Abstract
Spinal cord injury (SCI) affects over 17,000 individuals in the United States per year, resulting in sudden motor, sensory and autonomic impairments below the level of injury. These deficits may be due at least in part to the loss of oligodendrocytes and demyelination of spared axons as it leads to slowed or blocked conduction through the lesion site. It has long been accepted that progenitor cells form new oligodendrocytes after SCI, resulting in the acute formation of new myelin on demyelinated axons. However, the chronicity of demyelination and the functional significance of remyelination remain contentious. Here we review work examining demyelination and remyelination after SCI as well as the current understanding of oligodendrocyte lineage cell responses to spinal trauma, including the surprisingly long-lasting response of NG2+ oligodendrocyte progenitor cells (OPCs) to proliferate and differentiate into new myelinating oligodendrocytes for months after SCI. OPCs are highly sensitive to microenvironmental changes, and therefore respond to the ever-changing post-SCI milieu, including influx of blood, monocytes and neutrophils; activation of microglia and macrophages; changes in cytokines, chemokines and growth factors such as ciliary neurotrophic factor and fibroblast growth factor-2; glutamate excitotoxicity; and axon degeneration and sprouting. We discuss how these changes relate to spontaneous oligodendrogenesis and remyelination, the evidence for and against demyelination being an important clinical problem and if remyelination contributes to motor recovery.
Keywords: axon regeneration, differentiation, glutamate, myelination, NG2 cells, proliferation
1 |. NG2 CELLS—A BRIEF BACKGROUND ON THE FOURTH MAJOR CNS GLIAL CELL
In the early 1980s, William Stallcup’s lab was intensely investigating intriguing central nervous system (CNS) cells that appeared to be a unique population of immature glia expressing the NG2 proteoglycan (Stallcup, 1981, 2002). Soon after, Raff and colleagues discovered a population of progenitor cells in the rat optic nerve that they named O2-A cells since the cells could give rise to oligodendrocytes and type-2 astrocytes in vitro (Raff, Miller, & Noble, 1983). It did not take long for Stallcup’s group to verify that the NG2 cells they detected were the same as Raff’s O2-A cells. These cells are now recognized in the field as the fourth major CNS glial cell (Stallcup, 2002).
NG2 cells are distributed evenly throughout the brain and spinal cord, occupying nonoverlapping domains throughout the entire gray and white matter (Nishiyama, Boshans, Goncalves, Wegrzyn, & Patel, 2016; Nishiyama, Chang, & Trapp, 1999; Nishiyama, Lin, Giese, Heldin, & Stallcup, 1996). These are the most proliferative cells in the adult CNS and quickly divide to replace a neighboring NG2 cell or oligodendrocyte if it dies (Dawson, Polito, Levine, & Reynolds, 2003; Horner et al., 2000; Hughes, Kang, Fukaya, & Bergles, 2013). NG2 cell bodies and their processes constantly surveil and move within their domain, which is likely how they sense changes in neighboring cells (Hughes et al., 2013). Because they can replace oligodendrocytes, NG2 cells have come to be known as oligodendrocyte progenitor cells (OPCs), among other names.
Not only do NG2 cells monitor neighboring oligodendrocyte lineage cells, they also physically interact with neurons and axons. The first example of this was demonstrated by the Bergles’ lab in 2000 when they revealed that NG2 cells make “bona fide” synapses with glutamatergic neurons in the hippocampus (Bergles, Roberts, Somogyi, & Jahr, 2000). Specifically, NG2 cell processes form close appositions or synapses with glutamate-loaded axon terminals and exhibit AMPA receptor-mediated inward currents upon stimulation of excitatory hippocampal axons. Since that discovery, NG2 cell/neuron synapses have been identified in several gray matter locations and, intriguingly, in white matter tracts such as the corpus callosum. There unmyelinated axons display periodic swellings along their shafts where glutamate-containing synaptic vesicles accumulate. At many of these swellings, NG2 cell processes bearing AMPA receptors form “synapses” with the axons (Kukley, Capetillo-Zarate, & Dietrich, 2007; Ziskin, Nishiyama, Rubio, Fukaya, & Bergles, 2007). When the axons fire action potentials, glutamate is released from the axons and induces a calcium wave in the NG2 cell processes, thus showing active communication between these glial cells and axons. The ultimate function of these neuron/glia synapses remains unclear, but a reasonable hypothesis is that they form the basis for activity-dependent myelination, discussed in more detail below (Bakiri et al., 2009; Fields, 2015; Gautier et al., 2015; Sahel et al., 2015). Interestingly, depolarization of oligodendrocytes seems to in turn reduce impulse latency in the axons they ensheath suggesting bidirectional communication may modulate circuit activity (Fields, 2008).
To determine the ability of NG2 cells to serve as a pool for oligodendrocyte replacement in adult CNS, much work has focused on in vivo demyelination models. As first shown by the Nishiyama lab, NG2 cells surrounding demyelinated lesions undergo rapid proliferation, migrate into the lesions and differentiate into new remyelinating oligodendrocytes within ~3 weeks of chemically-induced demyelination (Watanabe, Toyama, & Nishiyama, 2002). Much less attention has been paid to oligodendrocyte lineage cell responses to traumatic CNS injury, such as spinal cord injury (SCI) where oligodendrocytes are also lost in large numbers (Grossman, Rosenberg, & Wrathall, 2001; McTigue, Wei, & Stokes, 2001; Rabchevsky, Sullivan, & Scheff, 2007; Tripathi & McTigue, 2007). Here, we will give a background on white matter damage after SCI and then discuss what is known about the robust response of NG2+ OPCs, including long-term proliferation and differentiation into remyelinating oligodendrocytes.
2 |. AN UNSETTLED ISSUE: IS DEMYELINATION AN IMPORTANT CLINICAL TARGET AFTER SCI?
Over 17,000 new SCI cases occur each year in the United States, where ~300,000 individuals are currently living with SCI (National Spinal Cord Injury Statistical Center, 2018). Human and preclinical animal studies reveal that the amount and integrity of white matter at the injury site largely determines the extent of functional recovery (Basso, Beattie, & Bresnahan, 1995; Blight, 1983a, 1983b; Blight & Decrescito, 1986; Bresnahan, Beattie, Todd, & Noyes, 1987; Bresnahan, King, Martin, & Yashon, 1976; Kelley et al., 2014; Kim et al., 2010; Loy et al., 2007). However, the presence of spared tissue does not always translate to preserved motor or sensory function. In fact, studies of human SCI cases showed that at least half of the individuals with complete loss of motor and sensory function below the injury (a.k.a. clinically complete) were anatomically incomplete (Bunge, Puckett, Becerra, Marcillo, & Quencer, 1993; National Spinal Cord Injury Statistical Center, 2018; World Health Organization, 2013; W. Young, 2002). That is, these individuals had tissue preserved, typically white matter, at the injury site, yet they still had complete loss of motor and sensory function below the injury. Clearly, this spared tissue was nonfunctional and could not transmit signals between the brain and distal spinal cord.
This phenomenon of spared but nonfunctional tissue has garnered significant attention (as it should) from the research community. It is widely thought that if this pathological tissue could be made functional again, SCI individuals would gain at least some meaningful recovery. Indeed, several animal and human studies have shown that some level of motor function is possible with surprisingly few axons. For instance, Windle et al. showed in 1958 that cats with 1–10% spared axons at the lesion site retained some hindlimb motor control (Windle, Smart, & Beers, 1958). Later work by Blight using quantitative morphometric analyses of contused cat spinal cords confirmed that only ~10% sparing of myelinated fibers is needed in key descending motor tracts for some locomotor recovery after SCI (Blight, 1983a, 1983b; Blight & Decrescito, 1986). Studies in rats demonstrated that sparing of 12% of axons, particularly in the rubrospinal, raphespinal and vestibulospinal tracts, was associated with significant hindlimb recovery (Fehlings & Tator, 1995). Similarly, work by Schucht et al. in rats showed that preservation of only a small number of ventral or ventrolateral axons was required for hindlimb motor function (Schucht, Raineteau, Schwab, & Fouad, 2002). Lastly, in humans, quantification of surviving corticospinal tract (CST) axons in patient samples showed that an individual with just 6% spared CST axons had some voluntary movement below the injury (Kakulas, 2004). Thus, it is evident that small changes in the amount or integrity of residual spared tissue at spinal injury sites could have a significant impact on functional outcome.
The question then turns to why is spared white matter nonfunctional in some cases? An obvious and seemingly straightforward hypothesis is that demyelination of spared axons leads to aberrantly slowed conduction or complete conduction block through the lesion site (Waxman, 1989). Notably, demyelinated axons are a major pathological feature in preclinical models of acute traumatic SCI, including mice, rats, dogs, and cats (Balentine, 1978; Blight, 1983a, 1983b; Gledhill, Harrison, & WI, 1973; Griffiths & McCulloch, 1983; Harrison, Gledhill, & McDonald, 1975; Lasiene, Shupe, Perlmutter, & Horner, 2008; McDonald, 2008; Powers et al., 2012; Siegenthaler, Berchtold, Cotman, & Keirstead, 2008; Smith & Jeffery, 2006; Tripathi & McTigue, 2007). Furthermore, axons speculated to be electrically silent have been identified after rodent SCI in multiple studies (for summary, see Guest, Hiester, & Bunge, 2005).
The consistency of extensive chronic demyelination and its contribution to functional impairment in humans or animal models, however, is surprisingly not settled. For instance, one study of human cervical SCI cases detected axon demyelination in four out of seven cases, with long-term demyelination “at a few sites” along the lesion cavity in a 22 years postinjury case (Guest et al., 2005). In other studies of human tissue, however, the presence of demyelination has been described as “uncommon” (Kakulas, 2004) or extensive only when chronic compression is present (Bunge et al., 1993). Preclinical animal models have not settled the issue either. Most studies describing acute demyelination also show robust remyelination by oligodendrocytes and Schwann cells within 3–4 weeks postinjury (Gledhill, Harrison, & WI, 1973; Griffiths & McCulloch, 1983; Harrison & McDonald, 1977; Smith & Jeffery, 2006). However, some studies do mention chronic demyelination, such as a time course study in rats which stated that >60% of axons were “nonmyelinated” at 2 months postinjury, which declined to 30% at 12 m postinjury suggesting substantial demyelination was still present chronically (Salgado-Ceballos et al., 1998). Other studies have described less extensive chronic demyelination. For instance, Smith and Jeffery’s work (Smith & Jeffery, 2006) documented “occassional” demyelination at 9–12 weeks postinjury in dogs and cats. In our own work, we have routinely detected what look to be spared demyelianted axons in chronic rodent moderate SCI tissue, but they are scattered and appeared much less numerous than remyelinated axons (Hesp, Goldstein, Miranda, Kaspar, & McTigue, 2015). A separate study claimed that demyelination progresses chronically after SCI, with a remarkable increase in demyelinated axons between 4 and 15 months postinjury; unfortunately, histological examples of this robust demyelination were not included in the manuscript (Totoiu & Keirstead, 2005).
Electrophysiology also has been used in attempt to identify connected but nonfunctional axons crossing SCI lesions. Several studies have documented decreased conduction velocity and reduced excitability of axons spared at the lesion site (Blight, 1983a; James et al., 2011; Nashmi & Fehlings, 2001a) and that cooling the preps enabled ~20% more fibers to fire, suggesting demyelination on a subset of axons (James et al., 2011). At least one of these studies, however, claimed selective sparing of smaller axons was the major reason for slowed conduction (Blight, 1983a). Additional “evidence” of demyelination has been benefits observed after treatment with the potassium channel blocker 4-aminopyridine (4-AP), which can increase conduction through demyelinated axons. While this drug has shown promise in clinical trials for MS (Dunn & Blight, 2011), results in SCI clinical trials showed positive but inconsistent results (Cardenas et al., 2007; Cardenas et al., 2014; Hansebout, Blight, Fawcett, & Reddy, 1993; Hayes et al., 1994) leading to the conclusion that 4-AP may be beneficial in “a select group” of SCI patients (Hayes et al., 1994)—perhaps those that happen to have substantial demyelination?
A separate intriguing study using a lateral hemisection model in rats documented a delayed loss of conduction in spared tissue contralateral to the injury (Arvanian et al., 2009). This “decay in transmission” contralateral to the injury reached a significantly reduction at 2 weeks postinjury and remained consistently low for another 12 weeks. This was associated with sodium channel spreading along the axons, a common feature of demyelinated axons; the presence of bare axons contralateral to the injuries was verified using electron microscopy (EM) (Hunanyan et al., 2011). A similar chronic spreading of potassium channels after SCI also has been noted (Nashmi, Jones, & Fehlings, 2000).
While there are several indications from studies above that demyelination may be a chronic problem in SCI tissue, other work suggests the contribution of spared but bare axons to functional impairment is minimal. For instance, when spared rubrospinal tracts were identified in SCI mouse cords (and differentiated from injured axons), they were shown to get completely remyelinated (Lasiene et al., 2008). In this study, any demyelinated rubrospinal axons were characterized as dystrophic, calling into question the viability of demyelinated axons identified in other work. Follow up work by this group in rats verified complete remyelination of spared rubrospinal axons as well as spared CST axons after SCI (Powers et al., 2012). This work again suggested that any demyelinated axons were proximal stumps of injured axons and not intact surviving axons. Importantly, no clear examples of widespread demyelination of confirmed healthy axons after SCI have been documented in preclinical work or in studies of human SCI tissue.
Improved recovery following transplantation of myelinating cells also has been used as evidence to suggest demyelination is a consistent problem after SCI (for thorough review, see Plemel et al., 2014). For instance, a series of transplant studies by the Fehlings’ lab has suggested demyelination is a chronic problem after SCI and that repairing it improves functional recovery. When cells capable of forming oligodendrocytes were transplanted around subacute (~2 weeks) SCI lesions, transplanted cells formed oligodendrocytes that myelinated spinal axons and motor recovery was subsequently improved (Hawryluk et al., 2014; Karimi-Abdolrezaee, Eftekharpour, & Fehlings, 2004; Karimi-Abdolrezaee, Eftekharpour, Wang, Morshead, & Fehlings, 2006; Nagoshi et al., 2018). Work transplanting human embryonic stem cell-derived OPCs into rat spinal contusion sites at 7 days postinjury also showed increased oligodendrocyte remyelination and significantly improved hindlimb locomotor function (Keirstead et al., 2005). Notably, this study also documents a sizable number of demyelinated axons in injured control rat spinal cords at 2- and 10-months postinjury. Importantly, this study was used as justification for an ongoing clinical trial transplanting OPCs in acute SCI patients. Functional improvements in transplantation studies have been put forth as evidence of post-SCI demyelination but these studies can be complicated by immunosuppressants and/or transplant cells secreting factors that facilitate repair beyond pure remyelination (Plemel et al., 2014). Furthermore, the ability of transplanting myelinating cells to “conclusively and significantly … improve functional recovery” has not been unequivocally demonstrated (Myers, Bankston, Burke, Ohri, & Whittemore, 2016).
Thus, it is not easy to rectify the various studies and come to a solid conclusion that there for sure are or are not substantial numbers of demyelinated axons after SCI. The presence, extent and chronicity of demyelination after SCI likely depends on multiple factors, such as location, severity of injury and duration of post-SCI compression, which can cause myelin pathology and/or overt myelin loss and impaired axon conduction (Bunge et al., 1993; Helweg-Larsen & Laursen, 1998; Nashmi & Fehlings, 2001a). The species examined may also influence results. For instance, while countless studies show rats and mice rapidly remyelinate following chemical demyelination, nonhuman primate spinal cords remain completely demyelinated at least 6 months after chemical demyelination (Sasaki, Lankford, Radtke, Honmou, & Kocsis, 2011). Furthermore, myelin loss in degenerating tracts appears to be prolonged in humans versus rodent SCI tissue (Buss et al., 2004), which is relevant as myelin debris can impair remyelination (Kotter, 2006). Thus, despite ~40 years of research using rats, mice, guinea pigs, dogs, cats, nonhuman primates and human samples, it is still unclear if repairing demyelination after SCI should be a universal clinical goal. Given the wide variation in human SCI pathology and the large number of studies seeing at least some demyelination, it would seem reasonable that a subset of patients has myelin loss around spared axons and that these axons could persist indefinitely and contribute to impaired function. Clearly more work is needed, particularly using a variety of preclinical SCI models and clinical subjects when possible, to nail down the consistency and extent of chronic demyelination after SCI. Also needed are ways to screen for patients who may have substantial demyelination, such as with 4-AP or electrical stimulation across the lesion site, and who may therefore benefit from remyelination therapies. This would be especially exciting for the large population of individuals living with chronic SCI.
3 |. EARLY POST-SCI CELL LOSS AND ROBUST OPC PROLIFERATION IN THE ACUTE LESION ENVIRONMENT (0–3 DAYS POSTINJURY)
While the consistency of chronic demyelination after SCI is still unsettled, what is clear, at least in preclinical work, is that the spinal cord can mount a spontaneous myelin repair response that is surprisingly robust and long-lasting. Considering how much the intraspinal tissue milieu changes from acute to chronic times postinjury (detailed below), it is notable that OPCs continue to proliferate and differentiate into new oligodendrocytes that myelinate axons in the varying postinjury environments. Understanding what drives the long-lasting myelin repair response of OPCs and oligodendrocytes could provide important insight and guidance into how to promote this response in injuries in which it fails, and in other conditions with oligodendrocyte loss and white matter pathology, such as chronic MS plaques and neurodegenerative conditions such as Alzheimer’s disease (Nasrabady, Rizvi, Goldman, & Brickman, 2018).
