Abstract
Background
This is the first update of a Cochrane review published in Issue 5, 2010 on transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Pain may present in a body part that has been amputated (phantom pain) or at the site of amputation (stump pain), or both. Phantom pain and stump pain are complex and multidimensional and the underlying pathophysiology remains unclear. The condition remains a severe burden for those who are affected by it. The mainstay treatments are predominately pharmacological, with increasing acknowledgement of the need for non‐drug interventions. TENS has been recommended as a treatment option but there has been no systematic review of available evidence. Hence, the effectiveness of TENS for phantom pain and stump pain is currently unknown.
Objectives
To assess the analgesic effectiveness of TENS for the treatment of phantom pain and stump pain following amputation in adults.
Search methods
For the original version of the review we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, AMED, CINAHL, PEDRO and SPORTDiscus (February 2010). For this update, we searched the same databases for relevant randomised controlled trials (RCTs) from 2010 to 25 March 2015.
Selection criteria
We only included RCTs investigating the use of TENS for the management of phantom pain and stump pain following an amputation in adults.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We planned that where available and appropriate, data from outcome measures were to be pooled and presented as an overall estimate of the effectiveness of TENS.
Main results
In the original review there were no RCTs that examined the effectiveness of TENS for the treatment of phantom pain and stump pain in adults. For this update, we did not identify any additional RCTs for inclusion.
Authors' conclusions
There were no RCTs to judge the effectiveness of TENS for the management of phantom pain and stump pain. The published literature on TENS for phantom pain and stump pain lacks the methodological rigour and robust reporting needed to confidently assess its effectiveness. Further RCT evidence is required before an assessment can be made. Since publication of the original version of this review, we have found no new studies and our conclusions remain unchanged.
Keywords: Adult, Humans, Amputation Stumps, Pain Management, Phantom Limb, Phantom Limb/therapy, Transcutaneous Electric Nerve Stimulation
Plain language summary
Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults
Pain may present in a body part that has been amputated (phantom pain) or at the site of amputation (stump pain), or both. Phantom pain and stump pain are complex conditions and affect up to 80% of amputees.The underlying causes are not fully understood. Drug therapy is the most common treatment yet the condition remains poorly managed. The need for non‐drug interventions has been recognised and TENS may have an important role to play.
TENS is an inexpensive, safe and easy to use analgesic technique. TENS is administered using a battery‐powered portable device, which generates electrical currents that are delivered to the skin to activate underlying nerves.
An updated search of various databases in March 2015 found no studies that met the eligibility criteria for inclusion in this review.
It was not possible to judge the effectiveness of TENS for phantom pain and stump pain.
It was not possible to assess the risk of harm from using TENS for phantom pain and stump pain.
A large, multicentre randomised controlled trial of TENS for phantom pain and stump pain is needed.
Background
This review is the first update of a previously published review in the Cochrane Database of Systematic Reviews (2010, Issue 5) (Mulvey 2010).
Description of the condition
Following amputation up to 80% of patients report pain that affects quality of life and hinders rehabilitation, including the use of prosthetic limbs (Ephraim 2005; Nikolajsen 2001). Pain may present in a body part that has been amputated (phantom pain) or at the site of amputation (stump pain), or both (Wilson 2008). Non‐painful sensations may also present in a phantom body part or a stump, or both (Nikolajsen 2001). Often patients present with a unique combination of symptoms (Nikolajsen 2001; Wiffen 2006). The underlying pathophysiology is unclear, although it is generally accepted that nociceptive and neuropathic processes are involved and that neuropathic changes include reorganisation and adaptation within the peripheral and central nervous systems (Flor 2002). Multimodal treatment strategies are used including analgesics, muscle relaxants, vasodilators, sympathetic blocks, sympathectomies, surgical revision of the stump, stimulation‐induced analgesic techniques and mirror box therapy (Flor 2002; Hanling 2010; Sherman 1994; Sindrup 1999). Despite a multitude of treatments, a study of 92 amputees revealed that only 9% were pain free (Smith 1999). In 2002, a systematic review of available treatment regimes concluded that it was not possible to determine optimal treatments for the management of phantom limb pain based on available evidence (Halbert 2002).
Description of the intervention
Transcutaneous electrical nerve stimulation (TENS) is a technique that delivers pulsed electrical currents across the intact surface of the skin to stimulate peripheral nerves (Johnson 2014; Walsh 1997). TENS is principally used to relieve pain and is administered using a 'standard TENS device' that consists of a battery‐powered portable machine that generates electrical currents, which are delivered through the skin via electrodes attached to the skin surface. TENS is safe, inexpensive and can be self administered. TENS is contraindicated for patients with electronic implants, such as cardiac pacemakers and implantable cardioverter defibrillators and precautions include pregnancy, epilepsy, active malignancy, deep‐vein thrombosis, and frail or damaged skin (Houghton 2010). TENS is used as a stand‐alone treatment and in combination with other treatments for a wide variety of acute and chronic pains, including phantom pain and stump pain.
How the intervention might work
TENS can be used to stimulate large diameter A‐beta afferents to elicit segmental analgesia (conventional TENS) or to stimulate smaller diameter A‐delta afferents to elicit extrasegmental analgesia (acupuncture‐like TENS) (Charlton 2005; Johnson 2008; Vance 2014). Physiological research suggests that TENS inhibits second order nociceptive neurons (Garrison 1994; Garrison 1996; Sdrulla 2015), increases blood flow (Chen 2007; Cramp 2001), and reduces muscle spasms (Avdic 2000). It is plausible that these actions could alleviate phantom pain, stump pain, or both.
