Abstract
Background
Natural killer (NK) cells are immune cells capable of killing virally infected cells and tumor cells without the need for antigen stimulation. Tumors, however, can create a suppressive microenvironment that decreases NK function. A feature of many tumors is hypoxia (low oxygen perfusion), which has been previously shown to decrease NK function. A high affinity NK (haNK) cell has been engineered to express a high affinity CD16 receptor as well as internal interleukin (IL)-2 for increased antibody-dependent cellular cytotoxicity (ADCC) and activation, respectively. We sought to investigate the tolerance of NK cells versus haNK cells to hypoxia.
Methods
We exposed healthy donor (HD) NK and X-irradiated haNK cells to normoxia (20% oxygen) as well as hypoxia (0% oxygen) and investigated their ability to kill prostate, breast and lung tumor cell lines after 5 hours. We also used monoclonal antibodies cetuximab (anti-EGFR) or avelumab (antiprogrammed death-ligand 1) to investigate the effects of hypoxia on NK ADCC. Genomic and proteomic analyzes were done to determine the effect of hypoxia on the expression of factors important to NK cell function.
Results
While HD NK cell cytolytic abilities were markedly and significantly impaired under hypoxic conditions, haNK cells maintained killing capacity under hypoxic conditions. NK killing, serial killing and ADCC were maintained under hypoxia in haNK cells. IL-2 has been previously implicated in serial killing and perforin regeneration and thus the endogenous IL-2 produced by haNK cells is likely a driver of the maintained killing capacity of haNK cells under hypoxic conditions. Activation of signal transducer and activator of transcription 3 (STAT3) is not seen in haNKs under hypoxia but is significant in HD NK cells. Pharmaceutical activation of STAT3 in haNKs led to reduced killing, implicating active STAT3 in reduced NK cell function.
Conclusions
In contrast to HD NK cells, haNK cells are resistant to acute hypoxia. The potent cytolytic function of haNK cells was maintained in an environment comparable to what would be encountered in a tumor. The data presented here provide an additional mechanism of action for haNK cells that are currently being evaluated in clinical trials for several tumor types.
Keywords: immunology, oncology, tumors
Background
Natural killer (NK) cells are a type of immune cell possessing cytolytic abilities independent of antigen stimulation.1 NK cells play an important role in the anticancer response2 and favorable prognosis has been correlated with increased tumor NK cell infiltration and function.2 3 NK cells recognize target cells through lack of major histocompatibility complex class I, which is often downregulated by tumors.4 After ligation of activating receptors such as NKG2D, NK cells kill target cells through release of perforin and granzyme granules.5 NK cells can also recognize target cells through antibody-dependent cellular cytotoxicity (ADCC), when NK CD16 binds to the Fc region of immunoglobulins bound to target cells and leads to NK cell degranulation and target lysis.6 In humans, it has been noted that patients with the V/V polymorphism at position 158 of CD16 had greater responses to therapies using monoclonal antibodies (mAbs), suggesting enhanced binding to IgG1 and therefore greater ADCC.7–9
While NK cells can be effective against tumor cells, the tumor microenvironment (TME) is suppressive to NK cells. Tumors often have very low (<0.1%) levels of oxygen perfusion10 due to increased cellular demands as well as abnormal vasculature.11 NK cytolytic function has been previously shown to be impaired under hypoxic conditions,12 13 suggesting that when NK cells infiltrate a tumor their function is likely diminished.
Interleukin 2 (IL-2) is critical to NK activation and function14 and can rejuvenate exhausted NK cells.15 IL-2 has also been shown to overcome hypoxia-induced NK impairment.13 However, recombinant IL-2 given systemically to patients with cancer can result in significant toxicity and may not be clinically feasible for most tumor types.16
We have previously extensively described the clinical potential of high affinity NK (haNK) cells.17–21 These cells are based on NK-92 (non-Hodgkin’s lymphoma) engineered to express high avidity CD16 (V158) for increased ADCC activity and IL-2 for an internal autocrine loop. In addition, these cells do not express the inhibitor molecule killer immunoglobulin receptor. haNK cells can be grown in large numbers for adoptive transfer (post 10 Gy irradiation) and are a potential universal therapy as no recipient matching is required. haNK cells are currently in clinical trials for pancreatic cancer (NCT03586869, NCT03387098, NCT03329248), triple negative breast cancer (NCT03387085), squamous cell carcinoma (NCT03387111) and metastatic colorectal cancer (NCT03563157) with promising clinical results.22–24
While haNK cells are a promising treatment, their function under hypoxic conditions (and thus in the TME) remains to be determined. In the present study, we investigated the effects of normoxia (20% oxygen) and hypoxia (0% oxygen) on healthy donor (HD) NK cells as well as haNK cells. Here for the first time, we show that haNK cells maintain NK killing, ADCC and serial killing21 under hypoxia while HD NK cells and NK cells from patients with cancer were significantly inhibited under these conditions. The mechanism for haNK cell resistance to hypoxia appears to be IL-2-mediated prevention of signal transducer and activator of transcription 3 (STAT3) activation in haNK cells, preserving their function under hypoxia.
Methods
Tumor cell lines
Human prostate cancer (PC3) cells were grown in FK-12 media, human lung cancer (H460) cells were grown in Roswell Park Memorial Institute (RPMI) and human breast cancer (MCF-7) cells were grown in Dulbecco’s Modified Eagle Medium with 2.5 µg/mL insulin. All media was supplemented with 10% fetal bovine serum, 2 mM glutamine, 0.1 mM sodium pyruvate, 1 mM HEPES, 0.1 mM non-essential amino acids, 1× penicillin/streptomycin and 50 µg/mL gentamicin sulfate. All cell lines were obtained from American Type Culture Collection, mycoplasma-free, used at low passage numbers and cultured at 37°C with 5% CO2.
NK cell preparation
Blood samples were obtained from normal HDs and processed as previously described.25 Briefly, blood was washed and frozen at a concentration of 5×107 cells/mL with fetal bovine serum (Atlanta Biologicals) containing 10% dimethyl sulfoxide. NK cells were isolated using the Human NK Cell Isolation (negative selection) Kit (130-092-657 Miltenyi Biotech), according to the manufacturer’s protocol. Purified NK cells were incubated overnight in RPMI-1640 medium (Mediatech) containing the supplements listed above. NK cells were de-identified and assigned NIH internal donor numbers. HD NK cells were designed HD***, where *** is the NIH internal donor number. NK cells from patients with cancer were obtained in a similar manner and depicted as CP***, where *** is the NIH internal donor number.
haNK cells
haNK cells are an NK cell line, NK-92, which expresses IL-2 and the high affinity (V) CD16 allele, as previously described.20 haNK cells were supplied by NantBioScience (Culver City, California, USA) through a Cooperative Research and Development Agreement with the National Cancer Institute and cultured at a concentration of 5×105 cells/mL in X-Vivo-10 medium (Lonza) supplemented with 5% heat-inactivated human AB serum (Omega Scientific).
Immunofluorescence staining
haNK and HD NK cells were divided into two T-25 flasks for each oxygen condition (normoxia at 20% and hypoxia at 0% O2). For pimonidazole staining, cells in each flask were incubated with 100 μM pimonidazole. Cells in the 0% O2 group were placed in the hypoxia chamber with oxygen sensor and flushed with 0% O2 for 7 min, tubes were clamped and the chamber was placed back into the incubator in standard culture conditions with the 20% O2 group. The hypoxia chamber was flushed every 2 hours and placed back into the incubator. After 5 hours, flasks were removed from their O2 conditions, counted and adjusted to 5×106 cells per mL in PBS; 100 µL of cells were added to Cell-Tak-treated slides (100 µL/section) and allowed to incubate at room temperature (RT) for 20 min. Slides were tilted to remove excess cell suspension and were immediately fixed with 3% paraformaldehyde (100 µL) for 20 min and washed with PBS. Fixed slides were stored overnight at 4°C in PBS. Cells were permeabilized with 0.05% Triton X-100 for 20 min at RT and then washed three times for 5 min each with PBS. Cells were then blocked with 3% PBS/bovine serum albumin (BSA) for 30 min and washed with PBS. For pimonidazole staining, the hypoxyprobe fluorescein isothiocyanate-labeled antibody (Hypoxyprobe-Green Kit, Hypoxyprobe) was added (5 µL/mL in 3% PBS/BSA) for 45 min. For perforin staining, the primary antiperforin (eBiosciences) or isotype antibodies were diluted 1:250 in 3% PBS/BSA and added 3 hours, then washed with PBS. The secondary antibody (antimouse Alexa fluor 488) was diluted 1:1000, added to cells for 45 min in the dark, then washed three times for 5 min each with PBS; 30 µL antifade+DAPI was added to each section for mounting each coverslip. Slides were allowed to dry completely and stored away from light before analysis. Image quantification was assessed by ImageJ.26
Flow cytometry
haNK cells were incubated in 20% or 0% O2 as described above. After 5 hours, cells were harvested, washed with FACS buffer (PBS+1% BSA) and counted. Human Fc block (BD Pharmingen, catalog #564219) was added to 1×106 cells and incubated for 10 min on ice. Cells were stained with PE-labeled antibodies (listed in online supplementary table S1), fixed with Becton Dickinson (BD) Cytofix (catalog #554655) and analyzed with the BD FACSCalibur. Each sample was gated on an isotype used on the same cells under the same conditions.
