The Amazon region is a major tropical forest region that is being deforested at an alarming rate to create space for cattle ranching and agriculture. Diazotrophs (nitrogen-fixing microorganisms) play an important role in supplying soil N for plant growth in tropical forests. It is unknown how diazotrophs respond to deforestation and whether they can recover in secondary forests that establish after agriculture is abandoned. Using high-throughput sequencing of nifH genes, we characterized the response of diazotrophs’ β-diversity and identified major drivers of changes in diazotrophs from forest-to-pasture and pasture-to-secondary-forest conversions. Studying the impact of land use change on diazotrophs is important for a better understanding of the impact of deforestation on tropical forest ecosystem functioning, and our results on the potential recovery of diazotrophs in secondary forests imply the possible restoration of ecosystem functions in secondary forests.
KEYWORDS: diazotrophs, functional distance decay, recovery of diazotrophs in secondary forest
ABSTRACT
Biological nitrogen fixation can be an important source of nitrogen in tropical forests that serve as a major CO2 sink. Extensive deforestation of the Amazon is known to influence microbial communities and the biogeochemical cycles they mediate. However, it is unknown how diazotrophs (nitrogen-fixing microorganisms) respond to deforestation and subsequent ecosystem conversion to agriculture, as well as whether they can recover in secondary forests that are established after agriculture is abandoned. To address these knowledge gaps, we combined a spatially explicit sampling approach with high-throughput sequencing of nifH genes. The main objectives were to assess the functional distance decay relationship of the diazotrophic bacterial community in a tropical forest ecosystem and to quantify the roles of various factors that drive the observed changes in the diazotrophic community structure. We observed an increase in local diazotrophic diversity (α-diversity) with a decrease in community turnover (β-diversity), associated with a shift in diazotrophic community structure as a result of the forest-to-pasture conversion. Both diazotrophic community turnover and structure showed signs of recovery in secondary forests. Changes in the diazotrophic community were primarily driven by the change in land use rather than differences in geochemical characteristics or geographic distances. The diazotroph communities in secondary forests resembled those in primary forests, suggesting that at least partial recovery of diazotrophs is possible following agricultural abandonment.
IMPORTANCE The Amazon region is a major tropical forest region that is being deforested at an alarming rate to create space for cattle ranching and agriculture. Diazotrophs (nitrogen-fixing microorganisms) play an important role in supplying soil N for plant growth in tropical forests. It is unknown how diazotrophs respond to deforestation and whether they can recover in secondary forests that establish after agriculture is abandoned. Using high-throughput sequencing of nifH genes, we characterized the response of diazotrophs’ β-diversity and identified major drivers of changes in diazotrophs from forest-to-pasture and pasture-to-secondary-forest conversions. Studying the impact of land use change on diazotrophs is important for a better understanding of the impact of deforestation on tropical forest ecosystem functioning, and our results on the potential recovery of diazotrophs in secondary forests imply the possible restoration of ecosystem functions in secondary forests.
INTRODUCTION
The Amazon rain forest acts as a major sink of CO2 by absorbing 0.4 to 0.6 Pg C annually, which represents a quarter of the absorption by all forests globally (1–4). Global emission of the greenhouse gas carbon dioxide (CO2) is a serious environmental concern. The uptake of CO2 requires significant input of nitrogen, which comes from the mineralization of organic material, atmospheric deposition, and biological nitrogen fixation (BNF). In terrestrial nonmanaged ecosystems, 97% of natural nitrogen input comes from biological nitrogen fixation (5, 6) carried out by diazotrophs (N2-fixing Bacteria and Archaea) (7, 8). To assess variations in the diazotrophic species, the marker gene nifH has been widely used as a target gene (9–17), and alterations in its composition and abundance have been directly correlated with nitrogen fixation rates (18–22).
The Amazon is the largest equatorial forest in the world, and it controls vital biogeochemical cycles. Changes in its land use can significantly influence a wide range of ecosystem processes (23, 24), such as the N cycle, in which decreases in mineralization, nitrification, and denitrification processes have been observed in response to deforestation (25–27). Currently, Amazon forests are being cleared for cattle ranching and soybean fields at an alarming rate, and about 20% of the area of the Brazilian Amazon rain forest was cleared between 1970 and 2015 (28). Ramankutty et al. (29) estimated that most of the deforested area is being used for pasture (62%) and crop production (6%), with the remaining area being secondary forests (32%) that were established after pasture abandonment. Owing to the extent of deforested area (7,418,000 ha) (30) that is currently pasture and secondary forest, it is important to assess how changes in land use have influenced microbial functional groups and whether restoration of microbial communities in secondary forests occurs. Previous studies, primarily focused on plants and animals, have demonstrated that secondary forests invariably have much lower biodiversity than undisturbed primary forests (31, 32) and that the effects of losing primary forests are irreparable (32). So far, detailed information on the recovery of microbial communities in general and those responsible for specific ecological functions (e.g., diazotrophs) is limited.
Previous molecular microbial studies in the Amazon region have mainly focused on the effect of deforestation on bacterial communities as a whole (33, 34), with a limited number of studies focused on specific functional groups (35–37). These studies have generally been limited in terms of both the number of soil samples collected across different land use systems and depth of sequencing (i.e., the number of DNA sequences per sample using Sanger DNA sequencing) (14, 38–40). Previously, Mirza et al. (14) reported alterations of diazotrophic community structure in the Amazon following deforestation and land use change, but they did not include large-scale spatial analyses. Therefore, in the present study, we used a spatially explicit, nested-sampling approach to understand the spatial variation in diazotrophic community structure and the factors that influence it over a wide geographical area (up to 10 km2). Second, we investigated the extent of their recovery in secondary forests that were established after the abandonment of pasture. High-throughput sequencing was used to generate 443,600 nifH gene sequences across 89 soil samples for the detailed comparison of diazotrophic community structure across three different land use types.
The specific objectives of the current study were (i) to evaluate a functional distance decay relationship of the diazotrophic bacterial community in a tropical forest ecosystem and (ii) to quantify the roles of various factors that drive the changes in the diazotrophic community structure and play a major role in the recovery of diazotrophs in secondary-forest ecosystems.
RESULTS AND DISCUSSION
Diazotrophic α-diversity and spatial turnover or β-diversity.
