Abstract
Introduction
We have previously found that papillary histopathology differs greatly between calcium oxalate and brushite stone formers (SF); the latter have much more papillary mineral deposition, tubular cell injury, and tissue fibrosis.
Methods
In this study, we applied unbiased orthogonal omics approaches on biopsied renal papillae and extracted stones from patients with brushite or calcium oxalate (CaOx) stones. Our goal was to discover stone type-specific molecular signatures to advance our understanding of the underlying pathogenesis.
Results
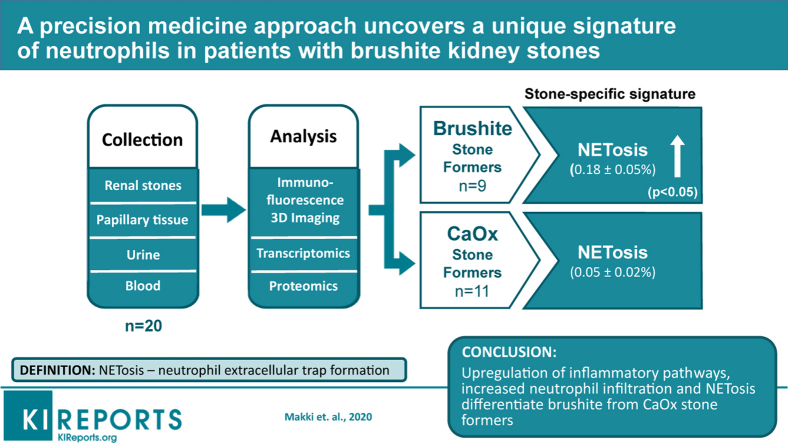
Brushite SF did not differ from CaOx SF with respect to metabolic risk factors for stones but did exhibit increased tubule plugging in their papillae. Brushite SF had upregulation of inflammatory pathways in papillary tissue and increased neutrophil markers in stone matrix compared with those with CaOx stones. Large-scale 3-dimensional tissue cytometry on renal papillary biopsies showed an increase in the number and density of neutrophils in the papillae of patients with brushite versus CaOx, thereby linking the observed inflammatory signatures to the neutrophils in the tissue. To explain how neutrophil proteins appear in the stone matrix, we measured neutrophil extracellular trap (NET) formation—NETosis—and found it significantly increased in the papillae of patients with brushite stones compared with CaOx stones.
Conclusion
We show that increased neutrophil infiltration and NETosis is an unrecognized factor that differentiates brushite and CaOx SF and may explain the markedly increased scarring and inflammation seen in the papillae of patients with brushite stones. Given the increasing prevalence of brushite stones, the role of neutrophil activation in brushite stone formation requires further study.
Keywords: kidney stones, nephrolithiasis, neutrophil extracellular trap
Graphical abstract
Nephrolithiasis is a clinical syndrome that likely arises from multiple diseases, each with a distinct pathobiology.1 For example, the papillary pathologies of patients who form idiopathic CaOx stones differ from that of those whose stones contain brushite.2 The former show mainly interstitial plaque with very modest tubule plugging and no apparent tissue injury, whereas in the latter, plugs are large and involve obvious tissue injury such as interstitial fibrosis and loss of tubule epithelial cells.2
To date, the tissues from these 2 highly contrasting forms of kidney stone disease have not been studied using modern techniques to disclose mechanisms of injury. A role for inflammation and oxidative injury has been proposed based on animal models of crystal-induced injury or in vitro studies.3,4 However, definitive evidence for such a role in human disease is limited. A recent study by Taguchi et al., using transcriptomic studies on papillary tissue, established an association between proinflammatory genes and development of Randall’s plaque in CaOx SFs.5 However, the papillary tissues of brushite SF, which have much more evidence for injury, have not been studied in similar fashion.
Significant advancement in molecular interrogation of tissue specimens presents a unique opportunity to uncover important pathways that are differentially activated in these 2 forms of kidney stone disease. These techniques, such as transcriptomics, proteomics, and large-scale quantitative 3-dimensional (3D) imaging, can provide an unbiased and comprehensive approach to discovery.6, 7, 8, 9, 10 We present here a detailed study contrasting CaOx and brushite clinical stone phenotypes, using these orthogonal omics approaches.
Because these approaches are costly and require specific expertise of interpretation and integration, our cohort is necessarily limited. Even so, our findings demonstrate that compared with CaOx SF, brushite SF have a unique signature of inflammation and neutrophil activation, which translates into increased abundance of neutrophils in the tissue and neutrophil proteins in the stones.
These findings were further extended by determining that these papillae are characterized by increased neutrophil extracellular trap formation (a process known as neutrophil extracellular trap [NET]osis),11,12 whereby neutrophils expel their intracellular content. In addition to the pathophysiological implications of this unique neutrophil infiltration and activation in brushite SF, this work supports that NETosis, an important pathway hitherto undescribed in stone disease, could contribute to tissue injury in patients who form brushite stones.
Methods
Subjects and Specimen Collection
Patients who were undergoing either percutaneous nephrolithotomy or ureteroscopic removal of renal stones were consented.13,14 Patients were included in this study if they had CaOx or brushite stones, defined as stone mineral content >50% of the specified material, and also available specimens (stone or tissue) that could be used experimentally. The entire endoscopic procedure was recorded on video; papillary visual appearance was graded as previously described;15 and papillary biopsies were taken, when possible. The study was approved by the Institutional Review Board Committee for Indiana University Health Partners (#98-073). Specific details of the specimens used for each patient are described in Table 1. The use of specific specimen in each technology was based on availability and the suitability of the samples to the corresponding technologies.
Table 1.
Clinical characteristics of the patients and the assays performed on each sample
| Patient no. | Stone mineral (%) |
Sex | Age at surgery, yr | Previous ESWL | Surgery type | Urine culture growth | BMI | Comorbidities | Assays used on each tissue or stone specimen |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaOx | Ap | Br | Tissue 3DTC |
Tissue Trans |
Stone Prot | Stone micro-computed tomography | ||||||||
| Brushite patients | ||||||||||||||
| 1 | 4 | 8a | 88 | M | 56 | Yes | PN | No | 21.4 | No | – | – | + | + |
| 2 | 5 | 6 | 89 | M | 46 | Yes | PN | Yes | 24.8 | No | – | – | + | + |
| 3 | 5 | 15 | 80 | M | 54 | Yes | PN | No | 31.9 | No | – | – | + | + |
| 4 | 26 | 1 | 73 | M | 40 | No | PN | No | 28.6 | No | – | – | + | + |
| 5 | 0 | 6 | 94 | M | 43 | Yes | PN | No | 25.1 | No | + | + | – | + |
| 6 | 1 | 5 | 94 | M | 25 | No | PN, URS | No | 25.0 | No | + | + | – | + |
| 7 | 3 | 2 | 95 | F | 44 | No | URS | No | 19.1 | No | + | – | – | + |
| 8 | 0 | 0 | 100 | M | 74 | No | PN | NA | 22.4 | No | + | + | – | + |
| 9 | 0 | 0 | 100 | M | 29 | Yes | PN | No | 33.4 | No | + | + | + | + |
| Calcium oxalate patients | ||||||||||||||
| 10 | 91 | 9 | 0 | M | 58 | No | PN | NA | 28.9 | No | – | – | + | + |
| 11 | 85 | 15 | 0 | M | 65 | No | PN | No | 36.2 | HTN,TD, CA | – | – | + | + |
| 12 | 94 | 6 | 0 | M | 21 | No | PN, URS | No | 27.8 | No | – | – | + | + |
| 13 | 82 | 18 | 0 | M | 47 | No | PN | No | 28.2 | HTN | + | – | + | + |
| 14 | 80 | 21 | 0 | F | 56 | Yes | PN | No | 25.5 | DM, TD, DP | + | + | – | + |
| 15 | 97 | 3 | 0 | F | 66 | Yes | PN, URS | NA | 40.3 | GSb | + | – | – | + |
| 16 | 85 | 15 | 0 | F | 50 | No | PN | No | 27.1 | No | + | – | – | + |
| 17 | 85 | 15 | 0 | M | 50 | No | PN | NA | 40.7 | No | + | + | – | + |
| 18 | 97 | 3 | 0 | M | 38 | Yes | PN | No | 47.4 | No | + | + | – | + |
| 19 | 99 | 1 | 0 | M | 53 | Yes | PN | No | 25.8 | No | – | + | – | + |
| 20 | 92 | 8 | 0 | M | 39 | Yes | PN | No | 31.0 | No | – | – | + | + |
3DTC, 3-dimensional tissue cytometry; Ap, apatite; BMI, body mass index; Br, brushite; CA, cancer; CaOx, calcium oxalate; DM, diabetes mellitus; DP, depression; ESWL, extracorporel shock-wave lithotripsy; GS, gastric surgery; HTN, hypertension; NA, not available; PN, percutaneous nephrolithotomy; Prot, proteomics; TD, thyroid disease; Trans, transcriptomics; URS, uretroscopy.
