Abstract
Background
Peak oxygen consumption (VO2) is reduced in women with a history of breast cancer (BC). We measured leg blood flow, oxygenation, bioenergetics, and muscle composition in women with BC treated with anthracycline chemotherapy (n = 16, mean age: 56 years) and age‐ and body mass index–matched controls (n = 16).
Materials and Methods
Whole‐body peak VO2 was measured during cycle exercise. 31Phosphorus magnetic resonance (MR) spectroscopy was used to measure muscle bioenergetics during and after incremental to maximal plantar flexion exercise (PFE). MR imaging was used to measure lower leg blood flow, venous oxygen saturation (SvO2), and VO2 during submaximal PFE, and abdominal, thigh, and lower leg intermuscular fat (IMF) and skeletal muscle (SM).
Results
Whole‐body peak VO2 was significantly lower in BC survivors versus controls (23.1 ± 7.5 vs. 29.5 ± 7.7 mL/kg/minute). Muscle bioenergetics and mitochondrial oxidative capacity were not different between groups. No group differences were found during submaximal PFE for lower leg blood flow, SvO2, or VO2. The IMF‐to‐SM ratio was higher in the thigh and lower leg in BC survivors (0.36 ± 0.19 vs. 0.22 ± 0.07, p = .01; 0.10 ± 0.06 vs. 0.06 ± 0.02, p = .03, respectively) and were inversely related to whole‐body peak VO2 (r = −0.71, p = .002; r = −0.68, p = .003, respectively). In the lower leg, IMF‐to‐SM ratio was inversely related to VO2 and O2 extraction during PFE.
Conclusion
SM bioenergetics and oxidative capacity in response to PFE are not impaired following anthracycline treatment. Abnormal SM composition (increased thigh and lower leg IMF‐to‐SM ratio) may be an important contributor to reduced peak VO2 during whole‐body exercise among anthracycline‐treated BC survivors.
Implications for Practice
Peak oxygen consumption (peak VO2) is reduced in breast cancer (BC) survivors and is prognostic of increased risk of cardiovascular disease‐related and all‐cause mortality. Results of this study demonstrated that in the presence of deficits in peak VO2 1 year after anthracycline therapy, skeletal muscle bioenergetics and oxygenation are not impaired. Rather, body composition deterioration (e.g., increased ratio of intermuscular fat to skeletal muscle) may contribute to reduced exercise tolerance in anthracycline BC survivors. This finding points to the importance of lifestyle interventions including caloric restriction and exercise training to restore body composition and cardiovascular health in the BC survivorship setting.
Keywords: Breast cancer, Anthracyclines, Skeletal muscle, Intermuscular fat, Oxygen uptake, Magnetic resonance imaging, Muscle bioenergetics
Short abstract
Women with a history of breast cancer are at a greater risk for cardiovascular disease. This article reports on the relationship between anthracycline chemotherapy and peak oxygen consumption in breast cancer survivors.
Introduction
Advances in prevention, early detection, and treatment of early‐stage breast cancer (BC) have dramatically improved survival, and consequently, other conditions including cardiovascular disease (CVD) now compete with BC as the primary cause of mortality 1, 2. Women with a history of BC are at greater risk for CVD and related mortality relative to women without BC. It is well established that following treatment for BC, cardiorespiratory fitness, measured as peak oxygen uptake (peak VO2) during whole‐body exercise, is ≈20% lower than age‐ and sex‐matched controls 3, 4, 5, 6, 7, 8. Low cardiorespiratory fitness is associated with increased heart failure incidence, cardiovascular events, and both CVD‐related and all‐cause mortality 9.
Several BC therapies can cause cardiovascular injury, including anthracycline chemotherapy, which is associated with a dose‐dependent, progressive, myocardial injury 10. Accordingly, impairments in peak VO2 could be attributed to “central” limitations that reduce peak exercise cardiac output and left ventricular ejection fraction—the current clinical standard measure for detecting and monitoring cardiotoxicity 7, 11, 12. However, “peripheral” impairments in exercise blood flow, citrate synthase activity, and relative reductions in oxidative muscle fiber and capillarity have been reported in individuals after systemic cancer therapy 11, 13, 14, 15. Abnormalities in skeletal muscle composition may also limit exercise tolerance; Reding et al. reported peak VO2 was inversely related to the ratio of intermuscular fat (IMF) to skeletal muscle (SM) within the paraspinal muscles in cancer survivors 16. Given that O2 consumption during cycle exercise occurs predominantly in the muscles of the thigh and lower leg, skeletal muscle composition in the leg is likely a more important determinant of peak VO2. Prior reports studied populations heterogeneous for both cancer type and treatment, or only assessed acute changes during active treatment. Accordingly, the aims of this study were as follows: (a) to comprehensively compare noninvasive measures of leg SM composition, rest and exercise blood flow, O2 extraction, VO2, and oxidative metabolism during plantar flexion exercise (PFE) using magnetic resonance (MR) imaging, 31P spectroscopy, and whole‐body (cycle) peak VO2 in BC survivors approximately 1 year after anthracycline treatment and age‐ and body mass index (BMI)‐matched noncancer controls; and (b) to evaluate the relationship between thigh, lower leg, and paraspinal SM composition and whole‐body peak VO2. We hypothesized that BC survivors would have attenuated exercise leg blood flow, impaired SM oxidative capacity, and unfavorable SM composition compared with controls and that IMF‐to‐SM ratio in the leg would be inversely related to peak VO2. An exploratory objective was to assess underlying effects of the IMF‐to‐SM ratio by examining its relationship with measures of exercise metabolism, blood flow, VO2, and O2 extraction in the lower leg of anthracycline‐treated BC survivors and noncancer controls.
Materials and Methods
Participants
Thirty‐two women participated in this cross‐sectional study, with 16 BC survivors and 16 age‐ and BMI‐matched noncancer controls. The women with BC were recruited from a randomly selected cohort of 75 BC survivors who received anthracycline treatment at the Cross Cancer Institute (Edmonton, Canada) in the previous year, by mailing an invitation to participate. The women with BC who responded to the letter were then further screened for eligibility, including an additional inclusion criterion of a minimum of 3.5 months since receipt of their last anthracycline‐based chemotherapy treatment to avoid effects of acute cardiotoxicity 12. The control participants were recruited from the local community via word of mouth and recruitment posters. Exclusion criteria for both groups included a history of cardiovascular disease, diabetes, or lung disease, and contraindications to MR imaging (MRI). Controls were also excluded if they had a history of cancer of any type. Informed, written consent was obtained from all participants, and the study was approved by the University of Alberta Research Ethics Board.
