Abstract
Manganese (Mn) is an essential micronutrient required for the normal development of many organs, including the brain. Although its roles as a cofactor in several enzymes and in maintaining optimal physiology are well-known, the overall biological functions of Mn are rather poorly understood. Alterations in body Mn status are associated with altered neuronal physiology and cognition in humans, and either overexposure or (more rarely) insufficiency can cause neurological dysfunction. The resultant balancing act can be viewed as a hormetic U-shaped relationship for biological Mn status and optimal brain health, with changes in the brain leading to physiological effects throughout the body and vice versa. This review discusses Mn homeostasis, biomarkers, molecular mechanisms of cellular transport, and neuropathological changes associated with disruptions of Mn homeostasis, especially in its excess, and identifies gaps in our understanding of the molecular and biochemical mechanisms underlying Mn homeostasis and neurotoxicity.
Keywords: metal, metal homeostasis, manganese, neurodegeneration, neurodegenerative disease, neurodevelopment, homeostasis, toxicology, neurotoxin, brain, neurotransmitter
Introduction
Manganese is essential for numerous vital process including nerve and brain development and cognitive functioning. For most people, dietary consumption generally fulfills the requisite Mn intake (1). Even though Mn is crucial for maintaining optimal physiology, several aspects of the biology of the homeostatic control and toxicity of Mn remain unclear (2, 3). Unanswered questions include the subcellular and organelle distribution of Mn and the nature of cellular events that occur when deviations from Mn homeostasis occur (e.g. with exposure to chronic low levels of Mn). Mn has been implicated in the metabolism of proteins, lipids, and carbohydrates and acts as a cofactor for numerous kinases and other enzymes (4, 5). Because magnesium (Mg) and Mn share a resemblance in their physicochemical properties, a vast majority of enzymes can use Mg in lieu of Mn for their activation (3).
Mn also plays unique roles that cannot be replaced by other metals, such as in Mn-dependent enzymes, including arginase, agmatinase, glutamine synthetase, and Mn superoxide dismutase (MnSOD).3 As a result, its presence at optimal levels to support these functions is required, and the lack of this essential micronutrient can give rise to cognitive deficits (6). Excess cellular levels of Mn are also detrimental, and this aspect of Mn-induced disease has gained attention in the field of toxicology. Mn accumulates in specific regions of the brain to selectively alter neurophysiology. Elevated brain Mn levels usually occur only following overexposure, which can result from numerous sources, including environmental sources, occupational exposures, or dietary exposures to Mn such as from contaminated drinking water.
The consequences of Mn overexposure occur throughout the nervous system and can affect both motor function and higher-order cognitive functions. Motor control is disrupted via disruption of dopaminergic (DAergic) function (7). This includes clinical expression of parkinsonism in occupationally exposed workers (8, 9). Occupational exposure to Mn has been linked to other unfavorable outcomes, including learning deficits and neurodegeneration (10). The consequences of Mn overexposure occur throughout the nervous system and affect motor functions. This review is focused on neurotoxicity and presents evidence that Mn plays a key role in the maintenance of brain physiological homeostasis and is a primary target of this metal (11). Recent identification of genetic disorders of Mn metabolism combined with studies providing insights into the mechanisms of Mn neurotoxicity make a comprehensive review on this topic timely. This review illuminates the unfavorable outcomes Mn exposure causes. There is urgency to explore and address the potential changes that are occurring at a molecular level, as these unfavorable outcomes are leading to increased risk of diseases. This attempt requires an interdisciplinary approach where scientists with expertise in neuroscience, biology, and chemistry to come together to think about the problem at hand.
Routes of manganese exposure and accumulation in the brain
Beyond occupational exposures, excessive dietary or drinking water uptake of Mn is another source of overexposure. The World Health Organization recommends that the daily Mn consumption for an adult be between 0.7 and 10.9 mg (12). Although use of Mn dietary supplementation containing greater than 20 mg of Mn has been reported in cases of osteoarthritis and osteoporosis (13, 14), to our knowledge, naturally occurring Mn deficiency has never been reported in humans. Ingested Mn has an absorption rate of 3–5% through the gastrointestinal tract and is subject to tight homeostatic control in vivo (15, 16). Systemic homeostatic control of Mn is a balance between transport across the enterocytes lining the intestinal wall and removal by the liver (17). Several factors can influence the oral uptake of Mn, including iron (Fe) status, dietary matrix, bioavailability, and existing body burden of Mn (18). Following oral intake, Mn is distributed widely to tissues, and its metabolism may also involve cycling between Mn2+ and Mn3+, although only a small fraction is found in the 3+ oxidation state (19). The oxidation state of Mn exposure is a critical determinant of Mn toxicokinetics, tissue toxicodynamics, and toxicity (20). Contaminated drinking water is another source of Mn exposure (21, 22). Metal concentrations in water vary by location, with Mn ranging from 0.0001 to 0.1 mg/liter (18). Although the United States Environmental Protection Agency does not consider Mn to be a primary drinking water contaminant, the standard concentration of allowable Mn is 0.05 mg/liter (23, 24). There are arguments that the current Mn reference concentration guidelines need to be re-evaluated to consider Mn as a potential contaminant (25), specifically when using water for infant formulas that already contain high levels of Mn (26). Inhalation is the predominant route of exposure associated with the toxic effects of Mn in adults. Current data suggest that Mn exposure via drinking water in children/adolescents is as important as inhalation exposure under environmental and occupational settings (27). Excess environmental exposure may arise from air pollution (28–31), gasoline enhanced with methylcyclopentadienyl manganese tricarbonyl (MMT) (32, 33), and agricultural fungicides (34, 35). Many of the initial epidemiologic studies of Mn and health outcomes examined occupational inhalation exposures within industries such as ferromanganese welding, mining, and refining (36–40). Inhaled Mn enters the circulatory system through the nasal mucosa, bypasses the biliary excretion mechanism, and can cross the blood-brain barrier via several pathways, including facilitated diffusion and active transport from the olfactory bulb to the cerebral cortex (41–44). Mn accumulates in Fe-rich brain regions of the basal ganglia: caudate, putamen, globus pallidus, substantia nigra, and subthalamic nuclei of the brain (43, 45, 46). Under normal conditions, estimated concentrations of Mn in the human brain range from 5.32 to 14.03 ng of Mn/mg of protein, with 15.96–42.09 ng of Mn/mg of protein being the estimated pathophysiological threshold (47). Based on occupational studies of Mn exposure, the Occupational Safety and Health Administration exposure limit for general industry, construction industry, and shipyard employment is 5 mg/m3 (RRID:SCR_018203). However, even with Mn airborne levels near the Environmental Protection Agency's reference concentration of 0.05 μg/m3 (23), exposure can result in deficits in postural balance and neuropsychological and motor functions (49–51) and increased risk for physician diagnosis of Parkinson's disease (PD) (52). Deficits in neuromotor function are similar among adult Mn-exposed workers (53, 54) and older PD patients (55). Longitudinal and cross-sectional environmental exposure studies link low-level Mn exposure to deficits in intellectual development (6), neurobehavior (56), neuromotor function (57), and neuropsychiatric changes with respect to attention and mood (58).
Using magnetic resonance imaging (MRI), several studies have shown that significant brain accumulation of Mn in both humans and other animal models is associated with increased risk of neurotoxicity. These subjects often show a characteristic accumulation of manganese in the basal ganglia, particularly in the globus pallidus (59–61). Mn accumulation was also observed in the frontal cortex (Fig. 1) (59). Although recovery upon cessation is possible (62), this rarely happens in cases of occupational exposures, which are prolonged and cumulative. In rodent studies, areas of notable accumulation include the olfactory bulb, cerebellum, hippocampus and dentate gyrus, and pituitary gland in addition to the basal ganglia (63, 64). Other studies in nonhuman primates have shown that inhaled ultrafine Mn particles translocate and accumulate in the olfactory bulb in addition to the striatum, frontal cortex, and cerebellum (43). In addition to using MRI to analyze brain Mn levels, other noninvasive methods of measuring total body Mn burden based on bone levels are being used based on neutron activation analysis (65). The next section details the biomarkers that are currently being used to assess Mn biological status.
Figure 1.
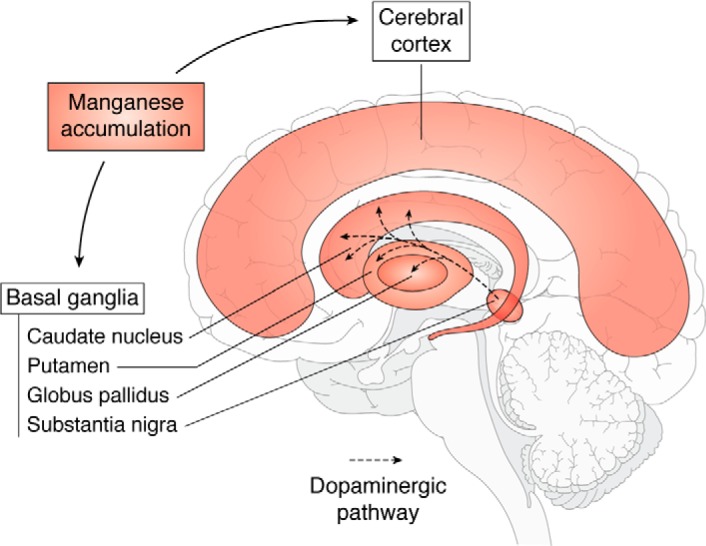
Schematic showing the sagittal section of human brain showing the brain regions where Mn predominantly accumulates (48, 200). Dopamine is a key neurotransmitter that is produced in the substantia nigra, and the dopaminergic neurotransmitters project to the basal ganglia region.
Biomarkers of brain and body Mn status
Blood Mn levels are the most used indicator of exposure and can characterize the difference between exposed and unexposed subjects (66). Blood Mn is reflective of recent exposure rather than total body burden due to the short half-life of Mn in blood, which is less than 2 h owing to rapid hepatic clearance (67–69). Hair Mn levels have been widely used to quantify chronic low levels of exposure, such as those commonly associated with deficits in cognition in children (28–30, 70, 71), but evidence that the hair Mn levels reflect internal exposure dose versus external exposure dose is lacking. Mn levels in dentin of deciduous teeth have also been used to quantify both prenatal and postnatal accumulation (34, 35). Fingernails and toenails are noninvasive indicators of internal Mn exposure and hold the potential to quantify long-term exposure for up to a year (72, 73). Due to the high rate of Mn elimination through bile to feces (>95%) and short half-life, urine Mn concentration is not recommended as an optimal medium for internal Mn exposure assessment (69, 73, 74). Only limited research is available on saliva and the hormone prolactin as alternate biomarkers for Mn exposure (68, 74, 75).
Biphasic relationship
Mn exhibits negative health effects at both deficient and excess exposures (6, 76, 77). In 1999, Mergler et al. (78) coined the term “a continuum of dysfunction” to describe the manifestations of Mn neurotoxicity along three toxicological outcomes: manganism, a neurological disorder in response to high exposures of Mn; neuropsychological abnormalities; and idiopathic Parkinson's disease. Vollet et al. (6) describe this dose-response relationship of Mn focusing on neurocognitive outcomes (Fig. 2). In recent years, an inverse U-shaped association has been reported between Mn exposure and adverse neurodevelopmental effects in infants and children, suggesting adverse effects of both low and high Mn exposures (Fig. 2). This pattern is consistent with Mn acting as both an essential nutrient and a toxicant (6, 28, 79). This biphasic dose response between Mn exposure and neurotoxic effects is observed in both human and animal studies (28, 80, 81). Additional studies are needed to determine the full extent of Mn exposure effects on health outcomes. Future prospective field-based exposure studies can provide substantial insights regarding chronic low-level impacts and eventual progression in terms of disability and disease (78). Future biochemical research should include examination of mechanisms of nonlinear relationships and cell/molecular consequences of exposures across the lifespan, particularly during critical developmental windows.
Figure 2.
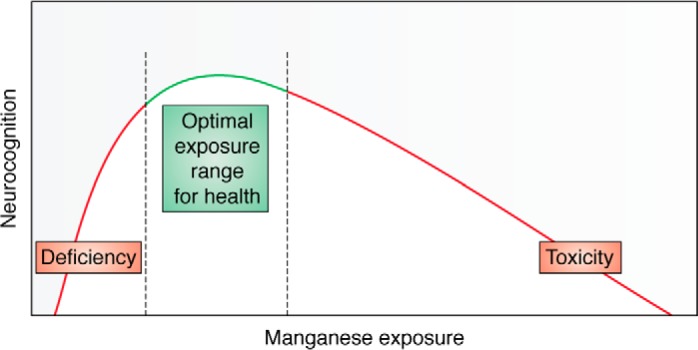
Mn exhibits hormetic dose response, which means an inverted “U-shaped” curve. Deficits in neurocognition are seen at both lower and higher doses, with maximum function being at the top of the inverted U-shaped curve. Adapted from Vollet et al. (6). This research was originally published in Current Environmental Health Reports. Vollet, K., Haynes, E. N., and Dietrich, K. N. Manganese exposure and cognition across the lifespan: contemporary review and argument for biphasic dose-response health effects. Curr. Environ. Health Rep. 2016; 3:392–404. © Springer.
Effects of Mn on neurotransmitters
Accumulation of Mn in the brain could potentially alter multiple neurotransmitter systems and their activity in the brain. Mn predominantly accumulates in the basal ganglia region of the brain, including the substantia nigra, striatum, and pallidum, when in excess (82–85). The basal ganglia have an intricate network of neurotransmitters that could potentially be altered and cause deviations in optimal physiology and behavior.
γ-Aminobutyric acid (GABA) is an inhibitory neurotransmitter seen in abundance in globus pallidus and substantia nigra pars reticulata that receives inputs from the striatum (caudate nucleus and putamen) (86, 87). Mn exposures, even at levels that are not cytotoxic to neurons (e.g. 100 μm), cause early and profound changes in neurite length and integrity, thus subsequently altering GABA levels (86). These deviations in GABAergic neurotransmission cause disinhibition of excitatory neurotransmitters. Stanwood et al. (86) showed that acute Mn treatment was neurotoxic in vitro, causing death in tyrosine hydroxylase (TH)-positive, presumptive dopamine (DA) neurons, along with loss of glutamic acid decarboxylase–positive neurons in the basal ganglia. Dopaminergic neurons present in the basal ganglia express TH, which is the rate-limiting enzyme for the synthesis of dopamine. Their study indicates that Mn toxicity acts on the neurocircuitry of this brain region to alter homeostasis and mediate neurodegeneration in the brain (86). Mn also inhibited GABA transport in the rat forebrain, confirming that Mn neurotoxicity perturbs the GABAergic neurotransmission (88).
Glutamate is the primary excitatory neurotransmitter and a critical signaling molecule. Mn can trigger neurotoxicity by release of excessive amounts of glutamate in the extracellular space, potentially leading to activation of glutamate receptors and subsequent downstream processes (89). Mn exposure leads to altered synaptic neurotransmission via increased sensitivity of postsynaptic glutamate receptors and activation of neurons in the globus pallidus (90–92). Mn is also a cofactor for glutamine synthetase, and Mn disruption of astrocyte metabolism also leads to unavailability of the necessary components for neurotransmitter and GSH metabolism (93).
The effects of Mn on brain cholinergic systems could add to the understanding of Mn neurotoxicity. Mn binds to the choline transporter and reduces choline uptake. Additionally, Mn influences regional choline uptake in the hippocampus, the frontal and parietal cortices, the caudate, and the putamen. These results suggest that choline uptake across the blood-brain barrier is likely inhibited by Mn (94). The deficit of choline, a key component needed for the synthesis of the neurotransmitter acetylcholine, could potentially contribute to deficits in both behavior and physiology. Mn has a greater neurotoxic effect on cholinergic neurons in the developing brain, as there is decrease in the enzyme choline acetyltransferase specifically in the midbrain and the cerebellar region (95). In adult rats, Mn may further contribute to regional specific (e.g. striatum and cerebellum) increases in the acetylcholine-degrading enzyme acetylcholinesterase (95–97). Further study is needed to corroborate the neurotoxic effects of Mn on cholinergic neurons and the interactions it has with the other neurotransmitter systems in the brain.
