Abstract
The human 22q11.2 chromosomal deletion is one of the strongest identified genetic risk factors for schizophrenia. Although the deletion spans a number of known genes, the contribution of each of these to the 22q11.2 deletion syndrome (DS) is not known. To investigate the effect of individual genes within this interval on the pathophysiology associated with the deletion, we analyzed their role in sleep, a behavior affected in virtually all psychiatric disorders, including the 22q11.2 DS. We identified the gene LZTR1 (night owl, nowl) as a regulator of night-time sleep in Drosophila. In humans, LZTR1 has been associated with Ras-dependent neurological diseases also caused by Neurofibromin-1 (Nf1) deficiency. We show that Nf1 loss leads to a night-time sleep phenotype nearly identical to that of nowl loss and that nowl negatively regulates Ras and interacts with Nf1 in sleep regulation. Furthermore, nowl is required for metabolic homeostasis, suggesting that LZTR1 may contribute to the genetic susceptibility to obesity associated with the 22q11.2 DS. Knockdown of nowl or Nf1 in GABA-responsive sleep-promoting neurons elicits the sleep phenotype, and this defect can be rescued by increased GABAA receptor signaling, indicating that Nowl regulates sleep through modulation of GABA signaling. Our results suggest that nowl/LZTR1 may be a conserved regulator of GABA signaling important for normal sleep that contributes to the 22q11.2 DS.
Author summary
Schizophrenia is a devastating mental disorder with a large genetic component to disease predisposition. One of the strongest genetic risk factors for this disorder is a relatively small genetic deletion of 43 genes on the 22nd chromosome, called 22q11.2, which confers about a 25% risk of schizophrenia development. However, it is likely that only some of these deleted genes affect disease risk, so we tested most of them individually. One of the main symptoms of schizophrenia is disturbed sleep. Sleep is an evolutionarily conserved behavior that can be easily studied in the fruit fly Drosophila melanogaster, so we investigated the effect on sleep of blocking expression of the fly homologs of most of the 22q11.2 genes and identified the gene LZTR1 (night owl, nowl) as an important sleep regulator. We found that Nowl/LZTR1 is required for inhibition of the Ras pathway and interacts genetically with the Ras inhibitor NF1. Nowl/LZTR1 appears to function in sleep by modulating inhibitory GABA signaling, which is affected in schizophrenia. Thus, this gene may underlie some of the phenotypes of the human schizophrenia-risk deletion.
Introduction
Recent genome-wide association studies have identified chromosomal deletions and duplications that confer elevated risk of neuropsychiatric disorders [1, 2]. One of these, the 22q11.2 deletion, which spans 43 genes and occurs in 1 of every 2–4,000 births worldwide [3], is the most prominent known genetic risk factor for development of schizophrenia and is associated with high risk of neuropsychiatric disease [4, 5]. This deletion leads to a variety of phenotypes, including clinical manifestations such as congenital defects of the palate and heart, learning and cognitive disabilities, and sleep disturbances [6]. Sleep abnormalities are among the most common manifestations of neuropsychiatric disease [7], with up to 80% of autism and schizophrenia patients experiencing sleep disturbances [8, 9]. Individuals affected by the 22q11.2 deletion have a roughly 25–30% chance of developing schizophrenia or other psychiatric disorders that include sleep disturbances [10, 11]. However, the possible contribution of each of the individual genes spanned by this deletion to this array of symptoms is not clear. Although certain disease phenotypes have been linked to single genes, such as TBX1, found to account for cardiac defects observed in 22q11.2 deletion carriers [12], the genes contributing to the 22q11.2-linked behavioral phenotypes such as sleep disturbance are not characterized.
Many schizophrenic patients exhibit abnormal sleep patterns such as delayed sleep onset, difficulty in maintaining sleep, and a reduced overall amount of sleep [9]. Thus, genes that might play a role in schizophrenia and other behavioral symptoms of the 22q11.2 deletion syndrome (DS) can potentially be identified by their sleep phenotypes. Sleep is an evolutionarily ancient conserved behavior that can be studied in organisms such as the fruit fly Drosophila melanogaster, a commonly used invertebrate model. Drosophila sleep has been rigorously examined and found to exhibit several of the mammalian hallmarks of sleep, such as sustained periods of quiescence, increased arousal threshold, and circadian and homeostatic regulation [13, 14]. Indeed, recent studies in Drosophila have identified genes associated with human neurodevelopmental and psychiatric disorders that disrupt sleep and circadian rhythm, such as the candidate autism-spectrum-disorder gene Cullin-3 (Cul3) [15, 16] and the gene alicorn (alc) linked to the 1q21.1 deletion, another deletion that confers schizophrenia risk [17].
To identify genes that contribute to the behavioral deficits of the 22q11.2 DS, we examined the role in sleep of individual genes within this interval by knockdown of their Drosophila homologs in the nervous system of the fly. We identified night owl (nowl, Lztr1, CG3711), an ortholog of the human LZTR1 (leucine-zipper-like transcription regulator 1), as a modulator of night-time sleep in Drosophila. In humans, mutations in LZTR1 cause neurofibromatosis, a genetic disorder characterized by the growth of benign nervous-system tumors, that is most commonly known to result from mutations in Neurofibromin-1 (Nf1) [18, 19]. Here, we show that neuronal knockdown or mutation of nowl causes sleep disturbances including highly increased fragmentation of sleep and reduced total night-time sleep, similar to neuronal loss of Neurofibromin-1 (Nf1) in Drosophila. Nf1 is a negative regulator of the Ras signaling pathway and an activator of cAMP signaling [20, 21]. On the other hand, little is known about the function of LZTR1, except that its loss has been associated with neurofibromatosis. Interestingly, we find that nowl and Nf1 inhibit Ras signaling and interact genetically in the regulation of sleep in Drosophila. Loss of nowl affects metabolism and may contribute to the obesity risk associated with the 22q11.2 DS. We find that nowl acts through gamma-aminobutyric acid (GABA)-responsive neurons expressing the ionotropic GABA receptor Rdl to maintain sleep. Furthermore, the sleep fragmentation exhibited by nowl mutant flies can be rescued by increased inhibitory signaling through this receptor. Because nowl knockdown phenocopies the sleep disturbances observed with neuronal Cul3 knockdown, we suggest that Nowl may interact with Cul3 and Nf1 in GABA-responsive neurons to regulate sleep. Thus, we have identified the 22q11.2-linked gene nowl, orthologous to human LZTR1, as a regulator of GABAergic signaling and night-time sleep in Drosophila. We suggest that LZTR1 is a promising candidate gene that may contribute to the sleep disturbances and behavioral symptoms seen in patients carrying the 22q11.2 deletion.
Results
Drosophila screening identifies an ortholog of human LZTR1 as a regulator of sleep
Sleep disturbance is a common manifestation of neuropsychiatric disorders in general and of the 22q11.2 DS. To determine which, if any, of the individual genes within this deletion might be required for normal sleep, we first identified the Drosophila orthologs of the human genes within the 22q11.2 interval. From the 43 genes lying within the 22q11.2 deletion, we identified 26 highly conserved orthologs in Drosophila (with a DIOPT score ≥ 6) [22] (Fig 1A). Next, we conducted an RNA-interference (RNAi)-based screen to investigate whether the neuronal function of these Drosophila genes might be important for sleep, using the Drosophila Activity Monitor (DAM) system (TriKinetics), an automated beam-crossing locomotion assay that is the widely accepted standard for Drosophila sleep studies. In these assays, locomotor quiescence of longer than 5 minutes is taken to indicate a sleep-like state, comparable to mammalian sleep [13, 14]. To determine whether each identified Drosophila ortholog might play a role in sleep, we knocked each gene down using the nervous system-specific elav-GAL4 (elav>) line to drive RNAi expression. When possible, two independent RNAi lines targeting each gene were tested. The effectiveness of RNAi knockdown was enhanced by the co-expression of Dicer-2 (Dcr-2) [23]. Some RNAi constructs induced lethality when expressed pan-neuronally or displayed a non-inflating wing phenotype due to a gene disruption at the insertion site of the transgene [24]. These constructs were excluded from further analysis.
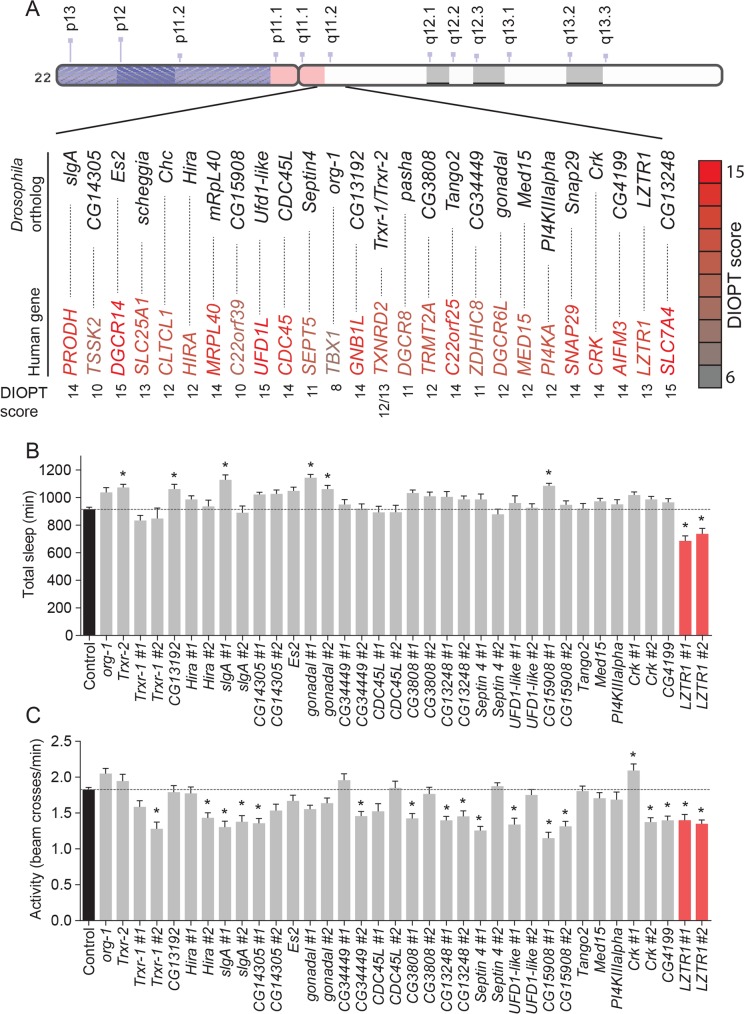
Fig 1. Screening Drosophila orthologs of human genes within the 22q11.2 deletion identifies LZTR1 as a modulator of sleep and activity in Drosophila.
(A) Schematic diagram of the human 22q11.2 deletion. For the 43 genes spanned by the 22q11.2 deletion, 26 orthologs were identified in Drosophila melanogaster with a DIOPT conservation score ≥ 6. (B, C) Total sleep (B) and activity (C) in adult males with pan-neuronal RNAi-mediated knockdown of each of these Drosophila orthologs. The elav-GAL4 (elav>) pan-neuronal driver line was crossed to UAS-RNAi transgenes targeting individual gene orthologs, with n = 16 flies for each genotype. For controls, elav> was crossed to w1118, the genetic background for the RNAi lines, with n = 140 flies. When possible, two independent UAS-RNAi transgenes were used against each gene. Total sleep (minutes) of 3-to-7-day-old males was recorded over a 24-hour period. Transformant ID numbers for RNAi lines are listed in S1 Table. Genes for which knockdown caused lethality or a non-inflating-wing phenotype were not analyzed. Average activity was measured as the number of beam crosses per minute. Knockdown of LZTR1 in the nervous system reduces both total sleep and locomotor activity. Graphs represent means with SEM of data pooled from one to five independent experiments. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (* p<0.05).
Pan-neuronal knockdown of several of the Drosophila 22q11.2 gene orthologs caused changes in total sleep and activity in adult males (Fig 1B and 1C). Most of these manipulations led to a decrease in locomotor activity, with unchanged or increased sleep amount. Nervous system-specific knockdown of CG15908 (C22orf39), gonadal (DGCR6L), or slgA (PRODH) increased the total amount of sleep exhibited by males, while only the knockdown of CG3711, the Drosophila ortholog of LZTR1, caused a decrease in total sleep (Fig 1A). In contrast, in females, neuronal knockdown of several genes led to decreased sleep, including CDC45L (human CDC45), CG13192 (GNB1L), Es2 (DGCR14), and Septin4 (SEPT5) (S1A Fig). Locomotor activity was affected upon knockdown of several 22q11.2 genes, with most leading to hypoactive phenotypes in both males and females (Fig 1C and S1B Fig). We decided to focus our further studies on LZTR1, the only tested gene whose knockdown led to reduced total sleep in combination with decreased locomotor activity in males.
Neuronal nowl function is required for night-time sleep
To investigate the role of LZTR1 in sleep, we examined the sleep patterns of animals with neuronal knockdown of LZTR1 (the LZTR1 #1 RNAi line was used for further experiments; see S1 Table). In light of the reduced-night-time-sleep phenotype displayed by these animals, we chose to refer to this gene as night owl (nowl) (Fig 2A). We found that nervous-system-specific knockdown of nowl (elav>nowl-RNAi) reduced night-time sleep compared to controls [elav>+ and UAS-nowl-RNAi (nowl-RNAi)/+, i.e., elav> and nowl-RNAi lines crossed to the w1118 genetic background for the RNAi line]. To further investigate the effect of nowl loss, we examined the sleep phenotype of a homozygous-viable transposon-insertion mutant of nowl, Mi{ET1}CG3711MB12128 (for simplicity we hereafter refer to this allele as nowl1), which carries a 6-kb insertion into the third exon of nowl [25] (Fig 2B–2D). Transcript levels of nowl have previously been shown to be drastically reduced in this mutant [26]. Like animals with neuronal knockdown, nowl1 mutants exhibited decreased sleep, primarily during the second part of the night, compared with the genetic-background controls [w1118/Y and +/Y, where “w1118” denotes an X chromosome with a w- mutation (the genetic background for most Drosophila lines), “+” a wild-type X chromosome carrying w+, and “Y” a wild-type Y chromosome]. When sleep data were binned into day and night periods, it showed that nowl loss-of-function had a much larger effect on sleep during the night than during the day (Fig 2E–2G). Night-time sleep dropped dramatically in both males and females. Loss of one copy of nowl (on the X chromosome) in females did not elicit a phenotype, suggesting that the nowl1 mutation is a recessive loss-of-function allele, consistent with the transcript-level defect previously reported [26]. To further confirm these results, we generated a CRISPR/Cas9-mediated deletion of the nowl coding sequence. Like neuronal knockdown and the nowl1 mutation, this deletion mutant (nowlKO) showed reduced night-time sleep (S2A–S2C Fig). Taken together, these data indicate that nowl is important for normal sleep behavior, mainly during the night.
