Abstract
Although the hypotheses, analyses, and results of this study are novel, other study findings from this project have been published. Please see references numbered 6–7,9, and 16 for more information.
1.0. Introduction
Chronic pain is associated with elevated negative emotions [1,10]. Negative emotion experiences (e.g., sadness, anxiety and anger) are ordinarily considered aversive and motivate avoidance behavior, but they can also be functional and motivate adaptive behavior [12,19]. Such a process requires adaptive regulation of negative emotions, but evidence suggests that the resources needed to adaptively regulate emotions can be depleted during prolonged pain and fatigue [1,25]. Wide or frequent fluctuations in negative emotions -- negative emotion variability – may be a consequence of diminished emotion regulation means, and may be related to maladaptive outcomes for people suffering from chronic pain [25,27].
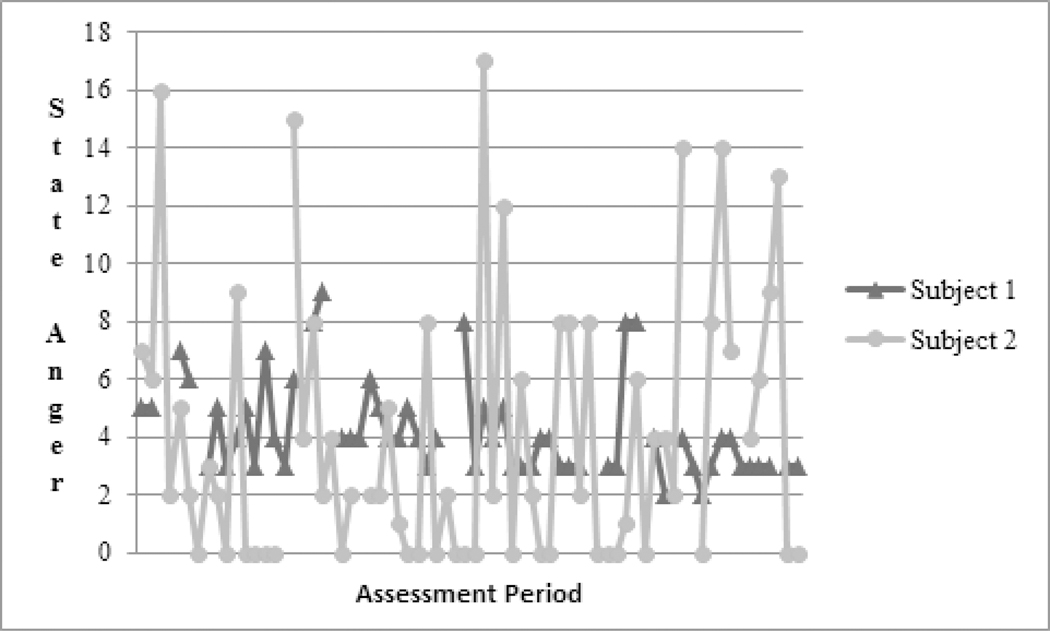
Studies of links between pain, function and negative emotions in people with chronic pain have focused mostly on relationships among mean levels of these factors. That is, these studies report only that higher levels of pain are related to higher levels of negative emotions [28]. Indexes that may reflect aspects of emotion regulation have typically not been analyzed, with rare exceptions [13, 27]. We propose that variability in emotion over time, as opposed to average emotion over time, is an index more sensitive to the detrimental effects of chronic pain on emotional regulatory capacity. Electronic daily diary methods employing repeated assessments over the course of hours or days, can be used to capture measures of central tendency (e.g., mean scores for negative emotion collapsed over observations), and indexes of variability (e.g., variance of negative emotions across many observations, viz., Stone, 2012[30]). Figure 1 illustrates the distinction between level and variability. Both subjects depicted in Figure 1 have mean state anger scores of approximately 4, but differ considerably in the variability of their anger over time. Although these individuals report similar average levels of anger, the regularity of their anger is quite different. Indeed, levels of depressive symptoms can predict variability in pain intensity beyond the prediction of simple mean levels of pain intensity, suggesting that variability may relate independently to the well-being of people with chronic pain [27].
Figure 1.
Raw State Anger Scores of Two Subjects. Although both subjects have similar mean state anger scores, there is substantially more variability in the state anger scores reported by subject 2.
The current study is based on daily electronic diary data. People with chronic low back pain (CLBP; n = 104) and their pain-free spouses (n = 104) completed diary measures five times per day for 14 consecutive days, producing 70 observations per subject from which we estimated relationships between within-subject variability in negative emotions and both pain intensity and function (pain-related impairments in ability to engage in physical activities). Because people with chronic pain may experience continuing erosion of emotion regulation resources, we hypothesized that individuals with CLBP would not only report significantly greater mean levels of negative emotions, but would also evidence significantly greater variability in negative emotions (i.e., greater variances) than their pain-free spouses. We further hypothesized that both patient-reported and spouse-observed pain and pain interference would be significantly associated with greater variability in negative emotions among patients with CLBP. Following Schneider et al. [28], we also tested the converse - whether mean levels of negative emotions were significantly related to variability in pain and pain interference.
2.0. Materials and methods
2.1. Subjects
The study methods have been reported previously [6–9,20] One hundred and twenty-one married couples were recruited through referrals from staff at the pain clinics of Rush University Medical Center in Chicago, IL, Duke University Medical Center in Durham, NC, Memorial Hospital in South Bend, IN, and through advertisements in local newspapers and flyers provided at various health care agencies. Each participant received $150. The protocol was approved by the Institutional Review Boards at Rush University Medical Center, Duke University Medical Center, and University of Notre Dame.