jitc-2019-000246supp001.pdf (107.7KB, pdf)
Western analysis
haNK cells were incubated in 20% or 0% O2 as described above. After 5 hours, cells were lysed using Cell Lysis Buffer (catalog #9803; Cell Signaling Technology). Phenylmethane sulfonyl fluoride (PMSF) (1 mM; Cell Signaling Technology) and Halt protease/phosphatase inhibitor cocktail (10 µL; Santa Cruz Biotechnology) were added to cells which were then incubated on ice for 10 min before vortexing and centrifugation for 10 min at 14,000 × g; 60 µg protein was added to Bolt 4%–12% Bis-Tris Gels and run with Bolt MES SDS running buffer (Invitrogen). Samples were transferred to a nitrocellulose membrane using the iBlot system (Invitrogen). Blots were probed with anti-IL-2 (catalog #12 239S; Cell Signaling Technology) at a 1:1000 dilution or anti-β-actin (catalog #3700S; Cell Signaling Technology) at a 1:1000 dilution. Secondary antibodies were used at a 1:10 000 dilution. Images were quantified using ImageJ.27 Capillary western blot analysis for phospho (Y705)-STAT3 was performed by the NIH Collaborative Protein Technology Resource. Briefly, samples were prepared in the same manner as described above and 20–40 ng protein was loaded into capillaries. After separation, proteins were immobilized with UV light and capillaries were probed for proteins of interest. A chemiluminescent reaction was captured, and a digital image was created and quantified.
Human IL-2 ELISA
Supernatant media was taken from haNK cells incubated at 20% or 0% O2 and analyzed with the Abcam high sensitivity human IL-2 ELISA kit (#ab46054; Cambridge, Massachusetts, USA) according to the manufacturer’s instructions. Sensitivity was <0.97 pg/mL, standard range was 1.87–60 pg/mL.
NK lysis assays
Tumor cell lines were harvested with trypsin, washed, counted and adjusted to appropriate concentrations; 2×105 total cells were labeled with 20 µL/mL of 111In at 37°C for 25 min. Cells were washed and plated with haNK or HD NK cells at a 20:1 effector to target (E:T) ratio and incubated at 20% or 0% O2 as described above. After 5 hours, supernatant media was assayed for 111In and used to calculate per cent lysis based on the formula: per cent lysis=((actual 111In–spontaneous 111In release)/(maximum 111In–spontaneous 111In release))×100. Spontaneous release was determined by incubating 111In-labeled cells without effectors (haNK or HD NK). Maximum release was calculated by lysing labeled cells with 2% Triton X-100. To calculate serial killing, multiple E:T ratios were plated and killing frequency was calculated as: killing frequency=# killed targets/# effectors cells plated (as previously described15).
RNAseq
haNK and HD NK cells were incubated for 5 hours at 37°C under 20% and 0% O2. Cells were harvested, washed and RNA was isolated using the Qiagen RNeasy Mini Kit (Qiagen, catalog #74104) according to the manufacturer’s instructions. Purified RNA was stored at −80°C until analysis. RNA was sent to NantOmics sequencing facility (Culver City). RNAseq libraries were prepared using the KAPA Stranded RNA-Seq Kit with RiboErase (Kapa Biosystems) and sequenced to a target depth of 200M reads on the Illumina HiSeq platform (Illumina). Samples were aligned to RefSeq build 73 transcriptomes using Bowtie2 V.2.2.6 and quantified using RSEM V.1.2.25 to transcripts per million. Downstream analysis was done in Python V.2.7.6 using numpy V.1.11.1, scipy V.0.17.1 and pandas V.0.18.1.
Proteomic analysis
In haNK and HD NK cells cultured under 20% and 0% O2, 63 clinically validated protein biomarkers were simultaneously measured using selected reaction monitoring mass spectrometry as previously described.28 Briefly, cells were solubilized to tryptic peptides using Liquid Tissue digestion. The total protein concentration of each peptide mixture was measured with a Micro BCA Assay Kit (Thermo Fisher Scientific, San Jose, California, USA). Quantitative proteomic analysis was performed with a TSQ Quantiva triple quadrupole mass spectrometer (Thermo Fisher Scientific) using stable isotope-labeled internal standard peptides for quantitation of target proteins. Each sample was run in triplicate and the %coefficients of variation calculated.
STRING analysis
Protein interactions and predictions were created using STRING software.29 Proteins identified in proteomic analysis were used as input and a STRING map was created with other associated proteins.
Statistics
Statistics were calculated using GraphPad Prism software (GraphPad, San Diego, California, USA) unless otherwise stated. Significant differences are denoted by an asterisk (*) if p≤0.05.
Results
Induction of acute hypoxia of HD NK cells or haNK cells exposed to 0% oxygen
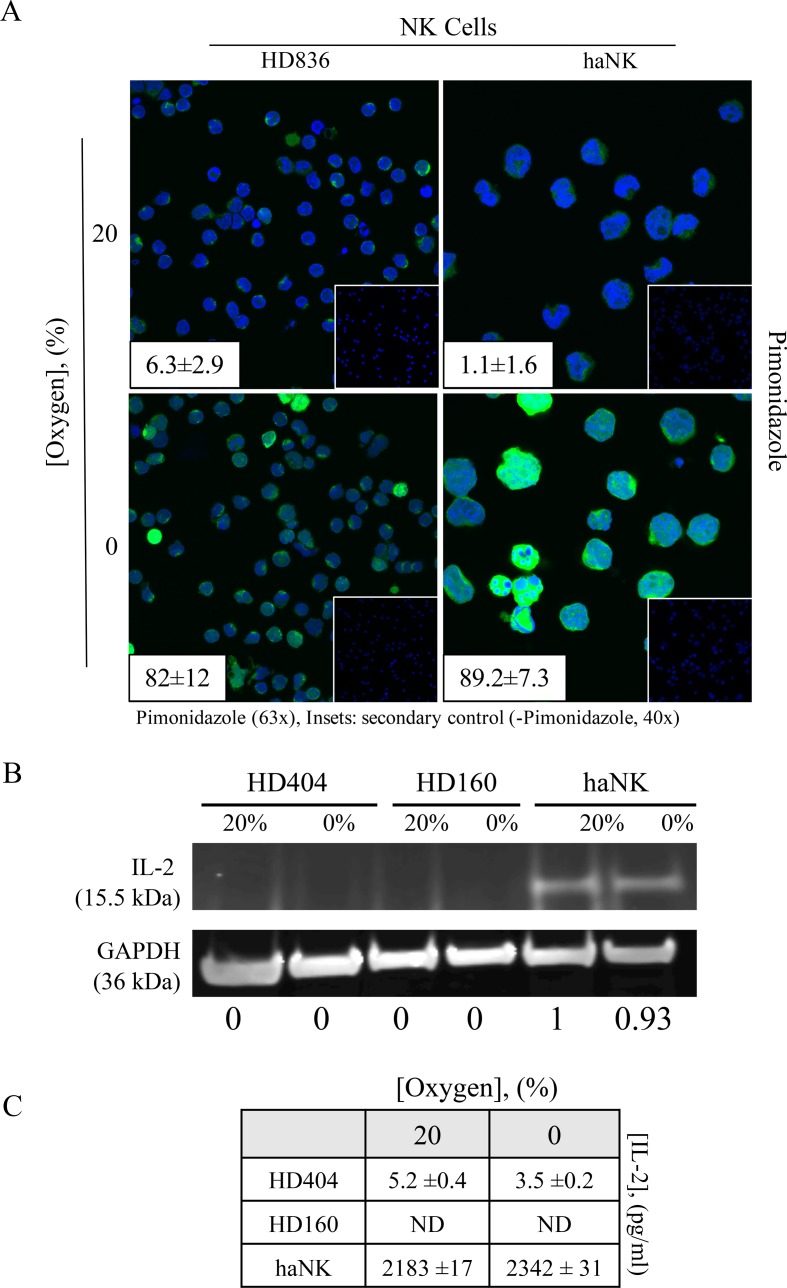
Hypoxia is a common feature of tumors and has been demonstrated in most solid tumor types11 30 and is often associated with poorer outcomes.31 We sought to investigate the effects of hypoxia on HD NK and X-irradiated haNK cells by culturing them in either normoxic (20% oxygen) or hypoxic (0% oxygen) conditions for 5 hours. We first demonstrated hypoxic conditions through staining with pimonidazole, a marker of hypoxia (figure 1A).32 Healthy donor NK cells typically do not produce their own IL-2.33 We examined endogenous IL-2 production from two healthy NK donors under normoxia or hypoxia by western blot analysis (figure 1B) and ELISA (figure 1C). There was no significant IL-2 produced from human NK cells in either condition. We also demonstrated no effect of acute (5 hours) hypoxia on endogenous expression of IL-2 by haNK cells through a western blot analysis (figure 1B) and confirmed these findings by ELISA of culture medium (figure 1C).
