Abstract
Background
Hypertensive disorders of pregnancy (HDP) are associated with increased risks for cardiovascular disease (CVD) later in life. The HDP incidence is commonly assessed using diagnostic codes, which are not reliable; and typically are expressed per-pregnancy, which may underestimate the number of women with a HDP history after their reproductive years.
Objective
We sought to determine the incidence of HDP expressed as both per-pregnancy and per-woman, and to establish their associations with future chronic conditions and multimorbidity, a measure of accelerated aging, in a population-based cohort study.
Methods
Using the Rochester Epidemiology Project medical record-linkage system, we identified residents of Olmsted County, Minnesota, who delivered between 1976 and 1982. We classified pregnancies into normotensive, gestational hypertension, preeclampsia, eclampsia, preeclampsia superimposed on chronic hypertension, and chronic hypertension using a validated electronic algorithm, and calculated the incidence of HDP both per-pregnancy and per-woman. The risk of chronic conditions between women with versus those without a history of HDP (age and parity 1:2 matched) was quantified using the hazard ratio (HR) and corresponding 95% confidence interval (CI) estimated from a Cox model.
Results
Among 9,862 pregnancies, we identified 719 (7.3%) with HDP and 324 (3.3%) with preeclampsia. The incidence of HDP and preeclampsia doubled when assessed on a per-woman basis: 15.3% (281/1839) and 7.5% (138/1839), respectively. Women with a history of HDP were at increased risk for subsequent diagnoses of stroke (HR 2.27; 95% CI 1.37–3.76), coronary artery disease (1.89; 1.26–2.82), cardiac arrhythmias (1.62; 1.28–2.05), chronic kidney disease (2.41; 1.54–3.78), and multimorbidity (1.25; 1.15–1.35).
Conclusions
The HDP population-based incidence expressed per-pregnancy underestimates the number of women affected by this condition during their reproductive years. A history of HDP confers significant increase in risks for future chronic conditions and multimorbidity.
Keywords: hypertensive disorders of pregnancy, cardiovascular disease, incidence, multimorbidity
Condensed abstract
Using a population-based study, we reported that the incidence of HDP expressed per pregnancy (7.3%) underestimated the number of affected women after their reproductive years (15.3%). Women with a history of HDP, compared to those with normotensive pregnancies, demonstrated significant increased risks for both kidney and CVD, even at a relatively young median age of 60 years, as well as multimorbidity, a measure of accelerated aging.
Introduction
Hypertensive disorders of pregnancy (HDP) include four categories - preeclampsia/eclampsia, gestational hypertension, chronic hypertension, and preeclampsia/eclampsia variants superimposed on chronic hypertension (1). HDP remain one of the leading causes of maternal and fetal morbidity and mortality worldwide. In addition, a history of HDP confers an elevated risk for future cardiovascular events, and is now incorporated into guidelines for risk assessment and the prevention of stroke and cardiovascular disease (CVD) for women (2,3). Over the last decade, several concerning trends have become apparent. The prevalence of both MI (4) and self-reported stroke (5) have increased compared to similarly aged men. Further, coronary heart disease death rates have increased in women 35–54 years of age (3) and in patients with severe forms of preeclampsia, who are at risk for CV death as early as the first decade after their affected pregnancies (6). These alarming developments, along with the recent evidence that the rates of HDP have increased over the last 3 decades (7,8), suggest that the role of HDP as a sex-specific CVD risk factor may become even more important in the years to come. Despite the acknowledged importance of HDP in risk assessment and prevention, the incidence of HDP is not well understood. Considerable variations in the incidence of HDP, ranging from 4–25%, and of preeclampsia, ranging from 1–9%, (7–9) make it difficult to estimate the attributable risk of CVD due to HDP. Studies of HDP incidence to date have primarily utilized registry or International Classification of Diseases (ICD) code approaches, which have been shown to be unreliable (10,11). A complete medical record abstraction of blood pressure and other individual-level data spanning the time before pregnancy through post-partum visits to ascertain severity and type of HDP has not yet been conducted. In addition, when assessing the subsequent risk of chronic disease beyond reproductive age in women with versus without a history of HDP, specifically preeclampsia, the number of affected women (incidence per woman) is more informative than the number of affected pregnancies (incidence per pregnancy). However, the medical literature has focused on the per-pregnancy incidence of HDP and the per-woman incidence is not quantified or appreciated.
The overall aim of this study was to determine the incidence rates of HDP (total and by subtype) per-pregnancy and per-woman in a population-based cohort by using a validated electronic algorithm based on accepted clinical criteria for subtypes of HDP. We postulated that the incidence of HDP, as estimated per number of pregnancies, underestimates the number of women with a history of this condition after their reproductive age. In addition, we aimed to compare the risk of common chronic conditions designated by the US Department of Health and Human Services between women with versus those without a history of HDP. As women with a history of HDP develop these conditions not only at higher rates, but years earlier than women who have had normotensive pregnancies (12), we postulated that women with a history of HDP will demonstrate accelerated aging, as demonstrated by accumulation of multimorbidity, a clinical proxy measure for accelerated aging (13).
Methods
Study Design and Population
The Rochester Epidemiology Project (REP) medical record-linkage system was used to establish a cohort consisting of all women who were residents of Olmsted County and delivered between January 1, 1976 and December 31, 1982 (liveborn or stillborn). The record-linkage system of the REP has been described comprehensively elsewhere (14,15). Briefly, the REP links all of the medical records from all providers in Olmsted County (encompassing Mayo Clinic and Olmsted Medical Center and their affiliated hospitals and medical facilities) using a unit medical record system whereby all outpatient, inpatient, emergency room, and nursing home information is kept in the same unit record. We selected the 1976–1982 time period for HDP assessment so that there was sufficient time for the women i) to complete their reproductive years, which would allow for the study of HDP incidence per woman and ii) to develop age-related chronic conditions in order to examine the relationship between HDP and adverse future outcomes, including cardiovascular and renal disease. This study was approved by the Institutional Review Boards at the Mayo Clinic and Olmsted Medical Center.
