Abstract
Perhaps the most salient behaviors that individuals engage in involve the avoidance of aversive experiences and the pursuit of pleasurable experiences. Engagement in these behaviors is regulated to a significant extent by an individual’s hormonal milieu. For example, glucocorticoid hormones are produced by the hypothalamic-pituitary-adrenocortical (HPA) axis, and influence most aspects of behavior. In turn, many behaviors can influence HPA axis activity. These bidirectional interactions not only coordinate an individual’s physiological and behavioral states to each other, but can also tune them to environmental conditions thereby optimizing survival. The present review details the influence of the HPA axis on many types of behavior, including appetitively-motivated behaviors (e.g., food intake, drug use), aversively-motivated behaviors (e.g., anxiety-related, depressive-like) and cognitive behaviors (e.g., learning, and memory). Conversely, the manuscript also describes how engaging in various behaviors influences HPA axis activity. Our current understanding of the neuronal and/or hormonal mechanisms that underlie these interactions is also summarized.
Introduction
HPA axis overview
The HPA axis (Figure 1) originates with the hypophysiotropic neurons located in the medial parvocellular subdivision of the paraventricular nucleus of the hypothalamus (PVH; see Table 1 for a list of all abbreviations) (please also see (560) for a general overview of the HPA axis). These neurons send projections to the median eminence, and when activated, they discharge corticotropin releasing hormone (CRH) and other releasing factors into the hypophyseal portal vasculature. These releasing factors are carried to the anterior pituitary, where they stimulate corticotrophs to release adrenocorticotropic hormone (ACTH) into systemic circulation. ACTH acts on cells in the zona fasciculata of the adrenal cortex to stimulate the production and secretion of glucocorticoid hormones (e.g., cortisol in humans and corticosterone in rodents) into general circulation.
Figure 1).
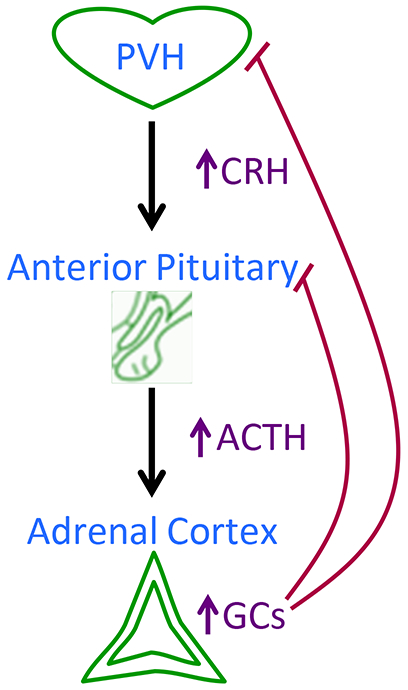
Schematic summarizing the organization of the hypothalamic-pituitary-adrenocortical (HPA) axis. Upon activation, neurons in the paraventricular nucleus of the hypothalamus (PVH) release corticotropin releasing hormone (CRH) and other releasing factors into portal circulation. These releasing factors act on the anterior pituitary to stimulate the release of adrenocorticotropin hormone (ACTH) into systemic circulation. ACTH acts on the adrenal cortex to stimulate the release of glucocorticoids (GCs; cortisol for people and corticosterone for rodents) into the bloodstream. Additionally, the glucocorticoids exert negative feedback effects at the level of the brain/hypothalamus and anterior pituitary to constrain further HPA axis activity.
Table 1.
List of abbreviations
| ACTH – adrenocorticotropin hormone |
| BLA – basolateral amygdala |
| BNST – bed nucleus of the stria terminalis |
| CMS – chronic mild stress |
| CRH – corticotropin releasing hormone |
| CRH-R1 – type 1 CRH receptor |
| CRH-R2 – type 2 CRH receptor |
| CR – conditioned response |
| CS – conditioned stimulus |
| CVS – chronic variable stress |
| DSM-5 – Diagnostics and Statistical Manual of Mental Disorders version 5 |
| FST – forced swim test |
| GR – glucocorticoid receptor |
| HPA axis – hypothalamic-pituitary-adrenocortical axis |
| icv – intracerebroventricular |
| LSI – limited sucrose intake |
| LTP – long-term potentiation |
| MAOI – monoamine oxidase inhibitor |
| MR – mineralocorticoid receptor |
| pCamKII -- phospho-calcium/calmodulin-dependent protein kinase II alpha |
| pCREB -- phospho-cAMP response element-binding protein |
| PFC – prefrontal cortex |
| PTSD – posttraumatic stress disorder |
| PVH – paraventricular nucleus of the hypothalamus |
| SAM – sympathomedullary branch of the autonomic nervous system |
| SCN – suprachiasmatic nucleus of the hypothalamus |
| SNP – single-nucleotide polymorphism |
| SSRI – selective serotonin reuptake inhibitor |
| SPT – sucrose preference test |
| TCA – tricyclic antidepressant |
| TeCA – tetracyclic antidepressant |
| TST – tail suspension test |
| UR – unconditioned response |
| US – unconditioned stimulus |
| VTA – ventral tegmental area |
Importantly, CRH neurotransmission is not limited to communication between the PVH and anterior pituitary. CRH is also expressed in other stress-regulatory brain regions, including the central amygdala and bed nucleus of the stria terminalis (BNST) (337, 376, 385, 529). Moreover, it has two receptors (CRH receptors 1 (CRH-R1) and 2 (CRH-R2)), and the affinity of CRH for CRH-R1 is much greater than for CRH-R2 (whereas another CRH-related peptide, urocortin, binds preferentially to CRH-R2 versus CRH-R1) (92, 96, 295, 399, 571). Both CRH-R1 and CRH-R2 are broadly expressed in brain and periphery (91, 414). Together this suggests that interventions that target CRH signaling have the capacity to influence the HPA axis, as well as other stress-related outcomes, through actions at multiple receptor types in multiple brain regions.
Circadian regulation of the HPA axis
The HPA axis displays a low level of basal activity that varies with time of day. ACTH and glucocorticoid levels are higher near the onset of the waking period (i.e., morning in people, and evening in nocturnal rodents like rats and mice), and are lower near the onset of the inactive period (please see (517) for a detailed review of HPA axis rhythmicity). This circadian regulation is largely set by the suprachiasmatic nucleus of the hypothalamus (SCN) (517), the central circadian pacemaker, and results in both elevated circulating ACTH and increased adrenal responsiveness to ACTH, that combine to increase circulating glucocorticoids near the circadian peak (554). This rise in glucocorticoids may then act on peripheral tissues to entrain their inherent circadian pacemakers, thereby keeping them in phase with the SCN (26, 120, 179, 273, 507).
Stress regulation of the HPA axis
Stress is often defined as a real or perceived threat to homeostasis or well-being. During exposure to stress, information about the stressor is processed by a complex network of brain circuitry that includes the PVH. This brain network ultimately evokes physiological responses to the stress, including activation of the HPA axis. The HPA axis has the capacity to exert widespread, long-term effects via the increased circulating glucocorticoids. Glucocorticoids mobilize energy (e.g., increasing liver gluconeogenesis to promote hyperglycemia (35)) and promote survival during an acute stress challenge.
