Abstract
The pancreas has physiologically important endocrine and exocrine functions; secreting enzymes into the small intestine to aid digestion and releasing multiple peptide hormones via the islets of Langerhans to regulate glucose metabolism, respectively. Insulin and glucagon, in combination with ghrelin, pancreatic polypeptide and somatostatin, are the main classical islet peptides critical for the maintenance of blood glucose. However, pancreatic islets also synthesis numerous ‘nonclassical’ peptides that have recently been demonstrated to exert fundamental effects on overall islet function and metabolism. As such, insights into the physiological relevance of these nonclassical peptides have shown impact on glucose metabolism, insulin action, cell survival, weight loss, and energy expenditure. This review will focus on the role of individual nonclassical islet peptides to stimulate pancreatic islet secretions as well as regulate metabolism. In addition, the more recognised actions of these peptides on satiety and energy regulation will also be considered. Furthermore, recent advances in the field of peptide therapeutics and obesity-diabetes have focused on the benefits of simultaneously targeting several hormone receptor signalling cascades. The potential for nonclassical islet hormones within such combinational approaches will also be discussed.
Keywords: β-cell, insulin secretion, nonclassical islet peptides, dual agonist, triagonist
Introduction
Metabolic abnormality can occur due to a range of genetic or acquired disorders including diabetes and obesity.1 These disorders involve a disturbance of metabolomics involved in energy balance regulation.2 Unfortunately, the prevalence of obesity and related type 2 diabetes mellitus (T2DM) is consistently increasing.3 The underlying pathophysiological feature of T2DM, and other related forms of diabetes, is primarily associated with a loss in function and/or mass of insulin secreting beta-cells (β-cells) of the endocrine pancreas.4 The pancreas is situated behind the peritoneum and consists of a head, body, and tail that is close to the duodenum. Most pancreatic mass (90%) is made of exocrine cells grouped in lobules and separated by connective tissue.5 The product of exocrine cells, an alkaline liquid concentrated with digestive enzymes, is drained via a pancreatic duct and enters the small intestine to aid digestion.6 However, throughout the exocrine tissue clusters of endocrine cells called ‘islets of Langerhans’ exist, these islets are known to play a vital role in controlling metabolism, and especially blood glucose equilibrium (Figure 1).7 The main hormones secreted from pancreatic islet cells are glucagon from alpha (α) cells, insulin from β-cells, somatostatin from delta (Δ) cells and pancreatic polypeptide (PP), with release believed to be under strict endocrine, paracrine, and neuronal control.8 However, several nonclassical islet peptides have also been described and shown to possess similarly important effects on islet function (Table 1). This is epitomised by recent acceptance of ghrelin as an islet-derived peptide secreted from epsilon (ε) cells with established intra-islet actions,9 as well as effects on metabolism beyond the pancreas.10 As such, although much research on pancreatic islets and T2DM treatments has focused on the classic peptide hormones,11 the role and therapeutic applicability of nonclassic islets peptide hormones also needs to be considered.
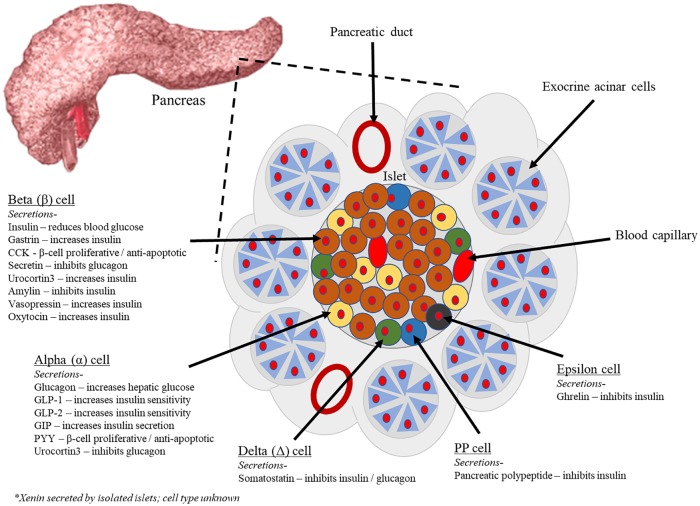
Figure 1.
Endocrine pancreatic cell types and their peptide secretions. Exocrine pancreatic acinar cells constitute most of the pancreatic tissue, these cells produce digestive enzymes which are transported via the pancreatic ducts. The endocrine pancreas is illustrated with all cell types; alpha, beta, delta, PP, and epsilon. The cells are arranged in compact islets and secrete a number of classical and ‘nonclassical’ peptides, as depicted. PP indicates pancreatic polypeptide.
Table 1.
Classical and ‘nonclassical’ peptides secreted by the pancreas. Table includes the peptides main origin tissue (including gut and CNS) and cells of origin. The key secretory initiators are stated and the effect each peptide has on the pancreatic islet.
| Peptide | Origin | Regulation of secretion | Effects on Islet | |
|---|---|---|---|---|
| Classical | Tissue | Cell | ||
| Insulin | Pancreas | β-cell | Plasma glucose, FFA, glucagon | Inhibits glucagon |
| Glucagon | Pancreas | α-cell | Amino acids released by digestion | Stimulates insulin secretion |
| Somatostatin | Pancreas | Δ-cell | Glucagon | Inhibits release of insulin and glucagon |
| Pancreatic polypeptide | Pancreas | PP-cell | To be determined | Inhibits insulin |
| Ghrelin | Pancreas | epsilon cell | Dietary intake of nutrients | Inhibits insulin |
| Nonclassical | ||||
| GLP-1 | Pancreas/Gut | α-cell/Intestinal L-cell | Ingested glucose load | Increases insulin secretion, β-cell mass, inhibits glucagon, apoptosis |
| GLP-2 | Pancreas/Gut | α-cell/L-cell | Dietary intake of nutrients | Positive effects on β-cell proliferation and apoptosis |
| GIP | Pancreas/Gut | α-cell/Intestinal K-cell | Ingested glucose load | Causes insulin secretion, and β-cell proliferation |
| PYY | Pancreas/Gut | α-cell/Intestinal L-cell | Dietary intake of nutrients | β-cell proliferative and anti-apoptotic effects |
| Gastrin | Pancreas/Gut | β-cell/G-cell | Stomach distension, digested proteins | Causes insulin secretion |
| CCK | Pancreas/Gut | β-cell/EEC cell | Stomach distension, digested proteins | β-cell proliferative and anti-apoptotic effects |
| Xenin-25 | Pancreas/Gut | Islets/K-cell | Dietary intake of nutrients | Increases GIP-mediated insulin secretion |
| Secretin | Pancreas/Gut | β-cell/S-cells | Dietary intake of nutrients/Stomach acid | Inhibits glucagon |
| Urocortin3 | Pancreas | α-cell/β-cell | Plasma glucose (co-secreted with insulin) | Promotes insulin secretion |
| Amylin | Pancreas | β-cell | Dietary intake of nutrients | Inhibits insulin secretion and β-cell proliferation |
| Vasopressin | Pancreas/CNS | β-cell/M-cell | Response to hypertonicity | Promotes Insulin secretion, β-cell proliferative and anti-apoptotic effects |
| Oxytocin | Pancreas/CNS | β-cell/M-cell | Related to reproductive function | Promotes insulin secretion, β-cell proliferative and anti-apoptotic effects |
Abbreviations: CCK, cholecystokinin; CNS, central nervous system; EEC, enteroendocrine cells; FFA, free fatty acid; GIP, glucose dependent insulinotropic peptide; GLP, glucagon-like peptide; PYY, Peptide Tyrosine Tyrosine.