Identification of per-pregnancy cohort and per-woman sub-cohort for HDP incidence
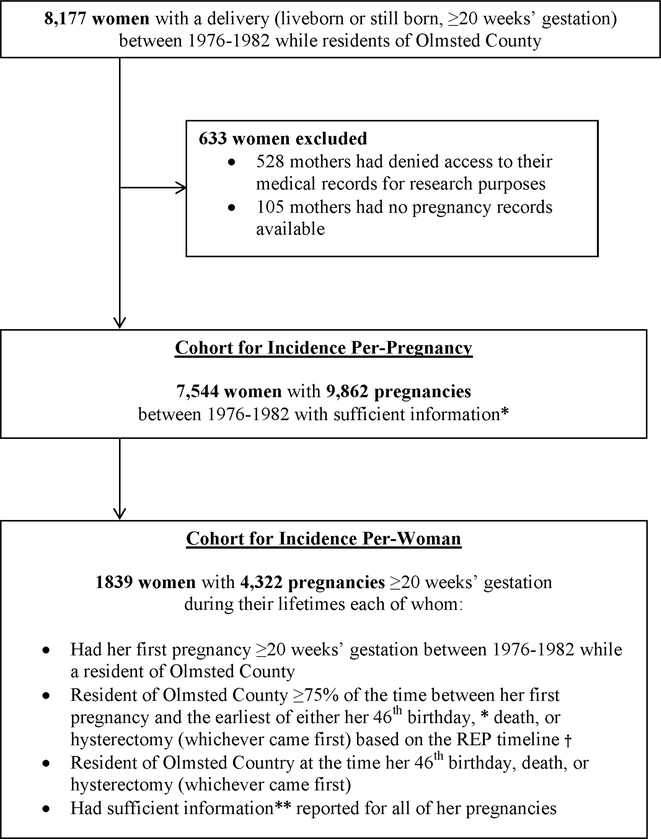
Using the REP medical records linkage system, we identified 8,177 women with a delivery (liveborn or stillborn, ≥20 weeks’ gestation) between January 1, 1976 and December 31, 1982 while residents of Olmsted County, Minnesota (Figure 1). We excluded women who either did not consent to the use of their medical records for research or with insufficient pregnancy information reported in the medical record. A pregnancy was classified as having sufficient information to determine HDP status if there was at least one blood pressure measurement from a prenatal visit and at least one blood pressure measurement from admission for delivery. Per-pregnancy HDP incidence was estimated based on all pregnancies (n=9,862) among 7,544 residents of Olmsted County who delivered during 1976–1982. For the incidence of HDP per-woman, a sub-cohort of 1,839 women was identified. These women had their first deliveries between1976 and 1982 while residents of Olmsted County, were residents of Olmsted County by the end of their childbearing years, and had sufficient information reported for all of their pregnancies.
Figure 1. Inclusion criteria and study cohorts.
Using a population-based study, two cohorts of women were identified with the goal to compute and compare the HDP incidence per-pregnancy versus the HDP incidence per woman. * The cut-off of 46 years was chosen as this was the oldest age at which a pregnancy was documented in this cohort. **A pregnancy was classified as having sufficient information to determine HDP status if there was at least one blood pressure measurement from a prenatal visit and at least one blood pressure measurement from admission for delivery. † St. Sauver JL, Grossardt BR, Yawn BP, Melton LJr, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–68. REP: Rochester Epidemiology Project
We first identified all women with diagnostic codes indicative of a possible HDP occurring between January 1, 1976 and December 31, 1982, and their charts were fully abstracted (a list of codes is shown in Supplemental Table 1). We then screened every chart of each remaining woman in the cohort without a code suggestive of a possible HDP. A positive screen was defined as two elevated blood pressures, either systolic blood pressures (SBP) >140 mmHg and/or diastolic blood pressures (DBP) >90 mmHg at any prenatal visit, during admission for delivery, or postnatally before leaving the hospital. All screen positive charts were then fully abstracted. Screen-negative charts were categorized as normotensive pregnancies.
Inter- and Intra-rater Reliability
There were six abstractors involved in retrieving data from the medical records over the course of the project. We assessed both inter-rater and intra-rater reliability for the diagnosis of normotensive pregnancy versus HDP and HDP type. For example, there were 21 pregnancies abstracted by the same nurse on different days, several months apart. The diagnosis of normotensive versus HDP and type were the same for all 21 pregnancies, resulting in 100% intra-rater agreement. Additionally, 128 pregnancies were accessed by two different abstractors, of which 126 (98.4%) had the same diagnosis of normotensive versus HDP and HDP type.
Assignment of HDP Exposure Status
From the first prenatal visit through 12 weeks post-partum, all data regarding blood pressures, dipstick protein, and hypertensive medication use at each visit were recorded and dated. The following laboratory values were collected between the first prenatal visit and up to 72 hours post-partum: 24-hour protein, serum creatinine, platelet count, and liver function tests. We used a validated electronic diagnostic algorithm to determine the presence of any HDP and type (16) based on accepted clinical criteria and the diagnosis of hypertension, which required blood pressure elevations in greater than 50% of blood pressure readings (the “50% rule”). The algorithm was validated by comparison of algorithm-based diagnoses to the gold standard, i.e., physician-made diagnoses. The algorithm-based approach demonstrated significant improvements in sensitivity and specificity in the classification of exposure (i.e., HDP) compared to methods that utilized diagnostic codes only (16). The definition of each HDP type used in the algorithm is described in Supplemental Table 2. In addition to the data that were required to classify pregnancies as normotensive vs. HDP, demographic, prenatal and intrapartum data were also collected. All abstracted data were first recorded on paper forms and subsequently entered into a database for future analysis.
Identification of Cohort for Outcome Analysis
For each woman with a pregnancy complicated by HDP, we defined the index date as the date when she first met criteria for HDP. We then randomly identified two referent women with normotensive pregnancies from the women in the birth cohort matched for the date of delivery (±1 year), maternal age (±1 year), and parity at index pregnancy (1 or >1) who had not met criteria for HDP before the index date. Women who first met criteria for HDP based on a delivery prior to 1976 were excluded from this analysis. Women with more than one normotensive pregnancy during 1976–1982 could have been identified as a matched referent for more than one HDP woman.
Ascertainment of Chronic Conditions
We considered 16 of the 20 chronic conditions recommended by the US Department of Health and Human Services (DHHS) (17–19) to study long-term multimorbidity (Supplemental Table 3). The following 4 DHHS conditions were excluded because they were rare in our population: human immunodeficiency virus infections, autism spectrum disorders, schizophrenia, and hepatitis. The six primary outcomes of the study were cardiac arrhythmias, coronary artery disease (CAD), congestive heart failure (CHF), stroke, chronic kidney disease (CKD) and dementia. Secondary outcomes included a number of multimorbidities accumulated over the time of follow up and death from any cause obtained from death certificates. These sixteen conditions were ascertained electronically by retrieving diagnosis codes from inpatient and outpatient visits to REP-affiliated providers from the index date through the women’s last visits.
