Abstract
Introduction:
The black-legged tick, Ixodes scapularis (I scapularis), is now recognized as the deadliest tick vector in the United States. The Upper Midwest, particularly Wisconsin and Minnesota, are endemic to a diversity of tick-transmitted infectious diseases. Although Borrelia burgdorferi, the agent of Lyme disease, still accounts for the majority of diagnosed infections, I scapularis is known to transmit other bacterial, viral, and parasitic agents.
Objective:
To provide an overview of the array of pathogenic microorganisms carried by I scapularis ticks in the Upper Midwest.
Methods:
A literature review was conducted to collect and analyze current information about I scapularis lifestyle, transmission, microorganisms carried by the arthropod vector, and the diseases that occur as a result of infections with these microorganisms in the Upper Midwest.
Results:
Diagnosis of co-infection from tick-borne zoonosis in humans has increased over the last 2 decades. Since I scapularis can transmit multiple pathogens, it is clinically important because different diagnostic testing and treatment strategies may need to be implemented for a patient with I scapularis-borne infection(s).
Conclusions:
This review has concentrated on I scapularis-transmitted diseases affecting the Upper Midwest and has explored the ecology of the I scapularis vector and its role in pathogen transmission.
INTRODUCTION
In 1982, the black-legged tick, Ixodes scapularis (I scapularis) was recognized as the vector for transmission of Borrelia burgdorferi (B burgdorferi), the causative agent of Lyme disease.1 Lyme disease is the most frequently reported vector-borne infection in the United States, with more than 300,000 estimated cases occurring in the United States.2 Besides B burgdorferi, I scapularis also can transmit Anaplasma phagocytophilum (human granulocytic anaplasmosis), Borrelia miyamotoi (B miyamotoi disease), Borrelia mayonii (Lyme disease), Babesia microti (babesiosis), Ehrlichia muris subsp eauclairensis (ehrlichiosis), and Powassan virus/deer tick virus (viral encephalitis).3–5
Human disease caused by I scapularis-transmitted pathogens correlates with vector abundance and pathogen endemicity. Vector transmission is spreading beyond known endemic areas due to climate change and the dispersion from source populations by hosts.6,7 Pathogen hosts acquire, maintain, and transmit pathogens, spreading them to naïve and already infected tick populations.8 The growing prevalence of host co-infection in endemic areas allows for black-legged tick-based transmission of multiple pathogens to susceptible populations. The risk of human infection with I scapularis-borne pathogens depends on complex factors, including the tick distribution in an ecosystem, reforestation, the attachment time of ticks on humans, and the prevalence of pathogens in ticks and reservoir hosts.6,8,9
DISTRIBUTION OF I SCAPULARIS
