Abstract
α-Synucleinopathies are neurodegenerative diseases that are characterized pathologically by α-synuclein inclusions in neurons and glia. The pathologic contribution of glial α-synuclein in these diseases is not well understood. Glial α-synuclein may be of particular importance in multiple system atrophy (MSA), which is defined pathologically by glial cytoplasmic α-synuclein inclusions. We have previously described Drosophila models of neuronal α-synucleinopathy, which recapitulate key features of the human disorders. We have now expanded our model to express human α-synuclein in glia. We demonstrate that expression of α-synuclein in glia alone results in α-synuclein aggregation, death of dopaminergic neurons, impaired locomotor function, and autonomic dysfunction. Furthermore, co-expression of α-synuclein in both neurons and glia worsens these phenotypes as compared to expression of α-synuclein in neurons alone. We identify unique transcriptomic signatures induced by glial as opposed to neuronal α-synuclein. These results suggest that glial α-synuclein may contribute to the burden of pathology in the α-synucleinopathies through a cell type-specific transcriptional program. This new Drosophila model system enables further mechanistic studies dissecting the contribution of glial and neuronal α-synuclein in vivo, potentially shedding light on mechanisms of disease that are especially relevant in MSA but also the α-synucleinopathies more broadly.
Keywords: Drosophila, glia, multiple system atrophy, Parkinson’s disease, α-Synuclein
1 |. INTRODUCTION
The α-synucleinopathies are a family of neurodegenerative diseases characterized by pathologic accumulation of misfolded α-synuclein (Fujiwara et al., 2002; Uversky, 2008; Vilar et al., 2008). Postmortem studies demonstrate that α-synuclein inclusions can be found in neurons and glia, to varying extents, in all of the α-synucleinopathies (Brück, Wenning, Stefanova, & Fellner, 2015). Specifically, in Parkinson’s disease (PD) and dementia with Lewy bodies (DLB), inclusions are predominantly identified in neurons in the form of Lewy bodies and Lewy neurites (Baba et al., 1998; Beyer & Ariza, 2007) but also to a lesser extent in astrocytes (Braak, Sastre, & Del Tredici, 2007; Song et al., 2009; Wakabayashi, Hayashi, Yoshimoto, Kudo, & Takahashi, 2000), whereas in multiple system atrophy (MSA) inclusions are found in oligodendrocytes in the form of glial cytoplasmic inclusions, but also in neurons and astrocytes (Cykowski et al., 2015; Gai, Power, Blumbergs, & Blessing, 1998; Papp & Lantos, 1994). Despite these consistent pathologic observations, whether glial α-synuclein serves as a pathologic driving force or is merely a bystander in the development or progression of these diseases remains unclear.
Drosophila offers many advantages as a model system and has been used successfully to model multiple diseases with prominent or exclusive glial pathology, including gliomas (Kim et al., 2014; Read et al., 2013; Witte, Jeibmann, Klämbt, & Paulus, 2009), glial tauopathies (Colodner & Feany, 2010), Alexander disease (Wang, Colodner, & Feany, 2011), and complex I deficiency (Hegde, Vogel, & Feany, 2014). Similar to mammalian glia, Drosophila glia include multiple specialized cell types (Kremer, Jung, Batelli, Rubin, & Gaul, 2017) and are essential for neuronal development (Booth, Kinrade, & Hidalgo, 2000; Sepp, Schulte, & Auld, 2001) and maintenance (Xiong & Montell, 1995). In the adult nervous system, they serve many of the same specialized functions as mammalian glia, including phagocytic clearance of cellular debris (Doherty, Logan, Taşdemir, & Freeman, 2009; MacDonald et al., 2006), participation in innate immunity (Kounatidis & Chtarbanova, 2018), blood–brain barrier formation (DeSalvo et al., 2014), glutamate recycling (Farca Luna, Perier, & Seugnet, 2017; Rival et al., 2004), protection of axons in white matter (Logan et al., 2012), and protection of neurons from reactive oxygen species through lipid droplet formation (L. Liu, MacKenzie, Putluri, Maletić -Savatić, & Bellen, 2017; L. Liu et al., 2015).
The Feany laboratory has previously published Drosophila models of neuronal α-synucleinopathy (Feany & Bender, 2000; Ordonez, Lee, & Feany, 2018). These flies recapitulate many features of human α-synucleinopathies, including progressive locomotor impairment, neurodegeneration (including death of dopaminergic neurons), and accumulation of α-synuclein inclusions. Here we expand on this model to investigate the pathologic contribution of glial α-synuclein. We make use of two bipartite expression systems, the UAS–GAL4 system (Brand & Perrimon, 1993) and the Q system (C. J. Potter, Tasic, Russler, Liang, & Luo, 2010) to independently express human α-synuclein in glia or neurons using the pan-glial driver repo-GAL4 or the pan-neuronal driver Syb-QF2, respectively. We determine that glial α-synuclein forms inclusions, impairs locomotion, causes autonomic dysfunction, and induces death of dopaminergic neurons. Additionally, flies expressing α-synuclein in both neurons and glia develop more α-synuclein inclusions in neurons than those expressing α-synuclein in neurons alone. Finally, we identify unique transcriptional programs induced by glial and neuronal α-synuclein, suggesting that the cellular context of α-synuclein matters for gene expression. Importantly, many of the differentially expressed genes we identify in Drosophila have orthologs that have been recognized as causally important in mammalian models of MSA or in patients, supporting the applicability of this model for uncovering human disease mechanisms. Our work represents a novel model system for studying glial α-synucleinopathy and uncovering glial-based therapeutic targets.
2 |. METHODS
2.1 |. Drosophila
All fly crosses and aging were performed at 25°C. All experiments include flies in which wild type human α-synuclein is expressed in either neurons or glia using the pan-neuronal driver synaptobrevin (Syb) or the pan-glial driver reversed polarity (repo), respectively. Control flies include the drivers but lack transgenic human α-synuclein. The exact genotypes for all experiments are as follows: (a) Control = Syb-QF2, repo-GAL4/+, (b) Glia = Syb-QF2, repo-GAL4/UAS-α-synuclein, (c) Neurons = Syb-QF2, repo-GAL4, QUAS-α-synuclein/+, and (d) Both = Syb-QF2, repo-GAL4, QUAS-α-synuclein/UAS-α-synuclein. All experiments were performed at 10 days post-eclosion unless otherwise noted in the figure legends.
2.2 |. Immunohistochemistry and immunofluorescence
Flies were fixed in formalin and embedded in paraffin. Either 2 or 4 μm serial frontal sections were prepared through the entire fly brain. Slides were processed through xylene, ethanols, and into water. For neuron counts, slides were stained with hematoxylin. For immunohistochemistry, microwave antigen retrieval with 10 mM sodium citrate, pH 6.0, was performed. Slides were blocked in 2% milk in PBS with 0.3% Triton X-100 for 1 hr then incubated with appropriate primary antibody in 2% milk in PBS with 0.3% Triton X-100 at room temperature overnight. Primary antibodies used include repo (1:5, mouse, Developmental Studies Hybridoma Bank, Iowa City, IA), elav (1:5, mouse, Developmental Studies Hybridoma Bank), tyrosine hydroxylase (1:200 to 1:500, mouse, Immunostar, Hudson, WI), α-synuclein hSA-2 (1:1000, rabbit, provided as a kind gift from Dr. Michael Schlossmacher, Boston, MA), and α-synuclein (1:1,000 to 1:10,000, rat, provided as a kind gift from Biolegend, San Diego, CA). α-Synuclein hSA-2 recognizes both monomeric and oligomeric α-synuclein by immunoblotting as well as α-synuclein aggregates by immunostaining. The α-Synuclein antibody from Biolegend was raised against aggregated α-synuclein and recognizes aggregates and total α-synuclein by immunostaining. For immunohistochemistry, slides were incubated in biotin-conjugated secondary antibodies in 2% milk in PBS with 0.3% Triton X-100 for 1 hr (1:200, Southern Biotech, Birmingham, AL) followed by avidin–biotin–peroxidase complex (Vectastain Elite) in PBS for 1 hr. Histochemical detection was performed with diaminobenzidine (ImmPACT DAB, Vector, Torrance, CA). For immunofluorescence, slides were incubated with fluorophore-conjugated secondary antibodies in 2% milk in PBS with 0.3% Triton X-100 for 1 hr (1:200, Alexa 488 or Alexa 555, Invitrogen, Waltham, MA) then mounted with DAPI-containing Fluoromount medium (Southern Biotech). Immunofluorescence microscopy was performed on an Olympus FV1000 confocal microscope through the Harvard Neurobiology Imaging Facility or on a Zeiss LSM 800 confocal microscope. Images were processed using Fiji.
2.3 |. Western blotting
Fly heads were homogenized in 2× Laemmli buffer, boiled for 10 min, and centrifuged. SDS-PAGE was performed (Lonza, Basel, Switzerland) followed by transfer to nitrocellulose membrane (Bio-Rad, Hercules, CA) and microwave antigen retrieval in PBS. Membranes were blocked in 2% milk in PBS with 0.05% Tween-20 for 1 hr, then immunoblotted with appropriate primary antibody in 2% milk in PBS with 0.05% Tween-20 overnight at 4°C. Primary antibodies used include α-synuclein H3C (1:10,000 to 1:100,000, mouse, Developmental Studies Hybridoma Bank), GAPDH (mouse, 1:25,000 to 1:100,000, Invitrogen), GFP N86/8 (mouse, 1:100, Neuromab, Davis, CA). Membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:50,000) in 2% milk in PBS with 0.05% Tween-20 for 3 hr. Signal was developed with enhanced chemiluminescence (Thermo Scientific, Waltham, CA). Anti-GAPDH was used to demonstrate equivalent protein loading.
2.4 |. Locomotion assay
Adult flies were aged in vials containing 9–14 flies per vial. At days 1, 4, 7, 10, 13, 16, and 21 of life, flies were transferred to a clean vial (without food) and given 1 min to acclimate to the new vial. The vial was then gently tapped three times to trigger the startle-induced locomotion response, then placed on its side for 15 s. The percentage of flies still in motion was then recorded. Differences between genotypes at specific time-points were measured and statistical significance assessed by two-way ANOVA. The global difference between genotypes was assessed by using linear regression to fit a linear curve to the data and to determine whether the slopes were statistically different (Prism GraphPad, San Diego, CA).
2.5 |. Constipation assay
Standard cornmeal-agar Drosophila medium was melted by microwaving briefly and then mixed with blue food coloring (AmeriColor, Placentia, CA) at a ratio of approximately 1:10 volumes to create uniformly dark blue food. The same batch of food was used for experimental and control groups. Adult flies were aged to 10 days, then transferred to vials with blue food for 24 hr. Flies were then transferred back to standard food, and the percent of blue excrement to total excrement was measured on an hourly basis for 8 hr. Statistical significance was determined by one-way ANOVA of the area under the curve for each genotype. Additionally, flies were photographed at 0, 2, and 4-hr timepoints to demonstrate delayed gut transit of the blue food.
2.6 |. Statistics
All statistical analysis aside from that used for RNAseq data analysis was performed using GraphPad Prism version 7.0a. In cases of multiple comparisons, Tukey’s multiple comparisons test was used to determine statistical significance.
2.7 |. RNA-Seq
Adult flies were aged to 10 days. Four biological replicates per genotype, each consisting of six fly heads (three male, three female), were used. Fly heads were homogenized in Qiazol (Qiagen, Germantown, MD) and phase separated with chloroform. The aqueous phase was mixed with 100% ethanol at 1:1 ratio then purified on RNeasy columns (Qiagen) per the manufacturer’s protocol. Stranded libraries for next-generation sequencing were prepared in the Harvard Biopolymers core facility by depleting total RNA of ribosomal RNA using the Directional RNA-Seq Wafergen system (Wafergen, Fremont, CA). All RNA samples were run on Agilent 2100 TapeStation D1000 High Sensitivity ScreenTape to assess concentration and size distribution prior to library creation and again after library creation. Libraries were also subjected to qPCR analysis for quality control. Libraries were then paired-end sequenced on an Illumina NextSeq 500 instrument.
2.8 |. RNA-Seq data analysis
Computational analysis was performed on the Harvard Medical School O2 High-Performance Research Computing Cluster. The four raw sequence (.fastq) files generated by the NextSeq were concatenated for each library, then analyzed with FastQC (Babraham Bioinformatics, Cambridgeshire, UK). Count matrices were generated in R Studio Version 1.1.423 using the Bioconductor (Huber et al., 2015) package called bcbioRNAseq, an open source python framework that aggregates other best-practice pipelines for RNA-Seq analysis developed by the Harvard Chan Bioinformatics Core in the Harvard Chan School of Public Health (Steinbaugh et al., 2018). Within the bcbioRNAseq package, STAR (Dobin et al., 2013) was used to align the sequence reads to the Drosophila melanogaster Release 6 reference genome (BDGP6), and Salmon (Patro, Duggal, Love, Irizarry, & Kingsford, 2017) and featureCounts (Liao, Smyth, & Shi, 2014) were used to generate counts associated with known genes. Gene annotations were obtained from Ensembl. Quality of the RNA-Seq data was assessed with MultiQC (Ewels, Magnusson, Lundin, & Käller, 2016). This quality control includes total reads, mapping rate, genes detected, gene saturation, counts per gene, gene count distributions, principal component analysis (Jolliffe, 2002), and sample similarity. Plots were generated by ggplot2 (Wickham, 2016) and heatmaps were generated by pheatmap (Kolde, 2015). Principle component analysis demonstrated the strongest clustering by genotype (accounting for 43% of the variance). Based on the clustering analysis, one sample each from the Control, Glia, and Neuron conditions was excluded from further analysis, leaving a minimum of three remaining biological replicates per genotype. Transcript quantification files produced by Salmon were imported into the DESeq2 package (Love, Huber, & Anders, 2014) and pair-wise differential expression across conditions was analyzed using a generalized linear model implemented in DESeq2. A pseudo-count of 1 was added to all genes with an expression count of 0. Expression counts for all mapped genes are included in Data File S3. Differentially expressed genes had a fold-change between conditions of ≥2 and FDR <0.05. Gene ontology analysis (release August 9, 2018) (Ashburner et al., 2000; Mi et al., 2017; The Gene Ontology Consortium, 2017) and PANTHER classification (version 13.1; Mi, Muruganujan, & Thomas, 2013; Thomas et al., 2003) was performed to identify enriched terms among differentially expressed genes and annotate genes. Mammalian orthologs for Drosophila genes were determined using the Drosophila RNAi Screening Center (DRSC) Integrative Ortholog Prediction Tool (DIOPT) version 7.1 (Hu et al., 2011).
2.9 |. qRT-PCR
Selected genes identified as differentially expressed in the RNA-Seq analysis were validated by qRT-PCR. Primers for these genes were chosen from the DRSC FlyPrimerBank (Hu et al., 2013). Twenty brains per genotype were dissected in PBS and pooled. Total RNA was prepared with Qiazol (Qiagen) per the manufacturer’s instructions then treated with DNase for 15 min. cDNA was prepared using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), then amplified with SYBR green on a StepOne Plus machine (Applied Biosystems). Relative expression was determined using the ΔΔCt method, with normalization to RPL32 used as a housekeeping gene.
2.10 |. Single cell transcriptome atlas
Selected genes identified as differentially expressed in the RNA-Seq analysis were mapped to a recently published Drosophila single-cell transcription atlas (Davie et al., 2018) using a publicly available tool: scope.aertslab.org. Marker lists for glial subpopulations were downloaded from this tool and used to annotate gene lists.
2.11 |. Data availability
Raw and final RNA-Seq data is available through Gene Expression Omnibus (GEO), accession number GSE128120. All other data that support the findings of this study are available from the corresponding author upon reasonable request (Olsen et al., 2019).
3 |. RESULTS
3.1 |. Independent expression of α-synuclein in neurons and glia
We have recently published a Drosophila model of neuronal α-synucleinopathy in which wild-type human α-synuclein is expressed under the control of a pan-neuronal driver, Syb-QF2, using the Q binary expression system (C. J. Potter et al., 2010; Riabinina et al., 2015). These flies have widespread neurodegeneration, α-synuclein aggregation, death of dopaminergic neurons, and impaired motor function (Ordonez et al., 2018). To examine the effects of glial α-synuclein expression, we employed the similar GAL4 binary expression system (Brand & Perrimon, 1993) to drive expression of wild type human α-synuclein under the pan-glial driver, repo-GAL4 (Sepp et al., 2001). These systems are independent of one another, allowing us to express human α-synuclein in neurons (elav positive cells), glia (repo positive cells), or both cell types (Figure S1a,c,d). Of note, when examined at the whole brain protein level by immunoblotting, expression of α-synuclein is not appreciably higher when expressed in neurons and glia as opposed to neurons alone (Figure S1b), which may reflect strong expression driven by Syb-QF2.
3.2 |. Glial α-synuclein impairs locomotion
Motor symptoms are the defining clinical feature of parkinsonism, and motor dysfunction has been previously demonstrated in Drosophila models of Parkinson’s disease in the form of impaired climbing (Feany & Bender, 2000; Ordonez et al., 2018), walking (Chen, Wilburn, Hao, & Tully, 2014; Pokrzywa et al., 2017), and proboscis extension (Cording et al., 2017). Using our model of glial α-synucleinopathy, we compared locomotor behavior in control flies to flies expressing α-synuclein in glia, neurons, or both cell types. Specifically, we developed a novel walking-based locomotion assay. Briefly, flies are transferred to clean, empty vials, allowed to acclimate for 1 min, and then gently tapped three times to evoke the startle-induced locomotion response (Liao, Morin, & Ahmad, 2014; Ma, Stork, Bergles, & Freeman, 2016; Riemensperger et al., 2013; Yamamoto et al., 2008). The percentage of flies still walking after a 15-s delay is recorded, and differences between control and neuronal α-synuclein flies are highly reproducible over time (Figure S2a) and correlate with our previously published climbing assay (Figure S2b).
