Highlights
-
•
Executive function deficit impairs functional outcome in Autism spectrum (ASD)
-
•
ASD showed normal brain activity in a working memory task but abnormal connectivity
-
•
A sub-set of the ASD group had highly variable patterns of activity
-
•
This may have implications for treatment targeting or diagnostic biotypes
Keywords: Functional neuroimaging, Executive function deficits, autism, Youth and young adults, Spatial working memory, Individual variability
Abstract
Background
Individuals with autism spectrum disorder (ASD) often present with executive functioning (EF) deficits, including spatial working memory (SWM) impairment, which impedes real-world functioning. The present study examined task-related brain activity, connectivity and individual variability in fMRI-measured neural response during an SWM task in older youth and young adults with autism and clinically significant EF impairment.
Methods
Neuroimaging was analyzed in 29 individuals with ASD without intellectual disability who had clinically significant EF impairment on the Behavior Rating Inventory of Executive Function, and 20 typically developing controls (participant age range=16-34). An SWM N-Back task was performed during fMRI. SWM activity (2-Back vs. 0-Back) and task-related dorsolateral prefrontal cortex (DLPFC) connectivity was examined within and between groups. Variability of neural response during SWM was also examined.
Results
During SWM performance both groups activated the expected networks, and no group differences in network activation or task-related DLPFC-connectivity were found. However, greater individual variability in the pattern of SWM activity was found in the ASD versus the typically developing control group.
Conclusions
While there were no group differences in SWM task-evoked activity or connectivity, fronto-parietal network engagement was found to be more variable/idiosyncratic in ASD. Our results suggest that the fronto-parietal network may be shifted or sub-optimally engaged during SWM performance in participants with ASD with clinically significant EF impairment, with implications for developing targeted interventions for this subgroup.
1. Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder affecting ∼1-2% of the population (Lyall et al., 2017). Although outcomes are mixed among affected individuals, the majority of adults with autism struggle with functional impairment and require support (Alvares et al., 2019; Tillmann et al., 2019). Evidence-based interventions that improve long term outcome in ASD are limited, particularly for youth and young adults (Warren et al., 2013). The majority of interventions with evidence of efficacy in older children and youth target specific symptom domains that contribute to impairment within subgroups of individuals with ASD (e.g., atypical antipsychotics for irritability, cognitive behavioural therapy for treatment of anxiety in ASD(Ameis et al., 2018). Executive functioning (EF) deficits are often (but not universally) present in individuals with ASD (Demetriou et al., 2018; Kenworthy et al., 2008), are associated with mental health symptoms (Lawson et al., 2015), adaptive functioning (Bertollo and Yerys, 2019; Gilotty et al., 2002), and functional independence in adulthood (Szatmari et al., 1989). Thus far, a cognitive behavioural intervention that targets EF has shown promise for improving EF domains (e.g., planning/organization, flexibility) in children with ASD with positive impact on everyday functioning (Kenworthy et al., 2014). EF enhancement may therefore be a promising interventional target for further development and refinement in ASD, particularly in the subgroup of individuals with significant impairment in this domain.
EF is an umbrella term that includes multiple subdomains, including working memory, set-shifting, planning and response inhibition skills (Diamond, 2013). The fronto-parietal network is comprised of the dorsolateral prefrontal cortex (DLPFC) and parietal cortex (near/along intraparietal sulcus). This network has been implicated in EF deficits across different mental health disorders, including schizophrenia and depression (Marek and Dosenbach, 2018). The fronto-parietal network is hypothesized to serve as a functional hub that flexibly integrates engagement of distributed brain networks to coordinate the brain-wide communication needed for higher-order cognitive ability, including EF (Cole et al., 2013).
Repetitive transcranial magnetic stimulation (rTMS) is an approved treatment for depression (Blumberger et al., 2018) that can stimulate focal cortical regions by a train of magnetic field pulses at typical frequencies between 1-20Hz (George et al., 2009). Efficacy studies in depression have also found improvement on secondary cognitive measures with rTMS to DLPFC (Guse et al., 2010). It has therefore been proposed that brain stimulation targeting the DLPFC could have broad clinical relevance as an intervention to enhance EF ability, potentially through augmentation of frontoparietal network function. rTMS to the DLPFC may then have potential as an intervention option to enhance EF functions in ASD.
In considering developing a directed biological intervention for EF deficits in individuals with autism, visuospatial working memory (SWM) may be a promising cognitive outcome to target as SWM impairment is often present in children and adults with ASD (Y. Wang et al., 2017). Deficits in SWM performance in children and youth with ASD have been linked to adaptive functioning; therefore improvement in this domain could have meaningful downstream effects on functioning (Rosa et al., 2017). Further, published functional MRI (fMRI) studies have helped to characterize fronto-parietal network response to SWM performance in participants with ASD versus typically developing participants using pairwise comparisons. Studies indicate that the overall pattern of local brain response to SWM performance is similar in autism as in controls (i.e., frontoparietal activation and deactivation in default mode network regions) (Kenworthy et al., 2008; Koshino et al., 2005; Luna et al., 2002; Lynch et al., 2017; Vogan et al., 2014). However, some studies have shown subtle regional differences in local activation in ASD versus control groups, often including the DLPFC, and particularly with increased working memory load, though exact locations and patterns differ between studies (Kenworthy et al., 2008; Koshino et al., 2005; Luna et al., 2002; Lynch et al., 2017; Vogan et al., 2014). Failure to modulate distributed network connectivity during EF performance has also been shown at the group level in participants with autism compared to controls, though not consistently (Koshino et al., 2005; Lynch et al., 2017). Of note, large-scale alterations in resting-state connectivity has recently been demonstrated reproducibly across four different ASD samples compared to controls, including hyperconnectivity of fronto-parietal executive control networks (Holiga et al., 2019), though findings across the broader resting-state connectivity literature in autism remains inconsistent (Vasa et al., 2016). Impaired connectivity involving the frontoparietal network in ASD may then disrupt rapid and flexible top down control of downstream networks (de Lacy et al., 2017), influencing EF ability (Marek and Dosenbach, 2018). Modulation of frontoparietal network properties through DLPFC stimulation may then have interventional relevance for improving EF in autism.
A key issue for development of brain stimulation protocols that target specific cortical regions is that the topography of organized neural networks varies substantially between individuals. This variability is prominent within the frontoparietal control network (Laumann Neuron 2015). In line with the considerable phenotypic and genetic heterogeneity that is inherent to the current clinical classification of autism (Lombardo et al., 2019), fMRI studies indicate that individualized alterations in the functional organization of neural networks are increased in ASD compared to control groups (Dickie et al., 2018; Hahamy et al., 2015; Poulin-Lord et al., 2014). Conventional targeting to a specific topographical location, such as the DLPFC, may not be suitable in autism as this network may be shifted or altered. Thus, an intervention aimed at modulating the frontoparietal network to enhance EF in autism may need to account for network variability.
The purpose of the present study was to further examine neural activity, functional connectivity, and individual variability during SWM performance in a subgroup of individuals diagnosed with ASD with clinically significant EF impairment, compared to a typically developing control group. Part of our longer-term objectives are to understand how to better use MRI-based targeting to enhance (and individualize) target engagement using rTMS in individuals with ASD. Therefore, we examined: (1) whether prior differences found in task-related fronto-parietal activity and DLPFC-connectivity were present in participants with ASD and EF impairment, relative to typically developing controls, and (2) whether participants with ASD and EF impairment would feature greater individual variability in brain response during SWM performance, relative to controls. We hypothesized that in individuals with ASD and clinically significant EF impairment, altered DLPFC activity and connectivity, and greater variability in brain response during task performance would be found compared to typically developing controls. Improving our understanding of the nature of DLPFC alterations during EF performance, and how it can vary across individuals, is essential to optimizing treatment approaches targeting this functional domain.
2. Methods
2.1. Participants
The MRI data analyzed here are from n=40 baseline scans of older youth and young adults diagnosed with ASD without intellectual or language impairment. MRI in participants with ASD was acquired during baseline assessment as part of a randomized, double-blind, sham-controlled rTMS pilot trial stimulating bilateral DLPFC for EF enhancement in ASD (clinicaltrials.gov ID: NCT02311751, (Ameis et al., 2017, Ameis et al., 2020). Participants with ASD were recruited from outpatient services at the Centre for Addiction and Mental Health (CAMH) in Toronto, Canada, community clinics, agencies providing services for individuals with ASD, and through advertisements at local colleges and universities. All ASD participants self-identified as having difficulties with EF performance (described as thinking problems in study advertisements). Potential participants with ASD were screened for eligibility based on age (16-35 years), presence of an ASD diagnosis, confirmed using the Autism Diagnostic Observation Schedule-2, Module 4 (Lord et al., 2003), IQ>or=70, and the presence of clinically significant EF deficits based on a T score >65 on any subscale of the Behavior Rating Inventory of Executive Functioning (BRIEF), using age-appropriate self-report versions. Twenty typically developing control participants were also recruited through local advertisements. Typically developing participants were included if they were between 16-35 years of age and fluent in English. Control participants were excluded if they had a diagnosed learning disorder or impaired academic or adaptive functioning on history, were pregnant, had full scale IQ<70, or psychiatric diagnosis on diagnostic interview via the Mini International Neuropsychiatric Interview; MINI (Lecrubier et al., 1998). All potential participants were excluded if they had a history of substance abuse or dependence in the last 6 months or a positive urine toxicology screen, or had a major medical or neurological illness. All participants were voluntary and competent to consent based on ability to provide a spontaneous narrative description of the key elements of the study. Written consent was provided by all participants. The study was approved by the local ethics review board and conducted in accordance with the declaration of Helsinki.