There was a significant increase in the diazotrophic α-diversity (for both taxonomic and phylogenetic metrics), based on the nifH gene sequences, in response to the forest-to-pasture conversion (Fig. 1; also, see Fig. S1 in the supplemental material) (P < 0.01). The increase in diazotrophic α-diversity at the pasture sites established after the deforestation of the primary forests can be due to the enhanced availability of slowly released labile carbon (41) or an enhanced supply of easily degradable C sources in the form of root exudates of fast-growing grass species (Urochloa brizantha and Panicum maximum) in the pastures (42). On average, we observed higher soil C content in the pasture sites (mean ± 95% confidence interval [CI], 2.93 ± 0.65 g kg of soil−1) than in the forest sites (1.52 ± 0.42 g kg of soil−1), which suggests that higher soil C content might have selectively favored a diverse diazotrophic community. Consistent with our observation, Cenciani et al. (43) also reported an increase in soil C content in pasture soil compared to primary-forest sites in the Amazon region.
FIG 1.
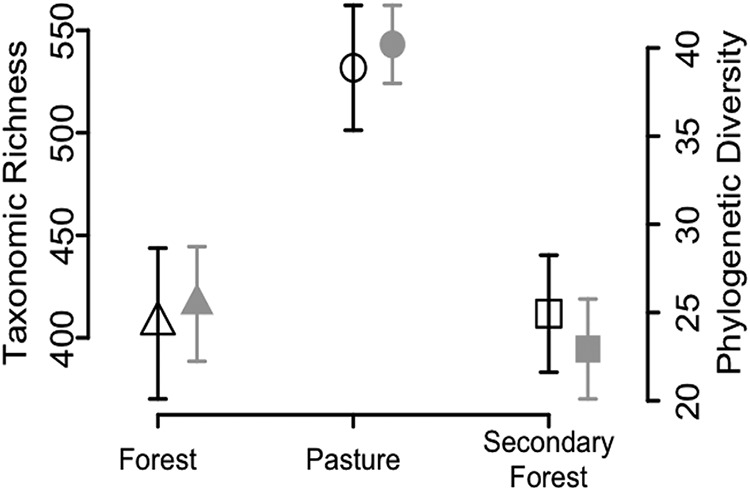
Differences in the taxonomic (black) and phylogenetic (gray) α-diversity of the nifH genes at 97% DNA identity in response to land use change in the Amazon. Taxonomic diversity was calculated as the number of unique genes, and phylogenetic diversity was calculated as Faith’s phylogenetic diversity (66). Error bars represent 95% CI.
Previous studies (44–46) have suggested that the presence of high plant diversity supports the growth of diverse microbial populations in soil mainly because of the differences in the nature organic material produced by different plants. In contrast, several microbial studies have also observed that despite the presence of high plant diversity, bacterial α-diversity was low in soil (47–50) and it increased after deforestation (33, 34, 48). Here, we observed a similar trend for the diazotrophic community, which we assessed using a genetic marker for nitrogen fixation. Furthermore, we also observed a decrease in the diazotrophic α-diversity at the secondary-forest sites, which had higher plant diversity than the pasture sites (Fig. 1; also, see Fig. S1) (P < 0.05) (51).
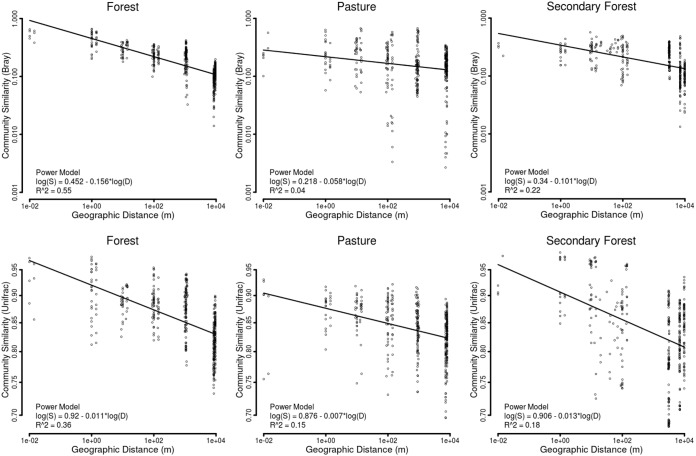
We also examined patterns of β-diversity by comparing the degree of community turnover between samples using both taxonomic and phylogenetic data. We found that the diazotrophic rate of turnover based on taxonomic diversity was the lowest in the pastures (slope = 0.22 ± 0.01 [95% bootstrap CI]; R2 = 0.04) (Fig. 2), intermediate in the secondary-forest sites (slope = 0.34 ± 0.02; R2 = 0.22), and highest in the primary-forest sites (slope = 0.45 ± 0.03; R2 = 0.55). This indicates that the pasture diazotrophic community was more homogenous, whereas the communities in the forested sites displayed greater patchiness. This trend was consistent at the different DNA and protein similarity levels (see Fig. S2A to C) as well as for the phylogenetic analysis (Fig. 2). A conceivable justification of the observed spatial patterns of diazotrophic species can be the presence of unique ecological niches or patchiness in the primary forests. Similar outcomes for general bacterial community turnover rates have been noticed in response to the deforestation of primary forest for pasture (34, 52). The present study not only extends this observation to a specific functional group but also shows that the spatial turnover rates partially recovered in secondary forest (Fig. 2).
FIG 2.
Diazotrophic community β-diversity turnover at 97% DNA identity, representing a distance decay relationship in response to land use change in the Amazon. Differences in the slopes across different land use systems were compared by a power model. The top row shows the variations in the taxonomic β-diversity and the bottom row shows the phylogenetic β-diversity across different land use systems.
The driver of changes in the diazotrophic community structure.
Overall, there was a significant shift in the diazotrophic microbial community composition at 97% DNA similarity across three different land use systems for both complete data sets (Fig. 3) (PERMANOVA, F(2,87) = 11.81; P = 0.001) and subsampled data sets (see Fig. S3 and S4) (PERMANOVA, F(2,87) = 6.92; P = 0.001). Similarly, an increase in abundance of the diazotrophic community, quantified through quantitative PCR (qPCR), was also significantly altered as a result of forest-to-pasture conversion (see Fig. S5) (P < 0.001). However, this abundance was not different between primary and secondary forests (see Fig. S5) (P > 0.05).