Using this locomotion assay, we performed a 21-day time course and determined that glial α-synuclein alone causes a locomotor deficit, and it also exacerbates the effect of neuronal α-synuclein (Figure 1). To ensure that this effect is specific to glial α-synuclein and not simply due to overexpression of an exogenous protein in glia, we repeated this experiment at Day 10, expressing green fluorescent protein (GFP) rather than α-synuclein in glia. GFP expression had no effect on locomotion (Figure S3). We then went further, expressing the R79H mutant of glial fibrillary acidic protein (GFAP), which causes the human astrogliopathy Alexander disease. We have previously shown that R79H GFAP expression in Drosophila glia causes noncell-autonomous toxicity to glutamatergic and other neurons in an Alexander disease model (L. Wang et al., 2011), but at the 10-day timepoint examined glial R79H GFAP expression did not enhance neuronal α-synuclein toxicity as measured by the locomotion assay (Figure S3), demonstrating specificity of glial α-synuclein in exacerbating the neuronal α-synuclein phenotype.
FIGURE 1.
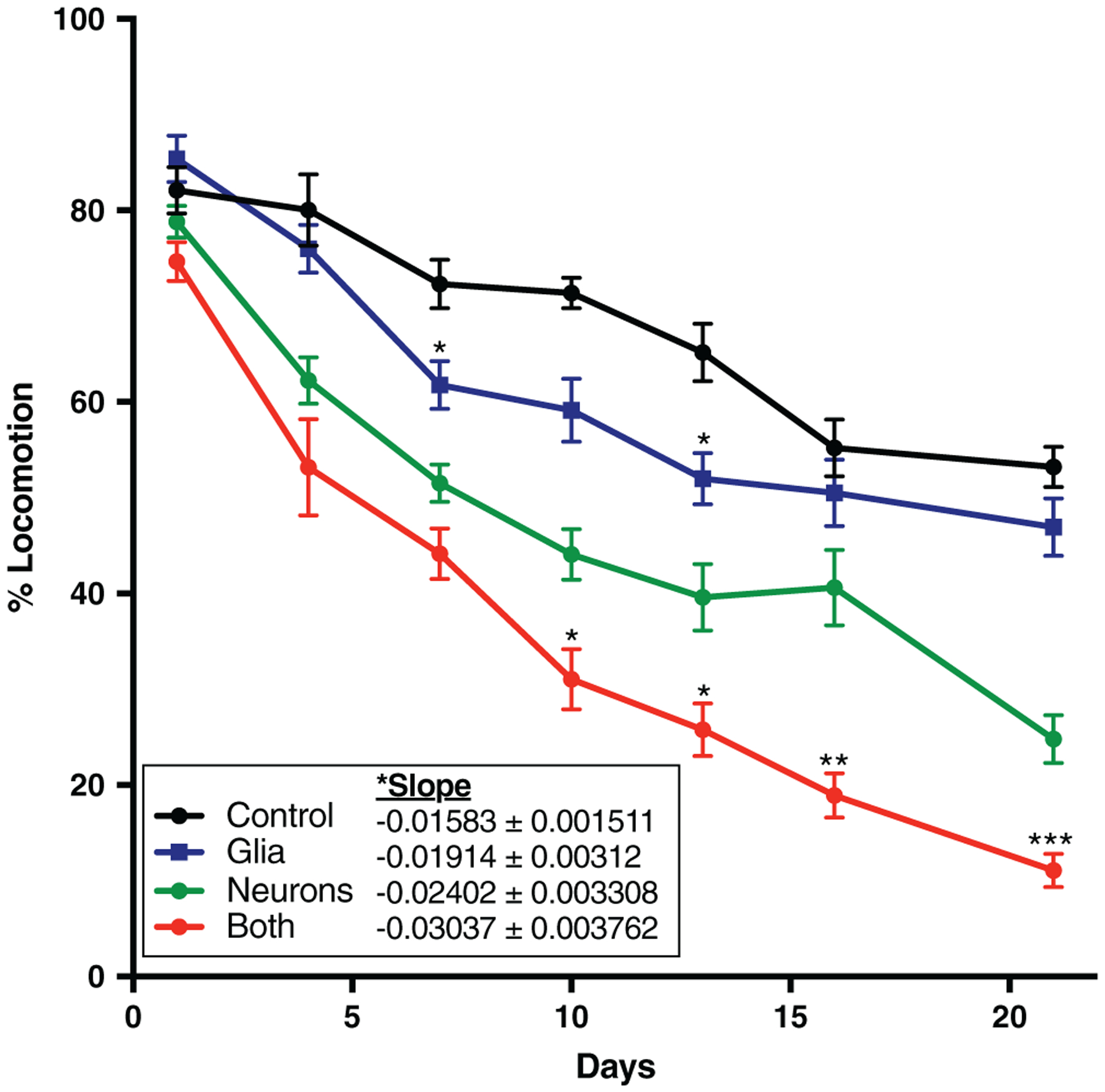
Glial α-synuclein impairs locomotion. Flies were subjected to a gentle tapping stimulus followed by a 15-s delay. The percentage of flies still in motion (% locomotion) following the delay was recorded and averaged over six technical replicates. Symbols above the “Glia” and “Both” curves represent statistically significant difference compared to the “Control” and “Neurons” curves, respectively, at a given time point. Slope of the line was determined by linear regression analysis and was also globally statistically significantly different between the four conditions. *p < .05, **p < .01, ***p < .005. n = minimum of 60 flies per genotype per time point (six biological replicates of 10 flies each)
3.3 |. Glial α-synuclein causes constipation
Autonomic nervous system dysfunction is common in all of the α-synucleinopathies, and constipation, in particular, may predate the onset of motor symptoms by many years (Adams-Carr et al., 2016). Nonmotor symptoms, including constipation, are a significant clinical problem, being more highly correlated with impaired patient quality of life than are motor symptoms (Estrada-Bellmann, Camara-Lemarroy, Calderon-Hernandez, Rocha-Anaya, & Villareal-Velazquez, 2016; Müller, Assmus, Herlofson, Larsen, & Tysnes, 2013; Prakash, Nadkarni, Lye, Yong, & Tan, 2016). The innervation of the gut in Drosophila is similar to that in mammals in that it is complex, involving efferent and afferent neurons, with contribution from both the central and peripheral nervous system (Cognigni, Bailey, & Miguel-Aliaga, 2011). We assessed whether glial α-synuclein contributes to constipation in Drosophila. In these experiments, 10-day-old files were housed in vials with blue food for 24 hr, then transferred to vials with regular food. Photographs of individual representative flies were taken at time 0, 2, and 4 hr (Figure 2a) demonstrating delayed gut transit for α-synuclein expressing flies compared to control, and markedly delayed gut transit for flies expressing α-synuclein in both neurons and glia. Additionally, the ratio of blue excrement to total excrement was measured per hour up to 8 hr (Figure 2b), also demonstrating the same phenomenon at a population level. That is, glial α-synuclein alone induces constipation to a similar extent as neuronal α-synuclein, and it exacerbates that induced by neuronal α-synuclein.
FIGURE 2.
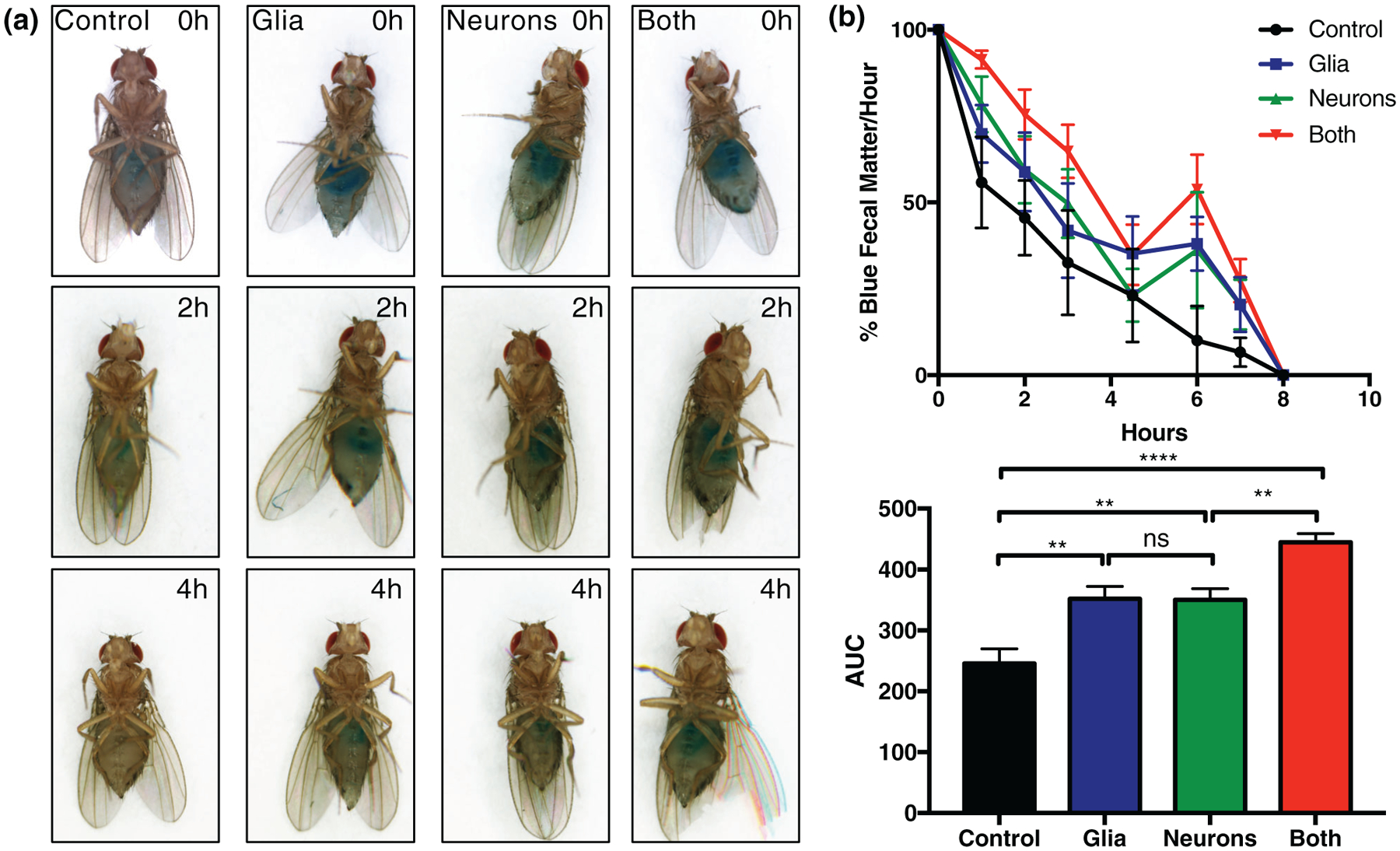
Glial α-synuclein causes constipation. Flies were aged to 10 days of life, transferred to blue colored food for 24 hr, then returned to regular food. (a) Photographs were taken at 0, 2, and 4 hr after return to regular food. (b) The % of blue to total fecal matter was counted on an hourly basis for 8 hr after return to regular food (left). Area under the curve is statistically significantly different between conditions as measured by one-way ANOVA (right). **p < .01, ****p < .001. n = minimum six biological replicates of 10 flies each
3.4 |. Glial α-synuclein causes death of total and dopaminergic neurons, but not glial cells
Neuronal death in varying cortical and subcortical regions occurs to differing extents in all of the α-synucleinopathies, and death of dopaminergic neurons in the substantia nigra pars compacta is a defining pathologic feature of Parkinson’s disease. We therefore sought to determine whether glial α-synuclein contributes to neuronal death generally and specifically to death of dopaminergic neurons. We first examined vacuolization, which is seen in patients with dementia with Lewy bodies (Sherzai et al., 2013) and is a common consequence of neurodegeneration in Drosophila (Kretzschmar, 2009; Sunderhaus & Kretzschmar, 2016; Wittmann et al., 2001). Glial α-synuclein caused infrequent, large vacuoles, whereas neuronal α-synuclein led to more numerous smaller vacuoles (Figure 3a). Glial α-synuclein alone induced neuron loss (quantified in Figure 3c) and exacerbated the loss of neurons when added to neuronal α-synuclein. More strikingly, glial α-synuclein alone caused loss of dopaminergic neurons and dopaminergic neurons were markedly reduced when both glial and neuronal were present (Figure 3b,d). Interestingly, the degree of loss of dopaminergic neurons due to glial α-synuclein was out of proportion to the total neuron loss (compare degree of change between Glia and Control or Both and Neuron in Figure 3c,d), consistent with differential vulnerability of dopaminergic neurons to glial α-synuclein. In contrast, quantitative examination of repo-stained sections did not reveal a clear difference in the number of glial cells between conditions (Figure S4), suggesting that there is no marked loss of this population but rather that glial dysfunction is responsible for the pathogenic effects of glial α-synuclein.
FIGURE 3.
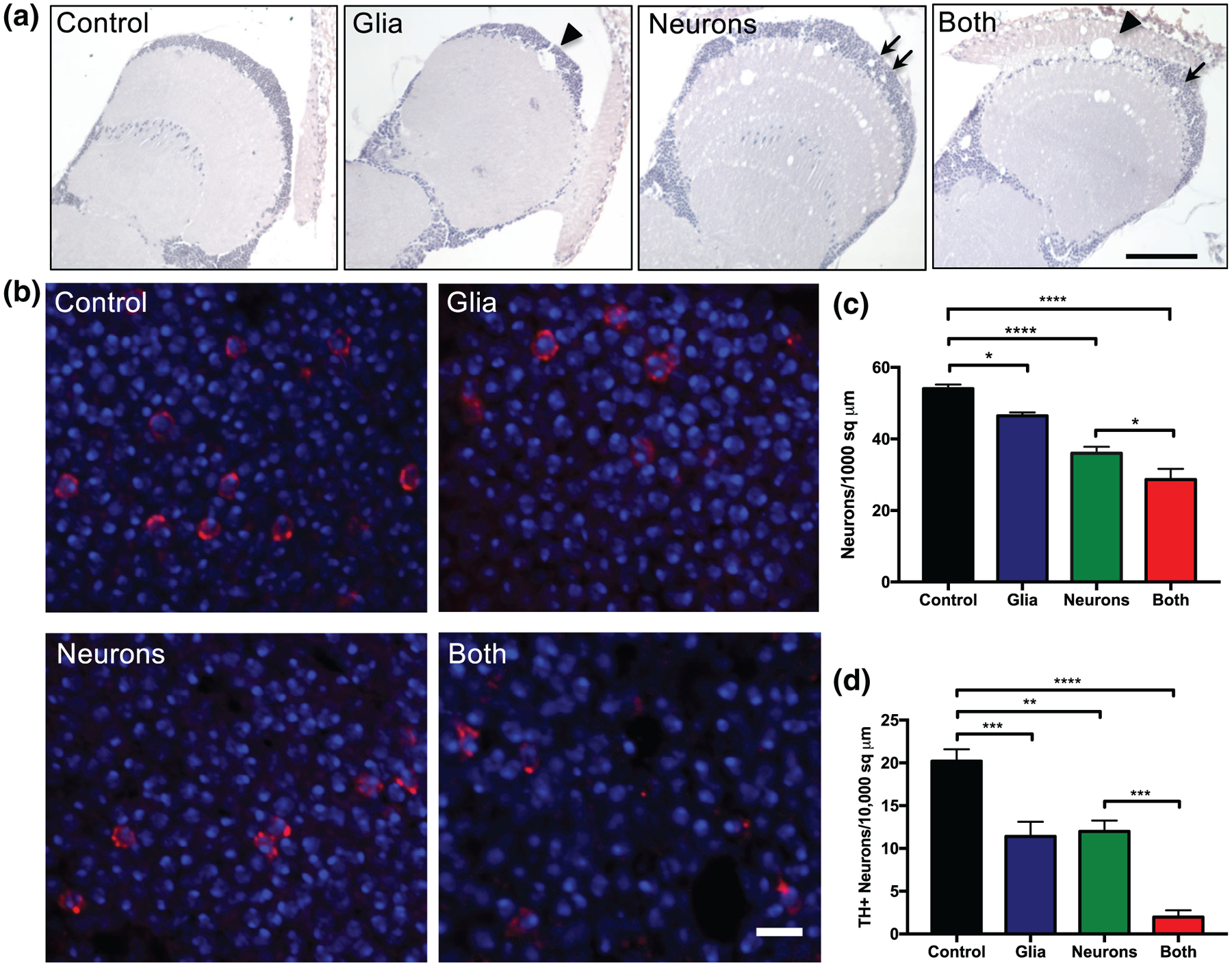
Glial α-synuclein causes neurodegeneration. (a) Optic lobe sections stained with hematoxylin demonstrating vacuolization, an indicator of neurodegeneration. Glial α-synuclein caused infrequent large vacuoles (arrowhead) whereas neuronal α-synuclein caused frequent small vacuoles (arrows). Scale bar = 100 μm. (b) Representative anterior medulla sections stained with DAPI (blue) and tyrosine hydroxylase antibody (red, mouse, 1:200, Immunostar) to indicate dopaminergic neurons. Scale bar = 5 μm. (c) Quantification of total neurons from hematoxylin stained slides of anterior medulla (not shown), n = 6 replicates per genotype. (d) Quantification of dopaminergic neurons from anterior medulla, n = 6 replicates per genotype. *p < .05, **p < .01, ***p < .005, ****p < .001, determined with one-way ANOVA
3.5 |. α-Synuclein aggregates in both neurons and glia
α-Synucleinopathies are, by definition, diseases of pathologic α-synuclein aggregation, which occurs to varying extents in neurons and glia depending on the specific disease. We identified α-synuclein aggregates in both neurons and glia in the conditions in which it was expressed in those cell types. Figure 4a demonstrates α-synuclein aggregates in neurons (identified by the marker elav), and Figure 4b demonstrates α-synuclein aggregates in glia (identified by the marker repo). Total α-synuclein aggregates were quantified from low power sections of cortex surrounding the optic lobe (Figure 4c). Interestingly, α-synuclein inclusions in dopaminergic neurons were increased when α-synuclein was present in both neurons and glia as opposed to in neurons alone (Figure 4d), suggesting that the presence of glial α-synuclein is able to perpetuate further α-synuclein aggregation in dopaminergic neurons in a noncell-autonomous manner. Such noncell-autonomous effects have been seen previously in a mouse model of MSA, in which overexpression of human α-synuclein in oligodendrocytes was shown to induce aggregation of endogenous mouse α-synuclein in neurons (Yazawa et al., 2005).