2.2. Clinical and Behavioral Assessments
All ASD participants who consented to the protocol were assessed using the ADOS-2, Module 4 (Lord et al., 2003), a semi-structured interview administered by an experienced child psychiatrist (SA) trained on the measure, to confirm diagnosis. Review of prior clinical assessments was also conducted to corroborate prior clinical diagnosis of ASD. Psychiatric disorders were assessed in typically developing controls and participants with ASD using the MINI (>18 years) or MINI for Children and Adolescents (MINI-KID) (16–18 years). The Wechsler Abbreviated Scale of Intelligence (WASI-II) or Wechsler Adult Intelligence Scale (WAIS-IV) were used to assess cognitive level in typically developing and ASD participants, respectively (Benson et al., 2010). In participants with ASD, the Vineland Adaptive Behavior Scale-II (VABS-II) (Sparrow and Cicchetti, 1985) and Social Responsiveness Scale (SRS-2) (Frazier et al., 2014) were used to assess adaptive functioning and dimensional autism symptoms, respectively. The self-report BRIEF (for participants aged 16–18) or BRIEF-A (adult version, for participants 18 and older), was used to assess impairment on everyday tasks requiring EF skills on 8 subscales. Two indices are derived from BRIEF subscales, the metacognitive index (including initiate, working memory, plan/organize, organization of materials and monitor subscales) and the behavior regulation index (comprised of inhibit, shift, and emotional control subscales), as well as a Global Executive Composite score. The behavior regulation index measures the ability to modulate both behavior and emotional control and move flexibly between different activities. The metacognitive index measures the ability to engage in active problem solving and initiate, organize and monitor one's actions (Gioia et al., 2002). The BRIEF can be considered a clinical measure of EF impairment with T scores>65 indicative of the presence of clinically significant EF deficits (McAuley et al., 2010). Clinically significant EF impairment on either index of the BRIEF (i.e., behavioral regulation or metacognitive index) has been associated with behavioral dysregulation and functional impairment, while impairment on the metacognitive index may be more closely linked with academic performance (McAuley et al., 2010). EF was also assessed using the computerized Cambridge Neuropsychological Test Automated Battery (CANTAB, www.cambridgecognition.com), including the Intradimensional/Extradimensional (IED) Set Shift task (cognitive flexibility), the Spatial Working Memory task and the Stop Signal Task (SST) (response inhibition).
2.3. fMRI Spatial Working Memory N-back Task
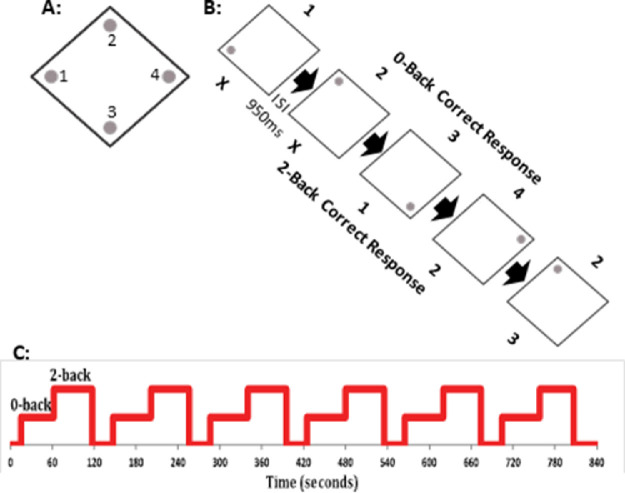
During MRI scanning participants performed an SWM N-Back task (Callicott et al., 2003, 2000), consisting of a 0-Back condition (a matched sensory-motor control condition with no working memory requirements) as well as a 2-Back condition (a moderately high working memory condition). The experiment was divided into alternating blocks of 20 0-Back trials, 20 2-Back trials, and 30 seconds of rest, repeated six times (i.e. six blocks per condition). Each 0-Back or 2-Back block was preceded by an instruction screen, while the words ‘rest’ were displayed on the screen during the rest session. Participants were instructed to keep their eyes open during the rest block. On each trial, participants were presented with a diamond shape including a dot/circle in one of the four corners of the diamond (Figure 1). Trials were presented for 1500ms with a 950ms inter-stimulus interval. Each position of the dot was associated with a specific button (index or middle finger of each hand). During the 0-Back condition, participants indicated the button corresponding to the current position of the dot. During the 2-Back condition, participants indicated the position of the dot two trials prior. In this way, each trial represented a target trial (i.e. there were no non-target trials). Participants were instructed to refrain from responding to the first two trials on the 2-Back condition, and that if they lost their memory set (i.e., the location of the dot from two trials prior) they should ‘start over’ mid-block. The task was practiced immediately prior to the MRI session to familiarize participants. Practice was repeated if participants scored less than 10 on the 2-back practice block.
Figure 1.
Task design for the fMRI N-Back SWM task. A: Dots are presented at one of four locations, within a diamond shape; each location was associated with a response button (1, left middle finger, 2, left index finger, 3, right index finger, 4, right middle finger). B: Task design, with images shown for 1500ms with an ISI of 950ms. For the 0-Back trials, participants indicate the position of the dot on the current image. For 2-Back, participants indicate the position on the image from two trials prior (thus, there is no response for trials 1 and 2). C: Schematic of the block design of condition presentations, with alternating 0-Back, 2-Back, 30 seconds rest, for six repetitions. Each 0-Back or 2-Back block included 20 trials. An instruction screen was presented before each block.
2.4. MRI Scan
Scanning was undertaken using a 3 Tesla GE MR750 (General Electric, Milwaukee, WI) research-dedicated scanner at CAMH. The multi-modal MRI session (90 minutes total scan time) included a T1 anatomical MRI (Sagittal BRAVO, TR=6.736ms, TE=3ms, flip angle=8, voxel size 0.9mm isotropic, duration 4.5 minutes), followed by a 7-minute eyes closed resting state fMRI (data not shown) and then the 14 minute N-back fMRI task (N-back: Echo planar imaging, TR=2000ms, TE=30ms, flip angle=90, voxel size 3.125 × 3.125 × 4mm).
2.5. fMRI Preprocessing
MRI preprocessing was performed using FMRIPREP (Esteban et al., 2019), a Nipype (Gorgolewski et al., 2011) based tool. Each T1w (T1-weighted) volume was corrected for INU (intensity non-uniformity) using N4BiasFieldCorrection v2.1.0 (Tustison et al., 2010) and skull-stripped using antsBrainExtraction.sh v2.1.0 (using the OASIS template). Brain surfaces were reconstructed using recon-all from FreeSurfer v6.0.1 (Dale et al., 1999), and the brain mask was refined with a custom variation of the method to reconcile ANTs-derived and FreeSurfer-derived segmentations of the cortical gray-matter of Mindboggle (Klein et al., 2017). Brain tissue segmentation of cerebrospinal fluid (CSF), white-matter (WM) and gray-matter (GM) was performed on the brain-extracted T1w using fast (Zhang et al., 2001). Functional data was slice time corrected using 3dTshift from AFNI v16.2.07 (Cox, 1996) and motion corrected using mcflirt (FSL v5.0.9 (Jenkinson et al., 2002). "Fieldmap-less" distortion correction was performed by co-registering the functional image to the same-subject T1w image with intensity inverted (Huntenburg, 2014; S. Wang et al., 2017) constrained with an average fieldmap template (Treiber et al., 2016), implemented with antsRegistration (ANTs). Functional data was then coregistered to the corresponding T1w using Freesurfer with boundary-based registration (Greve and Fischl, 2009) with 9 degrees of freedom. Functional data were transformed onto the cortical surface and converted to CIFTI format using the Ciftify toolbox (Dickie, Anticevic, et al., 2018; https://github.com/edickie/ciftify), which includes a non-linear transform to the MNI152 template calculated via FSL's FNIRT function using the T1w image and then applied to the functional data. Smoothing was then performed on the cortical surface with a FWHM of 8mm using tools in the Ciftify package.
