Abstract
Purpose of Review
We reviewed the most recent literature examining the associations between the Mediterranean-style diet (MD), neurodegenerative diseases, and markers and mechanisms of neurodegeneration.
Recent Findings
Most, but not all, epidemiologic studies report a protective association between MD adherence, cognitive impairment, and brain health. Data from clinical trials supporting these observational findings are also emerging. Limited evidence suggests that MD adherence may be protective for Parkinson’s disease risk. Mechanistically, plant polyphenols may activate similar molecular pathways as caloric restriction diets, which helps explain the neuroprotective properties of the MD.
Summary
Evidence for cognitive disorders is abundant, but there is a dearth of literature for other neurodegenerative disorders and for markers of neurodegeneration. Further research is needed to elucidate the protective role of MD on neurodegeneration, the most salient components of the MD, and the most sensitive time periods over the lifecourse at which the MD may exert its effects.
Keywords: Mediterraneandiet, Neurodegeneration, Neurodegenerativedisease, Alzheimer disease, Dementia, Epidemiology, Brain health
Introduction
Neurodegenerative diseases constitute a significant proportion of the neurological public health burden worldwide [1], with Alzheimer’s and Parkinson’s diseases predominantly affecting the aging population. Alzheimer’s disease (AD) is the most common neurodegenerative disease and is expected to affect 13.8 million people by 2050, approximately a threefold increase from current-day prevalence estimates [2]. Parkinson’s disease (PD) is the second most common neurodegenerative disorder and currently affects approximately 315 people per 100,000 [3]. With currently no curative therapies available for the treatment of neurodegenerative diseases, research efforts have shifted to identifying targets for prevention.
Modification of lifestyle and health behaviors, such as diet, are particularly effective public health targets for disease prevention. Given the association between cardiovascular and brain health [4], researchers posit that well-established cardioprotective dietary patterns, such as the Mediterranean-style diet (MD), may be protective for neurodegenerative diseases. This review examines the most current literature on the association of the MD and neurodegeneration.
Defining and Measuring the Mediterranean-Style Diet
The MD reflects the dietary habits common among populations bordering the Mediterranean Sea and is characterized by a relatively high intake of fruits, vegetables, monounsaturated fat, fish, whole grains, legumes, and nuts, as well as moderate alcohol consumption, and a low intake of red meat, dairy, saturated fat, and refined grains. There are no prescribed cutpoints or recommended levels of the MD components. Instead, when used in research studies, participants are compared within the sample to identify those that adhere to this dietary pattern more or less than the rest of the sample. Typically, in epidemiological studies, a score is derived to represent MD adherence relative to the cohort, using an approach developed by Trichopoulou et al., such that individuals are assigned value of 1 for each beneficial component (fruits, vegetables, legumes, cereals, fish) consumed at or above the median, for each detrimental component (meat, dairy) consumed below the median, for a ratio of monounsaturated fats to saturated fats above the median, and for mild to moderate alcohol consumption. The scores in the food categories are summed (range 0–9), with a greater score indicating greater similarity to the MD [5]. Other studies have also constructed MD adherence scores based on absolute values, as opposed to relative values compared to the sample studied [6, 7].
Adherence to the MD is often ascertained in epidemiological studies based on self-report using a validated semi-quantitative food frequency questionnaire that is typically intended to represent dietary habits over the previous year. A few limitations are noteworthy. First, the early pre-clinical phase of neurodegenerative diseases, like dementia, may impact diet behavior as well as the ability to accurately recall. Therefore, there is the potential for bias due to reverse confounding as well as misclassification. These biases underscore the need for prospective longitudinal studies, with sensitivity analyses excluding the first few years of follow-up. Some studies base their MD score from a 1-week food intake diary, which is less susceptible to recall bias, but may be less representative of long-term dietary habits.
Mediterranean-Style Diet and Cognitive Disorders
Associations with Dementia or Alzheimer’s Disease Risk
Adherence to the MD is one of the top five modifiable protective factors against AD and cognitive decline in the literature [8]. In 2013, a review of the association between the MD and neurodegenerative diseases described eight prospective cohort studies and one case-control study with cognition-related outcomes, including AD, dementia, mild cognitive impairment (MCI), and cognitive function [9]. All but three of these studies showed significant positive relationships between adherence to the MD and cognitive health, indicating a suggestive but inconclusive protective effect [9]. A 2014 meta-analysis included five prospective studies and showed that those in the highest MD tertile had a 33% lower risk of AD or MCI compared to those in the lowest tertile, and greater MD adherence was protective against the progression from MCI to AD [10].
Table 1 describes observational studies published over the past 5 years examining the association between adherence to the MD and risk of dementia, including AD. Though there are many studies examining the association of the MD and cognitive performance [11–26], there are fewer that have examined the risk of dementia, cognitive impairment, or AD [10, 27•, 28, 29•, 30–34]. Overall, greater adherence to the MD was associated with lower risk of dementia or AD. However, this was not consistent throughout all studies. Notably, a cross-sectional study in Greece sought to use cutpoints to represent adherence to the MD in the native region, rather than relative to the study sample [27•]. Researchers defined adherence to the MD a priori and found that in the Hellenic Longitudinal Investigation of Aging and Diet population-based cohort (N = 1865, 90 with dementia and 223 with MCI), greater adherence was associated with a decreased risk of dementia and better cognition, with the strongest association observed for memory, but also notable for language and visuospatial perception. In this study, the MD component most strongly protective against prevalent dementia was fish consumption [27•]. In contrast, another recent report from the Women’s Health Initiative Memory Study with a median follow-up of 9 years showed no association between four well-established healthy diet patterns, including a modified version of the MD, with incident MCI or dementia [29•]. Taken together, evidence is suggestive but not definite for a protective effect of the MD on dementia risk.
Table 1.