I scapularis is endemic to parts of the Midwest, Northeast, West, Southeast, and Southern United States, and its distribution is correlated with complex ecological factors. The availability of mammalian hosts, landscape, vegetation, and climate indices are all linked to where the vector can survive and thrive. The most important host for black-legged tick survival and reproduction is the white-tailed deer (Odocoileus virginianus). Approximately 50% to 95% of the adult female ticks feed on white-tailed deer.9,10 Deer are the primary host of mating ticks, supporting high populations of black-legged ticks.10
Although white-tailed deer can be hosts, the majority of larvae and nymphs feed on small vertebrate mammals. The most abundant and important host for all I scapularis-acquired pathogens is the white-footed mouse, Peromyscus leucopus (P leucopus).11 While P leucopus eradication efforts have led to decreased vector abundance, mouse populations are not linked to I scapularis survival in all habitats.12,13
An abundance of size-appropriate vertebrate mammals is a critical factor in establishing a tick population, but an appropriate landscape and vegetation also are needed for survival. Since the late 19th century, repurposing of landscapes from farmland to forest led to more land suitable for black-legged tick establishment.13 These second-growth forests (forests re-grown after a timber harvest) have created appropriate “edge” habitats for black-legged ticks and hosts, resulting in increased tick populations.10 The type of forest associated with I scapularis can vary; however, second-growth forests are the most suitable for black-legged ticks. Leaf litter maintains moisture while providing cover that is important for I scapularis survival. Blacklegged tick populations can be reduced 72% to 100% by removal of leaf litter.10 During active life stages, I scapularis is most abundant in woods but also can be found in shaded areas of lawn. Black-legged ticks do not survive in strictly agricultural cropland, and the repurposing of even small tracts of farmland to deciduous forest can foster I scapularis proliferation and maintain a transmission cycle of pathogens.14
Besides the landscape, climate factors—particularly high relative humidity—can increase I scapularis survival. Moisture availability, specifically > 82% relative humidity in leaf litter, increases I scapularis activity and abundance and is a limiting factor to survival if the leaf litter cannot retain enough moisture.15 Temperature is also important in areas with I scapularis. As climate change trends warmer, higher temperatures would allow immature black-legged tick larvae more opportunities for feeding and increase the probability of becoming a mating adult in regions once inhospitable for black-legged ticks.16
LIFE CYCLE OF I SCAPULARIS
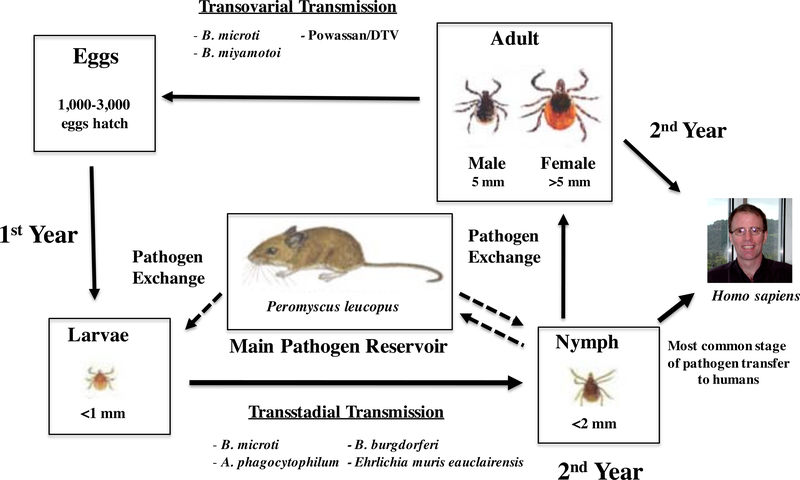
I scapularis has a 2-year life cycle and is a 3-host tick, taking a blood meal from different size-appropriate hosts to transition from larva to nymph and nymph to adult life stages (Figure). This feeding allows ticks to acquire and transmit pathogens between a variety of hosts, increasing the opportunities for transmission and pathogen maintenance.17 Larvae feed in the summer after hatching, nymphs feed in the late spring or summer of the second year, and adults feed in the fall. Larvae and nymph-stage ticks are known to feed on small animals and become infected by larvae and nymphs.12,17
Figure.