(–) or (+) imply assay not performed or performed, respectively.
Included 4.3% apatite, 2.8% octacalcium phosphate, and 0.5% whitlockite.
The type of surgery was unspecified. Mineral measurements were performed by volume through segmentation of 3D micro-computed tomography image stacks.
Stones were rinsed in normal saline and air dried at room temperature for further analysis by micro-computed tomography,16 using voxel sizes of 3–10 μm for the final reconstructions. Micro-computed tomography stone analysis was always confirmed using conventional Fourier transform infrared spectroscopic method.16
Clinical laboratory studies
All subjects collected two 24-hour urine samples postoperatively while eating a free-choice diet and off medications that could affect stone formation. Stone-risk analytes were measured and urine supersaturation (SS) calculated using methods detailed elsewhere.14,17 The mean of the 2 samples is reported in Table 2. Routine blood measurements were made for clinical purposes.
Table 2.
Urine and serum analytes
| Patient no. | Urine volume (L/d) | Urine pH | Urine calcium (mg/d) | Urine citrate (mg/d) | Urine oxalate | SS CaOx | SS CaP | Serum creatinine (mg/dl) | Serum calcium (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|
| Brushite patients | |||||||||
| 1 | 3.19 | 6.69 | 252.6 | 671.0 | 52.3 | 5.55 | 0.87 | 1.12 | 10.13 |
| 2 | 1.94 | 6.37 | 371.6 | 697.8 | 41.8 | 8.55 | 3.32 | 0.83 | 9.54 |
| 3 | 1.63 | 7.74 | 122.4 | 567.6 | 57.5 | 6.18 | 2.06 | 0.83 | 9.78 |
| 4 | 0.97 | 6.28 | 260.3 | 958.5 | 26.0 | 9.94 | 4.20 | 1.30 | 9.93 |
| 5 | 2.13 | 6.67 | 295.0 | 225.0 | 47.0 | 7.52 | 2.69 | – | – |
| 6 | 1.70 | 6.31 | 279.4 | 297.4 | 38.6 | 7.62 | 2.19 | 0.75 | 9.39 |
| 7 | 1.58 | 6.25 | 290.3 | 447.0 | 29.4 | 8.91 | 2.22 | 0.91 | 9.56 |
| 8 | – | – | – | – | – | – | – | – | – |
| 9 | 1.71 | 6.52 | 191.7 | 949.7 | 65.5 | 8.84 | 2.29 | 0.92 | 9.00 |
| Mean ± SD | 1.84 ± 0.68 | 6.31 ± 0.20 | 241.7 ± 63.0 | 648.7 ± 267.4 | 45.2 ± 14.6 | 7.80 ± 1.57 | 2.35 ± 0.98 | 0.97 ± 0.20 | 9.63 ± 0.40 |
| Calcium oxalate patients | |||||||||
| 10 | 1.69 | 5.97 | 145.5 | 425.0 | 45.6 | 6.42 | 0.81 | 0.62 | 9.47 |
| 11 | 2.11 | 6.51 | 256.7 | 278.9 | 50.0 | 8.03 | 2.32 | 0.79 | 8.83 |
| 12 | – | – | – | – | – | – | – | – | – |
| 13 | 1.33 | 6.01 | 315.9 | 881.6 | 30.2 | 8.65 | 3.73 | 1.04 | 9.24 |
| 14 | 2.79 | 6.55 | 494.3 | 899.9 | 40.0 | 8.17 | 2.64 | 0.72 | 9.65 |
| 15 | 1.55 | 6.28 | 91.1 | 253.7 | 56.1 | 7.89 | 0.77 | 0.92 | 7.99 |
| 16 | 0.91 | 5.89 | 310.5 | 972.3 | 28.5 | 16.11 | 3.52 | – | – |
| 17 | 1.55 | 5.63 | 293.8 | 670.5 | 51.4 | 15.51 | 0.96 | 0.92 | 8.31 |
| 18 | 1.25 | 5.45 | 528.5 | 949.8 | 64.9 | 26.47 | 2.25 | 0.84 | 9.42 |
| 19 | 1.59 | 5.90 | 479.2 | 940.2 | 41.4 | 12.52 | 2.23 | – | – |
| 20 | – | – | – | – | – | – | – | – | – |
| Mean ± SD | 1.67 ± 0.51 | 6.22 ± 0.42 | 328.7 ± 144.8 | 697.0 ± 282.9 | 45.0 ± 11.2 | 6.14 ± 6.14 | 2.25 ± 1.10 | 0.83 ± 0.13 | 9.05 ± 0.61 |
| P value | 0.55 | 0.59 | 0.15 | 0.74 | 0.97 | 0.11 | 0.84 | 0.15 | 0.07 |
CaOx, calcium oxalate; CaP, calcium phosphate as brushite; SS, supersaturation.
Dashes denote that data are not available.
Tissue
Whenever possible, papillary biopsies included both those that were immediately fixed in 4% paraformaldehyde, buffered to pH 7.4, or immediately immersed in optimal cutting temperature medium and frozen on dry ice. Biopsies fixed in paraformaldehyde were embedded in paraffin, sectioned, and stained. Biopsies that were frozen were cut in a cryostat (Leica Biosystems, Wetzlar, Germany) into 50-μm sections and immediately placed in 4% paraformaldehyde for fixation overnight for large-scale 3D imaging. The frozen tissue left over, if any, after sectioning was used for RNA extraction and transcriptomic analysis described here. Therefore, all tissues used in transcriptomics had also tissue cytometry performed, except for patient 17, for whom the tissue was too small to be sectioned for imaging and was used entirely for RNA analysis.
Immunofluorescence Staining and Large-Scale 3D Confocal Imaging
For immunofluorescence analysis, 50-μm sections were washed in phosphate-buffered saline and then blocked in 10% normal donkey serum for 2 hours at RT, followed by primary antibody incubation at room temperature overnight, and permeabilization with Triton X (Santa Cruz Biotechnology, Inc., Dallas, TX) at 0.2%.18,19 We used the following antibodies for detecting the target proteins: aquaporin 1 (Santa Cruz Biotechnology, Inc., sc-9878), myeloperoxidase (Abcam, Cambridge, MA; ab5690), CD68 (Agilent Technologies, Santa Clara, CA; M087601), Citrulline H3 (OriGene Technologies Inc., Rockville, MD; AM10179PU-N). After washing with phosphate-buffered saline, the sections were probed with Alexa (ThermoFisher Scientific, Waltham, MA) dye-conjugated secondary antibodies: donkey anti-goat-488 or anti-mouse-568 and anti-rabbit-647. 4′,6-Diamidino-2-phenylindole (Abcam) was used for staining the nuclei. Subsequently, sections were washed for 5 times 1 hour each and then fixed in 4% paraformaldehyde for an additional 15 minutes. After a final wash for 30 minutes, sections were mounted on a glass slide using fluoromount medium (Sigma Aldrich, St. Louis, MO; F-4680). Images were sequentially acquired in 4 separate channels using Leica SP8 confocal microscope as whole volume stacks using 20X NA 0.75 objective with 1.0-μm spacing. Stacks were stitched using Leica LAS X software to generate large-scale 3D images. 3D-image rendering was performed using Voxx V2.09 software (http://www.indiana.edu/∼voxx/). A negative control without primary antibody was used to ensure the absence of nonspecific binding of secondary antibodies. Microscope settings were identical among imaging sessions of each specimen.
