Abstract
Background
Older people taking multiple medications represent a large and growing proportion of the population. Managing multiple medications can be challenging, and this is especially the case for older people, who have higher rates of comorbidity and physical and cognitive impairment than younger adults. Good medication‐taking ability and medication adherence are necessary to ensure safe and effective use of medications.
Objectives
To evaluate the effectiveness of interventions designed to improve medication‐taking ability and/or medication adherence in older community‐dwelling adults prescribed multiple long‐term medications.
Search methods
We searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, CINAHL Plus, and International Pharmaceutical Abstracts from inception until June 2019. We also searched grey literature, online trial registries, and reference lists of included studies.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs. Eligible studies tested interventions aimed at improving medication‐taking ability and/or medication adherence among people aged ≥ 65 years (or of mean/median age > 65 years), living in the community or being discharged from hospital back into the community, and taking four or more regular prescription medications (or with group mean/median of more than four medications). Interventions targeting carers of older people who met these criteria were also included.
Data collection and analysis
Two review authors independently reviewed abstracts and full texts of eligible studies, extracted data, and assessed risk of bias of included studies. We conducted meta‐analyses when possible and used a random‐effects model to yield summary estimates of effect, risk ratios (RRs) for dichotomous outcomes, and mean differences (MDs) or standardised mean differences (SMDs) for continuous outcomes, along with 95% confidence intervals (CIs). Narrative synthesis was performed when meta‐analysis was not possible. We assessed overall certainty of evidence for each outcome using Grades of Recommendation, Assessment, Development and Evaluation (GRADE). Primary outcomes were medication‐taking ability and medication adherence. Secondary outcomes included health‐related quality of life (HRQoL), emergency department (ED)/hospital admissions, and mortality.
Main results
We identified 50 studies (14,269 participants) comprising 40 RCTs, six cluster‐RCTs, and four quasi‐RCTs. All included studies evaluated interventions versus usual care; six studies also reported a comparison between two interventions as part of a three‐arm RCT design.
Interventions were grouped on the basis of their educational and/or behavioural components: 14 involved educational components only, 7 used behavioural strategies only, and 29 provided mixed educational and behavioural interventions. Overall, our confidence in results regarding the effectiveness of interventions was low to very low due to a high degree of heterogeneity of included studies and high or unclear risk of bias across multiple domains in most studies.
Five studies evaluated interventions for improving medication‐taking ability, and 48 evaluated interventions for improving medication adherence (three studies evaluated both outcomes).
No studies involved educational or behavioural interventions alone for improving medication‐taking ability. Low‐quality evidence from five studies, each using a different measure of medication‐taking ability, meant that we were unable to determine the effects of mixed interventions on medication‐taking ability.
Low‐quality evidence suggests that behavioural only interventions (RR 1.22, 95% CI 1.07 to 1.38; 4 studies) and mixed interventions (RR 1.22, 95% CI 1.08 to 1.37; 12 studies) may increase the proportions of people who are adherent compared with usual care. We could not include in the meta‐analysis results from two studies involving mixed interventions: one had a positive effect on adherence, and the other had little or no effect. Very low‐quality evidence means that we are uncertain of the effects of educational only interventions (5 studies) on the proportions of people who are adherent.
Low‐quality evidence suggests that educational only interventions (SMD 0.16, 95% CI ‐0.12 to 0.43; 5 studies) and mixed interventions (SMD 0.47, 95% CI ‐0.08 to 1.02; 7 studies) may have little or no impact on medication adherence assessed through continuous measures of adherence. We excluded 10 studies (4 educational only and 6 mixed interventions) from the meta‐analysis including four studies with unclear or no available results. Very low‐quality evidence means that we are uncertain of the effects of behavioural only interventions (3 studies) on medication adherence when assessed through continuous outcomes.
Low‐quality evidence suggests that mixed interventions may reduce the number of ED/hospital admissions (RR 0.67, 95% CI 0.50 to 0.90; 11 studies) compared with usual care, although results from six further studies that we were unable to include in meta‐analyses indicate that the intervention may have a smaller, or even no, effect on these outcomes. Similarly, low‐quality evidence suggests that mixed interventions may lead to little or no change in HRQoL (7 studies), and very low‐quality evidence means that we are uncertain of the effects on mortality (RR 0.93, 95% CI 0.67 to 1.30; 7 studies).
Moderate‐quality evidence shows that educational interventions alone probably have little or no effect on HRQoL (6 studies) or on ED/hospital admissions (4 studies) when compared with usual care. Very low‐quality evidence means that we are uncertain of the effects of behavioural interventions on HRQoL (1 study) or on ED/hospital admissions (2 studies). We identified no studies evaluating effects of educational or behavioural interventions alone on mortality.
Six studies reported a comparison between two interventions; however due to the limited number of studies assessing the same types of interventions and comparisons, we are unable to draw firm conclusions for any outcomes.
Authors' conclusions
Behavioural only or mixed educational and behavioural interventions may improve the proportion of people who satisfactorily adhere to their prescribed medications, but we are uncertain of the effects of educational only interventions. No type of intervention was found to improve adherence when it was measured as a continuous variable, with educational only and mixed interventions having little or no impact and evidence of insufficient quality to determine the effects of behavioural only interventions. We were unable to determine the impact of interventions on medication‐taking ability. The quality of evidence for these findings is low due to heterogeneity and methodological limitations of studies included in the review. Further well‐designed RCTs are needed to investigate the effects of interventions for improving medication‐taking ability and medication adherence in older adults prescribed multiple medications.
Plain language summary
Interventions for helping older adults prescribed multiple medications to use and take their medications
Background: Older people are often prescribed multiple medications, which can be challenging to manage. Medication‐taking errors and non‐adherence (under‐use or over‐use of medication) can lead to negative health outcomes. Assisting older people to better use and adhere to their medications could reduce adverse medication events, such as medication‐related hospital admissions, and could improve health outcomes.
Question: What are the findings of studies testing ways to improve older people's ability to use and adhere to multiple medications?
Search strategy: To find relevant studies, we searched seven online databases, trial registries, and the reference lists of previous reviews, retrieving studies published until June 2019.
Selection criteria: We included randomised controlled trials (RCT) or studies of similar design comparing a group of people receiving an intervention to improve medication‐taking ability or medication adherence with a group receiving usual care (no intervention) or receiving a different intervention. We included trials that studied older adults (≥ 65 years) living at home (or being discharged from hospital back to home) who were using four or more regular prescription medications.
Main results: We identified 50 studies, involving 14,269 participants. All studies tested interventions versus usual care, with six studies also comparing two different types of interventions.
Fourteen studies tested educational interventions whereby people received education regarding their medications or a health professional reviewed their medications. Seven studies tested behavioural interventions such as changing dosing times, re‐packaging medications into multi‐compartment pill boxes to make medication regimens easier to take, or sending text message adherence reminders. Twenty‐nine studies tested mixed educational and behavioural interventions.
The studies identified were very different in terms of what interventions people received, where interventions were delivered, and how and when people's medication‐taking ability or adherence was measured. Due to these differences and problems with how the trials were conducted, the quality of the evidence was considered low or very low overall.
Low‐quality evidence means that the impact of mixed interventions on medication‐taking ability could not be determined, and no studies were identified that assessed educational only or behavioural only interventions for improving medication‐taking ability.
Low‐quality evidence suggests that compared with usual care, behavioural only and mixed interventions may improve the proportions of people who satisfactorily adhere to their prescribed medication, but very low‐quality evidence means that the effects of educational only interventions are uncertain. Low‐ and very low‐quality evidence means that no interventions were found to be effective in improving medication adherence when assessed by continuous measures such as percentage of medications consumed.
Low‐quality evidence also suggests that mixed interventions may reduce the number of emergency department visits or hospital admissions, and may lead to little or no change in health‐related quality of life (HRQoL). Moderate‐quality evidence shows that educational interventions alone probably have little or no effect on HRQoL or on emergency department or hospital admissions. The effects of behavioural interventions alone on HRQoL or emergency department or hospital admissions are uncertain because of very low‐quality evidence. We are uncertain of the effects of behavioural, educational, or mixed interventions on mortality.
Studies comparing one type of intervention with another were limited in number, and we are unable to draw firm conclusions for any key outcomes.
Authors' conclusions: Interventions varied greatly among studies, and there were problems regarding how the trials were conducted, which may have affected their results. We were unable to determine the impact of interventions on medication‐taking ability. Low‐quality evidence suggests that behavioural only and mixed educational and behavioural interventions may improve the proportions of people who adhere to their prescribed medication regimen. Low‐ and very low‐quality evidence found no type of intervention to be effective in improving medication adherence when this was assessed by a continuous measure. High‐quality studies are necessary to identify the most effective way to improve medication‐taking ability and medication adherence among older adults prescribed multiple medications.
Summary of findings
Background
Description of the condition
Older people, conventionally defined as those aged 65 years and older, often have multiple chronic health problems that require ongoing healthcare interventions (Hilmer 2007; WHO 2000). Increasing multi‐morbidity and an expanding evidence base supporting multi‐drug regimens in the management of many chronic diseases mean that polypharmacy (use of multiple medications) is often unavoidable in older people. Polypharmacy has a range of definitions but is commonly defined as the use of four or more medications (Department of Health (UK) 2001; Patterson 2014). The prevalence of polypharmacy is increasing. For example, in the United Kingdom, the number of adults aged over 65 years and taking five or more medications daily has quadrupled from 12% to 49% over the past two decades (Gao 2017). There is also a substantial subgroup of the older population who are prescribed an average of 10 or more different medications, which is sometimes referred to as hyperpolypharmacy (Elliott 2014).
Medication‐taking ability refers to a person’s ability to accurately follow a prescribed medication regimen. It includes knowing what medications to take and when to take them and being able to correctly administer the medication (Maddigan 2003). Managing multiple long‐term medications can be a complex and challenging task, especially for older people, who may experience a decline in the cognitive and physical abilities required for taking medication (Barbas 2001; Beckman 2005). More than a quarter of older people experience difficulties when opening medication packages, including opening bottles and removing medication from blister packs (Philbert 2014). Older people with visual impairment are more than twice as likely to require help in managing their medication as those without visual impairment (McCann 2012). Many older people receive assistance from informal or non‐professional carers when taking medication (ACSQHC 2012). Thus, interventions that aim to improve medication‐taking ability in older adults may need to target carers as well as consumers.
Medication adherence refers to the extent to which a person’s medication‐taking behaviour corresponds with agreed upon treatment recommendations from a healthcare provider (WHO 2003). Non‐adherence refers to deviations from that agreed upon treatment and includes under‐utilisation, over‐utilisation, and incorrect use of medication. There are two broad types of non‐adherence: unintentional non‐adherence – which may be due to factors such as forgetfulness, lack of understanding, physical problems, or the complexity of the regimen; and intentional non‐adherence – which occurs when a person decides not to take his or her treatment as instructed (Wroe 2002). A person is generally considered adherent if he or she takes between 80% and 120% of prescribed medication over a given time period (WHO 2003). Non‐adherence to medications has been reported in up to 50% of older people in different countries and settings (George 2006; Gilbert 1993;Gray 2001; Hemminki 1975;Lau 1996; Lee 2010; Mansur 2008; McElnay 1997;Okuno 1999;Sewitch 2008; Spagnoli 1989;Stoehr 2008; Thorpe 2009; Vik 2006). The World Health Organization (WHO) has recognised the importance of enhancing adherence as a strategy to tackle chronic health conditions effectively (WHO 2003).
Consequences of poor medication‐taking ability and of non‐adherence may include suboptimal response to treatment, recurrence of illness, adverse drug events (ADEs), increased healthcare service utilisation, unplanned hospitalisations, increased morbidity and mortality, and increased healthcare costs (Balkrishnan 2003; Col 1990;DiMatteo 2002;Howard 2003; Leendertse 2008; Tafreshi 1999). Among older adults, ADEs are a significant and increasing problem (Burgess 2005;Elliott 2014). Almost a quarter of preventable ADEs in older people are attributable to consumer error (Field 2007; Gurwitz 2003). Between US$100 and US$300 billion of avoidable healthcare costs has been attributed to non‐adherence in the United States annually (IMS 2013).
Medication‐taking ability and adherence are influenced by a range of factors related to healthcare consumers, their therapies, their medical conditions, social factors, and healthcare provider‐, and health system‐related factors (Balkrishnan 1998; Jin 2008; WHO 2003). Medication‐taking ability and adherence can be inter‐related. For example non‐adherence may result from a patient being unable to follow instructions or remove medications from packaging. Age itself is generally not an independent predictor of poor medication‐taking ability nor of non‐adherence (DiMatteo 2004; Vik 2004). Nevertheless, the prevalence of risk factors for medication use problems increases with age (Col 1990). These include polypharmacy (Gray 2001;Vik 2006), medication regimen complexity (Corsonello 2009; Jansa 2010; Vik 2006), cognitive and functional decline (Gray 2001; Hutchison 2006; Spiers 1995;Vik 2006), inadequate contact with health professionals (George 2006), depressive symptoms (Vik 2006), poor social support (DiMatteo 2000; Spiers 1995), and absence of assistance with administration of medications (Vik 2006). The risk factors for suboptimal use of medications by older people have been studied extensively in cross‐sectional studies (George 2006; Gilbert 1993; Gray 2001; Hemminki 1975; Jerant 2011; Lau 1996; McElnay 1997; Okuno 1999; Sears 2012; Spagnoli 1989; Tavares 2013; Vik 2006). Many adverse health outcomes may be preventable if appropriate measures are taken to address these risk factors and to optimise medication‐taking ability and adherence (George 2008; Jokanovic 2016; Sorensen 2004).
Description of the intervention
A range of simple to complex behavioural and educational interventions, given alone or in combination, have been tested for improving the medication‐taking ability and adherence of consumers (George 2008). Behavioural strategies include:
alarm/beeper;
calendar/diary;
reminder chart/medication list;
large print labels;
packaging change;
multi‐compartment pillbox/calendar pack/compliance aid (also known as dose administration aid (DAA));
contracting (verbal or written agreement);
adherence monitoring with or without feedback;
reminders (mail, telephone, email);
inpatient programs of self‐administration of medications;
simplification of medication regimens;
skill building (supervised, group);
tailoring (routinisation); and
follow‐up (home visit, scheduled clinic visit, video/teleconferencing).
Educational strategies comprise group (inpatient, family, and support group) and/or individual (verbal, audiovisual, visual, written, telephone, mail) education provided by physicians, pharmacists, nurses, allied health professionals, and others.
How the intervention might work
Behavioural and educational interventions, used alone or in combination, are intended to improve the ability of older people (and/or the ability of their carers) to manage medications and adhere to medication regimens. Interventions may target medication‐taking ability, medication adherence, or both.
These interventions may also lead to improvements in knowledge about medications and in confidence regarding medication management; greater satisfaction with treatment; better health‐related quality of life (HRQoL); reductions in the incidence of ADEs; and reductions in health service utilisation.
Why it is important to do this review
Older people taking multiple medications represent a large and growing proportion of consumers seen by health professionals in clinical practice. They are also the group most likely to experience ADEs. Evidence from well‐designed studies testing interventions to improve medication‐taking ability and adherence among older people prescribed multiple long‐term medications could provide valuable information for practitioners, researchers, and consumers to help optimise medication use among older people living in the community.
Interventions to improve medication adherence have been widely investigated (Nieuwlaat 2014; Ryan 2014). However previous reviews of interventions focusing on medication adherence in older people taking multiple medications are more than 10 years old (George 2008), or they have identified few studies for inclusion (Patton 2017; Zelko 2016). Older people form a heterogeneous population in terms of their medication consumption and disease patterns; therefore studies recruiting relatively homogenous samples of people experiencing one specific disease or consuming one type of medication have limited generalisability.
To date, no systematic review has included measures of medication‐taking other than adherence, such as ability to manage medications. Standardised methods for measuring the ability of people to manage medications have been developed (Elliott 2009; Elliott 2015), some of which have been used in studies of medication use in older people (Lam 2011). Two of the best‐studied assessment tools for evaluating medication‐taking are the Drug Regimen Unassisted Grading Scale (DRUGS) (Edelberg 1999), which utilises a person’s own medications, and the Medication Management Ability Assessment (MMAA) (Patterson 2002), which uses a simulated medication regimen.
Our review will focus on interventions to improve medication‐taking ability or adherence, or both, in older adults who are prescribed multiple medications, or their carers (who are not health professionals).
This review will complement previous Cochrane reviews looking at interventions for improving medication adherence in the general population (Nieuwlaat 2014), including the impact of dose reminder packaging (Mahtani 2011), and interventions for improving clinical outcomes in people with multi‐morbidities (Smith 2016). The appropriateness of people's medication regimens is dependent on a number of factors and will not be considered as part of this review, but only their ability to take (or use) the medications and their adherence to the agreed regimen. There has been a previous Cochrane review of interventions targeted at health professionals, designed to improve the appropriateness of prescribing and polypharmacy (Patterson 2014).
Objectives
To evaluate the effectiveness of interventions designed to improve medication‐taking ability and/or medication adherence in older community‐dwelling people (or their carers) whose treatment consists of multiple long‐term prescribed medications.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), cluster‐RCTs, and quasi‐RCTs, as specified by the Cochrane Consumers and Communication Group (CCCRG 2014).
Types of participants
We included studies in which:
most participants (≥ 80%) were aged 65 years and over, or the mean/median age was over 65 years. Studies were identified that did not meet these criteria but had relevant data regarding older people that could be extracted separately; these were also included;
participants were living in the community or were discharged from a hospital or other healthcare facility to the community (living in the community includes in a person’s own home or retirement village/independent living unit, with or without additional support; it does not include situations in which professional carers or nurses administer the person's medications, such as in nursing homes, residential care facilities or full nursing care in the home); and
participants used at least four long‐term regular prescription medications, or the group mean/median was greater than four (irrespective of participants’ number of medical conditions).
Studies that involved carers of consumers who met these criteria were also included. Carers were defined as “people who provide unpaid care and support to family members and friends who have disability, mental illness, a chronic condition, terminal illness, or general frailty” (ACSQHC 2012).
Types of interventions
We included studies that tested single interventions or combinations of interventions directed at the consumer or the carer that sought to improve medication‐taking ability and/or adherence by the consumer.
Examples included:
support for behaviour change;
provision of medication aids (e.g. DAAs, medication lists);
medication regimen simplification;
remote monitoring of medication use with or without feedback;
facilitation of communication and decision‐making about medications;
provision of information or education; and
acquisition of skills and competencies.
This list of interventions is not exhaustive. Therefore the search strategy (see Appendix 1) also focused on terms that described the outcomes of interest to avoid missing potentially relevant studies that tested novel interventions.
We included the following comparisons.
Interventions to improve medication‐taking ability and/or adherence versus standard or usual care.
One form of intervention to improve medication‐taking ability and/or adherence versus another − including simple versus complex interventions.
In future updates, we will consider including interventions to improve medication‐taking ability and/or adherence versus no intervention, but for this review, we identified no studies of this nature.
Types of outcome measures
Primary outcomes
This review focused on two outcomes directly related to medication‐taking behaviour of older adults (or their carers): ability to manage medications and adherence to medication regimens. To be eligible for inclusion, studies had to have assessed at least one of these outcome measures for at least four regular prescribed medications (which could be the person’s own medications or, for assessment of ability to manage medication, a validated, simulated medication regimen instrument) (Elliott 2009). These two outcomes were evaluated separately.
Ability to manage medications
This outcome assessed participants' (or carers') ability to manage medications using objective and/or subjective measures.
Objective measures: direct observation using standardised assessment instruments/methods (e.g. Drug Regimen Unassisted Grading Scale (DRUGS), Medication Management Ability Assessment (MMAA), device technique checklists (Elliott 2006; Elliott 2009; Patterson 2002)).
Subjective measures: self‐reported ability or self‐efficacy (e.g. Self‐Efficacy for Appropriate Medication Use Scale (SEAMS)) (Risser 2007).
Adherence to medication regimens
This outcome assessed consumer adherence to prescribed medication regimens, using objective and/or subjective measures.
Objective measures: refill data, pharmaceutical claims data, electronic monitoring, biological assay, measure of used/unused medications (e.g. pill count).
Subjective measures: self‐report of missed/used doses, validated questionnaires (e.g. Morisky scale (Morisky 1986)).
If an included study measured adherence and/or ability by using more than one type of outcome measure, review authors extracted the most reliable measure (i.e. objective measures were preferentially reported over subjective measures).
Secondary outcomes
We analysed the following secondary outcomes from studies that also measured at least one of the primary outcomes listed above.
Consumer (or carer) knowledge about their medications.
Consumer (or carer) satisfaction with the intervention.
Health‐related quality of life (HRQoL).
Adverse clinical health outcomes (e.g. unplanned hospital or emergency department presentations, general practitioner visits, ADEs).
Condition‐specific outcomes (e.g. cardiovascular events, blood pressure, blood glucose levels, lung function).
Cost‐effectiveness of the intervention.
Timing of outcome assessment
For adherence outcomes, the minimum duration of follow‐up was four weeks. For medication‐taking ability outcomes, a follow‐up period of at least 48 hours after the intervention was required.
If an included study measured adherence and/or ability more than once, we extracted the outcome measure with the longest follow‐up.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases in June 2019, using search strategies tailored to each database.
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library (to 2019).
MEDLINE (OvidSP) (1966 to 2019).
Embase (OvidSP) (1973 to 2019).
PsycINFO (OvidSP) (1967 to 2019).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus (EBSCOhost) (1981 to 2019).
International Pharmaceutical Abstracts (IPA) (ProQuest) (1971 to 2019).
The search strategies are presented in Appendix 1.
We applied no language restrictions (provided title and abstract were in English).
Searching other resources
We searched grey literature and online trial registries in November 2017.
For grey literature, we searched:
Joanna Briggs Institute Evidence Based Practice Database; and
conference proceedings (Scopus).
We checked the reference lists of included studies and of previously published relevant systematic reviews to locate potentially eligible studies that were not identified via electronic searches.
We also searched the following online trial registries for ongoing and recently completed studies.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
ClinicalTrials.gov.
ClinicalTrials.com.
TrialsCentral.
Australian New Zealand Clinical Trials Registry (ANZCTR).
United Kingdom Clinical Research Network (UKCRN).
Networked Digital Library of Theses and Dissertations (NDLTD).
International Standard Randomised Controlled Trial Number (ISRCTN) registry.
Non‐English language studies were translated and included if they met the eligibility criteria. Studies that were translated are noted in the Characteristics of included studies tables.
Data collection and analysis
Selection of studies
Two review authors (AC and KP, LK, or JG) independently screened abstracts and retrieved the full text of any papers identified as potentially relevant by at least one review author. Two review authors independently screened full‐text articles for inclusion or exclusion (AC and RE, KP, LK, or JG), with discrepancies resolved by discussion and by consulting a third review author if necessary to reach consensus (RE or JG). Review authors were not responsible for screening studies in which they were involved or that they were associated with. We listed as excluded studies all potentially relevant papers excluded from the review and provided reasons in the Characteristics of excluded studies table. We also provided citation details and any available information about ongoing studies, and we collated and reported details of duplicate publications, so that each study (rather than each report or manuscript) was the unit of interest in the review. We reported the screening and selection process in an adapted PRISMA flow chart (Liberati 2009).
Data extraction and management
Two review authors extracted data independently from included studies (AC and, RE, KP, LK, or JG). We resolved any discrepancies by discussion until consensus was reached, or through consultation with a third review author when necessary (RE or JG). We developed and piloted a data extraction form using the Cochrane Consumers and Communication Review Group Data Extraction Template (cccrg.cochrane.org/author-resources). Data extracted included the following study details: aim of the intervention, study design, study population, intervention details, control/comparison group(s), outcome(s), and follow‐up period(s). One review author (AC) entered all extracted data into Review Manager version 5.3 (RevMan 2014), and an independent person checked these data for accuracy against the data extraction sheets.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risks of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and according to Cochrane Consumers and Communication Group guidelines (CCCRG 2014), which recommend explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting; and other sources of bias. Other sources of bias included concerns related to sample size, fidelity, potential conflict of interest (e.g. influence of funding bodies), changes to methods (e.g. trial being ceased early), or trial results not published in a peer‐reviewed journal (e.g. thesis). We considered blinding separately for different outcomes when appropriate (e.g. blinding may have the potential to differently affect subjective versus objective outcome measures). We judged each item as being at high, low, or unclear risk of bias, as set out in the criteria provided by Higgins 2011, and we provided a quote or information from the study report and a justification for our judgement for each item in the "Risk of bias" table.
We deemed studies to be at highest risk of bias if they were scored at high or unclear risk of bias for the sequence generation or the allocation concealment domain, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011).
We assessed and reported quasi‐RCTs as being at high risk of bias on the random sequence generation item of the "Risk of bias" tool. For cluster‐RCTs, we also assessed and reported risk of bias associated with another domain: selective recruitment of cluster participants.
In all cases, two review authors independently assessed the risk of bias of included studies, with disagreements resolved by discussion to reach consensus. We contacted study authors for additional information about the included studies, or for clarification of study methods as required. We incorporated results of the "Risk of bias" assessment into the review through standard tables and systematic narrative description and commentary about each of the elements, leading to an overall assessment of the risk of bias of included studies and a judgement about the internal validity of review results.
Measures of treatment effect
We considered the primary outcomes as dichotomous variables when possible, that is, the person (or the carer) was assessed as able to manage medications or not, and similarly to have satisfactory adherence (80% to 120%) or not (< 80% or > 120%). If a study did not report its outcome as dichotomous, we extracted and analysed continuous outcomes.
For dichotomous outcomes, we analysed data based on the number of events and the number of people assessed in the intervention and comparison groups. We used these data to calculate the risk ratio (RR) and the 95% confidence interval (CI). Given heterogeneity in study measures, we analysed data for continuous measures using the standardised mean difference (SMD) and 95% CI approach via the inverse variance method in Review Manager 5.
Unit of analysis issues
For included cluster‐RCTs, we checked for unit of analysis errors. If errors were found, and if sufficient information was available, we re‐analysed data using the appropriate unit of analysis by taking into account the intracluster correlation coefficient (ICC). We obtained estimates of the ICC by contacting authors of included studies. When this was not possible, we reported effect estimates and annotated "unit of analysis errors." For future updates, we may impute missing ICCs using estimates from external sources, but this was not required for any of the trials included in this review.
Of the six cluster‐RCTs, three reported ICCs but did not report effective sample sizes. We recalculated effective sample sizes based on information reported in each study and divided the reported sample size by the design effect (Higgins 2011). We reported the adjusted sample sizes in meta‐analyses and reduced the weightings given to these studies.
Muth 2016 reported an ICC of 0.00; thus no adjustment was required.
Moral 2015 reported an ICC of 0.05 and had an average cluster size of 5.4 for intervention and 6.0 for control. There were 70 participants in the intervention group and 84 participants in the control group, which we adjusted to 57 for intervention and 67 for control for all outcomes.
Willeboordse 2017 reported an ICC of 0.08 but was not included in any meta‐analyses due to alternate reporting methods; thus effective sample sizes were not calculated.
For the three studies that did not report an ICC (Bernsten 2001;Volume 2001; Wood 1992), we contacted study authors for further information. As we received no response, we could make no adjustments. We conducted sensitivity analyses while excluding these studies to adjust for possible unit of analysis errors.
Dealing with missing data
We attempted to contact study authors to obtain missing data (participant, outcome, or summary data). When possible, we conducted analyses of participant data on an intention‐to‐treat (ITT) basis; otherwise we analysed data as reported. We reported on levels of loss to follow‐up and assessed this as a potential source of bias.
For missing outcome or summary data, we planned to impute missing data when possible and to report any assumptions, but this was not required or possible for any included studies.
We conducted sensitivity analyses that excluded studies presenting data with loss to follow‐up greater than 20% for the primary outcomes (medication‐taking ability and/or medication adherence) including total reported lost to follow‐up and differential loss to follow‐up between groups. This was due to potential serious threats to validity associated with high proportions of participants lost to follow‐up (Sackett 2000).
Assessment of heterogeneity
We identified substantial variations in types of interventions, populations studied, and study designs and settings. When studies were considered similar enough to enable data pooling via meta‐analysis, we assessed the degree of heterogeneity by visually inspecting forest plots and examining the Chi² test for heterogeneity. We quantified heterogeneity by using the I² statistic. We considered an I² value of 50% or more to represent substantial levels of heterogeneity, but we interpreted this value in light of the size and direction of effects and the strength of evidence for heterogeneity, based on the P value from the Chi² test (Higgins 2011). When heterogeneity was present in pooled effect estimates, we explored possible reasons for variability by conducting subgroup analyses.
When we detected substantial heterogeneity, particularly in relation to types of outcome measures or methods of reporting outcome measures, we did not report pooled results from meta‐analysis but instead used a narrative approach to data synthesis.
Assessment of reporting biases
We assessed reporting bias qualitatively based on the characteristics of included studies (e.g. if only small studies with positive findings were identified for inclusion), and based on information obtained by contacting authors of studies which suggested there might have been relevant unpublished data or studies.
We did not construct funnel plots to investigate publication bias because we found insufficient studies per outcome and intervention type and because multiple studies were not suitable for inclusion in meta‐analyses.
For future updates, if we should identify sufficient studies (at least 10) for inclusion in the review, we will construct a funnel plot to investigate small‐study effects and to formally test for funnel plot asymmetry, with test selection based on advice provided in Higgins 2011, while bearing in mind there may be several reasons for funnel plot asymmetry when results are interpreted.
Data synthesis
We conducted meta‐analyses on extracted data for some outcomes. Due to variability in the interventions of included studies, we used a random‐effects model for all meta‐analyses.
For studies not included in meta‐analyses, and for outcomes for which we were unable to pool data, we have presented data in tables and have narratively summarised the results for each outcome.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses to investigate heterogeneity of mixed interventions for medication adherence. We conducted three planned subgroup analyses.
Duration of intervention (short versus long).
Type of outcome measure (objective versus subjective).
Health professional group/system delivering the intervention (e.g. pharmacist versus nurse versus medical professional versus automated).
We were not able to conduct additional planned subgroup analyses to investigate heterogeneity because we found insufficient studies or poor reporting of participant characteristics. For future updates, should more studies be included (especially for other outcomes or other intervention types), we plan to also look at the following.
Duration of follow‐up (short, medium, and long term) as described under "Timing of outcome assessment."
Person managing the medication (consumer versus carer).
Number of medications (up to 10 versus 11 or more medications).
Frailty and/or functional ability (e.g. level of home assistance required) and/or cognitive function/ability.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes that excluded studies assessed to have losses to follow‐up greater than 20%, and that excluded studies with unit of analysis errors. We had planned to also conduct sensitivity analyses that excluded studies with high risk of bias; however there were too few included studies assessed as having low risk of bias. Future updates of this review should conduct these sensitivity analyses if sufficient studies with low risk of bias are identified.
"Summary of findings" table
We prepared "Summary of findings" tables to present results of meta‐analyses based on methods described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We presented results of meta‐analyses for the major comparisons of the review and for each of the primary outcomes, as outlined under Types of outcome measures. We provided a source and a rationale for each assumed risk cited in the tables and used Grades of Recommendation, Assessment, Development and Evaluation (GRADE) criteria to rank the quality of evidence using GRADEprofile (GRADEpro) software (Schünemann 2011). When meta‐analysis was not possible, we present results in a narrative "Summary of findings" table format, such as that used by Chan 2011.
Ensuring relevance to decisions in health care
Authors of this review received feedback from a consumer referee and a health professional as part of the standard editorial process of the Cochrane Consumers and Communication Group.
Results
Description of studies
See Characteristics of included studies,Characteristics of excluded studies,Characteristics of ongoing studies, and Characteristics of studies awaiting classification.
Results of the search
The database search yielded 27,854 titles. We found 65 additional records through a search of grey literature. After removing duplicates, we screened 19,192 studies and reviewed 482 full‐text articles. We excluded 373 studies that did not meet the inclusion criteria and recorded our reasons for exclusion. We included 50 independent studies (from 68 citations); 40 were randomised controlled trials (RCTs), four were considered quasi‐RCTs (Begley 1997; Shimp 2012; Volume 2001; Winland‐Brown 2000), and six were cluster‐RCTs (Bernsten 2001; Moral 2015; Muth 2016; Volume 2001; Willeboordse 2017; Wood 1992). Eight studies (from 12 citations) are ongoing (see Characteristics of ongoing studies). Twenty‐six studies (from 29 citations) are awaiting classification; eight have no published results; 15 may be eligible for inclusion but provide insufficient information to allow determination of eligibility; and three will be included in the next update of this review (see Characteristics of studies awaiting classification) (Char 2017; Marusic 2018; Muth 2018). Refer to Figure 1 for a PRISMA diagram.
1.
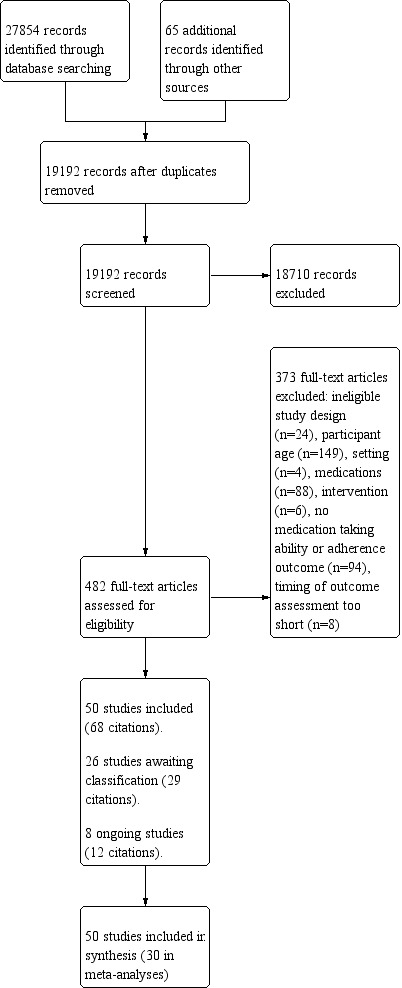
Study flow diagram.
Included studies
Participants
A total of 14,269 participants were included in the 50 studies. Fifteen studies involved fewer than 100 participants, and six studies involved more than 500 participants. In 38 studies, the intervention was directed at patients; in one study, the intervention was directed at family caregivers (George 2016); and in 11 studies, the intervention involved both patients and caregivers. The mean/median age of included patients ranged from 65.6 to 87.0 years, and 52.4% (6893/13,143) of patients were female (three studies did not provide clear details on gender). The mean/median number of medications ranged from 4.2 to 16.3, but the definition of 'medication' varied greatly between studies and was poorly described in 24 studies (48%). Eighteen studies clearly referred to prescribed medications, but many restricted the count to regular and/or oral medications only. Non‐prescription/over‐the‐counter (OTC) medications were included in the total count in five studies (Haag 2016; Khdour 2009; Krska 2001; Lingler 2016; Marek 2013); these were reported separately in three studies (Begley 1997; Chrischilles 2014; Volume 2001). Four studies did not provide mean/median values but were included based on the published range of the number of medications being taken (Winland‐Brown 2000), or on the fact that inclusion criteria ‐ Hale 2016 ‐ or additional information provided by study authors ‐ Blalock 2010, Shively 2013 ‐ indicated that the mean/median number of medications would be greater than four. One study was included because the subgroup of people taking more than eight medications met our inclusion criteria (Truelove 2015).
Sixteen studies reported some measure of frailty and/or functional ability for included participants, but variation in the scales used prevented comparison. Twenty studies included a measure of cognitive function of participants or listed the proportion of people with cognitive impairment, but the heterogeneous nature of reporting cognitive impairment prevented comparison. Seventeen studies excluded people with cognitive impairment, and 13 did not specify any details. The total mean/median number of chronic conditions (or co‐morbidities) was mentioned in only 10 studies and ranged from three to nine chronic conditions.
Setting
Included studies were carried out across four continents: North America, Europe, Asia, and Australia (see Table A). Most studies were conducted in the USA (21), the UK (8), Canada (5), and Australia (3). Ten studies were conducted in European countries: Spain (4), Croatia (1), Denmark (1), Germany (1), the Netherlands (1), Portugal (1), and Switzerland (1). Two studies were conducted in Asian countries: China (1) and Singapore (1). One study was conducted across seven countries (Bernsten 2001).
Study healthcare settings were categorised according to where the interventions were initiated during the patient's healthcare journey. Twenty‐six studies were initiated at the interface between hospital and community: in hospital (2), immediately before discharge (11), post discharge (6), or in hospital outpatient clinics (7). Twenty‐four studies were initiated in the community/primary care setting including general practice/medical clinics/centres (11), community pharmacies (5), home healthcare services (2), a university clinic (1), an independent living facility (1), and in the home (4, with 2 delivered online and 2 involving visiting health professionals).
Table A. Study design, setting, and participants
| Study ID | Study design | Target participants | Country | Stage of the patient's healthcare journey/healthcare setting where the intervention was initiated | |
| Hospital/Communityinterface | Community/Primary care | ||||
| Al‐Rashed 2002 | RCT | Patient | UK | Discharge | |
| Begley 1997 | RCT | Patient | UK | Post discharge | |
| Bernsten 2001 | Cluster‐RCT | Patient | 7 countries | Pharmacy | |
| Blalock 2010 | RCT | Patient | USA | Pharmacy | |
| Bond 2007 | RCT | Patient | UK | Pharmacy | |
| Cargill 1992 | RCT | Patient + Carer | USA | Outpatient clinic* | |
| Chrischilles 2014 | RCT | Patient | USA | Home (online) | |
| Cohen 2011 | RCT | Patient | USA | Medical centre | |
| Cossette 2015 | RCT | Patient | Canada | ED discharge | |
| George 2016 | RCT | Carer | USA | Online (home) | |
| Grymonpre 2001 | RCT | Patient | Canada | Health clinic | |
| Haag 2016 | RCT | Patient | USA | Post discharge | |
| Hale 2016 | RCT | Patient | USA | Post discharge | |
| Hanlon 1996 | RCT | Patient + Carer | USA | General medicine clinic* | |
| Holland 2007 | RCT | Patient + Carer | UK | Post discharge | |
| Khdour 2009 | RCT | Patient | UK | Outpatient clinic | |
| Krska 2001 | RCT | Patient | UK | General practice clinic | |
| Lee 2006 | RCT | Patient | USA | Outpatient pharmacy* | |
| Lim 2004 | RCT | Patient | Singapore | Outpatient clinic | |
| Lingler 2016 | RCT | Patient + Carer | USA | Community | |
| Lipton 1994 | RCT | Patient + Carer | USA | Discharge | |
| Lopez Cabezas 2006 | RCT | Patient | Spain | Discharge | |
| Manning 2007 | RCT | Patient | USA | Discharge | |
| Marek 2013 | RCT | Patient | USA | Home healthcare service | |
| Marusic 2013 | RCT | Patient | Croatia | Discharge | |
| Messerli 2016 | RCT | Patient | Switzerland | Pharmacy | |
| Moral 2015 | Cluster‐RCT | Patient | Spain | General practice | |
| Morales Suarez‐Vurela 2009 | RCT | Patient + Carer | Spain | Home healthcare service | |
| Murray 1993 | RCT | Patient | USA | Outreach centre | |
| Muth 2016 | Cluster‐RCT | Patient | Germany | General practice | |
| Nascimento 2016 | RCT | Patient + Carer | Portugal | Diabetes clinic | |
| Naunton 2003 | RCT | Patient + Carer | Australia | Post discharge | |
| Nazareth 2001 | RCT | Patient + Carer | UK | Discharge | |
| Olesen 2014 | RCT | Patient | Denmark | Community | |
| Pandey 2017 | RCT | Patient | Canada | Post discharge | |
| Pereles 1996 | RCT | Patient | Canada | Inpatient | |
| Rich 1996 | RCT | Patient | USA | Discharge | |
| Saez de la Fuente 2011 | RCT | Patient + Carer | Spain | Discharge | |
| Shimp 2012 | RCT | Patient | USA | University clinic | |
| Shively 2013 | RCT | Patient | USA | Primary care clinic | |
| Taylor 2003 | RCT | Patient | USA | General practice clinic | |
| Truelove 2015 | RCT | Patient | Australia | General practice clinic | |
| Vinluan 2015 | RCT | Patient | USA | Discharge | |
| Volume 2001 | Cluster‐RCT | Patient | Canada | Pharmacy | |
| Willeboordse 2017 | Cluster‐RCT | Patient | Netherlands | General practice clinic | |
| Williams 2012 | RCT | Patient | Australia | Outpatient clinic | |
| Winland‐Brown 2000 | RCT | Patient | USA | Independent living facility | |
| Wood 1992 | Cluster‐RCT | Patient | UK | Inpatient | |
| Wu 2006 | RCT | Patient + Carer | China | Outpatient clinic | |
| Young 2016 | RCT | Patient | USA | Discharge | |
*Unclear whether health service provided primary or secondary care or both.
ED: emergency department.
RCT: randomised controlled trial.
Interventions
A range of simple to complex interventions were used across the included studies. Due to the heterogeneous nature of the interventions, we categorised them into three broad groups: educational interventions, behavioural interventions, and mixed interventions (both educational and behavioural). These categories have been used in a previous systematic review of interventions to improve medication‐taking in elderly patients prescribed multiple medications (George 2008).
Fourteen studies involved educational interventions comprising medication/health education (provided in writing or verbally) and/or medication review only; seven studies involved behavioural interventions only; and 29 studies had both educational and behavioural elements.
Educational interventions were identified in 38 studies delivering patient or carer education regarding medications and/or health conditions, and in 26 studies involving a review of patient medications.
A range of behavioural interventions were used (either alone or in combination) in 24 studies utilising follow‐up or monitoring; in seven studies providing regimen simplification (Begley 1997;Bernsten 2001;Lim 2004;Lipton 1994;Murray 1993;Olesen 2014; Rich 1996); in five studies practising motivational interviewing (Khdour 2009;Moral 2015;Olesen 2014;Shively 2013;Williams 2012); and in two studies implementing three‐step self‐administration of medications (Pereles 1996; Wood 1992). All participants in six studies utilised DAAs including simple pill boxes (Lee 2006; Marek 2013; Morales Suarez‐Vurela 2009; Winland‐Brown 2000), unit of use packages (Murray 1993), automated dosing devices (Marek 2013; Winland‐Brown 2000), and remotely monitored electronic devices (Hale 2016). Two studies utilised DAAs for some participants as required (Cargill 1992; Naunton 2003), and two studies provided participants with electronic pill reminder devices (Olesen 2014; Young 2016). Other types of interventions included text message adherence reminders (Pandey 2017), a four‐ingredient poly‐pill (Truelove 2015), use of online personal health records (Chrischilles 2014), and a three‐dimensional (3D ‐ durable display at discharge) medication discharge tool that involved patients affixing a tablet/capsule of each medication onto the 3D tool (Manning 2007).
Most interventions were delivered by pharmacists (31 studies), nurses (17 studies), and physicians (15 studies), either alone (31 studies) or in multi‐disciplinary teams of two or more health professionals (15 studies). Two interventions were delivered online (Chrischilles 2014; George 2016); one study involved text message reminders (Pandey 2017); and one study involved a remotely monitored electronic device (Hale 2016). Interventions varied in duration, ranging from one‐off ‐ Al‐Rashed 2002, Blalock 2010, Haag 2016, Lim 2004, Manning 2007, Marusic 2013, Muth 2016, Nascimento 2016, Naunton 2003, Saez de la Fuente 2011, Willeboordse 2017 ‐ to two years ‐ Wu 2006, and were most commonly delivered in the home (face‐to‐face or by phone calls), hospital, medical centre, or community pharmacy. Four studies were delivered across two settings (Lipton 1994; Moral 2015; Nazareth 2001; Rich 1996), and 11 studies involved both face‐to‐face meetings and phone calls (Cargill 1992; Cossette 2015; Khdour 2009; Lingler 2016; Lipton 1994; Lopez Cabezas 2006; Olesen 2014; Shively 2013; Vinluan 2015; Williams 2012; Young 2016).
An additional table summarising the intervention features of all included studies is located at https://latrobe.figshare.com/articles/Additional_tables_Cross_et_al_2020_docx/12247385.
Primary outcomes
Medication‐taking ability was measured in five studies. Four studies used objective measures including a five‐item dexterity test that assessed skills such as ability to open child‐resistant closures on containers (Begley 1997), a medication‐taking behaviour score (Cargill 1992), the Medication Management Instrument for Deficiencies in the Elderly (MedMaIDE) (Lingler 2016), performance in an inpatient self‐administration of medications programme, and/or pharmacist assessment (with input from other team members) of ability to self‐administer medications (Pereles 1996). One study used a subjective measure ‐ a self‐reported assessment of safety in taking medication (Manning 2007). Medication‐taking ability was typically measured at short follow‐up points (e.g. 7 to 14 days; Manning 2007), except for one study, which had an extended measure at 12 months (Begley 1997).
Medication adherence was measured in 48 studies (Table C). Twenty studies used an objective measure of adherence such as pill count (Begley 1997;Cargill 1992;Cohen 2011;Lee 2006;Lopez Cabezas 2006;Marusic 2013;Moral 2015;Murray 1993;Olesen 2014;Pereles 1996;Rich 1996;Williams 2012;Winland‐Brown 2000;Wood 1992), prescription claims/refills (Al‐Rashed 2002;Grymonpre 2001;Messerli 2016;Shimp 2012;Vinluan 2015), or machine‐recorded correct doses (Marek 2013). Twenty‐eight studies used a subjective measure of adherence; 16 of these used an original or modified version of the Morisky Medication Adherence Scale ‐ a validated measure of adherence (Bernsten 2001;Chrischilles 2014;Cossette 2015;George 2016;Haag 2016;Hale 2016;Khdour 2009;Morales Suarez‐Vurela 2009;Muth 2016;Nascimento 2016;Saez de la Fuente 2011;Volume 2001), the Medication Adherence Rating Scale (Bond 2007;Holland 2007;Muth 2016), the Brief Medication Questionnaire (Blalock 2010), and the Medical Outcome Study‐Specific Adherence Scale (Shively 2013). Four studies used structured interviews to enquire about adherence (Hanlon 1996;Lipton 1994;Nazareth 2001;Willeboordse 2017), and six studies asked participants a single question about forgotten or missed doses (Lim 2004;Naunton 2003;Taylor 2003;Truelove 2015;Wu 2006;Young 2016). One study used a patient‐completed daily log‐book of medication consumption (Pandey 2017), and one study included pharmacist review for pharmaceutical care issues including potential or actual adherence issues (Krska 2001). The longest follow‐up time points of post‐intervention adherence outcomes ranged from 1 month to 18 months, with the median time point of 6 months.
Secondary outcomes
Knowledge about medications was measured in 13 studies. Knowledge was assessed most often by asking participants about one or more of the following: name of medication, appearance of medication, purpose of medication, dose, dose frequency/interval, side effects, drug interactions, and special comments or cautions (Al‐Rashed 2002; Begley 1997; Bernsten 2001; Grymonpre 2001; Hanlon 1996; Lim 2004; Manning 2007; Messerli 2016; Nazareth 2001; Pereles 1996; Taylor 2003). One study asked participants on a 5‐point Likert scale if they "knew more about their medicines compared to a year ago" (Bond 2007), and another study assessed medication knowledge as part of a larger chronic obstructive pulmonary disease (COPD) knowledge questionnaire (Khdour 2009).
Satisfaction with the intervention was measured in 13 studies. Six studies used a previously validated measure (George 2016;Hanlon 1996;Lopez Cabezas 2006;Nazareth 2001;Volume 2001;Willeboordse 2017), but no two studies used the same measure. Satisfaction was most commonly assessed on a 5‐point Likert‐type scale (Bernsten 2001;Bond 2007;Hanlon 1996;Manning 2007), or on a 7‐point Likert‐type scale (George 2016;Volume 2001;Willeboordse 2017), which included between one ‐ Manning 2007, Willeboordse 2017 ‐ and 15 items (Bond 2007). Lopez Cabezas 2006 used a 0 to 10 analogue scale, and four studies did not adequately describe the measure used (Holland 2007;Lingler 2016;Naunton 2003;Taylor 2003).
Health‐related quality of life (HRQoL) was measured in 14 studies. The two most common measures were the validated Short Form Health Survey involving 36 items (SF‐36) used in eight studies ‐ Bernsten 2001, Bond 2007, Cohen 2011, Hanlon 1996, Krska 2001, Marek 2013, Taylor 2003, Volume 2001 ‐ and the European Quality of Life 5‐Dimension Instrument (EQ‐5D) used in five studies ‐ Bond 2007, Holland 2007, Lopez Cabezas 2006, Muth 2016, Willeboordse 2017. Other measures used by individual studies included the 12‐item Short Form Health Survey (SF‐12; Willeboordse 2017), as well as disease‐specific quality of life measures including the Minnesota Living With Heart Failure Questionnaire (MLHFQ) ‐ Hale 2016, Holland 2007 ‐ and St George's Respiratory Questionnaire (SGRQ) ‐ Khdour 2009.
Adverse clinical health outcomes were measured in 28 studies and included measures such as emergency department (ED) and/or hospital admissions (Al‐Rashed 2002; Bernsten 2001; Cossette 2015; Haag 2016; Hale 2016; Holland 2007; Khdour 2009; Lipton 1994; Lopez Cabezas 2006; Marusic 2013; Messerli 2016; Muth 2016; Naunton 2003; Nazareth 2001; Olesen 2014; Rich 1996; Saez de la Fuente 2011; Shively 2013; Taylor 2003; Vinluan 2015; Winland‐Brown 2000; Wu 2006; Young 2016), mortality (Holland 2007; Lopez Cabezas 2006; Naunton 2003; Nazareth 2001; Olesen 2014; Saez de la Fuente 2011; Vinluan 2015; Wu 2006), adverse drug reactions (Chrischilles 2014; Hanlon 1996; Lim 2004; Marusic 2013; Murray 1993; Willeboordse 2017), and physician visits (Al‐Rashed 2002; Khdour 2009; Nazareth 2001; Winland‐Brown 2000).
Condition‐specific outcomes were measured in seven studies and included changes in blood pressure (Lee 2006;Taylor 2003;Williams 2012), diabetes control ‐ glycosylated haemoglobin (HbA1c)/blood glucose (Nascimento 2016;Taylor 2003;Williams 2012), low‐density lipoprotein (LDL) cholesterol (Lee 2006;Taylor 2003), falls (Blalock 2010), international normalised ratio (INR) of time taken for blood to clot (Taylor 2003), and renal function (Williams 2012). Two studies reported composite measures of reaching multiple 'health' targets (Bond 2007;Cohen 2011).
Cost‐effectiveness of the intervention was measured in four studies; three studies used costs of the intervention, medicines, hospitalisations, and/or health consultations (Bernsten 2001;Bond 2007;Lopez Cabezas 2006), and one study used US Medicare Part B costs and total hospital inpatient costs (Lipton 1994).
Other outcome measures extracted included medication management problems from a list of eight problems (Chrischilles 2014), a medication deficiency checklist (Lingler 2016), medication errors defined as both prescriber and patient errors (Moral 2015), and medication misadventures defined as one or more medication errors, adverse drug events, or adverse drug reactions (Taylor 2003).
An additional table summarising the type and timing of primary and secondary outcomes assessed by included studies is located at https://latrobe.figshare.com/articles/Additional_tables_Cross_et_al_2020_docx/12247385.
Excluded studies
We excluded 373 studies in total (see Characteristics of excluded studies). We excluded 24 studies because study design did not meet Cochrane criteria for an RCT, a cluster‐RCT, or a quasi‐RCT. We excluded 149 studies on the basis of the age of participants; 88 studies based on the number of regular prescription medications (including 13 studies for which the number of medications was unknown and attempts to contact study authors were unsuccessful); and 10 studies because study authors did not collect information on the number of medications. We excluded 94 studies because they did not include a measure of medication‐taking ability or medication adherence as an outcome, and 8 studies because the follow‐up period for outcome measures was too short (i.e. < 48 hours for medication‐taking ability, < 4 weeks for adherence). We excluded 6 studies because the intervention did not target consumers, along with 4 studies because participants were not community‐dwelling.
Risk of bias in included studies
See Characteristics of included studies table, Figure 2, and Figure 3 for a summary assessment of the risk of bias of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of bias for random sequence generation was low in 22 studies (44%), unclear in 24 studies (48%), and high in four studies (8%). For concealment of allocation, risk of bias was low in 17 studies (34%), unclear in 30 studies (60%), and high in three studies (6%). Selective recruitment of cluster participants was assessed for the six cluster‐RCTs ‐ three were considered at high risk (Moral 2015;Willeboordse 2017;Volume 2001), two were considered at low risk (Muth 2016;Wood 1992), and one was considered at unclear risk of recruitment bias (Bernsten 2001).
Blinding
Blinding of both participants and personnel could not be achieved through the study design in 49 of the 50 studies (98%), leading to high risk of performance bias. One study was considered to have low risk of performance bias, as the intervention was delivered online and both intervention and control participants viewed the same interface and thus were unaware of their allocation (George 2016).
Seventeen studies (34%) stated that there was no blinding of outcome assessment; we considered these studies to have high risk of detection bias. Twenty studies (40%) were assessed as having 'unclear' risk of detection bias due to insufficient details regarding the method of outcome assessment. Studies with unclear detection bias included one study for which data collection was performed "where possible" by a member of staff other than the intervention pharmacist (Bernsten 2001), one study involving caregiver‐reported patient adherence when caregivers were assumed to be unaware of allocation (George 2016), and two studies in which assessors were reported as blinded but contamination from unblinded participants was thought to be highly likely (Saez de la Fuente 2011; Young 2016). We assessed 13 studies (26%) as having low risk of detection bias; five involved an objective measure of the primary outcome (Grymonpre 2001;Marusic 2013;Messerli 2016;Rich 1996; Williams 2012), and eight involved subjective measures but data were collected/analysed by blinded investigators (Bond 2007;Chrischilles 2014;Cossette 2015;Haag 2016;Hanlon 1996;Lipton 1994;Manning 2007;Nazareth 2001).
Incomplete outcome data
Twenty‐two (44%) studies were considered to have incomplete outcome data and therefore high risk of attrition bias – 19 of these cases were due to high loss to follow‐up. A further three (6%) studies were assessed as having high risk of attrition bias due to inconsistency between the average number of medicines and the number assessed for adherence (Grymonpre 2001), inconsistency between the number of patients with adherence reported and the number who saved their medication boxes enabling accurate pill count (Williams 2012), and lack of details regarding attrition of the control group (Shimp 2012). Thirteen studies were assessed as having unclear risk of bias mainly due to low to moderate attrition, which may have had an impact on the results, or insufficient details on number of, or reasons for, attrition. Fifteen studies reported minimal incomplete outcome data and/or adequately addressed this (low risk of bias).
Selective reporting
Fourteen studies (28%) were considered to have high risk of reporting bias ‐ 12 due to missing outcome data (Begley 1997;Blalock 2010;Cohen 2011;Krska 2001;Lim 2004;Lopez Cabezas 2006;Messerli 2016;Morales Suarez‐Vurela 2009;Murray 1993;Olesen 2014;Shimp 2012; Winland‐Brown 2000), one because study authors did not clearly specify how data were obtained (Vinluan 2015), and one because researchers changed the inclusion criteria mid‐way through the study to increase recruitment (Williams 2012).
Twelve studies (24%) were considered to have unclear risk of reporting bias due to minor deviations from study methods (Bond 2007;Grymonpre 2001;Lipton 1994;Wood 1992), missing information in the methods section (Bernsten 2001;Willeboordse 2017), missing baseline data (Lee 2006), and unclear reporting of results (Cargill 1992;Lingler 2016;Marek 2013;Moral 2015;Willeboordse 2017).
Although 24 studies (48%) were assessed as having low risk of selective reporting, 15 of these did not have a published protocol nor trial registration; thus it was difficult to accurately assess reporting bias.
Other potential sources of bias
Four studies were identified as having high risk of other types of bias. One study was assessed as having high risk because it was a research thesis and had not been published in a peer‐reviewed journal (George 2016), two studies because of poor intervention fidelity (Chrischilles 2014;Nazareth 2001), and one study because researchers measured adherence only for the first three medications mentioned by the patient (Lipton 1994).
Twenty‐seven studies were considered to have unclear risk of other types of bias. Sixteen studies did not reach their specified target sample size (Bernsten 2001;Blalock 2010;Bond 2007;Cossette 2015;Hale 2016;Khdour 2009;Lopez Cabezas 2006;Marek 2013;Messerli 2016;Moral 2015;Morales Suarez‐Vurela 2009;Pereles 1996;Truelove 2015;Volume 2001;Willeboordse 2017; Wu 2006); three studies had unbalanced participant groups likely influencing outcomes (Haag 2016; Murray 1993; Winland‐Brown 2000); and four studies had potential conflicts due to funding arrangements (Holland 2007; Shimp 2012) or participant compensation (Messerli 2016; Shively 2013), which may have biased results of the study. Two studies provided limited information regarding intervention fidelity (Manning 2007; Nascimento 2016), and four studies expressed concerns regarding the appropriateness of the adherence assessment (Nascimento 2016; Rich 1996; Pandey 2017; Williams 2012).
Trial authors also noted that 10 studies did not declare a funding source. However, given the differences in journal requirements and the age of those studies, this was not considered to introduce risk of bias for this review.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Summary of findings: mixed interventions.
| Mixed educational and behavioural interventions aimed at improving medication‐taking ability and/or medication adherence compared with usual care for older community‐dwelling patients taking multiple medications | |||
|
Patient or population: older patients using at least 4 regular prescription medications (and/or their carers) Settings: community setting (including discharge from a hospital or other healthcare facility to the community) Intervention: interventions involving both educational and behavioural components Comparison: usual care | |||
| Outcomes | Impacts | No of Studies | Quality of the evidence (GRADE) |
|
Medication‐taking ability Follow‐up: 2 weeks to 12 months |
The effects of mixed interventions on medication‐taking ability were unable to be determined. Meta‐analysis was not possible due to all 5 studies using different outcome measures. Of the 5 studies, 1 demonstrated significant improvement in medication‐taking ability, 2 showed no significant impact, 1 did not test for differences between groups, and 1 did not report results | 5 | Lowa,b |
|
Medication adherence (dichotomous) Follow‐up: 1 to 18 months |
Mixed interventions may improve the proportion of people who are adherent (dichotomous adherence outcome) Twelve studies (3147 participants) were included in a meta‐analysis. Risk ratio was 1.22 (95% CI 1.08 to 1.37), indicating interventions increased the absolute number of adherent participants by 12.8% (4.6% to 21.5%) Two studies were excluded from the meta‐analysis due to alternate reporting of outcome data: 1 study reported the intervention increased the number of medications taken correctly; 1 study showed no differences between groups |
14 | Lowa,b |
|
Medication adherence (continuous) Follow‐up: 1 to 12 months |
Mixed interventions may have little or no impact on medication adherence measured by continuous adherence outcomes (e.g. proportion of pills dispensed or taken) Seven studies (1825 participants) were included in a meta‐analysis. Standardised mean difference was 0.47 (95% CI ‐0.08 to 1.02), indicating that the mean adherence score in the intervention group was 0.47 standard deviations higher (0.08 lower to 1.02 higher) than in the usual care group Four studies were excluded from the meta‐analysis due to alternate reporting of outcome data: 1 study showed fewer medication errors as a proportion of total doses with the intervention; 3 studies showed no significant effect on adherence. Two additional studies were excluded due to unclear reporting of results |
13 | Lowb,c |
|
Health‐related quality of life Follow‐up: 6 to 18 months |
Mixed interventions may lead to little or no change in health‐related quality of life. Six of 7 studies showed no significant impact on this outcome. One study reported the intervention may improve both physical and mental summary scores on the SF‐36 at 12 months. Meta‐analysis was not possible due to differences in scales used and differences in reporting of results | 7 | Lowa,b |
|
Emergency department (ED)/Hospital admissions Follow‐up: 1 to 24 months |
Mixed interventions may reduce the number of emergency department (ED) and/or hospital admissions. Eleven studies (1827 participants) were included in meta‐analysis. Risk ratio was 0.67 (95% CI 0.50 to 0.90), indicating mixed interventions may reduce the absolute number of patients admitted to ED/hospital by 12.3% (18.7% to 3.7% fewer). Six studies were excluded from the meta‐analysis due to alternate reporting of outcome data; none of these studies reported differences between groups in ED/hospital admissions | 17 | Lowa,b |
|
Mortality Follow‐up: 3 to 24 months |
We are uncertain of the effects of mixed interventions on mortality. Seven studies (1776 participants) were included in a meta‐analysis. Risk ratio was 0.93 (95% CI 0.67 to 1.30), with an anticipated absolute effect of 0.9% fewer deaths (4.1% fewer to 3.8% more). One study was excluded from meta‐analysis due to incomplete information | 8 | Very lowa,b,d |
| CI: confidence interval; SF‐36: Short Form Health Survey‐36. | |||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||
aOne mark deducted due to high or unclear risk of bias across multiple domains including sequence generation and allocation concealment.
bOne mark deducted due to variations in intervention, provider, setting, duration, and outcome measures, and because of high levels of heterogeneity in results.
cOne mark deducted due to high or unclear risk of bias across multiple domains and inclusion of studies at risk of attrition bias in meta‐analysis.
dOne mark deducted due to imprecision with limits of confidence intervals including both substantial potential benefit and harm.
Summary of findings 2. Summary of findings: educational interventions alone.
| Educational interventions aimed at improving medication‐taking ability and/or medication adherence compared with usual care for older community‐dwelling patients taking multiple medications | |||
|
Patient or population: older patients using at least 4 regular prescription medications (and/or their carers) Settings: community setting (including discharge from a hospital or other healthcare facility to the community) Intervention: interventions involving educational components only Comparison: usual care | |||
| Outcomes | Impacts | No of studies | Quality of the evidence (GRADE) |
|
Medication‐taking ability Follow‐up: N/A |
No studies that evaluated medication‐taking ability were found | ‐ | ‐ |
|
Medication adherence (dichotomous) Follow‐up: 1 to 6 months |
We are uncertain of the effects of educational interventions on the proportion of people who are adherent Two studies (182 participants) using dichotomous measures of adherence were included in a meta‐analysis. Risk ratio was 1.66 (95% CI 1.33 to 2.06), indicating that educational interventions increased the absolute number of adherent participants by 31.1% (15.6% to 50.1% more) Three studies were excluded from the meta‐analysis due to alternate reporting of outcome data: 1 study reported that the intervention increased the number of resolved medication issues (including non‐adherence); 2 studies reported no significant effect on adherence |
5 | Very lowa,b,c |
|
Medication adherence (continuous) Follow‐up: 1 to 12 months |
Educational interventions may have little or no impact on medication adherence measured by continuous adherence outcomes (e.g. proportion of pills dispensed or taken) Five studies (1165 participants) using continuous measures of adherence were included in a meta‐analysis. Standardised mean difference was 0.16 (95% CI ‐0.12 to 0.43), indicating that the mean adherence score in the intervention group was 0.16 standard deviations higher (0.12 lower to 0.43 higher) than in the usual care group Four studies were excluded from the meta‐analysis: 2 due to alternate reporting of outcome data (neither showed a difference between groups); 2 did not report results |
9 | Lowa,b |
|
Health‐related quality of life Follow‐up: 3 to 12 months |
Educational interventions probably have little or no effect on health‐related quality of life, with all 6 studies reporting no differences between groups. Meta‐analysis was not possible due to differences in scales used and differences in reporting of results | 6 | Moderatea |
|
ED/Hospital admissions Follow‐up: 4 to 28 weeks |
Educational interventions probably have little or no effect on ED/hospital admissions. Three studies (554 participants) were included in a meta‐analysis. Risk ratio was 1.02 (95% CI 0.71 to 1.48), indicating no change in the number of patients admitted to ED/hospital. One further study not included in meta‐analysis, reporting mean number of days in hospital, found no differences between groups | 4 | Moderatea |
|
Mortality Follow‐up: N/A |
No studies that evaluated the effects of educational interventions on mortality were found | ‐ | ‐ |
| CI: confidence interval; ED: emergency department. | |||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||
aOne mark deducted due to high or unclear risk of bias across multiple domains including sequence generation and allocation concealment.
bOne mark deducted due to variations in intervention, provider, setting, duration, and outcome measures, and because of high levels of heterogeneity in results.
cOne mark deducted due to imprecision ‐ small total number of participants and only two studies in meta‐analysis (one had very wide confidence interval and low events) plus the number of adherent patients (i.e. events) were not clearly reported in the two studies excluded from meta‐analysis.
Summary of findings 3. Summary of findings: behavioural interventions alone.
| Behavioural interventions aimed at improving medication‐taking ability and/or medication adherence compared with usual care for older community‐dwelling patients taking multiple medications | |||
|
Patient or population: older patients using at least 4 regular prescription medications (and/or their carers) Settings: community setting (including discharge from a hospital or other healthcare facility to the community) Intervention: interventions involving behavioural components only Comparison: usual care | |||
| Outcomes | Impacts | No of studies | Quality of the evidence (GRADE) |
|
Medication‐taking ability Follow‐up: N/A |
No studies that evaluated medication‐taking ability were found | ‐ | ‐ |
|
Medication adherence (dichotomous) Follow‐up: 3 to 18 months |
Behavioural interventions may improve the proportion of people who are adherent (dichotomous adherence outcome) Four studies (528 participants) were included in a meta‐analysis. Risk ratio was 1.22 (95% CI 1.07 to 1.38), indicating behavioural interventions increased the absolute number of adherent participants by 10.5% (3.3% to 18.1% more) |
4 | Lowa,b |
|
Medication adherence (continuous) Follow‐up: 6 to 12 months |
We are uncertain of the effects of behavioural interventions on medication adherence when continuous measures of adherence are used Three studies were identified, but results could not be pooled in a meta‐analysis due to differences in reporting. All 3 reported significant impact on medication adherence, 2 showed large effects on adherence based on pill count, and 1 showed moderate improvement in self‐reported adherence using daily logbooks to calculate percentage of days adherent |
3 | Very lowa,b,c |
|
Health‐related quality of life Follow‐up: 3 months |
We are uncertain of the effects of behavioural interventions on health‐related quality of life. Only 1 study was identified, which found that the intervention resulted in worsening quality of life using the Minnesota Living With Heart Failure Questionnaire | 1 | Very lowa,d,e |
|
ED/Hospital admissions Follow‐up: 3 to 6 months |
We are uncertain of the effects of behavioural interventions on ED/hospital admissions. Two studies (70 participants) were included in a meta‐analysis. Risk ratio was 0.21 (95% CI 0.08 to 0.55), indicating behavioural interventions may reduce the absolute number of patients admitted to ED/hospital by 42.9% (49.9% to 24.4% fewer) | 2 | Very lowa,f |
|
Mortality Follow‐up: N/A |
No studies that evaluated the effects of behavioural interventions on mortality were found | ‐ | ‐ |
| CI: confidence interval; ED: emergency department. | |||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||
aOne mark deducted due to high or unclear risk of bias across multiple domains including sequence generation and allocation concealment.
bOne mark deducted due to variations in intervention, provider, setting, duration, and outcome measures.
cOne mark deducted due to low participant numbers.
dOne mark deducted due to indirectness of evidence as the Minnesota Living With Heart Failure Questionnaire is specific for heart failure populations and results may not be generalisable to general population of older people.
eOne mark deducted due to low participant numbers from a single small study.
fTwo marks deducted due to low participant numbers and low number of events.
See "Summary of findings" tables for the main comparison.
COMPARISON 1. Intervention versus usual care
Primary outcome ‐ medication‐taking ability
Educational interventions
No studies were identified.
Behavioural interventions
No studies were identified.