Classical Islet Peptides
As noted above, the endocrine pancreas secretes 5 peptide hormones, namely, glucagon, insulin, somatostatin, PP, and ghrelin, from specific cell types, that play a critical role in glucose homeostasis and energy metabolism8 (Table 1). A secretory interplay between glucagon and insulin balances blood glucose in healthy individuals.12 Glucagon is released in times of low glucose to promote hepatic glycogenolysis,13 whereas insulin is released in response to raised glucose to promote glycogenesis and lipogenesis.14 The underlying molecular processes of insulin exocytosis in response to glucose is well known and involves the closure of KATP channels, β-cell membrane depolarisation, calcium influx, and subsequent secretion of insulin.15 During the onset of diabetes this relationship is negatively affected and insulin secretion rates decrease,16 this is often combined with desensitisation of the insulin receptor (IR) located on peripheral tissues.17 As such, treatment of diabetes has focused predominantly on addressing the deficit in insulin secretion through administration of direct insulin secretagogues or insulin replacement therapy,18 as well as therapies aimed at resensitising the IR. In this regard, there are a variety of nonclassical islet peptides that can trigger or potentiate insulin secretion, improve β-cell survival or increase insulin sensitivity through modulation of G-protein coupled receptors (GPCRs)8 (Figure 1).
Nonclassical Islet Peptides – Pancreatic Effects
Incretin and related peptides
As well as the classical islet peptides, there are a number of other peptide hormones known to be synthesised and released within pancreatic islets, often termed ‘nonclassical’ islet peptides8 (Figure 1). The incretin hormones, namely glucagon-like peptide (GLP)-1 and glucose dependent insulinotropic peptide (GIP), are the most comprehensively studied nonclassical islet peptides.19 Typically, GLP-1 and GIP are considered to be endocrine L- and K-cell derived peptides released in response to nutrient ingestion.20 Elegant studies have described the secretory dynamics of these 2 hormones from the gut.21 However, both GIP and GLP-1 have also been shown to be synthesised and released locally within pancreatic islets, with important paracrine effects.22 Glucagon-like peptide-1 has direct and indirect actions on α-cells, directly inhibiting glucagon23 and indirectly increasing intra-islet regulation via somatostatin,24 GIP effects on glucagon are glucose dependent. As such, at low levels of glycaemia, glucagon release is increased by GIP with minimal effect on insulin, with this glucagonotropic effect disappearing at higher glucose levels.25 Mechanistically, GLP-1 and GIP potentiate insulin secretion by binding to specific GPCRs located on pancreatic β-cells to increase cAMP26 and activate Epac and protein kinase A (PKA).20 The process of GIP and GLP-1 induced insulin secretion is well described, primarily due to increased interest in the ‘incretin effect’ over the past decades, clearly defining how the intestinally released hormones potentiate insulin release following meals.27 These mechanisms supplement glucose-mediated effects, closing K+ATP and Kv channels and promoting depolarisation of the β-cell. Calcium channels are also sensitised via PKA and Epac.20 Together this facilitates intracellular calcium stores to be released, resulting in insulin secretion. Glucagon-like peptide-2 (GLP-2) is another proglucagon derived peptide, its principle role is to maintain intestinal mucosal villus epithelium and aid gut absorption.28 In addition, GLP-2 acts as an intestinal growth factor, with clinical application for short bowel syndrome.29 Glucagon-like peptide-2 receptors are expressed on rodent α-cells 30 and linked to increased glucagon secretion.31 Notably, GLP-2 also has been demonstrated to possess proliferative and anti-apoptotic effects in β-cells,32 highlighting a role in glucose homeostasis. However, GLP-2 has no effect on insulin secretion from β-cells, or isolated mouse islets.31
Peptide Tyrosine Tyrosine
Peptide Tyrosine Tyrosine (PYY) is a 36 amino acid peptide hormone best known as being released postprandially from enteroendocrine L-cells, similar to GLP-1.33 Moreover, both hormones are expressed within pancreatic islets and released locally (Figure 1).34 Peptide Tyrosine Tyrosine and neuropeptide Y (NPY) both activate neuropeptide Y receptors (NPYRs) and represent promising antidiabetes targets.34 Although the peptides activate the same family of receptors, their effects on islet secretions differ due to differences in receptor affinity and specificity. Early rodent studies indicated plasma insulin levels were reduced or unaffected by PYY.35 Further studies in isolated islets and human β-cells also revealed minimal effect of PYY on insulin secretion, although β-cell anti-apoptotic and proliferative effects were noted.36 As such, transgenic mouse models overexpressing PYY exhibited enhanced glucose facilitated insulin responses and amplified islet number.37 Consistent with these findings, knock out of PYY in the gut and pancreas causes disruption of islet morphology and decreased β-cell mass.38 In addition, streptozotocin-induced insulin deficiency and β-cell destruction is linked to reduced islet PYY levels, whereas hydrocortisone-induced increases of β-cell mass are linked to elevated PYY levels.36 Furthermore, in the developing foetal pancreas PYY is detectable at a very early stage,39 indicating potential effects on pancreatic islet development. For PYY effects, it is also important to note the distinct receptor specificity of the 2 main circulating molecules and pancreatic expression of these receptors. As such, PYY (1-36) binds and activates all NPYRs,40 whereas PYY (3-36) is an NPYR2-specific molecule. PYY can prevent glucagon release from isolated islets, although NPY receptors are thought to be specifically on β-cells.41 This would suggest PYY modulation of glucagon occurs via indirect pathways and could involve insulin or somatostatin release.42 The ability of PYY to restore deranged insulin and glucagon secretions in T2DM, is of clinical significance and these findings justify further investigation.43 Similar to PYY, the neurotransmitter NPY has also been linked to decreased insulin and glucagon release.44,45
Gastrin and cholecystokinin
Receptors for gastrin and cholecystokinin (CCK), namely CCK1 receptors, are highly expressed on α- and β-cells and both are synthesised and secreted by islets and effect islet function.46 Hypergastrinaemia is linked to excessive insulin production and low blood glucose,47 and both hormones induce secretion with or without glucose.48 There is conflicting evidence on the effect of gastrin on glucagon secretion, with some models highlighting a stimulatory effect 49 whereas others conflict these findings.50 Disruption of the gastrin gene in mice does not affect islet morphology or basal glucagon levels, but does cause substandard glucagon secretion in response to insulin caused hypoglycaemia.51 Gastrin in combination with epidermal growth factor has been found to cause β-cell regeneration in mice after near total destruction by alloxan.52 In respect to CCK, little effect on glucagon release has been recorded,53 but a close relationship to GLP-1 has been identified.54 Research has focused on an intra-islet loop based on local islet secretion of these peptides, whether GLP-1 and CCK originating from the gut have the same islet effects is unknown. Sulphation of CCK at a tyrosine located 7 residues from the C-terminus is crucial for CCK bioactivity,55 this post-translational modification allows for strengthened protein-protein interaction.56 Glucagon-like peptide-1 secreted from the α-cell targets the β-cell to stimulate CCK production, while CCK can also stimulate α-cells to release GLP-1 increasing β-cell survival and proliferation, in a positive feedback loop within β-cells.54,57 Mechanistically, CCK1 receptor stimulation phosphorylates PKA and phospholipase C (PLC) increasing intracellular calcium concentrations via the L-channel, this causes positive bias on PLC activity. Increased calcium and diacylglycerol produced by PLC activates protein kinase C58 which ultimately opens gated calcium channels.59 Further work is required to fully assess the therapeutic promise of both gastrin and CCK, especially in relation to potential off-target side effects.47