Figure 1.
Induction of acute hypoxia of healthy donor (HD) natural killer (NK) cells or high affinity NK (haNK) cells exposed to 0% oxygen. (A) HD NK cells or haNK cells were incubated in ambient (20%) oxygen or 0% oxygen in the presence of the hypoxia indicator pimonidazole for 5 hours at 37°C. Cells were stained for Hypoxyprobe (green, 63×), DAPI nuclear stain (blue) and visualized by microscopy. Insets: average pimonidazole positive staining cells positive per high power field; (right) secondary antibody control (40×); (B) haNK cells continue to produce endogenous interleukin (IL)-2 under hypoxic conditions. Cells were incubated in 20% oxygen or 0% oxygen for 5 hours at 37°C. IL-2 expression was assessed by western blot analysis and quantified using band densitometry analysis normalizing to GAPDH (inset panels), and (C) quantified by ELISA. This experiment was repeated three times with similar results.
Acute hypoxia inhibits killing by HD NK cells while haNK cell function was preserved
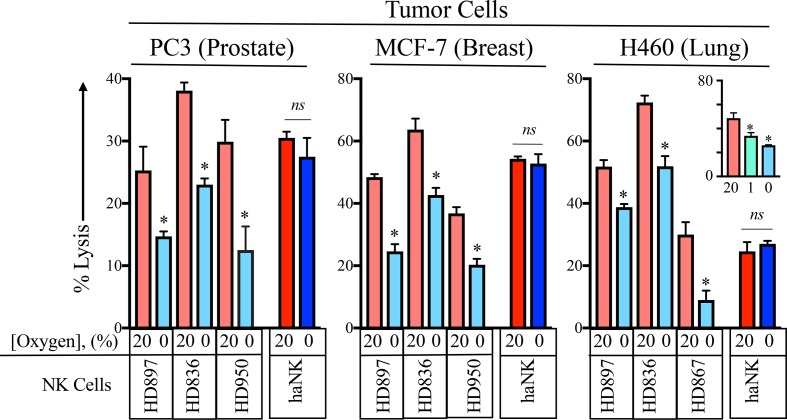
Decreased NK function under hypoxic conditions has been previously reported.13 34 There, the authors examined the function donor NK cells cultured at 21%, 1%, 0.2% and 0% concentrations of oxygen. The significant inhibition of NK function was seen at 1%. For our studies, we focused on the worst case possibility on NK function; a 0% oxygen environment. In order to investigate the function of HD NK as well as haNK cells under hypoxic conditions, prostate (PC3), breast (MCF-7) and lung (H460) human cancer cell lines were labeled with 111In and incubated with either HD NK or haNK cells in 20% or 0% oxygen for 5 hours at 37°C (figure 2). As it had been previously demonstrated that pre-incubation of NK or target cells at 0% oxygen did not affect NK activity,13 cells were not exposed to 0% oxygen before co-incubation. Under 0% oxygen, HD897 NK cells killing ability was decreased by 41% (p=0.02). HD836 NK cells displayed a 40% decrease (p=0.0008) and HD950 NK cells killing decreased by 58% (p=0.028). In contrast, haNK cells maintained killing ability under hypoxic conditions (p=0.39). Similar patterns were observed in MCF-7 and H460 cell lines. Additionally, HD NK killing was significantly decreased by 23% between 1% and 0% O2 in H460 cells (p=0.039, figure 2 inset). Although there is substantial variability among HD NK cells to kill prostate, breast and lung cancer tumor cells, an analysis of 13 independent donors indicated that 12 of 13 (92%) exhibited a significant loss of lytic capability after exposure to 0% oxygen (online supplementary figure 1).
Figure 2.
Acute hypoxia inhibits killing by healthy donor (HD) natural killer (NK) but not high affinity NK (haNK) cells. Prostate (PC3), breast (MCF-7) and lung (H460) cancer cell lines were labeled with 111In and co-incubated with either of three HD (HD897, HD836, HD950) NK or haNK cells in 20% or 0% oxygen for 5 hours at 37°C. 111In release was measured and used to calculate per cent lysis of target cells. Inset: killing of H460 cells was done at 1% oxygen in addition to 20% and 0% oxygen. *P≤0.05 using Student’s t-test. This experiment was repeated two or more times with similar results. ns, not significant.
