Abstract
The development of C–H activation reactions that use inexpensive and practical oxidants remains a significant challenge. Until our recent disclosure of the β-lactonization of free aliphatic acids, the use of peroxides in C–H activation reactions directed by weakly coordinating native functional groups was unreported. Herein we report C(sp3)–H β-acetoxylation and γ-, δ-, and ε-lactonization reactions of free carboxylic acids enabled by a novel cyclopentane-based mono-N-protected β-amino acid (MPAA) ligand. Notably, tert-butyl hydrogen peroxide (TBHP) is used as the sole oxidant for these reactions. This reaction has several key advantages over other C–H activation protocols: (1) exclusive mono-selectivity was observed in the presence of two α-methyl groups; (2) aliphatic carboxylic acids containing α-hydrogens are compatible with this protocol; (3) lactonization of free acids, affording γ-, δ-, or ε-lactones, has been achieved for the first time.
Graphical Abstract
1. Introduction
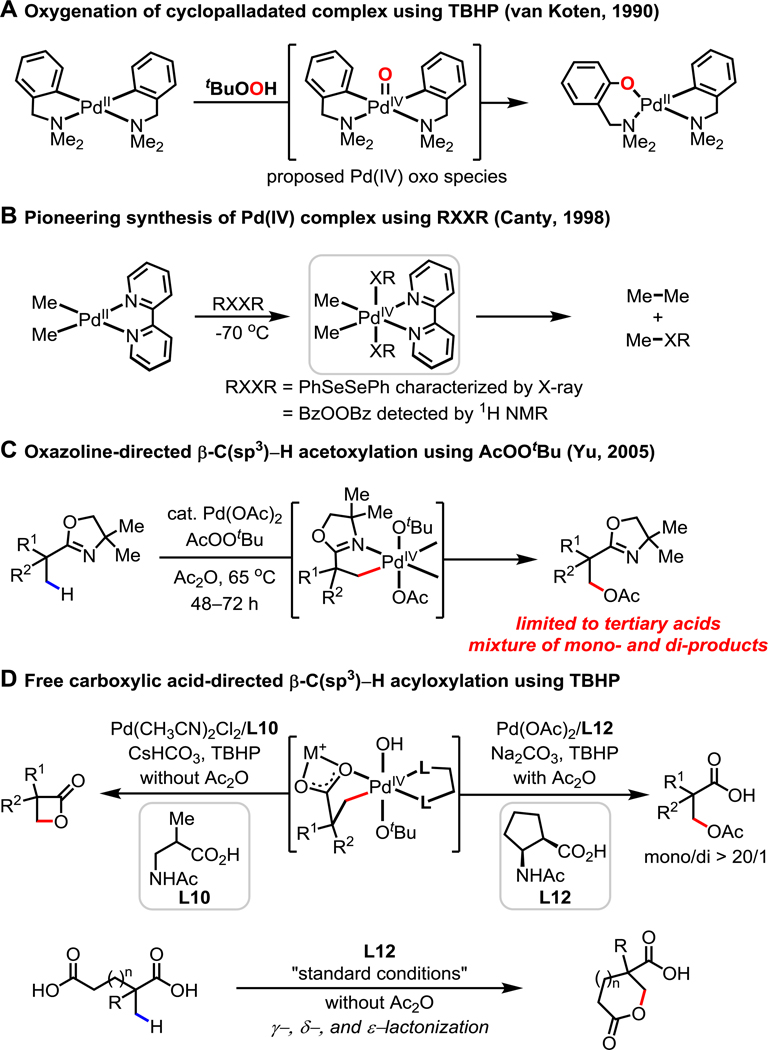
The past two decades have witnessed significant advances in the development of carbon–carbon and carbon–heteroatom bond-forming C–H activation reactions based on Pd(0)/Pd(II), Pd(II)/Pd(IV), Pd(II)/Pd(0), and Pd(II)/Pd(II) catalysis.1 From a practical perspective, each catalytic cycle has inherent advantages and limitations. For example, Pd(II)/Pd(0) catalysis enables transformations with a wide range of coupling partners, such as organotin2a and organoboron2b,c reagents. Meanwhile in Pd(II)/Pd(IV) catalysis, the relatively facile reductive elimination from Pd(IV) allows the reaction of C–H bonds with a variety of electrophiles such as I2, Cl2, IOAc, PhSSPh, PhSeSePh, and BzOOBz.3 Interestingly, PhI(OAc)2 has been shown to be an effective oxidant for both C(sp2)–H4a,b and C(sp3)–H4c activation reactions. Among a range of strong oxidants that are capable of oxidizing Pd(II) to Pd(IV), inexpensive, practical, and readily available group 16 oxidants such as peroxides have received special attention. In 1990, van Koten’s group reported that cyclopalladated N,N-dimethylbenzylamine complexes could be oxygenated with tert-butyl hydrogen peroxide (TBHP).5 An unstable Pd(IV) oxo intermediate was proposed to give oxygenated product by oxygen insertion into the Pd–C bond. Pioneering work from Canty’s group in 1998 showed that the stable Pd(IV) complex from the oxidation of Pd(II) by PhSeSePh could be isolated and characterized (Scheme 1B).6 At higher temperatures, this Pd(IV) complex decomposed to form a carbon– selenium bond-containing product and ethane. The analogous Pd(IV) intermediate from BzOOBz could also be detected by 1H NMR, but was too unstable to be isolated; its decomposition yielded the corresponding oxidation products. In our early work on chiral oxazoline-directed asymmetric β-C(sp3)–H acetoxylation (Scheme 1C),7 we proposed that the oxidative addition of AcOOtBu forms a Pd(IV) intermediate, which undergoes subsequent reductive elimination to afford acetoxylated product. However, despite the great value such reactions might have for synthetic chemistry, in the past decade further efforts to develop C–H activation reactions using peroxides as the sole oxidant have led to little success outside of C(sp2)–H functionalization reactions.8 Indeed, the use of peroxides for C–H activation reactions directed by weakly coordinating native functional groups was unprecedented until our recent disclosure of the β-lactonization of free aliphatic acids (Scheme 1D).9
Scheme 1.
