Abstract
Epidemiological studies suggest that women are not only at a higher risk for developing knee osteoarthritis (KOA), but also report greater symptom severity compared to men. One potential underlying mechanism of these sex differences may be exaggerated inflammatory responses to pain among women compared to men. The present study examined sex differences in interleukin-6 (IL-6) response over time following experimental pain testing. We hypothesized that women, when compared to men, would show greater IL-6 reactivity when exposed to acute pain in a human laboratory setting. Eighty-four participants (36 men and 48 women) with KOA scheduled for total knee arthroplasty underwent a quantitative sensory testing (QST) battery. A total of seven IL-6 measurements were taken, twice at baseline, once immediately after QST, and every 30 minutes up to 2 hours after QST. Consistent with our hypothesis, women, when compared to men, showed accelerated increases in IL-6 levels following laboratory-evoked pain, even after controlling for body mass index, marital status, clinical pain, evoked pain sensitivity, and situational pain catastrophizing. Given that KOA is a chronic condition, and individuals with KOA frequently experience pain, these sex differences in IL-6 reactivity may contribute to the maintenance and/or exacerbation of KOA symptoms.
Keywords: knee osteoarthritis, sex differences, interleukin-6, quantitative sensory testing, chronic pain
Perspectives:
The present study demonstrates that women, when compared to men, exhibit greater IL-6 reactivity after exposure to laboratory-evoked pain. Such sex differences may explain the mechanisms underlying women’s higher chronic pain risk and pain perception, as well as provide further insight in developing personalized pain interventions.
1. Introduction
Knee osteoarthritis (KOA) is a chronic pain condition that is one of the leading causes of physical impairment and disability across the world40. The prevalence of KOA is expected to continuously escalate due to population aging and increases in obesity rates63. Women are particularly at-risk for developing chronic pain and KOA20,43. Further, women generally report higher pain-related symptoms (including KOA symptoms57), evoked-pain sensitivity, and lower pain tolerance compared to men20,43.
Despite substantial evidence of sex differences in pain, the mechanisms underlying women’s higher chronic pain risk and pain perception remain unclear. One potential mechanism is sex differences in inflammatory responses52. Specifically, interleukin-6 (IL-6), one of the key inflammatory cytokines, warrants further investigation. Pre-clinical evidence suggests that IL-6 serves a critical role in central sensitization, chronic inflammation, and autoimmunity29,62,66. Repeated exposure to acute stressors can enhance individuals’ IL-6 reactivity27,42, which might contribute to the maintenance and exacerbation of existing pain. In human studies, IL-6 has been linked to the development of KOA, progression of structural abnormalities in the knee, and poor physical function among individuals with KOA53,58. Furthermore, maladaptive IL-6 responses may potentially be modifiable. Some therapeutic effects of an IL-6 receptor inhibitor on various autoimmune and musculoskeletal disorders have reported62. Lastly, in our previous study with KOA patients37, we found that among various inflammatory markers (i.e., C-reactive protein, IL-6, IL-1β, and tumor necrosis factor α), IL-6 was the most sensitive marker in response to acute pain testing. Hence, IL-6 may be a particularly important marker to focus on when employing a similar acute pain testing research paradigm.
Several previous studies of healthy subjects show sex differences in IL-6 response to mental and/or physical acute stressors13,16,59, as well as systemic inflammation induced by an endotoxin17,64. For instance, IL-6 reactivity was significantly higher in post-menopausal women exposed to a mental stressor compared to older men16. Similarly, cytokine reactivity to the bacterial endotoxin lipopolysaccharide was more pronounced in women than men64. Despite some inconsistencies13,19, the majority of studies to date suggest that compared to men, women show higher IL-6 reactivity to an acute mental stressor13,16, physical stressor65, and pharmacological inflammatory stimulation17,64.
As sex cannot be randomly assigned to participants, to increase the certainty of sex differences, covariates that have significant sex differences or are associated with IL-6 levels need to be considered in a statistical model. Studies have shown that individual differences in body mass index (BMI)47, socio-demographics19, anxiety46, and pain experiences37 are associated with IL-6 levels. In our previous studies, we also found that both trait and situational pain catastrophizing were significantly associated with IL-6 response over and above the effect of anxiety, negative mood, and pain severity14,34. Sleep disturbance, an important factor that can explain development and maintenance of chronic pain21, has also been found to be associated with IL-6 response among individuals with chronic pain24,25,49.
Building upon these previous findings, we aimed to test whether the sex differences in inflammatory reactivity observed in healthy individuals are seen when KOA patients are exposed to acute pain. Specifically, the present study examined whether laboratory-evoked pain was associated with a significant sex difference in IL-6 reactivity among KOA patients. We hypothesized that (1) laboratory-evoked pain would be associated with increased IL-6 level as reported by a number of previous studies5,12,14,34,37,50; (2) relative to men, women would show significantly higher IL-6 reactivity after exposure to evoked pain; and (3) the sex difference would remain significant even after controlling for a number of important covariates that have been associated with IL-6 levels14,19,46,47.
2. Materials and Methods
The present study is based upon a larger observational parent study with the goal of investigating bio-behavioral risk factors associated with persistent pain after total knee arthroplasty. The parent study collected data including psychophysical pain testing, self-report questionnaires, daily diaries, and prescription opioid use during hospitalization. A number of papers were published using the parent study2,35,44, and the main outcome paper is currently in progress. One paper examined sex differences in negative affect and postoperative pain using the parent study data44, however, none of these previously published studies overlap with the current study.
