Abstract
Hutchinson–Gilford progeria syndrome (HGPS), commonly called progeria, is an extremely rare disorder that affects only one child per four million births. It is characterized by accelerated aging in affected individuals leading to premature death at an average age of 14.5 years due to cardiovascular complications. The main cause of HGPS is a sporadic autosomal dominant point mutation in LMNA gene resulting in differently spliced lamin A protein known as progerin. Accumulation of progerin under nuclear lamina and activation of its downstream effectors cause perturbation in cellular morphology and physiology which leads to a systemic disorder that mainly impairs the cardiovascular system, bones, skin, and overall growth. Till now, no cure has been found for this catastrophic disorder; however, several therapeutic strategies are under development. The current review focuses on the overall progress in the field of therapeutic approaches for the management/cure of HGPS. We have also discussed the new disease models that have been developed for the study of this rare disorder. Moreover, we have highlighted the therapeutic application of extracellular vesicles derived from stem cells against aging and aging-related disorders and, therefore, suggest the same for the treatment of HGPS.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00167-3) contains supplementary material, which is available to authorized users.
Keywords: Hutchinson–Gilford progeria syndrome, Aging, Progerin, Therapeutics, Stem cells, Extracellular vesicles
Introduction
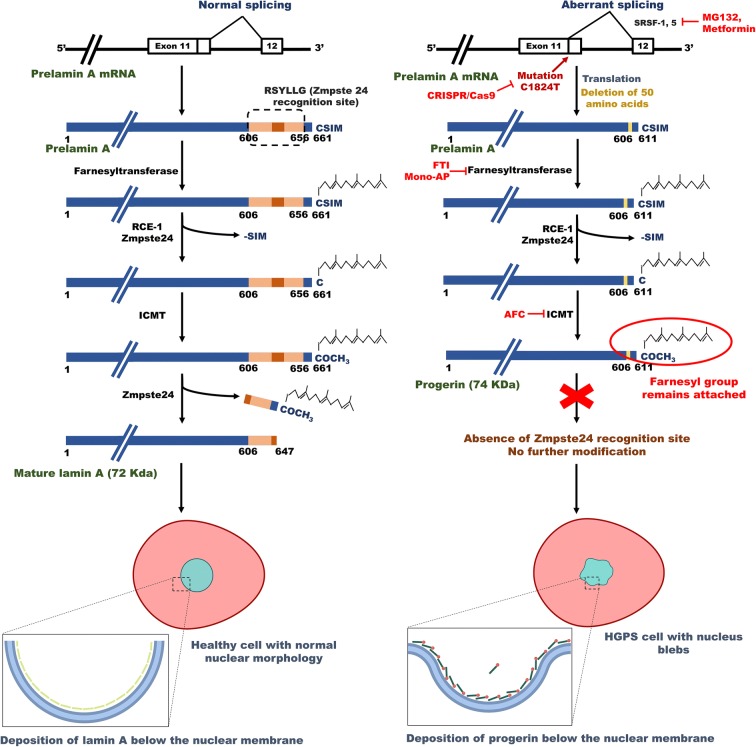
Hutchinson–Gilford progeria syndrome (HGPS), commonly called progeria, is a segmented laminopathy (McKenna et al. 2013) that occurs due to an autosomal dominant mutation (1824C>T, p. G608G) in the exon 11 of LMNA gene located at chromosome 1q21.2-q21.3 (De Sandre-Giovannoli et al. 2003; Eriksson et al. 2003; Goldman et al. 2004). This mutation generates a cryptic splice site that leads to deletion of 50 amino acids near the C terminus of prelamin A, resulting in the loss of recognition site (RSYLLG motif) of ZMPSTE24 endoprotease (or FACE-1 in human). This enzyme cleaves 14 amino acids at the C-terminal of wild-type prelamin A to produce functional lamin A protein. The deletion of ZMPSTE24 endoprotease recognition site in mutated prelamin A leads to the generation of a partially processed protein, “progerin,” that retains the farnesylated as well as carboxymethylated –CAAX motif at its C-terminal. This farnesylated end causes the accumulation of progerin underneath the inner nuclear membrane. Figure 1 demonstrates the molecular mechanism of progerin synthesis in the cell.
Fig. 1.
Biogenesis of lamin A and progerin in the cell. The left picture depicts the expression of normal lamin A protein from the LMNA gene. The normal prelamin A protein undergoes extensive modifications like farnesylation and carboxymethylation at the C-terminal. Finally, the C-terminal is removed by the activity of Zmpste24 endopeptidase to produce mature lamin A. Therefore, the mature lamin A protein does not contain the farnesyl group. The right picture shows the formation of progerin from mutant LMNA gene. Mutation in LMNA gene in exon 11 (1824 C>T) leads to generation of an aberrant splicing site. Splicing at this abnormal site causes deletion of 50 amino acids (607–656) in the prelamin A protein. As a result, prelamin A Δ50 loses the Zmpste24-recognition site, which causes the retention of modified C-terminal with the farnesyl group. This protein is now called as progerin. Accumulation of progerin at nuclear lamina leads to defects in nuclear morphology and function. Various therapeutic interventions may interfere with the biogenesis of progerin. The CRISPR–Cas9 system can correct the causative mutation, whereas MG132 and metformin can inhibit the aberrant splicing. FTIs (farnesyl transferase inhibitors) and mono-AP (mono-aminopyrimidines) can thwart farnesylation of progerin and AFC (N-acetyl-S-farnesyl-l-cysteine) inhibits methylation of the C-terminal of progerin. Abbreviations: ICMT, isoprenylcysteine carboxyl methyltransferase; RCE-1, Ras-converting enzyme; SRSF-1 and -5, serine/arginine-rich splicing factor-1 and -5
The aggregation of progerin renders the nucleus susceptible for mechanical damage, nuclear blebbing, congregation of nuclear pore complex, and stiffness and thickening of nuclear lamina (Dahl et al. 2006; Goldman et al. 2004; Verstraeten et al. 2007). Progerin interacts with several proteins of nuclear envelop (NE) and LINC (linker of the nucleoskeleton and cytoskeleton) complex that alter the mechano-sensitivity of NE as well as morphology (Chen et al. 2014; Lu and Djabali 2018). There are several other cellular and molecular consequences that result in the pathophysiology of this disease at the cellular and systemic levels. These changes include disruption of nucleocytoplasmic Ran gradient (Kelley et al. 2011), disturbance in nuclear export (García-Aguirre et al. 2019), alteration in microtubular network (Larrieu et al. 2018), defective polarity (Chang et al. 2019), reduction in autophagic proteolysis (Lu and Djabali 2018), mitochondrial dysfunction and oxidative stress (Kubben et al. 2016; Rivera-Torres et al. 2013; Sieprath et al. 2015), lowered PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) expression level (Xiong et al. 2016), stress in the endoplasmic reticulum (ER) and unfolded protein response (Hamczyk et al. 2019), DNA damage (Gonzalo and Kreienkamp 2015; Liu et al. 2005), shortening of telomeres (Gonzalez-Suarez et al. 2009), defects in purine synthesis (Mateos et al. 2018), loss of peripheral heterochromatin (Gesson et al. 2016), impaired epigenetic regulation (Shumaker et al. 2006), chromatin remodeling (Pegoraro et al. 2009), alteration in cell signaling pathways (Aliper et al. 2015), inhibition of cell cycle progression and increased apoptosis (Bridger and Kill 2004), leakage of DNA into cytoplasm (Kreienkamp et al. 2018), upregulation of inflammatory pathways (Osorio et al. 2012), depletion of stem cell reserves for tissue repairing (Rosengardten et al. 2011), defects in self-renewal and differentiation capacity of stem cells, and disbalance in the synthesis of extracellular matrix (ECM) components and remodeling enzymes (Csoka et al. 2004; Vidak et al. 2015).
The prevalence of HGPS is one in 20 million people; however, the incidence of HGPS among newborns is one in four million births (Gordon 2019c). There is no gender, geographical, or ethnic predisposition (Sinha et al. 2014). Till 01 December 2019, there were 162 known live children with progeria, i.e., 125 with classical HGPS and 37 with progeroid laminopathy (Gordon 2019b). Since HGPS occurs due to a sporadic de novo mutation, which is extremely rare, there are no known risk factors associated with this disorder. However, if the parents have a child with LMNA pathogenic variant, the chance of having a second baby with HGPS increases by 2–3% due to parental germline mosaicism (Gordon 2019c; Gordon et al. 2003).
Apart from observed heterozygous point mutation (C1824T) in classical HGPS, other mutations in LMNA gene cause “atypical progeria syndrome” with symptoms similar to classical HGPS but of varying severity (Doubaj et al. 2012; Ghosh and Zhou 2014; Serebryannyy and Misteli 2018). Mutation in ZMPSTE24 is also reported to cause accumulation of farnesylated prelamin A leading to a more severe form of HGPS—restrictive dermatopathy (Mazereeuw-Hautier et al. 2007; Moulson et al. 2005; Navarro et al. 2005; Navarro et al. 2004; Navarro et al. 2014; Wang et al. 2016). Zmpste24-deficient mice also share phenotypic similarities with that of HGPS human patients (Fong et al. 2004). LMNA aberrant splicing also occurs in the naturally aged wild-type cells, resulting in accumulation of progerin at a low level (Scaffidi and Misteli 2006). Therefore, HGPS may share certain common mechanistic pathways with aging.
Clinical manifestation of HGPS
HGPS is a systemic disorder that affects the majority of the organs of the body including the skin, bone, skeletal muscle, adipose tissues, heart, and large and small arteries; however, certain tissues and organs like bones, joints, and the blood vascular system are more prominently affected (Gordon et al. 2003, 2014; Hamczyk and Andrés 2018; Lowenstein and Bennett 2019; Vidak and Foisner 2016). The children affected with HGPS start exhibiting the symptoms of the disease at the age of 1–2. The affected children present growth reduction, alopecia, loss of hairs of eyebrows and eyelashes, prominent scalp veins, sclerodermatous skin, loss of subcutaneous fat, high pitched voice, horseman stance, protruding ears lacking ear lobes, reduction in hearing capability, sharp nose, cyanotic face, micrognathia, retarded and aberrant dentition, increased platelet number, prolonged prothrombin time, lean body, absence of puberty, alteration in metabolic pathways, extra-skeletal calcification, short dystrophic clavicles, pyriform thorax, thin legs, and stiff joints (Domingo et al. 2009; Espandar et al. 2012; Gordon et al. 2007, 2019; Gordon 2019c; Guardiani et al. 2011; Gustavo Monnerat et al. 2019; Kieran et al. 2007; Merideth et al. 2008; Rork et al. 2014; Ullrich and Gordon 2015). In addition, patients experience angina, hypertension, and hip dislocation. The patients die at a mean age of 14.5 from stroke or myocardial infarction due to the complications of atherosclerosis of coronary and cerebrovascular arteries (Gordon 2019c; Merideth et al. 2008). Mental and cognitive abilities of the patients remain unaffected, the probable reason of which might be the presence of a microRNA (miR-9) in the brain that negatively regulates the expression of progerin/lamin A in neural cells (Nissan et al. 2012).
Diagnosis of HGPS
The median age for diagnosis of HGPS is 2.9 years (Hennekam 2006). Classic HGPS is identified on the basis of clinical symptoms and the medical history of the patient. Also, its presence can be confirmed on the basis of genetic testing for the mutation (c.1824C>T) in the LMNA gene (Gordon 2019c; Gordon et al. 2003). However, there is no kit available for its early detection. The identification of HGPS on the basis of clinical features includes recognition of abnormalities in serum lipid level, sclerodermatous skin, alopecia, prominent scalp veins, insulin resistance, thin limbs, low bone density, coxa valga, receding mandible, osteolysis of the terminal phalanges, etc. (Gordon et al. 2003). The histopathological analysis of a biopsy sample from abdominal skin shows abnormal nuclear morphology. Radiological analyses can demonstrate defects in the skull, long bones, thorax, mandible, distal phalanges, etc. (Gordon 2019c; Gordon et al. 2003).
Atypical HGPS is diagnosed on the basis of clinical features similar to classic HGPS, resulting from a mutation in intron 11 (c.1968+1G>A, c.1968+2T>A, c.1968+2T>C, and c.1968+5G>C) of LMNA gene (Gordon et al. 2003). It can also be identified on the basis of c.1822G>A (p. Gly608Ser) variation in exon 11 of the LMNA gene (Gordon et al. 2003).
Contemporary and emerging disease models to study HGPS
To understand the molecular mechanism and cellular pathophysiology of HGPS and the effects of drug treatment, mainly cell culture and animal model studies are conducted (Piekarowicz et al. 2019; Vidak and Foisner 2016). For cell culture studies, mostly the tissues from HGPS patients (primary culture/cell lines) and mouse embryonic fibroblasts (MEFs) from progeroid mice are used (Piekarowicz et al. 2019), and a number of markers based on the phenotype of HGPS cells are observed. The biomarkers include nuclear blebbing, cellular senescence (measured by the status of β-galactosidase), progerin level, ratio of progerin to lamin A/C/B, mitochondrial function, nuclear trafficking, cellular proliferation, ROS (reactive oxygen species) generation and antioxidant status, epigenetic markers (viz. H3K9me3), LAP2 (lamina-associated polypeptide) levels, DNA double strand breaks (γ-H2AX foci), intracellular signaling pathways (e.g., mTOR, ERK1–3, Akt, NF-κB pathways), etc. (Piekarowicz et al. 2019). More than 200 cell lines are available now for the study of HGPS and progeroid laminopathies (Gordon 2019d). However, cell culture-based assays can only provide limited information regarding the pharmacological profile of a drug like information on molecular/biochemical pathways, pharmacodynamics, etc. (Ghanemi 2015; Gordon et al. 2012a). Therefore, to evaluate the effect of the drugs at the preclinical level, appropriate animal models with specific genetic mutation are required.
Several transgenic progeroid mouse models have been developed with mutations in LMNA gene or the processing enzymes of lamin A biosynthesis (Mayoral et al. 2018). These mice differ in genotype with varied molecular pathways and phenotypic characteristics but share certain common features like reduced lifespan and aberrant nuclear morphology (Carrero et al. 2016; Piekarowicz et al. 2019). The first progeroid mouse model (Zmpste24−/− or Zmpste24-null) was created in 2002 by Pendás et al. (2002) and Bergo et al. (2002). These mice also accumulate farnesylated prelamin A isoform and exhibit classical features of progeria like slow growth, lipodystrophy, muscular dystrophy, premature death, abnormal body posture, cardiac dysfunction, loss of body weight, alopecia, fragile bones, and hypoglycemia (Mayoral et al. 2018). As discussed earlier, similar mutation in humans causes restrictive dermatopathy. Although Zmpste24−/− mice represent many typical features of HGPS, the mutation in their genome is not the same as that in HGPS. Therefore, transgenic mice with mutation c.1827C>T; p.G609G, which is equivalent to c.1824C>T; p.G608G mutation among humans, were developed (Osorio et al. 2011). As a result of this mutation, progerin expression and accumulation starts in the mouse cells. These mice (LmnaG609G/G609G) phenocopy most of the clinical manifestations of HGPS viz. short life span, growth retardation, loss of body weight, cervicothoracic lordokyphosis, loss of subcutaneous fat, attrition of hair follicles, involution of thymus and spleen, depletion of vascular smooth muscle cell (VSMC), cardiovascular defects, hypoglycemia, weakness of bones, etc. The altered molecular pathway at the cellular level had a striking similarity with HGPS patients. The defects in LmnaG609G/+ were less severe as compared to LmnaG609G/G609G (Osorio et al. 2011).