The current body of literature has predominantly focused on the effects of Mn neurotoxicity on DA. As discussed earlier in this work, Mn accumulates in the basal ganglia, a DA-rich region. This accumulation has been shown to correlate inversely with DA levels in both neonates (98) and adult rats (99). Research has predominantly focused on DA subtype 2 receptor (D2R) and the DA transporter (DAT) responsible for Mn transport into DA neurons (100). Nelson et al. showed that D2R are involved in Mn toxicity, at the receptor level or at a point downstream in the signaling cascade that does not involve adenylyl cyclase (101). MnCl2 exposure can lead to DA depletion and blunt the efficacy of DAergic neurotransmission, leading to up-regulation of the post-synaptic dopamine receptors and causing behavioral alterations including hypoactivity, cognitive impairments, and altered sensorimotor function (102). DA release kinetics in rat striatum are also impacted by sub-acute exposure to Mn due to accumulation within the striatum, with decreased basal levels and lower stimulated release observed up to 3 weeks post-treatment (103).
DAT density is highest in the caudate, putamen, and nucleus accumbens, and this density increases with age under normal physiological conditions. Examination of DAT in Mn-intoxicated patients revealed a slight decrease in DAT density (104). The authors also noted significantly greater DAT in striatum of patients with Parkinson's disease compared with those with Mn intoxication. Nonhuman primate studies have shown that DAT in striatum is a target for Mn and could potentially indicate an early event in the damage of DAergic neurons by increased uptake of DA and/or Mn (105). Low doses of Mn do not kill DAergic neurons in vitro but can impair TH activity through activation of protein kinase Cδ and protein phosphatase 2A even in the absence of frank toxicity (106). Mn exposure in vivo increased DA and the DA metabolite 3,4-dihydroxyphenylacetic acid in adult rats (107). Tran et al. (108) showed that dietary Mn exposure caused disruption to the DA system that subsequently altered executive function. Developmental exposure of Mn caused cognitive deficits that implicate DA and brain-derived neurotrophic factor (109). Mn accumulation in the DAergic cells of the substantia nigra pars compacta in a rodent model points to a biological basis for deficits in motor skills seen in association with manganism (84). Reported effects of Mn neurotoxicity on DA include decreased DA levels (110–112), increased DA levels (113), or no modification (114) in the substantia nigra or striatum. These differences may be due to the specific exposure paradigm, including route of exposure, concentration, duration, Mn compound, animal model, species age, and sex of the subjects (115). An alternate mode of cytotoxicity is by promoting oxidation of DA and other catecholamines and altering the cellular protective mechanisms that cause the formation of ROS due to higher Mn dosages (116). Mn may directly contribute to dopaminergic cell death by disturbing mitochondrial respiration and antioxidant systems following accumulation within mitochondria (117). Cellular DA also potentiates the cytotoxicity caused by Mn through the oxidative stress–dependent NF-κB signaling cascade (118). Related to this, antioxidants like taurine may provide neuroprotective effects against Mn neurotoxicity (119).
Mn transporters and deviations of Mn homeostasis
Nongenetic influences on Mn toxicity
Mn and Fe share common absorption and transport pathways (120). The absorption of Mn is closely linked with absorption of Fe (121–123). Fe-deficient diets lead to increased absorption of Mn (124), and conversely, large amounts of dietary Fe have been shown to inhibit Mn absorption (125–127). This observation was confirmed by a study that showed that as Fe content increased, absorption of Mn decreased (128). Fe supplementation of 60 mg/day over 4 months was associated with decreased blood Mn levels and an overall reduction in Mn nutritional status as shown by decreased Mn superoxide dismutase activity in white blood cells (129). In addition, individual Fe body concentrations affect Mn bioavailability. Fe deficiency increases intestinal absorption of Mn, and increased Fe storage measured by ferritin levels is associated with a decrease in Mn absorption (122). Men generally have higher Fe stores than women, in part due to the loss of Fe during menstruation. This may be why men generally absorb less Mn than women (15). Further, Fe deficiency has been linked to increased risk of Mn accumulation in the brain (130).
Genetic modifiers of Mn toxicity
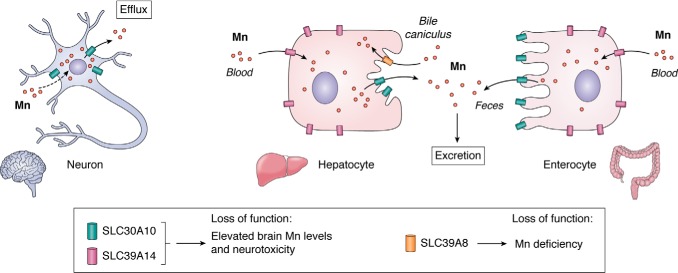
Whereas some mammalian Mn transporters were characterized over the last 2 decades, the recent identification of three hereditary disorders of Mn metabolism is beginning to provide a clearer understanding of the mechanisms that regulate Mn homeostasis in mammalian cells and organisms. Loss-of-function mutations in the Mn efflux transporter SLC30A10 or the Mn influx transporter SLC39A14 increase Mn levels in the body to induce neurotoxicity. In contrast, loss-of-function mutations in another Mn influx transporter, SLC39A8, induce Mn deficiency. The function of these transporters and the underlying disease mechanisms are described below (Fig. 3).
Figure 3.
Function of SLC30A10, SLC39A14, and SLC39A8. SLC30A10 and SLC39A14 synergistically mediate Mn excretion. SLC39A14 transports Mn from blood into hepatocytes and enterocytes for subsequent excretion by SLC30A10 into bile and feces. SLC30A10 also mediates Mn efflux from neuronal cells. In contrast, SLC39A8 reclaims Mn lost in bile. Elevated brain Mn levels and neurotoxicity evident on loss-of-function of SLC30A10 or SLC39A14 is a consequence of an inhibition of Mn excretion and, for SLC30A10, a block in Mn efflux from neurons. Loss-of-function of SLC39A8, in contrast, produces Mn deficiency.
SLC30A10
Results from human patients
A detailed clinical description of a patient who harbored homozygous loss-of-function mutations in SLC30A10 was first reported in 2008 (131). The patient was born to consanguineous parents, which was suggestive of an autosomal recessive disorder. On clinical examination, notable findings were that the patient exhibited motor abnormalities that influenced gait and fine movements of hands and dystonia that affected all four limbs. Blood Mn levels were ∼10-fold higher than normal. MRI was indicative of basal ganglia Mn deposition. Additionally, there was evidence of cirrhosis and increased liver Mn levels on biopsy. The patient was not environmentally exposed to high Mn, and plasma copper and zinc (Zn) levels were normal (131).
Subsequent studies reported on additional individuals with the above-described clinical features (132, 133). MRI provided evidence for the accumulation of manganese in numerous brain regions, including the caudate and lentiform nuclei, thalamus, cortico-spinal tract, and substantia nigra (132). Accumulation in areas beyond the basal ganglia may reflect the substantial elevations in body manganese levels in the genetic disease. Through whole-genome homozygosity mapping and exome sequencing, homozygous mutations in SLC30A10 were identified in affected patients. As expected, the disease exhibited an autosomal recessive form of inheritance (132, 133). Additional cases of patients harboring homozygous mutations in SLC30A10 and suffering from Mn toxicity were later identified (134–136). Autopsy findings from a patient with homozygous SLC30A10 mutations revealed marked elevations in Mn levels in the brain and liver with normal brain Zn and Fe levels (137). There was loss of neurons in the globus pallidus, which is also a characteristic feature of Mn toxicity secondary to occupational overexposure (60, 138); depigmentation without neuronal loss in the substantia nigra; and hepatomegaly and cirrhosis (139). Overall, the major implication of the human studies is that loss-of-function mutations in SLC30A10 alter Mn homeostasis in a manner that leads to the retention of Mn in the body. Accumulation of Mn in the brain, particularly in the basal ganglia, and liver likely cause neurotoxicity and hepatic damage, respectively. Notably, whereas the above discussion relates to rare homozygous mutations in SLC30A10, more recently, widely prevalent SNPs in SLC30A10 associated with altered blood manganese and neurological function have been identified (140, 141), suggesting that changes in SLC30A10 function likely influence Mn neurotoxicity in the general population as well.
Characterization of SLC30A10 as a Mn efflux transporter from cell culture assays
Determination of the molecular mechanism of action of SLC30A10 came from studies in cell culture. SLC30A10 is a member of the SLC30 family of metal transporters that usually transport Zn (142). Initial studies also characterized SLC30A10 as a Zn efflux transporter (143). However, doubts were raised by the fact that human patients had elevated Mn, but not Zn, levels. Evidence generated over the last few years indicates that SLC30A10 is a specific Mn efflux transporter. In multiple different cell types, WT SLC30A10 localized to the cell surface, decreased cellular Mn levels, and protected against Mn-induced toxicity (144). Disease-causing SLC30A10 mutants were retained in the endoplasmic reticulum and did not mediate Mn efflux (144). In GABAergic primary mouse midbrain neurons, expression of SLC30A10WT, but not a disease-causing mutant, protected against Mn-induced neurotoxicity (144). In neuronal AF5 cells, knockdown of SLC30A10 elevated cellular Mn levels and increased sensitivity to Mn toxicity (144). Importantly, overexpression of SLC30A10WT did not impact intracellular Zn levels or protect against Zn-induced cell death (144, 145). Mechanisms that confer Mn transport specificity to SLC30A10 are unclear, but analyses of the predicted structure of SLC30A10 and mutational assays suggest that the metal-binding site within its transmembrane domain is substantially different from that of related Zn transporters (145, 146). Orientation of amino acids within the transmembrane domain of SLC30A10 may favor Mn binding while simultaneously disfavoring association of other metals, such as Zn. Notably, mutations in residues required for, or adjacent to those required for, the Mn transport activity of SLC30A10 have been identified to induce disease in humans (136, 145). Liposome-based transport assays and crystallization may provide the information necessary to better-understand the mechanisms that confer specific Mn transport capability to SLC30A10.
Understanding the function of SLC30A10 at the organism level using mouse, nematode, and zebrafish models
Full-body Slc30a10 knockout mice were generated and characterized in 2017 (147). Knockout animals had ∼20–60-fold increases in brain, liver, and blood Mn levels (147). Other essential metals (Zn, Cu, and Fe) were largely unaffected (147). The full-body knockouts exhibited a failure-to-thrive phenotype and died between 7 and 8 weeks of age (147). Histological analyses provided signs of thyroid dysfunction, and hormone assays demonstrated that the animals suffered from severe hypothyroidism (147). Further analyses revealed that the knockouts accumulated high levels of Mn in the thyroid, which blocked thyroxine biosynthesis (147, 148). The phenotype was rescued when animals were fed a low-Mn diet, which decreased tissue Mn levels (147). Additionally, knockout of the Mn importer SLC39A14 in the Slc30a10 knockouts (i.e. Slc30a10/Slc39a14 double knockouts; see below for a more detailed description of these double knockouts) reduced thyroid Mn levels and also rescued the hypothyroidism phenotype (148). The novel phenotype of Slc30a10 knockout mice raises the hypothesis that thyroid dysfunction may be an understudied aspect of Mn-induced disease that exacerbates the direct neurotoxic effects of Mn. At least one human patient with homozygous SLC30A10 mutations has now been reported to also have hypothyroidism (149).
As detailed below, further understanding of the mechanisms leading to Mn retention upon loss-of-function of SLC30A10 are derived from analyses of tissue-specific Slc30a10 knockout mice. Mn is excreted by the liver and intestines into bile and feces, with biliary excretion being the predominant route of Mn elimination (17, 150–153). In mice and humans, strong expression of SLC30A10 was detectable in the liver and intestines (132, 139, 147, 148, 154, 155). Moreover, SLC30A10 localized to the apical/canalicular aspect of polarized HepG2 cells that model hepatocytes (148) and CaCo2 cells that model enterocytes (154), raising the hypothesis that the transport activity of SLC30A10 mediates hepatic and intestinal Mn excretion. Consistent with this, tissue-specific Slc30a10 knockout mice lacking SLC30A10 in both the liver and intestines (generated using an endoderm-specific Cre) exhibited marked increases in blood and brain Mn levels and had reduced fecal Mn levels (154). The above data suggest that loss-of-function of SLC30A10 blocks Mn excretion, which leads to a build-up of Mn within the body, and the retained Mn accumulates in the brain to induce neurotoxicity (Fig. 3). The excretory function of SLC30A10 was validated in 2019 using radioactive Mn excretion and surgical approaches (156).
In addition to its role in Mn excretion, SLC30A10 has an additional neuroprotective function in the brain (Fig. 3) (154). Robust expression of SLC30A10 was detected in the human and mouse brain, including neurons of the basal ganglia (132, 139, 147, 148, 154, 155). Mn levels in the basal ganglia of pan-neuronal/glial Slc30a10 knockout mice, lacking SLC30A10 in all neurons and glia, were comparable with littermate controls (154). However, exposure to a sub-chronic Mn regimen produced larger increases in basal ganglia Mn levels of the pan/neuronal-glial knockouts than littermates. These findings suggest that activity of SLC30A10 in the brain is likely important to reduce Mn levels and protect against neurotoxicity when body Mn levels become elevated (Fig. 3). In totality, the data available to date suggest that neurotoxicity on loss-of-function of SLC30A10 is a consequence of an inhibition of hepatic and intestinal Mn excretion combined with a block in the efflux of Mn from vulnerable basal ganglia neurons (Fig. 3).
Results in other organisms support findings obtained in mice. As examples, in Caenorhabditis elegans, SLC30A10WT protected DAergic neurons against Mn toxicity, rescued a Mn-induced behavioral defect, and increased viability on exposure to elevated levels of Mn, whereas a disease-causing mutant failed to exert these protective effects (144). There was no effect of SLC30A10WT expression on Zn toxicity in the nematodes (157). Additionally, depletion of SLC30A10 in zebrafish also induced Mn toxicity (158).
SLC39A14
Results from human patients
In 2016, homozygous loss-of-function mutations in SLC39A14 were reported to induce another inherited form of Mn neurotoxicity (159). SLC39A14 is a member of the SLC39 family of metal transporters (160). Unlike SLC30 proteins, members of the SLC39 family mediate metal influx; most members mediate Zn influx, but SLC39A14 can also mediate influx of Mn, Fe, and cadmium (Cd). Within the first decade of life, affected patients exhibited severe neurological dysfunction, including developmental deficits, dystonia, bulbar defects, spasticity, scoliosis, and loss of independent ambulatory activity (159). Parkinsonian features were evident in some (159). Blood Mn levels of patients were elevated, but importantly, Fe, Zn, and Cd in blood were within normal limits when tested (159). MRI indicated that there was accumulation of Mn in the brain, including in the globus pallidus and striatum (159). An important distinction from disease induced by SLC30A10 mutations was the lack of deposition of Mn in the liver and, consequently, lack of liver dysfunction (159). This clinical finding provided the first clue that activity of SLC39A14 may be necessary to transport Mn from blood into hepatocytes (Fig. 3). Post-mortem analyses of one patient showed evidence of neuronal degeneration in the globus pallidus (159). Additional descriptions of patients suffering from Mn toxicity due to mutations in SLC39A14 were reported in 2018 (161, 162).
Mechanistic in vivo and in vitro assays
Studies performed prior to discovery of the genetic disease had already demonstrated that SLC39A14 could mediate influx of Zn, Mn, Fe, and Cd (160, 163–168). SLC39A14WT and disease-causing mutants localized to the cell surface, but the Mn transport capacity of the mutants was lower than for the WT protein (159). Combined with the fact that levels of Fe, Cd, and Zn were unaltered in patients, these results suggested that a defect in Mn influx underlies the observed disease phenotype.