3D Tissue Cytometry
3D tissue cytometry was performed on image volumes using volumetric tissue exploration and analysis (VTEA) software, which allows volumetric cell identification on the basis of 3D nuclear segmentation.7 Segmentation settings were adjusted to yield the best quality, which was visually verified by sampling random fields within each image stack. Fluorescence from myeloperoxidase, CD68, or H3-citrulline associated with nuclei by a 3D morphological grow routine were displayed on a scatterplot as individual points. This method allows gating of specific cell populations based on fluorescence intensities and mapping of the gated cells directly on the image with the corresponding statistics (Supplementary Figure S1). Direct visualization of the gated cells on the image also allows validation of the gates, which was performed individually on random fields for each large-scale image volume. For spatial distribution analysis, regions of interest (ROIs) were manually drawn over collecting duct identified by morphological features on the stained tissue. Distance maps corresponding to the ROIs were built using ImageJ (National Institutes of Health, Bethesda, MD) Euclidian distance map API, and neutrophil position coordinates were correlated to corresponding locations on the distance map. Randomly generated position coordinates using a random number generator function (Gaussian) were used for comparison. A 2-sample Student's t test was used to compare the histogram distributions.
RNA Extraction, Library Preparation, and Sequencing
Total RNA extraction was performed using RNeasy Plus Micro Kit (Qiagen, Hilden, Germany). Remaining optimal cutting temperature–frozen biopsies were dissolved in RLT buffer (Qiagen) and lysed. Equal volume of 75% ethanol was added, and the rest of the protocol was followed according to the manufacturer’s instructions. Extracted RNA was dissolved in RNase free water. The concentration and quality of total RNA samples were first assessed using Agilent 2100 Bioanalyzer. An RNA Integrity Number of 5 or higher was required to pass the quality control. One nanogram of RNA per sample was used to prepare dual-indexed strand-specific cDNA libraries, using Clontech SMARTer RNA Pico Kit v2 (Clontech, Mountain View, CA). The resulting libraries were assessed for quantity and size distribution using Qubit and Agilent 2100 Bioanalyzer. Two-hundred picomolar pooled libraries were used per flow cell for clustering amplification on cBot using HiSeq 3000/4000 PE Cluster Kit and sequenced with 2 × 75 bp paired-end configuration on HiSeq4000 (Illumina, San Diego, CA), using HiSeq 3000/4000 PE SBS Kit. A Phred quality score (Q score) was used to measure the quality of sequencing. More than 90% of the sequencing reads reached Q30 (99.9% base call accuracy).
Sequence Alignment and Gene Counts
The sequencing data were first assessed using FastQC (Babraham Bioinformatics, Cambridge, UK) for quality control. Then all sequenced libraries were mapped to the human genome (UCSC mm 10), using STAR RNA-seq aligner20 with the following parameter: --outSAMmapqUnique 60. The reads distribution across the genome was assessed using bamutils (from ngsutils).21 Uniquely mapped sequencing reads were assigned to hg38 refGene genes, using featureCounts (from subread),22 with the following parameters: –s 2 –p –Q 10. Quality control of sequencing and mapping results was summarized using MultiQC (SciLifeLab, Solna, Sweden).23 Genes with read count per million > 0.5 in more than 4 of the samples were kept. The data were normalized using trimmed mean of M values method. A multidimensional scaling plot visualizing all the specimens was also constructed (Supplementary Figure S2). Differential expression analysis was performed using edgeR.24,25 False discovery rate was computed from P values, using the Benjamini-Hochberg procedure. Differentially expressed genes (uncorrected P < 0.01) were subjected to ingenuity pathway analysis (IPA) to determine significant gene clustering (P < 0.05) within signaling pathways or other disease annotations (Ingenuity Systems, http://www.ingneuity.com). Volcano plots were generated using the Shiny Transcriptome Analysis Resource Tool (START).26 Heat maps were generated using R software (version 3.4.2)
Proteomic Studies
Kidney stone samples were processed as 2 technical replicates when available. A total of 10 brushite samples (5 patients) and 9 CaOx samples (5 patients) were analyzed. Kidney stone samples, each >100 mg dry weight, were pulverized, and proteins were isolated as previously described and analyzed using a label-free liquid chromatography tandem mass spectrometry method.6 Briefly, stones were powdered to the consistency of fine flour; 100 mg of stone powder was combined with 1.0 ml of freshly prepared 8 M urea and 10 mM dithiothreitol. Mixture was sonicated, and tubes were incubated overnight at room temperature on orbital shaker (200 rpm). After centrifugation, the supernatant was collected, and the protein concentration was determined by Bradford assay. A 100-μg aliquot of each sample was concentrated, and volume was adjusted to 200 μl (4 M urea) and then reduced and alkylated by triethylphosphine and iodoethanol. Reduced/alkylated sample was dried by SpeedVac (ThermoScientific) and reconstituted in 100 μl of NH4HCO3. A 20-μg/ml trypsin solution was added into 150 μl aliquot for overnight digestion. Digested samples were inspected using a ThermoScientific Orbitrap Velos Pro hybrid ion trap-Orbitrap mass spectrometer coupled with a Surveyor auto sampler and MS HPLC system (ThermoScientific). The data were collected in the data-dependent MS/MS mode of Fourier transform-ion trap (MS-MS/MS), with the electrospray ionization interface, using normalized collision energy of 35%. The acquired data were searched against the UniProt protein sequence database of HUMAN using SEQUEST algorithm in the Bioworks software package (ThermoScientific). Identified proteins were validated by PeptideProphet and ProteinProphet using TransProteomic Pipeline. After validation protein probability of ≥ 0.9000 and peptide probability of ≥ 0.8000 were reported. Protein quantification was determined using a label-free quantification software package, IdentiQuantXL.6 Data were analyzed using the statistical package in MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/MetaboAnalyst/faces/home.xhtml). Heat maps were generated using log-transformed normalized data. In addition, uncorrected P values < 0.05 were applied to filter affected genes/proteins for function, pathway, and network analysis using IPA to determine significant enrichment of canonical pathways and gene clustering (P < 0.05) between the 2 groups.
Statistical analysis
The results are presented as the mean ± SD. Unless specified otherwise, the significant difference between the experimental groups was assessed by 2-tailed Student's t test. For the cytometry results, statistical analyses and graphing were performed using GraphPad Prism (La Jolla, CA), considering P values < 0.05 as statistically significant.
Results
Patient Characteristics
The 2 patient groups (Table 1) were similar in age (45.7 years ± 14.2 years vs. 49.4 years ± 12.7 years, mean ± SD, brushite and CaOx respectively, P = 0.55) and sex distributions (89% vs. 73% male, brushite and CaOx, respectively; P = 0.59), but CaOx patients had higher body mass index (25.7 ± 4.8 vs. 32.6 ± 7.4, brushite and CaOx, respectively, P < 0.05). Extracorporeal shock-wave lithotripsy rates were similar (55.6% vs. 45.5%, brushite and CaOx, respectively, P = 0.99). Few patients had comorbidities, and only 1 patient with brushites had an infected urine culture. Stone cultures were negative (not shown). With respect to metabolic evaluation, excretions of calcium, oxalate and citrate, and SS, with respect to CaOx and CaP, were also the same in both groups of patients (Table 2). This differs slightly from our previous work, in which calcium excretion and CaP SS were higher in patients with brushite stones than those with CaOx stones.27
Kidney Stone Characterization
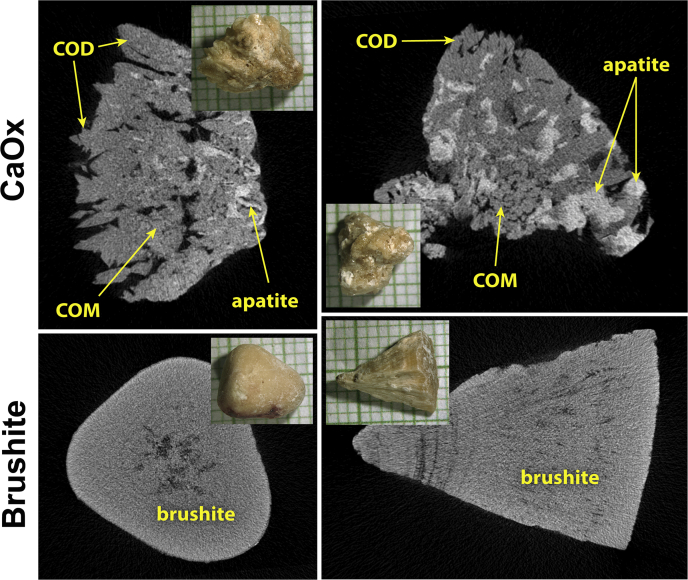
By micro-computed tomography analysis, CaOx stones were heterogeneous. In a majority, mineral type was calcium oxalate monohydrate, and all contained some apatite (Figure 116 [top] and Table 1). The average specimen composition (by volume) for the CaOx SF was 58.6% ± 30.1% calcium oxalate monohydrate, 31.1% ± 29.1% calcium oxalate dihydrate, and 10.3% ± 6.4% apatite. In contrast, the brushite specimens were homogeneous (95.4% ± 4.7% brushite, 1.7% ± 3.6% calcium oxalate dihydrate, and 2.9% ± 4.1% apatite (Figure 1 [bottom] and Table 1).
