Summary
Mucosal surfaces are key interfaces between the host and its environment, but also constitute ports of entry for numerous pathogens. The gut and lung mucosae act as points of nutrient and gas exchange, respectively, but the physiological purpose of the female reproductive tract (FRT) is to allow implantation and development of the fetus. Our understanding of immune responses in the FRT has traditionally lagged behind our grasp of the situation at other mucosal sites, but recently reproductive immunologists have begun to make rapid progress in this challenging area. Here, we review current knowledge of immune responses in the human FRT and their heterogeneity within and between compartments. In the commensal‐rich vagina, the immune system must allow the growth of beneficial microbes, whereas the key challenge in the uterus is allowing the growth of the semi‐allogeneic fetus. In both compartments, these objectives must be balanced with the need to eliminate pathogens. Our developing understanding of immune responses in the FRT will help us develop interventions to prevent the spread of sexually transmitted diseases and to improve outcomes of pregnancy for mothers and babies.
Keywords: ILC, macrophages, mucosa, T‐cells, uterus
Our understanding of immune responses in the FRT has traditionally lagged behind our grasp of the situation at other mucosal sites, but recently reproductive immunologists have begun to make rapid progress in this challenging area. Here, we review current knowledge of immune responses in the human FRT and their heterogeneity within and between compartments.
Abbreviations
- APC
antigen presenting cell
- EVT
extravillous trophoblast
- FRT
female reproductive tract
- HCMV
human cytomegalovirus
- HIV
human immunodeficiency virus
- HPV
human papilloma virus
- HSV
herpes simplex virus
- IFN
interferon
- IL
interleukin
- ILC
innate lymphoid cell
- KIR
killer immunoglobulin‐like receptor
- LC
Langerhans cells
- LP
lamina propria
- MAIT
mucosal‐associated invariant T cell
- NK
natural killer
- TGF
transforming growth factor
- TNF
tumour necrosis factor
- VT
villous trophoblast
Introduction
Mucosal barriers are critical interfaces between the host and the environment. Over the past decades, much effort has been dedicated to understanding immune protection in the intestinal and pulmonary mucosae, but our grasp of immune mechanisms in the female reproductive tract (FRT) has lagged behind. Although this partly reflects a climate in which research into women’s health has been underfunded,1 it is also a result of the not inconsiderable difficulties of studying the FRT.
The immune system encounters vastly different challenges in the various compartments of the FRT, and as a result differs between the lower (vagina and ectocervix) and upper (uterus and endocervix) FRT. Like the gut, the vagina is populated by a commensal flora, and here the immune system must allow the growth of beneficial microbes while preventing that of pathogens. The cervix acts as a gatekeeper, preventing the entry of microbes into the uterus, while permitting the passage of sperm. Finally, the immune system in the uterus faces the challenge of allowing the fetus, which is immunologically distinct from its mother, to co‐exist with her for 9 months, while simultaneously eliminating any pathogens that may enter. Every site within the FRT must mediate a fine balance between protection from pathogens and maintenance of tissue integrity and function, allowing fertilization, implantation and pregnancy to occur. The two are not necessarily at odds; however, because infections are among the common causes of infertility2 and late pregnancy failure.3
Another challenge of studying the FRT is the additional complexity of hormone‐driven alterations over the course of the menstrual cycle. It has long been appreciated that the uterine mucosa and cervix change over the cycle, allowing sperm to enter the uterus at roughly the time of ovulation, and the conceptus to implant about 9 days later. The uterine immune system, too, changes with the menstrual cycle and pregnancy. It is now coming to be appreciated that cyclical changes also occur in the vagina, and that these could have an impact on susceptibility to disease.
The vagina
Physical and chemical barriers in the lower reproductive tract
In contrast with the upper reproductive tract, which is lined by a monolayer of columnar epithelial cells, the ectocervix and vagina are lined by protective layers of non‐keratinized stratified squamous epithelium. In addition to the physical barrier that a stratified epithelium constitutes, chemical and biological barriers form a first line of defence, with mucus and antimicrobial peptides protecting the vagina from pathogens.4, 5 The epithelial layer may also play a role in the success of human immunodeficiency virus (HIV) containment using antiretroviral therapy, as FRT epithelial cells, as well as the underlying fibroblasts, can deliver and store antiretrovirals, promoting sustained protection of vaginal CD4+ T‐cells from viral infection.6
Another contributor to protection from pathogens in the lower reproductive tract is the population of commensal bacteria. The human vaginal microbiome can be classified into five core microbial communities, of which four are dominated by species belonging to the genus Lactobacillus, and one is characterized by higher levels of strict anaerobes, including Prevotella, Gardnerella, Dialister and Atopobium.7, 8, 9 The abundance of the latter microbial community is higher among Black and Hispanic populations9 and individuals displaying a microbiome characterized by dominant strict anaerobes other than Gardnerella display higher vaginal inflammation and an increased susceptibility to HIV‐1 infection.10, 11 Nevertheless, the beneficial effects of specific bacterial species in protecting the FRT are well established. In particular, lactobacilli produce lactic acid, establishing an acidic environment that limits colonization by other microorganisms.12 Lactobacilli also produce bacteriostatic compounds,13 compete with opportunistic pathogens for attachment to the vaginal epithelium14 and secrete antimicrobial peptides.15 Overall, the vaginal microbiome has, over the past decade, emerged as a critical modulator of inflammation in the reproductive tract, and the full extent of its impact on susceptibility versus protection against infection is an active area of research.
At the cervix, mucus serves as a physical barrier, changing its consistency over the course of the menstrual cycle to allow the passage of sperm at ovulation.16 Cervical mucus also contains immune mediators, including antibodies, complement and cytokines.17, 18 In addition to these barriers, the lower FRT is populated by immune cells, which participate in its protection and regulation. The barrier and immune mechanisms protecting the reproductive tract are depicted in Fig. 1.
Figure 1.
