Abstract
Noisy environment often occurs in hospitals. We set out to determine whether noisy environment induces neuroinflammation and impairment of learning and memory and whether the effects of noise contribute to the development of neuroinflammation and impairment of learning and memory during the perioperative period. Seven-week old CD-1 male mice were exposed to noisy environment in the presence or absence of surgery (right carotid artery exposure). Noisy environment was 75 db, 6 h/day, for 3 days or 5 days. Minocycline (40 mg/kg), an antibiotic with anti-inflammatory property, was administered intraperitoneally 1 h before surgery or each episode of noise. The learning and memory of mice were assessed by Barnes maze and fear conditioning tests. Brain was harvested for the determination of interleukin (IL)-1β and IL-6 and for immunohistochemical staining. We found that noise induced learning and memory impairment. Noise also increased IL-1β, IL-6 and ionized calcium binding adapter molecule 1 (Iba-1) in the hippocampus. The combination of noisy environment and surgery induced dysfunction of additional domains of learning and memory and a higher expression of Iba-1 in the hippocampus. The effects of noisy environment or the combination of noisy environment and surgery were attenuated by minocycline. These findings suggest that noisy environment induces neuroinflammation and impairment of learning and memory. These effects may contribute to the development of neuroinflammation and dysfunction of learning and memory during the perioperative period. Neuroinflammation may be an underlying pathophysiological process for cognitive dysfunction induced by noise or the combination of noise and surgery. Minocycline may be effective in attenuating these noise-induced effects.
Keywords: Hippocampus, mice, minocycline, neuroinflammation, noisy environment, postoperative cognitive dysfunction
1. Introduction
Postoperative cognitive dysfunction (POCD) is a common clinical syndrome that includes disturbance of learning, memory, and information processing after anesthesia and surgery (Bedford, 1955, Monk et al., 2008, Terrando et al., 2011a). POCD is associated with an increase of postoperative morbidity, mortality, length hospital stay and cost of care (Moller et al., 1998, Monk et al., 2008, Steinmetz et al., 2009). The pathogenesis of POCD is not fully understood and may involve many perioperative factors (Newman et al., 2007, Monk et al., 2008, Tang et al., 2011). Thus, it is imperative to identify risk factors and determine effective strategies to reduce the occurrence of POCD.
Many studies have shown that comfortable environment improves sleep quality and reduces pain and anxiety during the postoperative period (Salimpoor et al., 2011, Croom, 2012, Dobek et al., 2014). Comfortable music and good sleep help learning and memory function in hospitalized patients (Xu et al., 2010, Zhao et al., 2010, Aleisa et al., 2011). However, noisy environment often occur in critical care unit or general ward, especially in countries and regions with limited medical resource (Basner et al., 2014). It is clear that noisy environment causes dysfunction of learning and memory in healthy humans (Basner et al., 2014, Wright et al., 2016). Noisy environment also induces learning and memory impairment in senescence-accelerated prone mice (Cui et al., 2018). However, the role of noisy environment in the development of POCD is not known.
Neuroinflammation may be an underlying pathophysiological process for POCD (Cibelli et al., 2010, Cao et al., 2012). These neuroinflammatory responses include increased proinflammatory cytokines, such as interleukin (IL)-1β and IL-6, and ionized calcium binding adapter molecule 1 (Iba-1), a microglial marker (Cao et al., 2012, Zhang et al., 2014b). Sleep disturbance can induce neuroinflammation and dysfunction of learning and memory (Zhu et al., 2012). Since noisy environment can cause sleep disturbance (Basner et al., 2014, Wright et al., 2016), it is possible that noisy environment can cause neuroinflammation. However, it is not known yet whether noisy environment induces widespread neuroinflammation.
Based on the above information, we hypothesize that noisy environment induces neuroinflammation and dysfunction of learning and memory, which aggravates these pathological changes induced by surgery. This study was aimed to address this hypothesis. We subjected mice to noisy environment and surgery. Their learning and memory were determined by Barnes maze and fear conditioning tests. Minocycline, an anti-inflammatory agent (Tan et al., 2015), was used to determine the role of neuroinflammation in the learning and memory dysfunction after being exposed to noisy environment.
2. Materials and Methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80–23) revised in 2011.
2.1. Animal Groups
Seven week-old male CD-1 mice weighing 28 – 32 g from Charles River Laboratories International Inc. (Wilmington, MA, USA) were randomly assigned in the experiment 1 to: 1) control group (not being exposed to surgery or noise), 2) surgery group (right carotid artery exposure), 3) noise group (exposed to noisy environment for 5 days, 6 h/day), 4) surgery + 3 day noise group (noisy environment for 3 days after surgery), and 5) surgery + 5 day noise group (noisy environment for 2 days before surgery and 3 days after surgery) or in the experiment 2 to: 1) control group, 2) noise group, 3) noise + minocycline group, 4) surgery + 5 day noise group, and 5) surgery + 5 day noise + minocycline group. Each group had 15 mice for learning and memory tests and 6 mice for ELISA and immunohistochemistry assay.
2.2. Anesthesia and Surgery
The surgery was a right carotid artery exposure. As we described before (Fan et al., 2016), mice were anesthetized by 2% isoflurane. During the procedure, mice were spontaneously breathing and their rectal temperature was monitored and maintained at 37°C with the aid of a heating blanket (TCAT-2LV, Physitemp instruments Inc., Clifton, NJ). A 1.5-cm midline neck incision was made after the mouse was exposed to isoflurane for at least 10 min. Soft tissues over the trachea were retracted gently. One centimeter long right common carotid artery was carefully dissected free from adjacent tissues without any damage on vagus nerve. The wound was then irrigated and closed by using surgical suture. The surgical procedure was performed under sterile conditions and lasted around 10 min. After the surgery, all animals received a subcutaneous injection of 3 mg/kg bupivacaine. The total duration of anesthesia was 1 h. No response to toe pinching was observed during the anesthesia.
2.3. Noisy Environment
Noisy environment was for 6 h (12:00 to 18:00) each day in the following way. Aversive noise was controlled at 75 db and the audio was different from those during Barnes maze and Fear conditioning tests. The animals were housed under standard environment (quiet, 12-h light/dark cycle with ad libitum access to food and water) during the rest of the time each day.
2.4. Minocycline Application
Minocycline (catalog number: M 9511, Sigma-Aldrich, St. Louis, MO) at 40 mg/kg in normal saline (NS) was administered intraperitoneally 1 h before surgery or each episode of noisy environment. The dosage was chosen based on a previous study (Xue et al., 2007). The same volume of NS was given intraperitoneally to the other groups that did not receive minocycline in the study.