Figure 1.
Micro-computed tomography of kidney stones. Kidney stones are shown in insets, on mm paper. Upper panels show 2 typical calcium oxalate (CaOx) specimens, both of which show polyhedral crystals of calcium oxalate dihydrate (COD) at the surface, calcium oxalate monohydrate (COM; COM and COD can often be distinguished by micro-computed tomography by a combination of crystal shape and apparent x-ray density16) and apatite, which is the most x-ray dense of the minerals commonly found in stones. Specimen in the upper right contained the largest fraction of apatite of any of the specimens used (21% apatite by volume). The lower panels show brushite specimens, both of which were quite pure, which was typical for all the brushite samples.
Surgical and Tissue Phenotyping
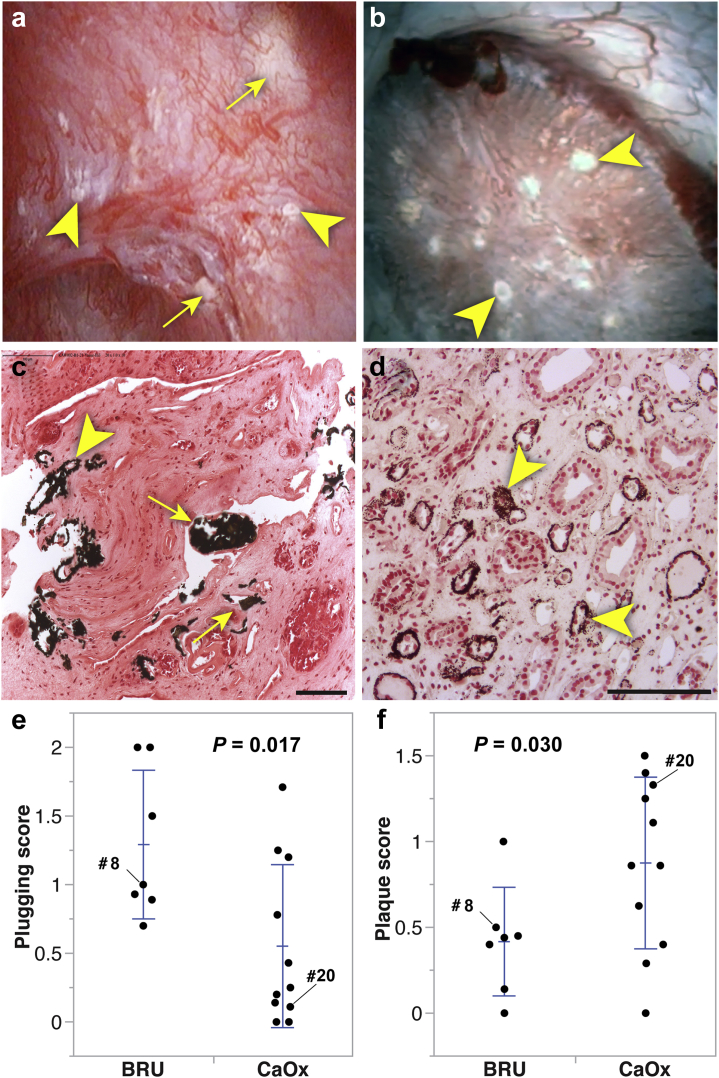
Surgery disclosed variable amounts of plugging and Randall’s plaque in both groups of patients (Figure 2a and b), which were confirmed histologically (Figure 2c and d). Plugs and plaque in both types of stone formers had been shown to be composed of apatite.27 Papillary grading (Table 3) showed higher plugging but lower Randall’s plaque scores in brushite versus CaOx (Figure 2e and f), which is consistent with earlier reports.27 When assessed using surface area measurements (Table 3), only the amount of plugging differed between stone types.
Figure 2.
Endoscopic and histologic analysis of brushite and calcium oxalate (CaOx) papillae. (a,b) endoscopic appearance of papillae in brushite (patient #8) and CaOx (patient #20) patients, respectively. (c,d) histological images (Yasue stain) of biopsies from these same patients. Arrowheads mark Randall’s plaque, and arrows point to regions of ductal plugging; bars in lower right indicate 100 μm. (e,f) papillary scores for plugging and Randall’s plaque for all the patients except for 2 brushite patients, 1 of whom had advanced scarring precluding scoring and another with low quality endoscopic video. In the scatter plots presented, each point represents the mean score per category from a single patient. Error bars show SD around the group mean. P values shown are from Student's t tests with Welch correction. BRU, brushite.
Table 3.
Papillary grading scores
| Patient | Papillaea | Plugging | Pitting | LoC | RP | % Plugging | % RP |
|---|---|---|---|---|---|---|---|
| Brushite patients | |||||||
| 1 | 2 | 1.5 | 0 | 0.5 | 1 | 2.39 | 2.70 |
| 2 | 9 | 0.89 | 0.78 | 0.56 | 0.44 | 0.97 | 3.26 |
| 3 | 15 | 0.93 | 0.47 | 1.47 | 0.4 | 0.71 | 0.64 |
| 4 | All papillae scarred with no recognizable features | ||||||
| 5 | 6 | 2 | 0.67 | 0.67 | 0 | 3.24 | 0.04 |
| 6 | 20 | 0.7 | 0.15 | 0.4 | 0.45 | 0.02 | 2.40 |
| 7 | 7 | 2 | 1.71 | 0.57 | 0.14 | 3.57 | 0.50 |
| 8 | 6 | 1 | 0.17 | 0.17 | 0.5 | 1.52 | 0.50 |
| 9 | Video too dark to discern details for grading | ||||||
| Mean ± SD | 9.3 ± 6.1 | 1.3 ± 0.5 | 0.6 ± 0.6 | 0.6 ± 0.4 | 0.4 ± 0.3 | 1.8 ± 1.3 | 1.4 ± 1.3 |
| Calcium oxalate patients | |||||||
| 10 | 6 | 0 | 0 | 0 | 1.5 | 0 | 4.51 |
| 11 | 4 | 0.25 | 0.5 | 0.25 | 1.25 | 0.06 | 1.47 |
| 12 | 8 | 0 | 0.375 | 0 | 0.625 | 0 | 4.39 |
| 13 | 7 | 0.14 | 0 | 0.29 | 0.29 | 0.05 | 0.45 |
| 14 | 5 | 1.2 | 0 | 0 | 0.4 | 0.79 | 1.29 |
| 15 | 9 | 0.78 | 0.33 | 0 | 1.11 | 0 | 2.68 |
| 16 | 7 | 0.43 | 0.43 | 0.71 | 0.86 | 0.15 | 2.17 |
| 17 | 7 | 1.71 | 0.43 | 0.14 | 0.86 | 3.20 | 1.77 |
| 18 | 5 | 0.2 | 0.4 | 0.8 | 1.4 | 0.052 | 3.34 |
| 19 | 4 | 1.25 | 0.25 | 0 | 0 | 0.47 | 0.15 |
| 20 | 9 | 0.11 | 0 | 0.33 | 1.33 | 0 | 6.56 |
| MEAN ± SD | 6.5 ± 1.8 | 0.6 ± 0.6 | 0.3 ± 0.2 | 0.2 ± 0.3 | 0.9 ± 0.5 | 0.4 ± 1.0 | 2.6 ± 1.9 |
| P value | 0.277 | 0.017 | 0.207 | 0.052 | 0.030 | 0.044 | 0.141 |
LoC, loss of papillary contour; RP, Randall's plaque.
Plugging, Pitting, LoC (loss of papillary contour), and RP (Randall’s plaque) are mean scores for papillae graded.15
% Plugging and % RP are obtained by measuring surface areas of plugging (yellow plaque) or RP on still frames from each papilla.55
P values are Student's t tests assuming unequal variances, comparing brushite and CaOx.
Bold font indicates P < 0.05.
Number of papillae from each patient that were able to be graded.
