Abstract
Elevated levels of serum uric acid (SUA) have been suggested to associate with cardiovascular disease, diabetes and metabolic syndrome (MetS). However, information is limited on the association between SUA and MetS in general adults. This study aimed to assess the relationship of SUA with MetS and its components in general adults in Bangladesh. A total of 420 participants were enrolled in this study and biochemical parameters including SUA, fasting blood glucose (FBG) and lipid profile were analyzed using standard methods. The NECP criteria were applied to define MetS. The association of SUA with MetS and its components were evaluated by multinomial logistic regression models. The overall prevalence of MetS was 22% with 21.9% in males and 22.1% in female participants. Male subjects had a high prevalence of elevated components of MetS than in the female subjects (p < 0.05 for all cases). The mean concentration of SUA was significantly higher in subjects of the MetS group compared to the non-MetS group (p < 0.05). The components of MetS were raised with the increasing concentrations of SUA across the quartiles. In regression analysis, SUA was significantly associated with the prevalence of MetS in Bangladeshi adults. In conclusion, elevated SUA was significantly associated with the prevalence of MetS and its components.
Subject terms: Metabolic disorders, Metabolic syndrome
Introduction
Metabolic syndrome (MetS) consist of several risk factors including central obesity, elevated blood pressure, hyperglycemia, high triglycerides, and reduced high-density lipoprotein cholesterol1. MetS is associated with the increased risk of type 2 diabetes, cardiovascular disease (CVD) and mortality2,3. Several population-based studies showed an increased risk of CVD in individuals with MetS compared to those who do not have the syndrome4,5. Besides the traditional risk factors, other factors including microalbuminuria, inflammatory markers and hyperuricemia have been suggested to be involved in the MetS6–8. Along with MetS, obesity has also been found as an important risk factor for CVD. Furthermore, a link has been found between obesity and hyperuricemia in various studies9–11. The prevalence of MetS is increasing at an alarming rate both in developed and developing countries. MetS is highly prevalent among Bangladeshi adults and has been increased rapidly in the last few decades. A recent review reported a high prevalence of MetS (30%) in the Bangladeshi population with 32% in females and 25% in males12.
Uric acid in serum is the final oxidation product of purine metabolism in human13. Recent epidemiological studies have demonstrated an association of serum uric acid (SUA) with MetS and its components in different populations14–19. Some other studies have also found that elevated SUA levels are an independent predictors of the components of MetS, such as high blood pressure and hyperglycemia20. However, information is limited regarding the relationship of SUA with MetS in general adults. Moreover, no study has been conducted to examine the association between SUA and MetS in Bangladeshi adults. Given the increased prevalence of MetS in the Bangladeshi population, this cross-sectional study aimed to investigate the relationship of SUA with MetS and its components in general adults. This study also aimed to assess whether SUA is an additional component of MetS in this population.
Materials and methods
Study population
This study was a cross-sectional design conducted between November 2017 and May 2019 at the Department of Biochemistry and Molecular Biology of Shahjalal University of Science and Technology, Sylhet, Bangladesh. A total of 420 general adults (aged ≥ 18 years) were enrolled from university students, academic and non-academic staff and local city people of the Sylhet and Dhaka region of Bangladesh. The inclusion criteria were: both gender, aged above 18 years, free from severe chronic illness and willing to participate. Exclusion criteria were: pregnant women, lactating mother and participants with a history of hepatotoxic drug intake, kidney disease, alcohol intake and self-reported evidence of acute or chronic hepatitis. We also excluded participants with missing anthropometric data or blood samples. This study was approved by the Internal Ethics Committee at the Department of Biochemistry and Molecular Biology of Shahjalal University of Science and Technology, Bangladesh. All participants provided written informed consent before inclusion in the study. All steps in the methods section were performed in accordance with the relevant guidelines and regulations.
General data collection
A standard questionnaire was used to collect demographic and lifestyle information from the participants. Individual anthropometric data such as age, gender, weight and height were recorded in the questionnaire form followed a standard procedure described elsewhere21–26. Briefly, systolic and diastolic blood pressure (SBP and DBP) were measured twice in the left arm of the participants with an automated sphygmomanometer (Omron M10, Omron Corporation, Tokyo, Japan) in the seated position after at least 10 minutes of rest. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). Waist circumference (WC) was measured using general tape that was placed midway between the lowest border of the ribs and iliac crest. Hip circumference (HC) was measured at the largest circumference of the buttocks to the nearest 0.5 cm. Waist-hip ratio (WHR) was measured as waist circumference divided by hip circumference.