Why it is important to do this review
Systematic reviews of TENS for acute pain have reported positive outcomes for primary dysmenorrhoea (Proctor 2002), conflicting outcomes for postoperative pain (Bjordal 2003; Carroll 1996), and inconclusive outcomes for labour pain (Dowswell 2009). A Cochrane Review of TENS for acute pain concluded that there is insufficient evidence to make any definitive conclusions about the effectiveness of TENS for acute pain in adults (Walsh 2009). Systematic reviews of TENS for chronic pain have reported positive outcomes for chronic recurrent headache (Bronfort 2004), and musculoskeletal pain (Johnson 2007), and inconclusive outcomes for low back pain (Khadilkar 2008), knee osteoarthritis (Bjordal 2007; Rutjes 2009), rheumatoid arthritis of the hand (Brosseau 2003), post‐stroke shoulder pain (Price 2000), cancer‐related pain (Hurlow 2012), and whiplash and mechanical neck disorders (Kroeling 2013). A Cochrane Review of TENS for chronic pain concluded that the lack of methodological rigour and robust reporting of published literature prevents confident assessment of the role of TENS in chronic pain management (Nnoaham 2008). This review has now been withdrawn to be replaced by reviews on TENS for neuropathic pain in adults (protocol in press) and TENS for fibromyalgia (protocol in press). Methodological weaknesses in randomised controlled trials (RCTs) have been shown to contribute to low fidelity (Bennett 2011), with more positive outcomes reported when adequate TENS techniques are taken into account (Bennett 2011; Bjordal 2003; Bjordal 2007; Sluka 2013). Criteria and operational guidelines for the design of a robust RCT on TENS have been published by Bennett 2011. TENS has been recommended as a treatment option for phantom pain and stump pain (Black 2009; Jensen 2006). Published case series and controlled clinical trials suggest that TENS may be of benefit (Carabelli 1985; Finsen 1988; Gyory 1977; Katz 1989; Katz 1991; Kawamura 1997; Thorsteinsson 1977; Wartan 1997). Prior to 2010 there was no systematic review evidence available upon which to judge the effectiveness of TENS for phantom pain and stump pain. The original Cochrane Review in 2010 concluded that there was insufficient evidence to make a judgement of effectiveness. An analysis of excluded studies from the original Cochrane Review was published in 2014 (Mulvey 2014). This update seeks to identify new randomised controlled trials published since 2010.
Objectives
To assess the analgesic effectiveness of TENS for the treatment of phantom pain and stump pain following amputation in adults.
Methods
Criteria for considering studies for this review
Types of studies
We sought all cross‐over or parallel‐group randomised controlled trials (RCTs) investigating the use of TENS for the management of pain following amputation. We excluded the following: letters, abstracts and reviews (unless they provided additional information from published RCTs that met the criteria); studies using experimental pain; case reports; clinical observations; trials that were non‐randomised.
Types of participants
Adult participants (16 years or above) with any limb amputation resulting in any type of pain in a phantom or stump, or both. Participants whose amputation had occurred for any reason were eligible for inclusion in this review.
Types of interventions
We only included trials that evaluated surface electrical nerve stimulation for the management of phantom pain or stump pain, or both, following amputation (i.e. transcutaneous as opposed to percutaneous electrical stimulation). We included trials only if they:
used a TENS device that delivered biphasic or monophasic pulsed electrical currents in the mA range. This included delivery of currents using the following devices: standard TENS device, Neuromuscular Electrical Stimulation devices (NMES), Functional Electrical Stimulation (FES), Interferential Current devices (IFC) and single electrode probes (i.e. TENS pens);
administered TENS at pulse amplitudes that produced 'strong and comfortable' paraesthesia that was felt by the participant (i.e. conventional TENS or acupuncture‐like TENS, or both)(TENS delivered at intensities reported to be 'barely perceptible’, 'faint' or 'mild' was excluded);
administered TENS in an area of the body that was sensate either at i) the site of pain, ii) over nerve bundles proximal to the site of pain, iii) on the contralateral limb at the mirror site to the phantom limb pain, iv) known acupuncture points;
used any parameters of stimulation providing they met the above criteria.
The planned intervention comparisons were the following.
TENS versus no treatment controls.
TENS versus sham controls. Sham controls are defined as any electrotherapeutic device that has been modified so that there is no active output (i.e. dummy device).
TENS versus a pharmacological intervention.
TENS versus a non‐pharmacological intervention.
It was intended that trials would be excluded from the analysis if TENS was administered in combination within another intervention as part of the formal trial design; for example additional analgesics or exercise. It was intended that trials where participants continued with their usual medications would be included as well as trials where participants were given rescue medication because the potential impact on pain scores was thought to be minimal.
Types of outcome measures
Primary outcomes
Patient‐reported pain using standard subjective validated scales (e.g. visual analogue scales (VAS) or numerical rating scales (NRS)).
Secondary outcomes
Any other related pain measure designed to capture data pertaining to the characteristics and quality of pain (e.g. McGill Pain Questionnaire)
Patient reported non‐painful phantom sensations using validated scales
Patient satisfaction
Activities of daily living and ambulation
Range of movement*
Quality of life
Anxiety/depression
Use of pain coping strategies
Sleep**
Analgesic consumption
Hospital attendance
Other healthcare interventions, e.g. physiotherapy visits, hospice admissions, day care etc
Any adverse effects
* Range of movement may not measure the actual range of movement possible but the range of movement that is comfortable.
** If 'sleep' outcomes are reported these may be heterogeneous and we planned subcategories in the analysis rather than combining all sleep outcomes together ‐ we identified no sleep trials so this was not an issue.
Search methods for identification of studies
For the original version of the review we searched for relevant trials to February 2010 (Appendix 1). For this update we tailored searches to individual databases and adapted them from those used in the original review. This update searched for relevant trials from 2010 to 25 March 2015 (Appendix 2).
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL2015, Issue 2);
MEDLINE (OVID) 1950 to 25 March 2015;
EMBASE (OVID) 1980 to 25 March 2015;
PsycINFO (OVID) 1806 to 25 March 2015;
AMED (OVID) 1985 to 25 March 2015;
CINAHL (EBSCO) 1982 to 25 March 2015;
PEDRO 1929 to 25 March 2015;
SPORTDiscus (EBSCO) 1975 to 25 March 2015.
We identified trials for inclusion using detailed search strategies developed for each electronic database. These were based on the search strategy developed for MEDLINE and we revised them accordingly for each database. We used medical subject headings (MeSH) or equivalent and text word terms. For the MEDLINE search, we ran the subject search with the following filter: Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised controlled trials in MEDLINE (via OVID): sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.a of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011). We searched reference lists of all eligible trials, key textbooks and previous systematic reviews for additional trials.
Language
The search strategy attempted to identify all relevant trials irrespective of language. We assessed non‐English papers and translated them if necessary.
Data collection and analysis
Selection of studies
From the titles, abstracts and descriptors, two independent review authors (MJ and MM) reviewed the results of the literature searches to identify potentially relevant trials for the full review. We resolved disagreements through discussion with a third review author (A‐MB). Review authors were not blinded to the authors' names and institutions, journal of publication, or trial results at this or any stage of the review.
Data extraction and management
It was intended that the following trial characteristics would be extracted for entry into RevMan 2014, version 5.3 (RevMan 2014): authors, participants, trial design, characteristics of interventions (TENS settings, application, treatment schedules, concurrent interventions), adverse effects and baseline and end of trial outcomes. It was intended that two out of three review authors would complete data extraction (MJ, MM) independently. Disagreements were to be resolved by consensus. Where necessary, we sought additional information from trial authors of relevant trials.