2.5. Barnes Maze
One week after surgery (for surgery group and surgery + noise groups) or the last episode of noise (for noise group), mice were subjected to Barnes maze to test their spatial learning and memory as previously described (Cao et al., 2012). The Barnes maze is a circular platform with 20 equally spaced holes (SD Instruments, San Diego, CA, USA). One of the holes was connected to a dark chamber that was called target box. The test was started by placing animals in the middle of the Barnes maze. Aversive noise (85 db) and bright light from a 200-W bulb shed on platform were used to induce mice to find and enter the target box. Animals were trained in a spatial acquisition phase that took 4 days with 3 min per trial, four trials per day, and 15-min interval between each trial. The memory test was carried out on day 5 (short-term retention) and day 12 (long-term retention). No test was performed during the period from day 5 to day 12. The latency to enter the target box during each trial was recorded by an ANY-Maze video tracking system (SD Instruments).
2.6. Fear Conditioning
Fear conditioning test was performed 24 h after the Barnes maze test as previously described (Tan et al., 2015). Mice were placed in a test chamber wiped with 70% alcohol and subjected to three tone-foot shock pairings (tone 2000 Hz, 85 db, 30 s; foot shock 0.7 mA, 2 s) with 1-min intertrial interval in a relatively dark room. Mice were removed from the test chamber 30 s after training and returned to their regular cages. Mice were placed back 24 h later to the same chamber for 8 min without tone and shock. The freezing behavior was recorded in an 8-s interval. Two hours later, mice were placed in a new test chamber that had different context and smell from the first test chamber. This chamber was wiped with 1% acetic acid and was in a relatively light room. Freezing behavior was recorded for 3 min without any stimuli. The tone stimulus was then turned on for three cycles with each cycle for 30 s followed by 1-min inter-cycle interval (total 4.5 min). The freezing behavior in this 4.5 min was also recorded. These testes were recorded by a camera, and the freezing behavior was scored by an observer who was blinded to the group assignment of animals.
2.7. Brain Tissue Harvesting
Mice were deeply anesthetized with isoflurane. They were perfused with normal saline. The bilateral cerebral cortex and hippocampus were dissected out at 6 h after surgery or the last episode of noise for ELISA assay of interleukin (IL)-1β or IL-6. A coronal brain slice between Bregma −2 and −4 mm was harvested for immunofluorescent staining. These slices containing hippocampus and cortex were fixed with 4% paraformaldehyde. All dissection procedures were performed on ice.
2.8. Quantification of IL-1β and IL-6
Brain tissues were homogenized on ice in 20 mM Tris-HCl buffer (pH = 7.3) containing protease inhibitors (10 mg/ml aproteinin, 5 mg/ml peptastin, 5 mg/ml leupeptin, and 1 mM phenylmethanesulfonylfluoride). Homogenates were centrifuged at 13,000 rpm for 20 min at 4°C. The supernatant was saved and Bradford protein assay of the supernatant was performed for each sample. ELISA kits for measuring mouse IL-1β and IL-6 (catalogue number: MLB00C and M6000B, respectively; R&D Systems, Minneapolis, MN) were used to quantify the concentrations of these cytokines in the samples according to the manufacturer’ instructions. The quantity of IL-1β and IL-6 in each brain sample was standardized to the protein contents.
2.9. Immunohistochemistry
As we described previously (Fan et al., 2016), brains were harvested and fixed by 4% paraformaldehyde in 0.1 M phosphate buffered saline at 4°C for 18 h, rehydrated, and embedded in paraffin. Coronal 5-μm sections of the cerebral hemisphere were cut sequentially and mounted on superfrost plus microscope slides. Antigen retrieval was performed with Tris/EDTA buffer (10 mM Tris Base, 1 mM EDTA, 0.05% Tween 20, pH 9.0) at 95 – 100°C for 20 min. After being washed in Tris-buffered saline (TBS) containing 0.025% triton-X 100, sections were blocked in 10% donkey serum plus 1% bovine serum albumin in TBS for 2 h at room temperature and then incubated at 4°C overnight with goat polyclonal anti-ionized calcium binding adapter molecule 1 (Iba-1) antibody (1:1000 dilution, catalogue number: ab107159; Abcam, Cambridge, MA). Sections were rinsed in TBS with 0.025% triton-X 100. The donkey anti-goat IgG antibody conjugated with Alexa Fluor 488 (1:200 dilution, catalogue number: A11055; Invitrogen) was incubated with the sections for 1 h at room temperature in the dark. After being washed in TBS, sections were mounted and cover-slipped with Vectashield mounting medium (H-1000; Vector Labs, Burlingame, CA). As we described previously (Tan et al., 2015), the density of Iba-1 staining was quantified in three non-overlapping fields randomly acquired in the hippocampal CA1 and CA3 sub-regions or cerebral cortex at ×100 magnification on an Olympus DP70 microscope (Olympus Corporation, Tokyo, Japan). Three sections per mouse were imaged. The number of pixels per image with intensity above a predetermined threshold level was quantified using ImageJ 1.47n software (National Institutes of Health, Bethesda, MD). The immunoreactivity was reflected by the percentage pixels with intensity above a threshold level in the total pixels of the imaged field. All quantitative analyses were performed in a blind manner. The nine measurements from one mouse were averaged to represent the value of the mouse.
2.10. Statistical Analysis
Parametric results in normal distribution are presented as mean ± S.E.M. (n ≥ 6). The data from the training sessions of Barnes maze test between groups were tested by two-way repeated measures analysis of variance followed by Tukey test. The data from the training sessions of Barnes maze test within one group were tested by one-way repeated measures analysis of variance followed by Tukey test. All other data were analyzed by one-way analysis of variance followed by the Tukey test if the data were normally distributed or by one-way analysis of variance on ranks followed by the Tukey test if the data were not normally distributed. Differences were considered significant at P < 0.05 based on two-tailed hypothesis testing. All statistical analyses were performed with SigmaStat (Systat Software, Point Richmond, CA).
