Abstract
Background
Meningioma, a most common brain tumor, has a high rate of recurrence. Tumor-associated macrophages (TAMs) are the most abundant immune cell type in meningioma. TAMs display functional phenotypic diversity and may establish either an inflammatory and anti-tumoral or an immunosuppressive and pro-tumoral microenvironment. TAM subtypes present in meningioma and potential contribution to growth and recurrence is unknown.
Methods
Immunofluorescence staining was used to quantify M1 and M2 TAM populations in tissues obtained from 30 meningioma patients. Associations between M1 and M2 cells, M1:M2 cell ratio to tumor characteristics, WHO grade, recurrence, size, location, peri-tumoral edema, and patient demographics such as age and sex were examined.
Results
TAM cells accounted for ~18% of all cells in meningioma tissues. More than 80% of infiltrating TAMs were found to be of pro-tumoral M2 phenotype and correlated to tumor size (P = .0409). M1:M2 cell ratio was significantly decreased in WHO grade II, compared to grade I tumors (P = .009). Furthermore, a 2.3-fold difference in M1:M2 ratio between primary (0.14) and recurrent (0.06) tumors was observed (n = 18 and 12 respectively, P = .044).
Conclusion
This study is the first to confirm existence of pro-tumoral M2 TAMs in the meningioma microenvironment, emphasizing its potential role in tumor growth and recurrence.
Keywords: macrophage, meningioma, microenvironment, M1:M2 TAM ratio, tumor recurrence
Importance of the Study.
Meningioma, often considered a benign brain tumor, has a lifetime recurrence rate of 30% for WHO grade I, 50% for WHO grade II, and 95% for WHO grade III. In addition, on recurrence, meningioma may transform to a higher grade. In this study, we identified for the first time that tumor-associated macrophages (TAMs) in meningioma microenvironment are predominantly of the M2 subtype (not M1). TAM quantities were positively associated with tumor size but did not correlate with tumor recurrence. Instead, the M1:M2 ratio was found to correlate with tumor recurrence and which indeed has been validated as a novel prognostic indicator for many cancers. Accordingly, the M1:M2 ratio may be an important biomarker of meningioma behavior and propensity for recurrence and progression.
Key Points.
Macrophages in meningioma are predominantly of M2 subtype.
The M1:M2 ratio may well be an indicator of tumor recurrence.
Meningioma is the most common primary brain tumor in adults, with incidences increasing with age.1 Often labeled as benign, the lifetime tumor recurrence rates for WHO grade I, II, and III meningioma are 30%, 50%, and 95%, respectively.2 Additional risk factors for tumor recurrence include tumor size, multiplicity, extent of resection, and genetics.3–6 However, pathological mechanisms that contribute to tumor recurrences and progression are incompletely understood.7 Tumor microenvironment plays a critical role in recurrence and prognosis for many solid tumors8,9 and is the focus of rigorous investigation for evolving cancer treatments.10,11 How the tumor microenvironment contributes to meningioma behavior is incompletely understood. Tumor and immune cell markers indicative of an immune suppressive environment are consistently identified in meningioma tissues,12–17 including the expression of immune checkpoint proteins B7-H3, CTLA-4, LIM, PD-1, PD-L1, PD-L2, and Tim-3 on tumor cells and infiltrating lymphocytes.12–18 The composition of infiltrating immune cells in meningioma microenvironment supporting immune suppression has also been noted. Tumor-associated macrophages (TAMs) are the most abundant immune cell type present in large numbers in the meningioma microenvironment..12–14,16,18 TAM infiltration varies noticeably between cases although a trend toward higher numbers of cells in tumors with monosomy for 22 (NF2 tumors)19 and with higher WHO grade is reported. TAMs may be scattered in single or groups of cells and may be dense in number at the tumor-brain interface often with a component of brain invasion.19,20 The composition of TAM populations in meningioma is not known.
TAMs are highly plastic, existing in a spectrum of polymerized states, but often dichotomized by an M1 or M2 phenotype.21–23 M1 TAMs have anti-tumoral effects but M2 macrophages are pro-tumoral.21,22 M2 TAMs are associated with poor prognosis and decreased time to recurrence for a number of cancers.24 This contrasts with M1 TAMs that are linked to improved prognosis.25–27 Increasing TAM numbers correlate to poor outcome for most cancers including brain tumors as does high M2:M1 cell ratio.28–32
In this immunofluorescence staining study of meningioma, we identified TAMs in the meningioma microenvironment to be predominantly of the M2 subtype. TAM quantities were positively associated with tumor size and increased with WHO grade but were not correlated with tumor recurrences. Instead, the M1:M2 ratio was found to correlate with tumor recurrence and indeed has been validated as a novel prognostic indicator for many cancers.28–32 Accordingly, this may also be an important biomarker of meningioma behavior and propensity for recurrence and progression.
Methods
Patient Characteristics
This study was approved by the Conjoint Health Research Ethics Board—University of Calgary. Informed written consent was obtained from each patient. A total of 30 patients (16 Grade I, 12 Grade II, and 2 Grade III) were included. For each case, histopathologic subtype and final WHO grading were obtained from Neuropathology report. Patient information (age, sex) and detailed medical history (past resections, comorbidities including cancer, other treatment, radiotherapy), baseline cognitive data and information specific to the tumor (size, location, surgical procedure, pre- and post-surgery MR imaging, extent of resection and severity of peri-tumoral edema) were also acquired (Table 1). Tumor size was estimated from MR images as the maximal cross-sectional distance in cm. A semi-quantitative assessment of the severity of peri-tumoral edema was performed. No peri-tumoral edema (scale = 0), mild peri-tumoral edema (scale = 1), was classified as edema adjacent to the tumor less than 1 cm in margin. In moderate peri-tumoral edema (scale = 2), edema had a margin of 1-2 cm. Peri-tumoral edema with a margin greater than 3 cm was classified as severe (scale = 3). Tumors were characterized as recurrent if the tissue was collected from resection of a recurrent tumor.