However, repeated or sustained activation of the physiological stress responses, as occurs during chronic stress, evokes a number of long term adaptations to these systems (please see (330, 425) for detailed reviews of chronic stress effects). Altered gene expression and synaptic plasticity in stress-regulatory brain regions lead to persistent changes in stress system function (560). For instance, chronic stress leads to dendritic atrophy in stress-inhibitory brain regions like the hippocampus and medial prefrontal cortex (PFC) (304, 426), and dendritic branching in stress-excitatory brain regions like the amygdala (574). This is accompanied by a shift in PVH inputs to favor neuronal excitation (157), elevated CRH mRNA expression in the PVH (156, 213), and adrenal hypertrophy (157, 213, 557). The net result of these various changes is increased HPA axis excitability, leading to elevated basal and stress-evoked glucocorticoid levels that evoke physiological changes that include atrophy of the thymus gland (11, 189, 562).
Glucocorticoid signaling
Glucocorticoids signal via binding to their receptors, both the type I mineralocorticoid receptors (MR) and the type II glucocorticoid receptors (GR). MR has a high affinity for glucocorticoids, and is nearly fully occupied at relatively low glucocorticoid concentrations (258, 336). MR is located in the hippocampus, amygdala, lateral septum, olfactory nucleus, cortex, and brainstem (258). In contrast, GR has a lower affinity for glucocorticoids and is occupied when glucocorticoids concentrations are high, such as after stress (431). GR is widely expressed throughout the brain and body, including in the anterior pituitary and brain regions that regulate HPA axis activity (201, 350, 432). This anatomical arrangement allows glucocorticoids to act on GR and/or MR in the PVH, pituitary and other stress-regulatory brain circuitry to exert negative feedback (259, 357, 358). By limiting their own production, glucocorticoids contribute to the termination of the HPA axis stress response.
The widespread expression of GR and MR throughout the brain also includes regions that are critical for regulating behavior including the PFC, amygdala, and hippocampus. This suggests that the HPA axis is well-positioned to modulate a wide variety of behavioral processes, including stress-related behaviors (e.g., depression-like and anxiety-related), rewarding behaviors (e.g., use of drugs of abuse and eating highly-palatable foods), and learning and memory. This contention is supported by a large body of scientific research, which is summarized below.
HPA axis interactions with stress-related behaviors
Exposure to stress or trauma is linked with the development of several neuropsychiatric disorders in humans, including depression, anxiety disorders and posttraumatic stress disorder (PTSD), as well as the onset of depression-like and anxiety-related behaviors in rodents (42, 49, 54, 78, 116, 187, 188, 215, 227, 256, 266, 272, 327, 329, 393, 409, 468, 566, 579, 617). In the following sections, we first provide a brief overview of the evidence for HPA involvement in various stress-related neuropsychiatric disorders; this information is provided as a framework for the subsequent descriptions of the related rodent-based research. Readers are encouraged to consult the Comprehensive Physiology review by Lauren Jacobson (231) for a more thorough review of these relationships in people.
Depression
Association of HPA axis dysfunction with depressive illness in humans
Depression is a leading cause of disability, with more than 350 million people suffering from depression worldwide (316, 598). Depression is marked by mood and behavioral disturbances. Affected persons feel discouraged, sad, hopeless, unmotivated, or disinterested in life in general. Depression interferes with the patient’s ability to complete daily activities and to find enjoyment in once pleasurable pursuits, and can even lead to suicidal thoughts or actions. Importantly, chronic stress is a primary risk factor for the development of depression. Patients who have a history of stressful life events are more likely to become depressed, with stress contributing to both the initial onset of depression as well as recurrence of depressive bouts (116, 256, 327, 393).
The contribution of stress to the pathobiology of depression has been related in part to the dysregulation of the HPA axis activity that accompanies chronic stress exposure. Many patients with depressive disorder show signs of HPA axis hyperactivity at multiple levels of the axis, including the brain, pituitary, and adrenal glands. First, several studies have indicated that CRH hypersecretion occurs in depression. The concentration of CRH in cerebrospinal fluid is elevated during depression (27, 362, 363, 439, 594). Also, post-mortem studies reveal that the hypothalami of depressed patients have a greater number of CRH positive neurons (28, 424), as well as increased expression of CRH mRNA (423). Second, depression is associated with indices of increased activation of pituitary corticotrophs. Circulating ACTH levels are elevated in many patients with depression (388, 402) and congruently pituitary size, as measured by MRI, is increased as well (269). Third, depression is linked with signs of increased adrenocortical tone. For example, many depressed patients have elevated circulating glucocorticoids (69, 177, 221, 222, 362, 382, 388), including disruption of the normal glucocorticoid circadian rhythm. More specifically, depressed patients often have elevated cortisol secretion during the late evening and night, a time of day corresponding to the nadir of the circadian rhythm, suggesting a possible disinhibition of normal HPA axis activity (86, 123, 180, 325, 427, 458). Consistent with elevated glucocorticoid production, the adrenal glands are enlarged in many depressed patients (17, 361, 532). Taken together, this work suggests that patients with depression often have signs of increased HPA axis tone that can span multiple levels of the axis.
Further, evidence suggests that this sustained HPA axis over-activity is often accompanied by impaired glucocorticoid negative feedback. For example, the dexamethasone suppression test has been used to assess glucocorticoid negative feedback in depressed patients and healthy controls. In this test, the ability of the synthetic glucocorticoid dexamethasone to reduce circulating cortisol is measured. In many depressed patients the extent of cortisol suppression by dexamethasone is blunted (85, 177, 214, 220, 362, 382). This limited capacity for glucocorticoid negative feedback can then contribute to the maintenance of HPA axis hyperactivity in depression. Importantly, this impaired negative feedback appears to result largely from reduced GR function, rather than overall reductions in GR number (reviewed in (100, 387)).
It is important to also note that depression is a heterogeneous disorder, with some depressive patients exhibiting HPA axis over-activity, others under-activity, and still others normal activity. This has led some to classify depression into various subtypes that are associated with distinctive HPA axis profiles. For example, the melancholic or typical subtype of depression affects approximately 30% of depressed patients, is associated with anxiety and fear of the future, and is linked with hyperactivity of the HPA axis (176). (Note that in the Diagnostic and Statistical Manual of Mental Disorders version 5 (DSM-5), varying presentations of major depression are classified by modifiers that are applied to the primary diagnosis (16)). In contrast, the atypical subtype of depression also affects approximately 30% of depressed patients, but is associated with lethargy and fatigue, and is primarily linked with CRH deficiency and down-regulation of the HPA axis (176). Other patients may present with a combination of features rather than pure melancholic or atypical depression (176).
