Abstract
Social Safety Theory hypothesizes that developing and maintaining friendly social bonds is a fundamental organizing principle of human behavior and that threats to social safety are a critical feature of psychological stressors that increase risk for disease. Central to this formulation is the fact that the human brain and immune system are principally designed to keep the body biologically safe, which they do by continually monitoring and responding to social, physical, and microbial threats in the environment. Because situations involving social conflict, isolation, devaluation, rejection, and exclusion historically increased risk for physical injury and infection, anticipatory neural–immune reactivity to social threat was likely highly conserved. This neurocognitive and immunologic ability for humans to symbolically represent and respond to potentially dangerous social situations is ultimately critical for survival. When sustained, however, this multilevel biological threat response can increase individuals’ risk for viral infections and several inflammation-related disease conditions that dominate present-day morbidity and mortality.
Keywords: belonging, affiliation, evolution, inflammation, health, disease
INTRODUCTION
The question of how the social environment shapes human health and behavior is one of the most well-studied topics in the social and health sciences. Emerging from this large body of research is substantial evidence that exposure to life stress and adversity can negatively affect human functioning, disease risk, and longevity (Lupien et al. 2009, Shields & Slavich 2017). Despite clear evidence of these detrimental effects, however, there is little agreement about the types of social-environmental adversity that are most deleterious and no consensus regarding the social-psychological characteristics that make particular stressors stressful (Cohen et al. 2019, Epel et al. 2018). As a result of these basic conceptual and definitional problems caused in part by stress-nology (Slavich 2019), some theorists have gone so far as to argue that the term stress should be abandoned altogether (see Kagan 2016).
The purpose of this review is to address these fundamental issues in stress research by viewing the construct of life stress through a psychoneuroimmunological lens. By doing so, I aim to shed light on the specific dimensions of social-environmental adversity that are most important for health and behavior, the evolutionary reasons for why certain stressors likely have a greater impact than others, and the psychological and biological processes underlying these effects. Emerging from this analysis is the take-home message that, contrary to what classic theories of stress have suggested (e.g., Selye 1976), not all stressors are equally likely to impact health. Rather, forming and maintaining friendly social bonds appears to be a fundamental organizing principle of human behavior, and threats to what I have called “social safety,” in turn, are likely a key ingredient of stressors that most strongly affect psychological, biological, and clinical outcomes.
The idea that social attachment is important and that threats to social bonds can strongly affect people is not new. Indeed, a rich literature exists showing that interpersonal relationships are critical for survival (Ainsworth et al. 1978), that humans are fundamentally motivated to socially connect (Baumeister & Leary 1995), and that this basic motivation structures social cognition and behavior (Gilbert 2005). Several reviews have also explained how threats to social standing can affect health (Dickerson 2008, Kemeny 2009) and why social rejection and exclusion may be particularly potent in this regard (Allen & Badcock 2003, Williams 2007). Oddly, however, these social-psychological perspectives have not been well integrated into existing theories of stress, leading instead to two largely distinct literatures: one that conceptualizes stress in high resolution but less frequently assesses biological mechanisms or concrete health outcomes (e.g., social psychology) and a second that conceptualizes stress in lower resolution but more commonly assesses biological mechanisms and clinical diagnoses (e.g., psychiatry, clinical psychology).
To integrate these literatures in a manner that could help advance our understanding of life stress, health, and behavior, I first describe existing theories of stress that have sought to identify the most significant dimensions of this critically important construct. Second, I review key aspects of the human immune system and brain to help provide a biologically grounded perspective on life stress. Third, I build upon this understanding to propose a new framework called Social Safety Theory, which posits that forming and maintaining friendly social bonds (i.e., fostering social safety) is a fundamental organizing principle of human behavior and that threats to social safety (e.g., social conflict, isolation, rejection, exclusion) are a key feature of psychological stressors that most strongly impact health and behavior. Fourth, I summarize existing evidence for this theory and discuss several factors that should be taken into account in future research on the topic. Finally, I highlight some individual and collective strategies that may help promote social safety and reduce social threat.
HISTORICAL PERSPECTIVES ON LIFE STRESS
Theorists and philosophers have long grappled with the concept of life stress (Slavich 2016). In early Greek Mythology, for example, Sisyphus was condemned to eternally push a boulder up a hill only to have it roll back down every time he neared the top, thus conjuring up images of how a relentless stressor can cause endless frustration (Monroe & Slavich 2016). In more recent times, Charles Darwin (1859) and Claude Bernard (1865) detailed how organisms must adapt to ever-changing environmental circumstances to survive, and Sir Clifford Allbutt (1895) described how modernization and the industrial revolution were causing nervousness, disability, hysteria, and frightfulness. Then, during the twentieth century, Walter Cannon (1929) discussed for the first time how emotions have specific physiologic consequences that help the body maintain homeostasis during different social-environmental situations.
This early thinking set the stage for the more systematic study of how different stressors affect human physiology and health (Weiner 1992). A central figure in this work was the endocrinologist Hans Selye, who published more than 1,700 articles and 39 books on stress. Selye systematically investigated how different provocations affect physiology, and he is famous in part for having argued that stress is “the nonspecific response of the body to any demand” (Selye 1976, p. 74) and that a stressor is “that which produces stress” (Selye 1976, p. 78). This nonspecific view of social-environmental adversity had a tremendous impact on stress research, as most articles published over the past century subsequently conceptualized life stress as a single, unitary construct, with little attention paid to how different stressors might have varying consequences for health (Hammen 2005, Monroe 2008, Slavich et al. 2010a).
What Makes Stressors Stressful?
Several alternatives to Selye’s nonspecific view of life stress have since been proposed. One perspective, described by Holmes & Rahe (1967), argues that stressors can be ranked by the degree of change or upheaval that they typically cause, with greater change or upheaval in turn requiring greater adaptation. From this viewpoint, positive life events (e.g., marriage) and negative life events (e.g., divorce) are both stressful insofar as they each require adaptation. A second perspective, advanced by Maier & Seligman (2016) and Weiss & Simson (1985), focuses on the extent to which stressors are controllable or escapable. Stressors that are difficult to control or escape include chronic housing and interpersonal difficulties, and have been called entrapment stressors by others (see Brown et al. 1995). A third perspective, described by Lazarus & Folkman (1984), argues that stress arises when situational demands exceed an individual’s ability to adequately cope—for example, when a person has high job demands but little actual or perceived support or coping ability.
A fourth formulation, proposed by Clark & Beck (1999), is that stressors can be sorted into different life domains, such as “interpersonal” (e.g., intimate relationship stressors) and “achievement” (e.g., work-related stressors). A stressor’s impact, in turn, is heightened when a stressor matches the “content” of an individual’s cognitive vulnerability (e.g., a person with a rejection-sensitive negative cognitive schema gets fired). A fifth hypothesis, described by Gilbert & Allan (1998), argues that direct social conflict or competition that reduces social status, rank, value, or regard (i.e., defeat) is the most deleterious quality of stressors. This could occur when an individual has a major argument with a spouse or is subjected to persistent subordination or bullying. Finally, a sixth perspective, offered by Brown & Harris (1978), is that stressors are most impactful when they cause substantial cognitive upheaval or disruption to an individual’s goals, plans, or aspirations for the future (see also Carver & Scheier 1999). From this viewpoint, getting fired would be a severe stressor if (for example) a person worked for a company for a long time, had several coworkers as confidants, or had few other financial resources or job prospects (Brown & Harris 1978).
Conceptual Conflict and Confusion
Although these theories have guided thousands of studies on life stress, several problems are readily evident. First, with the exception of some minor similarities, these formulations provide very different, largely nonoverlapping explanations for why certain stressors are most impactful. Second, given how expensive and difficult it is to collect extensive biological data on humans as they experience different stressors, these theories are largely based on behavioral and clinical data, with researchers in turn assuming how different stressors might affect underlying biological processes. Instead of going from psychology to biology in this way, which has not been particularly fruitful, I propose we try the opposite. Let’s begin by asking what our biological systems care most about and then use this information to inform the conceptualization of life stress. To do this, I focus first on the human immune system and then on the brain.
THE HUMAN IMMUNE SYSTEM
Humans inhabit a world that is populated with numerous pathogenic microbes and toxins that are constantly evolving and challenging our homeostasis. The primary goal of the immune system, in turn, is to keep the body biologically safe and protected from these foreign invaders and from physical injuries that could cause illness or death if left unaddressed (Slavich & Irwin 2014). To accomplish this challenging task, the immune system relies on a complex regulatory logic that evolved over millennia and that fine-tunes itself based on input from the surrounding social, physical, and microbial environment. Surviving such an environment requires the immune system to be well calibrated to the specific types of threats that are most likely to be present. Consequently, the human immune system starts off relatively undifferentiated during infancy and becomes increasingly educated as a person ages. This process refines the functional capacity and regulation of each individual’s immune system and provides humans with a highly evolved biological defense network that is critical for survival (Rook et al. 2017).
Innate and Adaptive Immunity
The two interconnected branches of the immune system are the innate immune system and adaptive immune system. The innate immune system dates back to the origin of multicellularity and provides humans with an extremely rapid, highly conserved immunologic defense response that gets activated under conditions of microbial infection and tissue damage (Travis 2009). Protection from this first type of threat (i.e., microbial infection) is mediated by monocytes, macrophages, neutrophils, and dendritic cells that use invariant receptors to recognize conserved features of microbes, called pathogen-associated molecular patterns (PAMPs), which include lipopolysaccha-ride (LPS) (also known as endotoxin), unmethylated cytosine–guanine dinucleotide sequences in bacterial and viral genomes, and double-stranded RNA viruses (e.g., rotaviruses). This recognition strategy is termed pattern recognition, and innate immune receptors that use this strategy are called pattern recognition receptors (PRRs).
One of the most well-characterized families of PRRs is that of toll-like receptors (TLRs), which recognize molecular components of many different bacteria, viruses, and fungi. When an extracellular pathogen (e.g., bacteria) is detected, TLRs respond by initiating a highly conserved signaling cascade that activates the intracellular transcription factors nuclear factor-κB (NF-κB) and activator protein 1 (AP-1). These transcription factors induce the expression of proinflammatory immune response genes leading to the production of several inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α). In contrast, if the immunologic threat involves an intracellular pathogen (e.g., virus), then TLRs activate the transcription factors NF-κB, ATF2–c-Jun, interferon-regulatory factor 3, and interferon-regulatory factor 7, which induce antiviral immune response genes, particularly type I interferon genes, leading to the production of the inflammatory cytokines interferon-β and interferon-α (Kawai & Akira 2006). Together, these responses protect humans from a variety of microbes that can cause widespread infection or death if not addressed.
As noted above, the innate immune system also detects and responds to tissue damage. This response is mediated by endogenous molecules called damage-associated molecular patterns (DAMPs) that are released following cellular stress or death caused by tissue damage, bodily trauma, and ischemia. Similar to PAMPs, DAMPs can bind to and activate PRRs, especially TLR2 and TLR4, and in turn initiate a very rapid inflammatory response that is characterized by NF-κB-mediated increases in several inflammatory cytokines, especially IL-1β (Schaefer 2014). This type of innate immune response can occur in the absence of a pathogenic infection, which is why some have called it sterile inflammation, and it plays a critical role in helping individuals recover from tissue damage sustained during physical injury or bodily trauma (Crişan et al. 2016). Importantly, DAMPs are now recognized as a key promoter of systemic chronic inflammation, meaning that they may be especially relevant for understanding how life stress exerts persistent effects on human health, behavior, and age-related disease risk (i.e., in the absence of infection) (see Furman et al. 2019).
When these innate immune system defenses are insufficient for addressing a particular threat, the body engages the evolutionarily younger, 450-million-year-old adaptive immune system (Travis 2009). Compared with the innate immune response, which is nonspecific and occurs quickly, adaptive immunity develops over several days and involves the proliferation of microbial-specific lymphocytes that neutralize or eliminate microbes based on an immunological memory of having responded to a specific pathogen or antigen in the past. This response is initiated by antigen-presenting cells (APCs), such as macrophages or dendritic cells, that help the system differentiate between the host’s own cells (i.e., self) and those of invading bacteria or viruses (i.e., nonself or foreign). These APCs are attracted to sites in the body where they ingest and process invading antigens. Once processed, APCs migrate from the infection site to local lymph nodes where they present antigen peptides to T helper cells, resulting in the release of several cytokines including IL-2, IL-4, IL-5, and interferon gamma (IFN-γ) (Slavich 2020).
