Abstract
Objectives
Many studies reported structural brain changes in patients with alcohol dependence (PADs). However, no studies identified structural correlates of apathy that might aggravate alcohol misuse. Here, we explored regional differences in cortical thickness in PADs relative to healthy controls (HCs), and examined the potential correlation of regional thickness with the severity of apathy.
Methods
Magnetic resonance imaging data were collected from 33 male PADs and 35 male age- and education-matched HCs. We used the FreeSurfer software to investigate group differences in cortical thickness across 148 regions. Apathy was evaluated using the Lille Apathy Rating Scale-Informant (LARS-I). Regression analyses examined the relationship between cortical thickness of regions of interest and apathy score in PADs.
Results
Compared to HCs, PADs showed significant decreases in the cortical thickness of occipito-temporal cortex (OTC), including the left middle occipital gyrus and occipital pole, right superior occipital gyri, and bilateral lingual gyrus; bilateral superior parietal cortex (SPC), including the right intraparietal sulcus; and bilateral inferior parietal cortex (IPC). Furthermore, the cortical thickness of all of the three regions was negatively correlated with the apathy total scores. The cortical thickness of the IPC was also negatively correlated with the action initiation subscore of the LARS-I.
Conclusions
The current results suggest the thickness of bilateral parietal and occipital temporal cortices as neural markers of apathy in PADs. These findings add to the literature by identifying the neural bases of a critical clinical feature of individuals with alcoholism.
Keywords: alcohol dependence, apathy, occipito-temporal cortex, superior parietal cortex, inferior parietal cortex
Introduction
Alcohol dependence (AD) is a chronic illness characterized by poor treatment outcomes and high relapse rates (1). Individuals with AD frequently demonstrate motivational deficits, exacerbating personal and interpersonal health (2). Brain imaging was widely used to investigate the consequences of alcohol misuse, which included atrophy of the gray and white matters, sulcal widening, and ventricular enlargement. The structural changes resulted from excessive alcohol consumption (3–7). These deficits involved multiple brain networks that support executive control, salience response, and emotion regulation, which may directly or indirectly contribute to cognitive and motivational dysfunction in patients with AD (8, 9).
The main clinical feature of apathy is lack of motivation (10) and reduced initiative, interest, and/or concern (11). It is also proposed that apathy be defined as dysfunction of volitional processes or a reduction in self-generated, voluntary, and purposeful behavior (i.e., goal-directed behavior) (12). Difficulties in goal-directed behavior, as a key feature of apathy, was significantly associated with symptoms of alcohol dependence (13). Almost all patients with severe alcohol dependence show diminished or lack of living practices except for drinking-related events, and executive dysfunction on alcohol abstinence (2, 14, 15). Importantly, patients undergoing treatment for AD often relapse despite better knowledge and their intention to remain abstinent, reflecting impairments in volition and goal-directed behavior (16). It is thus critical to investigate the neural correlates of apathy in AD.
In imaging studies of various brain disorders, apathy has been associated with structural and function deficits of multiple brain regions (17), particularly the dorsal anterior cingulate cortex (dACC) (18). Other studies implicated the fronto-subcortical circuits and lateral parietal cortex (19). Numerous studies have also shown structural and functional deficits of a wide range of cortical and subcortical circuits in individuals with AD (20). However, no studies to our knowledge have specifically examined the cerebral structural bases of apathy in AD.
The current study addressed this gap in research. On the basis of the literature that goal-directed behaviors require top-down attentional, cognitive and affective control as well as execution of complex motor movements (21), we hypothesized that the frontal and parietal cortical thickness would be diminished and may represent a neurobiological marker of apathy in AD.