Stages of Tick Development and Pathogen Transfer
P leucopus is the main reservoir host of all I scapularis pathogens in the environment.11,12 Mice can become infected from larvae or nymphs, depending on the pathogen transmitted (Figure). For example, B burgdorferi passes only transstadially and not transovarially in tick vectors. Transstadially transmitted pathogens can pass from the larval to the adult stage, while transovarially transmitted pathogens can pass from the adult female to the eggs. P leucopus has bacteremia without symptomatic infection, allowing for efficient and sustainable transmission of pathogens to Ixodes vectors.11
Nymphs are the most infectious vector stage to humans as they are more likely to carry pathogens and less likely to be observed than adults. Adult ticks feed on white-tailed deer or other large mammalian hosts, including humans. Deer and humans are deadend hosts because the microbes are not further transmitted. While deer are dead-end hosts, they allow increased distribution and survival of black-legged ticks.10,18
Nymphs molt into adults during the fall of the second year after which their sex can be first differentiated. I scapularis are hard-bodied and adults are dark brown to black in color. At the adult stage, the female body becomes orange-red as opposed to the larger grey to black color of the male. Once they become adults, males may attach to deer seeking female mates. Only female ticks take significant blood meals – mainly from white-tailed deer – acquiring and transmitting disease. Reproduction between adult male and female ticks occurs on white-tailed deer or in vegetation.10,19 Male black-legged ticks die after copulation. Females release 1000 to 3000 eggs in the leaf litter, typically in late May, and die soon afterwards.10
ACQUISITION AND TRANSMISSION OF PATHOGENS BY I SCAPULARIS
The small size of black-legged tick nymphs, the potential to carry pathogens, and the ability to feed on larger hosts, make nymphs the most probable tick life cycle stage for pathogen transmission to humans.17,19 Nymphs and adults use their front legs to attach to a host. After attachment, the tick finds a feeding spot and takes a blood meal. Pathogen acquisition and transmission is due to saliva transfer and gut regurgitation. Ticks can acquire and transmit pathogens consecutively or simultaneously over the course of their lives.20
Adult I scapularis ticks have the highest level of multiple pathogen infections, in part because they feed on 2 hosts.21 Coprevalence of 2 or more pathogens was observed in 26% of black-legged ticks from Westchester, New York.22 Pathogen carriage by ticks in the Upper Midwest includes B burgdorferi as the most common (26%), followed by A phagocytophilum (8.9%), B miyamotoi (7.1%), B microti (5%), and Powassan/deer tick virus (POWV/DTV) 2.9%).23–26
Pathogen transmission from tick to host depends on the microbe, the tick attachment time, and the tick’s infectious status. Infection risk after a single tick bite is low (1.3%−3.0%), even in endemic areas.27 In animal models, B burgdorferi transmission exponentially increased after 72 hours, correlating with an 18% to 25% incidence of Lyme disease.27 Transmission of A phagocytophilum and B microti take 12 to 24 hours and 36 to 48 hours,28 respectively. B miyamotoi is transmitted in approximately 24 hours,29 whereas POWV/DTV can transfer in as little as 15 minutes.30 B mayonii transmission times are similar to B burgdorferi. Little research has been done on transmission times for E muris ssp eauclairensi (EME) bacteria. Acquisition and transmission of pathogens does not seem to be affected by the prevalence of multiple pathogens.20 Co-infection of I scapularis-transmitted pathogens in humans is becoming a prominent clinical concern, making the symptoms, diagnostics, and treatment of each pathogen infection critical.
B burgdorferi (Lyme Disease)
Lyme disease was first recognized in 1975 in Lyme, Connecticut. William Burgdorfer isolated and linked the etiological agent, named B burgdorferi, to I scapularis ticks.1 Lyme disease is the most common black-legged tick-associated illness.2 Around 70% to 80% of patients will exhibit a “bulls-eye” pattern rash—known as erythema chronicum migrans—about 7 days post-exposure (Table).31 Fever, headaches, myalgia, stiff neck, arthralgia, and lymphadenopathy are often seen during early infection.28,31 The bacteria may also spread to regional lymph nodes, the brain, and the heart.32 In the last stage of Lyme disease, the spirochetes can penetrate tight joint spaces, causing arthritis in about 10% of patients.32
Table 1.