Assessment of risk of bias in included studies
In the original review it was intended that the risk of bias of any included trials would be assessed independently by the review authors. In this update it was intended that two authors (MJ, MM) would independently assess risk of bias for each trial, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We planned to complete a 'Risk of bias' table for each included trial using the 'Risk of bias tool in RevMan (RevMan 2014). In this update it was intended that we would assess the following for each trial:
Random sequence generation (checking for possible selection bias). We planned to assess the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). Trials using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number) would be excluded.
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We planned to assess the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); unclear risk of bias (method not clearly stated). Trials that do not conceal allocation (e.g. open list) would be excluded.
Blinding of outcome assessment (checking for possible detection bias). We planned to assess the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We planned to assess the methods as: low risk of bias (trial report states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (trial report states that it was blinded but does not provide an adequate description of how it was achieved). Trials that were not double‐blind would be excluded.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We planned to assess the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study and/or used 'baseline observation carried forward' analysis); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
Size of trial (checking for possible biases confounded by small size). We planned to assess studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
It was planned that where available and appropriate, data from outcome measures were to be pooled and presented as an overall estimate of the effectiveness of TENS. It was intended that the appropriateness of pooling would first have been assessed on the basis of clinical heterogeneity in terms of participants, settings, interventions and comparisons, dose intensity, outcomes measured and timing of outcome measurements; and on the basis of methodological heterogeneity. For each trial, risk ratio (RR) with 95% confidence intervals (CI) would be calculated for dichotomous outcomes. For continuous outcomes reported using the same scale, pooled results would be presented as mean difference (MD). Standardised mean differences (SMD) would be calculated where results for the same continuous outcome had been measured using different scales. The number needed to treat to benefit (NNTB) or number needed to treat to harm (NNTH) for treatment effect would be calculated where appropriate.
Unit of analysis issues
It was intended that if categorical data could not be split into dichotomous outcomes, they would not be included in a meta‐analysis but would be reported in tables and in the text. In the case of cross‐over trial designs, it was anticipated that the data reported would not permit analysis of paired within‐patient data. Cross‐over trials were intended to be analysed as if they were parallel‐group trials, combining data from all treatment periods. If a carry‐over effect was found and data were reported by period, then the analysis was to be restricted to period‐one data only. In those rare cases in which complete data were reported, within‐patient improvement scores were to be calculated. It was intended that if combining trials in a meta‐analysis was not possible, a narrative description of included trials would be provided.
Dealing with missing data
It was intended that if trials reported outcomes that could not be included in the meta‐analysis, either for reasons already mentioned, or because there was missing summary data (e.g. absent standard deviations) or the report showed that the data evidently came from a skewed distribution, the trial findings were to be reported in tables and in the text under the appropriate headings.
Assessment of heterogeneity
It was planned that estimates of effectiveness (both SMD and RR) were to be tested for statistical homogeneity, by visual inspection of the forest plot and by using the Chi2 test and I2 statistic. The I2 statistic value would be interpreted according to the following thresholds (Higgins 2011): 0% to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; and 75% to 100%, considerable heterogeneity. We planned to investigate any evidence of heterogeneity to determine if there were obvious differences in the trials that were likely causes of the heterogeneity. If effect estimates were consistent with homogeneity, they were to be combined using a fixed‐effect model. If statistical heterogeneity was present, an attempt would be made to explain the differences based on the clinical and methodological characteristics of the included trials. Trials thought to be the cause of statistical heterogeneity would be excluded from the analysis. Clinically dissimilar trials would not be statistically combined. However, if a group of trials with heterogeneous results appeared to be clinically similar, the trial estimates would be combined using a random‐effects model and the results interpreted with caution.
Subgroup analysis and investigation of heterogeneity
Where the data allowed, it was planned to separate the outcome analyses to test the following null hypotheses.
There is no difference in patient‐reported amputee pain for different causes of amputation.
There is no difference in patient‐reported amputee pain for different levels of amputation.
There is no difference in patient‐reported amputee pain for different TENS application technique.
Sensitivity analysis
It was planned that a sensitivity analysis would be performed when indicated to investigate the effects of allocation concealment, overall methodological quality and use of intention‐to‐treat (ITT) analysis. It was intended that trials with high attrition rates (i.e. more than 50%) would have been removed from the meta‐analysis to see if the results were significantly different without them.
Results
Description of studies
There were no trials that met the eligibility criteria in the original review. Since publication of the original version of this review, we have found no new trials. In total 72 published reports were identified by the searches in the original review in 2010 and we found an additional 85 published reports in this update (Figure 1). We assessed 14 full‐text reports for eligibility in the original review and we assessed an additional six reports in this update. None of these met the eligibility criteria for the review (see 'Characteristics of excluded studies').
1.
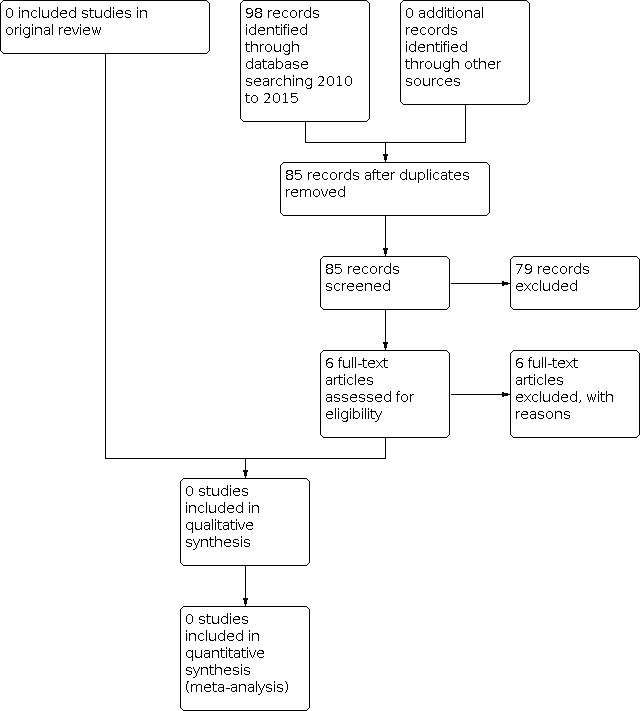
# of records identified through database searching. Study flow diagram.
In the original review there were four case reports (Giuffrida 2010; Gyory 1977; Hirano 1988; Katz 1989), eight case series (Carabelli 1985; Heidenreich 1988; Kawamura 1997; Miles 1978; Salim 1997; Sindou 1980; Stolke 1978; Winnem 1982), and two placebo‐controlled non‐randomised trials (Finsen 1988; Katz 1991). The two placebo‐controlled non‐randomised trials found beneficial effects from TENS when compared with placebo (no current) TENS but neither implemented adequate randomisation procedures and both failed to report methods of sequence generation (Finsen 1988; Katz 1991). Katz 1991 reported a "modest reduction" in phantom limb pain after 10 minutes of auricular TENS although no statistical analysis was reported for active versus sham TENS. Finsen 1988 reported that low frequency (2 Hz) segmental TENS reduced healing times and re‐amputation rates when compared to sham TENS but found there was no difference in analgesic consumption between the groups. No direct measure of pain was made. Finsen 1988 claimed to have randomised patients to one of three treatments. However, the authors report that after 18 months there was unequal distribution of amputation levels between the three groups and recruitment and randomisation was "...improved by taking into account the amputation level". It was felt that the adjustment of recruitment and randomisation procedures compromised randomisation and the possibility of purposive sampling cannot be discounted. In this update the searches found one new case report that investigated invasive peripheral nerve stimulation (Rauck 2012), one case series that investigated invasive peripheral nerve stimulation (Rauck 2014), one case series on TENS for phantom pain and stump pain in adult amputees (Mulvey 2013), and three reviews (Hu 2014; Lenggenhager 2014; Mulvey 2014).