Findings from Slc39a14 knockout mice were first reported in 2010 and 2011 (169, 170), but changes in Mn homeostasis were identified in 2017 by three independent groups (171–173). Consistent with observations in human patients, knockout mice exhibited increased Mn levels in blood and brain, but this increase was not evident in the liver (171–173). These findings were also consistent with results in zebrafish depleted in SLC39A14 in which Mn levels in the brain, but not abdominal viscera, were elevated (159).
A straightforward hypothesis emerged from the above-described findings in human patients and model systems lacking SLC30A10 or SLC39A14: SLC39A14 and SLC30A10 likely function cooperatively to mediate Mn excretion, with SLC39A14 transporting Mn from blood into hepatocytes and enterocytes and SLC30A10 transporting Mn into bile and feces (Fig. 3). This hypothesis supported two important predictions. First, SLC39A14 and SLC30A10 should localize to the basolateral and apical domains of polarized hepatocytes and enterocytes, respectively (Fig. 3). Second, liver and intestine Mn levels of Slc30a10/Slc39a14 double knockouts should not be elevated (148). As mentioned above, in polarized HepG2 cells, SLC30A10 was detected in the apical/luminal domain (148). In the same system, SLC39A14 localized to the basolateral aspect (148). Basolateral localization of SLC39A14 in polarized HepG2 cells was consistent with earlier observations in rat liver sections (174). A similar localization of SLC30A10 and SLC39A14 was also reported in Caco-2 enterocytes (154, 175). Analyses of Slc30a10/Slc39a14 double-knockout mice provided direct support for the above hypothesis. In Slc30a10/Slc39a14 double knockouts, liver Mn levels were not elevated (148). This effect was specific because, in the same experiment, liver Mn levels of Slc30a10 single knockouts were substantially higher than WT, but no change was evident in Slc39a14 single knockouts (148). Furthermore, blood and brain Mn levels of the double knockouts were higher than WT controls and both single knockouts (148). Results from the double knockouts imply that activity of SLC39A14 is necessary to transport Mn into hepatocytes for subsequent excretion by SLC30A10 (Fig. 3). Whereas intestinal Mn levels were not analyzed in the double knockouts, the differential localization of SLC30A10 and SLC39A14 in enterocytes described above, data from tissue-specific Slc30a10 knockout mice (154, 156), and recent findings showing that SLC39A14 is required for the transport of Mn from blood into enterocytes (176), put together, suggest that SLC30A10 and SLC39A14 likely also act cooperatively to mediate intestinal Mn excretion (Fig. 3). We note an additional interesting feature of the Slc30a10/Slc39a14 double knockouts. SLC39A14, but not SLC30A10, was detected in the thyroid (148). Consequently, thyroid Mn levels of Slc39a14 single and Slc30a10/Slc39a14 double knockouts were lower than Slc30a10 single knockouts, and both Slc39a14 single and Slc30a10/Slc39a14 double knockouts had functioning thyroid hormone (148). In sum, available data support the model presented in Fig. 3 and suggest that the neurotoxicity evident in patients with SLC39A14 mutations is an effect of a defect in Mn excretion.
SLC39A8
Results from human patients
In 2015, two companion papers reported that mutations in another member of the SLC39 family, SLC39A8, induce an inherited disorder of Mn deficiency (177, 178). SLC39A14 mediates influx of several metals: Zn, Mn, Fe, and Cd as well as cobalt (160, 164, 179). One of the papers reported detailed findings from an infant who had cranial asymmetry, severe infantile spasms with hypsarrhythmia, and disproportionate dwarfism (178). Atrophic changes were seen in the brain on computerized tomography and MRI (178). Plasma and urine Mn levels were below detection, but serum Zn levels and Fe metabolism parameters were unaffected (178). As part of the clinical analyses, glycosylation of serum transferrin (a common biomarker used to screen for congenital disorders of glycosylation) was found to be defective (178). Sequencing revealed that the patient carried homozygous mutations in SLC39A8. Subsequent examination of additional patients with unexplained defects in transferrin glycosylation led to the identification of a second infant with homozygous mutations in SLC39A8, severe neurological deficits, and undetectable Mn levels in whole blood and urine (178).
The second paper described findings from six children who belonged to the genetically isolated Hutterite ethnoreligious group and two other siblings born to consanguineous parents (177). Clinical features included severe intellectual and developmental disabilities, atrophy of the cerebellum, cross-eyed features, and hypotonia with signs being evident as early as birth (177). Sequencing identified homozygous mutations in SLC39A8 (177). Of the eight patients, Mn levels in blood or erythrocytes were decreased in four, at the lower end of the normal range in three, and not determined in one (177). Authors of the first paper performed glycosylation assays on some of the patients described in the second study and discovered that glycosylation of transferrin was defective (178). Overall, the clinical studies suggest that mutations in SLC39A8 induce Mn deficiency, which leads to deficits in glycosylation as several Golgi-localized enzymes involved in the glycosylation pathway require Mn for activity (180–182). Defective glycosylation, in turn, induces neurological disease.
Mechanistic in vivo and in vitro assays
In HeLa cells, SLC39A8WT localized to the cell surface (183). In contrast, disease-causing mutations trapped the transporter in the endoplasmic reticulum and blocked its capability to mediate Mn influx (183). Further mechanistic insights came from analyses of Slc39a8 knockout mice. Full-body depletion of Slc39a8, using a tamoxifen-inducible system, decreased tissue Mn levels and induced glycosylation defects, but did not impact Zn or Fe levels (184). Constitutive liver-specific Slc39a8 knockouts exhibited reductions in Mn levels not only in the liver but also in other tissues, such as the brain and intestines, and overexpression of Slc39a8 in the liver increased Mn levels in the liver as well as in extrahepatic organs (184). Microscopy assays revealed that SLC39A8 localized to the apical surface of polarized hepatocytes (184). Together, these results suggest that SLC39A8 regulates Mn homeostasis by primarily acting in the liver, where it transports Mn from bile into hepatocytes, thereby reclaiming Mn that would otherwise be excreted (Fig. 3). When SLC39A8 function is compromised, Mn is lost in bile, which subsequently induces Mn deficiency and leads to glycosylation deficits that result in neurological dysfunction (Fig. 3) (177, 178). The means by which blocking glycosylation produces specific neurological changes observed in patients with SLC39A8 mutations remain to be elucidated. Overall, SLC30A10, SLC39A14, and SLC39A8 have emerged as the critical regulators of Mn homeostasis in mammals. Much of the on-going work relates to using tissue-/cell-specific knockouts of these genes in mice to better understand how Mn homeostasis in specific neuronal populations and other peripheral organs is regulated and how changes in the homeostatic control of Mn induce neurotoxicity. This line of research, as well as work on other transporters that mediate transport of Mn into intracellular organelles (e.g. SPCA1 into the Golgi apparatus (185)), is expected to substantially improve understanding of Mn-induced neurological disease.
Pathogenic conditions linked to alteration in brain Mn status
Exposure to Mn can have detrimental effects on the brain at any stage of life, causing neurobehavioral and neuromotor deficits and/or neuropsychiatric illness (6, 78). Early life exposure to Mn has been shown to cause neurotoxic effects of concern in susceptible subgroups such as children and adolescents as their brains are undergoing developmental processes involving differentiation, apoptosis, and pathway direction. Each of these processes is vulnerable to deviations from normal physiology caused by exposure to Mn (186). A South Korean cohort–based study showed an inverted U-shaped association between blood Mn concentrations and infant mental development (187). A Taiwanese study showed a significant inverse association between Mn and Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT) scores at 2 years of age (188). Yet another cohort showed decreased cognitive scores on Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) when they were subjected to pre- and postnatal exposure (81, 189).
Adolescence is the next critical period where the brain is undergoing transformative changes. Exposure to Mn during this time can potentially alter continuing myelination and may affect intellectual and cognitive abilities, as adolescence is a crucial time for brain maturation and pruning (190, 191). Additionally, adolescence is also a time when there are structural and functional changes happening in the prefrontal cortex (192–194). A study in children aged 8–11 years showed that both lower and higher concentration of Mn altered cognition and other behavioral measures on a battery of tests (81). A study in a cohort of children living near a ferromanganese factory in Ohio showed a nonlinear U-shaped relationship between two Mn biomarkers, hair and blood, with pediatric intelligence quotient (28).
Adult populations are also vulnerable to inhaled Mn that enters the brain through the olfactory system (195). This can happen in both occupational and nonoccupational settings. Biomarkers, including scalp hair, axillary hair, fingernails, and saliva, were examined in 89 Brazilian men and women and showed that elevated levels of Mn were associated with lower visual scores in visual working memory (196). Another Brazilian study of 82 mothers and children showed that high Mn concentration in hair was associated with poor cognitive performance, particularly in the verbal domain (197). Studies that investigated biomarkers in people exposed to Mn in an occupational setting also showed deficits in cognition, specifically in working memory, compared with the control subjects (198). A cohort of Mn-exposed workers and their control counterparts were subjected to the Montreal Cognitive Assessment (MoCA) test, where the control group showed better cognition than the Mn-exposed group. This study also investigated the effects of Mn exposure on plasma brain-derived neurotrophic factor, which was lower in the Mn-exposed group (199). Increased Mn exposure in these human studies was investigated further with imaging studies to better identify the brain regions where Mn accumulated. These studies consistently identified the globus pallidus, frontal cortex, and striatum as brain regions that showed higher accumulation of Mn in exposed populations (59, 200, 201).
Neurobiology and neuronal metabolic pathways linked to Mn status
As an essential metal, it follows that deficient or excessive levels of Mn could result in biological dysfunction and disease. For example, enzymes that require Mn for their function may be expected to become dysfunctional in cases of insufficiency or overexposure. Key enzymes such as arginase and agmatinase, which are a part of the urea cycle in the brain, require Mn as a cofactor, providing a direct link between Mn status and enzymatic function (18). An Mn-deficient diet in rats caused decreased insulin production mimicking a diabetic-like state, and pancreatic insulin following glucose stimulus was also affected (202–205). A Mn-deficient diet also led to decreased circulating IGF-1 and insulin (206). Mn and physiologically relevant levels of IGF synergistically regulate IGF/insulin activity in the cell lines tested (207). Taken together, these studies point to a connection between Mn and the regulation of IGF-1/insulin level. The reader is referred to recent reviews that discuss changes in the neuronal urea cycle, insulin signaling, and the cellular process of autophagy that are linked to Mn health and dysfunction associated with changes in brain Mn levels in depth (105, 132, 208–213).
Mn neurotoxicity and parkinsonism
PD is a progressive motor disorder characterized by selective neurodegeneration of DAergic neurons in the substantia nigra (214). Under conditions of Mn neurotoxicity, Mn has been shown to accumulate in the subcortical structures of the basal ganglia, particularly the substantia nigra pars compacta, pallidum, and striatum (60, 215–217). As these brain regions are particularly sensitive to oxidative injury, a supported mechanism for Mn neurotoxicity, it is thought that this combination explains the relative involvement of these brain structures in Mn neurotoxicity (117, 217–220). The accumulation of Mn in basal ganglia occurs across multiple routes of exposure and genetic bases for elevated systemic Mn levels, including chronic oral exposure from contaminated water (218), inhalation of Mn particulates from ferromanganese plants (28), patients receiving parenteral nutrition (221, 222), or direct intravenous delivery (i.e. ephedrone users), as well as genetic alterations in the Mn efflux transporters as described earlier in this review (219, 223–226). In recently identified cases, manganese toxicity was caused by ephedrone abuse, which leads to manifestation of parkinsonian symptoms due to accumulation of Mn (227, 228). To further understand the underlying mechanism by which ephedrone leads to parkinsonian symptoms, imaging studies were performed in humans and nonhuman primates. These studies consistently show a lack of degeneration of dopamine neurons (224, 227, 228). Even though there is minimal degeneration of the neurons, ephedrone acts as a stimulant and induces the release of monoamines, thus causing a deviation from the normal physiology (229).
Excess brain Mn accumulation causes manganism (60, 216, 217, 230). Given that there are some similarities between the symptoms of manganism and idiopathic PD (231), although key differences exist as well (217, 232), research has predominantly focused on dysfunction and degeneration of dopaminergic neurons of the substantia nigra, although arguments for the involvement of other brain regions are also substantiated (138, 217, 233–235). A 1-week systemic Mn exposure trial in mice showed sex-dependent changes in dendritic spine density for medium spiny neurons (a subtype of GABAergic inhibitory neurons) of the striatum 3 weeks after exposure (Fig. 4). This may be evidence of damage either to these neurons directly or to the cortical glutamatergic or nigral dopaminergic presynaptic neurons that synapse onto the medium spiny neurons (83). In favor of a role for the presynaptic nigral dopaminergic neurons, Mn exposure is associated with a decrease in striatal dopamine and dopamine metabolite levels, although other neurotransmitters are also affected (82). It is noteworthy that deficits that are seen in motor function are associated with a marked decrease of in vivo DA release (103, 212, 236). Whereas these data do not report significant deficits in levels of TH or other structural markers of DA terminals, it is possible that chronic exposures and/or repeated deficits of in vivo DA release have long-term detrimental effects on DA structural markers. However, these data also suggest key differences between idiopathic PD and the neurotoxic effects of Mn. Further investigations with long-term exposure studies are needed to definitively determine the role of changes to DA systems in chronic Mn neurotoxicity.
Figure 4.
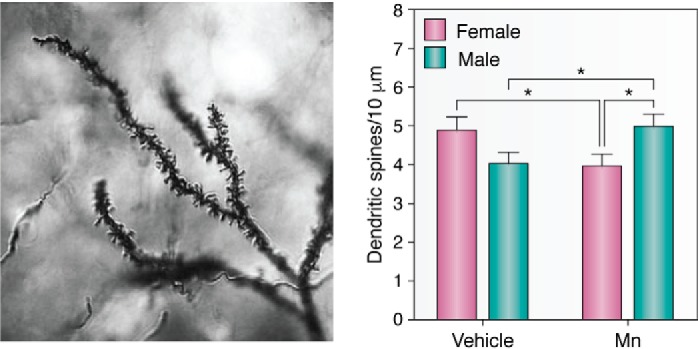
Mn exposure alters synaptic spine density in striatal MSNs. Left, representative mouse MSN dendritic segment impregnated by the rapid Golgi method, reproduced here with permission (83). Dendritic branching is clearly visualized as well as dendritic spines. Image obtained in 2010 by Jennifer Madison, Ph.D. in the Aaron Bowman laboratory at Vanderbilt University. Right, total spine density was the only measure of neuron morphology to have a significant gender difference 3 weeks post-exposure. Mn-exposed (Mn) male mice had a higher total spine density than Mn-exposed female mice, whereas Mn exposure decreased spine density in female mice. More studies need to be done to establish the spine density levels between sexes under control conditions to consistently better-establish the differences caused by exposure to contaminants. Data are plotted as mean ± S.E. (error bars); *, p < 0.05, post hoc t test. Data were originally published in Ref. 83. This research was originally published in Neurotoxicology. Madison, J. L., Wegrzynowicz, M., Aschner, M., and Bowman, A. B. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011; 32:896–906.
There is strong evidence of a decrease of in vivo DA release for both acute and chronic Mn exposure and limited evidence showing lack of degeneration of D1R and D2R in various models. Furthermore, studying the long-term effect on DA neuron viability with chronic Mn overexposure to understand the neurotoxic effects of Mn is vital. Long-term consequences of decreasing DA release on the neurons of the substantia nigra remain to be addressed. There are limited studies suggesting that changes in D2R occur with increasing Mn exposure (237).