Statistical Analyses
Per-pregnancy HDP incidence (per 100 pregnancies) was calculated considering each pregnancy as a distinct event. Per-pregnancy incidence rates were calculated overall, by HDP subtype, and stratified by calendar year and age (<20, 20–24, 25–29, 30–34, and ≥35 years) within each subtype of HDP. The denominators used to determine the incidence, by age and calendar year, are shown in Supplemental Table 4. For the per-pregnancy incidence rates, 95% confidence intervals (CI) were constructed using a Wilson score interval appropriate for a proportion estimated from clustered binary data given that women could have multiple pregnancies in the cohort (20). The 95% CIs for the per-woman incidence rates were constructed using an exact method for a binomial proportion. Per-woman HDP incidence (per 100 women) was calculated based on classifying each woman using the following hierarchy: eclampsia>preeclampsia superimposed on chronic hypertension>preeclampsia>chronic hypertension>gestational hypertension>normotensive.
Each of the 16 chronic conditions was evaluated separately, and women with the condition prior to the index date (ie for each matched set, the date of the exposed woman’s first pregnancy complicated by HDP) were excluded from each analysis in order to evaluate de novo conditions. The duration of follow-up was calculated from the index date to the date of the condition diagnosis, last visit to a REP-affiliated provider prior to the end of the study (December 31, 2017), or subsequent HDP diagnosis for the referent women. Cumulative incidence curves were estimated using the Kaplan–Meier method. Cox proportional hazards models (21) were used to estimate hazard ratios (HRs) and corresponding 95% CIs using age as the time scale, with women entering the risk set at their respective index ages. The proportional hazards assumptions for the Cox models were checked using martingale residuals using a Kolmogorov-type supremum test based on a sample of 1,000 simulated residual patterns. The accumulation of chronic conditions was calculated as the mean number of conditions accumulated over the follow-up after the index date and was represented graphically using Aalen-Johansen curves. Hazard ratios were computed using Anderson-Gill regression models with age as the time scale(22–24). Robust sandwich covariance estimates were used to account for either women included in both cohorts (e.g. referent women with subsequent HDP) or women with multiple pregnancies who were selected as referents more than once. Both unadjusted models and models adjusted for education, smoking, and obesity were fit. Tests of statistical significance were conducted at the two-tailed alpha level of 0.05. Bonferroni correction was used to adjust for type 1 error due to multiple comparisons for six primary outcomes (0.05/6=0.0083). Statistical analyses were performed using the SAS version 9.4 software package (SAS Institute, Inc.; Cary, NC) and R software v3.4.2.
Results
We identified 7, 544 mothers who had 9,862 pregnancies of ≥20 weeks’ gestation between January 1, 1976 and December 31, 1982 while residents of Olmsted County, MN for the calculation of incidence per-pregnancy (Figure 1). During the six-year assessment period, we identified 659 women with a total of 719 HDP pregnancies (Supplemental Table 5). Pregnancies with preeclampsia or preeclampsia superimposed on chronic hypertension - compared to pregnancies with gestational hypertension- were less frequently carried to term (302 (86.3%) vs 286 (95.3%), p<0.001), resulted more frequently in small-for-gestational age infants (80 (23.0%) vs 31 (10.5%), p<0.001), and had higher frequencies of stillbirths (10 (2.9%) vs 2 (0.7%), p=0.04).
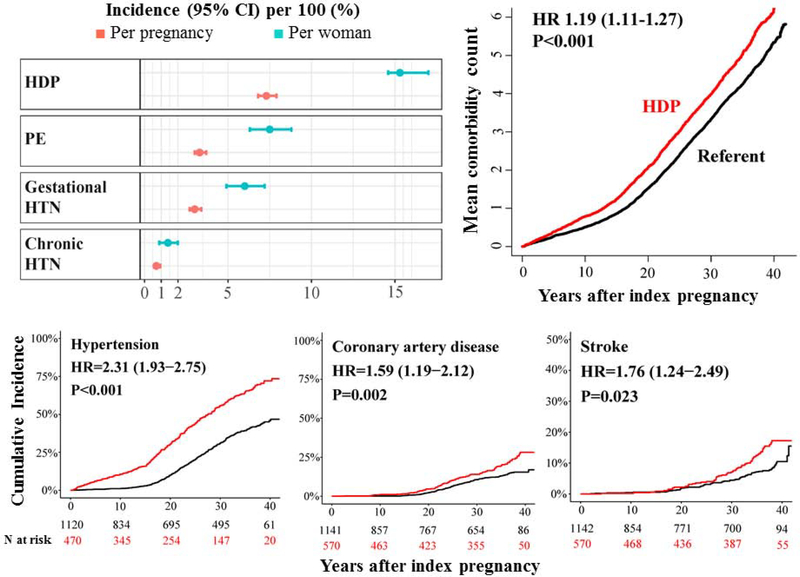
The per-pregnancy incidence rate was 7.3% (95% CI 6.8–7.9%) for HDP, including, 0.04% for eclampsia and 3.3% for preeclampsia (Table 1). The incidence per-woman calculation was based on a sub-cohort of 1,839 women for whom we had sufficient information on all of their pregnancies ≥20 weeks’ gestation (Figure 1), with a median [IQR] number of pregnancies 2 [2, 3] per woman. Of note, demographic and perinatal characteristics between women with and those without sufficient information on all of their pregnancies were similar (Supplemental Table 6). The per-woman incidence was twice that of their incidence rates observed per-pregnancy: 15.3% and 7.5% for HDP and preeclampsia, respectively (Table 1).
Table 1.