2.1. Participants
The present study includes a sample of patients with advanced KOA who were enrolled and assessed prior to undergoing total knee replacement. The Institutional Review Board at Johns Hopkins School of Medicine and Brigham and Women’s Hospital approved the study protocol. Written informed consent was obtained from all participants before initiating study procedures. Participants were recruited in the Baltimore, MD and Boston, MA areas via posted flyers, letters mailed to patients scheduled for joint replacement surgeries, advertisements in orthopedic clinics, and regular announcements on Hospital and University clinical research websites. Patients were also directly recruited from orthopedic surgery clinics. Patients were eligible for the study if they met the following inclusion criteria: (1) ≥ 45 years of age to reduce risk of including younger individuals with potential atypical KOA etiologies39; (2) diagnosis of knee osteoarthritis (OA) meeting the American College of Rheumatology criteria for knee OA; (3) scheduled for an upcoming total knee replacement surgery; and (4) eligible to undergo blood sampling procedures (i.e., participants had to have accessible veins for blood sampling). Patients taking nonsteroidal anti-inflammatory drugs or acetaminophen needed to be on a stable dose at least 1 month prior to participation in the study. Exclusion criteria included: (1) use of opioids for the past 30 days; (2) recent history of substance abuse or dependence; (3) presence of certain sleep disorders such as restless leg syndrome; (4) systematic inflammatory or autoimmune disorders; (5) pregnancy; (6) history of Raynaud’s disease; (7) current infection; (8) moderate-to-severe peripheral neuropathy; (9) history of myocardial infarction or other serious cardiovascular condition in the past 12 months; (10) current use of oral steroids; (11) demonstration of delirium, dementia, psychosis, or other cognitive impairments that would prevent the completion of study procedures. A total of 89 individuals met the inclusion criteria and participated in a laboratory visit consisting of quantitative sensory testing and blood sampling. Of those, 4 participants were unable to provide blood data due to difficulty in IV placement. Hence, the present study used a final sample of 85 participants who provided blood samples that were suitable for blood assays.
2.2. Procedures
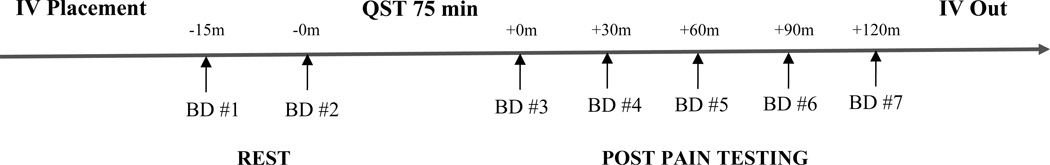
Following a brief telephone screening to preliminarily determine eligibility, potential participants were scheduled for their initial visit approximately 3 to 4 weeks prior to their scheduled total knee replacement surgery. During the visit, verbal and written informed consent were obtained prior to the start of any study procedures. Research assistants reminded participants to abstain from taking NSAIDs and pain medications 24 hours before the visit. To reduce circadian influences on IL-6 levels45, all QST sessions started between 11 to 11:30 AM. At seven different time points throughout the visit, the collection of blood samples took place. Following catheter placement, subjects rested comfortably in a recliner for 15 minutes. The first baseline blood draw took place approximately 15 minutes after IV placement and prior to quantitative sensory testing (QST). The second baseline blood sample was collected immediately prior to the start of QST. The total duration of QST was approximately 75 minutes. At the remaining time points (3rd to 7th blood draws), the blood samples were collected at 30-minute intervals beginning immediately post-QST and ending at 120 minutes post-QST. A visual summary of this timeline is shown in Figure 1. Blood samples were collected in 10 mL EDTA, purple top vacutainers. They were centrifuged immediately when possible (or were placed on ice and centrifuged within 30 minutes if needed). Plasma was removed and aliquoted for storage in a −81°C freezer. To assess serum levels of IL-6, a standard high-sensitivity enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA; lower limit of detection: 0.16 pg/mL; sensitivity: 0.04 pg/mL; intra-assay coefficient of variation: <5%) was conducted on all blood samples.
Figure 1.
Blood draw procedure timeline Note. m = minutes, IV = Intravenous, BD = Blood Draw, QST = Quantitative Sensory Testing.
2.3. Quantitative sensory testing (QST) procedures
2.3.1. Pressure Pain Testing
For the assessment of pressure pain thresholds (PPT), a SBmedic digital pressure algometer (1 cm2 hard rubber probe) was used. PPT was determined by two series of ascending pressure intensities (30 kPa per second) until the participant indicated that the pressure was first perceived as painful. PPT was measured bilaterally at the medial and lateral joint line of the knee, patella, middle insertion point of the quadriceps, trapezius, and thumb. To assess tonic deep-tissue pressure pain, a Hokanson rapid cuff inflator was applied to the calf (i.e., gastrocnemius muscle). It rapidly inflated and maintained a pressure that is individually tailored for each participant to produce a pain intensity rating of 40/100. Cuff inflation was maintained for up to two minutes.
2.3.2. Mechanical Pain Testing
Nylon monofilament probes were used to assess mechanical temporal summation (MTS). The testing sites were the dorsal surface of the nondominant middle finger and the patella. The probes were a set of weighted pinprick stimulators with fixed stimulus intensities (flat contact area of 0.2 mm diameter) that exerted forces of 256 to 512 mN. The probes were applied perpendicular to the skin. Participants rated on a 0–100 numerical pain rating scale (0 = no pain; 100 = worst pain imaginable) of the first tap of the probe. Then 10 repeated punctuate stimuli were administered at a rate of one tap per second. Participants were asked to rate their peak amount of pain from the train of 10 stimuli. An MTS difference score was calculated by subtracting initial stimulus pain rating from peak pain rating for each probe weight.