Studies published in the last 5 years (2012–2017) examining the association between the Mediterranean-style diet (and related diets/measures) and cognitive impairment risk
| First author (year) | Sample size (N) | Study type | Years of follow-up number, mean (SD) or range | Age (years) Mean (SD) or range | Main outcome(s) | Main results |
|---|---|---|---|---|---|---|
| Anastasiou (2017) | 1864 | Cross-sectional | N/A | 73(6) | Prevalent dementia | Highest quartile of Mediterranean diet scores were associated with lower odds of dementia relative to the lowest quartile (fully adjusted OR [95% CI] 0.449 [0.208, 0.969]). Mediterranean diet scores modeled continuously were associated with lower odds of dementia (fully adjusted OR [95% CI] 0.920 [0.870, 0.974]). |
| Gardener (2012) | 723 controls, 98 MCI, 149 AD | Cross-sectional | N/A | 72(8) | Prevalent MCI or AD | Greater adherence to the Mediterranean diet was associated with lower odds of MCI (fully adjusted OR [95% CI] 0.866 [0.75, 1.00]) or AD (0.806 [0.71, 0.92]). |
| Haring (2016) | 6425 | Prospective cohort | 9.1 | 65–79 | Mild cognitive impairment, probable dementia | The alternate Mediterranean diet was not associated with mild cognitive impairment or probable dementia (fully adjusted HR [95% CI] for highest quintile 0.82 [0.59, 1.14]). |
| Olsson (2015) | 1138 | Prospective cohort | 12 | 70 | Alzheimer dementia, all-type dementia, all-type cognitive impairment | The Mediterranean-like diet was not associated with dementia or cognitive impairment (OR [95% CI] 0.82 [0.65, 1.05]). |
| Morris (2015) | 923 | Prospective cohort | 4.5 | 58–98 | Alzheimer disease, mortality | Highest tertile of Mediterranean diet score was associated with decreased hazard of Alzheimer disease (fully adjusted HR [95% CI] 0.49 [0.29, 0.85]). |
| Tsivgoulis (2013) | 17,478 | Prospective cohort | 4(1.5) | 64(9) | Incident cognitive impairment | Higher adherence to the Mediterranean diet was associated with lower odds of cognitive impairment (fully adjusted OR [95% CI] 0.87 [0.76, 1.0]). |
| Cao (2016) | 43 studies | Meta-analysis | 1–34 | 20–100 | Dementia | The Mediterranean diet was associated with decreased risk of dementia (summary RR [95% CI] = 0.69 [0.57, 0.84]). |
| Singh (2014) | 5 studies | Meta-analysis | 2–8 | 76–86 | Cognitive impairment | Highest tertile of Mediterranean diet score was associated with lower risk of cognitive impairment (summary HR [95% CI] 0.67 [0.55, 0.81]). Results were similar in cognitively normal individuals. |
| Psaltopoulou (2013) | 22 | Meta-analysis | N/A | N/A | Cognitive impairment, stroke, depression | High adherence to the Mediterranean diet was associated with lower risk of cognitive impairment (summary RR [95% CI] 0.60 [0.43, 0.83]). |
Associations with Cognitive Performance and Decline
Greater adherence to the MD may also protect against age-related cognitive decline. In a large study of older adults in Utah, greater MD adherence was associated with consistently higher Mini-Mental State Examination (MMSE) scores over 11 years of follow-up [21]. A more recent 2016 qualitative review covered 18 longitudinal and prospective studies on a MD in relation to cognition [35]. The authors noted that 13 of these studies reported associations between MD adherence with improved cognitive function or decreased decline over time, while 5 of the studies failed to demonstrate these associations. Adherence to the MD has been positively associated with specific cognitive domains, including memory, delayed recognition, executive function, long-term working memory, and visual constructs [27•, 36]. Further research is needed to better understand how the MD may differentially impact cognitive domains, and whether different components of the MD may have varying effects across cognitive domains.
Associations with Vascular Disease
Researchers posit that the neuroprotective effects of the MD are likely due to the increased consumption of antioxidants and anti-inflammatory agents it provides. Indirect evidence for the protective role of the MD against dementia and cognitive decline comes from the large body of evidence linking the MD with many well-established vascular risk factors for dementia [8]. Most notably, adherence to the MD has been shown to be protective against obesity, hypertension, and diabetes [6, 37–41]. Many well-conducted epidemiological studies have also shown that adherence to the MD is an important protective factor for stroke [42, 43], which is associated with dementia risk. Growing evidence suggests that vascular and neurodegenerative pathways interact to contribute to dementia risk [4, 44]. Therefore, vascular disease may mediate the association between the MD and neurodegeneration.
Mediterranean-Style Diet in Clinical Trials
Randomized trials represent the gold standard in epidemiological studies and are important for inferring causality. Adherence to the MD has been tested in a recent clinical trial called the Prevención con Dieta Mediterránea (PREDIMED) trial, which examined the effect of the MD on primary cardiovascular prevention in older adults at high cardiovascular risk [43]. Participants were randomized to one of two MD treatment arms (supplemented with extra virgin olive oil or nuts) vs. a low-fat diet control group. Subsets of this large clinical trial have been studied to assess effects on cognitive health. In PREDIMED-NAVARRA, 522 participants received a cognitive assessment, including a MMSE and Clock Drawing Test (CDT) after 6.5 years of nutritional intervention (mean age of 75 at cognitive assessment). Both of the groups assigned to a MD (with either olive oil or nuts) had better performance on the MMSE and the CDT as compared to the low-fat diet control group [45].
A random subpopulation that included 285 participants in PREDIMED-NAVARRA was evaluated by neurologists to assess cognitive performance and status (normal, MCI, dementia) [46]. Participants in the MD plus olive oil arm demonstrated better post-intervention performance on fluency and memory tasks and a 66% lower risk of MCI (95% CI 3–88%) in comparison to the low-fat control group, whereas participants in the MD plus nuts group were not different from the low-fat control group in cognitive performance and status. This PREDIMED-NAVARRA randomized trial provided important confirmation of the value of a MD in preserving cognitive health. However, it did not examine change in cognitive performance over time. In 2015, another PREDIMED parallel-group randomized clinical trial was conducted among cognitively healthy adults at high cardiovascular risk in Barcelona, Spain (mean age 67 years) [13]. This study included multiple cognitive assessments in 334 participants to quantify change over time with a median of 4 years after intervention. Cognitive change was assessed based on an extensive neuropsychological battery including the MMSE and tests of memory, attention, executive function, and global cognition. Both of the MD treatment arms exhibited improved cognitive function over time, while those allocated to the low-fat control diet exhibited cognitive decline. In particular, as compared to the control arm, those in the MD plus nuts group had improvements in the memory domain, and those in the MD plus olive oil group had improvements in attention and executive function and global cognition.