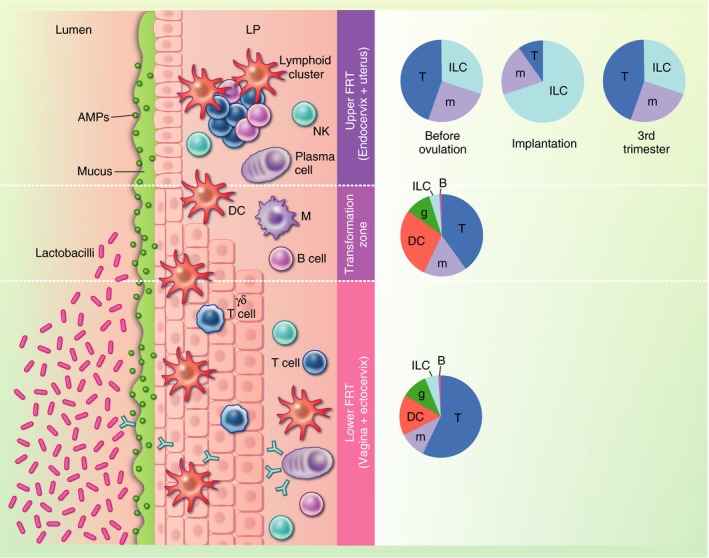
Immune and non‐immune barriers in the female reproductive tract (FRT). The FRT can be broadly divided into an upper section, comprising the uterus and the endocervix, and a lower section, which includes the vagina and ectocervix. The upper reproductive tract is lined by a single layer of columnar epithelium, while the lower reproductive tract is lined by stratified squamous epithelium. The zone where the two types of epithelia meet is called the transformation zone. Several non‐immune barriers form a first line of defence against pathogen invasion: the presence of tight junctions constitutes a physical barrier, mucus and antimicrobial peptides form a chemical barrier, and the Lactobacillus‐rich vaginal milieu creates a biological barrier. This multi‐layered defence strategy is further reinforced by a variety of immune cells that reside within the epithelium and the lamina propria (LP), patrolling for invading microorganisms. Pie charts indicate the composition of immune cells along the FRT. In the upper FRT, the composition differs by stage of the menstrual cycle or pregnancy where ‘Implantation’ represents the composition in the secretory phase of the menstrual cycle and early pregnancy. AMPs, antimicrobial peptides; DC, dendritic cell; FRT, female reproductive tract; g, granulocyte; ILC, innate lymphoid cell; LP, lamina propria; m, monocyte/macrophage; NK, natural killer.
Myeloid cells in the vagina
The lower FRT is populated by a variety of immune cells, which constitute between 6% and 20% of all cells and protect against invading pathogens.19 Whilst immune cell composition in the upper FRT changes over the menstrual cycle in response to hormonal changes, it does not seem to fluctuate in the lower FRT.20 However, there is emerging evidence that immune function in this region, and thus disease susceptibility, may vary across the menstrual cycle.21, 22
Four main subsets of antigen‐presenting cells (APCs) are present in the lower FRT: (i) intraepithelial Langerhans cells (LCs); (ii) lamina propria (LP) CD14− dendritic cells (DCs); (iii) CD14+ DCs; and (iv) macrophages. These comprise between 10% and 50% of leucocytes in the lower FRT. The vaginal mucosa does not contain mucosal‐associated lymphoid tissue so priming of adaptive responses takes place in draining lymph nodes. Upon infection, APCs are mobilized to the draining lymph nodes to prime naïve T‐cells. Functional and transcriptomic analysis indicates specialization among FRT myeloid populations.23 LP CD14− DCs and LCs are skewed towards Th2 cell activation and regulatory functions.24 In contrast, CD14+ DCs and macrophages resemble classical innate cells, which respond to pathogen‐derived molecules via TLRs and contribute to priming of Th1 responses.24
Further immune cell populations can populate the reproductive tract during an infectious challenge, and their protective roles in the context of HIV are beginning to emerge. Neutrophils were recently shown to release neutrophil extracellular traps in response to co‐culture with HIV viral‐like particles, contributing to viral inactivation.25 While myeloid cells serve critical functions in the surveillance of the FRT, inflammation may also increase susceptibility to HIV infection. LCs and CD14+ DCs support HIV‐1 infection,26 and HIV‐1 DNA has been detected in LCs and CD14+ cells isolated from HIV‐infected women.27, 28 Critically, HIV‐1 was detected in immune cells in individuals starting antiretroviral therapy as early as 10 days after the onset of symptoms of primary infection, and replicative virus was detectable in immune cells despite undetectable blood viremia.29 Therefore, targeting mucosal immune cells may be key to achieving sterilizing immunity to HIV.
Innate lymphoid cells and innate‐like T‐cells in the vagina
In addition to APCs, several innate and innate‐like lymphocyte populations contribute to immune surveillance and protection of the lower FRT. Natural killer (NK) cells, which are members of the innate lymphoid cell (ILC) family,30 are present in the vagina. In contrast to those present in the upper FRT, NK cells in the lower FRT resemble blood NK cells31 and play an important role in limiting viral infections. NK‐deficient individuals have an increased risk of herpes virus infection32 and a higher incidence of cervical cancer resulting from human papilloma virus (HPV) infection.33
Additional innate‐like lymphocyte populations, including γδ and mucosal‐associated invariant T‐(MAIT) cells, may play important roles in local protection against sexually transmitted infections (STIs). γδ T‐cells in the FRT predominantly express a Vδ1 TCR, in contrast with the Vγ9Vδ2+ γδ T‐cell subset predominant in peripheral blood.34 The balance between Vδ1 and Vδ2 cells is altered during bacterial vaginosis, with endocervical Vδ2 cells expressing CD4 and CCR5, which are required for HIV cell entry, increasing in frequency.35 This points to a potential link between microbial dysbiosis in the FRT and HIV transmission. Female genital MAIT cells produce interleukin (IL)‐17 and IL‐22 in response to Escherichia coli stimulation, indicating a potential role in protection against bacterial infections.36 The location of these unconventional T‐cell populations at key sites of infection and cellular transformation, together with their acute sensitivity to tissue perturbation, makes them an attractive target for immune intervention. Defining the cues underlying their activation and their contributions to protection will be central to capture the complexity of protective responses within the FRT.
