Abstract
Posttraumatic stress disorder (PTSD) has increasingly been linked to heightened systemic inflammation. Whether this association is causal and either bidirectional or unidirectional, or correlational matters. Investigators have hypothesized that chronic systemic low-grade inflammation may contribute to greater risk of developing PTSD after experiencing trauma and/or serve as a mechanism linking PTSD to adverse physical health outcomes. However, if the PTSD-inflammation relation is correlational, it may not warrant further research aimed at understanding inflammation as a PTSD risk factor or as a pathway linking PTSD with poor health. In this review, first we assess the longitudinal evidence related to PTSD and inflammation to understand more clearly the directionality and causal nature of this relation. Overall, few longitudinal studies rigorously assess the direction of the PTSD-inflammation relation. Some of the evidence indicates elevated inflammation assessed pre-trauma or in the acute aftermath of trauma increases risk for developing PTSD. Fewer studies evaluate the influence of PTSD on subsequent inflammation levels, and findings are mixed. Sample characteristics and study designs, and also type of inflammation-related measure, vary widely across studies. Based on current evidence, we then recommend several statistical and study design approaches that may help untangle issues of bidirectionality and aid in determining the direction of causality between PTSD and inflammation. Finally, we conclude with future research directions and consider potential implications for interventions or treatment approaches based on this growing body of literature.
Keywords: Trauma, posttraumatic stress disorder, inflammation, inflammatory markers, bidirectionality, vulnerability
Posttraumatic stress disorder (PTSD) is the quintessential trauma-related mental disorder, characterized by re-experiencing the trauma, avoidance of trauma reminders, negative alterations in cognition and mood, and hyperarousal(1). Although PTSD has long been recognized as a negative psychological response to trauma(2), why only some individuals develop PTSD remains unclear. Moreover, PTSD has been linked to development of chronic disease and other adverse physical health outcomes(3–8). A substantial literature suggests inflammation—a key component of the immune response—may play a role in the link between trauma and subsequent PTSD and/or in the relation between PTSD and subsequent poor physical health, with some investigators hypothesizing a bidirectional relation between PTSD and inflammation(9–10). However, whether inflammation increases PTSD risk or results from PTSD, or is simply a correlate of PTSD, remains unclear. Elucidating the directional and causal nature of the PTSD-inflammation relation is critical for improving understanding of the potential role of inflammation in vulnerability to PTSD and/or in linkages between PTSD and chronic disease. Furthermore, such information could have implications for PTSD prevention and treatment.
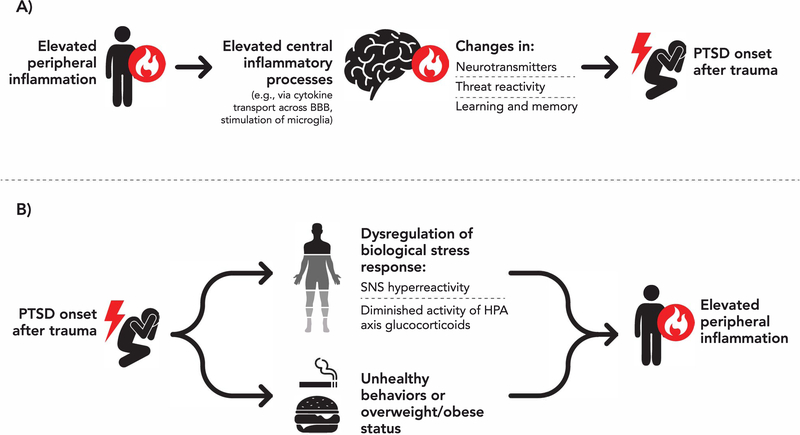
Numerous plausible mechanisms may underlie associations between PTSD and inflammation(11–12). Elevated peripheral inflammation could affect the brain in ways that increase the likelihood of developing PTSD (Figure 1A)(13–19). Peripheral cytokines can cross the blood-brain barrier and initiate processes that produce cytokines in the central nervous system(14–16) which, in turn, can affect cortico-amygdala functioning(13,17,18) and cognitive processes like learning and memory consolidation(19). PTSD could also activate downstream behavioral (e.g., sedentary behavior, smoking) and physiological processes that increase inflammation (Figure 1B)(20–31). On the physiological side, PTSD is characterized by dysregulation of biological stress response systems, including hyper-reactivity of the sympathetic nervous system and diminished activity of glucocorticoids produced by the hypothalamic-pituitary-adrenal axis [see Pitman et al.(23)], processes that contribute to heightened inflammation. In fact, in many studies evaluating if PTSD increases chronic disease risk(5–8), individuals are disease-free at baseline and there are many years between the time PTSD onsets to disease development. These features add support to the idea that PTSD can lead to elevated inflammation (via physiological and behavioral pathways).
Figure 1.
Potential mechanisms linking A) elevated inflammation with posttraumatic stress disorder (PTSD) risk and B) PTSD with elevated peripheral inflammation. BBB=blood-brain barrier. SNS=sympathetic nervous system. HPA axis=hypothalamic-pituitary-adrenal axis.
A 2015 meta-analysis of 20 studies consistently found elevated levels of several inflammatory markers, including interleukin-6 (IL-6), IL-1 beta (IL-1ß), interferon gamma (IFNγ), and tumor necrosis factor-alpha (TNFα), in individuals with versus without PTSD(32). However because all studies were cross-sectional, we cannot determine the direction of effects or assess if the relation is causal due to potential confounding and reverse causation. A cross-sectional PTSD-inflammation relation could result because PTSD triggers physiological dysregulation that promotes elevated inflammation and/or because inflammation increases risk for PTSD after trauma. A PTSD-inflammation relation could also be due to shared risk factors such as depression.
In this review, we conduct a comprehensive examination of evidence for a bidirectional association of PTSD with elevated inflammation. First, we evaluate empirical support for the hypothesis that PTSD leads to elevated inflammation and vice versa, focusing on publications since the 2015 meta-analysis(32) and prioritizing longitudinal studies. Second, we identify methodological frameworks that may help with untangling directional associations and address the extent to which these approaches have been (or could be) employed to understand the PTSD-inflammation relation. Third, we discuss how a clearer understanding of the directionality of this relation may inform PTSD prevention and treatment, particularly given interest in inflammation-related interventions to address mental health problems.
Evaluating the Evidence
Rather than conducting an exhaustive review of studies, we sought to identify longitudinal studies that provided the strongest possible evidence for causality by considering baseline inflammation as a predictor of subsequent PTSD or baseline PTSD as a predictor of change in inflammation. We searched MEDLINE and EMBASE for studies on “PTSD and inflammation” published from 2015 to September 12, 2019, plus Web of Science for papers citing the Passos et al. meta-analysis(32). To gather all possible longitudinal studies, we considered those identified in search results and ones that came up in references of relevant articles even if the study of interest was published before 2015. Together, we identified 15 longitudinal studies on inflammation-to-PTSD (Table 1) and 8 longitudinal studies on PTSD-to-inflammation (Table 2).
Table 1.