FIGURE 4.
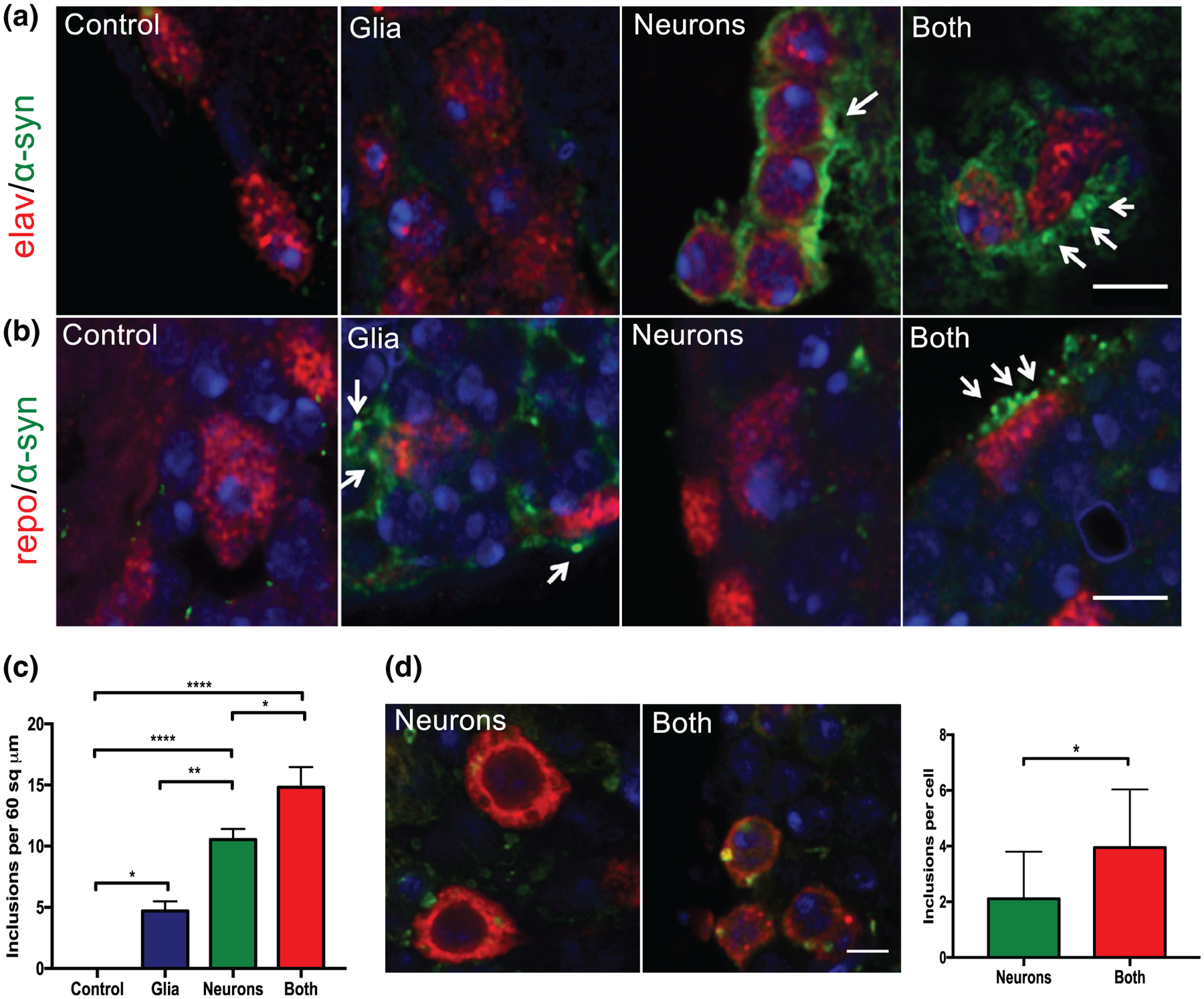
α-Synuclein aggregates in neurons and glia. (a) Immunofluoresence for DAPI (blue), elav (red, mouse 1:5, DSHB), and α-synuclein (green, rabbit 1:1000) by confocal microscopy (3 μm scale). Arrows indicate α-synuclein inclusions in neurons. (b) Immunofluoresence for DAPI (blue), repo (red, mouse 1:5, DSHB), and α-synuclein (green, rabbit 1:1000) by confocal microscopy (3 μm scale). Arrows indicate α-synuclein inclusions in glia. (c) Quantification of total aggregates from optic lobe cortex, n = 5–six flies per genotype. (d) Representative immunofluorescence for tyrosine hydroxylase (red, mouse, 1:200, Immunostar), α-synuclein (green, rat, 1:10,000, Biolegend), and DAPI. Scale bar = 5 μm. Inclusions are quantified in the right panel
3.6 |. α-Synuclein induced transcriptional changes depend on cellular context
Having demonstrated that glial α-synuclein enhances both the clinical phenotype and pathologic hallmarks of the α-synucleinopathies, we next sought to identify whether it altered gene expression. We expressed human wild type α-synuclein in glia, neurons, or both cell types and performed RNA-Seq on whole heads. Differentially expressed genes were identified by pair-wise comparisons of each α-synuclein condition compared to negative control, hereafter referred to as Glia: Control, Neuron:Control, or Both:Control. Interestingly, the effects of α-synuclein on transcription were markedly different depending on whether the protein was expressed in glia or neurons. Glial α-synuclein resulted in 158 differentially expressed genes compared to control flies lacking α-synuclein (Figure 5a, Data File S1). Nearly all the differentially expressed genes were upregulated (144/157, 92%). In contrast, neuronal α-synuclein resulted in 128 differentially expressed genes compared to control flies, and nearly all of these were downregulated (125/128, 98%; Figure 5A, Data File S1). Furthermore, there is very little overlap between the differentially expressed genes induced by glial versus neuronal α-synuclein (Figure 5b), suggesting that the cellular context of α-synuclein expression matters significantly for gene expression. When α-synuclein was expressed in both neurons and glia, 359 transcripts were differentially expressed (Figure 5a, Data File S1). The majority of these were downregulated (350/358 = 98%) and they include the vast majority of transcripts that were downregulated with neuronal α-synuclein alone, as well as many additional transcripts (Figure 5c). Of the overlapping transcripts that were downregulated with neuronal α-synuclein alone as well as when α-synuclein was present in both neurons and glia, they were downregulated to a greater degree with both glial and neuronal α-synuclein (Figure 5d).
FIGURE 5.
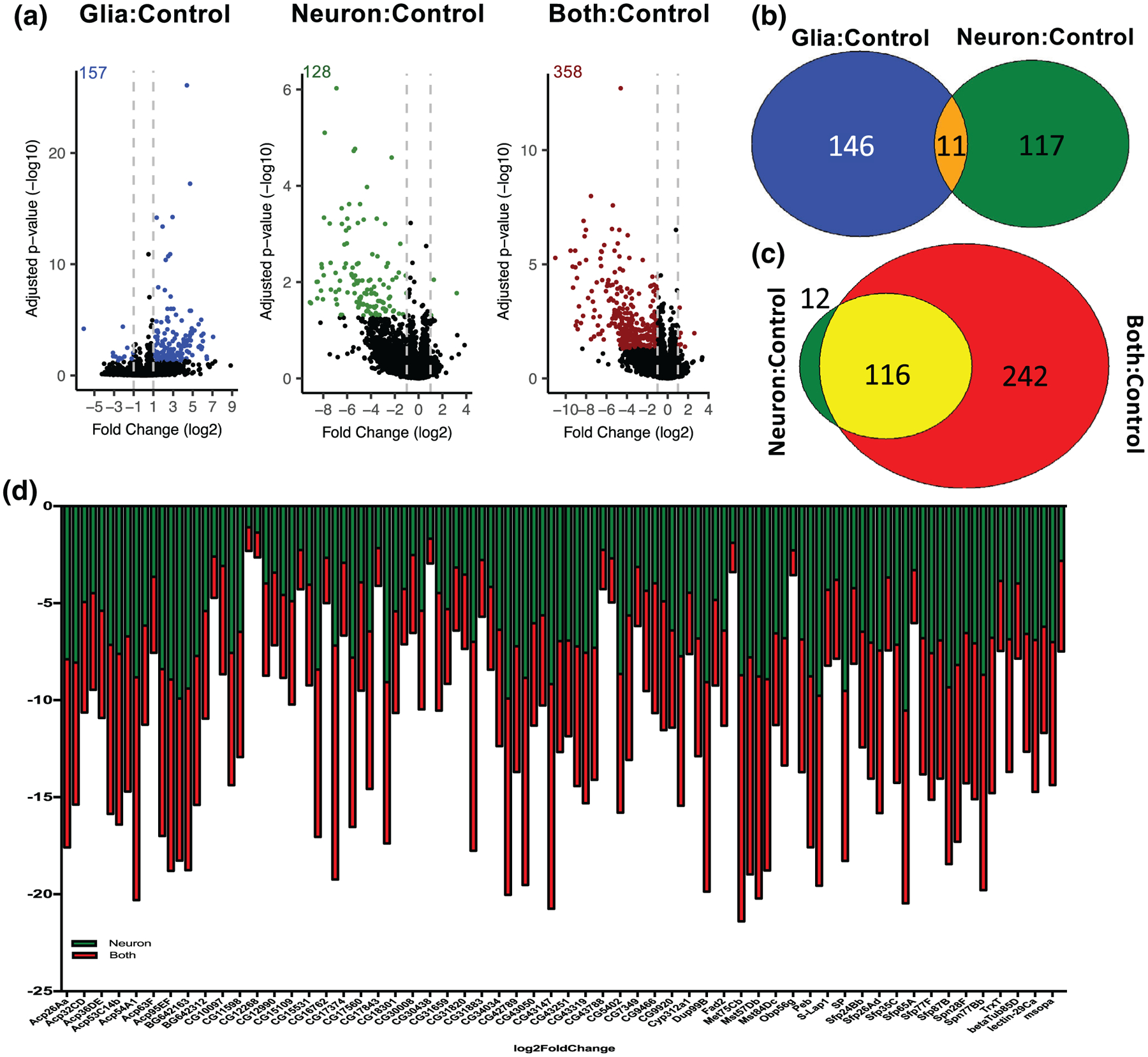
Transcriptional changes induced by α-synuclein depend on its cellular context. Bulk RNA-Seq from whole brains was performed on 10-day old flies. (a) Volcano plots demonstrating transcript expression changes. Colored dots (and numbers) represent statistically significant (padjust < .05 after correction for multiple comparisons) differentially expressed transcripts with ≥|1| log2fold change. (b) Venn diagram demonstrating little overlap between differentially expressed genes induced by glial and neuronal α-synuclein. (c) Venn diagram demonstrating significant similarity in differentially expressed genes with both glial and neuronal α-synuclein compared to neuronal α-synuclein alone. (d) When both glial and neuronal α-synuclein are present a common set of transcripts are further downregulated as compared to neuronal α-synuclein alone
We performed gene ontology analysis for all conditions (see Data File S2 for full gene ontology results). With glial α-synuclein, enriched biological process terms included proteolysis and cell surface receptor signaling (Figure 6a). The proteolysis term is enriched due to expression of many extracellular proteases as well as two caspases (Table 1, Figure 6b). Many of these proteases were not previously known to be expressed in the brain, and their upregulation may reflect a glial response to injury (Purice et al., 2017) or alternatively might represent the senescence-associated secretory phenotype, which may contribute to neurodegeneration (Bussian et al., 2018). The cell surface receptor signaling term is enriched partly due to expression of several tetraspanins (Table 2, Figure 6b), which are of particular interest given that their human orthologs (CD9, CD81, and TSPAN2) are markers of oligodendrocytes or oligodendrocyte precursors (Terada et al., 2002). We confirmed expression of a subset of both the proteases and cell signaling receptor transcripts by qRT-PCR (Figure S5a). Since differentially expressed transcripts could be either neuronal or glial in origin, and we are particularly interested in glial changes, we used a newly created single-cell Drosophila brain transcriptome atlas (Davie et al., 2018) to annotate transcripts that were identified in that study as markers of various glial subpopulations (Table 3). In the Glia:Control comparison, we identified 10 upregulated glial markers. We further investigated one of these, Ance, which is also one of the proteolysis-related genes (Figure 6b) and is of particular interest as it is the Drosophila ortholog of angiotensin-converting enzyme (ACE), and ACE inhibitors have been explored as possible therapeutics in Parkinson’s disease (Reardon, Mendelsohn, Chai, & Horne, 2000; Sonsalla et al., 2013). Ance expression is enriched in (but not limited to) subperineurial glia, as shown in silico using the single cell transcriptome atlas (Figure S5b). Additionally, we used Ance-GAL4 to drive GFP expression to assess further the pattern of Ance expression. These flies demonstrated GFP expression in the head (Figure S5c), and we confirmed a glial expression pattern by immunohistochemistry (Figure S5d).
FIGURE 6.
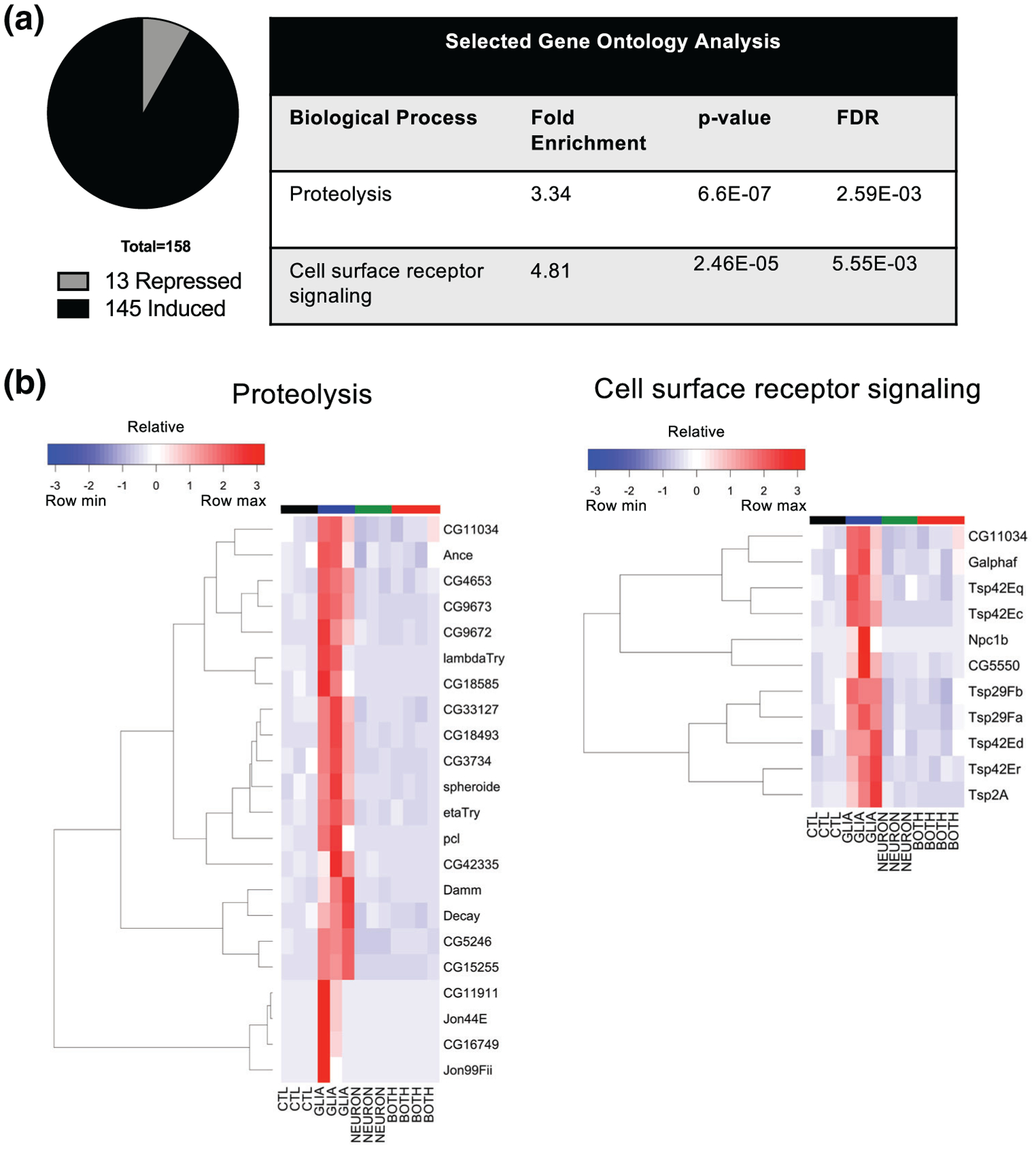
Transcriptional changes induced by glial α-synuclein include upregulation of proteolysis and cell surface receptor signaling. Bulk RNA-Seq from whole brains was performed on 10-day old flies. (a) Gene ontology analysis for upregulated transcripts demonstrates enrichment of the terms “proteolysis” and “cell surface receptor signaling”. (b) Hierarchical clustering of proteolysis and cell surface receptor signaling related transcripts
TABLE 1.