Contribution of HPA axis dysregulation to depressive illness in humans
Given associations between the HPA axis abnormalities and depression in some patients, it raises the possibility that HPA axis abnormalities may contribute to the pathophysiology of this disease. Indeed some clinical evidence supports this contention. First, diseases or genetic factors that affect glucocorticoid levels can also impact depression outcomes. For example, individuals with Cushing’s disease have chronically elevated glucocorticoids and are also at a greater risk for developing depression (203, 512). Also, Claes et al. (99) found a significant association between two single-nucleotide polymorphisms (SNP) within the CRH-binding protein gene (CRH binding protein is a secreted glycoprotein that has a high affinity for CRH and acts as a negative regulator of CRH activity (95)) and depressive disorder, suggesting that altered CRH signaling can contribute to the development of depressive behaviors. Another study found a protective SNP haplotype that may influence gene expression of the arginine vasopressin 1B receptor gene (vasopressin can act through this receptor to modulate ACTH release from the pituitary) whereby individuals carrying this gene were less likely to develop depression (581). Second, effective treatment of depression with an antidepressant, such as imipramine, also improves HPA axis function and regulation (435, 458, 615). Adrenal enlargement subsides upon recovery from the disease (455), and HPA axis normalization during antidepressant treatment is associated with a reduced rate of remission (387). Third, drug interventions that target the HPA axis may help to improve depression symptoms. More specifically, investigators have used several different approaches to reduce HPA axis tone (e.g., by reducing either the amount of glucocorticoids present and/or by limiting glucocorticoid actions at GR) and have seen beneficial effects in some patients. These approaches include treatment with ketoconazole, a drug that inhibits glucocorticoid biosynthesis and antagonizes GR (593); metyrapone, a drug that blocks production of steroid hormones including glucocorticoids (369, 429); and RU486, a GR antagonist (37, 38, 356, 607). Likewise, a CRH-R1 antagonist can also improve depression symptoms (219, 614). When taken as a whole, the normalization of HPA axis tone by some antidepressant therapies, the increased occurrence of depression in patients with HPA axis-based diseases (e.g., Cushing’s) or HPA axis-regulatory SNPs, and the effectiveness of pharmacological treatments targeting the HPA axis, suggests that HPA axis dysfunction may play a causative role in development of depression in some patients.
However, once again it is important to note that not all depressed patients show signs of HPA axis hyperactivity. This indicates that any causal role that HPA axis over-activity plays in the development of depression is not universal; leading to critical questions regarding how and when HPA axis dysfunction contributes to depression. Thus, many investigators have been using non-human animal models to provide additional mechanistic insights into the interactions between HPA axis tone and depressive-like behaviors.
Depressive-like behavior in rodents
Common assessments of depression-like behavior in rodents
Rodents have been used extensively to study the role of the HPA axis in promoting depression-like symptoms. One of the core features of depressive illness is altered mood, in which patients feel helplessness and dread about future prospects due to feelings of personal deficiency and failure (176). In rodents, behavioral despair is often inferred during tests of learned-helplessness, including the forced swim test and the tail suspension test (119) (Table 2). Another primary feature of depressive illness is the inability to enjoy previously pleasurable experiences (anhedonia), and this is often assessed in rodents with a sucrose preference test (119, 246, 585) (Table 2). These common tests are summarized below:
Table 2.
Tests that are commonly used to assess depression-like behavior in rodents.
| Behavioral paradigm |
Description | Indication of depressive behavior |
Responsive to anti- depressant treatment |
References |
|---|---|---|---|---|
| Forced Swim Test (FST) | Rodent briefly placed in a deep inescapable container of water | Increased time spent immobile suggests behavioral despair | Yes | (298, 409, 412, 413) |
| Tail Suspension Test (TST) | Mouse briefly suspended by the tail | Increased time spent immobile suggests behavioral despair | Yes | (409, 522) |
| Sucrose Preference Test (SPT) | Rodents offered free access to water and sucrose drink | Reduced consumption of sucrose (vs. water) suggests anhedonia | Yes | (246, 481, 585, 586) |
The typical forced swim test (298, 412, 413) occurs over two consecutive days, with rats or mice being placed into a container of water that is sufficiently deep to prevent standing on the bottom, and from which there is no way to escape. On the second day, a trained observer records the amount of time spent actively swimming (i.e., searching for an escape route) vs. immobile (i.e., doing only the minimal movements necessary to prevent drowning). Increased time spent immobile is considered as a depressive-like behavior similar to behavioral despair, with antidepressant treatment reducing immobility (409, 412).
For the tail suspension test, a mouse is briefly suspended by the tail, and the amount of time spent immobile is used as an indication of depressive-like behavior (522). Again, immobility in the tail suspension test is decreased by antidepressant treatment (409, 522).
For the sucrose preference test, rodents are generally offered free access to both normal water and a modestly sweet sucrose drink (such as 1%-2% sucrose solution), and the intake of both is monitored (481, 585). Sucrose preference is expressed as the percentage of total drink intake that was derived from the sucrose solution, with reduced sucrose preference being considered a sign of anhedonia (585, 586). Chronic stress typically reduces sucrose preference, and a wide range of antidepressant treatments are effective at reversing and/or preventing stress-induced reductions in sucrose preference (87, 524, 525, 585, 586).
Chronic stress promotes depressive-like behaviors in rodents
Homotypic chronic stress paradigms:
Consistent with the association of stressful life events and depression in humans, chronic stress paradigms in rodents elicit depression-like behaviors in these behavioral tests (Table 3). A wide variety of chronic stress paradigms have been used to induce depression-like behaviors in rodents. Among the most commonly used are repeated homotypic stress paradigms, in which a single type of stress is repeatedly administered over a multiple days (215, 409). For example, repeated footshock stress in adult rodents induces immobility in the forced swim test, and these effects can be reversed by treatment with the antidepressant citalopram (271, 409). Similarly, repeated restraint stress increases immobility in the forced swim test and reduces preference for sucrose, and these effects can be prevented by antidepressant drugs such as reboxetine and desipramine (66, 197). Chronic social defeat stress increases immobility in the forced swim test, and this is prevented with reboxetine treatment (197). It is clear that many repeated homotypic stress paradigms induce behavioral indices of depression that are generally reversed by antidepressant treatment. However, it should also be noted that the HPA axis typically undergoes habituation (22), a process in which the glucocorticoid response to a particular stressor is reduced following a history of prior exposures to that same stressor. This has led some investigators to use other chronic stress paradigms that are designed to limit HPA axis habituation, in order to explore whether they similarly evoke depressive-like behaviors.
Table 3.
