Abstract
Objective
To identify cell types in the male and female reproductive systems at risk for SARS-CoV-2 infection because of the expression of host genes and proteins used by the virus for cell entry.
Design
Descriptive analysis of transcriptomic and proteomic data.
Setting
Academic research department and clinical diagnostic laboratory.
Patient(s)
Not applicable (focus was on previously generated gene and protein expression data).
Intervention(s)
None.
Main Outcome Measure(s)
Identification of cell types coexpressing the key angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) genes and proteins as well as other candidates potentially involved in SARS-CoV-2 cell entry.
Result(s)
On the basis of single-cell RNA sequencing data, coexpression of ACE2 and TMPRSS2 was not detected in testicular cells, including sperm. A subpopulation of oocytes in nonhuman primate ovarian tissue was found to express ACE2 and TMPRSS2, but coexpression was not observed in ovarian somatic cells. RNA expression of TMPRSS2 in 18 samples of human cumulus cells was shown to be low or absent. There was general agreement between publicly available bulk RNA and protein datasets in terms of ACE2 and TMPRSS2 expression patterns in testis, ovary, endometrial, and placental cells.
Conclusion(s)
These analyses suggest that SARS-CoV-2 infection is unlikely to have long-term effects on male and female reproductive function. Although the results cannot be considered definitive, they imply that procedures in which oocytes are collected and fertilized in vitro are associated with very little risk of viral transmission from gametes to embryos and may indeed have the potential to minimize exposure of susceptible reproductive cell types to infection in comparison with natural conception.
Key Words: COVID-19, fertility, testis, ovary, IVF
Abstract
Enfermedad por coronavirus-19 y fertilidad: expresión en los tejidos reproductivos masculino y femenino de la proteína de entrada viral en el huésped
Objetivo
Identificar los tipos celulares con riesgo de infección por SARS-CoV-2 en los sistemas reproductivos masculino y femenino debido a la expresión de proteínas y genes utilizados por el virus para la entrada en la célula.
Diseño
Análisis descriptivo de datos transcriptómicos y proteómicos.
Entorno
Departamento académico de investigación y laboratorio de diagnóstico clínico.
Paciente(s)
Ninguno, el enfoque fue sobre datos de expresión génica y proteica previamente generados.
Intervención(es)
Ninguna.
Principal(es) medida(s) de resultado(s)
Identificación de los tipos celulares que coexpresan los genes y las proteínas claves, la enzima convertidora de angiotensina 2 (ACE2) y proteasa de serina transmembrana 2 (TMPRSS2), así como otros candidatos potencialmente implicados en la entrada del SARS-CoV-2 en la célula.
Resultado(s)
Basándose en los datos de secuenciación de RNA en célula única, no se detectó la coexpresión de ACE2 y TMPRSS2 en células del testículo, incluyendo a los espermatozoides. Se encontró la expresión de ACE2 y TMPRSS2 en una subpoblación de ovocitos de tejido ovárico de primates no humanos, pero la coexpresión no se observó en las células somáticas ováricas. En 18 muestras humanas de células del cúmulo se mostró que la expresión de RNA de TMPRSS2 era baja o ausente. En la mayoría de las bases de datos de RNA y proteínas públicamente disponibles hubo consenso en cuanto a los patrones de expresión de ACE2 y TMPRSS2 en las células del testículo, del ovario del endometrio y de la placenta.
Conclusión(es)
Estos análisis sugieren que es improbable que la infección por SARS-CoV-2 tenga efectos a largo plazo sobre la función reproductiva masculina y femenina. Aunque estos resultados no pueden considerarse definitivos, implican que los procedimientos en los cuales se obtienen ovocitos y se fecundan in vitro, se asocian con un riesgo muy bajo de transmisión viral desde los gametos a los embriones y, de hecho, tienen el potencial de minimizar la exposición de los tipos celulares reproductivos susceptibles a la infección, comparados con la concepción natural.
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/30350
As of April 26, 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is known to have infected 2,804,796 persons globally, and 193,710 have had coronavirus disease 2019 (COVID-19) at the time of death (1). Investigations into the molecular details of SARS-CoV-2 infection have been rapidly initiated, and several key facts are already known. Viral entry requires the binding of SARS-CoV-2 spike (S) glycoprotein to the host receptor angiotensin-converting enzyme 2 (ACE2) (2, 3, 4). Host proteases such as transmembrane serine protease 2 (TMPRSS2) are then needed to cleave the viral S protein to induce a conformational change to S that allows for permanent fusion of the viral and host cell membranes (2, 4). The importance of TMPRSS2 has been confirmed and is evident in studies showing that its inhibition blocks SARS-CoV-2 entry and spread in targeted lung cells (4).