Proteolysis related genes upregulated in Glia:Control
| Gene | PANTHER protein class | Best human ortholog |
|---|---|---|
| Jon44E | Unclassified | C1S, PROC |
| Jon99Fii | Unclassified | PROC |
| CG11911 | Serine protease | PRSS36, PRSS53 |
| pcl | Aspartic protease | REN, CTSE, CTSD, NAPSA |
| CG16749 | Serine protease | KLK3 |
| CG15255 | Metalloprotease | MEP1B, MEP1A |
| lambdaTry | Serine protease | PRSS53, PRSS36 |
| CG18493 | Serine protease | PRSS16 |
| CG42335 | Metalloprotease | TRHDE, LVRN, ANPEP |
| CG18585 | Metalloprotease | CPB1 |
| Damm | Cysteine protease (caspase) | CASP3, CASP7, CASP6 |
| CG9672 | Serine protease | PRSS56, F10, F9, F7, PROZ, PROC |
| etaTry | Serine protease | PRSS36, PRSS53 |
| CG5246 | Unclassified | KLK14, OVCH1, PRSS3, CFD, OVCH2 |
| CG9673 | Serine protease | TPSD1 |
| CG4653 | Serine protease | PRSS36, PRSS53 |
| CG3734 | Serine protease | PRSS16 |
| Decay | Cysteine protease (caspase) | CASP3 |
| Spheroide | Serine protease | PRSS36, PRSS53, PRSS38 |
| CG33127 | Serine protease | None |
| CG11034 | Serine protease | DPP4 |
| Ance | Metalloprotease | ACE |
TABLE 2.
Cell surface receptor signaling related genes upregulated in Glia:Control
| Gene | Best human ortholog |
|---|---|
| Npc1b | NPC1 |
| Tsp42Ec | TSPAN2, CD81, CD9, TSPAN19, CD63 |
| CG5550 | FCN3, FCN1 |
| Tsp2a | UPK1B, CD37, TSPAN8, TSPAN4, UPK1A, CD82, TSPAN18, TSPAN19, TSPAN1, TSPAN9, CD53 |
| Tsp42Eq | CD63 |
| Tsp42Er | TSPAN2, CD81, CD9 |
| Tsp29Fa | CD63 |
| CG11034 | DPP4 |
| Galphaf | GNAL |
| Tsp29Fb | CD63 |
| Tsp42Ed | CD63 |
TABLE 3.
Differentially expressed genes identified as glial markers in Davie et al
| Gene Glia:Control | Glial population | Best human ortholog |
|---|---|---|
| Pdxk | Chiasm glia, astrocyte like glia, ensheathing glia, cortex glia, perineurial glia | PDXK |
| Jheh3 | Chiasm glia, astrocyte-like glia, ensheathing glia, cortex glia | EPHX1 |
| CG4562 | Subperineurial glia | ABCC4 |
| Tsp42Ed | Chiasm glia, astrocyte-like glia, cortex glia, supperineurial glia, perineurial glia | CD63 |
| Ance | Subperineurial glia | ACE |
| CG8785 | Subperineurial glia | SLC36A4 |
| scb | Perineurial glia | ITGA4 |
| Oatp33Ea | Cortex glia | SLCO2A1 |
| CG4301 | Subperineurial glia, cortex glia, perineurial glia | ATP11B |
| CG32368 | Ensheathing glia, subperineurial glia, cortex glia, perineurial glia, chiasm glia | None |
| Neuron:Control | ||
| CG9507 | Astrocyte like glia, ensheathing glia, cortex glia | KEL |
| Obp44a | Chiasm glia, astrocyte like glia, Ensheathing glia, cortex glia | None |
| Both:Control | ||
| CG31272 | Subperineurial glia | SV2A |
| CG11892 | Ensheathing glia | None |
| Zasp52 | Cortex glia | LDB3 |
| CG1208 | Subperineurial glia | SLC2A8 |
| CG34423 | Astrocyte like glia, chiasm glia, ensheathing glia, subperineurial glia, perineurial glia | ATP5IF1 |
| CG8630 | Subperineurial glia | SCD |
| Lectin-46Cb | Chiasm glia | CLEC9A |
| CG44243 | Ensheathing glia, perineurial glia | LIPT1 |
| CG2765 | Ensheathing glia, cortex glia | THRSP |
| CG1674 | Subperineurial glia | SYNPO, SYNPO2, SYNPO2L |
| Mlp84B | Subperineurial glia | CSRP3 |
| CG14401 | Subperineurial glia | None |
In contrast to glial α-synuclein, neuronal α-synuclein led to downregulation of many transcripts (Figures 5a and 7a). Gene ontology on these transcripts revealed many terms regulated to mating and hormones (Figure 7a), which is explained due to downregulation of several members of four families of genes: Accessory gland protein (Acp), seminal fluid protein (Sfp), serpin (Spn), and odorant binding protein (Obp) families (Data File S1). Although the Acp and Sfp protein families are named for their expression in the male accessory gland and seminal fluid, respectively, they along with the Spn superfamily are composed mostly of protease inhibitors, raising the possibility of their being repurposed in the brain for protein homeostasis. Indeed, after the mating-related terms, the next enriched term by gene ontology analysis was negative regulation of endopeptidase activity (Figure 7a). We validated expression of several of these genes in the brain either by qRT-PCR (Figure 7b) or in silico analysis using the single cell transcriptome atlas (Figure 7c), where importantly, there were no sex-specific differences in their expression (Figure 7c). The fourth family contributing to the enrichment of mating-related terms is the Obp family. Obp proteins transport odorants to olfactory receptors. This is of some interest given the known olfactory deficits in the α-synucleinopathies, with PD pathology thought to start early in the olfactory bulbs (Del Tredici & Braak, 2016). Following mating-related terms and negative regulation of endopeptidase activity, the third biological process that was over-represented involved genes regulated to lipid metabolism (Table 4, Figure 7d). This finding is consistent with prior studies by our group (Scherzer, Jensen, Gullans, & Feany, 2003) and others (Don et al., 2014; Schafferer et al., 2016) that also identified changes in lipid metabolism related genes due to α-synuclein.
FIGURE 7.
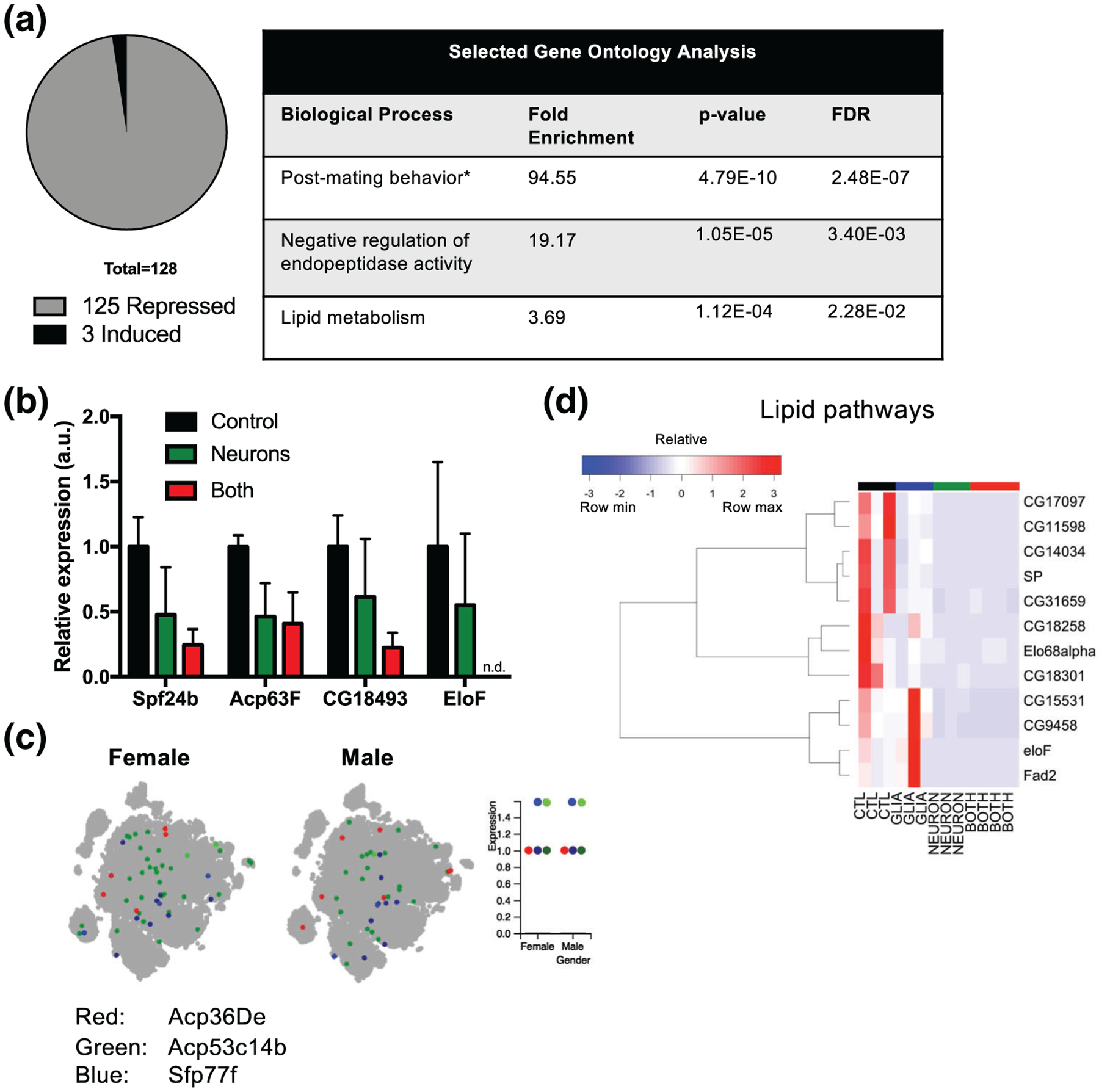
Confirmation of transcriptional changes induced by neuronal α-synuclein. (a) Gene ontology analysis. *Other reproduction-related terms beyond “post-mating behavior” were also enriched (Data file S2). (b) qRT-PCR for male and lipid-related genes. Values in Neuron and Both are normalized to Control. n = 2–3 biological replicates. (c) Visualization of selected Acp and Sfp gene expression in single cell transcriptome atlas. The dot plot represents expression. For both Sfp77f and Acp53c14b, there is a high and low expressing population, indicated by dots that are the same color but different intensity. (d) Hierarchical clustering of lipid related genes. All genes were significantly differentially expressed with adjusted p-value < .05
TABLE 4.
Lipid metabolism related genes reduced in Neuron:Control
| Gene | PANTHER protein class | Best human ortholog |
|---|---|---|
| SP | NONE | |
| CG18258 | Esterase, lipase, storage protein | LIPG, LIPL, LIPC |
| CG17097 | LIPK, LIPJ, LIPF, LIPN, LIPA, LIPM | |
| eloF | Acyltransferase | ELOVL1 |
| CG11598 | Lipase, serine protease | LIPK, LIPJ, LIPF, LIPN, LIPA, LIPM |
| Fad2 | SCD, SCD5 | |
| CG18301 | LIPM | |
| CG31659 | APOD | |
| Elo68alpha | Acyltransferase | ELOVL4 |
| CG14034 | LPL | |
| CG9458 | Acyltransferase | ELOVL7 |
| CG15531 | SCD |
As mentioned above, in the Both:Control comparison, the list of differentially expressed genes contains nearly all of the genes found Control in the Neuron:Control comparison (Figure 5c), and similarly, gene ontology analysis reveals several enriched terms that are mating-related (Data File S2). Beyond these shared genes and terms, however, there are an additional 242 differentially expressed genes in the Both:Control comparison that are not seen in the Neuron:Control comparison (Figure 5c). Additionally, gene ontology analysis in the Both:Control group reveals numerous additional enriched terms related to fatty acid metabolism (Data File S2). Among the fatty acid metabolism-related genes, there are 6 fatty acyl-CoA reductases, 5 fatty acid elongases, 9 genes known to localize to the peroxisome, and several additional enzymes with oxidoreductase activity (Table 5). Of note, there are two orthologs of stearoyl CoA desaturase (SCD), recently implicated as a therapeutic target for PD (Fanning et al., 2018; Vincent et al., 2018). The majority (though not all) of these fatty acid metabolism genes are downregulated, and they are also downregulated in the Neuron:Control comparison (Figure 8a), although only 10/29 reached statistical significance in that condition. In addition to the many terms related to fatty acid metabolism, gene ontology analysis also revealed other enriched terms, including myofibril assembly, muscle α-actinin binding, and sperm flagellum. The component transcripts responsible for these terms being enriched are cytoskeletal genes (Figure 8b, Table 6), which is of interest given our work (Ordonez et al., 2018) as well as the work of others (Chung et al., 2017; Esposito, Dohm, Kermer, Bähr, & Wouters, 2007; Khurana et al., 2017; Sousa et al., 2009) implicating dysfunction of the actin cytoskeleton in α-synuclein neurotoxicity. Similar to the fatty acid metabolism-related genes, the cytoskeletal genes were also downregulated in Neuron:Control (Figure 8b), though none reached statistical significance in that condition. Collectively, these data suggest that glial α-synuclein both potentiates the transcriptional effects of neuronal α-synuclein and also induces unique transcriptional changes.
TABLE 5.
Fatty acid metabolism and peroxisome genes reduced in Both:Control
| Gene | GO notes | Best human ortholog |
|---|---|---|
| Cyp6a18 | Oxygenase | TBXAS1 |
| eloF | Fatty acid elongation/acyltransferase | ELOVL1 |
| CG16904 | Fatty acid elongation/acyltransferase | ELOVL7 |
| Cyp4g1 | Oxygenase | CYP4V2 |
| Uro | Uricase | Nonea |
| CG6690 | Oxidase | QSOX2 |
| CG17560 | Fatty acyl-CoA reductase, localizes to peroxisome | FAR2 |
| CG10097 | Fatty acyl-CoA reductase, localizes to peroxisome | FAR2 |
| CG17562 | Fatty acyl-CoA reductase, localizes to peroxisome | FAR2 |
| CG5103 | None | |
| CG15531 | SCD | |
| CG13091 | Fatty acyl-CoA reductase, localizes to peroxisome | FAR2 |
| CG9458 | Fatty acid elongation/acyltransferase | ELOVL7 |
| CG5122 | Acetyltransferase, acyltransferase, localizes to peroxisome | CRAT |
| CG6432 | Dehydrogenase | ACSS3 |
| CG9459 | Fatty acid elongation/acyltransferase | ELOVL7 |
| Elo68alpha | Fatty acid elongation/acyltransferase | ELOVL4 |
| CG8517 | Oxidoreductase | None |
| CG31413 | Oxidase | QSOX2 |
| Cyp312a1 | Oxygenase | CYP4B1, CYP4F11, CYP4F22, CYP4F12 |
| CG4020 | Fatty acyl-CoA reductase, localizes to peroxisome | FAR1 |
| CG10096 | Fatty acyl-CoA reductase, localizes to peroxisome | FAR2 |
| CG17843 | QSOX2 | |
| CG9314 | Peroxidase, localizes to peroxisome | CAT |
| CG34172 | Oxidase | COX7A1 |
| Gld | CHDH | |
| CG8630 | SCD | |
| Se | GSTO1 | |
| Lsp1beta | Oxidase, Oxygenase | None |
Mouse ortholog is urate oxidase. This gene has been inactivated in humans due to mutation. Lack of the enzyme means that urate is the end product of purine metabolism in humans and represents a major antioxidant, with lower urate levels serving as a potential biomarker of Parkinson’s disease (Cipriani, Chen, & Schwarzschild, 2010).
FIGURE 8.