2.6. N-Back Task-Activation Analysis
Preprocessed N-BACK data were analyzed using a general linear model (GLM) approach as implemented in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). A first level design matrix was created using a mixed block and event-related design. 0-Back and 2-Back blocks were coded as blocks of 49 seconds. We also coded incorrect trials and misses as events in order to compensate for impacts of behavioral performance on the 0-Back and 2-Back block regressors. Specifically, incorrect trials and misses were coded as events as we anticipated reductions in BOLD activity when participants missed trials. Therefore, these regressors were included to account for such effects and to allow a more accurate fit of task block regressors when performance was poor. Task regressors were convolved with the canonical hemodynamic response function and the derivative and dispersion functions. Additional confound regressors were added to the model to account for sources of noise variance in the model, including white matter mean signal, cerebrospinal fluid mean signal, mean framewise displacement (Power et al., 2014, 2012), DVARS (Power et al., 2012), the six motion parameters from mcflirt (three rotations and three translations), and global mean signal. Given the controversies over the use of global mean signal as a regressor (Gotts et al., 2020; Liu et al., 2017), the GLM analyses were repeated without GSR as a regressor. A contrast of 2-Back - 0-Back was run at the single subject level, using the beta weights associated with the task block regressors, and contrast beta values were used for group analysis performed using 1000 permutations with threshold free clustering enhancement (FWE) (Winkler et al., 2014) as implemented in FSL's PALM. Group results were thresholded at p<0.05 FWE-corrected for the number of vertices in each hemisphere, and further corrected for separate runs of PALM for each hemisphere (i.e. the critical TFCE-corrected threshold was set to p<0.025). Group analyses were run comparing activity in the ASD group to typically developing controls (CON - ASD), as well as separately for each group for visual comparison to facilitate interpretation of group differences. Age, Gender, and IQ were considered as potential covariates, but were not included in the group analysis as they were not found to relate to brain measures (Supplemental Tables 1-2).
2.7. Task-Based Connectivity Analysis - Psychophysical Interactions (PPI)
To test the interaction between working memory load and task-based functional connectivity, the generalized form of psychophysiological interaction (gPPI) was run (https://www.nitrc.org/projects/gppi). gPPi measures change across task conditions in the connectivity of a ‘seed’ region of interest (ROI). Based on the central role of the DLPFC in the frontoparietal network and prior DLPFC alterations found in ASD versus control groups (Kenworthy et al., 2008; Koshino et al., 2005; Luna et al., 2002; Lynch et al., 2017; Vogan et al., 2014), ROIs were selected posteriori as the ROIs within the right and left DLPFC with the highest t-statistic from the task-activation GLM (above), considered across both groups. As PALM produces very large ROIs with homogeneous p-values at the cluster level, a more circumscribed DLPFC ROI included in the PALM cluster with highest t-statistic from the task-activation was selected from the Glasser human connectome project (HCP) parcellation (Glasser et al., 2016), where cortical ROIs were determined based on task activation, resting state functional connectivity, structural connectivity, and topography. The ROIs defined by this parcellation are therefore regions found to be functionally relevant at the group level. Group analyses were run in PALM, as described above. The PPI analysis was constructed as the N-Back task-activation analysis above, including the task regressors and the same confound regressors, but with the addition of the seed time series and the PPI regressors to model the interaction between the seed time series and the task (McLaren et al., 2012).
2.8. Individual Variability in N-Back Task Activity via Distance Metrics
We performed pairwise distance calculations between each pair of participants (including ASD and CON) to measure individual variability for task-evoked N-BACK activation (2-Back - 0-Back) for our sample, providing a metric of similarity or difference of the pattern of brain response between participants (Finn et al., 2015; Hawco et al., 2019, 2018). Squared Euclidean Distance and Correlational Distance (which is equal to one minus the absolute value of the correlation) were calculated for each participant pair. Euclidean and Correlational Distance are both affected by the spatial pattern of activity, however Euclidean distance is sensitive to magnitude effects (e.g. greater overall positivity or negativity) while correlational distance is invariant to magnitude. For each participant, contrast beta values (2-Back - 0-Back) for all cortical vertices were reshaped into a vector, creating a ‘spatial series’ for each participant (Hawco et al., 2019, 2018). Individual variability was quantified as the average distance from a given participant to all other participants. Participants with patterns of task-related activation more similar to the overall group (‘typical’ activators) feature lower distances, while those with more idiosyncratic patterns feature higher mean distance. Data was assessed for normality via a Shapiro-Wilk's test p < 0.05, (Shapiro and Wilk, 1965), and group differences were assessed via a nonparametric Mann-Whitney's U test for non-equal distributions.
Differences in variability of the activation patterns across each group were visualized by examining overlap in activity from the first-level (single subject) t-maps. First level t-maps for each participant for the 2-Back - 0-Back contrasts were calculated. The t-maps were binarized showing t-values with significance of p<0.001 uncorrected at the single vertex level, separately for positive and negative t-values. Overlap was calculated as a sum of the binarized maps, within each group.
2.9. Exploratory Analysis of Frontoparietal Network Properties and Variability
Mean distance was correlated with left or right DLPFC activity (beta weights for 2-Back - 0-Back for each participant) to examine whether individual variability in the pattern of brain activity was related to localized DLPFC activity. In accordance with the RDoC framework laid out by the NIMH (Insel et al., 2010), relationships were examined across participants with ASD and typically developing controls to determine whether relationships were present along a continuum, regardless of group membership. To address potential confounds related to differences between groups, relationships were also examined in the ASD group alone. All p-values were FDR corrected.
2.10. Code Sharing
Code used in the analysis of this dataset has been made available (https://github.com/colinhawco/ASDD). Statistical maps of the results reported below are also available for download and viewing.
3. Results
3.1. Participant Inclusion and Characteristics
Of the 40 participants with ASD, one failed preprocessing QC due to poor data quality, and five ASD participants were excluded for excessive motion (mean framewise displacement >0.5mm). No control participants met motion exclusion criteria. Motion was compared for each group. For mean framewise displacement (FD), there was a significantly higher mean FD in the ASD (mean FD = 0.130) versus typically developing control group (mean FD = 0.906) (U = 176.5, n1 = 29, n2 = 20, P = 0.021). Motion was also examined separately in the 0-Back or 2-Back blocks via a repeated measures ANOVA, with block (0-back and 2-Back) as a within-subject factor and group as between subjects. We found a main effect of block, F(1,47) = 8.1, p = 0.006 (motion was higher in the 0-Back, mean FD = 0.106, as opposed to 2-Back, mean 0.097), but no group by block interaction, F(1,47) = 0.44, p=0.835. Upon analyzing performance on the N-back task, it was found that some ASD participants performed very poorly on 2-Back trials. Five additional participants in the ASD group were excluded due to very poor (near chance) 2-Back performance, defined as less than 30 total correct trials out of 108 (chance accuracy=25%). Data from 29 participants with ASD were included in further analysis. Participants with ASD that were excluded for motion or poor 2-Back performance were generally more impaired on CANTAB SWM, had lower IQ, and greater clinical EF impairment on the BRIEF relative to the included ASD group (data not shown). Included participant demographics are shown in Table 1.
Table 1.
Baseline Demographics.
| Group | Controls | ASD | |
|---|---|---|---|
| (n=20) | Included (n=24) | Excluded (n=10) | |
| Age in years | 23.90 ± 4.3 | 22.50 ± 4.7 | 23.30 ± 5.1 |
| Gender | 14M (70%) | 18M (75%) | 6M (60%) |
| Handedness | 17 Right | 21 Right | 9 Right |
| Years of Education | 15.65 ± 2.3 | 14.38 ± 3.2 | 13.50 ± 2.0 |
| Inclusion IQ | 112.16 ± 9.9 | 116.67 ± 16.9 | 95.90 ± 13.5 |
| WAIS FSIQ | 106.79 ± 15.2 | 86.60 ± 12.9 | |
| Documented Comorbidity | 15 (62.5%) | 8 (80%) | |
| On Psychiatric Medications | 16 (66.7%) | 6 (60%) | |
| ADOS-2 | |||
| Communication + RSI | 6.00 ± 2.1 | 7.00 ± 1.1 | |
| Stereotyped Behaviours & Restricted Interests | 1.58 ± 1.3 | 2.56 ± 1.3 | |
| Adaptive Functioning | 77.63 ± 10.1 | 69.20 ± 6.9 | |
| Social Responsiveness Scale | 71.83 ± 7.5 | 71.30 ± 11.7 | |
| BRIEF Behaviour Regulation Index | 41.95 ± 5.9 | 61.96 ± 9.2 | 67.10 ± 10.8 |
| BRIEF Metacognition Index | 45.60 ± 6.6 | 72.88 ± 9.5 | 72.50 ± 7.4 |
| CANTAB | |||
| SST (reaction time) | 164.54 ± 44.5 | 173.41 ± 44.7 | 246.03 ± 125.7 |
| SWM (total errors) | 13.58 ± 13.0 | 15.63 ± 16.6 | 37.20 ± 17.4 |
| SWM (between errors) | 13.53 ± 13.0 | 15.33 ± 16.4 | 36.50 ± 17.3 |
| IED (stages completed) | 8.79 ± 0.6 | 8.92 ± 0.4 | 8.30 ± 0.9 |
| IED (total raw) | 13.42 ± 7.4 | 11.42 ± 7.5 | 23.20 ± 14.4 |
Note. Where appropriate variables are presented as Mean ± SD.