In further support of a link between Mn biology and PD, exposure to Mn has been suggested as a PD environmental risk factor (238–240). Further, a dose dependence between Mn-containing welding fumes and advancement of parkinsonism-like features has been observed (40). Indeed, parkinsonism is clinically distinguishable only by age of onset (46 versus 63 years, respectively) in welders versus nonwelders, and there is an increased prevalence of PD among welders compared with an age-matched population (9, 233, 241). A study done in Taiwanese ferromanganese factory workers showed that there was Mn accumulation in the globus pallidus and substantia nigra par reticulata, caudate nucleus, and putamen with minimal effects on the sub-thalamic nucleus and substantia nigra pars compacta. One of the points to keep in mind regarding the studies that point to the lack of degeneration of the neuronal terminals is that the imaging studies were done immediately after exposure. A better understanding of long-term and delayed effects of Mn neurotoxicity is still needed to provide clarity on the mechanisms at play (40, 232, 234). Last, transporters such as DMT-1 (divalent metal transporter-1) have been implicated in parkinsonian-like neurodegeneration in animal models (242). More investigation is needed to understand how transporters like DMT-1 could also give rise to parkinsonian symptoms due to Mn neurotoxicity as DMT-1 is involved in transport of Mn (243).
Potential role for Mn dyshomeostasis in Alzheimer's disease (AD) and other degenerative disorders
The relationship between Mn toxicology and AD needs to be further explored. Importantly for AD, Mn is required as a co-factor in enzymatic reactions including neurotransmitter metabolism (glutamine synthetase) and antioxidant status (MnSOD) (18). Glutamine synthetase is required for recycling of glutamate to glutamine within the astrocyte and as such plays a key role in glutamate clearance from the synapse, whereas MnSOD protects oxidative status in the mitochondria. Whereas Mn deficiency could thus impair glutamine synthetase enzymatic function, Mn exposure in cultured astrocytes was associated with increased intra- and extracellular glutamate, impairment of gap junctions, and cell death (244). As such, Mn sufficiency is key to brain health and protection from excitotoxicity and oxidative stress, particularly in neurodegenerative disorders (245). Nevertheless, evidence suggests that both deficiency and excess Mn can contribute to the development of AD, although more work is necessary to clarify potentially contradictory findings in the literature.
In a study of >2,000 Chinese elders, serum Mn levels were lower in AD and mild cognitive impairment (MCI) cases than in healthy controls (246). Epidemiological data from human AD and MCI studies are more equivocal and dependent on methods and sample type but support the role for altered Mn accumulation in AD brains (247, 248). In earlier studies, decreased Mn in AD and MCI were closely related to changes in other transition metals, including Fe and aluminum (249), and were dependent on sample type (e.g. serum but not erythrocytes (250)), which may also explain the lack of clarity in the literature. MnSOD RNA expression was decreased in lymphocytes in AD patients (251). In contrast, MnSOD expression was determined to be 3–11-fold greater in the hippocampus of AD patients than controls (252). In post-mortem AD tissue, MnSOD was localized to astrocytes associated with β-amyloid plaques in cortex and hippocampus, suggesting a response to a pathological stressor (253). In the Tg19959 mouse model of AD, decreased expression of MnSOD exacerbated β-amyloid pathology, whereas increased expression led to a 33% decrease in β-amyloid plaque burden and was associated with improvement in spatial memory (254, 255). MnSOD, a key antioxidant enzyme, is one target of tyrosine nitration causing degradation of the enzyme. Activity but not overall expression decreased in 5-month-old APP and PSEN1 knock-in mice due to increased nitration in transgenic mice, which worsened with age and β-amyloid accumulation (256) and contributed to decreased mitochondrial respiration. Even though studies have demonstrated that altered Mn exposure or handling may directly impact neuropathology through altered enzymatic function, there is no evidence that this happens at this age.
Exposure to Mn and other potential neurotoxicants through particulate matter in air pollution has been studied in post-mortem tissues from young adults in Mexico City (257) compared with controls in low-pollution cities. Although neither lung Mn levels nor lung nor cortical Fe levels increased significantly, an ∼50% increase in frontal cortex Mn was observed by MS in the pollution-exposed population. Mn was significantly correlated with IL-1β gene expression, suggesting inflammatory response changes. Neuropathological changes were noted in young adults from the Mexico City cohort that were not evident in control brains, including diffuse amyloid plaques (51%) and hyperphosphorylated tau (40%), suggesting a direct impact on development of AD even in young adults (258). The role of chronic exposure to modest levels of Mn in the development of AD pathology was assessed directly in cynomologous macaques (236, 259). Mn sulfate (3.3–5.0 mg/kg) given intravenously for 40 weeks increased Mn levels in the frontal cortex nearly 50% with no concomitant change in Fe levels. Strikingly, expression of β-amyloid–like protein 1 mRNA (APLP1, a member of the amyloid precursor protein family) was strongly up-regulated in the frontal cortex in Mn-treated animals, and this was confirmed with immunohistochemistry (236). Mn-treated animals also showed evidence of diffuse β-amyloid plaques that were not evident in control animals, as well as degenerating and apoptotic cells. A similar duration of Mn treatments also led to a modest decrease in cognitive function, including impaired spatial working memory and fine motor skills, and increased compulsive-like behaviors (259). Together, these results suggest a direct role for Mn in activating a cellular stress response and in driving AD neuropathology. Most AD cases cannot be attributed to a single gene or mutation. AD age of onset and speed of decline of both cognitive and pathological markers of disease is therefore far more susceptible to environmental influences, likely including changes in brain Mn status. An important role for dysfunction in Mn homeostasis is suggested by Mn exposure studies in rodents and nonhuman primates and correlational studies in human populations, as elucidated in this review. Both Mn deficiency and toxicity are implicated in AD-related changes through inflammatory response, oxidative stress, and degenerative pathways as well as direct effects on neuropathological markers. Nevertheless, the role(s) of altered Mn needs to be clarified in AD, particularly regarding genetic variation and Mn exposure levels within different human populations or communities.
Summary
Although essential for cellular metabolic signaling, exposures to high Mn levels cause neurotoxicity. This review of the available data suggests that Mn toxicity is multifaceted, yet genetic modulation of its transport (uptake and efflux) plays a major role in governing both health and disease. Numerous studies have pointed to a link between Mn homeostasis and Fe, and the ensuing oxidative stress secondary to impaired mitochondrial function and energy failure is a proven mediator of Mn-induced neurotoxicity. Despite growing awareness of the association between Mn exposure and adverse neurological outcomes, studies have yet to fully elucidate the underlying molecular mechanisms involved in its neurotoxicity. Mn also has an important role as an enzymatic cofactor, especially in insulin and insulin growth factor signaling pathways, along the continuum of biological Mn deficiency through neurotoxic Mn overexposure. Multiple kinases and phosphatases are known Mn-dependent (or Mn-preferring) enzymes, and as such, future experimental studies should identify their role in the etiology of neurodegenerative diseases vis-à-vis alterations in Mn brain levels. Whereas studies carried out in the last few decades have been highly instrumental in adding novel information on molecular aspects involved in Mn-induced neurotoxicity, further research on the role of Mn in health and disease as well as effective treatment strategies to reverse its neurological consequences are warranted. Additionally, it is important to draw attention to the fact that there are also differences in how individual research studies (sometimes with conflicting findings) have utilized either different animal species or formulations of Mn to treat/expose the subjects. The inconsistencies in reported observations could also be due to difference in the duration of exposure. Whereas standardizing protocols may eliminate the variation in results, it is nonetheless still important to learn the effects of Mn caused by different exposure durations and compositions. It would also be pertinent to model studies based on mixtures of other toxicants with Mn that human populations are exposed to in the environment, reflecting real-life scenarios of exposure. Importantly, these future studies should also consider age and sex, in addition to genetic factors, in modulating Mn-induced neurotoxicity.
Author contributions
R. C. B., S. M., D. M., J. V., F. E. H., E. N. H., and A. B. B. writing-original draft; R. C. B., S. M., D. M., M. A., E. N. H., and A. B. B. writing-review and editing; A. B. B. conceptualization; A. B. B. data curation; A. B. B. supervision; A. B. B. funding acquisition.
Acknowledgments
We thank Dr. Jennifer Madison for the rapid Golgi stain image and Dr. Andrey Selyunin (University of Texas, Austin) for initial drafts of Fig. 3.
This work was supported by NIEHS, National Institutes of Health, Grants R01 ES024812 (to S. M.), RO1 ES016931 (to A. B. B.), RO1 ES010563 (to A. B. B. and M. A.), R01 ES031401 (to F. E. H. and A. B. B.), and R01 ES026446 (to E. N. H.) and by Veterans Affairs Grant I01 CX001610-01 (to F. E. H.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- SOD
- superoxide dismutase
- DAergic
- dopaminergic
- PD
- Parkinson's disease
- MRI
- magnetic resonance imaging
- GABA
- γ-aminobutyric acid
- TH
- tyrosine hydroxylase
- DA
- dopamine
- D1R and D2R
- DA subtype 1 and 2 receptor, respectively
- DAT
- dopamine transporter
- IGF
- insulin-like growth factor
- AD
- Alzheimer's disease.
References
- 1. Aschner J. L., and Aschner M. (2005) Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 26, 353–362 10.1016/j.mam.2005.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Underwood M. J., More R. S., Thompson M. M., and Gershlick A. H. (1993) Reduction of vein graft intimal hyperplasia by ex vivo treatment with desferrioxamine manganese. J. Vasc. Res. 30, 239–240 10.1159/000158999 [DOI] [PubMed] [Google Scholar]
- 3. Takeda A. (2003) Manganese action in brain function. Brain Res. Rev. 41, 79–87 10.1016/S0165-0173(02)00234-5 [DOI] [PubMed] [Google Scholar]
- 4. Wedler F. C. (1993) Biological significance of manganese in mammalian systems. Prog. Med. Chem. 30, 89–133 10.1016/S0079-6468(08)70376-X [DOI] [PubMed] [Google Scholar]
- 5. Keen C. L., Ensunsa J. L., and Clegg M. S. (2000) Manganese metabolism in animals and humans including the toxicity of manganese. Met. Ions Biol. Syst. 37, 89–121 [PubMed] [Google Scholar]
- 6. Vollet K., Haynes E. N., and Dietrich K. N. (2016) Manganese exposure and cognition across the lifespan: contemporary review and argument for biphasic dose-response health effects. Curr. Environ. Health Rep. 3, 392–404 10.1007/s40572-016-0108-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guilarte T. R. (2013) Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front. Aging Neurosci. 5, 23 10.3389/fnagi.2013.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez-Cuyar L. F., Nelson G., Criswell S. R., Ho P., Lonzanida J. A., Checkoway H., Seixas N., Gelman B. B., Evanoff B. A., Murray J., Zhang J., and Racette B. A. (2014) Quantitative neuropathology associated with chronic manganese exposure in South African mine workers. Neurotoxicology 45, 260–266 10.1016/j.neuro.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Racette B. A., Tabbal S. D., Jennings D., Good L., Perlmutter J. S., and Evanoff B. (2005) Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology 64, 230–235 10.1212/01.WNL.0000149511.19487.44 [DOI] [PubMed] [Google Scholar]
- 10. Caito S., and Aschner M. (2015) Neurotoxicity of metals. Handb. Clin. Neurol. 131, 169–189 10.1016/B978-0-444-62627-1.00011-1 [DOI] [PubMed] [Google Scholar]
- 11. Roh E., Song D. K., and Kim M. S. (2016) Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 48, e216 10.1038/emm.2016.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization (2011) Manganese in drinking-water: background document for development of WHO Guidelines for Drinking-water Quality, pp. 5–6, WHO Press, Geneva [Google Scholar]
- 13. Lehmann T., and Lämmle B. (1999) IFNα treatment in systemic mastocytosis. Ann. Hematol. 78, 483–484 10.1007/s002770050604 [DOI] [PubMed] [Google Scholar]
- 14. Das A. Jr., and Hammad T. A. (2000) Efficacy of a combination of FCHG49 glucosamine hydrochloride, TRH122 low molecular weight sodium chondroitin sulfate and manganese ascorbate in the management of knee osteoarthritis. Osteoarthritis Cartilage 8, 343–350 10.1053/joca.1999.0308 [DOI] [PubMed] [Google Scholar]
- 15. Finley J. W., Johnson P. E., and Johnson L. K. (1994) Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am. J. Clin. Nutr. 60, 949–955 10.1093/ajcn/60.6.949 [DOI] [PubMed] [Google Scholar]
- 16. Davis C. D., Zech L., and Greger J. L. (1993) Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc. Soc. Exp. Biol. Med. 202, 103–108 10.3181/00379727-202-43518 [DOI] [PubMed] [Google Scholar]
- 17. Papavasiliou P. S., Miller S. T., and Cotzias G. C. (1966) Role of liver in regulating distribution and excretion of manganese. Am. J. Physiol. 211, 211–216 10.1152/ajplegacy.1966.211.1.211 [DOI] [PubMed] [Google Scholar]
- 18. Horning K. J., Caito S. W., Tipps K. G., Bowman A. B., and Aschner M. (2015) Manganese is essential for neuronal health. Annu. Rev. Nutr. 35, 71–108 10.1146/annurev-nutr-071714-034419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibbons R. A., Dixon S. N., Hallis K., Russell A. M., Sansom B. F., and Symonds H. W. (1976) Manganese metabolism in cows and goats. Biochim. Biophys. Acta 444, 1–10 10.1016/0304-4165(76)90218-X [DOI] [PubMed] [Google Scholar]
- 20. Reaney S. H., Bench G., and Smith D. R. (2006) Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol. Sci. 93, 114–124 10.1093/toxsci/kfl028 [DOI] [PubMed] [Google Scholar]
- 21. Wasserman G. A., Liu X., Parvez F., Ahsan H., Levy D., Factor-Litvak P., Kline J., van Geen A., Slavkovich V., LoIacono N. J., Cheng Z., Zheng Y., and Graziano J. H. (2006) Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 114, 124–129 10.1289/ehp.8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan K., Wasserman G. A., Liu X., Ahmed E., Parvez F., Slavkovich V., Levy D., Mey J., van Geen A., Graziano J. H., and Factor-Litvak P. (2012) Manganese exposure from drinking water and children's academic achievement. Neurotoxicology 33, 91–97 10.1016/j.neuro.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Center for Environmental Assessment (2012) Manganese (CASRN 7439-96-5). in Integrated Risk Information System, Environmental Protection Agency, Washington, D. C. [Google Scholar]
