Abstract
Background
The addition of bevacizumab to chemotherapy improved outcomes for patients with metastatic colon cancer. E5204 was designed to test whether the addition of bevacizumab to mFOLFOX6, following neoadjuvant chemoradiation and definitive surgery, could improve overall survival (OS) in patients with stage II/III adenocarcinoma of the rectum.
Subjects, Materials, and Methods
Patients with stage II/III rectal cancer who had completed neoadjuvant 5‐fluorouracil‐based chemoradiation and had undergone complete resection were enrolled. Patients were randomized to mFOLFOX6 (Arm A) or mFOLFOX6 with bevacizumab (Arm B) administered every 2 weeks for 12 cycles.
Results
E5204 registered only 355 patients (17% of planned accrual goal) as it was terminated prematurely owing to poor accrual. At a median follow‐up of 72 months, there was no difference in 5‐year overall survival (88.3% vs. 83.7%) or 5‐year disease‐free survival (71.2% vs. 76.5%) between the two arms. The rate of treatment‐related grade ≥ 3 adverse events (AEs) was 68.8% on Arm A and 70.7% on Arm B. Arm B had a higher proportion of patients who discontinued therapy early as a result of AEs and patient withdrawal than did Arm A (32.4% vs. 21.5%, p = .029).The most common grade 3–4 treatment‐related AEs were neutropenia, leukopenia, neuropathy, diarrhea (without prior colostomy), and fatigue.
Conclusion
At 17% of its planned accrual, E5204 did not meet its primary endpoint. The addition of bevacizumab to FOLFOX6 in the adjuvant setting did not significantly improve OS in patients with stage II/III rectal cancer.
Implications for Practice
At 17% of its planned accrual, E5204 was terminated early owing to poor accrual. At a median follow‐up of 72 months, there was no significant difference in 5‐year overall survival (88.3% vs. 83.7%) or in 5‐year disease‐free survival (71.2% vs. 76.5%) between the two arms. Despite significant advances in the treatment of rectal cancer, especially in improving local control rates, the risk of distant metastases and the need to further improve quality of life remain a challenge. Strategies combining novel agents with chemoradiation to improve both distant and local control are needed.
Keywords: Bevacizumab, Avastin, Adjuvant therapy, Rectal cancer, Antiangiogenesis
Short abstract
Despite advances in the treatment of rectal cancer, the risk of distant metastases and poor quality of life remain a challenge. Trial E5204 was designed to test whether the addition of bevacizumab to mFOLFOX6, following neoadjuvant chemoradiation and definitive surgery, could improve overall survival in patients with stage II/III adenocarcinoma of the rectum.
Introduction
5‐fluorouracil (5‐FU)‐based neoadjuvant chemoradiation has become the preferred approach to the treatment of patients with stage II/III rectal cancer 1. Following surgery, patients go on to complete adjuvant chemotherapy. The use of FOLFOX in the treatment of colon cancer has evolved based on the results of several large randomized trials including MOSAIC 2 and National Surgical Adjuvant Breast and Bowel Project (NSABP) C‐07 3. These results form the foundation of this study.
The standard of care for the adjuvant treatment of stage II/III colon cancer changed from 6 months of adjuvant 5‐FU‐based chemotherapy alone to adjuvant FOLFOX (infusional 5‐FU, leucovorin, and oxaliplatin) based on two large randomized trials including MOSAIC and NSABP C‐07 3. Various modifications of the combination of oxaliplatin with 5‐FU plus leucovorin regimens have been evaluated and have simplified the schedule of administration as well as improved patient convenience without compromising the efficacy of the regimen.
Given the improved survival seen with irinotecan and oxaliplatin in patients with metastatic colorectal cancer, E3201 explored this in the adjuvant setting in patients with rectal cancer. Following either pre‐ or postoperative 5‐FU‐based chemoradiation (50.4 Gy), patients were randomized to three different adjuvant treatment options: (a) FOLFOX, (b) FOLFIRI, and (c) 5‐FU/leucovorin. There were no significant differences in toxicity between those patients treated with pre‐ versus postoperative 5‐FU/radiation. Follow‐up data on the 123 patients who did complete treatment showed that adjuvant FOLFOX could be given safely in patients with rectal cancer following completion of neoadjuvant chemoradiation 4.
Vascular endothelial growth factor (VEGF) has been identified as a crucial regulator of both normal and pathologic angiogenesis. Bevacizumab is a humanized monoclonal antibody against the VEGF‐A ligand. Combining targeted agents such as bevacizumab with chemotherapy resulted in high response rates and improved overall survival (OS) in patients with metastatic colon cancer 5. In a phase I study of six patients with locally advanced rectal cancer who were treated with a single dose of bevacizumab, serial biopsies revealed a decrease in the number of viable, circulating endothelial and progenitor cells 6.
Subjects, Materials, and Methods
E5204 was designed as a phase III trial that randomized patients following completion of standard‐of‐care neoadjuvant 5‐FU‐based chemoradiation and surgery to adjuvant FOLFOX with or without bevacizumab administered for 12 cycles. Based on the MOSAIC data 2, 12 cycles of FOLFOX (± bevacizumab) was planned. The trial was approved by the local institutional review boards of all participating centers. This trial was registered with http://clinicaltrials.gov (identifier NCT00303628).