Progeroid mouse models are extremely useful for the study of HGPS. To demonstrate the efficacy of various treatments on progeria, most of the experimental work has been carried out on Zmpste24−/− and LmnaG609G/G609G mice (Table S1) (Mayoral et al. 2018). Besides these two, a number of other mouse models of premature aging have been established. One example of such mice is LmnaHG mouse model that has its intron 10 and 11 and the last 150 nucleotides of exon 11 deleted due to which it produces only progerin in the cell in homozygous condition (Yang et al. 2005). However, in heterozygous mouse, a low level of lamin A and C was also detected. This model phenocopies most of the HGPS clinical features except cardiovascular alterations (Mayoral et al. 2018; Yang et al. 2005, 2006). Further modifications were made in this mouse model to produce two new types of transgenic mice that had the CAAX motif modified into SAAX (LmnanHG mice) or CAX (LmnacsmHG mice) (Yang et al. 2008a, 2011). Both transgenic models produced unfarnesylated progerin and demonstrated clinical manifestation of progeria at less severe level, specifying the significance of farnesylation in the pathology of HGPS. However, LmnacsmHG mice had progeroid symptoms of much less intensity as compared to LmnanHG mice, indicating that cysteine (C) to serine (S) substitution at CAAX motif is also toxic (Yang et al. 2011).
Another mouse model that carried the mutated human LMNA gene (p.G608G) on a bacterial artificial chromosome (BAC) was developed (Varga et al. 2006). These mice recapitulated the arterial defects including progressive medial loss of VSMCs (vascular smooth muscle cells), but pathological features outside the cardiovascular system were absent. Older mice demonstrated arterial calcification and adventitial thickening with severe VSMC loss and ECM deposition (Varga et al. 2006). Further, mosaic Zmpste24−/− mice were produced to study the effect of prelamin A on cancer (de la Rosa et al. 2013). These mice did not exhibit progeroid phenotype and had normal life span that emphasized the role of cell-extrinsic mechanism in HGPS pathology. Similarly, other progeroid mice have been developed (keratin 14-progerin, tetop-LAG608G, etc.) to study the effect of progerin expression under specific conditions (Mayoral et al. 2018; Piekarowicz et al. 2019).
Progeroid mouse models are considered gold standard for the understanding of pathophysiology of HGPS and the outcomes of various treatment strategies at the cellular and systemic levels. The most common biomarkers evaluated for the efficacy of the drug involve life span, body weight and size, muscle strength, bone density and mineralization, progerin expression, nuclear abnormalities, cardiovascular function, and subcutaneous fat level (Table S1). However, none of these models displays all the characteristics of the disorder and some demonstrate additional features that are not found in progeria. Therefore, a cautious selection of an appropriate model is required for the study (Harkema et al. 2016).
Because of inadequate availability of human samples and limitations of existing models of progeria, there is a dire need of improved and more human-like disease model for testing of novel drugs. New advancements have turned the focus toward HGPS-derived iPSCs as a good model for its study (Compagnucci and Bertini 2017). HGPS-iPSCs (induced pluripotent cells derived from the cells of HGPS patients) neither accumulate progerin nor demonstrate the symptoms of the disease. HGPS-iPSC are like control cells in terms of pluripotency and nuclear membrane integrity, as well as transcriptional and epigenetic profiles (Chen et al. 2017). However, their differentiation into VSMCs triggers progerin expression and phenotype similar to that of HGPS (Chen et al. 2017; Liu et al. 2011a). Several other groups also successfully differentiated HGPS-iPSCs into different cell types to investigate the underlying mechanism of progeria and pharmacological testing (Blondel et al. 2014; Ho et al. 2011; Matrone et al. 2019; Nissan et al. 2011, 2012; Petrini et al. 2017; Zhang et al. 2011). Interestingly, progerin expression as a hallmark of disease phenotype was found more predominantly in VSMCs, MSCs, and fibroblasts as compared to others (Zhang et al. 2011). Recently, Matrone et al. (2019) revealed that endothelial cells differentiated from the iPSCs derived from fibroblasts of HGPS patients (HGPS-iPSC-ECs) exhibit several phenotypic and physiological features similar to HGPS patient cells in vivo and in vitro with high progerin expression. Thus, these cells can serve as a useful model for the study of the pathophysiology of the disease and therapeutics.
Further progress has been made in this field by Atchison et al. (2017), who developed a 3D model of HGPS that replicates an arteriole-scale tissue-engineered blood vessel (TEBV) using iPSC-derived smooth muscle cells (iSMCs) from an HGPS patient. This 3D culture can be used to examine functional responses of pharmacological interventions analogous to those performed clinically. The TEBV constructed from iSMCs derived from HGPS cells exhibited pathological condition similar to HGPS patient cardiovascular system viz. increased calcification, thicker medial walls, enhanced apoptosis, and escalated fibronectin deposition. The treatment of rapamycin analog, RAD001 (everolimus), to this 3D culture demonstrated increased expression of contractile proteins, progerin clearance, and restoration of nuclear morphology (Atchison et al. 2017).
Ribas et al. (2017) have also developed a progeria-on-a-chip model from HGPS-iPS-SMCs (SMCs derived from human-induced pluripotent stem cells of HGPS donors) for understanding the disease pathophysiology. They used a microfluidic polydimethylsiloxane (PDMS) device on top of which the cells can be cultured. The cells can be stretched by applying vacuum at the bottom of the PDMS membrane. This system recapitulates the cyclic mechanical stretching experienced by the vascular smooth muscle cells in the arterial wall of the blood vascular system and can be used to study cellular response under various physiological and pathological strains (Ribas et al. 2017). Using this system, they demonstrated that HGPS-iPS-SMCs demonstrate an exacerbated inflammatory response under 16% mechanical strain as compared to healthy iPS-SMCs. Treatment with lonafarnib and lovastatin could ameliorate this inflammatory response in HGPS-iPS-SMCs (Ribas et al. 2017).
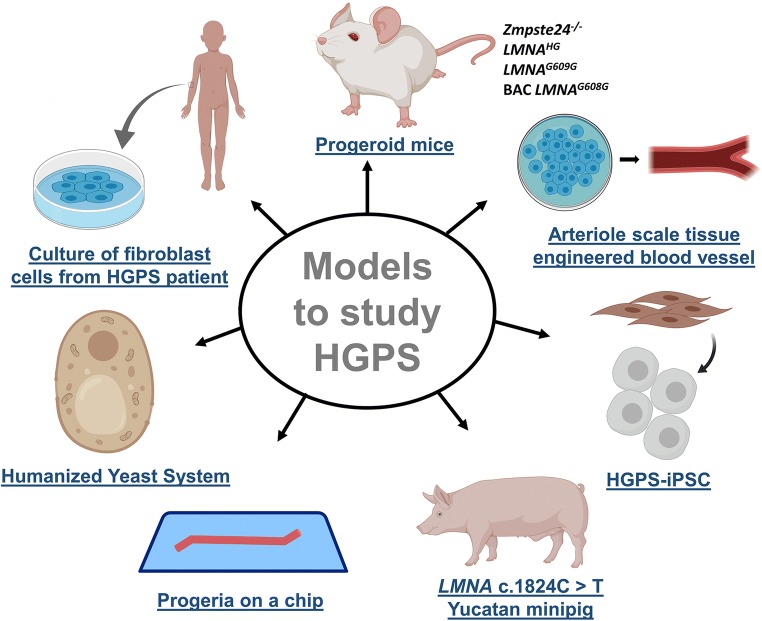
Recently, a humanized yeast system has also been developed to study prelamin A cleavage by Zmpste24 (Spear et al. 2019). Two versions of this system have been developed. Version 1.0 contains a mutated ZMPSTE24 gene, whereas version 2.0 is meant to analyze mutation in LMNA gene. This humanized yeast system can help in understanding the interaction of Zmpste24 with prelamin A and in developing pharmacological approaches for the treatment of certain types of progeria including HGPS. Further, Dorado et al. (2019) have produced the first large animal model of HGPS, i.e., a Yucatan minipig carrying heterozygous LMNA c.1824C>T mutation. This model was developed by using the CRISPR–Cas gene editing system. Like HGPS patients, HGPS minipig cells co-express progerin and normal lamin A/C. These pigs exhibit growth retardation, lipodystrophy, wrinkled and taut skin with patchy punctate pigmentation, large areas of alopecia, thin legs with joint stiffness, sculpted nose, thin lips, abnormal dentition, prominent eyes, and severe cardiovascular perturbations (viz. loss of VSMCs, disturbed electrical activity, systolic and diastolic myocardial dysfunction, microvascular dysfunction, and fibrosis). These symptoms are drastically similar to human HGPS patients. The HGPS pig also die near puberty, comparable to the human counterpart (Dorado et al. 2019). Therefore, HGPS minipigs appear to be a good model for preclinical trials and testing human size interventional devices for HGPS therapeutics. These novel disease models have addressed research gaps and created new opportunities to further clarify the molecular mechanism of HGPS. Figure 2 represents the current models in use for the study of HGPS.
Fig. 2.
Various models to study HGPS. Abbreviation: HGPS-iPSC, induced pluripotent cells derived from the cells of HGPS patients
Current therapeutic strategies for the treatment of HGPS
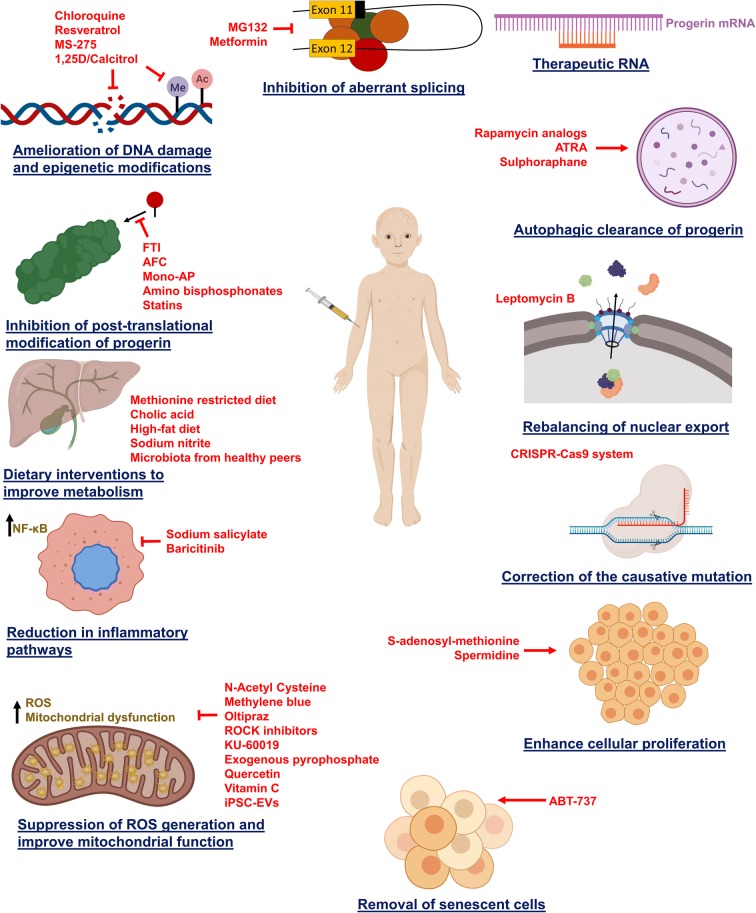
To date, no cure for HGPS has been found. Various treatments available can only improve the clinical conditions and reduce complications. Children affected with HGPS must be given a high-calorie diet on a regular basis and should be supplemented with multivitamin tablets to reduce weight loss and proper nutrition (Gordon 2019c; Gordon et al. 2003). Adequate oral hydration is advocated because stiff vasculature may be less tolerant to dehydration. Electrocardiogram (ECG) is used regularly for the diagnosis of atherosclerosis of the coronary artery. Children are also treated with a low dose of aspirin occasionally to decrease the chances of heart attack and stroke (Gordon et al. 2003). Nitroglycerin is used if angina develops. Due to coxa valga, hip dislocation is common in HGPS patients. Therefore, physical therapy, body bracing, and surgical procedures are recommended (Gordon et al. 2003). Patients should be accompanied because of vulnerability to fractures. Primary tooth extraction is performed to avoid dental crowding. Fluoride supplementation is also recommended for reducing dental problems. Regular physical and occupation therapy is suggested to maintain the range of motion in joints (Gordon 2019c; Gordon et al. 2003). Patients are advised to avoid dehydration and large crowds with taller peers. Also, their physical activity should be self-restricted (Gordon et al. 2003). Figure 3 illustrates the major potential therapeutic approaches against HGPS with their mechanism of action. Further, Table S1 (Supplementary information) summarizes the overall remedial strategies with their target molecule/pathways, models studied, and the therapeutic outcome.
Fig. 3.
The potential therapeutic strategies (red font) for the treatment of HGPS with their target molecular/physiological pathways. Abbreviations: 1,25D, 1α,25-dihydroxy vitamin D3; AFC, N-acetyl-S-farnesyl-l-cysteine; ATRA, all-trans retinoic acid; CRISPR, clustered regularly interspaced short palindromic repeats; FTI, farnesyl transferase inhibitors; ICMT, isoprenylcysteine carboxyl methyltransferase; iPSC-EVs, extracellular vesicles derived from induced pluripotent cells; mono-AP, mono-aminopyrimidines; NF-κB, nuclear factor kappa B; ROS, reactive oxygen species; ROCK, Rho-associated protein kinase
Pharmacological treatment
Inhibition of posttranslational modification of prelamin A
Various strategies are being tested for being a potential therapeutic against HGPS. Farnesyltransferase inhibitors (FTIs), that were initially used for the treatment of cancer, have shown efficacy against disease phenotype in progeria fibroblast culture as well as progeroid mouse model (Capell et al. 2005, 2008; Fong et al. 2006a; Glynn and Glover 2005; Pacheco et al. 2014; Rivera-Torres et al. 2013; Toth et al. 2005; Yang et al. 2005, 2006, 2008b; Young et al. 2013). FTI treatment has been reported to restore nuclear morphology in these models of HGPS. These are small molecules, which bind to the enzyme farnesyltransferase at the site of the CAAX binding domain. FTIs also improve lamin A-Rb signaling and DSB (double strand breaks of DNA) repair in HGPS fibroblasts (Constantinescu et al. 2010; Marji et al. 2010). Pacheco et al. (2014) reported that FTI treatment ameliorates self-renewal capacity in progerin-expressing stem cells. FTI treatment of HGPS cells can also increase telomere mobility (De Vos et al. 2010) and correct chromosome positioning (Bikkul et al. 2018; Mehta et al. 2011). Further, mesenchymal stem cells (MSC) originated from induced pluripotent cells (iPSCs) derived from the cells of HGPS patients (HGPS-iPSC-MSCs) were used for the screening of a number of small molecules for their farnesylation inhibitor activity (Blondel et al. 2016). It was found that mono-aminopyrimidines (mono-APs), which target farnesyl pyrophosphate synthase and farnesyl transferase, serve as both FTI and prenylation inhibitor. Administration of mono-APs to HGPS-iPSC-MSCs rescued progeria phenotypes viz. nuclear defects and premature differentiation into osteoblastic lineage (Blondel et al. 2016).