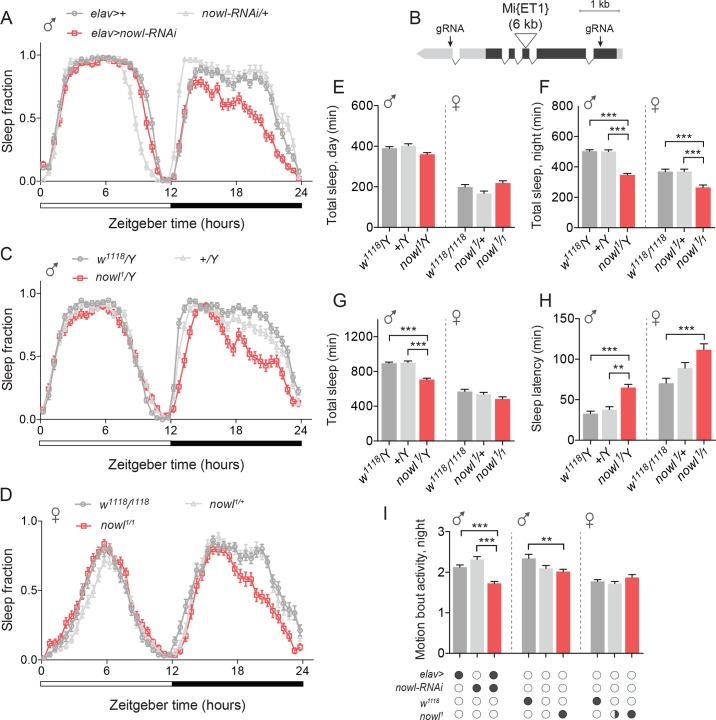
Fig 2. Neuronal loss of the LZTR1 homolog night owl (nowl) reduces night-time sleep in Drosophila.
(A) Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for controls (elav>+ and nowl-RNAi/+) compared to elav>nowl-RNAi. Sleep data are binned into 30-minute intervals. (B) Schematic of the nowl locus. The gene comprises 5′ and 3′ untranslated regions (light grey) and a multi-exon coding region (dark grey). The locations of the 6-kb Mi{ET1} Minos transposable element inserted in the nowl1 allele and the guide-RNA (gRNA) target sites used to induce the nowlKO CRISPR/Cas9-mediated lesion are indicated. (C, D) Sleep profiles for (C) male nowl1/Y and (D) female nowl1/nowl1 mutant flies, compared to control genotypes [in C: w1118 nowl+/Y (w1118/Y) and w+ nowl+/Y (+/Y); in D: w1118/1118 and nowl1/+]. (E-F) Total day- and night-time sleep (minutes) in male and female flies. (E) Total daytime sleep in male and female nowl1 mutant flies. (F) Total nighttime sleep is significantly decreased in hemizygous male and homozygous female nowl1 mutants, but not in heterozygous females. (G) Overall total sleep (min.) in male and female nowl1 mutant flies compared to controls. Loss of nowl in male flies significantly decreases total sleep compared to controls. (H) Sleep latency measured for male and female nowl1 mutant flies compared to controls. Loss of nowl in both male and female flies significantly increases sleep latency compared to controls. (I) Quantification of motion-bout activity (min.) during night. Motion-bout activity during the night is significantly reduced in nowl-knockdown flies. Graphs represent means with SEM (n = 32–92) of data pooled from one to three independent experiments. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (* p<0.05, ** p<0.01, *** p<0.001).
Although we observed a decrease in sleep levels overall, sleep levels in these flies still dropped in anticipation of light-dark and dark-light transitions, consistent with an intact circadian clock. To investigate whether nowl mutant males do maintain a normal circadian rhythm, adult animals entrained to a 12/12-hour light-dark cycle were kept under constant darkness (free-running conditions) for 8 days and analyzed from 2 h after lights-off transition for 6 days. The average circadian period length of nowl1 mutants during these days was 23.5 hours, which is very similar to the corresponding controls’ free-running periods of 23.6 and 23.5 hours (S3A and S3B Fig). This indicates that the nowl mutants can entrain and maintain a normal circadian rhythm with a period similar to the controls. However, when looking at the strength of the circadian clock, we found a reduction in total percentage of rhythmic flies and reduced strength of rhythmicity in flies lacking nowl in the nervous system (S3C and S3D Fig). This indicates that these animals have a weaker circadian rhythm, although the nowl mutants can maintain a normal period.
Loss of nowl disrupts sleep architecture and phenocopies lack of Cul3 and Nf1
Sleep-onset difficulties are common in psychiatric disorders [27]. Interestingly, male and female nowl mutants exhibit increased sleep-onset latency, defined as the time between lights-off and the first sleep bout; that is, they take longer to fall asleep than controls (Fig 2H and S2D Fig). Control males’ latency was 40 minutes, while hemizygous nowl mutants on average took 70 minutes to fall asleep after lights-off. Homozygous nowl-mutant females also exhibited delayed sleep onset and on average remained active for 110 minutes before their first sleep episode, while control females fell asleep after 70 minutes. However, no sleep-latency phenotype was observed in animals with RNAi-mediated knockdown of nowl in the nervous system, suggesting that maybe stronger loss of nowl function than provided by RNAi is required to affect sleep onset (Fig 2A).
To confirm that the observed phenotype was related to sleep per se and not to altered activity patterns, we analyzed average activity during periods in which the animals were active in the night. Motion-bout activity was significantly reduced when nowl was knocked down in the nervous system compared to driver- and UAS-alone control genotypes (Fig 2I), while nowl mutant flies also displayed reduced or unchanged motion-bout activity levels compared to control flies. This confirms that the observed sleep phenotype in animals lacking nowl is not the result of hyperactivity but rather is a specific sleep-disruption phenotype.
We next examined sleep fragmentation, which is associated with many psychiatric disorders and characterized by multiple short periods of sleep, i.e., an increased number of shorter sleep bouts. In the initial screen of genes within the 22q11.2 deletion, we found that knockdown of some genes including slgA (PRODH) and nowl/LZTR1 also altered the number and duration of sleep bouts (S4A–S4D Fig). To further investigate whether loss of nowl plays a role in sleep maintenance following sleep initiation, we analyzed average sleep-bout number and length during day and night in males lacking nowl. Knockdown of nowl in the nervous system using either of the two pan-neuronal drivers elav> and nSyb-GAL4 (nSyb>) caused a strong increase in fragmentation of night-time sleep, with a large decrease in sleep-bout duration combined with an increase in the number of sleep bouts, that was not observed during daytime (Fig 3A–3D and S5A–S5C Fig). Mutant flies carrying the nowl1 loss-of-function allele or the CRISPR-induced nowlKO deletion exhibited similar night-time sleep-fragmentation patterns (Fig 3A–3D and S2E–S2H Fig).
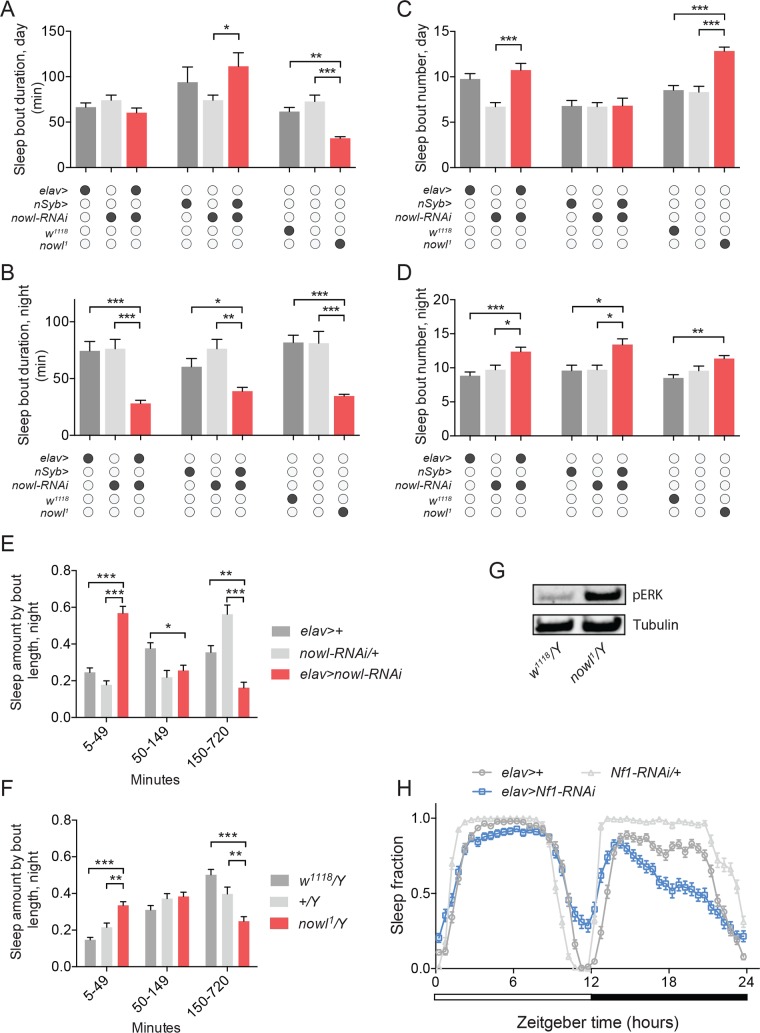
Fig 3. Loss of nowl in the nervous system causes sleep fragmentation.
(A-D) Quantification of average sleep-bout duration (A and B) and number (C and D) during day and night for males with pan-neuronal knockdown of nowl using the stronger (elav>) and weaker (nSyb>) GAL4 drivers and the nowl1 insertional mutant. Neuronal knockdown of nowl using elav> (elav>nowl-RNA) and nSyb> (nSyb>nowl-RNAi) or mutation disrupting nowl (nowl1/Y) result in decreased sleep-bout length and increased sleep-bout number, with a stronger effect during night-time, compared to controls (elav>+, nowl-RNAi/+, nSyb>/+, w1118/Y, and +/Y, which does not carry any transgene or the w1118 mutation and therefore is denoted by open circles). (E-F) Fraction of time male animals spent sleeping in short (5-49-min.), medium (50-149-min.) or long (150-720-min.) sleep bouts during night for controls (elav>+ and nowl-RNAi/+) compared to elav>nowl-RNAi animals and for nowl1 (nowl1/Y) mutants compared to controls (w1118/Y and +/Y). Flies carrying a nowl mutation or in which nowl has been knocked down neuronally spend significantly less time in long consolidated sleep episodes, compared to controls. (G) Western-blot analysis shows increased levels of phospho-ERK (pERK) in nowl mutant males compared to controls (w1118/Y), standardized to alpha-Tubulin. (H) Sleep pattern of Neurofibromin-1 (Nf1) knockdown males under a 12-hour light/dark (white and black bars) cycle in 30-minute intervals shows effects on sleep compared to controls (elav>+ and Nf1-RNAi/+) similar to animals lacking nowl, with decreased sleep mainly during the night. Graphs represent means with SEM (n = 32–92) of data pooled from one to three independent experiments. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (* p<0.05, ** p<0.01, *** p<0.001).
To more closely examine the sleep changes associated with night-time sleep fragmentation, we analyzed the distribution of sleep episodes by binning them by duration and calculating the time spent in each bin, for flies with reduced nowl expression in the nervous system and for nowl mutants, compared to control genotypes. Neuronal knockdown of nowl significantly increased the amount of time spent in short sleep bouts of 5–49 minutes, compared to control flies, which consolidated most of their night-time sleep into longer bouts of 150–720 minutes (Fig 3E). A similar change was observed in nowl mutant flies (Fig 3F). These data indicate that nowl is required for maintenance of proper sleep architecture in Drosophila.
Human LZTR1 was recently shown to function through the Cul3-based ubiquitin ligase complex, as a component of which it is suggested to mediate substrate specificity [28, 29]. Interestingly, Cul3 has also been implicated as a causative gene in psychiatric disorders [30] and has been shown to play an important role in Drosophila sleep [15, 16, 31]. We wondered whether nowl, like its ortholog LZTR1, might exert its effects on sleep through an interaction with Cul3. We therefore knocked down Cul3 pan-neuronally and analyzed sleep patterns, and found that Cul3 silencing in the nervous system caused a significant decrease in average sleep-bout duration and a significant increase in average sleep-bout number (S6A–S6E Fig), during both day and night. This phenotype is similar to the sleep disruption caused by neuronal knockdown of nowl, which is consistent with an interaction between the two genes.
