Abstract
The optimal conditions required for chitinase production from Bacillus licheniformis B307 strain, obtained from Syrian soil, were studied. Optimization experiments were carried out under submerged fermentation conditions, and colloidal chitin was the source of carbon. Luria broth medium supplied with 0.5% colloidal chitin was the optimum medium for chitinase production. The maximum chitinase yield was obtained at 30 °C, pH6, incubation time 14 days, and 150 rpm. The optimum chitinase activity was achieved at 60 °C and pH6. The chitinase activity with unmodified medium was 1.9 U/mL which then enhanced about eight folds to reach 14.2 U/mL under optimized submerged fermentation conditions. An extracellular chitinase of Bacillus licheniformis B307 was partially purified using ammonium sulfate precipitation followed by concentration with various sizes of concentrator tubes. The chitinase was partially purified 8.24 fold and specific enzyme activity increased 2.08 fold (2 U/mg). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of partial purified chitinase exhibited a molecular weight (Mr) near to 36 and 42kDa. These results make it possible to invest in this strain to produce chitinase to be used as antifungal, food additives and other applications.
Keywords: Biochemistry, Biotechnology, Microbiology, Molecular biology, Plant biology, Chitinase, Bacillus licheniformis, Optimization, Submerged culture, Purification
Biochemistry; Biotechnology; Microbiology; Molecular biology; Plant biology; Chitinase; Bacillus licheniformis; Optimization; Submerged culture; Purification
1. Introduction
Insoluble chitin is linear polymer of β-1,4-Nacetylglucosamine (GlcNAc), and it considers from the most naturally abundant polysaccharide and it is one of the major component of most fungal cell walls (Sadfi et al., 2001). Therefore, degradation of chitin is important step for recycling it as a nutrients in the nature. Hydrolytic enzymes Chitinases (EC 3.2.1.14) break down glycosidic bonds in chitin. Hence, Chitinases that are produced by microorganisms have crucial roles and wide range of applications. Chitinases can be used for bio-conversion of chitin into useful products in biotechnology, waste management, pharmaceuticals, biomedical applications, single-cell protein, isolation of protoplasts from fungi and yeast, drug delivery, and enzyme industry (Kumar et al., 2018; Patel et al., 2017; Patel and Goyal, 2017). In addition, there are many other important potential applications of chitinase, for instance it can be recruited as potential biocontrol agents against many fungal pathogens (Downing and Thomson, 2000; Gomaa, 2012), and possible future applications as food additives to increase shelf life (Hamid et al., 2013).
Bacillus species are one of the largest sources of bioactive natural compounds (Emmert and Handelsman, 1999), they form endospores and produce large amount of secondary metabolites, and classified as safe andbeneficial to grops and environment (Shoda, 2000). Several species of Bacillus have shown chitinolytic activities, such as B. pumilus (Agarwal et al., 2017), B. subtilis (Ashwini and Srividya, 2014; Narasimhan and Shivakumar, 2012; Saber et al., 2015), B. licheniformis strain LHH100 (Laribi-Habchi et al., 2015), B. licheniformis (Xiao et al., 2009), B. thuringiensis (Gomaa, 2012).
The production of extracellular chitinase, which has gained more attention across the world (Wang et al., 2006), can be enhanced by improvement of culture medium composition and fermentation conditions. Improved production conditions are critical to achieve optimal yield, productivity, and to reduce production costs (Abdel-Fattah et al., 2005). Currently, submerged fermentation (SmF) methods, which involve in production of enzymes by microorganisms in a liquid nutrient medium, are widely practiced to decrease infection and increase the possibility of greater yield of enzymes. Compared to solid state fermentation, SmF facilitates handling and controlling environmental factors such as pH and temperature. The fermentation medium optimization can be carried out through several strategies. One of them is the classical technique “one-factor-at-a-time” for chitinase production (Singh et al., 2017; Sukalkar et al., 2018).
This study aimed to optimize the culture and fermentation conditions of chitinase produced by Bacillus licheniformis B307 strain, that has chitinolytic and antifungal activity, by using SmF method. Partial purification for extracellular chitinase from the crude enzyme extract was also performed.
2. Materials and methods
2.1. Microorganism
Bacillus licheniformis B307 was isolated from Syrian soil (Salamiyah, N:34⁰ 57⁰ 48.5⁰) and it showed chitinolytic and antifungal activity against Botrytis cinerea (Akeed et al., 2019). The strain was maintained on nutrient agar (NA) at +4 °C and 20% glycerol at -80 °C. The inoculum of Bacillus strain was prepared by inoculating 20 mL of sterilized nutrient broth (NB) in 100 mL flask with loop full of pure Bacillus licheniformis B307 and incubated overnight at 30 °C and 200 rpm until the optical density at 600nm reached 0.15 which equals to 2 × 108 CFU/mL (Ammoneh et al., 2014).
2.2. Colloidal chitin preparation
The colloidal chitin was prepared according to the method described by Rodriguez-Kabana et al. (1983) with addition of 20g of chitin in 500mL of concentrated HCl. The chitin was added to the acid with vigorous stirring at 25 °C until it dissolved (1.5–2 h). The mixture was incubated in a water bath at 37 °C with gentle stirring till the mixture became transparent (0.5 h). The mixture then was filtered by using glass wool to remove impurities and undissolved particles. The filtrate was added to 5L cooled distilled water with stirring for 0.5 h, then it was placed at 4 °C without stirring for 24 h. The precipitate was collected and washed with distilled water by centrifuging until pH reached 5–6, and stored in the dark at 4 °C until used. 10 mL of colloidal chitin was taken and dried at 80 °C for 24 h to calculate the dry weight and determine the concentration of chitin.