Patient Eligibility
Patients with Eastern Cooperative Oncology Group (ECOG) performance status 0–1, ≥18 years of age, with histologically confirmed, nonmetastatic, clinically staged T3–4N0M0, TanyN1–2M0, adenocarcinoma of the rectum who had completed neoadjuvant therapy consisting of concurrent 5‐FU‐based chemoradiation to a total dose of 40–55.8 Gy were eligible. Patients were staged according to the American Joint Committee on Cancer 6th edition. Patients had to undergo definitive surgical resection. Adjuvant treatment had to begin between 28 and 56 days postoperatively. Patients who participated in NSABP R‐04 (NCT00058474) were eligible. Exclusion criteria included history of stroke, transient ischemic attacks, myocardial infarction/unstable angina, significant peripheral vascular disease, bleeding diathesis, uncontrolled hypertension, grade > 1 neuropathy, and allergy to platinum compounds. The ethics committees of the participating centers approved the study, and all patients provided written informed consent.
Treatment Plan
Eligible patients were randomized 1:1 to mFOLFOX with or without bevacizumab (5 mg/kg on Day 1). Four stratification factors were used in randomization: (a) ECOG performance status 0 versus 1; (b) clinical staging—high risk (T3N + M0, T4Nany M0) versus low risk (T1–2N+, T3N0M0); (c) preoperative oxaliplatin (yes vs. no); and (d) preoperative radiation dose (40–50 Gy vs. 50–55.8 Gy). Figure 1 displays the study schema. Treatment was repeated every 2 weeks for a total of 12 cycles. Patients (including NSABP R‐04 patients) who had received preoperative oxaliplatin had the following modification: nine cycles of FOLFOX6 followed by three cycles of 5‐FU and leucovorin. All doses were based on actual weight. Two levels of dose reductions were allowed for oxaliplatin and 5‐FU; no dose reduction was allowed for bevacizumab. Postoperative chemotherapy needed to begin within 28–56 days after surgery. The addition of bevacizumab as well as the duration of adjuvant therapy was designed to mirror ongoing studies of adjuvant bevacizumab in colon cancer.
Figure 1.
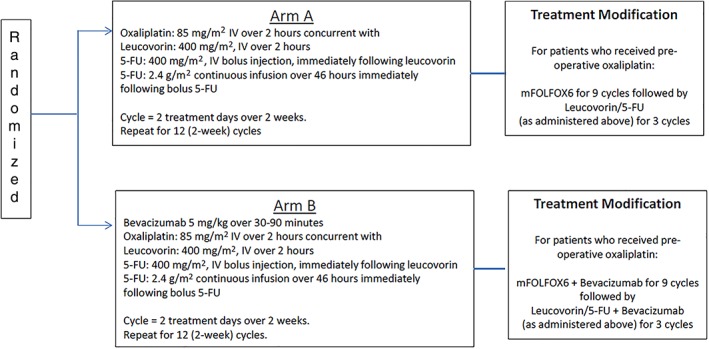
Treatment schema.Stratification factors: • Eastern Cooperative Oncology Group performance score (0 vs. 1) • Clinical stage (high risk vs. low risk) • Preoperative oxaliplatin (yes vs. no) • Preoperative radiation dose (40–50 Gy vs. 50–55.8 Gy)
Adverse events and dose modifications were defined using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 7. For both treatment arms, patient were to receive 12 cycles of protocol therapy unless the patient developed disease recurrence or unacceptable toxicity. All patients were followed for recurrence and for survival until 10 years from date of registration.
Endpoints
The primary endpoint of the trial was OS, defined as time from randomization to death from any cause. Secondary endpoints include disease‐free survival (DFS), adverse events, and health‐related quality of life (HRQoL). DFS was defined as time from randomization to recurrence, second cancer, or death, whichever came first.
Quality of Life
There were three HRQoL objectives in the study: (a) prospectively assess long‐term rectal function using the Bowel function/uniscale questionnaire and Functional Assessment of Cancer Therapy (FACT) Diarrhea subscale; (b) validate the FACT Diarrhea subscale; and (c) prospectively assess long‐term symptoms of oxaliplatin‐related neurotoxicity using the FACT/Gynecologic Oncology Group (GOG) Neurotoxicity (Ntx) scale (FACT/GOG‐Ntx). HRQoL endpoints were assessed at randomization, at end of treatment, 12 months after treatment, and then annually for 5 years after treatment. The primary endpoint for the quality of life (QOL) objective was the change in score from baseline to the assessments 12 months after chemotherapy between the two treatment groups. Rectal function was measured in patients with a functioning rectum using the Bowel Function Questionnaire and Functional Assessment of Cancer Therapy‐General (FACT‐G) 8. A key difference between the FACT Diarrhea Subscale and the Bowel Function Questionnaire is that the FACT Diarrhea Subscale uses a Likert scale, whereas the bowel function questionnaire uses a dichotomous scale for most questions. The total score for FACT Diarrhea is the sum of the scores for all 11 individual items (score range 0–44), and the total score for the Bowel Function Questionnaire is the number of problems with bowel function (score range 0–11).
Oxaliplatin‐related neurotoxicity was measured using the FACT/GOG‐Ntx‐13 subscale in all patients 9. Lower values of the FACT/GOG‐Ntx‐13 score indicate higher neurotoxicity (score range 0–44). Scale composite score was calculated if more than 50% of the items were answered.
Global quality of life was measured in patients with a functioning rectum using a Uniscale questionnaire, which was a single‐item score from 0 to 100. Higher scores indicate better QOL.
