Abstract
Humans and their gut bacteria have evolved multiple ways to communicate with and regulate one another. Psychological stress and depression can promote consumption of highly palatable foods, influencing which gut bacteria thrive. Additionally, stress and depression can reshape the gut bacteria’s composition through stress hormones, inflammation, and autonomic alterations. In turn, the gut bacteria release metabolites, toxins, and neurohormones that can alter eating behavior and mood. Some bacterial species may encourage dysregulated eating. The gut bacteria may also upregulate stress responsiveness and heighten the risk for depression, which probiotic supplementation may attenuate. This review focuses on human studies to address the bidirectional links among diet, stress, and the gut bacteria, and their impact on immune function and health.
Introduction
The brain and the gut have a lively ongoing dialog through the gut–brain axis. Most people have had firsthand experience with the unpleasant ways that negative emotions and stress can perturb gut motility. The gut– brain axis is relevant not only to these transient states, but also to longer-lasting conditions. Digestive disorders such as irritable bowel syndrome commonly coincide with mood disorders [1], and both may reflect a dysfunctional composition of gut bacteria, viruses, and fungi (the gut microbiota) and related chronic inflammation [2]. As such, manipulating the gut microbiota and their functions via probiotics and health behaviors is a promising therapeutic strategy. The goal of this review is to examine how diet and stress reciprocally interact with the gut microbiota and inflammation.
Independently and mutually, diet, stress, and mood can substantially influence which gut microbes thrive. Indeed, environmental factors and health behaviors explain more microbiota variability than do host genetics [3•]. Of all the gut microbes, bacteria are most often studied in relation to human stress, mood, and diet. Many modern practices such as antibiotic use, a Western diet, and high-stress lifestyles promote gut bacterial imbalances, called dysbiosis, as well as low diversity, referring to a smaller count and uneven distribution of bacterial species. Although there is no agreed-upon measure of a healthy gut, a diverse and well-balanced gut bacterial composition is a strong candidate. Dysbiosis and low diversity may alter food cravings, metabolism, stress reactivity, and mood, compromising immune function and health. This review focuses on a recent research examining these dynamic and reinforcing relationships.
Stress and depression facilitate dysbiosis and a leaky gut
Stress can affect health through its impact on gut bacteria. The autonomic and circulatory systems carry distress signals to the gut. Additionally, a new bone marrowmediated pathway was recently discovered [4], highlighting the role that immune cells play as messengers that convey psychological stress to the gut. The heightened inflammation that frequently accompanies stress and depression triggers blooms of pathogenic bacteria that encourage dysbiosis and a leaky gut [5].
Both chronic and acute stressors can shift the gut bacteria in multiple regions and habitats — both the inside (lumen) and border (mucosal lining) of the gut [6–8]. Rodent research demonstrates that stress can rapidly affect the gut bacteria’s composition [7,9]. Furthermore, an in vitro work shows that catecholamines can elevate certain bacterial levels 10 000-fold and intensify their infectiousness in 14 hours [10]. These pathogenic species may crowd out beneficial species. In line with an early work linking academic stress with immune dysregulation [11], Knowles et al. found that as university students’ stress increased throughout the semester, certain health-promoting bacteria decreased [12]. Even so, people under stress have unpredictable patterns of dysbiosis [13]. Gut bacteria can regulate the immune system, but dysbiosis can increase risk for infection or autoimmune disease [14].
Stress and mood disorders compound one another. Several studies show differences in the gut microbiota’s composition and function in individuals with major depressive disorder, compared to healthy controls. Some data suggest that proinflammatory species can dominate at the expense of health-promoting species in depressed individuals [15]. Comparison of depressed and non-depressed individuals revealed 279 different bacteria-synthesized proteins, primarily related to glucose and amino acid metabolism [16], possible inflammatory correlates.