Blood sample collection and laboratory measurements
Venous blood samples were collected after an overnight fast from each subject. The blood samples were centrifuged and stored the isolated serum at −20 °C until laboratory analysis. Serum uric acid (SUA), fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured by colorimetric methods with a semi-auto biochemistry analyzer (Humalyzer 3000, USA) described elsewhere21,22,26. The diagnostic kits were purchased from Human Diagnostic (Germany) for analysis of the above clinical parameters. The measurements were carried out according to the standard manufacturer’s protocols provided within the kit. The precision of the measurements was maintained regularly by method standard calibration.
Diagnostic criteria
In present study, hyperuricemia was defined as SUA concentration >7.0 mg/dL (416.4 µmol/L) in men or >6.0 mg/dL (356.9 µmol/L) in women27,28. All volunteers were stratified into four quartiles based on SUA concentrations (Q1: ≤ 243.9, 244–309.3, 309.4–380.7 and> 380.7 µmol/L). Metabolic syndrome (MetS) was diagnosed according to the National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATP III) criteria29. The components of the MetS were defined as following: i) Elevated BP (SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg or intake of an antihypertensive medication); ii) raised WC (> 102 cm for males and> 88 cm for females); iii) hyperglycemia (FBG ≥ 100 mg/dL). iv) hypertriglyceridemia (TG ≥ 150 mg/dL); v) low HDL-C (< 40 mg/dL for males and <50 mg/dL for females) and subjects with at least three of the above components were identified as having MetS.
Statistical analysis
Statistical data analyses were performed using IBM SPSS version 23. Data are presented as mean ± SD and quartile ranges. The tests applied during data analysis already described in our previous studies21,26. In brief, the baseline characteristics of the volunteers in the SUA quartiles were compared by one-way ANOVA. A Chi-square test was applied to differentiate the proportions of the categorical variables. Differences in the anthropometric and baseline characteristics between the gender groups were done by an independent sample t-test. Pearson’s correlation coefficient test was performed to assess the relationships between baseline variables and SUA concentrations. The association between MetS and SUA levels was evaluated by multinomial logistic regression models. MetS was categorized as yes (presence) and no (absence). In regression analysis, MetS (yes) was considered as the dependent variable and SUA as the independent variable. SUA and other covariates were used as continuous variables in the regression models. We applied three models in the regression analysis. Model 1 was adjusted for age (years) and gender (male and female). Model 2 was adjusted for age, gender and BMI (kg/m2), Model 3 was further adjusted for variables used in model 1 and 2 and LDL-C (mg/dL). A p-value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the participants in the MetS and non-MetS group
In total, 420 participants (257 male and 163 female) were enrolled in the present study. The baseline characteristics of the participants in the MetS and non-MetS groups are presented in Table 1. Among the participants, 93 subjects were diagnosed with MetS according to the diagnostic criteria. There were significant differences in the mean of age, WC, HC, WHR, BMI, SBP, DBP, FBG, TG, TC, LDL-C (p < 0.001 for all cases) between the MetS and non-MetS groups. The average level of SUA was also higher in the MetS group compared to the non-MetS group (p < 0.05). In contrast, subjects in the non-MetS group had a higher level of HDL-C level than the subjects in the MetS group (p < 0.05).
Table 1.
Baseline characteristics of the participants based on the presence of MetS.
