Abstract
Background
Physical inactivity, sedentary lifestyles, and low functional outcome are thought to impact the level of physical fitness in patients with aneurysmal subarachnoid hemorrhage (a-SAH). However, changes in fitness over time and associated factors have not been studied in a-SAH.
Objective
The objective was to evaluate the level of physical fitness in the first year after a-SAH and explore longitudinal relations with physical activity, sedentary behavior, and functional outcome. Additionally, we evaluated whether physical fitness could be predicted by disease-related characteristics (ie, severity of a-SAH, location of the aneurysm, treatment procedure, pituitary dysfunction, and complications).
Design
This was a prospective 1-year follow-up study.
Methods
Fifty-two participants performed exercise testing at 6 and 12 months after a-SAH. Cardiopulmonary exercise testing and isokinetic dynamometry were applied to determine the peak oxygen uptake 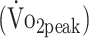 and the peak torque of the knee extensors (PText) and flexors (PTflex). In addition, physical activity and sedentary behavior were evaluated by accelerometer-based activity monitoring. The functional outcome was assessed by the Functional Independence Measure and Functional Assessment Measure. Disease-related characteristics were collected at hospital intake.
and the peak torque of the knee extensors (PText) and flexors (PTflex). In addition, physical activity and sedentary behavior were evaluated by accelerometer-based activity monitoring. The functional outcome was assessed by the Functional Independence Measure and Functional Assessment Measure. Disease-related characteristics were collected at hospital intake.
Results
At both 6 and 12 months, all fitness parameters were lower compared with predicted values (ranging from 18% to 28%). Physical activity is related to both 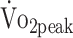 and PTflex. The Functional Independence Measure and Functional Assessment Measure scores was related to PText and PTflex. Further, participants who underwent surgical clipping had lower
and PTflex. The Functional Independence Measure and Functional Assessment Measure scores was related to PText and PTflex. Further, participants who underwent surgical clipping had lower 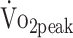 and PTflex.
and PTflex.
Limitations
Longitudinal observations cannot confirm causality.
Conclusions
Levels of physical fitness remain low over the first year after a-SAH. Participants who were physically more active had higher levels of physical fitness, whereas participants with impaired functional outcome or who were treated with surgical clipping were at risk of low physical fitness. Exercise interventions are warranted and should focus on the promotion of physical activity and target patients with impaired functional outcome or those who have been treated with surgical clipping.
Aneurysmal subarachnoid hemorrhage (a-SAH) is a life-threatening condition and accounts for 3% to 5% of all stroke cases.1 Depending on its severity, a-SAH is associated with a mortality rate of 8.3% to 66.7% within the first month.2 The incidence ranges from 4 to 10 per 100,000 persons per year.3 Most patients regain independence in daily functioning.4 However, more than two-thirds have an impaired functional outcome such that they experience restrictions in daily life and cannot regain premorbid levels of functioning.5,6 Because the mean age at which a-SAH occurs is a relatively young 55 years,3 these restrictions can have a devastating and long-lasting impact on daily living.
Because most patients with a-SAH experience restrictions in daily living, patients can be predisposed to inactive and sedentary lifestyles. This can lead to a negative circle of physical deconditioning (a conceptual framework is shown in eFig. 1, available at https://academic.oup.com/ptj). As a consequence, patients can be at risk of low physical fitness.6 Physical fitness refers to a set of physiological attributes that a person has or achieves and confers the ability to carry out daily activities without undue fatigue.7 Cardiorespiratory fitness and knee muscle strength are important aspects of physical fitness and indicative of independent daily living.8,9 Previous cross-sectional studies showed impaired cardiorespiratory fitness (62% to 77% of controls) and knee muscle strength (64% to 78% of controls) at 6 months after a-SAH.10 However, longitudinal studies are warranted to evaluate changes in fitness and related factors over time, which would provide important clinical information and could help to target therapeutic interventions.
Studies in patients with stroke, other than a-SAH, showed long-lasting impairments in cardiorespiratory fitness and knee muscle strength over time, ranging from 26% to 87%, and from 25% to 83% of controls, respectively.11,12 These deficits were found in the acute, subacute, and chronic phase after stroke (observed up to 8 years after onset).13 Patients with stroke who are less physically active, more severely disabled, or functionally more compromised are at risk of low physical fitness.14–16 Because the origin of brain damage differs between patients with ischemic or hemorrhagic stroke and patients with a-SAH (focal vs diffuse brain damage), it is not clear whether such factors play a similar role in fitness after a-SAH. In a-SAH, the severity of a-SAH (determined by Glasgow Coma Scale [GCS] score), treatment procedure (surgical clipping vs endovascular coiling), location of aneurysm (anterior vs posterior), and pituitary dysfunction are known predictors of long-term outcome.3,4,17 Therefore, we investigated whether these disease-related characteristics play a role in physical fitness as well.
The primary goal was to evaluate the level of physical fitness over the first year after a-SAH and to explore longitudinal relations with physical activity, sedentary behavior, and functional outcome. Our secondary aim was to evaluate whether physical fitness could be predicted by the above-mentioned disease-related characteristics to identify patients at risk of low fitness. Repeated measurements of physical fitness, physical activity, sedentary behavior, and functional outcome were performed at 6 and 12 months after a-SAH. We hypothesized that physical fitness remains low over the first year and that inactive and sedentary lifestyles are related to low physical fitness. Further, we hypothesized that patients with lower functional outcome, more severe a-SAH, and those who had been treated with surgical clipping are at risk of low physical fitness.
Methods
Participants and Design
This study, entitled HIPS-Rehab, was part of the Hypopituitarism In Patients after Subarachnoid hemorrhage (HIPS) study.18 Data collection, clinical definitions of a-SAH, and inclusion criteria have been published previously.10,18 Personal and disease-related characteristics were collected at hospital intake, and measures of physical fitness, physical activity, sedentary behavior, and functional outcome were assessed at 6 and 12 months after onset. This study was approved by the Medical Ethics Committee of the Erasmus MC University. All participants provided written informed consent.
Primary Outcome
Physical fitness was assessed by analyzing cardiorespiratory fitness and isokinetic knee muscle strength. Safety procedures were implemented prior to exercise testing. First, participants filled in the Physical Activity Readiness Questionnaire, which is a self-directed assessment to screen for health complications during exercise.19 Thereafter, participants were screened for medical contraindications to exercise testing by a treating neurologist. Exercise testing was not carried out if there was any suspicion of an underlying cardiopulmonary pathology that increased the risk of complications during exercise testing.