Symptoms, Diagnosis, and Treatment of Ixodes scapularis Tick-borne Diseases
| Organism | Symptoms | Treatment | |
|---|---|---|---|
| B. burgdorferi | - Myalgia | - Arthralgia | - 100 mg doxycycline b.i.d. for 10-21 days or amoxicillin t.i.d. for 10–21 days if doxycycline is contraindicated |
| - Fever | - Headache | ||
| B. mayonii | - Myalgia | - Arthralgia | - 100 mg doxycycline 2 times/day for 10–21 days |
| - High fever | - Headache | ||
| B. miyamotoi | - Myalgia | - Arthralgia | - 100 mg doxycycline 2 times/day for 10–21 days |
| - Chills | - Headache | ||
| - High fever (potentially relapsing) | |||
| A. phagocytophilum | - Myalgia | - Fever | - 100 mg doxycycline 2 times/day for 10–21 days |
| - Arthralgia | - Headache | ||
| Ehrlichia muris-like | - Fever | - Fatigue | - 100 mg doxycycline 2 times/day for 10–21 days |
| - Headache | - Malaise | ||
| - Nausea | - Vomiting | ||
| Babesia microti | - Malaise | - Myalgia | - Combination of atovaquone plus azithromycin or clindamycin plus quinine for 7–10 days |
| - Fatigue | - Fever | ||
| Powassan virus/DTV | - Fever | - Lethargy | - Supportive care including hydration therapy and pain management |
| - Rash | - Myalgia | ||
| - Stiff neck | - Headache | ||
B miyamotoi Disease
Another tickborne Borrelia pathogen is B miyamotoi. The first US patient infected was identified in 2013. The organism has been isolated from numerous Ixodes tick species in North America.33 Infection rates vary greatly in the United States by region and locality, with a ~12% prevalence of B miyamotoi from ticks in Indiana and a 0% to 6.8% prevalence on the East Coast.34 Unlike B burgdorferi, this pathogen is able to pass transovarially in the tick (Figure).
Patients with B miyamotoi disease have muscle aches, fever, and headache similar to tick-borne relapsing fever symptoms, but Lyme disease symptoms including erythema migrans, facial palsy, and arthritis are uncommon.35 B miyamotoi is part of the relapsing fever group of Borrelia (unlike B burgdorferi), with multiple cycles of high fever, chills, marked headache, and myalgia or arthralgia. Blood tests will show elevated liver enzyme levels, neutropenia, and thrombocytopenia indicative of a shock-like condition.
Borrelia mayonii Disease
Borrelia mayonii is the most recent tick-borne pathogen identified in the Upper Midwest regions of the United States, remaining centered in Wisconsin and Minnesota.5,36 Eventual identification occurred in 2016 after irregular polymerase chain reaction results were seen from blood and synovial fluid samples at the Mayo Clinic in Rochester, Minnesota.37 Of 100,545 clinical samples tested during 2003–2014, six specimens received after 2012 yielded melting temperature results outside normal limits for other Borrelia species. Furthermore, the species was not detected in specimens tested from states in the Northeast. B mayonii was found to have 93.83% sequence homology to B burgdorferi.38
The newly identified species is transmitted by I scapularis ticks, eliciting a Lyme-like borreliosis in humans.38 Some studies have confirmed that white-footed mice and the American red squirrel serve as B mayonii hosts.39,40 Given the absence of B mayonii disease in the Northeast, where equally suitable tick vectors and potential reservoirs are abundant,36,40 other factors besides tick vectors and animal reservoirs may be in play.