Mutations in human LZTR1 have been linked with schwannomatosis, a form of neurofibromatosis, a neurological disorder associated with sleep problems, mental disabilities, and psychiatric disorders that can also be caused by mutation of the Nf1 gene [18]. Nf1 encodes a GTPase-activating protein that negatively regulates the Ras signaling pathway. We therefore asked whether nowl, like Nf1, affects Ras signaling, by measuring the levels of phosphorylated ERK (pERK), a downstream effector of Ras and an indicator of Ras-pathway activation. We found increased levels of pERK in heads from nowl mutants (Fig 3G), indicating that the Ras pathway is overactivated, consistent with previous findings [29]. Thus, the Nowl protein does appear to be required for normal Ras-pathway inhibition, either directly, like Nf1, as a negative regulator of Ras, or indirectly. We next investigated whether Nf1 regulates sleep and found that neuronal knockdown of Nf1 reduces night-time sleep nearly identically with loss of nowl (Fig 3H). These similarities are consistent with a sleep-regulating genetic interaction between nowl and Nf1. To exclude the possibility that the effect of nowl and Nf1 on sleep is associated with increased neuronal proliferation or tumor formation, we next tested whether loss of these genes causes cell proliferation in the nervous system. Staining for phospho-Histone H3, a marker of mitosis, showed an absence of proliferating cells in the central nervous system of animals with knockdown of nowl or Nf1 (S7A Fig). In humans, neurofibromatosis often leads to tumors in peripheral nerves, so we also tested whether loss of nowl or Nf1 affects growth of peripheral nerves in Drosophila to exclude tumor formation in these neurons. Knockdown of nowl or Nf1 in peripheral class-IV dendritic arborization (da) neurons did not lead to morphological abnormalities in these neurons, including their dendritic and axonal structures (S7B Fig). Together, this shows that knockdown of nowl or Nf1 does not cause increased proliferation in the nervous system, suggesting that the associated phenotypes are caused by specific requirements for these genes in sleep-regulatory pathways.
In addition to circadian regulation, sleep is under homeostatic control, which allows recovery of lost sleep after insufficient sleep [32]. To analyze whether nowl or Nf1 influences homeostatic sleep drive, we sleep-deprived animals and measured recovery sleep. To assess recovery sleep, we mechanically sleep-deprived flies for the final 6 hours during the night of the second day and measured sleep rebound. This treatment resulted in sleep loss in both controls and animals with neuronal knockdown of nowl or Nf1 (Fig 4A and 4B). Following sleep deprivation, animals with neuronal knockdown of nowl or Nf1 exhibited rebound sleep and recovered similarly to controls, showing the ability to recover lost sleep (Fig 4C). These results suggest that homeostatic recovery sleep is not altered by neuronal loss of nowl or Nf1. Animals increased their average sleep-bout length during the first two hours following sleep deprivation (Fig 4D). However, this was less pronounced in flies with neuronal knockdown of nowl or Nf1, indicating impaired sleep consolidation with increased fragmentation.
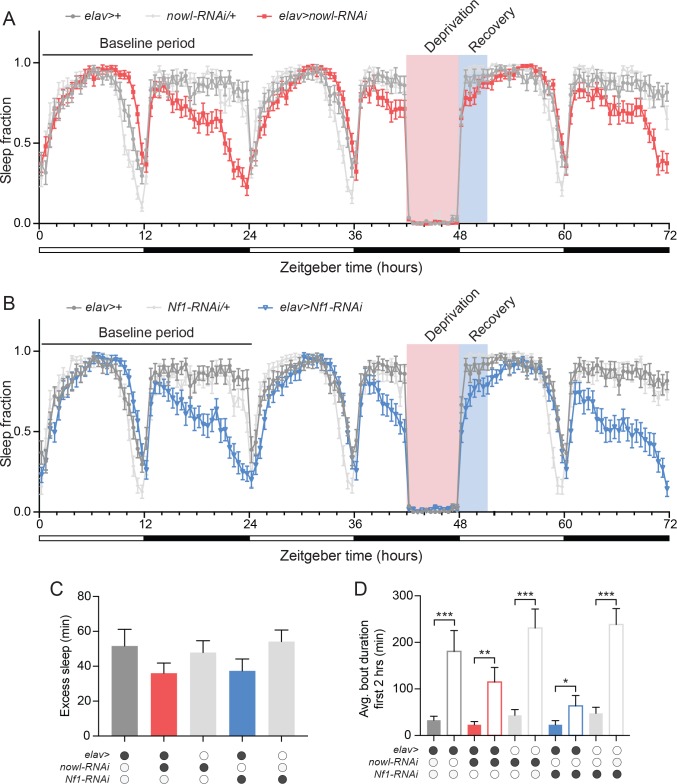
Fig 4. Homeostatic sleep recovery is not affected by neuronal loss of nowl or Nf1.
(A and B) Daily sleep profiles of males under a 12-hour light/dark (white and black bars) cycle in 30-minute intervals for 72 hours for the first (baseline), second (deprivation–flies were subjected to mechanical sleep deprivation for the final 6 hours during night), and third (recovery) days in controls (elav>+, nowl-RNAi/+, and Nf1-RNAi/+) and animals with neuronal knockdown of nowl or Nf1 (elav>nowl-RNAi and elav>Nf1-RNAi). Pink indicates sleep deprivation, while blue indicates recovery periods. (C) Quantification of recovery sleep (excess sleep) during the first 3 hours following sleep deprivation compared to the same period the first baseline day, shows rebound sleep for controls and animals with neuronal knockdown of nowl and Nf1. No significant differences were observed between genotypes. (D) Average duration of sleep-bouts initiated within the first 2 hours of the light phase was increased on the recovery day immediately following sleep deprivation compared to the first baseline day to a greater extent in controls than in animals with neuronal knockdown of nowl or Nf1. Filled bars represent the baseline day, and hollow bars represent the recovery day. Graphs represent means with SEM (n = 23–32) of data from one experiment. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05, ** p < 0.01, *** p < 0.001).
Interactions between nowl and Nf1 suggest that they act on a common sleep-regulatory pathway
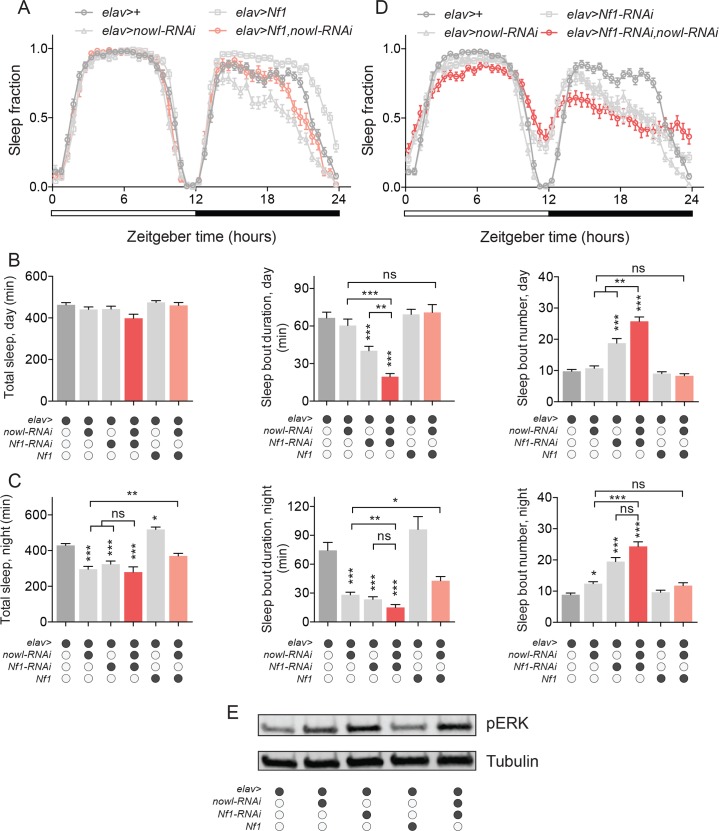
To investigate the possibility of genetic interaction between nowl and Nf1 in the regulation of sleep, we overexpressed Nf1 in nowl-RNAi animals. In this test, a rescue of the nowl short-sleep phenotype would indicate that Nf1 functions downstream of nowl in a sleep-regulatory pathway. Neuronal overexpression of Nf1 partially rescued the loss of night-time sleep and sleep fragmentation caused by nowl knockdown (Fig 5A–5C and S8 Fig), suggesting that nowl and Nf1 may function in the same sleep-regulatory pathway, with Nf1 working downstream of Nowl. Next, we analyzed interaction by comparing effects of the single-hit knockdown compared to the double-hit knockdown of nowl and Nf1. As an initial step, we determined the efficiency and specificity of the RNAi-mediated knockdown using qPCR, and validated that the RNAi lines against nowl and Nf1 both significantly reduced target-gene expression (S9A and S9B Fig). In animals with simultaneous knockdown of nowl and Nf1 in the nervous system, sleep fragmentation was greater than in animals with knockdown of either nowl or Nf1 alone and increased compared to the control, indicating that nowl and Nf1 interact in the regulation of daytime sleep (Fig 5B and 5D). In animals with double knockdown of nowl and Nf1, total night-time sleep was reduced, similar to animals with single knockdown of nowl or Nf1, compared to the control (Fig 5C). Simultaneous knockdown of nowl and Nf1 also increased night-time sleep fragmentation compared to the control. However, the effect of the double knockdown on night-time sleep parameters largely resembled the effect of Nf1 knockdown alone, suggesting an interaction between nowl and Nf1 in night-time sleep regulation that may be epistatic. The ability of Nf1 overexpression to partially rescue the overall loss of night-time sleep and the reduced sleep-bout duration during the night supports the notion that these two genes are functionally related and may converge on a common sleep-regulatory pathway. To exclude indirect developmental effects, we next asked whether nowl and Nf1 influence sleep through effects occurring during development or whether these genes have acute effects on sleep in the adult brain. Using the RU486-activated GeneSwitch system [33] to restrict knockdown to the adult stage and thus to avoid indirect developmental effects, we induced neuronal knockdown of nowl and Nf1 in adults. Adult-specific neuronal knockdown of nowl or Nf1 decreased night-time sleep compared to controls (S10A and S10B Fig) and caused an increased number of short sleep bouts during the night (S10C–S10F Fig). These adult-specific manipulations suggest that the sleep phenotypes caused by loss of nowl and Nf1 are unlikely due to developmental effects and that adult-specific function of Nowl and Nf1 is important for normal sleep.
Fig 5. nowl and Nf1 interact in the regulation of sleep.
(A) Daily sleep profiles of males under a 12-hour light/dark (white and black bars) cycle in 30-minute intervals. In elav>Nf1, nowl-RNAi animals (with neuronal overexpression of Nf1 and simultaneous knockdown of nowl), night-time sleep is partially restored compared to nowl knockdown alone. (B and C) Quantification of daytime (B) and nighttime (C) total sleep, sleep-bout duration, and sleep-bout numbers in males. The double knockdown (elav>nowl-RNAi, Nf1-RNAi) shows significant interactions between nowl and Nf1 in daytime total sleep and sleep-bout duration and number, indicating an additive effect in the daytime. The effects of double knockdown on total nighttime sleep and nighttime sleep-bout duration and number are not different from Nf1-RNAi alone, suggesting an epistatic relationship between nowl and Nf1 in night-time sleep regulation. Nf1 overexpression partially rescues the nowl-knockdown effect on sleep. Controls are elav>+. Additional effector line controls (nowl-RNAi/+, Nf1-RNAi/+, and Nf1/+) are shown in S8 Fig Genotypes that are indicated as different from the elav>+ controls are also statistically different (P< 0.05) from controls shown in S8 Fig (D) Daily sleep profiles of double-knockdown male animals (elav>Nf1-RNAi, nowl-RNAi) display a pronounced decrease in sleep. Controls are elav>+. (E) Western blotting against phosphorylated ERK (pERK) in males shows that single and double knockdowns of nowl and Nf1 cause increased pERK levels, standardized to alpha-Tubulin (controls are elav>+). Western blot is representative of two independent experiments. Graphs represent means with SEM (n = 32–81) of data pooled from one to three independent experiments. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05, ** p < 0.01, *** p < 0.001).
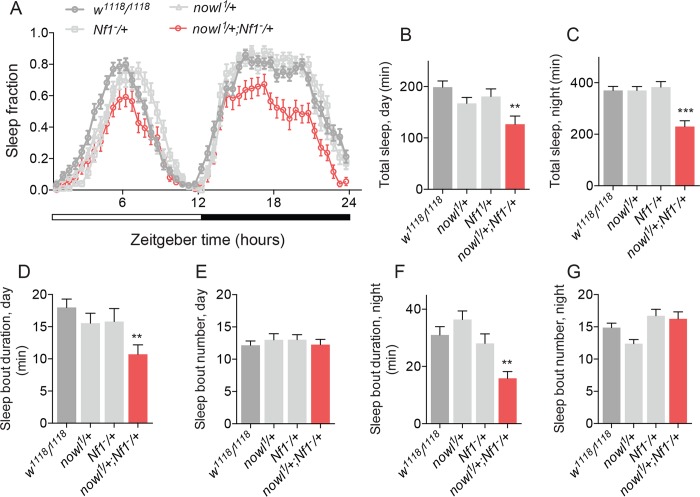
Since our results indicated that Nowl inhibits the Ras pathway (Fig 3G), we investigated the combined effect of nowl and Nf1 loss on the activation of the Ras pathway. Our data show that silencing nowl or Nf1 individually in the nervous system results in Ras overactivation, reflected in increased pERK levels (Fig 5E). Consistent with this and with their genetic interaction in sleep regulation, the simultaneous knockdown of nowl and Nf1 activates the Ras pathway to an extent similar to knockdown of Nf1 alone. Together these effects indicate that nowl and Nf1 interact to suppress Ras-pathway activity. To investigate the genetic interaction between nowl and Nf1 further, we compared the phenotypes of Nf1 and nowl trans-heterozygous mutants with those of the individual heterozygous mutants. The nowl gene is on the X chromosome, and males are therefore hemizygous; heterozygous effects can only be studied in females. Female animals heterozygous for either nowl or Nf1 displayed similar sleep patterns, with no significant loss of night-time sleep compared to the control (Fig 6A–6C), whereas trans-heterozygous nowl+/- Nf1+/- mutant females exhibited a strong reduction in total sleep. Furthermore, trans-heterozygous nowl+/- Nf1+/- mutants displayed shorter sleep bouts, while animals heterozygous for mutations in either nowl or Nf1 alone displayed no change in sleep-bout duration compared to controls (Fig 6D–6G). This indicates that trans-heterozygous loss of both nowl and Nf1 causes sleep fragmentation, further supporting the existence of a genetic interaction between nowl and Nf1 and a functional relationship between these two genes in the regulation of sleep.