3. Results
3.1. The combination of noisy environment and surgery induced learning and memory impairment
All mice survived until the end of the study and all of their data were included in the analysis. During the Barnes maze training, the time to identify the target box for mice in all groups was decreased with increased training sessions. The time to identify the target box in the training sessions on day 4 was shorter than that on day 1 in all five groups (Fig 1A). Noise and the combination of surgery and noise were significant factors to affect the time needed to identify the target box in the training sessions [F(1,28) = 4.533, P = 0.042 for noise alone; F(1,28) = 4.756, P = 0.038 for surgery plus 5 day noise]. In addition, the time needed for mice with surgery plus 5 day noise to identify the target box was longer than control mice on training day 3 and day 4 (Fig. 1A). Mice in the surgery + 3 day noise group and surgery + 5 day noise group took longer than mice in control group to identify the target box one day after the training sessions [F(4,70) = 3.045, P = 0.023 for the overall comparisons on the 5 groups with using one-way ANOVA; P = 0.033 and 0.037, respectively, for the comparisons between control and surgery + 3 day noise or control and surgery + 5 day noise] (Fig. 1B). No difference was noted between surgery + 3 day noise group and surgery + 5 day noise group (Fig. 1B). Similarly, freezing behavior of the surgery group, noise group, surgery + 3 day noise group and surgery + 5 day noise group was less than that of the control group in the context-related fear conditioning test [F(4,70) = 3.737, P = 0.008 for the overall comparisons on the 5 groups with using one-way ANOVA; P = 0.042, 0.049, 0.008 and 0.011, respectively, for the comparisons between control and 5 day noise, control and surgery, control and surgery + 3 day noise or control and surgery + 5 day noise]. However, the tone-related freezing behavior was not affected by any experimental conditions (Fig. 1C). These results suggest that surgery or noise can induce hippocampus-dependent (context-related) but not hippocampus-independent (tone-related) learning and memory impairment in mice. The combination of noise and surgery induces impairment in additional domains of learning and memory (spatial learning and memory impairment in Barnes maze). Because no difference in learning and memory impairment was noted between surgery + 3 day noise group and surgery + 5 day noise group, we choose surgery + 5 day noise as the combination condition in the following experiments.
Fig. 1. Effects of noisy environment and surgery on learning and memory.
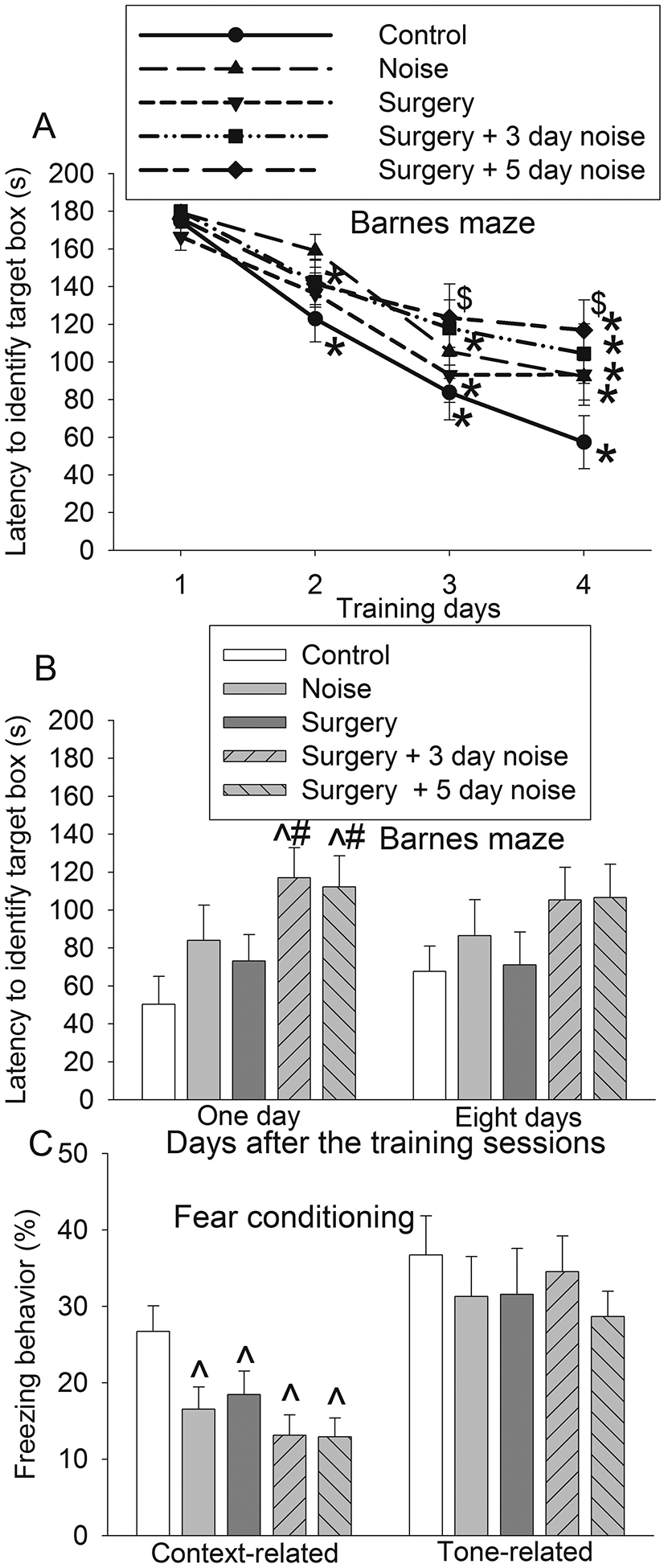
Seven-week old mice were subjected to right carotid artery exposure and 75 db noisy environments. They were started to be tested by Barnes maze and fear conditioning at one week after being exposed to various experimental conditions. A: Performance during the training sessions of Barnes maze test. B: Performance during the memory phase of Barnes maze test. C: Performance in fear conditioning test. Results are means ± S.E.M. (n = 15). * P < 0.05 compared with the corresponding data on day 1, $ P < 0.05 compared with control on corresponding day, ^ P < 0.05 compared with control, # P < 0.05 compared with surgery group.
3.2. The combination of noisy environment and surgery induced neuroinflammation
The concentrations of IL-1β in the hippocampus of noise group and surgery + noise group were higher than those of control group. The combination of noise and surgery induced a higher increase in the concentration of IL-1β than surgery alone [F(3,20) = 5.025, P = 0.009 for the overall comparisons on the 4 groups with using one-way ANOVA; P = 0.049 and 0.041, respectively, for the comparisons between control and 5 day noise or surgery and surgery + 5 day noise]. The concentrations of IL-6 in the hippocampus of noise group, surgery group and surgery + noise group were higher than those of control group. However, the concentrations of IL-1β and IL-6 in the cerebral cortex were not different among the groups (Fig. 2).
