Abstract
Combining biological and small-molecule catalysts under a chemoenzymatic manifold presents a series of significant advantages to the synthetic community. We report herein the successful development of a two-step/single flask synthesis of γ-lactones through the merger of Umpolung catalysis with a ketoreductase-catalyzed dynamic kinetic resolution, reduction, and cyclization. This combined approach delivers highly enantio- and diastereoenriched heterocycles and demonstrates the feasibility of integrating NHC catalysis with enzymatic processes.
Keywords: chemoenzymatic catalysis, sequential catalysis, ketoreductase enzyme, N-heterocyclic carbene catalysis, photoredox catalysis
Graphical Abstract
Herein we report the development of a two-step-one-pot synthesis of γ-lactones through the unification of a robust NHC-catalyzed Stetter reaction with a ketoreductase-catalyzed dynamic kinetic resolution, reduction, and cyclization. The process sets two stereogenic centers and provides rapid access to densely-functionalized privileged heterocycles.

Natural biological systems utilize a wide variety of chemical reactions catalyzed by enzymes. Poly-enzyme cascades harmoniously exchange substrates, sequestering otherwise incompatible chemical transformations to permit the synthesis of complex molecular scaffolds from simple building blocks.[1] Seeking to mimic these advantages, synthetic chemists have devised multi-catalyst systems to conduct cooperative, domino, and tandem catalytic processes that, ideally, shepherd a substrate through multiple chemical transformations.[2] These reactions are often challenging to develop, as conditions amenable for all catalysts and transformations may be elusive and/or fully incompatible.[3] Such a challenge is especially apparent in the case of paired chemical and enzymatic systems, since traditional reaction conditions suitable for small-molecule catalysts are characteristically incompatible with enzymes (e.g., solvent, pH, temperature). The challenges inherent in designing such processes notwithstanding, these systems remain attractive for reasons beyond the powerful bond transformations they enable, such as reduced intermediate purification and isolation requirements, decreased operation and production costs, and diminished waste generation.[3a]
Previous studies to merge chemical and biological catalysts have traditionally focused on chemoenzymatic dynamic kinetic resolutions (DKRs) of racemic alcohols and amines.[4] Only recently have examples of merging organocatalysis, transitionmetal catalysis, and photocatalysis with enzymatic catalysis and cascades emerged.[5] Recent work from Garg demonstrated cooperative nickel-catalyzed Suzuki-Miyaura coupling with enzymatic reduction for access to enantioenriched diaryl tertiary alcohols.[5h] Similarly, the Hyster and Hartwig labs have reported photocatalytic cooperativity with enzymatic processes for enantioselective deacetoxylation and access to α–substituted succinic esters and related species, respectively.[5c, 5d] Despite these impressive reports, intermolecular reactions, particularly carbon-carbon bond forming reactions, have received less attention in chemoenzymatic catalysis.
Our research group has long been driven by the synthetic utility found in leveraging the Umpolung, or reversal of polarity, of functional groups to facilitate valuable carbon-carbon bond-forming reactions.[6] Of particular use has been N-heterocyclic carbenes (NHCs) as organocatalysts due to their ability to induce the Umpolung of carbonyl species (e.g., classical reactions such as the Stetter reaction, Fig. 1A), as well as recent exploration of photochemically-enabled Umpolung of unsaturated malonate species.[7] We envisioned a chemoenzymatic sequence[8] that leveraged the powerful carbon-carbon bond-forming capabilities of NHC- or acyl radical chemistries[9] with the selectivity and specificity of engineered enzymes[10] (e.g., Fig. 1B) would present convenient and rapid access to a variety of scaffolds, notably γ-lactones, from readily-available commercial chemicals.
Figure 1.
Solvent and buffer screening for the enzymatic DKR/reduction and cyclization. a”Biphasic solvent” is composed of X% organic solvent (5, 15, 30, 50%) with the remaining balance occupied by aqueous buffer (phosphate [PO4], TRIS, triethanolamine [ETA]) and 10% IPA. See SI for screening details.
