Abstract
Objectives
Genome-wide meta-analyses of clinically defined gout were performed to identify subtype-specific susceptibility loci. Evaluation using selection pressure analysis with these loci was also conducted to investigate genetic risks characteristic of the Japanese population over the last 2000–3000 years.
Methods
Two genome-wide association studies (GWASs) of 3053 clinically defined gout cases and 4554 controls from Japanese males were performed using the Japonica Array and Illumina Array platforms. About 7.2 million single-nucleotide polymorphisms were meta-analysed after imputation. Patients were then divided into four clinical subtypes (the renal underexcretion type, renal overload type, combined type and normal type), and meta-analyses were conducted in the same manner. Selection pressure analyses using singleton density score were also performed on each subtype.
Results
In addition to the eight loci we reported previously, two novel loci, PIBF1 and ACSM2B, were identified at a genome-wide significance level (p<5.0×10–8) from a GWAS meta-analysis of all gout patients, and other two novel intergenic loci, CD2-PTGFRN and SLC28A3-NTRK2, from normal type gout patients. Subtype-dependent patterns of Manhattan plots were observed with subtype GWASs of gout patients, indicating that these subtype-specific loci suggest differences in pathophysiology along patients’ gout subtypes. Selection pressure analysis revealed significant enrichment of selection pressure on ABCG2 in addition to ALDH2 loci for all subtypes except for normal type gout.
Conclusions
Our findings on subtype GWAS meta-analyses and selection pressure analysis of gout will assist elucidation of the subtype-dependent molecular targets and evolutionary involvement among genotype, phenotype and subtype-specific tailor-made medicine/prevention of gout and hyperuricaemia.
Keywords: gout/hyperuricaemia, genome-wide association study (GWAS), Japanese, subtype specific locus, selection pressure analysis
Key messages.
What is already known about this subject?
Our previous genome-wide association study (GWAS) was performed on broad subtypes of gout with only 945 gout cases. A recent study has revealed genetic adaptive evolution of gout in the Japanese population.
What does this study add?
This is the first GWAS meta-analyses of clinically defined gout with more finely differentiated subtypes using two GWAS platforms with larger samples (3055 cases and 4554 controls). We identified multiple subtype-specific loci including four novel loci such as CD2, which encodes a well-known surface antigen found on all peripheral blood T-cells.
The present study showed significant enrichment of selection pressure on two genes, ABCG2 and ALDH2, for gout susceptibility in the Japanese population over the last 2000–3000 years.
Key messages.
How might this impact on clinical practice or future developments?
Our subtype GWASs of gout enabled us to develop subtype-dependent molecular targets that will lead to novel subtype-specific genome tailor-made therapies for gout/hyperuricaemia.
The present study also elucidates the Japanese genetic evolution of susceptibility to gout/hyperuricaemia and its subtypes.
Introduction
Gout is a well-known disease that manifests as acute and severe non-infectious arthritis.1 2 According to patients’ clinical parameters which reflect its causes,2–5 gout can be classified into four distinct subtypes: the renal underexcretion (RUE) type, renal overload (ROL) type, combined type and normal type, as shown in table 1 and online supplementary figure S1. Because these subtypes reflect causes of gout, genome-wide association studies (GWASs) of these subtypes are also likely to indicate its various genetic and pathophysiological backgrounds. While dividing patients into these subtypes is helpful for understanding patients’ pathophysiology, GWASs of these subtypes have only rarely been conducted, partly because clinical data, including time-consuming urinary collection, are necessary to categorise these subtypes. We previously performed a GWAS with clinically defined gout patients,6 followed by another with broader subtypes:7 RUE gout and ROL gout (table 1 and online supplementary figure S1), that revealed their specific loci. Although we were able to show these associations, this process has its limitations, including the use of a custom chip for replication studies that did not provide comprehensive genetic association searching. We use finely differentiated subtypes in daily clinical settings but there were not sufficient numbers of patients in the previous study7 to enable a GWAS with these finely differentiated subtypes. This prompted us to conduct, for the first time, GWASs with four distinct subtypes using meta-analysis across two GWAS platforms with a larger number of patients. We additionally conducted selection pressure analysis of the Japanese population on gout subtypes with the risk loci identified in the present study in order to investigate the evolutionary selective pressure on the Japanese population over the last 2000–3000 years.
Table 1.
Subtypes of gout* used in the present study
| Subtype | Clinical parameters |
| Differentiated subtype | |
| RUE type gout | FEUA <5.5% and UUE ≤25 |
| ROL type gout | FEUA≥5.5% and UUE >25 |
| Combined type gout | FEUA <5.5% and UUE >25 |
| Normal type gout | FEUA≥5.5% and UUE ≤25 |
| Broader subtype | |
| RUE gout (RUE type gout +combined type gout) | FEUA <5.5% |
| ROL gout (ROL type gout +combined type gout) | UUE >25 |
*Subtypes of hyperuricaemia can be classified in the same manner.
FEUA, fractional excretion of uric acid (unit: %); ROL, renal overload; RUE, renal underexcretion; UUE, urinary urate excretion (unit: mg/h/1.73 m2).
annrheumdis-2019-216644supp001.pdf (461.6KB, pdf)
Methods
Study subjects and patients involvement
We performed subtype genome-wide meta-analyses based on two case–control data sets for gout that included the Japonica Array8 and Illumina Array platforms. Patients with known clinical parameters were recruited from Japanese male outpatients at gout clinics (see online supplementary methods). All 3104 cases were clinically diagnosed as having primary gout according to the criteria established by the American College of Rheumatology,9 and their subtypes were also diagnosed along with their clinical parameters as described previously3 5 6 (table 1 and online supplementary figure S1). As controls, 6081 individuals were assigned from Japanese male participants in the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study).10 11 This research was done without patient involvement (see online supplementary methods).
Genotyping and imputation for the Japonica Array data set
A total of 1048 male clinically defined gout cases and 1179 male controls from the J-MICC Study10 11 were genotyped with the use of a Japonica SNP Array.8 The detail of quality control is described in online supplementary methods. This quality control filtering resulted in the selection of 1028 case subjects and 1167 control subjects as well as 603 009 single-nucleotide polymorphisms (SNPs). Prephasing and imputation were performed using SHAPEIT212 and Minimac3,13 respectively. Postimputation quality control was also performed as described in the online supplementary methods. Ultimately, 1028 case subjects and 952 control subjects as well as 7 529 176 SNPs remained for the GWAS analysis.
Genotyping and imputation for the Illumina Array data set
As case data, 2056 male gout cases subjects were genotyped with the use of HumanOmniExpress or HumanOmniExpressExome BeadChip Arrays (Illumina, San Diego, CA, USA). The detail of quality control is described in the online supplementary methods. This quality control filtering resulted in the selection of 2032 case subjects and 4901 control subjects as well as 553 321 SNPs. Postimputation quality control was also performed as described in the online supplementary methods. Ultimately, 2025 case subjects and 3602 control subjects as well as 7 356 207 SNPs remained for the GWAS analysis.