Patient inclusion criteria were: a) pain of the lower back stemming from degenerative disk disease, spinal stenosis, or disk herniation (radiculopathy subcategory), or muscular or ligamentous strain (chronic myofascial pain subcategory); b) pain duration of at least 6 months with an average intensity of at least 3/10 (with 0 being “no pain” and 10 “the worst pain possible”); and c) age between 18 and 70 years. The inclusion criterion for spouses was age between 18 and 70 years.
Exclusion criteria for both patients and spouses were: a) current alcohol or substance abuse problems, or meeting DSM-IV criteria for alcohol or substance abuse or dependence (within the past 12 months); b) past or current psychotic or bipolar disorders; c) inability to understand English well enough to complete questionnaires; d) acute suicidality; and e) meeting criteria for obsessive-compulsive disorder or posttraumatic stress disorder within the past 2 years. A further exclusion criterion for patients was if their pain complaint was due to malignant conditions (e.g., cancer, rheumatoid arthritis), migraine or tension headache, fibromyalgia, or complex regional pain syndrome. A further exclusion criterion for spouses was if they reported currently suffering from a condition that caused recurrent episodes of acute pain (e.g., migraine headaches) or reported a history of chronic pain within the past 12 months.
Inclusion and exclusion criteria were assessed through a medical and psychosocial history, including administration of the Mood Disorder, Psychotic Screening, and Substance Use Disorders modules of the Structured Clinical Interview for DSM-IV Axis I Disorders - Non-Patient Edition (SCID-IV/NP) [18].
Of the 121 couples recruited, eight couples declined to participate, three couples withdrew before completing 14 days of data collection, four couples lost data due to PDA malfunctions, and one couple’s data were lost due to failure to upload it from the PDA at an appropriate time. Thus, the final sample included 105 couples. Women patients comprised 48.6% of the sample (n = 51). Demographic characteristics of couples not included in this investigation did not differ significantly from those who were included (see Table 1). The majority of patients (91.4%) reported taking pain medications during at least one diary assessment. On average, patients reported taking pain medications during approximately one-third of assessment waves.
Table 1.
Demographic Characteristics
| Patient | Spouse | |
|---|---|---|
| Gender (female) | 48.6% (n = 51) | 51.4% (n = 54) |
| Age in years (M, SD) | 46.30 (12.1) | 45.96 (13.2) |
| Hispanic | 4.8% (n = 5) | 5.7% (n= 6) |
| African American | 15.2% (n = 16) | 18.1% (n = 19) |
| Caucasian | 80.0% (n = 84) | 76.2% (n = 80) |
| Employed | 40.0% (n = 42) | 63.8% (n = 67) |
| Disability Insurance | 34.3% (n = 36) | 13.3% (n = 14) |
| Length of Marriage (M, SD) | 14.30 (14.0) | --- |
| Pain Duration (M, SD) | 9.04 years (7.8) | --- |
2.2. Electronic Diary
The PDA program signaled participants to complete five assessments each day, starting at 8:50 am and occurring every three hours until 8:50 pm. Frequent assessments helped minimize retrospective bias in ratings [27]. Daily diary data obtained in this manner also appear to suffer little from reactivity effects that are sometimes caused by monitoring [22,26]. Variability in ratings within the day is also captured well by this method [26]. Previous studies support the reliability, validity, and compliance with electronic diary strategies when used to assess pain, affect, and behavior [16,22,26,31]. Electronic diaries with time-stamped entries also allowed us to accurately assess when ratings were made, something that cannot be done with paper diary methods [26]. Both patients and spouses completed electronic diary measures for 14 consecutive days. We used the Experience Sampling Program (ESP) [4] on handheld Palm® Zire 22 PDAs, running the Palm OS platform. The PDA program blocked participants from altering the items or alarm times.
2.3. Measures
2.3.1. Patient-reported pain-related variables.
At each assessment, patients rated pain intensity (“how intense was your pain”), pain interference (“to what degree did your pain interfere with you being physically active”) and downtime (“how much did you rest (sit, lie down) because of your pain”) during the past 3 hours. Responses were made on 9-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 8 (extremely). Mean pain intensity was correlated with mean pain interference r = .67 (p < .001), and with mean downtime, r = .35 (p < .001), while mean pain interference and mean downtime (rest) were correlated r = .39 (p < .001) with each other. Although all items were significantly intercorrelated, the magnitude of these associations suggests that each item also carries sufficient distinct variance to warrant being treated separately in subsequent analyses.
2.3.2. Patient and spouse state negative emotions.
At each assessment, patients and spouses rated the extent to which they felt anxious, on edge, uneasy, sad, helpless, discouraged, irritated, annoyed and angry during the past 3 hours [15]. Responses were made on 9-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 8 (extremely). A principal components analysis extracted a single negative emotions factor that accounted for 66% of the total variance in the nine patient-reported negative emotion items, and 60% of the total variance in spouse-reported negative emotion items. As a result, planned analyses focused on a composite measure of negative emotion reflecting the sum of these items.
2.3.3. Spouse-observed patient pain-related variables.
At each assessment, spouses were asked, “Did you observe your spouse during the past 3 hours?” If they responded, “yes,” then the diary software activated a branching algorithm through which spouses were asked about aspects of the patients’ pain. These items were: “how much pain did he/she appear to be in,” “how physically active was he/she,” “how many ‘pain behaviors’ (complaining, grimacing, etc.) did you hear or see?” [7]. Responses were made on 9-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 8 (extremely), except for the pain behavior item which used anchors, 0 (none), 2 (a few), 4 (some), 6 (many), and 8 (very many).