B mayonii infected patients have unique manifestations of disease compared to traditional Lyme disease.5,37 B mayonii causes 1 to 3 cases of Lyme-like disease within Minnesota per year.30,41 Clinical presentation in infected patients is largely congruent with Lyme disease: myalgia, headache, arthralgia, neck pain, and fatigue (Table).38 The familiar “bulls-eye” erythema migrans rash is sometimes associated with B mayonii disease, but more frequently there is a diffuse unconventional rash over a wider area.5,37 B mayonii infections may be differentiated based on acute onset unusually high fever, as well as nausea and vomiting not seen in other Borrelia-induced illness.41 Markers such as thrombocytopenia, lymphopenia, and increased levels of hepatic enzymes also have been seen.30
A phagocytophilum (Human Granulocytic Anaplasmosis)
A phagocytophilum causes human granulocytic anaplasmosis, first identified in Minnesota and Wisconsin. In Wisconsin, seroepide-miological data suggest that 15% of the population was infected.42 Human granulocytic anaplasmosis (HGA) causes disease mainly in older patients (median age 50–60 years).43 The case-fatality rate of HGA is <1% in the United States. Human granulocytic anaplasmosis cases have increased every year. According to the Centers for Disease Control and Prevention, there were 1,761 cases of HGA in the United States in 2010.43 Originally, A phagocytophilum was named the human granulocytic ehrlichiosis (HGE) agent. The formerly named HGE agent, along with the ruminant and equine pathogens, E phagocytophila and E equi, are now called A phagocytophilum.42 A phagocytophilum invades granulocytes, mainly neutrophils, creating vacuoles of replicating bacteria called morulae.44
Humans infected with A phagocytophilum display fever (89%), headache (82%), and fatigue (84%). (See Table.) Myalgia, chills, and shaking are also common symptoms. Nausea, arthralgia, vomiting, abdominal pain, and cough are less common symptoms of HGA. Severe symptoms may include hemorrhage, renal failure, or neurologic problems.45
Ehrlichia muris eauclairensis (Ehrlichiosis)
Until recently, only E chaffeensis and E ewingii were thought to cause ehrlichiosis in humans in the United States. In 2009, a novel pathogen was recognized in patients from Wisconsin and Minnesota. Genetic analyses revealed that this new Ehrlichia species is closely related to E muris. Formerly called Ehrlichia murislike, EME infection produces symptoms closely resembling those observed in A phagocytophilum and E chaffeensis infections. Males are infected more often than females (1.7 to 1), and the average patient age is 61 years. In 2014, there were 12 confirmed cases of EME in Wisconsin, bringing the total to 39 confirmed cases in Wisconsin since 2009.46 I scapularis ticks found in Minnesota and Wisconsin appear to be the only vector, since ticks from other states were negative.
The most common symptoms of EME infection include fever, malaise, fatigue, headache, nausea, and vomiting (Table). A rash is not commonly reported with EME infections compared to E chaffeensis infections. Clinical laboratory findings in patients include increased liver enzyme levels, thrombocytopenia, and reduced numbers of lymphocytes.47
Babesia microti (Babesiosis)
The apicomplexan parasite B microti is an emerging zoonotic intraerythrocytic organism of humans. The majority of cases (97%) come from 5 Northeastern states, Minnesota, and Wisconsin. Of 1,762 reported cases in 2013, 57% of patients were 60 years or older.43 Human disease is usually self-limiting, and most patients recover without treatment. Immunosuppressed (especially asplenic) patients, as well as patients with co-infection, are at risk for symptomatic disease. The mortality rate for clinically apparent infections is 5% in the United States.4 Babesia species may be transmitted transovarially, as well as transstadially in the tick (Figure).
Human babesiosis has a broad spectrum of symptoms dependent on the level of parasitemia. Low level parasitemia is more typical and often self-limiting. However, a high level of parasitemia may lead to a fulminating malaria-like infection characterized by malaise, chills, myalgia, anemia, fatigue, and fever that is more often observed in asplenic patients (Table).4
Powassan/Deer Tick Virus (Viral Encephalitis)
Deer tick virus, a variant of Powassan virus, is a positive-sense RNA Flavivirus. The name deer tick virus (DTV) has been established recently to differentiate this distinct Powassan virus lineage found only in I scapularis from the prototypical Powassan virus lineage found in I cookei, also known as the ground hog tick.48 Genetic sequencing can separate the 2 lineages. Disease caused by this arbovirus is very rare but is often fatal in those who are symptomatic. Currently, there has been only 1 case of sequence-confirmed DTV in Minnesota. Wisconsin has had 3 cases of confirmed POWV/DTV, in 2003, 2006, and 2007, respectively.49
A POWV/DTV infection may include a fever with headache, myalgia, arthralgia, and possible accompaniment of skin rash, lymphadenopathy, or central nervous system disease (meningitis or encephalitis) (Table). Severe symptoms that begin 8 to 34 days after POWV/DTV infection include respiratory distress, tremors, seizures, paralysis, and coma.48 Most symptomatic patients with POWV/DTV infection develop meningoencephalitis.50
TREATMENT OF BLACK-LEGGED TICKBORNE DISEASES
Patients with Lyme disease, B mayonii disease, anaplasmosis or ehrlichiosis are typically treated with 100 mg of doxycycline twice a day for 10 to 21 days (Table).43 Babesiosis is treated with a combination of atovaquone plus azithromycin or clindamycin plus quinine for 7 to 10 days, and supportive care with hydration is used for POWV/DTV infections.