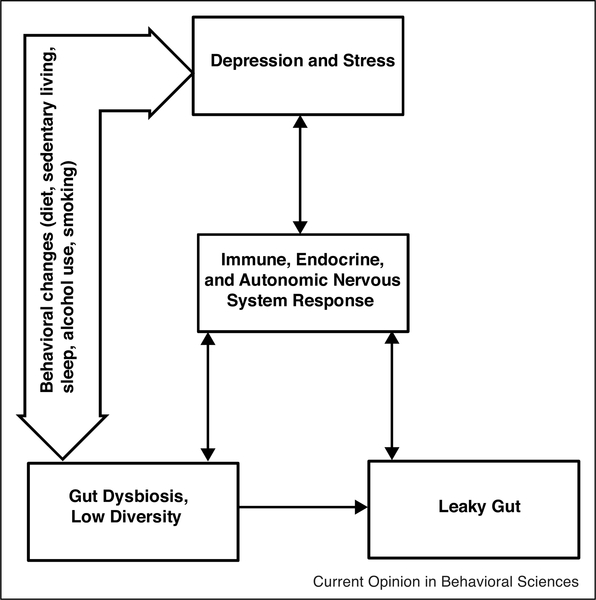
Stress and depression can increase gut barrier permeability. The result, a ‘leaky gut,’ allows bacteria to seep into circulation, producing an inflammatory response. Indeed, both depression and stress can provoke heightened inflammation [17,18] and gut leakiness [19••,20]. For example, a troubled marriage is a chronic stressor. Most hostile couples had greater gut permeability than their less hostile counterparts [19••]. Even a laboratory speech stressor increased intestinal permeability in healthy adults, and subgroup analysis revealed that this effect was only present in those who had elevated cortisol [21]. Along with cortisol, mast cells helped to weaken stressed participants’ gut barriers. Bacteria can leak through the stress-remodeled gut barrier, thereby boosting inflammation — illustrating stress’s multifaceted physiological assault (Figure 1).
Figure 1.
Gut environment’s hypothesized role in psychoneuroimmunology. Depression and stress get under the skin via behavioral and physiological changes, including altered immune function. These adverse changes promote the survival and replication of pathogenic gut bacteria and weaken the gut barrier. Gut dysbiosis itself increases gut permeability, and it may also influence health behaviors like diet. These physiological and behavioral changes can ultimately further dysregulate key stress-responsive systems, including the immune, endocrine, and autonomic nervous systems, thereby fueling the chronicity of depression and stress.
Stress and depression prompt unhealthy food choices and poor metabolic responses
Diet functions as a major pathway from stress to gut dysbiosis. Even mild stressors can encourage unhealthy eating. For example, saturated fat and caloric intake rose on Monday in cities whose NFL football team lost on Sunday, compared to declines in cities whose team won; intake remained stable in those cities whose NFL team did not play, or those without an NFL team. Furthermore, cities with the most committed fans showed the greatest change [22]. Relatedly, in a recent ecological momentary assessment study, emotional eaters’ hedonic, taste-based eating increased with negative emotion [23]. Functional neuroimaging evidence suggests that stress deactivates executive function in response to food cues and elicits a bias toward comfort foods [24]. As discussed below, stress-related microbiota shifts may also impact food cravings.
Stress and depression not only influence food choices, they can also alter metabolic responses to food. Following a fast-food type meal, women who reported prior day stressors had lower fat oxidation, higher insulin, and lower resting energy expenditure than those reporting no prior day stressors — with lower caloric expenditure that could potentially fuel 7–11 pounds of weight gain per year [25•]. Similarly, women with a history of depression had higher postprandial cortisol and fat oxidation, compared to women without a depression history [25•]. These kinds of metabolic changes could have downstream effects on the gut microbiota, and the reverse is also possible.
Diet shapes the gut bacteria
Diet has emerged as one of the most powerful predictors of gut bacteria composition — above and beyond one’s genotype [26]. Diet determines which bacteria will thrive in the gut, and the gut bacteria in turn aid digestion. Although long-term diets form the gut community’s structure, dietary modification can produce detectible shifts in some bacterial species within 24 hours [27].
Macronutrient profiles predict unique gut microbiota populations. A recent systematic review highlighted the emerging convergence in the field of nutrition that is facilitated by a focus on macronutrients’ impact on the gut microbiota [28]. Broadly speaking, plant protein, unsaturated fats, and fiber support a pro-health gut microbiota — in contrast to excessive consumption of animal protein, saturated fats, and simple or artificial sugars.