TMPRSS2 is more broadly expressed in human tissues than is ACE2, indicating that ACE2 may be one of the principal determinants of whether a given cell type is susceptible to viral infection (5). Single-cell RNA sequencing (scRNAseq) in human and nonhuman primate respiratory tissues has shown coexpression of ACE2 and TMPRSS2 in pneumocytes in the lungs and goblet secretory cells in the nose (6), indicating that these cell types may serve as foci for infection and potentially explaining the range of respiratory symptoms associated with COVID-19. Coexpression has also been reported in other tissue types, including the ileum, heart, and kidney, for which there are also established COVID-19 symptoms (5, 7, 8, 9, 10, 11). Detection of the virus in blood, feces, and possibly urine suggests that the impact of SARS-CoV-2 on cardiac, enteric, and renal function may be the direct result of infection of cells within these tissues rather than being secondary to acute respiratory distress syndrome (12, 13, 14).
Given that the clinical features of COVID-19 appear to be largely determined by the tissues with coexpression of ACE2 and TMPRSS2 in their constituent cells, it is conceivable that viral infection could have an impact on reproductive function if cells of the male and/or female reproductive systems also express these genes. Expression of ACE2 and TMPRSS2 has been shown in testicular, endometrial, and placental cells, with varying interpretations (7, 11). To our knowledge, expression of SARS-CoV-2 host entry proteins has not been assessed in human or nonhuman primate ovaries, specifically the outer ovarian cortex, which houses the germ cells. Reproductive indices are not typically assessed in the intensive care unit setting, and any effects of COVID-19 on fertility may not be readily apparent until epidemiological data become available. Therefore, we used publicly available scRNAseq data, our own unpublished transcriptomic data, and publicly available bulk RNA and proteomics data to assess the expression patterns of viral host entry proteins in reproductive cell populations. We also considered expression of the receptor basigin (BSG/CD147), inasmuch as limited data suggest that it may have the capacity to mediate viral entry (15), and also the cysteine protease cathepsin L (CatL; gene symbol, CTSL), which potentially cleaves the viral S protein (2, 4). Cathepsin L does not appear to be essential for viral infection, but it is possible that residual infection in cells treated with camostat mesylate, a TMPRSS2 inhibitor, may reflect S protein priming by CatL (4, 16).
Inasmuch as the expression patterns of ACE2 and TMPRSS2 in tissues studied to date are cell-type specific (5), scRNAseq analyses, which reveal the coexpression of these genes within individual cells, are expected to be particularly valuable to uncovering the causes of disease. Cell types in the reproductive system have variable lifespans, and cells that live for shorter periods may pose less of a threat to an individual's long-term reproductive potential if infected. For instance, somatic spermatogonial stem cells in the testes are continuously self-renewing cells that propagate throughout male reproductive life, whereas differentiated spermatocytes are cleared from the reproductive system within approximately 60 days (17). In the ovary, a cohort of ∼2,000,000 oocytes exist at birth, a diminishing subset of which will persist throughout female reproductive life—a cellular lifespan measured in decades. No new oocytes are produced. By contrast, cells of the decidua (outermost lining of the endometrium) differentiate and shed in successive menstrual cycles (regularly every 21–40 days). Cell type–specific expression patterns of genes that produce viral host entry proteins, and identification of potential loci of infection within the reproductive system, are therefore necessary to predict whether SARS-CoV-2 is likely to have any impact on fertility. Although not definitive, findings from studies such as this may also help to predict the likelihood of human embryos becoming infected with SARS-CoV-2. In the midst of the pandemic, fertility treatments in many countries have been curtailed, canceled, or even banned outright. Given the declining chances of treatment success with advancing female age, there is an urgent need to reestablish such treatments as soon as this can be safely accomplished. Understanding whether SARS-CoV-2 has the capacity to infect gametes and embryos produced in vitro is of great importance when considering the risks of natural and assisted reproduction during the COVID-19 pandemic.
Materials and methods
Preprocessing and Data Download
Protocols for amplification, sequencing, and alignment were previously described in the respective studies (18, 19). In brief, Guo et al. (18) generated scRNAseq data in testicular tissue from three healthy male individuals of peak reproductive age (17, 24, and 25 years old). Wang et al. (19) generated scRNAseq data in ovarian tissue from three young (4–5 years old) cynomolgus monkeys, a genus of Macaca. The combined expression matrices were downloaded from GSE112013 (testicular) and GSE130664 (ovarian).
Clustering Analysis and Cell Type Annotation Using Seurat
The combined expression matrices from each study were converted to a sparse matrix using the Matrix package in R (CRAN) and written out using writeMM. The sparse matrix was then loaded into R using the Read10X function in the Seurat V3.0 package (https://satijalab.org/seurat/9), and an object was created using the CreateSeuratObject function. Cells from each experiment were kept only if >500 genes were expressed, and <20% of reads mapped to the mitochondrial genome. The top 5,000 highly variable genes were identified using the FindVariableGenes function. Data were then normalized and scaled using the SCTransform function by regressing out the percentage of mitochondrial gene content. Principal components analysis was then performed on the scaled data using the RunPCA function. To visualize “dimensionality” of the dataset, we plotted the principal components using an elbow plot. This indicated that PC 1 to 13 explained the majority of the variance in both datasets. Downstream tSNE and clustering analyses were therefore performed on PC 1 to 13. The FindNeighbors and FindClusters function were used for the clustering analyses. In brief, FindNeighbors constructs a K-nearest neighbor graph and then determines the Jaccard similarity between any two cells. We manually assigned cell types to clusters with well-known markers from the original studies (18, 19). Expression of an “average” cell in each cluster was calculated on the basis of the normalized, scaled, and centered data. The average expression reflects the mean expression in each cluster compared with all other cells.