2.3. Chitinase assay
Chitinolytic activity was estimated by dinitrosalicylic acid (DNS) method using colloidal chitin as a substrate according to the method described by Sadfi et al. (2001) with some modification. The reaction matrix had 0.5 mL of 1% colloidal chitin suspension in 0.1 M sodium acetate buffer pH5, 0.4 mL of enzyme solution. The mixture then incubated 30 min at 50 °C and then the reaction was terminated by 1mL DNS (NaOH 10 g/L, dinitrosalicylic acid C7H4N2O7 10 g/L, phenol C6H6O 2 g/L, and adding Na2SO30.05g/100mL and sodium potassium tartrate C4H4KNaO6.4H2O 20 g/100mL when using). The color of the mixture was developed by incubating it for 10 min at 100 °C. Centrifugation was performed at 7500 ×g for 10 min, then the supernatant adsorption was measured at 540nm. A standard curve was plotted using N-acetyl glucosamine (NAG, Sigma). One unit (U) of chitinase activity represent the amount of released enzyme of 1μmol N-acetyl glucosamine of colloidal chitin per min under reaction conditions.
2.4. Optimization of chitinase production
SmF experiments were performed in 100 mL flasks that contain 20 mL of the culture medium, and inoculated with 1 mL of bacterial inoculum. Various chemical and physical parameters were studied and their effect on production of the enzyme was recorded.
2.5. Effect of medium composition on chitinase production
Different types of media were implemented for optimal production of chitinase including NB medium, consists of (g/L): 1 beef extract, 2 yeast extract, 5 peptone, 5 sodium chloride; LB medium, consists of (g/L): 10tryptone, 5 yeast extract, 5 sodium chloride; M medium, consists of (g/L): 0.5 yeast extract, 1 (NH4)2SO4, 1 KH2PO4, 0.3 MgSO4.7H2O; and Y medium, consists of (g/L): 5casein, 5 galactose, 1 KH2PO4, 0.3 MgSO4.7H2O; Z medium, consists of (g/L):0.5 yeast extract, 2 Na2HPO4, 1 K2HPO4, 1 NH4Cl, 0.5 CaCl2.2H2O, 0.5 NaCl, 0.5 MgSO4.7H2O. Colloidal chitin was added to each of the mentioned media to a final concentration of 0.5%. All the culture media were adjusted at pH 7. The cultivation experiments were carried out in shaking incubator 150 rpm, 30 °C, for 5 days.
2.6. Effect of colloidal chitin concentration on chitinase production
Production medium was prepared with different concentrations of colloidal chitin (0.1, 0.3, 0.5, 0.7, 0.9 and 1%), and incubated under shaking conditions (150 rpm) at 30 °C.
2.7. Effect of incubation temperature on chitinase production
To determine the optimum incubation temperature for chitinase production, cultivation was carried out at different incubation temperatures ranged from 20 to 40 °C with variation of 5 °C.
2.8. Effect of pH on chitinase production
The influence of pH on chitinase production was monitored, where the pH of production mediums were adjusted before sterilization, from pH4 to pH9 at intervals of 1 unit, using NaOH 1M and HCl 1M.
2.9. Effect of incubation time on chitinase production
The optimum incubation time was determined for optimal chitinase production, inoculated flasks were incubated in a rotary shaker at 150 rpm and 30 °C. Every 24 h the culture filtrate was harvested and the enzyme activity was measured for 15 days of incubation time.
2.10. Effect of agitation speed on chitinase production
Optimum agitation speed was determined by incubating the production medium in Erlenmeyer flasks 100 mL at different agitation speed in shaker incubator ranging from 50 to 200 rpm with interval unit of 50 rpm.
3. Enzyme characterization
3.1. Effects of both pH and temperature on chitinase activity
To determine the optimal temperature for chitinase activity, the reaction mixtures incubated at different temperatures ranged from 20 to 80 °C and then assayed the enzyme's activity under standard conditions using colloidal chitin as a substrate. Also, optimal pH for chitinase activity was determined by measuring the activity at 60 °C within a range of pH from 4 to 9 using sodium-acetate buffer (pH4.0–6.0), sodium-phosphate buffer (pH7.0–8.0) and glycine-NaOH buffer (pH 9.0).
3.2. Partial purification of chitinase
The crude enzyme was partially purified from the culture supernatant using saturated ammonium sulfate (SAS). The precipitation was carried out at 4 °C in stirring conditions using different percentage of SASs 45%, 55%, 65%, 75%, and 85%. Precipitates were collected by centrifugation at 11500 ×g for 10 min at 4 °C and dissolved in a volume of buffer sodium-acetate pH6 equal to the size of crude enzyme. Protein determination in each fraction was performed according to Bradford (1976) and bovine serum albumin was used as a standard. An estimate of the chitinase activity was carried out according to the assay method described above. The optimum specific chitinase activity at a specific concentration of ammonium sulfate reflected the best concentration to attain maximum enzyme recovery. The fraction, which gave the highest specific chitinase activity, was taken to second partial purification using various concentrator tubes (Sartorius, vivaspin 2) MWCO 10kDa (MWCO: Molecular Weight Cut-off), MWCO 30kDa, and MWCO 50kDa. The best precipitation product was taken, first separated using 10kDa membrane and centrifugation 2500 ×g for 30 min, then the supernatant was separated using 30kDa membrane and centrifugation 2500 ×g for 30 min, after that the supernatant was separated using 50KDa membrane and 2500 ×g for 30 min finally the total protein and enzyme activity were determined for every fractions, and each were also subjected to electrophoresis on 12% polyacrylamide gel. The gel was stained using 0.5% Coomassie Brilliant Blue R250 (Sigma).