Urocortin3 and islet amyloid polypeptide
Urocortin3 (Ucn3) is a peptide hormone expressed by mature β-cells, early work revealed insulin and glucagon release to increase in response to Ucn3 treatment.60 Interestingly, Ucn3 may also have benefits on plasma glucose61 and glucose tolerance.60 This has driven new studies to define the physiological role of Ucn3, and it is known that Ucn3 amplifies somatostatin release to modulate insulin and glucagon secretion.62 Somatostatin receptors (SSTRs) have been acknowledged on α- and β-cells.63 Rodent studies reveal inhibition of glucagon and insulin is facilitated through SSTRs 5 and 2, respectively; knockout of these receptors results in variation of basal and glucose stimulated insulin section.64 In T2DM, Ucn3 expression is reduced in β-cells and causes an unstable glycaemic state.65 Islet amyloid polypeptide (IAPP, amylin) is a peptide co-secreted from β-cells with insulin in response to glucose and fatty acids at a ratio of 15:1 (insulin:amylin).66 Currently, 3 amylin receptors have been identified, namely AMY1, AMY2, and AMY367 and activation by amylin is thought to regulate insulin secretion under certain disease states or conditions.68 Knockout studies in β-cells have revealed reduced amylin causes increased glucose-induced insulin secretion, whereas treatment of amylin at physiological levels inhibits insulin secretion.69 Amylin also controls β-cell proliferation in a glucose dependent manner. As such, at high glucose concentrations amylin inhibits proliferation and at low levels promotes β-cell proliferation.70
Xenin-25 and neurotensin
Xenin-25 is a peptide hormone traditionally characterised as being secreted from endocrine K-cells with GIP,71 but xenin-25, as well as the related neurotensin hormone, have also been found in pancreatic islets, alongside neurotensin receptors (NTSRs).72 The main pancreatic effects of xenin include increasing PP secretion and amplifying the effects of GIP,73 indirectly effecting insulin and glucagon release. Xenin-25 interacts with acetylcholine signalling pathways to increase β-cell insulin secretion, and this is thought to be independent of NTSRs.74 This may explain slight differences in the pancreatic effect of xenin-25 and neurotensin, despite both being NTSR ligands. Proliferation in rodent and human β-cells is enhanced by xenin-25 and neurotensin, suggesting the peptides as promising antidiabetic agents.44 The signalling mechanisms involved in insulin and glucagon secretion for xenin-25, differ between human and mouse models in response to co-treatment with GIP.74 In mice, positive effects of co-treatment were seen under hyperglycaemic conditions. In humans, effects on insulin and glucagon where specifically recorded in patients with reduced glucose tolerance, but not in patients with T2DM.74 Cholinergic signalling may act as a regulatory mechanism to increase insulin secretion, and failure is thought to lead to the development of T2DM.75 In mice, atropine treatment inhibited the effect of xenin on GIP-mediated insulin and glucagon secretion implicating cholinergic signalling73 whereas in humans there is no inhibition.74
Vasopressin, oxytocin, and secretin
Vasopressin (AVP) and oxytocin (OT) are neuropeptides and components of a cohesive system,76 with synthesis also evidenced within the pancreas.77,78 Oxytocin receptor knockout (OTKO) mice have increased sensitivity to AVP79 and AVP causes a significant concentration dependent increase of insulin secretion in rodent (BRIN BD11) and human (1.1B4) β-cells,77 but has no effect on glucagon secretion. AVP receptors; V1a, V1b, V2 are highly abundant on β-cells and antagonism of V1a, V1b has a negative effect on AVP-mediated insulin secretion.77 AVP also has positive effects on β-cell proliferation and survival.77 Oxytocin (OT) is known to stimulate insulin and glucagon secretion and have positive effects on insulin action, glucose intolerance and islet hypertrophy.78 Calcium-calmodulin kinase (Ca-CAMKK), phosphoinositide-3-kinase (PI3K) and AMP-activated protein kinase (AMPK) pathways may mediate these effects80 and serotonin (5-HT) and dopamine may have supplementary properties.76 Secretin is produced in the duodenum by S-cells and in the pancreas by secretin producing islets,81 it consists of 27 amino acids with a sequence similar to glucagon and GIP.82 Following an oral glucose load, secretin maintains blood glucose control through initiating insulin release,83 while other work has detailed suppressive effects on α-cell function and glucagon release.84 Interestingly, secretin has also been found to enhance the effects of CCK to increase β-cell proliferation and mass.85
Nonclassical Islet Peptides – Appetite Regulation
The peptide hormones discussed above are considered to be nonclassical islet peptides generated within the pancreas, that mediate numerous local biological actions (Table 1). However, as well as direct pancreatic effects, many of these hormones have well characterised effects outside of the pancreas, that could be linked to pancreatic or extra pancreatic sites of synthesis.
Glucagon-like peptide-1 has a major effect on energy balance and appetite via a complex relationship that is linked to centrally mediated actions.86 Physiological effects of GLP-1 include decreased food intake, inhibited gastric emptying and reduced gastric motility.87 These effects are facilitated by binding to the GLP-1R and signalling the central nervous system (CNS) via the vagal nervous system.88 Glucagon-like peptide-1 is secreted by GCG neurons, which allow direct central activation of the GLP-1R in the NTS of the hypothalamus. GCG neurons signal afferent nerves to relay satiety information to the hypothalamus.89 Glucose dependent insulinotropic peptides’ physiological effects on appetite are somewhat disputed, in vitro work using mouse 3T3L1 adipocytes found GIP increases adipogenesis,90 whereas chronically reduced GIP in mice attenuates weight gain.91 However, transgenic overexpression of GIP, as well as GIP agonist treatment in mice reduces obesity related traits.92 Moreover, recent clinical studies employing co-activation of GLP-1 and GIP receptors have revealed that GIP can augment the body weight reducing effects of GLP-1.93
As noted above, PYY circulates in distinct bioactive forms, with PYY (3-36) recognised as the foremost circulating PYY peptide postprandially. As well as pancreatic secretion, PYY is released from L-cells of the gut, 15 minutes after food intake, secretion is proportional to calories consumed.94 This would indicate that PYY release occurs prior to nutrients reaching the distal small intestine, suggesting secretion is mediated centrally. In addition, there is evidence of enteroendocrine PYY releasing cells in the upper ileum, with studies revealing such cells can express multiple gut hormones,29 which may also be important in this regard.
