Abstract
Mental disorders frequently begin in childhood or adolescence. Psychotropic medications have various indications for the treatment of mental disorders in this age group and are used not infrequently off‐label. However, the adverse effects of these medications require special attention during developmentally sensitive periods of life. For this meta‐review, we systematically searched network meta‐analyses and meta‐analyses of randomized controlled trials (RCTs), individual RCTs, and cohort studies reporting on 78 a priori selected adverse events across 19 categories of 80 psychotropic medications – including antidepressants, antipsychotics, anti‐attention‐deficit/hyperactivity disorder (ADHD) medications and mood stabilizers – in children and adolescents with mental disorders. We included data from nine network meta‐analyses, 39 meta‐analyses, 90 individual RCTs, and eight cohort studies, including 337,686 children and adolescents. Data on ≥20% of the 78 adverse events were available for six antidepressants (sertraline, escitalopram, paroxetine, fluoxetine, venlafaxine and vilazodone), eight antipsychotics (risperidone, quetiapine, aripiprazole, lurasidone, paliperidone, ziprasidone, olanzapine and asenapine), three anti‐ADHD medications (methylphenidate, atomoxetine and guanfacine), and two mood stabilizers (valproate and lithium). Among these medications with data on ≥20% of the 78 adverse events, a safer profile emerged for escitalopram and fluoxetine among antidepressants, lurasidone for antipsychotics, methylphenidate among anti‐ADHD medications, and lithium among mood stabilizers. The available literature raised most concerns about the safety of venlafaxine, olanzapine, atomoxetine, guanfacine and valproate. Nausea/vomiting and discontinuation due to adverse event were most frequently associated with antidepressants; sedation, extrapyramidal side effects, and weight gain with antipsychotics; anorexia and insomnia with anti‐ADHD medications; sedation and weight gain with mood stabilizers. The results of this comprehensive and updated quantitative systematic meta‐review of top‐tier evidence regarding the safety of antidepressants, antipsychotics, anti‐ADHD medications and mood stabilizers in children and adolescents can inform clinical practice, research and treatment guidelines.
Keywords: Safety, tolerability, children, adolescents, psychopharmacology, antidepressants, antipsychotics, mood stabilizers, psychostimulants, meta‐review
Childhood and adolescence are a crucial time of biopsychosocial development 1 . Many, if not most, severe mental disorders have their onset prior to age 18 2 . Early intervention is a cornerstone of modern psychiatry which has demonstrated superior outcomes, for example, in psychotic disorders and bipolar disorder3, 4. In addition to psychotherapeutic and psychosocial interventions, psychotropic medications are often necessary to treat severe mental disorders that result in subjective distress and/or significant dysfunction in youth.
Several antidepressants, antipsychotics, anti‐attention‐deficit/hyperactivity disorder (ADHD) medications and mood stabilizers indicated in adults have received regulatory approval for use in children and/or adolescents 5 , and many are used off‐label6, 7, 8, 9, 10. However, despite evidence for the efficacy of a number of psychotropic medications in youth, the duration of untreated illness in depressive disorder 11 , bipolar disorder12, 13, schizophrenia 14 , obsessive‐compulsive disorder 15 , anxiety disorders 16 , and other mental disorders 17 is often long18, 19, which adversely affects long‐term outcomes14, 20, 21, 22, 23, 24. Such delay can be related to several factors. These certainly include reduced access to care due to stigma and self‐stigma surrounding mental illness25, 26, 27, but stigma‐derived or data‐based concerns about the safety of psychotropic medications in children and adolescents are also relevant28, 29, 30, 31, 32, 33, 34.
The poor quality of data on safety of psychotropic medications can potentially induce a delay or refusal of treatment, despite evidence that medications used in psychiatry are generally not less effective than those prescribed in other fields of medicine 35 . For instance, poor reporting of adverse events in available randomized controlled trials (RCTs) may have led to inaccurate estimates of some serious events, such as suicidality with antidepressants 36 . In addition, regulatory agencies may issue boxed warnings for adverse events of medications, such as for antidepressants increasing suicidality in children, adolescents and young adults 37 , which can impact prescribing habits in everyday clinical practice 38 , but whose validity may then be questioned39, 40. At the same time, evidence‐based safety concerns and warnings are essential to inform treatment guidelines and clinical care and are crucial to protect patients according to the primum non nocere principle.
The evidence on safety of psychotropic agents in children and adolescents with mental disorders has been rapidly growing 41 , but remains fragmented. The available network meta‐analyses (NMAs) and meta‐analyses (MAs) have generally considered efficacy as their primary outcome, while safety is usually not prioritized in the primary RCTs and related evidence syntheses. Moreover, NMAs and MAs only include RCTs, usually concerning one or, rarely, few related mental disorders.
While RCTs minimize the influence of several sources of bias on estimates of medication effects in a specific population, they also apply strict selection criteria, which reduces the generalizability and external validity of their findings. Moreover, RCTs are often relatively small and short in duration, which precludes the adequate identification of rare but serious or long‐term adverse events 42 . Furthermore, NMAs and MAs generally focus on the use of medications in disorders for which they are indicated, excluding evidence about off‐label use. Therefore, a comprehensive summary of the evidence concerning the safety of psychotropic medications for all the mental health conditions for which they are used in children and adolescents, based on RCTs as well as on large cohort studies including more generalizable samples and reflecting real‐world use patterns, is important to inform clinical practice.
To the best of our knowledge, no systematic meta‐review exists to date that has focused on the safety of psychotropic drugs in children and adolescents as its primary outcome, summarizing data from NMAs, MAs, largest individual RCTs, and well‐designed matched cohort studies across all relevant mental disorders. The aim of the present meta‐review was to provide the largest and most comprehensive evidence synthesis on the safety of four major psychotropic medication classes (antidepressants, antipsychotics, anti‐ADHD drugs, mood stabilizers) in children and adolescents with mental disorders, in order to inform clinical decision making and guideline development, and to identify areas needing further research.
METHODS
Search, inclusion and exclusion criteria
This systematic meta‐review followed an a priori protocol (available upon request). We conducted a systematic search in PubMed and PsycINFO, from database inception up to September 7, 2019, using an exhaustive combination of key words for both psychotropic medications and adverse health outcomes (full search string available upon request). Additional manual searches were performed on reference lists of included articles. Pairs of authors conducted title/abstract screening and full‐text assessment, and extracted data into a pre‐defined excel spreadsheet. A third author resolved any conflict.
Inclusion criteria were: a) NMAs, MAs, individual RCTs, and cohort studies controlling for confounding by indication (i.e., medication vs. placebo/no medication in subjects affected by the same disorder); b) data on the association between antidepressants, antipsychotics, anti‐ADHD medications, or mood stabilizers and adverse health outcomes; c) population of children and/or adolescents with any mental disorder.
Exclusion criteria were: a) studies on conditions other than mental disorders for which psychotropic medications are indicated or used (e.g., epilepsy); b) confounding by indication (i.e., comparing patients on medications with healthy controls), even if they adjusted analyses for covariates; c) designs other than those indicated in inclusion criteria; d) no data on the association between the targeted medications and adverse health outcomes.
Included adverse events and psychotropic medications
The 78 a priori selected adverse events were subdivided into the following 19 categories: central nervous system (agitation, anxiety, asthenia, irritability, cognitive impairment, depression, dizziness, headache, mania, psychosis, sedation, insomnia, seizures, suicidal ideas/behaviors/attempts); nutritional and metabolic (anorexia, binge eating/increased appetite, increased cholesterol, increased triglycerides, metabolic syndrome, glucose dysregulation/diabetes, insulin resistance, increased waist circumference, weight gain/increased body mass index, weight loss); cardiovascular (arrhythmias/tachycardia, cardiomyopathy, cerebrovascular disease, coronary heart disease, hypertension, hypotension, myocarditis, QT prolongation, sudden cardiac death); gastrointestinal (abdominal pain, constipation, diarrhea, gastrointestinal symptoms, liver damage, nausea/vomiting); genitourinary (enuresis, kidney disease/failure, menstrual cycle alterations, polycystic ovarian syndrome, sexual dysfunction); movement disorders (akathisia, any extrapyramidal side effect, tremor, dystonia, tardive dyskinesia); impulse dyscontrol and risky behavior (criminal behavior, gambling, substance abuse, non‐suicidal self‐injury behaviors); endocrine (gynecomastia/galactorrhea, hypo/hyperprolactinemia, hypo/hyperthyroidism); hematologic (anemia, leukocytopenia, thrombocytopenia); mouth (dental caries, dry mouth, sialorrhea); respiratory (acute respiratory failure, asthma, nasopharyngitis/upper respiratory tract infection/pneumonia); venous thromboembolism (deep vein thrombosis, pulmonary embolism); bone health (osteopenia/osteoporosis, fractures); accidents (any accident, fall); neuroleptic malignant syndrome (neuroleptic malignant syndrome/fever/creatine phosphokinase elevation); any cancer; discontinuation due to adverse event; serious adverse events; and mortality (all‐cause, due to natural causes, due to suicide).
The 80 psychotropic medications were subdivided into the four categories of antidepressants, antipsychotics, anti‐ADHD medications, and mood stabilizers. The category of antidepressants included nine classes: monoamine oxidase inhibitors (I‐MAOs) (bifemelane, hydracarbazine, isocarboxazid, moclobemide, nialamide, phenelzine, pirlindole, rasagiline, safinamide, selegiline, toloxatane and tranylcypromine); tricyclics (TCAs) and tetracyclics (TeCAs) (amitriptyline, amoxapine, clomipramine, desipramine, doxepine, imipramine, maprotiline, nortriptyline, protriptyline and trimipramine); selective serotonin reuptake inhibitors (SSRIs) (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline); serotonin‐noradrenaline reuptake inhibitors (SNRIs) (desvenlafaxine, duloxetine, levomilnacipran, milnacipran and venlafaxine); serotonin partial agonist and reuptake inhibitors (SPARIs) (nefazodone, trazodone and milazodone); noradrenergic and specific serotoninergic antidepressants (NASSAs) (mianserin and mirtazapine); noradrenaline reuptake inhibitors (NRIs) (reboxetine); noradrenaline and dopamine reuptake inhibitors (NDRIs) (buproprion); others (agomelatine, esketamine, S‐adenosyl‐methionine and vortioxetine). The category of antipsychotics included two classes: first‐generation antipsychotics (FGAs) (chlorpromazine, fluphenazine, haloperidol, loxapine, molindone, perphenazine, promazine and trifluoperazine) and second‐generation antipsychotics (SGAs) (amisulpride, aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone and ziprasidone). Anti‐ADHD medications included psychostimulants (d‐amphetamine, lisdexamphetamine and methylphenidate) and medications with other mechanisms (atomoxetine, clonidine, guanfacine and modafinil). Mood stabilizers included antiepileptics (carbamazepine, gabapentin, lamotrigine, pregabalin, oxcarbazepine, topiramate and valproate) and lithium.
Primary and secondary outcomes
The primary outcome was the safety/coverage ratio (i.e., the number of adverse events significantly worse than placebo/no treatment over the number of adverse events covered by literature) for those psychotropic medications for which ≥20% of the 78 a priori selected events were covered by the literature. The secondary outcomes were the list of adverse events associated with each medication, their effect size ± 95% CI, and the study quality.
The magnitude of associations of each medication with the main adverse events was classified as small (≤0.5), medium (between >0.5 and <0.8) and large (≥0.8) for continuous outcomes (effect sizes >0) and inverse thresholds for effect sizes <0. For categorical outcomes, the magnitude of associations was classified as small (<3), medium (between ≥3 and <5) and large (≥5) for equivalent odds ratios (eORs) >1, and reciprocal thresholds for eORs <1 43 .
Quality of evidence
The quality of MAs and NMAs was measured with a modified version of the A Measurement Tool for the Assessment of Multiple Systematic Reviews (AMSTAR)‐PLUS 44 , which allows to measure both the quality of the methodology of (N)MAs, and the quality of the studies included in (N)MAs (AMSTAR‐Content). AMSTAR quality was considered low when the final score was <4, medium when it was 4‐7, and high when >7 45 . For AMSTAR‐Content, quality was considered low when the final score was <4, medium when it was 4‐6, and high when >6. The overall quality of (N)MAs was rated choosing the lower score of either AMSTAR or AMSTAR‐Content.
The quality of RCTs was assessed with the Risk of Bias tool 2 46 , assigning high risk, low risk, or some concerns. The quality of cohort studies was measured with the Newcastle‐Ottawa Scale (NOS) 47 , and high quality was assigned when the NOS score was ≥7.
Statistical analysis
We extracted random effects effect sizes ± 95% CIs for the difference in the incidence of specific adverse events between individual medications and placebo (RTCs), or between treated vs. untreated youth with mental disorders (cohort studies). We considered ORs, log ORs or risk ratios (RRs) with respective numbers‐needed‐to‐harm (NNH) for categorical outcomes, and standardized mean differences (SMDs) or mean differences (MDs) for continuous outcomes.