Table 1.
Patient characteristics
| Case ID |
Age | Gender | Location | Size - cm | Edema | WHO grade | Simpson grade | Histological subtype | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| M2 | 63 | F | R. mid parafalx | 1.8 | Moderate | I | 1 | Meningo/Angio | N |
| M3 | 62 | F | L. cerebellopontine angle | 3.5 | Nil | I | 4 | Secretary | N |
| M4 | 33 | F | R. Frontoparietal convexity | 4.4 | Moderate | II | 1 | Atypical | N |
| M5 | 71 | F | L. frontotemporal convexity | 2.2 | Moderate | III | 1 | Anaplastic/Rhabdoid | Y |
| M8 | 52 | M | R. convexity | 6.7 | Mild | II | 4 | Atypical | Y |
| M9 | 58 | F | L. Frontal-parietal Parafalx | 6.6 | Nil | II | 2 | Transitional | N |
| M11 | 79 | F | L. cerebellopontine angle | 4.8 | Mild | I | 4 | Meningothelial | N |
| M12 | 68 | M | L. frontal medial sphenoid wing | 5 | Mild | I | 4 | Psammomatous | N |
| M13 | 57 | F | L. sphenoid wing | 2.6 | Mild | I | 1 | Meningiothelial | N |
| M14 | 58 | F | Tuberculum sellae | 1.8 | Nil | I | 1 | Psammomatous | Y |
| M15 | 75 | F | L. mid posterior parafalx | 7.4 | Moderate | II | 4 | Atypical | Y |
| M17 | 46 | F | L. parietal parafalx | 1.6 | Nil | I | 4 | Fibrous | N |
| M18 | 28 | F | Midline Parafalx (R. > L.) | 7.5 | Moderate | II | 2 | Atypical | Y |
| M19 | 28 | F | L. sphenoid wing | 3 | Moderate | I | 4 | Meningothelial | N |
| M23 | 59 | F | Posterior-frontal parafalx | 2.9 | Nil | I | 1 | Transitional | N |
| M27 | 64 | F | L. frontal convexity | 4.4 | Mild | I | 1 | Transitional | Y |
| M28 | 41 | M | Posterior Parafalx (R. > L.) | 6.5 | Moderate | I | 4 | Transitional | Y |
| M29 | 74 | M | Petroclival | 6.5 | Moderate | I | 4 | Meningothelial | Y |
| M30 | 56 | M | Olfactory groove | 8.5 | Nil | II | 4 | Chordoid | Y |
| M31 | 62 | F | R. paritotemporal convexity | 3.9 | Nil | II | 1 | Atypical | N |
| M32 | 81 | F | Tuberculum sellae | 2.9 | Nil | I | 4 | Transitional | N |
| M34 | 68 | F | L. frontal convexity | 3.9 | Mild | II | 1 | Atypical | N |
| M35 | 62 | M | R. tentorial meningioma | 2 | Nil | I | 4 | Transitional | N |
| M38 | 51 | M | R. CP angle meningioma | 1.5 | Nil | I | 1 | Transitional | N |
| M39 | 70 | M | L. Cerebellar Convexity | 2.7 | Nil | I | 1 | Unknown | N |
| M40 | 71 | M | R. tentorial | 3.2 | Moderate | II | 1 | Atypical | N |
| M45 | 36 | F | R. & L. parietal meningioma | 7.5 | Nil | II | 4 | Atypical | Y |
| M46 | 83 | M | R. frontal convexity | 6.4 | Severe | II | 4 | Atypical | N |
| M47 | 73 | F | Post-midline parafalx | 4.3 | Severe | II | 1 | Atypical | Y |
| M65 | 69 | F | L. frontal convexity | 7.8 | Moderate | III | 1 | Rhabdoid | N |
Immunofluorescence Staining
Immunofluorescence staining was performed as described previously.17 Briefly, 4 µm tissue sections were cut in series from formalin-fixed paraffin-embedded tissue blocks for each meningioma case. Standard H&E staining was performed for each tumor and histological features of each section were reviewed by a neuropathologist establishing the WHO grade (Neuropathology report). For immunofluorescence, sections were stained overnight with primary antibodies; mouse anti-CD68 antibodies (1:200, (KP1) conjugated to Alexa-488 Santa Cruz Biotechnology), mouse anti-CD80 (1:100, (R&D Systems), mouse anti-CD86 (1;100, D-6 Santa Cruz Biotechnology together with either rabbit anti-CD163 (1:200, EPR19518, Abcam), rabbit anti-CD206 (1:1000, ab64693, Abcam), or rabbit anti-iNOS (1:200, Abcam). Secondary goat anti-rabbit or anti-mouse Alexa-555 & -488 antibodies (Molecular probes) were applied to sections and tissue auto-fluorescence was blocked with 30-min incubation in 0.02% Sudan black in 70% EtOH. Sections were then coverslipped using hard-set mounting media containing DAPI (Vectashield).
