Abstract
BACKGROUND
Prehospital plasma improves survival in severely injured patients transported by air ambulance. We hypothesized that prehospital plasma would be associated with a reduction in immune imbalance and endothelial damage.
METHODS
We sampled blood from 405 trauma patients enrolled in the Prehospital Air Medical Plasma (PAMPer) trial upon hospital admission (0 hours) and 24 hours post admission across 6 U.S. sites. We assayed samples for 21 inflammatory mediators and 7 markers associated with endothelial function and damage. We performed hierarchical clustering analysis (HCA) of these biomarkers of the immune response and endothelial injury. Regression analysis was used to control for differences across study and to assess any association with prehospital plasma resuscitation.
RESULTS
HCA distinguished two patient clusters with different injury patterns and outcomes. Patients in cluster A had greater injury severity and incidence of blunt trauma, traumatic brain injury, and mortality. Cluster A patients that received prehospital plasma showed improved 30-day survival. Prehospital plasma did not improve survival in cluster B patients. In an adjusted analysis of the most seriously injured patients, prehospital plasma was associated with an increase in adiponectin, IL-1β, IL-17A, IL-23, and IL-17E upon admission, and a reduction in syndecan-1, TM, VEGF, IL-6, IP-10, MCP-1, and TNF-α, and an increase in IL-33, IL-21, IL-23, and IL-17E 24 hours later.
CONCLUSION
Prehospital plasma may ameliorate immune dysfunction and the endotheliopathy of trauma. These effects of plasma may contribute to improved survival in injured patients.
TRIAL REGISTRATION
FUNDING
Department of Defense; National Institutes of Health, U.S. Army.
Keywords: Immunology, Inflammation
Keywords: Cellular immune response, Cytokines, endothelial cells
Introduction
Injury and the associated hemorrhagic shock are leading causes of death (1). Emerging evidence suggests that damage-control resuscitation strategies — which prioritize blood-component products, including plasma, platelets, and packed RBCs (PRBCs) over crystalloid fluids — improve survival following severe injury (2, 3). It is hypothesized that hemostatic resuscitation may prevent downstream complications of trauma, including coagulopathy, irreversible shock, and derangements to the immune response (4). Studies from military settings demonstrate the importance of intervening early (5). Thus, it was hypothesized that the administration of plasma in civilian prehospital settings would improve survival as compared with conventional crystalloid resuscitation. The Prehospital Air Medical Plasma (PAMPer) trial, a pragmatic, multicenter, cluster-randomized, phase 3 superiority trial, demonstrated that prehospital plasma fluid resuscitation reduces 30-day mortality in severely injured trauma patients at risk for hemorrhagic shock and transported by air ambulance (6). However, the biological mechanisms conferring this survival benefit remain uncertain, making it challenging to identify patients who are at the greatest risk of death and those who would benefit from early and targeted interventions.
Tissue injury induced by massive trauma results in damage and stress to the endothelium (7). Damage-associated molecular patterns (DAMPs) released by damaged and stressed tissues activate innate immune responses, including the extracellular release of immune mediators (8–10). While most inflammatory mediators are pleiotropic in their functions, some can be defined as predominantly inflammatory, antiinflammatory, or reparative (11). Abrupt changes in the levels of circulating markers of immune and endothelial cell activation occur quickly after injury and correspond to quantitative and qualitative aspects of the human response to injury (10, 12–15). For example, the dysregulation of immune responses leads to worsening short-term (<24 hours) and long-term (>30 days) outcomes related to infection (16), persistent illness (17), and mortality (18). The dynamic patterns of immune mediator levels have been correlated with injury characteristics (10), patient demographics (19, 20), and outcomes (16, 21). Likewise, endothelial proteins such as syndecan-1 and thrombomodulin (TM) have been used to assess the degree of endothelial glycocalyx damage and to predict mortality following trauma (22–24). The immune system and endothelium operate in concert in the host response to injury; therefore, an impairment in one of these systems would be expected to manifest as detectable alterations in the other. Principal component analysis (PCA) and hierarchical clustering analysis (HCA) of markers of inflammation in trauma patients may distinguish important molecular and clinical patterns (16).
The host response to injury can manifest as a cycle of barrier stress, inflammation, and danger signaling, but whether prehospital plasma mitigates these processes is uncertain. Previous research in laboratory experiments and animal models has suggested that plasma may have protective properties (12), acting to restore the glycocalyx (25), reduce endotheliopathy (26), and decrease vascular hyperpermeability (27, 28). Endothelial dysfunction is thought to underlie imbalances in perfusion, coagulation (12), and inflammation and has been implicated as a unifying force in biological responses to injury (15, 29). However, no human study has assessed the effect of plasma on the endotheliopathy of trauma (12, 30). Therefore, the extent to which prehospital plasma alters aberrant immune or endothelial responses in humans is not known.
In a post hoc analysis of the PAMPer study, we sought to determine whether circulating markers of inflammation and endothelial damage are associated with prehospital plasma administration and clinical outcomes. We hypothesized that trauma patients who receive prehospital plasma would be less likely to suffer from dysregulated immune responses and have reduced endothelial damage. Here, we present evidence in humans suggesting a favorable effect of prehospital plasma on the evolution of inflammatory responses and the endotheliopathy of trauma.
Results
Sampled cohort mirrors the PAMPer trial cohort.
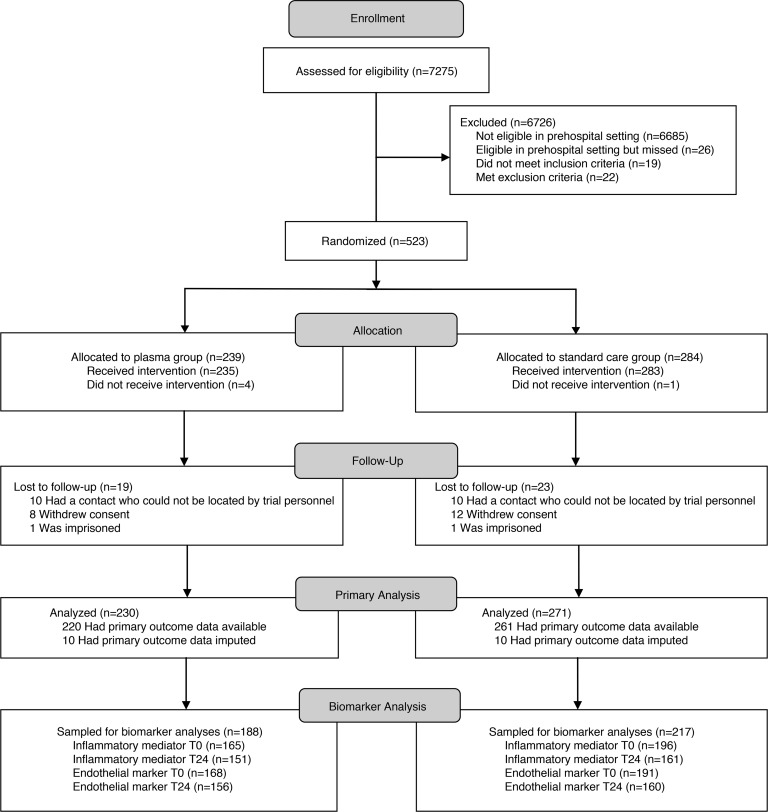
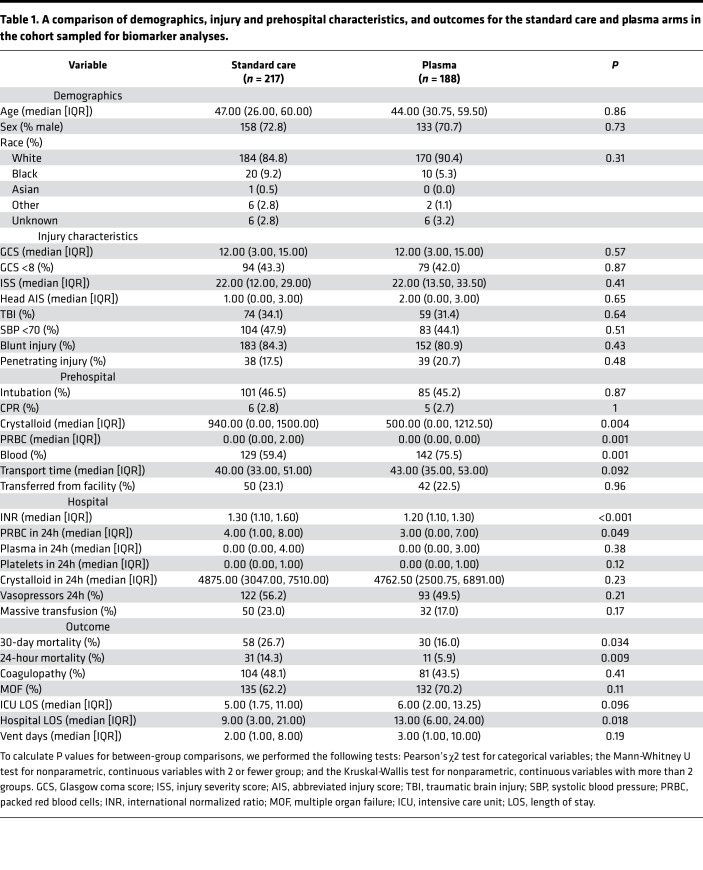
We obtained blood samples from patients enrolled the PAMPer trial (Figure 1). Sampling was not feasible in some patients due to time-sensitive procedures or early death. These patients without blood samples did not differ in injury severity score (ISS) but did have higher 24-hour mortality. Missing samples did not vary across randomized arms, and the comparison of patients in the standard care and plasma arms mirrors the comparison of patients in the overall PAMPer cohort (Table 1) (6).