The γ-lactone is found present in nearly 10% of natural products, and methods for the enantio- and diastereoselective construction of this privileged heterocycle are much desired.[11] From a reaction design standpoint, the 1,4-dicarbonyl products of an NHC-facilitated Stetter reaction could be intercepted by a ketoreductase enzyme, furnishing the desired lactone by catalytic reduction and intramolecular cyclization.[12] Strategic implementation of this process would permit the enzyme to set multiple stereogenic centers through a dynamic kinetic resolution, obviating NHC catalyst stereocontrol (Fig. 1C; Fig. 1D).
Given the challenges inherent to the development of chemoenzymatic processes, we initially decoupled and optimized each component of the two-step process, affecting a sequential chemoenzymatic process. Due to the sensitivity of enzymes to traditional small-molecule catalysis conditions, we sought especially robust operating conditions for the DKR/reduction portion of the overall reaction. We therefore initiated our enzyme studies by surveying a panel of commercially available KREDs from Codexis Inc. against ketone 1a for reduction and DKR activity (see electronic Supporting Information [SI] for screening details). The enzyme KRED-P2-C02 handily afforded γ-lactone 2a and was subsequently subjected to a broader selection of biphasic solvent conditions (Figure 1).[13] Scale-up of the screening hits found, with minor additional optimization, biphasic solvent conditions with a clear partition between organic and aqueous phases predominated. Such biphasic conditions are synthetically tractable for enzymatic catalysis due to the capability of the organic solvent to overcome the low water solubility of many organic compounds and mitigate substrate/product enzyme inhibition.
Our screening analysis identified that the enzymatic reaction proceeded with high levels of efficiency in a 10:45:45 isopropanol:aqueous buffer:organic solvent mixture with particular efficacy in clearly-defined phase separation. Scale-up efforts generally ruled out ethereal solvents due to diminished yields, establishing 1:3:6 isopropanol:1-chlorododecane:0.1 M tris buffer at pH = 9 as the optimal solvent conditions for the enzymatic DKR/reduction.
With optimized conditions for the enzymatic DKR/reduction portion of the chemoenzymatic process established, we set out to explore the capabilities of the reaction (Table 1). All products reported herein were observed in >20:1 dr as determined from 1H NMR spectral analysis of the unpurified reaction mixtures. This observation, together with the high stereochemical fidelity of the resulting products, strongly validates our DKR/reduction approach to γ-lactones. Aryl substrates bearing electron-neutral to electron-poor substituents produced products in good-to-excellent yield and excellent er (2a-2i), though rigid scaffolds of high steric bulk (2j) induced a reduction in yield and minor erosion in enantioselectivity. Heterocycles (2k) were well tolerated, as were alkyl substrates (2l, 2m) in good to excellent yield and enantioselectivity. Electron-rich substrates (e.g., 4-OMe, not shown) were largely unreactive under this manifold, requiring extended reaction times with poor yield and selectivity. This discrepancy may be due to cation-π interactions of the electron-rich arene in the enzyme active site.[14] Crystals of 2a suitable for X-ray crystallographic analysis confirm both the absolute and relative trans stereochemical relationship of the lactone substituents.
Table 1.
Substrate scope of enzymatic reduction.