The Western diet, high in saturated fat, processed foods, and refined sugar, starkly contrasts with fiber-rich, plantbased diets of indigenous cultures. The Western diet fosters a distinct gut microbiota signature with low gut microbiota diversity and greater gut leakiness, which may contribute to metabolic syndrome and chronic disease onset [29]. In particular, imbalanced macronutrient intake may be to blame. For instance, low fiber consumption may dysregulate immune function, as short-chain fatty acids (SCFAs) resulting from bacterial fermentation of complex carbohydrates are important for healthy immune function [30].
Likely due in part to the Western diet, 60–70 million Americans suffer from digestive disorders, costing $100 billion annually [31]. Importantly, some digestive medications impact the gut bacteria. For example, proton pump inhibitors (e.g. omeprazole), widely used to treat acid reflux, reduce diversity and affect 20% of species, creating an unhealthy gut microbiota that may predispose to gastrointestinal infection [32]. Indeed, PPIs may negatively impact the gut bacteria population even more so than do antibiotics [32].
The gut bacteria impact food cravings and eating behaviors
The number of bacterial cells in the human body parallels the number of human cells [33], and thus, whether they support or undermine health is of critical importance. In many instances, bacteria are clearly beneficial, but they can also compete with each other as well as their human host for resources. Thus, microbiota diversity ensures that one species’ interests do not eclipse the human’s. However, it has been hypothesized that dysbiosis and low diversity may prompt dysregulated eating behavior in line with the dominant bacterial species’ needs [34].
Mechanistic data support the notion that the gut bacteria influence food choices. The gut bacteria produce molecules that mimic or interfere with human appetite-regulating peptides and hormones [35,36]. Also, the gut bacteria can modify reward pathways [37], communicate with the appetite-modulating vagus nerve [38], and may even influence the expression of taste receptors [39]. Lastly, through their release of neurotransmitters, such as serotonin, acetylcholine, and norepinephrine, the gut bacteria may indirectly influence eating behavior through mood changes [40].
Evidence for this bottom-up pathway from gut bacteria to eating behavior has recently emerged. In the stool samples of healthy adults, specific microbiota abundances tracked with eating behaviors, including eating frequency and overnight-fast duration [41••]. Targeted delivery of a bacteria-produced SCFA, propionate, to the colon over 24 weeks reduced meal size, weight gain, belly fat, and increased postprandial satiety signals, but did not reduce subjective appetite in overweight adults [42]. Thus, there is accumulating evidence that the gut bacteria and their products affect appetite.
Diet modulates stress reactivity and depression
Clinical investigation of the role of macronutrient intake in stress reactivity remains largely inconclusive. Much of the relevant research is cross-sectional, assessing stress levels and food consumption at a single time point. An exception: following a dietary intervention, in which women were assigned to drink either sugar-sweetened or aspartame-sweetened beverages three times a day for two weeks, sugar consumption was associated with higher activity in the left hippocampus and reduced cortisol in response to stress [43]. If sugar indeed attenuates physiological stress responding, stressed individuals may preferentially consume sugar. In contrast, fat intake may enhance stress reactivity; healthy, normotensive individuals had greater cardiovascular reactivity to stress following a high-fat meal than they did after a low-fat meal [44].
Healthier diets can reduce the risk of depression. A metaanalysis showed that adherence to high-quality diets such as the Mediterranean diet was associated with a lower risk of depressive symptoms over time [45]. Inflammation elevates risk for depression, and the Mediterranean diet’s anti-inflammatory benefits have been demonstrated across multiple studies [46,47]. Mounting evidence suggests that these anti-inflammatory effects are mediated by the microbiome. Although multiple components of the Mediterranean diet have synergistic effects on inflammation, high dietary fiber and low levels of saturated fat sculpt the gut microbiota’s composition and its production of metabolites that reinforce the gut barrier [46–48]. Thus, fewer gut bacteria leak into the bloodstream, lessening inflammatory burden and depression risk.