We calculated the overall proportional coverage of the a priori selected adverse events for each of the individual psychotropic medications using descriptive statistics, and divided the covered adverse events into those with and without significantly higher frequencies vs. placebo or matched subjects. Furthermore, we identified medications with the best or worst safety/coverage ratio among those that had results for ≥20% of the adverse events.
RESULTS
Search results
The flow chart of the search process for the three systematic searches is presented in Figure 1. At title and abstract level, we screened 1,309 hits for NMAs and MAs, 5,716 hits for individual RCTs and 8,518 hits for cohort studies. We assessed full texts of 292 articles for NMAs and MAs, 519 for individual RCTs, and 173 for cohort studies. We ultimately extracted data from nine NMAs, 39 MAs, 90 individual RCTs, and eight cohort studies, including 337,686 children and adolescents (120,637 for antidepressants, 66,764 for antipsychotics, 148,664 for anti‐ADHD medications, and 1,621 for mood stabilizers).
Figure 1.
PRISMA flow chart for inclusion of studies. Search 1: network meta‐analyses (NMA) and meta‐analyses (MA); Search 2: individual randomized controlled trials (RCTs); Search 3: cohort studies controlling for confounding by indication
For antidepressants, we included four NMAs40, 48, 49, 50, 15 MAs36, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 27 individual RCTs65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 also covered in those NMA/MAs, six additional RCTs92, 93, 94, 95, 96, 97, and three cohort studies98, 99, 100. There were 120,637 youth on antidepressants, including 24,659 across 139 RCTs after eliminating duplicated RCTs in multiple NMA/MAs (22,704 in NMA/MAs, 1,955 in additional RCTs), and 95,978 in three cohort studies.
For antipsychotics, we included three NMAs101, 102, 103, 11 MAs104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 25 individual RCTs115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139 also included in those NMA/MAs, three additional RCTs140, 141, 142, and two cohort studies99, 143. There were 66,764 youth on antipsychotics, including 7,712 across 53 RCTs after eliminating duplicated RCTs in multiple NMA/MAs (6,725 in NMA/MAs, 987 in additional RCTs), and 59,052 in two cohort studies.
For anti‐ADHD medications, we included three NMAs49, 144, 145, 11 MAs146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 12 RCTs157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168 also included in those NMA/MAs, five additional RCTs169, 170, 171, 172, 173, and five cohort studies99, 174, 175, 176, 177. There were 148,664 youth on anti‐ADHD medications, including 28,834 across 298 RCTs after eliminating duplicated RCTs in multiple NMA/MAs (27,188 in NMA/MAs, 1,646 in additional RCTs), and 119,830 in five cohort studies.
For mood stabilizers, we included four MAs107, 112, 178, 179, seven RCTs180, 181, 182, 183, 184, 185, 186 also included in those NMA/MAs, and five additional RCTs187, 188, 189, 190, 191. There were 1,621 youth across 23 RCTs after eliminating duplicated RCTs in multiple NMA/MAs (1,244 in NMA/MA, 377 in additional RCTs).
Quality of included evidence
Among nine NMAs, the median AMSTAR score was 10 (interquartile range, IQR=9‐11) and the median AMSTAR‐Content score was 5 (IQR=5‐7). The quality was moderate in two (22.2%) NMAs, and high in the remaining seven NMAs (77.8%). The RCTs included in NMAs had moderate quality in six (66.7%) NMAs, and high quality in three (33.3%). The overall quality of the evidence from included NMAs was moderate in six (66.7%) and high in three (33.3%).
Among 39 MAs, the median AMSTAR score was 9 (IQR=7‐10) and the median AMSTAR‐Content was 5 (IQR=4‐6). The quality was moderate in 11 MAs (28.2%), and high in the remaining 28 (71.8%). The RCTs included in MAs had low quality in nine (23.1%) MAs, moderate quality in 23 (59.0%), and high in seven (17.9%). The overall quality of the evidence from included MAs was low in nine (23.1%), moderate in 25 (64.1%) and high in five (12.8%).
Among 90 individual RCTs, 26 (28.6%) had high risk of bias, 43 (47.3%) raised some concerns, and 22 (24.2%) had low risk of bias.
Among eight cohort studies, six (75%) had a high quality according to the Newcastle‐Ottawa scale, and the median quality score was 7 (IQR=7‐8).
Overall safety of classes of psychotropic medications in children and adolescents with mental disorders
Antidepressants
Out of 44 antidepressants, 18 (40.9%) had adverse event data covered in the literature. The available antidepressant literature covered 0‐24.4% (mean: 5.6%, median: 0%) of the reviewed adverse events. Details on the proportion of the 78 adverse events covered in the literature and of the adverse events that were significantly worse with individual antidepressants vs. placebo/controls are reported in Table 1 and Figure 2.
Table 1.
Safety of antidepressants in children and adolescents with any mental illness (adverse events significantly worse than with placebo/controls)
| Medication | Adverse events covered by literature | Adverse events worse than placebo | Adverse event | Type of effect size | Effect size | 95% CI | Source | Quality | N |
|---|---|---|---|---|---|---|---|---|---|
| Mixed antidepressants | 12 (15.4%) | 6 (7.7%) | Anorexia 48 | OR | 4.01 | 1.63‐10.17 | NMA | M | 26,114 |
|
Discontinuation due to adverse event 59 |
RR | 1.66 | 1.20‐2.28 | MA | M | 6,778 | |||
| Fractures 98 | HR | 1.03 | 1.00‐1.06 | C | H | 50,673 | |||
| Insomnia 63 | RR | 2.16 | 1.42‐3.27 | MA | M | 1,500 | |||
| Nausea/vomiting 63 | RR | 1.88 | 1.44‐2.45 | MA | M | 2,101 | |||
| Suicidality 56 | RR | 1.95 |
1.28‐2.98 |
MA | M | 3,930 | |||
| Mixed serotonin‐noradrenaline reuptake inhibitors | 9 (11.5%) | 3 (3.8%) | Headache 63 | RR | 1.52 | 1.09‐2.13 | MA | M | 688 |
| Nausea/vomiting 63 | RR | 1.97 | 1.36‐2.87 | MA | M | 688 | |||
| Serious adverse events 59 | RR | 2.10 | 1.19‐3.69 | MA | M | NA | |||
| Mixed selective serotonin reuptake inhibitors | 14 (17.9%) | 4 (5.1%) |
Discontinuation due to adverse event 49 |
Log OR | –1.8 | –3.4 to –0.4 | NMA | H | 2,623 |
| Headache 63 | RR | 1.27 | 1.03‐1.56 | MA | M | 2,297 | |||
|
Nausea/vomiting 63 |
OR | 1.89 | 1.42‐2.52 | MA | M | 831 | |||
| Serious adverse events 59 | RR | 1.72 | 1.12‐2.63 | MA | M | NA | |||
| Mixed tricyclics | 12 (15.4%) | 4 (5.1%) | Dry mouth 63 | RR | 3.28 | 1.82‐5.90 | MA | M | 232 |
| Hypotension 64 | OR | 6.78 | 2.06‐22.26 | MA | L | 324 | |||
| Tremor 64 | OR | 6.29 | 1.78‐22.17 | MA | L | 308 | |||
| Suicidality 49 | Log OR | 25.1 | 4.5‐57.4 | NMA | H | 2,623 | |||
| Amitriptiyline | 2 (2.6%) | 1 (1.3%) | Anorexia 65 | NA | Sig | Sig | RCT | M | 31 |
| Bupropion | 8 (10.3%) | 0 (0.0%) | |||||||
| Citalopram | 8 (10.3%) | 0 (0.0%) | |||||||
| Clomipramine | 8 (10.3%) | 1 (1.3%) | Any extrapyramidal side effects 97 | RR | 9.35 | 1.28‐68.6 | RCT | M | 60 |
| Desipramine | 6 (7.7%) | 0 (0.0%) | |||||||
| Desvenlafaxine | 9 (11.5%) | 0 (0.0%) | |||||||
| Duloxetine | 13 (16.7%) | 3 (3.8%) | Diarrhea 93 | OR | 3.26 | 1.09‐9.71 | RCT | H | 556 |
|
Discontinuation due to adverse event 40 |
OR | 2.80 | 1.20‐9.42 | NMA | H | 5,260 | |||
| Nausea/vomiting 93 | OR | 1.93 | 1.15‐3.25 | RCT | H | 556 | |||
| Escitalopram | 17 (21.8%) | 1 (1.3%) | Weight gain 87 | OR | 2.30 | 1.01‐5.25 | RCT | L | 312 |
| Fluoxetine | 16 (20.5%) | 1 (1.3%) | Weight loss 79 | MD | –1.2 |
–1.85 to –0.55 |
RCT | M | 103 |
| Fluvoxamine | 11 (14.1%) | 1 (1.3%) | Abdominal pain 89 | RR | 1.70 | 1.06‐2.71 | RCT | M | 128 |
| Imipramine | 15 (19.2%) | 5 (6.4%) | Any extrapyramidal side effects 90 | OR | 7.35 | 1.62‐33.3 | RCT | M | 182 |
|
Discontinuation due to adverse event 40 |
OR | 5.49 | 1.96‐20.9 | NMA | H | 5,260 | |||
| Dry mouth 62 | RR | 3.81 | 1.25‐11.6 | MA | M | 56 | |||
| Hypotension 90 | OR | 13.6 | 1.74‐107 | RCT | M | 182 | |||
| Sedation 90 | OR | 4.44 | 1.22‐16.2 | RCT | M | 182 | |||
| Mirtazapine | 2 (2.6%) | 0 (0.0%) | |||||||
| Nefazodone | 8 (10.3%) | 3 (3.8%) | Headache 91 | NA | Sig | Sig | RCT | L | 528 |
| Nausea/vomiting 91 | NA | Sig | Sig | RCT | L | 528 | |||
| Sedation 91 | NA | Sig | Sig | RCT | L | 528 | |||
| Nortriptyline | 3 (3.8%) | 1 (1.3%) | Hypertension 67 | NA | Sig | Sig | RCT | M | 50 |
| Paroxetine | 16 (20.5%) | 3 (3.8%) | Any extrapyramidal side effects 90 | OR | 5.12 | 1.09‐24.1 | RCT | M | 180 |
| Insomnia 82 | OR | 2.68 | 1.20‐6.00 | RCT | M | 319 | |||
| Nausea/vomiting 69 | OR | 3.69 | 1.01‐13.5 | RCT | L | 319 | |||
| Sertraline | 19 (24.4%) | 4 (5.1%) | Diarrhea 68 | OR | 3.04 | 1.25‐7.38 | RCT | H | 376 |
| Insomnia 84 | OR | 4.05 | 1.94‐8.49 | RCT | L | 189 | |||
| Nausea/vomiting 68 | OR | 2.65 | 1.03‐6.77 | RCT | H | 189 | |||
| Weight gain 68 | NA | Sig | Sig | RCT | H | 376 | |||
| Venlafaxine | 16 (20.5%) | 7 (9.0%) | Abdominal pain 70 | OR | 2.36 | 1.29‐4.32 | RCT | M | 367 |
| Anorexia 72 | OR | 4.25 | 1.55‐11.63 | RCT | M | 323 | |||
|
Discontinuation due to adverse event 40 |
OR | 3.19 | 1.01‐18.70 | NMA | H | 5,260 | |||
| Headache 72 | OR | 0.56 | 0.35‐0.92 | RCT | M | 313 | |||
| Hypertension 70 | NA | Sig | Sig | RCT | M | 367 | |||
| Serious adverse events 70 | OR | 4.14 | 1.15‐14.9 | RCT | M | 367 | |||
| Suicidality 40 | OR | 0.13 | 0.00‐0.55 | NMA | H | 5,260 | |||
| Vilazodone | 16 (20.5%) | 2 (2.6%) |
Discontinuation due to adverse event 94 |
OR | 8.55 | 1.13‐64.8 | RCT | H | 526 |
| Nausea/vomiting 94 | OR | 4.40 | 2.43‐9.76 | RCT | H | 526 | |||
OR – odds ratio, RR – risk ratio, Log OR – log odds ratio, HR – hazard ratio, MD – mean difference, NMA – network meta‐analysis, MA – meta‐analysis, RCT – randomized controlled trial, C – cohort study, NA – not available, H – high quality, M – medium quality, L – low quality (lower score of either AMSTAR or AMSTAR‐Content), Sig – significant difference between medication and placebo without effect size available
Figure 2.
