Abstract
Emerging technologies allow broad profiling of the cancer genome for differential DNA methylation relative to benign cells. Herein, bisulfite-modified DNA from lymph nodes with either reactive hyperplasia or follicular lymphoma (FL) were analyzed using a commercial C/UpG genotyping assay. Two hundred fifty-nine differentially methylated targets (DMT) distributed among 183 unique genes were identified in FL. Comparison of matched formalin-fixed, paraffin-embedded and frozen surgical pathology replicates showed the complete preservation of the cancer methylome among differently archived tissue specimens. Analysis of the DMT profile is consistent with a pervasive epigenomic remodeling process in FL that affects predominantly nonlymphoid genes.
Introduction
Global patterning of CpG methylation occurs during cellular differentiation in concert with immunophenotypic and functional maturation. Such patterning has been particularly well studied in CpG islands (CGI)—genomic motifs of higher CpG density often proximal to promoter regions of embryoexpressed and housekeeping genes and reported to undergo changes in methylation status during embryogenesis, germ cell development, and carcinogenesis (1, 2). The elucidation of cancer-related methylation divergences on a broad scale is a promising avenue for individualized cancer classification and therapy.
The current portrait of the cancer methylome is a composite derived from divergent sample types and methodologies. This portrait depicts a globally demethylated genome with locoregional promoter CGI hypermethylation. Many array-based methods of methylation profiling are biased toward detection of regions with high CpG density (3). Bisulfite sequencing and genotyping permit large-scale analysis of cancer tissue methylation, inclusive of high-density and low-density CpG genome space.
Archival formalin-fixed, paraffin-embedded (FFPE) specimens comprise the vast majority of human cancer tissues available for research studies and often may be the only available tissue source. FFPE archives have been used previously for genome-scale copy number profiling (4) and gene expression analysis (5). The demonstration of preserved DNA methylation signatures and a suitable method for their recovery would increase the value of FFPE archives for biomarker discovery.
The purposes of the present analysis are (a) to test the feasibility of differential-methylation profiling in FFPE samples compared with frozen samples; (b) to derive a balanced genomic profile of on-CGI and off-CGI differential methylation in a cancer, specifically follicular lymphoma (FL) relative to reactive lymph nodes selected for a predominance of follicular hyperplasia (FH); and (c) to investigate methylation–gene expression correlation in FL versus FH.
Materials and Methods
Tissue and Cell Specimens
Analysis set of samples
Twelve cases of FL grades 1 and 2 and 10 reactive lymph nodes selected for a significant component of FH were retrieved from the archives of the National Cancer Institute Laboratory of Pathology. For 10 cases, each of FL and FH matched FFPE and cryopreserved adjacent tissues were analyzed.
Validation set
Eleven cases of FL grades 1 and 2 and 14 reactive lymph nodes, of ages 10 to 14 y, were analyzed. All anatomic pathology diagnoses were rendered by an expert hematopathologist according to WHO criteria (6).
Cells
Peripheral blood lymphocytes (PBL) were isolated from fresh commercial leukopaks (Blood Systems Research Institute). CD19+ B cells and CD4+ and CD8+ T cells were isolated from PBLs using immunomagnetic beads (Miltenyi). Lymphoblastoid B-cell DNA samples NA06999, NA07033, NA10923, and NA10924 were purchased from Coriell Institute for Medical Research.
DNA Methylation Profiling Using Bead Arrays
Tissue was scraped from entire 5-µm tissue sections from either FFPE unstained slides or frozen sections of cryopreserved tissue blocks (69 total tissue samples). Tissue lysis and DNA extraction were performed using proteinase K digestion (Qiagen). DNAs were bisulfite modified using the Zymo Methylation Gold kit. Bisulfite (250 ng)-modified DNA was used for the Illumina bead array methylation assay. Methylation detection for 1505 CpG sites was performed, as described previously (7), using the standard cancer panel according to the manufacturer’s instructions. Image processing and intensity data extraction were performed with Illumina-supplied equipment. Each methylation data point is represented by fluorescent signals from the methylated (M) and unmethylated (U) alleles.
Gene Expression Profiling
RNA was isolated using Trizol reagent and subsequently purified over Qiagen columns. Complementary DNA was generated from total RNA by reverse transcription and linear amplifications (in vitro transcription) to generate biotinylated cRNA targets that were hybridized to Illumina Human Ref8 BeadChip arrays according to the manufacturer’s protocols.
