1410–5 [DOI] [PubMed] [Google Scholar]
- 105.Barni T, Vannelli BG, Sadri R, Pupilli C, Ghiandi P, Rizzo M, Selli C, Serio M, Fiorelli G (1994) Insulin-like growth factor-I (IGF-I) and its binding protein IGFBP-4 in human prostatic hyperplastic tissue: gene expression and its cellular localization. J Clin Endocrinol Metab 78: 778–83 [DOI] [PubMed] [Google Scholar]
- 106.Figueroa JA, Lee AV, Jackson JG, Yee D (1995) Proliferation of cultured human prostate cancer cells is inhibited by insulin-like growth factor (IGF) binding protein-1: evidence for an IGF-II autocrine growth loop. J Clin Endocrinol Metab 80: 3476–82 [DOI] [PubMed] [Google Scholar]
- 107.Figueroa JA, De Raad S, Tadlock L, Speights VO, Rinehart JJ (1998) Differential expression of insulin-like growth factor binding proteins in high versus low Gleason score prostate cancer. J Urol 159: 1379–83 [PubMed] [Google Scholar]
- 108.Pietrzkowski Z, Mulholland G, Gomella L, Jameson BA, Wernicke D, Baserga R (1993) Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Res 53: 1102–6 [PubMed] [Google Scholar]
- 109.Boudon C, Rodier G, Lechevallier E, Mottet N, Barenton B, Sultan C (1996) Secretion of insulin-like growth factors and their binding proteins by human normal and hyperplastic prostatic cells in primary culture. J Clin Endocrinol Metab 81: 612–7 [DOI] [PubMed] [Google Scholar]
- 110.Cohen P, Peehl DM, Lamson G, Rosenfeld RG (1991) Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J Clin Endocrinol Metab 73: 401–7 [DOI] [PubMed] [Google Scholar]
- 111.Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R (1992) Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res 52: 6447–51 [PubMed] [Google Scholar]
- 112.Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG (1992) Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab 75: 1046–53 [DOI] [PubMed] [Google Scholar]
- 113.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M (1998) Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 279: 563–6 [DOI] [PubMed] [Google Scholar]
- 114.Chokkalingam AP, Pollak M, Fillmore CM, Gao YT, Stanczyk FZ, Deng J, Sesterhenn IA, Mostofi FK, Fears TR, Madigan MP, Ziegler RG, Fraumeni JF Jr., Hsing AW (2001) Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev 10: 421–7 [PubMed] [Google Scholar]
- 115.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB, Baltimore Longitudinal Study on A (2000) Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. J Clin Endocrinol Metab 85: 4258–65 [DOI] [PubMed] [Google Scholar]
- 116.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R (2000) Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst 92: 1910–7 [DOI] [PubMed] [Google Scholar]
- 117.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D (1998) Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst 90: 911–5 [DOI] [PubMed] [Google Scholar]
- 118.Shaneyfelt T, Husein R, Bubley G, Mantzoros CS (2000) Hormonal predictors of prostate cancer: a meta-analysis. J Clin Oncol 18: 847–53 [DOI] [PubMed] [Google Scholar]
- 119.Travis RC, Appleby PN, Martin RM, Holly JMP, Albanes D, Black A, Bueno-de-Mesquita HBA, Chan JM, Chen C, Chirlaque MD, Cook MB, Deschasaux M, Donovan JL, et al. (2016) A Meta-analysis of Individual Participant Data Reveals an Association between Circulating Levels of IGF-I and Prostate Cancer Risk. Cancer Res 76: 2288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jones JI, Clemmons DR (1995) Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16: 3–34 [DOI] [PubMed] [Google Scholar]
- 121.Grzmil M, Hemmerlein B, Thelen P, Schweyer S, Burfeind P (2004) Blockade of the type I IGF receptor expression in human prostate cancer cells inhibits proliferation and invasion, up-regulates IGF binding protein-3, and suppresses MMP-2 expression. J Pathol 202: 50–9 [DOI] [PubMed] [Google Scholar]
- 122.Burfeind P, Chernicky CL, Rininsland F, Ilan J, Ilan J (1996) Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci U S A 93: 7263–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu JD, Odman A, Higgins LM, Haugk K, Vessella R, Ludwig DL, Plymate SR (2005) In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res 11: 3065–74 [DOI] [PubMed] [Google Scholar]
- 124.Hartog H, Wesseling J, Boezen HM, van der Graaf WT (2007) The insulin-like growth factor 1 receptor in cancer: old focus, new future. Eur J Cancer 43: 1895–904 [DOI] [PubMed] [Google Scholar]
- 125.Rodriguez-Berriguete G, Fraile B, Martinez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M (2012) MAP Kinases and Prostate Cancer. J Signal Transduct 2012: 169170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liao RS, Ma S, Miao L, Li R, Yin Y, Raj GV (2013) Androgen receptor-mediated non-genomic regulation of prostate cancer cell proliferation. Transl Androl Urol 2: 187–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lim W, Jeong M, Bazer FW, Song G (2017) Coumestrol Inhibits Proliferation and Migration of Prostate Cancer Cells by Regulating AKT, ERK1/2, and JNK MAPK Cell Signaling Cascades. J Cell Physiol 232: 862–71 [DOI] [PubMed] [Google Scholar]
- 128.Majumder PK, Sellers WR (2005) Akt-regulated pathways in prostate cancer. Oncogene 24: 7465–74 [DOI] [PubMed] [Google Scholar]
