Abstract
Generalized severe junctional epidermolysis bullosa (GS-JEB) is an incurable and fatal autosomal recessively inherited blistering skin disease caused by mutations in the LAMA3, LAMB3, or LAMC2 genes. Most of these mutations are nonsense mutations that create premature termination codons that lead to impaired production of functional laminin 332, a protein needed for epidermal-dermal adherence. Gentamicin induces readthrough of nonsense mutations and restores the full-length protein in various genetic diseases. Using primary keratinocytes from three GS-JEB patients, we showed that gentamicin induced functional laminin 332 that reversed a JEB-associated, abnormal cell phenotype. In a subsequent open-label trial involving the same patients, we examined whether 0.5% gentamicin ointment applied topically to open skin wounds could promote nonsense mutation readthrough and create new laminin 332 in the patients’ skin. Gentamicin-treated wounds exhibited increased expression of laminin 332 at the dermal-epidermal junction for at least 3 months and were associated with improved wound closure. There were no untoward side effects from topical gentamicin. The newly induced laminin 332 did not generate anti-laminin 332 autoantibodies in either the patients’ blood or skin. Gentamicin readthrough therapy may be a treatment for GS-JEB patients with nonsense mutations.
Graphical Abstract
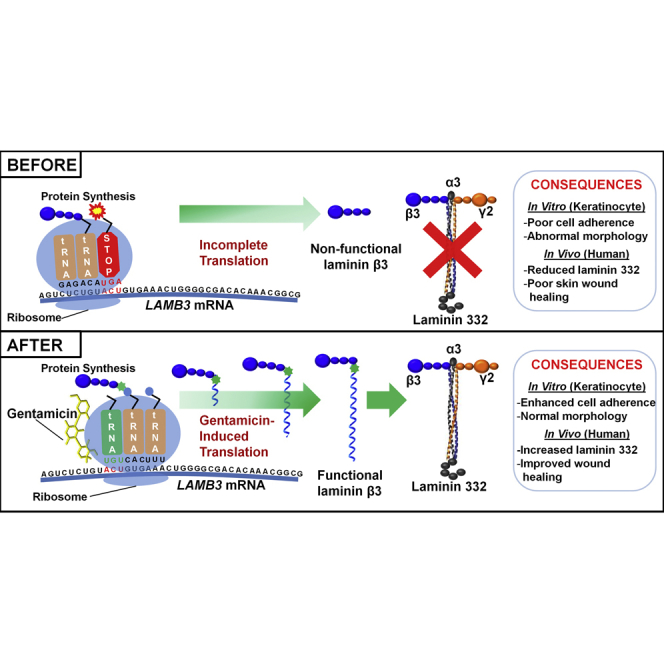
Many patients with junctional epidermolysis bullosa, a genetic skin disease, lack laminin 332 protein due to nonsense mutations in laminin 332 genes. This paper reports that topical gentamicin suppresses nonsense mutations, induces new laminin 332, and improves wound closure. Gentamicin may provide a novel, readily available therapy for these patients.
Introduction
Generalized severe junctional epidermolysis bullosa (GS-JEB), previously called Herlitz type JEB, is a lethal, autosomal recessive genetic skin-fragility disorder caused by loss-of-function mutations in LAMA3, LAMB3, or LAMC2, genes that encode the laminin α3, β3, or γ2 chain, respectively. In human skin, these three chains combine to form laminin 332, a heterotrimeric macromolecule that is an essential component of structures called anchoring filaments. Functional anchoring filaments localize to the dermal-epidermal junction (DEJ) where they bind to basal keratinocyte hemidesmosomes to mediate the adherence of the epidermis to the dermis.1,2 GS-JEB patients have an absence or a paucity of functional laminin 332, resulting in severe skin and mucosal blistering, water/nutrient loss, and poor wound healing,3 which frequently leads to refractory anemia, failure to thrive, respiratory failure, sepsis, and death. The mortality rate in GS-JEB is 73%, with few patients surviving past the first year of life.4, 5, 6
There is no cure for GS-JEB. Treatment is largely palliative.2,5 For the few GS-JEB patients who survive past infancy, management includes prompt treatment of skin wound infections, periodic iron or blood transfusions to treat the anemia, nutritional supplementation, pain control, and meticulous bandaging of open erosive skin wounds. Patients suffer a significantly reduced quality of life. The disease is a great emotional and economic burden on families, with average annual healthcare costs in excess of $50,000.7 Several experimental therapeutic strategies are in development or have been attempted such as protein replacement therapy, bone marrow stem cell transplantation (SCT), and gene-corrected keratinocyte autografts.2,8, 9, 10, 11, 12
Approximately 83% of GS-JEB patients have a nonsense mutation in at least one allele of their LAMA3, LAMB3, or LAMC2 genes.2 The LAMB3 gene is the most commonly affected gene, accounting for 80% of all GS-JEB cases.13 Of these LAMB3 gene mutations, 95% are nonsense mutations that generate premature termination codons (PTCs) and prevent the synthesis of the laminin β3 protein or create a truncated protein incapable of forming a functional laminin 332 heterotrimer.13 Given the high prevalence of nonsense mutations in GS-JEB patients, PTC readthrough may be a valid treatment approach.
Aminoglycoside antibiotics, such as gentamicin, are able to suppress nonsense mutations by binding to a specific site on mammalian ribosomal RNA, impairing codon/anticodon recognition at the aminoacyl transfer RNA site, and restore the full-length functional protein.14,15 To date, aminoglycoside-induced PTC readthrough has been demonstrated in several other genetic disorders such as cystic fibrosis (CF) and Duchenne’s muscular dystrophy (DMD).16, 17, 18 Furthermore, aminoglycoside-induced PTC readthrough has been successful in genetic skin diseases such as xeroderma pigmentosum and recessive dystrophic epidermolysis bullosa (RDEB).19, 20, 21 RDEB is another form of EB, and we demonstrated that administration of topical and intradermally injected gentamicin in these patients created robust and sustained new type VII collagen (C7) and anchoring fibrils at the DEJ, improved wound closure, and decreased new blister formation.21
Recently, we showed that gentamicin was capable of inducing PTC readthrough in GS-JEB cultured keratinocytes harboring LAMB3 nonsense mutations to produce full-length laminin β3, restore laminin 332 secretion and assembly, and generate proper localization of the α6β4 integrin in a three-dimensional in vitro skin equivalent model. It also reversed the JEB-associated abnormal keratinocyte morphology, poor growth potential, hypermotility, and faulty matrix attachment.22
In the current study, we extended our in vitro findings to an open-labeled clinical trial of three GS-JEB patients with nonsense mutations. With these three JEB patients, we first placed their keratinocytes into culture and showed that the administration of gentamicin to the cultures generated new laminin α3 or β3 chains, and reversed the abnormal cellular parameters (morphology and matrix attachment) characteristic of JEB keratinocytes. We then enrolled these patients into an open-label clinical trial whereby selected open wounds were treated with topical 0.5% gentamicin twice a day for 2 weeks. We found that topical gentamicin induced new and continuous laminin 332 that was properly located at the DEJ of patients’ skin and was sustained for 3 months. Furthermore, the newly induced laminin 332 in the topical gentamicin-treated wounds resulted in improved and more durable wound closure.
Results
Patients
Three GS-JEB patients (Table 1) with at least one nonsense mutation in LAMA3 or LAMB3 were recruited from August 2018 through June 2019 for this open-labeled interventional clinical study. All three patients met the inclusion criteria described in Materials and Methods, completed the study, and were assessed for primary and secondary endpoints (Figure 1). There were two primary endpoints: (1) new expression of laminin 332 within the DEJ of selected wounds treated with topical gentamicin, and (2) an assessment of safety parameters, including clinical symptoms, potential laboratory abnormalities (blood urea nitrogen [BUN], creatinine, calculated creatinine clearance), the development of anti-laminin 332 auto-antibodies, and audiometry. Specifically, we wanted to screen for the potential known gentamicin side effects of nephrotoxicity and ototoxicity. The secondary endpoint was an assessment of wound closure of wounds treated with topical gentamicin. In each of the three patients, three open erosive skin wounds of various sizes were treated with topical gentamicin 0.5% ointment twice a day for 2 weeks. All three patients had follow-up visits at 1 and 3 months after treatment. Table 1 shows the baseline clinical data of the three patients and their pre-treatment baseline expression of laminin 332 compared with those of a normal human skin (NHS) control. Patient 1 (PT1) has a nonsense mutation in LAMB3, while patient 2 (PT2) and patient 3 (PT3) have nonsense mutations in LAMA3. Immunofluorescence (IF) staining with monoclonal antibodies to each of the three chains of laminin 332 was performed to detect laminin 332 expression. PT1 and PT3 had less than 2% of the laminin 332 expression observed in the DEJ of NHS. PT2 had 12% of the laminin 332 expression observed in the DEJ as detected by antibody to the laminin α3 chain.