3.3. Statistical analysis
STATISTIC program (version 6 Stat soft, Inc. 2003) was used for statistical analyses at 5% level (P = 0.05). The data was subjected to analysis of variance (ANOVA; Tukey's HSD test) for the determination of differences in means between treatments. The percentages were analyzed by applying normal approximation test (analysis of proportions). All the experiments were repeated three times.
4. Results and discussion
4.1. Obtaining a new strain producing chitinases and antifungal activity
Chitinase has been previously isolated and characterized from a repertoire of Bacillus species (Khan et al., 2018; Oyeleye and Normi, 2018; Veliz et al., 2017). However, it is important to obtain chitinases with new characterization, and meet the application needs of these enzymes. Indeed, the chitinase that has been obtained in this study from the strain B307 has shown new properties compared to the ones in the literature (Table 1). In addition, the strain B307 possesses an antifungal property attributed to the chitinase activity which may broaden the use of this strain to biocontrol of some plant pathogens (Akeed et al., 2019). The strain B307 showed enzyme activity 14 ± 1.075 U/mL with temperature tolerance up to 60 °C. Comparing to other strains (Table 1) the strain B307 from this study has an increase in enzyme activity at 60 °C which makes it a potential candidate for industry.
Table 1.
Comparison between chitinase activity from B. licheniformis B307 and other works.
| Strain | Optimal condition for chitinase activity |
Chitinase activity U/mL | Reference | |
|---|---|---|---|---|
| pH | Temperature (°C) | |||
| Bacillus licheniformis A2 | 5 | 70 | 25.3 | Khiyami and Masmali (2008) |
| Bacillus licheniformis B307 | 6 | 60 | 14 ± 1.075 | Our work |
| Bacillus licheniformis JP2 | 6 | 60 | 0.05 | Keliat et al. (2016) |
| Bacillus licheniformis Mb-2 | 6 | 70 | 3.5 | Toharisman et al. (2005) |
| Bacillus cereus SV1 | 7 | 55 | 0.08 | Ghorbel-Bellaaj et al. (2011) |
| B. licheniformis | 8 | 40 | 23 | Gomaa (2012) |
4.2. Effect of medium composition on chitinase production
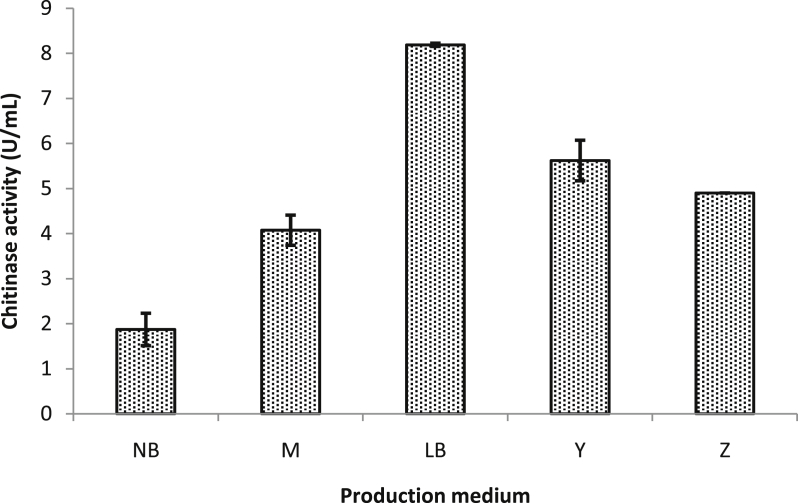
The components of the medium have an essential role in the growth and metabolism of microorganisms. Therefore, different broth media have been tested to optimize the culture medium for chitinase production. The results clearly showed that maximum chitinase production of 8.2 ± 0.035 U∖mL was observed when LB medium was used (Figure 1). In contrast, NB medium showed minimum chitinase yield (1.9 ± 0.35 U∖mL) (DF = 4, F-value = 178.256, P˂0.0001). It is known that the LB nutrient medium is commonly used in the cultivation of many species of bacteria because it permits fast and good growth yields for them, and the addition of colloidal chitin to this medium has stimulated the developing bacteria to produce chitinase. Similar works reported that colloidal chitin in LB has yielded maximum chitinase production by Bacillus subtilis, from all the tested media, LB with colloidal chitin showed also more productive in both Aeromonas hydrophila and Aeromonas punctate strains (Karunya, 2011; Kuddus and Ahmad, 2013).
Figure 1.
Effect of medium composition on chitinase production (30 °C and 5 days incubation with agitation 150rpm).
4.3. Effect of colloidal chitin concentration on chitinase production
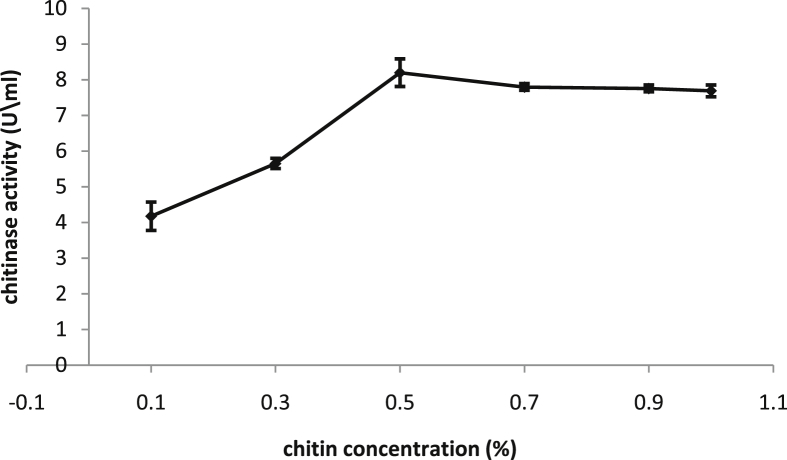
Production medium optimization is an essential step not only to maximize the yield and productivity, but also to minimize the production costs (Singh et al., 2017). Therefore, several concentrations of colloidal chitin were tested. Results showed that colloidal chitin concentration influenced the production of chitinase by B. licheniformis B307. There were obvious coloration between enzyme production and colloidal chitin concentration where the maximum enzyme production of 8,19 ± 0.655U∖mL (DF = 5, F-value = 40.287, P˂0.0001) was observed when 0.5% of colloidal chitin was used (Figure 2). However, no significant increase in enzyme yield beyond 0.5% was noticed. These results are in agreement with earlier findings of chitin which is a vital factor in inducing high chitinase production from microorganisms (Soiuza et al., 2005). Wen et al., (2002) reported that the addition of 0.5% colloidal chitin or more has induced the maximum chitinase production in Bacillus sp. NCTV2, as well, Abirami et al., (2016) reported that colloidal chitin concentration between 0.5-1% enhanced the chitinase production considerably by B. licheniformis SSCL10. In addition, the highest chitinase production by B. pumilus was obtained by using a medium supplemented with 0.5% chitin (Bhattacharya et al., 2016).