Descriptions of 15 longitudinal studies addressing the inflammation-to-PTSD relation.
| Authors and Year | Sample Description | Inflammatory Markers or Inflammation-related Measure | Study Designa | Covariates | Results | Direction of Findingb |
|---|---|---|---|---|---|---|
| Pre-Trauma Inflammation Predicting PTSD | ||||||
| Breen et al., 2015 (33) | 142 male U.S. Marines in the MRS with and without post-deployment PTSD (none with pre-deployment PTSD) assessed at 1 month pre- and 3 months post-deployment | Transcriptome-wide gene expression profiling from peripheral leukocytes at 1 month pre- and 3 months post-deployment | Pre-post trauma exposure (1) | Groups matched on combat exposure, age, and ethnicity | Pre-deployment differentially expressed gene modules between PTSD cases and controls at post-deployment included overexpressed genes involved in innate immune responses, interferon signaling, and monocyte specificity | Inflammation predicted PTSD (+) |
| Eraly et al., 2014 (10) | 1,719 male U.S. Marines in the MRS assessed at 1 month pre- and 3 months post-deployment | hsCRP at pre-deployment | Pre-post trauma exposure (2); baseline inflammation with repeated PTSD | Baseline PTSD symptoms, combat exposure, post-battle experiences, and Marine cohort assignment | High hsCRP increased risk of PTSD symptoms at post-deployment | Inflammation predicted PTSD (+) |
| Glatt et al., 2013 (34) | 48 male U.S. Marines in the MRS with and without post-deployment PTSD (none with pre-deployment PTSD) assessed at 1 month pre-and 3 months post-deployment | Transcriptome-wide gene expression profiling from peripheral blood samples at pre-deployment | Pre-post trauma exposure (2); baseline inflammation and repeated PTSD | Groups matched by age, deployment history, ethnicity, Marine cohort assignment, and level of exposure to putative traumas | Pre-deployment differentially expressed genes between PTSD cases and controls at post-deployment indicated genes involved in immune-related processes, protein domains involved in response to viral infection, and genes involved in Type-1 interferon signaling | Inflammation predicted PTSD (+) |
| Sumner et al., 2018 (9)c | 525 community-dwelling women with no trauma exposure, trauma with no PTSD, and PTSD that onset in between two blood draws 10–16 years apart | hsCRP, TNFRII, ICAM-1, VCAM-1 at both blood draws | Repeated measures (3) | Age, race, anti-hypertensive medication, anti-inflammatory medication, cholesterol-lowering medication, hormone therapy, and menopausal status | TNFRII and ICAM-1 levels at blood draw 1 (prior to PTSD onset) were higher among women who would have later PTSD onset, relative to women with no trauma; blood draw 1 biomarker levels were not associated with later PTSD symptom severity | Inflammation predicted PTSD (+) |
| van Zuiden et al., 2011 (36) | 1,023 Dutch male military personnel assessed pre- and 1 and 6 months post-deployment | CD2/CD28-induced T-cell cytokine production (IL-2, IL4, IL-5, IL-6, IL-10, TNFα, MCP-1, IFNγ, IFNγ-induced protein-10, RANTES) and LPS-stimulated monocyte cytokine production (IL-1α, IL-1β, IL-6, IL-8, IL-10, TNFα) at pre-and 1 and 6 months post-deployment | Pre-post trauma exposure (2); repeated inflammation with follow-up PTSD | Depression at post-deployment | Cytokine production at pre- and post-deployment was not associated with PTSD symptoms at 6 months post-deployment | Inflammation did not predict PTSD |
| van Zuiden et al., 2012 (35) | 526 Dutch male military personnel assessed pre- and 1 and 6 months post-deployment | DEX-sensitivity of T-cell mitogen-induced proliferation and DEX sensitivity of LPS-induced TNFα production at pre-deployment | Pre-post trauma exposure (2); baseline inflammation with follow-up PTSD | Baseline PTSD symptoms and depression and fatigue at follow-up | Greater pre-deployment DEX-sensitivity of T-cell mitogen-induced proliferation (which may relate to lower cytokine levels) was associated with elevated PTSD symptoms at 6 months post-deployment only for those without elevated depressive symptoms; no association observed for DEX-sensitivity of LPS-induced TNFα production | Inflammation predicted PTSD (−) |
| Inflammation in the Acute Aftermath of Trauma Predicting PTSD | ||||||
| Bielas et al., 2018 (37) | 183 acute MI patients participating in an RCT for post-MI psychological counseling assessed at hospital admission and 3 months later | CRP at hospital admission and 3 months later | Longitudinal (4); repeated inflammation with follow-up PTSD | Gender, age, education, BMI, smoking, alcohol intake, physical activity, ST-elevation MI, GRACE risk score, LVEF, troponin T, WBC count, social support, ASD symptoms at admission, antidepressants, and type of counseling intervention | Lower reduction or increased CRP from admission to follow-up predicted PTSD at follow-up | Inflammation predicted PTSD (+) |
| Cohen et al., 2011 (38) | 61 patients hospitalized for traumatic injuries assessed at hospitalization and 1 month later | IL-6, IL-8, TGF-β, IL-4, IL-10 at hospitalization | Longitudinal (4); baseline inflammation with follow-up PTSD | Age and severity of injury | IL-8 was positively associated and TGF-β was negatively associated with PTSD symptoms at 1 month post-hospitalization | Inflammation predicted PTSD (+) |
| Gandubert et al., 2016 (39) | 123 trauma-exposed adults assessed in emergency departments and 1, 4, and 12 months later (n=89 at 1 month, n=85 at 4 months, n=57 at 12 months) | hsCRP in emergency departments | Longitudinal (4); baseline inflammation and repeated PTSD | Age and sex | hsCRP levels were not associated with development of PTSD at 1, 4 and 12 months post-trauma | Inflammation did not predict PTSD |
| Michopoulos et al., 2019 (42) | 505 trauma-exposed adults assessed in emergency rooms and 1, 3, 6, and 12 months later | Proinflammatory cytokines (IL-1β, IL-6, TNFα, IFNγ), anti-inflammatory factors (IL-10, IL-1RA), other cytokines (IL-5, IL-15, IL-2, IL-13, IL-4, IL-9, IL-12, IL-17), chemokines (eotaxin, MIP-1α, IFNγ-induced protein-10, MCP-1, IL-8, MIP-1β, RANTES), and growth factors (GM-CSF, bFGF, IL-7, PDGF-BB) in the emergency department | Longitudinal (4); baseline inflammation and repeated PTSD | Sex, BMI, time elapsed between trauma exposure and blood sampling, time of day of blood sampling, incident and premorbid interpersonal trauma exposure | Participants exhibiting a chronic PTSD trajectory over follow-up had lower levels of TNFα and IFNγ in the emergency department compared to those exhibiting trajectories consistent with recovery (i.e., decreasing symptoms) or resilience (i.e., no symptoms); no significant differences observed for the other proinflammatory cytokines, anti-inflammatory factors, other cytokines, chemokines, or growth factors | Inflammation predicted PTSD (−) |
| Pervanidou et al., 2007 (40) | 88 children aged 7–18 following a motor vehicle accident and trauma-unexposed age and BMI-matched controls assessed at hospitalization and 1 and 6 months later | IL-6 at hospitalization and 1 and 6 months later | Repeated measures (3) | Injury severity, gender, age, BMI, and serum cortisol | Higher IL-6 levels at hospitalization was associated with PTSD at 6 months but not 1 month | Inflammation predicted PTSD (+) |
| Segman et al., 2005 (41) | 24 trauma-exposed adults assessed in emergency rooms and 4 months later | Gene expression from PBMCs at emergency room admission and/or 4 month follow-up | Repeated measures (3); for some of the sample | None | Differentially expressed transcripts from emergency room and 4 month samples were found between those with PTSD and no PTSD across follow-up; expression patterns from emergency room samples distinguished PTSD versus no PTSD at follow-up; differential expression signatures were involved in immune activation, signal transduction, and apoptosis | Inflammation predicted PTSD (+) |