Cardiorespiratory Fitness
Exercise testing was performed on an electrically braked upright cycle ergometer (Ergoselect II 1200; Ergoline, Bitz, Germany) using a staged protocol that started at 20 watts (W) and increased by 15 W every minute until volitional exhaustion. Expired gas was collected and analyzed using a commercially available metabolic measurement system (Encore229 Vmax; SensorMedics, Yorba Linda, CA) and the highest 20‐second average was used as whole‐body peak VO2. Heart rate (lead II electrocardiogram) was monitored continuously while rating of perceived exertion (RPE) was recorded every 2 minutes and at peak exercise.
MR Imaging and 31P Magnetic Resonance Spectroscopy
Skeletal muscle function, resting and exercise metabolism, and composition were evaluated using a 3.0 T MRI system (PRISMA; Siemens Healthineers, Erlangen, Germany).
Incremental PFE: 31P Metabolism
Subjects performed an incremental to maximal, unilateral (left leg), supine PFE test using a commercially available MRI ergometer (Trispect Module; Ergospect, Innsbruck, Austria; Fig. 1) inside the scanner. Plantar flexion exercise uses relatively small muscle mass, where the limiting role of the heart is minimized 17, and elicits a metabolic response similar to the common daily activity of stair climbing 18. The initial power output was set at 4 W and increased by 2 W every minute until volitional exhaustion. A metronome provided a consistent cadence of 30 contractions/minute and a study member provided feedback and encouragement from inside the scanner room to ensure test consistency. Tests were terminated at volitional exhaustion or technique breakdown (involvement of nonplantar flexor muscle groups or inability to perform 30 contractions/minute).
Figure 1.
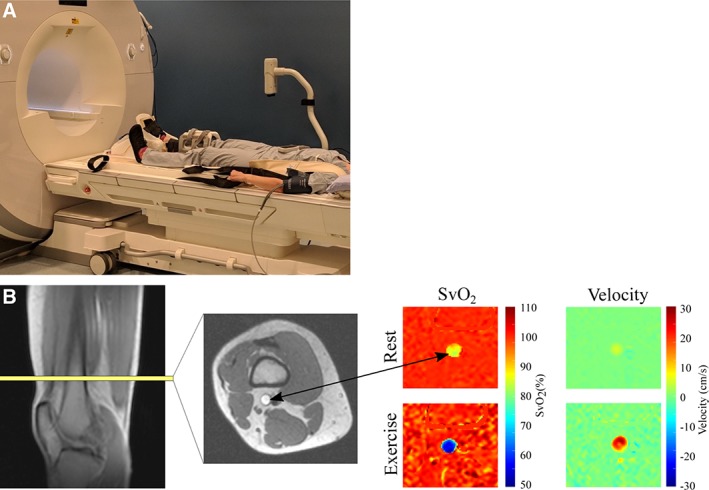
Experimental MRI plantar flexion setup and image acquisition. (A): Experimental setup for plantarflexion exercise device. (B): Image acquisition for determination of lower leg VO2, showing the typical location for imaging of the superficial femoral vein and representative SvO2 and blood velocity images, at rest and immediately after exercise. In this subject, SvO2 ≈ 86% at rest and 57% after exercise, with corresponding blood velocities of 7 cm/second and 25 cm/second. Abbreviations: SvO2, venous oxygen saturation; VO2, oxygen consumption.
31P spectra, averaged over 12‐second intervals, were acquired continuously from rest, throughout exercise, and for 4 minutes of recovery after cessation of exercise (repetition time = 1.5 seconds, flip angle = 30 degrees, 8‐cm‐diameter 31P radiofrequency coil was used for excitation and signal reception). Relative concentrations (area under the individual spectra) of phosphocreatine (PCr) and inorganic phosphate (Pi) were measured to generate Pi‐to‐PCr ratios, and intracellular pH was calculated from the chemical shift difference between the Pi and PCr signals 19. The Pi‐to‐PCr ratio and pH were compared at 60% of peak power output as a representative moderate‐intensity workload that occurred prior to a marked reduction in pH for all participants. From the time of exercise completion, a mono‐exponential curve was fit to the PCr recovery data to calculate the PCr recovery time constant, τ(PCr). The Pi‐to‐PCr ratio at the moderate exercise intensities and the PCr recovery τ were measured as indicators of skeletal muscle oxidative metabolism 20. 31P spectra were analyzed using custom, in‐house‐developed MATLAB code (MATLAB; MathWorks, Natick, MA).
Submaximal PFE Leg Blood Flow, Oxygen Extraction, and VO2
Following 15–20 minutes of recovery after the incremental test, participants performed 4 minutes of constant workload submaximal PFE using the right leg, at 60% of peak power output. Blood flow and venous oxygen saturation were measured in the right superficial femoral vein just superior to the knee at rest (prior to exercise), and immediately (<1 second) after cessation of exercise, using a previously described method 17, 21, 22, 23. Briefly, MR susceptometry‐based oximetry was used to estimate venous oxygen saturation (SvO2) based on the magnetic field shift associated with the concentration of deoxyhemoglobin 22. The same MRI acquisition included simultaneous quantification of blood flow (mL/minute) in the superficial femoral vein. Images were acquired perpendicular to the superficial femoral vein (axial image orientation) proximal to the knee (Fig. 1). Together with arterial oxygen saturation (SaO2, finger pulse oximetry) and hemoglobin (Hb) concentration (obtained from blood tests immediately prior to the MRI scan), lower leg VO2 and O2 extraction were calculated in accordance with the Fick Principle:
Lower leg O2 extraction was calculated as follows:
Skeletal Muscle and Fat Composition
At each of the lower leg, thigh, and abdomen regions, a multislice multiecho acquisition was used to reconstruct water and fat separated images using the modified Dixon approach (Fig. 2) 24. Typical image parameters include a 4‐mm slice thickness, axial slice orientation, 1.0–1.3‐mm in‐plane resolution, with 30 slices covering the lower leg, 50 slices covering the thigh, and 12 slices in the abdomen, with a subset of images selected for analysis at each location. For the lower leg, slices were analyzed from 5 cm below the proximal end of tibial plateau, as identified on a sagittal localizer image of the lower leg, to the distal elimination of the gastrocnemius. For the thigh, 10 consecutive slices were analyzed starting 40 mm from the most distal point of the femur, as identified using the sagittal localizer image of femur. In the abdomen, three slices centered at the middle of the third lumbar vertebrae were identified using sagittal localizer images of the spine.