Transcriptomic Profiling Uncovers an Immune Activation Molecular Response in Brushite Papillary Biopsies
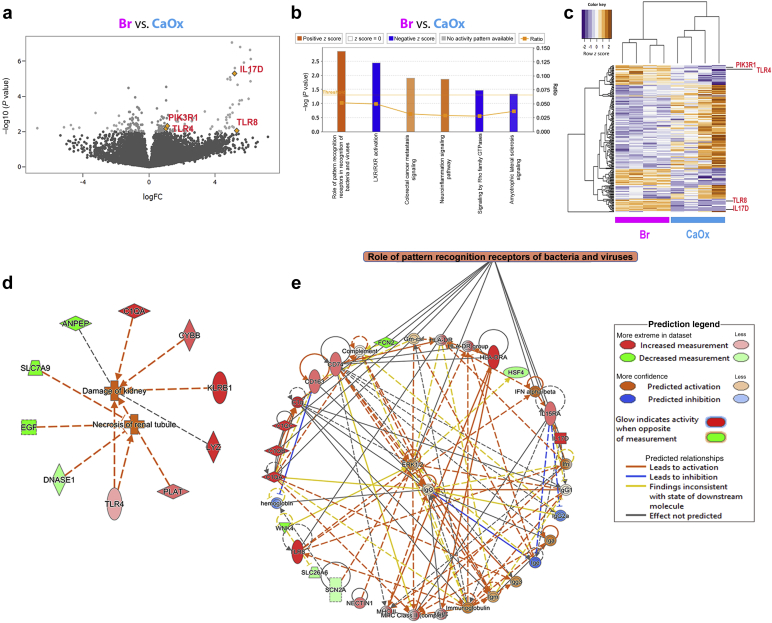
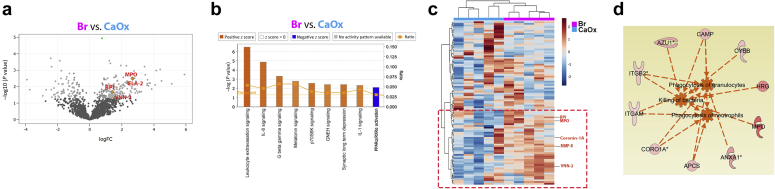
We extracted RNA from cryopreserved papillae (n = 4 each group) and performed RNA sequencing analysis as described in the methods. Differential expression analysis is presented in Supplementary Data File S1. Bioinformatic pathway analysis on differentially expressed genes showed that the pathway with most significant gene clustering (P < 0.01, Fisher’s exact test) and highest z-score for activation (z score >2) was that of pathogen recognition by the immune system (Figure 3a–c), which included proinflammatory genes such as TLR4, TLR8, IL17D, and PIK3R1. These were among the top upregulated genes in the brushite versus CaOx papillae (Figure 3c). Additional disease pathway and network analysis also confirmed a heightened injury and inflammatory activation signature in brushite versus CaOx (Figure 3d and e). Notably, many genes in the inflammatory pathways derived from this analysis are involved in inflammation and neutrophil activation (Figure 3d and e).
Figure 3.
Transcriptomic analysis of papillary biopsies. (a) Total RNA obtained from 4 calcium oxalate (CaOx) and 4 brushite (Br) papillary biopsies were individually sequenced. Volcano plot was made using negative log10 transformed P values against the log ratio, using values from brushite versus CaOx. Few genes that are part of the top significantly upregulated pathway are shown. (b) Differentially expressed genes as described in the Methods (brushite vs. CaOx) were analyzed for pathway enrichment, using ingenuity pathway analysis (IPA), and significantly altered pathways (P < 0.05) are shown with absolute z score >1 (orange, activation; blue, inhibition). The top pathway was the role of pathogen recognition receptors in recognition of bacteria and viruses with P = 0.00142 and z score for activation of 2.236. (c) Unsupervised hierarchical clustering was constructed for the top 300 genes sorted by P value, showing also genes from the top upregulated pathway. (d) Disease pathway analysis differentially activated in brushite versus CaOx, supporting heightened injury in the brushite papillae. (e) Signaling pathway analysis consistent with inflammatory activation signature in brushite versus CaOx. Color code is indicated in the legend. The orange-colored boxes indicate predicted activation, based on also on IPA. FC, fold change; GTP, guanosine triphosphate.
Label-Free Quantitative Mass Spectrometry of Kidney Stones Reveals a Unique Signature of Neutrophil Activation in Brushite Stones
In stones from 5 patients from each group, we identified 1947 proteins available across the sample types (Supplementary Data File S2). Bioinformatics analysis on differentially abundant proteins in brushite versus CaOx identified neutrophil-specific pathways such as leukocyte extravasation and IL-18 signaling among the top significantly enriched pathways (P < 0.01, Fisher’s exact test) with highest activation z scores (>2) (Figure 4). We identified 96 proteins in our data as definite neutrophil proteins (Supplementary Data File S3).28, 29, 30, 31, 32, 33, 34 Heat map visualization (Figure 4c) indicated clustering and increased abundance of neutrophil activation proteins (e.g., myeloperoxidase fold change 5.53, false discovery rate–adjusted P value = 0.006), vascular non-inflammatory molecule-2, fold change 3.52, false discovery rate–adjusted (P value = 0.02) in brushite stones compared with CaOx stones.
Figure 4.
Proteomic analysis of kidney stones. (a) Volcano plot showing P values (–log10) versus protein ratio of brushite (Br) versus calcium oxalate (CaOx) for all 1716 proteins, fulfilling strict quantification criteria. Gene name of few proteins involved in inflammatory signaling are indicated with myeloperoxidase (MPO) and vascular noniinflammatory molecule 2 (VNN2) significantly increased by false discovery rate (FDR). (b) The top canonical pathways enriched by ingenuity pathway analysis (IPA) are shown in a design similar to Figure 3. (c) Unsupervised hierarchical clustering of neutrophil specific proteins is shown, and the red box underscores proteins involved in neutrophil activity, and few examples are indicated to the right. (d) Disease pathways predicted by IPA. The proteins indicated are related to activation of neutrophil specific pathways such as phagocytosis of granulocytes, killing of bacteria, and phagocytosis of neutrophils in brushite versus CaOx. Orange, activation; blue, inhibition. FC, fold change; GNRH, gonadotropin releasing hormone; IL, interleukin; RXR, retinoid X receptor; BPI, bactericidal increasing permeability protein; VNN, vascular non-inflammatory molecule.
Large-Scale 3D Tissue Cytometry Maps the Molecular Signature of Neutrophil Activation to Cell Biology In Situ
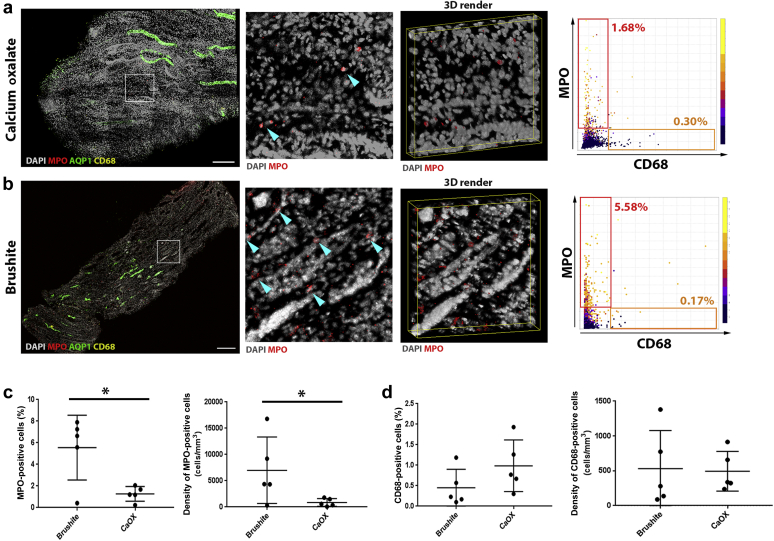
Large-scale 3D confocal imaging was performed on frozen papillary biopsies (patients with brushite and CaOx), stained for myeloperoxidase, CD68, aquaporin 1, and 4′,6-diamidino-2-phenylindole, to label neutrophils,35 activated macrophages,36 thin descending limbs,37 and nuclei, respectively. Quantitative 3D tissue cytometry was performed using the volumetric tissue cytometry and analysis software (VTEA) (Figure 5a and b).7
Figure 5.