Mediterranean-Style Diet and Other Neurodegenerative Diseases
A growing but still very limited body of literature suggests that diet plays a role in the etiology of other neurodegenerative diseases. Evidence for PD is particularly limited in relation to overall dietary patterns, as most of the literature has focused on individual foods, food groups, and vitamins or nutrients. A recent study compared 600 PD cases in Italy with 600 matched controls and found no difference between the groups in terms of overall MD adherence. However, cases consumed less alcohol, fish, and milk and more fruit, cooked vegetables, and cereals compared to controls, all of which are components that contribute to the MD score [47]. In contrast, another smaller European case-control study showed that PD cases reported increased consumption of vegetables, fruits, and fish compared to controls [48]. A USA-based case-control study with 257 cases and 198 controls showed that adherence to the MD was inversely associated with the odds of PD and was associated with a later age of onset [49]. However, because PD can affect dietary habits, particularly with dysphagia as the disease progresses, cross-sectional studies like these are of limited value in elucidating how the MD and its components may impact PD risk [47].
A large prospective study found that a diet characterized by high intake of fruits, vegetables, legumes, whole grains, nuts, fish, and poultry, as well as limited intake of saturated fat, and moderate alcohol consumption was significantly associated with a lower risk of PD [50]. Other studies that have looked at individual foods and nutrients in relation to PD have yielded mixed results [51–53]. More research is needed in longitudinal cohort studies to examine how dietary patterns, particularly in early and mid-life, may impact the incidence and progression of PD. The sensitive period for determining PD risk is most likely many decades before diagnosis, which represents a challenge in this field, but also underscores the need for further study.
Other than PD, literature reporting the association of the MD with other neurodegenerative diseases is very limited. Few studies have examined the role of diet in predicting amyotrophic lateral sclerosis (ALS), though two small studies have suggested that fruit consumption may have the potential to play a protective role [54, 55]. Another recent study reported no significant association of MD adherence with phenoconversion in Huntington Disease [56], but other reports suggest that MD adherence in Huntington Disease may be related to better quality of life [57, 58]. Though ALS and other neurodegenerative disorders are less prevalent than AD or PD, their impact on quality of life is still substantial, and more research examining these associations is warranted.
Mediterranean-Style Diet and Markers of Neurodegeneration
Research examining associations of the MD with biomarkers of neurodegeneration may inform prevention and risk stratification strategies, as well as help elucidate etiology. Common markers of neurodegeneration include structural markers of brain atrophy and cortical thinning and the detection of specific proteins in the blood or cerebrospinal fluid (CSF). Because neurodegeneration is thought to originate from protein misfolding and dysregulation intra- or extracellularly, leading to axonal and synaptic loss and cell death [59], protein-based markers have increasingly become important potential indicators for future disease risk. Several biomarkers (both pathological and in vivo) of neurodegeneration and neurodegeneration-related processes have been explored in animal and human studies.
PET Imaging Studies
Specific proteins detectable in the CSF have been implicated as markers of neurodegeneration in the context of AD and PD [60, 61]. Though examining the CSF is one of the most direct methods for detecting protein levels in the central nervous system, obtaining CSF via lumbar punctures is not ideal in a research or prevention setting. As a result, positron emission tomography (PET) imaging has been utilized to examine correlates of pathology and brain function in vivo. Amyloid-PET imaging has been increasingly utilized since its invention [62], and Tau-PET imaging is gradually emerging as an important marker of neurodegeneration [63]. A small, multi-modal study in cognitively normal, middle-aged adults found increased beta-amyloid plaque burden and decreased glucose metabolism in those who adhered to the MD [64]. Similar results were found in a study examining amyloid and tau pathology through PET imaging in older adults with subjective memory complaints and MCI; in this study, greater adherence to the MD was associated with less pathology in both groups [65]. If results can be replicated in larger samples, these data may support using PET imaging markers as outcome measures in clinical trials.
Structural MRI Markers
Gray Matter Volumetric and Cortical Thickness Measures
Investigators have used magnetic resonance imaging (MRI)-derived measures of brain volumes and cortical thicknesses to examine the association of MD and structural brain aging (Fig. 1). Hippocampal atrophy and cortical thinning of AD-signature regions have been identified as in vivo imaging markers of AD [66, 67] and are thought to reflect downstream consequences of beta-amyloid plaque and tau tangle deposition [68].
Fig. 1.