3.2. Behavioral Cognitive Performance
Accuracy and misses across blocks for both 0-Back and 2-Back were visualized, confirming no drop off in task performance over time (Supplemental Figure 1). As the majority of behavioral variables were found to be non-normally distributed (Shapiro-Wilk's test p < 0.05, (Shapiro and Wilk, 1965), a nonparametric Mann-Whitney's U test for non-equal distributions was used, with two-tailed significance testing. A trending but non-significant between-group difference was found in 2-back accuracy (U = 383.5, n1 = 29, n2 = 20, P = 0.057). Incorrect and missed responses were also examined. We did not find differences in the distribution of incorrect responses between groups (U = 304, n1 = 29, n2 = 20, P = 0.77), but a significant difference in the number of misses was found (U = 191, n1 = 29, n2 = 20, P = 0.044), indicating that individuals with ASD and EF impairment had an increased number of missed responses (i.e., no response) during the 2-Back task compared to typically developing controls. No group differences were found in 2-Back correct reaction time (U = 225, n1 = 29, n2 = 20, P = 0.19), 0-Back accuracy (U = 360, n1 = 29, n2 = 20, P =0.16), or 0-Back correct reaction time (U = 227, n1 = 29, n2 = 20, P = 0.20), however variability in performance was consistently higher in ASD versus typically developing participants.
3.3. N-BACK fMRI Group-level Task-Activation
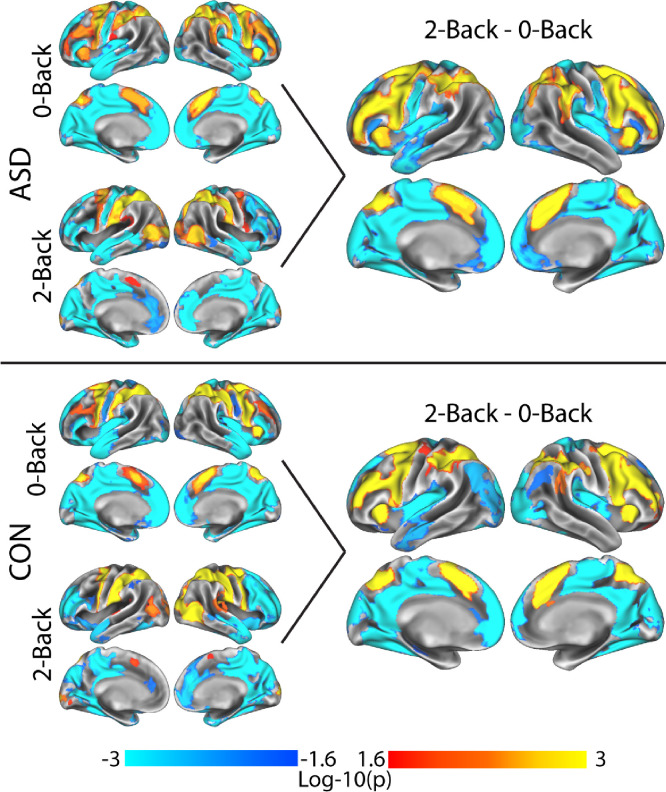
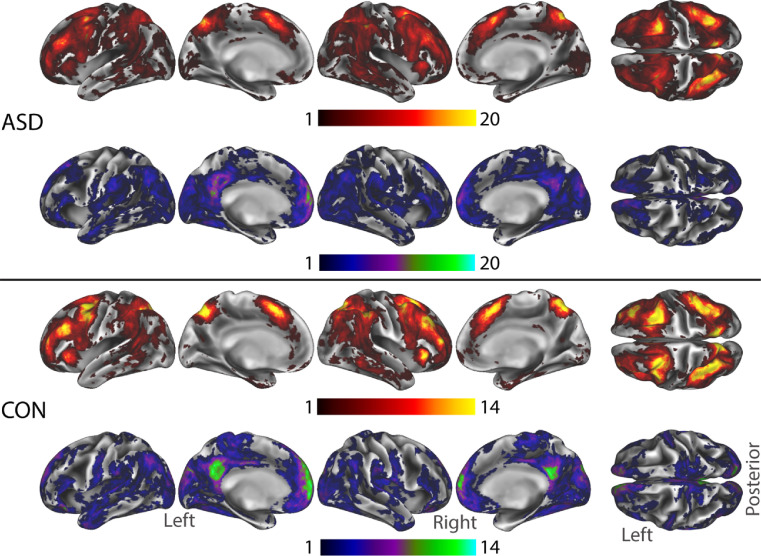
Group-level activity for the 2-Back - 0-Back contrast (Figure 2) demonstrated the expected pattern of activity in fronto-parietal and dorsal attention networks, deactivation/suppression in regions of the default mode network (DMN), and deactivation in visual and sensory-motor networks across both groups. These results were driven by greater activity or suppression in the 2-Back versus 0-Back condition (i.e. the observed ‘deactivations’ were greater suppression during 2-Back as opposed to greater positive activity during 0-Back). No group differences in activation were observed between participants with ASD and EF impairment versus typically developing participants. Task activity results without GSR as a regressor are presented in Supplemental Figure 2.
Figure 2.
Group GLM analysis of activity during the N-Back task for typically developing controls, CON, (top), ASD (bottom). 0-Back and 2-Back main effect contrasts are shown on the left to show the direction of effects in the 2-Back - 0-Back contrast (right). Both groups showed a similar pattern of activation during N-Back performance. A group contrast (CON vs. ASD) showed no significant group differences. Results showing in log-10(p), corrected at the cluster level for multiple comparisons via TFCE.
3.4. N-Back Task-Based DLPFC Connectivity
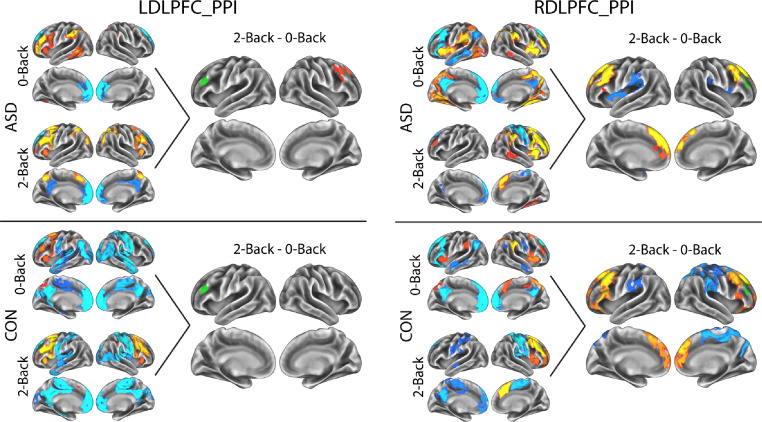
For the PPI analysis of task-based connectivity to the left DLPFC (Figure 3, left), the ASD group showed greater connectivity between the left DLPFC seed and the right DLPFC in 2-back than in 0-Back. The typically developing group did not show any significant differences between 2-back and 0-Back. For the PPI analysis of task-based connectivity to the right DLPFC (Figure 3, right), both ASD and typically developing participants showed increased connectivity with left and right DLPFC during 2-Back over 0-Back. Ipsilateral connectivity (right) was related to increased connectivity in the 2-Back condition, while contralateral connectivity (left) was related to reduced/negative connectivity in the 0-Back. The typically developing control group also showed connectivity with inferior frontal regions in 2-Back > 0-Back, related to reduced/negative connectivity in the 0-Back. Both groups showed 2-back > 0-back differences in medial prefrontal cortex related to reduced/negative connectivity in the 0-Back condition. The ASD group showed some regions of increased connectivity in 0-Back in the left insula, while the typically developing control group showed some regions of greater negative connectivity in parietal areas, particularly in the right hemisphere. No between-group differences in PPI task-related DLPFC-connectivity analysis for 2-Back - 0-Back were observed (i.e., in either left or right DLPFC seed region). PPI results without including GSR as a regressor in the model are presented in Supplemental Figure 3.
Figure 3.
Results of DLPFC PPI analysis (task-based connectivity changes, 2-Back - 0-Back). Results of PPI to left DLPFC seed (left), and right DLPFC seed (right) are shown. Seed regions are shown in green. Main effects of 0-Back and 2-Back PPI are shown for visualization only, to allow interpretation of the patterns in the 2-Back - 0-Back contrast. Results showing in log-10(p), corrected at the cluster level for multiple comparisons via TFCE.