2.4. Procedure
Patients and spouses who inquired about participation underwent screening procedures over the telephone. Eligible patients and spouses attended an initial session, during which they signed consent forms to participate and completed questionnaires. Patients and spouses were instructed to carry the PDAs with them throughout the day for 14 consecutive days. Research assistants described and defined terms contained in the diary items for participants and provided them with printed instructions as well. Participants were also given printed versions of these instructions for later reference and were asked to phone the research assistants with any problems or questions.
Starting at 8:50 am, and then again every three hours until 8:50 pm, participants were prompted by the PDA alarm to complete assessments. Participants had 15 minutes following this alert in which to respond to the PDA and the diary items. After the initial alarm, the PDA would emit a signal every 30 seconds until participants responded. Participants were also given the option to tap the screen to dismiss the alarms and delay the signals as long as they completed the assessment within 15 minutes. If participants did not respond in any way within 15 minutes of the original prompt, the time period was coded as missing data. The data for each assessment session was time stamped. After 14 days of data collection, participants returned the PDA, data were downloaded, and participants were debriefed.
2.5. Data Preparation
All item responses submitted past the 15-minute response interval were discarded. After deleting these responses, out of the 7,350 total possible diary responses, 80.01 – 87.06% (across measures) were complete. This amount of complete data is in the range typically found in other electronic diary studies involving pain patients [29]. Spouses reported interacting with patients during 72.4% of answered intervals. All analyses involving spouse-observed variables used data obtained when the spouse reported interacting with the patient.
2.6. Statistical Analysis
Data were modeled using Location Scale models within the Mixregls package for R [21]. “Mixregls” uses maximum likelihood estimation to estimate both means and variances in nested data. For the current study, “variability” was operationalized as the within-subject variances in negative emotion (composite variable), pain and function. We again refer to Figure 1. In this figure, values in state anger of two subjects (from the diary data analyzed in this study) are displayed. Each subject provided 70 observations over 14 consecutive days. Both subjects have mean anger scores of approximately 4, yet these participants differ considerably in the variability of their anger over time. The variance in anger scores for subject 1 is 2.69, and the variance for subject 2 is 21.44 indicating that the latter subject has considerably more anger variability than the former subject.
To ascertain whether within-person variability in negative emotion is indicative of abrupt successive change, the Mean Square of Successive Difference (MSSD) was computed for each study variable (see Rost [27]). Correlation coefficients were generated between MSSD values and the within-person standard deviation (WSSD). MSSD and WSSD values for patient negative emotion, pain intensity, pain interference, and downtime were strongly correlated (rs = .86 to .92, ps < .001). MSSD and WSSD values for spouse reports were also strongly correlated (rs = .62 to .69, ps < .001). Thus, greater within-person variability in these variables tends to reflect greater abrupt change. To analyze this within-person variability as a dependent variable, Location Scale models have been developed as an application of mixed modeling [21]. This analytic strategy can be used to simultaneously models means, between-subject variability, and within-subject variability as dependent variables. Thus, the Location Scale approach may offer a benefit over MSSD in that it can be used to characterize the relationships of pain-related variables not only with regard to variability in negative emotion but also with regard to mean levels in negative emotion, and the manner in which means and variability in negative emotion are related (e.g. negative emotion may become more variable at higher average levels). We examined whether patients and spouses differed on emotional variability by regressing within-person variances in negative emotions on a dichotomous patient/spouse variable. We next examined whether mean levels of patient pain and function were related to variability in patient negative emotions by regressing within-subject variances in negative emotions (emotional variability) on mean levels of pain intensity, pain interference and downtime. Estimates of effect size, expressed as percentages of increased variability per unit of increases in pain intensity, pain interference and downtime, were computed by exponentiating beta coefficients for the within-person models. These analyses were repeated for spouse observations of patient pain intensity, pain behaviors and physical activity predicting variability in patient negative emotion. We then examined the converse models for patients only. That is, we tested whether mean levels of patient negative emotion were related to variability in pain and function by regressing within-subject variances in pain intensity, pain interference and downtime on mean levels of negative emotions. Time since the start of the study was included as a covariate in each model to account for possible reactivity or trending over the course of the two-week study period.
The Location Scale Models also provide estimates of two random effects. A Random Location effect is computed to evaluate whether the mean level of a variable is related to the variability of this variable. For instance, for reasons described previously it is possible that participants with higher average levels of negative emotions might also experience greater variability in those emotions. Finally, a Random Scale Standard Deviation effect is computed to ascertain whether individuals vary with regard to how their scores are distributed after accounting for other effects in the model. A significant Random Scale Standard Deviation indicates that individual differences in variation remain to be accounted for.
3.0. Results
3.1. Comparison of Negative Emotion Variability between Patients and Spouses
Table 2 presents results of Model 2 (Covariate) and Model 3 (Covariate and Random Effects) Location Scale models that regressed means and within-subject variances in negative emotions on patient/spouse status. Model 1 (Intercept-Only) was omitted from presentation to simplify the tables. In all models at all stages, longer time in study was associated with lower mean levels and slightly less variability (~−1%) in negative emotions. In the second stage model patients reported both significantly higher levels of negative emotion (p<.001) and 46.7% more variability in negative emotion (p = .05) than their spouses. Review of Random Location coefficients shown under the Random Effects heading in Table 2 also provides information about the association of mean levels of each negative emotion with its variability. Although average levels of negative emotion were significantly related to greater variability in these emotions (B = .92, p <.001), Random Scale effects indicate that significant variability in each negative emotion remained unaccounted for by the current models. In sum, patients demonstrated greater variability in negative emotion compared to their spouses even after accounting for differences in their mean levels of negative emotions.
Table 2.