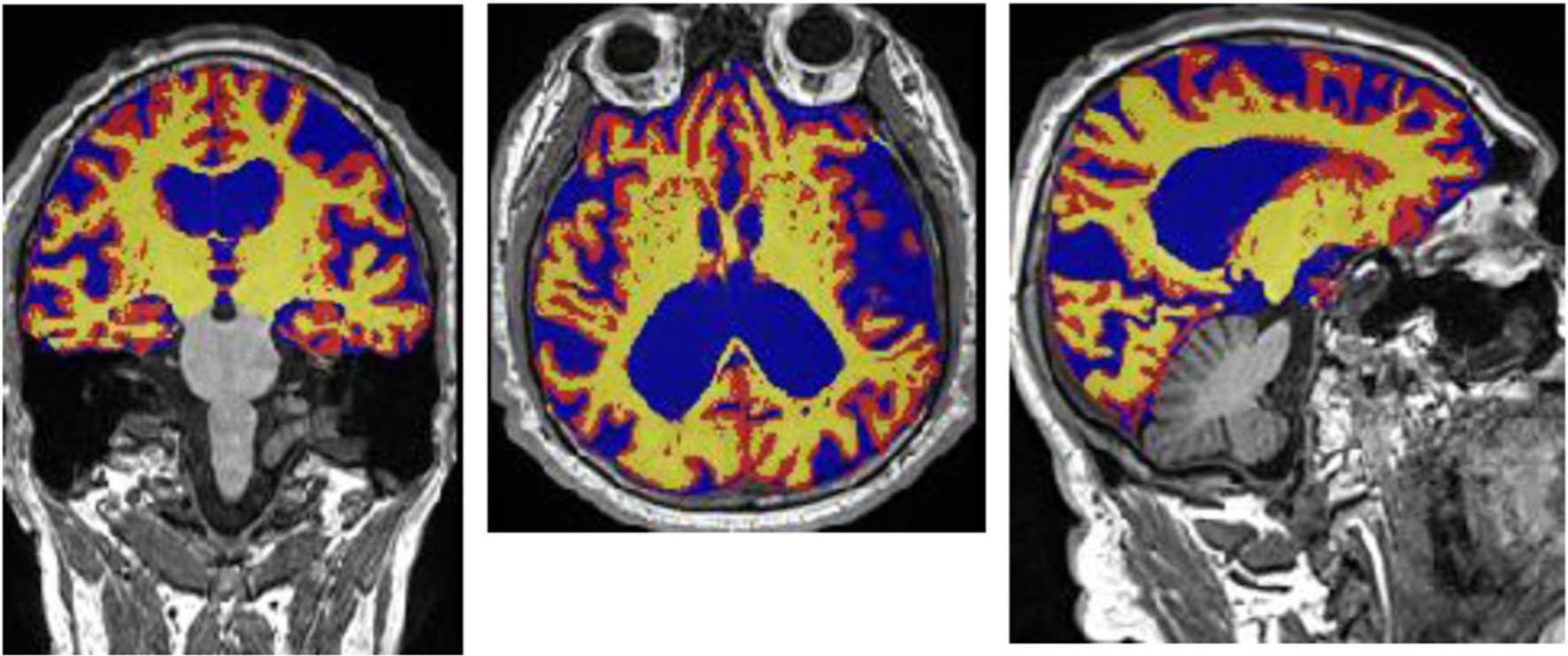
Example of a brain magnetic resonance image exhibiting severe brain atrophy. Segmentation done in FSL. Yellow: segmented white matter; Red: segmented cortical volume; Blue: cerebrospinal fluid
There are limited studies examining the association between MD and structural brain aging, and most current studies are cross-sectional, limiting causal inference. Smaller-scale studies have found that cognitively normal adults with greater MD adherence had thicker cortices in AD-signature regions and larger brain volumes vs. those with lower MD adherence [22, 69]. In a larger, epidemiological cohort composed of racially and ethnically diverse older adults in Northern Manhattan, Gu et al. found that greater adherence to the MD was associated with greater total brain, gray, and white matter volumes and greater mean cortical thickness [70]. This study also found significant cross-sectional associations between MD adherence and greater regional gray matter volumes, specifically for the cingulate, parietal and temporal lobes, and hippocampus, as well as greater superior-frontal cortical thickness [70]. Another epidemiological study of older adults in Rochester, MN showed generally consistent findings with Gu et al., though associations of MD with specific cortical regions and associations of specific MD components with cortical thickness differed between these studies [71]. Greater MD adherence in the Rochester-based study was associated with frontal, parietal, and occipital cortical thicknesses, and legume, fish, and carbohydrate intake were specifically identified as important components of the MD [71]. This is consistent with a prospective study from the Lothian Birth Cohort of 1936, which examined the association of MD on change in structural brain aging metrics [72••]. Luciano et al. showed that greater MD adherence was not cross-sectionally associated with brain volumes or cortical thickness but was associated with reduction in gray matter volumes over a 3-year follow up period [72••]. This longitudinal study provides stronger evidence that the MD can impact brain structure over time.
Markers of Cerebral Small Vessel Disease
Cerebral small vessel disease markers are commonly derived from analysis of brain MR images, and include white matter hyperintensities, subclinical brain infarcts, perivascular spaces, cerebral microbleeds, and diffusion tensor imaging markers (fractional anisotropy and mean diffusivity) [73]. Markers of cerebral small vessel disease have been associated with smaller brain volumes, thinner cortices, and cognitive impairment in large epidemiological studies [74–76]. Cerebral small vessel disease is common in old age and theorized to result from cumulative cardiovascular risk factor burden and subclinical cerebral ischemia over the life course [77]. Given current evidence suggesting that vascular disease and neurodegeneration may interact or influence each other to cause disease [4, 44], subclinical vascular pathology should be considered as a parallel marker for potential neurodegeneration or marker for risk stratification in those at high risk for neurodegeneration.
Despite well-known associations between the MD and vascular health [42], very few studies have examined the relationship between the MD and cerebral small vessel disease markers. Two cross-sectional studies from similar racially and ethnically diverse cohorts have shown that adherence to MD is associated with less cerebral small vessel burden [78, 79]. Scarmeas et al. showed in the Washington Heights Inwood Columbia Aging Project (WHICAP) that MD adherence was associated with reduced silent infarcts but not associated with white matter hyperintensities, while Gardener et al. showed MD adherence was related to lower white matter hyperintensity volume in the Northern Manhattan Study [79]. This discrepancy may be explained by the heterogeneous etiology of white matter hyperintensities, which includes cerebral small vessel disease as well as blood-brain barrier disruption and demyelination [80]. Though several studies have associated individual components of MD to cerebral small vessel disease burden, such as fish intake and alcohol consumption [81, 82], more research elucidating the association between the MD and other markers of cerebral small vessel disease is warranted.
Potential Mechanisms
Animal and in vitro models allow for mechanistic investigation into the protective effects of MD on neurodegeneration. Several recent studies have explored the specific compounds in the components of MD that may act as neuroprotective factors. Evidence suggests that specific compounds may act as “caloric restriction mimickers,” activating similar Sirtuin pathways as caloric restriction diets [83]. Though several compounds have been studied, plant polyphenols found in extra virgin olive oil and red wine have been of particular interest [83].