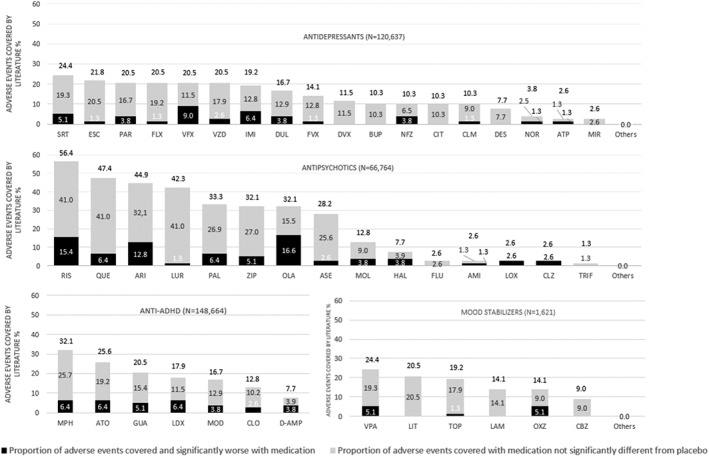
Proportion of adverse events covered by the literature that were significantly worse or non‐significantly different from placebo, for antidepressants, antipsychotics, anti‐attention‐deficit/hyperactivity (ADHD) medications, and mood stabilizers in children and adolescents with mental illness. AMI – amisulpride, ATP – amitriptyline, ARI – aripiprazole, ASE – asenapine, ATO – atomoxetine, BUP – bupropion, CBZ – carbamazepine, CIT – citalopram, CLM – clomipramine, CLO – clonidine, CLZ – clozapine, DES – desipramine, DVX – desvenlafaxine, D‐AMP – d‐amphetamine, DUL – duloxetine, ESC – escitalopram, FLX – fluoxetine, FLU – fluphenazine, FVX – fluvoxamine, GUA – guanfacine, HAL – haloperidol, IMI – imipramine, LAM – lamotrigine, LIT – lithium, LDX – lisdexamphetamine, LOX – loxapine, LUR – lurasidone, MPH – methylphenidate, MIR – mirtazapine, MOD – modafinil, MOL – molindone, NFZ – nefazodone, NOR – nortriptyline, OLA – olanzapine, OXZ – oxcarbazepine, PAL – paliperidone, PAR – paroxetine, QUE – quetiapine, RIS – risperidone, SRT – sertraline, TOP – topiramate, TRIF – trifluoperazine, VPA – valproate, VFX – venlafaxine, VZD – vilazodone, ZIP – ziprasidone
Among antidepressants with ≥20% of adverse events covered, the safety/coverage ratio was the best for escitalopram (1/17 adverse events covered significantly worse) and fluoxetine (1/16), progressively decreasing through vilazodone (2/16), paroxetine (3/16), sertraline (4/19), to venlafaxine, which had the worst safety/coverage ratio (7/16).
Five antidepressants were associated with significantly worse nausea/vomiting (duloxetine, nefazodone, paroxetine, sertraline, vilazodone), four with discontinuation due to adverse event (duloxetine, imipramine, venlafaxine, vilazodone), three with any extrapyramidal side effect (clomipramine, imipramine, paroxetine), two each with sedation (imipramine, nefazodone), diarrhea (duloxetine, sertraline), headache (nefazodone, venlafaxine), anorexia (amitriptyline, venlafaxine), and weight gain/increased body mass index (escitalopram, sertraline), and one each with weight loss (fluoxetine), and suicidality (venlafaxine).
Antipsychotics
Out of 21 antipsychotics, 15 (71.4%) had adverse event data covered in literature. The antipsychotic literature covered a range of 0‐56.4% (mean: 16.6%, median: 2.6%) of the reviewed adverse events. Details of the proportion of the 78 adverse events covered in the literature and of adverse events that were significantly worse with individual antipsychotics vs. placebo/controls are reported in Table 2 and Figure 2.
Table 2.
Safety of antipsychotics in children and adolescents with any mental illness (adverse events significantly worse than with placebo/controls)
| Medication | Adverse events covered by literature | Adverse events worse than placebo | Adverse event | Type of effect size | Effect size | 95% CI | Source | Quality | N |
|---|---|---|---|---|---|---|---|---|---|
| Mixed antipsychotics | 3 (3.8%) | 2 (2.6%) | Discontinuation due to adverse event 104 | RR | 2.40 | 1.10‐5.30 | MA | M | 942 |
| Weight gain 104 | SMD | 0.60 | 0.30‐0.90 | MA | M | 625 | |||
| Mixed second‐generation antipsychotics | 17 (21.8%) | 10 (12.8%) | Akathisia 107 | NNH | 20.4 | 14.1‐36.5 | MA | M | 1,118 |
| Any extrapyramidal side effects 107 | NNH | 7.5 | 5.7‐11.0 | MA | M | 1,118 | |||
| Diabetes 143 | IRR | 10.5 | 2.06‐33.2 | C | H | 37,866 | |||
| Discontinuation due to adverse event 107 | NNH | 20.4 | 13.4‐47.5 | MA | M | 1,118 | |||
| Dystonia 105 | OR | 3.90 | 1.70‐8.40 | MA | M | 666 | |||
| Hyperprolactinemia 107 | NNH | 7.9 | 6.10‐11.1 | MA | M | 1,118 | |||
| Sedation 107 | NNH | 4.7 | 3.90‐6.0 | MA | M | 1,118 | |||
| Tardive dyskinesia 105 | OR | 3.90 | 1.10‐14.1 | MA | M | 666 | |||
| Tremor 105 | OR | 3.49 | 1.50‐8.0 | MA | M | 666 | |||
| Weight gain 107 | NNH | 10.0 | 7.50‐14.8 | MA | M | 1,118 | |||
| Amisulpride | 2 (2.6%) | 1 (1.3%) | Any extrapyramidal side effects 124 | OR | 9.60 | 1.48‐62 | RCT | L | 27 |
| Aripiprazole | 35 (44.9%) | 10 (12.8%) | Akathisia 102 | OR | 3.10 | 1.0‐9.0 | NMA | M | 2,158 |
| Any extrapyramidal side effects 103 | OR | 3.80 | 2.20‐6.20 | NMA | M | 3,258 | |||
| NNH | 4.1 | 3.1‐6.2 | MA | M | 296 | ||||
| Asthenia 109 | OR | 8.54 | 2.59‐28.1 | MA | M | 405 | |||
| Anorexia 109 | OR | 5.11 | 1.14‐23.0 | MA | M | 308 | |||
| Increased cholesterol 108 | RR | 2.50 | 1.40‐4.40 | MA | L | 120 | |||
| Fever 109 | OR | 5.89 | 1.23‐28.2 | MA | M | 308 | |||
| Sedation 103 | OR | 6.10 | 2.80‐12.2 | NMA | M | 3,348 | |||
| Sialorrhea 109 | OR | 10.5 | 1.30‐84.2 | MA | M | 314 | |||
| Tremor 109 | OR | 11.5 | 1.40‐91.6 | MA | M | 313 | |||
| Weight gain 103 | OR | 4.40 | 2.0‐8.90 | NMA | M | 3,401 | |||
| Asenapine | 22 (28.2%) | 2 (2.6%) | Increased body mass index 136 | NA | Sig | Sig | RCT | M | 306 |
| Increased glucose 141 | NA | Sig | Sig | RCT | M | 403 | |||
| Clozapine | 2 (2.6%) | 2 (2.6%) | Sedation 103 | OR | 54.8 | 3.9‐260 | NMA | M | 3,348 |
| Weight gain101, 103 | OR | 13.8 | 2.20‐49.2 | NMA | M | 3,401 | |||
| SMD | –0.92 |
–1.61 to –0.22 |
NMA | M | 3,003 | ||||
| Fluphenazine | 2 (2.6%) | 0 (0.0%) | |||||||
| Haloperidol | 6 (7.7%) | 3 (3.8%) | Any extrapyramidal side effects 131 | OR | 59.1 | 6.66‐525 | RCT | L | 50 |
| Hyperprolactinemia 101 | SMD | 1.0 | 0.2‐1.8 | NMA | M | 3,003 | |||
| Sedation 101 | Log OR | –1.3 |
–2.3 to –0.3 |
NMA | M | 3,003 | |||
| Loxapine | 2 (2.6%) | 2 (2.6%) | Any extrapyramidal side effects 131 | OR | 62.4 | 7.05‐553 | RCT | L | 50 |
| Sedation 101 | Log OR | –1.9 |
–3.1 to – 0.7 |
NMA | M | 3,003 | |||
| Lurasidone | 33 (42.3%) | 1 (1.3%) | Nausea/vomiting 142 | OR | 3.1 | 1.50‐6.60 | RCT | M | 343 |
| Molindone | 10 (12.8%) | 3 (3.8%) | Akathisia 102 | OR | 24.1 | 5.70‐102 | NMA | M | 2,158 |
| Any extrapyramidal side effects 102 | OR | 10.4 | 3.0‐35.6 | NMA | M | 2,158 | |||
| Sedation 102 | OR | 10.9 | 2.40‐50.2 | NMA | M | 2,158 | |||
| Olanzapine | 25 (32.1%) | 13 (16.6%) | Akathisia 102 | OR | 3.70 | 1.10‐12.7 | NMA | M | 2,158 |
| Anemia 119 | NA | Sig | Sig | RCT | L | 107 | |||
| Any extrapyramidal side effects 103 | OR | 6.40 | 2.40‐13.8 | NMA | M | 3,258 | |||
| Increased cholesterol 103 | MD | 4.5 | 1.2‐7.7 | NMA | M | 1,784 | |||
| Increased creatine phosphokinase 119 | NA | Sig | Sig | RCT | L | 107 | |||
| Increased glucose 103 | MD | 2.1 | 0.1‐4.3 | NMA | M | 1,784 | |||
| Hyperprolactinemia101, 103 | OR | 15.6 | 4.40‐41.1 | NMA | M | 3,348 | |||
| SMD | 0.7 | 0.3‐1.1 | NMA | M | 3,003 | ||||
| Hypertension 130 | NA | Sig | Sig | RCT | L | 107 | |||
| Liver damage 113 | OR | 18.7 | 3.60‐96.4 | MA | H | 265 | |||
| Sexual adverse events 108 | MD | 11.5 | 8.80‐14.1 | MA | L | 241 | |||
| Sedation 103 | OR | 8.50 | 4.0‐16.6 | NMA | M | 3,348 | |||
| Increased triglycerides103, 113 | OR | 5.10 | 2.80‐9.40 | MA | M | 268 | |||
| MD | 20.2 | 9.8‐30.5 | NMA | H | 1,655 | ||||
| Weight gain 103 | OR | 15.1 | 6.60‐31.1 | NMA | M | 3,401 | |||
| Paliperidone | 26 (33.3%) | 5 (6.4%) | Akathisia 102 | OR | 5.60 | 1.80‐17.7 | NMA | M | 2,158 |
| Any extrapyramidal side effects 102 | OR | 6.30 | 2.30‐16.8 | NMA | M | 2,158 | |||
| Hyperprolactinemia 101 | SMD | 0.61 | 0.35‐0.86 | NMA | M | 3,003 | |||
| Sedation 101 | Log OR | –2.4 |
–4.4 to –0.3 |
NMA | M | 3,003 | |||
| Weight gain 101 | SMD | –0.7 |
–1.0 to –0.5 |
NMA | M | 3,003 | |||
| Quetiapine | 37 (47.4%) | 5 (6.4%) | Increased cholesterol 103 | MD | 10.8 | 6.6‐145 | NMA | M | 1,784 |
| Hyperprolactinemia 101 | SMD | 0.4 | 0.1‐0.7 | NMA | M | 3,003 | |||
| Sedation 103 | OR | 5.40 | 2.90‐9.30 | NMA | M | 3,348 | |||
| Increased triglycerides 103 | MD | 19.5 | 11.8‐27.2 | NMA | M | 1,655 | |||
| Weight gain101, 103 | OR | 6.20 | 2.60‐13.6 | NMA | M | 3,401 | |||
| SMD | –0.85 |
–1.09 to –0.61 |
NMA | M | 3,003 | ||||
| Risperidone | 44 (56.4%) | 12 (15.4%) | Akathisia 102 | OR | 4.0 | 1.40‐10.9 | NMA | M | 2,158 |
| Any extrapyramidal side effects 103 | OR | 3.70 | 2.20‐6.0 | NMA | M | 3,258 | |||
| Asthenia 109 | OR | 3.89 | 1.77‐8.53 | MA | M | 179 | |||
| Constipation 109 | OR | 3.42 | 1.33‐8.80 | MA | M | 179 | |||
| Gastrointestinal symptoms 115 | OR | 3.74 | 1.15‐12.2 | RCT | H | 168 | |||
| Increased glucose 103 | MD | 3.70 | 1.10‐6.40 | NMA | M | 1,784 | |||
| Hyperprolactinemia101, 103 | OR | 38.6 | 8.60‐126 | NMA | M | 1,180 | |||
| SMD | 1.40 | 0.80‐2.0 | NMA | M | 3,003 | ||||
| Increased appetite 109 | OR | 4.82 | 2.35‐9.88 | MA | M | 179 | |||
| Nasopharyngitis/upper respiratory tract infection 109 | OR | 3.14 | 1.26‐7.80 | MA | M | 179 | |||
| Sedation 103 | OR | 7.30 | 4.60‐11.2 | NMA | M | 3,348 | |||
| Tachycardia 109 | OR | 6.87 | 1.49‐31.7 | MA | M | 179 | |||
| Weight gain101, 103 | OR | 6.0 | 3.0‐11.0 | NMA | M | 3,401 | |||
| SMD | –0.61 |
–0.89 to –0.32 |
NMA | M | 3,003 | ||||
| Trifluoperazine | 1 (1.3%) | 0 (0.0%) | |||||||
| Ziprasidone | 25 (32.1%) | 4 (5.1%) | Any extrapyramidal side effects 103 | OR | 20.6 | 3.50‐69.0 | NMA | M | 3,258 |
| Dizziness 135 | OR | 9.15 | 1.20‐69.7 | RCT | L | 283 | |||
| Nausea/vomiting 135 | OR | 4.80 | 1.10‐21.1 | RCT | L | 283 | |||
| Sedation 103 | OR | 8.70 | 2.70 ‐22.0 | NMA | M | 3,348 |
OR – odds ratio, RR – risk ratio, Log OR – log odds ratio, SMD – standardized mean difference, IRR – incidence rate ratio, NNH – number needed to harm, NMA – network meta‐analysis, MA – meta‐analysis, RCT – randomized controlled trial, C – cohort study, NA – not available, H – high quality, M – medium quality, L – low quality (lower score of either AMSTAR or AMSTAR‐Content), Sig – significant difference between medication and placebo without effect size available
Among antipsychotics with ≥20% of adverse events covered, lurasidone had the best safety/coverage ratio (1/33 covered adverse events significantly worse), progressively decreasing through asenapine (2/22), quetiapine (5/37), ziprasidone (4/25), paliperidone (5/26), risperidone (12/44), aripiprazole (10/35), to olanzapine, which had the worst safety/coverage ratio (13/25).
