Abstract
Hematopoietic stem/progenitor cell (HSPC) mobilization from the bone marrow to the bloodstream is a required step for blood cell renewal and HSPC motility is a clinically relevant standard for peripheral blood stem cell transplantation. Individual HSPCs exhibit considerable heterogeneity in motility behaviors, which are subject to complex intrinsic and extrinsic regulatory mechanisms. Motility based cell sorting is then demanded to fulfill the study of such mechanism complexity. However, due to the HSPC heterogeneity and difficulty in monitoring cell motility, such a platform is still not available. With the recent development of microfluidics technology, motility-based monitoring, sorting, collecting, and analysis of HSPCs behaviors are highly possible and achievable if fluid channels and structures are correctly engineered. Here, we present a new design of microfluidic arrays for single-cell trapping, enabling high-throughput analysis of individual HSPC motility and behavior. Using these arrays, we observe that HSPC motility is positively correlated with CD34 asymmetric inheritance and cell differentiation. Transcriptomic analysis of HSPCs sorted according to motility reveals changes in expression of genes associated with the regulation of stem-cell maintenance. Ultimately, our novel physical cell-sorting system can facilitate the screening of HSPC mobilization compounds and the analysis of signals driving HSPC fate decisions.
Keywords: microfluidics, single cell trap, hematopoietic stem cell, motility, stem-cell maintenance
1. Introduction
Hematopoietic stem/progenitor cells (HSPCs) are multipotent progenitor stem cells that give rise to all blood cells through the process of hematopoiesis[1]. A growing body of evidence suggests striking functional heterogeneity among individual HSPCs, with distinct properties regulated by complicated intrinsic and extrinsic mechanisms[2]. Throughout adult life, most HSPCs are located within specialized niches in the bone marrow, where the activity of the HSPCs is tightly controlled[3]. However, under certain circumstances, HSPCs can mobilize from the bone marrow into peripheral circulation[4]. A better understanding of HSPC mobilization could improve peripheral blood stem-cell transplantation in a clinical setting[5].
High-resolution confocal microscopy and two-photon video imaging have been combined previously to track motility of individual HSPCs in a mouse model[6]. However, the collection and analysis of the target cells in such an experimental setup is hindered by the complexity of the in vivo system. Integrated microfluidic chips with microstructures and nanostructures have been designed to rapidly monitor cell motility and dynamics[7]. For example, bone marrow-on-a-chip, developed to replicate the hematopoietic niche physiology in vitro, has been used to study cell proliferation. This system revealed considerable heterogeneity among individual HSPCs[8]. Hence, high-throughput microfluidic platforms for monitoring, sorting, collecting, and analyzing individual HSPCs based on motility behaviors is highly desirable.
Here, we developed microfluidic cell trapping arrays to perform high-throughput analysis of individual HSPC motility. When trapped using this device, HSPCs demonstrated heterogeneity in their motility behaviors. Trapped HSPCs also showed enhanced motility after treatment with the mobilization reagent AMD3100. Following on-chip sorting and analysis, we found that HSCs and HPCs may exhibit different motile behaviors. During extended culturing, highly motile HSPCs exhibited higher levels of CD34 asymmetric inheritance than did HSPCs with lower motility. Moreover, RNA sequencing (RNA-seq) analysis of the sorted HSPC populations revealed that genes associated with signaling pathways controlling stem-cell maintenance were down-regulated in motile HSPCs. Ultimately, the novel physical cell-sorting system described here can accelerate the screening of HSPC mobilization compounds and the investigation of signals modulating HSPC fate decisions.
2. Experimental Section
2.1. Chip Design and Fabrication
The microfluidic pattern was designed with AutoCAD (Autodesk). Each chip consisted of four parallel channels branching from a single inlet and merging into a single outlet. The chamber of each microfluidic channel was designed to contain 160 single-cell trapping structures. Three size variations of the cup-shaped trapping structures were designed: the S, M, and L trapping structures contained openings for cell entry of 6 μm, 8 μm, and 10 μm, respectively. Regardless of size, all traps were 10 μm high and contained 2-μm outlet channels along the central axis. The microfluidic device was fabricated according to standard photolithography and soft lithography procedures. The permanent epoxy negative photoresist SU8–3025 (MicroChem) pattern on the silicon wafer was fabricated using a photomask. The silicon wafer was then silanized with trimethylchlorosilane (Thermo Scientific) to facilitate polydimethylsiloxane (PDMS) mold release. PDMS prepolymer (Dow Corning) was poured onto the silicon wafer and cured at 80°C for 1 h. Holes were punched in the PDMS, and oxygen plasma treatment was used to chemically bond the PDMS mold to a glass slide.