FIG 3.
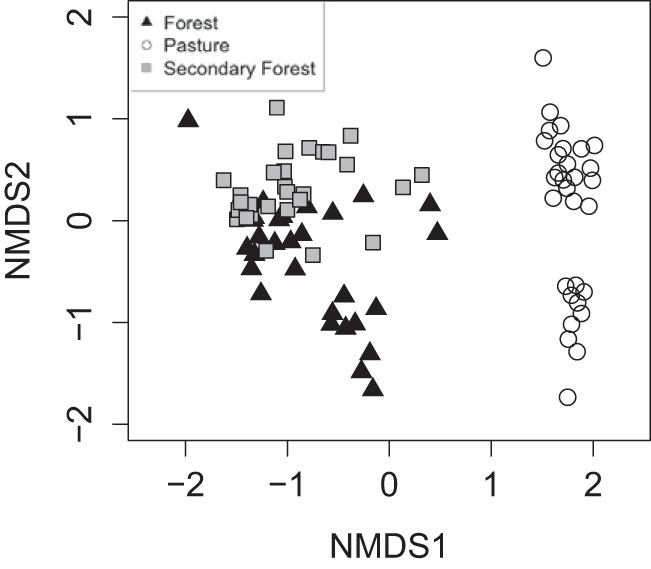
Ordination based on a dissimilarity matrix of the nifH gene sequences comparing three different land use systems in the Amazon. The nonmetric multidimensional scaling representation was based on 97% DNA identity. The complete data set contains a total of 443,600 nifH gene sequences, with the following abundance distribution of sequences retrieved from different treatments: primary-forest samples, 158,261; pasture samples, 143,700; secondary-forest samples, 131,640.
To further explore the question of what factors would have caused this variation in the diazotrophic community structure, we performed variance-partitioning analysis, in which three matrices of explanatory variables (the factors land use, physicochemical characteristics, and geographic distance) were evaluated. Using this approach, we were able to assess the influence of each factor while controlling the confounding influence of the other two factors. Results of variation partitioning analysis showed that about 46% of the total variation in the diazotrophic community structure was explained by these three matrices (Fig. 4). Land use or plant species was the primary driver of the observed changes in the diazotrophic community and explained about 29% of the variation through its individual influence (16.3%) and shared influences with the geographic distance (9.5%) and soil characteristics (2.3%). Geographic distance and soil properties had similar individual fractions of explained variation (approximately 10%), and their effects were much smaller than the effect of land use.
FIG 4.
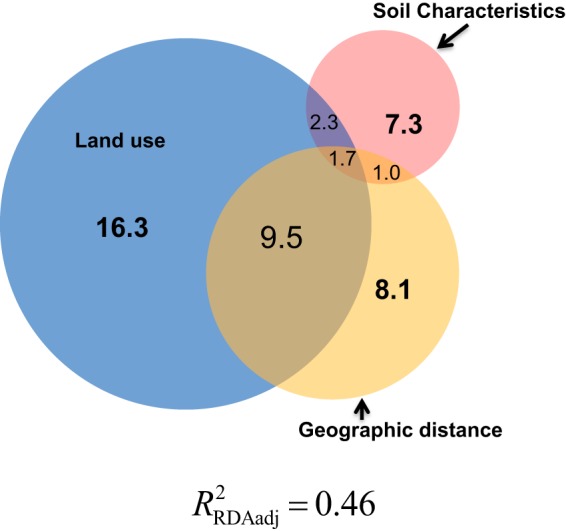
Variance partitioning analysis to determine how land use, geochemical characteristics, and geographic distance explain the variation in the diazotrophic community. Each circle represents the portion of variation accounted for by each of the three factors.
This change in the diazotrophic community in response to land use change could be due to the direct influence of plant species across different systems, i.e., plant root exudates and/or their detritus controlling diazotrophic community rather than their indirect effect by altering geochemical characteristics. Soil characteristics explained only about 7% of the variation in the diazotrophic community (Fig. 4). In contrast, Jesus et al. (33) found in the western part of the Amazon that soil attributes explained about 31% of the variation observed in general microbial communities from forest-to-pasture conversion, and the influence of land use was not significant. In our study, geographic distance explained only about 8% of variations in the diazotrophic community (Fig. 4). The geochemical characteristics, including organic matter content, pH, potential acidity (H+Al), potential cation exchange capacity (T), N, P, K, S, Ca, Mg, Al, Zn, Fe, Mn, Cu, and B (see Table S2), were measured in both studies. Most of the soil characteristics measured in this study were same as those measured by Jesus et al. (33), with a few exceptions, such as soil texture, base saturation, Al saturation, and equilibrium phosphorus. Other differences between these two studies were as follows: (i) the sampling depth in our study was 0 to 10 cm, whereas in the study by Jesus et al. (33), it was 0 to 20 cm, and (ii) we analyzed 108 individual soil samples, whereas Jesus et al. (33) analyzed 26 soil samples, and each was a composite of 12 individual soil samples. It is not possible to point out a factor or factors that contributed to the differences between the study results. One possible explanation for our results could be that several other factors as mentioned above (33), along with the available iron (53), light, and other variables (54) that were not measured in this study, might have contributed to the observed variations in the diazotrophic community. In contrast, several previous studies suggested that soil characteristics are the main driver of the changes in general microbial community compositions (33, 48, 55, 56).
The changes in the plant composition predominantly caused significant changes in the diazotrophic community structure. The most diverse forest sites may contain up to 300 different plant species per hectare (57), secondary forests exhibited an intermediate level of plant diversity, up to 15 to 18% of woody plant species (51), and pasture sites were least diverse, with only two fast-growing grass species. Previously, higher plant diversity has often been associated with higher N mineralization rates, which lead to a higher net N supply (58, 59), and significantly higher N mineralization rates have been observed upon forest-to-pasture conversion in this region (25). This was also consistent with the lower diazotrophic community abundance quantified in the primary forest in comparison to pasture (see Fig. S5). About 80% of the N requirements of the primary forests is fulfilled by the mineralization of diverse plant materials (60), and a small amount of N in forest soil is also contributed by atmospheric N depositions (61). Hence, tropical forests seem less dependent on the diazotrophic community to fulfill their N requirements. In contrast, pastures containing two fast-growing grass species (Urochloa brizantha and Panicum maximum) obtain about 40% of their N requirements through biological N2 fixation (42). N deficiency caused by continued depletion due to grazing of aboveground biomass and the presence of high-C resources supplied by the grass species in the form of root exudates may provide selectively favorable conditions for the diazotrophic community and enhance BNF. Future in situ estimates of BNF in the Amazon rain forest could help to improve our understanding of N flux in this terrestrial ecosystem and also indicate that high nifH gene abundance truly represents high N fixation rates, as were reported previously (21).