TAM Quantification
Tissue sections were imaged at 10× and 40× magnifications with a fluorescence microscope (Olympus BX51) with developer’s software. M1 and M2 distribution and association with tissue structures was assessed qualitatively at 10× and 40×. M1 and M2 cells were quantified in 5 regions of interest (ROI) per stained meningioma tissue section, under 40× magnification using a cell counter plugin in ImageJ (https://fiji.sc/). TAM quantifications were performed by 2 blinded independent observers. M2 macrophages were classified as CD68+/CD163+ double-stained cells. M1 macrophages were classified as CD68+-only cells in CD68 and CD163 co-stained sections or as CD68+/iNOS+ double-positive cells. Total number of cells in an ROI was calculated with the analyze particles plugin in Image J to count DAPI-labeled nuclei. Cell nuclei counts were used to calculate percentage of TAMs. TAM infiltration density was assessed in each ROI by correcting for regions and structures associate with vasculature. This was done by normalizing TAM counts according to total numbers of cells (DAPI-stained nuclei) in each ROI. Additional staining of M1 markers, CD80, and CD86 as well as an M2 marker CD206 was assessed in a small selection of cases in the study (n = 6).
Statistical Analysis
Correlations between TAM number (M1 + M2, ie, CD68+ cells) or TAM percentage of total cells or M1/M2 ratio (M2 = CD68+/CD163+ cells) to clinical data variables: tumor size, peri-tumoral edema severity, and age were evaluated and a Spearman rank correlation test was used in correlation analyses. A Welch’s t-test was used for comparing means between 2 groups for TAM number (CD68+ cells) or TAM percentage of total cells or M1:M2 ratio according to WHO grade, tumor location (skull base or convexity), gender, and recurrence status. One-way ANOVA was performed to evaluate differences in TAM number (CD68+ cells) between histological subtypes. Data in bar graphs are represented as group means ± SEM. Statistical significance was achieved with P-value ≤ .05 and provided as an absolute number. We excluded WHO grade III group from analyses as only 2 cases were included in the study. However, individual data points for these 2 cases are still included in results graphs.
Results
TAM Subtypes and Distribution in Meningioma
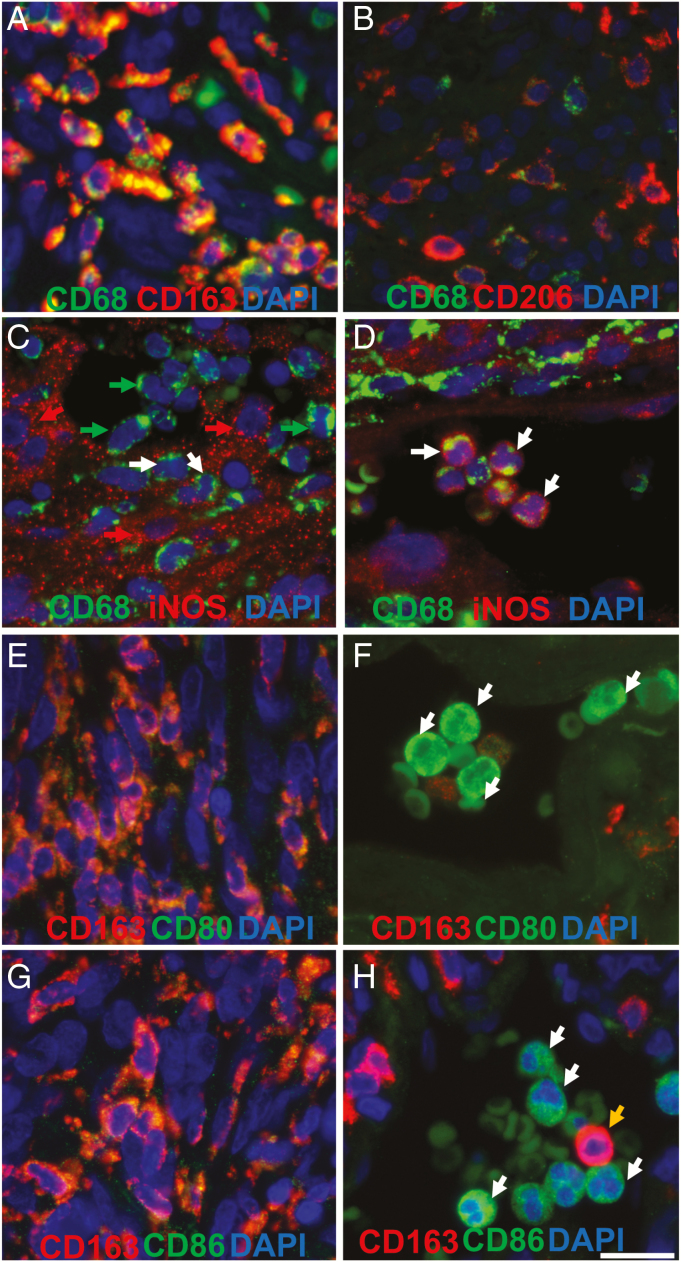
The TAM subtypes present in the tumor microenvironment are presented in Figures 1 and 2. M2 TAMs as indicated by (CD68+/CD163+ staining) were identified in all tissues studied, whereas a few M1, (CD68+/iNOS+) cells, were present in most (Figures 1 and 2 and Supplementary Figures S1–S2). Staining of additional M2 marker (CD206) and M1 markers (CD80 and CD86) was performed on 6 cases and showed comparable results to CD163 and iNOS staining. Majority of TAMs had high CD163 and CD206 staining and low CD80, CD86, and iNOS staining (Figure 1 and Supplementary Figures S4–S6). TAMs (CD68+ cells) accounted for on average 18 ± 2% of all cells (Figure 2K). CD68+/CD163+ double-positive cells were the most abundant TAM cell-type in tissues (89 ± 2% of TAMs). Fewer CD68+/CD206+ double labeled cells were present than CD68+/CD163+ cells leading to a higher M1:M2 ratio 0.2 to 0.1 respectively in n = 6 examined (Supplementary Table 1). Although a large discrepancy between CD163- and CD206-labeled cells in patient M65 largely accounted for this difference. CD68–/iNOS+ and CD68–/CD163+ positive cells were also present in tissues (Supplementary Figure S3). For most cases, TAM infiltration was observed throughout the tissue section (Figures 2A–C). Infiltration of single and groups of cells was present (Figures 1 and A–C, G, H). A high density of perivascular macrophages around vessels was common (Figure 1DSupplementary Figure S7). TAM infiltration was observed in whorls, but many whorls with limited TAM cell infiltration were also encountered (Figure 2C). Interestingly, in tumors with high numbers of CD68+/CD163– M1 cells, a regional distinction between populations of M1 and M2 cells was commonly observed (Figure 2A).