jitc-2019-000246supp002.pdf (761.4KB, pdf)
Acute hypoxia inhibits ADCC in HD NK cells but not haNK cells
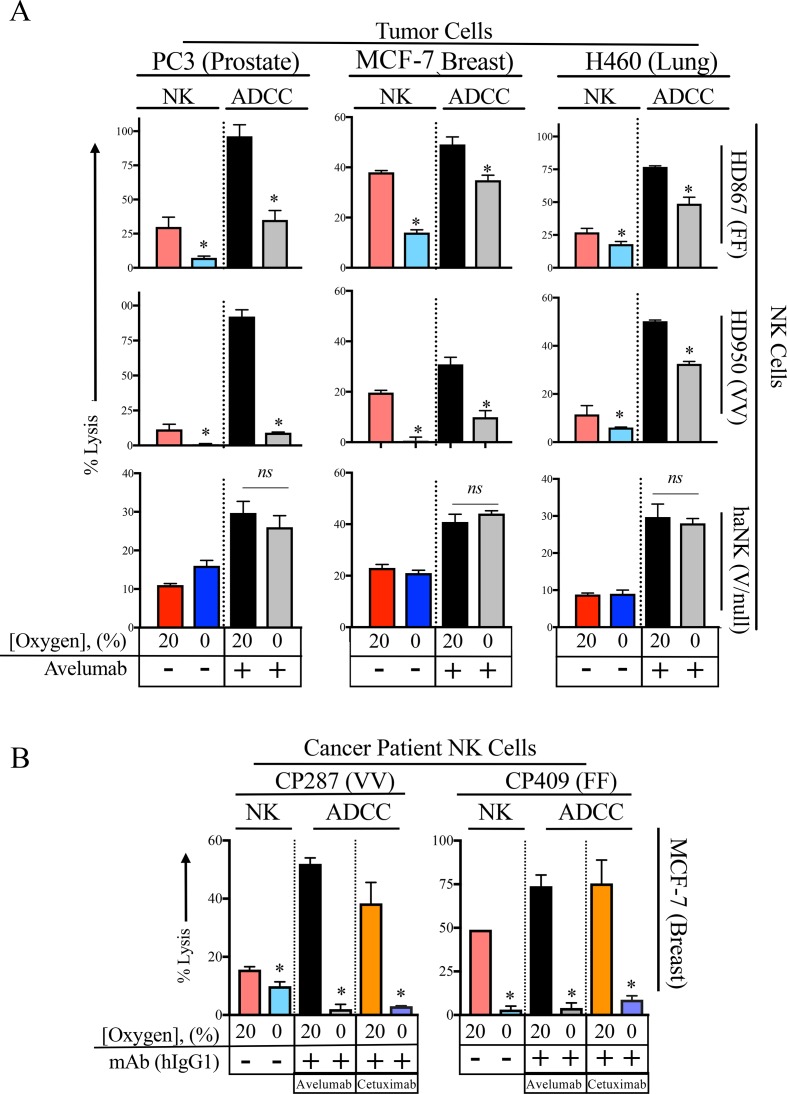
It has been previously reported that hypoxia did not affect ADCC capabilities of human NK cells, which can be partially attributed to the fact that hypoxia did not affect CD16 protein expression.34 To determine the effects of hypoxia on HD NK cells and haNK cells, 111In-labeled PC3, MCF-7 and H460 cells were incubated in 20% or 0% oxygen. ADCC was demonstrated using a human IgG1 against programmed death-ligand 1 (PD-L1) (avelumab 2 µg/mL). Confirming results from figure 2, hypoxia significantly inhibited HD NK killing while haNK killing remained constant (figure 3A). The addition of avelumab led to increased killing of PC3 through ADCC by HD and haNK compared with NK killing. Under normoxic conditions, NK cells from HD867 and HD950 killed at a higher level than haNK cells (figure 3A). Compared with normoxia, hypoxia led to decreases in killing of 63% (p=0.005) and 90% (p<0.0001) in HD867 and HD950, respectively. haNK cells did not show diminished ADCC capacity under hypoxic conditions (p=0.43). Additionally, ADCC was independent of CD16 polymorphism (F or V) as HD867 and HD950 NK cells killed at similar levels despite having different polymorphisms. Similar results were obtained in MCF-7 and H460 cells. Interestingly, haNK cell killing of certain tumor cells was lower than select HD NK cells, although HD NK cells are not being suggested as an adoptive cell therapy alternative to haNK cells. Importantly, when combined with a human IgG1 mAb to mediate ADCC, killing by haNK cells was superior to that of HD NK cells in all examined donors.
Figure 3.
Acute hypoxia inhibits antibody-dependent cellular cytotoxicity (ADCC) in healthy donor (HD) natural killer (NK) cells but not high affinity NK (haNK) cells. 111In-labeled prostate (PC3), breast (MCF-7) and lung (H460) cancer cell lines were incubated in 20% or 0% oxygen at 37°C with either HD (HD867 and HD950) NK cells or haNK cells. ADCC was demonstrated using an antiprogrammed death-ligand 1 (anti-PD-L1) antibody (avelumab, hIgG1, 2 µg/mL). After 5 hours, 111In was measured to determine per cent lysis of target cells. (A) Lysis and ADCC by HD NK and haNK cells. CD16 polymorphism is indicated for each donor. (B) Killing by NK cells from patients with cancer (CP1 and CP2). ADCC was determined using an anti-PD-L1 antibody (avelumab, hIgG1, 2 µg/mL) or an anti-EGFR antibody (cetuximab, hIgG1, 2 µg/mL). *P≤0.05 using Student’s t-test. ns, not significant.
NK cells have been reported to have an exhausted phenotype in patients with cancer.35 36 To determine if NK cells from patients with cancer displayed any altered NK killing ability, NK cells from pretreatment patients with cancer were used in the same assay described above using MCF-7 cells (figure 3B). In addition to avelumab, a human IgG1 against EGFR (cetuximab) was used to demonstrate ADCC. Compared with 20% oxygen, under 0% oxygen CP1 NK killing ability was decreased by 37% (p=0.034). Furthermore, ADCC was decreased by 96% (p>0.0001) and 92% (p=0.008) using avelumab and cetuximab, respectively. CP2 NK inhibition under 0% oxygen was comparable, with decreases in target lysis of 94% (p<0.0001), 95% (p=0.0006) and 88% (p=0.0079) in baseline killing and ADCC with avelumab and cetuximab, respectively.
Perforin expression is maintained in haNK cells in response to acute hypoxia
Previous reports on hypoxia-induced NK inhibition have suggested decreased expression of certain proteins important to NK function and killing as a possible mechanism.13 34 There was also the possibility that hypoxia could modulate tumor cell phenotype to render the tumor more difficult to target by NK cells. To interrogate this possibility, HD NK and haNK cells as well as PC3, MCF-7 and H460 tumor cell lines were evaluated for protein expression by flow cytometry after 5 hours of exposure to 20% or 0% oxygen (table 1). PC3 cells increased MICA/B expression by 47%, MCF-7 increased CD112 expression by 39% and H460 cells increased B7-H6 expression by 43%; however, there was no consistent change among all three lines in proteins evaluated (table 1). For HD NK cells, it was notable that there was no significant loss of viability by incubating at 0% oxygen (table 1). HD NK cells displayed more uniform changes in gene expression with three donors tested showing decreases in expression of activating receptors NKG2D37 and NKp4438 as well as perforin, while haNK only showed a decrease in NKp44 expression but no change in the other proteins (table 1).
Table 1.
Surface expression, % positive cells (mean fluorescent intensity)
| A. Tumor cells | |||||||||
| Tumor | % O2 | Viability (%) | HLA-ABC | MICA/B | B7-H6 | CD112 | CD155 | PD-L1 | EGFR |
| PC3 | 20 | 97.5 | 94.5 (135) | 28.8 (42) | 2.6 (38) | 9.1 (50) | 97.1 (410) | 93.1 (56) | 97.0 (98) |
| 0 | 97.1 | 95.6 (141) | 42.2 (45) | 2.4 (63) | 9.6 (42) | 98.7 (425) | 88.0 (50) | 94.2 (57) | |
| MCF-7 | 20 | 93.5 | 60.0 (63) | 47.9 (55) | 2.9 (42) | 15.1 (48) | 96.9 (154) | 39.0 (101) | 63.0 (52) |
| 0 | 92.6 | 50.9 (59) | 49.9 (57) | 2.5 (44) | 24.9 (43) | 96.5 (152) | 43.1 (144) | 60.1 (13) | |
| H460 | 20 | 98.7 | 95.9 (115) | 4.7 (23) | 6.9 (20) | 3.6 (27) | 99.1 (226) | 91.2 (47) | 94.9 (44) |
| 0 | 98.2 | 94.2 (108) | 5.6 (27) | 12.1 (24) | 3.3 (26) | 99.6 (233) | 96.4 (41) | 92.6 (49) | |
| B. NK cells | |||||||||
| NK cells | % O2 | Viability (%) | NKG2D | CD16 | NKp44 | NKp46 | DNAM-1 | Granzyme | Perforin |
| HD897 | 20 | 89.7 | 32.6 (440) | 55.2 (82) | 13.7 (95.5) | 58.6 (126) | 19.0 (69) | 69.3 (1946) | 17.4 (489) |
| 0 | 87.6 | 25.0 (337) | 63.8 (74) | 10.1 (141) | 47.8 (97.3) | 23.7 (70) | 77.1 (1781) | 3.6 (764) | |
| HD836 | 20 | 93.2 | 15.5 (690) | 69.5 (236) | 20.3 (207) | 39.0 (117) | 19.3 (215) | 99.0 (2716) | 94.7 (1119) |
| 0 | 91.5 | 9.9 (561) | 45.5 (162) | 2.1 (84) | 35.2 (84.0) | 12.1 (103) | 80.4 (2665) | 14.5 (309) | |
| HD950 | 20 | 91.1 | 9.2 (511) | 68.9 (339) | 25.4 (274) | 53.0 (177) | 22.7 (119) | 91.5 (1101) | 64.2 (276) |
| 0 | 90.6 | 6.2 (458) | 53.6 (193) | 2.1 (119) | 46.5 (132) | 8.5 (160) | 85.8 (787) | 51.4 (220) | |
| haNK | 20 | 90.7 | 22.1 (229) | 58.7 (433) | 2.5 (122) | 3.1 (274) | 59.3 (169) | 99.2 (455) | 55.6 (373) |
| 0 | 90.5 | 17.7 (208) | 54.7 (403) | 1.8 (124) | 3.4 (183) | 67.3 (200) | 98.4 (486) | 60.3 (629) | |
Bold type indicates marked modulation (≥20% change in % positive cells or ≥50% change not observed in isotype control).
haNK, high affinity NK; NK, natural killer; PD-L1, programmed death-ligand 1.