2.3.3. Thermal Pain Testing
Participants completed a series of computer-controlled Medoc 9-cm2 Contact Heat-Evoked Potential Stimulator (CHEPS) tasks. The thermal tasks involved assessment of threshold and tolerance, suprathreshold thermal pain stimuli, as well as temporal summation. Heat Pain Threshold (HPTh) was assessed twice on the dominant forearm near wrist and index kneecap (the knee that will go through operation) with 0.5°C/second rate of temperature rise. Participants indicated when they first felt pain via pressing a button that turned the device off. Heat Pain Tolerance (HPTo) was assessed by participants indicating when they could no longer tolerate the pain from the thermal device with 0.5°C/second rate of rise. To assess suprathreshold thermal pain sensitivity, brief noxious thermal stimuli were administered to the dominant ventral forearm. The temperatures were set at 44 and 46°C, and each stimulus was delivered for 3 seconds. Participants were asked to provide pain ratings for the brief thermal stimuli using the numerical pain rating scale, similar to prior studies investigating heat pain sensitivity as a predictor of post-surgical outcomes1,7. In addition, to measure after-sensations, a pain rating was also obtained at 15 seconds following offset of the heat stimulus. To measure after sensations, an additional pain rating was also obtained at 15 seconds following the heat stimulus. Lastly, Thermal Temporal Summation (TTS) was measured by response to three series (temperatures: 43, 45 and 47°C) of 10 heat pulses with each temperature rated on a 0–100 numerical pain rating scale. The CHEPS thermode was applied to the dominant ventral forearm. A 2.5 second inter stimulus interval and a 70°C/second rate of temperature rise was used. A TTS difference score was calculated for each temperature by subtracting pain rating of the first pulse of the series from pain rating after the train of heat stimuli. In order to characterize after sensations, an additional pain rating was obtained 15 seconds following the final pulse in each series.
2.3.4. Cold Pressor Testing
Participants immersed their dominant hand in a circulating water bath maintained at 4°C. Participants were allowed to withdraw their hand prior to 3 minute time limit if the pain became unbearable. Numerical pain ratings were obtained at every 20 seconds.
2.3.5. Conditioned Pain Modulation
Baseline PPT measurements were taken prior to initiating conditioned pain modulation (CPM) trials. The dominant hand was immersed in a 4°C circulating cold water bath and PPT was re-administered on the non-dominant trapezius muscle at the 20 second mark. Participants then removed their hand from the water bath for a two-minute rest period. We obtained three CPM trials, creating a difference score of PPT to pre-immersion baseline and averaged these three responses together for consistency.
2.4. Covariates
A number of covariates potentially associated with IL-6 response to QST were included in the present study: (1) socio-demographic variables, (2) body mass index (BMI), (3) trait pain catastrophizing (measured by the Pain Catastrophizing Scale total score60), (4) situational pain catastrophizing (measured right after each laboratory pain testing and we computed the average score15); (5) anxiety (measured by 7-item PROMIS Anxiety Short Form V1.08), (6) sleep disturbance (measured by the Pittsburgh Sleep Quality Index global score3); (7) baseline clinical pain intensity (measured by average of worst, least, average, and current pain using Brief Pain Inventory—Short Form11), and (8) an evoked-pain sensitivity summary score. To create the evoked-pain sensitivity summary score, each QST-derived parameter (i.e., PPT, deep-tissue pressure pain, MTS, HPTh, HPTo, TTS, suprathreshold thermal pain sensitivity, average pain intensity from cold pressor test, CPM, and after sensations) was first standardized into z-scores. Then, we computed the mean of these z-scores to calculate the summary score, an index of overall pain sensitivity. Note that higher scores indicate greater evoked-pain sensitivity, and thus z-scores of some QST-parameters (i.e., threshold measures and CPM) were reversed by multiplying by −1. A number of previous studies employed this approach to create a pain sensitivity index from various QST methods4,6,41.
2.5. Data analytic plan
First, outlier analysis of IL-6 responses was conducted based upon z-scores greater than 3.5 or less than −3.526. Second, descriptive statistics (i.e., mean, standard deviation, and bi-variate correlations) of all study variables were assessed. Third, potential sex differences in socio-demographics, covariates, and baseline IL-6 levels were examined using t-test for continuous variables and the chi-square test for categorical variables. Finally, multi-level modeling (MLM; also known as mixed modeling or hierarchical linear modeling) was employed to test our main hypotheses, as it best captures our hierarchical data structure that includes repeated IL-6 assessments nested in each participant. MLM has a number of advantages over traditional repeated-measures ANOVA. It is robust to homoscedasticity and sphericity51. It is also robust to missing data51, and thus, can more effectively handle the full range of data that was collected multiple times without discarding useful data. Note that the two baseline time points were not reduced to a single time point by taking the average because reduction of a time point can result in decrease in statistical power55.
A systematic multi-level model building approach was used to test our hypotheses. We first tested for potential significant increases in IL-6 response over time by adding time as a predictor. This addressed our first hypothesis (i.e., does IL-6 reactivity significantly increase over time due to acute pain testing?). Second, we tested whether inclusion of a quadratic time term (incorporating a non-linear trend) improved model fit using the likelihood ratio test. Third, a time random slope was included to see if the relationship between time and IL-6 response significantly varied across participants. Again, we used the likelihood ratio test to determine whether inclusion of the random slope significantly improved the model fit. Fourth, we included sex in the model to see if it moderated the association between time and IL-6 response, addressing our second hypothesis (i.e., does change in IL-6 reactivity over time differ between sexes?).
We included a number of covariates in the model to see if the sex moderation effect remained significant, which addressed our last hypothesis (i.e., is there a significant sex difference in IL-6 response above and beyond individuals’ socio-demographic, psychosocial, and pain sensitivity levels?). As there were a large number of baseline covariates relative to the current sample size, we used a systematic and parsimonious approach to include covariates in the final model. We first included covariates that exhibited significant sex differences. After confirming the sex moderation effect remained significant, we included additional covariates that were significantly associated with baseline IL-6 levels. It should be noted that we also included covariates and time interaction terms (e.g., time*covariate#1 + time*covariate#2…) so as to more conservatively control for the covariates’ potential influence on IL-6 reactivity over time. For instance, it is possible that higher IL-6 reactivity over time is due to individual differences in evoked-pain sensitivity rather than sex differences.