3.5.1. Distance Metrics of Individual Variability
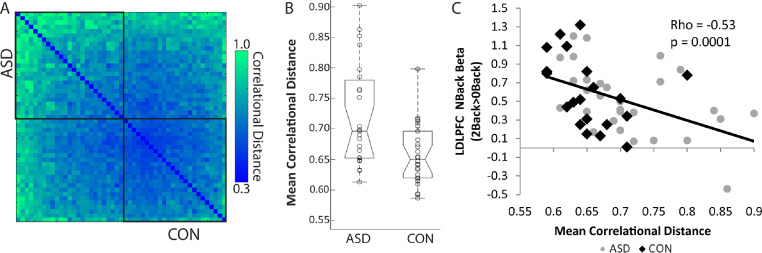
Mean squared Euclidean distance was significantly correlated with motion (mean framewise displacement), Rho = 0.44, p = 0.002, while there was no significant relationship between correlational distance and mean FD, Rho = 0.16, p = 0.27. There was also no significant association between correlational distance and temporal signal to noise ratio (tSNR) (rho p 0.054, p=0.71). Euclidean distance was therefore no longer considered. Correlational distances are plotted in Figure 4A. Mean distance did not follow a normal distribution, so a Mann-Whitney U test was performed to assess differences between groups. The distribution of responses across ASD and typically developing control participants was significantly different (U = 191, n1 = 29, n2 = 20, P = 0.044), indicating greater overall individual variability among ASD versus control participants. A subgroup of ASD participants with higher variability seemed to be driving this group difference (see Figure 4B). Variability in the spatial patterns of activity between groups are displayed using overlap maps of active vertices at the single-subject level (Figure 5). ASD participants showed reduced overlap in activity. Overlap was also calculated for PPI activity for the RDLPFC seed region (supplemental Figure 4)
Figure 4.
Correlational distances across participants. A: Pairwise correlational distance between participant pairs (each row/column represents a single participant, ASD top/Left, HC bottom/right). Participants in each group are ordered by mean distance, with ASD descending and typically developing controls (CON) in ascending order in order to position the participants with lowest mean distances (i.e. the most typical participants) in the center of the graph. B: Boxplots showing between-group differences in mean distance. C: Correlation between left DLPFC (LDLPFC) activity during N-Back (the beta weight for the 2-Back - 0-Back contrast) and mean correlational distance. Higher mean correlational distance was associated with lower LDLPFC activity during 2-Back - 0-Back performance across the sample.
Figure 5.
Overlap maps in activity at the single-subject level for the 2-Back - 0-Back contrast. Each single-subject t-map was binarized at a threshold of p<0.001 uncorrected at the single vertex, and binarized masks were summed to show the number of participants with activity at each vertex. Maps were calculated separately for positive (red-yellow) and negative (blue-green) t-values, for ASD (n=29) and typically developing (CON) (n=20) groups. The right-most brain image shows a dorsal (top-down) view, to allow better visualization of overlap in regions such as the intraparietal sulcus. Color maps for each group were thresholded to ∼70% of the sample size for improved visualization.
Demographic and clinical data of the highest variability ASD cases were explored. Nine ASD participants were identified as having high variability (i.e., variability in these individuals was outside the range of all controls except one). Demographic and clinical information for the “high” and “low” variability ASD cases is presented in Supplementary Table 4, which also includes comorbidities. Due to the small sample size of the high variability group (N=9), we did not perform statistical assessment; data is presented for descriptive purposes only. There were no clear trends indicating that high variability participants featured distinct patterns on clinical variables or cognitive measures.
3.5.2. Exploring Associations Between Frontoparietal Network Properties and Variability
There was an FDR corrected significant negative correlation between mean distance and activity in the left DLPFC, Rho = -0.53, p = 0.0001 (Figure 4C). The right DLPFC was not significant after FDR correction, Rho = -0.30, p = 0.039. The relationship between mean correlational distance and left DLPFC activity remained significant if we considered only ASD participants, Rho = -0.52, p=0.004, or only CON, Rho = -0.59, p=0.006. As such participants who show greater activity in the DLPFC during 2-Back vs 0-Back performance also feature less variable patterns of task-related brain activity.
4. Discussion
In the present study, we examined DLPFC activity and connectivity during SWM task performance in older youth and young adults with ASD with clinically significant EF impairment compared to a sample of typically developing controls. Participants with ASD exhibited poorer performance overall in N-Back performance metrics, including increased variability of performance compared to the control group and more missed responses. However, 2-Back accuracy and reaction time to correct responses did not differ significantly at the group level. Contrary to our hypotheses, neither task-related local brain activation nor DLPFC-connectivity differed at the group level during SWM performance in the current sample. Participants with ASD and clinical EF impairment did however feature more variable spatial patterns of task-related activity during N-Back performance (i.e., greater variability of task-evoked brain activation) on pairwise comparison. Increased variability of task-evoked brain activation was also associated with local DLPFC activation during N-Back performance across the entire sample, suggesting that highly variable participants may have been making use of altered or shifted neural networks for SWM performance. Increased variability in spatial patterns of task-related activity in participants with ASD and clinical EF impairment suggests that ‘idiosyncratic’ activation may be an important biological feature of EF performance in ASD, with implications for therapeutic (e.g. brain stimulation) studies and optimization of target engagement.
Our analysis suggested that activity and DLPFC-connectivity during the N-Back task was similar across our ASD and typically developing control groups. A number of prior task-fMRI studies have shown differences in activation or connectivity during SWM performance in participants with ASD compared to a control group, though there has been little consistency across studies in the presence, direction or location of between-group results (Koshino et al., 2005; Luna et al., 2002; Lynch et al., 2017; Vogan et al., 2018). A recent resting-state study which applied a rigorous denoising procedure to remove motion artifacts did not find group differences in intrinsic network connectivity in a sample comparing children with primary clinical diagnoses of ASD, attention-deficit/hyperactivity disorder (ADHD) and typically developing children (n=43/group) (Dajani et al., 2019). Of particular relevance to the current work, Dajani et al. (2019) also found no differences between groups when a data-driven subgroup classification was applied to their sample that grouped participants transdiagnostically based on high average, average and impaired performance on EF measures (where subgroup differentiation was largely based on the BRIEF). In contrast, another recent resting-state study found hyperconnectivity affecting the fronto-parietal network across four different ASD versus control datasets, with samples ranging in size from n∼50 to n∼700. Interestingly, effect sizes ranged from small to large across different datasets and significant overlap between controls and ASD participants was present (Holiga et al., 2019). Inconsistent results between fMRI studies confirm that conventional pairwise comparisons applied to small samples is inherently problematic in complex disorders such as ASD. Inconsistencies in the current literature may therefore be attributable to false positives in small samples (that could be driven by motion artifacts), or heterogeneity between samples (i.e., based on age, co-occurring mental health conditions, medications), or other factors (methodological differences).
Greater variability in the pattern of brain activity during SWM task performance found in ASD participants with clinical EF impairment may be driven by several factors. Importantly, increased correlational distance found in ASD did not share a significant relationship with in-scanner motion, suggesting increased variability found in participants with ASD was not driven by motion/noise. A number imaging studies have now found greater variability of brain response in ASD samples compared to typically developing controls both at rest and during task performance (Chen et al., 2017; Dickie et al., 2018; Easson and McIntosh, 2019; Hahamy et al., 2015; Haigh et al., 2016, 2015; Müller et al., 2003; Nunes et al., 2019; Poulin-Lord et al., 2014). Similar to the present study, the prior study by Gilbert and colleagues found no ASD versus control group differences in activation during mentalizing or attention task performance, but relationships between the spatial distribution of PFC activation and task condition differed across control participants versus participants with ASD (Gilbert et al., 2009). Less consistent patterns of activity have also been found in participants with schizophrenia compared to controls (Maïza et al., 2010). Increased variability in neural network response and organization found in neuropsychiatric disorders may be driven by compensatory mechanisms (Livingston and Happé, 2017), such that when a given neural system is dysfunctional, other systems/regions/networks may engage in an attempt to support task performance. It has been suggested that the PFC may have a specific role in influencing adaptation and compensation for suboptimal functioning in particular neural pathways during development (Johnson, 2012). Specifically, it has been hypothesized that optimal PFC function (and strong EF skills) in early development may serve as a protective factor for later emergence of neurodevelopmental disorders like ASD and ADHD, potentially through adaptive/compensatory processes (Johnson, 2012). Participants with greater individual variability in our sample tended to have reduced brain activation in the DLPFC during the N-BACK task, suggesting that the hub of the frontoparietal network may not be optimally functioning or engaged during SWM processing. Compensation for disrupted neural systems is frequently seen in healthy aging (Reuter-Lorenz and Park, 2014), such as a shift towards greater frontal activation and decreased posterior activity (Davis et al., 2007; Grady et al., 1994). While compensatory mechanisms in relation to aging tend to produce somewhat stereotypical changes which can be observed at the group level, such as generally increased activity (Reuter-Lorenz and Park, 2014) or specific patterns of group-level altered activity (Davis et al., 2007; Grady et al., 1994), in the current study atypical participants featured highly idiosyncratic variations in brain activity. If these idiosyncratic brain responses during SWM performance represent compensation for dysfunctional networks, this has important implications for efforts to develop biologically directed interventions using brain stimulation. For example, if idiosyncratic spatial patterns of activation represent compensation for dysfunctional networks, is it better to target the disrupted systems, or is it better to emphasize activity in compensatory networks? Prior studies evaluating interventions focused on improving EF domains have found greater effects/improvement in those with increased EF impairment on lab-based measures (Diamond and Ling, 2016). It remains to be seen whether brain stimulation approaches for EF impairment may be more effective in those with more impairment but also more typical brain response which can be reinforced, or amongst those with more pronounced disruptions in both cognition and patterns of brain response.