Cellular Soldiers Awaiting Deployment
Many factors determine the ultimate effectiveness of the human immune response, but few are more critical than timing. In metaphorical terms, the immune system is a highly trained “biological army,” and immune cells are cellular soldiers that hang out in their “barracks” (i.e., the spleen, bone marrow, and lymph nodes) until they are called to duty. Once they receive word of a threat, they leave their barracks and enter the “boulevards” (i.e., the bloodstream) and migrate to the “battlefield” (i.e., the site of tissue damage or infection), which could be anywhere in the body (Dhabhar et al. 2012). With an actual army, the earlier soldiers are deployed, the more likely they are to contain the invading enemy, retain control of their territory, and survive. I have argued that the same is true of the immune system (Slavich 2020, Slavich & Auerbach 2018, Slavich & Cole 2013, Slavich & Irwin 2014). Historically, individuals who regularly deployed immune cells to relevant bodily compartments in advance of tissue damage occurring would have been most likely to survive. Consequently, the capacity to carefully monitor the surrounding environment, detect early signs of social threats that could have increased risk for physical conflict or injury, and mount an anticipatory inflammatory response to such threats was likely highly conserved.
NEURAL NETWORKS REPRESENTING SOCIAL SAFETY
Because the immune system cannot directly detect social circumstances that may increase risk for physical conflict or injury, it relies on the brain. Mounting an anticipatory inflammatory response to potential physical danger requires continually monitoring the extent to which the body is in a socially safe environment, and four neural networks support this capacity: the amygdala network, mentalizing network, empathy network, and mirror neuron system (Kennedy & Adolphs 2012). The amygdala network plays a central role in detecting, decoding, and interpreting social signals (e.g., eye gaze, facial and group identity, social rank, trustworthiness); motivating prosocial and affiliative behaviors like cooperation, altruistic behavior, and interpersonal connection; and modulating apprehensiveness and aversion to untrustworthy, uncooperative, and unfair individuals (Bickart et al. 2014). The amygdala represents the hub of this network and projects to several other brain regions, including the ventromedial prefrontal cortex, superior temporal sulcus, caudal and rostral anterior cingulate cortex, insula, fusiform gyrus, temporal pole, striatum, hypothalamus, medial temporal lobe, and brainstem nuclei. The mentalizing network, in turn, subserves thinking about the thoughts, feelings, intentions, and beliefs of others and includes the dorsomedial prefrontal cortex, medial prefrontal cortex, precuneus/posterior cingulate cortex, temporoparietal junction, and posterior superior temporal sulcus (Frith & Frith 2006). The empathy network underpins prosocial concern, communication, and behavior and includes the brainstem, amygdala, hypothalamus, striatum, insula, anterior cingulate cortex, and orbitofrontal cortex (Decety 2015). Finally, the mirror neuron system enables individuals to simulate others’ behavior to better understand their goals, emotions, and actions and includes the dorsal and ventral premotor cortex, inferior frontal gyrus, and inferior and superior parietal lobule (Rizzolatti & Sinigaglia 2016).
Together, these neural networks form the so-called social brain (Dunbar & Shultz 2007). The social brain has enabled humans to create and survive in complex social networks, and it does so by subserving two complementary, evolutionarily adaptive goals. First, it motivates individuals to foster social safety by developing and maintaining social bonds with people who are friendly, helpful, predictable, dependable, sincere, and trustworthy (Fiske et al. 2007). Second, it enables humans to detect and mount an anticipatory inflammatory response to social threat, defined as actual or perceived social circumstances that historically increased risk for physical danger (e.g., social conflict, aggression, devaluation, isolation, rejection, exclusion) (Slavich & Irwin 2014).
NEURAL–IMMUNE COMMUNICATION
Multiple bidirectional pathways enable the brain to signal the peripheral immune system and vice versa during times of social threat. These pathways include the sympathetic nervous system (SNS), hypothalamic–pituitary–adrenal (HPA) axis, vagus nerve, and meningeal lymphatic vessels.
Sympathetic Nervous System
The SNS can upregulate proinflammatory cytokine activity by releasing the neurotransmitter norepinephrine into peripheral tissues, primary and secondary lymphoid organs, and most visceral organs and musculoskeletal structures (Irwin & Cole 2011). Activation of the SNS also causes the adrenal glands to release epinephrine into the circulation. Epinephrine and norepinephrine in turn modulate immune response gene transcription via β-adrenergic receptor (βAR) and, possibly, α-adrenergic signaling. This signaling cascade suppresses transcription of antiviral type I interferon genes (e.g., IFNA, IFNB) and upregulates transcription of proinflammatory immune response genes (e.g., IL1B, IL6, TNF) via NF-κB, AP-1, and CREB and GATA family transcription factors, leading to increased inflammatory and decreased antiviral activity (Irwin & Slavich 2017).
Hypothalamic–Pituitary–Adrenal Axis
The HPA axis regulates peripheral proinflammatory cytokine activity primarily via the glucocorticoid cortisol. Cortisol can reduce both inflammatory gene expression (e.g., IL1B, IL6, TNF) and antiviral gene expression (e.g., IFNA, IFNB) by suppressive binding to gene promoter sequences, inhibiting the proinflammatory function of NF-κB, increasing the expression of the inhibitor of NF-κB (IκB), and antagonizing proinflammatory gene transcription via protein–protein interactions between the glucocorticoid receptor and NF-κB in the nucleus of immune cells (Irwin & Cole 2011). However, cortisol can also lead to increased inflammatory activity by priming heightened inflammatory reactivity, stimulating immune cell trafficking, and promoting glucocorticoid insensitivity/resistance (Sorrells et al. 2009).
Vagus Nerve
In addition to regulating heart rate, dietary intake and metabolism, gastrointestinal activity, and other visceral functions, the vagus nerve governs systemic inflammatory activity. The efferent arm of the vagus nerve originates from four nuclei in the medulla oblongata (i.e., the nucleus ambiguus, solitary tract nucleus, dorsal motor nucleus of the vagus nerve, and spinal trigeminal nucleus) and is activated by muscarinic acetylcholine receptor–mediated mechanisms. It outputs to several organs including the heart, liver, gastrointestinal tract, intestine, and (possibly) the spleen, where it downregulates the production of the inflammatory cytokines IL-1β, IL-18, and TNF-α via alpha7 nicotinic receptors that inhibit NF-κB activity (Thayer & Sternberg 2010). In contrast, the afferent arm of the vagus senses peripheral inflammatory molecules via afferent vagus nerve fibers and conveys an anti-inflammatory signal to neurons residing in the nodose and jugular ganglia that terminate in the nucleus tractus solitarius and medulla oblongata. These regions in turn project to the brainstem nuclei, hypothalamus, and forebrain, which integrate visceral sensory information and coordinate autonomic and behavioral responses (Pavlov & Tracey 2012).
Meningeal Lymphatic Vessels
Finally, although the brain and peripheral immune system have historically been thought of as physically separate systems, two landmark studies recently revealed that the brain is directly connected to the periphery via meningeal lymphatic vessels that were previously not known to exist (Aspelund et al. 2015, Louveau et al. 2015). These vessels help drain macromolecules and excess fluid from the central nervous system (CNS). However, they also represent a physical path along which immune cells can travel from the CNS to the periphery, where they can upregulate systemic inflammatory activity.
Considered together, these four biological pathways (i.e., the SNS, HPA axis, vagus nerve, and meningeal lymphatic vessels) enable what we have previously referred to as social signal transduction—namely, the transmission of signals from the external social environment into the internal biological environment that shapes human health and behavior (Slavich & Cole 2013, Slavich & Irwin 2014, Slavich & Sacher 2019).
SOCIAL SAFETY THEORY
When combined, this information regarding the structure and function of the human immune system and brain can help refine our understanding of life stress and form the basis for a new perspective on stress, behavior, and health, called Social Safety Theory. From this viewpoint, humans have long inhabited a world filled with physical and microbial threats—including dangerous conspecifics, viruses, and bacteria—that can cause death and reduce reproductive success if not avoided or dealt with effectively. Physical wounding threatens survival directly (e.g., through injury-induced bleeding) but also indirectly by enabling pathogens to enter the body through wounds created during social conflict. Individuals who fostered greater social safety and who mounted anticipatory inflammatory responses to social threat would have been most likely to survive these challenging circumstances and pass on their genes. Consequently, a fundamental drive to develop friendly social bonds, and to mount a systemic inflammatory response to social cues historically associated with increased physical danger, was likely highly conserved.
Based on this understanding, we can reconceptualize impactful life stressors as acute or chronic exposures that were most likely to have increased individuals’ risk for physical danger over the course of evolution. Atop this list would be actual physical danger, such as being physically attacked, abused, or neglected. However, there are many other social circumstances a bit lower on the severity gradient that are also relevant, including social conflict, aggression, devaluation, discrimination, isolation, rejection, and exclusion (Slavich & Irwin 2014, Slavich et al. 2010a). Each of these experiences threatens social safety in a different way and can therefore engage the social signal transduction pathways described above, leading to substantial changes in disease risk, cognition, and behavior. The theory is graphically depicted in Figure 1.
Figure 1.
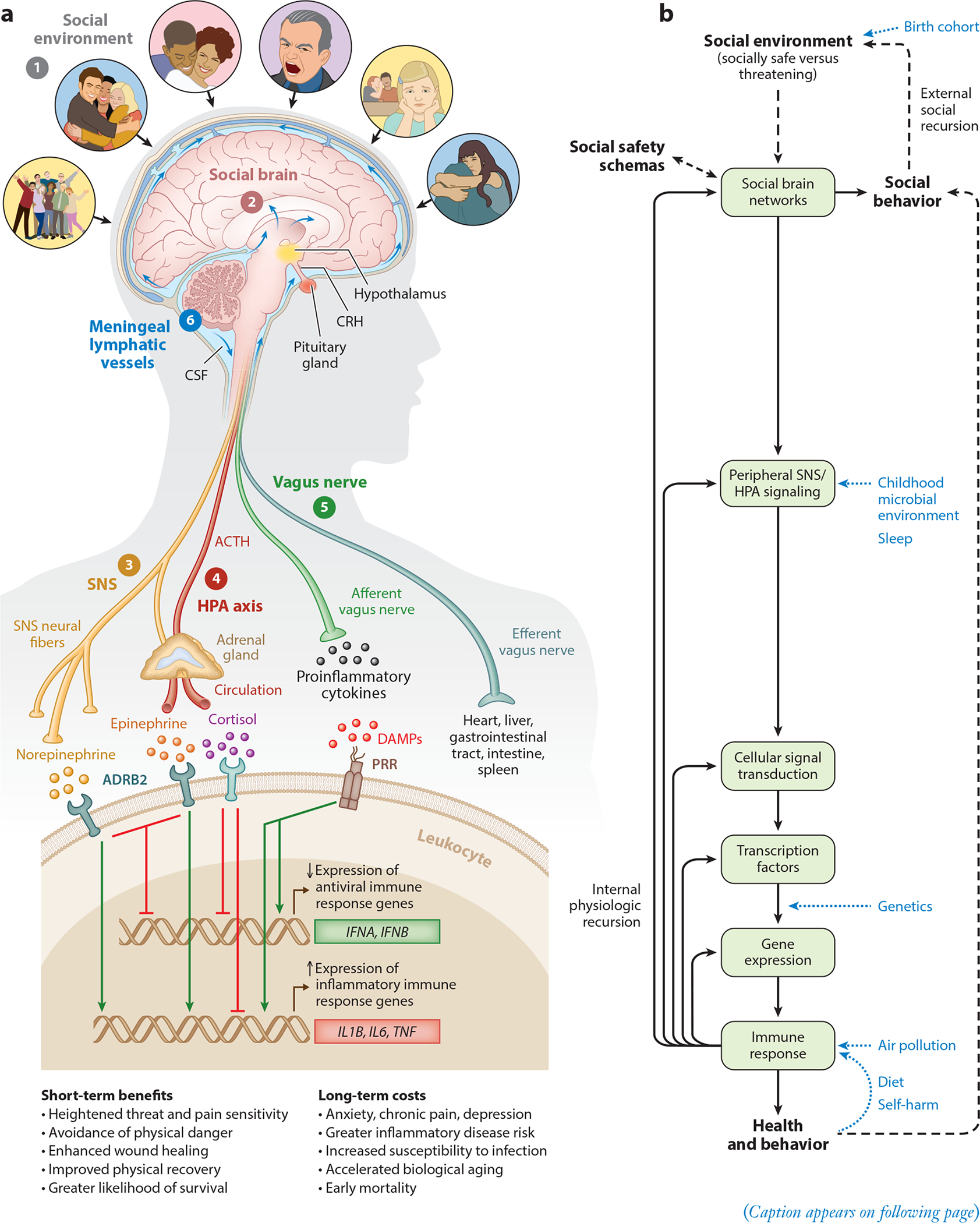
Social Safety Theory is grounded in the understanding that the primary purpose of the human brain and immune system is to keep the body biologically and physically safe. To accomplish this challenging task, humans developed a fundamental drive to create and maintain friendly social bonds and to mount anticipatory biobehavioral responses to social, physical, and microbial threats that increased risk for physical injury and infection over the course of evolution. (a) Accordingly, the brain continually monitors the (❶) social environment, interprets social signals and behaviors, and judges the extent to which its surroundings are socially safe versus threatening. These appraisals are subserved by the (❷) amygdala network, mentalizing network, empathy network, and mirror neuron system (i.e., the social brain). When a potential social threat is perceived, the brain activates a multilevel response that is mediated by several social signal transduction pathways—namely, the (❸) SNS, (❹) HPA axis, (❺) vagus nerve, and (❻) meningeal lymphatic vessels. These pathways enable the brain to communicate with the peripheral immune system and vice versa. Whereas the main end products of the SNS (i.e., epinephrine and norepinephrine) suppress transcription of antiviral type I interferon genes (e.g., IFNA, IFNB) and upregulate transcription of proinflammatory immune response genes (e.g., IL1B, IL6, TNF), the main end product of the HPA axis (i.e., cortisol) generally reduces both antiviral and inflammatory gene expression but also can lead to increased inflammatory gene expression under certain physiologic circumstances (e.g., glucocorticoid insensitivity/resistance). The vagus nerve in turn plays a putative role in suppressing inflammatory activity, whereas meningeal lymphatic vessels enable immune mediators originating in the CNS to traffic to the periphery, where they can exert systemic effects. (b) This multilevel “Biobehavioral Response to Social Threat” is critical for promoting well-being and survival. However, it can also increase risk for negative health and behavioral outcomes when it is sustained by internal physiologic or external social recursion. Several factors can also moderate these effects, including birth cohort, childhood microbial environment, sleep, genetics, air pollution, diet, and self-harm behavior. A person’s developmentally derived social safety schemas play a particularly important role in this multilevel process as they shape how social-environmental circumstances are appraised. Social safety schemas thus influence neurocognitive dynamics that initiate the full range of downstream biological interactions that ultimately structure disease risk and human behavior. Abbreviations: ACTH, adrenocorticotropin hormone; ADRB2, β2-adrenergic receptor; CNS, central nervous system; CRH, corticotropin-releasing hormone; CSF, cerebrospinal fluid; DAMPs, damage-associated molecular patterns; HPA, hypothalamic– pituitary–adrenal; PRR, pattern recognition receptor; SNS, sympathetic nervous system. Adapted with permission from Slavich & Cole (2013), SAGE Publishing; Slavich & Irwin (2014), American Psychological Association; and Slavich & Sacher (2019), Springer Nature.