Upon traumatic injury to the spinal cord, the parenchymal environment is immediately and drastically disrupted. As stated above, contusion is the most common type of human SCI and causes axonal stretching and tissue shearing resulting in rapid bruising and petechial hemorrhage formation (Blight, 1983a; Blight & Decrescito, 1986). In some instances, hemorrhage continues and spreads for at least 24 hr, expanding tissue damage and lesion size (Figure 1; Simard et al., 2007). Notably, the acute distribution of red blood cells closely predicts the long-term morphology of the lesion (Hill, Beattie, & Bresnahan, 2001). Components of blood likely have diverse actions on tissue parenchyma, including oligodendrocyte lineage cells (Sahinkaya, Milich, & McTigue, 2014). For instance, platelet-rich plasma may be neuroprotective due to its plethora of growth factors (Chen et al., 2018), but other components are deleterious for myelin repair. The blood-derived clotting factor fibrinogen activates bone morphogenetic protein (BMP) signaling in OPCs, which inhibits their differentiation into oligodendrocytes and could impair remyelination (Petersen et al., 2017). Acute tissue damage also leads to rapid cell death, including significant neuron and oligodendrocyte loss within 15 min of injury, which continues for at least 24–48 hr at the epicenter (Grossman et al., 2001; Rabchevsky et al., 2007). Astrocyte death begins slightly later, with the first significant loss detected at 4 hr postinjury (Grossman et al., 2001).
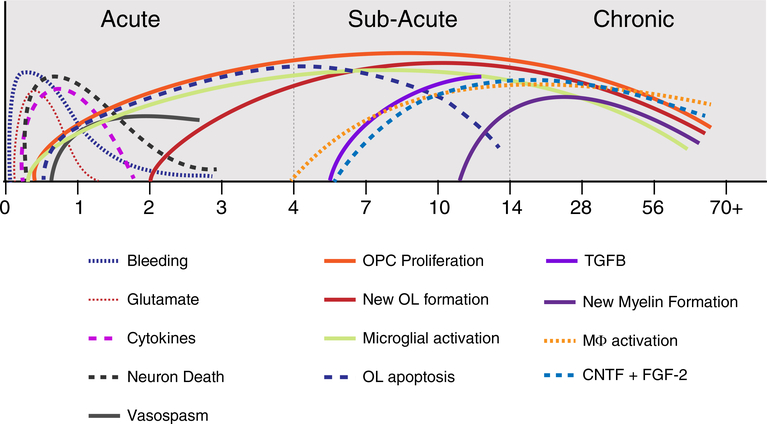
FIGURE 1.
Summary time line diagram of different events after spinal cord injury, which can be generally divided into acute, subacute and chronic, as indicated in the diagram. Acute time points include intraspinal bleeding, glutamate release, microglial activation cytokine expression such as TNFα and IL-1β. There is also neuron death over the first 1–2 days and vasospasm in spared tissue leading to ischemic damage. OPCs begin proliferating almost immediately upon injury and differentiate into new oligodendrocytes within 3 days. In the subacute time, OPC proliferation and differentiation continue, while early cytokines decline as others such as TGFβ increase. Growth factor expression such as CNTF and FGF-2 continues to rise and oligodendrocyte apoptosis is ongoing in degenerating axon tracts. This is also a time of robust monocyte infiltration and macrophage (mϕ) activation (derived from monocytes and microglia). In the chronic period, new oligodendrocyte and new myelin formation continues, and growth factors remain at levels significantly above naive
Acute cell loss likely results from a combination of factors in addition to bleeding, such as vasospasm-induced ischemia and oxidative injury in spared tissue (Figure 1; Almad, Sahinkaya, & McTigue, 2011; Anthes, Theriault, & Tator, 1996; Benton & Hagg, 2011; Dohrmann & Allen, 1975; Fassbender, Whittemore, & Hagg, 2011; Guha, Tator, & Piper, 1985; Koyanagi, Tator, & Theriault, 1993; Mautes, Weinzierl, Donovan, & Noble, 2000; Muradov & Hagg, 2013; Sun et al., 2010; Wallace, Tator, & Frazee, 1986). Proinflammatory cytokines harmful to oligodendrocytes also increase acutely after SCI (Donnelly & Popovich, 2008). For instance, intraspinal interleukin-1α (IL-1α) is increased 4 hr after SCI in mice, and knockout of IL-1α reduces oligodendrocyte death and decreases lesion size (Bastien et al., 2015). Enhanced oligodendrocyte survival in IL-1α knockout mice is thought to be mediated by upregulating the survival factor TOX high mobility group box family member 3 (TOX3) in oligodendrocytes (Bastien et al., 2015). Another potent cytokine elevated acutely after SCI is tumor necrosis factor-α (TNFα) (Donnelly & Popovich, 2008). Studies have shown that TNFα can kill oligodendrocytes through the TNF receptor p55 and apoptosis-inducing factor (AIF)-induced cell death (Akassoglou et al., 1998; Jurewicz et al., 2005). In addition, TNFα can synergize with IL-1β and C1q, which are also upregulated after SCI (for review, see Peterson & Anderson, 2014). The combination of TNFα, IL-1β and C1q can induce a cytotoxic “A1” astrocyte phenotype which can kill neurons and oligodendrocytes (Liddelow et al., 2017). Interestingly, the toxicity of TNFα to oligodendrocytes is exacerbated if the oligodendrocytes are iron-loaded (Zhang et al., 2005). This is notable because oligodendrocytes are already the highest iron-containing cells in the adult CNS (Cheepsunthorn, Palmer, & Connor, 1998; Thorburne & Juurlink, 1996) and iron levels in the spinal cord rise within 12 hr after SCI (Sauerbeck, Schonberg, Laws, & McTigue, 2013), which could exacerbate oligodendrocyte iron exposure.
Another potent mechanism for acute death of oligodendrocytes and other cells after SCI is glutamate-mediated excitotoxicity. Extracellular glutamate concentration rises to toxic levels within 40 min of SCI (Xu, Hughes, Ye, Hulsebosch, & McAdoo, 2004). Microinjecting comparable glutamate levels into naive spinal cord tissue causes significant oligodendrocyte death (Xu et al., 2004). The toxicity of post-SCI glutamate was verified by injecting an AMPA antagonist into acute SCI lesions which was neuroprotective and increased oligodendrocyte survival (Rosenberg, Teng, & Wrathall, 1999; Wrathall, Choiniere, & Teng, 1994; Wrathall, Teng, & Choiniere, 1996; Wrathall, Teng, & Marriott, 1997; Xu et al., 2004). Similar to IL-1β and C1q, glutamate can synergize with TNFα. For instance, microinjecting normally nontoxic levels of glutamate and TNFα together into the intact spinal cord evokes significant cell death at and around the injection site (Hermann, Rogers, Bresnahan, & Beattie, 2001). Thus, even though peak glutamate and TNFα levels subside acutely after injury, the combination of lower levels of these factors may prolong cell loss.
OPCs and oligodendrocytes are vulnerable to other pathological processes in the first days after SCI, such as ATP-induced excitotoxicity, sphingomyelinase/ceramide-induced pathology, cytotoxic molecules from acutely infiltrating neutrophils, and basic oxidative injury (for review, see Almad et al., 2011; Tripathi & McTigue, 2008). Thus, developing strategies that are oligo-protective for this acute time point could be quite challenging.
Neurons and astrocytes are not typically lost beyond 24 hr postinjury but oligodendrocytes continue to die by apoptosis (Figure 1), especially in distal axon tracts undergoing degeneration (Crowe, Bresnahan, Shuman, Masters, & Beattie, 1997; Warden et al., 2001). However, concomitant with this cell death is a robust, spontaneous oligodendrogenic response. Our prior work and that of others shows OPCs proliferate robustly within the first 3 days postinjury, especially in gray matter bordering the lesion (Horky, Galimi, Gage, & Horner, 2006; Lytle & Wrathall, 2007; McTigue et al., 2001; Tripathi & McTigue, 2007; Zai & Wrathall, 2005). Surprisingly, at least to us at the time, some newly divided OPCs differentiate into oligodendrocytes within 3 days postinjury (Tripathi & McTigue, 2007). This rapid pace of OPC proliferation and differentiation illustrates that the typically prolonged mitotic cycle of nonactivated adult OPCs (Hughes et al., 2013; Wang & Young, 2014; Wolswijk & Noble, 1989) can be markedly accelerated in vivo in response to CNS injury, as seen previously in vitro (Moyon et al., 2015). Clearly, exposure to the injury environment reduces the cell cycle length and accelerates differentiation of adult OPCs. In our study (Tripathi & McTigue, 2007), proliferating OPCs and new oligodendrocytes born within the first 3 days postinjury were mainly located in tissue directly bordering the lesion.
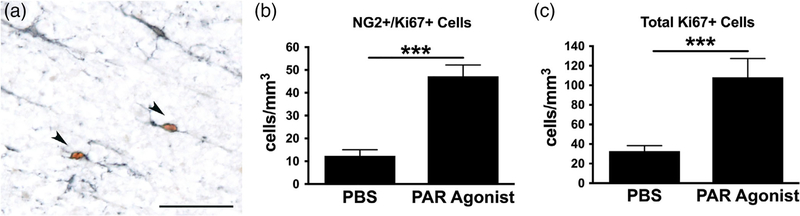
There are several candidate mechanisms for stimulating acute OPC proliferation and differentiation. First is the nature of OPCs themselves, which seem to adhere to homeostatic rules that once an OPC dies, a neighboring OPC detects its loss and divides to replace it (Hughes et al., 2013). A second factor is the abundant intraparenchymal blood. We showed previously that intraspinal bleeding alone (without trauma) is sufficient to promote OPC proliferation within 24 hr (Sahinkaya et al., 2014). Blood contains many potential bioactive factors, one of which is thrombin, a ligand for protease activated receptors (PARs), which are highly expressed on OPCs (Shavit et al., 2008; Wang, Richter-Landsberg, & Reiser, 2004; Yoon, Radulovic, Drucker, Wu, & Scarisbrick, 2015). Prior work from our lab showed that microinjecting a protease-activated receptor-1 (PAR1) agonist (TFLLR-NH2) into intact spinal white matter induced significant proliferation of OPCs within 24 hr (Figure 2), suggesting that PAR ligands from the blood may be early activators of OPCs around the lesion.
FIGURE 2.
Microinjections of a protease-activated receptor-1 (PAR1) agonist into the intact spinal white matter induces cell proliferation including OPCs within 24 hr. (a) Injection site of PAR agonist (TFLLR-NH2, 60 nmol/1.5 μL, Abgent, San Diego, California) immunolabeled for NG2 (for OPCs; black) and Ki67 (brown) to label dividing cells. Two double-labeled NG2 cells are indicated by arrowheads. Vehicle (phosphate-buffered saline, PBS) injection sites contained no Ki67+ cells. (b) The number of Ki67+ cells expressing NG2 were quantified in the injection site. PAR1 agonist injection significantly increased the number in dividing NG2 cells. (c) The total number of Ki67+ cells was also quantified in the injection sites revealing a significant increase in overall proliferation in PAR agonist-injected tissue compared to PBS control. ***p < .001 t-tailed t test
Many other factors could contribute to expanding OPC and oligodendrocyte numbers acutely after injury, such as the oxidative nature of the injury milieu itself. OPCs with higher (but not toxic) levels of reactive oxygen species (ROS) are more likely to differentiate than those with low ROS (Noble, Smith, Power, & Mayer-Pröschel, 2003; Olguín-Albuerne & Morán, 2018). Elevated glutamate could also influence OPCs surviving the injury; depending on the situation, glutamate can promote OPC migration, proliferation or differentiation (for review, see Spitzer, Volbracht, Lundgaard, & Káradóttir, 2016). Furthermore, the mixture of cytokines and chemokines that spike in the injured spinal cord over the first 24 hr could have varying effects (Donnelly & Popovich, 2008; McTigue et al., 1998; Streit et al., 1998). For instance, upregulated C-X-C motif chemokine ligand 1 (CXCL1) may limit OPC migration while acute TNFα and IL-1β could promote early OPC proliferation and differentiation (Arnett et al., 2001; Mason, Suzuki, Chaplin, & Matsushima, 2001; Robinson, Tani, Strieter, Ransohoff, & Miller, 1998).
OPC and oligodendrocyte survival is also affected by the Toll-like receptor 4 (TLR4) signaling pathway, which is prominently expressed in microglia and macrophages. Absence of TLR4 signaling enhances acute oligodendrocyte and OPC loss after SCI, suggesting that TLR4 activation acutely postinjury is protective for oligodendrocytes (Church, Kigerl, Lerch, Popovich, & McTigue, 2016). Enhanced oligodendrocyte loss in these mice may be due in part to elevated iron-induced toxicity since TLR4-deficient injured spinal cords had fewer ferritin+ macrophages, suggesting reduced iron uptake and more free iron. As stated above, iron is markedly increased after SCI, due to cell death and red blood cell extravasation (Kroner et al., 2014; Rathore et al., 2008; Sauerbeck et al., 2013). Iron is highly reactive and promotes oxidative cell death through the Fenton reaction (Winterbourn, 1995). TLR4 activation stimulates iron uptake and storage by macrophages in vivo (Goldstein et al., 2017; Schonberg & McTigue, 2009; Schonberg, Popovich, & McTigue, 2007), and there are many TLR4 ligands within the injury site, such as heme, high mobility group box 1 (HMGB1) and fibronectin (Kigerl et al., 2009; Kigerl, de Rivero Vaccari, Dietrich, Popovich, & Keane, 2014). Thus, acute TLR4 signaling after SCI appears to enhance iron sequestration in the injury site which would be protective to oligodendrocytes and other cells.
Despite this “inhospitable” environment full of blood, activated macrophages, iron, glutamate, free radicals, etc., surviving OPCs seem to thrive. They undergo significant proliferation acutely, especially in gray matter, and begin accumulating within and along the lesion edge (Horky et al., 2006; McTigue, Tripathi, & Wei, 2006; Tripathi & McTigue, 2007; Zai & Wrathall, 2005), suggesting that lesion-derived factors provide mitogenic and tropic signals for them. Our prior work also showed that OPCs readily migrate into and proliferate within clusters of TLR4-activated macrophages within the spinal cord (Schonberg et al., 2007, 2012), indicating that macrophages may induce OPC migration and proliferation after SCI. The identity of the macrophage-derived signals may be hard to nail down, however, as TLR4-activated macrophages produce hundreds of molecules. Macrophage phenotype may also play a role. Macrophages are often classified as pro-inflammatory M1 (more deleterious) or anti-inflammatory M2 (more pro-reparative) phenotypes, both of which are present acutely in SCI tissue (Kigerl et al., 2009). Although this terminology is oversimplified as it does not represent the complexity of macrophage activation, it does provide convenience when discussing macrophage contributions. For in-depth reviews on macrophage activation, we refer the reader to Ginhoux, Schultze, Murray, Ochando, & Biswas, 2016 and Martinez & Gordon, 2014. Macrophages consistent with an M2-like phenotype release activin-A which, in vitro, promotes oligodendrocyte differentiation (Miron et al., 2013). Thus, factors produced by acute TLR4- and/or M2-activated macrophages may contribute to driving acute OPC proliferation and oligodendrogenesis.
4 |. ROBUST OPC PROLIFERATION AND OLIGODENDROGENESIS CONTINUE IN THE SUBACUTE SCI ENVIRONMENT DESPITE A MARKEDLY CHANGING TISSUE MILIEU (~3–14 DAYS POSTINJURY)
The tissue microenvironment gradually changes over the first week post-SCI (Figure 1). Hemorrhage subsides, although the blood–brain barrier remains permeable to small molecules for at least 1 month after SCI (Popovich, Horner, Mullin, & Stokes, 1996). Over the first 7 days postinjury, edema at the epicenter resolves and monocyte-derived macrophages accumulate in large numbers (Popovich & Hickey, 2001). Microglia and macrophages engulf red blood cells (Rathore et al., 2008) and become loaded with intracellular iron and ferritin (Sahinkaya et al., 2014; Sauerbeck et al., 2013; Schonberg et al., 2012), which can alter their functional state (Goldstein et al., 2017; Schonberg et al., 2012; Schonberg & McTigue, 2009; Zhang, Surguladze, Slagle-Webb, Cozzi, & Connor, 2006). For instance, iron-loaded microglia enhance oligodendrocyte survival, while iron-loaded lipopolysaccharide-activated microglia kill oligodendrocytes, revealing that the iron status of microglia modulates their effects on other cells (Zhang et al., 2006).
By 3 dpi, myelin debris is abundant, the clearance of which occurs much more slowly compared to peripheral nervous system injury. Myelin debris prevents oligodendrocyte differentiation (Kotter, 2006; Plemel, Manesh, Sparling, & Tetzlaff, 2013), therefore its presence in and around the injury site may hamper oligodendrocyte differentiation, especially within the SCI lesion cavity where OPCs accumulate but rarely differentiate into oligodendrocytes (McTigue et al., 2001).