Location Scale Models Comparing Levels and Variability in Negative Emotions across Patients and Spouses
| Negative Affect Model 2: Inclusion of Covariates | ||||
| B | SE | z | p | |
| Means | ||||
| Intercept | 6.28 | .83 | 7.55 | .000 |
| Time | .00 | .00 | −6.87 | .000 |
| Patient | 4.98 | 1.16 | 4.28 | .000 |
| Between Subject Variance | ||||
| Intercept | 4.25 | .10 | 42.93 | .000 |
| Within Subject Variance | ||||
| Intercept | 4.17 | .03 | 145.33 | .000 |
| Time | .00 | .00 | −12.04 | .000 |
| Patient | .26 | .03 | 10.46 | .000 |
| Negative Affect Model 3: Inclusion of Random Effects | ||||
| B | SE | z | p | |
| Means | ||||
| Intercept | 5.83 | .80 | 7.27 | .000 |
| Time | .00 | .00 | −8.04 | .000 |
| Patient | 4.83 | 1.13 | 4.27 | .000 |
| Between Subject Variance | ||||
| Intercept | 4.20 | .10 | 41.22 | .000 |
| Within Subject Variance | ||||
| Intercept | 3.48 | .14 | 24.54 | .000 |
| Time | <.01 | .00 | −15.64 | .000 |
| Patient | .39 | .20 | 1.95 | .051 |
| Random Effects | ||||
| Random Location (mean and variability) | .92 | .09 | 10.33 | .000 |
| Random Scale (remaining variability) | 1.08 | .06 | 19.53 | .000 |
3.2. Associations Between Patient-Reported Mean Levels of Pain Intensity, Interference and Downtime and Negative Emotion Variability
Using Location Scale modeling, we regressed both mean levels and variability in Negative Emotion scores on measures of mean levels of pain intensity, pain interference and downtime for the patient sample (see Table 3). Random Location effects for each model indicated that higher negative emotion among patients was related significantly to greater variability in negative emotion (B range .75 to .85; p’s <.001). Results also show that mean levels of pain intensity ( B = 5.91, p < .001), pain interference ( B = 4.61, p < .001), and downtime ( B = 5.45, p < .001) were related to both higher mean levels of Negative Emotion, and also to higher levels of variability in Negative Emotion scores (Table 3, Model 3). Thus, the coefficients under the Within Subject Variance Model heading in Table 3 reflect the association of the Negative Emotion variability with mean levels of pain intensity, pain interference and downtime after accounting for mean levels of Negative Emotion scores. Specifically, a standard unit increase in mean pain intensity was associated with a 79% (exp(B = .58) = 1.79, p < .001) increase in Negative Emotion variability, a standard unit increase in mean pain interference was associated with a 65% ( exp(B = .50) = 1.65, p<.001) increase in Negative Emotion variability, and a standard unit increase in mean downtime was associated with a 55% (exp (B = .44) = 1.55, p < .001) increase in Negative Emotion variability. Thus, greater mean levels of pain intensity, pain interference and downtime were associated with greater fluctuations in negative emotions over and above their association with mean levels of negative emotions.
Table 3.
Location Scale Models of Negative Emotion as a Function of Pain Intensity, Pain Interference and Downtime
| DV: Negative Affect | DV: Negative Affect | DV: Negative Affect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | z | p | B | SE | z | p | B | SE | z | p | |||
| Means | ||||||||||||||
| Intercept | 10.26 | .81 | 12.71 | .000 | Intercept | 10.28 | .89 | 11.59 | .000 | Intercept | 10.28 | .85 | 12.13 | .000 |
| Time | −.33 | .04 | −7.29 | .000 | Time | −.33 | .04 | −7.31 | .000 | Time | −.33 | .04 | −7.30 | .000 |
| Intensity | 5.91 | .80 | 7.42 | .000 | Interference | 4.61 | .88 | 5.23 | .000 | Downtime | 5.45 | .88 | 6.18 | .000 |
| Between Variance | ||||||||||||||
| Intercept | 4.21 | .14 | 29.58 | .000 | Intercept | 4.40 | .14 | 30.87 | .000 | Intercept | 4.29 | .15 | 29.36 | .000 |
| Within Variance | ||||||||||||||
| Intercept | 3.47 | .12 | 28.04 | .000 | Intercept | 3.48 | .13 | 27.26 | .000 | Intercept | 3.48 | .13 | 26.74 | .000 |
| Time | −.24 | .02 | −12.02 | .000 | Time | −.25 | .02 | −12.06 | .000 | Time | −.24 | .02 | −12.01 | .000 |
| Pain Intensity | .58 | .12 | 4.71 | .000 | Pain Interference | .50 | .13 | 3.88 | .000 | Downtime | .44 | .13 | 3.31 | .001 |
| Random Effects | ||||||||||||||
| Random Location (mean and variability) | .75 | .11 | 6.52 | .000 | Random Location | .81 | .12 | 6.97 | .000 | Random Location | .85 | .12 | 7.20 | .000 |
| Random Scale (remaining variability) | 1.01 | .07 | 13.80 | .000 | Random Scale | 1.01 | .07 | 13.80 | .000 | Random Scale | 1.01 | .07 | 13.75 | .000 |
Note. DV indicates the dependent variable for the model. Means and within-person variance in negative emotions were regressed on Pain Intensity, Pain Interference and Downtime (Rest) in separate models. Model parameters for Pain Intensity, Pain Interference, and Downtime predicting within-person variance are bolded for ease of interpretation.