Methods
Participants and Consents
All patients with AD (PADs) were males, of Han Chinese descent, and recruited from Beijing Huilongguan Hospital between 2017 and 2018. Inclusion criteria were as follows: a) age between 18 and 65 years; b) diagnosis of AD in accordance with Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria (22); c) right-handed; d) abstinent for 14 to 28 days after completion of medically assisted withdrawal, with no obvious withdrawal symptoms and a Clinical Institute Withdrawal Assessment for Alcohol (CIWA) (23) score of less than 3. Patients with other axis I mental disorders, including dependence on substances other than alcohol and nicotine, or severe medical conditions (e.g., unstable hypertension, poorly controlled diabetes, myocardial infarction, liver cirrhosis) were excluded. In addition, we recruited 35 male age- and education-matched healthy controls (HCs) from the general medical clinics of the hospital and local community. Prior to study inclusion, all participants provided written informed consent in accordance with the Declaration of Helsinki and a protocol approved by the ethics committee of Beijing Huilongguan Hospital.
Assessments
All PADs were evaluated with the Alcohol Use Disorder Identification Test (AUDIT) (24), a semi-structured questionnaire to investigate the severity of alcohol consumption (25). Lifetime Drinking History assessment to determine the number of daily drinks (one drink = 10 g of ethanol), age at first drink, and age at onset of alcohol dependence. The extent of apathy was assessed using the Lille Apathy Rating Scale-Informant (LARS-I) (11), which comprised 33 items across nine domains, each corresponding to a specific clinical dimension of apathy. Each sub-scale score was from −4 to +4, with higher positive scores indicating more severe symptoms. In brief, a negative or positive score indicates less or more apathy, and zero represents moderate apathy in everyday productivity and interests domains or non-applicable or non-classifiable reply in other domains. In this study, we focused on the apathy total score (T score) and action initiation (AI) subscore in data analyses.
MRI Data Acquisition and Analyses
All PADs (after detoxification) and HCs underwent MRI. MRIs were obtained by using a Siemens 3T MRI scanner. Head motion was minimized using foam pads. Structural MR images were acquired for the whole brain with a sagittal 3D-magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence, using the follow parameters: echo time (TE) = 2.98 ms, inversion time (TI) = 900 ms, repetition time (TR) = 2,300 ms, flip angle (FA) = 9°, field-of-view (FOV) = 240 mm×256 mm, matrix size = 256×240, thickness/gap = 1/0 mm.
Measures of cortical thickness (mm) of 148 cortical regions (bilateral; 74 x 2; Desikan-Killiany Atlas), total subcortical thickness, and total gray matter volume (TGV) were obtained using FreeSurfer (26) (http://surfer.nmr.mgh.harvard.edu). TGV was used as a covariate in all analyses to account for differences in head size. We followed the ENIGMA pipeline (http://enigma.ini.usc.edu/) for quality control of the images: All cortical regions with a thickness >1.5 or <1.5 times the interquartile range were identified and visually inspected by overlaying their segmentations on the participants’ anatomical images. Only imaging data for which segmentation was judged to be accurate were subjected to statistical analyses.
Statistical Analysis
As all participants were male, continuous demographic variables were analyzed using independent samples t-tests and categorical data were analyzed with Chi-square (χ2) tests to compare PADs and HCs. The level of statistical significance was set at p < 0.05 (two-tailed).
Cortical thickness values of the 148 regions were compared between PADs and HCs using univariate linear regression analysis, where the cortical thickness of each region was used as the dependent variable, and group (PADs/HCs), age, smoking status, education, and TGV were entered as independent variables. The threshold for statistical significance was set at p < 0.00033784 (i.e., 0.05/148) to correct for multiple comparisons (Bonferroni correction).
We identified 12 cortical regions of interest (ROIs) that differed significantly between PADs and HCs in thickness, and these regions belong to the occipital temporal cortex (OTC) (lateral cortex including the left middle occipital gyrus, bilateral lingual gyrus, left occipital pole and right superior occipital gyrus), superior parietal cortex (SPC) (lateral cortex including the right intraparietal sulcus and bilateral superior parietal lobule), and inferior parietal cortex (IPC) (lateral cortex including the bilateral angular gyrus and supramarginal gyrus) (27). We performed bivariate correlation analyses to explore the relationship between cortical thickness of the OTC, SPC and IPC and apathy total score (T score), and action initiation subscore (AI score) in PADs. Statistical significance was tested at p < 0.05/6, two-sided, to correct for multiple comparisons. As many alcohol drinkers are heavy smokers, we performed two sets of regression, one without smoking status as a covariate, so that the results best reflect the typical populations of alcoholic participants, and the other with smoking status as a covariate, so the effects of smoking could be controlled for.
