Highlights
-
•
The world's population is becoming older, more obese, and more physically inactive, increasing the likelihood of pandemics such as coronavirus disease-2019.
-
•
Aging, obesity, and physical inactivity adversely impact immune function and host defense.
-
•
Regular moderate-intensity physical activity improves immunosurveillance against pathogens and reduces morbidity and mortality from respiratory illnesses.
-
•
Coronavirus disease-2019 is a wake-up call, a tocsin, to the world to focus on primary prevention countermeasures.
Keywords: COVID-19, Exercise, Immunology, Infection, Obesity
Abstract
Acute and chronic respiratory illnesses cause widespread morbidity and mortality, and this class of illness now includes the novel coronavirus severe acute respiratory syndrome that is causing coronavirus disease-2019 (COVID-19). The world is experiencing a major demographic shift toward an older, obese, and physically inactive populace. Risk factor assessments based on pandemic data indicate that those at higher risk for severe illness from COVID-19 include older males, and people of all ages with obesity and related comorbidities such as hypertension and type 2 diabetes. Aging in and of itself leads to negative changes in innate and adaptive immunity, a process termed immunosenescence. Obesity causes systemic inflammation and adversely impacts immune function and host defense in a way that patterns immunosenescence. Two primary prevention strategies to reduce the risk for COVID-19 at both the community and individual levels include mitigation activities and the adoption of lifestyle practices consistent with good immune health. Animal and human studies support the idea that, in contrast to high exercise workloads, regular moderate-intensity physical activity improves immunosurveillance against pathogens and reduces morbidity and mortality from viral infection and respiratory illnesses including the common cold, pneumonia, and influenza. The odds are high that infectious disease pandemics spawned by novel pathogens will continue to inflict morbidity and mortality as the world's population becomes older and more obese. COVID-19 is indeed a wake-up call, a tocsin, to the world that primary prevention countermeasures focused on health behaviors and hygiene demand our full attention and support.
Graphical abstract
1. Introduction
Acute and chronic respiratory illnesses are the most common infectious diseases on earth and cause widespread morbidity and mortality. According to the World Health Organization, lower respiratory tract infections and pneumonia account for more than 4 million deaths annually.1 The World Health Organization also estimates that influenza causes respiratory tract infections in 5%–15% of the world's population and severe illness in 3–5 million people.1 The Global Burden of Diseases, Injuries, and Risk Factors Study ranks upper respiratory infections as the leading incident disease in the world (17.1 billion per year).2
There are many types of viruses that cause acute upper and lower respiratory illnesses in humans. Virus identification has improved by means of real-time reverse-transcription polymerase chain reaction testing. Multiplex polymerase chain reaction can detect multiple pathogens at the same time. Reverse-transcription polymerase chain reaction has shown that predominant viruses causing acute respiratory illnesses include human rhinoviruses, influenza A and B viruses, 4 types of parainfluenza viruses, respiratory syncytial virus, adenoviruses, and coronaviruses.3,4
Coronaviruses are enveloped RNA viruses with a zoonotic origin and include 4 types—229E, OC43, NL63, and HKU1—that typically cause common cold symptoms. Coronaviruses have a sharp seasonality, with high infection frequency during the winter and spring.5 Three new types of coronaviruses have emerged. In 2003, a previously unrecognized coronavirus caused severe acute respiratory syndrome (SARS) before being stopped by rigorous infection control measures (8422 cases, with a case fatality rate of 11%).6 Another novel coronavirus of animal origin appeared in 2012 as the causative agent of Middle East Respiratory Syndrome (MERS) (2519 cases, with a case fatality rate of 34%).7
The impact of SARS and MERS was limited in comparison to the global disorder unleashed by the coronavirus SARS-CoV-2 that is causing coronavirus disease 2019 (COVID-19).8,9 The causal virus was identified on January 7, 2020, with the genome sequence of SARS-CoV-2 released on January 10, allowing work on potential vaccines and therapeutics to commence.8 Additional research showed that SARS-CoV-2 (1) is primarily transmitted through airborne droplets when infected individuals cough, sneeze, breathe deeply, or talk; (2) infects cells lining the air passageways by locking a spike (S) protein onto angiotensin-converting enzyme 2 receptors; and (3) typically causes modest symptoms such as coughing, fever/chills, shortness of breath, myalgia, and diarrhea before being eliminated by immune-generated neutralizing antibodies.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 The SARS-CoV-2 can cause severe symptoms in some individuals when it infects the lungs, instigating an intense inflammatory response of the immune system and a cytokine storm.17 This hyperinflammation state is characterized by increases in many cytokines, including those that are elevated after prolonged and intense exercise, such as interleukin-6, monocyte chemoattractant protein 1, and granulocyte-colony stimulating factor. SARS symptoms occur with SARS, MERS, and COVID-19, but the estimated case fatality rate of COVID-19 (2.3%) is substantially lower than SARS (11.0%) and MERS (34.0%).20,21 When compared with SARS and MERS, COVID-19 has spread more rapidly, perhaps due to increased globalization and adaptation of the virus in nearly every environment.20,21
2. COVID-19 epidemiology: focus on age, obesity, and comorbidities
The SARS-CoV-2 is highly transmissible and may be spread by infected individuals prior to the development of symptoms. On average, each infected person spreads the infection to an additional 2 or 3 persons, and the virus has a mean incubation period of about 6 days.18 SARS-CoV-2 RNA has been detected in upper and lower respiratory tract specimens and in blood and stool specimens.22 The virulence of SARS-CoV-2 appears to lie between SARS-CoV and community-acquired human coronarviruses.17 A vaccine should be developed soon, but may not be completely effective against COVID-19. The vaccine will be targeted initially to healthcare workers and vulnerable individuals.23
Within this context, the 2 most promising strategies to reduce the risk for COVID-19 at both the community and individual level are (1) mitigation activities and (2) the adoption of lifestyle practices consistent with good immune health. Mitigation approaches have been described extensively and adopted worldwide. They include personal protective measures (e.g., handwashing, cough etiquette, and face coverings), social distancing (e.g., maintaining physical distance between persons in community settings and staying at home), and environmental surface cleaning. This article provides an overview of the role of weight management and physical activity in improving immunosurveillance and viral defense.