Figure 1.
Representative CD68, CD163, CD206, iNOS, CD80, and CD86 staining in meningioma tissue sections. (A) CD68 (green), CD163 (red) tissue section double staining. CD68+/CD163+ cells are characterized as M2 tumor-associated macrophages (TAMs) (B) CD68 (green), CD206 (red) tissue section double staining. (C) CD68 (green), iNOS (red) tissue section double staining. Red and white arrows depict CD68–/iNOS+ tumor cells and CD68+/iNOS+ M1 cells respectively. CD68+/iNOS– M2 cells are labeled by green arrows. (D) Example CD68+/iNOS+ monocytes are labeled by white arrows. CD68+/iNOS– perivascular M2 cells are also present. (E and F) CD80 (green), CD163 (red) tissue section double staining. White arrows in F depict CD80+ monocytes. (G and H) CD86 (green), CD163 (red) tissue section double staining. White arrows in H depict CD86+ monocytes and a CD163+ monocyte is labeled by an orange arrow. Scale bars represent 15 µm.
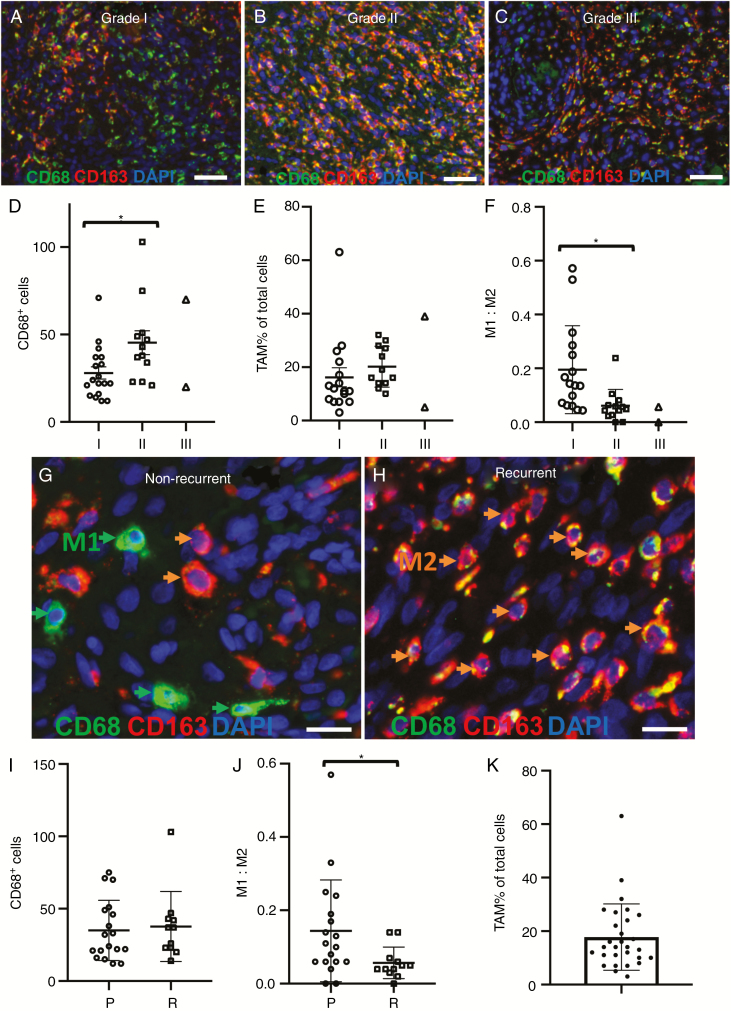
Figure 2.
Representative fluorescence immunohistochemistry images of tumor-associated macrophages in meningioma tissue sections according to WHO grades I–III, primary and recurrent tumor groups. (A–C) Representative images of CD68 (green) and CD163 (red) staining according to WHO grades I (n = 16), II (n = 12), and III (n = 2). (D) Mean tumor-associated macrophage (TAM) cell counts (CD68+ cells) for each case are plotted according to WHO grade. (E) TAMs as a percentage of total cells for each case are quantified according to WHO grade. (F) M1:M2 ratios for each case are quantified according to WHO grade. (G and H) Representative TAM cell staining in primary (n = 18) and recurrent (n = 12) meningioma tissue sections. Example M1 and M2 cells are labeled by arrows (I) Total TAMs (CD68+ cells) for each case are quantified according to recurrence status. (J) M1:M2 ratios for each case according to recurrence group. (K) Average value of TAMs as a percentage of total cells for each case. A whorl with little TAM infiltration is present in top left section of image in panel C. Bar represent mean TAM percentage (n = 30). Error bars in each graph represent ± SEM. Scale bars represent 200 µm (A–C) and 15 µm (G and H). *P < .05.