Another complementary hypothesis for greater variability in ASD is that idiosyncratic patterns of brain activity relate to changes in cognitive strategy to facilitate task performance. Individuals with ASD have been proposed to use alternate cognitive strategies as a means to compensate for neural circuit deficits (Craig et al., 2017; Hesling et al., 2010; Hubl et al., 2003; Ring et al., 1999), or show a deficit in strategy use (Bramham et al., 2009). In patients with schizophrenia, memory deficits can be reduced when effective cognitive strategies are provided via the structure of the task (Guimond and Lepage, 2016). The use of such cognitive strategies has also been related to activity in the DLPFC (Guimond et al., 2017; Hawco et al., 2013a, 2013b). Given that participants with greater variability showed less DLPFC activity, it is possible they are failing to engage optimal neuro-cognitive strategies during the N-Back task. Improving DLPFC engagement in these individuals may lead to improved working memory performance, suggesting targeted brain stimulation may be more effective in individuals with more idiosyncratic spatial patterns of brain activation during SWM performance.
Several limitations of our study should be noted. Data was collected from 40 individuals with ASD with clinical EF impairment, therefore our results are specific to this subgroup rather than the broader ASD population. Further, 29/40 participants with ASD and EF impairment were retained in the final analysis following QC for fMRI and task performance data. While it is necessary to exclude such participants due to potential confounds related to motion or severely impaired performance, this smaller and more circumscribed sample limits the generalizability of our findings further. While our total sample of individuals with ASD and clinical EF impairment was impaired, on average, on N-Back SWM performance, following exclusion of participants that performed very poorly on the task, accuracy and reaction time during 2-Back performance did not differ between our ASD and typically developing control groups. This minimizes the impact of task performance on between-group differences in brain activation and connectivity. Other studies have likewise removed many cases in order to avoid such confounds (Vogan et al., 2014). However, removal of these participants limits the ability to generalize findings in the current results to individuals with more significant EF impairment. It also may be important to consider that there was a higher percentage of individuals with co-occurring ADHD among participants with ASD and EF impairment that also featured high variability of brain response. Future research in this area should account for the common presence of co-occurring ADHD in ASD (Lai et al., 2019) and greater EF deficits in this subgroup (Dajani et al., 2016) and the potential influence on variability metrics.
The main goal of the present study was to further elucidate network activation and connectivity differences during SWM performance to better evaluate the DLPFC as a potential target to improve EF in a subgroup of individuals with ASD with clinically significant EF impairment. While we did not find differences between our groups in local activation or connectivity, we did find greater variability in the spatial patterns of brain activity in participants with ASD and EF impairment during SWM performance. Although the issue of heterogeneity is well documented (Chen et al., 2017; Dickie et al., 2018; Easson and McIntosh, 2019; Hahamy et al., 2015; Nunes et al., 2019), the extant literature has yet to consider the potential relevance of variability of brain response for development of targeted biological interventions. While our findings require further exploration, the patterns of variability observed in the clinical sample examined here may have implications for group-based strategies for targeting the brain in therapeutic intervention studies. The upshot of these findings is that therapeutic response may be made more likely by evaluating individualized neural activation patterns at baseline to optimize targeting (Wang et al., 2014). The current study supports further examination of whether targeted brain stimulation to the DLPFC changes local activation or DLPFC-connectivity in individuals with ASD and clinically significant EF impairment and whether baseline variability in task-evoked brain response influences brain changes following targeted intervention. These results will help to set the foundation for developing more personalized approaches to brain stimulation across clinical samples with EF impairment.
Acknowledgements
We would like to thank Yisheng (Ethan) Zhu for his help with data analysis. This publication was made possible through the American Academy of Child and Adolescent Psychiatry (AACAP) Pilot Research Award for Child and Adolescent Psychiatry Residents and Junior Faculty (to SHA), supported by CFAK; its contents are the responsibility of the authors and do not necessarily reflect the official views of AACAP. This research was also supported by: the University of Toronto, Faculty of Medicine, Dean's Fund New Staff Grant, the Innovation Fund from the Alternate Funding Plan of the Academic Health Sciences Centres of Ontario, an Ontario Mental Health Foundation (OMHF) Project A Grant and New Investigator Fellowship (to SHA). SHA receives support from the Canadian Institutes of Health Research (CIHR), and the National Institutes of Mental Health (NIMH). SHA and M-CL are supported by the O'Brien Scholars Program within the Child and Youth Mental Health Collaborative at the Centre for Addiction and Mental Health (CAMH) and The Hospital for Sick Children, Toronto, the Academic Scholars Award from the Department of Psychiatry, University of Toronto, the Slaight Family Child and Youth Mental Health Innovation Fund and The Catherine and Maxwell Meighen Foundation (both via CAMH Foundation). CH receives research support through the CAMH foundation. M-CL is supported by the Ontario Brain Institute via the Province of Ontario Neurodevelopmental Disorders (POND) Network. DMB receives research support from CIHR, NIH, Brain Canada and the Temerty Family Foundation through the CAMH Foundation and the Campbell Research Institute. ANV receives funding from the National Institute of Mental Health, Canadian Institutes of Health Research, Canada Foundation for Innovation, CAMH Foundation, and the University of Toronto. ZJD was supported by the OMHF, CIHR, NIMH and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute. PEC was supported by R01 MH113700. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PS is supported by the Patsy and Jamie Anderson in Child and Youth Mental Health.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102260.
Appendix. Supplementary materials
References
- Alvares G.A., Bebbington K., Cleary D., Evans K., Glasson E.J., Maybery M.T., Pillar S., Uljarević M., Varcin K., Wray J., Whitehouse A.J. The misnomer of “high functioning autism”: Intelligence is an imprecise predictor of functional abilities at diagnosis. Autism. 2019 doi: 10.1177/1362361319852831. 1362361319852831. [DOI] [PubMed] [Google Scholar]
- Ameis S.H., Daskalakis Z.J., Blumberger D.M., Desarkar P., Drmic I., Mabbott D.J., Lai M.-C., Croarkin P.E., Szatmari P. Repetitive Transcranial Magnetic Stimulation for the Treatment of Executive Function Deficits in Autism Spectrum Disorder: Clinical Trial Approach. J. Child Adolesc. Psychopharmacol. 2017;27:413–421. doi: 10.1089/cap.2016.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis S.H., Kassee C., Corbett-Dick P., Cole L., Dadhwal S., Lai M.-C., Veenstra-VanderWeele J., Correll C.U. Systematic review and guide to management of core and psychiatric symptoms in youth with autism. Acta Psychiatr. Scand. 2018;138:379–400. doi: 10.1111/acps.12918. [DOI] [PubMed] [Google Scholar]
- Ameis S.H., Blumberger D.M., Croarkin P.E., Mabbott D.J., Lai M.-C., Desarkar P., Szatmari P., Daskalakis Z.J. Treatment of Executive Function Deficits in autism spectrum disorder with repetitive transcranial magnetic stimulation: A double-blind, sham-controlled, pilot trial. Brain Stimulation. 2020;13:539–547. doi: 10.1016/j.brs.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N., Hulac D.M., Kranzler J.H. Independent examination of the Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV): what does the WAIS-IV measure? Psychol. Assess. 2010;22:121. doi: 10.1037/a0017767. [DOI] [PubMed] [Google Scholar]