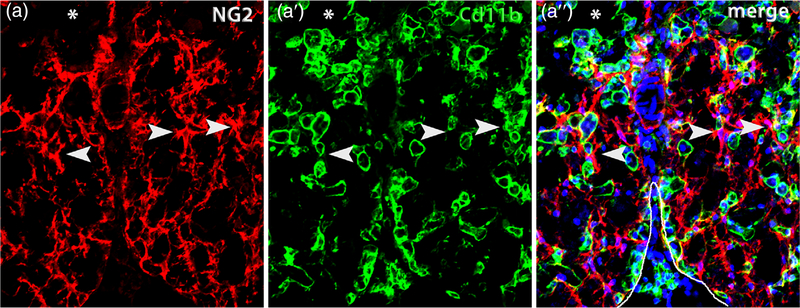
OPC proliferation remains high between 3 and 14 days post-SCI (Hesp et al., 2015; McTigue et al., 2001; Zai & Wrathall, 2005), especially in the surviving gray matter (Tripathi & McTigue, 2007). OPCs begin accumulating among astrocytes along the lesion border in a dense network (Church et al., 2016; Hesp et al., 2015; Hesp et al., 2018; Zai & Wrathall, 2005). Interestingly, this overlapping OPC accumulation implies that the density-dependent, self-avoidance feedback that typically inhibits OPC over-expansion is overruled along SCI lesion borders (Hughes et al., 2013; Zhang & Miller, 1996). OPCs also closely intermingle with activated macrophages along the lesion borders and in spared tissue (Figure 3), which means they are in position to be directly influenced by the numerous macrophage-release products.
FIGURE 3.
OPCs closely intermingle with macrophages after spinal cord injury. Single channel and merged confocal images from ventral white matter bordering the lesion (*) immunolabeled for NG2 (red) (a) and Cd11b (green) (a’). The merged image (a”) is counterstained with Dapi (blue) and the ventral pial border is outlined in white. NG2+ OPCs and their processes are commonly adjacent to macrophages (examples indicated by arrowheads). This section is taken from the lesion epicenter at 14 days postinjury
OPC differentiation persists in the first 2 weeks postinjury, resulting in a continuous rise in oligodendrocyte numbers mainly along lesion borders and in spared gray matter (Horky et al., 2006; Rabchevsky et al., 2007; Sellers, Maris, & Horner, 2009; Tripathi & McTigue, 2007; Zai & Wrathall, 2005). This robust oligodendrogenesis pushes total oligodendrocyte numbers from below normal at 3 dpi to ~threefold greater than naive levels at 14 dpi (McTigue & Tripathi, 2008; Tripathi & McTigue, 2007). This clearly more than compensates for ongoing oligodendrocyte apoptosis, which peaks ~2 weeks postinjury (Crowe et al., 1997). The preferential distribution of dividing OPCs and new oligodendrocytes in spared gray matter is interesting in light of data showing that white matter-derived OPCs normally have a higher maturation rate than those in gray matter (Viganò, Möbius, Götz, & Dimou, 2013).
As referred to above, classic studies from the 1970s showed clear evidence that some spared axons become demyelinated over the first 2 weeks post-SCI (Gledhill et al., 1973; Harrison & McDonald, 1977). While surviving mature oligodendrocytes are unable remyelinate axons (Crawford, Chambers, & Franklin, 2013), the robust OPC response to SCI provides an pool of new cells that can remyelinate axons. However, despite having new oligodendrocytes present as early as 3 dpi (Tripathi & McTigue, 2007), remyelination has not been definitively detected until 14 dpi or later, revealing that the time to associate with spared axons and form detectable compact myelin still requires ~2 weeks.
Despite the shifting tissue environment over 2 weeks postinjury (Figure 1), robust oligodendrocyte formation continues, indicating that the dynamic tissue milieu remains overall pro-reparative for oligodendrocyte replacement. As stated above, microglia and monocyte-derived macrophages accumulate during this time, although they are not a homogenous population. Instead, during the first week postinjury, microglia and macrophages are a mixture of so-called M1 and M2 phenotypes. Over the second week postinjury, M2 cells decline and the M1 phenotype predominates (Gensel & Zhang, 2015; Kigerl et al., 2009; Wang et al., 2015). This is relevant to oligodendrogenesis as both phenotypes promote OPC proliferation but only the M2 phenotype has been shown to promote oligodendrocyte differentiation and remyelination in vitro (Miron et al., 2013). In addition, transplantation of M2 macrophages into the SCI site increased myelination and promoted functional recovery in rats (Ma et al., 2015). Therefore, pushing macrophage population toward the “M2-type” phenotype in the injured spinal cord may be a promising strategy to promote oligodendrogenesis and tissue repair.
While cytokine expression declines over this time, certain growth factors increase, including transforming growth factor β (TGFβ) and ciliary neurotrophic factor (CNTF), both of which promote oligodendrocyte differentiation. TGFβ and its receptors increase over the first 7–10 days postinjury (McTigue, Popovich, Morgan, & Stokes, 2000; Semple-Rowland et al., 1995). TGFβ RNA is mainly expressed by macrophages within the lesion, suggesting its greatest influence would be on OPCs within or directly around the lesion (McTigue et al., 2000). CNTF is predominantly produced by astrocytes; its expression is low in the intact CNS, due in part to αvβ5 integrin signaling induced by physical contact with neurons (Kang, Keasey, Cai, & Hagg, 2012; Keasey, Kang, Lovins, & Hagg, 2013). After SCI, CNTF levels increase significantly by 14 days compared to naive and continue to rise throughout the first month postinjury (Figure 1; Tripathi & McTigue, 2008). Since CNTF promotes oligodendrocyte survival, maturation and myelination (Hackett et al., 2016; Steelman et al., 2016; Yokogami, Wakisaka, Avruch, & Reeves, 2000), this rich CNTF environment may contribute to the oligodendrogenic nature of the early post-SCI tissue environment.
Other extracellular factors could influence OL lineage cell responses from 3 days to 14 dpi as well. For instance, fibroblast growth factor-2 (FGF-2) expression rises over the first 2 weeks, especially in spared gray matter and along lesion borders (Follesa, Wrathall, & Mocchetti, 1994; Koshinaga, Sanon, & Whittemore, 1993; Mocchetti, Rabin, Colangelo, Whittemore, & Wrathall, 1996; Tripathi & McTigue, 2007). Interestingly, this effect may be enhanced by CNTF, as expression of FGF-2 and the FGF receptor-1 are both stimulated by CNTF (Albrecht, Dahl, Stoltzfus, Levenson, & Levison, 2002; Jiang, Levison, & Wood, 1999). FGF-2 is produced by astrocytes and microglia (Albrecht et al., 2002; Miron, 2017) and promotes OPC migration, proliferation and expression of PDGFRα; it can also prevent oligodendrocyte differentiation suggesting its effects may be predominantly on OPCs (Armstrong, Le, Frost, Borke, & Vana, 2002; Engel & Wolswijk, 1996; Goddard, Berry, Kirvell, & Butt, 2001; McKinnon, Matsui, Dubois-Dalcq, & Aaronsont, 1990; Wolswijk & Noble, 1992). Notably, insulin-like growth factor-1 (IGF-1) also increases acutely after SCI and can synergize with FGF-2 to enhance OPC proliferation (Frederick & Wood, 2004; F. Jiang, Frederick, & Wood, 2001; Hawryluk et al., 2012; Yao et al., 1995). Similarly, bone morphogenetic protein 4 (BMP4), which pushes progenitors toward an astrocytic rather than oligodendrocyte fate, is increased at 7 dpi (Sellers et al., 2009). However, since oligodendrogenesis is robust over the first 2 weeks after SCI, the combination of pro-differentiation factors must override inhibitory effects of FGF-2 or BMP4 on new oligodendrocyte formation.
Astrocytes produce many additional molecules after injury that can affect OPC fate and function (for an excellent review, see Burda & Sofroniew, 2014). One interesting factor is tissue inhibitor of metalloproteinase-1 (TIMP-1), which is an endogenous regulator of matrix metalloproteinases normally expressed at low levels in the adult CNS. After SCI, however, TIMP levels rise by 3 days and continue increasing for at least 28 dpi (Sandhir, Gregory, He, & Berman, 2011; Wu et al., 2010). In vitro, TIMP-1 stimulates maturation of OPCs into oligodendrocytes and in vivo, TIMP-1 knockout worsens myelin recovery in experimental allergic encephalomyelitis (EAE), a preclinical model of MS (Crocker et al., 2006; Moore et al., 2011). Thus, astrocyte-derived TIMP-1 may be an early inducer of oligodendrocyte formation in the traumatized spinal cord.
Oligodendrocyte lineage cell fate and function are also strongly modified by intracellular signals, including transcription factors, epigenetic modification and miRNAs (for a thorough review, see Emery & Lu, 2015). Two well-recognized intracellular inhibitors of OPC maturation into oligodendrocytes are inhibitor of differentiation (Id)2 and Id4. Prior work revealed that Id2 mRNA is upregulated at 3 dpi in tissue distal to the SCI site (Tzeng, Bresnahan, Beattie, & De Vellis, 2001). In our work, we did not observe a change in Id2 mRNA at 3 dpi, but we did detect a significant reduction in Hes Family BHLH transcription factor 5 (Hes5) and SRY-box 11 (Sox11) from 3 to 28 days postinjury (Hesp et al., 2015), both of which block OPC differentiation into oligodendrocytes (Li, He, Richardson, & Casaccia, 2009; Swiss et al., 2011). Therefore, reducing their levels would be prooligodendrogenic. Another intriguing intracellular molecule is peroxisome proliferator-activated receptor-δ (PPAR-δ). PPAR-δ is a ligand-activated transcription factor in the nuclear hormone receptor family (Qi, Zhu, & Reddy, 2000), and is implicated in oligodendrocyte development and myelination. For instance, PPAR-δ stimulates myelin protein expression, and PPAR-δ knockout mice display hypomyelination of the corpus callosum (Granneman, Skoff, & Yang, 1998; Peters et al., 2000; Saluja, Granneman, & Skoff, 2001). After SCI, PPAR-δ expression increases in OPCs and oligodendrocytes over the first 2 weeks and thus may contribute to oligodendrocyte formation (Almad & McTigue, 2010).
Collectively, studies examining the first 2 weeks after SCI reveal concomitant OPC proliferation and differentiation. This indicates that OPCs must integrate the multiple fluctuating signals in the acute SCI microenvironment resulting in cell division with a subset maturing into new oligodendrocytes.
5 |. OPC PROLIFERATION, DIFFERENTIATION INTO NEW OLIGODENDROCYTES AND PRODUCTION OF NEW MYELIN CONTINUE FOR MONTHS AFTER RODENT SCI (>14 DAYS POSTINJURY)
The robust oligodendrocyte formation in the first 2 weeks after SCI begins to pay off with axon remyelination visible by the third week. Using different reporter mice, several labs have demonstrated new oligodendrocyte myelin can be detected at this time, (Figure 4; Assinck et al., 2017; Hesp et al., 2015; Lasiene et al., 2008; Powers et al., 2013), which is consistent with earlier EM work that shows axon remyelination beginning 3 weeks postinjury (Gledhill et al., 1973; Harrison & McDonald, 1977). In contrast to typical chemical demyelination models which have a single wave of OPC proliferation, differentiation and typically complete remyelination (Blakemore & Franklin, 2008; Franklin & ffrench-Constant, 2017), OPC proliferation and new oligodendrocyte formation continue for weeks after SCI. For instance, our work revealed that OPC proliferation remains markedly elevated for at least 10 weeks after SCI in tissue at and distal to the epicenter (Hesp et al., 2015). We also detected a significant jump in new oligodendrocyte numbers between 4 and 5 weeks postinjury with continued oligodendrocyte formation throughout the third month after SCI (Hesp et al., 2015). This is consistent with work by Assinck et al. who showed a significant increase in oligodendrocyte numbers between 3 and 12 weeks postinjury such that >50% of oligodendrocytes present at 12 weeks were formed after injury (Assinck et al., 2017). These late-born oligodendrocytes continue to produce myelin around spinal axons during the third month postinjury revealing a surprisingly long-lasting endogenous repair response initiated by spinal trauma (Hesp et al., 2015). While it is true that even uninjured mice produce new oligodendrocytes and myelin throughout adulthood (Young et al., 2013), the post-SCI response is 6- to 10-fold greater than age-matched uninjured controls (Assinck et al., 2017), revealing a robust injury-specific effect.
FIGURE 4.
New myelin (GFP+) is present extensively throughout white matter after SCI. Reporter mice (Pdgfra-CreERT2:Tau-mGFP) were treated with tamoxifen during the second week postinjury and spinal cords examined at 5 weeks postinjury. In these mice, tamoxifen induces membrane GFP expression by newly formed oligodendrocytes. Since the Tau promoter is only active after OPCs differentiate into oligodendrocytes, only oligodendrocytes born after tamoxifen treatment are labeled (and not OPCs). These results indicate the new oligodendrocytes and myelin were formed between 3 and 5 weeks after SCI. Single channel confocal images are from sections immunolabeled for GFP (myelin, a) and the oligodendrocyte cell body marker GSTpi (red, a’); merged confocal images are shown in a”. Since the GFP is membrane labeled, myelin profiles are visible (arrowheads). Asterisk on image refers to the SCI lesion cavity
The majority of P0+ Schwann cells that accumulate in injured spinal cords also may be derived intraspinally from OPCs (Assinck et al., 2017; Bartus et al., 2016; Duncan et al., 2018). Intriguingly, if oligodendrocyte remyelination after SCI is prevented, Schwann cells do not compensate to myelinate the bare axons (Duncan et al., 2018). Similarly, if post-SCI Schwann cell myelination is blocked, oligodendrocytes do not myelinate the dorsal column axons typically remyelinated by Schwann cells (Bartus et al., 2016). Thus, if oligodendrocytes and Schwann cells are derived from the same progenitor population (e.g., OPCs) as suspected, the progenitors must respond to highly localized cues that direct them toward one cell fate or the other.
Although the chronic SCI microenvironment is quite different from the acute setting where robust OPC proliferation and new oligodendrogenesis are initiated, it still clearly promotes and supports formation of oligodendrocytes and new myelin. This may be due in part to continued expression of pro-oligogenic factors such as CNTF and FGF-2, which are elevated for at least 3 m postinjury (Figure 1; Hesp et al., 2015). Accordingly, phosphorylated signal transducer and activator of transcription 3 (pSTAT3) is expressed in NG2+ OPCs for over 1 month after SCI, suggesting active continued signaling by CNTF (or other family members) (Hesp et al., 2015). Other promyelinating factors, including leukemia inhibitory factor (LIF), IGF-1, and TGF-β1, also have been detected chronically at the injury epicenter in mice (Hawryluk et al., 2012) and in human SCI patients as well (Ferbert et al., 2017; Moghaddam et al., 2016; Resnick et al., 2004). LIF and CNTF are part of the same cytokine family and exert similar functions on oligodendrocyte lineage cells (Kerr & Patterson, 2005; Stankoff et al., 2002).
In addition to pro-myelin signals in chronic SCI tissue, factors that inhibit oligodendrocyte differentiation and could possibly push progenitors to an astrocytic fate also rise with time. One example is BMPs, members of the TGF-β superfamily that, as stated above, suppress OPC differentiation and instead push OPCs toward an astrocytic fate (Grinspan et al., 2000; Mabie et al., 1997; Petersen et al., 2017; See et al., 2004). Early during development, a population of OPCs gives rise to astrocytes (Masahira et al., 2006; Zhu et al., 2011), but this behavior is eliminated during normal adulthood (Simon, Götz, & Dimou, 2011; Zhu et al., 2011). However, OPCs are highly sensitive to their environment and it is possible that OPCs revert back to a developmental state in the injured CNS milieu. Indeed, genetic fate mapping studies show a small fraction of OPCs generate astrocytes after focal demyelination (Zawadzka et al., 2010) and SCI (Barnabé-Heider et al., 2010). After SCI, BMP4 mRNA reaches a significant peak at 14–28 days postinjury (Hesp et al., 2015). BMP4 effects on oligodendrocyte lineage cells are mediated, at least in part, by upregulating the transcription factors Id2 and Id4, which sequester the pro-oligodendrocyte transcription factors (Olig)1 and Olig2 in the cytoplasm and prevent their translocation to the nucleus (Cheng et al., 2007; Samanta, 2004; Songli Wang, Sdrulla, Johnson, Yokota, & Barres, 2001). Similar to BMP4, Id2 and Id4 mRNA are elevated at 14–28 days postinjury, which, if expressed by OPCs, would impair their ability to differentiate into new oligodendrocytes (Hesp et al., 2015). Thus, reducing BMP4 signaling in chronic SCI tissue may effectively release a “brake” on oligodendrogenesis and maintain production of new oligodendrocytes at a higher level.
The presence of new myelin formed at 2- and 3-month postinjury begs the question of which axons are becoming myelinated. Are they axons that were demyelinated acutely and for whatever reason were not remyelinated while nearby axons were? Or are there axons that become newly demyelinated chronically and are subsequently remyelinated? It is also possible they are regenerating axons, although this is less likely as the more chronically formed myelin was mostly on axons somewhat distal from the epicenter in spared white matter bordering the pia (Hesp et al., 2015); the axons did not have the morphology of growing axons and it has been shown that regenerating axons typically do not become myelinated (see below). The identity of the chronically myelinated axons after SCI requires further study.