Mixed educational and behavioural interventions
Mixed educational and behavioural interventions were identified in five studies, which showed mixed impact on medication‐taking ability (Table 4; low‐certainty evidence). One study involving an educational intervention combined with telephone follow‐up directed at both patients and caregivers (group 3) showed slightly greater improvement in medication‐taking ability compared to usual care (group 1), as measured by a behaviour score at four to six weeks (mean scores presented visually; mean 86/100 versus 74/100; P = 0.01) (Cargill 1992). Another study, also involving an educational intervention plus follow‐up, demonstrated significant decreases in medication management problems, as measured by the Management Instrument for Deficiencies in the Elderly (MedMaIDE), in both intervention and usual care groups but did not report between‐group comparisons (Lingler 2016). Two studies showed no significant difference in patients' medication‐taking ability (Manning 2007; Pereles 1996). In Manning 2007, a medication chart with tablets/capsules affixed and medication discharge education had no significant impact on the number of self‐reported mistakes in taking medication. In Pereles 1996, an inpatient self‐administration of medication programme was reported to have no impact on the number of participants able to self‐administer their medications; however, different methods of assessing the outcome were used for intervention and control groups. One study involving patient‐focused education and regimen simplification did not report results (Begley 1997).
1. Primary outcome ‐ medication‐taking ability.
| Study | Measure of medication‐taking ability | Outcome |
| Begley 1997 | Objective measure: 5‐task dexterity test (e.g. opening child‐resistant closure), 1 point awarded for each successfully completed activity. Note: no difference across groups at baseline ‐ mean (SD) group A: 7.8 (1.3), group B: 7.5 (1.5), group C: 8.0 (1.4) | Objective measure: follow‐up results not reported |
| Cargill 1992 | Objective measure: behaviour score/100 for congruency between supply of medications on hand and prescribed medications (/40), verbalising correct regimen (/30), maintaining each prescribed med (/20), appropriate use of OTC (/10). Points deducted for sequestering old scripts, inappropriate use of alternative medications, or mixing medications together | Mean read from graph: Control: 74 vs intervention (group 3); 86 vs intervention (group 2); 84 vs intervention (group 3): 86 |
| Lingler 2016 | Objective measure: Medication Management Instrument for Deficiencies in the Elderly (MedMaIDE). MedMaiDE uses interview and observation to assess ability to self‐administer medications in 3 areas: knowledge of medications, how to take medications, and how to procure medications. Each medication is reviewed during administration. Scores 0 to 13, max total deficiency score is 13 | Baseline: mean ± SD intervention 0.833 ± 0.745 vs control 0.692 ± 0.768 Unpublished follow‐up results: mean ± SD: intervention 0.595 ± 0.725 vs control 0.297 ± 0.777; both groups showed significant decreases in number of medication management problems at 2 months (P < 0.01) |
| Manning 2007 | Subjective measure: self‐reported safety. Since discharge, how many mistakes have you made taking your medications (score 0 to 4)? | Mean ± SD: intervention 0.78 ± 0.4187 (n = 72) vs control 0.79 ± 0.4113 (n = 57) |
| Pereles 1996 | Objective measure: assessed differently for each group: intervention = pharmacist assessment with input from other team members, primarily based on having made 2 or fewer errors at stage 2 of the inpatient self‐medication programme ‐ considered able to self‐medicate at discharge. Control = pharmacist assessment with input from other team members at time of discharge counselling. YES/NO ‐ self‐medicating at discharge (note: there could be reasons other than failing the SMP that might explain why they were not self‐medicating at discharge, such as patient preference) | n (%): intervention 39 (76.5%) vs control 39 (69.6%) |
OTC: over‐the‐counter.
SD: standard deviation.
Subgroup analysis was not possible due to the small number of eligible studies.
Overall,
no studies were identified that evaluated the impact of educational or behavioural interventions alone on medication‐taking ability; and
the effect of mixed interventions on medication‐taking ability was unable to be determined (low‐quality evidence). The evidence was downgraded due to high or unclear risk of bias across multiple domains (‐1) and for inconsistency (‐1) (variations in interventions, outcome measures, settings, duration, etc.).
Primary outcome ‐ adherence
Forty‐eight studies included a measure of adherence and were analysed based on the type of intervention provided: educational (n = 14 studies), behavioural (n = 7 studies), or mixed (n = 27 studies).
Meta‐analyses were possible for 31 studies: 18 involving dichotomous measures (Analysis 1.1), and 13 involving continuous measures (Analysis 1.2). The 17 studies not included in meta‐analyses are briefly summarised in Table 5 and in the following subsections.
1.1. Analysis.

Comparison 1: Interventions versus usual care, Outcome 1: Primary outcome: adherence, grouped by types of interventions (dichotomous)
1.2. Analysis.

Comparison 1: Interventions versus usual care, Outcome 2: Primary outcome: adherence, grouped by types of interventions (continuous)
2. Primary outcome ‐ adherence (studies not included in meta‐analyses).
| Study | Measure of adherence | Outcome |
| Al‐Rashed 2002 | Objective measure: percentage compliance using home medicines stocks and refill prescriptions between visits 1 and 2 | Intervention: 70% (n = 342 medications) vs control 15.8% (n = 328 medications) |
| Blalock 2010 | Subjective measure: Brief Medication Questionnaire (5‐item regimen screen that assesses how medication is used) | Not reported |
| Bond 2007 | Subjective measure: Extended Medication Adherence Report Scale (MARS) questionnaire (12 statements about medicine‐taking; score range 12 to 60) | Med (IQR): intervention 59 (57 to 60) vs control 59 (57 to 60) |
| Cargill 1992 | Objective measure: pill count, percentage of pills taken compared to those prescribed | Mean scores: control: 74.5; intervention (group 3): 76.2; intervention (group 2): 74; intervention (group 3): 76.2 |
| Cohen 2011 | Objective measure: medication possession ratios | Not clearly reported |
| Hanlon 1996 | Subjective measure: self‐report proportion of medications for which the patient's response agreed with the directions for use on the action profile | Intervention 77.4% (n = 86 people) vs usual care 76.1% (n = 83 people) |
| Holland 2007 | Subjective measure: Medication Adherence Report Scale (MARS) scores from 5 (very poor adherence) to 25 (perfect adherence) | Mean (median): 23.74 (25), n = 101 vs 23.55 (25), n = 103 |
| Krska 2001 | Subjective measure: pharmaceutical care issues including potential or actual compliance issues, number of baseline issues resolved at 3 months | 51 of 74 issues resolved (n = 168) vs 21 of 69 issues resolved (n = 164) |
| Lim 2004 | Subjective measure: self‐reported; patients asked if they 'forgot to take medication as directed'. Then categorised as least compliant (compliant base, not at 2 months), not compliant (not compliant at base or at 2 months), compliant (compliant at 2 months) | Not clearly reported; unadjusted OR 1.50, 90% CI 0.73 to 3.08 |
| Marek 2013 | Objective measure: machine recorded or nurse pill count, average percentage of correct doses per month | Not reported for control group MD.2: 98.8% (SD 0.32), planner: 97.4% (SD 5.19) |
| Pandey 2017 | Subjective measure: participants used a logbook to record name and timing of medications taken on a daily basis. Absolute medication adherence calculated as percentage of total prescribed doses that were actually taken each month. 12 months adherence calculated as the mean of each of the 12 monthly measurements. Adherence outcome is % of days covered | Intervention: 91% (n = 9), control: 73% (n = 8) |
| Pereles 1996 | Objective measure: patients discharged with 40 days worth of medication, pill count conducted in home at 40 days. Number of medication errors as a proportion of the total doses administered | Not clearly reported After controlling for age and MMSE ‐ I: 0.045, C: 0.086; P < 0.001 |
| Shimp 2012 | Objective measure: medication possession ratios defined as sum of all days of medication supply received during year divided by total numbers of days supply needed ‐ calculated for top 8 drug classes for chronic conditions | Not reported ‐ MPRs were very high for both groups (range 0.84 to 0.96), and no clinically meaningful changes were observed over time for either group. Fewer patients reported missed doses after the intervention |
| Taylor 2003 | Subjective measure: self‐reported number of medication doses missed. Presented as % adherence | Intervention mean 100 vs control mean 88.9 (± SD 6.3) |
| Volume 2001 | Subjective measure: Morisky Adherence, scores 0 to 4; lower scores = better adherence | Mean SD: 0.56 ± 0.75 vs 0.47 ± 0.69; number of participants in each group unclear |
| Willeboordse 2017 | Subjective measure: self‐reported adherence problems | Persistence of adherence problems = OR 0.83 (0.54 to 1.27) (P = 0.38) (unpublished = adherence worsened or persisted: 65 vs 54; adherence improved or remained the same: 143 vs 144) |
| Winland‐Brown 2000 | Objective measure: pill count, average number of missed doses (unclear over what time period). Please note: group 2 vs group 3 was used for comparison of intervention vs usual care; group 1 vs group 2 was used for comparison of intervention vs intervention | Mean: group 1 = 15.1, group 2 = 1.7, control = 19.7 |
Outcome results presented as intervention group vs usual care group unless otherwise stated.
C: control.
CI: confidence interval.
I: intervention.
IQR: interquartile ratio.
MMSE: Mini‐Mental State Examination.
MPR: medication possession ratio.
OR: odds ratio.
SD: standard deviation.
Educational interventions
Educational interventions were identified in 14 studies; seven studies were included in meta‐analyses. Two studies ‐ Haag 2016; Marusic 2013 ‐ involving dichotomous measures of adherence and five studies ‐ George 2016; Grymonpre 2001; Messerli 2016; Muth 2016; Nascimento 2016 ‐ involving continuous measures of adherence were included in the meta‐analyses. Analysis 1.1.1 indicated that educational interventions increase the proportion of patients who are adherent (risk ratio (RR) 1.66, 95% confidence interval (CI) 1.33 to 2.06); however results were strongly influenced by Marusic 2013. Three additional studies reported dichotomous data (Table 5), which could not be included in the meta‐analyses due to incomplete data or reporting of results in a format that could not be meta‐analysed. Krska 2001 increased the number of participants who had pharmaceutical care issues (e.g. non‐adherence) resolved (intervention: 68.9% versus usual care: 30.4% resolved), and two other studies reported no differences between groups (Hanlon 1996; Willeboordse 2017).
Overall, the quality of evidence was rated as very low, meaning we are uncertain of the effects of educational interventions on adherence measured dichotomously. We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains including sequence generation and allocation concealment, (by ‐1) for inconsistency (high I² and variations in intervention, providers, settings, duration, and outcome measures), and (by ‐1) for imprecision (only two studies in the meta‐analysis; one with very wide confidence interval and low events).
Analysis 1.2.1 showed that educational interventions may have little or no impact on adherence when assessed via continuous measures (standardised mean difference (SMD) 0.16, 95% CI ‐0.12 to 0.43), but heterogeneity was substantial (I² = 74%). Sensitivity analysis performed after one study with high attrition ‐ George 2016 ‐ was removed did not substantially alter the result (SMD 0.16, 95% CI ‐0.14 to 0.47). Four further studies reported continuous measures of adherence and could not be included in the meta‐analysis (Table 5) due to incomplete data or reporting of results in a format that could not be meta‐analysed (e.g. median, interquartile ratio (IQR)). Two studies reported no differences between groups (Bond 2007; Volume 2001), and two studies had no clear results available (Blalock 2010; Shimp 2012), but one study did report that a high medication possession ratio across both intervention and usual care groups meant that no clinically meaningful differences were observable (Shimp 2012).
Overall, educational interventions may have little or no effect on medication adherence measured by continuous adherence outcomes, with quality of evidence assessed as low. We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains and (by ‐1) for inconsistency (high I² and variations in interventions, providers, settings, duration, and outcome measures).
In total, 3 of the 14 educational interventions had positive effects on adherence, and all three were delivered as one‐off interventions. In Krska 2001 and Nascimento 2016, pharmacists provided individualised medication management education and medication reviews at home; Nascimento 2016 also provided therapeutic education on diabetes care. In Marusic 2013, physicians who were specialists in clinical pharmacology provided pre‐discharge counselling (e.g. medication indications, dosages, administration, importance of compliance, possible adverse drug reactions (ADRs)) to participants 24 hours before discharge. Adherence was measured through different measures and at different time points in each study; pharmacist assessment of pharmaceutical care issues included actual or potential adherence issues at three months (Krska 2001), pill count at 30 days (Marusic 2013), and subjective use of a Portuguese/Spanish variation of the Morisky adherence measure at six months (Nascimento 2016).
Behavioural interventions
Behavioural interventions were identified in seven studies, four of which were suitable for inclusion in meta‐analyses. Four studies used a dichotomous measure of adherence, and the meta‐analysis showed that behavioural interventions increased the proportion of adherent patients (Analysis 1.1.2; RR 1.22, 95% CI 1.07 to 1.38) (Hale 2016; Moral 2015; Morales Suarez‐Vurela 2009; Truelove 2015). Sensitivity analysis after removal of one study with high attrition ‐ Truelove 2015 ‐ did not alter the result substantially (RR 1.22, 95% CI 1.02 to 1.45).
Three studies were unable to be included in the meta‐analyses, one due to non‐reporting of standard deviations ‐ Winland‐Brown 2000 ‐ and one due to reporting of the percentage of days covered rather than the percentage of participants adherent (Pandey 2017). Both reported positive effects on adherence. Winland‐Brown 2000 reported fewer missed doses assessed via pill count among participants in the intervention group (1.7 intervention group versus 19.7 usual care), and Pandey 2017 reported a higher percentage of days adherent (91% absolute adherence intervention versus 73% usual care). The remaining single study that used a continuous measure reported a large effect on adherence (mean difference (MD) 13.60, 95% CI 7.78 to 19.42) (Murray 1993).
Overall, behavioural interventions may increase the proportion of people who are adherent to medications, but we are uncertain of the effects of behavioural interventions on medication adherence measured via continuous adherence outcomes. Quality of evidence was assessed as low for dichotomous outcomes and very low for continuous outcomes. We downgraded the evidence for both outcomes (by ‐1) due to high or unclear risk of bias across multiple domains and (by ‐1) for inconsistency (high I² and variations in interventions, providers, settings, duration, and outcome measures). We also downgraded the evidence for continuous outcomes (by ‐1) for imprecision (low participant numbers).
In total, five of the seven behavioural interventions had an individual positive impact on adherence. Two studies involved DAAs: Murray 1993 involved pharmacist‐led regimen simplification to twice‐daily dosing intervals and provided medications in unit of use packaging (translucent plastic cups with lids containing all medications for that dosing time), and Winland‐Brown 2000 used an automated dispenser with audible reminders. The remaining three studies all used different interventions: Truelove 2015 involved a cardiovascular four‐ingredient poly‐pill, Pandey 2017 sent once‐daily text message adherence reminders to participants, and Moral 2015 involved physician/nurse‐led motivational interviewing and follow‐up. Adherence was measured objectively at six months via pill count in three studies (Moral 2015; Murray 1993; Winland‐Brown 2000), and it was measured subjectively via patient log‐book records at 12 months (Pandey 2017), or by self‐reported use of medication at 18 months (Truelove 2015).
Mixed educational and behavioural interventions
Mixed educational and behavioural interventions were identified in 27 studies; 19 studies were included in meta‐analyses. Twelve studies used a dichotomous measure of adherence, and meta‐analysis of data from these studies shows that mixed interventions may increase the proportion of adherent patients (Analysis 1.1.3; RR 1.22, 95% CI 1.08 to 1.37; I² = 77%) (Bernsten 2001; Cossette 2015; Khdour 2009; Lopez Cabezas 2006; Naunton 2003; Olesen 2014; Rich 1996; Saez de la Fuente 2011; Vinluan 2015; Wood 1992; Wu 2006; Young 2016). A sensitivity analysis was conducted after removal of two cluster‐RCTs that had potential unit of analysis errors (Bernsten 2001; Wood 1992), but this had little impact on the risk ratio (RR 1.25, 95% CI 1.09 to 1.44). A second sensitivity analysis performed after removal of six studies with high attrition further strengthened the above finding further (RR 1.33, 95% CI 1.20 to 1.49) (Bernsten 2001; Cossette 2015; Lopez Cabezas 2006; Olesen 2014; Vinluan 2015; Wood 1992).
Seven studies used a continuous measure of adherence, and meta‐analysis of data from these studies shows no significant impact on adherence (Analysis 1.2.3; SMD 0.47, 95% CI ‐0.08 to 1.02; I² = 95%) (Begley 1997; Chrischilles 2014; Lee 2006; Lipton 1994; Nazareth 2001; Shively 2013; Williams 2012). A sensitivity analysis after removal of three studies with high attrition did not substantially alter the findings (SMD 0.70, 95% CI ‐0.25 to 1.65) (Lipton 1994; Nazareth 2001; Williams 2012).
Of the eight studies not included in meta‐analyses, two reported a positive impact on adherence with the intervention (Al‐Rashed 2002; Pereles 1996), four reported no differences between intervention and usual care groups (Cargill 1992; Holland 2007; Lim 2004; Taylor 2003), and two did not have clearly reported results (Cohen 2011; Marek 2013). Of the two studies showing positive impact, Al‐Rashed 2002 reported a significantly higher number of medications taken correctly in the intervention group (70% versus 15.8%) and Pereles 1996 reported a significantly lower number of medication errors in the intervention group, as a proportion of total doses administered (P < 0.001).
Overall, mixed interventions may increase the proportion of people who are adherent to medications but may have little or no impact on medication adherence measured via continuous adherence outcomes. Quality of evidence was assessed as low for both outcomes ‐ downgraded (by ‐1) due to high or unclear risk of bias across multiple domains, and (by ‐1) for inconsistency (high I² and variations in interventions, outcome measures, settings, duration, etc.).
In total, 11 of the 27 mixed interventions had a positive impact on adherence. All 11 studies were conducted at the hospital‐community interface (e.g. in hospital, at discharge, post discharge, at outpatient clinics) and involved elements of education/counselling. Five studies involved pharmacist medication review (Khdour 2009; Lee 2006; Lipton 1994; Naunton 2003; Rich 1996), and three involved regimen simplification (Begley 1997; Lipton 1994; Rich 1996). Interventions varied in duration from one‐off (Al‐Rashed 2002; Naunton 2003; Saez de la Fuente 2011), to within three months (Lipton 1994; Pereles 1996; Young 2016), to 6 to 12 months (Begley 1997; Khdour 2009; Lee 2006), to two years (Wu 2006). Duration of the intervention in one study was unclear (Rich 1996). Other behavioural interventions in these studies included blister‐packed DAAs (Lee 2006, and as needed in Naunton 2003), motivational interviewing (Khdour 2009), and use of a medication reminder card (Al‐Rashed 2002).
We identified sufficient mixed intervention studies to conduct three of the planned subgroup analyses.
Duration of intervention: short (≤ 3 months) versus long (> 3 months). When considered separately by subgroups based on intervention duration, there was no difference in adherence between those receiving interventions of a short duration and those receiving a long duration intervention when measured either as a dichotomous outcome (Analysis 1.3; RR 1.4 versus 1.11; test for subgroup differences P = 0.06; I² = 70.7%) or as a continuous outcome (Analysis 1.4; SMD 0.18 versus 0.70; test for subgroup differences P = 0.39; I² = 0%). Heterogeneity remained high, and it is not possible to state whether intervention duration is a major contributing factor to heterogeneity in effects on adherence.
Type of outcome measure: objective versus subjective. When considered separately by subgroups based on type of outcome measure, there was no difference in adherence between those studies using an objective measure of adherence and those using a subjective measure of adherence when measured either as a dichotomous outcome (Analysis 1.5; RR 1.13 versus 1.26; test for subgroup differences P = 0.38; I² = 0%) or as a continuous outcome (Analysis 1.6; SMD 0.82 versus 0.21; test for subgroup differences P = 0.39; I² = 0%). Heterogeneity remained high, and it is not possible to state whether type of outcome measure is a major contributing factor to heterogeneity in effects on adherence.
Health professional delivering the intervention: when considered separately by subgroups based on health professional delivering the intervention, there was no difference in adherence between those interventions delivered by pharmacists, nurses, or two or more health professionals when measured either as a dichotomous outcome (Analysis 1.7; RR 1.21 versus 1.19 versus 1.38; test for subgroup differences P = 0.83; I² = 0%) or as a continuous outcome (Analysis 1.8; SMD 1.38 versus ‐0.13 versus 0.42; test for subgroup differences P = 0.08; I² = 61.4%). Heterogeneity remained high, and it is not possible to state whether the type of health professional delivering the intervention is a major contributing factor to heterogeneity in effects on adherence.
1.3. Analysis.

Comparison 1: Interventions versus usual care, Outcome 3: Primary outcome: adherence, mixed interventions, grouped by intervention duration (dichotomous)
1.4. Analysis.

Comparison 1: Interventions versus usual care, Outcome 4: Primary outcome: adherence, mixed interventions, grouped by intervention duration (continuous)
1.5. Analysis.

Comparison 1: Interventions versus usual care, Outcome 5: Primary outcome: adherence, mixed interventions, grouped by subjective or objective outcome measures (dichotomous)
1.6. Analysis.

Comparison 1: Interventions versus usual care, Outcome 6: Primary outcome: adherence, mixed interventions, grouped by subjective or objective outcome measure (continuous)
1.7. Analysis.

Comparison 1: Interventions versus usual care, Outcome 7: Primary outcome: adherence, mixed interventions, grouped by provider (dichotomous)
1.8. Analysis.

Comparison 1: Interventions versus usual care, Outcome 8: Primary outcome: adherence, mixed interventions, grouped by provider (continuous)
Secondary outcome ‐ medication knowledge
Thirteen studies included a measure of medication knowledge (Table 6). Meta‐analysis was not possible due to large variations in outcome measures and reporting.
3. Secondary outcome ‐ medication knowledge.
| Study | Measure of medication knowledge | Outcome |
| Al‐Rashed 2002 | Pharmacist‐delivered questionnaire; percentage scores for correct answers (drug use, dose, dosage interval) | Drug use: 97.4% vs 69.5%; dosage interval: 97.4% vs 86.0%; dose: 98.5% vs 91.5% |
| Begley 1997 | Patients asked about name, purpose, dose, dosage frequency, and side effects. Reported as percentage of correct answers. Accuracy compared to hospital discharge or GP instructions | Group A 70%, Group B 68%, Group C 66% (usual care) |
| Bernsten 2001 | Interview‐based questionnaire calculating percentage correct (looking at 4 areas: indication, number of dosage units taken per dose, number of doses per day, and awareness of potential adverse effects). Higher scores = better knowledge | Mean ± SD change at 18 months: +3.19 ± 15.18 (n = 704) vs +3.16 ± 16.19 (n = 636) |
| Bond 2007 | Patients were asked whether they "knew more about their medicines compared with a year ago" on 5‐point Likert scale. Those who said agree/strongly agree | Trial report: 73% vs 65% |
| Grymonpre 2001 | Knows purpose of prescribed drugs (yes/no), expressed as number and percentage of drugs correct | 304/327 (93%) vs 335/373 (90%) |
| Hanlon 1996 | Self‐report knowledge of 'how they took each analysed medication and what the medication was for'; percentage of correct responses | 89.4% (n = 86) vs 90.6% (n = 83) |
| Khdour 2009 | COPD knowledge questionnaire (validated) ‐ effectiveness of education in helping persons with COPD. 16 T/F questions, correct response = 1, range 0 to 16, higher score = better knowledge | Median (IQR): 75.0 (32.0) vs 59.3 (33.0) |
| Lim 2004 | Composite knowledge of dose (D), frequency (F), and indication (I), percentage correct | Not reported |
| Manning 2007 | Assessment of knowledge of indication, dosage frequency, and special comments or cautions. 0 (for no correct responses) to 3 (all correct responses) | Mean ± SD: 1.96 ± 0.7561 vs 1.66 ± 0.6851 |
| Messerli 2016 | Knowledge of medicines and daily use ‐ phone questionnaire. 58 questions ‐ included assessing knowledge | Not reported |
| Nazareth 2001 | Prescription medicine interview ‐ patient's knowledge of prescribed drugs. Validated self‐report semi‐structured interview (knowledge score is out of 1, with 1 being 'total/highest' knowledge). Mean (SD) out of 1 | Mean ± SD: 0.69 ± 0.35 (n = 65) vs 0.68 ± 0.32 (n = 68) |
| Pereles 1996 | "Short medication knowledge questionnaire" = Patients asked to name and describe appearance and purpose of their medication, to describe their regimen and any potential side effects or drug interactions. Percentage of correct responses in each knowledge category | Discharge: name: 69% vs 55%; appearance: 77% vs 66%; times: 80% vs 69%; purpose: 77% vs 72%; side effects: 6% vs 4% Follow‐up: name: 77% vs 68%; appearance: 85% vs 83%; time: 87% vs 78%; purpose: 84% vs 85%; side effects: 5% vs 4% |
| Taylor 2003 | Self‐reports used to assess medication knowledge. Score determined by dividing the number of medications for which a patient reported the correct name, purpose, dose, and frequency by the total number of medications and multiplying by 100 | Mean ± SD: 92.6 ± 3.4 vs 42.9 ± 12.8 |
Outcome results presented as intervention group vs usual care group unless otherwise stated.
COPD: chronic obstructive pulmonary disease.
GP: general practitioner.
IQR: interquartile ratio.
SD: standard deviation.
T/F: true/false.
Educational interventions
Educational interventions were identified in four studies. In Bond 2007, pharmacist‐led medication management review (including assessment of medication appropriateness, adherence, lifestyle, and social support) conducted in the community pharmacy resulted in more patients agreeing that they knew more about their medications compared to what they knew one year ago (intervention 73% versus usual care 65%). Two studies showed no significant impact on medication knowledge, potentially due to the high levels of knowledge reported in both intervention and usual care groups (knowledge of medications was around 90% in all groups in both studies) (Grymonpre 2001; Hanlon 1996). In Grymonpre 2001, home medication histories were obtained by trained staff and were reviewed by pharmacists; pharmacists then sent a letter summarising the information and providing recommendations to the patient's general practitioner. In Hanlon 1996, the intervention involved pharmacist education provided to the participant and medication review at a general medicine clinic before/after physician appointments. One study involving pharmacist medication review and counselling in the pharmacy did not report results (Messerli 2016).
Overall, educational interventions may have little or no impact on medication knowledge (4 studies; low‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains including sequence generation, allocation, attrition, and outcome reporting, and (by ‐1) for inconsistency (variations in settings, providers, duration, and outcome measures).
Behavioural interventions
We identified no behavioural interventions that included a measure of medication knowledge.
Mixed educational and behavioural interventions
Mixed educational and behavioural interventions were identified in nine studies. Five studies reported a positive impact on medication knowledge; four reported small to moderate effects (Al‐Rashed 2002; Khdour 2009; Manning 2007; Pereles 1996), and one reported a large effect (mean ± standard deviation (SD) knowledge score/100: intervention 92.6 ± 3.4 versus usual care 42.9 ± 12.8) (Taylor 2003). Two studies used one‐off pre‐discharge education: Al‐Rashed 2002 involved a 30‐minute pharmacist counselling session (focusing on indications, side effects, dose, dosage times, importance of adherence, provision of a medication card etc.), and Manning 2007 involved nurse education via a three‐dimensional (3D) medication discharge education tool, whereby participants could affix a tablet or capsule of each medication onto the tool to assist with tablet identification. Three studies involved follow‐up/monitoring: Pereles 1996 involved a three‐stage self‐administration of medications programme in hospital, Khdour 2009 involved medication review and motivational interviewing conducted four times over nine months in an outpatient clinic or via phone, and Taylor 2003 involved pharmacist education and medication review at scheduled medical clinic visits over 12 months. Three studies reported no impact on medication knowledge ‐ one involving medication review and regimen simplification conducted at home post discharge for 12 months (Begley 1997), one involving medication review and regimen simplification conducted continuously for 18 months in the community pharmacy (Bernsten 2001), and one involving hospital pharmacist medication review with community pharmacist home visit follow‐up 7 to 14 days post discharge (Nazareth 2001). One study did not report any follow‐up results on medication knowledge (Lim 2004).
Overall, mixed interventions may improve medication knowledge but to a variable degree (9 studies; low‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains including sequence generation, allocation, and outcome reporting and (by ‐1) for inconsistency (variations in settings, providers, duration, and outcome measures).
Secondary outcome ‐ consumer satisfaction
Thirteen studies included a measure of consumer satisfaction, but only 10 studies measured the outcome in both intervention and control groups (Bernsten 2001;Bond 2007;George 2016;Hanlon 1996;Lopez Cabezas 2006;Manning 2007;Nazareth 2001;Taylor 2003;Volume 2001;Willeboordse 2017). Three studies measured satisfaction in the intervention group only (Holland 2007;Lingler 2016;Naunton 2003). Meta‐analysis was not possible due to large variation in outcome measures (Table 7).
4. Secondary outcome ‐ satisfaction.
| Study | Measure of satisfaction | Outcome (intervention vs usual care) |
| Bernsten 2001 | Self‐reported rating of services provided, satisfaction with services, and general opinion of pharmaceutical care. Questionnaire administered by pharmacist. Results presented as percentage who agree/mainly agree | Rating of services as excellent: 73.8% vs 64.6%; satisfaction with services: 93.9% vs > 90%; general opinion: 77% (intervention group only) |
| Bond 2007 | Overall score on 15 positive and negative statements of most recent pharmacy visit (total score 15 to 75, higher scores better) | Median (IQR): 46 (40 to 55) vs 43 (38 to 49) |
| George 2016 | User satisfaction regarding use of the computer programme questionnaire (USUCPQ): an 8‐item measure based on 7‐point Likert score (max score 56, higher scores better) | Mean ± SD total satisfaction: 45.33 ± 7.81 vs 44.68 ± 6.75 |
| Hanlon 1996 | Health Care Attitude Questionnaire: 3 questions on pharmacy‐related healthcare satisfaction (directions received, explanation of SES, numbers/types of drugs) based on 5‐point Likert scale (lower scores better) | Mean ± SD total score: 5.2 ± 1.5 vs 5.4 ± 1.7 |
| Holland 2007 | Satisfaction questionnaire; usefulness of community pharmacist visits | 75 (64%) considered the visits to have been extremely or very useful |
| Lingler 2016 | Acceptability of the intervention using a set of Likert scale questions and eliciting open‐ended comments | 88% of caregivers reported intervention topics useful and relevant; 92% reported that the intervention was helpful for managing the patient's treatment plan |
| Lopez Cabezas 2006 | Catalan Health Department satisfaction survey, asking participants about the care and information received and asking them to provide a global scoring (0 to 10) | Mean ± SD 8.9 ± 1.3 vs 8.8 ± 1.5 |
| Manning 2007 | Level of satisfaction using 5‐point Likert scale (5 = highest): "How satisfied were you with the form you received from the nurse when she/he was talking to you about your medications?" | Mean ± SD 4.24 ± 0.6986 vs 4.26 ± 0.8768 |
| Naunton 2003 | Survey of intervention group only | 94% very satisfied; 84% stated information they were given 'helped a great deal' |
| Nazareth 2001 | Validated patient satisfaction questionnaire, each item scored 1 to 4, mean score per item calculated (higher = better) | Mean ± SD 3.4 ± 0.6 (n = 62) vs 3.2 ± 0.6 (n = 61) |
| Taylor 2003 | Mean ± SD number of patients with pharmacy‐related satisfaction (details unclear) | Mean ± SD 81.9 ± 4.8 (n = 33) vs 89.0 ± 6.2 (n = 36) |
| Volume 2001 | Satisfaction with pharmacy services using 34‐item instrument and 7‐point Likert scale (lower scores = better). General satisfaction extracted | Mean ± SD 1.53 ± 0.77 vs 1.62 ± 0.88 |
| Willeboordse 2017 | Medication satisfaction questionnaire assessed on a 7‐point Likert scale | B (95% CI): 0.11 (‐0.08 to 0.30) (P = 0.25) |
Outcome results presented as intervention group vs usual care group unless otherwise stated.
CI: confidence interval.
IQR: interquartile ratio.
SD: standard deviation.
Educational interventions
Educational interventions were identified in five studies, four of which reported no impact on satisfaction (George 2016;Hanlon 1996;Volume 2001;Willeboordse 2017). Bond 2007, which involved community pharmacists providing consultations on medications, medication adherence, lifestyle, and social support, reported that participants in the intervention group had greater satisfaction compared to those in the usual care group; however, the effect size was small.
In summary, educational interventions may have little or no impact on consumer satisfaction (5 studies; low‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains including sequence generation, allocation, incomplete outcome reporting, and other sources of bias and (by ‐1) for inconsistency (variations in settings, providers, duration, and outcome measures).
Behavioural interventions
We identified no behavioural interventions that included a measure of satisfaction.
Mixed educational and behavioural interventions
Mixed educational and behavioural interventions were identified in eight studies. Three studies ‐ Holland 2007; Lingler 2016; Naunton 2003 ‐ measured participant satisfaction only in the intervention group, with satisfaction levels ranging from 64% in Holland 2007 to 94% in Naunton 2003. Five studies measured participant satisfaction in both intervention and usual care groups; between‐group differences were non‐significant in four studies (Bernsten 2001; Lopez Cabezas 2006; Manning 2007; Nazareth 2001), and one study reported slightly higher pharmacy‐related satisfaction in the group receiving usual care (mean 89.0, SD 6.2) compared with the intervention (mean 81.9, SD 4.8); however, the satisfaction measure used was poorly described (Taylor 2003).
Overall, we are uncertain of the effects of mixed interventions on consumer satisfaction (8 studies; very low‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains including sequence generation, allocation, and incomplete outcome reporting, (by ‐1) for inconsistency (variations in interventions, settings, providers, duration, and outcome measures), and (by ‐1) due to imprecision (three of eight studies reported satisfaction only in the intervention group).
Secondary outcome ‐ HRQoL
Fourteen studies included a measure of HRQoL (Table 8). Meta‐analysis was not possible due to differences in scales used and differences in reporting of results.
5. Secondary outcome ‐ HRQoL.
| Study | Measure | Time point | Outcome |
| Bernsten 2001 | SF‐36 | 18 months | Change: GH: +0.28 vs ‐0.66, MH: ‐0.80 vs ‐1.34, PF: ‐0.95 vs‐0.68 |
| Bond 2007 | SF‐36 | 12 months | Med (IQR): GH: 52 (35 to 65) vs 50 (35 to 70), MH: 80 (64 to 88) vs 80 (64 to 88), PF: 60 (35 to 80) vs 65 (35 to 85) |
| EQ‐5D | 12 months | Med (IQR): 0.73 (0.7 to 0.9) vs 0.73 (0.7 to 0.9) | |
| Cohen 2011 | VR‐36 (Veterans SF‐36) | 6 months | Change: Med (IQR): MH: 0.48 (‐3.37 to 4.32), C: 0.78 (‐2.67 to 4.23), PF: 1.65 (‐5.21 to 1.31), C: ‐1.95 (‐5.21 to 1.31) |
| Hale 2016 | MLHFQ | 90 days | Mean ± SD: 62.2 ± 20.6 vs 28.2 ± 22.3 |
| Hanlon 1996 | SF‐36 | 12 months | Mean ± SD: GH: 37.4 ± 1.6 vs 35.2 ± 1.7, MH: 61.1 ± 1.8 vs 60.4 ± 1.8, PF: 44.1 ± 2.0 vs 42.2 ± 2.0 |
| Holland 2007 | EQ‐5D, VAS | 6 months | Mean ± SD: EQ‐5D: 0.58 ± 0.29 vs 0.52 ± 0.34, VAS: 58.2 ± 19.6 vs 58.6 ± 19.8 |
| MLHFQ | Mean ± SD: 47.7 ± 26.3 vs 44.5 ± 27.9 | ||
| Khdour 2009 | SGRQ | 12 months | Mean (confidence interval): 61.8 (57.9 to 65.6) vs 65.3 (61.0 to 69.6) |
| Krska 2001 | SF‐36 | 3 months | No significant differences ‐ values not reported |
| Lopez Cabezas 2006 | EQ‐5D (Spanish and Catalan) | 12 months | Mean ± SD: 64 ± 15.4 vs 60.6 ± 17.8, subgroup > 70 years: 63.8 ± 15.3 vs 58.4 ± 15.9 |
| Marek 2013 | SF‐36 | 12 months | Comparison 1 ‐ Mean (confidence interval): planner (intervention) vs control (usual care) = PCS: 1.390 (0.816 to 1.963), MCS: 1.686 (0.949 to 2.423) |
| Comparison 2 ‐ Mean (confidence interval): MD.2 (intervention 1) vs planner (intervention 2) = PCS: 0.095 (‐0.450 to 0.640), MCS: 0.241 (‐0.459 to 0.940) | |||
| Muth 2016 | EQ‐5D | 12 weeks | Mean ± SD: change: ‐0.6 ± 19.61 vs ‐1.0 ± 13.66 |
| Taylor 2003 | SF‐36 | 12 months | Mean SD: GH: 57.0 ± 19.6 vs 50.1 ± 15.9, MH: 73.1 ± 21.2 vs 72.3 ± 17.1, PF: 68.6 ± 24.0 vs 56.1 ± 27.5 |
| Volume 2001 | SF‐36 | 12 to 13 months | Mean ± SD: MCS: 56.14 ± 8.30 vs 54.55 ± 8.65, PCS: 36.87 ± 11.62 vs 38.39 ± 11.44 |
| Willeboordse 2017 | SF‐12 | 6 months | Regression coefficients adjusted for baseline: PCS: ‐0.06 (‐3.19 to 3.06), MCS: 0.16 (‐2.89 to 3.22) |
| EQ‐5D‐3L | Regression coefficients adjusted for baseline: utility: 0.02 (‐0.02 to 0.05), VAS: 2.30 (‐0.16 to 4.76) |
Outcome results presented as intervention group vs usual care group unless otherwise stated.
C: control.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions.
GH: general health.
I: intervention.
MH: mental health.
MCS: mental components summary.
MLHFQ: Minnesota Living With Heart Failure Questionnaire (21 items, coded 0 to 5; higher scores indicate adverse impact on life).
PCS: physical components summary.
PF: physical function.
SD: standard deviation.
SF‐36: Short Form‐36 Health Survey.
SGRQ: St. George's Respiratory Questionnaire (76 items, total score 100; higher = better).
VAS: visual analogue scale.
Educational interventions
Educational interventions were identified in six studies (Bond 2007;Hanlon 1996;Krska 2001;Muth 2016;Volume 2001;Willeboordse 2017); none of these had a significant impact on HRQoL.
Overall, educational interventions probably have little or no effect on HRQoL (6 studies; moderate‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains.
Behavioural interventions
Behavioural interventions were identified in only one study (Hale 2016). The intervention involved a remotely monitored electronic medication dose administration aid with alerts and follow‐up calls if medications were missed. HRQoL was measured via the MLHFQ, with results showing that the intervention group had worse HRQoL (higher MLHFQ scores) both at baseline and at 90‐day follow‐up compared to the usual care group (baseline: mean ± SD: 43.7 ± 25.9 versus 26.2 ± 23.1; 90 days: mean ± SD: 62.2 ± 20.6 versus 28.2 ± 22.3).
Overall, we are uncertain of the effects of behavioural interventions on HRQoL (1 study; very low‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains, (by ‐1) for indirectness (MLHFQ specific to heart failure populations), and (by ‐1) for imprecision (low participant numbers).
Mixed educational and behavioural interventions
Mixed educational and behavioural interventions were identified in seven studies, with six showing no significant impact on HRQoL (Bernsten 2001;Cohen 2011;Holland 2007;Khdour 2009;Lopez Cabezas 2006;Taylor 2003). Marek 2013 showed that nurse education, follow‐up, and weekly nurse‐filled DAAs resulted in improved physical and mental summary scores on Short Form (SF)‐36 at 12 months compared to usual care (mean change: physical 1.390, 95% CI 0.816 to 1.963; mental 1.686, 95% CI 0.949 to 2.423; P < 0.0001).
Overall, mixed interventions may have little or no effect on HRQoL (7 studies; low‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains and (by ‐1) for inconsistency (variations in interventions, outcome measures, settings, duration, etc.).
Secondary outcome ‐ adverse clinical health outcomes
Twenty‐eight studies included a measure of adverse clinical health outcomes (Table 9).
6. Secondary outcome ‐ adverse clinical health outcomes.
| Study | Time point | ED/Hospital admissions | Mortality | Adverse drug reactions | GP visits |
| Al‐Rashed 2002 | 3 months | Patients re‐admitted to hospital: 8/43 v 28/40 | Total unplanned visits: 43 (n = 43) vs 59 (n = 40) | ||
| Bernsten 2001 | 18 months | Self‐reported: 35.6% vs 40.4%; n values unclear | |||
| Chrischilles 2014 | 3 months | Self‐reported: 100/802 (12.9%) vs 33/273 (12.2%) | |||
| Cossette 2015 | 30 days | ED visits: 18% (n = 108) vs 20% (n = 95) | |||
| Haag 2016 | 30 days | ED or hospital re‐admission: 2/11 (18%) vs 1/11 (9%) | |||
| Hale 2016 | 90 days | No. participants: ED: 3/11 (27%) vs 6/14 (43%); hospitalisation: 1/11 (9%) vs 7/14 (50%); total no.: ED 4 vs 7, hospital 2 vs 8 | |||
| Hanlon 1996 | 12 months | Self‐reported: 30.2% (n = 86) vs 40% (n = 83) (P = 0.19) | |||
| Holland 2007 | 6 months | Total number of ED admissions (not number of participants admitted): 134 (n = 148) vs 112 (n = 143) | 30/148 vs 24/143 | ||
| Khdour 2009 | 12 months | n = 71 and 72; ED: 40 vs 80, hospital: 26 vs 64; total hospital days: 164 vs 466 | Unscheduled: 28 (n = 71) vs 47 (n = 72) | ||
| Lim 2004 | 2 months | Self‐reported and assessed by physician, total ADRs at 2 months: 13 vs 6; residual ADRs from baseline: 4/13 vs 4/8 | |||
| Lipton 1994 | 6 months | Total days in hospital: mean ± SD 2.29 ± 5.96, n = 350 vs 2.02 ± 5.83, n = 356 | |||
| Lopez‐Cabezas 2016 | 12 months | Patients with re‐admission: 23/70 (32.9%) vs 31/64 (48.4%) | > 70 years subgroup: 7/53 (13.2%) vs 13/50 (26.0%) |
||
| Marusic 2013 | 30 days | Re‐admission or ED: 20/80 (25%) vs 27/80 (33.8%) | ? Self‐reported: 24/80 (30%) vs 30/80 (37.5%) (P = 0.315) | ||
| Messerli 2016 | 28 weeks | Self‐reported unplanned GP visit or hospitalisation: total during study: 110 vs 99, n unclear? ‐ 181 vs 191 | * | ||
| Murray 1993 | 6 months | Self‐reported side effects, ill effects, or other problems with medication: C1: 2/12, C2: 2/10, Int: 1/9 | |||
| Muth 2016 | 12 weeks | Days in hospital: mean ± SD (T1 + T2) ‐ T0 = ‐0.4 ± 0.73 vs ‐0.2 ± 0.69, n unclear | |||
| Naunton 2003 | 90 days | 1 or more unplanned re‐admissions: 16/57 (28%) vs 29/64 (45%) | 3/57 (5%) vs 5/64 (8%) | ||
| Nazareth 2001 | 6 months | Re‐admissions: 38/136 (27.9%) vs 43/151 (28.4%) Outpatient department: 39/137 vs 40/151 |
22/137(16.1%) vs 19/151 (12.6%) | 76/107 vs 82/116 | |
| Olesen 2014 | 24 months | Unplanned admissions: 77/253 (30%) vs 73/264 (28%) | 19/253 (7.5%) vs 14/264 (5%) | ||
| Rich 1996 | 90 days | Re‐admissions: 18/80 (22.5%) vs 22/76 (28.9%) | |||
| Saez de la Fuente 2011 | 50 days | Total re‐admissions: 5 (n = 26) vs 7 (n = 24); ED: 7 (n = 26) vs 9 (n = 24) (note percentages listed in paper do not match n values) | 2 (? n = 26) vs 1 (? n = 24) | ||
| Shively 2013 | 6 months | Hospital: mean (SD): 0.21 (0.409) vs 0.32 (0.475)
ED: 0.33 (0.478) vs 0.37 (0.489) n = 39 vs 37 |
|||
| Taylor 2003 | 12 months | Hospital: 2/33 vs 11/36; ED: 4/33 and 6/36 | |||
| Vinluan 2015 | 90 days | Hospital admissions: 2/7 vs 2/7 | 0/7 vs 2/7 | ||
| Willeboordse 2017 | 6 months | DRPs: baseline: 4.4 ± 1.9 vs 3.7 ± 1.7 % solved: 20.2 (12.2 to 28.1) |
|||
| Winland‐Brown 2000 | 6 months | Hospitalisations G1: 4/16, G2: 3/24, C: 12/21 | Physician visits: G1: 1.5/month, G2: 1/month, C: 1/month | ||
| Wu 2006 | 2 years | Med (IQR): n = 219 vs 223 ED visits: 0 (‐1 to 2) vs 0 (‐1 to 2) Hospital visits: 0 (‐1 to 2) vs 1 (‐1 to 2) Days in hospital: 0 (‐4 to 10) vs 3 (‐2 to 17.5) | 25/219 vs 38/223 | ||
| Young 2016 | 180 days | Hospital: 18/51 (35.3%) vs 20/49 (40.8%); ED visits: 12/51 (23.5%) vs 11/49 (22.4%) |
Outcome results presented as intervention group vs usual care group unless otherwise stated.
ADR: adverse drug reaction.
C: control.
C1: control group 1.
C2: control group 2.
DRP: drug‐related problem.
ED: emergency department.
G1: group 1.
G2: group 2.
GP: general practitioner.
Int: intervention.
SD: standard deviation.
Emergency department (ED)/Hospital admissions
ED/Hospital admissions were measured in 23 studies, and 16 studies were included in the meta‐analysis (Analysis 1.9). Four studies reported hospital and ED admissions separately, but only hospital admissions were chosen to be included in the meta‐analysis to avoid duplication of participants in the analysis (Hale 2016;Khdour 2009;Taylor 2003;Young 2016).
1.9. Analysis.

Comparison 1: Interventions versus usual care, Outcome 9: Secondary outcome: ED/Hospital admissions, grouped by type of intervention (dichotomous)
Educational interventions
Educational interventions had no significant impact on admissions (RR 1.02, 95% CI 0.71 to 1.48) (Haag 2016; Marusic 2013; Messerli 2016). One additional study that could not be included in meta‐analysis reported mean number of days in hospital but reported no effect of an educational intervention (Muth 2016).
Overall, educational interventions probably have little or no effect on ED/hospital admissions (RR 1.02, 95% CI 0.71 to 1.48; moderate‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains.
Behavioural interventions
Behavioural interventions reduced the risk of admissions (RR 0.21, 95% CI 0.08 to 0.55) (Hale 2016;Winland‐Brown 2000), although overall we are uncertain about the effects of behavioural interventions on admissions because of the very low quality of evidence for this outcome. We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains and (by ‐2) for imprecision (low participant numbers and low numbers of events).
Mixed educational and behavioural interventions
Meta‐analysis of 11 studies shows that mixed interventions reduced the risk of admissions (RR 0.67, 95% CI 0.50 to 0.90); however the level of heterogeneity was high (I² = 73%) (Al‐Rashed 2002;Cossette 2015;Khdour 2009;Lopez Cabezas 2006;Naunton 2003;Nazareth 2001;Olesen 2014;Rich 1996;Taylor 2003;Vinluan 2015;Young 2016).
A further six studies were not included in the meta‐analysis (Bernsten 2001;Holland 2007;Lipton 1994;Saez de la Fuente 2011;Shively 2013;Wu 2006). Two studies had unclear participant numbers (Bernsten 2001;Saez de la Fuente 2011), one reported only total admissions and not the number of participants admitted (Holland 2007), one reported the mean (SD) number of days in hospital (Lipton 1994), one reported mean (SD) admissions (Shively 2013), and one reported median (IQR) hospital visits (Wu 2006). None of these studies reported a difference between intervention and usual care groups for ED/hospital admissions.
Overall, mixed interventions may reduce the number of ED/hospital admissions (RR 0.67, 95% CI 0.50 to 0.90; low‐quality evidence). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains and (by ‐1) for inconsistency (variations in interventions, outcome measures, settings, duration, and effect estimates across studies).
In total, four out of 23 individual studies had results favouring the intervention ‐ one behavioural ‐ Winland‐Brown 2000 ‐ and three mixed interventions (Al‐Rashed 2002;Khdour 2009;Taylor 2003). Two studies involved pharmacist‐led education and medication review with ‐ Khdour 2009 ‐ or without ‐ Taylor 2003 ‐ motivational interviewing conducted in an outpatient clinic ‐ Khdour 2009 ‐ or a medical clinic ‐ Taylor 2003. One study involved an automated medication dispenser with audible adherence reminders in the participant's home (Winland‐Brown 2000). Another study involved pharmacist pre‐discharge counselling and provision of a medicine reminder card in hospital (Al‐Rashed 2002).
Mortality
Mortality was reported in eight studies, all involving mixed behavioural and educational interventions led by pharmacists. No studies were identified that evaluated the impact of educational or behavioural interventions alone on mortality. Meta‐analysis involving seven studies shows that we are uncertain of the impact of mixed interventions on mortality (Analysis 1.10; RR 0.93, 95% CI 0.67 to 1.30; very low‐quality evidence) (Holland 2007;Lopez Cabezas 2006;Naunton 2003;Nazareth 2001;Olesen 2014;Vinluan 2015;Wu 2006). No individual study had a significant impact on mortality; three appeared to favour the intervention, and four appeared to favour usual care. We excluded one further study the meta‐analysis due to unclear participant numbers (Saez de la Fuente 2011). We downgraded the evidence (by ‐1) due to high or unclear risk of bias across multiple domains, (by ‐1) for inconsistency (variations in interventions, outcome measures, settings, duration, etc.), and (by ‐1) for imprecision (limits of 95% confidence intervals include both potential benefit and potential harm).
1.10. Analysis.

Comparison 1: Interventions versus usual care, Outcome 10: Secondary outcome: mortality, mixed interventions
Adverse drug reactions (ADRs)
Adverse drug reactions (ADRs) were reported in six studies.
Educational interventions
Three studies providing educational interventions included a measure of ADRs; two reported no differences between groups (Hanlon 1996; Marusic 2013), and one found that the percentage of solved medication‐related problems (including ADRs) was significantly higher in the intervention group (regression coefficient 22.6, 95% CI 14.1 to 31.1; P < 0.001) (Willeboordse 2017).
Behavioural interventions
One study providing behavioural interventions was identified but reported no differences between groups in self‐reported ADRs (Murray 1993).
Mixed educational and behavioural interventions
Two studies providing mixed educational and behavioural interventions were identified. One study reported no differences between intervention and usual care groups (Chrischilles 2014), and the other found that total ADRs were higher at two months following an intervention involving education, medication review, and regimen simplification (total 13 versus 6) but that residual ADRs from baseline were lower (4/13 versus 4/8) (Lim 2004).
Physician visits
Physician visits were reported in four studies.
Mixed educational and behavioural interventions
Two studies, both involving mixed educational and behavioural interventions, reported reductions in the number of unplanned physician visits with the intervention over usual care (43 versus 59 total visits and 39% versus 65% of participants, respectively) (Al‐Rashed 2002; Khdour 2009).
Two studies ‐ one behavioural ‐ Winland‐Brown 2000 ‐ and one mixed ‐ Nazareth 2001 ‐ reported no between‐group differences for the number of physician visits.
Secondary outcome ‐ condition‐specific outcomes
Seven studies included a condition‐specific outcome measure (Table 10). Meta‐analysis was not possible due to variations in outcome measures.
7. Secondary outcome ‐ condition‐specific outcomes.
| Study | Measure | Outcome |
| Blalock 2010 | Falls (self‐reported) in 12 months (ITT analysis) | ≥ 1 fall: 53/93 vs 52/93 |
| Bond 2007 | Total score (/8) for reaching targets at 12 months (aspirin, lipid, BP, smoking, alcohol, physical activity, diet, BMI) | 4.6 ± 1.2 vs 4.6 ± 1.1 |
| Cohen 2011 | Percentage achieving targets at 6 months (SBP < 130, LDL < 100, HbA1c < 7%) | 16% (n = 50) vs 4.1% (n = 49) |
| Lee 2006 | Systolic and diastolic blood pressure (mmHg) and LDL‐cholesterol (mg/dL) at 6 months post phase 1 | SBP: 124.4 ± 14.0 vs 133.3 ± 21.5
DBP: 67.5 ± 9.9 vs 68.6 ± 10.5 LDL: 87.5 ± 24.2 vs 88.4 ± 21.0 |
| Nascimento 2016 | Fasting blood glucose and HbA1c at 6 months | FBG: 117.3 ± 26.8 vs 142.2 ± 32.9 HbA1C: 7.7 ± 0.8 vs 7.99 ± 0.67 |
| Taylor 2003 | Number of people reaching goal level at 12 months (BP ≤ 140/90, HbA1c ≤ 7.5%, INR 2 to 3, LDL) | BP: 22 (91.7%) vs 8 (27.6%)
Diabetes: 13 (100%) vs 5 (26.7%) INR: 4 (100%) vs 1 (16.7%) LDL: 14 (77.8%) vs 1 (5.9%) (Note: calculated mean across all 4 measures: 92% vs 19%) |
| Williams 2012 | Blood pressure, HbA1c, eGFR, and creatinine levels at 12 months (9 months post intervention) | SBP: mean (CI) ‐6.9 (‐13.8 to 0.02) vs ‐3.0 (‐8.4 to 2.4) HbA1c: med (IQR): 7 (7 to 9) vs 8 (7 to 9) eGFR: med (IQR): 48 (38 to 76) vs 46 (32 to 72) Creatinine: med (IQR): 117 (82 to 144) vs 108 (89 to 171) |
Outcome results presented as intervention group vs usual care group unless otherwise stated and presented as mean ± SD unless otherwise stated.
BMI: body mass index.
BP: blood pressure.
DBP: diastolic blood pressure.
eGFR: estimated glomerular filtration rate.
FBG: fasting blood glucose.
HbA1c: glycosylated haemoglobin.
INR: international normalised ratio.
IQR: interquartile ratio.
ITT: intention‐to‐treat.
LDL: low‐density lipoprotein.
SBP: systolic blood pressure.
Educational interventions
Three studies involved educational interventions: Blalock 2010 reported no difference between groups in terms of the number of participants experiencing one or more falls; Bond 2007 found no between‐group differences in the number of participants reaching health targets (total score for eight targets; e.g. physical activity, diet, weight); and Nascimento 2016 reported greater reductions in fasting blood glucose and glycosylated haemoglobin (HbA1c) levels in intervention participants compared with those receiving usual care.
Behavioural interventions
No studies that involved only behavioural interventions were identified.
Mixed educational and behavioural interventions
Four studies involved mixed interventions. Two studies involving multidisciplinary ‐ Cohen 2011 ‐ or pharmacist ‐ Taylor 2003 ‐ education in a medical clinic with follow‐up reported higher numbers of people reaching goal levels for blood pressure, HbA1c, and low‐density lipoprotein (LDL) cholesterol with the intervention over usual care (16% versus 4% and 92% versus 19%, respectively). Two additional studies measured multiple health targets including blood pressure, reporting that although results favoured the intervention, they were not significantly different from those attained with usual care (Lee 2006;Williams 2012).
Secondary outcome ‐ cost‐effectiveness
Four studies ‐ one providing educational intervention ‐ Bond 2007 ‐ and three providing mixed interventions ‐ Bernsten 2001;Lipton 1994;Lopez Cabezas 2006 ‐ included a measure of cost‐effectiveness (see Table 11).
8. Secondary outcome ‐ cost effectiveness.
| Study | Measure of costs | Outcome |
| Bernsten 2001 | Direct costs of the study, including additional time spent by pharmacists, costs associated with contacts with other health professionals, costs of hospitalisation and drugs | Average cost per patient (saving): Denmark: 1298.13 vs 1419.88 (+121.75) Germany: 2992.25 vs 3167.25 (+175.00) Northern Ireland: 735.22 vs 750.01 (+14.79) Sweden: 1266.76 vs 1250.34 (‐16.42) |
| Bond 2007 | Total NHS‐related study costs, including costs of intervention and other treatment (e.g. medicines, hospital, other health consultations) | Median cost (IQR): 970.5 (667.0 to 1489.0) vs 835.2 (534.4 to 1396.3) Median (IQR) cost of intervention alone (pharmacist time and training): 90 (60 to 118) |
| Lipton 1994 | Medicare Part B charges, total hospital inpatient charges | Total charges: mean ± SD 2769 ± 4789 vs 2598 ± 3722 Inpatient charges: mean ± SD 5472 ± 10904 vs 5263 ± 11478 |
| Lopez Cabezas 2006 | Hospitalisation costs, adding in intervention direct costs, delivered materials and time spent by the pharmacist | Average cost per patient: 997 vs 1575 |
Outcome results presented as intervention group vs usual care group unless otherwise stated.
IQR: interquartile ratio.
NHS: National Health Service.
SD: standard deviation.
Educational interventions
In one study (Bond 2007), the educational intervention involved a one‐off pharmacist medication review; total National Health Service (NHS)‐related study costs (medicines plus NHS visits plus intervention costs) were higher in the intervention group compared with the usual care group (median 970.5 versus 835.2), although the main difference was the cost of the intervention itself (median 90, IQR 60 to 118).
Mixed interventions
Two mixed interventions involving pharmacist medication review with repeated/continuous follow‐up for 18 months, either in the pharmacy ‐ Bernsten 2001 ‐ or in hospital with home follow‐up ‐ Lopez Cabezas 2006 ‐ reported that the intervention resulted in a reduction in mean/median costs per patient, although cost savings were variable. The third mixed intervention ‐ Lipton 1994 ‐ involving one‐off face‐to‐face medication review and education in hospital with telephone follow‐up post discharge for three months shows that intervention patients had higher Medicare Part B charges and total hospital inpatient charges as measured at six months compared to usual care patients.
Secondary outcome ‐ other
Four studies included other outcome measures potentially related to measures of medication‐taking ability, medication adherence, or adverse drug events (see Table 12).
9. Secondary outcome ‐ other.
| Study | Measure | Outcome (Intervention vs usual care) |
| Chrischilles 2014 | Mean (SD) number of medication management problems from a list of 8 problems, including questions on multiple prescribers, multiple pharmacies, mail order prescriptions, confusion whether medication was taken, taking medication without knowing indication, problems affording medications, feeling that medications are not working, and feeling that medications are not doing what they were intended to do | Mean ± SD 1.4 ± 1.4 vs 1.6 ± 1.5 |
| Lingler 2016 | Medication deficiency checklist: a 15‐item, investigator‐developed instrument that uses caregiver interviews to assess for the presence of errors and problems (e.g. incorrectly chewing pills or capsules, taking at the wrong time, repeating doses, patient refuses/unco‐operative) | Mean ± SD 2.19 ± 1.52 vs 2.36 ± 1.51 |
| Moral 2015 | Average number of medication errors, defined as both patient errors (e.g. omission of dose) and prescriber errors (e.g. dose too high or too low, duplicate therapy) (as reported in Perula de Torres 2014 paper) | Mean 0.429 vs 1.145 |
| Taylor 2013 | Number of participants with at least 1 medication misadventure (defined as medication errors, adverse drug events, and/or adverse drug reactions) | 2.8% (n = 33) vs 3.0% (n = 36) |
Outcome results presented as intervention group vs usual care group unless otherwise stated.
SD: standard deviation.
Behavioural interventions
One study, involving a behavioural intervention comprising motivational interviewing and follow‐up by physicians and nurses, found that the average number of medication errors, defined as both patient errors (e.g. omission of dose) and prescriber errors (e.g. dose too high or too low, duplicate therapy), was significantly lower in the intervention group compared to the usual care group (0.429 vs 1.145; P = 0.047) (Moral 2015).
Mixed interventions
Three studies involving mixed interventions reported no differences between intervention and usual care groups in medication management problems (Chrischilles 2014), medication errors and problems (Lingler 2016), and medication errors and adverse drug events or reactions (Taylor 2003).
COMPARISON 2. Intervention versus intervention
Six studies involved comparison between interventions as part of a three‐arm RCT design (Begley 1997;Cargill 1992;Marek 2013;Murray 1993;Olesen 2014;Winland‐Brown 2000).
Primary outcome ‐ medication‐taking ability
Two studies included measures of medication‐taking ability.
Cargill 1992 compared a mixed intervention versus an educational intervention. In this comparison, both groups received a 20‐minute nurse education session and review of medications (educational intervention); one of the groups also received follow‐up telephone calls (mixed intervention). No difference in medication‐taking behaviour between groups was reported at four to six weeks (score out of 100 read from a graph: mean 84 versus 86).
Begley 1997 compared a mixed intervention involving pharmacist home interview with counselling versus a behavioural intervention of home interview only without counselling (modified usual care). This study reported no difference in dexterity between groups at 12 months (data were not reported).
Primary outcome ‐ adherence
Six studies involved a measure of medication adherence.
Two studies compared two behavioural interventions. In Murray 1993, pill count adherence was slightly higher in the group that received regimen simplification and unit of dose medication packaging than in the group given regimen simplification alone (92.6% versus 82.6%; P = 0.02). In Winland‐Brown 2000, nurse‐filled pillbox DAAs were associated with a higher number of missed pills as measured via pill count than via an automated dispenser with audible adherence reminders (mean 15.1 versus 1.7; P < 0.001); however the time interval is unclear.
Cargill 1992 compared a mixed intervention versus an educational intervention and found no difference in pill count adherence between the group that received nurse teaching with additional telephone follow‐ups versus the group receiving a nurse teaching session without follow‐up (mean 76% versus 74%).
Two studies compared mixed interventions versus behavioural interventions. Begley 1997 found that pharmacist home interview with counselling had slightly greater impact on pill count adherence than the home interview alone (mean ± SD percentage 86 ± 19 versus 75 ± 21). Olesen 2014 measured adherence using different methods in different groups, and thus data are not comparable.
Marek 2013 compared two mixed interventions and found that pill count adherence was similar across both medication dispensing machine and simple medication box groups (98.8% versus 97.4%).
Secondary outcome ‐ medication knowledge
Begley 1997 compared a mixed intervention involving pharmacist home interview with counselling versus a behavioural intervention of home interview only without counselling (modified usual care). This study reported no difference in medication knowledge between groups at 12 months (mean 70% versus 68%), as measured by comparison of patient answers to hospital discharge and general practitioner (GP) instructions regarding medication name, purpose, dose, dosage frequency, and side effects.
Secondary outcome ‐ satisfaction
No studies were identified.
Secondary outcome ‐ HRQoL
Marek 2013 compared two mixed interventions ‐ a medication‐dispensing machine with audio and visual prompts for adherence versus nurse‐filled simple weekly medication boxes. There was no difference in improvement in participant physical or mental summary scores between the two groups as measured via SF‐36 (mean physical 0.095, 95% CI ‐0.450 to 0.640; mean mental 0.241, 95% CI ‐0.459 to 0.940).
Secondary outcome ‐ adverse clinical health outcomes effects
Three studies reported measures of adverse clinical health outcomes.
Two studies compared two behavioural interventions. In Winland‐Brown 2000, hospitalisations (4/16 (25%) versus 3/24 (12.5%) patients) and mean number of physician visits (1.5/month versus 1/month) were higher in the weekly pre‐filled pill box DAA group than in the group using an automated dispenser with audible reminders. In Murray 1993, the main intervention group (regimen simplification and unit of use DAA packages) and the modified usual care group (group C2; regimen simplification without unit of use medication DAA packages) had similar numbers of self‐reported side effects over six months of follow‐up (1/9 versus 2/10).
Olesen 2014 compared a mixed intervention versus a behavioural intervention (electronic reminder device), but study authors were contacted and reported that data on unplanned admissions and mortality were not collected for the electronic reminder device group.
Secondary outcome ‐ condition‐specific outcomes
No studies were identified.
Secondary outcome ‐ cost‐effectiveness
No studies were identified.
Discussion
Summary of main results
Our systematic review identified a range of simple to complex interventions for improving medication‐taking ability and medication adherence in older adults prescribed multiple medications. All included studies evaluated interventions versus usual care.
No studies of educational only or behavioural only interventions for improving medication‐taking ability were identified. We were unable to determine the impact of mixed interventions on medication‐taking ability due to the small number of eligible studies, variations in effects of the interventions across studies, and large variations in design and quality of those studies.
Our findings suggest that interventions with behavioural components alone and mixed educational and behavioural interventions compared with usual care may improve the proportion of people who are adherent to their prescribed medication. However, we are uncertain of the effects of educational only interventions on the proportion of people who are adherent to their prescribed medication.
When adherence is measured as a continuous variable (e.g. percentage of pills taken), our findings suggest that educational only and mixed interventions may have little or no impact on adherence, and we are uncertain of the effects of behavioural only interventions.
These results must be interpreted with a degree of caution, given the variations in intervention design, duration, follow‐up, and risk of bias of included studies, along with the overall low or very low quality rating of evidence for these outcomes.
Within mixed interventions for improving medication adherence, all of the individual studies that had a significant impact on medication adherence (11 out of 27 studies) were conducted at the hospital‐community/primary care interface (e.g. in hospital, at discharge, post discharge, at outpatient clinics). Three formal subgroup analyses performed on studies involving mixed interventions found no significant differences between subgroups based on intervention duration, type of outcome measure, or health provider delivering the intervention. Studies involving behavioural only interventions were too few to enable subgroup analyses.
Several secondary outcomes were evaluated, but heterogeneity and concerns regarding risk of bias limited our ability to draw firm conclusions. Large variations in outcome measures also limited our ability to pool results via meta‐analyses for most secondary outcomes such as medication knowledge and consumer satisfaction, and many secondary outcomes were reported in only a limited number of studies (e.g. condition‐specific outcomes ‐ 7 studies, adverse drug reactions (ADRs) ‐ 6 studies, and cost‐effectiveness of interventions ‐ 4 studies).
Overall, we found that mixed educational and behavioural interventions may improve medication knowledge but to a variable degree across studies. Pooled results suggest that mixed interventions may reduce the number of emergency department (ED)/hospital admissions compared with usual care, although studies unable to be included in the meta‐analysis suggest that the interventions may have little or no effect on these outcomes. Mixed interventions may lead to little or no change in health‐related quality of life (HRQoL), and we are uncertain about effects on mortality, consumer satisfaction, and other secondary outcomes such as ADRs, physician visits, and costs for these interventions.
Educational interventions delivered alone and compared with usual care may have little or no effect on medication knowledge and probably have little or no impact on most secondary outcomes, including HRQoL and ED/ hospital admissions. We are uncertain of the effects of behavioural interventions delivered alone on the above outcomes (HRQoL, ED/hospital admissions, knowledge) and of the effects of educational or behavioural interventions on a range of other secondary outcomes including satisfaction, ADRs, physician visits, and costs. We identified no studies evaluating the effects of educational or behavioural interventions delivered alone on mortality.
Six studies reported a comparison between two interventions as part of a three‐arm randomised controlled trial (RCT) design; however, due to the limited number of studies assessing the same types of interventions and comparisons, we were unable to draw firm conclusions for any primary or secondary outcomes.
Overall completeness and applicability of evidence
Most of the studies included in this review are relatively new, with almost half (24/50) published since 2010. This trend most likely reflects the increasing prevalence of multiple medication use in older adults and increasing efforts to improve medication adherence. In contrast, three of the five interventions evaluating medication‐taking ability were published last century.
Studies from four continents were identified. Most studies were from high‐income countries, and the greatest proportions emanated from the USA (21), the UK (8), and Canada (5). The results of this review may be more applicable to older adults residing in developed countries, mostly Western countries, with only two studies identified in non‐Western countries (one each from China and Singapore).