It is now known that after treatment of FTIs, prelamin A can still be modified by an alternate prenylation pathway, i.e., geranylgeranylation (Varela et al. 2008). This explains the reduced efficacy of FTIs in ameliorating progeroid phenotype in mouse models. They also demonstrated that statins (molecules that impair HMG-CoA reductase activity) and amino bisphosphonates (inhibit farnesyl pyrophosphate synthase and isopentenyl pyrophosphate isomerase activity) can prevent the formation of precursors of geranylgeranyl and farnesyl, preventing posttranslational modification of prelamin A and progerin. In this study, the use of pravastatin (statin) and zoledronate (amino bisphosphonate) ameliorated the characteristic phenotypes of the progeroid mouse (Zmpste24-deficient) viz. growth reduction, weight loss, lipodystrophy, loss of hair, and bone weakness. Also, treatment with statin and zoledronate in HGPS fibroblast and Zmpse24-deficient cells ameliorated nuclear architecture defects and formation of nucleoplasmic aggregates of lamin A and C isoforms. Addition of farnesol or geranylgeraniol abolished these therapeutic effects, suggesting that the possible mechanism of pravastatin–zoledronate treatment underlies the inhibition of geranylgeranylation and farnesylation of the precursor of progerin (Varela et al. 2008). An improvement in mitochondrial function in “mouse adult fibroblast (MAF) derived from Zmpste24−/− and LmnaG609G/G609G mice” was also observed after zoledronate + pravastatin treatment (Rivera-Torres et al. 2013), which was evident from enhanced ATP synthesis and COX/CS (cytochrome c oxidase/citrate synthase) ratio in these cells after treatment.
Combined treatment of HGPS skin fibroblasts with mevinolin (statin) and trichostatin A (TSA, histone deacetylase inhibitor) can also lower progerin accumulation in the cell, restore heterochromatin organization, and reorganize transcripts in HGPS fibroblasts (Columbaro et al. 2005). However, treatment with TSA only did not affect progerin level in the cell. This suggests that the defarnesylation with FTI destabilizes progerin, and TSA interferes with its interaction with other proteins, leading to its degradation. According to Mattioli et al. (2019), TSA enhances the lamin A/C–HDAC2 complex and weakens the progerin–HDAC2 interaction. Mevinolin interferes with lamin A/C–HDAC2 (histone deacetylase 2), but a combined treatment of both strengthens lamin A/C–HDAC2 interaction, indicating another possible therapeutic approach.
Isoprenylcysteine carboxyl methyltransferase (ICMT) is the enzyme responsible for the addition of methyl group at –CAAX motif at the C-terminal of prelamin A. Administration of N-acetyl-S-farnesyl-l-cysteine (AFC), an ICMT inhibitor, to HGPS fibroblast enhanced cell proliferation (Ibrahim et al. 2013). Similarly, inhibition of ICMT with short hairpin RNA (shRNA) in HGPS cells also delayed senescence and increased proliferation rate. Moreover, an expression of a hypomorphic allele of ICMT in progeroid mice has shown to increase body weight, normalize grip strength, reduce bone fractures, and enhance life span in an AKT-mTOR dependent manner (Ibrahim et al. 2013).
Autophagic clearance of progerin
Autophagy is an important cellular process that clears and recycles the damaged organelles (Mizushima and Komatsu 2011). The lack of nutrients and growth factors as well as environmental stress are major stimuli for autophagy. The mTOR (mammalian target of rapamycin) pathway plays a significant role in the regulation of autophagy (Kim and Guan 2015). It has been found that mTORC1 (mTOR complex 1) suppresses the autophagy-initiating UNC-5 like autophagy-activating kinase (ULK) complex by phosphorylating its components viz. autophagy-related gene 13 (ATG13) and ULK1/2 (Kim and Guan 2015). It also phosphorylates components of VPS34 (vacuolar protein sorting 34) complex, preventing its lipid kinase activity (Yuan et al. 2013). Further, mTORC1 inactivates the transcription factor EB (TFEB), which is a master regulator of the expression of genes of lysosomes biogenesis and autophagy (Settembre et al. 2013). These changes result in a series of events that finally lead to the inhibition of autophagy, and inhibition of the mTOR pathway with pharmacological interventions has shown to induce autophagy in experimental conditions (Kim and Guan 2015).
Rapamycin is an mTOR inhibitor drug that was initially used as an immunosuppressant among transplant patients (Chueh and Kahan 2005). It has also been found useful in the treatment of aging and related disorders (viz. neurodegenerative disorders, atherosclerosis) in various animal models (Bjedov et al. 2010; Ehninger et al. 2014; Elloso et al. 2003; Evangelisti et al. 2016; Harrison et al. 2009; Miller et al. 2011, 2014; Mueller et al. 2008; Vellai et al. 2003; Waksman et al. 2003; Zhang et al. 2013). Rapamycin has also shown beneficial effects on lamin A/C-deficient (Lmna−/−) mice by rescuing altered mTORC1 signaling (Liao et al. 2016; Ramos et al. 2012). Treatment with rapamycin and its analog (temsirolimus, everolimus) on human HGPS fibroblast has been reported to ameliorate nuclear blebbing, enhance progerin clearance by autophagy, restore chromatin dynamics, maintain histone methylation levels, re-establish BAF and LAP2α distribution patterns, partially ameliorate DNA damage, and extend cellular life span (Cao et al. 2011; Cenni et al. 2011; DuBose et al. 2018; Gabriel et al. 2016). However, the use of temsirolimus had no effect on mitochondrial function and ROS production (Gabriel et al. 2016). Rapamycin treatment in MDSPCs (muscle-derived stem/progenitor cells) derived from Zmpste24−/− mice reduces apoptosis, decreases senescence, augments myogenic capacity, and improves the chondrogenic differentiation capacity (Kawakami et al. 2019).
Further, administration of FTIs and rapamycin as a combinatorial treatment strategy in HGPS fibroblasts could correct the aberrant genome organization and reduce DNA damage (Bikkul et al. 2018). The use of rapamycin analog (everolimus) in combination with FTI (lonafarnib) is currently under clinical trial (NCT02579044). Discussion of the potential side effects of rapamycin and whether they should hinder human use is a continued area of debate in the geroscience community. In the context of HGPS, it would be helpful to know if the doses of rapamycin are similar to those given to transplant patients or closer to those of other mTOR inhibitors given in studies that show benefits in elderly humans (Mannick et al. 2014, 2018).
The progerin promoter contains retinoic acid response elements, and all-trans retinoic acid (ATRA) is known to induce autophagy in the cell (Rajawat et al. 2010, 2011). Therefore, ATRA has been studied in combination with rapamycin on HGPS fibroblast cells and a synergistic effect on progerin degradation was observed (Pellegrini et al. 2015). Sulforaphane (SFN), an antioxidant derived from crucifer vegetables, has also been reported to induce clearance of progerin from cells by micro-autophagy. Treatment with SFN abolished phenotypic changes in HGPS fibroblast culture (Gabriel et al. 2015). In addition, combined treatment of FTI (lonafarnib) with SFN separately (intermittent) on HGPS fibroblasts showed a synergistic and additive effect on progerin clearance, but induced cytotoxicity when given together (Gabriel et al. 2017). However, intermittent treatment with FTI and SFN rescued the cellular phenotype of progeria viz. reduction in ROS and increase in cellular ATP level, decreased DNA damage, and lowered the number of dysmorphic nuclei (Gabriel et al. 2017).
Downregulation of aberrant splicing of LMNA
Intramuscular injection of MG132 (an autophagy-activating drug) in LmnaG609G/G609G mice HGPS fibroblasts has been found to reduce progerin synthesis and improve cell viability in HGPS fibroblast, vascular smooth muscle cells, and HGPS-iPSC-derived mesenchymal cells through downregulation of SRSF-1 (serine/arginine-rich splicing factor-1) and SRSF-5, which are RNA binding protein that favors aberrant splicing of prelamin A into progerin (Harhouri et al. 2017). SRSF-1 has also been found to be regulated by an antidiabetic drug, metformin (Larsson et al. 2012), that is also known to possess anti-aging properties (Barzilai et al. 2016). Metformin downregulates specificity protein (Sp) transcription factors, Sp-regulated genes, mTOR signaling, and epidermal growth factor (EGFR)-dependent activation of Ras (Nair et al. 2014). Treatment of HGPS-iPSC-MSCs with metformin decreased SRSF-1 and progerin expression and ameliorated nuclear defects in the cells and premature osteoblastic differentiation. Remedial effects of metformin were also observed in human HGPS fibroblasts and LmnaG609G/G609G mouse fibroblasts (Egesipe et al. 2016; Park and Shin 2017).
Inhibition of progerin–lamin A interaction
Progerin interacts with lamin A with great affinity, disrupting nuclear lamina and membrane integrity. Therefore, progerin–lamin A inhibitors (JH1, JH4, and JH13) have also been proposed as a therapeutic against HGPS (Lee et al. 2016b). The use of these molecules, particularly JH4, alleviated the hallmarks of HGPS viz. distortion of the nucleus, senescence-associated β gal (SA-β-gal) activity, reduced H3K9me3 level, and cell cycle arrest. Anti-senescence effects of JH4 were observed in aging cells as well. It reduces the half-life of progerin in a proteasome-dependent manner. Further, it was observed that JH4 improves the life span of LmnaG609G/G609G-mutant mice, which are phenotypically similar to human HGPS, but does not affect progerin-independent progeric mice (Zmpste24−/−), which suggests that it selectively binds and inhibits progerin (Lee et al. 2016b).
Ameliorating mitochondrial dysfunction and oxidative stress
Mitochondrial dysfunction, increased oxidative stress, and lowered antioxidant levels are hallmarks of HGPS (Kubben et al. 2016; Richards et al. 2011; Rivera-Torres et al. 2013). Treatment of HGPS cells with antioxidants like N-acetyl cysteine (NAC) and methylene blue (MB) improves HGPS phenotype. NAC administration to progeroid fibroblasts reduces ROS generation, decreases unrepairable DSB, and improves cell proliferation (Richards et al. 2011). MB treatment in HGPS skin fibroblasts also alleviates mitochondrial as well as nuclear defects along with improvement in heterochromatin organization and restoration of expression of misregulated genes (Xiong et al. 2016). By using an in vitro reconstructed 3D human skin model, Xiong et al. (2017) demonstrated that MB treatment improves skin viability, stimulates wound healing, improves skin hydration, and extends dermis thickness. Additionally, it was found safe for application at high concentrations and for a longer period.
NRF2 is a critical redox sensor that interacts with ARE (antioxidant response element) motifs and activates an array of antioxidant and cytoprotective genes in response to oxidative stress (Lewis et al. 2010). It is sequestered by progerin in HGPS cells, resulting in its subnuclear mislocalization, which leads to oxidative stress (Kubben et al. 2016). Introduction of constitutively active NRF2 (caNRF2) in HGPS fibroblasts reduced ROS levels, increased expression of NRF2-regulated antioxidant genes, lowered the number of apoptotic cells, revoked HGPS-associated nuclear defects, and re-established the in vivo viability of HGPS-iPSC-MSCs in an animal model (Kubben et al. 2016). Similar effects were observed after treatment of cells with mild NRF2 activator oltipraz, suggesting that the NRF2 pathway has a major role in the presentation of HGPS phenotype (Kubben et al. 2016).
ROCK (Rho-associated protein kinase) phosphorylates Rac1b, which facilitates its interaction with cytochrome c to increase mitochondrial ROS production in a cytochrome c oxidase (COX)-dependent manner (Kang et al. 2017). Y-27632, a ROCK (Rho-associated protein kinase) inhibitor that prevents mitochondrial ROS generation, has also been found to ameliorate nuclear abnormalities, restore mitochondrial function (as demonstrated by recovering mitochondrial membrane potential and increased COX activity), and reduce DSB of DNA in HGPS fibroblasts (Kang et al. 2017). Similarly, treatment with Y-27632 and fasudil (another ROCK inhibitor) invoked mitochondrial function recovery, increased cell proliferation, and alleviated cellular senescence, and enhanced expression of chromatin remodeling genes was also observed in HGPS as well as normal senescent cells (Park et al. 2018). ROCK inhibitors could also aggravate wound healing in aged mice. These results indicate toward the potential of ROCK inhibitors against physiological and pathological aging.
Recently, Kuk et al. (2019) studied the effect of KU-60019, an ATM inhibitor and anti-aging agent (Kang et al. 2017), on HGPS skin fibroblast. Administration of KU-60019 reduced ROS level and improved mitochondrial membrane potential with metabolic reprogramming. It also led to the reduced accumulation of progeria, restoration of nuclear morphology, and alleviation of senescence. This provides evidence for a possible therapeutic approach by controlling ATM activity for age-related disorders.
Villa-Bellosta et al. (2013) conducted a study on knock-in LmnaG609G/+ mice and found that their capacity of inhibiting vascular calcification was drastically reduced. This defect is mainly because of the increased expression and activity of tissue-nonspecific alkaline phosphatase which leads to impaired mitochondrial function and ATP synthesis. This further causes impairment in the accumulation of extracellular pyrophosphate, which plays a key role as an inhibitor of vascular calcification. Administration of pyrophosphate ameliorated vascular calcification in LmnaG609G/G609G mice.
Modulation of inflammatory pathways
Hyperactivation of NF-κB has been linked to aging and several age-related disorders (Adler et al. 2007; Tilstra et al. 2011). Its inhibition has also been reported to delay aging in mice (Tilstra et al. 2012). Osorio et al. (2012), while working with LmnaG609G/G609G and Zmpste24-deficient mice, reported that the NF-κB signaling pathway is activated in progeroid syndrome via ATM (ataxia-telangiectasia-mutated) and NEMO (NF-kappa-B essential modulator) proteins, and its blocking with sodium salicylate [a nonsteroidal anti-inflammatory drug that binds with conserved IKK (IκB kinase) complex] prevents progeroid features and prolongs life span.
Recently, using a text mining approach, Liu et al. identified 17 proinflammatory marker genes that are associated with all the main pathological features of HGPS, i.e., alopecia, lipodystrophy, arthritis, and vascular disease (Liu et al. 2019a). Fourteen of these genes are linked to the JAK1/2–STAT1/3 signaling pathway. JAK–STAT signaling was also found to be overactivated in HGPS and normally aged cells (Liu et al. 2019a). Moreover, these 14 genes were also found to be directly regulated by NF-κB transcription factor. By treating HGPS fibroblast primary culture with baricitinib—an inhibitor of JAK1/2 that weakens STAT1/3 activation, Liu and colleagues (Liu et al. 2019a) found an improved growth rate, reduced senescence, decreased ROS level, enhanced ATP levels, and induced autophagy and proteasome activity. Prolonged baricitinib administration significantly reduced progerin level, the number of dysmorphic nuclei, and the expression of proinflammatory factors in HGPS cells. Further, it was elucidated that a high level of DNA damage in senescent/progeric cells is responsible for triggered JAK–STAT signaling within (Liu et al. 2019a).
