Abstract
Resisting cell death is a hallmark of cancer. Disturbances in the execution of cell death programs promote carcinogenesis and survival of cancer cells under unfavorable conditions, including exposition to anti-cancer therapies. Specific modalities of regulated cell death (RCD) have been classified based on different criteria, including morphological features, biochemical alterations and immunological consequences. Although melanoma cells are broadly equipped with the anti-apoptotic machinery and recurrent genetic alterations in the components of the RAS/RAF/MEK/ERK signaling markedly contribute to the pro-survival phenotype of melanoma, the roles of autophagy-dependent cell death, necroptosis, ferroptosis, pyroptosis, and parthanatos have recently gained great interest. These signaling cascades are involved in melanoma cell response and resistance to the therapeutics used in the clinic, including inhibitors of BRAFmut and MEK1/2, and immunotherapy. In addition, the relationships between sensitivity to non-apoptotic cell death routes and specific cell phenotypes have been demonstrated, suggesting that plasticity of melanoma cells can be exploited to modulate response of these cells to different cell death stimuli. In this review, the current knowledge on the non-apoptotic cell death signaling pathways in melanoma cell biology and response to anti-cancer drugs has been discussed.
Keywords: autophagy, differentiation, drug resistance, ferroptosis, melanoma, necroptosis, parthanatos, pyroptosis, reactive oxygen species (ROS), targeted therapy
1. Introduction
Cell death is an essential biological process that controls development and homeostasis in the organism [1,2]. Deregulation of cell death execution is a hallmark of cancer that promotes cancer development and progression, and is implicated in the resistance of cancer cells to therapies [3,4]. Regulated cell death (RCD) involves the activation of precisely controlled machinery that transmits signals promoting either cell survival or death. As RCD is executed by specific proteins, pharmacological targeting of these regulators can be exploited therapeutically [5,6,7,8]. For decades, apoptosis has been investigated as a major RCD modality involved in the survival of cancer cells and their response to anti-cancer drugs [9]. Apoptosis is initiated via one of two pathways: Extrinsic or intrinsic (mitochondrial). The extrinsic route of apoptosis couples the interactions between death receptors and death-promoting ligands, while intrinsic apoptosis is predominantly governed by the proteins from the B-cell leukemia/lymphoma 2 (BCL-2) family. This family consists of three groups of proteins: (i) Multi-domain pro-survival proteins such as BCL-2, myeloid cell leukemia 1 (MCL-1), B-cell lymphoma-extra large (BCL-XL), BCL-w, and BFL-1; (ii) multi-domain pro-apoptotic proteins BAX and BAK that execute apoptosis by compromising the outer mitochondrial membrane; (iii) a single-domain pro-apoptotic proteins, e.g., BCL-2-interacting mediator of cell death (BIM), that interact with either pro-survival or BAX/BAK proteins [7,8,10,11]. It is now evident that cell death signaling pathways are far more complex, and specific routes of cell death have been classified based on different criteria, including morphological features, biochemical alterations and immunological consequences (Table 1). However, the detailed molecular mechanisms of these modes of cell death are still poorly understood.
Table 1.
The comparison of major morphological and biochemical features and markers of apoptosis and non-apoptotic cell death modalities [12,13,14,15].
| Apoptosis | Autophagy | Necroptosis | Ferroptosis | Pyroptosis | |
|---|---|---|---|---|---|
| morphology | membrane blebbing | formation of intracellular vesicles | cell swelling | shrunken mitochondria with reduced crista | cell swelling; pore formation |
| DNA fragmentation | + | - | + (random) |
- | + |
| caspase activation | + | - | - | - | + |
| positive staining with Annexin-V | + | - | + | + | + |
| inflammation | - | - | + | + | + |
| other typical features | PARP cleavage | LC3-I to LC3-II conversion; p62/SQSTM1 degradation | RIPK3/MLKL activation; drop in ATP level | iron accumulation and lipid peroxidation | osmotic lysis of cell |
Melanoma is the deadliest form of skin cancer with increasing incidence worldwide [16]. The hyperactivation of the RAS/RAF/MEK/ERK signaling pathway (hereafter MAPK), which is assessed in the vast majority of melanomas as a result of genetic alterations in BRAF, RAS, or NF1 among others [17,18,19,20,21], contributes to the pro-survival phenotype of melanoma cells. A negative regulation of pro-apoptotic molecules (e.g., BIM) by oncogenic MAPK signaling has been reported [22], while anti-apoptotic proteins involved in the regulation of extrinsic and intrinsic apoptotic routes are largely overexpressed in melanoma [23,24]. Other signaling pathways [25], melanoma-specific transcriptional regulators [26] and post-transcriptional control [27] also extensively contribute to the capability of melanoma cells to counteract unfavorable conditions, including exposition to anti-cancer therapies. In addition, microenvironment-mediated regulation of expression of pro-survival molecules, including MCL-1, BCL-XL, and BFL-1 [28,29,30], supports a remarkable adaptive capabilities of melanoma cells. Despite a tremendous advances in the therapeutic options for melanoma patients (Figure 1), inability or limited vulnerability of melanoma cells to induction of apoptosis in response to inhibitors of BRAFmut (BRAFi) and MEK (MEKi) [31,32,33,34,35,36,37,38], and escape from immunotherapy [39,40,41] are the reasons for re-growth of drug-resistant disease. In this respect, research on the mechanisms of the non-apoptotic cell death modalities is attractive in melanoma.
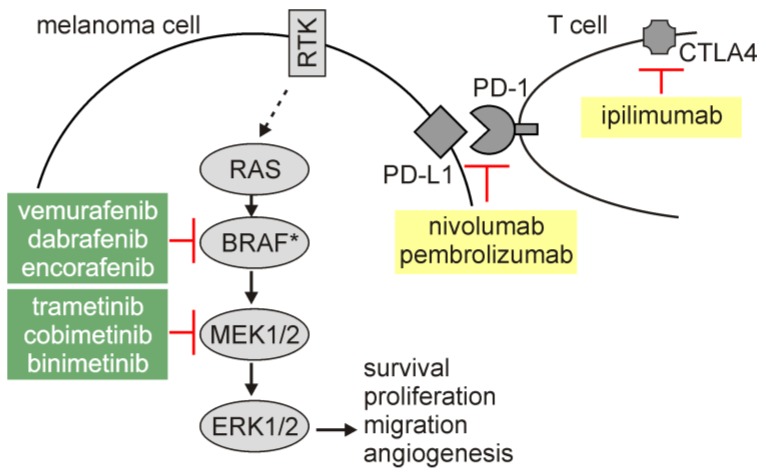
Figure 1.
Targeted therapeutics and immunotherapy used in the treatment of melanoma patients. Melanoma cells exert hyperactivation of the RAS/RAF/MEK/ERK signaling pathway that regulates different cellular programs, including survival. Targeted therapeutics (shown in green background) inhibit activity of either mutated BRAF (BRAF*, V600E is the most frequent amino acid substitution) or MEK1/2. BRAFi and MEKi are used as a combinatory treatment regimen. Immunotherapy (shown in yellow background) includes checkpoint inhibitors: antibodies blocking either PD-1 (programmed death-1) or CTLA4 (cytotoxic T-lymphocyte associated protein 4). Both targets for immunotherapy are physiological inhibitors of T cell-mediated immune response. RTK, receptor tyrosine kinase.
This review summarizes current knowledge on the role of non-apoptotic cell death signaling pathways in melanoma development and progression, as well as in response of melanoma cells to currently used therapeutics, i.e., BRAFi and MEKi, and immunotherapy.
2. Autophagy
2.1. An Overview of Autophagy and Autophagy-Dependent Cell Death
Autophagy is a catabolic process, in which proteins, bulk cytoplasm, and/or organelles are incorporated into double-membrane intracellular vesicles to be recycled within lysosomes. Thus, autophagy maintains cellular homeostasis by the removal of unfolded proteins and damaged organelles [42,43,44,45]. Autophagy can be executed either non-selectively (macroautophagy or autophagy) or in a selective manner to remove specific organelles, e.g., damaged mitochondria (mitophagy) [46] and peroxisomes (pexophagy) [47]. Autophagy is sustained at a low level in the majority of cells, while its efficiency can be affected by a number of stimuli [48]. Autophagy involves five stages: (1) Initiation, (2) nucleation of the double-membrane vesicles (phagophores, further extended to the autophagosomes), (3) expansion and elongation, (4) closure and fusion of the autophagosomes with the lysosomes, and (5) degradation of intravesicular content (Figure 2) [42,49]. Autophagy-related genes (ATGs) extensively contribute to different stages of autophagy execution. The formation of autophagosome is initiated by the protein complex composed of Unc51-like autophagy activating kinase 1 (ULK1) and ULK2 [50,51], a scaffolding protein 200 kDa focal adhesion kinase family-interacting protein (FIP200) [52], and ATG13 that serves as an adaptor protein [53]. The ULK complex transmits stress signals, such as nutrient or energy deprivation, to the autophagosome-forming sites as it regulates phosphorylation of downstream proteins. This protein complex is negatively regulated by the mechanistic target of rapamycin (mTOR) signaling pathway. The vesicle nucleation is proceeded by the phosphoinositide 3-kinase (PI3K) complex that includes VPS34/PIK3C3 and Beclin-1. Beclin-1, a BH3-only protein from BCL-2 family of proteins, is phosphorylated by ULK1 and acts as a scaffold facilitating localization of other autophagic proteins [54]. Beclin-1 is also a mechanistic link between autophagy and apoptosis as anti-apoptotic proteins BCL-2 and BCL-XL can interact with Beclin-1 to interrupt both the Beclin-1/VPS34 complex and the interaction with ultraviolet radiation resistance associated gene (UVRAG) [55]. Phosphorylation of VPS34 restricts its interaction with Beclin-1, while activating molecule in Beclin-1-regulated autophagy (AMBRA) stabilizes the PI3K complex by binding Beclin-1. In addition, the hypoxia-responsive gene encodes BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3), which is contextually implicated in the regulation of apoptosis and autophagy [56]. BNIP3 can displace Beclin-1 from BCL-2 and activate autophagy (Figure 2) [57]. Nucleation is followed by the expansion and elongation of the autophagosome membrane, and this stage is regulated by microtubule-associated protein light chain 3 (LC3) protein. Processing and conjugation of LC3-I with phosphatidylethanolamine (PE) is catalyzed by ATG4B and ATG7, and leads to the formation of LC3B-II. While LC3-I remains in the cytosol, LC3-II is incorporated into both the outer and the inner membrane of the autophagosome, and therefore LC3-II is largely considered as a marker of autophagy induction [54]. Also, an adaptor protein sequestosome 1 (SQTM1, known as p62), which directs substrates to the autophagosomes, is degraded with its cargo proteins and can be used as a read-out of the autophagic flux [58,59]. Another conjugation system is under control of ATG7 and ATG10, and regulates the formation of the ATG5/ATG12 conjugate. Autophagosomes subsequently fuse with lysosomes that is facilitated by syntaxin 17 (STX17). This is followed by the formation of autophagolysosomes, in which the cargo is digested by hydrolases that demand low pH for their activity. This stage of autophagy can be inhibited by chloroquine, hydroxychloroquine and bafilomycin A1 that disturb acidification of the lysosomes (Figure 2) [55]. The role of autophagy still remains elusive as it can be either a cytoprotective response or followed by induction of different modalities of cell death [60,61,62]. According to the most current recommendations of nomenclature, the term “autophagy-dependent cell death” can be used exclusively when observed cell death is strictly reliant on the autophagic machinery [5,63].
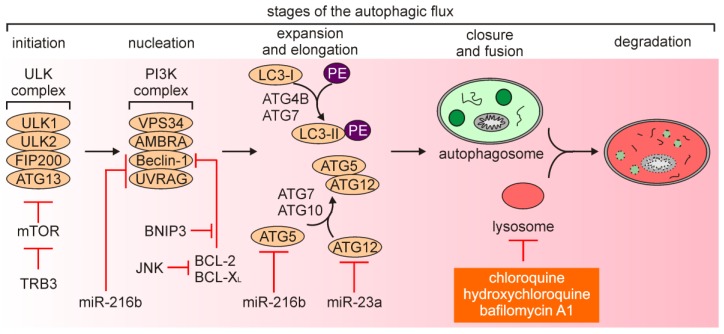
Figure 2.
Stages and machinery of autophagy. Under nutrient starvation and other autophagy-inducing conditions, the autophagy flux is initiated by formation of the Unc51-like autophagy activating kinase (ULK) complex. The activated ULK complex participates in the recruitment of other proteins that constitute the phosphoinositide 3-kinase (PI3K) complex and proceed assembly of an initial double-membrane structure (phagophore). This stage of autophagy can be additionally regulated by the apoptosis-related proteins such as B-cell leukemia/lymphoma 2 (BCL-2) and B-cell lymphoma-extra large (BCL-XL) as they can sequester Beclin-1. Elongation is mediated to form autophagosomes by two ubiquitin-like systems: (1) Autophagy-related genes(ATG)5/ATG12 conjugation system, and (2) ATG4B- and ATG7-mediated processing and phosphatidylethanolamine (PE) conjugation to light chain 3 (LC3)-I (LC3-I:LC3-II conversion). PE-conjugated LC3-II associates with the autophagosome. The maturation of autophagosome is associated with the delivery of misfolded proteins and/or damaged organelles, and then autophagosome is fused with the lysosomes. The cargo is exposed to the lysosomal hydrolases, which are active under acidic conditions. Compounds that affect the autophagic flux by disturbing lysosome acidification are shown in orange background. Mechanisms of autophagy regulation that have been shown in melanoma are additionally included.
2.2. Autophagy in Melanoma
In general, autophagy has been widely considered as a tumor suppressor mechanism at the stage of carcinogenesis, while tumor-promoting role of autophagy has been associated with progression of cancer, including melanoma [55,64,65,66]. It has been demonstrated that autophagy suppressed melanomagenesis by promoting senescence, and decreased expression of ATG5 was sufficient to preclude this process [67]. In addition, exposure to ultraviolet A (UVA) upregulated p62/SQSTM1 and triggered p62-dependent response that involved nuclear factor erythroid 2-related factor 2 (NRF-2 encoded by NFE2L2) activity to counteract oxidative stress [68], while simulated sunlight irradiation was associated with induction of mitophagy [69]. In turn, low efficiency of autophagy execution promoted survival of melanoma cells exposed to UV irradiation [70]. On the contrary, others have demonstrated that loss of ATG7 in a BRAFV600E/PTENnull model of melanoma was associated with increased oxidative stress and senescence that prevented melanoma tumorigenesis [71], suggesting that inhibition of autophagy may also play a tumor-suppressing role. Notably, an oncogenic activation of the MAPK signaling pathway has been associated with the regulation of autophagy in melanoma [72,73]. BRAFV600E has been shown to increase endoplasmic reticulum (ER) stress that was responsible for high basal autophagy in melanoma [74]. Mechanistically, c-JUN N-terminal kinase (JNK)-mediated phosphorylation of BCL-2 and BCL-XL released Beclin-1, while Tribbles pseudokinase 3 (TRB3) additionally inhibited mTOR signaling (Figure 2) [74]. A hyperactive MAPK signaling pathway regulated cAMP-responsive element binding protein (CREB)-dependent expression of NOXA, which promoted autophagy in melanoma cells upon nutrient deprivation [75]. BRAFV600E-mediated starvation-induced autophagy was also shown in uveal melanoma [76].