Fig 6. Interactions between nowl and Nf1 suggest that they act on a common sleep-regulatory mechanism.
(A) Sleep patterns of heterozygous nowl1/+ and Nf1-/+ females show that one copy of each is sufficient for maintaining proper sleep, while the trans-heterozygous (nowl1/+; Nf1-/+) mutants exhibit decreased sleep during both day and night. (B-G) Quantification of sleep parameters shows reduced average sleep-bout duration of trans-heterozygous nowl1/+; Nf1-/+ females, indicating that nowl and Nf1 interact to maintain sleep. Graphs represent means with SEM (n = 32) of data from one experiment. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05, ** p < 0.01, *** p < 0.001).
Neuronal Nf1 and nowl are required to maintain metabolic homeostasis
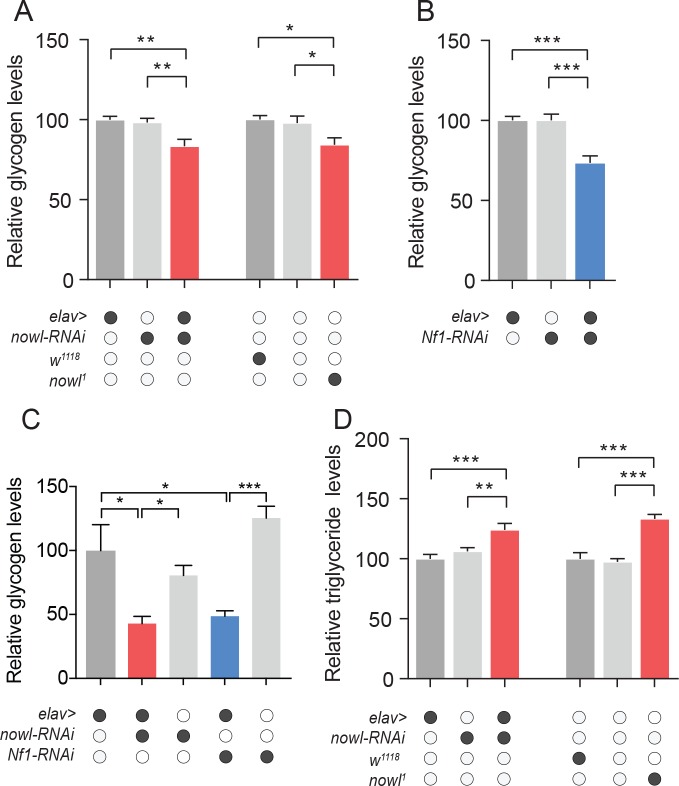
Emerging evidence suggests that sleep is important for energy homeostasis and that insufficient sleep can lead to obesity [34]. A proposed main function of sleep is to provide an opportunity to replenish brain supplies of glycogen, which are depleted during periods of wakefulness [35, 36]. Since nowl and Nf1 loss decrease night-time sleep and thereby increase wakefulness, we analyzed glycogen levels in these animals. Consistent with the notion that sleep is important for maintaining glycogen levels, we found that indeed whole-body glycogen levels were reduced in animals with neuronal knockdown of nowl and Nf1 and in nowl mutants (Fig 7A and 7B), which exhibits reduced night-time sleep. These data further suggest that neuronal function of nowl and Nf1 is essential for maintenance of glycogen stores, which may be related to their importance for getting enough sleep. Since sleep deprivation reduces brain glycogen [37], we asked whether neuronal knockdown of nowl or Nf1, which causes reduced night-time sleep, affects the glycogen content of the head. Heads of animals with neuronal knockdown of nowl or Nf1 showed reduced glycogen levels (Fig 7C), suggesting that nowl and Nf1 play a role in the regulation of brain glycogen metabolism. We next analyzed whole-body triglyceride levels, since the 22q11.2 deletion is associated with increased risk of obesity, which is also linked to insomnia [38, 39]. We found increased organismal levels of triglycerides in flies with neuronal knockdown of nowl and in nowl mutants (Fig 7D), suggesting that they exhibit increased adiposity, like human 22q11.2 carriers. Taken together our results suggest that nowl is required in the nervous system to maintain organismal energy homeostasis, a function that might be related to its role in sleep, considering the vital role of sleep in the regulation of energy homeostasis.
Fig 7. Loss of nowl and Nf1 leads to physiological changes that affect energy homeostasis.
(A-C) Quantification of whole-body glycogen (A, B) and triglyceride (D) content, and head glycogen (C), in adult male flies. Neuronal knockdown of nowl (elav>nowl-RNAi) or Nf1 (elav>Nf1-RNAi) or mutation disrupting nowl (nowl1/Y) results in reduced whole-body glycogen levels (A, B) compared to controls (elav>+, nowl-RNAi/+, Nf1-RNAi/+, w1118/Y, and +/Y). (C) Effect of neuronal nowl and Nf1 knockdown on glycogen content of the head. (D) Whole-body lipid content is increased in animals with neuronal knockdown of nowl or a mutation disrupting nowl. Graphs represent means with SEM (n = 5–20) of data from one experiment. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05, ** p < 0.01, *** p < 0.001).
Nf1 and nowl are required in GABA-responsive cells for night-time sleep
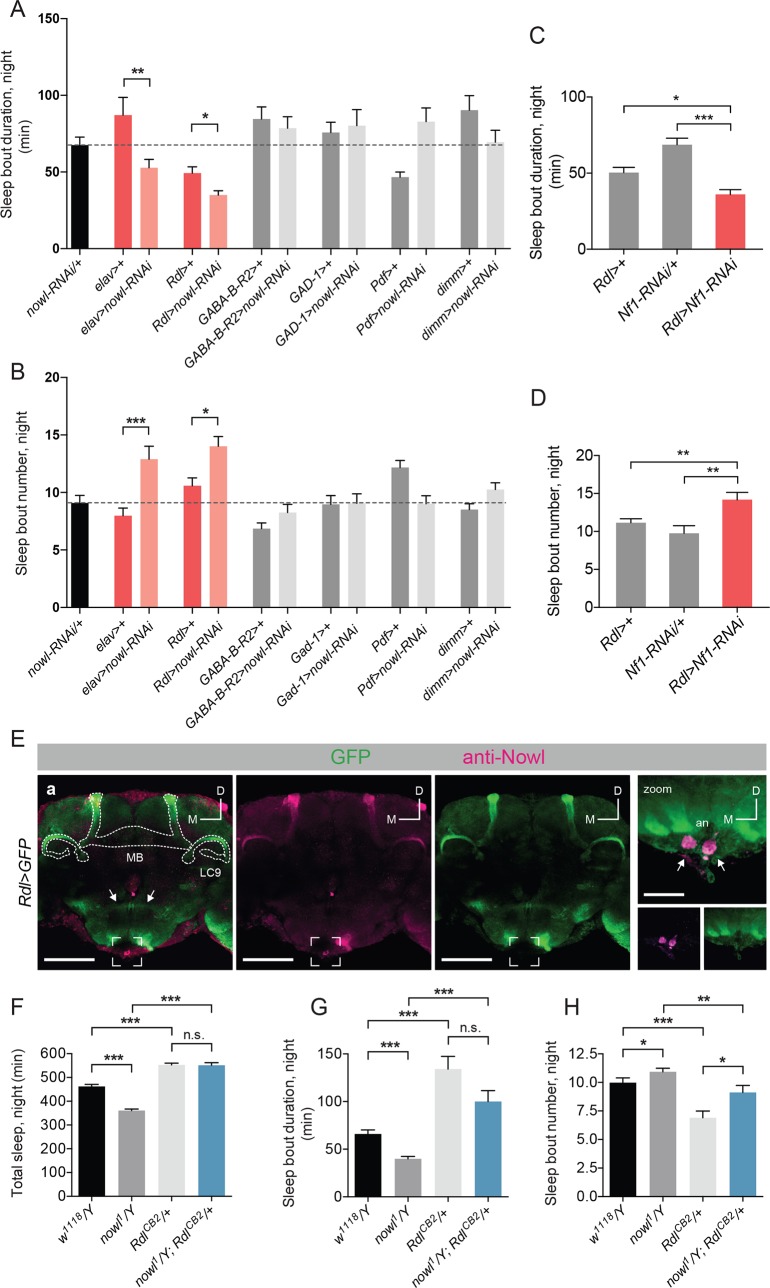
Changes in neuronal circuitry and neurotransmitter systems have been proposed to underlie several neuropsychiatric disorders. Signaling through GABA, in particular, has been found to be an important contributor to sleep in mammals [40]. GABAergic signaling has likewise been found to play a role in Drosophila night-time sleep initiation and maintenance [41–43]. Because we observed a strong disruption of both of these parameters in nowl knockdown and mutant flies, we wondered whether this gene might have a function in GABAergic signaling. To investigate this, we knocked down nowl in GABA-producing or GABA-responsive neurons using a panel of GAL4 driver lines. GABA-producing neurons were targeted using Gad1-GAL4 (Gad1>), which drives expression in neurons expressing the GABA-biosynthetic enzyme glutamate decarboxylase 1 (GAD 1). Rdl-GAL4 (Rdl>) was used to drive expression in neurons expressing the ionotropic GABAA receptor, while GABA-B-R2-GAL4 (GABA-B-R2>) was used to target neurons expressing the metabotropic GABAB receptor subtype 2. Furthermore, GABAergic innervation has been shown to regulate “clock” neurons expressing the Pigment Dispersing Factor (PDF) peptide, comprising a limited number of wake-promoting neurons, the small and large ventral lateral neurons (s-LNv and l-LNv, respectively) [32, 44]. To assess the function of nowl in these neurons, the Pdf-GAL4 (Pdf>) driver was used to drive expression in all PDF-expressing neurons, while the dimm-GAL4 (dimm>) driver allowed for knockdown in a broad array of peptidergic neurosecretory cells, including the l-LNvs, suggested to be the only sleep-regulatory neurons in this subset of cells [45]. Since global or neuronal loss of nowl leads to highly fragmented night-time sleep, we asked whether knockdown of nowl in any of these restricted neuronal subsets could elicit a similar effect on sleep maintenance. Interestingly, we found that knockdown of nowl using the Rdl> driver, thus specifically in GABAA-receptor-expressing neurons, caused a shortening of sleep bouts and an increase in their number during night, with an effect size similar to that seen with pan-neuronal knockdown (Fig 8A and 8B). Rdl encodes the Drosophila GABAA receptor, a ligand-gated chloride channel that mediates the fast inhibitory effects of GABA, and it has been shown to affect several behaviors such as learning and sleep [43, 46]. Knockdown of nowl in other neuronal populations using the other drivers–targeting the GABA-producing cells, the GABAB-receptor-expressing neurons, and the PDF-expressing clock neurons–had no significant effect on night-time sleep-bout number or duration.
Fig 8. nowl is required in GABA-responsive neurons for sleep maintenance, and increased GABA signaling rescues the sleep phenotype of nowl mutants.
(A-D) Quantification of average male night-time sleep-bout duration and length. (A) Sleep-bout duration is decreased, and (B) sleep-bout number is increased during the night when nowl is knocked down pan-neuronally (elav>nowl-RNAi) or specifically in GABAA-receptor Rdl-expressing neurons (Rdl>nowl-RNAi) compared to controls (elav>+ and Rdl>+), respectively. Knockdown animals were compared to the GAL4 driver line crossed to w1118 (the genetic background for the RNAi), and elav>nowl-RNAi was used as a positive control. (C) Sleep-bout duration is decreased and (D) sleep-bout number increased in animals with knockdown of Nf1 in Rdl-expressing neurons compared to controls (Rdl>+ and Nf1-RNAi/+). (E) Anti-Nowl staining (purple) in males is prominent in Rdl-expressing neurons (Rdl>GFP, green) of the mushroom body (MB), among other sites. The neurons of the α′/β′ lobes of the MB (outlined; “MB”) and the neurons projecting to the LC9 optic glomerulus (outlined; “LC9”) express both Rdl>GFP and Nowl. Interneurons connecting the paired olfactory lobes (arrows) express Rdl>GFP but not Nowl, and a pair of neuronal clusters at the tip of the subesophageal ganglion (bracketed and zoomed in right panel) strongly express Nowl but not Rdl>GFP, indicating independent expression and staining. (F-H) Quantification of male night-time total sleep and average sleep-bout duration and number for w1118/Y, nowl1/Y, RdlCB2/+, and nowl1/Y; RdlCB2/+. (F) The reduced night-time sleep exhibited by nowl1 mutant flies is rescued by introducing one copy of the RdlCB2 allele. (G, H) Sleep-bout duration during the night is significantly decreased in nowl mutants, and sleep-bout number is significantly increased. Introducing one copy of the RdlCB2 allele partially rescues both defects. Graphs represent means with SEM (n = 32–156) of data pooled from one to five independent experiments. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (* p<0.05, ** p<0.01, *** p<0.001).
Since our data show that nowl interacts genetically with Nf1 in the regulation of night-time sleep (Figs 5 and 6), we asked whether Nf1, like nowl, also regulates sleep maintenance through specific effects in the GABAA-receptor Rdl-expressing neurons. Consistent with the interaction of nowl and Nf1 in sleep regulation, knockdown of Nf1 in Rdl-expressing neurons produced a sleep-fragmentation phenotype characterized by more frequent sleep bouts of decreased duration, similar to loss of nowl in these GABA-responsive neurons (Fig 8C and 8D). Together these data suggest that both nowl and Nf1 are specifically required in GABA-responsive Rdl-expressing neurons to maintain sleep after sleep onset. The lack of knockdown effect in the PDF-expressing neurons further supports the independence of the night-time sleep phenotype of nowl-knockdown flies from circadian clock function.