2.2. Device Operation
Before cell loading, the single-cell trapping device was pretreated with 10% basal membrane extracts (BME) in phosphate-buffered saline (PBS) for 1h by applying negative pressure at the outlet. BME represents a more physiological microenvironment than the native PDMS surface. The channels were then washed with HSPC culture medium StemSpan™ SFEM. Cells were suspended in culture medium at a density of 106 cells/ml, placed in the inlet and pumped into the channels. Flow was maintained by connecting the outlets to a negative pressure control system, and flow rates were kept to less than 100 μm/s to avoid damaging the cells. The inlet reservoir was 3 mm in diameter to facilitate the addition or removal of cells and reagents. After cells were anchored by the traps, unanchored cells were washed away by replacing the cell suspension with culture medium. After single cells were captured, different flow rates were tested for flow effects on target cell collection. The optimal flow rate was defined as the lowest flow rate that could evacuate the highly motile HSPCs from the chip. We chose flow rate at 2 μl/min to collect target cells after on-chip culture for 3 hours with continuous medium flow. The chip was operated in the device incubator system in a humidified atmosphere of 5% CO2/95% air at 37°C. High-resolution time-lapse images of HSPCs growing in continuous medium flow were obtained with a CCD camera (DP72; Olympus) connected to an inverted microscope (IX-81; Olympus) equipped with image acquisition software (cellSens; Olympus) and EVOS auto cell imaging system (Life Technologies). The escaped cells refer to the cell samples that were collected from the outlet during 3 hours. The trapped cells refer to the cell samples that remained in the chip after 3 hours culture with continuous medium flow and collected from the inlet by applying positive pressure at the outlet. For each device, we could collect ~90 escaped cells and ~60 trapped cells after 3 hours culture at the flow rate of 2 μl/min. The escaped and trapped cells were collected for further experiments.
2.3. Materials and Reagents
SPR 220–7 photoresist was purchased from Rohm and Haas Electronic Materials. PDMS (GE 615 RTV) was purchased from Fisher Scientific. Tygon tubing was purchased from Saint-Gobain. Flat steel pins were purchased from New England Small Tubes. StemSpanTMSFEM and StemSpanTMC110 were purchased from StemCells Technologies for culture and expansion of the HSPCs. CHIR, Rapamycin and p38 inhibitor were purchased from Sigma.
2.4. Cell Culture and Colony-forming Unit Assays
Primary human CD34+ HSPCs (STEMCELL Technologies) were grown in StemSpan™ SFEM with 1% StemSpan™ CC110, 0.5% L-glutamine, and 0.5% penicillin-streptomycin in a humidified atmosphere of 5% CO2/95% air at 37°C. For colony-forming unit (CFU) assays, CD34+ HSPCs were cultured for 18 days in StemMACS™ HSC-CFU complete with Epo (Miltenyi Biotec), and hematopoietic progenitor CFUs were quantitated and classified according to morphology.
2.5. Flow Cytometry
HSPCs sorted using the microfluidic device were stained with FITC anti-human CD34, Alexa Fluor® 647 anti-human CD38, Brilliant Violet 421™ anti-human CD90, and PE/Cy7 anti-human CD45RA antibodies (BioLegend). Stained HSPCs were analyzed using a BD LSRFortessa cell analyzer.
2.6. RNA-seq
The retrieved HSPCs (~50 intact cells) were processed according to the SMART-Seq v4 protocol (SMART-Seq v4 Ultra Low Input RNA Kit, Clontech). In short, first-strand cDNA synthesis was performed on the lysed cells, followed by preamplification. The obtained polymerase chain reaction (PCR) products were then purified by AMPure XP magnetic beads (Beckman Coulter). Final libraries were prepared and amplified using Nextera XT library preparation kit (Illumina). The obtained libraries were then purified with AMPure XP magnetic beads. The quality of the libraries was assessed by the Bioanalyzer (Agilent 2100) before DNA sequencing on NextSeq 500 and subsequent bioinformatics analysis.
2.7. Statistical Analysis
The data are presented as the mean ± standard deviation (s.d.) from three different experiments. Error bars indicate s.d. A two-tailed Student’s t test was used for comparisons of two groups (n=3, measured three times, for each group). P values less than 0.01 were considered significant. Statistical analyses were performed using the GraphPad Prism 6.0 software (Graphpad.com., San Diego, CA, USA).