Melanoma cells employ autophagy as an adaptive mechanism to the microenvironment insults, including acidic conditions [77], while inhibition of autophagy has been shown when low pH was accompanied with glucose deprivation [78] or combined glucose starvation and hypoxia [79]. Autophagy was also essential for survival of melanoma cells exposed to fluctuating oxygen pressure [80], suggesting an important role of autophagy during metastasis. In addition, induction of autophagy in melanoma cells can modulate tumor microenvironment. The comparison of secretome of autophagylow and autophagyhigh melanoma cells revealed that several proteins with established roles in inflammation and tumorigenesis, including interleukin-1β (IL-1β), chemokine C-X-C motif ligand 8 (CXCL8), leukemia inhibitory factor (LIF), family with sequence similarity 3 member C (FAM3), and dickkopf 3 (DKK3), were secreted at higher levels by melanoma cells with high activity of autophagy [81]. Notably, also patient-derived serum samples from melanoma patients with high level of autophagy flux have shown high levels of these proteins [81], suggesting that they can serve as surrogates for intra-tumoral autophagy dynamics. Elevated autophagy accompanying melanoma progression has been associated with loss of galectin-3 [82], increased levels of sirtuin 1 (SIRT1) [83], SIRT6 [84,85], BNIP3 [86], and long non-coding RNA ZNNT1 [87], and enhanced activity of signaling pathways such as AKT, independent of the mutational status of BRAF [88]. A heterozygous loss of ATG5 enhanced melanoma metastasis and predicted poor overall patient survival [89]. In addition, miR-23a has been identified as a negative regulator of ATG12 (Figure 2), while ATG12 regulated melanoma cell invasion and migration through AMP-activated protein kinase-RAS homolog family member A (AMPK-RhoA) pathway [90]. Accordingly, expression of miR-23a was decreased in metastatic melanoma cell lines, and miR-23a level was significantly lower in serum of patients with metastatic melanoma [90]. An autophagy-independent role of p62/SQSTM1 has been ascribed to the control of melanoma metastasis by recruiting RNA-binding proteins in cooperation with insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) to stabilize transcripts of a number of pro-metastatic factors [91]. Notably, expression of several genes related to autophagy such as SQSTM1, AMBRA1, WD repeat domain phosphoinositide-interacting 1 (WIPI-1), peroxisomal biogenesis factor 3 (PEX3) and BCL-2-associating athanogene-1 (BAG1) has been indicated as prognostic in melanoma [92,93,94,95], and single nucleotide polymorphisms (SNPs) have been found in others [96]. Certain regulators relevant for melanoma can inhibit autophagy as shown for neural cell adhesion molecule (NCAM) [97], minichromosome maintenance protein 7 (MCM7) [98] and Wingless-type (WNT)/β-catenin cascade [99]. In line with this, high level of WNT5a, which regulates the activity of non-canonical WNT signaling pathway, and low level of β-catenin were associated with increased basal autophagy in melanoma cells [100]. In turn, downregulation of ATG5 in WNT5ahigh melanoma cells was associated with lower level of WNT5a and increased level of β-catenin [100], supporting the conclusion that autophagy is inversely correlated with the activity of canonical WNT/β-catenin signaling pathway.
The impact of autophagy on immune response has also been investigated. In a mouse model of melanoma, T cell-mediated response was found equivalent in autophagy-competent mice and mice with the loss of autophagy-associated genes or pharmacological inhibition of autophagy by chloroquine [101]. It has also been demonstrated, however, that targeting Beclin-1 in melanoma cells increased the infiltration of natural killer (NK) cells into melanoma tumors that was dependent on increased expression of the C-C motif chemokine ligand 5 (CCL5). Induction of CCL5 was also reported upon inhibition of other autophagy proteins, including ATG5 and p62/SQSTM1, or pharmacological inhibition of autophagy. In addition, a positive correlation between CCL5 and attraction of NK cells was shown in melanoma patients, and a high expression of CCL5 was correlated with improved patient survival [102,103]. This suggests that blocking autophagy may induce beneficial immune response, although it has been demonstrated that loss of BNIP3 significantly reduced phagocytic clearance of melanoma cells undergoing cell death [104].
In particular, autophagy is used by melanoma cells to counteract drug activity and drug-induced changes in tumor microenvironment, thus autophagy correlates with clinical outcome and largely contributes to resistance of melanoma cells to therapeutics [105,106,107,108]. Autophagy assessed in tumor specimens obtained from melanoma patients enrolled in a phase II trial of temozolomide and sorafenib has revealed that patients whose tumors displayed high autophagy activity had a worse clinical outcome [109]. Increased level of BNIP3 accompanying activation of autophagy was correlated with poor efficacy of pembrolizumab in melanoma patients [110]. In addition, patients with BRAF-mutant melanoma that exhibited high level of autophagy were characterized with poorer response to BRAFi [111]. It has been shown that BRAFi and combined BRAFi and MEKi upregulated autophagy as a cytoprotective mechanism [111]. Autophagy accompanied melanoma cell senescence induced by encorafenib [37,38], while loss of LC3 protein substantially prevented from encorafenib-induced senescence [38]. It has also been demonstrated that treatment with dabrafenib more markedly inhibited growth and induced senescence in tumors derived from ATG7-deficient melanoma cells [71], further indicating that efficient autophagic flux limits the response of melanoma cells to anti-cancer drugs. Autophagy was also assessed at a higher level in BRAFi-resistant melanomas, and significant tumor regression was reported when drug-resistant tumors were treated with a combination of BRAFmut and autophagy inhibitors [111]. Others have shown, however, that either pharmacological inhibition or ATG5 knockdown was insufficient to re-sensitize vemurafenib-resistant melanoma cells to this BRAFi, although higher activity of autophagy was assessed in resistant cells compared with drug-naïve counterparts [112]. As MEK-mediated resistance to vemurafenib was found in these cells, a combination of MEKi and autophagy inhibitor efficiently increased cell death [112], suggesting that specific mechanisms of resistance may affect melanoma cell dependence on autophagy.
Several mechanisms on drug-induced autophagy have been reported. It has been shown that vemurafenib reduced miR-216b level that resulted in upregulation of Beclin-1, ATG5 and UVRAG [113]. Dabrafenib induced autophagy by downregulation of miR-26a following upregulation of HMGB1 in melanoma [114]. In addition, it has been shown that combined vemurafenib and trametinib treatment induced SEC61-dependent translocation of components of the MAPK signaling pathway to endoplasmic reticulum [115]. Following this translocation, ERK was reactivated by protein kinase RNA-like endoplasmic reticulum kinase (PERK)-mediated phosphorylation. Re-phosphorylated ERK induced activating transcription factor 4 (ATF4) to trigger cytoprotective autophagy [115]. Moreover, it has been shown that vemurafenib-resistant melanoma cells employed autophagosomes to secret ATP and enhance cell migration and invasion [116]. Compromising autophagy by downregulation of several autophagy-related genes significantly reduced secretion of ATP and, consequently, cell invasion [116]. Inhibition of AMPK-α1 in vemurafenib-resistant melanoma cells impaired autophagy that was associated with decreased formation of the autophagosomes [117]. Drug resistance of melanoma cells can be additionally supported by elevated levels of other autophagy-related proteins, including BAG3 [118]. Available data suggest, however, that not all melanoma patients may benefit from inhibition of autophagy. Mechanistic study on induction of autophagy upon inhibition of BRAFV600E has shown that BRAFi disrupted ERK-mediated phosphorylation of transcription factor EB (TFEB), allowing its nuclear translocation and activation of a cellular program that controls lysosome biogenesis [119]. This was, surprisingly, followed by tumor suppression and decreased metastasis in a mouse model of melanoma [119]. On the other hand, suppression of Yin Yang 1 (YY1), which is a cofactor of TFEB and contributes to the regulation of genes related to autophagy and lysosome biogenesis, evoked enhanced anti-melanoma efficiency of vemurafenib both in vitro and in vivo [120]. Interestingly, another transcription factor that is related to TFEB, microphthalmia-associated transcription factor (MITF) has been shown to bind to the promoters of genes encoding lysosomal and autophagosomal proteins in melanoma. MITF-regulated genes were, however, distinct from these activated by TFEB and TFE3 [121]. Modulating the level of MITF largely affected the response of melanoma cells to starvation-induced autophagy [121]. Considering an important role of MITF in the regulation of melanoma cell phenotype and response to targeted drugs, including diverse execution of MITF-dependent programs in drug-resistant melanoma cells [122,123,124], further research is necessary to delineate a relationship between activity of MITF and TFE transcription factors and consequences of manipulation of autophagy-lysosomal signaling in melanoma cells.
3. Necroptosis
3.1. An Overview of Necroptotic Signaling Pathway
Necroptosis has been first described in 2005 as a caspase-independent cell death modality induced by perturbations of extra- or intra-cellular homeostasis when the apoptotic machinery is inhibited [125]. Necroptosis shares morphological features with necrosis, including membrane permeabilization, cell swelling and loss of cell integrity (Table 1), and in part, structural organization with extrinsic pathway of apoptosis (Figure 3) [126]. Necroptosis is associated with a substantial production of reactive oxygen species (ROS), hyperactivation of poly(ADP-ribose) polymerase 1 (PARP1) and depletion of ATP [127,128]. The cell rupture accompanying necroptotic cell death leads to the release of damage-associated molecular patterns (DAMPs) and cytokines, including IL-1α, IL-1β, high mobility group box 1 (HMGB1) and IL-33, thus triggers robust inflammatory response (Table 1). Necroptosis is largely reliant on sequential activation of receptor-interacting protein kinase 1 (RIPK1), RIPK3 and mixed lineage kinase domain-like (MLKL) [5], and necrostatin-1 (Nec-1) and necrostatin-1s (Nec-1s), which selectively stabilize an inactive conformation of RIPK1, inhibit the necroptotic cell death pathway [129,130]. Mechanistically, necroptosis is activated by a plethora of different ligands, including tumor necrosis factor alpha (TNF-α), FAS ligand (FASL), TNF-related apoptosis-inducing ligand (TRAIL), interferon gamma (IFN-γ) and lipopolysaccharide (LPS) that engage members of the tumor necrosis factor receptor (TNFR) family, FAS, T cell receptors (TCRs), and pattern recognition receptors (PRRs) [126,131]. TNF-α-mediated necroptosis has been largely investigated so far (Figure 3). Following binding TNF-α, trimers of TNFR1 recruit multiple proteins to form membrane-bound complex I. This complex includes TNFR-associated death domain (TRADD), RIPK1, cellular inhibitor of apoptosis protein 1 and 2 (cIAP1 and 2), TNFR-associated factor 2 and 5 (TRAF2 and 5), and linear ubiquitin chain assembly complex (LUBAC), in addition to TNFR [132]. RIPK1 is a member of serine-threonine kinase family, and consists of three major domains: (i) C-terminal domain that interacts with death domain receptors (DRs) and/or adaptor protein TRADD; (ii) central region that is responsible for nuclear factor kappa B (NF-κB) activation and includes receptor-interacting protein homotypic interaction motif (RHIM) responsible for the interaction with RIPK3; and (iii) a regulatory N-terminal fragment (T-loop) that contains Asp-Leu-Gly residues [133]. Within complex I, cIAPs can ubiquitinate RIPK1 and induce the pro-survival NF-κB signaling pathway. On the other hand, ligand-bound TNFR1 can be internalized, and RIPK1 is deubiquitinated by cylindromatosis (CYLD) that switches the pathway towards activation of cell death. This is associated with the formation of a cytoplasmic multimeric complex comprising RIPK1, TRADD, caspase-8, and FAS-associated death domain protein (FADD), which is known as complex II. Complex II links the activation of apoptotic (complex IIa and IIb) and necroptotic (complex IIc; necrosome) pathways. Activated caspase-8 cleaves and inactivates RIPK1 at Asp324, RIPK3, and CYLD, and leads to the execution of apoptosis, while necroptosis is proceeded upon inhibition of caspase-8 [131,133]. This is followed by an activatory autophosphorylation of RIPK1 at its N-terminal Ser161. Activated RIPK1 interacts with RIPK3, and subsequently RIPK3 phosphorylates MLKL. MLKL is recruited to the plasma membrane, where it interacts with phospholipids to enhance membrane permeability and execute necroptosis (Figure 3) [126,131]. The contribution of heat shock protein 90 (HSP90) to MLKL oligomerization and translocation has been ascribed [134,135]. Loss of plasma membrane integrity has been associated with the influx of calcium ions and exposure of phosphatidylserine, and both processes are executed in an MLKL-dependent manner [136]. This is also associated with ESCRT-II-dependent generation of the plasma membrane “bubbles”, which are however, dissimilar to the apoptotic bodies as they do not contain cytosolic remnants [136]. In addition, phosphorylated MLKL can be released within exosomes that has been suggested as a mechanism for cell self-restricting activity of MLKL in the execution of necroptosis [137]. It has also been demonstrated that necroptosis can be induced in certain cells independently of RIPK1, but still reliant on RIPK3 and MLKL [138], suggesting that activation of RIPK3 and MLKL is more universal biomarkers of necroptosis.
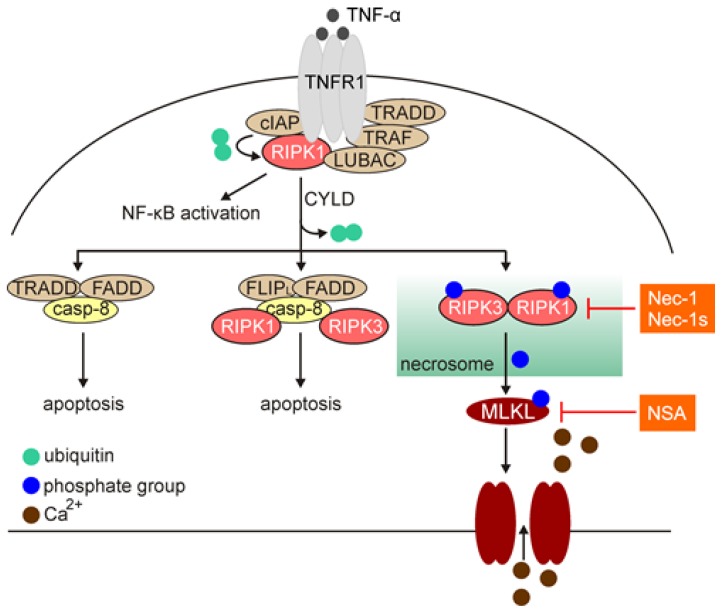
Figure 3.