Recovery of diazotrophs in the secondary forest.
Diazotrophic communities in approximately 12- to 17-year-old secondary forests showed signs of partial recovery in terms of α-diversity, turnover rates, and community structure (Fig. 1 to 3). First, as we described above, there was a decrease in the diazotrophic α-diversity at the secondary-forest sites (Fig. 1; also, see Fig. S1) (P < 0.05) (51). Second, we observed an intermediate level of turnover in the secondary forest (i.e., a lower level than in primary forests and higher than in pasture), which indicates the onset of potential restoration of confined ecological niches in the secondary forests that might have been lost in the pasture lands due to biotic homogenization (34). Third, the distributions of diazotrophic community composition of primary and secondary forests were significantly different (P < 0.05) at 97% DNA similarity. Nevertheless, the secondary-forest diazotrophic community composition was more closely related to that of the primary forest than that of pastures (Fig. 3). At other DNA (95% and 90%) and amino acid (99%, 97%, and 95%) base similarity levels, the differences in diazotrophic community composition between the primary and secondary forests were nonsignificant (see Fig. S4) (P > 0.05), while both sites were significantly different from pastures. Similarly, nifH gene abundance was also similar between primary forests and secondary forests (see Fig. S5) (P > 0.05). Lastly, phylogenetic analysis (Fig. S6) also indicated a high similarity in the diazotrophic groups at the primary- and secondary-forest sites, which were different from those detected in the pasture sites. Likewise, the overall diversity estimated by the accumulation of taxa with the number of soil samples also suggested a similarity between primary- and secondary-forest ecosystems compared to pasture sites (see Fig. S7).
It was evident from these results that the diazotrophic community was influenced by changes in land use. However, we observed potential recovery of the diazotrophic community in 12- to 17-year-old secondary forests, indicating their high resilience. This suggests that changes in land use from forest to pasture did not cause permanent loss of most of the diazotrophic species (extinction); rather, it caused a shift in their relative abundance in the pasture lands. These results highlight the need for more comprehensive spatial-scale studies to understand the true impact of land use on functional microbial communities and have improved our understanding of the effect of the transition from forest to pasture lands and from pasture to secondary forest on the diazotrophic community. Overall, this outcome is very encouraging; it indicates that, in parallel to the recovery of a few plant species, unique ecological niches for diazotrophic community can be restored and may eventually lead to the recovery of functions. To verify the complete recovery of function in the secondary forests, studies focused on the in situ estimates of BNF in the Amazon rain forest, pasture lands, and secondary forest would improve our understanding of this process and of the extent to which the recovery of diazotrophs translates into a function. This is a detailed study in this region that indicates the recovery of diazotrophic species in 12- to 17-year-old secondary forests in tropical regions.
Overall, we observed a decrease in β-diversity in response to the forest-to-pasture conversion. Land use was the single most important driver of changes in community structure (composition and abundance) relative to differences in geography and soil. We also observed the recovery in the diazotrophic β-diversity and community structure in the 12- to 17-year-old secondary forests, which has important ecological implications about the possible restoration of ecosystem function in secondary forests. Future in situ estimates of BNF in the Amazon rain forest could help to improve our understanding of N flux in this terrestrial ecosystem and of the extent to which the recovery of diazotrophs translates into a function.
MATERIALS AND METHODS
Sampling design and total soil DNA extraction.
Soil samples were collected from the state of Rondônia (10°10ʹ5ʺS, 62°49ʹ27ʺW), Brazil, in 2010. This site was selected because it was one of the three states (Rondônia, Mato Grosso, and Pará) which accounted for more than 85% of all Amazon deforestation from 1996 to 2005 (62). Primary forest had been cleared by removing large trees for timber and burning the remaining vegetation to create pasture lands that were vegetated with two fast-growing grasses (Urochloa brizantha and Panicum maximum). Once pastures became unproductive, they were abandoned, allowing secondary vegetation to grow. These secondary forests have much lower plant diversity than the primary forests (51, 63).
A spatially explicit sampling design was established for three plots within each of the primary-forest, adjacent pasture (38 years old), and secondary-forest (12 to 17 years old) areas (see Fig. S8). Totals of nine sites, three per land use system, and 12 samples per site were used. The nested-quadrat sampling design established 0.01-, 0.1-, 1.0-, and 10-m2 quadrats (shown as a series of squares in Fig. S8) nested within a 100-m2 quadrat, and 12 samples were collected within each 100-m2 plot. Overall, three 100-m2 quadrats were established 0, 1, and 10 km apart within both primary-forest and pasture sites (36 samples per land use system). No secondary-forest site was available at exactly a 1-km distance, so the second 100-m2 sampling plot for secondary forest was at about 3 km (0, 3, and 10 km). Soil cores (5-cm diameter) collected at a 10-cm depth were used for DNA extraction and physicochemical analysis as described previously (34).
Amplification and sequencing of a functional gene.
The nifH gene was amplified using the PCR primers PolF and PolR, developed by Poly et al. (64), and products were sequenced using the 454 GS FLX Sequencer (Michigan State University Genomics Facility, East Lansing, MI). We used the primers PolF and PolR because these are the most widely used primers to assess the diversity of diazotrophs in terrestrial ecosystems. Previously (14), we tested three other nifH gene-specific PCR primer sets (Ueda19F-Ueda470R, PicenoF-PicenolR, and Z-primer) along with PolF and PolR on 15 soil samples from the Amazon. Only PolF and PolR resulted in successful amplification of the nifH gene from all soil samples along with a single band of the appropriate size. Therefore, in this study, we used PolF and PolR. It is important to mention that like any other PCR primers, PolF and PolR may have some biases toward certain specific diazotrophic groups, such as Alpha-, Beta-, and Gammaproteobacteria, Firmicutes, and Actinobacteria (64).