Figure 1. Consort diagram.
Screening, randomization, follow-up, and biomarker sampling.
Table 1. A comparison of demographics, injury and prehospital characteristics, and outcomes for the standard care and plasma arms in the cohort sampled for biomarker analyses.
Unadjusted comparison of inflammatory mediators and endothelial damage markers.
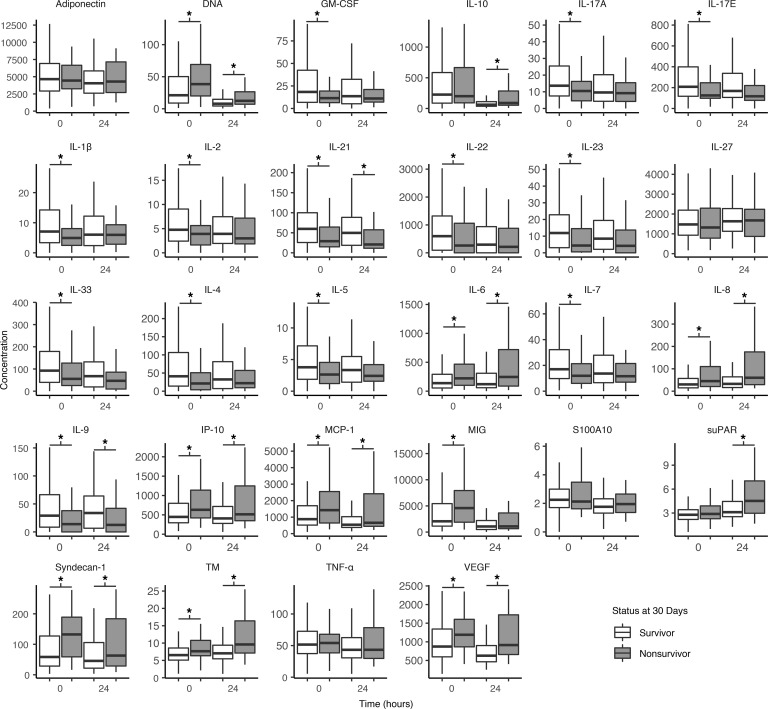
We first compared circulating levels of 21 inflammatory mediators and 7 endothelial markers for all sampled patients enrolled in the PAMPer trial at 0 and 24 hours as a function of survival. In this unadjusted analysis, mean concentrations did not differ across the plasma and standard care arms. However, immune mediators and endothelial markers were associated with 24-hour (not shown) and 30-day mortality (Figure 2). Most markers differed for survivors at admission (18 immune mediators, 4 endothelial markers), while most endothelial markers also differed 24 hours later (7 immune mediators, 5 endothelial markers). Survivors had lower concentrations of some proinflammatory mediators including IL-6 and several endothelial damage markers such as syndecan-1. Survivors were associated with higher concentrations of other mediators, including IL-1β, IL-17A, IL-23, and IL-33.
Figure 2. Circulating inflammatory and endothelial marker concentrations measured at 0 and 24 hours for 30-day survivors and nonsurvivors.
Lines within the bars represent medians. The lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles). The whiskers extend from the hinge to the smallest and largest values, no further than 1.5× IQR from the hinge (where IQR is the interquartile range). The asterisks denote significantly different (P < 0.05) time points as calculated by the Mann-Whitney U test. All inflammatory mediators are reported in pg/mL, except IL-23, which is reported in ng/mL. We report adiponectin, S100A10, suPAR, syndecan-1, and TM, in ng/mL, and VEGF in pg/mL. DNA (histone-complexed) is reported as relative units. 0h inflammatory mediators, n = 361; 24h inflammatory mediators, n = 312; 0h endothelial markers n = 359; 24h endothelial markers, n = 316.
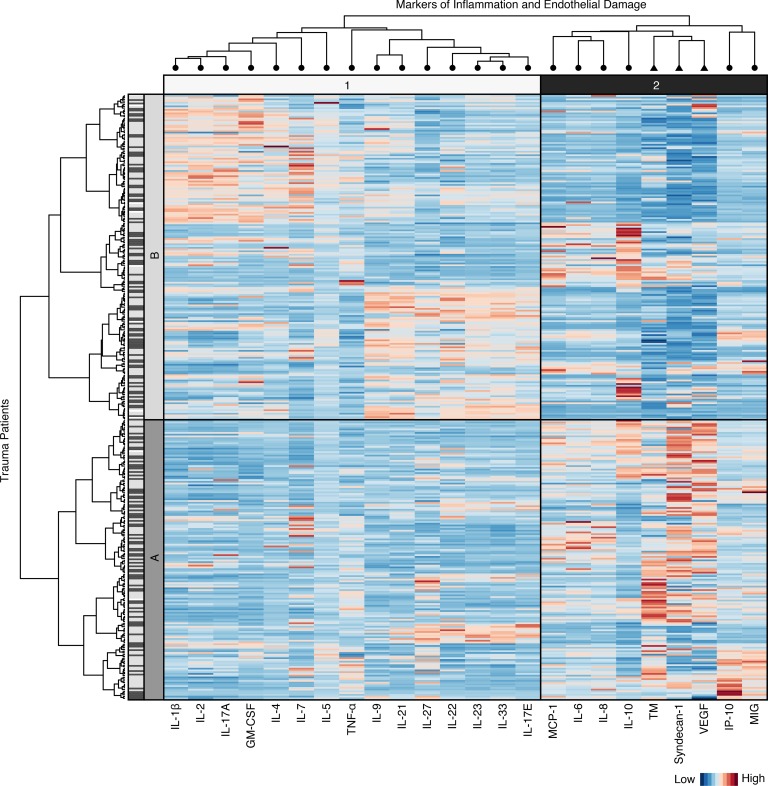
Clustering analysis based on early biomarker concentrations stratifies patients with different injury patterns and outcomes following prehospital plasma resuscitation.