Classical adaptive responses in the vagina
Antigen‐specific lymphocytes participate in the containment of numerous infections in the FRT, with T‐cells comprising between 35% and 50% of leucocytes, and B‐cells representing under 1% of all immune cells. Indeed, orchestration of a Th1 and cytotoxic T‐cell response is critical for containment of Chlamydia trachomatis, which accounts for a third of all new STI cases worldwide.37 Chlamydia is often asymptomatic, but 10% of women develop pelvic inflammatory disease with ascending infection often leading to lasting sequelae, including ectopic pregnancy, infertility and chronic pelvic pain.37 The quality of the immune response elicited by Chlamydia correlates with pathology, with elevated levels of cervical type I interferons (IFNs) and decreased IFN‐γ associated with severe ascending infection.38
Persistent herpes simplex virus (HSV)‐2 infection is associated with formation of lymphoid clusters in the vagina and cervix of both humans and mice.39, 40 These aggregates of CD4+ and CD8+ T‐cells, B‐cells, DCs and macrophages can persist for months to years after viral clearance, potentially conferring lasting protection against reinfection. In mice, CD8+ T‐cells are recruited to peripheral nerve endings following HSV‐2 reactivation, where they remain after viral containment.41 Subsequent viral reactivation at sites where CD8+ cells were present did not result in lesion formation, suggesting a role for local CD8+ T‐cells in limiting reactivation.
In the FRT, antibodies contribute to pathogen clearance through mechanisms including pathogen neutralization, opsonization and complement‐driven lysis. Plasma cells secreting IgG and IgA can be detected in the LP of both the cervix and the vagina,42 although in contrast with other mucosal sites, IgG, rather than IgA, is the predominant antibody isotype in the lower FRT.
The uterus
Immune interfaces in the uterus
In contrast to the lower FRT, commensal microbes have long been thought to be absent from the upper FRT. Some reports suggest that a small commensal population may be present in the uterus,43, 44 although a recent study that carefully controlled for the effects of contamination could find no evidence of commensals in placenta and uterine lining from healthy pregnancies.45 However, infections of the uterus with pathogens are widely recognized, occurring both during and outside of pregnancy. This is commonly a result of ascent of bacteria from the vagina, as is the case for infection with sexually transmitted Neisseria gonorrhoeae or C. trachomatis. The immune system responds to these infections in broadly the same way that it would in any other tissue, recruiting immune cells from the blood to produce an inflammatory response which, in pregnancy, may result in miscarriage or preterm birth.3, 46
The primary foreign cells with which immune cells in the healthy uterus interact are those of the placenta. The placenta invades through the uterine lining, so that the placental villi, which are the site of nutrient and gas exchange between mother and fetus, are bathed in the mother’s blood. This means that significant numbers of circulating immune cells are present. The villi are covered in villous trophoblast (VT) cells, whereas a population of extravillous trophoblast (EVT) cells invade into and transform the spiral arteries of the uterus, allowing blood to flow to the placenta at low pressure. Both trophoblast populations are exposed to circulating maternal immune cells, and have several features to prevent their recognition and elimination by these cells.47, 48, 49, 50 Another interface between the placenta and the maternal immune system occurs within the uterine lining (the endometrium), which in pregnancy is called the decidua. In early pregnancy, the decidua basalis lies beneath the site of placental implantation, the decidua capsularis encloses the fetal membranes, and the decidua parietalis lines the opposite wall of the uterus. By the fourth month of pregnancy, the decidua capsularis and parietalis fuse. Studies that have compared the basalis and the parietalis have found the frequencies of immune cells to differ slightly between them,51, 52 with evidence of greater immune activation in the basalis towards term. In the decidua basalis, interstitial EVT cells encounter specialized immune cells present at this mucosal site. The three interfaces between fetal trophoblast and maternal immune cells are depicted in Fig. 2.
Figure 2.
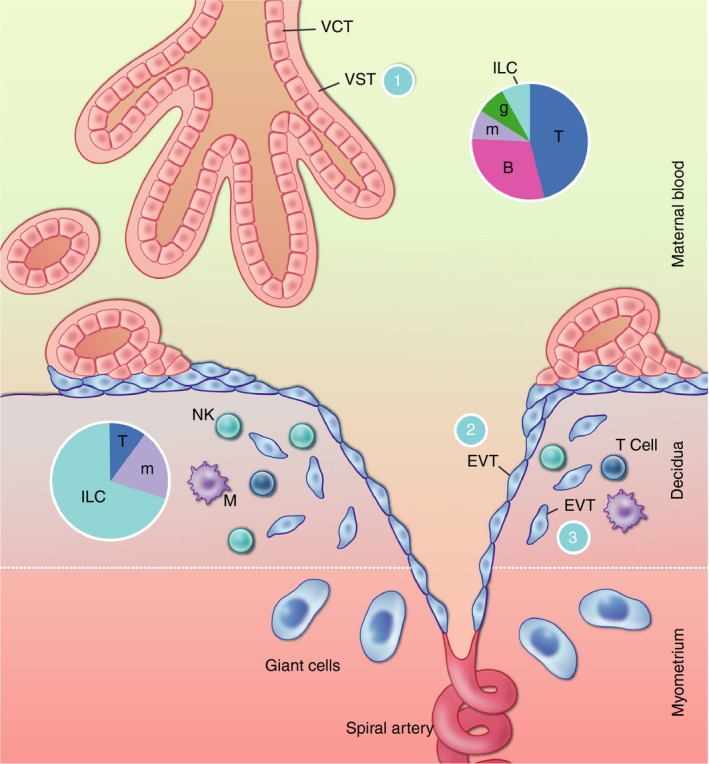
Interfaces between the placenta and the maternal immune system. The placental villi are bathed in the maternal blood. The outer layer of the villi is covered by VST with an underlying layer of mononuclear VCT. Villous trophoblast (VT) is protected from recognition by T‐cells, which are frequent in the maternal blood, by their complete lack of MHC expression (interface 1). They are further protected by immune cell recognition by their syncytial nature and thick glycocalyx. The inner layer of VCT grows out of the villi to anchor the placenta to the maternal decidua. VCT differentiates to extravillous trophoblast (EVT), some of which migrates down the spiral arteries, replacing the endothelial cells as far as the inner third of the myometrium. This process aids in the transformation of the spiral arteries, allowing blood to flow to the placenta at low pressure. These cells are in contact with the maternal blood (interface 2). Some EVT cells are also present in the decidua, where they interface with the unique immune cells present in this microenvironment (interface 3). EVT is largely protected from T‐cell recognition because they do not express the major TCR ligands HLA‐A and ‐B, but they do express the natural killer (NK) cell ligands HLA‐C, ‐E and G. At interface 2, this may protect EVT from recognition by blood NK cells. At interface 3, the expression of HLA‐C is likely to allow recognition by decidual NK cells, which are not cytotoxic but rather seem to have a role in tissue remodelling. Pie charts indicate the composition of maternal immune cells in the blood and decidua. VCT, villous cytotrophoblast; VST, villous syncytiotrophoblast; EVT, extravillous trophoblast; T, T‐cells; B, B‐cells; m, monocytes/macrophages; g, granulocytes; ILC, innate lymphoid cells.