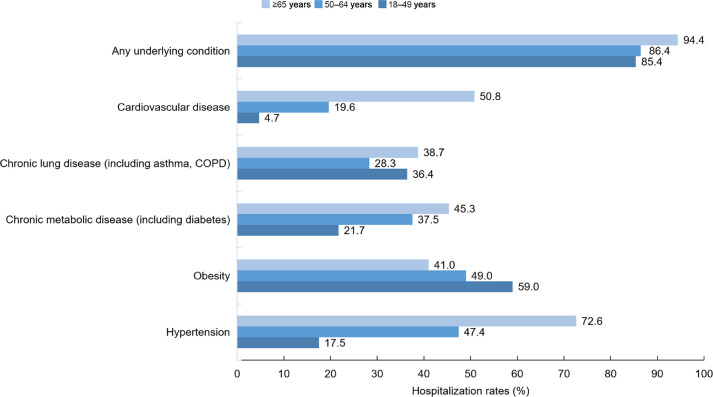
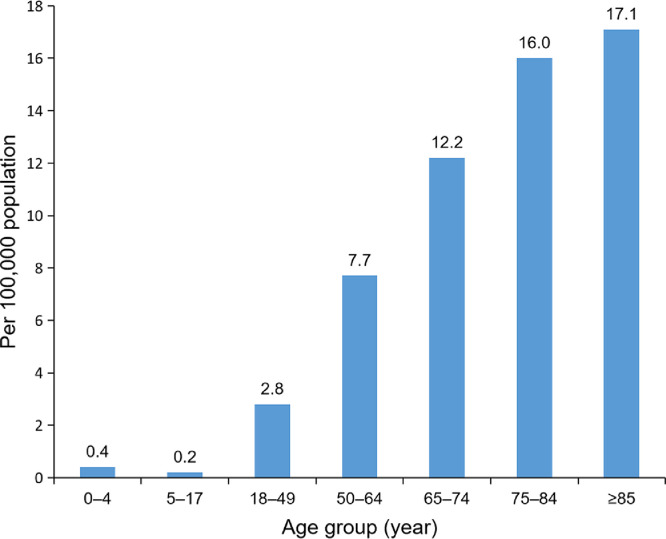
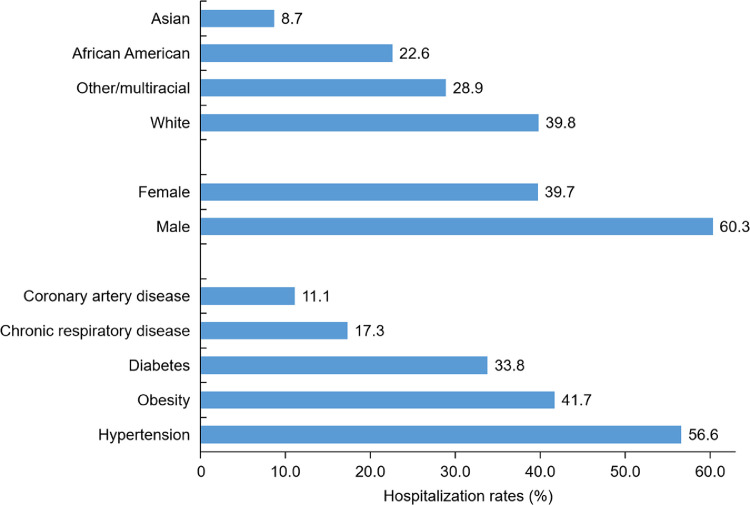
Risk factor assessments based on data from China, Europe, and the United States indicate that those at greater risk for severe illness from COVID-19 include older males, and people of all ages with obesity and underlying medical conditions such as hypertension, cardiovascular disease, chronic lung disease, and chronic metabolic diseases such as type 2 diabetes.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Fig. 1, Fig. 2, and Fig. 3 summarize the effect of age, demographics, and comorbidities for patients hospitalized in March 2020 with laboratory-confirmed COVID-19 in the United States.12,13 Among patients with data on underlying conditions, the most common were hypertension, obesity, chronic lung disease, diabetes mellitus, and cardiovascular disease. COVID-19 patients in hospitals tended to be older, non-white males. These findings underscore the importance of mitigation measures to protect older adults and those with underlying medical conditions. Additionally, this information supports the value of lifestyle approaches, such as physical activity, to reduce obesity prevalence in bolstering host antiviral, immune defense.14
Fig. 1.
Percentage of coronavirus disease-2019–associated hospitalizations, by age and comorbidity, in the United States (14 states) during March 2020.12 COPD = chronic obstructive pulmonary disease.
Fig. 2.
Rates for age groups for coronavirus disease-2019–associated hospitalizations in the United States (14 states) during March 2020.12
Fig. 3.