Fig. 2. Effects of noisy environment and surgery on proinflammatory cytokine concentrations in the brain.
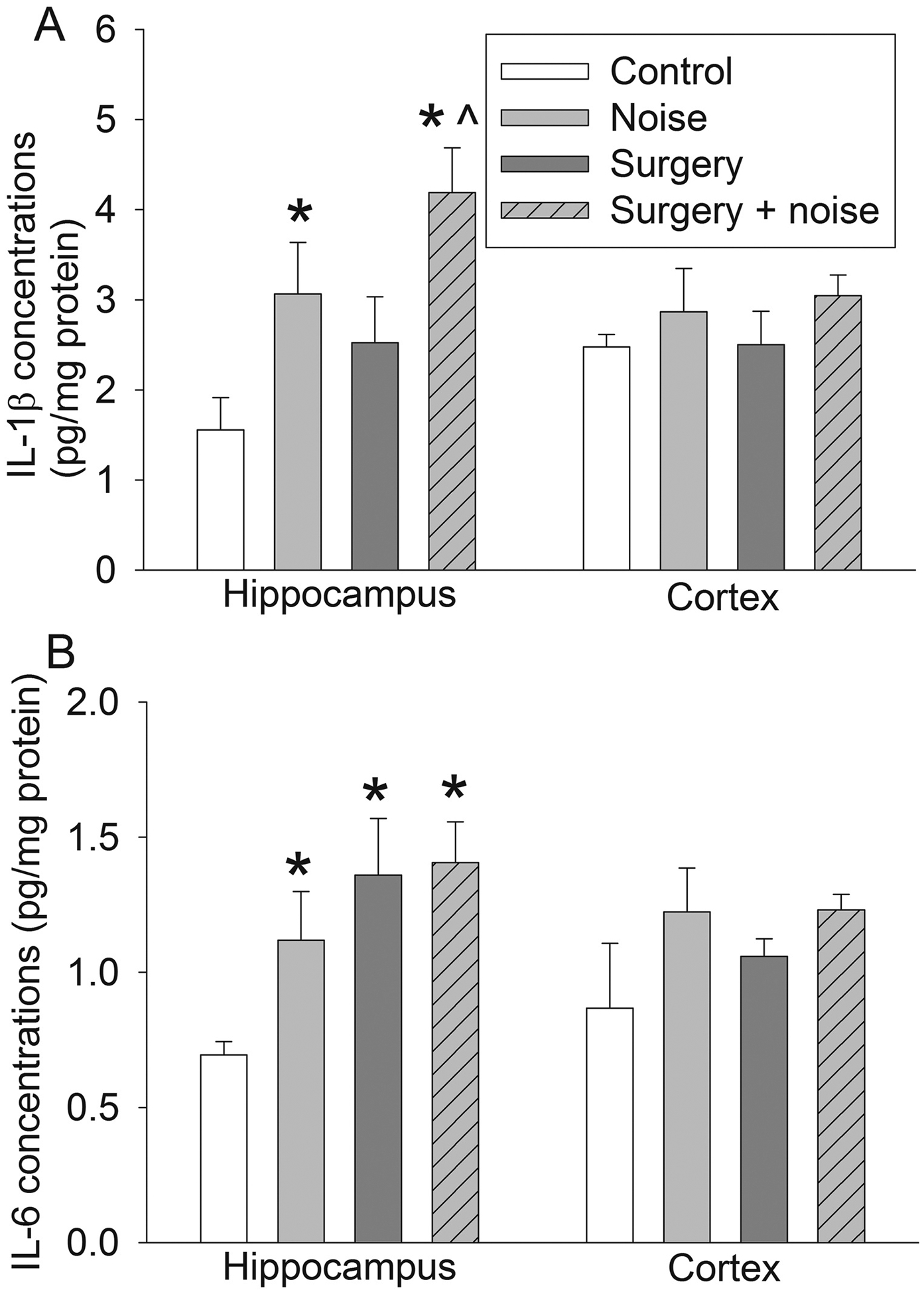
Seven-week old mice were subjected to right carotid exploration and 75 db noisy environments. Hippocampus and cortex were harvested at 6 h after surgery or the last episode of noise. A: IL-1β. B: IL-6. Results are means ± S.E.M. (n = 6). * P < 0.05 compared with control, ^ P < 0.05 compared with surgery group.
Compared with control group, the immune intensity of Iba-1, a microglial marker (Tan et al., 2015), in the CA1 and CA3 of hippocampus was increased by surgery or noise. This increase was even higher in the presence of the combination of noisy environment and surgery [For example, in CA1, F(3,20) = 11.350, P < 0.001 for the overall comparisons on the 4 groups with using one-way ANOVA; P = 0.003 and 0.003, respectively, for the comparisons between noise and surgery + 5 day noise or surgery and surgery + 5 day noise] (Fig. 3). However, the immune intensity of Iba-1 in the cerebral cortex was not affected by any experimental conditions (Fig. 3).
Fig. 3. Effects of noisy environment and surgery on Iba-1 expression in the brain.
