Abstract
Background
Mitochondrial DNA (mtDNA) has been reported to play a critical role in the progression of systemic inflammatory response syndrome (SIRS). The pathophysiology of acute respiratory distress syndrome (ARDS) is mainly attributed to the diffuse injury of alveolar epithelial cells caused by dysregulated inflammation upon direct or indirect insults. We hypothesized that plasma mtDNA may serve as an early biomarker that can predict the outcome of patients with ARDS.
Methods
This study was conducted in the Department of Critical Care Medicine, Zhongda Hospital, Southeast University, a tertiary teaching hospital, from 1 May 2016 to 31 January 2017. Patients diagnosed with ARDS at admission were screened. The levels of plasma mtDNA on Day 1, Day 3 and Day 7 were detected by real-time quantitative PCR (RT-qPCR). The patients were followed-up, and all-cause mortality was recorded. The prognostic values of plasma mtDNA were evaluated in ARDS patients using receiver operating characteristic (ROC) analysis.
Results
In total, 136 patients with ARDS were prospectively screened, and 73 patients were finally enrolled, with a 28-day mortality of 39.7% (29 of 73 patients). The plasma mtDNA levels at Day 7 of mild, moderate and severe ARDS patients were 1,230 (588–22,387), 5,370 (628–13,052) and 15,792 (1,623–186,814), respectively (copies/µL, P<0.05) Compared with the survivors, the level of plasma mtDNA in the nonsurvivors was significantly higher on Day 7 [67,608 (19,498–346,736) vs. 7,585 (1,717–15,792) copies/µL; P<0.05]. The AUROC of plasma mtDNA on Day 7 for predictive mortality in patients with ARDS was 0.74, and the optimal cut-off value was 18,640 copies/µL.
Conclusions
Plasma mtDNA levels were positively associated with the severity of ARDS. Higher plasma mtDNA levels on Day 7 indicated a poor outcome in ARDS patients.
Keywords: Mitochondrial DNA (mtDNA), acute respiratory distress syndrome (ARDS), 28-day mortality, prognosis
Introduction
Acute respiratory distress syndrome (ARDS) remains a great challenge in the intensive care unit (ICU). Despite improvements in therapeutic management, including lung-protective mechanical ventilation strategies (1-3), prone positioning and extracorporeal membrane oxygenation (4), the mortality of ARDS remains approximately 40% (5). The fundamental pathophysiology of ARDS is the diffuse injury of alveolar epithelial cells in the form of necrosis or apoptosis (6-8) caused by dysregulated inflammation in response to either infectious pathogens or noninfectious irritants (9).
Microbial pathogen-associated molecular patterns (PAMPs) activate immune cells through pattern recognition receptors. Then, the injured cells release endogenous damage-associated molecular patterns (DAMPs) that also activate the host immune system (10). DAMPs are reported to play a critical role in the development of SIRS. A large number of studies have suggested that mitochondria are also a major source of DAMPs (11) by the release of mitochondria-associated molecules when cells are under various stress conditions. Mitochondria-derived DAMPs mainly include mtROS, mtDNA fragments, N-formy1 peptides, ATP, cytochrome C, cardiolipin and carbamoyl phosphate synthetase. Recent studies have shown that mitochondrial DAMPs could induce SIRS and organ dysfunction. Moreover, markedly increased levels of circulating free mtDNA fragments have been observed in both sepsis and trauma conditions (12-16).
Similar to bacterial DNA, extracellular mtDNA can activate signaling pathways and promulgate inflammation (11). A prospective study of adult patients with severe sepsis and septic shock revealed that an increase in the concentration of plasma mtDNA by 1 ng/mL is associated with an increase in the fatality rate by 0.7% (17). Another study reported that plasma mtDNA is a novel DAMP in pediatric sepsis and appears to be associated with multiple organ failure (MOF) (18). In addition, Nakahira et al. demonstrated that the 28-d mortality was high in medical ICU patients when the plasma mtDNA level was ≥3,200 copies/µL (19). However, it is unknown whether plasma mtDNA is a novel biomarker for patients with ARDS. Consequently, the prognostic role of mtDNA in ARDS was investigated in this study.
Methods
The present study was a single-centered prospective observational study conducted in a 60-bed ICU in Zhongda Hospital, a tertiary hospital, from 1 May 2016 to 31 January 2017. The study was approved by the Ethics Committees for Clinical Research of Zhongda Hospital (No. 2016ZDSYLL033.0) and registered in ClinicalTrials.gov (NCT02883231). Informed consent was obtained from the patients or their next of kin.
Patients
Patients diagnosed with ARDS according to the Berlin definition from May 2016 through January 2017 were screened. Patients were excluded if at least one of the following non-inclusion exclusion criteria was met: age <18 years old or pregnancy; death or discharge within 24 hours after admission; advanced malignant tumor; immune deficiency, such as from neutropenia, long-term use of corticoids or diseases such as HIV infection; chronic lung disease, such as rheumatic autoimmune disease or exacerbation of chronic obstructive pulmonary disease, active tuberculosis, bronchiectasis, bronchial asthma, interstitial lung disease, etc.