Cytokines as Critical Mediators
Cytokines play a central role in structuring these social safety–related effects, as they mediate numerous physiologic, immunologic, cognitive, and behavioral processes. In addition to coordinating immune cell mobilization, signaling, and trafficking, for example, cytokines can affect threat and pain sensitivity, exploratory and pleasure-seeking behavior, ingestive behavior, sleep, learning, attention, memory, impulsivity, temporal focus, emotion regulation, executive function, and cognitive control (Larson & Dunn 2001, Shields et al. 2017). Indeed, cytokine receptors are present on virtually all neural cell types, and recent animal model work has shown that they play a critical mechanistic role in linking neuronal connectivity, immunity, and social behavior (Filiano et al. 2016).
Social Safety Schemas
The ability to recall past social interactions and to imagine potential future events is both a blessing and a curse in this regard. On the one hand, this unique neurocognitive capacity is what enabled humans to develop highly complex social networks based on coordination and cooperation in order to realize collective achievements that nonhuman primates never could (Barrett 2017, Silk 2007). On the other hand, though, symbolic, imagined, and perceived social threats can activate the exact same immunologic defense programs that evolved to combat actual physical threats, meaning that our neurocognitive ability to imagine threat can initiate biologically powerful processes even when they are not needed for survival (Slavich & Cole 2013). These activations can occur intermittently—for example, when engaged by occasional negative automatic thoughts about a potential threat in the environment—but they can also become prolonged, mediated by one’s social safety schemas about the self, social world, and future (see the sidebar titled Social Safety Schemas).
SOCIAL SAFETY SCHEMAS.
Social safety schemas are hypothesized to develop during childhood and adolescence based on a person’s perceptions of the self, social world, and future. Such perceptions are shaped by the actual situations that people encounter (e.g., abuse, bullying, social exclusion) and by the meaning and messages that individuals and their caregivers attribute to socially salient events (e.g., “You can’t handle it,” “Other people can’t be trusted,” “You’re going to be alone”). These schemas in turn play critical roles in structuring attitudes, expectations, beliefs, and behaviors across the life span. Most relevant for Social Safety Theory are individuals’ beliefs regarding whether other people generally are friendly versus hostile, predictable versus unreliable, supportive versus critical, helpful versus hurtful, and sincere versus manipulative (i.e., social world schemas). Beliefs regarding one’s own ability to cope with threats (i.e., self schemas) are also important, as are people’s expectations regarding their likelihood of experiencing future social isolation, failure, and danger (i.e., future schemas). Together, these beliefs shape not just occasional thoughts and emotions but also how individuals navigate their social worlds, the types of relationships people develop, and how their brains and immune systems respond to positive, negative, and ambiguous social circumstances.
Adaptive Versus Maladaptive Responsivity
The question of whether these responses are helpful versus harmful ultimately comes down to the issue of timing and regulation (Slavich 2015). Although upregulating inflammatory activity is highly beneficial in response to actual physical or microbial danger, negatively biased perceptions of the self, social world, or future can unnecessarily activate and/or prolong this response, as can navigating the social environment in a manner that engenders chronic interpersonal stress (i.e., stress generation) (Hammen 2005). In addition, several stress-induced biological changes can sustain the activation of these pathways. First, for example, past adversity can cause epigenetic modification of the glucocorticoid receptor gene in the neural transcriptome, especially in the hypothalamus and amygdala, thereby degrading HPA axis regulation of the inflammatory response (McGowan et al. 2009). Second, adversity can lead to epigenetic reprogramming of innate immune cells (Crişan et al. 2016) and alter the hematopoietic output of these cells from the bone marrow (Hanke et al. 2012), skewing them toward a more proinflammatory state. Finally, adversity can trigger increased arborization of SNS fibers in the lymph node, which expands the neural–immune regulatory pipeline and may promote neuro-inflammatory sensitization to adversity that sustains perceptions of social threat for months or years after an initial triggering event has passed (see Slavich & Irwin 2014). For optimum benefit, this multilevel “Biobehavioral Response to Social Threat” should only be engaged intermittently and during actual threat. Otherwise, the response can heighten a person’s risk for viral infections and numerous inflammation-related conditions including anxiety, depression, asthma, heart disease, stroke, and cancer, as well as several metabolic, autoimmune, and neurodegenerative disorders (Furman et al. 2019).
EVIDENCE SUPPORTING SOCIAL SAFETY THEORY
Current broad support for Social Safety Theory comes from several fields, including anthropology, psychology, sociology, epidemiology, and public health. Below, I summarize existing evidence for its three main tenets:
Humans evolved to foster social safety.
Social safety is beneficial for human health and behavior.
Social threat is harmful for human health and behavior.
Tenet 1: Humans Evolved to Foster Social Safety
Research on social support represents one of the messiest literatures in psychology, as there is virtually no agreement regarding what humans evolved to want or need from others, or what type of support is most beneficial. Is it emotional support to bolster our sense of self-worth, instrumental support to assist with tangible tasks, or informational support to increase knowledge? From the perspective of Social Safety Theory, the answer is straightforward: What matters most is a stable sense of social inclusion, belonging, and connection to others who are friendly, emotionally supportive, and dependable. Put simply, immune cells care about physical and social safety, not about a person’s financial resources or the size of his or her social network (what good is a large social network if it includes numerous dangerous conspecifics?).
As it turns out, comparative, developmental, neurobiological, and paleoanthropological research provides highly compelling, converging evidence that humans evolved to foster friendly, empathic relationships with one another (Decety 2015, Hare 2017). Evidence of natural selection for prosociality first emerged about 2.6 million years ago in the Paleolithic period and it drove human self-domestication, enabling people to live, hunt, and gather together in small bands (Henrich 2015). This so-called human self-domestication is subserved by several abilities that Homo sapiens developed over time, including the capacity to mentalize, perspective take, cooperatively communicate, experience others’ distress, tolerate social conflict, and exert self-control over hostile and aggressive impulses (Hare 2017). The neural mentalizing network and neuropeptide oxytocin played particularly important roles in generating prosocial behavior, as they enabled humans to experience compassionate feelings and empathy toward others (Carter 2014, Kennedy & Adolphs 2012). Ultimately, these abilities led to a strong preference for others who are friendly, emotionally warm, and socially safe (Hare 2017).
Humans can also act antisocially, of course. Rather than being contradictory, though, such aggression is believed to be the by-product of natural selection for in-group prosociality (Choi & Bowles 2007). Indeed, humans have a well-known affinity for those most like themselves and a tendency to be hostile toward strangers (Bloom 2013), which historically enhanced their reproductive success and helped ensure their survival (Silk 2007).
Tenet 2: Social Safety Is Beneficial for Human Health and Behavior
In addition to research demonstrating that fostering and maintaining social safety is highly conserved, there is a large literature showing that this tendency to connect confers substantial benefits for human health and behavior. In a landmark meta-analysis that reviewed data from 148 studies and 308,849 participants who were followed for an average of 7.5 years, for example, being socially well integrated was associated with a 91% increase in odds of survival [odds ratio (OR) =1.91; 95% confidence interval (CI) [1.63, 2.23]] (Holt-Lunstad et al. 2010). These beneficial effects are similar in magnitude to quitting smoking and exceed those associated with traditional risk factors for mortality, such as obesity and physical inactivity. Consistent with the immunologic mechanisms identified by Social Safety Theory, a subsequent meta-analysis examined data from 41 studies and 73,037 participants and found that being socially well integrated was associated with lower levels of the inflammatory markers TNF-α, IL-6, C-reactive protein (CRP), and fibrinogen (Zr = −0.076; 95% CI [−0.11, −0.04]) (Uchino et al. 2018). Beneficial effects such as these are often strongest for social integration and belonging but also extend to other indicators of social safety including socioeconomic status and subjective social status, which are strong predictors of all-cause mortality (see Adler & Rehkopf 2008, Muscatell et al. 2018).
Such beneficial effects are evident in a diverse array of clinical contexts as well and are often independent of other risk factors affecting health (Vogt et al. 1992). For example, individuals with greater social safety exhibit better outcomes in the context of acute myocardial infarction (Mookadam & Arthur 2004), amputation (Hawkins et al. 2016), acquired brain injury (Jones et al. 2011), substance dependence treatment (Haslam et al. 2019), HIV/AIDS (van Luenen et al. 2018), stroke (Haslam et al. 2008), and heart disease and cancer (Valtorta et al. 2016). In one study of 168 women with histologically confirmed epithelial ovarian cancer, for example, having a socially safe relationship characterized by a stable sense of emotional security was associated with a 34% lower hazard of death during the 7-year study period (Lutgendorf et al. 2012).
Finally, with respect to behavior, a wealth of research has shown that social safety is associated with greater occupational and scholastic achievement, perseverance, and productivity (Holt-Lunstad 2018, Strayhorn 2018) as well as with more volunteering and fewer sick days (Babey et al. 2019). Social safety is also related to practicing better health behaviors, which in turn predicts numerous behavioral and health-related outcomes including longevity (Umberson et al. 2010). The relevance of social safety for human health and behavior is thus difficult to overestimate (Kiecolt-Glaser & Wilson 2017, Pietromonaco & Collins 2017).