3. Results and Discussion
3.1. Design of a Novel Single-cell Trapping Device
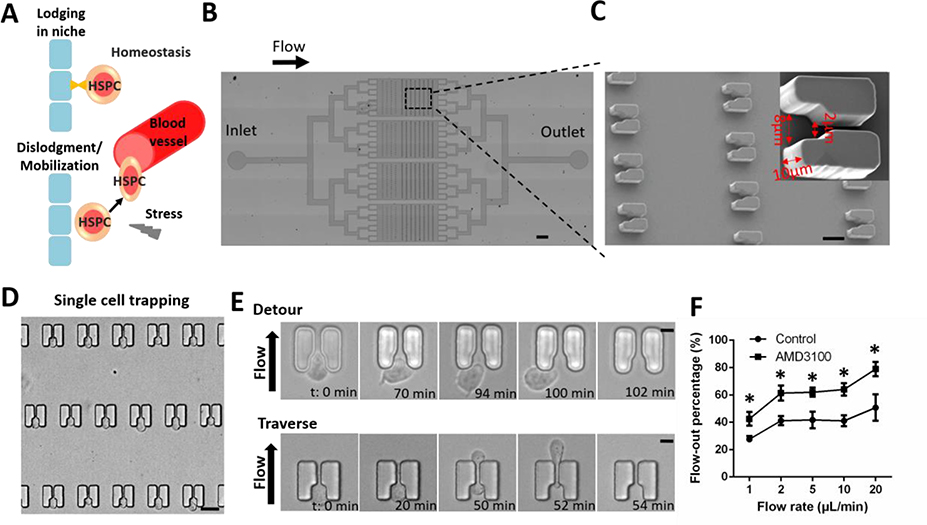
The response to transitions from homeostatic hematopoiesis to hematopoietic stress requires HSPC trafficking between distinct niches in the bone marrow[9] (Fig. 1A). Emerging evidence suggests that mechanical forces in the local microenvironment, such as blood flow and shear stress, are critical for HSPC fate decisions[10]. To study HSPC behaviors under stiff conditions, we designed microfluidic cell trapping arrays for high-throughput single-HSPC monitoring (Fig. 1B, S1A). Devices were fabricated using standard PDMS microfluidics technology. Each chip consisted of four parallel channels, each containing 160 single-cell trapping structures. The cup-shaped trappings structures were designed with an inlet opening for initial cell capture and a narrower outlet opening of 2 μm for cell trapping with fluid flow (Fig. 1C). To optimize the single-cell capture capacity of the device, three sizes of inlet openings (S, M, and L corresponding to 6 μm, 8 μm, and 10 μm, respectively) were tested. The M trap with an 8-μm opening demonstrated the highest single-cell capture efficiency at ~96.5% and was chosen as the final design for downstream analyses (Fig. 1C, S1B).
Figure 1. High-throughput analysis of single-HSPC motility using microfluidic cell trapping arrays.
(A) Model of hematopoietic progenitor cell or HSPC trafficking in the endosteal niche. HSPCs are located within specialized niches in the bone marrow. Certain circumstances induce the mobilization of HSPCs from the bone marrow into peripheral circulation. (B) Structure of the microfluidic device. The arrow indicates flow direction from the inlet to the outlet. Scale bar: 0.5 mm. (C) Scanning electron micrograph of single-cell trapping structures. Scale bar: 15 μm. Inset shows an individual 10 μm-high trap structure with an 8-μm inlet and a 2-μm outlet. Trap dimensions are indicated with red arrows. (D) Cell trapping was visualized by microscopy after HSPCs were loaded into the device. Scale bar: 15 μm. (E) HSPCs exhibited motility at the trap sites and exited the structures either by detouring at the inlet (Detour) or traversing the structure through the outlet (Traverse). Scale bar: 5 μm. (F) Percentage of loaded HSPCs flowing out of the chip in the absence or presence of AMD3100 (20 μg/ml). For each experiment, a total of ~150 cells in each device were analyzed to calculate the flow-out percentage in the absence or presence of AMD3100. Data are presented as mean ± s.d. from three different experiments (n=3, measured three times, for each group). *P < 0.01 determined by a two-tailed Student’s t test.
Following single-cell trapping (Fig. 1D, S1C), the microfluidic device was used to analyze the behavior of the trapped cells under conditions of continuous medium flow. Interestingly, we observed different HSPC behaviors following trapping. At the time scale of hours, some HSPCs exited the traps either by detouring at the inlet or by traversing the structure through the outlet (Fig. 1E). Prior to escaping, the cells continuously moved within the trapping structures (Movie S1 and S2). The HSPCs did not escape at identical rates; during specific observational time periods, some HSPCs rapidly evacuated the chip, while others remained trapped. The escaped cells refer to the cell samples that were collected from the outlet during 3 hours. The trapped cells refer to the cell samples that remained in the chip after 3 hours culture with continuous medium flow and collected from the inlet by applying positive pressure at the outlet. The escaped motile HSPCs contains two groups of cells. It is interesting to further distinguish the cells squeeze through the trap and those migrate around the trap.