Large-scale 3D imaging and tissue cytometry analysis of inflammatory cells in papillary biopsies. Fifty-micron-thick calcium oxalate (CaOx) (a) or Brushite (b) papilla biopsy sections were immunolabeled with anti-myeloperoxidase (MPO) (red), anti–aquaporin 1 (AQP1) (green) and anti-CD68 (yellow) antibodies and 4′,6-diamidino-2-phenylindole (DAPI) (gray). At left, large-scale maximum projection images of entire biopsies with insets as indicated and a 3D rendering. Arrowheads indicate neutrophils. At right, representative scatter plots from volumetric tissue exploration and analysis (VTEA) are shown with the percentage of positive cells in the gated areas as indicated. Bar = 100 μm. Quantifications obtained from VTEA analysis were plotted (c,d) showing percent (out of total number of cells) and density of MPO+ and CD68+ cells, respectively. Each dot represents the value from a single patient’s biopsy. ∗Two-tailed Student's t test, P < 0.05.
Both neutrophil abundance (5.55% ± 2.68% vs. 1.27% ± 0.61%, P < 0.05) and density (6.99 × 103 ± 5.6 × 103/mm3 vs. 0.87 × 103 ± 0.67 × 103/mm3; P < 0.05) were significantly higher in brushite versus CaOx biopsies, respectively (Figure 5c). Importantly, we did not notice any difference in the abundance or density of CD68+ cells in brushite compared with CaOx (Figure 5d). Therefore, these findings confirm a specific increase in neutrophil cell infiltration in the papillae of brushite patients.
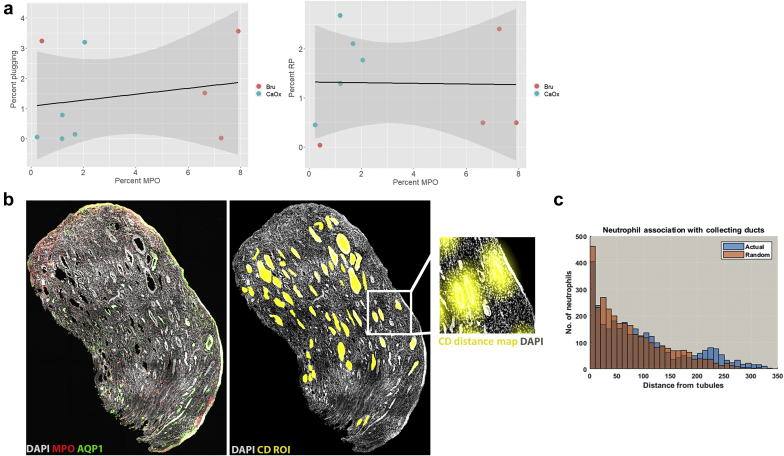
As plugging could cause inflammation, we asked if there is a link between neutrophil infiltration and the extent of papillary plugging. Figure 6a shows no correlation between neutrophil infiltration and plugging or Randall’s plaque formation. Furthermore, spatial correlation analysis, which was performed in 1 of the brushite biopsies, showed no distance correlation of neutrophil distribution to the location of the collecting duct (Figure 6b and c). These findings support that increased neutrophil infiltration in the brushite papillae is diffuse and does not cluster around a particular nidus of pathology.
Figure 6.
Correlation analysis between neutrophil distribution and papillary pathology. (a) Linear regression analysis was performed between neutrophil abundance in brushite (Bru) and calcium oxalate (CaOx) papilla biopsies compared with papillary endoscopic scoring of plugging (left) or Randall’s plaque (RP) (right). No correlation was observed between these events (P = 0.60 and 0.94, respectively). (b) Maximum projection of a papillary biopsy labeled with myeloperoxidase (MPO) (neutrophils) or aquaporin 1 (AQP1) (thin descending limb) shows diffuse pattern of neutrophil distribution (left). Region of interests (ROI) marking collecting ducts were manually drawn (yellow) and distance maps were built for every ROI (example of a distance map shown in the enlarged image in inset). The positions of all the neutrophils surveyed using volumetric tissue exploration and analysis were correlated to locations on the distance maps. Neutrophil distance distribution was nearly identical to a randomly generated distribution (Gaussian), shown in the histograms depicted in (c) (P = 1.0), suggesting no spatial correlation between neutrophil distribution and collecting ducts.
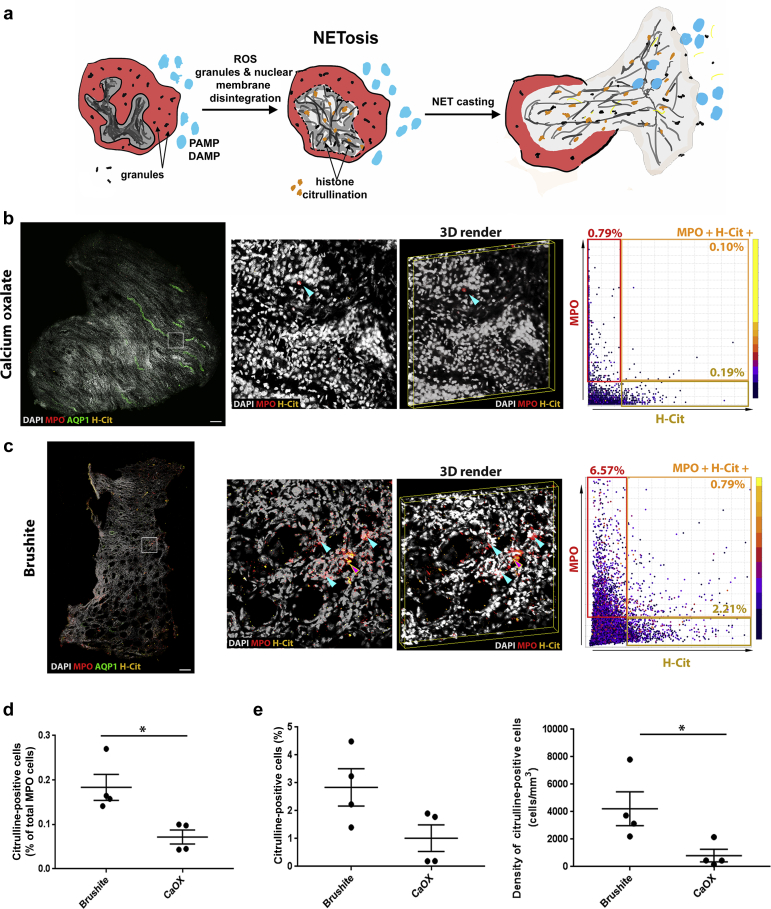
Increased NETosis in the Papilla Could Explain the Abundance of Neutrophil Proteins in Brushite Stones
In the brushite tissue specimens, many nuclei of neutrophils were poorly demarcated, which led us to hypothesize the presence of NETosis. Because citrullination of histone is a marker of NETosis (Figure 7a), we probed papillary biopsies for citrulline and performed 3D imaging and cytometry to quantify NETosis (Figure 7b and c, left). Using VTEA analysis, we confirmed that NETosis (citrulline+ neutrophils) was significantly increased in brushite versus CaOx papillae (0.18% ± 0.05% vs. 0.0%5 ± 0.02%; P < 0.05) (Figure 7d). Interestingly, there was also a significant increase in the citrullination of cells other than neutrophils in the brushite papillae (Figure 7e), which is also consistent with an inflammatory state.38
Figure 7.
Neutrophil extracellular trap formation (NETosis) in papillary biopsies. (a) Figure shows an illustration of neutrophils undergoing NETosis, triggered by invading pathogen associated molecular patterns (PAMP) or disease associated molecular patterns (DAMP). NETosis is initiated by intracellular calcium influx and production of reactive oxygen species (ROS). The intracellular signaling induces histone citrullination (yellow), chromatin condensation and disintegration of nuclear and granular membranes, which lead to the formation of NETs. These NETs consist of DNA packed with nuclear (e.g., histones) and granule (e.g., myeloperoxidase [MPO], elastase) proteins. (b,c) Fifty-micron-thick calcium oxalate (CaOx) (b) or brushite (c) papilla biopsy sections were immunolabeled with anti-MPO (red), anti–aquaporin 1 (AQP1) (green) and anticitrullinated histone (H-Cit) (yellow) antibodies and 4′,6-diamidino-2-phenylindole (DAPI, gray). At left, large-scale maximum projection images of entire biopsies with insets as indicated and a 3D rendering. Blue arrowheads indicate neutrophils, whereas red arrowhead point to neutrophils that also stain for H-Cit.At right, representative scatter plots from volumetric tissue exploration and analysis (VTEA) are shown with the percentage of positive cells in the gated areas as indicated. Bar = 200 μm. (d,e) Quantifications obtained from VTEA analysis were plotted (d,e), showing the percentage of H-Cit+ neutrophils undergoing NETosis (d) and percentage and density of non-neutrophil cells with citrullinated histones. Each dot represents the value from a single patient’s biopsy. ∗2-tailed Student's t test, P < 0.05.