Understanding the sequelae that lead to chronic myelin formation after SCI should yield important insights into how the axons became demyelinated and what mechanisms drive myelination in this chronic injury environment (which are likely different from the acute setting). This knowledge could potentially prove useful in other conditions with chronically bare axons such as MS. These results also emphasize that the field still has much to learn about the endogenous capacity of the adult CNS to induce “self-repair” and to determine if this robust reparative response in rodents is also present in humans. This is especially true since, as mentioned above, chemical demyelination lesions that remyelinate within weeks in rodents remain demyelinated for at least 6 months in nonhuman primates (Sasaki et al., 2011). As discussed above, we still have little understanding of the extent of demyelination routinely present in chronic SCI in humans and basically no information on the possibility that spontaneous chronic remyelination occurs. Since some human subjects show recovery throughout the first-year postinjury (Kakulas, 2004), it is plausible that ongoing remyelination contributes to the process. Having better insight into the mechanisms of myelin replacement in chronic SCI as well as more thorough knowledge of the prevalence and consistency of chronic demyelination in human SCI should allow better and more targeted therapies to potentially improve recovery of patients in which chronic demyelination is present.
6 |. IS REMYELINATION NEEDED FOR MOTOR RECOVERY?
As discussed above, oligodendrocyte remyelination begins within the first few weeks after SCI and continues for several months in rodents. The contribution of this new myelin to motor recovery, however, was recently called into question. Transgenic mice in which the myrf gene was deleted in OPCs were used to prevent new oligodendrocyte remyelination from occurring after thoracic SCI (Duncan et al., 2018). Remyelination after injury was almost completely prevented in these mice, yet their level of motor function recovery was the same as control mice that did remyelinate. The authors concluded that their study “raises doubts whether remyelination is a validated target for clinical translation following moderate spinal cord contusion.” Furthermore, the title of the paper pointedly states that locomotor recovery after SCI “does not require oligodendrocyte remyelination”.
The experiments were well-designed and the results straightforward. However, some caveats should be considered before completely discounting the relevance of oligodendrocyte remyelination after SCI. First, the net difference in myelinated axon number between the experimental groups may not have been sufficient to negatively impact gross hindlimb locomotion. Specifically, SCI control mice had ~19% of the total number of myelinated axons compared to naïve mice, while myrf knockout (myrf-KO) mice had 10%. As stated above, several studies show motor recovery is possible with just ~10% sparing (Blight, 1983a, 1983b; Blight & Decrescito, 1986; Fehlings & Tator, 1995; Windle et al., 1958). Furthermore, two different studies showed that locomotor recovery after SCI is not linear with respect to tissue sparing and that there are thresholds of sparing that are associated with certain levels of recovery. First, Schucht et al. (2002) showed that a range of white matter sparing can produce similar locomotor recovery. Furthermore, they showed that preservation of a small number of axons in the ventrolateral white matter allowed recovery of hindlimb plantar stepping and forelimb-hindlimb coordination (but not parallel paw placement, BBB of ~14) (Schucht et al., 2002). This is the same level of recovery obtained by the myrf-KO mice, suggesting that animals from both studies surpassed this threshold of recovery but did not reach the next threshold. Second, careful work by the Basso lab (Kloos, Fisher, Detloff, Hassenzahl, & Basso, 2005) showed that 10% white matter sparing also could produce the same level of recovery noted in the Duncan et al. study. To get greater recovery (e.g., parallel paw position), a minimum of 30% white matter sparing was needed. Thus, 10–19% sparing in the Duncan study places both groups of mice in the lower threshold of recovery range, which is likely why they displayed similar recovery. This is consistent with classic work by Blight that also concluded that motor recovery occurs within a wide range of tissue sparing and that there is “no simple relation … between lesion morphometry and behavioral recovery, except at the extremes of injury intensity” (Blight & Decrescito, 1986). Moreover, Schucht et al. (2002) showed that locomotor recovery correlates best with sparing of the reticulospinal tract in ventrolateral white matter, which appears to be spared in the Duncan work. Thus, these studies suggest that there is redundancy in spinal pathways such that sparing of ~10% of spinal white matter allows substantial recovery of function. When taken together, it is feasible that there were enough myelinated axons to surpass the threshold for the achieved level of hindlimb recovery in control and myrf-KO mice, and that tissue sparing would need to drop below 10% to have a better chance of revealing if lack of remyelination hampers motor recovery.
Another interesting point about myrf-KO mice having the same locomotor recovery as controls is that 95% of ventrolateral white matter axons were myelinated in control mice while only 65% were myelinated in myrf-KO (Duncan et al., 2018). In order to significantly affect locomotor function, however, it is likely that almost complete demyelination of this region would be needed, as was shown in work by Loy et al. (2002). Their study revealed that demyelination of the entire ventrolateral and ventral column white matter is necessary to significantly impair locomotor function. Their results suggested that a diffuse arrangement of multiple descending motor pathways control hindlimb locomotor functions, and that almost all these pathways need to be demyelinated before a large difference in BMS locomotor score would be observed.
Duncan et al. also highlight the dissociation between locomotor recovery, which plateaued by 2 weeks postinjury, and remyelination, which is not visible until after 2 weeks. This is said to be further evidence that remyelination does not play a role in recovery (Duncan et al., 2018). It is possible, however, that fine features of motor control would be more sensitive to altered remyelination. For instance, rodent studies characterizing cervical SCI show a much more protracted recovery of forelimb function, with substantial increases in recovery between 2 weeks and 6–8 weeks postinjury (Dunham, Siriphorn, Chompoopong, & Floyd, 2010; Gensel et al., 2006; Martinez, Brezun, Bonnier, & Xerri, 2009). Furthermore, given that human SCI patients can recover function over the first year postinjury (Kakulas, 2004), the association between remyelination and recovery may be different between rodents and humans. Notably, a nonhuman primate SCI study showed that the number of new oligodendrocytes increases 3.5-fold between 7 and 29 weeks postinjury, indicating that the process of cell replacement after SCI in primates is quite protracted (Yang et al., 2006).
Finally, clinical data showing that 4-aminopyridine or epidural stimulation can improve function in chronic SCI subjects, including return of some voluntary motor control, suggest that there are indeed intact but functionally silent axons present in some patient populations (Angeli et al., 2018; Angeli, Edgerton, Gerasimenko, & Harkema, 2014; Harkema et al., 2011; Hayes et al., 1993; Hayes et al., 1994; Rejc, Angeli, Atkinson, & Harkema, 2017). This variability in human SCI cases brings to mind discussions raised at the recent NIH SCI2020 meeting: Launching a Decade for Disruption in SCI research (https://videocast.nih.gov/summary.asp?live=30194&bhcp=1), which emphasized that strict control of animal models to reduce variability may be limiting our ability to accurately model all aspects of the human condition, including those who could potentially benefit from myelin-promoting therapies. Thus, while the Duncan et al. study is thought-provoking and suggests that oligodendrocyte remyelination may not improve hindlimb recovery after moderate thoracic SCI, it would seem prudent to proceed with caution until the idea that remyelination has no role in motor recovery from SCI is more widely tested and/or disproven.
7 |. POTENTIAL EFFECTS OF OLIGODENDROCYTES AND MYELIN ON AXON STRUCTURE AND FUNCTION AFTER SCI
Saltatory conduction of action potentials relies on properly formed nodes of Ranvier, which include axonal potassium Kv1.2 channels in the juxtaparanode, Contactin associated protein (Caspr) in the paranode, and sodium Nav1.6 channels in the node. When ion channels are properly organized in this way, action potentials “jump” from node to node, thereby significantly increasing conduction velocity. This highly structured organization of axonal proteins depends on physical associations between axonal proteins and proteins expressed by myelin at the paranodal loops, revealing that myelin plays an active role in axon structure (Chang et al., 2014; Dupree, Girault, & Popko, 1999; Marin et al., 2016; C. Zhang & Rasband, 2016). When axons lose their myelin sheath, nodal proteins diffuse, intermingle and lose their highly organized distribution on the axon, thereby preventing saltatory conduction. Notably, disrupted nodal protein organization has been detected in acute and chronic SCI tissue (Nashmi & Fehlings, 2001b; Powers et al., 2012); indeed we have noted nodal disruption on axons as late as 6 months postinjury in mice (Figure 5). These axons will have a drastically reduced ability to transmit signals between the brain and spinal cord. Fortunately, remyelination reestablishes proper nodal organization and therefore assists in recovery of conduction velocity (Marin et al., 2016). Notably, some nodal reorganization has been documented on remyelinated spinal axons after SCI (Hesp et al., 2015; Lasiene et al., 2008).
FIGURE 5.
Axonal node of Ranvier disruption is present chronically in the injured mouse spinal cord. Nodes are identified by Kv1.2+ segments (green) flanking Caspr+ segments (red). Axon neurofilaments are labeled with blue. (a) Confocal z-stacks of longitudinal sections from naïve spinal cords showing normal nodes of Ranvier. (b) White matter bordering SCI lesion sites at 6 months postinjury reveal marked spreading of Caspr and Kv1.2, an indication of demyelination. Pial border is at the top of the section in b-b”. Box in a,b shown at higher power in a’, b’. Boxes in a’, b’ shown at higher power in a” and b”. (a’-b”) Ion channel and Caspr spreading indicated by arrowheads in b”. Scale bars (a-b’) 25 μm, (a”, b”) 10 μm
Myelination also alters the axonal cytoskeleton, including the composition of neurofilaments and promoting phosphorylation of their side chains, which effectively spreads neurofilaments apart and widens the axon diameter (Brady et al., 1999). Axon demyelination results in a loss of side chain phosphorylation leading to axon thinning, which reduces action potential conduction speed (Powers et al., 2012). Demyelination-induced axon thinning is also thought to contribute to frank axon transection (Bjartmar, Yin, & Trapp, 1999; Waxman, 1989). Thus demyelination could make spared axons vulnerable to damage after SCI as well.
An interesting idea for which there is some evidence is that oligodendrocytes provide metabolic support for neurons (Lee et al., 2012). Since some axons are literally meters in length, it is implausible that the neuronal soma provides all the energy and nutrients necessary for the entire axon. Since oligodendrocytes wrap large segments of axons, the idea arose that perhaps oligodendrocytes supply energy substrates to axons. This oligodendrocyte to axon signaling could be initiated by what has been called the “axo-myelinic synapse” (reviewed in Micu, Plemel, Caprariello, Nave, & Stys, 2018). The myelin layer facing the axon expresses NMDA receptors, and when axons fire, these receptors are activated by glutamate released into the adaxonal space. This increases calcium locally in the oligodendrocyte process, which in turn is thought to promote lactate release from the myelin (Micu et al., 2018). Axons take up the lactate and use it for local energy production, which would efficiently couple axon activity with metabolic support from oligodendrocytes (Micu et al., 2018). Interestingly, this signaling is a two-way street that also benefits oligodendrocyte function due to glutamate-stimulated local MBP translation (Wake, Lee, & Fields, 2011). Other means of metabolic supply to axons may also exist, such as glucose uptake by axons at nodes of Ranvier and molecules released by nearby astrocytes, both of which may be sufficient for axonal energy production (Harris & Attwell, 2012; Waitt, Reed, Ransom, & Brown, 2017). Thus, long-term or even short-term demyelination of spinal axons after injury could not only impair action potential signaling but may also compromise the health and sustainability of the bare axons.
There are still other ways in which oligodendrocyte myelin facilitates axon health and function, although the cellular mechanisms are not completely understood (Nave & Trapp, 2008). For instance, deletion of CNP-1, a gene encoding an oligodendrocyte myelin protein, causes axon degeneration but does not impair myelin formation (Lappe-Siefke et al., 2003). Similarly, mice missing myelin proteolipid protein (PLP) have compact myelin and normal appearing oligodendrocytes, but undergo axon swelling and progressive axon loss over time (Griffiths et al., 1998). Interestingly, axon pathology even occurs when nonmyelin proteins are deleted in oligodendrocytes, such as Pex5, which codes for the peroxisomal protein peroxin-5. When Pex5 is deleted specifically in oligodendrocytes, mice develop progressive motor dysfunction and premature death, which is associated with extensive axon degeneration but not oligodendrocyte loss (Kassmann et al., 2007). Thus, oligodendrocytes serve a neuroprotective role that apparently requires them to have optimally functional peroxisomes. Oligodendrocytes can also release glial-derived neurotrophic factor (GDNF), which promotes neuron survival (Wilkins, Majed, Layfield, Compston, & Chandran, 2003). Clearly, oligodendrocyte/axon interactions are more complex than first meets the eye, which emphasizes that clarifying myelination issues after SCI will go beyond simply restoring conduction velocity. It may also facilitate preserving axons in a functioning and healthy state.
8 |. POTENTIAL ROLE OF AXON ACTIVITY IN POST-SCI REMYELINATION
It is well established that axon activity can drive developmental myelination, as first shown in rat pups where decreasing retinal ganglion cell activity led to reduced OPC proliferation in the optic nerve (Barres & Raff, 1993). It has since been shown that neural activity in the adult CNS can induce OPC proliferation, differentiation, myelin sheath synthesis and increased oligodendrocyte survival (Becker, Sadowsky, & McDonald, 2003; Fannon, Tarmier, & Fulton, 2015; Fields, 2015; Gautier et al., 2015; Gibson et al., 2014; Kougioumtzidou et al., 2017; Wake et al., 2011). Oligodendrocytes and OPCs rely on growth factor and neurotransmitter receptors to detect changes in neural activity (Káradóttir & Attwell, 2007). In particular, glutamate is widely studied in the context of myelination due to its multifaceted effects.
As stated above, depending on the situation, glutamate can promote OPC migration, facilitate OPC-axon contact and/or initiate OPC differentiation into myelinating oligodendrocytes (Gautier et al., 2015; Gudz, Komuro, & Macklin, 2006). Contrary to popular belief, vesicular neurotransmitter release is not restricted to the synaptic cleft; as discussed above glutamate-containing vesicles are stored and released along axon shafts (Kukley et al., 2007; Ziskin et al., 2007). Neural activity stimulates axonal glutamate release via vesicle exocytosis, after which glutamate binds to and activates AMPA receptors on adjacent OPC processes (Kukley et al., 2007; Ziskin et al., 2007).
It is plausible that glutamate also regulates OPCs after SCI. As mentioned above, excess glutamate causes excitotoxicity acutely after SCI, which may prompt surviving OPCs to migrate, proliferate and replace the lost cells (Xu et al., 2004). Based on recent evidence showing glutamate is necessary for remyelination after chemical demyelination (Gautier et al., 2015; Sahel et al., 2015), it may also be a mediator of remyelination after SCI. For instance, after moderate SCI, spared rubrospinal tract axons become demyelinated and then remyelinated by oligodendrocytes (Lasiene et al., 2008; Powers et al., 2012). Since over 95% of these axons are glutamatergic (Du Beau et al., 2012), axon-derived glutamate may signal to local OPCs and promote remyelination. While this mechanism may raise concerns of excitotoxicity, levels of released glutamate likely mimic those released at neuron/neuron synapses, which do not cause pathology. Future studies are needed to identify the role of glutamate (and other neurotransmitters) in endogenous repair after SCI.
9 |. ADDITIONAL FACTORS TO CONSIDER IN OPC RESPONSES AND MYELINATION AFTER SCI
9.1 |. Regenerating axons typically do not get myelinated
Despite the robust myelination of spared axons after SCI, axons induced to regenerate typically fail to become myelinated. For instance, ascending sensory axons can be stimulated to regenerate beyond a cervical SCI and reinnervate the appropriate brainstem nuclei; however, sensory function is not returned because the new axons lack myelin (Alto et al., 2009). This is similar to regenerated optic nerve axons reconnecting to their appropriate target in the superior colliculus but failing to restore visual function due to lack of myelination (Bei et al., 2016). Similarly, only a quarter of axons growing out from intraspinal neural progenitor transplants become myelinated (Hunt, Lu, & Tuszynski, 2017). Thus, understanding mechanisms that drive remyelination of spared axons after SCI may provide insight into enticing oligodendrocyte lineage cells (or Schwann cells) to also myelinate growing axons. This is not a trivial problem as there are hundreds of studies and numerous laboratories currently working to enhance axon regrowth after SCI, not to mention countless individuals with SCI placing their hopes for recovery on axon regeneration. It would be a shame if methods to recreate descending and intraspinal circuits were successful yet functional improvements remain absent because myelination of the new axons did not occur.
9.2 |. Physical activity levels and environmental changes
Physical activity is obviously reduced after SCI, especially during the acute phase. This likely has detrimental effects on overall recovery, as demonstrated by the fact that SCI rodents placed in “rat wheelchairs” recover significantly less locomotor abilities compared to controls (Caudle et al., 2011). SCI-induced restriction of patients to hospital beds could potentially be viewed as being placed in an “impoverished” environment, given that patients typically go from a life with unlimited mobility to lying in a hospital room for days. While enriched environments promote myelination in rodents (Markham, Herting, Luszpak, Juraska, & Greenough, 2009; Pusic & Kraig, 2014; Yang et al., 2013; Zheng, Ding, Li, & Yang, 2017), social isolation has the reverse effect. Just 2 weeks of social isolation is sufficient to significantly reduce myelin thickness in the prefrontal cortex of adult mice (Liu et al., 2012). A comparable effect may take place in the spinal cord in that reduced mobility could impair spontaneous remyelination. Reassuringly, reintroducing animals into a social environment reversed myelin loss in the brain (Liu et al., 2012). For the SCI patient, these results suggest that getting as much social interaction and physical activity as soon as their condition allows may be critically important for recovery.