Tenet 3: Social Threat Is Harmful for Human Health and Behavior
Conversely, perceived forms of social threat like loneliness—and actual social threats like social conflict, isolation, devaluation, rejection, and exclusion—are among the most powerful predictors of poor health and harmful behaviors. The literature on this topic is sizable and has shown that the increased likelihood of mortality over time is around 26% for low socioeconomic status (OR =1.26; 95% CI [1.21, 1.32]), 26% for perceived loneliness (OR = 1.26; 95% CI [1.04, 1.53]), 29% for social isolation (OR = 1.29; 95% CI [1.06, 1.56]), and 32% for living alone (OR = 1.32; 95% CI [1.14, 1.53]) in adjusted models that control for relevant demographic and clinical risk factors (Holt-Lunstad et al. 2015, Stringhini et al. 2017). These social-environmental effects translate into a rather striking reduced life expectancy of around 2.1 years by age 40, thus exceeding the mortality risk associated with high alcohol intake, obesity, and hypertension (Stringhini et al. 2017).
Epidemiologic studies are not designed to discern exactly what about social threat is most harmful for health, but psychological research has pointed to a combination of interpersonal loss and social rejection as being particularly deleterious (Slavich et al. 2010a). Consistent with Social Safety Theory, these so-called targeted rejection stressors hasten onset of depression (Slavich et al. 2009), promote suicidal ideation (Massing-Schaffer et al. 2019), upregulate molecular signaling pathways that increase inflammatory activity (Murphy et al. 2013), and downregulate molecular signaling pathways that reduce inflammatory activity (Murphy et al. 2015). Likewise, being exposed to sustained social threat characterized by verbal attacks, social exclusion, devaluation, and aggression heightens inflammatory reactivity to social stress (Giletta et al. 2018), promotes an increased proinflammatory/reduced antiviral skewing of the leukocyte basal transcriptome that is structured by the social signal transduction pathways described previously (i.e., NF-кB, AP-1, CREB, and the glucocorticoid receptor) (Thames et al. 2019), and accelerates biological aging as indexed by telomere length (Guarneri-White et al. 2018).
Social threat also greatly impacts behavior. For example, it can promote anger, procrastination, aggression, and withdrawal as well as a variety of externalizing behaviors, such as fighting, cursing, cheating, rule breaking, and stealing (Leary 2001). Actual or perceived social threat can also reduce prosocial behaviors, including helping, donating, and cooperating (Twenge et al. 2007). Therefore, whereas social safety confers numerous benefits, social threat can degrade mental and physical health, lead to a variety of negative behaviors, and increase risk for biological aging and early mortality (see the sidebar titled Social Safety and Psychopathology).
SOCIAL SAFETY AND PSYCHOPATHOLOGY.
Social Safety Theory hypothesizes that maximizing social safety and minimizing social threat made humans exquisitely sensitive to social information and created a deep motivation to foster, maintain, and restore social safety whenever possible. Positive social safety schemas provide individuals with a favorable sense of the self, social world, and future that promotes a stable feeling of social connection, affiliation, inclusion, and belonging. In contrast, negative social safety schemas give rise to thoughts and feelings about the self, social world, and future that can oscillate or change in response to varying social feedback and circumstances. Whereas positive social safety schemas enable normative psychosocial development and the formation of healthy interpersonal relationships, negative social safety schemas promote pathological thoughts and attempts to maintain social safety and relevance and are a core social-cognitive characteristic of many forms of severe psychopathology—whether it be an individual with borderline personality disorder who seeks excessive reassurance from others, a person with narcissistic personality disorder who continually exaggerates their import, or someone experiencing delusions who places him or herself at the center of a never-ending conspiracy theory or investigation. Indeed, disturbances in one’s social safety schemas may be key to understanding abnormal cognitive, emotional, and behavioral patterns that are central to a variety of different psychiatric disorders.
FACTORS AFFECTING SOCIAL SAFETY
Looking forward, research on social safety will need to account for several factors that can moderate the activity of the social signal transduction pathways described above, which may in turn have implications for experiences of social safety and threat. These factors include several usual suspects like genetics, sleep, and diet, but also a handful of less obvious social-environmental and behavioral determinants such as childhood microbial environment, birth cohort, air pollution, and self-harm behavior.
Genetics
As reviewed by Slavich & Cole (2013), variation in the human genome plays a critical role in determining the likelihood that neurocognitive perceptions of social safety and threat get converted into health- and behavior-altering biology. So far, this research on human social genomics has identified several genetic polymorphisms that moderate individual differences in biological sensitivity to social context that have downstream consequences for human disease risk and behavior. In a recent study of 420 adolescents, for example, we found that youths’ likelihood of developing depression following a socially painful targeted rejection life event was moderated by a functional single nucleotide polymorphism (SNP) in the μ-opioid receptor gene (OPRM1, rs1799971) that causes an amino acid change (N40D) that governs central OPRM1 expression, leading to differences in social and physical pain sensitivity (Slavich et al. 2014). Whereas G allele carriers were twice as likely to meet criteria for major depressive disorder following a recent severe targeted rejection life event (e.g., because they exhibit less opioid receptor expression and signaling efficiency), A/A homozygotes were completely unaffected by such stress.
In another study, Cole et al. (2010) examined a functionally active regulatory SNP in the human IL6 promoter (−174G>C, rs1800795) that is known to affect inflammatory dynamics. First, they examined the molecular signaling pathways that might transduce experiences of social threat and identified the GATA1 transcription factor as a mediator of SNS/βAR signaling. Based on this in vitro finding, the researchers then turned to a longitudinal dataset and found that older adults experiencing high levels of social adversity who were homozygous for the GATA1-sensitive G allele died 2.8 years sooner (on average) than their counterparts bearing the GATA1-insensitive C allele. Moreover, this SNS/βAR-driven IL6 gating effect on mortality was evident only for inflammation-related causes of death—namely, cardiovascular disease, Alzheimer’s disease, and cancer. Differences in IL6 genotype thus appear to significantly moderate the social threat–health link. Whereas high SNP-related DNA binding affinity facilitates social signal transduction and the upregulation of IL6 gene expression with corresponding consequences for inflammatory disease– related mortality, low binding affinity caused by this SNP effectively eliminates the negative impact that social threat would otherwise have on health (for a discussion, see Slavich & Cole 2013).
These studies provide illustrative examples of how certain SNPs can moderate the effects that friendly and threatening social environments have on human health and behavior. Other potentially relevant functional SNPs include those in the promoters of the inflammatory cytokine genes IL1B, IL8, and TNF, which help combat bacteria and other extracellular pathogens, and the antiviral immune response genes IFNA and IFNB, which target intracellular pathogens such as viruses. Yet other relevant SNPs include those in BDNF such as Val66Met, which codes for the brain-derived neurotrophic factor protein and has been implicated in numerous cognitive, emotional, and neuropsychiatric outcomes, and in CRHR1 and FKBP5, which govern corticotropin-releasing hormone activity and glucocorticoid receptor sensitivity and are therefore essential for social signal transduction.
Ultimately, this summary provides only a very limited glimpse into genetic factors that are relevant for Social Safety Theory. Other genetic loci and epigenetic processes that affect transcription factor binding affinity (e.g., methylation, histone modification) are also relevant. Moreover, given that anticipating and preemptively deploying biological defenses is critical for survival, it is possible that much of the genetic influence governing human transcriptional responses to social threat now resides in nonpolymorphic regions of the human genome (Slavich & Cole 2013).
Sleep
It will also be important to take sleep into account in studies of social safety. Sleep is a critical human behavior that plays a central role not just in everyday life but in regulating biological processes that affect social cognition, health, and behavior. Sleep disturbance (e.g., difficulty falling or staying asleep, nonrestorative sleep, early-morning awakening) is a strong predictor of both inflammation-related disease risk and all-cause mortality (Irwin 2019). Although sleep disturbance is relatively common in general, with up to 48% of adults reporting at least some sleep complaints, it is especially prevalent among persons exposed to stressors that threaten social safety, such as interpersonal violence and aggression (Gallegos et al. 2019).
Sleep quality and duration can affect experiences of the social environment by influencing the activity of the HPA axis and SNS, which in turn regulate the immune system. Whereas high-quality sleep is typically associated with less HPA axis and SNS activity—with corresponding reductions in cortisol, epinephrine, and norepinephrine—poor sleep disrupts this natural “relaxing” of these two major axes, leading to elevations in HPA axis and SNS activity that promote NF-κB-and β2-adrenergic receptor–mediated proinflammatory gene expression and decreased antiviral gene expression (Irwin 2019). The resulting release of IL-1β, IL-6, and TNF-α heightens threat sensitivity that can degrade experiences of social safety and increase perceptions of social threat.
Poor sleep can thus have direct biological effects that shape social safety. However, sleep may also interact with naturally occurring social threats to regulate biological pathways that influence health and behavior. In a recent prospective study that measured sleep duration and proinflammatory and antiviral gene expression in 87 adolescents, for example, greater daily experiences of social threat (e.g., arguments with parents, family members, teachers, or friends; punishment; being insulted or threatened) interacted with shorter sleep duration to predict reduced expression of 511 genes and increased expression of 1,894 genes including several that promote inflammatory activity, such as TNF, IL1RAP, IL2RB, and IL15RA. Promoter-based bioinformatics analyses in turn indicated that these gene expression changes were structured by increased NF-κB activity (Chiang et al. 2019).
Links between sleep, inflammatory biology, and social safety are ultimately highly interactive and complex. For example, poor sleep (e.g., staying up all night to finish a project) can upregulate inflammatory activity, leading to greater experiences of social threat that further disrupt sleep (e.g., poor sleep → inflammation → social threat → poor sleep). However, experiences of social threat (e.g., ruminating about a hostile boss) can also prevent sleep onset and/or degrade sleep quality, leading to increased inflammatory activity and experiences of social threat (e.g., social threat → poor sleep → inflammation → social threat). Research on Social Safety Theory will thus benefit from longitudinal studies that can discern the relative ordering of changes in these behavioral, immunologic, and cognitive processes.
Childhood Microbial Environment
Two other factors that can affect social safety are the frequency and diversity of early-life microbial exposures, which are largely determined by childhood living environment. Over the course of history, humans were exposed to a plethora of healthy microbes from other people, animals, feces, and soil, which helped educate the immune system and strengthen its regulatory capacity (Rook 2013). Today, however, 55% of people worldwide—and 82% of those in North America—live in relatively sanitized urban environments characterized by advanced sanitation methods, treated drinking water, excessive antibiotics use, cesarean section births, formula (instead of breast) milk, and infrequent contact with farm animals, mud, and feces (United Nations 2018).
As described by McDade (2012), these characteristics of the early living environment have critical implications for how people respond to both pathogens and psychological stressors throughout the life span. More specifically, hygienic urban environments minimize the frequency and diversity of microbial exposures and thus provide fewer opportunities for the immune system to practice upregulating inflammatory activity when it is needed and downregulating inflammation when it is no longer required. In contrast, less hygienic early-life environments increase the frequency, intensity, and diversity of microbial exposures occurring during critical periods of immune system development, leading to more frequent acute inflammatory responses and opportunities to develop a tightly controlled, counter-regulatory anti-inflammatory response. Like other neural and physiological systems, the benefit-to-cost ratio of the inflammatory response is greatest when it upregulates quickly and then downregulates shortly after a threat has passed. Growing up in a more sanitary, low-infectious environment may hamper such a tightly regulated response from developing, giving social threat the ability to promote an inflammatory state that fails to subside over time (i.e., chronic or nonresolving inflammation).
Supporting this model, a recent cohort study of 1,622 young adults in the Philippines examined whether the effects of social adversity on inflammatory activity are moderated by early-life microbial environment. For individuals exposed to low levels of animal feces during infancy, those who experienced a core threat to social safety in the form of prolonged parental absence during childhood (e.g., resulting from divorce, separation, or death) exhibited 47% higher CRP levels in young adulthood relative to their no-stress counterparts. In contrast, parental absence was associated with 17% lower CRP levels for persons exposed to high levels of feces during infancy. Experiencing social threat in childhood therefore predicted greater inflammation in adulthood, but only for individuals born into a relatively hygienic microbial environment (McDade et al. 2013).
A more recent study administered the socially stressful Trier Social Stress Test (TSST) to 20 young adults who grew up in an urban environment in the absence of farm animals and to 20 who grew up in a rural environment in the presence of animals. Those who grew up in a rural environment found the TSST to be more anxiety provoking, challenging, and threatening, and they also mounted greater HPA axis, SNS, and cardiovascular responses to the task. Nevertheless, it was the participants who grew up in an urban environment who exhibited the greatest TSST-induced increases in IL-6. Moreover, peripheral blood mononuclear cells (PBMCs) from these participants secreted more IL-6 in response to an inflammatory challenge (i.e., concanavalin A) in vitro, and PBMC secretion of the anti-inflammatory cytokine IL-10 was suppressed by two different inflammatory challenges (i.e., LPS and concanavalin A), but only for urban participants (Böbel et al. 2018). Together, these data provide strong converging evidence that relative to individuals who are raised in rural environments, those who grow up in urban environments exhibit greater inflammatory responses to acute social threat in addition to poorer immunoregulatory/anti-inflammatory capability following such threat (see the sidebar titled Social Safety Theory and Psychosocial Resilience).