To investigate the expression of Nowl in the brain, particularly in GABAA-receptor Rdl-expressing neurons, we generated an anti-Nowl antibody. Colocalization of mCD8::GFP expression in the Rdl-positive neurons with staining against Nowl shows that the mushroom bodies (MBs) express both the GABAA-receptor Rdl and Nowl (Fig 8E). Interestingly, downregulation of Rdl in the α′/β′ neurons of the MBs causes sleep loss similar to knockdown of nowl [47]. Together these data are consistent with a model in which Nowl promotes night-time sleep via modulation of GABA signaling that regulates the activity of the Rdl-expressing mushroom-body α′/β′ lobes.
Increased inhibitory signaling through the GABAA receptor rescues nowl mutant sleep phenotype
Knockdown of nowl in GABAA-receptor Rdl-expressing neurons led to disruptions in sleep architecture, similar to the effects observed when decreasing inhibitory GABAergic signaling via Rdl [47]. We therefore hypothesized that loss of nowl might affect sleep by reducing inhibitory GABAergic signaling effects in these neurons, thus disinhibiting them, which should promote wakefulness and reduce sleep [42, 43]. To address this possibility, we asked whether the RdlCB2 mutation would rescue the nowl mutant sleep phenotype. RdlCB2 mutant flies express a form of the GABAA receptor Rdl that is thought to exhibit reduced desensitization, leading to longer channel-opening duration, increased channel flux, and therefore increased neuronal inhibition downstream of GABA reception [41]. We rationalized that if loss of nowl increases neuronal excitability due to loss of GABAA-receptor signaling in Rdl-expressing neurons, the mutant RdlCB2 receptor might compensate for this and thereby rescue the sleep disturbance observed in nowl loss-of-function flies. Compared to control flies, introduction of one copy of the RdlCB2 allele significantly increased total sleep during the night, consistent with the expected effect of increased inhibitory GABA signaling. As we previously observed, nowl mutant flies displayed significantly decreased total sleep during the night. This effect was completely rescued in flies carrying both nowl1 and RdlCB2 (Fig 8F and S11 Fig). The addition of RdlCB2 caused a decreased number of longer night-time sleep bouts, eliminating the night-time sleep fragmentation induced by the loss of nowl function (Fig 8G and 8H). Taken together, these data indicate that increased inhibitory signaling through the GABAA receptor Rdl largely rescues the sleep disturbances observed in nowl loss-of-function flies. We thus propose that nowl affects sleep by modulating GABA signaling in Rdl-expressing neurons.
Discussion
Schizophrenia is a highly heritable disorder with a prevalence of 1% in the population [48, 49]. It encompasses a wide spectrum of symptoms, of which a major one is sleep disturbance [9]. The human 22q11.2 chromosomal deletion spans more than 40 genes, and children carrying this deletion are 20 to 25 times more prone to developing schizophrenia during adolescence than their peers [50]. To identify which 22q11.2 genes might underlie the syndrome and development of schizophrenia in human carriers, we screened for conserved genes within this deletion that are required for sleep in Drosophila by RNAi-induced knockdown in the nervous system. Neuronal knockdown of several genes each caused abnormal sleep patterns and activity in flies. In females, silencing of several genes including CDC45L (human CDC45), CG13192 (GNB1L), Es2 (DGCR14), and Septin4 (SEPT5) resulted in short-sleep phenotypes. Some of these genes have not previously been linked to behavioral deficits in schizophrenia. However, we observed that neuronal knockdown of CG13192 (human GNB1L) and slgA (PRODH) resulted in an increased number of sleep episodes that have a shorter length. PRODH catalyzes a step in the conversion of proline to glutamate, while GNB1L encodes a G-protein beta-subunit-like polypeptide [51, 52]; both genes have also been associated with schizophrenia and other mental disorders [51, 53–55]. In males, neuronal knockdown of several genes increased total sleep, while only nowl knockdown caused a decrease in total sleep.
We show here that nowl, the conserved Drosophila homolog of the human LZTR1 gene located within the 22q11.2 deletion, is required for night-time sleep initiation and maintenance. Mutation or neuronal knockdown of nowl decreased total sleep amount and caused highly fragmented sleep, especially during night-time, which is a phenotype frequently seen in mental illness [27]. Schizophrenia is a neurodevelopmental disorder believed to involve developmental pathology [56]. Our findings show that knockdown of nowl or Nf1 both during development and only in the adult stage results in reduced and fragmented night-time sleep. Specific knockdown in the adult stage also results in similar sleep phenotypes, indicating that nowl and Nf1 are required in the adult stage for normal night-time sleep. However, developmental effects cannot be completely excluded by these experiments with knockdown at the time of adult eclosion, since some changes within the nervous system occur post-eclosion.
In addition, nowl mutants exhibit highly delayed sleep onset (i.e., increased sleep-onset latency). Sleep-onset latency is a widely used measure in the study of human sleep disorders and psychiatric illnesses, including schizophrenia [27]. Accumulating evidence also indicates that sleep sufficiency is important for the maintenance of energy balance and that reduced or poor sleep increases the risk of developing obesity [34]. Sleep is tightly connected to metabolic processes, and one proposed function of sleep is that it serves a key role in replenishing glycogen stores, which are depleted during wakefulness [35, 36]. Consistent with this notion, brain glycogen levels are highest during periods of sleep and decrease following rest deprivation in Drosophila [37]. Our finding of reduced glycogen levels in whole body and heads of flies lacking nowl or Nf1 function is in alignment with the view that sleep disruption is associated with depletion of glycogen stores, although further studies are needed to determine whether this effect is linked to alterations in sleep or to more-direct effects on metabolism. Furthermore, our results show increased adiposity associated with loss of nowl in flies, suggesting that LZTR1 loss may contribute to the increased genetic susceptibility to develop obesity found in human 22q11.2 carriers [38]. Taken together the defects in sleep initiation and maintenance observed in nowl mutants suggest that LZTR1 loss may contribute to the symptoms observed in the 22q11.2 DS and in schizophrenia, including the increased incidence of obesity.
Delayed sleep onset and the short-sleep phenotype could be caused either by alterations in sleep homeostasis or by disturbances in circadian rhythm. Our data indicate that animals lacking nowl function exhibit a normal circadian period length and suggests that their reduced night-time sleep is not related to problems with homeostatic sleep regulation. Animals with neuronal loss of nowl or Nf1 have a normal capacity to recover lost sleep at beginning of the day, although this does not rule out night-time effects. Furthermore, the night-time short sleep of animals with loss of nowl function is not a consequence of hyperactivity, which demonstrates that the sleep fragmentation observed in these animals is a specific sleep-disturbance phenotype, likely associated with inability to sustain long periods of sleep. Since nowl encodes a highly conserved protein (51% identity and 67% similarity to the human LZTR1 protein), Nowl may govern an evolutionarily conserved mechanism that regulates night-time sleep in animals.
Human LZTR1 has been shown to interact with Cul3 in the Cul3 ubiquitin-ligase complex [28]. Cul3 in Drosophila has been associated with sleep and interacts physically in this regulation with Insomniac, another BTB/POZ-domain protein (like Nowl) that functions as a substrate adaptor in the Cul3 complex [16, 31]. Although Cul3 knockdown and nowl knockdown are phenotypically similar, Cul3 knockdown induces broader phenotypes including disruption of the circadian clock [15]. Future studies should determine whether Nowl regulates sleep through a physical interaction with Cul3. Recent studies have found that LZTR1 may function in the Cul3 ubiquitin ligase complex to ubiquitinate Ras [28, 29, 57], which has previously been implicated in sleep regulation [58]. Nf1 is another important regulator of Ras signaling, and mutations in Nf1 and LZTR1 both give rise to types of neurofibromatosis [18, 59]. We show that nowl is required for proper negative regulation of Ras signaling, similar to Nf1, and that nowl and Nf1 interact in the regulation of sleep. Heterozygotes for either nowl or Nf1 mutations exhibit normal sleep patterns, while nowl Nf1 trans-heterozygous animals display disrupted sleep architecture, including sleep fragmentation, indicating a genetic interaction. Furthermore, the reduction in total sleep and the fragmentation of night-time sleep caused by loss of nowl are partially rescued by Nf1 overexpression. Thus, these two genes may function in the same pathway, or both of them may act on a common element involved in sleep maintenance. Whether nowl and Nf1 regulate sleep through their effects on Ras activity will be an interesting question for future studies.
GABA neurotransmission is often altered in schizophrenic patients, and it is the main system that regulates night-time sleep [41, 44, 60, 61]. Since disruption of GABA signaling has also been shown to reduce night-time sleep in Drosophila, we asked whether nowl and Nf1 might function in GABA-producing or -responsive cells. We found that knockdown of nowl in Rdl-expressing GABA-responsive neurons led to strongly increased sleep fragmentation, similar to the effect of pan-neuronal nowl knockdown. The wake-promoting l-LNv clock neurons express Rdl and play a major role in regulating sleep [43]. When these neurons are artificially hyper-excited by the expression of the sodium channel NaChBac, which allows membrane depolarization to occur more readily, flies showed decreased levels of sleep and increased levels of arousal, especially during night-time [45]. Knockdown of Rdl in these cells increases sleep-onset latency [42], while reduced GABAB-R-2 metabotropic receptor expression leads to reduced sleep during the late night [44]. Using RNAi-induced knockdown driven by the Pdf> and dimm> constructs, we found that nowl does not appear to be required in these s-LNv clock neurons to regulate sleep, indicating that other Rdl-expressing neurons are involved in sleep-regulatory nowl activity. The mushroom bodies (MBs), which are required for olfactory associative learning and memory [62] and which also regulate sleep [63, 64], strongly express Rdl (Fig 7E and [46]). A single pair of dorsal paired medial (DPM) neurons have been found to strongly promote sleep via GABAergic and serotonergic innervation of the mushroom bodies [47]. The MB α′/β′ neuronal population in particular are wake-promoting neurons that receive GABAergic input from the DPM neurons. Increased activation of the α′/β′ population decreases night-time sleep and increases sleep fragmentation [47]. Knockdown of Rdl in the α′/β′ neurons results in night-time sleep-loss phenotypes almost identical to those seen with knockdown of nowl either pan-neuronally or specifically in the Rdl-expressing neurons, inducing increased sleep-bout numbers and reduced bout lengths, with a stronger effect during night-time [47]. Furthermore, we found strong enrichment of Nowl in Rdl-expressing MB neurons, consistent with Nowl’s functioning in the regulation of sleep via wake-promoting neurons in the mushroom body. Interestingly, nowl was also identified in an olfactory-learning screen, in which it was found to inhibit electric-shock-reinforced associative learning, consistent with a role for nowl in mushroom-body neuronal function [26]. Furthermore, we observed that Nf1 knockdown in Rdl-expressing neurons caused sleep reduction and fragmentation of night-time sleep similar to nowl knockdown, further supporting an interaction between Nf1 and nowl in the regulation of sleep. Investigating whether nowl and Nf1 do indeed regulate sleep via effects on GABA signaling in the mushroom bodies will be an interesting subject for follow-up studies.
It has been suggested that mutations in ion channels can lead to disrupted sleep by altering overall neuronal excitability [65]. One example of this is the gene Shaker, which encodes an α-subunit of a potassium channel that functions to regulate membrane repolarization after neuronal depolarization [66]. Loss-of-function mutations in this gene, and in its mouse orthologs, cause reduced sleep and shorter sleep episodes, without affecting circadian or homeostatic sleep drive [67]. Since sleep loss and fragmentation due to loss of nowl were partially rescued by increased GABAergic inhibition of Rdl-expressing neurons, we suggest that loss of nowl may modulate GABA signaling in wake-promoting Rdl-expressing neurons. It will be interesting to determine whether nowl directly affects the Rdl receptor or other substrates via Cul3 to regulate postsynaptic GABA signaling. Cul3 and its interaction partner Insomniac are recruited to the postsynaptic compartment within minutes of acute glutamate-receptor inhibition [68]. These proteins mediate local mono-ubiquitination, which is important for homeostatic signaling in the postsynaptic compartment. The number of Rdl receptors expressed in a neuron, beyond the mere presence or absence of these receptors, has been suggested to be important in sleep regulation [43]. Furthermore, in humans, agonists of GABAA-type receptors are common treatments for insomnia [69], and a loss-of-function mutation affecting a GABAA receptor subunit causes a heritable type of insomnia [70]. Altered GABAergic signaling is believed to play a role in many neurodevelopmental disorders, and a mechanism that involves GABAA-receptor signaling may be a key factor underlying the pathophysiology of the 22q11.2 DS. Our findings suggest a contribution of nowl/LZTR1 –a 22q11.2 gene–in altered GABAergic neurotransmission and thus may provide a new direction for understanding the mechanisms underlying the observed neurodevelopmental phenotypes in the 22q11.2 DS and for developing therapeutic interventions against them.
Essentially all psychiatric disorders are associated with sleep disturbances. We have identified nowl as regulator of sleep in Drosophila. Mutations in its human ortholog LZTR1 have been implicated in several human diseases, such as schwannomatosis, glioblastoma, and the psychiatric disorders linked to the 22q11.2 DS. We suggest that the nowl/LZTR1 gene encodes a conserved regulator of sleep that interacts with Nf1 and that may contribute to the overall pathophysiology of the 22q11.2 DS. Further studies of Nowl may help understand the molecular mechanisms underlying both mental disorders and Schwann-cell tumors.