Cardiorespiratory fitness was assessed by cardiopulmonary exercise testing (CPET) on an upright cycle ergometer (Jaeger ER800; Jaeger Toennies, Breda, the Netherlands). CPET was preceded by a 4-minute warm-up without resistance after which the resistance increased automatically every 10 seconds to ensure that voluntary exhaustion was reached within 10 to 14 minutes (increment for women: 12 W/min; for men: 16 W/min). The test stopped when the participants were not able to maintain target pedal rate (60–70 rpm). CPET could also be terminated because of medical complications, as prescribed by the guidelines of the American College of Sports Medicine.20 During CPET, gas exchanges were analyzed by indirect calorimetry (Oxycon Pro; ViaSys Healthcare, Houten, the Netherlands). Peak oxygen uptake 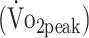 was measured at peak physical work rate.
was measured at peak physical work rate. 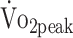 was defined as the highest mean peak value during 30 seconds of exercise and expressed in
was defined as the highest mean peak value during 30 seconds of exercise and expressed in 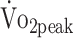 per kilogram body mass (mL/kg/min). To determine whether participants reached maximal physical exertion we used the following objective criteria: (1) respiratory exchange ratio (RER) > 1.0, or (2) peak heart rate (HRpeak) within 10 bpm of age-predicted maximum heart rate (HRmax) using the formula: HRmax = 208 − (0.7 × age). Because beta-blocker medication can reduce HRmax by approximately 30%, we adjusted the formula for participants receiving beta-blocker medication: HRmax = 0.7[208 − (0.7 × age)].10,21,22
per kilogram body mass (mL/kg/min). To determine whether participants reached maximal physical exertion we used the following objective criteria: (1) respiratory exchange ratio (RER) > 1.0, or (2) peak heart rate (HRpeak) within 10 bpm of age-predicted maximum heart rate (HRmax) using the formula: HRmax = 208 − (0.7 × age). Because beta-blocker medication can reduce HRmax by approximately 30%, we adjusted the formula for participants receiving beta-blocker medication: HRmax = 0.7[208 − (0.7 × age)].10,21,22
Isokinetic knee muscle strength was assessed by dynamometry using the Biodex Dynamometer (Biodex, Shirley, NY, USA). Adjustable seatbelts were used to minimize body movements. The lateral femoral epicondyle was aligned with the rotational axis of the dynamometer. Peak torque of the knee extensors (PText) and flexors (PTflex) was recorded (newton-meters) and corrected for body mass (N m/kg). The test protocol involved 5 maximal knee extension and flexion contractions at 60°/s. Peak torque was considered as the maximum torque generated throughout 1 series of repetitions. We calculated the average peak torque of both limbs because there were no significant differences in peak torque between the left and right lower limbs.
Secondary Outcome
Objective measures of physical activity and sedentary behavior were evaluated by accelerometer-based activity monitoring (VitaMove; 2M Engineering, Veldhoven, the Netherlands).23 The VitaMove consists of 3 individual body-fixed recorders (attached to sternum and both legs) (eFig. 2, available at https://academic.oup.com/ptj). The recorders are wirelessly connected and synchronized every 10 seconds. Each recorder has its own accelerometer (MMA7260Q; Freescale, Denver, CO, USA). The VitaMove demonstrates validity for quantifying body postures and movements in healthy subjects, and in different patient populations.23,24 Activity monitoring measurement started one day after the measurement visit, and participants wore the VitaMove on consecutive weekdays, except during swimming, bathing, and sleeping. The intended duration of measurement was 3 consecutive days, with a minimum of 1 day.25 Participants were instructed to continue their ordinary daily activities. The principles of measurement were explained after all measurements were completed in order to avoid measurement bias. Participants kept activity diaries to report reasons for nonwear periods. Accelerometer data were uploaded to a computer for kinematic analyses using VitaScore (VitaScore BV, Gemert, the Netherlands).23 The following outcome measures were calculated as the mean of available measurement days: duration of physical activity (including walking, cycling, running, and noncyclic movements; expressed as a percentage of a 24-hour period) and duration of sedentary behavior (including lying and sitting activities; expressed as a percentage of waking hours).
Functional outcome was assessed by the treating neurologist using the widely used questionnaire Functional Independence Measure and Functional Assessment Measure (FIM + FAM).26 The FIM + FAM consists of 30 items and evaluates functional independence by examining self-care, transfers and mobility, communication, and cognitive and psychosocial daily functioning. FIM + FAM scores range from 1 (total dependence) to 7 (complete independence). The FIM + FAM shows excellent validity and reliability in patients with stroke.26
The following disease-related characteristics were collected at hospital intake: (1) severity of a-SAH by using the GCS score; (2) location of the aneurysm (anterior vs posterior); (3) treatment procedure (surgical clipping vs endovascular coiling); (4) a-SAH complications (rebleeding, delayed cerebral ischemia, hyponatremia, and hydrocephalus); and (5) pituitary dysfunction. Methods of endocrine testing and definitions of pituitary dysfunction have been extensively described elsewhere.18 Endovascular coiling is the preferred treatment modality to close a ruptured aneurysm. Sometimes clipping is the designated treatment modality because coiling cannot be performed (eg, the location of the aneurysm is unreachable via a catheter, the aneurysm is too small, the neck of the aneurysm is too wide, or the shape of the aneurysm is not suitable for coiling).
Data Analysis
All data are expressed as mean [SD] unless otherwise indicated. Differences in clinical characteristics (including sex, age, GCS score, treatment procedure, and location of the aneurysm) between participants in HIPS-Rehab (n = 52) and those who did not participate in the current study (but included in HIPS; n = 32) were verified by independent t tests for interval variables and by χ2 tests for categorical variables. Descriptive statistics were used to describe personal and disease-related characteristics. Parametric tests were used because the Shapiro-Wilk test showed that fitness data were normally distributed: 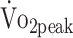 (W = 0.979; P = .325), PText (W = 0.984; P = .453), and PTflex (W = 0.972; P = .082).
(W = 0.979; P = .325), PText (W = 0.984; P = .453), and PTflex (W = 0.972; P = .082).
Linear mixed models were created to analyze changes in the estimated mean 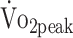 , PText, and PTflex over follow-up time. The visit (6 or 12 months) was entered in the model as a predictor of the dependent outcome. Linear mixed models were created to explore time-dependent relationships between physical fitness (separate models for
, PText, and PTflex over follow-up time. The visit (6 or 12 months) was entered in the model as a predictor of the dependent outcome. Linear mixed models were created to explore time-dependent relationships between physical fitness (separate models for  , PText, and PTflex) and 1 of the following factors: physical activity (% 24 hours), sedentary behavior (% waking hours), and functional outcome (FIM + FAM scores). The following fixed factors were entered to study whether disease-related characteristics can predict the level of physical fitness: severity of a-SAH (GCS score, range = 1–15), location of the aneurysm (0 = anterior, 1 = posterior), treatment procedure (0 = clipping, 1 = coiling), pituitary dysfunction (0 = no, 1 = yes), and complications (0 = no, 1 = yes).
, PText, and PTflex) and 1 of the following factors: physical activity (% 24 hours), sedentary behavior (% waking hours), and functional outcome (FIM + FAM scores). The following fixed factors were entered to study whether disease-related characteristics can predict the level of physical fitness: severity of a-SAH (GCS score, range = 1–15), location of the aneurysm (0 = anterior, 1 = posterior), treatment procedure (0 = clipping, 1 = coiling), pituitary dysfunction (0 = no, 1 = yes), and complications (0 = no, 1 = yes).