Baseline comorbidities and demographics for 5700 coronavirus disease-2019 patients (median age, 63 years) admitted to 12 hospitals in the New York City area, USA, during March 1 to April 4, 2020.13
3. Aging and obesity both impair host viral defense
The world is experiencing a major demographic shift toward an older, obese population. By 2050, the world's population aged 60 years and older is expected to total 2 billion, up from 900 million in 2015.2 Aging in and of itself leads to negative changes in innate and adaptive immunity, a process termed immunosenescence.24 The function of nearly every type of immune cell is negatively affected with increase in age, resulting in increased susceptibility to infectious diseases, reduced antibody responses to vaccinations, systemic inflammation, and decreased immune surveillance against cancer.24, 25, 26 Influenza and COVID-19 infections are associated with high rates of complicated illness, including pneumonia, among the elderly.10, 11, 12, 13, 14, 15, 16,27
If recent trends continue, nearly 50% of adults in the United States will be obese, and 60% of all adults worldwide will be overweight and obese, by 2030.28,29 Obesity markedly increases the risk for hypertension, type 2 diabetes, and cardiovascular disease, three of the most important underlying conditions for COVID-19.30,31 Obesity is a central component of the metabolic syndrome that undergirds many of the prevalent chronic diseases.31
Obesity also causes systemic inflammation and adversely impacts immune function and host defense in a way that patterns immunosenescence.32,33 Obese patients have higher rates of nosocomial infections following surgery and experience altered pharmacokinetics of antimicrobial drugs.32, 33, 34, 35, 36 Obesity emerged as an important risk factor for increased hospitalization and infection severity during both the 2009 influenza A virus H1N1 and COVID-19 pandemics.14,37, 38, 39, 40, 41, 42 The antibody response to the seasonal influenza vaccine is impaired in obese individuals, and virus shedding is prolonged during influenza illness.43 Compared to vaccinated normal-weight adults, vaccinated obese adults have twice the risk of influenza or influenza-like illness.33 Animal-based studies suggest that obesity increases the severity and duration of viral infections, increasing the potential for the evolution of pathogenic viral variants.44
The cytokine storm has been identified as the excessive immune response coupled with hyperinflammation in the lungs of the most severe COVID-19 cases.45 Limited data suggest that obesity-induced systemic inflammation primes the immune system to generate an even more intense cytokine storm when elicited by infection.46
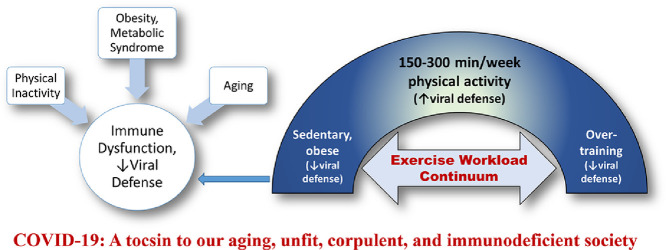
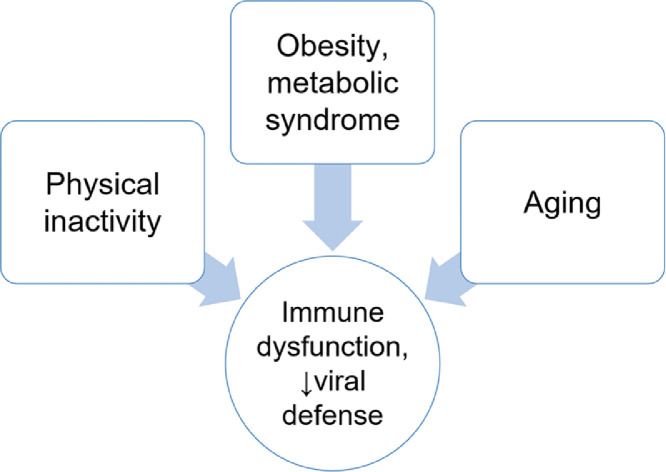
All projections indicate that the world's population will be older, more obese, and therefore increasingly more immunodeficient in the approaching decades. This shift is likely to increase the odds that infectious disease pandemics spawned by novel pathogens will continue to inflict widespread morbidity and mortality (Fig. 4).
Fig. 4.
Linkage between physical inactivity, aging, and obesity and the metabolic syndrome with immune dysfunction and diminished viral defense.
4. Viral illness and the exercise workload continuum
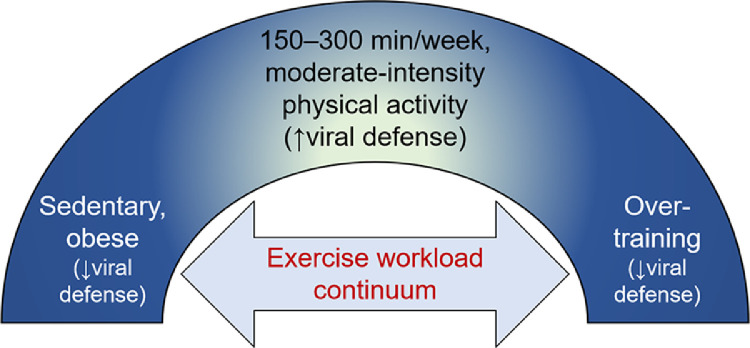
Patterns of physical activity vary widely in the general population, ranging from nearly complete inactivity to overtraining. Animal and human studies support a linkage between the range of physical activity behaviors and viral defense effectiveness (Fig. 5).47
Fig. 5.
The exercise workload benefit-risk continuum with viral defense.
4.1. Heavy exertion and decreased viral and immune defense
The earliest studies on heavy exertion and decreased viral and immune defense focused on muscular fatigue and pathogen resistance and were reviewed in 1932 by Baetjer,48 who concluded that exhaustive exercise before or immediately following infection predisposed animals to a more rapid and fatal attack. This observation has been supported by numerous animal- and human-based studies conducted using a variety of pathogens.49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 The virulence of influenza, herpes simplex virus, and the Coxsackie virus, for example, is increased when mice are inoculated before or after being forced to swim or run to exhaustion.52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 Equine respiratory disease, especially from the influenza virus, is a common infection requiring veterinary medical attention, and intense exercise has been linked to increased susceptibility to influenza disease among horses.71 Exhaustive exercise leading to increased morbidity and mortality from viral infection in animals has been linked to immune dysfunction, including a decrease in macrophage antiviral resistance and antigen presentation, natural killer cell cytotoxicity, and neutrophil oxidative burst activity.54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65
Clinicians since the 1940s have observed that certain patients with paralytic poliomyelitis gave a history of severe exertion immediately preceding or during the onset of paralysis.49, 50, 51 The clinical data were strongest for polio patients who reported continuing physical activity after the onset of symptoms. This finding was confirmed in a study with infected rhesus monkeys that were subjected to exhausting exercise.50
These data imply that exercising heavily before or while infected with a systemic infection such as influenza or COVID-19 may lead to a more severe and prolonged illness and, in some instances, death. Numerous case reports have been published of protracted illness, fatigue, and death in young healthy people who engaged in vigorous-intensity exercise during an acute viral illness.66, 67, 68, 69, 70, 71, 72, 73 Acute respiratory infections are especially challenging in military settings because demanding physical training regimens are combined with crowded living conditions, physical and psychological stresses, and environmental challenges.73 Military recruits often feel pressured to train even when they are sick. Clinical and autopsy records of 19 sudden cardiac deaths that occurred among Air Force recruits during basic training showed that the most frequent underlying etiology was myocarditis.74 Viral illness is endemic in barrack-residing recruits, and exertion may have exacerbated viral-induced subclinical cases of myocarditis. A study of 20 male soldiers in the Israel Defense Forces who had died suddenly and unexpectedly within 24 h of strenuous exertion also reasoned that febrile disease might have been a cause of death in some of the subjects.75
Human epidemiologic studies indicate that the odds of acute respiratory illness following marathon or ultramarathon events climb sharply, especially when combined with mental stress, sleep disruption, and travel.47 Intense acute and chronic exercise workloads cause varying levels of physiological, metabolic, and psychological stress leading to immune perturbations, inflammation, oxidative stress, muscle damage, and increased illness risk.76, 77, 78, 79 In particular, heavy exercise workloads result in an extensive downturn in the function of innate immune system cells including macrophages, neutrophils, and natural killer cells.77, 78, 79, 80, 81 These data have been reviewed previously.47
Taken together, existing data support the viewpoints that (1) overtraining is not recommended in areas of the world where risk of COVID-19 transmission is high and (2) intense exercise should be avoided when the person is infected with COVID-19 or other systemic viruses.