Clinical assessment and treatment
The pathological information of the patients was collected on Days 1, 3 and 7, including demographic data, Acute Physiology and Chronic Health Evaluation (APACHE) II score (20), number of organ failures included in the Sequential Organ Failure Assessment (SOFA) score (21) and Murray score. The levels of lactate and inflammatory mediators (i.e., plasma C-reactive protein and procalcitonin) were detected on Days 1, 3 and 7. All patients were followed up for 28 days, and all-cause mortality was recorded. The durations of mechanical ventilation and ICU stay were also recorded. The primary outcome was mortality on Day 28. Secondary outcomes included the ventilator-free days and ICU length of stay.
Sample collection and plasma mtDNA measurements
Peripheral blood samples (2 mL) were collected with EDTA-containing tubes on Day 1, Day 3 and Day 7 after admission to the ICU and centrifuged for 30 min at 3,000 rpm. Then, the supernatant plasma was collected and stored at −80 °C for the following analyses.
Plasma mitochondrial DNA was isolated using a DNeasy Blood and Tissue Kit (#69504; Qiagen), as previously reported (12). The concentration of plasma DNA was evaluated by RT-qPCR. The mitochondrial genome was amplified with primers MT-ND2-45F 5'-CGCAATGGCATTCCTAA-3' and MT-ND2-199R 5'-TAGATGTGGCGGGTTTT-3' by StepOne (7500). The amplicon was detected using the primer sequences and verified in the GenBank database (accession number NC012920.1). β-actin (forward, 5'-CGGGAAATCGTGCGTGACAT-3'; reverse, 5'-GAAGGAAGGCTGGAAGAGTG-3') was used as the control. The PCR parameters were 94 °C for 3 min, followed by 40 cycles for 10 s, 60 °C for 10 s, and 72 °C for 20 s.
Statistical analysis
Continuous variables with normal distributions are shown as the means ± standard deviations (SDs) and were compared by t tests between two groups or one-way analysis of variance (ANOVA) for three or more groups; variables with skewed distribution are presented as the medians (interquartile ranges, IQRs) and were compared by Mann-Whitney U tests; post hoc tests were used for pairwise comparisons. The ROC curve was employed for estimating plasma mtDNA count in the prediction of 28-day mortality. The area under the curve (AUROC) was used to evaluate the predictive accuracy. The optimal cut-off values of different parameters for mortality was decided based on the maximum Youden index (sensitivity + specificity − 1). Comparison of the AUC between mtDNA and the PaO2/FiO2 ratio was adopted using the DeLong method. The Kaplan-Meier method was used to calculate the survival rate and generate the survival curve. Judgment of the prognostic value was determined with the log-rank test. A P value less than 0.05 was considered statistically significant. The statistical package IBM SPSS Statistics (ver. 23.0) was used for the computations and analysis.
Results
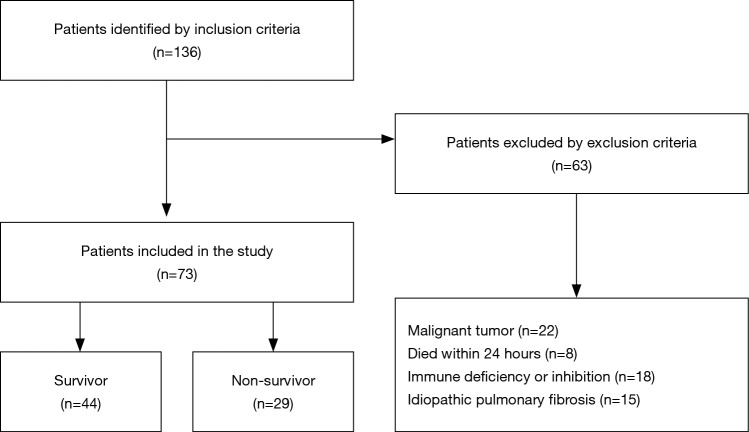
Baseline characteristics
A total of 136 patients who met the initial criteria were identified. After elimination for meeting the exclusion criteria, 73 ARDS patients were finally included. Among them, 29 died within the 28-day follow-up period (Figure 1), and 17 patients died or were discharged from the ICU before day 7. The baseline characteristics of the patient cohort are presented in Table 1. No significant differences were observed between survivors and non-survivors in age, gender, or the severity or etiology of ARDS. Compared with the survivors, the APACHE II scores of the non-survivors increased significantly [27 (22–30) vs. 23.5 (16–29), P<0.05]. The PaO2/FiO2 ratio of the non-survivors on Day 3 or Day 7 decreased significantly compared with the survivors (both P<0.05). Next, we adjusted the variables with logistic regression (i.e., age, gender, severity of ARDS, APACHE II score, SOFA score and Murray score on admission) (Table S1) and found that only age independently affected mortality [P=0.04, HR 0.97 (95% CI, 0.94–1.0)].