Necroptotic signaling pathway exemplified by tumor necrosis factor alpha (TNF-α)-mediated activation of tumor necrosis factor receptor (TNFR)1. Activated TNFR1 forms a multi-protein complex I that transmit either pro-survival response (NF-κB activation) or cell death response, which is mediated by cylindromatosis (CYLD)-dependent deubiquitylation of receptor-interacting protein kinase 1 (RIPK1). Complexes-IIa/b are formed when the activation of caspase-8 can proceed apoptosis. Upon inhibition of caspase-8, complex IIc (necrosome) mediates activation of mixed lineage kinase domain-like (MLKL) by phosphorylation at Ser358 that results in pore formation in the plasma membrane and Ca2+ influx. Compounds that inhibit necroptosis, including necrostatin-1 (Nec-1), necrostatin-1s (Nec-1s), and necrosulfonamide (NSA) are shown in orange background.
3.2. Necroptosis in Melanoma
Several studies have indicated that inducing necroptosis in melanoma may be limited as it was found that both CYLD [139] and RIPK3 were poorly expressed in melanoma cell lines [140,141], although melanocytes and benign nevi expressed RIPK3 at a high level [140]. This suggests that low expression of essential regulators of the necroptotic pathway may be an intrinsic feature of melanoma cells to evade necroptosis. It has also been reported that low level of RIPK1 in melanoma cells promoted induction of apoptosis [142], and RIPK1 regulated autophagy in melanoma cells upon ER stress [143]. The transforming growth factor β-activated kinase 1 (TAK1) could prevent from cell death by suppressing RIPK1 activity even if RIPK3 and MLKL were intrinsically expressed [144]. TAK1 inhibition sensitized melanoma cells to apoptosis induced by a combination of TNF-α and TRAIL [144], indicating that TAK1 regulated apoptosis-related role of RIPK1 in melanoma. In addition, inhibition of RIPK1 and loss of RIPK3 did not reduce lung metastasis in a mouse model of melanoma [145]. In another study, however, pre-treatment of mice with necrostatin-1 or a novel RIPK1 inhibitor, PK68, significantly suppressed lung metastasis by melanoma cells [146], suggesting that further research is needed to clarify the role of RIPK1 in melanoma progression.
In contrast to vemurafenib, it has been shown that dabrafenib inhibited RIPK3 activity in an ATP-competitive manner, and irrespectively of inhibition of BRAFmut [140,147]. Dabrafenib disrupted RIPK3 and MLKL interactions that was associated with decreased levels of MLKL phosphorylated at Ser358 [147], and prevented from induction of necroptosis in RIPK3-expressing melanoma cells [140]. It has been demonstrated that dabrafenib could also protect normal cells from necroptosis-inducing compounds as evidenced for hepatocytes [147] and neurons [148]. These observations suggest that loss of necroptotic machinery in the majority of melanomas and inhibition of RIPK3 activity by drugs used in the clinic contribute to protection of melanoma cells from necroptosis. This also justifies the successful transition to dabrafenib administration in patients with toxic epidermal necrolysis induced after treatment with vemurafenib [149], and indicates that use of specific BRAFmut inhibitor can be important when overall toxicity of the therapy is considered.
Strategies that allow to manipulate and reactivate the necroptosis-related machinery can be still relevant for triggering this type of cell death in melanoma cells. It has been shown that RIPK3-expressing melanoma cells were sensitive to necroptosis-inducing agents and cell death could be efficiently inhibited by necrostatin-1 [141], although gaining competence to trigger necroptosis upon overexpression of RIPK3 has been questioned by others [144]. Reconstitution of RIPK3 was sufficient to phosphorylate MLKL and induce necroptosis in response to FASL/IAP antagonist [140]. In addition, a pan-caspase inhibitor combined with radiotherapy, dacarbazine and hyperthermia significantly reduced melanoma growth by inducing necroptosis and increasing dendritic cell (DC) and CD8+ T-cell infiltration in the tumor microenvironment [150]. Necroptosis was induced in response of melanoma cells to photothermal therapy, but only within a narrow range of temperature [151]. Necroptosis was also reported as a way of dying in response of BRAFV600E melanoma cells to inhibition of the mitochondrial complex I, and this was associated with augmented production of ROS [152]. In addition, combined re-establishment of p19Arf and IFN-β signaling pathways was associated with induction of necroptosis in melanoma, and activation of immune response involving NK cells, T cells and neutrophils [153]. An interesting study published more recently has evidenced that intra-tumor delivery of MLKL-encoding mRNA elicited a potent anti-tumor immunity, even if tumors were defective for proteins that acted upstream of MLKL. Mechanistically, MLKL mRNA-induced T cell response required migration of DCs and interferon signaling. This strategy was effective at inhibiting both primary tumor growth and metastasis in a mouse model of melanoma, while it also complemented anti-melanoma activity of anti-PD1 immunotherapy [154]. Both UVB and UVC have also been shown to increase MLKL protein level [70], and vapor nanobubble photoporation-mediated delivery of MLKL protein to melanoma cells effectively induced necroptosis [155].
4. Ferroptosis
4.1. An Overview of Ferroptosis
Ferroptosis is a regulated caspase-independent cell death modality characterized with the overwhelming production of ROS and accumulation of iron-dependent lipid peroxides [156,157,158]. Cells that undergo ferroptosis exhibit a typical necrotic morphology and dysmorphic mitochondrial phenotype, including shrunken mitochondria, increased mitochondrial membrane density and reduced level of intracellular NADH, but not ATP (Table 1) [159,160]. Several metabolic signaling pathways and related regulators have been shown to modulate cell sensitivity to ferroptosis in different experimental settings [159,161,162,163]. The immunomodulatory role of ferroptosis has also been investigated and discussed [164]. A member of the BCL-2 family, BH3-interacting domain death agonist (BID), which is truncated (tBID) during extrinsic apoptosis has been required for ferroptosis in neurons [165], suggesting an interconnection between ferroptotic and apoptotic cell death signaling pathways. More universally, ferroptosis is triggered in response to inhibition of glutathione (GSH) biosynthesis or inhibition of selenoprotein glutathione peroxidase 4 (GPX4) (Figure 4). It has also been reported that both transferrin (an iron-carrier protein) and glutaminolysis control ferroptosis that is triggered by deprivation of amino acids or cystine alone [161]. More recently, it has been demonstrated that cell death through ferroptosis can be antagonized by glutathione-independent ferroptosis suppressor protein 1 (FSP1) known also as apoptosis-inducing factor mitochondrial 2 (AIFM2), which acts in parallel to GPX4 [166,167]. It has been shown that FSP1 is recruited to the plasma membrane upon myristoylation to reduce coenzyme Q10 (CoQ10), which functions as a lipophilic antioxidant and prevents from the propagation of lipid peroxides [166]. Inhibition of FSP1 substantially synergized with inhibitors of GPX4 in ferroptosis induction in cancer cells [167]. Fine-tuned iron homeostasis interconnects autophagy and ferroptosis as selective degradation of ferritin, referred to as ferritinophagy, potentiates cell sensitivity to ferroptosis. Ferritinophagy promotes the increase of content of free intracellular iron, which is known as labile iron portion (LIP) [161,168,169], in a ROS-dependent manner [170]. Nuclear receptor coactivator 4 (NCOA4) mediates ferroptinophagy, and inhibition of autophagy or knockdown of NCOA4 prevented from the accumulation of LIP and ROS [171].
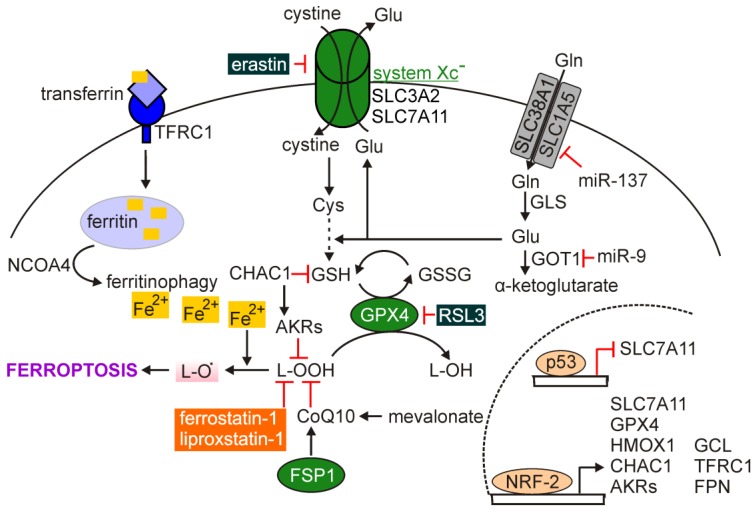
Figure 4.
Regulation of ferroptosis. Transmembrane system XC¯ composed of SLC3A2 and SLC7A11 subunits sustains the glutathione (GSH) homeostasis. GPX4 catalyzes the glutathione-dependent reduction of lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH) that prevents from the formation of highly reactive lipid alkoxy radicals (L-O•) and induction of ferroptosis. This process can also be prevented by the activity of FSP1, while Fe2+ released from ferritin in NCOA4-mediated ferritinophagy accelerates ferroptosis. Several genes encoding ferroptosis-regulating proteins are under transcriptional control of p53 and nuclear factor erythroid 2-related factor 2 (NRF-2). Compounds that inhibit ferroptosis are shown in orange background, and activators of ferroptosis are shown in dark green background. Mechanisms of ferroptosis regulation that have been demonstrated in melanoma are additionally included.
The disturbances in the synthesis of glutathione can be associated with inhibition of the cystine/glutamate antiporter named system XC−, which is composed of the twelve-pass transmembrane protein solute carrier family 7 member 11 (SLC7A11; xCT, light chain) connected to a single-pass transmembrane regulatory protein SLC3A2 (4F2, heavy chain) [13]. Inside the cell, reduction of cystine to cysteine occurs providing a substrate for glutathione synthesis and maintenance of intracellular redox homeostasis. Reduced glutathione is an essential intracellular antioxidant synthesized from amino acids: glycine, glutamate and cysteine in an ATP-dependent process involving cytosolic enzymes: Glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS), while cysteine availability is a rate-limiting step in GSH synthesis [172]. In turn, GPX4 has been recognized as the main endogenous negative regulator of ferroptosis that acts by catalyzing the glutathione-dependent reduction of lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH) (Figure 4) [173]. This prevents from the formation of markedly reactive lipid alkoxy radicals (L-O•) from lipid hydroperoxides [174]. When phospholipid hydroperoxides are not efficiently quenched by GPX4, they can trigger a reaction, which involves the transition metals such as iron (Fe(II)) [175]. Peroxidation of lipids can occur through autoxidation or enzyme activity, especially lipoxygenases (LOXs) [176]. It has been demonstrated that ferroptosis is induced upon preferential oxidation of polyunsaturated fatty acids (PUFAs) containing phosphatidylethanolamine, including arachidonic acid and adrenic acid [13]. Peroxidation of lipids reduces their ability to form fully functional cellular membranes that leads to the loss of membrane integrity. Accordingly, inhibition of acyl-CoA synthetase long chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3), which contribute to the insertion and remodeling of long PUFAs into membrane phospholipids, prevented from induction of ferroptosis [13]. Several activators of ferroptosis have been identified, and this class of agents includes inhibitors of GPX4 (e.g., (1S,3R)-RSL3) and inhibitors of system XC− (e.g., erastin) among others [173].
The majority of the proteins that regulate ferroptosis are under transcriptional control of NRF-2. NRF-2 regulates expression of SLC7A11, GPX4, and GCL, as well as genes related to iron metabolism, including transferrin receptor 1 (TFRC1) ferroportin (FPN encoded by SLC40A1) heme oxygenase 1 (HMOX1) and ferritin (FTL) [177,178]. Thus, NRF-2 modulates both basal redox homeostasis as NRF-2 is negatively regulated by Kelch-like ECH-associated protein 1 (KEAP-1), and antioxidant response during an excessive oxidative stress when KEAP1 is dissociated from NRF-2 and degraded [179].
4.2. Ferroptosis in Melanoma
Cancer cells are largely susceptible to perturbations of thiol metabolism and an excess of iron supply [180]. In melanoma, withstanding oxidative stress accompanying transient metabolic alterations has been recognized as a determinant of successful metastasis [181], suggesting that sensitivity of melanoma cells to ferroptosis may vary during melanoma development and progression. It has also been suggested that ferroptosis may be triggered upon DNA damage in melanoma cells [182]. In addition, several oncogenic pathways relevant for melanoma have been shown to render cancer cells susceptible to ferroptosis via the modulation of essential regulators of this type of cell death.