Rodent receptor KO studies reveal PYY (3-36) acts on the arcuate nucleus (ARC) in the CNS via the NPY2 receptor.94 Activation reduces appetite by increasing POMC/α-MSH activity95 while suppressing NPY neurons.96 Glucagon-like peptide-1 administration is known to cause nausea and gastric complications,97 and clinical studies have revealed such side-effects are heightened after PYY(3-36) administration,98 indicating related effects on energy regulation. Although CCK increases gallbladder contraction, inhibits gastric emptying and reduces food intake,99 many of its effects on energy balance are due to activation of peripheral vagal afferent fibres.100 However, CCK is also believed to penetrate the blood brain barrier (BBB) to act directly in the CNS to effect appetite and digestion.101
Xenin-25 decreases food intake when administered centrally,102 or peripherally at elevated doses,103 indicating action as a satiety factor. In animal studies, xenin-25 causes anorexia via activation of central NTRS1.104 This has been supported by work in NTSR1 KO mice, which were nonresponsive to xenin.105 The mechanisms involved in xenin appetite regulatory effects are poorly described, the extracellular signal-regulated kinases signalling cascade has been implicated, but is not essential for feeding suppression.105 In addition, a long acting xenin-25 analogue, xenin-25[Lys13PAL], lacked effects on energy intake and body weight, but did induce significant benefits on glucose homeostasis in mice.106
Oxytocin and vasopressin secretion are controlled by signals that regulate appetite,107 and both hormones are released acutely following a meal.108 Oxytocin is well established to influence energy balance in vivo, suppressing carbohydrate intake109 and promoting movement and thermogenesis.110 In rodent brain tissue, OT cells express IRs and glucokinase, and can be activated by insulin and glucose.111 Further research identified leptin receptors, as well as receptors allied with other anorectic peptides such as α-MSH, on OT producing cells.112 Ligands of these receptors signal the NTS113 including noradrenergic A2 cells,114 and these cells directly interact with OT cells.115 Other peptides, such as CCK, may support the energy balance effects of OT at central sites, but further studies are needed to elucidate exact mechanisms.
Therapeutic Potential of Multiagonist Nonclassical Islet Peptides
Dual agonists with nonclassical islet hormone components
Recent novel peptide-based approaches for the treatment of T2D and obesity have focused on simultaneous activation of multiple receptors, many of which are related to nonclassical islet peptide hormones116 (Table 2). Synthetic peptides acting as dual or triagonists at GPCRs have been predicted to have positive effects on energy balance expenditure, glucose homeostasis and appetite.117 Early dual compounds focused on GLP-1/glucagon co-agonism and activity was based on the naturally occurring peptide oxyntomodulin.118 Treatment improved maximum insulin secretion compared with exendin-4119 and resulted in large decreases in food intake, adiposity and increased lipolysis.120 Weight loss effects have been linked to significant leptin sensitisation121 and was successfully translated into phase II clinical trials.122 It has been concluded that these peptides have a greater effect than either single hormone. MEDI0382 is a GLP-1/glucagon agonist displaying significant promise in numerous clinical studies, reducing fasting and postprandial glucose and body weight.122 MEDI0382 has consistently exhibited a mild adverse event profile.123 Another dual GLP-1/glucagon agonist (SAR425899) also has significant clinical promise, reducing HbA1c and body weight over 28 days, with mild adverse events124 (Table 2).
Table 2.
Dual and triagonist peptides with classical/‘nonclassical’ islet peptide components. Table includes peptide hormone components, compound name, main effects and relevant citation(s).
| Peptides hormone components | Compound name | Main effects | Reference |
|---|---|---|---|
| Dual agonists | |||
| GLP-1/glucagon | MEDI0382 | Reduces fasting and postprandial glucose/body weight | 123,124 |
| GLP-1/glucagon | SAR425899 | Reduces HbA1c/body weight | 125 |
| GIP/GLP-1 | N-ac (DAla2) GIP/GLP-1-exe | Increases insulin secretion/sensitivity; reduces body weight | 128 |
| GLP-1/GIP | NNC0090-2746 | Reduces HbA1c/body weight | 117 |
| GLP-1/GIP | LY3298176 | Increases insulin secretion; Reduces HbA1c/body weight | 93,129 |
| GLP-1/xenin | Exendin-4/Xenin-8-Gln | Increases insulin secretion/GIP action, reduces appetite | 131 |
| GLP-1/gastrin | ZP3022 | Lowers HbA1c, improves glucose tolerance and β-cell neogenesis | 132 |
| GLP-1/PYY | EP45 | Lowers blood glucose, stimulates insulin secretion | 133,134 |
| Triagonists | |||
| GIP/glucagon/GLP-1 | GIP-Oxm | Improves glucose homeostasis, insulin secretion and body weight | 135 |
| YAG-glucagon | Y1-dA2-I12-N17-V18-I27-G28,29-glucagon | Reduces plasma glucose, food intake and body weight; increases plasma insulin and insulin sensitivity | 136 |
| GLP-1/GIP/glucagon | HM15211 | Improves weight loss, anti-inflammatory and anti-oxidative stress, neuroprotective | 142,143 |
| GLP-1/gastrin/xenin | Exendin-4(Lys27 PAL)/gastrin/xenin-8-Gln | Decreases nonfasting HbA1c, food intake. Enhances insulin levels, glucose tolerance | 144 |
| Glugagon/GLP-1/NPY | GGP817 | Unvalidated (FRET analysis) | 145 |
Abbreviations: FRET, fluorescence resonance energy transfer; GIP, glucose dependent insulinotropic peptide; GLP, glucagon-like peptide; PYY, Peptide Tyrosine Tyrosine.
In recent years, dual incretin agonists have been developed,125 these have focused on manipulating GIPR and GLP-1R signalling to reduce food intake and adiposity, and promote combined benefits on pancreatic β-cells.126 A novel GIP/GLP-1 agonist (N-ac(DAla2)GIP/GLP-1-exe) activates both incretin receptors to cause concentration dependent insulin secretion from BRIN BD11 β-cells and isolated islets.127 Dual receptor activation results in increased weight loss and improved insulin sensitivity compared with mono treatment in mice.125 These results have been confirmed in further preclinical work and in clinical studies.116 In a recent Phase 2A clinical trial a GLP-1/GIP dual agonist (NNC0090-2746) with balanced affinity for GLP-1R and GIPR significantly reduced HbA1c and weight over 8 weeks in T2D patients compared with placebo.116 LY3298176 is a fatty acid adapted peptide with GLP-1 and GIP receptor activity, designed to be administered once weekly.128 Preclinically, LY3298176 causes glucose-dependent insulin secretion from islets isolated from wild-type, GIPR−/− and GLP-1R−/− mice, indicative of activity at both receptors.128 LY3298176 has successfully translated preclinical findings into human studies, imparting meaningful enhancement of glycaemic control and body weight93 specifically, reducing Hba1c and body weight greater than a benchmark group taking Dulaglutide. This highlights the importance of balancing receptor interaction when developing dual or triagonists.129 It is worth noting that there was a 30% nonresponse rate, but results would indicate that dual incretin agonists are more potent than established mono treatments93 (Table 2).
A novel GLP-1/xenin hybrid also indicated significant therapeutic potential in mice, increasing insulin secretion, reducing appetite and improving GIP action.130 A GLP-1/gastrin dual agonist, ZP3022, also displayed multiple antidiabetic effects in vivo. These included lowering HbA1c, improving glucose tolerance and increasing β-cell neogenesis.131 Dual treatment of GLP-1 and PYY led to a synergistic reduction in food intake.132 A recent study characterised a chimeric peptide (EP45) that consists of sequences from exendin-4 (Ex-4) and PYY(3-36), multiple organs can be targeted with this peptide133 (Table 2). Fluorescence resonance energy transfer (FRET) assays demonstrate EP45 stimulates cAMP production via the GLP-1R, while inhibiting cAMP production via the NPY2R.133 These findings would indicate EP45 potentiates blood glucose lowering effects by activating GLP-1R on β-cells, thus triggering glucose stimulated insulin secretion and upregulating insulin gene expression.133 EP45 could also activate receptors centrally to affect appetite and would have relevance for the treatment of T2D.