The gut microbiota can influence stress reactivity and mood
Through their communication with the vagus nerve and neurotransmitter release, the gut microbiota may play a role in stress responding. In a functional neuroimaging study of 40 women, certain bacterial profiles tracked with patterns of brain activation following exposure to emotional stimuli [48]. Randomized controlled trials featuring probiotics suggest a causal link between the gut microbiota and stress responding. Probiotic supplementation improved sleep, autonomic balance, and bowel habits and reduced stress and cortisol levels in Japanese medical students [49]. Moreover, after one month of drinking a probiotic-containing fermented milk product, healthy women had less activity in emotion and sensation brain loci when exposed to emotional stimuli [50••].
Relatedly, the gut microbiota may contribute to depressive symptoms. In an elegant experimental design, the transfer of fecal matter from depressed humans to microbe-depleted rats induced depressive-like behavior in the rats [51]. Moreover, a large epidemiological study in the United Kingdom revealed that one course of antibiotics, which reliably destabilize the gut microbiota, increased risk for anxiety or depression by about 20%, while multiple courses increased risk by almost 50% [52]. Antibiotic use may program a new ‘set point’ of gut bacteria that is relatively stable — even though it may facilitate poorer mental and physical health [53]. In contrast, probiotic supplementation may reduce inflammation [54] and have positive effects on depression, anxiety, and stress, although some studies have not found this effect [55].
Putting it all together: a dynamic human-microbe cycle
Diet and stress modulate the gut microbiota, but the investigation of their combined effect among humans is just beginning. Existing evidence suggests bidirectional relationships among stress/mood, diet, and the gut microbiota, which ultimately form either a vicious or virtuous cycle. These mind–body, human–bacterial relationships help to explain both resilience and chronic disease. Today the top-down pathway from human behavior and mood to gut microbiota is better understood than the highly intriguing but less explored bottom-up pathway.
The field is dominated by rodent research, but emerging human evidence has begun to corroborate preclinical findings. However, humans diverge from rodents in many relevant ways, such as neuronal expression [56] and even gut microbiota [57], underscoring the need for more clinical research to replicate and extend preclinical research. Greater use of longitudinal designs will tease out directionality of these complex relationships, and isolating specific bacterial species, dietary components, and types of stressors (e.g. bereavement, abuse) will add clarity.
This science is young and requires interdisciplinary collaborations across populations – human, animal, bacteria – and specialties, such as medicine, immunology, nutrition, and psychology. Adapting lifestyles – including stress and diet – to steward gut bacteria populations that support healthy immune function will ultimately foster both mental and physical health.
Acknowledgement
Work on this project was supported in part by National Institutes of Health grants CA172296, CA186251, CA186720, and AG057032.
Footnotes
Conflict of interest statement Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L: Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014, 264:651–660. [DOI] [PubMed] [Google Scholar]
- 2.Collins SM: A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol 2014, 11:497. [DOI] [PubMed] [Google Scholar]
- 3.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N: Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555:210.•Comparing the gut microbiota and genotypes of 1056 healthy, genetically dissimilar individuals who shared a similar living environment revealed that genetic factors play a minor role in determining the gut microbiota’s composition, and instead, diet, drugs, and anthropometrics together explain 20% of gut microbiota variability. Moreover, compared to genetic and environmental data, microbiota data function as a better predictor of host metabolic traits (e.g. glucose and obesity measures). This study illustrates the strong bi-directional relationship between the gut microbiota and human phenotype.