Figure 2.
Effect of colloidal chitin concentration on chitinase production by B. licheniformis B307.
4.4. Effect of incubation temperature on chitinase production
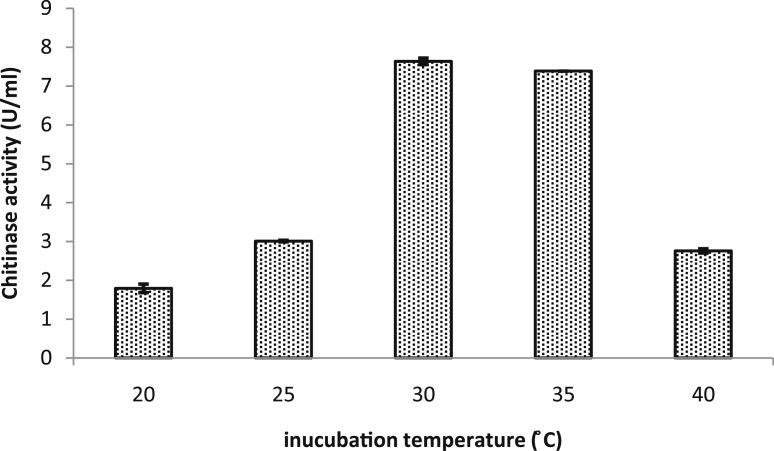
The changes in incubation temperature within the studied range (20–40 °C) showed significant effect on the chitinase yield produced by B. licheniformis strain B307. The maximum production was found to be 7.6 ± 0.145 and 7.3 U/mL (DF = 4, F-value = 1706.641, P˂0.0001) at 30 °C and 35 °C respectively, while significant reduction in the production was observed when the temperature increased or decreased from the previous values (Figure 3). Temperature has an impact on various biological processes, therefore the growth of bacteria and the production of enzymes are influenced by the incubation temperature modification. Narasimhan and Shivakumar (2012) reported maximum chitinase production from B. subtilis at 30 °C, while a number of researchers reported that the optimum temperature for production of chitinase from Bacillus licheniformis, B. laterosporus, B. pumilus and B. subtilis is 35 °C (Jholapara et al., 2013; Shanmugaiah et al., 2008; Bhattacharya et al., 2016; Karunya, 2011).
Figure 3.
Effect of incubation temperature on chitinase production by B. licheniformis B307. Error bars represent the standard error of three replicates.
4.5. Effect of pH on chitinase production
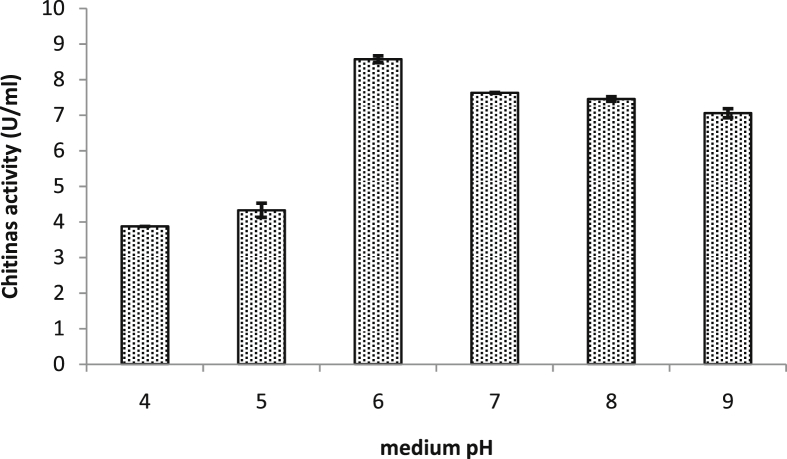
The effect of different initial pH of fermentation medium on the yield of chitinase produced by B. licheniformis B307 was tested. The maximum chitinase production was found to be at pH6 with the activity of 8.56 ± 0.165U∖mL, while the minimum chitinase activity 3.87 U∖mL (DF = 5, F-value = 319.445, P˂0.0001) was observed at pH4. However, yield at pH7 to pH9 has not decreased significantly (Figure 4). Culture medium pH has a great effect on microbe growth and metabolism (Pan et al., 2019), therefore the pH should be adjusted when preparing the fermentation medium. Similar researches referred to the variation in the optimum pH for the production of chitinase from Bacillus. For example, pH6 was optimal for chitinase production from Bacillus subtilis W-118 (Wang et al., 2006), pH7 from B. thuringiensis (Gomaa, 2012) and pH8 from B. laterosprous (Shanmugaiah et al., 2008).
Figure 4.