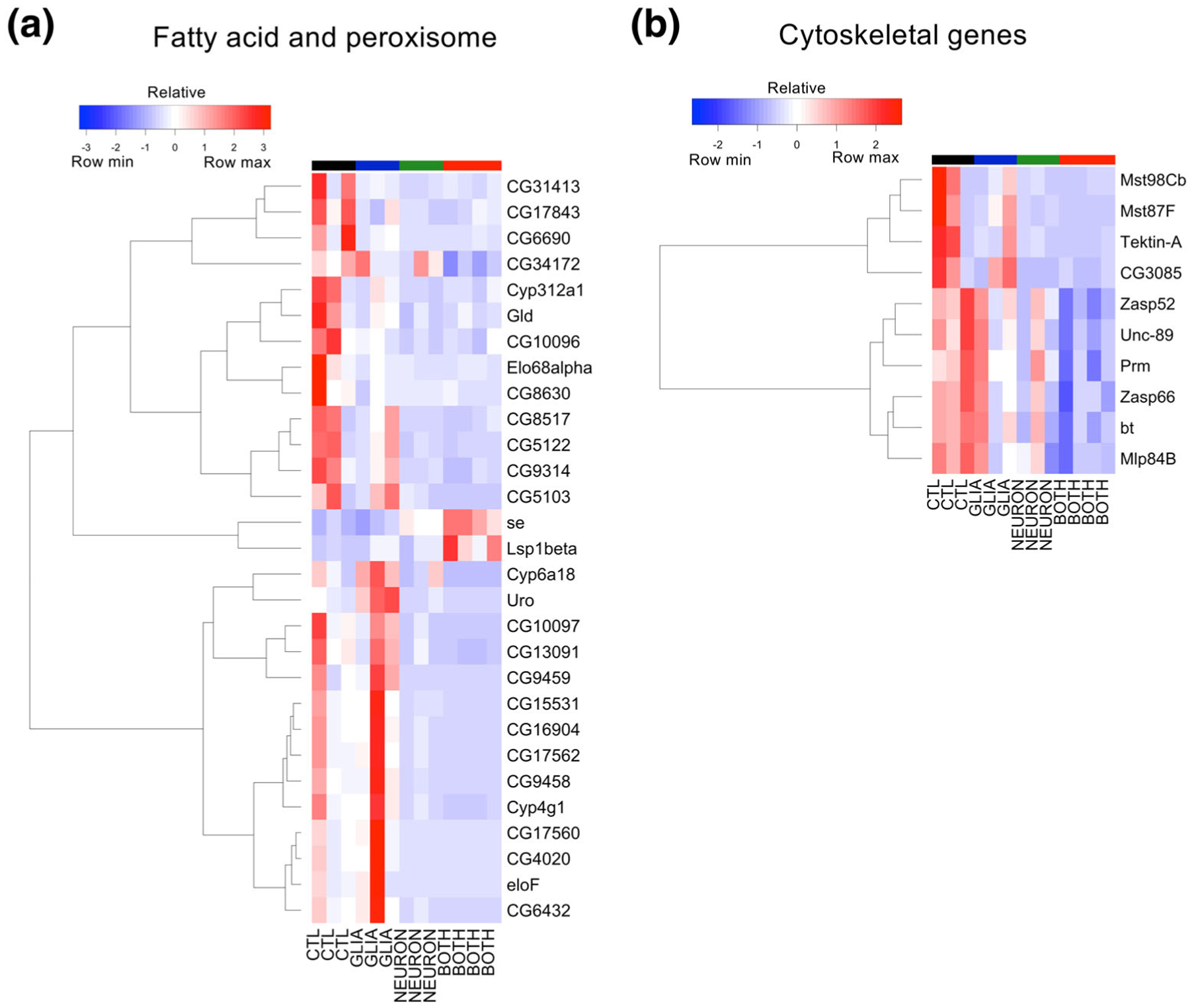
Fatty acid metabolism and cytoskeletal genes downregulated by glial and neuronal α-synuclein. (a) Hierarchical clustering of fatty acid metabolism and peroxisome related genes. (b) Hierarchical clustering of cytoskeletal related genes. The top six that cluster together are those that contribute to the GO terms “myofibril assembly” and “muscle alpha-actinin binding,” whereas the lower four contribute to the GO term “sperm flagellum assembly”
TABLE 6.
Cytoskeletal genes reduced in Both:Control
| Gene | GO notes | Best human ortholog |
|---|---|---|
| Mst87F | None | |
| Mst98Cb | None | |
| Tektin-A | Nonmotor microtubule binding protein | TEKT4 |
| CG3085 | Nonmotor microtubule binding protein | TEKT2 |
| Zasp52 | Actin family cytoskeletal protein | LDB3 |
| Zasp66 | Alpha-actinin binding | PDLIM1, PDLIM3 |
| Unc-89 | Tropomyosin binding, calcium-dependent kinase | SPEG |
| Prm | Paramyosin | MYH1 |
| bt | Myosin binding | TTN |
| Mlp84B | Actin family cytoskeletal protein | CSRP3 |
3.7 |. Relevance of the Drosophila model for mammalian α-synucleinopathy models and human disease
Drosophila serves as a powerful model organism for investigating human neurodegeneration in large part due to the high conservation of disease-related genes (McGurk, Berson, & Bonini, 2015; Rubin et al., 2000). To explore the relevance of our differentially expressed genes, we identified their rat, mouse, and human orthologs and cross-referenced this list with additional publicly available lists of differentially expressed genes identified in transcriptomic studies of MSA animal models (Kaji et al., 2018; Schafferer et al., 2016) or human post-mortem MSA brains (Langerveld, Mihalko, DeLong, Walburn, & Ide, 2007; Mills, Ward, Kim, Halliday, & Janitz, 2016). We also compared the ortholog list to genes that have been identified as candidate risk genes for any human α-synucleinopathy by examining genome-wide association or whole exome sequencing studies from MSA (X. Gu et al., 2018; Sailer et al., 2016), PD (Chang et al., 2017; Guo et al., 2018; Jansen et al., 2017; Li et al., 2018; Quadri et al., 2015; Robak et al., 2017; Sandor et al., 2017; Schormair et al., 2018; Shulskaya et al., 2018; Siitonen et al., 2017; Ylönen et al., 2017), or DLB (Guerreiro et al., 2018; Keogh et al., 2016; Peuralinna et al., 2015). In total, we found 30 transcripts with a mammalian ortholog that had also been identified in one or more of these studies (Table 7). This list includes orthologs that fell into nine pathways that have been implicated in pathogenesis of human α-synucleinopathies (Figure 9 and Discussion), suggesting that our model can be used to study relevant aspects of human disease pathophysiology.
TABLE 7.
Genes implicated in MSA or PD pathogenesis in humans or animal models
| Gene | Condition | Relevant ortholog | Gene name | Identified by | Ortholog score | Notes/potential mechanisms |
|---|---|---|---|---|---|---|
| Veil | Glia:Control | Nt5e (mouse) | 5′-Nucleotidase Ecto | Schafferer et al. | 14 | Nt5e converts AMP to adenosine. Caffeine, an adenosine antagonist, is inversely associated with risk of development of PD (Hernán, Takkouche, Caamaño-lsorna, & Gestal-Otero, 2002; Noyce et al., 2012). Adenosine A2A receptor knockout mice are protected in animal models of PD (Kachroo & Schwarzschild, 2012; Xu et al., 2016), and adenosine antagonists are in clinical used for Parkinson’s disease in Japan (Kondo, Mizuno,, & Japanese Istradefylline Study Group, 2015). Nt5e expression is altered in PD post-mortem brains (Garcia-Esparcia, Hernandez-Ortega, Ansoleaga, Carmona, & Ferrer, 2015). |
| Fa2h | Glia:Control | Fa2h (mouse) | Fatty acid 2-hydroxylase | Schafferer et al. | 13 | Mutations in FA2H are associated with neurologic diseases including familial leukodystrophy, levodopa-responsive hereditary spastic paraplegia SPG35, and neurodegeneration with brain iron accumulation (NBIA; Kruer et al., 2010; Scheid et al., 2013; Schneider & Bhatia, 2010; Soehn et al., 2016). The Fa2h knockout mouse (K. A. Potter et al., 2011) has demyelination, axon loss, cerebellar abnormalities, and memory deficits. |
| CG9314 | Both:Control | CAT (human) | Catalase | Langerveld et al. | 12 | Catalase protects cells from ROS by metabolizing H2O2. It is downregulated in A53T α-synuclein mice (Yakunin et al., 2014). α-synuclein induced H2O2 induces microglial migration toward aggregates (S. Wang et al., 2015). PD patients have reduced catalase activity in the substantia nigra (Ambani, Van Woert, & Murphy, 1975), and catalase containing nanoparticle delivery to brain has been explored as a therapeutic strategy in PD animal models (Klyachko et al., 2017). |
| Hsc70–1 | Both:Control | HSPA8 (human) | Heat shock cognate 71 kDa protein | Langerveld et al. | 11 | Hsp70 is a chaperone protein that breaks down α-synuclein fibrils in vitro (Gao et al., 2015) and is upregulated in mouse models of PD (Mak, McCormack, Manning-Bog, Cuervo, & Di Monte, 2010). It may also increase extracellular release of α-synuclein (Fontaine et al., 2016). |
| CG5703 | Both:Control | NDUFV2 (human) | NADH:Ubiquinone Oxidoreductase Core subunit V2 | Langerveld et al. | 11 | NDUFV2 is a subunit of the mitochondrial complex 1 respiratory chain. Rare mutations have been reported as a cause of familial PD (Nishioka et al., 2010), and variants are associated with idiopathic PD in small studies (Hattori, Yoshino, Tanaka, Suzuki, & Mizuno, 1998; Mizuta et al., 2008; Swerdlow et al., 2006). The transcript was downregulated in PD patient CSF (Hossein-Nezhad et al., 2016). |
| Npc1b | Glia:Control | NPC1 (human) | NPC intracellular cholesterol transporter 1 | Shulskaya et al. | 10 | Mutations in NPC1 cause the lysosomal storage disease Niemman Pick type Cl, which may predispose to α-synuclein pathology (Saito, Suzuki, Hulette, & Murayama, 2004). |
| CG30438 | Glia:Control | Ugt8a (mouse) | UDP galactosyltransferase 8A | Schafferer et al. | 10 | Ugta8a knockout mice have unstable myelin, progressive demyelination and severe motor coordination deficits (Coetzee et al., 1996). |
| Neuron:Control | ||||||
| Both:Control | ||||||
| Pci | Glia:Control | CTSD (rat), CTSD (human) | Cathepsin D | Kaji et al. (Ctsd), Robak et al. (CTSD) | 6a | Mutations in CTSD cause neuronal ceroid lipofuscinosis (Myllykangas et al., 2005). CTSD cleaves α-synuclein and protects against α-synuclein aggregation and toxicity (Cullen et al., 2009; Kiely et al., 2018; Qiao et al., 2008). |
| Itgbetanu | Glia:Control | Itgb1 (rat) | Integrin beta-1 | Kaji et al. | 6 | Beta 1 integrin is a subunit of many integrin receptors. It promotes microglial migration toward α-synuclein (Kim et al., 2014). It also promotes oligodendrocyte adhesion to fibronectin (Tsuboi et al., 2005), myelin formation (Camara et al., 2009), and dopaminergic neurite outgrowth (Izumi et al., 2017). |
| CG5278 | Glia:Control | ELOVL7 (human) | Elongation of very long chain fatty acids protein 7 | Sailer et al., Chang et al. | 6 | ELOVL7 is a fatty acid elongase. Mutations in yeast orthologs of fatty acid elongases enhance α-synuclein toxicity (Lee, Wang, Slone, Yacoubian, & Witt, 2011). Inhibiting the fatty acid desaturase SCD or its yeast ortholog OLE1 reduces levels of oleic acid and rescues α-synuclein toxicity in model organisms (Fanning et al., 2018; Vincent et al., 2018). |
| Neuron:Control | ||||||
| Both:Control | ||||||
| CG16904 | Both:Control | ELOVL7 (human) | Elongation of very long chain fatty acids protein 7 | Sailer et al., Chang et al. | 6 | |
| CG9458 | Neuron:Control | ELOVL7 (human) | Elongation of very long chain fatty acids protein 7 | Sailer et al., Chang et al. | 5 | |
| Both:Control | ||||||
| CG30008 | Neuron:Control | ELOVL7 (human) | Elongation of very long chain fatty acids protein 7 | Sailer et al., Chang et al. | 5 | |
| Both:Control | ||||||
| CG9459 | Both:Control | ELOVL7 (human) | Elongation of very long chain fatty acids protein 7 | Sailer et al., Chang et al. | 5 | |
| Npc2e | Glia:Control | Npc2 (mouse) | Npc intracellular cholesterol transporter 2 | Schafferer et al. | 5 | Mutations in NPC2 cause the lyosomal storage disease Niemann Pick type C2, but heterozygotes have been reported to have a parkinsonism syndrome (Kluenemann, Nutt, Davis, & Bird, 2013). |
| Oatp33Ea | Glia:Control | Slco2a1 (mouse) | Solute carrier organic anion transporter family member 2A1 | Schafferer et al. | 4 | Slco2a1 is a prostaglandin receptor expressed on microglia and endothelial cells that may play a role in neuroinflammation (Nakamura etal., 2018). |
| CGI5534 | Glia:Control | SMPD1 (human) | Sphingomyelin phosphodiesterase 1 | Robak et al. | 4 | SMPD1 mutations cause Niemann-Pick disease type A and B. Rare variants have been associated with PD in many small genetic studies prior to Robak et al. (reviewed in [Deng, Xiu, & Jankovic, 2015]). |
| Decay | Glia:Control | Casp3 (rat) | Caspase 3 | Kaji et al. | 4 | Caspases regulate apoptosis. Inhibiting caspase 3 is protective in rat PD models (Y. Liu et al., 2013; Yuan, Ren, Wang, He, & Zhao, 2016). |
| Damm | Glia:Control | Casp3 (rat) | Caspase 3 | Kaji et al. | 4 | |
| Spn38F | Neuron:Control | Serpinbla (mouse) | Serine (or cysteine) peptidase inhibitor, clade B, member 1a | Schafferer et al. | 4a | See below for further discussion on Serpin family members. |
| Both:Control | ||||||
| Cyp312al | Neuron:Control | CYP4F12 (human) | Cytochrome P450 4F12 | Mills et al. | 4a | CYP4F12 is a cytochrome P450 family member that localizes to the endoplasmic reticulum and oxidizes arachidonic acid. |
| Both:Control | ||||||
| CG14034 | Neuron:Control | Lpl (mouse) | Lipoprotein lipase | Schafferer et al. | 2 | Lipoprotein lipases hydrolyze long-chain triglycerides. Lpl knockout mice develop α-synuclein aggregates (Yang et al., 2015). |
| Both:Control | ||||||
| CG18258 | Neuron:Control | Lpl (mouse) | Liprotein lipase | Schafferer et al. | 1a | |
| Both:Control | ||||||
| Spn77Bb | Neuron:Control | SERPINA3 (human), SERPINA1 (human), Serpinbla (mouse) | Serpin family A member 3, Serpin family A member 1, serine (or cysteine) peptidase inhibitor, clade B, member 1a | Mills etal. (SERPINA3); Schafferer et al. (Serpinbla); Siitonen et al. (SERPINA1) | 1a | Serpin family members are protease inhibitors that participate in a wide variety of biological processes including inflammatory signaling cascades. Modified serpinA1 may be a biomarker for PD dementia (Halbgebauer et al., 2016). |
| Both:Control | ||||||
| Spn77Bc | Both:Control | SERPINA3 (human), SERPINA1 (human), Serpinbla (mouse) | Serpin family A member 3, Serpin family A member 1, serine (or cysteine) peptidase inhibitor, clade B, member 1a | Mills etal. (SERPINA3); Schafferer et al. (Serpinbla); Siitonen et al. (SERPINA1) | 1a | |
| CG9568 | Glia:Control | Cd59a (mouse) | CD59a antigen | Schafferer et al. | 1a | CD59a is a complement receptor. Complement is used by microglia to prune synapses in development (Schafer 2012) and disease (Hong et al., 2016) and causes formation of neurotoxic astrocytes (Liddelow et al., 2017). |
| Both:Control | ||||||
| CG15635 | Neuron:Control | MAGED4 (human) | MAGE family member D4 | Langerveld et al. | 1a | MAGED4 enhances E3 ubiquitin ligase activity. |
| Both:Control | ||||||
| CG9672 | Glia:Control | Prss56 (mouse) | Serine protease 56 | Schafferer et al. | 1a | Prss56 is a serine protease important for eye development. |
| CG43897 | Both:Control | PDLIM2 (human), Pdlim2 (mouse) | PDZ and LIM domain 2 | Chang et al. (PDLIM2), Schafferer et al. (Pdlim2), | 1a | PDLIM2 interacts with the actin cytoskeleton and promotes anchorage-independent growth and cell migration. |
| Ptp52F | Glia:Control | PTPRH (human) | Protein tyrosine phosphatase, receptor type H | Jansen et al. | 1a | PTPRH is a transmembrane phosphatase. Loss of function variants enhances α-synuclein toxicity in Drosophila (Jansen et al., 2017). |
Abbreviations: H2O2: hydrogen peroxide; ROS: reactive oxygen species.
There are multiple equally ranked orthologs for this gene. Those genes with mechanistic evidence supporting a causal role in α-synucleinopathy pathogenesis are indicated in bold.
FIGURE 9.