- 24. Environmental Protection Agency (2020) Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. Environmental Protection Agency, Washington, D. C. [Google Scholar]
- 25. Ljung K., and Vahter M. (2007) Time to re-evaluate the guideline value for manganese in drinking water? Environ. Health Perspect. 115, 1533–1538 10.1289/ehp.10316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collipp P. J., Chen S. Y., and Maitinsky S. (1983) Manganese in infant formulas and learning disability. Ann. Nutr. Metab. 27, 488–494 10.1159/000176724 [DOI] [PubMed] [Google Scholar]
- 27. Leonhard M. J., Chang E. T., Loccisano A. E., and Garry M. R. (2019) A systematic literature review of epidemiologic studies of developmental manganese exposure and neurodevelopmental outcomes. Toxicology 420, 46–65 10.1016/j.tox.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 28. Haynes E. N., Sucharew H., Kuhnell P., Alden J., Barnas M., Wright R. O., Parsons P. J., Aldous K. M., Praamsma M. L., Beidler C., and Dietrich K. N. (2015) Manganese exposure and neurocognitive outcomes in rural school-age children: the communities actively researching exposure study (Ohio, U.S.A.). Environ. Health Perspect. 123, 1066–1071 10.1289/ehp.1408993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torres-Agustín R., Rodríguez-Agudelo Y., Schilmann A., Solís-Vivanco R., Montes S., Riojas-Rodríguez H., Cortez-Lugo M., and Ríos C. (2013) Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res. 121, 39–44 10.1016/j.envres.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 30. Carvalho C. F., Menezes-Filho J. A., de Matos V. P., Bessa J. R., Coelho-Santos J., Viana G. F., Argollo N., and Abreu N. (2014) Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology 45, 301–308 10.1016/j.neuro.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 31. Solís-Vivanco R., Rodríguez-Agudelo Y., Riojas-Rodríguez H., Ríos C., Rosas I., and Montes S. (2009) Cognitive impairment in an adult Mexican population non-occupationally exposed to manganese. Environ. Toxicol. Pharmacol. 28, 172–178 10.1016/j.etap.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 32. Davis J. M. (1998) Methylcyclopentadienyl manganese tricarbonyl: health risk uncertainties and research directions. Environ. Health Perspect. 106, 191–201 10.1289/ehp.98106s1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gulson B., Mizon K., Taylor A., Korsch M., Stauber J., Davis J. M., Louie H., Wu M., and Swan H. (2006) Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline–preliminary results. Environ. Res. 100, 100–114 10.1016/j.envres.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 34. Mora A. M., Arora M., Harley K. G., Kogut K., Parra K., Hernández-Bonilla D., Gunier R. B., Bradman A., Smith D. R., and Eskenazi B. (2015) Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ. Int. 84, 39–54 10.1016/j.envint.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gunier R. B., Arora M., Jerrett M., Bradman A., Harley K. G., Mora A. M., Kogut K., Hubbard A., Austin C., Holland N., and Eskenazi B. (2015) Manganese in teeth and neurodevelopment in young Mexican-American children. Environ. Res. 142, 688–695 10.1016/j.envres.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodier J. (1955) Manganese poisoning in Moroccan miners. Br. J. Ind. Med. 12, 21–35 10.1136/oem.12.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zoni S., Albini E., and Lucchini R. (2007) Neuropsychological testing for the assessment of manganese neurotoxicity: a review and a proposal. Am. J. Ind. Med. 50, 812–830 10.1002/ajim.20518 [DOI] [PubMed] [Google Scholar]
- 38. Iregren A. (1990) Psychological test performance in foundry workers exposed to low levels of manganese. Neurotoxicol. Teratol. 12, 673–675 10.1016/0892-0362(90)90085-Q [DOI] [PubMed] [Google Scholar]
- 39. Roels H., Lauwerys R., Buchet J. P., Genet P., Sarhan M. J., Hanotiau I., de Fays M., Bernard A., and Stanescu D. (1987) Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am. J. Ind. Med. 11, 307–327 10.1002/ajim.4700110308 [DOI] [PubMed] [Google Scholar]
- 40. Racette B. A., Searles Nielsen S., Criswell S. R., Sheppard L., Seixas N., Warden M. N., and Checkoway H. (2017) Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology 88, 344–351 10.1212/WNL.0000000000003533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aschner M. (2006) The transport of manganese across the blood-brain barrier. Neurotoxicology 27, 311–314 10.1016/j.neuro.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 42. Davis J. M. (1999) Inhalation health risks of manganese: an EPA perspective. Neurotoxicology 20, 511–518 [PubMed] [Google Scholar]
- 43. Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., Potter R., Maynard A., Ito Y., Finkelstein J., and Oberdörster G. (2006) Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 114, 1172–1178 10.1289/ehp.9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dorman D. C., Struve M. F., and Wong B. A. (2002) Brain manganese concentrations in rats following manganese tetroxide inhalation are unaffected by dietary manganese intake. Neurotoxicology 23, 185–195 10.1016/S0161-813X(01)00075-4 [DOI] [PubMed] [Google Scholar]
- 45. Bock N. A., Paiva F. F., Nascimento G. C., Newman J. D., and Silva A. C. (2008) Cerebrospinal fluid to brain transport of manganese in a non-human primate revealed by MRI. Brain Res. 1198, 160–170 10.1016/j.brainres.2007.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eriksson H., Gillberg P. G., Aquilonius S. M., Hedström K. G., and Heilbronn E. (1992) Receptor alterations in manganese intoxicated monkeys. Arch. Toxicol. 66, 359–364 10.1007/BF01973632 [DOI] [PubMed] [Google Scholar]
- 47. Williams M., Todd G. D., Roney N., Crawford J., Coles C., McClure P. R., Garey J. D., Zaccaria K., and Citra M. (2012) Toxicological Profile for Manganese, Agency for Toxic Substances and Disease Registry, Atlanta, GA: [PubMed] [Google Scholar]
- 48. Blomlie V., Sivanandan R., and Jynge P. (2020) Manganese uptake and accumulation in the human brain. AJNR Am. J. Neuroradiol. 41, E3 10.3174/ajnr.A6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hernández-Bonilla D., Schilmann A., Montes S., Rodríguez-Agudelo Y., Rodríguez-Dozal S., Solís-Vivanco R., Ríos C., and Riojas-Rodríguez H. (2011) Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology 32, 615–621 10.1016/j.neuro.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 50. Standridge J. S., Bhattacharya A., Succop P., Cox C., and Haynes E. (2008) Effect of chronic low level manganese exposure on postural balance: a pilot study of residents in southern Ohio. J. Occup. Environ. Med. 50, 1421–1429 10.1097/JOM.0b013e3181896936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lucchini R. G., Guazzetti S., Zoni S., Donna F., Peter S., Zacco A., Salmistraro M., Bontempi E., Zimmerman N. J., and Smith D. R. (2012) Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology 33, 687–696 10.1016/j.neuro.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finkelstein M. M., and Jerrett M. (2007) A study of the relationships between Parkinson's disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ. Res. 104, 420–432 10.1016/j.envres.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 53. Huang C. C. (2007) Parkinsonism induced by chronic manganese intoxication—an experience in Taiwan. Chang Gung Med. J. 30, 385–395 [PubMed] [Google Scholar]
- 54. Huang C. C., Chu N. S., Lu C. S., Chen R. S., Schulzer M., and Calne D. B. (2007) The natural history of neurological manganism over 18 years. Parkinsonism Relat. Disord. 13, 143–145 10.1016/j.parkreldis.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 55. Revilla F. J., Larsh T. R., Mani A., Duker A. P., Cox C., Succop P., Gartner M., Jarrin Tejada C., and Bhattacharya A. (2013) Effect of dopaminergic medication on postural sway in advanced Parkinson's disease. Front. Neurol. 4, 202 10.3389/fneur.2013.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oulhote Y., Mergler D., Barbeau B., Bellinger D. C., Bouffard T., Brodeur M. È., Saint-Amour D., Legrand M., Sauvé S., and Bouchard M. F. (2014) Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ. Health Perspect. 122, 1343–1350 10.1289/ehp.1307918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rugless F., Bhattacharya A., Succop P., Dietrich K. N., Cox C., Alden J., Kuhnell P., Barnas M., Wright R., Parsons P. J., Praamsma M. L., Palmer C. D., Beidler C., Wittberg R., and Haynes E. N. (2014) Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in Southeastern Ohio. Neurotoxicol. Teratol. 41, 71–79 10.1016/j.ntt.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Laohaudomchok W., Lin X., Herrick R. F., Fang S. C., Cavallari J. M., Shrairman R., Landau A., Christiani D. C., and Weisskopf M. G. (2011) Neuropsychological effects of low-level manganese exposure in welders. Neurotoxicology 32, 171–179 10.1016/j.neuro.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma R. E., Ward E. J., Yeh C. L., Snyder S., Long Z., Gokalp Yavuz F., Zauber S. E., and Dydak U. (2018) Thalamic GABA levels and occupational manganese neurotoxicity: association with exposure levels and brain MRI. Neurotoxicology 64, 30–42 10.1016/j.neuro.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olanow C. W. (2004) Manganese-induced parkinsonism and Parkinson's disease. Ann. N.Y. Acad. Sci. 1012, 209–223 10.1196/annals.1306.018 [DOI] [PubMed] [Google Scholar]
- 61. Criswell S. R., Perlmutter J. S., Huang J. L., Golchin N., Flores H. P., Hobson A., Aschner M., Erikson K. M., Checkoway H., and Racette B. A. (2012) Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup. Environ. Med. 69, 437–443 10.1136/oemed-2011-100119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han J. H., Chung Y. H., Park J. D., Kim C. Y., Yang S. O., Khang H. S., Cheong H. K., Lee J. S., Ha C. S., Song C. W., Kwon I. H., Sung J. H., Heo J. D., Kim N. Y., Huang M., et al. (2008) Recovery from welding-fume-exposure-induced MRI T1 signal intensities after cessation of welding-fume exposure in brains of cynomolgus monkeys. Inhal. Toxicol. 20, 1075–1083 10.1080/08958370802116634 [DOI] [PubMed] [Google Scholar]
- 63. Finkelstein Y., Zhang N., Fitsanakis V. A., Avison M. J., Gore J. C., and Aschner M. (2008) Differential deposition of manganese in the rat brain following subchronic exposure to manganese: a T1-weighted magnetic resonance imaging study. Isr. Med. Assoc. J. 10, 793–798 [PMC free article] [PubMed] [Google Scholar]
- 64. Fitsanakis V. A., Zhang N., Anderson J. G., Erikson K. M., Avison M. J., Gore J. C., and Aschner M. (2008) Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol. Sci. 103, 116–124 10.1093/toxsci/kfn019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y., Rolle-McFarland D., Mostafaei F., Zhou Y., Li Y., Zheng W., Wells E., and Nie L. H. (2018) In vivo neutron activation analysis of bone manganese in workers. Physiol. Meas. 39, 035003 10.1088/1361-6579/aaa749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baker M. G., Simpson C. D., Sheppard L., Stover B., Morton J., Cocker J., and Seixas N. (2015) Variance components of short-term biomarkers of manganese exposure in an inception cohort of welding trainees. J. Trace Elem. Med. Biol. 29, 123–129 10.1016/j.jtemb.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baker M. G., Simpson C. D., Stover B., Sheppard L., Checkoway H., Racette B. A., and Seixas N. S. (2014) Blood manganese as an exposure biomarker: state of the evidence. J. Occup. Environ. Hyg. 11, 210–217 10.1080/15459624.2013.852280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. O'Neal S. L., and Zheng W. (2015) Manganese toxicity upon overexposure: a decade in review. Curr. Environ. Health Rep. 2, 315–328 10.1007/s40572-015-0056-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith D., Gwiazda R., Bowler R., Roels H., Park R., Taicher C., and Lucchini R. (2007) Biomarkers of Mn exposure in humans. Am. J. Ind. Med. 50, 801–811 10.1002/ajim.20506 [DOI] [PubMed] [Google Scholar]
- 70. Betancourt Ó., Tapia M., and Méndez I. (2015) Decline of general intelligence in children exposed to manganese from mining contamination in Puyango River Basin, Southern Ecuador. Ecohealth 12, 453–460 10.1007/s10393-015-1027-2 [DOI] [PubMed] [Google Scholar]
- 71. Rink S. M., Ardoino G., Queirolo E. I., Cicariello D., Mañay N., and Kordas K. (2014) Associations between hair manganese levels and cognitive, language, and motor development in preschool children from Montevideo, Uruguay. Arch. Environ. Occup. Health 69, 46–54 10.1080/19338244.2012.725229 [DOI] [PubMed] [Google Scholar]
- 72. Hassani H., Golbabaei F., Shirkhanloo H., and Tehrani-Doust M. (2016) Relations of biomarkers of manganese exposure and neuropsychological effects among welders and ferroalloy smelters. Ind. Health 54, 79–86 10.2486/indhealth.2014-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Laohaudomchok W., Lin X., Herrick R. F., Fang S. C., Cavallari J. M., Christiani D. C., and Weisskopf M. G. (2011) Toenail, blood and urine as biomarkers of manganese exposure. J. Occup. Environ. Med. 53, 506–510 10.1097/JOM.0b013e31821854da [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zheng W., Fu S. X., Dydak U., and Cowan D. M. (2011) Biomarkers of manganese intoxication. Neurotoxicology 32, 1–8 10.1016/j.neuro.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marreilha Dos Santos A. P., Lopes Santos M., Batoréu M. C., and Aschner M. (2011) Prolactin is a peripheral marker of manganese neurotoxicity. Brain Res. 1382, 282–290 10.1016/j.brainres.2011.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kang J. O. (2001) Chronic iron overload and toxicity: clinical chemistry perspective. Clin. Lab. Sci. 14, 209–219 [PubMed] [Google Scholar]
- 77. Ghosh K. (2006) Non haematological effects of iron deficiency—a perspective. Indian J. Med. Sci. 60, 30–37 10.4103/0019-5359.19676 [DOI] [PubMed] [Google Scholar]
- 78. Mergler D., Baldwin M., Bélanger S., Larribe F., Beuter A., Bowler R., Panisset M., Edwards R., de Geoffroy A., Sassine M. P., and Hudnell K. (1999) Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology 20, 327–342 [PubMed] [Google Scholar]
- 79. Claus Henn B., Ettinger A. S., Schwartz J., Téllez-Rojo M. M., Lamadrid-Figueroa H., Hernández-Avila M., Schnaas L., Amarasiriwardena C., Bellinger D. C., Hu H., and Wright R. O. (2010) Early postnatal blood manganese levels and children's neurodevelopment. Epidemiology 21, 433–439 10.1097/EDE.0b013e3181df8e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Beaudin S. A., Strupp B. J., Strawderman M., and Smith D. R. (2017) Early postnatal manganese exposure causes lasting impairment of selective and focused attention and arousal regulation in adult rats. Environ. Health Perspect. 125, 230–237 10.1289/EHP258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bhang S. Y., Cho S. C., Kim J. W., Hong Y. C., Shin M. S., Yoo H. J., Cho I. H., Kim Y., and Kim B. N. (2013) Relationship between blood manganese levels and children's attention, cognition, behavior, and academic performance—a nationwide cross-sectional study. Environ. Res. 126, 9–16 10.1016/j.envres.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 82. Madison J. L., Wegrzynowicz M., Aschner M., and Bowman A. B. (2012) Disease-toxicant interactions in manganese exposed Huntington disease mice: early changes in striatal neuron morphology and dopamine metabolism. PLoS ONE 7, e31024 10.1371/journal.pone.0031024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Madison J. L., Wegrzynowicz M., Aschner M., and Bowman A. B. (2011) Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology 32, 896–906 10.1016/j.neuro.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Robison G., Sullivan B., Cannon J. R., and Pushkar Y. (2015) Identification of dopaminergic neurons of the substantia nigra pars compacta as a target of manganese accumulation. Metallomics 7, 748–755 10.1039/C5MT00023H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Park J. D., Chung Y. H., Kim C. Y., Ha C. S., Yang S. O., Khang H. S., Yu I. K., Cheong H. K., Lee J. S., Song C. W., Kwon I. H., Han J. H., Sung J. H., Heo J. D., Choi B. S., et al. (2007) Comparison of high MRI T1 signals with manganese concentration in brains of cynomolgus monkeys after 8 months of stainless steel welding-fume exposure. Inhal. Toxicol. 19, 965–971 10.1080/08958370701516108 [DOI] [PubMed] [Google Scholar]