Figure 1.
Selection of patients for inclusion in this analysis.
Table 1. Main characteristics of 73 patients with ARDS.
| Variable | Overall (n=73) | Survivors (n=44) | Non-survivors (n=29) | P value |
|---|---|---|---|---|
| Age (year) | 64 | 59 [46–78.5] | 75 [60–79] | 0.05 |
| Male | 57 (78.08) | 36 (81.8) | 21 (72.4) | 0.34 |
| Height (cm) | 169 | 170 [168–175] | 170 [165–172] | 0.25 |
| Weight (kg) | 69 | 70 [60–80] | 70 [65–70] | 0.78 |
| Primary cause of ARDS | ||||
| Pneumonia | 42 (57.53) | 25 (56.8) | 18 (62.1) | 0.66 |
| Aspiration | 8 (10.96) | 7 (15.9) | 1 (3.4) | 0.10 |
| Severe trauma | 5 (6.85) | 4 (9.1) | 1 (3.4) | 0.35 |
| Drowning | 2 (2.74) | 2 (4.5) | 0 | 0.24 |
| Sepsis | 12 (16.44) | 4 (9.1) | 7 (24.1) | 0.33 |
| Others | 4 (5.48) | 2 (4.6) | 2 (6.9) | 0.56 |
| ARDS severity | ||||
| Mild | 13 (17.81) | 8 (18.2) | 5 (17.2) | 0.92 |
| Moderate | 36 (49.32) | 22 (50.0) | 13 (44.8) | 0.67 |
| Severe | 24 (32.88) | 14 (31.8) | 11 (38) | 0.59 |
| Mechanically ventilated | 62 (84.9) | 37 (84.1) | 25 (86.2) | 0.80 |
| PaO2/FiO2 Day 1 (mmHg) | 170 [130–229] | 174 [126–231] | 187 [140–227] | 0.60 |
| PaO2/FiO2 Day 3 (mmHg) | 231 [183–310] | 267 [202–341] | 217 [148–274] | 0.03 |
| PaO2/FiO2 Day 7 (mmHg) | 241 [180–323] | 294 [213–357] | 205 [78–260] | 0.01 |
| APACHE II score | 25 [19–30] | 23.5 [16–29] | 27 [22–30] | 0.04 |
| SOFA score | 13 [10–17] | 13 [9–16] | 14 [11–19] | 0.14 |
| Murray score | 2 [1.6–2.7] | 2 [1.55–2.67] | 2.3 [1.67–3.0] | 0.50 |
| Ventilator days | 7 [3–12.5] | 6 [3–10.5] | 8 [3–17] | 0.34 |
| ICU stay | 13 [8.5–27] | 14.5 [9.5–28] | 11 [6–17] | 0.09 |
Continuous variables are presented as mean ± SD or median [interquartile range] if not normally distributed; categorical variables are presented as No. (%). ARDS, acute respiratory distress syndrome; APACHE II, acute physiology and chronic health evaluation II; SOFA, sepsis-related organ failure assessment; FiO2, fraction of inspired oxygen; ICU, intensive care unit; PaO2, partial pressure of oxygen in arterial blood.
Table S1. shows the adjusted analysis of covariates.
| Variable | Number (percent) or median (interquartile range) | P value | Adjusted P value | Adjusted HR (95% CI) | ||
|---|---|---|---|---|---|---|
| Overall (n=73) | Survivors (n=44) | Non-survivors (n=29) | ||||
| Age (year) | 64 | 59 [46–78.5] | 75 [60–79] | 0.05 | 0.04 | 0.97 [0.94–1.0] |
| Man (male) | 57 (78.08) | 36 (81.8) | 21 (72.4) | 0.34 | 0.30 | |
| Sepsis | 12 (16.44) | 4 (9.1) | 7 (24.1) | 0.33 | 0.78 | |
| Septic shock | 4 (5.48) | 2 (4.6) | 2 (6.9) | 0.56 | 0.28 | |
| ARDS severity | ||||||
| Mild | 13 (17.81) | 8 (18.2) | 5 (17.2) | 0.92 | 0.72 | |
| Moderate | 36 (49.32) | 22 (50.0) | 13 (44.8) | 0.67 | 0.96 | |
| Severe | 24 (32.88) | 14 (31.8) | 11 (38) | 0.59 | 0.50 | |
| APACHE II score | 25 [19–30] | 23.5 [16–29] | 27 [22–30] | 0.04 | 0.11 | |
| SOFA score | 13 [10–17] | 13 [9–16] | 14 [11–19] | 0.14 | 0.60 | |
| Murray score | 2 [1.6–2.7] | 2 [1.55–2.67] | 2.3 [1.67–3.0] | 0.50 | 0.28 | |
Continuous variables are presented as mean ± SD or median [interquartile range] if not normally distributed; categorical variables are presented as No. (%). ARDS, acute respiratory distress syndrome; APACHE II, acute physiology and chronic health evaluation II; SOFA, sepsis-related organ failure assessment.