Improvement in DNA damage repair machinery and epigenetic modifications
Kreienkamp et al. (2016) reported that progerin accumulation in HGPS leads to decreased levels of vitamin D receptors (VDR) and DNA repair factors such as BRCA1 (breast cancer type 1) and 53BP1 (p53 binding protein 1). Restoration of VDR signaling with 1α,25-dihydroxy vitamin D3 (1,25D) in HGPS fibroblasts could ameliorate pathological features viz. nuclear defects, premature senescence, and DSB repair defects. The underlying mechanism of action of this drug might involve correction of replicative stress and DNA damage in progeric cells (Kreienkamp et al. 2018).
Another promising molecule is resveratrol, a SIRT1 activator that associates with lamin A. In the presence of progerin, SIRT1 partially interacts with the nuclear matrix and decreases deacetylase activity that causes rapid depletion of adult stem cells in progeroid (Zmpste24−/−) mouse model. Resveratrol administration in these mice improves SIRT1–lamin A interaction and decreases adult stem cell depletion. At the systemic level, it results in improved body weight and bone density and decelerated weight loss with a significant increase in the life span (Liu et al. 2012). Mattioli et al. (2019) suggested the use of HDAC inhibitors (TSA and MS-275) as therapeutics in HGPS cells as those weaken progerin–HDAC2 interactions and augment lamin A/C–HDAC2 binding with improved histone acetylation status of the cells. A recent report also gave evidence of a progressive decline of ATM-centered DNA damage response (DDR) with aging (Qian et al. 2018). Treatment with a low dose of chloroquine (CQ, a malaria treatment drug) induces ATM, clears DNA damage, relieves age-related metabolic shift, and prolongs life span in aging cells. ATM stabilizes SIRT6, which is a longevity enzyme, by phosphorylating SIRT6 deacetylase enzyme that mediates proteasomal degradation of SIRT6. Prolonged administration of CQ also enhanced life span in progeroid (Zmpste24−/−) mouse model and ameliorated hallmarks of progeria phenotype.
Farnesyl diphosphate synthase (FDPS) or farnesyl pyrophosphate synthase is a key enzyme in isoprenoid biosynthetic pathway that helps in farnesylation and geranylgeranylation of proteins. Human skin fibroblasts derived from HGPS patients exhibit increased expression of FDPS. Pamidronate, a FDPS inhibitor and a bisphosphonate drug, reduced senescence and increased proliferation in these cells. Pamidronate treatment also rescued misshapen morphology of the nucleus (Griveau et al. 2018). Further, phospholipase A2 receptor (PLA2R1) is a transmembrane protein that regulates senescence in a p53-, JAK/STAT-, and ERRα-dependent fashion (Augert et al. 2009; Griveau et al. 2016). The expression of PLA2R1 increases in progerin overexpressing cells. It was found that knocking down of PLA2R1 with shRNA can ameliorate progerin-induced senescence and increase proliferation in these cells in a DDR/p53/FDPS-dependent pathway (Griveau et al. 2018). However, some more insight is required for understanding the mechanism of action of FDPS as it is an important enzyme in isoprenoid biosynthetic pathway that helps in the formation of progerin; on the other hand, it serves as a downstream effector of progerin in a PLA2R1-dependent manner.
HGPS cells also exhibit increased shortening of telomeres and heterogeneity in their lengths (Decker et al. 2009; Gonzalo and Kreienkamp 2015; Li et al. 2019). The telomere attrition induces DDR that leads to proliferation arrest and senescence. Recently, it has been demonstrated that transient expression of human telomerase (hTERT) mRNA in HGPS cell lines can extend telomere length. hTERT mRNA administration could also enhance the proliferative capacity, reduce the number of senescent cells, exacerbate senescence-associated secretory phenotype (SASP), and rescue nuclear morphology. Combined treatment of FTI + hTERT mRNA had a synergistic effect on cell proliferation (Li et al. 2019).
According to Finley (2018), the drugs that alleviate the pathological defects of HGPS (viz. rapamycin, metformin, methylene blue, all-trans retinoic acid, MG132, 1α,25-dihydroxy vitamin D3, sulforaphane, and oltipraz) by improving mitochondrial function, enhancing Nrf2 activity, promoting autophagy, or by altering gene splicing act through a common mechanism, i.e., by modulating AMPK pathway.
Pharmacological removal of senescent cells
Senescent endothelial cells and VSMCs play a major role in the development and progression of atherosclerotic plaque in chronologically aged people (Katsuumi et al. 2018). Elimination of senescent cells in progeroid mice has been shown to increase survival and reduce the incidences of age-related disorders (Baar et al. 2017). Senescent cells also upregulate the expression of anti-apoptotic proteins BCL-W and BCL-XL, the inhibition of which with siRNA or small molecules ABT-737 or ABT-263 (senolytic drugs) leads to apoptosis (Chang et al. 2016; Yosef et al. 2016). Ovadya et al. (2018) demonstrated an extensive accumulation of senescent cells in Lmna+/G609G progeroid mice, which results in a short life span. Pharmacological treatment with ABT-737 in these mice significantly reduced the number of senescent cells in the liver, pancreas, lungs, and skin of these mice and partially ameliorated progeric phenotype (Ovadya et al. 2018). This gives new drug targets for therapeutics of age-related disorders. Administration of senolytic drugs (dasatinib and quercetin) on aged/atherosclerotic mice has also been found effective in improving the vasomotor functions and reduce the aortic calcification by inducing nitric oxide signaling (Roos et al. 2016). It also reduced the senescent cell burden and DNA damage.
Growth hormone treatment
Somatotroph signaling [i.e., growth hormone (GH)/insulin-like growth factor 1 (IGF-1)] has been found to be one of the major regulators of aging in a variety of organisms including humans (Russell and Kahn 2007). An extreme reduction in plasma IGF-1 level has been observed in the murine model of HGPS (Zmpste24−/− mice) (Mariño et al. 2010). Treatment of these mice with recombinant human IGF-1 (rIGF-1) restored the proper balance between IGF-1 and GH and extended life span. It also ameliorated several phenotypic features of HGPS like improved body weight, increase in the amount of subcutaneous fat, reduced level of kyphosis, and alopecia (Mariño et al. 2010). In a previous study (Abdenur et al. 1997), patients with HGPS demonstrated increased growth hormone (GH) resistance. Treatment with GH and high-calorie diet improved growth velocity in progeric patients (Abdenur et al. 1997; Merideth et al. 2008).
Targeting other downstream actions of progerin
Chemical screening of several molecules resulted in the finding of a new molecule “remodelin” that could improve nuclear architecture, chromatin organization, and health of progeric as well as lamin A/C-depleted cells (Larrieu et al. 2014). Remodelin targets an acetyl transferase protein, NAT10 (N-acetyltransferase 10), which restores nuclear shape in laminopathic cells via rearrangement of microtubules (Larrieu et al. 2014). Remodelin administration to HGPS and aged cells restored normal nucleocytoplasmic transport and rebalanced gene expression at a large scale in a Transportin-1 (TNPO1)-dependent manner. Similar effects were seen after treatment with siRNA-mediated depletion of NAT10 in these cells (Larrieu et al. 2018). Furthermore, chemical inhibition of NAT10 by oral administration of remodelin to homo- and heterozygous LmnaG609G HGPS mouse model led to the augmentation in health span of animals as demonstrated by improvement in age-dependent body weight loss, cardiac function, curvature, fitness, and genomic instability. Remodelin works in a progerin-independent manner as the progerin level remains similar in remodelin-treated and control mice (Balmus et al. 2018).
Geng et al. (2018) screened a number of natural products on Werner syndrome (adult progeria) human mesenchymal stem cells (WS-hMSC) to test their effectiveness as a geroprotective agent. They identified 10 natural compounds for their geroprotective role, out of which, quercetin had the most profound effect. Quercetin attenuated WS-hMSC senescence by promoting proliferation, differentiation, and self-renewal capabilities in addition to re-establishing heterochromatin structure via modulating multiple cellular pathways (viz. cell cycle, chromosome condensation, and antioxidant gene expression). It was also found to improve telomere length and reduce the percentage of abnormal nuclei significantly. In the same study, it was demonstrated that quercetin and vitamin C also reduce accelerated cellular senescence in HGPS-hMSC (LMNAG608G/+) with a synergistic function. Both molecules lowered progerin expression in the cell, increased proliferation rate, enhanced clonal expansion ability, and decreased SA-β-gal-positive cells (Geng et al. 2018).
A next-generation sequencing (RNAseq) and high-resolution quantitative proteomics (iTRAQ) techniques-based study (Mateos et al. 2018) demonstrated a reduction in expression level of ribose-phosphate pyrophosphokinase 1 (PRPS1), a protein that is essential for purine synthesis in the cell. Supplementation of HGPS cells with S-adenosyl-methionine (SAMe), which is a methyl donor and alternative source of purine in living cells (Chiang et al. 1996), resulted in increased proliferation capability and reduced senescence. According to Mateos et al. (2018), SAMe supplementation enhances AMP production in the cell, which could also convert into GMP, and help in restoring cellular energy status and biosynthetic pathways to partially rescue progeria phenotype.
Treatment with TUDCA, a low-abundance bile acid in human and a chemical chaperon, has been shown to ameliorate vascular defects and increase life span in progeroid mice (Hamczyk et al. 2019). The molecular mechanism of this molecule involves enhancement of genes related to protein folding and modification, which could ameliorate endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in this model of progeria.
Casein kinase 2 (CK2) is a serine/threonine protein kinase that binds to nuclear lamina and nuclear matrix with the help of lamin A. It regulates various important cellular processes like proliferation and survival, differentiation and development, DDR, and apoptosis. Lamin A serves as endogenous inhibitor of CK2; however, it was found that mutated prelamin A (with additional 18 amino acids) can further enhance this inhibitory action in Zmpste-24-deficient mice (Ao et al. 2019). This “prelamin A with additional amino acids” is not equivalent to progerin in this regard as progerin contains a deletion of extra 50 amino acids from lamin A. Spermidine, a polybasic compound, serves as a CK2 activator. Spermidine supplementation to Zmpste-24-deficient mice extended life span. In Zmpste24−/− MEFs, spermidine treatment could alleviate senescence and improve DDR.
The overexpression of a protein exportin-1 or chromosomal region maintenance 1 (CRM1) caused by progerin expression leads to a disbalance in nuclear protein export, which is one of the central regulatory features of progeric phenotype (García-Aguirre et al. 2019). Pharmacological inhibition of CRM1 with leptomycin B (LMB) can alleviate almost all significant pathophysiological features of HGPS in the cell viz. nuclear blebbing, reduced lamin B1 expression, cellular senescence, heterochromatin loss, expansion of nucleoli, etc. Since enhanced nuclear export due to enhanced CRM1 activity is now considered as key converging mechanism in pathological and normal aging, targeting this molecule can significantly attenuate the progression of the disorder (García-Aguirre et al. 2019).
In LmnaG609G/G609G mice, it was noted that those exhibit several characteristics of hematopoietic aging viz. expanded hematopoietic stem cells (HSCs) with reduced engraftment, lymphoid deficiency, and myeloid differentiation into platelets. The microenvironment of bone marrow (BM) is considered as the major factor for premature hematopoietic aging in these mice. Treatment of BRL37344, a β3-adrenergic receptor (β3-AR) agonist that targets the BM microenvironment, for over 2 months can reduce HSCs, normalize the association of HSCs to megakaryotes, and correct lympho-myeloid skewing in these mice (Ho et al. 2019).
Dietary interventions and modulation of gut microbiota
In a study by Bárcena et al. (2018), the use of 0.1% cholic acid (a primary bile acid) in the diet delayed aging in Zmpste24−/− mice along with improvement in progeria-associated phenotype such as hind limb stiffness, loss of body weight, loss of body hair, and cervicothoracic lordokyphosis. Further, it was found that methionine-restricted (MR) diet can increase life span in HGPS mouse model by reversing the transcriptome alterations in inflammation and DNA damage response genes present in this condition. Also, the MR diet could improve lipid profile and bile acid status and conjugation in progeroid and control rats. These results cumulatively indicate a key role of bile acid metabolism in progeroid syndromes. The possible mechanism of MR diet may underlie the activation of autophagy-mediated progerin removal by targeting the mTOR pathway (Bárcena et al. 2019a). Dietary supplementation of nitrite (sodium nitrite) in progeroid (LmnaG609G/G609G) mice also improved vascular defects, i.e., protecting them from vascular stiffness and inward remodeling (del Campo et al. 2019). High-fat diet (HFD) in G609G mice has also shown promising results against HGPS (Kreienkamp et al. 2019). HFD nearly doubled the life span of mice and significantly improved organismal decline as compared to control with a significantly distinctive metabolic response. As discussed earlier, the Progeria Research Foundation also recommends a high-calorie diet for HGPS patients.
In a recent novel study, Bárcena et al. (2019b) found that progeroid mice as well as progeria patients present intestinal dysbiosis. They examined two mouse models of progeria, i.e., LmnaG609G/G609G mice and Zmpste24−/− mice, and found that these mice have a high prevalence of Proteobacteria and Cyanobacteria and a reduced abundance of Verrucomicrobia in the gut. Similar patterns were observed in human HGPS and NGPS (Nestor–Guillermo progeria syndrome) patients. On the other hand, it was discovered that centenarians/long-lived humans have a high prevalence of Verrucomicrobia but low Proteobacteria in their gut (Bárcena et al. 2019b). The gut microbiota genera Klebsiella, Lactobacillus, Parabacteroides, and Akkermansia were mainly abundant among centenarians. LmnaG609G/G609G mice, when orally supplemented with fecal microbiota from wild-type mice, demonstrated a median 13.5% increase in life span. Also, they displayed a delayed loss of body weight, ameliorated body temperature, and improved blood glucose levels. The treatment of Zmpste24−/− mice with fecal microbiota from wild type enhanced the median life span of 13.4%. An improvement in health of these mice was also observed. Further, supplementation of Akkermansia muciniphila only to the LmnaG609G/G609G mice also extended the life span of these mice with thickening of intestinal mucosa and expression of wound-healing factors. The possible mechanism of fecal microbiota transplantation-induced improvement in health and life span may involve restoration of secondary bile acids and other metabolites like arabinose, ribose, and inosine (Bárcena et al. 2019b).
Downregulation of progerin expression with therapeutic RNA
Few studies have shown that silencing of progerin expression can completely reverse the disease phenotype in skin and bones of HGPS transgenic animal models, even after the disease phenotype is fully developed (Sagelius et al. 2008; Strandgren et al. 2015). It was first demonstrated by Scaffidi and Misteli (2005), who administered morpholino antisense oligonucleotide against activated cryptic splice site in HGPS fibroblasts. Correction of splicing led to lowering of mutant lamin A protein, restoration of the typical nuclear structure, normal expression of misregulated genes, re-establishment of cellular levels of lamin-associated proteins, and amelioration of the defects in heterochromatin-specific histone modifications.