Prediction equations, established in the general population, were used to predict individual 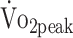 , and served as a comparison.27 The following equations were used27:
, and served as a comparison.27 The following equations were used27:
 |
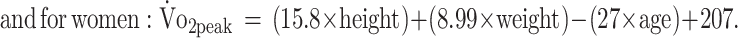 |
To better interpret individual levels of 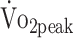 , peak values were additionally classified according to norm data derived from the Cooper Institute.28 These norm data were gathered in a norm population and classified (reference values for
, peak values were additionally classified according to norm data derived from the Cooper Institute.28 These norm data were gathered in a norm population and classified (reference values for  classification are presented in the eTable, available at https://academic.oup.com/ptj). Norms were specified by sex and age category, and
classification are presented in the eTable, available at https://academic.oup.com/ptj). Norms were specified by sex and age category, and 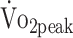 values below the 20th percentile of the norm population were considered “very poor.” For maximal isokinetic PText and PTflex we used the normative data gathered by Sunnerhagen et al.29
values below the 20th percentile of the norm population were considered “very poor.” For maximal isokinetic PText and PTflex we used the normative data gathered by Sunnerhagen et al.29
Linear mixed models are flexible in handling missing values, and these models take into account the covariance between measurements within patients. Each model was adjusted for sex (0 = women, 1 = men) and age. Statistically, sex and age were not always significant confounders, but were kept in each model because these factors are considered of fundamental importance in research on physical fitness,30 leading to the following linear mixed model equation:
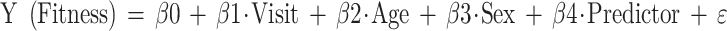 |
We reported estimated β coefficients, effect sizes (Cohen d), 95% confidence intervals, and P values. The significance level was set at P < .05. Bonferroni correction was applied to adjust for type I error for multiple testing (Statistical Package for the Social Sciences (SPSS), version 22, Inc, Chicago, IL, USA).
Role of the Funding Source
The funders had no role in the design, analysis, write-up, or decision to submit for publication.
Results
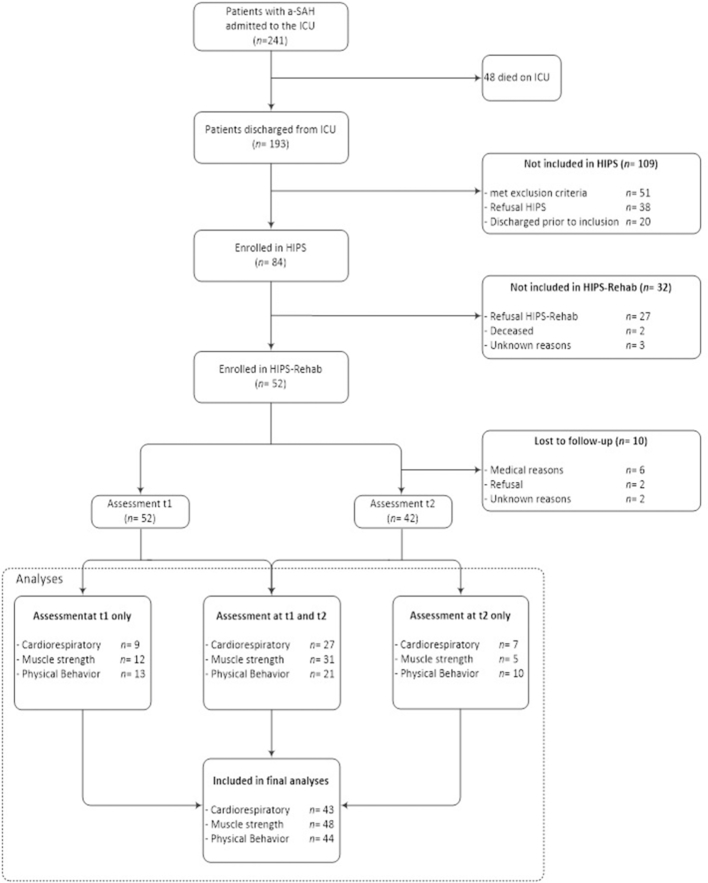
In total, 241 patients were admitted to the intensive care unit with a diagnosis of a-SAH18; of the 84 eligible patients 52 participated in the present study. Participants in HIPS-Rehab (n = 52) did not differ from those who did not participate in the current study (but included in HIPS; n = 32) with respect to: age (t(84) = −0.005; P = .996), sex (χ2(1) = 0.066; P = .291), GCS score (t(84) = 1.505; P = .136), location of the aneurysm (χ2(1) = 0.092; P = .469), and treatment procedure (χ2(1) = 0.086; P = .489). Fifty-two participants were assessed at 6 months, and 42 were assessed at 12 months after onset (Tab. 1). Patients with complete follow-up (n = 42) did not differ from those lost to follow-up (n = 10) with respect to: age (t(52) = 1.269; P = .210), sex (χ2(1) = 0.495; P = .264), GCS score (t(52) = −1.282; P = .230), location of the aneurysm (χ2(1) = 0.001; P = .634), treatment procedure (χ2(1) = 0.581; P = .353), or complications (χ2(1) = 0.288; P = .464). Because we used linear mixed model analyses, data of most patients could be included in the final analyses because this method allows inclusion of patients in the analyses for whom some of the data are missing (Fig. 1 presents a detailed flow diagram).
Table 1.
Characteristics of 52 Participants With Aneurysmal Subarachnoid Hemorrhagea
| Characteristic | Value for Participantsb |
|---|---|
| Mean [SD] age, y, | 56.1 [13.5] |
| Male sex | 16 (31) |
| Mean [SD] GCS score | 13.5 [2.1] |
| Location of aneurysm | |
| Anterior | 31 (60) |
| Posterior | 21 (40) |
| Treatment procedure | |
| Endovascular coiling | 47 (90) |
| Surgical clipping | 11 (21) |
| Secondary complications | |
| Rebleeding | 0 |
| Delayed cerebral ischemia | 7 (13) |
| Hyponatremia | 6 (12) |
| Hydrocephalus | 13 (25) |
| Pituitary dysfunction | 24 (46) |
GCS = Glasgow Coma Scale.
Data are presented as number (percentage) of participants unless otherwise indicated.
Figure 1.
Flow diagram. a-SAH = aneurysmal subarachnoid hemorrhage; HIPS = Hypopituitarism In Patients after Subarachnoid hemorrhage (study); ICU = intensive care unit.
Data were not available for all participants. CPET data for 43 patients were included in the final analyses (83% of the sample). In total, 6 patients were not able to perform CPET due to contraindications (n = 3), logistical reasons (n = 2), or because of an additional injury (n = 1). Furthermore, CPET data of 3 patients did not meet the objective criteria for maximal physical exertion and were excluded. In total, 9 patients with successful CPET were receiving beta-blocker medication. Isokinetic dynamometry data of 48 patients were included (92% of the sample); 3 were not able to perform isokinetic dynamometry because of medical reasons and 1 because of logistical reasons. Activity monitoring data of 44 patients were included (85% of the sample); measurements in 4 patients were lost due to technical failure, 3 because of logistical reasons, and 1 because of refusal.