Raw DNA sequences (456,211) were initially processed for quality control using the functional gene pipeline of the Ribosomal Database Project II (RDP) (65) (http://rdp.cme.msu.edu). Chimeric sequences were identified and removed using the USEARCH 6 chimera check. Frameshift errors were adjusted by running FrameBot, and protein sequences were aligned through the hidden Markov model HMMER3 aligner. After initial filtering, high-quality sequences (443,600), both DNA and translated proteins, were aligned and clustered into operation taxonomic units (OTUs) at 99, 97, 95, and 90% for DNA and operational protein units at 99, 97, and 95% for translated proteins by complete linkage clustering using RDP’s mcClust tool. Identification of the nifH gene sequences was carried out by running NCBI local BLAST and comparing against a nifH gene reference database of about 1,100 sequences obtained from Wang et al. (16). A total of 19 soil samples resulted in <1,500 nifH gene sequences that were not included in this study. The details of these 19 samples are provided in the supplemental material (Table S2).
Diversity metrics.
Because analyses of α- and β-diversities can be influenced by differences in the number of DNA sequences retrieved per sample, we subsampled the data set with a total of 1,500 nifH gene sequences per sample (45,000 per land use type) to evaluate differences in the diazotrophic diversity in response to land use change. Subsamples were generated by randomly selecting 1,500 DNA sequences per sample, based on the sample with the fewest DNA sequences, using a Python script developed in-house. Differences in the α-diversity across the three different land use systems were compared by calculating both taxonomic and phylogenetic diversity. Taxonomic diversity was calculated as total species richness, Shannon diversity, and Margalef species richness using OTU abundance data (97%). Phylogenetic diversity was calculated as Faith’s phylogenetic diversity (66) by constructing a phylogenetic tree of representative nifH gene sequences using FastTree 2.15 (67). The significance of the differences was assessed through one-way analysis of variance (ANOVA).
Species turnover and variance partitioning.
We examined spatial variation in diazotrophic community turnover using three distinct approaches: (i) distance decay analysis (68) to compare the patterns and rates of decay in diazotrophic community similarity across the three land uses, (ii) nonmetric multidimensional scaling (NMDS) (69) to explore variability in taxonomic composition across all three land uses simultaneously, and (iii) variation partitioning (70) to estimate the individual and shared components of variation in species turnover due to land use, geography, and soil physicochemistry. For NMDS and variation partitioning analyses, we used both the complete data set (containing 443,600 nifH gene sequences) and a subsampled data set (135,000 nifH gene sequences) to assess the changes in the diazotrophic community composition. We used the abundance-based Bray-Curtis metric as a measure of taxonomic similarity, which was calculated using the function vegdist in the R package vegan (71). For phylogenetic similarity, the Fast Uni-Frac distance metric was used (72).
We computed the distance decay relationship (DDR) separately for each type of land use by estimating the rate of change in taxonomic and phylogenetic community similarity as a function of geographic distance. To estimate the rate of distance decay, we fitted the two most common models of the DDR: the exponential and power models (73). To test the differences between distance decay slopes, we compared the 95% bootstrapped confidence intervals for the slope parameters using 10,000 bootstrap iterations, which avoids distributional assumptions about the test statistic (i.e., the slope). If the confidence intervals did not overlap, the slopes were considered significantly different. We computed the DDR for 97, 95, and 90% cutoffs for defining OTUs (see the supplemental material). We tested whether the sample centroids were located in significantly different locations of multidimensional space via PERMANOVA (74) using the function adonis in R (71). We carried out the variation partitioning analysis using the varpart function in R (71).
Data availability.
The nifH sequences were deposited in the NCBI Sequence Read Archive (SRA) under BioProject no. PRJNA329012.
Supplementary Material
ACKNOWLEDGMENTS
B.J.M.B., K.N., J.M.T., and J.L.M.R. designed research; B.S.M. performed research; J.L.M.R. and J.M.T contributed reagents; B.S.M. and D.J.M. analyzed data; and B.S.M. wrote the manuscript. All authors critically revised and approved the final manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Baker TR, Phillips OL, Malhi Y, Almeida S, Arroyo L, Di Fiore A, Erwin T, Higuchi N, Killeen TJ, Laurance SG, Laurance WF, Lewis SL, Monteagudo A, Neill DA, Vargas PN, Pitman NC, Silva JN, Martínez RV. 2004. Increasing biomass in Amazonian forest plots. Philos Trans R Soc Lond B Biol Sci 359:353–365. doi: 10.1098/rstb.2003.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson EA, de Araújo AC, Artaxo P, Balch JK, Brown IF, Bustamante CMM, Coe MT, DeFries RS, Keller M, Longo M, Munger JW, Schroeder W, Soares-Filho BS, Souza CM Jr, Wofsy SC. 2012. The Amazon basin in transition. Nature 481:321–328. doi: 10.1038/nature10717. [DOI] [PubMed] [Google Scholar]
- 3.Phillips OL, Aragão LEOC, Lewis SL, Fisher JB, Lloyd J, López-González G, Malhi Y, Monteagudo A, Peacock J, Quesada CA, van der Heijden G, Almeida S, Amaral I, Arroyo L, Aymard G, Baker TR, Bánki O, Blanc L, Bonal D, Brando P, Chave J, de Oliveira ACA, Cardozo ND, Czimczik CI, Feldpausch TR, Freitas MA, Gloor E, Higuchi N, Jiménez E, Lloyd G, Meir P, Mendoza C, Morel A, Neill DA, Nepstad D, Patiño S, Peñuela MC, Prieto A, Ramírez F, Schwarz M, Silva J, Silveira M, Thomas AS, Steege HT, Stropp J, Vásquez R, Zelazowski P, Alvarez Dávila E, Andelman S, Andrade A, Chao K-J, Erwin T, Di Fiore A, Honorio C E, Keeling H, Killeen TJ, Laurance WF, Peña Cruz A, Pitman NCA, Núñez Vargas P, Ramírez-Angulo H, Rudas A, Salamão R, Silva N, Terborgh J, Torres-Lezama A. 2009. Drought sensitivity of the Amazon rainforest. Science 323:1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D. 2011. A large and persistent carbon sink in the world’s forests. Science 333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 5.Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 6.Reed SC, Cleveland CC, Townsend AR. 2011. Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512. doi: 10.1146/annurev-ecolsys-102710-145034. [DOI] [Google Scholar]