Figure 2.
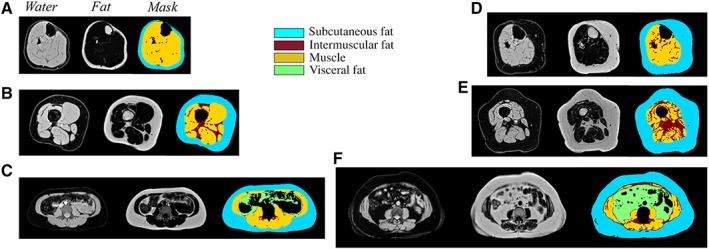
Magnetic resonance body composition acquisition and analysis by the modified Dixon fat–water separation approach in the lower leg (A), thigh (B), and abdomen (C) of a representative participant with low intermuscular fat and in the lower leg (D), thigh (E), and abdomen (F) of a representative participant with high intermuscular fat.
Absolute volumes of muscle (SM), intermuscular fat (IMF), and subcutaneous fat were measured in the lower leg, thigh, and abdomen. Visceral fat (VF) was also measured in the abdomen. For image segmentation, custom MATLAB code was used to perform semiautomated analysis to identify the boundaries of subcutaneous fat, SM, IMF, VF, and bones. All regions were manually checked and adjusted in each slice to ensure accuracy of the automated delineation. Volumes for each component were quantified using the disk summation method at each of the three regions (lower leg, thigh, and abdomen). The SM and IMF volumes were used to calculate the ratio of IMF to SM as well as the SM fat fraction (IMF/(IMF + SM)*100%) in each region.
Descriptive Information
Cardiovascular risk factors and usual physical activity in the past month were collected by researcher‐developed questionnaire and the Godin Leisure‐Time Exercise Questionnaire 25. Diagnosis and treatment history were extracted from medical records.
Statistical Analysis
Categorical descriptive characteristics were compared between groups using chi‐square tests or Fisher's exact test for variables with cell size above or below n = 5, respectively. Student's t tests for independent samples were used to compare continuous variables between groups. In the case of nonparametric distribution of continuous variables (confirmed by the Shapiro‐Wilk test), the Mann‐Whitney U test was used instead. Bivariate associations between IMF‐to‐SM ratio or SM fat fraction in each region to relative peak VO2 were characterized with Pearson's product‐moment correlations within both groups combined and confirmed within the BC group alone. Pearson's correlations were also used to characterize associations between our parameters of lower leg function and metabolism and the IMF‐to‐SM ratio in the lower leg. Continuous data are presented as mean ± SD.
Results
Participants
Twenty‐two women with BC responded to recruitment letters, and after further screening, six were excluded owing to a history of diabetes (n = 2), MRI incompatibility (n = 2), and failure to respond to follow‐up calls regarding scheduling (n = 2). Of the remaining 16 BC survivors who enrolled, 15 received epirubicin, 1 received doxorubicin, 15 received radiation therapy, and none received trastuzumab (Table 1). On average, BC survivors were studied 12.8 ± 4.7 months after administration of the final anthracycline treatment. The groups were well matched for age, weight, body mass index, hemoglobin, cardiovascular risk factors, and self‐reported exercise (Table 1).
Table 1.
Participant characteristics
| Parameter | BC (n = 16) | Control (n = 16) | p value |
|---|---|---|---|
| Age, years | 56 (10) | 56 (10) | .95 |
| Weight, kg | 75.6 (12.0) | 74.9 (11.7) | .87 |
| Body mass index, kg/m2 | 29.0 (3.6) | 27.9 (4.9) | .47 |
| Self‐reported exercise, minutes/week | 225 (275) | 313 (208) | .16a |
| Hemoglobin, g/L | 133 (11) | 134 (8) | .67 |
| Hematocrit, % | 40 (3) | 41 (2) | .51 |
| Cardiovascular risk factors, % | |||
| Sedentary | 25 | 13 | .65 |
| Hypertension | 13 | 0 | .48 |
| Hypercholesterolemia | 0 | 13 | .48 |
| Former smoker | 44 | 50 | .72 |
| Current smoker | 0 | 0 | — |
| Diabetes | 0 | 0 | — |
| Overweight | 31 | 50 | .47 |
| Obese | 44 | 44 | 1 |
| Other comorbid conditions, % | |||
| Arthritis | 31 | 44 | .72 |
| Osteoporosis | 13 | 6 | 1 |
| Thyroid disorder | 13 | 19 | 1 |
| Lung disease | 0 | 0 | 1 |
| Breast cancer characteristics | |||
| Stage II/III/IV, n | 8/7/1 | — | — |
| Epirubicin/doxorubicin, n | 15/1 | — | — |
| Dose, mg/m2 | 307 (75) | — | — |
| Does reduction, n | 3 | — | — |
| No surgery/lumpectomy/mastectomy, n | 1/6/9 | — | — |
| Radiation therapy, n | 15 | — | — |
| Hormone therapy, n | 11 | — | — |
Results are presented as mean (SD).
Abbreviations: —, not applicable; BC, breast cancer.
Nonparametric distribute, Mann‐Whitney U/ test used.
Cardiorespiratory Fitness
Peak cycle ergometer power output (132 ± 31 vs. 165 ± 30 W, p < .01), absolute peak VO2 (1.69 ± 0.37 vs. 2.13 ± 0.41 L/minute, p < .01), and peak VO2 indexed to body mass (23.1 ± 7.5 vs. 29.5 ± 7.7 mL/kg/minute, p = .02) were significantly lower in BC survivors compared with controls. Peak exercise heart rate, SaO2, respiratory exchange ratio, and rating of perceived exertion did not differ between groups (Table 2).
Table 2.
