Abstract
G protein-coupled receptor 68 (GPR68) is a proton sensor that is activated upon binding to extracellular protons. We have previously found that GPR68 induces a proapoptotic pathway in bone marrow (BM) cells from the patients with myelodysplastic syndromes (MDS) after treated with lenalidomide. However, the function of GPR68 in normal hematopoietic cells remains unclear. With genetic loss of function approach, we found reduced frequency and number of B lymphocytes in the peripheral blood (PB) of whole body Gpr68-/- mice compared to control littermates upon aging. During hematopoietic regeneration, such as in response to fluorouracil (5-FU), we also found reduced frequency and number of B lymphocytes in Gpr68-/- mice compared to wild type mice. Mechanism studies revealed that Gpr68 expression was upregulated in B lymphocytes of BM during aging and in hematopoietic progenitor cells after treatment with 5-FU. In addition, activation of Gpr68 by its activators increased the frequency and number of B lymphocytes. Our studies indicate that Gpr68 expression is upregulated in hematopoietic cells upon aging and during hematopoietic regeneration that ends up with increased number of B lymphocytes.
Keywords: G protein-coupled receptors, GPR68 agonist, GPR68 allosteric modulator, hematopoiesis, stress hematopoiesis, hematologic regeneration
Introduction
The bone marrow (BM) microenvironment has long been known to have low oxygen levels [1]. Local acidosis is frequently observed in areas of ischemia or hypoxia [2]. This prompts us to ask whether local acidosis regulates hematopoiesis and the molecular mechanism involved. The G protein-coupled receptor 68 (GPR68), also known as ovarian cancer G protein-coupled receptor 1 (OGR1), responds to extracellular acidosis, i.e. protons [3]. Upon activation, GPR68 couples to G protein q/11 (Gq/11), leading to activation of the phospholipase C/calcium (Ca2+) pathway [3,4]. In addition, GPR68 can also couple to G protein s (Gs), leading to activation of the adenylyl cyclase/cAMP pathway [5]. GPR68 is implicated in pleotropic pathophysiological processes, such as cancer, inflammation, bone absorption and steer stress response [6-9]. We have recently demonstrated that GPR68 expression is induced by lenalidomide [4], an immunomodulatory drug used for patients with myelodysplastic syndromes (MDS) and multiple myeloma [10,11]. Upregulation of GPR68 mediates a calcium/calpain proapoptotic pathway in MDS cells [4]. Despite GPR68’s various pathophysiological functions, genetic deletion of Gpr68 in mice (i.e. whole body Gpr68-/- mice) results in only a mild phenotype [12]. In particular, the Gpr68-/- mice display very marginal phenotypes in hematopoietic tissues, indicating a dispensable role of Gpr68 in hematopoiesis under steady state conditions. However, the potential roles of Gpr68 during aging and/or under stressed conditions are essentially unknown. In the present study, we examined the hematopoietic phenotype of Gpr68-/- mice during aging and under stressed conditions, i.e. during hematopoietic regeneration.
Materials and methods
Mice
Gpr68 knockout (KO, i.e. Gpr68-/-) mice (on a C57Bl/6 background) [12,13] and wild type (WT) mice (on a C57Bl/6 background) were bred, housed and handled in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility of University of South Carolina. Peripheral blood (PB) cells were collected from retro-orbital veins and measured with the VetScan HM5 (Abaxis). Bone marrow (BM) cells were harvested from tibia, femur and pelvic bones, and maintained in IMDM with 2% fetal bovine serum and 100 U/mL penicillin and streptomycin.
Injection
To study hematopoiesis under stress, a single dose of fluorouracil (5-FU, 50 mg/kg) was injected intraperitoneally into WT and Gpr68 KO mice [14]. To examine the effect of Gpr68 activators on hematopoiesis, Ogerin (10 mg/kg in saline) or 3,5-disubstituted isoxazole (Isx, 16 mg/kg in 20% w/v 2-hydroxypropyl-β-cyclodextrin) were injected intraperitoneally to WT mice for five consecutive days [15,16].