Plasma mtDNA levels associated with ARDS severity
Patients were divided into mild, moderate, and severe ARDS groups according to the Berlin definition based on the degree of hypoxemia. Interestingly, the three groups had different plasma mtDNA levels from one another on Day 7. Patients in the severe ARDS group had the highest plasma levels, followed by those in the moderate and mild ARDS groups [1,230 (588–22,387) vs. 5,370 (628–13,052) vs. 15,792 (1,623–186,814) copies/µL, respectively, P=0.03] (Table S2).
Table S2. The plasma mtDNA levels among patients with different degrees of ARDS.
| Time point | mtDNA (copies/μL) | P value | ||
|---|---|---|---|---|
| Mild ARDS | Moderate ARDS | Severe ARDS | ||
| Day 1 | 5,128 (1,659–10,719) | 16,982 (5,744–48,977) | 45,005 (4,692–298,557) | 0.3 |
| Day 3 | 18,197 (2,951–112,201) | 31,622 (3,432–155,250) | 85,678 (18,633–465,058) | 0.2 |
| Day 7 | 1,230 (588–22,387) | 5,370 (628–13,052) | 15,792 (1,623–186,814) | 0.03 |
Data are presented as median (interquartile range) for variables that are not normally distributed. mtDNA, mitochondria DNA. See Table 1 legend for expansion of other abbreviation. ARDS, acute respiratory distress syndrome.
Plasma mtDNA levels and 28-day mortality in ARDS
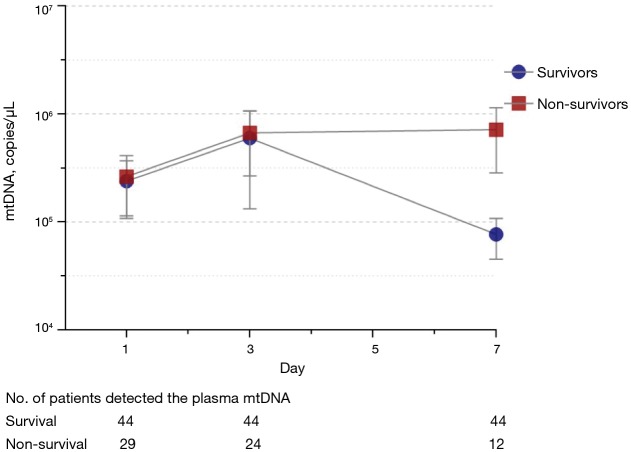
Plasma mtDNA levels were higher in non-survivors than in survivors on Day 7 [67,608 (19,498–346,736) vs. 7,585 (1,717–15,792) copies/µL, P=0.005], and no significant differences were observed on Day 1 (P=0.24) or Day 3 (P=0.88) (Table S3). The plasma mtDNA levels of the survivors had decreased by Day 7, while the mtDNA levels of the non-survivors were still high on Day 7 (Figure 2). Patients with elevated mtDNA always had higher mortality than those with decreased mtDNA over time (Table S4).
Table S3. The plasma mtDNA levels between survivors and non-survivors.
| Time point | mtDNA (copies/μL) | P value | |
|---|---|---|---|
| Survivors | Non-survivors | ||
| Day 1 | 15,135 (3,507–54,616) | 18,197 (5,754–245,470) | 0.24 |
| Day 3 | 38,018 (4,897–161,497) | 37,153 (6,918–239,883 | 0.88 |
| Day 7 | 7,585 (1,717–15,792) | 67,608 (19,498–346,736) | 0.005 |
Data are presented as median (interquartile range) for variables that are not normally distributed. mtDNA, mitochondria DNA.
Figure 2.
mtDNA levels in patients with ARDS during the first week in the ICU. mtDNA levels peaked by Day 3 and remained elevated in non-survivors but was reduced in survivors. n=29 for non-survivors, n=44 for survivors.
Table S4. shows the variation trend of plasma mtDNA associated with ARDS outcomes.
| Variable | Overall | Declined | Elevated | P value |
|---|---|---|---|---|
| Number | 21 | 14 | 7 | |
| Ventilator-free days | 14±10 | 18±8 | 6±9 | 0.03 |
| ICU stay | 20±9 | 20±8 | 20±11 | 0.55 |
| Mortality (%) | 4 (19.0%) | 1 (7.1%) | 3 (42.9%) | 0.09 |
Data are presented as mean ± SD or median (interquartile range) if not normally distributed. mtDNA, mitochondria DNA. Decrease Group means patients’ mtDNA levels declined by time (Day 1, Day 3 and Day 7); Increase Group means patients’ mtDNA levels rose by time (Day1, Day3 and Day7). See Table 1 legend for expansion of other abbreviation.