Discussion
In this work, we determined that patients with different types of stone disease exhibit distinct cellular and molecular signatures. Our main finding is that tissue from brushite, but not CaOx SF shows abundant neutrophil activation, including NETosis, a neutrophil response to bacteria, and other potential pathogens. In addition, neutrophil-derived proteins are abundant in brushite, but not CaOx, stone matrix.
The marked difference between brushite and CaOx SF with respect to papillary and stone matrix findings is especially striking, given the fact that in this series demographic and metabolic characteristics of the 2 patient groups are so similar. Brushite stones carry a worse prognosis than CaOx stones in certain respects, as brushite stones have increased risk of recurrence,39 are frequently large and bilateral,40 and require more extracorporeal lithotripsy procedures41 compared with CaOx stones. Of concern, brushite stones appear to be increasing in prevalence, 42 including among pediatric SF.43 In some cases, patients who initially make CaOx stones may later convert to making stones formed predominantly of calcium phosphate.44,45 In a previous publication, we documented that a significant fraction of our brushite SF had initially made stones composed mainly of CaOx.27 The association of papillary inflammatory activation with brushite stone formation suggests that conversion may involve more than merely simple factors related to relative SS with respect to calcium phosphate, as some have proposed.46 At this time, it is not possible to tell whether the papillary inflammatory findings preceded brushite stone formation or followed it. We propose that finding of neutrophil related proteins preferentially in brushite stone matrix may suggest the former.
The signal stimulating the inflammatory response in brushite SF is unknown. Crystals are known to induce inflammation and cause NET formation when exposed to neutrophils in vitro.3,47,48 Although crystal-induced inflammation is quite possible in the brushite papilla, the widespread infiltration and the lack of clustering of neutrophils in specific areas of deposits argue against this, as does the fact that there was no significant relationship between tubule plugging and the signal for NETosis in the patients as a whole (Figure 6). Ascending infections can trigger neutrophil infiltration and NETosis.11 It is possible that patients with brushites may be uniquely susceptible to subclinical infections of papillary tissue and/or maladaptively respond to the presence of pathogens, thereby linking infection to brushite stone formation in potentially predisposed patients. Although there were no obvious signs of increased urinary infection rate in the brushite group, not all bacteria residing in urine may be amenable to detection by typical culture methods.49 Additional studies are needed to test this hypothesis, knowing that the therapeutic implications of a positive finding would be immense: treating an infection to prevent stone formation in a specific patient population.
Metabolic abnormalities such as oxidative stress deserve consideration.50,51 Oxidative stress can trigger a proinflammatory signaling that promotes cell injury, apoptosis, and inflammation. Our findings, especially of increased overall citrullination (Figure 7e) and altered expression of multiple histone genes in brushite papillary tissue, raise this possibility52 and will require future testing. Likewise, injury from SWL could have led to our findings, but both groups had similar exposure to SWL, making this a less plausible explanation.
The released neutrophil proteins were a major component of the brushite stone matrix. Whether neutrophil proteins are a nidus of brushite crystal nucleation, or stabilize brushite crystals to prevent their spontaneous transformation to apatite,27 are hypotheses beyond the current study.
At present, it is not known if brushite SF are unique in having these inflammatory findings in papillary tissue, and whether there is also a more systemic neutrophil activation response in these patients. Other types of stones are associated with tubule plugs and scarring,1 and future studies will be needed to determine if similar inflammatory pathways are upregulated in these stone phenotypes as well.
From a methodological standpoint, this study demonstrates how to integrate clinical phenotyping with large multimodal omics datasets and quantitative 3D imaging and tissue cytometry. Characterization of stone composition and tissue mineral deposits was confirmed with high precision using micro-computed tomography. The ability to map pathways discovered, using an unbiased approach to the cell biology within the tissue, is a demonstration of the strength and potential uses of such an integrated multidimensional approach.
Our study has limitations. Despite the important insights and relevance gained by working on human tissue, direct causal relationships cannot be inferred from this work. The representativeness of the small cohort of patients, particularly for each technology used—and the generalizability of our findings—are weaknesses, pointing to the need to validate these findings in a larger cohort. However, the ranges of many of the clinical variables in the patients from our study, such as urine pH and mineral supersaturation, were comparable with other studies,27,40,53 suggesting that the cohort used here is composed of patients with common stone presentations. The fact that not all interrogation techniques could be performed on specimens from the same patients is another limitation. However, the fact that the same findings were detected in different patients using orthogonal techniques could be viewed as a strength.
Despite the small number of patients, the depth provided by the molecular interrogation techniques was still sufficient to clearly detect specific molecular signals. The demonstration that this approach can extract meaningful results from a small cohort of patients is another strength of this study. Although we did not observe differences in all the proteins associated with neutrophils between the 2 types of stones, it is unlikely that all proteins released in the urine are equally incorporated into stones. Also, some neutrophil proteins, such as S-100, are made by other cells, such as collecting duct cells, which will mask the specific contribution of neutrophils.54
In conclusion, we demonstrate the presence of a stone-specific molecular signature of neutrophil activation and NETosis that informs on the pathogenesis of brushite stone formation. In the process, we showcase the strength of a multimodal approach consisting of thorough clinical phenotyping, integrated with high-resolution cellular and molecular interrogation, and linked to the biology in situ, using large-scale 3D imaging. In addition to the overall implications for the pathogenesis of nephrolithiasis, our study could serve as a model and a proof of concept for large precision medicine initiatives that are seeking to implement such multidimensional approaches to better classify and understand human disease.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (P01DK056788 and P30DK079312) and the National Institutes of Health Director’s Office (S10OD016208).
Author Contributions
MSM, SW, FW, JCW, and TME-A conducted the experiment design. JEL, AK, SB, and JCW conducted the surgical studies. EMW, FC, JEL, AK, KB, SB, JCW, and TME-A conducted the clinical studies. MSM, SW, JEL, AK, FW, SB, KB, JCW, and TME-A collected data. MSM, SW, SK, FW, EMW, FC, APE, KB, DB, JCW, and TME-A analyzed data. All authors prepared and edited the manuscript.
Translational Statement
Kidney stone disease is very common and causes significant morbidity. The main goal of this study was to uncover stone-type specific molecular signatures using a precision medicine approach that combines unbiased transcriptomics, proteomics, and innovative 3-dimensional imaging strategies. We found that increased neutrophil infiltration and NETosis are unrecognized new factors specifically associated with brushite stone formation. These discoveries underscore the unequivocal presence of inflammation in human stone disease and the divergence of cellular and molecular pathways in distinct forms of nephrolithiasis.
Footnotes
Figure S1. Volumetric tissue exploration and analysis (VTEA) tool for 3D cytometry. (A) 50-μm thick papilla section was imaged using confocal microscope. (B) Four channel-imaged volume was loaded into Image/FIJI. 3D nuclei segmentation was performed by VTEA and (C) VTEA generates scattered plot, where each dot represents measured parameters for a single cell. Cytometric events were gated using rectangle or free hand tool, which was able to identify subpopulation of cells. Gated cells were highlighted as nuclear overlays in the image volume to identify their spatial distribution (D). This allows immediate validation of the gates used on the scatter plots.
Figure S2. Multidimensional scaling (MDS) plot of the transcriptomic data by sample. An MDS plot for the papillary transcriptomic data visualization with dimensionality reduction shows clustering of the brushite specimens, but variability in the calcium oxalate specimens. Ca, calcium oxalate; Br, brushite.
Data File S1. Summary file for transcriptomics with differential expression analysis and summary statistics.
Data File S2. Summary file for raw data from label-free quantitative proteomics for proteins identified in each sample.
Data File S3. Neutrophil proteins identified in this study and 5 other publications.
Contributor Information
James C. Williams, Jr., Email: jwillia3@iupui.edu.
Tarek M. El-Achkar, Email: telachka@iu.edu.