Triagonists With Nonclassical Islet Hormone Components
The positive results from dual agonist studies have driven the development of triagonists, such as GIP-oxyntomodulin (GIP-Oxm)134 and an adapted glucagon peptide (YAG-glucagon).135 Most recent work has focused on the incretin/glucagon system and triagonists have been developed to activate the GLP-1/GIP/glucagon receptors.136 Glucagon-like peptide-1/glucose dependent insulinotropic peptide and glucagon independently stimulate complementary mechanisms in vivo to cause glucose-mediated insulin secretion,137 and reductions in body weight and cholesterol are greater than a dose matched liraglutide treatment.138,139 In a diabetic rodent model GLP1R-GCGR-GIPR tri-agonism had beneficial pancreatic effects, animals displayed improved glucose tolerance and reduced ab libitum blood glucose, with reduced insulin levels suggesting improved insulin sensitivity140 (Table 2). Peptide characterisation of triple-acting entities in nonhuman primates has been completed by Sanofi, Novo Nordisk and Hanmi, with Novo Nordisk and Hanmi conducting phase 1 clinical trials.129 A long acting GLP-1/GIP/glucagon agonist (HM15211) has a unique pharmacological profile, demonstrating benefits on weight loss and lipid profiles, while exhibiting no risk of hyperglycaemia. Interestingly, in mice HM15211 has also shown promise in treating nonalcoholic steatohepatitis (NASH), fibrosis141 and alzheimers disease142 (Table 2).
Other triagonists include an enzyme resistant GLP-1/gastrin/xenin peptide which has antidiabetic potential in respect to insulin secretion and enhancement of GIP action,143 as well as a triagonist (GGP817) that activates the GluR, GLP-1R and NPY2R.144 This peptide has not been validated in vivo, but it is thought agonism of GluR would cause increased energy expenditure,145 GLP-1R activation would result in enhanced β-cell function and improved glucose homeostasis146 and NPY2R agonism would suppress appetite.95
Conclusion
Signalling processes within pancreatic islets are highly complicated,147,148 and our recent knowledge of an important role for numerous nonclassical islet peptides is these processes requires further detailed study. Understanding their physiology, and potential pathophysiology in diabetes, could open up new promising avenues for drug effective candidates.19 Both preclinical and clinical evidence demonstrate that metabolic profile can be improved by synergistic action of multiple peptide hormone receptor targets, many of which include nonclassical islet peptides.149 Such strategies hold great promise for obesity and related forms of diabetes, especially given the optimism generated through early clinical observations.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was supported by the EFSD/Lilly European Diabetes Research Programme 2017.
Declaration of Conflicting Interest:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AE: Wrote the first draft of the manuscript.
AE,NE: Jointly developed the structure of the paper.
AE, NE: Made critical revisions and appoved final version.
All authers reviewed and approved the final maniscript.
ORCID iD: Andrew English  https://orcid.org/0000-0003-1232-5585
https://orcid.org/0000-0003-1232-5585
References
- 1. Lopes LL, Bressan J, Peluzio MDCG, Hermsdorff HHM. LINE-1in obesity and cardiometabolic diseases: a systematic review. J Am Coll Nutr. 2019;38:478-484. [DOI] [PubMed] [Google Scholar]
- 2. Jmel H, Romdhane L, Ben Halima Y, et al. Pharmacogenetic landscape of Metabolic Syndrome components drug response in Tunisia and comparison with worldwide populations. PLoS ONE. 2018;13:e0194842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organisation. Global report on diabetes, 2016. http://www.who.int/diabetes/global-report/en/. Accessed 12 July, 2017.
- 4. Baynes H. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metab. 2015;6:541. [Google Scholar]
- 5. Longnecker DS. Anatomy and Histology of the Pancreas. Pancreapedia: The Exocrine Pancreas Knowledge Base. 2014;4:1-26. [Google Scholar]
- 6. Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52:1-17. [DOI] [PubMed] [Google Scholar]
- 7. Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2:163-214. [DOI] [PubMed] [Google Scholar]
- 8. Khan D, Moffet CR, Flatt PR, Kelly C. Role of islet peptides in beta cell regulation and type 2 diabetes therapy. Peptides. 2018;100:212-218. [DOI] [PubMed] [Google Scholar]
- 9. Andralojc K, Mercalli A, Nowak K, et al. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52:486-493. [DOI] [PubMed] [Google Scholar]
- 10. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908-913. [DOI] [PubMed] [Google Scholar]
- 11. Unger RH, Dobbs R, Orci L. Insulin, glucagon, and somatostatin secretion in the regulation of metabolism. Annu Rev Physiol. 1978;40:307-343. [DOI] [PubMed] [Google Scholar]
- 12. Samols E, Marri G, Marks V. Interrelationship of glucagon, insulin and glucose. Diabetes. 1966;15:855-866. [DOI] [PubMed] [Google Scholar]
- 13. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13:572-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Titchenell PM, Lazar MA, Birnbaum MJ. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28:497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seino S, Sugawara K, Yokoi N, Takahashi H. β-Cell signalling and insulin secretagogues: a path for improved diabetes therapy. Diabetes Obes Metab. 2017;19:22-29. [DOI] [PubMed] [Google Scholar]
- 16. Vilsboll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47:357-366. [DOI] [PubMed] [Google Scholar]
- 17. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964-973. [DOI] [PubMed] [Google Scholar]
- 19. Brunton S. GLP-1 receptor agonists vs. DPP-4 inhibitors for Type 2 Diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;68:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [DOI] [PubMed] [Google Scholar]
- 21. Kuhre RE, Gribble FM, Hartmann B, et al. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol. 2014;306:G622-G630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marchetti P, Lupi R, Bugliani M, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55:3262-3272. [DOI] [PubMed] [Google Scholar]
- 23. Piro S, Mascali LG, Urbano F, et al. Chronic exposure to GLP-1 increases GLP-1 synthesis and release in a pancreatic alpha cell line (α-TC1): evidence of a direct effect of GLP-1 on pancreatic alpha cells. PLoS ONE. 2014;9:e90093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Heer J, Rasmussen C, Coy D, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263. [DOI] [PubMed] [Google Scholar]
- 25. Meier J, Gallwitz B, Siepmann N, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798-801. [DOI] [PubMed] [Google Scholar]
- 26. Baggio LL, Kim JG, Drucker DJ. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes. 2004;53:S205-S214. [DOI] [PubMed] [Google Scholar]
- 27. Kazafeos K. Incretin effect: GLP-1, GIP, DPP4. Diabetes Res Clin Pract. 2011;93:S32-S36. [DOI] [PubMed] [Google Scholar]
- 28. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egerod KL, Engelstoft MS, Grunddal KV, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt W, Siegel E, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704-707. [DOI] [PubMed] [Google Scholar]
- 31. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439. [DOI] [PubMed] [Google Scholar]
- 32. Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161-171. [DOI] [PubMed] [Google Scholar]