Results
Demographic and Clinical Characteristics
There were no significant differences in age (t=1.257, p = 0.213) or years of education (t=−0.883, p=0.381) between PADs and HCs. More PADs were smokers than HCs (χ2 = 11.87, p=0.001). Smoking status was thus included as a covariate in the following data analyses. Compared to HCs, PADs showed higher LARS total or T score (t=5.85, p < 0.001) and action initiation or AI subscore (t=5.43, p < 0.001). The demographic and clinical characteristics of the two groups are presented in Table 1.
Table 1.
Demographic and clinical characteristics in patients with alcohol dependence (PADs) and healthy controls (HCs) (mean ± SD).
| PAD (n = 33) | HCs (n = 35) | t/χ2-value | p-value | |
|---|---|---|---|---|
| Age, years | 44.8 ± 9.8 | 41.6 ± 11.5 | 1.257 | 0.213 |
| Education, years | 11.8 ± 3.3 | 12.3 ± 2.4 | −0.883 | 0.381 |
| Smoker/non-smoker a | 26/7 | 13/22 | 11.87 | 0.001 |
| Action initiation (AI) | −1.21 ± 0.86 | −2.19 ± 0.61 | 5.43 | 0.000 |
| Total score of LARS | −5.85 ± 6.87 | −14.78 ± 5.70 | 5.85 | 0.000 |
| Age at onset of AD, years | 32.5 ± 10.1 | N/A | N/A | N/A |
| Age at first drink, years | 19.9 ± 5.5 | N/A | N/A | N/A |
| AUDIT total score | 21.7 ± 5.2 | N/A | N/A | N/A |
| Mean daily drinks b | 19.1 ± 8.7 | N/A | N/A | N/A |
PADs, patients with alcohol dependence; HCs, healthy controls; LARS, Lille Apathy Rating Scale; AUDIT, Alcohol Use Disorders Identification Test; N/A, not applicable.
Chi-square (χ2) test: smoker = current or previous smoker; non-smoker: never smoker
One drink contains 10 g of pure alcohol.
Cortical Thickness
The cortical thickness of the bilateral lingual gyrus in the OTC was significantly higher in PADs as compared with HCs; however, relative to HCs, PADs exhibited significant decreases in overall cortical thickness in the OTC (consisting of the left middle occipital gyrus and occipital pole, right superior occipital gyri, and bilateral lingual gyrus), SPC, as well as IPC, (all p’s <0.00033784, Table 2) accounting for age, years of education, smoking status, and TGV. The mean cortical thickness and statistics for all 148 regions were shown in Supplementary Table 1.
Table 2.
Brain regions with significant differences in cortical thickness between patients with alcohol dependence (PADs) and healthy controls (HCs).
| Cortical thickness of Destrieux Atlas (mm) | PADs (n 33) | HCs (n = 35) | F-value | p-value |
|---|---|---|---|---|
| L-Middle occipital gyrus | 2.165 ± 0.297 | 2.613 ± 0.123 | 29.545 | 9.768E−07 |
| L-Lingual gyrus | 2.118 ± 0.142 | 1.879 ± 0.131 | 44.499 | 7.960E−09 |
| L-Angular gyrus | 2.152 ± 0.329 | 2.705 ± 0.139 | 26.065 | 3.378E−06 |
| L-Supramarginal gyrus | 2.371 ± 0.271 | 2.741 ± 0.141 | 14.574 | 3.138E−04 |
| L-Superior parietal lobule | 1.931 ± 0.301 | 2.461 ± 0.117 | 32.666 | 3.351E−07 |
| L-Occipital pole | 1.672 ± 0.219 | 1.947 ± 0.145 | 20.949 | 2.321E−05 |
| R-Superior occipital gyrus | 1.881 ± 0.302 | 2.261 ± 0.145 | 17.749 | 8.313E−05 |
| R-Lingual gyrus | 2.137 ± 0.129 | 1.967 ± 0.156 | 16.656 | 1.303E−04 |
| R-Angular gyrus | 2.167 ± 0.324 | 2.704 ± 0.131 | 41.123 | 2.208E−08 |
| R-Supramarginal gyrus | 2.389 ± 0.299 | 2.741 ± 0.118 | 16.734 | 1.262E−04 |
| R-Superior parietal lobule | 1.929 ± 0.318 | 2.445 ± 0.118 | 38.473 | 5.044E−08 |
| R-Intraparietal sulcus and transverse parietal sulci | 1.892 ± 0.211 | 2.191 ± .089 | 20.290 | 3.004E−05 |
One-way ANOVA, Bonferroni corrected at p<0.00033784.