Physical Fitness
The estimated mean  in patients increased between 6 and 12 months by + 6.2% (β = 1.417 mL/kg/min; P = .027), and there was a nonsignificant trend for an increase of + 5.1% in PT ext (β = 0.071 N·m/kg; P = .061) (Tab. 2). The estimated mean PTflex did not change over time (β = 0.026 N·m/kg; P = .281). Although patients managed to exercise toward acceptable cardiorespiratory limits at both follow-up times, the estimated mean peak respiratory exchange ratio at 6 months was lower compared with 12 months: 1.13 (SE = 0.01) versus 1.19 (SE = 0.02), respectively ( P < .001). The estimated mean
in patients increased between 6 and 12 months by + 6.2% (β = 1.417 mL/kg/min; P = .027), and there was a nonsignificant trend for an increase of + 5.1% in PT ext (β = 0.071 N·m/kg; P = .061) (Tab. 2). The estimated mean PTflex did not change over time (β = 0.026 N·m/kg; P = .281). Although patients managed to exercise toward acceptable cardiorespiratory limits at both follow-up times, the estimated mean peak respiratory exchange ratio at 6 months was lower compared with 12 months: 1.13 (SE = 0.01) versus 1.19 (SE = 0.02), respectively ( P < .001). The estimated mean 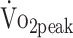 was 28% higher in men than in women (β = 6.606; P = .001), and
was 28% higher in men than in women (β = 6.606; P = .001), and 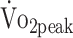 decreased by 8% per 10 years additional age (β = −1.601; P = .021). Further,
decreased by 8% per 10 years additional age (β = −1.601; P = .021). Further, 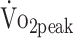 in patients who were receiving beta-blocker medication did not differ from those who were not receiving beta-blockers (β = −2.333; P = .214).
in patients who were receiving beta-blocker medication did not differ from those who were not receiving beta-blockers (β = −2.333; P = .214).
Table 2.
Estimated Values for 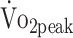 , Knee Extensor and Flexor Strength, and Daily Physical Activity and Sedentary Behavior and Significance Level of Change Over Timea
, Knee Extensor and Flexor Strength, and Daily Physical Activity and Sedentary Behavior and Significance Level of Change Over Timea
| Physical Fitness | Mean (SE) at: | Change From t1 to t2b | 95% CI for Change | Cohen dc | P | |
|---|---|---|---|---|---|---|
| 6 mo (t1) | 12 mo (t2) | |||||
| Cardiorespiratory fitness | ||||||
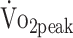 , mL/kg/min , mL/kg/min |
22.79 (0.94) | 24.20 (0.91) | +1.417 | 0.170 to 2.664 | 0.257 | .027d |
| HRpeak, % of predicted HRmax | 88.59 (2.37) | 90.42 (2.32) | +1.824 | −1.398 to 5.054 | 0.132 | .255 |
RERpeak, 
|
1.13 (0.01) | 1.19 (0.02) | +0.058 | 0.034 to 0.081 | 0.653 | <.001d |
| Knee muscle strength | ||||||
| PText, N·m/kg | 1.38 (0.06) | 1.45 (0.06) | +0.071 | −0.004 to 0.146 | 0.185 | .061e |
| PTflex, N·m/kg | 0.61 (0.04) | 0.64 (0.04) | +0.026 | −0.022 to 0.073 | 0.119 | .281 |
All determinants were entered separately with adjustment for sex and age. CI = confidence interval; HRmax = maximum predicted heart rate; HRpeak = peak heart rate; PText = peak torque of the knee extensors; PTflex = peak torque of the knee flexors; RERpeak = peak respiratory exchange ratio; 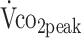 = peak carbon dioxide uptake;
= peak carbon dioxide uptake; 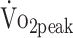 = peak oxygen uptake.
= peak oxygen uptake.
Estimated mean change over follow-up time.
Cohen d =  . μ2 = standardized mean t2; μ1 = standardized mean t1; Σσ = pooled standardized standard deviation.
. μ2 = standardized mean t2; μ1 = standardized mean t1; Σσ = pooled standardized standard deviation.
Significant difference (P < .05).
Nonsignificant trend for a difference (P < .10).
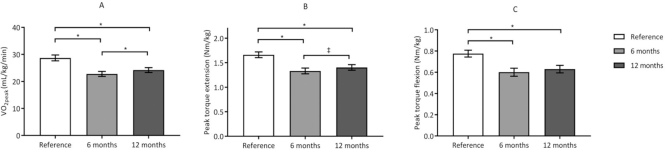
Figure 2 shows a graphical presentation of the differences in the estimated values of 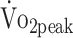 , PText, and PTflex of the participants and predicted values. The estimated mean
, PText, and PTflex of the participants and predicted values. The estimated mean 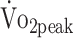 values at 6 and 12 months were significantly lower than the predicted values calculated from the formulas established by Fairbarn et al27: 26% lower at 6 months (β = −7.865 mL/kg/min;, P < .001) and 21% lower at 12 months (β = −6.537 mL/kg/min; P < .001).27 According to the classifications made by the Cooper Institute, at 6 and 12 months, the
values at 6 and 12 months were significantly lower than the predicted values calculated from the formulas established by Fairbarn et al27: 26% lower at 6 months (β = −7.865 mL/kg/min;, P < .001) and 21% lower at 12 months (β = −6.537 mL/kg/min; P < .001).27 According to the classifications made by the Cooper Institute, at 6 and 12 months, the  was considered very poor in, respectively, 43% and 39% of participants.28 Analyzing individual change (criterion: ± 2.0 mL/kg/min),31 showed that the
was considered very poor in, respectively, 43% and 39% of participants.28 Analyzing individual change (criterion: ± 2.0 mL/kg/min),31 showed that the 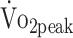 improved in 30%, remained stable in 48%, and deteriorated in 22% of the patients. At both 6 and 12 months, the knee muscle strength was significantly lower than predicted values.29 At 6 months, PText was 22% lower (β = −0.344 N·m/kg; P < .001) and PTflex was 28% lower than predicted values (β = −0.282 N·m/kg; P < .001); at 12 months, PText was 18% lower (β = −0.216 N·m/kg; P < .001) and PTflex was 22% lower than predicted values (β = −0.186 N·m/kg; P < .001).29
improved in 30%, remained stable in 48%, and deteriorated in 22% of the patients. At both 6 and 12 months, the knee muscle strength was significantly lower than predicted values.29 At 6 months, PText was 22% lower (β = −0.344 N·m/kg; P < .001) and PTflex was 28% lower than predicted values (β = −0.282 N·m/kg; P < .001); at 12 months, PText was 18% lower (β = −0.216 N·m/kg; P < .001) and PTflex was 22% lower than predicted values (β = −0.186 N·m/kg; P < .001).29
Figure 2.