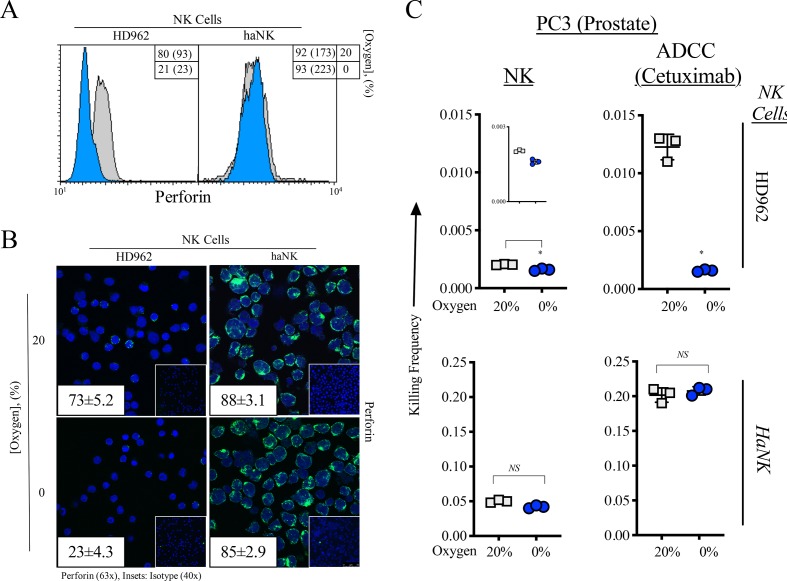
As perforin is the principal mechanism by which NK cells kill,39 we first confirmed that perforin expression was not modulated in haNK cells under hypoxia while HD NK cells experience a significant decrease in perforin levels (figure 4A). Furthermore, immunofluorescent staining for perforin in HD NK and haNK cells revealed higher levels of perforin in haNK cells compared with HD NK cells and that these levels were not affected by hypoxia in haNK cells while staining decreased in HD NK cells (figure 4B).
Figure 4.
Perforin expression is maintained in high affinity natural killer (haNK) cells in response to acute hypoxia. (A) Perforin expression as measured by flow cytometry in healthy donor (HD) NK and haNK cells incubated in 20% (gray) and 0% (blue) oxygen for 5 hours at 37°C. Inset: % positive cells (mean fluorescent intensity). (B) Cells were incubated as in (A) and perforin expression was measured by immunofluorescence (63×). Insets: average perforin positive staining cells positive per high power field; (right) secondary antibody control (40×). (C) 111In-labeled prostate (PC3) cells were co-incubated with HD NK or haNK cells at 37°C for 5 hours under 20% or 0% oxygen. Lysis of target (PC3) cells was determined and used to calculate killing frequency (killing frequency=# target cells killed/# effector cells plated), a measure of the serial killing ability of NK cells. Results are normalized to HD NK at 20% oxygen. *P≤0.05 using Student’s t-test. This experiment was repeated two times with similar results.
NK cells become exhausted after perforin granule release and killing but are able to kill more than one cell before exhaustion occurs (serial killing).15 As we have observed that perforin expression is unchanged in haNK cells with 0% oxygen, we next examined their serial killing ability under hypoxia (figure 4C). HD NK serial killing was significantly decreased under hypoxia by 21% (p=0.015). The addition of cetuximab increased serial killing by sixfold at 20% oxygen compared with baseline NK serial killing and 0% oxygen brought serial killing down to levels similar to those without cetuximab. haNK cells displayed a 24.6-fold increase in serial killing compared with HD962 under normoxia and this was significantly decreased by 16% (p=0.02) under hypoxia, displaying a 20.6-fold increase over HD962 at 20% O2. The addition of cetuximab increased haNK serial killing 99-fold over HD962 at 20% oxygen and was not significantly decreased under 0% oxygen.
Differential gene and protein expression in HD NK and haNK cells is induced by acute hypoxia
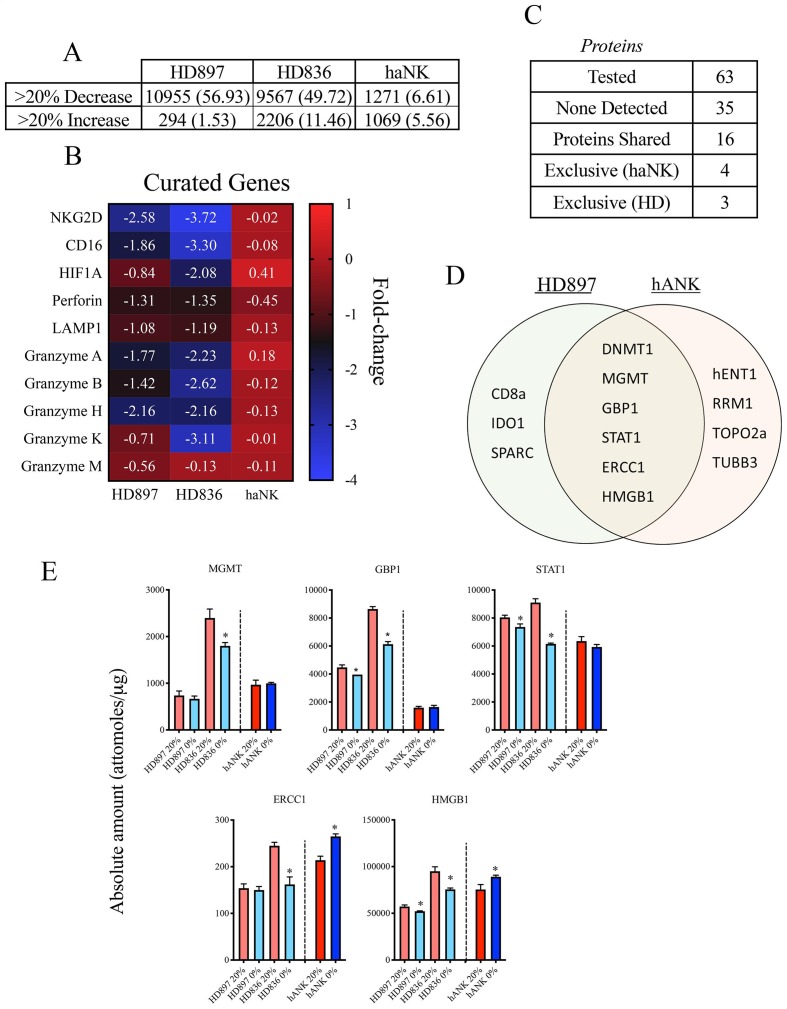
Markers such as NKp44 and NKG2D are important with respect to activating NK cells.38 40 Other markers related to lytic ability include lysosomal-associated membrane protein 1 (LAMP1; CD107a), required for perforin delivery41 and protection from self-induced perforin damage,42 as well as perforin and granzyme, both of which are involved in lysis of target cells.39 43 We examined the effect that hypoxia has on the expression of these and other genes in HD NK and irradiated haNK cells. RNA was isolated from cells cultured at 20% and 0% oxygen for 5 hours and RNAseq was performed. With respect to genes involved in NK activation, lytic function and ADCC capabilities, much more marked changes were observed in HD NK compared with haNK cells (figure 5A). Indeed, under acute hypoxia, expression of NKG2D, NKp44, CD16, perforin, LAMP1 and multiple granzyme genes decreased in HD NK cells while displaying only modest changes in haNK cells (figure 5B).
Figure 5.