Cell culture
Lineage negative (Lin-) BM cells were enriched with EasySepTM Mouse Hematopoietic Progenitor Cell Enrichment Kit (StemCell Technologies, 19756) according to the manufacture’s recommendation. Antibodies labeling lineage positive cells include CD3, B220, CD11b, Gr1 and Ter119. Lin- cells were cultured in RPMI1640 media, supplemented with 10% FBS and 100 U/mL penicillin/streptomycin, 10 ng/mL mouse stem cell factor (Peprotech, 250-03), 10 ng/mL mouse interleukin 3 (Peprotech, 213-13), and 10 ng/mL human interleukin 6 (Peprotech, 200-06). Lin- cells were treated with 5-FU (10 μM) for 24 hours, followed by examination of Gpr68 mRNA.
Flow cytometry
For immunophenotypic analysis of lymphoid and myeloid cells, 20 μL PB samples were treated with 1 mL 1× red blood cell (RBC) lysis buffer (BD Biosciences, 555899) at 37° for 30 minutes. The cells were washed and incubated with antibodies, including CD11b (eBioscience, 15-0112-83), Gr1 (eBioscience, 48-5931-82), CD3 (eBioscience, 12-0031-83), B220 (eBioscience, 17-0452-81), at 4° for 30 minutes. The cells were then washed again before analysis with flow cytometer. Alternatively, BM cells and splenocytes were stained with CD3, B220, CD11b, Gr1 and Ter119 (eBioscience, 25-5921-82). To analyze Gpr68 expression, PB cells and BM cells were incubated with Gpr68 antibody (Alomone Labs, AGR-042), followed by staining with secondary antibody (Jackson ImmunoResearch, 111-096-144). Analysis was performed using NovoCyte Flow Cytometer with NovoExpress software.
Quantitative RT-PCR
Total RNA was extracted and purified using Quick-RNA MiniPrep (Zymo research, R1055) and reverse transcription was carried out using SuperScript VILO cDNA Synthesis Kit (Invitrogen). Quantitative PCR was performed with Taqman Master Mix (Life Technologies) for mGapdh (Cat 4331182, Assay ID Mm99999915_g1, Applied Biosystems) and mGpr68 (Cat 4331182, Assay ID Mm00558545_s1, Applied Biosystems).
Statistical analysis
Results are shown the mean ± s.e.m. Student’s t-test was used for all the results with GraphPad Prism (v7, GraphPad).
Results
Deletion of Gpr68 reduces the number of B lymphocytes in older mice
Previous studies have shown that whole body Gpr68 knockout (KO, Gpr68-/-) mice on a mixed genetic background exhibit similar hematopoietic output as compared to the wild type (WT, Gpr68+/+) mice [12]. To address the potential influence from genetic background, we characterized the hematopoietic phenotype of whole body Gpr68 KO mice on a congenic genetic background (i.e. C57Bl/6). Successful deletion of Gpr68 and reduced Gpr68 expression was confirmed by genotyping and quantitative RT-PCR (Figure S1A, S1B). Complete blood count (CBC) revealed comparable levels of white blood cells (WBC) in peripheral blood (PB) from Gpr68 KO and WT mice at either younger (2-month old) or older age (12-month old, Figure 1A). Analysis of cell surface immunophenotype revealed reduced frequencies of B220+ B lymphocytes but increased frequencies of CD11b+ myeloid cells (including neutrophils and monocytes) in PB from Gpr68 KO mice than those from WT mice at older age (12-month old, Figure 1B, 1C). Particularly, the number of B lymphocytes largely reduced in PB from Gpr68 KO mice than those from WT mice at older age (12-month old, Figure 1D). Consistently, the frequencies of B lymphocytes were also reduced in bone marrow (BM) and spleens of Gpr68 KO mice compared with WT mice at 12-month of age (Figure 1E). In contrast, the number of myeloid cells only slightly increased in PB from Gpr68 KO mice than those from WT mice at older age (12-month old, Figure 1D). The levels of CD3+ T lymphocytes, red blood cells (RBC), hemoglobin or platelets were comparable in PB from WT and Gpr68 KO mice (Figures 1B-D, S1C-E). These data suggest that Gpr68 increases the number of B lymphocytes in PB during aging.
Figure 1.