| Additional Longitudinal Studies of Inflammation Predicting PTSD | ||||||
| Eswarappa et al., 2019 (44) | 700 veterans assessed annually for 4 years; categorized as no PTSD, resolved PTSD, developed PTSD, and chronic PTSD | WBC count, hsCRP, fibrinogen, platelet count, ESR, IL-6 at baseline | Longitudinal (4); baseline inflammation and repeated PTSD | Age, sex, physical activity | Higher WBC, CRP, fibrinogen, and ESR levels predicted worse course of PTSD adjusting for age and sex, but effects besides WBC were fully attenuated when adjusting for physical activity | Inflammation did not predict PTSD |
| Glaus et al., 2018 (43)c | 2,573 Swiss adults with current, remitted, or no PTSD assessed at baseline and 5.5 years later | hsCRP, IL-6, TNFα at baseline and follow-up | Repeated measures (3) | Length of follow-up, sex, age, SES, race, marital status, psychotropic medication, aspirin, statins, NSAIDs, smoking, physical inactivity, BMI, diabetes, dyslipidemia, and hypertension | Baseline inflammation did not predict lifetime PTSD at follow-up, adjusting for PTSD at baseline, or incident PTSD that developed over follow-up | Inflammation did not predict PTSD |
| Smid et al., 2015 (45) | 814 Dutch military personnel assessed pre- and 1, 6, 12, and 24 months post-deployment (n=693 at 1 month, n=644 at 6 months, n=465 at 12 months and n=433 at 24 months) | CD2/CD28-induced T-cell cytokine production (IL-2, IL-4, IL-5, IL-6, IL-10, TNFα, MCP-1, IFNγ, IFNγ-induced protein-10, RANTES) and LPS-stimulated monocyte cytokine production (IL-1α, IL-1β, IL-6, IL-8, IL-10, TNFα) at 1-month post-deployment | Pre-post trauma exposure (2); repeated PTSD with follow-up inflammation | Gender, age, education, rank, deployment history, early life trauma, smoking, BMI, oral anti-contraceptives, non-systemic glucocorticoids, antihistamines, cholesterol, and anti-hypertensive drugs | Three-way interaction effect of combat exposure, post-deployment SLEs, and cytokine production from both T-cell and monocytes on change in PTSD symptoms over follow-up; in presence of high combat exposure and high T-cell or monocyte cytokine production, more post-deployment SLEs were associated with increased PTSD symptoms, whereas fewer post-deployment SLEs were associated with decreased PTSD symptoms | Inflammation predicted PTSD (+) |
Study designs in order from strongest to weakest evidence for causality:
1. Pre-post trauma exposure – assessments both before and following trauma exposure for both PTSD and inflammation
2. Pre-post trauma exposure – assessments both before and following trauma exposure, but either PTSD or inflammation only assessed at one timepoint
3. Repeated measures (with or without PTSD treatment) – repeated assessments for both PTSD and inflammation
4. Longitudinal – repeated assessments for either PTSD or inflammation, and either PTSD or inflammation only assessed at one timepoint
Positive associations are indicated by “+,” negative associations are indicated by “−,” and both positive and negative associations are indicated by “mixed.”
Study is included as evidence for both the inflammation-to-PTSD relation and the PTSD-to-inflammation relation.
Abbreviations: PTSD = posttraumatic stress disorder; MRS = Marine Resiliency Study; hsCRP = high sensitivity C-reactive protein; TNFRII = tumor necrosis factor alpha-receptor II; ICAM = intercellular adhesion molecule; VCAM = vascular cell adhesion molecule; IL = interleukin; TNFα = tumor necrosis factor alpha; MCP = monocyte chemoattractant protein; IFNγ = interferon gamma; RANTES = regulated upon activation, normal T cell expressed, and secreted; LPS = lipopolysaccharide; DEX = dexamethasone; MI = myocardial infarction; RCT = randomized control trial; CRP = C-reactive protein; BMI = body mass index; GRACE = Global Registry of Acute Coronary Events; LVEF = left ventricular ejection fraction; WBC = white blood cell; ASD = acute stress disorder; TGF-β = transforming growth factor beta; MIP = macrophage inflammatory protein; GM-CSF = granulocyte macrophage-colony stimulating factor; bFGF = basic fibroblast growth factor; PDGF-BB = platelet-derived growth factor BB; PMBC = peripheral blood mononuclear cell; ESR = erythrocyte sedimentation rate; SES = socio-economic status; NSAID = nonsteroidal anti-inflammatory drugs; SLE = stressful life events
Table 2.
Descriptions of 8 longitudinal studies addressing the PTSD-to-inflammation relation.
| Authors and Year | Sample Description | Inflammatory Markers or Inflammation-related Measure | Study Designa | Covariates | Results | Direction of Findingb |
|---|---|---|---|---|---|---|
| PTSD Onset or Diagnosis and Changes in Inflammation | ||||||
| Glaus et al., 2018 (43)c | 2,573 Swiss adults with current, remitted, or no PTSD assessed at baseline and 5.5 years later | hsCRP, IL-6, TNFα at baseline and follow-up | Repeated measures (3) | Length of follow-up, sex, age, SES, race, marital status, major depressive disorder, bipolar disorder, substance use disorders, psychotropic medication, aspirin, statins, NSAIDs, smoking, physical inactivity, BMI, diabetes, dyslipidemia, and hypertension | Current PTSD at baseline was associated with lower IL-6 levels at follow-up, adjusting for baseline IL-6; remitted PTSD was not associated with inflammatory markers at follow-up | PTSD predicted inflammation (−) |
| Jergović et al., 2015 (46) | 47 combat exposed male veterans with chronic PTSD assessed at baseline and 3 months later | CRP, IFNγ, IL-1β, IL-2, IL-4, IL-6, TNFα, sPECAM-1, sICAM-1, MIP-1α, IL-8, sCD40L, NGF, and leptin at baseline and 3 months later | Longitudinal (4); baseline PTSD with repeated inflammation | Age, smoking, alcohol use, and medication use | Veterans with PTSD exhibited decreases in sCD40L and sPECAM-1 and increases in IL-8 and IL-1β over follow-up | PTSD predicted inflammation (mixed) |
| Solomon et al., 2017 (47) | 101 Yom Kippur War ex-POW combat veterans assessed in 1991, 2003, 2008, and 2015 assigned to chronic, resilient, and delayed PTSD trajectories | WBC, CRP assessed in 2015 | Longitudinal (4); repeated PTSD with follow-up inflammation | Captivity stressors and depression | Delayed and chronic PTSD was associated with high CRP among ex-POWs, compared to resilient trajectories | PTSD predicted inflammation (+) |
| Sumner et al., 2017 (48) | 524 community-dwelling women with no trauma exposure, trauma but no PTSD, and chronic PTSD; two blood draws conducted 10–16 years apart | hsCRP, TNFRII, ICAM-1, VCAM-1 at both blood draws | Repeated measures (3) | Time between blood draws, age, race, anti-hypertensive medication, anti-inflammatory medication, cholesterol-lowering medication, hormone therapy, and menopausal status | Women with chronic PTSD had higher hsCRP, TNFRII, and ICAM-1 levels averaged across follow-up compared to those with no trauma; women with chronic PTSD had greater increases in VCAM-1 over time compared to women with no trauma | PTSD predicted inflammation (+) |
| Sumner et al., 2018 (9)c | 525 community-dwelling women with no trauma exposure, trauma with no PTSD, and PTSD that onset in between two blood draws 10–16 years apart | hsCRP, TNFRII, ICAM-1, VCAM-1 at both blood draws | Repeated measures (3) | Time between blood draws, age, race, anti-hypertensive medication, anti-inflammatory medication, cholesterol-lowering medication, hormone therapy, menopausal status, BMI, smoking, alcohol consumption, physical activity, and diet quality | Women with PTSD onset had larger increases in VCAM-1 over follow-up compared to those with no trauma exposure | PTSD predicted inflammation (+) |
| PTSD Treatment and Inflammation | ||||||