Per-pregnancy and per-woman incidence (per 100) of hypertensive disorders of pregnancy
| Per-pregnancy* | N | Incidence (%) | 95% CI † |
| Any hypertensive disorder of pregnancy | 719 | 7.3 | 6.8–7.9 |
| Preeclampsia | 324 | 3.3 | 3.0–3.7 |
| Eclampsia | 4 | 0.04 | 0.04–0.14 |
| Preeclampsia superimposed on chronic HTN | 22 | 0.22 | 0.17–0.38 |
| Gestational HTN | 300 | 3.0 | 2.7–3.4 |
| Chronic HTN | 69 | 0.70 | 0.56–0.94 |
| Normotensive pregnancies | 9143 | 92.7 | 92.2–93.3 |
| Per-woman‡ | N | % | 95% CI § |
| Any hypertensive disorder of pregnancy | 281 | 15.3 | 14.6–17.0 |
| Preeclampsia | 138 | 7.5 | 6.3–8.8 |
| Eclampsia | 0 | 0 | 0–0 |
| Preeclampsia superimposed on chronic HTN | 8 | 0.44 | 0.19–0.86 |
| Gestational HTN | 110 | 6.0 | 4.9–7.2 |
| Chronic HTN | 25 | 1.4 | 0.88–2.0 |
| Normotensive | 1558 | 84.7 | 83.0–86.3 |
CI, confidence interval; HTN, hypertension
Per-pregnancy incidence based on 9,862 pregnancies among 7,544 residents of Olmsted County who delivered during 1976–1982.
95% CIs for the per-pregnancy incidence rates were constructed using a Wilson score interval appropriate for a proportion estimated from clustered binary data.
Per-woman incidence based on 1,839 residents of Olmsted County considering all of their pregnancies. Each woman was classified using the following hierarchy: Eclampsia>Preeclampsia superimposed on chronic HTN>Preeclampsia>Chronic HTN>Gestational HTN>Normotensive.
95% CIs for the per-woman incidence rates were constructed using an exact method for a binomial proportion.
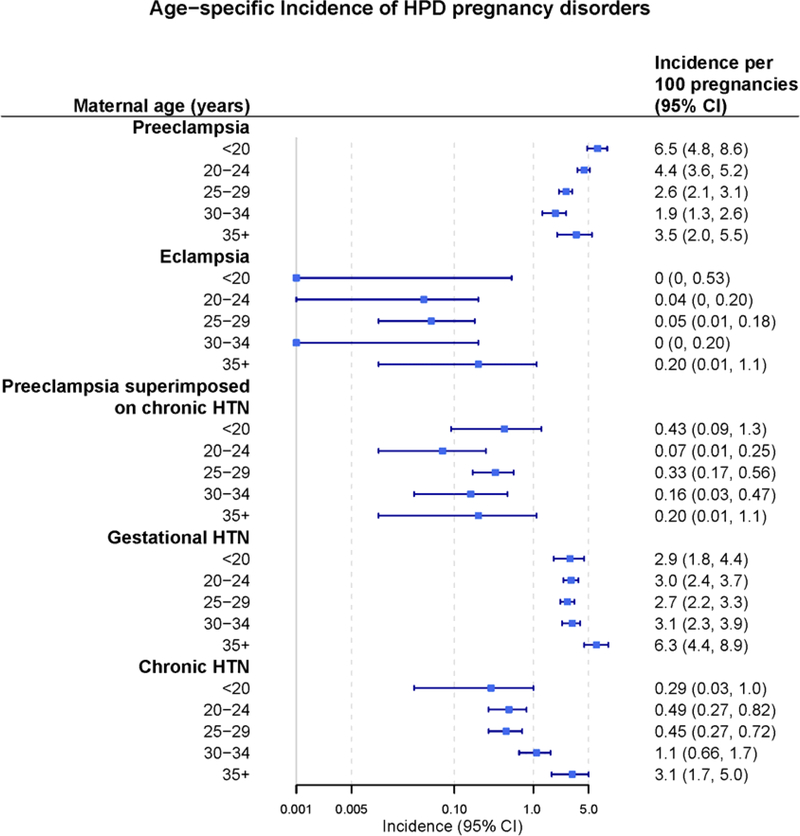
The age-specific incidence (per 100 pregnancies) of each HDP subtype is shown in Figure 2 and in Supplemental Table 7. The per-pregnancy incidence of preeclampsia with respect to age was U-shaped, such that the youngest women (i.e., <20 years and 20–24 years) and those ages ≥35 years had the highest incidence. The per-pregnancy incidence of preeclampsia in women less than 20 years of age was higher than in women 20–34 years of age (6.5 [95% CI 4.8–8.6] vs 3.0 [95% CI 2.7–3.4] per 100 pregnancies). Per-pregnancy incidence of gestational hypertension was higher in women ages ≥35 compared to other age groups (6.3 [95% CI 4.4–8.9] vs 2.9 [95% CI 2.5–3.2-] per 100 pregnancies.
Figure 2. Age-specific per-pregnancy incidence of hypertensive disorders of pregnancy among 9,862 pregnancies during 1976–1982 for residents of Olmsted County, Minnesota.