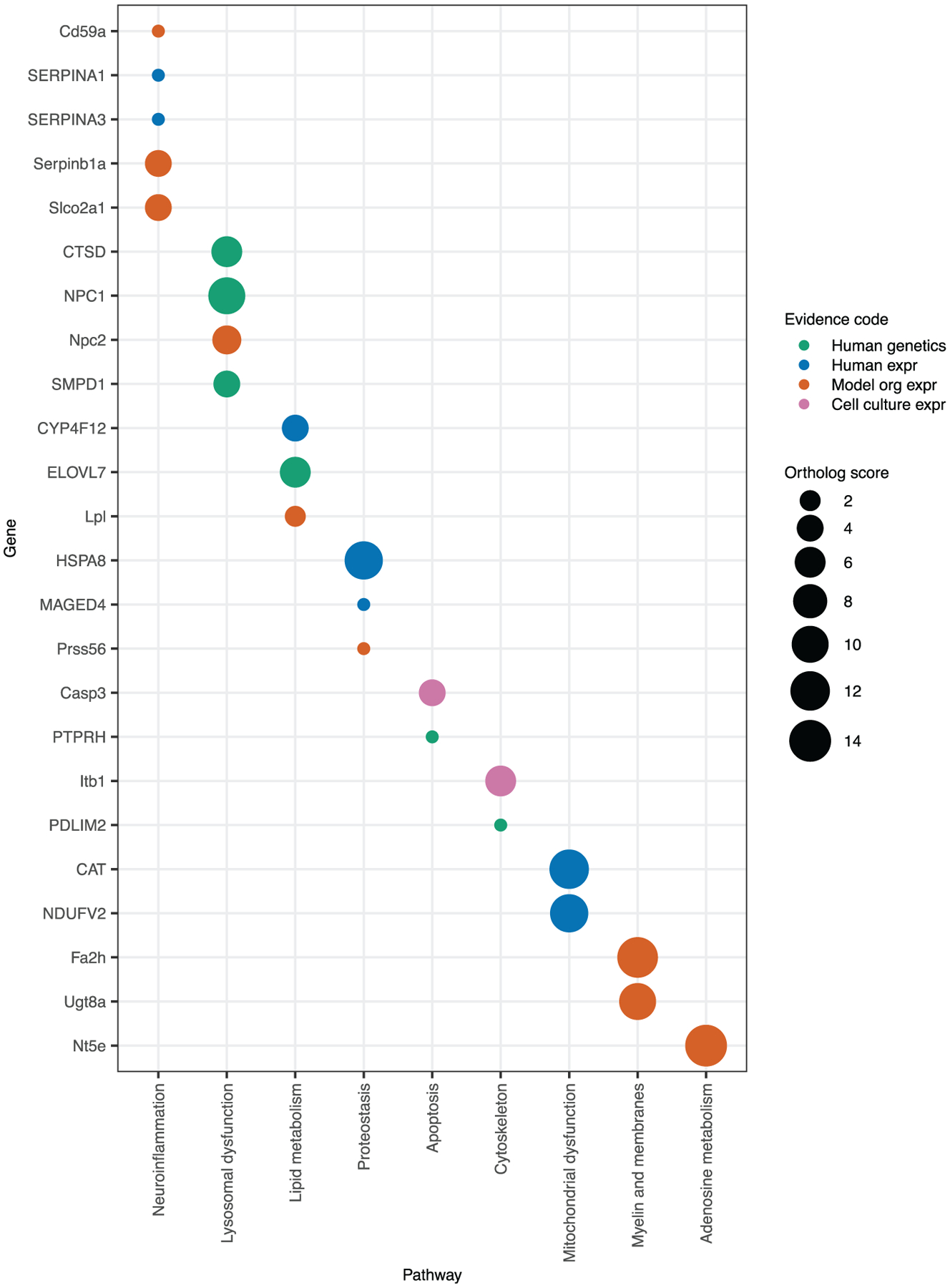
Drosophila RNAseq identifies conserved targets and essential pathways in α-synucleinopathy pathogenesis. Mammalian orthologs of Drosophila genes were identified using DRSC Integrative Ortholog Prediction Tool. Orthologs have a ranked score from 1 to 14 indicating the degree of conservation (with 14 being the best). Orthologs that have been previously reported in MSA transcriptomic studies or in human α-synucleinopathy genome wide association studies (GWAS) or whole exome sequencing (WES) studies are shown. The type of evidence is indicated by the color of the circle. Human genetics, human α-synucleinopathy GWAS or WES; Human expr, expression is changed in human MSA patients; Model org expr, expression is changed in a mouse model of MSA; Cell culture expr, expression is changed in a rat oligodendrocyte model of MSA. Orthologs fall into nine pathways of known relevance to human α-synucleinopathies
4 |. DISCUSSION
Here we describe a novel Drosophila model of glial α-synucleinopathy. We demonstrate that glial α-synuclein forms inclusions, increases aggregation of α-synuclein within dopaminergic neurons, impairs locomotion, causes constipation, and triggers neurodegeneration of both dopaminergic and nondopaminergic neurons. Furthermore, we demonstrate that α-synuclein can induce unique transcriptional programs depending on the cell type in which it is expressed. One striking finding from our RNA-Seq results was how different the transcriptional signatures are between the three conditions, including not only the specific genes that were differentially expressed but also the fact that glial α-synuclein alone led to upregulation of many genes, whereas many genes were downregulated in the other conditions. In fact, only three transcripts were upregulated in the Neuron:Control condition and two of these are known glial markers (Table 3). We have previously reported that nuclear α-synuclein inhibits histone acetylation (Kontopoulos, Parvin, & Feany, 2006), and this inhibition would be expected to decrease transcription. Others have reported direct interactions between α-synuclein and histones (Goers et al., 2003) or DNA (Siddiqui et al., 2012), as well as wide-ranging transcriptional deregulation (Pinho et al., 2019). α-Synuclein-induced transcriptional changes to remain a ripe area for future study, particularly as single cell RNA-Seq becomes more accessible.
Among the differentially expressed genes that we identified across all conditions, we found 30 transcripts that have been reported either as having altered expression in mammalian models of MSA or human patients with MSA or reported as being genetic risk factors for MSA or PD (Table 7). These transcripts are involved in pathways known to be essential for α-synucleinopathy pathogenesis, including mitochondrial function, lysosomal function, myelin synthesis, cytoskeletal function, fatty acid metabolism, apoptosis, and adenosine metabolism (Figure 9). There are six genes meeting the highest level of evidence for a causal role in human disease, having been identified in human GWAS or by WES: CTSD, ELOVL7, NPC1, SMPD1, PTPRH, and PDLIM2 (Figure 9). Additionally, there is direct mechanistic evidence in animal models to support a causative role for several genes in α-synucleinopathy pathogenesis. Genes with animal model evidence are bolded in Table 7 and include CAT, HSPA8, CTSD, Itgb1, Casp3, Lpl, and PTPRH. Furthermore, while there is not yet direct evidence for ELOVL7 in α-synucleinopathy pathogenesis, loss of function mutants in yeast orthologs of ELOVL7 have been shown to exacerbate α-synuclein toxicity (Lee et al., 2011). Beyond these 30 transcripts, we identified an additional novel 298 differentially expressed genes with human orthologs in the Glia:Control or Both:Control conditions, leaving many candidates for future studies.
In addition to being one of very few published transcriptomic studies of glial α-synuclein expression, our model reproduces important α-synuclein induced phenotypes. Autonomic dysfunction is a core clinical feature underlying all of the α-synucleinopathies and includes bladder dysfunction, constipation, cardiovascular abnormalities, sexual dysfunction, sialorrhea, dry eyes, excessive sweating, and altered thermoregulation. These symptoms are a greater contributor to loss of quality of life than are the motor symptoms in PD (Estrada-Bellmann et al., 2016; Martinez-Martin, Rodriguez-Blazquez, Kurtis, Chaudhuri,, & NMSS Validation Group, 2011; Müller et al., 2013; Prakash et al., 2016; Tibar et al., 2018), yet have been challenging to investigate in model organisms. Although common to all of the α-synucleinopathies, autonomic dysfunction is particularly important in MSA (Fanciulli & Wenning, 2015), where it is required to make the diagnosis (Gilman et al., 2008). Only one mouse model of MSA has been reported to have autonomic features (Boudes et al., 2013), and our Drosophila model represents a valuable new model further investigating autonomic dysfunction in vivo.
Another interesting pathologic finding in our model is that dopaminergic neurons were lost at a disproportionate rate to total neurons when glial α-synuclein was present, either alone or in conjunction with neuronal α-synuclein. There are several hypotheses as to why dopaminergic neurons are uniquely susceptible to injury in the α-synucleinopathies (Surmeier, Obeso, & Halliday, 2017), including their long, thin, unmyelinated axons with extensive arborization (Matsuda et al., 2009), reliance on calcium homeostasis and susceptibility to oxidative stress (Duda, Pötschke, & Liss, 2016; Tabata et al., 2018), and the inherent toxicity of dopamine (Burbulla et al., 2017; Mor et al., 2017). Additionally, dopaminergic neurons are highly reliant on support from astrocytes (Datta, Ganapathy, Razdan, & Bhonde, 2018; Du, Yu, Chen, Chen, & Yan, 2018; Kuter, Olech, Głowacka, & Paleczna, 2019). However, neither the intrinsic characteristics of dopaminergic neurons nor the general phenomenon of astrocyte dysfunction fully explains why dopaminergic neurons degenerate specifically in α-synucleinopathies, as these features are also present in other neurodegenerative diseases that do not specifically affect dopaminergic neurons. This raises the possibility of noncell-autonomous toxic effects that are due specifically to glial α-synuclein, as has recently been shown with astrocyte LRRK2 G2019S (di Domenico et al., 2019). In accordance with this, we demonstrate that glial α-synuclein increases aggregation of α-synuclein in dopaminergic neurons. This finding could be explained by a noncell-autonomous effect of glial α-synuclein on neuronal proteostasis or alternatively, by direct spread of α-synuclein from glia to dopaminergic neurons. We have not observed spread of α-synuclein from glia to neurons when it is expressed in glia alone (data not shown). However, we cannot exclude the possibility of spread in the condition in which α-synuclein is expressed in both neurons and glia, as it is possible that neurons must first express α-synuclein themselves in order to be receptive to spread from glia.
In contrast to the marked loss of dopaminergic neurons, glial numbers were not significantly changed between conditions (Figure S4). Prior mammalian studies of glial α-synuclein expression have reported varied results in terms of whether glial α-synuclein expression induces glial cell death. For example, α-synuclein expression in astrocyte cell lines induces apoptosis (M. Liu et al., 2018; N. Stefanova, Klimaschewski, Poewe, Wenning, & Reindl, 2001), whereas a transgenic astrocytic A53T α-synuclein expressing mouse model demonstrated not loss of astrocytes but rather impaired astrocyte function including decreased glutamate transporter expression and blood–brain barrier disruption, leading to marked neurodegeneration (X.-L. Gu et al., 2010). Likewise, of the three transgenic mouse models of oligodendrocyte α-synuclein expression, only one demonstrates loss of oligodendrocytes (Yazawa et al., 2005). The others display mitochondrial abnormalities in oligodendrocytes without frank oligodendrocyte loss (Shults et al., 2005), or absence of oligodendrocyte loss (Kahle et al., 2002) unless the mice are additionally treated with the mitochondrial toxin 3-nitroprinoic acid (Nadia Stefanova et al., 2005). These varied effects of glial α-synuclein expression on glial cell death may stem from different expression levels and reflect the complexity of modeling glial α-synucleinopathy (Bleasel, Halliday, & Kim, 2016).
In summary, our work represents the first report of independent manipulation of gene expression in neurons and glia by combining the Q and GAL4 bipartite expression systems. This is a robust and flexible model that can be used to mechanistically analyze the genetic contributions of neurons and glia to neurodegenerative diseases in vivo at scale. Here we have used these genetic tools to create the first Drosophila model of glial α-synucleinopathy, demonstrating in the process that glial α-synuclein induces neurodegeneration and that cell context is critical for α-synuclein induced transcriptional changes. We further demonstrate that this system can be used to identify processes of known relevance to human diseases, including MSA. Beyond investigating the effects of glial α-synuclein, combining the Q and GAL4 expression systems represents a powerful method for dissecting glial and neuronal interactions in vivo in neurodegenerative diseases broadly.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. John Hutchinson of the Harvard Chan Bioinformatics Core, Harvard T.H. Chan School of Public Health, Boston, MA for assistance with the RNAseq analysis. The project was conducted with the support of Harvard Catalyst and the Harvard Catalyst (NIH award #UL1 RR 025758 and financial contributions from participating institutions). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We also thank Dr. Joanna DiSpirito for assistance with the RNAseq analysis. We thank the Neurobiology Department and the Neurobiology Imaging Facility for consultation and instrument availability that supported this work. This facility is supported in part by the Neural Imaging Center as part of an NINDS P30 Core Center grant #NS072030. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The elav-7E8A10 monocolonal antibody developed by G.M. Rubin of HHMI/Janelia Farm Research and the repo-8D12 monoloclonal antibody developed by C. Goodman of the University of California, Berkeley were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. The GFP N86/8 antibody was from the University of California, Davis/NIH NeuroMab Facility. Drosophila stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Funding information
Congressionally Directed Medical Research Programs, Grant/Award Number: PD W81XWH1810395; National Institute of Neurological Disorders and Stroke, Grant/Award Numbers: K08-NS109344-01, LRP L30NS108208-01, R01 NS098821, R21 NS0105151, R25 NS065743
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Adams-Carr KL, Bestwick JP, Shribman S, Lees A, Schrag A, & Noyce AJ (2016). Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery, and Psychiatry, 87(7), 710–716. 10.1136/jnnp-2015-311680 [DOI] [PubMed] [Google Scholar]
- Ambani LM, Van Woert MH, & Murphy S (1975). Brain peroxidase and catalase in Parkinson disease. Archives of Neurology, 32(2), 114–118. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, … Sherlock G (2000). Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nature Genetics, 25(1), 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, … Iwatsubo T (1998). Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. The American Journal of Pathology, 152(4), 879–884. [PMC free article] [PubMed] [Google Scholar]
- Beyer K, & Ariza A (2007). Protein aggregation mechanisms in synucleinopathies: Commonalities and differences. Journal of Neuropathology and Experimental Neurology, 66(11), 965–974. 10.1097/nen.0b013e3181587d64 [DOI] [PubMed] [Google Scholar]
- Bleasel JM, Halliday GM, & Kim WS (2016). Animal modeling an oligodendrogliopathy—Multiple system atrophy. Acta Neuropathologica Communications, 4, 12 10.1186/s40478-016-0279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth GE, Kinrade EF, & Hidalgo A (2000). Glia maintain follower neuron survival during Drosophila CNS development. Development (Cambridge, England), 127(2), 237–244. [DOI] [PubMed] [Google Scholar]
- Boudes M, Uvin P, Pinto S, Voets T, Fowler CJ, Wenning GK, … Stefanova N (2013). Bladder dysfunction in a transgenic mouse model of multiple system atrophy. Movement Disorders, 28(3), 347–355. 10.1002/mds.25336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Sastre M, & Del Tredici K (2007). Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathologica, 114(3), 231–241. 10.1007/s00401-007-0244-3 [DOI] [PubMed] [Google Scholar]
- Brand AH, & Perrimon N (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development (Cambridge, England), 118(2), 401–415. [DOI] [PubMed] [Google Scholar]
- Brück D, Wenning GK, Stefanova N, & Fellner L (2015). Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiology of Disease, 85, 262–274. 10.1016/j.nbd.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, … Krainc D (2017). Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science (New York, N.Y.), 357(6357), 1255–1261. 10.1126/science.aam9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, & Baker DJ (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature, 562(7728), 578–582. 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Câmara J, Wang Z, Nunes-Fonseca C, Friedman HC, Grove M, Sherman DL, … ffrench-Constant C (2009). Integrin-mediated axoglial interactions initiate myelination in the central nervous system. The Journal of Cell Biology, 185(4), 699–712. 10.1083/jcb.200807010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, … Graham RR (2017). A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nature Genetics, 49(10), 1511–1516. 10.1038/ng.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AY, Wilburn P, Hao X, & Tully T (2014). Walking deficits and centrophobism in an α-synuclein fly model of Parkinson’s disease. Genes, Brain, and Behavior, 13(8), 812–820. 10.1111/gbb.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Khurana V, Yi S, Sahni N, Loh KH, Auluck PK, … Lindquist S (2017). In situ peroxidase Labeling and mass-spectrometry connects alpha-synuclein directly to endocytic trafficking and mRNA metabolism in neurons. Cell Systems, 4(2), 242–250.e4. 10.1016/j.cels.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Chen X, & Schwarzschild MA (2010). Urate: A novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomarkers in Medicine, 4(5), 701–712. 10.2217/bmm.10.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, … Popko B (1996). Myelination in the absence of galactocerebroside and sulfatide: Normal structure with abnormal function and regional instability. Cell, 86(2), 209–219. [DOI] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, & Miguel-Aliaga I (2011). Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metabolism, 13(1), 92–104. 10.1016/j.cmet.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colodner KJ, & Feany MB (2010). Glial fibrillary tangles and JAK/-STAT-mediated glial and neuronal cell death in a Drosophila model of glial tauopathy. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(48), 16102–16113. 10.1523/JNEUROSCI.2491-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cording AC, Shiaelis N, Petridi S, Middleton CA, Wilson LG, & Elliott CJH (2017). Targeted kinase inhibition relieves slowness and tremor in a Drosophila model of LRRK2 Parkinson’s disease. NPJ Parkinson’s Disease, 3, 34 10.1038/s41531-017-0036-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, … Tyynelä J (2009). Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Molecular Brain, 2, 5 10.1186/1756-6606-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, … Parisi JE (2015). Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain: A Journal of Neurology, 138(Pt 8), 2293–2309. 10.1093/brain/awv114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta I, Ganapathy K, Razdan R, & Bhonde R (2018). Location and number of astrocytes determine dopaminergic neuron survival and function under 6-OHDA stress mediated through differential BDNF release. Molecular Neurobiology, 55(7), 5505–5525. 10.1007/s12035-017-0767-0 [DOI] [PubMed] [Google Scholar]
- Davie K, Janssens J, Koldere D, De Waegeneer M, Pech U, Kreft Ł, … Aerts S (2018). A single-cell Transcriptome atlas of the aging Drosophila brain. Cell, 174(4), 982–998.e20. 10.1016/j.cell.2018.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K, & Braak H (2016). Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathology and Applied Neurobiology, 42(1), 33–50. 10.1111/nan.12298 [DOI] [PubMed] [Google Scholar]
- Deng H, Xiu X, & Jankovic J (2015). Genetic convergence of Parkinson’s disease and lysosomal storage disorders. Molecular Neurobiology, 51(3), 1554–1568. 10.1007/s12035-014-8832-4 [DOI] [PubMed] [Google Scholar]