The main predictor sex variable was a level-2 variable that was dummy coded (0 = male; 1 = female). The time variable was centered at the first assessment time point, as this is typical in the linear growth model framework which we used in the present study. The rest of the level-2 covariates were grand mean centered (i.e., age, BMI, pain catastrophizing, evoked pain sensitivity) or dummy coded (i.e., race, marital status). For all analyses, the threshold for statistical significance was set at p < .05 (two-tailed). All analyses were conducted in SPSS version 25 using the MIXED command.
3. Results
3.1. Results of preliminary analyses
The outlier analysis revealed one case that showed higher than 3.5 standard deviation IL-6 responses on all 7 assessments. Although this outlier case did not significantly change the overall result, the inclusion of this outlier slightly intensified the sex moderation effect. In order to increase the generalizability of our findings, we excluded this case from our analyses. Hence, the total sample size that was used in the following analyses was 84. Among these participants, 48 were females and 36 were males. Table 1 shows comparisons of socio-demographics, baseline covariates, and baseline IL-6 levels by sex. There were significant sex differences in the proportion of marital status and QST-evoked pain sensitivity. A much higher proportion of men (88.9%) reported that they were either married or currently living with a partner than women (58.3%). Consistent with previous findings [e.g.,20,43], women exhibited significantly higher evoked-pain sensitivity than men.
Table 1.
Differences among socio-demographic and psychosocial characteristics based on sex
| Variables | Men (SD) | Women (SD) | p |
|---|---|---|---|
| Age | 67.7 (9.0) | 65.0 (8.4) | .17 |
| Education | .33 | ||
| Some high school or less | 0% | 6% | |
| High School Graduate/GED | 8.3% | 12.5% | |
| Some College | 25.0% | 29.2% | |
| College graduate or higher education | 28.6% | 29.8% | |
| Race | .68 | ||
| White | 80.6% | 87.5% | |
| Black | 16.7% | 10.4% | |
| Others | 2.8% | 2.1% | |
| Marital status | < .05 | ||
| Married or living with partner | 88.9% | 58.3% | |
| Single | 5.6% | 14.6% | |
| Separated or divorced | 2.8% | 14.6% | |
| Widowed | 2.8% | 12.5% | |
| Employment | .18 | ||
| Full-time | 33.3% | 35.4% | |
| Part-time | 8.3% | 2.1% | |
| Homemaker | 0% | 8.3% | |
| Retired | 52.8% | 39.6% | |
| Unemployed | 0% | 4.2% | |
| Disability | 5.6% | 10.4% | |
| BMI | 32.0 (6.2) | 31.0 (6.2) | .48 |
| Pain catastrophizing | 15.1 (13.0) | 17.2 (14.7) | .54 |
| Anxiety | 13.2 (5.4) | 14.7 (5.2) | .22 |
| Pain intensity (NRS) | 4.0 (2.1) | 4.2 (2.0) | .66 |
| Evoked pain sensitivity | −0.1 (0.3) | 0.2 (0.4) | < .01 |
| Baseline IL-6 Time 1 (−15 min) | 3.6 (2.5) | 3.8 (2.6) | .73 |
| Baseline IL-6 Time 2 (−0 min) | 3.1 (1.8) | 3.7 (2.4) | .21 |
Note. For comparisons between men and women, ANOVA was used for continuous covariates and the chi-square test was used for categorical covariates.
Table 2 summarizes bi-variate correlations of all baseline variables that were used in the present study. Sex was significantly associated with pain sensitivity, indicating that women reported higher pain sensitivity than men. BMI, baseline clinical pain intensity, and situational pain catastrophizing were also significantly and positively associated with baseline IL-6.
Table 2.
Bi-variate correlations of baseline study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sex | — | |||||||||||
| 2 | Age | −.15 | — | ||||||||||
| 3 | Race | −.10 | −.07 | — | |||||||||
| 4 | Education | .15 | −.12 | .37** | — | ||||||||
| 5 | BMI | −.08 | −.22 | .23* | .22* | — | |||||||
| 6 | Pain catastrophizing | .07 | −.11 | .27* | .37** | .16 | — | ||||||
| 7 | Anxiety | .14 | −.17 | .06 | .30** | .04 | .47** | — | |||||
| 8 | Baseline pain intensity | .05 | −.06 | .30* | .36** | .28* | .56** | .18 | — | ||||
| 9 | Evoked pain sensitivity | .38** | −.08 | .07 | .16 | .05 | .15 | .18 | .12 | — | |||
| 10 | Sleep disturbance | .10 | −28* | .19 | .42** | .20 | .43** | .24* | .46** | .21 | — | ||
| 11 | Situational pain catastrophizing | .15 | −.04 | .38** | .25* | .13 | .61** | .25* | .47** | .29** | .24* | — | |
| 12 | Baseline IL-6 | .11 | .15 | .00 | .16 | .36** | .16 | .05 | .26** | .13 | .15 | .28* | — |
Note. Baseline IL-6 is an average score of IL-6 level at Time 1 and Time 2;
p < .05,
p < .01.
3.2. Findings of multi-level models
3.2.1. IL-6 levels following laboratory-evoked pain testing
In the first step, we found a significant time effect (β = .42, SE = .04, p < .001) indicating that participants’ overall IL-6 level increased over time following QST. Second, a quadratic term (i.e., time-squared) variable was included. The likelihood ratio test revealed that inclusion of the quadratic term significantly improved the model fit [χ(1) = 8. 97, p < .01], indicating that allowing for non-linearity improved model fit.
3.2.2. Sex differences in IL-6 reactivity following laboratory-evoked pain testing
Prior to testing the sex difference, we first tested whether the time slope varied across individuals. We found a significant time random slope, suggesting that the rate of change significantly varied across individuals [χ(1) = 213.20, p < .001]. When the sex and time interaction term was included in the model, we found a significant sex moderation effect (β = .04, SE = .02, p < .05), indicating that the trajectory of IL-6 reactivity differed by women and men. Figure 2 provides a visual illustration of the sex difference on IL-6 response over time. In brief, women showed an earlier and greater acceleration of IL-6 level over the period of measurement compared to men.