Effect of initial pH of the fermentation media on chitinase production by B. licheniformis B307. Error bars represent the standard error of three replicates.
4.6. Effect of incubation time on chitinase production
The maximum chitinase yield was found after incubation for 14 days (9.54 ± 0.04 U/mL) (DF = 14, F-value = 102.24, P˂0.0001), after that, the production began to decline. In many SmF processes, when the production reaches the highest yield, further increase in incubation time can reduce the yield due to the degradation of metabolites (Hao et al., 2012).
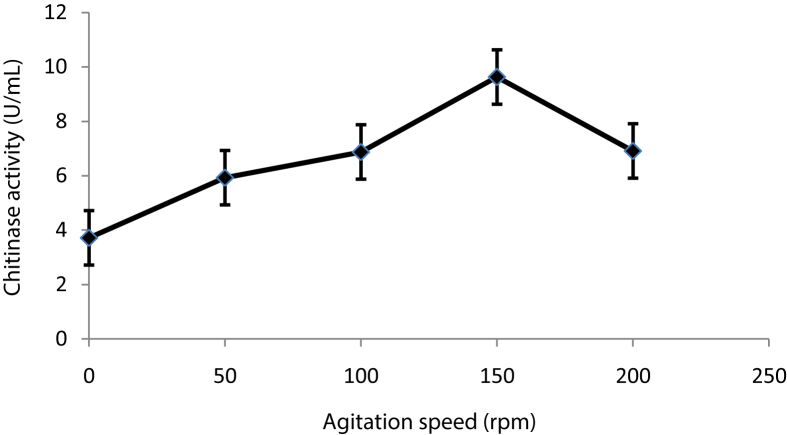
4.7. Effect of agitation speed on chitinase production
Chitinase production by shaking microorganisms under SmF is better than using static condition (Shivalee et al., 2018). The maximum chitinase production by B. licheniformis B307 was observed at agitation speed of 150 rpm (9.63 ± 0.88 U∖mL) (DF = 4, F-value = 41.715, P˂0.0001). The chitinase production was increased as there were increases in the agitation speeds up to 150 rpm, then decreased with increased speed (Figure 5). Similarly, maximum chitinase production by Bacillus sp. was found at 150 rpm (Gupta et al., 2017).
Figure 5.
Effect of agitation speed on chitinase production by B. licheniformis B307. Error bars represent the standard error of three replicates.
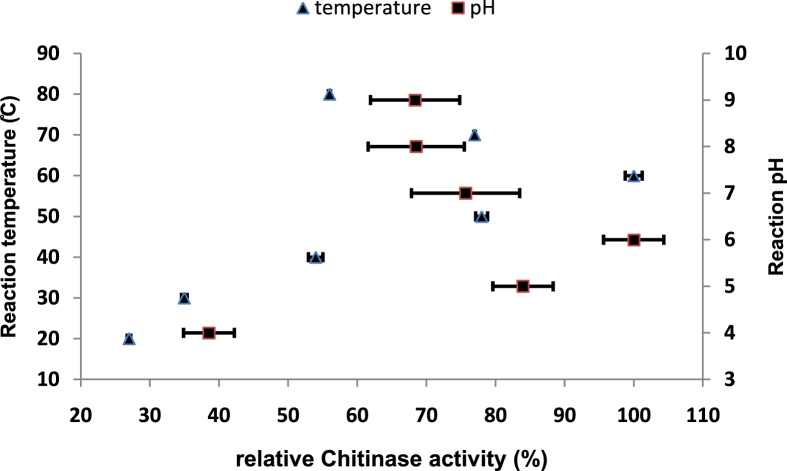
4.8. Effect of temperature on chitinase activity
Reaction temperature is one of the determining factors in the effectiveness of enzyme activities. In this study, results showed that the optimum temperature for chitinase activity produced by B. licheniformis B307 strain was at 60 °C (10.1 ± 0.215 U/mL) (DF = 6, F-value = 1287, P˂0.0001). So, when the temperature increased or decreased from 60 °C, the activity of chitinase gradually reduced. The relative activity of chitinase was 80% at 50 and 70 °C (Figure 6). In Table 1, the optimal temperature for chitinase activity from B. licheniformis B307 was compared to chitinases produced from other strains in similar works.
Figure 6.
Effect of temperature and different pH values on chitinase activity produced by B. licheniformis B307. Error bars represent the standard error of three replicates.
4.9. Effect of pH on chitinase activity
Enzymes activities are affected by changes in the pH value of the reaction, and the point that achieves the highest enzymatic efficiency is known as the optimum pH. Therefore, the pH- relative activity of B. licheniformis B307 chitinase was determined in the range pH4.0- pH9.0 (Figure 6), and the optimum pH was found to be at pH6 (14 ± 1.075 U/mL) (DF = 5, F-value = 12.34, P˂0.0002). In Table 1, the optimal pH for chitinase activity from B. licheniformis B307 was compared to chitinases produced from other strains in similar works.