Cardiopulmonary exercise performance during cycle exercise and lower leg muscle metabolism and oxygenation at rest and after plantar flexion exercise in BC survivors and controls
| Parameter | BC | Control | p value |
|---|---|---|---|
| Peak cycle ergometry | |||
| Power output, W | 132 (31) | 165 (30) | <.01 |
| Oxygen uptake, L/minute | 1.69 (0.37) | 2.13 (0.41) | <.01 |
| Oxygen uptake, mL/kg/minute | 23.1 (7.5) | 29.5 (7.7) | .02 |
| Respiratory exchange ratio | 1.29 (0.09) | 1.27 (0.08) | .60 |
| SaO2, % | 96 (2) | 94 (2) | .08 |
| Heart rate, bpm | 166 (12) | 161 (14) | .35 |
| Rating of perceived exertion, 0–10 scale | 9 (1) | 9 (1) | .68 |
| Rest, lower leg | |||
| Pi‐to‐PCr ratio | 0.10 (0.03) | 0.11 (0.03) | .24 |
| pH | 7.06 (0.06) | 7.08 (0.10) | .44 |
| Blood flow, mL/minute | 55 (23) | 77 (29) | .02 |
| Pulse oximeter SaO2, % | 97 (2) | 98 (2) | .36 |
| SvO2, % | 74 (5) | 80 (5) | <.01 |
| O2 extraction ratio | 0.24 (0.06) | 0.18 (0.05) | .01 |
| VO2, mL/minute | 2.1 (0.84) | 2.3 (0.54) | .64 |
| Submaximal (60% of peak) plantar flexion exercise | |||
| Power output, W | 10.9 (1.2) | 12.1 (2.4) | .09 |
| Blood flow, mL/minute | 526 (171) | 581 (198) | .41 |
| SvO2, % | 55 (7) | 54 (5) | .81 |
| O2 extraction ratio | 0.44 ± 0.07 | 0.45 ± 0.06 | .72 |
| Peak VO2, mL/minute | 40 (15) | 45 (15) | .31 |
| Peak plantar flexion exercise | |||
| Power output, W | 18.0 (2.4) | 19.6 (3.4) | .13 |
| Heart rate, bpm | 102 (16) | 100 (20) | .84 |
| Rating of perceived exertion, 0–10 for lower leg | 10 (1) | 10 (0) | .28 |
| Pi‐to‐PCr ratio | 0.98 (0.32) | 0.90 (0.24) | .41 |
| pH | 6.46 (0.22) | 6.45 (0.28) | .39 |
| Recovery, lower leg | |||
| PCr τ, seconds | 36 (11) | 33 (9) | .33 |
Data are presented as mean (SD). Bolded p values are statistically significant.
Abbreviations: BC, breast cancer; PCr, phosphocreatine; Pi, inorganic phosphate; SaO2, arterial oxygen saturation; SvO2, venous oxygen saturation; VO2, oxygen consumption.
Skeletal Muscle Oxidative Capacity
Resting Pi‐to‐PCr ratio and pH were similar in both groups (Table 2). Peak PFE power output, heart rate, rating of perceived exertion, Pi‐to‐PCr ratio, and pH were not different between groups (Table 2). Skeletal muscle oxidative capacity, measured as Pi‐to‐PCr ratio at a moderate‐intensity submaximal exercise workloads and by postexercise PCr recovery τ, did not differ between BC and controls (Table 2), with similar results for pH.
Rest and Submaximal Exercise Lower Leg VO2 and Its Determinants
Resting lower leg SvO2 was lower in the BC as compared with the control group (74 ± 5 vs. 80 ± 5%, p < .01), corresponding to a higher O2 extraction ratio (0.24 ± 0.06 vs. 0.18 ± 0.05, p = .01), whereas leg blood flow was lower in the BC group (55 ± 23 vs. 77 ± 29 mL/minute, p = .02), resulting in no difference between groups for resting leg VO2 (Table 2). Power output, leg VO2, blood flow, SvO2, and O2 extraction were similar between groups during submaximal PFE (Table 2).
Body Composition
Subcutaneous fat volume did not differ between groups for the abdomen, thigh, or lower leg regions (Table 3). In the abdomen, visceral fat volume was higher (BC 406 ± 217 vs. 273 + 123 mL, p = .04), paraspinal SM volume was lower (313 ± 39 vs. 340 ± 32 mL, p = .04), but absolute IMF volume, IMF‐to‐SM ratio, and SM fat fraction did not differ between groups. In the thigh, SM volume did not differ between groups, but IMF volume (82 ± 31 vs. 58 ± 17 mL, p = .01), IMF‐to‐SM ratio (0.36 ± 0.19 vs. 0.22 ± 0.07, p = .01), and SM fat fraction (25 ± 10 vs. 18 ± 5%, p = .02) were all significantly higher in BC compared with controls. In the lower leg, SM was reduced (752 ± 149 vs. 903 ± 162 mL, p = .01; Table 3) and IMF volume did not differ, whereas the IMF‐to‐SM ratio (0.10 ± 0.06 vs. 0.06 ± 0.02, p = .03) and the SM fat fraction (9 ± 5 vs. 5 ± 2%, p = .02; Table 3) were significantly higher in BC.
Table 3.
Body composition in the thigh, lower leg, and abdomen
| Variable | BC (n = 16) | Controls (n = 16) | p value |
|---|---|---|---|
| Thigha | |||
| Total volume, mL | 754 ± 83 | 771 ± 147 | .70 |
| Subcutaneous fat, mL | 397 ± 83 | 420 ± 127 | .55 |
| Muscle, mL | 249 ± 53 | 266 ± 37 | .27 |
| Intermuscular fat, mL | 82 ± 31 | 58 ± 17 | .01 |
| IMF‐to‐SM ratio | 0.36 ± 0.19 | 0.22 ± 0.07 | .01 |
| SM fat fraction, % | 25 ± 10 | 18 ± 5 | .02 |
| Lower legb | |||
| Total volume, mL | 1,599 ± 285 | 1,884 ± 395 | .03 |
| Subcutaneous fat, mL | 659 ± 189 | 787 ± 264 | .13 |
| Muscle, mL | 752 ± 149 | 903 ± 162 | .01 |
| Intermuscular fat, mL | 65 ± 32 | 51 ± 23 | .06 |
| IMF‐to‐SM ratio | 0.10 ± 0.06 | 0.06 ± 0.02 | .03 |
| SM fat fraction, % | 9 ± 5 | 5 ± 2 | .02 |
| Abdomenc | |||
| Total volume, mL | 1,684 ± 471 | 1,490 ± 473 | .25 |
| Subcutaneous fat, mL | 897 ± 258 | 823 ± 379 | .52 |
| Muscle, mL | 313 ± 39 | 340 ± 32 | .04 |
| Intermuscular fat, mL | 69 ± 27 | 53 ± 28 | .13 |
| IMF‐to‐SM ratio | 0.22 ± 0.10 | 0.16 ± 0.09 | .06 |
| SM fat fraction, % | 18 ± 6 | 13 ± 6 | .06 |
| Visceral fat | 406 ± 217 | 273 ± 123 | .04 |
Bolded p values are statistically significant.