Cumulus Cell RNA Extraction and RNA Sequencing
Cumulus cells associated with 18 independent oocytes from nine patients undergoing routine assisted reproductive treatment were collected (ethical approval: WIRB 20031397). Total RNA was extracted from each sample using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions, and samples were eluted in 14 μL of RNAse-free water. cDNA was synthesized and amplified using the SMART-Seq v4 Ultra Low Input RNA Kit for Sequencing (TaKaRa Bio) according to the standard workflow. Sequencing library preparation was performed using the Nextera XT DNA Library Prep Kit (Illumina) according to the manufacturer’s instructions. Briefly, purified double-stranded cDNA samples were fragmented, indexed, cleaned, normalized, and pooled. The final denatured and diluted pooled libraries were sequenced on a NextSeq 550 (Illumina) instrument with 75 cycle paired-end synthesis, using a NextSeq 150 Cycle High Output Kit. An average of >50,000,000 sequencing reads were obtained per sample. Relative gene expression levels were given in transcripts per million (TPM), calculated by dividing the number of mapped reads corresponding to an individual gene by the length of the gene in kilobases (this gives the number of reads per kilobase: RPK). All the RPK values for a sample were summed together, and the resulting number was divided by 1,000,000. The RPK values for individual genes were divided by this number to give TPM.
Protein and Bulk RNA Expression
Protein and bulk RNA expression data were retrieved from the Human Protein Atlas (proteinatlas.org) and the Human Proteome Map (humanproteomemap.org). Of note, these publicly available bulk RNA and protein datasets were generated independently from the previously described scRNAseq datasets. RNA expression was categorized into groups by the following schema, consistent with that used by Hikmet et al. (20). Low expression = 1% to 10% of the maximum value, medium expression = 11% to 50% of the maximum value, and high expression = 51% 100% of the maximum value. Categorizations for protein expression correspond to the categorizations given by the Human Protein Atlas and the Human Proteome Map.
Results
Viral Entry RNA Expression
Published scRNAseq datasets in human testicular (18) and nonhuman primate ovarian (19) tissue were used to assess the cell-type specific expression pattern of SARS-CoV-2 entry receptor ACE2 and entry-associated protease TMPRSS2 and their coexpression. The expression and coexpression of alternative receptor BSG and protease CTSL were also investigated. Additionally, a novel RNAseq dataset was available for human cumulus cells and provided supplementary data on gene expression levels in that cell type.
Human testicular cells
From scRNAseq datasets, we annotated 11 previously identified cell types of the testes, including somatic niche cells (Leydig cells, endothelial cells, myoid cells, and macrophages) and germ cells (differentiating spermatogonia, early primary spermatocytes, late primary spermatocytes, round spermatids, elongated spermatids, and sperm (Fig. 1A and B ). We were also able to annotate a cluster of spermatogonial stem cells (Fig. 1A and B). Sertoli cells are underrepresented owing to cell size filtering in the original study (18) and therefore did not cluster separately.
Figure 1.
Clustering of distinct testicular cell populations and transcriptional signatures. (A) Clustering was performed on the normalized and scaled data, and (B) clusters were manually assigned cell types based on well-known markers. Legend bars reflect the normalized and log-transformed gene expression values for each cell based on the raw expression matrix (mRNA transcript counts) and therefore contain zero or positive values. (C) The dot plot depicts the scaled (Pearson residuals) and centered (mean zero) expression of an average cell in each cluster and therefore contains negative and positive values. The average expression reflects the mean expression in each cluster compared with all other cells. The size of the dot reflects the percentage of cells with mRNA transcripts detected. VIM = somatic cell marker, CD14 = macrophage marker, VWF = endothelial cell marker, DLK1 = Leydig cell marker, ACTA2 = myoid cell marker, STRA8 = retinoic acid target gene that marks the transition between differentiating spermatogonia and spermatocytes, SYCP3 = meiosis marker, ZPBP = spermatid structure protein, PRM2 = nuclear condensation/protamine repackaging factor, ID4 = spermatogonial stem cell marker, S’cytes = spermatocytes, S’gonial = spermatogonial, ACE2 = angiotensin-converting enzyme 2, TMPRSS2 = transmembrane serine protease 2, BSG = receptor basigin, CTSL = cysteine protease cathepsin L.