3.2. Validation of The Microfluidic Single-cell Trapping Device
Differences in the motility behaviors of human HSCs and hematopoietic progenitor cells have been observed at the osteoblastic niche[11]. Many mobilizing agents have been developed to enhance stem-cell transplantation, including granulocyte colony-stimulating factor, small molecule C-X-C chemokine receptor type 4 (CXCR4) inhibitors, and very late antigen-4 inhibitors[12]. To validate the use of the microfluidic cell trapping arrays for single-cell motility analysis, we assessed the numbers of cells escaping the traps in the absence or presence of the CXCR4 antagonist AMD3100. Different flow rates were tested for flow effects on target cell collection (Table S1). The evacuation rate did not show an obvious change as the flow speed raised from 2 μl/min to 10 μl/min. The optimal flow rate was defined as the lowest flow rate that could evacuate the highly motile HSPCs from the chip. To minimize the effects of the flow speed, we chose flow rate at 2 μl/min for the following experiments. Cells became more motile after treatment with AMD3100 by ~1.5-fold increase in the percentage of highly motile HSPCs escaping the chip traps at all flow rates (Fig. 1F). These results validated the use of our platform for rapid screening of HSPC mobilization agents.
3.3. Correlation Between HSPC Motility and CD34 Asymmetric Inheritance
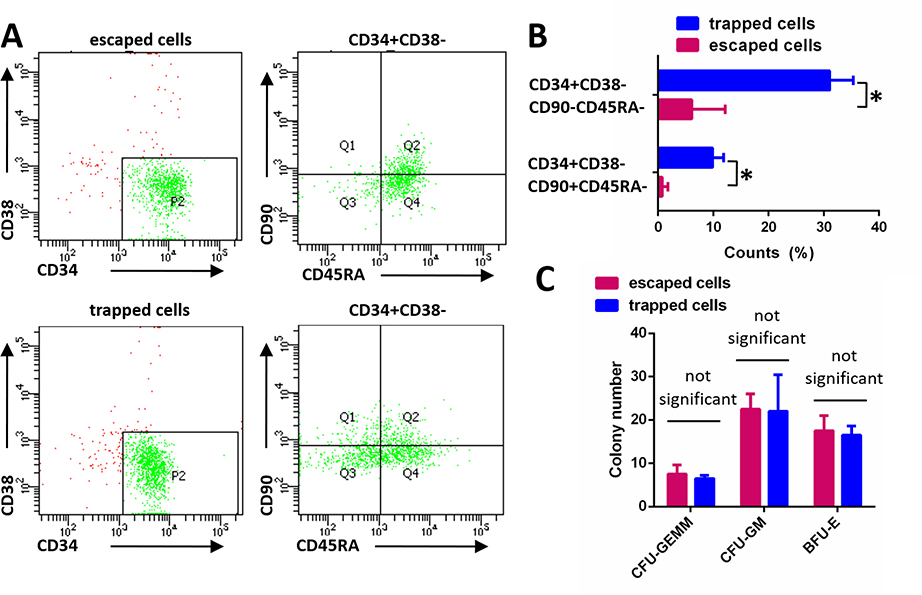
The observed differences in the motility behaviors of HSPCs prompted us to investigate the correlation between motility and biological functions of the cells. To exclude the effects of the device treatment to HSPCs, we collected cells flowed through the chip without trapping. The cell viability assay showed that there were no detectable effects on cell viability with the device treatment to HSPCs (Fig. S2). We stained both HSPCs with and without device treatment using FITC-labeled anti-CD34 antibodies and did not detect an obvious difference (Fig. S3). In addition, the cells were cultured for another 6 days and the percentage of CD34-positive cells was similar (Fig. S4). The expression of CD34, CD38, CD90, and CD45RA are commonly used to indicate the primitivity of HSPCs. The populations of CD34+CD38-CD90+CD45RA- and CD34+CD38-CD90-CD45RA- cells are high progenitor cells. Then we collected the trapped and escaped cells from the same chip and assessed them for the expression of the HSPC markers CD34, CD38, CD90, and CD45RA. Flow cytometry analysis revealed differences in the percentages of CD34+CD38-CD90+CD45RA- and CD34+CD38-CD90-CD45RA- populations between the two groups of collected cells (Fig. 2A). The percentage of high progenitor cells was significantly lower in the escaped cell group relative to the trapped cell group by ~34% decrease (Fig. 2B), which implied HSCs and HPCs may exhibit different motile behaviors. The Colony-forming unit (CFU) assays is most commonly used to detect multipotential and lineage-restricted progenitors of the erythroid, granulocytic and macrophage lineages. To compare the differences in multipotency, CFU assay were performed between the two group of cells (Fig. S5) and we did not detect obvious differences in formed colonies between the two groups of collected cells (Fig. 2C). Based on these experiments, we thought there could be other factors that affect HSPC multipotency besides cell primitivity and motility.