Figure 2.
Trajectories of IL-6 levels by sex Note. min = minutes, QST = quantitative sensory testing, pre = prior to QST, post = after QST. The sex differences in IL-6 trajectory was statistically significant (p < .05). † < .10, * < .05
3.2.3. Sex differences in IL-6 reactivity controlling for baseline covariates
As described in the analytic approach, in order to systematically control for a number of baseline covariates, we first included variables that significantly differed by sex (i.e., marital status and evoked pain sensitivity; see Table 1) in the model. The likelihood ratio difference test revealed that the model that included these covariates (−2LogLikelihood = 2018.74) had a significantly better model fit than that without any covariates (−2LogLikelihood = 2143.90) [χ(4) = 125.16, p < .001]. As hypothesized, the sex moderation effect remained significant (β = .04, SE = .02, p < .05) even after controlling for these covariates and their interaction with time. Table 3 provides all the regression estimates in this model.
Table 3.
Multi-level model parameter estimates
| Parameters | Est. | SE | t | p |
|---|---|---|---|---|
| Intercept | 3.01 | .92 | 3.25 | .002 |
| Time | .07 | .11 | .63 | .530 |
| Time2 | −.02 | .03 | −.55 | .586 |
| Sex | .39 | .58 | .67 | .508 |
| Marital Status | −.95 | .58 | −1.62 | .109 |
| Evoked Pain Sensitivity | .73 | .64 | 1.15 | .254 |
| Sex * Time2 | .04 | .02 | 2.08 | .041 |
| Marital Status * Time2 | .002 | .02 | .09 | .931 |
| Evoked Pain Sensitivity * Time2 | −.03 | .02 | −1.47 | .147 |
| Intercept Variance | 4.65 | .83 | ||
| Time2 Slope Variance | .004 | .00 | ||
| Intercept- Time2 Slope Covariance | .02 | .02 | ||
| Residual Variance | 1.19 | .09 | ||
Note. Wald tests are invalid for variance estimates in multi-level model and are omitted from the table.
The Sex Time2 interaction was statistically significant even before entering all covariates.
We then included three additional covariates (i.e., BMI, baseline clinical pain intensity, and situational pain catastrophizing) that were significantly associated with baseline IL-6 levels into the model. Again, we found significant model fit improvement when these additional covariates were included in the model (−2LogLikelihood = 1579.27) [χ(6) = 439.47, p < .001]. The results of this final model are shown in Table 4. We found that there was still a significant sex moderation effect (β = .05, SE = .02, p < .05). BMI was positively associated with mean IL-6 levels across time. Evoked pain sensitivity and the time interaction emerged to be statistically significant in the model. However, this likely represents a suppression effect56 because this interaction effect was non-significant in the previous model that only included marital status and evoked pain sensitivity as covariates. As this interaction finding is not robust and prone to potentially erroneous conclusion, we elected not to further interpret these findings.
Table 4.
Final multi-level model parameter estimates
| Parameters | Est. | SE | t | p |
|---|---|---|---|---|
| Intercept | −2.59 | 1.76 | −1.47 | .147 |
| Time | −.08 | .13 | −.58 | .561 |
| Time2 | −.03 | .07 | −.41 | .685 |
| Sex | 1.26 | .59 | 2.12 | .039 |
| Marital Status | −2.24 | .62 | −3.61 | .001 |
| BMI | .13 | .04 | 3.01 | .004 |
| Clinical Pain Severity | .004 | .15 | .03 | .977 |
| Evoked Pain Sensitivity | .35 | .66 | .54 | .592 |
| Situational Pain Catastrophizing | .84 | .37 | 2.27 | .027 |
| Sex * Time2 | .05 | .02 | 2.03 | .047 |
| Marital Status * Time2 | −.01 | .02 | −.45 | .653 |
| BMI * Time2 | .004 | .00 | .29 | .774 |
| Clinical Pain Severity * Time2 | −.003 | .01 | −.56 | .578 |
| Evoked Pain Sensitivity * Time2 | −.06 | .03 | −2.25 | .029 |
| Situational Pain Catastrophizing * Time2 | .02 | 0.01 | 1.16 | .251 |
| Intercept Variance | 3.39 | .74 | ||
| Time2 Slope Variance | .004 | .00 | ||
| Intercept- Time2 Slope Covariance | −.01 | .02 | ||
| Residual Variance | 1.21 | .10 | ||
Note. Wald tests are invalid for variance estimates in multi-level model and are omitted from the table.
The Sex Time2 interaction was statistically significant even before entering all covariates.
The model that included time and sex effect accounted for 64.3% of within-person (level-1) and 32.5% of between-person (level-2) IL-6 variability, respectively. With inclusion of all covariates, additional 3% and 20% of level-1 and level-2 variability were explained, respectively.
3.2.4. Results of Sensitivity Analysis
We found that the IL-6 outcome variable was moderately skewed (skewness = 1.67), indicating non-normality. Hence, we conducted a sensitivity analysis using a transformed IL-6 variable. In terms of transformation, we first tried log-transformation but found that the variable was still non-normally distributed while the direction of skewness changed from positive to negative direction (skewness = −1.28). Thus, we used square root transformation, which is a more appropriate transformation for moderately skewed data61. The variable became normally distributed (skewness = 0.40) after the square root transformation. We found that even when the IL-6 variable was transformed, the main results of sex moderation remained statistically significant (β = .01, SE = .004, p < .05).
4. Discussion
Chronic pain negatively affects women at a higher rate than men43,57, particularly among individuals with KOA40. Mechanisms contributing to sex disparities in the prevalence and severity of chronic pain remain unclear. One potential biological mechanism is divergence of inflammatory reactivity to psychophysical stressors52, such as pain. From our knowledge, this is one of the first studies examining sex differences in IL-6 responses following laboratory-evoked pain testing among KOA patients. We found that laboratory-evoked pain was generally associated with increased IL-6 levels and was moderated by sex, so that women showed significantly higher IL-6 reactivity after exposure to evoked pain relative to men, even when controlling for germane covariates.