COBRA Assays
Differential methylation between FL and reactive hyperplasia was confirmed for numerous targets using COBRA (combined bisulfite restriction analysis; ref. 8) assays. Eight independent target CpG COBRA assays were developed (four hypomethylated and four hypermethylated targets in FL), which confirmed differential methylation in accordance with array analyses. Supplementary Table S3 provides target IDs and PCR primer sequences for COBRA assays. Figure 3 illustrates COBRA assays in representative cases of FL and reactive hyperplasia.
Figure 3.
Representative COBRA assays for validation of array analyses. PCR primers for each of the eight CpG targets are given in Supplementary Table S4. One half of each PCR product was run on 3% agarose gel neat (−) and the other half after Taq1 restriction digest (+). AIM2, CARD15, CHI3L2, and EPHX1 show hypomethylation in FL samples relative to FH, indicated by diminished Taq1 cutting of PCR product. EPHA7, ETV1, PITX2, and TFAP2C show hypermethylation in FL relative to FH, indicated by greater Taq1 restriction digestion of FL-derived PCR products. These results are in accordance with array measures.
Data Analysis
CGI determination
The number of on-island and off-island targets varied with the details of the criterion used to define CGI targets. A conservative computational criteria definition based on C, G, and GC density in genomic sequence, mirroring the criteria used by the University of California-Santa Cruz (UCSC) genome browser CGI track, classified 649 of the 1,505 targets as within-CGI. The definition used in this study differs in that it uses a target centric region; a 200-bp region centered around the probed CpG target is analyzed rather than the running window used in UCSC’s definition for entire genomes. The other criteria are unchanged. The region is called a CGI if the number of CpG dinucleotides is >60% of what is expected by the frequency of individual Cs and Gs and their combined frequency exceeds 50% of the nucleotides in the region.
Calculation of CpG target β
A nonlinear transformation was used to map the raw Cy3 and Cy5 BeadArray fluorescent intensities R,G to methylation β levels.
FFPE versus frozen methylation profiles
Three of the 1,505 targets were identified by significance analysis of microarrays (SAM; ref. 9) as statistically different between the tissue-processing classes. However, in these three instances, the difference between frozen and FFPE classes was only 1% to 2% methylation. Further analysis showed that the identification of these three targets was most probably a mathematical artifact of the nonlinear transformation used to convert raw fluorescent intensities to methylation β levels.
Differentially methylated target identification: FLversus reactive hyperplasia
SAM (9) was used to identify CpG target methylation β values as statistically different between FL and reactive hyperplasia. The additional criterion group average target β difference between FL and reactive hyperplasia of >0.15 led to the exclusion of additional targets, resulting in the final list of 259 differentially methylated targets (DMT).
Classification accuracy: FL versus reactive hyperplasia
After exclusion of samples FL1 and FH9 (see text), the prediction analysis of microarrays (PAM) package was used to identify the most robust DMT classifiers of FL versus reactive hyperplasia.
Activity matrix
The methylation of targets in the reference (reactive hyperplasia) samples were categorized into unmethylated (β < 0.2), hemimethylated (0.2 ≤ β ≤ 0.8), and methylated (β > 0.8). The compartment (CGI or non-CGI) activity for hypermethylation in FL was then defined as
wherein #unmethylated and #hemimethylated are the numbers of unmethylated and hemimethylated array targets in reactive hyperplasia, respectively. The factor 2 in the denominator accounts for the number of unmethylated alleles (two in unmethylated and only one in hemimethylated targets).
Compartmental hypomethylation activity is correspondingly defined as
Correlation of gene expression with DMTs
Differentially expressed genes between FL and reactive hyperplasia were ordered by t test value, and the DMT list was aligned to it. This analysis showed that DMTs were significantly enriched in those genes with low expression levels in both FL and reactive hyperplasia.
Results and Discussion
DNA methylation profiling was performed on matched pairs of FFPE and frozen lymph node tissues, EBV-transformed B-lymphoblastoid cell lines, and purified PBL populations of CD19+ B cells, CD4+ T cells, and CD8+ T cells using a BeadArray bisulfite epityping platform (7). Differential methylation between FL and reactive hyperplasia lymph node groups was further correlated with gene expression.