Statistical Analysis
The distributions for OS and DFS were estimated using the Kaplan‐Meier method 10, with 95% confidence intervals (CIs) calculated using Greenwood's formula. Stratified log‐rank tests and Cox proportional hazards model 11 were used to compare OS and DFS between the two groups. The incidence of treatment‐related grade 3 or higher adverse events (AEs) were summarized for each arm using the binomial proportion and exact 95% confidence intervals and compared between arms using Fisher's exact test. Descriptive statistics for QOL endpoints were summarized for each arm and compared between the two arms using two sample t test or Wilcoxon rank sum test.
The trial was designed to enroll 2,100 patients to have 85% power to detect a hazard ratio (HR) for Arm A/Arm B = 1.30 (5‐year OS of 62% vs. 69% under the assumption of exponential distribution of OS) using a stratified log‐rank test with overall one‐sided type I error rate of 0.025. Assuming an annual accrual rate of 600 patients, it would take 3.5 years for accrual and additional 2 years of follow‐up to reach the full information of 553 deaths. The trial was terminated before reaching its accrual goal, and no interim analysis was performed prior to termination. The significance level was set at one‐sided .025 for the primary endpoint of OS and two sided .05 for all other endpoints.
Results
From February 17, 2006, to April 29, 2009, 355 patients were enrolled. In April 2009, the ECOG Data and Safety Monitoring Committee closed the study as a result of low accrual rate. As of accrual closure, there were 65 (18.3%) patients still on protocol treatment, 36 (20.4%) on Arm A (FOLFOX) and 29 (16.2%) on Arm B (FOLFOX/bevacizumab). Patients have remained under follow‐up for recurrence and survival. This report is based on data available as of April 08, 2015. By then, 52 patients had died, and the median follow‐up time was 72.0 (range: 0.2–101.4) months for the 303 patients alive.
Of the 355 randomized patients, 7 (5 patients on Arm A and 2 patients on Arm B) were ineligible and 8 did not start treatment (3 patients on Arm A and 5 on Arm B). Figure 2 displays the Consolidated Standards of Reporting Trials (CONSORT) diagram.
Figure 2.
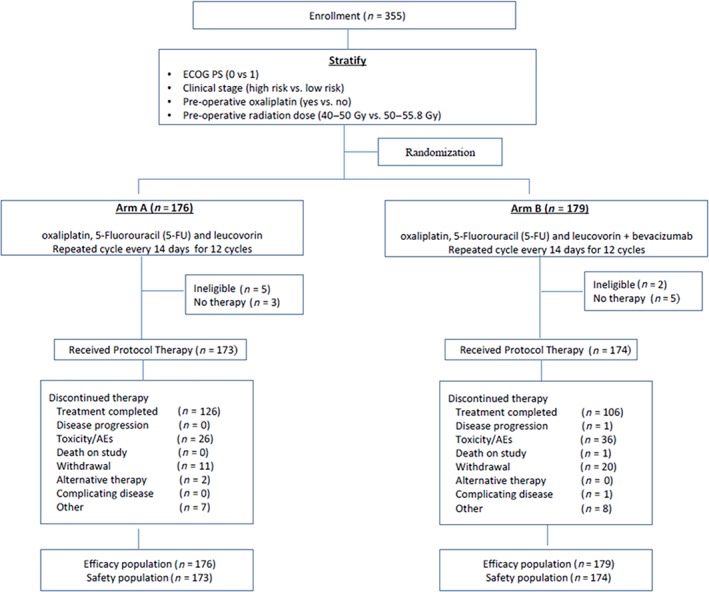
CONSORT diagram.Abbreviations: AE, adverse event; ECOG PS, Eastern Cooperative Oncology Group performance status.
Patient demographics and tumor characteristics were evenly distributed between the two treatment arms (Table 1). Prior neoadjuvant chemoradiation treatments were also well balanced between the two arms, with nearly 25% having received oxaliplatin as a component of treatment. The types of surgery—low anterior resection and abdominoperineal resection—were well balanced between the two groups. More than half of patients received continuous infusion 5‐FU as a component of neoadjuvant therapy, and 86% of patients received more than 50 Gy radiation preoperatively.
Table 1.