- 86. Stanwood G. D., Leitch D. B., Savchenko V., Wu J., Fitsanakis V. A., Anderson D. J., Stankowski J. N., Aschner M., and McLaughlin B. (2009) Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J. Neurochem. 110, 378–389 10.1111/j.1471-4159.2009.06145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fitsanakis V. A., Au C., Erikson K. M., and Aschner M. (2006) The effects of manganese on glutamate, dopamine and γ-aminobutyric acid regulation. Neurochem. Int. 48, 426–433 10.1016/j.neuint.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 88. Wong P. C. L., Lai J. C. K., Lim L., and Davison A. N. (1981) Selective inhibition of l-glutamate and gammaaminobutyrate transport in nerve ending particles by aluminium, manganese, and cadmium chloride. J. Inorg. Biochem. 14, 253–260 10.1016/S0162-0134(00)80005-7 [DOI] [PubMed] [Google Scholar]
- 89. Danbolt N. C. (2001) Glutamate uptake. Prog. Neurobiol. 65, 1–105 10.1016/S0301-0082(00)00067-8 [DOI] [PubMed] [Google Scholar]
- 90. Spadoni F., Stefani A., Morello M., Lavaroni F., Giacomini P., and Sancesario G. (2000) Selective vulnerability of pallidal neurons in the early phases of manganese intoxication. Exp. Brain Res. 135, 544–551 10.1007/s002210000554 [DOI] [PubMed] [Google Scholar]
- 91. Takeda A., Sotogaku N., and Oku N. (2002) Manganese influences the levels of neurotransmitters in synapses in rat brain. Neuroscience 114, 669–674 10.1016/S0306-4522(02)00353-6 [DOI] [PubMed] [Google Scholar]
- 92. Takeda A., Sotogaku N., and Oku N. (2003) Influence of manganese on the release of neurotransmitters in rat striatum. Brain Res. 965, 279–282 10.1016/S0006-8993(02)04157-4 [DOI] [PubMed] [Google Scholar]
- 93. Zwingmann C., Leibfritz D., and Hazell A. S. (2003) Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J. Cereb. Blood Flow Metab. 23, 756–771 10.1097/01.WCB.0000056062.25434.4D [DOI] [PubMed] [Google Scholar]
- 94. Eriksson H., Morath C., and Heilbronn E. (1984) Effects of manganese on the nervous system. Acta Neurol. Scand. Suppl. 100, 89–93 [PubMed] [Google Scholar]
- 95. Lai J. C. K., Leung T. K. C., and Lim L. (1984) Differences in the neurotoxic effects of manganese during development and aging—some observations on brain regional neurotransmitter and non-neurotransmitter metabolism in a developmental rat model of chronic manganese encephalopathy. Neurotoxicology 5, 37–47 [PubMed] [Google Scholar]
- 96. Yousefi Babadi V., Sadeghi L., Shirani K., Malekirad A. A., and Rezaei M. (2014) The toxic effect of manganese on the acetylcholinesterase activity in rat brains. J. Toxicol. 2014, 946372 10.1155/2014/946372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lai J. C. K., Chan A. W. K., Leung T. K. C., Minski M. J., and Lim L. (1992) Neurochemical changes in rats chronically treated with a high-concentration of manganese chloride. Neurochem. Res. 17, 841–847 10.1007/BF00993259 [DOI] [PubMed] [Google Scholar]
- 98. Tran T. T., Chowanadisai W., Crinella F. M., Chicz-DeMet A., and Lönnerdal B. (2002) Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology 23, 635–643 10.1016/S0161-813X(02)00091-8 [DOI] [PubMed] [Google Scholar]
- 99. Seth P. K., and Chandra S. V. (1984) Neurotransmitters and neurotransmitter receptors in developing and adult rats during manganese poisoning. Neurotoxicology 5, 67–76 [PubMed] [Google Scholar]
- 100. Ingersoll R. T., Montgomery E. B. Jr., and Aposhian H. V. (1999) Central nervous system toxicity of manganese. II: Cocaine or reserpine inhibit manganese concentration in the rat brain. Neurotoxicology 20, 467–476 [PubMed] [Google Scholar]
- 101. Nelson M., Adams T., Ojo C., Carroll M. A., and Catapane E. J. (2018) Manganese toxicity is targeting an early step in the dopamine signal transduction pathway that controls lateral cilia activity in the bivalve mollusc Crassostrea virginica. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 213, 1–6 10.1016/j.cbpc.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vezér T., Kurunczi A., Náray M., Papp A., and Nagymajtényi L. (2007) Behavioral effects of subchronic inorganic manganese exposure in rats. Am. J. Ind. Med. 50, 841–852 10.1002/ajim.20485 [DOI] [PubMed] [Google Scholar]
- 103. Khalid M., Aoun R. A., and Mathews T. A. (2011) Altered striatal dopamine release following a sub-acute exposure to manganese. J. Neurosci. Methods 202, 182–191 10.1016/j.jneumeth.2011.06.019 [DOI] [PubMed] [Google Scholar]
- 104. Huang C. C., Weng Y. H., Lu C. S., Chu N. S., and Yen T. C. (2003) Dopamine transporter binding in chronic manganese intoxication. J. Neurol. 250, 1335–1339 10.1007/s00415-003-0214-1 [DOI] [PubMed] [Google Scholar]
- 105. Chen M. K., Lee J. S., McGlothan J. L., Furukawa E., Adams R. J., Alexander M., Wong D. F., and Guilarte T. R. (2006) Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology 27, 229–236 10.1016/j.neuro.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 106. Zhang D., Kanthasamy A., Anantharam V., and Kanthasamy A. (2011) Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol. Appl. Pharmacol. 254, 65–71 10.1016/j.taap.2010.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Amos-Kroohs R. M., Davenport L. L., Gutierrez A., Hufgard J. R., Vorhees C. V., and Williams M. T. (2016) Developmental manganese exposure in combination with developmental stress and iron deficiency: effects on behavior and monoamines. Neurotoxicol. Teratol. 56, 55–67 10.1016/j.ntt.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tran T. T., Chowanadisai W., Lönnerdal B., Le L., Parker M., Chicz-Demet A., and Crinella F. M. (2002) Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology 23, 645–651 10.1016/S0161-813X(02)00068-2 [DOI] [PubMed] [Google Scholar]
- 109. Bailey R. A., Gutierrez A., Kyser T. L., Hemmerle A. M., Hufgard J. R., Seroogy K. B., Vorhees C. V., and Williams M. T. (2019) Effects of preweaning manganese in combination with adult striatal dopamine lesions on monoamines, BDNF, TrkB, and cognitive function in Sprague-Dawley rats. Neurotox. Res. 35, 606–620 10.1007/s12640-018-9992-1 [DOI] [PubMed] [Google Scholar]
- 110. Autissier N., Rochette L., Dumas P., Beley A., Loireau A., and Bralet J. (1982) Dopamine and norepinephrine turnover in various regions of the rat brain after chronic manganese chloride administration. Toxicology 24, 175–182 10.1016/0300-483X(82)90055-5 [DOI] [PubMed] [Google Scholar]
- 111. Eriksson H., Mägiste K., Plantin L.-O., Fonnum F., Hedström K.-G., Theodorsson-Norheim E., Kristensson K., Stålberg E., and Heilbronn E. (1987) Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch. Toxicol. 61, 46–52 10.1007/BF00324547 [DOI] [PubMed] [Google Scholar]
- 112. Sistrunk S. C., Ross M. K., and Filipov N. M. (2007) Direct effects of manganese compounds on dopamine and its metabolite Dopac: an in vitro study. Environ. Toxicol. Pharmacol. 23, 286–296 10.1016/j.etap.2006.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tomás-Camardiel M., Herrera A. J., Venero J. L., Cruz Sánchez-Hidalgo M., Cano J., and Machado A. (2002) Differential regulation of glutamic acid decarboxylase mRNA and tyrosine hydroxylase mRNA expression in the aged manganese-treated rats. Mol. Brain Res. 103, 116–129 10.1016/S0169-328X(02)00192-4 [DOI] [PubMed] [Google Scholar]
- 114. Gwiazda R. H., Lee D., Sheridan J., and Smith D. R. (2002) Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology 23, 69–76 10.1016/S0161-813X(02)00002-5 [DOI] [PubMed] [Google Scholar]
- 115. Avila Costa M. R., Gutierrez-Valdez A., Anaya V., Ordoñez Librado J., Sanchez Betancourt J., Montiel-Flores E., Aley-Medina P., Reynoso-Erazo L., Espinosa-Villanueva J., Tron-Alvarez R., and Rodriguez Lara V. (2018) Manganese inhalation induces dopaminergic cell loss: relevance to Parkinson's disease, pp. 59–83. In Dopamine: Health and Disease, InTechOpen Ltd., London [Google Scholar]
- 116. Stredrick D. L., Stokes A. H., Worst T. J., Freeman W. M., Johnson E. A., Lash L. H., Aschner M., and Vrana K. E. (2004) Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology 25, 543–553 10.1016/j.neuro.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 117. Smith M. R., Fernandes J., Go Y. M., and Jones D. P. (2017) Redox dynamics of manganese as a mitochondrial life-death switch. Biochem. Biophys. Res. Commun. 482, 388–398 10.1016/j.bbrc.2016.10.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Prabhakaran K., Ghosh D., Chapman G. D., and Gunasekar P. G. (2008) Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res. Bull. 76, 361–367 10.1016/j.brainresbull.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 119. Ommati M. M., Heidari R., Ghanbarinejad V., Abdoli N., and Niknahad H. (2019) Taurine treatment provides neuroprotection in a mouse model of manganism. Biol. Trace Elem. Res. 190, 384–395 10.1007/s12011-018-1552-2 [DOI] [PubMed] [Google Scholar]
- 120. Fitsanakis V. A., Zhang N., Garcia S., and Aschner M. (2010) Manganese (Mn) and iron (Fe): interdependency of transport and regulation. Neurotox. Res. 18, 124–131 10.1007/s12640-009-9130-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sandstrom B., Davidsson L., Cederblad A., Eriksson R., and Lonnerdal B. (1986) Manganese absorption and metabolism in man. Acta Pharmacol. Toxicol. (Copenh.) 59, 60–62 10.1111/j.1600-0773.1986.tb02708.x [DOI] [PubMed] [Google Scholar]
- 122. Finley J. W. (1999) Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 70, 37–43 10.1093/ajcn/70.1.37 [DOI] [PubMed] [Google Scholar]
- 123. Thomson A. B., Olatunbosun D., and Valverg L. S. (1971) Interrelation of intestinal transport system for manganese and iron. J. Lab. Clin. Med. 78, 642–655 [PubMed] [Google Scholar]
- 124. Rehnberg G. L., Hein J. F., Carter S. D., Linko R. S., and Laskey J. W. (1982) Chronic ingestion of Mn3O4 by rats: tissue accumulation and distribution of manganese in two generations. J. Toxicol. Environ. Health 9, 175–188 10.1080/15287398209530153 [DOI] [PubMed] [Google Scholar]
- 125. Schroeder H. A., Balassa J. J., and Tipton I. H. (1966) Essential trace metals in man: manganese. A study in homeostasis. J. Chronic Dis. 19, 545–571 10.1016/0021-9681(66)90094-4 [DOI] [PubMed] [Google Scholar]
- 126. Lutz T. A., Schroff A., and Scharrer E. (1993) Effects of calcium and sugars on intestinal manganese absorption. Biol. Trace Elem. Res. 39, 221–227 10.1007/BF02783192 [DOI] [PubMed] [Google Scholar]
- 127. McDermott S. D., and Kies C. (1987) Manganese usage in humans as affected by use of calcium supplements. in Nutritional Bioavailability of Manganese (Kies C., ed) pp. 146–151, American Chemical Society, Washington, D. C. [Google Scholar]
- 128. Keen C. L., and Zidenberg-Cherr S. (1990) Manganese. In Present Knowledge in Nutrition (Brown M. L., ed) pp. 279–286, International Life Science Institute, Nutrition Foundation, Washington, D. C. [Google Scholar]
- 129. Davis C. D., and Greger J. L. (1992) Longitudinal changes of manganese-dependent superoxide dismutase and other indexes of manganese and iron status in women. Am. J. Clin. Nutr. 55, 747–752 10.1093/ajcn/55.3.747 [DOI] [PubMed] [Google Scholar]
- 130. Aschner M., and Dorman D. C. (2006) Manganese: pharmacokinetics and molecular mechanisms of brain uptake. Toxicol. Rev. 25, 147–154 10.2165/00139709-200625030-00002 [DOI] [PubMed] [Google Scholar]
- 131. Tuschl K., Mills P. B., Parsons H., Malone M., Fowler D., Bitner-Glindzicz M., and Clayton P. T. (2008) Hepatic cirrhosis, dystonia, polycythaemia and hypermanganesaemia—a new metabolic disorder. J. Inherit. Metab. Dis. 31, 151–163 10.1007/s10545-008-0813-1 [DOI] [PubMed] [Google Scholar]
- 132. Quadri M., Federico A., Zhao T., Breedveld G. J., Battisti C., Delnooz C., Severijnen L. A., Di Toro Mammarella L., Mignarri A., Monti L., Sanna A., Lu P., Punzo F., Cossu G., Willemsen R., et al. (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 90, 467–477 10.1016/j.ajhg.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tuschl K., Clayton P. T., Gospe S. M. Jr., Gulab S., Ibrahim S., Singhi P., Aulakh R., Ribeiro R. T., Barsottini O. G., Zaki M. S., Del Rosario M. L., Dyack S., Price V., Rideout A., Gordon K., et al. (2012) Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 90, 457–466 10.1016/j.ajhg.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gulab S., Kayyali H. R., and Al-Said Y. (2018) Atypical neurologic phenotype and novel SLC30A10 mutation in two brothers with hereditary hypermanganesemia. Neuropediatrics 49, 72–75 10.1055/s-0037-1608778 [DOI] [PubMed] [Google Scholar]
- 135. Quadri M., Kamate M., Sharma S., Olgiati S., Graafland J., Breedveld G. J., Kori I., Hattiholi V., Jain P., Aneja S., Kumar A., Gulati P., Goel M., Talukdar B., and Bonifati V. (2015) Manganese transport disorder: novel SLC30A10 mutations and early phenotypes. Mov. Disord. 30, 996–1001 10.1002/mds.26202 [DOI] [PubMed] [Google Scholar]
- 136. Zaki M. S., Issa M. Y., Elbendary H. M., El-Karaksy H., Hosny H., Ghobrial C., El Safty A., El-Hennawy A., Oraby A., Selim L., and Abdel-Hamid M. S. (2018) Hypermanganesemia with dystonia, polycythemia and cirrhosis in 10 patients: six novel SLC30A10 mutations and further phenotype delineation. Clin. Genet. 93, 905–912 10.1111/cge.13184 [DOI] [PubMed] [Google Scholar]
- 137. Gospe S. M. Jr., Caruso R. D., Clegg M. S., Keen C. L., Pimstone N. R., Ducore J. M., Gettner S. S., and Kreutzer R. A. (2000) Paraparesis, hypermanganesaemia, and polycythaemia: a novel presentation of cirrhosis. Arch. Dis. Child. 83, 439–442 10.1136/adc.83.5.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Perl D. P., and Olanow C. W. (2007) The neuropathology of manganese-induced parkinsonism. J. Neuropathol. Exp. Neurol. 66, 675–682 10.1097/nen.0b013e31812503cf [DOI] [PubMed] [Google Scholar]
- 139. Lechpammer M., Clegg M. S., Muzar Z., Huebner P. A., Jin L. W., and Gospe S. M. Jr. (2014) Pathology of inherited manganese transporter deficiency. Ann. Neurol. 75, 608–612 10.1002/ana.24131 [DOI] [PubMed] [Google Scholar]
- 140. Wahlberg K. E., Guazzetti S., Pineda D., Larsson S. C., Fedrighi C., Cagna G., Zoni S., Placidi D., Wright R. O., Smith D. R., Lucchini R. G., and Broberg K. (2018) Polymorphisms in manganese transporters SLC30A10 and SLC39A8 are associated with children's neurodevelopment by influencing manganese homeostasis. Front. Genet. 9, 664 10.3389/fgene.2018.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wahlberg K., Kippler M., Alhamdow A., Rahman S. M., Smith D. R., Vahter M., Lucchini R. G., and Broberg K. (2016) Common polymorphisms in the solute carrier SLC30A10 are associated with blood manganese and neurological function. Toxicol. Sci. 149, 473–483 10.1093/toxsci/kfv252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Huang L., and Tepaamorndech S. (2013) The SLC30 family of zinc transporters: a review of current understanding of their biological and pathophysiological roles. Mol. Aspects Med. 34, 548–560 10.1016/j.mam.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 143. Bosomworth H. J., Thornton J. K., Coneyworth L. J., Ford D., and Valentine R. A. (2012) Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 4, 771–779 10.1039/c2mt20088k [DOI] [PubMed] [Google Scholar]