Thigh data is for 4 cm of transverse slices starting 4 cm from the distal end of femur.
Lower leg data is for the whole lower leg starting 5 cm below the tibial plateau until the distal end of the gastrocnemius.
Lumbar spine data is 1.2‐cm section of transverse images at the center of the third lumbar vertebrae.
Abbreviations: IMF, intermuscular fat; SM, skeletal muscle.
Relationship of Skeletal Muscle and Intermuscular Fat with Whole‐Body Peak VO2
On analysis of all study participants combined, the IMF‐to‐SM ratio in the paraspinal, thigh, and lower leg muscle groups each had a strong, inverse relationship to whole‐body relative peak VO2 (r = −0.70, p < .001, r = −0.72, p < .001, and r = −0.66, p < .001 respectively; Fig. 3). These relationships were similarly strong in the BC group (paraspinal: r = −0.70, p = .003; thigh: r = −0.71, p = .002; lower leg: r = −0.68, p = .003). The SM fat fraction had a slightly stronger relationship with whole‐body peak VO2 than the IMF‐to‐SM ratio, in all regions (paraspinal: r = −0.73, p < .001; thigh: r = −0.78, p < .001; lower leg: r = −0.71, p < .001).
Figure 3.
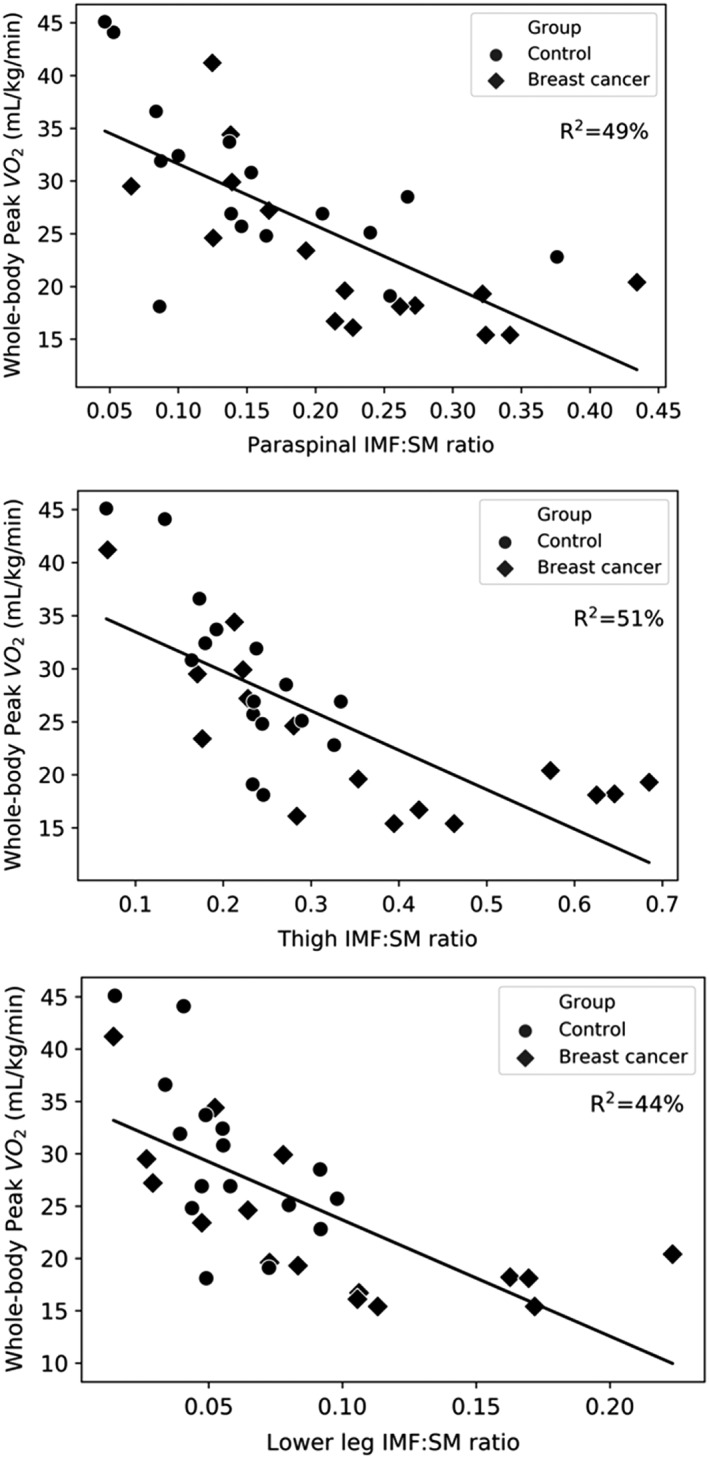
Relationship between IMF‐to‐SM ratio and whole‐body peak VO2 for the paraspinal, thigh, and lower leg muscle regions. Abbreviations: IMF, intermuscular fat; SM, skeletal muscle; VO2, oxygen consumption.
Relationship of Lower Leg Muscle Composition, Resting and Exercise Metabolism, Blood Flow, Oxygen Consumption, and Extraction
The lower leg IMF‐to‐SM ratio and absolute muscle mass were not related to lower leg peak power output, Pi‐to‐PCr ratio, or pH (Table 4). Lower leg IMF‐to‐SM ratio correlated strongly and positively with lower leg submaximal exercise SvO2 (r = 0.70, p < .001). The IMF‐to‐SM ratio also had a strong inverse relationship with lower leg submaximal exercise VO2 and O2 extraction (r = −0.64, p = .008; r = −0.67, p < .001) and a moderate inverse relationship with lower leg exercise blood flow (r = −0.35, p = .05; Table 4). These relationships were similarly evident within the BC group alone, except for blood flow, where the correlation effect size was similar but no longer significant owing to greater variability (r = −0.37, p = .16).