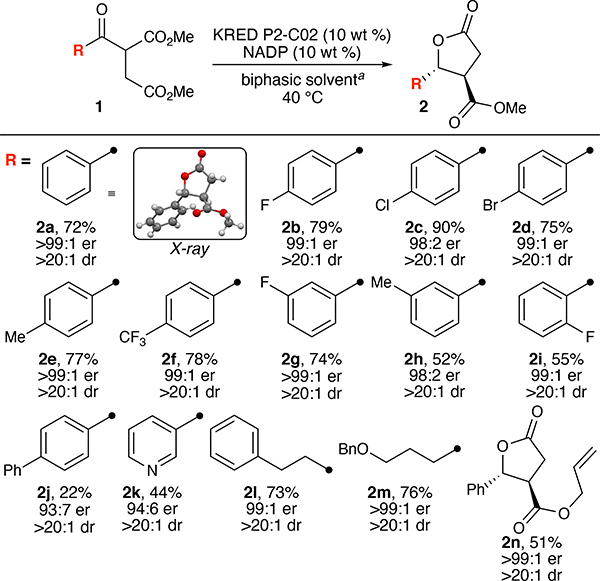 |
See SI for reaction protocol and details. Yields reported are isolated; er determined by chiral-phase SFC/HPLC analysis; dr determined from unpurified 1H NMR spectra. Absolute stereochemistry of 2a determined by X-ray crystallographic analysis; stereochemistry of all other lactone products assigned by analogy.
Solvent: 1:3:6 isopropanol:1-chlorododecane:tris buffer (0.1 M, pH = 9).
This enzymatic DKR/reduction and cyclization also permitted access to differentially-protected lactones such as 2n in high selectivity and moderate yield. Together with methyl ester lactone 2a, these products were further processed through amidation (3), yielding a densely-functionalized β-hydroxy ester, and palladiumcatalyzed deprotection (4). Such a strategy permits differential access to both the ester and lactone functionality, enabling further transformations. For example, scaffolds such as 4 present useful functionality for elaboration through classical two-electron and radical decarboxylative pathways.[15]

With efficacious conditions for the enzymatic portion of our chemoenzymatic process identified, we turned our attention to the Umpolung reaction sequence. Despite the widespread use of NHCs in organocatalysis, the usage of such catalysts under reaction conditions suitable for enzymatic catalysis is comparatively rare.[16] Also surprisingly atypical are Stetter reactions conducted on the α,β-unsaturated ester substrates considered precursors for the desired acetylated ketones 1.[17] A reaction traditionally performed neat, in ethanol, or DMF at elevated temperatures, the Stetter reaction and other NHC-catalyzed reactions can be made more amenable to conditions favorable to enzymes.[12a,12b,18] We have previously demonstrated the viability of a decarboxylation-enabled Stetter reaction under buffered aqueous conditions, though the elevated temperatures required for the generation of the Breslow intermediate would be deleterious to paired enzymes.[16a] Indeed, we observed significant drop-off in enzyme activity with prolonged exposure to temperatures above 50 °C, which validates the sequential approach to this chemistry. However, an approach utilizing Glorius’s Isa-NHC catalyst[19] proved fruitful, furnishing desired acetylated β-ketoesters 1 in good to excellent yields over a variety of substrates (Table 2).[12c]
Table 2.
Scope of NHC-catalyzed Stetter reaction of aldehydes and fumarates.
 |
Reaction conducted under air atmosphere.
This Stetter reaction proved modestly efficient under air and in 1:1 THF:0.1 M tris @ pH = 9. Additionally, the reaction was operable at enzyme-friendly temperatures (40 °C), though with reduced reaction rate. Slight reductions in yield were noted when THF was replaced by the enzyme-optimal biphasic solvent conditions (see SI). Control reactions wherein elements of the Stetter reaction were introduced to the enzyme portion of the process revealed the use of potassium bases entirely inhibited enzyme activity, an outcome not well documented to date or anticipated (see SI). However, substituting potassium carbonate with sodium carbonate and magnesium carbonate basic—metal counterions amenable to KRED catalysis—restored activity, albeit in diminished yield (54%). With the identification of a Stetter reaction to produce the substrates for enzymatic reduction, we successfully realized a two-step chemoenzymatic process for the synthesis of diastereo- and enantioenriched γ-lactones. Additionally, the utilization of an NHC catalyst in this synthesis of γ-lactones represents access to substrates previously inaccessible in the NHC catalytic domain. Though NHC-catalyzed syntheses of γ-lactones are known to the literature, these preparations both preferentially produce the cis product and are not extendable to the ester-substituted class of compounds 2. The oxobutanoate substrate required for this bond disconnection preferentially undergoes NHC-catalyzed β-protonation/dimerization through the enolate pathway to afford 5, not the expected homoenolate product 6 in the presence of benzaldehyde (see SI, references). [7a,20]

Given the comparatively mild conditions we identified for the Stetter reaction producing species 1, we conducted the full chemoenzymatic cascade in a single operation. Directly combining the Stetter reaction with KRED catalysis furnished a modest yield of 27% of the desired lactone with full consumption of the starting 4-chlorobenzaldehyde and the intermediate ketone 1c (Scheme 2A). Analysis of the unpurified reaction indicates the presence of multiple species containing the 4-chlorophenyl moiety, suggesting unproductive side reactions of both the NHC and KRED. Significantly more success was identified under a solvent-swap manifold, whereby the Stetter reaction was run to completion in THF before replacement with the optimized biphasic solvent conditions. This one-pot chemoenzymatic procedure produced 75% yield of the desired lactone product with a single purification and minimal handling.