The per-pregnancy incidence of preeclampsia with respect to age was U-shaped, such that the youngest women (i.e., <20 years and 20–24 years) and those ages ≥35 years had the highest incidence. HTN: hypertension
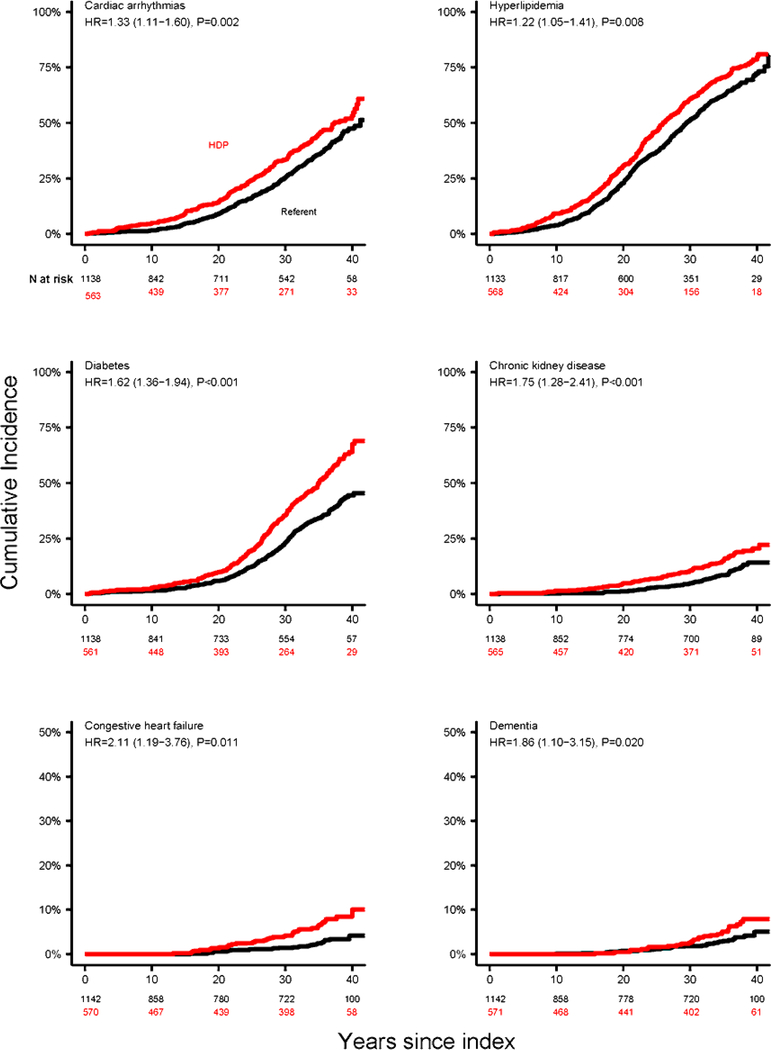
The development of chronic conditions after a pregnancy complicated by HDP was studied in 571 women with pregnancies complicated by HDP, and 1142 age- and parity-matched referents (Table 2). The median length of follow-up was 36.2 years (IQR, 23.5–38.2) and 35.8 years (IQR, 13.7–37.9) for women with a history of HDP and referent women, respectively. Women with a history of HDP compared to referent women demonstrated increased risks of CVD events and risk factors, including cardiac arrhythmias CAD, CHF, stroke, CKD, dementia, hyperlipidemia, hypertension, and diabetes, and in analyses both unadjusted and adjusted for education, smoking, and obesity (Table 2, Figure 3, and Central Illustration). Women with a history of HDP also experienced accelerated rates of accumulation of the 16 chronic conditions considered together (Table 2 and Central Illustration). The difference in the multimorbidity burden remained similar after excluding hypertension (Table 2). However, all-cause death rates were not different between the groups. A sub-analysis of women with a history of preeclampsia/eclampsia/preeclampsia superimposed on chronic hypertension showed that the magnitude of the effect of a history of preeclampsia was similar to that of HDP for most of the outcomes (Table 3). After Bonferroni correction for multiple comparisons for the cohort as a whole (Table 2), all primary endpoints except dementia were still statistically significant in unadjusted analyses; and cardiac arrhythmia, CAD, and CKD were still significant in adjusted analyses. In a sub-group of women with a history of preeclampsia/eclampsia/preeclampsia superimposed on chronic hypertension (Table 3), CAD and CKD were significant in both the unadjusted and adjusted analyses after Bonferroni correction.
Table 2.
Comparison of the incidence of chronic conditions first occurring after the index date, between women with hypertensive disorders of pregnancy and 1:2 age- and parity-matched referent women
| HDP (N=571) | Referent (N=1,142) | Unadjusted models | Adjusted models* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual Condition | N at risk† | Person-years | N events | N at risk‡ | Person-years | N events | HR (95% CI) | P‡ | HR (95% CI) | P‡ |
| Cardiac Arrhythmias | 563 | 13803 | 214 | 1138 | 26840 | 315 | 1.35 (1.13–1.61) | <0.001 | 1.33 (1.11–1.60) | 0.002 |
| Coronary Artery Disease | 570 | 15765 | 101 | 1141 | 29246 | 111 | 1.73 (1.32–2.28) | <0.001 | 1.59 (1.19–2.12) | 0.002 |
| Congestive Heart Failure | 570 | 16530 | 34 | 1142 | 30287 | 24 | 2.72 (1.60–4.64) | <0.001 | 2.11 (1.19–3.76) | 0.011 |
| Stroke | 570 | 16333 | 65 | 1142 | 29834 | 65 | 1.87 (1.32–2.63) | <0.001 | 1.76 (1.24–2.49) | 0.023 |
| Chronic Kidney Disease | 565 | 15861 | 79 | 1138 | 29755 | 86 | 1.78 (1.31–2.43) | <0.001 | 1.75 (1.28–2.41) | <0.001 |
| Dementia | 571 | 16630 | 28 | 1142 | 30237 | 28 | 1.85 (1.10–3.12) | 0.021 | 1.86 (1.10–3.15) | 0.020 |
| Depression | 555 | 12463 | 213 | 1112 | 23582 | 345 | 1.16 (0.98–1.38) | 0.078 | 1.15 (0.97–1.37) | 0.12 |
| Substance abuse | 568 | 15587 | 84 | 1134 | 28877 | 121 | 1.30 (0.98–1.71) | 0.068 | 1.37 (1.04–1.82) | 0.028 |
| Hyperlipidemia | 568 | 11695 | 324 | 1133 | 23045 | 513 | 1.31 (1.14–1.51) | <0.001 | 1.22 (1.05–1.41) | 0.008 |
| Hypertension | 470 | 9763 | 247 | 1120 | 26061 | 319 | 2.45 (2.06–2.91) | <0.001 | 2.31 (1.93–2.75) | <0.001 |
| Diabetes | 561 | 13982 | 248 | 1138 | 27191 | 292 | 1.77 (1.50–2.10) | <0.001 | 1.62 (1.36–1.94) | <0.001 |
| Arthritis | 569 | 13184 | 304 | 1138 | 24868 | 486 | 1.20 (1.04–1.39) | 0.014 | 1.17 (1.00–1.36) | 0.024 |
| Cancer | 566 | 15392 | 124 | 1132 | 27827 | 224 | 0.97 (0.78–1.21) | 0.81 | 0.99 (0.79–1.24) | 0.91 |
| Asthma | 562 | 14505 | 100 | 1123 | 27463 | 155 | 1.22 (0.94–1.57) | 0.13 | 1.19 (0.92–1.53) | 0.20 |
| COPD | 526 | 9695 | 236 | 1075 | 19153 | 422 | 1.11 (0.94–1.31) | 0.22 | 1.07 (0.90–1.27) | 0.45 |
| Osteoporosis | 571 | 15857 | 95 | 1142 | 28647 | 204 | 0.81 (0.63–1.03) | 0.085 | 0.83 (0.64–1.08) | 0.17 |
| Accumulation of Multimorbidity | ||||||||||
| Considering all of the above | 400 | 11722 | - | 987 | 26225 | - | 1.22 (1.15–1.30) | <0.001 | 1.19 (1.11–1.27) | <0.001 |
| All of the above, except HTN | 479 | 14102 | - | 1001 | 26509 | - | 1.18 (1.11–1.26) | <0.001 | 1.15 (1.08–1.22) | <0.001 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease, HR, hazard ratio.