4.2. Moderate-intensity physical activity and enhanced viral and immune defense
In contrast to high exercise workloads, animal and human data support the position that regular moderate-intensity physical activity improves immunosurveillance against pathogens and reduces morbidity and mortality from viral infection and acute respiratory illness.47,78 As reviewed previously,47 regular aerobic exercise similar to 30–60 min of near-daily brisk walking improves overall surveillance against pathogens by stimulating the ongoing exchange of important types of white blood cells between the circulation and tissues.82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94 Stress hormones and inflammatory responses remain at low levels while neutrophils, natural killer cells, cytotoxic T cells, immature B cells, and monocytes circulate at a higher rate than normal during exercise and a few hours of recovery. When repeated in regular fashion, these transient, exercise-induced increases in antipathogenic leukocytes enhance immunosurveillance, reduce illness risk, and lower systemic inflammation. Thus, regular exercise training can be viewed as an immune system adjuvant and is of particular clinical value for obese individuals with comorbidities, as well as for older individuals.95, 96, 97, 98, 99, 100, 101, 102, 103
Cross-sectional studies comparing immune function in lean, fit individuals versus sedentary elderly individuals report that many aspects of immunosenescence are moderated.24,87,88,101 Other data support the idea that habitual exercise improves regulation of the immune system in the elderly and delays the onset of immunosenescence because exercise provides a decrease in senescent T cells; an increase in neutrophils, natural killer cells, and T-cell function; and a reduction in systemic inflammation.24,96, 97, 98, 99, 100, 101 Exercise training has also been shown to improve vaccine efficacy for a variety of diseases, including influenza.96,97
Epidemiologic and randomized clinical trials support a 40%–45% reduction in illness days stemming from acute respiratory infections, such as the common cold, in younger and older adults who engage in near-daily aerobic activity compared to sedentary behavior.47,104 A group of 1002 adults (aged 18–85 years) were monitored for acute respiratory illness symptoms and severity, using a validated survey, for 12 weeks during the winter and fall seasons.104 The number of days with illness during the 12-week period was significantly reduced by 43% in those reporting 5 or more days per week of aerobic exercise compared to those who were largely sedentary. Illness severity and symptomatology were also reduced 32%–41% between high and low aerobic activity and physical fitness tertiles. These data indicate that physical fitness and frequency of aerobic exercise are important correlates of reduced days with acute respiratory illness and severity of symptoms during the winter and fall seasons, when common colds are more frequent. This study also showed that eating 3 or more servings of fruit per day was independently related to fewer days with respiratory illness.104
Physical fitness status and exercise training have also been linked to a reduced risk of stress-induced latent viral reactivation in astronauts.85 Findings from other studies support a consistent and impressive reduction in pneumonia and influenza incidence and mortality with regular physical activity (Table 1).105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115 The risk for severe influenza increased in communities reporting a high prevalence of physical inactivity and obesity, as well as for low intake of fruits and vegetables.114 In contrast, regular exercise training was associated with reduced influenza mortality in elderly individuals.110 Infectious disease mortality risk (primarily pneumonia and sepsis) was 40% lower in adults participating in physical activity more than 150 min per week compared to those who were inactive in a cohort of 97,844 men and women followed for more than 9 years.113 Whether or not these data indicating reduced systemic infectious illnesses in physically active individuals are applicable to COVID-19 remains to be determined.
Table 1.
Epidemiologic research on the relationship between physical activity, influenza, and pneumonia.