Table 1.
Summary of the Mutations, Baseline Laminin 332 Expression, and Demographics of the Three Study Patients
| PT1 | PT2 | PT3 | |
|---|---|---|---|
| Sex | male | female | female |
| Age (years) | 6 | 9 | 1 |
| Gene affected | LAMB3 | LAMA3 | LAMA3 |
| Allele 1/allele 2 | C325X/c.629-12T>A | Y63X/Y63X | Q625X/c.4909+1G>A |
| Laminin 332 at DEJ (%)a | <2.0 | 12 | <1.0 |
Assessed by immunofluorescence staining using monoclonal antibodies to laminin β3 (PT1) and α3 (PT2 and PT3) chains of laminin 332. Expression levels at the dermal-epidermal junction were calculated from comparison to normal human skin set at 100% (see Materials and Methods).
Figure 1.
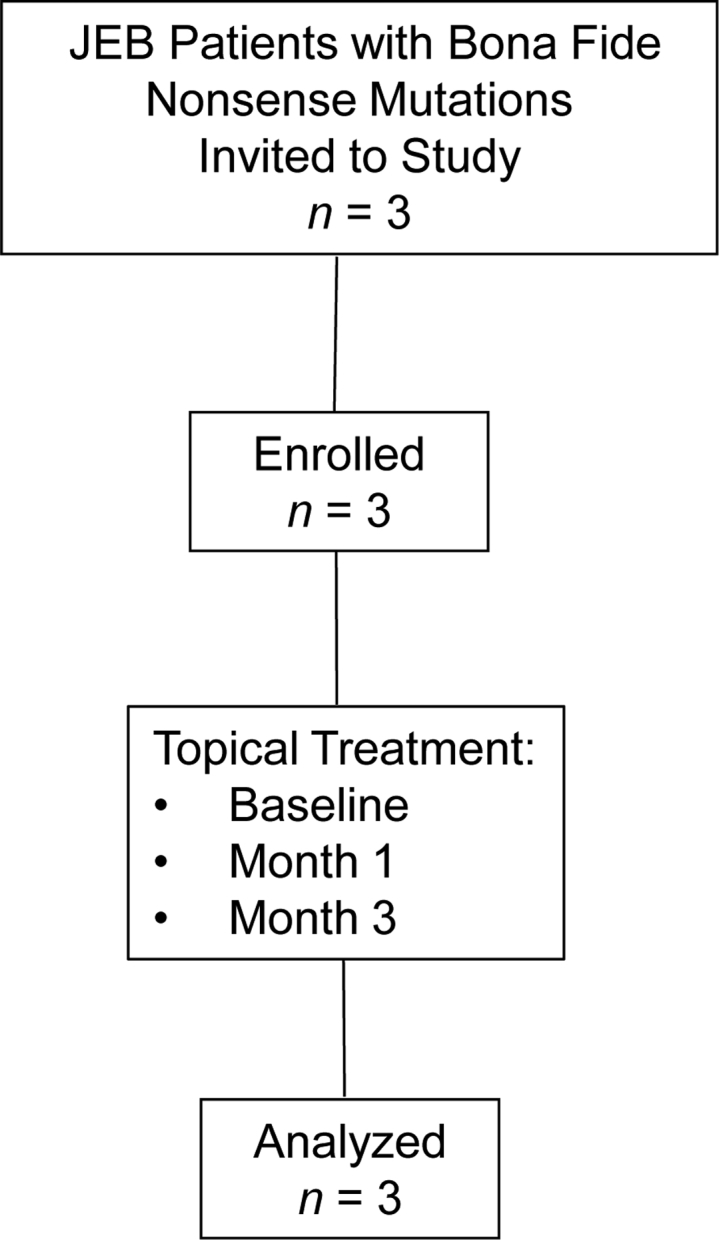
CONSORT Flow Diagram
Flowchart summarizing GS-JEB patient enrollment and completion of the trial.
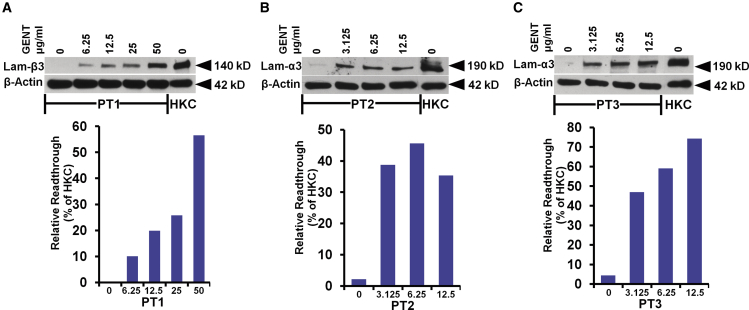
Gentamicin Induced Dose-Dependent Full-Length Laminin α3 and β3 in Primary GS-JEB Keratinocytes
To investigate the efficacy of gentamicin in vitro, we isolated primary keratinocytes from skin biopsies from each of the three GS-JEB patients prior to any gentamicin treatment, as previously described.23 Cells were treated with increasing concentrations of gentamicin once daily for 96 h, and cell lysates were prepared and subjected to immunoblot analysis. As shown in Figure 2, gentamicin mediated a dose-dependent induction of the full-length 140-kDa laminin β3 chain for PT1 and the full-length 190-kDa laminin α3 chain for PT2 and PT3. In contrast, untreated cells displayed no or negligible expression of laminin β3 or α3, respectively. The optimal gentamicin concentration for inducing readthrough was 50 μg/mL for PT1 and 12.5 μg/mL for PT2 and PT3. Under optimal gentamicin concentrations that maximized laminin chain production without significantly impairing cell viability (Figure S1), quantification with ImageJ showed that in the GS-JEB keratinocyte cultures gentamicin induced a maximum expression of laminin β3 at 57% (PT1) or laminin α3 at 45% (PT2) and 74% (PT3) of the levels observed in normal human keratinocyte (HKC) cultures. These data indicate that gentamicin is capable of increasing laminin α3 and β3 expression in primary GS-JEB keratinocytes carrying nonsense mutations, in accordance with our previous publication.22
Figure 2.
Gentamicin Mediates Dose-Dependent Induction of Full-Length α3 or β3 in Primary Keratinocytes Derived from GS-JEB Patients
(A–C) The primary keratinocytes isolated from PT1 (A), PT2 (B), and PT3 (C) were either untreated or treated with increasing concentrations of gentamicin (GENT), as indicated, for 96 h. Cell lysates were prepared and then subjected to 4%–12% SDS-PAGE, along with control lysates from normal keratinocytes (HKCs), followed by immunoblot analysis with a monoclonal antibody against the defective laminin subunit (either α3 or β3) for each respective patient in addition to a loading control, β-actin. Before treatment, all patient cells expressed minimal to no laminin α3 or β3. Treatment with gentamicin induced full-length laminin α3 or β3 in a dose-dependent manner. Three independent experiments were performed with similar results. ImageJ analysis of laminin α3 or β3 expression normalized with β-actin is shown below the respective blots. Results are displayed as a percentage of HKC expression (100%).