Comparison of FFPE and frozen specimens
Precision and accuracy are two variables commonly used to characterize the quality of array measurements; precision estimates how close measured values are to their corresponding “true” values and is shifted by systematic biases, whereas accuracy reflects how well technical replicates agree and essentially captures stochastic noise (10). Because formalin fixation may alter DNA through fragmentation, sequence modification, and cross-linking, the methylomes of 20 surgical specimens (Table 1)—parallel-processed with both FFPE and cryopreservation—were compared to identify potential random or systematic alterations.
Table 1.
Pathology specimens included in study
| ID | Age | Sex | Pathology group | Clinical condition | Archive time (mo) |
|---|---|---|---|---|---|
| FH1* | 43 | M | FH | HIV | 28 |
| FH2* | 14 | F | FH | RA | 26 |
| FH3* | 39 | M | FH | HIV | 24 |
| FH4* | 12 | F | FH | ALPS | 14 |
| FH5* | 52 | F | FH | RA | 13 |
| FH6* | 15 | F | FH | Hyper IgE, sepsis | 12 |
| FH7* | 13 | M | FH | Suspected ALPS | 11 |
| FH8* | 31 | F | FH | ALPS | 7 |
| FH9*,† | 64 | M | MCD | MCD | 3 |
| FH10* | 61 | F | FH | Lymphoma, remission | 3 |
| FL1† | 53 | M | FH | Lymphoma | 22 |
| FL2 | 54 | M | FL | Lymphoma | 22 |
| FL3* | 50 | M | FL | Lymphoma | 22 |
| FL4* | 50 | F | FL | Lymphoma | 21 |
| FL5* | 47 | F | FL | Lymphoma | 19 |
| FL6* | 35 | M | FL | Lymphoma | 18 |
| FL7* | 62 | F | FL | Lymphoma | 13 |
| FL8* | 68 | M | FL | Lymphoma | 13 |
| FL9* | 42 | M | FL | Lymphoma | 11 |
| FL10* | 27 | F | FL | Lymphoma | 9 |
| FL11* | 36 | F | FL | Lymphoma | 7 |
| FL12* | 54 | F | FL | Lymphoma | 2 |
Abbreviations: M, male; F, female; RA, rheumatoid arthritis; ALPS, autoimmune lymphoproliferative syndrome.
Matched FFPE and cryopreserved tissue.
Removed from DMT selection after pathology review (see text).
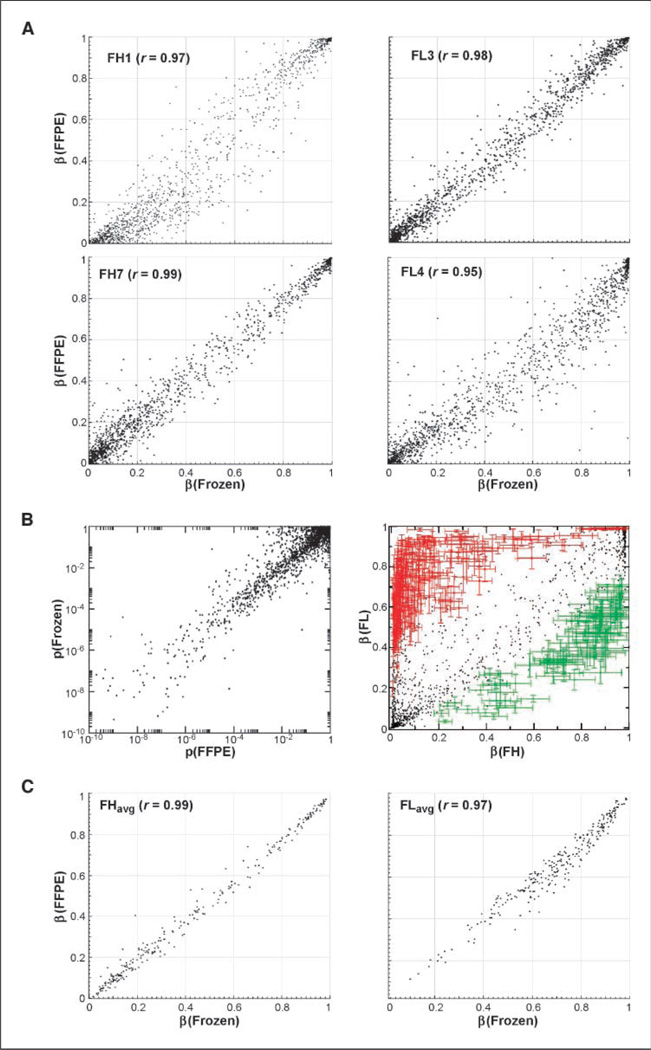
As a first step of data analysis, correlations were plotted between matched pairs of FFPE and frozen samples for each individual (Fig. 1A). Next, the 20 FH samples were arranged into two groups based on the processing method (frozen versus FPPE) and evaluated for any target CpG methylation β differences indicative of processing bias. The methylation β is calculated from the ratio of Cy3 and Cy5 signal at each CpG target (see Materials and Methods) and is a measure of target methylation. A total of 1,502 of the 1505 targets measured showed no significant difference between the FFPE and frozen sample processing classes after correction for multiple comparisons; three outlier targets showed a minute difference (<0.02β) between FFPE and frozen and were most likely artifacts of the nonlinear data normalization rather than errors introduced by sample processing (see Materials and Methods).