Differential gene and protein expression in healthy donor (HD) NK and high affinity natural killer (haNK) cells in response to hypoxia. HD NK and haNK cells were incubated in 20% or 0% oxygen for 5 hours at 37°C. RNA from two HD NK and haNK cells was extracted and RNAseq was performed in triplicate to analyze changes in gene expression in response to hypoxia. (A) Number of genes (and per cent) changed by <20% or >20% in HD NK and haNK cells. (B) Changes in RNA expression of genes important to NK cell function are presented as the ratio of the gene RNA transcripts per million (TPM) of expression under 0% O2 vs the 20% O2 in log2 space. (C) Number of proteins assayed and detected for both HD NK and haNK samples. (D) Diagram of proteins exclusive to HD NK or haNK cells as well as selected proteins shared between both cell types. Quantification of selected proteins is shown in (E). Each sample was run in triplicate and results are presented as mean±%coefficients of variation.
Proteomic analysis was also done to identify hypoxia-related changes at the protein level. Of the 63 proteins assayed, 35 were not detected in any cell type. Four proteins were expressed in haNK but not NK cells (hENT1, RPM1, TOP2A, TUBB3), 3 were expressed in NK cells but not in haNK cells (CD8a, IDO1, SPARC) and 16 were expressed in both cell types (figure 5C, D). The two proteins with the largest relative increases in expression under hypoxia in haNK cells were the DNA repair protein ERCC1 which increased by 19.2% (p=0.0009) and the chromatin-associated non-histone protein known as high-mobility group box 1 protein (HMGB1) which increased by 15.2% (p=0.0135). Expression levels of these proteins decreased under hypoxia in healthy NK cells (figure 5E). Hypoxic expression values for methylated-DNA-protein-cysteine methyltransferase (a DNA repair protein) and guanylate binding protein 1 (GBP1, induced by interferon γ (IFNγ)) remained constant in haNK cells while decreasing in healthy NK cells.
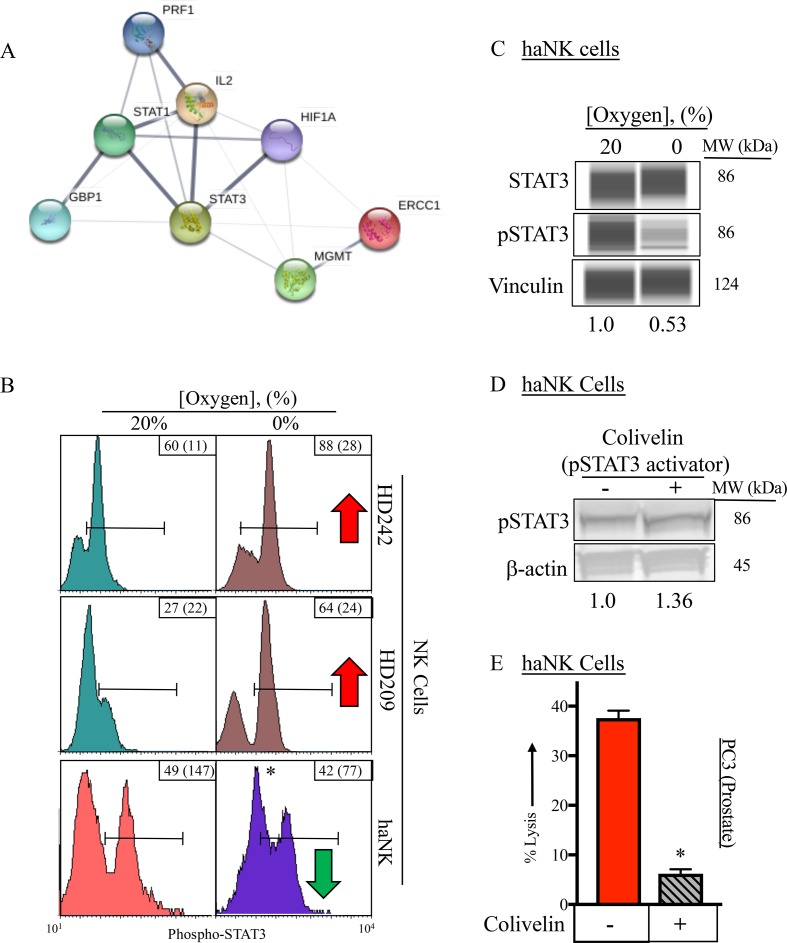
Phospho-STAT3 reduces NK killing capacity
We used STRING analysis to determine relationships of proteins elucidated by the proteomic analysis.29 Using selected proteins from figure 5B as input, we created a STRING protein association network (figure 6A). STAT3 emerged from this analysis connected to multiple proteins of interest. STAT3 has previously been reported to regulate multiple functions of NK activity, with activated (phosphorylated) STAT3 (pSTAT3) leading to decreased NK activity.44 We next investigated pSTAT3 expression by flow cytometry on HD NK and haNK cells cultured under 20% or 0% oxygen (figure 6B). While hypoxia led to increased STAT3 in both HD NK cells, haNK cells showed a slight decrease in STAT3. A twofold decrease in pSTAT3 protein expression in haNK under hypoxia was confirmed by western blot analysis while no change in total STAT3 was observed (figure 6C). Finally, to pharmacologically recapitulate a hypoxic environment, we used an activator of STAT3 (colivelin45), which caused a 36% increase in pSTAT3 in haNK (figure 6D). Pretreatment of haNK cells with colivelin ultimately led to haNK killing inhibition as demonstrated by a sixfold decrease in lysis of PC3 cells at 20% oxygen (figure 6E), further confirming pSTAT3 in NK impairment under hypoxic conditions.
Figure 6.
Phospho-STAT3 (pSTAT3) reduces natural killer (NK) killing capacity. (A) STRING protein network depicting the connections between multiple proteins identified as having an important role in the NK and high affinity (ha)NK response to hypoxia. (B) Flow cytometry analysis of pSTAT3 in healthy donor (HD) NK cells and haNK cells incubated in 20% or 0% oxygen. (C) Capillary western blot analysis of total STAT3 and pSTAT3 in haNK cells incubated in 20% or 0% oxygen. Quantification of pSTAT3 normalized to vinculin loading control is given under each lane. (D) Western blot analysis of pSTAT3 in haNK following the addition of colivelin (100 µM), a pSTAT3 activator. (E) Per cent lysis of PC3 target cells by haNK cells with the addition of colivelin (100 µM). Cells were incubated for 5 hours at 20% or 0% oxygen at 37°C. *P≤0.05 using Student’s t-test.
Discussion
It has been previously reported that hypoxia can impair NK function through mechanisms including, but not limited to, downregulation of CD16, NKG2D, perforin and granzyme.34 As tumors often have very low levels of oxygen, reaching below 0.1%,10 we investigated the effects of short-term hypoxia (at 0% oxygen) on HD NK as well as haNK cells, an NK cell line engineered to express IL-2 as well as a high affinity CD16 receptor.20 Our studies confirmed previous findings that hypoxia does decrease NK function (figures 2–4) and occurs with concomitant decreases in NKG2D and perforin expression (table 1) (figure 5).
While it has been previously reported that ADCC was unaffected in HD NK cells under hypoxic conditions,34 the studies reported here demonstrate significantly impaired NK ADCC under 0% oxygen (figure 3). However, the aforementioned study was done at 1% oxygen with continuous IL-2 supplementation. IL-2 has been demonstrated to partially restore NK function under hypoxia13 and could explain the enhanced NK function relative to our study, in which we did not supplement with IL-2. To this point, the haNK cells, with an internal, continuous supply of IL-2, do not display inhibited lytic or ADCC function under acute hypoxia. Additionally, there are significant differences observed in NK function between 0% and 1% oxygen (figure 2 inset).46
NK cells can kill multiple cells before becoming exhausted and losing function, a capability termed serial killing.15 20 The studies here demonstrate a significant hypoxia-induced impairment of serial killing ability of HD NK (figure 4C). haNK cells demonstrated a much more robust serial killing ability compared with HD NK that was greatly enhanced by the addition of cetuximab, which was not inhibited under 0% oxygen. Because NK cytotoxicity is largely mediated by granule release,47 the enhanced serial killing abilities of haNK cells may be partially explained by the significantly higher perforin levels and their persistence in hypoxia (figure 4A, B). Bhat and Watzl demonstrated the ability of IL-2 to restore the cytolytic ability of exhausted NK cells,15 further implicating IL-2 in the resistance of haNK to hypoxia-induced impairment.