Graphical presentation of the estimated mean (SE) for (A) peak oxygen uptake 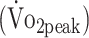 , (B) peak torque knee extension (PText), and (C) peak torque knee flexion (PTflex) at 6 and 12 months after aneurysmal subarachnoid hemorrhage compared with reference values. Reference values for
, (B) peak torque knee extension (PText), and (C) peak torque knee flexion (PTflex) at 6 and 12 months after aneurysmal subarachnoid hemorrhage compared with reference values. Reference values for  were calculated from Fairbarn et al,27 and reference values for PText and PTflex were calculated from Sunnerhagen et al.29 Asterisks denote significant difference (P < .05).
were calculated from Fairbarn et al,27 and reference values for PText and PTflex were calculated from Sunnerhagen et al.29 Asterisks denote significant difference (P < .05).
Determinants of Physical Fitness
Table 3 presents the results of the linear mixed models, evaluating the determinants of physical fitness. Total physical activity (% 24 hours) was positively associated with 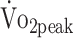 (β = 0.638 mL/kg/min; P = .006) and PTflex (β = 0.018 N·m/kg; P = .037), indicating that patients who were physically more active had greater cardiorespiratory fitness and knee flexion strength. The functional outcome (FIM + FAM score) was significantly related to PText (β = 0.125 N·m/kg; P = .004) and PTflex (β = 0.057 N·m/kg; P = .042), indicating that patients with lower functional outcome had lower knee extension and flexion strength. There was no evidence for a relationship between sedentary behavior and physical fitness.
(β = 0.638 mL/kg/min; P = .006) and PTflex (β = 0.018 N·m/kg; P = .037), indicating that patients who were physically more active had greater cardiorespiratory fitness and knee flexion strength. The functional outcome (FIM + FAM score) was significantly related to PText (β = 0.125 N·m/kg; P = .004) and PTflex (β = 0.057 N·m/kg; P = .042), indicating that patients with lower functional outcome had lower knee extension and flexion strength. There was no evidence for a relationship between sedentary behavior and physical fitness.
Table 3.
Determinants of Cardiorespiratory Fitness 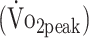 and knee muscle strength (PText and PTflex)a
and knee muscle strength (PText and PTflex)a
| Linear Mixed Model |
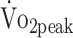 , mL/kg/min (n = 43) , mL/kg/min (n = 43) |
PText, N·m/kg (n = 48) | PTflex, N·m/kg (n = 48) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| βb | 95% CI for β | P | βb | 95% CI for β | P | βb | 95% CI for β | P | |
| Time | +1.417 | 0.170–2.664 | .027 | +0.071 | −0.004 to 0.146 | .061 | +0.026 | −0.022 to 0.073 | .281 |
| Sex | +6.606 | 3.062–10.150 | .001c | +0.340 | 0.120–0.561 | .003c | +0.214 | 0.075–0.353 | .003c |
| Age | −0.160 | −0.295 to −0.025 | .021 | −0.012 | −0.020 to −0.003 | .010 | −0.009 | −0.015 to −0.004 | .002c |
| Physical behavior and functioning | |||||||||
| Physical activity | +0.638 | 0.193–1.082 | .006 | +0.023 | −0.009 to 0.056 | .147 | +0.018 | 0.001–0.036 | .037 |
| Sedentary behavior | −0.030 | −0.201 to 0.142 | .726 | −0.004 | −0.013 to 0.006 | .459 | −0.002 | −0.004 to 0.008 | .556 |
| Functional outcomed | +0.469 | −1.031 to 1.969 | .531 | +0.125 | 0.041–0.209 | .004c | +0.057 | 0.002–0.112 | .042 |
| Baseline characteristics | |||||||||
| GCS score | +0.103 | −0.708 to 0.914 | .799 | +0.037 | −0.010 to 0.084 | .116 | +0.022 | −0.008 to 0.053 | .146 |
| Location of aneurysm | +1.191 | −2.059 to 4.441 | .463 | +0.105 | −0.101 to 0.310 | .311 | +0.048 | −0.082 to 0.179 | .459 |
| Treatment | −4.946 | −8.528 to −1.365 | .008 | −0.135 | −0.396 to −0.126 | .302 | −0.176 | −0.333 to −0.018 | .029 |
| Pituitary dysfunction | −1.312 | −4.531 to 1.908 | .415 | −0.009 | −0.216 to 0.197 | .928 | −0.066 | −0.195 to 0.063 | .306 |
| Complications | −0.524 | −3.809 to 2.762 | .749 | −0.110 | −0.324 to 0.104 | .305 | −0.071 | −0.205 to 0.064 | .295 |
Each of the following determinants was included in a separate linear mixed model with adjustment for sex (0 = female; 1 = male) and age (y): time (0 = 6 mo; 1 = 12 mo); physical activity (% of 24 h); sedentary behavior (% of waking h); Functional Independence Measure and Functional Assessment Measure (range = 1–7); Glasgow Coma Scale (GCS) score (range = 1–15); location of aneurysm (0 = anterior; 1 = posterior); treatment (0 = coiling; 1 = clipping); pituitary dysfunction (0 = no; 1 = yes); and secondary complications (0 = no; 1 = yes). CI= confidence interval; PText = peak torque of the knee extensors; PTflex = peak torque of the knee flexors; 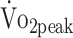 = peak oxygen uptake (mL/kg/min).
= peak oxygen uptake (mL/kg/min).
Estimated β coefficient.
Significant predictor of physical fitness after Bonferroni correction: P = .05/10 = .005.
Functional outcome was determined with the Functional Independence Measure and Functional Assessment Measure questionnaires.
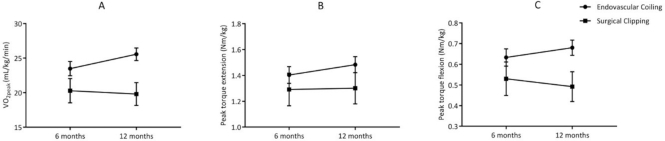
Further, patients who had been treated with surgical clipping had 22% lower 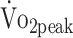 (β = −4.946 mL/kg/min; P = .008) and 29% lower PText (β = −0.176 N·m/kg; P = .029) compared with those who underwent endovascular coiling. Figure 3 depicts the change in physical fitness over the first year, specified by treatment procedure (surgical clipping vs endovascular coiling). Other baseline characteristics of interest such as severity of a-SAH (GCS score), location of the aneurysm, and pituitary dysfunction were not associated with physical fitness.
(β = −4.946 mL/kg/min; P = .008) and 29% lower PText (β = −0.176 N·m/kg; P = .029) compared with those who underwent endovascular coiling. Figure 3 depicts the change in physical fitness over the first year, specified by treatment procedure (surgical clipping vs endovascular coiling). Other baseline characteristics of interest such as severity of a-SAH (GCS score), location of the aneurysm, and pituitary dysfunction were not associated with physical fitness.
Figure 3.