| Total | MetS | Non-MetS | p-value | |
|---|---|---|---|---|
| N | 420 | 93 | 327 | — |
| Gender, m/f | 420 | 57/36 | 200/127 | — |
| Age, year | 30.5 ± 12.4 | 39.5 ± 14.1 | 27.8 ± 10.4 | 0.000 |
| WC, cm | 83.9 ± 10.6 | 90.5 ± 12.3 | 81.4 ± 8.6 | 0.000 |
| HC, cm | 92.8 ± 8.2 | 96.0 ± 8.6 | 91.5 ± 7.6 | 0.000 |
| WHR | 0.68 ± 0.39 | 0.91 ± 0.19 | 0.62 ± 0.41 | 0.000 |
| BMI, kg/m2 | 23.9 ± 3.8 | 26.3 ± 3.8 | 23.3 ± 3.6 | 0.000 |
| SBP, mmHg | 118.2 ± 15.1 | 128.8 ± 18.1 | 115.1 ± 12.5 | 0.000 |
| DBP, mmHg | 73.6 ± 14.9 | 81.6 ± 8.4 | 71.4 ± 15.6 | 0.000 |
| SUA, µmol/L | 319.2 ± 107.8 | 332.0 ± 111.8 | 308.2 ± 106.6 | 0.026 |
| FBG, mg/dL | 98.8 ± 37.5 | 130.4 ± 66.2 | 89.9 ± 17.7 | 0.000 |
| TG, mg/dL | 135.1 ± 101.6 | 211.6 ± 107.2 | 113.5 ± 88.9 | 0.000 |
| TC, mg/dL | 157.7 ± 63.9 | 189.2 ± 75.7 | 148.8 ± 56.3 | 0.000 |
| HDL-C, mg/dL | 31.9 ± 17.1 | 29.6 ± 11.1 | 33.4 ± 18.5 | 0.046 |
| LDL-C, mg/dL | 93.3 ± 58.3 | 115.9 ± 73.2 | 85.9 ± 50.4 | 0.000 |
MetS: Metabolic Syndrome. Values are presented as mean ± SD. P-values are obtained from independent sample t-test.
Prevalence of MetS and its components in gender and hyperuricemic group
The prevalence of MetS and its components are presented in Table 2 and Fig. 1. Overall, the prevalence of MetS was 22% with 21.9% in males and 22.1% in female subjects. The MetS components such as raised WC, high blood pressure, hyperglycemia and high TG were significantly higher in male than in the female subjects (p < 0.01 for all cases). In contrast, there was no significant difference for low HDL-C between the male-female groups. The prevalence of hyperuricemia was 16.6% with significant differences between the male (21.3%) and female (8.3%) group (p < 0.01). All components of MetS were significantly higher in subjects in the hyperuricemic group compared to the subjects in the non-hyperuricemic group (p < 0.05 for all cases).
Table 2.
Prevalence of MetS and its components in the male-female group.
| Parameters | Total | Male | Female | P-value |
|---|---|---|---|---|
| MetS, % | 22.0 | 21.9 | 22.1 | >0.05 |
| Raised WC, % | 13.3 | 4.6 | 30.9 | <0.01 |
| Elevated SBP, % | 17.9 | 24.1 | 8.6 | <0.01 |
| Elevated DBP, % | 17.4 | 18.4 | 15.9 | <0.05 |
| Hyperglycemia, % | 25.7 | 23.4 | 29.2 | <0.05 |
| Elevated TG, % | 40.4 | 52.2 | 22.9 | <0.01 |
| Reduced HDL-C, % | 80.7 | 79.5 | 82.5 | >0.05 |
| Hyperuricemia | 16.6% | 21.3 | 8.3 | <0.01 |
The difference between male and female is expressed as p-value. P-values are obtained from Chi-square test.
Figure 1.
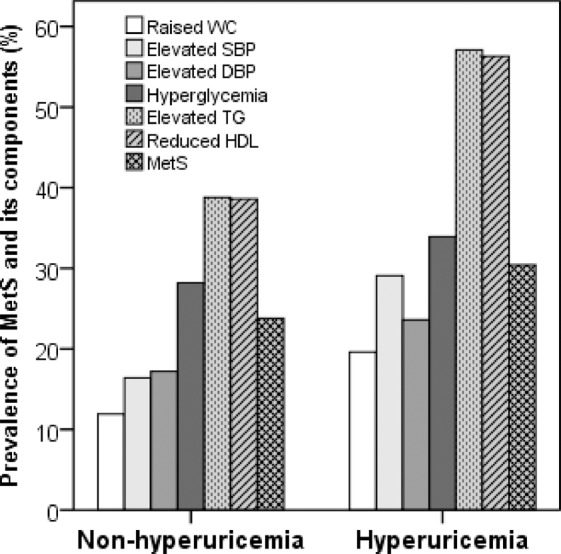
Prevalence of MetS and its components in the hyperuricemic and non-hyperuricemic group. P < 0.05 for all cases when the prevalence of MetS and its component are compared between the groups. P-values are obtained from the Chi-square test.