Before pregnancy, the frequency of immune cells in the endometrium varies over the menstrual cycle. ILCs are sparse before ovulation, in the proliferative phase of the cycle. They increase rapidly following ovulation, in the secretory phase, and at the time of implantation represent about 70% of the immune cells present.53, 54 Like ILCs, macrophage numbers increase over the course of the menstrual cycle,55, 56 whereas T‐cells remain roughly constant.53, 54 If pregnancy occurs, the high frequency of ILC characteristic of the secretory phase of the menstrual cycle is maintained into the first trimester of pregnancy, with approximately 70% of decidual immune cells varieties of ILC. Macrophages are the next most frequent immune cell in first‐trimester decidua (20%), followed by T‐cells (10%).57, 58, 59
Some studies have been unable to locate DCs in the human decidua,59 while others have described CD209+ cells that could represent immature DCs occurring at a low frequency,60 and evidence from mice suggests that the rarity of DCs in the decidua may be among the mechanisms preventing the initiation of classical immune responses to the placenta.61 B‐cells are also largely absent.59 The number and frequency of ILCs declines over the course of pregnancy, although significant numbers are still detectable at term. Meanwhile, the numbers of macrophages and T‐cells remain roughly constant, although the decline in ILCs means that the proportion of decidual immune cells accounted for by these cells increases.51, 60 These changes in the composition of the endometrial/decidual immune system are depicted in Fig. 1.
ILC in the uterus
The ILC family is divided into five groups: NK cells, ILC1, ILC2, ILC3 and lymphoid tissue inducer cells.30 A large population of cells that resemble NK cells is present in the decidua, with smaller populations of ILC1 and ILC3. ILC2 are rare.59 Unlike circulating NK cells, decidual NK cells are not cytotoxic,62, 63, 64, 65, 66 and their presence in large numbers at the time and site of implantation led to the suggestion that they might have a role promoting placentation. In support of this, decidual NK cells produce pro‐angiogenic and trophoblast‐chemoattractant factors, as well as factors that may promote tissue remodelling by their action on macrophages.64, 67 Most suggestively, women who have genes (KIR2DS1, KIR2DS5 or KIR2DS4) for receptors that activate NK cells when they bind to HLA‐C2 expressed by fetal EVT are less likely to be affected by pre‐eclampsia, fetal growth restriction and recurrent miscarriage,72, 73, 74, 75] pointing to the importance of decidual NK cell activation in successful pregnancy.
Single‐cell RNAseq approaches have recently been used to show that decidual NK cells consist of three subpopulations, which have been called dNK1, ‐2 and ‐3.59 Among these, dNK1 have the highest levels of killer immunoglobulin‐like receptors (KIRs) and LILRB1, which recognize HLA molecules expressed on EVT. Therefore, this subset is likely to be the one that responds to EVT. dNK3 are phenotypically similar to ILC1 identified in human lymph nodes,76 and may represent uterine ILC1. Intriguingly, dNK2 display some features that are intermediate between dNK1 and dNK3 (or ILC1), which could indicate a certain level of plasticity between the NK and ILC1 lineages within the uterus.
It is not yet clear how decidual NK cells fit into our current scheme for understanding ILC: are they NK cells, ILC1 or something else? Their low cytotoxicity suggests that they should perhaps be rebranded innate helper cells of pregnancy, rather than killers. Nonetheless, our understanding of circulating NK cells has influenced the kinds of questions that we ask about decidual NK cells. Circulating NK cells have a role in the control of HIV infection and decidual NK cells may act similarly, as they can inhibit HIV infection of decidual macrophages in vitro.77, 78, 79 Circulating NK cells are also a major defence against human cytomegalovirus (HCMV), which can cross the placenta. Although they are usually not cytotoxic, decidual NK cells can kill HCMV‐infected fibroblasts.66 Circulating NK cells produce a memory‐like response to HCMV,80 and there is some evidence for a memory‐like phenomenon in decidual NK cells during second pregnancies.81 This could perhaps account for the longstanding observation that second and subsequent pregnancies are more likely to be successful.82 However, it is not yet clear if the reported phenotype in fact arises in response to HCMV infection.83
In contrast to decidual NK cells, ILC3 are present at a relatively low frequency in the uterine mucosa.59, 84 They produce IL‐22, which maintains homeostasis at mucosal sites,87 and evidence from mice suggests that it may help to maintain pregnancy in the face of infection.88 Decidual ILC3 also produce neutrophil‐attractive chemokines and their ability to do this may be associated with better outcomes in early pregnancy.71
Macrophages in the uterus
Macrophages are the second most prominent group of immune cells in the uterine mucosa and are present in non‐pregnant endometrium throughout the menstrual cycle, with their numbers climbing as menstruation begins.55, 56 They play a role in breakdown of the endometrium in early menstruation, in part by the release of matrix metalloproteinases, as well as repair of the tissue and clearance of debris during the final phase of menstruation.55, 89
If pregnancy occurs, macrophage numbers stabilize and remain about 20% of the leucocyte population throughout pregnancy.59 Implantation has been proposed to be an inflammatory process, shaped by macrophage cytokine production.90 In support of this, inflammatory genes are upregulated in the endometrium during the window of implantation, although some anti‐inflammatory genes, such as transforming growth factor (TGF)‐β, are also overexpressed.91 In the first trimester, this continues as macrophages produce IL‐6 and IL‐8, which can promote placental invasion, in response to EVT.92, 93
Although macrophages have historically been classified as M1 and M2, with M1 displaying a pro‐inflammatory and M2 a pro‐repair phenotype,94 they are now known to be highly heterogeneous and capable of specialization in different tissues,95 throwing into question whether this is an appropriate way of classifying decidual macrophages. Indeed, in the uterus, an alternative macrophage grouping based on CD11c expression has been suggested.96 Unbiased RNAseq approaches have also defined two populations of decidual macrophages, dM1 and dM2, whose gene expression profiles match the CD11chi and CD11clo profiles, respectively.59