Materials and methods
Drosophila lines and maintenance
Drosophila larvae and adults of mixed sexes were raised on standard cornmeal medium (Nutri-Fly “Bloomington” formulation) at 25°C under a 12:12-hour light/dark cycle with 60% humidity. The following fly lines were obtained from Bloomington Drosophila Stock Center (BDSC; Bloomington, IL): elav-GAL4; UAS-Dicer-2 (#25750), elav-GS-Gal4 (#43642), nSyb-GAL4 (#51635), Gad1-GAL4/CyO (#51630), Rdl-GAL4/CyO (#66509), Pdf-GAL4 (#6899), dimm-GAL4 (#25373), UAS-Dicer-2 (#24651), Mi{ET1}CG3711MB12128 (#29940), RdlCB2/TM6B, Tb1 (#35493), UAS-mCD8::GFP (#4776), and ppk-Gal4 (#32078). UAS-RNAi lines against orthologs of human 22q11.2 CNV-linked genes were ordered from Vienna Drosophila Resource Center (VDRC; Vienna, Austria) and are listed in S1 Table. UAS-Cul3-RNAi (#109415), UAS-Nf1-RNAi (#109637), and the w1118 (#60000) genetic background line were also obtained from VDRC.
The GABA-B-R2-GAL4::p65 line carries a bacterial artificial chromosome (BAC) containing ~80 kb of genomic sequence surrounding the GABA-B-R2 gene, in which the first coding exon of this gene was replaced with GAL4::p65 and associated terminator sequences; the remaining exons and introns are still present, with the assumption that they contain regulatory sequences, but they are no longer transcribed. This construct was built using recombineering techniques [71] in P[acman] BAC clone CH321-95N09 (Children’s Hospital Oakland Research Institute, Oakland, CA). Selectable markers (conferring kanamycin resistance and streptomycin sensitivity) were cloned from pSK+KanaRpsL [72] (AddGene plasmid #20871) and flanked with upstream GAL4 and downstream HSP70-UTR arms cloned from pBPGUw [73], a gift of G. Rubin. GABA-B-R2-specific homology arms were added by PCRing this landing-site cassette with the following primers (genomic sequence is in lower case): GABA-B-R2-GAL4-F: cgatatgcgc tattcacatt tagaatcgtt ttacagccca cgcggtcaac ATGAAGCTAC TGTCTTCTAT CGAACAAGC; GABA-B-R2-UTR-R: catcatcaga gattcactta atgaaatctt caagctaaac cctaactcac GATCTAAACG AGTTTTTAAG CAAACTCACT CCC. The homology-flanked landing-site cassette was recombined into the BAC (with kanamycin selection), and then full-length GAL4::p65-HSP70 amplified from pBPGAL4.2::p65Uw [74] (a gift of G. Rubin) was recombined into this site in a second recombination (with streptomycin selection). The recombined regions of the BAC were sequenced, and the sequence-verified BAC was integrated into the Drosophila genome at attP40 [75] by Genetic Services, Inc. (Cambridge, MA).
Generation of CRISPR mutant
The CRISPR/Cas9 method was used to disrupt the nowl locus. Two guide RNAs (gRNAs) were designed using the Shigen Cas9 Target Finder (https://shigen.nig.ac.jp/fly/nigfly/cas9) and checked for potential off-target sites using FlyCRISPR Optimal Target Finder tool (http://targetfinder.flycrispr.neuro.brown.edu/). One gRNA was designed to target exon 1 and the second one to target exon 6, thus removing almost the entire nowl open reading frame. Oligonucleotides containing these gRNA sequences (gRNA1: CTT CGT GCT GGG CGT CTA AGC AGC; gRNA2: CTT CGA CCT AGA GTA GGA TTC ATC), designed with complementary Bbs1 restriction-site overhangs, were cloned into the pBFvU6.2 vector. Four μl of the forward and reverse oligos (100 μM) were annealed in 2 μl of 5x Phusion PH Buffer by heating the reaction at 95°C for 5 min and then leaving it to cool down to room temperature. One microgram of the pBFv-U6.2 vector was cut using BbsI enzyme at 37°C for 1 h, and the enzyme was inactivated by raising the temperature to 65°C for 20 min. The cut vector was purified through gel electrophoresis, and the QIAquick gel-extraction kit (QIAGEN) was used to extract the DNA. The annealed oligonucleotides were then ligated into cut pBFv-U6.2. One μl of the digested vector, 1 μl of annealed oligonucleotides, 0.5 μl of T4 ligase, and 2 μl of 10X T4 ligase buffer (Thermo Scientific) was mixed to a final volume of 10 μl. For ligation, the reactions were incubated for 1 h at 37°C and transformed into 50 μl of Stellar competent cells (Clonetech) that were grown overnight at 37°C on LB plates containing ampicillin (100 μg/ml). Several colonies were selected and grown into liquid culture overnight. Colony PCR was conducted to identify the colonies containing properly configured plasmid using one gRNA oligo in combination with the M13 reverse primer. Plasmid DNA was extracted and sequenced to confirm the correct insertion of gRNA sequence. Plasmids were then injected into y1w1118; attP2(nos-Cas9)/TM6, Sb, Tb embryos by BestGene Inc (Chino Hills, CA).
Identification of Drosophila orthologs
Drosophila orthologs of human 22q11.2 CNV-linked genes were identified using the “Drosophila RNAi Screening Center Integrative Ortholog Prediction Tool” (DIOPT), available at the website of the Drosophila RNAi Screening Center (DRSC), Harvard Medical School [22].
Sleep assays and analysis
The Drosophila Activity Monitor (DAM) system (TriKinetics, Inc., Waltham, MA) was used to measure locomotion. Adult flies were sorted by sex and collected in groups of 30 soon after eclosion. Collected flies were housed under standard conditions until the start of the experiment. Three-to-seven-day-old flies (either male only or virgin female only, as indicated) were used for experiments, during which they were housed in 65-mm-long glass tubes containing a plug of food medium (5% sucrose and 2% agar in water) at one end and a cotton stopper at the other. For experiments using the GeneSwitch system, newly eclosed adult flies were maintained on normal food containing 1 mM RU486 (Sigma #M8086) dissolved in ethanol, or normal food containing a similar amount of ethanol, for 5–7 days. Flies were then loaded into tubes containing 5% sucrose and 2% agar in water with or without 1 mM RU486 in ethanol. Control flies were fed equal amounts of ethanol in their food. Experiments were run in a behavioral incubator under a 12-hour light/12-hour dark cycle, and flies were allowed 12–24 hours to acclimatize prior to experimental start. Activity, determined as the number of beam crosses per fly per minute, was measured in one-minute periods, and episodes of sleep were defined as at periods of least 5 minutes of uninterrupted quiescence. Flies exhibiting less than 10 minutes of activity during either the entire light or dark phase were flagged as dead. The pySolo software package [76] and a custom MATLAB script (MATLAB R2016b, The MathWorks Inc, Natick, Massachusetts) were used to analyze sleep dynamics. Sleep latency was calculated as the period from lights-off to the first sleep episode. Locomotor activity of male flies kept on a normal LD cycle for several days was monitored in constant darkness (DD) for 8 days to determine the length of the free-running circadian period. Data were aggregated into 30-minute bins and analyzed using the FaasX software package [77], which uses Chi-Square tests to calculate the period length. For mechanical sleep deprivation, flies in DAM monitors were stimulated using a vortexer plate (TriKinetics) for 2 seconds every minute during a 6-hour period before lights-on. Recovery sleep was determined during the first three hours following sleep deprivation.
Western blotting
For Western-blot analysis, ten male flies per genotype were frozen at -80°C, vortexed, and sieved to obtain samples of heads. Heads were homogenized in 50 μl Laemmli Sample Buffer (Bio-Rad) containing 2-mercaptoethanol. Samples were denatured for 5 minutes at 95°C and then centrifuged at maximum speed (17,000 g) for 5 minutes. Samples (20 μl) were loaded on a 4–20% gradient polyacrylamide gel (Bio-Rad), and proteins were separated at 150 V for 30 minutes. Proteins were transferred onto PVDF membrane (Millipore), and the membrane was blocked for one hour in Odyssey Blocking Buffer (LI-COR). Primary antibodies (rabbit anti-phospho-ERK, Cell Signaling Technology #9101, 1:1000; mouse anti-α-tubulin, Sigma-Aldrich #T9026, 1:5000) were diluted in Odyssey Blocking Buffer containing 0.2% Tween 20, and the membrane was incubated overnight in this solution. Membranes were then rinsed and incubated for 40 minutes with secondary antibodies conjugated to infrared fluorophores (anti-mouse IRDye 680RD and anti-rabbit 800CW, LI-COR, 1:10,000), and staining was imaged using an Odyssey Fc scanner (LI-COR).
Anti-Nowl antiserum development, immunostaining and class IV neuron visualization
To generate a Nowl antibody, a peptide corresponding to residues 123–141 of the protein (LRSSFKSSKRNKARKSAST) was used in a custom immunization protocol carried out by Genosphere Biotechnologies (Paris, France). Epitope specificity of the antiserum was confirmed by comparing wild-type (Rdl>GFP) and elav>nowl-RNAi animals by immunostaining (S9C Fig) as well as by co-application of the pre-immune serum. For immunocytochemistry, primary antibodies used were polyclonal rabbit α-Nowl (1:200), α-phospho-Histone H3 (αPH3; 1:500; EMD Millipore #06–570), and FITC-conjugated polyclonal goat anti-GFP (1:200; Abcam, #ab6662). Biotinylated anti-rabbit was applied (1:200; Vector Laboratories, #BA-1100), followed by Cy3-streptavidin (1:200; Jackson ImmunoResearch Laboratories, #016-160-084). Tissues were mounted on poly-L-lysine-coated 35-mm glass-bottomed dishes (MatTek Corporation, MA, USA) in Vectashield (Vector Laboratories Inc., CA, USA), and image acquisition was performed on an inverted Zeiss LSM800 confocal microscope with AiryScan (Zeiss, Oberkochen, Germany). Images were processed in CorelDraw X8 (Corel, Ottawa, Canada). For visualization of class-IV da neurons, wandering third-instar larvae expressing GFP driven by the class-IV-specific ppk> driver were anesthetized by exposure to ether for five minutes in a sealed container. For visualization of dendritic arbors, live larvae were mounted in 90% glycerol and imaged for GFP. Imaging of class-IV axonal terminals was performed on dissected brains with ventral nerve cords, mounted in Vectashield.
Transcript analysis by quantitative PCR
The efficiency of nowl and Nf1 RNAi-mediated gene knockdown was analyzed using quantitative RT-PCR (qPCR). Heads from adult male flies expressing neuronal RNAi against nowl or Nf1, along with driver- and RNAi-alone controls, were collected, and total RNA was extracted from 10 heads per sample using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was reverse-transcribed from total RNA using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher #4368814) and used as a template for qPCR with the QuantiTect SYBR Green PCR Kit (Fisher Scientific #204145) and an Mx3005P qPCR System (Agilent Technologies). Levels of target-gene expression were normalized against RpL32. Precomputed oligo pairs from the FlyPrimerBank were used: GGAGGATCGGGATGTAGTATGG and CGTGTAGGAGGTGAAGTCCAC for nowl; AGTATCTGATGCCCAACATCG and CAATCTCCTTGCGCTTCTTG for Nf1; and CTTTTGGCACGTTTCGAGGAT and GGTAGCGCGATATGTGGATCAG for RpL32.
Measurements of glycogen and triglycerides
For triglyceride and glycogen assays, one-week-old adult males were collected and frozen at -80 oC in groups of three animals. Animals were homogenized in 80 μl PBS + 0.5% Tween using a Tissue Lyser LT (Qiagen) with small steel beads. For triglyceride measurements, 8 μl of the homogenate was added to 80 μl Free Glycerol Reagent (Sigma, F6428) and 20 μl Triglyceride Reagent (Sigma, T2449), and reactions were incubated at 37 oC for 10 minutes with agitation, before being read at 540-nm absorbance. For glycogen measurements, 6 μl homogenate was mixed with 94 μl Glucose Oxidase (GO) reagent from the Glucose (GO) Assay Kit (Sigma, GAGO20) containing 1 μl (0.3 U) of low-glucose Amyloglucosidase (Sigma, A7420-5MG) to determine total glucose + glycogen. Another 6 μl homogenate was mixed with 94 μl GO reagent alone (without the Amyloglucosidase) to determine free glucose. For analysis of glycogen content of heads, each sample was based on 5 adult heads homogenized in 40 μl PBS + 0.5% Tween, of which 4 μl homogenate was used with 25 ul GO reagent with and without the Amyloglucosidase. Reactions were incubated at 37 oC for 20 minutes, after which 12-N sulfuric acid was added to stop and complete the reaction (66 μl for whole-body and 12 μl for head samples) before absorbance was measured at 540 nm. Absorbance was measured using an EnSight multimode plate reader (PerkinElmer), and values were standardized against readings for a range of known concentrations.
Statistics
GraphPad Prism software was used for statistical analysis. For single comparisons, significance was analyzed by Mann-Whitney U tests. When comparing multiple samples, Kruskal-Wallis tests with Dunn's post-hoc testing were used. Statistical significance was set at p≤0.05.
Supporting information
(A, B) Quantification of total sleep (A) and average activity measured as the number of beam crosses per minute (B) in 3-to-7-day-old females, measured over a 24-hour period. UAS-RNAi constructs were expressed under the control of the pan-neuronal elav-GAL4 driver. n = 140 flies for controls (elav> crossed to w1118, the genetic background for the RNAi lines); n = 16 flies for each RNAi genotype. When possible, two distinct UAS-RNAi constructs were used against each gene. RNAi efficiency was enhanced by co-expressing the processing enzyme Dicer-2 (UAS-Dcr-2). Graphs represent means with SEM of data pooled from one to five independent experiments. Statistical significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (* p<0.05).
(PDF)
(A) Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male controls (w1118/Y) compared to nowlKO mutants (nowlKO/Y). Sleep data are binned into 30-minute intervals. (B, C) Total day- and night-time sleep (minutes) in males. (C) Total night-time sleep is decreased in nowlKO mutants. (D) Sleep latency in males in nowlKO mutant males is increased compared to the control. (E-H) Quantification of average sleep-bout duration and number during day and night for male nowlKO mutants compared to controls. (G, H) Night-time sleep is fragmented in nowlKO mutant males compared to the control, as indicated by increased sleep-bout number (G) and reduced sleep-bout duration (H). Graphs represent mean and SEM (n = 32–75) of data pooled from one to three experiments. Significance was tested by Mann-Whitney U tests (*** p < 0.001).