Association analysis for SNPs and gout
The association of SNPs with gout was assessed using logistic regression analysis (generalised linear model); the dependent variable was gout label (case=1, control=0), and the independent variables included imputed genotypes of each SNP and covariates. The covariates comprised the first four principal component scores. The effect sizes and standard errors estimated in logistic regression analysis were used in the subsequent meta-analysis. The association analysis was performed with the use of Efficient and Parallelizable Association Container Toolbox (EPACTS). https://genome.sph.umich.edu/wiki/EPACTS).
Meta-analyses
The meta-analyses were performed using a total of 3053 cases and 4554 controls from the two data sets (online supplementary table S1–S3). The association results for each SNP across the studies were combined with METAL software14 using the fixed-effects inverse-variance-weighted method. Heterogeneity of effect sizes was assessed via the I 2 index. The meta-analysis included 7 206 774 SNPs and the results from both the Japonica and Illumina Arrays. The genome-wide significance level α was set to a p value of <5×10–8.
Genetic correlation analysis
Genetic correlation analysis using linkage disequilibrium score (LDSC) regression analysis15 was conducted to examine the potential genetic overlap between gout subtypes and between each subtype and serum uric acid (SUA) levels. For the regression, we used the 1000 genomes phase_3 East Asian LDSC and summary statistics for high-quality common SNPs present in the HapMap 3 reference panel for each analysis.
Selection pressure analysis
The details of genome-wide recent natural selection signature using singleton density score (SDS)16 from high-depth whole genome sequence data of the Japanese population had been described in the previous study.17 Using the same approach,17 we calculated the SDS of gout-risk variants identified in the present study, and evaluated overlaps between enrichment of the natural selection signatures and these variants from each subtype.
Results
Subtype GWASs of gout
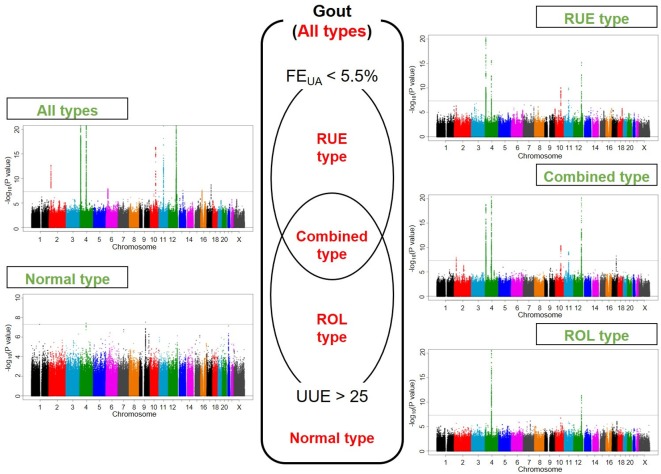
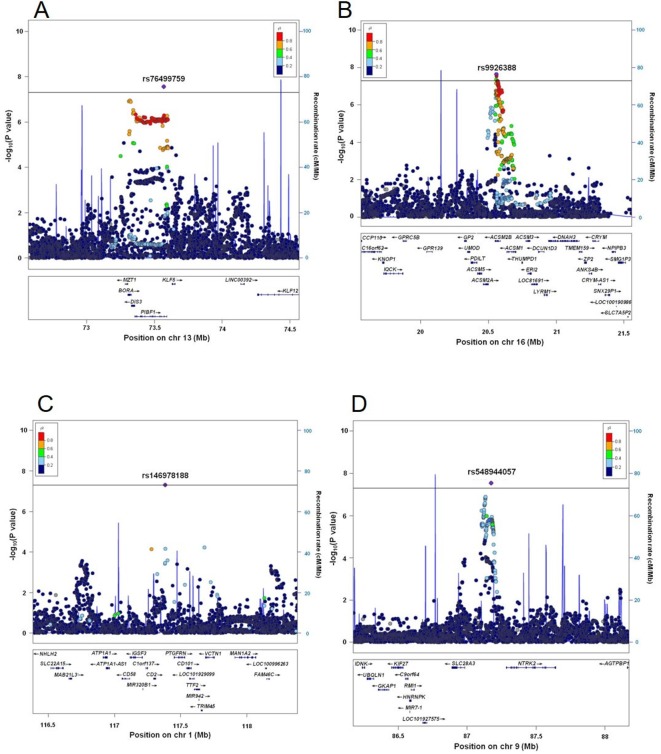
Figure 1 displays Manhattan plots of all and four clinical subtypes of gout, and figure 2 shows regional plots of novel loci. Compared with the plotted pattern for all clinically defined gout patients, each gout subtype (RUE type, ROL type, combined type and normal type) shows a subtype-specific plotted pattern and indicates the presence of cause-specific associated genes such as SLC2A9 and ABCG2. Table 2 lists the genome-wide significant loci from subtype GWASs. In total, 10, five, two, seven and three loci were identified at the genome-wide significant level to be associated with all, the RUE type, ROL type, combined type and normal type gout, respectively. Of these, two loci from all gout types, rs76499759 of PIBF1 and rs9926388 of ACSM2B, and another two intergenic loci from normal type gout, rs146978188 of CD2-PTGFRN and rs548944057 of SLC28A3-NTRK2, were detected as novel loci.
Figure 1.
Manhattan plots of GWASs of subtypes of gout. Clinical subtypes and Manhattan plots of GWASs of all gout types, RUE type gout, combined type gout, ROL type gout and normal type gout are shown. The x-axis represents chromosomal positions and the y-axis shows −log10 p values. The dotted lines indicate the genome-wide significance threshold (p=5.0×10–8). FEUA, fractional excretion of uric acid (%); GWASs, genome-wide association studies; ROL, renal overload; RUE, renal underexcretion; UUE, urinary urate excretion (mg/h/1.73 m2). See table 1 and online supplementary figure S1 for a detailed classification of gout/hyperuricaemia.
Figure 2.
Regional association plots of novel gout loci. Two loci were revealed to exceed the genome-wide significance level from the meta-analysis of GWASs from all gout patients, and another two loci from normal type gout patients. The highest association signal in each panel is located on (A) PIBF1, (B) ACSM2B, (C) CD2-PTGFRN and (D) SLC28A3-NTRK2. The region within 1 Mb from the single-nucleotide polymorphism (SNP) indicating the lowest p value is shown. (Upper panel) Plots of −log10 p values for the test of SNP association with gout. The SNP showing the lowest p value in the meta-analysis is depicted as a purple diamond. Other SNPs are colour-coded according to the extent of linkage disequilibrium (measured in r2) with the SNP showing the lowest p value. Recombination rates (centimorgans per Mb) estimated from HapMap Phase II data are also plotted. (Lower panel) RefSeq genes. Genomic coordinates are based on NCBI human genome reference sequence build hg19. The r2 data were calculated with 1000 Genomes Project Phase_3 JPT samples.45 GWASs, genome-wide association studies.