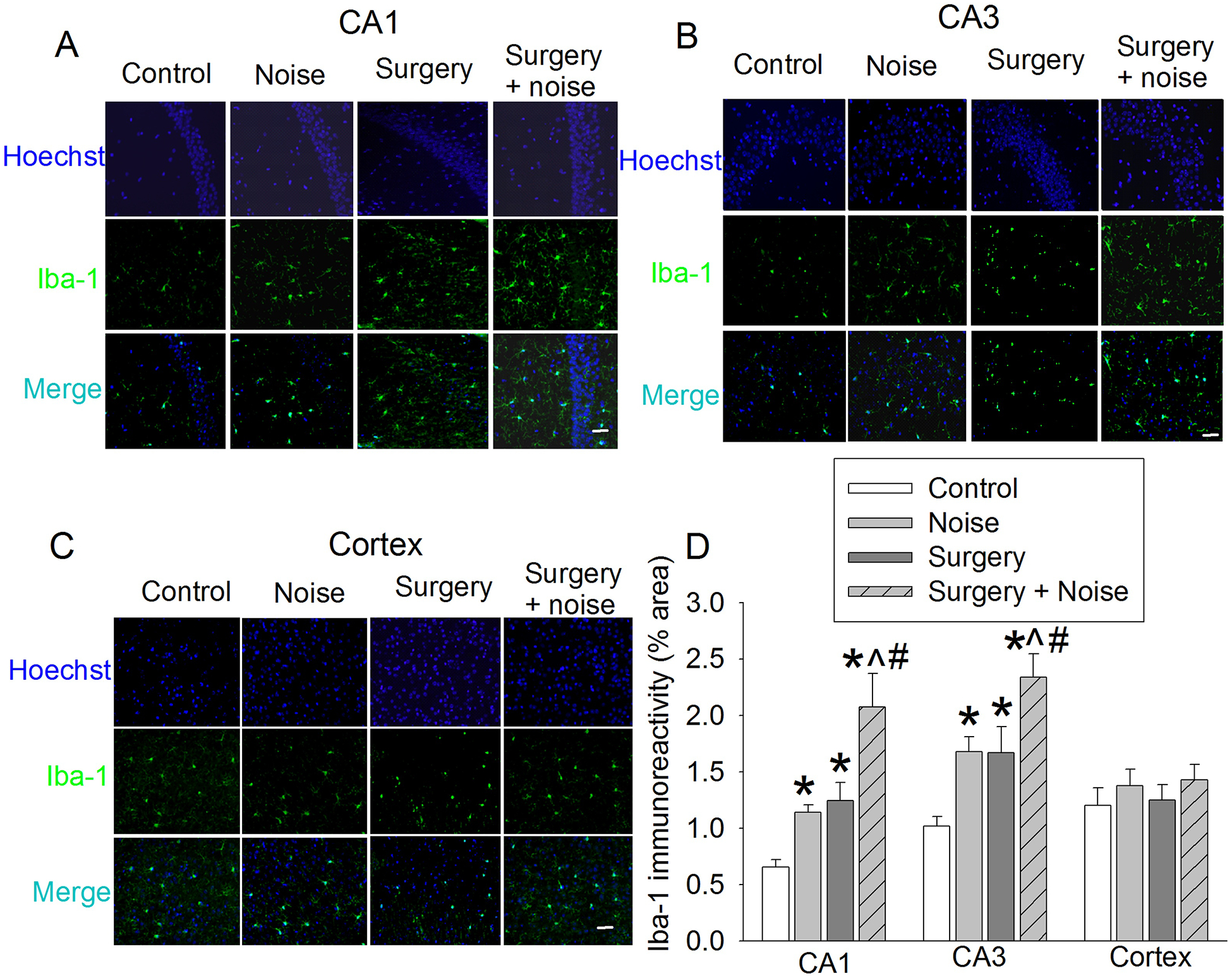
Seven-week old mice were subjected to right carotid exploration and 75 db noisy environments. Hippocampus and cortex were harvested at 6 h after surgery or the last episode of noise. A: Representative immunostaining images of Iba-1 in CA1. B: Representative immunostaining images of Iba-1 in CA3. C: Representative immunostaining images of Iba-1 in cerebral cortex. D: Graphic presentation of the percentage area that is Iba-1-postive staining in CA1, CA3, and cerebral cortex. Scale bar = 30 μm. Results are means ± S.E.M. (n = 6). * P < 0.05 compared with control, ^ P < 0.05 compared with noise group, # P < 0.05 compared with surgery group.
These results suggest that surgery or noise induces neuroinflammation and microglial activation in the hippocampus and that this effect was enhanced by the presence of noisy environment during perioperative period.
3.3. Minocycline attenuated learning and memory impairment induced by surgery and noisy environment
The time for mice to identify the target box in all groups was decreased with increased training sessions of Barnes maze (Fig. 4A). The time needed for mice to identify the target box on day 1 and day 8 after the training sessions in noise group and surgery + noise group was increased compared with that in control group. Minocycline attenuated this increase induced by noisy environment or the combination of noisy environment plus surgery [For example, on day 8 after the training sessions, F(4,70) = 9.486, P < 0.001 for the overall comparisons on the 5 groups with using one-way ANOVA; P = 0.036 and < 0.001, respectively, for the comparisons between 5 day noise and 5 day noise + minocycline or surgery + 5 day noise and surgery + 5 day noise + minocycline] (Fig. 4B). Similar to the pattern of Barnes maze results, freezing behavior in the context-related fear conditioning test was decreased by noisy environment or the combination of noisy environment and surgery and this decrease was attenuated by minocycline. The tone-related freezing behavior was not affected by any experimental conditions (Fig. 4C). These results suggest that minocycline attenuates the learning and memory impairment induced by surgery and noisy environment.
Fig. 4. Minocycline attenuated learning and memory impairment induced by noise or the combination of surgery and noisy environment.
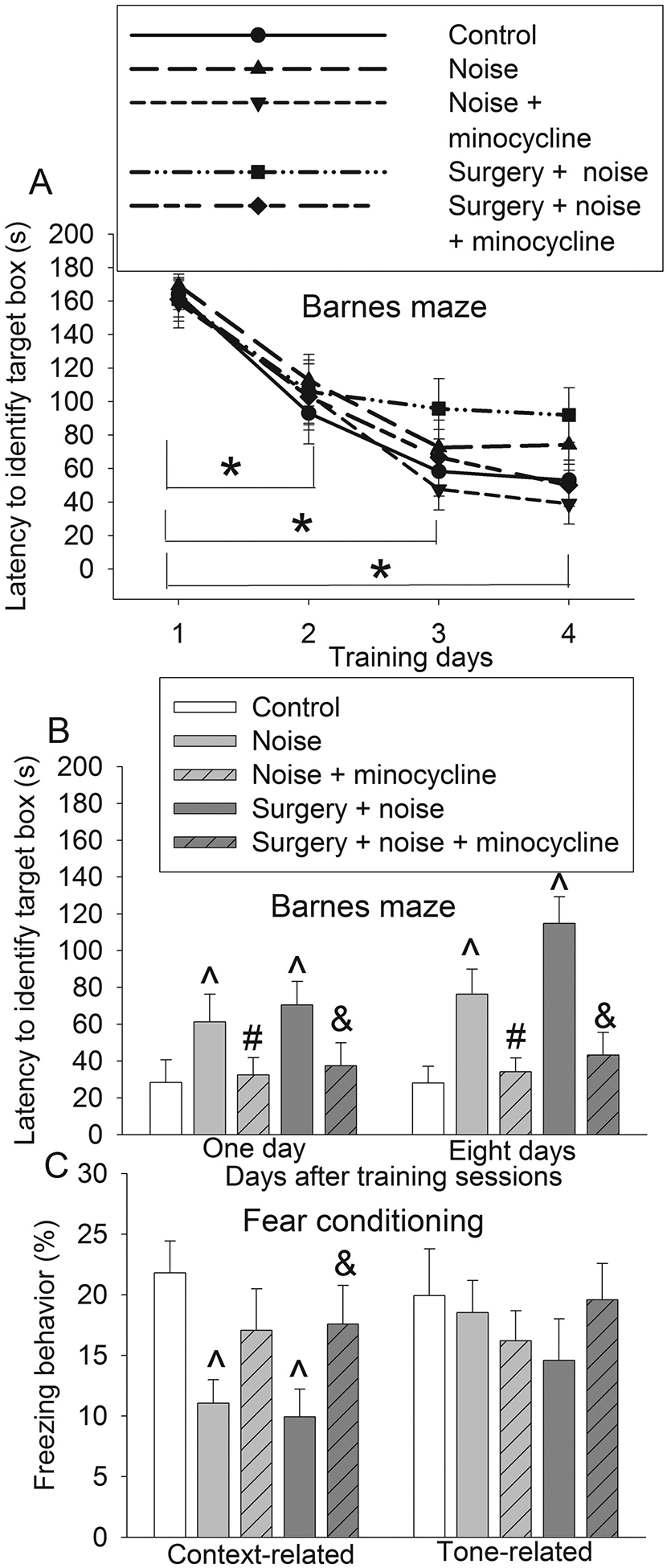
Seven-week old mice were subjected to right carotid artery exposure and 75 db noisy environments in the presence or absence of minocycline. They were started to be tested by Barnes maze and fear conditioning at one week after being exposed to various experimental conditions. A: Performance during the training sessions of Barnes maze test. B: Performance during the memory phase of Barnes maze test. C: Performance in fear conditioning test. Results are means ± S.E.M. (n = 15). * P < 0.05 compared with the corresponding data on day 1 in all five groups, ^ P < 0.05 compared with control, # P < 0.05 compared with noise group, & P < 0.05 compared with surgery plus noise group.