Baseline demographic and disease characteristics
| Variable | Arm A (n = 176) | Arm B (n = 179) | ||
|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |
|
Age at randomization (mean, SD), years |
54.3 | 11.7 | 53.9 | 9.9 |
| Gender | ||||
| Male | 114 | 64.8 | 112 | 62.6 |
| Female | 62 | 35.2 | 67 | 37.4 |
| Race | ||||
| White | 159 | 90.9 | 161 | 91.5 |
| Black | 5 | 2.9 | 5 | 2.8 |
| Other | 11 | 6.3 | 10 | 5.7 |
| Ethnicity | ||||
| Hispanic | 8 | 4.6 | 20 | 11.2 |
| Non‐Hispanic | 160 | 90.9 | 146 | 81.6 |
| Unknown | 8 | 4.6 | 13 | 7.2 |
| ECOG PS | ||||
| 0 | 106 | 60.2 | 109 | 60.9 |
| 1 | 70 | 39.8 | 70 | 39.1 |
| Clinical bowel obstruction | ||||
| No | 154 | 87.5 | 165 | 92.2 |
| Yes | 22 | 12.5 | 14 | 7.8 |
| Bowel perforation | ||||
| No | 171 | 99.4 | 173 | 98.3 |
| Yes | 1 | 0.6 | 3 | 1.7 |
| Clinical T stage | ||||
| 1 | 1 | 0.6 | 0 | 0 |
| 2 | 7 | 4 | 13 | 7.4 |
| 3 | 161 | 91.5 | 159 | 89.8 |
| 4 | 7 | 4 | 5 | 2.8 |
| Clinical N stage | ||||
| 0 | 72 | 40.9 | 66 | 37.1 |
| 1 | 92 | 52.3 | 98 | 55.1 |
| 2 | 12 | 6.8 | 14 | 7.9 |
| Histologic grade | ||||
| Well (grade 1) | 20 | 12.5 | 31 | 18.8 |
| Moderate (grade 2) | 122 | 76.3 | 111 | 67.3 |
| Poor (grade 3) | 17 | 10.6 | 22 | 13.3 |
| Undifferentiated (grade 4) | 1 | 0.6 | 1 | 0.6 |
| Prior chemotherapy | ||||
| Prior oxaliplatin therapy | 45 | 26 | 43 | 25 |
| Prior capecitabine therapy | 71 | 41.5 | 44 | 26 |
| Prior continuous 5‐FU infusion | 95 | 54.9 | 119 | 69.6 |
| Prior 5‐FU and leucovorin | 8 | 4.7 | 7 | 4.2 |
|
Prior RT total dose, mean, SD |
50.5 | 2.8 | 50.4 | 2.7 |
| Prior RT total dose, median, range | 50.4 | 43.2–55.8 | 50.4 |
41.4– 55.8 |
| Primary surgery type | ||||
| Low anterior resection | 63 | 35.8 | 71 | 39.7 |
| Abdominal perineal resection | 59 | 33.5 | 60 | 33.5 |
| Low anterior resection/coloanal anastomosis | 43 | 24.4 | 38 | 21.2 |
| Other | 11 | 6.3 | 10 | 5.6 |
| Complete resection | ||||
| No | 6 | 3.4 | 4 | 2.2 |
| Yes | 170 | 96.6 | 175 | 97.8 |
| Ostomy created | ||||
| No | 35 | 20.2 | 29 | 16.9 |
| Yes, colostomy | 71 | 41 | 73 | 42.4 |
| Yes, ileostomy | 67 | 38.7 | 70 | 40.7 |
| Sphincter preserved | ||||
| No | 62 | 35.4 | 65 | 36.7 |
| Yes | 113 | 64.6 | 112 | 63.3 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; RT, radiation therapy.
Treatment Tolerance
Overall, 67% of patients (232/347, 95% CI: 61.6–71.8) completed the 12 cycles of therapy and 35% completed all 12 cycles of oxaliplatin (121/347, 95% CI: 29.9–40.1). Of the 174 patients on Arm B, 58% (101/174, 95% CI: 50.3–65.5) completed all 12 cycles of bevacizumab. Reasons for therapy discontinuation are displayed in Figure 2. There was a higher proportion of patients who discontinued protocol therapy because of AEs or patient withdrawal in Arm B, (21.5% vs. 32.4%, Fisher's exact p = .029).
Treatment‐Related Adverse Events
Table 2 summarizes the number of patients with grade 3 or higher adverse events. Overall, the incidence of grade 3 or higher toxicities were similar in Arm A 68.8% (119/173, 95% CI: 61.3–75.6) and Arm B 70.7% (123/174, 95% CI: 63.3–77.3). The most common treatment‐related grade 3 or 4 toxicities included neutropenia, leukopenia, neuropathy, diarrhea, and fatigue, which were equally distributed in both arms. Of the two deaths during treatment, both occurred on Arm B. One involved a patient with lower gastrointestinal hemorrhage and was deemed treatment related. The other death involved a patient with known cardiac disease who died of myocardial ischemia and was thought unlikely to be related to the treatment.
Table 2.
Grade 3 and 4 treatment‐related adverse events by treatment arm
| Adverse event | Arm A (n = 173), % | Arm B (n = 174), % | ||
|---|---|---|---|---|
| 3 | 4 | 3 | 4 | |
| Allergic reaction | 1 | — | 2 | 1 |
| Hemoglobin | 1 | — | 1 | — |
| Leukocytes | 27 | 1 | 21 | 1 |
| Lymphopenia | 9 | — | 5 | 1 |
| Neutrophils | 28 | 8 | 25 | 5 |
| Platelets | 6 | 1 | 1 | — |