Although we attempted to pool interventions under three broad categories (educational only, behavioural only, or mixed), a large degree of heterogeneity remained within each category, which impacted both our confidence in, and the potential applicability of, our findings. For example, among the mixed interventions that showed positive effects on medication adherence, interventions varied from one‐off to two years in duration, were delivered by various health professionals face‐to‐face and/or via telephone, and involved one or more behavioural components (e.g. regimen simplification, motivational interviewing, adherence aids, reminder cards). Furthermore, although interventions were primarily compared with usual care, variation in definitions of 'usual care' likely influenced the size of the effect for some interventions. Interventions that were compared to usual care that involved some form of medication counselling, education, or monitoring may have been less likely to impact medication‐taking ability and medication adherence than interventions that were compared to usual care that more closely resembled a pure control (no intervention) group. However, the often poor description of usual care made this difficult to assess. Our review found only a limited number of studies comparing one intervention versus another intervention. Further research assessing this comparison in different ways may help to identify the most clinically effective and cost‐effective interventions, without the potential ethical dilemmas inherent when health‐related interventions are compared to a pure control consisting of minimal or no intervention.
Reporting of medication use was generally poor and inconsistent. This may have resulted in exclusion of potentially eligible studies for which authors did not collect and/or clearly report the number of medications participants were taking. We also noted variation in the types of medications reported across studies, with some reporting only prescription medication, some reporting only regular medication, and some reporting all medications (e.g. regular, when required, prescription, non‐prescription medications). It was also unclear at times whether the interventions targeted all medications taken by participants, and whether assessment of medication‐taking ability or medication adherence applied to all medications or only to a subset. There is a need for clearer reporting of medication use in intervention studies targeting medication‐taking ability or medication adherence.
Reporting of risk factor(s) for poor medication‐taking ability and/or medication adherence was also inconsistent or non‐existent. Common risk factors such as number of medications, frailty, and cognitive impairment were extracted when possible, but subgroup analysis based on such factors was not possible. There is a need for clearer identification and reporting of patient, therapy, condition, system, and environmental factors that influence both medication‐taking ability and medication adherence, and that may be targeted by interventions to improve medication‐taking ability and adherence. Clearer reporting may also highlight and clarify the sometimes inter‐related nature of medication‐taking ability and medication adherence, as many of the interventions described within this review likely targeted aspects of medication‐taking ability (e.g. regimen simplification) in an attempt to improve medication adherence but did not directly measure medication‐taking ability as an outcome.
A limited number of studies evaluated clinical health outcomes, including condition‐specific outcomes and adverse events. Clinical health outcomes should be measured for any intervention that may result in changes in a person's medication intake, including any changes (decrease or increase) in medication adherence. ED and hospital admissions were reported in 23 studies; however, it is often unclear how many were unplanned admissions and/or medication‐related admissions. Only six studies reported the number of participants experiencing ADRs, and four studies reported the number of primary care physician visits, which may be a surrogate for minor to moderate medication‐related problems. Condition‐specific outcomes such as blood pressure or blood glucose were reported in seven studies, meaning the clinical impact of changes in medication‐taking ability or medication adherence was omitted from most studies. Improving clinical outcomes and preventing adverse health outcomes should be the priority of any intervention aimed at improving medication use. This is particularly important among older adults prescribed multiple medications, as this population is at increased risk of adverse events. Evidence of clinical impact and reduction in adverse health outcomes is also necessary to drive translation of research into clinical practice and policy.
Studies that evaluated the cost‐effectiveness of interventions to improve medication‐taking ability or medication adherence are scarce. Future studies should include appropriate measures of cost‐effectiveness of interventions to assist decision‐makers in allocating healthcare resources efficiently.
Quality of the evidence
We evaluated the certainty of the body of evidence using the GRADE approach for the two primary outcomes ‐ medication‐taking ability and medication adherence ‐ and for three key secondary health outcomes ‐ HRQoL, ED/hospital admissions, and mortality. Overall there were serious concerns related to risk of bias and inconsistencies, resulting in low‐ or very low‐quality evidence for most outcomes, except for the effects of educational interventions on HRQoL and ED/hospital admissions, for which evidence was considered of moderate quality.
Most studies had unclear or high risk of bias across multiple domains, particularly related to random sequence generation and allocation concealment. Nearly all studies (98%) also had high risk of performance bias, but we acknowledge that blinding of participants and clinicians (providers of the intervention) is often impossible to achieve in pragmatic health services research. More than half of the included studies were also rated as having unclear or high risk of detection, attrition, and/or reporting biases. Studies were commonly given an ‘unclear’ risk of bias rating due to poorly described methods or results. Future studies should strongly consider prospective registration of the trials in registries, publication of study protocols with detailed methods and description of outcome measures, and more rigorous study methods and reporting.
Serious concerns related to inconsistencies were identified due to the heterogeneous and complex nature of the interventions (components, providers, settings, duration) and variations in outcome measures. Although interventions were broadly grouped as educational, behavioural, or mixed interventions, moderate to high heterogeneity was evident in meta‐analyses and in most cases contributed to our limited certainty in the results.
Concerns related to imprecision were also present for behavioural interventions, for which participant numbers and event rates were often low and/or confidence intervals were wide.
Potential biases in the review process
Differing terminologies for medication‐taking ability and medication adherence may have limited the number of studies found, despite our use of broad search terms and our search of grey literature and the reference lists of included studies. As mentioned previously, poor reporting of medication use may have caused us to miss potentially eligible studies.
Agreements and disagreements with other studies or reviews
Our review is the first to evaluate interventions for improving medication‐taking ability in older adults prescribed multiple medications. Reviews of instruments to assess medication‐taking ability ‐ Elliott 2009 ‐ and self‐efficacy for medication management ‐ Lamarche 2018 ‐ have been published, but no reviews of interventions utilising these instruments have been published to date.
Several reviews have investigated medication adherence, but only four reviews to date have evaluated interventions for improving medication adherence in older people taking multiple medications. Two of these reviews were conducted over a decade ago (George 2008;Williams 2008), and two were published more recently ‐ one focusing on theory‐based interventions only (Patton 2017), and the other including both cross‐sectional analyses of prevalence of medication adherence and clinical trials/systematic reviews of interventions targeting adherence (Zelko 2016). Both Patton 2017 and Zelko 2016 included far fewer intervention studies than are included in our systematic review because of their different inclusion criteria and search strategies. Our findings are consistent with the findings of these four previous reviews, all of which concluded that high‐quality evidence is scarce, and that inconsistencies in methods, interventions, and outcome measures have made it difficult to draw firm conclusions regarding the most effective interventions for improving medication adherence.
Our concerns regarding risk of bias of included studies were also consistent with a previous Cochrane systematic review investigating interventions for enhancing medication adherence (Nieuwlaat 2014), which evaluated all RCTs of interventions to improve adherence with prescribed medications (i.e. not restricted to older adults or multiple medications) and found that only 17 of 182 included studies had the lowest risk of bias for study design features and their primary clinical outcome.
Authors' conclusions
Implications for practice.
Our review highlights a significant gap in the literature regarding high‐quality evidence on the effects of interventions for improving medication‐taking ability and medication adherence in older adults prescribed multiple medications. Low‐quality evidence suggests that healthcare providers might best utilise behavioural interventions alone to improve medication adherence or mixed educational and behavioural interventions to improve medication adherence while reducing the number of ED/hospital admissions. Given that the causes of non‐adherence and medication‐taking errors vary among patients, healthcare providers should aim to tailor interventions to the individual to address their specific medication adherence barriers.
Healthcare providers and policy‐makers may want to consider our findings that interventions initiated at the hospital‐community interface may be most likely to influence medication adherence, although effects were variable. Medication errors and non‐adherence place a significant cost burden on the healthcare system (Cutler 2018), and interventions that are effective in reducing medication errors and non‐adherence are needed to reduce avoidable healthcare costs. However, implementation of complex multi‐faceted interventions may require system‐, policy‐ and/or funding‐related changes, and our review has found that currently evidence related to cost‐effectiveness is insufficient to inform these changes.
Implications for research.
Further well‐designed RCTs are needed to investigate the effects of interventions for improving medication‐taking ability and medication adherence in older adults prescribed multiple medications. Priority should be given to adequately powered trials using validated, objective measures of medication‐taking ability and medication adherence. The effects of interventions on clinical outcomes, particularly adverse medication events, and cost‐effectiveness should be evaluated. Researchers should strongly consider prospective trial registration and publication of protocols using standard reporting checklists such as the Standard Protocol Items: Recommendations for Interventional Trials (An‐Wen 2013). This will help to ensure clearer and more consistent reporting of outcome variables and participant characteristics that may impact medication‐taking ability and medication adherence.
Greater recognition of the complexity of medication‐taking and medication adherence is needed. There remains no 'gold standard' measure of medication‐taking ability or medication adherence, and the two behaviours are sometimes inter‐related and influenced by a range of patient, therapy, condition, system, and environmental factors. Given that reasons for non‐adherence and medication‐taking errors are different among individuals, 'one size fits all' interventions are unlikely to be effective; this may be why many existing studies and reviews have reported that it is difficult to consistently improve medication‐taking and adherence. Future studies should report the specific factors targeted by their interventions and should include as participants patient groups for which those factors are relevant. Mixed methods research that aims to qualitatively understand participant experiences with interventions, as well as barriers to and enablers of these interventions, may also be useful.
History
Protocol first published: Issue 10, 2016 Review first published: Issue 5, 2020
Acknowledgements
We would like to acknowledge support and assistance received from the Cochrane Consumers and Communication editors and staff, particularly John Kis‐Rigo, Rebecca Ryan, Ann Parkill, Sophie Hill, Bronwen Merner, and Ann Jones.
Amanda Cross and Kate Petrie were supported by Australian Government Research Training Program scholarships during their involvement in this review.
Appendices
Appendix 1. Search strategies
MEDLINE (Ovid SP) search strategy
| 1. exp aged/ |
| 2. ((old or older or aged or senior) adj2 (person? or people or adult? or men or women or patient* or consumer* or carer* or caregiver* or care giver*)).ti,ab,kw. |
| 3. (late life or ag?ing or old age or seniors).ti,ab,kw. |
| 4. (elder* or geriatr* or gerontol* or geropsych* or veteran*).mp. |
| 5. or/1‐4 |
| 6. exp Pharmaceutical Preparations/ |
| 7. (medication* or medicine? or medicament* or pharmac* or drug? or polypharmac*).ti,ab,kw. |
| 8. exp drug therapy/ |
| 9. exp pharmaceutical services/ |
| 10. exp therapeutic uses/ |
| 11. or/6‐10 |
| 12. 5 and 11 |
| 13. primary health care/ |
| 14. (primary adj2 (care or healthcare)).ti,ab,kw. |
| 15. ambulatory care/ |
| 16. ambulatory.ti,ab,kw. |
| 17. exp general practice/ |
| 18. general practitioners/ |
| 19. gp?.ti,ab,kw. |
| 20. physicians primary care/ |
| 21. physicians family/ |
| 22. ((general or family) adj practi*).ti,ab,kw. |
| 23. exp ambulatory care facilities/ |
| 24. home care services/ |
| 25. exp community health services/ |
| 26. patient discharge/ |
| 27. (hospital adj3 discharge).ti,ab,kw. |
| 28. continuity of patient care/ |
| 29. aftercare/ |
| 30. (community or home* or domicil* or outreach or out‐reach or postdischarge or post‐discharge or postacute or post‐acute or discharge plan* or aftercare or after care).ti,ab,kw. |
| 31. or/13‐30 |
| 32. 12 and 31 |
| 33. patient education as topic/ |
| 34. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).mp. |
| 35. exp counseling/ |
| 36. information services/ |
| 37. drug information services/ |
| 38. (inform* adj5 (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).ti,ab,kw. |
| 39. reminder systems/ |
| 40. drug packaging/ |
| 41. drug prescriptions/ |
| 42. medication therapy management/ |
| 43. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) and (manag* adj5 (medication* or medicines))).ti,ab,kw. |
| 44. pharmac* care.ti,ab,kw. |
| 45. or/33‐44 |
| 46. medication adherence/ or patient compliance/ |
| 47. ((medication or treatment) adj (complian* or adheren* or noncomplian* or nonadheren*)).ti,ab,kw. |
| 48. self efficacy/ |
| 49. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) and (competen* or confident or confidence or abilit* or capacit* or skill* or self‐efficacy or cope? or coping or complian* or noncomplian* or adher* or nonadher* or underadheren* or concordan* or nonconcordan* or persisten* or nonpersist*)).ti,ab,kw. |
| 50. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) adj5 (error* or mistak* or misus* or mismanag*)).ti,ab,kw. |
| 51. (patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*).ti,ab,kw. and medication errors/ |
| 52. self‐administration/ |
| 53. or/46‐52 |
| 54. 32 and (45 or 53) |
| 55. randomized controlled trial.pt. |
| 56. controlled clinical trial.pt. |
| 57. randomized.ab. |
| 58. placebo.ab. |
| 59. clinical trials as topic.sh. |
| 60. randomly.ab. |
| 61. trial.ti. |
| 62. or/55‐61 |
| 63. 54 and 62 |
Cochrane Central Register of Controlled Trials (CENTRAL) (via Ovid)
1. aged:kw
2. ((old or older or aged or senior) near/2 (person* or people or adult* or men or women or patient* or consumer* or carer* or caregiver* or care‐giver*)):ti,ab,kw
3. ("late life" or ag*ing or "old age" or seniors):ti,ab,kw
4. (elder* or geriatri* or gerontol* or geropsych* or veteran*):ti,ab,kw
5. {or 1‐4}
6. [mh "pharmaceutical preparations"]
7. [mh "drug therapy"]
8. [mh "pharmaceutical services"]
9. [mh "therapeutic uses"]
10. (medication* or medicine* or medicament* or pharmac* or drug or drugs or polypharmac*):ti,ab,kw
11. {or 6‐10}
12. 5 and 11
13. ((primary near/2 *care) or primary‐nursing):ti,ab,kw
14. ambulatory:ti,ab,kw
15. (((general or family) next (practi* or physician* or doctor*)) or gp or gps or family‐medicine):ti,ab,kw
16. [mh "ambulatory care facilities"]
17. outpatient‐department:ti,ab,kw
18. [mh "community health services"]
19. ((patient* or hospital*) near/3 discharg*):ti,ab,kw
20. (continu* near/3 care):ti,ab,kw
21. (community or home* or domicil* or outreach or out‐reach or postdischarge or post‐discharge or postacute or post‐acute or discharge‐plan* or aftercare or after‐care):ti,ab,kw
22. {or 13‐21}
23. 12 and 22
24. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care‐giver*)):ti,ab,kw
25. counseling:kw
26. information‐services:kw
27. ((medical or drug) next information):ti,ab,kw
28. (inform* near/5 (patient* or client* or consumer* or user* or carer* or caregiver* or care‐giver*)):ti,ab,kw
29. reminder‐system*:ti,ab,kw
30. (drug next (packaging or label*)):ti,ab,kw
31. medication‐therapy‐management:ti,ab,kw
32. ((patient* or client* or consumer* or user* or subject or subjects or carer* or caregiver* or care‐giver*) and (manag* near/5 (medication* or medicine*))):ti,ab,kw
33. pharmac*‐care:ti,ab,kw
34. {or 24‐33}
35. ((medication or treatment) next (complian* or adheren* or noncomplian* or nonadheren*)):ti,ab,kw
36. (self next (efficacy or concept)):ti,ab,kw
37. ((patient* or client* or consumer* or user* or subject or subjects or carer* or caregiver* or care‐giver*) and (competen* or confident or confidence* or abilit* or capacit* or skill* or self‐efficacy or cope* or coping or complian* or noncomplian* or adher* or nonadher* or underadheren* or concordan* or nonconcordan* or persisten* or nonpersist*)):ti,ab,kw
38. ((patient* or client* or consumer* or user* or subject or subjects or carer* or caregiver* or care‐giver*) near/5 (error* or mistak* or misus* or mismanag*)):ti,ab,kw
39. (patient* or client* or consumer* or user* or subject or subjects or carer* or caregiver* or care‐giver*):ti,ab,kw and medication‐error*:kw
40. self‐administ*:ti,ab,kw
41. {or 35‐40}
42. 23 and (34 or 41) in Trials
CINAHL Plus (via EBSCOhost)
| Line # | Query |
| S43 | s42 |
| S42 | s31 and s41 |
| S41 | S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 |
| S40 | TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*) |
| S39 | AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*) |
| S38 | AB (random* or trial or placebo*) or TI (random* or trial or placebo*) |
| S37 | MH Quantitative Studies |
| S36 | MH Placebos |
| S35 | MH Random Assignment |
| S34 | MH Clinical Trials+ |
| S33 | PT Clinical Trial |
| S32 | "randomi?ed controlled trial" or PT randomized controlled trial |
| S31 | s16 and (s24 or s30) |
| S30 | s25 or s26 or s27 or s28 or s29 |
| S29 | "self administ*" |
| S28 | (patient* or client* or consumer* or user* or subject* or carer* or caregiver* or "care giver*") and (error* or mistak* or misus* or mismanag* or (inappropriate* N2 prescri*) |
| S27 | ((patient* or client* or consumer* or user* or subject* or carer* or caregiver* or "care giver*") and (competen* or confident or confidence or abilit* or capacit* or skill* or "self‐efficacy" or cope* or coping or complian* or noncomplian* or adher* or nonadher* or underadheren* or concordan* or nonconcordan* or persist* or nonpersist*) |
| S26 | "self efficacy" |
| S25 | (patient or medication or treatment) N5 (complian* or adheren* or noncomplian* or nonadheren*) |
| S24 | s17 or s18 or s19 or s20 or s21 or s22 or s23 |
| S23 | (patient* or client* or consumer* or user* or subject* or carer* or caregiver* or "care giver*") and (manag* N5 (medication* or medicine*) |
| S22 | reminder* or "drug packaging" or "drug label*" or "pharmac* care" |
| S21 | inform* N5 (patient* or client* or consumer* or user* or carer* or caregiver* or "care giver*") |
| S20 | MW information services |
| S19 | MH counseling |
| S18 | (educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or "care giver*") |
| S17 | MH patient education+ |
| S16 | s5 and s10 and s15 |
| S15 | s11 or s12 or s13 or s14 |
| S14 | MH continuity of patient care |
| S13 | community or home* or domicil* or outreach or "out‐reach" or "post‐discharge" or "post‐acute" or "patient discharge" or "discharge plan*" or (hospital N3 discharg*) or aftercare or "after care" |
| S12 | ((general or family) N1 (practi* or physician* or doctor*)) or "family medicine" or gp or gps |
| S11 | (primary N2 (care or healthcare or nursing)) or ambulatory or "nurse‐managed center*" |
| S10 | s6 or s7 or s8 or s9 or s10 |
| S9 | MH "Pharmacy and Pharmacology+" |
| S8 | medication* or medicine* or medicament* or pharmac* or drug* or polypharmac* |
| S7 | MH drug therapy+ |
| S6 | MH "miscellaneous drugs and agents+" |
| S5 | s1 or s2 or s3 or s4 |
| S4 | MH health services for the aged |
| S3 | "late life" or ag#ing or "old age" or seniors or elder* or geriatr* or gerontol* or geropsych* or veteran* |
| S2 | (old or older or aged or senior) N2 (person* or people or adult* or men or women or patient* or consumer* or carer* or caregiver* or "care giver*") |
| S1 | MH aged+ |
Embase (via Ovid)
| 1. exp aged/ |
| 2. ((old or older or aged or senior) adj2 (person? or people or adult? or men or women or patient* or consumer* or carer* or caregiver* or care giver*)).ti,ab,kw. |
| 3. (late life or ag?ing or old age or seniors).ti,ab,kw. |
| 4. (elder* or geriatr* or gerontol* or geropsych* or veteran*).mp. |
| 5. or/1‐4 |
| 6. exp drug/ |
| 7. (medication* or medicine? or medicament* or pharmac* or drug? or polypharmac*).ti,ab,kw. |
| 8. exp drug therapy/ |
| 9. pharmacy/ |
| 10. exp pharmaceutics/ |
| 11. or/6‐10 |
| 12. 5 and 11 |
| 13. exp primary health care/ |
| 14. (primary adj2 (care or healthcare)).ti,ab,kw. |
| 15. exp ambulatory care/ |
| 16. ambulatory.ti,ab,kw. |
| 17. general practice/ |
| 18. general practitioner/ |
| 19. gp?.ti,ab,kw. |
| 20. family medicine/ |
| 21. ((general or family) adj practi*).ti,ab,kw. |
| 22. outpatient department/ |
| 23. exp home care/ |
| 24. exp community care/ |
| 25. hospital discharge/ |
| 26. (hospital adj3 discharge).ti,ab,kw. |
| 27. aftercare/ |
| 28. (community or home* or domicil* or outreach or out‐reach or postdischarge or post‐discharge or postacute or post‐acute or discharge plan* or aftercare or after care).ti,ab,kw. |
| 29. or/13‐28 |
| 30. 12 and 29 |
| 31. patient education/ |
| 32. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).mp. |
| 33. counseling/ or patient counseling/ |
| 34. patient information/ or medical information/ |
| 35. drug information/ |
| 36. (inform* adj5 (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).ti,ab,kw. |
| 37. reminder system/ |
| 38. drug packaging/ or drug labeling/ |
| 39. medication therapy management/ |
| 40. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) and (manag* adj5 (medication* or medicines))).ti,ab,kw. |
| 41. pharmaceutical care/ |
| 42. pharmac* care.ti,ab,kw. |
| 43. or/31‐42 |
| 44. medication compliance/ or patient compliance/ |
| 45. ((medication or treatment) adj (complian* or adheren* or noncomplian* or nonadheren*)).ti,ab,kw. |
| 46. self concept/ |
| 47. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) and (competen* or confident or confidence or abilit* or capacit* or skill* or self‐efficacy or cope? or coping or complian* or noncomplian* or adher* or nonadher* or underadheren* or concordan* or nonconcordan* or persisten* or nonpersist*)).ti,ab,kw. |
| 48. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) adj5 (error* or mistak* or misus* or mismanag*)).ti,ab,kw. |
| 49. (patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*).ti,ab,kw. and exp medication error/ |
| 50. drug self‐administration/ |
| 51. or/44‐50 |
| 52. 30 and (43 or 51) |
| 53. randomized controlled trial/ |
| 54. controlled clinical trial/ |
| 55. single blind procedure/ or double blind procedure/ |
| 56. crossover procedure/ |
| 57. random*.tw. |
| 58. placebo*.tw. |
| 59. ((singl* or doubl*) adj (blind* or mask*)).tw. |
| 60. (crossover or cross over or factorial* or latin square).tw. |
| 61. (assign* or allocat* or volunteer*).tw. |
| 62. or/53‐61 |
| 63. 52 and 62 |
IPA (via ProQuest)
1. ab,ti((old or older or aged or senior) n/2 (person$1 or people or adult$1 or patient* or consumer* or carer* or caregiver* or “care giver*”))
2. ab,ti(late life or ag$1ing or old age or seniors or elder* or geriatr* or gerontol* or geropsych* or veteran*)
3. 1 or 2
4. su(dosage forms or prescription drugs or drug utilization)
5. ab,ti(medication* or medicine$1 or medicament* or pharmac* or pharmacotherap* or drug$1 or polypharmac*)
6. su(combined therapy or polypharmacy)
7. su(pharmacy services)
8. or/4‐7
9. 3 and 8
10. su(primary care)
11. ab,ti((primary) n/2 (care or healthcare))
12. su(ambulatory care)
13. ab,ti(ambulatory)
14. ab,ti((general or family) n/1 (practi*))
15. ab,ti(gp$1)
16. su(physicians)
17. su(health centers)
18. su(home health care)
19. ab,ti((hospital) n/3 (discharge))
20. su(patient care; continuity)
21. su(after hours service)
22. ab,ti(community or home or domicil* or outreach or out‐reach or postdischarge or post‐discharge or postacute or post‐acute or discharge plan* or aftercare or “after care”)
23. OR/10‐22
24. 9 and 23
25. su(patient education)
26. ab,ti((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn* or remind*) and (patient* or client* or consumer* or user* or carer* or caregiver* or “care giver*”))
27. su(counseling)
28. su(drug information)
29. ab,ti((inform*) n/5 (patient* or client* or consumer* or user* or carer* or caregiver* or “care giver*”))
30. ab,ti((remind*) and (system* or aid* or service* or package* or message* or call*))
31. su(drugs; packaging)
32. su(collaborative drug therapy management)
33. ab,ti((patient* or client* or consumer* or user* or subject$1 or carer* or caregiver* or “care giver*”) and ((manag*) n/5 (medication* or medicine*)))
34. ab,ti(pharmac* care)
35. or/25‐34
36. su(compliance)
37. su(self‐medication)
38. ab,ti((medication or treatment) n/1 (complian* or adheren* or noncomplian* or nonadheren*))
39. ab,ti((patient* or client* or consumer* or user* or subject$1 or carer* or caregiver* or “care giver*”) and (competen* or confident or confidence or abilit* or capacit* or skill* or self‐efficacy or cope$1 or coping or complian* or noncomplian* or adher* or nonadher* or underadheren* or concordan* or nonconcordan* or persisten* or nonpersist*))
40. ab,ti((patient* or client* or consumer* or user* or subject$1 or carer* or caregiver* or “care giver*”) n/5 (error* or mistak* or misus* or mismanag*))
41. ab,ti(patient* or client* or consumer* or user* or subject$1 or carer* or caregiver* or “care giver*”) and su(medication errors)
42. OR/36‐41
43. 24 and (35 or 42)
44. ab,ti(random* or controlled or control or placebo)
45. 43 and 44
PsycINFO (via Ovid)
| 1. aged.id. |
| 2. ((old or older or aged or senior) adj2 (person? or people or adult? or men or women or patient* or consumer* or carer* or caregiver* or care giver*)).ti,ab,id. |
| 3. (late life or ag?ing or old age or seniors).ti,ab,hw,id. |
| 4. (elder* or geriatric* or gerontolog* or geropsych* or veteran*).mp. |
| 5. ("380" or "390").ag. |
| 6. or/1‐5 |
| 7. exp drugs/ |
| 8. exp pharmacology/ |
| 9. (medication* or medicine? or medicament* or pharmac* or drug? or polypharmac*).ti,ab,hw,id. |
| 10. exp drug therapy/ |
| 11. or/7‐10 |
| 12. 6 and 11 |
| 13. primary health care/ |
| 14. (primary adj2 (care or healthcare)).ti,ab,id. |
| 15. exp outpatient treatment/ |
| 16. ambulatory.ti,ab,id. |
| 17. family medicine/ |
| 18. general practitioners/ |
| 19. gp?.ti,ab,id. |
| 20. family physicians/ |
| 21. ((general or family) adj practi*).ti,ab,id. |
| 22. home care/ |
| 23. exp community services/ |
| 24. long term care/ |
| 25. aftercare/ |
| 26. exp facility discharge/ |
| 27. ((patient or hospital) adj3 discharge).ti,ab,hw,id. |
| 28. "continuum of care"/ |
| 29. (community or home* or domicil* or outreach or out‐reach or postdischarge or post‐discharge or postacute or post‐acute or discharge plan* or aftercare or after care).ti,ab,hw,id. |
| 30. or/13‐29 |
| 31. 12 and 30 |
| 32. client education/ |
| 33. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).mp. |
| 34. exp counseling/ |
| 35. information services/ |
| 36. information/ |
| 37. (inform* adj5 (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).ti,ab,id. |
| 38. (reminder? or reminder system*).ti,ab,id. |
| 39. warning labels/ |
| 40. "prescribing (drugs)"/ |
| 41. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) and (manag* adj5 (medication* or medicines))).ti,ab,hw,id. |
| 42. pharmac* care.ti,ab,id. |
| 43. or/32‐42 |
| 44. treatment compliance/ |
| 45. ((medication or treatment) adj (complian* or adheren* or noncomplian* or nonadheren*)).ti,ab,id. |
| 46. self efficacy/ |
| 47. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) and (competen* or confident or confidence or abilit* or capacit* or skill* or self‐efficacy or cope? or coping or complian* or noncomplian* or adher* or nonadher* or underadheren* or concordan* or nonconcordan* or persisten* or nonpersist*)).ti,ab,hw,id. |
| 48. ((patient* or client* or consumer* or user* or subject? or carer* or caregiver* or care giver*) and (error* or mistak* or misus* or mismanag*)).ti,ab,hw,id. |
| 49. drug self‐administration/ |
| 50. or/44‐49 |
| 51. 31 and (43 or 50) |
| 52. random*.ti,ab,hw,id. |
| 53. trial*.ti,ab,hw,id. |
| 54. controlled stud*.ti,ab,hw,id. |
| 55. placebo*.ti,ab,hw,id. |
| 56. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id. |
| 57. (cross over or crossover or factorial* or latin square).ti,ab,hw,id. |
| 58. (assign* or allocat* or volunteer*).ti,ab,hw,id. |
| 59. treatment effectiveness evaluation/ |
| 60. mental health program evaluation/ |
| 61. exp experimental design/ |
| 62. "2100".md. |
| 63. or/52‐62 |
| 64. 51 and 63 |
Data and analyses
Comparison 1. Interventions versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Primary outcome: adherence, grouped by types of interventions (dichotomous) | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Educational interventions | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.33, 2.06] |
| 1.1.2 Behavioural interventions | 4 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.38] |
| 1.1.3 Mixed educational and behavioural interventions | 12 | 3147 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.37] |
| 1.2 Primary outcome: adherence, grouped by types of interventions (continuous) | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 Educational interventions | 5 | 1165 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.12, 0.43] |
| 1.2.3 Mixed educational and behavioural interventions | 7 | 1825 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.08, 1.02] |
| 1.3 Primary outcome: adherence, mixed interventions, grouped by intervention duration (dichotomous) | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Short duration (≤ 3 months) | 6 | 486 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.13, 1.74] |
| 1.3.2 Long duration (> 3 months) | 5 | 2505 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.97, 1.27] |
| 1.4 Primary outcome: adherence, mixed interventions, grouped by intervention duration (continuous) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Short duration (≤ 3 months) | 3 | 398 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.52, 0.88] |
| 1.4.2 Long duration (> 3 months) | 4 | 1427 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.25, 1.65] |
| 1.5 Primary outcome: adherence, mixed interventions, grouped by subjective or objective outcome measures (dichotomous) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Objective outcome measure | 5 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.93, 1.38] |
| 1.5.2 Subjective outcome measure | 7 | 2385 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.09, 1.46] |
| 1.6 Primary outcome: adherence, mixed interventions, grouped by subjective or objective outcome measure (continuous) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 Objective outcome measure | 3 | 360 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [‐0.49, 2.13] |
| 1.6.2 Subjective outcome measure | 4 | 1465 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.23, 0.66] |
| 1.7 Primary outcome: adherence, mixed interventions, grouped by provider (dichotomous) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Provider: pharmacist | 8 | 2672 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.04, 1.41] |
| 1.7.2 Provider: nurse | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.38] |
| 1.7.3 Provider: 2 or more health professionals | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.88, 2.16] |
| 1.8 Primary outcome: adherence, mixed interventions, grouped by provider (continuous) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 Provider: pharmacist | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | 1.38 [0.01, 2.75] |
| 1.8.2 Provider: nurse | 2 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.53, 0.27] |
| 1.8.3 Provider: 2 or more health professionals | 2 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.38, 1.21] |
| 1.9 Secondary outcome: ED/Hospital admissions, grouped by type of intervention (dichotomous) | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.9.1 Educational interventions | 3 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.48] |
| 1.9.2 Behavioural interventions | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.55] |
| 1.9.3 Mixed educational and behavioural interventions | 11 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.50, 0.90] |
| 1.10 Secondary outcome: mortality, mixed interventions | 7 | 1776 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.67, 1.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Rashed 2002.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate whether pharmaceutical counselling pre‐discharge from hospital (in combination with a medication and information discharge summary (MIDS) and a medicine reminder card) can improve a patient’s therapeutic management post discharge and reduce unnecessary visits to the doctor or hospital re‐admission; to investigate whether a pharmaceutical domiciliary visit can reinforce inpatient counselling Study design: RCT (2 inpatient wards: 1 intervention, 1 control) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: UK Setting: hospital discharge Inclusion criteria: > 65 years, prescribed 4 or more regular items, discharged to own home, abbreviated mental score > 7 (/10), first language English, assessed by pharmacist as at risk for problems with prescribed medicines when discharged home Number of participants randomised: 89 (45 intervention, 44 control) Number of participants included in analysis: 83 (43 intervention, 40 control) Age: mean (SD): 80.2 (5.7) years intervention, 81.1 (5.8) years control Gender: female: 36% (n = 16) intervention, 45% (n = 20) control Ethnicity: not specified Number of medications: regular medications at discharge mean (SD): 7.1 (1.8) intervention, 7.1 (2.3) control Frailty/Functional impairment: not specified Cognitive impairment: all participants had abbreviated mental score > 7/10 Co‐morbidities: not specified |
|
| Interventions |
Group 1 ‐ Pharmaceutical counselling pre‐discharge: approximately 30 minutes pre‐discharge, counselling by clinical ward pharmacist, including indication, side effects, doses, dosage times, and importance of compliance. Counselling conducted regarding medicine reminder card Group 2 ‐ Usual care: nurse went through discharge medication at point of discharge Co‐intervention: both groups received MIDS and medicine reminder card and 14 days of medication. All patients received home visit by pharmacists at 15 to 22 days and were questioned on medication use. Any incorrect information provided to participants was corrected Provider: pharmacist (hospital) Where: hospital (inpatient ward) When and how often: intervention provided once, within 24 hours of discharge Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 3 months post discharge Medication adherence (objective) : percentage of total medications with which participants are compliant (compliance considered 85% to 115%), using home medicines stock and refill prescriptions Knowledge about medicines (objective): percentage of correct answers on a pharmacist‐delivered questionnaire (drug use, dose, dosage interval) Adverse clinical health outcomes (objective) : numbers of general practitioner visits and hospital re‐admissions |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: not specified Dropout: before first visit: intervention = 2 (1 died, 1 withdrew), control = 4 (2 died, 1 nursing home, 1 withdrew) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Of the 2 care of the elderly wards, 1 was randomly chosen for study group patients and the other for control group patients. Unclear how this was chosen |
| Allocation concealment (selection bias) | Unclear risk | Unclear how patients were allocated to wards and if this was affected by the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and research staff unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research pharmacist not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excluded/declined patients not reported; 6/89 attrition (6.7%) |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in methods reported |
| Other bias | Low risk | None apparent |
Begley 1997.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the influence of domiciliary pharmacist visits on medication management in a sample of elderly people recently discharged from hospital to their own homes Study design: RCT (3 hospitals; unit of allocation: individual) Number of arms/groups: 3 |
|
| Participants |
Description: patient/consumer Geographic location: UK Setting: community (post discharge) Inclusion criteria: ≥ 75 years, ≥ 3 prescribed drugs, ≥ 2 doses/d, under care of participating consultant, consented to participate, discharged to own home Number of participants randomised: 222 (A: 74, B: 75, C: 73) Number of participants included in analysis: 190 (A: 61, B: 63, C: 66) Age: median (range) = A: 84 (75 to 94), B: 81 (75 to 96), C: 82 (76 to 92) Gender: female A: 61%, B: 65%, C: 56% Ethnicity: not specified Number of medications: mean (SD) prescribed: A: 4.6 (1.8), B: 4.8 (1.6), C: 5.5 (1.9); mean (SD) OTC: A: 2.6 (0.7), B: 4.1 (1.4), C: 2.2 (1.8) Frailty/Functional impairment: not specified Cognitive impairment: abbreviated mental test mean (SD): A: 8.4 (1.5), B: 8.8 (1.1), C: 7.9 (1.3) Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Domiciliary pharmacy medication management visit: interview consisted of 6 sections: patient information, drug knowledge, patient dexterity, abbreviated mental test, medication management, compliance. Following interview, intervention group (A) received structured counselling on correct use, storage, and compliance (including simplifying regimen, emphasising importance of compliance, positive reinforcement) Group 2‐ Group (B): control, with home interview but no counselling Group 3 ‐ Group (C): control with no home visit (i.e. usual care) Co‐intervention: N/A Provider: pharmacist (investigator) Where: home (post discharge) When and how often: home visits for A and B occurred at baseline, 2 weeks, 1 month, 3 months, and 12 months Intervention personalised: yes ‐ counselling tailored to needs of the patient |
|
| Outcomes |
Timing of outcome assessment: 12 months Medication adherence (objective): percentage of medications with which participant is compliant using pill count. Researcher counted remaining tablets and measured volume of liquid. To improve reliability and accuracy, patients were asked to retain all used medicine containers for removal by the investigator Medication‐taking ability (objective): dexterity medication management. 5‐task dexterity test (e.g. opening child‐resistant closure) assessed at baseline and at 12 months; 1 point awarded for each successfully completed activity Knowledge about medicines (subjective): drug knowledge. Percentage of correct answers. Patients asked about name, purpose, dose, dosage frequency, and side effects. Accuracy compared to hospital discharge or GP instructions |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: not specified Dropout: 4 withdrew (refused), 28 were lost to follow‐up (7 death, 7 hospitalised, 10 nursing home, 4 moved out of area) For this review, group A vs group C was considered under Comparison 1 ‐ Intervention vs control; group A vs group B was considered under Comparison 2 ‐ Intervention vs intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Recruitment staff blinded to identity of groups; allocated patients consecutively to group A, B, or C. No random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocated sequentially by blinded recruiter |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants; patients knew what they were getting (respondent bias) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Intervention delivered by same pharmacist taking outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up; reported only completed patients; rates seem similar across groups but breakdown of reasons not available for each group |
| Selective reporting (reporting bias) | High risk | Some outcomes not reported at follow‐up (e.g. dexterity). Unclear whether results for adherence are patient‐reported or pill count; gives only 'difference' ‐ not raw scores for both |
| Other bias | Low risk | Required sample size achieved (61 per group) |
Bernsten 2001.
| Study characteristics | ||
| Methods |
Aim of study: to investigate the impact of a co‐ordinated community pharmacy‐based pharmaceutical care programme for elderly patients on a range of health and economic outcomes Study design: cluster‐RCT with repeated measures (unit of allocation: pharmacy) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: 7 European countries (Denmark, Germany, the Netherlands, Northern Ireland, Portugal, Republic of Ireland, Sweden) Sturgess 2003 paper describes subgroup of Ireland data only Setting: community pharmacy Inclusion criteria: ≥ 65 years; ≥ 4 prescribed medications; oriented with respect to self, time, and place; community dwelling; regular visitors to a recruited pharmacy Exclusion criteria: housebound, resident in a nursing/residential home Number of participants randomised: intervention: 104 pharmacies, 1290 patients; control: 86 pharmacies, 1164 patients; Ireland subgroup: 191 patients (110 vs 81) Number of participants included in analysis: 18 months: 1340 (704 intervention, 636 control); Ireland subgroup: 110 patients (75 vs 35) Age: median (IQR): I: 74 (8); C: 74 (8); Ireland subgroup: mean ± SD 73.1 ± 5.0 vs 74.2 ± 6.3 Gender: female: 57.9% intervention, 57.3% control; Ireland subgroup: female 63.6% vs 61.0% Ethnicity: not specified Number of medications: prescribed medications mean ± SD: intervention: 7.1 ± 2.5, control: 7.0 ± 2.5, Ireland subgroup: 5.87 ± 1.86 vs 6.66 ± 1.99 Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Structured community pharmaceutical care: intervention pharmacists attended ≥ 1 study day and were given a study manual. Pharmacists assessed participants to identify actual and potential DRPs (e.g. poor compliance, poor knowledge, ADRs, interactions suboptimal) using a structured approach. Pharmacists then formulated an intervention (education, implementing compliance strategies, simplification, etc.) and monitoring plan per patient Group 2: ‐ Usual care: control pharmacist provided normal services Co‐intervention: N/A Provider: pharmacist (community) Where: community pharmacy When and how often: assessment and intervention was a continuous process throughout the 18 months Intervention personalised: personalised intervention and monitoring plan |
|
| Outcomes |
Timing of outcome assessment: 18 months Medication adherence (subjective): percentage self‐reported compliant. Self‐completed questionnaire using 4‐item, 4‐point Likert scale (forgetting doses, choosing not to take a dose because feeling well, choosing not to take a dose because of perceived non‐benefit, and choosing to take more of a medicine than prescribed because of feeling the need for it) ‐ based on previously validated Morisky scale. Patients compliant if never experienced any aspects of non‐compliance Medication adherence (objective): Ireland subgroup only: refill compliance. Refill compliance rates calculated from patient medication records, percentage of patients compliant Knowledge about medicines (objective): percentage correct knowledge. Interview‐based questionnaire calculating % correctness (looking at 4 areas: indication, number of dosage units taken per dose, number of doses per day, and awareness of potential adverse effects). Higher scores = better knowledge Satisfaction with intervention (subjective): self‐reported by investigator‐administered questionnaire ‐ satisfaction with services and general opinion of pharmaceutical care. % who agree/mainly agree Health‐related quality of life (subjective): SF‐36 (validated) ‐ 8 dimensions Adverse clinical health outcomes (subjective): 1 or more hospitalisations in past 18 months, self‐reported via investigator‐administered questionnaire Cost‐effectiveness (objective): health care‐related resource usage. Direct costs of the study including additional time spent by pharmacists; contacts with GPs, specialists, and nurses; and costs of hospitalisation and medications |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: European Commission (BIOMED 2)‐funded co‐ordination of RCT (+ lots of others for financial/logistic support), Ireland subgroup: Northern Pharmacies Trust, Northern Ireland and European Commission Dropout: at 18 months: 1114 (45%) patients (586 intervention, 528 control) due to unwillingness, illness, moving away, pharmacy withdrawal, and death. Ireland subgroup: withdrew: 41 (15 and 26), illness: 2 (1 and 1), pharmacy withdrew: 30 (15 and 15), patient death: 8 (4 and 4) ICC value unclear, study authors contacted for further information but no response. Thus unit of analysis error exists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pharmacies assigned as control or intervention; "where possible control and intervention sites were matched as closely as possible" ‐ limited details Sturgess 2003: restricted randomisation technique to match community pharmacies in similar pairs. Half participating pharmacies, then randomly assigned as intervention sites; other half as control sites. No details on randomisation technique |
| Allocation concealment (selection bias) | High risk | Pharmacies randomised; participants attended their normal pharmacy. Unclear whether allocation was concealed from pharmacies until after randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind; patients and pharmacies aware. Non‐blinding may have influenced service delivery by pharmacists |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collection interviews performed, when possible, by a member of staff other than the pharmacist (e.g. pharmacy assistant). Suspect staff would still be aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two countries did not complete 18‐month follow‐up (stopped at 6 months); 1 country conducted 18‐month follow up at 24 months. High withdrawal; those who withdrew were older and had poorer QoL Sturgess 2003: large attrition ‐ 191 at baseline, 147 at 6 months, 119 at 12 months, 110 at 18 months |
| Selective reporting (reporting bias) | Unclear risk | Reported as per methods. However methods did not discuss how to deal with large attrition |
| Other bias | Unclear risk | Target sample size was 480 per country ‐ not reached. Large variance in follow‐up Recruitment bias (selective recruitment of cluster participants): unclear whether allocation was concealed from pharmacies until after recruitment. Potential for risk of bias as patients with good relationships with pharmacists and knowledge of intervention may have preferentially joined the study |
Blalock 2010.
| Study characteristics | ||
| Methods |
Aim of study: to assess effects of a community pharmacy‐based falls prevention programme targeting high‐risk older adults on rates of recurrent falls, recurrent injurious falls, and filling prescriptions for medications that have been associated with increased risk of falls Study design: RCT (1 year look back and 1 year follow‐up after RCT; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: community pharmacy Inclusion criteria: those at high risk of falling (≥ 65 years, ≥ 1 fall not attributable to syncope within the 1 year preceding, ≥ 4 long‐term prescription medications, ≥ 1 CNS‐active medication) Exclusion criteria: housebound, in long‐term care facility, not able to read and write English, exhibited significant cognitive impairment Number of participants randomised: 186 (93 intervention, 93 control) Number of participants included in analysis: 93 and 93 (ITT), 73 and 113 (as treated) Age: mean (SD): I: 75.5 (7.0) vs C: 74.1 (6.8) Gender: female: 78.5% intervention vs 65.4% control Ethnicity: white: 91.4% intervention vs 86.0% control Number of medications: unclear (inclusion criteria ≥ 4 long‐term prescriptions) Frailty/Functional impairment: use of cane or walker: 37 (39.8%) vs 43 (46.2%) (P = 0.37) Cognitive impairment: not specified Comorbidities: high‐risk conditions (dizziness, diabetes, incontinence, arthritis, Parkinson, stroke): mean (SD): 1.65 ± 1.19 intervention vs 1.58 ± 1.06 control |
|
| Interventions |
Group 1 ‐ Enhanced pharmacologic care. Invitation to participate in free face‐to‐face medication consultation (~ 45 minutes) with community pharmacy resident. Pharmacist reviewed medications and identified potential problems (emphasis on CNS‐active medications) using structured algorithms. If problem identified and patient interested in making a change, pharmacist contacted physician to seek prescriber approval of the recommended changes Group 2 ‐ Usual care: no medication consultation Co‐intervention: both groups received 2 brochures on prevention of falls Provider: pharmacist (community) Where: local health care centre When and how often: once Intervention personalised: yes, personalised medication review |
|
| Outcomes |
Timing of outcome assessment: baseline and 12 months Medication adherence (subjective): BMQ (validated), 5‐item regimen screen that assesses how medication is used Condition‐specific outcomes (subjective): 1 or more falls. Calculated using monthly fall calendar; patients recorded each fall "a sudden, accidental change in position where you land on the ground, floor, or an object" |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention Dropout: 20 did not receive intervention, 27 dropped out (17 vs 10), 9 unable to contact (6 vs 3), 5 died (3 vs 2) Further information required: BMQ results (email correspondence with trial author ‐ unsuccessful) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to either intervention or control ‐ unclear how |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind; patients knew if they had the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT, although no BMQ data, so difficult to assess if/how ITT was done |
| Selective reporting (reporting bias) | High risk | "BMQ was readministered at 4 monthly intervals, ending 12 months after the baseline assessment" ‐ results not listed. However BMQ listed as a data source ‐ not an outcome in methods |
| Other bias | Unclear risk | Initial sample size was 262; "interim power analyses were conducted when it became apparent that it would be difficult to reach target sample size" Sample size changed to 95 per group. Sample size based on falls risk |
Bond 2007.
| Study characteristics | ||
| Methods |
Aim of study: to test the hypothesis that a comprehensive MEDMAN service would increase the proportion of patients receiving treatment according to the National Service Framework in England and Wales; would improve overall patient health status; and would be cost‐effective Study design: RCT (pharmacy/GP; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: UK Setting: community pharmacy (+ primary care (GP)) Inclusion criteria: patients registered with GP > 17 years and with CHD (previous MI, angina, CABG, and/or angioplasty); pharmacies (only pharmacies with private consultation area were eligible to participate) Exclusion criteria: illiterate/innumerate, history of alcohol/drug misuse, terminal/serious illness, severe mental illness, unable to provide informed consent or otherwise unsuitable for the trial as determined by GP Number of participants randomised: 1493 (I: 980, C: 513) Number of participants included in analysis: questionnaires analysed: 712 vs 373, clinical records analysed: 868 vs 466 Age: mean ± SD intervention 68.7 ± 9.2 vs control 68.8 ± 9.1 Gender: F: 307 (32.6%) vs 147 (29.4%) Ethnicity: not specified Number of medications: prescribed medications median (IQR) of 738, intervention: 7 (5 to 10) Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Community Pharmacy Medicines Management (MEDMAN): patients received a study registration card and a letter asking them to visit their nominated pharmacy to initiate service. Initial consultation informed by extracted medical data supplied by researchers. Further consultations provided according to pharmacist‐determined patient need. Consultations included assessments of the following: therapy, medication compliance, lifestyle (e.g. smoking, exercise, diet), and social support (e.g. difficulties collecting prescriptions and opening bottles). Recommendations were recorded on a referral form, which was sent to the GP, who returned annotated copies to pharmacists Group 2 ‐ Usual care from GP and community pharmacy Co‐intervention: N/A Provider: pharmacist (community) Where: community pharmacy When and how often: initial consultation, then as pharmacist‐determined need Intervention personalised: yes ‐ assessment of therapy, compliance, lifestyle, social ‐ and further consultations as needed |
|
| Outcomes |
Timing of outcome assessment: baseline and 12 months Medication adherence (subjective): 12 statements about medicine‐taking were summated to derive self‐reported compliance score (range 12 to 60). 12‐Item scale extended scope of MARS questionnaire, introduced a time dimension, and rephrased some questions to make them more patient friendly Knowledge about medicines (subjective): patients were asked whether they "knew more about their medicines compared with a year ago" on a 5‐point Likert scale. Dichotomous; those who said agree/strongly agree Satisfaction with intervention (subjective): responses to 15 positive and negative statements regarding their most recent pharmacy visit. Overall score 15 to 75 (higher = better) Health‐related quality of life (subjective): SF‐36 and EuroQoL‐5D Condition‐specific outcomes (objective): patients reaching CHD targets. Total score for patients reaching 8 targets (aspirin, lipid, BP, smoking, alcohol, physical activity, diet, and BMI) Cost‐effectiveness (objective): health economics analysis. Total NHS‐related study cost: NHS resource use based on information extracted from GP‐held records at baseline and at follow‐up. NHS costs included costs of intervention and other treatment (e.g. medicines, hospital, other health consultations) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Department of Health for England and Wales, managed by National Pharmaceutical Association, Royal Pharmaceutical Society of Great Britain, Company Chemist Association, and Co‐operative Pharmacy Technical Panel, led by PSNC Dropout: before intervention: 3 died, 49 withdrew (total 52, 39 vs 13); post intervention: 38 vs 9 withdrew, 20 vs 19 died Unpublished data included: full trial report provided by trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised 2:1 (intervention:usual care) independently of research team using a password‐protected computer programme in permuted blocks stratified by practice |
| Allocation concealment (selection bias) | Low risk | Patients consented before randomisation; randomisation done independent of research team |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants nor staff to intervention. Pharmacies not told which control patients had nominated their pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Audit clerks and researchers conducting statistical analyses were blinded to patient randomisation. Self‐reported data were collected by postal questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% and 79% of questionnaires analysed. Intention‐to‐treat, but patients with missing data excluded. Potential selection bias resulting from loss to follow‐up or missing data was tested and adjusted for, using the Heckman selection correction. When evidence of selection bias was found, unbiased effect of the intervention was reported. 98 and 99% of clinical record forms analysed |
| Selective reporting (reporting bias) | Unclear risk | Krska paper; not as per protocol |
| Other bias | Unclear risk | Did not reach required sample size. Sample size calculation: 1920 (1280 vs 640) |
Cargill 1992.
| Study characteristics | ||
| Methods |
Aim of study: to provide information on identification of patients at highest risk for problems related to medication non‐compliance and problematic behaviours in the home setting and their response to teaching interventions; to optimise medication‐taking compliance of elderly patients by strengthening the home medication administration system; to reinforce the nursing role as facilitator of maximum health status Study design: RCT (unit of allocation: individual) Number of arms/groups: 3 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: USA Setting: outpatient clinic (general medicine servicing Veterans Administration) Inclusion criteria: ≥ 60 years, metropolitan area accessible to home visits Number of participants randomised: 70 Number of participants included in analysis: 70 Age: range 62 to 97 years, mean 72 Gender: not specified Ethnicity: not specified Number of medications: prescription, non‐topical, non‐inhalant, non‐liquid; mean: 7.5 Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: not specified |
|
| Interventions |
Group 2 ‐ Nurse teaching session: 20‐minute teaching session, including review of medications timed to patient's schedule and any allowed flexibility. A pill cassette was dispensed if feasible for the patient Group 3 ‐ Nurse teaching session and follow‐up phone call: 20‐minute teaching session (as above) plus additional follow‐up telephone call 1 to 2 weeks after visit in which the nurse reviewed the medication regimen verbally with the patient Group 1‐ Usual care Co‐intervention: N/A Provider: nurse Where: home ± phone call (group 3) When and how often: once (+ follow‐up at 1 to 2 weeks in group 3) Intervention personalised: personalised review of medications was timed to patient's schedule; pill cassette was dispensed if feasible |
|
| Outcomes |
Timing of outcome assessment: baseline, 4 to 6 weeks Medication adherence (objective): pill count percentage compliance. Percentage of pills taken vs those prescribed to be taken using pill count Medication taking ability (objective): behaviour score/100 for congruency between supply of medications on hand and prescribed medications (/40), verbalising correct regimen (/30), maintaining each prescribed med (/20), appropriate use of OTC (/10). Points deducted for sequestering old scripts, using alternative medications inappropriately, or mixing medications together |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: not specified Dropout: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding; assume patients and nurses knew allocation to perform intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of attrition |
| Selective reporting (reporting bias) | Unclear risk | Raw data not reported; only in graph format |
| Other bias | Low risk | None apparent |
Chrischilles 2014.
| Study characteristics | ||
| Methods |
Aim of study: to examine the impact of a personal health record (PHR) on medication use safety among older adults Study design: RCT (single‐centre open‐label parallel group study with unequal randomisation (3:1); unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: patient's home (online) Inclusion criteria: age 65+, used a computer in past month to visit websites or to send/receive email, responded to questionnaire Number of participants randomised: 1163 Number of participants included in analysis: 1075 (802 vs 273) Age: mean ± SD 72.5 ± 6.0 vs 72.0 ± 6.3 Gender: female: 461 (57.5%) vs 150 (54.9%) Ethnicity: non‐Hispanic white: 782 (99%) vs 267 (98.2%) Number of medications: mean ± SD: prescription: 4.1 ± 3.2 vs 4.2 ± 3.2, OTC: 4.1 ± 2.8 vs 4.3 ± 3.1 Frailty/Functional impairment: physical health (SF‐12): 45.9 ± 10.6 vs 46.1 ± 10.3 Cognitive impairment: memory problems: 80 (10%) vs 31 (11.4%) Comorbidities: medical conditions (from list of 19): 3.6 ± 2.3 vs 3.6 ± 2.2 |
|
| Interventions |
Group 1 ‐ Personal Health Records (PHRs): participants sent an invitation to use study PHR for a period of 1 year and a quick‐start guide. Users could enter, view, and print their current and past medicines, allergies, health conditions, and health event tracking over time. PHR also had user‐friendly medication safety messages based on the Assessing Care of Vulnerable Elders project (ALCOVE‐3) medication use quality indicators. PHR displayed a message when a user entered a medication with an associated ALCOVE‐3 safety concern (drug‐drug interactions e.g. warfarin; dosage concerns e.g. acetaminophen; important lab monitoring e.g. loop diuretics; risk awareness e.g. NSAIDs, bleeding; drugs that should be avoided e.g. barbiturates). Three levels of increasing detail ‐ brief alert, summary level, and detailed explanation. Participants who did not log in were sent a reminder letter 3 to 4 weeks after initial invitation Group 2‐ Usual care: no access to study PHR Co‐intervention: N/A Provider: online Where: online (with baseline and follow‐up questionnaires mailed) When and how often: continuous for 1 year at patient discretion Intervention personalised: individual medication‐specific messages |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (subjective): modified Morisky self‐reported adherence. Answers never, rarely, sometimes, often, always (instead of original yes/no). Mailed questionnaire Adverse clinical health outcomes (subjective): experienced medication side effects in past 3 months (yes/no ‐ reported as percentage of participants experiencing side effects) Other (subjective): medication management problems: mean (SD) number of medication management problems. List of 8 problems including questions on multiple prescribers, multiple pharmacies, mail‐order prescriptions, confusion whether medication was taken, taking medication without knowing indication, problems affording medications, feeling that medications are not working, feeling that medications are not doing what they were intended to do |
|
| Notes | Trial registration: NCT02012712 Consumer involvement: PHR was developed and refined using participatory design and focus group sessions with older adults, as well as evaluation in a usability laboratory Funding source: Agency for Healthcare Research and Quality grant and National Institutes of Health grant Dropout: 23 before intervention, 65 lost to follow‐up Fidelity: 61.2% attempted to log on to PHR; 55.2% performed some activity with PHR. More than 40% entered at least 1 medication into PHR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in a 3:1 ratio using computerised random numbers. Groups comparable |
| Allocation concealment (selection bias) | Low risk | Notification of study group assignment was sent by mail to all trial participants by an investigator with no clinical involvement in the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded ‐ participants knew allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mailed questionnaires and online results; no outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1075 of 1163 included in analysis; ITT |
| Selective reporting (reporting bias) | Low risk | Reported as per methods |
| Other bias | High risk | 61.2% attempted to log on to PHR; 55.2% performed some activity with PHR. More than 40% entered at least 1 medication. ‐ so poor fidelity of intervention. Reimbursed for completing baseline and follow‐up questionnaires |
Cohen 2011.
| Study characteristics | ||
| Methods |
Aim of study: to assess whether Veterans Affairs Multi‐disciplinary Education and Diabetes Intervention for Cardiac Risk Reduction Extended for 6 Months could improve attainment of target goals for hypertension, hyperglycaemia, hyperlipidaemia, and tobacco use in patients with type 2 diabetes compared to primary care after 6 months of intervention Study design: RCT (1:1 randomisation; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: medical centre (Veterans Affairs medical centre) Inclusion criteria: veterans with type 2 diabetes HbA1c > 7%, LDL‐C > 100 mg/dL; coronary artery disease LDL > 70 mg/dL, BP > 130/80 in previous 6 months Exclusion criteria: gestational diabetes, unable to attend group sessions, psychiatric instability or organic brain injury that precluded diabetes self‐care Number of participants randomised: 103 Number of participants included in analysis: 99 (50 and 49) Age: mean ± SD I: 69.8 ± 10.7, C: 67.2 ± 9.4 Gender: female: 0% (n = 0) vs 4% (n = 2) Ethnicity: not specified Number of medications: total not available (added means = 4.20 vs 4.15). Hypertension: 2.02 ± 1.09 vs 1.86 vs 1.12, diabetes: 1.38 ± 0.81 vs 1.47 ± 0.82, cholesterol: 0.80 ± 0.49 vs 0.82 vs 0.53 Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: heart failure 16% vs 10.2%, smoker 14% vs 8.2%, stroke 4% vs 4.1%, coronary heart disease 48% vs 46.9%, COPD 14% vs 20.4%, mood disorder 14% vs 14.3% |
|
| Interventions |
Group 1 ‐ VA MEDIC‐E: 4 weekly group sessions followed by 5 monthly booster group sessions. Each 2‐hour session included 1 hour multi‐disciplinary diabetes‐specific healthy lifestyle education and 1 hour pharmacotherapeutic intervention performed by a clinical pharmacist (diabetes educator). Family/friends encouraged to participate. 90‐minute booster sessions were less structured Group 2 ‐ Usual care (clinic visits with primary care providers; average once every 4 months) Co‐intervention: N/A Provider: weekly multi‐disciplinary (pharmacist, dietician, pharmacist/PT, nurse) + monthly booster clinical pharmacist Where: medical centre room When and how often: 4 once‐weekly + 5 monthly boosters Intervention personalised: sessions were group based; however pharmacist sessions were more informal and allowed for open discussion about each individual's risk factor control, obstacles, solutions |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (objective): medication possession ratios: total days supply of medication received divided by total number of expected medication intake days Health‐related quality of life (subjective): change in VR‐36 (SF‐36 for veterans) Condition‐specific outcomes (objective): achievement of glycaemic and cardiac risk factor goals. Percentage of participants achieving SBP < 130, LDL < 100, A1c < 7% |
|
| Notes | Trial registration: NCT00409240 Consumer involvement: not specified Funding source: Sandra A. Daugherty Foundation Dropout: 4 before intervention, 3 died Further information required: total number of medications and complete data regarding medication adherence (email correspondence with trial author ‐ successful, but authors had no further data available) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned in a 1:1 ratio; no details on randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants/personnel; assumed intervention would impact behaviour |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 103 randomised, 99 included in analysis; 4 revoked consent (3 vs 1); 3.8% attrition |
| Selective reporting (reporting bias) | High risk | Raw data missing for adherence. MPR for total medications quoted but total number of medications not reported |
| Other bias | Low risk | None noted |
Cossette 2015.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effectiveness of an ED‐based nursing intervention; to report the impact of an intervention on the secondary outcomes of perceived continuity of care, illness perceptions, self‐care capacities, psychological symptoms, and medication adherence Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Canada Setting: hospital discharge (emergency department) Inclusion criteria: at risk for ED return because ≥ 1 ED visit in past year, ≥ 6 medications Exclusion criteria: inability to speak French or English, cognitive problems (e.g. dementia), already receiving regular follow‐up (e.g. at a specialised clinic in hospital) Number of participants randomised: 265 (132 vs 133) Number of participants included in analysis: 203 (108 vs 95) Age: mean ± SD: 67.06 ± 10.42 vs 67.33 ± 9.11 Gender: female: 38.9% vs 48.4% Ethnicity: not specified Number of medications: medications on arrival in ED: mean (SD) 9.2 (2.79) vs 9.95 (3.47) Frailty/Functional impairment: not specified Cognitive impairment: cognitive impairment (e.g. dementia) excluded Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Nursing ED intervention: 3 encounters: 1 discharge, 2 telephone follow‐ups at 2 to 4 days and 7 to 10 days post discharge. Potential patient concerns assessed by a 19‐item clinical disease management tool (worries about returning home, disease management, treatment management, ADL/iADL, emotions/cognition, informal resources, healthcare system). If patients rated as 'at risk', they received tailored nurse intervention (e.g. teaching, advice, feedback, referring to external resources). Patients could also call the nurse between planned encounters if they had questions or concerns Group 2‐ Usual care + project nurse repeated advice given by bedside nurse that patients should contact regular healthcare resources if needed (e.g. hotlines, GPs, cardiologists) Co‐intervention: N/A Provider: nurse (project nurse: bachelors degree + 5 years experience in clinical cardiac care) Where: face‐to‐face in ED and telephone follow‐up When and how often: 3 times (discharge, 2 to 4 days and 7 to 10 days) Intervention personalised: yes ‐ each person received different package care |
|
| Outcomes |
Timing of outcome assessment: 30 days post discharge Medication adherence (subjective): Morisky self‐reported medication‐taking scale (validated). Patients indicated whether (1) or not (0) they forgot (item 1), omitted (item 2), were careless (item 3), or stopped their medication when feeling better (item 4). In this study, results were dichotomised as 0 (never miss Rx) vs 1 or more (1 or more missing Rx) Adverse clinical health outcomes (objective): ED revisits. Percentage of participants re‐admitted to emergency department |
|
| Notes | Trial registration: ISRCTN88422298 Consumer involvement: not specified Funding source: Fonds de la Recherche Quebec Sante, Quebec Network on Nursing Intervention Research, Montreal Heart Institute Foundation and Research Centre Dropout: 62 (24/38) lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generated by independent statistician using PROC PLAN procedure |
| Allocation concealment (selection bias) | Low risk | Statistician provided opaque envelopes containing assignment to project nurse who was blinded until opening envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind ‐ patient and nurse unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant who collected outcome measure data by telephone was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing patients ‐ 203/265 reached at time of outcome assessment. Unbalanced losses also 24 (18%) vs 38 (29%) |
| Selective reporting (reporting bias) | Low risk | Reported as per methods |
| Other bias | Unclear risk | Trial ceased early due to unlikely achievement of primary outcome. Sample size not reached |
George 2016.
| Study characteristics | ||
| Methods |
Aim of study: to examine the efficacy of a web‐based intervention that utilised Bandura's theory of self‐efficacy and targeted dementia family caregivers Study design: RCT (substudy of larger study; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: carer Geographic location: USA Setting: community Inclusion criteria: carer: (a) women ≥ 18 years (b) assisting a community‐dwelling biological or “chosen” earlier‐generation relative by (c) accompanying/providing transportation to a medical appointment of this relative ≥ 1×/year, (d) engaging in ≥ 1 caregiving activities related to prescription drugs: ordering, retrieving, organising, or administering medication, routinely reminding the older adult to take medications, or sharing in decision‐making with care recipient and physician to begin, hold, increase, decrease, or discontinue a medication, and who (e) endorsed a score ≥ 2, “somewhat distressed”, on 2 items of the Family Caregiver. Care recipients (i.e. patients) were required to have a caregiver‐reported diagnosis of dementia Exclusion criteria: care recipients: lifetime‐reported history of (a) schizophrenia, (b) bipolar disorder, (c) suicide attempts, (d) Huntington’s disease, (e) Korsakoff’s disease, (f) multiple sclerosis, (g) HIV, (h) traumatic brain injury, or (i) drug/alcohol dependence Medication Administration Hassles Scale Number of participants randomised: 53 (28 vs 25) Number of participants included in analysis: 35 (18 vs 17) completed programme Age: mean age years ± SD: carers: 53 ± 10.7 vs 53.92 ± 9.05, patients: 83.03 ± 9.12 vs 83.00 ± 6.83 Gender: all carers female (n = 53; 100%), patients: female: 22 (78.6%) vs 19 (76%) Ethnicity: carers: Caucasian: 25 (89.3%) vs 15 (60%), African American: 2 (7.1%) vs 6 (24%), Latina: 0 (0%) vs 1 (4%), multi‐racial: 1 (3.6%) vs 3 (12%) Number of medications: total prescription medications: 7.03 ± 3.47 vs 7.76 ± 3.91 Frailty/Functional impairment: ADL score: 1.29 ± 1.61 vs 0.80 ± 1.15 Cognitive impairment: all had carer‐reported dementia (CRD): 1.46 ± 0.81 vs 1.44 ± 0.79 Comorbidities: total not specified |
|
| Interventions |
Group 1 ‐ Narrative health education/Narrative online education: participants entering the experimental condition’s website also encountered a still screen shot with 4 clickable content areas. In the centre of the page, they saw a clickable section titled “introduction” ‐ which linked to a video. When participants entered any content area, they saw another screen containing 2 columns ‐ 1 with resources and a single video of an “expert” (pharmacist, nurse, psychologist, or social worker) providing brief supplementary information, the second column titled “story” including brief video episodes, each less than 4 minutes in length, showing ethnically diverse care dyads encountering various medication‐related challenges as the weeks progressed Group 2 ‐ Narrative health education/Didactic online education: still screen shot with 4 clickable content areas. When participants entered any content area, they saw another screen containing 1 column. This column was titled “resources” and contained PDF didactic handouts with information about that content area and a single video of an “expert” (pharmacist, nurse, psychologist, or social worker) providing brief supplementary information Co‐intervention: N/A Provider: online delivery Where: online delivery When and how often: continuous for 1 month Intervention personalised: no |
|
| Outcomes |
Timing of outcome assessment: baseline and 1 month Medication adherence (subjective) : Morisky medication adherence: 8‐item self‐report by caregiver (caregiver answered questions about care recipient's level of adherence) = Yes/No answers, Yes = 1 indicating non‐adherent behaviour, No = 0 indicating good medication adherence. Higher scores = poorer adherence Satisfaction with intervention (subjective): user satisfaction regarding use of the computer programme questionnaire (USUCPQ): 8‐item measure that assesses user satisfaction with online health‐based interventions. This measure is based on a 7‐point Likert scale (0 = very unsatisfied, 7 = very satisfied). Explores following domains of caregiver satisfaction: (a) convenience, (b) entertainment, (c) how interesting the content was, (d) speed of the modules, (e) usefulness, (f) practicality, (g) tolerability, and (h) how much information was presented. Maximum score = 56 |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Express Scripts Research Award (July 2013) Dropout: 7 and 11 did not complete the programme Unpublished data: full manuscript of thesis. Some data, particularly related to the larger study, are not yet published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online random number generator used to evenly divide ID numbers into 2 groups before the study began |
| Allocation concealment (selection bias) | Low risk | A lab member unaffiliated with the project placed randomly assigned condition types into sealed envelopes with an ID number on the front. Once an individual was determined to be in the dementia group, the envelope with the correct participant number in the group was opened, and the individual was placed in the condition identified inside the envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Project co‐ordinator accessed pre‐intervention survey to determine dementia status; thus random assignment was not blind to project co‐ordinator. Of note, all intervention materials and contact points were pre‐determined, and thus there was no possibility of differing participant assignment based on project co‐ordinator knowledge of the intervention condition. Both groups viewed the same online interface, thus may have been unaware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Caregiver self‐reported adherence but likely blinded, so unsure of the impact this would have on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition: a third considered non‐completers. No difference in dropout rates between groups. Participants who dropped out were reporting poorer medication management adherence and higher level of overall medication‐related hassles. No significant differences in dropout rates emerged between didactic and narrative vignettes |
| Selective reporting (reporting bias) | Low risk | Results as per methods |
| Other bias | High risk | Thesis only ‐ not published in peer‐reviewed journal. Poor fidelity, as 33% did not log on to complete the programme |
Grymonpre 2001.