| Investigators, year published | Study population | Research design | Key findings |
|---|---|---|---|
| Baik et al., 2000105 | 26,429 men (40–79 years old), 78,062 women (27–44 years old) | 290 new pneumonia cases in men (6-year follow-up), 305 in women (2-year follow-up), questionnaire responses entered into a Cox proportional hazards model | 34% reduction in risk for developing pneumonia for women, but not men, in the highest vs. lowest quintile of physical activity |
| Inoue et al., 2007106 | 110,792 adults (40–79 years old) | 1,112,747 person-years, 1246 pneumonia deaths, health screenings, questionnaire responses entered into a Cox proportional hazards model | Walking regularly for 0.5–1.0 h/day and more than 1.0 h/day decreased risk for pneumonia mortality by 20%–30% compared with 0.5 h/day |
| Wong et al., 2008107 | 24,656 adults (≥30 years old) who died in 1998 in Hong Kong, China | Families interviewed for lifestyle habits of deceased, sera influenza virus detection entered into multinomial logistic regression analysis | Excess risk of influenza-associated mortality reduced for low/moderate physical activity but not frequent physical activity |
| Neuman et al., 2010108 | 83,165 women (27–44 years old) | 965,168 person-years, 1265 new cases of pneumonia, questionnaire responses entered into a Cox proportional hazards model | 28% reduction in risk for developing pneumonia for women in the highest vs. lowest quintile of physical activity, but lowered to 16% reduction after adjustment for body mass index, smoking, and alcohol use |
| Williams, 2014109 | 109,352 runners, 40,798 walkers; age (mean ± SD), male runners (40.4 ± 10.9, walkers (61.2 ± 13.1); female runners (38.2 ± 10.1, walkers (50.4 ± 10.9) | 11.4-year average follow-up for running and walking history, lifestyle habits entered into a Cox proportional hazards model | Risk for respiratory disease mortality decreased 7.9% and for pneumonia 13.1% per metabolic equivalent of task hours per day run or walked |
| Wong et al., 2014110 | 66,820 elderly adults (≥56 years old) | 12-year follow-up, lifestyle habits entered into a time-dependent Cox proportional hazards model | Excess risk of influenza mortality was lower for frequent exercisers |
| Wu et al., 2016111 | 13,003 adults (≥18 years old) | Self-reported influenza-like illness and demographic data entered into a multivariate logistic regression model | Regular physical activity associated with a 20% reduction in likelihood of reporting influenza-like illness |
| Ukawa et al., 2019112 | 22,280 elderly adults (65–79 years old) | 1203 pneumonia deaths, 11.9-year follow-up, health screenings, questionnaire responses entered into an inverse probability weighting Cox proportional hazards model | 10%–35% reduction in risk for pneumonia mortality among elderly with or without underlying cardiovascular disease who walked regularly for ≥1.0 h/day compared with 0.5 h/day |
| Hamer et al., 2019113 | 97,844 adults (47.1 ± 17.7 years old) | 9027 deaths from infectious disease, 9.4-year follow-up, questionnaire responses entered into a Cox proportional hazards regression model | 40% reduced risk for infectious disease mortality with physical activity compared with physical inactivity |
| Charland et al., 2013114 | Data records used from 274 counties with total population of 116,146,020. A total of 3,076,699 hospitalizations for influenza-like illness (all ages) | Data used to regress log-transformed age-sex influenza-related hospitalization rates with lifestyle factors after adjustment for covariates | A 5% increase in the prevalence of physical inactivity was associated with an 11% and 19% increase in influenza-related hospitalization rates for adults and children, respectively, after adjustment for potential confounders |
The robust reduction in mortality risk for community-acquired bloodstream infections has been reported by several other research groups.116, 117, 118 A 15-year follow-up study of 64,027 individuals showed that bloodstream infections were nearly 5 times more likely in obese and physically inactive individuals who smoked tobacco compared to normal weight, physically active individuals who did not smoke.116
Animal studies support the linkage between physical exercise, augmented immunity, and reduced risk of influenza and pneumonia.83,119, 120, 121, 122 In one study using obese and lean mice, 8 weeks of exercise training followed by influenza viral infection decreased disease severity in both groups.83 Chronic moderate exercise for 8–14 weeks followed by influenza infection in mice resulted in reduced symptoms coupled with lowered virus load and levels of inflammatory cytokines and chemokines.83,95 Aerobic exercise training inhibited lipopolysaccharides-induced acute respiratory distress syndrome in mice by attenuating inflammatory cytokines and oxidative stress markers through inhibition of nuclear factor kappa B signaling, reduced neutrophil infiltration, and enhanced interleukin-10 production.82,123 These data are of high interest for their potential relevance to COVID-19.
5. Physical activity, lifestyle habits, viral defense capacity, and COVID-19
Taken together, these studies support the viewpoint that regular physical activity and the avoidance of obesity maintain immune health while reducing the risk for several types of respiratory illnesses.47 These primary prevention strategies against respiratory illnesses are particularly important in aging societies with a high prevalence of obesity and related comorbidities and are essential adjuvants to mitigation practices.
COVID-19 took just a few months to spread to nearly every nation on earth while causing widespread morbidity and mortality. The data support the belief that severe cases of COVID-19 are more likely in older and obese individuals. This is indeed a wake-up call, a tocsin, to the world that primary prevention countermeasures focused on health behaviors and hygiene demand our full attention and support. Secondary and tertiary prevention approaches centered on vaccine and therapeutics development will take time and may not be fully effective, giving even more urgency to staying fixated on primary prevention.
The relationship between varying exercise workloads and viral defense effectiveness can be placed on a continuum (Fig. 5).47,78 Recommended amounts of moderate-intensity physical activity typically range between 150 and 300 min per week124 and are consistent with enhanced immunosurveillance and lowered risk for respiratory illness. Physical inactivity, overtraining, and exercising while infected with a respiratory pathogen, on the other hand, have been linked to immune dysfunction and elevated risk for respiratory illness. Until more is known, this model can be applied to COVID-19, with the high likelihood that risk of morbidity and mortality is moderated in lean, physically active individuals of all ages.
Competing interests
The author declares that he has no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix. Supplementary materials
References
- 1.Forum of International Respiratory Societies. The global impact of respiratory disease – second edition. Available at: https://www.mdedge.com/chestphysician/article/140055/society-news/global-impact-respiratory-disease-second-edition. [accessed 15.04.2020].