Figure 2. Sorted hematopoietic progenitor cell populations exhibit different motile behaviors.
(A) HSPC marker expression in trapped and escaped cells was assessed using flow cytometry. (B) The percentages of CD34+CD38-CD90+CD45RA- and CD34+CD38-CD90-CD45RA- high progenitor cell populations in the trapped and escaped cell groups were assessed using flow cytometry. Data are presented as mean ± s.d. from three different experiments (n=3, measured three times, for each group). *P < 0.01 determined by a two-tailed Student’s t test. (C) Trapped and escaped cells were cultured in StemMACS™ HSC-CFU for 18 days, and hematopoietic progenitor CFUs were classified based on morphology and quantitated. CFU-GEMM: common myeloid progenitor; CFU-GM: granulocyte-macrophage progenitor; BFU-E: erythroid burst-forming units. Data are presented as mean ± s.d. from three different experiments (n=3, measured three times, for each group).
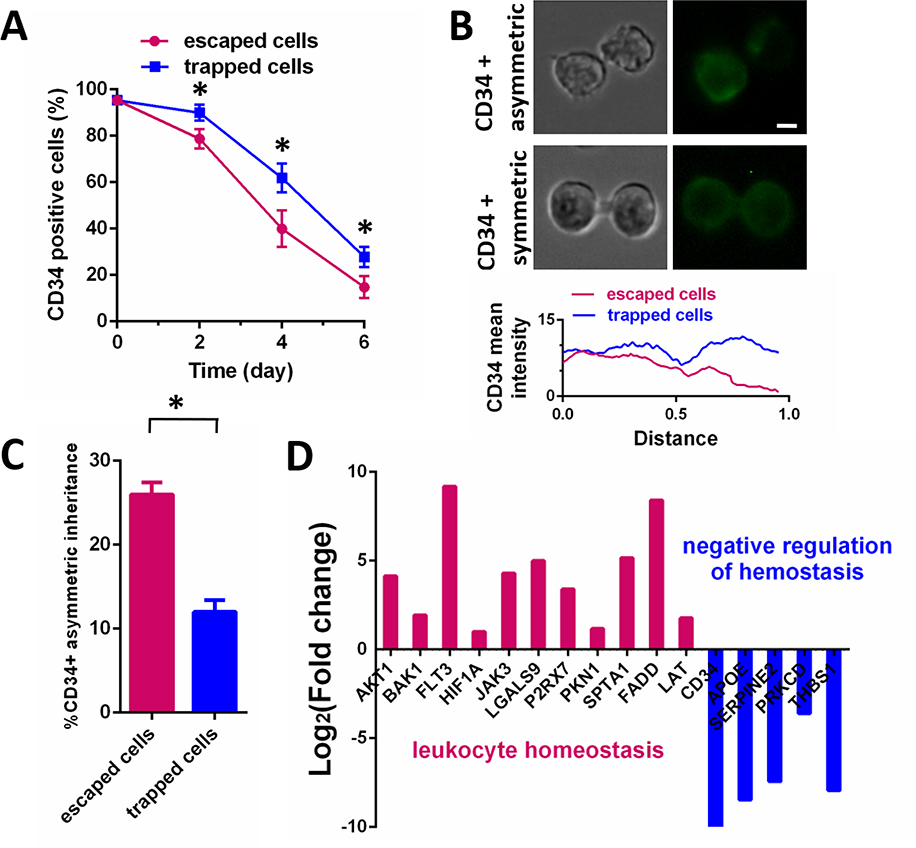
Our microfluidic cell-sorting platform allows for the collection of sorted cells for continuous culturing and analysis. To monitor the stemness of the trapped and escaped HSPCs, the cells were cultured for 6 days and stained with anti-CD34-FITC antibodies every 2 days (Fig. S6). The percentage of CD34-positive cells was higher in the trapped HSPC group than in the escaped HSPC group at all time points tested, with a ~2-fold change after culture cells for 6 days (Fig. 3A). These results suggested that trapped HSPCs more readily maintain stemness than escaped HSPCs and that motile cells lose stemness more rapidly.
Figure 3. HSPC mobilization is associated with CD34 asymmetric inheritance and cell differentiation.