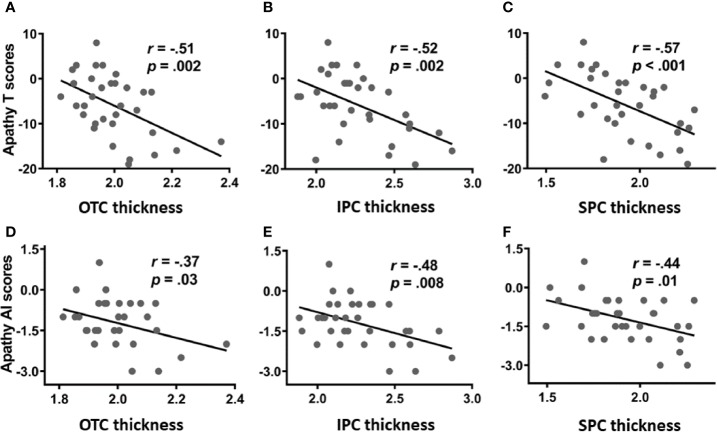
For PADs, we focused on the OTC, SPC, and IPC and performed Pearson correlation analyses to evaluate the relationship between cortical thickness and apathy (T score and AI subscore). Evaluated at p < 0.0083 (i.e., 0.05/6, Bonferroni correction), the results revealed negative correlations between the thickness of the OTC, SPC as well as IPC and both apathy T score. The cortical thickness of the IPC was also negatively correlated with the AI subscore. These results are shown in Figure 1.
Figure 1.
Correlation of cortical thickness of the occipital temporal cortex (OTC), inferior parietal cortex (IPC), and superior parietal cortex (SPC) with apathy total score (T score; upper panel: A–C) and action initiation subscore (AI subscore; lower panel: D–F).
Because many more PADs are smokers, we performed covariance analyses with smoking status as a covariate. The results showed that apathy T score remained negatively correlated with the thickness of the OTC (r=−0.44, p=0.011), IPC (r=−0.47, p=0.007), and SPC (r=−0.58, p < 0.001); and the apathy AI subscore showed negative correlation with thickness of the IPC (r=−0.41, p=0.019) and SPC (r=−0.44, p=0.012) but not the OTC (r=−0.32, p=0.078). The findings suggest that after controlling for smoking status, the thickness of the parietal, but not that of occipital temporal cortex, was correlated with difficulties in action initiation in PADs.
Discussion
We hypothesized that PADs would exhibit reduced thickness in the frontal and parietal cortex, and the extent of the reduction would be correlated with the severity of apathy in PADs. To this end, we determined differences in regional cortical thickness between PADs and HCs and investigated how these differences are related to apathy in PADs. The results suggest that AD was associated with reduced thickness in the bilateral occipital temporal cortex, the superior and inferior parietal cortices, and the extent of the reduction in cortical thickness was correlated with the severity of apathy. Although the cortical thickness of the bilateral lingual gyrus showed peculiar increases in PADs, this change, contrary to that shown in most of the previous studies (5, 28), did not affect the reduction of the whole OTC region in PADs compared with HCs. The mechanism of compensatory hypertrophy might be a potential explanation to this change (29) because a previous study found similar increases in alcohol abuse individuals, regardless of sex and brain regions (30). Greater reduction of parietal cortical thickness appears to be more specifically related to action initiation deficits in PADs. Together, these findings support structural changes of posterior cortical regions as a neural marker of apathy in alcoholism.