| Study characteristics | ||
| Methods |
Aim of study: to measure the impact of a community‐based geriatric pharmaceutical care model on specific process measures Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Canada Setting: primary care clinic (interdisciplinary community health clinic) Inclusion criteria: ≥ 65 years, non‐institutionalised, ≥ 2 medications (prescribed or non‐prescribed) Number of participants randomised: 135 (69 intervention vs 66 control) Number of participants included in analysis:114 (56 vs 58) Age: mean ± SD 76.9 ± 8.4 vs 77.2 ± 8.8 Gender: female: 75% vs 83% Ethnicity: 100% Caucasian Number of medications: prescribed medications mean ± SD 5.9 ± 3.1 vs 6.5 ± 3.4 Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Geriatric pharmaceutical care model: home medication history (HMH) conducted by trained staff or volunteers using standardised instrument, reviewed by pharmacist to identify and document potential and actual drug‐related issues. Pharmacist letter summarising info and recommendations provided to physician Group 2 ‐ Usual care with home medication history but no pharmacist intervention: HMH was reviewed by pharmacist for any immediate concerns, and those with "life‐threatening" drug‐related problems were required to withdraw Co‐intervention: N/A Provider: pharmacist Where: private office or at home When and how often: once (and then as required) Intervention personalised: yes ‐ depending on nature of drug‐related problems |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (objective): prescription refill adherence: refill adherence was based on provincial prescription claims database; medication percentage adherence by comparing 1 year before and 1 year after intervention date prescription claims database. Percentage adherence = sum of days supply in interval × 100/actual number of days in interval between first and last fills Knowledge about medicines (objective):knowledge of purpose: knows purpose of prescribed drugs (yes/no), expressed per prescribed drug |
|
| Notes | Trial registration: not specified Consumer involvement: not specified Funding source: not specified Dropout: 15 withdrew (10 vs 5), 4 died (2 vs 2), 1 NH, 1 unable to contact Fidelity: pharmacist was able to evaluate and make recommendations on 66 of the 69 test patients at baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | All patients were informed, in a letter, of their allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind; patients were informed by letter of their allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Home medication history re‐administered at 6 months by blinded, trained volunteers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adherence calculated per drug ‐ although number of drugs does not match up to mean number of prescribed drugs used by patients nor to number of drugs used to assess knowledge |
| Selective reporting (reporting bias) | Unclear risk | Outcomes specified in methods reported. Specific data comparisons not specified in methods ‐ may have been searching for significant outcomes (e.g. % hoarded, mean hoarded) |
| Other bias | Low risk | Sample size based on number of drugs ‐ "100 test drugs and 100 control drugs" ‐ reached |
Haag 2016.
| Study characteristics | ||
| Methods |
Aim of study: to assess the impact of comprehensive pharmacist‐provided telephonic MTM on care quality in an outpatient care transition programme (CTP) for high‐risk adults aged ≥ 60 years Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: outpatient clinic (primary care work group at tertiary care academic medical centre) Inclusion criteria: ≥ 60 years, independent living, enrolled in local care transition programme (CTP). Enrolled in CTP during hospitalisation if in primary care work group, resided within 20 minutes drive, and predicted high risk of health utilisation) Number of participants randomised: 25 Number of participants included in analysis: 22 Age: median (IQR): 81 (78 to 85) intervention vs 86 (79.5 to 87) control Gender: female: 4 (31%) vs 2 (17%) Ethnicity: white: 13 (100%) vs 11 (92%) Number of medications: all medications listed on home medication list (prescription, non‐prescription, and herbal); median (IQR): 17 (12 to 20) intervention vs 15.5 (13 to 18.5) control Frailty/Functional impairment: Elder Risk Assessment Index score: median (IQR) 18 (17 to 20) vs 20 (17.5 to 22.5) Cognitive impairment: dementia excluded Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Medication therapy management (MTM): MTM consultation with a pharmacist by telephone 3 to 7 business days after hospital discharge. Intervention complemented existing CTP (care transition programme). Pharmacist completed comprehensive review of all prescription, non‐prescription, and herbal medications to identify, resolve, and prevent DRPs (e.g. PIM, ADEs, prescribing omissions). Recommendations sent via secure messaging function within electronic medical record to CTP provider Group 2 ‐ Usual care ‐ defined as pre‐existing CTP without pharmacist intervention Co‐intervention: pre‐existing CTP programme: home visit by nurse practitioner within 3 business days after discharge. As part of the visit, the nurse practitioner reviewed the patient's medications and made changes as deemed appropriate. Changes were implemented directly or were discussed with the patient's primary care provider, depending on clinical judgement Provider: pharmacist Where: by telephone (phone call to patient's home) When and how often: once, 3 to 7 days after discharge Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: baseline and 5 weeks (or 30 days) Medication adherence (subjective) : adapted Morisky Medication Adherence Scale (MMAS): self‐reported by questionnaire over phone. 6 yes/no questions ‐ number of no's (no = indicating good adherence behaviour). Validated Adverse clinical health outcomes (objective): ED visits or hospital re‐admissions: re‐admissions assessed by blinded, independent pharmacists |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Grant # UL1 TR000135 from National Center for Advancing Translational Sciences, NIH, and US DHHS Dropout: 1 withdrew, 2 died |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study statistician used a random number generator to determine allocation sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation completed during phone call with study co‐ordinator, who opened a sealed envelope that contained an indication of which group the patient was assigned to |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was unblinded (participants and investigators) ‐ but was unable to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes were assessed while blinded to intervention or usual care group allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost ‐ balanced across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, including NS outcomes |
| Other bias | Unclear risk | 2 of the 12 patients who were randomised to the usual care group had participated in MTM in the past 12 months |
Hale 2016.
| Study characteristics | ||
| Methods |
Aim of study: to compare the MedSentry remote medication monitoring system vs usual care in older HF adult patients who recently completed an HF telemonitoring programme Study design: RCT of people who had recently completed hospital‐based heart failure telemonitoring (individual allocation) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: community dwelling (hospital telemonitoring into the home) Inclusion criteria: using 3 to 10 different medications daily, no more than 4 specified times each day; able to sort and manage own medications; had a telephone/mobile phone; live in greater Boston area; speak, read, and write English Exclusion criteria: vision or hearing impaired (i.e. unable to hear an alarm), dementia, or other conditions precluding informed consent; awaiting revascularisation, cardiac resynchronisation, or heart transplant; terminal illness Number of participants randomised: 29 (13 vs 16) Number of participants included in analysis: 25 (11 vs 14) Age: mean ± SD = 68.4 ± 11.8 vs 74.4 ± 10.4 Gender: female: 4 (36%) vs 5 (36%) Ethnicity: white: 9 (82%) vs 13 (93%) Number of medications: not specified, but all participants taking min 3 and max 10 different medications/d Frailty/Functional impairment: NYHA functional classification: Class II or higher: 10 (91%) vs 2 (16%) Cognitive impairment: dementia excluded Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ MedSentry Medication Management System: (1) a remotely monitored electronic device (“device”) that alerts participants when it is time to take their medications, and (2) a monitoring centre with advisors who contact participants and caregivers when medications are not taken. The device is installed in the participant’s home, and data are transmitted to the monitoring centre via the Internet. The device is approximately the size of a small microwave oven. The top of the device consists of a series of small, removable bins arranged in a 7 × 4 configuration (7 days of the week and 4 medication times per day). A lid on the top of each bin detects when a bin is opened. The bottom of each bin is clear plastic. Cameras located under the bins transmit an image of the contents to the monitoring centre. First, the device provides a visual cue (blue lights around a bin) and an audio alarm to alert a participant when it is time to take medication. If a dose is not taken within 30 minutes, an advisor at the monitoring centre calls the participant. After 3 attempts over a 45‐minute time span to contact the participant, a voice message is left and a call is placed to an optional caregiver who has agreed to be contacted and to follow up with the participant. Participants were responsible for refilling the device and communicating medication changes to the monitoring centre Group 2 ‐ Usual care Co‐intervention: N/A Provider: MedSentry device (with telephone calls if non‐adherent to device‐administered medication) Where: home When and how often: continuous ‐ 90 days Intervention personalised: alerts based on individual medication regimens ‐ otherwise no |
|
| Outcomes |
Timing of outcome assessment: baseline and 90 days Medication adherence (subjective) : Morisky Medication Adherence: 8‐item questionnaire, scored from 0 to 8. 0 = high adherence, 1 to 2 = medium adherence, 3 or more = low adherence Health‐related quality of life (subjective): Minnesota Living With Heart Failure Questionnaire: 21 items that assess the impact of HF and HF treatment. Responses coded from 0 = does not apply to 5 = very much. Higher scores indicate greater impact (worse) Adverse clinical health outcomes (mixed objective/subjective): all‐cause unplanned hospitalisations and ED visits. Electronic medical records and patient questionnaires |
|
| Notes | Trial registration: NCT01814696 Consumer involvement: not specified Funding source: Presentcare, Inc. Dropout: 3 (2 vs 1) did not complete enrolment, 1 control withdrew, 1 control was excluded for randomisation error. Adherence measure also had missing participants not described (9 vs 13 baseline, 10 vs 12 follow‐up) Further information required: mean/median number of medications (email correspondence with trial author ‐ unsuccessful) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Patients who agreed to participate were randomised during the screening phone call. No further details were provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Missing cases for some comparisons is because of incomplete responses on the closeout questionnaire" 3 did not complete enrolment, 1 withdrew, 1 was excluded for randomisation error |
| Selective reporting (reporting bias) | Low risk | Appears to present results as planned |
| Other bias | Unclear risk | "recruitment was slow and the study was ended early before achieving the original goal of 35 participants per study arm" |
Hanlon 1996.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effects of sustained clinical pharmacist interventions involving elderly outpatients with polypharmacy and their physicians Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: USA Setting: primary care clinic (General Medicine Clinic (GMC) at Veterans Affairs Medical Center) Inclusion criteria: ≥ 65, evidence of polypharmacy (≥ 5), received primary care in GMC Exclusion criteria: living in nursing home. Patients with cognitive impairment eligible only if a caregiver was available to be involved Number of participants randomised: 208 Number of participants included in analysis: 172 (88 intervention vs 84 control) Age: mean ± SD: 69.7 ± 3.5 vs 69.9 ± 4.1 Gender: female: 1.9% vs 0% Ethnicity: % white: 79.0 vs 74.8 Number of medications: Veterans Affairs prescribed medications 7.6 ± 2.8 vs 8.2 ± 2.7 Frailty/Functional impairment: not specified Cognitive impairment: percentage of participants: 7.6% vs 12.6% Comorbidities: mean chronic medical conditions: 9.2 ± 3.7 vs 9.0 ± 3.0 |
|
| Interventions |
Group 1 ‐ Sustained clinical pharmacist involvement: usual and clinical pharmacist care. Pharmacist monitored drug therapy outcomes and medication list and identified DRPs before every scheduled GMC visit by reviewing medical records and meeting with patients/caregivers. Pharmacists provided written recommendations to primary physician. After physician visit, pharmacist educated patients regarding any medication changes. Pharmacist also used compliance‐enhancing strategies and written patient education materials to assist compliance. Clinical pharmacist also reviewed with patients and caregivers general principles of safe medicine use in the elderly and the importance of discussing their medications with physicians Group 2 ‐ Usual care (GMC) ‐ clinic nurse reviewing medications before visit, physician/nurse reviewing medications after visit. Clinical pharmacist neither spoke with nor gave advice to control patients or their physicians during the study period. Written drug therapy recommendations for control patients prepared before randomisation were not discussed nor given to the primary physician but were filed for review at the end of the study Co‐intervention: N/A Provider: pharmacist Where: medical centre When and how often: before/after GMC visits Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: baseline and 12 months Medication adherence (subjective) : self‐reported compliance: proportion of medications for which patients' adherence response agreed with directions for their use on their action profile, obtained during telephone interviews Knowledge about medicines (subjective): self‐report knowledge of 'how they took each analysed medication and what the medication was for', proportion of correct responses Satisfaction with intervention (subjective): Health Care Attitude Questionnaire: 5‐point Likert scales to rate 3 questions on pharmacy‐related healthcare satisfaction: (1) directions received for taking medication, (2) explanation of SEs, (3) numbers and types of drugs they were taking Health‐related quality of life (subjective): assessed via SF‐36 by blinded interviewers Adverse clinical health outcomes (subjective): patients asked if they had or had not had any possible ADEs (side effects, unwanted reactions, or other problems with medications) |
|
| Notes | Trial registration: not specified Consumer involvement: not specified Funding source: National Institute on Aging grant and academic award; supported by Claude D. Pepper Older Americans Independence Center Dropout: lost to follow‐up 36 (17 vs 19) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by a separate blinded clinical pharmacist |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up (19%). Methods state ITT but not done for adherence outcomes? |
| Selective reporting (reporting bias) | Low risk | Appears to be as per methods |
| Other bias | Unclear risk | Physicians not randomised; potential to be differentially influenced. Sample size 100 per group "to detect an effect of 0.4" or 84 per group "to detect an effect size of 0.5" |
Holland 2007.
| Study characteristics | ||
| Methods |
Aim of study: to test whether a drug review and symptom self‐management and lifestyle advice intervention by community pharmacists could reduce hospital admissions or mortality in heart failure patients Study design: RCT (unit of allocation: individual stratified) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: UK Setting: community pharmacy (after discharge from hospital) Inclusion criteria: > 18 years, admitted as an emergency in which HF was an important ongoing clinical condition, prescribed ≥ 2 medications on discharge Exclusion criteria: residential or nursing home, awaiting surgery for heart disease/transplant, had terminal malignancy Number of participants randomised: 339 (169 intervention vs 170 control) Number of participants included in analysis: 291 (148 vs 143) Age: mean ± SD: 77.6 ± 9.0 vs 76.4 ± 9.5 Gender: female: 54 (36.2%) vs 53 (36.7%) Ethnicity: not specified Number of medications: number of prescribed items taken daily: mean ± SD 7.9 ± 2.6 vs 7.7 ± 2.3 Frailty/Functional impairment: not specified Cognitive impairment: abbreviated mental test: 9.2 ± 1.0 vs 9.3 ± 1.0 Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ HeartMed (visits from community pharmacist): community pharmacist received discharge letter and arranged home visit within 2 weeks of discharge to meet with patient or carer. As appropriate, educated about heart failure, drugs, exercise, diet, smoking; provided signs and symptoms daily cards; removed discontinued drugs; fed recommendations back to GP. Intervention delivered in line with advice from British Heart Foundation's booklet "Living With Heart Failure", which was also given to the patient. A follow‐up visit occurred at 6 to 8 weeks to reinforce Group 2 ‐ Usual care Co‐intervention: N/A Provider: pharmacist (community) Where: patient's home When and how often: twice ‐ at 2 weeks and at 6 to 8 weeks post discharge Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (subjective) : Medication Adherence Report Scale (MARS) scores from 5 (very poor adherence) to 25 (perfect adherence). Questionnaires mailed to patients Satisfaction with intervention (subjective): satisfaction questionnaire at 3 months Health‐related quality of life (subjective): EQ‐5D ‐ self‐assessed quality of life: 1 (perfect health) to ‐0.59 (worst imaginable health state) Adverse clinical health outcomes (objective): mortality ‐ number of deaths Adverse clinical health outcomes (objective): emergency admissions ‐ emergency admission data from Hospital Episode Statistics Condition‐specific outcomes (subjective): Minnesota Living With Heart Failure Questionnaire: 21 questions of 0 to 5 giving total score from 0 to 105. Higher scores implying worse condition. Change of 5 points is significant |
|
| Notes | Trial registration: ISRCTN59427925 Consumer involvement: not specified Funding source: British Heart Foundation project grant; Great Yarmouth and Southern Norfolk Primary Care Trusts covered excess treatment costs. Pfizer supported pharmacist training Dropout: 46 (20 vs 26) excluded before intervention, 2 (1 vs 1) lost to follow‐up Fidelity: of 149 intervention patients ‐ 136 received first visit, 119 received second visit, 13 did not receive intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Third party telephone randomisation based on a computer‐generated random allocation sequence. Stratified by New York Heart Association class and recruitment site |
| Allocation concealment (selection bias) | Unclear risk | Participants told allocation after baseline; concealment unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo possible, so participants were told which group they were in |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of failure to complete 6‐month assessments (only 101/169 intervention and 103/170) |
| Selective reporting (reporting bias) | Low risk | As per methods |
| Other bias | Unclear risk | Pharmacist training funded by Pfizer ‐ unclear what, if any, influence Pfizer had on content of training. Of 149 intervention patients ‐ 136 received first visit, 119 received second visit, 13 did not receive intervention |
Khdour 2009.
| Study characteristics | ||
| Methods |
Aim of study: to investigate the impact of a pharmacy‐led disease and medicine management programme (with a strong focus on self‐management) in patients with COPD on clinical and humanistic outcomes Study design: RCT (1 clinic; allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Ireland Setting: outpatient clinic (COPD hospital clinic) Inclusion criteria: confirmed diagnosis of COPD by hospital consultant for ≥ 1 year, FEV1 of 30% to 80% of predicted normal value, > 45 years old Exclusion criteria: CHF, moderate to severe learning difficulties (judged by hospital consultant), attended pulmonary rehab programme in last 6 months, severe mobility problems, terminal illness Number of participants randomised: 173 (86 vs 87) Number of participants included in analysis: 143 (71 vs 72) Age: mean ± SD: 65.63 ± 10.1 intervention vs 67.3 ± 9.2 control Gender: female: 55.8% vs 56.3% Ethnicity: not specified Number of medications: combined prescription and non‐prescription: 8.3 ± 2.9 vs 8.0 ± 3.8 Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: comorbid conditions: n = 41 (47.7%) vs n = 44 (50.5%) |
|
| Interventions |
Group 1 ‐ Pharmacy‐led COPD disease and medicine management programme: preliminary assessment with pharmacist to determine individual needs (data on disease knowledge, smoking, medication adherence, self‐efficacy, exercise, and diet). Intervention pharmacist then discussed drug therapy with consultant and provided education (adherence, inhaler technique, home exercises, management of COPD symptoms). Pharmacist demonstrated techniques and then observed patients carrying out the techniques (a booklet on these techniques was given to take home). Pharmacist provided advice using motivational interviewing technique (e.g. quit smoking) and provided customisable action plan for exacerbations (include advice to GPs about antibiotics). Initial intervention lasted for approximately 1 hour (slightly longer for smokers) Group 2 ‐ Usual care (medical and nursing staff only ‐ no pharmacist involvement) Co‐intervention: N/A Provider: pharmacist Where: outpatient clinic When and how often: baseline and 6 months in person, phone call at 3 and 9 months Intervention personalised: yes ‐ tailored according to preliminary assessment |
|
| Outcomes |
Timing of outcome assessment: baseline and 12 months Medication adherence (subjective) : Morisky Adherence (measures adherence with 4 Yes/No response items: forgetting, carelessness, stopping when feeling better, and stopping when feeling worse); yes = 1, no = 0; high adherence (score 0 to 1) vs low adherence (score 2 to 4) Knowledge about medicines (objective): COPD knowledge questionnaire (validated) ‐ effectiveness of education in helping persons with COPD. 16 T/F questions, correct response = 1, range 0 to 16, higher score = better knowledge Health‐related quality of life (subjective): St George's Respiratory Questionnaire (SGRQ) total score = SGRQ is a 76‐item supervised self‐administered survey; scores for symptoms, activity, and impact to give global view of respiratory health. Scores 0 to 100; high = poor health Adverse clinical health outcomes (objective): ED visits, hospital admissions, and unscheduled GP visits, assessed via questionnaire and computer records for past year |
|
| Notes | Trial registration: not specified Consumer involvement: not specified Funding source: Chest Heart and Stroke (N Ireland) financial support Dropout: 13 (7 vs 6) withdrew, 8 died (3 vs 5), 9 were lost to follow‐up (5 vs 4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation carried out via the minimisation method (see reference). Groups matched as closely as possible |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and research staff unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research pharmacist not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up described and balanced |
| Selective reporting (reporting bias) | Low risk | Reported results for all outcome measures listed in methods section |
| Other bias | Unclear risk | Sample size based on SQRG ‐ aimed for 180 patients (90 vs 90) ‐ not reached |
Krska 2001.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effects of pharmacist‐led medication review on outcomes such as presence of pharmaceutical care issues (PCIs), hospitalisation, medication costs, and HRQoL Study design: RCT (6 general medical clinics; individual patients randomised) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Scotland Setting: primary care clinic (general medical practices) Inclusion criteria: ≥ 65 years, ≥ 4 medications (via computerised repeat prescribing system), ≥ 2 chronic conditions (Note: a maximum of 70 patients from each practice were invited to participate) Exclusion criteria: dementia, GP considered patient unable to cope with study Number of participants randomised: 381 Number of participants included in analysis: 332 (168 and 164) Age: mean ± SD (range): 74.8 ± 6.2 (65 to 90) intervention vs 75.2 ± 6.6 (65 to 93) control Gender: female: 95 (56.5%) vs 106 (64.6%) Ethnicity: not specified Number of medications: medications actually being taken (prescription and non‐prescription): mean ± SD (range) 7.3 ± 2.7 (3 to 16) vs 7.6 ± 2.7 (3 to 17) Frailty/Functional impairment: not specified Cognitive impairment: dementia excluded Comorbidities: chronic diseases: 3.9 ± 1.4 (2 to 8) vs 3.8 ± 1.4 (2 to 9) |
|
| Interventions |
Group 1 ‐ Pharmacist medication review and pharmaceutical care planning: pharmaceutical care plan drawn up via medical notes and home interview (actual and potential PCIs, actions planned, desired outputs). Copies of plan put in medical notes and given to GP. GP asked to indicate level of agreement with each PCI identified and with actions. Pharmacist then implemented agreed actions Group 2 ‐ Interviewed and PCIs identified: no pharmaceutical care plan written or implemented, just usual care (but if serious PCI identified, independent medical assessor decided whether to withdraw patient, n = 1) Co‐intervention: patients interviewed at home about medications, health services, SF‐36, medication costs Provider: pharmacist Where: GP practice/Home When and how often: 1 home visit Intervention personalised: Yes ‐ individualised care plan |
|
| Outcomes |
Timing of outcome assessment: baseline and 3 months Medication adherence (subjective): potential/actual compliance ‐ pharmacist review identified pharmaceutical care issues, including potential or actual compliance issues. Results as total number of issues at baseline and number resolved at 3 months Health‐related quality of life (subjective): SF‐36 (data not reported in paper) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Grampians Healthcare NHS Trust Dropout: 24 and 25 (excluded after randomisation ‐ hospital, ill health, holidays), 1 withdrew |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After stratification by number of drugs, number of CV drugs and presence of NSAIDs, patients allocated randomly to intervention or control |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal explained. No differences in demography or medicine use between groups |
| Selective reporting (reporting bias) | High risk | Not all results listed (e.g. HRQoL just says not significant) |
| Other bias | Low risk | None apparent |
Lee 2006.
| Study characteristics | ||
| Methods |
Aim of study: to test the efficacy of a comprehensive pharmacy care programme to improve medication adherence and its associated effects on BP and LDL‐C Study design: RCT (multi‐phase prospective study with observational and RCT components) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: outpatient pharmacy clinics (outpatient medicine service of Army Medical Centre and Armed Forces Retirement Home (independently living military healthcare beneficiaries)) Inclusion criteria: ≥ 65 years, ≥ 4 long‐term medications daily, at increased risk for non‐adherence Exclusion criteria: not living independently (assisted living or nursing home residents excluded), had serious medical condition with unlikely 1‐year survival Number of participants randomised: 159 (83 vs 76) enrolled in RCT phase Number of participants included in analysis: 159 (83 vs 76) Age: mean SD 77 ± 10.5 vs 78 ± 6.2 Gender: female: 21 (25.3%) vs 20 (26.3%) Ethnicity: white: 51 (61.4%) vs 43 (56.6%), black: 29 (34.9%) vs 31 (40.8%) Number of medications: long‐term medications: 9.1 ± 3.2 vs 8.3 ± 2.8 Frailty/Functional impairment: not specified Cognitive impairment: taking medication for memory problems: 6 (3.8%) vs 2 (1.3%) Comorbidities: ≥ 4 health problems: 52 (62.7%) vs 38 (50%) |
|
| Interventions |
Group 1 ‐ Comprehensive pharmacy care programme: clinical pharmacist meeting every 2 months; medications continued to be blister‐packed (phase 2) Group 2 ‐ Return to usual care (no adherence aid, new pill bottles with 90‐day supply and 1 refill prescription given) Co‐intervention: run‐in: (months 1 and 2) = baseline data collection (adherence, BP, LDL‐C); phase 1 (months 3 to 8): prospective observational study of comprehensive pharmacy care programme including individualised medication education, medications dispensed via adherence aid, and regular follow‐up with clinical pharmacist every 2 months Provider: pharmacist Where: pharmacy clinics at outpatient medical centre and retirement home When and how often: 2 monthly clinical pharmacist follow‐ups Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: baseline (end of phase 1; 8 months) and conclusion (end of phase 2; 14 months) Medication adherence (objective):pill count adherence: sustained mean medication adherence Condition‐specific outcomes (objective): blood pressure: change in systolic and diastolic blood pressure ‐ mmHg Condition‐specific outcomes (objective): LDL cholesterol: change in LDL‐C ‐ mg/dL |
|
| Notes | Trial registration: NCT00393419 Consumer involvement: not specified Funding source: competitive junior investigator grant from American Society of Health‐System Pharmacists Research and Education Foundation Dropout: 13 lost to follow‐up (6 and 7), last observation carried forward |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in 1:1 ratio via a computer‐generated random number sequence. Patients randomised in blocks based on level of baseline medication adherence (above or below 55%) |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed to both patients and study personnel and was revealed at end of phase 1 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible to blind pharmacists assessing outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure 1 shows participant flow, last observation carried forward for analysis |
| Selective reporting (reporting bias) | Unclear risk | Results at baseline not split based on intervention or control; hard to compare intervention effect |
| Other bias | Low risk | None apparent |
Lim 2004.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the impact of a pharmacist consult clinic on health‐related outcomes of elderly outpatients in a local setting Study design: RCT (randomised in blocks of 2 participants) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Singapore Setting: outpatient clinic (geriatric medicine hospital outpatient clinic) Inclusion criteria: participants who required drug therapy monitoring, evidence of polypharmacy (> 3 regular meds or > 9 doses per day), documented non‐compliance, self‐administered drugs that require psychomotor skill and co‐ordination, on nasogastric tube feeding, > 1 doctor managing care OR hospitalised within last 6 months Exclusion criteria: stable on follow‐up, cognitive impairment and no caregiver to participate, life expectancy < 6 months, medications supervised by other healthcare personnel Number of participants randomised: 136 (68 and 68) Number of participants included in analysis: 126 (64 and 62) Age: mean ± SD: 79.6 ± 7.7 vs 80.5 ± 8.1 Gender: female: 60.9% vs 69.4% Ethnicity: Chinese 73.4% vs 83.9%, Malay 6.3% vs 6.5%, Indian 12.5% vs 6.5%, Other 7.8% vs 3.2% Number of medications: regularly scheduled medicines: median (range): 6 (3 to 16) vs 7 (3 to 10) Frailty/Functional impairment: ADL independent: 50.8 % vs 40.3% Cognitive impairment: impaired cognition: 20.3% vs 21.0% Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Pharmacist consult in clinic (10 to 30 minutes) ‐ evaluate patients for MRPs by reviewing medical records and medication list and by interviewing patient and caregiver. Recommendations to simplify, reduce ADEs, decrease cost, etc., discussed with primary physician, and accepted recommendations implemented. Pharmacist also counselled on medication knowledge, administration, etc. Group 2: assumed usual care Co‐intervention: N/A Provider: pharmacist Where: outpatient clinic When and how often: once at baseline Intervention personalised: yes ‐ Individualised based on medications and MRPs |
|
| Outcomes |
Timing of outcome assessment: baseline and 2 months Medication adherence (subjective) : self‐reported compliance: patients asked if they 'forgot to take medication as directed'. Then categorised as compliant or not. Participants then classified as least compliant (compliant at base, not at 2 months), not compliant (not compliant at base or 2 months), compliant (compliant at 2 months) Knowledge about medicines (objective): composite % knowledge of dose (D), frequency (F), and indication (I), reported as percentage correct. Adverse clinical health outcomes (subjective): reported ADRs. Asking patients if they experience side effects or unwanted reactions with their medications. Patients asked to name medication involved; this was assessed by primary care physician to ascertain whether symptoms were indeed ADRs of the implicated medicine |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: National Healthcare Group research grant NHG‐RPR/01027 Dropout: 10 excluded before intervention (4 and 6), 9 withdrew (5 and 4), 17 lost‐to follow up (8 and 9) Further information required: raw data on adherence and medication knowledge at follow‐up (email correspondence with trial author successful, but further data not available) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned via computer‐generated numbers in blocks of 2 |
| Allocation concealment (selection bias) | Unclear risk | Randomisation carried out before consent (Zelen design) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Figure 2 shows study profile; ITT concluded patients only |
| Selective reporting (reporting bias) | High risk | Raw values for outcomes not listed; 90% CI?; no sample size calculation |
| Other bias | Low risk | None apparent. Sample size 60/arm "to achieve a power of 80% to detect a 10% difference between the 2 groups in the knowledge outcome" |
Lingler 2016.
| Study characteristics | ||
| Methods |
Aim of study: to develop and examine the efficacy of a tailored, problem‐solving intervention on informal caregivers' management of medications for community‐dwelling persons with memory loss Study design: RCT (unit of allocation: dyad) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carers (recruited as dyads) Geographic location: USA Setting: participant's home Inclusion criteria: PATIENT: self‐ or caregiver‐reported memory loss necessitating help with medication‐taking, ≥ 2 comorbid conditions requiring medication, living in community, provided informed consent. INFORMAL CAREGIVERS: family members or kin‐like friends, ≥ 18 years, participating in management of patient's medications, exhibiting ≥ 1 deficiency on any of 3 measures of ability to effectively manage the patient's medications, living within 75 miles of the University Exclusion criteria: paid caregivers, living in residential care setting Number of participants randomised: 83 pairs (42 vs 41) Number of participants included in analysis: 76 pairs (37 and 39) Age: mean ± SD: patients 79.67 ± 9.19 vs 80.15 ± 8.48, caregivers 66.00 ± 12.8 vs 67.80 ± 11.2 Gender: female = patients 28 (67%) vs 22 (54%), caregivers 29 (69%) vs 29 (71%) Ethnicity: white: patients 34 (81%) vs 37 (90%), caregivers 34 (81%) vs 37 (90%); black: patients 3 (7%) vs 3 (7%), caregivers 4 (10%) vs 3 (7%); other patients 5 (12%) vs 1 (2%), caregivers 4 (9%) vs 1 (2%) Number of medications: total medications (including OTC, supplements, etc.): 10.79 ± 5.52 vs 10.61 ± 5.89 Frailty/Functional impairment: not specified Cognitive impairment: baseline sample (n = 91) patients: MMSE = 17.62, carer blessed = 2.97 Comorbidities: number of comorbidities: patients 8.691 ± 3.57 vs 9.024 ± 4.21, carers 7.86 ± 3.69 vs 6.44 ± 3.59 |
|
| Interventions |
Group 1 ‐ Maximising Medication Management by Caregivers of Persons With Memory Loss: guided by intervention manual; sessions with nurse or social worker interventionist addressed 7 basic aspects of the caregiver's role in managing medications during home/telephone discussions. Caregivers provided with self‐study version of intervention manual. Initial 8‐week intervention, then 4 bi‐weekly calls over next 8 weeks (note: mean length of home visits ‐ 40.05 minutes (SD 13.22) ‐ and telephone sessions ‐ 13.42 minutes (SD 6.34)) Group 2‐ Usual care: at baseline, received pamphlet on medication safety. Received home visits for purpose of data collection only (medication errors were corrected). At completion of study, caregivers received intervention manual Co‐intervention: for safety, if any errors were noted during medication reconciliation, they were brought to the attention of both caregivers and prescribers regardless of group assignment. In addition, all participants received care as usual from their healthcare providers Provider: nurse or social worker Where: patient's home When and how often: 2 or 3 home visits 2 weeks apart, followed by 2 or 3 telephone sessions 7 to 10 days apart for 8 weeks; then 4 bi‐weekly phone calls for 8 weeks Intervention personalised: Yes ‐ guided by intervention manual but individualised based on caregivers needs/queries and patients medication |
|
| Outcomes |
Timing of outcome assessment: baseline and 2 months post intervention Medication‐taking ability (objective): MedMaIDE: Medication Management Instrument for Deficiencies in the Elderly: uses interview and observation to assess ability to self‐administer medications using 3 areas: knowledge of medications, how to take medications, and how to procure medications. Each medication is reviewed during administration. Scores 0 to 13; max total deficiency score is 13 Satisfaction with intervention (subjective): set of Likert scaled questions (not specified), eliciting open‐ended comments during an exit interview at study completion Other (objective): Medication Deficiency Checklist (MDC): 15‐item, investigator‐developed instrument; uses caregiver interviews to assess for the presence of errors and problems (e.g. taking at the wrong time). Investigator‐developed tool ‐ not validated |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: programme project grant: NIH/NINR P01 NR010949 Dropout: 3 pairs withdrew (2 vs 1), 4 pairs were lost to follow‐up (3 vs 1) Fidelity: good ‐ independent rater randomly selected 10% of cases for an audit of protocol fidelity. Percentage of agreement 91.6%, quality of interaction 4.5/5 Unpublished data included successful communication with trial authors; follow‐up MedMAIDE results provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random group assignments were computer generated with permuted blocks within strata to ensure balance of nurse/social worker, relationship of caregiver, and race/ethnicity |
| Allocation concealment (selection bias) | Unclear risk | Order of consent/allocation not specified; no details on allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded ‐ necessary because it was not feasible to blind participating caregivers to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding specified; unclear who did outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal loss to follow‐up; clearly detailed. Erlen paper: includes instances where data are missing, so number of participants may be different. We did not impute data for these participants |
| Selective reporting (reporting bias) | Unclear risk | MedMaIDE results presented only in visual format; raw results not included in results text |
| Other bias | Low risk | None apparent |
Lipton 1994.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate whether the intervention would (1) enhance patients' compliance with drug regimens, (2) reduce polypharmacy, (3) lower healthcare expenditures for physician visits, ED visits, and hospitalisations, and (4) reduce hospital re‐admission rates Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: USA Setting: hospital discharge (in hospital and post discharge via telephone or face‐to‐face in hospital or at home) Inclusion criteria: ≥ 65 years, covered by Medicare, admitted to non‐psychiatric ward, residing within 35 miles, English‐speaking (or proxy), mentally competent (or proxy), access to telephone, discharged not to nursing home or hospice, ≥ 3 medications taken for chronic conditions at hospital discharge Number of participants randomised: 719 (not clearly stated; 52% of 1383 eligible) Number of participants included in analysis: 706 (350 vs 356) Age: mean: 74.6 vs 74.4 Gender: not specified Ethnicity: not specified Number of medications: long‐term medications at second compliance assessment: 5.16 ± 2.62 vs 6.75 ± 2.92 Frailty/Functional impairment: at least 1 sensory deficit: 29% vs 32% Cognitive impairment: those not mentally competent had to have proxy Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Clinical pharmacist review and face‐to‐face consultations: pharmacists review hospital records and drug regimens, then conduct face‐to‐face consultation with patient. Post‐discharge consultations at 1 week, 2 to 4 weeks, 2 months, and 3 months post discharge, via telephone or in pharmacy at hospital or in home. Medication regimen simplification by discussion with physician for prescription, or with patient for non‐prescription Group 2: usual care Co‐intervention: both groups: booklets given at discharge to record medication information (e.g. drug purpose, dosage, schedule) Provider: pharmacist (clinical hospital pharmacist) Where: in hospital + hospital, home or telephone When and how often: baseline, post‐discharge consultations at 1 week, 2 to 4 weeks, 2 months, and 3 months post discharge Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 6 to 8 weeks and 12 to 14 weeks post discharge Medication adherence (subjective) : structured telephone interviews with a subsample. Only data for antiarrhythmics, antihypertensives, anticoagulants, cardiac anticonvulsants, antidiabetic NSAIDs, respiratory and GI drugs collected, and only for first 3 medications mentioned by patient. Adherence asked for the first 3 such medications mentioned by patient. 4 behavioural questions (excluding purpose) ‐ calculated as total compliance score out of 100. Perfect compliance = 100. Results as (1) mean compliance scores (SD), (2) mean proportion with perfect (100%) scores Cost‐effectiveness (objective): Medicare Part B charges: total days in hospital, total hospital inpatient charges |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: John A. Hartford Foundation (NYC) Dropout: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients drew a folded slip of paper from a box containing equal numbers of experimental and control‐designated slips |
| Allocation concealment (selection bias) | Low risk | Patients drew a folded slip of paper from a box containing equal numbers of experimental and control‐designated slips |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers not study pharmacists; interviews conducted by investigators. Both interviewers were blinded to study group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Subgroup selected for substudy on adherence; no details on how selected. States no demographic differences but demographics not listed. 274 selected for interview, but only 206 completed second assessment (25% attrition) |
| Selective reporting (reporting bias) | Unclear risk | Hospital re‐admission data not clear; compliance reported ‐ but additional analyses that looked at compliance (excluding purpose) not specified in methods |
| Other bias | High risk | Adherence measure only for first 3 medications mentioned by patient ‐ patients may have preferentially selected medications they were more familiar with (thus more adherent with) |
Lopez Cabezas 2006.
| Study characteristics | ||
| Methods |
Aim of study: to assess the efficacy of multi‐factorial educational intervention carried out by a pharmacist in patients with heart failure Study design: RCT (2 hospitals; allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Spain Setting: hospital discharge Inclusion criteria: patients admitted for definite heart failure (Framingham criteria ‐ 2 major or 1 major + 2 minor criteria met simultaneously) Falces 2008 describes subgroup > 70 years Exclusion criteria: living out of the area of influence of the hospital, living in old people's home, moved to a social‐health centre or other centre for acute patients, suffering any type of dementia or psychiatric disease, refusing participation Number of participants randomised: 134 (70 and 64), subgroup > 70 years: 103 (53 vs 50) Number of participants included in analysis: 134 (70 and 64), except adherence 63 (40 vs 23), subgroup > 70 years: 82 (45 vs 37) Age: 75.3 ± 8.4 vs 76.1 ± 9.4, subgroup > 70 years: 79.0 ± 4.9 vs 80.1 ± 5.5 Gender: female: 41 (58.6%) vs 34 (53.1%), subgroup > 70 years: 60.4% vs 56% Ethnicity: not specified Number of medications: type not specified, 7.1 ± 3.0 vs 7.1 ± 2.5, subgroup > 70 years: 7.5 ± 3.1 intervention vs 7.0 ± 2.1 control Frailty/Functional impairment: New York Heart Association Functional Classification: I to II: 58 (84.1%) vs 54 (87.1%) Cognitive impairment: dementia excluded Comorbidities: total not reported |
|
| Interventions |
Group 1 ‐ Active information programme: active information programme (run by a pharmacist from the research team) consisted of a personal interview at the time of discharge and subsequent telephone reinforcement. Intervention included information about the disease, diet education, and information about the medications. Simple language was used, adapted to the cultural level of patients, with support of audiovisual and written didactic materials. Monthly during first 6 months of follow‐up, and subsequently every 2 months, patients were called to reinforce the intervention and to solve doubts or problems that may have arisen Group 2 ‐ Usual care Co‐intervention: N/A Provider: pharmacist Where: in person at discharge; telephone to home When and how often: discharge, monthly follow‐up for 6 months, then 2‐monthly follow‐up for 6 months Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 12 months Medication adherence (objective) : pill count/tablet accountability: % of reliable patients (95% to 100% compliance) Satisfaction with intervention (subjective): Catalan Health Department satisfaction survey, asking patients about care and information received and asking them to provide a global score from 0 to 10 Health‐related quality of life (subjective): EuroQol, validated in Spanish and in Catalan Adverse clinical health outcomes (objective): hospital re‐admissions, percentage of patients with re‐admission, subgroup > 70 years: mortality Adverse clinical health outcomes (objective): number of deaths Cost‐effectiveness (objective): financial evaluation: hospitalisation costs calculated for both groups, with intervention direct costs, delivered materials, and time spent by the pharmacist added in |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Health Research Fund and European Regional Development Fund Dropout: 12 months of data for adherence only available for 40 and 23 patients, subgroup > 70 years: 20 died (7 vs 13) Language translation: yes ‐ Falces 2008 was translated to English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generation of the randomisation sequence was the responsibility of the clinical epidemiology unit. Randomisation lists were generated by software and in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | Allocation controlled by admissions department; recruitment carried out by cardiology department |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. Neither the physician nor the nurse responsible for the patient knew the allocation until the educational intervention on the day of discharge |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Compliance assessed by pharmacist. Pharmacist responsible for active info programme knew allocation; this could have generated contamination problems |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No mention of reason for attrition; only 47% completed 12‐month compliance Falces paper: compliance data available for only 49 patients (59%). Methods stated that those who did not attend follow‐up were to be considered non‐compliant, but results were not presented this way. P value figure not listed |
| Selective reporting (reporting bias) | High risk | Reported only 'reliable patients' ‐ but compliance had 3 levels: reliable, partially reliable, not reliable. Financial evaluation not a planned outcome Falces paper: no reasons given for chosen variables in hazard ratio calculation |
| Other bias | Unclear risk | Sample size calculation: 67/group not reached; 3 years from final data to publication |
Manning 2007.
| Study characteristics | ||
| Methods |
Aim of study: to determine whether the 3D tool is better than the Medication Discharge Worksheet in terms of patient satisfaction, understanding, and safety Study design: RCT (exploratory RCT; 4 medical units; individual allocation) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: hospital discharge Inclusion criteria: > 20 years, ≥ 3 discharge medications, returning to self‐care at home (or to care of a relative) Exclusion criteria: discharge to nursing home, hospital, or assisted living facility; unable to speak or read English; unable to hear over the telephone to participate in follow‐up; pregnant Number of participants randomised: 337 Number of participants included in analysis: 138 (78 and 60) Age: mean ± SD 68.1 ± 5.65 intervention vs 67.6 ± 13.06 control (total range 24 to 100) Gender: unclear: % or mean (SD): Table 1: 0.51 ± 0.50 vs 0.38 ± 0.49 Ethnicity: not specified Number of medications: not specified (discharge medications): 10.0 ± 4.42 vs 8.7 ± 3.93 (total range 4 to 31) Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ 3D (durable display at discharge) medication discharge education tool: 3D tool including purpose, time to take medications, comments and cautions, and space for durable display (patients encouraged to affix tablet/capsule of each medication onto the 3D tool in column labelled "Display"). Plus a section for "home medications you should no longer take". Participants randomised to 3D upon returning home and after filling any new prescriptions were encouraged to affix (with clear adhesive tape) a tablet or capsule of each medication onto the 3D adjacent to the medication name and under the column labelled "Display" Group 2 ‐ Usual care ‐ medication discharge worksheet (MDW) Co‐intervention: before hospital dismissal, the primary nurse conducted her/his usual patient education session including usage of either MDW or 3D (per randomisation) Provider: 3D medication sheets (generated by study recruiter, reviewed by principal investigator or pharmacist co‐investigator. Nurse provided patient education) Where: hospital discharge When and how often: once Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 7 to 14 days after discharge Medication taking ability (subjective) : self‐reported safety in taking medications: "since discharge, how many mistakes have you made taking your medications (score 0‐4)?" Knowledge about medicines (objective): assessment of knowledge of indication, dosage frequency, and special comments or cautions: 0 (for no correct responses) to 3 (all correct responses) Satisfaction with intervention (subjective): how satisfied were you with the form you received from the nurse when she/he was talking to you about your medications? 5‐point Likert scale: 1 (low) to 5 (high) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Mayo Clinic Rochester MIDAS Grant; Mayo Foundation for Education and Research, small grants programme Dropout: 38 (did not remember form ‐ so were not interviewed), 126 lost to follow‐up (93 could not be reached, 12 excluded post discharge, 4 could not hear during call, 5 incorrect phone number, 2 did not receive MDW, 5 too ill, 4 refused, 1 no English) Fidelity: compliance with affixing medications to 3D is uncertain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number algorithm |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant conducting follow‐up call was blinded to both study hypotheses and participant randomisation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up high. 176/337 randomised were contacted by telephone, 38 did not remember the tool so were excluded from analysis, only 41% of randomised patients (138/337) were included in analysis. No statistically significant differences in patient loss at each level |
| Selective reporting (reporting bias) | Low risk | Hypotheses and outcomes listed as per methods |
| Other bias | Unclear risk | "Patient compliance with affixing medications to 3D is uncertain" ‐ "analysed on intention‐to‐influence basis with knowledge that any non‐compliance might diminish the apparent 3D benefit" |
Marek 2013.
| Study characteristics | ||
| Methods |
Aim of study: the purpose of this study was to evaluate health status outcomes of frail older adults receiving a home‐based nurse support programme that emphasised self‐management of medications using both care co‐ordination and technology Study design: RCT (3 home healthcare agencies, individual allocation) Number of arms/groups: 3 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: discharge from home health care Inclusion criteria: ≥ 60, Medicare primary payer, impaired ability to manage medications and/or impaired cognitive functioning, working telephone and electricity Exclusion criteria: terminal diagnosis or hospice care that would make attrition likely, use of other device for medications (e.g. pager) Number of participants randomised: 456 Number of participants included in analysis: 414 (152, 137, 125); completed 12‐month follow‐up: 301 (98, 102, 101) Age: mean ± SD: MD.2: 79.6 ± 7.92 vs Planner: 79.6 ± 7.64 vs Control: 78.2 ± 7.25 Gender: n (%) female: 104 (68.4) vs 93 (67.9) vs 77 (61.6) Ethnicity: n (%): white: 124 (81.6) vs 114 (83.2) vs 113 (90.4); black: 28 (18.4) vs 22 (16.1) vs 12 (9.6); Hispanic: 2 (1.3) vs 6 (4.4) vs 3 (2.4) Number of medications: all medications: mean ± SD: 11.01 ± 4.466; range 2 to 27 (as listed in Lancaster 2014) Frailty/Functional impairment: physical performance test: 14.6 ± 5.06 vs 14.2 ± 5.16 vs 15.8 ± 6.14 Cognitive impairment: MMSE: 25.5 ± 3.33 vs 25.0 ± 3.65 vs 26.3 ± 3.17 Comorbidities: total comorbidities not listed |
|
| Interventions | Both groups 1 and 2: received nurse care co‐ordination ‐ education, tools for participants to manage their chronic conditions, enhanced communication with health professionals, monitoring signs and symptoms of disease. Nurse visited at least every 2 weeks + additional visits if change in medication or if hospitalised Group 1MD.2: medication‐dispensing machine (releases preloaded medication in plastic reusable cups; at pre‐programmed intervals, user presses large red button and a plastic cup containing medications in a chute. Audible and visual prompt for 45 minutes; if medication not taken, then notification to identified responder, e.g. family member, nurse) Group 2Medplanner: Medplanner (simple weekly medication box). Nurses filled Medplanners and recorded number of medications remaining in the Medplanners before refilling. Group 3Usual care Co‐intervention: each participant received a pharmacy screen on admission (pharmacist and advanced practice nurse), which was sent to prescribing provider(s). Main purpose was to ensure medications were not harmful Provider: nurse care co‐ordinators + medication device Where: in home When and how often: 12 months: contact minimum every 2 weeks as per intervention Intervention personalised: individualised |
|
| Outcomes |
Timing of outcome assessment: monthly for 12 months Medication adherence (objective) : percentage correct doses/month: average percentage of correct doses per month in 2 intervention groups. Machine recorded medication doses or nurse counted medications left in planner Health‐related quality of life (subjective): HRQoL: quarterly improvement in SF‐36 |
|
| Notes | Trial registration: NCT01321853 Consumer involvement: not specified Funding source: National Institute of Nursing Research & University of Wisconsin‐Milwaukee Self‐Management Science Center Dropout: excluded before baseline (22, 17, 3), did not receive intervention (22, 11, 0), lost to follow‐up (32, 24, 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer programme developed by a study statistician |
| Allocation concealment (selection bias) | Low risk | Randomised before staff contacted potential patients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind providers or patients. Higher attrition rate from MD.2 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible to blind providers and data collection. Research data collectors, however, did not deliver the intervention, and interrater reliability among data collectors was monitored closely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysed based on intention‐to‐treat. Only 72.7% completed 12‐month follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Adherence results not reported clearly; no MD.2 vs control for HRQoL |
| Other bias | Unclear risk | Sample size 100 per group reached (but close) ‐ noted actually completed numbers 98 vs 102 vs 101 |
Marusic 2013.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effect of hospital pharmacotherapeutic counselling on rates and causes of 30‐day post‐discharge hospital re‐admissions and ED visits Study design: RCT (individual allocation) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Croatia Setting: hospital discharge Inclusion criteria: ≥ 65 years, hospital discharge to community with prescription for ≥ 2 medications for treatment of chronic disease Exclusion criteria: cognitive or perceptual problems, diagnosis of terminal illness with life expectancy < 1 month, discharge to long‐term care facility, inability to be followed up Number of participants randomised: 160 Number of participants included in analysis: 160 (80 and 80) Age: mean ± SD (range): 74.0 ± 6.7 (65 to 88) vs 73.9 ± 5.5 (65 to 87) Gender: female n (%): 43 (53.8%) vs 47 (58.8%) Ethnicity: not specified Number of medications: prescribed medication mean ± SD (range): 6.6 ± 2.4 (2 to 13) vs 6.2 ± 2.6 (2 to 13) Frailty/Functional impairment: not specified Cognitive impairment: cognition problems excluded Comorbidities: number of discharge diagnoses: 4.4 ± 1.6 (1 to 8) vs 3.9 ± 1.5 (2 to 8) |
|
| Interventions |
Group 1 ‐ Pharmacotherapeutic discharge counselling: pre‐discharge counselling (30 minutes) by qualified physician, specialist in clinical pharmacology, provided within 24 hours before discharge. Counselling included indications, dosage and admin times, importance of compliance, possible consequences of non‐compliance, possible ADRs Group 2 ‐ Usual care (including discharge letter to be handed to GP) Co‐intervention: N/A Provider: physician (specialist in clinical pharmacology) Where: hospital When and how often: once within 24 hours of discharge Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 30 days Medication adherence (objective) : pill count ‐ patients asked to bring all remaining medications and empty packaging to follow‐up visit. Compliance = total number of doses taken by the patient since discharge/total number of doses to be taken since discharge × 100. Reported as percentage of participants who are compliant (80% to 110%). If participants could not attend hospital, then visit was arranged at home Adverse clinical health outcomes (objective): hospital re‐admission/ED visit: number of patients with re‐admission or ED visit Adverse clinical health outcomes (objective): number of patients with ADRs: the probability that an ADR was drug related was estimated using the Naranjo ADR probability scale. ADRs that were fatal or life threatening or required hospital admission were considered serious ADRs |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: no external funding Dropout: nil mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Manual shuffle of 80 intervention and 80 control cards in envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed, unmarked envelope contained card with 'intervention' or 'control'. Unclear if opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and physicians were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by research assistant blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Reported as per methods |
| Other bias | Low risk | Sample size 80/group |
Messerli 2016.
| Study characteristics | ||
| Methods |
Aim of study: to investigate the impact of the polymedication check (PMC) on patients on polypharmacy Study design: RCT (individual allocation) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Switzerland Setting: community pharmacy Inclusion criteria: > 18 years, ≥ 4 prescribed drugs over ≥ 3 months Exclusion criteria: living in retirement home, prior PMC, receiving weekly dosing aids filled by the pharmacy or another person, cognitive impairment, move or death, insufficient knowledge of written and spoken German or French Number of participants randomised: 450 (218, 232) Number of participants included in analysis: 372 completed; 450 in analysis Age: mean ± SD 67.2 ± 11.52 vs 67.1 ± 11.56 Gender: female: 118 (54.1%) vs 125 (53.9%) Ethnicity: not specified. Number of medications: long‐term oral medications (excluding on‐demand and self‐medication): 6.8 ± 2.92 (range 1 to 19) Frailty/Functional impairment: Disabilities of the Arm, Shoulder and Hand (DASH‐4) score: 4.9 ± 2.01 vs 4.9 ± 1.83 Cognitive impairment: cognitive impairment excluded Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Polymedication check (PMC): face‐to‐face counselling with pharmacist. Pharmacist screened all meds, checked for knowledge gaps and pharmaceutical care issues (e.g. handling, adherence). Pharmacist documented all resulting interventions (e.g. GP consultations, implementation of weekly dose reminder systems). Education and medication plan could also be provided when necessary. PMC occurred at T0 and T28 (28 weeks = study end) Group 2 ‐ Usual care: no intervention or T0 documentation. Did receive PMC at 28 weeks (study end) Co‐intervention: N/A Provider: pharmacist (appropriately trained) Where: community pharmacy (separate area, i.e. consulting room) When and how often: T0 (intervention) and T28 (both) Intervention personalised: yes ‐ personalised because medication specific |
|
| Outcomes |
Timing of outcome assessment: baseline (200 days before T0) and 28 weeks (T0 = T28 = 196 days) Medication adherence (objective) : medication possession ratio (MPR) ‐ calculated by dividing the days supply of a medication dispensed by the number of days in the time interval of interest Knowledge about medicines (objective): knowledge of medicines and daily use ‐ phone questionnaire; 58 questions ‐ included assessing knowledge Adverse clinical health outcomes (subjective): GP/Hospital visits: self‐reported patient's unplanned visits at the general practitioner or hospital |
|
| Notes | Trial registration: NCT01739816 Consumer involvement: unclear ‐ the PMC (polymedication check) is standardised so potential consumers can be involved in the original development of the Swiss PMC Funding source: investigator initiated project; funded in part by Swiss Pharmacists Association, pharmaSuisse Dropout: 18 withdrew, 60 were lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned by 2 × 4 block randomisation to intervention or control group. Initially, each study pharmacist received 2 blocks containing 8 dossiers (4 intervention and 4 control), each packed in sealed and unlabelled envelopes. Unclear if envelopes opaque |
| Allocation concealment (selection bias) | Unclear risk | Once the first patient had consented, the study pharmacist opened 1 envelope out of the first block to reveal which arm of the study the patient had been randomised to. Once all 8 envelopes of block No. 1 had been assigned, the next block was used. Upon request, further blocks were available. Pharmacist would know allocation of some (e.g. if already opened 4 intervention, then would know remaining were control) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind; Hawthorne effect |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients filled out questionnaire, sealed in envelope, and returned to pharmacy. Interviewers blinded to intervention and without knowledge of the content of the PMC or the patient questionnaire at T0 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Table 2 summarises reasons for dropout: 34 lost because pharmacist revoked study participation because underestimated time commitment. Missing patients from both T0 and T28 analyses unexplained |
| Selective reporting (reporting bias) | High risk | MPR for antiplatelets and PPI listed, not mentioned in methods; no results presented for medication knowledge |
| Other bias | Unclear risk | Sample size calculation: 780 at T0 and 252 at T28 (not reached for adherence). Also study pharmacists received compensation for delivery of each complete patient data set, and patients paid for time spent on telephones |
Moral 2015.
| Study characteristics | ||
| Methods |
Aim of study: to determine whether a face‐to‐face communicative strategy based on motivational interviewing (MI), used by health practitioners (family physicians and nurses) in a primary care setting, and aimed at patients over 65 years old with a chronic disease who are being treated by polypharmacy and who have poor medication adherence can achieve better results than the usual approach based on an informative model of providing education and advice Study design: cluster‐RCT (2 arms, 16 health centres, stratified by professional) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Spain Setting: primary care clinic (health centre) Inclusion criteria: > 65, chronic disease, polypharmacy (≥ 5 medicines or ≥ 12 daily doses for a period ≥ 6 months), high probability for non‐adherence (Haynes‐Sackett yes, inconsistent answers to at least 1 of the 4 Morisky‐Green Qs) Exclusion criteria: serious psychiatric and neurological diseases, difficulties coping with basic daily activities (Barthel Index < 60), those who had cognitive impairment (Pfeiffer's test), those admitted to hospital at least twice in last year, patients under carer's supervision Number of participants randomised: 32 (16 and 16) health professionals, 70 vs 84 patients Number of participants included in analysis: 66 vs 81 (but included in analysis 70 vs 84) Age: 75.6 ± 5.9 vs 76.1 ± 5.8 Gender: female: 49 (70%) vs 57 (67.9%) Ethnicity: not specified Number of medications: medication consumption: 8.7 ± 2.5 vs 9.0 ± 3.1 Frailty/Functional impairment: not specified; < 60 Barthel Index ADLs excluded Cognitive impairment: cognitive impairment excluded Comorbidities: mean ± SD: chronic disease: 4.9 ± 2.1 vs 5.1 ± 2.6 |
|
| Interventions |
Group 1 ‐ Motivational interviewing (MI): intervention health professionals attended an additional 20‐hour workshop taught by family doctor who is expert in the field. Intervention professionals focused on motivational interviewing. Strategies of EMot are based on a collaborative, evocative style and respect for autonomy of the patient. The practice of EMot is based on 4 basic principles grouped under the acronym RULE: R (resist) resist the redirect reflex, U (understand) understand and explore the motivations of the patient himself, L (listen) listen empathically, and E (empower) empower the patient, favouring hope and optimism Group 2 ‐ Usual care: control patients received routine clinical attention based on transmission of info and persuasive advice Co‐intervention: before intervention, healthcare providers in both groups attended a 15‐hour workshop on patient safety and medication adherence. Intervention: (1) initial assessment of medication status, (2) detection of critical incidents and possible medication errors, (3) providing information, (4) developing customised action plan, (5) proposal for implementation Provider: physician or nurse (trained health professionals ‐ 16 physicians and 11 nurses) Where: patient's home or health centre When and how often: V0 baseline in healthcare setting (15 m), V1 at 15 to 20 days at home (45 to 60 m), V2 at 3 months in healthcare setting (15 m), V3 at 6 months at home (45 to 60 m) Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (objective):pill count: number of tablets presumably consumed/Number of tablets that should be consumed × 100. Adherent if average adherence > 80% and < 110% Other (objective) : average medication errors according to group. Errors including subtherapeutic dose, omission of administration, deteriorated drug, duplicate therapy, higher doses and other (reported in Perula de Torres 2014 paper) |
|
| Notes | Trial registration: NCT01291966 Consumer involvement: not specified Funding source: supported by Spanish Society of Family and Community Medicine and Andalusian Society of Family and Community Medicine research grant, and the Ministry of Health of the Government of Andalusia, Spain Dropout: 5 (3 vs 2) health professionals did not recruit any participants, 2 patients withdrew, 4 were lost to follow‐up (Perula de Torres paper says 5 lost to follow‐up) Language translation: yes ‐ Perula de Torres 2014 paper translated to English Exact ICC value not reported. Paper states that "ICC in cRCT in primary care generally less than 0.05". Thus 0.05 was used to recalculate sample sizes, 57 intervention vs 67 control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We performed blinded randomization to one of the two study arms (C4‐Study Design Pack; Glaxo S.A.)" 32 professionals assigned randomly and stratified by type of professional |
| Allocation concealment (selection bias) | Unclear risk | Allocation based on clusters and stratified by profession (nurse or physician). Unclear if/how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to mask the intervention, either to patients or to providers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if assessors blinded. Final results were evaluated by a methodology expert of the investigation, who remained at all times blind to the status of patients |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear who is in final results; states ITT |
| Selective reporting (reporting bias) | Unclear risk | Unclear how they assessed medication adherence at baseline (i.e. how did they do pill count). Medication adherence mean only at baseline |
| Other bias | Unclear risk | Sample size calculation: 78 per group not reached Recruitment bias (selective recruitment of cluster participants): high risk. Participants recruited by consecutive sampling. "Time between the training program and patient recruitment and intervention was about two weeks"; thus health professionals were aware of randomisation during participant recruitment stage |
Morales Suarez‐Vurela 2009.
| Study characteristics | ||
| Methods |
Aim of study: to assess the utility of the pillbox, individualized dispensing system, and practical dosing, to improve therapeutic compliance in polymedicated patients with diminution of mobility capacity Study design: RCT (open‐label; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: Spain Setting: community (home) Inclusion criteria: ineffective management of medications due to > 70 years and > 3 prescribed medications and limited mobility Exclusion criteria: cognitive impairment (Pfeiffer test < 3 if person can read and write and < 4 if person cannot read or write), mentally incapacitated patients, hospitalised patients at start of study Number of participants randomised: 182 (89 vs 93) Number of participants included in analysis: 182 (89 vs 93) Age: mean (CI)= 77.08 (76.224 to 77.936), vs 77.39 (76.646 to 78.134); range (min to max) = 61 to 93 vs 20 to 70 Gender: female: 64 (71.9%) vs 64 (68.8%) Ethnicity: not specified Number of medications: (type unclear) mean (CI) 8.35 (7323 to 9377) vs 7.83 (7403 to 8257); range (min to max) 3 to 60 vs 3 to 18. Number of medications/day = 9.22 (8.701 to 9.739) vs 10.60 (9.946 to 11.254), range (min to max) 0 to 23 vs 3 to 22 Frailty/Functional impairment: not reported Cognitive impairment: cognitive impairment excluded Comorbidities: no total comorbidity score |
|
| Interventions |
Group 1 ‐ Practidose Pillbox: a reusable pillbox ‐ plastic container with 7 compartments (7 days of the week). Name of patient written on the outside of the container and also included treatment control sheet (medication list/chart). Contains only solid dose forms ‐ this fact is rectified by introducing a cardboard pictogram of a jar of syrup, spoon, etc., in the corresponding space, at the time of administration, which reminds the patient that he/she should also take this medication. It is not clear who filled the pillbox as the intervention is not well described Group 2 ‐ Not specified ‐ presumably usual care without pillbox Co‐intervention: N/A Provider: nurse (district link nurse) and pillbox Where: unclear When and how often: in person baseline and 2 months, phone call at 14 days Intervention personalised: no (aside from individual medications in the box) |
|
| Outcomes |
Timing of outcome assessment: baseline and 2 months Medication adherence (subjective) : Morisky‐Green Medication Compliance: nurse‐administered survey. Unclear how results are reported ‐ appears to be reported as % patients who are compliant, but it is not defined) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: not specified Dropout: nil Language translation: yes ‐ translated to English Further information required: more detail on randomisation, allocation, recruitment, and adherence measure (email correspondence ‐ unsuccessful) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The selection of patients was made by assignment randomized by blocks" It appears these were blocks of 10 (5 intervention and 5 control for each nurse), but it is unclear how they were generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All patients aware of allocation, unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded ‐ appears nurses administered intervention and follow‐up on their own patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition specified |
| Selective reporting (reporting bias) | High risk | Multi‐variate analyses not reported in table ‐ unclear what was done. Morisky‐Green individual questions not reported ‐ unclear how adherence summarised (suspect answer no to all questions) |
| Other bias | Low risk | Sample size of 83 in each group |
Murray 1993.
| Study characteristics | ||
| Methods |
Aim of study: to determine the effect of unit‐of‐use packaging on medication compliance among elderly outpatients treated with complex medication regimens Study design: RCT (unit of allocation: individual) Number of arms/groups: 3 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: primary care clinic (geriatric outreach centres located in urban public housing units for the elderly and disabled (people living independently)) Inclusion criteria: ≥ 60 years, ≥ 3 medications Exclusion criteria: medication pharmacologic and pharmacokinetic properties considered unfeasible for twice‐daily regimen, nursing home Number of participants randomised: 36 Number of participants included in analysis: 31 (control 1: 12, control 2: 10, intervention: 9) Age: mean (range) = C1: 71.3 (64 to 81), C2: 72.5 (60 to 87), I: 72.9 (63 to 81) Gender: female: C1: 9 (75%), C2: 8 (80%), I: 6 (67%) Ethnicity: black (not white): C1: 8 (75%), C2: 9 (90%), I: 6 (67%) Number of medications: type not specified: mean ± SD: C1: 4.8 ± 2.2, C2: 3.8 ± 1.1, I: 5.1 ± 2.1 Frailty/Functional impairment: Medical Outcomes Study General Health Survey: physical function mean ± SD = C1: 52.8 ± 34.0, C2: 43.3 ± 29.6, I: 37.9 ± 31.7 Cognitive impairment: mean ± SD MMSE: C1: 27.2 ± 2.2, C2: 28.5 ± 1.0, I: 27.7 ± 1.8 Comorbidities: not specified |
|
| Interventions |
Group 1 (intervention) ‐ Unit‐of‐use medication packaging and regimen simplification: medications in unit‐of‐use packages with twice‐daily dosing intervals (morning and evening). Medications in translucent plastic cups with translucent plastic snap‐on lids. Yellow label for AM, blue label for PM Group 2 (control 1) ‐ Usual care: medications in conventional packaging and no change to dosing interval Group 3 (control 2) ‐ Regimen simplification: medications in conventional packaging but dosing intervals made twice daily (morning and night) using 2 clear plastic zip‐lock bags. Co‐intervention: all medications packaged individually by study pharmacist and dispensed monthly (33 days supply) Provider: pharmacist Where: ambulatory clinic/home When and how often: monthly (medications resupplied monthly) Intervention personalised: no |
|
| Outcomes |
Timing of outcome assessment: 6 months (assessed monthly) Medication adherence (objective) : pill count: percentage compliant (note over‐adherence expressed as under‐adherence, e.g. 90% not 110%). Scale 0 to 100 Adverse clinical health outcomes (subjective): asked: "Have you had any side effects, ill effects, or any other problems caused by medications you have taken? (yes/no)" |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Health Foundation of Greater Indianapolis Dropout: 4 withdrew, 1 was lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of pharmacist who delivered the intervention and collected outcome data |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of pharmacist who delivered the intervention and collected outcome data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 people missing (16%): 3 from intervention group (25%), 1 NH, 3 returned to prior regimen, 1 disliked unit‐of‐use packaging |
| Selective reporting (reporting bias) | High risk | Compliance measured in 4 ways; subjective not reported at follow‐up; results in abstract not matching main paper |
| Other bias | Unclear risk | No power calculation. Small sample size in each group. Groups not particularly well matched (e.g. mean number of drugs). Pill counts occurred in the pharmacy ‐ patients may not have returned all meds, and this be more of an issue with unit‐of‐use packaging (a bag for empty containers was provided, but it is possible that containers were discarded and % containers returned was not reported) |
Muth 2016.