Risk of bias in included studies
There were no trials included in this review so risk of bias could not be evaluated.
Effects of interventions
There were no trials included in this review so effects could not be evaluated.
Discussion
No randomised controlled trials (RCTs) examining the clinical efficacy of transcutaneous electrical nerve stimulation (TENS) for the treatment of phantom pain and stump pain in adults were identified by the searches in the original and this updated review. Therefore it was not possible to make a judgement about clinical efficacy or effectiveness. The lack of RCTs in this area was identified in a systematic review by Halbert 2002 and no RCTs have been published since. The positive trend towards pain relief in some of the excluded case reports, case series and non‐randomised trials suggests that TENS may be beneficial for some individuals and that a large, multicentre, adequately powered RCT is needed. Careful consideration should be given to randomisation, allocation concealment, blinding, adequacy of TENS, and the timing and appropriateness of the outcome measures because evidence suggests that there are significant sources of potential bias in both directions in previous RCTs on TENS in other conditions (Bennett 2011). Suboptimal dosing of TENS and inappropriate assessment of pain outcomes are particularly prevalent. Criteria for judging directions of bias in RCTs on TENS, developed by Bennett 2011, can be adapted to design future trials. In particular, it is important that TENS is administered to skin with normal sensation and functional nerves to produce a strong, non‐painful TENS sensation within the receptive field of the area of pain. Attempts should also be made to report blinding procedures and whether blinding was maintained.
Authors' conclusions
Implications for practice.
Since publication of the original version of this review, we have found no new trials. There is insufficient evidence from RCTs to judge whether TENS should, or should not, be used in the management of phantom pain and stump pain in adults.
Implications for research.
A large, multicentre, adequately powered, randomised, placebo‐controlled trial with appropriate procedures for sequence generation, allocation concealment and blinding is needed. Data provided in the reports of the excluded studies may prove useful in calculating sample size. Future studies need to ensure that TENS is delivered at a strong, non‐painful intensity within or close to the site of pain (Bjordal 2003), using an appropriate technique in line with best practice (Johnson 2014). Pain outcomes should be measured whilst the TENS device is switched on, rather than before and after TENS, and the duration and frequency of each treatment recorded when TENS is used at home. Means and standard deviations for continuous data should be reported as standard to enable data extraction for subsequent meta‐analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 11 March 2021 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 5, 2010
| Date | Event | Description |
|---|---|---|
| 11 August 2015 | Review declared as stable | The authors and editors have agreed that this review will be assessed for further updating in 2020, or earlier if new evidence becomes available. |
| 22 May 2015 | New citation required but conclusions have not changed | We identified 85 published reports in this update. None met the eligibility criteria for inclusion in the review. |
| 22 May 2015 | New search has been performed | This review has been updated to include the results of a new search. |
| 3 March 2013 | Amended | No new trials available. To be assessed for updating in 2015. |
Notes
Following a full updated search in March 2021, we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We would like to acknowledge the following individuals for their assistance in this update: Anna Hobson, Managing Editor, Cochrane Pain Palliative and Supportive Care (PaPaS) Review Group for her invaluable support throughout the entire process of this update; Joanne Abbott, Trials Search Co‐ordinator (PaPaS) for her assistance in conducting the search strategy for this update. We would like to acknowledge the following individuals for their assistance in the original review: Paul Marchant (PM) who contributed to the original review but did not participate in the update; Jessica Thomas (former Managing Editor PaPaS), Caroline Struthers, (former Trials Search Co‐ordinator) and Phil Wiffen (former Co‐ordinating Editor of PaPaS).
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategies for original review
MEDLINE via Ovid search (1950 to February 2010)
[mp=title, original title, abstract, name of substance word, subject heading word]
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
4. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
5. ("transcutaneous electric*" adj4 stimulat*).mp.
6. (amputat* or amputee*).mp.
7. (postamputation* or post‐amputation*).mp.
8. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
9. (#4 or #1 or #3 or #2 or #5).
10. (#8 or #6 or #7).
11. (#10 and #9).
Cochrane highly sensitive strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format
12. randomized controlled trial.pt.)
13. controlled clinical trial.pt.
14. randomized.ab.
15. placebo.ab.
16. drug therapy.fs.
17. randomly.ab.
18. trial.ab.
19. groups.ab.
20. or/12‐19
21. (animals not (humans and animals)).sh.
22. 20 not 21
23. 22 and 11
The Cochrane Library search (2010, Issue 1)
1. "tens" or "al‐tens" or "tns" or "ens" or "tes":ti,ab,kw.
2. "transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation":ti,ab,kw.
3. "electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*":ti,ab,kw.
4. "electric* nerve therap*" or electroanalgesi* or electro‐analgesi*:ti,ab,kw.
5. "transcutaneous electric*" NEAR stimulation:ti,ab,kw.
6. (#1 OR #2 OR #3 OR #4 OR #5).
7. (amputat* or amputee*):ti,ab,kw.
8. (post‐amputation* or postamputation*):ti,ab,kw.
9. (phantom‐limb or (phantom NEAR limb) or stump* ):ti,ab,kw.
10. (fantom‐limb or (fantom NEAR limb)):ti,ab,kw.
11. (#7 OR #8 OR #9 OR #10).
12. (#6 AND #11).
13. #12 Records from CENTRAL.
EMBASE search via Ovid (1980 to Feb 2010)
[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
4. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
5. ("transcutaneous electric*" adj4 stimulat*).mp.
6. (amputat* or amputee*).mp.
7. (postamputation* or post‐amputation*).mp.
8. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
9. (#4 or #1 or #3 or #2 or #5).
10. (#8 or #6 or #7).
11. (#10 and #9).
Cochrane highly sensitive strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format
12. random*.ti,ab.