Structural imaging studies reported significant volume losses in the prefrontal cortex, insula, and hippocampus in AD (5, 31, 32); however, volumetric deficits of the posterior cortical regions have received less attention (30). For instance, parietal-occipital gray matter volumes were decreased in patients with AD and can be used to predict shorter time of any alcohol use and relapse to heavy drinking (33). A recent meta-analysis of 3,240 individuals with drug and alcohol dependence indicated that the most severe volumetric deficits are found in not only the frontal but also occipital, temporal, and parietal cortices in those with alcohol dependence (34). Consistently, we observed a significant reduction in cortical thickness in the occipital and parietal cortices. However, it is unclear why structural changes cannot be detected in the frontal cortex. One possibility is that the current work only focused on cortical thickness, a volumetric measure different from that as employed in the earlier work. This discrepancy clearly warrants further studies.
Many studies examined the neural mechanisms underlying cognitive and emotional deficits (35) but few focused on apathy in individuals with AD (36). In accordance with previous studies we observed more significant apathy symptoms, as reflected in the apathy total score in PADs than in HCs, suggesting motivational dysfunction in individuals with alcoholism (37). The cortical thickness of the occipital-temporal as well as superior and inferior parietal cortices was negatively correlated with the severity of apathy, consistent with earlier work associating apathy with structural changes in the parietal cortex, in addition to the prefrontal cortex (PFC) and basal ganglia (38). Research has indicated that the superior parietal lobule, including the intraparietal sulcus, play a key role in goal-directed behaviors and volitional processes (39). In addition, an [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) study in a rat model of alcoholism revealed that alcohol significantly reduced whole-brain glucose metabolism, and that the effect was most pronounced in the parietal cortex (40). Together, the current along with these earlier studies support the importance of posterior cortical structures in mediating cognitive and emotional deficits (41), which may manifest as apathy in AD. Importantly, as apathy may impact the motivation for behavioral changes, the current findings are also consistent with our earlier work showing that volumetric deficits in the posterior-occipital region predicted relapse in AD patients undergoing treatment (42).
Limitations
A few limitations of present study included: 1) there were more smokers in PADs than in HCs. Although we have accounted for the potential effects of smoking in data analyses, one cannot completely rule out the effects of chronic smoking on the findings. On the other hand, compared to smoking, alcohol consumption appears to be an overriding factor leading to alterations in brain structures and functions (43). Further, alcohol dependent individuals are typically smokers; thus, one may argue that the current findings reflect that general alcohol dependent populations; 2) as a specialist hospital in Beijing for the PADs, we were exposed to patients with more serious clinical symptoms and social dysfunction and difficult to enroll those patients who have mild symptoms and no obvious apathy. This is the reason of correlation analysis without setting apathy-free patients as control in this study. 3) because there were much fewer women with alcohol dependence in China and no women were included in the present study, we were unable to examine the effects of gender on cortical thickness in relation to the impact of alcohol misuse. As sex differences in the etiological processes or consequences of alcohol misuse have been demonstrated in numerous studies (44, 45). We hope to study more women to examine sex differences, including the finding that neurotoxicity may be more severe among women with alcoholism. Lastly, the sample size of the present study is moderate and the findings would warrant replication.
Conclusions
The present study demonstrated significant differences in occipital, temporal, and parietal cortical thickness in individual with alcohol dependence as compared with healthy controls. These structural changes were related to the severity of apathy in alcohol dependent individuals. Future research with functional imaging may provide an opportunity to understand in more details how the posterior cortical regions participate in motivated behavior and how these processes may be compromised in alcohol dependent individuals with apathy.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Huilongguan Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YT designed the project, and took responsibility for the integrity of the data and the accuracy of the data analysis. KY, QY, and YN were responsible for recruiting the patients, performing the clinical rating, and collecting the samples. KY wrote the paper. Critical revision of the manuscript for important intellectual content was drafted by YT and C-SL. C-SL, XL, ST, ZW, JT, SC, FF, FY, and TL were invited in evolving the ideas, statistical analysis, and editing the manuscript. All authors have contributed to and have approved the final manuscript.