Administration of antisense morpholino oligonucleotide in HGPS (LmnaG609G/G609G) mouse model revoked pathogenic alternate splicing and ameliorated progeroid phenotype, promoting life span and improved body weight (Osorio et al. 2011). Lee et al. (2016a) also showed that the delivery of antisense oligonucleotide against a 30 nucleotide stretch in exon 11 of LMNA gene in in vitro (HGPS fibroblasts) and in vivo (LmnaG09G/G09G mouse) system blocked SRSF2 binding site and enhanced the synthesis of lamin C protein at the cost of prelamin A. Since the production of both lamin A and C is not required and the mouse model expressing only lamin C is also disease free, this could also be a possible strategy for the treatment of HGPS (Coffinier et al. 2010; Fong et al. 2006b).
Another strategy is the use of RNA interference. Huang et al. (2005) developed short hairpin RNA (shRNA) against mutated LMNA mRNA and delivered it with the help of lentivirus into the HGPS fibroblast. As a result, the protein expression level of mutant lamin A was reduced to 26% that significantly improved proliferative ability and nuclear structure in progeroid cells (Huang et al. 2005). In some cases, a mutation at exon 11 of LMNA gene produced progerin and other truncated or wild-type prelamin A (Barthélémy et al. 2015). Patients carrying such type of mutations are called as “HGPS like patients.” Harhouri et al. (2016) conducted a study on HGPS, HGPS-like, and MAD-B (mandibuloacral dysplasia type B) patients. They showed that administration of morpholino antisense oligonucleotide into the cells derived from these patients reduced the formation of truncated prelamin A isoform significantly. The use of microRNA has also been found to be effective in the treatment of HGPS along with other premature aging syndromes and laminopathies, as reviewed by Frankel et al. (2018). However, the lack of techniques of tissue-specific delivery of RNA and poor cellular uptake are the limitations for the use of therapeutic RNA in humans (Yong-Fu 2016). Moreover, the development of an animal model and clinically characterized patient model poses another challenge for preclinical and clinical studies (Kole et al. 2012).
NANOG overexpression
HGPS cells are characterized by defects in ECM synthesis and remodeling. It has been found that it is a major factor responsible for reduced proliferative capacity, and the progeric cells grown on wild-type ECM components have normal proliferation (Hernandez et al. 2010). An overexpression of Nanog homeobox (NANOG), which is a transcription factor, can re-establish TGF-β1 and ROCK pathways and induce SMAD2/3-dependent type III collagen (COL3) synthesis (Rong et al. 2019). The restoration of COL3 in these cells can reduce senescence, induce proliferation, and lower DNA damage (Rong et al. 2019). NANOG expression can also increase actin polymerization and restore contractile activity in HGPS cells (Mistriotis et al. 2017).
Treatment with healthy and functional stem cells
Depletion of functional stem cells, leading to the decline of regenerative ability of tissues, has been considered as a hallmark of aging (Kirkwood 2005). Similarly, MSCs recovered from the bone marrow of HGPS patients tend to lose their differentiation ability (Scaffidi and Misteli 2008). Lavasani et al. (2012) showed that transplantation of muscle-derived stem/progenitor cells (MDSPCs), isolated from young wild-type mice, in progeroid mice improved life span and health. It also promoted the growth of the animals with improved brain and muscle tissue health. It ameliorated phenotypic defects in tissues where stem cells were not injected indicating secretion of factors with paracrine action. However, treatment with cells has certain disadvantages in general, viz. lack of immediate action, teratoma formation, and low survival rates of implanted cells.
Cellular reprogramming
Cellular reprogramming can induce tissue regeneration and can be achieved by inducing Yamanaka transcription factors or OSKM (i.e., Oct4, Sox2, Klf4, and c-myc) in progeroid mice (Ocampo et al. 2016). Partial cellular reprogramming with Yamanaka factors in LAKI 4F (LmnaG609G/G609G mice crossed to mice carrying an OSKM polycystronic cassette and a rtTA trans-activator) mice extended their life span and ameliorated age-related histological changes in multiple tissues like the skin, spleen, kidney, and stomach. Induction of Yamanaka factors in these mice also partially rescued the degeneration of VSMCs and progressive development of bradycardia (Ocampo et al. 2016). In vitro studies also revealed an anti-senescence effect of OSKM induction in tail tip fibroblast culture from progeroid mice (Ocampo et al. 2016). There was a significant reduction in several age-associated markers, ROS generation, and DNA damage. Epigenetic modifications implicated in heterochromatin maintenance were also restored (Ocampo et al. 2016).
Correction of the causative mutation
There is an emerging area of gene therapy using clustered regularly interspaced short palindromic repeats/Cas protein (CRISPR/Cas) genome editing system (Arancio et al. 2015). This approach permits genome editing at specific loci. This system consists of a guide RNA with Cas (double-stranded DNA endonuclease) protein that can generate DSB at a desired location in the genome with high accuracy. The alterations done by this system are permanent. The CRISPR/Cas system can be used mainly in two ways: (i) by gene disruption, creating and indel (small insertion/deletion), or by (ii) sequence-specific gene editing approach. This technique, in combination with adeno-associated virus-derived vector delivery system, has shown promising future possibilities in permanent genome editing for the treatment of various genetic disorders. Adeno virus-derived vectors have been used once to correct the mutation in LMNA gene of HGPS in patient-specific iPSCs with homologous recombination-based gene editing approach (Liu et al. 2011b).
Recently, two independent studies (Beyret et al. 2019; Santiago-Fernández et al. 2019) have demonstrated the potential of this technique in an animal model of HGPS. Beyret et al. (2019) demonstrated a single-dose systemic delivery (intravenous injection) of adeno-associated virus-delivered CRISPR–Cas9 components in a mouse model of HGPS. Administration of CRISPR–Cas9 components reduced lamin A/progerin level, attenuated weight loss, extended life span, and improved health status in mice. In another independent study, Santiago-Fernández et al. (2019)) used lentiviral vector-based delivery of Staphylococcus aureus Cas9 and gRNAs in human (LMNAG608G/+ fibroblasts from HGPS patients and LMNA+/+ fibroblasts) and murine fibroblast (Lmna+/+ and LmnaG609G/G609G) and found that it leads to a reduction in progerin and lamin A protein level. Adeno-associated virus systemic delivery (intraperitoneal injection) of Staphylococcus aureus Cas9 and gRNA into LmnaG609G/G609G mice as an HGPS animal model also reduced progerin expression, increased mean survival time, and improved health status in these animals. In vivo CRISPR/Cas treatment has been demonstrated in mice with promising outcomes in other disease models also (Cheng et al. 2014; Ding et al. 2014). Moreover, researchers have claimed the use of the CRISPR/Cas9 system in human preimplantation embryo for correction of MYBPC3 mutation (Ma et al. 2017), demonstrating a possible use of this technology in humans in the near future.
There are many factors that affect the efficacy and specificity of the CRISPR–Cas9 system as a therapeutic approach. It may produce off-target effects that can generate undesired mutations at random sites in the genome (Keep off-target effects in focus 2018). A pre-existing humoral and cell-mediated immunity against the Cas9 orthologs derived from Staphylococcus aureus and Streptococcus pyogenes, which are the most frequently used Cas9 orthologs, has been reported (Charlesworth et al. 2019). The presence of a pre-existing adaptive immune response against the CRISPR–Cas9 system may cause severe immune responses in the treated patients and also interfere with the systemic delivery of the system. The other challenges that affect the efficacy of the CRISPR–Cas9 system include low incidences of HDR (homology-directed repair)-mediated DNA repair, target DNA site selection and sgRNA design, and selection of Cas9 orthologs with appropriate PAM (protospacer adjacent motif) sequences (Lino et al. 2018).
Clinical trials for the treatment of HGPS
Currently, four major clinical trials for the treatment of HGPS are ongoing. Administration of lonafarnib, an FTI, to 26 HGPS patients in phase II clinical trial (NCT00425607) from 2007 to 2009 demonstrated remarkable improvement in weight gain, vascular architecture, and bone structure along with a decreased frequency of headaches and strokes (Gordon et al. 2012b, 2014). Furthermore, a recent clinical study conducted by Gordon et al. (2018) demonstrated an improvement in the life span of lonafarnib-treated patients as compared to no treatment (NCT00879034 and NCT00916747). Pravastatin (statin) and zoledronic acid (bisphosphonates) have also been used in combination with lonafarnib in clinical trials but failed to show any significant beneficial effect on cardiovascular defects as compared to lonafarnib monotherapy; however, an improvement in bone mineral density was noted (Gordon et al. 2016). Moreover, lonafarnib treatment (115–150 mg/m2 for 24 to 29 months) has been reported to reduce the prevalence of stroke, TIA, and headache in HGPS patients (Ullrich et al. 2013). A clinical study on the combined efficacy of lonafarnib, zoledronic acid, and pravastatin is being conducted with estimated 85 participants and is expected to be completed in July 2020 (NCT00916747).
Another phase I–II clinical trial involving two drugs, i.e., lonafarnib and everolimus (mTOR kinase inhibitor, rapamycin analog), began in April 2016 (NCT02579044). Phase I was completed in June 2017, which registered 17 patients. Phase II clinical trial started in July 2017 and 41 patients have been registered till February 2018 (Gordon 2019a). Despite the beneficial effects of lonafarnib, its effects on mortality and morbidity of the patients are still not very much known. Therefore, long-term studies are required to evaluate lonafarnib on these parameters. Another clinical study on the effect of umbilical cord blood transfusion on HGPS patients has started in March 2019 (NCT03871972).
Extracellular vesicles as a therapeutic candidate for HGPS?
The use of stem cell-derived extracellular vesicles (EVs) for the treatment of aging and aging-related disorders is nowadays drawing a lot of attention. EVs are membrane-bound carriers of bioactive molecular cargos (viz. RNA, proteins, growth factors) that are released from the cells and can fuse with a target cell to make changes in it (van Niel et al. 2018). The EVs derived from healthy/stem cells are therefore extensively studied for their therapeutic actions against various clinical disorders (Park et al. 2019; Reiner et al. 2017). Treatment with EVs also confers a therapeutic effect on multiple tissues/organs associated with aging (Panagiotou et al. 2018; Robbins 2017). Despite this, there are no reports of application of EVs on HGPS yet. The EVs derived from stem cells mediate the communication between the stem cells and the target damaged cells, which alters the functioning of the target cell. Recently, it has been reported that EVs derived from healthy MSCs (MSC-EVs) and human iPSC-EVs (iPSCs derived from human-induced pluripotent cells) can ameliorate aging phenotype in senescent MSCs and enhance proliferation (Liu et al. 2019b). However, human iPSC-EVs were more effective than MSC-EVs. They also demonstrated that under a defined culture condition, human iPSCs produce > 16-fold higher number of EVs as compared to MSCs. These highly purified nano-sized human iPSC-EVs carry an intracellular antioxidant enzyme “peroxiredoxin” in high concentration, which can alleviate cellular senescence by reducing oxidative stress and DNA damage in the cells. Moreover, human iPSC-EVs and MSCs could also attenuate premature aging in progerin-induced senescent model of MSCs that mimics HGPS at the cellular level (Liu et al. 2019b). In another recent report, the EVs containing extracellular nicotinamide phosphoribosyl transferase (eNAMPT) were isolated from young mice and cultured adipocytes and injected into aged mice (Yoshida et al. 2019). This treatment extended the life span of aged mice with enhanced physical activity in an increased NAD+ biosynthesis-dependent manner. There is one clinical trial being conducted on the efficacy of umbilical cord blood transfusion in HGPS patients as a stem cell therapy (NCT03871972). Treatment with this cell-free system (EVs) is safer, highly stable in circulation, high transport capability to target cells, and with fewer complications as compared to cell therapy (Adamiak et al. 2018; Villa et al. 2019).
There are several reports on the treatment of aging-related bone and cardiovascular disorders with EVs in in vitro and in vivo conditions (Alibhai et al. 2018; Chen et al. 2019; Yuan et al. 2018). EVs derived from a variety of stem cell and even nonstem cell sources have shown potential for preventing post-myocardial infarction (MI) remodeling in animal models. EVs have shown to repair and rejuvenate heart cells after MI. It reduces apoptosis, enhances cell proliferation, attenuates inflammatory responses, and induces angiogenesis (Alibhai et al. 2018; Yang et al. 2019). Several microRNAs (miR-126, 210, 146a, 19a, 21, 22) and bioactive molecules have been identified for such an action of the EVs (Alibhai et al. 2018; Yang et al. 2019). Further, microRNA-containing EVs have demonstrated atheroprotective activity. EVs derived from Krupple-like factor-2 (KLF2)-transduced or physiological shear stress-induced endothelial cells are rich in miR-143 and 145. These microRNA-enriched vesicles could prevent the formation of atherosclerotic lesions in Apo−/− mice fed on a high-fat diet (Hergenreider et al. 2012). Moreover, delivery of EVs enriched in miR-126 into the mice with endothelial damage can protect from atherosclerosis and promote vascular endothelial cell repair by inducing CXCL12/CXCR4 and suppressing SPRED1 pathway (Jansen et al. 2013; Zernecke et al. 2009). Further, endothelial progenitor cell (EPC)-derived microparticles (MPEs) have been shown to reduce the development of atherosclerotic lesions in hypertensive-hypercholesterolemic hamster model by alleviating accumulation of lipids and macrophages in the liver/arterial wall, reducing proinflammatory molecules, restoring cytokine/chemokine profiles, re-establishing cholesterol and triglyceride concentrations, improving blood pressure and heart rate, and ameliorating arterial dysfunction (Georgescu et al. 2016, 2017). It was also found that the mechanism of atheroprotective activity of MPEs involves the transfer of microRNAs such as miR-21, miR-126, miR-146a, and miR-223 to late EPCs (Georgescu et al. 2017).
In a recent study, stem cells obtained from human urine (USCs) were used for the production of EVs (USC-EVs) that could prevent bone loss and promote osteogenesis in a mouse model of osteoporosis (Chen et al. 2019). USC-EVs have also demonstrated wound-healing ability in diabetic mice (Chen et al. 2018). Intravenous injection of EVs isolated from human umbilical cord blood plasma (UCB-EVs) into aged osteoporotic mice reduced bone loss, increased osteogenesis, and reduced osteoclast activities. This effect of UCB-EVs was mediated by the delivery of miR-3960 into osteoblasts and osteoclasts, thereby inducing osteogenic differentiation but reducing the same in osteoclasts (Hu et al. 2019). EVs derived from MSCs and macrophages are also directly associated with bone repair and mineralization (Chu et al. 2019). Stem cell-derived EVs have also demonstrated high capacity of skin repair, wound healing, and attenuation of signs of skin aging (Ferreira and Gomes 2018).