Pd(IV) Chemistry Enabled by Group 16 Oxidants
Hydroxy fatty acids represent an important class of lipids that have broad applications in dietary supplements and pharmaceuticals.10 The distance between the hydroxyl and carboxylic acid functional groups has a remarkable effect on biological activity.11 It is therefore highly desirable to develop synthetic methods to selectively oxidize parent fatty acids.12,13 Recent advances in the C(sp3)–H activation of aliphatic acid derivatives represent a powerful approach to the direct oxidation of aliphatic acids at the β position (Scheme 1C).7 However, these reaction protocols always require exogenous directing groups (DGs) to promote cyclometallation; thus two or three additional steps are required to install and remove the DG.4c,7,14,15a–c Additionally, reported oxidants used in C–H oxidation are largely limited to hypervalent iodine reagents such as PhI(OAc)2.4,14b–g,15,16 Despite the early successes in β-arylations of free acids through the reactivity of sodium or potassium carboxylates,2c analogous acetoxylation reactions were only recently reported by van Gemmeren’s group.16 However, this protocol requires 2.5 equivalents of excess acid substrate and PhI(OAc)2 as the limiting reagent. In addition, aliphatic acids containing α-hydrogens are not reactive. Despite significant advances in developing diverse C–H activation transformations for free aliphatic acids,17 scalable and practical β-C(sp3)–H oxidations of free acids remain a significant challenge.
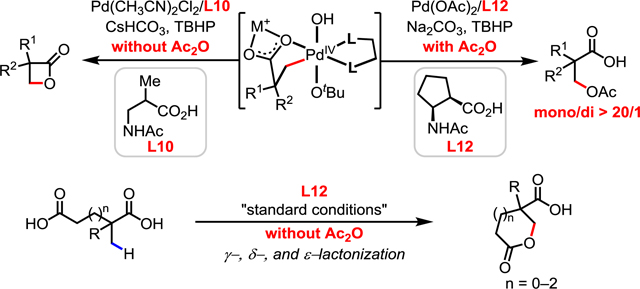
Herein we report β-C(sp3)–H acyloxylation of free carboxylic acids enabled by a newly developed mono-N-protected β-amino acid (MPAA) ligand (Scheme 1D). This method features relatively mild temperatures (60 °C), an inexpensive and practical oxidant (TBHP in water, $5/mol), and uses two equivalents of Ac2O as the crucial promoter. A broad range of aliphatic acids containing α-hydrogens or α-quaternary centers are compatible with this catalysis. Exclusive mono-selectivity was observed for acids bearing multiple α-methyl groups. The C(sp3)–H γ-, δ-, and ε-lactonization of free carboxylic acids has also been realized for the first time.
2. Results and Discussion
Following our earlier disclosure of the asymmetric β-acetoxylation of aliphatic acids using chiral oxazoline directing groups,7 we have extensively investigated the possibility of extending this practically appealing catalytic system to free carboxylic acids without success for the past 15 years. Prompted by our recent discovery that C(sp3)–H activation of free carboxylic acids could be promoted by a bidentate ligand,17 we began to test various ligands for reactivity using TBHP (70% in water) as the oxidant. We discovered that a β-amino acid ligand promoted an unprecedented β-lactonization reaction.9 This finding prompted us to further tune the ligand and reaction conditions such that the reductive elimination pathway of the Pd(IV) intermediate might favor an acyloxylation pathway (Scheme 1D). We selected 2-methyl butyric acid 1a as a model substrate for ligand design and reaction development (Table 1). While no desired acetoxylation product was detected in the absence of ligand, we were pleased to observe a 13% 1H NMR yield of β-acetoxylation product 1b when using the thioether-based bidentate ligand L2.17d However, thioether ligand L2 was completely oxidized to sulfoxide after the reaction, which may have contributed to the low yield of the reaction. Guided by MPAA ligand-enabled arylation17b,c,f and lactonization9 reactions of free aliphatic acids, a series of commercially available MPAA ligands (L4–L8) that are stable in the presence of peroxide were investigated. To our delight, the simple β-amino acid ligand L8 further improved the yield to 44%. Further modifications to the backbone of the β-amino acid ligand (L9–L11, with L10 being the optimal ligand from the previously reported β-lactonization reaction), led to no further improvement in yield. Aiming to test the effect of a more rigid conformation and bite angle on ligand reactivity,18 a series of cycloalkane-based cis-bidentate β-amino acid ligands (L12–L14) were prepared. To our delight, the yield was improved to 61% using the cyclopentane-based ligand L12. Furthermore, the corresponding methyl ester of the desired acetoxylation product could be isolated in 72% yield when using TBHP in decane. However, lower catalysis loading led to lower yield: using 5 mol% and 2 mol% Pd only gave 31% and 7% 1H NMR yield, respectively. A control experiment showed that no reaction occurred in the presence of the γ-amino acid ligand (L15), indicating the importance of six-membered chelation for the observed reactivity.
Table 1.