CONCLUSION
Multiple tick-borne infections from the tick vector I scapularis are endemic in the Upper Midwest and can present as undifferentiated febrile illness. Age, immunocompetence, and co-infection play important roles in disease severity. Patients with underlying medical conditions that require immunosuppressive medication have an increased likelihood of opportunistic and novel infection that can be lethal. The diversity of pathogens carried by I scapularis found in the Upper Midwest makes it imperative that a proper diagnosis followed by treatment is carried out.
Acknowledgement:
The authors wish to thank Bobbi Pritt for critical reading of the manuscript.
Funding/Support: Dr Schwan was supported by a grant AI065432 from the National Institutes of Health.
Footnotes
Financial Disclosures: None declared.
REFERENCES
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis?. Science. 1982;216(4552):1317–1319. doi: 10.1126/science.7043737 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. Surveillance for Lyme disease - United States, 2008–2015. MMWR Surveill Summ. 2017;66(22):1–12. doi: 10.15585/mmwr.ss6622a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gugliotta J, Goethert H, Berardi V, Telford SR III. Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368(3):240–245. doi: 10.1056/NEJMoa1209039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homer MJ, Aguilar-Delfin I, Telford SR 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13(3):451–469. doi: 10.1128/cmr.13.3.451-469.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritt BS, Respicio-Kingry LB, Sloan LM, et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol. 2016;66(11):4878–4880. doi: 10.1099/ijsem.0.001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownstein JS, Holford TR, Fish D. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ Health Perspect. 2003;111(9):1152–1157. doi: 10.1289/ehp.6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Glowacki MN, Hoet AE, et al. Emergence of Ixodes scapularis and Borrelia burgdorferi, the Lyme disease vector and agent, in Ohio. Front Cell Infect Microbiol. 2014;4:70. doi: 10.3389/fcimb.2014.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walk ST, Xu G, Stull JW, Rich SM. Correlation between tick density and pathogen endemicity, New Hampshire. Emerg Infect Dis. 2009;15(4):585–587. doi: 10.3201/eid1504.080940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy DC, Campbell SR, Clark D, DiMotta C, Gurney S. Ixodes scapularis (Acari: Ixodidae) deer tick mesoscale populations in natural areas: effects of deer, area, and location. J Med Entomol. 1994;31(1):152–158. doi: 10.1093/jmedent/31.1.152 [DOI] [PubMed] [Google Scholar]
- 10.Stafford KC III, Williams SC. Deer, ticks, and Lyme disease: deer management as a strategy for the reduction of Lyme disease. 2014. The Connecticut Agricultural Experiment Station. Accessed August 1, 2019 https://www.beaconfalls-ct.org/sites/beaconfallsct/files/uploads/deer_ticks_fact_sheet.pdf. [Google Scholar]
- 11.Hersh MH, LaDeau SL, Previtali MA, Ostfeld RS. When is a parasite not a parasite? Effects of larval tick burdens on white-footed mouse survival. Ecology. 2014;95(5):1360–1369. doi: 10.1890/12-2156.1 [DOI] [PubMed] [Google Scholar]
- 12.Lindsay LR, Mathison SW, Barker IK, McEwen SA, Surgeoner GA. Abundance of Ixodes scapularis (Acari: Ixodidae) larvae and nymphs in relation to host density and habitat on Long Point, Ontario. J Med Entomol. 1999;36(3):243–254. doi: 10.1093/jmedent/36.3.243 [DOI] [PubMed] [Google Scholar]