Consistent with previous findings5,14,34,37,50, the present study showed increased IL-6 responses across KOA participants of both genders after acute pain testing. There are a number of factors that influence IL-6 levels, including circadian effects45. A recent study34 addressed confounding effects on IL-6 by asking participants to complete both standard and non-painful acute pain testing sessions. IL-6 reactivity patterns were similar between standard vs. non-painful pain testing procedures across both chronic pain and healthy groups, with a trend for higher IL-6 reactivity during the painful testing session34. To make a stronger claim that acute pain is directly associated with increased inflammatory levels beyond confounding factors, future studies should include a control group that either does not complete standard pain testing or undergoes non-painful sensory testing. Another avenue for future studies is to determine whether KOA disease stage influences IL-6 responses to acute pain challenges. Compared to healthy controls, OA patients exhibited overall higher serum IL-6 levels37,38,50 and a trend toward greater IL-6 reactivity after QST37. Although our study consisted only of late-stage KOA waiting for total knee replacement, future studies should incorporate both healthy adults and early-stage KOA patients to investigate whether IL-6 is upregulated systemically in KOA, particularly as the disease progresses. This approach will help determine whether acute pain-related changes in IL-6 levels reflects primed (sensitized) inflammatory responses as KOA progresses.
In line with previous work demonstrating a tendency toward higher IL-6 reactivity in response to acute psychological and physical stressors among women13,16,65, we found that women showed a greater non-linear (accelerated) increase in IL-6 response compared to men following laboratory pain testing. This result remained after controlling for baseline individual difference characteristics. Combined with previous findings, these data further support the claim that sex is an important biological factor that potentially modulates IL-6 reactivity in response to pain which is a psychophysical stressor characterized by an unpleasant sensory and emotional experience.
Female participants’ IL-6 levels showed an increasing trend even two hours after acute pain testing. Given that the current study includes KOA patients who experience pain almost daily, this robust sex difference in acute pain-related IL-6 reactivity might help explain sex disparities in KOA symptom severity. Higher levels of IL-6 are associated with mechanical hyperalgesia and have direct relevance to arthritic pain (e.g., joint movement-evoked pain)54. Similarly, inflammation induction via endotoxins is associated with decreased pain thresholds and conditioned pain modulation, a measure of endogenous pain inhibition28,64, and might have greater influence on pain among women 28. It is plausible that heightened IL-6 reactivity in response to pain, when cumulated over time, could contribute to robustly documented sex disparities in KOA-related symptom severity.
Cautiously, there are several possible mechanisms responsible for the observed sex difference in IL-6 reactivity. First, sex chromosomes and gonadal hormones potentially influence activity in cytokine-producing cells and immune system regulation31. For instance, research suggests that even with relative absence of estrogen (e.g., in childhood or post-menopause), differences in sex chromosomes can modulate immune responses among men and women9,22. Our findings support this argument because the majority of our sample (mean age = 66) included post-menopausal women. Higher estrogen levels can also result in a greater immune response to injury comparing women to men, leading to greater likelihood of peripheral sensitization in women52. While estrogen increases levels of pro-inflammatory cytokines released from macrophages33, testosterone has anti-inflammatory effects via IL-6 reduction23. Perhaps, sex differences in immune response can be further enhanced by the effect of gonadal hormones9. Future studies should evaluate hormonal influence on IL-6 reactivity following noxious stimulation by comparing menstruating women versus post-menopausal women.
Second, observed sex difference in inflammatory reactivity might concern evolutionary factors. Females evolved to serve certain discrete social roles that require greater immune response9. For instance, most female mammals carry and deliver a fetus, lactate, and protect their developing child9. To safely protect, nourish, and raise their children, females evolved to have a stronger immune systems, enabling more efficient protection from infections9. Indeed, females exhibit greater immune response to antigenic challenges including infection, injury, and vaccination30,32,48. This evolutionarily protective, heightened immune response in women is, balanced with facilitation of autoimmune damage36. In fact, autoimmune disorders are substantially more prevalent in women18.
4.1. Future implications
Our findings highlight viable future research avenues and clinical implications. First, taken together with previous findings, it appears that both psychological (e.g., anxiety) and psychophysical (i.e., pain) stressors are associated with elevation of cytokine responses. To gain further insight into individual differences in development and maintenance of chronic pain, it would be important to investigate the potentially distinct role of pain in inflammatory cytokine responses over and above other psychological stressors. For instance, future studies can compare various cytokine reactivities in response to QST versus acute psychological stress induction.
Second, although the present study only examined whether there was a significant sex difference in IL-6 reactivity following a laboratory-evoked pain testing, researchers can replicate and further extend our findings in longitudinal studies. For instance, as most of the studies on IL-6 and central sensitization are based on animal models, human studies should investigate how sex differences in IL-6 reactivity relate to widespread pain and central pain sensitivity. Also, future work should examine whether sex differences in IL-6 reactivity after exposure to evoked-pain can reliably predict future pain, disability, and emotional distress among individuals with KOA.
Third, exploring sex as a biological variable is an important endeavor in pain research and clinical care overall. Traditionally, many fields have ignored sex differences in disease research. Collapsing findings across sexes has possibly diminished a comprehensive understanding of disease etiology and concomitant treatment development. Researchers should always consider sex differences to enhance personalized or precision pain medicine, given that sex chromosomes and hormones can affect an array of biological functions, disease manifestation, and treatment response10. For example, previous work has demonstrated that IL-6 receptor inhibitors can be effective in reducing joint pain and bone erosion54, presenting a potential area of research for developing chronic pain prevention programs and/or more effective pain treatment strategies for women.