- 129.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E (2002) Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst 94: 1099–106 [DOI] [PubMed] [Google Scholar]
- 130.Li L, Yu H, Schumacher F, Casey G, Witte JS (2003) Relation of serum insulin-like growth factor-I (IGF-I) and IGF binding protein-3 to risk of prostate cancer (United States). Cancer Causes Control 14: 721–6 [DOI] [PubMed] [Google Scholar]
- 131.Lee YC, Hashibe M (2014) Tobacco, alcohol, and cancer in low and high income countries. Ann Glob Health 80: 378–83 [DOI] [PubMed] [Google Scholar]
- 132.Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Bruffaerts R, de Girolamo G, Gureje O, Huang Y, Karam A, Kostyuchenko S, Lepine JP, et al. (2008) Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med 5: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nelson DE, Jarman DW, Rehm J, Greenfield TK, Rey G, Kerr WC, Miller P, Shield KD, Ye Y, Naimi TS (2013) Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health 103: 641–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sesso HD, Paffenbarger RS Jr., Lee IM (2001) Alcohol consumption and risk of prostate cancer: The Harvard Alumni Health Study. Int J Epidemiol 30: 749–55 [DOI] [PubMed] [Google Scholar]
- 135.Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D (2010) Alcoholic beverages and prostate cancer in a prospective US cohort study. Am J Epidemiol 172: 773–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hayes RB, Brown LM, Schoenberg JB, Greenberg RS, Silverman DT, Schwartz AG, Swanson GM, Benichou J, Liff JM, Hoover RN, Pottern LM (1996) Alcohol use and prostate cancer risk in US blacks and whites. Am J Epidemiol 143: 692–7 [DOI] [PubMed] [Google Scholar]
- 137.Dagnelie PC, Schuurman AG, Goldbohm RA, Van den Brandt PA (2004) Diet, anthropometric measures and prostate cancer risk: a review of prospective cohort and intervention studies. BJU Int 93: 1139–50 [DOI] [PubMed] [Google Scholar]
- 138.Longnecker MP (1995) Alcohol consumption and risk of cancer in humans: an overview. Alcohol 12: 87–96 [DOI] [PubMed] [Google Scholar]
- 139.Morton MS, Griffiths K, Blacklock N (1996) The preventive role of diet in prostatic disease. Br J Urol 77: 481–93 [DOI] [PubMed] [Google Scholar]
- 140.Dennis LK (2000) Meta-analysis for combining relative risks of alcohol consumption and prostate cancer. Prostate 42: 56–66 [DOI] [PubMed] [Google Scholar]
- 141.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, et al. (2015) Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 112: 580–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Middleton Fillmore K, Chikritzhs T, Stockwell T, Bostrom A, Pascal R (2009) Alcohol use and prostate cancer: a meta-analysis. Mol Nutr Food Res 53: 240–55 [DOI] [PubMed] [Google Scholar]
- 143.Rota M, Scotti L, Turati F, Tramacere I, Islami F, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C, Bagnardi V (2012) Alcohol consumption and prostate cancer risk: a meta-analysis of the dose-risk relation. Eur J Cancer Prev 21: 350–9 [DOI] [PubMed] [Google Scholar]
- 144.Zhao J, Stockwell T, Roemer A, Chikritzhs T (2016) Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer 16: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Papa NP, MacInnis RJ, Jayasekara H, English DR, Bolton D, Davis ID, Lawrentschuk N, Millar JL, Pedersen J, Severi G, Southey MC, Hopper JL, Giles GG (2017) Total and beverage-specific alcohol intake and the risk of aggressive prostate cancer: a case-control study. Prostate Cancer Prostatic Dis 20: 305–10 [DOI] [PubMed] [Google Scholar]
- 146.McGregor SE, Courneya KS, Kopciuk KA, Tosevski C, Friedenreich CM (2013) Case-control study of lifetime alcohol intake and prostate cancer risk. Cancer Causes Control 24: 451–61 [DOI] [PubMed] [Google Scholar]
- 147.Sawada N, Inoue M, Iwasaki M, Sasazuki S, Yamaji T, Shimazu T, Tsugane S (2014) Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center-based prospective study. Int J Cancer 134: 971–8 [DOI] [PubMed] [Google Scholar]
- 148.Gong Z, Kristal AR, Schenk JM, Tangen CM, Goodman PJ, Thompson IM (2009) Alcohol consumption, finasteride, and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer 115: 3661–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang ET, Hedelin M, Adami HO, Gronberg H, Balter KA (2005) Alcohol drinking and risk of localized versus advanced and sporadic versus familial prostate cancer in Sweden. Cancer Causes Control 16: 275–84 [DOI] [PubMed] [Google Scholar]
- 150.Fowke JH, Howard L, Andriole GL, Freedland SJ (2014) Alcohol intake increases high-grade prostate cancer risk among men taking dutasteride in the REDUCE trial. Eur Urol 66: 1133–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Humans IWGotEoCRt (2012) Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 100: 1–538 [PMC free article] [PubMed] [Google Scholar]
- 152.Balbo S, Brooks PJ (2015) Implications of acetaldehyde-derived DNA adducts for understanding alcohol-related carcinogenesis. Adv Exp Med Biol 815: 71–88 [DOI] [PubMed] [Google Scholar]
- 153.Fang JL, Vaca CE (1997) Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis 18: 627–32 [DOI] [PubMed] [Google Scholar]