Consistent with independent analyses (7), we found ACE2 expression in myoid cells (average expression = 2.2, 5.3% of cells with mRNA transcripts), spermatogonial stem cells (average expression = 1.0, 4.8% of cells with mRNA transcripts), and Leydig cells (average expression = 1.0, 2.3% of cells with mRNA transcripts (Fig. 1C). TMPRSS2 expression was detected predominantly in elongated spermatids (average expression = 2.5, 15.0% of cells with mRNA transcripts) and to a lesser extent in spermatogonial stem cells (average expression = 1.5, 9.3% of cells with mRNA transcripts (Fig. 1C). Despite a small proportion of spermatogonial stem cells expressing ACE2 and TMPRSS2, cells coexpressing both genes were extremely rare (0.05% of cells (Fig. 1C). We did not observe a correlation between ACE2 and TMPRSS2 expression in any of the annotated cell types or testicular cells broadly (Pearson correlation value = −0.01). Alternative receptors and proteases may mediate viral entry in these cells. BSG was more broadly expressed across testicular cell types than ACE2 and was coexpressed with CSTL in early and late primary spermatocytes (78.7% and 90.8% of cells with mRNA transcripts, respectively). Lower levels of BSG and CTSL coexpression were also detected in Leydig cells, myoid cells, endothelial cells, and differentiating spermatogonia (Fig. 1C).
Nonhuman primate ovarian cells
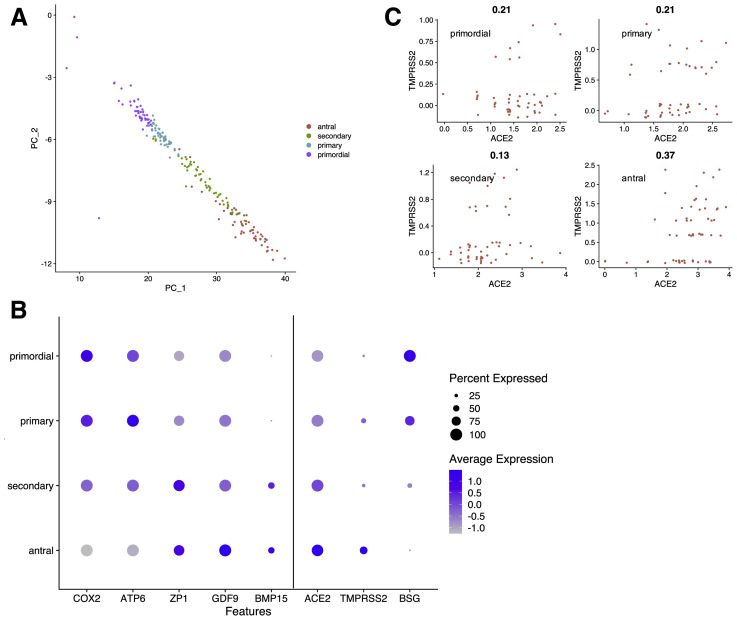
To our knowledge, scRNA data in human ovaries (specifically the outer ovarian cortex) are not yet readily available; however, data in nonhuman primate ovaries have been made public. Consistent with previous analyses (19), we identified seven ovarian cell types, including oocytes and six somatic cell types (stromal cells, granulosa cells, smooth muscle cells, natural killer cells, macrophages, and endothelial cells (Fig. 2A and B ). BSG was expressed in somatic ovarian cells, and ACE2 and TMPRSS2 expression was restricted to germ cells (Fig. 2C). A moderate correlation between ACE2 and TMPRSS2 in ovarian cells (Pearson correlation value = 0.51) was driven entirely by expression in oocytes (Fig. 2D). As in the original study (19), a principle component analysis within the oocyte cluster was used to identify four subtypes of oocytes with distinct gene expression profiles (Fig. 3 A). These four clusters represented sequential developmental stages of folliculogenesis (i.e., oocytes within primordial, primary, secondary, and antral follicles), as is shown by the distribution of markers known to be involved in follicular development (Fig. 3B). The degree of ACE2 and TMPRSS2 coexpression increased with oocyte maturity: primordial (17.0% of cells, Pearson correlation value = 0.21), primary (39.0%, Pearson correlation value = 0.21), secondary (25.0%, Pearson correlation value = 0.13), and antral (62.1%, Pearson correlation value = 0.37 (Fig. 3B and C). Unlike lung tissue (5), ACE2 (average expression = 2.3, 100% of cells) is more broadly expressed in oocytes than TMPRSS2 (average expression = 2.2, 37.0% of cells), suggesting that the latter may be a potential limiting factor for infection of the female gamete (Fig. 3B). Expression of the alternative CTSL could not be interrogated because it was not captured in the original study (absent from combined expression matrix) (19). BSG transcripts were detected in 100% of primordial oocytes, with diminishing expression across maturing oocytes (Fig. 3B).
Figure 2.
Clustering of distinct ovarian cell populations and transcriptional signatures. (A) Clustering was performed on the normalized and scaled data, and (B) clusters were manually assigned cell types based on well-known markers. Legend bars reflect the normalized and log-transformed gene expression values for each cell based on the raw expression matrix (mRNA transcript counts) and therefore contain zero or positive values. (C) The dot plot depicts the scaled (Pearson residuals) and centered (mean zero) expression of an average cell in each cluster and therefore contains negative and positive values. The average expression reflects the mean expression in each cluster compared with all other cells. The size of the dot reflects the percentage of cells with mRNA transcripts detected. (D) The feature scatterplot shows the Pearson correlation value for expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) in all ovarian cells with color-coded cell types.