Initially, ferroptosis was postulated to be relevant exclusively in RAS mutant cancer cells, but it is now evident that this modality of cell death can be induced irrespectively of the mutational status of RAS [183,184,185]. Indeed, it has been demonstrated that mutated BRAF negatively regulated oxidative metabolism in melanoma cells [186], while BRAF inhibitors enhanced dependence of melanoma cells on the oxidative phosphorylation (OXPHOS) [186,187]. It has been hypothesized that chemosensitivity of OXPHOShigh cancer cells could rely on ROS accumulation and might be potentially executed by induction of ferroptosis [188]. This suggests that a metabolic switch induced by BRAFi can sensitize melanoma cells to ferroptosis-inducing agents, and SCL7A11 has been suggested as a marker of the susceptibility of metastatic melanoma cells to ferroptosis [189]. In line with this, acute treatment with vemurafenib or trametinib reduced transcript level of SLC7A11 in BRAFV600E melanoma cells [190]. Others have identified changes of the lipid metabolism, such as accumulation of PUFAs, in melanoma cells exposed to vemurafenib [191]. BAY 87-2243, an inhibitor that impairs OXPHOS by targeting the mitochondrial complex I, cooperated with vemurafenib in attenuation of melanoma tumor growth in vivo [187], although this could be related to induction of both ferroptosis and necroptosis as reported by others [152]. The metabolic switch of melanoma cells towards OXPHOS can be further extended by more complex phenotypic alterations accompanying acquired resistance to BRAFi and MEKi. It has been found that the acquisition of resistance was associated with an elevated dependence on the glutamine metabolism as BRAFi-resistant melanoma cells promoted glucose-derived glutamate synthesis and increase in glutathione content. In addition, drug-resistant cells exhibited a substantial activation of NRF-2 that was associated with activation of the pentose phosphate pathway involved in the regeneration of GSH, as well as increased expression of a component of system XC− [192]. In another study, however, basal levels of GSH were significantly lower in vemurafenib-resistant cell lines than in matched drug-naïve cells [193]. It has been further demonstrated for different types of cancer, including melanoma, that persisting drug-resistant cells that exhibited a gene signature of mesenchymal-like state were more susceptible to inhibition of GPX4 [194]. A non-genetic addiction to GPX4 accompanied drug-resistant patient-derived melanoma cells reliant on transforming growth factor beta (TGF-β) [195]. In addition, GPX4 knockout was lethal in chemoresistant, but not drug-naive, A375 melanoma cells, while this could be prevented by chemical inhibitors of ferroptosis such as ferrostatin-1. Ferrostatin-1 promoted tumor growth of A375GPX4-/- cells xenografted into mice, which were treated with a combination of dabrafenib and trametinib, and withdrawal of ferrostatin-1 was associated with inhibition of the growth of GPX4-/- tumors [196]. Furthermore, drug-resistant melanoma cells can dedifferentiate to different extent that is associated with loss of melanoma-specific transcription factor, MITF [123,193], and several studies have suggested that the dedifferentiation status can dictate sensitivity of cancer cells to ferroptosis. A large pharmacogenomics integration approach confirmed an inverse association between differentiation state of melanoma and its vulnerability to ferroptosis. BRAFi resistance-associated dedifferentiation increased the sensitivity to ferroptosis inducers, erastin and RSL3. In addition, levels of GSH were significantly correlated with the stage of cell dedifferentiation [193]. This suggests that glutathione can be a metabolic link between drug-induced dedifferentiation and sensitivity to ferroptosis-inducing agents, and this was confirmed by supplementation of the culture medium with GSH that rescued melanoma from ferroptosis [193]. Recently, cerebellar degeneration-related 1 antisense (CDR1as) has been identified as a novel marker of melanoma cell differentiation state, and depletion of CDR1as was associated with the metastatic potential of melanoma [197]. It has been found that CDR1ashigh cells were substantially more sensitive to inhibitors of GPX4, thus induction of ferroptosis. In addition, high levels of CDR1as have been preferentially associated with melanoma cell state marked by low MITF level and high AXL level [197]. Thus, expression of CDR1as may serve as a determinant of phenotypic state of melanoma cells that may be targeted by inducers of ferroptosis.
Ferroptosis is also relevant in melanoma cell response to immunotherapy. Reflecting the inflammatory microenvironment by supplementation of IFN-γ or TNF-α to melanoma cell culture has provoked cell dedifferentiation, and was associated with increased susceptibility to ferroptosis induction [193]. It has been further demonstrated that CD8+ T cells activated by immunotherapy enhance lipid peroxidation in tumor cells, including melanoma. This contributed to anti-melanoma effect of immunotherapy as efficacy of anti-CTLA4 and anti-PD-1 antibodies was efficiently inhibited by liproxstatin-1, an inhibitor of ferroptosis. This indicated that immunotherapy-activated T cells can employ ferroptosis as a cytotoxic mechanism in melanoma cells [198]. Mechanistically, T cell-derived IFN-γ reduced expression of SLC3A2 and SLC7A11, thus impairing the cystine uptake and affecting intracellular GSH supply. Analysis of transcriptomes of melanoma patients before and during nivolumab treatment revealed that clinical benefit was associated with reduced expression of SLC3A2, enhanced CD8+ T cell signature and expression of IFNG that was associated with improved overall patient survival [198].
The role of other regulators of ferroptosis have been investigated in melanoma. p53, encoded by the TP53, is frequently mutated in cancer, but very infrequent loss-of-function genetic alterations of TP53 are found in melanoma [17], suggesting that activity of p53 may be exploited to induce ferroptosis. The tumor suppressor activity of p53 has been linked to inhibition of cell cycle, as well as induction of senescence and apoptosis, while the contribution of p53 to the control of cellular redox state and metabolism may be associated with the regulation of ferroptosis by this transcription factor [199,200]. It has been postulated that two divergent p53 functions can be exhibited depending on the stimuli. When DNA damage occurs, p53 functions as a regulator of cell cycle arrest and apoptosis, whereas accumulation of ROS beyond the state of reversal implicates p53 in cell response to this insult [201]. This suggests that p53 might act as a rheostat that prevents from ferroptosis under low oxidative stress, whereas it promotes ferroptosis under high ROS stress. This has been supported by reports showing that p53 repressed SLC7A11 and eventually limited cystine uptake (Figure 4) [202]. The contribution of p53 to the regulation of expression of several other genes encoding proteins involved in redox homeostasis has been established [201]. Interestingly, it has also been shown that p53 activated expression of nerve growth factor receptor (NGFR, also known as CD271), and negative feedback was driven by NGFR to inactivate p53 by both promoting mouse double minute 2 (MDM2)-dependent proteolysis of p53 and inhibiting interaction between p53 and DNA [203]. As enhanced NGFR/CD271 level is associated with the neural crest-like state of melanoma cells [193], and vemurafenib- or trametinib-resistant cell lines frequently exhibit higher percentages of NGFRhigh cells compared with matched drug-naïve cell lines [124], further research is necessary to delineate whether and how p53 contributes to increased sensitivity of drug-resistant melanoma cells to ferroptosis.
Also the role of NRF-2 has been investigated in melanoma (Figure 4). Enhanced activity of NRF-2 in melanoma was connected to genetic alterations in KEAP1, and was responsible for intrinsic resistance of melanoma cells to cisplatin and dacarbazine [204]. In a recent study, the role of NRF-2 has been more extensively investigated in melanoma cells resistant to ferroptosis [189]. It has been shown that ferroptosis-resistant cells upregulated NRF-2, which induced expression of an early ferroptotic marker glutathione-specific gamma-glutamylcyclotransferase 1 (CHAC1), and the aldo-keto reductases (AKRs) such as AKR1C1, AKR1C2, and AKR1C3 to degrade the lipid peroxides and prevent from ferroptotic cell death. Inhibition of AKR activity sensitized melanoma cells to ferroptosis confirming the role of these enzymes in prevention of ferroptosis induction [189]. In addition, NRF-2 has been implicated in UV-induced expression of PD-L1 in melanoma. It has been shown that depletion of NRF-2 was associated with tumor infiltration by CD8+ and CD4+ T cells, whereas combined inhibition of NRF-2 and PD-1 demonstrated enhanced anti-melanoma effect [205]. This suggests that NRF-2 may also be considered as an alternative target to inhibit the PD-1/PD-L1 signaling. Others reported that both tumor growth and lung metastasis of melanoma were enhanced in Nrf2-null mice [206], and reduced mRNA level of NRF-2 was observed during melanomagenesis [207].
The role of microRNAs in the regulation of ferroptosis has also been demonstrated in melanoma (Figure 4). miR-137 negatively regulated ferroptosis, which was shown both in vitro and in vivo, as it directly inhibited glutamine transporter SLC1A5 in melanoma cells [208]. It has been demonstrated that ectopic expression of miR-137 suppressed SLC1A5 that was associated with reduced uptake of glutamine and accumulation of malondialdehyde [208]. Furthermore, it has been shown that miR-9 inhibited ferroptosis by targeting glutamicoxaloacetic transaminase 1 (GOT1), which mediates the conversion of glutamate to α-ketoglutarate [209]. Inhibition of miR-9 led to lipid peroxidation and iron accumulation [209].
5. Pyroptosis
5.1. An Overview of Pyroptosis
Pyroptosis is a type of a lytic cell death associated with the damage of cellular membrane by members of the gasdermin protein family, and it is usually triggered by the activation of inflammation-related caspases (Table 1) [5]. The term pyroptosis was originally coined to define a modality of RCD dependent on inflammatory caspase-1, and the role of pyroptosis was assigned to innate immunity as a way of clearance of bacterial and viral infections [210]. Growing evidence, however, has indicated that pyroptosis can also be induced in cancer cells in response to chemotherapy [211]. Pyroptosis is usually executed by two proteins gasdermin D (GSDMD) and gasdermin E (GSDME), which is encoded by DFNA5. Other members of the gasdermin family of proteins include GSDMA, GSDMB, GSDMC, and DFNB59, and they all exhibit membrane perforation activity, which is associated with induction of pyroptosis. Gasdermin proteins form pores, which have a diameter of 10–14 nm and contain sixteen protomers [15,212].
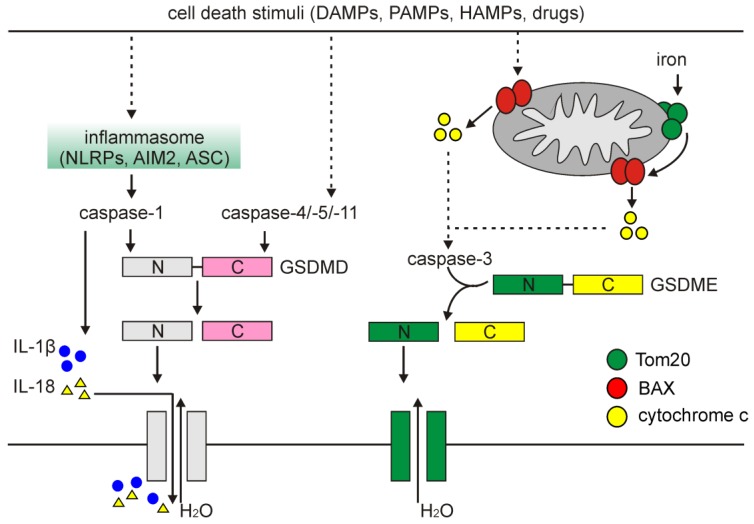
A classical pathway of pyroptosis is triggered when cells activate inflammasomes, which are cytoplasmic multi-protein platforms that control maturation and secretion of cytokines. Inflammasomes are composed of the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family: NLRP1, NLRP3, and NLRC4, absent in melanoma 2 (AIM2), and pyrin proteins through the action of pathogen-associated molecular patterns (PAMPs), DAMPs and homeostasis-altering molecular processes (HAMPs), which are diversely involved under different conditions and stimuli [213]. Inflammasomes activate caspase-1, which promotes both the maturation and secretion of pro-inflammatory cytokines, and cleaves GSDMD (Figure 5). Notably, while NLRP3-dependent activation of caspase-1 requires the contribution of apoptosis-associated speck-like (ASC) protein containing a caspase recruitment domain (CARD), NLRP1 activates caspase-1 in an ASC-independent manner due to the presence of a C-terminal CARD domain that facilitates direct association with caspase-1 and its activation via CARD–CARD interaction [214]. GSDMD has a highly conserved site between C-terminal (auto-inhibitory) and N-terminal domains recognized and cleaved by caspase-1, -4, -5, and -11. Following cleavage by caspases, the 30 kDa N-terminal (cNT) fragment of GSDMD (GSDMD-cNT) is released and binds to the components of the lipid bilayer, including phosphatidic acid, phosphatidylinositol and phosphatidylserine that results in pore formation to execute cell swelling and rupture [211,215]. In addition, caspase-1-processed IL-1β and IL-18 are released through pores formed by GSDMD-cNT (Figure 5) [214].
Figure 5.
Signaling pathways that regulate pyroptosis. Pyroptosis-inducing stimuli activate inflammatory caspases, and this is followed by (i) processing of gasdermin D (GSDMD) by cleavage of the hinge region between C- and N-terminal domains to generate fragments that form pores in the plasma membrane, and (ii) processing of inflammatory cytokines, e.g., IL-1β and IL-18. In addition, depolarization of the mitochondrial membrane associated with iron-mediated oligomerization of Tom20 or other cell death signals leads to the release of cytochrome c and ultimately results in activation of caspase-3. Active caspase-3 can mediate cleavage of the N-terminal domains of gasdermin E (GSDME) that form pores, similarly to the N-terminal fragments of GSDMD.
It has also been demonstrated that a non-classical pyroptosis can be triggered in response to activation of caspase-4, -5, and -11, which can produce a similar cleavage product of GSDMD followed by channel formation in the cellular membrane (Figure 5) [215]. Although caspase-4, -5, and -11 can interact with and activate caspase-1 in the presence of NLRP3 and ASC, caspase-1 is dispensable for cleavage of GSDMD in a non-classical pyroptosis [216]. More recently, it has been also demonstrated that caspase-3, involved in apoptosis, can cleave GSDME after Asp270, and generate an N-terminal fragment that mediates pyroptosis in a fashion similar to GSDMD-cNT-mediated cell death [217]. This was independently confirmed by others, and caspase-3-dependent cleavage of GSDME has been implicated as a mechanism underlying cancer cell response to several chemotherapeutics (Figure 5) [218].
5.2. Pyroptosis in Melanoma
As GSDME is expressed in the majority of melanomas [218], it may be an opportunity for exploiting pyroptosis as an alternative anti-melanoma therapeutic strategy. The abundance of GSDME can determine whether cells undergo either apoptosis or pyroptosis. Cell lines with high expression of DFNA5 were committed to pyroptosis in response to different chemotherapeutics, while DFNA5-null cancer cells underwent apoptosis upon treatment with these drugs [218]. It has been shown that GSDME mediated doxorubicin-induced pyroptosis in melanoma cells [219]. Pyroptosis was further enhanced in cells exposed to doxorubicin when autophagy was inhibited, while the crosstalk between these two modalities of cell death was dictated by eukaryotic elongation factor-2 kinase (eEF-2K) [219]. In turn, etoposide-resistant melanoma cells expressed DFNA5 at low level, but upregulation of DFNA5 expression enhanced sensitivity of melanoma cells to etoposide [220]. It has also been found that pyroptosis was a way of oncolytic activity of herpes simplex virus type 2 (HSV-2) mutant ΔPK in a number of melanoma cell lines. HSV-2 mutant ΔPK activated calpain and caspase-3 and -7, and involved TNF-α, which activated NLRP3- and caspase-1-dependent pyroptosis [221]. More recently, it has been demonstrated that a combination of BRAFi and MEKi induced pyroptosis in melanoma cells. Following combined treatment, cleavage of GSDME, and increased expression and release of immunostimulatory molecules such as HMGB1 were reported. As a consequence, enhanced expression of major histocompatibility complex (MHC) class II on tumor-resident DCs was followed by tumor infiltration with T cells. Mechanistically, gasdermin E was indispensable for melanoma cell response as GSDME deficiency resulted in more frequent tumor re-growth when administration of drugs was ceased. Notably, BRAFi- and MEKi-resistant cells were still sensitive to pyroptosis-inducing compounds [222].