In order to assess the earliest dynamic molecular immune responses occurring within hours of injury and prehospital plasma administration, we employed HCA based on principal components (16). Clusters were determined using only the earliest, hospital admission values of key markers of immune function and endothelial damage. HCA resulted in 2 primary clusters, cluster A (n = 158) and cluster B (n = 179), each with different injury severity and type, biomarker patterns, and clinical outcomes (Figure 3). Cluster A patients are defined by lower levels of some mediators, including IL-22 and IL-33, and higher levels of other mediators, including proinflammatory IL-6 and endothelial damage markers syndecan-1, TM, and VEGF. Cluster B patients exhibit less endothelial damage and more of a reparative, T cell–mediated immune response. Additionally, most reparative or T cell mediators clustered together, and most damage or innate immune mediators clustered together, although not every biomarker fits this distinction (e.g., TNF-α and GM-CSF).
Figure 3. Heatmap of scaled hospital admission marker concentrations corresponding to patients (n = 337) in the cluster dendrogram.
Clusters are denoted by A and B. Dark gray lines next to patients correspond to patients who received prehospital plasma, and light gray lines correspond to patients who received standard care resuscitation. Markers of inflammation (circles) and endothelial damage (triangles) form clusters (denoted 1 and 2) along the top and are labeled along the bottom of the heatmap. Higher-scaled values are represented by darker red lines, and lower-scaled values are represented by darker blue lines.
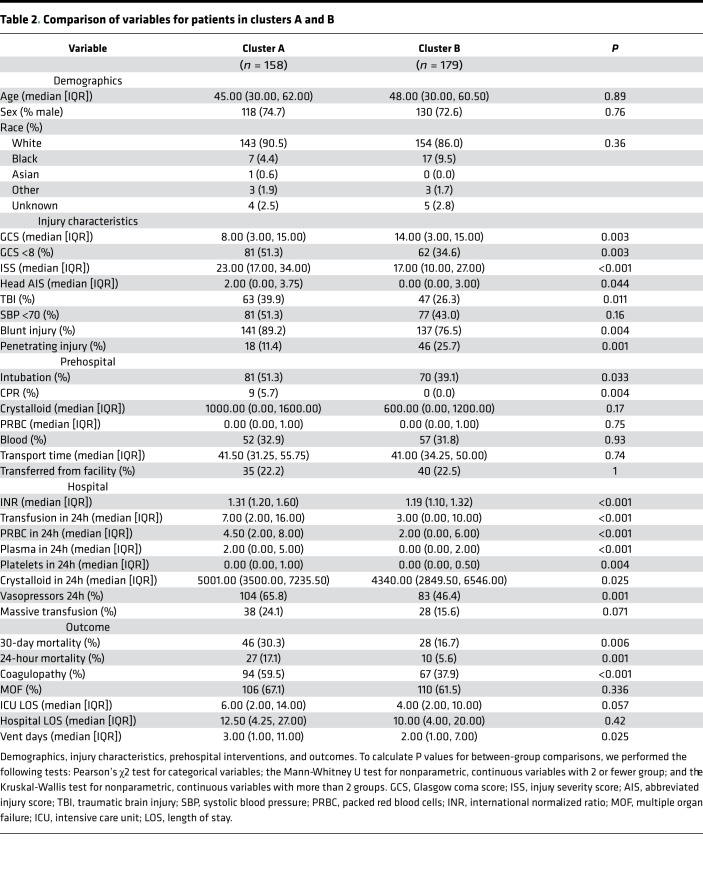
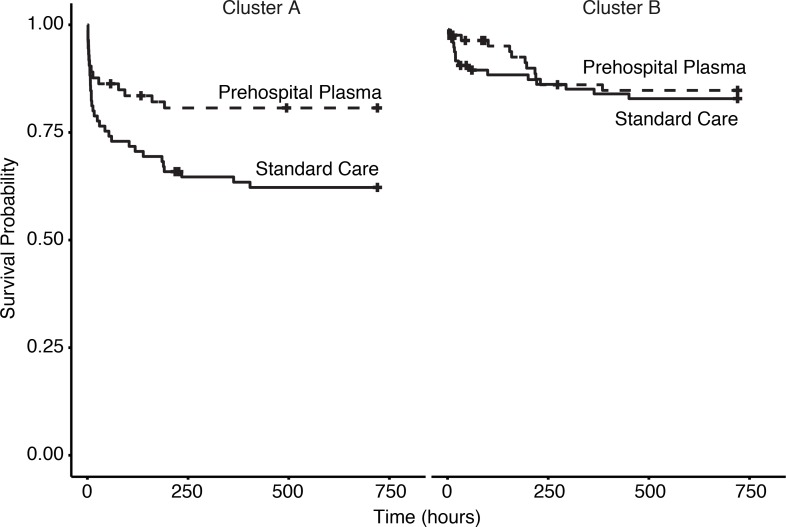
Overall, patient demographics did not differ across clusters, and neither cluster included more prehospital plasma patients. However, HCA did distinguish injury patterns. Patients in cluster A had higher injury severity (median ISS, 23.00 [interquartile range (IQR) 17.00, 34.00] versus 17.00 [IQR 10.00, 27.00], P < 0.001). Patients in cluster A were also more likely to suffer blunt trauma and traumatic brain injury (TBI), while patients in cluster B were more likely to suffer penetrating trauma. Mortality (24-hour and 30-day), incidence of coagulopathy, ventilator days, and 24-hour transfusion requirements were greater for cluster A (Table 2). HCA also identified a group of patients associated with more severe injury and a survival benefit following prehospital plasma. Cluster A patients who received prehospital plasma showed improved 30-day survival (P = 0.016), while prehospital plasma did not alter survival in cluster B patients (P = 0.66) (Figure 4). Patients grouped by neutrophil to platelet ratio (NPR) as a proxy for systemic inflammation (not shown) did not differ in injury characteristics or outcomes. HCA provides a unique method by which to understand the dynamic molecular immune patterns and responses to interventions in injured patients.
Table 2. Comparison of variables for patients in clusters A and B.
Figure 4. Kaplan Meier survival curves for cluster A and cluster B.
Cluster A, n = 158, log-rank, P = 0.016. Cluster B, n = 179, log-rank, P = 0.66. Time is in hours (to 30 days).
Prehospital plasma is associated with modified immune mediator patterns and reduced endothelial damage in the most severely injured patients.