- 33. Jorsal T, Rhee NA, Pedersen J, et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018;61:284-294. [DOI] [PubMed] [Google Scholar]
- 34. Jackerott M, Oster A, Larsson L-I. PYY in developing murine islet cells: comparisons to development of islet hormones, NPY, and BrdU incorporation. J Histochem Cytochem. 1996;44:809-817. [DOI] [PubMed] [Google Scholar]
- 35. Bertrand G, Gross R, Roye M, Ahren B, Ribes G. Evidence for a direct inhibitory effect of PYY on insulin secretion in rats. Pancreas. 1992;7:595-600. [DOI] [PubMed] [Google Scholar]
- 36. Khan D, Vasu S, Moffett RC, Irwin N, Flatt PR. Islet distribution of peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta-cell function and survival. Mol Cell Endocrinol. 2016;436:102-113. [DOI] [PubMed] [Google Scholar]
- 37. Shi YC, Loh K, Bensellam M, et al. Pancreatic PYY is critical in the control of insulin secretion and glucose homeostasis in female mice. Endocrinology. 2015;156:3122-3136. [DOI] [PubMed] [Google Scholar]
- 38. Loh K, Shi Y-C, Bensellam M, Lee K, Laybutt DR, Herzog H. Y1 receptor deficiency in β-cells leads to increased adiposity and impaired glucose metabolism. Scientific Reports. 2018;8:11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Portela-Gomes GM, Johansson H, Olding L, Grimelius L. Co-localization of neuroendocrine hormones in the human fetal pancreas. Eur J Endocrinol. 1999;141:526-533. [DOI] [PubMed] [Google Scholar]
- 40. Wahlestedt C, Regunathan S, Reis DJ. Identification of cultured cells selectively expressing Y1-, Y2-, or Y3-type receptors for neuropeptide Y/peptide YY. Life Sci. 1992;50:PL7-PL12. [DOI] [PubMed] [Google Scholar]
- 41. Whim MD. Pancreatic beta cells synthesize neuropeptide Y and can rapidly release peptide co-transmitters. PLoS ONE. 2011;6:e19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bado A, Cloarec D, Moizo L, Laigneau J-P, Bataille D, Lewin M. Neurotensin and oxyntomodulin-(30-37) potentiate PYY regulation of gastric acid and somatostatin secretions. Am J Physiol. 1993;265:G113-G117. [DOI] [PubMed] [Google Scholar]
- 43. Ramracheya RD, McCulloch LJ, Clark A, et al. PYY-dependent restoration of impaired insulin and glucagon secretion in type 2 diabetes following Roux-En-Y gastric bypass surgery. Cell Rep. 2016;15:944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khan D, Vasu S, Moffett RC, Irwin N, Flatt PR. Influence of neuropeptide Y and pancreatic polypeptide on islet function and beta-cell survival. Biochim Biophys Acta Gen Subj. 2017;1861:749-758. [DOI] [PubMed] [Google Scholar]
- 45. Myrsen-Axcrona U, Karlsson S, Sundler F, Ahren B. Dexamethasone induces neuropeptide Y (NPY) expression and impairs insulin release in the insulin-producing cell line RINm5F. J Biol Chem. 1997;272:10790-10796. [DOI] [PubMed] [Google Scholar]
- 46. Innis RB, Snyder SH. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980;77:6917-6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rehfeld JF. CCK, gastrin and diabetes mellitus. Biomark Med. 2016;10:1125-1127. [DOI] [PubMed] [Google Scholar]
- 48. Rehfeld JF. Why cholecystokinin and gastrin are also incretins. Cardiovasc Endocrinol Metab. 2016;5:99-101. [Google Scholar]
- 49. Rehfeld JF, Holst JJ, Kuhl C. The effect of gastrin on basal and aminoacid-stimulated insulin and glucagon secretion in man. Eur J Clin Invest. 1978;8:5-9. [DOI] [PubMed] [Google Scholar]
- 50. Hansky J, Soveny C, Korman M. The effect of glucagon on serum gastrin: I Studies in normal subjects. Gut. 1973;14:457-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boushey RP, Abadir A, Flamez D, et al. Hypoglycemia, defective islet glucagon secretion, but normal islet mass in mice with a disruption of the gastrin gene. Gastroenterology. 2003;125:1164-1174. [DOI] [PubMed] [Google Scholar]
- 52. Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47:259-265. [DOI] [PubMed] [Google Scholar]
- 53. Ahren B, Holst JJ, Efendic S. Antidiabetogenic action of cholecystokinin-8 in type 2 diabetes. J Clin Endocrinol Metab. 2000;85:1043-1048. [DOI] [PubMed] [Google Scholar]
- 54. Linnemann AK, Davis DB. Glucagon-like peptide-1 and cholecystokinin production and signaling in the pancreatic islet as an adaptive response to obesity. J Diabetes Investig. 2016;7:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hunter R, Carpenter A, Swiger E, et al. Discovery of novel, potent and long acting CCK analogs. Paper presented 24th American Peptide Symposium (eds Srivastava V, Yudin A, Lebl M.). American Peptide Society; 2015. https://pdfs.semanticscholar.org/10fc/54dab5a30e230672c6026bd1243becff9323.pdf. [Google Scholar]
- 56. Klement É, Hunyadi-Gulyás É, Medzihradszky KF. Biological significance and analysis of tyrosine sulfation. In: Griffiths JR, Unwin RD, eds. Analysis of Protein Post-Translational Modifications by Mass Spectrometry. Hoboken, NJ: John Wiley & Sons; 2016:333-349. [Google Scholar]
- 57. Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB. Glucagon-like peptide-1 regulates cholecystokinin production in β-cells to protect from apoptosis. Mol Endocrinol. 2015;29:978-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams JA. Cholecystokinin (CCK) regulation of pancreatic acinar cells: physiological actions and signal transduction mechanisms. Compr Physiol. 2019;9:535-564. [DOI] [PubMed] [Google Scholar]
- 59. Lippo BR, Batista TM, de Rezende LF, et al. Low-protein diet disrupts the crosstalk between the PKA and PKC signaling pathways in isolated pancreatic islets. J Nutr Biochem. 2015;26:556-562. [DOI] [PubMed] [Google Scholar]
- 60. Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci U S A. 2007;104:4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li C, Chen P, Vaughan J, et al. Urocortin III is expressed in pancreatic β-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216-3224. [DOI] [PubMed] [Google Scholar]
- 62. van der Meulen T, Donaldson CJ, Caceres E, et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med. 2015;21:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Portela-Gomes GM, Stridsberg M, Grimelius L, Oberg K, Janson ET. Expression of the five different somatostatin receptor subtypes in endocrine cells of the pancreas. Appl Immunohistochem Mol Morphol. 2000;8:126-132. [DOI] [PubMed] [Google Scholar]
- 64. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111-117. [DOI] [PubMed] [Google Scholar]
- 65. Neelankal John A, Ram R, Jiang FX. RNA-seq analysis of islets to characterise the dedifferentiation in type 2 diabetes model mice db/db. Endocr Pathol. 2018;29:207-221. [DOI] [PubMed] [Google Scholar]
- 66. Stridsberg M, Sandler S, Wilander E. Cosecretion of islet amylid polypeptide (IAPP) and insulin from isolated rat pancreatic islets following stimulation or inhibition of β-cell function. Regulatory Peptides. 1993;45:363-370. [DOI] [PubMed] [Google Scholar]
- 67. Hay D, Christopoulos G, Christopoulos A, Sexton P. Amylin receptors: molecular composition and pharmacology. Biochem Soc Trans. 2004;32:865-867. [DOI] [PubMed] [Google Scholar]
- 68. Grizzanti J, Corrigan R, Servizi S, Casadesus G. Amylin signaling in diabetes and Alzheimer’s disease: therapy or pathology? J Neurol Neuromedicine 2019;4:12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riddle MC, Nahra R, Han J, et al. Control of postprandial hyperglycemia in type 1 diabetes by 24-hour fixed-dose coadministration of pramlintide and regular human insulin: a randomized, two-way crossover study. Diabetes Care. 2018;41:2346-2352. [DOI] [PubMed] [Google Scholar]