| Himmerich et al., 2016 (49) | 38 male combat soldiers with PTSD randomly assigned to inpatient psychotherapy or control outpatient psychotherapy for 6 weeks | TNFα and sTNFR p55 and p75 at baseline and 6 weeks follow-up | Repeated measures with PTSD treatment (3) | None | TNFα increased, sTNFR decreased, and PTSD symptoms decreased by follow-up among all participants; PTSD symptom changes were not related to inflammatory changes | PTSD did not predict inflammation |
| Toft et al., 2018 (51) | 124 psychiatric patients (39 with PTSD) in 12-week inpatient psychotherapy and psychoeducation treatment | IL-1β, MCP-1, and TNFα at baseline, 6 weeks and 12 weeks of follow-up | Repeated measures with PTSD treatment (3) | None | Individuals with PTSD had increasing levels of IL-1β, MCP-1, and TNFα across follow-up, compared to other psychiatric diagnoses | PTSD predicted inflammation (+) |
| Tucker, et al., 2004 (50) | 58 individuals with PTSD randomized to two SSRIs or placebo for 10 weeks; 21 trauma-exposed controls for baseline comparison | IL-1β and IL-2R at baseline and 10 weeks follow-up | Repeated measures with PTSD treatment (3) | None | Those with PTSD had higher IL-1β and lower IL-2R levels compared to controls at baseline; over treatment, PTSD symptoms decreased, IL-1β decreased, and IL-2R increased by follow-up, regardless of treatment status | PTSD did not predict inflammation |
Study designs in order from strongest to weakest evidence for causality:
1. Pre-post trauma exposure – assessments both before and following trauma exposure for both PTSD and inflammation
2. Pre-post trauma exposure – assessments both before and following trauma exposure, but either PTSD or inflammation only assessed at one timepoint
3. Repeated measures (with or without PTSD treatment) – repeated assessments for both PTSD and inflammation
4. Longitudinal – repeated assessments for either PTSD or inflammation, and either PTSD or inflammation only assessed at one timepoint
Positive associations are indicated by “+,” negative associations are indicated by “−,” and both positive and negative associations are indicated by “mixed.”
Study is included as evidence for both the inflammation-to-PTSD relation and the PTSD-to-inflammation relation.
Abbreviations: PTSD = posttraumatic stress disorder; hsCRP = high sensitivity C-reactive protein; IL = interleukin; TNFα = tumor necrosis factor alpha; SES = socio-economic status; NSAID = nonsteroidal anti-inflammatory drugs; BMI = body mass index; CRP = C-reactive protein; IFNγ = interferon gamma; sPECAM = soluble platelet-endothelial cell adhesion molecule; sICAM = soluble intercellular adhesion molecule; MIP = macrophage inflammatory protein; sCD40L = soluble CD40 ligand; NGF = nerve growth factor; POW = prisoner of war; WBC = white blood cell; TNFRII = tumor necrosis factor alpha-receptor II; ICAM = intercellular adhesion molecule; VCAM = vascular cell adhesion molecule; sTNFR = soluble tumor necrosis factor receptor; MCP = monocyte chemoattractant protein; SSRI = selective serotonin reuptake inhibitors
Evidence for the Inflammation-to-PTSD Relation
Studies addressing the extent to which inflammation increases vulnerability to PTSD utilized several designs. For example, some studies in longitudinal cohorts (particularly military samples) investigated whether pre-trauma inflammation predicted PTSD. Some also considered whether inflammation in the acute aftermath of trauma predicted subsequent PTSD. In these studies, inflammation was measured prior to PTSD diagnosis, which cannot be determined until one month after trauma. Additional longitudinal studies examined inflammation as a predictor of change in PTSD over time.
Pre-Trauma Inflammation Predicting PTSD
Five studies of military personnel examined whether pre-deployment inflammation, measured in various ways, predicted PTSD symptoms after deployment(10,33–36). A strength of these studies is that the trauma associated with PTSD (deployment-related trauma) occurred after inflammation measurement. Moreover, most studies queried pre-existing PTSD before deployment(10,33–35), with some adjusting for pre-deployment symptoms(10,35) and others only analyzing those with first-onset PTSD post-deployment(33,34).
Overall, large-scale military studies yielded mixed results (for this review, “large-scale” refers to studies with N≥500; small studies have N<50). In male U.S. Marines, higher pre-deployment high sensitivity C-reactive protein (hsCRP) levels were associated with increased likelihood of presence versus absence of any PTSD symptoms post-deployment, but not with severity of symptoms when present(10). Investigations of Dutch military personnel considering in vitro stimulated measures of inflammatory cytokine- and/or chemokine-producing immune cells at pre-deployment as predictors of post-deployment PTSD symptoms did not support a role for heightened inflammatory cytokine levels leading to PTSD. For instance, pre-deployment T-cell and monocyte cytokine production capacity did not predict PTSD symptoms at 6 months post-deployment(36). In another investigation, greater glucocorticoid-sensitivity of T-cells (which could produce lower levels of pro-inflammatory cytokines), but not monocytes, at pre-deployment was associated with elevated PTSD symptoms at 6 months post-deployment(35). Notably, greater pre-deployment glucocorticoid-sensitivity of T-cells was present only in soldiers who developed high PTSD, and not depressive, symptoms post-deployment. PTSD and depression frequently co-occur, and these results offer preliminary evidence that greater glucocorticoid inhibition of T-cell proliferation might be associated with vulnerability to features unique to PTSD.
Two studies using gene expression to assess pre-deployment inflammation-related processes are also suggestive. In a small study of male U.S. Marines without PTSD at baseline, pre-deployment functional annotation of genes indicated up-regulation of immune-related genes in men who later did versus did not develop PTSD(34). In a larger study of male U.S. Marines without PTSD at baseline, men who did versus did not develop PTSD exhibited overexpression of genes in networks associated with innate immune response and interferon signaling at pre-deployment(33).
Although most studies of pre-trauma inflammation and PTSD onset used a deployment framework and examined largely men, we investigated these associations in a large sample of women experiencing various trauma types. Using retrospective dating of trauma exposure/PTSD onset in a longitudinal female cohort [the Nurses’ Health Study II (NHSII)], we identified women who developed PTSD during study follow-up and had measures of inflammation before and after PTSD onset(9). Compared to women without trauma, women who later developed PTSD had higher TNFα-receptor II (TNFRII) and intercellular adhesion molecule-1 (ICAM-1), but not hsCRP or vascular cell adhesion molecule-1 (VCAM-1), levels before PTSD onset. Similar to findings in U.S. Marines(10), greater pre-PTSD-onset inflammation did not predict the extent of symptoms in women who developed PTSD.
Inflammation in the Acute Aftermath of Trauma Predicting PTSD
Six studies examined whether inflammation measured within hours to days after trauma predicted subsequent PTSD(37–42). This approach may indicate whether heightened inflammatory responses to trauma increase vulnerability to PTSD. However, without assessing inflammation before and immediately after trauma, one cannot disentangle whether it is pre-trauma inflammation or the acute posttraumatic inflammatory response that predicts PTSD. Furthermore, because inflammation is measured after trauma, inflammation levels could reflect aspects of trauma exposure, particularly for injury or medical traumas.