Total Number of TAMs and TAM Profiling According to WHO Grade and Histological Subtype
M1 and M2 cell infiltration was evident in tissues of each tumor grade. Mean total number of TAMs (CD68+ cells) was statistically higher in grade II (45 ± 7 cells/ROI, n = 12) compared with grade I tumors (28 ± 4 cells/ROI, n = 16; P = .0195; Figure 2D). However, when we corrected TAM infiltration according to total numbers of cells in each ROI (to account for tissue regions and structures associated with vasculature), no significant difference was observed (Figure 2E). The composition of TAM phenotypes (represented as M1:M2 cell ratio) according to WHO grade was next examined. The M1:M2 cell ratio differed significantly between grade I (0.20 ± 0.04/ROI, n = 16) compared with grade II tumors (0.06 ± 0.02/ROI, n = 12; P = .009; Figure 2F). No differences in total TAM numbers were recorded between histological subtype groups (P = .46; Supplementary Figure S8).
TAM Profiles and Meningioma Recurrence
The number of CD68+ TAM cells was compared between primary and recurrent tumor groups (n = 18 and 12 cases respectively). Figure 2G and H demonstrates representative M1 and M2 cell infiltration staining for each group. TAM numbers (CD68+ cells) between these 2 groups were not statistically significant (P = .75; Figure 2I). However, the M1:M2 cell ratio differed significantly between primary tumor (0.14 ± 0.03/ROI, n = 18) and recurrent tumor groups (0.06 ± 0.01/ROI, n = 12; P = .0448; Figure 2J). No effect of extent of resection (Simpson grade) on recurrence was observed (P = .39; Supplementary Figure S9).
TAM Profiles According to Clinical Data and Patient Demographics (Age, Sex, Tumor Location, Size, Edema)
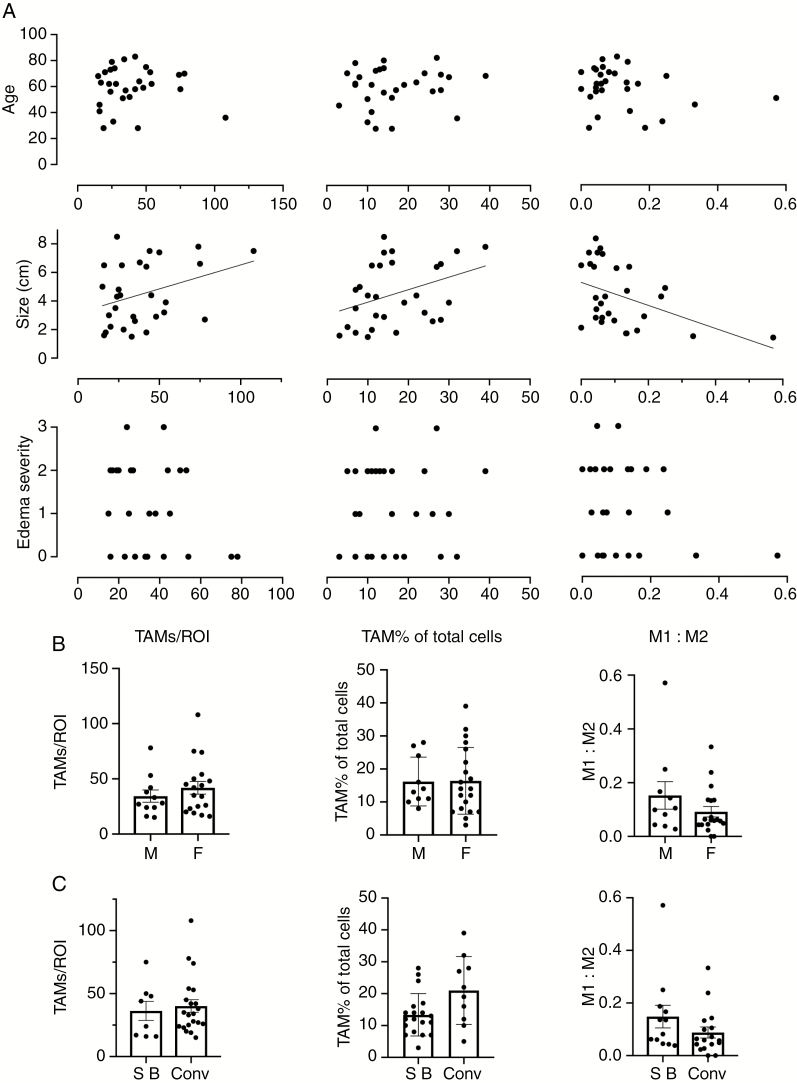
Several clinical variables and patient demographics have been associated with tumor recurrence in meningioma. We investigated the potential association between TAM subtype profiles (M1:M2 ratio), total number of TAMs and the percentage of TAMs of total cells to patient age and gender as well as tumor location, size, and severity of peri-tumoral edema (Figure 3). Numbers of TAMs, TAM percentage of total cells and M1:M2 ratio each correlated to tumor size (r = .3819, P = .0409, r = .3677, P = .0497, and r = –.4366, P = .0179, respectively; Figure 3A). No correlation was observed between TAM profile variables and age (Figure 3A) and TAM profiles did not differ significantly between male or female patients (Figure 3B). Similarly, no association was observed between TAMs and severity of peri-tumoral edema and no statistically significant differences were measured between skull base and convexity tumor location groups (Figures 3A and C).
Figure 3.