P-values in bold denote statistical significance at the 0.05 alpha level.
Age was used as the time scale for all models in the table.
Adjusted for education (categories include: <high school, high school or GED; some college; ≥college; or unknown), smoking (y/n), and obesity (defined as BMI based on weight taken closest to conception date within 6 months prior and up to 20 gestational weeks, categorized as: <25; 25–29; 30+; or unknown)
The number of women at risk varied across conditions because we excluded women with that specific condition prior to the index date, based on the diagnosis codes considered for each condition. For hypertension, we also excluded those who had hypertension prior to pregnancy based on chart review or chronic hypertension based on algorithm. For diabetes, we also excluded those who had diabetes prior to pregnancy based on chart review. For multimorbidity, we excluded those with any of the 16 chronic conditions prior to pregnancy.
Using a Bonferroni correction, all primary endpoints except dementia were statistically significant in unadjusted analyses; cardiac arrhythmia, CAD, and CKD were significant in adjusted analyses.
Figure 3. Cumulative incidence curves for cardiovascular and metabolic conditions in women with HDP compared with 1:2 age- and parity-matched referent women.
The reported HRs and corresponding 95% CIs and p-values were estimated from Cox models adjusted for education, smoking, and obesity. Age was used as the time scale. Women with HDP compared to referent women demonstrated increased risks of CVD risk factors and events. HDP: hypertensive disorders of pregnancy. HR: hazard ratio. CI: confidence intervals.
Central Illustration: Hypertension in pregnancy: incidence per-pregnancy and per-woman, outcomes and multimorbidity later in life.
Conventional per pregnancy incidence values underestimate the number of women experiencing HDP by half. Women with a history of HDP compared with referent women, have increased risks for developing multimorbidity, CVD risk factors and events earlier in life.
Table 3.
Comparison of the incidence of chronic conditions first occurring after the index date, between women with a history of preeclampsia/eclampsia-superimposed preeclampsia and 1:2 age- and parity-matched referent women
| Preeclampsia (N=298) | Referent (N=596) | Unadjusted models | Adjusted models* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual Condition | N at risk† | Person-years | N events | N at risk‡ | Person-years | N events | HR (95% CI) | P‡ | HR (95% CI) | P‡ |
| Cardiac Arrhythmias | 293 | 7247 | 110 | 595 | 14119 | 163 | 1.37 (1.08–1.75) | 0.010 | 1.38 (1.07–1.77) | 0.012 |
| Coronary Artery Disease | 297 | 8377 | 51 | 595 | 15334 | 51 | 1.91 (1.30–2.82) | 0.001 | 1.85 (1.23–2.78) | 0.003 |
| Congestive Heart Failure | 297 | 8748 | 14 | 596 | 15833 | 11 | 2.48 (1.14–5.39) | 0.022 | 2.07 (0.93–4.60) | 0.08 |
| Stroke | 297 | 8655 | 33 | 596 | 15535 | 40 | 1.48 (0.94–2.33) | 0.09 | 1.40 (0.89–2.22) | 0.15 |
| Chronic Kidney Disease | 293 | 8230 | 43 | 594 | 15492 | 46 | 1.85 (1.22–2.80) | 0.004 | 1.84 (1.19–2.83) | 0.006 |
| Dementia | 298 | 8759 | 13 | 596 | 15771 | 17 | 1.39 (0.68–2.83) | 0.37 | 1.24 (0.62–2.47) | 0.54 |
| Depression | 292 | 6614 | 111 | 580 | 12086 | 191 | 1.06 (0.84–1.33) | 0.65 | 1.03 (0.81–1.30) | 0.83 |
| Substance abuse | 296 | 8239 | 43 | 590 | 15036 | 68 | 1.16 (0.79–1.68) | 0.45 | 1.28 (0.88–1.88) | 0.20 |
| Hyperlipidemia | 296 | 6272 | 165 | 588 | 12080 | 259 | 1.25 (1.03–1.53) | 0.028 | 1.19 (0.97–1.47) | 0.09 |
| Hypertension | 258 | 5744 | 118 | 582 | 13569 | 169 | 1.83 (1.44–2.33) | <0.001 | 1.75 (1.37–2.24) | <0.001 |
| Diabetes | 293 | 7428 | 123 | 593 | 14290 | 149 | 1.67 (1.31–2.12) | <0.001 | 1.53 (1.20–1.96) | <0.001 |
| Arthritis | 296 | 6824 | 163 | 595 | 13183 | 246 | 1.38 (1.13–1.69) | 0.002 | 1.37 (1.11–1.68) | 0.003 |
| Cancer | 297 | 8233 | 58 | 592 | 14579 | 121 | 0.81 (0.59–1.12) | 0.20 | 0.81 (0.59–1.13) | 0.21 |
| Asthma | 292 | 7652 | 54 | 588 | 14237 | 88 | 1.13 (0.81–1.59) | 0.47 | 1.11 (0.78–1.58) | 0.56 |
| COPD | 273 | 5177 | 124 | 564 | 10015 | 217 | 1.11 (0.88–1.39) | 0.38 | 1.09 (0.86–1.37) | 0.49 |
| Osteoporosis | 298 | 8269 | 53 | 596 | 15148 | 85 | 1.15 (0.81–1.62) | 0.44 | 1.27 (0.89–1.81) | 0.19 |
| Accumulation of Multimorbidity | ||||||||||
| Considering all of the above | 220 | 6523 | - | 517 | 13640 | - | 1.18 (1.08–1.29) | <0.001 | 1.17 (1.07–1.28) | <0.001 |
| All of the above, except HTN | 248 | 7440 | - | 526 | 13801 | - | 1.16 (1.06–1.27) | <0.001 | 1.15 (1.05–1.25) | 0.003 |
| Death | ||||||||||
| All-cause | 298 | 8922 | 12 | 596 | 15949 | 27 | 0.80 (0.41–1.57) | 0.51 | 0.77 (0.40–1.50) | 0.44 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease, HR, hazard ratio.
Age was used as the time scale for all models in the table.
P-values in bold denote statistical significance at the 0.05 alpha level.