Graphical presentation of the estimated mean (SE) for (A) peak oxygen uptake 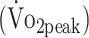 , (B) peak torque extension, and (C) peak torque flexion at 6 and 12 months for participants who underwent surgical clipping or endovascular coiling. Surgical clipping was negatively associated with
, (B) peak torque extension, and (C) peak torque flexion at 6 and 12 months for participants who underwent surgical clipping or endovascular coiling. Surgical clipping was negatively associated with 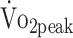 (P = .008) (A) and peak torque flexion (P = .029) (C).
(P = .008) (A) and peak torque flexion (P = .029) (C).
The estimated β coefficients are presented in Table 3 and reflect the change in fitness that is associated with a 1-unit change in the predictor. We highlight the finding that participants who were physically more active had higher 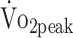 (β = 0.638 mL/kg/min); participants with + 1% higher PA had a higher
(β = 0.638 mL/kg/min); participants with + 1% higher PA had a higher  of + 0.638 mL/kg/min. This finding indicates that 3.1% greater physical activity levels (45 min/24 h) are associated with a + 2.0 mL/kg/min higher
of + 0.638 mL/kg/min. This finding indicates that 3.1% greater physical activity levels (45 min/24 h) are associated with a + 2.0 mL/kg/min higher 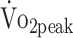 .
.
Discussion
This 1-year follow-up study showed that participants with a-SAH have low physical fitness over the first year.27,28 More than one-third of the participants had 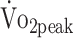 values that were considered very poor at both 6 and 12 months after onset. Participants who were physically more active had higher peak oxygen uptake and knee flexion strength, and participants with lower functional outcome had lower knee extension and flexion strength. Further, participants who had been treated with surgical clipping were at risk of low physical fitness. Our findings indicate that exercise interventions are warranted. Such interventions should involve both cardiorespiratory endurance and muscle strengthening components and should consider the promotion of physical activity. Further, these interventions should target patients with impaired functional outcome or those who have been treated with surgical clipping.
values that were considered very poor at both 6 and 12 months after onset. Participants who were physically more active had higher peak oxygen uptake and knee flexion strength, and participants with lower functional outcome had lower knee extension and flexion strength. Further, participants who had been treated with surgical clipping were at risk of low physical fitness. Our findings indicate that exercise interventions are warranted. Such interventions should involve both cardiorespiratory endurance and muscle strengthening components and should consider the promotion of physical activity. Further, these interventions should target patients with impaired functional outcome or those who have been treated with surgical clipping.
The procedure to close the aneurysm was found to be a predictor of physical fitness. The 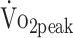 of participants who had been treated with surgical clipping was 22% lower compared with those who underwent endovascular coiling; the deficit was 29% for knee flexor strength. Our findings indicate that endovascular coiling gives better longterm follow-up compared with surgical clipping, which is in line with a meta-analysis showing that endovascular coiling yields optimal clinical outcomes compared with surgical clipping.32 Professionals should be aware that patients who have been treated with surgical clipping are at risk of a poor health outcome. This finding could help to target future interventions in patients with a-SAH.
of participants who had been treated with surgical clipping was 22% lower compared with those who underwent endovascular coiling; the deficit was 29% for knee flexor strength. Our findings indicate that endovascular coiling gives better longterm follow-up compared with surgical clipping, which is in line with a meta-analysis showing that endovascular coiling yields optimal clinical outcomes compared with surgical clipping.32 Professionals should be aware that patients who have been treated with surgical clipping are at risk of a poor health outcome. This finding could help to target future interventions in patients with a-SAH.
The observed deficits in physical fitness (18% to 28% lower than reference) were smaller compared with patients with other types of stroke; where fitness parameters were 13% to 75% lower than healthy controls.11,12 An explanation is that patients with ischemic or hemorrhagic stroke are often more disabled due to focal brain damage and neuromotor lesions and therefore less likely to maintain active lifestyles. Consistent with our findings, studies in stroke showed relationships between lower functional outcome and decreased knee muscle strength.33 Further, the reported deficits in our group are comparable with patients with transient ischemic attack and patients with minor ischemic stroke (21% to 35% lower than controls).14,34
Physical activity has been related to improved physical fitness across different patient groups (eg, coronary heart disease, diabetes mellitus, obesity, and stroke).9,15 In our sample, we also found that participants who were physically more active had higher 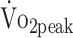 values. The observed relations between activity and fitness in a-SAH are consistent with research in patients with stroke, in whom higher levels of physical activity were positively associated with higher levels of fitness.35 The estimated β coefficient for physical activity showed that participants with higher physical activity (45 min/24 h) had a 2.0 mL/kg/min higher
values. The observed relations between activity and fitness in a-SAH are consistent with research in patients with stroke, in whom higher levels of physical activity were positively associated with higher levels of fitness.35 The estimated β coefficient for physical activity showed that participants with higher physical activity (45 min/24 h) had a 2.0 mL/kg/min higher 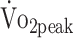 (reflects minimal clinical important difference in stroke).31 Intervention studies are warranted to investigate whether increased levels of physical activity lead to improved
(reflects minimal clinical important difference in stroke).31 Intervention studies are warranted to investigate whether increased levels of physical activity lead to improved 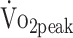 in patients with a-SAH. Because physical activity in our study was based on total physical activity time, regardless of intensity of activities, walking or household activities are already associated with higher
in patients with a-SAH. Because physical activity in our study was based on total physical activity time, regardless of intensity of activities, walking or household activities are already associated with higher 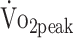 . However, research in sports and exercise shows that the intensity of physical activity plays a decisive role in fitness.7,15 Future studies are warranted to investigate optimal treatment paradigms to improve fitness in patients with a-SAH.
. However, research in sports and exercise shows that the intensity of physical activity plays a decisive role in fitness.7,15 Future studies are warranted to investigate optimal treatment paradigms to improve fitness in patients with a-SAH.
Sedentary behavior and physical activity are 2 different constructs, in that sedentary behavior is more than merely a lack of physical activity (too little exercise); it is rather a behavioral entity that can have distinct physiological effects independent of the amount of physical activity.36 Physically active individuals who satisfy recommendations for optimal physical activity can still be sedentary for their remaining waking hours. In our study, we did not observe any relationship between sedentary behavior and physical fitness. Although associations between physical inactivity and low physical fitness are well established,9 studies are warranted to better understand relations between sedentary behavior and physical fitness.
The longitudinal models were corrected for sex and age. According to the estimated β coefficients, female sex and older age were negatively associated with physical fitness. However, the findings that  in women was approximately 28% lower than in men, and that
in women was approximately 28% lower than in men, and that 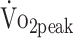 decreased by 8% per 10 years of age, are similar to findings in the general population.37,38
decreased by 8% per 10 years of age, are similar to findings in the general population.37,38
Studies in patients with non–a-SAH stroke showed that exercise training can improve cardiorespiratory fitness by 9% to 23%.39 Furthermore, exercise training can reduce depressive symptoms, prevent complications associated with physical inactivity, and decrease the likelihood of recurrent stroke.15,40 There could also be important health benefits from exercise training for patients with a-SAH. However, exercise interventions are lacking in a-SAH. Intervention studies are warranted to investigate the beneficial effects of exercise training in patients with a-SAH.