Using mouse models of beta-amyloid plaque deposition, studies have shown that administration of extra virgin olive oil improved cognitive performance and reduced beta-amyloid load and tau [84], with similar results after dietary administration of oleuropein aglycone and oleocanthal, which are polyphenols found in extra virgin olive oil [85–87]. Further in vitro work has implicated potential role of oleocanthal in attenuating amyloid-beta oligomer-mediated astrocyte inflammation and synaptic proteins [88]. Hydroxytyrosol has also been shown to improve insulin sensitivity in a cultured astrocytic model of AD [89], which is relevant given epidemiological evidence that insulin resistance and diabetes are related to AD development [90].
Resveratrol, a polyphenol found in red wine, has also been of interest as a potential compound for the treatment of AD [91, 92]. Recent in vitro work has shown that resveratrol protected PC12 cells from the apoptotic effects of Aβ23–35 peptides [93]. A recent randomized clinical trial recently reported the safety and tolerability of resveratrol in AD patients [94••]. This study also reported declines in mean levels of CSF and plasma Aβ40 concentrations between the experimental and placebo groups, while brain volume decreased in the experimental vs. placebo groups [94••]. These data support resveratrol as a possible treatment strategy for AD.
Conclusions
Although the past 5 years have yielded growing evidence in support of the MD in the prevention of cognitive impairment and dementia, the evidence remains inconclusive, with many questions left to be answered. Inconsistencies in the literature may be due to differences in the cognitive outcomes included (age-related cognitive decline, MCI, overall dementia, and AD). Cognitive decline and dementia are etiologically heterogeneous outcomes, with vascular and non-vascular origins, which makes the identification of protective factors challenging. Also, adherence to the MD is typically a cohort-dependent variable, and a given individual may score high in one study population and low in another population where objective adherence to a MD is more prevalent. Differences in study populations by country of original, race/ethnicity, sex, and associated risk factors may also play a role, as there is limited information about effect modifiers for the relationship between MD and cognition. Other factors that may account for inconsistencies across the literature include [1] bias due to residual confounding by measured and unmeasured associated lifestyle factors (e.g., physical activity, sleep, social isolation, depression), [2] differential and non-differential misclassification of diet based on self-report, and [3] limited follow-up and small sample size resulting in insufficient statistical power. Some studies on cognition are also lacking multiple neuropsychological assessments over time, needed to understand how diet can impact cognitive trajectories.
Evidence is mounting that the etiologically sensitive period for determining late-life cognitive health is mid-life, and perhaps even early-life [95]. Dietary behavior in late-life, a few years before the age of diagnosis, may be too late to attenuate neurodegeneration. Mid-life obesity, particularly, abdominal obesity, has been shown to be a well-established risk factor for dementia and cognitive decline, while the data for late-life obesity is inconsistent and inconclusive, underscoring the fact that mid-life may be the critical period during which diet may impact future neurodegeneration [96]. Late-life dietary habits may only be associated with cognitive outcomes to the extent that they are highly correlated with mid-life dietary habits. Ideally, future studies are needed that measure diet in mid-life (or even early-life), with repeated diet assessments over time, and follow participants for several decades until they reach the age when cognitive decline is measurable and dementia is diagnosed. In other words, long-term longitudinal follow-up studies are needed to help identify when during the lifecourse diet may have the greatest impact on late-life cognition and whether improvements in diet during mid-life or late-life have the potential to protect brain health and minimize neurodegeneration. These are the crucial questions that need to be answered in future studies.
There remains ambiguity regarding the components of the MD that may be driving the protection against neurodegeneration. Fish consumption has emerged as a component of the MD that may be a driving factor in its protection against cognitive decline and dementia [97]. However, other components have also received particular attention. A small prospective cohort study in Sweden showed no association between adherence to a MD overall in relation to dementia or brain volume, but increased meat consumption was associated with an increased risk of dementia and lower brain volume [22]. Further studies are needed to elucidate which components of the MD are most important for the population to focus on, and whether these components vary by age, race/ethnicity, or other population characteristics.
Observational studies are threatened by bias due to confounding from lifestyle factors associated with dietary patterns that can impact cognitive health, like physical activity, education, engagement in mentally stimulating activities, social isolation, depression, and smoking. Though randomized trials are expensive and challenging, the large body of observational evidence showing varied health benefits of a MD support the value of conducting clinical trials to examine its effects. However, there are also important disadvantages when using randomized trials to study diet in relation to neurological outcomes. Most importantly, adhering to a prescribed diet for a long follow-up period is extremely difficult to achieve. As participants decrease adherence to their randomized diets over time, biases including confounding and misclassification are introduced. Alternatively, application of causal inference methods to these research questions may leverage existing observational data to help infer causal effects of the MD on neurodegenerative disease. Finally, translational efforts should be encouraged between mechanistic animal studies and epidemiological studies in humans to facilitate better interventions in the clinic and population.
Taken together, these data suggest that the MD can be beneficial for protection against neurodegenerative diseases. Though AD and dementia are large threats to public health, the relation between the MD and neurodegenerative diseases should also be studied, as these conditions confer poor quality of life and increased disability. Future studies should consider improved methodology to investigate these associations and inform translation to clinical and public health practice.