| Cardiac‐ischemia | — | 1 | 1 | 1 |
| Hypertension | — | — | 6 | 1 |
| Cardiomyopathy, restrictive | — | — | 1 | — |
| Fatigue | 7 | — | 11 | — |
| Fever without neutropenia | 1 | 1 | — | — |
| Weight gain | 1 | — | — | — |
| Weight loss | 1 | — | — | — |
| INR | — | — | 1 | — |
| Hand–foot reaction | — | — | 3 | — |
| Wound—noninfectious | — | — | 1 | — |
| Anorexia | 2 | — | 3 | — |
| Dehydration | 4 | — | 5 | — |
| Diarrhea without prior colostomy | 9 | — | 11 | — |
| Dysphagia | — | — | 1 | — |
| Enteritis | 1 | — | — | — |
| Fistula, colon/cecum/appendix | — | — | 1 | — |
| Fistula, rectum | — | — | 1 | 1 |
| Dyspepsia | — | — | 1 | — |
| Ileus | 1 | — | 1 | — |
| Leak, including anastomotic, rectum | — | — | 1 | 1 |
| Muco/stomatitis oral cavity | 1 | — | 2 | — |
| Nausea | 3 | 1 | 3 | — |
| Obstruction, small bowel | 1 | — | 1 | — |
| Proctitis | 1 | — | — | — |
| Vomiting | 3 | 1 | — | 1 |
| GI—other | — | — | 1 | — |
| Lower GI, hemorrhage | 2 | — | 1 | — |
| Febrile neutropenia | 1 | — | 2 | — |
| Infection with grade 3–4 neutropenia | 1 | — | 2 | 1 |
| Infection with grade 0–2 neutropenia | 1 | — | 5 | — |
| Infection with unknown ANC | 1 | — | 2 | 1 |
| LFTs (AST, ALT) | — | — | 2 | — |
| Hypocalcemia | — | 1 | 1 | — |
| Creatinine | — | — | 1 | — |
| Hyperglycemia | 2 | — | 2 | — |
| Hypophosphatemia | 1 | — | — | — |
| Hypokalemia | 2 | — | 2 | — |
| Proteinuria | — | — | 1 | — |
| Hyponatremia | 1 | — | 2 | 1 |
| Fracture | 1 | — | — | — |
| Avascular necrosis | — | — | 1 | — |
| Ataxia/dizziness | — | — | 2 | — |
| Laryngeal nerve dysfunction | 1 | — | — | — |
| Leukoencephalopathy | — | — | 1 | — |
| Depression | — | — | — | 1 |
| Neuropathy—sensory/motor | 16 | — | 19 | — |
| Syncope | 1 | — | 1 | — |
| Neurologic—other | — | — | — | 1 |
| Tearing | — | — | 1 | — |
| Ocular—other | — | — | 1 | — |
| Abdomen, pain | 1 | — | 2 | — |
| Pain, musculoskeletal | — | 1 | 3 | — |
| Gallbladder, pain | 1 | — | — | — |
| Head/headache | 1 | — | 1 | — |
| Pain, musculoskeletal | 3 | 1 | 7 | — |
| Dyspnea | 1 | — | 3 | 1 |
| Hypoxia | — | — | 1 | — |
| Prolonged intubation post pulmonary resection | — | — | — | 1 |
| Voice changes/dysarthria | — | — | 1 | — |
| Pulmonary/upper respiratory | — | — | — | 1 |
| Incontinence urinary | — | — | 1 | — |
| Obstruction—ureteral | — | — | 1 | — |
| Renal failure | — | — | 1 | — |
| Urinary frequency/urgency | — | — | 1 | — |
| Urinary retention | — | — | 1 | — |
| Secondary malignancy | — | 1 | — | — |
| Thrombosis/embolism | 3 | 1 | 2 | 4 |
| WORST DEGREE | 55 | 13 | 57 | 13 |
Abbreviations: ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; GI, gastrointestinal; INR, international normalized ratio; LFTs, liver function tests.
A total of 19 patients developed second primary cancers during the study period, including two patients with acute nonlymphocytic leukemia and one with myelodysplastic syndrome.
Survival
At a median follow‐up of 72 months, 70 patients had experienced disease recurrence, 16 patients had developed second invasive primary cancer, and 52 patients had died. In total, 52 OS events (23 on Arm A and 29 on Arm B) and 93 DFS events (52 on Arm A and 41 on Arm B) had occurred. There was no significant difference in 5‐year OS, which was 88.3% (95% CI: 82.3–92.4) in Arm A and 83.7% (95% CI: 77.1–88.6) in Arm B (Fig. 3). There was also no significant difference in 5‐year DFS rate, which was 71.2% (95% CI: 63.4–77.6) in Arm A and 76.5% (95% CI: 69.2–82.3) in Arm B (Fig. 4). When patients were evaluated by pretreatment clinical stage into low‐risk (T1‐3, node negative) or high‐risk (T4 and/or node positive) disease, there was no difference in 5‐year OS or DFS. On the other hand, patients who achieved a complete pathologic response had a significant improvement in both 5‐year OS (p < .001) and DFS (p < .001).
Figure 3.
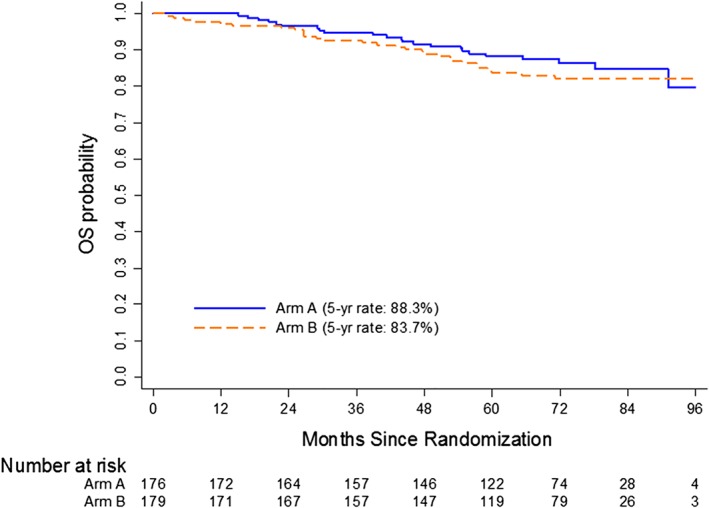
Kaplan‐Meier estimates of overall survival.Abbreviation: OS, overall survival.
Figure 4.