Scheme 2.
One-pot chemoenzymatic syntheses of γ-lactones. aFumarate+NHC+K2CO3, THF, then solvent swap then enzyme; see SI for reaction details. bAll components present at start of reaction. cPhotoredox catalysis then enzyme addition in the same flask; see SI for reaction details.
In parallel with our exploration of Stetter reactivity for the generation of β-ketoesters1, we examined the possibility of pairing our enzymatic DKR/reduction with a photocatalytic generation of 1 (Scheme 2B). The combination of photocatalysis with enzymatic catalysis is appealing: photocatalytic reactions typically occur at or near ambient temperature and proceed through water- and protein-stable reactive intermediates. Accordingly, accessing acyl radicals by way of photoredox-induced decarboxylation enables a Giese-type addition to dimethyl maleate, producing 1a on way to the desired lactone in 49% one-pot yield.[16a· 21] By delaying the addition of enzyme until the photochemical reaction is complete the yield of the desire lactone increases to 73%. However, attempts to generalize the substrate scope of this process has not been realized yet and efforts are ongoing to realize this process.
We have developed an efficient, mild, and highly selective two-step-one-pot chemoenzymatic protocol to access densely functionalized γ-lactones inaccessible to date with established NHC catalysis. The process, accomplished through the concomitant application of a compatible Stetter reaction and KRED-catalyzed DKR/reduction, permits rapid access to these privileged heterocycles from simple building blocks. Given the synthetic ease and high selectivity of this approach, we anticipate such processes merging the synthetic breadth of small-molecule catalytic manifolds with highly-selective biocatalysts represent the future of synthetic catalysis.
Supplementary Material
Scheme 1.