Table 2.
Significant gout loci identified in the present genome-wide meta-analyses
| SNP* | Locus | Chr. | Position (bp)† |
Gene‡ | Alleles | Illumina Array | Japonica Array | Meta-analysis | ||||||||||
| Risk | Non- risk | RAF | OR (95% CI) | P value | RAF | OR (95% CI) | P value | OR (95% CI) | P value | I2 | HetP | |||||||
| Case | Control | Case | Control | |||||||||||||||
| All gout patients | ||||||||||||||||||
| rs1260326 | 2p23.3 | 2 | 27 730 940 | GCKR | T | C | 0.623 | 0.545 | 1.33 (1.22 to 1.44) | 8.75×10–12 | 0.613 | 0.565 | 1.22 (1.07 to 1.39) | 3.61×10–3 | 1.30 (1.21 to 1.39) | 2.07×10–13 | 10.3 | 0.291 |
| rs3775946 | 4p16.1 | 4 | 9 995 256 | SLC2A9 | G | A | 0.677 | 0.569 | 1.62 (1.49 to 1.76) | 4.55×10–29 | 0.672 | 0.555 | 1.67 (1.46 to 1.92) | 9.42×10–14 | 1.63 (1.52 to 1.75) | 3.73×10–41 | 0 | 0.669 |
| rs4148155 | 4q22.1 | 4 | 89 054 667 | ABCG2 | G | A | 0.454 | 0.277 | 2.18 (2.00 to 2.38) | 1.05×10–70 | 0.462 | 0.264 | 2.38 (2.06 to 2.75) | 8.13×10–33 | 2.23 (2.08 to 2.41) | 1.81×10–101 | 3.8 | 0.308 |
| rs2817188 | 6p22.2 | 6 | 25 807 603 | SLC17A1 | G | A | 0.874 | 0.825 | 1.33 (1.18 to 1.51) | 5.03×10–6 | 0.871 | 0.836 | 1.40 (1.16 to 1.69) | 5.03×10–4 | 1.35 (1.22 to 1.50) | 1.06×10–8 | 0 | 0.660 |
| rs3129500 | 10q23.2 | 10 | 88 915 107 | SHLD2/FAM35A | G | A | 0.423 | 0.358 | 1.40 (1.29 to 1.53) | 2.36×10–14 | 0.423 | 0.371 | 1.30 (1.13 to 1.50) | 2.71×10–4 | 1.37 (1.28 to 1.48) | 4.34×10–17 | 0 | 0.355 |
| rs145954970 | 11q13.1 | 11 | 64 273 830 | SLC22A11 | C | G | 0.995 | 0.971 | 10.43 (5.83 to 18.66) | 2.78×10–15 | 0.997 | 0.967 | 25.09 (7.83 to 80.37) | 5.75×10–8 | 12.43 (7.39 to 20.92) | 2.25×10–21 | 42.8 | 0.186 |
| rs671 | 12q24.12 | 12 | 112 241 766 | ALDH2 | G | A | 0.823 | 0.725 | 1.89 (1.71 to 2.08) | 8.65×10–36 | 0.821 | 0.684 | 2.04 (1.75 to 2.37) | 2.78×10–20 | 1.93 (1.78 to 2.10) | 3.19×10–54 | 0 | 0.412 |
| rs76499759 | 13q22.1 | 13 | 73 568 511 | PIBF1 | A | G | 0.219 | 0.180 | 1.30 (1.17 to 1.43) | 2.56×10–7 | 0.212 | 0.183 | 1.20 (1.02 to 1.41) | 2.62×10–2 | 1.27 (1.17 to 1.38) | 2.79×10–8 | 0 | 0.418 |
| rs9926388 | 16p12.3 | 16 | 20 558 441 | ACSM2B | A | G | 0.301 | 0.252 | 1.24 (1.14 to 1.36) | 1.05×10–6 | 0.315 | 0.277 | 1.22 (1.06 to 1.42) | 6.46×10–3 | 1.24 (1.15 to 1.33) | 2.30×10–8 | 0 | 0.861 |
| rs1010269 | 17q23.2 | 17 | 59 448 945 | BCAS3 | G | A | 0.558 | 0.503 | 1.24 (1.14 to 1.34) | 6.06×10–7 | 0.583 | 0.528 | 1.26 (1.10 to 1.43) | 7.68×10–4 | 1.24 (1.16 to 1.33) | 1.81×10–9 | 0 | 0.836 |
| RUE type gout patients | ||||||||||||||||||
| rs3775948 | 4p16.1 | 4 | 9 995 182 | SLC2A9 | C | G | 0.705 | 0.573 | 1.83 (1.57 to 2.14) | 2.60×10–14 | 0.717 | 0.556 | 2.04 (1.61 to 2.59) | 3.82×10–9 | 1.89 (1.66 to 2.15) | 8.01×10–22 | 0 | 0.448 |
| rs4148155 | 4q22.1 | 4 | 89 054 667 | ABCG2 | G | A | 0.388 | 0.277 | 1.66 (1.43 to 1.93) | 2.72×10–11 | 0.382 | 0.264 | 1.74 (1.39 to 2.19) | 1.73×10–6 | 1.69 (1.49 to 1.91) | 2.54×10–16 | 0 | 0.737 |
| rs9420434 | 10q23.2 | 10 | 88 843 209 | GLUD1 (SHLD2) | C | T | 0.316 | 0.245 | 1.49 (1.27 to 1.74) | 6.39×10–7 | 0.342 | 0.243 | 1.65 (1.31 to 2.09) | 2.37×10–5 | 1.54 (1.35 to 1.75) | 8.62×10–11 | 0 | 0.462 |
| rs76741582 | 11q13.1 | 11 | 64 247 850 | SLC22A11 | T | C | 0.028 | 0.009 | 3.57 (2.16 to 5.88) | 6.31×10–7 | 0.036 | 0.008 | 4.87 (2.31 to 10.25) | 3.09×10–5 | 3.93 (2.59 to 5.95) | 1.06×10–10 | 0 | 0.497 |
| rs4646776 | 12q24.12 | 12 | 112 230 019 | ALDH2 | G | C | 0.819 | 0.722 | 1.88 (1.57 to 2.26) | 1.73×10–11 | 0.796 | 0.680 | 1.82 (1.4 to 2.36) | 6.66×10–6 | 1.86 (1.60 to 2.16) | 5.80×10–16 | 0 | 0.839 |
| ROL type gout patients | ||||||||||||||||||
| rs4148155 | 4q22.1 | 4 | 89 054 667 | ABCG2 | G | A | 0.515 | 0.277 | 2.87 (2.43 to 3.39) | 5.14×10–35 | 0.493 | 0.264 | 2.61 (2 to 3.41) | 2.05×10–12 | 2.79 (2.42 to 3.22) | 9.75×10–46 | 0 | 0.559 |
| rs11066008 | 12q24.12 | 12 | 112 140 669 | ACAD10 (ALDH2) | A | G | 0.724 | 0.643 | 1.67 (1.38 to 2.04) | 2.49×10–7 | 0.751 | 0.600 | 2.12 (1.56 to 2.89) | 1.57×10–6 | 1.79 (1.52 to 2.11) | 4.20×10–12 | 39 | 0.200 |
| Combined type gout patients | ||||||||||||||||||