Pyroptosis can also be induced in melanoma by ROS that links pyroptosis and other cell death modalities. It has been demonstrated that iron-induced ROS signaling caused the oxidation and oligomerization of the subunit of mitochondrial import receptor, translocase of outer mitochondrial membrane (Tom20). Activated Tom20 facilitated recruitment of BAX to release cytochrome c and activate caspase-3, which eventually cleaved GSDME and induced pyroptosis (Figure 5) [223]. To validate the role of pyroptosis in melanoma, A375 cells either GSDMEwt or GSDME-deficient were separately injected subcutaneously into nude mice. It has been demonstrated that treatment of GSDMEwt mice with iron dextran promoted cleavage of GSDME and significantly decreased the tumor growth. In contrast, iron supplementation was ineffective in the reduction of tumor weight in GSDME-knockdown mice [223]. Thus, it has been suggested that supplementation of iron can maximize the anti-tumor effect of drugs that induce ROS to inhibit growth of melanoma tumor via GSDME-dependent pyroptosis [223].
Several studies have investigated the role of inflammasome components in melanoma. In general, RNA sequencing data of 458 melanoma specimens have revealed that low expression of inflammasome-related mediators are predictive of poor prognosis in melanoma patients [224]. It has been demonstrated that both primary and metastatic melanoma cells possessed activated inflammasomes that was associated with co-expression of ASC and NLRP3, whereas self-dependence of melanoma cells on IL-1β signaling was shown to increase with the stage of disease [225]. A melanoma-specific role of other inflammasome-related protein NLRP1 has also been envisaged [226]. It has been shown that NLRP1 exhibited a dual role as it promoted melanoma growth by activating inflammasome and suppressing apoptosis. Inhibition of apoptosis by NLRP1 was evidenced by immunoprecipitation that revealed interaction between CARD-containing NLRP1 with similar domains in caspase-2 and caspase-9. In addition, NLRP1 was mainly confined in the cytosol of melanoma cells, suggesting that cellular localization of NLRP1 might also promote its interaction with caspases. Downregulation of NLRP1 promoted apoptosis in both primary and metastatic melanoma. However, further study revealed an additional function of NLRP1 assessed exclusively in metastatic melanoma cells. Mechanistic studies showed that cells employed NLRP1-dependent inflammasomes, and NLRP1 knockdown was associated with reduced activity of caspase-1, diminished secretion of IL-1β, and decreased activity of NF-κB in metastatic melanoma cells [226]. Interestingly, also other inflammasome-related proteins have been shown to exert melanoma stage-dependent function. Expression of ASC was downregulated in metastatic melanoma compared with primary tumor specimens. In contrast, elevated level of ASC inhibited NF-κB activity and affected processing of IL-1β in primary melanoma cells [227]. This suggests that the function of ASC switches from anti-cancer activity in primary melanoma cells to pro-tumorigenic in metastatic melanoma. Other members of the gasdermin family of proteins have also been detected in melanoma as shown for GSDMC that was not expressed in the normal epidermis, but its elevated level in melanoma cells correlated with their metastatic potential [228].
6. Conclusions and Future Directions
It is evident that melanoma cells utilize multiple mechanisms to counteract cell death-inducing signals, and the intersections between different signaling pathways involved in the regulation of cell survival and death are presumably a reason for a failure in melanoma response to potentially pro-death triggers. The landscape of regulated cell death modalities is still enlarging, and the role of non-apoptotic cell death signaling pathways have been given increasing attention in melanoma. This is further exemplified by parthanatos, which is a modality of cell death initiated by hyperactivation of PARP1 that results in depletion of both NAD+ and ATP, and accumulation of poly(ADP-ribosyl)ated proteins in the mitochondria [5,229,230]. This is followed by the release of apoptosis-inducing factor (AIF) into the cytosol and its subsequent translocation to the nucleus, where it mediates DNA fragmentation in cooperation with macrophage migration inhibitory factor (MIF) [231,232], but without contribution of caspases and endonuclease G (ENDOG) [233]. Parthanatos has been indicated as a cell death modality in melanoma cells that acquired resistance to BRAFi and/or MEKi, and it was preferentially induced upon drug withdrawal in cell lines characterized with high levels of active ERK1/2 rebound in response to drug cessation [234].
The reports discussed in the present review largely suggest that melanoma cell vulnerability to induction of cell death markedly depends on the microenvironment insults, including anti-cancer drugs, and can extensively evolve during acquisition of drug resistance. This implicates non-apoptotic types of regulated cell death to be considered as alternative therapeutic targets when induction of apoptosis is impaired. However, this also raises a question how uniform the sensitivity of melanoma cells to induction of particular type of cell death is, considering large patient-to-patient variability of drug resistance mechanisms, which can be both genetic and non-genetic [124,235,236]. In this respect, searching of molecular markers that correlate the phenotype of melanoma cells with vulnerability to different death-inducing triggers, and identifying transcriptional regulators responsible for controlling acquisition of this phenotype should be the directions of current research.
Abbreviations
| ATGs | autophagy-related genes |
| BCL-2 | B-cell leukemia/lymphoma 2 |
| BNIP3 | BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3 |
| BRAF | B-Raf proto-oncogene serine/threonine kinase |
| BRAFi | inhibitors of mutated BRAF |
| CDR1as | cerebellar degeneration-related 1 antisense |
| DAMPs | damage-associated molecular patterns |
| DC | dendritic cell |
| GPX4 | glutathione peroxidase 4 |
| GSDM | gasdermin |
| GSH | glutathione (reduced) |
| HMGB1 | high mobility group box 1 |
| HSP90 | heat shock protein 90 |
| IFN | interferon |
| IL-1β | interleukin-1β |
| LC3 | microtubule-associated protein light chain 3 |
| MEKi | inhibitors of MEK1/2 |
| MITF | microphthalmia-associated transcription factor |
| MLKL | mixed lineage kinase domain-like |
| NF-κB | nuclear factor-kappa B |
| NGFR | nerve growth factor receptor |
| NK | natural killer |
| NRF-2 | nuclear factor erythroid 2-related factor 2 |
| PD-L1 | programmed death-ligand 1 |
| OXPHOS | oxidative phosphorylation |
| RCD | regulated cell death |
| RIPK | receptor-interacting protein kinase |
| ROS | reactive oxygen species |
| SQSTM1/p62 | sequestosome 1 |
| TNF-α | tumor necrosis factor alpha |
| UV | ultraviolet |
| WNT | Wingless-type |
Funding
This research was funded by National Science Centre (Poland), grant number 2016/23/D/NZ7/03904.
Conflicts of Interest
The author declares no conflict of interest. The funder had no role in the writing of the manuscript.
References
- 1.Vaux D.L., Korsmeyer S.J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/S0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 2.Opferman J.T., Korsmeyer S.J. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 2003;4:410–415. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 3.Fouad Y.A., Aanei C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L., Vitale I., Aaronson S., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulda S. Targeting apoptosis for anticancer therapy. Semin. Cancer Biol. 2015;31:84–88. doi: 10.1016/j.semcancer.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Fulda S. Targeting extrinsic apoptosis in cancer: Challenges and opportunities. Semin. Cell Dev. Biol. 2015;39:20–25. doi: 10.1016/j.semcdb.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Hartman M.L., Czyz M. Pro-apoptotic activity of BH3-only proteins and BH3 mimetics: From theory to potential cancer therapy. Anticancer Agents Med. Chem. 2012;12:966–981. doi: 10.2174/187152012802650084. [DOI] [PubMed] [Google Scholar]
- 9.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 10.Adams J.M., Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartman M., Czyz M. BCL-w: Apoptotic and non-apoptotic role in health and disease. Cell Death Dis. 2020;11:260. doi: 10.1038/s41419-020-2417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klöditz K., Fadeel B. Three cell deaths and a funeral: Macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 2019;5:65. doi: 10.1038/s41420-019-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang D., Kang R., Berghe T.V., Vandenabeele P., Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z., Li G. Mechanisms and therapeutic regulation of pyroptosis in inflammatory diseases and cancer. Int. J. Mol. Sci. 2020;21:1456. doi: 10.3390/ijms21041456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. C.A. Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Network Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman M.L., Sztiller-Sikorska M., Czyz M. Whole-exome sequencing reveals novel genetic variants associated with diverse phenotypes of melanoma cells. Mol. Carcinog. 2019;58:588–602. doi: 10.1002/mc.22953. [DOI] [PubMed] [Google Scholar]
- 19.Hodis E., Watson I.R., Kryukov G.V., Arold S.T., Imielinski M., Theurillat J.P., Nickerson E., Auclair D., Li L., Place C., et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kontogianni G., Piroti G., Maglogiannis I., Chatziioannou A., Papadodima O. Dissecting the mutational landscape of cutaneous melanoma: An omic analysis based on patients from Greece. Cancers (Basel) 2018;10:96. doi: 10.3390/cancers10040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T., Dutton-Regester K., Brown K.M., Hayward N.K. The genomic landscape of cutaneous melanoma. Pigment Cell Melanoma Res. 2016;29:266–283. doi: 10.1111/pcmr.12459. [DOI] [PubMed] [Google Scholar]
- 22.Cartlidge R.A., Thomas G.R., Cagnol S., Jong K.A., Molton S.A., Finch A.J., McMahon M. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–544. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman M.L., Czyz M. Anti-apoptotic proteins on guard of melanoma cell survival. Cancer Lett. 2013;331:24–34. doi: 10.1016/j.canlet.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Mielczarek-Lewandowska A., Hartman M.L., Czyz M. Inhibitors of HSP90 in melanoma. Apoptosis. 2020;25:12–28. doi: 10.1007/s10495-019-01577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies M.A. The role of the PI3K-AKT pathway in melanoma. Cancer J. 2012;18:142–147. doi: 10.1097/PPO.0b013e31824d448c. [DOI] [PubMed] [Google Scholar]
- 26.Hartman M.L., Czyz M. Pro-survival role of MITF in melanoma. J. Investig. Dermatol. 2015;135:352–358. doi: 10.1038/jid.2014.319. [DOI] [PubMed] [Google Scholar]
- 27.Bennett P.E., Bemis L., Norris D.A., Shellman Y.G. miR in melanoma development: miRNAs and acquired hallmarks of cancer in melanoma. Physiol. Genom. 2013;45:1049–1059. doi: 10.1152/physiolgenomics.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman M.L., Talar B., Gajos-Michniewicz A., Czyz M. MCL-1, BCL-XL and MITF are diversely employed in adaptive response of melanoma cells to changes in microenvironment. PLoS ONE. 2015;10:e0128796. doi: 10.1371/journal.pone.0128796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman M.L., Talar B., Noman M.Z., Gajos-Michniewicz A., Chouaib S., Czyz M. Gene expression profiling identifies microphthalmia-associated transcription factor (MITF) and Dickkopf-1 (DKK1) as regulators of microenvironment-driven alterations in melanoma phenotype. PLoS ONE. 2014;9:e95157. doi: 10.1371/journal.pone.0095157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calance D.N., Steixner C., Gross S., Schuler-Thurner B., Knoll G., Ehrenschwender M. Hypertonicity primes malignant melanoma cells for apoptosis. Apoptosis. 2018;23:201–209. doi: 10.1007/s10495-018-1446-y. [DOI] [PubMed] [Google Scholar]
- 31.Rohrbeck L., Gong J.N., Lee E.F., Kueh A.J., Behren A., Tai L., Lessene G., Huang D.C., Fairlie W.D., Strasser A., et al. Hepatocyte growth factor renders BRAF mutant human melanoma cell lines resistant to PLX4032 by downregulating the pro-apoptotic BH3-only proteins PUMA and BIM. Cell Death Differ. 2016;23:2054–2062. doi: 10.1038/cdd.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Søndergaard J.N., Nazarian R., Wang Q., Guo D., Hsueh T., Mok S., Sazegar H., MacConaill L.E., Barretina J.G., Kehoe S.M., et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J. Transl. Med. 2010;8:39. doi: 10.1186/1479-5876-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serasinghe M.N., Missert D.J., Asciolla J.J., Podgrabinska S., Wieder S.Y., Izadmehr S., Belbin G., Skobe M., Chipuk J.E. Anti-apoptotic BCL-2 proteins govern cellular outcome following B-RAF(V600E) inhibition and can be targeted to reduce resistance. Oncogene. 2015;34:857–867. doi: 10.1038/onc.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedini F., De Luca G., Felicetti F., Puglisi R., Boe A., Arasi M.B., Fratini F., Mattia G., Spada M., Caporali S., et al. Joint action of miR-126 and MAPK/PI3K inhibitors against metastatic melanoma. Mol. Oncol. 2019;13:1836–1854. doi: 10.1002/1878-0261.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalesna I., Osrodek M., Hartman M.L., Rozanski M., Sztiller-Sikorska M., Niewinna K., Nejc D., Czyz M. Exogenous growth factors bFGF, EGF and HGF do not influence viability and phenotype of V600EBRAF melanoma cells and their response to vemurafenib and trametinib in vitro. PLoS ONE. 2017;12:e0183498. doi: 10.1371/journal.pone.0183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartman M.L., Rozanski M., Osrodek M., Zalesna I., Czyz M. Vemurafenib and trametinib reduce expression of CTGF and IL-8 in V600EBRAF melanoma cells. Lab. Investig. 2017;97:217–227. doi: 10.1038/labinvest.2016.140. [DOI] [PubMed] [Google Scholar]
- 37.Hartman M.L., Gajos-Michniewicz A., Talaj J., Mielczarek-Lewandowska A., Czyz M. Selective BH3 mimetics potentiate encorafenib activity in inducing apoptosis in BRAFV600E melanoma cells. Cell Death Dis. 2020 doi: 10.1016/j.canlet.2020.11.036. in press. [DOI] [PubMed] [Google Scholar]