Plasma mtDNA levels and 28-day mortality prediction
ROC analysis (Figure 3) showed that mtDNA level on Day 7 was a reliable predictor of 28-day mortality compared with the PaO2/FiO2 ratio in ARDS, with AUROCs of 0.74 (95% CI, 0.59–0.89) and 0.68 (95% CI, 0.54–0.82), respectively. Seventeen patients died or were discharged from the ICU before Day 7, so only the data from 56 patients on Day 7 were available. The optimal cut-off value for mtDNA for predicting 28-day mortality was 18,640 copies/µL (76.5% sensitivity and 76.9% specificity). Kaplan-Meier survival curves showed significant differences between patients with plasma mtDNA ≥18,640 copies/µL and patients with plasma mtDNA <18,640 copies/µL on Day 7 (P=0.002 by log-rank test) (Figure 4).
Figure 3.
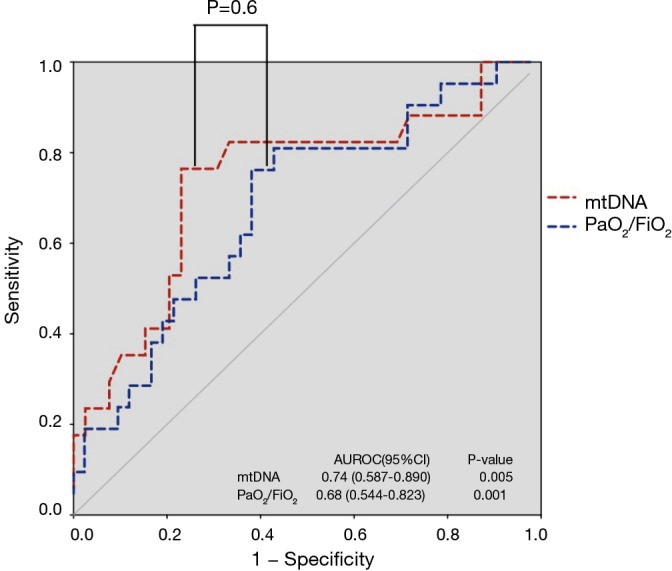
The ROC curves for both plasma mtDNA levels and PaO2/FiO2. The areas under the ROC curve on day 7 for the two measures were 0.74 and 0.68, respectively.
Figure 4.
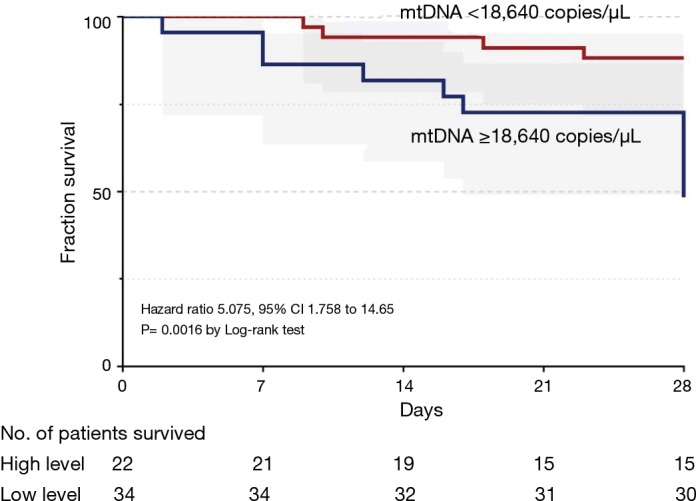
Kaplan-Meier plot of the 28-day mortality of ARDS patients stratified by plasma mtDNA level. Patients stratified with a ROC-determined cutoff point of 18,640 copies/µL of plasma mtDNA level. The Kaplan-Meier curves showed that survival in patients with plasma mtDNA levels above 18,640 copies/µL was significantly less than that of patients with plasma mtDNA levels less than 18,640 copies/µL (P<0.01).
Plasma mtDNA levels were associated with ventilator-free days
Patients were grouped according to the cut-off value of plasma mtDNA, and the results revealed that patients with plasma mtDNA <18,640 copies/µL on Day 7 had longer ventilator-free days compared with those with plasma mtDNA ≥18,640 copies/µL (17±10 vs. 7±11 days, P=0.04) (Table S5). In addition, patients with increasing plasma mtDNA levels over time also had shorter ventilator-free days (18±8 vs. 6±9 days, P=0.03) (Table S4).