Figure 3.
Clustering of distinct stages of folliculogenesis. (A) Principal components analysis (PCA) revealed four stages of folliculogenesis within the oocyte cluster. PC1 is plotted against PC2, and clusters were manually assigned cell types using known markers of follicular development. (B) The dot plot depicts the expression distribution of follicular development markers and expression of transmembrane serine protease 2 (TMPRSS2) and angiotensin-converting enzyme 2 (ACE2) across oocyte subclusters. Mitochondrially encoded ATP synthase membrane subunit 6 (ATP6) and cyclooxygenase 2 (COX2) are markers of primordial follicles and are progressively downregulated during folliculogenesis. Zona pellucida glycoprotein 1 (ZP1), growth differentiation factor 9 (GDF9), and bone morphogenetic protein 15 (BMP15) are promoters of follicular development and are progressively upregulated during folliculogenesis. Average expression represents the scaled (Pearson residuals) and centered (mean zero) expression of an average cell in each cluster and therefore contains negative and positive values. The size of the dot reflects the percentage of cells with detected mRNA transcripts. Primordial = oocytes within primordial follicles (also known as the ovarian reserve). Primary = oocytes within primary follicles. Secondary = oocytes within secondary follicles. Antral = oocytes within antral follicles.
Human cumulus cells
Analysis of the entire transcriptome of 18 human cumulus cell samples using RNAseq allowed evaluation of the expression of ACE2, TMPRSS2, CTSL, and BSG. Of note, each sample was composed of all of the cumulus cells associated with a single oocyte, and consequently it was not possible to determine whether genes were coexpressed in individual cells. Consistent with the nonhuman primate scRNAseq results, ACE2 was shown to have wide expression. Transcripts from this gene were detectable in 18 of 18 samples, and a TPM score of 24.03 was recorded. Also concordant with the primate results, expression of TMPRSS2 was found to be very low in human cumulus cells. Transcripts were completely undetectable in 15 of 18 samples and expressed only at low levels in the other three samples (averaging 0.13 TPM). By contrast, BSG and CTSL transcripts were detectable in all samples (18/18) and had expression levels of 374.19 TPM and 105.45 TPM, respectively.
Viral Entry Protein Expression
Proteomic expression of ACE2 (Fig. 4 A), TMPRSS2 (Fig. 4B), BSG (Fig. 4C), and CTSL (Fig. 4D) was queried from two publicly available databases, the Human Protein Atlas (HPA) and the Human Proteome Map. The HPA measures protein expression by immunohistochemistry (IHC), and the Human Proteome Map uses a mass spectrometry–based profiling platform. Protein expression in ovarian, testicular, endometrial, and placental tissue was correlated with bulk cell RNA expression data from four publicly available datasets: the HPA and GTEx datasets, which both use RNA-seq; the FANTOM5 dataset, which uses cap analysis gene expression; and the HPA consensus dataset, a consensus dataset of the other three RNA datasets. The low sequencing depth of scRNAseq datasets means that many cells will have no expression by chance; therefore, bulk RNAseq datasets, sequenced at higher depths, can serve as a useful point of comparison.
Figure 4.
Protein expression of viral host entry proteins in human reproductive tissues. Proteomic expression of (A) transmembrane serine protease 2 (ACE2), (B) transmembrane serine protease 2 (TMPRSS2), (C) receptor basigin (BSG), and (D) cysteine protease cathepsin L (CTSL) was queried from two publicly available databases, the Human Protein Atlas and the Human Proteome Map and correlated with RNA expression data from four different publicly available datasets. Expression was categorized as no expression (white space), low (small circle), medium (medium circle), or high (large circle). The expression of some proteins was not assessed in certain tissues, denoted by N/A. HPA = Human Protein Atlas, GTEx = genome-based tissue expression, CAGE = cap analysis gene expression, FANTOM5 = functional annotation of the mammalian, IHC = immunohistochemistry, Protein MS = protein mass spectrometry.
There was general agreement between the expression data from the two protein datasets and the RNA datasets. In ovarian tissue, mass spectrometry found high levels of ACE2 protein expression in ovarian cells, whereas IHC did not detect the protein in either follicular or stromal ovarian cells (Fig. 4A). In ovarian cells, TMPRSS2 was not detected by either protein profiling platform, which was supported by the external RNA datasets (Fig. 4B). These results are consistent with our internal RNAseq results in human cumulus cells showing broad expression of ACE2 but not TMPRSS2. BSG expression on a protein level was detected by mass spectrometry but not by IHC in ovarian cells, which was supported by RNA expression data (Fig. 4C). CTSL showed moderate and high RNA expression but no protein expression (Fig. 4D).