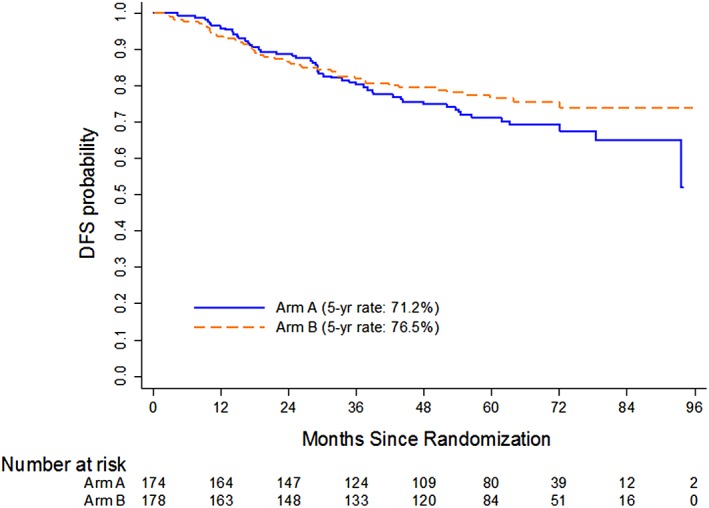
Kaplan‐Meier estimates of disease‐free survival.
Bowel Function
Of the 355 patients who were enrolled on study, colostomy was created for 144 patients and ileostomy was created for 137 patients. No ostomy was created for 74 patients. A total of 127 patients submitted Bowel Function Questionnaire at one or more time points, 59 at baseline and 76 at 12 months' visit. In total, 32 patients (15 on Arm A and 17 on Arm B) reported Bowel Function Questionnaire data at both baseline and 12 months after chemotherapy, and 23 of them reported all 11 items in the form. Overall, the number of reported problems with bowel function slightly increased over time on both arms, but there was no statistically significant difference between the two arms.
For the FACT Diarrhea subscale, a total of 129 patients reported data at one or more time points, 58 patients at baseline and 74 patients at 12 months' visit. In total, 30 patients (14 on Arm A and 16 on Arm B) reported FACT Diarrhea data at both baseline and 12‐month time points. On both arms, FACT Diarrhea score slightly reduced between randomization and 12 months after chemotherapy (i.e., increase in diarrhea), but there was no statistically significant difference between the two arms in the score change.
Oxaliplatin‐Related Neurotoxicity
A total of 214 patients submitted FACT/GOG‐Ntx form at one or more time points, 199 patients at baseline and 119 patients at 12 months' visit. In total, 116 patients (53 on Arm A and 63 on Arm B) reported FACT/GOG‐Ntx data at both baseline and 12‐month time points. Of the 116 patients, 115 of them answered at least six question items at both visits, and summary score for FACT/GOG‐Ntx was calculated for them. On both arms, FACT/GOG‐Ntx score reduced between baseline and 12 months after chemotherapy (i.e., more severe neurotoxicity), and there was no statistically significant difference between the two arms.
Overall Quality of Life
A total of 127 patients answered the Uniscale question at one or more time points. In both arms, patients’ QOL worsened slightly after starting protocol therapy, improved after end of treatment, and then remained stable 1 year after treatment. There was no statistically significant difference between the two arms in QOL.
Discussion
Several phase III studies had shown an improvement in survival with the addition of bevacizumab to fluoropyrimidine‐oxaliplatin or irinotecan‐based chemotherapy regimens in the patients with metastatic colorectal cancer. Despite these encouraging findings in the metastatic setting, our study found that this treatment paradigm was not successful in the adjuvant treatment of rectal cancer. Drug development in oncology has traditionally relied on first establishing efficacy in the metastatic setting and then moving these drugs to the adjuvant setting. The only exception was irinotecan, where three randomized trials showed no improvement in DFS or OS in the adjuvant setting 12, 13, 14 despite positive results in the metastatic setting 5. Unfortunately, this strategy has not held up in the era of molecularly targeted therapies such as for antibodies against VEGF and epidermal growth factor receptor (cetuximab) 15, 16.
These seemingly contradictory findings of benefit in the metastatic setting but no benefit in the adjuvant setting were also seen in the adjuvant treatment of colon cancer. The NSABP C‐08 trial combined bevacizumab with a modified FOLFOX6 regimen for 6 months and continued thereafter as monotherapy for another 6 months. The trial did not meet its endpoint of prolonging DFS after 3 years 17. A second study—the Adjuvant FOLFOX4 Versus Bevacizumab and FOLFOX4 Versus Bevacizumab, Oxaliplatin, and Capecitabine in Patients With High‐Risk Stage II or Stage III Colon Cancer (AVANT) trial—was designed to evaluate a similar question in nearly 3,500 patients 18. This study showed that bevacizumab not only did not prolong disease‐free survival when added to adjuvant chemotherapy in resected stage III colon cancer, it resulted in a potential detrimental effect with bevacizumab plus oxaliplatin‐based adjuvant therapy in these patients 18. In both of these studies, there was a decrease in early relapse in the first 12 months of treatment but an excess of relapses when the drug was stopped. In a third trial (QUASAR2; n = 1,941), there was no benefit to adding bevacizumab to capecitabine for the adjuvant treatment of stage III (62%) and high‐risk stage II colon cancers 19. Although the final results of ongoing studies of adjuvant bevacizumab were not reported until later, the lack of benefit to bevacizumab in the adjuvant setting in the treatment of colon cancer trials (NSABP C‐08, AVANT) was being discussed, which may have resulted in the slow accrual that led to the early closure of this trial.