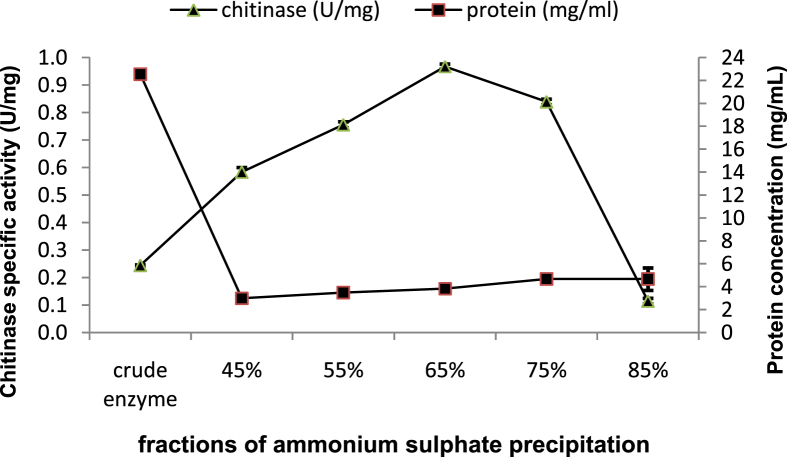
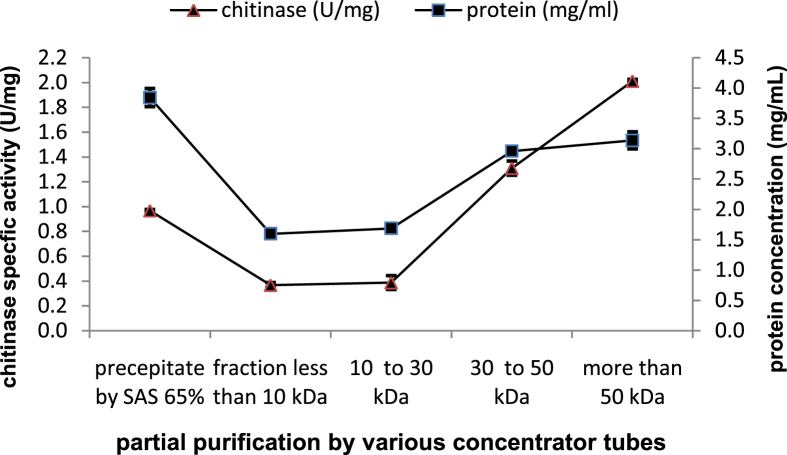
4.10. Partial purification of chitinase
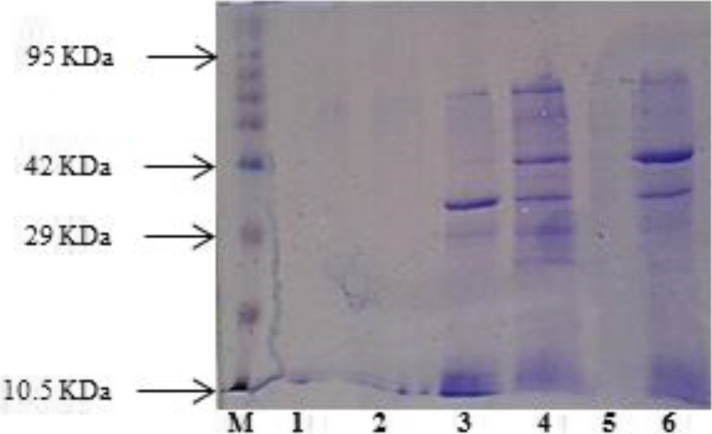
In the first step, the enzyme was partially purified by precipitation with different concentrations of SAS. Maximum specific enzyme activity 0.97U/mg was observed in the fraction containing 65% of SAS, while in the crude enzyme it was 0.24U/mg (Figure 7). It was found to increase 3.96 fold enzyme activity with 67.52% yield after precipitation with 65% of SAS. After that, in the second step for partial purification of chitinase from B. licheniformis B307, the fraction of 65% SAS which gave the highest specific chitinase activity was concentrated by various concentrator tubes. The fraction containing the supernatant of tube MWCO 50kDa showed the highest chitinase specific activity 2U/mg and the specific enzyme activity was increased 2.08 fold with 169% yield from 65% SAS (Figure 8). SDS-PAGE of denatured partial purified chitinase exhibited an Mr near to 36 and 42kDa (Figure 9). Many researches have recorded isolation and purification of chitinases produced by Bacillus of different molecular weights: 89, 76, 72, 66, 62, 59, 53, 49, 42, and 36kDa (Kudan and Pichyangkura, 2009; Liu et al., 2010; Takayanagi et al., 1991; Trachuk et al., 1996).
Figure 7.
Chitinase activity and protein concentration of various fractions after precipitation crude enzyme with different concentrations of SAS. Error bars represent the standard error of three replicates.
Figure 8.
Chitinase activity and protein concentration of various fractions after concentration of 65% SAS by various concentrator tubes. Error bars represent the standard error of three replicates.
Figure 9.
SDS-PAGE analysis for partial purification of chitinase from Bacillus licheniformis B307. 1: Flow-throw of MWCO 10kDa tube, 2: fraction between 10kDa and 30kDa, 3: fraction between 30kDa and 50kDa, 4: supernatant of MWCO 50kDa tube, 5: nothing, 6: product of precipitation by 65% SAS. See supplementary Figure 1 for full image.
5. Conclusion
In this study, the optimal conditions for chitinase production from B. licheniformis B307strain were determined using SmF method. A partial characterization was also performed, where the optimum temperature and pH were determined to obtain the best chitinase activity. A partial purification of the extracellular chitinase in the crude extract was also performed, where chitinase was purified to reach 8.24 fold comparing to the crude extract with specific activity 2U/mg for the partial purified enzyme.
Depending on its properties, the chitinase obtained by B. licheniformis B307 strain, has a potential use in the industries that have obstacles in using chitinase enzymes at pH6 and/or temperature up to 50–70 °C degrees such as pharmaceuticals, biocontrol and biotechnology applications among others.
Declarations
Author contribution statement
Yasser Akeed: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Faiza Atrash: Conceived and designed the experiments; Analyzed and interpreted the data.
Walid Naffaa: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Dr. Muhammad Hawat and Mrs. Tasneem Malla for their linguistic review of the manuscript.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplement _Fig1.