Ten antipsychotics were associated with significantly worse sedation (aripiprazole, clozapine, haloperidol, loxapine, molindone, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone), nine with any extrapyramidal side effect (amisulpride, aripiprazole, haloperidol, loxapine, molindone, olanzapine, paliperidone, risperidone, ziprasidone), seven with weight gain/increased body mass index (aripiprazole, asenapine, clozapine, olanzapine, paliperidone, quetiapine, risperidone), five with hyperprolactinemia (haloperidol, olanzapine, paliperidone, quetiapine, risperidone), and three each with increased cholesterol (aripiprazole, olanzapine, quetiapine) and glucose increase/diabetes (asenapine, olanzapine, risperidone).
Anti‐ADHD medications
All seven anti‐ADHD medications had adverse event data covered in the literature. The available literature covered 7.7‐32.1% (mean: 19.0%, median: 17.9%) of the reviewed adverse events. Details of the proportion of the 78 adverse events covered in the literature and of adverse events that were significantly worse with individual anti‐ADHD medications vs. placebo/controls are reported in Table 3 and Figure 2.
Table 3.
Safety of anti‐attention‐deficit/hyperactivity (ADHD) medications in children and adolescents with any mental illness (adverse events significantly worse than with placebo/controls)
| Medication | Adverse events covered by literature | Adverse events worse than placebo | Adverse event | Type of effect size | Effect size | 95% CI | Source | Quality | N |
|---|---|---|---|---|---|---|---|---|---|
| Mixed anti‐ADHD medications | 19 (24.4%) | 7 (9.0%) | Abdominal pain 155 | RR | 1.44 | 1.03‐2.00 | MA | H | 2,155 |
| Anorexia 155 | RR | 6.31 | 2.58‐15.5 | MA | H | 2,467 | |||
|
Discontinuation due to adverse event 144 |
OR | 2.30 | 1.36‐3.89 | NMA | H | 14,346 | |||
| Hypertension 144 | SMD | 0.09 | 0.01‐0.18 | NMA | H | 14,346 | |||
| Insomnia 155 | RR | 3.80 | 2.12‐6.83 | MA | H | 2,429 | |||
| Nausea/vomiting 155 | RR | 1.63 | 1.04‐2.56 | MA | H | 1,579 | |||
| Weight loss 144 | SMD | –0.71 | –1.15 to –0.27 | NMA | H | 14,346 | |||
| Mixed α‐2 agonists | 5 (6.4%) | 1 (1.3%) |
Discontinuation due to adverse event 49 |
Log OR | –29.6 | –95.5 to –2.6 | NMA | M | 2,623 |
| Atomoxetine | 20 (25.6%) | 5 (6.4%) | Anorexia 147 | RR | 2.51 | 1.77‐3.57 | MA | M | 2,179 |
| Gastrointestinal symptoms 147 | RR | 1.76 | 1.51‐2.07 | MA | M | 3,712 | |||
| Hypertension 144 | SMD | 0.12 | 0.02‐0.22 | NMA | H | 14,346 | |||
| Nausea/vomiting 156 | RR | 1.91 | 1.24‐2.94 | MA | L | 193 | |||
| Weight loss 144 | SMD | –0.84 | –1.16 to –0.52 | NMA | H | 14,346 | |||
| Clonidine | 10 (12.8%) | 2 (2.6%) | Hypotension 149 | Hedges’ g | 0.52 | 0.15‐0.89 | MA | M | 119 |
| Sedation 164 | OR | 7.67 | 2.92‐20.1 | RCT | M | 230 | |||
| d‐amphetamine | 6 (7.7%) | 3 (3.8%) | Anorexia 170 | NA | Sig | Sig | RCT | L | 81 |
| Insomnia 170 | NA | Sig | Sig | RCT | L | 81 | |||
| Irritability 170 | NA | Sig | Sig | RCT | L | 81 | |||
| Guanfacine | 16 (20.5%) | 4 (5.1%) | Abdominal pain 166 | OR | 4.51 | 1.34‐15.2 | RCT | M | 455 |
|
Discontinuation due to adverse event 144 |
OR | 2.64 | 1.20‐5.81 | NMA | H | 14,346 | |||
| QT prolongation 149 | Hedges’ g | 0.33 | 0.12‐0.54 | MA | M | 785 | |||
| Sedation 149 | RR | 2.43 | 1.06‐5.58 | MA | M | 1,059 | |||
| Lisdexamphetamine | 14 (17.9%) | 5 (6.4%) | Anorexia 155 | RR | 9.83 | 5.08‐19.0 | MA | H | 1,081 |
|
Discontinuation due to adverse event 145 |
RR | 3.11 | 1.20‐3.76 | NMA | M | 6,931 | |||
| Dry mouth 169 | OR | 8.63 | 1.13‐66.0 | RCT | H | 547 | |||
| Hypertension 144 | SMD | 0.14 | 0.03‐0.25 | NMA | H | 14,346 | |||
| Insomnia 155 | RR | 5.91 | 2.84‐12.3 | MA | H | 1,081 | |||
| Methylphenidate | 25 (32.1%) | 5 (6.4%) | Abdominal pain 154 | RR | 1.50 | 1.26‐1.79 | MA | M | 5,983 |
| Anorexia 154 | RR | 3.21 | 2.61‐3.94 | MA | M | 5,983 | |||
| Insomnia 148 | OR | 4.66 | 1.99‐10.9 | MA | M | 749 | |||
| Nausea/vomiting 154 | RR | 1.38 | 1.04‐1.84 | MA | M | 2,630 | |||
| Weight loss 144 | SMD | –0.77 | –1.09 to –0.45 | NMA | H | 14,346 | |||
| Modafinil | 13 (16.7%) | 3 (3.8%) | Anorexia 153 | RR | 5.02 | 2.55‐9.89 | MA | M | 921 |
| Insomnia 153 | RR | 6.16 | 3.40‐11.2 | MA | M | 921 | |||
| Weight loss 144 | SMD | –0.93 | –1.59 to –0.26 | NMA | H | 14,346 |
OR – odds ratio, RR – risk ratio, Log OR – log odds ratio, SMD – standardized mean difference, NMA – network meta‐analysis, MA – meta‐analysis, RCT – randomized controlled trial, NA – not available, H – high quality, M – medium quality, L – low quality (lower score of either AMSTAR or AMSTAR‐Content), Sig – significant difference between medication and placebo without effect size available
Among anti‐ADHD medications with ≥20% of adverse events covered, methylphenidate had the best safety/coverage ratio (5/25 adverse events covered significantly worse), while guanfacine and atomoxetine had the worst safety/coverage ratio (4/16 and 5/20, respectively).
Five anti‐ADHD medications were associated with significantly worse anorexia (atomoxetine, d‐amphetamine, lisdexamphetamine, methylphenidate, modafinil), four with insomnia (d‐amphetamine, lisdexamphetamine, methylphenidate, modafinil), three with weight loss (atomoxetine, methylphenidate, modafinil), two each with abdominal pain (methylphenidate, guanfacine), discontinuation due to adverse event (lisdexamphetamine, guanfacine), hypertension (atomoxetine, lisdexamphetamine), and sedation (clonidine, guanfacine), and one with QT prolongation (guanfacine).
Mood stabilizers
Out of eight mood stabilizers, six (75.0%) had adverse event data covered in the literature. The mood stabilizer literature covered 0‐24.4% (mean: 12.7%, median: 14.1%) of the reviewed adverse events. Details on the proportion of the 78 adverse events covered in the literature and of adverse events that were worse with individual mood stabilizers vs. placebo/controls are reported in Table 4 and Figure 2.
Table 4.
Safety of mood stabilizers in children and adolescents with any mental illness (adverse events significantly worse than with placebo/controls)
| Medication | Adverse events covered by literature | Adverse events worse than placebo | Adverse event | Type of effect size | Effect size | 95% CI | Source | Quality | N |
|---|---|---|---|---|---|---|---|---|---|
| Mixed mood stabilizers | 4 (5.1%) | 1 (1.3%) | Sedation 107 | NNH | 9.5 | 6.3‐23.5 | MA | L | 469 |
| Carbamazepine | 7 (9.0%) | 0 (0.0%) | |||||||
| Lamotrigine | 11 (14.1%) | 0 (0.0%) | |||||||
| Lithium | 16 (20.5%) | 0 (0.0%) | |||||||
| Oxcarbazepine | 11 (14.1%) | 4 (5.1%) |
Discontinuation due to adverse event 181 |
OR | 6.19 | 1.31‐29.3 | RCT | M | 116 |
| Nausea/vomiting 181 | OR | 3.66 | 1.33‐10.1 | RCT | M | 116 | |||
| Sedation 181 | OR | 6.89 | 1.47‐32.4 | RCT | M | 116 | |||
| Weight gain 181 | NA | Sig | Sig | RCT | M | 116 | |||
| Topiramate | 15 (19.2%) | 1 (1.3%) | Anorexia 182 | OR | 21.7 | 1.19‐398 | RCT | M | 56 |
| Valproate | 19 (24.4%) | 4 (5.1%) | Leukocytopenia 180 | NA | Sig | Sig | RCT | H | 150 |
| Sedation 107 | NNH | 7.8 | 5.3‐15.0 | MA | L | 231 | |||
| Thrombocytopenia 180 | NA | Sig | Sig | RCT | H | 150 | |||
| Weight gain 107 | Effect size | 0.4 | 0.07‐0.73 | MA | L | 231 | |||
OR – odds ratio, RR – risk ratio, NNH – number needed to harm, MA – meta‐analysis, RCT – randomized controlled trial, NA – not available, H – high quality, M – medium quality, L – low quality (lower score of either AMSTAR or AMSTAR‐Content), Sig – significant difference between medication and placebo without effect size available
Among mood stabilizers with ≥20% of adverse events covered, the best safety/coverage ratio emerged for lithium (0/16 adverse events covered significantly worse), while valproate showed the worst safety/coverage ratio (4/19).
Two mood stabilizers were associated with significantly worse sedation (oxcarbazepine, valproate), and weight gain/increased body mass index (oxcarbazepine, valproate), and one each with weight loss or anorexia (topiramate), thrombocytopenia and leucocytopenia (valproate), and nausea/vomiting (oxcarbazepine).
Evidence from studies lasting ≥6 months
For antidepressants, no RCT lasted ≥6 months, while one cohort studies lasted 6 to 12 months 100 , and two ≥12 months (range: 12‐130 months)98, 99. Significant associations emerged between current mixed antidepressants and fractures (small effect size, ≥12 months), but this association became non‐significant when considering past exposure to antidepressants. Also, while antidepressants had a small association (≥12 months) with increased risk of any cancer in the first version of the analyses from a large cohort study, additional analyses from the same database did not confirm such association when removing mixed medications 99 .