- 38.Li Z., Jiang K., Zhu X., Lin G., Song F., Zhao Y., Piao Y., Liu J., Cheng W., Bi X., et al. Encorafenib (LGX818), a potent BRAF inhibitor, induces senescence accompanied by autophagy in BRAFV600E melanoma cells. Cancer Lett. 2016;370:332–344. doi: 10.1016/j.canlet.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Mattia G., Puglisi R., Ascione B., Malorni W., Carè A., Matarrese P. Cell death-based treatments of melanoma: Conventional treatments and new therapeutic strategies. Cell Death Dis. 2018;9:112. doi: 10.1038/s41419-017-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J., Powis de Tenbossche C.G., Cané S., Colau D., van Baren N., Lurquin C., Schmitt-Verhulst A.M., Liljeström P., Uyttenhove C., Van den Eynde B.J. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat. Commun. 2017;8:1404. doi: 10.1038/s41467-017-00784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santana-Magal N., Farhat-Younis L., Gutwillig A., Gleiberman A., Rasoulouniriana D., Tal L., Netanely D., Shamir R., Blau R., Feinmesser M., et al. Melanoma-secreted lysosomes trigger monocyte-derived dendritic cell apoptosis and limit cancer immunotherapy. Cancer Res. 2020 doi: 10.1158/0008-5472.CAN-19-2944. [DOI] [PubMed] [Google Scholar]
- 42.Mulcahy Levy J.M., Thorburn A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27:843–857. doi: 10.1038/s41418-019-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Duve C., Wattiaux R. Functions of lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 44.Ashford T.P., Porter K.R. Cytoplasmic components in hepatic cell lysosomes. J. Cell. Biol. 1962;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soengas M.S. Mitophagy or how to control the Jekyll and Hyde embedded in mitochondrial metabolism: Implications for melanoma progression and drug resistance. Pigment Cell Melanoma Res. 2012;25:721–731. doi: 10.1111/pcmr.12021. [DOI] [PubMed] [Google Scholar]
- 47.Germain K., Kim P.K. Pexophagy: A model for selective autophagy. Int. J. Mol. Sci. 2020;16:578. doi: 10.3390/ijms21020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towers C.G., Thorburn A. Therapeutic targeting of autophagy. EBioMedicine. 2016;14:15–23. doi: 10.1016/j.ebiom.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Condello M., Pellegrini E., Caraglia M., Meschini S. Targeting autophagy to overcome human diseases. Int. J. Mol. Sci. 2019;20:725. doi: 10.3390/ijms20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egan D.F., Chun M.G., Vamos M., Zou H., Rong J., Miller C.J., Lou H.J., Raveendra-Panickar D., Yang C.C., Sheffler D.J., et al. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang F., Hu P., Yang Z., Xue C., Gong J., Sun S., Shi L., Zhang S., Li Z., Yang C., et al. SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways. Oncol. Rep. 2017;37:3449–3458. doi: 10.3892/or.2017.5635. [DOI] [PubMed] [Google Scholar]
- 52.Bae H., Guan J.L. Suppression of autophagy by FIP200 deletion impairs DNA damage repair and increases cell death upon treatments with anticancer agents. Mol. Cancer Res. 2011;9:1232–1241. doi: 10.1158/1541-7786.MCR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallot-Hieke N., Verma N., Schlütermann D., Berleth N., Deitersen J., Böhler P., Stuhldreier F., Wu W., Seggewiß S., Peter C., et al. Systematic analysis of ATG13 domain requirements for autophagy induction. Autophagy. 2018;14:743–763. doi: 10.1080/15548627.2017.1387342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demirsoy S., Martin S., Maes H., Agostinis P. Adapt, recycle, and move on: Proteostasis and trafficking mechanisms in melanoma. Front. Oncol. 2016;6:240. doi: 10.3389/fonc.2016.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulcahy Levy J.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J., Ney P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maes H., Agostinis P. Autophagy and mitophagy interplay in melanoma progression. Mitochondrion. 2014;19:58–68. doi: 10.1016/j.mito.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Islam M.A., Sooro M.A., Zhang P. Autophagic regulation of p62 is critical for cancer therapy. Int. J. Mol. Sci. 2018;19:1405. doi: 10.3390/ijms19051405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lalaoui N., Lindqvist L.M., Sandow J.J., Ekert P.G. The molecular relationships between apoptosis, autophagy and necroptosis. Semin. Cell Dev. Biol. 2015;39:63–69. doi: 10.1016/j.semcdb.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Bialik S., Vasari S.K., Kimchi A. Autophagy-dependent cell death—Where, how and why a cell eats itself to death. J. Cell. Sci. 2018;131:jcs215152. doi: 10.1242/jcs.215152. [DOI] [PubMed] [Google Scholar]
- 62.Kroemer G., Levine B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arakawa S., Tsujioka M., Yoshida T., Tajima-Sakurai H., Nishida Y., Matsuoka Y., Yoshino I., Tsujimoto Y., Shimizu S. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ. 2017;24:1598–1608. doi: 10.1038/cdd.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai M., La Rocca V., Amato R., Freer G., Pistello M. Sphingolipid/ceramide pathways and autophagy in the onset and progression of melanoma: Novel therapeutic targets and opportunities. Int. J. Mol. Sci. 2019;20:3436. doi: 10.3390/ijms20143436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Towers C.G., Wodetzki D., Thorburn A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations. J. Cell Biol. 2020;219:e201909033. doi: 10.1083/jcb.201909033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Leo L., Bodemeyer V., De Zio D. The complex role of autophagy in melanoma evolution: New perspectives from mouse models. Front. Oncol. 2020;9:1506. doi: 10.3389/fonc.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu H., He Z., Simon H.U. Autophagy suppresses melanoma tumorigenesis by inducing senescence. Autophagy. 2014;10:372–373. doi: 10.4161/auto.27163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sample A., Zhao B., Wu C., Qian S., Shi X., Aplin A., He Y.Y. The autophagy receptor adaptor p62 is up-regulated by UVA radiation in melanocytes and in melanoma cells. Photochem. Photobiol. 2018;94:432–437. doi: 10.1111/php.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanchetta L.M., Garcia A., Lyng F., Walsh J., Murphy J.E. Mitophagy and mitochondrial morphology in human melanoma-derived cells post exposure to simulated sunlight. Int. J. Radiat. Biol. 2011;87:506–517. doi: 10.3109/09553002.2011.556175. [DOI] [PubMed] [Google Scholar]
- 70.Konstantakou E.G., Velentzas A.D., Anagnostopoulos A.K., Giannopoulou A.F., Anastasiadou E., Papassideri I.S., Voutsinas G., Tsangaris G.T., Stravopodis D.J. Unraveling the human protein atlas of metastatic melanoma in the course of ultraviolet radiation-derived photo-therapy. J. Proteom. 2018;188:119–138. doi: 10.1016/j.jprot.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 71.Xie X., Koh J.Y., Price S., White E., Mehnert J.M. Atg7 overcomes senescence and promotes growth of BrafV600E-driven melanoma. Cancer Discov. 2015;5:410–423. doi: 10.1158/2159-8290.CD-14-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maddodi N., Huang W., Havighurst T., Kim K., Longley B.J., Setaluri V. Induction of autophagy and inhibition of melanoma growth in vitro and in vivo by hyperactivation of oncogenic BRAF. J. Investig. Dermatol. 2010;130:1657–1667. doi: 10.1038/jid.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferretta A., Maida I., Guida S., Azzariti A., Porcelli L., Tommasi S., Zanna P., Cocco T., Guida M., Guida G. New insight into the role of metabolic reprogramming in melanoma cells harboring BRAF mutations. Biochim. Biophys. Acta. 2016;1863:2710–2718. doi: 10.1016/j.bbamcr.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Corazzari M., Rapino F., Ciccosanti F., Giglio P., Antonioli M., Conti B., Fimia G.M., Lovat P.E., Piacentini M. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ. 2015;22:946–958. doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y.L., Lai F., Wilmott J.S., Yan X.G., Liu X.Y., Luan Q., Guo S.T., Jiang C.C., Tseng H.Y., Scolyer R.A., et al. Noxa upregulation by oncogenic activation of MEK/ERK through CREB promotes autophagy in human melanoma cells. Oncotarget. 2014;30:11237–11251. doi: 10.18632/oncotarget.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y., Wang W., Min I., Wyrwas B., Moore M., Zarnegar R., Fahey T.J. BRAF V600E-dependent role of autophagy in uveal melanoma. J. Cancer Res. Clin. Oncol. 2017;143:447–455. doi: 10.1007/s00432-016-2317-y. [DOI] [PubMed] [Google Scholar]
- 77.Marino M.L., Pellegrini P., Di Lernia G., Djavaheri-Mergny M., Brnjic S., Zhang X., Hägg M., Linder S., Fais S., Codogno P., et al. Autophagy is a protective mechanism for human melanoma cells under acidic stress. J. Biol. Chem. 2012;287:30664–30676. doi: 10.1074/jbc.M112.339127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsuo T., Sadzuka Y. Extracellular acidification by lactic acid suppresses glucose deprivation-induced cell death and autophagy in B16 melanoma cells. Biochem. Biophys. Res. Commun. 2018;496:1357–1361. doi: 10.1016/j.bbrc.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 79.Matsuo T., Daishaku S., Sadzuka Y. Lactic acid promotes cell survival by blocking autophagy of B16F10 mouse melanoma cells under glucose deprivation and hypoxic conditions. Biol. Pharm. Bull. 2019;42:837–839. doi: 10.1248/bpb.b18-00919. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Wang Y., Wu J., Wang W., Zhang Y. Oxygen partial pressure plays a crucial role in B16 melanoma cell survival by regulating autophagy and mitochondrial functions. Biochem. Biophys. Res. Commun. 2019;510:643–648. doi: 10.1016/j.bbrc.2019.01.135. [DOI] [PubMed] [Google Scholar]
- 81.Kraya A.A., Piao S., Xu X., Zhang G., Herlyn M., Gimotty P., Levine B., Amaravadi R.K., Speicher D.W. Identification of secreted proteins that reflect autophagy dynamics within tumor cells. Autophagy. 2015;11:60–74. doi: 10.4161/15548627.2014.984273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bustos S.O., da Silva Pereira G.J., de Freitas Saito R., Gil C.D., Zanatta D.B., Smaili S.S., Chammas R. Galectin-3 sensitized melanoma cell lines to vemurafenib (PLX4032) induced cell death through prevention of autophagy. Oncotarget. 2018;9:14567–14579. doi: 10.18632/oncotarget.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun T., Jiao L., Wang Y., Yu Y., Ming L. SIRT1 induces epithelial-mesenchymal transition by promoting autophagic degradation of E-cadherin in melanoma cells. Cell Death Dis. 2018;9:136. doi: 10.1038/s41419-017-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garcia-Peterson L.M., Ndiaye M.A., Singh C.K., Chhabra G., Huang W., Ahmad N. SIRT6 histone deacetylase functions as a potential oncogene in human melanoma. Genes Cancer. 2017;8:701–712. doi: 10.18632/genesandcancer.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Guo W., Ma J., Dai W., Liu L., Guo S., Chen J., Wang H., Yang Y., Yi X., et al. Aberrant SIRT6 expression contributes to melanoma growth: Role of the autophagy paradox and IGF-AKT signaling. Autophagy. 2018;14:518–533. doi: 10.1080/15548627.2017.1384886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang Z., Yu F., Li M. Upregulation of BCL2 19 kD Protein-Interacting Protein 3 (BNIP3) is predictive of unfavorable prognosis in uveal melanoma. Med. Sci. Monit. 2018;24:4711–4717. doi: 10.12659/MSM.907679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li P., He J., Yang Z., Ge S., Zhang H., Zhong Q., Fan X. ZNNT1 long noncoding RNA induces autophagy to inhibit tumorigenesis of uveal melanoma by regulating key autophagy gene expression. Autophagy. 2019:1–14. doi: 10.1080/15548627.2019.1659614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maes H., Martin S., Verfaillie T., Agostinis P. Dynamic interplay between autophagic flux and Akt during melanoma progression in vitro. Exp. Dermatol. 2014;23:101–106. doi: 10.1111/exd.12298. [DOI] [PubMed] [Google Scholar]
- 89.García-Fernández M., Karras P., Checinska A., Cañón E., Calvo G.T., Gómez-López G., Cifdaloz M., Colmenar A., Espinosa-Hevia L., Olmeda D., et al. Metastatic risk and resistance to BRAF inhibitors in melanoma defined by selective allelic loss of ATG5. Autophagy. 2016;12:1776–1790. doi: 10.1080/15548627.2016.1199301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo W., Wang H., Yang Y., Guo S., Zhang W., Liu Y., Yi X., Ma J., Zhao T., Liu L., et al. Down-regulated miR-23a contributes to the metastasis of cutaneous melanoma by promoting autophagy. Theranostics. 2017;7:2231–2249. doi: 10.7150/thno.18835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karras P., Riveiro-Falkenbach E., Cañón E., Tejedo C., Calvo T.G., Martínez-Herranz R., Alonso-Curbelo D., Cifdaloz M., Perez-Guijarro E., Gómez-López G., et al. p62/SQSTM1 fuels melanoma progression by opposing mRNA decay of a selective set of pro-metastatic factors. Cancer Cell. 2019;35:46–63.e10. doi: 10.1016/j.ccell.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 92.D’Arcangelo D., Giampietri C., Muscio M., Scatozza F., Facchiano F., Facchiano A. WIPI1, BAG1, and PEX3 autophagy-related genes are relevant melanoma markers. Oxid. Med. Cell. Longev. 2018;2018:1471682. doi: 10.1155/2018/1471682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang D.Y., Ellis R.A., Lovat P.E. Prognostic impact of autophagy biomarkers for cutaneous melanoma. Front. Oncol. 2016;6:236. doi: 10.3389/fonc.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scatozza F., D’Arcangelo D., Giampietri C., Facchiano F., Facchiano A. Melanogenesis and autophagy in melanoma. Melanoma Res. 2019 doi: 10.1097/CMR.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 95.Wan Q., Jin L., Su Y., Liu Y., Li C., Wang Z. Development and validation of autophagy-related-gene biomarker and nomogram for predicting the survival of cutaneous melanoma. IUBMB Life. 2020 doi: 10.1002/iub.2258. [DOI] [PubMed] [Google Scholar]
- 96.White K.A., Luo L., Thompson T.A., Torres S., Hu C.A., Thomas N.E., Lilyquist J., Anton-Culver H., Gruber S.B., From L., et al. Variants in autophagy-related genes and clinical characteristics in melanoma: A population-based study. Cancer Med. 2016;5:3336–3345. doi: 10.1002/cam4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J., Yang R., Yang H., Chen S., Wang L., Li M., Yang S., Feng Z., Bi J. NCAM regulates the proliferation, apoptosis, autophagy, EMT, and migration of human melanoma cells via the Src/Akt/mTOR/cofilin signaling pathway. J. Cell. Biochem. 2020;121:1192–1204. doi: 10.1002/jcb.29353. [DOI] [PubMed] [Google Scholar]
- 98.Yang Y., Ma S., Ye Z., Zhou X. MCM7 silencing promotes cutaneous melanoma cell autophagy and apoptosis by inactivating the AKT1/mTOR signaling pathway. J. Cell. Biochem. 2020;121:1283–1294. doi: 10.1002/jcb.29361. [DOI] [PubMed] [Google Scholar]