13. factorial*.ti,ab.
14. (crossover* or cross over* or cross‐over*).ti,ab.
15. placebo*.ti,ab.
16. (doubl* adj blind*).ti,ab.
17. (singl* adj blind*).ti,ab.
18. assign*.ti,ab.
19. allocat*.ti,ab.
20. volunteer*.ti,ab.
21. CROSSOVER PROCEDURE.sh.
22. DOUBLE‐BLIND PROCEDURE.sh.
23. RANDOMIZED CONTROLLED TRIAL.sh.
24. SINGLE BLIND PROCEDURE.sh.
25. or(/#12‐#24).
26. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/.
27. HUMAN/.
28. (#26 and #27).
29. (#26 not #28).
30. (#25 not #29).
31. (#11 and #30)
PsycINFO search via Ovid (1806 to February 2010)
[mp=title, abstract, heading word, table of contents, key concepts]
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
4. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
5. ("transcutaneous electric*" adj4 stimulat*).mp.
6. (amputat* or amputee*).mp.
7. (postamputation* or post‐amputation*).mp.
8. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
9. (#4 or #1 or #3 or #2 or #5).
10. (#8 or #6 or #7).
11. (#10 and #9).
AMED via Ovid (Allied and Complementary Medicine) search (1985 to February 2010)
[mp=abstract, heading words, title]
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
4. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
5. ("transcutaneous electric*" adj4 stimulat*).mp.
6. (amputat* or amputee*).mp.
7. (postamputation* or post‐amputation*).mp.
8. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
9. (#4 or #1 or #3 or #2 or #5).
10. (#8 or #6 or #7).
11. (#9 and #10)
CINAHL search (1982 to February 2010)
[ti,ab = title, abstract]
1. transcutaneous electrical nerve stimulation.
2. (tens OR al‐tens OR tns OR ens OR tes).ti,ab.
3. ("transcutaneous electric* nerve stimulation" OR "transcutaneous nerve stimulation").ti,ab.
4. ("electric* nerve stimulation" OR "electrostimulation therap*" OR "electro‐stimulation therap*").ti,ab.
5. ("electric* nerve therap*" OR electroanalgesi* OR electro‐analgesi*).ti,ab.
6. ("transcutaneous electric*" adj4 stimulat*).ti,ab.
7. (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR # OR #7).
8. exp AMPUTATION/ OR AMPUTATION STUMPS/.
9. (amputat* OR amputee*).ti,ab.
10. (postamputation* OR post‐amputation*).ti,ab.
11. ((phantom adj6 limb) OR phantom‐limb OR stump*).ti,ab.
12. PHANTOM LIMB/ OR PHANTOM PAIN/.
13. (#8 OR #9 OR #10 OR #11 OR #13).
14. (#7 AND #13).
PEDro search (1929 to February 2010)
[mp=title, abstract]
1. "transcutaneous electric* nerve stimulation".
2. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
3. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
4. (amputat* or amputee*).mp.
5. (postamputation* or post‐amputation*).mp.
6. “pain”
7. (#1 or #4 or #6).
8. (#1 and #4 and #6)
SPORTDiscus search (1975 to February 2010)
1. "tens" or "al‐tens" or "tns" or "ens" or "tes":TX
2. "transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation”:TX
3. "electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*":TX
4. "electric* nerve therap*" or electroanalgesi* or electro‐analgesi*:TX
5. "transcutaneous electric* stimulation":TX
6. (#1 OR #2 OR #3 OR #4).
7. (amputat* or amputee*):TX
8. (post‐amputation* or postamputation*):TX
9. (phantom‐limb or (phantom NEAR limb) or stump* ):TX
10. (fantom‐limb or (fantom NEAR limb)):TX
11. (#7 OR #8 OR #9 ).
12. (#6 AND #11).
13. (#6 AND #11) and “control trial”: TX
SPORTDiscus search (1975 to February 2010)
1. "tens" or "al‐tens" or "tns" or "ens" or "tes":TX
2. "transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation”:TX
3. "electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*":TX
4. "electric* nerve therap*" or electroanalgesi* or electro‐analgesi*:TX
5. "transcutaneous electric* stimulation":TX
6. (#1 OR #2 OR #3 OR #4).
7. (amputat* or amputee*):TX
8. (post‐amputation* or postamputation*):TX
9. (phantom‐limb or (phantom NEAR limb) or stump* ):TX
10. (fantom‐limb or (fantom NEAR limb)):TX
11. (#7 OR #8 OR #9 ).
12. (#6 AND #11).
13. (#6 AND #11) and “control trial”: TX
Appendix 2. Search strategies for update
MEDLINE (OVID) 2010 to March week 3 2015
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. Transcutaneous Electric Nerve Stimulation/
4. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
5. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
6. ("transcutaneous electric*" adj4 stimulat*).mp.
7. or/1‐6
8. exp Amputation/
9. Amputees/
10. (amputat* or amputee*).mp.
11. (postamputation* or post‐amputation*).mp.
12. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
13. or/8‐12
14. 7 and 13
15. randomized controlled trial.pt.
16. controlled clinical trial.pt.
17. randomized.ab.
18. placebo.ab.
19. drug therapy.fs.
20. randomly.ab.
21. trial.ab.
22. groups.ab.
23. 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24. exp animals/ not humans.sh.
25. 23 not 24
26. 14 and 25
CENTRAL 2015, Issue 2 (searched 2010 to 2015)
#1 (tens or al‐tens or tns or ens or tes):ti,ab,kw (Word variations have been searched)
#2 ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation"):ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Transcutaneous Electric Nerve Stimulation] this term only
#4 ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*"):ti,ab,kw (Word variations have been searched)
#5 ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*):ti,ab,kw (Word variations have been searched)
#6 ("transcutaneous electric*" near/4 stimulat*):ti,ab,kw (Word variations have been searched)
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Amputation] explode all trees
#9 MeSH descriptor: [Amputees] this term only
#10 (amputat* or amputee*):ti,ab,kw (Word variations have been searched)
#11 (amputat* or amputee*):ti,ab,kw (Word variations have been searched)
#12 ((phantom near/6 limb) or phantom‐limb or stump*):ti,ab,kw (Word variations have been searched)
#13 #8 or #9 or #10 or #11 or #12
#14 #7 and #13 Publication Year from 2010 to 2015
EMBASE (OVID) 2010 to 2015 March 24
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. Transcutaneous Nerve Stimulation/
4. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
5. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
6. ("transcutaneous electric*" adj4 stimulat*).mp.
7. or/1‐6
8. exp Amputation/
9. (amputat* or amputee*).mp.
10. (postamputation* or post‐amputation*).mp.
11. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
12. or/8‐11
13. 7 and 12
14. random$.tw.