Decidual macrophages may also play an important role in the initiation of childbirth, a process in which increased expression of inflammatory mediators promotes uterine contraction, delivery and placental detachment.97 Macrophages increase in the decidua of rats prior to labour and, in humans, decidual samples from labouring women have greater numbers of macrophages compared with samples from non‐labouring women.98 Macrophages are also recruited to the cervix during ripening, a tissue remodelling process that occurs prior to birth.99
T‐cells in the uterus
T‐cells are present in non‐pregnant endometrium with CD8+ T‐cells the major population throughout the menstrual cycle.54 This contrasts with peripheral blood, in which the proportion of CD4+ T‐cells is greater than that of CD8+ T‐cells.100 In early pregnancy, T‐cells account for a minority of decidual immune cells, but by term just over half of the leucocytes are T‐cells,101 with most of the increase accounted for by CD4+ T‐cells.60
CD8+ T‐cells in early pregnancy express reduced levels of cytotoxic molecules,102, 103 a phenomenon that may occur under hormonal control.104 They do not degranulate in response to EVT, but are nevertheless capable of activation and killing.103, 105 This may suggest that they stand ready to act as a defence should a viral infection occur, a hypothesis supported by the observation that HCMV‐specific decidual CD8+ T‐cells expand and express increased granzyme B.103 On the other hand, it has also been proposed that, like decidual NK cells and macrophages, decidual CD8+ T‐cells produce cytokines, such as IL‐8 and IFN‐γ, that may promote EVT invasion.105 CD4+ T‐cells in the uterine lining have an effector memory phenotype and are better able to produce cytokines than their counterparts in the peripheral blood.106, 107 Their ability to produce IFN‐γ decreases as pregnancy progresses, while their ability to produce IL‐4 goes up.108
Tregs are present in the lining of the uterus at a higher frequency than in peripheral blood both before and throughout pregnancy.109 This is likely to be a consequence of high levels of TGFβ in the decidua,59, 110 and they may also be induced by the immunomodulatory enzyme IDO produced by decidual macrophages and/or by interactions with EVT cells.111, 112 The regulatory environment is also supported by decidual γδ T‐cells, which are enriched in first‐trimester decidua and produce high levels of IL‐10 and TGFβ.113, 114 In addition to classical FoxP3+ Treg, the decidua contains two FoxP3‐negative populations of CD4+ T‐cells, which express the regulatory molecules PD‐1 or TIGIT.112 All three of these can suppress T‐cell proliferation, but they differ in their ability to impact cytokine production, with FoxP3+ Tregs most effective at inhibiting IFN‐γ and tumour necrosis factor (TNF‐α) production, while PD‐1+ CD4+ T‐cells promote the production of IL‐10. It has been proposed that decidual Tregs may promote tolerance to the placenta, as a subset of FoxP3+ Treg is reduced in decidua from miscarriages compared with healthy pregnancies.115 However, it is difficult to determine if this represents a cause or an effect of the miscarriage. Another possibility is that Tregs in the decidua have a role in tissue repair and regeneration, as is seen in other organs.116 This is in line with current ideas about the roles of the two other major immune cell populations in the decidua, decidual NK cells and macrophages.
A key question is whether decidual T‐cells can recognize allogeneic proteins expressed by trophoblast. Such T‐cells have been described in mice,49 but in humans this has not been an easy question to address. The approaches that have most often been used to investigate decidual T‐cell reactivity have looked at their responses to umbilical cord blood cells or HY antigens being presented on HLA‐A or ‐B, but neither of these is representative of the molecules expressed by trophoblast. However, an expansion of trophoblast‐specific Tregs is indirectly suggested by the finding that particular Treg clones are expanded in the decidua compared with the blood.117 In the future, better defining the reactivity of these T‐cells will be a key challenge in properly understanding the decidual immune response to trophoblast.
Perspectives and opportunities
Although our understanding of immune responses in the FRT has lagged behind our understanding of those at other mucosal surfaces, we are beginning to make progress in this area. It is an exciting time to be a reproductive immunologist.
The discovery that immune responses in the lower FRT fluctuate over the menstrual cycle has led to the proposal of a ‘window of susceptibility’ for sexually‐transmitted diseases such as HIV21 and chlamydia.22 This may have an impact on public health recommendations and vaccination strategies for these diseases. Likewise, our emerging understanding that vaginal dysbiosis and inflammation may be harmful in the context of HIV transmission35 will help shape therapeutic and preventative approaches. A better understanding of local protective immune responses will also be important in the design of interventions against emerging sexually transmitted diseases, such as multidrug‐resistant N. gonorrhoea and Zika virus.
In the upper FRT, the development of novel multiparametric approaches means that we now have a better understanding of the diverse populations of immune cells that are present, including which are mucosal and which come from the blood.59 This means that several questions that had been considered resolved will have to be reopened, but we have the opportunity to make rapid progress. The recent development of trophoblast118 and endometrial119 organoid cultures means that we will be able use in vitro approaches to understand how immune cells interact with these cells. This will have a significant impact on our understanding of the immunology of pregnancy, and will help in the design of interventions to improve outcomes for mothers and babies.
Disclosure
The authors declare no competing interests.
Acknowledgements
LM is supported by the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska‐Curie grant agreement (number 792383). EMW is supported by the Borne Foundation. VM is supported by the Borne Foundation and a Royal Society and Wellcome Trust‐funded Sir Henry Dale Fellowship (WT105677).