Figure 1.
Comparison between FFPE and frozen archiving samples and groups for target differential methylation measurement. A, representative correlations ofFFPE and frozen sample array measures for various individuals. Top left, oldest FH sample pair (r = 0.97; 28 mo); top right, oldest FL sample pair (r = 0.98; 22 mo); bottom left, best correlation (r = 0.99); bottom right, worst correlation sample pair (r = 0.95). B, pairwise comparison of target differential methylation significances (P values) between FL and FH for 1,505 targets, as measured separately for FFPE and frozen archive groups (left). Differentially methylated CpG targets show hypermethylation (red, n = 184) and hypomethylation (green, n = 75) in FL relative to reactive hyperplasia (right). C, comparison of FFPE versus frozen archive groups for uniformity of measurement of the identified 259 DMTs in FH (left) and FL (right).
Next, noise levels in FFPE versus frozen samples were compared based on the uniformity of their DMT profiles in malignancy. To generate a phenotype-dependent DMT profile to use as a metric for uniformity, P values were calculated for each of the 1,505 dinucleotide CpG targets for which cytosine methylation is measured by the array, separately for frozen and FFPE samples of reactive hyperplasia (FH) and grade 1 FL (ref. 6; total number of P value calculations, 3,010). The resultant P values were then compared CpG-by-CpG between the two independent analyses (Fig. 1B). The points lay on a diagonal line, and the dynamic range of P values was the same on both axes, indicating that both archiving methods generate equivalent sensitivity to identify DMTs broadly across the genome. Very few targets exhibited apparent discrepancies. The comparable success of DMT discovery in FFPE and frozen groups is likely because the bisulfite-modified DNA target regions of the BeadArray assay are short fragments of roughly 55 bp in length.
DNA methylation classifiers of FLare pervasive
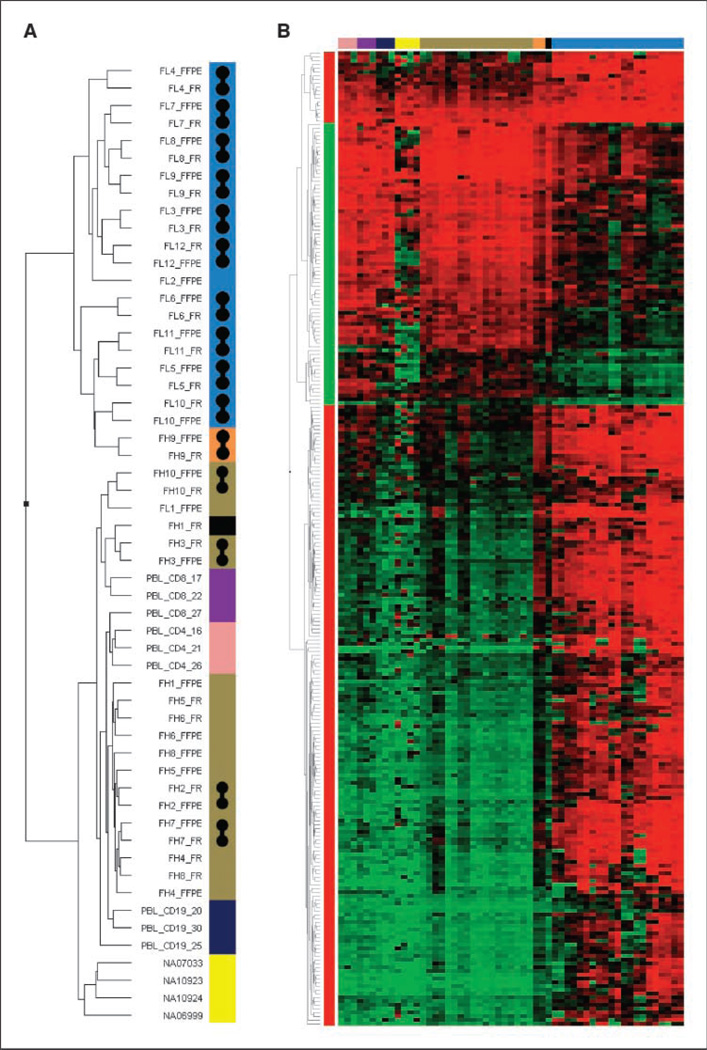
Of the 1,505 targets (806 genes) analyzed, 259 targets distributed among 183 unique genes were identified as significantly differentially methylated between hyperplasia and FL (see Materials and Methods for DMT identification). Of the 259 DMTs, 184 were hypermethylated in FL (Supplementary Table S1), whereas 75 were hypomethylated (Supplementary Table S2; Figs. 1C and 2). Numerous DMTs were validated using the COBRA (8) assay, which showed correspondence with array analyses (Fig. 3). The PAM (11) software package was used to develop DMT classifiers discriminating FL and FH; it was found that these classifiers had perfect generalization properties (100% sensitivity and specificity) in PAM cross-validation tests. The PAM algorithm allows controlling the number of targets used in the final classifier