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monto AS, Malosh RE, Petrie JG, Thompson MG, Ohmit SE. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. J Infect Dis. 2014;210:1792–1799. doi: 10.1093/infdis/jiu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao X, Hu Z, Liu W, Lu Y, Chen D, Chen M. New epidemiological and clinical signatures of 18 pathogens from respiratory tract infections based on a 5-year study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monto AS, DeJonge P, Callear AP, Bazzi LA, Capriola S, Malosh RE. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9–16. doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 7.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 8.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A. Lille Intensive Care COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients withCoronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 17.Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC COVID-19 Response Team Severe outcomes among patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020 doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen WH, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020 doi: 10.1007/s40475-020-00201-6. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity. Nat Rev Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 25.van Beek AA, Van den Bossche J, Mastroberardino PG, de Winther MPJ, Leenen PJM. Metabolic alterations in aging macrophages: ingredients for inflammaging. Trends Immunol. 2019;40:113–127. doi: 10.1016/j.it.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H. Exercise and the aging immune system. Ageing Res Rev. 2012;11:404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 27.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–388. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 29.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 30.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clin North Am. 2018;102:13–33. doi: 10.1016/j.mcna.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green WD, Beck MA. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14(Suppl. 5):S406–S409. doi: 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honce R, Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huttunen R, Karppelin M, Syrjänen J. Obesity and nosocomial infections. J Hosp Infect. 2013;85:8–16. doi: 10.1016/j.jhin.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Hainer V, Zamrazilová H, Kunešová M, Bendlová B, Aldhoon-Hainerová I. Obesity and infection: reciprocal causality. Physiol Res. 2015;64(Suppl. 2):S105–S119. doi: 10.33549/physiolres.933130. [DOI] [PubMed] [Google Scholar]
- 37.Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57:759–764. doi: 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A (H1N1) disease. PLoS One. 2010;5:e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Wang Q, Yang G, Lin C, Zhang Y, Yang P. Weight and prognosis for influenza A (H1N1) infection during the pandemic period between 2009 and 2011: a systematic review of observational studies with meta-analysis. Infect Dis (Lond) 2016;48:813–822. doi: 10.1080/23744235.2016.1201721. [DOI] [PubMed] [Google Scholar]
- 40.Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA. WHO working group for risk factors for severe H1N1 infection. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter SJ, Baranauskas MN, Fly AD. Considerations for obesity, vitamin D, and physical activity amidst the COVID-19 pandemic. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22838. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maier HE, Lopez R, Sanchez N, Ng S, Gresh L, Ojeda S. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218:1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honce R, Karlsson EA, Wohlgemuth N, Estrada LD, Meliopoulos VA, Yao J. Obesity-related microenvironment promotes emergence of virulent influenza virus strains. mBio. 2020;11 doi: 10.1128/mBio.03341-19. e03341–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos Muniz MG, Palfreeman M, Setzu N, Sanchez MA, Saenz Portillo P, Garza KM. Obesity exacerbates the cytokine storm elicited by Francisella tularensis infection of females and is associated with increased mortality. Biomed Res Int. 2018;2018 doi: 10.1155/2018/3412732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baetjer A. The effect of muscular fatigue upon resistance. Physiol Rev. 1932;12:453–468. [Google Scholar]
- 49.Horstmann DM. Acute poliomyelitis: relation of physical activity at the time of onset to the course of the disease. JAMA. 1950;142:236–241. doi: 10.1001/jama.1950.02910220016004. [DOI] [PubMed] [Google Scholar]
- 50.Levinson SO, Milzer A, Lewin P. Effect of fatigue, chilling and mechanical trauma on resistance to experimental poliomyelitis. Am J Hygiene. 1945;42:204–213. [Google Scholar]
- 51.Weinstein L. Poliomyelitis: a persistent problem. N Engl J Med. 1973;288:370–371. doi: 10.1056/NEJM197302152880714. [DOI] [PubMed] [Google Scholar]
- 52.Reyes MP, Lerner AM. Interferon and neutralizing antibody in sera of exercised mice with Coxsackievirus B-3 myocarditis. Proc Soc Exp Bio Med. 1976;151:333–338. doi: 10.3181/00379727-151-39204. [DOI] [PubMed] [Google Scholar]
- 53.Ilbäck NG, Friman G, Beisel WR, Johnson AJ, Berendt RF. Modifying effects of exercise on clinical course and biochemical response of the myocardium in influenza and tularemia in mice. Infect Immun. 1984;45:498–504. doi: 10.1128/iai.45.2.498-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis JM, Murphy EA, McClellan JL, Carmichael MD, Gangemi JD. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol Regul Integr Comp Physiol. 2008;295:R505–R509. doi: 10.1152/ajpregu.90319.2008. [DOI] [PubMed] [Google Scholar]
- 55.Murphy EA, Davis JM, Carmichael MD, Gangemi JD, Ghaffar A, Mayer EP. Exercise stress increases susceptibility to influenza infection. Brain Behav Immun. 2008;22:1152–1155. doi: 10.1016/j.bbi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Murphy EA, Davis JM, Brown AS, Carmichael MD, Carson JA, Van Rooijen N. Benefits of oat beta-glucan on respiratory infection following exercise stress: role of lung macrophages. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1593–R1599. doi: 10.1152/ajpregu.00562.2007. [DOI] [PubMed] [Google Scholar]
- 57.Shi Y, Shi H, Nieman DC, Hu Q, Yang L, Liu T. Lactic acid accumulation during exhaustive exercise impairs release of neutrophil extracellular traps in mice. Front Physiol. 2019;10:709. doi: 10.3389/fphys.2019.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chao CC, Strgar F, Tsang M, Peterson PK. Effects of swimming exercise on the pathogenesis of acute murine Toxoplasma gondii Me49 infection. Clin Immunol Immunopathol. 1992;62:220–226. doi: 10.1016/0090-1229(92)90075-y. [DOI] [PubMed] [Google Scholar]