- 154.Castro GD, Delgado de Layno AM, Costantini MH, Castro JA (2001) Cytosolic xanthine oxidoreductase mediated bioactivation of ethanol to acetaldehyde and free radicals in rat breast tissue. Its potential role in alcohol-promoted mammary cancer. Toxicology 160: 11–8 [DOI] [PubMed] [Google Scholar]
- 155.Castro GD, Delgado de Layno AM, Costantini MH, Castro JA (2002) Rat ventral prostate microsomal biotransformation of ethanol to acetaldehyde and 1-hydroxyethyl radicals: its potential contribution to prostate tumor promotion. Teratog Carcinog Mutagen 22: 335–41 [DOI] [PubMed] [Google Scholar]
- 156.Gomez MI, de Castro CR, Fanelli SL, Quintans LN, Costantini MH, Castro JA, Castro GD (2007) Biochemical and ultrastructural alterations in the rat ventral prostate due to repetitive alcohol drinking. J Appl Toxicol 27: 391–8 [DOI] [PubMed] [Google Scholar]
- 157.Castro GD, Costantini MH, Castro JA (2009) Rat ventral prostate xanthine oxidase-mediated metabolism of acetaldehyde to acetyl radical. Hum Exp Toxicol 28: 203–8 [DOI] [PubMed] [Google Scholar]
- 158.Sutcliffe S, Giovannucci E, Leitzmann MF, Rimm EB, Stampfer MJ, Willett WC, Platz EA (2007) A prospective cohort study of red wine consumption and risk of prostate cancer. Int J Cancer 120: 1529–35 [DOI] [PubMed] [Google Scholar]
- 159.Chao C, Haque R, Van Den Eeden SK, Caan BJ, Poon KY, Quinn VP (2010) Red wine consumption and risk of prostate cancer: the California men's health study. Int J Cancer 126: 171–9 [DOI] [PubMed] [Google Scholar]
- 160.Schoonen WM, Salinas CA, Kiemeney LA, Stanford JL (2005) Alcohol consumption and risk of prostate cancer in middle-aged men. Int J Cancer 113: 133–40 [DOI] [PubMed] [Google Scholar]
- 161.Aherne SA, O'Brien NM (2002) Dietary flavonols: chemistry, food content, and metabolism. Nutrition 18: 75–81 [DOI] [PubMed] [Google Scholar]
- 162.Yang CS, Landau JM, Huang MT, Newmark HL (2001) Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr 21: 381–406 [DOI] [PubMed] [Google Scholar]
- 163.Kampa M, Hatzoglou A, Notas G, Damianaki A, Bakogeorgou E, Gemetzi C, Kouroumalis E, Martin PM, Castanas E (2000) Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer 37: 223–33 [DOI] [PubMed] [Google Scholar]
- 164.Bylsma LC, Alexander DD (2015) A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr J 14: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gathirua-Mwangi WG, Zhang J (2014) Dietary factors and risk for advanced prostate cancer. Eur J Cancer Prev 23: 96–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lijinsky W (1999) N-Nitroso compounds in the diet. Mutat Res 443: 129–38 [DOI] [PubMed] [Google Scholar]
- 167.Phillips DH (1999) Polycyclic aromatic hydrocarbons in the diet. Mutat Res 443: 139–47 [DOI] [PubMed] [Google Scholar]
- 168.Sugimura T, Wakabayashi K, Nakagama H, Nagao M (2004) Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci 95: 290–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Felton JS, Jagerstad M, Knize MG, Skog K, and Wakabayashi K (2000) Contents in foods, beverages and tobacco In Food Borne. Carcinogens Heterocyclic Amines (Nagao M, and Sugimura T, Eds.) pp 31–71. John Wiley & Sons Ltd, Chichester, England [Google Scholar]
- 170.Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, Hasegawa R, Imaida K, Matsumoto K, Wakabayashi K, Sugimura T, Ito N (1997) The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res 57: 195–8 [PubMed] [Google Scholar]
- 171.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K (2015) Carcinogenicity of consumption of red and processed meat. Lancet Oncol 16: 1599–600 [DOI] [PubMed] [Google Scholar]
- 172.Bogen KT, Keating GA (2001) U.S. dietary exposures to heterocyclic amines. J Expo Anal Environ Epidemiol 11: 155–68 [DOI] [PubMed] [Google Scholar]
- 173.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R (1999) Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res 59: 5704–9 [PubMed] [Google Scholar]
- 174.Tappel A (2007) Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med Hypotheses 68: 562–4 [DOI] [PubMed] [Google Scholar]
- 175.Sinha R, Park Y, Graubard BI, Leitzmann MF, Hollenbeck A, Schatzkin A, Cross AJ (2009) Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol 170: 1165–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jakszyn PG, Allen NE, Lujan-Barroso L, Gonzalez CA, Key TJ, Fonseca-Nunes A, Tjonneland A, Fons-Johnsen N, Overvad K, Teucher B, Li K, Boeing H, Trichopoulou A, et al. (2012) Nitrosamines and heme iron and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 21: 547–51 [DOI] [PubMed] [Google Scholar]
- 177.Dietrich M, Block G, Pogoda JM, Buffler P, Hecht S, Preston-Martin S (2005) A review: dietary and endogenously formed N-nitroso compounds and risk of childhood brain tumors. Cancer Causes Control 16: 619–35 [DOI] [PubMed] [Google Scholar]
- 178.Mirvish SS (1995) Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett 93: 17–48 [DOI] [PubMed] [Google Scholar]
- 179.Mende P, Spiegelhalder B, Preussmann R (1991) Trace analysis of nitrosated foodstuffs for nitrosamides. Food Chem Toxicol 29: 167–72 [DOI] [PubMed] [Google Scholar]
- 180.Cross AJ, Pollock JR, Bingham SA (2003) Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res 63: 2358–60 [PubMed] [Google Scholar]
- 181.Hughes R, Cross AJ, Pollock JR, Bingham S (2001) Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis 22: 199–202 [DOI] [PubMed] [Google Scholar]