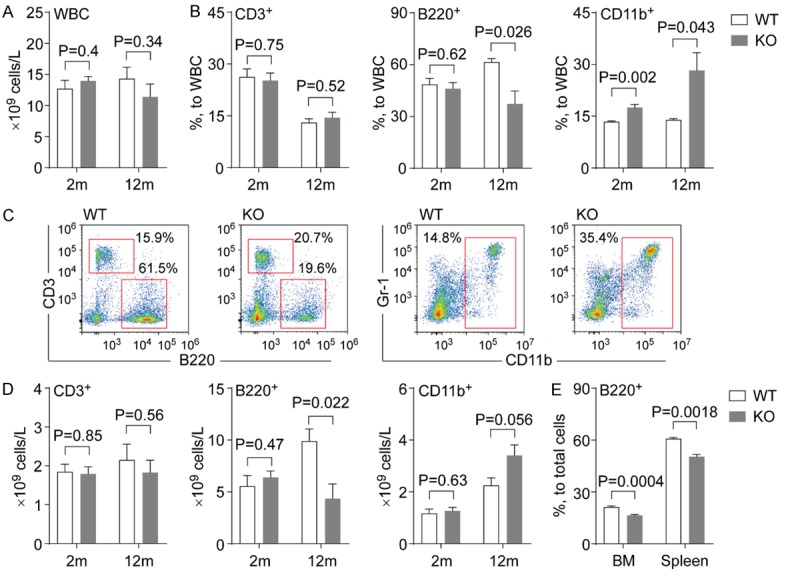
Deletion of Gpr68 reduces the number of B lymphocytes. A. Number of WBC in PB from WT and Gpr68 KO mice at 2-month old (2 m) and 12-month old (12 m) (n=4~9). B. Frequencies of CD3+ T cells (left), B220+ B cells (middle) and CD11b+ myeloid cells (right) in PB from WT and Gpr68 KO mice at 2-month old (2 m) and 12-month old (12 m) (n=4~5). C. Representative flow cytometric analysis of CD3+ T cells, B220+ B cells and CD11b+ myeloid cells in PB from WT and Gpr68 KO mice at 12-month old. D. Number of CD3+ T cells (left), B220+ B cells (middle) and CD11b+ myeloid cells (right) in PB from WT and Gpr68 KO mice at 2-month old (2 m) and 12-month old (12 m) (n=4~5). E. Frequencies of B220+ B cells in BM and spleens from WT and Gpr68 KO mice at 12-month old (n=3~5).
Deletion of Gpr68 reduces the number of B lymphocytes in response to 5-FU
Fluorouracil (5-FU), a chemotherapeutic agent, mediates cytotoxicity of hematopoietic progenitor cells, and has been commonly used to study hematopoietic regeneration after stress [14]. To explore a potential function of Gpr68 during hematopoietic regeneration, a single dose of 5-FU (50 mg/kg) was injected into WT or Gpr68 KO mice. As expected, 5-FU reduced the number of WBC, followed by recovery of WBC count a week after 5-FU injection, indicating hematopoietic regeneration (Figure 2A). Compared to WT mice, Gpr68 KO mice had a slower regeneration rate as evidenced by lower numbers of WBC at day 3 and day 10 post 5-FU injection (Figure 2A). We also examined the frequencies of the lymphoid and myeloid lineages in WT and Gpr68 KO mice after 5-FU injection. At day 7 post 5-FU injection, the frequencies of B lymphocytes were lower, while the frequencies of myeloid cells were higher in PB from Gpr68 KO mice compared to those from WT mice (Figure 1B, 1C). In particular, the number of B cells was reduced in PB from Gpr68 KO than those from WT mice day 10 post 5-FU injection (Figure 2D). The levels of T lymphocytes, RBC, hemoglobin or platelets were comparable in PB from WT and Gpr68 KO mice after 5-FU injection (Figures 2B-D, S2A-C). Our findings suggest that Gpr68 increases the number of B cells in PB during hematopoietic regeneration.
Figure 2.