| Study characteristics | ||
| Methods |
Aim of study: to test the feasibility of an intervention and cluster‐RCT study design for an intervention designed to improve medication appropriateness and adherence in elderly patients with multi‐morbidity Study design: cluster‐RCT (20 GP practices; unit of allocation: practice) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Germany Setting: primary care clinic (GP practices) Inclusion criteria: GPs: provision of primary care within German statutory health insurance system, healthcare assistant could access Internet. Patients: ≥ 65 years, ≥ 3 chronic conditions, ≥ 5 long‐term prescriptions, ≥ 1 practice visit in past quarter, ability to fill in questionnaire and participate in telephone interviews Exclusion criteria: Patients: MMSE < 26, life expectancy ≤ 6 months, alcohol and drug abuse (based on GP assessment) Number of participants randomised: 100 Number of participants included in analysis: 100 ITT (94 as per abstract) Age: mean ± SD 75.8 ± 6.7 vs 75.2 ± 5.88 Gender: female: 28 (56%) vs 24 (48%) Ethnicity: not specified Number of medications: long‐term prescriptions: 9.5 ± 2.67 vs 8.7 ± 2.66 Frailty/Functional impairment: falls: 7 (14%) vs 6 (12%), 94% intervention and 92% control were "fending for themselves", which presumably means they were independently functioning Cognitive impairment: excluded MMSE < 26, MMSE data not reported Comorbidities: mean ± SD Schafer et al, count of chronic diseases: 8.4 ± 2.52 vs 7.0 ± 2.62; Charlson score: 4.5 ± 2.64 vs 4.5 ± 2.46 |
|
| Interventions |
Group 1 ‐ Prioritising Multimedication in Multimorbidity in general practices (PRIMUMpilot): intervention group received a brown bag review and a checklist‐based pre‐consultation interview with patient and healthcare assistant to detect potential medication issues and non‐adherence; then a computer‐assisted medication review was carried out by GP along with GP‐patient consultation Group 2 ‐ Usual care (not described) Co‐intervention: both groups of GPs received practice guidelines for older patients Provider: GP with assistance from healthcare assistants Where: GP clinic When and how often: once at baseline Intervention personalised: yes ‐ tailored to individual medications |
|
| Outcomes |
Timing of outcome assessment: baseline (0 weeks) and 12 weeks Medication adherence (subjective) : Morisky adherence: validated questionnaire; 4 items resulting in sum scores of 0 to 4 with low scores indicating good adherence Medication adherence (subjective) : Medication Adherence Rating Scale (MARS): validated questionnaire; 5 items resulting in sum score 5 to 25, with high scores indicating good adherence Medication adherence (subjective) : discrepancy between medicines patients reported actually taking (at patient interview) and medicines prescribed (reported by GP). Three domains: drug score, dose score, and regimen score. Scores outside of 0.8 to 1.2 considered deviant. Unsure if validated Health‐related quality of life (subjective): EQ‐5D Adverse clinical health outcomes (subjective): number of days in hospital |
|
| Notes | Trial registration: ISRCTN99691973 Consumer involvement: not specified Funding source: German Federal Ministry of Education and Research, Grant no. 01GK0702 Dropout: 1 hospitalised, 7 lost to follow‐up (5 and 2) ICC for adherence reported as 0.000; thus no need to recalculate results |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation sequence generated by an external researcher using the random number generator of Microsoft Excel |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed to practices and patients until data collection at baseline had been completed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded ‐ participants could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ‐ adherence assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant quantity of missing data for adherence measures; reasons not adequately explained |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per methods |
| Other bias | Low risk | Aim for 100 patients (50:50), 10 GPs, 5 patients per cluster Recruitment bias (selective recruitment of cluster participants): low risk; treatment allocation was concealed to practices and patients until data collection at baseline (and thus recruitment) had been completed |
Nascimento 2016.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate improvement in diabetes self‐care (including adherence) after an individualised pharmacotherapy management service (home medication review and therapeutic education) in elderly patients Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: Portugal Setting: diabetes care clinic and patient's home Inclusion criteria: T2DM, ≥ 65 years, HbA1c ≥ 7.5% Exclusion criteria: (unclear) cancer, cognitive impairment or other condition that could "hinder communication" unless they could submit a caregiver Number of participants randomised: 90 Number of participants included in analysis: 87 (44 and 43) Age: mean ± SD: 74.2 ± 5.4 vs 72.3 ± 4.5 Gender: female: 43.2% vs 41.9% Ethnicity: not specified Number of medications: type not specified: 6.86 ± 3.32 vs 5.84 ± 2.76 Frailty/Functional impairment: not specified Cognitive impairment: cognitive impairment ‐ needed carer Comorbidities: no total score given |
|
| Interventions |
Group 1 ‐ Pharmacotherapy management service for T2DM elderly patients: individualized pharmacotherapy management service at home, including analysis of necessity, safety, and effectiveness of medications taken. Also received individualised therapeutic education on diabetes care especially pharmacotherapy. Unclear whether med review and education were limited to diabetes medications only or included all medications Group 2 ‐ Usual care: standard medical care consultation (no details) Co‐intervention: N/A Provider: not specified ‐? Pharmacist Where: home When and how often: once at baseline (no details) Intervention personalised: yes ‐ individual medication review |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (subjective) : Medida de Adesao aos Traamentos (ref 68), a validated Portuguese/Spanish measure based on Morisky Green Test: average level of adherence to drug therapy ‐ 7 questions, on 0 to 6 scale, with 6 being highest adherence Condition specific outcomes (objective): fasting blood glucose in mg/dL and glycosylated haemoglobin (HbA1c) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: funded in part by DGS Dropout: 3 lost to follow‐up Unpublished data: mean medications: control = 5.84 ± 2.76, intervention = 6.86 ± 3.32; adherence assessed for all medications, not just diabetes medications; adherence measured using a Spanish tool that is based on Morisky |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not explicitly reported, but the way it is described raises suspicion that it could have been alternating allocation, hence not random ("were randomised into a control and an intervention group, for a consecutive sampling") |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinical data accessed by an Independent clinical laboratory ‐ unsure if blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 randomised, 87 completed. No reasons given, but attrition small |
| Selective reporting (reporting bias) | Unclear risk | Methods unclear; method of assessing adherence mentioned at end, near conclusion |
| Other bias | Unclear risk | Method is very brief, making assessment of rigour and bias very difficult. Adherence assessment and analysis methods are unclear |
Naunton 2003.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate pharmacist‐conducted post‐discharge follow‐up at home of high‐risk elderly patients on various outcomes (including adherence) Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: Australia Setting: home (post discharge) Inclusion criteria: ≥ 60 years, ≥ 2 chronic medical conditions requiring medication (≥ 1 of HF, IHD, COPD, or DM), ≥ 4 prescribed regular medications Exclusion criteria: lived in domiciliary care facility or beyond greater Hobart area, were to be visited at home by a community nurse within 5 days of discharge, had terminal malignancy, were unable to provide informed consent Number of participants randomised: 136 Number of participants included in analysis: 121 (57 and 64) (unclear as number of participants alive at 90 days: 54 vs 59) Age: median (range): 74 (65 to 90) vs 77 (60 to 91) Gender: female: 56% vs 69% Ethnicity: not specified Number of medications: regular medications on discharge: median (range): 8 (3 to 15) vs 8 (3 to 16) Frailty/Functional impairment: nursing assistance at home: 28% vs 21% Cognitive impairment: not specified Comorbidities: chronic medical conditions: median (range) = 5 (2 to 9) vs 5 (2 to 13) |
|
| Interventions |
Group 1 ‐ Pharmacist post‐discharge home visit: 5 days after discharge, patients visited at home by study pharmacists. Objective of visit was to educate, answer any queries, optimise medication management (e.g. Dosette or Webster, if necessary), improve compliance, detect DRPs, and improve liaison with community‐based health services. Brief letter composed in the patient's home was given to the patient to present to the doctor. Study pharmacist also called GP and community pharmacy to inform of study Group 2 ‐ Usual care (no specific post‐discharge follow‐up for this group) Co‐intervention: 89% in both groups were seen by hospital pharmacist before discharge Provider: pharmacist Where: home When and how often: once, 5 days after discharge Intervention personalised: yes ‐ based on adherence assessment at home visit; specific strategies were offered such as compliance aids, carer assistance, community nursing, etc. |
|
| Outcomes |
Timing of outcome assessment: 90 days post discharge Medication adherence (subjective) : self‐reported missing doses: compliance defined as 'never miss medication'. Non‐compliance ‐ therefore any self‐reported missed doses (from 'rarely' to 'once a day'). Self‐reported 'never' forget to take their medication. 1 question ‐ "How often would you say you miss taking your pills?", with 7 response options from never to once a day. Presented as dichotomous variable adherent or not adherent. ? Not validated Satisfaction with intervention (subjective): satisfaction survey ‐ intervention group only Adverse clinical health outcomes (objective): deaths: % patients who died within 90 days of discharge. Retrospective medical record review and contact with family and/or GP Adverse clinical health outcomes (objective): unplanned hospital re‐admissions: % patients with 1 or more unplanned re‐admissions within 90 days of discharge (patients asked and retrospective medical records checked) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Abbott Australasia Pharmacy Research Grant, through SHPA Dropout: 2 withdrew (1 I, 1 C); 13 were lost to follow‐up (3 C died, 3 I and 2 C were uncontactable, 2 I and 1 C were admitted to nursing home, 2 I was admitted to intensive nursing care) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by study pharmacist using a computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred after discharge from hospital and collection of baseline data |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded post randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Good breakdown of excluded patients |
| Selective reporting (reporting bias) | Low risk | As described in methods |
| Other bias | Low risk | None apparent |
Nazareth 2001.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effectiveness of a co‐ordinated hospital and community pharmacy discharge care plan for elderly patients (75+) on ≥ 4 medications discharged from hospital Study design: RCT (4 hospitals; individual block randomisation) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: UK Setting: hospital discharge (hospital and patient's home) Inclusion criteria: 75+ years, ≥ 4 medications, discharged home from elderly care wards (3 acute general and 1 long‐stay hospital) to a catchment area of the 4 participating hospitals Exclusion criteria: could not speak English, too ill (no definition) Number of participants randomised: 362 (181 and 181) Number of participants included in analysis: 6 months: 306 (149 vs 157), interviewed: 132 vs 135 Age: mean ± SD: 84 ± 5.2 vs 84 ± 5.4 Gender: female: 62% vs 66% Ethnicity: 97% white; not reported for individual groups but not significantly different Number of medications: oral prescribed medications at discharge: mean 6, SD 2 overall (not reported for individual groups, but not significantly different) Frailty/Functional impairment: not specified Cognitive impairment: MMSE ≤ 15 (n = 39) excluded from interview process Comorbidities: mean 3 chronic medical conditions (not reported for individual groups, but not significantly different) |
|
| Interventions |
Group 1 ‐ Co‐ordinated hospital and community pharmacy discharge: hospital pharmacist pre‐discharge intervention: assessment of medication, rationalisation of drug treatment, assessment of patients' ability to manage their medication, provision of information on current drugs, and liaison with carers and community professionals (pharmacy, GP, etc., where appropriate). Written discharge plan given to patient, community pharmacist, and GP. Community pharmacist intervention: home visit at days 7 to 14 to check for discrepancies between what patient is taking vs that prescribed on discharge, assessment of patient knowledge ad adherence, patient counselling, removal of excess medications, and additional visits prn Group 2 ‐ Usual care: standard procedures. Discharge letter to GP. Pharmacists did not provide review of discharge medications nor community follow‐up (Unclear what services the hospital pharmacy did provide ‐ presumably some discharge counselling) Co‐intervention: N/A Provider: pharmacist (hospital and community) Where: discharge and home When and how often: pre‐discharge in hospital and at home 7 to 14 days after discharge Intervention personalised: yes (mostly standardised, but intervention tailored to address individual patient's medication management problems) |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (subjective) : self‐reported adherence: obtained through prescription medicine interview; adherence to prescribed drugs in the previous week; validated self‐report semi‐structured interview (adherence score is out of 1, with 1 being 'total/highest' adherence); mean (SD) out of 1 Knowledge about medicines (subjective): self‐reported medication knowledge: prescription medicine interview ‐ patient's knowledge of prescribed drugs; validated self‐report semi‐structured interview (knowledge score is out of 1, with 1 being 'total/highest' knowledge); mean (SD) out of 1 Satisfaction with intervention (subjective): validated patient satisfaction questionnaire ‐ each item scored 1 to 4; mean score per item calculated Adverse clinical health outcomes (objective): service usage: hospital re‐admission, death, outpatient department attendance, GP attendance. Data from hospital and GP surveys |
|
| Notes | Trial registration: ISRCTN66700837 Consumer involvement: not specified Funding source: National Health Service Research and Development programme on the primary/secondary care interface Dropout: 32 vs 24 died Fidelity: discharge plans were 'misplaced' for 36/181 patients, and 52/181 patients did not receive the home visit |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After consent, independently randomised by health authority's central community pharmacy office using computer‐generated random numbers. Block randomisation, stratified by trial centre |
| Allocation concealment (selection bias) | Low risk | Randomisation done by an independent group after consent obtained |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant remained blinded to allocation or patient |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up ‐ only 44% answered; 6‐month adherence |
| Selective reporting (reporting bias) | Low risk | As per methods |
| Other bias | High risk | Poor fidelity of the intervention: discharge plans were 'misplaced' for 36/181 patients, and 52/181 patients did not receive the home visit Sample size: "195 patients were required in each group" ‐ not reached |
Olesen 2014.
| Study characteristics | ||
| Methods |
Aim of study: this paper: to investigate the impact of pharmaceutical care on medication adherence, hospitalisation, and mortality of home‐living elderly (65+) patients prescribed polypharmacy. Overall study aim also included a third arm designed to assess the impact of an electronic reminder device on adherence Study design: RCT (2‐arm results from 3‐arm RCT; individual allocation) Number of arms/groups: 3 (2 discussed) |
|
| Participants |
Description: patient/consumer Geographic location: Denmark Setting: patient's home (community) Inclusion criteria: ≥ 65 years, ≥ 5 current prescription drugs taken without assistance Exclusion criteria: residence in a nursing home, terminal illness, cognitive disorders such as dementia, medication supervised by healthcare providers, immigration to Denmark after January 2005, and severe motor impairment. Patients hospitalised longer than 7 days during the study were excluded before the final adherence evaluation Number of participants randomised: 630 (315, 315) Number of participants included in analysis: 517 (253, 264) Age: median (IQR, range): 74 (70 to 80, 65 to 94) vs 74 (70 to 80, 65 to 91) Gender: female: 133 (53%) vs 134 (51%) Ethnicity: not specified (recent immigrants excluded) Number of medications: oral prescription medications: median (IQR, range): 7 (5 to 8, 1 to 16) vs 7 (5 to 8, 3 to 18) Note: only meds taken throughout the 12‐month study period were included in the adherence assessment Frailty/Functional impairment: not specified Cognitive impairment: cognitive impairment such as dementia excluded Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Pharmaceutical care: pharmacist home visit to deliver patient education, motivation, and regimen simplification. There was also a medication review to identify DRPs, but this was a minor component. Pharmacist examined medicines list with regard to possible side effects, interactions, and administration, then tried to make the regimen less complex, informed patients meanwhile about the drugs, listened to questions concerning the drugs, handed over information leaflets, and motivated adherence. Phone call at 3, 6, and 9 months to inquire about patient's condition and changes in the medicine, to uncover problems, and to answer questions Group 2 ‐ Reminder device: patient was given an e‐reminder device the size of a mobile phone that beeps when medications are due, and patient presses a button to indicate medications are taken Group 3 ‐ Usual care: no intervention (not described) Co‐intervention: all groups had regular nurse home visits to collect data for medication counts Provider: pharmacist Where: home and telephone When and how often: home at baseline, phone call at 3, 6, and 9 months Intervention personalised: yes ‐ personalised based on medication, but broad intervention the same |
|
| Outcomes |
Timing of outcome assessment: 12 months (adherence), 24 months (health outcomes) Medication adherence (objective) : pill count of oral prescription drugs. Nurse visited patients at baseline, 6 months, and 12 months to photograph pills, which were counted later by a 'counter pen' (combination of a marker and a digital camera). Adherence rate (%) per drug calculated as mean adherence rate during 1 year. < 80% pills taken as prescribed = non‐adherence (> 100% pills taken was regarded as 100%, i.e. adherent) Adverse clinical health outcomes (objective): unplanned hospital admissions to medical departments obtained from Danish e‐Health Portal Adverse clinical health outcomes (objective): mortality data obtained from hospital e‐journal (electronic hospital record that automatically records information on all deceased patients) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: supported by the Danish Ministry of Health and the Association of Danish Pharmacies Dropout: excluded before intervention 31 and 31 (hospitalised or medications administered), withdrew 15 and 5 (lack of interest), lost to follow‐up 16 and 15 (outside region, no adherence count, died) Fidelity: poor ‐ adherence measured for only 48% of medications. No data provided regarding whether pharmacists delivered the intervention exactly as intended, nor whether all phone follow‐ups occurred Further information required:hospitalisation, mortality, and adherence data for electronic reminder group (email correspondence ‐ successful, but did not collect hospitalisation or mortality data; adherence was measured by a different method ‐ see Harbig 2012) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 945 envelopes prepared, with each containing a study inclusion code. Patients selected an envelope at first home visit ‐ inadequate details provided re randomisation method |
| Allocation concealment (selection bias) | Unclear risk | At first home visit by a project nurse, patients were asked to select 1 envelope. Unclear if opaque envelopes, or order, or if nurse had knowledge |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to conceal the identity of patients in the pharmaceutical care group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Does not specify any blinding ‐ project nurse photographed pills to be counted later by a counter pen |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Able to assess adherence for only 48% of medications; insufficient detail as to why hospitalised patients were excluded |
| Selective reporting (reporting bias) | High risk | Three‐arm study but only 2 arms described. Outcomes mostly per methods, although additional adherence calculations were conducted that were not specified in methods (e.g. within‐group comparisons). Harbig paper describes third arm adherence by a different method |
| Other bias | Low risk | None noted ‐ adherence was very high in the control group, leaving little room for improvement |
Pandey 2017.
| Study characteristics | ||
| Methods |
Aim of study: to assess the impact of text message reminders on adherence to medications and exercise in patients recently discharged from the hospital after a myocardial infarction (MI) Study design: RCT (pilot single centre; individual allocation) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Canada Setting: hospital discharge to community Inclusion criteria: ≥ 18, discharged from hospital after MI in preceding 2 weeks and enrolled in a structured cardiac rehabilitation programme. Patients receiving treatment with medications from all 4 of the following classes: antiplatelets, BB, ACEI/A2RA, and statins Exclusion criteria: patients taking medications in dosing regimens > once daily, no mobile phone, unable to read and write in English or provide informed consent, those incarcerated Number of participants randomised: 34 Number of participants included in analysis: 33 (17 and 16) Age: 64.6 ± 11.5 vs 62.1 ± 11.0; subgroup > 65: 7 (41%) vs 8 (50%) Gender: 11 (65%) vs 2 (12%); not reported for subgroup Ethnicity: not specified Number of medications: cardiac (post‐MI) prescription medication: 10.1 ± 4.5 vs 8.0 ± 5.2; not reported for older subgroup Frailty/Functional impairment: not specified Cognitive impairment: dementia: 3 (18%) vs 2 (13%) Comorbidities: no total comorbidity score |
|
| Interventions |
Group 1 ‐ Text message reminder: once‐daily text message at the time patient preferred to take medications. Text messages simply indicated that patient should remember to take medications and contained no identifiable information such as medication names or classes (e.g. "Please remember to take your morning medications now") Group 2 ‐ Usual care (no text message) Co‐intervention: all participants received outpatient cardiac rehab programme for 3 months and follow‐up assessment at 12 months Provider: automated (set up by cardiac rehab nurses, then automated to send daily) Where: via text message When and how often: daily for 12 months Intervention personalised: not really ‐ standard wording: "Please remember to take your morning medications now"; time of day was modified for patient |
|
| Outcomes |
Timing of outcome assessment: 12 months Medication adherence (subjective) : self‐reported adherence: participants asked to use a logbook to record name and timing of medications taken on a daily basis. Logbooks were collected monthly. Absolute medication adherence was calculated as percentage of total prescribed doses that were actually taken each month. 12‐month adherence calculated as the mean of each of the 12 monthly measurements. Adherence outcome is % of days covered |
|
| Notes | Trial registration: NCT02783287 Consumer involvement: not specified Funding source: unclear; Brigham and Women's Hospital and University of Waterloo listed under sponsors and collaborators Dropout: 1 control withdrew |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a web‐based random number generator in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and their healthcare providers were aware of the arm to which they had been randomised |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial; no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 1 withdrew ‐ but may be relevant given low patient numbers, especially in older people subgroup |
| Selective reporting (reporting bias) | Low risk | As described in methods |
| Other bias | Unclear risk | Persistence with the 4 post‐MI medications not reported. Typically this is well below 100%, plus some medications may be stopped due to ADRs, etc. It is unlikely that no medications were stopped for any patients over 12 months. It is unclear how this was accounted for in the study |
Pereles 1996.
| Study characteristics | ||
| Methods |
Aim of study: to determine the effect of an inpatient self‐medication programme (SMP) on the ability to self‐medicate, patient medication knowledge, compliance, and patient morale Study design: RCT (2 inpatient geriatric units, individual allocation) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Canada Setting: hospital (before discharge from inpatient geriatric units) Inclusion criteria: discharge home (community living), patient responsible for administration of own medication, MMSE ≥ 20, ability to give informed consent, medically stable condition Number of participants randomised: 107 (51 and 56) Number of participants included in analysis: unclear (107 for baseline, 74 for follow‐up) Age: mean SD 80 ± 7 vs 80 ± 7 Gender: female: 37 (73%) vs 48 (86%) Ethnicity: not specified Number of medications: medications/d: inpatient: 4.8 ± 3 vs 4.7 ± 2; discharge: 4.7 ± 3 vs 5.1 ± 4 Frailty/Functional impairment: not specified Cognitive impairment: MMSE < 20 excluded, MMSE 26 ± 3 Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Inpatient self‐medication programme: three‐stage programme in which patient is given increasing responsibility for administration of his/her medications. (1) Patient is counselled by pharmacist and patient requests medications from nurses at appropriate times; (2) patient is given 24‐hour supply of medications to self‐administer. Advances to next stage if no errors after 3 to 5 days; (3) patient is given several days supply of medication. Average duration of program = 21.6 days (SD 19) Group 2 ‐ Usual care ‐ medications administered by nursing staff Co‐intervention: 72 hours before discharge, both groups received pharmacist assessment of knowledge and functional ability to manage medicines and pre‐discharge medication education from a pharmacist; both groups had a 20‐minute counselling session with a pharmacist Provider: pharmacist and nurse Where: inpatient geriatric unit (subacute, average LOS = 40 days) When and how often: before discharge, continuously for an average of 21.6 days Intervention personalised: yes ‐ to some extent, e.g. 41% intervention patients discharged with a Dosette (and 34% control patients) |
|
| Outcomes |
Timing of outcome assessment: discharge and 40 days post discharge Medication adherence (objective) : pill count: patients discharged with 40 days worth of medication, pill count conducted in home at 40 days. Proportion of medication errors (? assumed missed doses but not explained) of total doses administered Medication‐taking ability (objective) : assessed differently for each group: intervention = 2 or fewer errors on stage 2 of SMP considered able to self‐medicate at discharge. Control = Pharmacist assessment at time of discharge counselling with input from other team members. YES/NO ‐ self‐medicating at discharge (note: there could be reasons other than failing the SMP that might explain why patients were not self‐medicating at discharge, such as patient preference) Knowledge about medicines (objective): "short medication knowledge questionnaire" = Patients asked to name and describe appearance and purpose of their medication, to describe their regimen and any potential side effects or drug interactions. % correct responses in each knowledge category |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: not specified Dropout: 1 refusal, 32 lost to follow‐up (19 C, 14 I) (5 deaths, 2 lost to follow‐up, 21 RACF, 4 other) Fidelity: 33 did not complete study protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded (unable to blind) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Medication ability not blinded (groups assessed differently); unclear whether adherence was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33 did not complete the study; they were older, had lower MMSE, and had longer hospital stays |
| Selective reporting (reporting bias) | Low risk | Reported as per methods |
| Other bias | Unclear risk | Power analysis "suggested 48 patients in each group would be required"; loss to follow‐up meant required sample size was not maintained. 33 did not complete study protocol ‐ poor fidelity |
Rich 1996.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effect of a multi‐disciplinary treatment strategy on compliance rates for patients hospitalised with congestive heart failure Study design: RCT (substudy of larger RCT (Rich 1995); unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: hospital, discharge and post discharge in the home Inclusion criteria: aged 70+, admitted to hospital with CHF, at least 1 risk factor for early re‐admission Exclusion criteria: severe dementia (inability to assist with self‐care) or other serious psychiatric illness, limited life expectancy (3 months), discharge to RACF living outside catchment area Number of participants randomised: unclear Number of participants included in analysis: 156 (80 and 76) Age: mean ± SD 80.5 ± 5.7 vs 78.4 ± 6.1 Gender: female: 74% vs 59% Ethnicity: Caucasian: 40% vs 29% Number of medications: medications at discharge: 5.2 ± 2.4 vs 5.2 ± 2.5 Frailty/Functional impairment: ADL: 5.7 ± 1.1 vs 5.7 ± 0.8 Cognitive impairment: severe dementia excluded Comorbidities: no total comorbidity score |
|
| Interventions |
Group 1 ‐ Multi‐disciplinary intervention for elderly people with CHF: education from study nurse about CHF and its management using a 15‐page teaching guide prepared by the study team. Patients seen daily by a study nurse throughout the remainder of their hospital stay; the importance of compliance with both medications and diet was emphasised repeatedly. Also visited by dietician and social service representative (who assisted in arranging appropriate post‐discharge care). Shortly before discharge, geriatric cardiologist reviewed medications and made recommendations to primary physician regarding simplification and consolidation. Following discharge, all patients seen by hospital's home care department and contacted regularly by study nurse Group 2 ‐ Usual care: conventional medical care + standard hospital services, including dietary teaching and pre‐discharge medication instructions Co‐intervention: N/A Provider: nurse (+ input from dietician and geriatric cardiologist) Where: hospital and home When and how often: during hospital, reinforced daily. Duration post discharge unclear Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 30 ± 2 days after discharge Medication adherence (objective) : pill count: performed for all current medications, presented dichotomously as compliant (≥ 80%) or non‐compliant Adverse clinical health outcomes (objective): hospital re‐admissions: number of people re‐admitted to hospital within 30 days of discharge |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: National Heart, Lung and Blood Institute grant Dropout: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated based on terminal digit of a computer‐generated sequence of random numbers (i.e. even or odd) |
| Allocation concealment (selection bias) | Low risk | Neither patients nor investigators were aware of treatment assignment until after randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pill count by experienced clinical pharmacist or trained pharmacy assistant blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported; ITT |
| Selective reporting (reporting bias) | Low risk | Reported as per methods, except for additional measure of adherence (> 80%) |
| Other bias | Unclear risk | Unclear whether adherence assessment was for all medications or only for cardiac medications, which were the focus of this study |
Saez de la Fuente 2011.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the utility of a pharmacotherapeutic information programme at hospital discharge in polymedicated patients and the profile of modifications in treatment of the patient at 30 to 50 days of hospital discharge Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: Spain Setting: hospital discharge Inclusion criteria: using prescription medications for 3 or more months, ≥ 4 active ingredients at discharge Exclusion criteria: transfer to geriatric residence, dementia and/or psychiatric illness, incapacitated in the absence of a caregiver responsible for medication at the time of the interview, Barthel Index < 20 Number of participants randomised: 59 (29 and 30) Number of participants included in analysis: 50 (26 and 24) (but methods says ITT) Age: median (range): 73 (28 to 93) vs 75 (14 to 96) Gender: female n (%): 10 (34.5%) vs 11 (36.7%) Ethnicity: not specified Number of medications: median (IQR): active ingredients/patient at discharge: 8.3 (7.4 to 9.3) vs 7.6 (6.5 to 8.7); pharmaceutical forms/patient: 7.9 (7.1 to 8.8) vs 7.1 (6.0 to 8.1) Frailty/Functional impairment: Barthel Index: median (IQR) 100 (85 to 100) vs 100 (65 to 100) Cognitive impairment: dementia/psychiatric illness: 8 (26.7%) vs 8 (26.7%) Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Pharmacotherapeutic discharge information programme: verbal and written information about treatment at hospital discharge ‐ unclear who provided information, suspect pharmacist (as pharmacist conducted interview). Format and content unclear Group 2 ‐ Usual care ‐ no discharge information provided Co‐intervention: N/A Provider: unclear ‐? Pharmacist Where: discharge from hospital When and how often: once, pre‐discharge Intervention personalised: yes (info provided presumably tailored to individual medication regimen) |
|
| Outcomes |
Timing of outcome assessment: discharge and 30 to 50 days (mean 42.1, SD 9.6 days) Medication adherence (subjective): Morisky‐Green Medication Compliance: 4 questions: (1) Do you ever forget to take the medications, yes or no? (2) Take the medicines at the indicated time, yes or no? (3) When you feel better, do you stop taking the medication, yes or no? (4) If you sometimes feel worse when taking your medication, do you stop taking the medication, yes or no? ==> To consider good adherence, the response of all questions must be adequate (no, yes, no, no) Adverse clinical health outcomes (objective): deaths: number of deaths during follow‐up Adverse clinical health outcomes (subjective): ED and hospital admissions: telephone questionnaire ‐ number of ED or hospital re‐admissions during follow‐up |
|
| Notes | Trial registration: not specified Consumer involvement: not specified Funding source: not specified Dropout: 9 lost to follow up (3 died, 5 in hospital, 1 moved) Note: caregiver at discharge: 15 (57.7%) vs 17 (70.8%) Language translation: yes ‐ translated to English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | At the time of discharge, patients were distributed randomly in 2 groups by a block method with a 1: 1 ratio between groups |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients not blinded; unclear about personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Pharmacist was interviewer. At the time of the telephone interview, the interviewer was unaware of treatment of the patient, as well as the previous result of adherence to discharge |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal dropout; unclear why those in hospital were not counted as re‐admissions |
| Selective reporting (reporting bias) | Low risk | Appears per protocol |
| Other bias | Low risk | None apparent |
Shimp 2012.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate a patient‐centred employer‐based medication therapy management (MTM) programme Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: community (University) Inclusion criteria: University of Michigan beneficiaries (employees, retirees, and their dependents), taking ≥ 7 prescription medications. Patients with a University of Michigan primary care provider were preferentially invited Number of participants randomised: 133 (intervention); "a similar number who consented but were not invited formed control" Number of participants included in analysis: 128 (intervention) Age: mean age: 70 years (intervention) Gender: 55% female (intervention) Ethnicity: not specified Number of medications: prescription medications: 9.2 ± 3.2 (intervention) Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: 3 ± 1.4 medical conditions (intervention) |
|
| Interventions |
Group 1 ‐ Focus on Medicines (FOM) Medication Therapy Management (MTM): 2 face‐to‐face meetings with University of Michigan clinical pharmacists. First visit was comprehensive review of all medications. Patients with DM, HT, dyslipidaemia, asthma, arthritis, chronic pain, and OP were asked disease‐specific questions. Patient questions were answered. Second visit patient and pharmacist discussed recommendations and a medication action plan (MAP). This detailed DRPs, recommended actions, and person responsible Group 2 ‐ No intervention Co‐intervention: N/A Provider: pharmacist Where: unclear ‐ University or home ? When and how often: twice, unsure of timing Intervention personalised: yes ‐ patient‐centred medication action plan |
|
| Outcomes |
Timing of outcome assessment: baseline (1 year pre‐study) and final (1 year post study) Medication adherence (objective) : medication possession ratio: MPR defined as sum of all days of medication supply received during 1 year pre‐study and 1 year post‐study periods, divided by the total number of days supply needed during 365 days. MPR calculated for top 8 drug classes for chronic conditions |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: University of Michigan Dropout: 5 withdrew Further information required: raw data on MRPs (author correspondence successful, but no further data were available; study authors said "results showed no significant change") |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not specified; those who agreed to participate compared with similar number of individuals meeting selection criteria but not invited to participate (control group) |
| Allocation concealment (selection bias) | High risk | Not specified ‐ patients randomly selected by study team ? |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details on attrition of control group, etc. Would assume some people would change jobs |
| Selective reporting (reporting bias) | High risk | Adherence outcomes ‐ both BMQ and Treatment Satisfaction Questionnaire for Medication ‐ not included despite being a main outcome and listed in methods |
| Other bias | Unclear risk | Funded by University of Michigan; preference given to people with primary practitioner who worked at University of Michigan |
Shively 2013.
| Study characteristics | ||
| Methods |
Aim of study: to determine the efficacy of a patient activation (Heart PACT) intervention compared with usual care on activation, self‐care management, hospitalisations, and emergency department visits in patients at high risk of re‐admission/hospitalisation for HF Study design: RCT (repeated measure design; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: Veterans Affairs Healthcare System (single site) Inclusion criteria: document clinical HF stage C, incident hospitalisation or ED visit for HF treatment within previous 12 months, ≥ 18 years, live in San Diego County, read and speak English, telephone access, has a primary care provider Exclusion criteria: inability to provide written consent, acute medical problems within previous month, considered by investigators to be medically unstable, enrolled in speciality HF programme or telehealth or had long‐term follow‐up by cardiology after hospital admission, severe medical problems, life expectancy < 1 year, acute substance abuse, psychiatric problems, homelessness Number of participants randomised: 84 (43 vs 41) Number of participants included in analysis: 6 months: 68 (34 vs 34) Age: grouped: mean ± SD 66.1 ± 10.76, range 42 to 89 (intervention: 63.4 ± 9.10, control: 68.9 ± 11.73) Gender: 1 female (1.2%) (0 vs 1) Ethnicity: white: 76.7% vs 78.0% (other races not extracted) Number of medications: trial author reported all > 4 medications (unpublished) Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: 71% reported ≥ 3 comorbidities |
|
| Interventions |
Group 1 ‐ Patient activation (Heart PACT): 6‐month activation/Heart PACT programme developed to enhance self‐management. Intervention used activation theory and was tailored to each participant's activation level. At each meeting/telephone call, goals and progress toward attaining these were discussed (Figure 2). Also received a self‐management tool kit (BP cuff, weight scale, pedometer, HF self‐management DVD, and educational booklet) Group 2‐ Usual care ‐ general medical care and any HF‐specific clinical care from primary care provider. Received self‐management toolkit after final assessment (6 months) Co‐intervention: 2‐hour baseline outcome assessment Provider: nurse (advanced practice nurse) Where: telephone and F2F. Unclear location ‐ assume at clinic When and how often: 6 sessions over 6 months Intervention personalised: yes ‐ personalised based on activation level |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (subjective) : self‐reported medication adherence: medication adherence (as part of MOS‐specific adherence scale). "Took medications as prescribed (on time without skipping dose) in the past 4 weeks". Responses from 0 to 5, transformed to a 0 to 100 scale Adverse clinical health outcomes (subjective): self‐reported hospitalisations: patients asked to report any hospitalisations, ED visits, and other unscheduled visits including reason for visit and treatment. Results reported as mean (SD) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service, project 04‐252 Dropout: no details provided Unpublished data: trial author reported that participants were on > 4 medications; most had minimum of 12 medications and 3 comorbid conditions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified block randomisation approach, based on baseline activation, was used to ensure patients were equally distributed. No other details regarding randomisation were provided |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned after baseline assessment ‐ unclear who was allocated or if concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing completely at random (MCAR) analysis completed but not reported. Attrition rate 19% |
| Selective reporting (reporting bias) | Low risk | As per methods |
| Other bias | Unclear risk | Participants received $10 at baseline and at 3 months, $20 at 6 months |
Taylor 2003.
| Study characteristics | ||
| Methods |
Aim of study: to determine the effect of pharmaceutical care on prevention, detection, and resolution of drug‐related problems in high‐risk patients in a rural community Study design: RCT (3 clinics; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: primary care clinic (family medicine clinics ‐ rural) Inclusion criteria: adults (18+) at high risk for medication‐related adverse events (presence of 3 or more risk factors: ≥ 5 medications, ≥ 12 doses/d, ≥ 4 medication changes in previous year, ≥ 3 concurrent diseases, history of non‐compliance, presence of drugs requiring therapeutic monitoring) attending a participating clinic Exclusion criteria: significant cognitive impairment, history of missed office visits, scheduling conflicts, life expectancy < 1 year Number of participants randomised: 81 Number of participants included in analysis: 69 (33 and 36) Age: 64.4 ± 13.7 vs 66.7 ± 12.3 (P = 0.467) Gender: female: 63.6% vs 72.2% Ethnicity: white: 60.6% vs 61.1% Number of medications: type not specified, 6.3 ± 2.2 vs 5.7 ± 1.7 Frailty/Functional impairment: not specified Cognitive impairment: significant cognitive impairment excluded Comorbidities: ≥ 3 concurrent diseases ‐ inclusion criterion |
|
| Interventions |
Group 1 ‐ Outpatient pharmaceutical care: standard medical care + pharmaceutical care. Pharmaceutical care (~ 20 minutes) occurred with a pharmacist, before seeing physician at regularly scheduled office visits. Pharmacists evaluated indication, effectiveness, dosage, correctness and practicality of directions, drug‐drug interactions, drug‐disease interactions, therapeutic duplication, duration of treatment, untreated indications, and expense. Pharmacist reviewed medical record, conducted a chart review, and examined medication history to determine compliance with and complications of medications and provided comprehensive individualised patient education that included a brief review of the disease, important lifestyle modifications, and basic drug information. Therapeutic recommendations were communicated to physicians through discussions and progress notes Group 2 ‐ Usual care: standard medical care (assume no pharmaceutical care during clinic visits) Co‐intervention: both groups received baseline and follow‐up interviews with pharmacist. Information collected included compliance, presence of medication misadventures, and medication knowledge Provider: pharmacist Where: medical clinic When and how often: continuous for 1 year at scheduled clinic visits Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: baseline and 12 months Medication adherence (subjective) : self‐reported compliance: percentage of patients with medication compliance scores of 80% to 100%. Calculated by asking the patient the number of medication doses missed during the past week or month and dividing the estimated number of doses taken by the total number of doses prescribed Knowledge about medicines (objective): self‐reports used to assess medication knowledge during each pharmacist‐patient encounter. A knowledge score was determined by dividing the number of medications for which a patient reported the correct name, purpose, dose, and frequency by the total number of medications and multiplying by 100 Satisfaction with intervention (subjective): patient satisfaction with pharmacy‐related services, survey Health‐related quality of life (subjective): SF‐36 health survey Adverse clinical health outcomes (objective): ED and hospital visits: numbers of emergency department visits and hospitalisations for each patient during preceding year ‐ medical record audit Condition‐specific outcomes (objective): clinical markers of disease: number of people reaching goal level of BP ≤ 140/90, DM:HbA1c ≤ 7.5%, INR 2 to 3, and lipids LDL concentrations Other (subjective): patients with at least 1 medication misadventure |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: ASHP Research and Education Foundation Dropout: 3 intervention refused, 6 lost to follow‐up (3 and 3), 3 died (2 and 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned to a control group or an intervention group; no details on randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Not specified; order of consent/allocation unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding; may have impacted reporting of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 (14.8%) lost to follow‐up; no mention if characteristics similar |
| Selective reporting (reporting bias) | Low risk | Reported as per methods |
| Other bias | Low risk | None apparent |
Truelove 2015.
| Study characteristics | ||
| Methods |
Aim of study: to determine whether fixed‐dose combinations of generic drugs ('polypills') would promote use of optimal preventative drugs Study design: RCT Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Australia Setting: primary care clinic (general practices or aboriginal medical services) Inclusion criteria: established CVD (MI, stroke, or PVD = secondary prevention group) or 5‐year CVD risk ≥ 15% using Framingham‐based calculation (=primary prevention group), enrolling doctor had to be satisfied that each medication was clearly indicated and no contraindication Exclusion criteria: contraindication to any component of polypill, responsible clinician feels change to current therapy will place patient at risk Number of participants randomised: 623 (311 and 312) Number of participants included in analysis: 609 (304 and 305), subgroup of more than 8 meds: 202 Age: more than 8 meds subgroup = 66.4 years ± 11.4 years Gender: 41.6% female Ethnicity: not specified Number of medications: > 8 prescription medications subgroup Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Polypill: all previous CV medications stopped and started on a polypill (Version 1 aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, atenolol 50 mg; Version 2 aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, HCT 12.5 mg) Group 2 ‐ Same medications but no polypill (i.e. usual care) Co‐intervention: N/A N/A ‐ Doctors given complete treatment autonomy after randomisation. Both polypill and usual medications dispensed from designated pharmacies with same out‐of‐pocket costs Provider: GP prescribed Where: general practice or aboriginal medical centre When and how often: continuous unless stopped by GP or patient Intervention personalised: not really ‐ 2 versions of polypill. Otherwise no personalisation |
|
| Outcomes |
Timing of outcome assessment: baseline, 1 month, then at 6 monthly intervals for 18 months Medication adherence (subjective) : self‐reported use of combination treatment (≥ 2 BP‐lowering medications: an antiplatelet and a statin). Patient must have reported taking each component medication on at least 4 of the 7 preceding days. This captures combined effect of changes by healthcare provider and patient adherence |
|
| Notes | Trial registration: ACTRN126080005833347 Consumer involvement: not specified Funding source: NHMRC of Australia Fidelity: 62.4% still taking polypill at end of study (suggesting there had been many GP/patient changes during study) Dropout: 6 refused (3 and 3), 2 died (1 and 1), 6 missing/unable to contact (3 and 3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central, computer‐based randomisation service. Stratified by study centre, indication, and prescription of all appropriate therapies at baseline (yes vs no) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind; designed as an open trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinded endpoints but adherence was self‐reported, so unable to blind this outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 97.7% and 97.8% completed, but only 62.4% taking polypill at end of study |
| Selective reporting (reporting bias) | Low risk | As per methods |
| Other bias | Unclear risk | Trial study sample size calculation 1000; only 623 recruited due to insufficient resources. Only 62.4% taking polypill at end may indicate poor fidelity |
Vinluan 2015.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effect of pharmacist discharge counselling on patient adherence to heart failure (HF) therapy among an elderly US population and to assess hospital re‐admission rates Study design: RCT Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: hospital discharge Inclusion criteria: ≥ 65 years admitted with a new diagnosis of HF or already had diagnosis and were re‐admitted with HF exacerbation, prescribed ≥ 5 medications at discharge Exclusion criteria: living in long‐term care facility, hearing or cognitive impairment, could not communicate in English, did not manage own medications, lacked a telephone, unable to give informed consent Number of participants randomised: 16 (7 and 9) Number of participants included in analysis: adherence: 2 and 2, hospitalisation: 7 and 9 Age: 74 ± 5.9 vs 71 ± 6.9 Gender: female 86% vs 78% Ethnicity: not specified Number of medications: long‐term/regular medications: 8.1 ± 2.9 vs 8.3 ± 2.2 Frailty/Functional impairment: not specified Cognitive impairment: cognitive impairment excluded Comorbidities: total number not specified |
|
| Interventions |
Group 1 ‐ Pharmaceutical discharge HF counselling: individual inpatient counselling by a pharmacist and telephone call follow‐up with review of current medications and HF counselling after discharge at days 3, 30, 60, and 90 Group 2 ‐ Usual care: regular care by nurses (including discharge counselling using generated d/c med list and HF handout) Co‐intervention: pharmacist performed a comprehensive review of inpatient HF medication regimen for all patients to ensure appropriate therapy was initiated; reviewed drug interactions, drug‐disease interactions, and duplicate therapy Provider: pharmacist Where: hospital and telephone When and how often: hospital at baseline, telephone days 3, 30, 60, and 90 Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 90 days post discharge Medication adherence (objective) : prescription refill history from community pharmacy, percentage of participants adherent Adverse clinical health outcomes (subjective): rehospitalisation: patient asked number of hospital admissions within 90 days after hospital discharge. Number of deaths also collected (source ?) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: nil funding Dropout: details not specified, but for adherence 14 dropouts (7 and 7) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation was performed using envelopes created by an outside affiliate with an assigned number inside |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened in front of only patient and pharmacist once patient agreed to participate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind; unclear if this would impact outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported patient outcomes; unclear if blinded or not for pharmacist and refill history |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Massive attrition ‐ unable to reach by phone. Also said 7 and 7 lost to follow‐up but still reported 2 and 2 (should be 0 and 2) ? |
| Selective reporting (reporting bias) | High risk | Stated rehospitalisation data obtained from patients during phone calls. However, researchers have data for all participants. Also unclear how death data were obtained |
| Other bias | Low risk | None apparent |
Volume 2001.
| Study characteristics | ||
| Methods |
Aim of study: to describe changes in patients' adherence to therapy regimens, patients' expectations of the care they receive from their pharmacist, patients' satisfaction with pharmacy services, and patients' HRQoL after provision of pharmaceutical care Study design: cluster‐RCT (16 pharmacies; cluster unit of allocation: pharmacy) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Canada Setting: community pharmacy Inclusion criteria: coverage of medications under Alberta Health & Wellness senior drug benefit plan (age ≥ 65 years), ≥ 3 medications according to dispensing records, able to complete telephone interviews, residing in Alberta for 12 of 15 study months, agree to get prescriptions from study pharmacy (Kassam 2001) Exclusion criteria: communication and language barriers, terminal disease, unable to provide informed consent Number of participants randomised: 16 pharmacies (8 vs 8), 363 patients (159 vs 204) Number of participants included in analysis: 292 completed Age: 73.89 ± 6.09 vs 73.18 ± 6.11 Gender: female: 63.5% vs 69.6% Ethnicity: not specified Number of medications: prescription: 4.67 ± 2.82 vs 3.90 ± 2.49. Non‐prescription: 0.63 ± 0.92 vs 0.73 ± 1.17 Frailty/Functional impairment: not specified Cognitive impairment: not specified Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Pharmaceutical care research and education project (PREP): comprehensive pharmaceutical care. Treatment pharmacists (enrolled in an intensive education programme) used the Pharmacist's Management of Drug‐Related Problems (PMDRP) instrument to summarise information collected during patient interview and used SOAP (subjective, objective, assessment, and plan) record to document actions and follow‐ups Group 2 ‐ Usual care: control pharmacists not told which patients had or had not agreed to participate Co‐intervention: N/A Provider: pharmacists (community) Where: community pharmacies When and how often: 1 interview with frequent follow‐up Intervention personalised: yes ‐ based on interview and required pharmaceutical care |
|
| Outcomes |
Timing of outcome assessment: baseline and 12 to 13 months Medication adherence (subjective) : Morisky self‐reported adherence: adherence to medication regimens assessed using a 4‐item self‐report Morisky (validated) measure. Summing numerical values for each answer provides a summary adherence score ranging from 0 to 4, with lower scores indicating better adherence Satisfaction with intervention (subjective): patient satisfaction with pharmacy services using 34‐item instrument based on work by MacKeidan and Larson and Johnson et al; 7‐point Likert scale used, with 1 indicating highest satisfaction. Only general satisfaction extracted Health‐related quality of life (subjective): SF‐36 health survey, validated |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: Alberta Ministry of Health, Alberta Pharmaceutical Association, University of Alberta Central Research Fund, Merck Frosst Canada, Hoechst Marion Roussel, Alberta Health‐Health Services Research Innovation Fund; drug references for pharmacists were provided by Bristol‐Myers Squibb Dropout: unclear: 5 treatment and 7 control pharmacies supplied patient data; 3 treatment and 1 control never recruited patients ICC not reported, numbers of participants in intervention and control groups at follow‐up unclear. Trial authors contacted for more information but no response. Thus unit of analysis error exists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pharmacies paired based on Statistics Canada median income for first 3 digits of pharmacy's postal code. Study statistician did not know the identity of pharmacies and randomly assigned pharmacies to treatment or control within the pair. One pair were very closely located, thus assigned to same treatment/control to minimise contamination. Unclear why groups still balanced (8 and 8) |
| Allocation concealment (selection bias) | Unclear risk | Statistician did not know identity of pharmacies but took steps to minimise contamination and match characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind patients to intervention; control pharmacists not told which patients had or had not agreed to participate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Telephone survey by Population Research Lab at the University ‐ unclear whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only patients with data at all 3 time points were included in the analysis; T1 = 363, T2 = 292 |
| Selective reporting (reporting bias) | Low risk | Appears to be presented as per methods |
| Other bias | Unclear risk | "We intended that each pharmacy would identify enough to produce sample of 50 participants" ‐ not reached, sample size based on HRQoL Recruitment bias (selective recruitment of cluster participants): high risk, patient recruitment occurred after allocation of clusters, resulting in potential for recruitment bias; "it is possible that pharmacists selected patients whom they believed would benefit from the intervention or who had a more positive attitude toward pharmaceutical care" |
Willeboordse 2017.
| Study characteristics | ||
| Methods |
Aim of study: to investigate effectiveness of clinical medication reviews (CMRs) for quality of life and geriatric problems in comparison with usual care in older patients with geriatric problems in general practice Study design: cluster‐RCT (22 general practices; cluster unit of allocation: GP practice) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: the Netherlands Setting: primary care clinic (general practice clinics) Inclusion criteria: PRACTICE: inclusion = all GP practices members of Academic Network of GPs. Practice not performing CMRs on a regular basis and would not start doing if randomised to control. PARTICIPANTS: inclusion = ≥ 65, newly presented with a geriatric problem in general practice, and used ≥ 1 prescribed drug long term (≥ 3 months). Geriatric problems identified by screening electronic records and by completing screening questionnaire. Geriatric problems included mobility, dizziness, fear of falling, urinary incontinence, and cognitive impairment. Patients were included if they scored ≥ 5 on VAS scales (range 1 to 10) for geriatric problems or reported ≥ 1 fall in preceding 6 months Exclusion criteria: PARTICIPANTS: recorded dementia diagnosis, GP excluded patients who had recent CMR or deemed unable to participate Number of participants randomised: 518 (275 and 243) Number of participants included in analysis: T0 = 270 vs 239, T2 = 215 vs 211 (unpublished adherence results for 208 vs 198) Age: 77.8 ± 7.7 vs 77.8 ± 8.0 Gender: female: 177 (64.4%) vs 159 (65.4%) Ethnicity: born Dutch or other European: 91.7% vs 93.6% Number of medications: number of drugs reported by patient: 6.1 ± 3.1 vs 5.6 ± 3.2 Frailty/Functional impairment: mobility problems (≥ 5 VAS) = 57.9% vs 62.6% Cognitive impairment: cognitive problems (≥ 5 VAS) = 25.5 vs 26.9%, diagnosed dementia excluded Comorbidities: chronic diseases: 2.77 ± 1.76 vs 3.23 ± 2.19 |
|
| Interventions |
Group 1 ‐ Optimised clinical medication reviews (Opti‐Med): (1) preparation: info from EMRs, pharmacy, and screening questionnaire collected including drug use, medication history, potential DRPs, medical problems, recent lab results, and non‐lab measurements; (2) clinical medication review: expert team of GPs/nursing home physicians and community pharmacists performed review using adapted systematic tool to reduce inappropriate prescribing (STRIP) method; (3) pharmacotherapeutic treatment plan (PTP): PTP sent to patient's GP; (4) implementation of PTP: patients invited for consultation with GP in which PTP was discussed and was determined together with the patient Group 2 ‐ Usual care: expert team also performed CMR analyses, but GPs and patients did not receive the results Co‐intervention: N/A Provider: independent expert team (doctor and pharmacist) and patient's GP Where: primary care clinic When and how often: once ‐ baseline Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: baseline and 6 months Medication adherence (subjective): self‐reported adherenceproblems assessed in the screening and follow‐up questionnaire Satisfaction with intervention (subjective): Medication Satisfaction Questionnaire: assessed on a 7‐point Likert scale. A 1‐point change in MSQ score was considered clinically meaningful Health‐related quality of life (subjective): SF‐12 and EQ‐5D‐3L Adverse clinical health outcomes (objective): drug‐related problems: number of DRPs per patient ‐ using the DOCUMENT checklist. Assessed by expert team at baseline and by 1 researcher at follow‐up |
|
| Notes | Trial registration: NTR4264 Consumer involvement: not specified Funding source: Dutch Organisation for Health Research and Development Dropout: 9 excluded before intervention (5 and 4), 51 withdrew (33 and 18), 42 were lost to follow‐up (27 and 15) Fidelity: 274 of 275 received CMR; 247 discussed with patient Unpublished data: adherence worsened or persisted: 65 vs 54; adherence improved or remained the same: 143 vs 144 ICC value: 0.08. Effective sample sizes were not calculated, as this study was not included in any meta‐analyses due to alternate reporting methods |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of practices performed by statistician blinded to characteristics of practices using a computer‐generated list of random numbers |
| Allocation concealment (selection bias) | High risk | Randomisation done at practice level before patients recruited ‐ could have influenced recruitment of patients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, but blinding to treatment allocation not possible due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective outcomes unblinded; unclear if researcher blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher dropout and loss to follow‐up in intervention group: 51 withdrew (33 and 18), 42 were lost to follow‐up (27 and 15) |
| Selective reporting (reporting bias) | Unclear risk | Raw data not reported |
| Other bias | Unclear risk | Sample size 500 patients, not maintained during follow‐up. Based on EQ‐5D. 274 of 275 received CMR, 247 discussed with patient Recruitment bias (selective recruitment of cluster participants): high risk, randomisation carried out before patients were recruited, thus potential for selective recruitment based on practice knowledge of being in intervention or control group |
Williams 2012.
| Study characteristics | ||
| Methods |
Aim of study: to test the feasibility and impact of a multi‐factorial Medication Self‐Management Intervention (MESMI) to improve blood pressure control and medication adherence in adults with coexisting diabetes and CKD disease Study design: RCT (single hospital; unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: Australia Setting: outpatient clinic Inclusion criteria: ≥ 18 years, comprehended English, mentally competent (AMT), type 1 or 2 diabetes, CKD (modified diet in renal disease; eGFR > 15 or diabetic kidney disease), systolic hypertension ≥ 130 mmHg Exclusion criteria: > 50 km from city centre Number of participants randomised: 80 (39 vs 41) Number of participants included in analysis: 75 (36 vs 39), adherence 74 (35 vs 39) as per trial author correspondence Age: 68 ± 8.3 vs 66 ± 10.8 Gender: female: 17 (42.6%) vs 26 (63.4%) Ethnicity: Australia born: 14 (35.9%) vs 15 (36.6%) Number of medications: prescribed medications (excluding insulin and OTC) 7.6 ± 2.6 vs 7.2 ± 3.3 Frailty/Functional impairment: not specified Cognitive impairment: AMT: 10 (10 to 11) vs 11 (11 to 10), cognitive impairment excluded Comorbidities: other long term illnesses: 7.7 ± 2.5 vs 8.2 ± 2.5 |
|
| Interventions |
Group 1 ‐ Medication Self‐Management Intervention (MESMI): multi‐factorial intervention consisting of self‐monitoring of BP, individualised medication review, 20‐minute DVD, and fortnightly motivational interviewing follow‐up telephone contact for 12 weeks to support BP control and optimal medication self‐management. Patients taught how to take BP correctly and recorded BP daily for 3 months. Medication review involved drawing up a medication chart (generic name, indication, dose, targets). DVD had 3 sections: how BP affects body, benefits and safety of prescribed medications, tips to help take medications as prescribed. Motivational interviewing: open‐ended questions used to prompt discussion about the participant's well‐being, BP, and medications Group 2 ‐ Usual care: standard outpatient care Co‐intervention: N/A Provider: nurse (renal specialist, doctoral qualification, and motivational training) Where: home and telephone When and how often: home intervention, then 12 weeks of support and follow‐up phone calls Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: 9 months post intervention (12 months post enrolment) Medication adherence (objective) : pill count: percentage medication adherence to all long‐term prescribed medications measured by pill counts. Insulin and OTC (vitamins) not included in pill count Condition‐specific outcomes (objective): change in BP (checked by research assistant), HbA1c, eGFR, creatine |
|
| Notes | Trial registration: ACTRN12607000044426 Consumer involvement: not specified Funding source: Australian Research Council grant (LP0774989), Sigma Theta Tau International Small grant, Nurses Memorial Centre Australian legion of Ex‐servicemen and ‐women scholarship, Mona Menzies Nurses Board of Victoria Grant Dropout: 1 withdrew, 3 died, 1 was lost to follow‐up Fidelity: accuracy of pill count confounded by participants unable to recall when they started their new prescription. Only 30 participants saved their medication boxes to enable a full pill count to be performed Unpublished data: trial authors contacted regarding number of people included in adherence follow‐up; total people included in calculation was 35 vs 39 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by off‐site statistician; stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | Following recruitment, participants were allocated code numbers before enrolment and randomisation by an off‐site statistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded and were asked not to disclose group allocation to research assistant |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Pill count not possible for most (only 30 saved all pill boxes, enabling complete count) |
| Selective reporting (reporting bias) | High risk | Sample size calculated as 108 participants ‐ did not reach target sample size and modified inclusion criteria to try to increase recruitment |
| Other bias | Unclear risk | Accuracy of pill count confounded by participants unable to recall when they started their new prescription. Only 30 participants saved their medication boxes to enable a full pill count to be performed. Sample size calculated as 108 participants ‐ did not reach target sample size |
Winland‐Brown 2000.
| Study characteristics | ||
| Methods |
Aim of study: to investigate the effects of 3 medication management approaches on medication adherence. To examine the relationship between medication adherence and utilisation of healthcare resources, including numbers of physician office visits, hospitalisations, and home health visits Study design: RCT (unit of allocation: individual) Number of arms/groups: 3 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: independent living facility Inclusion criteria: capable of following simple directions, had a medication mismanagement episode, had hospitalisation for medication non‐adherence or an illness in which therapeutic accuracy was necessary for its management Exclusion criteria: not specified Number of participants randomised: 61 (16, 24, 21) Number of participants included in analysis: 61 (16, 24, 21) Age: mean: 87 years, range 70 to 100 Gender: 35 F, 26 M Ethnicity: primarily Jewish Number of medications: type not specified, range 3 to 15 Frailty/Functional impairment: not specified Cognitive impairment: 17.5% had dementia Comorbidities: total number not specified |
|
| Interventions |
Group 1 ‐ Pre‐poured pillbox: medication management pack, no voice activation Group 2 ‐ Automated voice‐activated dispenser: voice‐activated message that audibly reminded and automatically dispensed individual doses of medication to the participant Group 3 ‐ Usual care: self‐administration of own medications Co‐intervention: nurses visited patients each week to refill medication packs and address any questions or concerns (unclear whether control group also visited) Provider: nurse filled medication packs Where: independent living facility (patient homes) When and how often: weekly for 6 months Intervention personalised: no |
|
| Outcomes |
Timing of outcome assessment: baseline (for healthcare utilisation) and 6 months Medication adherence (objective) : pill count: average number of missed doses via pill count Adverse clinical health outcomes (objective): healthcare utilisation: medical records examined for numbers of physician visits, hospital admissions, and home visits, and transition to a higher level of care Condition‐specific outcomes (objective): biochemical markers of adherence: measured by the impact on medical diagnosis. Hypertensive group defined as adherent if sustained normotensive, cardiac adherent by INR 2.0 to 3.0, antipsychotic adherent by stable blood levels and mood stabilisation, diabetes by stable blood glucose and periodic HbA1c < 9% |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: not specified Dropout: nil specified Further information required: biomedical markers of adherence as per protocol (trial author correspondence ‐ successful, no further results available) Please note: group 2 vs group 3 was used for comparison of intervention vs usual care, group 1 vs group 2 was used for comparison of intervention vs intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients "were randomly assigned to 1 of 2 medication management programs" (details unclear) and "clients of similar age, gender, and cognition were assigned to a control group" (not randomised) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding; unclear whether nurses who refilled pack each week also conducted adherence assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition mentioned |
| Selective reporting (reporting bias) | High risk | Missing data on impact on medical diagnoses |
| Other bias | Unclear risk | Groups unbalanced; G2 all had been prolonged hospitalisation compared to 7/16 in G1 and none in usual care group. Also, half of G2 participants had 2 hours home health services/d compared to none in the G1 and usual care groups |
Wood 1992.
| Study characteristics | ||
| Methods |
Aim of study: to determine the effects of an inpatient self‐administration of medication programme on compliance post discharge among elderly patients Study design: cluster‐RCT (2 wards intervention, 2 wards control, 1 intervention and 1 control per hospital) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: UK Setting: hospital inpatient (pre‐discharge) from rehabilitation wards Inclusion criteria: rehabilitating with the ultimate aim of discharge to own home to live alone Exclusion criteria: nil mentioned Number of participants randomised: 33 (18 and 15) Number of participants included in analysis: 22 (11 and 11) Age: 85.4 ± 6.0 vs 84.8 ± 5.8 Gender: M:F = 1:8 (88.8% F) vs 1:6.5 (86.7%) Ethnicity: not specified Number of medications: number of dose‐taking events per day: 8.1 ± 5.0 vs 6.17 ± 5.0 Frailty/Functional impairment: not specified Cognitive impairment: abbreviated mental test score: 9.1 vs 9.4 Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Inpatient self‐administration: 3 distinct phases ‐ phase 1: medicine containers labelled as for discharge, drugs handed to patient at appropriate times, and full supervision of medication selection and ingestion. After 7 days, or earlier if appropriate, patient moved to phase 2; phase 2: patient required to request medication at appropriate times. After 7 error‐free days, patient moves on; phase 3: patient becomes totally responsible for his/her own medication. Medicines stored in locked cupboard. Compliance checked by tablet count Group 2 ‐ Usualcare: discharge medicines issued by nursing staff immediately before discharge Co‐intervention: N/A Provider: care team ‐ including pharmacist and nurse Where: hospital inpatient (pre‐discharge) When and how often: up to 3 weeks, inpatient Intervention personalised: yes ‐ moved through phases only if able to self‐administer medications |
|
| Outcomes |
Timing of outcome assessment: 2 weeks and 3 months post discharge Medication adherence (objective) : pill count: average percentage of non‐compliance calculated by pill count. No errors made, few errors (1% to 15% non‐compliance), and many errors (> 15%) |
|
| Notes | Trial registration: N/A Consumer involvement: not specified Funding source: not specified Dropout: 8 lost to follow‐up (4 and 4) plus 3 intervention could not be analysed ICC value unclear; unsuccessful contact with study authors likely due to age of study. Thus unit of analysis error exists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Wards allocated ‐ randomisation method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. Blinding was not possible for the type of intervention provided unless the researcher was not involved in providing the intervention; unclear if this had any impact on the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified whether home visit staff were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 lost to follow‐up from both groups, 3 intervention patients excluded because they had received new medications but transferred them to original containers = potentially unbalanced analysis |
| Selective reporting (reporting bias) | Unclear risk | Additional data in results that are not specified in methods (e.g. errors sufficient to be detrimental to health) |
| Other bias | Low risk | Recruitment bias (selective recruitment of cluster participants): low risk, recruitment occurred after allocation but all patients on the ward were included in the study (no exclusions), thus limited risk of recruitment bias |
Wu 2006.
| Study characteristics | ||
| Methods |
Aim of study: to investigate the effects of compliance and periodic telephone counselling by a pharmacist on mortality in patients receiving polypharmacy Study design: RCT (unit of allocation: individual) Number of arms/groups: 2 |
|
| Participants |
Description: both patient/consumer and carer Geographic location: China Setting: specialist medical clinic at a hospital Inclusion criteria: non‐compliant (pharmacist assessed medical clinic records), ≥ 5 drugs on ≥ 2 consecutive visits to the clinic Exclusion criteria: non‐Cantonese dialects or a different language, had conditions that prevented effective communication (deaf, mute, dementia, psychological disorders), living in nursing homes with supervised treatment Number of participants randomised: 442 (219 and 223) Number of participants included in analysis: 442 (219 and 223) (but 43 died by follow‐up) Age: 71.2 ± 9.4 vs 70.5 ± 11.1 Gender: female 51% vs 52% Ethnicity: not specified ‐ all spoke Cantonese Number of medications: drugs for chronic illnesses: 6.0 ± 1.3 vs 5.9 ± 1.2 Frailty/Functional impairment: not specified Cognitive impairment: dementia excluded Comorbidities: not specified |
|
| Interventions |
Group 1 ‐ Telephone counselling by a pharmacist: patients received a 10‐ to 15‐minute telephone call from pharmacist at midpoint between clinic visits (6 to 8 calls over 2 years). Pharmacist asked about patient's treatment regimens, clarified any misconceptions, explained side effects, reminded of next clinic appointment, reinforced importance of compliance Group 2 ‐ Usual care: no telephone intervention Co‐intervention: all patients received 10‐ to 15‐minute educational talk by pharmacist during screening. Pharmacist determined compliance using structured questionnaire Provider: pharmacist Where: telephone to home When and how often: 6 to 8 telephone calls for 2 years Intervention personalised: yes |
|
| Outcomes |
Timing of outcome assessment: screening or enrolment and 2 years Medication adherence (subjective) : patient asked if he or she had missed any doses; changed regimens in terms of dose, frequency, and timing; or had drugs left over. Compliant 80% to 120%. This information was checked against dispensing information Adverse clinical health outcomes (objective): all‐cause mortality Adverse clinical health outcomes (objective): hospitalisations: rate of admission to hospital, number of emergency department visits, and hospital stay 2 years before and after screening |
|
| Notes | Trial registration: SRCTN48076318 Consumer involvement: not specified Funding source: Hong Kong Government Health Care and Promotion Fund and MSD international grant Dropout: 25 and 38 died |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacist blinded to randomisation codes, which were computer‐generated by statistician and sealed in envelopes labelled with consecutive numbers |
| Allocation concealment (selection bias) | Low risk | Envelopes opened by clinic nurse in an ascending manner, and patients allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible because the intervention was complex and caregivers were involved |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not possible; could have had blinded research review of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT; no loss to follow‐up, but 60 defaulters before randomisation, 31 of whom died |
| Selective reporting (reporting bias) | Low risk | Baseline reports as compliant/non‐compliant, follow‐up reports who remains compliant/non‐compliant (number can be computed); otherwise as per methods |
| Other bias | Unclear risk | Sample size 1067 to account for non‐compliance and achieve significant reduction in mortality ‐ not reached |
Young 2016.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effects of patient activation intervention on self‐management (SM) adherence, hospital re‐admission, and ED visit rates at 30 days, 3 months, and 6 months Study design: RCT (block randomisation of 4 to 6) Number of arms/groups: 2 |
|
| Participants |
Description: patient/consumer Geographic location: USA Setting: 2 rural critical access hospitals Inclusion criteria: ≥ 21 years, HF as one of discharge diagnoses, New York Heart Association class II to IV or class I symptoms and ≥ 1 HF‐related hospitalisation/ED visit in past year, discharged to home, passed mini‐cognitive screen, understood English, had access to a phone Exclusion criteria: scheduled procedures/surgeries, depressive symptoms (score ≥ 3 on Patient Health Questionnaire, liver cirrhosis, renal failure, end‐stage and/or terminal illness that affected ability to perform SM behaviours Number of participants randomised: 105 (54 and 51) Number of participants included in analysis: 100 (51 vs 49) Age: 68.7 ± 11.8 vs 71.8 ± 12.6 Gender: female: 52.9% vs 75.5% Ethnicity: Caucasian: 94.1% vs 95.9% Number of medications: medications per day (type unknown) 16.4 ± 10.0 vs 15.9 ± 7.4 Frailty/Functional impairment: not specified Cognitive impairment: had to pass mini‐cognitive assessment to be included Comorbidities: total comorbidities 7.8 ± 2.5 vs 8.0 ± 2.7 |
|
| Interventions |
Group 1 ‐ Patient AcTivated Care at Home Model (PATCH): usual care + 12 weeks of PATCH intervention. Intervention comprised 2 phases in which the in‐hospital discharge education session was followed by 12 weeks of post‐discharge educational sessions delivered by telephone. Patients received SM toolkit (calendar for weight and salt, scales, electronic pill organiser reminder alarm). Each intervention session lasted 45 to 50 minutes. Booster sessions for patients struggling at home Group 2 ‐ Usual care (standard discharge teaching for HF and scheduled follow‐up doctor appointments) Co‐intervention: N/A Provider: unclear ‐ nurse ? Where: phase 1: in hospital; phase 2: post discharge via telephone When and how often: post‐discharge phone calls twice a week for first 2 weeks, then weekly 3 to 6 weeks, then every second week 7 to 12) Intervention personalised: yes ‐ tailored based on activation level, pre‐set goals, and specific SM needs; booster sessions given to those struggling with SM |
|
| Outcomes |
Timing of outcome assessment: hospital discharge and 6 months (180 days) Medication adherence (subjective) : self‐reported adherence: number of days when any medication doses were missed in past 7 days, grouped as 0 or ≥ 1 days (dichotomous) Adverse clinical health outcomes (objective): all‐cause re‐admissions and ED visits ‐ self‐report and primary care provider report |
|
| Notes | Trial registration: NCT01964053 Consumer involvement: not specified Funding source: National Institutes Nursing Research of the National Institutes of Health Dropout: 3 intervention withdrew before intervention, 2 control withdrew/were lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Project statistician used an on‐line pseudo‐random number generator to create an allocation schedule; random ordering of block sizes 4 and 6 was used to maintain even accrual throughout the study |
| Allocation concealment (selection bias) | Low risk | Group assignments placed in sealed envelope and opened sequentially as patients were enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of subject and intervention is impossible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collector was blinded to treatment assignment. Unclear whether this blinding was completely possible as patients were aware of their treatment group and may have told assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition; sample calculations allowed for 15% but it was much less |
| Selective reporting (reporting bias) | Low risk | As per methods |
| Other bias | Low risk | None apparent; sample size 96 |
A2RA: angiotensin 2 receptor antagonist.