(A) The percentage of CD34-positive cells in cultured trapped and escaped HSPC groups was monitored over time by flow cytometry. Data are presented as mean ± s.d. from three different experiments (n=3, measured three times, for each group). *P < 0.01 determined by a two-tailed Student’s t test. (B) CD34 staining of dividing HSPCs was performed to monitor for CD34 asymmetric inheritance. The bottom panel demonstrates the measurement of fluorescence intensity along the membrane contour of dividing cells, with distance normalized relative to total cell length. Scale bar: 2 μm. (C) Rates of CD34 asymmetric inheritance in the trapped and escaped cell groups were determined by monitoring CD34 staining of dividing cells. For each experiment, equal number of trapped and escaped cells (n=30) was analyzed. Data are presented as mean ± s.d. from three different experiments (n=3, measured three times, for each group). *P < 0.01 determined by a two-tailed Student’s t test. (D) Fold-change (log2) in expression of homeostasis regulation genes in the escaped relative to trapped cells was determined using RNA-seq analysis.
Asymmetric inheritance of CD34+ cells, producing one stem cell and one cell destined for differentiation, is involved in HSPC maintenance. To investigate the relationship between HSPC motility and HSPC maintenance, we assessed the incidence of asymmetric cell inheritance in the two cell populations sorted by our microfluidic device. We detected a higher percentage of CD34 asymmetric inheritance by ~2.2-fold, as evidenced by asymmetric distribution of CD34 staining between dividing cells, in the escaped cells relative to trapped cells (Fig. 3B, 3C). These findings suggested a positive correlation between HSPC motility and differentiation. RNA-seq analysis confirmed that the highly motile cultured HSPCs expressed differentiation markers at higher levels than the trapped HSPCs. For example, markers associated with the regulation of leukocyte homeostasis were highly expressed in escaped HSPCs, while those associated with the negative regulation of hemostasis were highly expressed in trapped HSPCs (Fig. 3D). Collectively, the data demonstrated that HSPC mobilization is associated with CD34 asymmetric inheritance and cell differentiation.
3.4. Gene Expression Analysis of Sorted HSPCs
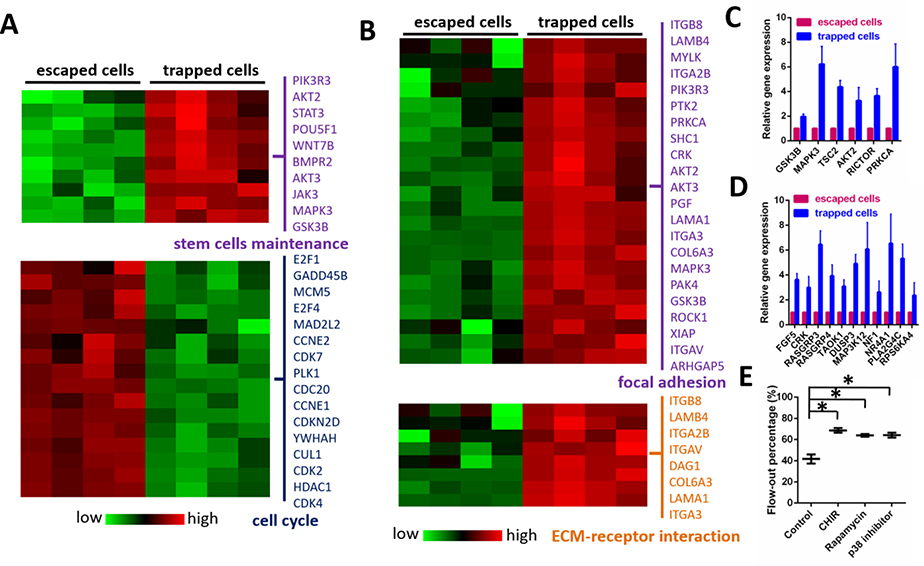
Considering the distinct phenotypes of trapped and escaped HSPCs, we concluded that cell motility is closely associated with HSPC maintenance. To validate these findings, we performed motility-based HSPC sorting and subjected freshly sorted cells to RNA-seq analysis. As expected, the markers representing stem-cell maintenance were expressed at higher levels in the trapped HSPCs than in the escaped cells (Fig. 4A). Markers associated with cell-cycle regulation were up-regulated in the highly motile HSPCs, indicating that these cells were undergoing cell-cycle progression (Fig. 4A, S7). Genes associated with the regulation of focal adhesion and extracellular matrix-receptor interactions were highly expressed in trapped cells, verifying their roles in HSPC lodgment and maintenance (Fig. 4B).
Figure 4. Sorted HSPCs exhibit differences in gene expression associated with the regulation of HSPC mobilization and maintenance.