Association of total tumor-associated macrophages (TAM) number, TAM percentage of total cells and M1/M2 profiles to patient demographics and clinical variables. (A) Scatter plots for patient age, tumor size, and peri-tumoral edema severity according to TAM profile variables. Peri-tumoral edema severity in A; (0 = nil, 1 = mild, 2 = moderate, 3 = severe). (B and C) TAM profile variables according to sex (M—male, F—female) and tumor location (SB—skull base, Conv—convexity) are quantified in bar graphs. Bars represent mean. Error bars in each graph represent ± SEM.
Discussion
This study is the first to confirm and quantify the existence and abundance of pro-tumoral M2 TAMs in meningioma microenvironment. Interestingly, we found greater than 80% of infiltrating TAMs were of polarized M2-like phenotype and that the number of TAMs was positively associated with tumor size. Of note, TAM subtype profiles differed significantly between primary and recurrent meningioma. Specifically, the M1:M2 cell ratio was decreased in recurrent tumors. Accordingly, the results identify a potential role for infiltrating TAM cells in promoting meningioma growth and recurrence.
Infiltrating immune cells have an integral role in the tumor microenvironment, influencing the growth and progression of most solid tumors and the functional phenotype of immune cells is often a reliable indicator of tumor behavior. Macrophages are the most abundant infiltrating immune cell type in meningioma and are likely to be a major contributor to an apparent immunosuppressive environment.12–14,16,18 Interestingly, TAMs isolated from meningioma were demonstrated to have an immunosuppressive effect on in vitro T cell proliferation and also express PD-L1 immune checkpoint protein.18 Total number of TAMs has been correlated to poor prognosis for many solid tumors28–30 and a positive association between numbers of TAMs and tumor grade has been reported for meningioma.19 Adding to this, we have now shown that different TAM phenotypes may contribute to tumor behavior and recurrences.
Previous studies showed meningioma tissues stain positive for the M2 marker CD163, suggesting TAMs of an M2 phenotype are likely present.33–35 CD163 expression was reported in cells of macrophage-like morphology in the stroma of some meningioma tissues33 and in 2 cases of chordoid meningioma.35 However, these findings were not confirmed, and previous work on meningioma has mainly focused on CD163 expression in tumor cells. Moreover, mRNA patterns in flow-cytometry harvested immune cells have indicated TAMs present in meningioma tissues are predominantly of the M1 type, where infiltration patterns may differ according to genetic subtype.19 However, no confirmatory method such as immunohistochemistry was performed to substantiate the transcript findings.
In this investigation, we have confirmed that both M1 and M2 TAMs are present in the meningioma microenvironment using a well-established and accepted dual macrophage marker immunostaining method for differentiating TAM subtypes. Indeed, TAMs accounted for ~18% of all cells and their numbers increased with WHO grade. These finding were consistent with previous reports.16,36 We did not observe any significant difference between TAM infiltration in skull base and convexity meningioma groups despite reported differences in genetics and tumor behavior in these locations. The number of TAMs in meningioma did positively correlate to tumor size. This is in line with recent findings in other cancers, and current classification of M2 TAMs as tumorigenic, capable of supporting tumor cell growth.35, 37–40 However, it was the reduced M1:M2 ratio, and not total number of TAMs that was associated with tumor recurrence.
The M1:M2 cell ratio has been demonstrated to have prognostic value in several tumor types, including glioma, melanoma, ovarian, colorectal, and liver cancer.28–32 A higher M1:M2 ratio has been associated with decreased tumor severity and grade26,38 and an association between higher M1:M2 ratios and longer progression-free survival has been observed.31,32 Improved progression-free survival is attributable to M1 TAMs, which may function as tumoricidal cells by promoting inflammation via phagocytosis and cytotoxic cytokine release and recruiting immunostimulating leukocytes to impede growth.25–27 Thus, higher ratios of M1 to M2 TAMs likely decreases risk of recurrence.26 Conversely, an inverse correlation between M2 TAM density and 5-year overall survival has been reported.24 Overall, our findings in meningioma indicate that M1:M2 TAM ratio may prove to be an effective indicator of meningioma growth and recurrence. However, to establish this more conclusively, a larger patient cohort with long-term clinical outcome that includes robust statistical analyses would be necessary.
Investigators have reported an association between TAM density in meningioma tissues and WHO grade.16,41 Although we also detected higher numbers of TAMs in grade II tumors compared to grade I, when correcting for TAM infiltration by area of tissue in each ROI removing regions and structures associate with vasculature, no significant difference was observed. Furthermore, TAM numbers did not differ between primary and recurrent tumors. These findings are consistent with previous reports on other tumor types, showing little association between the number of M1 or M2 or total TAM numbers to tumor behavior.28–32 A more specific approach to the quantification of TAM infiltration by tumor region has shown TAM abundance in tumor parenchyma, and this may be a reliable predictor for tumor progression.37,42 It will be important in future studies on meningioma tissues to quantify numbers of TAMs in stromal and tumor parenchyma compartments.
A number of biological events are described in meningioma that could regulate TAM behavior and explain the profiles and distribution patterns of TAMs in these tumors. In many tumors including glioblastoma, hypoxia has been considered a key contributor to induction of TAMs to an M2 subtype.43–46 The spatiotemporal distribution of TAMs throughout a solid tumor is often thought to be a direct result of high density of TAMs in hypoxic regions.45,47 Similarly, TAMs are also attracted to necrotic regions.44,48 Although necrosis is not a common pathology of meningioma, it can be a feature of high-grade and aggressive subtypes.49 The number of hypoxic regions in meningioma also increases with grade.50,51 These events could explain the increase in density of TAMs with tumor grade. The location of these pathological features may also contribute to the variable TAM distribution observed in meningioma, that is from single and sparse infiltration to dense groups of cells when in close proximity to hypoxic tissue.