by adjusting the threshold. By adjusting and reducing the target set size, it turned out that even very small sets of only two CpG targets were sufficient for perfect classification in the cross-validation tests. This excellent generalization property of the classifier was confirmed in a validation set of samples, as discussed in Materials and Methods. The successful classification of samples using a relatively small number of array CpG targets is promising for the future development of DMT-based assays for clinical molecular diagnostic application.
Figure 2.
A, dendrogram of unsupervised hierarchical classification of all samples in the study based on 259 DMT profile. Blue, FL; orange, MCD; yellow, lymphoblastoid cell lines; pink, PBL CD4+ T cells; purple, PBL CD8+ T cells; dark blue, PBL B cells; brown, reactive lymph nodes; black, noncancerous lymph node tissue adjacent lymphoma. Gray barbells, matched FFPE and frozen lymph node pairs that cosegregate. B, heatmap of unsupervised hierarchical cluster of 259 DMT β values from above cases. Same color code applies.
One limitation of methyl affinity–based methods (such as MDIP), as used in prior profiling studies, is their bias toward measurement of high-density CpG regions of the genome. Thus, not surprisingly, the present study identified many off-CGI DMT in genes not previously reported in any cancer, including FL. In addition, the present study identified numerous hypermethylated CGIs not revealed in prior CGI microarray studies of FL (12).
Initially, two additional samples (FL1 and FH9) were included in the analysis that did not generalize well in the classification. In these two cases wherein the methylation profile conflicted with the group index of FL or reactive hyperplasia, further analysis of pathologic features explained these discrepancies. The sample labeled FL1 was a noncancerous lymph node obtained from a patient with a history of FL; microscopic FL was present in a lymph node adjacent to the one that was profiled (13). FH9 was an abnormal lymph node diagnosed as consistent with the plasma cell variant of multicentric Castleman’s disease (MCD), negative for HHV-8. The idiopathic form of MCDis not well understood but is an immunosecretory disorder characterized by an excessive plasma cell proliferation in a background of reactive FH (14). These two samples were subsequently excluded from PAM identification of DMTs.
Finally, the cosegregation in the hierarchical cluster of a greater number of FFPE/frozen pairs of FL than FH samples (Fig. 2A) is consistent with a greater overall uniformity in the FH than FL group. In other words, the DMT profiles of FL lymph nodes are more individualized. Correlation of such individual variations in DMT profile in FL patients with clinical phenotype is warranted in future studies.