- 70. Kiriyama Y, Nochi H. Role and cytotoxicity of amylin and protection of pancreatic islet β-cells from amylin cytotoxicity. Cells. 2018;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martin CM, Parthsarathy V, Pathak V, Gault VA, Flatt PR, Irwin N. Characterisation of the biological activity of xenin-25 degradation fragment peptides. J Endocrinol. 2014;221:193-200. [DOI] [PubMed] [Google Scholar]
- 72. Khan D, Vasu S, Moffett RC, Gault VA, Flatt PR, Irwin N. Locally produced xenin and the neurotensinergic system in pancreatic islet function and β-cell survival. Biological Chemistry. 2017;399:79-92. [DOI] [PubMed] [Google Scholar]
- 73. Wice BM, Wang S, Crimmins DL, et al. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem. 2010;285:19842-19853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang S, Oestricker LZ, Wallendorf MJ, et al. Cholinergic signaling mediates the effects of xenin-25 on secretion of pancreatic polypeptide but not insulin or glucagon in humans with impaired glucose tolerance. PLoS ONE. 2018;13:e0192441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren P-O, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63:2714-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Baribeau DA, Anagnostou E. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci. 2015;9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mohan S, Moffett RC, Thomas KG, Irwin N, Flatt PR. Vasopressin receptors in islets enhance glucose tolerance, pancreatic beta-cell secretory function, proliferation and survival. Biochimie. 2019;158:191-198. [DOI] [PubMed] [Google Scholar]
- 78. Mohan S, Khan D, Moffett RC, Irwin N, Flatt PR. Oxytocin is present in islets and plays a role in beta-cell function and survival. Peptides. 2018;100:260-268. [DOI] [PubMed] [Google Scholar]
- 79. Carter CS. The oxytocin-vasopressin pathway in the context of love and fear. Front Endocrinol. 2017;8:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Elabd S, Sabry I. Two birds with one stone: possible dual-role of oxytocin in the treatment of diabetes and osteoporosis. Front Endocrinol. 2015;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nishitani J, Rindi G, Lopez M, Upchurch B, Leiter A. Transcriptional regulation of secretin gene expression. J Clin Gastroenterol. 1995;21:S50-S55. [PubMed] [Google Scholar]
- 82. DiGregorio N, Sharma S. Physiology, Secretin. Treasure Island, FL: Statpearls Publishing; 2018. [PubMed] [Google Scholar]
- 83. Kraegen E, Chisholm D, Young J, Lazarus L. The gastrointestinal stimulus to insulin release. II. A dual action of secretin. J Clin Invest. 1970;49:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Santeusanio F, Faloona GR, Unger RH. Suppressive effect of secretin upon pancreatic alpha cell function. J Clin Invest. 1972;51:1743-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chey WY, Lee K, Chang T, Chen Y-F, Millikan L. Potentiating effect of secretin on cholecystokinin-stimulated pancreatic secretion in dogs. Am J Physiol. 1984;246:G248-G252. [DOI] [PubMed] [Google Scholar]
- 86. Krieger J-P, Santos da, Conceicao EP, Sanchez-Watts G, et al. Glucagon-like peptide-1 regulates brown adipose tissue thermogenesis via the gut-brain axis in rats. Am J Physiol Regul Integr Comp Physiol. 2018;315:R708-R720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aaboe K, Krarup T, Madsbad S, Holst JJ. GLP-1: physiological effects and potential therapeutic applications. Diabetes Obes Metab. 2008;10:994-1003. [DOI] [PubMed] [Google Scholar]
- 88. Krieger J-P, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65:34-43. [DOI] [PubMed] [Google Scholar]
- 89. Lefort S, Tschop MH, Garcia-Caceres C. A synaptic basis for GLP-1 action in the brain. Neuron. 2017;96:713-715. [DOI] [PubMed] [Google Scholar]
- 90. Getty-Kaushik L, Song DH, Boylan MO, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity. 2006;14:1124-1131. [DOI] [PubMed] [Google Scholar]
- 91. Nasteska D, Harada N, Suzuki K, et al. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 2014;63:2332-2343. [DOI] [PubMed] [Google Scholar]
- 92. Irwin N, McClean P, O’harte F, Gault V, Harriott P, Flatt P. Early administration of the glucose-dependent insulinotropic polypeptide receptor antagonist (Pro 3) GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia. 2007;50:1532-1540. [DOI] [PubMed] [Google Scholar]
- 93. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180-2193. [DOI] [PubMed] [Google Scholar]
- 94. Blevins J, Chelikani P, Haver A, Reidelberger R. PYY (3-36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides. 2008;29:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. De Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6:10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jones ES, Nunn N, Chambers AP, Ostergaard S, Wulff BS, Luckman SM. Modified peptide YY molecule attenuates the activity of NPY/AgRP neurons and reduces food intake in male mice. Endocrinology. 2019;160:2737-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Degen L, Oesch S, Casanova M, et al. Effect of peptide YY3-36 on food intake in humans. Gastroenterology. 2005;129:1430-1436. [DOI] [PubMed] [Google Scholar]
- 99. Muurahainen N, Kissileff HR, Derogatis AJ, Pi-Sunyer FX. Effects of cholecystokinin-octapeptide (CCK-8) on food intake and gastric emptying in man. Physiol Behav. 1988;44:645-649. [DOI] [PubMed] [Google Scholar]
- 100. Schwartz GJ. Roles for gut vagal sensory signals in determining energy availability and energy expenditure. Brain Res. 2018;1693:151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Austin J, Marks D. Hormonal regulators of appetite. Int J Pediat Endocrin. 2008;2009:141753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Craig SL, Gault VA, Irwin N. Emerging therapeutic potential for xenin and related peptides in obesity and diabetes. Diabetes Metab Res Rev. 2018;34:e3006. [DOI] [PubMed] [Google Scholar]
- 103. Taylor AI, Irwin N, McKillop AM, Patterson S, Flatt PR, Gault VA. Evaluation of the degradation and metabolic effects of the gut peptide xenin on insulin secretion, glycaemic control and satiety. J Endocrinol. 2010;207:87-93. [DOI] [PubMed] [Google Scholar]
- 104. Bhavya S, Lew PS, Mizuno TM. Central action of xenin affects the expression of lipid metabolism-related genes and proteins in mouse white adipose tissue. Neuropeptides. 2017;63:67-73. [DOI] [PubMed] [Google Scholar]
- 105. Kim ER, San Lew P, Spirkina A, Mizuno TM. Xenin-induced feeding suppression is not mediated through the activation of central extracellular signal-regulated kinase signaling in mice. Behav Brain Res. 2016;312:118-126. [DOI] [PubMed] [Google Scholar]
- 106. Gault VA, Martin C, Flatt PR, Parthsarathy V, Irwin N. Xenin-25 [Lys 13 PAL]: a novel long-acting acylated analogue of xenin-25 with promising antidiabetic potential. Acta Diabetologica. 2015;52:461-471. [DOI] [PubMed] [Google Scholar]