- Bertollo J.R., Yerys B.E. More Than IQ: Executive Function Explains Adaptive Behavior Above and Beyond Nonverbal IQ in Youth With Autism and Lower IQ. Am. J. Intellect. Dev. Disabil. 2019;124:191–205. doi: 10.1352/1944-7558-124.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberger D.M., Vila-Rodriguez F., Thorpe K.E., Feffer K., Noda Y., Giacobbe P., Knyahnytska Y., Kennedy S.H., Lam R.W., Daskalakis Z.J., Downar J. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–1692. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- Bramham J., Ambery F., Young S., Morris R., Russell A., Xenitidis K., Asherson P., Murphy D. Executive functioning differences between adults with attention deficit hyperactivity disorder and autistic spectrum disorder in initiation, planning and strategy formation. Autism. 2009;13:245–264. doi: 10.1177/1362361309103790. [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Bertolino A., Mattay V.S., Langheim F.J., Duyn J., Coppola R., Goldberg T.E., Weinberger D.R. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Egan M.F., Mattay V.S., Bertolino A., Bone A.D., Verchinksi B., Weinberger D.R. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am. J. Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Chen H., Nomi J.S., Uddin L.Q., Duan X., Chen H. Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Hum. Brain Mapp. 2017;38:5740–5755. doi: 10.1002/hbm.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Reynolds J.R., Power J.D., Repovs G., Anticevic A., Braver T.S. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig A.B., Grossman E., Krichmar J.L. Investigation of autistic traits through strategic decision-making in games with adaptive agents. Sci. Rep. 2017;7:5533. doi: 10.1038/s41598-017-05933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani D.R., Burrows C.A., Odriozola P., Baez A., Nebel M.B., Mostofsky S.H., Uddin L.Q. Investigating functional brain network integrity using a traditional and novel categorical scheme for neurodevelopmental disorders. Neuroimage Clin. 2019;21 doi: 10.1016/j.nicl.2019.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani D.R., Llabre M.M., Nebel M.B., Mostofsky S.H., Uddin L.Q. Heterogeneity of executive functions among comorbid neurodevelopmental disorders. Sci. Rep. 2016;6:36566. doi: 10.1038/srep36566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis S.W., Dennis N.A., Daselaar S.M., Fleck M.S., Cabeza R. Que PASA? The posterior–anterior shift in aging. Cereb. Cortex. 2007;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacy N., Doherty D., King B.H., Rachakonda S., Calhoun V.D. Disruption to control network function correlates with altered dynamic connectivity in the wider autism spectrum. Neuroimage Clin. 2017;15:513–524. doi: 10.1016/j.nicl.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou E.A., Lampit A., Quintana D.S., Naismith S.L., Song Y.J.C., Pye J.E., Hickie I., Guastella A.J. Autism spectrum disorders: a meta-analysis of executive function. Mol. Psychiatry. 2018;23:1198–1204. doi: 10.1038/mp.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Ling D.S. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev. Cogn. Neurosci. 2016;18:34–48. doi: 10.1016/j.dcn.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie E.W., Ameis S.H., Shahab S., Calarco N., Smith D.E., Miranda D., Viviano J.D., Voineskos A.N. Personalized Intrinsic Network Topography Mapping and Functional Connectivity Deficits in Autism Spectrum Disorder. Biol. Psychiatry. 2018;84:278–286. doi: 10.1016/j.biopsych.2018.02.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easson A.K., McIntosh A.R. BOLD signal variability and complexity in children and adolescents with and without autism spectrum disorder. Dev. Cogn. Neurosci. 2019;36 doi: 10.1016/j.dcn.2019.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., Kent J.D., Goncalves M., DuPre E., Snyder M., Oya H., Ghosh S.S., Wright J., Durnez J., Poldrack R.A., Gorgolewski K.J. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., Papademetris X., Constable R.T. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18:1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier T.W., Ratliff K.R., Gruber C., Zhang Y., Law P.A., Constantino J.N. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the social responsiveness scale-2. Autism. 2014;18(1):31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- George M.S., Padberg F., Schlaepfer T.E., O'Reardon J.P., Fitzgerald P.B., Nahas Z.H., Marcolin M.A. Controversy: Repetitive transcranial magnetic stimulation or transcranial direct current stimulation shows efficacy in treating psychiatric diseases (depression, mania, schizophrenia, obsessive-complusive disorder, panic, posttraumatic stress disorder) Brain Stimul. 2009;2:14–21. doi: 10.1016/j.brs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Meuwese J.D.I., Towgood K.J., Frith C.D., Burgess P.W. Abnormal functional specialization within medial prefrontal cortex in high-functioning autism: a multi-voxel similarity analysis. Brain. 2009;132:869–878. doi: 10.1093/brain/awn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilotty L., Kenworthy L., Sirian L., Black D.O., Wagner A.E. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychol. 2002;8:241–248. doi: 10.1076/chin.8.4.241.13504. [DOI] [PubMed] [Google Scholar]
- Gioia G.A., Isquith P.K., Retzlaff P.D., Espy K.A. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 2002;8:249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Ugurbil K., Andersson J., Beckmann C.F., Jenkinson M., Smith S.M., Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts S.J., Gilmore A.W., Martin A. Brain networks, dimensionality, and global signal averaging in resting-state fMRI: Hierarchical network structure results in low-dimensional spatiotemporal dynamics. Neuroimage. 2020;205 doi: 10.1016/j.neuroimage.2019.116289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C.L., Maisog J.M., Horwitz B., Ungerleider L.G., Mentis M.J., Salerno J.A., Pietrini P., Wagner E., Haxby J.V. Age-related changes in cortical blood flow activation during visual processing of faces and location. The Journal of Neuroscience. 1994 doi: 10.1523/jneurosci.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond S., Hawco C., Lepage M. Prefrontal activity and impaired memory encoding strategies in schizophrenia. J. Psychiatr. Res. 2017;91:64–73. doi: 10.1016/j.jpsychires.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Guimond S., Lepage M. Cognitive training of self-initiation of semantic encoding strategies in schizophrenia: A pilot study. Neuropsychol. Rehabil. 2016;26:464–479. doi: 10.1080/09602011.2015.1045526. [DOI] [PubMed] [Google Scholar]
- Guse B., Falkai P., Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J. Neural Transm. 2010;117:105–122. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A., Behrmann M., Malach R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat. Neurosci. 2015;18:302–309. doi: 10.1038/nn.3919. [DOI] [PubMed] [Google Scholar]
- Haigh S.M., Gupta A., Barb S.M., Glass S.A.F., Minshew N.J., Dinstein I., Heeger D.J., Eack S.M., Behrmann M. Differential sensory fMRI signatures in autism and schizophrenia: Analysis of amplitude and trial-to-trial variability. Schizophr. Res. 2016;175:12–19. doi: 10.1016/j.schres.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh S.M., Heeger D.J., Dinstein I., Minshew N., Behrmann M. Cortical variability in the sensory-evoked response in autism. J. Autism Dev. Disord. 2015;45:1176–1190. doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawco C., Armony J.L., Lepage M. Neural activity related to self-initiating elaborative semantic encoding in associative memory. Neuroimage. 2013;67:273–282. doi: 10.1016/j.neuroimage.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Hawco C., Berlim M.T., Lepage M. The dorsolateral prefrontal cortex plays a role in self-initiated elaborative cognitive processing during episodic memory encoding: rTMS evidence. PLoS One. 2013;8:e73789. doi: 10.1371/journal.pone.0073789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawco C., Buchanan R.W., Calarco N., Mulsant B.H., Viviano J.D., Dickie E.W., Argyelan M., Gold J.M., Iacoboni M., DeRosse P., Foussias G., Malhotra A.K., Voineskos A.N., SPINS Group Separable and Replicable Neural Strategies During Social Brain Function in People With and Without Severe Mental Illness. Am. J. Psychiatry appiajp. 2019 doi: 10.1176/appi.ajp.2018.17091020. 201817091020. [DOI] [PubMed] [Google Scholar]
- Hawco C., Viviano J.D., Chavez S., Dickie E.W., Calarco N., Kochunov P., Argyelan M., Turner J.A., Malhotra A.K., Buchanan R.W., Voineskos A.N., Group SPINS. A longitudinal human phantom reliability study of multi-center T1-weighted, DTI, and resting state fMRI data. Psychiatry Res Neuroimaging. 2018;282:134–142. doi: 10.1016/j.pscychresns.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesling I., Dilharreguy B., Peppé S., Amirault M., Bouvard M., Allard M. The integration of prosodic speech in high functioning autism: a preliminary FMRI study. PLoS One. 2010;5:e11571. doi: 10.1371/journal.pone.0011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holiga Š., Hipp J.F., Chatham C.H., Garces P., Spooren W., D'Ardhuy X.L., Bertolino A., Bouquet C., Buitelaar J.K., Bours C., Rausch A., Oldehinkel M., Bouvard M., Amestoy A., Caralp M., Gueguen S., Ly-Le Moal M., Houenou J., Beckmann C.F., Loth E., Murphy D., Charman T., Tillmann J., Laidi C., Delorme R., Beggiato A., Gaman A., Scheid I., Leboyer M., d'Albis M.-A., Sevigny J., Czech C., Bolognani F., Honey G.D., Dukart J. Patients with autism spectrum disorders display reproducible functional connectivity alterations. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aat9223. [DOI] [PubMed] [Google Scholar]