Table 4.
Pearson correlations between lower leg IMF‐to‐SM ratio and skeletal muscle function, resting, and exercise metabolism
| BC group (n = 16) | All participants (n = 32) | |||
|---|---|---|---|---|
| Parameter | r | p value | r | p value |
| Peak plantar flexion power output | −0.24 | .38 | −0.29 | .11 |
| Peak plantar flexion Pi‐to‐PCr ratio | −0.20 | .46 | −0.17 | .35 |
| Peak plantar flexion pH | 0.42 | .11 | 0.22 | .21 |
| Pi‐to‐PCr ratio at ~60% peak power | 0.21 | .44 | 0.07 | .71 |
| pH at ~60% peak power | 0.12 | .66 | 0.17 | .35 |
| PCr recovery tau | 0.03 | .92 | 0.14 | .43 |
| Lower leg exercise blood flow | −0.37 | .16 | −0.35 | .05 |
| Lower leg exercise VO2 | −0.63 | .008 | −0.64 | .008 |
| Lower leg exercise SvO2 (%) | 0.76 | <.001 | 0.70 | <.001 |
| Lower leg exercise O2 extraction | −0.71 | .002 | −0.67 | <.001 |
Bolded p values are statistically significant.
Abbreviations: BC, breast cancer; PCr, phosphocreatine; Pi, inorganic phosphate; SvO2, venous oxygen saturation; VO2, oxygen consumption.
Discussion
Peak VO2 is a strong independent predictor of CVD, disability, and mortality in healthy and clinical populations 26. Following BC treatment, women have a peak VO2 that is on average 20% lower than age‐matched healthy sedentary women 3, 4, 5, 6, 7, 8. The mechanisms responsible for the reduced exercise tolerance are not well understood; however, recent evidence suggests that “noncardiac” peripheral abnormalities associated with chemotherapy may be important contributors to reduced exercise tolerance 13, 16. The major new finding of this study is that despite a significantly lower peak VO2 during whole‐body exercise, BC survivors had preserved lower leg blood flow, leg VO2, and mitochondrial oxidative capacity relative to controls during unilateral PFE. A second novel finding is that the IMF‐to‐SM ratio and SM fat fraction in the thigh and lower leg are strongly related to peak VO2, explaining approximately 50% of the variability in cardiorespiratory fitness. We also uncovered potential mediating mechanisms for the impact of the IMF‐to‐SM ratio on local VO2. Specifically, a higher IMF‐to‐SM ratio in the lower leg was associated with increased SvO2, reduced muscle VO2, blood flow, and O2 extraction with moderate‐intensity unilateral PFE. Taken together, these results suggest that the primary peripheral impairments evident approximately 1 year after anthracyclines are abnormalities in skeletal muscle composition, namely, increased IMF, which is an important contributor to the reduced whole‐body VO2 and O2 extraction in BC survivors.
Skeletal Mitochondrial Oxidative Capacity in BC Survivors
Contrary to our hypothesis, we did not observe impaired muscle function or reduced oxidative capacity in our group of BC survivors. Specifically, in response to unilateral PFE for which maximal blood flow is not limited by convective O2 delivery, the peak PFE workloads achieved by the BC survivors did not differ from the controls. This was unexpected given the significant and marked reduction in cycle ergometer power output achieved by the BC group relative to the control group. Furthermore, the Pi‐to‐PCr ratio, which reflects coupling of adenosine triphosphate (ATP) use and resynthesis, did not differ between groups at rest or at moderate‐ and peak‐intensity exercise, indicating similar patterns of aerobic and anaerobic metabolism during exercise (Table 2). Notably, the peak pH at the end of exercise was similar between groups, indicating comparable states of metabolic acidosis, allowing for comparison of postexercise PCr recovery 27. The PCr postexercise recovery τ, a measure of mitochondrial ATP synthesis 28, 29, 30, was not different between groups, nor was blood flow, a potential confounding factor (Table 2). Taken together, these results suggest that, on average, women who received anthracyclines for BC do not have impaired SM mitochondrial function in response to small muscle mass exercise where the limiting role of the heart is minimized.
Using vastus lateralis skeletal muscle biopsy, Mijwel et al. showed that during receipt of anthracycline chemotherapy, women with BC experienced a reduction in oxidative muscle fibers, citrate synthase, and capillarity 13. These deteriorations were attributed to deconditioning rather than a result of chemotherapy as a 16‐week exercise intervention consisting of high‐intensity aerobic and resistance interval training during adjuvant chemotherapy attenuated the decline in mitochondrial function 13. In the present study, the women with BC were on average 13 months post‐anthracycline treatment, and 75% self‐reported performing some physical activity in the previous month. It is possible that this length of time since treatment and/or the physical activity performed may have contributed to recovery or preservation of mitochondrial function.
Skeletal Muscle Composition and Its Relationship to VO2
Haykowsky et al. previously reported that the reduced peak VO2 in patients with heart failure and preserved ejection fraction (HFpEF) was associated with a higher thigh IMF‐to‐SM ratio 31. Similarly, Reding et al. found inverse correlations between peak VO2 and paraspinal IMF‐to‐SM ratio in 14 cancer survivors with mixed diagnoses and timing since treatment 16. However, Reding et al. did not find differences in absolute amounts of IMF or the IMF‐to‐SM ratio of the paraspinal muscles between cancer survivors and noncancer controls. In our study, we used a similar approach to assess the paraspinal muscle and found a similarly strong relationship between IMF‐to‐SM ratio and whole‐body peak VO2. We also assessed composition of the thigh and lower leg muscles, as the primary muscles driving oxygen uptake during cycle and PFE. We extended prior noncancer and cancer studies by demonstrating that BC survivors previously treated with anthracycline chemotherapy have increased thigh IMF, IMF‐to‐SM ratio, and SM fat fraction, reduced lower leg SM, and increased lower leg IMF‐to‐SM and SM fat fraction compared with controls. Moreover, although the average IMF‐to‐SM ratio and SM fraction varied among the paraspinal, thigh, and lower leg muscles, it was inversely related to peak whole‐body VO2 during cycle exercise in all three regions, implicating IMF in the pathophysiology of anthracycline‐related VO2 deterioration. A further illustration of the extent of impairment in SM composition among women with BC is that the IMF‐to‐SM ratio in our BC group was similar to that reported by Haykowsky et al. in patients with HFpEF 31. To our knowledge, no study has examined the time course or cause of IMF deposition in BC survivors. However, an observational study of 3,241 women found that sarcopenia occurs in one third of BC at diagnosis 32. Furthermore, SM loss and weight gain (increased adiposity) are reported over the course of adjuvant therapy, related to a small reduction in physical activity without an adjustment in caloric intake 33.