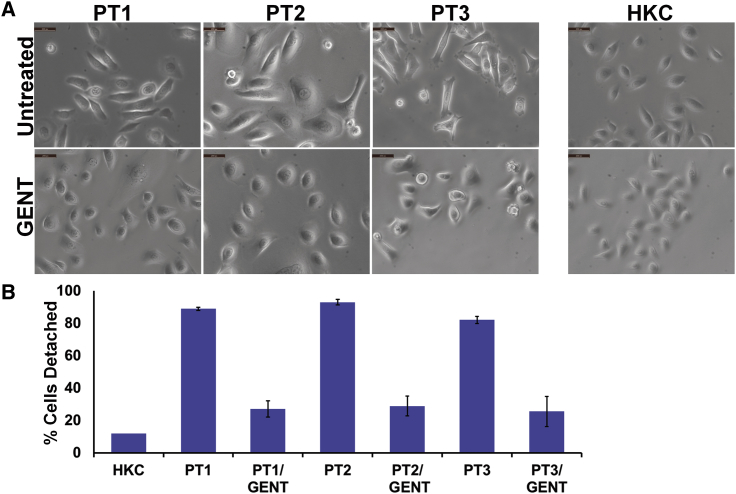
Gentamicin Normalized the Morphology and Poor Cell-Matrix Adhesion of GS-JEB Keratinocytes
Gentamicin-induced PTC readthrough is mediated by mispairing between the stop codon and the near-cognate aminoacyl tRNA, resulting in the insertion of a random amino acid instead of chain termination.14,15 It is possible that the random amino acid inserted at the PTC could lead to an impaired, dysfunctional readthrough product compared with the normal, wild-type protein. Therefore, we wanted to determine whether the gentamicin-induced laminin 332 produced by the GS-JEB keratinocytes was functional. As a measure of functionality, we determined whether the gentamicin induction of laminin 332 would correct the characteristic GS-JEB cellular phenotype including abnormal cellular morphology and poor cell-substratum adhesion.24 As seen in Figure 3A, light microscopy demonstrated that 86%–100% of untreated keratinocytes from all three GS-JEB patients were morphologically abnormal with polymorphic elongated spindle-shaped cells and large round cells. In cultures of normal keratinocytes, only 2% of the cells exhibited this abnormal morphology. Treatment with gentamicin significantly reduced the percentage of GS-JEB keratinocytes with abnormal morphology to 7% (PT1), 6% (PT2), and 18% (PT3). This indicates that gentamicin treatment can correct the abnormal morphology of GS-JEB cells.
Figure 3.
Gentamicin Normalizes the Cell Morphology and Substratum Attachment of GS-JEB Cells
(A) Under light microscopy, cultured normal HKCs show a compact, typical epithelial cell morphology. Untreated GS-JEB keratinocytes, in contrast, exhibit a mixture of cell morphologies, with some cells displaying an elongated, spindle-shaped morphology while other cells have an enlarged cytoplasm. After 48 h of treatment with 50 μg/mL gentamicin for PT1 and 12.5 μg/mL for PT2 and PT3, the treated GS-JEB keratinocytes show a more typical epithelial cell morphology reminiscent of normal human keratinocytes (HKCs; scale bars, 100 μm). (B) Primary GS-JEB keratinocytes were seeded onto tissue culture plates and treated with an effective, non-toxic dose of GENT as above for 48 h. All cells were trypsinized, and the number of cells detached after 5 min was determined and expressed as a percentage of the total number of cells. After 5 min, the primary GS-JEB keratinocytes were almost completely detached, while gentamicin-treated GS-JEB keratinocytes exhibited substantially reduced detachment that approached that observed in HKCs. Each value is the mean ± SD (n = 4).
Next, we evaluated whether gentamicin treatment could correct the faulty cell-substratum adhesion seen in GS-JEB cells by subjecting normal and primary GS-JEB keratinocytes derived from PT1, PT2, and PT3 (either untreated or treated with gentamicin) to a well-established kinetic cell-detachment assay.25 Figure 3B depicts the percentage of cells detached from the substratum 5 min after the addition of trypsin. Untreated primary GS-JEB cells exhibited poor cell-substratum adhesion, with up to 92% of cells detached at 5 min compared to 12% detachment observed in HKCs. In contrast, gentamicin-treated GS-JEB cells exhibited improved cell-matrix adhesion to a degree approaching that of HKCs. These data indicate that the laminin 332 induced by gentamicin in GS-JEB keratinocyte cultures is functional and able to correct the faulty substratum attachment inherent in GS-JEB cells.
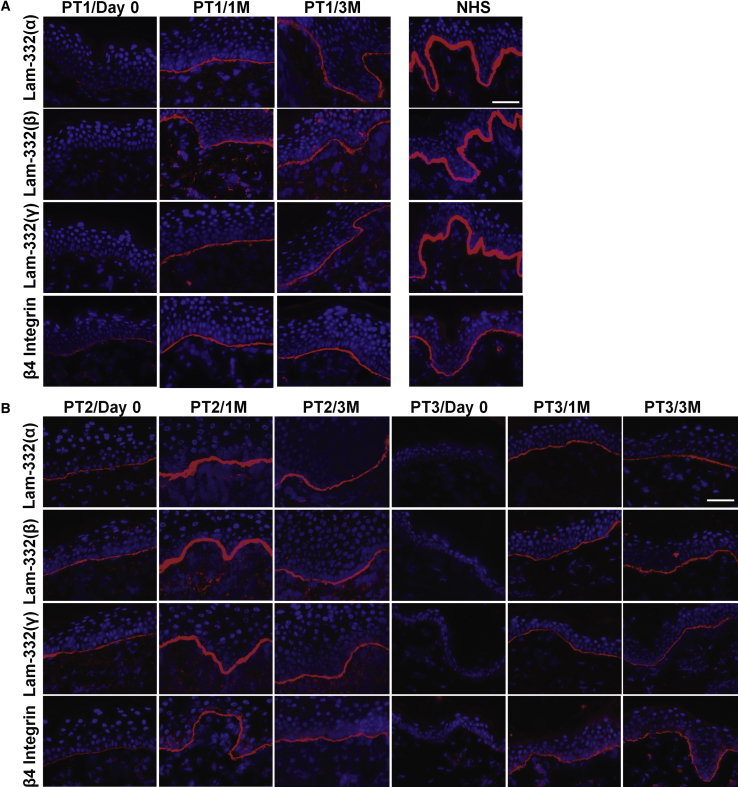
Topical Gentamicin Induced New Laminin 332
After verifying the efficacy of gentamicin in patients’ keratinocytes in vitro, we proceeded to conduct an open-label clinical trial by applying gentamicin topically twice a day to three open erosive skin wounds for 2 weeks. Biopsies were taken at baseline and at months 1 and 3 after treatment and evaluated for laminin 332 expression at the DEJ by IF staining with antibodies against the three chains of laminin 332 (α3, β3, and γ2) as well as with an antibody against the β4 integrin. It is known that the β4 integrin combines with the α6 integrin to form the α6β4 integrin heterodimer within the hemidesmosomes of basal keratinocytes in NHS. Studies have shown that laminin 332 is a prerequisite for correct localization of α6β4 integrin to the DEJ.26 Figure 4 shows the laminin 332 expression by IF of patients’ skin samples taken at baseline (day 0) and at months 1 and 3 after gentamicin treatment with three separate antibodies targeting the α3, β3, and γ2 subunits. PT1 and PT3 had less than 2% detectable laminin 332 expression by antibodies to each laminin chain, as well as 19% and 3% β4 integrin expression at the DEJ at baseline when compared to NHS. At baseline prior to treatment, PT2 had 12% (α3 chain), 34.5% (β3 chain), 37.6% (γ2 chain), and 25% β4 integrin of that observed in NHS.
Figure 4.
Topical Gentamicin Generated New Laminin 332 in GS-JEB Patients
(A and B) IF staining of skin biopsy specimens from PT1 (A), PT2 (B), and PT3 (B) taken prior to treatment and 1 month (1M) and 3 months (3M) after gentamicin treatment using monoclonal antibodies that specifically target the three chains (α3, β3, or γ2) of laminin 332 or the β4 integrin. Note that within the treated sites, gentamicin induced continuous expression of all three chains of laminin 332 at the DEJ of all three patients. Additionally, β4 integrin is increased and polarized along the entire DEJ after treatment. All images were obtained using the same camera and identical exposure times. Scale bars, 50 μm.