Adjusted for education (categories include: <high school, high school or GED; some college; ≥college; or unknown), smoking (y/n), and obesity (defined as BMI based on weight taken closest to conception date within 6 months prior and up to 20 gestational weeks, categorized as: <25; 25–29; 30+; or unknown)
The number of women at risk varied across conditions because we excluded women with that specific condition prior to the index date, based on the diagnosis codes considered for each condition. For hypertension, we also excluded those who had hypertension prior to pregnancy based on chart review or chronic hypertension based on algorithm. For diabetes, we also excluded those who had diabetes prior to pregnancy based on chart review. For multimorbidity, we excluded those with any of the 16 chronic conditions prior to pregnancy.
Using a Bonferroni correction, CAD and CKD were significant in both the unadjusted and adjusted analyses.
Discussion
Our present study reports several novel findings regarding HDP incidence and related long-term outcomes (Central Illustration). First, it assessed the incidence of HDP in a population based cohort by medical record review and chart abstraction, an approach superior to the use of discharge diagnoses or registry data that have been previously used for US populations. Second, it clearly demonstrated that the incidence of HDP expressed per pregnancy (7.3%) underestimated the number of affected women after their reproductive years (15.3%). Third, women with a history of HDP, compared to those with normotensive pregnancies, demonstrated significant increased risks for both kidney and heart disease, including cardiac arrhythmias, CAD, and stroke that can be, at least in part, explained by higher rates of CVD risk factors, such as hyperlipidemia, hypertension, and diabetes. Fourth, a history of HDP identified women at risk for multimorbidity, as these women experienced accelerated rates of accumulation of chronic conditions, primarily those related to CVD risks and events, compared to women with normotensive pregnancies. Taken together, our findings indicate that the risks for kidney and heart disease in women with HDP histories have been underestimated. The proportion of women who may be at risk based on their HDP histories (15.3%) is similar to the proportions of women at risk for CVD based on the presence of traditional risk factors such as, smoking (13.7%) (25), hyperlipidemia (14.8%) (26), and diabetes (12%) (27). Inclusion of HDP history may substantially reduce misclassification using current CVD risk scores, which are particularly inaccurate in women (28).
The wide variations in previously reported rates of HDP and preeclampsia may be attributable to differences in study design, population characteristics and setting, the definition of HDP and type, years of assessment, or to geographical and/or natural variation. Most of the reports of US population-based rates of HDP have used national registries or databases.(7–8), (29– 34). A comparison of a registry based diagnosis with a “gold standard” chart review in the Medical Birth Registry of Norway for the diagnosis of preeclampsia showed a specificity of 99.2%, but a sensitivity of only 43.0% (35). In particular, mild cases of preeclampsia were most often missed. The use of ICD codes to establish HDP diagnoses have been reported to be no better, with high specificity, but low sensitivity due to significant underreporting of milder disease forms (10,11, 36). Given the unreliability of the ICD codes, we have developed and validated an electronic algorithm for the retrospective diagnoses of HDP (16). The electronic diagnostic algorithm was superior to diagnostic codes and allowed for the consistent application of objective criteria, thus reducing the risk for bias that may be introduced by individual medical experts, while allowing for the analyses of large datasets. However, with respect to long-term outcomes, our results indicate risk estimates that are comparable to previously published studies. For example, we report that women with a history of HDP have twice the risk for CAD compared to women with normotensive pregnancies, similar to the risk that was reported in a prospective, observational UK Biobank study in which HDP was confirmed using diagnostic codes or self-report at enrollment (37).
Most of the prior incidence studies have reported the incidence of HDP and preeclampsia per-pregnancy because this is the most useful information for obstetricians when estimating the risk of HDP and preeclampsia among pregnant women and the related maternal and fetal complications. However, reports of the incidence of HDP per- pregnancy may underestimate the number of affected women, who may have more than one pregnancy and who may be assessed based on their normotensive, rather than their preeclamptic pregnancies. Our previous study that reported CVD outcomes after HDP, reported similar HDP rates per-woman: 643 of 4064 women (13%), with a HDP in at least one of their pregnancies (12). Our current data suggest that 1 in 6 women may have increased risks for CVD and renal disease based on their reproductive histories of HDP. Furthermore, and to the best of our knowledge, none of the published studies that examined the prevalence or incidence of CVD events identified HDP using accepted clinical criteria, but rather, most commonly used diagnostic codes. As these commonly lead to misclassification, our study provides more accurate estimates of the risks for CVD and renal disease that may be attributed to HDP. In addition, we report that women with a history of HDP are at higher risks for multiple chronic conditions which may necessitate sex-specific screening, preventive, and treatment programs. Finally, our results indicate that women with a history of HDP are at risk of multimorbidity, a measure of accelerated aging, which, in turn, may be reflective of multi-system involvement, a key feature of preeclampsia. The underlying mechanism may be cellular senescence - an irreversible cell-cycle arrest mechanism characterized by release of pro-inflammatory markers, commonly referred to as the senescence-associated secretory phenotype (SASP). We (38) and others (39) have demonstrated a role for senescence in the pathophysiology of preeclampsia, which, once established, may persist for years. We hypothesize that senescent cell burden and elevated SASP components may lead to an accelerated aging-like state, and related chronic conditions in women with histories of preeclampsia. Future research should address the role of premature/accelerated aging as a possible mechanism for CVD after pregnancies affected by HDP. This may lead to identification of new biomarkers for early detection of women at risk, and novel therapeutic approaches using drugs that target fundamental aging and senescence processes.
Study Limitations
Our study has following limitations. First, it defined the incidence of HDP in a population which is predominantly v Caucasian. Recent studies have shown that African-American women not only have higher rates of HDP, but also higher risks for CVD compared to white women (40). Taken together, these observations call for studies that address the role of sex-specific risk factors in racially diverse cohorts. A second limitation is that we did use codes to ascertain long-term outcomes. To decrease the risk of false-positive diagnoses, only persons who received two codes for a given condition separated by more than 30 days were considered prevalent for this condition. Finally, our study defines the incidence of HDP in a population of women with pregnancies four decades ago. With the higher rates of HDP risk factors, such as obesity and higher maternal age at gestation, the incidence of HDP is expected to be higher today than between 1976 to 1982. However, the identification of a pregnancy cohort from 4 decades ago facilitated our research seeking to characterize the association between HDP and CVD outcomes later in life.