For antipsychotics, no RCT lasted ≥6 months, no cohort study lasted 6‐12 months, while two cohort studies lasted ≥12 months (range: 84‐130 months)99, 143. A large association was found between mixed SGAs and diabetes (≥12 months).
For anti‐ADHD medications, no RCT lasted ≥6 months, no cohort study 6‐12 months, while five cohort studies lasted ≥12 months (range: 12‐130 months)99, 174, 175, 176, 177. A large protective association was found between methylphenidate and any cancer (≥12 months), which survived after additional analyses from the same database removing mixed medications 99 .
For mood stabilizers, no RCT lasted ≥6 months and no cohort studies were identified, so there was no long‐term data on adverse events for any mood stabilizer.
DISCUSSION
This meta‐review of 80 psychotropic medications summarized data on 78 preselected adverse events in children and adolescents with mental illness, quantifying data for 18 antidepressants (N=120,637), 15 antipsychotics (N=66,764), seven anti‐ADHD medications (N=148,664) and six mood stabilizers (N=1,621).
Overall, the amount of coverage of the preselected adverse events was 0‐24.4% for antidepressants (no data for 26 antidepressants), 0‐56.4% for antipsychotics (no data for six antipsychotics), 7.7‐32.1% for anti‐ADHD medications (data for all anti‐ADHD medications), and 0‐24.4% for mood stabilizers (no data for two mood stabilizers).
Data were reported on ≥20% of the preselected adverse events for only six antidepressants (sertraline, escitalopram, paroxetine, fluoxetine, venlafaxine, vilazodone), eight antipsychotics (risperidone, quetiapine, aripiprazole, lurasidone, paliperidone, ziprasidone, olanzapine, asenapine), three anti‐ADHD medications (methylphenidate, atomoxetine, guanfacine), and two mood stabilizers (valproic acid, lithium).
Thus, the present meta‐review shows that the evidence on adverse events of psychotropic medications in children and adolescents is modest overall, and that psychostimulants are the drugs which have been most studied up to now.
The main adverse events for antidepressants were (in descending order of number of medications associated with the specific event): nausea/vomiting, discontinuation due to adverse event, extrapyramidal side effects, weight gain, sedation, diarrhea, headache and anorexia. Based on the safety/coverage ratio among agents with ≥20% adverse event coverage, the safest profile emerged for escitalopram and fluoxetine, and the worst for venlafaxine. These data confirm, and put in a more comprehensive framework, the findings of a previous NMA on antidepressants in children and adolescents 40 (focusing, however, on efficacy as its primary outcome), which showed that both fluoxetine and escitalopram were not associated with more drop‐outs than placebo, while venlafaxine was, with a moderate effect size (OR=3.19). In the same NMA, fluoxetine was found to be the only antidepressant significantly superior to placebo with respect to its impact on depressive symptoms (SMD=–0.51). Merging the safety results of the present meta‐review with the available evidence on efficacy from that NMA 40 , fluoxetine probably has the best harm‐benefit ratio among all antidepressants for youth, and might be proposed as the first‐line treatment for depressive disorders in children and adolescents.
The main adverse events for antipsychotics were (in descending order of number of medications associated with the specific event): sedation, extrapyramidal side effects, weight gain, hyperprolactinemia, increased cholesterol, and glucose increase. Based on the safety/coverage ratio among agents with ≥20% adverse event coverage, the safest profile emerged for lurasidone, and the worst for olanzapine. These data confirm in part, and put in a more comprehensive framework, the findings of the largest NMA of antipsychotics in children and adolescents with schizophrenia 101 (which, however, focused on efficacy as primary outcome). In the same NMA, the only antipsychotic superior to all others in terms of efficacy was clozapine, and no further difference emerged among other antipsychotics, except for ziprasidone being inferior to molindone, olanzapine and risperidone, and fluphenazine being inferior to all other antipsychotics.
Merging the safety results of the present meta‐review with available evidence on efficacy 101 , lurasidone might be proposed as the first‐line treatment for schizophrenia spectrum disorders in children and adolescents. Less tolerable yet effective medications can be used as second‐line treatments, tailoring the choice to each individual patient’s expectations and safety priorities (e.g., sexually active subjects might prefer agents not increasing prolactin). Importantly, clozapine should be considered only for treatment‐resistant cases, given the lack of evidence regarding its safety in children and adolescents, and its poor safety profile in adults 192 , which can be expected to be similar in children and adolescents, if not worse.
The main adverse events for anti‐ADHD medications were (in descending order of number of medications associated with the specific event): anorexia, insomnia, weight loss, abdominal pain, hypertension, and sedation. Based on safety/coverage ratio among agents with ≥20% adverse event coverage, the safest profile emerged for methylphenidate, and the worst for atomoxetine and guanfacine. Our comprehensive meta‐review provides a finer‐grained insight into the adverse events of anti‐ADHD medications, while the largest NMA to date 144 did not reveal differences among these drugs concerning tolerability. Somewhat surprisingly, methylphenidate was also protective against cancer when long follow‐up was considered, with such protective association surviving additional analyses excluding mixed medications 99 . Further research is warranted on this protective effect.
Our meta‐review shows that both atomoxetine and methylphenidate induce weight loss, consistent with previous findings 144 . Sedation was only observed with the alpha‐2 agonists clonidine and guanfacine. Clinically, this effect can sometimes be exploited to counter insomnia, but residual daytime sedation may impair cognitive performance in subjects with ADHD. In terms of efficacy, in the above‐mentioned NMA 144 , only methylphenidate outperformed placebo (SMD=–0.82) according to teachers’ ratings. Moreover, methylphenidate was superior to atomoxetine (SMD=0.22). Considering the available safety and efficacy data, methylphenidate might be considered the first‐line treatment for ADHD in children and adolescents.
The main adverse events for mood stabilizers were (with the same number of medications associated with the specific event) sedation and weight gain. Based on the safety/coverage ratio among agents with ≥20% adverse event coverage, the safest event profile emerged for lithium, and the worst for valproate. While the lack of any association between lithium and thyroid/kidney damage 188 as well as weight gain 190 is likely due to the small sample size of the included RCTs (N=124 and N=31, respectively), and the short duration of one RCT (3 months) 188 , significant lithium‐induced weight gain would have emerged during the six‐month RCT 190 . Considering the well‐established efficacy of lithium, which is the first‐line treatment in adolescent bipolar disorder according to international guidelines 193 , currently available data on the harm‐benefit ratio favor the choice of lithium among mood stabilizers in youth. However, long‐term cohort studies in this age group are clearly warranted. All antipsychotics have more adverse events than lithium according to this meta‐review, except for lurasidone, which seems to have a comparably safe profile and could be preferred to lithium for the treatment of bipolar depression193, 194.
The results of this meta‐review need to be interpreted considering some limitations. First, data for adverse events are lacking for some, and limited for many of the reviewed psychotropic medications. Absence of evidence for certain adverse events cannot be taken as evidence of their absence. Therefore, a more comprehensive reporting of adverse events is strongly recommended in studies concerning the use of psychotropic medications in children and adolescents.
Second, information on adverse events is mostly based on spontaneous reports. While these will underestimate the frequency of such events, the use of rating scales might increase the level of noise. Interviews and/or self‐report scales would assure a more comprehensive capturing of adverse events, and applying appropriate thresholds for severity and frequency could enhance the signal‐to‐noise ratio.
Third, long‐term and rare adverse events are likely underrepresented in the reviewed data, that are based mostly on short‐ and medium‐term RCTs, with only eight cohort studies of sufficient methodological quality providing longer‐term data. Fourth, we did not differentiate the adverse events based on dose effects due to limited data. Fifth, we took a transdiagnostic approach in order to capture all available information. Although certain adverse events could possibly differ across the various mental disorders, no clear evidence exists for this possibility, and other patient‐ and medication‐related factors that are transdiagnostic (e.g., age, treatment‐naiveté, dose, co‐medications) are likely much more important than diagnosis.
Of course, safety of medications needs to be considered along with their efficacy. This was not a focus of this large‐scale meta‐review, but we discussed our findings in the context of efficacy data from the largest and most recent NMA or MA for the respective medication class for its main indication. Finally, this meta‐review does not include data on strategies to prevent or mitigate adverse events of psychotropic medications in youth. While this is clearly an important area, this topic is beyond the scope of the present review and needs to be considered on the basis of targeted reviews and studies focusing on specific adverse events of individual medications195, 196, 197, 198, 199, 200, 201.
In summary, the results of this meta‐review have several clinical implications, which can guide the use of psychotropic medications in children and adolescents. First, for some medications, there are no or very insufficient high‐quality adverse event data in this age group, which should caution their use. Second, within each of the four major classes, we provide a hierarchy of medications on the basis of the available safety evidence: the preferred agents are likely to be fluoxetine and escitalopram among antidepressants, lurasidone among antipsychotics, methylphenidate among anti‐ADHD medications, and lithium among mood stabilizers. By contrast, potentially least preferred agents based on safety are likely to be venlafaxine among antidepressants, olanzapine among antipsychotics, atomoxetine and guanfacine among anti‐ADHD medications, and valproate among mood stabilizers.
Together with the efficacy data for these medications, the results of this comprehensive and updated meta‐review of top‐tier evidence regarding the safety of antidepressants, antipsychotics, anti‐ADHD medications and mood stabilizers in children and adolescents can inform clinical practice, research and treatment guidelines.
ACKNOWLEDGEMENTS
E.G. Ostinelli is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility and the NIHR Oxford Health Biomedical Research Centre (grant BRC‐1215‐20005).