- 7.Eady RR. 1992. The dinitrogen-fixing bacteria, p 534–553. In Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (ed), The prokaryotes—a handbook on the biology of bacteria: ecophysiology, isolation, identification, application, 2nd ed Springer-Verlag, New York, NY. [Google Scholar]
- 8.Young JPW. 1992. Phylogenetic classification of nitrogen-fixing organisms, p 43–86. In Stacy G, Burris RH, Evans HJ (ed), Biological nitrogen fixation. Chapman and Hall, New York, NY. [Google Scholar]
- 9.Bednarz VN, van de Water J, Rabouille S, Maguer JF, Grover R, Ferrier‐Pagès C. 2019. Diazotrophic community and associated dinitrogen fixation within the temperate coral Oculina patagonica. Environ Microbiol 21:480–495. doi: 10.1111/1462-2920.14480. [DOI] [PubMed] [Google Scholar]
- 10.Berthrong S, Yeager CM, Gallegos-Graves L, Steven B, Eichorst SA, Jackson RB, Kuske CR. 2014. Nitrogen fertilization has a stronger effect on soil N-fixing bacterial communities than elevated atmospheric CO2. Appl Environ Microbiol 80:3103–3112. doi: 10.1128/AEM.04034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collavino MM, Tripp HJ, Frank IE, Vidoz ML, Calderoli PA, Donato M, Zehr JP, Aguilar OM. 2014. nifH pyrosequencing reveals the potential for location-specific soil chemistry to influence N2-fixing community dynamics. Environ Microbiol 16:3211–3223. doi: 10.1111/1462-2920.12423. [DOI] [PubMed] [Google Scholar]
- 12.Gaby JC, Rishishwar L, Valderrama-Aguirre LC, Green SJ, Valderrama-Aguirre A, Jordan IK, Kostka JE. 2017. Diazotroph community characterization via a high-throughput nifH amplicon sequencing and analysis pipeline. Appl Environ Microbiol 84:e01512-17. doi: 10.1128/AEM.01512-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng H, Zhou Z, Wu R, Wang Y, Gu JD. 2019. Diazotrophic microbial community and abundance in acidic subtropical natural and re-vegetated forest soils revealed by high-throughput sequencing of nifH gene. Appl Microbiol Biotechnol 103:995–1005. doi: 10.1007/s00253-018-9466-7. [DOI] [PubMed] [Google Scholar]
- 14.Mirza BS, Potisap C, Nüsslein K, Bohannan BJM, Rodrigues JLM. 2014. Response of free-living nitrogen-fixing microorganisms to land use change in the Amazon rainforest. Appl Environ Microbiol 80:281–288. doi: 10.1128/AEM.02362-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu Q, Deng Y, Yan Q, Shen L, Lin L, He Z, Wu L, Van Nostrand JD, Buzzard V, Michaletz ST, Enquist BJ, Weiser MD, Kaspari M, Waide RB, Brown JH, Zhou J. 2016. Biogeographic patterns of soil diazotrophic communities across six forests in the North America. Mol Ecol 25:2937–2948. doi: 10.1111/mec.13651. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Quensen JF, Fish JA, Lee TK, Sun Y, Tiedje JM, Cole JR. 2013. Ecological patterns of nifH genes in four terrestrial climatic zones explored with targeted metagenomics using FrameBot, a new informatics tool. mBio 4:e00592-13. doi: 10.1128/mBio.00592-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zehr JP, Jenkins BD, Short SM, Steward GF. 2003. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5:539–554. doi: 10.1046/j.1462-2920.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 18.Großkopf T, Mohr W, Baustian T, Schunck H, Gill D, Kuypers MMM, Lavik G, Schmitz RA, Wallace DWR, LaRoche J. 2012. Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488:361–364. doi: 10.1038/nature11338. [DOI] [PubMed] [Google Scholar]
- 19.Hsu SF, Buckley DH. 2009. Evidence for the functional significance of diazotroph community structure in soil. ISME J 3:124–136. doi: 10.1038/ismej.2008.82. [DOI] [PubMed] [Google Scholar]
- 20.Moseman SM, Zhang R, Qian PY, Levin LA. 2009. Diversity and functional responses of nitrogen fixing microbes to three wetland invasions. Biol Invasions 11:225–239. doi: 10.1007/s10530-008-9227-0. [DOI] [Google Scholar]
- 21.Reed SC, Townsend AR, Cleveland CC, Nemergut DR. 2010. Microbial community shifts influence patterns in tropical forest nitrogen fixation. Oecologia 164:521–531. doi: 10.1007/s00442-010-1649-6. [DOI] [PubMed] [Google Scholar]
- 22.Yeager CM, Kornosky JL, Housman DC, Grote EE, Belnap J, Kuske C. 2004. Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado Plateau and Chihuahuan Desert. Appl Environ Microbiol 70:973–983. doi: 10.1128/aem.70.2.973-983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr CH, James A, Leifert C, Cooper JM, Cummings SP. 2011. Diversity and activity of free-living nitrogen-fixing bacteria and total bacteria in organic and conventionally managed soils. Appl Environ Microbiol 77:911–919. doi: 10.1128/AEM.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakelin SA, Gregg AL, Simpson RJ, Li GD, Riley IT, McKay AC. 2009. Pasture management clearly affects soil microbial community structure and N-cycling bacteria. Pedobiologia 52:237–251. doi: 10.1016/j.pedobi.2008.10.001. [DOI] [Google Scholar]
- 25.Neill C, Piccolo MC, Cerri CC, Steudler PA, Melillo JM, Brito M. 1997. Net nitrogen mineralization and net nitrification rates in soils following deforestation for pasture across the southwestern Brazilian Amazon Basin landscape. Oecologia 110:243–252. doi: 10.1007/s004420050157. [DOI] [PubMed] [Google Scholar]
- 26.Neill C, Piccolo MC, Melillo JM, Steudler PA, Cerri CC. 1999. Nitrogen dynamics in Amazon forest and pasture soils measured by 15N pool dilution. Soil Biol Biochem 31:567–572. doi: 10.1016/S0038-0717(98)00159-X. [DOI] [Google Scholar]
- 27.Neill C, Piccolo MC, Steudler PA, Melillo JM, Feigl BJ, Cerri CC. 1995. Nitrogen dynamics in soils of forests and active pastures in the western Brazilian Amazon basin. Soil Biol Biochem 27:1167–1175. doi: 10.1016/0038-0717(95)00036-E. [DOI] [Google Scholar]
- 28.Macedo MN, DeFries RS, Morton DC, Stickler CM, Galford GL, Shimabukuro YE. 2012. Decoupling of deforestation and soy production in the southern Amazon during the late 2000s. Proc Natl Acad Sci U S A 109:1341–1346. doi: 10.1073/pnas.1111374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramankutty N, Gibbs HK, Achard F, DeFries R, Foley JA, Houghton RA. 2007. Challenges to estimating carbon emissions from tropical deforestation. Global Change Biol 13:51–66. doi: 10.1111/j.1365-2486.2006.01272.x. [DOI] [Google Scholar]
- 30.Butler RA. 2015. Brazil forest infomation and data. Tropical rainforests: deforestation rates tables and charts. Mongabay https://rainforests.mongabay.com/deforestation/2000/Brazil.htm. Accessed 1 July 2019.