We have further added to the understanding of the impact of IMF on SM metabolism by using novel MRI techniques to noninvasively assess lower leg local VO2 and O2 extraction during submaximal PFE. We did not find any relationship between IMF‐to‐SM ratio and SM exercise metabolism as assessed by 31P spectroscopy. However, lower leg IMF‐to‐SM ratio was inversely related to local exercise VO2 and O2 extraction in both groups (Table 4). Specifically, the greater the amount of IMF relative to the amount of SM, the less O2 consumed or extracted with exercise. That we found this relationship between IMF‐to‐SM ratio and VO2 for both whole‐body and small muscle mass exercise indicates that the impact of IMF on VO2 is independent of potential “central” limitations. A potential explanation for this IMF effect can be drawn from a study demonstrating that blood flow to subcutaneous fat adjacent to exercising muscle increases in an intensity‐dependent manner 34. This study of subcutaneous fat proposed potential mechanisms of vasodilation of adipose tissue vessels including (a) heat conduction from working muscle; (b) adenosine formation that occurs in fat during exercise; and (c) conductive vasodilation up the arterial tree. It is possible that a similar effect occurs in IMF where blood flow is shunted through the metabolically inactive IMF, resulting in increased mixed venous O2 content 34. Consequently, this could explain the strong relationship between lower leg IMF‐to‐SM ratio and both SvO2 and O2 extraction (Table 4). Another potential mechanism for the impact of IMF on oxygen extraction is that the deposits of adipose tissue in the muscle could reduce muscle oxygen diffusive conductance as a result of a greater distance for O2 to travel from the capillaries to muscle mitochondria and a limited ability to locally restrict vascular conductance in adipose tissue in close proximity to active SM 31. Finally, others have suggested that IMF may result in lower overall blood flow to a tissue 35; indeed, we observed a negative correlation between exercise blood flow to the entire lower leg (i.e., both fat and lean tissue) and IMF‐to‐SM in both groups combined (Table 4).
In other populations, increased levels of IMF are associated with inflammation, insulin resistance, reduced muscular activation, and reduced physical function 36. In combination with the findings from the current study, prevention and reduction of IMF accumulation should be an important goal for individuals with cancer. Both exercise and dietary interventions can reduce IMF, but in either case, it appears that weight loss is necessary to achieve reductions in IMF 36. Weight loss induced by exercise may more effectively reduce IMF compared with weight loss induced by caloric restriction 37, 38, 39, 40. Furthermore, the combination of caloric restriction and aerobic exercise appears to be substantially more effective for reducing IMF compared with caloric restriction and resistance exercise in premenopausal obese women 40.
Limitations
A limitation of this study is that lower leg VO2 and its determinants were only measured at rest and submaximal PFE. Although caution is warranted in extending these measures to maximal PFE, it is unlikely that group differences would exist, as the Pi‐to‐PCr ratio and intracellular pH during and after incremental to maximal exercise were not different between groups. Finally, blood flow was measured in the popliteal vein and was assumed to be representative of lower leg venous return. Future studies may consider using positron emission tomography or arterial spin labeling techniques to measure skeletal muscle perfusion during both isolated and whole‐body exercise.
Conclusion
In women with BC treated with anthracycline chemotherapy 1 year earlier, peak VO2 and power output during whole body (cycle) exercise are significantly reduced relative to age‐ and BMI‐matched controls. However, during small muscle mass exercise that is not limited by cardiac reserve, women with BC were able to perform a similar amount of work with a similar lower leg VO2, blood flow, O2 extraction, and oxidative capacity to controls. The primary peripheral anomaly in BC compared with controls was in SM composition, with an increased IMF‐to‐SM ratio and SM fat fraction in the thigh and lower leg among the women with BC. This is an important difference because a higher IMF‐to‐SM ratio was associated with lower whole‐body peak VO2 and less lower leg blood flow, VO2, and O2 extraction. Therefore, IMF appears to play an important role in the impaired cardiorespiratory fitness reported following completion of BC treatment. Diet and exercise interventions targeted to reduce the relative amount of IMF and increase the absolute amount of SM should be investigated as possible therapies to improve cardiorespiratory fitness following cancer therapy.
Author Contributions
Conception/design: Rhys I. Beaudry, Amy A. Kirkham, Richard B. Thompson, John R. Mackey, Mark J. Haykowsky
Provision of study material or patients: Rhys I. Beaudry, Amy A. Kirkham, John R. Mackey
Collection and/or assembly of data: Rhys I. Beaudry, Amy A. Kirkham, Richard B. Thompson, Justin G. Grenier
Data analysis and interpretation: Rhys I. Beaudry, Amy A. Kirkham, Richard B. Thompson, Justin G. Grenier, John R. Mackey, Mark J. Haykowsky
Manuscript writing: Rhys I. Beaudry, Amy A. Kirkham, Richard B. Thompson, John R. Mackey, Mark J. Haykowsky
Final approval of manuscript: Rhys I. Beaudry, Amy A. Kirkham, Richard B. Thompson, Justin G. Grenier, John R. Mackey, Mark J. Haykowsky
Disclosures
The authors indicated no financial relationships.
Acknowledgments
M.J.H. is funded by the Moritz Chair in Geriatrics in the College of Nursing and Health Innovation, University of Texas Arlington. A.A.K. is funded by Postdoctoral Fellowships from Susan G. Komen, Canadian Institutes of Health Research and Alberta Innovates Health Solutions. This study was funded in part by the Susan G. Komen Foundation (PDF17483149).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society . Cancer treatment & survivorship: Facts & figures 2016‐2017. Available at https://www.cancer.org/cancer/breast-cancer.html. Accessed August 2019.