- DeSalvo MK, Hindle SJ, Rusan ZM, Orng S, Eddison M, Halliwill K, & Bainton RJ (2014). The Drosophila surface glia transcriptome: Evolutionary conserved blood-brain barrier processes. Frontiers in Neuroscience, 8, 346 10.3389/fnins.2014.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico A, Carola G, Calatayud C, Pons-Espinal M, Muñoz JP, Richaud-Patin Y, … Consiglio A (2019). Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson’s disease. Stem Cell Reports, 12, 213–229. 10.1016/j.stemcr.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, … Gingeras TR (2013). STAR: ultrafast universal RNA-Seq aligner. Bioinformatics (Oxford, England), 29(1), 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Taşdemir OE, & Freeman MR (2009). Ensheathing glia function as phagocytes in the adult Drosophila brain. The Journal of Neuroscience, 29(15), 4768–4781. 10.1523/JNEUROSCI.5951-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don AS, Hsiao J-HT, Bleasel JM, Couttas TA, Halliday GM, & Kim WS (2014). Altered lipid levels provide evidence for myelin dysfunction in multiple system atrophy. Acta Neuropathologica Communications, 2, 150 10.1186/s40478-014-0150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Yu Q, Chen A, Chen D, & Yan SS (2018). Astrocytes attenuate mitochondrial dysfunctions in human dopaminergic neurons derived from iPSC. Stem Cell Reports, 10(2), 366–374. 10.1016/j.stemcr.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda J, Pötschke C, & Liss B (2016). Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease. Journal of Neurochemistry, 139(Suppl 1), 156–178. 10.1111/jnc.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito A, Dohm CP, Kermer P, Bähr M, & Wouters FS (2007). Alpha-Synuclein and its disease-related mutants interact differentially with the microtubule protein tau and associate with the actin cytoskeleton. Neurobiology of Disease, 26(3), 521–531. 10.1016/j.nbd.2007.01.014 [DOI] [PubMed] [Google Scholar]
- Estrada-Bellmann I, Camara-Lemarroy CR, Calderon-Hernandez HJ, Rocha-Anaya JJ, & Villareal-Velazquez HJ (2016). Non-motor symptoms and quality of life in patients with Parkinson’s disease in Northeastern Mexico. Acta Neurologica Belgica, 116(2), 157–161. 10.1007/s13760-015-0544-7 [DOI] [PubMed] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, & Käller M (2016). MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics (Oxford, England), 32(19), 3047–3048. 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanciulli A, & Wenning GK (2015). Multiple-system atrophy. The New England Journal of Medicine, 372(3), 249–263. 10.1056/NEJMra1311488 [DOI] [PubMed] [Google Scholar]
- Fanning S, Haque A, Imberdis T, Baru V, Barrasa MI, Nuber S, … Selkoe D (2018). Lipidomic analysis of α-Synuclein neurotoxicity identifies Stearoyl CoA Desaturase as a target for Parkinson treatment. Molecular Cell, 73, 1001–1014.e8. 10.1016/j.molcel.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farca Luna AJ, Perier M, & Seugnet L (2017). Amyloid precursor protein in Drosophila glia regulates sleep and genes involved in glutamate recycling. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(16), 4289–4300. 10.1523/JNEUROSCI.2826-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, & Bender WW (2000). A Drosophila model of Parkinson’s disease. Nature, 404(6776), 394–398. 10.1038/35006074 [DOI] [PubMed] [Google Scholar]
- Fontaine SN, Zheng D, Sabbagh JJ, Martin MD, Chaput D, Darling A, … Dickey CA (2016). DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. The EMBO Journal, 35(14), 1537–1549. 10.15252/embj.201593489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, … Iwatsubo T (2002). Alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biology, 4(2), 160–164. 10.1038/ncb748 [DOI] [PubMed] [Google Scholar]
- Gai WP, Power JH, Blumbergs PC, & Blessing WW (1998). Multiple-system atrophy: A new alpha-synuclein disease? Lancet (London, England), 352(9127), 547–548. [DOI] [PubMed] [Google Scholar]
- Gao X, Carroni M, Nussbaum-Krammer C, Mogk A, Nillegoda NB, Szlachcic A, … Bukau B (2015). Human Hsp70 Disaggregase reverses Parkinson’s-linked α-Synuclein amyloid fibrils. Molecular Cell, 59(5), 781–793. 10.1016/j.molcel.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Esparcia P, Hernández-Ortega K, Ansoleaga B, Carmona M, & Ferrer I (2015). Purine metabolism gene deregulation in Parkinson’s disease. Neuropathology and Applied Neurobiology, 41(7), 926–940. 10.1111/nan.12221 [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, … Vidailhet M (2008). Second consensus statement on the diagnosis of multiple system atrophy. Neurology, 71(9), 670–676. 10.1212/01.wnl.0000324625.00404.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, … Fink AL (2003). Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry, 42(28), 8465–8471. 10.1021/bi0341152 [DOI] [PubMed] [Google Scholar]
- Gu X, Chen Y, Zhou Q, Lu Y-C, Cao B, Zhang L, … Asian Multiple System Atrophy Consortium (AMSAC). (2018). Analysis of GWAS-linked variants in multiple system atrophy. Neurobiology of Aging, 67, e201. e1–e201.e4. 10.1016/j.neurobiolaging.2018.03.018 [DOI] [PubMed] [Google Scholar]
- Gu X-L, Long C-X, Sun L, Xie C, Lin X, & Cai H (2010). Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Molecular Brain, 3, 12 10.1186/1756-6606-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Ross OA, Kun-Rodrigues C, Hernandez DG, Orme T, Eicher JD, … Bras J (2018). Investigating the genetic architecture of dementia with Lewy bodies: A two-stage genome-wide association study. The Lancet Neurology, 17(1), 64–74. 10.1016/S1474-4422(17)30400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-F, Zhang L, Li K, Mei J-P, Xue J, Chen J, … Tang B-S (2018). Coding mutations in NUS1 contribute to Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America, 115(45), 11567–11572. 10.1073/pnas.1809969115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbgebauer S, Nagl M, Klafki H, Haußmann U, Steinacker P, Oeckl P, … Otto M (2016). Modified serpinA1 as risk marker for Parkinson’s disease dementia: Analysis of baseline data. Scientific Reports, 6, 26145 10.1038/srep26145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Yoshino H, Tanaka M, Suzuki H, & Mizuno Y (1998). Geno-type in the 24-kDa subunit gene (NDUFV2) of mitochondrial complex I and susceptibility to Parkinson disease. Genomics, 49(1), 52–58. 10.1006/geno.1997.5192 [DOI] [PubMed] [Google Scholar]
- Hegde VR, Vogel R, & Feany MB (2014). Glia are critical for the neuropathology of complex I deficiency in Drosophila. Human Molecular Genetics, 23(17), 4686–4692. 10.1093/hmg/ddu188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Takkouche B, Caamaño-Isorna F, & Gestal-Otero JJ (2002). A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Annals of Neurology, 52(3), 276–284. 10.1002/ana.10277 [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, … Stevens B (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science (New York, NY), 352(6286), 712–716. 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein-Nezhad A, Fatemi RP, Ahmad R, Peskind ER, Zabetian CP, Hu S-C, … Faghihi MA (2016). Transcriptomic profiling of extracellular RNAs present in cerebrospinal fluid identifies differentially expressed transcripts in Parkinson’s disease. Journal of Parkinson’s Disease, 6(1), 109–117. 10.3233/JPD-150737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Flockhart I, Vinayagam A, Bergwitz C, Berger B, Perrimon N, & Mohr SE (2011). An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics, 12, 357 10.1186/1471-2105-12-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Sopko R, Foos M, Kelley C, Flockhart I, Ammeux N, … Mohr SE (2013). FlyPrimerBank: An online database for Drosophila melanogaster gene expression analysis and knockdown evaluation of RNAi reagents. G3 (Bethesda, Md.), 3(9), 1607–1616. 10.1534/g3.113.007021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, … Morgan M (2015). Orchestrating high-throughput genomic analysis with bioconductor. Nature Methods, 12(2), 115–121. 10.1038/nmeth.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Wakita S, Kanbara C, Nakai T, Akaike A, & Kume T (2017). Integrin α5β1 expression on dopaminergic neurons is involved in dopaminergic neurite outgrowth on striatal neurons. Scientific Reports, 7, 42111 10.1038/srep42111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen IE, Ye H, Heetveld S, Lechler MC, Michels H, Seinstra RI, … Heutink P (2017). Discovery and functional prioritization of Parkinson’s disease candidate genes from large-scale whole exome sequencing. Genome Biology, 18(1), 22 10.1186/s13059-017-1147-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe IT (2002). Principal component analysis (2nd ed.). New York: Springer-Verlag. [Google Scholar]
- Kachroo A, & Schwarzschild MA (2012). Adenosine A2A receptor gene disruption protects in an α-synuclein model of Parkinson’s disease. Annals of Neurology, 71(2), 278–282. 10.1002/ana.22630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, … Haass C (2002). Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Reports, 3(6), 583–588. 10.1093/embo-reports/kvf109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji S, Maki T, Kinoshita H, Uemura N, Ayaki T, Kawamoto Y, … Takahashi R (2018). Pathological endogenous α-Synuclein accumulation in Oligodendrocyte precursor cells potentially induces inclusions in multiple system atrophy. Stem Cell Reports, 10(2), 356–365. 10.1016/j.stemcr.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh MJ, Kurzawa-Akanbi M, Griffin H, Douroudis K, Ayers KL, Hussein RI, … Chinnery PF (2016). Exome sequencing in dementia with Lewy bodies. Translational Psychiatry, 6, e728 10.1038/tp.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V, Peng J, Chung CY, Auluck PK, Fanning S, Tardiff DF, … Lindquist S (2017). Genome-scale networks link neurodegenerative disease genes to α-synuclein through specific molecular pathways. Cell Systems, 4(2), 157–170.e14. 10.1016/j.cels.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely AP, Miners JS, Courtney R, Strand C, Love S, & Holton JL (2018). Exploring the putative role of kallikrein-6, calpain-1 and cathepsin-D in the proteolytic degradation of α-synuclein in multiple system atrophy. Neuropathology and Applied Neurobiology, 45, 347–360. 10.1111/nan.12512 [DOI] [PubMed] [Google Scholar]
- Kim SN, Jeibmann A, Halama K, Witte HT, Wälte M, Matzat T, … Klämbt C (2014). ECM stiffness regulates glial migration in Drosophila and mammalian glioma models. Development (Cambridge, England), 141 (16), 3233–3242. 10.1242/dev.106039 [DOI] [PubMed] [Google Scholar]
- Kluenemann HH, Nutt JG, Davis MY, & Bird TD (2013). Parkinsonism syndrome in heterozygotes for Niemann-pick C1. Journal of the Neurological Sciences, 335(1–2), 219–220. 10.1016/j.jns.2013.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko NL, Polak R, Haney MJ, Zhao Y, Gomes Neto RJ, Hill MC, … Batrakova EV (2017). Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials, 140, 79–87. 10.1016/j.biomaterials.2017.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde R (2015). pheatmap: Pretty heatmaps. Retrieved from https://CRAN.R-project.org/package=pheatmap
- Kondo T, Mizuno Y, & Japanese Istradefylline Study Group. (2015). A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clinical Neuropharmacology, 38(2), 41–46. 10.1097/WNF.0000000000000073 [DOI] [PubMed] [Google Scholar]
- Kontopoulos E, Parvin JD, & Feany MB (2006). Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Human Molecular Genetics, 15(20), 3012–3023. 10.1093/hmg/ddl243 [DOI] [PubMed] [Google Scholar]
- Kounatidis I, & Chtarbanova S (2018). Role of glial immunity in lifespan determination: A Drosophila perspective. Frontiers in Immunology, 9, 1362 10.3389/fimmu.2018.01362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer MC, Jung C, Batelli S, Rubin GM, & Gaul U (2017). The glia of the adult Drosophila nervous system. Glia, 65(4), 606–638. 10.1002/glia.23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D (2009). Swiss cheese et allii, some of the first neurodegenerative mutants isolated in Drosophila. Journal of Neurogenetics, 23 (1–2), 34–41. 10.1080/01677060802471635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruer MC, Paisán-Ruiz C, Boddaert N, Yoon MY, Hama H, Gregory A, … Hayflick SJ (2010). Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA). Annals of Neurology, 68(5), 611–618. 10.1002/ana.22122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuter K, Olech Ł, Głowacka U, & Paleczna M (2019). Astrocyte support is important for the compensatory potential of the nigrostriatal system neurons during early neurodegeneration. Journal of Neurochemistry, 148(1), 63–79. 10.1111/jnc.14605 [DOI] [PubMed] [Google Scholar]
- Langerveld AJ, Mihalko D, DeLong C, Walburn J, & Ide CF (2007). Gene expression changes in postmortem tissue from the rostral pons of multiple system atrophy patients. Movement Disorders, 22(6), 766–777. 10.1002/mds.21259 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Wang S, Slone SR, Yacoubian TA, & Witt SN (2011). Defects in very long chain fatty acid synthesis enhance alpha-synuclein toxicity in a yeast model of Parkinson’s disease. PLoS One, 6 (1), e15946 10.1371/journal.pone.0015946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Cui S, Du J, Liu J, Zhang P, Fu Y, … Chen S (2018). Association of GALC, ZNF184, IL1R2 and ELOVL7 with Parkinson’s disease in southern Chinese. Frontiers in Aging Neuroscience, 10, 402 10.3389/fnagi.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Morin LW, & Ahmad ST (2014). Methods to characterize spontaneous and startle-induced locomotion in a rotenone-induced Parkinson’s disease model of Drosophila. Journal of Visualized Experiments, 90 10.3791/51625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, & Shi W (2014). featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics (Oxford, England), 30(7), 923–930. 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, … Barres BA (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, & Bellen HJ (2017). The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/-D. Cell Metabolism, 26(5), 719–737.e6. 10.1016/j.cmet.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, … Bellen HJ (2015). Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell, 160(1–2), 177–190. 10.1016/j.cell.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Qin L, Wang L, Tan J, Zhang H, Tang J, … Wang C (2018). α-Synuclein induces apoptosis of astrocytes by causing dysfunction of the endoplasmic reticulum-Golgi compartment. Molecular Medicine Reports, 18(1), 322–332. 10.3892/mmr.2018.9002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Guo Y, An S, Kuang Y, He X, Ma H, … Jiang C (2013). Targeting caspase-3 as dual therapeutic benefits by RNAi facilitating brain-targeted nanoparticles in a rat model of Parkinson’s disease. PLoS One, 8(5), e62905 10.1371/journal.pone.0062905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan MA, Hackett R, Doherty J, Sheehan A, Speese SD, & Freeman MR (2012). Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury. Nature Neuroscience, 15(5), 722–730. 10.1038/nn.3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, & Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15(12), 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Stork T, Bergles DE, & Freeman MR (2016). Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature, 539(7629), 428–432. 10.1038/nature20145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, & Freeman MR (2006). The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron, 50(6), 869–881. 10.1016/j.neuron.2006.04.028 [DOI] [PubMed] [Google Scholar]
- Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, & Di Monte DA (2010). Lysosomal degradation of alpha-synuclein in vivo. The Journal of Biological Chemistry, 285(18), 13621–13629. 10.1074/jbc.M109.074617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, & NMSS Validation Group. (2011). The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Movement Disorders, 26(3), 399–406. 10.1002/mds.23462 [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, & Kaneko T (2009). Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. The Journal of Neuroscience, 29(2), 444–453. 10.1523/JNEUROSCI.4029-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk L, Berson A, & Bonini NM (2015). Drosophila as an in vivo model for human neurodegenerative disease. Genetics, 201(2), 377–402. 10.1534/genetics.115.179457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, & Thomas PD (2017). PANTHER version 11: Expanded annotation data from gene ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Research, 45(D1), D183–D189. 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, & Thomas PD (2013). PANTHER in 2013: Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Research, 41(Database issue), D377–D386. 10.1093/nar/gks1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JD, Ward M, Kim WS, Halliday GM, & Janitz M (2016). Strand-specific RNA-sequencing analysis of multiple system atrophy brain transcriptome. Neuroscience, 322, 234–250. 10.1016/j.neuroscience.2016.02.042 [DOI] [PubMed] [Google Scholar]
- Mizuta I, Tsunoda T, Satake W, Nakabayashi Y, Watanabe M, Takeda A, … Toda T (2008). Calbindin 1, fibroblast growth factor 20, and alpha-synuclein in sporadic Parkinson’s disease. Human Genetics, 124(1), 89–94. 10.1007/s00439-008-0525-5 [DOI] [PubMed] [Google Scholar]