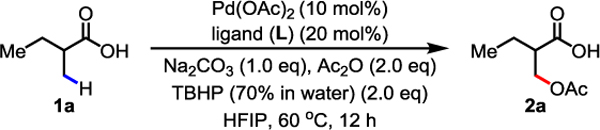 |
|---|
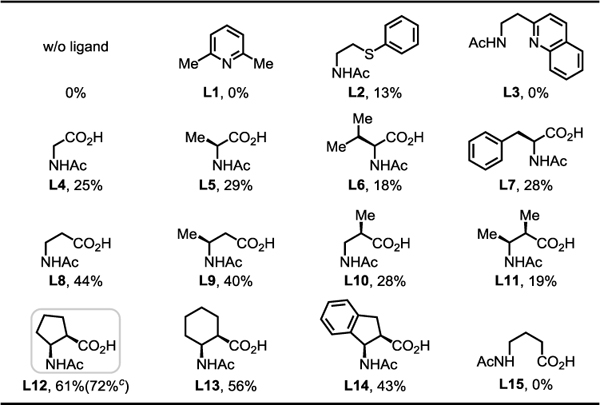 |
Conditions: 1a (0.1 mmol), Pd(OAc)2 (10 mol%), ligand (L) (20 mol%), Na2CO3 (1.0 eq), Ac2O (2.0 eq), TBHP (70% in water) (2.0 eq), HFIP, 60 °C, 12 h.
The yields were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard.
TBHP (~5.5 M in decane) (2.0 eq), isolated yield of the corresponding methyl ester.
With the optimal ligand and reaction conditions in hand, the scope of aliphatic carboxylic acids was evaluated (Table 2). Compared to the β-lactonization reaction,9 this protocol showed broader scope since a wide range of aliphatic carboxylic acids containing α-hydrogens were compatible with the current conditions. Commercially available 2-methyl aliphatic acids (1a–1e) could undergo β-C(sp3)–H acetoxylation in good yields. Less reactive propionic acid (1f) also provided the acetoxylation product in moderate yield (51%). Aliphatic carboxylic acids bearing cyclic rings, including six- (1g–1j), five- (1k), four- (1l), and three- (1m and 1u) membered rings, were well tolerated. Among these acids, potentially reactive functional groups including carbonyl (1h) and tert-butyloxycarbonyl (Boc) (1j) groups were untouched under these mild conditions. Phenyl (2n–2p, 2v, 2y, and 2ab) groups were also compatible with the TBHP system, and remained intact despite the potentially reactive aryl or benzylic C–H bonds. A range of synthetically versatile functionalities such as benzoyl (Bz) protected hydroxyl (2q), ester (2r), chloro (2s), fluoro (2w), and trifluoromethyl (2x) were all well tolerated. Aliphatic carboxylic acids bearing α-quaternary centers (1t–1ab) consistently afforded the desired acetoxylation products in useful yields. Notably, compared to other β-C(sp3)–H functionalization reactions, this protocol displayed exclusive mono-selectivity (2b and 2t–2y) in the presence of two α-methyl groups, likely due to catalysis deactivation via bidentate chelation by the installed OAc group and carboxylate group. It is noteworthy that the low yield for several cases is due to low conversion; remaining unreacted acid substrates could be detected from crude 1H NMR, while by-products such as β-lactone and β-hydroxy acid weren’t observed during the reaction. Besides acetic anhydride, other aliphatic acid anhydrides including propionic, isobutyric, and pivalic anhydrides are also compatible with the reported protocol but with low efficiency (see Supporting Information Table S7).
Table 2.
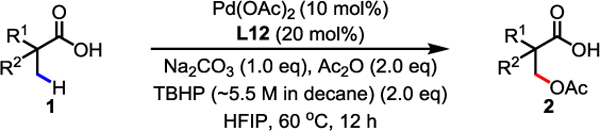 |
|---|
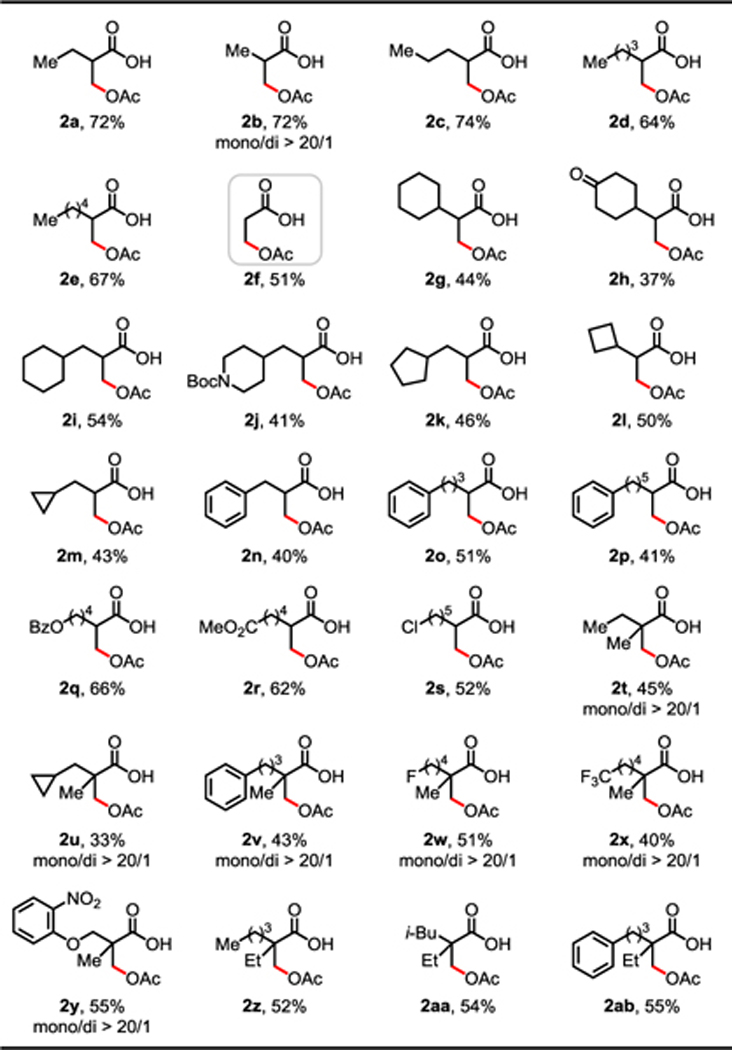 |
Conditions: 1 (0.1 mmol), Pd(OAc)2 (10 mol%), L12 (20 mol%), Na2CO3 (1.0 eq), Ac2O (2.0 eq), TBHP (~5.5 M in decane) (2.0 eq), HFIP, 60 °C, 12 h.