Several [but not all(39,42)] studies found elevated inflammation levels after trauma predicted subsequent PTSD onset(37,38,40). For example, in child motor vehicle accident survivors, higher IL-6 levels within 24 hours of the accident predicted PTSD diagnosis 6 months post-accident(40). Additionally, a small study of gene expression patterns within hours of trauma identified differential expression patterns in genes related to immune activation in individuals who did and did not develop PTSD(41). However, in a large sample of adults assessed in the emergency department within hours of trauma exposure and then followed for up to 12 months, participants who exhibited a chronic PTSD trajectory over follow-up had lower TNFα and IFNγ levels in the emergency department compared to those exhibiting symptom trajectories consistent with recovery or resilience(42). No significant differences were observed for numerous other inflammation-related markers.
Additional Longitudinal Studies of Inflammation Predicting PTSD
Additional longitudinal studies of the inflammation-to-PTSD relation yielded mixed results. For example, in a large Swiss sample, neither hsCRP, IL-6, nor TNFα levels at baseline predicted incident PTSD 5.5 years later, but findings may be limited as few participants developed PTSD over follow-up(43). In a predominantly male U.S. veteran sample, higher baseline levels of hsCRP, white blood cell (WBC) count, fibrinogen, and erythrocyte sedimentation rate were related to a worse course of PTSD (e.g., having ongoing or new-onset PTSD versus no PTSD) over four years of follow-up(44). Notably, most associations were attenuated after adjusting for physical activity. Preliminary support for a role for elevated inflammation in heightened stress sensitivity and PTSD risk after deployment comes from another large study of Dutch soldiers. Among soldiers reporting high combat exposure, high stimulated T-cell cytokine production and monocyte cytokine production at one month post-deployment were each associated with greater increases in PTSD symptoms in response to stressors over the first two years after deployment(45).
Evidence for the PTSD-to-Inflammation Relation
Using varied approaches, several longitudinal studies have examined whether PTSD leads to inflammation. One approach investigates whether PTSD onset results in changes in inflammation from pre- to post-PTSD onset, whereas another considers whether PTSD at baseline predicts changes in inflammation over time. PTSD treatment studies measuring inflammation pre- and post-treatment, along with symptom change, also address this relation.
PTSD Onset or Diagnosis and Changes in Inflammation
Although rare, some studies include inflammation measures pre- and post-PTSD onset. In the NHSII, we demonstrated that women whose PTSD onset between two blood draws 10–16 years apart had greater increases in VCAM-1 levels (but not other inflammatory markers) from before to after PTSD onset compared to women without trauma(9). Four studies evaluated whether PTSD diagnosis predicted changes in inflammatory markers over time(43,46–48). Findings suggest that PTSD is associated with increases in inflammation, although results are inconsistent across different markers. For example, among Croatian men, combat veterans with chronic PTSD showed increases in IL-1ß and IL-8 over a 3-month period and decreases in soluble platelet-endothelial cell adhesion molecule-1 and soluble CD40 ligand(46). However, numerous other inflammation-related markers were unchanged, and this small study did not include comparisons to veterans without PTSD. Another study in the NHSII found women with chronic PTSD versus no trauma had higher hsCRP, TNFRII, and ICAM-1 levels averaged across two time points over 10–16 years(48). Women with chronic PTSD also exhibited greater increases in VCAM-1 over follow-up. In contrast, in the large Swiss sample described above, baseline PTSD was associated with lower IL-6 approximately 5.5 years later, adjusting for baseline IL-6(43).
PTSD Treatment and Inflammation
Three treatment studies—two randomized controlled trials (RCTs) using intent-to-treat analyses(49,50) and one single-arm trial(51)—measured PTSD and/or inflammatory markers at pre- and post-treatment. Findings are mixed. One study randomized German veterans to a 6-week inpatient psychotherapy or control outpatient psychotherapy and measured TNFα and its receptors (sTNFR p55 and p75)(49). In both conditions, PTSD symptoms and sTNFR p55 and p75 decreased over treatment, and TNFα increased over treatment. Thus, a non-specific effect of psychotherapy increasing inflammation was observed, with no significant correlations between changes in PTSD symptoms and inflammatory markers. In the second RCT, PTSD patients were assigned to 10 weeks of citalopram, sertraline, or placebo, with levels of IL-1ß and soluble IL-2 receptor (IL-2R; an indicator of cell-mediated immunity) measured pre- and post-treatment(50). At baseline, patients with PTSD had higher IL-1ß and lower IL-2R levels than trauma-exposed controls. Patients with PTSD in all three groups exhibited decreases in PTSD symptoms and IL-1ß and increases in IL-2R from pre- to post-treatment. Thus, PTSD symptoms declined and cytokine levels normalized with either antidepressants or placebo, perhaps reflecting a powerful placebo response. The investigators did not evaluate change in PTSD symptoms in relation to change in cytokines. In a 12-week inpatient treatment study of patients with PTSD or with mood, anxiety, or eating disorders, both mental distress (as measured by Global Severity Index scores) and inflammation changed over time(51). However, as PTSD symptom changes were not measured, whether inflammation changes paralleled changes in PTSD is unknown.
Summary
A number of investigations, including large-scale studies, present positive findings suggesting that inflammation may increase susceptibility to PTSD after trauma or that PTSD may lead to altered inflammatory processes, hinting the association may be bidirectional. However, taken together, the research presents a mixed picture. Even when studying large samples and using the strongest designs available to assess the PTSD-inflammation association in either direction, results are inconsistent. Even within the same study, results were often inconsistent across multiple inflammatory markers(9,38,44,46,48). These studies were conducted using widely different samples and inflammation-related markers and paradigms, making comparisons difficult. Thus, despite plausible biological mechanisms for the PTSD-inflammation relation, existing evidence precludes strong conclusions regarding directionality and causality. Future work will benefit not only from using the strongest study designs possible but also by using common data elements in studies of the PTSD-inflammation relation to facilitate comparisons across investigations. Furthermore, comprehensively accounting for potential confounding factors is critical(3). As seen in Tables 1 and 2, covariates included in the reviewed studies were wide-ranging. Several of these are particularly important, including socioeconomic status, childhood maltreatment, and depression [see Koenen et al.(3)], and it will be critical to evaluate carefully whether they contribute to the PTSD-inflammation relation.
Methodological Approaches for Untangling Directional Associations
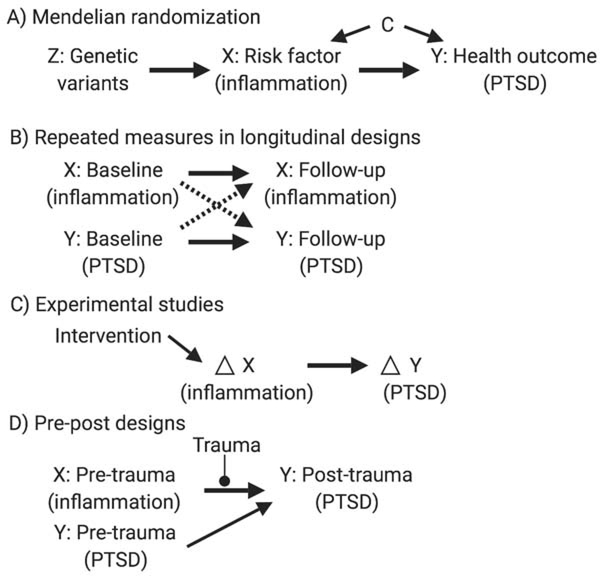
Here, we review several methodological frameworks useful for ascertaining potential causal associations and untangling directionality (Figure 2). Additionally, we address the extent to which these approaches can be employed to understand the PTSD-inflammation relation. Also worth noting is the importance of incorporating the highest quality measures of PTSD and inflammation. For example, single measures of inflammatory markers can be noisy; repeated measures may reduce noise in this signal.