4.2. Limitations
The present study has a number of limitations. First, previous work has shown that IL-6 levels are sensitive to circadian effects45. To try to control for these effects, all of our experimental session started at a similar time (i.e., between 11:00 AM and 11:30 AM). However, future studies should consider including a control group that does not undergo QST to more rigorously disentangle circadian and pain effects on IL-6 response. Second, our study focused on IL-6 alone. Although IL-6 is one of the key pro-inflammatory cytokines related to pain, future studies should explore sex differences within the full cytokine reactivity cascade (e.g., IL-1β, TNF-α, NGF) to more comprehensively understand how men and women differ in pain-related inflammatory responses. Third, we did not collect sex-related serum markers such as estradiol and testosterone. Future studies should replicate and expand on our present findings by evaluating the effects of sex hormones on pain-related immune responses. Last, our study sample consisted of predominantly White patients and only included patients with advanced osteoarthritis scheduled for total knee arthroplasty. The generalizability of our findings to broader KOA patient populations may be limited.
4.3. Conclusions
The present study demonstrated divergent inflammatory responses following nociceptive stimulation among women and men. Even after controlling for covariates, women still displayed more pronounced IL-6 reactivity after exposure to laboratory-evoked pain. Given that KOA is a chronic condition, and individuals with KOA experience pain almost every day, such sex differences in inflammatory response may lead to maintenance or further exacerbation of KOA, especially in women. Future research examining mechanisms contributing to chronic pain and its amelioration need to account for sex as a biological variable52 to promote the generation of targeted pain interventions.
Acknowledgments
Source of Funding: Funding for this research was supported by the National Institute on Aging (R01AG034982 to RRE), National Heart, Lung, and Blood Institute (F32HL143941 to JEL), and National Institute of Neurological Disorders and Stroke (T32NS070201 for CJM’s postdoctoral training). MCM served on the advisory board of Axial Healthcare and Alosa Health, owned stock options in Axial Healthcare, and received support from the Foundation for Anesthesia Education and Research. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest in conducting this research.
References
- 1.Aasvang EK, Gmaehle E, Hansen JB, Gmaehle B, Forman JL, Schwarz J, Bittner R, Kehlet H: Predictive risk factors for persistent postherniotomy pain. Anesthesiol; 112:957–69, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Abrecht CR, Cornelius M, Wu A, Jamison RN, Janfaza D, Urman RD, Campbell C, Smith M, Haythornthwaite J, Edwards RR: Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: psychophysical and psychosocial factors. Pain Med; 20:161–71, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ: The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res; 28:193–213, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT: Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res; 67:1387–96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell CM, Carroll CP, Kiley K, Han D, Haywood C, Lanzkron S, Swedberg L, Edwards RR, Page GG, Haythornthwaite JA: Quantitative sensory testing and pain-evoked cytokine reactivity: Comparison of patients with sickle cell disease to healthy matched controls. Pain 157:949–56, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell CM, Moscou-Jackson G, Patrick Carroll C, Kiley K, Haywood C Jr, Lanzkron S, Hand M, Edwards RR, Haythornthwaite JA: An evaluation of central sensitization in patients with sickle cell disease. J Pain 17:617–27, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho B, Zheng M, Aiono-Le Tagaloa L: Evaluation of experimental pain tests to predict labour pain and epidural analgesic consumption. Br J Anaesth; 110:600–6, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn E a, Lai J, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R: Initial adult health item banks and first wave testing of the Patient-Reported Outcomes Measurement Information System (PROMIS) Network: 2005–2008. J Clin Epidemiol 63:1179–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrousos GP: Stress and sex versus immunity and inflammation. Sci Signal 3:, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Clayton JA: Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav; 187:2–5, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS, Ryan KM: Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore Annals, Academy of Medicine, Singapore; 23:129–38, 1994. [PubMed] [Google Scholar]
- 12.Cruz-Almeida Y, Aguirre M, Sorenson HL, Tighe P, Wallet SM, Riley JL III: Age differences in cytokine expression under conditions of health using experimental pain models. Exp Gerontol; 72:150–6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards KM, Burns VE, Ring C, Carroll D: Sex differences in the interleukin-6 response to acute psychological stress. Biol Psychol; 71:236–9, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG: Association of catastrophizing with interleukin-6 responses to acute pain. Pain; 140:135–44, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA: Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain; 22:730–7, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Endrighi R, Hamer M, Steptoe A: Post-menopausal Women Exhibit Greater Interleukin-6 Responses to Mental Stress Than Older Men. Ann Behav Med; 50:564–71, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S: Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav Immun; 52:18–26, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Fairweather D, Frisancho-Kiss S, Rose NR: Sex differences in autoimmune disease from a pathological perspective. Am J Pathol; 173:600–9, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson JF, Patel PN, Shah RY, Mulvey CK, Gadi R, Nijjar PS, Usman HM, Mehta NN, Shah R, Master SR, Propert KJ, Reilly MP: Race and gender variation in response to evoked inflammation. J Transl Med 11:1–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillingim RB, King CD, Ribeiro-Dasilva MC, Riley JL III: Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10:447–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finan PH, Goodin BR, Smith MT: The association of sleep and pain: an update and a path forward. J Pain; 14:1539–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM: Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci; 111:869–74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganesan K, Balachandran C, Manohar BM, Puvanakrishnan R: Effects of testosterone, estrogen and progesterone on TNF-α mediated cellular damage in rat arthritic synovial fibroblasts. Rheumatol Int; 32:3181–8, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Heffner KL, France CR, Ashrafioun L, Quiñones M, Walsh P, Maloney MD, Giordano BD, Pigeon WR: Clinical pain-related outcomes and inflammatory cytokine response to pain following insomnia improvement in adults with knee osteoarthritis. Clin J Pain; 34:1133–40, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffner KL, France CR, Trost Z, Ng HM, Pigeon WR: Chronic low back pain, sleep disturbance, and interleukin-6. Clin J Pain; 27:35–41, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglewicz B, Hoaglin DC: How to detect and handle outliers. Asq Press; 1993. [Google Scholar]