Footnotes
This article is part of the Topical Collection on Neurological Disease and Cognitive Function
Conflict of Interest Hannah Gardener and Michelle R. Caunca declare they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Neurology. 2017;16(11):877–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83. 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Movement Disorders : official Journal of the Movement Disorder Society. 2014;29(13):1583–90. 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 4.Gardener H, Wright CB, Rundek T, Sacco RL. Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. 2015;11(11):651–7. 10.1038/nrneurol.2015.195. [DOI] [PubMed] [Google Scholar]
- 5.Trichopoulou A, Costacou T, Bamia C, Adherence TD. To a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. 10.1056/EJMoa025039. [DOI] [PubMed] [Google Scholar]
- 6.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44(4): 335–40. 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Panagiotakos DB, Milias GA, Pitsavos C, Stefanadis C. MedDietScore: a computer program that evaluates the adherence to the Mediterranean dietary pattern and its relation to cardiovascular disease risk. Comput Methods Prog Biomed. 2006;83(1):73–7. 10.1016/j.cmpb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimer’s & Dementia : the Journal of the Alzheimer’s Association. 2015;11(6):718–26. 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Sofi F, Macchi C, Casini A. Mediterranean Diet and Minimizing Neurodegeneration. Current Nutrition Reports. 2013;2(2):75–80. 10.1007/s13668-013-0041-7. [DOI] [Google Scholar]
- 10.Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, et al. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Journal of Alzheimer’s Disease : JAD. 2014;39(2):271–82. 10.3233/JAD-130830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEvoy CT, Guyer H, Langa KM, Yaffe K. Neuroprotective diets are associated with better cognitive function: the health and retirement study. J Am Geriatr Soc. 2017;65(8):1857–62. 10.1111/jgs.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Welsh-Bohmer K, Browndyke JN, et al. Lifestyle and Neurocognition in older adults with cardiovascular risk factors and cognitive impairment. Psychosom Med. 2017;79(6):719–27. 10.1097/PSY.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martinez-Gonzalez MA, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175(7):1094–103. 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- 14.Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C, et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr. 2015;54(8):1311–21. 10.1007/s00394-014-0811-z. [DOI] [PubMed] [Google Scholar]
- 15.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimer’s & Dementia : the Journal of the Alzheimer’s Association. 2015;11(9):1015–22. 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DR, et al. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci. 2015;70(3):354–9. 10.1093/gerona/glu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardener SL, Rainey-Smith SR, Barnes MB, Sohrabi HR, Weinborn M, Lim YY, et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol Psychiatry. 2015;20(7):860–6. 10.1038/mp.2014.79. [DOI] [PubMed] [Google Scholar]
- 18.Galbete C, Toledo E, Toledo JB, Bes-Rastrollo M, Buil-Cosiales P, Marti A, et al. Mediterranean diet and cognitive function: the SUN project. J Nutr Health Aging. 2015;19(3):305–12. 10.1007/s12603-015-0441-z. [DOI] [PubMed] [Google Scholar]
- 19.Tangney CC, Li H, Wang Y, Barnes L, Schneider JA, Bennett DA, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410–6. 10.1212/WNL.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, healthy eating index 2005, and cognitive function in middle-aged and older Puerto Rican adults. Journal of the Academy of Nutrition and Dietetics. 2013;113(2):276–81.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263–71. 10.3945/ajcn.112.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Titova OE, Ax E, Brooks SJ, Sjogren P, Cederholm T, Kilander L, et al. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol. 2013;48(12):1443–8. 10.1016/j.exger.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, et al. Mediterranean diet and cognitive function in older age. Epidemiology. 2013;24(4):490–9. 10.1097/EDE.0b013e318294a065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samieri C, Okereke OI, ED E, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr. 2013;143(4):493–9. 10.3945/jn.112.169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, et al. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97(2):369–76. 10.3945/ajcn.112.047993. [DOI] [PubMed] [Google Scholar]
- 26.Katsiardanis K, Diamantaras AA, Dessypris N, Michelakos T, Anastasiou A, Katsiardani KP, et al. Cognitive impairment and dietary habits among elders: the Velestino study. J Med Food. 2013;16(4):343–50. 10.1089/jmf.2012.0225. [DOI] [PubMed] [Google Scholar]
- 27.•.Anastasiou CA, Yannakoulia M, Kosmidis MH, Dardiotis E, Hadjigeorgiou GM, Sakka P, et al. Mediterranean diet and cognitive health: initial results from the Hellenic longitudinal investigation of ageing and diet. PloS One. 2017;12(8):e0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the most recent cross-sectional reports of the Mediterranean diet and cognition in a large epidemiologic study focusing on diet.
- 28.Cao L, Tan L, Wang HF, Jiang T, Zhu XC, Lu H, et al. Dietary patterns and risk of dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol. 2016;53(9):6144–54. 10.1007/s12035-015-9516-4. [DOI] [PubMed] [Google Scholar]
- 29.•.Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, et al. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women’s Health Initiative memory study. Journal of the academy of nutrition and dietetics. 2016;116(6):921–30.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]; A longitudinal study in a well-characterized cohort that reported null associations with the MD and incident cognitive impairment.