- 99.Brown K., Yang P., Salvador D., Kulikauskas R., Ruohola-Baker H., Robitaille A.M., Chien A.J., Moon R.T., Sherwood V. WNT/β-catenin signaling regulates mitochondrial activity to alter the oncogenic potential of melanoma in a PTEN-dependent manner. Oncogene. 2017;36:3119–3136. doi: 10.1038/onc.2016.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ndoye A., Budina-Kolomets A., Kugel C.H., Webster M.R., Kaur A., Behera R., Rebecca V.W., Li L., Brafford P.A., Liu Q., et al. ATG5 mediates a positive feedback loop between Wnt signaling and autophagy in melanoma. Cancer Res. 2017;77:5873–5885. doi: 10.1158/0008-5472.CAN-17-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Starobinets H., Ye J., Broz M., Barry K., Goldsmith J., Marsh T., Rostker F., Krummel M., Debnath J. Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment. J. Clin. Investig. 2016;126:4417–4429. doi: 10.1172/JCI85705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mgrditchian T., Arakelian T., Paggetti J., Noman M.Z., Viry E., Moussay E., Van Moer K., Kreis S., Guerin C., Buart S., et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl. Acad. Sci. USA. 2017;114:E9271–E9279. doi: 10.1073/pnas.1703921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noman M.Z., Paggetti J., Moussay E., Berchem G., Janji B. Driving Natural Killer cells toward the melanoma tumor battlefield: Autophagy as a valuable therapeutic target. Oncoimmunology. 2018;7:e1452583. doi: 10.1080/2162402X.2018.1452583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romano E., Rufo N., Korf H., Mathieu C., Garg A.D., Agostinis P. BNIP3 modulates the interface between B16-F10 melanoma cells and immune cells. Oncotarget. 2018;9:17631–17644. doi: 10.18632/oncotarget.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ndoye A., Weeraratna A.T. Autophagy-an emerging target for melanoma therapy. F1000Resesrch. 2016;5:1000. doi: 10.12688/f1000research.8347.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meng X.X., Yao M., Zhang X.D., Xu H.X., Dong Q. ER stress-induced autophagy in melanoma. Clin. Exp. Pharmacol. Physiol. 2015;42:811–816. doi: 10.1111/1440-1681.12436. [DOI] [PubMed] [Google Scholar]
- 107.Antunes F., Pereira G.J.S., Saito R.F., Buri M.V., Gagliardi M., Bincoletto C., Chammas R., Fimia G.M., Piacentini M., Corazzari M., et al. Effective synergy of sorafenib and nutrient shortage in inducing melanoma cell death through energy stress. Cells. 2020;9:640. doi: 10.3390/cells9030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu X., Wu J., Qin H., Xu J. The role of autophagy in the resistance to BRAF inhibition in BRAF-mutated melanoma. Target. Oncol. 2018;13:437–446. doi: 10.1007/s11523-018-0565-2. [DOI] [PubMed] [Google Scholar]
- 109.Ma X.H., Piao S., Wang D., McAfee Q.W., Nathanson K.L., Lum J.J., Li L.Z., Amaravadi R.K. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin. Cancer Res. 2011;17:3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buart S., Terry S., Noman M.Z., Lanoy E., Boutros C., Fogel P., Dessen P., Meurice G., Gaston-Mathé Y., Vielh P., et al. Transcriptional response to hypoxic stress in melanoma and prognostic potential of GBE1 and BNIP3. Oncotarget. 2017;8:108786–108801. doi: 10.18632/oncotarget.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma X.H., Piao S.F., Dey S., McAfee Q., Karakousis G., Villanueva J., Hart L.S., Levi S., Hu J., Zhang G., et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Investig. 2014;124:1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin S., Dudek-Perić A.M., Maes H., Garg A.D., Gabrysiak M., Demirsoy S., Swinnen J.V., Agostinis P. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in vemurafenib-resistant melanoma cells. Biochem. Pharmacol. 2015;93:290–304. doi: 10.1016/j.bcp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Luo M., Wu L., Zhang K., Wang H., Wu S., O’Connell D., Gao T., Zhong H., Yang Y. miR-216b enhances the efficacy of vemurafenib by targeting Beclin-1, UVRAG and ATG5 in melanoma. Cell. Signal. 2018;42:30–43. doi: 10.1016/j.cellsig.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 114.Yu Y., Xiang N., Lin M., Huang J.W., Zhang J., Cheng B., Ji C. miR- 26a sensitizes melanoma cells To dabrafenib via targeting HMGB1-dependent autophagy pathways. Drug Des. Devel. Ther. 2019;13:3717–3726. doi: 10.2147/DDDT.S225671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ojha R., Leli N.M., Onorati A., Piao S., Verginadis I.I., Tameire F., Rebecca V.W., Chude C.I., Murugan S., Fennelly C., et al. ER Translocation of the MAPK pathway drives therapy resistance in BRAF-mutant melanoma. Cancer Discov. 2019;9:396–415. doi: 10.1158/2159-8290.CD-18-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin S., Dudek-Peric A.M., Garg A.D., Roose H., Demirsoy S., Van Eygen S., Mertens F., Vangheluwe P., Vankelecom H., Agostinis P. An autophagy-driven pathway of ATP secretion supports the aggressive phenotype of BRAFV600E inhibitor-resistant metastatic melanoma cells. Autophagy. 2017;13:1512–1527. doi: 10.1080/15548627.2017.1332550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Y.Y., Wu C., Shah S.S., Chen S.M., Wangpaichitr M., Kuo M.T., Feun L.G., Han X., Suarez M., Prince J., et al. Degradation of AMPK-α1 sensitizes BRAF inhibitor-resistant melanoma cells to arginine deprivation. Mol. Oncol. 2017;11:1806–1825. doi: 10.1002/1878-0261.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guerriero L., Palmieri G., De Marco M., Cossu A., Remondelli P., Capunzo M., Turco M.C., Rosati A. The anti-apoptotic BAG3 protein is involved in BRAF inhibitor resistance in melanoma cells. Oncotarget. 2017;8:80393–80404. doi: 10.18632/oncotarget.18902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li S., Song Y., Quach C., Guo H., Jang G.B., Maazi H., Zhao S., Sands N.A., Liu Q., In G.K., et al. Transcriptional regulation of autophagy-lysosomal function in BRAF-driven melanoma progression and chemoresistance. Nat. Commun. 2019;10:1693. doi: 10.1038/s41467-019-09634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du J., Ren W., Yao F., Wang H., Zhang K., Luo M., Shang Y., O’Connell D., Bei Z., Wang H., et al. YY1 cooperates with TFEB to regulate autophagy and lysosomal biogenesis in melanoma. Mol. Carcinog. 2019;58:2149–2160. doi: 10.1002/mc.23105. [DOI] [PubMed] [Google Scholar]
- 121.Möller K., Sigurbjornsdottir S., Arnthorsson A.O., Pogenberg V., Dilshat R., Fock V., Brynjolfsdottir S.H., Bindesboll C., Bessadottir M., Ogmundsdottir H.M., et al. MITF has a central role in regulating starvation-induced autophagy in melanoma. Sci. Rep. 2019;9:1055. doi: 10.1038/s41598-018-37522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Müller J., Krijgsman O., Tsoi J., Robert L., Hugo W., Song C., Kong X., Possik P.A., Cornelissen-Steijger P.D., Geukes Foppen M.H., et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat. Commun. 2014;5:5712. doi: 10.1038/ncomms6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Czyz M., Sztiller-Sikorska M., Gajos-Michniewicz A., Osrodek M., Hartman M.L. Plasticity of drug-naïve and vemurafenib- or trametinib-resistant melanoma cells in execution of differentiation/pigmentation program. J. Oncol. 2019;2019:1697913. doi: 10.1155/2019/1697913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hartman M.L., Sztiller-Sikorska M., Gajos-Michniewicz A., Czyz M. Dissecting mechanisms of melanoma resistance to BRAF and MEK inhibitors revealed genetic and non-genetic patient- and drug-specific alterations and remarkable phenotypic plasticity. Cells. 2020;9:142. doi: 10.3390/cells9010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 126.Gong Y., Fan Z., Luo G., Yang C., Huang Q., Fan K., Cheng H., Jin K., Ni Q., Yu X., et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer. 2019;23:100. doi: 10.1186/s12943-019-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 128.Florean C., Song S., Dicato M., Diederich M. Redox biology of regulated cell death in cancer: A focus on necroptosis and ferroptosis. Free Radic. Biol. Med. 2019;134:177–189. doi: 10.1016/j.freeradbiomed.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 129.Degterev A., Maki J.L., Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20:366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Degterev A., Hitomi J., Germscheid M., Ch’en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G., et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khoury M.K., Gupta K., Franco S.R., Liu B. Necroptosis in the pathophysiology of disease. Am. J. Pathol. 2020;190:272–285. doi: 10.1016/j.ajpath.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seifert L., Miller G. Molecular pathways: The ncrosome-a target for cancer therapy. Clin. Cancer Res. 2017;23:1132–1136. doi: 10.1158/1078-0432.CCR-16-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ofengeim D., Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 134.Jacobsen A.V., Lowes K.N., Tanzer M.C., Lucet I.S., Hildebrand J.M., Petrie E.J., van Delft M.F., Liu Z., Conos S.A., Zhang J.G., et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7:e2051. doi: 10.1038/cddis.2015.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao X.M., Chen Z., Zhao J.B., Zhang P.P., Pu Y.F., Jiang S.H., Hou J.J., Cui Y.M., Jia X.L., Zhang S.Q. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis. 2016;7:e2089. doi: 10.1038/cddis.2015.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gong Y.N., Guy C., Olauson H., Becker J.U., Yang M., Fitzgerald P., Linkermann A., Green D.R. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169:286–300.e16. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yoon S., Kovalenko A., Bogdanov K., Wallach D. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity. 2017;47:51–65.e7. doi: 10.1016/j.immuni.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 138.Kaiser W.J., Sridharan H., Huang C., Mandal P., Upton J.W., Gough P.J., Sehon C.A., Marquis R.W., Bertin J., Mocarski E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ke H., Augustine C.K., Gandham V.D., Jin J.Y., Tyler D.S., Akiyama S.K., Hall R.P., Zhang J.Y. CYLD inhibits melanoma growth and progression through suppression of the JNK/AP-1 and β1-integrin signaling pathways. J. Investig. Dermatol. 2013;133:221–229. doi: 10.1038/jid.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geserick P., Wang J., Schilling R., Horn S., Harris P.A., Bertin J., Gough P.J., Feoktistova M., Leverkus M. Absence of RIPK3 predicts necroptosis resistance in malignant melanoma. Cell Death Dis. 2015;10:e1884. doi: 10.1038/cddis.2015.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rossi A., Pakhomova O.N., Pakhomov A.G., Weygandt S., Bulysheva A.A., Murray L.E., Mollica P.A., Muratori C. Mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors. Sci. Rep. 2019;9:431. doi: 10.1038/s41598-018-36527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ivanov V.N., Hei T.K. Regulation of viability, differentiation and death of human melanoma cells carrying neural stem cell biomarkers: A possibility for neural trans-differentiation. Apoptosis. 2015;20:996–1015. doi: 10.1007/s10495-015-1131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luan Q., Jin L., Jiang C.C., Tay K.H., Lai F., Liu X.Y., Liu Y.L., Guo S.T., Li C.Y., Yan X.G., et al. RIPK1 regulates survival of human melanoma cells upon endoplasmic reticulum stress through autophagy. Autophagy. 2015;11:975–994. doi: 10.1080/15548627.2015.1049800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Podder B., Guttà C., Rožanc J., Gerlach E., Feoktistova M., Panayotova-Dimitrova D., Alexopoulos L.G., Leverkus M., Rehm M. TAK1 suppresses RIPK1-dependent cell death and is associated with disease progression in melanoma. Cell Death Differ. 2019;26:2520–2534. doi: 10.1038/s41418-019-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel S., Webster J.D., Varfolomeev E., Kwon Y.C., Cheng J.H., Zhang J., Dugger D.L., Wickliffe K.E., Maltzman A., Sujatha-Bhaskar S., et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. 2020;27:161–175. doi: 10.1038/s41418-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hou J., Ju J., Zhang Z., Zhao C., Li Z., Zheng J., Sheng T., Zhang H., Hu L., Yu X., et al. Discovery of potent necroptosis inhibitors targeting RIPK1 kinase activity for the treatment of inflammatory disorder and cancer metastasis. Cell Death Dis. 2019;10:493. doi: 10.1038/s41419-019-1735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J.X., Feng J.M., Wang Y., Li X.H., Chen X.X., Su Y., Shen Y.Y., Chen Y., Xiong B., Yang C.H., et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 2014;5:e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cruz S.A., Qin Z., Stewart A.F.R., Chen H.H. Dabrafenib, an inhibitor of RIP3 kinase-dependent necroptosis, reduces ischemic brain injury. Neural. Regen. Res. 2018;13:252–256. doi: 10.4103/1673-5374.226394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tahseen A.I., Patel N.B. Successful dabrafenib transition after vemurafenib-induced toxic epidermal necrolysis in a patient with metastatic melanoma. JAAD Case Rep. 2018;4:930–933. doi: 10.1016/j.jdcr.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Werthmöller N., Frey B., Wunderlich R., Fietkau R., Gaipl U.S. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 2015;14:e1761. doi: 10.1038/cddis.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Y., Zhan X., Xiong J., Peng S., Huang W., Joshi R., Cai Y., Liu Y., Li R., Yuan K., et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018;8:8720. doi: 10.1038/s41598-018-26978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Basit F., van Oppen L.M., Schöckel L., Bossenbroek H.M., van Emst-de Vries S.E., Hermeling J.C., Grefte S., Kopitz C., Heroult M., Hgm Willems P., et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Medrano R.F.V., Hunger A., Catani J.P.P., Strauss B.E. Uncovering the immunotherapeutic cycle initiated by p19Arf and interferon-β gene transfer to cancer cells: An inducer of immunogenic cell death. Oncoimmunology. 2017;6:e1329072. doi: 10.1080/2162402X.2017.1329072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Van Hoecke L., Van Lint S., Roose K., Van Parys A., Vandenabeele P., Grooten J., Tavernier J., De Koker S., Saelens X. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat. Commun. 2018;9:3417. doi: 10.1038/s41467-018-05979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Hoecke L., Raes L., Stremersch S., Brans T., Fraire J.C., Roelandt R., Declercq W., Vandenabeele P., Raemdonck K., Braeckmans K., et al. Delivery of mixed-lineage kinase domain-like protein by vapor nanobubble photoporation induces necroptotic-like cell death in tumor cells. Int. J. Mol. Sci. 2019;20:4254. doi: 10.3390/ijms20174254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: Process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fearnhead H.O., Vandenabeele P., Vanden Berghe T. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017;24:1991–1998. doi: 10.1038/cdd.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hassannia B., Vandenabeele P., Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 160.Li J., Cao F., Yin H.L., Huang Z.J., Lin Z.T., Mao N., Sun B., Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chiang S.K., Chen S.E., Chang L.C. A dual role of heme oxygenase-1 in cancer cells. Int. J. Mol. Sci. 2018;20:39. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dai C., Chen X., Li J., Comish P., Kang R., Tang D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020 doi: 10.1038/s41417-020-0170-2. [DOI] [PubMed] [Google Scholar]