Figure 2.
Diagrams outlining methodological approaches for untangling directionality that can be applied to the posttraumatic stress disorder (PTSD)-inflammation relation. These directed acyclic graphs (DAGs) are visual representations of causal assumptions. DAGs are shown to represent the following statistical approaches and study designs: A) Mendelian randomization (Z=genetic variants; X=risk factor such as inflammation; Y=health outcome such as PTSD; C=confounders), B) Repeated measures in longitudinal designs [dashed arrows=cross-lagged paths representing effects of baseline variable Y (PTSD) on follow-up variable X (inflammation) and of baseline variable X (inflammation) on follow-up variable Y (PTSD); solid arrows=autoregressive effects representing stability in variables X and Y over time], C) Experimental studies [intervention leads to change (Δ) in variable X (inflammation), which is then related to Δ in variable Y (PTSD)], D) Pre-post designs [pre-trauma measure of variable X (inflammation) is then related to a post-trauma measure of variable Y (PTSD), accounting for a pre-trauma measure of variable Y (PTSD)].
Mendelian Randomization
Mendelian randomization (MR) is an instrumental variable (IV) analytic method (Figure 2A) that rigorously accounts for confounding (measured and unmeasured) in observational studies. By using an instrument—a variable that is related to a risk factor, is not related to confounders, and influences an outcome only through the risk factor—the design mimics that of an experiment with individuals randomly assigned to be exposed or unexposed to the risk factor(52). Because genetic variants are assigned and fixed at conception akin to a randomized experiment(52), MR leverages genetic variants as IVs for risk factors associated with health outcomes(53). By examining the extent to which genetic variants are associated with both risk factor and health outcome, the MR estimate provides evidence for a causal relation(52). However, several stringent assumptions must be met for MR studies to be valid [see Davies et al.(52)], and the genetic IV needs to account for a substantial proportion of variance in the risk factor to be useful.
One-sample MR requires measures of genotype, risk factor, and outcome in the same sample, whereas two-sample MR can obtain estimations of the genetic variants-risk factor association and genetic variants-outcome association from two different sets of participants(54). With two-sample MR, because the risk factor and outcome do not need to be measured in the same study, this method can use summary results from genome-wide association studies (GWAS)(52). Consortia are now pooling GWAS data and publicly sharing summary statistics(55,56), thereby offering more precise estimates based on large samples. Furthermore, GWAS summary statistics can be used to generate a genetic IV that incorporates information from multiple genetic variants and thus accounts for more variance in the phenotype of interest, providing a stronger genetic instrument.
Bidirectional two-sample MR could be implemented to examine whether inflammation is a causal risk factor for PTSD (using genetic variants associated with inflammation as an IV) and whether PTSD is a causal risk factor for inflammation (using genetic variants associated with PTSD as an IV). This approach has yet to be used to investigate the PTSD-inflammation relation. However, with summary statistics now available from large (N≥200,000) GWAS for PTSD(57) and inflammatory markers(58), new opportunities to apply this approach are available. Perhaps worth noting is that studies using non-genetic IVs are possible (e.g., using natural experiments) but have presented challenges with regard to finding appropriate instruments.
Repeated Measures in Longitudinal Studies
When studies include only one measure of PTSD or inflammation, concerns about reverse causality are difficult to mitigate. Only a few studies have incorporated repeated measures of both inflammatory markers and PTSD(40,43), and two examined updated PTSD status over time (although using retrospectively reported timing of trauma/PTSD onset) with change in inflammatory biomarkers(9,48). Repeated measures studies also benefit from rigorous statistical methods that directly examine bidirectionality. For example, cross-lagged panel analyses simultaneously evaluate longitudinal effects of two variables on each other, while controlling for baseline levels (Figure 2B). A recent study using this approach found support for bidirectionality in the relation between depressive symptoms and inflammation (IL-6 and hsCRP) over 7 years(59). Baseline IL-6 positively predicted depressive symptoms, whereas baseline depressive symptoms did not predict IL-6. Conversely, baseline hsCRP did not predict depressive symptoms, but baseline depressive symptoms predicted increased hsCRP. Future studies should determine longitudinal courses of PTSD symptoms in conjunction with changes in inflammation and leverage these kinds of rigorous analytic techniques.
Experimental Studies
Experimentally inducing inflammation and assessing subsequent changes in PTSD symptoms may be a useful study design for examining causal effects of inflammation on PTSD (Figure 2C). Little work has examined the impact of anti-inflammatory agents on PTSD, although meta-analytic evidence supports the effectiveness of anti-inflammatory treatment (e.g., cytokine antagonists, non-steroidal anti-inflammatory drugs) for improving depressive symptoms(60,61). Experimental studies using anti-inflammatory agents in patients with depression also provide evidence that inflammation may influence subsequent psychopathology. For example, in patients with treatment-resistant depression, pre-treatment hsCRP levels predicted response to a novel treatment for depression, administering a TNF-antagonist versus placebo(62). TNF-antagonist treatment was not effective in improving depressive symptoms overall. However, differential effects were observed whereby individuals with high (>5mg/L) but not low (≤5mg/L) baseline hsCRP showed significantly improved depressive symptoms after treatment with TNF-antagonist. These findings suggest there are psychiatric disease subtypes characterized partly by inflammation levels and, as a result, some are likely to be more or less responsive to inflammation-targeted pharmacotherapy.
Experimental work has also explored acute induction of inflammation and subsequent psychological symptoms, again largely focused on depression(63). Experimental inflammation inductions (e.g., typhoid vaccination, endotoxin injection) provide more rigorous tests of the causal role of inflammation on psychological experience. Generally consistent evidence suggests that inflammation inductions impact neural reactivity to negative stimuli (e.g., heightening threat vigilance), reward processing (e.g., blunting reactivity to rewards), and somatic symptoms (e.g., increasing sleep disturbances)(63), which are characteristic of PTSD(1,64). Research using these experimental paradigms of inflammation is warranted in PTSD.
Although PTSD is difficult to manipulate experimentally, treatment studies for PTSD may provide evidence regarding how change in PTSD impacts change in inflammation. As noted, a few psychotherapy or pharmacotherapy studies of PTSD assessed changes in inflammation(49–51). Although these trials indicate that inflammation levels may change in response to intervention (albeit at times with increases), it remains unclear if these changes are due to change in PTSD. Furthermore, no studies have examined gold-standard trauma-focused psychotherapies (e.g., Prolonged Exposure, Cognitive Processing Therapy), and prior studies may not have been adequately powered to test whether changes in PTSD related to changes in inflammation. Future investigations should directly incorporate hypotheses regarding change in inflammatory markers to test more robustly whether effective PTSD treatment could influence subsequent inflammation.
Pre-Post Designs
Prospective observational studies with assessments of inflammation before and after trauma and PTSD onset can provide important evidence regarding the extent to which elevated inflammation may increase PTSD risk (Figure 2D). As noted, studies of military personnel have collected pre- and post-deployment measures(10,33–36). Although less common, pre-post designs have also been implemented in non-military samples(9). Pre-post designs examining inflammation and PTSD could also be considered for individuals before entering situations with heightened risk of trauma exposure (e.g., trainee firefighters or police officers studied during training and after being on the job). These kinds of samples have been leveraged to identify PTSD risk factors but have yet to examine risk associated with pre-trauma inflammation(65,66).