(A) RNA-seq analysis was used to generate a heat map demonstrating expression levels of genes associated with the regulation of stem-cell maintenance and cell-cycle progression in trapped and escaped HSPCs. (B) RNA-seq analysis was used to generate a heat map showing the expression levels of genes mediating focal adhesion and ECM-receptor interactions in trapped and escaped HSPCs. (C) Expression of genes associated with GSK3 and mTOR pathways was analyzed in trapped and escaped HSPCs. Data are presented as mean ± s.d. from three different experiments (n=3, measured three times, for each group). (D) Expression of genes associated with MAPK signaling was analyzed in trapped and escaped HSPCs. Data are presented as mean ± s.d. from three different experiments. (n=3, measured three times, for each group) (E) Percentage of HSPCs escaping the traps and flowing out of the chip in the presence or absence of indicated inhibitors. CHIR (1 μM), Rapamycin (1 μM) and p38 inhibitor (1 μM) were used to inhibit GSK3, mTOR and MAPK signaling pathways respectively. For each experiment, a total of ~150 cells in each device were analyzed to calculate the flow-out percentage. Data are presented as mean ± s.d. from three different experiments (n=3, measured three times, for each group). *P < 0.01 determined by a two-tailed Student’s t test.
Moreover, in the two sorted HSPC groups, there was differential expression in genes associated with signaling pathways regulating HSPC maintenance, including the glycogen synthase kinase-3 (GSK3) pathway, the mechanistic target of rapamycin (mTOR) pathway and the AMP-activated protein kinase (AMPK) pathway[13]. Genes associated with all three signaling pathways were up-regulated in trapped HSPCs, confirming their roles in controlling HSPC stemness and maintenance (Fig. 4C–D). Disruption of these pathways with specific inhibitors increased the percentage of highly motile HSPCs escaping the chip traps by 1.65-fold, 1.53-fold and 1.54-fold respectively (Fig. 4E and Table S2). It is very interesting to further investigate the effects and mechanisms of activation of GSK3, mTOR and MAPK signaling pathways on HSPC motility. Taken together, these data showed an inverse correlation between HSPC mobilization and maintenance.
4. Concluding Remarks
In conclusion, our microfluidic cell trapping arrays, designed for high-throughput monitoring, sorting, and collection of cells, have been validated through the analysis of single-HSPC phenotypes. By combining these analyses with transcriptional profiling, we uncovered a novel link between HSPC motility and maintenance. These findings can facilitate the identification of novel genes and signaling pathways controlling HSPC maintenance. Ultimately, our unique platform provides a novel, rapid, and efficient strategy for the screening and discovery of HSPC mobilization compounds, which is critical for clinical applications such as peripheral blood stem cell transplantation.
Supplementary Material
Significance Statement.
Because of its specific clinical relevance, the field of HSPC mobilization has received broad attention, owing mainly to the belief that pharmacologic stem cell mobilization might provide clues as to how stem cells are retained and regulated in their natural environment. Although high-resolution microscopy and video imaging effectively track HSPC motility in vivo, collection and analysis of the target cells is hindered by the complexity of the in vivo systems. Our microfluidic platform enables simultaneous monitoring and motility-based sorting of cells, thereby facilitating the analysis of the molecular mechanisms underlying the motility differences. Our microfluidic cell trapping arrays, designed for high-throughput monitoring, sorting, and collection of cells, have been validated through the analysis of single-HSPC phenotypes. By combining these analyses with transcriptional profiling, we uncovered a novel link between HSPC motility and maintenance. These findings can facilitate the identification of novel genes and signaling pathways controlling HSPC maintenance. Ultimately, our unique platform provides a novel, rapid, and efficient strategy for the screening and discovery of HSPC mobilization compounds.
Acknowledgements
We would like to thank Oscar Quintana-Bustamante (Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas) for suggestions regarding HSPC culturing and identification, Ricardo Miguel de Castro (Houston Methodist Research Institute) for critical discussion of the manuscript. This study was funded by National Institutes of Health grants R21 CA191179.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Xin Han, Department of Nanomedicine, Houston Methodist Research Institute, 6670 Bertner Ave, Houston, TX 77030, USA; Department of Cell Biology and Medical Genetics, Nanjing University of Chinese Medicine, Nanjing, 210023, PR China.
Yuan Ma, Department of Nanomedicine, Houston Methodist Research Institute, 6670 Bertner Ave, Houston, TX 77030, USA.
Kai Zhang, Department of Nanomedicine, Houston Methodist Research Institute, 6670 Bertner Ave, Houston, TX 77030, USA.
Pengchao Zhang, Department of Nanomedicine, Houston Methodist Research Institute, 6670 Bertner Ave, Houston, TX 77030, USA.
Ning Shao, Department of Nanomedicine, Houston Methodist Research Institute, 6670 Bertner Ave, Houston, TX 77030, USA.