| rs1260326 | 2p23.3 | 2 | 27 730 940 | GCKR | T | C | 0.647 | 0.545 | 1.45 (1.27 to 1.66) | 4.92×10–8 | 0.615 | 0.565 | 1.23 (1.03 to 1.48) | 2.50×10–2 | 1.37 (1.23 to 1.53) | 1.05×10–8 | 50.5 | 0.155 |
| rs3775948 | 4p16.1 | 4 | 9 995 182 | SLC2A9 | C | G | 0.690 | 0.573 | 1.70 (1.48 to 1.95) | 9.54×10–14 | 0.672 | 0.556 | 1.63 (1.35 to 1.97) | 2.58×10–7 | 1.67 (1.50 to 1.87) | 1.43×10–19 | 0 | 0.748 |
| rs74904971 | 4q22.1 | 4 | 89 050 026 | ABCG2 | A | C | 0.474 | 0.276 | 2.42 (2.11 to 2.78) | 2.40×10–36 | 0.474 | 0.264 | 2.56 (2.11 to 3.09) | 5.87×10–22 | 2.46 (2.20 to 2.76) | 1.53×10–56 | 0 | 0.646 |
| rs6586063 | 10q23.2 | 10 | 88 949 045 | SHLD2/FAM35A | G | A | 0.457 | 0.368 | 1.60 (1.39 to 1.85) | 1.60×10–10 | 0.436 | 0.393 | 1.28 (1.04 to 1.58) | 1.94×10–2 | 1.49 (1.32 to 1.68) | 4.30×10–11 | 65.6 | 0.088 |
| rs11231879 | 11q13.1 | 11 | 64 581 645 | CDC42BPG (SLC22A12) | G | A | 0.360 | 0.295 | 1.38 (1.19 to 1.60) | 2.44×10–5 | 0.375 | 0.282 | 1.56 (1.29 to 1.89) | 4.39×10–6 | 1.44 (1.28 to 1.62) | 7.66×10–10 | 4.4 | 0.307 |
| rs116873087 | 12q24.13 | 12 | 112 511 913 | NAA25 (ALDH2) | G | C | 0.828 | 0.749 | 2.21 (1.80 to 2.72) | 5.86×10–14 | 0.845 | 0.683 | 2.61 (2.06 to 3.31) | 2.69×10–15 | 2.38 (2.03 to 2.78) | 1.88×10–27 | 6.1 | 0.302 |
| rs9905274 | 17q23.2 | 17 | 59 450 441 | BCAS3 | C | T | 0.558 | 0.482 | 1.34 (1.18 to 1.53) | 1.20×10–5 | 0.581 | 0.499 | 1.42 (1.19 to 1.70) | 1.03×10–4 | 1.37 (1.23 to 1.52) | 5.62×10–9 | 0 | 0.596 |
| Normal type gout patients | ||||||||||||||||||
| rs146978188 | 1p13.1 | 1 | 117 383 166 | CD2 - PTGFRN | A | G | 0.065 | 0.015 | 6.53 (3.09 to 13.77) | 8.35×10–7 | 0.086 | 0.021 | 5.58 (1.33 to 23.46) | 1.90×10–2 | 6.31 (3.26 to 12.24) | 4.93×10–8 | 0 | 0.849 |
| rs4148155 | 4q22.1 | 4 | 89 054 667 | ABCG2 | G | A | 0.473 | 0.277 | 2.38 (1.71 to 3.33) | 3.35×10–7 | 0.438 | 0.264 | 2.14 (1.05 to 4.36) | 3.67×10–2 | 2.34 (1.73 to 3.16) | 3.64×10–8 | 0 | 0.787 |
| rs548944057 | 9q21.33 | 9 | 87 174 107 | SLC28A3 - NTRK2 | T | A | 0.125 | 0.046 | 3.89 (2.22 to 6.83) | 2.13×10–6 | 0.160 | 0.047 | 4.58 (1.63 to 12.83) | 3.83×10–3 | 4.04 (2.47 to 6.62) | 2.91×10–8 | 0 | 0.788 |
*dbSNP rs number.
†SNP positions are based on NCBI human genome reference sequence Build hg19.
‡Novel loci are shown in bold.
Chr, chromosome; RAF, risk allele frequency; ROL, renal overload; RUE, renal underexcretion; SNP, single-nucleotide polymorphism.
For all gout cases (table 2), 10 loci showed association at the genome-wide significance level: rs4148155 of ABCG2 (pmeta=1.81×10–101; ORs=2.23), rs671 of ALDH2 (pmeta=3.19×10–54; OR=1.93), rs3775946 of SLC2A9 (pmeta=3.73×10–41; OR=1.63), rs145954970 of SLC22A11 (pmeta=2.25×10–21; OR=12.43), rs3129500 of FAM35A (recently renamed as SHLD2, pmeta=4.34×10–17; OR=1.37), rs1260326 of GCKR (pmeta=2.07×10–13; OR=1.30), rs1010269 of BCAS3 (pmeta=1.81×10–9; OR=1.24), rs2817188 of SLC17A1 (pmeta=1.06×10–8; OR=1.35), rs9926388 of ACSM2B (pmeta=2.30×10–8; OR=1.24) and rs76499759 of PIBF1 (pmeta=2.79×10–8; OR=1.27). Among these 10 loci, PIBF1 and ACSM2B (table 2 and figure 2A, B). were identified for the first time as gout-risk loci at the genome-wide significance level. BCAS3 was identified here for the first time by the GWAS approach with Japanese individuals, while Li et al18 reported that rs11653176, another SNP of BCAS3, is associated with gout based on a GWAS with a Han Chinese population. We also replicated its association with gout in a Japanese population using a candidate gene approach.19 Other loci have been previously reported to have an association with gout in our previous GWASs6 7 and association studies.20 21 As suggestive loci for gout, seven loci: PDZK1, TACR1-EVA1A, LOC100128993, ARID5B, TOLLIP-AS1-BRSK2, SLC38A1 and MLXIP, were detected (see online supplementary table S4). Of these, PDZK1, a gene encoding a scaffolding protein22 23 such as for urate transporters SLC22A12/URAT1 and ABCG2, was reported to have an association with SUA by a GWAS approach24 25 and with gout as a result of candidate gene approach studies.26–28
As shown in online supplementary table S2, all the 3053 cases were classified into RUE type gout (654 cases), ROL type gout (486 cases), combined type gout (905 cases) and normal type gout (92 cases) for GWASs of gout subtypes.