All three patients responded to gentamicin, but their responses were variable. At 1 month after topical gentamicin, for PT1, laminin 332 expression increased to 41% (α3 chain), 60% (β3 chain), and 57% (γ2 chain) of that seen in NHS using antibodies to α3, β3, and γ2, respectively. β4 integrin increased to 75%. For PT2, expression of the three laminin 332 chains increased to 93% (α3 chain), 120% (β3 chain), and 102% (γ2 chain) of that seen in NHS. β4 integrin expression also increased to 100%. For PT3, gentamicin treatment increased laminin 332 to 40% (α3 chain), 49% (β3 chain), and 55% (γ2 chain). After gentamicin treatment, the expression of the β4 integrin increased up to 91% of that seen in NHS. At 3 months for PT1 and PT3, the gentamicin-induced expression of laminin 332 and β4 integrin was maintained at a similar level, whereas there was a notable reduction in laminin 332 for PT2.
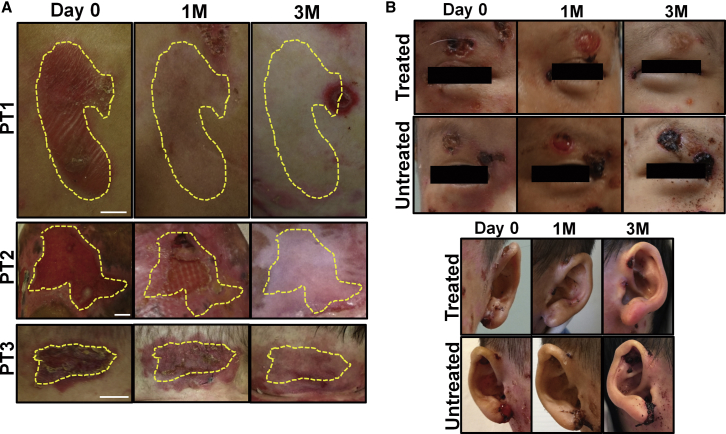
Topical Gentamicin Improved Wound Closure
GS-JEB patients have severe skin fragility, and slight trauma can easily result in new blisters and erosive wounds. During the study, patients were not restricted from performing their normal daily activities. During the baseline visit, each patient had three open wounds selected for treatment with topical gentamicin. As shown in Table 2, the wound areas decreased significantly from baseline based on image analysis of standardized photographs taken at baseline and during clinic visits at 1 and 3 months. Figure 5A shows representative images of erosive wounds treated with topical gentamicin in the study participants. All treated erosions were closed or reduced in wound size by 1 month and fully closed after 3 months. Figure 5B shows images of bilateral open erosions located above the eyes of PT1. These parallel wounds were created by ophthalmology speculums and had not healed for more than a year. One side was treated with gentamicin while the other was not. As seen, the side treated with topical gentamicin had fully closed by the end of the study, whereas the untreated side repeatedly reopened throughout the evaluation period. Similarly, the gentamicin-treated ear showed enhanced wound closure, while the untreated ear failed to heal.
Table 2.
Characteristics and Clinical Assessment of the Test Sites Treated Topically in the Three Study Patients
| Location | PT1 |
PT2 |
PT3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Left Middle Back | Left Upper Back | Face | Right Thigh | Right Flank | Left Thigh | Right Lower Abdomen | Right Buttock | Neck | |
| Treated area (cm2) | 9.93 | 11.75 | 2.23 | 16.65 | 6.21 | 20.10 | 7.25 | 5.20 | 2.45 |
| Wound closurea | |||||||||
| 1 month | +++ | +++ | ++ | ++ | +++ | ++ | +++ | + | ++ |
| 3 months | +++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
Percentage of wound closure based on clinical photographs taken at day 0, month 1, and month 3 (see Materials and Methods). Percentage of wound closure: +++, 85%–100%; ++, 50%–85%; +, <50%.
Figure 5.
Topical Gentamicin Improved Wound Closure
(A) Representative photographs of the open erosions prior to treatment (day 0), 1 month, and 3 months after treatment with topical gentamicin in PT1-PT3. Yellow dotted lines indicate the treated areas. (B) Representative photographs of matching treated and untreated wounds for PT1. The left side of the face including the eyebrow and ear were treated with topical gentamicin, while the right side was not. Note that the treated wounds exhibited full wound closure by 3 months whereas the untreated wounds showed minimal to no improvement. Scale bars, 1 cm.
Gentamicin Did Not Generate Any Adverse Effects or Anti-Laminin 332 Antibodies
Safety parameters that were measured prior to treatment and at follow-up visits included complete blood counts, BUN, creatinine, calculated creatinine clearance, electrolytes, liver function tests, and pure-tone audiometry. All of these laboratory values were normal at baseline and remained unchanged throughout the study.
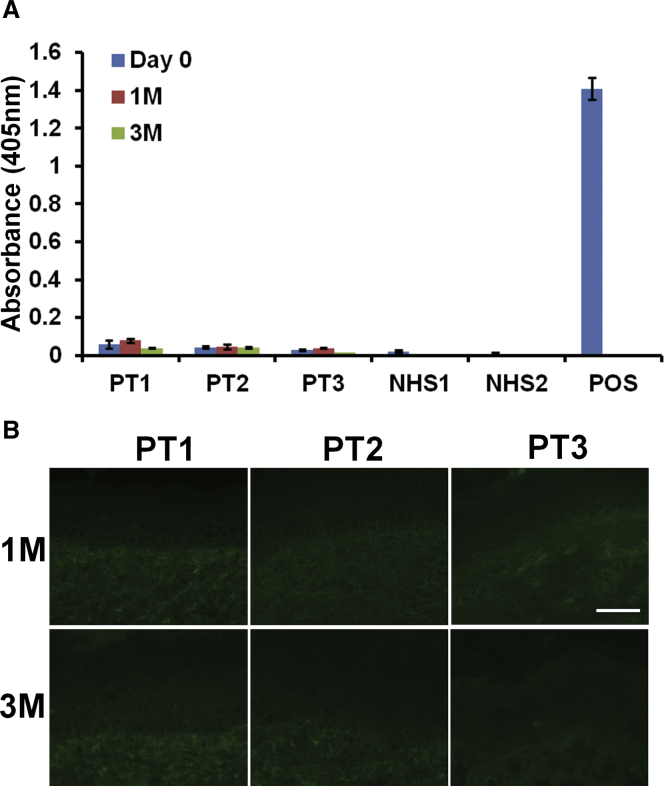
The introduction of new, full-length laminin 332 could potentially induce the patients to generate anti-laminin 332 autoantibodies because their immune system is exposed to new domains of laminin 332 that were previously not present prior to gentamicin-induced PTC readthrough. Anti-laminin 332 antibodies are a well-known cause of mucous membrane pemphigoid, a serious, acquired, autoimmune bullous disease.27,28 In order to detect gentamicin-induced autoantibodies against laminin 332, we obtained patients’ sera at baseline and at 1 and 3 months after gentamicin treatment and tested the sera for anti-laminin 332 antibodies using an ELISA.29 None of the patients’ sera exhibited detectable levels of anti-laminin 332 antibodies at 1 or 3 months following gentamicin treatment (Figure 6A). Additionally, direct IF staining performed on patient skin biopsies obtained during patient follow-up visits did not reveal any anti-laminin 332 antibody deposits in the DEJ of their skin (Figure 6B).
Figure 6.
Topical Gentamicin Did Not Induce Anti-laminin 332 Antibodies
(A) Sera were obtained from GS-JEB patients before treatment and 1 and 3 months after topical gentamicin treatment. These samples, along with appropriate normal human serum (NHS) and positive control sera, were subjected to ELISA on laminin 332-coated plates. Note that none of the patients’ sera exhibited any anti-laminin 332 antibodies at any time points during the study. Data represent the mean ± SD (n = 6). (B) Skin biopsies from the gentamicin-treated sites were obtained 1 and 3 months after treatment and subjected to direct IF using a FITC-conjugated goat anti-human IgG antibody. Note that IgG deposits were not detected at the DEJ for any of the patient samples at 1 and 3 months after treatment. Scale bar, 50 μm.