The effect of 5-FU on PB cells from WT and Gpr68 KO mice. A. Number of WBC in PB from WT and Gpr68 KO mice after 5-FU injection at the indicated time points (n=5). B. Frequencies of CD3+ T cells (left), B220+ B cells (middle) and CD11b+ myeloid cells (right) in PB from WT and Gpr68 KO mice after 5-FU injection at the indicated time points (n=5). C. Representative flow cytometric analysis of CD3+ T cells, B220+ B cells and CD11b+ myeloid cells in PB from WT and Gpr68 KO mice at day 7 post 5-FU injection. D. Number of CD3+ T cells (left), B220+ B cells (middle) and CD11b+ myeloid cells (right) in PB from WT and Gpr68 KO mice after 5-FU injection at the indicated time points (n=5). #, P<0.1; *, P<0.05.
Gpr68 expression is upregulated in older mice and in response to 5-FU
It has been demonstrated that overexpression of G protein-coupled receptors increases their basal level of activation [17]. This prompts us to examine the expression of Gpr68 in hematopoietic cells during aging and upon stress. Previous studies revealed that Gpr68 mRNA was detectable in PB cells [12]. As expected, Gpr68 protein was expressed at higher levels in PB cells (mean fluorescence intensity ~105), including T lymphocytes, B lymphocytes and myeloid cells (Figure 3A, 3B). In contrast, Gpr68 protein was expressed at much lower levels in BM hematopoietic cells (mean fluorescence intensity ~104), including T lymphocytes, B lymphocytes, myeloid cells and Ter119+ erythroid cells (Figure 3C). Notably, Gpr68 expression was upregulated in B lymphocytes and myeloid cells in BM of older mice (20-month old) than younger mice (5-month old) (Figure 3C). In addition, Gpr68 mRNA was also upregulated in BM lineage negative (Lin-) hematopoietic progenitor cells after treated with 5-FU (Figure S2D). These data suggest that aging and regeneration are two conditions to induce Gpr68 expression, indicating increased level of Gpr68 activation.
Figure 3.
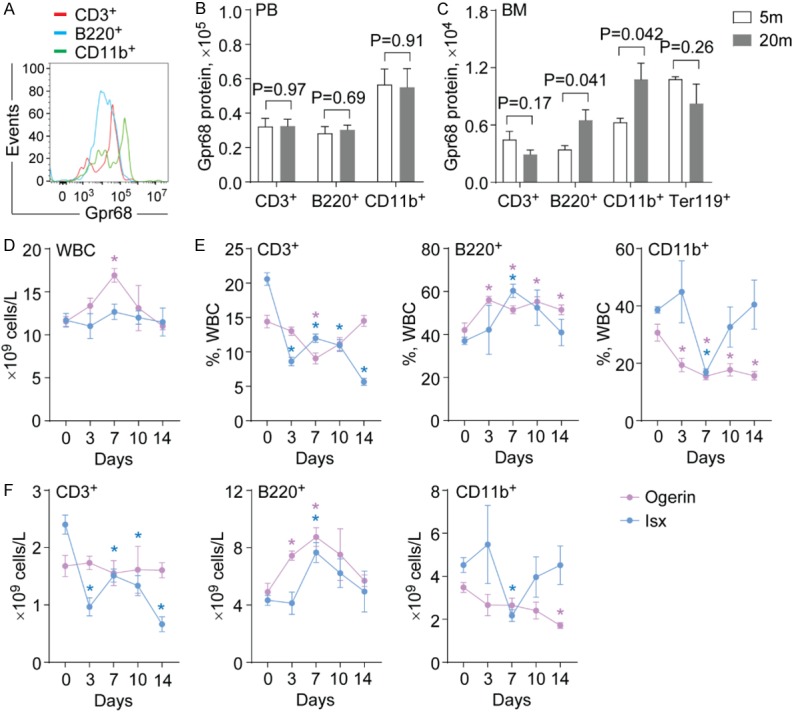
The effect of Gpr68 activators on hematopoietic output. (A) Representative flow cytometric analysis of Gpr68 expression in PB cells from WT mice. (B, C) Gpr68 protein expression in PB cells (B) and BM cells (C) from WT mice at 5-month old (5 m) and 20-month old (20 m) (n=4). (D) Number of WBC in PB from WT mice after injection with Ogerin (red) or Isx (blue) at the indicated time points (n=5). (E) Frequencies of CD3+ T cells (left), B220+ B cells (middle) and CD11b+ myeloid cells (right) in PB from WT mice after injection with Ogerin (red) or Isx (blue) at the indicated time points (n=5). (F) Number of CD3+ T cells (left), B220+ B cells (middle) and CD11b+ myeloid cells (right) in PB from WT mice after injection with Ogerin (red) or Isx (blue) at the indicated time points (n=5). *, P<0.05.