Summary of rodent studies exploring the link between the HPA axis and depression-like behavior. In general, manipulations that increase HPA axis tone promote depressive behaviors, whereas manipulations that limit HPA axis tone reduce or prevent them. FST = forced swim test; SPT = sucrose preference test; CRH = corticotropin releasing hormone; GC = glucocorticoid.
| HPA manipulation | Test | Behavioral Outcome |
References |
|---|---|---|---|
| Increased CRH or GC signaling (Receptor agonist, transgenic mice) | FST SPT |
↑ immobility ↓ sucrose intake |
(137, 168, 381) (181) |
| Chronic stress-induced increases in CRH and GC signaling (Homotypic or heterotypic stress paradigms) | FST SPT |
↑ immobility ↓ sucrose intake |
(1, 197, 271, 351, 352, 409, 534) (197, 215, 347, 409, 534, 585, 586) |
| Reduced CRH or GC signaling during/after chronic stress (Receptor antagonists, adrenalectomy) | FST SPT |
↓ immobility ↑ sucrose intake |
(511, 567, 600) (181, 599) |
Heterotypic chronic stress paradigms:
Chronic heterotypic stress paradigms, in which a variety of stressors are administered on an unpredictable schedule, are often used when investigators wish to minimize HPA axis habituation (215, 409). For example, in the commonly-used chronic mild stress (CMS) paradigm, rodents are subjected to an array of stressors, including overnight illumination, cage tilt, change of cage mate, and brief food or water deprivation, that are generally administered in an unpredictable order for approximately 4 weeks (585). CMS typically results in anhedonia, as assessed by the sucrose preference test, and this is prevented by treatment with a variety of antidepressants including tricyclic (TCAs) and tetracyclic (TeCAs) antidepressants, selective serotonin reuptake inhibitors (SSRIs), and monoamine oxidase inhibitors (MAOIs) (215, 347, 409, 585, 586). CMS also decreases sexual, aggressive and exploratory behavior, and causes deficits in reward system measures (586). Another closely-related heterotypic stress paradigm, chronic variable stress (CVS), consists of twice-daily exposure to one of a variety of brief stressors (e.g., hypoxia, restraint, cold exposure, placement on a slow orbital shaker platform, etc.) that are typically given over two weeks (213). CVS exposure induces anhedonia in the sucrose preference test and immobility in the forced swim test (534), suggesting that CVS induces depressive-like behaviors similar to CMS.
Early life stress paradigms:
Lastly, adverse early life events such as childhood abuse and neglect or prenatal stress have been implicated in predisposing people for depressive illness (116, 205, 218) and this has been modeled in rodents using paradigms such as maternal deprivation and prenatal stress (409). For maternal deprivation, pups are separated from their mother for designated periods of time (up to 4 hours per day) during the first few weeks of life, resulting in deprivation of maternal care, as well as changes in the behavior of the mother to the pups (409). For prenatal stress, common paradigms expose the pregnant mother to brief stressors on certain days of the pregnancy, for example, by temporarily placing her into a well-ventilated, clear plastic restraint tube (301, 409). These early life stress paradigms can lead to depression-like behaviors in the offspring later in life, including increased depression-like behavior in the forced swim test that can be reversed with antidepressant treatment (1, 301, 351, 352). Notably, early life stress can also cause HPA axis disturbances later in life, such as increased c-fos expression in the PVH (suggestive of enhanced PVH activation) and elevated glucocorticoid responses to an acute stress challenge (1, 212, 301, 406). Taken together, these data suggest that excessive HPA axis reactivity is correlated with the development of depressive-like behavior following early life stress.
Contribution of the HPA axis to depressive-like behaviors in rodents
As described above, the HPA axis undergoes changes in response to chronic stress exposure, including a shift in stress-regulatory inputs to the PVH that favors neuronal excitation (157, 304, 305, 426, 574), increased PVH expression of CRH mRNA (213, 308), adrenal hypertrophy (157, 213, 308, 558), and elevated basal and stress-evoked glucocorticoids. Notably, this shift towards greater HPA axis excitability mirrors that which occurs in a subset of depressed patients (as described above). However, despite the well-documented associations between altered HPA axis tone and depression-like behaviors, the specific role that the HPA axis plays in promoting depressive-like behaviors is not clear, particularly since only some depressive patients exhibit HPA axis over-activation.
Effect of manipulations that reduce CRH or glucocorticoid signaling on depressive-like behaviors:
Rodent models have proved useful for exploring the potential contributions of the HPA axis to depressive-like behaviors, as they provide opportunities to directly intervene in HPA axis function at multiple levels of the axis while assessing behavioral outcomes. First, interventions that limit CRH or glucocorticoid activity reduce depressive-like behaviors (Table 3). The CRH-R1 antagonist NBI30775 given following peripubertal stress reduces depression-like behaviors in the forced swim test in adulthood (567). Loss of glucocorticoids following adrenalectomy prevents the development of reduced sucrose preference following CMS (181). Likewise, the GR antagonist mifepristone prevents reductions in sucrose preference following CMS (599).
Effect of manipulations that increase CRH or glucocorticoid signaling on depressive-like behaviors:
Interventions that increase central (e.g., CRH) and/or peripheral (e.g., glucocorticoid) HPA axis tone generally promote depression-like behaviors (Table 3). For example, intracerebral administration of CRH to rats, mice and monkeys elicits depression-like behaviors such as increased immobility, behavioral despair, and distress (381). Transgenic mice that overexpress CRH mRNA (via the MT-1 promoter) have elevated plasma ACTH and corticosterone levels, and also display increased immobility in the forced swim test (168, 519). Similarly, chronic exogenous corticosterone administration reduces sucrose preference and social exploration in mice similar to CMS (181) and also increases immobility in the forced swim test in rats (137). Transgenic mice with reduced GR expression in neurons or specific brain regions generally exhibit increased glucocorticoids and increased depression-like behaviors (62, 166, 346). However, there are some conflicting behavioral results between mouse lines depending on sex, the extent of GR reduction and/or the particular brain regions targeted (167, 510, 549), indicating that further research is needed to fully understand the role of central GR signaling (and/or accompanying increases in circulating glucocorticoids) in depressive behavior. When taken as a whole, these results suggest that manipulations that increase HPA axis tone generally promote depressive behaviors, whereas manipulations that decrease HPA axis tone generally reduce them.
Mechanisms by which GR-signaling modulates depressive-like behavior:
Since evidence suggests that elevated HPA axis activity might increase depressive-like behavior, at least in part, via increased signaling at GR in brain, recent work has turned towards identifying potential downstream mechanism by which central GR could modify these behaviors. Some of this work has studied the effects of reduced GR function by using a transgenic mouse that overexpresses GR antisense RNA in neurons (reviewed in (324)). This manipulation results in markedly reduced GR expression in brain that is accompanied by increased HPA axis tone (presumably due to diminished glucocorticoid negative feedback). Notably, reduced central GR expression alters the expression of numerous other genes in the hippocampus and frontal cortex. For instance, the mRNA expression of Arc and NeuroD1 (genes that are linked with synaptic plasticity), is altered in the hippocampus of these mice. There is also decreased expression of several histone-related genes (e.g., H2AZ, HDAC2, HDAC5, MYST2, and NCor1), as well as increased expression of the CpG binding protein, MeCP2, which taken together are suggestive of altered transcriptional activity and epigenetic regulation. Consistent with the possibility that GR-signaling modulates depressive-like behavior, at least in part, via synaptic plasticity, treatment of male rats with the GR antagonist mifepristone prevents both CMS-induced alterations in hippocampal synapsin I expression, and reduced sucrose preference (599). Lastly, the expression of several clock genes (e.g., rev-ervB, Per 1, Per 3, NPAS2) is altered in the hippocampus ((26) and reviewed in (324)). Thus, it is possible that GR could impact depressive behavior through effects on multiple, and potentially overlapping, neurobiological mechanisms, including synaptic plasticity, epigenetic regulation, and clock/circadian gene regulation.