In order to assess genome-wide effects of hypoxia, RNAseq was done on samples from HD NK and haNK cells grown under 20% or 0% oxygen for 5 hours. Confirming results from flow cytometry analysis, perforin and NKG2D expression decreased under hypoxia in HD NK cells but displayed no change in haNK cells. Decreased CD16 expression only in HD NK cells could explain the differences in ADCC ability under hypoxia between HD NK and haNK cells. LAMP1 (also known as CD107a) expression was also decreased in HD NK but not haNK cells under hypoxia. LAMP1 is commonly used as a marker of degranulation,48 but is also fundamental to the cytotoxicity of NK cells. Decreased LAMP1 expression leads to a disruption in perforin trafficking to lytic granules, which then hinders granzyme B delivery and associated cytotoxicity.41 In general, haNK gene expression was altered significantly less in response to hypoxia compared with HD NK cells (figure 5B), suggesting that haNK cells are less susceptible to hypoxia-induced alterations in gene expression.
Proteomic analysis revealed a more pronounced response of haNK cells to hypoxia. Under hypoxia, HMGB1 increased and GBP1 remained constant in comparison to protein quantities under normoxia. HMGB1 secretion may induce immune response; loss of HMGB1 expression correlated with reduced quantities of all immune effectors in the tumor and TME of breast cancer models.49
Using relevant genes and proteins we identified as differentially affected by hypoxia in HD NK and haNK cells as input, we performed STRING analysis.29 This allowed us to visualize the connections involved in hypoxia responses and suggested other proteins which may be involved as well. STAT3 was identified as a nexus of the proteins critical to the haNK cell response to hypoxia. Our interrogation of the pSTAT3 demonstrated that unlike HD NK cells, haNK cells do not increase pSTAT3 under hypoxia. Conversely, they display decreased levels of pSTAT3 (figure 6). We demonstrated decreased haNK killing in the presence of a STAT3 activator (which recapitulated the effects of hypoxia on HD NK cells), implicating pSTAT3 in NK impairment under hypoxic conditions.
In part, this can be attributed to hypoxia-inducible factor 1-alpha (HIF1α) regulation as STAT3 has been shown to bind the HIF1α promoter50 as well as prolong the half-life of HIF1α in tumors by competing with phosphorylated von Hippel-Lindau for binding to HIF1α.51 There is also some evidence of HIF1α regulation of STAT3.52 Additionally, STAT3 and HIF1α cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells.53
As IL-2 appears to be integral to haNK cell resistance to hypoxia, it is reasonable to hypothesize that IL-2 influences STAT3 phosphorylation. Indeed, IL-2 has been previously shown to induce STAT3 phosphorylation in T cells54 as well as an NK cell line.55 However, haNK cells show a decrease in STAT3 phosphorylation in response to hypoxia. haNK cells have a continuous supply of IL-2 which is unaltered by hypoxia (figure 1), hence there is likely an alternative mechanism of STAT3 phosphorylation which is not directly linked to IL-2.
We showed (figure 6) that treatment of haNK cells with a STAT3 activator (colivelin) could recapitulate the effect of hypoxia in HD NK cells. This does not, however, confirm that colivelin exclusively mediated the phosphorylaiton of STAT3. While the exact mechanism of STAT3 activation in this setting needs further elucidation, it is apparent that decreased activation is favorable in relation to NK cell activity. Previous reports have demonstrated that the absence of total STAT3 in NK cells led to increased tumor killing by NK cells.56 Interestingly, the NK cells lacking STAT3 had higher levels of perforin and granzyme as well as the activating receptor DNAM-1. Additionally, it has been shown that STAT3 activation in NK cells leads to decreased NKG2D, IFNγ and TNFα, all of which can lead to decreased cytotoxicity.44
Conclusions
In conclusion, the studies reported here show for the first time that haNK cells are resistant to hypoxia-induced functional suppression. haNK cells possess high levels of perforin which is unaffected by hypoxic conditions and likely bolsters haNK serial killing abilities compared with HD NK cells. haNK cells generally did not display changes in expression of genes and proteins essential to their function and cytolytic abilities (including granzyme, CD16 and NKG2D) while HD NK cells downregulated expression of many of these genes and proteins, suggesting a greater resistance to hypoxia-induced alterations in haNK cells. Finally, we have shown that pSTAT3 is reduced in haNK cells under hypoxic conditions while HD NK cells display increased activation of STAT3. This is integral to the performance and function of haNK cells as their killing ability was severely inhibited when pSTAT3 was experimentally increased. Taken together, our data show that IL-2 and STAT3 are fundamental to the resistance of haNK cells to hypoxia.
As a cancer therapeutic, X-irradiated haNK cells are promising as they maintain activity in hypoxic environments that would render normal NK cells exhausted and non-functional, and they avert the issues involved with systemic IL-2 treatment. The data presented here provide an additional mechanism of action for haNK cells, which are currently being evaluated in clinical trials for several tumor types.22–24
Acknowledgments
The authors would like to thank Jake Griner and Andy Hausted for pilot studies; Marion Taylor for outstanding technical assistance; Sarit Schwartz and Kerry Scott for proteomic analysis; and Debra Weingarten for excellent editorial assistance in the preparation of this manuscript.
Footnotes
Contributors: Conception and design: JWH. Development of methodology: MRP, JWH. Acquisition of data: MRP, TH, SB, JWH. Analysis and interpretation of data: KS, MRP, SB, TH, JWH. Writing/review of manuscript: KS, MRP, SR, PS-S, JS, JWH. Administrative, technical or material support: JS, JWH. Study supervision: JWH.
Funding: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health as well as through a Cooperative Research and Development Agreement (CRADA) between NantBioScience and the National Cancer Institute.
Patient consent for publication: Not required.
Ethics approval: Blood samples were obtained from normal healthy donors on the NCI IRB approved NIH protocol 99-CC-0168. Research blood donors were provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Contact jh241d@nih.gov.