Limitations and Conclusion
While a small patient cohort, the findings presented here offer unique insight into the potential pathogenic processes that contribute to tumor growth, progression, and recurrence of meningioma. However, macrophage plasticity and that TAMs in meningioma may have phenotypes anywhere on the spectrum between the 2 extremes of the M1 and M2 dichotomy need to be recognized.21 The TAM subtype quantification was performed on only a single whole-tumor slice, and heterogeneity in patterns of immune cell infiltration in other regions of the tumor that may exist was not detected. Similarly, although we did not observe a difference in TAM infiltration between skull base and convexity meningioma, there may be differences between histological subtypes that we were not able to establish due to the small sample size.
We have used the commonly accepted marker of macrophages, CD68, and the iNOS and CD163 for identifying and quantifying M1 and M2 macrophages respectively.29,52,53 However, it should be noted that both iNOS and CD163 macrophage markers may also be expressed in tumor cells.33–35 Furthermore, high expression of CD68 in select tumor cells has been observed in a few cases of rare xanthomatous meningioma and also in a small number of atypical meningioma case reports.54–56 Indeed, the expression of CD68-/CD163+ tumor cells was commonly present in the cases studied, as was the expression of iNOS and CD206 in tumor cells. When differentiating and quantifying M1 and M2 cells in meningioma, these factors must be taken into consideration. Interestingly, the aberrant expression of macrophage markers in tumor cells has been observed in other cancers and is associated with more aggressive behavior of the tumor cell. Given immune marker expression in tumor cells was more frequently attributed to higher-grade cases, a similar aggressive behavior of these cells may be a characteristic of meningioma biology.
It may now be concluded that macrophages in meningioma are predominantly of M2 subtype, as evidenced by high expression of CD163 and CD206 and low expression of CD80, CD86, and iNOS. Furthermore, an M1:M2 ratio may be an indicator of tumor recurrence. These findings are important as the presence of M2 macrophages, considered to be pro-tumoral in nature, could well contribute to tumor progression and recurrence. Furthermore, this could account for immunosuppressive events present in meningioma, establishing the M2 macrophage as a novel target for future therapy.
Ethical Approval
Ethics Approval and Consent to Participate: The study was approved by University of Calgary Conjoint Health Research Ethics Board Ethics ID REB15-0413. All patients provided informed consent to participate.
Data Availability
All data are being securely held within the Project neuroArm Research Facility, University of Calgary. All data have been systematically cataloged and are readily available.
Supplementary Material
Acknowledgments
The authors thank the Division of Neurosurgery, Department of Clinical Neurosciences, University of Calgary-Foothills Medical centre, for patient care platform and access to clinical population.
Funding
The project is self-funded.
Conflict of interest statement: None of the authors have any conflict of interest.
Authorship statement: Lead molecular neuroscientist, responsible for study concept and design, sample processing, immunofluorescence staining, quality assurance, statistical analysis, and manuscript preparation: Dustin T. Proctor. Honors thesis on meningioma, responsible for immunofluorescence, and quantification of M1:M2 TAM ratio: Jordan Huang. Clinical data/operating room specimen collection, patient consent, provision of MR imaging information and histopathology data, manuscript editing: Sanju Lama. MR imaging review and clinical data archiving: Abdulrahman Albakr. Provision of molecular diagnostic lab facility, manuscript editing: Guido van Marle. Project Lead, responsible for original study concept, design, quality assurance, surgical care, and manuscript editing: Garnette R. Sutherland.
References
- 1. Sutherland GR, Florell R, Louw D, Choi NW, Sima AA. Epidemiology of primary intracranial neoplasms in Manitoba, Canada. Can J Neurol Sci. 1987;14(4):586–592. [PubMed] [Google Scholar]
- 2. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Proctor DT, Ramachandran S, Lama S, Sutherland GR. Towards molecular classification of meningioma: evolving treatment and diagnostic paradigms. World Neurosurg. 2018;119:366–373. [DOI] [PubMed] [Google Scholar]
- 4. Ildan F, Erman T, Göçer AI, et al. Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull Base. 2007;17(3):157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maillo A, Orfao A, Espinosa AB, et al. Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro Oncol. 2007;9(4):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, Guthikonda B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2017;126(1):201–211. [DOI] [PubMed] [Google Scholar]
- 7. Rohringer M, Sutherland GR, Louw DF, Sima AA. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71(5 Pt 1):665–672. [DOI] [PubMed] [Google Scholar]
- 8. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belli C, Trapani D, Viale G, et al. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22–32. [DOI] [PubMed] [Google Scholar]
- 11. Sounni NE, Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem. 2013;59(1):85–93. [DOI] [PubMed] [Google Scholar]
- 12. Du Z, Abedalthagafi M, Aizer AA, et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han SJ, Reis G, Kohanbash G, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016;130(3):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang L, Lowther DE, Meizlish ML, et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro Oncol. 2013;15(11):1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polyzoidis S, Koletsa T, Panagiotidou S, Ashkan K, Theoharides TC. Mast cells in meningiomas and brain inflammation. J Neuroinflammation. 2015;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Domingues PH, Teodósio C, Ortiz J, et al. Immunophenotypic identification and characterization of tumor cells and infiltrating cell populations in meningiomas. Am J pathol. 2012;181(5):1749–1761. [DOI] [PubMed] [Google Scholar]