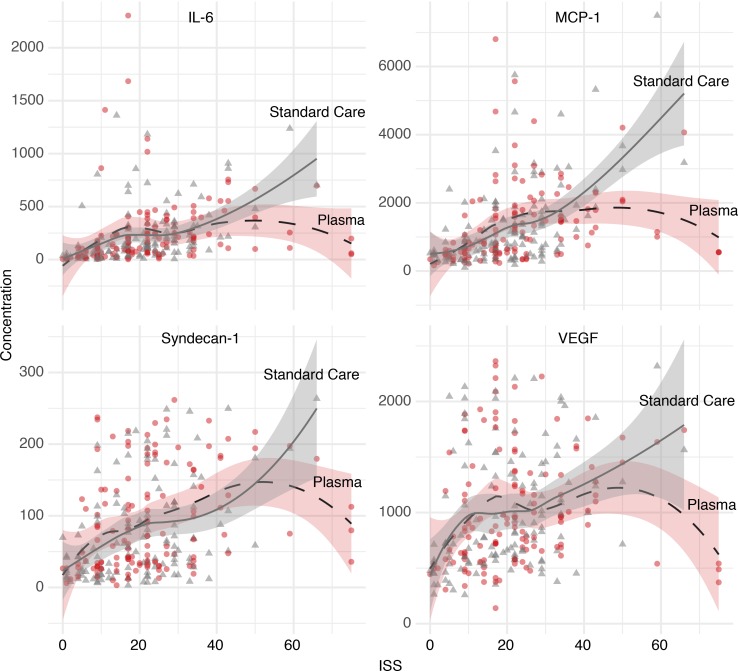
We hypothesized that injury severity may be associated with some of the differences observed between clusters A and B derived from our HCA results. We expanded our analysis in order to assess whether these differences vary across study arms among the most severely injured patients. We assessed polynomial regression curves using locally estimated scatter plot smoothing (LOESS) to explore and visualize possible relationships between ISS and admission biomarker concentrations among survivors. Figure 5 illustrates that circulating levels of immune mediators IL-6 and monocyte chemoattractant protein-1 (MCP-1), and endothelial damage markers syndecan-1 and VEGF, may increase with increasing ISS for the standard care group. However, concentrations of these biomarkers may decrease with increasing ISS (>30) for the plasma group, suggesting that there may be a greater response to plasma in patients with greater injury severity. These results are exploratory in nature but were robust across fitting parameters and consistent with HCA results.
Figure 5. Early (0 hours) inflammatory and endothelial marker concentrations plotted against injury severity score (ISS) in patients who survived to 30 days.
Gray triangles represent patients in the standard care arm, and red circles represent patients in the prehospital plasma arm. Shading represents the 95% CI. Inflammatory mediators IL-6 and MCP-1 are reported in pg/mL (n = 263). Endothelial marker syndecan-1 is reported in ng/mL, and VEGF is reported in pg/mL (n = 262).
Based on HCA and the above relationships, we hypothesized that injury patterns and severity may affect the observed biological responses to prehospital plasma. To further explore this relationship, we evaluated patterns of circulating inflammatory mediators and endothelial injury markers in the most severely injured subgroup (75th percentile ISS, >30) and adjusted for known differences across arms of the trial near the time of randomization.
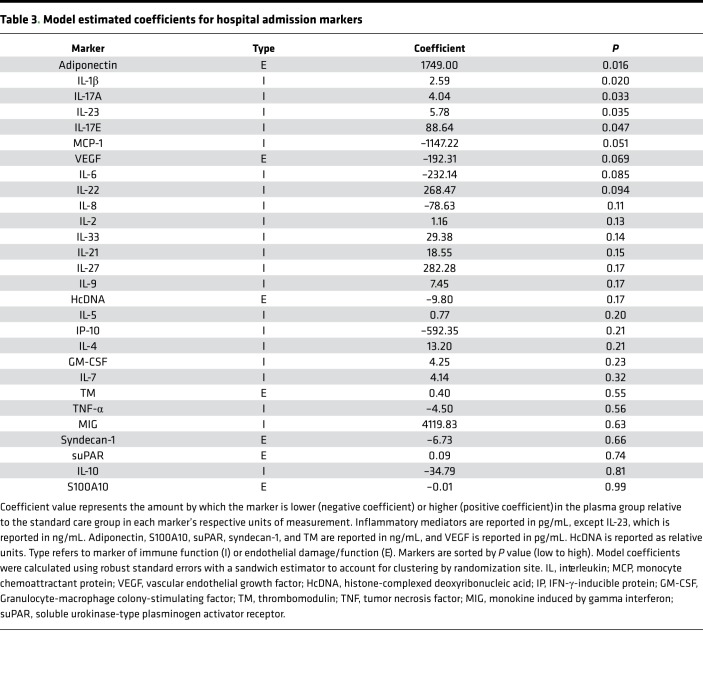
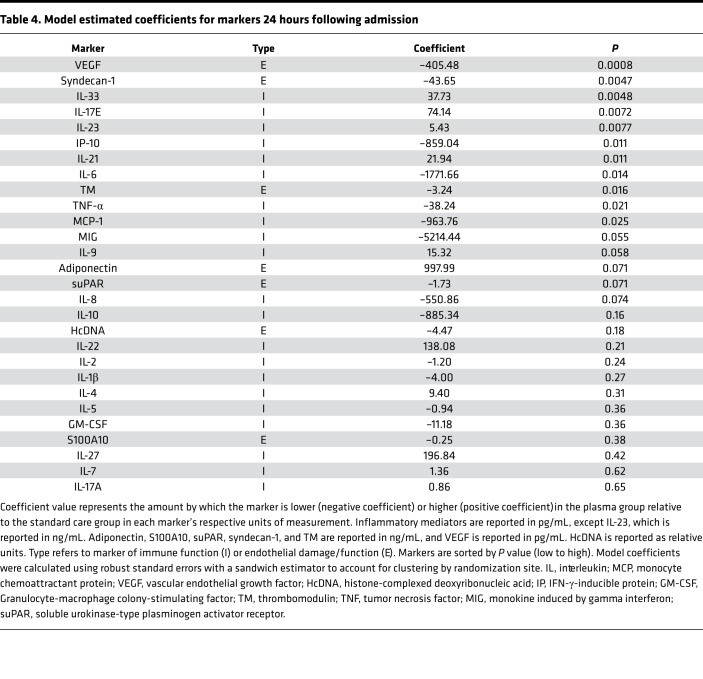
In this adjusted analysis, elevated admission levels of adiponectin, IL-1β, IL-17A, IL-23, and IL-17E were associated with prehospital plasma (Table 3). Except for adiponenctin, which did not differ for survivors and nonsurvivors, these markers were also higher for survivors at 0 hours. Levels of 10 biomarkers also differed at 24 hours following admission (Table 4). Estimated coefficients reveal that plasma was associated with a reduction in endothelial damage markers syndecan-1, TM, and VEGF; a reduction in proinflammatory mediators IL-6, IFN-γ–inducible protein 10 (IP-10), MCP-1, and TNF-α; and an increase in a subset of immune mediators that include IL-33, IL-21, IL-23, and IL-17E. Thus, after controlling for differences across arms within the patients with highest injury severity, plasma is associated with lower levels of certain proinflammatory mediators and higher levels of other mediators, some of which are associated with repair and regeneration (e.g., IL-33 and IL-17E). Coefficients for endothelial damage markers syndecan-1, TM, and VEGF were also lower in the plasma group (4).Therefore, among the most severely injured patients, prehospital plasma is associated with a change in inflammatory mediator expression patterns and a reduction in endothelial damage by 24 hours, some of which may be a result of early immune mediator differences.
Table 3. Model estimated coefficients for hospital admission markers.
Table 4. Model estimated coefficients for markers 24 hours following admission.