EVs containing miR-9 can also be helpful for the treatment of HGPS. Nissan et al. have demonstrated that microRNA miR-9 overexpression in HGPS-iPSC-MSC can reduce progerin level and alleviate nuclear blebbing (Nissan et al. 2012). Several studies have reported the collection of miR-9 in EVs from different types of cells in culture conditions (Lu et al. 2018; Roballo et al. 2019; Yang et al. 2018). However, the efficacy of these types of EVs in progeric cells or animal models has not been tested yet. Furthermore, EVs can be bioengineered to load therapeutic cargos such as siRNA or small molecules and targeted drug delivery. EVs derived from bone marrow-MSCs and loaded with siRNA targeting oncogenic Kras (siKrasG12D iExo) have shown efficacy against pancreas ductal adenocarcinoma (PDAC) in mouse models (Mendt et al. 2018). This strategy is currently under phase I clinical trial (NCT03608631). The E-selectin-targeted delivery of therapeutic microRNA to endothelial cell with the help of EVs has also been shown to alleviate endothelial inflammation and atherosclerosis (Ma et al. 2016). Further, packaging of the CRISPR–Cas9 system into EVs and delivery to target cells in vivo has been reported that demonstrate the efficiency of EVs as a delivery system (Gulei and Berindan-Neagoe 2019; Lainšček et al. 2018). EVs can be delivered to multiple organ systems via various administrative routes including intravenous injections and oral administration (Lamichhane and Jay 2018; Sil et al. 2019).
Globally, there are several clinical trials (https://clinicaltrials.gov/) going on using EVs as therapeutics against various disorders including cancer (NCT03608631), diabetes (NCT02138331), macular holes (NCT03437759), kidney damage (Nassar et al. 2016), cerebrovascular disorder acute ischemic stroke (NCT03384433), and cutaneous wound healing (NCT02565264). Exosomes derived from dendritic cells and loaded tumor antigen are also under clinical trials for their vaccination efficacy against nonsmall cell lung cancer (NCT01159288). Further, plant-derived EVs are also being clinically tested for their efficacy against diseases like “oral mucositis linked with chemo- and radio-therapy of head and neck cancer (NCT01668849)” and polycystic ovary syndrome (NCT03493984). In total, the attributes discussed above make EVs a very promising therapeutic approach for HGPS. There are many other clinical trials being conducted on EVs/exosomes for their potential as prognostic marker for disorders like various types of cancer, neurodegenerative disorders, sepsis, obstructive sleep apnea syndrome, diabetes, bronchopulmonary dysplasia, inflammatory/allergic diseases, liver cirrhosis, and cardiovascular diseases (https://clinicaltrials.gov/).
Conclusion
A number of the new therapeutic strategies for the treatment of HGPS are arising. However, a deeper knowledge of the mechanism is still required for an effective treatment strategy to emerge and its evaluation. Since HGPS is a systemic disorder that affects multiple organs in the body, it is now becoming clear that targeting HGPS at a single physiological level is not enough to alleviate the disease significantly and bring the patients back to normal life. Therefore, multiple combinations of drugs should be used to decrease accumulation and increase the removal of progerin and its downstream effectors.
The limited availability of HGPS patients poses a challenge to clinical trials for these drugs. Despite the growing list of possible therapeutic approaches for HGPS, there is a lack of sufficient preclinical data for application in humans. Further, because of the limited pool of patients available for clinical trials, it is very difficult to study many approaches at a given time. Because of the unavailability of sufficient control subjects, the trial endpoints have to be compared with historical controls, and randomized, double-blind, control studies cannot be performed. In all the preclinical studies related to HGPS, the ability of the drug to target the cardiovascular system should be preferably evaluated, since it is the major pathophysiological reason for premature deaths in the patients.
Biotechnology can play a very important role in the cure of HGPS. The majority of the drug-based approaches can only target the downstream effectors of progerin, but cannot correct the causative mutation. However, some of them can target the aberrant splicing mechanism. Approaches such as gene therapy, cellular reprogramming, and the CRISPR–Cas9 system can target the causative mutation and the formation of defective protein progerin. However, the current developments in this field have been limited to laboratory animals and cell culture studies only, and human in vivo studies at the systemic level are required to develop those into a remedy for HGPS. Further, the therapeutic EVs from various stem cell/nonstem cell sources have shown success in ameliorating aging or aging-related ailments. Therefore, its efficacy needs to be evaluated against HGPS in cell/animal disease models. Finally, this review article will help the researchers to evaluate existing potential therapeutic strategies for HGPS and disease models for their examination.
We also propose the use of EVs derived from healthy stem cells for the treatment of HGPS. EVs are stable in biological fluids, capable of multidrug delivery, less immunogenic, and less toxic. They can be administered by various physiological routes and can cross various physical barriers and extracellular matrix. EVs can be loaded with a variety of cargo molecules including RNAs, proteins, and drug molecules and can protect their enclosed cargos from enzymatic/nonenzymatic degradation (Murphy et al. 2019; Sil et al. 2019). Further, bioengineered EVs can be utilized for the targeted delivery of therapeutic RNA and drug molecules.
Electronic supplementary material
(PDF 263 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdenur JE, Brown WT, Friedman S, Smith M, Lifshitz F. Response to nutritional and growth hormone treatment in progeria. Metab Clin Exp. 1997;46:851–856. doi: 10.1016/S0026-0495(97)90069-X. [DOI] [PubMed] [Google Scholar]
- Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, Karnas E, Xuan YT, Skupien-Rabian B, Chen X, Jankowska U, Girgis M, Sekula M, Davani A, Lasota S, Vincent RJ, Sarna M, Newell KL, Wang OL, Dudley N, Madeja Z, Dawn B, Zuba-Surma EK. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ Res. 2018;122:296–309. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler AS, Sinha S, Kawahara TLA, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibhai FJ, Tobin SW, Yeganeh A, Weisel RD, Li R-K. Emerging roles of extracellular vesicles in cardiac repair and rejuvenation. Am J Phys Heart Circ Phys. 2018;315:H733–H744. doi: 10.1152/ajpheart.00100.2018. [DOI] [PubMed] [Google Scholar]
- Aliper AM, Csoka AB, Buzdin A, Jetka T, Roumiantsev S, Moskalev A, Zhavoronkov A. Signaling pathway activation drift during aging: Hutchinson-Gilford progeria syndrome fibroblasts are comparable to normal middle-age and old-age cells. Aging. 2015;7:26–37. doi: 10.18632/aging.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Y, et al. Lamin A buffers CK2 kinase activity to modulate aging in a progeria mouse model. Sci Adv. 2019;5:eaav5078–eaav5078. doi: 10.1126/sciadv.aav5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio W, Genovese SI, Pizzolanti G, Giordano C. Hutchinson Gilford progeria syndrome: a therapeutic approach via adenoviral delivery of CRISPR/cas genome editing system. J Genet Syndr Gene Ther. 2015;6:1. doi: 10.4172/2157-7412.1000256. [DOI] [Google Scholar]
- Atchison L, Zhang H, Cao K, Truskey GA. A tissue engineered blood vessel model of Hutchinson-Gilford progeria syndrome using human iPSC-derived smooth muscle cells. Sci Rep. 2017;7:8168. doi: 10.1038/s41598-017-08632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augert A, Payré C, de Launoit Y, Gil J, Lambeau G, Bernard D. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 2009;10:271–277. doi: 10.1038/embor.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar MP, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e116. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmus G, et al. Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome. Nat Commun. 2018;9:1700. doi: 10.1038/s41467-018-03770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárcena C, et al. Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell Rep. 2018;24:2392–2403. doi: 10.1016/j.celrep.2018.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárcena C, López-Otín C, Kroemer G. Methionine restriction for improving progeria: another autophagy-inducing anti-aging strategy? Autophagy. 2019;15:558–559. doi: 10.1080/15548627.2018.1533059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárcena C, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25:1234–1242. doi: 10.1038/s41591-019-0504-5. [DOI] [PubMed] [Google Scholar]
- Barthélémy F, et al. Truncated prelamin A expression in HGPS-like patients: a transcriptional study. Eur J Hum Genet. 2015;23:1051–1061. doi: 10.1038/ejhg.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo MO, et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci U S A. 2002;99:13049–13054. doi: 10.1073/pnas.192460799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyret E, et al. Single-dose CRISPR–Cas9 therapy extends lifespan of mice with Hutchinson–Gilford progeria syndrome. Nat Med. 2019;25:419–422. doi: 10.1038/s41591-019-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikkul MU, Clements CS, Godwin LS, Goldberg MW, Kill IR, Bridger JM. Farnesyltransferase inhibitor and rapamycin correct aberrant genome organisation and decrease DNA damage respectively, in Hutchinson-Gilford progeria syndrome fibroblasts. Biogerontology. 2018;19:579–602. doi: 10.1007/s10522-018-9758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel S, et al. Induced pluripotent stem cells reveal functional differences between drugs currently investigated in patients with Hutchinson-Gilford progeria syndrome. Stem Cells Transl Med. 2014;3:510–519. doi: 10.5966/sctm.2013-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel S, et al. Drug screening on Hutchinson Gilford progeria pluripotent stem cells reveals aminopyrimidines as new modulators of farnesylation. Cell Death Dis. 2016;7:e2105–e2105. doi: 10.1038/cddis.2015.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger JM, Kill IR. Aging of Hutchinson–Gilford progeria syndrome fibroblasts is characterised by hyperproliferation and increased apoptosis. Exp Gerontol. 2004;39:717–724. doi: 10.1016/j.exger.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- Capell BC, et al. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2005;102:12879–12884. doi: 10.1073/pnas.0506001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell BC, et al. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci U S A. 2008;105:15902–15907. doi: 10.1073/pnas.0807840105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero D, Soria-Valles C, López-Otín C. Hallmarks of progeroid syndromes: lessons from mice and reprogrammed cells. Dis Model Mech. 2016;9:719–735. doi: 10.1242/dmm.024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni V, et al. Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur J Histochem: EJH. 2011;55:e36–e36. doi: 10.4081/ejh.2011.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Wang Y, Luxton GWG, Östlund C, Worman HJ, Gundersen GG. Imbalanced nucleocytoskeletal connections create common polarity defects in progeria and physiological aging. Proc Natl Acad Sci. 2019;116:3578. doi: 10.1073/pnas.1809683116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth CT, et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25:249–254. doi: 10.1038/s41591-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-J, et al. Dysregulated interactions between lamin A and SUN1 induce abnormalities in the nuclear envelope and endoplasmic reticulum in progeric laminopathies. J Cell Sci. 2014;127:1792. doi: 10.1242/jcs.139683. [DOI] [PubMed] [Google Scholar]
- Chen Z, et al. Reprogramming progeria fibroblasts re-establishes a normal epigenetic landscape. Aging Cell. 2017;16:870–887. doi: 10.1111/acel.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8:1607–1623. doi: 10.7150/thno.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019;7:18–18. doi: 10.1038/s41413-019-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R, et al. Efficient gene editing in adult mouse livers via adenoviral delivery of CRISPR/Cas9. FEBS Lett. 2014;588:3954–3958. doi: 10.1016/j.febslet.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP. S-Adenosylmethionine and methylation. FASEB J. 1996;10:471–480. doi: 10.1096/fasebj.10.4.8647346. [DOI] [PubMed] [Google Scholar]
- Chu C, Wei S, Wang Y, Wang Y, Man Y, Qu Y. Extracellular vesicle and mesenchymal stem cells in bone regeneration: recent progress and perspectives. J Biomed Mater Res A. 2019;107:243–250. doi: 10.1002/jbm.a.36518. [DOI] [PubMed] [Google Scholar]
- Chueh S-CJ, Kahan BD. Clinical application of sirolimus in renal transplantation: an update. Transpl Int. 2005;18:261–277. doi: 10.1111/j.1432-2277.2004.00039.x. [DOI] [PubMed] [Google Scholar]
- Coffinier C, et al. Direct synthesis of lamin A, bypassing prelamin a processing, causes misshapen nuclei in fibroblasts but no detectable pathology in mice. J Biol Chem. 2010;285:20818–20826. doi: 10.1074/jbc.M110.128835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbaro M, et al. Rescue of heterochromatin organization in Hutchinson-Gilford progeria by drug treatment. Cellular and Molecular Life Sciences : CMLS. 2005;62:2669–2678. doi: 10.1007/s00018-005-5318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnucci C, Bertini E. The potential of iPSCs for the treatment of premature aging disorders. Int J Mol Sci. 2017;18:2350. doi: 10.3390/ijms18112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu D, Csoka AB, Navara CS, Schatten GP. Defective DSB repair correlates with abnormal nuclear morphology and is improved with FTI treatment in Hutchinson-Gilford progeria syndrome fibroblasts. Exp Cell Res. 2010;316:2747–2759. doi: 10.1016/j.yexcr.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Csoka AB, et al. Genome-scale expression profiling of Hutchinson–Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelerated atherosclerosis. Aging Cell. 2004;3:235–243. doi: 10.1111/j.1474-9728.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa J, et al. Prelamin A causes progeria through cell-extrinsic mechanisms and prevents cancer invasion. Nat Commun. 2013;4:2268–2268. doi: 10.1038/ncomms3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- De Vos WH, et al. Increased plasticity of the nuclear envelope and hypermobility of telomeres due to the loss of A–type lamins. Biochim Biophys Acta Gen Subj. 2010;1800:448–458. doi: 10.1016/j.bbagen.2010.01.002. [DOI] [Google Scholar]
- Decker ML, Chavez E, Vulto I, Lansdorp PM. Telomere length in Hutchinson-Gilford progeria syndrome. Mech Ageing Dev. 2009;130:377–383. doi: 10.1016/j.mad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- del Campo L, et al. Vascular smooth muscle cell-specific progerin expression in a mouse model of Hutchinson–Gilford progeria syndrome promotes arterial stiffness: therapeutic effect of dietary nitrite. Aging Cell. 2019;18:e12936. doi: 10.1111/acel.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res. 2014;115:488–492. doi: 10.1161/CIRCRESAHA.115.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo DL, et al. Hutchinson-Gilford progeria syndrome: oral and craniofacial phenotypes. Oral Dis. 2009;15:187–195. doi: 10.1111/j.1601-0825.2009.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorado B, et al. Generation and characterization of a novel knockin minipig model of Hutchinson-Gilford progeria syndrome. Cell Discovery. 2019;5:16. doi: 10.1038/s41421-019-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubaj Y, et al. An inherited LMNA gene mutation in atypical progeria syndrome. Am J Med Genet A. 2012;158A:2881–2887. doi: 10.1002/ajmg.a.35557. [DOI] [PubMed] [Google Scholar]
- DuBose AJ, Lichtenstein ST, Petrash NM, Erdos MR, Gordon LB, Collins FS. Everolimus rescues multiple cellular defects in laminopathy-patient fibroblasts. Proc Natl Acad Sci U S A. 2018;115:4206–4211. doi: 10.1073/pnas.1802811115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egesipe A-L, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson–Gilford progeria syndrome cells. NPJ Aging Mech Dis. 2016;2:16026. doi: 10.1038/npjamd.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci: CMLS. 2014;71:4325–4346. doi: 10.1007/s00018-014-1677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elloso MM, et al. Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in Apo E-deficient mice. Am J Transplant. 2003;3:562–569. doi: 10.1034/j.1600-6143.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- Eriksson M, et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espandar R, Eraghi AS, Mardookhpour S. Simultaneous shoulder and hip dislocation in a 12-year-old girl with Hutchinson-Gilford progeria syndrome. Acta Medica Iranica. 2012;50:439–443. [PubMed] [Google Scholar]
- Evangelisti C, Cenni V, Lattanzi G. Potential therapeutic effects of the MTOR inhibitors for preventing ageing and progeria-related disorders. Br J Clin Pharmacol. 2016;82:1229–1244. doi: 10.1111/bcp.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AF, Gomes DA. Stem cell extracellular vesicles in skin repair. Bioengineering. 2018;6:4. doi: 10.3390/bioengineering6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley J. Cellular stress and AMPK activation as a common mechanism of action linking the effects of metformin and diverse compounds that alleviate accelerated aging defects in Hutchinson-Gilford progeria syndrome. Med Hypotheses. 2018;118:151–162. doi: 10.1016/j.mehy.2018.06.029. [DOI] [PubMed] [Google Scholar]
- Fong LG, et al. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci U S A. 2004;101:18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong LG, Frost D, Meta M, Qiao X, Yang SH, Coffinier C, Young SG. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science. 2006;311:1621. doi: 10.1126/science.1124875. [DOI] [PubMed] [Google Scholar]
- Fong LG, et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel D, Delecourt V, Harhouri K, De Sandre-Giovannoli A, Lévy N, Kaspi E, Roll P. MicroRNAs in hereditary and sporadic premature aging syndromes and other laminopathies. Aging Cell. 2018;17:e12766–e12766. doi: 10.1111/acel.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel D, Roedl D, Gordon LB, Djabali K. Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts. Aging Cell. 2015;14:78–91. doi: 10.1111/acel.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel D, Gordon LB, Djabali K. Temsirolimus partially rescues the Hutchinson-Gilford progeria cellular phenotype. PLoS One. 2016;11:e0168988–e0168988. doi: 10.1371/journal.pone.0168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel D, Shafry DD, Gordon LB, Djabali K. Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson-Gilford progeria fibroblasts. Oncotarget. 2017;8:64809–64826. doi: 10.18632/oncotarget.19363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Aguirre I, et al. Enhanced nuclear protein export in premature aging and rescue of the progeria phenotype by modulation of CRM1 activity. Aging Cell. 2019;18:e13002. doi: 10.1111/acel.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein & Cell. 2018;10:417–435. doi: 10.1007/s13238-018-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu A, Alexandru N, Andrei E, Dragan E, Cochior D, Dias S. Effects of transplanted circulating endothelial progenitor cells and platelet microparticles in atherosclerosis development. Biol Cell. 2016;108:219–243. doi: 10.1111/boc.201500104. [DOI] [PubMed] [Google Scholar]
- Georgescu A, Alexandru N, Dragan E, Safciuc F, Daraban AM, Badila E. Endothelial progenitor cells - derived microparticles reproduce the favorable role of their parent cells of healthy origins in the treatment of atherosclerosis via microrna transfer. Atherosclerosis. 2017;263:e48. doi: 10.1016/j.atherosclerosis.2017.06.163. [DOI] [Google Scholar]
- Gesson K, Rescheneder P, Skoruppa MP, von Haeseler A, Dechat T, Foisner R. A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res. 2016;26:462–473. doi: 10.1101/gr.196220.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanemi A. Cell cultures in drug development: applications, challenges and limitations. Saudi Pharm J. 2015;23:453–454. doi: 10.1016/j.jsps.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Zhou Z. Genetics of aging, progeria and lamin disorders. Curr Opin Genet Dev. 2014;26:41–46. doi: 10.1016/j.gde.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Glynn MW, Glover TW. Incomplete processing of mutant lamin A in Hutchinson–Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum Mol Genet. 2005;14:2959–2969. doi: 10.1093/hmg/ddi326. [DOI] [PubMed] [Google Scholar]
- Goldman RD, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009;28:2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S, Kreienkamp R. DNA repair defects and genome instability in Hutchinson-Gilford progeria syndrome. Curr Opin Cell Biol. 2015;34:75–83. doi: 10.1016/j.ceb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB (2019a) Clinical trials. The Progeria Research Foundation https://www.progeriaresearch.org/clinical-trials/.