Validation set of FL and FH tissue samples
The matched FFPE and frozen samples included in the initial analysis were <3 years old. Because additional DNA damage may occur during routine archiving of FFPE specimens (e.g., oxidative damage), 25 additional FFPE samples including one B5-mercury fixed specimen, archived from 10 to 14 years (Supplementary Table S5), were subsequently analyzed. The additional cases included 11 FL and 14 FH and yielded comparable signal intensities and phenotype-dependent methylation profiles to the matched FFPE/frozen samples. The array methylation β values from this additional set were subjected to the PAM classifier, which resulted in correct classification of 24/25 (96%). This result indicates excellent generalizability of a DNA methylation–based PAM classifier. Additionally, unsupervised hierarchical clustering of the validation set based on the original 259 DMT profile (Supplementary Table S1 and Supplementary Table S2) resulted in correct segregation of all 25 samples according to pathologic status of FL versus FH (Supplementary Fig. S1).
Pattern of differential methylation in FLis divergent from normal lymphoid populations
Analysis of appropriate reference groups is an important consideration for comparative epigenetic research. In our study, we considered the possibility that FL DMTs could originate from enrichment for wild-type lineage-dependent epigenetic markings of the cell type of origin of the cancer, namely B cells. The FL DMT profile was, therefore, compared with that of purified populations of PBL B and T cells and transformed B-lymphoblastoid cell lines to assess for pathologic divergence of methylation as opposed to shifts in B-cell and T-cell composition. Pursuantly, the CGI hypermethylations observed in FL were absent in the B-cell and EBV-transformed B-cell line populations (Fig. 2B). Of further note, only 10 (<5%) of the 259 DMTs in FL versus FH show a significant difference in methylation between normal B and T cells, although 97 array targets are highly differentially methylated (Δβ >0.35; Supplementary Table S3) between B and T cells. Thus, the preponderance of hypomethylated and hypermethylated DMTs in FL represents epigenetic divergences from the wild-type lymphoid lineages analyzed and are not shifts in proportions of background T and B cells between FL and reactive lymph nodes. The analysis further showed that follicular lympho-magenesis and B-cell immortalization via EBV transformation are two largely distinct epigenetic processes. Whereas several hypo-methylations were shared by FL and EBV-transformed B cells, the majority of hypomethylations and nearly all hypermethylations in FL relative to FH are absent from B cells immortalized by EBV.
It is likely that the hypermethylations that distinguish FL from reactive hyperplasia are also divergences from bone marrow stem cells (BMSC). Bibikova and colleagues (15) recently reported a DNA methylation analysis of BMSC, differentiated somatic lineages and tissues, embryonic stem cells, and cancer cell lines. Globally, of the 370 genes profiled in their study, BMSC methylation was comparable with that of B-cell lines, and these two groups clustered together in an unsupervised analysis that included ES cells and numerous cancer cell lines. Roughly, 80% of the genes profiled in that study were also profiled in this study, albeit not at identical CpG target loci. Thus, comparison of the two data sets implies that the FL profile is largely divergent from BMSC.
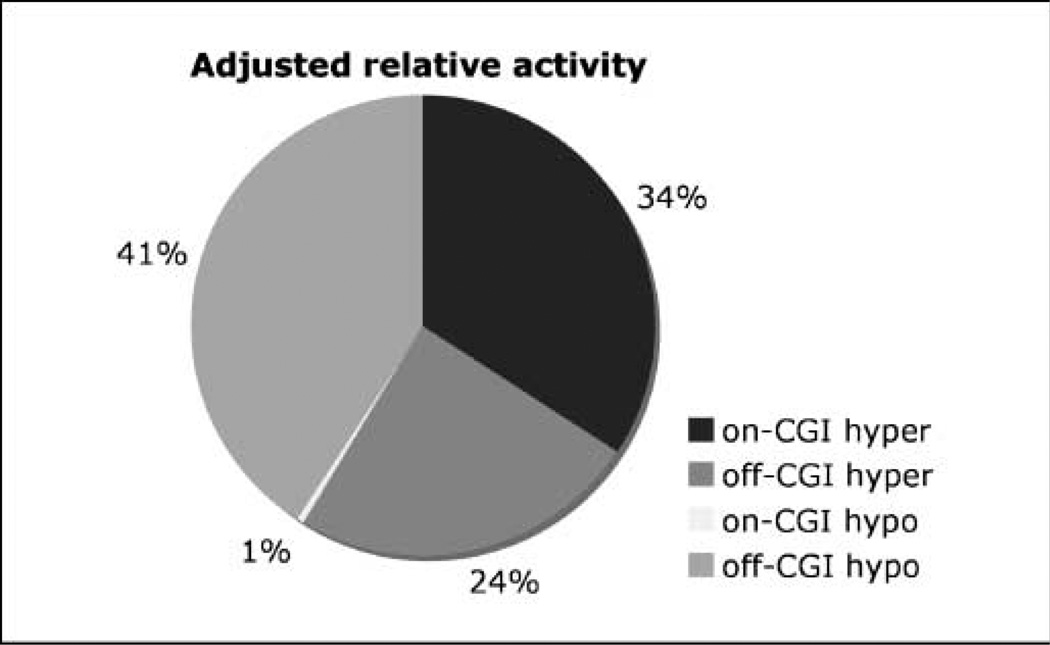