References
- 1. Fisk NM, Atun R. Systematic analysis of research underfunding in maternal and perinatal health. BJOG 2009; 116:347–56. [DOI] [PubMed] [Google Scholar]
- 2. Tsevat DG, Wiesenfeld HC, Parks C, Peipert JF. Sexually transmitted diseases and infertility. Am J Obstet Gynecol 2017; 216:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000; 342:1500–7. [DOI] [PubMed] [Google Scholar]
- 4. Lai SK, Wang Y‐Y, Wirtz D, Hanes J. Micro‐ and macrorheology of mucus. Adv Drug Deliv Rev 2009; 61:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol 2010; 10:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen Z, Rodriguez‐Garcia M, Patel MV, Bodwell J, Wira CR. Epithelial cells and fibroblasts from the human female reproductive tract accumulate and release TFV and TAF to sustain inhibition of HIV infection of CD4+ T cells. Sci Rep 2019; 9:1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Redondo‐Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis 1990; 12:856–72. [DOI] [PubMed] [Google Scholar]
- 8. Weinstein L, Bogin M, Howard JH. A survey of the vaginal flora at various ages, with special reference to the Döderlein bacillus. Am J Obstet Gynecol 1936; 32:211–8. [Google Scholar]
- 9. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL et al Vaginal microbiome of reproductive‐age women. Proc Natl Acad Sci USA 2011; 108(Suppl 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M et al Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu‐Ali G, Bowman BA et al Lactobacillus‐deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African Women. Immunity 2017; 46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R et al Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 2015; 2:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mijac VD, Dukić SV, Opavski NZ, Dukić MK, Ranin LT. Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur J Obstet Gynecol Reprod Biol 2006; 129:69–76. [DOI] [PubMed] [Google Scholar]
- 14. Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014; 289:479–89. [DOI] [PubMed] [Google Scholar]
- 15. Amabebe E, Anumba DOC. The vaginal microenvironment: the physiologic role of lactobacilli. Front Med 2018; 13:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martyn F, McAuliffe FM, Wingfield M. The role of the cervix in fertility: is it time for a reappraisal? Hum Reprod 2014; 29:2092–8. [DOI] [PubMed] [Google Scholar]
- 17. Kutteh WH, Prince SJ, Hammond KR, Kutteh CC, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol 1996; 104:538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kutteh WH, Moldoveanu Z, Mestecky J. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retroviruses 1998; 14:S51–5. [PubMed] [Google Scholar]
- 19. Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM et al Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol 1997; 38:350–9. [DOI] [PubMed] [Google Scholar]
- 20. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. J Low Genit Tract Dis 2001; 5:116. [PubMed] [Google Scholar]
- 21. Saba E, Origoni M, Taccagni G, Ferrari D, Doglioni C, Nava A et al Productive HIV‐1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol 2013; 6:1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wira CR, Rodriguez‐Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 2015; 15:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duluc D, Banchereau R, Gannevat J, Thompson‐Snipes L, Blanck J‐P, Zurawski S et al Transcriptional fingerprints of antigen‐presenting cell subsets in the human vaginal mucosa and skin reflect tissue‐specific immune microenvironments. Genome Med 2014; 6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duluc D, Gannevat J, Anguiano E, Zurawski S, Carley M, Boreham M et al Functional diversity of human vaginal APC subsets in directing T‐cell responses. Mucosal Immunol 2013; 6:626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barr FD, Ochsenbauer C, Wira CR, Rodriguez‐Garcia M. Neutrophil extracellular traps prevent HIV infection in the female genital tract. Mucosal Immunol 2018; 11:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen R, Richter HE, Smith PD. Early HIV‐1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011; 65:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pena‐Cruz V, Agosto LM, Akiyama H, Olson A, Moreau Y, Larrieux J‐R et al HIV‐1 replicates and persists in vaginal epithelial dendritic cells. J Clin Invest 2018; 128:3439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perez‐Zsolt D, Cantero‐Pérez J, Erkizia I, Benet S, Pino M, Serra‐Peinado C et al Dendritic cells from the cervical mucosa capture and transfer HIV‐1 via Siglec‐1. Front Immunol 2019; 30:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV‐1 infection. Proc Natl Acad Sci USA 1998; 95:8869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al Innate lymphoid cells: 10 years on. Cell 2018; 174:1054–66. [DOI] [PubMed] [Google Scholar]
- 31. Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR et al Unique characteristics of NK cells throughout the human female reproductive tract. Clin Immunol 2007; 124:69–76. [DOI] [PubMed] [Google Scholar]
- 32. Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect 2002; 4:1545–58. [DOI] [PubMed] [Google Scholar]
- 33. Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR et al GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014; 123:809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strbo N, Romero L, Alcaide M, Fischl M. Isolation and flow cytometric analysis of human endocervical gamma delta T cells. J Vis Exp 2017. 10.3791/55038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alcaide ML, Strbo N, Romero L, Jones DL, Rodriguez VJ, Arheart K et al Bacterial vaginosis is associated with loss of gamma delta T cells in the female reproductive tract in women in the Miami Women Interagency HIV Study (WIHS): a cross sectional study. PLoS One 2016; 11:e0153045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibbs A, Leeansyah E, Introini A, Paquin‐Proulx D, Hasselrot K, Andersson E et al MAIT cells reside in the female genital mucosa and are biased towards IL‐17 and IL‐22 production in response to bacterial stimulation. Mucosal Immunol 2016; 10:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Connell CM, Ferone ME. Chlamydia trachomatis genital infections. Microb Cell 2016; 3:390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poston TB, Lee DE, Darville T, Zhong W, Dong L, O’Connell CM et al Cervical cytokines associated with Chlamydia trachomatis susceptibility and protection. J Infect Dis 2019; 220:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang VA, Rosenthal KL. Intravaginal infection with herpes simplex virus type‐2 (HSV‐2) generates a functional effector memory T cell population that persists in the murine genital tract. J Reprod Immunol 2010; 87:39–44. [DOI] [PubMed] [Google Scholar]
- 40. Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C et al Persistence of HIV‐1 receptor‐positive cells after HSV‐2 reactivation is a potential mechanism for increased HIV‐1 acquisition. Nat Med 2009; 15:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10:524–30. [DOI] [PubMed] [Google Scholar]
- 42. Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol 1997; 36:23–50. [DOI] [PubMed] [Google Scholar]
