Abstract
Post-traumatic stress disorder (PTSD) is a disabling psychiatric condition that can develop following a physical, psychological, or sexual trauma. Despite the growing body of literature examining the psychological and biological factors involved in PTSD psychopathology, specific biomarkers that may improve diagnosis and treatment of PTSD have yet to be identified and validated. This challenge may be attributed to the diverse array of symptoms that individuals with the disorder manifest. Examining the interrelated stress and fear systems allows for a more comprehensive study of these symptoms, and through this approach, which aligns with the research domain criteria (RDoC) framework, neural and psychophysiological measures of PTSD have emerged. In this review, we discuss PTSD neurobiology and treatment within the context of fear and stress network interactions and elucidate the advantages of using an RDoC approach to better understand PTSD with fear conditioning and extinction paradigms.
Keywords: Post-traumatic stress disorder, stress, fear extinction, fear conditioning, amygdala, prefrontal cortex, hippocampus
Introduction
Post-traumatic stress disorder (PTSD) is an incapacitating psychiatric condition that some individuals develop after experiencing stressful or traumatic life events. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5), PTSD is diagnosed based on clusters of symptoms, such as the re-experiencing/having intrusive memories of the trauma, avoidance, negative cognition and mood, and hyperarousal.1 PTSD afflicts 7% to 8% of the adult population in the United States,2 and despite the many efforts to understand the pathophysiology of the disease, improved diagnosis and treatment options are still needed. The heterogeneous and complex nature of PTSD may contribute to the difficulty in achieving these improvements, and one approach to addressing this challenge is through the research domain criteria (RDoC) framework.3,4 Through this perspective, we are able to envision PTSD in a way that is more comprehensive, not limited by subjectively reported symptoms or DSM-5 criteria, and inclusive of neurobiological and physiological measures.
One underlying transdiagnostic feature of PTSD and other anxiety disorders is fear dysregulation. Accumulating evidence has demonstrated abnormalities in the brain circuits that mediate fear responses, and these abnormalities are associated with PTSD.5–8 Both preclinical and clinical data implicate the role of stress and fear circuit interactions in the development, maintenance, and treatment of PTSD. In this review, we examine the neurobiology of PTSD and its intersection with stress and fear response systems. Additionally, we discuss the advantages of using an RDoC approach (supplied by fear conditioning and extinction paradigms) in combination with the DSM-5 to improve our understanding and treatment of PTSD.
Neurobiology of Fear and PTSD
Individuals with PTSD have great difficulty regulating their fear of trauma-associated stimuli, making the study of fear integral to fully understanding the disorder.5,7,9–11 The behavioral and neurobiological responses to fear are critical to this understanding and have been investigated extensively using a paradigm dependent on a Pavlovian learning process called fear conditioning. During this type of learning, a subject is presented with a neutral stimulus (conditioned stimulus), which is typically a light, tone, image, or context. This neutral stimulus is paired with an unconditioned stimulus, which is usually a shock. As the association between the two stimuli is learned, the subject expresses a response that is called the conditioned response. In rodents, the conditioned response when measuring fear is usually freezing behavior or the fear-potentiated startle reflex. In humans, it is typically a psychophysiological index of fear including measures such as fear potentiation of the acoustic startle reflex,12,13 skin conductance response (SCR),5,14–16 or heart rate.17,18 Healthy individuals learn to reduce their fear response to the conditioned stimulus in the absence of a threat. The failure to reduce fear in this way often leads to the development of PTSD psychopathology.10,19–21
Psychophysiological measures have been valuable to the study of fear processing and the neurobiology and symptoms of PTSD.5,22–25 In one example of this, combat veterans, who were subthreshold for a full PTSD diagnosis but did express PTSD symptoms, showed abnormal psychophysiological responses to fear (i.e., heart rate, SCR, startle, and respiratory rate) that were similar to those with a full PTSD diagnosis.17 This suggests that despite not having a PTSD diagnosis, many individuals may still need treatment for the symptoms of PTSD and benefit from additional psychophysiological measures. Using fear paradigms in this way provides a venue in which specific attributes of PTSD, such as impaired safety learning, amplified fear responses to trauma- or stress-related stimuli, and fear generalization can be distinctly investigated. Moreover, these fear paradigm-centered findings support the idea that shifting focus towards the symptoms rather than relying solely on DSM diagnoses may foster more insight and effective treatments for PTSD. Considering this and the broad range of applications for studying fear, examining the neural correlates of fear in PTSD (or PTSD symptomatic but subthreshold) individuals may provide insight into the neurobiological mechanisms underlying the disorder.
Much of what we know about human fear neurocircuitry has correlates in rodents, enabling many preclinical findings to be translatable to human studies. The fear network has been extensively studied in rodents, with the prelimbic (PL) and infralimbic (IL) areas of the medial prefrontal cortex (mPFC), specific nuclei of the amygdala, and the hippocampus identified as important mediators of fear-related processes.26–32 One study delineating the roles of these regions in fear acquisition, extinction, and memory retrieval found that inactivation of the PL before extinction training reduced fear expression during early extinction training (where one would see evidence of fear memory) but had no effect on extinction memory.30 IL inactivation prior to extinction training left the fear memory intact, while extinction learning and memory were impaired.30 Moreover, stimulation of the PL during tone presentations increased fear expression and impaired extinction learning, whereas IL stimulation reduced freezing to the conditioned tone during extinction.33 Together, these findings suggest that PL activity is necessary to drive the expression of conditioned fear, and IL activity is important for its inhibition.