Discussion
In this study, we found that gentamicin increased laminin α3 and β3 chain levels in primary keratinocytes from three GS-JEB patients carrying nonsense mutations, resulting in the production of functional laminin 332 that was capable of correcting the intrinsic abnormal GS-JEB cellular phenotype. Consistent with our in vitro data, open erosive wounds from all three patients treated with topical gentamicin showed increased laminin 332 expression at the DEJ of their skin and proper polarization of the β4 integrin, results that persisted for up to 3 months after treatment. Also, the gentamicin-treated wounds exhibited enhanced wound closure and remained clinically improved during the 3-month course of the study. Importantly, treatment with gentamicin did not produce any untoward side effects or induce production of anti-laminin 332 antibodies in any of the patients.
We were surprised to discover that despite having two copies of the same mutated stop codon Y63X, which would predict no protein production, PT2 expressed small amounts of laminin 332 before gentamicin treatment (see Table 1). Similar findings have been reported in other genetic diseases caused by PTCs, such as RDEB, CF, and DMD.18,21,30 Previous reports showed that baseline protein expression appeared to predict a positive gentamicin response in patients with DMD and CF.18,30 In accordance with these studies, PT2, who had some baseline laminin 332 at her DEJ, responded more favorably in terms of gentamicin-induced readthrough and greater laminin 332 production than did the other two patients (PT1 and PT3) who had minimal to no detectable baseline laminin 332. As with RDEB, CF, and DMD, it is possible that the basal level of laminin 332 at the DEJ of skin can potentially predict which patient is more likely to respond to readthrough treatment.
It has been shown previously that the type of stop codon (UGA>UAG>UAA) as well as the nucleotides in the immediate vicinity of the codon determine the relative readthrough ability of any particular PTC mutation.31,32 As presented in Table S1, PT2 and PT3 harbor LAMA3 mutations with UAA stop codons and surrounding nucleotides that would predict a relatively poor response to gentamicin, while PT1 had a UGA stop codon in LAMB3, predicted to have the best readthrough, surrounded by a nucleotide context that would further support an optimal readthrough response in this particular stop codon.31 While this study was limited by a small sample size, our current study does not support an apparent correlation between in vitro readthrough and the type of stop codon or surrounding nucleotide context , in agreement with our previous studies.20,22
The potential for readthrough therapies to insert amino acids randomly at the site of the PTC could result in a protein with altered function. Therefore, we needed to assess gentamicin-induced laminin 332 for proper function and stability. There are several pieces of data generated in this study that strongly support the capability of gentamicin to produce fully functional laminin 332. First, there was continuous co-localization of all laminin 332 subunits and α6β4 integrin at the DEJ, which suggests that the laminin 332 protein was fully formed, properly functional, and appropriately deposited into the right position. Second, our in vitro work also presented evidence that the newly produced laminin 332 reversed the abnormal cellular phenotype (abnormal morphology and faulty substratum adhesion) characteristic of GS-JEB-cultured keratinocytes. Finally, the gentamicin-treated wounds demonstrated clinical improvement that persisted throughout the 3-month follow-up period. While this was not designed as a placebo-controlled study, we were still able to monitor similarly sized gentamicin-treated and untreated wounds with similar histories. For example, PT1 had bilateral wounds of the same size, same chronicity, and relative location above each eye that were iatrogenically created by ophthalmology speculums. The wound on the side of his face that was treated with topical gentamicin closed completely and remained closed for 3 months, while the untreated wound remained open throughout the study. Similarly, the ear on the treated side of his face showed remarkable improvement during the 3-month study, while a similar untreated wound on the other ear failed to heal.
In GS-JEB, most skin erosions and wounds are colonized with ambient bacteria, and any antibiotic that reduces bacterial colonization could theoretically improve wound closure. Thus, it is possible that the topical gentamicin improved wound closure due to its antimicrobial effects rather than its ability to increase laminin 332 at the DEJ. In this study, none of the treated wounds showed any signs of frank infection at day 0 or throughout the 3-month treatment period. In any future gentamicin clinical trials, baseline and follow-up bacterial cultures of the test site wounds should be done to better clarify the role of an altered microbiome as a factor for enhancing wound healing. Nevertheless, the generation of new laminin 332 at the DEJ at treated sites would also likely be a responsible factor for the observed improvement in wound healing.
Two major potential side effects of gentamicin are nephrotoxicity and ototoxicity. We found no evidence of either complication in this 3-month study with short-term topical gentamicin usage at 1 or 3 months after treatment. Another potential side effect of gentamicin-induced new laminin 332 would be the possible development of autoantibodies against new domains of laminin 332 that the patient’s immune system has not previously encountered. Since GS-JEB patients may lack laminin 332, any therapy aimed at restoring the full-length protein has the potential to induce autoantibodies. Autoantibodies to laminin 332 are not common, but they are present in anti-laminin 332 mucous membrane pemphigoid, an autoimmune disease with scarring blisters and erosions in mucosal sites (oral, conjunctival, anal, genital, esophageal), and are associated with an increased risk of an underlying malignancy.27,28 In this study, gentamicin therapy did not induce any new laminin 332 autoantibodies in the sera of these patients. We also did not detect any new anti-laminin 332 antibody deposits in the patients’ skin. These findings are similar to our work in RDEB where we found that topical gentamicin did not generate type VII collagen autoantibodies in RDEB patients carrying nonsense mutations.21 One possible explanation for the lack of reactivity in the current study is that all three patients already expressed detectable, albeit low, levels of laminin 332 at baseline, implying the occurrence of some basal readthrough. As shown in other studies, basal readthrough could protect these patients from developing autoimmune conditions targeting newly generated laminin 332.33
The definition for success of any therapy for JEB would be the generation of new functional laminin 322 in the DEJ of the patients’ skin that promotes epidermal-dermal adherence and diminishes skin fragility and skin blistering. Indeed, this was one of the primary endpoints of this pilot study. A study analyzing keratinocytes and skin biopsies from a GS-JEB patient carrying compound heterozygous nonsense mutations in LAMA3 (R943X/R1159X) suggests that the minimum amount of laminin 332 required to restore epidermal-dermal adherence, although unknown, may be significantly less than that produced by normal keratinocytes.34 In our current study, we found that gentamicin-induced laminin 332 expression was increased up to over 40% of that seen in NHS for all three patients and was durable out to 3 months. These data indicate that laminin 332, similar to some other basement membrane proteins such as type VII collagen,21 is a stable protein in vivo with a long half-life. This is consistent with a study where the half-life of laminin α3 in lung epithelium was determined to be 30–60 days.35 It is likely that laminin 332 would have a similar half-life. With data showing a robust response to short-term gentamicin treatment and the marked stability of laminin 332, we envision that gentamicin could be delivered as a short-term pulse therapy for JEB patients with nonsense mutations.
While our data demonstrated the safety and efficacy of gentamicin therapy in restoring laminin 332 to GS-JEB patients, there were several limitations to this open-label pilot study. First, the number of patients studied was small. Only three patients with bona fide GS-JEB caused by nonsense mutations in LAMA3 or LAMB3, but not LAMC2, were enrolled in the study. Also, note that nonsense mutations in LAMA3 and LAMB3 constitute the majority of GS-JEB cases and are associated with the most lethal forms of the disease.2 It is hard to recruit a large number of patients because GS-JEB is exceedingly rare (0.5 per million) and most patients die within their first year of life. The second limitation was that only three open wounds were treated with topical gentamicin and evaluated in our study. This limitation was due to the difficulty of designing a pilot study with a tolerable number of skin biopsies for the patients, who were all young, fragile, medically compromised, and required significant assistance from their parents. Finally, while there are at least 22 unique LAMA3 nonsense mutations, 39 LAMB3 nonsense mutations, and 14 LAMC2 nonsense mutations associated with GS-JEB,2 the present study only examined 3 nonsense mutations. Our data may not reflect all potential responses in GS-JEB patients harboring different nonsense mutations.