SOCIAL SAFETY THEORY AND PSYCHOSOCIAL RESILIENCE.
Numerous studies have suggested that life stress exerts beneficial effects on psychological, cognitive, and emotional outcomes when experienced in moderation and under manageable circumstances. This finding is based on data showing that stress is related to human functioning not in a linear fashion but, rather, in a curvilinear manner whereby low and high levels of stress exposure both portend worse outcomes. This phenomenon has been called many things including toughening, steeling, antifragility, immunization, and stress inoculation, but is it real? If so, what is the biological explanation?
As discussed in this review, research has shown that childhood environments possessing greater microbial diversity trigger inflammatory reactions more frequently and therefore promote a more tightly controlled counter-regulatory immunologic response that terminates inflammation when a microbial threat has passed. From the perspective of Social Safety Theory, exposure to moderate amounts of intermittent social threat may be beneficial for the same reason—namely, it gives the immune system more opportunities to upregulate inflammation when a social threat is present and to downregulate inflammation when the threat has passed, thus leading to a more tightly regulated, time-limited inflammatory response. This formulation, herein called the “Immunological Psychosocial Resilience Hypothesis,” does not replace psychological explanations for this effect but, rather, provides a complementary, biologically plausible framework for understanding individual differences in resilience to social stress and adversity.
Diet
Diet can also shape experiences of social safety in several ways, the first of which involves affecting the composition of the gut microbiota. The human gut contains approximately 150 times more genes than the human genome and is inhabited by 10 times more microorganisms than eukaryotic cells in the human body. These microorganisms belong to more than 1,000 different species and 7,000 strains and are largely defined by two bacterial phylotypes—Bacteroidetes and Firmicutes—which account for up to 75% of the microbiome. Diet strongly affects the bacterial composition of the microbiota that can in turn shape experiences of social safety and threat by influencing the brain–gut–microbiome axis, which includes the CNS, neuroendocrine and neuroimmune systems, autonomic nervous system, enteric nervous system, and intestinal microbiota (Dinan & Cryan 2012). In particular, high-fat diets cause compositional changes in the microbiome that heighten neuroendocrine reactivity and inflammatory activity, which in turn induce anxiety, hypervigilance, and experiences of social threat. Moreover, certain foodborne pathogens, such as Citrobacter rodentium and Campylobacter jejuni, can directly alter neuronal threat circuitry through the activation of vagal pathways (Foster & McVey Neufeld 2013). Such effects may also be self-promoting. For example, experiences of social threat can increase gut permeability, which enables bacteria to cross the epithelial barrier and activate a mucosal immune response that changes the microbiome, upregulates HPA axis and inflammatory activity, and heightens experiences of social threat (e.g., social threat → gut permeability → inflammation → social threat) (Dinan & Cryan 2012).
Diet can also affect experiences of social safety by promoting the accumulation of adipose tissue, which is a major storehouse of immune cells that release cytokines during social stress. Whereas adipocytes (i.e., fat cells) operate in a type 2 or T helper 2 state under lean conditions, obesity shifts this immunologic stance toward a type 1 or T helper 1 state in which proinflammatory (i.e., M1-polarized) macrophages accumulate and secrete large amounts of different cytokines, including IL-1β and TNF-α, under conditions of psychological stress (Reilly & Saltiel 2017). Adipocytes can also produce leptin, which can have proinflammatory effects by upregulating the secretion of TNF-α, IL-6, and IL-12 via IRS-1, PI3K/Akt, STAT-3, and NF-κB signaling (Iikuni et al. 2008).
Consistent with these pathways, several studies have demonstrated that diet-related increases in adiposity moderate the effects of early threats to social safety on inflammatory activity and health. In one prospective study of 600 Chilean youth who were followed since infancy, for example, greater exposure to serious interpersonal conflict in infancy (e.g., parental fights, family conflict, marital separation or divorce) interacted with youths’ BMI to predict higher basal serum CRP levels in adolescence. Moreover, these effects were specific to early-life stressors involving interpersonal conflict and did not extend to non-social-safety-related forms of stress (Reid et al. 2019). In a second three-wave longitudinal study of 91 older adolescents living in the United States, experiences of early-life conflict, violence, harsh discipline, and unaffectionate or emotionally neglectful parenting predicted youths’ IL-6 reactivity to the TSST but only for youth with greater total body and abdominal adiposity as measured by BMI and waist circumference, respectively (Chiang et al. 2017). Future research on Social Safety Theory should thus account for differences in diet and diet-related factors.
Birth Cohort
Birth cohort can also influence experiences of social safety, health, and behavior by affecting the social, medical, and microbial environments into which people are born and live. Indeed, a recent analysis documented substantial longitudinal changes that could impact experiences of social safety worldwide (OECD 2011). Across 35 countries, total fertility rates have dropped from an average of 2.7 children/woman in 1970 to 1.7 children/woman in 2009, leading to a corresponding decline in average household size and fewer available familial bonds. During this same period, marriage rates decreased from 8.1 marriages/1,000 people to 5.0 marriages/1,000 people. Consequently, the number of children born outside of marriage and without a solid family structure tripled from 11% in 1980 to nearly 33% in 2007. Divorce rates, in turn, doubled and are now at 2.4 divorces/1,000 people, meaning that more children than ever now live in reconstituted families (~10%) or sole-parent families (~15%) and may have degraded schemas of family-based social safety characterized by a lack of perceived social continuity, dependability, and security.
Explicit threats to social safety are also on the rise. For example, UNICEF (2014) estimated that across 62 countries, approximately 70% of children 2–14 years old have experienced psychological threats to social safety, such as verbal aggression, intimidation, humiliation, or ridicule. Moreover, approximately 60% have experienced physical threats, such as hitting, kicking, spanking, or shaking. In the United States, 43.43% of adults presently agree with the statement “Sometimes a child just needs a good, hard spanking,” and 24.3% strongly agree. Social networking platforms also now provide opportunities for children to be virtually bullied, with 28% of American teenagers reporting that they were bullied over the past year alone (UNICEF 2014). Among adults, inequality rates have generally increased over the past 50 years, and explicit threats to social safety are rampant for some groups. For instance, approximately 33% of women report having experienced lifetime partner or nonpartner physical violence, or nonpartner sexual violence (OECD 2011).
As bad as these dynamics are for social safety, countervailing effects are also present, driven by improvements in public health and hygiene, living standards, vaccine availability, and likelihood of exposure to inflammation-promoting pathogens (Finch & Crimmins 2004). Over the past 100 years, for example, better medical procedures and public health policies have led to corresponding advances in dental hygiene and care that have decreased levels of periodontal disease, which is a well-known driver of chronic inflammation. Likewise, cohort-related improvements in medical interventions and standards of living have reduced early-life infections of Escherichia coli and Helicobacter pylori and the likelihood of contracting chronic tuberculosis, diarrhea, and malaria, all of which can lead to chronic inflammation (Finch & Crimmins 2004). Cohort-based changes in influenza vaccination can also make a difference. In a notable example of this, Gostic et al. (2016) analyzed epidemiologic data from six countries over nearly 100 years and showed that an abrupt worldwide change in human infections of avian influenza A/H5N1 and A/H7N9 around birth year 1968 could be attributed solely to the widespread use of a new flu vaccine. When a person was born—and, therefore, what flu shot they got—can also thus affect their lifelong immunologic vulnerability and inflammatory activity, which in turn can have implications for their perceived social safety, health, and behavior.
There is also some evidence of even more granular cohort-based effects. For example, birth month has been found to independently predict longevity in several studies, with such effects being attributed to seasonal differences in the availability of healthy foods during pregnancy (which is generally better during spring and summer months) and to differences in infants’ likelihood of exposure to inflammation-promoting pathogens (which is generally worse in autumn and winter months) (Doblhammer & Vaupel 2001). Indeed, children born during winter seasons marked by higher rainfall or flooding, which can disperse pathogens widely, have been found to have greater inflammatory responses to psychological stress, as indexed by CRP (Yazawa et al. 2015). Therefore, birth month may also play a role in structuring inflammation-driven effects on experiences of social safety and threat.
Air Pollution
Air pollution may also make a difference. Air pollution is presently considered one of the greatest environmental threats to human health by the World Health Organization (WHO 2016). As we have reviewed elsewhere (Olvera Alvarez et al. 2018), ambient air pollution includes ozone, nitric oxide, sulfur dioxide, carbon monoxide, and particulate matter (PM) that is emitted from vehicles, commercial industries, heating units, and cooking with biofuels such as fuelwood and animal dung. Exposure to fine PM ≤2.5 μm in aerodynamic diameter (i.e., PM2.5) is especially harmful because these particles can be inhaled, enter the circulation, and reach the brain, where they influence serotonergic and dopaminergic activity and affect brain structure, function, and plasticity. These particles can also cause a local inflammatory response (e.g., in the lungs), a peripheral inflammatory response in the body, and neuroinflammation in the CNS that is initiated either indirectly (e.g., by reactive oxygen species, which activate NF-κB) or directly via the release of IL-1β and TNF-α. As a result, air pollution has been associated with increased risk for several inflammation-related health problems, including asthma, cardiovascular disease, stroke, cognitive impairment, depression, and suicide (Olvera Alvarez et al. 2018).
Given these dynamics, air pollution–mediated effects on perceptions of social safety may be greatest for persons living in urban environments, especially near highways or commercial zones, and for those in rural environments where cooking with biofuels is more common. Interactive effects with social stress are also possible. In a recent study of 60,925,443 adults living in the United States, for example, exposure to PM2.5 <12 μg/m3 was associated with a 13.6% increase in all-cause mortality (hazard ratio = 1.136; 95% CI [1.131, 1.141]). However, this mortality risk was significantly greater for persons most likely to experience discrimination in the United States—namely, those low in socioeconomic status and blacks, with the latter group exhibiting a PM2.5-related risk of death that was three times greater than that of the overall population (Di et al. 2017).
Self-Harm
Finally, self-harm, or nonsuicidal self-injury (NSSI) more specifically, can result from social threat but may also play a biologically mediated role in shaping such experiences. NSSI has a lifetime prevalence of up to 23% in adolescents and 4% in adults and is defined as the “direct, deliberate destruction of one’s own body tissue in the absence of intent to die” (Nock 2009, p. 78). Therefore, NSSI involves inflicting the exact same kinds of bodily injury (e.g., cuts or burns on the arms or legs) that trigger the release of DAMPs, which upregulate systemic inflammatory activity and promote chronic inflammation. It is well known that NSSI is more common in people who have experienced major threats to social safety, such as emotional, physical, or sexual abuse, thus making social threat a key risk factor for NSSI (Nock 2009, Stewart et al. 2019). Moreover, it has been hypothesized that inflammation may mediate these effects (e.g., social threat → inflammation → NSSI) (Brundin et al. 2015). The more novel question is whether cutting oneself induces an inflammatory response that in turn affects experiences of social safety and future risk for NSSI (e.g., NSSI → inflammation → social threat → NSSI).
No studies have examined within-person associations between NSSI, inflammation, and experiences of social safety or threat to test whether NSSI increases inflammatory activity that in turn modulates social cognition. Nevertheless, the mechanistic pathways linking tissue damage to systemic inflammation are well known. Specifically, bodily injury releases mitochondrial DAMPs into the circulation. In turn, mitochondrial DAMPs initiate innate and adaptive immune responses by activating intracellular receptors (e.g., TLR9, NLRP3) or cell surface receptors (e.g., P2X7R, FPRs) on immune cells, including microglia and astrocytes that regulate neuroinflammation. The resulting production of inflammatory cytokines increases neural threat sensitivity that can degrade perceptions of social safety and promote experiences of social threat (Zhang et al. 2010). NSSI may thus represent a behavioral trigger that induces neuroinflammation, which exacerbates a person’s perceptions of social conflict, criticism, or exclusion, in turn leading to heightened emotional distress and an even greater propensity to engage in NSSI (e.g., NSSI → inflammation → social threat → NSSI). Self-harm may therefore represent an outcome of low social safety but may also lead to such experiences by kindling the activity of social signal transduction pathways that promote perceptions of social threat.