Discussion
Prehospital plasma improved survival in trauma patients transported by air ambulance (6). The reasons for this survival benefit are unknown; however, several underlying mechanisms have been hypothesized (4, 31). For example, plasma may attenuate inflammation (32), immune dysfunction (28), and endothelial damage (12, 26). In this study, immune mediators and endothelial damage markers are altered in trauma patients who receive prehospital plasma and survive. An adjusted analysis of the most severely injured patients reveals that patients who received plasma had lower concentrations of markers of endothelial damage and proinflammatory mediators, suggesting a possible mechanism for the plasma benefit. Prehospital plasma may narrow the imbalance between proinflammatory and protective cytokines, and a reduction in endothelial cell damage may be a key factor in mitigating this response. This is the first translational evidence in humans to suggest that prehospital plasma may intervene on the underlying biology of trauma. We suggest that these markers of inflammation and tissue damage reveal associations that may improve our understanding of how prehospital plasma affects aberrant immune responses and improves survival following trauma.
In our analysis, PCA and HCA identified clinically relevant patterns in immune function and endothelial damage. Although raw circulating inflammatory mediator and endothelial damage marker concentrations did not differ across arms of the PAMPer trial, it has been shown that computational methods are necessary to distinguish patient phenotypes (33) and underlying dynamic responses of the immune system (16). In this study, survival following prehospital plasma differed for patients clustered by hospital admission values of inflammatory mediators and endothelial damage markers. In the more severely injured cluster, plasma was associated with improved survival, whereas mortality did not differ with plasma administration in the less severely–injured cohort. This stratification may reflect differences in treatment or host response to prehospital interventions following trauma. In prior studies, multiple measurements made over 24 hours were needed in order to establish patient clusters (16). Our unbiased clustering analysis demonstrates that a single measurement made upon admission distinguishes clinically relevant groups of patients. This approach may improve predictive capacity following injury or inform the prospective stratification of patients for early interventions.
Markers of inflammation and endothelial damage may also suggest mechanisms by which patients benefit from prehospital plasma. We found potentially important relationships among the 7 markers of endothelial damage and the 21 inflammatory mediators in our panel. Several mediators linked with the proinflammatory response were higher in severely injured patients who exhibited evidence of endothelial injury, while other mediators were significantly higher when endothelial injury markers were suppressed. Cytokines such as IL-33 and IL-17E (IL-25), known to be involved in epithelial cell repair (34, 35), were higher in the plasma group. A plasma-associated increase in IL-23, a driver of TH17 cell differentiation (18), was also observed. Based on these findings, it is reasonable that plasma had less of an effect in the cluster B patients, who had overall greater survival and less endothelial injury than cluster A. We hypothesize that plasma may suppress endothelial injury — and subsequent inflammation — and activate a reparative response. However, this requires further exploration in mechanistic models.
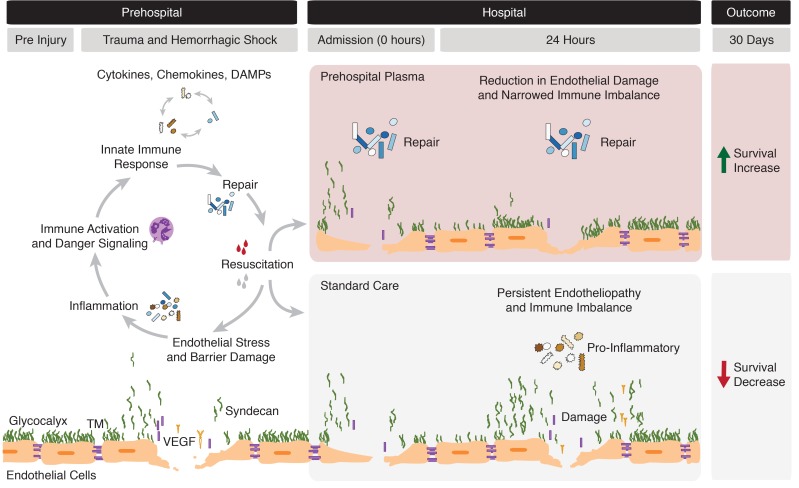
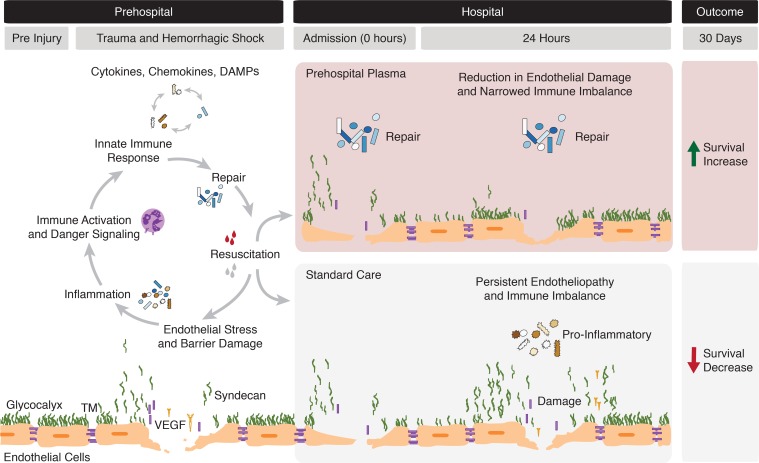
Prehospital plasma appears to have a beneficial, pleiotropic effect on endotheliopathy. In our analysis, plasma was associated with lower concentrations of circulating syndecan-1, TM, and VEGF, reflecting a reduction in damage to the glycocalyx, the protein C system, and the tight junctions, respectively (29). While the link between plasma and glycocalyx integrity remains poorly understood, our results are consistent with previous animal and laboratory studies that suggest that plasma may protect the glycocalyx following trauma (12, 36). Because the half lives of syndecan-1 and TM are short, persistently elevated levels may indicate sustained production (37). Our analysis also demonstrated a plasma-associated increase in adiponectin upon admission. It has been previously suggested that resuscitation with plasma increases adiponectin, which may protect vascular barrier function (38). These results suggest that prehospital plasma may protect or restore the endothelium following severe injury. The current analysis is the first to our knowledge to demonstrate any prehospital plasma–associated change in inflammatory mediators and endothelial markers in human trauma patients. Figure 6 illustrates the proposed relationships between the endothelium, immune response, and prehospital resuscitation following injury. Based on the results of HCA and adjusted analyses of the most severely injured patients, we depict a possible mechanism for the survival benefit following prehospital plasma.
Figure 6. Illustration depicting the dynamic immune and endothelial responses to traumatic injury.
The gray bars at the top of the figure define the time period, with hospital admission occurring within approximately 1 hour of injury or initial emergency response. The hypothesized effects of prehospital fluid administration as reported in this study are delineated by the red (plasma) and gray (standard care) panels. The endothelium is represented as previously depicted (10).
Our results suggest that the timing of marker expression patterns is important and warrants further investigation. Prehospital plasma was associated with reduced endothelial damage 24 hours following admission; however, this effect was not apparent at 0 hours. Although the timing of biological responses to trauma is poorly understood, immune markers change quickly following trauma (14), may decrease significantly within 24 hours (39, 40), and exhibit heterogeneous response times (41). We hypothesize that traumatic injury and fluid resuscitation trigger both immediate and delayed changes in immune and damage expression patterns. It is possible that there may also be a dose-dependent aspect to plasma resuscitation and that patients benefit after receiving a threshold fluid volume. The time course of endothelial damage differences in the plasma group paired with the improved survival add further weight to amelioration of endothelial damage as a potential mechanism of the survival benefit, rather than being simply an injury severity marker.