Conclusions
Our study provides a population based incidence of HDP and preeclampsia, both per pregnancy and per woman, and suggests that the former may underestimate the number of affected women with a history of this condition who may be at risk for future CVD and renal disease. These two measures are not mutually exclusive, but rather are complementary. Studies of HDP and preeclampsia should include incidence estimates, both per pregnancy and per woman, as these may facilitate assessments of risk for pregnancy-related complications and risks for future CVD and renal disease, respectively. Our data underscore the need for future research that will address the mechanisms of the multimorbidity burden in women with a history of HDP, as well as for sex-specific risk scores for prediction of CVD and renal disease that will include reproductive history.
PERSPECTIVES
Competency in Systems-Based Practice
Patient-level health economic analysis suggests that, to optimize value in relation to cost, PCSK9 inhibitor therapy should be directed toward patients at highest risk, such as those with baseline LDL-cholesterol levels ≥100 mg/dL.
Translational Outlook
Additional cost-effective strategies that reduce LDL-cholesterol and lower the risk of ischemic events and death are needed for patients in whom statin therapy is not sufficient.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health P50-AG044170, R01-AG034676, UL1TR002377, and R01-HL136348
Hypertensive disorders expressed per-pregnancy underestimate the number of affected women and confer increased risks for future multimorbidity
Abbreviations
- CAD
coronary artery disease
- CHF
congestive heart failure
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HDP
hypertensive disorders of pregnancy
- REP
Rochester Epidemiology Project
- SBP
systolic blood pressure
Footnotes
Disclosure: None of the authors declare competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 2.Bushnell C, McCullough LD, Awad IA et al. Guidelines for the Prevention of Stroke in Women: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosca L, Benjamin EJ, Berra K et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update. J Am Coll Cardiol. 2011. March 22;57(12):1404–23. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towfighi A, Zheng L, Ovbiagele B. Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med 2009;169:1762–6. [DOI] [PubMed] [Google Scholar]
- 5.Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology 2007;69:1898–904. [DOI] [PubMed] [Google Scholar]
- 6.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 2010;56:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009;113:1299–306. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Hauth JC, Curet LB et al. Trial of Calcium to Prevent Preeclampsia. N Engl J Med 1997;337:69–77. [DOI] [PubMed] [Google Scholar]
- 10.Geller SE, Ahmed S, Brown ML, Cox SM, Rosenberg D, Kilpatrick SJ. International Classification of Diseases-9th revision coding for preeclampsia: how accurate is it? Am J Obstet Gynecol 2004;190:1629–33; discussion 1633–4. [DOI] [PubMed] [Google Scholar]
- 11.Kattah AG, Scantlebury DC, Agarwal S et al. Preeclampsia and ESRD: The Role of Shared Risk Factors. Am J Kidney Dis 2017;69:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garovic VD, Bailey KR, Boerwinkle E et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens 2010;28:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. Journal of the American Medical Directors Association 2015;16:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St. Sauver JL, Grossardt BR, Yawn BP, Melton LJr, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Yawn BP et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milic NM, Codsi E, Butler Tobah YS et al. Electronic Algorithm Is Superior to Hospital Discharge Codes for Diagnoses of Hypertensive Disorders of Pregnancy in Historical Cohorts. Mayo Clin Proc 2018;93:1707–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JW, Cohen SB, Banthin JS. The medical expenditure panel survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care 2009;47:S44–50. [DOI] [PubMed] [Google Scholar]
- 19.Rocca WA, Gazzuola-Rocca L, Smith CY et al. Accelerated Accumulation of Multimorbidity After Bilateral Oophorectomy: A Population-Based Cohort Study. Mayo Clin Proc 2016;91:1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha KK, Miller D, Wang S. A Comparison of Some Approximate Confidence Intervals for a Single Proportion for Clustered Binary Outcome Data. Int J Biostat 2016;12. [DOI] [PubMed] [Google Scholar]
- 21.Therneau TM, Grambsch PM, Pankratz VS. Penalized Survival Models and Frailty. Journal of Computational & Graphical Statistics 2003;12:156–175. [Google Scholar]
- 22.Therneau TMGP. Modeling Survival Data: Extending the Cox Model. New York, NY, 2000. [Google Scholar]
- 23.CH J Multi-state models for panel data: The msm package for R. J Stat Softw 2011;38:1–28. [Google Scholar]
- 24.Al-Khalidi HR, Hong Y, Fleming TR, Therneau TM. Insights on the robust variance estimator under recurrent-events model. Biometrics 2011;67:1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll MD, Fryar CD, Nguyen DT. Total and High-density Lipoprotein Cholesterol in Adults: United States, 2015–2016. NCHS data brief, no. 290. Hyattsville, MD: National Center for Health Statistics, 2017. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 28.Michos ED, Nasir K, Braunstein JB et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis 2006;184:201–6. [DOI] [PubMed] [Google Scholar]
- 29.Rudra CB, Williams MA. Monthly variation in preeclampsia prevalence: Washington State, 1987–2001. J Matern Fetal Neonatal Med 2005;18:319–24. [DOI] [PubMed] [Google Scholar]
- 30.Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007;26:67–76. [DOI] [PubMed] [Google Scholar]
- 31.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008;21:521–6. [DOI] [PubMed] [Google Scholar]
- 32.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol 1990;163:460–5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 2003;22:203–12. [DOI] [PubMed] [Google Scholar]
- 34.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013;209:544 e1–544 e12. [DOI] [PubMed] [Google Scholar]
- 35.Klungsoyr K, Harmon QE, Skard LB et al. Validity of pre-eclampsia registration in the medical birth registry of norway for women participating in the norwegian mother and child cohort study, 1999–2010. Paediatr Perinat Epidemiol 2014;28:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of Preeclampsia-related Diagnoses Recorded in a National Hospital Registry and in a Postpartum Interview of the Women. Am J Epidemiol. 2007. July 15;166(2):117–24. Epub 2007 Jun 7. [DOI] [PubMed] [Google Scholar]
- 37.Honigberg MC, Zekavat SM, Aragam K et al. Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J Am Coll Cardiol 2019;74:2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suvakov S, Cubro H, White WM et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ 2019;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Londero AP, Orsaria M, Marzinotto S et al. Placental aging and oxidation damage in a tissue micro-array model: an immunohistochemistry study. Histochem Cell Biol 2016;146:191–204. [DOI] [PubMed] [Google Scholar]
- 40.Force USPST, Bibbins-Domingo K, Grossman DC et al. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317:1661–1667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.