3.4. Minocycline attenuated neuroinflammation induced by surgery and noisy environment
The concentrations of IL-1β and IL-6 in the hippocampus were increased by noisy environment or the combination of noisy environment and surgery. These increases were attenuated by minocycline [For example, for IL-1β, F(4,25) = 11.705, P < 0.001 for the overall comparisons on the 5 groups with using one-way ANOVA; P = 0.024 and 0.002, respectively, for the comparisons between 5 day noise and 5 day noise + minocycline or surgery + 5 day noise and surgery + 5 day noise + minocycline] (Fig. 5A). Similarly, the immune intensity of Iba-1 in the CA1 and CA3 of the hippocampus was increased by noisy environment or the combination of noisy environment and surgery and this increase was attenuated by minocycline. However, the immune intensity of Iba-1 in the cerebral cortex was not changed by any experimental conditions (Figs. 5B to 5E). These results suggest that minocycline inhibits neuroinflammation and microglial activation induced by noisy environment or the combination of noisy environment and surgery.
Fig. 5. Minocycline attenuated neuroinflammation induced by noise or the combination of surgery and noisy environment.
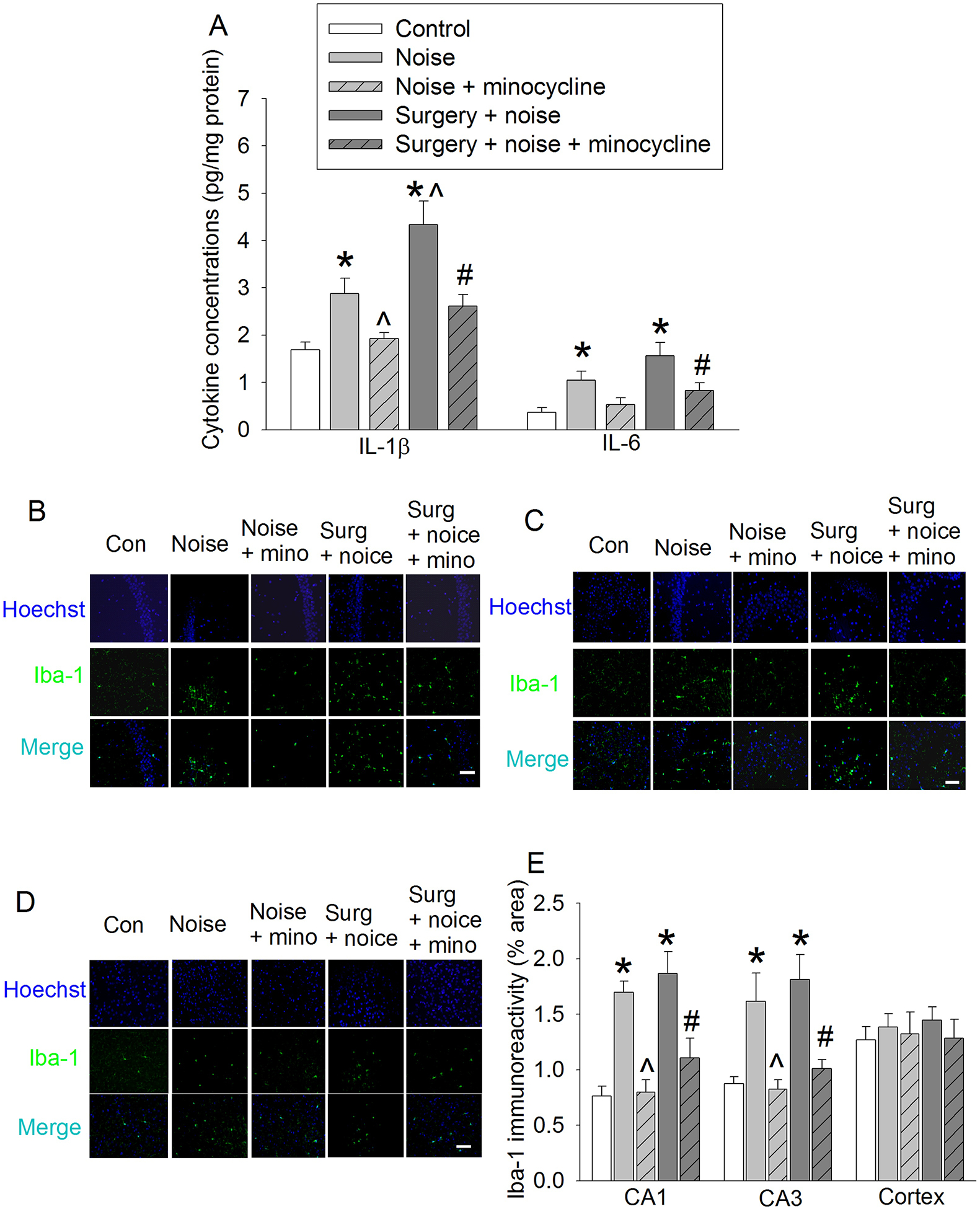
Seven-week old mice were subjected to right carotid exploration and 75 db noisy environments with or without the treatment of minocycline. Hippocampus was harvested at 6 h after surgery or the last episode of noise. A: The abundance of IL-1β and IL-6 in the hippocampus. B: Representative immunostaining images of Iba-1 in CA1. C: Representative immunostaining images of Iba-1 in CA3. D: Representative immunostaining images of Iba-1 in cerebral cortex. E: Graphic presentation of the percentage area that is Iba-1-postive staining in CA1, CA3 and cerebral cortex. Scale bar = 30 μm. Results are means ± S.E.M. (n = 6). * P < 0.05 compared with control group; ^ P < 0.05 compared with noise group; # P < 0.05 compared with surgery plus noise group.