Table S5. shows the plasma mtDNA levels associated with ventilator-free days and days of ICU stay.
| Variable | Overall | Plasma mtDNA level (copies/μL) | P value | |
|---|---|---|---|---|
| mtDNA ≥18,640 | mtDNA <18,640 | |||
| Number | 56 | 22 | 34 | |
| Ventilator-free days | 13±11 | 7±11 | 17±10 | 0.04 |
| ICU stay | 18±9 | 17±9 | 18±8 | 0.37 |
Data are presented as mean ± SD or median (interquartile range) if not normally distributed. mitochondria DNA. See Table 1 legend for expansion of other abbreviation.
Plasma mtDNA levels were correlated modestly with SOFA
Plasma mtDNA levels demonstrated a slightly but obviously positive correlation with severity of illness as measured by SOFA score on Day 7 in non-surgical ARDS patients in the linear regression. Patients with higher SOFA scores had greater plasma levels of mtDNA (r=0.26, P=0.04) (Figure S1).
Figure S1.
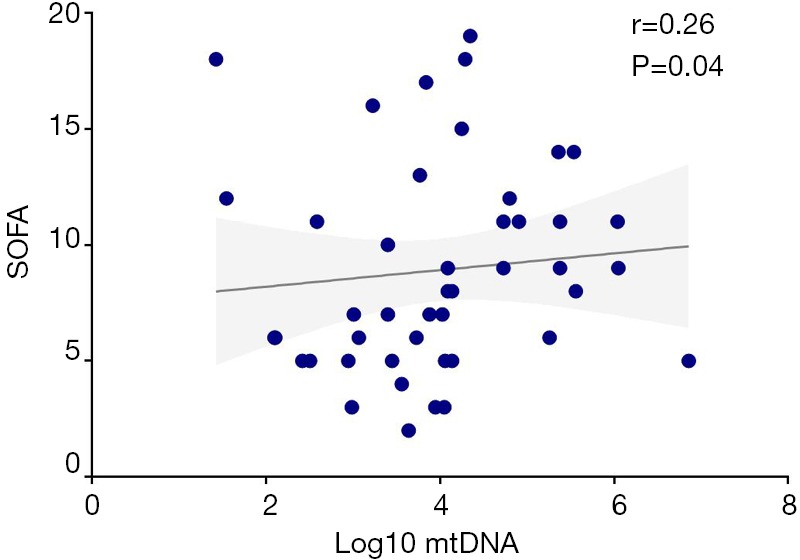
Log10 mtDNA level was correlated with SOFA score on Day 7 (Spearman rho=0.26, P<0.05). SOFA, sepsis-related organ failure assessment.
Plasma mtDNA levels correlate modestly with C-reactive protein (CRP)
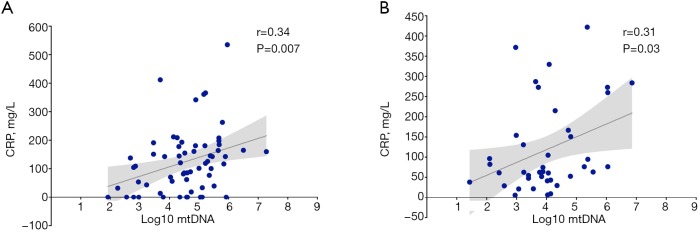
Since mtDNA may activate immunity and initiate SIRS, it may be correlated with markers of inflammation. We therefore determined whether plasma mtDNA was associated with CRP. Plasma mtDNA levels were found to be significantly positively correlated with CRP on Day 3 (r=0.34, P=0.007) (Figure S2A) and Day 7 (r=0.31, P=0.03) (Figure S2B) in the linear regression.
Discussion
ARDS is a severe pulmonary disease. Although more than thirty clinical trials have been conducted in the past decade, the efficacy of treatments for this life-threatening disease is still unsatisfactory.
The severity of ARDS is often assessed using the PaO2/FiO2 ratio, even though the prognostic value of this variable remains low to moderate, with an AUC of only 0.58 (95% CI, 0.56–0.59) in a recent large study (22). Novel biomarkers for ARDS severity are needed. In this prospective study of patients with ARDS, higher levels of plasma mtDNA were associated with the severity of ARDS. This association was independent of age, gender, and risk factors for ARDS. We measured plasma mtDNA levels at three points during the early phase of ARDS (Day 1, Day 3, Day 7), and indicated that plasma mtDNA levels at Day 7 could significantly judge the severity of ARDS. On the other hand, plasma mtDNA levels on Days 1 and 3 seemed to lack utility. In studies of sepsis, we found that there was also no difference in mtDNA levels between control subjects and patients with sepsis on Day 1 (23,24). Furthermore, the trend of mtDNA seems more important in sepsis, as another study also demonstrated that mtDNA changes in sepsis were correlated with survival outcome (25), which was consistent with our study.
We also found that age may be an independent factor for evaluating patient mortality after adjustment. In fact, mitochondrial function is closely associated with aging, partially due to the crucial role of mitochondria in modulating oxidative stress. For example, the number of mitochondria is lower in the liver or muscles in aged people than in young people (26,27). The level of mitochondrial protein is also reduced in older individuals (28). However, to date, the association between cell-free mtDNA level and age remains largely elusive.