Taken together, our results may partly explain clinical outcomes previously observed. The PAMPer trial showed a survival advantage for patients who received prehospital plasma (6); however, other studies have shown no survival benefit associated with prehospital plasma (42). This discordance may be due to differences in patient populations and injury patterns. In our analyses, the most severely injured trauma patients who experienced blunt trauma and TBI showed the greatest improvement in survival following prehospital plasma. This suggests that, with greater injury severity (and presumably greater shock severity and endothelial damage), there may be an increased benefit of plasma. Previous work has also suggested that injury severity may differentially affect inflammatory responses (43). Moreover, it has been previously shown that the most severely injured patients transported by air ambulance who received prehospital blood products have the greatest reduction in risk of death (5, 44). Therefore, it is possible that the PAMPer trial was enriched in an inherently more responsive subgroup of patients. Consistent with our HCA results, other secondary analyses of the PAMPer trial have shown that the survival benefit of prehospital plasma is principally in blunt, as compared with penetrating, trauma patients (45). Patients with penetrating trauma likely had more hemorrhage, while patients with blunt injuries likely had more endotheliopathy. Thus, we hypothesize that the patients who showed the greatest increase in survival following plasma were those who were most likely to experience endothelial dysfunction.
The clinical data collected for this study are from a multicenter randomized trial. However, this study involves a secondary analysis of prospective data and has several limitations. Samples were not collected specifically for characterizing underlying mechanisms involved in plasma resuscitation. Only a subset of cytokines and no endothelial cell markers were specified a priori. This exploratory analysis did not adjust for multiple comparisons. Microvascular dysfunction is a key component of shock, but our measurements are limited to circulating fluids. Systemic plasma level measurements may not reflect the local milieu, and the absence of a systemic change may not imply a lack of effect. The roles of the inflammatory mediators and endothelial damage markers are simplified in this study. Some markers may have nonspecific origins or functions, and more mechanistic studies are needed in order to determine the functional relationship between plasma, the mediators studied, and mortality. However, samples were taken immediately following acute, severe injury, presumably before other complex interactions have manifested in these patients. The LOESS curves are exploratory and may be sensitive to smoothing parameters and the small sample sizes at the highest ISS values. The limitations of sampling and variability in pre- and in-hospital factors (prehospital times and provider-level differences) introduce bias. The majority of hospital admission samples were collected within the first 3 hours following admission; however, markers of immune function and endothelial damage may be in different phases of the response to trauma. The source and age of donor plasma may be a potential confounder, but no unit of plasma was older than 4 days, and we found no differences in clinical outcome associated with plasma age in the PAMPer trial. Many of our results are hypothesis generating and require further mechanistic assessment. We cannot prove responsiveness or definitively ascertain an underlying mechanism. For instance, it is possible that prehospital plasma reduces the volume of prehospital crystalloid, thereby decreasing endothelial injury, as shown in sepsis patients (46). Conclusions of this study are also limited by survivor bias and by the fact that samples could not be collected from patients prior to injury or intervention. Finally, a spectrum bias exists, as some patients did not receive additional lab samples due to early death or time-sensitive interventions. However, this would tend to bias our findings to the null.
HCA based on inflammatory mediator and endothelial marker concentrations measured upon hospital admission defines groups of patients with different injury patterns, outcomes, and survival following the administration of prehospital plasma. Regression analysis reveals that the most severely injured patients who received prehospital plasma express different inflammatory mediator and endothelial marker patterns. Our results suggest that prehospital plasma may attenuate inflammation and reduce endothelial damage, thereby leading to a survival benefit. Future mechanistic studies will be important for understanding these differences.
Methods
Trial design and study population.
The PAMPer trial was a prospective, randomized trial designed to test the effect of administering prehospital plasma to severely injured trauma patients on air ambulances within approximately 1 hour of injury and prior to arrival at the hospital. We randomized patients by air ambulance base to receive either standard care fluid (crystalloid or crystalloid and PRBCs) resuscitation or 2 units of freshly thawed plasma, followed by the standard care fluid resuscitation. The full study protocol is publicly available (https://clinicaltrials.gov/ct2/show/NCT01818427).
Sample collection and measurement.
We collected blood samples from PAMPer trial patients upon hospital admission (the first blood draw, referred to as 0 hours) and at 24 hours after admission. We assayed plasma collected at 0 and 24 hours for 21 inflammatory mediators and 7 putative endothelial damage markers or markers hypothesized to be involved with endothelial function.
Inflammatory mediators were assayed using a Luminex MAGPIX (Luminex, Luminex Corp). GM-CSF, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-17A, IFN-γ–IP-10, MCP-1 (MilliporeSigma), and TNF-α were measured using a MilliporeSigma 13-plex kit (catalog HCYTOMAG-60K). IL-9, IL-17E/IL-25, IL-21, IL-22, IL-23, IL-27, and IL-33 (MilliporeSigma) were measured using a MilliporeSigma 7-plex kit (catalog HTH17Mag-14k). Monokine induced by IFN-γ (MIG) was measured using a separate MilliporeSigma kit (catalog HCYP3MAG-63K). We report all inflammatory markers in pg/mL except IL-23 (reported in ng/mL).
Damage markers adiponectin, histone-complexed DNA (HcDNA) fragments, human S100 calcium-binding protein A10 (S100A10), soluble urokinase receptor (suPAR), syndecan-1, TM, and vascular endothelial growth factor (VEGF) were assayed by commercially available immunoassays in EDTA plasma according to the manufacturer’s recommendations as previously reported (15). We analyzed soluble biomarkers representing damage to the glycocalyx (syndecan-1, catalog 950.640.192, lot no. 0138-62 + 0138-66, Nordic Biosite ApS), endothelium (TM, catalog 850.720.192, lot no. 0141-47, Nordic Biosite ApS), and endothelial tight-junction (VEGF-R1/Flt-1, catalog DVR100C, lot no. P186961, Bio-Techne). We also analyzed markers of cell death as cell-free DNA (HcDNA, catalog 11774425001, lot no. 29876600, Sigma-Aldrich), immunologically active endothelial cells (suPAR, catalog E001, lot no. XS2141, suPARnostic, ViroGates), mediators of fibrinolysis (S100A10, catalog abx152996, lot no. E1905813M, Abbexa Ltd.), and an adipokine related to endothelial function (adiponectin, catalog DRP300, lot no. P186579, Bio-Techne). We report adiponectin, S100A10, suPAR, syndecan-1, and TM in ng/mL, and we report VEGF in pg/mL. HcDNA is reported as relative units. Some of these markers (HcDNA, S100A10, suPAR) may be nonspecific markers of damage and derived from multiple cell types, but they all have a hypothesized association with endothelial cell damage or function (9, 47, 48). It is also hypothesized that adiponectin, produced by adipocytes, may play a restorative role in endothelial function (12, 15, 49). Syndecan-1, TM, and VEGF have been associated with endothelial damage following trauma (15, 23). Because of the potential relationship between these markers and endothelial function, we categorized these 7 markers as endothelial cell markers (as opposed to putative immune mediators) for the purposes of this study.
Clustering and regression analysis.
We applied unsupervised HCA to identify possible underlying biological patterns following trauma. We assessed standardized concentrations of circulating inflammatory mediators and endothelial markers measured upon admission to reduce differences associated with in-hospital care and to assess the value of the earliest laboratory values. Due to sampling limitations and to reduce the loss of data, we included patients in this analysis if they had marker concentrations for all inflammatory mediators and 3 endothelial damage markers (syndecan-1, TM, and VEGF) measured upon hospital admission, and we excluded 1 outlier (n = 346). We assessed clustering parameters and identified the optimal number of clusters using 30 indices in the NbClust v 3 (50) package. In order to improve the stability of clusters, we performed PCA as an initial step on standardized concentrations of circulating mediators. We kept 7 principal components, retaining approximately 75% variance. HCA was performed using a Spearman distance matrix and Ward D2 cluster analysis method. We used summary statistics to assess injury, demographic, and clinical differences across clusters for all patients with outcome information (n = 337). Kaplan-Meier survival curves were built for each cluster, and log-rank P values were calculated using the Survminer v 0.4.3 (51) package. We compared our results with an analysis of NPR as a proxy for systemic inflammation (52).