- 182.Lunn JC, Kuhnle G, Mai V, Frankenfeld C, Shuker DE, Glen RC, Goodman JM, Pollock JR, Bingham SA (2007) The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis 28: 685–90 [DOI] [PubMed] [Google Scholar]
- 183.Kuhnle GG, Bingham SA (2007) Dietary meat, endogenous nitrosation and colorectal cancer. Biochem Soc Trans 35: 1355–7 [DOI] [PubMed] [Google Scholar]
- 184.Saffhill R, Margison GP, O'Connor PJ (1985) Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta 823: 111–45 [DOI] [PubMed] [Google Scholar]
- 185.Belinsky SA, Devereux TR, Maronpot RR, Stoner GD, Anderson MW (1989) Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res 49: 5305–11 [PubMed] [Google Scholar]
- 186.Loechler EL, Green CL, Essigmann JM (1984) In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A 81: 6271–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, Boeing H, Del Giudice G, Palli D, Saieva C, Krogh V, Sacerdote C, Tumino R, et al. (2006) Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis 27: 1497–501 [DOI] [PubMed] [Google Scholar]
- 188.De Stefani E, Boffetta P, Mendilaharsu M, Carzoglio J, Deneo-Pellegrini H (1998) Dietary nitrosamines, heterocyclic amines, and risk of gastric cancer: a case-control study in Uruguay. Nutr Cancer 30: 158–62 [DOI] [PubMed] [Google Scholar]
- 189.Pobel D, Riboli E, Cornee J, Hemon B, Guyader M (1995) Nitrosamine, nitrate and nitrite in relation to gastric cancer: a case-control study in Marseille, France. Eur J Epidemiol 11: 67–73 [DOI] [PubMed] [Google Scholar]
- 190.La Vecchia C, D'Avanzo B, Airoldi L, Braga C, Decarli A (1995) Nitrosamine intake and gastric cancer risk. Eur J Cancer Prev 4: 469–74 [DOI] [PubMed] [Google Scholar]
- 191.Knekt P, Jarvinen R, Dich J, Hakulinen T (1999) Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int J Cancer 80: 852–6 [DOI] [PubMed] [Google Scholar]
- 192.Loh YH, Jakszyn P, Luben RN, Mulligan AA, Mitrou PN, Khaw KT (2011) N-Nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am J Clin Nutr 93: 1053–61 [DOI] [PubMed] [Google Scholar]
- 193.Humans IWGotEoCRt (2010) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum 92: 1–853 [PMC free article] [PubMed] [Google Scholar]
- 194.Mottier P, Parisod V, Turesky RJ (2000) Quantitative determination of polycyclic aromatic hydrocarbons in barbecued meat sausages by gas chromatography coupled to mass spectrometry. J Agric Food Chem 48: 1160–6 [DOI] [PubMed] [Google Scholar]
- 195.Boffetta P, Jourenkova N, Gustavsson P (1997) Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 8: 444–72 [DOI] [PubMed] [Google Scholar]
- 196.Pyy L, Makela M, Hakala E, Kakko K, Lapinlampi T, Lisko A, Yrjanheikki E, Vahakangas K (1997) Ambient and biological monitoring of exposure to polycyclic aromatic hydrocarbons at a coking plant. Sci Total Environ 199: 151–8 [DOI] [PubMed] [Google Scholar]
- 197.Grimmer G, Dettbarn G, Jacob J (1993) Biomonitoring of polycyclic aromatic hydrocarbons in highly exposed coke plant workers by measurement of urinary phenanthrene and pyrene metabolites (phenols and dihydrodiols). Int Arch Occup Environ Health 65: 189–99 [DOI] [PubMed] [Google Scholar]
- 198.Vu AT, Taylor KM, Holman MR, Ding YS, Hearn B, Watson CH (2015) Polycyclic Aromatic Hydrocarbons in the Mainstream Smoke of Popular U.S. Cigarettes. Chem Res Toxicol 28: 1616–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jagerstad M, Skog K (2005) Genotoxicity of heat-processed foods. Mutat Res 574: 156–72 [DOI] [PubMed] [Google Scholar]
- 200.Lee JG, Kim SY, Moon JS, Kim SH, Kang DH, Yoon HJ (2016) Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chem 199: 632–8 [DOI] [PubMed] [Google Scholar]
- 201.Viegas O, Novo P, Pinto E, Pinho O, Ferreira IM (2012) Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem Toxicol 50: 2128–34 [DOI] [PubMed] [Google Scholar]
- 202.Simko P (2005) Factors affecting elimination of polycyclic aromatic hydrocarbons from smoked meat foods and liquid smoke flavorings. Mol Nutr Food Res 49: 637–47 [DOI] [PubMed] [Google Scholar]
- 203.Chung SY, Yettella RR, Kim JS, Kwon K, Kim MC, Min DB (2011) Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chemistry 129: 1420–6 [Google Scholar]
- 204.Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N (2001) Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol 39: 423–36 [DOI] [PubMed] [Google Scholar]
- 205.Domingo JL, Nadal M (2015) Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Food Chem Toxicol 86: 144–53 [DOI] [PubMed] [Google Scholar]
- 206.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR (1996) Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res 56: 2979–84 [PubMed] [Google Scholar]
- 207.Williams JA, Martin FL, Muir GH, Hewer A, Grover PL, Phillips DH (2000) Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis 21: 1683–9 [DOI] [PubMed] [Google Scholar]
- 208.Martin FL, Cole KJ, Muir GH, Kooiman GG, Williams JA, Sherwood RA, Grover PL, Phillips DH (2002) Primary cultures of prostate cells and their ability to activate carcinogens. Prostate Cancer Prostatic Dis 5: 96–104 [DOI] [PubMed] [Google Scholar]