References
- 1.Abel AM, Yang C, Thakar MS, et al. . Natural killer cells: development, maturation, and clinical utilization. Front Immunol 2018;9:9. 10.3389/fimmu.2018.01869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol 2019;40:142–58. 10.1016/j.it.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Pasero C, Gravis G, Granjeaud S, et al. . Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget 2015;6:14360–73. 10.18632/oncotarget.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol 2003;195:346–55. 10.1002/jcp.10290 [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol 2017;8:1124. 10.3389/fimmu.2017.01124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth MJ, Cretney E, Kelly JM, et al. . Activation of NK cell cytotoxicity. Mol Immunol 2005;42:501–10. 10.1016/j.molimm.2004.07.034 [DOI] [PubMed] [Google Scholar]
- 7.Cartron G, Dacheux L, Salles G, et al. . Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002;99:754–8. 10.1182/blood.V99.3.754 [DOI] [PubMed] [Google Scholar]
- 8.Koene HR, Kleijer M, Algra J, et al. . Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997;90:1109–14. 10.1182/blood.V90.3.1109 [DOI] [PubMed] [Google Scholar]
- 9.Weng W-K, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003;21:3940–7. 10.1200/JCO.2003.05.013 [DOI] [PubMed] [Google Scholar]
- 10.Lewis DM, Park KM, Tang V, et al. . Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc Natl Acad Sci U S A 2016;113:9292–7. 10.1073/pnas.1605317113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muz B, de la Puente P, Azab F, et al. . The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015;3:83–92. 10.2147/HP.S93413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink T, Ebbesen P, Koppelhus U, et al. . Natural killer cell-mediated basal and interferon-enhanced cytotoxicity against liver cancer cells is significantly impaired under in vivo oxygen conditions. Scand J Immunol 2003;58:607–12. 10.1111/j.1365-3083.2003.01347.x [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Germeraad WTV, Rouschop KMA, et al. . Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS One 2013;8:e64835. 10.1371/journal.pone.0064835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann C, Zeis M, Uharek L. Activation of natural killer cells with interleukin 2 (IL-2) and IL-12 increases perforin binding and subsequent lysis of tumour cells. Br J Haematol 2001;114:660–5. 10.1046/j.1365-2141.2001.02995.x [DOI] [PubMed] [Google Scholar]
- 15.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells–enhancement by therapeutic antibodies. PLoS One 2007;2:e326. 10.1371/journal.pone.0000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Lotze MT, Muul LM, et al. . Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985;313:1485–92. 10.1056/NEJM198512053132327 [DOI] [PubMed] [Google Scholar]
- 17.Friedman J, Morisada M, Sun L, et al. . Inhibition of WEE1 kinase and cell cycle checkpoint activation sensitizes head and neck cancers to natural killer cell therapies. J Immunother Cancer 2018;6:59. 10.1186/s40425-018-0374-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman J, Padget M, Lee J, et al. . Direct and antibody-dependent cell-mediated cytotoxicity of head and neck squamous cell carcinoma cells by high-affinity natural killer cells. Oral Oncol 2019;90:38–44. 10.1016/j.oraloncology.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giles AJ, Hao S, Padget M, et al. . Efficient ADCC killing of meningioma by avelumab and a high-affinity natural killer cell line, haNK. JCI Insight 2019;4 10.1172/jci.insight.130688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jochems C, Hodge JW, Fantini M, et al. . An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016;7:86359–73. 10.18632/oncotarget.13411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jochems C, Hodge JW, Fantini M, et al. . ADCC employing an NK cell line (haNK) expressing the high affinity CD16 allele with avelumab, an anti-PD-L1 antibody. Int J Cancer 2017;141:583–93. 10.1002/ijc.30767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seery TE, Lee JH, Sender LS, et al. . NANT cancer vaccine an orchestration of immunogenic cell death by overcoming immune suppression and activating NK and T cell therapy in patients with third line or greater metastatic pancreatic cancer (abstr, ASCO 2019). J Clin Oncol 2019;37. [Google Scholar]
- 23.Carlson E, Kistler M, Soon-Shiong P, et al. . NANT Cancer Vaccine an orchestration of immunogenic cell death by overcoming immune suppression and activating NK and T cell therapy in patients with third line or greater TNBC and head & neck SCC (abstr, SITC 2018). J ImmunoTher Cancer 2018;6. [Google Scholar]
- 24.Seery TE, Shinde AM, Kistler M, et al. . NANT cancer vaccine (NCV): an orchestration of immunogenic cell death by overcoming immune suppression and activating natural killer (nk) and t cell therapy in patients with greater than 3rd line metastatic pancreatic cancer (poster, SITC 2018). J ImmunoTher Cancer 2018;6. [Google Scholar]
- 25.Fenerty KE, Padget M, Wolfson B, et al. . Immunotherapy utilizing the combination of natural killer- and antibody dependent cellular cytotoxicity (ADCC)-mediating agents with poly (ADP-ribose) polymerase (PARP) inhibition. J Immunother Cancer 2018;6:133. 10.1186/s40425-018-0445-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rueden CT, Schindelin J, Hiner MC, et al. . ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 2017;18:529. 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hembrough T, Thyparambil S, Liao W-L, et al. . Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J Mol Diagn 2013;15:454–65. 10.1016/j.jmoldx.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 29.Szklarczyk D, Franceschini A, Wyder S, et al. . STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 2015;43:D447–52. (Database issue). 10.1093/nar/gku1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marignol L, Coffey M, Lawler M, et al. . Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat Rev 2008;34:313–27. 10.1016/j.ctrv.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 31.Walsh JC, Lebedev A, Aten E, et al. . The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal 2014;21:1516–54. 10.1089/ars.2013.5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem 1998;253:743–50. 10.1046/j.1432-1327.1998.2530743.x [DOI] [PubMed] [Google Scholar]
- 33.Miller JS, Tessmer-Tuck J, Blake N, et al. . Endogenous IL-2 production by natural killer cells maintains cytotoxic and proliferative capacity following retroviral-mediated gene transfer. Exp Hematol 1997;25:1140–8. [PubMed] [Google Scholar]
- 34.Balsamo M, Manzini C, Pietra G, et al. . Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol 2013;43:2756–64. 10.1002/eji.201343448 [DOI] [PubMed] [Google Scholar]
- 35.Sun C, Xu J, Huang Q, et al. . High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2017;6:e1264562. 10.1080/2162402X.2016.1264562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platonova S, Cherfils-Vicini J, Damotte D, et al. . Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011;71:5412–22. 10.1158/0008-5472.CAN-10-4179 [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Messina L, Reyburn HT, Valés-Gómez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol 2012;3:299. 10.3389/fimmu.2012.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantoni C, Bottino C, Vitale M, et al. . NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med 1999;189:787–96. 10.1084/jem.189.5.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osińska I, Popko K, Demkow U. Perforin: an important player in immune response. Cent Eur J Immunol 2014;39:109–15. 10.5114/ceji.2014.42135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wensveen FM, Jelenčić V, Polić B. NKG2D: a master regulator of immune cell responsiveness. Front Immunol 2018;9:441. 10.3389/fimmu.2018.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krzewski K, Gil-Krzewska A, Nguyen V, et al. . LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood 2013;121:4672–83. 10.1182/blood-2012-08-453738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohnen A, Chiang SC, Stojanovic A, et al. . Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood 2013;122:1411–8. 10.1182/blood-2012-07-441832 [DOI] [PubMed] [Google Scholar]
- 43.Pardo J, Balkow S, Anel A, et al. . Granzymes are essential for natural killer cell-mediated and perf-facilitated tumor control. Eur J Immunol 2002;32:2881–6. [DOI] [PubMed] [Google Scholar]
- 44.Cacalano NA. Regulation of natural killer cell function by STAT3. Front Immunol 2016;7:128. 10.3389/fimmu.2016.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Li Z, Yin Y, et al. . Ghrelin inhibits the differentiation of T helper 17 cells through mTOR/STAT3 signaling pathway. PLoS One 2015;10:e0117081. 10.1371/journal.pone.0117081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loeffler DA, Juneau PL, Heppner GH. Natural killer-cell activity under conditions reflective of tumor micro-environment. Int J Cancer 1991;48:895–9. 10.1002/ijc.2910480617 [DOI] [PubMed] [Google Scholar]
- 47.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 2003;3:361–70. 10.1038/nri1083 [DOI] [PubMed] [Google Scholar]
- 48.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004;294:15–22. 10.1016/j.jim.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 49.Ladoire S, Enot D, Senovilla L, et al. . The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 2016;12:864–75. 10.1080/15548627.2016.1154244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui Y, Li Y-Y, Li J, et al. . STAT3 regulates hypoxia-induced epithelial mesenchymal transition in oesophageal squamous cell cancer. Oncol Rep 2016;36:108–16. 10.3892/or.2016.4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung JE, Kim HS, Lee CS, et al. . STAT3 inhibits the degradation of HIF-1alpha by pVHL-mediated ubiquitination. Exp Mol Med 2008;40:479–85. 10.3858/emm.2008.40.5.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paris S, Chambard JC, Pouysségur J. Tyrosine kinase-activating growth factors potentiate thrombin- and AIF4- -induced phosphoinositide breakdown in hamster fibroblasts. Evidence for positive cross-talk between the two mitogenic signaling pathways. J Biol Chem 1988;263:12893–900. [PubMed] [Google Scholar]
- 53.Pawlus MR, Wang L, Hu C-J. STAT3 and HIF1α cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene 2014;33:1670–9. 10.1038/onc.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell JD, Cook G, Robertson SE, et al. . Suppression of IL-2-induced T cell proliferation and phosphorylation of STAT3 and STAT5 by tumor-derived TGF beta is reversed by IL-15. J Immunol 2001;167:553–61. 10.4049/jimmunol.167.1.553 [DOI] [PubMed] [Google Scholar]
- 55.Frank DA, Robertson MJ, Bonni A, et al. . Interleukin 2 signaling involves the phosphorylation of Stat proteins. Proc Natl Acad Sci U S A 1995;92:7779–83. 10.1073/pnas.92.17.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gotthardt D, Putz EM, Straka E, et al. . Loss of STAT3 in murine NK cells enhances NK cell-dependent tumor surveillance. Blood 2014;124:2370–9. 10.1182/blood-2014-03-564450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2019-000246supp001.pdf (107.7KB, pdf)
jitc-2019-000246supp002.pdf (761.4KB, pdf)