The large number of both CGI-positive and CGI-negative gene promoters profiled in our study permitted evaluation for a pathologic CGI bias in target hypermethylation in FL. Preliminary analysis suggested such a bias: 74% (136 of 184) of the hypermethylated targets were located inside CGI, significantly more than their fraction of targets on the entire array (43%, n = 649). However, rather than being specifically targeted for methylation, it is possible that CGI hypermethylation in FL is a mere reflection of the underlying kinetics. In noncancerous lymph nodes, CpGs manifest lower methylation states inside CGI; therefore, more targets can be further methylated. Inversely, there is a greater methylation “preload” for off-CGI targets, so comparatively fewer of these targets can be hypermethylated. The “activity coefficients” introduced in this study (see Materials and Methods) take these kinetic factors into account. Even with this correction, the methylation activity inside CGIs is twice that found at off-CGI targets. However, the most pronounced difference between on-CGI and off-CGI targets is not in their hypermethylation activity but in demethylation; hypomethylation activity is over one order of magnitude lower inside CGIs (Fig. 4).
Figure 4.
Relative methylation activities of CGI and non-CGI targets for hypermethylated and hypomethylated DMTs. This plot, adjusted for the greater methylation preload of non-CGI targets, reflects a roughly 2-fold greater CGI hypermethylation versus non-CGI hypermethylation, equivalent non-CGI hypomethylation and hypermethylation, and significant deficit of CGI demethylation in FL.
Thus, remodeling of methylome during follicular lymphoma-genesis involves extensive CGI hypermethylation and a deficit in CGI demethylation. This is in contrast to other methylation remodeling processes, such as early embryogenesis and germ cell development, in which methylation imprints on CGI are erased on a genomic scale. The methylation pattern in FL could point to a dysfunctional methylation process acting globally on the genome or a normal methylation process acting on a structurally/ epigenetically altered chromatin template. Whether the deficit of CGI methylation erasure is unique to FL among cancers, as well as the biochemical basis for this process, requires further investigation.
Correlation of gene expression and differential methylation
Emerging information from genome-wide DNA methylation profiling studies reveals widespread cancer CGI hypermethylation that involves numerous genes not implicated in cell growth or tumorigenesis (16). To explore the relationship in FL between gene expression and differential methylation, gene expression analysis was performed on a subset of the cryopreserved lymphoma (n = 8) and hyperplasia (n = 5) samples for which sufficient RNA could be extracted (data are available; GEO accession number GSE14214). Two different tests were used to determine the correlation between differential methylation and differential expression for proximate genes. No agreement was observed between the differentially expressed genes and the differential methylation state, as assessed by Pearson’s correlation coefficients and gene set enrichment analyses. By contrast, hypermethylated DMTs preferentially link to genes showing the least differential expression. The absence of differences was in part driven by the fact that differentially methylated genes were expressed at lower levels than unaffected genes, regardless of pathologic state. A random permutation test revealed not a single instance of differential expression (P < 0.01) between the median expression of the differentially methylated genes and the median expression of 100,000 randomly selected gene sets of the same size.