- 59.Davis JM, Kohut ML, Colbert LH, Jackson DA, Ghaffar A, Mayer EP. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J Appl Physiol (1985) 1997;83:1461–1466. doi: 10.1152/jappl.1997.83.5.1461. [DOI] [PubMed] [Google Scholar]
- 60.Ceddia MA, Voss EW, Jr, Woods JA. Intracellular mechanisms responsible for exercise-induced suppression of macrophage antigen presentation. J Appl Physiol (1985) 2000;88:804–810. doi: 10.1152/jappl.2000.88.2.804. [DOI] [PubMed] [Google Scholar]
- 61.Woods JA, Ceddia MA, Kozak C, Wolters BW. Effects of exercise on the macrophage MHC II response to inflammation. Int J Sports Med. 1997;18:483–488. doi: 10.1055/s-2007-972668. [DOI] [PubMed] [Google Scholar]
- 62.Murphy EA, Davis JM, Brown AS, Carmichael MD, Van Rooijen N, Ghaffar A. Role of lung macrophages on susceptibility to respiratory infection following short-term moderate exercise training. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1354–R1358. doi: 10.1152/ajpregu.00274.2004. [DOI] [PubMed] [Google Scholar]
- 63.Frellstedt L, Waldschmidt I, Gosset P, Desmet C, Pirottin D, Bureau F. Training modifies innate immune responses in blood monocytes and in pulmonary alveolar macrophages. Am J Respir Cell Mol Biol. 2014;51:135–142. doi: 10.1165/rcmb.2013-0341OC. [DOI] [PubMed] [Google Scholar]
- 64.Kohut ML, Boehm GW, Moynihan JA. Prolonged exercise suppresses antigen-specific cytokine response to upper respiratory infection. J Appl Physiol (1985) 2001;90:678–684. doi: 10.1152/jappl.2001.90.2.678. [DOI] [PubMed] [Google Scholar]
- 65.Ceddia MA, Woods JA. Exercise suppresses macrophage antigen presentation. J Appl Physiol (1985) 1999;87:2253–2258. doi: 10.1152/jappl.1999.87.6.2253. [DOI] [PubMed] [Google Scholar]
- 66.Baron RC, Hatch MH, Kleeman K, MacCormack JN. Aseptic meningitis among members of a high school football team. JAMA. 1982;248:1724–1727. [PubMed] [Google Scholar]
- 67.Krikler DN, Zilberg B. Activity and hepatitis. The Lancet. 1966;2:1046–1047. doi: 10.1016/s0140-6736(66)92026-5. [DOI] [PubMed] [Google Scholar]
- 68.Roberts JA. Loss of form in young athletes due to viral infection. Br J Med. 1985;290:357–358. doi: 10.1136/bmj.290.6465.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts JA. Viral illnesses and sports performance. Sports Med. 1986;3:298–303. doi: 10.2165/00007256-198603040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharp JCM. Viruses and the athlete. Br J Sports Med. 1989;23:47–48. doi: 10.1136/bjsm.23.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Folsom RW, Littlefield-Chabaud MA, French DD, Pourciau SS, Mistric L, Horohov DW. Exercise alters the immune response to equine influenza virus and increases susceptibility to infection. Equine Vet J. 2001;33:664–669. doi: 10.2746/042516401776249417. [DOI] [PubMed] [Google Scholar]
- 72.Parker S, Brukner P, Rosier M. Chronic fatigue syndrome and the athlete. Sports Med Train Rehab. 1996;6:269–278. [Google Scholar]
- 73.Sanchez JL, Cooper MJ, Myers CA, Cummings JF, Vest KG, Russell KL. Respiratory infections in the U.S. military: recent experience and control. Clin Microbiol Rev. 2015;28:743–800. doi: 10.1128/CMR.00039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phillips M, Robinowitz M, Higgins JR, Boran KJ, Reed T, Virmani R. Sudden cardiac death in Air Force recruits. A 20-year review. JAMA. 1986;256:2696–2699. [PubMed] [Google Scholar]
- 75.Drory Y, Kramer MR, Lev B. Exertional sudden death in soldiers. Med Sci Sports Exerc. 1991;23:147–151. [PubMed] [Google Scholar]
- 76.Nieman DC, Lila MA, Gillitt ND. Immunometabolism: a multi-omics approach to interpreting the influence of exercise and diet on the immune system. Annu Rev Food Sci Technol. 2019;10:341–363. doi: 10.1146/annurev-food-032818-121316. [DOI] [PubMed] [Google Scholar]
- 77.Nieman DC. Immune response to heavy exertion. J Appl Physiol (1985) 1997;82:1385–1394. doi: 10.1152/jappl.1997.82.5.1385. [DOI] [PubMed] [Google Scholar]
- 78.Simpson RJ, Campbell JP, Gleeson M, Krüger K, Nieman DC, Pyne DB. Can exercise affect immune function to increase susceptibility to infection. Exerc Immunol Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- 79.Nieman DC, Groen AJ, Pugachev A, Simonson AJ, Polley K, James K. Proteomics-based detection of immune dysfunction in an elite adventure athlete trekking across the Antarctica. Proteomes. 2020;8:4. doi: 10.3390/proteomes8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 81.Nieman DC, Pence BD. Exercise immunology: future directions. J Sport Health Sci. 2020 doi: 10.1016/j.jshs.2019.12.003. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi Y, Liu T, Nieman DC, Cui Y, Li F, Yang L. Aerobic exercise attenuates acute lung injury through NET inhibition. Front Immunol. 2020;11:409. doi: 10.3389/fimmu.2020.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Warren KJ, Olson MM, Thompson NJ, Cahill ML, Wyatt TA, Yoon KJ. Exercise improves host response to influenza viral infection in obese and non-obese mice through different mechanisms. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta P, Bigley AB, Markofski M, Laughlin M, LaVoy EC. Autologous serum collected 1 h post-exercise enhances natural killer cell cytotoxicity. Brain Behav Immun. 2018;71:81–92. doi: 10.1016/j.bbi.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Agha NH, Mehta SK, Rooney BV, Laughlin MS, Markofski MM, Pierson DL. Exercise as a countermeasure for latent viral reactivation during long duration space flight. FASEB J. 2020;34:2869–2881. doi: 10.1096/fj.201902327R. [DOI] [PubMed] [Google Scholar]
- 86.Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:2187. doi: 10.3389/fimmu.2018.02187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 88.Nieman DC, Henson DA. Role of endurance exercise in immune senescence. Med Sci Sports Exerc. 1994;26:172–181. doi: 10.1249/00005768-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 89.Adams GR, Zaldivar FP, Nance DM, Kodesh E, Radom-Aizik S, Cooper DM. Exercise and leukocyte interchange among central circulation, lung, spleen, and muscle. Brain Behav Immun. 2011;25:658–666. doi: 10.1016/j.bbi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. 2014;39:160–171. doi: 10.1016/j.bbi.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 91.Nieman DC, Henson DA, Austin MD, Brown VA. Immune response to a 30-minute walk. Med Sci Sports Exerc. 2005;37:57–62. doi: 10.1249/01.mss.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- 92.Simpson RJ, Bigley AB, Agha N, Hanley PJ, Bollard CM. Mobilizing immune cells with exercise for cancer immunotherapy. Exerc Sport Sci Rev. 2017;45:163–172. doi: 10.1249/JES.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turner JE, Spielmann G, Wadley AJ, Aldred S, Simpson RJ, Campbell JP. Exercise-induced B cell mobilization: preliminary evidence for an influx of immature cells into the bloodstream. Physiol Behav. 