In addition to identifying these critical brain regions, numerous rodent studies have also revealed the role of specific cell types and synchrony of activity or connections between the regions. While the amygdala’s contributions to fear processes have long been recognized, further investigations have delineated specific microcircuits within its nuclei. For example, interactions between the lateral and central divisions of the amygdala have been shown to increase and decrease fear output based on the centrolateral amygdala’s inhibitory control of the centromedial amygdala.34 The connections between the amygdala and the prefrontal cortex have also been found to be critical in regulating fear responses.28,35–38 For example, rats exhibiting high anxiety-related behaviors showed impaired extinction with abnormal activations within the prefrontal-amygdala circuit (reduced prefrontal activity and increased centromedial amygdala activity).39 During safety learning, theta oscillation synchrony within this circuit also seems to modulate fear responding.38,40 Theta oscillations are neural signals that oscillate at a frequency range of 4 to 12 Hz, and synchrony of these oscillatory patterns between brain areas is important for learning and indicates communication between the regions during specific learned behaviors and/or stimuli presentations.40 Increased synchrony of theta oscillations between the mPFC and the basolateral amygdala was found to modulate discrimination between safety and fear learning, suggesting that basolateral amygdala responses (and their timing) to inputs from the mPFC determine increases and reductions in fear behaviors.38
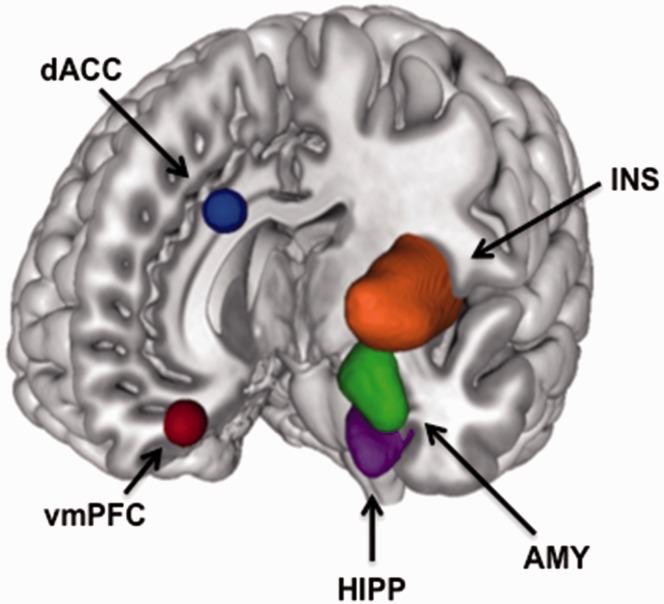
Fear conditioning and extinction studies in humans support the translatability of these preclinical findings, identifying critical nodes of the fear network, including the ventromedial prefrontal cortex (vmPFC), amygdala, dorsal anterior cingulate cortex (dACC), insula, and hippocampus (Figure 1).5,41–43 Brain activations within the dACC (homologous with the rodent PL) appear critical during fear acquisition, whereas vmPFC (homologous with the rodent IL) activation plays an important role in fear extinction.5,44 Additional brain regions, including parts of the cerebellum, insula, thalamus, and striatum, have also been found to be significantly activated during fear acquisition.43,45 As these regions are not only involved within the threat detection and fear processing circuitry, these findings suggest other networks should be investigated as well.
Figure 1.
Summary of brain activations commonly considered the fear network regions. In healthy subjects, the dACC, insula, and amygdala exhibit robust activations during fear acquisition. During fear extinction, the AMY and vmPFC show increased activations. The vmPFC and hippocampus are activated during extinction recall.
dACC: dorsal anterior cingulate cortex; vmPFC: ventromedial prefrontal cortex; AMY: amygdala; HIPP: hippocampus; INS: insula.
There is accumulating evidence indicating that individuals with PTSD have difficulty regulating their fear and learning safety signals.6,7,10,19–21,46–52 Studies have shown extinction learning and memory recall to be significantly impaired in patients with PTSD compared to trauma-exposed non-PTSD control participants.5,52,53 Milad et al.5 found that the impairment was restricted to the recall of the extinction memory, whereas conditioned fear acquisition and extinction learning were not disrupted in the PTSD group. While studies such as these report deficits in only the memory of extinction or extinction retention, other studies have also reported deficits in the learning of fear inhibition.8,48,54,55 For example, PTSD subjects exhibited greater fear-potentiated startle responses to the extinguished cue during extinction, showing an impairment in learning to extinguish their fear, in addition to heightened startle during fear conditioning.48
PTSD and anxiety literature also describe an inverse relationship between activations within frontal regions and the amygdala, i.e., vmPFC hypoactivation and amygdala hyperactivation; this suggests a loss of prefrontal control of the amygdala, which would normally regulate fear responses.7,56–58 Milad et al.5 reported that the extinction memory impairment in PTSD patients was associated with significant deactivation in the vmPFC and heightened activation within the dACC, suggesting less prefrontal inhibition, but more excitation, of the amygdala. A meta-analysis reported that reduced activation of the vmPFC was correlated with increased activation of the amygdala in PTSD patients,59 indicating the consistency of this brain activation pattern in the fear network in PTSD. Thus, alterations in neural connectivity or communication between these brain regions due to disproportionate activation may underlie some PTSD symptoms.57,60,61 In fact, amygdala hyperreactivity and differential cortical activity (top-down processing regions) in response to fearful versus neutral face stimuli was found to be predictive of the perseverance of PTSD symptoms.61 This was also observed for symptom severity in individuals below threshold for a PTSD diagnosis.61,62 Higher Clinician-Administered PTSD Scale scores appeared to be associated with reduced amygdala-vmPFC connectivity, suggesting that communication between these two structures may be compromised with more severe cases of PTSD.63 Moreover, structural differences in these areas have been reported in PTSD; reduced cortical thickness in the frontal lobes and hippocampal volume of soldiers with PTSD compared to those without PTSD were observed.64,65 Given that daily life stressors and other triggering stimuli are often unavoidable, the altered neurocircuitry in PTSD creates an ongoing inability to control fear. This is also true in instances when exposure to stress is prolonged and becomes chronic stress, which has been shown to exacerbate PTSD symptoms.66
Chronic Stress, Fear, and PTSD
Fear is an adaptive threat response, and as such, it is important for survival. A certain level of stress can be similarly beneficial, promoting alertness and energy in situations when a quick reaction is necessary.67 When a stress response is activated in these situations, it triggers components mediating the “fight-or-flight” response. These include the locus coeruleus/norepinephrine (NE) system, the corticotropin-releasing factor/hypothalamic-pituitary-adrenal (HPA) axis, as well as other neurobiological systems.68,69 Traumatic or chronic stress, which is similar to an exaggerated fear response that cannot be turned off, can alter functioning of these systems (often increasing responsivity to future stressors). This may contribute to PTSD symptoms and have serious consequences for mental and physical health.70–74 Chronic stress refers to not only normally occurring, daily life stressors and any subsequent trauma experienced after a PTSD-inducing event but also the repeated stress caused by re-experiencing trauma through recurring, intrusive thoughts, and/or nightmares. Prior traumatic stress exposure is a prerequisite for PTSD diagnosis according to DSM-5 criteria, but persistent alterations in the neurobiological mechanisms mediating the stress response also appear to underlie many PTSD symptoms,70,75,76 suggesting that consequent stressors following the diagnosis are also critical contributors to the disease. As the type of initial trauma can affect PTSD symptomatology, it may also be important to account for the type or source of chronic stress exposure in PTSD research.