References
- Abdel-Fattah Y.R., Saeed H.M., Gohar Y.M., El-Baz M.A. Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process Biochem. 2005;40(5):1707–1714. [Google Scholar]
- Abirami S., Yogalsakshmi K., Pushpa A.S.R., Kananan M. Screening and identification of chitin degrading bacteria from shrimp shell waste dumping soil environment and its media optimization for chitinase enzyme production. World J. Pharm. Pharmaceut. Sci. 2016;5(11):743–757. [Google Scholar]
- Agarwal M., Dheeman S., Dubey R.C., Kumar P., Maheshwari D.K., Bajpai V.K. Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol. Res. 2017;205:40–47. doi: 10.1016/j.micres.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Akeed Y., Atrash F., Naffaa W. Isolation and identification of Bacillus spp. Syrian soils and testing their antifungal activity against Botrytis cinerea in vitro. J. Arid Environ. 2019 (in press) [Google Scholar]
- Ammoneh H., Harba M., Akeed Y., Al-Halabi M., Bakri Y. Isolation and identification of local Bacillus isolates for xylanase biosynthesis. Iran. J. Microbiol. 2014;6(2):127. [PMC free article] [PubMed] [Google Scholar]
- Ashwini N., Srividya S. Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3 Biotech. 2014;4(2):127–136. doi: 10.1007/s13205-013-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Das A., Samadder S., Rajan S.S. Biosynthesis and characterization of a thermostable, alkali-tolerant chitinase from Bacillus pumilus JUBCH08 displaying antagonism against phytopathogenic Fusarium oxysporum. 3 Biotech. 2016;6(1):87. doi: 10.1007/s13205-016-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Downing K.J., Thomson J.A. Introduction of the Serratia marcescens chiA gene into an endophytic Pseudomonas fluorescens for the biocontrol of phytopathogenic fungi. Can. J. Microbiol. 2000;46(4):363–369. doi: 10.1139/w99-147. [DOI] [PubMed] [Google Scholar]
- Emmert E.A.B., Handelsman J. Biocontrol of plant disease: a (Gram-) positive perspective. FEMS Microbiol. Lett. 1999;171(1):1–9. doi: 10.1111/j.1574-6968.1999.tb13405.x. [DOI] [PubMed] [Google Scholar]
- Ghorbel-Bellaaj O., Manni L., Jellouli K., Hmidet N., Nasri M. Optimization of protease and chitinase production by Bacillus cereus SV1 on shrimp shell waste using statistical experimental design. Biochemical and molecular characterization of the chitinase. Ann. Microbiol. 2011;62(3):1255–1268. [Google Scholar]
- Gomaa E.Z. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: their potential in antifungal biocontrol. J. Microbiol. 2012;50(1):103–111. doi: 10.1007/s12275-012-1343-y. [DOI] [PubMed] [Google Scholar]
- Gupta N., Kumar A., Laksh S.A., Rana M. Process optimization of extracellular chitinase production from Bacillus sp. Isolated from fish waste Dumping site. Eur. J. Pharmaceut. Med. Res. 2017;4(9):474–480. [Google Scholar]
- Hamid R., Khan M.A., Ahmad M., Ahmad M.M., Abdin M.Z., Musarrat J., Javed S. Chitinases: an update. J. Pharm. BioAllied Sci. 2013;5(1):21. doi: 10.4103/0975-7406.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z., Cai Y., Liao X., Zhang X., Fang Z., Zhang D. Optimization of nutrition factors on chitinase production from a newly isolated Chitiolytic bacter meiyuanensis SYBC-H1. Braz. J. Microbiol. 2012;43(1):177–186. doi: 10.1590/S1517-838220120001000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jholapara R.J., Mehta R.S., Sawant C.S. Optimization of cultural conditions for chitinase production from chitinolytic bacterium isolated from soil sample. Int. J. Pharm. Biol. Sci. 2013;4(2):464–471. [Google Scholar]
- Karunya S.K. Optimization and purification of chitinase produced by Bacillus subtilis and its antifungal activity against plant pathogens. Int. J. Pharmaceut. Biol. Arch. 2011;2(6):1680–1685. [Google Scholar]
- Keliat J.M., Suryanto D., Munir E. Characterization of extraceluller chitinase produced by Bacillus licheniformis JP2 from Penen Hot Springs, North Sumatera. Front. Eng. Manag. 2016;2(5):24–27. [Google Scholar]
- Khan N., Martínez-Hidalgo P., Ice T.A., Maymon M., Humm E.A., Nejat N. Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front. Microbiol. 2018;9:2363. doi: 10.3389/fmicb.2018.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiyami M., Masmali I. Characteristics of thermostable chitinase enzymes of Bacillus licheniformis isolated from red Palm Weavil Gut. Aust. J. Basic Appl. Sci. 2008;2(4):943–948. [Google Scholar]
- Kudan S., Pichyangkura R. Purification and characterization of thermostable chitinase from Bacillus licheniformis SK-1. Biotechnol. Appl. Biochem. 2009;157:23. doi: 10.1007/s12010-008-8328-7. [DOI] [PubMed] [Google Scholar]
- Kuddus M., Ahmad I.Z. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol. 2013;11(1):39–46. [Google Scholar]