- 164.Friedmann Angeli J.P., Krysko D.V., Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer. 2019;19:405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 165.Neitemeier S., Jelinek A., Laino V., Hoffmann L., Eisenbach I., Eying R., Ganjam G.K., Dolga A.M., Oppermann S., Culmsee C. BID links ferroptosis to mitochondrial cell death pathways. Redox Biol. 2017;12:558–570. doi: 10.1016/j.redox.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Grocin A.G., Xavier da Silva T.N., Panzilius E., Scheel C.H., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 168.Hou W., Xie Y., Song X., Sun X., Lotze M.T., Zeh H.J., Kang R., Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou B., Liu J., Kang R., Klionsky D.J., Kroemer G., Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 170.Park E., Chung S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gao M., Monian P., Pan Q., Zhang W., Xiang J., Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bebber C.M., Müller F., Prieto Clemente L., Weber J., von Karstedt S. Ferroptosis in cancer cell biology. Cancers (Basel) 2020;12:164. doi: 10.3390/cancers12010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seiler A., Schneider M., Förster H., Roth S., Wirth E.K., Culmsee C., Plesnila N., Kremmer E., Rådmark O., Wurst W., et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 175.Shah R., Shchepinov M.S., Pratt D.A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Conrad M., Pratt D.A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019;15:1137–1147. doi: 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 177.Kajarabille N., Latunde-Dada G.O. Programmed cell-death by ferroptosis: Antioxidants as mitigators. Int. J. Mol. Sci. 2019;20:4968. doi: 10.3390/ijms20194968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hennig P., Garstkiewicz M., Grossi S., Di Filippo M., French L.E., Beer H.D. The crosstalk between Nrf2 and inflammasomes. Int. J. Mol. Sci. 2018;19:562. doi: 10.3390/ijms19020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Toyokuni S. Iron and thiols as two major players in carcinogenesis: Friends or foes? Front. Pharmacol. 2014;5:200. doi: 10.3389/fphar.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Z., Jin D., Ma D., Ji C., Wu W., Xu L., Zhang L. Ferroptosis suppressed the growth of melanoma that may be related to DNA damage. Dermatol. Ther. 2019;32:e12921. doi: 10.1111/dth.12921. [DOI] [PubMed] [Google Scholar]
- 183.Weïwer M., Bittker J.A., Lewis T.A., Shimada K., Yang W.S., MacPherson L., Dandapani S., Palmer M., Stockwell B.R., Schreiber S.L., et al. Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg. Med. Chem. Lett. 2012;22:1822–1826. doi: 10.1016/j.bmcl.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Haq R., Shoag J., Andreu-Perez P., Yokoyama S., Edelman H., Rowe G.C., Frederick D.T., Hurley A.D., Nellore A., Kung A.L., et al. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schöckel L., Glasauer A., Basit F., Bitschar K., Truong H., Erdmann G., Algire C., Hägebarth A., Willems P.H., Kopitz C., et al. Targeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growth. Cancer Metab. 2015;3:11. doi: 10.1186/s40170-015-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gentric G., Kieffer Y., Mieulet V., Goundiam O., Bonneau C., Nemati F., Hurbain I., Raposo G., Popova T., Stern M.H., et al. PML-regulated mitochondrial metabolism enhances chemosensitivity in human ovarian cancers. Cell Metab. 2019;29:156–173.e10. doi: 10.1016/j.cmet.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gagliardi M., Cotella D., Santoro C., Corà D., Barlev N.A., Piacentini M., Corazzari M. Aldo-keto reductases protect metastatic melanoma from ER stress-independent ferroptosis. Cell Death Dis. 2019;10:902. doi: 10.1038/s41419-019-2143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Osrodek M., Hartman M.L., Czyz M. Physiologically relevant oxygen concentration (6% O2) as an important component of the microenvironment impacting melanoma phenotype and melanoma response to targeted therapeutics in vitro. Int. J. Mol. Sci. 2019;20:4203. doi: 10.3390/ijms20174203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Talebi A., Dehairs J., Rambow F., Rogiers A., Nittner D., Derua R., Vanderhoydonc F., Duarte J.A.G., Bosisio F., Van den Eynde K., et al. Sustained SREBP-1-dependent lipogenesis as a key mediator of resistance to BRAF-targeted therapy. Nat. Commun. 2018;27:2500. doi: 10.1038/s41467-018-04664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Khamari R., Trinh A., Gabert P.E., Corazao-Rozas P., Riveros-Cruz S., Balayssac S., Malet-Martino M., Dekiouk S., Joncquel Chevalier Curt M., Patrice Maboudou G.G., et al. Glucose metabolism and NRF2 coordinate the antioxidant response in melanoma resistant to MAPK inhibitors. Cell Death Dis. 2018;9:325. doi: 10.1038/s41419-018-0340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsoi J., Robert L., Paraiso K., Galvan C., Sheu K.M., Lay J., Wong D.J.L., Atefi M., Shirazi R., Wang X., et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell. 2018;33:890–904.e5. doi: 10.1016/j.ccell.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu T., Ding W., Ji X., Ao X., Liu Y., Yu W., Wang J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019;23:4900–4912. doi: 10.1111/jcmm.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Viswanathan V.S., Ryan M.J., Dhruv H.D., Gill S., Eichhoff O.M., Seashore-Ludlow B., Kaffenberger S.D., Eaton J.K., Shimada K., Aguirre A.J., et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hangauer M.J., Viswanathan V.S., Ryan M.J., Bole D., Eaton J.K., Matov A., Galeas J., Dhruv H.D., Berens M.E., Schreiber S.L., et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hanniford D., Ulloa-Morales A., Karz A., Berzoti-Coelho M.G., Moubarak R.S., Sánchez-Sendra B., Kloetgen A., Davalos V., Imig J., Wu P., et al. Epigenetic silencing of CDR1as drives IGF2BP3-mediated melanoma invasion and metastasis. Cancer Cell. 2020;37:55–70.e15. doi: 10.1016/j.ccell.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang W., Green M., Choi J., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kaiser A.M., Attardi L.D. Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ. 2018;25:93–103. doi: 10.1038/cdd.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang S.J., Ou Y., Jiang L., Gu W. Ferroptosis: A missing puzzle piece in the p53 blueprint? Mol. Cell. Oncol. 2015;3:e1046581. doi: 10.1080/23723556.2015.1046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiang L., Kon N., Li T., Wang S.J., Su T., Hibshoosh H., Baer R., Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou X., Hao Q., Liao P., Luo S., Zhang M., Hu G., Liu H., Zhang Y., Cao B., Baddoo M., et al. Nerve growth factor receptor negates the tumor suppressor p53 as a feedback regulator. Elife. 2016;5:e15099. doi: 10.7554/eLife.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Miura S., Shibazaki M., Kasai S., Yasuhira S., Watanabe A., Inoue T., Kageshita Y., Tsunoda K., Takahashi K., Akasaka T., et al. A somatic mutation of the KEAP1 gene in malignant melanoma is involved in aberrant NRF2 activation and an increase in intrinsic drug resistance. J. Investig. Dermatol. 2014;134:553–556. doi: 10.1038/jid.2013.343. [DOI] [PubMed] [Google Scholar]
- 205.Zhu B., Tang L., Chen S., Yin C., Peng S., Li X., Liu T., Liu W., Han C., Stawski L., et al. Targeting the upstream transcriptional activator of PD-L1 as an alternative strategy in melanoma therapy. Oncogene. 2018;37:4941–4954. doi: 10.1038/s41388-018-0314-0. [DOI] [PubMed] [Google Scholar]
- 206.Zhu H., Jia Z., Trush M.A., Li Y.R. Nrf2 deficiency promotes melanoma growth and lung metastasis. React. Oxyg. Species (Apex) 2016;2:308–314. doi: 10.20455/ros.2016.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hämäläinen M., Teppo H.R., Skarp S., Haapasaari K.M., Porvari K., Vuopala K., Kietzmann T., Karihtala P. NRF1 and NRF2 mRNA and protein expression decrease early during melanoma carcinogenesis: An insight into survival and microRNAs. Oxid. Med. Cell. Longev. 2019;2019:2647068. doi: 10.1155/2019/2647068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Luo M., Wu L., Zhang K., Wang H., Zhang T., Gutierrez L., O’Connell D., Zhang P., Li Y., Gao T., et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25:1457–1472. doi: 10.1038/s41418-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang K., Wu L., Zhang P., Luo M., Du J., Gao T., O’Connell D., Wang G., Wang H., Yang Y. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol. Carcinog. 2018;57:1566–1576. doi: 10.1002/mc.22878. [DOI] [PubMed] [Google Scholar]
- 210.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 211.Wang Y.Y., Liu X.L., Zhao R. Induction of pyroptosis and its implications in cancer management. Front. Oncol. 2019;9:971. doi: 10.3389/fonc.2019.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.C., Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 213.Xue Y., Enosi Tuipulotu D., Tan W.H., Kay C., Man S.M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40:1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 214.Wang J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem. Biol. Interact. 2020:109052. doi: 10.1016/j.cbi.2020.109052. [DOI] [PubMed] [Google Scholar]
- 215.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 216.Kayagaki N., Stowe I.B., Lee B.L., O’Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 217.Rogers C., Fernandes-Alnemri T., Mayes L., Alnemri D., Cingolani G., Alnemri E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang Y., Gao W., Shi X., Ding J., Liu W., He H., Wang K., Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;6:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 219.Yu P., Wang H.Y., Tian M., Li A.X., Chen X.S., Wang X.L., Zhang Y., Cheng Y. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacol. Sin. 2019;40:1237–1244. doi: 10.1038/s41401-019-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lage H., Helmbach H., Grottke C., Dietel M., Schadendorf D. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells. FEBS Lett. 2001;6:54–59. doi: 10.1016/S0014-5793(01)02304-3. [DOI] [PubMed] [Google Scholar]
- 221.Colunga A.G., Laing J.M., Aurelian L. The HSV-2 mutant DeltaPK induces melanoma oncolysis through nonredundant death programs and associated with autophagy and pyroptosis proteins. Gene Ther. 2010;17:315–327. doi: 10.1038/gt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Erkes D.A., Cai W., Sanchez I.M., Purwin T.J., Rogers C., Field C.O., Berger A.C., Hartsough E.J., Rodeck U., Alnemri E.S., et al. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10:254–269. doi: 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhou B., Zhang J.Y., Liu X.S., Chen H.Z., Ai Y.L., Cheng K., Sun R.Y., Zhou D., Han J., Wu Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171–1185. doi: 10.1038/s41422-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Emran A.A., Tseng H.Y., Coleman M.C., Tiffen J., Cook S., McGuire H.M., Gallagher S., Feng C., Hersey P. Do innate killing mechanisms activated by inflammasomes have a role in treating melanoma? Pigment Cell Melanoma Res. 2020 doi: 10.1111/pcmr.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Okamoto M., Liu W., Luo Y., Tanaka A., Cai X., Norris D.A., Dinarello C.A., Fujita M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J. Biol. Chem. 2010;285:6477–6488. doi: 10.1074/jbc.M109.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhai Z., Liu W., Kaur M., Luo Y., Domenico J., Samson J.M., Shellman Y.G., Norris D.A., Dinarello C.A., Spritz R.A., et al. NLRP1 promotes tumor growth by enhancing inflammasome activation and suppressing apoptosis in metastatic melanoma. Oncogene. 2017;36:3820–3830. doi: 10.1038/onc.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu W., Luo Y., Dunn J.H., Norris D.A., Dinarello C.A., Fujita M. Dual role of apoptosis-associated speck-like protein containing a CARD (ASC) in tumorigenesis of human melanoma. J. Investig. Dermatol. 2013;133:518–527. doi: 10.1038/jid.2012.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Watabe K., Ito A., Asada H., Endo Y., Kobayashi T., Nakamoto K., Itami S., Takao S., Shinomura Y., Aikou T., et al. Structure, expression and chromosome mapping of MLZE, a novel gene which is preferentially expressed in metastatic melanoma cells. Jpn. J. Cancer Res. 2001;92:140–151. doi: 10.1111/j.1349-7006.2001.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Virág L., Robaszkiewicz A., Rodriguez-Vargas J.M., Oliver F.J. Poly(ADP-ribose) signaling in cell death. Mol. Aspects Med. 2013;34:1153–1167. doi: 10.1016/j.mam.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 230.Yu S.W., Wang H., Poitras M.F., Coombs C., Bowers W.J., Federoff H.J., Poirier G.G., Dawson T.M., Dawson V.L. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 231.Fatokun A.A., Dawson V.L., Dawson T.M. Parthanatos: Mitochondrial-linked mechanisms and therapeutic opportunities. Br. J. Pharmacol. 2014;171:2000–2016. doi: 10.1111/bph.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.David K.K., Andrabi S.A., Dawson T.M., Dawson V.L. Parthanatos, a messenger of death. Front. Biosci. (Landmark Ed.) 2009;14:1116–1128. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xu Z., Zhang J., David K.K., Yang Z.J., Li X., Dawson T.M., Dawson V.L., Koehler R.C. Endonuclease G does not play an obligatory role in poly(ADP-ribose) polymerase-dependent cell death after transient focal cerebral ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R215–R221. doi: 10.1152/ajpregu.00747.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hong A., Moriceau G., Sun L., Lomeli S., Piva M., Damoiseaux R., Holmen S.L., Sharpless N.E., Hugo W., Lo R.S. Exploiting drug addiction mechanisms to select against MAPKi-resistant melanoma. Cancer Discov. 2018;8:74–93. doi: 10.1158/2159-8290.CD-17-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fattore L., Ruggiero C.F., Liguoro D., Mancini R., Ciliberto G. Single cell analysis to dissect molecular heterogeneity and disease evolution in metastatic melanoma. Cell Death Dis. 2019;10:827. doi: 10.1038/s41419-019-2048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Luebker S.A., Koepsell S.A. Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front. Oncol. 2019;9:268. doi: 10.3389/fonc.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]