The literature on PTSD consistently reports increased NE and dysregulation of the HPA axis, a neurobiological system that modulates stress hormones and reactions to trauma, in individuals with PTSD.77–80 Compared to healthy controls, the pharmacological stressor yohimbine produced increased levels of plasma NE metabolite, as well as PTSD symptoms such as intrusive memories of trauma, emotional numbing, and grief.81 Geracioti et al.82 reported that combat-related film footage also elicited elevations in NE levels in the cerebrospinal fluid of combat-related PTSD patients compared to neutral film footage. In contrast, cerebrospinal fluid concentrations of corticotropin-releasing hormone, also known as corticotropin-releasing factor, were shown to be reduced in response to the traumatic film compared to the neutral film. Yehuda et al.80,83–86 have reported numerous findings on the relationship between low cortisol levels and the risk for PTSD. Urinary cortisol samples revealed that combat veteran PTSD patients had low levels of cortisol excretion compared to non-PTSD individuals, and these low levels were associated with PTSD symptom presentation.80 Subjects administered a low-dose dexamethasone test exhibited greater cortisol suppression compared to healthy participants, suggesting greater negative feedback sensitivity of the HPA axis and subsequently lower baseline cortisol levels in PTSD.86 Competing findings on baseline levels of cortisol remain, with some studies describing increased levels in PTSD, whereas others report blunted baseline levels as mentioned above.78,84,87 These mixed findings suggest another measure may be necessary to understand the role of stress hormones in PTSD.
Stress hormones also influence brain structure and function as many preclinical studies have indicated. The brain regions involved in fear and PTSD circuitry, such as the amygdala, prefrontal cortex, cingulate cortex, and hippocampus, are significantly affected by stress.79,88–90 Evidence in the animal literature describes dendritic remodeling in regions that are necessary for fear processing following stress exposure. In rodents, neuronal hypertrophy within the amygdala and dendritic atrophy in the hippocampus were observed following chronic restraint stress or prolonged periods of glucocorticoid exposure.91–95 Chronic restraint stress exposure for 21 days also reduced dendritic branching within the mPFC in rats,96 suggesting less synaptic plasticity and function within the mPFC and less control over the amygdala. Furthermore, in vivo single-unit recordings in rats that were chronically stressed revealed hyperexcitability in neurons within the lateral amygdala.97 These preclinical findings may contribute to the understanding of the hyper-responsivity of the amygdala that is observed in PTSD patients in response to fearful stimuli.98,99
The overlap in stress and fear neurocircuitry facilitates the use of fear behavioral paradigms to uncover neural and psychophysiological signatures of PTSD, which present symptoms related to both systems. In fact, one might consider chronic stress part of the PTSD symptomatology, as it is a typical byproduct of the nightmares, flashbacks, and recurring intrusive thoughts or reminders of the trauma common with the disorder. Investigating the role of stress in PTSD has revealed that stress exposure can enhance fear learning and impair fear extinction, with associated structural and functional changes within the amygdala and other nodes of the fear network.100,101 In rodents, chronic injections of stress hormone corticosterone enhanced fear memory and increased memory-related activity within the amygdala.102 Rats that were exposed to chronic restraint stress also exhibited improved fear acquisition, but poor extinction memory retrieval, which seemed to be associated with a stress-induced reduction in IL firing.103 Three exposures to an uncontrollable stressor before fear conditioning not only induced dendritic retraction within the IL but they also impaired extinction.104 The specificity of this effect of stress to impact only the IL, and not the PL, morphology is interesting to note given its critical role in extinction. Chronic stress also appeared to increase generalization of fear across contexts, disrupting fear extinction and increasing neuronal activity within the amygdala and hippocampus.105 These preclinical findings are consistent with the impaired extinction recall and decreased vmPFC (and increased amygdala) activation observed in people with PTSD.5,6,57
There are relatively few human studies examining the effects of stress exposure on extinction learning and memory, and within these, there appear to be inconsistencies. Some of these inconsistencies may be attributed to the timing of exposure to stress and sex differences.69,106,107 Studies have reported an impairment in extinction memory retrieval as measured by SCR following stress exposure.108,109 However, some studies also report stress-induced impairments in fear memory retrieval, which may actually enhance extinction processes.110 In one study using the cold pressor test, there was no effect of stress exposure on fear extinction learning; however, fear memory retrieval was found to be impaired in healthy men but not in women.111 Aside from the potential sex differences, this result may be attributed in part to the timing of the stressor exposure; HPA activity and stress hormone levels vary based on the time of day and as a function of time since the onset of the stressor.112 Thus, the distinction between acute and chronic stress effects necessary, as studies could yield opposing results on fear extinction learning and memory simply due to differences in this variable. Moreover, there is strong evidence that fear memory is enhanced when the timing of NE and cortisol release is synchronized but not when the timing is off.69 The interaction between the NE and cortisol systems has been shown to modify brain activations within the fear network during encoding of neutral and emotional stimuli, with deactivation within prefrontal areas specifically noted.113
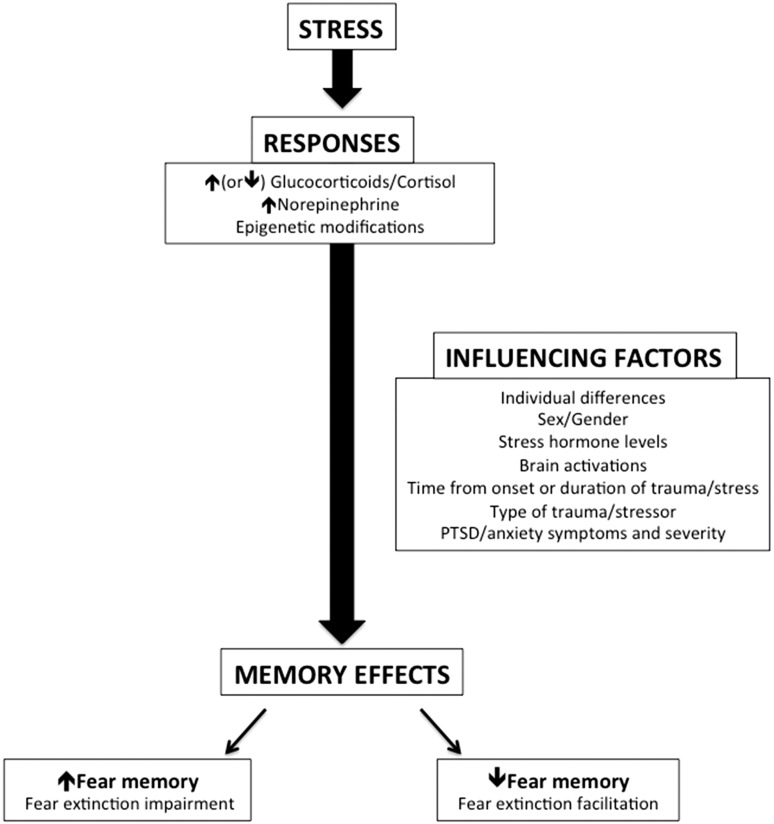
The mixed findings of stress effects on fear extinction may also be due to sex differences and/or the potential influence of gonadal hormones in stress and fear extinction.114–119 Indeed, there are reported interactions between the stress and gonadal hormone systems that can influence fear behaviors.120–124 Antov and Stockhorst114 examined the interaction between menstrual phase, stress, and fear in the effect of psychosocial stressor exposure on fear conditioning and extinction. No differences in the effects of stress on fear acquisition were observed, but women stressed in the high estrogen phase of their menstrual cycles exhibited enhanced extinction recall. Women stressed in their low estrogen phase, however, exhibited impaired extinction memory. Therefore, sex and gonadal hormones should be taken into consideration when using neural and/or psychophysiological fear responses as biomarkers of fear dysregulation in PTSD as indicated in Glover et al.115 and Zeidan et al.119 These and other factors potentially influencing the effects of stress on the brain and fear memory have been summarized in Figure 2.