ACEI: angiotensin‐converting enzyme inhibitor.
ADL: activity of daily living.
ADR: adverse drug reaction.
BB: beta‐blocker.
BMI: body mass index.
BMQ: Beliefs About Medicines Questionnaire.
BP: blood pressure.
C: control.
CABG: coronary artery bypass graft.
CHD: coronary heart disease.
CHF: coronary heart failure.
CI: confidence interval.
CKD: chronic kidney disease.
CMR: clinical medication review.
CNS: central nervous system.
COPD: chronic obstructive pulmonary disease.
CRD: carer‐reported dementia.
CTP: care transition programme.
CV: cardiovascular.
CVD: cardiovascular disease.
DASH: Disabilities of the Arm, Shoulder and Hand.
DM: diabetes mellitus.
DRP: drug‐related problem.
ED: emergency department.
eGFR: estimated glomerular filtration rate.
EMR: electronic medical record.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions.
EuroQoL‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions.
FEV1: forced expiratory volume in one second.
GMC: general medicine clinic.
GP: general practitioner.
HbA1c: glycosylated haemoglobin.
HF: heart failure.
HMH: home medication history.
HRQoL: health‐related quality of life.
I: intervention.
iADL: instrumental activity of daily living.
ICC: intracluster correlation coefficient.
IHD: ischaemic heart disease.
INR: international normalised ratio.
IQR: interquartile ratio.
ITT: intention‐to‐treat.
LDL‐C: low‐density lipoprotein cholesterol.
LOS: length of stay.
MAP: medication action plan.
MARS: Medication Adherence Rating Scale.
MD.2: medication dispenser machine
MDW: medication discharge worksheet.
MI: motivational interview; myocardial infarction.
MIDS: medication and information discharge summary.
MMAS: Morisky Medication Adherence Scale.
MOS: Medication Outcomes Study
MPR: medication possession ratio.
N/A: not applicable.
NH: nursing home
NHS: National Health Service.
NSAID: non‐steroidal anti‐inflammatory drug.
OTC: over‐the‐counter.
PCI: pharmaceutical care issue.
PHR: personal health record.
PIM: potentially inappropriate medication
PMC: polymedication check.
PPI: proton pump inhibitor.
PTP: pharmacotherapeutic treatment plan.
PVD: peripheral vascular disease
QoL: quality of life.
RACF: residential aged care facility
RCT: randomised controlled trial.
SBP: systolic blood pressure.
SD: standard deviation.
SE: standard error.
SF‐36: Short Form‐36 Health Survey.
SGRQ: St. George's Respiratory Questionnaire.
T2DM: type 2 diabetes mellitus.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12616001411437 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| ACTRN12617001352392 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (intervention focused on hypertension medications only) |
| Adams 2015 | EXCLUDE Medications = unable to determine if > 4 medications (trial authors did not collect numbers of medications) |
| Ahmad 2011 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Al‐Asseri 2001 | EXCLUDE Participant mean age ≤ 65 years |
| Al‐Khadra 2014 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Allen 1986 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Allen 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Altavela 2008 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Alvarez 2001 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Ansari 2017 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Ansari 2017a | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Antoniades 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Antonicelli 2008 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Artinian 2003 | EXCLUDE Medications = unable to determine if > 4 medications (no response from trial author) |
| Ascione 1984 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (intervention and outcome only at CV medications, average 2) |
| Bailey 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Barker 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Basger 2015 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Basheti 2016 | EXCLUDE Participant mean age ≤ 65 years |
| Bennett 2003 | EXCLUDE Participant mean age ≤ 65 years |
| Bhattacharya, 2016 | EXCLUDE Outcome = follow‐up too short (3 weeks) |
| Biese 2011 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (conference paper for 2014 paper) |
| Biese 2014 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Biese 2017 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Bilotta 2011 | EXCLUDE study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Birtwhistle 2004 | EXCLUDE Participant mean age ≤ 65 years |
| Biswas 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (numbers of medications data not collected) |
| Blenkinsopp 2000 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (antihypertensives only) |
| Bogner 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Bolas 2004 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Bolton 2004 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Bonetti 2018 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Boult 2013 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Branda 2013 | EXCLUDE Participant mean age ≤ 65 years |
| Braun 2009 | EXCLUDE Participant mean age ≤ 65 years |
| Broadbent 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (assessed respiratory medications only) |
| Bronson 1986 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Bryant 2011 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Bryson 2008 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Burrelle 1986 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Caetano 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (diabetes medications only) |
| Calvert 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Cedilnik 2016 | EXCLUDE Intervention = not directed at consumer nor carer (directed at GP) |
| Chan 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Chan 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Chau 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Chen 2016 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Choudhry 2008 | EXCLUDE Participant mean age ≤ 65 years |
| Chow 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Clarkesmith 2013 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Clemson 2004 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Clifford 2006 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Coleman 1999 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Coombes 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (looking only at adherence to medications for secondary prevention of stroke ‐ between 1 and 3 medications/person) |
| Costa 2008 | EXCLUDE Participant mean age ≤ 65 years |
| Cotterell 1992 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Coull 2004 | EXCLUDE Medications = unable to determine if > 4 medications (no contact with trial authors) |
| Criswell 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Crotty 2005 | EXCLUDE Setting = participants not living in community nor discharged from hospital to community |
| Crowley 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Ctri 2017 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Cummings 2019 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| D'Agostino 2006 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Damush 2011 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Damush 2016 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Day 1992 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Day 1998 | EXCLUDE Participant mean age ≤ 65 years |
| De Azevedo 2017 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| de Lusignan 2001 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Denneboom 2007 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Denneboom 2008 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Dickson 2011 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Dorje 2018 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Doucette 2009 | EXCLUDE Participant mean age ≤ 65 years |
| Doughty 2002 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Doyon 2009 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Drenth‐van 2013 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Drks 2014 | EXCLUDE Intervention = not directed at consumer nor carer |
| Drks 2015 | EXCLUDE Intervention = not directed at consumer nor carer |
| Dunn 1995 | EXCLUDE Participant mean age ≤ 65 years |
| Duong 1996 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Eggink 2010 | EXCLUDE Outcome = follow‐up too short (adherence 23 days) |
| Eikelenboom 2016 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Elliott 2008 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (new medicines only) |
| Elliott 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Ellis 2000 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Enguidanos 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Epstein 1990 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Esposito 1995 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (assumed given low MCI scores) |
| Evans 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Fabacher 1994 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Fan 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Farmer 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Fernandes 2012 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (intervention targeting hypertension only) |
| Fernando 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Fernley 1983 | EXCLUDE Outcome = follow‐up too short (adherence 7 to 10 days) |
| Ferrat 2018 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Figar 2006 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Fikri‐Benbrahim 2013 | EXCLUDE Participant mean age ≤ 65 years |
| Fikri‐Benbrahim 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Fincher 2009 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Finley 2003 | EXCLUDE Participant mean age ≤ 65 years |
| Fischer 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Fortney 2007 | EXCLUDE Participant mean age ≤ 65 years |
| Fortney 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Fortney 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Frennet 2011 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Frey 2001 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Friedman 1996 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Fugazzaro 2016 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT (non‐randomised as per 2018 paper) |
| Fulmer 1999 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (capped maximum 4 medications per person, hence average < 4) |
| Gabriel 1977 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Gamboa 2019 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Garcao 2002 | EXCLUDE Participant mean age ≤ 65 years |
| Garcia 2017 | EXCLUDE Participant mean age ≤ 65 years |
| Garza 2016 | EXCLUDE Participant mean age ≤ 65 years |
| Gellis 2014 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Gialamas 2009 | EXCLUDE Medications = unable to determine if > 4 medications (trial authors did not collect numbers of medications) |
| Gould 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Grant 2003 | EXCLUDE Participant mean age ≤ 65 years |
| Green 2008 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence to antihypertensives only) |
| Grice 2014 | EXCLUDE Setting = participants not living in community nor discharged from hospital to community |
| Gujral 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Gums 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Gwadry‐Sridhar 2005 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Han 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Hansen 2009 | EXCLUDE Participant mean age ≤ 65 years |
| Haramiova 2017 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (protocol only, blood pressure‐lowering medication only) |
| Harari 2008 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Hawe 1990 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Hayes 1998 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Hedegaard 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Heisler 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Heisler 2011 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability (conference abstract for 2012 paper) |
| Heisler 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Heisler 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Hesselink 2004 | EXCLUDE Participant mean age ≤ 65 years |
| Ho 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Hohmann 2014 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Holdford 2013 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Horton 2017 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Hsieh 2008 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (TB meds only) |
| Huang 2018 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Hugtenburg 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Hunter 1996 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Hyrkas 2014 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Insel 2016 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence to 1 medicine only) |
| IRCT20110728007143N | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| IRCT201306297531N | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| IRCT2014050617596N | EXCLUDE Participants mean age ≤ 65 years, or more than 20% aged 65 years |
| IRCT2015090513092N | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| IRCT2016082129448N | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| IRCT2016090629728N | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| IRCT2016120731290N | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| IRCT20171125037616N | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence not assessed for all medications) |
| IRCT20171213037859N | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| IRCT20180513039646N | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| ISRCTN03155973 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| ISRCTN12752680 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability. Note: secondary outcomes were changed 17/07/17; adherence was previously listed but was no longer included in the study protocol |
| ISRCTN18285541 | EXCLUDE Medications ‐ unable to determine if eligible as study never completed. "Investigators decided to close the clinical trial because the rate of inclusion of patients was being so slow that it was impossible to reach the necessary number in a reasonable time". Dr. José Luis González Guerrero (joselglezg@gmail.com) |
| Jager 2017 | EXCLUDE Intervention = not directed at consumer nor carer (GP is participant) |
| Jager 2017a | EXCLUDE Intervention = not directed at consumer nor carer |
| Jahangard‐Rafsanjani 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Jarab 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Jarab 2012a | EXCLUDE Participant mean age ≤ 65 years |
| Jensen 2003 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Jerant 2003 | EXCLUDE Medications = unable to determine if > 4 medications (trial authors did not collect numbers of medications) |
| Jiang 2007 | EXCLUDE Participant mean age ≤ 65 years |
| Johnson‐Warrington 2016 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (COPD only) |
| Johnston 2000 | EXCLUDE Medications = unable to determine if > 4 medications (no response from trial authors) |
| Junling 2015 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Kalichman 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Karagiannis 2016 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (diabetic medications only) |
| Kaukab 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Kaur 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Kavin 2010 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Kelly 1990 | EXCLUDE Participant mean age ≤ 65 years |
| Kempen 2017 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Keyserling 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Khan 2019 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Khonsari 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Khunti 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Kim 2014 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence to BP medications only) |
| Kim 2015 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Kim 2015a | EXCLUDE Participant mean age ≤ 65 years |
| Kimball 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Koberlein‐Neu 2016 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Kogos 2004 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Kono 2004 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Kotowycz 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Kozuki 2006 | EXCLUDE Participant mean age ≤ 65 years |
| Kraemer 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Kranker 2018 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Krass 2007 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Kripalani 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Kripalani 2012a | EXCLUDE Participant mean age ≤ 65 years |
| Krishnamurthi 2014 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (TB medications only) |
| Krishnaswami 1981 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Krishnaveni 2019 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Kutzleb 2006 | EXCLUDE Participant mean age ≤ 65 years |
| Lalonde 2017 | EXCLUDE Intervention = not directed at consumer nor carer |
| Lam 2011 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (antihypertensives only) |
| Lam 2017 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Lange 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Laramee 2003 | EXCLUDE Medications = unable to determine if > 4 medications (trial authors did not collect information) |
| Laufs 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence measure not for all medications) |
| Leavitt 2017 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Lei 2019 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Leiva‐Fernandez 2014 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (COPD only) |
| Lenaghan 2007 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Lerma 2017 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Levine 1979 | EXCLUDE Participant mean age ≤ 65 years |
| Levy 2004 | EXCLUDE Participant mean age ≤ 65 years |
| Li 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Li 2013 | EXCLUDE Medications = unable to determine if > 4 medications (no response from trial authors) |
| Li‐Hong 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Lin 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Lluch‐Canut 2006 | EXCLUDE Participant mean age ≤ 65 years |
| Lowe 1995 | EXCLUDE Outcome = follow‐up too short (adherence measured at 10 days) |
| Lowe 2000 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Lu 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Luttik 2014 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (total unknown, adherence for individual medication classes only) |
| Ma 2014 | EXCLUDE Participant mean age ≤ 65 years |
| MacDonald 1977 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Mackenzie 2013 | EXCLUDE Medications = unable to determine if > 4 medications (no response from trial authors) |
| Maduka 2013 | EXCLUDE Participant mean age ≤ 65 years |
| Magid 2011 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Maislos 2004 | EXCLUDE Participant mean age ≤ 65 years |
| Malet‐Larrea 2016 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Maly 1999 | EXCLUDE Participant mean age ≤ 65 years |
| Margolius 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Marin 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Marquez Contreras 2009 | EXCLUDE Participant mean age ≤ 65 years |
| Martin 1982 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (total not provided, low medication regimen complexity) |
| Martin 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Martinez 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Matsuyama 1993 | EXCLUDE Participant mean age ≤ 65 years |
| Mazzuca 1986 | EXCLUDE Participant mean age ≤ 65 years |
| McCarthy 2013 | EXCLUDE Participant mean age ≤ 65 years |
| McCarthy 2017 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| McGeoch 2006 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Mehos 2000 | EXCLUDE Participant mean age ≤ 65 years |
| Mehuys 2008 | EXCLUDE Participant mean age ≤ 65 years |
| Mehuys 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Meredith 2002 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Michiels 2017 | EXCLUDE Medications ‐ data on number of medications not collected, thus unable to determine eligibility |
| Miller 1988 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Miller 2008 | EXCLUDE Participant mean age ≤ 65 years |
| Mitchell 2014 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Moczygemba 2012 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Moorhead 2017 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Moreno 2016 | EXCLUDE Participant mean age ≤ 65 years |
| Morisky 1990 | EXCLUDE Participant mean age ≤ 65 years |
| Morrison 2016 | EXCLUDE Participant mean age ≤ 65 years |
| Mullan 2009 | EXCLUDE Participant mean age ≤ 65 years |
| Muniz 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Murray 2007 | EXCLUDE Participant mean age ≤ 65 years |
| NCT00838344 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (diabetic medication only) |
| NCT01144182 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| NCT01271985 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| NCT01404988 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence not measured for all medications) |
| NCT01914588 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| NCT02035566 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence not measured for all medications) |
| NCT02140619 | EXCLUDE Medications < 4 regular medications or group mean ≤4 (persistence to statin or antiplatelet only) |
| NCT02490423 | EXCLUDE Participant mean age ≤ 65 years (mean age 63.2 as per investigator, Dr. Horne) |
| NCT02697422 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| NCT02905474 | EXCLUDE Participant mean age ≤ 65 years (mean age 57 as per trial author email) |
| NCT03342729 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Nesari 2010 | EXCLUDE Participant mean age ≤ 65 years |
| Newman 2016 | EXCLUDE study design: not RCT, cluster‐RCT, nor quasi‐RCT |
| Nguyen 2017 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Nishita 2013 | EXCLUDE Participant mean age ≤ 65 years |
| Noureldin 2012 | EXCLUDE Participant mean age ≤ 65 years |
| NTR2288, 2010 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Obreli‐Neto 2011 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Ogedegbe 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Oliveira‐Filho 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Oonk 2017 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Ostbring 2014 | EXCLUDE Outcome = follow‐up too short (adherence measured 2 weeks after intervention) |
| Ostovaneh 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Park 2011 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Parker 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Pearl 2003 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Peng 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Perl 2016 | EXCLUDE Participant mean age ≤ 65 years |
| Persaud 2017 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Peters‐Klimm 2010 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Phumipamorn 2008 | EXCLUDE Participant mean age ≤ 65 years |
| Pitner 2004 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Pladevall 2010 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Pladevall 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Plant 2015, ACTRN12609000554268 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Polack 2008 | EXCLUDE Participant mean age ≤ 65 years |
| Ponnusankar 2004 | EXCLUDE Participant mean age ≤ 65 years |
| Powers 2011 | EXCLUDE Medications = unable to determine if > 4 medications (CVD adherence only; email sent to trial authors for actual number of medications but no response) |
| Pringle 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Pérez‐Escamilla 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Radini 2017 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Ramaekers 2009 | EXCLUDE Medications = unable to determine if > 4 medications (trial authors provided no additional information) |
| Ramanath 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Ravn‐Nielsen 2018 | EXCLUDE Setting = participants not all living in community or discharged from hospital to community (possibility of subgroup analyses not explored as adherence data were not available) |
| Raynor 1993 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Reed 2018 | EXCLUDE Medications ‐ unable to determine eligibility as data on number of medications not collected |
| Richmond 2010 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Roden 1985 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Rose 2016 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Rothschild 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Rozenfeld 1999 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence only to 1 or 2 CV medications) |
| Rubak 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Rytter 2010 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Safren 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Salisbury 2016 | EXCLUDE Medications = unable to determine whether > 4 medications (trials authors collected only info on CVD medications) |
| Samtia 2013 | EXCLUDE Participant mean age ≤ 65 years |
| Sanchez 2012 | EXCLUDE Outcome = follow‐up too short (adherence at 7 days only) |
| Sandler 1989 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Schneider 2008 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence to lisinopril only) |
| Schou 2013 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Schou 2014 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability (conference abstract to 2013 paper) |
| Schwalm 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Schwartz 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Schwartz 2017 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Scott 2004 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Selak 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Shah 2013 | EXCLUDE Outcome = follow‐up too short (adherence 7 to 14 days only) |
| Sherrard 2009 | EXCLUDE Participant mean age ≤ 65 years |
| Sherrard 2015 | EXCLUDE Participant mean age ≤ 65 years |
| Shuster 1998 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Sidel 1990 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Simkins 1986 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Simoni 2011 | EXCLUDE Participant mean age ≤ 65 years |
| Sit 2016 | EXCLUDE Medications = unable to determine whether > 4 medications (no response from trial authors) |
| Sledge 2006 | EXCLUDE Participant mean age ≤ 65 years |
| Smith 1997 | EXCLUDE Outcome = follow‐up too short (adherence measured at 7 to 10 days only) |
| Smith 2004 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Smith 2007 | EXCLUDE Medications = unable to determine whether > 4 medications (no information available from trial authors) |
| Soloman 1998 | EXCLUDE Medications = unable to determine whether > 4 medications (no response from trial authors) |
| Sookaneknun 2004 | EXCLUDE Participant mean age ≤ 65 years |
| Soong 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Souter 2017 | EXCLUDE Medications = unable to determine whether > 4 medications (no response from trial authors) |
| Stange 2013 | EXCLUDE Participant mean age ≤ 65 years |
| Stanhope 2013 | EXCLUDE Participant mean age ≤ 65 years |
| Strobach 2000 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Stromberg 2005 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Sweeney 1989 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Tai 2014 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Touchette, 2012 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Towfighi 2017 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Tu 1999 | EXCLUDE Setting = participants not living in community or discharged from hospital to community |
| Tu 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (hypertensive medications only) |
| Vaillant‐Roussel 2016 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Valimaki 2017 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Varleta 2017 | EXCLUDE Participant mean age ≤ 65 years, or more than 20% aged 65 years |
| Varma 1999 | EXCLUDE Medications = unable to determine whether > 4 medications (trial authors did not collect this information) |
| Verbeek 2016 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Via‐Sosa 2013 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Villani 2014 | EXCLUDE Medications = unable to determine whether > 4 medications (no response from trial authors) |
| Vinks 2009 | EXCLUDE Study design not RCT, cluster‐RCT, nor quasi‐RCT |
| Vivian 2002 | EXCLUDE Participant mean age ≤ 65 years |
| Vollmer 2014 | EXCLUDE Participant mean age ≤ 65 years |
| Wakefield 2011 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Wakefield 2012 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Wandless 1981 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 |
| Wang 2013 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Wild 2016 | EXCLUDE Participant mean age ≤ 65 years |
| Williams 2004 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (adherence to diabetic medication only) |
| Williford 1995 | EXCLUDE Participant mean age ≤ 65 years |
| Yatabe 2018 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (hypertension medications only) |
| Yu 2012 | EXCLUDE Participant mean age ≤ 65 years |
| Zermansky 2002 | EXCLUDE Outcome = not medication adherence nor medication‐taking ability |
| Zhao 2004 | EXCLUDE Participant mean age ≤ 65 years |
| Zhao 2015 | EXCLUDE Medications = unable to determine if > 4 medications (trial authors did not provide information regarding number of medications) |
| 李静, 2017 | EXCLUDE Medications < 4 regular medications or group mean ≤ 4 (hypertension medications only) |
BP: blood pressure.
COPD: chronic obstructive pulmonary disease.
CV: cardiovascular.
CVD: cardiovascular disease.
GP: general practitioner.
RCT: randomised controlled trial.
TB: tuberculosis.
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12606000247572.
| Methods | RCT |
| Participants |
Inclusion: (i) Australian veteran or war widow(er)s living in the community aged 39 to 97 years; (ii) receiving more than 5 medicines every day; or (iii) having 3 or more concurrent medical conditions. Participation restricted to veterans who are willing to use just 1 local medical officer (LMO) and 1 community pharmacy for the duration of the trial, and whose LMO and community pharmacy are willing to participate Exclusion: already using a DAA, residing in an aged care facility, or participating in other studies with similar aims |
| Interventions |
Intervention: involved the veteran's local medical officer (LMO) prescribing a DAA in which the veteran's pharmacy packed and dispensed the veteran's medication for the 12 months of the intervention phase Control: usual care |
| Outcomes | Change in adherence and number of medications found in the home, change in GP‐rated severity of illness |
| Notes | ACTRN12606000247572 First enrolment December 2000. No protocol nor results published Investigators could not be contacted |
ACTRN12611000452998.
| Methods | RCT |
| Participants |
Inclusion: aged 18 to 80 years and with a working diagnosis of acute coronary syndrome, who are admitted to 2 public, acute care hospitals, will be screened for enrolment into the trial Exclusion: not being discharged home, documented cognitive decline, non‐Medicare eligibility, presence of a terminal malignancy |
| Interventions |
Intervention: patients will be offered usual post‐discharge care and a directed home medicines review at 2 months post discharge (i.e. acute coronary syndrome as a referral trigger). The pharmacist review should occur at or near 2 months post discharge. Accredited pharmacists completing the reviews will be given additional training and a brief assessment quiz on evidence‐based management of acute coronary syndrome (ACS) and how to include this into an ACS‐specific home medicine review Control: usual care |
| Outcomes | Primary outcome will be the proportion of patients who are adherent to a complete, guideline‐based medication regimen (using medication possession ratio assessed by dispensing records). Secondary outcomes will include hospital re‐admission rates, length of hospital stays, changes in quality of life, smoking cessation rates, cardiac rehabilitation completion rates, and mortality |
| Notes | ACTRN12611000452998 First enrolment 25/04/2012. Protocol published 2012. Intending to publish results soon Investigator contacted: Dr. Bernal (email: ddbernal@utas.edu.au) confirmed participant mean age > 65 years and mean number of medications > 4 |
ACTRN12616000910404.
| Methods | Sequential mixed methods with a nested pilot RCT |
| Participants | Inclusion: 18 years or older; established diagnosis of cardiac disease and referred to cardiac rehabilitation; ≥ 1 cardioprotective medication; must have primary responsibility for taking medications; able to speak, read, and understand English; own a mobile phone (able to receive and reply to phone/text messages); willing to give written and oral consent; ≥ 1 medication non‐adherence factor |
| Interventions | Patients identified as non‐adherent based on the result of exploratory phases (phases 1 and 2) will be invited to participate in the pilot randomised controlled trial (RCT) phase Intervention: participants will receive usual care plus behavioural counselling about medication adherence using motivational interviewing (MINT) techniques and text message reminders (TM). Each patient will receive approximately 30 to 40 minutes of a single MINT counselling session by the researcher following recruitment. The MINT counselling will be delivered by the researcher, who is a registered nurse. TM will be sent to participants: 1 text message daily for 2 weeks, then on alternate days for 2 weeks, then on a weekly basis for the next 6 months Control: usual care |
| Outcomes | Self‐reported medication adherence rate ‐ assessed by Medication Adherence Questionnaire (MAQ) |
| Notes | ACTRN12616000910404 First enrolment: 1/09/2016. Last data collection: 03/07/17 Trial not completed due to lack of participants within inclusion criteria. Manuscript currently under review (January 2019) Investigator contacted: Dr. Ali Al‐Ganmi (email: ali.h.al‐ganmi@student.uts.edu.au) provided unpublished data: 120 participants = 82 (68.3%) aged 65 years or older, 66 (55%) used 4 or more medications |
ACTRN12617000665336.
| Methods | RCT |
| Participants |
Inclusion criteria: (1) attend medical follow‐up in Specialized Out‐Patient Clinic (SOPC) of the Department of Medicine, (2) are 65 years or older, (3) have hyperpolypharmacy (defined as 10 or more regular drugs, and (4) agree to provide oral informed consent Exclusion criteria: (1) patients who are cognitively impaired (defined as a clinical diagnosis of dementia or mild cognitive impairment and/or not communicable and do not have caregivers, (2) patients who had received pharmacist medication review within 6 months before randomisation |
| Interventions |
Intervention: patients receive a pharmacist medication chart review, which includes assessing the appropriateness of each of the regular medications based on laboratory findings, medication lists, consultation and discharge notes, procedures, and test results. Face‐to‐face interview (lasts around 30 to 45 minutes) will then be conducted with patients on the day before SOPC follow‐up. Clinical pharmacists will assess drug use history, identify drug‐related problems, and provide drug therapy interventions through written pharmacist note to physicians during SOPC follow‐up, based on the medication chart review and the above pharmaceutical assessments. Immediately after SOPC follow‐up, clinical pharmacist will provide education (which lasts about 15 minutes) on drug‐related problem identified before the visit, reinforce physician’s instruction, and encourage drug compliance using written patient educational leaflets. Phone follow‐up will be conducted 1 month after the pharmacist intervention Control: usual care at SOPC |
| Outcomes | Medication adherence measured by Morisky Medication Adherence Scale (MMAS‐4), change in number of drug‐related problems, 30‐day unplanned hospital admission, patient satisfaction |
| Notes | ACTRN12617000665336 Start date: 7 March 2017. Anticipated last data collection: 30 September 2017 Project proposal published on trial registry website. No published results; unsure if study ever completed Investigator Miss Heidi Chan (email: cyh123@ha.org.hk) contacted for further information – no response |
Ahmad 2010.
| Methods | RCT |
| Participants |
Inclusion: patients older than 60 years; using 5 or more prescription‐only chronically used drugs when admitted to the hospital; discharged from the department of cardiovascular disease, lung disease, or internal medicine Exclusion: discharged to nursing home, too confused to participate, terminally ill, unable to communicate in Dutch |
| Interventions |
Intervention: systematic medication review by community pharmacists. Pharmacy technicians will counsel patients at home at baseline and at 1, 3, 6, 9, and 12 months, using cognitive‐behavioural therapy according to the theory of planned behaviour Control: usual care |
| Outcomes | Persistence of drug adherence, quality of life, re‐hospitalisation related to medicines and cost adherence, occurrence of drug‐related problems, attitudes toward drugs |
| Notes | NTR1194 Protocol published 2010. Baseline (qualitative) published 2012. Conference abstract 2017 Investigator contacted for follow‐up adherence data ‐ no response |
Cao 2017.
| Methods | RCT |
| Participants |
Inclusion: (i) aged ≥18 years; (ii) diagnosed with coronary heart disease and admitted for the first time; (iii) lived in the central districts of Chengdu; (iv) returned to the home residence, not to long‐term care facilities, after discharge; (v) could be contacted by mobile phone after discharge; and (vi) agreed to participate in the study Exclusion: patients with visual or hearing impairment, mental disorder, or dementia |
| Interventions |
Intervention: transitional care programme. (1) Cardiologist and hospital nurse evaluated medications at admission; (2) cardiologist educated patients about medications during hospital stay and nurse provided written self‐management advice; (3) written and individualised discharge plan developed by cardiologist, nurse, patient, and caregiver day before discharge, (4) post‐discharge nurse sent discharge plan to home nurse, who created electronic health record and notified family physician. Nurse and physician provided structured telephone calls to patients during weeks after discharge Control: usual care |
| Outcomes | Medicine adherence using 8‐item Morisky Medication Adherence Scale, hospital re‐admission rates after discharge, chronic disease self‐efficacy, quality of care transitions |
| Notes | Results published 2017 Mean/median number of medications unclear ‐ investigator contacted, no response |
Char 2017.
| Methods | RCT |
| Participants |
Inclusion: ≥ 21 years old; taking ≥ 5 long‐term medications; self‐administering own medication (or accompanied by caregiver); able to speak English, Mandarin, or Malay; attending first follow‐up visit to clinic for chronic disease management following recent discharge from a local public hospital or emergency department short stay ward Exclusion: nursing home residents, attending clinic for acute condition, or unwilling to consent to 30‐day follow‐up phone call |
| Interventions |
Intervention: pharmacist conducted medication reconciliation with patient before physician consultation and best possible medication history was created Control: usual care Both groups underwent medication reconciliation with a different pharmacist after physician visits |
| Outcomes | Medication adherence using 8‐item Morisky Medication Adherence Scale (MMAS‐8), rehospitalisation |
| Notes | Not published – was retracted from journal; trial author (Dr. Kok Wai) contacted for unpublished manuscript and stated desire to change journal Note: mean age 74.8/73.7, thus eligible |
Cheema 2017.
| Methods | RCT |
| Participants | Patients will be identified from a larger study, TAPER (Team Approach to Polypharmacy Evaluation and Reduction) Inclusion: 70 years or older, currently taking 5 or more medications, did not have a recent comprehensive medication review, participating family doctor as most responsible provider, patient of the McMaster Family Health Team Exclusion: English language or cognitive skills inadequate to understand and respond to rating scales. Terminal illness or other circumstance precluding 13‐month study period |
| Interventions |
Intervention: a medication wallet card will be given to the intervention group. This will be personalised for each patient and will include the patient's medications, dosages, and medical conditions. It will be personally given to a patient after a medical appointment with the family physician Control (placebo intervention): a reminder card will be given to this group. The card will not be personal and will be mailed to patients. It will state, "Remember to keep an up‐to‐date listing of your medications and bring your medications to your doctor's appointments" |
| Outcomes | Quote: “quantification of medication discrepancies (including dosages and frequency, unidentified discontinued medications, and those prescribed by others not included in electronic medication record)” |
| Notes |
NCT02820129 Start date: July 2016. Estimated completion: December 2018 Conference abstract 2017; no other published results found Unclear whether primary outcome relevant. Investigator A/Prof Ainsley Moore (email: amoore@mcmaster.ca) contacted for further information – no response |
ChiCTR‐TRC‐12002106.
| Methods | RCT |
| Participants |
Inclusion: aged ≥ 65, with acute coronary syndrome in department of cardiology of West China Hospital, Sichuan University Exclusion: lacked self‐care ability; did not ensure buying medicine at the same site; refused to attend the test or sign the consent form |
| Interventions |
Intervention: patients given picture album of daily self‐questionnaires for improving medication adherence in addition to conventional drug education Control: usual care (conventional drug education) |
| Outcomes | Adherence rate (unclear how this is measured) |
| Notes |
http://www.chictr.org.cn/showproj.aspx?proj=7443 Investigators (email: hmaochen@vip.sina.com) contacted for results ‐ no response Need to determine mean/median number of medications to confirm eligibility |
Demers 2014.
| Methods | Pilot RCT |
| Participants | Inclusion: patients with heart failure |
| Interventions | Intervention: multi‐component intervention to enhance HF care after discharge, with or without the support of a CP for the patient. The 3‐month intervention included: (1) a talking scale, (2) a diuretic decision support tool, (3) literacy‐sensitive HF home‐based educational sessions, and (4) an HF‐specific hospital discharge summary sent immediately to the primary care physician |
| Outcomes | Medication adherence with the medication possession ratio; self‐care was assessed using the SCHFI for the patient and CP; HF knowledge was explored with the Knowledge Assessment questionnaire; CP burden was measured with the modified Oberst Scale |
| Notes | Conference abstract only Patient enrolment started in October 2012 with last follow‐up visit planned for July 2014. Full results plan for submission January 2019 Age and number of medications unknown (mean age of first 85 patients recruited was 76 years) Investigator contacted: Dr. Catherine Demers, Hamilton Health Sciences, Canada |
Flink 2016.
| Methods | RCT |
| Participants |
Inclusion: 18 years or older with COPD or congestive heart failure admitted at a short‐term medical ward and living in own private home Exclusion: diagnosis of dementia, need for an interpreter to communicate in Swedish |
| Interventions |
Intervention: included patients' transition to home will be bridged through a telephone call from a patient activation coach 2 days post discharge. Patients will thereafter have motivational interviewing sessions by the same patient activation coach with the goal that patients are motivated to acquire the knowledge, skills, and confidence needed to manage the 4 main activity areas: (1) medication management; (2) adherence to care plan/follow‐up visits according to the discharge plan; (3) recognition of indications (symptoms/signs) that the condition is worsening and how to respond; and (4) contact with and management of relations/encounters with healthcare providers. Patients in control group will receive standard care (i.e. discharge and follow‐up as in normal procedures) Control: usual care |
| Outcomes | Medication adherence (Morisky), rehospitalisation, healthcare usage, patient activation, health‐related quality of life, basic psychological needs, depression |
| Notes |
NCT02823795 Protocol published 2016. Estimated study completion date September 2018 Need to determine mean/median age and number of medications to confirm eligibility Investigator Dr. Maria Flink (email: maria.flink@ki.se) contacted for more information ‐ no response |
Madarshahian 2018.
| Methods | RCT |
| Participants | Inclusion: > 50 years, history of stroke longer than 1 year, more than 5 years of schooling, recruited from neurology and stroke clinic |
| Interventions | Intervention: key family members taught in small groups according to their educational needs Control: unclear (? usual care) |
| Outcomes | Medication adherence using Morisky Medication Adherence Scale (MMAS), cognition (MMSE) |
| Notes | Conference abstract only. Unable to find any published protocol or results Investigator F. Madarshahian contacted – no response Note: need to determine mean/median age and mean/median number of medications to confirm eligibility |
Marusic 2018.
| Methods | RCT |
| Participants |
Inclusion: ≥ 18 years old, T2DM diagnosis, hospital discharge to the community Exclusion: cognitive disorders, terminal illness (< 1 month life expectancy), transfer to other hospital or discharge to a long‐term care facility, refusal to participate |
| Interventions |
Intervention: participants received additional 30‐minute individual pre‐discharge pharmacotherapeutic education by a qualified physician about discharge medication (indications, dosage, administration, importance of adherence, and possible adverse drug reactions). Participants were given the same information in writing Control: usual care Both groups during the hospital stay received standardised diabetes education, including education about the disease, diet, physical activity, alcohol intake, smoking, diabetes medications, glucose self‐monitoring, and acute and chronic diabetes complications |
| Outcomes | Medication adherence using pill count, adverse drug reactions, hospital re‐admissions, emergency department visits, and mortality |
| Notes |
NCT03438162; completed trial Note: median age 72/71, mean number of prescribed drugs 7.5/7.3 |
Muth 2018.
| Methods | Cluster‐RCT |
| Participants |
Clinic inclusion: providing primary care under the German statutory health insurance system, at least 1 healthcare assistant staff member able to access the Internet Clinic exclusion: practices specialising in unconventional treatments or in special indications (e.g. HIV) Participant inclusion: ≥ 60 years old, ≥ 3 chronic conditions (affecting ≥ 2 different organ systems) under pharmacological treatment, ≥ 5 long‐term prescription drugs with systemic effects, made ≥ 1 practice visit during the past quarter, able to fill in questionnaires and participate in telephone interviews Participant exclusion: MMSE < 26, life expectancy ≤ 12 months, alcohol and drug abuse, participation in another clinical trial 30 days before inclusion |
| Interventions |
Intervention: 4 elements: (1) a brown bag review, and (2) a checklist‐based preconsultation interview with the patient conducted by the healthcare assistant, (3) a computerised decision support system (CDSS)‐assisted medication review carried out by the GP, and (4) a GP–patient consultation to optimise and prioritise medication Control: usual care Both groups: practice team received GP guidelines for ambulatory geriatric care |
| Outcomes | Medication adherence using Morisky and observed adherence (difference between patient‐reported and GP‐prescribed), hospitalisations, quality of life (EQ‐5D), functional status (Vulnerable Elderly Survey‐13 items) |
| Notes | ISRCTN99526053; NCT01171339 ‐ completed trial Note: this is the full report of the Muth 2016 pilot study |
NCT00560001.
| Methods | RCT |
| Participants |
Inclusion: coming up for regular review in case or medication management; requiring medication management services; having 2 or more doses of medication per day; having someone to fill MD.2; in independent living (may be assisted living with NO medication management services); expected to live through follow‐up period of 6 months; having an active phone line that can be utilised by the MD.2 system Exclusion: having someone available to administer medications for every dose; having someone in household who is likely to interfere with MD.2; blind and deaf; eligible for hospice; having an MD.2 currently |
| Interventions |
Intervention: MD.2 medication dispenser machine that provides verbal and auditory explicit reminders for individuals to take their medication Control: usual care (i.e. no MD.2 machine) |
| Outcomes | Medication adherence (unclear if just for intervention group), hospitalisations, emergency department visits |
| Notes |
NCT00560001 Start date: January 2006. Estimated completion: May 2008 No published protocol or results Investigator Karen Farris contacted (email: karen-farris@uiowa.edu) – no response Note: unable to confirm eligibility as mean/median number of medications unclear |
NCT00916214.
| Methods | RCT |
| Participants |
Inclusion: > 65 years taking > 2 medications Exclusion: living in nursing home, people receiving unit dose dispensing by medicine |
| Interventions |
Intervention: pharmaceutical care programme involving individualised medication education and regular follow‐up Control: unclear (? usual care) |
| Outcomes | Medication adherence (unclear how it was measured) |
| Notes |
NCT00916214 Start date: June 2009. End date: June 2011 No published protocol or results Investigator Muhammad Saeed not contactable Note: need to determine mean/median number of medications to confirm eligibility |
NCT01105104.
| Methods | RCT |
| Participants |
Inclusion: ≥ 55 years, coming in for routine outpatient visits, speaks and reads English, history of high blood pressure, systolic BP ≥ 130 mmHg, using antihypertensive medication, using ≥ 2 prescription medications, plans to stay in area for the 9 months of the study Exclusion: receives personal help or reminders to take medication, moderate to severe dementia (MMSE < 18), severe hearing or vision deficiency |
| Interventions |
Intervention: MedMinder System. Participants will receive a fully activated reminder unit as well as at least 1 call per month from a counsellor. The in‐home ReMinder will use a familiar pillbox layout (4 doses/d for 7 days) and will allow easy removal of medication cups by elderly, rheumatic fingers. The built‐in pager will continuously download remotely programmed visual and/or aural prompts and reminders from a central server (RemoteMind). It will continuously upload the date and time when each medication cup is removed, and when weekly refill is carried out, enabling remote adherence monitoring, alerts to caregivers, and follow‐up intervention(s) from personal and/or professional caregivers as needed Control: MedMinder deactivated. Participants will receive a 1‐way reminder unit that will remotely transmit information on medication adherence |
| Outcomes | Medication adherence, change in self‐efficacy about taking medication, self‐reported medication‐taking, change in systolic blood pressure |
| Notes |
NCT01105104 Start date: September 2011. Estimated completion: June 2013 Investigator Dr. Sundar Natarajan (sundar.natarajan@va.gov) contacted for results ‐ no response Note: need to determine mean/median participant age and number of medications to confirm eligibility |
NCT01534559.
| Methods | RCT |
| Participants | Inclusion: > 18 years admitted to hospital and meeting at least 1 of the following: ≥ 5 medications, ≥ 3 changes to medication in hospital, past history of medication‐related problems, patient referred to medicines management clinic service by hospital doctor or pharmacist due to concerns about ability to manage medicines |
| Interventions | Intervention: medicines management clinic within an outpatient setting as well as follow‐up telephone calls from a clinical pharmacist (extension of ongoing integrated medicines management programme (IMMP)) |
| Outcomes | Medication adherence (using medication adherence report scale and beliefs about medicines questionnaire), time to re‐admission to hospital, number of re‐admissions, number of GP consultations/home visits, number of accident and emergency visits, health‐related quality of life, cost utility analysis |
| Notes |
NCT01534559 Study start date: November 2014. Final data collection: December 2017 Manuscripts currently being drafted Investigator Anita Hogg (email: anita.hogg@northerntrust.hscni.net) contacted and confirmed participant mean age > 65 years and mean number of medications > 4 |
NCT02047448.
| Methods | RCT |
| Participants |
Inclusion: 18 years and older, admitted to hospital with heart failure or COPD, anticipated eventual discharge to home, agreeable to participate in monthly counselling sessions Exclusion: cognitive impairment, non‐English speaking, anticipated discharge to a long‐term care or skilled nursing facility, permanent long‐term care facility resident, surgical patient, hospice patient, patients who die within 30 days of initial hospitalisation |
| Interventions |
Intervention: hospital pharmacist will meet with the patient and complete medication reconciliation, assess patient's understanding of the medications, and identify medication‐related problems. Hospital pharmacist will complete a pharmacist discharge care plan, and a copy will be sent to the participating community pharmacist. Patients will be scheduled for the first meeting with their community pharmacist within 1 week of hospital discharge. Community pharmacist will interview the patient about his or her general health and any current symptoms of heart failure or COPD, identify any additional medication‐related problems, follow up on any issues as described in the pharmacist discharge care plan, and provide patient education. Patients will then meet with their community pharmacist for counselling and patient education at monthly intervals for 6 months following hospital discharge Control: usual care |
| Outcomes | Medication adherence (proportion of days covered calculation), medication‐related problems, patient satisfaction, hospital re‐admissions or ED visits |
| Notes |
NCT02047448 Note: need to determine mean/median age and mean/median number of medications to confirm eligibility Contacted investigator ‐ A/Prof Judith Kristeller, Wilkes University ‐ no response |
NCT02424786.
| Methods | RCT |
| Participants | Inclusion: patients > 65 years in dialysis treatment or with CKD stage 5 |
| Interventions | Medication lists from patients randomised to the intervention group will be evaluated by the research physician with the help of STOPP/START criteria Feedback on this screening will be given to the team responsible for patient treatment |
| Outcomes | Medication non‐adherence ‐ measured by Morisky Medication Adherence Scale and visual adherence scale Secondary outcomes: improvement in polypharmacy, associations between beliefs about medication/anxiety/depression with adherence and QoL, predictors of non‐adherence, risk factors for non‐adherence, changes in number of inappropriate medications |
| Notes |
NCT02424786 Start date: May 2015. Study completion: September 2017 Results manuscripts currently being prepared Investigator contacted ‐ Krystina Parker confirmed: 180 patients, mean number of medications 11.1 (range 4 to 19) |
NCT02842840.
| Methods | RCT |
| Participants |
Inclusion: participants aged > 65 years and able to give informed consent Exclusion: recurrent stroke, diagnosis of subarachnoid haemorrhage, significant impairments precluding participation, another condition likely to impact participation (e.g. life‐threatening condition), expected discharge to hospital/nursing home setting |
| Interventions |
Intervention: combined patient and family‐based intervention involving behavioural treatment and series of educational/motivational interventions Control: usual care |
| Outcomes | Patient‐reported medication adherence rating scale, changes in blood pressure, changes in intention to medication adherence, changes in action plan, changes in coping plan, changes in quality of life, changes in perceived behavioural control of medication adherence, changes in self‐monitoring of medication adherence, changes in illness perceptions (Brief Illness Perception Questionnaire) |
| Notes |
NCT02842840 Estimated study completion: November 2017 Investigator contacted (email: amir.pakpour@gmail.com) ‐ no results provided, currently being analysed Note: need to determine mean/median number of medications to confirm eligibility |
NCT03156348.
| Methods | RCT (3 groups) |
| Participants |
Inclusion: patients 60 years or older attended by the staff of internists of the internal medicine service of the Clinical Hospital of the University of Chile for acute condition or decompensation of chronic pathology; with estimated survival > 6 months; on pharmacological therapy; having a contact person or responsible caregiver willing to comply with the scheduled care plan; and having a contact telephone number Exclusion: cognitive impairment and no caregiver, any other condition that in the judgement of the research team affects the quality of the collection of information |
| Interventions |
Intervention: during hospitalisation and at discharge, a clinical pharmacist (CP) will monitor daily pharmacological safety and efficacy of the medication to assess and make appropriate recommendations. CP will explain the reasons for use of each of the drugs. At 30 days post discharge, CP will review the updated clinical record of the patient and will conduct a home visit to enhance and ask about adherence, self‐medication, medication use at that time, and possible results of laboratory tests performed and to clarify doubts regarding the use of current medications. The same activities will be conducted at 60 days by telephonic way, to reinforce the recommendations Parallel control: usual care by team with training on pharmacogeriatrics Historical control group: usual care (no training) |
| Outcomes | Adherence measured with Morisky and Green scale, adverse drug events, hospitalisations, prevalence of self‐medication (taking a drug without medical indications) |
| Notes |
NCT03156348 Start date: May 2015. Estimated completion: December 2017 Investigators contacted for more information (email: comiteetica@hcuch.cl) ‐ no response Note: need to determine mean/median number of medications to confirm eligibility |
NCT03162848.
| Methods | Pilot RCT |
| Participants | Inclusion: 50 years or older; heart failure diagnosis; prescribed diuretics; self‐administering medications; able to open an electronic cap; able to speak, hear, and understand English; not hospitalised; no cognitive impairment |
| Interventions |
Intervention: The SystemCHANGE™ intervention utilises the socioecological model and the Plan‐Do‐Check Act model as its framework and focuses on changing the individual's environment to change behaviour using small experiments with feedback. At initial home visit, the PI will work with the participant to identify important people for medication‐taking, routines, and cycles of routines. Possible solutions to incorporate medication‐taking into routines will be identified by the participant and the PI, and the participant will start to implement these solutions. Medication adherence will continuously be monitored via medication event monitoring systems. At 1 month, the participant will be sent a report on medication‐taking and a phone call with the PI will occur to discuss whether solutions improved medication adherence or whether other solutions need to be implemented. At month 2, the intervention will end but participants are urged to continue to use solutions long term Control: usual care with education at baseline, 1 month, and 2 months |
| Outcomes | Medication adherence using medication event monitoring systems, acceptability and feasibility using open‐ended questionnaire, systems thinking using questionnaire, Kansas City Cardiomyopathy questionnaire |
| Notes |
NCT03162848 No protocol or results published. Estimated study completion date: July 2018 Need to determine mean/median number of medications to confirm eligibility Investigator contacted for more information ‐ no response Investigator contacted: Angela Andrews, University of Missouri, Kansas City |
Ostbring 2018.
| Methods | RCT |
| Participants |
Inclusion: 18 years or older, admitted for angiography, verified coronary artery disease, planned for follow‐up at the outpatient clinic, Swedish speaking Exclusion: cognitive impairment or any other condition making interview or phone calls impossible, non‐participation in standard follow‐up, prior participation in this study (pilot) |
| Interventions |
Intervention: medication review and motivational interviewing (MI) for patients with coronary heart disease (CHD). Clinical pharmacists competent in MI and cardiology will conduct medication interviews and medication reviews at the outpatient clinic. Intervention will continue during 9 months, with interviews and reviews as needed. Follow‐up of results will take place 16 months after inclusion (corresponding to 4 months after end of intervention) Control: usual care |
| Outcomes | Cholesterol levels, percentage of patients adherent to individual medications (cholesterol, ACE inhibitors, acetylsalicylic acid, PSY‐12 antagonist, and beta‐blocker), blood pressure, quality of life, hospital re‐admissions, emergency department visits |
| Notes |
NCT02102503 Protocol published 2018. Extension of earlier pilot study (Ostbring 2014), which was excluded due to short follow‐up Unclear whether overall adherence measure will be provided. Age and number of medications unavailable (but pilot study met eligibility) Publication anticipated 2019 |
Prados‐Torres 2017.
| Methods | Cluster‐RCT |
| Participants |
Inclusion: 65 to 74 years, multi‐morbidity (3 or more chronic diseases), polypharmacy (5 or more drugs taken for at least 3 months), at least 1 visit to family physician in past year, agree to participate and provide written informed consent Exclusion: institutionalised at nursing home or similar, life expectancy < 12 months, mental and/or physical conditions considered by family physician to prevent fulfilment of study requirements |
| Interventions |
Intervention: family physicians receive training related to multi‐morbidity, appropriateness of prescribing, treatment adherence, Ariadne principles, and physician‐patient shared decision‐making. Then physicians conduct physician‐patient interviews based on Ariadne principles including structured review of treatment plan, inclusion of patient preferences, and a pharmacological treatment plan Control: usual care |
| Outcomes | Medication adherence using Morisky‐Green questionnaire and Haynes‐Sackett questionnaire, health‐related quality of life (EQ‐5D‐5L), use of health services (hospitalisations, emergency services, and primary care), medication safety (incidence of adverse drug reactions), and cost utility (time spent on training family physicians, cost of teaching staff, time spent on physician‐patient interviews, utilities measured using the EuroQol‐5D‐5L) |
| Notes |
NCT02866799 Start date: November 2016. End date: February 2018 Protocol published 2017. Results not yet published Investigator: Alexandra Prados‐Torres (email: sprados.iacs@aragon.es) |
Reilly 2016.
| Methods | Pilot RCT |
| Participants | Inclusion: 60 inpatients with congestive heart failure |
| Interventions | Intervention: multi‐component health education intervention that combines (a) an interactive computer‐based CHF education video viewed during hospital stay and geared toward helping patients understand CHF and its management, (b) educational weekly mailings to reinforce the Kognito video, and (c) 4‐weekly post‐discharge phone counselling using motivational interviewing techniques |
| Outcomes | Medication adherence using the Morisky Medication Adherence Scale, Atlanta HF Knowledge Score, HF‐specific quality of life using the Minnesota Living With Heart Failure questionnaire (HF‐specific), general quality of life using the SF‐36 physical component score, diet adherence using sodium intake estimated from 24‐hour dietary recall |
| Notes | Conference abstract published 2016. No full paper Investigator contacted: Dr. Natarajan Sundar (email: Sundar.Natarajan@nyulangone.org). Intention to publish, not published yet Age and number of medications unknown ‐ trial author believes > 65 years and > 4 medications. Need to confirm when results available |
ACS: acute coronary syndrome.
COPD: chronic obstructive pulmonary disease.
CP: clinical pharmacist.
DAA: dose administration aid.
GP: general practitioner.
HF: heart failure.
LMO: local medical officer.
MAQ: Medication Adherence Questionnaire.
MINT: motivational interviewing technique.
MMAS: Morisky Medication Adherence Scale.
MMSE: Mini‐Mental State Examination.
RCT: randomised controlled trial.
SCHFI: Self‐Care Heart Failure Index.
SOPC: Specialized Out‐Patient Clinic.
T2DM: type 2 diabetes mellitus.
TM: text message.
Characteristics of ongoing studies [ordered by study ID]
Adam 2019.
| Study name | OPtimising thERapy to Prevent Avoidable Hospital Admissions in the Multimorbid Older People (OPERAM) |
| Methods | Multi‐centre cluster‐RCT |
| Participants |
Inclusion: hospitalised patients ≥ 70 years with ≥ 3 chronic medical conditions and concurrent use of ≥ 5 long‐term medications at 1 of 4 participating study centres Exclusion: inability to provide informed consent, direct admission to palliative care (< 24 hours after admission), has passed or will pass a systematic structured drug review during this hospitalisation or within last 2 months |
| Interventions |
Intervention group: Systematic Tool to Reduce Inappropriate Prescribing (STRIP) intervention during initial hospital admission (index hospitalisation) or an equivalent situation for outpatients. STRIP intervention consists of 9 steps: (1) structured history‐taking of medication; (2) recording of medication and diagnoses in decision‐support software, (3) structured drug review based; (4) communication and discussion of structured drug review with prescribing physician; (5) shared decision‐making with patient; (6) optional revision based on new accumulating data during hospitalisation (e.g. new diagnoses, adverse drug reactions); (7) generation of GP report; (8) delivery of the report to the patient and to the GP; (9) follow‐up Control group: receive usual care and 1 questionnaire will be conducted by the intervention team in both arms, which is considered a sham intervention Patients will be followed up by phone at 2, 6, and 12 months |
| Outcomes | Drug compliance using Morisky Mediation Adherence Questionnaire (MMAS‐8), healthcare utilisation, hospitalisations, mortality, adverse medical events (including adverse drug events and number of falls), quality of life using EQ‐5D |
| Starting date | Start date: December 2016. Anticipated completion: 2019 |
| Contact information | Professor Nicolas Rodondi (email: nicolas.rodondi@insel.ch) |
| Notes |
NCT02986425 Protocol published 2019. Communication with investigator; results expected early 2020 |
Bailey 2017.
| Study name | A Universal Medication Schedule to Promote Adherence to Complex Drug Regimens |
| Methods | RCT Four groups: (1) enhanced usual care alone; (2) daily medication reminders via SMS text messages; (3) medication monitoring via a patient portal‐based assessment; (4) both SMS text message reminders and portal‐based medication monitoring |
| Participants |
Inclusion: (1) age 50 years and older, (2) English or Spanish speaking, (3) currently prescribed 3 or more medications used on a regular basis, (4) primary responsibility for administering one’s own medication, (5) owns a cell phone and feels comfortable receiving texts, (6) Internet access at home, (7) a personal email account, and (8) basic familiarity with text messaging and with using the Internet for email Exclusion: major cognitive, visual, or hearing impairment |
| Interventions |
Enhanced usual care (EHR): patients will receive EHR tools (e.g. patient‐friendly medication lists) EHR + Text: EHR tools + text message reminders telling them to take their medicine EHR + Portal: EHR tools + enrolment into their clinic’s portal. Patients prompted every other week to fill out an online survey asking if they filled their medication and about any side effects or concerns. Concerns and questions followed up by nurse EHR + Text + Portal: EHR tools, text reminders, and portal communication (i.e. all interventions) |
| Outcomes | Medication adherence (self‐report, pill count, and proportion of days covered, with medication based on pharmacy claims), clinical markers (HbA1c, blood pressure, and cholesterol) |
| Starting date | Start date: April 2017. Anticipated completion date: March 2020 |
| Contact information | Dr. Stacey Bailey (email: scbailey@unc.edu) |
| Notes |
NCT02820753 Protocol published 2017 Need to confirm mean/median age > 65 years when results are available |
Dodge 2018.
| Study name | Internet‐Based Conversational Engagement Clinical Trial (I‐CONNECT study) |
| Methods | RCT |
| Participants | Socially isolated participants aged 80 and older (Lubben's Social Network Scale Y 12 and other criteria) will be recruited from a local Meals on Wheels programme in Portland, Oregon, and Detroit, Michigan, the latter targeting African American participants Note: website states participants aged 75 and older |
| Interventions |
Intervention: face‐to‐face communications with trained interviewers using an Internet‐enabled link to a study‐dedicated home computer will be conducted 4 times per week for 6 months and twice per week for additional 6 months Control: brief weekly phone calls |
| Outcomes | Medication adherence monitored by an electronic pillbox (MedTracker), cognitive function in memory and executive domains |
| Starting date | Start: 2018. Results expected: 2023 |
| Contact information | Dr. Hiroko Dodge (email: dodgeh@ohsu.edu) |
| Notes | Two conference abstracts. Website: https://www.i-conect.org/ Investigator contacted ‐ results available 2023 |
ISRCTN13355076, 2018.
| Study name | An educational intervention to promote health‐related quality of life after an acute coronary syndrome |
| Methods | RCT |
| Participants | Inclusion: 21 years or older, hospitalised due to acute coronary syndrome, able to read and write in Portuguese, no diagnosed cognitive condition, able to provide informed consent |
| Interventions |
Intervention: educational intervention. First session 1 hour lecturing and take‐home individualised information booklet on their heart condition, lifestyle changes, medication, etc. Second session involving telephone contact 10 days after discharge to reinforce teaching. Third session involving clinic or home visit to monitor participant’s lifestyle and discuss returning to work Control: usual care Participants in both groups will receive a calendar to register daily activity, number of smoked cigarettes, forgotten medicine, and any complications that may occur |
| Outcomes | Adherence to therapy, number of rehospitalisations, health‐related quality of life (EQ‐5D), clinical markers (body weight, blood pressure, lipids) |
| Starting date | Start date: February 2014. Anticipated end date: July 2019 |
| Contact information | Maria Teresa Leal (email: tleal@esel.pt) |
| Notes | ISRCTN13355076 Investigator contacted for results – reported delay in start date, study still ongoing (September 2019) Need to determine mean/median age and number of medications to confirm eligibility once results are available |
NCT03511027.
| Study name | Medication dispenser to improve care at home for the elderly |
| Methods | RCT |
| Participants |
Inclusion: 18 years and older, manage medications independently at home, stabilised on medication, mild to moderate cognitive/physical impairments, Montreal Cognitive Assessment (MoCA) not less than 16, English speaking Exclusion: absent from community for longer than 1 month during study, inability to access study site pharmacy following discharge |
| Interventions | All participants will undergo screening for self‐medication readiness, will receive self‐medication education by a study occupational therapist and a 5‐day self‐medication performance assessment by a registered nurse before discharge Intervention: medication self‐management education + orientation to Karie Automated Medication Delivery by the study Occupational Therapist (SME + K). Participants will use the Karie device for all applicable medications for the study duration Control: medication self‐management education (SME) by an occupational therapist followed by a 5‐day self‐medication performance assessment by a registered nurse before discharge. Patients will fill prescriptions as usual for duration of the study |
| Outcomes | Medication adherence using the Medication Adherence Questionnaire (MAQ) and medication 7‐day recall, self‐medication behaviours using the Self‐Efficacy for Appropriate Medication Scale (SEAMS), quality of life (EQ‐5D), health care consumption (hospitalisations, GP visits, and emergency department visits), economic analysis |
| Starting date | Start date: December 2018. Anticipated completion: September 2019 |
| Contact information | Lee Verweel (email: lee.verweel@westpark.org) |
| Notes |
NCT03511027; study protocol available on trial registry Investigator contacted – enrolment ongoing, no available results Need to determine mean/median age to confirm eligibility once results are available |
NCT03722017.
| Study name | Drug reduction in older patients: the DROP trial (DROP) |
| Methods | RCT |
| Participants |
Inclusion: referred to short‐term stay, being discharged from Nashville VA hospital from a medicine or orthopaedics team, age 50 years or older, polypharmacy (> 5 medications), able to self‐consent or have a surrogate Exclusion: resides in long‐term care, on hospice, not expected to be discharged within 48 hours of referral |
| Interventions |
Intervention: pharmacist or nurse practitioner will ascertain indication and de‐prescribing rationale for each medication identified during medication history‐taking. De‐prescribing recommendations will be made as appropriate Control: usual care (including structured interview and chart review by study pharmacist or nurse practitioner) |
| Outcomes | Medication adherence using 12‐item Adherence to Refills Medication Scale (ARMS), unplanned healthcare utilisation, adverse drug withdrawal events, mortality, number of falls, health status (long‐term care, hospice, or death) |
| Starting date | Start date: October 2019. Estimated completion: November 2021 |
| Contact information | Dr. Sandra Simmons (email: Sandra.Simmons@Vanderbilt.edu) |
| Notes |
NCT03722017 Need to determine mean/median age to confirm eligibility once results are available |
Vasilevskis 2019.
| Study name | A randomized controlled trial to de‐prescribe for older patients with polypharmacy transferred from the hospital to skilled nursing facilities (Shed‐Meds) |
| Methods | Randomised controlled trial ‐ 1 hospital and 14 area skilled nursing facilities |
| Participants |
Inclusion: 50 years or older, hospitalised, Medicare‐eligible, discharged to a post‐acute care facility, > 5 medications, speaks English, primary home residence within one of 9 surrounding counties Exclusion: long‐term care, life expectancy < 6 months, enrolled in a clinical drug trial, stage IV cancer, incarcerated, homeless, unable to provide consent (and no surrogate) |
| Interventions |
Inclusion: participants will receive a clinical review of their prescribed medications by a research clinician (pharmacist, physician, and/or nurse practitioner) followed by a patient interview to assess their willingness to discontinue or reduce some of their medicines based on clinical recommendations of the team. Hospital and outpatient providers also will be part of the de‐prescribing decision process. De‐prescribing actions will be initiated in the hospital before discharge and will continue through the skilled nursing facility stay Control: usual care |
| Outcomes | Changes in total number of medications, change in functional health status, change in drug burden index, change in medication adherence (using adherence to refills and medication scale) |
| Starting date | Study start date: March 2017. Completion date: April 2021 |
| Contact information | Dr. Sandra Simmons, Vanderbilt University Medical Center (email:sandra.simmons@vanderbilt.edu) |
| Notes |
NCT02979353 Pilot study published ‐ did not assess adherence Full study ongoing |
Verweij 2018.
| Study name | Cardiac Care Bridge trial (CCB‐trial) |
| Methods | RCT |
| Participants |
Inclusion: 70 years and older, admitted to cardiology or cardiac surgery department, admission > 48 hours, high risk of functional decline Exclusion: MMSE < 15, congenital heart disease, terminal illness (< 3 months to live), transferred from or planned discharge to a nursing home, planned discharge to another hospital/department not participating in study, unable to communicate in Dutch, delirium confirmed by treating physician |
| Interventions |
Intervention: patients will receive a comprehensive geriatric assessment (CGA) performed by a cardiac nurse and care based on CGA integrated care plan, face‐to‐face handover from the cardiac nurse with the community care registered nurse (CCRN) before discharge, and 4 home visits post discharge. CCRNs will collaborate with physical therapists who will perform home‐based cardiac rehabilitation, and with a pharmacist who advises CCRNs in medication management Control: usual care |
| Outcomes | Medication adherence using questionnaire and pharmacy dispensing records, unplanned hospital re‐admission, mortality, health‐related quality of life (EQ‐5D), healthcare utilisation (GP visits, physical therapy, cardiac rehabilitation), clinical parameters (e.g. cholesterol, blood pressure), cost‐effectiveness |
| Starting date | Start date: June 2017. Anticipated completion: March 2020 |
| Contact information | Lotte Verweig (email: l.verweij@hva.nl) |
| Notes | NTR6316 Protocol published 2018 Investigator contacted to confirm eligibility regarding number of medications: at baseline, 74.5% and 81% of participants took 5 or more medications |
ARMS: Adherence to Refills Medication Scale.
CCRN: community care registered nurse.
CGA: comprehensive geriatric assessment.
EHR: electronic health record.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions.
GP: general practitioner.
HbA1c: glycosylated haemoglobin.
K: Karie Automated Medication Delivery.
MAQ: Medication Adherence Questionnaire.
MMSE: Mini‐Mental State Examination.
MoCA: Montreal Cognitive Assessment.
SEAMS: Self‐Efficacy for Appropriate Medication Scale.
SME: self‐management education.
SMS: short message service.
Differences between protocol and review
The MEDLINE search strategy was updated by John Kis‐Rigo, the information specialist with the Cochrane Consumers and Communication Group, after the protocol was published but before the database search was performed. The updated search strategy contained additional terms to minimise the potential for missed studies.
Interventions were grouped as educational, behavioural, or mixed, which was not specified in our protocol. We believe this grouping was necessary to help describe and understand the large number of heterogeneous studies, and this grouping had been used in a previous systematic review of interventions to improve medication adherence (George 2008).
Risk factor(s) for poor medication‐taking ability and/or medication adherence targeted by the intervention(s) were not extracted. In general, this information was poorly reported within the included studies, as medication‐taking ability and/or medication adherence was not always the primary outcome of the included studies. We attempted to extract potential participant factors that may affect medication use (e.g. number of medications used, frailty, cognitive impairment, number of comorbidities); however even this was limited by poor reporting in the included studies. Analysis of specific risk factors targeted by interventions should be considered in future updates of this review, when further high‐quality studies are identified.
We could not conduct several subgroup analyses as planned due to an insufficient number of studies identified within each comparison and each outcome.
Contributions of authors
Writing of protocol and review: AC, RE, JG
Screening of titles and abstracts: AC, RE, KP, LK, JG
Assessment of studies for inclusion: AC, RE, KP, LK, JG
Quality assessment: AC, RE, KP, LK, JG
Data extraction: AC, RE, KP, LK, JG
Data entry into RevMan: AC
Data analysis: AC in consultation with RE and JG
Disagreement resolution: AC, RE, JG
Declarations of interest
Amanda J Cross: none declared.
Rohan A Elliott: none declared.
Kate Petrie: none declared
Lisha Kuruvilla: none declared
Johnson George has received investigator‐initiated research grants from Boehringer Ingelheim (2014), from Pfizer through the Global Research Awards for Nicotine Dependence (2017), and from GSK through Medical Education Grants (2018) for unrelated projects. He has also provided consultancy services to GSK (review of educational materials ‐ 2018) and Pfizer (delivering education sessions as part of CPD – 2019; not for promoting any particular product or molecule). These grants have been largely interdisciplinary and involved multiple investigators. He has not used any part of the funding for his salary or for other personal benefits. He has a tenured academic appointment at Monash University. These funds were paid to his employer (Monash University) and were used to support staff working on those projects or for professional development (e.g. conference attendance).
Rohan Elliott was an author of one study that was potentially eligible for inclusion in this review (Elliott 2012). To avoid bias, two independent members of the review team (AC and JG) screened this study and deemed that it was ineligible for inclusion.