Baseline characteristics of the study subjects according to SUA quartiles
The baseline characteristics of the study participants were also evaluated according to SUA quartiles (Table 3). The volunteers were divided into 4 groups based on SUA levels (Q1: ≤ 243.9; Q2: 244–309.3; Q3: 309.4–380.7 and >380.7 µmol/L). No significant difference was observed in the mean age across the SUA quartiles. Participants in the fourth quartile of SUA had a significantly higher WC, WHR, BMI, SBP, DBP, SUA, TG, TC LDL-C and lower FBG and HDL-C than the subjects in the other quartiles of SUA (p < 0.05 for all cases).
Table 3.
Baseline characteristics of the participants according to SUA quartiles.
| Q1 ≤ 243.9 µmol/L | Q2 244–309.3 µmol/L | Q3 309.4–380.7 µmol/L | Q4> 380.7 µmol/L | p-value for trend | |
|---|---|---|---|---|---|
| N | 104 | 110 | 105 | 101 | — |
| Sex, m/f | 34/70 | 57/53 | 80/25 | 86/15 | — |
| Age, year | 34.1 ± 13.9 | 31.2 ± 11.9 | 31.2 ± 12.0 | 31.3 ± 12.3 | 0.338 |
| WC, cm | 81.9 ± 9.9 | 82.6 ± 9.7 | 83.8 ± 8.0 | 87.3 ± 13.5 | 0.010 |
| HC, cm | 91.7 ± 8.4 | 92.0 ± 8.5 | 93.2 ± 6.8 | 94.1 ± 8.7 | 0.282 |
| WHR | 0.72 ± 0.36 | 0.75 ± 0.34 | 0.78 ± 0.31 | 0.88 ± 0.22 | 0.010 |
| BMI, kg/m2 | 23.9 ± 3.7 | 24.2 ± 3.8 | 24.6 ± 3.5 | 25.4 ± 3.9 | 0.008 |
| SBP, mmHg | 114.7 ± 15.1 | 116. 7 ± 13.9 | 120.5 ± 13.0 | 124.9 ± 15.2 | 0.000 |
| DBP, mmHg | 69.2 ± 21.1 | 73.7 ± 12.0 | 76.0 ± 12.1 | 77.3 ± 11.9 | 0.003 |
| SUA, µmol/L | 196.2 ± 38.7 | 282.0 ± 19.7 | 347.1 ± 20.9 | 465.6 ± 85.1 | 0.000 |
| FBG, mg/dL | 108.7 ± 55.6 | 102.7 ± 44.4 | 90.7 ± 15.8 | 98.2 ± 28.6 | 0.025 |
| TG, mg/dL | 122.2 ± 73.9 | 145.9 ± 96.5 | 156.1 ± 92.4 | 171.6 ± 122.7 | 0.012 |
| TC, mg/dL | 139.4 ± 71.1 | 147.4 ± 47.3 | 164.1 ± 54.9 | 188.7 ± 77.6 | 0.000 |
| HDL-C, mg/dL | 35.9 ± 12.3 | 37.7 ± 14.6 | 31.9 ± 12.1 | 27.1 ± 13.9 | 0.000 |
| LDL-C, mg/dL | 80.6 ± 68.4 | 80.9 ± 42.1 | 98.9 ± 52.7 | 119.6 ± 66.6 | 0.000 |
Values are presented as mean ± SD. P-values are obtained from one-way ANOVA.
Association of SUA with the prevalence of MetS and its components
Taking SUA as the independent variable and MetS as the dependent variable, multinomial logistic regression was performed to assess the relationship between SUA and MetS. The detailed results are presented in Table 4. In regression analysis, a positive association was observed between SUA and MetS. In all regression models, SUA showed a significant association with the prevalence of MetS. We further assessed the relationship of SUA with the individual components of MetS (Table 5). After adjustment for age, a positive association was observed between SUA and the components of MetS except for hyperglycemia and low HDL-C.
Table 4.
Multinomial logistic regression analysis to evaluate the association between SUA levels and MetS.
| B | SE | Wald | df | OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Model 1 | 0.011 | 0.003 | 10.570 | 1 | 1.011 (1.004–1.017) | 0.001 |
| Model 2 | 0.008 | 0.004 | 5.727 | 1 | 1.008 (1.002–1.016) | 0.016 |
| Model 3 | 0.007 | 0.004 | 3.872 | 1 | 1.006 (1.000–1.013) | 0.042 |
Dependent variable is MetS (yes) and independent variable is SUA (µmol/L). Reference category is normal (non-MetS). Model 1: adjusted for age (years) and gender (male and female). Model 2: model 1+ BMI (kg/m2) Model 3: model 2+ LDL (mg/dL). OR, odds ratio; CI, confidence interval; SE, Standard error.