4. Discussion
Comfortable music can improve patient’s prognosis after surgery (Salimpoor et al., 2011, Croom, 2012, Dobek et al., 2014). Good sleep helps learning and memory function in hospitalized patients (Xu et al., 2010, Zhao et al., 2010, Aleisa et al., 2011). However, uncomfortable noisy environment often occurs in hospital because of noise from medical devices, human voices and others. Noisy environment is known to cause many non-auditory effects including sleep disturbance and impairment of learning and memory in humans (Basner et al., 2014, Wright et al., 2016). However, previous studies often measure learning and memory functions in the noisy environment (Wright et al., 2016, Clark and Paunovic, 2018) and rarely determine these functions after noise have been gone. Also, it is unclear whether noisy environment contributes to POCD. Our results provide evidence that surgery or noise alone can induce learning and memory impairment and that the combination of noisy environment and surgery may induce impairment of more domains of learning and memory. These results suggest that perioperative noisy environment is an important component contributing to the development of POCD. We placed the animals in noisy environment for 3 days after surgery to simulate clinical situation because patients often are connected to many medical devices with alarms and have frequent nursing activity during the first few days after surgery. We added another group of mice that received 2-day noise before the surgery and 3 day noise after surgery. This condition was used to simulate clinical situations of sick patients who may be in an intensive care unit before surgery. Our results showed that mice with surgery plus 3 post-surgery day noise and mice with surgery plus noise before and after surgery had a similar degree of learning and memory dysfunction, suggesting that a threshold may have reached by 3 post-surgery day noise to affect learning and memory functions.
We and others have shown an important role of neuroinflammation in the learning and memory impairment induced by anesthesia and surgery (Wan et al., 2007, Cibelli et al., 2010, Cao et al., 2012, Zhang et al., 2014a). Similarly, studies have shown that microglial activation leading to the production of the pro-inflammatory cytokines is crucial for the learning and memory impairment after surgery (van Gool et al., 2010, Xu et al., 2017, Cao et al., 2018). A surgery on peripheral tissues or organs induces systemic inflammation, which then is transmitted by permeation of proinflammatory factors or cells from blood into the brain to induce activation of brain cells including microglia (Terrando et al., 2011b, Zhang et al., 2014b, Zheng et al., 2017). Our previous study has shown that inflammatory cytokines can block protein trafficking that is necessary for learning and memory (Tan et al., 2014), which may be a mechanism for neuroinflammation to cause learning and memory dysfunction. Our results showed that noisy environment increased the expression of IL-1β, IL-6 and Iba-1 in the hippocampus. In addition, minocycline, a tetracycline derivative with anti-inflammatory property (Tan et al., 2015), attenuated neuroinflammation and dysfunction of learning and memory after exposure to noise or the combination of noise and surgery. These results suggest that neuroinflammation is an important underlying neuropathological process for the learning and memory dysfunction induced by noise or the combination of noise and surgery. These findings, along with previous results that minocycline attenuates neuroinflammation- and surgery-induced dysfunction of learning and memory (Tan et al., 2015, Wang et al., 2016), indicate a potential therapeutic role of minocycline in POCD. Interestingly, a recent study has shown that chronic noise exposure (88 or 98 dB noise, 4 h/day for 30 days) can induce gut dysbiosis (Cui et al., 2018), which may then induce neuroinflammation and impairment of learning and memory because our previous study has shown that gut dysbiosis can induce neuroinflammation and cognitive impairment (Liang et al., 2018).
Our mice showed impairment of context-related fear conditioning impairment after being exposed to noise or the combination of noise and surgery, suggesting hippocampus-dependent learning and memory dysfunction (Kitamura et al., 2009, Satomoto et al., 2009, Wiltgen et al., 2010). Also, the expression of IL-1β, IL-6 and Iba-1 was increased in the hippocampus but not in the cerebral cortex after being exposed to noise or the combination of noise and surgery. The mechanisms for the apparent brain region-specific change are not known. However, brain region-specific responses have been reported in the literature. For example, a previous study showed that neurotoxicity caused by paraquat treatment can induce the expression of pro-inflammatory cytokines in the hippocampus, but not in the cortex (Mitra et al., 2011). It is possible that hippocampus may be more susceptible to neuroinflammation and microglial activation. Another possibility is that the changes in the hippocampus and cerebral cortex have different time-courses and that the time points we used in the study caught the changes in the hippocampus but not those in the cerebral cortex.
Our findings may have clinical implication. Reducing noise may be an effective way to attenuate learning and memory dysfunction after surgery. Surgical patients often are monitored by various medical devices with alarm system. Frequent nursing activity also generates sound. Human voices from visitors, patients and staff all contribute to the noisy level in the hospital. Thus, patients during the perioperative period are especially prone to noisy environment. Careful monitoring the noisy level and designing methods to reduce noisy level shall be done to improve the outcome of our patients.
Our study has limitations. First, we have not done a careful dose-response study to determine safety level of noise for brain health. Rather, we tested a level of noisy that is relatively easy to achieve for its effects on the brain. Second, we showed that noise induced neuroinflammation to lead to learning and memory dysfunction. However, we have not determined how noise can cause neuroinflammation. Future studies are needed to understand these mechanisms.
Conclusion:
Our study showed that noisy environment can induce neuroinflammation and dysfunction of learning and memory in mice. These effects may contribute to postoperative cognitive dysfunction. Neuroinflammation may be an important neuropathological process for the learning and memory dysfunction induced by noise or the combination of noise and surgery.