15. factorial$.tw.
16. crossover$.tw.
17. cross over$.tw.
18. cross‐over$.tw.
19. placebo$.tw.
20. (doubl$ adj blind$).tw.
21. (singl$ adj blind$).tw.
22. assign$.tw.
23. allocat$.tw.
24. volunteer$.tw.
25. Crossover Procedure/
26. double‐blind procedure.tw.
27. Randomized Controlled Trial/
28. Single Blind Procedure/
29. or/14‐28
30. (animal/ or nonhuman/) not human/
31. 29 not 30
32. 13 and 31
PsycINFO (OVID) 2010 to March week 3 2015
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. Transcutaneous Nerve Stimulation/
4. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
5. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
6. ("transcutaneous electric*" adj4 stimulat*).mp.
7. or/1‐6
8. (amputat* or amputee*).mp.
9. (postamputation* or post‐amputation*).mp.
10. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
11. amputation/ or phantom limbs/
12. or/8‐11
13. 7 and 12
AMED (OVID) 2010 to March 2015
1. (tens or al‐tens or tns or ens or tes).mp.
2. ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation").mp.
3. Transcutaneous Electric Nerve Stimulation/
4. ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*").mp.
5. ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*).mp.
6. ("transcutaneous electric*" adj4 stimulat*).mp.
7. or/1‐6
8. (amputat* or amputee*).mp.
9. (postamputation* or post‐amputation*).mp.
10. ((phantom adj6 limb) or phantom‐limb or stump*).mp.
11. amputation/ or phantom limb/
12. or/8‐11
13. 7 and 12
CINAHL (EBSCO) 2010 to March 2015
S14 S7 AND S13
S13 S8 OR S9 OR S10 OR S11 OR S12
S12 ((phantom N6 limb) or phantom‐limb or stump*)
S11 (postamputation* or post‐amputation*)
S10 (amputat* or amputee*)
S9 (MH "Amputees")
S8 (MH "Amputation+")
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S6 ("transcutaneous electric*" n4 stimulat*)
S5 ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*)
S4 ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*")
S3 (MH "Transcutaneous Electric Nerve Stimulation")
S2 ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation")
S1 (tens or al‐tens or tns or ens or tes)
SPORTDiscus (EBSCO) 2010 to March 2015
S13 S7 AND S12
Limiters ‐ Published Date: 20100101‐20150331
S12 S8 OR S9 OR S10 OR S11
S11 ((phantom N6 limb) or phantom‐limb or stump*)
S10 (postamputation* or post‐amputation*)
S9 (amputat* or amputee*)
S8 DE "AMPUTEES"
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S6 ("transcutaneous electric*" n4 stimulat*)
S5 ("electric* nerve therap*" or electroanalgesi* or electro‐analgesi*)
S4 ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*")
S3 ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation")
S2 (tens or al‐tens or tns or ens or tes)
S1 DE "TRANSCUTANEOUS electrical nerve stimulation"
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carabelli 1985 | Case series |
| Finsen 1988 | Pain assessment was not a primary outcome. TENS was used with the intention of increasing peripheral vasodilation in order to decrease stump wound healing time and decrease re‐amputation rates. Randomisation was compromised by taking into account level of amputation prior to randomisation |
| Giuffrida 2010 | Report of 2 cases of contralateral TENS for phantom limb pain |
| Gyory 1977 | Case report of prosthetic socket TENS |
| Heidenreich 1988 | Case series of conservative management of phantom limb pain |
| Hirano 1988 | Case report |
| Hu 2014 | Review of case studies on acupuncture or TENS for phantom limb syndrome |
| Katz 1989 | Case report of contralateral TENS and stump skin conductance |
| Katz 1991 | Non‐randomised controlled trial of auricular TENS for phantom sensations and phantom pain |
| Kawamura 1997 | Case series of contralateral TENS for phantom limb pain |
| Lenggenhager 2014 | Review of advances in integrative therapies for pain and embodiment phantom limbs |
| Miles 1978 | Case series of electrical stimulation for phantom limb pain |
| Mulvey 2013 | Case series of TENS for phantom pain and stump pain in adult amputees |
| Mulvey 2014 | An extended analysis of excluded studies from the 2010 Cochrane systematic review of TENS for phantom pain and stump pain following amputation in adults |
| Rauck 2012 | Case report of peripheral nerve stimulation (invasive) |
| Rauck 2014 | Case series of peripheral nerve stimulation (invasive) |
| Salim 1997 | Case series of TENS for phantom limb pain |
| Sindou 1980 | Case series of TENS for neuropathic pain |
| Stolke 1978 | Case series of electrostimulation for stump and phantom limb pain |
| Winnem 1982 | Case series of TENS for phantom limb pain |
TENS: transcutaneous electrical nerve stimulation
Differences between protocol and review
There are no differences between the protocol and the review.
Contributions of authors
Writing protocol ‐ MM, A‐MB, MJ, PM.
Writing original review ‐ MM, A‐MB, MJ, PM.
Searching databases ‐ MJ, MM.
Study selection ‐ MJ, MM.
Assessment of methodological quality ‐ MJ, MM, A‐MB.
Data extraction ‐ MJ, MM.
Statistical analysis ‐ MJ, MM, A‐MB.
Writing updates ‐ MJ, MM, A‐MB.
Declarations of interest
Mark I Johnson has no conflicts of interest to declare.
Matthew R Mulvey has no conflicts of interest to declare.