The meta-analysis of a GWAS of the RUE type gout (table 2) showed significant SNPs in the following five loci: rs3775948 of SLC2A9 (pmeta=8.01×10–22; OR=1.89), rs4148155 of ABCG2 (pmeta=2.54×10–16; OR=1.69), rs4646776 of ALDH2 (pmeta=5.80×10–16; OR=1.86), rs9420434 of GLUD1 (pmeta=8.62×10–11; OR=1.54) and rs76741582 of SLC22A11 (pmeta=1.06×10–10; OR=3.93). Since GLUD1 is in LD with SHLD2/FAM35A, for which we previously showed a significant association with gout,7 all of these five loci were previously identified as having an association with gout.6 7 We also detected nine suggestive loci: SMYD3, GCKR-C2orf16, SMARCC1, FRMD4B-MITF, ARL4A-ETV1, C7orf66-EIF3IP1, ASB10, PXDNL and LOC100287896-POLD3, for RUE type gout as shown in online supplementary table S4.
From ROL type gout (table 2), rs4148155 of ABCG2 (pmeta=9.75×10–46; OR=2.79) and rs11066008 of ACAD10 (ALDH2) (pmeta=4.20×10–12; OR=1.79) were revealed to have an association. We had previously reported both to have a significant association with gout.6 7 20 21 Three suggestive loci, CNPY4, GRID1 and KCNJ2-CASC17, were also identified from ROL type gout (see online supplementary table S4).
Combined type gout displayed the following seven significant loci (table 2): rs74904971 of ABCG2 (pmeta=1.53×10–56; OR=2.46), rs116873087 of NAA25 (pmeta=1.88×10–27; OR=2.38), rs3775948 of SLC2A9 (pmeta=1.43×10–19; OR=1.67), rs6586063 of SHLD2/FAM35A (pmeta=4.30×10–11; OR=1.49), rs9905274 of BCAS3 (pmeta=5.62×10–9; OR=1.37), rs11231879 of CDC42BPG (pmeta=7.66×10–10; OR=1.44) and rs1260326 of GCKR (pmeta=1.05×10–8; OR=1.37). There are studies on these which report an association between CDC42BPG and hyperuricaemia from the Japanese exome-wide association study,29 and GWAS on SUA from a Korean population.30 The significance around rs11231879 of CDC42BPG was, however, no longer evident when conditioned on rs11231879 itself, nor when conditioned on the secondarily significant SNP, rs56093838 of SLC22A12/URAT1, demonstrating that these signals were from the same locus (see online supplementary figure S2). Because URAT1 is a well-known urate transporter that markedly affects SUA level, these findings indicate that the true associated gene for combined type gout on chromosome 11q13.1 locus is not CDC42BPG, but SLC22A12/URAT1. rs116873087 of NAA25 is in strong LD with rs671 of ALDH2 (r2=0.97 in the 1000 Genomes Project Phase_3: JPT samples). All of the seven loci had therefore been previously identified as having an association with gout.6 7 20 21 Two suggestive loci, NCKAP5-MIR3679 and PRDM8-FGF5, were also identified from combined type gout (see online supplementary table S4).
Three significant loci were found from normal type gout: rs548944057 of SLC28A3-NTRK2 (pmeta=2.91×10–8; OR=4.04), rs4148155 of ABCG2 (pmeta=3.64×10–8; OR=2.34) and rs146978188 of CD2-PTGFRN (pmeta=4.93×10–8; OR=6.31). Of these, two intergenic loci are novel susceptibility loci for gout (table 2 and figure 2C, D) There were eight suggestive loci: ZNF639-MFN1, RUNX2-CLIC5, DST, HGF, MED27-NTNG2, LINC00944-LINC02372, SV2B and GABPA, for normal type gout as shown in online supplementary table S4.
The LDSC regression analysis was performed to examine the potential genetic overlap between gout subtypes and between each subtype and SUA levels. Significant positive genetic correlations were observed among these subtypes as well as between these traits and SUA levels (see online supplementary figure S3).
Selection pressure analysis of gout susceptibility
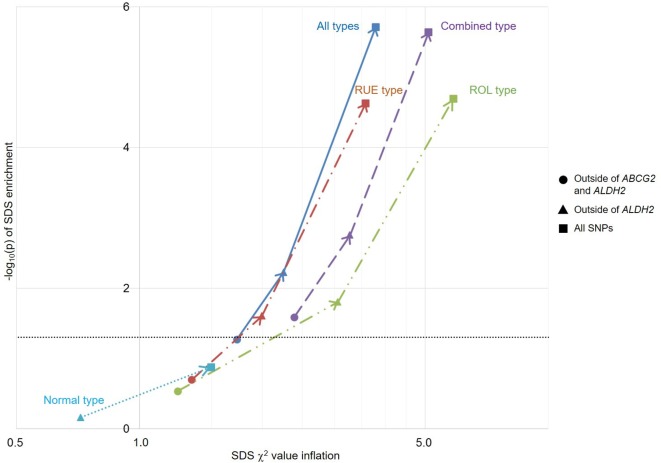
We also performed selection pressure analysis of gout on the basis of a previous report on the recent natural selection signature in the Japanese population.17 This analysis enables us to elucidate the genetic risks of gout characterised in the recent evolutionary history (2000–3000 years) of the Japanese population. Because ALDH2 was reported to be subjected to strong selection pressure in the Japanese population,17 and because ABCG2,31 32 as well as ALDH2,20 21 33 is a well-known strong susceptible gene for gout in Japanese, selection pressure analysis was initially performed outside of these two loci. As a result, only combined type gout showed significant enrichment of selection pressure (p=0.026; figure 3 and online supplementary table S5). When the ABCG2 locus was included in the analysis, all of these subtypes except for normal type gout then showed significant enrichment of selection pressure. As expected, analysis including ALDH2 and outside of ABCG2 showed significant enrichment of selection pressure except for normal type gout. This trend also persisted in the analysis with all associated SNPs (figure 3 and online supplementary table S5).
Figure 3.