An important potential limitation of pre-post studies to date is that prior unmeasured trauma exposure or psychopathology could influence baseline inflammation that, in turn, predicts subsequent PTSD. Although studies have adjusted for pre-existing PTSD symptoms(10,35), none comprehensively accounted for prior trauma history and psychopathology. Moreover, only a few studies examined inflammation-related processes as predictors of first-onset PTSD(33,34). Given links between trauma and inflammation(67) and between other psychopathology (e.g., depression, anxiety) and inflammation(68,69), it is critical to account for these exposures to rule out concerns that pre-existing factors unrelated to inflammation might explain findings.
Future Directions for Research and PTSD Prevention and Intervention
Going forward, research addressing the PTSD-inflammation relation will be strengthened by incorporating different biological levels of analysis in a single investigation. Studies have primarily measured inflammatory markers or gene expression. Future research investigating epigenetics, gene expression, metabolomics, and the microbiome, along with inflammatory markers, may shed light on mechanisms underlying the PTSD-inflammation association. For example, epigenetic processes influence gene expression(70), but these processes have not been considered within the PTSD-inflammation literature. Additionally, as the microbiome is a key regulator of inflammation and immune responses(71), considering these processes may be informative. An exploratory study found decreases in the relative abundances of Actinobacteria, Lentisphaere, and Verrucomicrobia in individuals with PTSD compared to trauma-exposed controls, which could contribute to immunoregulation deficiencies in those with PTSD(72). Additionally, early metabolomics research identified reduced unsaturated fatty acid levels in patients with versus without PTSD, which could increase cytokine production(73). Although promising, these studies are cross-sectional, precluding conclusions about directionality and causality.
If research continues to indicate that elevated inflammation predisposes individuals to develop PTSD, treatments that reduce inflammation in trauma-exposed individuals may be warranted for those who show heightened inflammation before or after trauma. Such interventions could mirror work conducted in those with depression(62), evaluating if anti-inflammatory interventions prevent PTSD onset and/or promote PTSD remission. Additional work is also needed to identify individuals who might benefit most from anti-inflammatory interventions.
If future evidence also supports a causal link between PTSD and elevated inflammation, then rigorous research (i.e., appropriately powered, using gold-standard treatments) that explicitly tests whether successfully treating PTSD leads to improvement in inflammatory markers is warranted. Notably, preliminary work comparing inflammation in individuals with remitted PTSD and without PTSD indicates that individuals with remitted disorder do not show elevated inflammation. For example, in one cross-sectional study, individuals with remitted PTSD did not differ from those without PTSD on hsCRP, fibrinogen, and WBC count(74). In the longitudinal study of Swiss adults discussed above, remitted PTSD at baseline was not associated with IL-6, hsCRP, or TNFα levels approximately 5.5 years later, adjusting for baseline inflammation(43). However, because studies did not measure inflammation before remission, it is unclear whether these individuals already exhibited lower levels of inflammation and if this contributed to their remission, or if the PTSD remission led to decreased inflammation. Evidence supporting the latter has implications for physical health consequences of PTSD.
Conclusions
Preliminary, but inconsistent, evidence suggests that elevated inflammation may increase risk of PTSD onset and that PTSD may lead to heightened inflammation. Conclusions regarding causality remain limited, and methodological limitations and differences in the inflammation measures and samples studied make cross-investigation comparisons challenging. Nevertheless, these initial findings suggest that targeted research is warranted to better understand these associations. Employing a broad range of methodological approaches will help elucidate whether the PTSD-inflammation relation is bidirectional and causal.
Acknowledgements
This work was supported by the National Institutes of Health (JAS, grant numbers K01HL130650, R01HL139614; KCK, LDK, grant number R01MH101269, KMN, grant number T32MH017119).
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 2.Monson CM, Friedman MJ, La Bash HA (2007): A psychological history of PTSD In: Friedman MJ, Keane T, Resick P, editors. Handbook of PTSD: Science and Practice. New York: Guilford Press, pp. 37–52. [Google Scholar]
- 3.Koenen KC, Sumner JA, Gilsanz P, Glymour MM, Ratanatharathorn A, Rimm EB, et al. (2017): PTSD and cardiometabolic disease: improving causal inference to inform practice. Psychol Med 47: 209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryder AL, Azcarate PM, Cohen BE (2018): PTSD and physical health. Curr Psychitry Rep 20: 116. [DOI] [PubMed] [Google Scholar]
- 5.Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais JC, Chen Q, et al. (2015): Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation 132: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, et al. (2015): Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry 72: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, Neylan TC (2015): Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry 77: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts AL, Malspeis S, Kubzanksy LD, Feldman CH, Chang SC, Koenen KC, et al. (2017): Association of trauma and posttraumatic stress disorder with incident systemic lupus erythematosus in a longitudinal cohort of women. Arthritis Rheumatol 69:2162–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. (2018): Posttraumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain Behav Immun 69: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, et al. (2014): Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 71: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hori H, Kim Y (2019): Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci 73: 143–153. [DOI] [PubMed] [Google Scholar]
- 12.Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017): Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42: 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI (2012): Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage 59: 3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin MR, Cole SW (2011): Reciprocal regulation of the neural and innate immune systems. Nature Rev Immunol 11: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AH, Haroon E, Raison CL, Felger JC (2013): Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan N, Banks WA (2007): Brain-immune communication pathways. Brain Behav Immun 21: 727–735. [DOI] [PubMed] [Google Scholar]
- 17.Swartz JR, Prather AA, Di Iorio CR, Bogdan R, Hariri AR (2017): A functional interleukin-18 haplotype predicts depression and anxiety through increased threat-related amygdala reactivity in women but not men. Neuropsychopharmacology 42: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swartz JR, Prather AA, Hariri AR (2017): Threat-related amygdala activity is associated with peripheral CRP concentrations in men but not women. Psychoneuroendocrinology 78: 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yirmiya R, Goshen I (2011): Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181–213. [DOI] [PubMed] [Google Scholar]
- 20.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. (2003): A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci 100: 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koenen KC, Hitsman B, Lyons MJ, Stroud L, Niaura R, McCaffery J, et al. (2006): Posttraumatic stress disorder and late-onset smoking in the Vietnam era twin registry. J Consult Clin Psychol 74: 186–190. [DOI] [PubMed] [Google Scholar]
- 22.Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, et al. (2014): The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry 71: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. (2012): Biological studies of post-traumatic stress disorder. Nature Rev Neurosci 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Southwick SM, Bremner JD, Rasmusson A, Morgan CA III, Arnsten A, Charney DS (1999): Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 46: 1192–1204. [DOI] [PubMed] [Google Scholar]
- 25.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM (2007): β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA-and NF-κB-independent mechanisms. Cell Signal 19: 251–260. [DOI] [PubMed] [Google Scholar]
- 26.van den Berk-Clark C, Secrest S, Walls J, Hallberg E, Lustman PJ, Schneider FD, et al. (2018): Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: a systematic review and meta-analysis. Health Psychol 37:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wingenfeld K, Whooley MA, Neylan TC, Otte C, Cohen BE (2015): Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinology 52: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winning A, Gilsanz P, Koenen KC, Roberts AL, Chen Q, Sumner JA et al. (2017): Post-traumatic stress disorder and 20-year physical activity trends among women. Am J Prev Med 52: 753–-760.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yehuda R, Boisoneau D, Lowy MT, Giller EL (1995): Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 52: 583–593. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R, Lowy MT, Southwick S, Shaffer D, Giller EJ (1991): Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am J Psychiatry 148: 499–504. [DOI] [PubMed] [Google Scholar]