2016;164:376–382. doi: 10.1016/j.physbeh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 94.Campbell JP, Riddell NE, Burns VE, Turner M, van Zanten JJ, Drayson MT. Acute exercise mobilizes CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 95.Sim YJ, Yu S, Yoon KJ, Loiacono CM, Kohut ML. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200:1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22:2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 97.Ledo A, Schub D, Ziller C, Enders M, Stenger T, Gärtner BC. Elite athletes on regular training show more pronounced induction of vaccine-specific T-cells and antibodies after tetravalent influenza vaccination than controls. Brain Behav Immun. 2020;83:135–145. doi: 10.1016/j.bbi.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 98.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 2020;128:87–99. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Araújo AL, Silva LC, Fernandes JR, Matias Mde S, Boas LS, Machado CM. Elderly men with moderate and intense training lifestyle present sustained higher antibody responses to influenza vaccine. Age (Dordr) 2015;37:105. doi: 10.1007/s11357-015-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 101.Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. 2018;17:e12750. doi: 10.1111/acel.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shanely RA, Nieman DC, Henson DA, Jin F, Knab AM, Sha W. Inflammation and oxidative stress are lower in physically fit and active adults. Scand J Med Sci Sports. 2013;23:215–223. doi: 10.1111/j.1600-0838.2011.01373.x. [DOI] [PubMed] [Google Scholar]
- 103.Wedell-Neergaard AS, Krogh-Madsen R, Petersen GL, Hansen AM, Pedersen BK, Lund R. Cardiorespiratory fitness and the metabolic syndrome: roles of inflammation and abdominal obesity. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nieman DC, Henson DA, Austin MD, Sha W. Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med. 2011;45:987–992. doi: 10.1136/bjsm.2010.077875. [DOI] [PubMed] [Google Scholar]
- 105.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 106.Inoue Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C. Risk and protective factors related to mortality from pneumonia among middle-aged and elderly community residents: the JACC Study. J Epidemiol. 2007;17:194–202. doi: 10.2188/jea.17.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong CM, Lai HK, Ou CQ, Ho SY, Chan KP, Thach TQ. Is exercise protective against influenza-associated mortality? PLoS One. 2008;3:e2108. doi: 10.1371/journal.pone.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neuman MI, Willett WC, Curhan GC. Physical activity and the risk of community-acquired pneumonia in US women. Am J Med. 2010;123:281. doi: 10.1016/j.amjmed.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams PT. Dose-response relationship between exercise and respiratory disease mortality. Med Sci Sports Exerc. 2014;46:711–717. doi: 10.1249/MSS.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wong CM, Chan WM, Yang L, Chan KP, Lai HK, Thach TQ. Effect of lifestyle factors on risk of mortality associated with influenza in elderly people. Hong Kong Med J. 2014;20(Suppl. 6):S16–S19. [PubMed] [Google Scholar]
- 111.Wu S, Ma C, Yang Z, Yang P, Chu Y, Zhang H. Hygiene behaviors associated with influenza-like illness among adults in Beijing, China: a large, population-based survey. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ukawa S, Zhao W, Yatsuya H, Yamagishi K, Tanabe N, Iso H, Tamakoshi A. Associations of daily walking time with pneumonia mortality among elderly individuals with or without a medical history of myocardial infarction or stroke: findings from the Japan Collaborative Cohort Study. J Epidemiol. 2019;29:233–237. doi: 10.2188/jea.JE20170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamer M, O'Donovan G, Stamatakis E. Lifestyle risk factors, obesity and infectious disease mortality in the general population: linkage study of 97,844 adults from England and Scotland. Prev Med. 2019;123:65–70. doi: 10.1016/j.ypmed.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Charland KM, Buckeridge DL, Hoen AG, Berry JG, Elixhauser A, Melton F. Relationship between community prevalence of obesity and associated behavioral factors and community rates of influenza-related hospitalizations in the United States. Influenza Other Respir Viruses. 2013;7:718–728. doi: 10.1111/irv.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song Y, Ren F, Sun D, Wang M, Baker JS, István B. Benefits of exercise on influenza or pneumonia in older adults: a systematic review. Int J Environ Res Public Health. 2020;17:E2655. doi: 10.3390/ijerph17082655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paulsen J, Å Askim, Mohus RM, Mehl A, Dewan A, Solligård E. Associations of obesity and lifestyle with the risk and mortality of bloodstream infection in a general population: a 15-year follow-up of 64 027 individuals in the HUNT Study. Int J Epidemiol. 2017;46:1573–1581. doi: 10.1093/ije/dyx091. [DOI] [PubMed] [Google Scholar]
- 117.Wang HE, Baddley J, Griffin RL, Judd S, Howard G, Donnelly JP. Physical inactivity and long-term rates of community-acquired sepsis. Prev Med. 2014;65:58–64. doi: 10.1016/j.ypmed.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams PT. Inadequate exercise as a risk factor for sepsis mortality. PLoS One. 2013;8:e79344. doi: 10.1371/journal.pone.0079344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lowder T, Padgett DA, Woods JA. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun. 2005;19:377–380. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 120.Sim YJ, Yu S, Yoon KJ, Loiacono CM, Kohut ML. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200:1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stravinskas Durigon T, MacKenzie B, Carneiro Oliveira-Junior M, Santos-Dias A, De Angelis K, Malfitano C. Aerobic exercise protects from Pseudomonas aeruginosa-induced pneumonia in elderly mice. J Innate Immun. 2018;10:279–290. doi: 10.1159/000488953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olivo CR, Miyaji EN, Oliveira ML, Almeida FM, Lourenço JD, Abreu RM. Aerobic exercise attenuates pulmonary inflammation induced by Streptococcus pneumoniae. J Appl Physiol (1985) 2014;117:998–1007. doi: 10.1152/japplphysiol.00290.2014. [DOI] [PubMed] [Google Scholar]
- 123.Rigonato-Oliveira NC, Mackenzie B, Bachi ALL, Oliveira-Junior MC, Santos-Dias A, Brandao-Rangel MAR. Aerobic exercise inhibits acute lung injury: from mouse to human evidence. Exercise reduced lung injury markers in mouse and in cells. Exerc Immunol Rev. 2018;24:36–44. [PubMed] [Google Scholar]
- 124.U.S. Department of Health and Human Services. Physical activity guidelines for Americans (2nd edition). Available at: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. [accessed 20.04.2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.