- 17. Proctor DT, Patel Z, Lama S, Resch L, van Marle G, Sutherland GR. Identification of PD-L2, B7-H3 and CTLA-4 immune checkpoint proteins in genetic subtypes of meningioma. Oncoimmunology. 2019;8(1):e1512943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinton L, Solito S, Masetto E, et al. Immunosuppressive activity of tumor-infiltrating myeloid cells in patients with meningioma. Oncoimmunology. 2018;7(7):e1440931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Domingues PH, Teodósio C, Otero Á, et al. Association between inflammatory infiltrates and isolated monosomy 22/del(22q) in meningiomas. PLoS One. 2013;8(10):e74798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Backer-Grøndahl T, Moen BH, Arnli MB, Torseth K, Torp SH. Immunohistochemical characterization of brain-invasive meningiomas. Int J Clin Exp Pathol. 2014;7(10):7206–7219. [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordon S, Martinez-Pomares L. Physiological roles of macrophages. Pflugers Arch. 2017;469(3–4):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung KY, Cho SW, Kim YA, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015;49(4):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lisi L, Ciotti GM, Braun D, et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci Lett. 2017;645:106–112. [DOI] [PubMed] [Google Scholar]
- 26. Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. [DOI] [PubMed] [Google Scholar]
- 28. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang M, He Y, Sun X, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herwig MC, Bergstrom C, Wells JR, Höller T, Grossniklaus HE. M2/M1 ratio of tumor associated macrophages and PPAR-gamma expression in uveal melanomas with class 1 and class 2 molecular profiles. Exp Eye Res. 2013;107:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lundholm M, Hägglöf C, Wikberg ML, et al. Secreted factors from colorectal and prostate cancer cells skew the immune response in opposite directions. Sci Rep. 2015;5:15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrillo M, Zannoni GF, Martinelli E, et al. Polarisation of tumor-associated macrophages toward M2 phenotype correlates with poor response to chemoradiation and reduced survival in patients with locally advanced cervical cancer. PLoS One. 2015;10(9):e0136654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanno H, Nishihara H, Wang L, et al. Expression of CD163 prevents apoptosis through the production of granulocyte colony-stimulating factor in meningioma. Neuro Oncol. 2013;15(7):853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guadagno E, Presta I, Maisano D, et al. Role of macrophages in brain tumor growth and progression. Int J Mol Sci. 2018;19(4):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Presta I, Guadagno E, Di Vito A, et al. Innate immunity may play a role in growth and relapse of chordoid meningioma. Int J Immunopathol Pharmacol. 2017;30(4):429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asai J, Suzuki R, Fujimoto T, et al. Fluorescence automatic cell sorter and immunohistochemical investigation of CD68-positive cells in meningioma. Clin Neurol Neurosurg. 1999;101(4):229–234. [DOI] [PubMed] [Google Scholar]
- 37. Jackute J, Zemaitis M, Pranys D, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu C, Kros JM, van der Weiden M, Zheng P, Cheng C, Mustafa DA. Expression site of P2RY12 in residential microglial cells in astrocytomas correlates with M1 and M2 marker expression and tumor grade. Acta Neuropathol Commun. 2017;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prosniak M, Harshyne LA, Andrews DW, et al. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19(14):3776–3786. [DOI] [PubMed] [Google Scholar]
- 41. Rossi ML, Cruz Sanchez F, Hughes JT, Esiri MM, Coakham HB. Immunocytochemical study of the cellular immune response in meningiomas. J Clin Pathol. 1988;41(3):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rakaee M, Busund LR, Jamaly S, et al. Prognostic value of macrophage phenotypes in resectable non-small cell lung cancer assessed by multiplex immunohistochemistry. Neoplasia. 2019;21(3):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA. Hypoxia and tumor-associated macrophages: a deadly alliance in support of tumor progression. Oncoimmunology. 2014;3(1):e27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104(8):2224–2234. [DOI] [PubMed] [Google Scholar]
- 45. Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo X, Xue H, Shao Q, et al. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016;7(49):80521–80542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167(3):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79(5–6):991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Góes P, Santos BFO, Suzuki FS, et al. Necrosis is a consistent factor to recurrence of meningiomas: should it be a stand-alone grading criterion for grade II meningioma? J Neurooncol. 2018;137(2):331–336. [DOI] [PubMed] [Google Scholar]
- 50. Yoo H, Baia GS, Smith JS, et al. Expression of the hypoxia marker carbonic anhydrase 9 is associated with anaplastic phenotypes in meningiomas. Clin Cancer Res. 2007;13(1):68–75. [DOI] [PubMed] [Google Scholar]
- 51. Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046–2056. [DOI] [PubMed] [Google Scholar]
- 52. Almatroodi SA, McDonald CF, Darby IA, Pouniotis DS. Characterization of M1/M2 Tumour-Associated Macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron. 2016;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suriano F, Santini D, Perrone G, et al. Tumor associated macrophages polarization dictates the efficacy of BCG instillation in non-muscle invasive urothelial bladder cancer. J Exp Clin Cancer Res. 2013;32:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ersoz S, Yilmaz ZS, Eyuboglu I, Yazar U. Xanthomatous meningioma: a case report. Turk Neurosurg. 2019;29(1):141–144. [DOI] [PubMed] [Google Scholar]
- 55. Ishida M, Fukami T, Nitta N, et al. Xanthomatous meningioma: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6(10):2242–2246. [PMC free article] [PubMed] [Google Scholar]
- 56. Liu L, Stone J, Hoffpauir JT, Xiong Z. Histiocytic meningioma: A distinctive subtype of meningioma? Intractable Rare Dis Res. 2014;3(2):57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are being securely held within the Project neuroArm Research Facility, University of Calgary. All data have been systematically cataloged and are readily available.