- Mor DE, Tsika E, Mazzulli JR, Gould NS, Kim H, Daniels MJ, … Ischiropoulos H (2017). Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nature Neuroscience, 20(11), 1560–1568. 10.1038/nn.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, Assmus J, Herlofson K, Larsen JP, & Tysnes O-B (2013). Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson’s disease. Parkinsonism & Related Disorders, 19(11), 1027–1032. 10.1016/j.parkreldis.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Myllykangas L, Tyynelä J, Page-McCaw A, Rubin GM, Haltia MJ, & Feany MB (2005). Cathepsin D-deficient Drosophila recapitulate the key features of neuronal ceroid lipofuscinoses. Neurobiology of Disease, 19(1–2), 194–199. 10.1016/j.nbd.2004.12.019 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nakanishi T, Shimada H, Shimizu J, Aotani R, Maruyama S, … Tamai I (2018). Prostaglandin transporter OATP2A1/SLCO2A1 is essential for body temperature regulation during fever. The Journal of Neuroscience, 38(24), 5584–5595. 10.1523/JNEUROSCI.3276-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Vilariño-Güell C, Cobb SA, Kachergus JM, Ross OA, Hentati E, … Farrer MJ (2010). Genetic variation of the mitochondrial complex I subunit NDUFV2 and Parkinson’s disease. Parkinsonism & Related Disorders, 16(10), 686–687. 10.1016/j.parkreldis.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, & Schrag A (2012). Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Annals of Neurology, 72(6), 893–901. 10.1002/ana.23687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AL, Feany MB; 2019. Glial and neuronal alpha synuclein induce unique transcriptional programs. GEO dataset, Accession number: GSE128120. [Google Scholar]
- Ordonez DG, Lee MK, & Feany MB (2018). α-Synuclein induces mitochondrial dysfunction through Spectrin and the actin cytoskeleton. Neuron, 97(1), 108–124.e6. 10.1016/j.neuron.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp MI, & Lantos PL (1994). The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain: A Journal of Neurology, 117(Pt 2), 235–243. [DOI] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, & Kingsford C (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nature Methods, 14(4), 417–419. 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuralinna T, Myllykangas L, Oinas M, Nalls MA, Keage HAD, Isoviita V-M, … Tienari PJ (2015). Genome-wide association study of neocortical Lewy-related pathology. Annals of Clinical Translational Neurology, 2(9), 920–931. 10.1002/acn3.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho R, Paiva I, Jercic KG, Fonseca-Ornelas L, Gerhardt E, Fahlbusch C, … Outeiro TF (2019). Nuclear localization and phosphorylation modulate pathological effects of alpha-synuclein. Human Molecular Genetics, 28(1), 31–50. 10.1093/hmg/ddy326 [DOI] [PubMed] [Google Scholar]
- Pokrzywa M, Pawełek K, Kucia WE, Sarbak S, Chorell E, Almqvist F, & Wittung-Stafshede P (2017). Effects of small-molecule amyloid modulators on a Drosophila model of Parkinson’s disease. PLoS One, 12(9), e0184117 10.1371/journal.pone.0184117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, & Luo L (2010). The Q system: A repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell, 141(3), 536–548. 10.1016/j.cell.2010.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter KA, Kern MJ, Fullbright G, Bielawski J, Scherer SS, Yum SW, … Hama H (2011). Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia, 59(7), 1009–1021. 10.1002/glia.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash KM, Nadkarni NV, Lye W-K, Yong M-H, & Tan E-K (2016). The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients: A longitudinal study. European Journal of Neurology, 23(5), 854–860. 10.1111/ene.12950 [DOI] [PubMed] [Google Scholar]
- Purice MD, Ray A, Münzel EJ, Pope BJ, Park DJ, Speese SD, & Logan MA (2017). A novel Drosophila injury model reveals severed axons are cleared through a Draper/MMP-1 signaling cascade. eLife, 6, e23611 10.7554/eLife.23611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, … Zhang J (2008). Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Molecular Brain, 1, 17 10.1186/1756-6606-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Yang X, Cossu G, Olgiati S, Saddi VM, Breedveld GJ, … Bonifati V (2015). An exome study of Parkinson’s disease in Sardinia, a Mediterranean genetic isolate. Neurogenetics, 16(1), 55–64. 10.1007/s10048-014-0425-x [DOI] [PubMed] [Google Scholar]
- Read RD, Fenton TR, Gomez GG, Wykosky J, Vandenberg SR, Babic I, … Thomas JB (2013). A kinome-wide RNAi screen in Drosophila glia reveals that the RIO kinases mediate cell proliferation and survival through TORC2-Akt signaling in glioblastoma. PLoS Genetics, 9 (2), e1003253 10.1371/journal.pgen.1003253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon KA, Mendelsohn FA, Chai SY, & Horne MK (2000). The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson’s disease. Australian and New Zealand Journal of Medicine, 30(1), 48–53. [DOI] [PubMed] [Google Scholar]
- Riabinina O, Luginbuhl D, Marr E, Liu S, Wu MN, Luo L, & Potter CJ (2015). Improved and expanded Q-system reagents for genetic manipulations. Nature Methods, 12(3), 219–222, 5 p following 222. 10.1038/nmeth.3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T, Issa A-R, Pech U, Coulom H, Nguyễn M-V, Cassar M, … Birman S (2013). A single dopamine pathway underlies progressive locomotor deficits in a Drosophila model of Parkinson disease. Cell Reports, 5(4), 952–960. 10.1016/j.celrep.2013.10.032 [DOI] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson M-T, Iché M, & Birman S (2004). Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Current Biology: CB, 14(7), 599–605. 10.1016/j.cub.2004.03.039 [DOI] [PubMed] [Google Scholar]
- Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, … Shulman JM (2017). Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain: A Journal of Neurology, 140(12), 3191–3203. 10.1093/brain/awx285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, … Lewis S (2000). Comparative genomics of the eukaryotes. Science (New York, N.Y.), 287(5461), 2204–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Scholz SW, Nalls MA, Schulte C, Federoff M, Price TR, … European Multiple System Atrophy Study Group and the UK Multiple System Atrophy Study Group. (2016). A genome-wide association study in multiple system atrophy. Neurology, 87(15), 1591–1598. 10.1212/WNL.0000000000003221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Suzuki K, Hulette CM, & Murayama S (2004). Aberrant phosphorylation of alpha-synuclein in human Niemann-pick type C1 disease. Journal of Neuropathology and Experimental Neurology, 63(4), 323–328. [DOI] [PubMed] [Google Scholar]
- Sandor C, Honti F, Haerty W, Szewczyk-Krolikowski K, Tomlinson P, Evetts S, … Wade-Martins R (2017). Whole-exome sequencing of 228 patients with sporadic Parkinson’s disease. Scientific Reports, 7, 41188 10.1038/srep41188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, … Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 74(4), 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafferer S, Khurana R, Refolo V, Venezia S, Sturm E, Piatti P, … Stefanova N (2016). Changes in the miRNA-mRNA regulatory network precede motor symptoms in a mouse model of multiple system atrophy: Clinical implications. PLoS One, 11(3), e0150705 10.1371/journal.pone.0150705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid I, Maruani A, Huguet G, Leblond CS, Nygren G, Anckarsäter H, … Delorme R (2013). Heterozygous FA2H mutations in autism spectrum disorders. BMC Medical Genetics, 14, 124 10.1186/1471-2350-14-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Jensen RV, Gullans SR, & Feany MB (2003). Gene expression changes presage neurodegeneration in a Drosophila model of Parkinson’s disease. Human Molecular Genetics, 12(19), 2457–2466. 10.1093/hmg/ddg265 [DOI] [PubMed] [Google Scholar]
- Schneider SA, & Bhatia KP (2010). Three faces of the same gene: FA2H links neurodegeneration with brain iron accumulation, leukodys-trophies, and hereditary spastic paraplegias. Annals of Neurology, 68(5), 575–577. 10.1002/ana.22211 [DOI] [PubMed] [Google Scholar]
- Schormair B, Kemlink D, Mollenhauer B, Fiala O, Machetanz G, Roth J, … Winkelmann J (2018). Diagnostic exome sequencing in early-onset Parkinson’s disease confirms VPS13C as a rare cause of autosomal-recessive Parkinson’s disease. Clinical Genetics, 93(3), 603–612. 10.1111/cge.13124 [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, & Auld VJ (2001). Peripheral glia direct axon guidance across the CNS/PNS transition zone. Developmental Biology, 238 (1), 47–63. 10.1006/dbio.2001.0411 [DOI] [PubMed] [Google Scholar]
- Sherzai A, Edland SD, Masliah E, Hansen L, Pizzo DP, Sherzai A, & Corey-Bloom J (2013). Spongiform change in dementia with Lewy bodies and Alzheimer disease. Alzheimer Disease and Associated Disorders, 27(2), 157–161. 10.1097/WAD.0b013e318256d507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulskaya MV, Alieva AK, Vlasov IN, Zyrin VV, Fedotova EY, Abramycheva NY, … Shadrina MI (2018). Whole-exome sequencing in searching for new variants associated with the development of Parkinson’s disease. Frontiers in Aging Neuroscience, 10, 136 10.3389/fnagi.2018.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, … Masliah E (2005). Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: Implications for multiple system atrophy. The Journal of Neuroscience, 25(46), 10689–10699. 10.1523/JNEUROSCI.3527-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, … Andersen JK (2012). Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: Implications for Parkinson’s disease. Free Radical Biology & Medicine, 53(4), 993–1003. 10.1016/j.freeradbiomed.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siitonen A, Nalls MA, Hernández D, Gibbs JR, Ding J, Ylikotila P, … Majamaa K (2017). Genetics of early-onset Parkinson’s disease in Finland: Exome sequencing and genome-wide association study. Neurobiology of Aging, 53, e195.e7–e195.e10. 10.1016/j.neurobiolaging.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehn AS, Rattay TW, Beck-Wödl S, Schäferhoff K, Monk D, Döbler-Neumann M, … Schöls L (2016). Uniparental disomy of chromosome 16 unmasks recessive mutations of FA2H/SPG35 in 4 families. Neurology, 87(2), 186–191. 10.1212/WNL.0000000000002843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YJC, Halliday GM, Holton JL, Lashley T, O’Sullivan SS, McCann H, … Revesz TR (2009). Degeneration in different Parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. Journal of Neuropathology and Experimental Neurology, 68 (10), 1073–1083. 10.1097/NEN.0b013e3181b66f1b [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Coleman C, Wong L-Y, Harris SL, Richardson JR, Gadad BS, … German DC (2013). The angiotensin converting enzyme inhibitor captopril protects nigrostriatal dopamine neurons in animal models of parkinsonism. Experimental Neurology, 250, 376–383. 10.1016/j.expneurol.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VL, Bellani S, Giannandrea M, Yousuf M, Valtorta F, Meldolesi J, & Chieregatti E (2009). {alpha}-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics. Molecular Biology of the Cell, 20(16), 3725–3739. 10.1091/mbc.e08-03-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Klimaschewski L, Poewe W, Wenning GK, & Reindl M (2001). Glial cell death induced by overexpression of alpha-synuclein. Journal of Neuroscience Research, 65(5), 432–438. 10.1002/jnr.1171 [DOI] [PubMed] [Google Scholar]
- Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, & Wenning GK (2005). Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. The American Journal of Pathology, 166(3), 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh MJ, Pantano L, Kirchner RD, Barrera V, Chapman BA, Piper ME, … Sui SH (2018). bcbioRNASeq: R package for bcbio RNA-seq analysis, (version 2; referees: 1 approved, 1 approved with reservations). F1000Research, 6, 1976. [Google Scholar]
- Sunderhaus ER, & Kretzschmar D (2016). Mass histology to quantify Neurodegeneration in Drosophila. Journal of Visualized Experiments, 118 10.3791/54809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, & Halliday GM (2017). Selective neuronal vulnerability in Parkinson disease. Nature Reviews. Neuroscience, 18(2), 101–113. 10.1038/nrn.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Weaver B, Grawey A, Wenger C, Freed E, & Worrall BB (2006). Complex I polymorphisms, bigenomic heterogeneity, and family history in Virginians with Parkinson’s disease. Journal of the Neurological Sciences, 247(2), 224–230. 10.1016/j.jns.2006.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata Y, Imaizumi Y, Sugawara M, Andoh-Noda T, Banno S, Chai M, … Okano H (2018). T-type calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson disease. Stem Cell Reports, 11(5), 1171–1184. 10.1016/j.stemcr.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Baracskay K, Kinter M, Melrose S, Brophy PJ, Boucheix C, … Trapp BD (2002). The tetraspanin protein, CD9, is expressed by progenitor cells committed to oligodendrogenesis and is linked to beta1 integrin, CD81, and Tspan-2. Glia, 40(3), 350–359. 10.1002/glia.10134 [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium. (2017). Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Research, 45(D1), D331–D338. 10.1093/nar/gkw1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, … Narechania A (2003). PANTHER: A library of protein families and subfamilies indexed by function. Genome Research, 13(9), 2129–2141. 10.1101/gr.772403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibar H, El Bayad K, Bouhouche A, Ait Ben Haddou EH, Benomar A, Yahyaoui M, … Regragui W (2018). Non-motor symptoms of Parkinson’s disease and their impact on quality of life in a cohort of Moroccan patients. Frontiers in Neurology, 9, 170 10.3389/fneur.2018.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K, Grzesiak JJ, Bouvet M, Hashimoto M, Masliah E, & Shults CW (2005). Alpha-synuclein overexpression in oligodendrocytic cells results in impaired adhesion to fibronectin and cell death. Molecular and Cellular Neurosciences, 29(2), 259–268. 10.1016/j.mcn.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Uversky VN (2008). Alpha-synuclein misfolding and neurodegenerative diseases. Current Protein & Peptide Science, 9(5), 507–540. [DOI] [PubMed] [Google Scholar]
- Vilar M, Chou H-T, Lührs T, Maji SK, Riek-Loher D, Verel R, … Riek R (2008). The fold of alpha-synuclein fibrils. Proceedings of the National Academy of Sciences of the United States of America, 105(25), 8637–8642. 10.1073/pnas.0712179105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent BM, Tardiff DF, Piotrowski JS, Aron R, Lucas MC, Chung CY, … Rhodes KJ (2018). Inhibiting Stearoyl-CoA Desaturase ameliorates α-Synuclein cytotoxicity. Cell Reports, 25(10), 2742–2754.e31. 10.1016/j.celrep.2018.11.028 [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, & Takahashi H (2000). NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathologica, 99(1), 14–20. [DOI] [PubMed] [Google Scholar]
- Wang L, Colodner KJ, & Feany MB (2011). Protein misfolding and oxidative stress promote glial-mediated neurodegeneration in an Alexander disease model. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(8), 2868–2877. 10.1523/JNEUROSCI.3410-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chu C-H, Stewart T, Ginghina C, Wang Y, Nie H, … Zhang J (2015). α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proceedings of the National Academy of Sciences of the United States of America, 112(15), E1926–E1935. 10.1073/pnas.1417883112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag. [Google Scholar]
- Witte HT, Jeibmann A, Klämbt C, & Paulus W (2009). Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia (New York, N.Y.), 11(9), 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, & Feany MB (2001). Tauopathy in Drosophila: Neurodegeneration without neurofibrillary tangles. Science (New York, N.Y.), 293(5530), 711–714. 10.1126/science.1062382 [DOI] [PubMed] [Google Scholar]
- Xiong WC, & Montell C (1995). Defective glia induce neuronal apoptosis in the repo visual system of Drosophila. Neuron, 14(3), 581–590. [DOI] [PubMed] [Google Scholar]
- Xu K, Di Luca DG, Orrú M, Xu Y, Chen J-F, & Schwarzschild MA (2016). Neuroprotection by caffeine in the MPTP model of Parkinson’s disease and its dependence on adenosine A2A receptors. Neuroscience, 322, 129–137. 10.1016/j.neuroscience.2016.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunin E, Kisos H, Kulik W, Grigoletto J, Wanders RJA, & Sharon R (2014). The regulation of catalase activity by PPAR γ is affected by α-synuclein. Annals of Clinical Translational Neurology, 1(3), 145–159. 10.1002/acn3.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Zwarts L, Callaerts P, Norga K, Mackay TFC, & Anholt RRH (2008). Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12393–12398. 10.1073/pnas.0804889105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhou T, Wang H, Liu T, Ueda K, Zhan R, … Chui D (2015). Lipoprotein lipase deficiency leads to α-synuclein aggregation and ubiquitin C-terminal hydrolase L1 reduction. Neuroscience, 290, 1–10. 10.1016/j.neuroscience.2014.12.068 [DOI] [PubMed] [Google Scholar]
- Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, … Lee VM-Y (2005). Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron, 45(6), 847–859. 10.1016/j.neuron.2005.01.032 [DOI] [PubMed] [Google Scholar]
- Ylönen S, Siitonen A, Nalls MA, Ylikotila P, Autere J, Eerola-Rautio J, … Majamaa K (2017). Genetic risk factors in Finnish patients with Parkinson’s disease. Parkinsonism & Related Disorders, 45, 39–43. 10.1016/j.parkreldis.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Ren J, Wang Y, He X, & Zhao Y (2016). Acteoside binds to Caspase-3 and exerts neuroprotection in the rotenone rat model of Parkinson’s disease. PLoS One, 11(9), e0162696 10.1371/journal.pone.0162696 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and final RNA-Seq data is available through Gene Expression Omnibus (GEO), accession number GSE128120. All other data that support the findings of this study are available from the corresponding author upon reasonable request (Olsen et al., 2019).