Overlap between natural selection signatures and genetic risk of gout and its subtypes in the Japanese population. For each trait, inflation of the selection χ2 value is indicated along the x-axis, and −log10(p) of enrichment is plotted along the y-axis. The horizontal grey line represents significance threshold (p<0.05). Because ABCG2 and ALDH2 are associated with a well-known genetic risk of gout in Japanese individuals, selection pressure analyses without these loci were performed initially (filled circle), and subsequent analyses were conducted with ABCG2 (filled triangle), as well as ABCG2 and ALDH2 (filled square). When calculated with ABCG2 (outside of ALDH2) (filled triangle), all but normal type gout showed significant selective pressure, indicating that ABCG2 is involved in adaptive evolution in Japanese for having higher SUA levels, which can result in gout. Finally, all but normal type gout also showed significant selective pressure with ABCG2 and ALDH2 loci (filled square). ROL, renal overload; RUE, renal underexcretion; SDS, singleton density score; SNPs, single-nucleotide polymorphisms.
Discussion
We previously performed GWASs of clinically defined gout cases in the Japanese population and found loci including ABCG2, SLC2A9, ALDH2 (CUX2), GCKR and SHLD2/FAM35A to be associated with gout at a genome-wide significant level.6 7 While our previous study was performed with broader subtypes, it is one of the unique points of this study that the present GWAS is the first to be conducted with four differentiated subtypes: the RUE type, ROL type, combined type and normal type gout (figure 1), which are commonly used in daily clinical settings. Two platforms for GWASs and meta-analyses between them were used to perform a comprehensive genetic association search.
The results allowed four novel loci to be identified from the present study. The pathophysiological associations of the two novel loci from all gout types, PIBF1 and ACSM2B with gout, are totally unknown. PIBF (progesterone-induced blocking factor) is induced by progesterone and is a mediator that exerts substantial antiabortive activities, including cytokine secretion.34 PIBF1 might be therefore involved in decreased urate production by female hormone and/or decreased inflammatory response to gout attack. ACSM2B (acyl-CoA synthetase medium chain family member 2B) is a predominant transcript in the human liver and an enzyme catalysing the activation of medium-chain length fatty acids.35 Because ASCM2B is involved in the production of ATP, a purine body metabolised to urate, ASCM2B might contribute to gout via that mechanism. While the present study showed significance at SNPs of two genes, it is of course possible that these are mere markers and that the true risk SNPs are present close by. For example, since UMOD, a causative gene of uromodulin-associated kidney disease (previously known as familial juvenile hyperuricemic nephropathy)36 is located 180 kb downstream from rs9926388 of ACSM2B, there might be a relationship between them.
Another two intergenic loci from normal type gout, rs146978188 of CD2-PTGFRN and rs548944057 of SLC28A3-NTRK2, were detected for the first time to have an association with gout. CD2 is well known as a surface antigen found on all peripheral blood T-cells, and PTGFRN/CD9P-1 encodes prostaglandin F2 receptor inhibitor. Neither of these was previously known to have an association with gout or uric acid. CD101, which is next to PTGFRN (150 kb downstream from rs146978188), is reported to be expressed on macrophages/monocytes and T-cells, to confer a modulatory/coregulatory function, and to be conspicuously downregulated in rheumatoid arthritis patients.37 Because macrophages are a chief contributor to gouty attack, CD101 might be the true susceptible gene for normal type gout. rs146978188 is also in LD with an SNP of SLC22A15/FLIPT1, an orphan transporter gene in the same family as SLC22A12/URAT1, a well-known urate transporter gene that is strongly associated with gout. This transporter gene might have an association with normal type gout. SLC28A3/CNT3 is reported to be an Na+-dependent pyrimidine-selective and purine-selective transporter found predominantly in the intestine and kidney,38 39 which are the main urate excretion pathways. Further analysis is needed to elucidate the relationship between this transporter gene and gout, including normal type gout.
While the present study revealed only suggestive loci from other subtype GWASs, some of these loci from subtype gout also suggest a relationship with RUE, extra-RUE and/or overproduction of urate, because gout subtypes reflect their clinical parameters (table 1). GWASs with these subtypes should therefore be useful for estimating the expression and function of proteins encoded by identified loci. rs557868370 of SLC38A1, a transporter gene, was detected as a suggestive locus (see online supplementary table S4). Since there is a study reporting the relationship between oxidative stress and SLC38A1/SNAT1,40 it might have a relationship with urate, which also has antioxidative stress effects. Because genetic variants in urate transporter genes such as ABCG2, SLC2A9 and SLC22A12 are well known to cause SUA variation and gout, it is also possible that the SLC38A1/SNAT1 transporter is involved in urate or purine transport.
Very recently, Tin et al41 performed transancestry GWAS meta-analysis of SUA and gout, including self-reported gout cases. We compared these SNPs and found five of the 10 loci detected here (GCKR, SLC2A9, ABCG2, SLC17A1 and BCAS3) to be associated with gout (see online supplementary table S6), indicating population differences in the genetic basis of gout.
Taking into account the evidence of shared genetic background among gout subtypes (see online supplementary figure S3) and the presence of subtype-specific genetic factors of gout (figure 1, table 2), these results will provide helpful information for the development of novel subtype-specific genome tailor-made medicines and/or prevention for gout and hyperuricaemia.
Adaptive evolution results from adaptation to environmental changes over generations. Selection pressure analysis using SDS in the present study has elucidated which genes have been involved in adaptive evolution over the last 2000–3000 years in the Japanese population. The results revealed that the Japanese population has evolved to have higher SUA levels, which can result in gout, due to the ABCG2 locus in addition to the already-known ALDH2 gene. ABCG2 is now a well-known susceptible gene for hyperuricaemia and gout, especially in the Japanese population.31 42 Few patients had gout before Westernisation of Japan about 150 years ago, which brought more purine-rich foods to Japan. The fact that ABCG2 also has a relationship with SUA levels might thus have caused few problems to Japanese people until recently. The upside of SUA elevation in the Japanese population might include resistance to antioxidative stress, lower cancer risk, neuroprotective effect and longevity.43 44 Selection pressure analysis with other populations will also generate more information on evolutionary association with gout to elucidate this hypothesis.
In summary, we performed GWASs of all gout as well as of distinct gout subtypes, and identified multiple subtype-specific loci including four novel loci. Selection pressure analysis revealed significant enrichment of selection for the ABCG2 and ALDH2 loci in Japanese gout patients of each subtype. These findings will lead to elucidation of the molecular pathophysiology of each gout/hyperuricaemia subtype and the development of novel subtype-specific genome tailor-made medicine/prevention of gout and hyperuricaemia.