New
References
References to studies included in this review
Al‐Rashed 2002 {published data only}
- Al-Rashed SA, Wright D J, Roebuck N, Sunter W, Chrystyn H. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. British Journal of Clinical Pharmacology 2002;54(6):657-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begley 1997 {published data only}
- Begley S, Livingstone C, Hodges N, Williamson V. Impact of domiciliary pharmacy visits on medication management in an elderly population. International Journal of Pharmacy Practice 1997;5(3):111-21. [Google Scholar]
Bernsten 2001 {published data only}
- Bernsten C, Bjorkman I, Caramona M, Crealey G, Frokjaer B, Grundberger E, et al. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs & Aging 2001;18(1):63-77. [DOI] [PubMed] [Google Scholar]
- Sturgess IK, McElnay JC, Hughes CM, Crealey G. Community pharmacy based provision of pharmaceutical care to older patients. Pharmacy World and Science 2003;25(5):218-26. [DOI] [PubMed] [Google Scholar]
Blalock 2010 {published data only}
- Blalock SJ, Casteel C, Roth MT, Ferreri S, Demby KB, Shankar V. Impact of enhanced pharmacologic care on the prevention of falls: a randomized controlled trial. American Journal of Geriatric Pharmacotherapy 2010;8(5):428-40. [DOI] [PubMed] [Google Scholar]
Bond 2007 {published and unpublished data}
- Bond C. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Family Practice 2007;24(2):189-200. [DOI] [PubMed] [Google Scholar]
- Krska J, Avery AJ, The Community Pharmacy Medicines Management Project Evaluation Team. Evaluation of medication reviews conducted by community pharmacists: a quantitative analysis of documented issues and recommendations. British Journal of Clinical Pharmacology 2008;65(3):386-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cargill 1992 {published data only}
- Cargill JM. Medication compliance in elderly people: influencing variables and interventions. Journal of Advanced Nursing 1992;17(4):422-6. [DOI] [PubMed] [Google Scholar]
Chrischilles 2014 {published data only}
- Chrischilles EA, Hourcade JP, Doucette W, Eichmann D, Gryzlak B, Lorentzen R, et al. Personal health records: a randomized trial of effects on elder medication safety. Journal of the American Medical Informatics Association 2014;21(4):679-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2011 {published data only}
- Cohen LB, Taveira TH, Khatana SAM, Dooley AG, Pirraglia PA, Wu WC. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Education 2011;37(6):801-12. [DOI] [PubMed] [Google Scholar]
Cossette 2015 {published data only}
- Cossette S, Frasure-Smith N, Vadeboncoeur A, McCusker J, Guertin MC. The impact of an emergency department nursing intervention on continuity of care, self-care capacities and psychological symptoms: secondary outcomes of a randomized controlled trial. International Journal of Nursing Studies 2015;52(3):666-76. [DOI] [PubMed] [Google Scholar]
George 2016 {unpublished data only}
- George NR. Medication non-adherence in community dwelling older adults with dementia: an educational intervention for family caregivers. Dissertation available from https://irl.umsl.edu/dissertation/163 2016:No Pagination Specified.
Grymonpre 2001 {published data only}
- Grymonpre RE, Williamson DA, Montgomery PR. Impact of a pharmaceutical care model for non-institutionalised elderly: results of a randomised, controlled trial. International Journal of Pharmacy Practice 2001;9(4):235-41. [Google Scholar]
Haag 2016 {published data only}
- Haag JD, Davis AZ, Hoel RW, Armon JJ, Odell LJ, Dierkhising RA, et al. Impact of pharmacist-provided medication therapy management on healthcare quality and utilization in recently discharged elderly patients. American Health & Drug Benefits 2016;9(5):259-67. [PMC free article] [PubMed] [Google Scholar]
Hale 2016 {published data only}
- Hale TM, Jethwani K, Kandola MS, Saldana F, Kvedar JC. A remote medication monitoring system for chronic heart failure patients to reduce readmissions: a two-arm randomized pilot study. Journal of Medical Internet Research 2016;18(3):e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanlon 1996 {published data only}
- Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. American Journal of Medicine 1996;100(4):428-37. [DOI] [PubMed] [Google Scholar]
Holland 2007 {published data only}
- Holland R, Brooksby I, Lenaghan E, Ashton K, Hay L, Smith R, et al. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ 2007;334(7603):1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khdour 2009 {published data only}
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. British Journal of Clinical Pharmacology 2009;68(4):588-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krska 2001 {published data only}
- Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age and Ageing 2001;30(3):205-11. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA 2006;296(21):2563-71. [DOI] [PubMed] [Google Scholar]
Lim 2004 {published data only}
- Lim WS, Low HN, Chan SP, Chen HN, Ding YY, Tan TL. Impact of a pharmacist consult clinic on a hospital-based geriatric outpatient clinic in Singapore. Annals Academy of Medicine Singapore 2004;33(2):220-7. [PubMed] [Google Scholar]
Lingler 2016 {published data only}
- Erlen JA, Lingler J, Sereika S, Tamres L, Happ MB, Tang F. Characterizing caregiver mediated medication management in patients with memory loss. Journal of Gerontological Nursing 2013;39(4):30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingler JH, Arida J, Happ MB, Sereika SMM, Tamres L, Tang F, et al. Medication errors and related deficiencies by caregivers of persons with memory loss: preliminary results of an intervention to maximize medication management (3M). Alzheimers & Dementia 2014;10:P737. [Google Scholar]
- Lingler JH, Sereika SM, Amspaugh CM, Arida JA, Happ ME, Houze MP, et al. An intervention to maximize medication management by caregivers of persons with memory loss: intervention overview and two-month outcomes. Geriatric Nursing 2016;37(3):186-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT. Improving caregiver mediated medication management - the 3M study. https://clinicaltrials.gov/show/nct03127930 2017.
Lipton 1994 {published data only}
- Lipton HL, Bird JA. The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: a randomized controlled trial. The Gerontologist 1994;34(3):307-15. [DOI] [PubMed] [Google Scholar]
Lopez Cabezas 2006 {published data only}
- Falces C, Lopez-Cabezas C, Andrea R, Arnau A, Ylla M, Sadurni J. [An educative intervention to improve treatment compliance and to prevent readmissions of elderly patients with heart failure]. Medicina Clinica 2008;131(12):452-6. [DOI] [PubMed] [Google Scholar]
- Lopez CC, Falces SC, Cubi QD, Arnau BA, Ylla BM, Muro PN, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farmacia Hospitalaria 2006;30(6):328-42. [DOI] [PubMed] [Google Scholar]
Manning 2007 {published data only}
- Manning DM, O'Meara JG, Williams AR, Rahman A, Myhre D, Tammel KJ, et al. 3D: a tool for medication discharge education. Quality & Safety in Health Care 2007;16(1):71-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marek 2013 {published data only}
- Lancaster R, Marek KD, Bub LD, Stetzer F. Medication regimens of frail older adults after discharge from home healthcare. Home Healthcare Nurse 2014;32(9):536-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek KD, Stetzer F, Ryan PA, Bub LD, Adams SJ, Schlidt A, et al. Nurse care coordination and technology effects on health status of frail older adults via enhanced self-management of medication: randomized clinical trial to test efficacy. Nursing Research 2013;62(4):269-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marusic 2013 {published data only}
- Marusic S, Gojo-Tomic N, Erdeljic V, Bacic-Vrca V, Franic M, Kirin M, et al. The effect of pharmacotherapeutic counseling on readmissions and emergency department visits. International Journal of Clinical Pharmacy 2013;35(1):37-44. [DOI] [PubMed] [Google Scholar]
Messerli 2016 {published data only}
- Messerli M, Blozik E, Vriends N, Hersberger KE. Impact of a community pharmacist-led medication review on medicines use in patients on polypharmacy - a prospective randomised controlled trial. BMC Health Services Research 2016;16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moral 2015 {published data only}
- Moral RR, Torres LA, Ortega LP, Larumbe MC, Villalobos AR, Garcia JA, et al. Effectiveness of motivational interviewing to improve therapeutic adherence in patients over 65 years old with chronic diseases: a cluster randomized clinical trial in primary care. Patient Education and Counseling 2015;98(8):977-83. [DOI] [PubMed] [Google Scholar]
- Perula de Torres LA, Pulido Ortega L, Perula de Torres C, Gonzalez Lama J, Olaya Caro I, Ruiz Moral R, et al. [Efficacy of motivational interviewing for reducing medication errors in chronic patients over 65 years with polypharmacy: results of a cluster randomized trial]. Medicina Clinica 2014;143(8):341-8. [DOI] [PubMed] [Google Scholar]
Morales Suarez‐Vurela 2009 {published data only}
- Morales Suarez-Varela MT, Gemecor. [Study on the use of a smart pillbox to improve treatment compliance]. Atencion Primaria 2009;41(4):185-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1993 {published data only}
- Murray MD, Birt JA, Manatunga AK, Darnell JC. Medication compliance in elderly outpatients using twice-daily dosing and unit-of-use packaging. Annals of Pharmacotherapy 1993;27(5):616-21. [DOI] [PubMed] [Google Scholar]
Muth 2016 {published data only}
- ISRCTN. Prioritising and optimising multi-medication in multimorbidity. http://www.who.int/trialsearch/trial2.aspx?Trialid=isrctn99526053 2010.
- Muth C, Harder S, Uhlmann L, Rochon J, Fullerton B, Guthlin C, et al. Pilot study to test the feasibility of a trial design and complex intervention on PRI oritising MU ltimedication in M ultimorbidity in general practices (PRIMUM pilot). BMJ Open 2016;6(7):e15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nascimento 2016 {published and unpublished data}
Naunton 2003 {published data only}
- Naunton M, Peterson GM. Evaluation of home-based follow-up of high-risk elderly patients discharged from hospital. Journal of Pharmacy Practice and Research 2003;33(3):176-82. [Google Scholar]
Nazareth 2001 {published data only}
- ISRCTN. A randomised controlled trial of a pharmacy care plan for elderly patients discharged from hospital. http://www.who.int/trialsearch/trial2.aspx?Trialid=isrctn66700837 2004.
- Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H. A pharmacy discharge plan for hospitalized elderly patients - a randomized controlled trial. Age & Ageing 2001;30(1):33-40. [DOI] [PubMed] [Google Scholar]
Olesen 2014 {published data only}
- Harbig P, Barat I, Damsgaard E. Suitability of an electronic reminder device for measuring drug adherence in elderly patients with complex medication. Journal of Telemedicine and Telecare 2012;18:352-6. [DOI] [PubMed] [Google Scholar]
- Olesen C, Harbig P, Buus KM, Barat I, Damsgaard EM. Impact of pharmaceutical care on adherence, hospitalisations and mortality in elderly patients. International Journal of Clinical Pharmacy 2014;36(1):163-71. [DOI] [PubMed] [Google Scholar]
Pandey 2017 {published data only}
- Pandey A, Choudhry N. Arest MI: adherence effects of a comprehensive reminder system for post-myocardial infarction secondary prevention. Journal of the American College of Cardiology 2015;65(10 Suppl 1):A1384. [Google Scholar]
Pereles 1996 {published data only}
- Pereles L, Romonko L, Murzyn T, Hogan D, Silvius J, Stokes E, et al. Evaluation of a self-medication program. Journal of the American Geriatrics Society 1996;44(2):161-5. [DOI] [PubMed] [Google Scholar]
Rich 1996 {published data only}
- NCT. Prevention of early readmission in elderly congestive heart failure patients. https://clinicaltrials.gov/show/nct00000475 1999.
- Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. New England Journal of Medicine 1995;333(18):1190-5. [DOI] [PubMed] [Google Scholar]
- Rich MW, Gray DB, Beckham V, Wittenberg C, Luther P. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. American Journal of Medicine 1996;101(3):270-6. [DOI] [PubMed] [Google Scholar]
Saez de la Fuente 2011 {published data only}
- Saez de la Fuente J, Granja Berna V, Lechuga Vazquez P, Otero Perpina B, Herreros de Tejada Lopez-Coterilla A, Medina Asensio J. [Efficiency of the information given at discharge and adherence of polymedicated patients]. Farmacia Hospitalaria 2011;35(3):128-34. [DOI] [PubMed] [Google Scholar]
Shimp 2012 {published data only}
- Shimp LA, Kucukarslan SN, Elder J, Remington T, Wells T, Choe HM, et al. Employer-based patient-centered medication therapy management program: evidence and recommendations for future programs. Journal of the American Pharmacists Association 2012;52(6):768-76. [DOI] [PubMed] [Google Scholar]
Shively 2013 {published and unpublished data}
- Shively MJ, Gardetto NJ, Kodiath MF, Kelly A, Smith TL, Stepnowsky C, et al. Effect of patient activation on self-management in patients with heart failure. Journal of Cardiovascular Nursing 2013;28(1):20-34. [DOI] [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. American Journal of Health-System Pharmacy 2003;60(11):1123-9. [DOI] [PubMed] [Google Scholar]
Truelove 2015 {published data only}
- Liu H, Patel A, Brown A, Eades S, Hayman N, Jan S, et al. Rationale and design of the Kanyini guidelines adherence with the polypill (Kanyini-GAP) study: a randomised controlled trial of a polypill-based strategy amongst Indigenous and non Indigenous people at high cardiovascular risk. BMC Public Health 2010;10:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Cass A, Peiris D, Usherwood T, Brown A, Jan S, et al. A pragmatic randomized trial of a polypill-based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. European Journal of Preventative Cardiology 2015;22(7):920-30. [DOI] [PubMed] [Google Scholar]
- Truelove M, Patel A, Bompoint S, Brown A, Cass A, Hillis GS, et al. The effect of a cardiovascular polypill strategy on pill burden. Cardiovascular Therapeutics 2015;33(6):347-52. [DOI] [PubMed] [Google Scholar]
Vinluan 2015 {published data only}
- Vinluan CM, Wittman D, Morisky D. Effect of pharmacist discharge counselling on medication adherence in elderly heart failure patients: a pilot study. Journal of Pharmaceutical Health Services Research 2015;6(2):103-10. [Google Scholar]
Volume 2001 {published data only}
- Kassam R, Farris KB, Burback L, Volume CI, Cox CE, Cave A. Pharmaceutical care research and education project: pharmacists' interventions. Journal of the American Pharmaceutical Association (Washington, DC 1996) 2001;41(3):401-10. [DOI] [PubMed] [Google Scholar]
- Volume CI, Farris KB, Kassam R, Cox CE, Cave A. Pharmaceutical care research and education project: patient outcomes. Journal of the American Pharmacists Association 2001;41(3):411-20. [DOI] [PubMed] [Google Scholar]
Willeboordse 2017 {published and unpublished data}
- Willeboordse F, Hugtenburg JG, Dijk L, Bosmans JE, Vries OJ, Schellevis FG, et al. Opti-Med: the effectiveness of optimised clinical medication reviews in older people with ‘geriatric giants’ in general practice; study protocol of a cluster randomised controlled trial. BMC Geriatrics 2014;14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeboordse F, Schellevis FG, Chau SH, Hugtenburg JG, Elders PJM. The effectiveness of optimised clinical medication reviews for geriatric patients: Opti-Med: a cluster randomised controlled trial. Family Practice 2017;34(4):437-45. [DOI] [PubMed] [Google Scholar]
Williams 2012 {published and unpublished data}
- Williams A, Manias E, Walker R, Gorelik A. A multifactorial intervention to improve blood pressure control in co-existing diabetes and kidney disease: a feasibility randomized controlled trial. Journal of Advanced Nursing 2012;68(11):2515-25. [DOI] [PubMed] [Google Scholar]
Winland‐Brown 2000 {published data only}
- Winland-Brown JE, Valiante J. Effectiveness of different medication management approaches on elders' medication adherence. Outcomes Management 2000;4(4):172-6. [PubMed] [Google Scholar]
Wood 1992 {published data only}
- Wood SI, Calvert RT, Acomb C, Kay EA. Self medication scheme for elderly patients improves compliance with their medication regimens. International Journal of Pharmacy Practice 1992:240-1. [Google Scholar]
Wu 2006 {published data only}
- Wu JY, Leung WY, Chang S, Lee B, Zee B, Tong PC, et al. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ 2006;333(7567):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2016 {published data only}
- Young L, Hertzog M, Barnason S. Effects of a home-based activation intervention on self-management adherence and readmission in rural heart failure patients: the PATCH randomized controlled trial. BMC Cardiovascular Disorders 2016;16(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Montgomery M, Barnason S, Schmidt C, Do V. A conceptual framework for barriers to the recruitment and retention of rural CVD participants in behavior intervention trials. GSTF Journal on Nursing and Health Care 2015;2(2):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12616001411437 {published data only}
- ACTRN12616001411437. A randomised controlled trial to evaluate the effect of preventive home visits on well being in elderly over 75 years. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364176 (first received 10 October 2016).
ACTRN12617001352392 {published data only}
- ACTRN12617001352392. A hypertension management program for community-dwelling older people with diabetes in China. http://www.who.int/trialsearch/trial2.aspx?Trialid=actrn12617001352392 (first received 26 September 2017).
Adams 2015 {published data only}
- Adams RP, Barton G, Bhattacharya D, Grassby PF, Holland R, Howe A, et al. Supervised pharmacy student-led medication review in primary care for patients with type 2 diabetes: a randomised controlled pilot study. BMJ Open 2015;5(11):e009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmad 2011 {published data only}
- Ahmad AL, Mast RM, Nijpels G, Dekker JM, Kostense PJ, Hugtenburg JG. Does medication review lead to a reduction of drug problems in elderly patients discharged from the hospital? Pharmacoepidemiology and Drug Safety 2011;20:S246-7. [Google Scholar]
Al‐Asseri 2001 {published data only}
- Al-Asseri AA, Al-Achi A, Greenwood R. Counseling and post-discharge care. Saudi Pharmaceutical Journal 2001;9(2):119-21. [Google Scholar]
Al‐Khadra 2014 {published data only}
- Al-Khadra S, Meisinger C, Amann U, Holle R, Kirchberger I. Secondary prevention medication after myocardial infarction: persistence in elderly people over the course of 1 year. Drugs & Aging 2014;31(7):513-25. [DOI] [PubMed] [Google Scholar]
Allen 1986 {published data only}
- Allen CM, Becker PM, McVey LJ. A randomized, controlled clinical trial of a geriatric consultation team. Compliance with recommendations. Journal of the American Medical Association 1986;255(19):2617-21. [PubMed] [Google Scholar]
Allen 2011 {published data only}
- Allen JK, Dennison-Himmelfarb CR, Szanton SL, Bone L, Hill MN, Levine DM, et al. Community Outreach and Cardiovascular Health (COACH) trial: a randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circulation: Cardiovascular Quality and Outcomes 2011;4(6):595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altavela 2008 {published data only}
- Altavela JL, Jones MK, Ritter M. A prospective trial of a clinical pharmacy intervention in a primary care practice in a capitated payment system. Journal of Managed Care Pharmacy 2008;14(9):831-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez 2001 {published data only}
- Alvarez de Toledo F, Arcos Gonzalez P, Eyaralar Riera T, Abal Ferrer FF, Dago Martinez A, Cabiedes Miragaya L, et al. [Pharmaceutical care in people who have had acute coronary episodes (TOMCOR study)]. Revista Espanola de Salud Publica 2001;75(4):375-87. [PubMed] [Google Scholar]
Ansari 2017 {published data only}
- Ansari S, Hosseinzadeh H, Dennis S, Zwar N. Empowering primary care patients with COPD in the context of multi-morbidity through the pilot APCOM self-management program. In: European Respiratory Journal. Conference: European Respiratory Society International Congress, ERS. Vol. 50. 2017.
Ansari 2017a {published data only}
- Ansari S, Hosseinzadeh H, Dennis SM, Zwar NA. Empowerment of primary care patients with chronic obstructive pulmonary disease (COPD) in the context of multi-morbidity by tailored self-management education in Sydney, Australia. In: American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS. Vol. 195. 2017.
Antoniades 2012 {published data only}
Antonicelli 2008 {published data only}
- Antonicelli R, Testarmata P, Spazzafumo L, Gagliardi C, Bilo G, Valentini M, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. Journal of Telemedicine & Telecare 2008;14(6):300-5. [DOI] [PubMed] [Google Scholar]
Artinian 2003 {published data only}
- Artinian NT, Harden JK, Kronenberg MW, Vander Wal JS, Daher E, Stephens Q, et al. Pilot study of a web-based compliance monitoring device for patients with congestive heart failure. Heart & Lung 2003;32(4):226-33. [DOI] [PubMed] [Google Scholar]
Ascione 1984 {published data only}
- Ascione FJ, Shimp LA. The effectiveness of four education strategies in the elderly. Drug Intelligence & Clinical Pharmacy 1984;18(11):926-31. [DOI] [PubMed] [Google Scholar]
Bailey 2012 {published data only}
- Bailey SC, Sarkar U, Chen AH, Schillinger D, Wolf MS. Evaluation of language concordant, patient-centered drug label instructions. Journal of General Internal Medicine 2012;27(12):1707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2012 {published data only}
- Barker A, Barlis P, Berlowitz D, Page K, Jackson B, Lim WK. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. International Journal of Cardiology 2012;159(2):139-43. [DOI] [PubMed] [Google Scholar]
Basger 2015 {published data only}
- Basger BJ, Moles RJ, Chen TF. Impact of an enhanced pharmacy discharge service on prescribing appropriateness criteria: a randomised controlled trial. International Journal of Clinical Pharmacy 2015;37(6):1194-205. [DOI] [PubMed] [Google Scholar]
Basheti 2016 {published data only}
- Basheti I A, Al-Qudah RA, Obeidat NM, Bulatova NR. Home medication management review in outpatients with chronic diseases in Jordan: a randomized control trial. International Journal of Clinical Pharmacy 2016;38(2):404-13. [DOI] [PubMed] [Google Scholar]
Bennett 2003 {published data only}
- Bennett JW, Glasziou P, Mar C, Looze F. A computerised prescribing decision support system to improve patient adherence with prescribing. A randomised controlled trial. Australian Family Physician 2003;32(8):667-71. [PubMed]
Bhattacharya, 2016 {published data only}
- Bhattacharya D, Aldus CF, Barton G, Bond CM, Boonyaprapa S, Charles IS, et al. The feasibility of determining the effectiveness and cost-effectiveness of medication organisation devices compared with usual care for older people in a community setting: systematic review, stakeholder focus groups and feasibility randomised controlled trial. Health Technology Assessment 2016;20(50):1-250. [DOI] [PMC free article] [PubMed]
Biese 2011 {published data only}
- Biese K, LaMantia M, McCall B, Kizer JS, Busby-Whitehead J. Impact of a phone call follow up on discharge instruction compliance and understanding in elderly patients after an emergency department (ED) visit. Journal of the American Geriatrics Society 2011;59:S7. [Google Scholar]
Biese 2014 {published data only}
- Biese K, Lamantia M, Shofer F, McCall B, Roberts E, Stearns SC, et al. A randomized trial exploring the effect of a telephone call follow-up on care plan compliance among older adults discharged home from the emergency department. Academic Emergency Medicine 2014;21(2):188-95. [DOI] [PubMed] [Google Scholar]
Biese 2017 {published data only}
- Biese K, Busby-Whitehead J, Cai J, Stearns S, Roberts E, Mihas P, et al. Telephone follow-up for older adults discharged to home from the emergency department: a randomized controlled trial. Journal of the American Geriatrics Society 2017;65(Suppl 1):S1. [DOI] [PubMed] [Google Scholar]
Bilotta 2011 {published data only}
- Bilotta C, Lucini A, Nicolini P, Vergani C. An easy intervention to improve short-term adherence to medications in community-dwelling older outpatients. A pilot non-randomised controlled trial. BMC Health Services Research 2011;11:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birtwhistle 2004 {published data only}
- Birtwhistle RV, Godwin MS, Delva MD, Casson RI, Lam M, MacDonald SE, et al. Randomised equivalence trial comparing three month and six month follow up of patients with hypertension by family practitioners. BMJ 2004;328(7433):204. [DOI] [PMC free article] [PubMed]
Biswas 2018 {published data only}
- Biswas A, Sinha N, Ray K, Tripathi SK. A study on drug use and medication management perspectives among elderly and the impact of professional oversight. Journal of Clinical and Diagnostic Research 2018;12(5):FC01-7. [Google Scholar]
Blenkinsopp 2000 {published data only}
- Blenkinsopp A, Phelan M, Bourne J, Dakhil N. Extended adherence support by community pharmacists for patients with hypertension: a randomised controlled trial. International Journal of Pharmacy Practice 2000;8(3):165-75. [Google Scholar]
Bogner 2012 {published data only}
- Bogner HR, Morales KH, Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Annals of Family Medicine 2012;10(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolas 2004 {published data only}
- Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharmacy World & Science 2004;26(2):114-20. [DOI] [PubMed] [Google Scholar]
Bolton 2004 {published data only}
- Bolton PGM, Parker SM. Impact of medication review by general practitioners and patient peer education. Journal of Pharmacy Practice and Research 2004;34(1):8-10. [Google Scholar]
Bonetti 2018 {published data only}
- Bonetti AF, Bagatim BQ, Mendes AM, Rotta I, Reis RC, Favero MLD, et al. Impact of discharge medication counseling in the cardiology unit of a tertiary hospital in Brazil: a randomized controlled trial. Clinics (Sao Paulo, Brazil) 2018;73:e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boult 2013 {published data only}
- Boult C, Leff B, Boyd CM, Wolff JL, Marsteller JA, Frick KD, et al. A matched-pair cluster-randomized trial of guided care for high-risk older patients. Journal of General Internal Medicine 2013;28(5):612-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branda 2013 {published data only}
- Branda ME, LeBlanc A, Shah ND, Tiedje K, Ruud K, Van Houten H, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Services Research 2013;13:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2009 {published data only}
- Braun E, Baidusi A, Alroy G, Azzam ZS. Telephone follow-up improves patients satisfaction following hospital discharge. European Journal of Internal Medicine 2009;20(2):221-5. [DOI] [PubMed] [Google Scholar]
Broadbent 2018 {published data only}
- Broadbent E, Garrett J, Jepsen N, Ogilvie VL, Ahn HS, Robinson H, et al. Using robots at home to support patients with chronic obstructive pulmonary disease: pilot randomized controlled trial. Journal of Medical Internet Research 2018;20(2):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bronson 1986 {published data only}
- Bronson DL, Costanza MC, Tufo HM. Using medical records for older patient education in ambulatory practice. Medical Care 1986;24(4):332-9. [DOI] [PubMed] [Google Scholar]
Bryant 2011 {published data only}
- Bryant LJ, Coster G, Gamble GD, McCormick RN. The General Practitioner-Pharmacist Collaboration (GPPC) study: a randomised controlled trial of clinical medication reviews in community pharmacy. International Journal of Pharmacy Practice 2011;19(2):94-105. [DOI] [PubMed] [Google Scholar]
Bryson 2008 {published data only}
- Bryson CL, Au DH, Sun HL, Williams EC, Bradley KA. Alcohol screening scores and medication nonadherence. Annals of Internal Medicine 2008;149(11):795-804. [DOI] [PubMed] [Google Scholar]
Burrelle 1986 {published data only}
- Burrelle TN. Evaluation of an interdisciplinary compliance service for elderly hypertensives. Journal of Geriatric Drug Therapy 1986;1(2):23-51. [Google Scholar]
Caetano 2018 {published data only}
- Caetano I, Santiago LM, Marques M. Impact of written information on control and adherence in type 2 diabetes. Revista Da Associacao Medica Brasileira 2018;64(2):140-7. [DOI] [PubMed] [Google Scholar]
Calvert 2012 {published data only}
- Calvert SB, Kramer JM, Anstrom KJ, Kaltenbach LA, Stafford JA, Allen Lapointe NM. Patient-focused intervention to improve long-term adherence to evidence-based medications: a randomized trial. American Heart Journal 2012;163(4):657-65.e1. [DOI] [PubMed] [Google Scholar]
Cedilnik 2016 {published data only}
- Cedilnik Gorup E, Petek-Ster M. Use of web-based application to improve prescribing in home-living elderly: a randomised controlled study protocol. European Geriatric Medicine 2013;4:S178-9. [Google Scholar]
- Selic P, Gorup EC, Gorup S, Ster MP, Rifel J, Ketis ZK. The effects of a web application and medical monitoring on the quality of medication, adverse drug events and adherence in the elderly living at home: a protocol of the study. Materia Socio-Medica 2016;28(6):432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan CW, Siu SC, Wong CK, Lee VW. A pharmacist care program: positive impact on cardiac risk in patients with type 2 diabetes. Journal of Cardiovascular Pharmacology & Therapeutics 2012;17(1):57-64. [DOI] [PubMed] [Google Scholar]
Chan 2015 {published data only}
- Chan B, Goldman LE, Sarkar U, Schneidermann M, Kessell E, Guzman D, et al. The effect of a care transition intervention on the patient experience of older multi-lingual adults in the safety net: results of a randomized controlled trial. Journal of General Internal Medicine 2015;30(12):1788-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chau 2012 {published data only}
- Chau JPC, Lee DTF, Yu DSF, Chow AYM, Yu WC, Chair SY, et al. A feasibility study to investigate the acceptability and potential effectiveness of a telecare service for older people with chronic obstructive pulmonary disease. International Journal of Medical Informatics 2012;81(10):674-82. [DOI] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen JH, Ou HT, Lin TC, Lai EC, Kao YH. Pharmaceutical care of elderly patients with poorly controlled type 2 diabetes mellitus: a randomized controlled trial. International Journal of Clinical Pharmacy 2016;38(1):88-95. [DOI] [PubMed] [Google Scholar]
Choudhry 2008 {published data only}
- Choudhry NK, Brennan T, Toscano M, Spettell C, Glynn RJ, Rubino M, et al. Rationale and design of the Post-MI FREEE trial: a randomized evaluation of first-dollar drug coverage for post-myocardial infarction secondary preventive therapies. American Heart Journal 2008;156(1):31-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chow 2015 {published data only}
- Chow EP, Hassali MA, Saleem F, Aljadhey H. Effects of pharmacist-led patient education on diabetes-related knowledge and medication adherence: a home-based study. Health Education Journal 2015;75(4):421-33. [Google Scholar]
Clarkesmith 2013 {published data only}
- Clarkesmith DE, Pattison HM, Lip GYH, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLoS ONE 2013;8(9):e74037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemson 2004 {published data only}
- Clemson L, Cumming RG, Kendig H, Swann M, Heard R, Taylor K. The effectiveness of a community-based program for reducing the incidence of falls in the elderly: a randomized trial. Journal of the American Geriatrics Society 2004;52(9):1487-94. [DOI] [PubMed] [Google Scholar]
Clifford 2006 {published data only}
- Clifford S, Barber N, Elliott R, Hartley E, Horne R. Patient-centred advice is effective in improving adherence to medicines. Pharmacy World & Science 2006;28(3):165-70. [DOI] [PubMed] [Google Scholar]
Coleman 1999 {published data only}
- Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a model of primary care for frail older adults. Journal of the American Geriatrics Society 1999;47(7):775-83. [DOI] [PubMed] [Google Scholar]
Coombes 2018 {published data only}
- Coombes JA, Rowett D, Whitty JA, Cottrell WN. Use of a patient-centred educational exchange (PCEE) to improve patient's self-management of medicines after a stroke: a randomised controlled trial study protocol.. BMJ Open 2018;8(8):e022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2008 {published data only}
- Costa e Silva R, Pellanda L, Portal V, Maciel P, Furquim A, Schaan B. Transdisciplinary approach to the follow-up of patients after myocardial infarction. Clinics (Sao Paulo, Brazil) 2008;63(4):489-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotterell 1992 {published data only}
- Cotterell C, Yee C, Nye M, Foster E. Pharmaceutical care in the elderly: evaluation of outcomes and costs-research in progress. ASHP Midyear Clinical Meeting 1992;27:P-155R.
Coull 2004 {published data only}
- Coull AJ, Taylor VH, Elton R, Murdoch PS, Hargreaves AD. A randomised controlled trial of senior lay health mentoring in older people with ischaemic heart disease: the Braveheart project. Age & Ageing 2004;33(4):348-54. [DOI] [PubMed] [Google Scholar]
Criswell 2010 {published data only}
- Criswell TJ, Weber CA, Xu Y, Carter BL. Effect of self-efficacy and social support on adherence to antihypertensive drugs. Pharmacotherapy: The Journal of Human Pharmacology & Drug Therapy 2010;30(5):432-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crotty 2005 {published data only}
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long term care facility? Results of a randomized, controlled trial. American Journal of Geriatric Pharmactherapy 2004;2(4):257-64. [DOI] [PubMed] [Google Scholar]
Crowley 2015 {published data only}
- Crowley MJ, Edelman D, McAndrew AT, Kistler S, Danus S, Webb JA, et al. Effectiveness of a scalable telemedicine intervention for veterans with persistent poor diabetes control. Diabetes 2015;64:A80. [Google Scholar]
Ctri 2017 {published data only}
- CTRI. Interventions to improve medication adherence among hypertensives aged 60 years and above. http://www.who.int/trialsearch/trial2.aspx?Trialid=ctri/2017/04/008405 2017.
Cummings 2019 {published data only}
- Cummings DM, Lutes LD, Littlewood K, Solar C, Carraway M, Kirian K, et al. Randomized trial of a tailored cognitive behavioral intervention in type 2 diabetes with comorbid depressive and/or regimen-related distress symptoms: 12-month outcomes from COMRADE. Diabetes Care 2019;42(5):841-8. [DOI] [PubMed] [Google Scholar]
D'Agostino 2006 {published data only}
- D'Agostino CS, Barry KL, Blow FC, Podgorski C. Community interventions for older adults with comorbid substance abuse: the Geriatric Addictions Program (GAP). Journal of Dual Diagnosis 2006;2(3):31-45. [Google Scholar]
Damush 2011 {published data only}
- Damush TM, Ofner S, Yu Z, Plue L, Nicholas G, Williams LS. Implementation of a stroke self-management program: a randomized controlled pilot study of veterans with stroke. Translational Behavioral Medicine 2011;1(4):561-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Damush 2016 {published data only}
- Damush TM, Myers L, Anderson JA, Yu Z, Ofner S, Nicholas G, et al. The effect of a locally adapted, secondary stroke risk factor self-management program on medication adherence among veterans with stroke/TIA. Translational Behavioral Medicine 2016;6(3):457‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 1992 {published data only}
- Day RA, Moore KN. Evaluation of a self-medication program: pilot project. Canadian Journal of Rehabilitation 1992;5(4):205-16. [Google Scholar]
Day 1998 {published data only}
- Day RA, Moore KN, Hodgins M. The effects of instruction and practice on medication knowledge and compliance. Canadian Journal of Rehabilitation 1998;12(1):15-24. [Google Scholar]
De Azevedo 2017 {published data only}
- De Azevedo MGB, Pedrosa RS, Aoqui CM, Martins RR, Nagashima Junior T. Effectiveness of home pharmaceutical interventions in metabolic syndrome: a randomized controlled trial. Brazilian Journal of Pharmaceutical Sciences 2017;53(2):e16089. [Google Scholar]
de Lusignan 2001 {published data only}
- Lusignan S, Wells S, Johnson P, Meredith K, Leatham E. Compliance and effectiveness of 1 year's home telemonitoring. The report of a pilot study of patients with chronic heart failure. European Journal of Heart Failure 2001;3(6):723-30. [DOI] [PubMed] [Google Scholar]
Denneboom 2007 {published data only}
- Denneboom W, Dautzenberg MGH, Grol R, De Smet PAGM. Treatment reviews of older people on polypharmacy in primary care: cluster controlled trial comparing two approaches. British Journal of General Practice 2007;57(542):723-31. [PMC free article] [PubMed] [Google Scholar]
Denneboom 2008 {published data only}
Dickson 2011 {published data only}
- Dickson V, Wong AL, Dillworth J, R'Bibo S, Katz S, Melkus GD. A community-based program to improve heart failure self-care. Circulation. Conference: American Heart Association's Scientific Sessions 2011;124(21):Suppl 1. [Google Scholar]
Dorje 2018 {published data only}
- Dorje T, Zhao G, Scheer A, Tsokey L, Wang J, Chen Y, et al. SMARTphone and social media-based Cardiac Rehabilitation and Secondary Prevention (SMART-CR/SP) for patients with coronary heart disease in China: a randomised controlled trial protocol. BMJ Open 2018;8(6):e021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doucette 2009 {published data only}
- Doucette WR, Witry MJ, Farris KB, McDonough RP. Community pharmacist-provided extended diabetes care. Annals of Pharmacotherapy 2009;43(5):882-9. [DOI] [PubMed] [Google Scholar]
Doughty 2002 {published data only}
- Doughty RN, Wright SP, Pearl A, Walsh HJ, Muncaster S, Whalley GA, et al. Randomized controlled trial of integrated heart failure management: the Auckland Heart Failure Management Study. European Heart Journal 2002;23(2):139-46. [DOI] [PubMed] [Google Scholar]
Doyon 2009 {published data only}
- Doyon O, Rouleau JL, Lemaire J, Raymond L, Ducharme A, Brophy J, et al. Effect of a nurse-led multidisciplinary heart failure clinic on professional practice: a randomized trial. European Heart Journal 2009;30:862. [Google Scholar]
Drenth‐van 2013 {published data only}
- Drenth-van Maanen AC, Wilting I, Jansen PA, Marum RJ, Egberts TC. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. Journal of the American Geriatrics Society 2013;61(3):456-8. [DOI] [PubMed] [Google Scholar]
Drks 2014 {published data only}
- DRKS. Use of START/STOP criteria in a web-based medication review application in community-dwelling elderly. http://www.who.int/trialsearch/trial2.aspx?Trialid=drks00005734 2014.
Drks 2015 {published data only}
- DRKS. Optimizing medication regimes for seniors by FORTA: a prospective, randomized multi-center study in primary care (Fit FOR The Aged). http://www.who.int/trialsearch/trial2.aspx? Trialid=drks00009236 2015.
Dunn 1995 {published data only}
- Dunn RB, Guy PM, Hardman CS, Lewis PA, Vetter NJ. Can a house call by a public health nurse improve the quality of the discharge process for geriatric patients? Clinical Performance & Quality Health Care 1995;3(3):151-5. [PubMed] [Google Scholar]
Duong 1996 {published data only}
- Duong PT, Baran RW, Wylie JD, Erwin WG. Improving outcomes of prescription drug use through a therapeutic outcome monitoring process implemented by pharmacists. ASHP Midyear Clinical Meeting 1996;31:P-423R.
Eggink 2010 {published data only}
- Eggink RN, Lenderink AW, Widdershoven JWMG, Van Den Bemt PMLA. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharmacy World and Science 2010;32(6):759-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eikelenboom 2016 {published data only}
- Eikelenboom N, Van Lieshout J, Jacobs A, Verhulst F, Lacroix J, Van Halteren A, et al. Effectiveness of personalised support for self-management in primary care: a cluster randomised controlled trial. British Journal of General Practice 2016;66(646):e354-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2008 {published data only}
- Elliott RA, Barber N, Clifford S, Horne R, Hartley E. The cost effectiveness of a telephone-based pharmacy advisory service to improve adherence to newly prescribed medicines. Pharmacy World & Science 2008;30(1):17-23. [DOI] [PubMed] [Google Scholar]
Elliott 2012 {published data only}
- Elliott RA, Martinac G, Campbell S, Thorn J, Woodward MC. Pharmacist-led medication review to identify medication-related problems in older people referred to an aged care assessment team: a randomized comparative study. Drugs & Aging 2012;29(7):593-605. [DOI] [PubMed] [Google Scholar]
Ellis 2000 {published data only}
- Ellis SL, Carter BL, Malone DC, Billups SJ, Okano GJ, Valuck RJ, et al. Clinical and economic impact of ambulatory care clinical pharmacists in management of dyslipidemia in older adults: the IMPROVE study. Pharmacotherapy 2000;20(12):1508-16. [DOI] [PubMed] [Google Scholar]
Enguidanos 2012 {published data only}
- Enguidanos S, Gibbs N, Jamison P. From hospital to home: a brief nurse practitioner intervention for vulnerable older adults. Journal of Gerontological Nursing 2012;38(3):40-50. [DOI] [PubMed] [Google Scholar]
Epstein 1990 {published data only}
- Epstein AM, Hall JA, Fretwell M, Feldstein M, DeCiantis ML, Tognetti J, et al. Consultative geriatric assessment for ambulatory patients. A randomized trial in a health maintenance organization. JAMA 1990;263(4):538-44. [PubMed] [Google Scholar]
Esposito 1995 {published data only}
- Esposito L. The effects of medication education on adherence to medication regimens in an elderly population. Journal of Advanced Nursing 1995;21(5):935-43. [DOI] [PubMed] [Google Scholar]
Evans 2010 {published data only}
- Evans CD, Eurich DT, Taylor JG, Blackburn DF. The Collaborative Cardiovascular Risk Reduction in Primary Care (CCARP) study. Pharmacotherapy: The Journal of Human Pharmacology & Drug Therapy 2010;30(8):766-75. [DOI] [PubMed] [Google Scholar]
Fabacher 1994 {published data only}
- Fabacher D, Josephson K, Pietruszka F, Linderborn K, Morley JE, Rubenstein LZ. An in-home preventive assessment program for independent older adults: a randomized controlled trial. Journal of the American Geriatrics Society 1994;42(6):630-8. [DOI] [PubMed] [Google Scholar]
Fan 2012 {published data only}
- Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial.. Annals of Internal Medicine 2012;156(10):673-83. [DOI] [PubMed] [Google Scholar]
Farmer 2012 {published data only}
- Farmer A, Hardeman W, Hughes D, Prevost AT, Youngsuk K, Craven A, et al. An explanatory randomised controlled trial of a nurse-led, consultation-based intervention to support patients with adherence to taking glucose lowering medication for type 2 diabetes. BMC Family Practice 2012;13(1):30-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandes 2012 {published data only}
- Fernandes M, Areias C, Souto D, Alarcao V, Nicola P, Rocha E. HyDia project: evaluation of a tailored intervention among medicated patients with uncontrolled hypertension, from Lisbon primary healthcare centers. European Journal of Epidemiology 2012;1:S87-8. [Google Scholar]
Fernando 2012 {published data only}
- Fernando TJ, Nguyen DD, Baraff LJ. Effect of electronically delivered prescriptions on compliance and pharmacy wait time among emergency department patients. Academic Emergency Medicine 2012;19(1):102-5. [DOI] [PubMed] [Google Scholar]
Fernley 1983 {published data only}
- Fernley D, Fitzpatrick RW. Improving compliance in the elderly. Pharmaceutical Journal (England) 1983;231:75-6.
Ferrat 2018 {published data only}
- Ferrat E, Bastuji-Garin S, Paillaud E, Caillet P, Clerc P, Moscova L, et al. Efficacy of nurse-led and general practitioner-led comprehensive geriatric assessment in primary care: protocol of a pragmatic three-arm cluster randomised controlled trial (CEpiA study). BMJ Open 2018;8(4):e020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figar 2006 {published data only}
- Figar S, Galarza C, Petrlik E, Hornstein L, Rodriguez Loria G, Waisman G, et al. Effect of education on blood pressure control in elderly persons: a randomized controlled trial. American Journal of Hypertension 2006;19(7):737-43. [DOI] [PubMed] [Google Scholar]
Fikri‐Benbrahim 2013 {published data only}
Fikri‐Benbrahim 2014 {published data only}
- Fikri-Benbrahim N, Sabater-Hernandez D, Fikri-Benbrahim O, Faus MJ, Martinez-Martinez F, Gonzalez-Segura Alsina D. Effect of pharmaceutical intervention on medication adherence and blood pressure control in treated hypertensive patients: rationale, design and methods of the AFenPA pilot study. ARS Pharmaceutica 2014;52(4):29-38. [Google Scholar]
Fincher 2009 {published data only}
- Fincher L, Ward C, Dawkins V, Magee V, Willson P. Using telehealth to educate Parkinson's disease patients about complicated medication regimens. Journal of Gerontological Nursing 2009;35(2):16-24. [DOI] [PubMed] [Google Scholar]
Finley 2003 {published data only}
- Finley PR, Rens HR, Pont JT, Gess SL, Louie C, Bull SA, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy 2003;23(9 I):1175-85. [DOI] [PubMed] [Google Scholar]
Fischer 2014 {published data only}
- Fischer MA, Choudhry NK, Bykov K, Brill G, Bopp G, Wurst AM, et al. Pharmacy-based interventions to reduce primary medication nonadherence to cardiovascular medications. Medical Care 2014;52(12):1050-4. [DOI] [PubMed] [Google Scholar]
Fortney 2007 {published data only}
- Fortney JC, Pyne JM, Edlund MJ, Williams DK, Robinson DE, Mittal D, et al. A randomized trial of telemedicine-based collaborative care for depression. Journal of General Internal Medicine 2007;22(8):1086-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortney 2011 {published data only}
- Fortney JC, Pyne JM, Edlund MJ, Stecker T, Mittal D, Robinson DE, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. Journal of Clinical Psychiatry 2011;72(6):827-34. [DOI] [PubMed] [Google Scholar]
Fortney 2015 {published data only}
- Fortney JC, Pyne JM, Kimbrell TA, Hudson TJ, Robinson DE, Schneider R, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 2015;72(1):58-67. [DOI] [PubMed] [Google Scholar]
Frennet 2011 {published data only}
- Frennet M, Kerger J, Cornette P, Humblet Y, Lesage V, De Saint-Hubert M. Impact of comprehensive geriatric assessment (CGA) during oncologic treatment in frail elderly patients. European Geriatric Medicine 2011;2:S35. [Google Scholar]
Frey 2001 {published data only}
- Frey D, Simmon WJ. Home health medications management model. ASHP Midyear Clinical Meeting 2001;36:HC-8.
Friedman 1996 {published data only}
- Friedman RH, Kazis LE, Jette A, Smith MB, Stollerman J, Torgerson J, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. American Journal of Hypertension 1996;9(4 Pt 1):285-92. [DOI] [PubMed] [Google Scholar]
Fugazzaro 2016 {published data only}
- Fugazzaro S, Bardelli R, Accogli MA, Denti M, Altavilla A, Piccinini M, et al. Defining therapeutic patient education in post-stroke rehabilitation: usual care and preliminary data of an Italian self-management early intervention for stroke survivors (LAY-look after yourself). Cerebrovascular Diseases 2016;41:111. [Google Scholar]
Fulmer 1999 {published data only}
- Fulmer TT, Feldman PH, Kim TS, Carty B, Beers M, Molina M, et al. An intervention study to enhance medication compliance in community-dwelling elderly individuals. Journal of Gerontological Nursing 1999;25(8):6-14. [DOI] [PubMed] [Google Scholar]
Gabriel 1977 {published data only}
- Gabriel M, Gagnon JP, Bryan CK. Improved patient compliance through use of a daily drug reminder chart. American Journal of Public Health 1977;67(10):968-9. [DOI] [PMC free article] [PubMed]
Gamboa 2019 {published data only}
- Gamboa ME, Mateo-Abad M, Ochoa de Retana Garcia L, Vrotsou K, Campo Pena E, Sanchez Perez A, et al. Efficacy of a self-management education programme on patients with type 2 diabetes in primary care: a randomised controlled trial. Primary Care Diabetes 2019;13(2):122-33. [DOI] [PubMed] [Google Scholar]
Garcao 2002 {published data only}
- Garcao JA, Cabrita J. Evaluation of a pharmaceutical care program for hypertensive patients in rural Portugal. Journal of the American Pharmaceutical Association 2002;42(6):858-64. [DOI] [PubMed] [Google Scholar]
Garcia 2017 {published data only}
Garza 2016 {published data only}
- Garza KB, Owensby JK, Braxton Lloyd K, Wood EA, Hansen RA. Pilot study to test the effectiveness of different financial incentives to improve medication adherence. Annals of Pharmacotherapy 2016;50(1):32-8. [DOI] [PubMed] [Google Scholar]
Gellis 2014 {published data only}
- Gellis ZD, Kenaley BL, Ten Have T. Integrated telehealth care for chronic illness and depression in geriatric home care patients: the integrated telehealth education and activation of mood (I-TEAM) study. Journal of the American Geriatrics Society 2014;62(5):889-95. [DOI] [PubMed] [Google Scholar]
Gialamas 2009 {published data only}
- Gialamas A, Yelland LN, Ryan P, Willson K, Laurence CO, Bubner TK, et al. Does point-of-care testing lead to the same or better adherence to medication? A randomised controlled trial: the PoCT in General Practice Trial. Medical Journal of Australia 2009;191(9):487-91. [DOI] [PubMed] [Google Scholar]
Gould 2011 {published data only}
- Gould KA. A randomized controlled trial of a discharge nursing intervention to promote self-regulation of care for early discharge interventional cardiology patients. Dimensions of Critical Care Nursing 2011;30(2):117-25. [DOI] [PubMed] [Google Scholar]
Grant 2003 {published data only}
- Grant RW, Devita NG, Singer DE, Meigs JB. Improving adherence and reducing medication discrepancies in patients with diabetes. Annals of Pharmacotherapy 2003;37(7-8):962-9. [DOI] [PubMed] [Google Scholar]
Green 2008 {published data only}
- Green BB, Ralston JD, Fishman PA, Catz SL, Cook A, Carlson J, et al. Electronic communications and home blood pressure monitoring (e-BP) study: design, delivery, and evaluation framework. Contemporary Clinical Trials 2008;29(3):376-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grice 2014 {published data only}
- Grice GR, Tiemeier A, Hurd P, Berry TM, Voorhees M, Prosser TR, et al. Student use of health literacy tools to improve patient understanding and medication adherence. Consultant Pharmacist 2014;29(4):240-53. [DOI] [PubMed] [Google Scholar]
Gujral 2014 {published data only}
Gums 2015 {published data only}
- Gums TH, Uribe L, Vander Weg MW, James P, Coffey C, Carter BL. Pharmacist intervention for blood pressure control: medication intensification and adherence. Journal of the American Society of Hypertension 2015;9(7):569-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwadry‐Sridhar 2005 {published data only}
- Gwadry-Sridhar FH, Arnold JMO, Zhang Y, Brown JE, Marchiori G, Guyatt G. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. American Heart Journal 2005;150(5):982.e1-9. [DOI] [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han HR, Kim J, Kim KB, Jeong S, Levine D, Li C, et al. Implementation and success of nurse telephone counseling in linguistically isolated Korean American patients with high blood pressure. Patient Education and Counselling 2010;80(1):130-4. [DOI] [PMC free article] [PubMed]
Hansen 2009 {published data only}
- Hansen RA, Dusetzina SB, Song L, Gaynes BN, Tu W, Murray MD. Depression affects adherence measurement but not the effectiveness of an adherence intervention in heart failure patients. Journal of the American Pharmacists Association 2009;49(6):760-8. [DOI] [PubMed] [Google Scholar]
Haramiova 2017 {published data only}
- Haramiova Z, Stasko M, Hulin M, Tesar T, Kuzelova M, Morisky DM. The effectiveness of daily SMS reminders in pharmaceutical care of older adults on improving patients' adherence to antihypertensive medication (SPPA): study protocol for a randomized controlled trial. Trials 2017;18(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harari 2008 {published data only}
Hawe 1990 {published data only}
- Hawe P, Higgins G. Can medication education improve the drug compliance of the elderly? Evaluation of an in hospital program. Patient Education and Counseling 1990;16(2):151-60. [DOI] [PubMed] [Google Scholar]
Hayes 1998 {published data only}
- Hayes KS. Randomized trial of geragogy-based medication instruction in the emergency department. Nursing Research 1998;47(4):211-8. [DOI] [PubMed] [Google Scholar]
Hedegaard 2015 {published data only}
- Hedegaard U, Kjeldsen LJ, Pottegard A, Henriksen JE, Lambrechtsen J, Hangaard J, et al. Improving medication adherence in patients with hypertension: a randomized trial. American Journal of Medicine 2015;128(12):1351-61. [DOI] [PubMed] [Google Scholar]
Heisler 2010 {published data only}
- Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Annals of Internal Medicine 2010;153(8):507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heisler 2011 {published data only}
- Heisler M, Hofer T, Schmittdiel JA, Selby JV, Bosworth H, Kerr EA. Blood pressure lowering among 'resistant' hypertensive diabetes patients in two high-performing health systems: the adherence and intensification of medication (AIM) cluster randomized controlled effectiveness trial. Journal of General Internal Medicine 2011;26:S306. [Google Scholar]
Heisler 2012 {published data only}
- Heisler M, Hofer TP, Schmittdiel JA, Selby JV, Klamerus ML, Bosworth HB, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: the adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation 2012;125(23):2863-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heisler 2014 {published data only}
- Heisler M, Choi H, Palmisano G, Mase R, Richardson C, Fagerlin A, et al. Comparison of community health worker-led diabetes medication decision-making support for low-income Latino and African American adults with diabetes using e-health tools versus print materials: a randomized, controlled trial. Annals of Internal Medicine 2014;161(10 Suppl):S13-22. [DOI] [PMC free article] [PubMed]
Hesselink 2004 {published data only}
- Hesselink AE, Penninx BW, Windt DA, Duin BJ, Vries P, Twisk JW, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Education & Counseling 2004;55(1):121-8. [DOI] [PubMed] [Google Scholar]
Ho 2014 {published data only}
- Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Internal Medicine 2014;174(2):186-93. [DOI] [PubMed] [Google Scholar]
Hohmann 2014 {published data only}
- Hohmann C, Neumann-Haefelin T, Klotz JM, Freidank A, Radziwill R. Providing systematic detailed information on medication upon hospital discharge as an important step towards improved transitional care. Journal of Clinical Pharmacy and Therapeutics 2014;39(3):286-91. [DOI] [PubMed] [Google Scholar]
Holdford 2013 {published data only}
- Holdford DA, Inocencio TJ. Adherence and persistence associated with an appointment-based medication synchronization program. Journal of the American Pharmacists Association 2013;53(6):576-83. [DOI] [PubMed] [Google Scholar]
Horton 2017 {published data only}
- Horton J. Improving self-management in patients with chronic conditions. Dissertation Abstracts International: Section B: The Sciences and Engineering 2017;77(10-B(E)):No Pagination Specified. [Google Scholar]
Hsieh 2008 {published data only}
- Hsieh CJ, Lin LC, Kuo BI, Chiang CH, Su WJ, Shih JF. Exploring the efficacy of a case management model using DOTS in the adherence of patients with pulmonary tuberculosis. Journal of Clinical Nursing 2008;17(7):869-75. [DOI] [PubMed] [Google Scholar]
Huang 2018 {published data only}
- Huang B, Li Z, Wang Y, Xia J, Shi T, Jiang J, et al. Effectiveness of self-management support in maintenance haemodialysis patients with hypertension: a pilot cluster randomized controlled trial. Nephrology 2018;23(8):755-63. [DOI] [PubMed] [Google Scholar]
Hugtenburg 2012 {published data only}
- Hugtenburg J, Ahmad A, Dekker JM, Kostense PJ, Nijpels G. Medication review in elderly patients discharged from hospital substantially decreased drug related problems. Diabetologia 2012;55:S412. [DOI] [PubMed] [Google Scholar]
Hunter 1996 {published data only}
- Hunter KA. Pharmaceutical care for the home-dwelling elderly: interim report. ASHP Midyear Clinical Meeting 1996;31:FGF-1.
Hyrkas 2014 {published data only}
- Hyrkas K, Wiggins M. A comparison of usual care, a patient-centred education intervention and motivational interviewing to improve medication adherence and readmissions of adults in an acute-care setting. Journal of Nursing Management 2014;22(3):350‐61. [DOI] [PubMed] [Google Scholar]
Insel 2016 {published data only}
- Insel KC, Einstein GO, Morrow DG, Koerner KM, Hepworth JT. Multifaceted prospective memory intervention to improve medication adherence. Journal of the American Geriatrics Society 2016;64(3):561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20110728007143N {published data only}
- IRCT20110728007143N. The effect of education based on self-management empowerment model on empowerment level of elderly patients with type 2 diabetes. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct20110728007143n5 (first received 18 June 2018).
IRCT201306297531N {published data only}
- IRCT201306297531N. The effects of cardiac rehabilitation on elderly with congestive heart failure. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct201306297531n2 (first received 13 September 2013).
IRCT2014050617596N {published data only}
- IRCT2014050617596N. Comparison of telephone and SMS follow up on treatment regimen adherence in patients with coronary artery bypass surgery. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct2014050617596n1 (first received 7 August 2014).
IRCT2015090513092N {published data only}
- IRCT2015090513092N. Effect of self management empowerment model on sense of coherence and self efficacy of retired elderly with chronic disease. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct2015090513092n9 (first received 20 April 2016).
IRCT2016082129448N {published data only}
- IRCT2016082129448N. Impact of per-discharge self-care behavior and readmission in heart failure patients. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct2016082129448n1 (first received 10 November 2016).
IRCT2016090629728N {published data only}
- IRCT2016090629728N. The effect of self-management program on quality of life of elderly patients with acute coronary syndrome. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct2016090629728n1 (first received 26 November 2016).
IRCT2016120731290N {published data only}
- IRCT2016120731290N. The effects of cardiac rehabilitation on self-esteem and self-efficacy in elder coronary patient. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct2016120731290n1 (first received 4 September 2017).
IRCT20171125037616N {published data only}
- IRCT20171125037616N. The effect of educational program based on health belief model on belief about medicines & medication adherence in the elderly patients with hypertension. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct20171125037616n1 (first received 5 January 2018).
IRCT20171213037859N {published data only}
- IRCT20171213037859N. Investigating the effect of multiple interventions on improving compliance with the treatment therapy and quality of life in dialysis patients. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct20171213037859n1 (first received 8 September 2018).
IRCT20180513039646N {published data only}
- IRCT20180513039646N. The effect of the family-centered educational program on medication management and quality of life in elderly patients with ischemic heart disease. http://www.who.int/trialsearch/trial2.aspx?Trialid=irct20180513039646n1 (first received 23 September 2018).
ISRCTN03155973 {published data only}
- ISRCTN03155973. Homebased medication review: evaluation of home-based medication review by community pharmacists in elderly people: a randomised controlled trial. http://www.who.int/trialsearch/trial2.aspx?Trialid=isrctn03155973 (first received 5 September 2007).
ISRCTN12752680 {published data only}
- ISRCTN12752680. Supporting medicines management in older adults with multiple medical conditions. https://doi.org/10.1186/ISRCTN12752680 (first received 26 August 2016).
ISRCTN18285541 {published data only}
- ISRCTN18285541. Effect of a dual intervention in elderly heart failure patients with cognitive impairment and their caregivers after hospital discharge: a randomized controlled trial. https://doi.org/10.1186/ISRCTN18285541 (first received 15 February 2016).
Jager 2017 {published data only}
- Jäger C, Freund T, Steinhäuser J, Stock C, Krisam J, Kaufmann-Kolle P, et al. Impact of a tailored program on the implementation of evidence-based recommendations for multimorbid patients with polypharmacy in primary care practices - results of a cluster-randomized controlled trial. Implementation Science 2017;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jager 2017a {published data only}
- Jager C, Steinhauser J, Freund T, Kuse S, Szecsenyi J, Wensing M. A tailored programme to implement recommendations for multimorbid patients with polypharmacy in primary care practices - process evaluation of a cluster randomized trial. Implementation Science 2017;12(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahangard‐Rafsanjani 2015 {published data only}
- Jahangard-Rafsanjani Z, Sarayani A, Nosrati M, Saadat N, Rashidian A, Hadjibabaie M, et al. Effect of a community pharmacist-delivered diabetes support program for patients receiving specialty medical care: a randomized controlled trial. Diabetes Educator 2015;41(1):127-35. [DOI] [PubMed] [Google Scholar]
Jarab 2012 {published data only}
- Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. International Journal of Clinical Pharmacy 2012;34(1):53-62. [DOI] [PubMed] [Google Scholar]
Jarab 2012a {published data only}
- Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. Journal of Managed Care Pharmacy 2012;18(7):516-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2003 {published data only}
- Jensen L. Self-administered cardiac medication program evaluation. Canadian Journal of Cardiovascular Nursing 2003;13(2):35-44. [PubMed] [Google Scholar]
Jerant 2003 {published data only}
- Jerant AF, Azari R, Martinez C, Nesbitt TS. A randomized trial of telenursing to reduce hospitalization for heart failure: patient-centered outcomes and nursing indicators. Home Health Care Services Quarterly 2003;22(1):1-20. [DOI] [PubMed] [Google Scholar]
Jiang 2007 {published data only}
Johnson‐Warrington 2016 {published data only}
- Johnson-Warrington V, Rees K, Gelder C, Morgan MD, Singh SJ. Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. International Journal of COPD 2016;11(1):1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2000 {published data only}
- Johnston B, Wheeler L, Deuser J, Sousa KH. Outcomes of the Kaiser Permanente tele-home health research project. Archives of Family Medicine 2000;9(1):40-5. [DOI] [PubMed] [Google Scholar]
Junling 2015 {published data only}
- Junling G, Yang L, Junming D, Pinpin Z, Hua F. Evaluation of group visits for Chinese hypertensives based on primary health care center. Asia-Pacific Journal of Public Health 2015;27(2):NP350-60. [DOI] [PubMed] [Google Scholar]
Kalichman 2011 {published data only}
- Kalichman SC, Kalichman MO, Cherry C, Swetzes C, Amaral CM, White D, et al. Brief behavioral self-regulation counseling for HIV treatment adherence delivered by cell phone: an initial test of concept trial. AIDS Patient Care & STDs 2011;25(5):303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karagiannis 2016 {published data only}
- Karagiannis T, Liakos A, Branda ME, Athanasiadou E, Mainou M, Boura P, et al. Use of the Diabetes Medication Choice Decision Aid in patients with type 2 diabetes in Greece: a cluster randomised trial. BMJ Open 2016;6:e012185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaukab 2015 {published data only}
- Kaukab I, Nasir B, Abrar MA, Muneer S, Murtaza G. Effect of pharmacist-led patient education on management of depression in drug resistance tuberculosis patients using cycloserine. a prospective study. Latin America Journal of Pharmacy 2015:1403-10.