- 4.Yang T, Ahmari N, Schmidt JT, Redler T, Arocha R, Pacholec K, Magee KL, Malphurs W, Owen JL, Krane GA: Shifts in the gut microbiota composition due to depleted bone marrow beta Adrenergic signaling are associated with suppressed inflammatory transcriptional networks in the mouse colon. Front Physiol 2017, 8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng M, Inohara N, Nuñ ez G: Mechanisms of inflammationdriven bacterial dysbiosis in the gut. Mucosal Immunol 2017, 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M: Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011, 25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT: Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 2014, 14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galley JD, Yu Z, Kumar P, Dowd SE, Lyte M, Bailey MT: The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes 2014, 5:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partrick KA, Chassaing B, Beach LQ, McCann KE, Gewirtz AT, Huhman KL: Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behav Brain Res 2018, 345:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M: Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in traumainduced sepsis. Shock 2002, 18:465–470. [DOI] [PubMed] [Google Scholar]
- 11.Kiecolt-Glaser JK, Glaser R, Strain EC, Stout JC, Tarr KL, Holliday JE, Speicher CE: Modulation of cellular immunity in medical students. J Behav Med 1986, 9:5–21. [DOI] [PubMed] [Google Scholar]
- 12.Knowles SR, Nelson EA, Palombo EA: Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biol Psychol 2008, 77:132–137. [DOI] [PubMed] [Google Scholar]
- 13.Zaneveld JR, McMinds R, Thurber RV: Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat Microbiol 2017, 2:17121. [DOI] [PubMed] [Google Scholar]
- 14.Wu H-J, Wu E: The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J: Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015, 48:186–194. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C, Hu Z, Wang H, Zhong X, Zeng L: Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 2018, 29:417–425. [DOI] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Derry HM, Fagundes CP: Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry 2015, 172:1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaser R, Kiecolt-Glaser JK: Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005, 5:243. [DOI] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, Fagundes CP, Malarkey WB, Laskowski B, Belury MA: Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98:52–60.••In this double-blind randomized crossover study, couples exhibiting more hostile conflict behavior toward their partner had greater lipopolysaccharide-binding protein, a marker of leaky gut, than less hostile couples. Additionally, these biomarkers tracked with inflammation. Although depression has been linked to greater intestinal permeability, this is among the first evidence in humans that chronic psychological stress – a distressed marriage – corresponds to leaky gut.
- 20.Maes M, Kubera M, Leunis J-C, Berk M: Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord 2012, 141:55–62. [DOI] [PubMed] [Google Scholar]
- 21.Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Rasoel SS, Tóth J, Holvoet L, Farré R: Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast celldependent mechanism. Gut 2013, 63:1293–1299. [DOI] [PubMed] [Google Scholar]
- 22.Cornil Y, Chandon P: From fan to fat? Vicarious losing increases unhealthy eating, but self-affirmation is an effective remedy. Psychol Sci 2013, 24:1936–1946. [DOI] [PubMed] [Google Scholar]
- 23.Reichenberger J, Kuppens P, Liedlgruber M, Wilhelm FH, Tiefengrabner M, Ginzinger S, Blechert J: No haste, more taste: an EMA study of the effects of stress, negative and positive emotions on eating behavior. Biol Psychol 2016, 131 10.1016/j.biopsycho.2016.09.00254-26. [DOI] [PubMed] [Google Scholar]
- 24.Tryon MS, Carter CS, DeCant R, Laugero KD: Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav 2013, 120:233–242. [DOI] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, Belury MA: Daily stressors, past depression, and metabolic responses to high-fat meals: a novel path to obesity. Biol Psychiatry 2015, 77:653–660.•Healthy women reporting greater numbers of prior daily stressors had lower post-prandial resting energy expenditure and fat oxidation and higher insulin, compared to their non-stressed peers. This study provides initial evidence for stress’s impact on human metabolism.
- 26.Carmody RN, Gerber GK, Luevano JM Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ: Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R: Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willson K, Situ C: Systematic review on effects of diet on gut microbiota in relation to metabolic syndromes. J Clin Nutr Metab 2017, 1. [Google Scholar]
- 29.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR: Regulation of immune cell function by short-chain fatty acids.Clin Transl Immunol 2016, 5:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma N, Tian Y, Wu Y, Ma X: Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr Protein Pept Sci 2017, 18:795–808. [DOI] [PubMed] [Google Scholar]
- 31.Imhann F, Bonder MJ, Vila AV, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ: Proton pump inhibitors affect the gut microbiome. Gut 2016, 65:740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sender R, Fuchs S, Milo R: Revised estimates for the number of human and bacteria cells in the body. PLoSBiol 2016,14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcock J, Maley CC, Aktipis C: Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 2014, 36:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemot J, Goichon A, Guérin C, Peltier J, Pestel-Caron M: Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab 2016, 23:324–334. [DOI] [PubMed] [Google Scholar]
- 35.Tennoune N, Chan P, Breton J, Legrand R, Chabane Y, Akkermann K, Järv A, Ouelaa W, Takagi K, Ghouzali I: Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide a-MSH, at the origin of eating disorders. Transl Psychiatry 2014, 4:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aarts E, Ederveen TH, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, Smeekens SP, Netea MG, Buitelaar JK, Franke B: Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One 2017, 12:e0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF: Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011, 108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swartz TD, Duca F, De Wouters T, Sakar Y, Covasa M: Upregulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br J Nutr 2012, 107:621–630. [DOI] [PubMed] [Google Scholar]
- 39.Cryan JF, Dinan TG: Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012, 13:701. [DOI] [PubMed] [Google Scholar]
- 40.Kaczmarek JL, Musaad SM, Holscher HD: Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr 2017, 106:1220–1231. [DOI] [PubMed] [Google Scholar]
- 41.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS: Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2014, 64:1744–1754.••In this double-blind, placebo-controlled trial, 60 overweight subjects were randomly assigned to either a 10 g/day inulin-control group or a 10 g/day inulin-propionate ester group for 24 weeks of supplementation. At the end of this novel trial, among those in the intervention group, food intake decreased and physiological satiety signals increased. As propionate is a product of gut bacteria, these findings demonstrate that gut bacteria play a role in appetite regulation.