- 209.Hruba E, Trilecova L, Marvanova S, Krcmar P, Vykopalova L, Milcova A, Libalova H, Topinka J, Starsichova A, Soucek K, Vondracek J, Machala M (2010) Genotoxic polycyclic aromatic hydrocarbons fail to induce the p53-dependent DNA damage response, apoptosis or cell-cycle arrest in human prostate carcinoma LNCaP cells. Toxicology Letters 197: 227–35 [DOI] [PubMed] [Google Scholar]
- 210.Kooiman GG, Martin FL, Williams JA, Grover PL, Phillips DH, Muir GH (2000) The influence of dietary and environmental factors on prostate cancer risk. Prostate Cancer Prostatic Dis 3: 256–8 [DOI] [PubMed] [Google Scholar]
- 211.Rybicki BA, Neslund-Dudas C, Bock CH, Rundle A, Savera AT, Yang JJ, Nock NL, Tang D (2008) Polycyclic aromatic hydrocarbon--DNA adducts in prostate and biochemical recurrence after prostatectomy. Clin Cancer Res 14: 750–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rybicki BA, Nock NL, Savera AT, Tang D, Rundle A (2006) Polycyclic aromatic hydrocarbon-DNA adduct formation in prostate carcinogenesis. Cancer Lett 239: 157–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rybicki BA, Rundle A, Savera AT, Sankey SS, Tang D (2004) Polycyclic aromatic hydrocarbon-DNA adducts in prostate cancer. Cancer Res 64: 8854–9 [DOI] [PubMed] [Google Scholar]
- 214.Tang D, Kryvenko ON, Wang Y, Jankowski M, Trudeau S, Rundle A, Rybicki BA (2013) Elevated polycyclic aromatic hydrocarbon-DNA adducts in benign prostate and risk of prostate cancer in African Americans. Carcinogenesis 34: 113–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.John K, Ragavan N, Pratt MM, Singh PB, Al-Buheissi S, Matanhelia SS, Phillips DH, Poirier MC, Martin FL (2009) Quantification of phase I/II metabolizing enzyme gene expression and polycyclic aromatic hydrocarbon-DNA adduct levels in human prostate. Prostate 69: 505–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weston A, Manchester DK, Poirier MC, Choi JS, Trivers GE, Mann DL, Harris CC (1989) Derivative fluorescence spectral analysis of polycyclic aromatic hydrocarbon-DNA adducts in human placenta. Chem Res Toxicol 2: 104–8 [DOI] [PubMed] [Google Scholar]
- 217.Nock NL, Tang D, Rundle A, Neslund-Dudas C, Savera AT, Bock CH, Monaghan KG, Koprowski A, Mitrache N, Yang JJ, Rybicki BA (2007) Associations between smoking, polymorphisms in polycyclic aromatic hydrocarbon (PAH) metabolism and conjugation genes and PAH-DNA adducts in prostate tumors differ by race. Cancer Epidemiol Biomarkers Prev 16: 1236–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xiao S, Guo J, Yun BH, Villalta PW, Krishna S, Tejpaul R, Murugan P, Weight CJ, Turesky RJ (2016) Biomonitoring DNA Adducts of Cooked Meat Carcinogens in Human Prostate by Nano Liquid Chromatography-High Resolution Tandem Mass Spectrometry: Identification of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine DNA Adduct. Anal Chem 88: 12508–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ni W, McNaughton L, LeMaster DM, Sinha R, Turesky RJ (2008) Quantitation of 13 heterocyclic aromatic amines in cooked beef, pork, and chicken by liquid chromatography-electrospray ionization/tandem mass spectrometry. J Agric Food Chem 56: 68–78 [DOI] [PubMed] [Google Scholar]
- 220.Jagerstad M, Skog K, Grivas S, Olsson K (1991) Formation of heterocyclic amines using model systems. Mutat Res 259: 219–33 [DOI] [PubMed] [Google Scholar]
- 221.Skog KI, Johansson MA, Jagerstad MI (1998) Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food Chem Toxicol 36: 879–96 [DOI] [PubMed] [Google Scholar]
- 222.Yoshida D, Matsumoto T, Yoshimura R, Matsuzaki T (1978) Mutagenicity of amino-alpha-carbolines in pyrolysis products of soybean globulin. Biochem Biophys Res Commun 83: 915–20 [DOI] [PubMed] [Google Scholar]
- 223.Matsumoto T, Yoshida D, Tomita H (1981) Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer Lett 12: 105–10 [DOI] [PubMed] [Google Scholar]
- 224.Manabe S, Tohyama K, Wada O, Aramaki T (1991) Detection of a carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), in cigarette smoke condensate. Carcinogenesis 12: 1945–7 [DOI] [PubMed] [Google Scholar]
- 225.Yoshida D, Matsumoto T (1980) Amino-alpha-carbolines as mutagenic agents in cigarette smoke condensate. Cancer Lett 10: 141–9 [DOI] [PubMed] [Google Scholar]
- 226.Smith CJ, Qian X, Zha Q, Moldoveanu SC (2004) Analysis of alpha- and beta-carbolines in mainstream smoke of reference cigarettes by gas chromatography-mass spectrometry. J Chromatogr A 1046: 211–6 [DOI] [PubMed] [Google Scholar]
- 227.Zhang L, Ashley DL, Watson CH (2011) Quantitative analysis of six heterocyclic aromatic amines in mainstream cigarette smoke condensate using isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Nicotine Tob Res 13: 120–6 [DOI] [PubMed] [Google Scholar]
- 228.Turesky RJ, Le Marchand L (2011) Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol 24: 1169–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Knize MG, Felton JS (2005) Formation and human risk of carcinogenic heterocyclic amines formed from natural precursors in meat. Nutr Rev 63: 158–65 [DOI] [PubMed] [Google Scholar]
- 230.Sinha R, Rothman N, Brown ED, Salmon CP, Knize MG, Swanson CA, Rossi SC, Mark SD, Levander OA, Felton JS (1995) High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo- [4,5-b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res 55: 4516–9 [PubMed] [Google Scholar]