- 13.Guerra M, Walker E, Jones C, et al. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north central United States. Emerg Infect Dis. 2002;8(3):289–297. doi: 10.3201/eid0803.010166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray JS, Kahl O, Lane RS, Stanek G, eds. Lyme Borreliosis: Biology, Epidemiology, and Control. CABI Publishing; 2002. [Google Scholar]
- 15.Berger KA, Ginsberg HS, Gonzalez L, Mather TN. Relative humidity and activity patterns of Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2014;51(4):769–776. doi: 10.1603/me13186 [DOI] [PubMed] [Google Scholar]
- 16.Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, Wu J. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ Health Perspect. 2014;122(6):631–638. doi: 10.1289/ehp.1307799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10(2):87–99. doi: 10.1038/nrmicro2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilpatrick HJ, LaBonte AM, Stafford KC. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. J Med Entomol. 2014;51(4):777–784. doi: 10.1603/me13232 [DOI] [PubMed] [Google Scholar]
- 19.Zung JL, Lewengrub S, Rudzinska MA, Spielman A, Telford SR, Piesman J. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can J Zoo. 1989; 67(7):1737–1748. doi: 10.1139/z89-249 [DOI] [Google Scholar]
- 20.Levin ML, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. 2000;68(4):2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerg Infect Dis. 1998;4(1):97–99. doi: 10.3201/eid0401.980113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J Med Entomol. 2014;51(1):226–236. doi: 10.1603/me13101 [DOI] [PubMed] [Google Scholar]
- 23.Han S, Hickling GJ, Tsao JI. High prevalence of Borrelia miyamotoi among adult blacklegged ticks from white-tailed deer. Emerg Infect Dis. 2016;22(2):316–318. doi: 10.3201/eid2202.151218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knox KK, Thomm AM, Harrington YA, Ketter E, Patitucci JM, Carrigan DR. Powassan/deer tick virus and Borrelia burgdorferi infection in Wisconsin tick populations. Vector Borne Zoonotic Dis. 2017;17(7):463–466. doi: 10.1089/vbz.2016.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalski TJ, Jobe DA, Dolan EC, Kessler A, Lovrich SD, Callister SM. The emergence of clinically relevant Babesiosis in southwestern Wisconsin. WMJ. 2015;114(4):152–157. [PubMed] [Google Scholar]
- 26.Murphy DS, Lee X, Larson SR, Johnson DK, Loo T, Paskewitz SM. Prevalence and distribution of human and tick infections with the Ehrlichia muris-like agent and Anaplasma phagocytophilum in Wisconsin, 2009–2015. Vector Borne Zoonotic Dis. 2017;17(4):229–236. doi: 10.1089/vbz.2016.2055 [DOI] [PubMed] [Google Scholar]
- 27.Costello CM, Steere AC, Pinkerton RE, Feder HM Jr. A prospective study of tick bites in an endemic area for Lyme disease. J Infect Dis. 1989;159(1):136–139. doi: 10.1093/infdis/159.1.136 [DOI] [PubMed] [Google Scholar]
- 28.Biesiada G, Czepiel J, Leśniak MR, Garlicki A, Mach T. Lyme disease: review. Arch Med Sci. 2012;8(6):978–982. doi: 10.5114/aoms.2012.30948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisen L Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis. 2018;9(3):535–542. doi: 10.1016/j.ttbdis.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg. 2004;71(3):268–271. doi: 10.4269/ajtmh.2004.71.3.0700268 [DOI] [PubMed] [Google Scholar]