Numerous therapeutic strategies have been posited for GS-JEB. These include protein replacement therapy, bone marrow SCT, and gene-corrected cultured keratinocyte autograft transplantation.2,8, 9, 10, 11, 12 Compared with these approaches, topical gentamicin therapy for nonsense mutations is significantly less arduous, invasive, and costly and has a few advantages. First, gentamicin readthrough therapy does not expose JEB patients to any live cells, exogenous DNA/RNA, or viral vectors. Second, it does not require extensive surgery for grafting gene-corrected cells or immunosuppression of patients for SCT. Third, topical gentamicin is commercially available, safe, inexpensive, and can be readily prescribed and administered easily at an outpatient clinic or at home. A recent retrospective study of systemic gentamicin treatment (intravenous or intramuscular) for GS-JEB infants showed a positive impact on skin fragility and quality of life in four out of five of the patients.36
In summary, our study demonstrated that gentamicin suppression of nonsense mutations in GS-JEB patients can restore sufficient levels of functional laminin 332 and improve wound healing. The future goals of our research will be to determine the dose of gentamicin, administration route, and frequency of gentamicin delivery that optimizes the generation of laminin 332 in the skin of GS-JEB patients. We will also determine whether gentamicin administered systemically to these patients can treat all skin wounds simultaneously, as well as address extracutaneous mucosal manifestations of GS-JEB including often life-threatening involvement of the respiratory tract. Our hope is that most JEB patients with nonsense mutations may benefit from gentamicin therapy. Finally, gentamicin-mediated readthrough therapy for suppressing PTCs may also be beneficial in other inherited skin diseases caused by nonsense mutations.
Materials and Methods
Patients and Interventions
Gentamicin is a well-studied, US Food and Drug Administration (FDA)-approved antibiotic. This investigation was an off-label use of gentamicin. The inclusion criteria for this study were as follows: (1) GS-JEB patients with at least one nonsense mutation in either of their LAMA3 or LAMB3 alleles, and (2) an absence or decrease in laminin 332 expression at their DEJ when compared to that of NHS. The exclusion criteria for this study were as follows: (1) pre-existing renal or auditory impairment, (2) allergies to aminoglycosides or sulfate compounds, (3) pregnancy, and (4) exposure to gentamicin within the past 6 weeks.
We treated three GS-JEB patients that had well-characterized nonsense mutations (for details, please see ClinicalTrials.gov: NCT03526159). All three patients were children who had severe skin disease. Concerning the weight of each patient, PT1’s weight was in the 10th percentile, PT2 was in the 25th percentile, and PT3’s weight fell below the 5th percentile. Both patients with LAMA3 mutations had extensive mucocutaneous involvement. PT2 had a tracheal tube in place prior to involvement in the study, while PT3 was placed on steroids and supplemental oxygen to prevent airway closure. In addition, PT2 was wheelchair-dependent and required a gastrointestinal tube. While PT1 had no impairment of the airways, he did have corneal and oropharyngeal involvement. None of these patients or their mutations has been described in the literature. The study was an open-label clinical trial. Patients applied a thin layer of topical 0.5% gentamicin ointment consisting of gentamicin sulfate (Professional Compounding Centers of America) in a white petroleum base (prepared by Pasadena City Pharmacy, Pasadena, CA, USA) twice a day to three open erosions for a 2-week period. The wounds selected for treatment were not clinically infected and were typically chronic in nature. Prior to treatment, patients were evaluated with baseline biopsies, audiometry, and laboratory blood tests. These were used to establish the baseline level of laminin 332 in the DEJ of their skin, their baseline auditory acuity, and baseline blood tests (vide infra). The patients then had follow-up visits at 1 and 3 months after treatment, and these same parameters were again measured.
Clinical and Safety Assessments
Each week, the patients completed a brief standardized telephone questionnaire. They also kept a wound healing diary and photographed their treated wounds once a week. Sites treated with topical gentamicin were assessed for the percentage of wound closure prior to treatment and at 1 and 3 months post-treatment. For assessment of wound closure, the areas of open wounds were measured using marked, matched photographs taken during clinic visits. Standardized digital photographs were taken of the treated sites, and open wound areas were determined with an image analyzer (AlphaEase FC version 4.1.0; Alpha Innotech) as described.21 The percentages of wound closure were graded as follows: 85%–100% (+++), 50%–85% (++), and <50% (+).
A number of safety parameters were also assessed at baseline and at 1- and 3-month follow-up visits, including complete blood count, BUN, creatinine, calculated creatinine clearance, electrolytes, liver function tests, and pure-tone audiometry.
Assessment of Laminin 332 in Patients’ Skin
Prior to the application of topical gentamicin, baseline levels of laminin 332 in the DEJ of each patient were assessed. Briefly, 8-mm punch biopsies were obtained from patients’ intact skin and then divided into two parts. One part was assessed with quantitative IF staining to evaluate the expression of laminin 332 subunits or α6β4 integrin, and the other part was used to initiate primary cell cultures, as described below. For PT3, two 4-mm punch biopsies were obtained from the patient’s intact skin and then one was used for IF analysis and another one was used for cell isolation. At 1- and 3-month follow-up visits, biopsies from the treated areas were obtained and processed for quantitative IF. A minimum of 60 vertical frozen sections throughout the entire specimen were prepared and subjected to IF staining with antibodies specifically targeting laminin 332 (laminin α3, laminin β3, laminin γ2) or β4 integrin. Immunolabeled vertical sections of NHS (positive control) and the gentamicin-treated samples were photographed using the same camera and identical exposure times. Quantitation of laminin 332 and β4 integrin expression at the DEJ was performed by computer-assisted image analysis using ImageJ (https://rsb.info.nih.gov/ij/). For each tissue sample, six random images from positively stained areas were taken and analyzed for mean fluorescence intensity. For each image, six measurements were taken at 20-μm intervals along the DEJ. Mean averages and standard errors were calculated for each sample. Normal human skin was included as a positive control, and the expression of each laminin 332 subunit and β4 integrin in the treated sites was compared to the expression in NHS (100%).
Assessment of Anti-Laminin 332 Autoantibodies
The production of serum circulating anti-laminin 332 antibodies was assessed by ELISA using recombinant laminin 332-coated ELISA plates, as previously described,29 using immobilized recombinant human laminin 332 (BioLamina). Evaluation for the presence of anti-laminin 332 antibody deposition in patient skin was assessed via direct IF staining of patient skin biopsy samples using fluorescein isothiocyanate (FITC)-conjugated goat anti-human immunoglobulin G (IgG) (Sigma-Aldrich), as previously described.37, 38, 39
Study Approval
The study protocol was approved by the Institutional Review Board (IRB) of the University of Southern California, and all investigations were conducted according to the Declaration of Helsinki principles. The study was registered (see ClinicalTrials.gov: NCT03526159. Written informed consent was obtained from all patients prior to enrollment into the study.
Cell Lines and Culture Conditions
Eight-millimeter punch biopsies were taken from intact skin sites of GS-JEB patients as described above and divided into half for initiating primary cells. Briefly, biopsies were stored in DMEM high-glucose, serum-free media (Corning Life Sciences) with 5 μg/mL amphotericin B and 500 U/mL penicillin/streptomycin. The biopsy was placed in 1× sterile Dispase with 2.5 μg/mL amphotericin and 500 U/mL penicillin/streptomycin for 2 h at 37°C and then placed into sterile PBS where the epidermis was removed from the dermis with sterile forceps. The epidermis was then placed into a sterile Petri dish containing 0.05% trypsin with 2% EDTA for incubation at 37°C for 20 min with frequent pipetting to release cells. Trypsin was neutralized by DMEM high-glucose media containing 10% FBS, and then cells were centrifuged at 300 × g for 5 min. Cell pellets were re-suspended in primary keratinocyte isolation medium (DMEM supplemented with 10% FBS, 0.05 μg/mL hydrocortisone, 10 μg/mL epidermal growth factor [EGF], 10−10 M [8.5 ng/mL] cholera toxin, 5 μg/mL insulin, 500 U/mL penicillin/streptomycin, and 2.5 μg/mL amphotericin B). The cell suspension was divided and plated on two 100-mm tissue culture dishes that had been pre-seeded with Swiss 3T3 cells (purchased from the European Collection of Authenticated Cell Cultures [ECACC]) that had been previously lethally treated with mitomycin C (MilliporeSigma) and plated at 30% confluence. Cells were left untouched for 5 days, after which they were grown in 1:1 Defined Keratinocyte SFM (serum-free medium)/Medium 154 (Gibco) with supplements.