Table 5.
Age-adjusted logistic regression analysis to evaluate the association between SUA and the components of MetS.
| B | SE | Wald | df | OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Abdominal obesity | 0.009 | 0.004 | 6.008 | 1 | 1.009 (1.002–1.017) | 0.014 |
| High blood pressure | 0.008 | 0.004 | 4.445 | 1 | 1.008 (1.001–1.016) | 0.035 |
| Hyperglycemia | −0.001 | 0.001 | 0.638 | 1 | 0.999 (0.997–1.001) | 0.425 |
| High TG | 0.11 | 0.003 | 17.956 | 1 | 1.011 (1.006–1.016) | 0.000 |
| Low-HDL-C | 0.002 | 0.002 | 1.024 | 1 | 1.002 (0.998–1.007) | 0.312 |
The dependent variable is MetS components (yes) and the independent variable is SUA (µmol/L). The reference category is normal. The model is adjusted for age (years). OR, odds ratio; CI, confidence interval; SE, Standard error.
Discussion
The present study investigates the relationship between SUA and MetS in general adults. Although, the association of SUA with MetS has been studied in diabetic and hypertensive subjects, however, limited studies have documented the information regarding the link of SUA with MetS in general adults. In this study, we first report a positive association of SUA with MetS and its components in general adults in Bangladesh.
In the present study, no significant difference was observed in the prevalence of MetS between the gender groups. Among the MetS components, high TG and reduced-HDL-C were the more common abnormalities found in the participants. Anthropometric variables, lipid profile, FBG and SUA were significantly higher in subjects with MetS than in the subjects who do not have this syndrome. In this study, we observed an increasing trend in the levels of individual components of MetS across the SUA quartiles. These results are consistent with the findings reported in Japanese30,31, Chinese19, Iranian17, the United States32,33, and European population34.
Several epidemiological studies demonstrated a positive association between SUA and the prevalence of MetS in human cohorts. However, it is still debated whether increased SUA concentration is a risk factor or only a biomarker in the progression and development of MetS19. Some studies reported that hyperuricemia may be an individual component of the MetS35,36, whereas, other works suggested to include hyperuricemia as an additional component of MetS37,38. In prospective studies, SUA at baseline level was associated with an increased risk of MetS in both genders27,39. A previous study reported that subjects with hyperuricemia have a higher chance of developing of MetS than in the non-hyperuricemic subjects40. This predictive role of SUA has been demonstrated in individuals who were free of all components of MetS at baseline27. Moreover, a recent clinical study reported that elevated levels of SUA can play a pathogenic role in MetS41. Due to controversial findings, NCEP could not include hyperuricemia as an individual component of MetS yet, and it seems that further longitudinal investigations are required to elucidate whether hyperuricemia is another component of the MetS or not17.
In regression models, SUA showed a significant association with MetS and several components of MetS. However, after adjusting some confounding factors, we did not find the significant association of SUA with hyperglycemia and low HDL-C. This might be happened because of the inverse relationship of SUA with the prevalence of diabetes in the Bangladeshi population26. On the other hand, we observed a very high prevalence of low HDL-C among the participants that could be a reason for the non-significant association of SUA with HDL-C.
The underline mechanisms between SUA and MetS are not fully understood yet. The possible mechanisms of SUA in inducing MetS are as follows: First, hyperuricemia has been demonstrated to cause endothelial dysfunction in human and animal models42,43. Second, SUA has been exhibited to hinder the production of NO44, which is considered as important for insulin function45,46. Deficiency of endothelial-formed NO is thought to reduce blood flow to cells leading to stop the insulin action and inducing hyperinsulinemia17. Thus, hyperuricemia may have a potential role in inducing or worsening insulin resistance by itself. In turn, the resistance of insulin is believed to play a vital role in the pathogenesis of MetS47. Although a positive association of hyperuricemia with MetS has been demonstrated through epidemiological and animal studies, the exact mechanisms by which SUA leads to this disorder are still at the beginning need to be explained. Thus, it is obvious that further prospective studies are required to determine the role of SUA in the development of MetS.
There were some limitations to the study. First, the sample size in the present study was relatively small. Second, the cross-sectional nature of the data did not allow us to draw a causal relationship between SUA and MetS. Moreover, we adjusted some probable confounding factors in determining the relationship between SUA and MetS; however, other confounding factors effects cannot be excluded. For example, we did not have information on renal function tests and diet habits that can affect SUA levels. Therefore, the present study findings should be generalized cautiously to other general populations.