Activation of Gpr68 increases the number of B lymphocytes
To determine whether GPR68 was functionally involved, we used two GPR68 modulators that have been shown to activate GPR68 function. Ogerin is demonstrated as a positive allosteric modulator for GPR68, activating either the cAMP or the Ca2+ pathway in the presence of extracellular protons [9,16]. 3,5-disubstituted isoxazole (Isx) acts as an agonist of GPR68 that activates the Ca2+ pathway irrespective of pH [15]. To examine the effects of Ogerin and Isx on hematopoiesis, we injected Ogerin (5 mg/kg daily) or Isx (16 mg/kg daily) into WT mice for 5 consecutive days. We found increased number of WBC in PB from WT mice after Ogerin injection (Figure 3D). In contrast, the number of WBC was unaltered in PB from WT mice treated with Isx (Figure 3D). The frequencies of B lymphocytes were increased, while the frequencies of T lymphocytes and myeloid cells were reduced in PB from WT mice after Ogerin injection (Figure 3E). Isx injection induced similar effects (Figure 3E). Accordingly, the number of B lymphocytes was increased in PB of WT mice after injection with Ogerin or Isx (Figure 3F). Injection with Isx but not Ogerin reduced the number of T cells (Figure 3F). Both Ogerin and Isx injection reduced the number of myeloid cells (Figure 3F). Consistent with Gpr68 overexpression data, our studies indicate that Gpr68 activation increases the number of B lymphocytes, possibly through the Ca2+ pathway.
Discussion
The bone marrow microenvironment has long been known as hypoxic that leads to local acidosis [1,2]. Whether local acidosis will regulate hematopoiesis is unclear. Given that GPR68 responds to extracellular protons [3], we examined whether GPR68 regulates hematopoiesis with a genetic loss of function approach. Under a mixed genetic background, deletion of Gpr68 resulted in a slight increase in the number of neutrophils under steady state [12]. Consistently, we found that deletion of Gpr68 under a pure genetic background also resulted in a slight increase in the number of myeloid cells in PB of older mice. More importantly, the number of B lymphocytes were significantly reduced in PB, BM and spleens from older Gpr68 KO mice compared to the age matched WT mice, indicating reduced development of B lymphocytes upon aging due to deletion of Gpr68. In addition, when we used 5-FU to stress hematopoietic cells, we also found reduced number of B lymphocytes in PB from Gpr68 KO mice compared to WT mice. These data suggest that Gpr68 increases the number of B lymphocytes under stressed conditions, such as aging and hematopoietic regeneration.
Intriguingly, we found increased Gpr68 expression in B lymphocytes and myeloid cells in BM from older WT mice compared to younger WT mice. We also found increased Gpr68 transcript in BM hematopoietic progenitor cells in response to 5-FU treatment. Given that overexpression of GPR68 will increase its activity [4,17], our data suggest that activation of Gpr68 promotes the development of B cells at the expense of myeloid cells. To further validate this observation, we treated WT mice with two Gpr68 modulators, Ogerin and Isx, that activates Gpr68 function. Consistent with Gpr68 overexpression, activation of Gpr68 in vivo increases the number of B lymphocytes in PB. Given that both Ogerin and Isx activates Grp68-mediated Ca2+ pathway, our data suggest that Gpr68 favors lymphopoiesis towards B cell lineage through activating a Ca2+ pathway. However, the downstream signaling events need further study.
Our studies suggest that local acidosis of the BM microenvironment could impact on hematopoiesis, partly through a proton sensor Gpr68. Overexpression or activation of Gpr68 increases the number of B lymphocytes, possibly through activating the Ca2+ pathway. The Gpr68 modulators are useful tools to understand the pathophysiological function of GPR68 in vivo. However, the cell and molecular mechanism of how Gpr68 regulates B cell development and whether Gpr68 regulates B cell function needs further study.