In order to explore and visualize possible relationships between marker concentration and injury severity, we generated local polynomial regression curves using LOESS. We regressed all markers of inflammation and endothelial damage against ISS across arms of the PAMPer trial. We evaluated whether relationships were robust across a range of fitting parameters, and we present figures for span = 0.9.
To more quantitatively assess marker concentrations among the most severely injured patients, we built a generalized linear model (GLM), adjusting for potential confounders across trial arms. We evaluated biomarkers measured at hospital admission (0 hours) and 24 hours following admission. We included the 75th percentile of ISS (ISS > 30) and controlled for differences across arms of the trial near the time of randomization and for known clinical confounders. We included ISS, Glasgow Coma Score (GCS), prehospital shock (systolic blood pressure < 70), prehospital fluid resuscitation (crystalloid, PRBCs, and plasma), and international normalized ratio (INR). We performed a sensitivity analysis to ensure our results were robust across a range of parameters, and we minimized the number of variables included to avoid overfitting our model. We analyzed the resulting model coefficients using robust standard errors with a sandwich estimator to account for clustering by randomization site.
Statistics.
We analyzed clinical and biomarker data and performed summary statistics using R Version 3.4.1 (53). The analysis code is publicly available (https://github.com/dgru/pamper-car; branch: master; commit ID: e729fd6). To calculate P values for between-group comparisons, we performed the following tests: Pearson’s χ2 test with continuity correction for categorical variables; the Mann-Whitney U test for nonparametric, continuous variables with 2 or fewer groups; the Kruskal-Wallis test for nonparametric, continuous variables with more than 2 groups; and the log-rank test for survival curves. Statistical significance was determined at the P < 0.05 level.
Study approval.
This study was registered with ClinicalTrials.gov (NCT01818427) and approved by the University of Pittsburgh (Pittsburgh, Pennsylvania, USA) IRB as previously described (6). The study was approved under an Emergency Exception From Informed Consent (EFIC) protocol from the Human Research Protection Office of the US Army Medical Research and Material Command.
Author contributions
DSG analyzed the data and wrote the manuscript. JBB, FXG, MDN, BJD, RSM, BGH, JAC, HAP, BSZ, TRB, and JLS designed the original study and sampling plan. YV, PIJ, JS, DAB, and JY analyzed samples. All authors contributed to the drafting and critical revision of the manuscript. PAMPer study authors contributed to patient enrollment and sample procurement.
Supplementary Material
Acknowledgments
DSG was supported by a NIH T32 Ruth L. Kirschstein Service Fellowship. The PAMPer study was supported by a grant (W81XWH-12-2-0023) from the US Army Medical Research and Materiel Command. YV and TRB are cofounders of and stakeholders in Immunetrics Inc. TRB was funded by a grant from the NIH R35. MDN is supported by 1R35GM119526-01 from the NIH. This work was also supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Defense Medical Research and Development Program under Award No. W81XWH-18-2-0051 and W81XWH-15-PRORP-OCRCA to TRB and YV. Opinions, interpretations, conclusions, and recommendations are those of the authors and not necessarily endorsed by the Department of Defense. The authors acknowledge all PAMPer study collaborators, prehospital providers, site personnel, and research staff (including MACRO) for enabling the collection of this data. JLS reports grants from Department of Defense. DSG reports grants from NIH. YV reports grants from the Department of Defense. BGH reports grants from Department of Defense and from University of Pittsburgh. MDN reports grants from NIH 1R35GM119526-01. See Supplemental Acknowledgments for consortium details.
Version 1. 03/31/2020
In-Press Preview
Version 2. 04/23/2020
Electronic publication
Footnotes
Conflict of interest: YV reports funding from Immunetrics Inc. BGH reports grants, personal fees, and nonfinancial support from Haemonetics; grants and personal fees from Janssen Pharmaceuticals; grants from Instrument Laboratories; grants from Noveome; support from Haima Therapeutics; and personal fees from CSL Behring.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: JCI Insight. 2020;5(8):e135350.https://doi.org/10.1172/jci.insight.135350.
Contributor Information
Danielle S. Gruen, Email: dsg40@pitt.edu.
Joshua B. Brown, Email: brownjb@upmc.edu.
Francis X. Guyette, Email: guyefx@UPMC.EDU.
Yoram Vodovotz, Email: vodovotzy@upmc.edu.
Jakob Stensballe, Email: Jakob.Stensballe@regionh.dk.
Derek A. Barclay, Email: barcdx2@UPMC.EDU.
Jinling Yin, Email: yinj@upmc.edu.
Brian J. Daley, Email: BDaley@utmck.edu.
Richard S. Miller, Email: richard.miller@vumc.org.
Brian G. Harbrecht, Email: briang.harbrecht@louisville.edu.
Jeffrey A. Claridge, Email: jclaridge@metrohealth.org.
Herb A. Phelan, Email: hphelaniii@gmail.com.
Matthew D. Neal, Email: nealm2@upmc.edu.
Timothy R. Billiar, Email: billiartr@upmc.edu.
Jason L. Sperry, Email: sperryjl@upmc.edu.