In testicular tissue, ACE2 (Fig. 4A) and BSG (Fig. 4C) both showed medium or high expression across all platforms. The underrepresentation of Sertoli cells in the scRNAseq data may account for some of the discrepancy in the ACE2 expression profile between these two datasets. It is also worth noting that baseline ACE2 expression was low, even in tissues known to be infected (5); therefore, given the low sequencing depth of scRNAseq platforms, detection of mRNA transcripts in few cells may still indicate functional activity (as is suggested by protein expression data). TMPRSS2 was found to be expressed only at a low level in the RNA datasets and was not detectable by either protein platform (Fig. 4B). CTSL was detected by mass spectrometry and all RNA analyses but not by IHC (Fig. 4D). In endometrial tissues, only BSG (Fig. 4C) was detected at a protein level. In placental tissues, there was both RNA and protein evidence of robust expression of BSG (Fig. 4C) and CTSL (Fig. 4D) but not ACE2 (Fig. 4A) or TMPRSS2 (Fig. 4B).
Human Reproductive Cell Lines
In addition to primary tissues, human cell lines may serve as valuable models for studying viral replication and effects on different tissue types. The Cell Atlas provides RNAseq expression data for 64 different human cell lines (21). Of those 64, three were of particular interest to this study: EFO-21, a metastatic ovarian serous cystadenocarcinoma cell line; AN3-Ca, an endometrial carcinoma line; and BEWO, a metastatic choriocarcinoma line. The disease states of the cell lines may perturb expression and thus undermine conclusions about expression in healthy human tissues. Even so, expression of SARS-CoV-2 viral entry proteins in the cell lines would establish whether or not they were susceptible to viral infection and were appropriate models for further study. RNAseq expression levels for ACE2, BSG, TMPRSS2, and CTSL was queried for all three lines. Expression data given by the Cell Atlas was in the form of normalized expression levels (NX), where the Cell Atlas takes an NX of 1 as the lower threshold for expression.
Of the three cell lines, only BEWO had detectable expression of all five proteins of interest, with low levels of ACE2 and TMPRSS2 expression (NX = 2.4 and 1.3, respectively) and higher levels of BSG and CTSL expression (NX = 87.4 and 15.4, respectively). EFO-21 and AN3-Ca both showed expression of BSG and CTSL (EFO-21: NX = 61.7 and 96, respectively; AN3-Ca: NX = 92.3 and 4.3, respectively) but not of ACE2 or TMPRSS2. As such, the BEWO cell line seems a good potential model system for investigating any impact(s) of SARS-CoV-2 infection on placental tissue. EFO-21 and AN3-Ca could be viable model lines if CTSL is determined capable of functionally replacing TMPRSS2 for viral S protein priming. These lines may serve as good candidates for investigating the potential functional interchangeability of TMPRSS2 and CTSL.
Discussion
It is not yet clear what effects, if any, the COVID-19 pandemic will have on male and female fertility. Therefore, we decided to look at the expression patterns of known viral host entry proteins to gain an insight into the possible biologic consequences of SARS-CoV-2 infection on reproduction. Our results from scRNAseq data suggest that sperm cells may not be at increased risk for viral entry and spread mediated by ACE2 and TMPRSS2, given the lack of coexpression in any testicular cell type, although we were unable to interrogate Sertoli cells. Separately, bulk RNAseq and protein platforms indicated moderate ACE2 expression in testicular tissue, whereas expression of TMPRSS2 was found to be low or undetectable. Alternative receptor BSG and protease capthesin L were broadly expressed in testicular cell clusters, as is true in other tissue types studied to date (7). However, it has not yet been empirically determined whether cathepsin L can functionally replace the key role of TMPRSS2 in viral S protein priming (4, 5). Our findings are consistent with those of a recent study showing an absence of SARS-CoV-2 RNA in semen and testicular biopsy specimens from infected men (22). Taken together, these results suggest that long-term effects of SARS-CoV-2 on male reproductive function are unlikely and that the risk of infection of sperm and subsequent transmission to embryos during fertilization is low.
At present, relatively little scRNAseq data are available from human ovarian tissue. Fan et al. (23) sequenced the inner cortex of ovaries from women undergoing ovarian tissue cryopreservation; however, the inner cortex is largely devoid of germ cells. More recently, Wagner et al. (24) performed scRNAseq of the outer cortex, containing both germinal and somatic cell types, producing data that could potentially serve as an external validation dataset if expression matrices are made available. As a result of the scarcity of scRNAseq data from the human ovary, this study and others have used information from closely related nonhuman primates as a proxy for expression in our own species (19). Naturally, such analyses have limitations and, although potentially indicative, cannot be considered conclusive.