In the ICU, the APACHE II score is widely used to evaluate disease severity, but it has limitations in predicting disease progression and prognosis in ARDS because it involves complex computations and subjective evaluation, which may introduce bias (29,30). Hence, it is urgent to develop novel molecular predictors for disease severity and patient outcome, especially plasma markers due to their high accessibility. In our study, we observed that plasma mtDNA levels could reflect disease severity, but the AUROC was no better than that obtained with the APACHE II score (0.74 vs. 0.63, P=0.27) (Figure S3). However, plasma mtDNA levels were associated with an increased risk of death in ARDS. These findings indicated that plasma mtDNA levels were superior to the APACHE II score for assessing severity and predicting patient outcome in ARDS.
Figure S3.
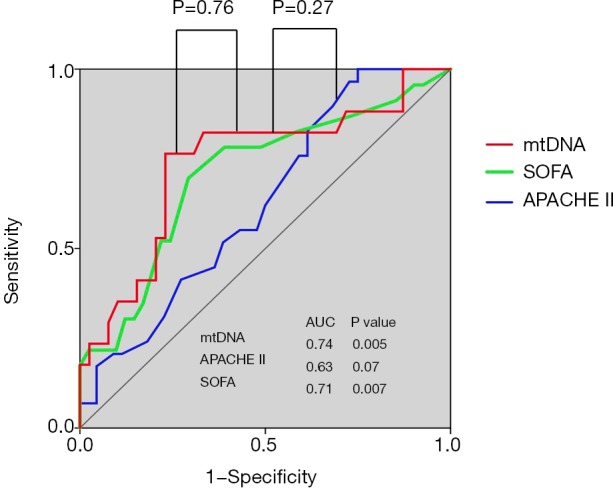
ROC curves for plasma mtDNA levels, SOFA scores and APACHE II scores. The areas under the ROC curve were 0.74, 0.70 and 0.63, respectively. SOFA, sepsis-related organ failure assessment; APACHE II, acute physiology and chronic health evaluation II.
The SOFA score is a scoring system used to assess disease severity and outcome prediction in critically ill patients (31,32). This score was designed to provide an objective approach to assess single or multiple organ failure (33). In our study, no correlation between plasma mtDNA level and SOFA score was observed on day 1 and day 3; however, a slightly but significant positive correlation was demonstrated between them when we excluded patients who underwent surgery. As with the APACHE II score, the plasma mtDNA level was no better a predictor for ARDS severity than the SOFA score in terms of the AUROC (0.74 vs. 0.71, P=0.36) (Figure S3). mtDNA is usually released from tissue or cells after surgery, so surgery may affect mtDNA levels in ARDS patients.
ARDS is a pulmonary inflammatory disorder that often occurs upon pulmonary or extrapulmonary injury. Recently, many researches (34-36) tried to clarify the role of plasma mediators and their relationship with the phenotype and outcome in ARDS patients. Inflammation and injury markers such as CRP (37,38) have been implicated in the prediction of the early onset and outcome of ARDS in cross-sectional studies. Many studies have shown that mtDNA derived from damaged cells functions as a DAMP and plays crucial roles in inflammation. In this study, we revealed that plasma mtDNA levels and CRP levels were significantly correlated on Day 3 and Day 7. A previous study showed that extracellular mtDNA could enhance endothelial permeability and promote neutrophil adhesion to the endothelium (39). Rats administered intravenous mitochondrial DAMPs showed marked evidence of lung injury and increased pulmonary albumin permeability (11). These findings may partly explain the pathological role of plasma mtDNA in ARDS. However, the molecular mechanisms of the effect of mtDNA need further investigation.
Our study has some limitations. First, this is a single-center study. The primary cause of ARDS in this study was pneumonia, and there were few patients with other sources of ARDS, so the results of this study may not represent all ARDS patients. Second, the number of patients enrolled in the present study was not large. Third, the proportion of patients with mild ARDS was small compared to the proportion of patients with moderate and severe ARDS, indicating that some ARDS patients may have received treatment for the disease before recruitment. This may have also influenced our study.
Conclusions
In summary, the mtDNA level in plasma was positively associated with the severity and could predict the outcome of ARDS. Therefore, plasma mtDNA levels may be applied in clinical practice as a new biomarker for ARDS.
Figure S2.
Log10 mtDNA level was correlated with CRP on Day 3 (Spearman rho=0.34, P<0.05) and Day 7 (Spearman rho=0.31, P<0.05).
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported in part by grants from the National Natural Science Foundation of China (grant number: 81571847), the Project of Jiangsu Province’s Medical Key Discipline (ZDXKA2016025), and the Key Research and Development Plan of Jiangsu Province (BE2018743). The funding sources had no role in the design and implementation of the study; the collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committees for Clinical Research of Zhongda Hospital (No. 2016ZDSYLL033.0). After discussing the study, parents who were eligible and interested in participating provided written informed consent.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.49). The authors have no conflicts of interest to declare.