References
- 1.Rhee P, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etchill EW, et al. Should All Massively Transfused Patients Be Treated Equally? An Analysis of Massive Transfusion Ratios in the Nontrauma Setting. Crit Care Med. 2017;45(8):1311–1316. doi: 10.1097/CCM.0000000000002498. [DOI] [PubMed] [Google Scholar]
- 4.Brown JB, et al. Taking the Blood Bank to the Field: The Design and Rationale of the Prehospital Air Medical Plasma (PAMPer) Trial. Prehosp Emerg Care. 2015;19(3):343–350. doi: 10.3109/10903127.2014.995851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shackelford SA, et al. Association of Prehospital Blood Product Transfusion During Medical Evacuation of Combat Casualties in Afghanistan With Acute and 30-Day Survival. JAMA. 2017;318(16):1581–1591. doi: 10.1001/jama.2017.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperry JL, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med. 2018;379(4):315–326. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 7.Naumann DN, et al. Endotheliopathy is associated with higher levels of cell-free DNA following major trauma: A prospective observational study. PLoS ONE. 2017;12(12):e0189870. doi: 10.1371/journal.pone.0189870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12(4-6):105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell RT, et al. Histone-Complexed DNA Fragments Levels are Associated with Coagulopathy, Endothelial Cell Damage, and Increased Mortality after Severe Pediatric Trauma. Shock. 2018;49(1):44–52. doi: 10.1097/SHK.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 10.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. 2018;19(4):327–341. doi: 10.1038/s41590-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 12.Barelli S, Alberio L. The Role of Plasma Transfusion in Massive Bleeding: Protecting the Endothelial Glycocalyx? Front Med (Lausanne) 2018;5:91. doi: 10.3389/fmed.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namas RA, Vodovotz Y. From static to dynamic: a sepsis-specific dynamic model from clinical criteria in polytrauma patients. Ann Transl Med. 2016;4(24):492. doi: 10.21037/atm.2016.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazeldine J, et al. Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study. PLoS Med. 2017;14(7):e1002338. doi: 10.1371/journal.pmed.1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson PI, et al. Traumatic Endotheliopathy: A Prospective Observational Study of 424 Severely Injured Patients. Ann Surg. 2017;265(3):597–603. doi: 10.1097/SLA.0000000000001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namas RA, et al. Individual-specific principal component analysis of circulating inflammatory mediators predicts early organ dysfunction in trauma patients. J Crit Care. 2016;36:146–153. doi: 10.1016/j.jcrc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mira JC, et al. The Epidemiology of Chronic Critical Illness After Severe Traumatic Injury at Two Level-One Trauma Centers. Crit Care Med. 2017;45(12):1989–1996. doi: 10.1097/CCM.0000000000002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abboud A, et al. Computational Analysis Supports an Early, Type 17 Cell-Associated Divergence of Blunt Trauma Survival and Mortality. Crit Care Med. 2016;44(11):e1074–e1081. doi: 10.1097/CCM.0000000000001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperry JL, et al. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64(3):572–579. doi: 10.1097/TA.0b013e3181650fdf. [DOI] [PubMed] [Google Scholar]
- 20.Lamparello AJ, Namas RA, Abdul-Malak O, Vodovotz Y, Billiar TR. Young and Aged Blunt Trauma Patients Display Major Differences in Circulating Inflammatory Mediator Profiles after Severe Injury. J Am Coll Surg. 2019;228(2):148–160.e7. doi: 10.1016/j.jamcollsurg.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankar-Hari M, Fan E, Ferguson ND. Acute respiratory distress syndrome (ARDS) phenotyping. Intensive Care Med. 2019;45(4):516–519. doi: 10.1007/s00134-018-5480-6. [DOI] [PubMed] [Google Scholar]
- 22.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson PI, Haase N, Perner A, Ostrowski SR. Association between sympathoadrenal activation, fibrinolysis, and endothelial damage in septic patients: a prospective study. J Crit Care. 2014;29(3):327–333. doi: 10.1016/j.jcrc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Wei S, et al. Elevated Syndecan-1 after Trauma and Risk of Sepsis: A Secondary Analysis of Patients from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial. J Am Coll Surg. 2018;227(6):587–595. doi: 10.1016/j.jamcollsurg.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozar RA, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diebel LN, Martin JV, Liberati DM. Microfluidics: A high-throughput system for the assessment of the endotheliopathy of trauma and the effect of timing of plasma administration on ameliorating shock-associated endothelial dysfunction. J Trauma Acute Care Surg. 2018;84(4):575–582. doi: 10.1097/TA.0000000000001791. [DOI] [PubMed] [Google Scholar]
- 27.Peng Z, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pati S, Peng Z, Wataha K, Miyazawa B, Potter DR, Kozar RA. Lyophilized plasma attenuates vascular permeability, inflammation and lung injury in hemorrhagic shock. PLoS One. 2018;13(2):e0192363. doi: 10.1371/journal.pone.0192363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson PI, Stensballe J, Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017;21(1):25. doi: 10.1186/s13054-017-1605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: The past, present, and future. J Thromb Haemost. 2019;17(6):852–862. doi: 10.1111/jth.14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pusateri AE, et al. The need for dried plasma - a national issue. Transfusion. 2019;59(S2):1587–1592. doi: 10.1111/trf.15261. [DOI] [PubMed] [Google Scholar]
- 32.Chang R, Holcomb JB, Johansson PI, Pati S, Schreiber MA, Wade CE. Plasma Resuscitation Improved Survival in a Cecal Ligation and Puncture Rat Model of Sepsis. Shock. 2018;49(1):53–61. doi: 10.1097/SHK.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seymour CW, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321(20):2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 2012;33(7):343–349. doi: 10.1016/j.it.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14(7):e1002365. doi: 10.1371/journal.pmed.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milford EM, Reade MC. Resuscitation Fluid Choices to Preserve the Endothelial Glycocalyx. Crit Care. 2019;23(1):77. doi: 10.1186/s13054-019-2369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naumann DN, et al. Endotheliopathy of Trauma is an on-Scene Phenomenon, and is Associated with Multiple Organ Dysfunction Syndrome: A Prospective Observational Study. Shock. 2018;49(4):420–428. doi: 10.1097/SHK.0000000000000999. [DOI] [PubMed] [Google Scholar]
- 38.Deng X, et al. Adiponectin in Fresh Frozen Plasma Contributes to Restoration of Vascular Barrier Function After Hemorrhagic Shock. Shock. 2016;45(1):50–54. doi: 10.1097/SHK.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Namas RA, et al. Temporal Patterns of Circulating Inflammation Biomarker Networks Differentiate Susceptibility to Nosocomial Infection Following Blunt Trauma in Humans. Ann Surg. 2016;263(1):191–198. doi: 10.1097/SLA.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaski GE, et al. Early Immunologic Response in Multiply Injured Patients With Orthopaedic Injuries Is Associated With Organ Dysfunction. J Orthop Trauma. 2019;33(5):220–228. doi: 10.1097/BOT.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 41.Minami T, Sugiyama A, Wu SQ, Abid R, Kodama T, Aird WC. Thrombin and phenotypic modulation of the endothelium. Arterioscler Thromb Vasc Biol. 2004;24(1):41–53. doi: 10.1161/01.ATV.0000099880.09014.7D. [DOI] [PubMed] [Google Scholar]
- 42.Moore HB, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392(10144):283–291. doi: 10.1016/S0140-6736(18)31553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billiar IM, Guardado J, Abdul-Malak O, Vodovotz Y, Billiar TR, Namas RA. Elevations in Circulating sST2 Levels Are Associated With In-Hospital Mortality and Adverse Clinical Outcomes After Blunt Trauma. J Surg Res. 2019;244:23–33. doi: 10.1016/j.jss.2019.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holcomb JB, et al. Prehospital Transfusion of Plasma and Red Blood Cells in Trauma Patients. Prehosp Emerg Care. 2015;19(1):1–9. doi: 10.3109/10903127.2014.923077. [DOI] [PubMed] [Google Scholar]
- 45.Reitz KM, et al. Prehospital plasma in injured patients is associated with survival principally in blunt injury: Results from two randomized prehospital plasma trials. J Trauma Acute Care Surg. 2020;88(1):33–41. doi: 10.1097/TA.0000000000002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hippensteel JA, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019;23(1):259. doi: 10.1186/s13054-019-2534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson PI, et al. High sCD40L levels early after trauma are associated with enhanced shock, sympathoadrenal activation, tissue and endothelial damage, coagulopathy and mortality. J Thromb Haemost. 2012;10(2):207–216. doi: 10.1111/j.1538-7836.2011.04589.x. [DOI] [PubMed] [Google Scholar]
- 48.Haupt TH, et al. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: a prospective observational study. Crit Care. 2012;16(4):R130. doi: 10.1186/cc11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halbgebauer R, et al. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J Crit Care. 2018;44:229–237. doi: 10.1016/j.jcrc.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 50.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J Stat Softw. 2014;61:11744–11750. [Google Scholar]
- 51. Kassambara A, Kosinski M. Survminer: Drawing Survival Curves using ’ggplot2’. R project. https://rpkgs.datanovia.com/survminer/index.html Accessed April 6, 2020.
- 52.He W, et al. High Neutrophil-to-Platelet Ratio Is Associated With Hemorrhagic Transformation in Patients With Acute Ischemic Stroke. Front Neurol. 2019;10:1310. doi: 10.3389/fneur.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. [No author listed]. R: A Language Environment for Statistical Computing. R Project. https://www.r-project.org/ Accessed April 6, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.