- 31.Chazdon RL. 2008. Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science 320:1458–1460. doi: 10.1126/science.1155365. [DOI] [PubMed] [Google Scholar]
- 32.Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, Peres CA, Bradshaw CJA, Laurance WF, Lovejoy TE, Sodhi NS. 2011. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378–381. doi: 10.1038/nature10425. [DOI] [PubMed] [Google Scholar]
- 33.Jesus ED, Marsh TL, Tiedje JM, Moreira F. 2009. Changes in land use alter the structure of bacterial communities in western Amazon soils. ISME J 3:1004–1011. doi: 10.1038/ismej.2009.47. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues JLM, Pellizari VH, Mueller R, Baek K, Jesus EDC, Paula FS, Mirza B, Hamaoui GS, Tsai SM, Feigl B, Tiedje JM, Bohannan BJM, Nüsslein K. 2013. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of bacterial microbial communities. Proc Natl Acad Sci U S A 110:988–993. doi: 10.1073/pnas.1220608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamaoui GS Jr, Rodrigues JLM, Bohannan BJM, Tiedje JM, Nüsslein K. 2016. Land-use change drives abundance and community structure alterations of thaumarchaeal ammonia oxidizers in tropical rainforest soils in Rondônia, Brazil. Appl Soil Ecol 107:48–56. doi: 10.1016/j.apsoil.2016.05.012. [DOI] [Google Scholar]
- 36.Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM. 2014. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8:1577–1587. doi: 10.1038/ismej.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paula FS, Rodrigues JLM, Zhou J, Wu L, Mueller RC, Mirza BS, Bohannan BJM, Nüsslein K, Deng Y, Tiedje JM, Pellizari VH. 2014. Land use change alters functional gene diversity, composition, and abundance in Amazon forest soil microbial communities. Mol Ecol 23:2988–2999. doi: 10.1111/mec.12786. [DOI] [PubMed] [Google Scholar]
- 38.Dörr N, Glaser B, Kolb S. 2010. Methanotrophic communities in Brazilian ferralsols from naturally forested, afforested, and agricultural sites. Appl Environ Microbiol 76:1307–1310. doi: 10.1128/AEM.02282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taketani RG, Tsai SM. 2010. The influence of different land uses on the structure of archaeal communities in Amazonian anthrosols based on 16S rRNA and amoA genes. Microb Ecol 59:734–743. doi: 10.1007/s00248-010-9638-1. [DOI] [PubMed] [Google Scholar]
- 40.Navarrete AA, Taketani RG, Mendes LW, Cannavan FS, Moreira FMS, Tsai SM. 2011. Land-use systems affect archaeal community structure and functional diversity in western Amazon soils. Rev Bras Ciênc Solo 35:1527–1540. doi: 10.1590/S0100-06832011000500007. [DOI] [Google Scholar]
- 41.Knoepp JD, Swank WT. 1997. Forest management effects on surface soil carbon and nitrogen. Soil Sci Soc Am J 61:928–935. doi: 10.2136/sssaj1997.03615995006100030031x. [DOI] [Google Scholar]
- 42.Reis VM, dos Reis FB, Quesada DM, de Oliveira OCA, Alves BJR, Urquiaga S. 2001. Biological nitrogen fixation associated with tropical pasture grasses. Functional Plant Biol 28:837–844. doi: 10.1071/PP01079. [DOI] [Google Scholar]
- 43.Cenciani K, Lambais MR, Cerri CC, Basilio de Azevedo LC, Feigl BJ. 2009. Bacteria diversity and microbial biomass in forest, pasture and fallow soils in the southwestern Amazon Basin. Rev Bras Ciênc Solo 33:907–916. doi: 10.1590/S0100-06832009000400015. [DOI] [Google Scholar]
- 44.Broughton LC, Gross KL. 2000. Patterns of diversity in plant and soil microbial communities along a productivity gradient in a Michigan old-field. Oecologia 125:420–427. doi: 10.1007/s004420000456. [DOI] [PubMed] [Google Scholar]
- 45.Kowalchuk GA, Buma DS, De Boer W, Klinkhamer PGL, van Veen JA. 2002. Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Anton Leeuw Int J 81:509–520. doi: 10.1023/A:1020565523615. [DOI] [PubMed] [Google Scholar]
- 46.Peay KG, Baraloto C, Fine PV. 2013. Strong coupling of plant and fungal community structure across western Amazonian rain forests. ISME J 7:1852–1861. doi: 10.1038/ismej.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastida F, García C, Bergen MV, Moreno JL, Richnow HH, Jehmlich N. 2015. Deforestation fosters bacterial diversity and the cyanobacterial community responsible for carbon fixation processes under semiarid climate: a metaproteomics study. Appl Soil Ecol 93:65–67. doi: 10.1016/j.apsoil.2015.04.006. [DOI] [Google Scholar]
- 48.Crowther TW, Maynard DS, Leff JW, Oldfield EE, McCulley RL, Fierer N, Bradford MA. 2014. Predicting the responsiveness of soil biodiversity to deforestation: a cross-biome study. Glob Chang Biol 20:2983–2994. doi: 10.1111/gcb.12565. [DOI] [PubMed] [Google Scholar]
- 49.Farias F, Orlando J, Bravo R, Guevera R, Carú M. 2009. Comparison of soil bacterial communities associated with actinorhizal, non-actinorhizal plants and the interspaces in the sclerophyllous matorral from Central Chile in two different seasons. J Arid Environ 73:1117–1124. doi: 10.1016/j.jaridenv.2009.06.010. [DOI] [Google Scholar]