Summary of depressive illness and depression-related behaviors
Chronic stress increases both HPA axis tone and risk for developing depressive illness and many, but not all, depressed patients exhibit signs of HPA axis over-activation. This has led some to speculate that HPA axis over-activation may contribute to the etiology of depression in at least a subset of patients. Chronic stress paradigms in experimental animals largely corroborate these clinical observations. Mechanistic studies in experimental animals are directed towards further understanding the nature of these interactions, for example by studying the possible roles of synaptic plasticity, epigenetics and/or clock genes to these interactions, in order to uncover novel therapeutic targets.
Anxiety- and Trauma-Related Disorders
In addition to evoking depressed mood, stressful experiences can also produce feelings of anxiety or fear. Anxiety and fear are related emotions that are often distinguished by whether one is referring to a general state of ‘heightened awareness’ that accompanies a common non-imminent threat (worry about potential future event – anxiety) versus a specific response to an overt, actual threat (reaction to real current event -- fear) (459). Anxiety itself can be an adaptive and can lead to beneficial behaviors to manage threat (459). Consider the adaptive anxiety response when walking alone down a dark alleyway in an unfamiliar part of town at night, where a heightened sense of awareness and vigilance could be advantageous should an actual threat materialize. In contrast, chronic feelings of anxiety in unwarranted surroundings may manifest as an anxiety disorder in which uncontrollable anxiety impairs an individual’s ability to manage daily tasks (16). The following section describes the relationship between HPA axis function and anxiety-related behaviors. Since the related psychological state of fear is generally studied in rodents by use of conditioning paradigms, research studying HPA interactions with fear processes will be summarized within the final section which is focused on behaviors related to learning and memory.
Association of HPA axis dysfunction with anxiety- and trauma-related disorders in humans
Anxiety- and trauma-related disorders are extremely common, affecting as much as 18% of the United States population each year (251, 252). The DSM-5 divides this large group of disorders into those that are anxiety-related, such as generalized anxiety disorder, panic disorder, social anxiety disorder and phobias, versus those that are trauma-related like PTSD (16).
PTSD is perhaps the most studied of these types of disorders, and is a condition that develops in a subset of people after experiencing severe emotional distress and trauma. As such, PTSD is highly prevalent in war fighters, first responders, and abuse survivors (42, 54, 468). PTSD patients face chronic, debilitating symptoms including intrusive memories or nightmares, such as reliving a traumatic event; increased anxiety, emotional arousal, irritability, physiological distress, guilt, shame and hopelessness; avoidance of reminders of trauma; memory problems; lack of interest in things once enjoyed; engagement in dangerous behaviors; and difficulty maintaining relationships (16). In terms of the HPA axis, PTSD appears to have a general HPA axis profile suggestive of overall HPA hypoactivity (despite increased CRH) (23, 54, 67, 223, 231, 503, 526). For instance, many, though not all, PTSD patients have enhanced HPA axis suppression in the dexamethasone suppression test (249, 518, 603), that is accompanied by reduced circadian glucocorticoid levels (223, 231, 323, 602-604, 606). Since the clinical research on PTSD is highly variable, readers are referred to the Comprehensive Physiology review by Lauren Jacobson (231) as well as other reviews (602, 604, 606) for more detailed descriptions of this complex literature.
In contrast to the trauma-related disorder PTSD, some evidence suggests that the broad class of anxiety-related disorders is generally linked with an increase in HPA axis tone (in at least some of these patients), and readers are again referred to the Jacobson review (231) for additional details on this topic. However, as the broad class of anxiety-related disorders contains several distinct psychiatric illnesses, which each have important, unique characteristics, it is perhaps not surprising that the relationship with the HPA axis can vary considerably among these specific disorders.
Thus, while HPA axis abnormalities are not uniform for all anxiety- and trauma-related disorders, this is likely consistent with the heterogeneity of these broad classes of neuropsychiatric diseases. HPA axis hyperactivity can occur in some anxiety disorders, while PTSD may be accompanied by HPA axis hypoactivity in some patients. These discordant observations have led to questions regarding the extent to which the HPA axis might contribute to the development of anxiety- and trauma-related disorders in people, particularly since many of these disorders are also highly comorbid with depressive illness (494).
Contribution of HPA axis dysfunction to anxiety- and trauma-related disorders in humans
As anxiety- and trauma-related disorders can be accompanied by altered HPA axis tone in some patients, this raises the possibility that the HPA axis may be a causative factor in the development of these disorders. Limited clinical evidence supports this idea. For instance, natural genetic variations in HPA-regulatory genes, including CRH, CRHR1, and FKBP5, may increase risk for developing an anxiety- or trauma-related disorder and/or predict treatment outcomes (54, 289, 292, 504). Further, PTSD may develop preferentially in people with low cortisol levels or an exaggerated glucocorticoid negative feedback effect (605, 606), possibly by interfering with appropriate sympathetic responses and memory consolidation (420, 602, 606). Taken together, these findings suggest that altered HPA axis tone may influence an individual’s risk for developing an anxiety disorder.
Furthermore, HPA axis-based interventions may be an effective therapeutic approach if the HPA axis contributes to the etiology of anxiety disorders. In support of this idea, treatment with glucocorticoids may reduce symptoms in those with phobias or PTSD, as well as reduce the likelihood of developing PTSD when administered following trauma (9, 239, 420, 421, 474, 513, 616). However, it should be noted that glucocorticoid treatment in non-anxious individuals (e.g., as treatment for inflammatory illnesses) can contribute to anxiety and panic disorder occurrence (70, 250, 286, 359, 428). Taken together, these findings indicate that it is critical to maintain an appropriate balance of HPA axis activity, as both insufficient and excessive glucocorticoid signaling may contribute to anxiety-and trauma-related behaviors. It should also be noted that some research has also explored whether the specific targeting of CRH signaling might be an effective therapeutic strategy. For instance, Zobel et al. (614) administered the CRH-R1 receptor antagonist R121919, a water-soluble pyrrolopyrimidine with high affinity for CRH-R1 receptors, to patients with major depression and found that patient- and clinician-rated anxiety and depression scores were significantly improved over the course of treatment. However, treatment with the selective CRH-R1 antagonist pexacerfont did not reduce anxiety symptoms in patients with generalized anxiety disorder (114). Such discordant clinical findings may indicate that the relative contribution of glucocorticoid and/or CRH signaling to anxiety varies markedly among the disparate types of anxiety- and trauma-related disorders, and/or among various sub-types of patients. This has further led some to speculate that HPA axis-based therapies may need to be targeted to appropriate patient sub-groups that have the corresponding HPA axis anomaly (231). The wide disparity among the various clinical findings underscores the fact that it is difficult to efficiently explore the many factors that influence the development of complex anxiety disorders through clinical research alone. As a result, preclinical research in experimental animals has provided additional insights into the complex relationships between stress, the HPA axis, and anxiety-related behaviors.