- 30.Olsson E, Karlstrom B, Kilander L, Byberg L, Cederholm T, Dietary SP. Patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. Journal of Alzheimer’s Disease : JAD. 2015;43(1):109–19. 10.3233/JAD-140867. [DOI] [PubMed] [Google Scholar]
- 31.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s & Dementia : the Journal of the Alzheimer’s Association. 2015;11(9):1007–14. 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74(4): 580–91. 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 33.Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. 2013;80(18):1684–92. 10.1212/WNL.0b013e3182904f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardener S, Gu Y, Rainey-Smith SR, Keogh JB, Clifton PM, Mathieson SL, et al. Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population. Transl Psychiatry. 2012;2(10):e164 10.1038/tp.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a Mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Frontiers in Nutrition. 2016;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loughrey DG, Lavecchia S, Brennan S, Lawlor BA, Kelly ME. The Impact of the Mediterranean Diet on the Cognitive Functioning of Healthy Older Adults: A Systematic Review and Meta-Analysis. Advances in Nutrition (Bethesda, Md). 2017;8(4): 571–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis CR, Hodgson JM, Woodman R, Bryan J, Wilson C, Mediterranean MKJA. Diet lowers blood pressure and improves endothelial function: results from the MedLey randomized intervention trial. Am J Clin Nutr. 2017;105(6):1305–13. 10.3945/ajcn.116.146803. [DOI] [PubMed] [Google Scholar]
- 38.Domenech M, Roman P, Lapetra J, Garcia de la Corte FJ, Sala-Vila A, de la Torre R, et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: one-year randomized, clinical trial. Hypertension. 2014;64(1):69–76. 10.1161/HYPERTENSIONAHA.113.03353. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Gonzalez MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, et al. adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336(7657):1348–51. 10.1136/bmj.39561.501007.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babio N, Bullo M, Salas-Salvado J. Mediterranean diet and metabolic syndrome: the evidence. Public Health Nutr. 2009;12(9a): 1607–17. 10.1017/S1368980009990449. [DOI] [PubMed] [Google Scholar]
- 41.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Olive TA. Oil, the Mediterranean diet, and arterial blood pressure: the Greek European prospective investigation into cancer and nutrition (EPIC) study. Am J Clin Nutr. 2004;80(4): 1012–8. [DOI] [PubMed] [Google Scholar]
- 42.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119 (8):1093–100. 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90. 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 44.Appelman AP, Exalto LG, van der Graaf Y, Biessels GJ, Mali WP, Geerlings MI. White matter lesions and brain atrophy: more than shared risk factors? A systematic review. Cerebrovasc Dis. 2009;28(3):227–42. 10.1159/000226774. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84(12):1318–25. 10.1136/jnnp-2012-304792. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Lapiscina EH, Clavero P, Toledo E, San Julian B, Sanchez-Tainta A, Corella D, et al. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging. 2013;17(6):544–52. 10.1007/s12603-013-0027-6. [DOI] [PubMed] [Google Scholar]
- 47.Cassani E, Barichella M, Ferri V, Pinelli G, Iorio L, Bolliri C, et al. Dietary habits in Parkinson’s disease: adherence to Mediterranean diet. Parkinsonism Relat Disord. 2017;42:40–6. 10.1016/j.parkreldis.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Okubo H, Miyake Y, Sasaki S, Murakami K, Tanaka K, Fukushima W, et al. Dietary patterns and risk of Parkinson’s disease: a case-control study in Japan. European Journal of Neurology : the Official Journal of the European Federation of Neurological Societies. 2012;19(5):681–8. 10.1111/j.1468-1331.2011.03600.x. [DOI] [PubMed] [Google Scholar]
- 49.Alcalay RN, Gu Y, Mejia-Santana H, Cote L, Marder KS, Scarmeas N. The association between Mediterranean diet adherence and Parkinson’s disease. Movement Disorders : Official Journal of the Movement Disorder Society. 2012;27(6):771–4. 10.1002/mds.24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, et al. prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86(5):1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang F, Wolk A, Hakansson N, Pedersen NL, Dietary WK. Antioxidants and risk of Parkinson’s disease in two population-based cohorts. Movement Disorders : Official Journal of the Movement Disorder Society. 2017;32(11):1631–6. 10.1002/mds.27120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherzai AZ, Tagliati M, Park K, Pezeshkian S, Sherzai D. Micronutrients and risk of Parkinson’s disease: a systematic review. Gerontology & Geriatric Medicine 2016;2:2333721416644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seidl SE, Santiago JA, Bilyk H, Potashkin JA. The emerging role of nutrition in Parkinson’s disease. Front Aging Neurosci. 2014;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Y, Oh K, Oh SI, Baek H, Kim SH, Park Y. Dietary intake of fruits and beta-carotene is negatively associated with amyotrophic lateral sclerosis risk in Koreans: a case-control study. Nutr Neurosci. 2014;17(3):104–8. 10.1179/1476830513Y.0000000071. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto K, Kihira T, Kobashi G, Washio M, Sasaki S, Yokoyama T, et al. Fruit and vegetable intake and risk of amyotrophic lateral sclerosis in Japan. Neuroepidemiology. 2009;32(4):251–6. 10.1159/000201563. [DOI] [PubMed] [Google Scholar]
- 56.Marder K, Gu Y, Eberly S, Tanner CM, Scarmeas N, Oakes D, et al. relationship of Mediterranean diet and caloric intake to phenoconversion in Huntington disease. JAMA Neurology. 2013;70(11):1382–8. 10.1001/jamaneurol.2013.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivadeneyra J, Cubo E, Gil C, Calvo S, Mariscal N, Martinez A. Factors associated with Mediterranean diet adherence in Huntington’s disease. Clinical Nutrition ESPEN. 2016;12:e7–e13. [DOI] [PubMed] [Google Scholar]
- 58.Cubo E, Rivadeneyra J, Armesto D, Mariscal N, Martinez A, Relationship CRJ. Between nutritional status and the severity of Huntington’s disease. A Spanish multicenter dietary intake study. Journal of Huntington’s Disease. 2015;4(1):78–85. [PubMed] [Google Scholar]
- 59.Hoglund K, Salter H. Molecular biomarkers of neurodegeneration. Expert Rev Mol Diagn. 2013;13(8):845–61. 10.1586/14737159.2013.850033. [DOI] [PubMed] [Google Scholar]
- 60.Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. The Lancet Neurology. 2016;15(7):673–84. 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 61.Halbgebauer S, Ockl P, Wirth K, Steinacker P, Protein OM. Biomarkers in Parkinson’s disease: focus on cerebrospinal fluid markers and synaptic proteins. Movement Disorders : Official Journal of the Movement Disorder Society. 2016;31(6):848–60. 10.1002/mds.26635. [DOI] [PubMed] [Google Scholar]
- 62.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55(3):306–19. 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 63.Bischof GN, Endepols H, van Eimeren T, Drzezga A. Tau-imaging in neurodegeneration. Methods (San Diego, Calif). 2017;130:114–23. [DOI] [PubMed] [Google Scholar]
- 64.Matthews DC, Davies M, Murray J, Williams S, Tsui WH, Li Y, et al. Physical activity, Mediterranean diet and biomarkers-assessed risk of Alzheimer’s: a multi-modality brain imaging study. Advances in Molecular Imaging. 2014;4(4):43–57. 10.4236/ami.2014.44006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merrill DA, Siddarth P, Raji CA, Emerson ND, Rueda F, Ercoli LM, et al. Modifiable risk factors and brain positron emission tomography measures of amyloid and tau in nondemented adults with memory complaints. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry. 2016;24(9):729–37. 10.1016/j.jagp.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jack CR Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42(1):183–8. 10.1212/WNL.42.1.183. [DOI] [PubMed] [Google Scholar]
- 67.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Update on hypothetical model of Alzheimer’s disease biomarkers. The Lancet Neurology. 2013;12(2):207–16. 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mosconi L, Murray J, Tsui WH, Li Y, Davies M, Williams S, et al. Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for Alzheimer’s disease. The Journal of Prevention of Alzheimer’s Disease. 2014;1(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 70.Gu Y, Brickman AM, Stern Y, Habeck CG, Razlighi QR, Luchsinger JA, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015;85(20):1744–51. 10.1212/WNL.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Staubo SC, Aakre JA, Vemuri P, Syrjanen JA, Mielke MM, Geda YE, et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017;13(2):168–77. 10.1016/j.jalz.2016.06.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.••.Luciano M, Corley J, Cox SR, Valdes Hernandez MC, Craig LC, Dickie DA, et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology. 2017;88(5):449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]; A longitudinal neuroimaging study in an epidemiologic cohort examining the association of the Mediterranean diet with change in brain structure.