- 231.Knize MG, Dolbeare FA, Carroll KL, Moore DH 2nd, Felton JS (1994) Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem Toxicol 32: 595–603 [DOI] [PubMed] [Google Scholar]
- 232.Skog K, Solyakov A, Arvidsson P, Jagerstad M (1998) Analysis of nonpolar heterocyclic amines in cooked foods and meat extracts using gas chromatography-mass spectrometry. J Chromatogr A 803: 227–33 [DOI] [PubMed] [Google Scholar]
- 233.Sinha R, Rothman N, Salmon CP, Knize MG, Brown ED, Swanson CA, Rhodes D, Rossi S, Felton JS, Levander OA (1998) Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol 36: 279–87 [DOI] [PubMed] [Google Scholar]
- 234.Keating GA, Bogen KT (2004) Estimates of heterocyclic amine intake in the US population. J Chromatogr B Analyt Technol Biomed Life Sci 802: 127–33 [DOI] [PubMed] [Google Scholar]
- 235.Crofts FG, Sutter TR, Strickland PT (1998) Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P4501A1, P4501A2 and P4501B1. Carcinogenesis 19: 1969–73 [DOI] [PubMed] [Google Scholar]
- 236.Boobis AR, Lynch AM, Murray S, de la Torre R, Solans A, Farre M, Segura J, Gooderham NJ, Davies DS (1994) CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res 54: 89–94 [PubMed] [Google Scholar]
- 237.Glatt H, Pabel U, Meinl W, Frederiksen H, Frandsen H, Muckel E (2004) Bioactivation of the heterocyclic aromatic amine 2-amino-3-methyl-9H-pyrido [2,3-b]indole (MeAalphaC) in recombinant test systems expressing human xenobiotic-metabolizing enzymes. Carcinogenesis 25: 801–7 [DOI] [PubMed] [Google Scholar]
- 238.Schut HA, Snyderwine EG (1999) DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis 20: 353–68 [DOI] [PubMed] [Google Scholar]
- 239.Turesky RJ, Vouros P (2004) Formation and analysis of heterocyclic aromatic amine-DNA adducts in vitro and in vivo. J Chromatogr B Analyt Technol Biomed Life Sci 802: 155–66 [DOI] [PubMed] [Google Scholar]
- 240.Turteltaub KW, Felton JS, Gledhill BL, Vogel JS, Southon JR, Caffee MW, Finkel RC, Nelson DE, Proctor ID, Davis JC (1990) Accelerator mass spectrometry in biomedical dosimetry: relationship between low-level exposure and covalent binding of heterocyclic amine carcinogens to DNA. Proc Natl Acad Sci U S A 87: 5288–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Turesky RJ, Box RM, Markovic J, Gremaud E, Snyderwine EG (1997) Formation and persistence of DNA adducts of 2-amino-3-methylimidazo[4,5-f]quinoline in the rat and nonhuman primates. Mutat Res 376: 235–41 [DOI] [PubMed] [Google Scholar]
- 242.Fukushima S, Wanibuchi H, Morimura K, Iwai S, Nakae D, Kishida H, Tsuda H, Uehara N, Imaida K, Shirai T, Tatematsu M, Tsukamoto T, Hirose M, et al. (2004) Existence of a threshold for induction of aberrant crypt foci in the rat colon with low doses of 2-amino-1-methyl-6-phenolimidazo[4,5-b]pyridine. Toxicol Sci 80: 109–14 [DOI] [PubMed] [Google Scholar]
- 243.Nauwelaers G, Bessette EE, Gu D, Tang Y, Rageul J, Fessard V, Yuan JM, Yu MC, Langouet S, Turesky RJ (2011) DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes. Chem Res Toxicol 24: 913–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Turesky RJ, Constable A, Richoz J, Varga N, Markovic J, Martin MV, Guengerich FP (1998) Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2. Chem Res Toxicol 11: 925–36 [DOI] [PubMed] [Google Scholar]
- 245.Turesky RJ, Parisod V, Huynh-Ba T, Langouёt S, Guengerich FP (2001) Regioselective differences in C(8)- and N-oxidation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline by human and rat liver microsomes and cytochromes P450 1A2. Chem Res Toxicol 14: 901–11 [DOI] [PubMed] [Google Scholar]
- 246.Totsuka Y, Fukutome K, Takahashi M, Takashi S, Tada A, Sugimura T, Wakabayashi K (1996) Presence of N2-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (dG-C8-MeIQx) in human tissues. Carcinogenesis 17: 1029–34 [DOI] [PubMed] [Google Scholar]
- 247.Dingley KH, Curtis KD, Nowell S, Felton JS, Lang NP, Turteltaub KW (1999) DNA and protein adduct formation in the colon and blood of humans after exposure to a dietary-relevant dose of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Epidemiol Biomarkers Prev 8: 507–12 [PubMed] [Google Scholar]
- 248.Friesen MD, Kaderlik K, Lin D, Garren L, Bartsch H, Lang NP, Kadlubar FF (1994) Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling. Chem Res Toxicol 7: 733–9 [DOI] [PubMed] [Google Scholar]
- 249.Turteltaub KW, Mauthe RJ, Dingley KH, Vogel JS, Frantz CE, Garner RC, Shen N (1997) MeIQx-DNA adduct formation in rodent and human tissues at low doses. Mutat Res 376: 243–52 [DOI] [PubMed] [Google Scholar]
- 250.Lightfoot TJ, Coxhead JM, Cupid BC, Nicholson S, Garner RC (2000) Analysis of DNA adducts by accelerator mass spectrometry in human breast tissue after administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and benzo[a]pyrene. Mutat Res 472: 119–27 [DOI] [PubMed] [Google Scholar]
- 251.Zhu J, Chang P, Bondy ML, Sahin AA, Singletary SE, Takahashi S, Shirai T, Li D (2003) Detection of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine-DNA adducts in normal breast tissues and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 12: 830–7 [PubMed] [Google Scholar]