Anxiety-related behaviors in rodents
Common assessments of anxiety-related behaviors in rodents
Much of the preclinical research in rodents has been directed towards understanding the neurobiological factors/mechanisms that regulate anxiety and its related behaviors. This work necessitated the development of various behavioral assays intended to measure an anxiety-like state in rodents (119) (Table 4). The most commonly used tests include:
Table 4.
Test that are commonly used to assess anxiety-related behaviors in rodents.
| Behavioral paradigm |
Description | Indication of anxiety-related behavior |
Responsive to anxiolytic and/or anxiogenic agents |
References |
|---|---|---|---|---|
| Open Field Test (OFT) | Rodent explores an open arena | Reduced time spent in center of arena; less exploratory locomotion | Yes | (141, 200, 418) |
| Elevated Plus Maze (EPM) | Rodent explores elevated platform that contains 2 open and 2 enclosed arms | Reduced time spent in open arms of maze; less exploratory behaviors | Yes | (106, 152, 154, 265, 397, 547) |
| Startle Reflex | Measures amplitude of the startle response to an unexpected stimulus | Increased amplitude of startle response | Yes | (88, 129, 187, 188, 210, 545) |
| Light-Dark Box | Rodent explores an apparatus that contains a well-lit and a dark compartment | Increased time spent in the dark compartment | Yes | (84, 117, 253) |
Open field test: The open field test is based on the observation that rodents display an innate avoidance of large open spaces (418). In this test, the subject (either a rat or mouse) is placed into an unfamiliar open arena and they are allowed to freely explore the space while their behavior is recorded (200). A low anxiety state is inferred when the rodent spends much of their time walking around the space, particularly the central portion of the arena (418). In contrast, a high anxiety state is inferred when the rodent displays less exploratory locomotion and avoids the center of the arena (418). Variations on this test involve placing a novel object, highly-palatable food, or a conspecific in the center of the field in order to provide a conflict between the desire to avoid open spaces and the desire to explore the item in the center; in these procedures the latency and time spent interacting with the central object are used as additional indices of anxiety-related behavior (141, 200). Some anxiolytic and anxiogenic agents either increase or decrease, respectively, exploratory behavior in the open field (for detailed review see (418)).
Elevated plus maze: The elevated plus maze is based on the observation that rodents display an innate avoidance of heights and/or exposed spaces (397). This test involves placing the subject (either rat or mouse) onto an elevated platform that is shaped like a “+”, in which two non-adjacent arms of the “+” have walls (enclosed arms), and the other two arms do not (open arms). The subject is allowed to move freely through the apparatus and the resulting behavior is monitored (397). A low anxiety state is inferred when the subject spends large amounts of time in the open arms of the platform, and/or engages in ethological indices of exploratory behavior (e.g., looking out over the edge of the maze) (152, 154, 397, 547). In contrast, a high anxiety state is inferred when the subject avoids the open arms of the platform (152, 154, 397, 547). Importantly, performance on the elevated plus maze can be modulated by treatment with known anxiolytic and anxiogenic drugs, which either reduce or enhance anxiety-like behaviors, respectively (106, 265).
Startle reflex: This test is based upon an innate reflex that causes rapid and abrupt movement (e.g., eyeblink, whole-body startle) in response to an unexpected stimulus, such as a sudden loud noise or puff of air (88, 187). The amplitude of the response, often measured as the magnitude of the reflexive movement, is modulated by an individual’s emotional state such that increased stress or anxiety potentiate this reflex (88, 187). Likewise, anxiogenic and anxiolytic drugs modulate startle responses (129, 187, 210, 545). Notably, this test can be used in both human and non-human experimental subjects and is therefore a valuable link between preclinical and clinical research (187, 188).
Light-dark box test: This test is based upon the innate inclination of rodents to prefer dark areas (117). For this test, the subject (rat or mouse) is placed into an apparatus that contains two connected compartments – one that is dark and the other that is well-lit. The subject is allowed to freely explore the apparatus and behavior is recorded (117). A low anxiety state is inferred when the subject spends large amounts of time in the illuminated compartment, whereas a high anxiety state is inferred when they spend greater time in the dark compartment (117). As would be expected, behavior in this test is sensitive to both anxiogenic and anxiolytic drugs that decrease and increase time spent in the well-lit compartment, respectively (84, 117, 253).
Collectively, these various rodent behavioral assays are routinely used in ongoing preclinical anxiety research. Much of this work has been directed towards understanding the role that stress and the HPA axis plays in promoting anxiety- and trauma-related behaviors.
Stress promotes anxiety-related behaviors in rodents
Acute stress paradigms:
Just as stressful or traumatic life experiences increase risk for developing an anxiety-related disorder in people, stress can also increase indices of anxiety-related behaviors in experimental animals (Table 5). A single acute stress exposure given prior to behavioral assessment is sufficient to increase anxiety-like behaviors. For instance, anxiety-related behaviors are increased in the elevated plus maze test when rodents experience a restraint, swim, isolation, or predator stress prior to testing (for detailed review see (266)). The anxiogenic effects of stress exposure can also persist for weeks after the acute stress exposure (266). Likewise, the startle reflex is potentiated by prior stress in both human and non-human subjects (187, 188). Moreover, the extent to which anxiety-related behaviors are potentiated by a prior acute stress appears to be directly related to the overall intensity of the stressful experience (4, 266, 335). For example, more severe behavioral anxiety is observed following stronger stressors (5, 321, 612); when the stressor is uncontrollable (265); or when minimal post-stress recovery times are permitted (265, 612).
Table 5.