Isolated yields of the corresponding methyl ester.
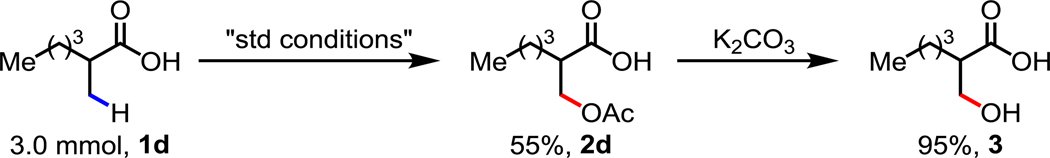
To demonstrate the utility of the herein reported method, we performed the reaction on a 3.0 mmol scale with substrate 1d using TBHP in water under the aforementioned reaction conditions, yielding the desired β-acetoxylated carboxylic acid in 55% yield. The acetyl protecting group could be removed in the presence of K2CO3 to generate the free β-hydroxy acid in near quantitative yield (95%). (Scheme 2)
Scheme 2.
Scale-up Reaction and Deprotection of Acetyl Group
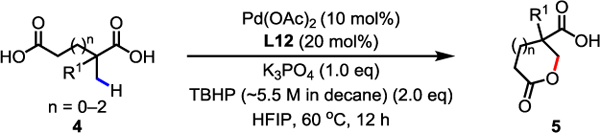
We were also interested in whether this protocol could be extended to form synthetically valuable lactones in an intramolecular fashion (Table 3). Although a single example of C(sp3)–H lactonization of acid derivatives has been reported,14f this method was limited to the syntheses of γ-lactones and the use of a directing group. By simply changing the base and removing Ac2O (see Supporting Information for details), we were delighted to find that the desired lactonization product could be obtained. Different sizes of lactones such as γ-lactones (5a), δ-lactones (5b and 5d), and ε-lactones (5c) could be formed in moderate to good yields. Less reactive free carboxylic acids containing α-hydrogen (4d) were also compatible with the standard conditions.
Table 3.
 |
|---|
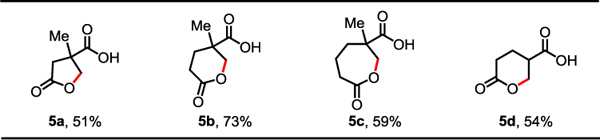 |
Conditions: 4 (0.1 mmol), Pd(OAc)2 (10 mol%), L12 (20 mol%), K3PO4 (1.0 eq), TBHP (~5.5 M in decane) (2.0 eq), HFIP, 60 °C, 12 h.
Isolated yields of the corresponding methyl ester.
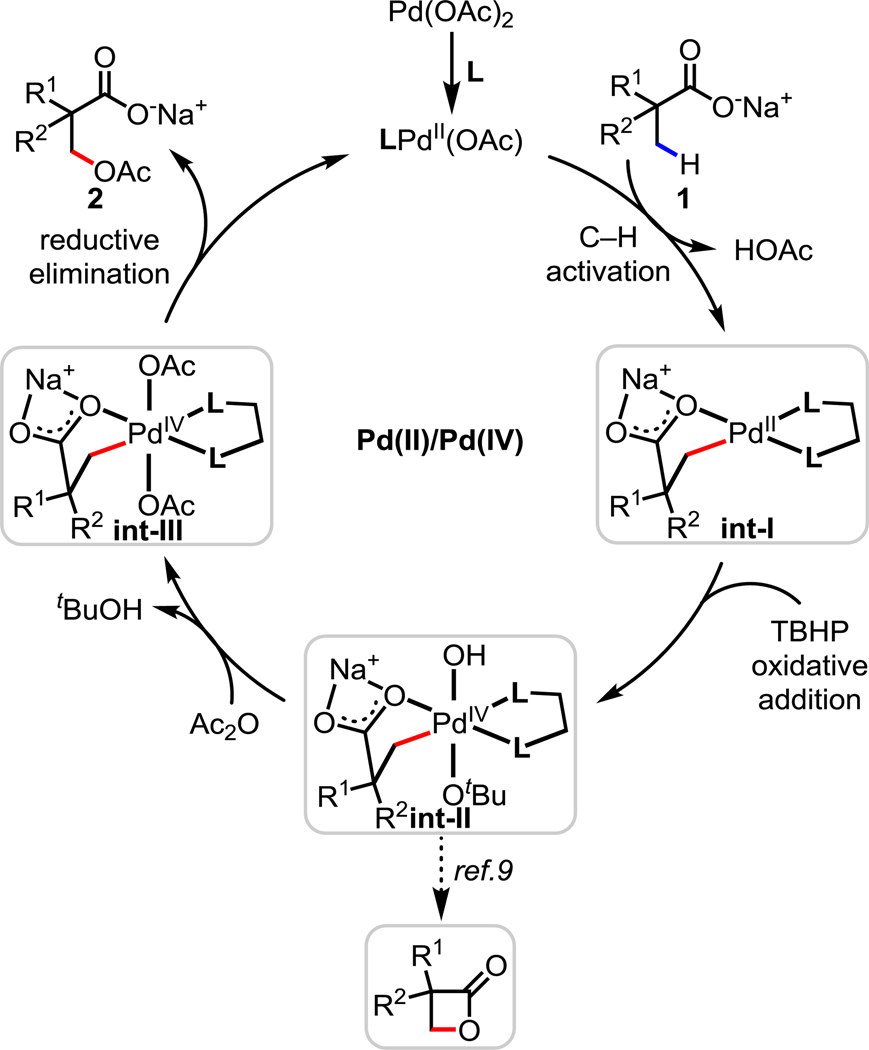
Based on previous literature6–9 and our results from the lactonization reaction, we propose that our transformation proceeds via the Pd(II)/Pd(IV) catalytic cycle outlined in Scheme 3. First, coordination of Pd(OAc)2 to an MPAA ligand generates the active LPd(II)(OAc) species. After coordination of the acid substrate 1 to Pd, both the counteranion Na+ and the MPAA ligand accelerate the cyclopalladation of the β-C(sp3)–H bond to form int-I. Next, oxidative addition of int-I by TBHP produces int-II. The direct involvement of TBHP without formation of AcOOtBu by Ac2O in the oxidation addition step is supported by 1H NMR studies of the reaction and the success of lactonization under the conditions without Ac2O. In the previously reported β-lactonization reaction, promoted by tBuO− and MPAA on Pd(IV) center, selective reductive elimination of int-II yields strained β-lactone product. In contrast, in this case, int-II undergoes subsequent ligand exchange by Ac2O to generate int-III. Finally, reductive elimination generates β-acetoxylation product 