This suggests that despite their similarities on routine pathologic evaluation, primary tumors and metastatic lesions may respond to different oncogenic drivers and thereby different targeted agents. Phases of tumor development may also result in changes in the tumor microenvironment and vasculature, which could lead to varying responses to the same targeted agent.
Although our study evaluated an aggressive treatment regimen with more than two thirds of patients having grade 3 side effects, these were equally distributed between the two arms, suggesting that the addition of bevacizumab was well tolerated. The longitudinal analysis of quality of life measures show that symptoms in both treatment groups worsen after adjuvant chemoradiation but then plateaued. There was no difference between quality of life metrics between those who did or did not receive bevacizumab. These results can be used to counsel patients on what to expect with regard to their quality of life and symptoms during long‐term follow‐up. Similar rates of grade 3 or greater toxicities were also seen in adjuvant trials of bevacizumab in colon cancer. Grade 3–5 adverse events occurred in 73% in the FOLFOX4 arm and 76% in the bevacizumab‐FOLFOX4 18. In another large adjuvant colon cancer trial, the overall rates of grade 4 or 5 toxicities were also found to be nearly identical in the FOLFOX6 and FOLFOX6 plus bevacizumab arms 20. Taken together, these studies have established FOLFOX as the adjuvant therapy in cancers of the colon and rectum.
Conclusion
Many large trials have been focusing on the neoadjuvant component of treatment using the short‐term surrogate of pathologic response rates to measure outcome. Therefore, the adjuvant therapy has been highly variable. This study was an effort to obtain more data to inform the use of adjuvant therapy following neoadjuvant therapy and to evaluate the long term sequelae of this approach. Comprehensive strategies aimed at improving both acute toxicity and persisting late side effects of treatment should continue to be explored. The total neoadjuvant approach results in higher complete clinical response rates, and therefore, nonoperative treatment strategies such as “watchful waiting,” which allows for sphincter preservation, can also be tested 21. The use of short‐course radiation (5 × 5 Gy) followed by delayed surgery is another novel method of reducing acute toxicities 22.
Author Contributions
Conception/design: Neal J. Meropol, Patrick J. Flynn, Lynne I. Wagner, Jeffrey Sloan, Robert B. Diasio, Edith P. Mitchell, Paul Catalano, Bruce J. Giantonio, Robert B. Catalano, Daniel G. Haller, Rashid A. Awan, Mary F. Mulcahy, Timothy E. O'Brien, Roger Santala, Christine Cripps, John R. Weis, James N. Atkins, Cynthia G. Leichman, Nicholas J. Petrelli, Frank A. Sinicrope, James D. Brierley, Joel E. Tepper, Peter J. O'Dwyer, Elin R. Sigurdson, Stanley R. Hamilton, David Cella, Al B. Benson, III
Provision of study material or patients: Neal J. Meropol, Patrick J. Flynn, Robert B. Diasio, Edith P. Mitchell, Bruce J. Giantonio, Robert B. Catalano, Daniel G. Haller, Rashid A. Awan, Mary F. Mulcahy, Timothy E. O'Brien, Roger Santala, Christine Cripps, John R. Weis
James N. Atkins, Cynthia G. Leichman, Nicholas J. Petrelli, Frank A. Sinicrope, James D. Brierley, Joel E. Tepper, Peter J. O'Dwyer, Elin R. Sigurdson, Stanley R. Hamilton, Al B. Benson, III
Collection and/or assembly of data: Fengmin Zhao, Paul Catalano
Data analysis and interpretation: Fengmin Zhao, Paul Catalano
Manuscript writing: A. Bapsi Chakravarthy, Fengmin Zhao, Neal J. Meropol, Patrick J. Flynn, Lynne I. Wagner, Jeffrey Sloan, Robert B. Diasio, Edith P. Mitchell, Paul Catalano, Bruce J. Giantonio, Robert B. Catalano, Daniel G. Haller, Rashid A. Awan, Mary F. Mulcahy, Timothy E. O'Brien, Roger Santala, Christine Cripps, John R. Weis, James N. Atkins, Cynthia G. Leichman, Nicholas J. Petrelli, Frank A. Sinicrope, James D. Brierley, Joel E. Tepper, Peter J. O'Dwyer, Elin R. Sigurdson, Stanley R. Hamilton, David Cella, Al B. Benson, III
Final approval of manuscript: A. Bapsi Chakravarthy, Fengmin Zhao, Neal J. Meropol, Patrick J. Flynn, Lynne I. Wagner, Jeffrey Sloan, Robert B. Diasio, Edith P. Mitchell, Paul Catalano, Bruce J. Giantonio, Robert B. Catalano, Daniel G. Haller, Rashid A. Awan, Mary F. Mulcahy, Timothy E. O'Brien, Roger Santala, Christine Cripps, John R. Weis, James N. Atkins, Cynthia G. Leichman, Nicholas J. Petrelli, Frank A. Sinicrope, James D. Brierley, Joel E. Tepper, Peter J. O'Dwyer, Elin R. Sigurdson, Stanley R. Hamilton, David Cella, Al B. Benson, III
Disclosures