Anne‐Marie Bagnall has no conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies excluded from this review
Carabelli 1985 {published data only}
- Carabelli RA, Kellerman WC. Phantom limb pain: relief by application of TENS to contralateral extremity. Archives of Physical Medicine and Rehabilitation 1985;66(7):466-7. [PMID: ] [PubMed] [Google Scholar]
Finsen 1988 {published data only}
- Finsen V, Persen L, Lovlien M, Veslegaard EK, Simensen M, Gasvann AK, et al. Transcutaneous electrical nerve stimulation after major amputation. Journal of Bone and Joint Surgery 1988;70(1):109-12. [DOI] [PubMed] [Google Scholar]
Giuffrida 2010 {published data only}
- Giuffrida O, Simpson L, Halligan PW. Contralateral stimulation, using TENS, of phantom limb pain: two confirmatory cases. Pain Medicine 2010;11(1):133-41. [DOI] [PubMed] [Google Scholar]
Gyory 1977 {published data only}
- Gyory AN, Caine DC. Electric pain control (EPC) of a painful forearm amputation stump. Medical Journal of Australia 1977;2(5):156-8. [DOI] [PubMed] [Google Scholar]
Heidenreich 1988 {published data only}
- Heidenreich EM, Hentschel R, Lange A. Experiment with the transcutaneous electric nerve stimulation for the treatment of acute and chronic conditions of pain. With 1 figure. Zeitschrift fur Physiotherapie 1988;40(6):389-96. [Google Scholar]
Hirano 1988 {published data only}
- Hirano K, Yamashiro H, Maeda N, Takeuchi T. A case of long-standing phantom limb pain: complete relief of pain. Masui. The Japanese Journal of Anesthesiology 1988;37(2):222-5. [PMID: ] [PubMed] [Google Scholar]
Hu 2014 {published data only}
- Hu X, Trevelyan E, Yang G, Lee MS, Lorenc A, Liu J, et al. The effectiveness of acupuncture or TENS for phantom limb syndrome. II: A narrative review of case studies. European Journal of Integrative Medicine 2014;6(3):365-81. [Google Scholar]
Katz 1989 {published data only}
- Katz J, France C, Melzack R. An association between phantom limb sensations and stump skin conductance during transcutaneous electrical nerve stimulation (TENS) applied to the contralateral leg: a case study. Pain 1989;36(3):367-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Katz 1991 {published data only}
- Katz J, Melzack R. Auricular transcutaneous electrical nerve stimulation (TENS) reduces phantom limb pain. Journal of Pain & Symptom Management 1991;6(2):73-83. [DOI] [PubMed] [Google Scholar]
Kawamura 1997 {published data only}
- Kawamura HIK, Yamamoto M, Yamamoto H, Yamamoto M, Ishida K, Kawakami T, et al. The transcutaneous electrical nerve stimulation applied to contralateral limbs for the phantom limb pain. Journal of Physical Therapies Science 1997;9:71-6. [Google Scholar]
Lenggenhager 2014 {published data only}
- Lenggenhager B, Arnold CA, Giummarra MJ. Phantom limbs: pain, embodiment, and scientific advances in integrative therapies. WIRES Cognitive Science 2014;5(2):221-31. [DOI] [PubMed] [Google Scholar]
Miles 1978 {published data only}
- Miles J, Lipton S. Phantom limb pain treated by electrical stimulation. Pain 1978;5(4):373-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mulvey 2013 {published data only}
- Mulvey MR, Radford HE, Fawkner HJ, Hirst L, Neumann V, Johnson MI. Transcutaneous electrical nerve stimulation for phantom pain and stump pain in adult amputees. Pain Practice 2013;13(4):289-96. [DOI] [PubMed] [Google Scholar]
Mulvey 2014 {published data only}
- Mulvey MR, Bagnall A-M, Marchant PR, Johnson MI. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults: an extended analysis of excluded studies from a Cochrane systematic review. Physical Therapy Reviews 2014;19(4):234-44. [Google Scholar]
Rauck 2012 {published data only}
- Rauck RL, Kapural L, Cohen SP, North JM, Gilmore CA, Zang RH, et al. Peripheral nerve stimulation for the treatment of postamputation pain--a case report. Pain Practice 2012;12(8):649-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rauck 2014 {published data only}
- Rauck RL, Cohen SP, Gilmore CA, North JM, Kapural L, Zang RH, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation 2014;17(2):188-97. [DOI] [PubMed] [Google Scholar]
Salim 1997 {published data only}
- Salim M. Transcutaneous electrical nerve stimulation in phantom limb pain. Alternative Therapies in Clinical Practice 1997;4(4):135-7. [Google Scholar]
Sindou 1980 {published data only}
- Sindou M, Keravel Y. Pain relief through transcutaneous electrical nerve stimulation (TENS). Results on painful neurological disorders in 180 cases (author's transl) [Analgesie par la methode d'electrostimulation transcutanee. Resultats dans les douleurs d'origine neurologique. A propos de 180 cas]. Neuro-Chirurgie 1980;26(2):153-7. [PMID: ] [PubMed] [Google Scholar]
Stolke 1978 {published data only}
- Stolke D, Winkelmuller W. Stump and phantom-limb pain in amputees: types and possibilities of treatment, with particular regard to electrostimulation. [German]. Nervenarzt 1978;49(2):116-9. [PubMed] [Google Scholar]
Winnem 1982 {published data only}
- Winnem MF, Amundsen T. Treatment of phantom limb pain with TENS. Pain 1982;12(3):299-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Avdic 2000
- Avdic D, Buljina A. TENS in the treatment of muscle spasm. Medicinski Arhiv (Sarajevo) 2000;54(1):49-51. [PubMed] [Google Scholar]
Bennett 2011
- Bennett MI, Hughes N, Johnson MI. Methodological quality in randomised controlled trials of TENS for pain: low fidelity may explain negative findings. Pain 2011;152(6):1226-232. [DOI] [PubMed] [Google Scholar]
Bjordal 2003
- Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. European Journal of Pain 2003;7:181-8. [DOI] [PubMed] [Google Scholar]
Bjordal 2007
- Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskeletal Disorders 2007;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2009
- Black LM, Persons RK, Jamieson B. Clinical inquiries. What is the best way to manage phantom limb pain? Journal of Family Practice 2009;58(3):155-8. [PubMed] [Google Scholar]
Bronfort 2004
- Bronfort G, Nilsson N, Haas M, Evans R, Goldsmith CH, Assendelft WJ. Non-invasive physical treatments for chronic/recurrent headache. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD001878. [DOI: 10.1002/14651858.CD001878.pub2] [DOI] [PubMed] [Google Scholar]
Brosseau 2003
- Brosseau L, Judd MG, Marchand S, Robinson VA, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation (TENS) for the treatment of rheumatoid arthritis in the hand. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD004377. [DOI: 10.1002/14651858.CD004377] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 1996
- Carroll D, Tramer M, McQuay H, Nye B, Moore A. Randomization is important in studies with pain outcomes: systematic review of transcutaneous electrical nerve stimulation in acute postoperative pain. British Journal of Anaesthesia 1996;77(6):798-803. [DOI] [PubMed] [Google Scholar]
Charlton 2005
- Charlton J. Core Curriculum for Professional Education in Pain. 3rd edition. Seattle: IASP Press, 2005. [Google Scholar]
Chen 2007
- Chen CC, Johnson MI, McDonough S, Cramp F. The effect of transcutaneous electrical nerve stimulation on local and distal cutaneous blood flow following a prolonged heat stimulus in healthy subjects. Clinical Physiology and Functional Imaging 2007;27(3):154-61. [DOI] [PubMed] [Google Scholar]