- 31.Marques AR. Laboratory diagnosis of Lyme disease: advances and challenges. Infect Dis Clin North Am. 2015;29(2):295–307. doi: 10.1016/j.idc.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halperin JJ. Chronic Lyme disease: misconceptions and challenges for patient management. Infect Drug Resist. 2015;8:119–128. doi: 10.2147/IDR.S66739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17(10):1816–1823. doi: 10.3201/eid1710.101474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowder CD, Carolan HE, Rounds MA, et al. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg Infect Dis. 2014;20(10):1678–1682. doi: 10.3201/eid2010.131583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdri HR, Gugliotta JL, Berardi VP, et al. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med. 2013;159(1):21–27. doi: 10.7326/0003-4819-159-1-201307020-00005 [DOI] [PubMed] [Google Scholar]
- 36.Eisen L, Breuner NE, Hojgaard A, et al. Comparison of vector efficiency of Ixodes scapularis (Acari: Ixodidae) from the Northeast and upper Midwest of the United States for the Lyme disease spirochete Borrelia mayonii. J Med Entomol. 2017; 54(1):239–242. doi: 10.1093/jme/tjw [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritt BS, Mead PS, Johnson DKH, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016; 16(5):556–564. doi: 10.1016/S1473-3099(15)00464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kingry LC, Batra D, Replogle A, Rowe LA, Pritt BS, Petersen JM. Whole genome sequence and comparative genomics of the novel Lyme borreliosis causing pathogen, Borrelia mayonii. PLoS One. 2016;11(12):e0168994. doi: 10.1371/journal.pone.0168994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolan MC, Breuner NE, Hojgaard A, et al. Duration of Borrelia mayonii infectivity in an experimental mouse model for feeding Ixodes scapularis larvae. Ticks Tick Borne Dis. 2017;8(1):196–200. doi: 10.1016/j.ttbdis.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 40.Johnson TL, Graham CB, Hojgaard A, et al. Isolation of the Lyme disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J Med Entomol. 2017; 54(4):1088–1092. doi: 10.1093/jme/tjx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingry LC, Anacker M, Pritt B, Bjork J, Respicio-Kingry L, Liu G, Sheldon S, Boxrud D, Strain A, Oatman S, Berry J, Sloan L, Mead P, Neitzel D, Kugeler KJ, Petersen JM. Surveillance for and discovery of Borrelia species in US patients suspected of tickborne illness. Clin Infect Dis. 2018; 66(12):1864–1871. doi: 10.1093/cid/cix1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11(12):1828–1834. doi: 10.3201/eid1112.050898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anaplasmosis. Centers for Disease Control and Prevention website. Reviewed February 19, 2019. Accessed August 1, 2019 https://www.cdc.gov/anaplasmosis/stats/index.html
- 44.Rikihisa Y Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24(3):469–489. doi: 10.1128/CMR.00064-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther. 2009;7(6):709–722. doi: 10.1586/eri.09.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson DK, Schiffman EK, Davis JP, Neitzel DF, Sloan LM, Nicholson WL, Fritsche TR, Steward CR, Ray JA, Miller TK, Feist MA, Uphoff TS, Franson JJ, Livermore AL, Deedon AK, Theel ES Pritt BS. Human infection with Ehrlichia muris-like pathogen, United States, 2007–2013. Emerg Infect Dis. 2015; 21(10):1794–1799. doi: 10.3201/eid2110.150143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365(5):422–429. doi: 10.1056/NEJMoa1010493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Khoury MY, Hull RC, Bryant PW, et al. Diagnosis of acute deer tick virus encephalitis. Clin Infect Dis. 2013;56(4):e40–e47. doi: 10.1093/cid/cis938 [DOI] [PubMed] [Google Scholar]
- 49.Johnson DK, Staples JE, Sotir MJ, Warshauer DM, Davis JP. Tickborne Powassan virus infections among Wisconsin residents. WMJ. 2010;109(2):91–97. [PubMed] [Google Scholar]
- 50.Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol. 2012;50(3):903–908. doi: 10.1128/JCM.05848-11 [DOI] [PMC free article] [PubMed] [Google Scholar]