Figure 2.
The effects of stress and influencing factors on fear memory. Stress exposure (acute or chronic) induces stress responses that include changes in cortisol, norepinephrine, and epigenetic mechanisms. These responses to stress can affect fear memory by either increasing or decreasing it during retrieval, and the direction of the effect is dependent on various influencing factors such as individual differences (accounting for personal experiences, coping styles, etc.), sex (or gonadal hormones), stress hormone levels at the time of the stressor/learning/memory recall, brain responses to the stressor, type of stressor, and the presence of PTSD/anxiety symptoms and severity. Disrupting retrieval of the fear memory may enhance extinction processes, suggesting that stress (or stress hormone) administration may improve response to extinction-based treatments such as prolonged exposure therapy.
RDoC and PTSD
When diagnosing medical problems, physicians run through a checklist of symptoms, but conditions can still be misdiagnosed if the proper follow-up questions are not asked. For example, chest pain is a shared symptom of multiple medical problems, including angina, aortic dissection, pneumonia, gastroesophageal reflux disorder, and costochondritis, the inflammation of the cartilage joining the upper rib with the breastbone. If a patient arrives at a hospital complaining of this symptom, the efficacy of their treatment hinges upon the medical professional’s ability to properly identify its root cause. Luckily, we have a strong understanding of how the heart functions, and through physical exams and history taking, physiological symptoms and genetics are taken into account to arrive at a more specific and effective treatment.125 Medical professionals treating mental illness follow a similar checklist of symptoms to help identify disorders. However, with the nature of the DSM-5 yielding 636,120 ways to have a PTSD diagnosis, as reported by Galatzer-Levy and Bryant,126 and the diagnosis further dependent on subjective reports of symptoms, effective diagnosis (and subsequent treatment) can be a challenge. The RDoC framework may be a valuable tool in this regard, supplementing the DSM-5 and enabling us to obtain more comprehensive and quantifiable diagnostic biomarkers that span across mental disorders.
Diagnoses determined via the DSM alone can often lead to the exclusion of individuals who experience symptoms of PTSD but are subsyndromal or subthreshold for the criteria for PTSD nosology. These people may be suffering from symptoms of mental illness but will not receive proper treatment simply because they do not present their condition in a way that aligns with the DSM-5. On the other hand, it may also be possible for an individual to present biomarkers (i.e., an aberrant functional activation of the amygdala) for a PTSD diagnosis, despite not having the symptoms/disorder. Identifying associations between RDoC’s specific units of analysis and research domains (biological targets and functional constructs) will provide a more complete diagnosis/treatment profile to improve detection and treatment.3,127,128 Moreover, as more data are collected, these constructs and categorizations will become more refined, reliable, and transdiagnostic.
Moving away from the restrictive nature of the DSM-5’s distinct and somewhat limited classifications, we can provide more accurate and targeted treatments for individuals with various mental illnesses and comorbidities.127 In fact, a recent study investigating the psychophysiological and neurobiological patterns during fear conditioning and extinction across anxiety disorders found no differences in SCR between the healthy and anxiety groups but did find differences within the vmPFC, rostral anterior cingulate cortex, and insula activations.129 In line with the RDoC initiative, Marin et al.129 also reported a negative correlation between trait anxiety levels and vmPFC activation within the anxiety group. This suggests a link between anxiety symptoms across different disorder diagnoses and a common neural correlate of fear.
In a recent assessment of the domains and constructs of the RDoC, fear conditioning paradigms were the recommended behavioral tasks to address the acute threat construct within the negative valence system domain.130 A recommended behavioral task has not yet been defined for the sustained threat construct (i.e., chronic stress or distress construct) due to potential ethical violations associated with prolonged stress exposure. Few human studies have examined stress exposure effects on fear extinction learning and memory. However, utilization of a fear conditioning and extinction task in combination with measures of chronic stress using functional magnetic resonance imaging, psychometric, and psychophysiological methods can allow for multidimensional diagnoses. Findings from these studies provide valuable neural and behavioral data to aid in the development of a new classification system enabling improved diagnosis and treatment for individuals who share stress and anxiety-related symptomatology.9
As chronic stress is a major contributor to the development and maintenance of symptoms related to PTSD and other psychiatric disorders such as anxiety, examining potential biomarkers of chronic stress (or “sustained threat”) may be critical to this paradigm. With the known biology of stress, we can measure stress hormones such as corticosterone in rodents and cortisol (i.e., chronic stress levels from hair samples) in humans; these measures have been extensively studied in PTSD research80,84,86,131 and may be valuable in improving diagnoses/treatment. Moreover, chronic stress not only affects cortisol and catecholamine release as reviewed above, but it can also influence cytokine signaling, alter levels of inflammation markers, and induce epigenetic modifications such as DNA methylation; these alterations have also been reported in PTSD.132,133 One study indicated that a specific glucocorticoid receptor gene polymorphism, the BcII GG genotype, was associated with lower levels of cortisol, more persistent traumatic memories, and increased PTSD symptoms,134 providing additional evidence of stress hormone effects in PTSD at the genetic level. Subjective measures of chronic stress, including chronic stress scales and questionnaires such as the perceived stress questionnaire, can elucidate the mechanisms that underlie the psychological impacts of stress, subsequently contribute to mental illness, and affect the neurobiology.135–137 The relationships and correlations between these psychological and biological markers of stress and the psychological, physiological, and neural substrates of fear can further aid classification of the symptoms and profiles of individuals suffering from stress- and anxiety-related disorders.