Acknowledgments
We would like to thank all the participants for their generous involvement in this study. We are grateful to the staff of the institutions participating in the J-MICC Study for their outstanding assistance in collecting samples and clinical information. We are indebted to M Miyazawa, K Morichika, M Sakiyama, M Nakajima, K Maehara and M Kirihara (National Defense Medical College) for genetic analysis and to N Hamajima at Nagoya University Graduate School of Medicine for sample collection. We are also indebted to H Fujiwara (Midorigaoka Hospital), M Senda and M Hamamura (Ryougoku East Gate Clinic).
Footnotes
Handling editor: Josef S Smolen
AN, MN, YK, KY and HN contributed equally.
Collaborators: Members of the Japan Gout Genomics Consortium (Japan Gout) are: Katsuhisa Inoue (Department of Biopharmaceutics, School of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Tokyo), Tomoya Yasujima, Hiroaki Yuasa (Department of Biopharmaceutics, Graduate School of Pharmaceutical Sciences, Nagoya City University, Nagoya, Aichi), Hiroaki Ikezaki, Masayuki Murata (Department of Environmental Medicine and Infectious Disease, Graduate School of Medical Sciences, Kyushu University), Keito Morimoto (Department of Pharmacy, the University of Tokyo Hospital, Tokyo), Mitsuhiro Yokota (Department of Medical Biochemistry, Kurume University School of Medicine, Kurume, Fukuoka), Sahoko Ichihara (Department of Environmental and Preventive Medicine, Jichi Medical University School of Medicine, Shimotsuke, Tochigi), Tatsuaki Matsubara (Department of Internal Medicine, School of Dentistry, Aichi Gakuin University, Nagoya, Aichi), Toshimitsu Ito (Department of Internal Medicine, Self-Defense Forces Central Hospital, Tokyo), Miki Ueno, Kimiko Hayano (Division of Nursing, National Defense Medical College, Tokorozawa, Saitama), Kunio Mizutari, Akihiro Shiotani (Department of Otolaryngology-Head and Neck Surgery, National Defense Medical College, Tokorozawa, Saitama), Yuka Miyoshi, Satoko Suzuki, Satoko Iwasawa (Department of Preventive Medicine and Public Health, National Defense Medical College, Tokorozawa, Saitama), Yuka Aoyagi, Yuka Aoki (Department of Integrative Physiology and Bio-Nano Medicine, National Defense Medical College, Tokorozawa, Saitama), Tatsuya Saitoh (Laboratory of Bioresponse Regulation, Graduate School of Pharmaceutical Sciences, Osaka University, Suita, Osaka).
Contributors: AN, MNakatochi and HMatsuo conceived and designed this study. KY, HNakaoka, II, YO and NS assisted with research design. TS, KO, HO, MNagase, YH, MKawaguchi, MTakao, RS, THosoya, KI and HMatsuo collected and analysed clinical data of cases. TK, AHishida, KK, MW, DM, TTamura, TNishiyama, CS, SKK, NT, NK, HMikami, TTakezaki, KM, SSuzuki and KW collected and analysed clinical data of controls. AN, YKawamura, KY, SShimizu, THigashino, YTakada, ID, YKamatani, MKubo, NS and HMatsuo performed genetic analysis. AN, MNakatochi, YKawamura, HNakaoka, SShimizu, YS, TNakamura, HNakashima and YO performed statistical analysis. HMatsuo organized this collaborative study. KY, HNakaoka, THigashino, MTsunoda, ID, AHozawa, KH, YToyoda, YKubota, TTakada, HS, BS, TM, TR, THosoya, YKamatani, MKubo, KI, KW, II, YO and NS provided intellectual input and assisted with the preparation of the manuscript. AN, MNakatochi, YKawamura, YO, NS and HMatsuo wrote the manuscript.
Funding: This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, including JSPS Kakenhi Grants (Nos. 16H06279, 16H01808, 17H04128, 18KK0247, 19K22786 and 25293145) and a Grant-in-Aid for Scientific Research on Innovative Areas (No. 221S0002), the Ministry of Defense, and the Kawano Masanori Memorial Foundation for the Promotion of Pediatrics and the Gout Research Foundation of Japan. The J-MICC Study was supported by Grants-in-Aid for Scientific Research from MEXT, including those for Priority Areas (No. 17015018) and Innovative Areas (No. 221S0001), as well as by a JSPS Kakenhi grant (No. 16H06277). The BioBank Japan Project was supported by grants from the Japan Agency for Medical Research and Development since April 2015, and the Ministry of Education, Culture, Sports, Science and Technology from April 2003 to March 2015.
Competing interests: TTakada, NS and HMatsuo have a patent pending based on the work reported in this paper.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the institutional ethical committees and written consent was obtained from all of its participants. All procedures involved were performed in accordance with the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
Contributor Information
On behalf of Japan Gout Genomics Consortium (Japan Gout):
Katsuhisa Inoue, Tomoya Yasujima, Hiroaki Yuasa, Hiroaki Ikezaki, Masayuki Murata, Keito Morimoto, Mitsuhiro Yokota, Sahoko Ichihara, Tatsuaki Matsubara, Toshimitsu Ito, Miki Ueno, Kimiko Hayano, Kunio Mizutari, Akihiro Shiotani, Yuka Miyoshi, Satoko Suzuki, Satoko Iwasawa, Yuka Aoyagi, Yuka Aoki, and Tatsuya Saitoh
References
- 1. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet 2016;388:2039–52. 10.1016/S0140-6736(16)00346-9 [DOI] [PubMed] [Google Scholar]
- 2. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers 2019;5:69 10.1038/s41572-019-0115-y [DOI] [PubMed] [Google Scholar]
- 3. Ichida K, Matsuo H, Takada T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun 2012;3:764 10.1038/ncomms1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wortmann RL. Disorders of purine and pyrimidine metabolism : Fauci AS, Braunwald E, Kasper D, et al., Harrison's Principles of Internal Medicine. 17th edn New York, N.Y: McGraw-Hill, 2008: 2444–9. [Google Scholar]
- 5. Matsuo H, Nakayama A, Sakiyama M, et al. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci Rep 2014;4:3755 10.1038/srep03755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsuo H, Yamamoto K, Nakaoka H, et al. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann Rheum Dis 2016;75:652–9. 10.1136/annrheumdis-2014-206191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakayama A, Nakaoka H, Yamamoto K, et al. GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Ann Rheum Dis 2017;76:869–77. 10.1136/annrheumdis-2016-209632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawai Y, Mimori T, Kojima K, et al. Japonica array: improved genotype imputation by designing a population-specific SNP array with 1070 Japanese individuals. J Hum Genet 2015;60:581–7. 10.1038/jhg.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900. 10.1002/art.1780200320 [DOI] [PubMed] [Google Scholar]
- 10. Hamajima N, J-MICC Study Group . The Japan multi-institutional collaborative cohort study (J-MICC study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev 2007;8:317–23. [PubMed] [Google Scholar]
- 11. Asai Y, Naito M, Suzuki M, et al. Baseline data of Shizuoka area in the Japan multi-institutional collaborative cohort study (J-MICC study). Nagoya J Med Sci 2009;71:137–44. [PMC free article] [PubMed] [Google Scholar]