- Hubl D., Bölte S., Feineis–Matthews S., Lanfermann H., Federspiel A., Strik W., Poustka F., Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Huntenburg J.M. Freie Universität Berlin; 2014. Evaluating nonlinear coregistration of BOLD EPI and T1w images. [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Executive function and developmental disorders: the flip side of the coin. Trends Cogn. Sci. 2012;16:454–457. doi: 10.1016/j.tics.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Kenworthy L., Anthony L.G., Naiman D.Q., Cannon L., Wills M.C., Luong-Tran C., Werner M.A., Alexander K.C., Strang J., Bal E., Sokoloff J.L., Wallace G.L. Randomized controlled effectiveness trial of executive function intervention for children on the autism spectrum. J. Child Psychol. Psychiatry. 2014;55:374–383. doi: 10.1111/jcpp.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L., Yerys B.E., Anthony L.G., Wallace G.L. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychol. Rev. 2008;18:320–338. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Ghosh S.S., Bao F.S., Giard J., Häme Y., Stavsky E., Lee N., Rossa B., Reuter M., Chaibub Neto E., Keshavan A. Mindboggling morphometry of human brains. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H., Carpenter P.A., Minshew N.J., Cherkassky V.L., Keller T.A., Just M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lai M.-C., Kassee C., Besney R., Bonato S., Hull L., Mandy W., Szatmari P., Ameis S.H. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:819–829. doi: 10.1016/S2215-0366(19)30289-5. [DOI] [PubMed] [Google Scholar]
- Lawson R.A., Papadakis A.A., Higginson C.I., Barnett J.E., Wills M.C., Strang J.F., Wallace G.L., Kenworthy L. Everyday executive function impairments predict comorbid psychopathology in autism spectrum and attention deficit hyperactivity disorders. Neuropsychology. 2015;29:445–453. doi: 10.1037/neu0000145. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D., Hergueta T., Weiller E. The mini international neuropsychiatric interview. European Psychiatry. 1998 doi: 10.1016/s0924-9338(99)80239-9. [DOI] [PubMed] [Google Scholar]
- Liu T.T., Nalci A., Falahpour M. The global signal in fMRI: Nuisance or Information? NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston L.A., Happé F. Conceptualising compensation in neurodevelopmental disorders: Reflections from autism spectrum disorder. Neurosci. Biobehav. Rev. 2017;80:729–742. doi: 10.1016/j.neubiorev.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Lai M.-C., Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry. 2019 doi: 10.1038/s41380-018-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P.C., Risi S. Western Psychological Services; Los Angeles, CA: 2003. Autism Diagnostic Observation Schedule: ADOS. [Google Scholar]
- Luna B., Minshew N.J., Garver K.E., Lazar N.A., Thulborn K.R., Eddy W.F., Sweeney J.A. Neocortical system abnormalities in autism. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Lyall K., Croen L., Daniels J., Fallin M.D., Ladd-Acosta C., Lee B.K., Park B.Y., Snyder N.W., Schendel D., Volk H., Windham G.C., Newschaffer C. The Changing Epidemiology of Autism Spectrum Disorders. Annu. Rev. Public Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C.J., Breeden A.L., You X., Ludlum R., Gaillard W.D., Kenworthy L., Vaidya C.J. Executive Dysfunction in Autism Spectrum Disorder Is Associated With a Failure to Modulate Frontoparietal-insular Hub Architecture. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:537–545. doi: 10.1016/j.bpsc.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maïza O., Mazoyer B., Hervé P.-Y., Razafimandimby A., Dollfus S., Tzourio-Mazoyer N., Andreassen O.A. Impact of cognitive performance on the reproducibility of fMRI activation in schizophrenia. J. Psychiatry Neurosci. 2010;35:378–389. doi: 10.1503/jpn.090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Dosenbach N.U.F. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. 2018;20:133–140. doi: 10.31887/DCNS.2018.20.2/smarek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley T., Chen S., Goos L., Schachar R., Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J. Int. Neuropsychol. Soc. 2010;16:495–505. doi: 10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R.-A., Kleinhans N., Kemmotsu N., Pierce K., Courchesne E. Abnormal variability and distribution of functional maps in autism: an FMRI study of visuomotor learning. Am. J. Psychiatry. 2003;160:1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- Nunes A.S., Peatfield N., Vakorin V., Doesburg S.M. Idiosyncratic organization of cortical networks in autism spectrum disorder. Neuroimage. 2019;190:182–190. doi: 10.1016/j.neuroimage.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Poulin-Lord M.-P., Barbeau E.B., Soulières I., Monchi O., Doyon J., Benali H., Mottron L. Increased topographical variability of task-related activation in perceptive and motor associative regions in adult autistics. Neuroimage Clin. 2014;4:444–453. doi: 10.1016/j.nicl.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A., Park D.C. How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol. Rev. 2014;24:355–370. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring H.A., Baron-Cohen S., Wheelwright S., Williams S.C.R., Brammer M., Andrew C., Bullmore E.T. Cerebral correlates of preserved cognitive skills in autismA functional MRI study of Embedded Figures Task performance. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Rosa M., Puig O., Lázaro L., Vallés V., Lera S., Sánchez-Gistau V., Calvo R. Broad Cognitive Profile in Children and Adolescents with HF-ASD and in Their Siblings: Widespread Underperformance and its Clinical and Adaptive Correlates. J. Autism Dev. Disord. 2017;47:2153–2162. doi: 10.1007/s10803-017-3137-x. [DOI] [PubMed] [Google Scholar]
- Shapiro S.S., Wilk M.B. An Analysis of Variance Test for Normality (Complete Samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Sparrow S.S., Cicchetti D.V. Diagnostic uses of the Vineland Adaptive Behavior Scales. J. Pediatr. Psychol. 1985;10:215–225. doi: 10.1093/jpepsy/10.2.215. [DOI] [PubMed] [Google Scholar]
- Szatmari P., Bartolucci G., Bremner R., Bond S., Rich S. A follow-up study of high-functioning autistic children. Journal of Autism and Developmental Disorders. 1989 doi: 10.1007/bf02211842. [DOI] [PubMed] [Google Scholar]
- Tillmann J., San José Cáceres A., Chatham C.H., Crawley D., Holt R., Oakley B., Banaschewski T., Baron‐Cohen S., Bölte S., Buitelaar J.K., Durston S., Ham L., Loth E., Simonoff E., Spooren W., Murphy D.G., Charman T., the EU‐AIMS LEAP group, Ahmad J., Ambrosino S., Auyeung B., Baumeister S., Beckmann C., Bourgeron T., Bours C., Brammer M., Brandeis D., Brogna C., de Bruijn Y., Chakrabarti B., Cornelissen I., Acqua F.D., Dumas G., Ecker C., Faulkner J., Frouin V., Garcés P., Goyard D., Hayward H., Hipp J., Johnson M.H., Jones E.J.H., Kundu P., Lai M., D'ardhuy X.L., Lombardo M., Lythgoe D.J., Mandl R., Mason L., Meyer‐Lindenberg A., Moessnang C., Mueller N., O'Dwyer L., Oldehinkel M., Oranje B., Pandina G., Persico A.M., Ruggeri B., Ruigrok A., Sabet J., Sacco R., Toro R., Tost H., Waldman J., Williams S.C.R., Wooldridge C., Zwiers M.P. Investigating the factors underlying adaptive functioning in autism in the EU‐AIMS Longitudinal European Autism Project. Autism Res. 2019;12:645–657. doi: 10.1002/aur.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber J.M., White N.S., Steed T.C., Bartsch H., Holland D., Farid N., McDonald C.R., Carter B.S., Dale A.M., Chen C.C. Characterization and Correction of Geometric Distortions in 814 Diffusion Weighted Images. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa R.A., Mostofsky S.H., Ewen J.B. The Disrupted Connectivity Hypothesis of Autism Spectrum Disorders: Time for the Next Phase in Research. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:245–252. doi: 10.1016/j.bpsc.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan V.M., Francis K.E., Morgan B.R., Smith M.L., Taylor M.J. Load matters: neural correlates of verbal working memory in children with autism spectrum disorder. J. Neurodev. Disord. 2018;10:19. doi: 10.1186/s11689-018-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan V.M., Morgan B.R., Lee W., Powell T.L., Smith M.L., Taylor M.J. The neural correlates of visuo-spatial working memory in children with autism spectrum disorder: effects of cognitive load. J. Neurodev. Disord. 2014;6:19. doi: 10.1186/1866-1955-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.X., Rogers L.M., Gross E.Z., Ryals A.J., Dokucu M.E., Brandstatt K.L., Hermiller M.S., Voss J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Peterson D.J., Gatenby J.C., Li W., Grabowski T.J., Madhyastha T.M. Evaluation of Field Map and Nonlinear Registration Methods for Correction of Susceptibility Artifacts in Diffusion MRI. Front. Neuroinform. 2017;11:17. doi: 10.3389/fninf.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Y.-B., Liu L.-L., Cui J.-F., Wang J., Shum D.H.K., van Amelsvoort T., Chan R.C.K. A Meta-Analysis of Working Memory Impairments in Autism Spectrum Disorders. Neuropsychol. Rev. 2017;27:46–61. doi: 10.1007/s11065-016-9336-y. [DOI] [PubMed] [Google Scholar]
- Warren, Z., Taylor, J.L., McPheeters, M.L., Worley, K., Veenstra-Vander Weele, J., 2013. Future Research Needs: Interventions for Adolescents and Young Adults With Autism Spectrum Disorders: Identification of Future Research Needs From Comparative Effectiveness Review No. 65. Agency for Healthcare Research and Quality (US), Rockville (MD). [PubMed]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.