Psychiatric disorders such as PTSD and anxiety disorders are often accompanied by disturbances in learning and memory processes and altered function within the brain regions modulating these processes. These brain regions are involved in fear circuitry and are affected by chronic stress as well.138,139 Fear conditioning and extinction protocols already contain a learning and memory component that allows for evaluating the cognitive effects of fear responding associated with PTSD and other psychiatric disorders. Further evaluating cognitive performance by combining these protocols with an additional and more general learning and/or memory task (i.e., working memory task) that has been extensively studied and has well-known neurocircuitry may provide more useful or comprehensive insight that is not limited to the fear network.45,140
PTSD Treatment
Current therapies for PTSD, such as exposure therapy, are based on extinction processes, and as a result, having a tool to evaluate how well an individual can learn safety signals (or learn not to fear once the threat is no longer present) is integral to the success of these treatments.50,141–143 For individuals with PTSD, trauma is continually re-experienced; living in a chronically stressed condition is the nature of the illness. Interestingly, it is commonly also the nature of their treatment in prolonged exposure therapy with repeated sessions of re-experiencing the trauma. If stress can modulate critical extinction processes, understanding how, when, and where this modulation occurs might offer insight into why treatments for PTSD work for some people and not for others. Among studies on the effect of stress and cortisol on fear extinction that report impairments, many findings also describe disrupted fear memory retrieval, which may enhance extinction memory.144 More research is necessary to determine how these effects of stress and stress hormones can modify fear extinction and the relevant neurocircuitry. Interestingly, some PTSD studies have used the fear memory-impairing effects of stress hormones by administering NE or cortisol during PTSD treatment to facilitate extinction by impairing retrieval of the trauma memory.145–149 One study examining military veterans with PTSD, who received hydrocortisone or placebo, revealed a reduction in PTSD symptoms with hydrocortisone treatment.149 This effect was particularly pronounced in those with glucocorticoid sensitivity, supporting the rationale for augmenting prolonged exposure therapy with glucocorticoids for combat-related PTSD.149 As mentioned previously, sex and gonadal hormones can also affect extinction processes critical for treatment of PTSD and its symptoms and should therefore be considered as factors in classification and treatment. An example of this can be seen in cortisol treatment after reactivation, which demonstrates differential effects on reconsolidation in healthy men and women, enhancing the reactivated fear memory in men and having no effect in women.150,151 This is important as these differences in the effect of cortisol on fear could subsequently produce differential PTSD treatment responses between the sexes.
Fear conditioning and extinction protocols also facilitate the ability to track neural changes and physiological responses with treatment progress in parallel.152 Helpman et al.152 examined a cohort of subjects with PTSD and trauma-exposed individuals without PTSD who underwent a two-day fear conditioning and extinction protocol before and after a 10-week long prolonged exposure treatment. It was found that from pre- to post-treatment, brain activations differed within specific brain regions, i.e., rostral anterior cingulate cortex and subgenual anterior cingulate cortex; reduced subgenual anterior cingulate cortex and parahippocampal activations were associated with reductions in PTSD symptom severity.
Similar to these post-treatment brain changes and improvements in PTSD symptoms in humans, there is also evidence in rodents that demonstrates rescued function of stress-affected brain areas, such as the mPFC and hippocampus, following rest after chronic stress.153,154 Moreover, Fucich et al.155 report therapeutic effects of extinction training in rats that were fear conditioned and then exposed to chronic unpredictable stress; extinction training improved performance on cognitive flexibility and coping behavior tasks, an improvement that was associated with molecular mechanisms that involved protein synthesis within the mPFC. These findings maybe translatable to humans as they indicate that allowing time for recovery may mitigate some of the negative effects of stress, such as exaggerated fear response in the brain. These changes may again be detected and assessed by examining fear learning and extinction during functional magnetic resonance imaging. These clinical and pre-clinical results support the idea that examining the processes that underlie extinction learning, and memory will enable us to understand how treatments can modify brain circuits and neurobiological mechanisms to improve mental health.
Conclusion
Identifying more specific diagnostic biomarkers of PTSD and other stress-related disorders is critical to the improvement of current diagnostic tools and treatment options. It is evident that there is no single biological marker for PTSD vulnerability, and there is a strong rationale for moving away from DSM diagnosis-centric research to focus more on biological underpinnings of the symptoms individuals present.78,128 Stress, whether in the acute or chronic sense, is a persistent catalyst for the development and exacerbation of not only PTSD symptoms but also depression and anxiety.107,156–159 As such, measurements of stress (i.e., subjective reports/ratings, stress hormone levels, genetics, epigenetics) should be included in the study of symptoms across fear-related disorders. Given that the inherent interactions between the fear and stress systems expand the ability to study both networks and their influences in PTSD symptomatology, the fear conditioning and extinction paradigms discussed here provide a transdiagnostic tool to examine both mechanisms in psychiatric disorders. This examination is not limited to PTSD research but rather intended to obtain measures of critical biological markers that correlate with stress- and anxiety-related symptoms for broad applications. In some studies, it has been useful to integrate DSM criteria and RDoC analyses (across several domains/constructs) to gain multidimensional perspectives in identifying biomarkers. Schmidt and Vermetten160 assessed the value of RDoC in PTSD research and found it useful (i.e., identification of amygdalar hyperactivity across domains) but still lacking in interpretation of findings and linking cellular and molecular mechanisms with symptomatology. As reviewed here, incorporating measures of chronic stress at both the psychological and biological levels within fear conditioning and extinction paradigms may help improve diagnostic efficacy and push forward the development of increasingly targeted treatments for stress- and fear-based disorders.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIMH grants 1R01MH097880-01 and 1R01MH097964-01 to Mohammed R Milad.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 2.Kilpatrick DG, Resnick HS, Milanak ME, et al. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 2013; 26: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013; 11: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167: 748–751. [DOI] [PubMed] [Google Scholar]
- 5.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wicking M, Steiger F, Nees F, et al. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol Learn Mem 2016; 136: 116–126. [DOI] [PubMed] [Google Scholar]
- 9.Briscione MA, Jovanovic T, Norrholm SD. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Front Psychiatry 2014; 5: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. [published online ahead of print December 1, 2011]. Am J Psychiatry. doi:10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed]
- 11.VanElzakker MB, Dahlgren MK, Davis FC, et al. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 2014; 113: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grillon C, Ameli R, Woods SW, et al. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 1991; 28: 588–595. [DOI] [PubMed] [Google Scholar]
- 13.Norrholm SD, Glover EM, Stevens JS, et al. Fear load: the psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int J Psychophysiol Off J Int Organ Psychophysiol 2015; 98: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43: 897–905. [DOI] [PubMed] [Google Scholar]