- Gordon LB (2019b) Find the children. Progeria Research Foundation https://www.progeriaresearch.org/find-the-children/.
- Gordon LB. The progeria handbook: a guide for families & health care providers of children with progeria. Peabody, MA: Progeria Research Foundation; 2019. [Google Scholar]
- Gordon LB (2019d) The Progeria Research Foundation Cell and Tissue Bank. Progeria Research Foundation https://www.progeriaresearch.org/wp-content/uploads/2019/11/PRF-AVAILABLE-CELL-LINES-1.pdf.
- Gordon LB, Brown WT, Collins FS (2003) Hutchinson-Gilford progeria syndrome. University of Washington. https://www.ncbi.nlm.nih.gov/books/NBK1121/ Accessed 03 April 2019 2019 [PubMed]
- Gordon LB, McCarten KM, Giobbie-Hurder A, Machan JT, Campbell SE, Berns SD, Kieran MW. Disease progression in Hutchinson-Gilford progeria syndrome: impact on growth and development. Pediatrics. 2007;120:824. doi: 10.1542/peds.2007-1357. [DOI] [PubMed] [Google Scholar]
- Gordon LB, Cao K, Collins FS. Progeria: translational insights from cell biology. J Cell Biol. 2012;199:9–13. doi: 10.1083/jcb.201207072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2012;109:16666–16671. doi: 10.1073/pnas.1202529109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, et al. Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation. 2014;130:27–34. doi: 10.1161/CIRCULATIONAHA.113.008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, et al. Clinical trial of the protein farnesylation inhibitors lonafarnib, pravastatin, and zoledronic acid in children with Hutchinson-Gilford progeria syndrome. Circulation. 2016;134:114–125. doi: 10.1161/CIRCULATIONAHA.116.022188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, et al. Association of lonafarnib treatment vs no treatment with mortality rate in patients with Hutchinson-Gilford progeria syndrome. JAMA. 2018;319:1687–1695. doi: 10.1001/jama.2018.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CM, et al. Extraskeletal calcifications in Hutchinson-Gilford progeria syndrome. Bone. 2019;125:103–111. doi: 10.1016/j.bone.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griveau A, et al. The PLA2R1-JAK2 pathway upregulates ERRα and its mitochondrial program to exert tumor-suppressive action. Oncogene. 2016;35:5033. doi: 10.1038/onc.2016.43. [DOI] [PubMed] [Google Scholar]
- Griveau A, et al. Targeting the phospholipase A2 receptor ameliorates premature aging phenotypes. Aging Cell. 2018;17:e12835–e12835. doi: 10.1111/acel.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiani E, et al. Otologic and audiologic manifestations of Hutchinson-Gilford progeria syndrome. Laryngoscope. 2011;121:2250–2255. doi: 10.1002/lary.22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulei D, Berindan-Neagoe I. Activation of necroptosis by engineered self tumor-derived exosomes loaded with CRISPR/Cas9. Mol Ther Nucleic Acids. 2019;17:448–451. doi: 10.1016/j.omtn.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavo Monnerat GPCE, Evaristo JAM, dos Santos CGM, Maciel GCL, Carvalho VO, Nogueira FCS, Domont GB, de Carvalho ACC. Metabolomic profiling suggests systemic signatures of premature aging induced by Hutchinson–Gilford progeria syndrome. Metabolomics. 2019;15:100. doi: 10.1007/s11306-019-1558-6. [DOI] [PubMed] [Google Scholar]
- Hamczyk MR, Andrés V. Accelerated atherosclerosis in HGPS. Aging. 2018;10:2555–2556. doi: 10.18632/aging.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamczyk MR, et al. Progerin accelerates atherosclerosis by inducing endoplasmic reticulum stress in vascular smooth muscle cells. EMBO Molecular Medicine. 2019;11:e9736. doi: 10.15252/emmm.201809736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhouri K et al. (2016) Antisense-based progerin downregulation in HGPS-like patients’ cells. Cells 5:31. doi:10.3390/cells5030031 [DOI] [PMC free article] [PubMed]
- Harhouri K, et al. MG132-induced progerin clearance is mediated by autophagy activation and splicing regulation. EMBO Mol Med. 2017;9:1294–1313. doi: 10.15252/emmm.201607315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema L, Youssef SA, de Bruin A. Pathology of mouse models of accelerated aging. Vet Pathol. 2016;53:366–389. doi: 10.1177/0300985815625169. [DOI] [PubMed] [Google Scholar]
- Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RCM. Hutchinson–Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140A:2603–2624. doi: 10.1002/ajmg.a.31346. [DOI] [PubMed] [Google Scholar]
- Hergenreider E, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- Hernandez L, et al. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev Cell. 2010;19:413–425. doi: 10.1016/j.devcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JC, et al. Generation of induced pluripotent stem cell lines from 3 distinct laminopathies bearing heterogeneous mutations in lamin A/C. Aging. 2011;3:380–390. doi: 10.18632/aging.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y-H, et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 2019;25:407–418.e406. doi: 10.1016/j.stem.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism. 2019;95:93–101. doi: 10.1016/j.metabol.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Huang S, Chen L, Libina N, Janes J, Martin GM, Campisi J, Oshima J. Correction of cellular phenotypes of Hutchinson-Gilford progeria cells by RNA interference. Hum Genet. 2005;118:444–450. doi: 10.1007/s00439-005-0051-7. [DOI] [PubMed] [Google Scholar]
- Ibrahim MX, Sayin VI, Akula MK, Liu M, Fong LG, Young SG, Bergo MO. Targeting isoprenylcysteine methylation ameliorates disease in a mouse model of progeria. Science. 2013;340:1330–1333. doi: 10.1126/science.1238880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen F, et al. Endothelial microparticle–mediated transfer of microRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- Kang HT, et al. Chemical screening identifies ROCK as a target for recovering mitochondrial function in Hutchinson-Gilford progeria syndrome. Aging Cell. 2017;16:541–550. doi: 10.1111/acel.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuumi G, Shimizu I, Yoshida Y, Minamino T. Vascular senescence in cardiovascular and metabolic diseases. Front Cardiovasc Med. 2018;5:18–18. doi: 10.3389/fcvm.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, et al. Rapamycin rescues age-related changes in muscle-derived stem/progenitor cells from progeroid mice. Molecular Therapy - Methods & Clinical Development. 2019;14:64–76. doi: 10.1016/j.omtm.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep off-target effects in focus Nat Med. 2018;24:1081–1081. doi: 10.1038/s41591-018-0150-3. [DOI] [PubMed] [Google Scholar]
- Kelley JB, et al. The defective nuclear lamina in Hutchinson-Gilford progeria syndrome disrupts the nucleocytoplasmic Ran gradient and inhibits nuclear localization of Ubc9. Mol Cell Biol. 2011;31:3378–3395. doi: 10.1128/MCB.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran MW, Gordon L, Kleinman M. New approaches to progeria. Pediatrics. 2007;120:834. doi: 10.1542/peds.2007-1356. [DOI] [PubMed] [Google Scholar]
- Kim YC, Guan K-L. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012;11:125–140. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienkamp R, et al. Vitamin D receptor signaling improves Hutchinson-Gilford progeria syndrome cellular phenotypes. Oncotarget. 2016;7:30018–30031. doi: 10.18632/oncotarget.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienkamp R, et al. A cell-intrinsic interferon-like response links replication stress to cellular aging caused by progerin. Cell Rep. 2018;22:2006–2015. doi: 10.1016/j.celrep.2018.01.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienkamp R, et al. Doubled lifespan and patient-like pathologies in progeria mice fed high-fat diet. Aging Cell. 2019;18:e12852. doi: 10.1111/acel.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben N, et al. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk MU, Kim JW, Lee Y-S, Kyung AC, Park JT, Park SC. Alleviation of senescence via ATM inhibition in accelerated aging models. Mol Cells. 2019;42:210–217. doi: 10.14348/molcells.2018.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainšček D, Kadunc L, Keber MM, Bratkovič IH, Romih R, Jerala R. Delivery of an artificial transcription regulator dCas9-VPR by extracellular vesicles for therapeutic gene activation. ACS Synth Biol. 2018;7:2715–2725. doi: 10.1021/acssynbio.8b00192. [DOI] [PubMed] [Google Scholar]
- Lamichhane TN, Jay SM. Production of extracellular vesicles loaded with therapeutic cargo. Methods Mol Biol. 2018;1831:37–47. doi: 10.1007/978-1-4939-8661-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science. 2014;344:527. doi: 10.1126/science.1252651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu D, Viré E, Robson S, Breusegem SY, Kouzarides T, Jackson SP. Inhibition of the acetyltransferase NAT10 normalizes progeric and aging cells by rebalancing the Transportin-1 nuclear import pathway. Sci Signal. 2018;11:eaar5401. doi: 10.1126/scisignal.aar5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O, Morita M, Topisirovic I, Alain T, Blouin M-J, Pollak M, Sonenberg N. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci U S A. 2012;109:8977–8982. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani M, et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, et al. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J Clin Invest. 2016;126:1592–1602. doi: 10.1172/JCI85908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-J, et al. Interruption of progerin-lamin A/C binding ameliorates Hutchinson-Gilford progeria syndrome phenotype. J Clin Invest. 2016;126:3879–3893. doi: 10.1172/JCI84164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Transient introduction of human telomerase mRNA improves hallmarks of progeria cells. Aging Cell. 2019;18:e12979–e12979. doi: 10.1111/acel.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-Y, et al. Rapamycin reverses metabolic deficits in lamin A/C-deficient mice. Cell Rep. 2016;17:2542–2552. doi: 10.1016/j.celrep.2016.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25:1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, et al. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11:780. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- Liu G-H, et al. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature. 2011;472:221. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G-H, et al. Targeted gene correction of laminopathy-associated LMNA mutations in patient-specific iPSCs. Cell Stem Cell. 2011;8:688–694. doi: 10.1016/j.stem.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012;16:738–750. doi: 10.1016/j.cmet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Liu C, Arnold R, Henriques G, Djabali K (2019a) Inhibition of JAK-STAT signaling with Baricitinib reduces inflammation and improves cellular homeostasis in progeria cells. Cells 8:1276. doi:10.3390/cells8101276 [DOI] [PMC free article] [PubMed]
- Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW, Cheng L. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells. 2019;37:779–790. doi: 10.1002/stem.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein CJ, Bennett JA. New vascular insights into premature aging. J Clin Invest. 2019;129:492–493. doi: 10.1172/JCI125616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Djabali K. Autophagic removal of farnesylated carboxy-terminal lamin peptides. Cells. 2018;7:33. doi: 10.3390/cells7040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, et al. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J Exp Clin Cancer Res. 2018;37:147–147. doi: 10.1186/s13046-018-0814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, et al. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep. 2016;6:22910. doi: 10.1038/srep22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–419. doi: 10.1038/nature23305. [DOI] [PubMed] [Google Scholar]
- Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179–268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Mannick JB, et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018;10:eaaq1564. doi: 10.1126/scitranslmed.aaq1564. [DOI] [PubMed] [Google Scholar]
- Mariño G, Ugalde AP, Fernández ÁF, Osorio FG, Fueyo A, Freije JM, López-Otín C. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc Natl Acad Sci. 2010;107:16268–16273. doi: 10.1073/pnas.1002696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marji J, et al. Defective lamin A-Rb signaling in Hutchinson-Gilford progeria syndrome and reversal by farnesyltransferase inhibition. PLoS One. 2010;5:e11132. doi: 10.1371/journal.pone.0011132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos J, et al. Next-generation sequencing and quantitative proteomics of Hutchinson-Gilford progeria syndrome-derived cells point to a role of nucleotide metabolism in premature aging. PLoS One. 2018;13:e0205878. doi: 10.1371/journal.pone.0205878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrone G, Thandavarayan RA, Walther BK, Meng S, Mojiri A, Cooke JP. Dysfunction of iPSC-derived endothelial cells in human Hutchinson–Gilford progeria syndrome. Cell Cycle. 2019;18:2495–2508. doi: 10.1080/15384101.2019.1651587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli E, et al. Statins and histone deacetylase inhibitors affect lamin A/C-histone deacetylase 2 interaction in human cells. Front Cell and Dev Biol. 2019;7:6. doi: 10.3389/fcell.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral P, Bárcena C, López-Otín C (2018) Chapter 51—progeria mouse models. In: Ram JL, Conn PM (eds) Conn’s handbook of models for human aging (2nd Edition). Academic, pp 689–701. doi: 10.1016/B978-0-12-811353-0.00051-8
- Mazereeuw-Hautier J, Wilson L, Mohammed S, Smallwood D, Shackleton S, Atherton D, Harper J. Hutchinson–Gilford progeria syndrome: clinical findings in three patients carrying the G608G mutation in LMNA and review of the literature. Br J Dermatol. 2007;156:1308–1314. doi: 10.1111/j.1365-2133.2007.07897.x. [DOI] [PubMed] [Google Scholar]
- McKenna TS, Baek J-H, Eriksson M. Laminopathies. In: Puiu M, editor. Genetic disorders. London: IntechOpen Limited; 2013. pp. 27–64. [Google Scholar]
- Mehta IS, Eskiw CH, Arican HD, Kill IR, Bridger JM. Farnesyltransferase inhibitor treatment restores chromosome territory positions and active chromosome dynamics in Hutchinson-Gilford progeria syndrome cells. Genome Biol. 2011;12:R74. doi: 10.1186/gb-2011-12-8-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendt M et al (2018) Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3. 10.1172/jci.insight.99263 [DOI] [PMC free article] [PubMed]