- 3. Jones LW, Courneya KS, Mackey JR et al. Cardiopulmonary function and age‐related decline across the breast cancer survivorship continuum. J Clin Oncol 2012;30:2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burnett D, Kluding P, Porter C et al. Cardiorespiratory fitness in breast cancer survivors. SpringerPlus 2013;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koelwyn GJ, Lewis NC, Ellard SL et al. Ventricular‐arterial coupling in breast cancer patients after treatment with anthracycline‐containing adjuvant chemotherapy. The Oncologist 2016;21:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khouri MG, Hornsby WE, Risum N et al. Utility of 3‐dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin‐containing adjuvant therapy. Breast Cancer Res Treat 2014;143:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones LW, Haykowsky M, Pituskin EN et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor ‐ Positive operable breast cancer. The Oncologist 2007;12:1156–1164. [DOI] [PubMed] [Google Scholar]
- 8. Beaudry R, Howden E, Foulkes S et al. Determinants of exercise intolerance in breast cancer patients prior to anthracycline chemotherapy. Physiol Rep 2019;7:e13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ozemek C, Laddu DR, Lavie CJ et al. An update on the role of cardiorespiratory fitness, structured exercise and lifestyle physical activity in preventing cardiovascular disease and health risk. Prog Cardiovasc Dis 2018;61:484–490. [DOI] [PubMed] [Google Scholar]
- 10. McGowan JV, Chung R, Maulik A et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther 2017;31:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foulkes S, Howden EJ, Bigaran A et al. Persistent impairment in cardiopulmonary fitness following breast cancer chemotherapy. Med Sci Sports Exerc 2019;51:1573–1581. [DOI] [PubMed] [Google Scholar]
- 12. Cardinale D, Colombo A, Bacchiani G et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 13. Mijwel S, Cardinale DA, Norrbom J et al. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J 2018;32:5495–5505. [DOI] [PubMed] [Google Scholar]
- 14. Didier KD, Ederer AK, Reiter LK et al. Altered blood flow response to small muscle mass exercise in cancer survivors treated with adjuvant therapy. J Am Heart Assoc 2017;6:e004784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howden E, Bigaran A, Beaudry R et al. Exercise as a diagnostic and therapeutic tool for the prevention of functional disability in breast cancer patients. Eur J Prev Cardiol 2019;26:305–315. [DOI] [PubMed] [Google Scholar]
- 16. Reding KW, Brubaker P, D'Agostino R et al. Increased skeletal intermuscular fat is associated with reduced exercise capacity in cancer survivors: A cross‐sectional study. Cardio‐oncology 2019;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson RB, Tomczak CR, Haykowsky MJ. Evaluation of cardiac, vascular, and skeletal muscle function with MRI: Novel physiological end points in cardiac rehabilitation research. Can J Cardiol 2016;32(10 suppl 2):S388–S396. [DOI] [PubMed] [Google Scholar]
- 18. Mancini DM, Ferraro N, Tuchler M et al. Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus‐31 nuclear magnetic resonance. Am J Cardiol 1988;62:1234–1240. [DOI] [PubMed] [Google Scholar]
- 19. Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem 1973;248:7276–7278. [PubMed] [Google Scholar]
- 20. McCully KK, Kakihira H, Vandenborne K et al. Noninvasive measurements of activity‐induced changes in muscle metabolism. J Biomech 1991;24(suppl 1):153–161. [DOI] [PubMed] [Google Scholar]
- 21. Mathewson KW, Haykowsky MJ, Thompson RB. Feasibility and reproducibility of measurement of whole muscle blood flow, oxygen extraction, and VO2 with dynamic exercise using MRI. Magn Reson Med 2015;74:1640–1651. [DOI] [PubMed] [Google Scholar]
- 22. Thompson RB, Pagano JJ, Mathewson KW et al. Differential responses of post‐exercise recovery of leg blood flow and oxygen uptake kinetics in HFpEF versus HFrEF. PLoS One 2016;11:e0163513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang E, Kirkham AA, Grenier J et al. Measurement and correction of the bulk magnetic susceptibility effects of fat: Application in venous oxygen saturation imaging. Magn Reson Med 2019;81:3124–3137. [DOI] [PubMed] [Google Scholar]
- 24. Hernando D, Kellman P, Haldar JP et al. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med 2010;63:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985;10:141–146. [PubMed] [Google Scholar]
- 26. Myers J, Prakash M, Froelicher V et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 27. Argov Z, Lofberg M, Arnold DL. Insights into muscle diseases gained by phosphorus magnetic resonance spectroscopy. Muscle Nerve 2000;23:1316–1334. [DOI] [PubMed] [Google Scholar]
- 28. Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J 1993;291:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forbes SC, Paganini AT, Slade JM et al. Phosphocreatine recovery kinetics following low‐ and high‐intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol 2009;296:R161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kemp GJ, Ahmad RE, Nicolay K et al. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: A quantitative review. Acta Physiol (Oxf) 2015;213:107–144. [DOI] [PubMed] [Google Scholar]
- 31. Haykowsky MJ, Kouba EJ, Brubaker PH et al. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014;113:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caan BJ, Cespedes Feliciano EM, Prado CM et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 2018;4:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Demark‐Wahnefried W, Peterson BL, Winer EP et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 2001;19:2381–2389. [DOI] [PubMed] [Google Scholar]
- 34. Heinonen I, Bucci M, Kemppainen J et al. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. J Appl Physiol (1985) 2012;112:1059–1063. [DOI] [PubMed] [Google Scholar]
- 35. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 36. Addison O, Marcus RL, Lastayo PC et al. Intermuscular fat: A review of the consequences and causes. Int J Endocrinol 2014;2014:309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christiansen T, Paulsen SK, Bruun JM et al. Comparable reduction of the visceral adipose tissue depot after a diet‐induced weight loss with or without aerobic exercise in obese subjects: A 12‐week randomized intervention study. Eur J Endocrinol 2009;160:759–767. [DOI] [PubMed] [Google Scholar]
- 38. Murphy JC, McDaniel JL, Mora K et al. Preferential reductions in intermuscular and visceral adipose tissue with exercise‐induced weight loss compared with calorie restriction. J Appl Physiol (1985) 2012;112:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avila JJ, Gutierres JA, Sheehy ME et al. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol 2010;109:517–525. [DOI] [PubMed] [Google Scholar]
- 40. Janssen I, Fortier A, Hudson R et al. Effects of an energy‐restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 2002;25:431–438. [DOI] [PubMed] [Google Scholar]