- 12. Delaneau O, Zagury J-F, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 2013;10:5–6. 10.1038/nmeth.2307 [DOI] [PubMed] [Google Scholar]
- 13. Das S, Forer L, Schönherr S, et al. Next-generation genotype imputation service and methods. Nat Genet 2016;48:1284–7. 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willer CJ, Li Y, Abecasis GR. Metal: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–1. 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bulik-Sullivan BK, Loh P-R, Finucane HK, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 2015;47:291–5. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Field Y, Boyle EA, Telis N, et al. Detection of human adaptation during the past 2000 years. Science 2016;354:760–4. 10.1126/science.aag0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okada Y, Momozawa Y, Sakaue S, et al. Deep whole-genome sequencing reveals recent selection signatures linked to evolution and disease risk of Japanese. Nat Commun 2018;9:1631 10.1038/s41467-018-03274-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C, Li Z, Liu S, et al. Genome-wide association analysis identifies three new risk loci for gout arthritis in Han Chinese. Nat Commun 2015;6:7041 10.1038/ncomms8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakiyama M, Matsuo H, Nakaoka H, et al. Common variant of BCAS3 is associated with gout risk in Japanese population: the first replication study after gout GWAS in Han Chinese. BMC Med Genet 2018;19:96 10.1186/s12881-018-0583-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakiyama M, Matsuo H, Nakaoka H, et al. Identification of rs671, a common variant of ALDH2, as a gout susceptibility locus. Sci Rep 2016;6:25360 10.1038/srep25360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakiyama M, Matsuo H, Akashi A, et al. Independent effects of ADH1B and ALDH2 common dysfunctional variants on gout risk. Sci Rep 2017;7:2500 10.1038/s41598-017-02528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anzai N, Miyazaki H, Noshiro R, et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem 2004;279:45942–50. 10.1074/jbc.M406724200 [DOI] [PubMed] [Google Scholar]
- 23. Shimizu T, Sugiura T, Wakayama T, et al. PDZK1 regulates breast cancer resistance protein in small intestine. Drug Metab Dispos 2011;39:2148–54. 10.1124/dmd.111.040295 [DOI] [PubMed] [Google Scholar]
- 24. Nakatochi M, Kanai M, Nakayama A, et al. Genome-wide meta-analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun Biol 2019;2:115 10.1038/s42003-019-0339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 2009;5:e1000504 10.1371/journal.pgen.1000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higashino T, Matsuo H, Sakiyama M, et al. Common variant of PDZ domain containing 1 (PDZK1) gene is associated with gout susceptibility: a replication study and meta-analysis in Japanese population. Drug Metab Pharmacokinet 2016;31:464–6. 10.1016/j.dmpk.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 27. Li M, Li Q, Li C-G, et al. Genetic polymorphisms in the PDZK1 gene and susceptibility to gout in male Han Chinese: a case-control study. Int J Clin Exp Med 2015;8:13911–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Ketharnathan S, Leask M, Boocock J, et al. A non-coding genetic variant maximally associated with serum urate levels is functionally linked to HNF4A-dependent PDZK1 expression. Hum Mol Genet 2018;27:3964–73. 10.1093/hmg/ddy295 [DOI] [PubMed] [Google Scholar]
- 29. Yasukochi Y, Sakuma J, Takeuchi I, et al. Identification of CDC42BPG as a novel susceptibility locus for hyperuricemia in a Japanese population. Mol Genet Genomics 2018;293:371–9. 10.1007/s00438-017-1394-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee J, Lee Y, Park B, et al. Genome-wide association analysis identifies multiple loci associated with kidney disease-related traits in Korean populations. PLoS One 2018;13:e0194044 10.1371/journal.pone.0194044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuo H, Takada T, Ichida K, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med 2009;1:5ra11 10.1126/scitranslmed.3000237 [DOI] [PubMed] [Google Scholar]
- 32. Matsuo H, Ichida K, Takada T, et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci Rep 2013;3:2014 10.1038/srep02014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawamura Y, Nakaoka H, Nakayama A, et al. Genome-wide association study revealed novel loci which aggravate asymptomatic hyperuricaemia into gout. Ann Rheum Dis 2019;78:1430–7. 10.1136/annrheumdis-2019-215521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Druckmann R, Druckmann M-A. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol 2005;97:389–96. 10.1016/j.jsbmb.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 35. Boomgaarden I, Vock C, Klapper M, et al. Comparative analyses of disease risk genes belonging to the acyl-CoA synthetase medium-chain (ACSM) family in human liver and cell lines. Biochem Genet 2009;47:739–48. 10.1007/s10528-009-9273-z [DOI] [PubMed] [Google Scholar]
- 36. Hart TC, Gorry MC, Hart PS, et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 2002;39:882–92. 10.1136/jmg.39.12.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jovanovic DV, Boumsell L, Bensussan A, et al. CD101 expression and function in normal and rheumatoid arthritis-affected human T cells and monocytes/macrophages. J Rheumatol 2011;38:419–28. 10.3899/jrheum.100676 [DOI] [PubMed] [Google Scholar]
- 38. Young JD, Yao SYM, Baldwin JM, et al. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med 2013;34:529–47. 10.1016/j.mam.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 39. Young JD. The SLC28 (CNT) and SLC29 (ENT) nucleoside transporter families: a 30-year collaborative odyssey. Biochem Soc Trans 2016;44:869–76. 10.1042/BST20160038 [DOI] [PubMed] [Google Scholar]
- 40. Ogura M, Takarada T, Nakamichi N, et al. Exacerbated vulnerability to oxidative stress in astrocytic C6 glioma cells with stable overexpression of the glutamine transporter slc38a1. Neurochem Int 2011;58:504–11. 10.1016/j.neuint.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 41. Tin A, Marten J, Halperin Kuhns VL, et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet 2019;51:1459–74. 10.1038/s41588-019-0504-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakayama A, Matsuo H, Nakaoka H, et al. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci Rep 2014;4:5227 10.1038/srep05227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matsuo H, Tomiyama H, Satake W, et al. ABCG2 variant has opposing effects on onset ages of Parkinson's disease and gout. Ann Clin Transl Neurol 2015;2:302–6. 10.1002/acn3.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cutler RG. Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch Gerontol Geriatr 1984;3:321–48. 10.1016/0167-4943(84)90033-5 [DOI] [PubMed] [Google Scholar]
- 45. Auton A, Brooks LD, Durbin RM, et al. A global reference for human genetic variation. Nature 2015;526:68–74. 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2019-216644supp001.pdf (461.6KB, pdf)