Neal J. Meropol: Flatiron Health (E), Flatiron Health, Roche (OI); Lynn I. Wagner: Celgene Inc. (C/A); Frank A. Sinicrope: Guardent Health, Roche/Ventana Medical Systems (C/A); Peter J. O'Dwyer: Genentech (C/A, RF); Stanley R. Hamilton: Bristol‐Myers Squibb, Guardant, LOXO‐Oncology (C/A), Merck & Co, Inc., Fred Hutchinson Cancer Research Center, Centers for Medicare and Medicaid Services, Halio DX, Thermo‐Fisher Scientific (SAB), MD Anderson Cancer Center (E); Al B. Benson, III: Bristol‐Myers Squibb, Guardant Health, Eli Lilly & Company, Exelixis, Purdue Pharma, inVentive Health Inc., Axio, Genentech, Bayer, Merck, Rafael Pharmaceuticals, Terumo, Taiho Pharmaceutical, TheraBionic, LSK, Incyte Corporation (C/A), Acerta, Celgene, Advanced Accelerator Applications, Novartis, Merck Sharp & Dohme, Taiho Pharmaceutical, Bristol‐Myers Squibb, Medimmune/AstraZeneca (RF), Astellas, Axio, Infinity Pharmaceuticals, Bristol‐Myers Squibb, Xencor, PreECOG, Amgen, ECOG‐ACRIN (Data Monitoring Committee). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was coordinated by the ECOG‐ACRIN Cancer Research Group (Peter J. O'Dwyer, M.D. and Mitchell D. Schnall, M.D., Ph.D., Group Co‐Chairs) and included participation from Alliance for Clinical Trials in Oncology, NRG Oncology, SWOG Cancer Research Network, Canadian Cancer Trials Group. It was supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA180794, CA180863, CA180888, CA180790, CA189863, CA180821,CA180838, CA180844, CA180847, CA180853, CA180818, CA180867, CA189872, CA189867, CA180868,CA189819, CA189828, CA189858, CA180858, CA180863, and the Canadian Cancer Society 704970. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. Oxaliplatin was provided by Sanofi‐Aventis Pharmaceuticals
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Sauer R, Liersch T, Merkel S et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO‐94 randomized phase III trial after a median follow‐up of 11 years. J Clin Oncol 2012;30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 2. André T, Boni C, Mounedji‐Boudiaf L et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–2351. [DOI] [PubMed] [Google Scholar]
- 3. Kuebler JP, Wieand HS, O'Connell MJ et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C‐07. J Clin Oncol 2007;25:2198–2204. [DOI] [PubMed] [Google Scholar]
- 4. Benson AB, Catalano P, Meropol NJ et al. ECOG E3201: Intergroup randomized phase III study of postoperative irinotecan, 5‐ fluorouracil (FU), leucovorin (LV) (FOLFIRI) vs oxaliplatin, FU/LV (FOLFOX) vs FU/LV for patients (pts) with stage II/ III rectal cancer receiving either pre or postoperative radiation (RT)/ FU. J Clin Oncol 2006;24(suppl 18):3526. [Google Scholar]
- 5. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 6. Willett CG, Boucher Y, di Tomaso E et al. Direct evidence that the VEGF‐specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004;10:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) . 2006. Available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 8, 2015.
- 8. Ward WL, Hahn EA, Mo F et al. Reliability and validity of the Functional Assessment of Cancer Therapy‐Colorectal (FACT‐C) quality of life instrument. Qual Life Res 1999;8:181–195. [DOI] [PubMed] [Google Scholar]
- 9. Kopec J, Land S, Cecchini R et al. Validation of a self‐reported neurotoxicity scale in patients with operable colon cancer receiving oxaliplatin. J Support Oncol 2006;4:W1–W8. [Google Scholar]
- 10. Kaplan E, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 11. Cox D. Regression models and life tables (with discussion). J Royal Stat Soc B 1972;34:187–220. [Google Scholar]
- 12. Van Cutsem E, Labianca R, Bodoky G et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC‐3. J Clin Oncol 2009;27:3117–3125. [DOI] [PubMed] [Google Scholar]
- 13. Saltz LB, Niedzwiecki D, Hollis D et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 2007;25:3456–3461. [DOI] [PubMed] [Google Scholar]
- 14. Ychou M, Raoul JL, Douillard JY et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high‐risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009;20:674–680. [DOI] [PubMed] [Google Scholar]
- 15. Van Cutsem E, Köhne CH, Hitre E et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–1417. [DOI] [PubMed] [Google Scholar]
- 16. Alberts SR, Sargent DJ, Nair S et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA 2012;307:1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allegra CJ, Yothers G, O'Connell MJ et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C‐08. J Clin Oncol 2011;29:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Gramont A, Van Cutsem E, Schmoll HJ et al. Bevacizumab plus oxaliplatin‐based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225–1233. [DOI] [PubMed] [Google Scholar]
- 19. Kerr RS, Love S, Segelov E et al. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): An open‐label, randomised phase 3 trial. Lancet Oncol 2016;17:1543–1557. [DOI] [PubMed] [Google Scholar]
- 20. Allegra CJ, Yothers G, O'Connell MJ et al. Initial safety report of NSABP C‐08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol 2009;27:3385–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Valk MJM, Hilling DE, Bastiaannet E et al. Long‐term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018;391:2537–2545. [DOI] [PubMed] [Google Scholar]
- 22. Erlandsson J, Holm T, Pettersson D et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non‐blinded, phase 3, non‐inferiority trial. Lancet Oncol 2017;18:336–346. [DOI] [PubMed] [Google Scholar]