Drug Treatment and Immunoblotting
To determine the cellular expression of laminin α3 and β3 proteins, the keratinocyte cultures were incubated without or with gentamicin (Sigma-Aldrich), whereas media were changed and supplemented daily at the doses 3.125–50 μg/mL) for 96 h. Cellular extracts were then prepared and subjected to 4%–15% SDS-PAGE. Proteins were transferred from the acrylamide gels onto a nitrocellulose membrane. The presence of laminin β3 monomer was detected with mouse monoclonal anti-laminin β3 antibodies (anti-kalinin B1, clone 17; BD Biosciences), and the laminin α3 monomer was detected with a monoclonal anti-laminin α3 antibody (a gift from the Dr. Vitali Alexeev, Thomas Jefferson University, Philadelphia, PA, USA) followed by secondary antibodies using horseradish peroxidase-conjugated goat anti-mouse IgG and an enhanced chemiluminescence detection reagent (GE Healthcare).
Cell Morphology and Cell Detachment Assay
We studied cellular morphology using a Leica DMIL LED inverted microscope (×10 lens) equipped with a Leica DFC340 FX digital camera to digitally capture the images. To determine the degree of gentamicin-induced cellular adherence, a trypsin-based detachment assay was used.25 Briefly, HKCs and GS-JEB keratinocytes were seeded on 24-well tissue culture plates at a density of 1 × 104 cells per well. Twenty-four hours after seeding, the medium was changed to one containing gentamicin at various concentrations depending on the cell line (PT1, 50 μg/mL; PT2 and PT3, 12.5 μg/mL). After 48 h, 250 μL of trypsin/EDTA was added to each well, and any detached cells were removed after 5 min and counted. An additional 250 μL of trypsin/EDTA was added, and all remaining cells were allowed to detach and were subsequently counted. The percentages of cells detached were obtained, and the averages and SDs from four independent wells for each condition/cell line were calculated.
Cell Viability
To evaluate gentamicin cytotoxicity, primary GS-JEB keratinocytes isolated from patient biopsy samples were seeded onto an uncoated, 96-well plate at a density of 1 × 104 cells per well in 100 μL of culture medium. Twenty-four hours later, media were changed and supplemented with gentamicin at the indicated concentrations from 0 to 400 μg/mL. Plates were allowed to incubate for 48 h. A freshly prepared solution of 4 mg of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-5[(phenylamino)carbonyl]- 2H-tetrazolium hydroxide (XTT; VWR) in 4 mL of culture medium was mixed with 10 μL of phenazine methosulfate (PMS; Sigma) solution (3 mg of PMS in 1 mL of PBS), and 25 μL of the combined XTT/PMS solution was directly added to each 100-μL cell culture.40 Cultures were incubated for 4 h at 37°C, and absorbance was read at 450 nm.
Author Contributions
Conceptualization: M.C. and D.T.W.; Funding Acquisition: M.C. and D.T.W.; Investigation: A.K., J.C., Y.H., R.A., M.H., V.L., G.K., Q.C., D.T.W., and M.C.; Project Administration: M.C.; Supervision: M.C. and D.T.W.; Validation: A.K., J.C., Y.H., and M.C.; Visualization: A.K., J.C., and M.C.; Writing – Original Draft Preparation: A.K., J.C., and M.C.; Writing – Review & Editing: A.K., J.C., R.A., M.H., V.L., G.K., D.T.W., and M.C.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported in part by grants from the Epidermolysis Bullosa Research Partnership and the Epidermolysis Bullosa Medical Research Foundation (to M.C. and D.T.W.), and the Congressionally Directed Medical Research Programs under award no. W81XWH-1810558 to M.C. We thank the patients for granting permission to publish this information. We thank Anita Jeredjian from Pasadena City Pharmacy for formulating the topical gentamicin. We gratefully acknowledge Carlos Beltran, Griselda Hagmaier, and Brenda Cornejo at the USC Department of Dermatology.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.03.006.
Contributor Information
David T. Woodley, Email: dwoodley@usc.edu.
Mei Chen, Email: chenm@usc.edu.
Supplemental Information
References
- 1.Kiritsi D., Has C., Bruckner-Tuderman L. Laminin 332 in junctional epidermolysis bullosa. Cell Adhes. Migr. 2013;7:135–141. doi: 10.4161/cam.22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammersen J., Has C., Naumann-Bartsch N., Stachel D., Kiritsi D., Söder S., Tardieu M., Metzler M., Bruckner-Tuderman L., Schneider H. Genotype, clinical course, and therapeutic decision making in 76 infants with severe generalized junctional epidermolysis bullosa. J. Invest. Dermatol. 2016;136:2150–2157. doi: 10.1016/j.jid.2016.06.609. [DOI] [PubMed] [Google Scholar]
- 3.Mühle C., Jiang Q.J., Charlesworth A., Bruckner-Tuderman L., Meneguzzi G., Schneider H. Novel and recurrent mutations in the laminin-5 genes causing lethal junctional epidermolysis bullosa: molecular basis and clinical course of Herlitz disease. Hum. Genet. 2005;116:33–42. doi: 10.1007/s00439-004-1210-y. [DOI] [PubMed] [Google Scholar]
- 4.Yan E.G., Paris J.J., Ahluwalia J., Lane A.T., Bruckner A.L. Treatment decision-making for patients with the Herlitz subtype of junctional epidermolysis bullosa. J. Perinatol. 2007;27:307–311. doi: 10.1038/sj.jp.7211694. [DOI] [PubMed] [Google Scholar]
- 5.Fine J.D., Johnson L.B., Weiner M., Suchindran C. Cause-specific risks of childhood death in inherited epidermolysis bullosa. J. Pediatr. 2008;152:276–280. doi: 10.1016/j.jpeds.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 6.Kelly-Mancuso G., Kopelan B., Azizkhan R.G., Lucky A.W. Junctional epidermolysis bullosa incidence and survival: 5-year experience of the Dystrophic Epidermolysis Bullosa Research Association of America (DebRA) nurse educator, 2007 to 2011. Pediatr. Dermatol. 2014;31:159–162. doi: 10.1111/pde.12157. [DOI] [PubMed] [Google Scholar]
- 7.Angelis A., Kanavos P., López-Bastida J., Linertová R., Oliva-Moreno J., Serrano-Aguilar P., Posada-de-la-Paz M., Taruscio D., Schieppati A., Iskrov G., BURQOL-RD Research Network Social/economic costs and health-related quality of life in patients with epidermolysis bullosa in Europe. Eur. J. Health Econ. 2016;17(Suppl 1):31–42. doi: 10.1007/s10198-016-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins P.B., Lin Q., Goodnough J.B., Tian H., Chen X., Khavari P.A. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc. Natl. Acad. Sci. USA. 2001;98:5193–5198. doi: 10.1073/pnas.091484998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavilio F., Pellegrini G., Ferrari S., Di Nunzio F., Di Iorio E., Recchia A., Maruggi G., Ferrari G., Provasi E., Bonini C. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat. Med. 2006;12:1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- 10.Igoucheva O., Kelly A., Uitto J., Alexeev V. Protein therapeutics for junctional epidermolysis bullosa: incorporation of recombinant β3 chain into laminin 332 in β3−/− keratinocytes in vitro. J. Invest. Dermatol. 2008;128:1476–1486. doi: 10.1038/sj.jid.5701197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Rosa L., Carulli S., Cocchiarella F., Quaglino D., Enzo E., Franchini E., Giannetti A., De Santis G., Recchia A., Pellegrini G., De Luca M. Long-term stability and safety of transgenic cultured epidermal stem cells in gene therapy of junctional epidermolysis bullosa. Stem Cell Reports. 2013;2:1–8. doi: 10.1016/j.stemcr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch T., Rothoeft T., Teig N., Bauer J.W., Pellegrini G., De Rosa L., Scaglione D., Reichelt J., Klausegger A., Kneisz D. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varki R., Sadowski S., Pfendner E., Uitto J. Epidermolysis bullosa. I. Molecular genetics of the junctional and hemidesmosomal variants. J. Med. Genet. 2006;43:641–652. doi: 10.1136/jmg.2005.039685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linde L., Kerem B. Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet. 2008;24:552–563. doi: 10.1016/j.tig.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Bidou L., Allamand V., Rousset J.P., Namy O. Sense from nonsense: therapies for premature stop codon diseases. Trends Mol. Med. 2012;18:679–688. doi: 10.1016/j.molmed.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Bedwell D.M., Kaenjak A., Benos D.J., Bebok Z., Bubien J.K., Hong J., Tousson A., Clancy J.P., Sorscher E.J. Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat. Med. 1997;3:1280–1284. doi: 10.1038/nm1197-1280. [DOI] [PubMed] [Google Scholar]