Existing strategies and proposed combined catalysis strategy
Acknowledgements
We thank Northwestern and the National Institute of General Medical Sciences (GM073072 and GM131431) for support of this work. The authors thank Keegan Fitzpatrick and Charlotte Stern (NU) for X-ray crystallography assistance, Ada Kwong (NU) for high-resolution mass spectrometry assistance, and Dr. Adam Csakai for helpful early discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) For reviews discussing multienzyme cascades and catalytic complexes, see: Reed LJ, Acc. Chem. Res 2002, 7, 40–46; [Google Scholar]; b) Walsh CT, Moore BS, Angew. Chem. Int. Ed 2019, 58, 6846–6879; For recent reviews on artificial multienzyme cascades, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Schrittwieser JH, Velikogne S, Hall M, Kroutil W, Chem. Rev 2018, 118, 270–348; [DOI] [PubMed] [Google Scholar]; d) Sperl JM, Sieber V, ACS Catal. 2018, 8, 2385–2396. [Google Scholar]
- [2].a) For reviews discussing sequential catalytic transformations, see: Tietze LF, Beifuss U, Angew. Chem. Int. Ed 1993, 32, 131–163; [Google Scholar]; b) Pellissier H, Adv. Synth. Catal 2012, 354, 237–294; [Google Scholar]; c) Allen AE, Macmillan DW, Chem. Sci 2012, 2012, 633–658; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pellissier H, Adv. Synth. Catal 2019, 361, 1733–1755; [Google Scholar]; e) Romiti F, Del Pozo J, Paioti PHS, Gonsales SA, Li X, Hartrampf FWW, Hoveyda AH, J. Am. Chem. Soc 2019, 141, 17952–17961. [DOI] [PubMed] [Google Scholar]
- [3].a) Rudroff F, Mihovilovic MD, Gröger H, Snajdrova R, Iding H, Bornscheuer UT, Nat. Catal 2018, 1, 12–22; [Google Scholar]; b) Schmidt S, Castiglione K, Kourist R, Chem. Eur. J 2018, 24, 1755–1768; [DOI] [PubMed] [Google Scholar]; c) Devine PN, Howard RM, Kumar R, Thompson MP, Truppo MD, Turner NJ, Nat. Rev. Chem 2018, 2, 409–421. [Google Scholar]
- [4].a) For reviews of enzymatic DKR for the production of stereoenriched alcohols and amines, see: Verho O, Backvall JE, J. Am. Chem. Soc 2015, 137, 3996–4009; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Aranda C, Oksdath-Mansilla G, Bisogno F, de Gonzalo G, Adv. Synth. Catal 2019; For reviews of DKR, see: [Google Scholar]; c) Trost BM, Crawley ML, Chem. Rev 2003, 103, 2921–2944; [DOI] [PubMed] [Google Scholar]; d) Pellissier H, Tetrahedron 2011, 67, 3769–3802; [Google Scholar]; e) Pellissier H, Tetrahedron 2016, 72, 3133–3150; Selected examples of metal- and small molecule-catalyzed DKR: [Google Scholar]; f) Noyori R, Ohkuma T, Kitamura M, Takaya H, Sayo N, Kumobayashi H, Akutagawa S, J. Am. Chem. Soc 1987, 109, 5856–5858; [Google Scholar]; g) Kitamura M, Ohkuma T, Tokunaga M, Noyori R, Tetrahedron: Asymmetry 1990, 1, 1–4; [Google Scholar]; h) Jurkauskas V, Buchwald SL, J. Am. Chem. Soc 2002, 124, 2892–2893; [DOI] [PubMed] [Google Scholar]; i) Hoffmann S, Nicoletti M, List B, J. Am. Chem. Soc 2006, 128, 13074–13075; [DOI] [PubMed] [Google Scholar]; j) Steward KM, Gentry EC, Johnson JS, J. Am. Chem. Soc 2012, 134, 7329–7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) For reviews of cooperative chemo/biocatalytic processes, see: Denard CA, Hartwig JF, Zhao H, AcsCatal. 