In the current study, based on scRNAseq data, a subpopulation of oocytes in nonhuman primate ovarian tissue was found to coexpress ACE2 and TMPRSS2, while coexpression was infrequent in other oocytes and absent in ovarian somatic cells. Coexpression of ACE2 and TMPRSS2 in oocytes appeared to increase as follicles progressed through development: oocytes in primordial follicles had minimal coexpression, while 62% of those in antral follicles had detectable expression of both ACE2 and TMPRSS2 (Pearson correlation value = 0.37). These results are relatively reassuring concerning the long-term effects of SARS-CoV-2 on female reproduction. Considering that the cells predicted to have the greatest susceptibility to infection are oocytes in antral follicles, which are either ovulated or atrophy within several days of appearance each cycle, a sustained impact on the female gamete seems unlikely. Additionally, it is important to note that oocytes within antral follicles are entirely surrounded within a mass of cumulus cells (a specialized class of granulosa cell). The nonhuman primate scRNAseq data suggest that such cells do not coexpress ACE2 and TMPRSS2 and may therefore provide a physical barrier to infection. Our transcriptomic data from human cumulus cells indicates that ACE2 is expressed in this cell type, findings which are concordant with those of previous research (25). However, transcripts of TMPRSS2 were found to be either entirely absent or exist at very low levels in the 18 sets of cumulus cells examined, and therefore a low risk of infection is predicted.
The documented lack of SARS-CoV (a coronavirus identified in 2003 that also uses ACE2 for viral entry) (4) replication in ovarian tissue, as determined by pathologic and immunohistochemical analyses, is supportive of our hypothesis that ovarian somatic tissues have little susceptibility to SARS-CoV-2 infection (12). As of yet, no autopsy reports in persons with laboratory-confirmed COVID-19 at the time of death have assessed SARS-Cov-2 replication in the reproductive system. Rather, infected tissues known to contribute to death in patients, such as the lungs and heart, have been the primary focus (26, 27). Moreover, current data on pathology are largely based on samples from postmenopausal women and therefore cannot address the potential activity of SARS-CoV-2 in mature oocytes (because postmenopausal women have none). Further experimental data in ovarian tissue from women of reproductive age with COVID-19 is needed to confirm whether the female germ cells can be infected. It is also worth noting that detection of ACE2 and TMPRSS2 mRNA transcripts in mature oocytes may not reflect active production of the associated proteins. Translation of mRNA gradually decreases during oocyte maturation, allowing a large pool of RNA transcripts to accumulate, necessary to sustain the embryo before genome activation (28).
Given that the impact of SARS-CoV-2 on preimplantation development, implantation, and early pregnancy is largely unknown, and considering that there is a theoretical possibility of viral infection of mature oocytes (due to coexpression of ACE2 and TMPRSS2), there may be concerns over the initiation of pregnancy during the COVID-19 pandemic. However, the data presented here suggest that procedures in which cumulus cell–enclosed oocytes are collected and fertilized outside the female reproductive tract (e.g., in vitro fertilization [IVF]) are likely to pose very little risk and may have the potential to reduce or entirely eliminate exposure of susceptible cell types to infection. The risk of infection for primordial follicles (the ovarian reserve) appears to be low (ACE2 and TMPRSS2 expression correlation value = 0.21), indicating that any possible effects of COVID-19 on fertility may be transient.
So far, published data also provide some reassurance concerning aspects of reproductive risk occurring at later stages, after initiation of a pregnancy. Publicly available developmental scRNAseq databases reveal no coexpression of ACE2 and TMPRSS2 in fetal tissues, including liver, thymus, skin, bone marrow, and yolk sac (6). Expression of ACE2 has been documented in the placenta, but coexpression with TMPRSS2 has not been reported, suggesting that placental cells may act as a barrier to vertical SARS-CoV-2 transmission (7). These conclusions are supported by the lack of ACE2 and TMPRSS2 protein expression in endometrial and placental tissue (HPA/IHC datasets) described in this study. The limited clinical data available have also indicated a lack of vertical transmission in newborns of pregnant women who had confirmed COVID-19 in the third trimester (29). Additionally, obstetric outcomes for babies born to mothers infected with SARS-CoV-2 have not shown any impact of COVID-19 on pregnancy (30).
In conclusion, the results presented here can only be considered indicative and require verification. Our results cannot rule out the possibility that proteases other than TMPRSS2 (including CTSL) may facilitate viral entry in some cells, such as primordial oocytes (100% of which have detected ACE2 and BSG transcripts). Nonetheless, they add to a growing body of evidence suggesting that most aspects of reproduction during the COVID-19 pandemic are unlikely to be associated with increased risks of clinical complications. The amount of data currently available is small, but thus far there is no indication of an increased incidence of severe disease among pregnant women, and obstetric outcomes for babies born to mothers infected with SARS-CoV-2 appear to be within the normal range (29). Furthermore, at the time of writing, viral RNA has not been detected in semen or testicular biopsy specimens from infected men (23). The results of the current study suggest that sperm and cumulus-enclosed oocytes are unlikely to be susceptible to infection by SARS-CoV-2; consequently, it should be possible to undertake oocyte collection and fertilization, followed by in vitro embryo culture and cryopreservation, with minimal risk to the embryos produced. Indeed, considering the lack of knowledge concerning the risk of infection during in vivo preimplantation development, a stage during which the potentially protective cumulus cells are shed, it could be argued that IVF might represent a safer reproductive strategy than natural conception at this time. Given that the success rates of IVF treatments decline at an ever-accelerating rate as the age of the female patient increases (falling by approximately 0.3% per month from the midthirties, according to data from the Society of Assisted Reproductive Technologies and the Human Fertilisation and Embryology Authority), it is imperative that delays to fertility treatments are minimized. Provided adequate precautions can be instituted to ensure the safety of patients attending fertility clinics, as well as that of clinical staff, it seems reasonable to consider the reintroduction of IVF treatments (at a minimum cycles involving cryopreservation of embryos) in countries where the response to COVID-19 currently includes a severe restriction or denial of patient access to fertility treatments.