- 252.Zhu J, Rashid A, Cleary K, Abbruzzese JL, Friess H, Takahashi S, Shirai T, Li D (2006) Detection of 2-amino-1-methyl-6-phenylimidazo [4,5-b]-pyridine (PhIP)-DNA adducts in human pancreatic tissues. Biomarkers 11: 319–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Magagnotti C, Pastorelli R, Pozzi S, Andreoni B, Fanelli R, Airoldi L (2003) Genetic polymorphisms and modulation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in human lymphocytes. Int J Cancer 107: 878–84 [DOI] [PubMed] [Google Scholar]
- 254.Malfatti MA, Dingley KH, Nowell-Kadlubar S, Ubick EA, Mulakken N, Nelson D, Lang NP, Felton JS, Turteltaub KW (2006) The urinary metabolite profile of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine is predictive of colon DNA adducts after a low-dose exposure in humans. Cancer Res 66: 10541–7 [DOI] [PubMed] [Google Scholar]
- 255.Tang D, Liu JJ, Rundle A, Neslund-Dudas C, Savera AT, Bock CH, Nock NL, Yang JJ, Rybicki BA (2007) Grilled meat consumption and PhIP-DNA adducts in prostate carcinogenesis. Cancer Epidemiol Biomarkers Prev 16: 803–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bessette EE, Spivack SD, Goodenough AK, Wang T, Pinto S, Kadlubar FF, Turesky RJ (2010) Identification of carcinogen DNA adducts in human saliva by linear quadrupole ion trap/multistage tandem mass spectrometry. Chem Res Toxicol 23: 1234–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim JK, McCormick MA, Gallaher CM, Gallaher DD, Trudo SP (2018) Apiaceous Vegetables and Cruciferous Phytochemicals Reduced PhIP-DNA Adducts in Prostate but Not in Pancreas of Wistar Rats. J Med Food 21: 199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dingley KH, Ubick EA, Chiarappa-Zucca ML, Nowell S, Abel S, Ebeler SE, Mitchell AE, Burns SA, Steinberg FM, Clifford AJ (2003) Effect of dietary constituents with chemopreventive potential on adduct formation of a low dose of the heterocyclic amines PhIP and IQ and phase II hepatic enzymes. Nutr Cancer 46: 212–21 [DOI] [PubMed] [Google Scholar]
- 259.Archer CL, Morse P, Jones RF, Shirai T, Haas GP, Wang CY (2000) Carcinogenicity of the N-hydroxy derivative of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, 2-amino-3, 8-dimethyl-imidazo[4,5-f]quinoxaline and 3, 2'-dimethyl-4-aminobiphenyl in the rat. Cancer Lett 155: 55–60 [DOI] [PubMed] [Google Scholar]
- 260.Stuart GR, Holcroft J, de Boer JG, Glickman BW (2000) Prostate mutations in rats induced by the suspected human carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res 60: 266–8 [PubMed] [Google Scholar]
- 261.Nakai Y, Nelson WG, De Marzo AM (2007) The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res 67: 1378–84 [DOI] [PubMed] [Google Scholar]
- 262.Li G, Wang H, Liu AB, Cheung C, Reuhl KR, Bosland MC, Yang CS (2012) Dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer Prev Res 5: 963–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chen C, Ma X, Malfatti MA, Krausz KW, Kimura S, Felton JS, Idle JR, Gonzalez FJ (2007) A comprehensive investigation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) metabolism in the mouse using a multivariate data analysis approach. Chem Res Toxicol 20: 531–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chen JX, Li G, Wang H, Liu A, Lee MJ, Reuhl K, Suh N, Bosland MC, Yang CS (2016) Dietary tocopherols inhibit PhIP-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer Lett 371: 71–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Oliveira DS, Dzinic S, Bonfil AI, Saliganan AD, Sheng S, Bonfil RD (2016) The mouse prostate: a basic anatomical and histological guideline. Bosn J Basic Med Sci 16: 8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Borowsky AD, Dingley KH, Ubick E, Turteltaub KW, Cardiff RD, Devere-White R (2006) Inflammation and atrophy precede prostatic neoplasia in a PhIP-induced rat model. Neoplasia 8: 708–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nakai Y, Nonomura N (2013) Inflammation and prostate carcinogenesis. Int J Urol 20: 150–60 [DOI] [PubMed] [Google Scholar]
- 268.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL (2004) Molecular determinants of resistance to antiandrogen therapy. Nat Med 10: 33–9 [DOI] [PubMed] [Google Scholar]
- 269.Richmond PJ, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M (1997) Aberrant E-cadherin and alpha-catenin expression in prostate cancer: correlation with patient survival. Cancer Res 57: 3189–93 [PubMed] [Google Scholar]
- 270.Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M (2000) p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 157: 1769–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ross JS, Figge HL, Bui HX, del Rosario AD, Fisher HA, Nazeer T, Jennings TA, Ingle R, Kim DN (1994) E-cadherin expression in prostatic carcinoma biopsies: correlation with tumor grade, DNA content, pathologic stage, and clinical outcome. Mod Pathol 7: 835–41 [PubMed] [Google Scholar]
- 272.Gupta-Elera G, Garrett AR, Robison RA, O'Neill KL (2012) The role of oxidative stress in prostate cancer. Eur J Cancer Prev 21: 155–62 [DOI] [PubMed] [Google Scholar]
- 273.Di Cristofano A, Pandolfi PP (2000) The multiple roles of PTEN in tumor suppression. Cell 100: 387–90 [DOI] [PubMed] [Google Scholar]