- 17.Wagner K.R., Hamed S., Hadley D.W., Gropman A.L., Burstein A.H., Escolar D.M., Hoffman E.P., Fischbeck K.H. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann. Neurol. 2001;49:706–711. [PubMed] [Google Scholar]
- 18.Linde L., Boelz S., Nissim-Rafinia M., Oren Y.S., Wilschanski M., Yaacov Y., Virgilis D., Neu-Yilik G., Kulozik A.E., Kerem E., Kerem B. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J. Clin. Invest. 2007;117:683–692. doi: 10.1172/JCI28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuschal C., DiGiovanna J.J., Khan S.G., Gatti R.A., Kraemer K.H. Repair of UV photolesions in xeroderma pigmentosum group C cells induced by translational readthrough of premature termination codons. Proc. Natl. Acad. Sci. USA. 2013;110:19483–19488. doi: 10.1073/pnas.1312088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cogan J., Weinstein J., Wang X., Hou Y., Martin S., South A.P., Woodley D.T., Chen M. Aminoglycosides restore full-length type VII collagen by overcoming premature termination codons: therapeutic implications for dystrophic epidermolysis bullosa. Mol. Ther. 2014;22:1741–1752. doi: 10.1038/mt.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodley D.T., Cogan J., Hou Y., Lyu C., Marinkovich M.P., Keene D., Chen M. Gentamicin induces functional type VII collagen in recessive dystrophic epidermolysis bullosa patients. J. Clin. Invest. 2017;127:3028–3038. doi: 10.1172/JCI92707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lincoln V., Cogan J., Hou Y., Hirsch M., Hao M., Alexeev V., De Luca M., De Rosa L., Bauer J.W., Woodley D.T., Chen M. Gentamicin induces LAMB3 nonsense mutation readthrough and restores functional laminin 332 in junctional epidermolysis bullosa. Proc. Natl. Acad. Sci. USA. 2018;115:E6536–E6545. doi: 10.1073/pnas.1803154115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choate K.A., Medalie D.A., Morgan J.R., Khavari P.A. Corrective gene transfer in the human skin disorder lamellar ichthyosis. Nat. Med. 1996;2:1263–1267. doi: 10.1038/nm1196-1263. [DOI] [PubMed] [Google Scholar]
- 24.Krueger J.G., Lin A.N., Leong I., Carter D.M. Junctional epidermolysis bullosa keratinocytes in culture display adhesive, structural, and functional abnormalities. J. Invest. Dermatol. 1991;97:849–861. doi: 10.1111/1523-1747.ep12491525. [DOI] [PubMed] [Google Scholar]
- 25.Löffek S., Hurskainen T., Jackow J., Sigloch F.C., Schilling O., Tasanen K., Bruckner-Tuderman L., Franzke C.W. Transmembrane collagen XVII modulates integrin dependent keratinocyte migration via PI3K/Rac1 signaling. PLoS ONE. 2014;9:e87263. doi: 10.1371/journal.pone.0087263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonkman M.F., de Jong M.C., Heeres K., Sonnenberg A. Expression of integrin alpha 6 beta 4 in junctional epidermolysis bullosa. J. Invest. Dermatol. 1992;99:489–496. doi: 10.1111/1523-1747.ep12616168. [DOI] [PubMed] [Google Scholar]
- 27.Kirtschig G., Marinkovich M.P., Burgeson R.E., Yancey K.B. Anti-basement membrane autoantibodies in patients with anti-epiligrin cicatricial pemphigoid bind the α subunit of laminin 5. J. Invest. Dermatol. 1995;105:543–548. doi: 10.1111/1523-1747.ep12323431. [DOI] [PubMed] [Google Scholar]
- 28.Lazarova Z., Yee C., Lazar J., Yancey K.B. IgG autoantibodies in patients with anti-epiligrin cicatricial pemphigoid recognize the G domain of the laminin 5 α-subunit. Clin. Immunol. 2001;101:100–105. doi: 10.1006/clim.2001.5091. [DOI] [PubMed] [Google Scholar]
- 29.Chiorean R., Danescu S., Virtic O., Mustafa M.B., Baican A., Lischka A., Hashimoto T., Kariya Y., Koch M., Sitaru C. Molecular diagnosis of anti-laminin 332 (epiligrin) mucous membrane pemphigoid. Orphanet J. Rare Dis. 2018;13:111. doi: 10.1186/s13023-018-0855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik V., Rodino-Klapac L.R., Viollet L., Wall C., King W., Al-Dahhak R., Lewis S., Shilling C.J., Kota J., Serrano-Munuera C. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol. 2010;67:771–780. doi: 10.1002/ana.22024. [DOI] [PubMed] [Google Scholar]
- 31.Floquet C., Hatin I., Rousset J.P., Bidou L. Statistical analysis of readthrough levels for nonsense mutations in mammalian cells reveals a major determinant of response to gentamicin. PLoS Genet. 2012;8:e1002608. doi: 10.1371/journal.pgen.1002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zingman L.V., Park S., Olson T.M., Alekseev A.E., Terzic A. Aminoglycoside-induced translational read-through in disease: overcoming nonsense mutations by pharmacogenetic therapy. Clin. Pharmacol. Ther. 2007;81:99–103. doi: 10.1038/sj.clpt.6100012. [DOI] [PubMed] [Google Scholar]
- 33.Marinkovich M.P., Tang J.Y. Gene therapy for epidermolysis bullosa. J. Invest. Dermatol. 2019;139:1221–1226. doi: 10.1016/j.jid.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Pacho F., Zambruno G., Calabresi V., Kiritsi D., Schneider H. Efficiency of translation termination in humans is highly dependent upon nucleotides in the neighbourhood of a (premature) termination codon. J. Med. Genet. 2011;48:640–644. doi: 10.1136/jmg.2011.089615. [DOI] [PubMed] [Google Scholar]
- 35.Urich D., Eisenberg J.L., Hamill K.J., Takawira D., Chiarella S.E., Soberanes S., Gonzalez A., Koentgen F., Manghi T., Hopkinson S.B. Lung-specific loss of the laminin α3 subunit confers resistance to mechanical injury. J. Cell Sci. 2011;124:2927–2937. doi: 10.1242/jcs.080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammersen J., Neuner A., Wild F., Schneider H. Attenuation of severe generalized junctional epidermolysis bullosa by systemic treatment with gentamicin. Dermatology (Basel) 2019;235:315–322. doi: 10.1159/000499906. [DOI] [PubMed] [Google Scholar]
- 37.Woodley D.T., Briggaman R.A., O’Keefe E.J., Inman A.O., Queen L.L., Gammon W.R. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N. Engl. J. Med. 1984;310:1007–1013. doi: 10.1056/NEJM198404193101602. [DOI] [PubMed] [Google Scholar]
- 38.Woodley D.T., Burgeson R.E., Lunstrum G., Bruckner-Tuderman L., Reese M.J., Briggaman R.A. Epidermolysis bullosa acquisita antigen is the globular carboxyl terminus of type VII procollagen. J. Clin. Invest. 1988;81:683–687. doi: 10.1172/JCI113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodley D.T., Cogan J., Wang X., Hou Y., Haghighian C., Kudo G., Keene D.R., Chen M. De novo anti-type VII collagen antibodies in patients with recessive dystrophic epidermolysis bullosa. J. Invest. Dermatol. 2014;134:1138–1140. doi: 10.1038/jid.2013.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.