2 and regenerates LPd(II)(OAc) species; however, an SN2-type reaction by AcO− that affords the final product cannot be ruled out. Similarly, for lactonization reaction, two possible pathways of Pd(IV) center (int-II) are proposed to generate lactone product: (1) carboxylic acid moiety on the side chain can replace OH or OtBu on Pd(IV) (int-II) by ligand exchange. Subsequent reductive elimination affords the lactonization product; (2) it is also possible that the other carboxylate from diacid can attack C–Pd(IV) bond of int-II by SN2-type reaction to yield lactone product. The possibility of forming the acetoxylated product from the corresponding β-lactone was ruled out by a control experiment: when the β-lactone analog of 2t was subjected to the standard conditions, no acetoxylated product was observed.
Scheme 3.
Proposed Mechanism for β-C(sp3)–H Acetoxylation
3. Conclusion
In summary, we have developed Pd(II)-catalyzed intra- and intermolecular C(sp3)–H acyloxylation of carboxylic acids using inexpensive TBHP as the sole oxidant. The key to this reaction’s success hinged on the design of a cyclopentane-based MPAA ligand. The use of the inexpensive oxidant TBHP (70% in water) renders this reaction practical and scalable. A wide range of α-methyl aliphatic carboxylic acids are compatible with the reported conditions and exclusive mono-selectivity is observed in the presence of multiple α-methyl groups. An efficient method for the C(sp3)–H γ-, δ-, and ε-lactonization of free carboxylic acids has also been realized for the first time.
4. Experimental Section
General procedure for β-C(sp3)–H acetoxylation.
In the culture tube, Pd(OAc)2 (10 mol%, 2.2 mg), ligand L12 (20 mol%, 3.4 mg), Na2CO3 (1.0 eq, 10.6 mg), and carboxylic acid 1 (0.1 mmol) in order were weighed in air and placed with a magnetic stir bar. Then HFIP (1.0 mL), Ac2O (2.0 eq, 19 μL), and TBHP (~5.5 M in decane) (2.0 eq, 36 μL) were added. The reaction mixture was stirred at rt for 3 minutes, and then heated to 60 °C for 12 hours (600 rpm). After being allowed to cool to room temperature, the mixture was treated with AcOH (0.05 mL) and concentrated in vacuo. The resulting mixture was dissolved in MeOH (1.0 mL), treated with TMSCHN2 (2.0 eq), and concentrated in vacuo after 1 hour. The crude mixture was purified by pTLC or column chromatography to afford corresponding methyl esters. Full experimental details and characterization of new compounds can be found in the Supporting Information.
Supplementary Material
Acknowledgements.
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, R01GM084019) for financial support.
Footnotes
Notes. The authors declare no competing financial interest.