Indeed, recent evidence suggests that increased physical activity after SCI may promote remyelination. Currently there is much excitement in the SCI field over the remarkable results being obtained with activity-based training and epidural stimulation in SCI individuals (Capogrosso et al., 2016; Formento et al., 2018; Gill et al., 2018; Moraud et al., 2016; Wagner et al., 2018; Wenger et al., 2014). The most common form of activity-based therapy is body weight supported treadmill training in which SCI subjects are placed in a harness and trainers move their limbs to provide sufficient input to the neuromuscular system (Dobkin et al., 2003). Activity-dependent training is associated with functional reorganization of spinal circuitry and enhanced motor recovery in animals and humans with chronic SCI (Fouad & Tetzlaff, 2012; Leblond, L’Esperance, Orsal, & Rossignol, 2003; Wernig & Müller, 1992). The cellular mechanisms driving activity-based recovery are not clear. However, active or passive exercise drives growth factor expression within the CNS, including factors that positively regulate myelination such as brain-derived neurotrophic factor (BDNF) (Keeler et al., 2012; Leech & Hornby, 2017; Wang et al., 2015; Ying et al., 2008; Ying, Roy, Edgerton, & Gómez-Pinilla, 2005). How activity-based training alters oligodendrocyte functions or myelination status after SCI is currently unknown.
As stated above, neuronal activity-induced myelination in the adult CNS is also emerging as an exciting approach. For instance, optogenetic stimulation of murine cortical neurons increases axon-OPC contacts, mature oligodendrocyte numbers, myelin sheath formation, and alters motor function (Gibson et al., 2014; Li & Li, 2017). This may also be true in the spinal cord. For instance, electrical stimulation of the motor cortex or medullary pyramids stimulates OPC proliferation and differentiation in the corticospinal tract (Li, Brus-Ramer, Martin, & McDonald, 2010; Li, Houdayer, Liu, & Belegu, 2017). After SCI, epidural stimulation of the spinal cord activates spinal circuitry to stimulate locomotor activity, and when combined with physical therapy, completely paralyzed animals and some human subjects experience locomotor recovery (Angeli et al., 2018; Carhart, He, Herman, D’Luzansky, & Willis, 2004; Ichiyama, Gerasimenko, Zhong, Roy, & Edgerton, 2005; Rejc et al., 2017). Excitingly, SCI subjects undergoing this treatment report additional important benefits, such as improved cardiovascular function (Angeli et al., 2018), demonstrating that measuring nonmotor functions is critical as well.
Since neural activity can facilitate OPC-axon contact through vesicular glutamate release, it is theorized that glutamate plays a role in neuromodulation-induced locomotion. Indeed, stimulating murine brainstem glutamatergic neurons in conjunction with gravity-assisted rehabilitation promotes extensive reorganization of neural projections, ultimately leading to long lasting motor recovery, even when epidural stimulation is discontinued (Asboth et al., 2018). Perhaps neuromodulation allows electrically silent neurons/axons to regain activity-induced glutamate release after SCI. This in turn could induce permanent rewiring and remyelination necessary for motor control. The power of this technique to reverse disability in some SCI subjects is quite profound. Therefore, it is essential that studies work toward understanding the cellular mechanisms, including potential remyelination induced as part of this response, so that it may be optimized and perhaps better translated to those individuals in which it does not work.
9.3 |. Common therapeutic and experimental SCI medications
Spinal cord injured individuals are often prescribed multiple medications for the many complications they routinely face. The potential off-target effect of these medicines, however, on endogenous cell responses is not always clear or considered, and these effects could be beneficial or detrimental. For instance, spasticity is a common problem after SCI, which is often managed by intrathecal administration of baclofen. While baclofen typically provides relief of excess spasticity, it may also affect oligodendrocyte lineage cells. OPCs express functional gamma-aminobutyric acid B (GABA(B)) receptors that, when activated by baclofen, promote OPC migration and proliferation (Luyt et al., 2007). This could theoretically impact their repair response by interfering with differentiation into oligodendrocytes. Another commonly prescribed medication is pregabalin or gabapentin (aka Neurontin) for neuropathic pain. Gabapentin is structurally similar to GABA, and while studies of its effects on CNS myelin have not been completed, it does increase MBP expression and myelination of peripheral nerves (Câmara et al., 2015). Higher doses of gabapentin have been associated with memory impairment and decreased executive function in a small cohort of SCI individuals (Shem, Barncord, Flavin, & Mohan, 2018), which could be mediated, at least in part, by effects on myelination of the prefrontal cortex.
Minocycline, an anti-inflammatory agent currently in Phase 3 clinical trials for SCI, is a tetracycline antibiotic that promotes white matter sparing in preclinical SCI models (Stirling et al., 2004). The mechanisms by which minocycline work could be varied, but may include reducing pro-nerve growth factor production by microglia, which is a known toxin for oligodendrocytes (Beattie et al., 2002; Yune et al., 2007). Minocycline is beneficial in in vitro models of oxygen–glucose deprivation of OPCs and in vivo demyelination models (Schmitz, Endesfelder, Chew, Zaak, & Bührer, 2012; Skripuletz et al., 2010). However, other work showed that minocycline can impair OPC responses after demyelination (Li, Setzu, Zhao, & Franklin, 2005), which may be mediated in part by reducing CNTF levels (Tanaka, Murakami, Bando, & Yoshida, 2013). While the collective actions of minocycline after SCI appear to be positive, oligodendrocyte-specific effects should be kept in mind when interpreting data.
A drug currently in clinical trials for SCI due to its axon growth-promoting effects is the Rho/ROCK inhibitor Cethrin (Dergham et al., 2002; Forgione & Fehlings, 2014). While Rho/ROCK actions on axon growth are well known, less recognized may be that oligodendrocyte lineage cells also use Rho/ROCK signaling (Harlow & Macklin, 2014). ROCK inhibition in cultured OPCs promotes differentiation and enhances myelination of neurons cocultured with the OPCs (Pedraza et al., 2014). Rho-ROCK signaling may also promote oligodendrocyte survival through a PPAR-mediated mechanism (Paintlia, Paintlia, Singh, & Singh, 2013). Thus, Cethrin treatment after SCI may not only increase recovery by enhancing axon growth, but also by inducing oligodendrocyte sparing and myelination of spared or regenerated axons, which, as stated above, typically do not undergo spontaneous myelination.
Lastly, a potentially promising therapeutic agent currently in clinical trials for SCI is riluzole. Riluzole is a neuroprotective agent that interferes with glutamate signaling and is currently FDA approved for ALS. While glutamate excitotoxicity is a recognized secondary injury mechanism after SCI, as discussed above, glutamate signaling also may be important for activity-dependent myelination (Gautier et al., 2015; Xu et al., 2004). Therefore, examining white matter changes after riluzole treatment will be important to help delineate mechanisms that contribute to improved recovery.
10 |. SUMMARY
Overall, it is clear that the injured spinal cord is a highly dynamic environment in which cell death and replacement continue long after the initial trauma, and in which circuit function may be “reawakened” chronically with neuromodulation. While SCI research has generated >33,000 manuscripts and dates back over 100 years, in many ways it is still in its infancy in understanding what actually occurs at a cellular level within and around the injury site, both acutely and chronically. Preclinical animal models have been perfected perhaps to a fault, in that they may not represent a large portion of SCI individuals and we may therefore miss potentially promising treatments for that set of patients.
With regard to myelination in particular, it is important to determine if the surprisingly robust and long-lasting oligodendrogenesis and remyelination observed in rodents also occurs in the clinical human population. It is also imperative that better assays be developed for SCI subjects to determine if there is a need for improved remyelination, which will likely depend on the variety of factors discussed above. It is also important to recognize that the cellular environment fluctuates after SCI, and therefore any therapeutic treatment targeting remyelination (or other repair mechanisms) should take into consideration possible concomitant environmental factors that could enhance or impair the therapy. Likewise, therapies targeting particular cellular mechanisms should be aware that a fairly robust endogenous reparative response may be occurring that should not be interfered with by the treatment.
While the ever-changing SCI environment makes studying cellular interactions and signaling mechanisms challenging, this could also be viewed as a strength of the model. SCI studies give the opportunity to examine OPC (and other cellular) responses during times of active hemorrhage, vascular shearing, excitoxicity and cell death in the acute phase; during times of angiogenesis, immune cell infiltration, initiation of growth factor expression, axon sprouting and Wallerian degeneration in the subacute phase; and during long-term tissue remodeling, glial and fibrotic scarring, and rising pro- and anti-myelinating factor expression in the chronic phase. SCI provides different tissue environments in terms of proximity to the injury site and injury responses in distal white matter and gray matter. Lets hope that it does not take the next 100 years of research to bring us clarity on cellular mechanisms and therapeutic strategies that allow rapid and tailored responses to SCI so that it is no longer the devastating event that it is today.
ACKNOWLEDGMENT
Funding for data included in this review came from National Institute of Neurological Disorders and Stroke (NINDS) R01NS100522.
Funding information
National Institute of Neurological Disorders and Stroke, Grant/Award Number: R01NS100522
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, & Probert L (1998). Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice. The American Journal of Pathology, 153(3), 801–813. 10.1016/S0002-9440(10)65622-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, & Levison SW (2002). Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Experimental Neurology, 173(1), 46–62. 10.1006/exnr.2001.7834 [DOI] [PubMed] [Google Scholar]
- Almad A, & McTigue DM (2010). Chronic expression of PPAR-delta by oligodendrocyte lineage cells in the injured rat spinal cord. The Journal of Comparative Neurology, 518(6), 785–799. 10.1002/cne.22242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almad A, Sahinkaya FR, & McTigue DM (2011). Oligodendrocyte fate after spinal cord injury. Neurotherapeutics, 8(2), 262–273. 10.1007/s13311-011-0033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto LT, Havton LA, Conner JM, Hollis ER, Blesch A, & Tuszynski MH (2009). Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nature Neuroscience, 12(9), 1106–1113. 10.1038/nn.2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, … Harkema SJ (2018). Recovery of over-ground walking after chronic motor complete spinal cord injury. New England Journal of Medicine, 379(13), 1244–1250. 10.1056/NEJMoa1803588 [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, & Harkema SJ (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain, 137(5), 1394–1409. 10.1093/brain/awu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthes DL, Theriault E, & Tator CH (1996). Ultrastructural evidence for arteriolar vasospasm after spinal cord trauma. Neurosurgery, 39(4), 804–814. 10.1097/00006123-199610000-00032 [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Frost EE, Borke RC, & Vana AC (2002). Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. The Journal of Neuroscience, 22(19), 8574–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, & Ting JP-Y (2001). TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nature Neuroscience, 4(11), 1116–1122. 10.1038/nn738 [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Schnell L, Lou L, Golshani R, Hunanyan A, Ghosh A, … Mendell LM (2009). Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Experimental Neurology, 216(2), 471–480. 10.1016/J.EXPNEUROL.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, … Courtine G (2018). Cortico–reticulo–spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nature Neuroscience, 21(4), 576–588. 10.1038/s41593-018-0093-5 [DOI] [PubMed] [Google Scholar]
- Assinck P, Duncan GJ, Plemel JR, Lee MJ, Stratton JA, Manesh SB, … Tetzlaff W (2017). Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. The Journal of Neuroscience, 37(36), 8635–8654. 10.1523/JNEUROSCI.2409-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri Y, Burzomato V, Frugier G, Hamilton NB, Káradóttir R, & Attwell D (2009). Glutamatergic signaling in the brain’s white matter. Neuroscience, 158(1), 266–274. 10.1016/j.neuroscience.2008.01.015 [DOI] [PubMed] [Google Scholar]
- Balentine JD (1978). Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Laboratory Investigation, 39(3), 254–266. [PubMed] [Google Scholar]
- Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, & Frisén J (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell, 7(4), 470–482. 10.1016/j.stem.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Barres BA, & Raff MC (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature, 361(6409), 258–260. 10.1038/361258a0 [DOI] [PubMed] [Google Scholar]
- Bartus K, Galino J, James ND, Hernandez-Miranda LR, Dawes JM, Fricker FR, … Bradbury EJ (2016). Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain, 139(5), 1394–1416. 10.1093/brain/aww039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, & Bresnahan JC (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma, 12(1), 1–21. 10.1089/neu.1995.12.1 [DOI] [PubMed] [Google Scholar]
- Bastien D, Bellver Landete V, Lessard M, Vallieres N, Champagne M, Takashima A, … Lacroix S (2015). IL-1 gene deletion protects oligodendrocytes after spinal cord injury through upregulation of the survival factor Tox3. The Journal of Neuroscience, 35, 10715–10730. 10.1523/JNEUROSCI.0498-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, … Yoon SO (2002). ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron, 36(3), 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Sadowsky CL, & McDonald JW (2003). Restoring function after spinal cord injury. The Neurologist, 9(1), 1–15. 10.1097/01.nrl.0000038587.58012.05 [DOI] [PubMed] [Google Scholar]
- Bei F, Lee HHC, Liu X, Gunner G, Jin H, Ma L, … He Z (2016). Restoration of visual function by enhancing conduction in regenerated axons. Cell, 164(1–2), 219–232. 10.1016/j.cell.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, & Hagg T (2011). Vascular pathology as a potential therapeutic target in SCI. Translational Stroke Research, 2(4), 556–574. 10.1007/s12975-011-0128-7 [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JDB, Somogyi P, & Jahr CE (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature, 405(6783), 187–191. 10.1038/35012083 [DOI] [PubMed] [Google Scholar]
- Bickenbach J, Officer A, Shakespeare T, von Groote Per, World Health Organization. et al. (2013). International perspectives on spinal cord injury / edited by Jerome Bickenbach. World Health Organization. https://apps.who.int/iris/handle/10665/94190 [Google Scholar]
- Bjartmar C, Yin X, & Trapp BD (1999). Axonal pathology in myelin disorders. Journal of Neurocytology, 28(4–5), 383–395. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, & Franklin RJM (2008). Remyelination in experimental models of toxin-induced demyelination. Current Topics in Microbiology and Immunology, 318, 193–212. [DOI] [PubMed] [Google Scholar]
- Blight AR (1983a). Axonal physiology of chronic spinal cord injury in the cat: Intracellular recording in vitro. Neuroscience, 10(4), 1471–1486. 10.1016/0306-4522(83)90128-8 [DOI] [PubMed] [Google Scholar]
- Blight AR (1983b). Cellular morphology of chronic spinal cord injury in the cat: Analysis of myelinated axons by line-sampling. Neuroscience, 10(2), 521–543. 10.1016/0306-4522(83)90150-1 [DOI] [PubMed] [Google Scholar]
- Blight AR, & Decrescito V (1986). Morphometric analysis of experimental spinal cord injury in the cat: The relation of injury intensity to survival of myelinated axons. Neuroscience, 19(1), 321–341. 10.1016/0306-4522(86)90025-4 [DOI] [PubMed] [Google Scholar]
- Brady ST, Witt AS, Kirkpatrick LL, de Waegh SM, Readhead C, Tu PH, & Lee VM (1999). Formation of compact myelin is required for maturation of the axonal cytoskeleton. The Journal of Neuroscience, 19(17), 7278–7288. 10.1242/jeb.089177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan JC, Beattie MS, Todd FD, & Noyes DH (1987). A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Experimental Neurology, 95(3), 548–570. 10.1016/0014-4886(87)90299-8 [DOI] [PubMed] [Google Scholar]
- Bresnahan JC, King JS, Martin GF, & Yashon D (1976). A neuroanatomical analysis of spinal cord injury in the rhesus monkey (Macaca mulatta). Journal of the Neurological Sciences, 28(4), 521–542. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, & Quencer RM (1993). Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Advances in Neurology, 59, 75–89. [PubMed] [Google Scholar]
- Burda JE, & Sofroniew MV (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron, 81(2), 229–248. 10.1016/j.neuron.2013.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Pech K, Merkler D, Kakulas BA, Martin D, Schoenen J, … Brook GA (2004). Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain, 128(2), 356–364. 10.1093/brain/awh355 [DOI] [PubMed] [Google Scholar]
- Câmara CC, Araújo CV, de Sousa KKO, Brito GAC, Vale ML, Raposo RDS, … Oriá RB (2015). Gabapentin attenuates neuropathic pain and improves nerve myelination after chronic sciatic constriction in rats. Neuroscience Letters, 607, 52–58. 10.1016/j.neulet.2015.09.021 [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot J-B, … Courtine G (2016). A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature, 539 (7628), 284–288. 10.1038/nature20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas DD, Ditunno J, Graziani V, Jackson AB, Lammertse D, Potter P, … Blight AR (2007). Phase 2 trial of sustained-release fampridine in chronic spinal cord injury. Spinal Cord, 45(2), 158–168. 10.1038/sj.sc.3101947 [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Ditunno JF, Graziani V, McLain AB, Lammertse DP, Potter PJ, … Blight AR (2014). Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal Cord, 52(1), 70–76. 10.1038/sc.2013.137 [DOI] [PubMed] [Google Scholar]