(PDF)
(A, B) Circadian rhythmicity of 3-to-8-day-old male flies kept in the dark for 6 days. During entrainment, lights-on occurred at Zeitgeber time (ZT) 0 and lights-off occurred at ZT12. (A) Actograms showing average activity during the light/dark entrainment period and during constant-darkness free-running cycles (ZT in hours). (B) Representative periodograms of nowl1 mutants indicate that it cycles similarly to controls (w1118/Y and +/Y) with a nearly 24-hour period, indicating that nowl is not necessary for entrainment or free running of the circadian clock. The data in the periodograms begins 24 hours after lights-OFF transition. (C and D) Strength of the clock and rhythmicity of male flies. (C) Representative graphs of individual rhythmic flies showing the strength of circadian clock which is defined by the main peak (the power is the height of the peak above the 5% significance level. (D) Quantification of the percentage of rhythmic flies, the average period, and the strength of rhythmicity (the power and the width of the peak, an additional measure of the strength of the circadian clock). The chi-squared analysis shows that the main peak is lower for nowl1 mutants compared to one of the controls. Similarly, the width of the peak is lower for nowl1 mutants than controls. Only the data of rhythmic flies were taken into account for data in (B-D). Data represents means (n = 17–28) of data from one experiment and was analysed by chi-square test in the FaasX software package [77].
(PDF)
Neuronal gene knockdown was induced by crossing elav> to each gene-specific UAS-RNAi line. As a control, the elav> line was crossed to w1118. (A-D) Sleep-episode duration and number during the day (A, B) and night (C, D) of 3-to-8-day-old male flies (n = 140 flies for controls; n = 16 flies for each RNAi genotype). Graphs represent mean and SEM of data pooled from one to five independent experiments. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05).
(PDF)
(A) Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male controls (nSyb>+ and nowl-RNAi/+) compared to nSyb>nowl-RNAi animals with knockdown of nowl in the nervous system. Sleep data are binned into 30-minute intervals. (B, C) Quantification of total day- and night-time sleep (minutes) in males. Graphs represent mean and SEM (n = 32) of data from one experiment.
(PDF)
(A) Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male controls (elav>+ and Cul3-RNAi/+) compared to elav>Cul3-RNAi animals with knockdown of Cul3 in the nervous system. (B, C) Quantification of average male daytime sleep-bout duration (B) and number (C) for elav>Cul3-RNAi flies compared to controls (elav>+ and UAS-Cul3-RNAi/+). Sleep-bout length was significantly decreased, and sleep-bout number was significantly increased during the day when Cul3 was knocked down in the nervous system. (D, E) Quantification of average night-time sleep-bout duration (D) and number (E) in males. Bout length was significantly decreased, and average bout numbers were significantly increased during the night upon pan-neuronal knockdown of Cul3. Graphs represent mean and SEM (n = 32) of data from one experiment, which is representative of two independent experiments. Statistical significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*** p<0.001).
(PDF)
(A) Representative images of anti-phospho-Histone H3 (α-PH3, red) and DAPI (blue) staining of adult brains of 5-7-day-old males show absence of cell proliferation in animals with knockdown of nowl or Nf1. Cell proliferation, as indicated α-PH3, was not observed in analysis of five independent brains of each genotype. (B) Representative images of five independent class-IV dendritic arborization (da) neurons from abdominal segment A3 in the peripheral nervous system and their axonal terminals in the ventral nerve cord. Knockdown of nowl or Nf1 does not alter the gross morphology of class-IV da neuronal structures.
(PDF)
Quantification of daytime (A) and nighttime (B) total sleep, sleep-bout duration, and sleep-bout numbers for nowl-RNAi/+, Nf1-RNAi/+ and UAS-Nf1/+ (Nf1/+) control males for main Fig 5. Graphs represent means with SEM (n = 32–81) of data pooled from one to three independent experiments.
(PDF)
(A, B) Efficiency of nowl (A) and Nf1 (B) gene knockdown. Gene expression was determined in adult male heads using qPCR. (C) Anti-Nowl staining features observed in male Rdl>GFP controls are not present in elav>nowl-RNAi brains with reduced expression of nowl in the nervous system, indicating that the anti-Nowl antibody specifically recognizes the Nowl protein. Graphs represent mean and SEM (n = 6) of data from one experiment. Statistical significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05, *** p < 0.001).
(PDF)
RU486 was used to induce RNAi restricted to the adult stage using the GeneSwitch (GS) system in flies carrying elav-GS-GAL4 (elavGS>). (A, B) Total sleep per night is decreased in male flies with adult-specific neuronal knockdown of nowl (elavGS>nowl-RNAi) or Nf1 (elavGS>Nf1-RNAi) induced by RU486 compared to vehicle-treated controls and elavGS>+ controls. (C-F) Night-time sleep is fragmented in adult males when knockdown of nowl or Nf1 is induced, as indicated by reduced sleep-bout duration (C, D) and increased sleep-bout number (E, F) primarily during the night. Graphs represent mean and SEM (n = 32) of data from one experiment. Significance was tested by Mann-Whitney U tests (*p < 0.05, ** p < 0.01, *** p < 0.001).
(PDF)
Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male flies of the genotypes w1118/Y, nowl1/Y, RdlCB2/+, and nowl1/Y; RdlCB2/+. The reduced night-time sleep produced by loss of nowl (nowl1 mutant) is rescued by introducing one copy of the RdlCB2 allele.
(PDF)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Danish Council for Independent Research, Medical Sciences (https://dff.dk/) grant 4183-00312 to K.R. T.K and K.A.H. were supported by Villum Foundation grant 15365 to K.A.H. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science (New York, NY). 2007;316(5823):445–9. Epub 2007/03/17. 10.1126/science.1138659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455(7210):232–6. Epub 2008/08/01. 10.1038/nature07229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vo OK, McNeill A, Vogt KS. The psychosocial impact of 22q11 deletion syndrome on patients and families: A systematic review. American journal of medical genetics Part A. 2018;176(10):2215–25. Epub 2018/03/27. 10.1002/ajmg.a.38673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–41. 10.1016/j.cell.2012.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Didriksen M, Fejgin K, Nilsson SR, Birknow MR, Grayton HM, Larsen PH, et al. Persistent gating deficit and increased sensitivity to NMDA receptor antagonism after puberty in a new mouse model of the human 22q11.2 microdeletion syndrome: a study in male mice. J Psychiatry Neurosci. 2017;42(1):48–58. 10.1503/jpn.150381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Developmental disabilities research reviews. 2008;14(1):3–10. Epub 2008/07/19. 10.1002/ddrr.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szelenberger W, Soldatos C. Sleep disorders in psychiatric practice. World psychiatry: official journal of the World Psychiatric Association (WPA). 2005;4(3):186–90. . [PMC free article] [PubMed] [Google Scholar]
- 8.Glickman G. Circadian rhythms and sleep in children with autism. Neurosci Biobehav Rev. 2010;34(5):755–68. 10.1016/j.neubiorev.2009.11.017 . [DOI] [PubMed] [Google Scholar]
- 9.Cohrs S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS drugs. 2008;22(11):939–62. 10.2165/00023210-200822110-00004 . [DOI] [PubMed] [Google Scholar]
- 10.Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. The American journal of psychiatry. 2003;160(9):1580–6. Epub 2003/08/29. 10.1176/appi.ajp.160.9.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moulding HA, Bartsch U, Hall J, Jones MW, Linden DE, Owen MJ, et al. Sleep problems and associations with psychopathology and cognition in young people with 22q11.2 deletion syndrome (22q11.2DS). Psychol Med. 2019:1–12. Epub 2019/05/31. 10.1017/S0033291719001119 . [DOI] [PubMed] [Google Scholar]
- 12.Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet (London, England). 2003;362(9393):1366–73. Epub 2003/10/31. 10.1016/s0140-6736(03)14632-6 . [DOI] [PubMed] [Google Scholar]
- 13.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25(1):129–38. 10.1016/s0896-6273(00)80877-6 . [DOI] [PubMed] [Google Scholar]
- 14.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science (New York, NY). 2000;287(5459):1834–7. Epub 2000/03/10. 10.1126/science.287.5459.1834 . [DOI] [PubMed] [Google Scholar]
- 15.Grima B, Dognon A, Lamouroux A, Chelot E, Rouyer F. CULLIN-3 controls TIMELESS oscillations in the Drosophila circadian clock. PLoS biology. 2012;10(8):e1001367 Epub 2012/08/11. 10.1371/journal.pbio.1001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stavropoulos N, Young MW. insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron. 2011;72(6):964–76. Epub 2011/12/27. 10.1016/j.neuron.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy S, Maurer GW, Hentze JL, Rose M, Werge TM, Rewitz K. AMPK signaling linked to the schizophrenia-associated 1q21.1 deletion is required for neuronal and sleep maintenance. PLoS Genet. 2018;14(12):e1007623 10.1371/journal.pgen.1007623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123(1):124–33. Epub 2009/01/02. 10.1542/peds.2007-3204 . [DOI] [PubMed] [Google Scholar]
- 19.Smith MJ, Isidor B, Beetz C, Williams SG, Bhaskar SS, Richer W, et al. Mutations in LZTR1 add to the complex heterogeneity of schwannomatosis. Neurology. 2015;84(2):141–7. 10.1212/WNL.0000000000001129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballester R, Marchuk D, Boguski M, Saulino A, Letcher R, Wigler M, et al. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63(4):851–9. Epub 1990/11/16. 10.1016/0092-8674(90)90151-4 . [DOI] [PubMed] [Google Scholar]
- 21.Hannan F, Ho I, Tong JJ, Zhu Y, Nurnberg P, Zhong Y. Effect of neurofibromatosis type I mutations on a novel pathway for adenylyl cyclase activation requiring neurofibromin and Ras. Hum Mol Genet. 2006;15(7):1087–98. Epub 2006/03/04. 10.1093/hmg/ddl023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Flockhart I, Vinayagam A, Bergwitz C, Berger B, Perrimon N, et al. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC bioinformatics. 2011;12:357 Epub 2011/09/02. 10.1186/1471-2105-12-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–6. Epub 2007/07/13. nature05954 [pii] 10.1038/nature05954 . [DOI] [PubMed] [Google Scholar]
- 24.Vissers JH, Manning SA, Kulkarni A, Harvey KF. A Drosophila RNAi library modulates Hippo pathway-dependent tissue growth. Nat Commun. 2016;7:10368 10.1038/ncomms10368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167(2):761–81. Epub 2004/07/09. 10.1534/genetics.104.026427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel M, Scholz CJ, Muller T, Dittrich M, Konig C, Bockstaller M, et al. Genome-Wide Association Analyses Point to Candidate Genes for Electric Shock Avoidance in Drosophila melanogaster. PLoS One. 2015;10(5):e0126986 Epub 2015/05/21. 10.1371/journal.pone.0126986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson KN, Bradley AJ. Sleep disturbance in mental health problems and neurodegenerative disease. Nat Sci Sleep. 2013;5:61–75. Epub 2013/06/14. 10.2147/NSS.S34842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steklov M, Pandolfi S, Baietti MF, Batiuk A, Carai P, Najm P, et al. Mutations in LZTR1 drive human disease by dysregulating RAS ubiquitination. Science (New York, NY). 2018;362(6419):1177–82. Epub 2018/11/18. 10.1126/science.aap7607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bigenzahn JW, Collu GM, Kartnig F, Pieraks M, Vladimer GI, Heinz LX, et al. LZTR1 is a regulator of RAS ubiquitination and signaling. Science. 2018;362(6419):1171–7. Epub 2018/11/18. 10.1126/science.aap8210 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderica-Romero AC, Gonzalez-Herrera IG, Santamaria A, Pedraza-Chaverri J. Cullin 3 as a novel target in diverse pathologies. Redox biology. 2013;1:366–72. Epub 2013/09/12. 10.1016/j.redox.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiffenberger C, Allada R. Cul3 and the BTB adaptor insomniac are key regulators of sleep homeostasis and a dopamine arousal pathway in Drosophila. PLoS Genet. 2012;8(10):e1003003 10.1371/journal.pgen.1003003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potdar S, Sheeba V. Lessons from sleeping flies: insights from Drosophila melanogaster on the neuronal circuitry and importance of sleep. Journal of neurogenetics. 2013;27(1–2):23–42. 10.3109/01677063.2013.791692 . [DOI] [PubMed] [Google Scholar]
- 33.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98(22):12596–601. 10.1073/pnas.221303298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97(6):1792–801. Epub 2012/03/24. 10.1210/jc.2012-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347–60. 10.1016/0301-0082(94)00057-o . [DOI] [PubMed] [Google Scholar]
- 36.Petit JM, Burlet-Godinot S, Magistretti PJ, Allaman I. Glycogen metabolism and the homeostatic regulation of sleep. Metab Brain Dis. 2015;30(1):263–79. 10.1007/s11011-014-9629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman JE, Mackiewicz M, Galante RJ, Zhang L, Cater J, Zoh C, et al. Glycogen in the brain of Drosophila melanogaster: diurnal rhythm and the effect of rest deprivation. J Neurochem. 2004;88(1):32–40. 10.1046/j.1471-4159.2003.02126.x . [DOI] [PubMed] [Google Scholar]
- 38.Voll SL, Boot E, Butcher NJ, Cooper S, Heung T, Chow EW, et al. Obesity in adults with 22q11.2 deletion syndrome. Genet Med. 2017;19(2):204–8. Epub 2016/08/19. 10.1038/gim.2016.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. Epub 2008/07/02. 10.1196/annals.1417.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111(2):231–9. Epub 2002/05/02. 10.1016/s0306-4522(02)00034-9 . [DOI] [PubMed] [Google Scholar]