Funding:
This study was supported by grants (R01 GM098308, R01 HD089999, R01 AG061047 and R21 AG056995 to Z Zuo) from the National Institutes of Health, Bethesda, MD, the Robert M. Epstein Professorship endowment (to Z Zuo), University of Virginia, Charlottesville, VA and a grant (AB18126061 to F Lin) from Guangxi science and technology key research and development project, Guangxi, China.
Abbreviations
- Iba-1
ionized calcium binding adapter molecule 1
- IL
interleukin
- NS
normal saline
- POCD
postoperative cognitive dysfunction
- TBS
Tris-buffered saline
Footnotes
Ethical Approval and Consent to participate: Not a clinical study. Animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA).
Consent for publication: All authors have approved the submission and publication of the findings. There is no need to get approval from funding agencies for publication.
Availability of supporting data: Data will be available upon request.
Competing interests: The authors declare no competing interests.
References
- Aleisa AM, Alzoubi KH, Alkadhi KA, 2011. Post-learning REM sleep deprivation impairs long-term memory: reversal by acute nicotine treatment. Neurosci Lett 499, 28–31. [DOI] [PubMed] [Google Scholar]
- Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, Stansfeld S, 2014. Auditory and non-auditory effects of noise on health. Lancet 383, 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford PD, 1955. Adverse cerebral effects of anaesthesia on old people. Lancet 269, 259–263. [DOI] [PubMed] [Google Scholar]
- Cao L, Li L, Lin D, Zuo Z, 2012. Isoflurane induces learning impairment that is mediated by interleukin 1beta in rodents. PLoS ONE [Electronic Resource] 7, e51431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Fang J, Wang X, Wang Y, Duan K, Ye F, Ouyang W, Tong J, 2018. Activation of AMP-activated protein kinase (AMPK) aggravated postoperative cognitive dysfunction and pathogenesis in aged rats. Brain Research 1684, 21–29. [DOI] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M, 2010. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol 68, 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Paunovic K, 2018. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Cognition. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croom AM, 2012. Music, neuroscience, and the psychology of well-being: a precis. Front Psychol 2, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Su D, Li W, She X, Zhang M, Wang R, Zhai Q, 2018. Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer’s disease. J Neuroinflammation 15, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobek CE, Beynon ME, Bosma RL, Stroman PW, 2014. Music modulation of pain perception and pain-related activity in the brain, brain stem, and spinal cord: a functional magnetic resonance imaging study. J Pain 15, 1057–1068. [DOI] [PubMed] [Google Scholar]
- Fan D, Li J, Zheng B, Hua L, Zuo Z, 2016. Enriched Environment Attenuates Surgery-Induced Impairment of Learning, Memory, and Neurogenesis Possibly by Preserving BDNF Expression. Molecular Neurobiology 53, 344–354. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K, 2009. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139, 814–827. [DOI] [PubMed] [Google Scholar]
- Liang P, Shan W, Zuo Z, 2018. Perioperative use of cefazolin ameliorates postoperative cognitive dysfunction but induces gut inflammation in mice. J Neuroinflammation 15, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Chakrabarti N, Bhattacharyya A, 2011. Differential regional expression patterns of alpha-synuclein, TNF-alpha, and IL-1beta; and variable status of dopaminergic neurotoxicity in mouse brain after Paraquat treatment. J Neuroinflammation 8, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS, 1998. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351, 857–861. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS, 2008. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108, 18–30. [DOI] [PubMed] [Google Scholar]
- Newman S, Stygall J, Hirani S, Shaefi S, Maze M, 2007. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology 106, 572–590. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ, 2011. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci 14, 257–262. [DOI] [PubMed] [Google Scholar]
- Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J, 2009. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110, 628–637. [DOI] [PubMed] [Google Scholar]
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, 2009. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110, 548–555. [DOI] [PubMed] [Google Scholar]
- Tan H, Bi J, Wang Y, Zhang J, Zuo Z, 2015. Transfusion of Old RBCs Induces Neuroinflammation and Cognitive Impairment. Critical Care Medicine 43, e276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Cao J, Zhang J, Zuo Z, 2014. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. Journal of neuroinflammation 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG, 2011. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology 115, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Brzezinski M, Degos V, Eriksson LI, Kramer JH, Leung JM, Miller BL, Seeley WW, Vacas S, Weiner MW, Yaffe K, Young WL, Xie Z, Maze M, 2011. Perioperative cognitive decline in the aging population. Mayo Clin Proc 86, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M, 2011. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 70, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool WA, van de Beek D, Eikelenboom P, 2010. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375, 773–775. [DOI] [PubMed] [Google Scholar]
- Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M, 2007. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 106, 436–443. [DOI] [PubMed] [Google Scholar]
- Wang HL, Liu H, Xue ZG, Liao QW, Fang H, 2016. Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med 20, 1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, Li W, Silva AJ, 2010. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol 20, 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Peters ER, Ettinger U, Kuipers E, Kumari V, 2016. Moderators of noise-induced cognitive change in healthy adults. Noise Health 18, 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dong H, Qian Q, Zhang X, Wang Y, Jin W, Qian Y, 2017. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behavioural Brain Research 332, 145–153. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Gao CY, Fang CQ, Zhou HD, Jiang XJ, 2010. The mechanism and characterization of learning and memory impairment in sleep-deprived mice. Cell Biochem Biophys 58, 137–140. [DOI] [PubMed] [Google Scholar]
- Xue QS, Sparks DL, Streit WJ, 2007. Microglial activation in the hippocampus of hypercholesterolemic rabbits occurs independent of increased amyloid production. J Neuroinflammation 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jiang W, Zuo Z, 2014. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor kappaB. Neuroscience 261, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan H, Jiang W, Zuo Z, 2014. Amantadine alleviates postoperative cognitive dysfunction possibly by increasing glial cell line-derived neurotrophic factor in rats. Anesthesiology 121, 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Huang L, Wu H, Li Y, Zhang L, Yin Y, Xiang Z, Zhao Z, 2010. Neuropeptide S mitigates spatial memory impairment induced by rapid eye movement sleep deprivation in rats. Neuroreport 21, 623–628. [DOI] [PubMed] [Google Scholar]
- Zheng B, Lai R, Li J, Zuo Z, 2017. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery. Brain, behavior, and immunity 61, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z, 2012. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiology of Disease 48, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]