Supplementary Material
References
- 1.Coe F.L., Evan A.P., Worcester E.M. Three pathways for human kidney stone formation. Urol Res. 2010;38:147–160. doi: 10.1007/s00240-010-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evan A.P., Lingeman J.E., Coe F.L. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 3.Khan S.R. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol. 2004;8:75–88. doi: 10.1007/s10157-004-0292-0. [DOI] [PubMed] [Google Scholar]
- 4.Joshi S., Wang W., Khan S.R. Transcriptional study of hyperoxaluria and calcium oxalate nephrolithiasis in male rats: inflammatory changes are mainly associated with crystal deposition. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taguchi K., Hamamoto S., Okada A. Genome-wide gene expression profiling of Randall's plaques in calcium oxalate stone formers. J Am Soc Nephrol. 2017;28:333–347. doi: 10.1681/ASN.2015111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witzmann F.A., Evan A.P., Coe F.L. Label-free proteomic methodology for the analysis of human kidney stone matrix composition. Proteome Sci. 2016;14:4. doi: 10.1186/s12953-016-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winfree S., Khan S., Micanovic R. Quantitative Three-Dimensional Tissue Cytometry to Study Kidney Tissue and Resident Immune Cells. J Am Soc Nephrol. 2017;28:2108–2118. doi: 10.1681/ASN.2016091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winfree S., Ferkowicz M.J., Dagher P.C. Large-scale 3-dimensional quantitative imaging of tissues: state-of-the-art and translational implications. Transl Res. 2017;189:1–12. doi: 10.1016/j.trsl.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaFavers K.A., Macedo E., Garimella P.S. Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hato T., Zollman A., Plotkin Z. Endotoxin preconditioning reprograms S1 tubules and macrophages to protect the kidney. J Am Soc Nephrol. 2018;29:104–117. doi: 10.1681/ASN.2017060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yipp B.G., Petri B., Salina D. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nature Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branzk N., Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol. 2013;35:513–530. doi: 10.1007/s00281-013-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coe F.L., Evan A.P., Lingeman J.E. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38:239–247. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evan A.P., Lingeman J.E., Coe F.L. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borofsky M.S., Paonessa J.E., Evan A.P. A proposed grading system to standardize the description of renal papillary appearance at the time of endoscopy in patients with nephrolithiasis. J Endourol. 2016;30:122–127. doi: 10.1089/end.2015.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams J.C., Jr., McAteer J.A., Evan A.P. Micro-computed tomography for analysis of urinary calculi. Urol Res. 2010;38:477–484. doi: 10.1007/s00240-010-0326-x. [DOI] [PubMed] [Google Scholar]
- 17.Parks J.H., Goldfisher E., Asplin J.R. A single 24-hour urine collection is inadequate for the medical evaluation of nephrolithiasis. J Urol. 2002;167:1607–1612. [PubMed] [Google Scholar]
- 18.Gildea J.J., Van Sciver R.E., McGrath H.E. Dopaminergic immunofluorescence studies in kidney tissue. Methods Mol Biol. 2017;1527:151–161. doi: 10.1007/978-1-4939-6625-7_12. [DOI] [PubMed] [Google Scholar]
- 19.El-Achkar T.M., Plotkin Z., Marcic B. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol. 2007;293:F1187–F1196. doi: 10.1152/ajprenal.00217.2007. [DOI] [PubMed] [Google Scholar]
- 20.Dobin A., Davis C.A., Schlesinger F. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breese M.R., Liu Y. NGSUtils: a software suite for analyzing and manipulating next-generation sequencing datasets. Bioinformatics. 2013;29:494–496. doi: 10.1093/bioinformatics/bts731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 23.Ewels P., Magnusson M., Lundin S. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy D.J., Chen Y., Smyth G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson J.W., Sklenar J., Barnes A.P. The START App: a web-based RNAseq analysis and visualization resource. Bioinformatics. 2017;33:447–449. doi: 10.1093/bioinformatics/btw624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evan A.P., Lingeman J.E., Worcester E.M. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec (Hoboken) 2014;297:731–748. doi: 10.1002/ar.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza Castro M., de Sa N.M., Gadelha R.P. Proteome analysis of resting human neutrophils. Protein Pept Lett. 2006;13:481–487. doi: 10.2174/092986606776819529. [DOI] [PubMed] [Google Scholar]
- 29.Jethwaney D., Islam M.R., Leidal K.G. Proteomic analysis of plasma membrane and secretory vesicles from human neutrophils. Proteome Sci. 2007;5:12. doi: 10.1186/1477-5956-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lominadze G., Powell D.W., Luerman G.C. Proteomic analysis of human neutrophil granules. Mol Cell Proteomics. 2005;4:1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Piubelli C., Galvani M., Hamdan M. Proteome analysis of rat polymorphonuclear leukocytes: a two-dimensional electrophoresis/mass spectrometry approach. Electrophoresis. 2002;23:298–310. doi: 10.1002/1522-2683(200202)23:2<298::AID-ELPS298>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Tomazella G.G., da Silva I., Laure H.J. Proteomic analysis of total cellular proteins of human neutrophils. Proteome Sci. 2009;7:32. doi: 10.1186/1477-5956-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uriarte S.M., Powell D.W., Luerman G.C. Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J Immunol. 2008;180:5575–5581. doi: 10.4049/jimmunol.180.8.5575. [DOI] [PubMed] [Google Scholar]
- 34.Xu P., Crawford M., Way M. Subproteome analysis of the neutrophil cytoskeleton. Proteomics. 2009;9:2037–2049. doi: 10.1002/pmic.200800674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley P.P., Priebat D.A., Christensen R.D. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 36.Thomsen L.L., Miles D.W., Happerfield L. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J., Lee G.S., Tisher C.C. Role of apoptosis in development of the ascending thin limb of the loop of Henle in rat kidney. Am J Physiol. 1996;271:F831–F845. doi: 10.1152/ajprenal.1996.271.4.F831. [DOI] [PubMed] [Google Scholar]
- 38.Makrygiannakis D., af Klint E., Lundberg I.E. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65:1219–1222. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan L.E., Enders F.T., Lieske J.C. Predictors of symptomatic kidney stone recurrence after the first and subsequent episodes. Mayo Clin Proc. 2019;94:202–210. doi: 10.1016/j.mayocp.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krambeck A.E., Handa S.E., Evan A.P. Profile of the brushite stone former. J Urol. 2010;184:1367–1371. doi: 10.1016/j.juro.2010.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parks J.H., Worcester E.M., Coe F.L. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–785. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 42.Daudon M., Bouzidi H., Bazin D. Composition and morphology of phosphate stones and their relation with etiology. Urol Res. 2010;38:459–467. doi: 10.1007/s00240-010-0320-3. [DOI] [PubMed] [Google Scholar]
- 43.Wood K.D., Stanasel I.S., Koslov D.S. Changing stone composition profile of children with nephrolithiasis. Urology. 2013;82:210–213. doi: 10.1016/j.urology.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 44.Mandel N., Mandel I., Fryjoff K. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol. 2003;169:2026–2029. doi: 10.1097/01.ju.0000065592.55499.4e. [DOI] [PubMed] [Google Scholar]
- 45.Parks J.H., Coe F.L., Evan A.P. Urine pH in renal calcium stone formers who do and do not increase stone phosphate content with time. Nephrol Dial Transplant. 2009;24:130–136. doi: 10.1093/ndt/gfn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siener R., Netzer L., Hesse A. Determinants of brushite stone formation: a case-control study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linkermann A., Stockwell B.R., Krautwald S. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14:759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 48.Mulay S.R., Kulkarni O.P., Rupanagudi K.V. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1 beta secretion. J Clin Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilt E.E., McKinley K., Pearce M.M. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi S., Wang W., Peck A.B. Activation of the NLRP3 inflammasome in association with calcium oxalate crystal induced reactive oxygen species in kidneys. J Urol. 2015;193:1684–1691. doi: 10.1016/j.juro.2014.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan S.R. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3:256–276. doi: 10.3978/j.issn.2223-4683.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero V., Fert-Bober J., Nigrovic P.A. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006869. 209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linnes M.P., Krambeck A.E., Cornell L. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013;84:818–825. doi: 10.1038/ki.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norman A.W., Vanaman T.C., Means A.R. Academic Press; San Diego, CA: 1987. Calcium-Binding Proteins in Health and Disease. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.