REFERENCES
- 1. Parellada M. Why psychogeriatrics starts right after adolescence. Eur Child Adolesc Psychiatry 2013;22:391‐3. [DOI] [PubMed] [Google Scholar]
- 2. Kessler RC, Berglund P, Demler O et al. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 2005;62:593‐602. [DOI] [PubMed] [Google Scholar]
- 3. Correll CU, Galling B, Pawar A et al. Comparison of early intervention services vs treatment as usual for early‐phase psychosis: a systematic review, meta‐analysis, and meta‐regression. JAMA Psychiatry 2018;75:555‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chia MF, Cotton S, Filia K et al. Early intervention for bipolar disorder – Do current treatment guidelines provide recommendations for the early stages of the disorder? J Affect Disord 2019;257:669‐77. [DOI] [PubMed] [Google Scholar]
- 5. Correll CU, Kratochvil CJ, March JS. Developments in pediatric psychopharmacology: focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry 2011;72:655‐70. [DOI] [PubMed] [Google Scholar]
- 6. Kornø KT, Aagaard L. Off‐label prescribing of antipsychotics in a Danish child and adolescent mental health center: a register‐based study. J Res Pharm Pract 2018;7:205‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panther SG, Knotts AM, Odom‐Maryon T et al. Off‐label prescribing trends for ADHD medications in very young children. J Pediatr Pharmacol Ther 2017;22:423‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braüner JV, Johansen LM, Roesbjerg T et al. Off‐label prescription of psychopharmacological drugs in child and adolescent psychiatry. J Clin Psychopharmacol 2016;36:500‐7. [DOI] [PubMed] [Google Scholar]
- 9. Sharma AN, Arango C, Coghill D et al. BAP Position Statement: Off‐label prescribing of psychotropic medication to children and adolescents. J Psychopharmacol 2016;30:416‐21. [DOI] [PubMed] [Google Scholar]
- 10. Shekelle P, Maglione M, Bagley S. Efficacy and comparative effectiveness of off‐label use of atypical antipsychotics. Agency Healthc Res Qual 2007;6. [PubMed] [Google Scholar]
- 11. Hung C, Yu NW, Liu CY et al. The impact of the duration of an untreated episode on improvement of depression and somatic symptoms. Neuropsychiatr Dis Treat 2015;11:2245‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dagani J, Signorini G, Nielssen O et al. Meta‐analysis of the interval between the onset and management of bipolar disorder. Can J Psychiatry 2017;62:247‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Meter AR, Burke C, Youngstrom EA et al. The bipolar prodrome: meta‐analysis of symptom prevalence prior to initial or recurrent mood episodes. J Am Acad Child Adolesc Psychiatry 2016;55:543‐55. [DOI] [PubMed] [Google Scholar]
- 14. Compton MT, Gordon TL, Goulding SM et al. Patient‐level predictors and clinical correlates of duration of untreated psychosis among hospitalized first‐episode patients. J Clin Psychiatry 2011;72:225‐32. [DOI] [PubMed] [Google Scholar]
- 15. Albert U, Barbaro F, Bramante S et al. Duration of untreated illness and response to SRI treatment in obsessive‐compulsive disorder. Eur Psychiatry 2019;58:19‐26. [DOI] [PubMed] [Google Scholar]
- 16. Benatti B, Camuri G, Dell’Osso B et al. Which factors influence onset and latency to treatment in generalized anxiety disorder, panic disorder, and obsessive‐compulsive disorder? Int Clin Psychopharmacol 2016;31:347‐52. [DOI] [PubMed] [Google Scholar]
- 17. Kisely S, Scott A, Denney J et al. Duration of untreated symptoms in common mental disorders: association with outcomes. Br J Psychiatry 2006;189:79‐80. [DOI] [PubMed] [Google Scholar]
- 18. Rubio JM, Correll CU. Duration and relevance of untreated psychiatric disorders, 1: Psychotic disorders. J Clin Psychiatry 2017;78:358‐9. [DOI] [PubMed] [Google Scholar]
- 19. Rubio JM, Correll CU. Duration and relevance of untreated psychiatric disorders, 2: Nonpsychotic psychiatric disorders and substance use disorders. J Clin Psychiatry 2017;78:464‐5. [DOI] [PubMed] [Google Scholar]
- 20. Penttilä M, Jaä¨skel¨ainen E, Hirvonen N et al. Duration of untreated psychosis as predictor of long‐term outcome in schizophrenia: systematic review and meta‐analysis. Br J Psychiatry 2014;205:88‐94. [DOI] [PubMed] [Google Scholar]
- 21. Ghio L, Gotelli S, Marcenaro M et al. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta‐analysis. J Affect Disord 2014;152‐154:45‐51. [DOI] [PubMed]
- 22. Compton MT, Gordon TL, Weiss PS et al. The “doses” of initial, untreated hallucinations and delusions: a proof‐of‐concept study of enhanced predictors of first‐episode symptomatology and functioning relative to duration of untreated psychosis. J Clin Psychiatry 2011;72:1487‐93. [DOI] [PubMed] [Google Scholar]
- 23. Hung CI, Liu CY, Yang CH. Untreated duration predicted the severity of depression at the two‐year follow‐up point. PLoS One 2017;12:e0185119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medeiros GC, Senço SB, Lafer B et al. Association between duration of untreated bipolar disorder and clinical outcome: data from a Brazilian sample. Rev Bras Psiquiatr 2016;38:6‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kular A, Perry BI, Brown L et al. Stigma and access to care in first‐episode psychosis. Early Interv Psychiatry 2019;13:1208‐13. [DOI] [PubMed] [Google Scholar]
- 26. Gronholm PC, Thornicroft G, Laurens KR et al. Mental health‐related stigma and pathways to care for people at risk of psychotic disorders or experiencing first‐episode psychosis: a systematic review. Psychol Med 2017;47:1867‐79. [DOI] [PubMed] [Google Scholar]
- 27. Gerlinger G, Hauser M, De Hert M et al. Personal stigma in schizophrenia spectrum disorders: a systematic review of prevalence rates, correlates, impact and interventions. World Psychiatry 2013;12:155‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ray WA, Stein CM, Murray KT et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry 2019;76:162‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galling B, Roldán A, Nielsen RE et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta‐analysis. JAMA Psychiatry 2016;73:247‐59. [DOI] [PubMed] [Google Scholar]
- 30. Isacsson G, Rich CL. Antidepressant drugs and the risk of suicide in children and adolescents. Pediatr Drugs 2014;16:115‐22. [DOI] [PubMed] [Google Scholar]
- 31. Hennissen L, Bakker MJ, Banaschewski T et al. Cardiovascular effects of stimulant and non‐stimulant medication for children and adolescents with ADHD: a systematic review and meta‐analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs 2017;31:199‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zito JM, Burcu M. Stimulants and pediatric cardiovascular risk. J Child Adolesc Psychopharmacol 2017;27:538‐45. [DOI] [PubMed] [Google Scholar]
- 33. Fish FA, Kannankeril PJ. Diagnosis and management of sudden death in children. Curr Opin Pediatr 2012;24:592‐602. [DOI] [PubMed] [Google Scholar]
- 34. Bell GS, Mula M, Sander JW. Suicidality in people taking antiepileptic drugs: what is the evidence? CNS Drugs 2009;23:281‐92. [DOI] [PubMed] [Google Scholar]
- 35. Dragioti E, Solmi M, Favaro A et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry 2019;76:1241‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma A, Guski LS, Freund N et al. Suicidality and aggression during antidepressant treatment: systematic review and meta‐analyses based on clinical study reports. BMJ 2016;352:i65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barbui C, Cipriani A, Geddes JR. Antidepressants and suicide symptoms: compelling new insights from the FDA’s analysis of individual patient level data. Evid Based Ment Health 2008;11:34‐6. [DOI] [PubMed] [Google Scholar]
- 38. Singh T, Prakash A, Rais T et al. Decreased use of antidepressants in youth after US Food and Drug Administration black box warning. Psychiatry 2009;6:30‐4. [PMC free article] [PubMed] [Google Scholar]
- 39. Fornaro M, Anastasia A, Valchera A et al. The FDA “black box” warning on antidepressant suicide risk in young adults: more harm than benefits? Front Psychiatry 2019;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cipriani A, Zhou X, Del Giovane C et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta‐analysis. Lancet 2016;388:881‐90. [DOI] [PubMed] [Google Scholar]
- 41. Cortese S, Tomlinson A, Cipriani A. Meta‐review: network meta‐analyses in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry 2019;58:167‐79. [DOI] [PubMed] [Google Scholar]
- 42. Solmi M, Correll CU, Carvalho AF et al. The role of meta‐analyses and umbrella reviews in assessing the harms of psychotropic medications: beyond qualitative synthesis. Epidemiol Psychiatr Sci 2018;27:537‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat ‐ Simul Comput 2010;39:860‐4. [Google Scholar]
- 44. Correll CU, Rubio JM, Inczedy‐Farkas G et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta‐analytic evidence. JAMA Psychiatry 2017;74:675‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shea BJ, Grimshaw JM, Wells GA et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Higgins JPT, Savovic J, Page MJ et al. Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2.
- 47. Wells G, Shea B, O’Connell J et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 48. Catalá‐López F, Hutton B, Núñez‐Beltrán A et al. The pharmacological and non‐pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta‐analyses of randomised trials. PLoS One 2017;12:e0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta‐analysis. J Clin Psychiatry 2019;80:17r12064. [DOI] [PubMed]
- 50. Uthman OA, Abdulmalik J. Comparative efficacy and acceptability of pharmacotherapeutic agents for anxiety disorders in children and adolescents: a mixed treatment comparison meta‐analysis. Curr Med Res Opin 2010;26:53‐9. [DOI] [PubMed] [Google Scholar]
- 51. Maneeton N, Srisurapanont M. Tricyclic antidepressants for depressive disorders in children and adolescents: a meta‐analysis of randomized‐controlled trials. J Med Assoc Thai 2000;83:1367‐74. [PubMed] [Google Scholar]
- 52. Hetrick SE, McKenzie JE, Cox GR et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev 2012;11:CD004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Julious SA. Efficacy and suicidal risk for antidepressants in paediatric and adolescent patients. Stat Methods Med Res 2013;22:190‐218. [DOI] [PubMed] [Google Scholar]
- 54. Bridge JA, Iyengar S, Salary CB et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta‐analysis of randomized controlled trials. JAMA 2007;297:1683‐96. [DOI] [PubMed] [Google Scholar]
- 55. Dubicka B, Hadley S, Roberts C. Suicidal behaviour in youths with depression treated with new‐generation antidepressants: meta‐analysis. Br J Psychiatry 2006;189:393‐8. [DOI] [PubMed] [Google Scholar]
- 56. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332‐9. [DOI] [PubMed] [Google Scholar]
- 57. Strawn JR, Welge JA, Wehry AM et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta‐analysis. Depress Anxiety 2015;32:149‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rohden AI, Benchaya MC, Camargo RS et al. Dropout prevalence and associated factors in randomized clinical trials of adolescents treated for depression: systematic review and meta‐analysis. Clin Ther 2017;39:971‐92. [DOI] [PubMed] [Google Scholar]
- 59. Locher C, Koechlin H, Zion SR et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin‐norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta‐analysis. JAMA Psychiatry 2017;74:1011‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Otasowie J, Castells X, Ehimare UP et al. Tricyclic antidepressants for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2014;9:CD006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ipser JC, Stein DJ, Hawkridge S et al. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2009;3:CD005170. [DOI] [PubMed] [Google Scholar]
- 62. Wang Z, Whiteside SPH, Sim L et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: a systematic review and meta‐analysis. JAMA Pediatr 2017;171:1049‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rojas‐Mirquez JC, Rodriguez‐Zuñiga MJM, Bonilla‐Escobar FJ et al. Nocebo effect in randomized clinical trials of antidepressants in children and adolescents: systematic review and meta‐analysis. Front Behav Neurosci 2014;8:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hazell P, O’Connell D, Heathcote D et al. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev 2002;2:CD002317. [DOI] [PubMed] [Google Scholar]
- 65. Kye CH, Waterman GS, Ryan ND et al. A randomized, controlled trial of amitriptyline in the acute treatment of adolescent major depression. J Am Acad Child Adolesc Psychiatry 1996;35:1139‐44. [DOI] [PubMed] [Google Scholar]
- 66. Conners CK, Casat CD, Gualtieri CT et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry 1996;35:1314‐21. [DOI] [PubMed] [Google Scholar]
- 67. Geller B, Cooper TB, Graham DL et al. Pharmacokinetically designed double‐blind placebo‐controlled study of nortriptyline in 6‐ to 12‐year‐olds with major depressive disorder. J Am Acad Child Adolesc Psychiatry 1992;31:34‐44. [DOI] [PubMed] [Google Scholar]
- 68. Wagner KD, Ambrosini P, Rynn M et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA 2003;290:1033‐41. [DOI] [PubMed] [Google Scholar]
- 69. Wagner KD, Berard R, Stein MB et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry 2004;61:1153‐62. [DOI] [PubMed] [Google Scholar]
- 70. Emslie GJ, Findling RL, Yeung PP et al. Venlafaxine ER for the treatment of pediatric subjects with depression: results of two placebo‐controlled trials. J Am Acad Child Adolesc Psychiatry 2007;46:479‐88. [DOI] [PubMed] [Google Scholar]
- 71. Emslie GJ, Prakash A, Zhang Q et al. A double‐blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 2014;24:170‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rynn M. Efficacy and safety of extended‐release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo‐controlled trials. Am J Psychiatry 2007;164:290‐300. [DOI] [PubMed] [Google Scholar]
- 73. Emslie GJ, Ventura D, Korotzer A et al. Escitalopram in the treatment of adolescent depression: a randomized placebo‐controlled multisite trial. J Am Acad Child Adolesc Psychiatry 2009;48:721‐9. [DOI] [PubMed] [Google Scholar]
- 74. March JS, Entusah AR, Rynn M et al. A randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry 2007;62:1149‐54. [DOI] [PubMed] [Google Scholar]
- 75. Pfizer . Double‐blind, placebo‐controlled study of venlafaxine ER in children and adolescents with generalized anxiety disorder. EMA Paediatric Web Synopsis, 2011.
- 76. Wagner KD, Jonas J, Findling RL et al. A double‐blind, randomized, placebo‐controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry 2006;45:280‐8. [DOI] [PubMed] [Google Scholar]
- 77. Von Knorring AL, Olsson GI, Thomsen PH et al. A randomized, double‐blind, placebo‐controlled study of citalopram in adolescents with major depressive disorder. J Clin Psychopharmacol 2006;26:311‐5. [DOI] [PubMed] [Google Scholar]
- 78. Emslie GJ, Heiligenstein JH, Wagner KD et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo‐controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 2002;41:1205‐15. [DOI] [PubMed] [Google Scholar]
- 79. Geller DA, Hoog SL, Heiligenstein JH et al. Fluoxetine treatment for obsessive‐compulsive disorder in children and adolescents: a placebo‐controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40:773‐9. [DOI] [PubMed] [Google Scholar]
- 80. Riddle MA, Reeve EA, Yaryura‐Tobias JA et al. Fluvoxamine for children and adolescents with obsessive‐compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40:222‐9. [DOI] [PubMed] [Google Scholar]
- 81. Emslie GJ, Wagner KD, Kutcher S et al. Paroxetine treatment in children and adolescents with major depressive disorder: a randomized, multicenter, double‐blind, placebo‐controlled trial. J Am Acad Child Adolesc Psychiatry 2006;45:709‐19. [DOI] [PubMed] [Google Scholar]
- 82. Geller DA, Wagner KD, Emslie G et al. Paroxetine treatment in children and adolescents with obsessive‐compulsive disorder: a randomized, multicenter, double‐blind, placebo‐controlled trial. J Am Acad Child Adolesc Psychiatry 2004;43:1387‐96. [DOI] [PubMed] [Google Scholar]
- 83. Robb AS, Cueva JE, Sporn J et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double‐blind, placebo‐controlled trial. J Child Adolesc Psychopharmacol 2010;20:463‐71. [DOI] [PubMed] [Google Scholar]
- 84. March JS, Biederman J, Wolkow R et al. Sertraline in children and adolescents with obsessive‐compulsive disorder: a multicenter randomized controlled trial. JAMA 1998;280:1752‐6. [DOI] [PubMed] [Google Scholar]
- 85. Biederman J, Baldessarini RJ, Wright V et al. A double‐blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry 1989;28:777‐84. [DOI] [PubMed] [Google Scholar]
- 86. Strawn JR, Prakash A, Zhang Q et al. A randomized, placebo‐controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 2015;54:283‐93. [DOI] [PubMed] [Google Scholar]
- 87. Findling RL, Robb A, Bose A. Escitalopram in the treatment of adolescent depression: a randomized, double‐blind, placebo‐controlled extension trial. J Child Adolesc Psychopharmacol 2013;23:468‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. March JS. Fluoxetine, cognitive‐behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 2004;292:807‐20. [DOI] [PubMed] [Google Scholar]
- 89. Pine DS, Walkup JT, Labellarte MJ et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 2001;344:1279‐85. [DOI] [PubMed] [Google Scholar]
- 90. Keller MB, Ryan ND, Strober M et al. Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2001;40:762‐72. [DOI] [PubMed] [Google Scholar]
- 91. Mosholder AD. Nefazodone hydrochloride (Serzone) ‐ Review and evaluation of clinical data. https://www.accessdata.fda.gov/drugsatfda_docs/pediatric/020152s032_nefazodone_Serzone_Clinical_BPCA.pdf.