Conclusions
The present study showed that the components of MetS are raised with the increasing concentrations of SUA across the quartiles. In this study, SUA was significantly associated with the prevalence of MetS and its components. Given the high prevalence of MetS among Bangladeshi adults, more studies are required to examine the role of SUA in the pathogenesis of MetS. Furthermore, attention should be paid to the increased risk of MetS and hyperuricemia for the general population.
Acknowledgements
The authors would like to thank all staff and volunteers for their active cooperation in the study.
Author contributions
N.A. played a major role in the conception and design of the study, data interpretation and wrote the manuscript. R.M., M.H., Z.B. and A.D.M. experimented and analyzed the data. J.M.H., A.D.T., and A.H. helped in sample collection and contributed to results analysis. F.I. helped in result analysis and revision of the manuscript draft. All authors read the manuscript and approved the final version.
Data availability
All related data are included in this paper. The datasets produced and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 2.Thomas GN, et al. Metabolic syndrome increases all-cause and vascular mortality: the Hong Kong Cardiovascular Risk Factor Study. Clin. Endocrinol. (Oxf.) 2007;66:666–671. doi: 10.1111/j.1365-2265.2007.02798.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 4.Butler J, et al. Metabolic Syndrome and the Risk of Cardiovascular Disease in Older Adults. J. Am. Coll. Cardiol. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Dekker JM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 6.Fu C-C, Wu D-A, Wang J-H, Yang W-C, Tseng C-H. Association of C-reactive protein and hyperuricemia with diabetic nephropathy in Chinese type 2 diabetic patients. Acta Diabetol. 2009;46:127–134. doi: 10.1007/s00592-008-0069-0. [DOI] [PubMed] [Google Scholar]
- 7.Guo L, et al. Association between microalbuminuria and cardiovascular disease in type 2 diabetes mellitus of the Beijing Han nationality. Acta Diabetol. 2012;49(Suppl 1):S65–71. doi: 10.1007/s00592-010-0205-5. [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishna V, Jailkhani R. Oxidative stress in non-insulin-dependent diabetes mellitus (NIDDM) patients. Acta Diabetol. 2008;45:41–46. doi: 10.1007/s00592-007-0018-3. [DOI] [PubMed] [Google Scholar]
- 9.Ali N, et al. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: A study on Bangladeshi adults. PLOS ONE. 2018;13:e0206850. doi: 10.1371/journal.pone.0206850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizaka N, et al. Changes in waist circumference and body mass index in relation to changes in serum uric acid in Japanese individuals. J. Rheumatol. 2010;37:410–416. doi: 10.3899/jrheum.090736. [DOI] [PubMed] [Google Scholar]
- 11.Tsushima Y, et al. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury MZI, et al. Prevalence of metabolic syndrome in Bangladesh: a systematic review and meta-analysis of the studies. BMC Public Health. 2018;18:308. doi: 10.1186/s12889-018-5209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glantzounis G, Tsimoyiannis E, Kappas A, Galaris D. Uric Acid and Oxidative Stress. Curr. Pharm. Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 14.Cicero AFG, et al. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci. Rep. 2018;8:11529. doi: 10.1038/s41598-018-29955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J-K, Ryoo J-H, Choi J-M, Park SK. Association of serum uric acid level and the development of metabolic syndrome in middle-aged Korean men: a 5-year follow-up study. J. Prev. Med. Pub. Health. 2014 doi: 10.3961/jpmph.14.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, et al. Association of Uric Acid with Metabolic Syndrome in Men, Premenopausal Women and Postmenopausal Women. Int. J. Environ. Res. Public. Health. 2014;11:2899–2910. doi: 10.3390/ijerph110302899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meshkani R, Zargari M, Larijani B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta Diabetol. 2011;48:79–88. doi: 10.1007/s00592-010-0231-3. [DOI] [PubMed] [Google Scholar]
- 18.Nagahama K, et al. Hyperuricemia predicts future metabolic syndrome: a 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens. Res. 2014;37:232–238. doi: 10.1038/hr.2013.137. [DOI] [PubMed] [Google Scholar]