Supporting Information Available. Full experimental details and characterization of new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1. For reviews on C–H functionalization, see:; (a) Daugulis O; Roane J; Tran L. D. Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon–Hydrogen Bonds. Acc. Chem. Res 2015, 48, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lyons TW; Sanford MS. Palladium-Catalyzed Ligand-directed C–H Functionalization Reactions. Chem. Rev 2010, 110, 1147–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) He J; Wasa M; Chan KSL; Shao Q; Yu J-Q Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev 2017, 117, 8754–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Chen X; Li J-J; Hao X-S; Goodhue CE; Yu J-Q Palladium-Catalyzed Alkylation of Aryl C– H Bonds with sp3 Organotin Reagents Using Benzoquinone as a Crucial Promoter. J. Am. Chem. Soc 2006, 128, 78–79. [DOI] [PubMed] [Google Scholar]; (b) Chen X; Goodhue CE; Yu J-Q Palladium-Catalyzed Alkylation of sp2 and sp3 C–H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C–H Activation Pathways. J. Am. Chem. Soc 2006, 128, 12634–12635. [DOI] [PubMed] [Google Scholar]; (c) Giri R; Maugel N; Li J-J; Wang D-H; Breazzano SP; Saunder LB; Yu J-Q Palladium-Catalyzed Methylation and Arylation of sp2 and sp3 C–H Bonds in Simple Carboxylic Acids. J. Am. Chem. Soc 2007, 129, 3510–3511. [DOI] [PubMed] [Google Scholar]
- 3. For reviews on Pd(IV) chemistry, see:; (a) Xu L-M; Li B-J; Yang Z; Shi Z-J Organopalladium(IV) Chemistry. Chem. Soc. Rev 2010, 39, 712–733. [DOI] [PubMed] [Google Scholar]; (b) Sehnal P; Taylor RJK; Fairlamb IJS Emergence of Palladium(IV) Chemistry in Synthesis and Catalysis. Chem. Rev 2010, 110, 824–889. [DOI] [PubMed] [Google Scholar]; (c) Engle KM; Mei T-S; Wang X; Yu J-Q Bystanding F+ Oxidants Enable Selective Reductive Elimination From High-Valent Metal Centers in Catalysis. Angew. Chem., Int. Ed 2011, 50, 1478–1491. For selected examples using aryl iodide for Pd(IV) chemistry, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li B; Seth K; Niu B; Pan L;Yang H; Ge H. Transient-Ligand-Enabled ortho-Arylation of Five-Membered Heterocycles: Facile Access to Mechanochromic Materials. Angew. Chem., Int. Ed 2018, 57, 3401–3405. [DOI] [PubMed] [Google Scholar]; (e) Li B; Lawrence B; Li G; Ge H. Ligand-Controlled Direct γ-C–H Arylation of Aldehydes. Angew. Chem., Int. Ed 2020, 59, 3078–3082. [DOI] [PubMed] [Google Scholar]; (f) Zhang F-L; Hong K; Li T-J; Park H; Yu J-Q Functionalization of C(sp3)–H Bonds Using a Transient Directing Group. Science 2016, 351, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen G; Gong W; Zhuang Z; Andrä MS; Chen Y-Q; Hong X; Yang Y-F; Liu T; Houk KN; Yu J-Q Ligand Accelerated Enantioselective Methylene C(sp3)–H Bond Activation. Science 2016, 353, 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Wu Q-F; Shen P-X; He J; Wang X-B; Zhang F; Shao Q; Zhu R-Y; Mapelli C; Qiao JX; Poss MA; Yu J-Q Formation of α-Chiral Centers by Asymmetric β-C(sp3)–H Arylation, Alkenylation, and Alkynylation. Science 2017, 355, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Yoneyama T; Crabtree RH. Pd(II) catalyzed Acetoxylation of Arenes with Iodosyl Acetate. J. Mol. Catal. A 1996, 108, 35–40. [Google Scholar]; (b) Dick AR; Hull KL; Sanford MS. A Highly Selective Catalytic Method for the Oxidative Functionalization of C−H Bonds. J. Am. Chem. Soc 2004, 126, 2300–2301. [DOI] [PubMed] [Google Scholar]; (c) Desai LV; Hull KL; Sanford MS. Palladium-Catalyzed Oxygenation of Unactivated sp3 C–H Bonds. J. Am. Chem. Soc 2004, 126, 9542–9543. [DOI] [PubMed] [Google Scholar]
- 5.(a) Alsters PL; Teunissen HT; Boersma J; van Koten G. The Oxygenation of Cyclopalladated N,N-Dimethylbenzylamine Derivatives with tert-Butyl Hydroperoxide. Recl. Trav. Chim. Pays-Bas 1990, 109, 487–489. [Google Scholar]; (b) Alsters PL; Teunissen HT; Boersma J; Spek AL; van Koten G. Oxygenation of Cyclopalladated N,N-Dimethylbenzylamine Complexes by Inorganic and Organic Peroxides: Oxygen Insertion into the Palladium–Carbon Bond. Organometallics 1993, 12, 4691–4696. [Google Scholar]
- 6.(a) Canty AJ; Jin H; Skelton BW; White AH. Oxidation of Complexes by (O2CPh)2 and (ER)2 (E = S, Se), Including Structures of Pd(CH2CH2CH2CH2)(SePh)2(bpy) (bpy = 2,2’-Bipyridine) and MMe2(SePh)2(L2) (M = Pd, Pt; L2 = bpy, 1,10-Phenanthroline) and C···O and C···E Bond Formation at Palladium(IV). Inorg. Chem 1998, 37, 3975–3981. [DOI] [PubMed] [Google Scholar]; (b) Byers PK; Canty AJ; Skelton BW; White AH. The Oxidative Addition of Iodomethane to [PdMe2(bpy)] and the X-ray Structure of the Organopalladium(IV) Product fac-[PdMe3(bpy)l](bpy = 2,2′-bipyridyl). J. Chem. Soc., Chem. Commun 1986, 1722–1724. [Google Scholar]