References
- 1.Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 2.Guérin C, Papazian L, Reignier J, et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care 2016;20:384. 10.1186/s13054-016-1556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. Jama 2010;303:865-73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 4.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 5.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. Jama 2016;315:788-800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 6.Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol 2002;161:1783-96. 10.1016/S0002-9440(10)64455-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon BA, Easley RB, Grigoryev DN, et al. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L851-61. 10.1152/ajplung.00463.2005 [DOI] [PubMed] [Google Scholar]
- 8.Matute-Bello G, Liles WC, Steinberg KP, et al. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol 1999;163:2217-25. [PubMed] [Google Scholar]
- 9.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol 2002;42:469-99. 10.1146/annurev.pharmtox.42.091901.115838 [DOI] [PubMed] [Google Scholar]
- 10.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994;12:991-1045. 10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464:104-7. 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 2011;12:222-30. 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rongvaux A, Jackson R, Harman CC, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 2014;159:1563-77. 10.1016/j.cell.2014.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012;36:401-14. 10.1016/j.immuni.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West AP, Khoury-Hanold W, Staron M, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015;520:553-7. 10.1038/nature14156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White MJ, McArthur K, Metcalf D, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 2014;159:1549-62. 10.1016/j.cell.2014.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kung CT, Hsiao SY, Tsai TC, et al. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J Transl Med 2012;10:130. 10.1186/1479-5876-10-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Caro V, Walko TD, 3rd, Bola RA, et al. Plasma Mitochondrial DNA--a Novel DAMP in Pediatric Sepsis. Shock 2016;45:506-11. 10.1097/SHK.0000000000000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakahira K, Kyung SY, Rogers AJ, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med 2013;10:e1001577; discussion e. 10.1371/journal.pmed.1001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793-800. 10.1097/00003246-199811000-00016 [DOI] [PubMed] [Google Scholar]
- 22.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [DOI] [PubMed] [Google Scholar]
- 23.Puskarich MA, Shapiro NI, Trzeciak S, et al. Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock 2012;38:337-40. 10.1097/SHK.0b013e318266a169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanouchi S, Kudo D, Yamada M, et al. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care 2013;28:1027-31. 10.1016/j.jcrc.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 25.Sursal T, Stearns-Kurosawa DJ, Itagaki K, et al. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock 2013;39:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 2000;526 Pt 1:203-10. 10.1111/j.1469-7793.2000.t01-1-00203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 2005;102:5618-23. 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojaimi J, Masters CL, Opeskin K, et al. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev 1999;111:39-47. 10.1016/S0047-6374(99)00071-8 [DOI] [PubMed] [Google Scholar]
- 29.Féry-Lemonnier E, Landais P, Loirat P, et al. Evaluation of severity scoring systems in ICUs--translation, conversion and definition ambiguities as a source of inter-observer variability in Apache II, SAPS and OSF. Intensive Care Med 1995;21:356-60. 10.1007/BF01705416 [DOI] [PubMed] [Google Scholar]
- 30.Polderman KH, Girbes AR, Thijs LG, et al. Accuracy and reliability of APACHE II scoring in two intensive care units Problems and pitfalls in the use of APACHE II and suggestions for improvement. Anaesthesia 2001;56:47-50. 10.1046/j.1365-2044.2001.01763.x [DOI] [PubMed] [Google Scholar]
- 31.Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 2009;37:1649-54. 10.1097/CCM.0b013e31819def97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SJ, Chao TF, Chiang MC, et al. Prediction of patient outcome from Acinetobacter baumannii bacteremia with Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE) II scores. Intern Med 2011;50:871-7. 10.2169/internalmedicine.50.4312 [DOI] [PubMed] [Google Scholar]
- 33.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 34.Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med 2007;35:2755-61. [PMC free article] [PubMed] [Google Scholar]
- 35.McClintock DE, Ware LB, Eisner MD, et al. Higher urine nitric oxide is associated with improved outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2007;175:256-62. 10.1164/rccm.200607-947OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons PE, Matthay MA, Ware LB, et al. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 2005;288:L426-31. 10.1152/ajplung.00302.2004 [DOI] [PubMed] [Google Scholar]
- 37.Komiya K, Ishii H, Teramoto S, et al. Plasma C-reactive protein levels are associated with mortality in elderly with acute lung injury. J Crit Care 2012;27:524.e1-6. 10.1016/j.jcrc.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Kim J, Kim K, et al. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care 2011;26:287-94. 10.1016/j.jcrc.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 39.Sun S, Sursal T, Adibnia Y, et al. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One 2013;8:e59989. 10.1371/journal.pone.0059989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as