- 92. Atkinson S, Lubaczewski S, Ramaker S et al. Desvenlafaxine versus placebo in the treatment of children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 2018;28:55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Emslie GJ, Wells TG, Prakash A et al. Acute and longer‐term safety results from a pooled analysis of duloxetine studies for the treatment of children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 2015;25:293‐305. [DOI] [PubMed] [Google Scholar]
- 94. Durgam S, Chen C, Migliore R et al. A phase 3, double‐blind, randomized, placebo‐controlled study of vilazodone in adolescents with major depressive disorder. Paediatr Drugs 2018;20:353‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Herscu P, Handen BL, Arnold LE et al. The SOFIA study: negative multi‐center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord (in press). [DOI] [PubMed] [Google Scholar]
- 96. Hollander E, Phillips A, Chaplin W et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005;30:582‐9. [DOI] [PubMed] [Google Scholar]
- 97. DeVeaugh‐Geiss J, Moroz G, Biederman J et al. Clomipramine hydrochloride in childhood and adolescent obsessive‐compulsive disorder – a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31:45‐9. [DOI] [PubMed] [Google Scholar]
- 98. Gracious BL, Fontanella CA, Phillips GS et al. Antidepressant exposure and risk of fracture among Medicaid‐covered youth. J Clin Psychiatry 2016;77:e950‐6. [DOI] [PubMed] [Google Scholar]
- 99. Steinhausen HC, Helenius D. The association between medication for attention‐deficit/hyperactivity disorder and cancer. J Child Adolesc Psychopharmacol 2013;23:208‐13. [DOI] [PubMed] [Google Scholar]
- 100. Valuck RJ, Libby AM, Sills MR et al. Antidepressant treatment and risk of suicide attempt by adolescents with major depressive disorder: a propensity‐adjusted retrospective cohort study. CNS Drugs 2004;18:1119‐32. [DOI] [PubMed] [Google Scholar]
- 101. Krause M, Zhu Y, Huhn M et al. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: a network meta‐analysis. Eur Neuropsychopharmacol 2018;28:659‐74. [DOI] [PubMed] [Google Scholar]
- 102. Pagsberg AK, Tarp S, Glintborg D et al. Acute antipsychotic treatment of children and adolescents with schizophrenia‐spectrum disorders: a systematic review and network meta‐analysis. J Am Acad Child Adolesc Psychiatry 2017;56:191‐202. [DOI] [PubMed] [Google Scholar]
- 103. Cohen D, Bonnot O, Bodeau N et al. Adverse effects of second‐generation antipsychotics in children and adolescents: a Bayesian meta‐analysis. J Clin Psychopharmacol 2012;32:309‐16. [DOI] [PubMed] [Google Scholar]
- 104. Stafford MR, Mayo‐Wilson E, Loucas CE et al. Efficacy and safety of pharmacological and psychological interventions for the treatment of psychosis and schizophrenia in children, adolescents and young adults: a systematic review and meta‐analysis. PLoS One 2015;10:e0117166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ardizzone I, Nardecchia F, Marconi A et al. Antipsychotic medication in adolescents suffering from schizophrenia: a meta‐analysis of randomized controlled trials. Psychopharmacol Bull 2010;43:45‐66. [PubMed] [Google Scholar]
- 106. Schneider‐Thoma J, Efthimiou O, Bighelli I et al. Second‐generation antipsychotic drugs and short‐term somatic serious adverse events: a systematic review and meta‐analysis. Lancet Psychiatry 2019;6:753‐65. [DOI] [PubMed] [Google Scholar]
- 107. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo‐controlled trials. Bipolar Disord 2010;12:116‐41. [DOI] [PubMed] [Google Scholar]
- 108. Seida JC, Schouten JR, Mousavi SS et al. First‐ and second‐generation antipsychotics for children and young adults. Rockville: Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
- 109. Fallah MS, Shaikh MR, Neupane B et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta‐analysis. J Child Adolesc Psychopharmacol 2019;29:168‐80. [DOI] [PubMed] [Google Scholar]
- 110. Maneeton B, Putthisri S, Maneeton N et al. Quetiapine monotherapy versus placebo in the treatment of children and adolescents with bipolar depression: a systematic review and meta‐analysis. Neuropsychiatr Dis Treat 2017;13:1023‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Maneeton N, Maneeton B, Putthisri S et al. Aripiprazole in acute treatment of children and adolescents with autism spectrum disorder: a systematic review and meta‐analysis. Neuropsychiatr Dis Treat 2018;14:3063‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fung LK, Mahajan R, Nozzolillo A et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta‐analysis. Pediatrics 2016;137 (Suppl. 2):S124‐35. [DOI] [PubMed] [Google Scholar]
- 113. Pringsheim T, Lam D, Ching H et al. Metabolic and neurological complications of second‐generation antipsychotic use in children: a systematic review and meta‐analysis of randomized controlled trials. Drug Saf 2011;34:651‐68. [DOI] [PubMed] [Google Scholar]
- 114. Kumar A, Datta SS, Wright SD et al. Atypical antipsychotics for psychosis in adolescents. Cochrane Database Syst Rev 2013;10:CD009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Aman MG, Bukstein OG, Gadow KD et al. What does risperidone add to parent training and stimulant for severe aggression in child attention‐deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry 2014;53:47‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Connor DF, McLaughlin TJ, Jeffers‐Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol 2008;18:140‐56. [DOI] [PubMed] [Google Scholar]
- 117. Hollander E, Wasserman S, Swanson EN et al. A double‐blind placebo‐controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol 2006;16:541‐8. [DOI] [PubMed] [Google Scholar]
- 118. Ichikawa H, Mikami K, Okada T et al. Aripiprazole in the treatment of irritability in children and adolescents with autism spectrum disorder in Japan: a randomized, double‐blind, placebo‐controlled study. Child Psychiatry Hum Dev 2017;48:796‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kryzhanovskaya L, Schulz SC, McDougle C et al. Olanzapine versus placebo in adolescents with schizophrenia: a 6‐week, randomized, double‐blind, placebo‐controlled trial. J Am Acad Child Adolesc Psychiatry 2009;48:60‐70. [DOI] [PubMed] [Google Scholar]
- 120. Loebel A, Brams M, Goldman RS et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord 2016;46:1153‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Marcus RN, Owen R, Kamen L et al. A Placebo‐controlled, fixed‐dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry 2009;48:1110‐9. [DOI] [PubMed] [Google Scholar]
- 122. McCracken JT, McGough J, Shah B et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314‐21. [DOI] [PubMed] [Google Scholar]
- 123. Owen R, Sikich L, Marcus RN et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 2009;124:1533‐40. [DOI] [PubMed] [Google Scholar]
- 124. Paillère‐Martinot ML, Lecrubier Y, Martinot JL et al. Improvement of some schizophrenic deficit symptoms with low doses of amisulpride. Am J Psychiatry 1995;152:130‐4. [DOI] [PubMed] [Google Scholar]
- 125. Pathak S, Findling RL, Earley WR et al. Efficacy and safety of quetiapine in children and adolescents with mania associated with bipolar I disorder. J Clin Psychiatry 2013;74:e100‐9. [DOI] [PubMed] [Google Scholar]
- 126. Remington G, Sloman L, Konstantareas M et al. Clomipramine versus haloperidol in the treatment of autistic disorder: a double‐blind, placebo‐controlled, crossover study. J Clin Psychopharmacol 2001;21:440‐4. [DOI] [PubMed] [Google Scholar]
- 127. Findling RL, Robb A, Nyilas M et al. A multiple‐center, randomized, double‐blind, placebo‐controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry 2008;165:1432‐41. [DOI] [PubMed] [Google Scholar]
- 128. Reyes M, Buitelaar J, Toren P et al. A randomized, double‐blind, placebo‐controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. Am J Psychiatry 2006;163:402‐10. [DOI] [PubMed] [Google Scholar]
- 129. Singh J, Robb A, Vijapurkar U et al. A randomized, double‐blind study of paliperidone extended‐release in treatment of acute schizophrenia in adolescents. Biol Psychiatry 2011;70:1179‐87. [DOI] [PubMed] [Google Scholar]
- 130. Tohen M, Kryzhanovskaya L, Carlson G et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry 2007;164:1547‐56. [DOI] [PubMed] [Google Scholar]
- 131. Pool D, Bloom W, Mielke DH et al. A controlled evaluation of loxitane in seventy five adolescent schizophrenic patients. Curr Ther Res Clin Exp 1976;19:99‐104. [PubMed] [Google Scholar]
- 132. Haas M, Unis AS, Armenteros J et al. A 6‐week, randomized, double‐blind, placebo‐controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol 2009;19:611‐21. [DOI] [PubMed] [Google Scholar]
- 133. Findling RL, Nyilas M, Forbes RA et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double‐blind, placebo‐controlled study. J Clin Psychiatry 2009;70:1441‐51. [DOI] [PubMed] [Google Scholar]
- 134. Findling RL, McKenna K, Earley WR et al. Efficacy and safety of quetiapine in adolescents with schizophrenia investigated in a 6‐week, double‐blind, placebo‐controlled trial. J Child Adolesc Psychopharmacol 2012;22:327‐42. [DOI] [PubMed] [Google Scholar]
- 135. Findling RL, Cavus¸ I, Pappadopulos E et al. Ziprasidone in adolescents with schizophrenia: results from a placebo‐controlled efficacy and long‐term open‐extension study. J Child Adolesc Psychopharmacol 2013;23:531‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Findling RL, Landbloom RP, Mackle M et al. Safety and efficacy from an 8 week double‐blind trial and a 26 week open‐label extension of asenapine in adolescents with schizophrenia. J Child Adolesc Psychopharmacol 2015;25:384‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Goldman R, Loebel A, Cucchiaro J et al. Efficacy and safety of lurasidone in adolescents with schizophrenia: a 6‐week, randomized placebo‐controlled study. J Child Adolesc Psychopharmacol 2017;27:516‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Haas M, Delbello MP, Pandina G et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double‐blind, placebo‐controlled study. Bipolar Disord 2009;11:687‐700. [DOI] [PubMed] [Google Scholar]
- 139. Hagman J, Gralla J, Sigel E et al. A double‐blind, placebo‐controlled study of risperidone for the treatment of adolescents and young adults with anorexia nervosa: A pilot study. J Am Acad Child Adolesc Psychiatry 2011;50:915‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Findling RL, Cavus¸ I, Pappadopulos E et al. Efficacy, long‐term safety, and tolerability of ziprasidone in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 2013;23:545‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Findling RL, Landbloom RL, Szegedi A et al. Asenapine for the acute treatment of pediatric manic or mixed episode of bipolar I disorder. J Am Acad Child Adolesc Psychiatry 2015;54:1032‐41. [DOI] [PubMed] [Google Scholar]
- 142. DelBello MP, Goldman R, Phillips D et al. Efficacy and safety of lurasidone in children and adolescents with bipolar i depression: a double‐blind, placebo‐controlled study. J Am Acad Child Adolesc Psychiatry 2017;56:1015‐25. [DOI] [PubMed] [Google Scholar]
- 143. Andrade SE, Lo JC, Roblin D et al. Antipsychotic medication use among children and risk of diabetes mellitus. Pediatrics 2011;128:1135‐41. [DOI] [PubMed] [Google Scholar]
- 144. Cortese S, Adamo N, Del Giovane C et al. Comparative efficacy and tolerability of medications for attention‐deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2018;5:727‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Joseph A, Ayyagari R, Xie M et al. Comparative efficacy and safety of attention‐deficit/hyperactivity disorder pharmacotherapies, including guanfacine extended release: a mixed treatment comparison. Eur Child Adolesc Psychiatry 2017;26:875‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Storebø OJ, Ramstad E, Krogh HB et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev 2015;11:CD009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Schwartz S, Correll CU. Efficacy and safety of atomoxetine in children and adolescents with attention‐deficit/hyperactivity disorder: results from a comprehensive meta‐analysis and metaregression. J Am Acad Child Adolesc Psychiatry 2014;53:174‐87. [DOI] [PubMed] [Google Scholar]