2013, 3, 2856–2864; [Google Scholar]; b) Hönig M, Sondermann P, Turner NJ, Carreira EM, Angew. Chem. Int. Ed 2017, 56, 8942–8973; Selected examples of cooperative chemo/biocatalytic processes: [DOI] [PubMed] [Google Scholar]; c) Litman ZC, Wang Y, Zhao H, Hartwig JF, Nature 2018, 560, 355–359; [DOI] [PubMed] [Google Scholar]; d) Biegasiewicz KF, Cooper SJ, Emmanuel MA, Miller DC, Hyster TK, Nat. Chem 2018, 10, 770–775; [DOI] [PubMed] [Google Scholar]; e) Burda E, Hummel W, Gröger H, Angew. Chem. Int. Ed 2008, 47, 9551–9554; [DOI] [PubMed] [Google Scholar]; f) Johnston EV, Bogár K, Backväll JE, J. Org. Chem 2010, 75, 4596–4599; [DOI] [PubMed] [Google Scholar]; g) Sarkale AM, Maurya V, Giri S, Appayee C, Org. Lett 2019, 21, 4266–4270; [DOI] [PubMed] [Google Scholar]; h) Dander JE, Giroud M, Racine S, Darzi ER, Alvizo O, Entwistle D, Garg NK, Commun. Chem 2019, 2, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Lettan RB, Reynolds TE, Galliford CV, Scheidt KA, J. Am. Chem. Soc 2006, 128, 15566–15567; [DOI] [PubMed] [Google Scholar]; b) Reynolds TE, Stern CA, Scheidt KA, Org. Lett 2007, 9, 2581–2584; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chan A, Scheidt KA, J. Am. Chem. Soc 2007, 129, 5334–5335; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Reynolds TE, Binkley MS, Scheidt KA, Org. Lett 2008, 10, 5227–5230; [DOI] [PubMed] [Google Scholar]; e) Lettan RB, Woodward CC, Scheidt KA, Angew. Chem. Int. Ed 2008, 47, 2294–2297; [DOI] [PubMed] [Google Scholar]; f) Lettan RB, Galliford CV, Woodward CC, Scheidt KA, J. Am. Chem. Soc 2009, 131, 8805–8814. [DOI] [PubMed] [Google Scholar]
- [7].a) For recent reviews on NHCs and NHC cooperative catalysis, see: Hopkinson MN, Richter C, Schedler M, Glorius F, Nature 2014, 510, 485–496; [DOI] [PubMed] [Google Scholar]; b) Wang MH, Scheidt KA, Angew. Chem. Int. Ed 2016, 55, 14912–14922; [DOI] [PubMed] [Google Scholar]; c) Murauski KJR, Jaworski AA, Scheidt KA, Chem. Soc. Rev 2018, 47, 1773–1782; Selected NHC-catalyzed processes from our group: [DOI] [PubMed] [Google Scholar]; d) Mattson AE, Bharadwaj AR, Scheidt KA, J. Am. Chem. Soc 2004, 126, 2314–2315; [DOI] [PubMed] [Google Scholar]; e) Hovey MT, Cohen DT, Walden DM, Cheong PH, Scheidt KA, Angew. Chem. Int. Ed 2017, 56, 9864–9867; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Murauski KJR, Walden DM, Cheong PH, Scheidt KA, Adv. Synth. Catal 2017, 359, 3713–3719; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Lee A, Zhu JL, Feoktistova T, Brueckner AC, Cheong PH, Scheidt KA, Angew. Chem. Int. Ed 2019, 58, 5941–5945; Selected photocatalytic Umpolung processes from our group: [DOI] [PMC free article] [PubMed] [Google Scholar]; h) McDonald BR, Scheidt KA, Org. Lett 2018, 20, 6877–6881; [DOI] [PubMed] [Google Scholar]; i) Betori RC, Scheidt KA, ACS Catal.2019, 9, 10350–10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Betori RC, May CM, Scheidt KA, Angew. Chem. Int. Ed 2019, 58, 16490–16494. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [9].a) For reviews on acyl and other carbonyl radicals, see: Chatgilialoglu C, Crich D, Komatsu M, Ryu I, Chem. Rev 1999, 99, 1991–2070; [DOI] [PubMed] [Google Scholar]; b) Raviola C, Protti S, Ravelli D, Fagnoni M, Green Chem. 2019, 21, 748–764; Selected acyl radical literature: [Google Scholar]; c) Chudasama V, Fitzmaurice RJ, Caddick S, Nat. Chem. 2010, 2, 592–596; [DOI] [PubMed] [Google Scholar]; d) Goti G, Bieszczad B, Vega-Penaloza A, Melchiorre P, Angew. Chem. Int. Ed. 2019, 58, 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) For reviews on engineered enzymes, see: Liang J, Lalonde J, Borup B, Mitchell V, Mundorff E, Trinh N, Kochrekar DA, Nair Cherat R, Pai GG, Org. Process Res. Dev 2010, 14, 193–198; [Google Scholar]; b) Noey EL, Tibrewal N, Jimenez-Oses G, Osuna S, Park J, Bond CM, Cascio D, Liang J, Zhang X, Huisman GW, Tang Y, Houk KN, PNAS 2015, 112, E7065–7072; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Prier CK, Zhang RK, Buller AR, Brinkmann-Chen S, Arnold FH, Nat. Chem 2017, 9, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) For reviews on ɣ-lactones, see: Seitz M, Reiser O, Curr. Opin. Chem. Biol 2005, 9, 285–292; [DOI] [PubMed] [Google Scholar]; b) Mao B, Fananas-Mastral M, Feringa BL, Chem. Rev 2017, 117, 10502–10566; Selected approaches to γ-lactones: [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hoffmann HMR, Rabe J, Angew. Chem. Int. Ed 1985, 24, 94–110; [Google Scholar]; d) Díaz-Rodríguez A, Borzçcka W, Lavandera I, Gotor V, ACS Catal. 2013, 4, 386–393; [Google Scholar]; e) Classen T, Korpak M, Schölzel M, Pietruszka J, ACS Catal. 2014, 4, 1321–1331; [Google Scholar]; f) Simon RC, Busto E, Schrittwieser JH, Sattler JH, Pietruszka J, Faber K, Kroutil W, Chem. Commun. 2014, 50, 15669–15672; [DOI] [PubMed] [Google Scholar]; g) Griswold JA, Johnson JS, ACS Catal. 2019, 11614–11618; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Singha S, Serrano E, Mondal S, Daniliuc CG, Glorius F, Nat. Catal. 2020, 3, 48–54; [Google Scholar]; i) Bourgeois F, Medlock JA, Bonrath W, Sparr C, Org. Lett 2020, 22, 110–115. [DOI] [PubMed] [Google Scholar]
- [12].a) Stetter H, Schreckenberg M, Angew. Chem. Int. Ed 1973, 12, 81–81; [Google Scholar]; b) Stetter H, Angew. Chem. Int. Ed 1976, 15, 639–647; [Google Scholar]; c) Ahire MM, Mhaske SB, Tetrahedron 2018, 74, 2079–2084. [Google Scholar]
- [13].Rosinha Grundtvig IP, Heintz S, Kruhne U, Gernaey KV, Adlercreutz P, Hayler JD, Wells AS, Woodley JM, Biotechnol. Adv 2018, 36, 1801–1814. [DOI] [PubMed] [Google Scholar]
- [14].Kumar K, Woo SM, Siu T, Cortopassi WA, Duarte F, Paton RS, Chem. Sci 2018, 9, 2655–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chu L, Ohta C, Zuo Z, MacMillan DW, J. Am. Chem. Soc 2014, 136, 10886–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Myers MC, Bharadwaj AR, Milgram BC, Scheidt KA, J. Am. Chem. Soc 2005, 127, 14675–14680; [DOI] [PubMed] [Google Scholar]; b) Iwamoto K.- i., Hamaya M, Hashimoto N, Kimura H, Suzuki Y, Sato M, Tetrahedron Lett. 2006, 47, 7175–7177. [Google Scholar]
- [17].Wurz NE, Daniliuc CG, Glorius F, Chem. Eur. J 2012, 18, 16297–16301. [DOI] [PubMed] [Google Scholar]
- [18].Beigi M, Waltzer S, Zarei M, Müller M, Biotechnol J 2014, 191, 64–68. [DOI] [PubMed] [Google Scholar]
- [19].Piel I, Pawelczyk MD, Hirano K, Fröhlich R, Glorius F, Eur. J. Org. Chem 2011, 2011, 5475–5484. [Google Scholar]
- [20].Hovey MT, Northwestern University 2017. [Google Scholar]
- [21].Wang GZ, Shang R, Cheng WM, Fu Y, Org. Lett 2015, 17, 4830–4833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