Previous studies have shown that cognitive impairments and psychological factors, such as mood and anxiety, lower the functional outcome after a-SAH.4 In this study we provide evidence for an association between physical fitness and functional outcome. Our results revealed that FIM + FAM scores were associated with knee extension and flexion strength, indicating that patients with less muscle strength had lower functional outcomes. From aging studies we know that improved knee muscle strength contributes to functional outcome and independent daily living.41 It could be argued that improved knee muscle strength can also improve functional outcome in a-SAH. However, intervention studies are warranted to investigate whether strengthening exercise improves functional outcome in a-SAH.
Interventions in stroke rehabilitation mainly focus on the performance of daily activities.15 As a result, there is a lack of interventions targeting fitness after stroke.40 Our findings indicate that exercise interventions in a-SAH should involve both cardiorespiratory endurance and strengthening exercise components. Interval training could be advantageous in a-SAH, to challenge the cardiorespiratory system without exhausting the muscular system.15 Further, because we found a positive relationship between physical activity and physical fitness, future exercise programs could also consider the promotion of daily physical activity in patients with a-SAH.
Limitations
Some critical reflections are warranted. First, not all measurements were available for all participants, which might have led to selection bias. However, the data of most participants could be included in the final analyses by estimating the mean outcome using linear mixed model analyses. This statistical method accounts for the covariance between measurements within participants. In total, 3 participants had absolute contraindications to exercise testing and did not perform fitness measurements. Because these participants were more likely to abstain from exercise, we could have underestimated fitness deficits in patients with a-SAH. Second, we could not confirm causality between parameters. However, the longitudinal relations provide important clinical information about the coexistence of problems and can help to direct therapeutic options in a-SAH. Third, interaction terms could not be studied because of insufficient statistical power (sample size n = 52). However, because fitness parameters changed only slightly or not at all over follow-up time, we do not expect to find interaction effects in our data. Ideally, a CPET practice trial should have been implemented because practice effects of CPET can lead to improvements in CPET performance.42 Therefore, the observed improvement in 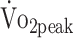 could be an overestimation of the actual improvement. However, implementing an additional practice trial was not feasible within our study. Activity monitoring measurements covered 3 consecutive weekdays and started the day after the visit. It remains questionable whether short measurement periods are representative of routine physical activity. However, 3-day measurement schedules have been frequently used to objectively determine physical activity in daily life.24 Finally, selection bias could have occurred toward patients who are interested in sports and exercise, which might have led to an underestimation of physical fitness deficits. However, participants did not differ from those who did not participate in HIPS-Rehab but were included in HIPS.
could be an overestimation of the actual improvement. However, implementing an additional practice trial was not feasible within our study. Activity monitoring measurements covered 3 consecutive weekdays and started the day after the visit. It remains questionable whether short measurement periods are representative of routine physical activity. However, 3-day measurement schedules have been frequently used to objectively determine physical activity in daily life.24 Finally, selection bias could have occurred toward patients who are interested in sports and exercise, which might have led to an underestimation of physical fitness deficits. However, participants did not differ from those who did not participate in HIPS-Rehab but were included in HIPS.
Conclusion
In summary, physical fitness remained low over the first year after a-SAH. More than one-third of the participants had very poor levels of 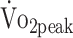 at 6 and 12 months after onset.28 Our findings revealed that participants who were physically more active had higher
at 6 and 12 months after onset.28 Our findings revealed that participants who were physically more active had higher 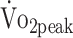 and knee flexion strength, whereas participants with lower functional outcome had lower knee extension and flexion strength. Further, participants who had been treated with surgical clipping were at risk of low physical fitness. Exercise interventions are warranted; they should consider the promotion of daily physical activity and should target patients with lower functional outcome or those who have been treated with surgical clipping. Research is warranted to investigate whether rehabilitation services can adapt their programs to meet the needs of patients with a-SAH.
and knee flexion strength, whereas participants with lower functional outcome had lower knee extension and flexion strength. Further, participants who had been treated with surgical clipping were at risk of low physical fitness. Exercise interventions are warranted; they should consider the promotion of daily physical activity and should target patients with lower functional outcome or those who have been treated with surgical clipping. Research is warranted to investigate whether rehabilitation services can adapt their programs to meet the needs of patients with a-SAH.
Supplementary Material
Author Contributions
Concept/idea/research design: L. Khajeh, G.M. Ribbers, F. van Kooten, S.J.C.M.M. Neggers, R.J. van den Berg-Emons
Writing: W.J. Harmsen, G.M. Ribbers, M.H. Heijnbrok-Kal, S.J.C.M.M. Neggers
Data collection: W.J. Harmsen, L. Khajeh, E. Sneekes, F. van Kooten, S.J.C.M.M. Neggers
Data analysis: W.J. Harmsen, M.H. Heijnbrok-Kal, E. Sneekes, S.J.C.M.M. Neggers
Project management: W.J. Harmsen, G.M. Ribbers, E. Sneekes, F. van Kooten, R.J. van den Berg-Emons
Fund procurement: R.J. van den Berg-Emons
Providing participants: L. Khajeh, G.M. Ribbers, F. van Kooten, S.J.C.M.M. Neggers
Providing facilities/equipment: G.M. Ribbers, E. Sneekes, R.J. van den Berg-Emons
Providing institutional liaisons: G.M. Ribbers
Consultation (including review of manuscript before submitting): L. Khajeh, G.M. Ribbers, E. Sneekes, F. van Kooten, R.J. van den Berg-Emons
Ethics Approval
This study was approved by the Medical Ethics Committee of the Medical Ethics Committee of the Erasmus MC University. All participants provided written informed consent.
Funding
Financial support for this study was provided by the Dutch Brain Foundation (grant no. 15F07.06).