3.3. Associations Between Patient-Reported Mean Levels of Negative Emotions and Variability in Pain Intensity, Interference and Downtime
Using Location Scale modeling, we tested the converse of the models tested in the analyses described previously. Specifically, we regressed variability in pain intensity, pain interference and downtime scores on mean levels of Negative Emotion scores (see Table 4). Random effects indicated that average levels of Negative Emotions were associated with greater variability in pain intensity, pain interference, and downtime (B’s = .26 to .56, p < .001). Results also show that mean levels of Negative Emotions were related significantly to variability in pain interference and downtime, but not to within-person variability in pain intensity. Again, note that these models control for mean levels of pain interference and downtime values. Thus, the coefficients under the Within Subject Variance Model heading in Table 4 reflect the associations of pain interference and downtime variability with mean levels of Negative Emotions after accounting for mean levels of pain interference and downtime. Specifically, a standard unit increase in mean Negative Emotion scores was associated with 19% (exp(B = .17) = 1.19, p <.001) more variability in pain interference, and 32% (exp(B = .28) = 1.32, p <.001) more variability in downtime. Thus, greater mean levels of negative emotions were associated with greater fluctuations in pain interference and downtime over and above their association with mean levels of pain interference and downtime.
Table 4.
Location Scales Models of Pain Intensity, Pain Interference, and Downtime as Functions of Negative Emotion
| DV: Pain Intensity | DV: Pain Interference | DV: Downtime | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | z | p | B | SE | z | p | B | SE | z | p | |||
| Means | ||||||||||||||
| Intercept | 3.06 | .13 | 23.92 | .000 | Intercept | 2.68 | .15 | 17.36 | .000 | Intercept | 2.43 | .13 | 18.74 | .000 |
| Time | −.05 | .02 | −3.57 | .000 | Time | −.07 | .02 | −4.43 | .000 | Time | −.03 | .01 | −3.08 | .002 |
| Negative Affect | .91 | .12 | 7.43 | .000 | Negative Affect | .94 | .15 | 6.30 | .000 | Negative Affect | .66 | .12 | 5.38 | .000 |
| Between Variance | ||||||||||||||
| Intercept | .52 | .14 | 3.68 | .000 | Intercept | .88 | .14 | 6.19 | .000 | Intercept | .53 | .14 | 3.72 | .000 |
| Within Variance | ||||||||||||||
| Intercept | .52 | .07 | 7.49 | .000 | Intercept | .65 | .08 | 8.07 | .000 | Intercept | .95 | .10 | 9.70 | .000 |
| Time | −.12 | .02 | −6.02 | .000 | Time | −.21 | .02 | −10.56 | .000 | Time | −.16 | .02 | −7.85 | .000 |
| Negative Affect | .01 | .07 | .16 | .870 | Negative Affect | .17 | .08 | 2.11 | .035 | Negative Affect | .28 | .09 | 3.02 | .003 |
| Random Effects | ||||||||||||||
| Random Location (mean and variability) | .26 | .07 | 3.78 | .000 | Random Location | .38 | .08 | 4.91 | .000 | Random Location | .56 | .09 | 6.24 | .000 |
| Random Scale (remaining variability) | .63 | .05 | 13.23 | .000 | Random Scale | .71 | .05 | 13.27 | .000 | Random Scale | .81 | .06 | 13.54 | .000 |
Note. DV indicates the dependent variable for the model. The parameters of negative affect predicting within-person variability are bolded to ease interpretation.
3.4. Associations Between Spouse-Observed Mean Levels of Pain Intensity, Interference and Downtime and Patient Negative Emotion Variability
Finally, Location Scale models were computed using spouse observations of patient pain and function. Patient-reported mean levels of Negative Emotion and within patient variability in Negative Emotion were regressed on spouse observations of patient pain intensity, frequency of pain behaviors, and physical activity. See Table 5. Results show that mean levels of spouse-observed pain intensity and pain behaviors were related to higher mean levels of patient-reported Negative Emotion (B = 4.48, p <.001 and B = 3.38, p<.001, respectively). Mean levels of spouse-observed physical activity were not associated with mean levels of patient Negative Emotion. Results also show that spouse-observed patient pain intensity and pain behaviors were related significantly to variability in patient-reported Negative Emotion scores (see Table 5, Model Model 3). A standard unit increase in spouse observed pain intensity was associated with a 51% (exp (B = .41) = 1.51, p =.002) increase in patient Negative Emotion variability, and a standard unit increase in spouse-observed pain behavior was associated with a 38% (exp (B = .32) = 1.38, p =.014) increase in patient Negative Emotion variability. Please note that the coefficients under the Within Subject Variance Model heading in Table 5 reflect the association between patient Negative Emotion variability and mean levels of spouse-observed pain intensity and pain behaviors after accounting for mean levels of patient Negative Emotion scores. Thus, greater mean levels of spouse-observed pain intensity and pain behaviors were associated with greater fluctuations in patient-reported negative emotions over and above their association with mean levels of patient negative emotions.
Table 5.