- 7.Giri R; Liang J; Lei J-G; Li J-J; Wang D-H; Chen X; Naggar IC; Guo C; Foxman BM; Yu J-Q Pd-Catalyzed Stereoselective Oxidation of Methyl Groups by Inexpensive Oxidants under Mild Conditions: A Dual Role for Carboxylic Anhydrides in Catalytic C–H Bond Oxidation. Angew. Chem., Int. Ed 2005, 44, 7420–7424. [DOI] [PubMed] [Google Scholar]
- 8.(a) Vickers CJ; Mei TS; Yu J-Q Pd(II)-Catalyzed o-C–H Acetoxylation of Phenylalanine and Ephedrine Derivatives with MeCOOOtBu/Ac2O. Org. Lett 2010, 12, 2511–2513. [DOI] [PubMed] [Google Scholar]; (b) Wei Y; Yoshikai N. Oxidative Cyclization of 2-Arylphenols to Dibenzofurans under Pd(II)/Peroxybenzoate Catalysis. Org. Lett 2011, 13, 5504–5507. [DOI] [PubMed] [Google Scholar]; (c) Duan S; Xu Y; Zhang X; Fan X. Synthesis of 2,2’-Biphenols through Direct C(sp2)–H Hydroxylation of [1,1’]-Biphenyl]-2-ols. Chem. Commun 2016, 52, 10529–10532. [DOI] [PubMed] [Google Scholar]; (d) Zhang Y; Feng J; Li C-J Palladium-Catalyzed Methylation of Aryl C–H Bond by Using Peroxides. J. Am. Chem. Soc 2008, 130, 2900–2901. [DOI] [PubMed] [Google Scholar]; (e) Sharma AK; Roy D; Sunoj RB. The Mechanism of Catalytic Methylation of 2-Phenylpyridine using Di-tert-butyl Peroxide. Dalton Trans. 2014, 43, 10183–10201. [DOI] [PubMed] [Google Scholar]; (f) Lv W; Wen S; Liu J; Cheng G. Palladium-Catalyzed ortho-C–H Methylation of Benzoic Acids. J. Org. Chem 2019, 84, 9786–9791. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang Z; Yu J-Q Lactonization as a General Route to β-C(sp3)–H Functionalization. Nature 2020, 577,656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Cao Y; Zhang X. Production of Long-Chain Hydroxy Fatty Acids by Microbial Conversion. Appl. Microbiol. Biotechnol 2013, 97, 3323–3331. [DOI] [PubMed] [Google Scholar]; (b) Offermanns S. Free Fatty Acid (FFA) and Hydroxy Carboxylic Acid (HCA) Receptors. Annu. Rev. Pharmacol. Toxicol 2014, 54, 407–434. [DOI] [PubMed] [Google Scholar]; (c) Kornhauser A; Coelho SG; Hearing VJ. Applications of Hydroxy acids: Classification, Mechanisms, and Photoactivity. Clin. Cosmet. Investig. Dermatol 2010, 3, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Kaspersen MH; Jenkins L; Dunlop J; Milligan G; Ulven T. Succinct Synthesis of Saturated Hydroxy Fatty Acids and in vitro Evaluation of all Hydroxylauric Acids on FFA1, FFA4 and GPR84. Med. Chem. Commun 2017, 8, 1360–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yore MM; Syed I; Moraes-Vieira PM; Zhang T; Herman MA; Homan EA; Patel RT; Lee J; Chen S; Peroni OD; Dhaneshwar AS; Hammarstedt A; Smith U; McGraw TE; Saghatelian A; Kahn BB. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabeticand Anti-inflammatory Effects Cell 2014, 159, 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. For reviews on C–H oxidation reactions, see:; (a) Moghimi S; Mahdavi M; Shafiee A; Foroumadi A. Transition-Metal-Catalyzed Acyloxylation: Activation of C(sp2)–H and C(sp3)–H Bonds. Eur. J. Org. Chem 2016, 3282–3299. [Google Scholar]; (b) Le Bras J; Muzart J. C–O Bonds from Pd-Catalyzed C(sp3)–H Reactions Mediated by Heteroatomic Groups. Eur. J. Org. Chem 2018, 1176–1203. [Google Scholar]; (c) Sterckx H; Morel B; Maes BUW Catalytic Aerobic Oxidation of C(sp3)–H Bonds. Angew. Chem., Int. Ed 2019, 58, 7946–7970. [DOI] [PubMed] [Google Scholar]
- 13. For reviews on biocatalytic oxidation reactions, see: [Google Scholar]; (a) Dong J; Fernandez-Fueyo E; Hollmann F; Paul C; Pasic M; Schmidt S; Wang Y; Younes S; Zhang W. Biocatalytic Oxidation Reactions: A Chemist’s Perspective. Angew. Chem., Int. Ed 2018, 57, 9238–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sheldon RA; Woodley JM. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev 2018, 118, 801–838. [DOI] [PubMed] [Google Scholar]