- 144. Leyva-Illades D., Chen P., Zogzas C. E., Hutchens S., Mercado J. M., Swaim C. D., Morrisett R. A., Bowman A. B., Aschner M., and Mukhopadhyay S. (2014) SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 34, 14079–14095 10.1523/JNEUROSCI.2329-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zogzas C. E., Aschner M., and Mukhopadhyay S. (2016) Structural elements in the transmembrane and cytoplasmic domains of the metal transporter SLC30A10 are required for its manganese efflux activity. J. Biol. Chem. 291, 15940–15957 10.1074/jbc.M116.726935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Nishito Y., Tsuji N., Fujishiro H., Takeda T. A., Yamazaki T., Teranishi F., Okazaki F., Matsunaga A., Tuschl K., Rao R., Kono S., Miyajima H., Narita H., Himeno S., and Kambe T. (2016) Direct comparison of manganese detoxification/efflux proteins and molecular characterization of ZnT10 protein as a manganese transporter. J. Biol. Chem. 291, 14773–14787 10.1074/jbc.M116.728014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hutchens S., Liu C., Jursa T., Shawlot W., Chaffee B. K., Yin W., Gore A. C., Aschner M., Smith D. R., and Mukhopadhyay S. (2017) Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice. J. Biol. Chem. 292, 9760–9773 10.1074/jbc.M117.783605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu C., Hutchens S., Jursa T., Shawlot W., Polishchuk E. V., Polishchuk R. S., Dray B. K., Gore A. C., Aschner M., Smith D. R., and Mukhopadhyay S. (2017) Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production. J. Biol. Chem. 292, 16605–16615 10.1074/jbc.M117.804989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Anagianni S., and Tuschl K. (2019) Genetic disorders of manganese metabolism. Curr. Neurol. Neurosci. Rep. 19, 33 10.1007/s11910-019-0942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Greenberg D. M., Copp D. H., and Cuthbertson E. M. (1943) Studies in mineral metabolism with the aid of artificial radioactive isotopes. 7. The distribution and execration, particularly by way of the bile, of iron, cobalt, and manganese. J. Biol. Chem. 147, 749–756 [Google Scholar]
- 151. Ballatori N., Miles E., and Clarkson T. W. (1987) Homeostatic control of manganese excretion in the neonatal rat. Am. J. Physiol. 252, R842–R847 10.1152/ajpregu.1987.252.5.R842 [DOI] [PubMed] [Google Scholar]
- 152. Klaassen C. (1974) Biliary excretion of manganese in rats, rabbits, and dogs. Toxicol. Appl. Pharmacol. 29, 458–468 10.1016/0041-008X(74)90117-3 [DOI] [PubMed] [Google Scholar]
- 153. Bertinchamps A. J., Miller S. T., and Cotzias G. C. (1966) Interdependence of routes excreting manganese. Am. J. Physiol. 211, 217–224 10.1152/ajplegacy.1966.211.1.217 [DOI] [PubMed] [Google Scholar]
- 154. Taylor C. A., Hutchens S., Liu C., Jursa T., Shawlot W., Aschner M., Smith D. R., and Mukhopadhyay S. (2019) SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity. J. Biol. Chem. 294, 1860–1876 10.1074/jbc.RA118.005628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C. A., et al. (2015) Proteomics: tissue-based map of the human proteome. Science 347, 1260419 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 156. Mercadante C. J., Prajapati M., Conboy H. L., Dash M. E., Herrera C., Pettiglio M. A., Cintron-Rivera L., Salesky M. A., Rao D. B., and Bartnikas T. B. (2019) Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J. Clin. Invest. 129, 5442–5461 10.1172/JCI129710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chen P., Bowman A. B., Mukhopadhyay S., and Aschner M. (2015) SLC30A10: a novel manganese transporter. Worm 4, e1042648 10.1080/21624054.2015.1042648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xia Z., Wei J., Li Y., Wang J., Li W., Wang K., Hong X., Zhao L., Chen C., Min J., and Wang F. (2017) Zebrafish slc30a10 deficiency revealed a novel compensatory mechanism of Atp2c1 in maintaining manganese homeostasis. PLoS Genet. 13, e1006892 10.1371/journal.pgen.1006892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tuschl K., Meyer E., Valdivia L. E., Zhao N., Dadswell C., Abdul-Sada A., Hung C. Y., Simpson M. A., Chong W. K., Jacques T. S., Woltjer R. L., Eaton S., Gregory A., Sanford L., Kara E., et al. (2016) Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 7, 11601 10.1038/ncomms11601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jeong J., and Eide D. J. (2013) The SLC39 family of zinc transporters. Mol. Aspects Med. 34, 612–619 10.1016/j.mam.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Juneja M., Shamim U., Joshi A., Mathur A., Uppili B., Sairam S., Ambawat S., Dixit R., and Faruq M. (2018) A novel mutation in SLC39A14 causing hypermanganesemia associated with infantile onset dystonia. J. Gene Med. 20, e3012 10.1002/jgm.3012 [DOI] [PubMed] [Google Scholar]
- 162. Marti-Sanchez L., Ortigoza-Escobar J. D., Darling A., Villaronga M., Baide H., Molero-Luis M., Batllori M., Vanegas M. I., Muchart J., Aquino L., Artuch R., Macaya A., Kurian M. A., and Dueñas P. (2018) Hypermanganesemia due to mutations in SLC39A14: further insights into Mn deposition in the central nervous system. Orphanet J. Rare Dis. 13, 28 10.1186/s13023-018-0758-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Girijashanker K., He L., Soleimani M., Reed J. M., Li H., Liu Z., Wang B., Dalton T. P., and Nebert D. W. (2008) Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol. Pharmacol. 73, 1413–1423 10.1124/mol.107.043588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jenkitkasemwong S., Wang C. Y., Mackenzie B., and Knutson M. D. (2012) Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25, 643–655 10.1007/s10534-012-9526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Liuzzi J. P., Aydemir F., Nam H., Knutson M. D., and Cousins R. J. (2006) Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. U.S.A. 103, 13612–13617 10.1073/pnas.0606424103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Liuzzi J. P., Lichten L. A., Rivera S., Blanchard R. K., Aydemir T. B., Knutson M. D., Ganz T., and Cousins R. J. (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. U.S.A. 102, 6843–6848 10.1073/pnas.0502257102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Pinilla-Tenas J. J., Sparkman B. K., Shawki A., Illing A. C., Mitchell C. J., Zhao N., Liuzzi J. P., Cousins R. J., Knutson M. D., and Mackenzie B. (2011) Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 301, C862–C871 10.1152/ajpcell.00479.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Taylor K. M., Morgan H. E., Johnson A., and Nicholson R. I. (2005) Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 579, 427–432 10.1016/j.febslet.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 169. Hojyo S., Fukada T., Shimoda S., Ohashi W., Bin B. H., Koseki H., and Hirano T. (2011) The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS ONE 6, e18059 10.1371/journal.pone.0018059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tang T., Li L., Tang J., Li Y., Lin W. Y., Martin F., Grant D., Solloway M., Parker L., Ye W., Forrest W., Ghilardi N., Oravecz T., Platt K. A., Rice D. S., et al. (2010) A mouse knockout library for secreted and transmembrane proteins. Nat. Biotechnol. 28, 749–755 10.1038/nbt.1644 [DOI] [PubMed] [Google Scholar]
- 171. Aydemir T. B., Kim M. H., Kim J., Colon-Perez L. M., Banan G., Mareci T. H., Febo M., and Cousins R. J. (2017) Metal transporter Zip14 (Slc39a14) deletion in mice increases manganese deposition and produces neurotoxic signatures and diminished motor activity. J. Neurosci. 37, 5996–6006 10.1523/JNEUROSCI.0285-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Jenkitkasemwong S., Akinyode A., Paulus E., Weiskirchen R., Hojyo S., Fukada T., Giraldo G., Schrier J., Garcia A., Janus C., Giasson B., and Knutson M. D. (2018) SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl. Acad. Sci. U.S.A. 115, E1769–E1778 10.1073/pnas.1720739115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Xin Y., Gao H., Wang J., Qiang Y., Imam M. U., Li Y., Wang J., Zhang R., Zhang H., Yu Y., Wang H., Luo H., Shi C., Xu Y., Hojyo S., et al. (2017) Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 3, 17025 10.1038/celldisc.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nam H., Wang C. Y., Zhang L., Zhang W., Hojyo S., Fukada T., and Knutson M. D. (2013) ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica 98, 1049–1057 10.3324/haematol.2012.072314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Guthrie G. J., Aydemir T. B., Troche C., Martin A. B., Chang S. M., and Cousins R. J. (2015) Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G171–G178 10.1152/ajpgi.00021.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Scheiber I. F., Wu Y., Morgan S. E., and Zhao N. (2019) The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 294, 9147–9160 10.1074/jbc.RA119.008762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Boycott K. M., Beaulieu C. L., Kernohan K. D., Gebril O. H., Mhanni A., Chudley A. E., Redl D., Qin W., Hampson S., Küry S., Tetreault M., Puffenberger E. G., Scott J. N., Bezieau S., Reis A., et al. (2015) Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am. J. Hum. Genet. 97, 886–893 10.1016/j.ajhg.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Park J. H., Hogrebe M., Grüneberg M., DuChesne I., von der Heiden A. L., Reunert J., Schlingmann K. P., Boycott K. M., Beaulieu C. L., Mhanni A. A., Innes A. M., Hörtnagel K., Biskup S., Gleixner E. M., Kurlemann G., et al. (2015) SLC39A8 deficiency: a disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 97, 894–903 10.1016/j.ajhg.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wang C. Y., Jenkitkasemwong S., Duarte S., Sparkman B. K., Shawki A., Mackenzie B., and Knutson M. D. (2012) ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 287, 34032–34043 10.1074/jbc.M112.367284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wagner R. R., and Cynkin M. A. (1971) Glycoprotein metabolism: a UDP-galactose-glycoprotein galactosyltransferase of rat serum. Biochem. Biophys. Res. Commun. 45, 57–62 10.1016/0006-291X(71)90049-0 [DOI] [PubMed] [Google Scholar]
- 181. Schachter H., McGuire E. J., and Roseman S. (1971) Sialic acids. 13. A uridine diphosphate d-galactose: mucin galactosyltransferase from porcine submaxillary gland. J. Biol. Chem. 246, 5321–5328 [PubMed] [Google Scholar]
- 182. Bendiak B., and Schachter H. (1987) Control of glycoprotein synthesis. Kinetic mechanism, substrate specificity, and inhibition characteristics of UDP-N-acetylglucosamine:α-d-mannoside β1–2 N-acetylglucosaminyltransferase II from rat liver. J. Biol. Chem. 262, 5784–5790 [PubMed] [Google Scholar]
- 183. Choi E. K., Nguyen T. T., Gupta N., Iwase S., and Seo Y. A. (2018) Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders. Sci. Rep. 8, 3163 10.1038/s41598-018-21464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lin W., Vann D. R., Doulias P. T., Wang T., Landesberg G., Li X., Ricciotti E., Scalia R., He M., Hand N. J., and Rader D. J. (2017) Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Invest. 127, 2407–2417 10.1172/JCI90896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mukhopadhyay S., and Linstedt A. D. (2011) Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc. Natl. Acad. Sci. U.S.A. 108, 858–863 10.1073/pnas.1013642108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Landrigan P. J., and Miodovnik A. (2011) Children's health and the environment: an overview. Mt. Sinai J. Med. 78, 1–10 10.1002/msj.20236 [DOI] [PubMed] [Google Scholar]
- 187. Chung S. E., Cheong H. K., Ha E. H., Kim B. N., Ha M., Kim Y., Hong Y. C., Park H., and Oh S. Y. (2015) Maternal blood manganese and early neurodevelopment: the Mothers and Children's Environmental Health (MOCEH) study. Environ. Health Perspect. 123, 717–722 10.1289/ehp.1307865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Takser L., Mergler D., Hellier G., Sahuquillo J., and Huel G. (2003) Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology 24, 667–674 10.1016/S0161-813X(03)00058-5 [DOI] [PubMed] [Google Scholar]
- 189. Rodrigues E. G., Bellinger D. C., Valeri L., Hasan M. O., Quamruzzaman Q., Golam M., Kile M. L., Christiani D. C., Wright R. O., and Mazumdar M. (2016) Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ. Health. 15, 44 10.1186/s12940-016-0127-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Frangou S., Chitins X., and Williams S. C. (2004) Mapping IQ and gray matter density in healthy young people. NeuroImage 23, 800–805 10.1016/j.neuroimage.2004.05.027 [DOI] [PubMed] [Google Scholar]
- 191. Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Evans A., Rapoport J., and Giedd J. (2006) Intellectual ability and cortical development in children and adolescents. Nature 440, 676–679 10.1038/nature04513 [DOI] [PubMed] [Google Scholar]
- 192. Lewis D. A. (1997) Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology 16, 385–398 10.1016/S0893-133X(96)00277-1 [DOI] [PubMed] [Google Scholar]
- 193. Weathers J. D., Stringaris A., Deveney C. M., Brotman M. A., Zarate C. A. Jr., Connolly M. E., Fromm S. J., LeBourdais S. B., Pine D. S., and Leibenluft E. (2012) A developmental study of the neural circuitry mediating motor inhibition in bipolar disorder. Am. J. Psychiatry 169, 633–641 10.1176/appi.ajp.2012.11081244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bourgeois J. P., Goldman-Rakic P. S., and Rakic P. (1994) Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex 4, 78–96 10.1093/cercor/4.1.78 [DOI] [PubMed] [Google Scholar]
- 195. Dorman D. C., Brenneman K. A., McElveen A. M., Lynch S. E., Roberts K. C., and Wong B. A. (2002) Olfactory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J. Toxicol. Environ. Health A 65, 1493–1511 10.1080/00984100290071630 [DOI] [PubMed] [Google Scholar]
- 196. Viana G. F., de Carvalho C. F., Nunes L. S., Rodrigues J. L., Ribeiro N. S., de Almeida D. A., Ferreira J. R., Abreu N., and Menezes-Filho J. A. (2014) Noninvasive biomarkers of manganese exposure and neuropsychological effects in environmentally exposed adults in Brazil. Toxicol. Lett. 231, 169–178 10.1016/j.toxlet.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 197. Menezes-Filho J. A., Novaes Cde O., Moreira J. C., Sarcinelli P. N., and Mergler D. (2011) Elevated manganese and cognitive performance in school-aged children and their mothers. Environ. Res. 111, 156–163 10.1016/j.envres.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Chang Y., Lee J. J., Seo J. H., Song H. J., Kim J. H., Bae S. J., Ahn J. H., Park S. J., Jeong K. S., Kwon Y. J., Kim S. H., and Kim Y. (2010) Altered working memory process in the manganese-exposed brain. NeuroImage 53, 1279–1285 10.1016/j.neuroimage.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 199. Zou Y., Qing L., Zeng X., Shen Y., Zhong Y., Liu J., Li Q., Chen K., Lv Y., Huang D., Liang G., Zhang W., Chen L., Yang Y., and Yang X. (2014) Cognitive function and plasma BDNF levels among manganese-exposed smelters. Occup. Environ. Med. 71, 189–194 10.1136/oemed-2013-101896 [DOI] [PubMed] [Google Scholar]
- 200. Long Z., Jiang Y. M., Li X. R., Fadel W., Xu J., Yeh C. L., Long L. L., Luo H. L., Harezlak J., Murdoch J. B., Zheng W., and Dydak U. (2014) Vulnerability of welders to manganese exposure—a neuroimaging study. Neurotoxicology 45, 285–292 10.1016/j.neuro.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pautler R. G., Mongeau R., and Jacobs R. E. (2003) In vivo trans-synaptic tract tracing from the murine striatum and amygdala utilizing manganese enhanced MRI (MEMRI). Magn. Reson. Med. 50, 33–39 10.1002/mrm.10498 [DOI] [PubMed] [Google Scholar]