- 19.Wang H-J, Shi L-Z, Liu C-F, Liu S-M, Shi S-T. Association between uric acid and metabolic syndrome in elderly women. Open Med. 2018;13:172–177. doi: 10.1515/med-2018-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi Y, et al. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: The Osaka Health Survey. J. Hypertens. 2001;19:1209–1215. doi: 10.1097/00004872-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ali N, et al. Relationship between serum uric acid and hypertension: a cross-sectional study in Bangladeshi adults. Sci. Rep. 2019;9:9061. doi: 10.1038/s41598-019-45680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali N, et al. The relationship between serum uric acid and lipid profile in Bangladeshi adults. BMC Cardiovasc. Disord. 2019;19:42. doi: 10.1186/s12872-019-1026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam, S. et al. Prevalence of elevated liver enzymes and its association with type 2 diabetes: A cross-sectional study in Bangladeshi adults. Endocrinol. Diabetes Metab, 10.1002/edm2.116 (2020). [DOI] [PMC free article] [PubMed]
- 24.Rahman S, Islam S, Haque T, Kathak RR, Ali N. Association between serum liver enzymes and hypertension: a cross-sectional study in Bangladeshi adults. BMC Cardiovasc. Disord. 2020;20:128. doi: 10.1186/s12872-020-01411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali, N. et al. Hypertension prevalence and influence of basal metabolic rate on blood pressure among adult students in Bangladesh. BMC Public Health18, (2018). [DOI] [PMC free article] [PubMed]
- 26.Haque T, Rahman S, Islam S, Molla NH, Ali N. Assessment of the relationship between serum uric acid and glucose levels in healthy, prediabetic and diabetic individuals. Diabetol. Metab. Syndr. 2019;11:49. doi: 10.1186/s13098-019-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008;57:845–852. doi: 10.1016/j.metabol.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You L, Liu A, Wuyun G, Wu H, Wang P. Prevalence of hyperuricemia and the relationship between serum uric acid and metabolic syndrome in the Asian Mongolian area. J. Atheroscler. Thromb. 2014;21:355–365. doi: 10.5551/jat.20529. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Nagahama K, et al. Hyperuricemia and cardiovascular risk factor clustering in a screened cohort in Okinawa, Japan. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2004;27:227–233. doi: 10.1291/hypres.27.227. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi N, et al. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur. J. Epidemiol. 2002;18:523–530. doi: 10.1023/A:1024600905574. [DOI] [PubMed] [Google Scholar]
- 32.Coutinho TA, et al. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am. J. Hypertens. 2007;20:83–89. doi: 10.1016/j.amjhyper.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertens. Dallas Tex 1979. 2007;49:298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 34.Bonora E, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 35.Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism. 2006;55:1293–1301. doi: 10.1016/j.metabol.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Yoo TW, et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ. J. Off. J. Jpn. Circ. Soc. 2005;69:928–933. doi: 10.1253/circj.69.928. [DOI] [PubMed] [Google Scholar]
- 37.Liou T-L, et al. Is hyperuricemia another facet of the metabolic syndrome? J. Chin. Med. Assoc. JCMA. 2006;69:104–109. doi: 10.1016/S1726-4901(09)70186-8. [DOI] [PubMed] [Google Scholar]
- 38.Sheu WHH, Tseng Y-H. Uric acid: an additional component of metabolic syndrome? J. Chin. Med. Assoc. JCMA. 2006;69:99–100. doi: 10.1016/S1726-4901(09)70184-4. [DOI] [PubMed] [Google Scholar]
- 39.Ryu S, et al. Incidence and risk factors for metabolic syndrome in Korean male workers, ages 30 to 39. Ann. Epidemiol. 2007;17:245–252. doi: 10.1016/j.annepidem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, et al. Relationship between hyperuricemia and metabolic syndrome. J. Zhejiang Univ. Sci. B. 2007;8:593–598. doi: 10.1631/jzus.2007.B0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanbay M, et al. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur. J. Intern. Med. 2016;29:3–8. doi: 10.1016/j.ejim.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khosla UM, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 43.Mercuro G, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am. J. Cardiol. 2004;94:932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 44.Kang D-H, Park S-K, Lee I-K, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. JASN. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 45.Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am. J. Physiol. 1998;274:E692–699. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- 46.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. BioFactors Oxf. Engl. 2009;35:21–27. doi: 10.1002/biof.3. [DOI] [PubMed] [Google Scholar]
- 47.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med. Clin. North Am. 2007;91:1063–1077. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All related data are included in this paper. The datasets produced and/or analyzed during the present study are available from the corresponding author upon reasonable request.