- Merideth MA, et al. Phenotype and course of Hutchinson–Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol: Series A. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistriotis P, et al. NANOG reverses the myogenic differentiation potential of senescent stem cells by restoring actin filamentous organization and SRF-dependent gene expression. Stem Cells. 2017;35:207–221. doi: 10.1002/stem.2452. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Moulson CL, Go G, Gardner JM, van der Wal AC, Smitt JHS, van Hagen JM, Miner JH. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Investig Dermatol. 2005;125:913–919. doi: 10.1111/j.0022-202X.2005.23846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR−/− mice despite severe hypercholesterolemia. Atherosclerosis. 2008;198:39–48. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:32. doi: 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Hoffman AR, Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem. 2014;289:27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar W, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21–21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro CL, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet. 2004;13:2493–2503. doi: 10.1093/hmg/ddh265. [DOI] [PubMed] [Google Scholar]
- Navarro CL, et al. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of lamin A precursors. Hum Mol Genet. 2005;14:1503–1513. doi: 10.1093/hmg/ddi159. [DOI] [PubMed] [Google Scholar]
- Navarro CL, et al. New ZMPSTE24 (FACE1) mutations in patients affected with restrictive dermopathy or related progeroid syndromes and mutation update. Eur J Hum Genet. 2014;22:1002. doi: 10.1038/ejhg.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissan X, Blondel S, Peschanski M (2011) In vitro pathological modelling using patient-specific induced pluripotent stem cells: the case of progeria, vol vol 39. Portland Press Limited. 10.1042/BST20110659 [DOI] [PubMed]
- Nissan X, et al. Unique preservation of neural cells in Hutchinson-Gilford progeria syndrome is due to the expression of the neural-specific miR-9 microRNA. Cell Rep. 2012;2:1–9. doi: 10.1016/j.celrep.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Ocampo A, et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–1733. e1712. doi: 10.1016/j.cell.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio FG, et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med. 2011;3:106ra107–106ra107. doi: 10.1126/scitranslmed.3002847. [DOI] [PubMed] [Google Scholar]
- Osorio FG, et al. Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012;26:2311–2324. doi: 10.1101/gad.197954.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadya Y, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco LM, Gomez LA, Dias J, Ziebarth NM, Howard GA, Schiller PC. Progerin expression disrupts critical adult stem cell functions involved in tissue repair. Aging (Albany NY) 2014;6:1049. doi: 10.18632/aging.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotou N, Neytchev O, Selman C, Shiels PG. Extracellular vesicles, ageing, and therapeutic interventions. Cells. 2018;7:110. doi: 10.3390/cells7080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson–Gilford progeria syndrome dermal fibroblasts. Exp Dermatol. 2017;26:889–895. doi: 10.1111/exd.13323. [DOI] [PubMed] [Google Scholar]
- Park JT, Kang HT, Park CH, Lee Y-S, Cho KA, Park SC. A crucial role of ROCK for alleviation of senescence-associated phenotype. Exp Gerontol. 2018;106:8–15. doi: 10.1016/j.exger.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Park K-S, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288. doi: 10.1186/s13287-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro G, Kubben N, Wickert U, Göhler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol. 2009;11:1261. doi: 10.1038/ncb1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini C, et al. All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype. Oncotarget. 2015;6:29914. doi: 10.18632/oncotarget.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendás AM, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase–deficient mice. Nat Genet. 2002;31:94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- Petrini S, et al. Aged induced pluripotent stem cell (iPSCs) as a new cellular model for studying premature aging. Aging (Albany NY) 2017;9:1453. doi: 10.18632/aging.101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarowicz K, Machowska M, Dzianisava V, Rzepecki R. Hutchinson-Gilford progeria syndrome—current status and prospects for gene therapy treatment. Cells. 2019;8:88. doi: 10.3390/cells8020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M, et al. Boosting ATM activity alleviates aging and extends lifespan in a mouse model of progeria. eLife. 2018;7:e34836. doi: 10.7554/eLife.34836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajawat Y, Hilioti Z, Bossis I. Autophagy: a target for retinoic acids. Autophagy. 2010;6:1224–1226. doi: 10.4161/auto.6.8.13793. [DOI] [PubMed] [Google Scholar]
- Rajawat Y, Hilioti Z, Bossis I. Retinoic acid induces autophagosome maturation through redistribution of the cation-independent mannose-6-phosphate receptor. Antioxid Redox Signal. 2011;14:2165–2177. doi: 10.1089/ars.2010.3491. [DOI] [PubMed] [Google Scholar]
- Ramos FJ, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C–deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103–144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner AT, et al. Concise review: Developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl Med. 2017;6:1730–1739. doi: 10.1002/sctm.17-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J, et al. Biomechanical strain exacerbates inflammation on a progeria-on-a-chip model. Small. 2017;13:1603737. doi: 10.1002/smll.201603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SA, Muter J, Ritchie P, Lattanzi G, Hutchison CJ. The accumulation of un-repairable DNA damage in laminopathy progeria fibroblasts is caused by ROS generation and is prevented by treatment with N-acetyl cysteine. Hum Mol Genet. 2011;20:3997–4004. doi: 10.1093/hmg/ddr327. [DOI] [PubMed] [Google Scholar]
- Rivera-Torres J, et al. Identification of mitochondrial dysfunction in Hutchinson–Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture. J Proteome. 2013;91:466–477. doi: 10.1016/j.jprot.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Roballo KCS, et al. Neurons-derived extracellular vesicles promote neural differentiation of ADSCs: a model to prevent peripheral nerve degeneration. Sci Rep. 2019;9:11213–11213. doi: 10.1038/s41598-019-47229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD. Extracellular vesicles and aging. Stem Cell Investigation. 2017;4:98. doi: 10.21037/sci.2017.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong N et al (2019) Restoring extracellular matrix synthesis in senescent stem cells. FASEB J 201900377R. 10.1096/fj.201900377R [DOI] [PMC free article] [PubMed]
- Roos CM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rork JF, Huang JT, Gordon LB, Kleinman M, Kieran MW, Liang MG. Initial cutaneous manifestations of Hutchinson-Gilford progeria syndrome. Pediatr Dermatol. 2014;31:196–202. doi: 10.1111/pde.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengardten Y, McKenna T, Grochová D, Eriksson M. Stem cell depletion in Hutchinson–Gilford progeria syndrome. Aging Cell. 2011;10:1011–1020. doi: 10.1111/j.1474-9726.2011.00743.x. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Sagelius H, Rosengardten Y, Schmidt E, Sonnabend C, Rozell B, Eriksson M. Reversible phenotype in a mouse model of Hutchinson–Gilford progeria syndrome. J Med Genet. 2008;45:794–801. doi: 10.1136/jmg.2008.060772. [DOI] [PubMed] [Google Scholar]
- Santiago-Fernández O, et al. Development of a CRISPR/Cas9-based therapy for Hutchinson–Gilford progeria syndrome. Nat Med. 2019;25:423–426. doi: 10.1038/s41591-018-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11:440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebryannyy L, Misteli T. Protein sequestration at the nuclear periphery as a potential regulatory mechanism in premature aging. J Cell Biol. 2018;217:21–37. doi: 10.1083/jcb.201706061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker DK, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieprath T, et al. Sustained accumulation of prelamin A and depletion of lamin A/C both cause oxidative stress and mitochondrial dysfunction but induce different cell fates. Nucleus. 2015;6:236–246. doi: 10.1080/19491034.2015.1050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil S, Dagur RS, Liao K, Peeples ES, Hu G, Periyasamy P, Buch S (2019) Strategies for the use of extracellular vesicles for the delivery of therapeutics. J NeuroImmune Pharmacol. 10.1007/s11481-019-09873-y [DOI] [PMC free article] [PubMed]
- Sinha JK, Ghosh S, Raghunath M. Progeria: a rare genetic premature ageing disorder. Indian J Med Res. 2014;139:667. [PMC free article] [PubMed] [Google Scholar]
- Spear ED, et al. A humanized yeast system to analyze cleavage of prelamin A by ZMPSTE24. Methods. 2019;157:47–55. doi: 10.1016/j.ymeth.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandgren C, et al. Transgene silencing of the Hutchinson-Gilford progeria syndrome mutation results in a reversible bone phenotype, whereas resveratrol treatment does not show overall beneficial effects. FASEB J. 2015;29:3193–3205. doi: 10.1096/fj.14-269217. [DOI] [PubMed] [Google Scholar]
- Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-κB in aging and disease. Aging Dis. 2011;2:449. [PMC free article] [PubMed] [Google Scholar]
- Tilstra JS, et al. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J Clin Invest. 2012;122:2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth JI, et al. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc Natl Acad Sci. 2005;102:12873–12878. doi: 10.1073/pnas.0505767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich NJ, Gordon LB (2015) Chapter 18—Hutchinson–Gilford progeria syndrome. In: Islam MP, Roach ES (eds) Handbook of clinical neurology, vol vol 132. Elsevier, pp 249–264. 10.1016/B978-0-444-62702-5.00018-4 [DOI] [PubMed]
- Ullrich NJ, et al. Neurologic features of Hutchinson-Gilford progeria syndrome after lonafarnib treatment. Neurology. 2013;81:427–430. doi: 10.1212/WNL.0b013e31829d85c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Varela I, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med. 2008;14:767–772. doi: 10.1038/nm1786. [DOI] [PubMed] [Google Scholar]
- Varga R, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Verstraeten V, Broers J, Ramaekers F, van Steensel M. The nuclear envelope, a key structure in cellular integrity and gene expression. Curr Med Chem. 2007;14:1231–1248. doi: 10.2174/092986707780598032. [DOI] [PubMed] [Google Scholar]
- Vidak S, Foisner R. Molecular insights into the premature aging disease progeria. Histochem Cell Biol. 2016;145:401–417. doi: 10.1007/s00418-016-1411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidak S, Kubben N, Dechat T, Foisner R. Proliferation of progeria cells is enhanced by lamina-associated polypeptide 2α (LAP2α) through expression of extracellular matrix proteins. Genes Dev. 2015;29:2022–2036. doi: 10.1101/gad.263939.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa F, Quarto R, Tasso R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics. 2019;11:557. doi: 10.3390/pharmaceutics11110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acín-Pérez R, Enriquez JA, López-Otín C, Andrés V. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2013;127:2442–2451. doi: 10.1161/CIRCULATIONAHA.112.000571. [DOI] [PubMed] [Google Scholar]
- Waksman R, et al. Oral rapamycin inhibits growth of atherosclerotic plaque in apoE knock-out mice. Cardiovasc Radiat Med. 2003;4:34–38. doi: 10.1016/S1522-1865(03)00121-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. A mutation abolishing the ZMPSTE24 cleavage site in prelamin A causes a progeroid disorder. J Cell Sci. 2016;129:1975–1980. doi: 10.1242/jcs.187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZM, et al. Methylene blue alleviates nuclear and mitochondrial abnormalities in progeria. Aging Cell. 2016;15:279–290. doi: 10.1111/acel.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z-M, O’Donovan M, Sun L, Choi JY, Ren M, Cao K. Anti-aging potentials of methylene blue for human skin longevity. Sci Rep. 2017;7:2475. doi: 10.1038/s41598-017-02419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson–Gilford progeria syndrome mutation. Proc Natl Acad Sci. 2005;102:10291–10296. doi: 10.1073/pnas.0504641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, et al. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest. 2006;116:2115–2121. doi: 10.1172/JCI28968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Andres DA, Spielmann HP, Young SG, Fong LG. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J Clin Invest. 2008;118:3291–3300. doi: 10.1172/JCI35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Qiao X, Fong LG, Young SG. Treatment with a farnesyltransferase inhibitor improves survival in mice with a Hutchinson–Gilford progeria syndrome mutation. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2008;1781:36–39. doi: 10.1016/j.bbalip.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, et al. Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Hum Mol Genet. 2011;20:436–444. doi: 10.1093/hmg/ddq490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, et al. Exosomal miR-9 released from HIV tat stimulated astrocytes mediates microglial migration. J NeuroImmune Pharmacol. 2018;13:330–344. doi: 10.1007/s11481-018-9779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhu J, Zhang C, Wang J, Yue F, Jia X, Liu H. Stem cell-derived extracellular vesicles for myocardial infarction: a meta-analysis of controlled animal studies. Aging. 2019;11:1129–1150. doi: 10.18632/aging.101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Fu L. In vivo delivery of morpholino oligos as therapeutics: what barriers still exist? Journal of Drug Discovery Development and Delivery. 2016;3:1017. [Google Scholar]
- Yosef R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M et al. (2019) Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metabolism 30:329–342.e325. doi: 10.1016/j.cmet.2019.05.015 [DOI] [PMC free article] [PubMed]
- Young SG, Yang SH, Davies BS, Jung H-J, Fong LG. Targeting protein prenylation in progeria. Sci Transl Med. 2013;5:171ps173–171ps173. doi: 10.1126/scitranslmed.3005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H-X, Russell RC, Guan K-L. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy. 2013;9:1983–1995. doi: 10.4161/auto.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F-L, et al. Osteoclast-derived extracellular vesicles: novel regulators of osteoclastogenesis and osteoclast–osteoblasts communication in bone remodeling. Front Physiol. 2018;9:628. doi: 10.3389/fphys.2018.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A: Biomed Sci Medi Sci. 2013;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 263 kb)