STRATEGIES FOR PROMOTING SOCIAL SAFETY
In addition to elucidating multilevel processes that mediate and moderate experiences of social safety and threat, additional research is needed to develop effective strategies for promoting social safety and reducing social threat as a means of improving the human condition. These approaches can focus on modifying the factors described above that indirectly shape perceptions of social safety and threat (e.g., by reducing inflammatory activity). In terms of more direct interpersonal and social-cognitive strategies, these will need to consider the fact that humans are embedded in a series of social safety circles that independently and interactively shape moment-to-moment and lifelong experiences of social safety and threat (see Figure 2). Because objective characteristics of the social environment influence the extent to which people generally regard their surroundings as socially safe versus threatening, strategies for enhancing social safety can focus on improving actual social environments (e.g., by promoting social inclusion, connection, and belonging). However, neurocognitive representations of social safety and threat are ultimately what regulate inflammatory dynamics that affect human health and behavior, so the most effective interventions for producing social safety–related benefits will likely be those that target modifiable social cognitions and behaviors that increase perceptions of social safety and reduce perceptions of social threat (Black & Slavich 2016, O’Donovan et al. 2013, Slavich et al. 2010b).
Figure 2:

Individuals are embedded in a variety of social safety circles that determine their moment-to-moment and lifelong experiences of social safety and threat. These social networks directly affect human health and behavior by influencing the extent to which people are exposed to objective forms of social safety (e.g., strong family cohesion, welcoming neighbors, inclusive public policy) and social threat (e.g., family conflict, hostile neighbors, divisive public policy). In addition, these networks indirectly affect health and behavior by exposing individuals to construals, messages, and meanings that shape their social safety schemas, which in turn influence their perceptions of the surrounding environment as being socially safe versus threatening. Strategies for promoting social safety can target any of these circles to promote social safety and reduce social threat as a means of improving human health and behavior.
Table 1 summarizes several strategies that are currently available for promoting social safety and reducing social threat by targeting social, psychological, emotional, or neurochemical processes. Included in this list are individual methods, such as cognitive behavior therapy and mindfulness meditation; family-based approaches, such as nurturant parenting and family cohesion training; school-based interventions, such as social belonging and identity safety interventions; and community- and society-based strategies, such as developing policies aimed at reducing bias, preventing bullying, and building empathy. Presently, however, acetaminophen administration is the only biologically informed strategy, and it is far from being an accepted approach for treating social threat. A lot more work is thus needed before we have effective psychosocial or psychopharmacological interventions that are informed by the biological pathways described in this review. To help promote this much-needed research, new intervention approaches will be catalogued online as they are identified (see https://www.socialsafetytheory.org).
Table 1.
Individual and collective strategies for promoting social safety and reducing social threat
| Intervention | Possible mechanism of action | Example reference(s) |
|---|---|---|
| Individual | ||
| Cognitive behavior therapy | Increase social engagement and reduce social threat–related thoughts and beliefs | Clark & Beck 1999, Hofmann & Otto 2017, Shields et al. 2020 |
| Acceptance and commitment therapy | Develop cognitive flexibility to notice, accept, and embrace past social threats | Hayes et al. 2009 |
| Mindfulness meditation | Bring awareness to the present, which is typically absent of social threat | Creswell et al.2014 |
| Loving-kindness and compassion meditation | Enhance positive emotional states of kindness and compassion toward others | Hofmann et al. 2011 |
| Forgiveness therapy | Reduce negative thoughts and emotions induced by offenders/aggressors | Akhtar & Barlow 2018, Worthington 2013 |
| Self-distancing | Reduce the negativity of past aversive events by increasing distance/perspective | Kross & Ayduk 2017 |
| Stress mindset interventions | View social threat as an enhancing rather than debilitating experience | Crum et al. 2017 |
| Acetaminophen administration | Reduce neurobiological signaling that subserves social pain and rejection | DeWall et al. 2010, Slavich et al. 2019 |
| Safety learning | Remodel neural networks underlying anxiety, fear, and threat | Meyer et al. 2019 |
| Family | ||
| Nurturant parenting training | Strengthen familial relationships | Miller et al. 2014 |
| Family cohesion training | Promote social caring, interpersonal connection, and family cohesion | King et al. 2019 |
| School | ||
| Identity safety interventions | Foster sense that one’s identity and values are accepted and welcome | Goyer et al.2019 |
| Social belonging interventions (a type of identity safety intervention) | Increase social connection, cohesion, belonging, affiliation, and inclusion; reappraise social cues and adversity | Allen et al. 2016, Borman et al. 2019, Patton et al. 2006, Walton & Cohen 2011 |
| Mere belonging interventions | Enhance social connection and belonging with minimal cues and socially shared goals | Gehlbach et al. 2016, Walton et al. 2012 |
| Community/society | ||
| Reduce bias | Decrease instances of prejudice and discrimination | Eberhardt 2019 |
| Prevent bullying | Reduce experiences of social aggression and exclusion | Williams & Nida 2014 |
| Promote social connection | Decrease loneliness and increase social interaction and belonging | Holt-Lunstad et al. 2017 |
| Build empathy | Promote social connection, caring, and empathy toward others | Zaki 2019 |
CONCLUSION
In conclusion, stress research has long been hampered by imprecise, behaviorally derived definitions that have caused countless conceptual, empirical, and reproducibility issues. To address these problems and begin to understand the true underlying structure of life stress, I propose that we need only look to our cellular selves, which evolved over millennia to monitor the surrounding environment, detect potential threats, and mount anticipatory biobehavioral responses that conferred the greatest adaptive advantage. Physical threats are the most impactful types of stressors from this evolutionary perspective, but such experiences are followed closely by social threats that historically increased risk for possible isolation, conflict, or wounding. Life stress has been a notoriously messy construct with little agreement about what actually matters (Cohen et al. 2019, Malat et al. 2017, Monroe & Slavich 2020). If we ask the human brain and immune system, though, they will tell us that social safety and social threat lie at the heart of life’s most impactful experiences.
Stressnology:
an approach to studying life stress exposure that involves measuring only the superficial contours of this very complex construct
Innate immune system:
evolutionarily older branch of the immune system, which provides very quick, first-line, nonspecific protection against various pathogens
Adaptive immune system:
evolutionarily younger branch of the immune system, which provides slower, second-line, pathogen-specific protection based on immunologic memory
Pathogen-associated molecular patterns (PAMPs):
highly conserved molecular motifs that, when recognized by pattern recognition receptors, activate innate immune responses
Pattern recognition:
innate immune system process that enables detection of infectious nonself molecules (via PAMPs) and cellular stress/death (via DAMPs)
Pattern recognition receptors (PRRs):
receptors expressed by immune cells that activate innate immune responses when they detect PAMPs or DAMPs
Toll-like receptors (TLRs):
transmembrane receptors that promote inflammation and adaptive immunity following recognition of pathogenic/nonpathogenic microbes, proteins, and nucleic acids
Damage-associated molecular patterns (DAMPs):
molecules released during cellular stress/death that strongly induce inflammation and appear to underlie the pathogenesis of inflammation-related diseases
Sterile inflammation:
DAMP-mediated increases in inflammation triggered by tissue damage, bodily trauma, ischemia, or cellular stress/death absent a pathogenic infection
Lymphocytes:
bone marrow–derived white blood cells found in lymph tissue that include B cells, T cells, and natural killer cells
Social brain:
network of brain structures that mediate social cognition and behavior and enable social interaction, communication, cooperation, and prediction
Social safety:
evolutionarily beneficial actual or perceived social circumstances characterized by social acceptance, affiliation, cohesion, belonging, interaction, inclusion, and connection
Social threat:
evolutionarily detrimental actual or perceived social circumstances characterized by social conflict, aggression, devaluation, discrimination, isolation, rejection, and exclusion
Social signal transduction:
process by which neurocognitive representations of the external social environment get transduced and converted into genome-wide transcriptional dynamics that affect health and behavior
Cytokines:
proteins produced by immune cells that coordinate immunity, inflammation, and hematopoiesis, and that have numerous physiologic, biobehavioral, and health effects
Social safety schemas:
cognitive representations of the self, social world, and future—including how socially safe versus threatening others are—which in turn influence human cognition, biology, behavior, and health
Neuro-inflammatory sensitization to adversity:
bidirectional neuroinflammatory interactions that promote physiologic recursion and cause persistently heightened sensitivity to social adversity
Targeted rejection:
social rejection that is directed at, and meant to affect, a single person, and that involves an active and intentional severing of relational ties
Human social genomics:
the study of how social processes affect the human genome, which in turn shapes health and behavior
Social safety circles:
social groups in which individuals are embedded, which determine the quality of daily and lifelong social interactions, and shape social safety schemas
SUMMARY POINTS.
Despite the centrality of life stress and adversity in most major theories of human development, health, and behavior, there is little agreement regarding the specific characteristics that make particular stressors stressful.
A principal function of the human immune system and brain is to keep the body biologically and physically safe, which these systems do by continually monitoring and responding to social, physical, and microbial threats in the environment. The immune system detects microbial infection and tissue damage and relies on the brain to scan the environment for cues indicating potential danger.
Over the course of evolution, individuals who fostered greater social safety and who mounted anticipatory inflammatory responses to social threat were most likely to survive and pass on their genes. Consequently, a drive to develop friendly social bonds, and to mount a systemic inflammatory response to social cues indicating an increased risk of physical danger, was likely highly conserved.
These dynamics, described by Social Safety Theory, are supported by extensive data showing that humans evolved to foster social safety (tenet 1), that social safety is beneficial for human health and behavior (tenet 2), and that social threat is harmful for human health and behavior (tenet 3).
Future research examining this theory should consider several factors that can alter the activity of the social signal transduction pathways that shape experiences of social safety, including genetics, sleep, childhood microbial environment, diet, birth cohort, air pollution, and self-harm behavior.
Existing strategies that may help promote social safety and reduce social threat include psychotherapy; nurturant parenting and family cohesion training; stress mindset and social belonging interventions; and public policies aimed at reducing bias and bullying, promoting social connection, and increasing individual and collective empathy.
Research on life stress and social support has historically been plagued by conceptual imprecision, conflict, and confusion, but the key characteristics of these critically important constructs become clearer when viewed through the lens of Social Safety Theory.
Additional research is needed to examine the core tenets and hypotheses derived from this theory of life stress, health, and behavior.
FUTURE ISSUES.
Among the many types of experiences that can be considered social threats (e.g., social conflict, aggression, devaluation, discrimination, isolation, rejection, exclusion), which are most detrimental for social safety and why? What is the hierarchical organization of these and other related forms of social adversity? What is the best way to measure social threat?
Among the many types of experiences that help promote social safety (e.g., social acceptance, affiliation, cohesion, belonging, interaction, inclusion, connection), which are most beneficial for social safety and why? What is the hierarchical organization of these and other salutatory social experiences? What is the best way to measure social safety?
What are the more granular neural, physiologic, immunologic, molecular, genetic, and genomic processes that underlie social signal transduction? How do these processes interact and combine to exert long-term effects on human health and behavior?
Several factors other than those described in this review can upregulate inflammation, including bisphenol A and phthalates (e.g., from water bottles and food storage containers), pesticides (e.g., from crop protection and food preservation), heavy metals (e.g., cadmium, lead), electromagnetic fields (e.g., from mobile phones and Wi-Fi networks), pollen (e.g., from grass and trees), and excessive noise (e.g., from construction and vehicle traffic). What role do these factors play in regulating social signal transduction pathways that shape experiences of social safety and threat?
What social, cultural, environmental, and biological processes underlie the intergenerational transmission of the propensity to view others and the world as a socially safe versus threatening place? What is the relative contribution of individual versus collective processes in the persistence of social safety and threat over time?
How do experiences of social safety and threat spread across social networks? Can these network dynamics help explain why certain complex phenotypes or diseases cluster in particular areas, groups, or populations? What role does culture play in shaping social safety schemas and social signal transduction?
What individual and collective interventions are most effective for reducing experiences of social threat, disrupting social signal transduction, promoting experiences of social safety, and enhancing individual and collective resilience to social threat? What social, psychological, and biological mechanisms underlie these effects?
Answering these important questions and others will require a transdisciplinary approach to studying Social Safety Theory that leverages ideas and methods from several relevant disciplines, including sociology, anthropology, epidemiology, public health, psychology, medicine, neuroscience, psychophysiology, immunology, genetics, and genomics. Studies that span multiple disciplines and levels of analysis will likely be most informative, especially if they include psychological, behavioral, and/or clinical outcomes.