- Carhart MR, He J, Herman R, D’Luzansky S, & Willis WT (2004). Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 12(1), 32–42. 10.1109/TNSRE.2003.822763 [DOI] [PubMed] [Google Scholar]
- Caudle KL, Brown EH, Shum-Siu A, Burke DA, Magnuson TSG, Voor MJ, & Magnuson DSK (2011). Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehabilitation and Neural Repair, 25(8), 729–723. 10.1177/1545968311407519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-J, Zollinger DR, Susuki K, Sherman DL, Makara MA, Brophy PJ, … Rasband MN (2014). Glial ankyrins facilitate paranodal axoglial junction assembly. Nature Neuroscience, 17(12), 1673–1681. 10.1038/nn.3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheepsunthorn P, Palmer C, & Connor JR (1998). Cellular distribution of ferritin subunits in postnatal rat brain. The Journal of Comparative Neurology, 400(1), 73–86. [DOI] [PubMed] [Google Scholar]
- Chen N-F, Sung C-S, Wen Z-H, Chen C-H, Feng C-W, Hung H-C, … Chen W-F (2018). Therapeutic effect of platelet-rich plasma in rat spinal cord injuries. Frontiers in Neuroscience, 12, 252 10.3389/fnins.2018.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Wang Y, He Q, Qiu M, Whittemore SR, & Cao Q (2007). Bone morphogenetic protein signaling and Olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells, 25(12), 3204–3214. 10.1634/stemcells.2007-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Kigerl KA, Lerch JK, Popovich PG, & McTigue DM (2016). TLR4 deficiency impairs oligodendrocyte formation in the injured spinal cord. The Journal of Neuroscience, 36(23), 6352–6364. 10.1523/JNEUROSCI.0353-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AH, Chambers C, & Franklin RJM (2013). Remyelination: The true regeneration of the central nervous system. Journal of Comparative Pathology, 149(2–3), 242–254. 10.1016/j.jcpa.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Whitmire JK, Frausto RF, Chertboonmuang P, Soloway PD, Whitton JL, & Campbell IL (2006). Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. American Journal of Pathology, 69 (6), 2104–2116. 10.2353/ajpath.2006.060626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, & Beattie MS (1997). Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Medicine, 3(1), 73–76. 10.1038/nm0197-73 [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, & Reynolds R (2003). NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Molecular and Cellular Neuroscience, 24(2), 476–488. 10.1016/S1044-7431(03)00210-0 [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, & McKerracher L (2002). Rho signaling pathway targeted to promote spinal cord repair. The Journal of Neuroscience, 22(15), 6570–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, … Spinal Cord Injury Locomotor Trial (SCILT) Group. (2003). Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabilitation and Neural Repair, 17(3), 153–167. 10.1177/0888439003255508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrmann GJ, & Allen WE (1975). Microcirculation of traumatized spinal cord: A correlation of microangiography and blood flow patterns in transitory and permanent paraplegia. Journal of Trauma, 15(11), 1003–1013. 10.1097/00005373-197511000-00011 [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, & Popovich PG (2008). Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Experimental Neurology, 209(2), 378–388. 10.1016/j.expneurol.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Beau A, Shakya Shrestha S, Bannatyne BA, Jalicy SM, Linnen S, & Maxwell DJ (2012). Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience, 227, 67–79. 10.1016/J.NEUROSCIENCE.2012.09.037 [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Manesh SB, Hilton BJ, Assinck P, Liu J, Moulson A, … Tetzlaff W (2018). Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nature Communications, 9(1), 3066 10.1038/s41467-018-05473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham KA, Siriphorn A, Chompoopong S, & Floyd CL (2010). Characterization of a graded cervical hemicontusion spinal cord injury model in adult male rats. Journal of Neurotrauma, 27(11), 2091–2106. 10.1089/neu.2010.1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J, & Blight A (2011). Dalfampridine: A brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis. Current Medical Research and Opinion, 27(7), 1415–1423. 10.1185/03007995.2011.583229 [DOI] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, & Popko B (1999). Axo-glial interactions regulate the localization of axonal paranodal proteins. Journal of Cell Biology, 147(6), 1145–1151. 10.1083/jcb.147.6.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, & Lu QR (2015). Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harbor Perspectives in Biology, 7(9), a020461 10.1101/cshperspect.a020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel U, & Wolswijk G (1996). Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: in vitro characteristics and response to PDGF, bFGF and NT-3. Glia, 16(1), 16–26. [DOI] [PubMed] [Google Scholar]
- Fannon J, Tarmier W, & Fulton D (2015). Neuronal activity and AMPAtype glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia, 63(6), 1021–1035. 10.1002/glia.22799 [DOI] [PubMed] [Google Scholar]
- Fassbender JM, Whittemore SR, & Hagg T (2011). Targeting microvasculature for neuroprotection after SCI. Neurotherapeutics, 8(2), 240–251. 10.1007/s13311-011-0029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings MG, & Tator CH (1995). The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Experimental Neurology, 132(2), 220–228. [DOI] [PubMed] [Google Scholar]
- Ferbert T, Child C, Graeser V, Swing T, Akbar M, Heller R, … Moghaddam A (2017). Tracking spinal cord injury: Differences in cytokine expression of IGF-1, TGF-B1, and sCD95l can be measured in blood samples and correspond to neurological remission in a 12-week follow-up. Journal of Neurotrauma, 34(3), 607–614. 10.1089/neu.2015.4294 [DOI] [PubMed] [Google Scholar]
- Fields RD (2008). Oligodendrocytes changing the rules: Action potentials in glia and oligodendrocytes controlling action potentials. The Neuroscientist, 14(6), 540–543. 10.1177/1073858408320294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2015). A new mechanism of nervous system plasticity: Activity-dependent myelination. Nature Reviews Neuroscience, 16(12), 756–767. 10.1038/nrn4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Wrathall JR, & Mocchetti I (1994). Increased basic fibroblast growth factor mRNA following contusive spinal cord injury. Brain Research. Molecular Brain Research, 22(1–4), 1–8. [DOI] [PubMed] [Google Scholar]
- Forgione N, & Fehlings MG (2014). Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurgery, 82(3–4), e535–e539. 10.1016/j.wneu.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Formento E, Minassian K, Wagner F, Mignardot JB, Le Goff-Mignardot CG, Rowald A, … Courtine G (2018). Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nature Neuroscience, 21(12), 1728–1741. 10.1038/s41593-018-0262-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, & Tetzlaff W (2012). Rehabilitative training and plasticity following spinal cord injury. Experimental Neurology, 235(1), 91–99. 10.1016/J.EXPNEUROL.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Franklin RJM, & ffrench-Constant C (2017). Regenerating CNS myelin—From mechanisms to experimental medicines. Nature Reviews Neuroscience, 18(12), 753–769. 10.1038/nrn.2017.136 [DOI] [PubMed] [Google Scholar]
- Frederick TJ, & Wood TL (2004). IGF-I and FGF-2 coordinately enhance cyclin D1 and cyclin E-cdk2 association and activity to promote G1progression in oligodendrocyte progenitor cells. Molecular and Cellular Neuroscience, 25, 480–492. 10.1016/j.mcn.2003.11.015 [DOI] [PubMed] [Google Scholar]
- Gautier HOB, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, … Káradóttir RT (2015). Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nature Communications, 6, 8518 10.1038/ncomms9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FPT, Deibert RJ, Beattie MS, & Bresnahan JC (2006). Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. Journal of Neurotrauma, 23(1), 36–54. 10.1089/neu.2006.23.36 [DOI] [PubMed] [Google Scholar]
- Gensel JC, & Zhang B (2015). Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Research, 1619, 1–11. 10.1016/j.brainres.2014.12.045 [DOI] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, … Monje M (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science (New York, N.Y.), 344(6183), 1252304 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, … Zhao KD (2018). Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nature Medicine, 24(11), 1677–1682. 10.1038/s41591-018-0175-7 [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Schultze JL, Murray PJ, Ochando J, & Biswas SK (2016). New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nature Immunology, 17(1), 34–40. 10.1038/ni.3324 [DOI] [PubMed] [Google Scholar]
- Gledhill RF, Harrison BM, & WI MD (1973). Demyelination and remyelination after acute spinal cord compression. Experimental Neurology, 38, 472–487. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Kirvell SL, & Butt AM (2001). Fibroblast growth factor-2 inhibits myelin production by oligodendrocytes in vivo. Molecular and Cellular Neuroscience, 18, 557–569. 10.1006/mcne.2001.1025 [DOI] [PubMed] [Google Scholar]
- Goldstein EZ, Church JS, Pukos N, Gottipati MK, Popovich PG, & McTigue DM (2017). Intraspinal TLR4 activation promotes iron storage but does not protect neurons or oligodendrocytes from progressive iron-mediated damage. Experimental Neurology, 298, 42–56. 10.1016/J.EXPNEUROL.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman J, Skoff R, & Yang X (1998). Member of the peroxisome proliferator-activated receptor family of transcription factors is differentially expressed by oligodendrocytes. Journal of Neuroscience Research, 51(5), 563–573. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, … Nave KA (1998). Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science, 280 (5369), 1610–1613. 10.1126/science.280.5369.1610 [DOI] [PubMed] [Google Scholar]
- Griffiths IR, & McCulloch MC (1983). Nerve fibres in spinal cord impact injuries. Part 1. Changes in the myelin sheath during the initial 5 weeks. Journal of the Neurological Sciences, 58(3), 335–349. 10.1016/0022-510X(83)90093-X [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, & Golden JA (2000). Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. Journal of Neurobiology, 43(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, & Wrathall JR (2001). Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Experimental Neurology, 168, 273–282. 10.1006/exnr.2001.7628 [DOI] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, & Macklin WB (2006). Glutamate stimulates oligodendrocyte progenitor migration mediated via an v integrin/myelin proteolipid protein complex. Journal of Neuroscience, 26(9), 2458–2466. 10.1523/JNEUROSCI.4054-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, & Bunge RP (2005). Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Experimental Neurology, 192(2), 384–393. 10.1016/j.expneurol.2004.11.033 [DOI] [PubMed] [Google Scholar]
- Guha A, Tator CH, & Piper I (1985). Increase in rat spinal cord blood flow with the calcium channel blocker, nimodipine. Journal of Neurosurgery, 63, 250–259. 10.3171/jns.1985.63.2.0250 [DOI] [PubMed] [Google Scholar]
- Hackett AR, Lee D-H, Dawood A, Rodriguez M, Funk L, Tsoulfas P, & Lee JK (2016). STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiology of Disease, 89, 10–22. 10.1016/j.nbd.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansebout RR, Blight AR, Fawcett S, & Reddy K (1993). 4-Aminopyridine in chronic spinal cord injury: A controlled, doubleblind, crossover study in eight patients. Journal of Neurotrauma, 10(1), 1–18. 10.1089/neu.1993.10.1 [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, … Edgerton VR (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. The Lancet, 377(9781), 1938–1947. 10.1016/S0140-6736(11)60547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow DE, & Macklin WB (2014). Inhibitors of myelination: ECM changes, CSPGs and PTPs. Experimental Neurology, 251, 39–46. 10.1016/j.expneurol.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, & Attwell D (2012). The energetics of CNS white matter. Journal of Neuroscience, 32(1), 356–371. 10.1523/JNEUROSCI.3430-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BM, Gledhill RF, & McDonald WJ (1975). Remyelination after transient compression of the spinal cord. Proceedings of the Australian Association of Neurologists, 12, 117–122. [PubMed] [Google Scholar]
- Harrison BM, & McDonald WI (1977). Remyelination after transient experimental compression of the spinal cord. Annals of Neurology, 1, 542–551. [DOI] [PubMed] [Google Scholar]
- Hawryluk GWJ, Mothe A, Wang J, Wang S, Tator C, & Fehlings MG (2012). An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells and Development, 21 (12), 2222–2238. 10.1089/scd.2011.0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk GWJ, Spano S, Chew D, Wang S, Erwin M, Chamankhah M, … Fehlings MG (2014). An examination of the mechanisms by which neural precursors augment recovery following spinal cord injury: A key role for remyelination. Cell Transplantation, 23(3), 365–380. 10.3727/096368912X662408 [DOI] [PubMed] [Google Scholar]
- Hayes KC, Blight AR, Potter PJ, Allatt RD, Hsieh JTC, Wolfe DL, … Hamilton JT (1993). Predinical trial of 4-aminopyridine in patients with chronic spinal cord injury. Paraplegia, 31(4), 216–224. 10.1038/sc.1993.40 [DOI] [PubMed] [Google Scholar]
- Hayes KC, Potter PJ, Wolfe DL, Hseih JTC, Delaney GA, & Blight AR (1994). 4-Aminopyridine-sensitive neurologic deficits in patients with spinal cord injury. Journal of Neurotrauma, 11(4), 433–446. 10.1089/neu.1994.11.433 [DOI] [PubMed] [Google Scholar]
- Helweg-Larsen S, & Laursen H (1998). Clinical and autopsy findings in spinal cord compression due to metastatic disease. European Journal of Neurology, 5(6), 587–592. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, Bresnahan JC, & Beattie MS (2001). Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiology of Disease, 8, 590–599. 10.1006/nbdi.2001.0414 [DOI] [PubMed] [Google Scholar]
- Hesp ZC, Goldstein EA, Miranda CJ, Kaspar BK, & McTigue DM (2015). Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. Journal of Neuroscience, 35(3), 1274–1290. 10.1523/JNEUROSCI.2568-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesp ZC, Yoseph RY, Suzuki R, Jukkola P, Wilson C, Nishiyama A, & McTigue DM (2018). Proliferating NG2-celldependent angiogenesis and scar formation alter axon growth and functional recovery after spinal cord injury in mice. The Journal of Neuroscience, 38(6), 1366–1382. 10.1523/JNEUROSCI.3953-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Beattie MS, & Bresnahan JC (2001). Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Experimental Neurology, 171(1), 153–169. 10.1006/exnr.2001.7734 [DOI] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, & Horner PJ (2006). Fate of endogenous stem/progenitor cells following spinal cord injury. Journal of Comparative Neurology, 498(4), 525–538. 10.1002/cne.21065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, … Gage FH (2000). Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. The Journal of Neuroscience, 20(6), 2218–2228. 10.1523/JNEUROSCI.20-06-02218.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, & Bergles DE (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neuroscience, 16(6), 668–676. 10.1038/nn.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunanyan AS, Alessi V, Patel S, Pearse DD, Matthews G, & Arvanian VL (2011). Alterations of action potentials and the localization of Nav1.6 sodium channels in spared axons after hemisection injury of the spinal cord in adult rats. Journal of Neurophysiology, 105(3), 1033–1044. 10.1152/jn.00810.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M, Lu P, & Tuszynski MH (2017). Myelination of axons emerging from neural progenitor grafts after spinal cord injury. Experimental Neurology, 296, 69–73. 10.1016/J.EXPNEUROL.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, & Edgerton VR (2005). Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neuroscience Letters, 383(3), 339–344. 10.1016/j.neulet.2005.04.049 [DOI] [PubMed] [Google Scholar]
- James ND, Bartus K, Grist J, Bennett DLH, McMahon SB, & Bradbury EJ (2011). Conduction failure following spinal cord injury: Functional and anatomical changes from acute to chronic stages. The Journal of Neuroscience, 31(50), 18543–18555. 10.1523/JNEUROSCI.4306-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Frederick TJ, & Wood TL (2001). IGF-I synergizes with FGF-2 to stimulate oligodendrocyte progenitor entry into the cell cycle. Developmental Biology, 232, 414–423. 10.1006/dbio.2001.0208 [DOI] [PubMed] [Google Scholar]
- Jiang FJ, Levison SW, & Wood TL (1999). Ciliary neurotrophic factor induces expression of the IGF type I receptor and FGF receptor 1 mRNAs in adult rat brain oligodendrocytes. Journal of Neuroscience Research, 57, 447–457. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Tybor K, Kilianek L, Raine CS, & Selmaj K (2005). Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain, 128(11), 2675–2688. 10.1093/brain/awh627 [DOI] [PubMed] [Google Scholar]
- Kakulas BA (2004). Neuropathology: The foundation for new treatments in spinal cord injury. Spinal Cord, 42, 549–563. 10.1038/sj.sc.3101670 [DOI] [PubMed] [Google Scholar]
- Kang SS, Keasey MP, Cai J, & Hagg T (2012). Loss of neuronastroglial interaction rapidly induces protective CNTF expression after stroke in mice. Journal of Neuroscience, 32, 9277–9287. 10.1523/JNEUROSCI.1746-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, & Attwell D (2007). Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience, 145(4), 1426–1438. 10.1016/j.neuroscience.2006.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, & Fehlings MG (2004). Temporal and spatial patterns of Kv1.1 and Kv1.2 protein and gene expression in spinal cord white matter after acute and chronic spinal cord injury in rats: Implications for axonal pathophysiology after neurotrauma. European Journal of Neuroscience, 19(3), 577–589. 10.1111/j.0953-816X.2004.03164.x [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, & Fehlings MG (2006). Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. Journal of Neuroscience, 26(13), 3377–3389. 10.1523/JNEUROSCI.4184-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassmann CM, Lappe-Siefke C, Baes M, Brügger B, Mildner A, Werner HB, … Nave KA (2007). Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nature Genetics, 39(8), 969–976. 10.1038/ng2070 [DOI] [PubMed] [Google Scholar]