- 15.Sevenster D, Beckers T, Kindt M. Fear conditioning of SCR but not the startle reflex requires conscious discrimination of threat and safety. [published online ahead of print February 28, 2014]. Front Behav Neurosci. doi:10.3389/fnbeh.2014.00032. [DOI] [PMC free article] [PubMed]
- 16.Tabbert K, Stark R, Kirsch P, et al. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. NeuroImage 2006; 32: 761–770. [DOI] [PubMed] [Google Scholar]
- 17.Costanzo M, Jovanovic T, Norrholm SD, et al. Psychophysiological investigation of combat veterans with subthreshold post-traumatic stress disorder symptoms. Mil Med 2016; 181: 793–802. [DOI] [PubMed] [Google Scholar]
- 18.Roy MJ, Costanzo ME, Jovanovic T, et al. Heart rate response to fear conditioning and virtual reality in subthreshold PTSD. Stud Health Technol Inform 2013; 191: 115–119. [PubMed] [Google Scholar]
- 19.Jovanovic T, Norrholm SD, Blanding NQ, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 2010; 27: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jovanovic T, Kazama A, Bachevalier J, et al. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 2012; 62: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milad MR, Orr SP, Lasko NB, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 2008; 42: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acheson DT, Geyer MA, Baker DG, et al. Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology 2015; 51: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McTeague LM, Lang PJ, Laplante M-C, et al. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry 2010; 67: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am 2002; 25: 271–293. [DOI] [PubMed] [Google Scholar]
- 25.Tanev KS, Orr SP, Pace-Schott EF, et al. Positive association between nightmares and heart rate response to loud tones: relationship to parasympathetic dysfunction in PTSD nightmares. J Nerv Ment Dis 2017; 205: 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenton GE, Pollard AK, Halliday DM, et al. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem 2014; 21: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002; 420: 70–74. [DOI] [PubMed] [Google Scholar]
- 28.Quirk GJ, Likhtik E, Pelletier JG, et al. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 2003; 23: 8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 2008; 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 2011; 36: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, et al. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 2012; 76: 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeng LY, Cover KK, Taha MB, et al. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res 2017; 95: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, et al. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem Cold Spring Harb N 2006; 13: 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciocchi S, Herry C, Grenier F, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010; 468: 277–282. [DOI] [PubMed] [Google Scholar]
- 35.Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast 2007; 2007: 30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Likhtik E, Paz R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci 2015; 38: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Likhtik E, Pelletier JG, Paz R, et al. Prefrontal control of the amygdala. J Neurosci 2005; 25: 7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Likhtik E, Stujenske JM, Topiwala MA, et al. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci 2014; 17: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muigg P, Hetzenauer A, Hauer G, et al. Impaired extinction of learned fear in rats selectively bred for high anxiety–evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur J Neurosci 2008; 28: 2299–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Likhtik E, Gordon JA. Circuits in sync: decoding theta communication in fear and safety. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 2014; 39: 235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 2012; 63: 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43: 897–905. [DOI] [PubMed] [Google Scholar]
- 43.Linnman C, Rougemont-Bücking A, Beucke JC, et al. Unconditioned responses and functional fear networks in human classical conditioning. Behav Brain Res 2011; 221: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fani N, King TZ, Brewster R, et al. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex J Devoted Study Nerv Syst Behav 2015; 64: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fullana MA, Harrison BJ, Soriano-Mas C, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry 2016; 21: 500–508. [DOI] [PubMed] [Google Scholar]
- 46.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res 2008; 167: 151–169. [DOI] [PubMed] [Google Scholar]
- 47.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 2013; 14: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norrholm SD, Jovanovic T, Olin IW, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry 2011; 69: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol Psychiatry 2006; 60: 376–382. [DOI] [PubMed] [Google Scholar]
- 50.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 2003; 1008: 112–121. [DOI] [PubMed] [Google Scholar]
- 51.Zuj DV, Palmer MA, Lommen MJJ, et al. The centrality of fear extinction in linking risk factors to PTSD: a narrative review. Neurosci Biobehav Rev 2016; 69: 15–35. [DOI] [PubMed] [Google Scholar]
- 52.Zuj DV, Palmer MA, Hsu C-MK, et al. Impaired fear extinction associated with PTSD increases with hours-since-waking. Depress Anxiety 2016; 33: 203–210. [DOI] [PubMed] [Google Scholar]
- 53.Garfinkel SN, Abelson JL, King AP, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci 2014; 34: 13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sijbrandij M, Engelhard IM, Lommen MJJ, et al. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). J Psychiatr Res 2013; 47: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 55.Steiger F, Nees F, Wicking M, et al. Behavioral and central correlates of contextual fear learning and contextual modulation of cued fear in posttraumatic stress disorder. Int J Psychophysiol 2015; 98: 584–593. [DOI] [PubMed] [Google Scholar]
- 56.Williams LM, Kemp AH, Felmingham K, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage 2006; 29: 347–357. [DOI] [PubMed] [Google Scholar]
- 57.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 2006; 1071: 67–79. [DOI] [PubMed] [Google Scholar]
- 58.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2012; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens JS, Jovanovic T, Fani N, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 2013; 47: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevens JS, Kim YJ, Galatzer-Levy IR, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. [published online ahead of print December 23, 2016]. Biol Psychiatry. doi:10.1016/j.biopsych.2016.11.015. [DOI] [PMC free article] [PubMed]
- 62.White SF, Costanzo ME, Blair JR, et al. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. NeuroImage Clin 2015; 7: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin C, Qi R, Yin Y, et al. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol Med 2014; 44: 1927–1936. [DOI] [PubMed] [Google Scholar]
- 64.O’Doherty DCM, Chitty KM, Saddiqui S, et al. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res 2015; 232: 1–33. [DOI] [PubMed] [Google Scholar]
- 65.Sussman D, Pang EW, Jetly R, et al. Neuroanatomical features in soldiers with post-traumatic stress disorder. BMC Neurosci 2016; 17: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Arch Gen Psychiatry 2004; 61: 481–488. [DOI] [PubMed] [Google Scholar]
- 67.Joëls M, Pu Z, Wiegert O, et al. Learning under stress: how does it work? Trends Cogn Sci. 2006, 10: 152–158. [DOI] [PubMed] [Google Scholar]
- 68.George SA, Knox D, Curtis AL, et al. Altered locus coeruleus-norepinephrine function following single prolonged stress. Eur J Neurosci 2013; 37: 901–909. [DOI] [PubMed] [Google Scholar]
- 69.Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn Sci 2011; 15: 280–288. [DOI] [PubMed] [Google Scholar]
- 70.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904. [DOI] [PubMed] [Google Scholar]
- 71.Morilak DA, Barrera G, Echevarria DJ, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 1214–1224. [DOI] [PubMed] [Google Scholar]
- 72.King LA, King DW, Fairbank JA, et al. Resilience-recovery factors in post-traumatic stress disorder among female and male Vietnam veterans: hardiness, postwar social support, and additional stressful life events. J Pers Soc Psychol 1998; 74: 420–434. [DOI] [PubMed] [Google Scholar]
- 73.King DW, King LA, Foy DW, et al. Posttraumatic stress disorder in a national sample of female and male Vietnam veterans: risk factors, war-zone stressors, and resilience-recovery variables. J Abnorm Psychol 1999; 108: 164–170. [DOI] [PubMed] [Google Scholar]