Funding
This work was supported by the Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding support (XMLX201834), Beijing Municipal Commission of Science and Technology (Z161100000516046), and the National Institutes of Health (AA021449). None of the funding sources influenced the study design, the collection, analysis, and interpretation of the data, approval of the manuscript, or the decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge all participants involved in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00364/full#supplementary-material
References
- 1. World Health Organization Global status report on alcohol and health. Geneva, Switzerland: World Health Organization; (2011). 286 p. [Google Scholar]
- 2. Day AM, Kahler CW, Ahern DC, Clark US. Executive Functioning in Alcohol Use Studies: A Brief Review of Findings and Challenges in Assessment. Curr Drug Abuse Rev (2015) 8(1):26–40. 10.2174/1874473708666150416110515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dupuy M, Chanraud S. Imaging the Addicted Brain: Alcohol. Int Rev Neurobiol (2016) 129:1–31. 10.1016/bs.irn.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 4. Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS. White matter volume in alcohol use disorders: a meta-analysis. Addict Biol (2013) 18(3):581–92. 10.1111/j.1369-1600.2012.00441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutherland GT, Sheedy D, Kril JJ. Neuropathology of alcoholism. Handb Clin Neurol (2014) 125:603–15. 10.1016/B978-0-444-62619-6.00035-5 [DOI] [PubMed] [Google Scholar]
- 6. Topiwala A, Ebmeier KP. Effects of drinking on late-life brain and cognition. Evid Based Ment Health (2018) 21(1):12–5. 10.1136/eb-2017-102820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, Pan P. Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend (2015) 153:22–8. 10.1016/j.drugalcdep.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 8. Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology (2014) 76 Pt B:370–82. 10.1016/j.neuropharm.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiat (2016) 3(8):760–73. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry (2008) 79(10):1088–92. 10.1136/jnnp.2007.136895 [DOI] [PubMed] [Google Scholar]
- 11. Sockeel P, Dujardin K, Devos D, Deneve C, Destee A, Defebvre L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson’s disease. J Neurol Neurosurg Psychiatry (2006) 77(5):579–84. 10.1136/jnnp.2005.075929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex (2006) 16(7):916–28. 10.1093/cercor/bhj043 [DOI] [PubMed] [Google Scholar]
- 13. Paulus DJ, Bakhshaie J, Lemaire C, Garza M, Ochoa-Perez M, Valdivieso J, et al. Negative Affectivity and Problematic Alcohol Use Among Latinos in Primary Care: The Role of Emotion Dysregulation. J Dual Diagn (2016) 12(2):137–47. 10.1080/15504263.2016.1172897 [DOI] [PubMed] [Google Scholar]
- 14. Sher KJ. The Oxford handbook of substance use and substance use disorders. Oxford University Press; (2016). 2 p. [Google Scholar]
- 15. Bosscher RJ, Smit JH. Confirmatory factor analysis of the General Self-Efficacy Scale. Behav Res Ther (1998) 36(3):339–43. 10.1016/s0005-7967(98)00025-4 [DOI] [PubMed] [Google Scholar]
- 16. Garbusow M, Sebold M, Beck A, Heinz A. Too difficult to stop: mechanisms facilitating relapse in alcohol dependence. Neuropsychobiology (2014) 70(2):103–10. 10.1159/000362838 [DOI] [PubMed] [Google Scholar]
- 17. Kos C, van Tol MJ, Marsman JB, Knegtering H, Aleman A. Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci Biobehav Rev (2016) 69:381–401. 10.1016/j.neubiorev.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 18. McIntosh RC, Rosselli M, Uddin LQ, Antoni M. Neuropathological sequelae of Human Immunodeficiency Virus and apathy: A review of neuropsychological and neuroimaging studies. Neurosci Biobehav Rev (2015) 55:147–64. 10.1016/j.neubiorev.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 19. Stella F, Radanovic M, Aprahamian I, Canineu PR, de Andrade LP, Forlenza OV. Neurobiological correlates of apathy in Alzheimer’s disease and mild cognitive impairment: a critical review. J Alzheimers Dis (2014) 39(3):633–48. 10.3233/JAD-131385 [DOI] [PubMed] [Google Scholar]