- Keasey MP, Kang SS, Lovins C, & Hagg T (2013). Inhibition of a novel specific neuroglial integrin signaling pathway increases STAT3-mediated CNTF expression. Cell Communication and Signaling, 11, 35 10.1186/1478-811X-11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, & Houlé JD (2012). Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Research, 1438, 8–21. 10.1016/j.brainres.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, & Steward O (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. Journal of Neuroscience, 25(19), 4694–4705. 10.1523/JNEUROSCI.0311-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Harel NY, Kim C-Y, Papademetris X, Coman D, Wang X, … Strittmatter SM (2014). Diffusion tensor imaging as a predictor of locomotor function after experimental spinal cord injury and recovery. Journal of Neurotrauma, 31, 1362–1373. 10.1089/neu.2013.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BJ, & Patterson PH (2005). Leukemia inhibitory factor promotes oligodendrocyte survival after spinal cord injury. Glia, 51(1), 73–79. 10.1002/glia.20177 [DOI] [PubMed] [Google Scholar]
- Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, & Keane RW (2014). Pattern recognition receptors and central nervous system repair. Experimental Neurology, 258, 5–16. 10.1016/j.expneurol.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, & Popovich PG (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. Journal of Neuroscience, 29, 13435–13444. 10.1523/JNEUROSCI.3257-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Loy DN, Wang Q, Budde MD, Schmidt RE, Trinkaus K, & Song S-K (2010). Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. Journal of Neurotrauma, 27, 587–598. 10.1089/neu.2009.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, & Basso DM (2005). Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Experimental Neurology, 191(2), 251–265. 10.1016/J.EXPNEUROL.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Koshinaga M, Sanon HR, & Whittemore SR (1993). Altered acidic and basic fibroblast growth factor expression following spinal cord injury. Experimental Neurology, 120(1), 32–48. 10.1006/exnr.1993.1038 [DOI] [PubMed] [Google Scholar]
- Kotter MR (2006). Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. Journal of Neuroscience, 26 (1), 328–332. 10.1523/JNEUROSCI.2615-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, … Richardson WD (2017). Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife, 6, e28080 10.7554/eLife.28080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi I, Tator CH, & Theriault E (1993). Silicone rubber microangiography of acute spinal cord injury in the rat. Neurosurgery, 32(2), 260–268. 10.1227/00006123-199302000-00015 [DOI] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, PassosdosSantos R, Gaestel M, & David S (2014). TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron, 83, 1098–1116. 10.1016/j.neuron.2014.07.027 [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, & Dietrich D (2007). Vesicular glutamate release from axons in white matter. Nature Neuroscience, 10(3), 311–320. 10.1038/nn1850 [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, … Nave K-A (2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genetics, 33(3), 366–374. 10.1038/ng1095 [DOI] [PubMed] [Google Scholar]
- Lasiene J, Shupe L, Perlmutter S, & Horner P (2008). No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. Journal of Neuroscience, 28, 3887–3896. 10.1523/JNEUROSCI.4756-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond H, L’Esperance M, Orsal D, & Rossignol S (2003). Treadmill locomotion in the intact and spinal mouse. The Journal of Neuroscience, 23(36), 11411–11419. 10.1523/JNEUROSCI.23-36-11411.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, … Rothstein JD (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature, 487(7408), 443–448. 10.1038/nature11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech KA, & Hornby TG (2017). High-intensity locomotor exercise increases brain-derived neurotrophic factor in individuals with incomplete spinal cord injury. Journal of Neurotrauma, 34(6), 1240–1248. 10.1089/neu.2016.4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DC, & Li Q (2017). Electrical stimulation of cortical neurons promotes oligodendrocyte development and remyelination in the injured spinal cord. Neural Regeneration Research, 12(10), 1613–1615. 10.4103/1673-5374.217330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, He Y, Richardson WD, & Casaccia P (2009). Two-tier transcriptional control of oligodendrocyte differentiation. Current Opinion in Neurobiology, 19, 479–485. 10.1016/j.conb.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brus-Ramer M, Martin JH, & McDonald JW (2010). Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neuroscience Letters, 479(2), 128–133. 10.1016/j.neulet.2010.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Houdayer T, Liu S, & Belegu V (2017). Induced neural activity promotes an oligodendroglia regenerative response in the injured spinal cord and improves motor function after spinal cord injury. Journal of Neurotrauma, 34(24), 3351–3361. 10.1089/neu.2016.4913 [DOI] [PubMed] [Google Scholar]
- Li WW, Setzu A, Zhao C, & Franklin RJM (2005). Minocyclinemediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. Journal of Neuroimmunology, 158, 58–66. 10.1016/j.jneuroim.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, … Barres BA (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, Deloyht JM, Pedre X, Kelkar D, Kaur J, … Casaccia P (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience, 15, 1621–1623. 10.1038/nn.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Kim JH, Xie M, Schmidt RE, Trinkaus K, & Song S-K (2007). Diffusion tensor imaging predicts hyperacute spinal cord injury severity. Journal of Neurotrauma, 24, 979–990. 10.1089/neu.2006.0253 [DOI] [PubMed] [Google Scholar]
- Loy DN, Magnuson DSK, Zhang YP, Onifer SM, Mills MD, Cao Q, … Whittemore SR (2002). Functional redundancy of ventral spinal locomotor pathways. Journal of Neuroscience, 22(1), 315–323. 10.1523/JNEUROSCI.22-01-00315.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt K, Slade TP, Dorward JJ, Durant CF, Wu Y, Shigemoto R, … Molnár E (2007). Developing oligodendrocytes express functional GABAB receptors that stimulate cell proliferation and migration. Journal of Neurochemistry, 100(3), 822–840. 10.1111/j.1471-4159.2006.04255.x [DOI] [PubMed] [Google Scholar]
- Lytle JM, & Wrathall JR (2007). Glial cell loss, proliferation and replacement in the contused murine spinal cord. European Journal of Neuroscience, 25(6), 1711–1724. 10.1111/j.1460-9568.2007.05390.x [DOI] [PubMed] [Google Scholar]
- Ma S-F, Chen Y-J, Zhang J-X, Shen L, Wang R, Zhou J-S, … Lü H-Z (2015). Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain, Behavior, and Immunity, 45, 157–170. 10.1016/J.BBI.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, & Kessler JA (1997). Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. The Journal of Neuroscience, 17(11), 4112–4120. 10.1523/JNEUROSCI.17-11-04112.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MA, de Lima S, Gilbert H-Y, Giger RJ, Benowitz L, & Rasband MN (2016). Reassembly of excitable domains after CNS axon regeneration. Journal of Neuroscience, 36(35), 9148–9160. 10.1523/JNEUROSCI.1747-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Herting MM, Luszpak AE, Juraska JM, & Greenough WT (2009). Myelination of the corpus callosum in male and female rats following complex environment housing during adulthood. Brain Research, 1288, 9–17. 10.1016/j.brainres.2009.06.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, & Gordon S (2014). The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000prime Reports, 6, 13 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Brezun J-M, Bonnier L, & Xerri C (2009). A new rating scale for open-field evaluation of behavioral recovery after cervical spinal cord injury in rats. Journal of Neurotrauma, 26(7), 1043–1053. 10.1089/neu.2008.0717 [DOI] [PubMed] [Google Scholar]
- Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, … Ikenaka K (2006). Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Developmental Biology, 293(2), 358–369. 10.1016/J.YDBIO.2006.02.029 [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, & Matsushima GK (2001). Interleukin-1β promotes repair of the CNS. The Journal of Neuroscience, 21 (18), 7046–7052. 10.1523/JNEUROSCI.21-18-07046.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautes AE, Weinzierl MR, Donovan F, & Noble LJ (2000). Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Physical Therapy, 80(7), 673–687. 10.1016/j.brainres.2014.04.033 [DOI] [PubMed] [Google Scholar]
- McDonald WI (2008). Mechanisms of functional loss and recovery in spinal cord damage. In Porter R & Fitzsimons DW (Eds.), Ciba foundation symposium 34 - outcome of severe damage to the central nervous system 10.1002/9780470720165.ch3 [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Dubois-Dalcq M, & Aaronsont SA (1990). FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron, 5(5), 603–614. 10.1016/0896-6273(90)90215-2 [DOI] [PubMed] [Google Scholar]
- McTigue DM, Popovich PG, Morgan TE, & Stokes BT (2000). Localization of transforming growth factor-β1 and receptor mRNA after experimental spinal cord injury. Experimental Neurology, 163(1), 220–230. 10.1006/EXNR.2000.7372 [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tani M, Krivacic K, Chernosky A, Kelner GS, Maciejewski D, … Stokes BT (1998). Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. Journal of Neuroscience Research, 53(3), 368–376. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, & Wei P (2006). NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. Journal of Neuropathology and Experimental Neurology, 65(4), 406–420. 10.1097/01.jnen.0000218447.32320.52 [DOI] [PubMed] [Google Scholar]
- McTigue DM, & Tripathi RB (2008). The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of Neurochemistry, 107 (1), 1–19. 10.1111/j.1471-4159.2008.05570.x [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, & Stokes BT (2001). Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. The Journal of Neuroscience, 21(10), 3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I, Plemel JR, Caprariello AV, Nave KA, & Stys PK (2018). Axo-myelinic neurotransmission: A novel mode of cell signalling in the central nervous system. Nature Reviews Neuroscience, 19(1), 49–58. 10.1038/nrn.2017.128 [DOI] [PubMed] [Google Scholar]
- Miron VE (2017). Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. Journal of Leukocyte Biology, 101 (5), 1103–1108. 10.1189/jlb.3RI1116-494R [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, … ffrench-Constant C (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature Neuroscience, 16(9), 1211–1218. 10.1038/nn.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Rabin SJ, Colangelo AM, Whittemore SR, & Wrathall JR (1996). Increased basic fibroblast growth factor expression following contusive spinal cord injury. Experimental Neurology, 141(1), 154–164. 10.1006/exnr.1996.0149 [DOI] [PubMed] [Google Scholar]
- Moghaddam A, Sperl A, Heller R, Kunzmann K, Graeser V, Akbar M, … Biglari B (2016). Elevated serum insulin-like growth factor 1 levels in patients with neurological remission after traumatic spinal cord injury. PLoS One, 11(7), e0159764 10.1371/journal.pone.0159764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, … Crocker SJ (2011). Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. Journal of Neuroscience, 31(16), 6247–6254. 10.1523/JNEUROSCI.5474-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraud EM, Capogrosso M, Formento E, Wenger N, DiGiovanna J, Courtine G, & Micera S (2016). Mechanisms underlying the neuromodulation of spinal circuits for correcting gait and balance deficits after spinal cord injury. Neuron, 89(4), 814–828. 10.1016/j.neuron.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, … Lubetzki C (2015). Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. Journal of Neuroscience, 35(1), 4–20. 10.1523/JNEUROSCI.0849-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradov JM, & Hagg T (2013). Intravenous infusion of magnesium chloride improves epicenter blood flow during the acute stage of contusive spinal cord injury in rats. Journal of Neurotrauma, 30(10), 840–852. 10.1089/neu.2012.2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SA, Bankston AN, Burke DA, Ohri SS, & Whittemore SR (2016). Does the preclinical evidence for functional remyelination following myelinating cell engraftment into the injured spinal cord support progression to clinical trials? Experimental Neurology, 283(Pt B), 560–572. 10.1016/j.expneurol.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi N, Khazaei M, Ahlfors J-E, Ahuja CS, Nori S, Wang J, … Fehlings MG (2018). Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cells Translational Medicine, 7 (11), 806–818. 10.1002/sctm.17-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, & Fehlings MG (2001a). Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience, 104(1), 235–251. [DOI] [PubMed] [Google Scholar]
- Nashmi R, & Fehlings MG (2001b). Mechanisms of axonal dysfunction after spinal cord injury: With an emphasis on the role of voltage-gated potassium channels. Brain Research Reviews, 38(1–2), 165–191. 10.1016/S0165-0173(01)00134-5 [DOI] [PubMed] [Google Scholar]
- Nashmi R, Jones OT, & Fehlings MG (2000). Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv1.2 K+ channels after chronic spinal cord injury. European Journal of Neuroscience, 12(2), 491–506. 10.1046/j.1460-9568.2000.00926.x [DOI] [PubMed] [Google Scholar]
- Nasrabady SE, Rizvi B, Goldman JE, & Brickman AM (2018). White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathologica Communications, 6(1), 22 10.1186/s40478-018-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. (2018). National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 10.1179/204577212X13237783484262 [DOI] [Google Scholar]
- Nave K-A, & Trapp BD (2008). Axon-glial Signaling and the glial support of axon function. Annual Review of Neuroscience, 31(1), 535–561. 10.1146/annurev.neuro.30.051606.094309 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, & Patel KD (2016). Lineage, fate, and fate potential of NG2-glia. Brain Research, 1638(Pt B), 116–128. 10.1016/j.brainres.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, & Trapp BD (1999). NG2+ glial cells: A novel glial cell population in the adult brain. Journal of Neuropathology and Experimental Neurology, 58(11), 1113–1124. 10.1097/00005072-199911000-00001 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, & Stallcup WB (1996). Co-localization of NG2 proteoglycan and PDGF α-receptor on O2A progenitor cells in the developing rat brain. Journal of Neuroscience Research, 43(3), 299–314. [DOI] [PubMed] [Google Scholar]
- Noble M, Smith J, Power J, & Mayer-Pröschel M (2003). Redox state as a central modulator of precursor cell function. Annals of the New York Academy of Sciences, 991, 251–271. [DOI] [PubMed] [Google Scholar]
- Olguín-Albuerne M, & Morán J (2018). Redox signaling mechanisms in nervous system development. Antioxidants & Redox Signaling, 28(18), 1603–1625. 10.1089/ars.2017.7284 [DOI] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Singh AK, & Singh I (2013). Modulation of Rho-Rock signaling pathway protects oligodendrocytes against cytokine toxicity via PPAR-α-dependent mechanism. Glia, 61(9), 1500–1517. 10.1002/glia.22537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza CE, Taylor C, Pereira A, Seng M, Tham CS, Izrael M, & Webb M (2014). Induction of oligodendrocyte differentiation and in vitro myelination by inhibition of Rho-associated kinase. ASN Neuro, 6(4), 175909141453813 10.1177/1759091414538134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Lee SST, Li W, Ward JM, Gavrilova O, Everett C, … Gonzalez FJ (2000). Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta (delta ). Molecular and Cellular Biology, 20(14), 5119–5128. 10.1128/MCB.20.14.5119-5128.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MA, Ryu JK, Chang KJ, Etxeberria A, Bardehle S, Mendiola AS, … Akassoglou K (2017). Fibrinogen activates BMP signaling in oligodendrocyte progenitor cells and inhibits remyelination after vascular damage. Neuron, 96(5), 1003–1012. 10.1016/j.neuron.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SL, & Anderson AJ (2014). Complement and spinal cord injury: Traditional and non-traditional aspects of complement cascade function in the injured spinal cord microenvironment. Experimental Neurology, 258, 35–47. 10.1016/J.EXPNEUROL.2014.04.028 [DOI] [PubMed] [Google Scholar]
- Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, & Tetzlaff W (2014). Remyelination after spinal cord injury: Is it a target for repair? Progress in Neurobiology, 117, 54–72. 10.1016/J.PNEUROBIO.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Plemel JR, Manesh SB, Sparling JS, & Tetzlaff W (2013). Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia, 61(9), 1471–1487. 10.1002/glia.22535 [DOI] [PubMed] [Google Scholar]
- Popovich PG, & Hickey WF (2001). Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. Journal of Neuropathology and Experimental Neurology, 60(7), 676–685. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, & Stokes BT (1996). A quantitative spatial analysis of the blood–spinal cord barrier I. Permeability changes after experimental spinal contusion injury. Experimental Neurology, 142(2), 258–275. [DOI] [PubMed] [Google Scholar]
- Powers BE, Lasiene J, Plemel JR, Shupe L, Perlmutter SI, Tetzlaff W, & Horner PJ (2012). Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. Journal of Neuroscience, 32(15), 5120–5125. 10.1523/JNEUROSCI.0002-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, … Horner PJ (2013). Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proceedings of the National Academy of Sciences, 110(10), 4075–4080. 10.1073/pnas.1210293110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic AD, & Kraig RP (2014). Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia, 62(2), 284–299. 10.1002/glia.22606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C, Zhu Y, & Reddy JK (2000). Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochemistry and Biophysics, 32, 187–204. 10.1385/CBB:32:1-3:187 [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Sullivan PG, & Scheff SW (2007). Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia, 55(8), 831–843. 10.1002/glia.20508 [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, & Noble M (1983). A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature, 303(5916), 390–396. 10.1038/303390a0 [DOI] [PubMed] [Google Scholar]
- Rathore KI, Kerr BJ, Redensek A, Lopez-Vales R, Jeong SY, Ponka P, & David S (2008). Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. Journal of Neuroscience, 28 (48), 12736–12747. 10.1523/JNEUROSCI.3649-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejc E, Angeli CA, Atkinson D, & Harkema SJ (2017). Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Scientific Reports, 7(1), 13476 10.1038/s41598-017-14003-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick DK, Schmitt C, Miranpuri GS, Dhodda VK, Isaacson J, & Vemuganti R (2004). Molecular evidence of repair and plasticity following spinal cord injury. Neuroreport, 15(5), 837–839. [DOI] [PubMed] [Google Scholar]