- 31.Zen AL, Whooley MA, Zhao S, Cohen BE (2012): Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 31: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. (2015): Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2: 1002–1012. [DOI] [PubMed] [Google Scholar]
- 33.Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, et al. (2015): Gene networks specific for innate immunity define post-traumatic stress disorder. Molecular Psychiatry 20: 1538–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glatt SJ, Tylee DS, Chandler SD, Pazol J, Nievergelt CM, Woelk CH, et al. (2013): Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: a pilot study. Am J Med Genet B Neuropsychiatr Genet 162: 313–326. [DOI] [PubMed] [Google Scholar]
- 35.van Zuiden M, Heijnen CJ, Maas M, Amarouchi K, Vermetten E, Geuze E, et al. (2012): Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: a prospective study. Psychoneuroendocrinology 37: 1822–1836. [DOI] [PubMed] [Google Scholar]
- 36.van Zuiden M, Heijnen CJ, van de Schoot R, Amarouchi K, Maas M, Vermetten E, et al. (2011): Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS One 6: e29142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bielas H, Meister-Langraf RE, Schmid J-P, Barth J, Znoj H, Schnyder U, et al. (2018): C-reactive protein as a predictor of posttraumatic stress induced by acute myocardial infarction. Gen Hosp Psychiatry 53: 125–130. [DOI] [PubMed] [Google Scholar]
- 38.Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S (2011): Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med 42: 117–131. [DOI] [PubMed] [Google Scholar]
- 39.Gandubert C, Scali J, Ancelin M, Carriere I, Dupuy AM, Bagnolini G, et al. (2016): Biological and psychological predictors of posttraumatic stress disorder onset and chronicity: a one-year prospective study. Neurobiol Stress 3: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, et al. (2007): Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 32: 991–999. [DOI] [PubMed] [Google Scholar]
- 41.Segman R, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev A (2005): Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry 10: 500–513. [DOI] [PubMed] [Google Scholar]
- 42.Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, Rothbaum BO, et al. (2019): Association of prospective risk for chronic PTSD symptoms with low TNFα and IFNγ concentrations in the immediate aftermath of trauma exposure. Am J Psychiatry doi: 10.1176/appi.ajp.2019.19010039. [DOI] [PubMed] [Google Scholar]
- 43.Glaus J, von Känel R, Lasserre AM, Strippoli MF, Vandeleur CL, Castelao E, et al. (2018): The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: results from a large longitudinal population-based study. Depress Anxiety 35: 360–371. [DOI] [PubMed] [Google Scholar]
- 44.Eswarappa M, Neylan TC, Whooley MA, Metzler TJ, Cohen BE (2019): Inflammation as a predictor of disease course in posttraumatic stress disorder and depression: a prospective analysis from the Mind Your Heart Study. Brain Behav Immun 75: 220–227. [DOI] [PubMed] [Google Scholar]
- 45.Smid GE, van Zuiden M, Geuze E, Kavelaars A, Heijnen CJ, Vermetten E (2015): Cytokine production as a putative biological mechanism underlying stress sensitization in high combat exposed soldiers. Psychoneuroendocrinology 51: 534–546. [DOI] [PubMed] [Google Scholar]
- 46.Jergović M, Bendelja K, Savić Mlakar A, Vojvoda V, Aberle N, Jovanovic T, et al. (2015): Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder–a 3-month follow-up study. Front Psychiatry 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon Z, Levin Y, Assayag EB, Furman O, Shenhar-Tsarfaty S, Berliner S, et al. (2017): The implication of combat stress and PTSD trajectories in metabolic syndrome and elevated C-reactive protein levels: a longitudinal study. J Clin Psychiat 78: e1180–e1186. [DOI] [PubMed] [Google Scholar]
- 48.Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. (2017): Cross-sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biol Psychiatry 82: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Himmerich H, Willmund GD, Zimmermann P, Wolf J-E, Bühler AH, Kirkby KC, et al. (2016): Serum concentrations of TNF-a and its soluble receptors during psychotherapy in German soldiers suffering from combat-related PTSD. Psychiatr Danub 28: 293–298. [PubMed] [Google Scholar]
- 50.Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP, et al. (2004): Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry 56: 121–128. [DOI] [PubMed] [Google Scholar]
- 51.Toft H, Bramness JG, Lien L, Abebe DS, Wampold BE, Tilden T, et al. (2018): PTSD patients show increasing cytokine levels during treatment despite reduced psychological distress. Neuropsychiatr Dis Treat 14: 2367–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies NM, Holmes MV, Smith GD (2018): Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362: k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Hinke S, Smith GD, Lawlor DA, Propper C, Windmeijer F (2016): Genetic markers as instrumental variables. J Health Econ 45: 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng J, Baird D, Borges M-C, Bowden J, Hemani G, Haycock P, et al. (2017): Recent developments in Mendelian randomization studies. Curr Epidemiol Rep 4: 330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan PF (2010): The psychiatric GWAS consortium: big science comes to psychiatry. Neuron 68: 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornelis MC, Agrawal A, Cole JW, Hansel NN, Barnes KC, Beaty TH, et al. (2010): The Gene, Environment Association Studies consortium (GENEVA): maximizing the knowledge obtained from GWAS by collaboration across studies of multiple conditions. Genet Epidemiology 34: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen C-Y, Choi KW, et al. (2019): Largest genome-wide association study for PTSD identifies genetic risk loci in European and African ancestries and implicates novel biological pathways. Nat Commun. In press. [Google Scholar]
- 58.Ligthart S, Vaez A, Võsa U, Stathopoulou MG, De Vries PS, Prins BP, et al. (2018): Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am J Hum Genet 103: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang M, Su S, Goldberg J, Miller AH, Levantsevych OM, Shallenberger L, et al. (2019): Longitudinal association of inflammation with depressive symptoms: a 7-year cross-lagged twin difference study. Brain Behav Immun 75: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. (2014): Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71: 1381–1391. [DOI] [PubMed] [Google Scholar]
- 61.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM (2018): Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 23: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. (2013): A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, Bower JE (2018): The role of inflammation in core features of depression: insights from paradigms using exogenously-induced inflammation. Neurosci Biobehav Rev 94: 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nawijn L, van Zuiden M, Frijling JL, Koch SB, Veltman DJ, Olff M (2015): Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev 51: 189–204. [DOI] [PubMed] [Google Scholar]
- 65.Bryant RA, Guthrie RM (2005): Maladaptive appraisals as a risk factor for posttraumatic stress: a study of trainee firefighters. Psychol Sci 16: 749–752. [DOI] [PubMed] [Google Scholar]
- 66.Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR (2009): Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol Psychiatry 65: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tursich M, Neufeld R, Frewen P, Harricharan S, Kibler JL, Rhind SG, et al. (2014): Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry 4: e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renna ME, O’Toole MS, Spaeth PE, Lekander M, Mennin DS (2018): The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress Anxiety 35: 1081–1094. [DOI] [PubMed] [Google Scholar]
- 69.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. (2010): A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 70.Gibney E, Nolan C (2010): Epigenetics and gene expression. Heredity 105: 4–13. [DOI] [PubMed] [Google Scholar]
- 71.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D (2017): Regulation of inflammation by microbiota interactions with the host. Nat Immunol 18: 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hemmings SM, Malan-Muller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, et al. (2017): The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 79: 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mellon SH, Bersani FS, Lindqvist D, Hammamieh R, Donohue D, Dean K, et al. (2019): Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS One 14: e0213839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Donovan A, Ahmadian AJ, Neylan TC, Pacult MA, Edmondson D, Cohen BE (2017): Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain Behav Immun 60: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]