ACKNOWLEDGMENTS
Preparation of this review was supported by a Society in Science-Branco Weiss Fellowship, NARSAD Young Investigator Grant 23958 from the Brain & Behavior Research Foundation, and National Institutes of Health grant K08 MH103443 to G.M.S.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adler NE, Rehkopf DH. 2008. U.S. disparities in health: descriptions, causes, and mechanisms. Annu. Rev.Public Health 29:235–52 [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, Wall SN. 1978. Patterns of Attachment: A Psychological Study of the Strange Situation Hillsdale, NJ: Lawrence Erlbaum [Google Scholar]
- Akhtar S, Barlow J. 2018. Forgiveness therapy for the promotion of mental well-being: a systematic review and meta-analysis. Trauma Violence Abuse 19:107–22 [DOI] [PubMed] [Google Scholar]
- Allbutt C 1895. Nervous diseases and modern life. Contemp. Rev 67:210–17 [Google Scholar]
- Allen KA, Vella-Brodrick D, Waters L. 2016. Fostering school belonging in secondary schools using a socioecological framework. Educ. Dev. Psychol 33:97–121 [Google Scholar]
- Allen NB, Badcock PB. 2003. The social risk hypothesis of depressed mood: evolutionary, psychosocial, and neurobiological perspectives. Psychol. Bull 129:887–913 [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, et al. 2015. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med 212:991–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babey SH, Wolstein J, Becker TL, Scheitler AJ. 2019. School Discipline Practices Associated with Adolescent School Connectedness and Engagement Los Angeles: UCLA Cent. Health Policy Res. [Google Scholar]
- Barrett LF. 2017. How Emotions Are Made: The Secret Life of the Brain New York: Houghton Mifflin Harcourt [Google Scholar]
- Baumeister RF, Leary MR. 1995. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull 117:497–529 [PubMed] [Google Scholar]; This landmark review provides a comprehensive synthesis of research describing the many benefits of social belonging.
- Bernard C 1865. Introduction à L’étude de la Médecine Expérimentale Paris: J.B. Baillière [Google Scholar]
- Bickart KC, Dickerson BC, Barrett LF. 2014. The amygdala as a hub in brain networks that support social life. Neuropsychologia 63:235–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Slavich GM. 2016. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann. N.Y. Acad. Sci 1373:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom P 2013. Just Babies: The Origins of Good and Evil New York: Crown [Google Scholar]
- Böbel TS, Hackl SB, Langgartner D, Jarczok MN, Rohleder N, et al. 2018. Less immune activation following social stress in rural versus urban participants raised with regular or no animal contact, respectively. PNAS 115:5259–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman GD, Rozek CS, Pyne J, Hanselman P. 2019. Reappraising academic and social adversity improves middle school students’ academic achievement, behavior, and well-being. PNAS 116:16286–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO. 1978. Social Origins of Depression: A Study of Psychiatric Disorder in Women New York: Free Press [Google Scholar]
- Brown GW, Harris TO, Hepworth C. 1995. Loss, humiliation and entrapment among women developing depression: a patient and non-patient comparison. Psychol. Med 25:7–21 [DOI] [PubMed] [Google Scholar]
- Brundin L, Erhardt S, Bryleva EY, Achtyes ED, Postolache TT. 2015. The role of inflammation in suicidal behaviour. Acta. Psychiatry Scand 132:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. 1929. Organization for physiological homeostasis. Physiol. Rev 9:399–431 [Google Scholar]
- Carter CS. 2014. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol 65:17–39 [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. 1999. Stress, coping, and self-regulatory processes In Handbook of Personality, ed. Pervin LA, John OP, pp. 553–75. New York: Guilford Press [Google Scholar]
- Chiang JJ, Bower JE, Irwin MR, Taylor SE, Fuligni AJ. 2017. Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. Brain Behav. Immun 66:146–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Cole SW, Bower JE, Irwin MR, Taylor SE, et al. 2019. Daily interpersonal stress, sleep duration, and gene regulation during late adolescence. Psychoneuroendocrinology 103:147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Bowles S. 2007. The coevolution of parochial altruism and war. Science 318:636–40 [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT. 1999. Scientific Foundations of Cognitive Theory of Depression New York: Wiley [Google Scholar]
- Cohen S, Murphy ML, Prather AA. 2019. Ten surprising facts about stressful life events and disease risk. Annu. Rev. Psychol 70:577–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, et al. 2010. Computational identification of gene-social environment interaction at the human IL6 locus. PNAS 107:5681–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Pacilio LE, Lindsay EK, Brown KW. 2014. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology 44:1–12 [DOI] [PubMed] [Google Scholar]
- Crişan TO, Netea MG, Joosten LA. 2016. Innate immune memory: implications for host responses to damage-associated molecular patterns. Eur. J. Immunol 46:817–28 [DOI] [PubMed] [Google Scholar]
- Crum AJ, Akinola M, Martin A, Fath S. 2017. The role of stress mindset in shaping cognitive, emotional, and physiological responses to challenging and threatening stress. Anxiety Stress Coping 30:379–95 [DOI] [PubMed] [Google Scholar]; This study illustrates how modifying “stress mindset” alters cognitive, affective, and physiologic reactivity to social threat.
- Darwin CR. 1859. On the Origin of Species London: John Murray [Google Scholar]
- Decety J 2015. The neural pathways, development and functions of empathy. Curr. Opin. Behav. Sci 3:1–6 [Google Scholar]
- DeWall CN, MacDonald G, Webster GD, Masten CL, Baumeister RF, et al. 2010. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol. Sci 21:931–37 [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS. 2012. Stress-induced redistribution of immune cells—from barracks to boulevards to battlefields: a tale of three hormones—Curt Richter Award Winner. Psychoneuroendocrinology 37:1345–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, et al. 2017. Air pollution and mortality in the Medicare population. N. Engl. J. Med 376:2513–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS. 2008. Emotional and physiological responses to social-evaluative threat. Soc. Personal. Psychol. Compass 2:1362–78 [Google Scholar]
- Dinan TG, Cryan JF. 2012. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37:1369–78 [DOI] [PubMed] [Google Scholar]
- Doblhammer G, Vaupel JW. 2001. Lifespan depends on month of birth. PNAS 98:2934–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. 2007. Evolution in the social brain. Science 317:1344–47 [DOI] [PubMed] [Google Scholar]
- Eberhardt JL. 2019. Biased: Uncovering the Hidden Prejudice That Shapes What We See, Think, and Do New York:Viking [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, et al. 2018. More than a feeling: a unified view of stress measurement for population science. Front. Neuroendocrinol 49:146–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, et al. 2016. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535:425–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. 2004. Inflammatory exposure and historical changes in human life-spans. Science 305:1736–39 [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJ, Glick P. 2007. Universal dimensions of social cognition: warmth and competence. Trends Cogn. Sci 11:78–83 [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. 2013. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–12 [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. 2006. The neural basis of mentalizing. Neuron 50:531–34 [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, et al. 2019. Chronic inflammation in the etiology of disease across the life span. Nat. Med 25:1822–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Trabold N, Cerulli C, Pigeon WR. 2019. Sleep and interpersonal violence: a systematic review. Trauma Violence Abuse In press. 10.1177/1524838019852633 [DOI] [PubMed] [Google Scholar]
- Gehlbach H, Brinkworth ME, King AM, Hsu LM, McIntyre J, Rogers T. 2016. Creating birds of similar feathers: leveraging similarity to improve teacher-student relationships and academic achievement. J. Educ. Psychol 108:342–52 [Google Scholar]
- Gilbert P 2005. Social mentalities: a biopsychosocial and evolutionary approach to social relationships In Interpersonal Cognition, ed. Baldwin MW, pp. 299–333. New York: Guilford Press [Google Scholar]
- Gilbert P, Allan S. 1998. The role of defeat and entrapment (arrested flight) in depression: an exploration of an evolutionary view. Psychol. Med 28:585–98 [DOI] [PubMed] [Google Scholar]
- Giletta M, Slavich GM, Rudolph KD, Hastings PD, Nock MK, Prinstein MJ. 2018. Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. J. Child Psychol. Psychiatry 59:129–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. 2016. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 354:722–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer JP, Cohen GL, Cook JE, Master A, Apfel N, et al. 2019. Targeted identity safety interventions cause lasting reductions in discipline citations among ethnic-minority boys. J. Personal. Soc. Psychol 117:229–59 [DOI] [PubMed] [Google Scholar]
- Guarneri-White ME, Arana AA, Boyd EQ, Jensen-Campbell LA. 2018. It’s more than skin-deep: the relationship between social victimization and telomere length in adolescence. Aggress. Behav 44:337–47 [DOI] [PubMed] [Google Scholar]
- Hammen C 2005. Stress and depression. Annu. Rev. Clin. Psychol 1:293–319 [DOI] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. 2012. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav. Immun 26:1150–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B 2017. Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annu. Rev. Psychol 68:155–86 [DOI] [PubMed] [Google Scholar]
- Haslam C, Best D, Dingle GA, Staiger PK, Savic M, et al. 2019. Social group membership before treatment for substance dependence predicts early identification and engagement with treatment communities. Addict. Res. Theory 27:363–72 [Google Scholar]
- Haslam C, Holme A, Haslam SA, Iyer A, Jetten J, Williams WH. 2008. Maintaining group memberships: social identity continuity predicts well-being after stroke. Neuropsychol. Rehabil 18:671–91 [DOI] [PubMed] [Google Scholar]
- Hawkins AT, Pallangyo AJ, Herman AM, Schaumeier MJ, Smith AD, et al. 2016. The effect of social integration on outcomes after major lower extremity amputation. J. Vasc. Surg 63:154–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. 2009. Acceptance and Commitment Therapy Washington, DC: Am. Psychol.Assoc. [Google Scholar]
- Henrich J 2015. The Secret of Our Success: How Culture Is Driving Human Evolution, Domesticating Our Species, and Making Us Smarter Princeton, NJ: Princeton Univ. Press [Google Scholar]
- Hofmann SG, Grossman P, Hinton DE. 2011. Loving-kindness and compassion meditation: potential for psychological interventions. Clin. Psychol. Rev 31:1126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Otto MW. 2017. Cognitive Behavioral Therapy for Social Anxiety Disorder: Evidence-Based and Disorder-Specific Treatment Techniques New York: Routledge; 2nd ed. [Google Scholar]
- Holmes TH, Rahe RH. 1967. The social readjustment rating scale. J. Psychosom. Res 11:213–21 [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J 2018. Fostering social connection in the workplace. Am. J. Health Promot 32:1307–12 [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Robles TF, Sbarra DA. 2017. Advancing social connection as a public health priority in the United States. Am. Psychol 72:517–30 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article argues that promoting social connection should be a top public health priority.
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. 2015. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect. Psychol. Sci 10:227–37 [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLOS Med 7:e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iikuni N, Lam K, Queenie L, Lu L, Matarese G, Cava AL. 2008. Leptin and inflammation. Curr. Immunol. Rev 4:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR. 2019. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol 19:702–15 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. 2011. Reciprocal regulation of the neural and innate immune systems. Nat. Rev.Immunol 11:625.– [DOI] [PMC free article] [PubMed] [Google Scholar]; This comprehensive review describes several bidirectional pathways linking neural and innate immune system activity.
- Irwin MR, Slavich GM. 2017. Psychoneuroimmunology In Handbook of Psychophysiology, ed. Cacioppo JT, Tassinary LG, Berntson GG, pp. 377–98. New York: Cambridge Univ. Press; 4th ed. [Google Scholar]
- Jones JM, Haslam SA, Jetten J, Williams WH, Morris R, Saroyan S. 2011. That which doesn’t kill us can make us stronger (and more satisfied with life): the contribution of personal and social changes to well-being after acquired brain injury. Psychol. Health 26:353–69 [DOI] [PubMed] [Google Scholar]
- Kagan J 2016. An overly permissive extension. Perspect. Psychol. Sci 11:442.– [DOI] [PubMed] [Google Scholar]; This perspective discusses the conceptual imprecision, conflict, and confusion plaguing contemporary life stress research.