- 74.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med 2011; 62: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charney DS, Deutch AY, Krystal JH, et al. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry 1993; 50: 295–305. [DOI] [PubMed] [Google Scholar]
- 76.Nutt DJ. The psychobiology of posttraumatic stress disorder. J Clin Psychiatry 2000; 61(Suppl 5): 24–29. [PubMed] [Google Scholar]
- 77.Cohen H, Zohar J, Gidron Y, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry 2006; 59: 1208–1218. [DOI] [PubMed] [Google Scholar]
- 78.Marshall RD, Garakani A. Psychobiology of the acute stress response and its relationship to the psychobiology of post-traumatic stress disorder. Psychiatr Clin North Am 2002; 25: 385–395. [DOI] [PubMed] [Google Scholar]
- 79.Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety 2002; 16: 14–38. [DOI] [PubMed] [Google Scholar]
- 80.Yehuda R, Teicher MH, Trestman RL, et al. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry 1996; 40: 79–88. [DOI] [PubMed] [Google Scholar]
- 81.Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry 1993; 50: 266–274. [DOI] [PubMed] [Google Scholar]
- 82.Geracioti TD, Baker DG, Kasckow JW, et al. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology 2008; 33: 416–424. [DOI] [PubMed] [Google Scholar]
- 83.Yehuda R. Clinical relevance of biologic findings in PTSD. Psychiatr Q 2002; 73: 123–133. [DOI] [PubMed] [Google Scholar]
- 84.Yehuda R. Neuroendocrine aspects of PTSD. Handb Exp Pharmacol 2005; 169: 371–403. [DOI] [PubMed] [Google Scholar]
- 85.Yehuda R, Giller EL, Southwick SM, et al. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry 1991; 30: 1031–1048. [DOI] [PubMed] [Google Scholar]
- 86.Yehuda R, Southwick SM, Krystal JH, et al. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 1993; 150: 83–86. [DOI] [PubMed] [Google Scholar]
- 87.Meewisse M-L, Reitsma JB, de Vries G-J, et al. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry J Ment Sci 2007; 191: 387–392. [DOI] [PubMed] [Google Scholar]
- 88.López JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry 1999; 46: 1461–1471. [DOI] [PubMed] [Google Scholar]
- 89.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci 2009; 10: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci 2009; 32: 289–313. [DOI] [PubMed] [Google Scholar]
- 91.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res 1990; 531: 225–231. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 1992; 588: 341–345. [DOI] [PubMed] [Google Scholar]
- 93.Vyas A, Mitra R, Shankaranarayana Rao BS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitra R, Jadhav S, McEwen BS, et al. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A 2005; 102: 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henckens MJAG, van der Marel K, van der Toorn A, et al. Stress-induced alterations in large-scale functional networks of the rodent brain. NeuroImage 2015; 105: 312–322. [DOI] [PubMed] [Google Scholar]
- 96.Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 2006; 16: 313–320. [DOI] [PubMed] [Google Scholar]
- 97.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry 2010; 67: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res 2008; 167: 151–169. [DOI] [PubMed] [Google Scholar]
- 99.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47: 769–776. [DOI] [PubMed] [Google Scholar]
- 100.Cacciaglia R, Nees F, Grimm O, et al. Trauma exposure relates to heightened stress, altered amygdala morphology and deficient extinction learning: implications for psychopathology. Psychoneuroendocrinology 2017; 76: 19–28. [DOI] [PubMed] [Google Scholar]
- 101.Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 2016; 41: 58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monsey MS, Boyle LM, Zhang ML, et al. Chronic corticosterone exposure persistently elevates the expression of memory-related genes in the lateral amygdala and enhances the consolidation of a pavlovian fear memory. PloS One 2014; 9: e91530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilber AA, Walker AG, Southwood CJ, et al. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience 2011; 174: 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 2006; 26: 5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffman AN, Lorson NG, Sanabria F, et al. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol Learn Mem 2014; 112: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci 2017; 18: 7–19. [DOI] [PubMed] [Google Scholar]
- 107.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 2007; 133: 25–45. [DOI] [PubMed] [Google Scholar]
- 108.Raio CM, Brignoni-Perez E, Goldman R, et al. Acute stress impairs the retrieval of extinction memory in humans. Neurobiol Learn Mem 2014; 112: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamacher-Dang TC, Merz CJ, Wolf OT. Stress following extinction learning leads to a context-dependent return of fear. Psychophysiology 2015; 52: 489–498. [DOI] [PubMed] [Google Scholar]
- 110.Antov MI, Melicherová U, Stockhorst U. Cold pressor test improves fear extinction in healthy men. Psychoneuroendocrinology 2015; 54: 54–59. [DOI] [PubMed] [Google Scholar]
- 111.Bentz D, Michael T, Wilhelm FH, et al. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology 2013; 38: 1186–1197. [DOI] [PubMed] [Google Scholar]
- 112.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 2007; 133: 25–45. [DOI] [PubMed] [Google Scholar]
- 113.Van Stegeren AH, Roozendaal B, Kindt M, et al. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem 2010; 93: 56–65. [DOI] [PubMed] [Google Scholar]
- 114.Antov MI, Stockhorst U. Stress exposure prior to fear acquisition interacts with estradiol status to alter recall of fear extinction in humans. Psychoneuroendocrinology 2014; 49: 106–118. [DOI] [PubMed] [Google Scholar]
- 115.Glover EM, Jovanovic T, Mercer KB, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry 2012; 72: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hwang MJ, Zsido RG, Song H, et al. Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry 2015; 15: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Merz CJ, Tabbert K, Schweckendiek J, et al. Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci 2012; 7: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Milad MR, Igoe SA, Lebron-Milad K, et al. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 2009; 164: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zeidan MA, Igoe SA, Linnman C, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 2011; 70: 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du X, Pang TY, Mo C, et al. The influence of the HPG axis on stress response and depressive-like behaviour in a transgenic mouse model of Huntington’s disease. Exp Neurol 2014; 263C: 63–71. [DOI] [PubMed] [Google Scholar]
- 121.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol 2014; 35: 197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu J, Wu X-Y, Zhu Q-B, et al. Sex differences in the stress response in SD rats. Behav Brain Res 2015; 284: 231–237. [DOI] [PubMed] [Google Scholar]
- 123.Maeng LY, Milad MR. Sex differences in anxiety disorders: interactions between fear, stress, and gonadal hormones. [published online ahead of print April 14, 2015]. Horm Behav. doi:10.1016/j.yhbeh.2015.04.002. [DOI] [PMC free article] [PubMed]
- 124.Toufexis D, Rivarola MA, Lara H, et al. Stress and the reproductive axis. J Neuroendocrinol 2014; 26: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lebo MS, Baxter SM. New molecular genetic tests in the diagnosis of heart disease. Clin Lab Med 2014; 34: 137–156. vii–viii. [DOI] [PubMed] [Google Scholar]
- 126.Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci J Assoc Psychol Sci 2013; 8: 651–662. [DOI] [PubMed] [Google Scholar]
- 127.Casey BJ, Craddock N, Cuthbert BN, et al. DSM-5 and RDoC: progress in psychiatry research? Nat Rev Neurosci. 2013, 14: 810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lupien SJ, Sasseville M, François N, et al. The DSM5/RDoC debate on the future of mental health research: implication for studies on human stress and presentation of the signature bank. Stress 2017; 20: 95–111. [DOI] [PubMed] [Google Scholar]
- 129.Marin M-F, Zsido RG, Song H, et al. Skin conductance responses and neural activations during fear conditioning and extinction recall across anxiety disorders. [published online ahead of print April 12, 2017]. JAMA Psychiatry. doi:10.1001/jamapsychiatry.2017.0329. [DOI] [PMC free article] [PubMed]
- 130.National Advisory Mental Health Council Workgroup on Tasks and Measures for Research Domain Criteria. Behavioral Assessment Methods for RDoC Constructs. Bethesda, MD. https://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/rdoc_council_workgroup_report_153440.pdf. Accessed May 22, 2017.