- 43. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014; 6:237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franasiak JM, Scott RT. Endometrial microbiome. Curr Opin Obstet Gynecol 2017; 29:146–52. [DOI] [PubMed] [Google Scholar]
- 45. de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ et al Human placenta has no microbiome but can contain potential pathogens. Nature 2019;572:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med 2015; 372:2039–48. [DOI] [PubMed] [Google Scholar]
- 47. Jones CJ, Carter CM, Aplin JD, Enders AC. Glycosylation at the fetomaternal interface in hemomonochorial placentae from five widely separated species of mammal: is there evidence for convergent evolution? Cells Tissues Organs 2007; 185:269–84. [DOI] [PubMed] [Google Scholar]
- 48. Trowsdale J, Moffett A. NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol 2008; 20:317–20. [DOI] [PubMed] [Google Scholar]
- 49. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 2013; 13:23–33. [DOI] [PubMed] [Google Scholar]
- 50. Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal‐fetal interface. J Clin Invest 2014; 124:1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol 2009; 82:24–31. [DOI] [PubMed] [Google Scholar]
- 52. Solders M, Gorchs L, Gidlöf S, Tiblad E, Lundell AC, Kaipe H. Maternal adaptive immune cells in decidua parietalis display a more activated and coinhibitory phenotype compared to decidua basalis. Stem Cells Int 2017; 2017:8010961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pace D, Morrison L, Bulmer JN. Proliferative activity in endometrial stromal granulocytes throughout menstrual cycle and early pregnancy. J Clin Pathol 1989; 42:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flynn L, Byrne B, Carton J, Kelehan P, O'Herlihy C, O'Farrelly C. Menstrual cycle dependent fluctuations in NK and T‐lymphocyte subsets from non‐pregnant human endometrium. Am J Reprod Immunol 2000; 43:209–17. [DOI] [PubMed] [Google Scholar]
- 55. Bonatz G, Hansmann ML, Buchholz F, Mettler L, Radzun HJ, Semm K. Macrophage‐ and lymphocyte‐subtypes in the endometrium during different phases of the ovarian cycle. Int J Gynaecol Obstet 1992; 37:29–36. [DOI] [PubMed] [Google Scholar]
- 56. Garry R, Hart R, Karthigasu KA, Burke C. Structural changes in endometrial basal glands during menstruation. BJOG 2010; 117:1175–85. [DOI] [PubMed] [Google Scholar]
- 57. Bulmer JN, Sunderland CA. Immunohistological characterization of lymphoid cell populations in the early human placental bed. Immunology 1984; 52:349–57. [PMC free article] [PubMed] [Google Scholar]
- 58. King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3‐ leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol 1991; 1:169–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vento‐Tormo R, Efremova M, Botting RA, Turco MY, Vento‐Tormo M, Meyer KB et al Single‐cell reconstruction of the early maternal‐fetal interface in humans. Nature 2018; 563:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bartmann C, Segerer SE, Rieger L, Kapp M, Sütterlin M, Kämmerer U. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am J Reprod Immunol 2014; 71:109–19. [DOI] [PubMed] [Google Scholar]
- 61. Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest 2009; 119:2062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. King A, Birkby C, Loke YW. Early human decidual cells exhibit NK activity against the K562 cell line but not against first trimester trophoblast. Cell Immunol 1989; 118:337–44. [DOI] [PubMed] [Google Scholar]
- 63. Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F et al Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 2003; 198:1201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, et al Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci USA 2005; 102:15 563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vacca P, Cantoni C, Prato C, Fulcheri E, Moretta A, Moretta L, et al Regulatory role of NKp44, NKp46, DNAM‐1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int Immunol 2008; 20:1395–405. [DOI] [PubMed] [Google Scholar]
- 66. Siewiera J, El Costa H, Tabiasco J, Berrebi A, Cartron G, Le Bouteiller P et al Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog 2013; 9:e1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hanna J, Goldman‐Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson‐Yaron S et al Decidual NK cells regulate key developmental processes at the human fetal‐maternal interface. Nat Med 2006; 12:1065–74. [DOI] [PubMed] [Google Scholar]
- 68. Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF et al Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol 2006; 80:572–80. [DOI] [PubMed] [Google Scholar]
- 69. Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD et al Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012; 26:4876–85. [DOI] [PubMed] [Google Scholar]
- 70. Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O et al Maternal uterine NK cell‐activating receptor KIR2DS1 enhances placentation. J Clin Invest 2013; 123:4264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Croxatto D, Micheletti A, Montaldo E, Orecchia P, Loiacono F, Canegallo F et al Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal Immunol 2016; 9:1372–83. [DOI] [PubMed] [Google Scholar]
- 72. Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CWG, Carrington M, Trowsdale J et al Combinations of maternal KIR and fetal HLA‐C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004; 200:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A et al Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA‐C2. J Clin Invest 2010; 120:4102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nakimuli A, Chazara O, Hiby SE, Farrell L, Tukwasibwe S, Jayaraman J et al A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre‐eclampsia. Proc Natl Acad Sci USA 2015; 112:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kennedy PR, Chazara O, Gardner L, Ivarsson MA, Farrell LE, Xiong S et al Activating KIR2DS4 is expressed by uterine NK cells and contributes to successful pregnancy. J Immunol 2016; 197:4292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD et al Intraepithelial type 1 innate lymphoid cells are a unique subset of IL‐12‐ and IL‐15‐responsive IFN‐γ‐producing cells. Immunity 2013; 38:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Quillay H, El Costa H, Marlin R, Duriez M, Cannou C, Chrétien F et al Distinct characteristics of endometrial and decidual macrophages and regulation of their permissivity to HIV‐1 infection by SAMHD1. J Virol 2015; 89:1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Quillay H, El Costa H, Duriez M, Marlin R, Cannou C, Madec Y et al NK cells control HIV‐1 infection of macrophages through soluble factors and cellular contacts in the human decidua. Retrovirology 2016; 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. El Costa H, Quillay H, Marlin R, Cannou C, Duriez M, Benjelloun F et al The local environment orchestrates mucosal decidual macrophage differentiation and substantially inhibits HIV‐1 replication. Mucosal Immunol 2016; 9:634–46. [DOI] [PubMed] [Google Scholar]
- 80. Beaulieu AM. Memory responses by natural killer cells. J Leukoc Biol 2018; 104:1087–96. [DOI] [PubMed] [Google Scholar]