- 27.Von Känel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE: Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun; 20:40–8, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Karshikoff B, Lekander M, Soop A, Lindstedt F, Ingvar M, Kosek E, Olgart Höglund C, Axelsson J: Modality and sex differences in pain sensitivity during human endotoxemia. Brain Behav Immun; 46:35–43, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki Y, Zhang L, Cheng J-K, Ji R-R: Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci; 28:5189–94, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein SL: The effects of hormones on sex differences in infection: From genes to behavior. Neurosci Biobehav Rev; 24:627–38, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Klein SL, Flanagan KL: Sex differences in immune responses. Nat Rev Immunol; 16:626–38, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Klein SL, Poland GA: Personalized vaccinology: One size and dose might not fit both sexes. Vaccine; 31:2599–600, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Kovats S: Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294:63–9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazaridou A, Martel MO, Cahalan CM, Cornelius MC, Franceschelli O, Campbell CM, Haythornthwaite JA, Smith M, Riley J, Edwards RR: The impact of anxiety and catastrophizing on interleukin-6 responses to acute painful stress. J Pain Res; 11:637–47, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazaridou A, Martel MO, Cornelius M, Franceschelli O, Campbell C, Smith M, Haythornthwaite JA, Wright JR, Edwards RR: The association between daily physical activity and pain among patients with knee osteoarthritis: the moderating role of pain catastrophizing. Pain Med; 20:916–24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KA: Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol 46:1000–15, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, Edwards RR: Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res; 63:320–7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FMK, Spector TD: Interleukin‐6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum; 60:2037–45, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Losina E, Thornhill TS, Rome BN, Wright J, Katz JN: The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Jt Surgery Am; 94:201–7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.March L, Smith EUR, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, Buchbinder R, Vos T, Woolf AD: Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol; 28:353–66, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Mathur VA, Kiley KB, Carroll CP, Edwards RR, Lanzkron S, Haythornthwaite JA, Campbell CM: Disease-related, nondisease-related, and situational catastrophizing in sickle cell disease and its relationship with pain. J Pain; 17:1227–36, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McInnis CM, Thoma M V, Gianferante D, Hanlin L, Chen X, Breines JG, Hong S, Rohleder N: Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun; 42:33–40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogil JS: Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat Rev Neurosci; 13:859–66, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Nandi M, Schreiber KL, Martel MO, Cornelius M, Campbell CM, Haythornthwaite JA, Smith MT, Wright J, Aglio LS, Strichartz G, Edwards RR: Sex differences in negative affect and postoperative pain in patients undergoing total knee arthroplasty. Biol Sex Differ; 10:1–8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilsonne G, Lekander M, Åkerstedt T, Axelsson J, Ingre M: Diurnal variation of circulating interleukin-6 in humans: A meta-analysis. PLoS One 11:1–17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin M-T, O’Farrelly C, Malone KM: Clinical anxiety, cortisol and interleukin-6: Evidence for specificity in emotion–biology relationships. Brain Behav Immun; 24:1074–7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HS, Park JY, Yu R: Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract; 69:29–35, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Plackett TP, Gamelli RL, Kovacs EJ: Gender-based differences in cytokine production after burn unjury: A role of interleukin-6. J Am Coll Surg 210:73–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quartana PJ, Finan PH, Page GG, Smith MT: Effects of insomnia disorder and knee osteoarthritis on resting and pain-evoked inflammatory markers. Brain Behav Immun; 47:228–37, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quartana PJ, Finan PH, Page GG, Smith MT: Effects of insomnia disorder and knee osteoarthritis on resting and pain-evoked inflammatory markers. Brain Behav Immun; 47:228–37, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quené H, Van Den Bergh H: On multi-level modeling of data from repeated measures designs: A tutorial. Speech Commun; 43:103–21, 2004. [Google Scholar]
- 52.Rosen S, Ham B, Mogil JS: Sex differences in neuroimmunity and pain. J Neurosci Res; 95:500–8, 2017. [DOI] [PubMed] [Google Scholar]
- 53.Santos M, Gomes WF, Pereira DS, Oliveira DMG, Dias JMD, Ferrioli E, Pereira LSM: Muscle strength, muscle balance, physical function and plasma interleukin-6 (IL-6) levels in elderly women with knee osteoarthritis (OA). Arch Gerontol Geriatr; 52:322–6, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Schaible H-G: Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther; 16:470, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultzberg M, Muthén B: Number of subjects and time points needed for multilevel time-series analysis: A simulation study of dynamic structural equation modeling. Struct Equ Model; 25:495–515, 2018. [Google Scholar]
- 56.Smith RL, Ager JW Jr, Williams DL: Suppressor variables in multiple regression/correlation. Educ Psychol Meas; 52:17–29, 1992. [Google Scholar]
- 57.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G: A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil; 13:769–81, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C: Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis; 72:535–40, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V: Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behav Immun; 16:774–84, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan MJL, Bishop SR, Pivik J: The Pain Catastrophizing Scale: Development and validation. Psychol Assess; 7:524–32, 1995. [Google Scholar]
- 61.Tabachnick BG, Fidell LS: Using multivariate statistics Boston. 5th Editio Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- 62.Tanaka T, Narazaki M, Kishimoto T : IL-6 in Inflammation, Immunity, and Disease; 6:1–16, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, Woods RJ, Lieberman DE: Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci 114:9332–36, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wegner A, Elsenbruch S, Rebernik L, Roderigo T, Engelbrecht E, Ja Ger M, Engler H, Schedlowski M, Benson S: Inflammation-induced pain sensitization in men and women: Does sex matter in experimental endotoxemia? Pain; 156:1954–64, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS: Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology; 133:2523–30, 1993. [DOI] [PubMed] [Google Scholar]
- 66.Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK: Interleukin-6: An emerging regulator of pathological pain. J Neuroinflammation; 13:1–9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]