- 73.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38. 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham offspring study. Stroke. 2010;41(4):600–6. 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikram MA, Vrooman HA, Vernooij MW, den Heijer T, Hofman A, Niessen WJ, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging. 2010;31(3):378–86. 10.1016/j.neurobiolaging.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, et al. Brain tissue volumes in the general elderly population. The Rotterdam scan study. Neurobiol Aging. 2008;29(6):882–90. 10.1016/j.neurobiolaging.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Pantoni L Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:680–701. [DOI] [PubMed] [Google Scholar]
- 78.Scarmeas N, Luchsinger JA, Stern Y, Gu Y, He J, DeCarli C, et al. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol. 2011;69(2):257–68. 10.1002/ana.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gardener H, Scarmeas N, Gu Y, Boden-Albala B, Elkind MS, Sacco RL, et al. Mediterranean diet and white matter hyperintensity volume in the northern Manhattan study. Arch Neurol. 2012;69(2): 251–6. 10.1001/archneurol.2011.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wardlaw JM, Valdes Hernandez MC, Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4(6):001140 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Virtanen JK, Siscovick DS, Longstreth WT Jr, Kuller LH, Mozaffarian D. Fish consumption and risk of subclinical brain abnormalities on MRI in older adults. Neurology. 2008;71(6):439–46. 10.1212/01.wnl.0000324414.12665.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukamal KJ, Longstreth WT Jr, Mittleman MA, Crum RM, Siscovick DS. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke. 2001;32(9):1939–46. 10.1161/hs0901.095723. [DOI] [PubMed] [Google Scholar]
- 83.Rigacci S, Stefani M. Nutraceutical properties of olive oil polyphenols. An itinerary from cultured cells through animal models to humans. Int J Mol Sci. 2016;17(6) 10.3390/ijms17060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qosa H, Mohamed LA, Batarseh YS, Alqahtani S, Ibrahim B, Le Vine H 3rd, et al. Extra-virgin olive oil attenuates amyloid-beta and tau pathologies in the brains of TgSwDI mice. J Nutr Biochem. 2015;26(12):1479–90. 10.1016/j.jnutbio.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grossi C, Rigacci S, Ambrosini S, Ed Dami T, Luccarini I, Traini C, et al. The polyphenol oleuropein aglycone protects TgCRND8 mice against AB plaque pathology. PLoS One. 2013;8(8):e71702 10.1371/journal.pone.0071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pantano D, Luccarini I, Nardiello P, Servili M, Stefani M, Casamenti F. Oleuropein aglycone and polyphenols from olive mill waste water ameliorate cognitive deficits and neuropathology. Br J Clin Pharmacol. 2017;83(1):54–62. 10.1111/bcp.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qosa H, Batarseh YS, Mohyeldin MM, El Sayed KA, Keller JN, Kaddoumi A. Oleocanthal enhances amyloid-beta clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem Neurosci. 2015;6(11):1849–59. 10.1021/acschemneuro.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Batarseh YS, Mohamed LA, Al Rihani SB, Mousa YM, Siddique AB, El Sayed KA, et al. Oleocanthal ameliorates amyloid-beta oligomers’ toxicity on astrocytes and neuronal cells: in vitro studies. Neuroscience. 2017;352:204–15. 10.1016/j.neuroscience.2017.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crespo MC, Tome-Carneiro J, Pintado C, Davalos A, Visioli F, Hydroxytyrosol B-RE. Restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. BioFactors (Oxford, England). 2017;43(4):540–8. 10.1002/biof.1356. [DOI] [PubMed] [Google Scholar]
- 90.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam study. Neurology. 2010;75(22):1982–7. 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sawda C, Moussa C, Resveratrol TRS. For Alzheimer’s disease. Ann N Y Acad Sci. 2017;1403(1):142–9. 10.1111/nyas.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmed T, Javed S, Javed S, Tariq A, Samec D, Tejada S, et al. Resveratrol and Alzheimer’s disease: mechanistic insights. Mol Neurobiol. 2017;54(4):2622–35. 10.1007/s12035-016-9839-9. [DOI] [PubMed] [Google Scholar]
- 93.Feng X, Liang N, Zhu D, Gao Q, Peng L, Dong H, et al. Resveratrol inhibits beta-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathway. PLoS One. 2013;8(3): e59888 10.1371/journal.pone.0059888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.••.Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]; A randomized clinical trial testing the safety and efficacy of resveratrol, a polyphenol abundant in the Mediterranean diet, for treatment of Alzheimer disease.
- 95.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48(10):e284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14–21. 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 97.Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s disease and late-life cognitive disorders: a systematic review. Journal of Alzheimer’s Disease : JAD. 2017;59(3):815–49. 10.3233/JAD-170248. [DOI] [PubMed] [Google Scholar]