- 81. Gamliel M, Goldman‐Wohl D, Isaacson B, Gur C, Stein N, Yamin R et al Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity 2018; 48:951–62. [DOI] [PubMed] [Google Scholar]
- 82. Goldman‐Wohl D, Gamliel M, Mandelboim O, Yagel S. Learning from experience: cellular and molecular bases for improved outcome in subsequent pregnancies. Am J Obstet Gynecol 2019; 221:183–93. [DOI] [PubMed] [Google Scholar]
- 83. Feyaerts D, van der Meer A, Joosten I, van der Molen RG. Selective expansion and CMV‐dependency in pregnancy trained human endometrial NK cells. Cell Mol Immunol 2019; 16:410–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L et al Composition, development, and function of uterine innate lymphoid cells. J Immunol 2015; 195:3937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vacca P, Montaldo E, Croxatto D, Loiacono F, Canegallo F, Venturini PL et al Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol 2015; 8:254–64. [DOI] [PubMed] [Google Scholar]
- 86. Montaldo E, Vacca P, Chiossone L, Croxatto D, Loiacono F, Martini S et al Unique eomes(+) NK cell subsets are present in uterus and decidua during early pregnancy. Front Immunol 2016; 6:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nikoopour E, Bellemore SM, Singh B. IL‐22, cell regeneration and autoimmunity. Cytokine 2015; 74:35–42. [DOI] [PubMed] [Google Scholar]
- 88. Dambaeva S, Schneiderman S, Jaiswal MK, Agrawal V, Katara GK, Gilman‐Sachs A et al Interleukin 22 prevents lipopolysaccharide‐ induced preterm labor in mice. Biol Reprod 2018; 98:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maybin JA, Barcroft J, Thiruchelvam U, Hirani N, Jabbour HN, Critchley HO. The presence and regulation of connective tissue growth factor in the human endometrium. Hum Reprod 2012; 27:1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011; 1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Giudice LC. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics 2004; 4:299–312. [DOI] [PubMed] [Google Scholar]
- 92. Li C, Houser BL, Nicotra ML, Strominger JL. HLA‐G homodimer‐induced cytokine secretion through HLA‐G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci USA 2009; 106:5767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Apps R, Sharkey A, Gardner L, Male V, Kennedy P, Masters L et al Ex vivo functional responses to HLA‐G differ between blood and decidual NK cells. Mol Hum Reprod 2011; 17:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014; 5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gordon S, Pluddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol 2017; 15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. Two unique human decidual macrophage populations. J Immunol 2011; 186:2633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 2009; 200:e1–11. [DOI] [PubMed] [Google Scholar]
- 98. Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A et al Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod 2012; 86:39. [DOI] [PubMed] [Google Scholar]
- 99. Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol 2005; 66:161–73. [DOI] [PubMed] [Google Scholar]
- 100. Lee S, Kim J, Jang B, Hur S, Jung U, Kil K et al Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol 2010; 185:756–62. [DOI] [PubMed] [Google Scholar]
- 101. Rinaldi SF, Makieva S, Saunders PT, Rossi AG, Norman JE. Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol Hum Reprod 2017; 23:708–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM et al Human decidual tissue contains differentiated CD8+ effector‐memory T cells with unique properties. J Immunol 2010; 185:4470–7. [DOI] [PubMed] [Google Scholar]
- 103. van der Zwan A, Bi K, Norwitz ER, Crespo ÂC, Claas FHJ, Strominger JL, et al Mixed signature of activation and dysfunction allows human decidual CD8+ T cells to provide both tolerance and immunity. Proc Natl Acad Sci USA 2018; 115:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA et al CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol 1997; 158:3017–27. [PubMed] [Google Scholar]
- 105. Scaife PJ, Bulmer JN, Robson SC, Innes BA, Searle RF. Effector activity of decidual CD8+ T lymphocytes in early human pregnancy. Biol Reprod 2006; 75:562–7. [DOI] [PubMed] [Google Scholar]
- 106. Zeng W, Liu Z, Liu X, Zhang S, Khanniche A, Zheng Y et al Distinct transcriptional and alternative splicing signatures of decidual CD4+ T cells in early human pregnancy. Front Immunol 2017; 8:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, et al Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J Immunol 2017; 199:3406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Saito S, Tsukaguchi N, Hasegawa T, Michimata T, Tsuda H, Narita N. Distribution of Th1, Th2, and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol 1999; 42:240–5. [DOI] [PubMed] [Google Scholar]
- 109. Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot‐Swings GM et al Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(‐) T‐cells in decidua and maternal blood during human pregnancy. Placenta 2006; 27:S47–53. [DOI] [PubMed] [Google Scholar]
- 110. Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD et al TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16‐ NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA 2007; 104:3378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D et al Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci USA 2010; 107:11 918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Salvany‐Celades M, van der Zwan A, Benner M, Setrajcic‐Dragos V, Bougleux Gomes HA, Iyer V et al Three types of functional regulatory T cells control T cell responses at the human maternal‐fetal interface. Cell Rep 2019; 27:2537–47. [DOI] [PubMed] [Google Scholar]
- 113. Fan DX, Duan J, Li MQ, Xu B, Li DJ, Jin LP. The decidual gamma‐delta T cells up‐regulate the biological functions of trophoblasts via IL‐10 secretion in early human pregnancy. Clin Immunol 2011; 141:284–92. [DOI] [PubMed] [Google Scholar]
- 114. Terzieva A, Dimitrova V, Djerov L, Dimitrova P, Zapryanova S, Hristova I et al Early pregnancy human decidua is enriched with activated, fully differentiated and pro‐inflammatory gamma/delta T cells with diverse TCR repertoires. Int J Mol Sci 2019; 20:E687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Inada K, Shima T, Ito M, Ushijima A, Saito S. Helios‐positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J Reprod Immunol 2015; 107:10–9. [DOI] [PubMed] [Google Scholar]
- 116. Li J, Tan J, Martino MM, Lui KO. Regulatory T‐cells: potential regulator of tissue repair and regeneration. Front Immunol 2018; 9:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tsuda S, Nakashima A, Shima T, Saito S. New paradigm in the role of regulatory T cells during pregnancy. Front Immunol 2019; 10:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS et al Trophoblast organoids as a model for maternal‐fetal interactions during human placentation. Nature 2018; 564:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Turco MY, Gardner L, Hughes J, Cindrova‐Davies T, Gomez MJ, Farrell L et al Long‐term, hormone‐responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 2017; 19:568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