Disclosures
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. van Gijn J, Kerr RS, Rinkel GJE. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. [DOI] [PubMed] [Google Scholar]
- 2. Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–642. [DOI] [PubMed] [Google Scholar]
- 3. Rinkel GJ, Algra A.. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349–356. [DOI] [PubMed] [Google Scholar]
- 4. Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–536. [DOI] [PubMed] [Google Scholar]
- 5. Huenges Wajer IM, Visser-Meily JM, Greebe P, Post MW, Rinkel GJ, van Zandvoort MJ. Restrictions and satisfaction with participation in patients who are ADL-independent after an aneurysmal subarachnoid hemorrhage. Top Stroke Rehabil. 2017;24:134–141. [DOI] [PubMed] [Google Scholar]
- 6. Kruisheer EM, Huenges Wajer IMC, Visser-Meily JMA, Post MWM. Course of participation after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2017;26:1000–1006. [DOI] [PubMed] [Google Scholar]
- 7. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 8. Wasserman K HJ, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. Including Pathophysiology and Clinical Applications. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 9. Durstine JL, Painter P, Franklin BA, Morgan D, Pitetti KH, Roberts SO. Physical activity for the chronically ill and disabled. Sports Med. 2000;30:207–219. [DOI] [PubMed] [Google Scholar]
- 10. Harmsen WJ, Ribbers GM, Zegers B, Sneekes EM, Heijenbrok-Kal MH, Khajeh L et al.. Impaired cardiorespiratory fitness after aneurysmal subarachnoid hemorrhage. J Rehabil Med. 2016;48:769–775. [DOI] [PubMed] [Google Scholar]
- 11. Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. Int J Stroke. 2012;7:499–510. [DOI] [PubMed] [Google Scholar]
- 12. Ada L, Dorsch S, Canning CG. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother. 2006;52:241–248. [DOI] [PubMed] [Google Scholar]
- 13. Saunders DH, Sanderson M, Brazzelli M, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2013;10:CD003316. doi:10.1002/14651858.CD003316.pub5 [DOI] [PubMed] [Google Scholar]
- 14. Baert I, Daly D, Dejaeger E, Vanroy C, Vanlandewijck Y, Feys H. Evolution of cardiorespiratory fitness after stroke: a 1-year follow-up study. Influence of prestroke patients' characteristics and stroke-related factors. Arch Phys Med Rehabil. 2012;93:669–676. [DOI] [PubMed] [Google Scholar]
- 15. Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM et al.. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. [DOI] [PubMed] [Google Scholar]
- 16. Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D et al.. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. [DOI] [PubMed] [Google Scholar]
- 17. Passier PE, Visser-Meily JM, Rinkel GJ, Lindeman E, Post MW. Determinants of health-related quality of life after aneurysmal subarachnoid hemorrhage: a systematic review. Qual Life Res. 2013;22:1027–1043. [DOI] [PubMed] [Google Scholar]
- 18. Khajeh L, Blijdorp K, Heijenbrok-Kal MH, Sneekes EM, van den Berg-Emons HJ, van der Lely AJ et al.. Pituitary dysfunction after aneurysmal subarachnoid haemorrhage: course and clinical predictors-the hips study. J Neurol Neurosurg Psychiatry. 2015;86:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cardinal BJ, Esters J, Cardinal MK. Evaluation of the revised physical activity readiness questionnaire in older adults. Med Sci Sports Exerc. 1996;28:468–472. [DOI] [PubMed] [Google Scholar]
- 20. Thompson PD, Arena R, Riebe D, Pescatello LS, American College of Sports Medicine. ACSM's new preparticipation health screening recommendations from ACSM's guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12:215–217. [DOI] [PubMed] [Google Scholar]
- 21. Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. [DOI] [PubMed] [Google Scholar]
- 22. van de Port IG, Kwakkel G, Wittink H. Systematic review of cardiopulmonary exercise testing post stroke: are we adhering to practice recommendations?. J Rehabil Med. 2015;47:881–900. [DOI] [PubMed] [Google Scholar]
- 23. Bussmann JB, Martens WL, Tulen JH, Schasfoort FC, van den Berg-Emons HJ, Stam HJ. Measuring daily behavior using ambulatory accelerometry: the activity monitor. Behav Res Methods Instrum Comput. 2001;33:349–356. [DOI] [PubMed] [Google Scholar]
- 24. van den Berg-Emons RJ, Bussmann JB, Stam HJ. Accelerometry-based activity spectrum in persons with chronic physical conditions. Arch Phys Med Rehabil. 2010;91:1856–1861. [DOI] [PubMed] [Google Scholar]
- 25. White DK, Wagenaar RC, Del Olmo ME, Ellis TD. Test-retest reliability of 24 hours of activity monitoring in individuals with Parkinson's disease in home and community. Neurorehabil Neural Repair. 2007;21:327–340. [DOI] [PubMed] [Google Scholar]
- 26. Nayar M, Vanderstay R, Siegert RJ, Turner-Stokes L. The UK functional assessment measure (UK Fim+Fam): psychometric evaluation in patients undergoing specialist rehabilitation following a stroke from the National UK Clinical Dataset. PLoS One. 2016;11:e0147288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fairbarn MS, Blackie SP, McElvaney NG, Wiggs BR, Pare PD, Pardy RL. Prediction of heart rate and oxygen uptake during incremental and maximal exercise in healthy adults. Chest. 1994;105:1365–1369. [DOI] [PubMed] [Google Scholar]
- 28. Heyward VH. Advance Fitness Assessment & Exercise Prescription. Dallas, TX: The Cooper Institute for Aerobics Research; 1998. [Google Scholar]
- 29. Sunnerhagen KS, Hedberg M, Henning GB, Cider A, Svantesson U. Muscle performance in an urban population sample of 40- to 79-year-old men and women. Scand J Rehabil Med. 2000;32:159–167. [PubMed] [Google Scholar]
- 30. Baert I, Vanlandewijck Y, Feys H, Vanhees L, Beyens H, Daly D. Determinants of cardiorespiratory fitness at 3, 6 and 12 months poststroke. Disabil Rehabil. 2012;34:1835–1842. [DOI] [PubMed] [Google Scholar]
- 31. Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP et al.. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013;44:29–37. [DOI] [PubMed] [Google Scholar]
- 33. Cohen JW, Ivanova TD, Brouwer B, Miller KJ, Bryant D, Garland SJ. Do performance measures of strength, balance and mobility predict quality of life and community reintegration after stroke?. Arch Phys Med Rehabil. 2018;99:713–719. [DOI] [PubMed] [Google Scholar]
- 34. Boss HM, Deijle I, Van Schaik S, de Melker E, van den Berg BJ, Weinstein H et al.. Cardiorespiratory fitness after transient ischemic attack and minor ischemic stroke: baseline data of the MoveIT study. J Stroke Cerebrovasc Dis. 2017;26:1114–1120. [DOI] [PubMed] [Google Scholar]
- 35. English C, Manns PJ, Tucak C, Bernhardt J. Physical activity and sedentary behaviors in people with stroke living in the community: a systematic review. Phys Ther. 2014;94:185–196. [DOI] [PubMed] [Google Scholar]
- 36. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG et al.. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. [DOI] [PubMed] [Google Scholar]
- 38. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. [DOI] [PubMed] [Google Scholar]
- 39. Ivey FM, Hafer-Macko CE, Macko RF. Exercise rehabilitation after stroke. NeuroRx. 2006;3:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dunn A, Marsden DL, Van Vliet P, Spratt NJ, Callister R. Independently ambulant, community-dwelling stroke survivors have reduced cardiorespiratory fitness, mobility and knee strength compared to an age- and gender-matched cohort. Top Stroke Rehabil. 2017;24:163–169. [DOI] [PubMed] [Google Scholar]
- 41. Cress ME, Meyer M.. Maximal voluntary and functional performance levels needed for independence in adults aged 65 to 97 years. Phys Ther. 2003;83:37–48. [PubMed] [Google Scholar]
- 42. Tang A, Sibley KM, Thomas SG, McIlroy WE, Brooks D. Maximal exercise test results in subacute stroke. Arch Phys Med Rehabil. 2006;87:1100–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.