Cramp 2001
- Cramp AF, Noble JG, Lowe AS, Walsh DM. Transcutaneous electrical nerve stimulation (TENS): the effect of electrode placement upon cutaneous blood flow and skin temperature. Acupuncture and Electro-Therapeutics Research 2001;26(1-2):25-37. [DOI] [PubMed] [Google Scholar]
Dowswell 2009
- Dowswell T, Bedwell C, Lavender T, Neilson JP. Transcutaneous electrical nerve stimulation (TENS) for pain relief in labour. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007214. [DOI: 10.1002/14651858.CD007214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ephraim 2005
- Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Archives of Physical Medicine and Rehabilitation 2005;86(10):1910-9. [DOI] [PubMed] [Google Scholar]
Flor 2002
- Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurology 2002;1(3):182-9. [DOI] [PubMed] [Google Scholar]
Garrison 1994
- Garrison DW, Foreman RD. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS). Pain 1994;58(3):309-15. [DOI] [PubMed] [Google Scholar]
Garrison 1996
- Garrison DW, Foreman RD. Effects of transcutaneous electrical nerve stimulation (TENS) on spontaneous and noxiously evoked dorsal horn cell activity in cats with transected spinal cords. Neuroscience Letters 1996;216(2):125-8. [DOI] [PubMed] [Google Scholar]
Halbert 2002
- Halbert J, Crotty M, Cameron ID. Evidence for the optimal management of acute and chronic phantom pain: a systematic review. Clinical Journal of Pain 2002;18(2):84-92. [DOI] [PubMed] [Google Scholar]
Hanling 2010
- Hanling SR, Wallace SC, Hollenbeck KJ, Belnap BD, Tulis MR. Preamputation mirror therapy may prevent development of phantom limb pain: a case series. Anesthesia & Analgesia 2010;110(2):611-4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Houghton 2010
- Houghton P, Nussbaum E, Hoens A. Electrophysical agents - Contraindications and precautions: an evidence-based approach to clinical decision making in physical therapy. Physiotherapy Canada 2010;62(5):1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurlow 2012
- Hurlow A, Bennett MI, Robb KA, Johnson MI, Simpson KH, Oxberry SG. Transcutaneous electric nerve stimulation (TENS) for cancer pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD006276. [DOI: 10.1002/14651858.CD006276.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2006
- Jensen T, Nikolajsen L. Phantom pain and other phenomena after amputation. In: Wall PD, Melzack R, editors(s). Textbook of Pain. 5th edition. London: Churchill Livingstone, 2006:799-814. [Google Scholar]
Johnson 2007
- Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain 2007;130(1-2):157-65. [DOI] [PubMed] [Google Scholar]
Johnson 2008
- Johnson MI. Transcutaneous electrical nerve stimulation. In: Watson T, editors(s). Electrotherapy: Evidence Based Practice. London: Churchill Livingstone, 2008:253-96. [Google Scholar]
Johnson 2014
- Johnson MI. Transcutaneous Electrical Nerve Stimulation (TENS). Research to Support Clinical Practice. 1st edition. Oxford, UK: Oxford University Press, 2014. [Google Scholar]
Khadilkar 2008
- Khadilkar A, Milne S, Brosseau L, Robinson V, Saginur M, Shea B. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003008. [DOI: 10.1002/14651858.CD003008.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroeling 2013
- Kroeling P, Gross A, Graham N, Burnie SJ, Szeto G, Goldsmith CH, et al. Electrotherapy for neck pain. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004251. [DOI: 10.1002/14651858.CD004251.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikolajsen 2001
- Nikolajsen L, Jensen TS. Phantom limb pain. British Journal of Anaesthesia 2001;87(1):107-16. [DOI] [PubMed] [Google Scholar]
Nnoaham 2008
- Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD003222. [DOI: 10.1002/14651858.CD003222] [DOI] [PubMed] [Google Scholar]
Price 2000
- Price CI, Pandyan AD. Electrical stimulation for preventing and treating post-stroke shoulder pain. Cochrane Database of Systematic Reviews 2000, Issue 4. Art. No: CD001698. [DOI: 10.1002/14651858.CD001698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2002
- Proctor ML, Smith CA, Farquhar CM, Stones RW. Transcutaneous electrical nerve stimulation and acupuncture for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD002123. [DOI: 10.1002/14651858.CD002123] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutjes 2009
- Rutjes AW, Nuesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD002823. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sdrulla 2015
- Sdrulla AD, Xu Q, He SQ, Tiwari V, Yang F, Zhang C, et al. Electrical stimulation of low-threshold afferent fibers induces a prolonged synaptic depression in lamina II dorsal horn neurons to high-threshold afferent inputs in mice. Pain 2015;156(6):1008-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 1994
- Sherman RA. Phantom limb pain. Mechanism-based management. Clinics in Podiatric Medicine and Surgery 1994;11(1):85-106. [PubMed] [Google Scholar]
Sindrup 1999
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain 1999;83(3):389-400. [DOI] [PubMed] [Google Scholar]
Sluka 2013
- Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Physical Therapy 2013;93(10):1397-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1999
- Smith DG, Ehde DM, Legro MW, Reiber GE, Aguila M, Boone DA. Phantom limb, residual limb, and back pain after lower extremity amputations. Clinical Orthopaedics and Related Research 1999;361:29-38. [DOI] [PubMed] [Google Scholar]
Thorsteinsson 1977
- Thorsteinsson G, Stonnington HH, Stillwell GK, Elveback LR. Transcutaneous electrical stimulation: a double-blind trial of its efficacy for pain. Archives of Physical Medicine and Rehabilitation 1977;58(1):8-13. [PubMed] [Google Scholar]
Vance 2014
- Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Management 2014;4(3):197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1997
- Walsh D. TENS. Clinical Application and Related Theory. London: Churchill Livingstone, 1997. [Google Scholar]
Walsh 2009
- Walsh DM, Howe TE, Johnson MI, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD006142. [DOI: 10.1002/14651858.CD006142.pub2] [DOI] [PubMed] [Google Scholar]
Wartan 1997
- Wartan SW, Hamann W, Wedley JR, McColl I. Phantom pain and sensation among British veteran amputees. British Journal of Anaesthesiology 1997;78(6):652-9. [DOI] [PubMed] [Google Scholar]
Wiffen 2006
- Wiffen P, Meynadier J, Dubois M, Thurel C, deSmet J, Harden RN. Diagnostic and treatment issues in postamputation pain after landmine injury. Pain Medicine 2006;7 Suppl 2:209-12. [DOI] [PubMed] [Google Scholar]
Wilson 2008
- Wilson JA, Nimmo AF, Fleetwood-Walker SM, Colvin LA. A randomised double blind trial of the effect of pre-emptive epidural ketamine on persistent pain after lower limb amputation. Pain 2008;135(1-2):108-18. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mulvey 2010
- Mulvey MR, Bagnall AM, Johnson MI, Marchant PR. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD007264. [DOI: 10.1002/14651858.CD007264.pub2] [DOI] [PubMed] [Google Scholar]