- 14. For C(sp3)–H oxidation of carboxylic acids using various directing groups, see:; (a) Reddy BVS; Reddy LR; Corey EJ. Novel Acetoxylation and C–C Coupling Reactions at Unactivated Positions in α-Amino Acid Derivatives. Org. Lett 2006, 8, 3391–3394. [DOI] [PubMed] [Google Scholar]; (b) Chen K; Zhang S-Q; Jiang H-Z; Xu J-W; Shi B-F Practical Synthesis of anti-β-Hydroxy-α-Amino Acids by PdII-Catalyzed Sequential C(sp3)–H Functionalization. Chem. -Eur. J 2015, 21, 3264–3270. [DOI] [PubMed] [Google Scholar]; (c) Rit RK; Yadav MR; Sahoo AK. Pd(II)-Catalyzed Primary-C(sp3)–H Acyloxylation at Room Temperature. Org. Lett 2012, 14, 3724–3727. [DOI] [PubMed] [Google Scholar]; (d) Chen F-J; Zhao S; Hu F; Chen K; Zhang Q; Zhang S-Q; Shi B-F Pd(II)-Catalyzed Alkoxylation of Unactivated C(sp3)–H and C(sp2)–H Bonds Using a Removable Directing Group: Efficient Synthesis of Alkyl Ethers. Chem. Sci 2013, 4, 4187–4192. [Google Scholar]; (e) Gong W; Zhang G; Liu T; Giri R; Yu J-Q Site-Selective C(sp3)–H Functionalization of Di-, Tri-, and Tetrapeptides at the N-Terminus. J. Am. Chem. Soc 2014, 136, 16940–16946. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Liu B; Shi B-F γ-Lactone Synthesis via Palladium(II)-Catalyzed Lactonization of Unactivated Methylene C(sp3)–H Bonds. Synlett 2016, 27, 2396–2400. [Google Scholar]; (g) Shan G; Yang X; Zong Y; Rao Y. An Efficient Palladium-Catalyzed C–H Alkoxylation of Unactivated Methylene and Methyl Groups with Cyclic Hypervalent Iodine (I3+) Oxidants. Angew. Chem., Int. Ed 2013, 52, 13606–13610. [DOI] [PubMed] [Google Scholar]; (h) Wu X; Zhao Y; Ge H. Copper-Promoted Site-Selective Acyloxylation of Unactivated C(sp3)–H Bonds. Chem. Asian J 2014, 9, 2736–2739. [DOI] [PubMed] [Google Scholar]; (i) Wang Z; Kuninobu Y; Kanai M. Copper-Mediated Direct C(sp3)–H and C(sp2)–H Acetoxylation. Org. Lett 2014, 16, 4790–4793. [DOI] [PubMed] [Google Scholar]; (j) Zhou L; Lu W. Palladium-Catalyzed β-Acyloxylation of Simple Amide via sp3 C–H Activation. Org. Lett 2014, 16, 508–511. [DOI] [PubMed] [Google Scholar]
- 15. For C–H oxidation of other classes of substrates using directing groups and PhI(OAc)2, see:; (a) Zhang S-Y; He G; Zhao Y; Wright K; Nack WA; Chen G. Efficient Alkyl Ether Synthesis via Palladium-Catalyzed, Picolinamide-Directed Alkoxylation of Unactivated C(sp3)–H and C(sp2)–H Bonds at Remote Positions. J. Am. Chem. Soc 2012, 134, 7313–7316. [DOI] [PubMed] [Google Scholar]; (b) Ye X; He Z; Ahmed T; Weise K; Akhmedov NG; Petersen JL; Shi X. 1,2,3-Triazoles as Versatile Directing Group for Selective sp2 and sp3 C–H Activation: Cyclization vs Substitution. Chem. Sci 2013, 4, 3712–3716. [Google Scholar]; (c) Ren Z; Mo F; Dong G. Catalytic Functionalization of Unactivated sp3 C–H Bonds via exo-Directing Groups: Synthesis of Chemically Differentiated 1,2-Diols. J. Am. Chem. Soc 2012, 134, 16991–16994. For C(sp2)–H oxidation of free mandelic acid using PhI(OAc)2, see: [DOI] [PubMed] [Google Scholar]; (d) Dastbaravardeh N; Toba T; Farmer ME; Yu J-Q Monoselective o-C–H Functionalizations of Mandelic Acid and α-Phenylglycine. J. Am. Chem. Soc 2015, 137, 9877–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh KK; Uttry A; Koldemir A; Ong M; van Gemmeren M. Direct β-C(sp3)–H Acetoxylation of Aliphatic Carboxylic Acids. Org. Lett 2019, 21, 7154–7157. [DOI] [PubMed] [Google Scholar]
- 17. For examples of C(sp3)–H activation reactions of free carboxylic acids, see:; (a) Chen G; Zhuang Z; Li G-C; Saint-Denis TG; Hsiao Y; Joe CL; Yu J-Q Ligand-Enabled β-C–H Arylation of α-Amino Acids without Installing Exogenous Directing Groups. Angew. Chem., Int. Ed 2017, 56, 1506–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu Y; Chen X; Yuan C; Li G; Zhang J; Zhao Y. Pd-Catalysed Ligand-Enabled Carboxylate-Directed Highly Regioselective Arylation of Aliphatic Acids. Nat. Commun 2017, 8, 14904–14911. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ghosh KK; van Gemmeren M. Pd-Catalyzed β-C(sp3)–H Arylation of Propionic Acid and Related Aliphatic Acids. Chem. -Eur. J 2017, 23, 17697–17700. [DOI] [PubMed] [Google Scholar]; (d) Zhuang Z; Yu C-B; Chen G; Wu Q-F; Hsiao Y; Joe CL; Qiao JX; Poss MA; Yu J-Q Ligand-Enabled β-C(sp3)–H Olefination of Free Carboxylic Acids. J. Am. Chem. Soc 2018, 140, 10363–10367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shen P-X; Hu L; Shao Q; Hong K; Yu J-Q Pd(II)-Catalyzed Enantioselective C(sp3)–H Arylation of Free Carboxylic Acids. J. Am. Chem. Soc 2018, 140, 6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hu L; Shen P-X; Shao Q; Hong K; Qiao JX; Yu J-Q PdII-Catalyzed Enantioselective C(sp3)–H Activation/Cross-Coupling Reactions of Free Carboxylic Acids. Angew. Chem., Int. Ed 2019, 58, 2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Jacobsen EN. Asymmetric Catalysis of Epoxide Ring-Opening Reactions. Acc. Chem. Res 2000, 33, 421–431. [DOI] [PubMed] [Google Scholar]; (b) Trost BM; Bunt RC; Lemoine RC; Calkins TL. Dynamic Kinetic Asymmetric Transformation of Diene Monoepoxides: a Practical Asymmetric Synthesis of Vinylglycinol, Vigabatrin, and Ethambutol. J. Am. Chem. Soc 2000, 122, 5968–5976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.