Location Scale Models of Negative Affect as a function of Spouse Observed Pain Intensity, Pain Behavior, and Activity
| DV: Negative Affect | DV: Negative Affect | DV: Negative Affect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | z | p | B | SE | z | p | B | SE | z | p | |||
| Means | ||||||||||||||
| Intercept | 10.34 | .90 | 11.53 | .000 | Intercept | 10.33 | .92 | 11.23 | .000 | Intercept | 10.40 | 1.00 | 10.45 | .000 |
| Time | −.33 | .04 | −7.31 | .000 | Time | −.33 | .04 | −7.32 | .000 | Time | −.33 | .04 | −7.31 | .000 |
| Pain Intensity | 4.48 | .89 | 5.02 | .000 | Pain Behavior | 3.88 | .90 | 4.31 | .000 | Activity | .61 | 1.01 | .60 | .548 |
| Between Variance | ||||||||||||||
| Intercept | 4.43 | .14 | 31.21 | .000 | Intercept | 4.48 | .14 | 31.60 | .000 | Intercept | 4.64 | .14 | 32.80 | .000 |
| Within Variance | ||||||||||||||
| Intercept | 3.48 | .13 | 26.71 | .000 | Intercept | 3.48 | .13 | 26.29 | .000 | Intercept | 3.48 | .14 | 25.67 | .000 |
| Time | −.25 | .02 | −12.05 | .000 | Time | −.25 | .02 | −12.07 | .000 | Time | −.25 | .02 | −12.12 | .000 |
| Pain Intensity | .41 | .13 | 3.12 | .002 | Pain Behavior | .32 | .13 | 2.47 | .014 | Activity | .08 | .14 | .61 | .540 |
| Random Effects | ||||||||||||||
| Random Location (mean and variability) | .85 | .12 | 7.29 | .000 | Random Location | .88 | .12 | 7.49 | .000 | Random Location | .94 | .12 | 7.82 | .000 |
| Random Scale (remaining variability) | 1.01 | .07 | 13.78 | .000 | Random Scale | 1.01 | .07 | 13.78 | .000 | Random Scale | 1.01 | .07 | 13.78 | .000 |
Note. DV indicates the dependent variable for the model.
3.5. Comparison of Average and Variability in Pain Factors Predicting Emotional Lability
To test the sensitivity of the findings, MSSDs of Negative Emotion were regressed on Means and MSSDs of the other study variables (See Table 6). Results indicated that after accounting for MSSDs or variability in each variable, patient reported pain intensity and down time, along with spouse observed pain intensity and pain behaviors were significantly associated with Negative Affect MSSDs. Patient reported Pain Intensity MSSDs and Pain Interference MSSDs were also significantly associated with Negative Affect MSSD. Thus, these sensitivity analyses support other findings that overall levels of pain and pain related factors are linked to greater Negative Emotion variability. Findings also show that variation in pain intensity and interference are significantly linked to Negative Emotion variability.
Table 6.
Relationships between Mean and MSSD in Pain Variables Predicting Negative Affect MSSD
| Model 1 | B | p |
| Mean Pain Intensity | .30 | .002 |
| Pain Intensity MSSD | .24 | .013 |
| Model 2 | ||
| Mean Pain Interference | .16 | .109 |
| Pain Interference MSSD | .42 | .000 |
| Model 3 | ||
| Mean Spouse Observed Activity | .12 | .245 |
| Spouse Observed Activity MSSD | .02 | .869 |
| Model 4 | ||
| Mean Spouse Observed Pain Behavior | .29 | .005 |
| Pain Behavior MSSD | .16 | .109 |
| Model 5 | ||
| Mean Spouse Observed Pain Intensity | .37 | .000 |
| Spouse Observed Pain Intensity MSSD | .14 | .168 |
| Model 6 | ||
| Mean Downtime | .28 | .008 |
| Downtime MSSD | .20 | .061 |
Note. Each model represents a separate regression model regressing Negative Affect MSSDs on mean and MSSDs for each pain related factor.
4.0. Discussion
Coping with chronic pain and accompanying function impairments may place strong demands on, and deplete emotion regulation resources [25]. The resulting frequent, sharp increases in anxiety, anger or sadness, may be indexed by wide variability of negative emotion states. Variability in negative emotion is an understudied process that may have important associations with pain and function. First, patients with CLBP not only reported higher mean levels of negative emotions than their pain-free spouses, but they also revealed greater variability in these negative emotions. Second, higher levels of pain intensity and functional impairment among these patients were related to greater variability in their negative emotions. Third, higher levels of patient pain intensity and pain behaviors observed by spouses were also related to greater variability in patient negative emotions. Fourth, high mean levels of patient negative emotions were related to greater variability in their pain interference and downtime. Finally the links between average pain intensity and variability in negative emotion was distinct from the relationship accounted for by variability in pain. These findings suggest that experiencing chronic pain is linked to greater emotional lability, and further suggest that elevations in key factors linked to chronic pain – pain intensity and impairment -- are related to increased variability in negative emotions.
Findings indicated that individuals with chronic pain experienced greater variability in negative emotion than their spouses, providing evidence that simply having chronic pain may be linked to greater lability in negative emotions. Differences in negative emotion variability between patients and spouses remained significant after controlling for differences in mean negative emotion levels, supporting the notion that variability is a unique construct. Thus, having chronic pain is related to both the degree to which patients experience negative emotions, and the regularity in these emotions.
Nes and colleagues argued that chronic pain may be conceptualized as a chronic stressor [25] that thereby presents challenges for the maintenance and renewal of adequate coping resources. If persistent high levels of pain intensity and functional impairment exhaust emotion regulation resources, then these patients would be more inclined to experience wide variability in negative emotions. Greater patient-reported pain intensity, pain interference and downtime were indeed significantly related to greater variability in negative emotions beyond any influence of mean negative emotion levels. Spouses’ impressions of patients’ pain and activity were important features of our daily diary data. Spouse observations of patient pain intensity and frequency of pain behaviors were related to both mean levels and variability in patient negative emotions. The findings based on an external observer strengthen the results, suggesting that greater pain and reduced function are related to impaired emotion regulation.
On one level, these results highlight the distinction between experiencing negative emotions and regulating them. An individual with a relatively low level of negative emotions but relatively high variability may experience troubling mood swings and wide fluctuations in emotional state. On a second level, results point toward another negative consequence of having prolonged pain and functional impairment. Pain and impairment may exhaust people’s ability to modulate negative emotions, and increase vulnerability to mood swings. Wide variability in emotions may not only be unpleasant for the person experiencing them, but it is also recognized as a predictor of poor prognosis in psychiatric contexts [5]. Thus, negative emotion variability could increase risk in chronic pain patients for developing or exacerbating psychiatric disorders. On a third level, emotion variability and mood swings may threaten interpersonal relationships and social support. Sudden and frequent outbursts of anger by patients may strain family and caregivers capacity and willingness to extend support. Indeed, family members may in turn respond with anger [6] or other aversive responses toward the person with chronic pain [33]. Deteriorating relationships among patients and their spouses might then negatively impact how patients, communicate about, and manage pain, as low relationship satisfaction has been associated with higher patient pain ratings and increased negative emotions among both patients and caregivers [11, 24].