- 50.Prober SM, Leff JW, Bates ST, Borer ET, Firn J, Harpole WS, Lind EM, Seabloom EW, Adler PB, Bakker JD, Cleland EE, DeCrappeo NM, DeLorenze E, Hagenah N, Hautier Y, Hofmockel KS, Kirkman KP, Knops JMH, La Pierre KJ, MacDougall AS, McCulley RL, Mitchell CE, Risch AC, Schuetz M, Stevens CJ, Williams RJ, Fierer N. 2015. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol Lett 18:85–95. doi: 10.1111/ele.12381. [DOI] [PubMed] [Google Scholar]
- 51.Feigl B, Cerri C, Piccolo M, Noronha N, Augusti K, Melillo J, Eschenbrenner V, Melo L. 2006. Biological survey of a low-productivity pasture in Rondonia State, Brazil. Outlook Agric 35:199–208. doi: 10.5367/000000006778536738. [DOI] [Google Scholar]
- 52.Navarrete AA, Venturini AM, Meyer KM, Klein AM, Tiedje JM, Bohannan BJ, Nüsslein K, Tsai SM, Rodrigues JL. 2015. Differential response of Acidobacteria subgroups to forest-to-pasture conversion and their biogeographic patterns in the western Brazilian Amazon. Front Microbiol 6:1443. doi: 10.3389/fmicb.2015.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bomfim B, Silva LCR, Doane TA, Horwath WR. 2019. Interactive effects of land-use change and topography on asymbiotic nitrogen fixation in the Brazilian Atlantic Forest. Biogeochem 142:137–153. doi: 10.1007/s10533-018-0525-z. [DOI] [Google Scholar]
- 54.Shay PE, Winder RS, Trofymow JA. 2015. Nutrient-cycling microbes in coastal Douglas-fir forests: regional-scale correlation between communities, in situ climate, and other factors. Front Microbiol 6:1097. doi: 10.3389/fmicb.2015.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fierer N, Jackson RB. 2006. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffiths R, Thomson B, James P, Bell T, Bailey M, Andrew S. 2011. The bacterial biogeography of British soils. Environ Microbiol 13:1642–1654. doi: 10.1111/j.1462-2920.2011.02480.x. [DOI] [PubMed] [Google Scholar]
- 57.Gentry AH. 1988. Tree species richness of upper Amazonian forests. Proc Natl Acad Sci U S A 85:156–159. doi: 10.1073/pnas.85.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. 2001. Diversity and productivity in a long-term grassland experiment. Science 294:843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- 59.Zak DR, Holmes WE, White DC, Peacock AD, Tilman D. 2003. Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84:2042–2050. doi: 10.1890/02-0433. [DOI] [Google Scholar]
- 60.Norby RJ, Iversen CM. 2006. Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2-enriched sweetgum forest. Ecology 87:5–14. doi: 10.1890/04-1950. [DOI] [PubMed] [Google Scholar]
- 61.Martinelli LA, Piccolo MC, Townsend AR, Vitousek PM, Cuevas E, McDowell W, Robertson GP, Santos OC, Treseder K. 1999. Nitrogen stable isotopic composition of leaves and soils: tropical versus temperate forests. Biogeochemistry 46:45–65. doi: 10.1007/BF01007573. [DOI] [Google Scholar]
- 62.Amigo I. 2020. When will the Amazon hit a tipping point? Nature 578:505–507. doi: 10.1038/d41586-020-00508-4. [DOI] [PubMed] [Google Scholar]
- 63.Pires JM, Prance GT. 1985. The vegetation types of the Brazilian Amazon, p 109–145. In Prance GT, Lovejoy TE (ed), Amazonia: key environments. Pergamon Press, Oxford, United Kingdom. [Google Scholar]
- 64.Poly F, Ranjard L, Nazaret S, Gourbière F, Monrozier LJ. 2001. Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262. doi: 10.1128/AEM.67.5.2255-2262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fish JA, Chai B, Wang Q, Sun Y, Brown CT, Tiedje JM, Cole JR. 2013. FunGene: the functional gene pipeline and repository. Front Microbiol 4:291. doi: 10.3389/fmicb.2013.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faith DP. 1992. Conservation evaluation and phylogenetic diversity. Biol Conserv 61:1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- 67.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nekola JC, White PS. 1999. The distance decay of similarity in biogeography and ecology. J Biogeography 26:867–878. doi: 10.1046/j.1365-2699.1999.00305.x. [DOI] [Google Scholar]
- 69.Minchin PR. 1987. An evaluation of relative robustness of techniques for ecological ordination. Vegetation 69:89–87. doi: 10.1007/BF00038690. [DOI] [Google Scholar]
- 70.Borcard D, Legendre P, Drapeau P. 1992. Partialling out the spatial component of ecological variation. Ecology 73:1045–1055. doi: 10.2307/1940179. [DOI] [Google Scholar]
- 71.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D. 2015. Vegan: Community Ecology Package 15. R package version 2.4-6 http://CRAN.R-project.org/package=vegan.
- 72.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nekola JC, McGill BJ. 2014. Scale dependency in the functional form of the distance decay relationship. Ecography 37:309–320. doi: 10.1111/j.1600-0587.2013.00407.x. [DOI] [Google Scholar]
- 74.Anderson MJ, Walsh DC. 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr 83:557–574. doi: 10.1890/12-2010.1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The nifH sequences were deposited in the NCBI Sequence Read Archive (SRA) under BioProject no. PRJNA329012.