- Kawai T, Akira S. 2006. Innate immune recognition of viral infection. Nat. Immunol 7:131–37 [DOI] [PubMed] [Google Scholar]
- Kemeny ME. 2009. Psychobiological responses to social threat: evolution of a psychological model in psychoneuroimmunology. Brain Behav. Immun 23(1):1–9 [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. 2012. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci 16:559–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ. 2017. Lovesick: how couples’ relationships influence health. Annu. Rev. Clin. Psychol 13:421–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Arango A, Kramer A, Busby D, Czyz E, et al. 2019. Association of the youth-nominated support team intervention for suicidal adolescents with 11- to 14-year mortality outcomes: secondary analysis of a randomized clinical trial. JAMA Psychiatry 76:492–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Ayduk O. 2017. Self-distancing: theory, research, and current directions. Adv. Exp. Soc. Psychol 55:81–136 [Google Scholar]
- Larson SJ, Dunn AJ. 2001. Behavioral effects of cytokines. Brain Behav. Immun 15:371–87 [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. 1984. Stress, Appraisal, and Coping Berlin: Springer [Google Scholar]
- Leary MR, ed. 2001. Interpersonal Rejection New York: Oxford Univ. Press [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, et al. 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10:434–45 [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, et al. 2012. Social influences on clinical outcomes of patients with ovarian cancer. J. Clin. Oncol 30:2885–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Seligman ME. 2016. Learned helplessness at fifty: insights from neuroscience. Psychol. Rev 123:349–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malat J, Jacquez F, Slavich GM. 2017. Measuring lifetime stress exposure and protective factors in life course research on racial inequality and birth outcomes. Stress 20:379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massing-Schaffer M, Helms SW, Rudolph KD, Slavich GM, Hastings PD, et al. 2019. Preliminary associations among relational victimization, targeted rejection, and suicidality in adolescents: a prospective study. J. Clin. Child Adolesc. Psychol 48:288–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW. 2012. Early environments and the ecology of inflammation. PNAS 109:17281–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C. 2013. Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav. Immun 31:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, et al. 2009. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci 12:342–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, et al. 2019. Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. PNAS 116:26970–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E. 2014. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. PNAS 111:11287–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM. 2008. Modern approaches to conceptualizing and measuring life stress. Annu. Rev. Clin. Psychol 4:33–52 [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM. 2016. Psychological stressors: overview In Stress: Concepts, Cognition, Emotion, and Behavior, ed. Fink G, pp. 109–15. Cambridge, MA: Academic; 1st ed. [Google Scholar]
- Monroe SM, Slavich GM. 2020. Major life events: a review of conceptual, definitional, measurement issues, and practices In The Oxford Handbook of Stress and Mental Health, ed. Harkness KL, Hayden EP, pp. 7–26. New York: Oxford Univ. Press [Google Scholar]
- Mookadam F, Arthur HM. 2004. Social support and its relationship to morbidity and mortality after acute myocardial infarction: systematic overview. Arch. Intern. Med 164:1514–18 [DOI] [PubMed] [Google Scholar]
- Murphy ML, Slavich GM, Chen E, Miller GE. 2015. Targeted rejection predicts decreased anti-inflammatory gene expression and increased symptom severity in youth with asthma. Psychol. Sci 26:111–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ML, Slavich GM, Rohleder N, Miller GE. 2013. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clin. Psychol. Sci 1:30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Brosso SN, Humphreys KL. 2018. Socioeconomic status and inflammation: a meta-analysis. Mol. Psychiatry In press. 10.1038/s41380-018-0259-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK. 2009. Why do people hurt themselves? New insights into the nature and functions of self-injury. Curr. Dir. Psychol. Sci 18:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Slavich GM, Epel ES, Neylan TC. 2013. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci. Biobehav. Rev 37:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organ. Econ. Co-operation Dev.). 2011. Doing Better for Families Paris: OECD [Google Scholar]
- Olvera Alvarez HA, Kubzansky LD, Campen MJ, Slavich GM. 2018. Early life stress, air pollution, inflammation, and disease: an integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci. Biobehav. Rev 92:226–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Bond L, Carlin JB, Thomas L, Butler H, et al. 2006. Promoting social inclusion in schools: a group-randomized trial of effects on student health risk behavior and well-being. Am. J. Public Health 96:1582–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. 2012. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat. Rev. Endocrinol 8:743–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco PR, Collins NL. 2017. Interpersonal mechanisms linking close relationships to health. Am. Psychol 72:531–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid BM, Doom JR, Argote RB, Correa-Burrows P, Lozoff B, et al. 2019. Pathways to inflammation in adolescence through early adversity, childhood depressive symptoms, and body mass index: a prospective longitudinal study of Chilean infants. Brain Behav. Immun In press. 10.1016/j.bbi.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, Saltiel AR. 2017. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol 13:633–43 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. 2016. The mirror mechanism: a basic principle of brain function. Nat. Rev. Neurosci 17:757–65 [DOI] [PubMed] [Google Scholar]
- Rook GA. 2013. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. PNAS 110:18360–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Bäckhed F, Levin BR, McFall-Ngai MJ, McLean AR. 2017. Evolution, human-microbe interactions, and life history plasticity. Lancet 390:521.– [DOI] [PubMed] [Google Scholar]; This enlightening review explains microbial diversity and its relation to human evolution, health, and behavior.
- Schaefer L 2014. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem 289:35237–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H 1976. The Stress of Life New York: McGraw-Hill; 2nd ed. [Google Scholar]
- Shields GS, Moons WG, Slavich GM. 2017. Inflammation, self-regulation, and health: an immunologic model of self-regulatory failure. Perspect. Psychol. Sci 12:588–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Slavich GM. 2017. Lifetime stress exposure and health: a review of contemporary assessment methods and biological mechanisms. Soc. Personal. Psychol. Compass 11(8):e12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Spahr CM, Slavich GM. 2020. Psychosocial interventions and immune system function: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB. 2007. Social components of fitness in primate groups. Science 317:1347–51 [DOI] [PubMed] [Google Scholar]
- Slavich GM. 2015. Understanding inflammation, its regulation, and relevance for health: a top scientific and public priority. Brain Behav. Immun 45:13–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM. 2016. Life stress and health: a review of conceptual issues and recent findings. Teach. Psychol 43:346–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM. 2019. Stressnology: the primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain Behav. Immun 75:3–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM. 2020. Psychoneuroimmunology of stress and mental health In The Oxford Handbook of Stress and Mental Health, ed. Harkness KL, Hayden EP, pp. 519–46. New York: Oxford Univ. Press [Google Scholar]
- Slavich GM, Auerbach RP. 2018. Stress and its sequelae: depression, suicide, inflammation, and physical illness In APA Handbook of Psychopathology, Vol. 1: Psychopathology: Understanding, Assessing, and Treating Adult Mental Disorders, ed. Butcher JN, Hooley JM, pp. 375–402. Washington, DC: Am. Psychol. Assoc. [Google Scholar]
- Slavich GM, Cole SW. 2013. The emerging field of human social genomics. Clin. Psychol. Sci 1:331–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull 140:774–815 [DOI] [PMC free article] [PubMed] [Google Scholar]; This review describes Social Signal Transduction Theory of Depression, which is the theoretical precursor to Social Safety Theory.
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. 2010a. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev 35:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Sacher J. 2019. Stress, sex hormones, inflammation, and major depressive disorder: extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology 236:3063–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Shields GS, Deal BD, Gregory A, Toussaint LL. 2019. Alleviating social pain: a double-blind, randomized, placebo-controlled trial of forgiveness and acetaminophen. Ann. Behav. Med 53:1045–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Tartter MA, Brennan PA, Hammen C. 2014. Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology 49:141–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH. 2009. Targeted rejection predicts hastened onset of major depression. J. Soc. Clin. Psychol 28:223–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. 2010b. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. PNAS 107:14817–22 [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first study to elucidate neurocognitive mechanisms underlying inflammatory reactivity to social stress.
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. 2009. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron 64:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JG, Shields GS, Esposito EC, Cosby EA, Allen NB, et al. 2019. Life stress and suicide in adolescents. J. Abnorm. Child Psychol 47:1707–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayhorn TL. 2018. College Students’ Sense of Belonging: A Key to Educational Success for All Students New York: Routledge [Google Scholar]
- Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, et al. 2017. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 389:1229–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Irwin MR, Breen EC, Cole SW. 2019. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology 106:277–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. 2010. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav.Immun 24:1223–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J 2009. On the origin of the immune system. Science 324:580–82 [DOI] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, DeWall CN, Ciarocco NJ, Bartels JM. 2007. Social exclusion decreases prosocial behavior. J. Personal. Soc. Psychol 92:56–66 [DOI] [PubMed] [Google Scholar]
- Uchino BN, Trettevik R, Kent de Grey RG, Cronan S, Hogan J, Baucom BR. 2018. Social support, social integration, and inflammatory cytokines: a meta-analysis. Health Psychol 37:462–71 [DOI] [PubMed] [Google Scholar]
- Umberson D, Crosnoe R, Reczek C. 2010. Social relationships and health behavior across the life course. Annu. Rev. Sociol 36:139–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF. 2014. Hidden in Plain Sight: A Statistical Analysis of Violence Against Children New York: UNICEF [Google Scholar]
- United Nations. 2018. 2018 Revision of World Urbanization Prospects New York: United Nations [Google Scholar]
- Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. 2016. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart 102:1009–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luenen S, Garnefski N, Spinhoven P, Spaan P, Dusseldorp E, Kraaij V. 2018. The benefits of psychosocial interventions for mental health in people living with HIV: a systematic review and meta-analysis. AIDS Behav 22:9–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt TM, Mullooly JP, Ernst D, Pope CR, Hollis JF. 1992. Social networks as predictors of ischemic heart disease, cancer, stroke and hypertension: incidence, survival and mortality. J. Clin. Epidemiol 45:659–66 [DOI] [PubMed] [Google Scholar]
- Walton GM, Cohen GL. 2011. A brief social-belonging intervention improves academic and health outcomes of minority students. Science 331:1447–51 [DOI] [PubMed] [Google Scholar]; This landmark RCT demonstrated that enhancing social belonging improves threat perception, academic performance, health, and well-being.
- Walton GM, Cohen GL, Cwir D, Spencer SJ. 2012. Mere belonging: the power of social connections. J. Pers. Soc. Psychol 102:513–32 [DOI] [PubMed] [Google Scholar]
- Weiner H 1992. Perturbing the Organism: The Biology of Stressful Experience Chicago, IL: Univ. Chicago Press [Google Scholar]; This book provides a groundbreaking summary of research demonstrating the impressive specificity of stress-physiology- health links.
- Weiss JM, Simson PG. 1985. Neurochemical mechanisms underlying stress-induced depression In Stress and Coping, ed. Field TM, McCabe PM, Schneiderman N, pp. 93–116. Hillsdale, NJ: Lawrence Erlbaum [Google Scholar]
- WHO (World Health Organ.). 2016. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease Geneva: WHO [Google Scholar]
- Williams KD. 2007. Ostracism. Annu. Rev. Psychol 58:425–52 [DOI] [PubMed] [Google Scholar]
- Williams KD, Nida SA. 2014. Ostracism and public policy. Policy Insights Behav. Brain Sci 1:38–45 [Google Scholar]
- Worthington EL Jr. 2013. Forgiveness and Reconciliation: Theory and Application New York: Routledge [Google Scholar]
- Yazawa A, Inoue Y, Stickley A, Li D, Du J, Watanabe C. 2015. The effects of season of birth on the inflammatory response to psychological stress in Hainan Island, China. PLOS ONE 10:e0139602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J 2019. The War for Kindness: Building Empathy in a Fractured World New York: Crown [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, et al. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
RELATED RESOURCES
- Cicchetti D 2016. Socioemotional, personality, and biological development: illustrations from a multilevel developmental psychopathology perspective on child maltreatment. Annu. Rev. Psychol 67:187–211 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M. 2019. Developmental adaptation to stress: an evolutionary perspective. Annu. Rev.Psychol 70:111–39 [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J 2018. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu. Rev. Psychol 69:437–58 [DOI] [PubMed] [Google Scholar]
- Lieberman MD. 2007. Social cognitive neuroscience: a review of core processes. Annu. Rev. Psychol 58:259–89 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK. 2014. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev 47:578–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. 2009. Health psychology: developing biologically plausible models linking the social world and physical health. Annu. Rev. Psychol 60:501–24 [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. 2013. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 18:15–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra DA, Coan JA. 2018. Relationships and health: the critical role of affective science. Emot. Rev 10:40–54 [Google Scholar]
- Schaller M 2006. Parasites, behavioral defenses, and the social psychological mechanisms through which cultures are evoked. Psychol. Inq 17:96–101 [Google Scholar]
- Slavich GM, Shields GS. 2018. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): an overview and initial validation. Psychosom. Med 80:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]