Kaur 2015 {published data only}
- Kaur R, Singh Kajal K, Kaur A, Singh P. Telephonic consultation and follow-up in diabetics: impact on metabolic profile, quality of life, and patient compliance. North American Journal of Medical Sciences 2015;7(5):1-9. [DOI] [PMC free article] [PubMed]
Kavin 2010 {published data only}
- Kavin M, Anel-Tiangco RM, Mauger DT, Gabbay RA. Development and pilot of a low-literacy diabetes education book using social marketing techniques. Diabetes Therapy 2010;1(2):93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 1990 {published data only}
- Kelly GR, Scott JE, Mamon J. Medication compliance and health education among outpatients with chronic mental disorders.[Erratum appears in Med Care 1991 Sep;29(9):889]. Medical Care 1990;28(12):1181-97. [DOI] [PubMed] [Google Scholar]
Kempen 2017 {published data only}
- Kempen TGH, Bertilsson M, Lindner KJ, Sulku J, Nielsen EI, Hogberg A, et al. Medication Reviews Bridging Healthcare (MedBridge): study protocol for a pragmatic cluster-randomised crossover trial. Contemporary Clinical Trials 2017;61:126-32. [DOI] [PubMed] [Google Scholar]
Keyserling 2014 {published data only}
- Keyserling TC, Sheridan SL, Draeger LB, Finkelstein EA, Gizlice Z, Kruger E, et al. A comparison of live counseling with a web-based lifestyle and medication intervention to reduce coronary heart disease risk: a randomized clinical trial. JAMA Internal Medicine 2014;174(7):1144-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2019 {published data only}
- Khan N, Walley JD, Khan SE, Hicks J, Sheikh FI, Khan MA, et al. Enhanced hypertension care through private clinics in Pakistan: a cluster randomised trial. BJGP Open 2019;3(1):bjgpopen18X101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khonsari 2015 {published data only}
- Khonsari S, Subramanian P, Chinna K, Latif LA, Ling LW, Gholami O. Effect of a reminder system using an automated short message service on medication adherence following acute coronary syndrome. European Journal of Cardiovascular Nursing 2015;14(2):170-9. [DOI] [PubMed] [Google Scholar]
Khunti 2012 {published data only}
- Khunti K, Gray LJ, Skinner T, Carey ME, Realf K, Dallosso H, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ 2012;344:e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim KB, Han HR, Huh B, Nguyen T, Lee H, Kim MT. The effect of a community-based self-help multimodal behavioral intervention in Korean American seniors with high blood pressure. American Journal of Hypertension 2014;27(9):1199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim M, Song M. [Effects of self-management program applying dongsasub training on self-efficacy, self-esteem, self-management behavior and blood pressure in older adults with hypertension]. Journal of Korean Academy of Nursing 2015;45(4):576-86. [DOI] [PubMed] [Google Scholar]
Kim 2015a {published data only}
- Kim MT, Kim KB, Huh B, Nguyen T, Han HR, Bone LR, et al. The effect of a community-based self-help intervention: Korean Americans with type 2 diabetes. American Journal of Preventative Medicine 2015;49(5):726-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimball 2010 {published data only}
- Kimball S, Buck G, Goldstein D, Largaespada E, Logan L, Stebbins D, et al. Testing a teaching appointment and geragogy-based approach to medication knowledge at discharge. Rehabilitation Nursing Journal 2010;35(1):31-40. [DOI] [PubMed] [Google Scholar]
Koberlein‐Neu 2016 {published data only}
- Köberlein-Neu J, Mennemann H, Hamacher S, Waltering I, Jaehde U, Schaffert C, et al. Interprofessional medication management in patients with multiple morbidities. Deutsches Arzteblatt International 2016;113(44):741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kogos 2004 {published data only}
- Kogos SC. Support groups and treatment adherence in a geriatric outpatient clinic. Journal of Clinical Psychology in Medical Settings 2004;11(4):275-82. [Google Scholar]
Kono 2004 {published data only}
Kotowycz 2010 {published data only}
- Kotowycz MA, Cosman TL, Tartaglia C, Afzal R, Syal RP, Natarajan MK. Safety and feasibility of early hospital discharge in ST-segment elevation myocardial infarction - a prospective and randomized trial in low-risk primary percutaneous coronary intervention patients (the Safe-Depart Trial). American Heart Journal 2010;159(1):117.e1-6. [DOI] [PubMed] [Google Scholar]
Kozuki 2006 {published data only}
- Kozuki Y, Schepp KG. Visual-feedback therapy for antipsychotic medication adherence. International Clinical Psychopharmacology 2006;21(1):57-61. [DOI] [PubMed] [Google Scholar]
Kraemer 2012 {published data only}
Kranker 2018 {published data only}
- Kranker K. The efficacy of using financial incentives to change unhealthy behaviors among a rural chronically Ill and uninsured population. American Journal of Health Promotion 2018;32(2):301-11. [DOI] [PubMed] [Google Scholar]
Krass 2007 {published data only}
- Krass I, Armour CL, Mitchell B, Brillant M, Dienaar R, Hughes J, et al. The Pharmacy Diabetes Care Program: assessment of a community pharmacy diabetes service model in Australia. Diabetic Medicine 2007;24(6):677-83. [DOI] [PubMed] [Google Scholar]
Kripalani 2012 {published data only}
- Kripalani S, Roumie CL, Dalal AK, Cawthon C, Businger A, Eden SK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Annals of Internal Medicine 2012;157(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kripalani 2012a {published data only}
- Kripalani S, Schmotzer B, Jacobson TA. Improving medication adherence through graphically enhanced interventions in coronary heart disease (IMAGE-CHD): a randomized controlled trial. Journal of General Internal Medicine 2012;27(12):1609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishnamurthi 2014 {published data only}
- Krishnamurthi R, Witt E, Barker-Collo S, McPherson K, Davis-Martin K, Bennett D, et al. Reducing recurrent stroke: methodology of the motivational interviewing in stroke (MIST) randomized clinical trial. International Journal of Stroke 2014;9(1):133-9. [DOI] [PubMed] [Google Scholar]
Krishnaswami 1981 {published data only}
- Krishnaswami KV, Somasundaram PR, Tripathy SP, Vaidyanathan B, Radhakrishna S, Fox W. A randomised study of two policies for managing default in out-patients collecting supplies of drugs for pulmonary tuberculosis in a large city in South India. Tubercle 1981;62(2):103-12. [DOI] [PubMed] [Google Scholar]
Krishnaveni 2019 {published data only}
- Krishnaveni K, Rajagopal SS. Impact of a pharmacist intervention on improving medication adherence and knowledge towards diabetes mellitus: a randomised controlled study. International Journal of Pharmaceutical Research 2019;11(1):416-20. [Google Scholar]
Kutzleb 2006 {published data only}
Lalonde 2017 {published data only}
- Lalonde L, Quintana-Barcena P, Lord A, Bell R, Clement V, Daigneault AM, et al. Community pharmacist training-and-communication network and drug-related problems in patients with CKD: a multicenter, cluster-randomized, controlled trial. American Journal of Kidney Diseases 2017;70(3):386-96. [DOI] [PubMed] [Google Scholar]
Lam 2011 {published data only}
- Lam P, Elliott R, George J. Impact of a self-administration of medications programme on elderly inpatients' competence to manage medications: a pilot study. Journal of Clinical Pharmacy and Therapeutics 2011;36(1):80-6. [DOI] [PubMed] [Google Scholar]
Lam 2017 {published data only}
- Lam AY, Nguyen JK, Parks JJ, Morisky DE, Berry DL, Wolpin SE. Addressing low health literacy with "Talking Pill Bottles": a pilot study in a community pharmacy setting. Journal of the American Pharmacists Association 2017;57(1):20-9.e3. [DOI] [PubMed] [Google Scholar]
Lange 2010 {published data only}
- Lange I, Campos S, Urrutia M, Bustamante C, Alcayaga C, Tellez A, et al. [Effect of a tele-care model on self-management and metabolic control among patients with type 2 diabetes in primary care centers in Santiago, Chile]. Revista Medica de Chile 2010;138(6):729-37. [DOI] [PubMed] [Google Scholar]
Laramee 2003 {published data only}
- Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Archives of Internal Medicine 2003;163(7):809-17. [DOI] [PubMed] [Google Scholar]
Laufs 2018 {published data only}
- Laufs U, Griese-Mammen N, Krueger K, Wachter A, Anker SD, Koehler F, et al. PHARMacy-based interdisciplinary program for patients with Chronic Heart Failure (PHARM-CHF): rationale and design of a randomized controlled trial, and results of the pilot study. European Journal of Heart Failure 2018;20(9):1350-9. [DOI] [PubMed] [Google Scholar]
Leavitt 2017 {published data only}
- Leavitt MAM. The Effect of the Heart Failure Nurse Navigator on 30-Day Hospital Readmissions of Older Adults. Boca Raton, FL: Florida Atlantic University, 2017. [Google Scholar]
Lei 2019 {published data only}
- Yang L. The effects of a strengths-based intervention based on salutogenic model on self-care behaviors and sense of coherence in older adults with type 2 diabetes. Dissertation Abstracts International: Section B: The Sciences and Engineering 2019;80(4-B(E)):No Pagination Specified. [Google Scholar]
Leiva‐Fernandez 2014 {published data only}
- Leiva-Fernandez J, Leiva-Fernandez F, Garcia-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulmonary Medicine 2014;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenaghan 2007 {published data only}
- Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care - The POLYMED randomised controlled trial. Age & Ageing 2007;36(3):292-7. [DOI] [PubMed] [Google Scholar]
Lerma 2017 {published data only}
- Lerma C, Badillo H, Lerma A. Treatment efficacy and adherence to combined enalapril and nifedipine compared to conventional treatment among hypertensive patients in primary care from Mexico City. Pharmacoepidemiology and Drug Safety 2017;26:516. [Google Scholar]
Levine 1979 {published data only}
- Levine DM, Green LW, Deeds SG, Chwalow J, Russell RP, Finlay J. Health education for hypertensive patients. JAMA 1979;241(16):1700-3. [PubMed]
Levy 2004 {published data only}
- Levy RW, Rayner CR, Fairley CK, Kong DC, Mijch A, Costello K, et al. Multidisciplinary HIV adherence intervention: a randomized study. AIDS Patient Care and STDS 2004;18(12):728-35. [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li J, Wang YL, Yu XQ, Wu J, Tang C, Wu W L, et al. Community-based management for chronic heart failure patients under the professional guidance of upper first-class hospital staff. [Chinese]. Chinese Journal of Cardiology 2012;40(11):939-44. [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li Y, Liebel DV, Friedman B. An investigation into which individual instrumental activities of daily living are affected by a home visiting nurse intervention. Age & Ageing 2013;42(1):27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li‐Hong 2018 {published data only}
- Wan LH, Zhang XP, You LM, Ruan HF, Chen SX. The efficacy of a comprehensive reminder system to improve health behaviors and blood pressure control in hypertensive ischemic stroke patients: a randomized controlled trial. Journal of Cardiovascular Nursing 2018;33(6):509-17. [DOI] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin EH, Von Korff M, Ciechanowski P, Peterson D, Ludman EJ, Rutter CM, et al. Treatment adjustment and medication adherence for complex patients with diabetes, heart disease, and depression: a randomized controlled trial. Annals of Family Medicine 2012;10(1):6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lluch‐Canut 2006 {published data only}
- Lluch-Canut MT, Checa-Peña F, García-Morales J, Márquez-Romero MI, Camarena-Pelegrí X, Beltrán-Megías JJ, et al. Effectiveness of a home care nursing program for patients with severe mental disorder: a controlled clinical trial. Enfermeria Clinica 2006;16(4):198-205. [Google Scholar]
Lowe 1995 {published data only}
- Lowe CJ, Raynor DK, Courtney EA, Purvis J, Teale C. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ 1995;310(6989):1229-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2000 {published data only}
- Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of a medicine review and education programme for older people in general practice. British Journal of Clinical Pharmacology 2000;50(2):172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2015 {published data only}
- Lu CH, Tang ST, Lei YX, Zhang MQ, Lin WQ, Ding SH, et al. Community-based interventions in hypertensive patients: a comparison of three health education strategies. BMC Public Health 2015;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luttik 2014 {published data only}
- Luttik ML, Jaarsma T, Geel PP, Brons M, Hillege HL, Hoes AW, et al. Long-term follow-up in optimally treated and stable heart failure patients: primary care vs. heart failure clinic. Results of the COACH-2 study. European Journal of Heart Failure 2014;16(11):1241-8. [DOI] [PubMed] [Google Scholar]
Ma 2014 {published data only}
- Ma C, Zhou Y, Zhou W, Huang C. Evaluation of the effect of motivational interviewing counselling on hypertension care. Patient Education & Counseling 2014;95(2):231-7. [DOI] [PubMed] [Google Scholar]
MacDonald 1977 {published data only}
- MacDonald ET, MacDonald JB, Phoenix M. Improving drug compliance after hospital discharge. BMJ 1977;2(6087):618-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackenzie 2013 {published data only}
- Mackenzie G, Ireland S, Moore S, Heinz I, Johnson R, Oczkowski W, et al. Tailored interventions to improve hypertension management after stroke or TIA--phase II (TIMS II). Canadian Journal of Neuroscience Nursing 2013;35(1):27-34. [PubMed] [Google Scholar]
Maduka 2013 {published data only}
- Maduka O, Tobin-West CI. Adherence counseling and reminder text messages improve uptake of antiretroviral therapy in a tertiary hospital in Nigeria. Nigerian Journal of Clinical Practice 2013;16(3):302-8. [DOI] [PubMed] [Google Scholar]
Magid 2011 {published data only}
- Magid DJ, Ho PM, Olson KL, Brand DW, Welch LK, Snow KE, et al. A multimodal blood pressure control intervention in 3 healthcare systems. American Journal of Managed Care 2011;17(4):e96-103. [PubMed] [Google Scholar]
Maislos 2004 {published data only}
Malet‐Larrea 2016 {published data only}
- Malet-Larrea A, Goyenechea E, García-Cárdenas V, Calvo B, Arteche JM, Aranegui P, et al. The impact of a medication review with follow-up service on hospital admissions in aged polypharmacy patients.. British Journal of Clinical Pharmacology 2016 Sep;82(3):831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maly 1999 {published data only}
- Maly RC, Bourque LB, Engelhardt RF. A randomized controlled trial of facilitating information giving to patients with chronic medical conditions: effects on outcomes of care. Journal of Family Practice 1999;48(5):356-63. [PubMed] [Google Scholar]
Margolius 2012 {published data only}
- Margolius D, Bodenheimer T, Bennett H, Wong J, Ngo V, Padilla G, et al. Health coaching to improve hypertension treatment in a low-income, minority population. Annals of Family Medicine 2012;10(3):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin 2015 {published data only}
- Marin GH, Risso P, Sbatella D, Haag G. Treatment adherence by personalizing the drug dispensing for diabetic patients in social vulnerable situation. Quality in Primary Care 2015;23(2):93-6. [Google Scholar]
Marquez Contreras 2009 {published data only}
- Márquez CE, Martel Claros N, Gil Guillén V, Martín De Pablos JL, De la Figuera Von Wichman M, Casado Martínez JJ, et al. Non-pharmacological intervention as a strategy to improve antihypertensive treatment compliance. Atencion Primaria 2009;41(9):501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1982 {published data only}
- Martin DC, Mead K. Reducing medication errors in a geriatric population. Journal of the American Geriatric Society 1982;30(4):258-60. [DOI] [PubMed] [Google Scholar]
Martin 2011 {published data only}
- Martin MY, Kim YI, Kratt P, Litaker MS, Kohler CL, Schoenberger YM, et al. Medication adherence among rural, low-income hypertensive adults: a randomized trial of a multimedia community-based intervention. American Journal of Health Promotion 2011;25(6):372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2014 {published data only}
- Martinez H, Palar K, Linnemayr S, Smith A, Derose KP, Ramirez B, et al. Tailored nutrition education and food assistance improve adherence to HIV antiretroviral therapy: evidence from Honduras. AIDS & Behavior 2014;18 Suppl 5:S566-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuyama 1993 {published data only}
- Matsuyama JR, Mason BJ, Jue SG. Pharmacists' interventions using an electronic medication-event monitoring device's adherence data versus pill counts. Annals of Pharmacotherapy 1993;27(7-8):851-5. [DOI] [PubMed] [Google Scholar]
Mazzuca 1986 {published data only}
McCarthy 2013 {published data only}
- McCarthy ML, Ding R, Roderer NK, Steinwachs DM, Ortmann MJ, Pham JC, et al. Does providing prescription information or services improve medication adherence among patients discharged from the emergency department? A randomized controlled trial. Annals of Emergency Medicine 2013;62(3):212-23.e1. [DOI] [PubMed]
McCarthy 2017 {published data only}
- McCarthy C, Clyne B, Corrigan D, Boland F, Wallace E, Moriarty F, et al. Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomised controlled trial protocol and pilot.. Implementation Science 2017;12(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGeoch 2006 {published data only}
- McGeoch GR, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006;11(5):611-8. [DOI] [PubMed] [Google Scholar]
Mehos 2000 {published data only}
- Mehos BM, Saseen JJ, MacLaughlin EJ. Effect of pharmacist intervention and initiation of home blood pressure monitoring in patients with uncontrolled hypertension. Pharmacotherapy 2000;20(11 I):1384-9. [DOI] [PubMed] [Google Scholar]
Mehuys 2008 {published data only}
- Mehuys E, Bortel L, Bolle L, Tongelen I, Annemans L, Remon JP, et al. Effectiveness of pharmacist intervention for asthma control improvement. European Respiratory Journal 2008;31(4):790-9. [DOI] [PubMed] [Google Scholar]
Mehuys 2011 {published data only}
Meredith 2002 {published data only}
- Meredith S, Feldman P, Frey D, Giammarco L, Hall K, Arnold K, et al. Improving medication use in newly admitted home healthcare patients: a randomized controlled trial. Journal of the American Geriatrics Society 2002;50(9):1484-91. [DOI] [PubMed] [Google Scholar]
Michiels 2017 {published data only}
- Michiels Y, Bugnon O, Chicoye A, Verges B, Moisan C, Mechin H, et al. Impact of a community pharmacy-based information program on type 2 diabetic patients' adherence: Iphodia, a cluster randomized study vs usual practice-12 month final results. Value in Health 2017;20 (9):A483. [Google Scholar]
Miller 1988 {published data only}
- Miller P, Wikoff R, McMahon M, Garrett MJ, Ringel K. Influence of a nursing intervention on regimen adherence and societal adjustments postmyocardial infarction. Nursing Research 1988;37(5):297-302. [PubMed]
Miller 2008 {published data only}
- Miller MJ, Abrams MA, McClintock B, Cantrell MA, Dossett CD, McCleeary EM, et al. Promoting health communication between the community-dwelling well-elderly and pharmacists: the Ask Me 3 program. Journal of the American Pharmacists Association 2008;48(6):784-92. [DOI] [PubMed] [Google Scholar]
Mitchell 2014 {published data only}
- Mitchell KE, Johnson-Warrington V, Apps LD, Bankart J, Sewell L, Williams JE, et al. A self-management programme for COPD: a randomised controlled trial. European Respiratory Journal 2014;44(6):1538-47. [DOI] [PubMed] [Google Scholar]
Moczygemba 2012 {published data only}
Moorhead 2017 {published data only}
- Moorhead P, Zavala A, Kim Y, Virdi NS. Efficacy and safety of a medication dose reminder feature in a digital health offering with the use of sensor-enabled medicines. Journal of the American Pharmacists Association 2017;57(2):155-61.e1. [DOI] [PubMed] [Google Scholar]
Moreno 2016 {published data only}
- Moreno EG, De Retana Garcia LO, Pena MEDC, Perez AS, Carazo CM, Ortiz JCA, et al. A pilot study to assess the feasibility of the Spanish diabetes self-management program in the Basque country. Journal of Diabetes Research 2016;2016:9145673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morisky 1990 {published data only}
Morrison 2016 {published data only}
- Morrison D, Wyke S, Saunderson K, McConnachie A, Agur K, Chaudhuri R, et al. Findings from a pilot Randomised trial of an Asthma Internet Self-management Intervention (RAISIN). BMJ Open 2016;6(5):e009254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullan 2009 {published data only}
- Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Archives of Internal Medicine 2009;169(17):1560-8. [DOI] [PubMed] [Google Scholar]
Muniz 2010 {published data only}
- Muñiz J, Gómez-Doblas JJ, Santiago-Pérez MI, Lekuona-Goya I, Murga-Eizagaetxebarría N, de Teresa-Galván ßE, et al. The effect of post-discharge educational intervention on patients in achieving objectives in modifiable risk factors six months after discharge following an episode of acute coronary syndrome, (CAM-2 Project): a randomized controlled trial. Health and Quality of Life Outcomes 2010;8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2007 {published data only}
- Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Annals of Internal Medicine 2007;146(10):714-25. [DOI] [PubMed] [Google Scholar]
NCT00838344 {published data only}
- NCT00838344. The Medication Adherence Program (MAP). https://clinicaltrials.gov/ct2/show/NCT00838344 (first received February 6, 2009).
NCT01144182 {published data only}
- NCT01144182. Veterans affairs lowering readmission in heart failure. https://clinicaltrials.gov/show/nct01144182 (first received June 15, 2010).
NCT01271985 {published data only}
- NCT01271985. Prevention of cardiovascular disease in middle-aged and elderly Iranians using a single polypill. https://clinicaltrials.gov/show/nct01271985 (first received January 7, 2011).
NCT01404988 {published data only}
- NCT01404988. Veterans intensive personalized treatment in heart failure. https://clinicaltrials.gov/show/nct01404988 (first received July 28, 2011).
NCT01914588 {published data only}
- NCT01914588. Multiparametric telemonitoring In elderly people with chronic heart failure. https://clinicaltrials.gov/show/nct01914588 (first received August 2, 2013).
NCT02035566 {published data only}
- NCT02035566. Telehome monitoring for chronic disease management. https://clinicaltrials.gov/show/nct02035566 (first received January 14, 2014).
NCT02140619 {published data only}
- NCT02140619. Affect of multiple health education on medication persistence and clinical prognosis of ischemic stroke patients. https://clinicaltrials.gov/ct2/show/NCT02140619 (first received May 16, 2014).
NCT02490423 {published data only}
- NCT02490423. Improvement in medication compliance through the implementation of personalized nudges: the ENCOURAGE Trial (ENCOURAGE). https://clinicaltrials.gov/ct2/show/NCT02490423 (first received July 3, 2015).
NCT02697422 {published data only}
- NCT02697422. Veteran peer coaches optimizing and advancing cardiac health. https://clinicaltrials.gov/show/nct02697422 (first received March 3, 2016).
NCT02905474 {published data only}
- NCT02905474. Mobile health technology for chronic kidney disease patients: medication management. https://clinicaltrials.gov/ct2/show/NCT02905474 (first received September 19, 2016).
NCT03342729 {published data only}
- NCT03342729. Aging Mastery Program® (AMP) evaluation. https://clinicaltrials.gov/show/nct03342729 (first received November 17, 2017).
Nesari 2010 {published data only}
- Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Japan Journal of Nursing Science 2010;7(2):121-8. [DOI] [PubMed] [Google Scholar]
Newman 2016 {published data only}
- Newman N, Porter B, Hoyt C, Southerland L, Payne K, Lehman A, et al. Impact of community pharmacy and home health care transitions of care services on 30-day emergency department revisit rates. Journal of the American Pharmacists Association 2016;56 (3):e92. [Google Scholar]
Nguyen 2017 {published data only}
- Nguyen T. Medication reconciliation following hospital discharge for high-risk elderly patients. In: Journal of the American Pharmacists Association Conference. Vol. 57. 2017.
Nishita 2013 {published data only}
- Nishita C, Cardazone G, Uehara DL, Tom T. Empowered diabetes management: life coaching and pharmacist counseling for employed adults with diabetes. Health Education & Behavior 2013;40(5):581-91. [DOI] [PubMed] [Google Scholar]
Noureldin 2012 {published data only}
- Noureldin M, Plake KS, Morrow DG, Tu W, Wu J, Murray MD. Effect of health literacy on drug adherence in patients with heart failure. Pharmacotherapy 2012;32(9):819-26. [DOI] [PubMed] [Google Scholar]
NTR2288, 2010 {published data only}
- NTR. Central Utrecht Elderly Care Project 'Om U': “somebody who knows what’s going on and can see things from my side”. http://www.who.int/trialsearch/trial2.aspx?Trialid=ntr2288 2010.
Obreli‐Neto 2011 {published data only}
- Obreli-Neto PR, Guidoni CM, Oliveira Baldoni A, Pilger D, Cruciol-Souza JM, Gaeti-Franco WP, et al. Effect of a 36-month pharmaceutical care program on pharmacotherapy adherence in elderly diabetic and hypertensive patients. International Journal of Clinical Pharmacy 2011;33(4):642-9. [DOI] [PubMed] [Google Scholar]
Ogedegbe 2012 {published data only}
- Ogedegbe GO, Boutin-Foster C, Wells MT, Allegrante JP, Isen AM, Jobe JB, et al. A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Archives of Internal Medicine 2012;172(4):322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliveira‐Filho 2014 {published data only}
- Oliveira-Filho AD, Morisky DE, Costa FA, Pacheco ST, Neves SF, Lyra-Jr DP. Improving post-discharge medication adherence in patients with CVD: a pilot randomized trial. Arquivos Brasileiros de Cardiologia 2014;103(6):503-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oonk 2017 {published data only}
- Oonk N, Munster E, Verbeek A, Ter Huurne K, Van Der Palen J, Movig K, et al. A study assessing the effect of a structured medication review on quality of life in patients with Parkinson's disease: an interim analysis. European Journal of Neurology 2017;24(Suppl 1):396. [Google Scholar]
Ostbring 2014 {published data only}
- Ostbring MJ, Eriksson T, Petersson G, Hellstrom L. Medication beliefs and self-reported adherence - results of a pharmacist's consultation: a pilot study. European Journal of Hospital Pharmacy 2014;21(2):102-7.
Ostovaneh 2015 {published data only}
- Ostovaneh MR, Poustchi H, Hemming K, Marjani H, Pourshams A, Nateghi A, et al. Polypill for the prevention of cardiovascular disease (PolyIran): study design and rationale for a pragmatic cluster randomized controlled trial. European Journal of Preventive Cardiology 2015;22(12):1609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park M. Effects of interactive pictorial education on community dwelling older adult's self efficacy and knowledge for safe medication. Journal of Korean Academy of Nursing 2011;41(6):795-804. [DOI] [PubMed] [Google Scholar]
Parker 2014 {published data only}
- Parker CP, Cunningham CL, Carter BL, Vander Weg MW, Richardson KK, Rosenthal GE. A mixed-method approach to evaluate a pharmacist intervention for veterans with hypertension. Journal of Clinical Hypertension 2014;16(2):133-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearl 2003 {published data only}
- Pearl A, Wright SP, Gamble GD, Muncaster S, Walsh HJ, Sharpe N, et al. The effect of an integrated care approach for heart failure on general practice. Family Practice 2003;20(6):642-5. [DOI] [PubMed] [Google Scholar]
Peng 2014 {published data only}
- Peng B, Ni J, Anderson CS, Zhu Y, Wang Y, Pu C, et al. Implementation of a structured guideline-based program for the secondary prevention of ischemic stroke in China. Stroke 2014;45(2):515-9. [DOI] [PubMed] [Google Scholar]
Pérez‐Escamilla 2015 {published data only}
- Pérez-Escamilla R, Damio G, Chhabra J, Fernandez ML, Segura-Pérez S, Vega-López S, et al. Impact of a community health workers-led structured program on blood glucose control among latinos with type 2 diabetes: the DIALBEST trial. Diabetes Care 2015;38(2):197-205. [DOI] [PMC free article] [PubMed]
Perl 2016 {published data only}
- Perl S, Niederl E, Kos C, Mrak P, Ederer H, Rakovac I, et al. Randomized evaluation of the effectiveness of a structured educational program for patients with essential hypertension. American Journal of Hypertension 2016;29(7):866-72. [DOI] [PubMed] [Google Scholar]
Persaud 2017 {published data only}
- Persaud N, Lee T, Ahmad H, Li W, Taglione M S, Rajakulasingam Y, et al. Protocol for a randomised controlled trial evaluating the effects of providing essential medicines at no charge: the Carefully seLected and Easily Accessible at No Charge Medicines (CLEAN Meds) trial. BMJ Open 2017;7(5):e015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters‐Klimm 2010 {published data only}
- Peters-Klimm F, Campbell S, Hermann K, Kunz CU, Muller-Tasch T, Szecsenyi J. Case management for patients with chronic systolic heart failure in primary care: the HICMan exploratory randomised controlled trial. Trials 2010;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phumipamorn 2008 {published data only}
- Phumipamorn S, Pongwecharak J, Soorapan S, Pattharachayakul S. Effects of the pharmacist's input on glycaemic control and cardiovascular risks in Muslim diabetes. Primary Care Diabetes 2008;2(1):31-7. [DOI] [PubMed] [Google Scholar]
Pitner 2004 {published data only}
- Pitner J. Specialty geriatric evaluation and management teams reduce adverse drug reactions. Consultant Pharmacist 2004;19(11):1042-9. [PubMed] [Google Scholar]
Pladevall 2010 {published data only}
- Pladevall M, Brotons C, Gabriel R, Arnau A, Suarez C, Figuera M, et al. Multicenter cluster-randomized trial of a multifactorial intervention to improve antihypertensive medication adherence and blood pressure control among patients at high cardiovascular risk (the COM99 study). Circulation 2010;122(12):1183-91. [DOI] [PMC free article] [PubMed]
Pladevall 2015 {published data only}
- Pladevall M, Divine G, Wells KE, Resnicow K, Williams LK. A randomized controlled trial to provide adherence information and motivational interviewing to improve diabetes and lipid control. Diabetes Educator 2015;41(1):136-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plant 2015, ACTRN12609000554268 {published data only}
- Plant N, Leeder S. A single-blinded randomised controlled trial comparing the effect of care navigation versus current standard care in patients presenting to the emergency department with serious chronic illness on the rate of re-presentation to the emergency department, re-admission to hospital and time spent in hospital. ACTRN12609000554268 .
Polack 2008 {published data only}
- Polack J, Jorgenson D, Robertson P. Evaluation of different methods of providing medication-related education to patients following myocardial infarction. Canadian Pharmacists Journal 2008;141(4):241-7. [Google Scholar]
Ponnusankar 2004 {published data only}
- Ponnusankar S, Surulivelrajan M, Anandamoorthy N, Suresh B. Assessment of impact of medication counseling on patients' medication knowledge and compliance in an outpatient clinic in South India. Patient Education and Counseling 2004;54(1):55-60. [DOI] [PubMed] [Google Scholar]
Powers 2011 {published data only}
- Powers BJ, Danus S, Grubber JM, Olsen MK, Oddone EZ, Bosworth HB. The effectiveness of personalized coronary heart disease and stroke risk communication. American Heart Journal 2011;161(4):673-80. [DOI] [PubMed] [Google Scholar]
Pringle 2014 {published data only}
- Pringle J, Boyer A, Conklin M, McCullough J, Aldridge A. Effect of a pharmacy-based intervention on medication adherence and health care costs. Journal of the American Pharmacists Association 2014;54 (2):e77-8. [Google Scholar]
Radini 2017 {published data only}
- Radini D, Apuzzo GM, Stellato K, Sola G, Delli Quadri N, Fragiacomo E, et al. Acceptance of home telemonitoring in patients with heart failure: the Ecare client impact survey in the European funded project Smartcare. European Journal of Heart Failure 2017;19(Suppl 1):109. [Google Scholar]
Ramaekers 2009 {published data only}
- Ramaekers BL, Janssen-Boyne JJ, Gorgels AP, Vrijhoef HJ. Adherence among telemonitored patients with heart failure to pharmacological and nonpharmacological recommendations. Telemed Journal and e-Health 2009;15(6):517-24. [DOI] [PubMed] [Google Scholar]
Ramanath 2011 {published data only}
- Ramanath KV, Santhosh YL. Impact of clinical pharmacist provided patient education on QOL outcome in type II diabetes mellitus in rural population. Asian Journal of Pharmaceutical and Clinical Research 2011;4(4):15-20. [Google Scholar]
Ravn‐Nielsen 2018 {published data only}
- Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Internal Medicine 2018;178(3):375-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raynor 1993 {published data only}
- Raynor DK, Booth TG, Blenkinsopp A. Effects of computer generated reminder charts on patients' compliance with drug regimens. BMJ 1993;306(6886):1158-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reed 2018 {published data only}
- Reed RL, Roeger L, Howard S, Oliver-Baxter JM, Battersby MW, Bond M, et al. A self-management support program for older Australians with multiple chronic conditions: a randomised controlled trial. Medical Journal of Australia 2018;208(2):69-74. [DOI] [PubMed] [Google Scholar]
Richmond 2010 {published data only}
- RESPECT Trial Team. Effectiveness of shared pharmaceutical care for older patients: RESPECT trial findings. British Journal General Practice 2010;60(570):e10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roden 1985 {published data only}
- Roden S, Harvey P, Mayer P, Spence L. Evaluation of two techniques to improve drug compliance in the elderly. Journal of Clinical & Experimental Gerontology 1985;7(1):71-82. [Google Scholar]
Rose 2016 {published data only}
- Rose O, Mennemann H, John C, Lautenschläger M, Mertens-Keller D, Richling K, Waltering I, et al. Priority setting and influential factors on acceptance of pharmaceutical recommendations in collaborative medication reviews in an ambulatory care setting - analysis of a cluster randomized controlled trial (WestGem-Study). PLoS One 2016;11(6):e0156304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothschild 2014 {published data only}
- Rothschild SK, Martin MA, Swider SM, Tumialán Lynas CM, Janssen I, Avery EF, et al. Mexican American trial of community health workers: a randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus. American Journal of Public Health 2014;104(8):1540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rozenfeld 1999 {published data only}
- Rozenfeld V, Pflomm J, Singh KK, Bazil MK, Cheng JWM. Assessing the impact of medication consultations with a medication event monitoring system. Hospital Pharmacy 1999;34(5):539-49. [Google Scholar]
Rubak 2011 {published data only}
- Rubak S, Sandbaek A, Lauritzen T, Borch-Johnsen K, Christensen B. Effect of "motivational interviewing" on quality of care measures in screen detected type 2 diabetes patients: a one-year follow-up of an RCT, ADDITION Denmark. Scandinavian Journal of Primary Health Care 2011;29(2):92-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rytter 2010 {published data only}
- Rytter L, Jakobsen HN, Ronholt F, Hammer AV, Andreasen AH, Nissen A, et al. Comprehensive discharge follow-up in patients' homes by GPs and district nurses of elderly patients. A randomized controlled trial. Scandinavian Journal of Primary Health Care 2010;28(3):146-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Safren 2014 {published data only}
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care 2014;37(3):625-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salisbury 2016 {published data only}
- Salisbury C, O'Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. BMJ 2016;353:i2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samtia 2013 {published data only}
- Samtia AM, Rasool MF, Ranjha NM, Usman F, Javed I. A multifactorial intervention to enhance adherence to medications and disease-related knowledge in type 2 diabetic patients in Southern Punjab, Pakistan. Tropical Journal of Pharmaceutical Research 2013;12(5):851-6. [Google Scholar]
Sanchez 2012 {published data only}
- Sanchez Ulayar A, Gallardo Lopez S, Pons Llobet N, Murgadella Sancho A, Campins Bernadas L, Merino Mendez R. Pharmaceutical intervention upon hospital discharge to strengthen understanding and adherence to pharmacological treatment. Farmacia Hospitalaria 2012;36(3):118-23. [DOI] [PubMed] [Google Scholar]
Sandler 1989 {published data only}
- Sandler DA, Mitchell JR, Fellows A, Garner ST. Is an information booklet for patients leaving hospital helpful and useful? BMJ 1989;298(6677):870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2008 {published data only}
- Schneider PJ, Murphy JE, Pedersen CA. Impact of medication packaging on adherence and treatment outcomes in older ambulatory patients. Journal of the American Pharmacists Association 2008;48(1):58-63. [DOI] [PubMed] [Google Scholar]
Schou 2013 {published data only}
- Schou M, Gislason GG, Videbaek L, Kober L, Tuxen CD, Torp-Pedersen C, et al. Long term adherence in primary care versus a specialized heart failure clinic for outpatients with systolic heart failure. European Heart Journal 2013;34:161-2. [Google Scholar]
Schou 2014 {published data only}
- Schou M, Gislason G, Videbaek L, Kober L, Tuxen C, Torp-Pedersen C, et al. Effect of extended follow-up in a specialized heart failure clinic on adherence to guideline recommended therapy: NorthStar Adherence Study. European Journal of Heart Failure 2014;16(11):1249-55. [DOI] [PubMed] [Google Scholar]
Schwalm 2015 {published data only}
Schwartz 2015 {published data only}
- Schwartz JK. Single-subject analysis of an occupational therapy intervention for medication nonadherence. Archives of Physical Medicine and Rehabilitation 2015;96 (10):e66. [Google Scholar]
Schwartz 2017 {published data only}
- Schwartz EJ, Turgeon J, Patel J, Patel P, Shah H, Issa AM, et al. Implementation of a standardized medication therapy management plus approach within primary care. Journal of the American Board of Family Medicine 2017;30(6):701-14. [DOI] [PubMed] [Google Scholar]
Scott 2004 {published data only}
- Scott JC, Conner DA, Venohr I, Gade G, McKenzie M, Kramer AM, et al. Effectiveness of a group outpatient visit model for chronically ill older health maintenance organization members: a 2-year randomized trial of the cooperative health care clinic. Journal of the American Geriatrics Society 2004;52(9):1463-70. [DOI] [PubMed] [Google Scholar]
Selak 2014 {published data only}
- Selak V, Elley CR, Bullen C, Crengle S, Wadham A, Rafter N, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ 2014;348:g3318. [DOI] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah RB, Desai SV, Gajjar BM, Shah AM. Factors responsible for noncompliance to drug therapy in the elderly and the impact of patient education on improving compliance. Drugs and Therapy Perspectives 2013;29(11):360-6. [Google Scholar]
Sherrard 2009 {published data only}
- Sherrard H, Struthers C, Kearns SA, Wells G, Chen L, Mesana T. Using technology to create a medication safety net for cardiac surgery patients: a nurse-led randomized control trial. Canadian Journal of Cardiovascular Nursing 2009;19(3):9-15. [PubMed]
Sherrard 2015 {published data only}
- Sherrard H, Duchesne L, Wells G, Kearns SA, Struthers C. Using interactive voice response to improve disease management and compliance with acute coronary syndrome best practice guidelines: a randomized controlled trial. Canadian Journal of Cardiovascular Nursing 2015;25(1):10-5. [PubMed] [Google Scholar]
Shuster 1998 {published data only}
- Shuster GF. A home based intervention reduced the frequency of hospital readmissions and out of hospital deaths after discharge [commentary on Stewart S, Pearson S, Luke CG, et al. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. J Am Geriatr Soc 1998 Feb;46:174-80]. Evidence Based Nursing 1998;1(4):120. [DOI] [PubMed] [Google Scholar]
Sidel 1990 {published data only}
- Sidel VW, Beizer JL, Lisi-Fazio D, Kleinmann K, Wenston J, Thomas C, et al. Controlled study of the impact of educational home visits by pharmacists to high-risk older patients. Journal of Community Health 1990;15(3):163-74. [DOI] [PubMed] [Google Scholar]
Simkins 1986 {published data only}
- Simkins CV, Wenzloff NJ. Evaluation of a computerized reminder system in the enhancement of patient medication refill compliance. Drug Intelligence & Clinical Pharmacy 1986;20(10):799-802. [DOI] [PubMed] [Google Scholar]
Simoni 2011 {published data only}
- Simoni JM, Chen WT, Huh D, Fredriksen-Goldsen KI, Pearson C, Zhao H, et al. A preliminary randomized controlled trial of a nurse-delivered medication adherence intervention among HIV-positive outpatients initiating antiretroviral therapy in Beijing, China. AIDS and Behavior 2011;15(5):919-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sit 2016 {published data only}
- Sit JWH, Chair SY, Choi KC, Chan CWH, Lee DTF, Chan AWK, et al. Do empowered stroke patients perform better at self-management and functional recovery after a stroke? A randomized controlled trial. Clinical Interventions in Aging 2016;11:1441-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sledge 2006 {published data only}
- Sledge WH, Brown KE, Levine JM, Fiellin DA, Chawarski M, White WD, et al. A randomized trial of primary intensive care to reduce hospital admissions in patients with high utilization of inpatient services. Disease Management 2006;9(6):328-38. [DOI] [PubMed] [Google Scholar]
Smith 1997 {published data only}
- Smith L, McGowan L, Moss-Barclay C, Wheater J, Knass D, Chrystyn H. An investigation of hospital generated pharmaceutical care when patients are discharged home from hospital. British Journal of Clinical Pharmacology 1997;44(2):163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2004 {published data only}
Smith 2007 {published data only}
- Smith GE, Lunde AM, Hathaway JC, Vickers KS. Telehealth home monitoring of solitary persons with mild dementia. American Journal of Alzheimers Disease & Other Dementias 2007;22(1):20-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soloman 1998 {published data only}
- Solomon DK, Portner TS, Bass GE, Gourley DR, Gourley GA, Holt JM, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. Journal of the American Pharmacists Association 1998;38(5):574-85. [DOI] [PubMed] [Google Scholar]
Sookaneknun 2004 {published data only}
- Sookaneknun P, Richards RME, Sanguansermsri J, Teerasut C. Pharmacist involvement in primary care improves hypertensive patient clinical outcomes. Annals of Pharmacotherapy 2004;38(12):2023-8. [DOI] [PubMed] [Google Scholar]
Soong 2014 {published data only}
- Soong C, Kurabi B, Wells D, Caines L, Morgan MW, Ramsden R, et al. Do post discharge phone calls improve care transitions? A cluster-randomized trial. PLoS ONE 2014;9(11):e112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souter 2017 {published data only}
- Souter C, Kinnear A, Kinnear M, Mead G. A pilot study to assess the practicality, acceptability and feasibility of a randomised controlled trial to evaluate the impact of a pharmacist complex intervention on patients with stroke in their own homes. European Journal of Hospital Pharmacy - Science and Practice 2017;24:101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stange 2013 {published data only}
- Stange D, Kriston L, von-Wolff A, Baehr M, Dartsch DC. Reducing cardiovascular medication complexity in a German university hospital: effects of a structured pharmaceutical management intervention on adherence. Journal of Managed Care Pharmacy 2013;19(5):396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanhope 2013 {published data only}
- Stanhope V, Ingoglia C, Schmelter B, Marcus SC. Impact of person-centered planning and collaborative documentation on treatment adherence. Psychiatric Services 2013;64(1):76-9. [DOI] [PubMed] [Google Scholar]
Strobach 2000 {published data only}
- Strobach D, Vetter-Kerkhoff C, Bogner J, Breugst W, Schlondorff D. [Patient medication counseling - patient counseling about discharge medication]. Medizinische Klinik 2000;95(10):548-51. [DOI] [PubMed] [Google Scholar]
Stromberg 2005 {published data only}
- Strömberg A, Dahlström U, Fridlund B. Computer-based education for patients with chronic heart failure. A randomised, controlled, multicentre trial of the effects on knowledge, compliance and quality of life. Patient Educationa and Counseling 2006;64(1-3):128-35. [DOI] [PubMed] [Google Scholar]
Sweeney 1989 {published data only}
- Sweeney SJ, Dixon JS, Sutcliffe I. Impact of the clinical pharmacist on compliance in a geriatric population. Pharmaceutical Journal 1989;242(Feb 18 Suppl):242:R4-6.
Tai 2014 {published data only}
- Tai B, Law A, LaRue C. Effect of focused education on functional health literacy and prescription label comprehension: a randomized controlled trial. Journal of the American Pharmacists Association 2014;54 (2):e111-2. [DOI] [PubMed] [Google Scholar]
Touchette, 2012 {published data only}
Towfighi 2017 {published data only}
- Towfighi A, Cheng EM, Ayala-Rivera M, et al. Randomized controlled trial of a coordinated care intervention to improve risk factor control after stroke or transient ischemic attack in the safety net: Secondary stroke prevention by Uniting Community and Chronic care model teams Early to End Disparities (SUCCEED). BMC Neurology 2017;17:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tu 1999 {published data only}
- Tu MS. The effect of health education on self-care behaviors and hypertension control in elderly hypertensive patients at a veterans home. [Korean]. Chinese Journal of Public Health 1999;18(1):54-65. [Google Scholar]
Tu 2018 {published data only}
- Tu Q, Xiao LD, Ullah S, Fuller J, Du H. Hypertension management for community-dwelling older people with diabetes in Nanchang, China: study protocol for a cluster randomized controlled trial. Trials [Electronic Resource] 2018;19(1):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaillant‐Roussel 2016 {published data only}
- Vaillant-Roussel H, Laporte C, Pereira B, De Rosa M, Eschalier B, Vorilhon C, et al. Impact of patient education on chronic heart failure in primary care (ETIC): a cluster randomised trial. BMC Family Practice 2016;17:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valimaki 2017 {published data only}
- Valimaki M, Kannisto KA, Vahlberg T, Hatonen H, Adams CE. Short text messages to encourage adherence to medication and follow-up for people With psychosis (Mobile.Net): randomized controlled trial in Finland. Journal of Medical Internet Research 2017;19(7):e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varleta 2017 {published data only}
- Varleta P, Acevedo M, Akel C, Salinas C, Navarrete C, Garcia A, et al. Mobile phone text messaging improves antihypertensive drug adherence in the community. Journal of Clinical Hypertension 2017;19(12):1276-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varma 1999 {published data only}
- Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: interventions and outcomes. Pharmacotherapy 1999;19(7):860-9. [DOI] [PubMed] [Google Scholar]
Verbeek 2016 {published data only}
- Verbeek A, Oonk N, Munster E, Movig K, Ter Huurne K, Nijmeijer H, et al. The effect of a structured medication review on quality of life in patients with Parkinson's disease. An interim analysis. Contempory Clinical Trials Communication 2016;31:S170-1.
Via‐Sosa 2013 {published data only}
- Via-Sosa MA, Lopes N, March M. Medication management in a geriatric care practice setting. BMC Family Practice 2013;14(1):96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villani 2014 {published data only}
- Villani A, Malfatto G, Compare A, Della Rosa F, Bellardita L, Branzi G, et al. Clinical and psychological telemonitoring and telecare of high risk heart failure patients. Journal of Telemedicine & Telecare 2014;20(8):468-75. [DOI] [PubMed] [Google Scholar]
Vinks 2009 {published data only}
- Vinks TH, Egberts TC, Lange TM, Koning FH. Pharmacist-based medication review reduces potential drug-related problems in the elderly: the SMOG controlled trial. Drugs & Aging 2009;26(2):123-33. [DOI] [PubMed] [Google Scholar]
Vivian 2002 {published data only}
- Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy 2002;22(12):1533-40. [DOI] [PubMed] [Google Scholar]
Vollmer 2014 {published data only}
- Vollmer WM, Owen-Smith AA, Tom JO, Laws R, Ditmer DG, Smith DH, et al. Improving adherence to cardiovascular disease medications with information technology. American Journal of Managed Care 2014;20:502-10. [PMC free article] [PubMed] [Google Scholar]
Wakefield 2011 {published data only}
- Wakefield BJ, Holman JE, Ray A, Scherubel M, Adams MR, Hillis SL, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemedicine Journal and e-Health 2011;17(4):254-61. [DOI] [PubMed] [Google Scholar]
Wakefield 2012 {published data only}
- Wakefield BJ, Holman JE, Ray A, Scherubel M, Adams MR, Hills SL, et al. Outcomes of a home telehealth intervention for patients with diabetes and hypertension. Telemedicine Journal and e-Health 2012;18(8):575-9. [DOI] [PubMed] [Google Scholar]
Wandless 1981 {published data only}
- Wandless I, Whitmore J. The effect of counseling by a pharmacist on drug compliance in elderly patients. Journal of Clinical & Hospital Pharmacy 1981;6(1):51-6. [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang CJ, Fetzer SJ, Yang YC, Wang JJ. The impacts of using community health volunteers to coach medication safety behaviors among rural elders with chronic illnesses. Geriatric Nursing 2013;34(2):138-45. [DOI] [PubMed] [Google Scholar]
Wild 2016 {published data only}
- Wild SH, Hanley J, Lewis SC, McKnight JA, McCloughan LB, Padfield PL, et al. Supported telemonitoring and glycemic control in people with type 2 diabetes: the Telescot diabetes pragmatic multicenter randomized controlled trial.. PLOS Medicine 2016;13(7):e1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2004 {published data only}
- Williams JW Jr, Katon W, Lin EH, Nöel PH, Worchel J, Cornell J, et al. The effectiveness of depression care management on diabetes-related outcomes in older patients. Annals of Internal Medicine 2004;140(12):1015-24. [DOI] [PubMed] [Google Scholar]
Williford 1995 {published data only}
- Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Military Medicine 1995;160(11):561-4. [PubMed] [Google Scholar]
Yatabe 2018 {published data only}
- Yatabe MS, Yatabe J, Asayama K, Staessen JA, Mujaj B, Thijs L, et al. The rationale and design of reduction of uncontrolled hypertension by Remote Monitoring and Telemedicine (REMOTE) study. Blood Pressure 2018;27(2):99-105. [DOI] [PubMed] [Google Scholar]
Yu 2012 {published data only}
- Yu M. The effectiveness of a heart failure disease management programme on clinical outcomes, health-related quality of life, and psychological status of patients with heart failure in China. Dissertation Abstracts International: Section B: The Sciences and Engineering 2012;73(6-B):3538. [Google Scholar]
Zermansky 2002 {published data only}
- Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freemantle N, Vail A. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial. Health Technology Assessment 2002;6(20):1-86. [DOI] [PubMed] [Google Scholar]
Zhao 2004 {published data only}
- Zhao Y. Effects of a discharge planning intervention for elderly patients with coronary heart disease in Tianjin, China: a randomized controlled trial. The Hong Kong Polytechnic University 2004;221.
Zhao 2015 {published data only}
- Zhao S, Zhao H, Du S, Qin Y. The impact of clinical pharmacist support on patients receiving multi-drug therapy for coronary heart disease in china: a long-term follow-up study. Indian Journal of Pharmaceutical Sciences 2015;22(6):323-7. [DOI] [PMC free article] [PubMed]
李静, 2017 {published data only}
- 李静, 李荣, 张会敏, 张瑞芹. 感恩干预对社区老年高血压病人自我管理水平的影响...Influence of gratitude intervention of self-management level of elderly patients with community hypertension. Chinese Nursing Research 2017;31(2):163-6. [Google Scholar]
References to studies awaiting assessment
ACTRN12606000247572 {published data only}
- ACTRN12606000247572. A randomised controlled trial to measure the effectiveness of dose administration aids (DAAs) in improving veteran adherence and health outcomes and to characterise which veterans have a beneficial outcome. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1391 (first received 20 June 2006).
ACTRN12611000452998 {published and unpublished data}
- ACTRN12611000452998. Medication Reviews ReDirected (MedReDi): acute coronary syndrome as an indication for home medicine review, a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000452998 (first received 3 May 2011).
- Bernal DD, Stafford L, Bereznicki LR, Castelino RL, Davidson PM, Peterson GM. Home medicines reviews following acute coronary syndrome: study protocol for a randomized controlled trial. Trials 2012;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12616000910404 {published data only}
- ACTRN12616000910404. The effectiveness of motivational interviewing (MINT) and text message reminders on medication adherence among patients with heart disease. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370973 (first received 8 July 2016).
ACTRN12617000665336 {published data only}
- ACTRN12617000665336. Impact of clinical pharmacist medication review on appropriate prescribing in elderly patients: a randomized, controlled trial. http://www.who.int/trialsearch/trial2.aspx?Trialid=actrn12617000665336 (first received 8 May 2017).
Ahmad 2010 {published data only}
- Ahmad A, Hugtenburg J, Welschen LM, Dekker JM, Nijpels G. Effect of medication review and cognitive behaviour treatment by community pharmacists of patients discharged from the hospital on drug related problems and compliance: design of a randomized controlled trial. Biomed Central Public Health 2010;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A. Beliefs about prescribed medicine and self-reported adherence among elderly patients discharged from hospital. International Journal of Person Centered Medicine 2013;2:818-82. [Google Scholar]
- Hugtenburg JG. The effectiveness of optimised clinical medication reviews for geriatric patients: a cluster randomized controlled trial. In: Pharmacoepidemiology and Drug Safety. Vol. 26(Suppl 2). 2017:194. [DOI] [PubMed]
Cao 2017 {published data only}
- Cao XY, Tian L, Chen L, Jiang XL. Effects of a hospital-community partnership transitional program in patients with coronary heart disease in Chengdu, China: a randomized controlled trial. Japan Journal of Nursing Science 2017;14(4):320-31. [DOI] [PubMed] [Google Scholar]
Char 2017 {unpublished data only}
- Char CWT, Yip AYF, Kee KW, Lee ES, Chua AHL. Effectiveness of pre-consultation medication reconciliation service in reducing medication discrepancies during transition of care from hospital discharge to primary care setting in Singapore - a randomised controlled trial. Unpublished (supplied by author) 2017.
Cheema 2017 {published data only}
- Cheema R, Kwolek S, Frias J, Mangin D, Moore A, Masrur M. Measurement of medication discrepancies and use of medication wallet cards to increase patient self-efficacy. In: Canadian Family Physician. Vol. 63. 2017:S108.
ChiCTR‐TRC‐12002106 {published data only}
- ChiCTR‐TRC‐12002106. Using the picture album of daily self-questionnaires to improve medication adherence of secondary prevention programs for elderly patients with acute coronary syndrome: a randomised controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-12002106 (date received 18 April 2012).
Demers 2014 {published data only}
- Demers C, Patterson C, Archer N, Coallier J, Strachan P, Keshavjee K, et al. A simple multi-component intervention improves self management in heart failure. In: Canadian Journal of Cardiology. Vol. 30. 2014:S201.
Flink 2016 {published data only}
- Flink M, Lindblad M, Frykholm O, Kneck A, Nilsen P, Årestedt K, et al. The Supporting Patient Activation in Transition to Home (sPATH) intervention: a study protocol of a randomised controlled trial using motivational interviewing to decrease re-hospitalisation for patients with COPD or heart failure. BMJ Open 2017;7:e014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madarshahian 2018 {published data only}
- Madarshahian F, Safaie YN, Safaie YA, Roohbakhsh FO, Madarshahian MR. Impact of family support on medication adherence and cognition in stroke patients. In: European Stroke Journal. Vol. 3. 2018:296.
Marusic 2018 {published data only}
- Marusic S, Melis P, Lucijanic M, Grgurevic I, Turcic P, Neto PRO, et al. Impact of pharmacotherapeutic education on medication adherence and adverse outcomes in patients with type 2 diabetes mellitus: a prospective, randomized study. Croatian Medical Journal 2018;59(6):290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muth 2018 {published data only}
- Muth C, Uhlmann L, Haefeli WE, Rochon J, den Akker M, Perera R, et al. Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open 2018;8(2):e017740. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00560001 {published data only}
- NCT00560001. MD.2 medication dispenser medication adherence study. https://clinicaltrials.gov/show/nct00560001 (first received November 19, 2007).
NCT00916214 {published data only}
- NCT00916214. Improving compliance among elderly polypharmacy users through community pharmacy based pharmaceutical care program. https://clinicaltrials.gov/show/nct00916214 (first received June 9, 2009).
NCT01105104 {published data only}
- NCT01105104. An enhanced medication monitoring program. https://clinicaltrials.gov/show/nct01105104 (first received April 16, 2010).
NCT01534559 {published data only}
- NCT01534559. Pharmacist-led medicines management outpatient service (MMC). https://clinicaltrials.gov/ct2/show/NCT01534559 (first received February 16, 2012).
NCT02047448 {published data only}
- NCT02047448. Improving medication adherence through a transitional care pharmacy practice model. https://clinicaltrials.gov/ct2/show/NCT02047448 (first received Janurary 28, 2014).
NCT02424786 {published data only}
- NCT02424786. Non-adherence and polypharmacy in elderly patients. https://clinicaltrials.gov/ct2/show/NCT02424786 (first received April 23, 2015).
NCT02842840 {published data only}
- NCT02842840. The effectiveness of multimodal educational intervention to improve adherence to treatment regimens in older stroke patients. https://clinicaltrials.gov/ct2/show/NCT02842840 (first received July 25, 2016).
NCT03156348 {published data only}
- NCT03156348. Impact of clinical pharmacist on adverse drug events in older adults. https://clinicaltrials.gov/show/nct03156348 (first received May 17, 2017).
NCT03162848 {published data only}
- NCT03162848. SystemCHANGE™ intervention on medication adherence in older adults with heart failure (ECHO). https://clinicaltrials.gov/ct2/show/NCT03162848 (first received May 22, 2017).
Ostbring 2018 {published data only}
- Östbring MJ, Eriksson T, Petersson G, Hellström L. Motivational Interviewing and Medication Review in Coronary heart disease (MIMeRiC): protocol for a randomized controlled trial investigating effects on clinical outcomes, adherence, and quality of life. Journal of Medical Internet Research, Research Protocols 2018;7(2):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prados‐Torres 2017 {published data only}
- Prados-Torres A, Cura-Gonzalez I, Prados-Torres D, López-Rodríguez JA, Leiva-Fernández F, Calderón-Larrañaga A, et al. Effectiveness of an intervention for improving drug prescription in primary care patients with multimorbidity and polypharmacy: study protocol of a cluster randomized clinical trial (Multi-PAP project). Implementation Science 2017;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reilly 2016 {published data only}
- Reilly K, West E, Jung S, Friedberg JP, Natarajan S. A multicomponent transitional care intervention to improve clinical outcomes among patients being discharged after a heart failure admission: a pilot randomized clinical trial. In: Journal of General Internal Medicine. Vol. 1. 2016:S99.
References to ongoing studies
Adam 2019 {published data only}
- Adam L, Moutzouri E, Baumgartner C, Loewe AL, Feller M, M'Rabet-Bensalah K, et al. Rationale and design of OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people (OPERAM): a cluster randomised controlled trial. BMJ Open 2019;9:e026769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02986425. OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly (OPERAM). https://clinicaltrials.gov/ct2/show/NCT02986425 (date received December 8, 2016).
Bailey 2017 {published data only}
- Bailey SC, Wismer GA, Parker RM, Walton SM, Wood AJJ, Wallia A, et al. Development and rationale for a multifactorial, randomized controlled trial to test strategies to promote adherence to complex drug regimens among older adults. Contemporary Clinical Trials 2017;62:21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodge 2018 {published data only}
- Dodge H, Goodrich E, Lindsley J, Hampstead B, Kaye J. Behavioral RCT using frequent social interaction to enhance cognitive reserve. In: Clinical Trials. Vol. 15. 2018:130-1.
- Dodge HH, Hampstead BM, Potempa K, Struble L, Lindsley J, Rooney WD, et al. Behavioral randomized controlled trial (RCT) using internet-based social interactions as a tool to enhance cognitive reserve. In: Alzheimer's & Dementia. Vol. 14. 2018:586.
ISRCTN13355076, 2018 {published data only}
- ISRCTN13355076. An educational intervention to promote health-related quality of life after an acute coronary syndrome. http://www.who.int/trialsearch/trial2.aspx?Trialid=isrctn13355076 (first received 8 August 2018).
NCT03511027 {published data only}
- NCT03511027. Medication dispenser to improve care at home for the elderly. https://clinicaltrials.gov/show/nct03511027 (first received April 27, 2018).
NCT03722017 {published data only}
- NCT03722017. Drug reduction in older patients: the DROP Trial. https://clinicaltrials.gov/show/nct03722017 (first received October 26, 2018).
Vasilevskis 2019 {published data only}
- NCT02979353. A randomized controlled trial to deprescribe for older patients with polypharmacy (Shed-Meds). https://clinicaltrials.gov/ct2/show/NCT02979353 (first received December 1, 2016).
- Vasilevskis EE, Shah AS, Hollingsworth EK, Shotwell MS, Mixon AS, Bell SP, et al. A patient-centered deprescribing intervention for hospitalized older patients with polypharmacy: rationale and design of the Shed-MEDS randomized controlled trial. BioMed Central Health Services Research 2019;19:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verweij 2018 {published data only}
- NTR6316. Cardiac care bridge trial. https://www.trialregister.nl/trial/6169 (first received 4 June 2006).
- Verweij L, Jepma P, Buurman BM, Latour CHM, Engelbert RHH, Ter Riet G, et al. The cardiac care bridge program: design of a randomized trial of nurse-coordinated transitional care in older hospitalized cardiac patients at high risk of readmission and mortality. BioMed Central Health Services Research 2018;18:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACSQHC 2012
- Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards. Available at www.safetyandquality.gov.au 2012.
An‐Wen 2013
- Chan A, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balkrishnan 1998
- Balkrishnan R. Predictors of medication adherence in the elderly. Clinical Therapy 1998;20(4):764-71. [DOI] [PubMed] [Google Scholar]
Balkrishnan 2003
- Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray FT, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clinical Therapy 2003;25(11):2958-71. [DOI] [PubMed] [Google Scholar]
Barbas 2001
- Barbas NR, Wilde EA. Competency issues in dementia: medical decision making, driving, and independent living. Journal of Geriatric Psychiatry and Neurology 2001;14(4):199-212. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beckman 2005
- Beckman A, Bernsten C, Parker MG, Thorslund M, Fastbom J. The difficulty of opening medicine containers in old age: a population-based study. Pharmacy World & Science 2005;27(5):393-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burgess 2005
- Burgess CL, Holman CD, Satti AG. Adverse drug reactions in older Australians, 1981-2002. Medical Journal of Australia 2005;182(6):267-70. [DOI] [PubMed] [Google Scholar]
CCCRG 2014
- Cochrane Consumers and Communication. Standard protocol text and additional guidance for review authors. cccrg.cochrane.org 2014.
Chan 2011
- Chan RJ, Webster J, Marquart L. Information interventions for orienting patients and their carers to cancer care facilities. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008273.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Col 1990
- Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Archives of Internal Medicine 1990;150(4):841-5. [PubMed] [Google Scholar]
Corsonello 2009
- Corsonello A, Pedone C, Lattanzio F, Lucchetti M, Garasto S, Carbone C, et al. Regimen complexity and medication nonadherence in elderly patients. Therapeutics and Clinical Risk Management 2009;5(1):209-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cutler 2018
- Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 2018;8(1):e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Department of Health (UK) 2001
- Department of Health. National service framework for older people. Available at www.gov.uk/government/ 2001.
DiMatteo 2000
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine 2000;160(14):2101-7. [DOI] [PubMed] [Google Scholar]
DiMatteo 2002
- DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Medical Care 2002;40:794-811. [DOI] [PubMed] [Google Scholar]
DiMatteo 2004
- DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Medical Care 2004;42:200-9. [DOI] [PubMed] [Google Scholar]
Edelberg 1999
- Edelberg HK, Shallenberger E, Wei JY. Medication management capacity in highly functioning community-living older adults: detection of early deficits. Journal of the American Geriatrics Society 1999;47(5):592-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Elliott 2006
- Elliott RA. Problems with medication use in the elderly: an Australian perspective. Journal of Pharmacy Practice and Research 2006;36(1):58-66. [Google Scholar]
Elliott 2009
- Elliott RA, Marriott JL. Standardised assessment of patients' capacity to manage medications: a systematic review of published instruments. BMC Geriatrics 2009;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2012
- Elliott RA, Martinac G, Campbell S, Thorn J, Woodward MC. Pharmacist-led medication review to identify medication-related problems in older people referred to an aged care assessment team: a randomized comparative study. Drugs & Aging 2012;29(7):593-605. [DOI] [PubMed] [Google Scholar]
Elliott 2014
- Elliott RA, Booth JC. Problems with medicine use in older Australians: a review of recent literature. Journal of Pharmacy Practice and Research 2014;44(4):258-71. [Google Scholar]
Elliott 2015
- Elliott RA, Goeman D, Beanland C, Koch S. Ability of older people with dementia or cognitive impairment to manage medicine regimens: a narrative review. Current Clinical Pharmacology 2015;10(3):213-21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 2007
- Field TS, Mazor KM, Briesacher B, Debellis KR, Gurwitz JH. Adverse drug events resulting from patient errors in older adults. Journal of the American Geriatric Society 2007;55(2):271-6. [DOI] [PubMed] [Google Scholar]
Gao 2017
- Gao L, Maidment I, Matthews F, Robinson L, Brayne C, Medical Research Council Cognitive Function and Ageing Study. Medication usage change in older people (65+) in England over 20 years: findings from CFAS I and CFAS II. Age Ageing 2017;47(2):220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2006
- George J, Munro K, McCaig DJ, Stewart DC. Prescription medications: beliefs, experiences, behavior, and adherence of sheltered housing residents. Annals of Pharmacotherapy 2006;40(12):2123-9. [DOI] [PubMed] [Google Scholar]
George 2008
- George J, Elliott RA, Stewart DC. A systematic review of interventions to improve medication taking in elderly patients prescribed multiple medications. Drugs & Aging 2008;25(4):307-24. [DOI] [PubMed] [Google Scholar]
Gilbert 1993
- Gilbert A, Luszcz M, Owen N. Medication use and its correlates among the elderly. Australian Journal of Public Health 1993;17(1):18-22. [DOI] [PubMed] [Google Scholar]
Gray 2001
- Gray SL, Mahoney JE, Blough DK. Medication adherence in elderly patients receiving home health services following hospital discharge. Annals of Pharmacotherapy 2001;35(5):539-45. [DOI] [PubMed] [Google Scholar]
Gurwitz 2003
- Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289(9):1107-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hemminki 1975
- Hemminki E, Heikkila J. Elderly people's compliance with prescriptions, and quality of medication. Scandinavian Journal of Social Medicine 1975;3(2):87-92. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 [updated July 2019]. Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hilmer 2007
- Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Archives of Internal Medicine 2007;167(8):781-7. [DOI] [PubMed] [Google Scholar]
Howard 2003
- Howard RL, Avery AJ, Howard PD, Partridge M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Quality and Safety in Health Care 2003;12(4):280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchison 2006
- Hutchison LC, Jones SK, West DS, Wei JY. Assessment of medication management by community-living elderly persons with two standardized assessment tools: a cross-sectional study. American Journal of Geriatric Pharmacotherapy 2006;4(2):144-53. [DOI] [PubMed] [Google Scholar]
IMS 2013
- International Medical Services Institute for Healthcare Informatics. Avoidable costs in U.S. healthcare. Available at www.imshealth.com 2013.
Jansa 2010
- Jansa M, Hernandez C, Vidal M, Nunez M, Bertran MJ, Sanz S, et al. Multidimensional analysis of treatment adherence in patients with multiple chronic conditions. A cross-sectional study in a tertiary hospital. Patient Education and Counseling 2010;81(2):161-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jerant 2011
- Jerant A, Chapman B, Duberstein P, Robbins J, Franks P. Personality and medication non-adherence among older adults enrolled in a six-year trial. British Journal of Health Psychology 2011;16(Pt 1):151-69. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 2008
- Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient's perspective. Therapeutics and Clinical Risk Management 2008;4(1):269-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jokanovic 2016
- Jokanovic N, Tan EC, den Bosch D, Kirkpatrick CM, Dooley MJ, Bell JS. Clinical medication review in Australia: a systematic review. Research in Social & Administrative Pharmacy 2016;12(3):384-418. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lam 2011
- Lam P, Elliott RA, George J. Impact of a self-administration of medications programme on elderly inpatients' competence to manage medications: a pilot study. Journal of Clinical Pharmacy and Therapeutics 2011;36(1):80-6. [DOI] [PubMed] [Google Scholar]
Lamarche 2018
- Lamarche L, Tejpal A, Mangin D. Self-efficacy for medication management: a systematic review of instruments. Patient Preference and Adherence 2018;12:1279-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 1996
- Lau HS, Beuning KS, Postma-Lim E, Klein-Beernink L, Boer A, Porsius AJ. Non-compliance in elderly people: evaluation of risk factors by longitudinal data analysis. Pharmacy World & Science 1996;18(2):63-8. [DOI] [PubMed] [Google Scholar]
Lee 2010
- Lee CY, George J, Elliott RA, Stewart K. Prevalence of medication-related risk factors among retirement village residents: a cross-sectional survey. Age and Ageing 2010;39(5):581-7. [DOI] [PubMed] [Google Scholar]
Leendertse 2008
- Leendertse AJ, Egberts AC, Stoker LJ, den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Archives of Internal Medicine 2008;168(17):1890-6. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddigan 2003
- Maddigan SL, Farris KB, Keating N, Wiens CA, Johnson JA. Predictors of older adults' capacity for medication management in a self-medication program: a retrospective chart review. Journal of Aging and Health 2003;15(2):332-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mahtani 2011
- Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD005025.pub3] [DOI] [PubMed] [Google Scholar]
Mansur 2008
- Mansur N, Weiss A, Hoffman A, Gruenewald T, Beloosesky Y. Continuity and adherence to long-term drug treatment by geriatric patients after hospital discharge: a prospective cohort study. Drugs and Aging 2008;25(10):861-70. [DOI] [PubMed] [Google Scholar]
McCann 2012
- McCann RM, Jackson AJ, Stevenson M, Dempster M, McElnay JC, Cupples ME. Help needed in medication self-management for people with visual impairment: case-control study. British Journal of General Practice 2012;62(601):e530-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McElnay 1997
- McElnay JC, McCallion CR, al-Deagi F, Scott M. Self-reported medication non-compliance in the elderly. European Journal of Clinical Pharmacology 1997;53(3-4):171-8. [DOI] [PubMed] [Google Scholar]
Morisky 1986
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care 1986;24(1):67-74. [DOI] [PubMed] [Google Scholar]
Nieuwlaat 2014
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD000011.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okuno 1999
- Okuno J, Yanagi H, Tomura S, Oka M, Hara S, Hirano C, et al. Compliance and medication knowledge among elderly Japanese home-care recipients. European Journal of Clinical Pharmacology 1999;55(2):145-9. [DOI] [PubMed] [Google Scholar]
Patterson 2002
- Patterson TL, Lacro J, McKibbin CL, Moscona S, Hughs T, Jeste DV. Medication management ability assessment: results from a performance-based measure in older outpatients with schizophrenia. Journal of Clinical Psychopharmacology 2002;22(1):11-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patterson 2014
- Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008165.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Patton 2017
- Patton DE, Hughes CM, Cadogan CA, Ryan CA. Theory-based interventions to improve medication adherence in older adults prescribed polypharmacy: a systematic review. Drugs Aging 2017;34(2):97-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philbert 2014
- Philbert D, Notenboom K, Bouvy ML, Geffen EC. Problems experienced by older people when opening medicine packaging. International Journal of Pharmacy Practice 2014;22(3):200-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.Software.
Risser 2007
- Risser J, Jacobson TA, Kripalani S. Development and psychometric evaluation of the Self-efficacy for Appropriate Medication Use Scale (SEAMS) in low-literacy patients with chronic disease. Journal of Nursing Measurement 2007;15(3):203-19. [DOI] [PubMed] [Google Scholar]
Ryan 2014
- Ryan R, Santesso N, Lowe D, Hill S, Grimshaw JM, Prictor M, et al. Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007768.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sackett 2000
- Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. Philadelphia: Churchill Livingstone, 2000. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sears 2012
- Sears K, Scobie A, Mackinnon NJ. Patient-related risk factors for self-reported medication errors in hospital and community settings in 8 countries. Canadian Pharmacists Journal 2012;145(2):88-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sewitch 2008
- Sewitch MJ, Cole M, McCusker J, Ciampi A, Dyachenko A. Medication use and nonadherence to psychoactive medication for mental health problems by community-living Canadian seniors with depression. Canadian Journal of Psychiatry 2008;53(9):609-20. [DOI] [PubMed] [Google Scholar]
Smith 2016
- Smith S, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD006560.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorensen 2004
- Sorensen L, Stokes JA, Purdie DM, Woodward M, Elliott RA, Roberts MS. Medication reviews in the community: results of a randomized, controlled effectiveness trial. British Journal of Clinical Pharmacology 2004;58(6):648-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spagnoli 1989
- Spagnoli A, Ostino G, Borga AD, D'Ambrosio R, Maggiorotti P, Todisco E, et al. Drug compliance and unreported drugs in the elderly. Journal of the American Geriatric Society 1989;37(7):619-24. [DOI] [PubMed] [Google Scholar]
Spiers 1995
- Spiers MV, Kutzik DM. Self-reported memory of medication use by the elderly. American Journal of Health-System Pharmacy 1995;52(9):985-90. [DOI] [PubMed] [Google Scholar]
Stoehr 2008
- Stoehr GP, Lu SY, Lavery L, Bilt JV, Saxton JA, Chang CC, et al. Factors associated with adherence to medication regimens in older primary care patients: the Steel Valley Seniors survey. American Journal of Geriatric Pharmacotherapy 2008;6(5):255-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tafreshi 1999
- Tafreshi MJ, Melby MJ, Kaback KR, Nord TC. Medication-related visits to the emergency department: a prospective study. Annals of Pharmacotherapy 1999;33(12):1252-7. [DOI] [PubMed] [Google Scholar]
Tavares 2013
- Tavares NU, Bertoldi AD, Thume E, Facchini LA, Franca GV, Mengue SS. Factors associated with low adherence to medication in older adults [Fatores associados a baixa adesao ao tratamento medicamentoso em idosos]. Revista de Saude Publica 2013;47(6):1092-101. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorpe 2009
- Thorpe CT, Bryson CL, Maciejewski ML, Bosworth HB. Medication acquisition and self-reported adherence in veterans with hypertension. Medical Care 2009;47(4):474-81. [DOI] [PubMed] [Google Scholar]
Vik 2004
- Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Annals of Pharmacotherapy 2004;38(2):303-12. [DOI] [PubMed] [Google Scholar]
Vik 2006
- Vik SA, Hogan DB, Patten SB, Johnson JA, Romonko-Slack L, Maxwell CJ. Medication nonadherence and subsequent risk of hospitalisation and mortality among older adults. Drugs and Aging 2006;23(4):345-56. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization Panel on Ageing and Social Development. Social development and ageing: crisis or opportunity? Available at www.who.int/ageing 2000.
WHO 2003
- World Health Organization. Adherence to long-term therapies: evidence for action. Available at www.who.int/chp/knowledge/publications/adherence_report/en 2003.
Williams 2008
- Williams A, Manias E, Walker R. Interventions to improve medication adherence in people with multiple chronic conditions: a systematic review. Journal of Advanced Nursing 2008;63(2):132-43. [DOI] [PubMed] [Google Scholar]
Wroe 2002
- Wroe AL. Intentional and unintentional nonadherence: a study of decision making. Journal of Behavioral Medicine 2002;25(4):355-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zelko 2016
- Zelko E, Klemenc-Ketis Z, Tusek-Bunc K. Medication adherence in elderly with polypharmacy living at home: a systematic review of existing studies. Materia Socio Medica 2016;28(2):129-32. [DOI] [PMC free article] [PubMed] [Google Scholar]