Summary of rodent studies exploring the link between the HPA axis and anxiety-related behaviors. In general, manipulations that increase HPA axis tone promote anxious behaviors, whereas manipulations that limit HPA axis tone reduce or prevent them. OFT= open field test (includes interaction tests); EPM= elevated plus maze; STR= startle reflex; LDB= light-dark box; CRH = corticotropin releasing hormone; GC = glucocorticoid.
| HPA manipulation | Test | Behavioral Outcome |
References |
|---|---|---|---|
| Increased CRH or GC signaling (Receptor agonist, transgenic mice) | OFT EPM STR LDB |
↓ open exploration ↓ open exploration ↑ magnitude ↓ light exploration ↑ light exploration (only in animals that are inherently high- anxiety) |
(68, 141, 192, 262, 381, 520) (141, 186, 192, 492, 520) (141, 262, 282, 381, 530) (168, 192, 381, 514) (514) |
| Acute stress-induced increases in CRH and GC signaling (diverse stressors) | OFT EPM STR LDB |
↓ open exploration ↓ open exploration ↑ magnitude ↓ light exploration |
(1, 566, 579) (5, 266, 321, 335, 566, 579, 612) (187) (579) |
| Chronic stress-induced increases in CRH and GC signaling (homotypic and heterotypic stress paradigms) | OFT EPM STR |
↓ open exploration ↓ open exploration ↑ magnitude |
(49, 207, 272, 409) (227, 272, 617) (207, 227, 272, 617) |
| Reduced CRH or GC signaling during/after chronic stress (or in animals that are inherently high-anxiety) (Receptor antagonists, adrenalectomy, transgenic mice) | OFT EPM STR LDB |
↑ open exploration ↑ open exploration ↓ magnitude ↑ light exploration |
(240, 501, 543, 579) (63, 208, 262, 501, 502, 549, 579) (262) (63, 502, 543, 549) |
Chronic stress paradigms:
Given that acute stress can increase multiple indices of anxiety-related behaviors in rodents, it is perhaps not surprising that chronic stress paradigms can elicit similar effects. Chronic social stress, in which a rodent is repeatedly exposed to an aggressor and recurrently experiences social defeat, can evoke anxiety-related behaviors (49, 409). This is particularly evident when behavioral anxiety is measured in subsequent social interaction tests, in which the defeated rodent engages in fewer social interactions with an unfamiliar conspecific (49). Similarly, when rats are given a chronic psychosocial stress paradigm that combines occasional predator exposure with daily changes of cage mate (i.e., ‘unstable housing conditions’), the rats spend less time in the open arms of the elevated plus maze and have an increased magnitude of acoustic startle, suggestive of enhanced anxiety (617).
Early life stress paradigms:
Importantly, evidence from preclinical research also suggests that stressful experiences early in life, such as prenatal and neonatal stress, can impact anxiety-related behaviors and that these effects can persist into adulthood. For example, adult rodents that previously experienced prenatal stress displayed increased behavioral anxiety in the elevated plus maze and open field tests (566, 579). The type of maternal care that neonatal rats receive can also predict anxiety-like behaviors later in life, with lower levels of maternal care predisposing for increased anxiety in the offspring when they reach adulthood. For instance, the adult offspring of mothers that inherently displayed low levels of licking, grooming, and arched-back nursing spend less time exploring the central portion of an open field and have increased latency to eat in a novel environment (79). Similarly, repeated, prolonged maternal separation stress in early life can increase freezing behavior in the open field test, decrease exploration of a novel environment, increase time spent in the closed arms of the elevated plus maze, and increase acoustic startle responses – all of which are suggestive of increased behavioral anxiety (78, 206, 227, 272). Some reports suggest that maternal separation does not reliably increase anxiety-related behaviors (341), possibly because a combination of stressful experiences early in life and during adulthood is more effective at inducing anxiety. For instance, a history of neonatal maternal separation and adult chronic restraint stress reduces time spent in the open arm of the elevated plus maze to a larger extent than either the maternal separation or chronic restraint alone (329). These studies suggest that early life stress can predispose an animal to an anxiety phenotype later in life. Taken together with observations that stress during adulthood similarly modulates anxiety end points, it follows that stressful life experiences in general can promote anxiety-like behaviors.
Contribution of the HPA axis to anxiety-related behaviors in rodents
Association between increased HPA axis tone and behavioral anxiety after stress exposure:
Given the clear relationship between stress and anxiety, considerable preclinical research has been focused on exploring whether/how the HPA axis mediates the anxiogenic effects of stress. Some of this work has been directed towards asking whether chronic stress induces parallel changes in both HPA axis and behavioral anxiety end points. Several of the stress–anxiety studies described in the preceding section also assessed HPA end points, and generally found that the increased anxiety-related behaviors were associated with indices of increased HPA axis activity. For example, while chronic psychosocial stress (i.e., occasional predator exposure combined with unstable housing conditions) increased anxiety-related behaviors in the elevated plus maze and acoustic startle tests, this was also accompanied by facilitated corticosterone responses to stress, adrenal hypertrophy and thymic involution – all indices of chronic HPA axis activation (617). Similarly, the degree of behavioral anxiety that is induced in adult rodents that previously received prenatal stress is related to the magnitude of their plasma corticosterone response to stress (566, 579). The anxiogenic effects of prior neonatal maternal separation stress are also linked with indices of increased HPA axis tone. Adult rats with a prior history of neonatal maternal separation stress had increased expression of both CRH mRNA and immunoreactive-CRH peptide in the PVH, central nucleus of the amygdala, BNST and locus coeruleus/parabrachial nucleus, and this was accompanied by a higher concentration of immunoreactive-CRH in the cerebrospinal fluid (408). CRH-R1 mRNA expression and receptor binding were also increased in the PVH and locus coeruleus/parabrachial nucleus (408), suggesting the potential for long-term increases in available CRH receptors. Likewise, the anxiogenic effect of receiving poor maternal care (i.e., having a mother with inherently low levels of licking, grooming and arched-back nursing) is associated with increased HPA axis stress responses, elevated CRH mRNA expression in the PVH, and reduced glucocorticoid negative feedback (79, 291). Collectively, these correlative results suggest that chronic stress could impact anxiety-related behaviors, at least in part, via stress-induced enhancements of HPA axis tone. Such increases in anxiety-related behaviors following stress might promote survival by reducing risk-taking and increasing vigilance when in a dangerous environment.