Lidong Qin, Department of Nanomedicine, Houston Methodist Research Institute, 6670 Bertner Ave, Houston, TX 77030, USA.
References
- [1].a) Doulatov S, Notta F, Laurenti E, Dick JE, Cell stem cell 2012, 10, 120–136; [DOI] [PubMed] [Google Scholar]; b) Rodriguez-Fraticelli AE, Wolock SL, Weinreb CS, Panero R, Patel SH, Jankovic M, Sun J, Calogero RA, Klein AM, Camargo FD, Nature 2018, 553, 212–216; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Carrelha J, Meng Y, Kettyle LM, Luis TC, Norfo R, Alcolea V, Boukarabila H, Grasso F, Gambardella A, Grover A, Hogstrand K, Lord AM, Sanjuan-Pla A, Woll PS, Nerlov C, Jacobsen SEW Nature 2018, 554, 106–111. [DOI] [PubMed] [Google Scholar]
- [2].a) Copley MR, Beer PA, Eaves CJ, Cell stem cell 2012, 10, 690–697; [DOI] [PubMed] [Google Scholar]; b) Ye H, Wang X, Li Z, Zhou F, Li X, Ni Y, Zhang W, Tang F, Liu B, Lan Y, Cell research 2017, 27, 1065–1068; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Alemany A, Florescu M, Baron CS, Peterson-Maduro J, van Oudenaarden A, Nature 2018, 556, 108–112. [DOI] [PubMed] [Google Scholar]
- [3].a) Crane GM, Jeffery E, Morrison SJ, Nature reviews. Immunology 2017, 17, 573–590; [DOI] [PubMed] [Google Scholar]; b) Peerani R, Zandstra PW, The Journal of clinical investigation 2010, 120, 60–70; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hoggatt J, Singh P, Tate TA, Chou BK, Datari SR, Fukuda S, Liu L, Kharchenko PV, Schajnovitz A, Baryawno N, Mercier FE, Boyer J, Gardner J, Morrow DM, Scadden DT, Pelus LM, Cell 2018, 172, 191–204 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Ugarte F, Forsberg EC, The EMBO journal 2013, 32, 2535–2547; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yamamoto R, Wilkinson AC, Nakauchi H, Science 2018, 362, 895–896. [DOI] [PubMed] [Google Scholar]
- [5].a) Bonig H, Papayannopoulou T, Leukemia 2013, 27, 24–31; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M, Science 2018, 359. [DOI] [PubMed] [Google Scholar]
- [6].Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT, Nature 2009, 457, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Han X, Liu Z, Zhao L, Wang F, Yu Y, Yang J, Chen R, Qin L, Angewandte Chemie 2016, 55, 8561–8565; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dance A, Nature 2017, 545, 511–514; [DOI] [PubMed] [Google Scholar]; Sackmann cE. K, Fulton AL, Beebe DJ, Nature 2014, 507, 181–189. [DOI] [PubMed] [Google Scholar]
- [8].a) Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE, Nature methods 2014, 11, 663–669; [DOI] [PubMed] [Google Scholar]; b) Lecault V, Vaninsberghe M, Sekulovic S, Knapp DJ, Wohrer S, Bowden W, Viel F, McLaughlin T, Jarandehei A, Miller M, Falconnet D, White AK, Kent DG, Copley MR, Taghipour F, Eaves CJ, Humphries RK, Piret JM, Hansen, Nature methods 2011, 8, 581–586. [DOI] [PubMed] [Google Scholar]
- [9].Raaijmakers MH, Haematologica 2010, 95, 1439–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Choi JS, Harley BA, Science advances 2017, 3, e1600455; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mahadik BP, Pedron Haba S, Skertich LJ, Harley BA, Biomaterials 2015, 67, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Foster K, Lassailly F, Anjos-Afonso F, Currie E, Rouault-Pierre K, Bonnet D, Stem cell reports 2015, 5, 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Greenbaum AM, Link DC, Leukemia 2011, 25, 211–217; [DOI] [PubMed] [Google Scholar]; b) To LB, Levesque JP, Herbert KE, Blood 2011, 118, 4530–4540; [DOI] [PubMed] [Google Scholar]; Broxmeyer cH. E, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF, The Journal of experimental medicine 2005, 201, 1307–1318; [DOI] [PMC free article] [PubMed] [Google Scholar]; Rettig dM. P, Ansstas G, DiPersio JF, Leukemia 2012, 26, 34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Huang J, Zhang Y, Bersenev A, O’Brien WT, Tong W, Emerson SG, l PS, The Journal of clinical investigation 2009, 119, 3519–3529; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS, Nature medicine 2012, 18, 1778–1785; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chan G, Gu S, Neel BG, Blood 2013, 121, 3594–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.