- Robinson S, Tani M, Strieter RM, Ransohoff RM, & Miller RH (1998). The chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation. The Journal of Neuroscience, 18(24), 10457–10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, & Wrathall JR (1999). 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. The Journal of Neuroscience, 19(1), 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel A, Ortiz FC, Kerninon C, Maldonado PP, Angulo MC, & Nait-Oumesmar B (2015). Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination. Frontiers in Cellular Neuroscience, 9, 77 10.3389/fncel.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahinkaya FR, Milich LM, & McTigue DM (2014). Changes in NG2 cells and oligodendrocytes in a new model of intraspinal hemorrhage. Experimental Neurology, 255, 113–126. 10.1016/j.expneurol.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Ceballos H, Guizar-Sahagun G, Feria-Velasco A, Grijalva I, Espitia L, Ibarra A, & Madrazo I (1998). Spontaneous long-term remyelination after traumatic spinal cord injury in rats. Brain Research, 782(1–2), 126–135. 10.1016/s0006-8993(97)01252-3 [DOI] [PubMed] [Google Scholar]
- Saluja I, Granneman JG, & Skoff RP (2001). PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia, 33(3), 191–204. [PubMed] [Google Scholar]
- Samanta J (2004). Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development, 131(17), 4131–4142. 10.1242/dev.01273 [DOI] [PubMed] [Google Scholar]
- Sandhir R, Gregory E, He Y-Y, & Berman NEJ (2011). Upregulation of inflammatory mediators in a model of chronic pain after spinal cord injury. Neurochemical Research, 36(5), 856–862. 10.1007/s11064-011-0414-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Lankford KL, Radtke C, Honmou O, & Kocsis JD (2011). Remyelination after olfactory ensheathing cell transplantation into diverse demyelinating environments. Experimental Neurology, 229(1), 88–98. 10.1016/J.EXPNEUROL.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Schonberg DL, Laws JL, & McTigue DM (2013). Systemic iron chelation results in limited functional and histological recovery after traumatic spinal cord injury in rats. Experimental Neurology, 248, 53–56. 10.1016/j.expneurol.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz T, Endesfelder S, Chew LJ, Zaak I, & Bührer C (2012). Minocycline protects oligodendroglial precursor cells against injury caused by oxygen–glucose deprivation. Journal of Neuroscience Research, 90 (5), 933–944. 10.1002/jnr.22824 [DOI] [PubMed] [Google Scholar]
- Schonberg DL, Goldstein EZ, Sahinkaya FR, Wei P, Popovich PG, & McTigue DM (2012). Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo. Journal of Neuroscience, 32(16), 5374–5384. 10.1523/JNEUROSCI.3517-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg DL, & McTigue DM (2009). Iron is essential for oligodendrocyte genesis following intraspinal macrophage activation. Experimental Neurology, 218(1), 64–74. 10.1016/j.expneurol.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg DL, Popovich PG, & McTigue DM (2007). Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. Journal of Neuropathology and Experimental Neurology, 66(12), 1124–1135. 10.1097/nen.0b013e31815c2530 [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, & Fouad K (2002). Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Experimental Neurology, 176(1), 143–153. [DOI] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, Mun S-B, Mamontov P, Golden JA, & Grinspan JB (2004). Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Molecular and Cellular Neuroscience, 26 (4), 481–492. 10.1016/j.mcn.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Sellers DL, Maris DO, & Horner PJ (2009). Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. Journal of Neuroscience, 29(20), 6722–6733. 10.1523/JNEUROSCI.4538-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple-Rowland SL, Mahatme A, Popovich PG, Green DA, Hassler G, STOKES BT, & STREIT WJ (1995). Analysis of TGF-β1 gene expression in contused rat spinal cord using quantitative RTPCR. Journal of Neurotrauma, 12(6), 1003–1014. 10.1089/neu.1995.12.1003 [DOI] [PubMed] [Google Scholar]
- Shavit E, Beilin O, Korczyn AD, Sylantiev C, Aronovich R, Drory VE, … Chapman J (2008). Thrombin receptor PAR-1 on myelin at the node of Ranvier: A new anatomy and physiology of conduction block. Brain, 131(Pt 4), 1113–1122. 10.1093/brain/awn005 [DOI] [PubMed] [Google Scholar]
- Shem K, Barncord S, Flavin K, & Mohan M (2018). Adverse cognitive effect of gabapentin in individuals with spinal cord injury: Preliminary findings. Spinal Cord Series and Cases, 4, 9 10.1038/s41394-018-0038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler MM, Berchtold NC, Cotman CW, & Keirstead HS (2008). Voluntary running attenuates age-related deficits following SCI. Experimental Neurology, 210(1), 207–216. 10.1016/j.expneurol.2007.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, … Gerzanich V (2007). Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. The Journal of Clinical Investigation, 117(8), 2105–2113. 10.1172/JCI32041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Götz M, & Dimou L (2011). Progenitors in the adult cerebral cortex: Cell cycle properties and regulation by physiological stimuli and injury. Glia, 59(6), 869–881. 10.1002/glia.21156 [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Miller E, Moharregh-Khiabani D, Blank A, Pul R, Gudi V, … Stangel M (2010). Beneficial effects of minocycline on cuprizone induced cortical demyelination. Neurochemical Research, 35(9), 1422–1433. 10.1007/s11064-010-0202-7 [DOI] [PubMed] [Google Scholar]
- Smith PM, & Jeffery ND (2006). Histological and ultrastructural analysis of white matter damage after naturally-occurring spinal cord injury. Brain Pathology (Zurich, Switzerland), 16(2), 99–109. 10.1111/j.1750-3639.2006.00001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer S, Volbracht K, Lundgaard I, & Káradóttir RT (2016). Glutamate signalling: A multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology, 110(Pt B), 574–585. 10.1016/j.neuropharm.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Stallcup WB (1981). The NG2 antigen, a putative lineage marker: Immunofluorescent localization in primary cultures of rat brain. Developmental Biology, 83(1), 154–165. 10.1016/S0012-1606(81)80018-8 [DOI] [PubMed] [Google Scholar]
- Stallcup WB (2002). The NG2 proteoglycan: Past insights and future prospects. Journal of Neurocytology, 31(6–7), 423–435. 10.1023/A:1025731428581 [DOI] [PubMed] [Google Scholar]
- Stankoff B, Aigrot M-S, Noël F, Wattilliaux A, Zalc B, & Lubetzki C (2002). Ciliary neurotrophic factor (CNTF) enhances myelin formation: A novel role for CNTF and CNTF-related molecules. The Journal of Neuroscience, 22(21), 9221–9227. 10.1523/JNEUROSCI.22-21-09221.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman AJ, Zhou Y, Koito H, Kim S, Payne HR, Lu QR, & Li J (2016). Activation of oligodendroglial Stat3 is required for efficient remyelination. Neurobiology of Disease, 91, 336–346. 10.1016/j.nbd.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, … Tetzlaff W (2004). Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. Journal of Neuroscience, 24(9), 2182–2190. 10.1523/JNEUROSCI.5275-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, & Stokes BT (1998). Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Experimental Neurology, 152(1), 74–87. 10.1006/exnr.1998.6835 [DOI] [PubMed] [Google Scholar]
- Sun F, Lin C-LG, Mctigue D, Shan X, Tovar CA, Bresnahan JC, & Beattie MS (2010). Effects of axon degeneration on oligodendrocyte lineage cells: Dorsal rhizotomy evokes a repair response while axon degeneration rostral to spinal contusion induces both repair and apoptosis. Glia, 58(11), 1304–1319. 10.1002/glia.21009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiss VA, Nguyen T, Dugas J, Ibrahim A, Barres B, Androulakis IP, & Casaccia P (2011). Identification of a gene regulatory network necessary for the initiation of oligodendrocyte differentiation. PLoS One, 6(4), e18088 10.1371/journal.pone.0018088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Murakami K, Bando Y, & Yoshida S (2013). Minocycline reduces remyelination by suppressing ciliary neurotrophic factor expression after cuprizone-induced demyelination. Journal of Neurochemistry, 127(2), 259–270. 10.1111/jnc.12289 [DOI] [PubMed] [Google Scholar]
- Thorburne SK, & Juurlink BH (1996). Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. Journal of Neurochemistry, 67(3), 1014–1022. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, & Keirstead HS (2005). Spinal cord injury is accompanied by chronic progressive demyelination. The Journal of Comparative Neurology, 486(4), 373–383. 10.1002/cne.20517 [DOI] [PubMed] [Google Scholar]
- Tripathi R, & McTigue DM (2007). Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia, 55(7), 698–711. 10.1002/glia.20491 [DOI] [PubMed] [Google Scholar]
- Tripathi RB, & McTigue DM (2008). Chronically increased ciliary neurotrophic factor and fibroblast growth factor-2 expression after spinal contusion in rats. Journal of Comparative Neurology, 510(2), 129–144. 10.1002/cne.21787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng SF, Bresnahan JC, Beattie MS, & De Vellis J (2001). Upregulation of the HLH id gene family in neural progenitors and glial cells of the rat spinal cord following contusion injury. Journal of Neuroscience Research, 66(6), 1161–1172. 10.1002/jnr.10089 [DOI] [PubMed] [Google Scholar]
- Viganò F, Möbius W, Götz M, & Dimou L (2013). Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nature Neuroscience, 16(10), 1370–1372. 10.1038/nn.3503 [DOI] [PubMed] [Google Scholar]
- Wagner FB, Mignardot J-B, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, … Courtine G (2018). Targeted neurotechnology restores walking in humans with spinal cord injury. Nature, 563 (7729), 65–71. 10.1038/s41586-018-0649-2 [DOI] [PubMed] [Google Scholar]
- Waitt AE, Reed L, Ransom BR, & Brown AM (2017). Emerging roles for glycogen in the CNS. Frontiers in Molecular Neuroscience, 10, 73 10.3389/fnmol.2017.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, & Fields RD (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science (New York, N.Y.), 333(6049), 1647–1651. 10.1126/science.1206998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MC, Tator CH, & Frazee P (1986). Relationship between posttraumatic ischemia and hemorrhage in the injured rat spinal cord as shown by colloidal carbon angiography. Neurosurgery, 18(4), 433–439. 10.1227/00006123-198604000-00007 [DOI] [PubMed] [Google Scholar]
- Wang H, Liu NK, Zhang YP, Deng L, Lu QB, Shields CB, … Xu XM (2015). Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Experimental Neurology, 271, 368–378. 10.1016/j.expneurol.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla A, Johnson JE, Yokota Y, & Barres BA (2001). A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron, 29(3), 603–614. 10.1016/S0896-6273(01)00237-9 [DOI] [PubMed] [Google Scholar]
- Wang S, & Young KM (2014). White matter plasticity in adulthood. Neuroscience, 276, 148–160. 10.1016/j.neuroscience.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, … Ren Y (2015). Macrophages in spinal cord injury: Phenotypic and functional change from exposure to myelin debris. Glia, 63(4), 635–651. 10.1002/glia.22774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Richter-Landsberg C, & Reiser G (2004). Expression of protease-activated receptors (PARs) in OLN-93 oligodendroglial cells and mechanism of PAR-1-induced calcium signaling. Neuroscience, 126 (1), 69–82. 10.1016/j.neuroscience.2004.03.024 [DOI] [PubMed] [Google Scholar]
- Warden P, Bamber NI, Li H, Esposito A, Ahmad KA, Hsu CY, & Xu XM (2001). Delayed glial cell death following wallerian degeneration in white matter tracts after spinal cord dorsal column cordotomy in adult rats.PG. Experimental Neurology, 168(2), 213–224. 10.1006/exnr.2000.7622 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, & Nishiyama A (2002). Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. Journal of Neuroscience Research, 69(6), 826–836. 10.1002/jnr.10338 [DOI] [PubMed] [Google Scholar]
- Waxman SG (1989). Demyelination in spinal cord injury. Journal of the Neurological Sciences, 91(1–2), 1–14. [DOI] [PubMed] [Google Scholar]
- Wenger N, Moraud EM, Raspopovic S, Bonizzato M, DiGiovanna J, Musienko P, … Courtine G (2014). Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Science Translational Medicine, 6(255), 255ra133 10.1126/scitranslmed.3008325 [DOI] [PubMed] [Google Scholar]
- Wernig A, & Müller S (1992). Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Spinal Cord, 30(4), 229–238. 10.1038/sc.1992.61 [DOI] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, & Chandran S (2003). Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: A novel role for oligodendrocytederived glial cell line-derived neurotrophic factor. The Journal of Neuroscience, 23(12), 4967–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle WF, Smart JO, & Beers JJ (1958). Residual function after subtotal spinal cord transection in adult cats. Neurology, 8(7), 518–521. 10.1212/wnl.8.7.518 [DOI] [PubMed] [Google Scholar]
- Winterbourn CC (1995). Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicology Letters, 82–83, 969–974. 10.1016/0378-4274(95)03532-X [DOI] [PubMed] [Google Scholar]
- Wolswijk G, & Noble M (1989). Identification of an adult-specific glial progenitor cell. Development, 105, 387–400. 10.1016/0922-3371(89)90618-7 [DOI] [PubMed] [Google Scholar]
- Wolswijk G, & Noble M (1992). Cooperation between PDGF and FGF converts slowly dividing O-2A(adult) progenitor cells to rapidly dividing cells with characteristics of O- 2A(perinatal) progenitor cells. Journal of Cell Biology, 118(4), 889–900. 10.1083/jcb.118.4.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall JR, Choiniere D, & Teng YD (1994). Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. The Journal of Neuroscience, 14(11 Pt 1), 6598–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall JR, Teng YD, & Choiniere D (1996). Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-D-aspartate receptors. Experimental Neurology, 137(1), 119–126. 10.1006/exnr.1996.0012 [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Teng YD, & Marriott R (1997). Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Experimental Neurology, 145(2 Pt 1), 565–573. 10.1006/exnr.1997.6506 [DOI] [PubMed] [Google Scholar]
- Wu J, Yoo S, Wilcock D, Lytle JM, Leung PY, Colton CA, & Wrathall JR (2010). Interaction of NG2+ glial progenitors and microglia/macrophages from the injured spinal cord. Glia, 58(4), 410–422. 10.1002/glia.20932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G-Y, Hughes MG, Ye Z, Hulsebosch CE, & McAdoo DJ (2004). Concentrations of glutamate released following spinal cord injury kill oligodendrocytes in the spinal cord. Experimental Neurology, 187(2), 329–336. 10.1016/J.EXPNEUROL.2004.01.029 [DOI] [PubMed] [Google Scholar]
- Yang H, Lu P, McKay HM, Bernot T, Keirstead H, Steward O, … Tuszynski MH (2006). Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. Journal of Neuroscience, 26(8), 2157–2166. 10.1523/JNEUROSCI.4070-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li C, Qiu X, Zhang L, Lu W, Chen L, … Tang Y (2013). Effects of an enriched environment on myelin sheaths in the white matter of rats during normal aging: A stereological study. Neuroscience, 234, 13–21. 10.1016/j.neuroscience.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Yao D-L, West NR, Bondy CA, Brenner M, Hudson LD, Zhou J, … Webster H (1995). Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor I and insulin-like growth factor binding protein 2 during myelin regeneration. Journal of Neuroscience Research, 40(5), 647–659. 10.1002/jnr.490400510 [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, & Gómez-Pinilla F (2005). Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Experimental Neurology, 193(2), 411–419. 10.1016/j.expneurol.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, & Gomez-Pinilla F (2008). BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience, 155(4), 1070–1078. 10.1016/j.neuroscience.2008.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogami K, Wakisaka S, Avruch J, & Reeves SA (2000). Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Current Biology, 10(1), 47–50. 10.1016/S0960-9822(99)00268-7 [DOI] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Drucker KL, Wu J, & Scarisbrick IA (2015). The thrombin receptor is a critical extracellular switch controlling myelination. Glia, 63(5), 846–859. 10.1002/glia.22788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn S-J, Cossell L, Attwell D, … Richardson WD (2013). Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron, 77(5), 873–885. 10.1016/j.neuron.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W (2002). Spinal cord contusion models. Progress in Brain Research, 137, 231–255. 10.1016/S0079-6123(02)37019-5 [DOI] [PubMed] [Google Scholar]
- Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, … Oh TH (2007). Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. Journal of Neuroscience, 27(29), 7751–7761. 10.1523/JNEUROSCI.1661-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai LJ, & Wrathall JR (2005). Cell proliferation and replacement following contusive spinal cord injury. Glia, 50(3), 247–257. 10.1002/glia.20176 [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SPJ, Zhao C, Tripathi R, Jamen F, … Franklin RJM (2010). CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell, 6(6), 578–590. 10.1016/j.stem.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, & Rasband MN (2016). Cytoskeletal control of axon domain assembly and function. Current Opinion in Neurobiology, 39, 116–121. 10.1016/j.conb.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, & Miller RH (1996). Density-dependent feedback inhibition of oligodendrocyte precursor expansion. The Journal of Neuroscience, 16(21), 6886–6895. 10.1523/JNEUROSCI.16-21-06886.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Haaf M, Todorich B, Grosstephan E, Schieremberg H, Surguladze N, & Connor JR (2005). Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia, 52(3), 199–208. 10.1002/glia.20235 [DOI] [PubMed] [Google Scholar]
- Zhang X, Surguladze N, Slagle-Webb B, Cozzi A, & Connor JR (2006). Cellular iron status influences the functional relationship between microglia and oligodendrocytes. Glia, 54(8), 795–804. 10.1002/glia.20416 [DOI] [PubMed] [Google Scholar]
- Zheng J, Ding W, Li B, & Yang Y (2017). Enriched environment promotes remyelination and motor function recovery through modulation of HDAC1/2 in mice. Neuroscience Letters, 655, 121–130. 10.1016/j.neulet.2017.06.039 [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, & Nishiyama A (2011). Age-dependent fate and lineage restriction of single NG2 cells. Development (Cambridge, England), 138(4), 745–753. 10.1242/dev.047951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, & Bergles DE (2007). Vesicular release of glutamate from unmyelinated axons in white matter. Nature Neuroscience, 10(3), 321–330. 10.1038/nn1854 [DOI] [PMC free article] [PubMed] [Google Scholar]