- 42.Tryon MS, Stanhope KL, Epel ES, Mason AE, Brown R, Medici V, Havel PJ, Laugero KD: Excessive sugar consumption may be a difficult habit to break: a view from the brain and body. J Clin Endocrinol Metab 2015, 100:2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakulj F, Zernicke K, Bacon SL, van Wielingen LE, Key BL, West SG, Campbell TS: A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J Nutr 2007, 137:935–939. [DOI] [PubMed] [Google Scholar]
- 44.Molendijk M, Molero P, Sánchez-Pedreñ o FO, Van der Does W, Martínez-González MA: Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord 2017, 226:346–354. [DOI] [PubMed] [Google Scholar]
- 45.Bailey MA, Holscher HD: Microbiome-mediated effects of the Mediterranean diet on inflammation. Adv Nutr 2018, 9:193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tosti V, Bertozzi B, Fontana L: Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol Ser A 2017, 73:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C: High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2015, 65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 48.Tillisch K, Mayer EA, Gupta A, Gill Z, Brazeilles R, Le Nevé B, van Hylckama Vlieg JE, Guyonnet D, Derrien M, Labus JS: Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom Med 2017, 79:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishida K, Sawada D, Kuwano Y, Tanaka H, Sugawara T, Aoki Y, Fujiwara S, Rokutan K: Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stressassociated symptoms in Japanese medical students. J Funct Foods 2017, 36:112–121. [Google Scholar]
- 50.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D,Legrain–Raspaud S,Trotin B,NaliboffB:Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144: 1394–1401.••To address the question of directionality in cross-sectional research, Kelly et al. transferred fecal microbiota from 34 depressed humans and 33 healthy controls to microbe-depleted rats and measured behavioral changes. The gut microbiota of depressed patients was less diverse than that of healthy controls, and when transferred to rats, caused anhedonia and anxiety-like behaviors as well as altered tryptophan metabolism.
- 51.Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G: Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016, 82:109–118. [DOI] [PubMed] [Google Scholar]
- 52.Lurie I, Yang Y-X, Haynes K, Mamtani R, Boursi B: Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry 2015, 76:15221528. [DOI] [PubMed] [Google Scholar]
- 53.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R: Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park C, Brietzke E, Rosenblat JD, Musial N, Zuckerman H, Ragguett R-M, Pan Z, Rong C, Fus D, McIntyre RS: Probiotics for the treatment of depressive symptoms: an anti-inflammatory mechanism? Brain Behav Immun 2018, 73:115–124. [DOI] [PubMed] [Google Scholar]
- 55.Wallace CJ, Milev R: The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 2017, 16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, Baka J, Borde S, Close JL, Diez-Fuertes F, Ding S-L: Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat Neurosci 2018, 21:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li D, Chen H, Mao B, Yang Q, Zhao J, Gu Z, Zhang H, Chen YQ, Chen W: Microbial biogeography and core microbiota of the rat digestive tract. Sci Rep 2017, 7:45840. [DOI] [PMC free article] [PubMed] [Google Scholar]