Footnotes
K.E.S. has nothing to disclose. E.T. has nothing to disclose. M.L. reports grants from IVI-RMA during the conduct of the study. D.W. reports personal fees from Juno Genetics, grants from IVI-RMA, outside the submitted work.
Supported in part by grants from IVI-RMA (to M.L). and by funding from the NIHROxford Biomedical Research Centre (to D.W).
References
- 1.World Health Organization Novel coronavirus (2019-nCoV): situation report 97. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200416-sitrep-87-covid-19.pdf?sfvrsn=9523115a_2 Available at:
- 2.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Dhindsa R., Povysil G., Zoghbi A., Motelow J., Hostyk J., et al. Transcriptional inhibition of host viral entry proteins as a therapeutic strategy for SARS-CoV-2. https://www.preprints.org/manuscript/202003.0360/v1 Available at:
- 6.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Med Epub. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sungnak W., Huang N., Bécavin C., Berg M., Network H.L.B. SARS-CoV-2 entry genes are most highly expressed in nasal goblet and ciliated cells within human airways. http://arxiv.org/abs/2003.06122 arXiv:200306122 [q-bio]. Available at:
- 8.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B., et al. ST-segment elevation in patients with Covid-19: a case series. N Engl J Med Epub. 2020 Apr 17 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabb H. Kidney diseases in the time of COVID-19: major challenges to patient care. https://www.jci.org/articles/view/138871/pdf Available at: [DOI] [PMC free article] [PubMed]
- 10.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med Epub. 2020 Apr 17 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi J., Zhou Y., Hua J., Zhang L., Bian J., Liu B., et al. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to COVID-19 infection. 2020. https://www.biorxiv.org/content/10.1101/2020.04.16.045690v1 Available at: [DOI] [PMC free article] [PubMed]
- 12.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. https://jamanetwork.com/journals/jama/fullarticle/2762997 Available at: [DOI] [PMC free article] [PubMed]
- 14.Peng L., Liu J., Xu W., Luo Q., Deng K., Lin B., et al. 2019 Novel coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples. 2020. https://www.medrxiv.org/content/10.1101/2020.02.21.20026179v1 Available at: [DOI] [PMC free article] [PubMed]
- 15.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. http://biorxiv.org/lookup/doi/10.1101/2020.03.14.988345 Available at:
- 16.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun Epub. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griswold M.D. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J., Grow E.J., Mlcochova H., Maher G.J., Lindskog C., Nie X., et al. The adult human testis transcriptional cell atlas. Cell Res. 2018;28:1141–1157. doi: 10.1038/s41422-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S., Zheng Y., Li J., Yu Y., Zhang W., Song M., et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180:585–600.e19. doi: 10.1016/j.cell.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Hikmet F., Méar L., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. 2020. https://www.biorxiv.org/content/10.1101/2020.03.31.016048v1 Available at: [DOI] [PMC free article] [PubMed]
- 21.Thul P.J., Åkesson L., Wiking M., Mahdessian D., Geladaki A., Ait Blal H., et al. A subcellular map of the human proteome. Science Epub. 2017:356. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 22.Song C., Wang Y., Li W., Hu B., Chen G., Xia P., et al. Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of COVID-19 patients. 2020. https://www.medrxiv.org/content/10.1101/2020.03.31.20042333v2 Available at:
- 23.Fan X., Bialecka M., Moustakas I., Lam E., Torrens-Juaneda V., Borggreven N.V., et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat Commun. 2019;10:3164. doi: 10.1038/s41467-019-11036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner M., Yoshihara M., Douagi I., Damdimopoulos A., Panula S., Petropoulos S., et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11:1147. doi: 10.1038/s41467-020-14936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavallo I.K., Dela Cruz C., Oliveira M.L., Del Puerto H.L., Dias J.A., Lobach V.N., et al. Angiotensin-(1-7) in human follicular fluid correlates with oocyte maturation. Hum Reprod. 2017;32:1318–1324. doi: 10.1093/humrep/dex072. [DOI] [PubMed] [Google Scholar]
- 26.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., et al. [A pathological report of three COVID-19 cases by minimally invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 27.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet Epub. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansova D., Tetkova A., Koncicka M., Kubelka M., Susor A. Localization of RNA and translation in the mammalian oocyte and embryo. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L., et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med Epub. 2020 Apr 17 doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]