An important aspect of cancer epigenetics is the elucidation of how DNA methylation changes contribute to the malignant phenotype, including the consequences for the transcription of biologically critical genes. Remarkably, integration of the methylation data with gene expression analysis found not a single randomly selected set of differentially expressed genes that correlated with differential DNA methylation in FL. In large part, DMT neighbor genes are weakly expressed in both FL and reactive hyperplasia. In a recent study of diffuse large B-cell lymphoma, Pike and colleagues made a similar observation (17). In addition, comparable with our findings, Keshet and colleagues found that CGI hypermethylation in cancer occurs frequently at genes that are already repressed (16). The present analysis of FL further reveals that preferential hypermethylation of weakly expressed genes is not related to CGI status. The uniformity of the DMT profile among FL cases and the significant enrichment for nonexpressed/ nonlymphoid genes among the DMTs argue against a purely random process of pathologic methylation. At the same time, the results underscore the emerging concept in cancer tissue epigenetics that differential methylation can operate at the genomic level and may not correlate with a change in gene expression for individual targets.
To summarize the portrait of the FL methylome that emerges from these analyses, (a) FL manifests a pervasive shift in genomic DNA methylation relative to reactive hyperplasia, T cells, B cells, or bone marrow stem cells; (b) FL does not show any significant hypomethylation of CGI targets, despite the presence of numerous normally methylated CGI in reactive hyperplasia and B cells; (c) in contrast, non-CGI genome space contains significant pathologic DMTs, including a relatively large hypomethylation activity; (d) microanatomic B-cell compositional enrichment in FL does not contribute substantially to the identified DMT profile; (e) hypermethylated targets in an unselected FL cohort are those with minimal or no expression at baseline in benign lymph nodes and include numerous nonlymphoid genes; and finally, (f) the ability to analyze complex genomic patterns of differential methylation in archival FFPE pathology specimens will be a useful tool for biomarker discovery.
Acknowledgments
Grant support: Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research.
We thank Marbin Pineda, Theresa Davies-Hill, and Dr. Lynn Sorbara for technical assistance; Julie Stewart for help with figures; and Art Glatfelter, Shannon Harmon, Shyam Kalavar CT, Rachel Dove HT, Marie Mueller CTR, and Dr. Gene Passamani for facilitating pathology archive research.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 2.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 3.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci U S A. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oosting J, Lips EH, van Eijk R, et al. High-resolution copy number analysis of paraffin-embedded archival tissue using SNP BeadArrays. Genome Res. 2007;17:368–376. doi: 10.1101/gr.5686107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibikova M, Talantov D, Chudin E, et al. Quantitative gene expression profiling in formalin-fixed, paraffin-embedded tissues using universal bead arrays. Am J Pathol. 2004;165:1799–1807. doi: 10.1016/S0002-9440(10)63435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe ES. World Health Organization. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon Oxford: IARC Press; Oxford University Press (distributor); 2001. [Google Scholar]
- 7.Bibikova M, Lin Z, Zhou L, et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res. 2006;16:383–393. doi: 10.1101/gr.4410706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naef F, Socci ND, Magnasco M. A study of accuracy and precision in oligonucleotide arrays: extracting more signal at large concentrations. Bioinformatics. 2003;19:178–184. doi: 10.1093/bioinformatics/19.2.178. [DOI] [PubMed] [Google Scholar]
- 11.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahmatpanah FB, Carstens S, Guo J, et al. Differential DNA methylation patterns of small B-cell lymphoma subclasses with different clinical behavior. Leukemia. 2006;20:1855–1862. doi: 10.1038/sj.leu.2404345. [DOI] [PubMed] [Google Scholar]
- 13.Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002;99:3376–3382. doi: 10.1182/blood.v99.9.3376. [DOI] [PubMed] [Google Scholar]
- 14.Kojima M, Nakamura S, Nishikawa M, Itoh H, Miyawaki S, Masawa N. Idiopathic multicentric Castleman’s disease. A clinicopathologic and immunohistochemical study of five cases. Pathol Res Pract. 2005;201:325–332. doi: 10.1016/j.prp.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Bibikova M, Chudin E, Wu B, et al. Human embryonic stem cells have a unique epigenetic signature. Genome Res. 2006;16:1075–1083. doi: 10.1101/gr.5319906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshet I, Schlesinger Y, Farkash S, et al. Evidence for an instructive mechanism of de novo methylation in cancer cells. Nat Genet. 2006;38:149–153. doi: 10.1038/ng1719. [DOI] [PubMed] [Google Scholar]
- 17.Pike BL, Greiner TC, Wang X, et al. DNA methylation profiles in diffuse large B-cell lymphoma and their relationship to gene expression status. Leukemia. 2008;22:1035–1043. doi: 10.1038/leu.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]