- 274.Wang X, McCullough KD, Franke TF, Holbrook NJ (2000) Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 275: 14624–31 [DOI] [PubMed] [Google Scholar]
- 275.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D (1997) Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 57: 4997–5000 [PubMed] [Google Scholar]
- 276.Tang D, Liu JJ, Bock CH, Neslund-Dudas C, Rundle A, Savera AT, Yang JJ, Nock NL, Rybicki BA (2007) Racial differences in clinical and pathological associations with PhIP-DNA adducts in prostate. Int J Cancer 121: 1319–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tang D, Kryvenko ON, Wang Y, Trudeau S, Rundle A, Takahashi S, Shirai T, Rybicki BA (2013) 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in benign prostate and subsequent risk for prostate cancer. Int J Cancer 133: 961–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yun BH, Xiao S, Yao L, Krishnamachari S, Rosenquist TA, Dickman KG, Grollman AP, Murugan P, Weight CJ, Turesky RJ (2017) A Rapid Throughput Method To Extract DNA from Formalin-Fixed Paraffin-Embedded Tissues for Biomonitoring Carcinogenic DNA Adducts. Chem Res Toxicol 30: 2130–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Norrish AE, Ferguson LR, Knize MG, Felton JS, Sharpe SJ, Jackson RT (1999) Heterocyclic amine content of cooked meat and risk of prostate cancer. J Natl Cancer Inst 91: 2038–44 [DOI] [PubMed] [Google Scholar]
- 280.Rohrmann S, Nimptsch K, Sinha R, Willett WC, Giovannucci EL, Platz EA, Wu K (2015) Intake of Meat Mutagens and Risk of Prostate Cancer in a Cohort of U.S. Health Professionals. Cancer Epidemiol Biomarkers Prev 24: 1557–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, John EM, Ingles SA, Stern MC (2012) Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer: results from a multiethnic case-control study. Carcinogenesis 33: 2108–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sharma S, Cao X, Wilkens LR, Yamamoto J, Lum-Jones A, Henderson BE, Kolonel LN, Le ML (2010) Well-done meat consumption, NAT1 and NAT2 acetylator genotypes and prostate cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 19: 1866–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sander A, Linseisen J, Rohrmann S (2011) Intake of heterocyclic aromatic amines and the risk of prostate cancer in the EPIC-Heidelberg cohort. Cancer Causes Control 22: 109–14 [DOI] [PubMed] [Google Scholar]
- 284.Takahashi S, Tamano S, Hirose M, Kimoto N, Ikeda Y, Sakakibara M, Tada M, Kadlubar FF, Ito N, Shirai T (1998) Immunohistochemical demonstration of carcinogen-DNA adducts in tissues of rats given 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP): detection in paraffin-embedded sections and tissue distribution. Cancer Res 58: 4307–13 [PubMed] [Google Scholar]
- 285.Bellamri M, Xiao S, Murugan P, Weight CJ, Turesky RJ (2018) Metabolic Activation of the Cooked Meat Carcinogen 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine in Human Prostate. Toxicol Sci 163: 543–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW (2001) Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res 61: 103–9 [PubMed] [Google Scholar]
- 287.Wang CY, Debiec-Rychter M, Schut HA, Morse P, Jones RF, Archer C, King CM, Haas GP (1999) N-Acetyltransferase expression and DNA binding of N-hydroxyheterocyclic amines in human prostate epithelium. Carcinogenesis 20: 1591–5 [DOI] [PubMed] [Google Scholar]
- 288.Glass-Holmes M, Aguilar BJ, Gragg RD, Darling-Reed S, Goodman CB (2015) Characterization of 2-amino-1-methyl-6-phenylimida zo[4,5b]pyridine at androgen receptor: mechanistic support for its role in prostate cancer. Am J Canc Res 5: 191–200 [PMC free article] [PubMed] [Google Scholar]
- 289.Creton SK, Zhu H, Gooderham NJ (2007) The cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine activates the extracellular signal regulated kinase mitogen-activated protein kinase pathway. Cancer Res 67: 11455–62 [DOI] [PubMed] [Google Scholar]
- 290.Cano E, Mahadevan LC (1995) Parallel signal processing among mammalian MAPKs. Trends Biochem Sci 20: 117–22 [DOI] [PubMed] [Google Scholar]
- 291.Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- 292.Petimar J, Wilson KM, Wu K, Wang M, Albanes D, van den Brandt PA, Cook MB, Giles GG, Giovannucci EL, Goodman GE, Goodman PJ, Hakansson N, Helzlsouer K, et al. (2017) A Pooled Analysis of 15 Prospective Cohort Studies on the Association between Fruit, Vegetable, and Mature Bean Consumption and Risk of Prostate Cancer. Cancer Epidemiol Biomarkers Prev 26: 1276–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ahn-Jarvis JH, Clinton SK, Grainger EM, Riedl KM, Schwartz SJ, Lee ML, Cruz-Cano R, Young GS, Lesinski GB, Vodovotz Y (2015) Isoflavone pharmacokinetics and metabolism after consumption of a standardized soy and soy-almond bread in men with asymptomatic prostate cancer. Cancer Prev Res (Phila) 8: 1045–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Grainger EM, Hadley CW, Moran NE, Riedl KM, Gong MC, Pohar K, Schwartz SJ, Clinton SK (2015) A comparison of plasma and prostate lycopene in response to typical servings of tomato soup, sauce or juice in men before prostatectomy. Br J Nutr 114: 596–607 [DOI] [PMC free article] [PubMed] [Google Scholar]