Because we were interested in testing associations between persistent pain and impaired function with negative emotion regulation, our primary analyses focused on the relationships between “mean pain and function” and “negative emotion variability.” We used state measures derived from diary data. Schneider and colleagues (2012) [28] took a different tack, and reported links between depressive symptoms and trait measures, such as self-efficacy and pain catastrophizing, and daily variability in pain, as well as happiness, frustration and quality of patients’ day. To follow their lead using diary data, we also examined the set of relationships between “mean negative emotion” and “variability in pain and function.” Consistent with their findings, we found that greater mean levels of negative emotions were related to greater variability in pain interference and downtime, but not pain intensity. On one level, results indicate that patients with relatively high levels of negative emotions may be prone to frequent “boom and bust” cycles of low interference with activity followed by high interference with activity. That is, much activity giving way rapidly to low activity; a pattern that may exacerbate pain and related fatigue [2,23]. Thus, individuals with chronic pain who have high levels of negative emotion may not only have overall high pain interference and downtime, but may also be characterized by poor activity pacing.
On a second level, it is important to note the difference in the magnitude of effects between the two sets of relationships. For the relationships between “mean negative emotion” and “variability in pain and function,” mean levels of negative emotions were associated with 1% to 32% increases in variability in pain and impairment, whereas for the “mean pain and function” and “negative emotion variability” relationships, mean levels of pain and function were associated with 54% to 74% increases in negative emotion variability. Although high levels of negative emotion may be associated with frequent fluctuations from relatively low to relatively high interference with performance of daily activities, high levels of pain and impairment appear related to a greater extent of fluctuation from low to high negative emotions. These findings underscore the degree to which persistent pain and impairment may undermine adaptive emotion regulation.
Results have clinical implications. Echoing Schneider and colleagues (2012) [28], our findings that pain and function are related uniquely to variability in negative emotions and that negative emotions are related uniquely to variability in pain and function suggest that such variability is a common and key feature of chronic pain. This emotional variability could in turn impact levels of disability and function [27]. Day to day and even hour by hour variability may also have to be assessed, to have a full grasp of a patient’s experience of and adjustment to chronic pain. If high mean levels of pain and impairment are linked strongly to an index of poor regulation of negative emotion (i.e., wide variability), then helping patients reduce negative emotion variability would appear to be an apt treatment goal. Applying, for instance, components of dialectical behavior therapy may be especially helpful for patients to preserve important interpersonal relationships, and to tolerate distress during pain flairs and abrupt increases in pain interference. Finally, as reported by Schneider and colleagues (2012) [28], clinicians should be aware that patients characterized by high mean levels of pain and impairment may also be at risk for experiencing negative emotion variability and poor regulation.
4.1. Limitations
Limitations of this investigation must be delineated. First, although the data presented here originated from a 14-day diary study, the analyses as described are cross-sectional. Data were collapsed across 70 assessment points to derive means and estimates of variability for each subject. Causal inferences cannot be drawn from these analyses. Lagged analyses would provide more information regarding the possibility of causal relationships, and we have reported lagged analyses of these dairy data [6–7,9,20]. However, a challenge of capturing lagged relationships in variability is identifying ample observation periods observation. Artificially parsing the data into assessment subgroups (e.g., within-day, within-week) reduces power. Using lagged analyses, causal inferences are limited when the precise time lag to identify a cause and effect may be unknown. Future research may experimentally manipulate pain intensity (e.g., engage in pain-provoking activities) to help establish whether high pain causes subsequent negative emotion variability. Such studies may also create opportunities to validate within-subject variance in relation to alternative measures of variability (e.g. self-reported emotional lability), as different indices of variability could yield different results. Our data were confined to patient and spouse self-report. Use of objective methods (e.g., actigraphy) would expand confidence that results are not driven by patient and spouse reporting biases. Spouse pain, which could impact spouse emotional lability, was not assessed via daily diary. The full participant flow from outreach to contact to enrollment was also unknown and could introduce selection bias.
5.0. Conclusion
In sum, our findings are consistent with the hypothesis that high levels of chronic pain and functional impairment may be associated with depleted coping resources needed to regulate negative emotions. Poorly regulated negative emotion marked by sharp and frequent increases and decreases, may threaten well-being, and undermine the quality of important interpersonal relationships.
Acknowledgements
This research was supported by NINR Grant R01 NR010777 (Burns: PI). We are grateful to Dr. Donald Hedeker for his assistance.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Contributor Information
James I. Gerhart, Rush University Medical Center.
John W. Burns, Rush University Medical Center.
Stephen Bruehl, Vanderbilt University Medical Center.
David A. Smith, University of Notre Dame.
Kristina M. Post, University of La Verne.
Laura S. Porter, Duke University Medical Center.
Erik Schuster, Rush University Medical Center.
Asokumar Buvanendran, Rush University Medical Center.
Anne Marie Fras, Duke University Medical Center.
Francis J. Keefe, Duke University Medical Center.