- 107. Freeman SM, Ngo J, Singh B, Masnaghetti M, Bales KL, Blevins JE. Effects of chronic oxytocin administration and diet composition on oxytocin and vasopressin 1a receptor binding in the rat brain. Neuroscience. 2018;392:241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ong ZY, Alhadeff AL, Grill HJ. Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing. Am J Physiol Regul Integr Comp Physiol. 2015;308:R800-R806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Klockars A, Levine AS, Olszewski PK. Central oxytocin and food intake: focus on macronutrient-driven reward. Front Endocrinol. 2015;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity. 2015;23:950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang B, Nakata M, Nakae J, Ogawa W, Yada T. Central insulin action induces activation of paraventricular oxytocin neurons to release oxytocin into circulation. Sci Rep. 2018;8:10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Engineer DR, Garcia JM. Leptin in anorexia and cachexia syndrome. Int J Pept. 2012;2012:287457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bassi M, Furuya WI, Zoccal D, et al. Control of respiratory and cardiovascular functions by leptin. Life Sci. 2015;125:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2010;300:R222-R235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhu L, Onaka T. Involvement of medullary A2 noradrenergic neurons in the activation of oxytocin neurons after conditioned fear stimuli. Eur J Neurosci. 2002;16:2186-2198. [DOI] [PubMed] [Google Scholar]
- 116. Frias JP, Bastyr EJ, 3rd, Vignati L, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26:343-352.e2. [DOI] [PubMed] [Google Scholar]
- 117. Moran BM, McKillop AM, O’Harte FP. Development of novel ligands for peptide GPCRs. Curr Opin Pharmacol. 2016;31:57-62. [DOI] [PubMed] [Google Scholar]
- 118. Pocai A, Carrington PE, Adams JR, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Farooq G, Jones B, Minnion JS, Bloom SR. Effect of biased GLP-1/Glucagon receptor co-agonists on insulin secretion. Diabetes. 2018;67:1100. [Google Scholar]
- 120. Day JW, Gelfanov V, Smiley D, et al. Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents. Biopolymers. 2012;98:443-450. [DOI] [PubMed] [Google Scholar]
- 121. Patel V, Joharapurkar A, Dhanesha N, et al. Co-agonist of glucagon and GLP-1 reduces cholesterol and improves insulin sensitivity independent of its effect on appetite and body weight in diet-induced obese C57 mice. Can J Physiol Pharmacol. 2013;91:1009-1015. [DOI] [PubMed] [Google Scholar]
- 122. Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607-2618. [DOI] [PubMed] [Google Scholar]
- 123. Ambery PD, Klammt S, Posch MG, et al. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, Phase 1 study. Br J Clin Pharmacol. 2018;84:2325-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tillner J, Posch MG, Wagner F, et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab. 2019;21:120-128. [DOI] [PubMed] [Google Scholar]
- 125. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. [DOI] [PubMed] [Google Scholar]
- 126. Skow MA, Bergmann NC, Knop FK. Diabetes and obesity treatment based on dual incretin receptor activation: ‘twincretins’. Diabetes Obes Metab. 2016;18:847-854. [DOI] [PubMed] [Google Scholar]
- 127. Pathak N, Pathak V, Gault VA, McClean S, Irwin N, Flatt P. Novel dual incretin agonist peptide with antidiabetic and neuroprotective potential. Biochem Pharmacol. 2018;155:264-274. [DOI] [PubMed] [Google Scholar]
- 128. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Brandt S, Muller TD, DiMarchi RD, Tschop MH, Stemmer K. Peptide-based multi-agonists: a new paradigm in metabolic pharmacology. J Intern Med. 2018;284:581-602. [DOI] [PubMed] [Google Scholar]
- 130. Hasib A, Ng MT, Khan D, Gault VA, Flatt PR, Irwin N. A novel GLP-1/xenin hybrid peptide improves glucose homeostasis, circulating lipids and restores GIP sensitivity in high fat fed mice. Peptides. 2018;100:202-211. [DOI] [PubMed] [Google Scholar]
- 131. Skarbaliene J, Rigbolt KT, Fosgerau K, Billestrup N. In-vitro and in-vivo studies supporting the therapeutic potential of ZP3022 in diabetes. Eur J Pharmacol. 2017;815:181-189. [DOI] [PubMed] [Google Scholar]
- 132. Neary NM, Small CJ, Druce MR, et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146:5120-5127. [DOI] [PubMed] [Google Scholar]
- 133. Chepurny OG, Bonaccorso RL, Leech CA, et al. Chimeric peptide EP45 as a dual agonist at GLP-1 and NPY2R receptors. Scientific Reports. 2018;8:3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bhat VK, Kerr BD, Flatt PR, Gault VA. A novel GIP-oxyntomodulin hybrid peptide acting through GIP, glucagon and GLP-1 receptors exhibits weight reducing and anti-diabetic properties. Biochem Pharmacol. 2013;85:1655-1662. [DOI] [PubMed] [Google Scholar]
- 135. Bhat V, Kerr B, Vasu S, Flatt P, Gault V. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice. Diabetologia. 2013;56:1417-1424. [DOI] [PubMed] [Google Scholar]
- 136. Jall S, Sachs S, Clemmensen C, et al. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol Metab. 2017;6:440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nauck M, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912-917. [DOI] [PubMed] [Google Scholar]
- 138. Gault VA, Bhat VK, Irwin N, Flatt PR. A novel glucagon-like peptide-1 (GLP-1)/glucagon hybrid peptide with triple-acting agonist activity at glucose-dependent insulinotropic polypeptide, GLP-1, and glucagon receptors and therapeutic potential in high fat-fed mice. J Biol Chem. 2013;288:35581-35591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Brandt SJ, Gotz A, Tschop MH, Muller TD. Gut hormone polyagonists for the treatment of type 2 diabetes. Peptides. 2018;100:190-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kaur K, Allahbadia G, Singh M. Are we at the verge of finding a new efficacious pharmacotherapy for obesity in the form of agonism at triple drug receptors: glucagon, Glucagon like peptide1 (GLP1), glucose dependent insulin tropic peptide (GIP). Int Phys Med Rehab J. 2019;3:22-27. [Google Scholar]
- 141. Choi IY, Kim JK, Lee JS, et al. Effect of a Novel Long-Acting GLP-1/GIP/Glucagon Triple Agonist (HM15211) in a NASH and Fibrosis Animal Model. Diabetes. 2018;67:1106. [Google Scholar]
- 142. Camins A, Ettcheto M, Busquets O, et al. Triple GLP-1/GIP/glucagon receptor agonists, a potential novel treatment strategy in Alzheimer’s disease. Expert Opin Investig Drugs. 2019;28:93-97. [DOI] [PubMed] [Google Scholar]
- 143. Hasib A, Ng MT, Khan D, Gault VA, Flatt PR, Irwin N. Characterisation and antidiabetic utility of a novel hybrid peptide, exendin-4/gastrin/xenin-8-Gln. Eur J Pharmacol. 2018;834:126-135. [DOI] [PubMed] [Google Scholar]
- 144. Chepurny OG, Matsoukas M-T, Liapakis G, et al. Nonconventional glucagon and GLP-1 receptor agonist and antagonist interplay at the GLP-1 receptor revealed in high-throughput FRET assays for cAMP. J Biol Chem. 2019;294:3514-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Szalowska E, te Meerman GJ, Hoek A, Vonk RJ. Adipokines and energy metabolism genes, but not proinflammatory genes are deregulated in patients with higher HOMA and lower HDL. In: Ewa S, ed. An Adipocentric View of the Development of Insulin Resistance. 2011:107 https://www.rug.nl/research/portal/files/2490617/thesis_ewa_new2.pdf.
- 146. Shin S, Le Lay J, Everett LJ, Gupta R, Rafiq K, Kaestner KH. CREB mediates the insulinotropic and anti-apoptotic effects of GLP-1 signaling in adult mouse β-cells. Mol Metab. 2014;3:803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Barker CJ, Leibiger IB, Berggren P-O. The pancreatic islet as a signaling hub. Adv Biol Regul. 2013;53:156-163. [DOI] [PubMed] [Google Scholar]
- 148. Aamodt KI, Powers AC. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes Metab. 2017;19:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Finan B, Yang B, Ottaway N, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27-36. [DOI] [PubMed] [Google Scholar]