- Kumar A., Kumar D., George N., Sharma P., Gupta N. A process for complete biodegradation of shrimp waste by a novel marine isolate Paenibacillus sp. AD with simultaneous production of Chitinase and chitin oligosaccharides. Int. J. Biol. Macromol. 2018;109:263–272. doi: 10.1016/j.ijbiomac.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Laribi-Habchi H., Bouanane-Darenfed A., Drouiche N., Pauss A., Mameri N. Purification, characterization, and molecular cloning of an extracellular chitinase from Bacillus licheniformis stain LHH100 isolated from waste water samples in Algeria. Int. J. Biol. Macromol. 2015;72:1117–1128. doi: 10.1016/j.ijbiomac.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Liu D., Cai J., Xie C.C., Liu C., Chen Y.H. Purification and partial characterization of a 36-kDa chitinase from Bacillus thuringiensis sub sp. colmeri, and its biocontrol potential. Enzym. Microb. Technol. 2010;46(3-4):252–256. [Google Scholar]
- Narasimhan A., Shivakumar S. Optimization of chitinase produced by a biocontrol strain of Bacillus subtilis using Plackett-Burman design. Eur. J. Exp. Biol. 2012;2(4):861–865. [Google Scholar]
- Oyeleye A., Normi Y.M. Chitinase: diversity, limitations, and trends in engineering for suitable applications. Biosci. Rep. 2018;38(4) doi: 10.1042/BSR20180323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R., Bai X., Chen J., Zhang H., Wang H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: a literature review. Front. Microbiol. 2019;10:294. doi: 10.3389/fmicb.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.K., Singhania R.R., Pandey A. Production, purification, and application of microbial enzymes. Enzym. Microb. Technol. 2017:13–41. [Google Scholar]
- Patel S., Goyal A. Chitin and chitinase: role in pathogenicity, allergenicity and health. Int. J. Biol. Macromol. 2017;97:331–338. doi: 10.1016/j.ijbiomac.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Kabana R., Godoy G., Morgan-Jones G., Shelby R.A. The determination of soil chitinase activity: conditions for assay and ecological studies. Plant Soil. 1983;75(1):95–106. [Google Scholar]
- Saber W.I., Ghoneem K.M., Al-Askar A.A., Rashad Y.M., Ali A.A., Rashad E.M. Chitinase production by Bacillus subtilis ATCC 11774 and its effect on biocontrol of Rhizoctonia diseases of potato. Acta Biol. Hung. 2015;66(4):436–448. doi: 10.1556/018.66.2015.4.8. [DOI] [PubMed] [Google Scholar]
- Sadfi N., Cherif M., Fliss I., Boudabbous A., Antoun H. Evaluation of bacterial isolates from salty soils and Bacillus thuringiensis strains for the biocontrol of Fusarium dry rot of potato tubers. J. Plant Pathol. 2001;83:101–117. [Google Scholar]
- Shanmugaiah V., Mathivanan N., Balasubramanian N., Manoharan P.T. Optimization of cultural conditions for production of chitinase by Bacillus laterosporous MML2270 isolated from rice rhizosphere soil. Afr. J. Biotechnol. 2008;7(15) [Google Scholar]
- Shivalee A., Lingappa K., Mahesh D. Influence of bioprocess variables on the production of extracellular chitinase under submerged fermentation by Streptomyces pratensis strain KLSL55. J. Genet. Eng. Biotechnol. 2018;16(2):421–426. doi: 10.1016/j.jgeb.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoda M. Bacterial control of plant disease. J. Biosci. Bioeng. 2000;89:515–521. doi: 10.1016/s1389-1723(00)80049-3. [DOI] [PubMed] [Google Scholar]
- Singh V., Haque S., Niwas R., Srivastava A., Pasupuleti M., Tripathi C.K.M. Strategies for fermentation medium optimization: an in-depth review. Front. Microbiol. 2017;7:2087. doi: 10.3389/fmicb.2016.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiuza R.F., Soares R.M., Nascimento R.P., Coelho R.R., Gomes R.C. Effect of different carbon sources on endochitinase production by Colletotrichum gloeosporioides. Curr. Microbiol. 2005;51:16–21. doi: 10.1007/s00284-005-4506-9. [DOI] [PubMed] [Google Scholar]
- Sukalkar S.R., Kadam T.A., Bhosale H.J. Optimization of chitinase production from Streptomyces macrosporeus m1. Res. J. Life Sci. Bioinf. Pharmaceut. Chem. Sci. 2018;4(1):106–114. [Google Scholar]
- Takayanagi T., Ajisaka K., Takiguchi Y., Shimahara K. Isolation and characterization of thermostable chitinases from Bacillus licheniformis X-7u. Biochim. Biophys. Acta BBA – Protein Struct. Mol. Enzymol. 1991;1078:404–410. doi: 10.1016/0167-4838(91)90163-t. [DOI] [PubMed] [Google Scholar]
- Trachuk L.A., Revina L.P., Shemyakina T.M., Chestukhina G.G., Stepanov V.M. Chitinases of Bacillus licheniformis B-6839: isolation and properties. Can. J. Microbiol. 1996;42:307–315. [Google Scholar]
- Toharisman A., Suhartono M.T., Spindler-Barth M., Hwang J.K., Pyun Y.R. Purification and characterization of a thermostable chitinase from Bacillus licheniformis Mb-2. Can. J. Microbiol. 2005;21(5):733–738. [Google Scholar]
- Veliz E.A., Martínez-Hidalgo P., M Hirsch A. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 2017;3(3):689–705. doi: 10.3934/microbiol.2017.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.L., Lin T.Y., Yen Y.H., Liao H.F., Chen Y.J. Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr. Res. 2006;341:2507–2515. doi: 10.1016/j.carres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Wen C.M., Tseng C.S., Cheng C.Y., Li Y.K. Purification, characterization and cloning of a chitinases from Bacillus sp. NCTU2. Biotechnol. Appl. Biochem. 2002;35:213–219. doi: 10.1042/ba20020001. [DOI] [PubMed] [Google Scholar]
- Xiao L., Xie C.C., Cai J., Lin Z.J., Chen Y.H. Identification and characterization of a chitinase-produced Bacillus showing significant antifungal activity. Curr. Microbiol. 2009;58(5):528–533. doi: 10.1007/s00284-009-9363-5. [DOI] [PubMed] [Google Scholar]