- 41.Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11(3):354–9. Epub 2008/01/29. 10.1038/nn2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60(4):672–82. 10.1016/j.neuron.2008.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol. 2009;19(5):386–90. 10.1016/j.cub.2009.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gmeiner F, Kolodziejczyk A, Yoshii T, Rieger D, Nassel DR, Helfrich-Forster C. GABA(B) receptors play an essential role in maintaining sleep during the second half of the night in Drosophila melanogaster. J Exp Biol. 2013;216(Pt 20):3837–43. 10.1242/jeb.085563 . [DOI] [PubMed] [Google Scholar]
- 45.Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, et al. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Current biology: CB. 2008;18(20):1537–45. Epub 2008/09/06. 10.1016/j.cub.2008.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Krause WC, Davis RL. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56(6):1090–102. Epub 2007/12/21. 10.1016/j.neuron.2007.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haynes PR, Christmann BL, Griffith LC. A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. Elife. 2015;4 10.7554/eLife.03868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Mol Psychiatry. 2005;10(1):6–13. Epub 2004/12/25. 10.1038/sj.mp.4001571 . [DOI] [PubMed] [Google Scholar]
- 49.Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85–94. Epub 1993/02/01. 10.1001/archpsyc.1993.01820140007001 . [DOI] [PubMed] [Google Scholar]
- 50.Bassett AS, Marshall CR, Lionel AC, Chow EW, Scherer SW. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Hum Mol Genet. 2008;17(24):4045–53. Epub 2008/09/23. 10.1093/hmg/ddn307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clelland CL, Read LL, Baraldi AN, Bart CP, Pappas CA, Panek LJ, et al. Evidence for association of hyperprolinemia with schizophrenia and a measure of clinical outcome. Schizophr Res. 2011;131(1–3):139–45. Epub 2011/06/08. 10.1016/j.schres.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong L, Liu M, Jen J, Yeh ET. GNB1L, a gene deleted in the critical region for DiGeorge syndrome on 22q11, encodes a G-protein beta-subunit-like polypeptide. Biochimica et biophysica acta. 2000;1494(1–2):185–8. Epub 2000/11/10. 10.1016/s0167-4781(00)00189-5 . [DOI] [PubMed] [Google Scholar]
- 53.Chen YZ, Matsushita M, Girirajan S, Lisowski M, Sun E, Sul Y, et al. Evidence for involvement of GNB1L in autism. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(1):61–71. Epub 2011/11/19. 10.1002/ajmg.b.32002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishiguro H, Koga M, Horiuchi Y, Noguchi E, Morikawa M, Suzuki Y, et al. Supportive evidence for reduced expression of GNB1L in schizophrenia. Schizophr Bull. 2010;36(4):756–65. Epub 2008/11/18. 10.1093/schbul/sbn160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams NM, Glaser B, Norton N, Williams H, Pierce T, Moskvina V, et al. Strong evidence that GNB1L is associated with schizophrenia. Hum Mol Genet. 2008;17(4):555–66. Epub 2007/11/16. 10.1093/hmg/ddm330 . [DOI] [PubMed] [Google Scholar]
- 56.Owen MJ, O'Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198(3):173–5. Epub 2011/03/02. 10.1192/bjp.bp.110.084384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abe T, Umeki I, Kanno SI, Inoue SI, Niihori T, Aoki Y. LZTR1 facilitates polyubiquitination and degradation of RAS-GTPases. Cell Death Differ. 2019. Epub 2019/07/25. 10.1038/s41418-019-0395-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams JA, Su HS, Bernards A, Field J, Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293(5538):2251–6. 10.1126/science.1063097 . [DOI] [PubMed] [Google Scholar]
- 59.Piotrowski A, Xie J, Liu YF, Poplawski AB, Gomes AR, Madanecki P, et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet. 2014;46(2):182–7. Epub 2013/12/24. 10.1038/ng.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. Epub 2001/05/30. 10.1016/S0893-133X(01)00225-1 . [DOI] [PubMed] [Google Scholar]
- 61.de Jonge JC, Vinkers CH, Hulshoff Pol HE, Marsman A. GABAergic Mechanisms in Schizophrenia: Linking Postmortem and In Vivo Studies. Front Psychiatry. 2017;8:118 Epub 2017/08/30. 10.3389/fpsyt.2017.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11(1):1–14. Epub 1993/07/01. 10.1016/0896-6273(93)90266-t . [DOI] [PubMed] [Google Scholar]
- 63.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441(7094):757–60. Epub 2006/06/09. 10.1038/nature04811 . [DOI] [PubMed] [Google Scholar]
- 64.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441(7094):753–6. Epub 2006/06/09. 10.1038/nature04739 . [DOI] [PubMed] [Google Scholar]
- 65.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nature reviews Neuroscience. 2009;10(8):549–60. Epub 2009/07/21. 10.1038/nrn2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salkoff L, Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981;293(5829):228–30. Epub 1981/09/17. 10.1038/293228a0 . [DOI] [PubMed] [Google Scholar]
- 67.Espinosa F, Marks G, Heintz N, Joho RH. Increased motor drive and sleep loss in mice lacking Kv3-type potassium channels. Genes, brain, and behavior. 2004;3(2):90–100. Epub 2004/03/10. 10.1046/j.1601-183x.2003.00054.x . [DOI] [PubMed] [Google Scholar]
- 68.Kikuma K, Li X, Perry S, Li Q, Goel P, Chen C, et al. Cul3 and insomniac are required for rapid ubiquitination of postsynaptic targets and retrograde homeostatic signaling. Nature communications. 2019;10(1):2998 Epub 2019/07/07. 10.1038/s41467-019-10992-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roth T. A physiologic basis for the evolution of pharmacotherapy for insomnia. The Journal of clinical psychiatry. 2007;68 Suppl 5:13–8. Epub 2007/08/19. . [PubMed] [Google Scholar]
- 70.Buhr A, Bianchi MT, Baur R, Courtet P, Pignay V, Boulenger JP, et al. Functional characterization of the new human GABA(A) receptor mutation beta3(R192H). Human genetics. 2002;111(2):154–60. Epub 2002/08/22. 10.1007/s00439-002-0766-7 . [DOI] [PubMed] [Google Scholar]
- 71.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33(4):e36 10.1093/nar/gni035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S, Zhao Y, Leiby M, Zhu J. A new positive/negative selection scheme for precise BAC recombineering. Mol Biotechnol. 2009;42(1):110–6. 10.1007/s12033-009-9142-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105(28):9715–20. 10.1073/pnas.0803697105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186(2):735–55. 10.1534/genetics.110.119917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104(9):3312–7. 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gilestro GF, Cirelli C. pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics (Oxford, England). 2009;25(11):1466–7. Epub 2009/04/17. 10.1093/bioinformatics/btp237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klarsfeld A, Leloup JC, Rouyer F. Circadian rhythms of locomotor activity in Drosophila. Behav Processes. 2003;64(2):161–75. Epub 2003/10/15. 10.1016/s0376-6357(03)00133-5 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B) Quantification of total sleep (A) and average activity measured as the number of beam crosses per minute (B) in 3-to-7-day-old females, measured over a 24-hour period. UAS-RNAi constructs were expressed under the control of the pan-neuronal elav-GAL4 driver. n = 140 flies for controls (elav> crossed to w1118, the genetic background for the RNAi lines); n = 16 flies for each RNAi genotype. When possible, two distinct UAS-RNAi constructs were used against each gene. RNAi efficiency was enhanced by co-expressing the processing enzyme Dicer-2 (UAS-Dcr-2). Graphs represent means with SEM of data pooled from one to five independent experiments. Statistical significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (* p<0.05).
(PDF)
(A) Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male controls (w1118/Y) compared to nowlKO mutants (nowlKO/Y). Sleep data are binned into 30-minute intervals. (B, C) Total day- and night-time sleep (minutes) in males. (C) Total night-time sleep is decreased in nowlKO mutants. (D) Sleep latency in males in nowlKO mutant males is increased compared to the control. (E-H) Quantification of average sleep-bout duration and number during day and night for male nowlKO mutants compared to controls. (G, H) Night-time sleep is fragmented in nowlKO mutant males compared to the control, as indicated by increased sleep-bout number (G) and reduced sleep-bout duration (H). Graphs represent mean and SEM (n = 32–75) of data pooled from one to three experiments. Significance was tested by Mann-Whitney U tests (*** p < 0.001).
(PDF)
(A, B) Circadian rhythmicity of 3-to-8-day-old male flies kept in the dark for 6 days. During entrainment, lights-on occurred at Zeitgeber time (ZT) 0 and lights-off occurred at ZT12. (A) Actograms showing average activity during the light/dark entrainment period and during constant-darkness free-running cycles (ZT in hours). (B) Representative periodograms of nowl1 mutants indicate that it cycles similarly to controls (w1118/Y and +/Y) with a nearly 24-hour period, indicating that nowl is not necessary for entrainment or free running of the circadian clock. The data in the periodograms begins 24 hours after lights-OFF transition. (C and D) Strength of the clock and rhythmicity of male flies. (C) Representative graphs of individual rhythmic flies showing the strength of circadian clock which is defined by the main peak (the power is the height of the peak above the 5% significance level. (D) Quantification of the percentage of rhythmic flies, the average period, and the strength of rhythmicity (the power and the width of the peak, an additional measure of the strength of the circadian clock). The chi-squared analysis shows that the main peak is lower for nowl1 mutants compared to one of the controls. Similarly, the width of the peak is lower for nowl1 mutants than controls. Only the data of rhythmic flies were taken into account for data in (B-D). Data represents means (n = 17–28) of data from one experiment and was analysed by chi-square test in the FaasX software package [77].
(PDF)
Neuronal gene knockdown was induced by crossing elav> to each gene-specific UAS-RNAi line. As a control, the elav> line was crossed to w1118. (A-D) Sleep-episode duration and number during the day (A, B) and night (C, D) of 3-to-8-day-old male flies (n = 140 flies for controls; n = 16 flies for each RNAi genotype). Graphs represent mean and SEM of data pooled from one to five independent experiments. Significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05).
(PDF)
(A) Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male controls (nSyb>+ and nowl-RNAi/+) compared to nSyb>nowl-RNAi animals with knockdown of nowl in the nervous system. Sleep data are binned into 30-minute intervals. (B, C) Quantification of total day- and night-time sleep (minutes) in males. Graphs represent mean and SEM (n = 32) of data from one experiment.
(PDF)
(A) Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male controls (elav>+ and Cul3-RNAi/+) compared to elav>Cul3-RNAi animals with knockdown of Cul3 in the nervous system. (B, C) Quantification of average male daytime sleep-bout duration (B) and number (C) for elav>Cul3-RNAi flies compared to controls (elav>+ and UAS-Cul3-RNAi/+). Sleep-bout length was significantly decreased, and sleep-bout number was significantly increased during the day when Cul3 was knocked down in the nervous system. (D, E) Quantification of average night-time sleep-bout duration (D) and number (E) in males. Bout length was significantly decreased, and average bout numbers were significantly increased during the night upon pan-neuronal knockdown of Cul3. Graphs represent mean and SEM (n = 32) of data from one experiment, which is representative of two independent experiments. Statistical significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*** p<0.001).
(PDF)
(A) Representative images of anti-phospho-Histone H3 (α-PH3, red) and DAPI (blue) staining of adult brains of 5-7-day-old males show absence of cell proliferation in animals with knockdown of nowl or Nf1. Cell proliferation, as indicated α-PH3, was not observed in analysis of five independent brains of each genotype. (B) Representative images of five independent class-IV dendritic arborization (da) neurons from abdominal segment A3 in the peripheral nervous system and their axonal terminals in the ventral nerve cord. Knockdown of nowl or Nf1 does not alter the gross morphology of class-IV da neuronal structures.
(PDF)
Quantification of daytime (A) and nighttime (B) total sleep, sleep-bout duration, and sleep-bout numbers for nowl-RNAi/+, Nf1-RNAi/+ and UAS-Nf1/+ (Nf1/+) control males for main Fig 5. Graphs represent means with SEM (n = 32–81) of data pooled from one to three independent experiments.
(PDF)
(A, B) Efficiency of nowl (A) and Nf1 (B) gene knockdown. Gene expression was determined in adult male heads using qPCR. (C) Anti-Nowl staining features observed in male Rdl>GFP controls are not present in elav>nowl-RNAi brains with reduced expression of nowl in the nervous system, indicating that the anti-Nowl antibody specifically recognizes the Nowl protein. Graphs represent mean and SEM (n = 6) of data from one experiment. Statistical significance was determined using Kruskal-Wallis test with Dunn's post-hoc testing (*p < 0.05, *** p < 0.001).
(PDF)
RU486 was used to induce RNAi restricted to the adult stage using the GeneSwitch (GS) system in flies carrying elav-GS-GAL4 (elavGS>). (A, B) Total sleep per night is decreased in male flies with adult-specific neuronal knockdown of nowl (elavGS>nowl-RNAi) or Nf1 (elavGS>Nf1-RNAi) induced by RU486 compared to vehicle-treated controls and elavGS>+ controls. (C-F) Night-time sleep is fragmented in adult males when knockdown of nowl or Nf1 is induced, as indicated by reduced sleep-bout duration (C, D) and increased sleep-bout number (E, F) primarily during the night. Graphs represent mean and SEM (n = 32) of data from one experiment. Significance was tested by Mann-Whitney U tests (*p < 0.05, ** p < 0.01, *** p < 0.001).
(PDF)
Daily sleep profiles across a 12-hour light, 12-hour dark (white and black bars) cycle for 3-to-7-day-old male flies of the genotypes w1118/Y, nowl1/Y, RdlCB2/+, and nowl1/Y; RdlCB2/+. The reduced night-time sleep produced by loss of nowl (nowl1 mutant) is rescued by introducing one copy of the RdlCB2 allele.
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.