- 202. Baly D. L., Lee I., and Doshi R. (1988) Mechanism of decreased insulinogenesis in manganese-deficient rats: decreased insulin mRNA levels. FEBS Lett. 239, 55–58 10.1016/0014-5793(88)80544-1 [DOI] [PubMed] [Google Scholar]
- 203. Baly D. L., Keen C. L., and Hurley L. S. (1986) Effects of manganese deficiency on pyruvate carboxylase and phosphoenolpyruvate carboxykinase activity and carbohydrate homeostasis in adult rats. Biol. Trace Elem. Res. 11, 201–212 10.1007/BF02795535 [DOI] [PubMed] [Google Scholar]
- 204. Baly D. L., Curry D. L., Keen C. L., and Hurley L. S. (1984) Effect of manganese deficiency on insulin secretion and carbohydrate heomostasis in rats. J. Nutr. 114, 1438–1446 10.1093/jn/114.8.1438 [DOI] [PubMed] [Google Scholar]
- 205. Baly D. L., Schneiderman J. S., and Garcia-Welsh A. L. (1990) Effect of manganese deficiency on insulin binding, glucose transport and metabolism in rat adipocytes. J. Nutr. 120, 1075–1079 10.1093/jn/120.9.1075 [DOI] [PubMed] [Google Scholar]
- 206. Clegg M. S., Donovan S. M., Monaco M. H., Baly D. L., Ensunsa J. L., and Keen C. L. (1998) The influence of manganese deficiency on serum IGF-1 and IGF binding proteins in the male rat. Proc. Soc. Exp. Biol. Med. 219, 41–47 10.3181/00379727-219-44314 [DOI] [PubMed] [Google Scholar]
- 207. Bryan M. R., Nordham K. D., Rose D. I. R., O'Brien M. T., Joshi P., Foshage A. M., Gonçalves F. M., Nitin R., Uhouse M. A., Aschner M., and Bowman A. B. (2020) Manganese acts upon insulin/IGF receptors to phosphorylate AKT and increase glucose uptake in Huntington's disease cells. Mol. Neurobiol. 57, 1570–1593 10.1007/s12035-019-01824-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Yamada M., Ohno S., Okayasu I., Okeda R., Hatakeyama S., Watanabe H., Ushio K., and Tsukagoshi H. (1986) Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 70, 273–278 10.1007/BF00686083 [DOI] [PubMed] [Google Scholar]
- 209. Bryan M. R., and Bowman A. B. (2017) Manganese and the insulin-IGF signaling network in Huntington's disease and other neurodegenerative disorders. Adv. Neurobiol. 18, 113–142 10.1007/978-3-319-60189-2_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Verity M. A. (1999) Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology 20, 489–497 [PubMed] [Google Scholar]
- 211. Pfalzer A. C., and Bowman A. B. (2017) Relationships between essential manganese biology and manganese toxicity in neurological disease. Curr. Environ. Health Rep. 4, 223–228 10.1007/s40572-017-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Guilarte T. R., Chen M. K., McGlothan J. L., Verina T., Wong D. F., Zhou Y., Alexander M., Rohde C. A., Syversen T., Decamp E., Koser A. J., Fritz S., Gonczi H., Anderson D. W., and Schneider J. S. (2006) Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol. 202, 381–390 10.1016/j.expneurol.2006.06.015 [DOI] [PubMed] [Google Scholar]
- 213. Aschner M., Guilarte T. R., Schneider J. S., and Zheng W. (2007) Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 221, 131–147 10.1016/j.taap.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Hisahara S., and Shimohama S. (2011) Dopamine receptors and Parkinson's disease. Int. J. Med. Chem. 2011, 403039 10.1155/2011/403039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Newland M. C., Ceckler T. L., Kordower J. H., and Weiss B. (1989) Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp. Neurol. 106, 251–258 10.1016/0014-4886(89)90157-X [DOI] [PubMed] [Google Scholar]
- 216. Cersosimo M. G., and Koller W. C. (2006) The diagnosis of manganese-induced parkinsonism. Neurotoxicology 27, 340–346 10.1016/j.neuro.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 217. Guilarte T. R. (2010) Manganese and Parkinson's disease: a critical review and new findings. Environ. Health Perspect. 118, 1071–1080 10.1289/ehp.0901748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lao Y., Dion L.-A. A., Gilbert G., Bouchard M. F., Rocha G., Wang Y., Leporé N., and Saint-Amour D. (2017) Mapping the basal ganglia alterations in children chronically exposed to manganese. Sci. Rep. 7, 41804 10.1038/srep41804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Aschner J. L., Anderson A., Slaughter J. C., Aschner M., Steele S., Beller A., Mouvery A., Furlong H. M., and Maitre N. L. (2015) Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. Am. J. Clin. Nutr. 102, 1482–1489 10.3945/ajcn.115.116285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Cordova F. M., Aguiar A. S. Jr., Peres T. V., Lopes M. W., Gonçalves F. M., Pedro D. Z., Lopes S. C., Pilati C., Prediger R. D., Farina M., Erikson K. M., Aschner M., and Leal R. B. (2013) Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch. Toxicol. 87, 1231–1244 10.1007/s00204-013-1017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Hsieh C. T., Liang J. S., Peng S. S., and Lee W. T. (2007) Seizure associated with total parenteral nutrition-related hypermanganesemia. Pediatr. Neurol. 36, 181–183 10.1016/j.pediatrneurol.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 222. Iwase K., Higaki J., Mikata S., Tanaka Y., Kondoh H., Yoshikawa M., Hori S., and Kamiike W. (2002) Manganese deposition in basal ganglia due to perioperative parenteral nutrition following gastrointestinal surgeries. Dig. Surg. 19, 174–183 10.1159/000064210 [DOI] [PubMed] [Google Scholar]
- 223. Rivera-Mancía S., Ríos C., and Montes S. (2011) Manganese accumulation in the CNS and associated pathologies. Biometals 24, 811–825 10.1007/s10534-011-9454-1 [DOI] [PubMed] [Google Scholar]
- 224. Sikk K., Haldre S., Aquilonius S. M., and Taba P. (2011) Manganese-induced parkinsonism due to ephedrone abuse. Parkinsons Dis. 2011, 865319 10.4061/2011/865319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ohtake T., Negishi K., Okamoto K., Oka M., Maesato K., Moriya H., and Kobayashi S. (2005) Manganese-induced parkinsonism in a patient undergoing maintenance hemodialysis. Am. J. Kidney Dis. 46, 749–753 10.1053/j.ajkd.2005.05.033 [DOI] [PubMed] [Google Scholar]
- 226. Brna P., Gordon K., Dooley J. M., and Price V. (2011) Manganese toxicity in a child with iron deficiency and polycythemia. J. Child Neurol. 26, 891–894 10.1177/0883073810393962 [DOI] [PubMed] [Google Scholar]
- 227. Selikhova M., Fedoryshyn L., Matviyenko Y., Komnatska I., Kyrylchuk M., Krolicki L., Friedman A., Taylor A., Jäger H. R., Lees A., and Sanotsky Y. (2008) Parkinsonism and dystonia caused by the illicit use of ephedrone—a longitudinal study. Mov. Disord. 23, 2224–2231 10.1002/mds.22290 [DOI] [PubMed] [Google Scholar]
- 228. Iqbal M., Monaghan T., and Redmond J. (2012) Manganese toxicity with ephedrone abuse manifesting as parkinsonism: a case report. J. Med. Case Rep. 6, 52 10.1186/1752-1947-6-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ares-Santos S., Granado N., and Moratalla R. (2013) The role of dopamine receptors in the neurotoxicity of methamphetamine. J. Intern. Med. 273, 437–453 10.1111/joim.12049 [DOI] [PubMed] [Google Scholar]
- 230. Calne D. B., Chu N. S., Huang C. C., Lu C. S., and Olanow W. (1994) Manganism and idiopathic parkinsonism: similarities and differences. Neurology 44, 1583–1586 10.1212/WNL.44.9.1583 [DOI] [PubMed] [Google Scholar]
- 231. Sulzer D. (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 30, 244–250 10.1016/j.tins.2007.03.009 [DOI] [PubMed] [Google Scholar]
- 232. Guilarte T. R., and Gonzales K. (2015) Manganese-induced parkinsonism is not idiopathic Parkinson's disease: environmental and genetic evidence. Toxicol. Sci. 146, 204–212 10.1093/toxsci/kfv099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Criswell S. R., Perlmutter J. S., Videen T. O., Moerlein S. M., Flores H. P., Birke A. M., and Racette B. A. (2011) Reduced uptake of [18F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology 76, 1296–1301 10.1212/WNL.0b013e3182152830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kwakye G. F., Paoliello M. M., Mukhopadhyay S., Bowman A. B., and Aschner M. (2015) Manganese-induced parkinsonism and Parkinson's disease: shared and distinguishable features. Int. J. Environ. Res. Public Health 12, 7519–7540 10.3390/ijerph120707519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Chen P., Chakraborty S., Peres T. V., Bowman A. B., and Aschner M. (2015) Manganese-induced neurotoxicity: from C. elegans to humans. Toxicol. Res. (Camb.) 4, 191–202 10.1039/C4TX00127C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Guilarte T. R., Burton N. C., McGlothan J. L., Verina T., Zhou Y., Alexander M., Pham L., Griswold M., Wong D. F., Syversen T., and Schneider J. S. (2008) Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J. Neurochem. 107, 1236–1247 10.1111/j.1471-4159.2008.05695.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Criswell S. R., Warden M. N., Searles Nielsen S., Perlmutter J. S., Moerlein S. M., Sheppard L., Lenox-Krug J., Checkoway H., and Racette B. A. (2018) Selective D2 receptor PET in manganese-exposed workers. Neurology 91, e1022–e1030 10.1212/WNL.0000000000006163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Gorell J. M., Johnson C. C., Rybicki B. A., Peterson E. L., Kortsha G. X., Brown G. G., and Richardson R. J. (1999) Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology 20, 239–247 [PubMed] [Google Scholar]
- 239. Gorell J. M., Peterson E. L., Rybicki B. A., and Johnson C. C. (2004) Multiple risk factors for Parkinson's disease. J. Neurol. Sci. 217, 169–174 10.1016/j.jns.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 240. Kim Y., Kim J. W., Ito K., Lim H. S., Cheong H. K., Kim J. Y., Shin Y. C., Kim K. S., and Moon Y. (1999) Idiopathic parkinsonism with superimposed manganese exposure: utility of positron emission tomography. Neurotoxicology 20, 249–252 [PubMed] [Google Scholar]
- 241. Racette B. A., Aschner M., Guilarte T. R., Dydak U., Criswell S. R., and Zheng W. (2012) Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology 33, 881–886 10.1016/j.neuro.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Salazar J., Mena N., Hunot S., Prigent A., Alvarez-Fischer D., Arredondo M., Duyckaerts C., Sazdovitch V., Zhao L., Garrick L. M., Nuñez M. T., Garrick M. D., Raisman-Vozari R., and Hirsch E. C. (2008) Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 105, 18578–18583 10.1073/pnas.0804373105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Garrick M. D., Dolan K. G., Horbinski C., Ghio A. J., Higgins D., Porubcin M., Moore E. G., Hainsworth L. N., Umbreit J. N., Conrad M. E., Feng L., Lis A., Roth J. A., Singleton S., and Garrick L. M. (2003) DMT1: a mammalian transporter for multiple metals. Biometals 16, 41–54 10.1023/A:1020702213099 [DOI] [PubMed] [Google Scholar]
- 244. Lu C., Meng Z., He Y., Xiao D., Cai H., Xu Y., Liu X., Wang X., Mo L., Liang Z., Wei X., Ao Q., Liang B., Li X., Tang S., and Guo S. (2018) Involvement of gap junctions in astrocyte impairment induced by manganese exposure. Brain Res. Bull. 140, 107–113 10.1016/j.brainresbull.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 245. Pajarillo E., Rizor A., Lee J., Aschner M., and Lee E. (2019) The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: potential targets for neurotherapeutics. Neuropharmacology 161, 107559 10.1016/j.neuropharm.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Du K., Liu M., Pan Y., Zhong X., and Wei M. (2017) Association of serum manganese levels with Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis. Nutrients 9, 231–243 10.3390/nu9030231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Tong Y., Yang H., Tian X., Wang H., Zhou T., Zhang S., Yu J., Zhang T., Fan D., Guo X., Tabira T., Kong F., Chen Z., Xiao W., and Chui D. (2014) High manganese, a risk for Alzheimer's disease: high manganese induces amyloid-β related cognitive impairment. J. Alzheimers Dis. 42, 865–878 10.3233/JAD-140534 [DOI] [PubMed] [Google Scholar]
- 248. Molina J. A., Jiménez-Jiménez F. J., Aguilar M. V., Meseguer I., Mateos-Vega C. J., González-Muñoz M. J., de Bustos F., Porta J., Ortí-Pareja M., Zurdo M., Barrios E., and Martínez-Para M. C. (1998) Cerebrospinal fluid levels of transition metals in patients with Alzheimer's disease. J. Neural Transm. (Vienna) 105, 479–488 10.1007/s007020050071 [DOI] [PubMed] [Google Scholar]
- 249. González-Domínguez R., García-Barrera T., and Gómez-Ariza J. L. (2014) Homeostasis of metals in the progression of Alzheimer's disease. Biometals 27, 539–549 10.1007/s10534-014-9728-5 [DOI] [PubMed] [Google Scholar]
- 250. Hare D. J., Faux N. G., Roberts B. R., Volitakis I., Martins R. N., and Bush A. I. (2016) Lead and manganese levels in serum and erythrocytes in Alzheimer's disease and mild cognitive impairment: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing. Metallomics 8, 628–632 10.1039/C6MT00019C [DOI] [PubMed] [Google Scholar]
- 251. De Leo M. E., Borrello S., Passantino M., Palazzotti B., Mordente A., Daniele A., Filippini V., Galeotti T., and Masullo C. (1998) Oxidative stress and overexpression of manganese superoxide dismutase in patients with Alzheimer's disease. Neurosci. Lett. 250, 173–176 10.1016/S0304-3940(98)00469-8 [DOI] [PubMed] [Google Scholar]
- 252. Marcus D. L., Strafaci J. A., and Freedman M. L. (2006) Differential neuronal expression of manganese superoxide dismutase in Alzheimer's disease. Med. Sci. Monit. 12, BR8–14 [PubMed] [Google Scholar]
- 253. Maeda M., Takagi H., Hattori H., and Matsuzaki T. (1997) Localization of manganese superoxide dismutase in the cerebral cortex and hippocampus of Alzheimer-type senile dementia. Osaka City Med. J. 43, 1–5 [PubMed] [Google Scholar]
- 254. Dumont M., Wille E., Stack C., Calingasan N. Y., Beal M. F., and Lin M. T. (2009) Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 23, 2459–2466 10.1096/fj.09-132928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Li F., Calingasan N. Y., Yu F., Mauck W. M., Toidze M., Almeida C. G., Takahashi R. H., Carlson G. A., Flint Beal M., Lin M. T., and Gouras G. K. (2004) Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J. Neurochem. 89, 1308–1312 10.1111/j.1471-4159.2004.02455.x [DOI] [PubMed] [Google Scholar]
- 256. Anantharaman M., Tangpong J., Keller J. N., Murphy M. P., Markesbery W. R., Kiningham K. K., and St Clair D. K. (2006) β-Amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a double knock-in mouse model of Alzheimer's disease. Am. J. Pathol. 168, 1608–1618 10.2353/ajpath.2006.051223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Calderón-Garcidueñas L., Serrano-Sierra A., Torres-Jardón R., Zhu H., Yuan Y., Smith D., Delgado-Chávez R., Cross J. V., Medina Cortina H., Kavanaugh M., and Guilarte T. R. (2013) The impact of environmental metals in young urbanites' brains. Exp. Toxicol. Pathol. 65, 503–511 10.1016/j.etp.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Calderón-Garcidueñas L., D'Angiulli A., Kulesza R. J., Torres-Jardón R., Osnaya N., Romero L., Keefe S., Herritt L., Brooks D. M., Avila-Ramirez J., Delgado-Chávez R., Medina-Cortina H., and González-González L. O. (2011) Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem auditory evoked potentials. Int. J. Dev. Neurosci. 29, 365–375 10.1016/j.ijdevneu.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Schneider J. S., Decamp E., Koser A. J., Fritz S., Gonczi H., Syversen T., and Guilarte T. R. (2006) Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 1118, 222–231 10.1016/j.brainres.2006.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]