- 131.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003; 160: 1554–1565. [DOI] [PubMed] [Google Scholar]
- 132.Hoge EA, Brandstetter K, Moshier S, et al. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 2009; 26: 447–455. [DOI] [PubMed] [Google Scholar]
- 133.Smith AK, Conneely KN, Kilaru V, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet Part B Neuropsychiatr Genet 2011; 156B: 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hauer D, Weis F, Papassotiropoulos A, et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit Care Med 2011; 39: 643–650. [DOI] [PubMed] [Google Scholar]
- 135.Fliege H, Rose M, Arck P, et al. The Perceived Stress Questionnaire (PSQ) reconsidered: validation and reference values from different clinical and healthy adult samples. Psychosom Med 2005; 67: 78–88. [DOI] [PubMed] [Google Scholar]
- 136.Levenstein S, Prantera C, Varvo V, et al. Development of the perceived stress questionnaire: a new tool for psychosomatic research. J Psychosom Res 1993; 37: 19–32. [DOI] [PubMed] [Google Scholar]
- 137.Schulz P, Schlotz W. Trierer Inventar zur Erfassung von chronischem Sre (TICS): Skalenkonstruktion, teststatistische Überprüfung und Validierung der Skala Arbeitsüberlastung. [The Trier Inventory for the Assessment of Chronic Stress (TICS). Scale construction, statistical testing, and validation of the scale work overload.]. Diagnostica 1999; 45: 8–19. [Google Scholar]
- 138.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism 2005; 54: 20–23. [DOI] [PubMed] [Google Scholar]
- 139.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 2001; 933: 265–277. [DOI] [PubMed] [Google Scholar]
- 140.LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry 2016; 173: 1083–1093. [DOI] [PubMed] [Google Scholar]
- 141.Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry 2000; 61(Suppl 5): 43–48. [PubMed] [Google Scholar]
- 142.Foa EB. Prolonged exposure therapy: past, present, and future. Depress Anxiety 2011; 28: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 143.Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Sci Public Interest J Am Psychol Soc 2013; 14: 65–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci 2017; 18: 7–19. [DOI] [PubMed] [Google Scholar]
- 145.Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry 2004; 161: 1488–1490. [DOI] [PubMed] [Google Scholar]
- 146.De Quervain DJ-F. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. In: Ronald De Kloet E, Melly S, Oitzl, Eric V. (eds). Progress in Brain Research, Amsterdam, The Netherlands: Elsevier, 2007, pp. 239–247. [DOI] [PubMed] [Google Scholar]
- 147.De Quervain DJ-F, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. Eur J Pharmacol 2008; 583: 365–371. [DOI] [PubMed] [Google Scholar]
- 148.Wolf OT, Atsak P, de Quervain DJ, et al. Stress and memory: a selective review on recent developments in the understanding of stress hormone effects on memory and their clinical relevance. [published online ahead of print August 15, 2016]. J Neuroendocrinol. doi:10.1111/jne.12353. [DOI] [PubMed]
- 149.Yehuda R, Bierer LM, Pratchett LC, et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology 2015; 51: 589–597. [DOI] [PubMed] [Google Scholar]
- 150.Drexler SM, Merz CJ, Hamacher-Dang TC, et al. Effects of cortisol on reconsolidation of reactivated fear memories. Neuropsychopharmacol 2015; 40: 3036–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meir Drexler S, Merz CJ, Hamacher-Dang TC, et al. Cortisol effects on fear memory reconsolidation in women. Psychopharmacology (Berl) 2016; 233: 2687–2697. [DOI] [PubMed] [Google Scholar]
- 152.Helpman L, Marin M-F, Papini S, et al. Neural changes in extinction recall following prolonged exposure treatment for PTSD: a longitudinal fMRI study. NeuroImage Clin 2016; 12: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 2004; 128: 667–673. [DOI] [PubMed] [Google Scholar]
- 154.Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res 2009; 1293: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fucich EA, Paredes D, Morilak DA. Therapeutic effects of extinction learning as a model of exposure therapy in rats. Neuropsychopharmacol 2016; 41: 3092–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Breslau N, Davis GC. Chronic stress and major depression. Arch Gen Psychiatry 1986; 43: 309–314. [DOI] [PubMed] [Google Scholar]
- 157.Davidson LM, Baum A. Chronic stress and posttraumatic stress disorders. J Consult Clin Psychol 1986; 54: 303–308. [DOI] [PubMed] [Google Scholar]
- 158.Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010; 35: 2–16. [DOI] [PubMed] [Google Scholar]
- 159.Van Praag HM. Can stress cause depression? World J Biol Psychiatry 2005; 6(Suppl 2): 5–22. [DOI] [PubMed] [Google Scholar]
- 160.Schmidt U, Vermetten E. Integrating NIMH research domain criteria (RDoC) into PTSD research. [published online ahead of print March 25, 2017]. Curr Top Behav Neurosci. doi:10.1007/7854_2017_1. [DOI] [PubMed]