- 20. Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex (2012) 22(1):158–65. 10.1093/cercor/bhr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallivan JP, McLean DA, Valyear KF, Pettypiece CE, Culham JC. Decoding action intentions from preparatory brain activity in human parieto-frontal networks. J Neurosci (2011) 31(26):9599–610. 10.1523/JNEUROSCI.0080-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Psychiatric Association Electronic DSM-IV-TR plus. Version 1.0. ed. Washington, D.C.: American Psychiatric Association; (2000), 1 p. [Google Scholar]
- 23. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict (1989) 84(11):1353–7. 10.1111/j.1360-0443.1989.tb00737.x [DOI] [PubMed] [Google Scholar]
- 24. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction (1993) 88(6):791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 25. Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend (1996) 42(1):49–54. 10.1016/0376-8716(96)01263-X [DOI] [PubMed] [Google Scholar]
- 26. Fischl B. FreeSurfer. Neuroimage (2012) 62(2):774–81. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage (2010) 53(1):1–15. 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res (2011) 35(10):1771–93. 10.1111/j.1530-0277.2011.01540.x [DOI] [PubMed] [Google Scholar]
- 29. Bansal R, Hellerstein DJ, Peterson BS. Evidence for neuroplastic compensation in the cerebral cortex of persons with depressive illness. Mol Psychiatry (2018) 23(2):375–83. 10.1038/mp.2017.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche JP, Ferrett H, et al. Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Res (2013) 214(1):1–8. 10.1016/j.pscychresns.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res (2013) 37(1):67–74. 10.1111/j.1530-0277.2012.01853.x [DOI] [PubMed] [Google Scholar]
- 32. Hoefer ME, Pennington DL, Durazzo TC, Mon A, Abe C, Truran D, et al. Genetic and behavioral determinants of hippocampal volume recovery during abstinence from alcohol. Alcohol (2014) 48(7):631–8. 10.1016/j.alcohol.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, et al. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry (2011) 168(2):183–92. 10.1176/appi.ajp.2010.10020233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, et al. Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry (2019) 176(2):119–28. 10.1176/appi.ajp.2010.10020233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol (2010) 15(2):169–84. 10.1111/j.1369-1600.2009.00194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tumati S, Martens S, de Jong BM, Aleman A. Lateral parietal cortex in the generation of behavior: Implications for apathy. Prog Neurobiol (2019) 175:20–34. 10.1016/j.pneurobio.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 37. Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology (2007) 21(3):346–62. 10.1037/0894-4105.21.3.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lundqvist T. Imaging cognitive deficits in drug abuse. Curr Top Behav Neurosci (2010) 3:247–75. 10.1007/7854_2009_26 [DOI] [PubMed] [Google Scholar]
- 39. Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron (2009) 63(5):568–83. 10.1016/j.neuron.2009.08.028 [DOI] [PubMed] [Google Scholar]
- 40. Gispert JD, Figueiras FP, Vengeliene V, Herance JR, Rojas S, Spanagel R. Changes in cerebral [(18)F]-FDG uptake induced by acute alcohol administration in a rat model of alcoholism. Behav Brain Res (2017) 327:29–33. 10.1016/j.bbr.2017.03.038 [DOI] [PubMed] [Google Scholar]
- 41. Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry (2008) 165(4):429–42. 10.1176/appi.ajp.2008.07111774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J, Fan Y, Dong Y, Ma M, Dong Y, Niu Y, et al. Combining gray matter volume in the cuneus and the cuneus-prefrontal connectivity may predict early relapse in abstinent alcohol-dependent patients. PloS One (2018) 13(5):e196860. 10.1371/journal.pone.0196860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Li CR. Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. NeuroImage: Clin (2017) 14:750–9. 10.1016/j.nicl.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ide JS, Zhornitsky S, Chao HH, Zhang S, Hu S, Wang W, et al. Thalamic Cortical Error-Related Responses in Adult Social Drinkers: Sex Differences and Problem Alcohol Use. Biol Psychiatry Cogn Neurosci Neuroimaging (2018) 3(10):868–77. 10.1016/j.bpsc.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A (2014) 111(47):16913–8. 10.1073/pnas.1415297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.