Acknowledgements
This work was supported by NIH (R01CA218076), NIH COBRE 1P20GM109091-01, St. Baldrick’s Foundation and Aplastic Anemia & MDS International Foundation. We thank the animal facility of University of South Carolina (USC), Center for Colon Cancer Research (supported by NIH 5 P30 GM103336) of USC, and the Instrumentation Resource Facility at USC School of Medicine for their help with mouse work. We thank Dr. Michael Wyatt for his advices on the manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine RL. Ischemia: from acidosis to oxidation. FASEB J. 1993;7:1242–1246. doi: 10.1096/fasebj.7.13.8405809. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Liu X, Bolanos L, Barker B, Rigolino C, Cortelezzi A, Oliva EN, Cuzzola M, Grimes HL, Fontanillo C, Komurov K, MacBeth K, Starczynowski DT. A calcium- and calpain-dependent pathway determines the response to lenalidomide in myelodysplastic syndromes. Nat Med. 2016;22:727–734. doi: 10.1038/nm.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomura H, Wang JQ, Komachi M, Damirin A, Mogi C, Tobo M, Kon J, Misawa N, Sato K, Okajima F. Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem. 2005;280:34458–34464. doi: 10.1074/jbc.M505287200. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza CA, Zhao FL, Li X, Xu Y, Dunn SE, Zhang L. OGR1/GPR68 modulates the severity of experimental autoimmune encephalomyelitis and regulates nitric oxide production by macrophages. PLoS One. 2016;11:e0148439. doi: 10.1371/journal.pone.0148439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieger NS, Yao Z, Kyker-Snowman K, Kim MH, Boyce BF, Bushinsky DA. Increased bone density in mice lacking the proton receptor OGR1. Kidney Int. 2016;89:565–573. doi: 10.1016/j.kint.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol. 2013;4:354. doi: 10.3389/fphys.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Mathur J, Vessieres E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J, Lee V, Xiang FL, Mainquist JK, Cahalan SM, Orth AP, Walker JR, Ma S, Lukacs V, Bordone L, Bandell M, Laffitte B, Xu Y, Chien S, Henrion D, Patapoutian A. GPR68 senses flow and is essential for vascular physiology. Cell. 2018;173:762–775. e716. doi: 10.1016/j.cell.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talati C, Sallman D, List A. Lenalidomide: myelodysplastic syndromes with del(5q) and beyond. Semin Hematol. 2017;54:159–166. doi: 10.1053/j.seminhematol.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Moreau P, Zamagni E, Mateos MV. Treatment of patients with multiple myeloma progressing on frontline-therapy with lenalidomide. Blood Cancer J. 2019;9:38. doi: 10.1038/s41408-019-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Wang D, Singh LS, Berk M, Tan H, Zhao Z, Steinmetz R, Kirmani K, Wei G, Xu Y. Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PLoS One. 2009;4:e5705. doi: 10.1371/journal.pone.0005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan L, Singh LS, Zhang L, Xu Y. Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene. 2014;33:157–164. doi: 10.1038/onc.2012.566. [DOI] [PubMed] [Google Scholar]
- 14.Hou Y, Li W, Sheng Y, Li L, Huang Y, Zhang Z, Zhu T, Peace D, Quigley JG, Wu W, Zhao YY, Qian Z. The transcription factor Foxm1 is essential for the quiescence and maintenance of hematopoietic stem cells. Nat Immunol. 2015;16:810–818. doi: 10.1038/ni.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell JL, Goetsch SC, Aguilar HR, Coe H, Luo X, Liu N, van Rooij E, Frantz DE, Schneider JW. Regulated expression of pH sensing G protein-coupled receptor-68 identified through chemical biology defines a new drug target for ischemic heart disease. ACS Chem Biol. 2012;7:1077–1083. doi: 10.1021/cb300001m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang XP, Karpiak J, Kroeze WK, Zhu H, Chen X, Moy SS, Saddoris KA, Nikolova VD, Farrell MS, Wang S, Mangano TJ, Deshpande DA, Jiang A, Penn RB, Jin J, Koller BH, Kenakin T, Shoichet BK, Roth BL. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature. 2015;527:477–483. doi: 10.1038/nature15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Ligt RA, Kourounakis AP, AP IJ. Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discovery. Br J Pharmacol. 2000;130:1–12. doi: 10.1038/sj.bjp.0703311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