References
- 1.Aldao A, Nolen-hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. 2010;30(2):217–37. [DOI] [PubMed] [Google Scholar]
- 2.Antcliff D, Keeley P, Campbell M, Woby S, McGowan L. Exploring patients’ opinions of activity pacing and a new activity pacing questionnaire for chronic pain and/or fatigue: a qualitative study. Physiotherapy. 2016. September 1;102(3):300–7. [DOI] [PubMed] [Google Scholar]
- 3.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–45. [DOI] [PubMed] [Google Scholar]
- 4.Barrett LF, Barrett DJ. An introduction to computerized experience sampling in psychology. Social Science Computer Review. 2001; 19(2):175–185. [Google Scholar]
- 5.Broome MR, Saunders KE, Harrison PJ, Marwaha S. Mood instability: significance, definition and measurement. Br J Psychiatry. 2015;207(4):283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns JW, Gerhart JI, Bruehl S, et al. Anger arousal and behavioral anger regulation in everyday life among people with chronic low back pain: Relationships with spouse responses and negative affect. Health Psychol. 2016;35(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns JW, Gerhart JI, Bruehl S, et al. Anger arousal and behavioral anger regulation in everyday life among patients with chronic low back pain: Relationships to patient pain and function. Health Psychol. 2015;34(5):547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns JW, Gerhart JI, Post KM, et al. The Communal Coping Model of Pain Catastrophizing in Daily Life: A Within-Couples Daily Diary Study. J Pain. 2015;16(11):1163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns JW, Peterson KM, Smith DA, et al. Temporal associations between spouse criticism/hostility and pain among patients with chronic pain: a within-couple daily diary study. Pain. 2013;154(12):2715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JW. Arousal of negative emotions and symptom-specific reactivity in chronic low back pain patients. Emotion. 2006;6(2):309–19. [DOI] [PubMed] [Google Scholar]
- 11.Corley AM, Cano A, Goubert L, Vlaeyen JW, Wurm LH. Global and situational relationship satisfaction moderate the effect of threat on pain in couples. Pain Medicine. 2016. March 19;17(9):1664–75. [DOI] [PubMed] [Google Scholar]
- 12.Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the society for research in child development. 1994; 1;59(2‐3):73–102. [PubMed] [Google Scholar]
- 13.Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007;131(1–2):162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corley AM, Cano A, Goubert L, Vlaeyen JW, Wurm LH. Global and situational relationship satisfaction moderate the effect of threat on pain in couples. Pain Medicine. 2016; 19;17(9):1664–75. [DOI] [PubMed] [Google Scholar]
- 15.Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change: can mood measures in diary studies detect change reliably?. Pers Soc Psychol Bull. 2006; 32(7):917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruise CE, Broderick J, Porter L, Keall A, Stone AA. Reactive effects of diary self-assessment in chronic pain patients. Pain.1996; 67:253–258. [DOI] [PubMed] [Google Scholar]
- 17.Deng Y, Chang L, Yang M, Huo M, Zhou R. Gender differences in emotional response: Inconsistency between experience and expressivity. PloS one. 2016. Jun 30;11(6):e0158666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders-Non-patient edition (SCID-I/NP, Version 2.0). New York: Biometrics Research Department; 1996. [Google Scholar]
- 19.Gardner FL, Moore ZE. Understanding clinical anger and violence: the anger avoidance model. Behav Modif. 2008;32(6):897–912. [DOI] [PubMed] [Google Scholar]
- 20.Gerhart JI, Burns JW, Post KM, et al. Relationships Between Sleep Quality and Pain-Related Factors for People with Chronic Low Back Pain: Tests of Reciprocal and Time of Day Effects. Ann Behav Med. 2017;51(3):365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedeker D, Mermelstein RJ, Demirtas H. Modeling between-subject and within-subject variances in ecological momentary assessment data using mixed-effects location scale models. Stat Med. 2012;31(27):3328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pain: 1 year validation study. Pain. 2001; 91:277–285. [DOI] [PubMed] [Google Scholar]
- 23.Kempke S, Luyten P, Claes S, et al. Self-critical perfectionism and its relationship to fatigue and pain in the daily flow of life in patients with chronic fatigue syndrome. Psychol Med. 2013;43(5):995–1002. [DOI] [PubMed] [Google Scholar]
- 24.Kindt S, Vansteenkiste M, Loeys T, Cano A, Lauwerier E, Verhofstadt LL, Goubert L. When is helping your partner with chronic pain a burden? The relation between helping motivation and personal and relational functioning. Pain Medicine. 2015. September 1;16(9):1732–44. [DOI] [PubMed] [Google Scholar]
- 25.Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med. 2009;37(2):173–83. [DOI] [PubMed] [Google Scholar]
- 26.Peters ML, Sorbi MJ, Kruise DA, Kerssens JJ, Verhaak PFM, Bensing JM. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain. 2000; 84:181–192. [DOI] [PubMed] [Google Scholar]
- 27.Rost S, Van Ryckeghem DM, Koval P, Sütterlin S, Vögele C, Crombez G. Affective instability in patients with chronic pain: a diary approach. Pain. 2016. August 1;157(8):1783–90. [DOI] [PubMed] [Google Scholar]
- 28.Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE. Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: associations with psychological variables. Pain. 2012;153(4):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 30.Stone AA, Broderick JE, Schneider S, Schwartz JE. Expanding options for developing outcome measures from momentary assessment data. Psychosom Med. 2012;74(4):387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994; 16: 199–202. [Google Scholar]
- 32.Urry HL, Gross JJ. Emotion regulation in older age. Current Directions in Psychological Science. 2010. December;19(6):352–7. [Google Scholar]
- 33.Wilson SJ, Martire LM, Keefe FJ, Mogle JA, Stephens MA, Schulz R. Daily verbal and nonverbal expression of osteoarthritis pain and spouse responses. PAIN. 2013. October 31;154(10):2045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]