- 148. Ching C, Eslick GD, Poulton AS. Evaluation of methylphenidate safety and maximum‐dose titration rationale in attention‐deficit/hyperactivity disorder: a meta‐analysis. JAMA Pediatr 2019;173:630‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hirota T, Schwartz S, Correll CU. Alpha‐2 agonists for attention‐deficit/hyperactivity disorder in youth: a systematic review and meta‐analysis of monotherapy and add‐on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 2014;53:153‐73. [DOI] [PubMed] [Google Scholar]
- 150. Coughlin CG, Cohen SC, Mulqueen JM et al. Meta‐analysis: reduced risk of anxiety with psychostimulant treatment in children with attention‐deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2015;25:611‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Schachter HM, Pham B, King J et al. How efficacious and safe is short‐acting methylphenidate for the treatment of attention‐deficit disorder in children and adolescents? A meta‐analysis. CMAJ 2001;165:1475‐88. [PMC free article] [PubMed] [Google Scholar]
- 152. Bangs ME, Wietecha LA, Wang S et al. Meta‐analysis of suicide‐related behavior or ideation in child, adolescent, and adult patients treated with atomoxetine. J Child Adolesc Psychopharmacol 2014;24:426‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang SM, Han C, Lee SJ et al. Modafinil for the treatment of attention‐deficit/hyperactivity disorder: a meta‐analysis. J Psychiatr Res 2017;84:292‐300. [DOI] [PubMed] [Google Scholar]
- 154. Holmskov M, Storebø OJ, Moreira‐Maia CR et al. Gastrointestinal adverse events during methylphenidate treatment of children and adolescents with attention deficit hyperactivity disorder: a systematic review with meta‐analysis and trial sequential analysis of randomised clinical trials. PLoS One 2017;12:e0178187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Punja S, Shamseer L, Hartling L et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2016;2:CD009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Patra S, Nebhinani N, Viswanathan A et al. Atomoxetine for attention deficit hyperactivity disorder in children and adolescents with autism: a systematic review and meta‐analysis. Autism Res 2019;12:542‐52. [DOI] [PubMed] [Google Scholar]
- 157. Newcorn JH, Kratochvil CJ, Allen AJ et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 2008;165:721‐30. [DOI] [PubMed] [Google Scholar]
- 158. Findling RL, Quinn D, Hatch SJ et al. Comparison of the clinical efficacy of twice‐daily Ritalin® and once‐daily EquasymTM XL with placebo in children with attention deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry 2006;15:450‐9. [DOI] [PubMed] [Google Scholar]
- 159. Greenhill LL, Biederman J, Boellner SW et al. A randomized, double‐blind, placebo‐controlled study of modafinil film‐coated tablets in children and adolescents with attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2006;45:503‐11. [DOI] [PubMed] [Google Scholar]
- 160. Kahbazi M, Ghoreishi A, Rahiminejad F et al. A randomized, double‐blind and placebo‐controlled trial of modafinil in children and adolescents with attention deficit and hyperactivity disorder. Psychiatry Res 2009;168:234‐7. [DOI] [PubMed] [Google Scholar]
- 161. Biederman J, Lopez FA, Boellner SW et al. A randomized, double‐blind, placebo‐controlled, parallel‐group study of SLI381 (Adderall XR) in children with attention‐deficit/hyperactivity disorder. Pediatrics 2002;110:258‐66. [DOI] [PubMed] [Google Scholar]
- 162. Biederman J, Swanson JM, Wigal SB et al. A comparison of once‐daily and divided doses of modafinil in children with attention‐deficit/hyperactivity disorder: a randomized, double‐blind, and placebo‐controlled study. J Clin Psychiatry 2006;67:727‐35. [DOI] [PubMed] [Google Scholar]
- 163. Hervas A, Huss M, Johnson M et al. Efficacy and safety of extended‐release guanfacine hydrochloride in children and adolescents with attention‐deficit/hyperactivity disorder: a randomized, controlled, Phase III trial. Eur Neuropsychopharmacol 2014;24:1861‐72. [DOI] [PubMed] [Google Scholar]
- 164. Jain R, Segal S, Kollins SH et al. Clonidine extended‐release tablets for pediatric patients with attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011;50:171‐9. [DOI] [PubMed] [Google Scholar]
- 165. Michelson D, Faries D, Wernicke J et al. Atomoxetine in the treatment of children and adolescents with attention‐deficit/hyperactivity disorder: a randomized, placebo‐controlled, dose‐response study. Pediatrics 2001;108:E83. [DOI] [PubMed] [Google Scholar]
- 166. Wilens TE, Bukstein O, Brams M et al. A controlled trial of extended‐release guanfacine and psychostimulants for attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012;51:74‐85.e2. [DOI] [PubMed] [Google Scholar]
- 167. Biederman J, Swanson JM, Wigal SB et al. Efficacy and safety of modafinil film‐coated tablets in children and adolescents with attention‐deficit/hyperactivity disorder: results of a randomized, double‐blind, placebo‐controlled, flexible‐dose study. Pediatrics 2005;116:e777‐84. [DOI] [PubMed] [Google Scholar]
- 168. Gittelman‐Klein R, Klein DF, Katz S et al. Comparative effects of methylphenidate and thioridazine in hyperkinetic children: I. Clinical results. Arch Gen Psychiatry 1976;33:1217‐31. [DOI] [PubMed] [Google Scholar]
- 169. Newcorn JH, Nagy P, Childress AC et al. Randomized, double‐blind, placebo‐controlled acute comparator trials of lisdexamfetamine and extended‐release methylphenidate in adolescents with attention‐deficit/hyperactivity disorder. CNS Drugs 2017;31:999‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Conners CK, Taylor E, Meo G et al. Magnesium pemoline and dextroamphetamine: a controlled study in children with minimal brain dysfunction. Psychopharmacologia 1972;26:321‐36. [DOI] [PubMed] [Google Scholar]
- 171. Daviss WB, Patel NC, Robb AS et al. Clonidine for attention‐deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry 2008;47:189‐98. [DOI] [PubMed] [Google Scholar]
- 172. Tumuluru R V., Corbett‐Dick P, Aman MG et al. Adverse events of atomoxetine in a double‐blind placebo‐controlled study in children with autism. J Child Adolesc Psychopharmacol 2017;27:708‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Spencer TJ, Abikoff HB, Connor DF et al. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of oppositional defiant disorder with or without comorbid attention‐deficit/hyperactivity disorder in school‐aged children and adolescents: a 4‐week, multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled, forced‐dose‐escalation study. Clin Ther 2006;28:402‐18. [DOI] [PubMed] [Google Scholar]
- 174. McAfee AT, Holdridge KC, Johannes CB et al. The effect of pharmacotherapy for attention deficit hyperactivity disorder on risk of seizures in pediatric patients as assessed in an insurance claims database. Curr Drug Saf 2008;3:123‐31. [DOI] [PubMed] [Google Scholar]
- 175. Winterstein AG, Gerhard T, Shuster J et al. Cardiac safety of central nervous system stimulants in children and adolescents with attention‐deficit/hyperactivity disorder. Pediatrics 2007;120:e1494‐501. [DOI] [PubMed] [Google Scholar]
- 176. Dalsgaard S, Kvist AP, Leckman JF et al. Cardiovascular safety of stimulants in children with attention‐deficit/hyperactivity disorder: a nationwide prospective cohort study. J Child Adolesc Psychopharmacol 2014;24:302‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hemmer SA, Pasternak JF, Zecker SG et al. Stimulant therapy and seizure risk in children with ADHD. Pediatr Neurol 2001;24:99‐102. [DOI] [PubMed] [Google Scholar]
- 178. Hirota T, Veenstra‐Vanderweele J, Hollander E et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta‐analysis. J Autism Dev Disord 2014;44:948‐57. [DOI] [PubMed] [Google Scholar]
- 179. Jochim J, Rifkin‐Zybutz R, Geddes J et al. Valproate for acute mania. Cochrane Database Syst Rev 2019; 10:CD004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wagner KD, Redden L, Kowatch RA et al. A double‐blind, randomized, placebo‐controlled trial of divalproex extended‐release in the treatment of bipolar disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 2009;48:519‐32. [DOI] [PubMed] [Google Scholar]
- 181. Wagner KD, Kowatch RA, Emslie GJ et al. A double‐blind, randomized, placebo‐controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Am J Psychiatry 2006;163:1179‐86. [DOI] [PubMed] [Google Scholar]
- 182. Delbello MP, Findling RL, Kushner S et al. A pilot controlled trial of topiramate for mania in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:539‐47. [DOI] [PubMed] [Google Scholar]
- 183. Belsito KM, Law PA, Kirk KS et al. Lamotrigine therapy for autistic disorder: a randomized, double‐blind, placebo‐controlled trial. J Autism Dev Disord 2001;31:175‐81. [DOI] [PubMed] [Google Scholar]
- 184. Hellings JA, Weckbaugh M, Nickel EJ et al. A double‐blind, placebo‐controlled study of valproate for aggression in youth with pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005;15:682‐92. [DOI] [PubMed] [Google Scholar]
- 185. Hollander E, Chaplin W, Soorya L et al. Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology 2010;35:990‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Rezaei V, Mohammadi MR, Ghanizadeh A et al. Double‐blind, placebo‐controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuro‐Psychopharmacology Biol Psychiatry 2010;34:1269‐72. [DOI] [PubMed] [Google Scholar]
- 187. Blader JC, Schooler NR, Jensen PS et al. Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry 2009;166:1392‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Yuan J, Song J, Zhu D et al. Lithium treatment is safe in children with intellectual disability. Front Mol Neurosci 2018;11:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cueva JE, Overall JE, Small AM et al. Carbamazepine in aggressive children with conduct disorder: a double‐blind and placebo‐controlled study. J Am Acad Child Adolesc Psychiatry 1996;35:480‐90. [DOI] [PubMed] [Google Scholar]
- 190. Findling RL, McNamara NK, Pavuluri M et al. Lithium for the maintenance treatment of bipolar I disorder: a double‐blind, placebo‐controlled discontinuation study. J Am Acad Child Adolesc Psychiatry 2019;58:287‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Findling RL, Chang K, Robb A et al. Adjunctive maintenance lamotrigine for pediatric bipolar I disorder: a placebo‐controlled, randomized withdrawal study. J Am Acad Child Adolesc Psychiatry 2015;54:1020‐31. [DOI] [PubMed] [Google Scholar]
- 192. Huhn M, Nikolakopoulou A, Schneider‐Thoma J et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi‐episode schizophrenia: a systematic review and network meta‐analysis. Lancet 2019;394:939‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Yatham LN, Kennedy SH, Parikh SV et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 2018;20:97‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Fornaro M, De Berardis D, Perna G et al. Lurasidone in the treatment of bipolar depression: systematic review of systematic reviews. Biomed Res Int 2017;2017:3084859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018;17:341‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ellul P, Delorme R, Cortese S. Metformin for weight gain associated with second‐generation antipsychotics in children and adolescents: a systematic review and meta‐analysis. CNS Drugs 2018;32:1103‐12. [DOI] [PubMed] [Google Scholar]
- 197. Correll CU, Sikich L, Reeves G et al. Metformin add‐on vs. antipsychotic switch vs. continued antipsychotic treatment plus healthy lifestyle education in overweight or obese youth with severe mental illness: results from the IMPACT trial. World Psychiatry 2020;19:69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry 2008;47:9‐20. [DOI] [PubMed] [Google Scholar]
- 199. Luft MJ, Lamy M, DelBello MP et al. Antidepressant‐induced activation in children and adolescents: risk, recognition and management. Curr Probl Pediatr Adolesc Health Care 2018;48:50‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Wigal SB. Efficacy and safety limitations of attention‐deficit hyperactivity disorder pharmacotherapy in children and adults. CNS Drugs 2009;23(Suppl. 1):21‐31. [DOI] [PubMed] [Google Scholar]
- 201. Montejo AL, Montejo L, Baldwin DS. The impact of severe mental disorders and psychotropic medications on sexual health and its implications for clinical management. World Psychiatry 2018;17:3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]