Association between inherent differences in anxiety-related behaviors and HPA axis tone:
Other preclinical research has explored the relationship between the HPA axis and anxiety by taking advantage of inherent differences in anxiety-related behaviors between individuals. For instance, rats can be divided into those that are naturally high responding (defined as those with the highest amount of exploratory locomotion when placed into an unfamiliar environment) versus those that are naturally low responding (those with the lowest amount of exploratory locomotion), such that the low responding rats resemble a risk-aversive, high-anxiety phenotype (240). Notably, the expression of GR varies with response status, such that low responding (high-anxiety) rats have increased GR mRNA expression in the hippocampus that is accompanied by reduced plasma corticosterone responses to some (e.g., the light-dark box test), but not all (e.g., restraint), acute stressors (240, 241). High responding rats have lower GR mRNA expression in the hippocampus (240, 241) and have higher plasma glucocorticoid levels during recovery from social stress or restraint stress (82, 241). Taken together, this suggests that intrinsic differences in corticosterone–GR signaling may influence inherent anxiety-related traits. In support of this idea, administration of a GR antagonist into the hippocampus of low responding rats (to decrease GR signaling) increases their exploratory behavior to levels that are comparable to the high responders, suggesting that this intervention reduced their behavioral anxiety (240). Similar results are seen if individual differences in anxiety outcomes are explored in mice. Selective breeding of CD-1 mice based on their performance in the elevated plus maze has been used to generate mice that are inherently high-, normal-, or low-anxiety, with high-anxiety mice typically having open arm times of less than 15%, normal-anxiety mice having open arm times of 35-40%, and low-anxiety mice having open arm times of over 60% (514). Mice that are inherently high-anxiety have higher GR mRNA expression in the PVH, hippocampus, and pituitary, as well as higher GR protein expression (assessed by western blot) in the PFC and amygdala (514). The high-anxiety mice also have a lower plasma corticosterone response to acute stress, as well as lower plasma corticosterone levels in a CRH/dexamethasone test, suggesting the possibility of increased glucocorticoid negative feedback (514). Chronic treatment with glucocorticoids normalizes anxiety-related behaviors in the high-anxiety mice, though this same treatment increases anxiety outcomes in those that were originally characterized as normal-anxiety (514). When taken together, both the research in rats and mice suggest that individuals that are prone to anxiety have increased GR mRNA expression that is linked with reduced HPA reactivity to stress, and that manipulation of corticosterone–GR signaling reduces anxiety measures in anxious individuals. Of note though, the same manipulations can have opposing effects in those that are otherwise normal-anxiety (i.e., non-anxious), suggesting that anxiety-related behaviors can be modulated by either excessive or insufficient GR signaling. Furthermore, there are other known stress-regulatory factors that differ between rodents that are inherently low- or high-anxiety. For example, high-anxiety is associated with elevated CRH mRNA expression in the PVH (240), lower CRH mRNA expression in the central nucleus of the amygdala (240) and lower CRH-R1 mRNA expression in the pituitary (514). This suggests the potential for additional factors, like intrinsic differences in CRH neurotransmission, to also contribute to inherent individual differences in anxiety-like behaviors.
The strong associations between altered HPA axis stress reactivity and enhanced anxiety-related behaviors (as described above) has led many to speculate that the HPA axis plays a causal role in promoting anxiety. If this is the case, then one might predict that modulation of HPA axis function at multiple levels of the axis could influence behavioral anxiety. Research of this nature has focused largely on assessing anxiety end points after manipulating either CRH or glucocorticoid signaling in brain.
Effect of manipulations that increase central CRH signaling on anxiety-related behaviors:
In general, experimental approaches that augment CRH actions in brain induce both elevated HPA axis activity and behavioral anxiety. Exogenous central (intracerebroventricular, icv) CRH administration to rats increases plasma ACTH and corticosterone levels (141). It also suppresses exploratory behavior in a novel environment, decreases social interaction, potentiates acoustic startle responses, and decreases open arm time on the elevated plus maze– all of which are suggestive of increased anxiety (68, 141, 262, 381, 493, 530). Similarly, transgenic mice that overexpress CRH exhibit signs of chronic HPA axis activation (e.g., elevated basal plasma corticosterone, adrenal hypertrophy, and blunted glucocorticoid negative feedback), as well as behavioral anxiety in the elevated plus maze and light-dark box (although interpretation of anxiety-related behavior in these mice is confounded by general locomotor deficits, and specific behavioral effects also vary somewhat between the different CRH-overexpressing mouse lines) (191, 192, 520). Taken together, this evidence suggests that experimental interventions that increase brain CRH signaling can increase behavioral anxiety.
The anxiogenic effects of CRH appear to be primarily mediated via its actions at CRH-R1 since interventions that limit CRH-R1 signaling are generally anxiolytic. First, knockdown of CRH-R1 expression in rat brain (via icv infusion of antisense oligodeoxynucleotides that are targeted against CRH-R1) prevents the anxiogenic effects of central CRH administration (501). Second, acute icv treatment with CRH-R1 antagonist decreases both anxiety-related behaviors and HPA axis activity in rats (208). Similarly, treatment of adult male rhesus macaques with the CRH-R1 antagonist antalarmin inhibits a constellation of anxiety-related behaviors following intense social stress, such as body tremors, grimacing, teeth gnashing, urination, and defecation, and also increases exploratory and sexual behaviors that are typically suppressed during stress (196). Third, genetic disruption of the CRH-R1 gene in mice decreases both anxiety-related behaviors and HPA axis activity (502, 543). Together these results suggest that endogenous CRH neurosignaling impacts anxiety outcomes, particularly via actions at CRH-R1. Of note, some evidence also suggests that CRH-R2 can influence anxiety-related behaviors and is perhaps anxiogenic; readers are referred to (25) for a detailed review of this topic.
As described in the previous two paragraphs, manipulations that alter CRH signaling generally impact both behavioral anxiety and HPA axis activity (with increased anxiety associating with increased HPA tone). However, the pervasive nature of CRH signaling in stress-regulatory pathways complicates interpretation of these results. First, it is difficult to determine the anatomical locus for the effects of CRH-based interventions on HPA end points, since they could act directly at the level of PVH – anterior pituitary communication, or at other brain sites that are positioned to modulate HPA axis tone indirectly. For example, CRH appears to promote anxiety, at least in part, via actions in the limbic forebrain, including the PFC, hippocampus, and basolateral amygdala (BLA) (354), which are each able to indirectly influence HPA tone (560). Second, it is difficult to determine the extent to which the anxiogenic effects of CRH depend on the HPA axis, since CRH signaling occurs in multiple brain regions that could directly impact behavior in ways that go beyond their ability to regulate the HPA axis. For instance, mice that are genetically-deficient for CRH binding protein have increased anxiety-related behaviors despite normal HPA axis tone (245), suggesting the CRH can modulate anxiety in ways that are distinct from its role in HPA axis regulation.
Effects of manipulations that alter GR signaling on anxiety-related behaviors:
In addition to CRH signaling, preclinical research has also focused on understanding whether/how glucocorticoids, as the primary physiological end products of the HPA axis, impact behavioral anxiety. Much of this research has employed transgenic mouse models in which GR has been constitutively deleted from various brain regions. Mice with a genetic deletion of GR throughout the nervous system exhibit decreased anxiety-related behaviors despite having elevated circulating glucocorticoid levels, which presumably result from impaired glucocorticoid negative feedback in brain (549) (although it should be noted that there is also a potential contribution of developmental effects due to GR deletion early in life). Further, a more specific constitutive deletion of GR that is limited to the limbic forebrain (e.g., cortex, hippocampus and BLA, but not the central nucleus of the amygdala) similarly leads to increased HPA axis responses to stress, as well as changes in some anxiety-related behaviors (63). However, these mice display a complicated mixture of behavioral responses in a battery of anxiety tests, including increased open arm time and locomotion in the elevated plus maze, unaltered behavior in the open field test, and increased exploration and time spent in the well-lit chamber in the light-dark test, suggesting that their behavior may indicate stress-induced impulsivity or agitation as opposed to altered anxiety per se (63). Taken together, this suggests that the loss of GR in limbic forebrain is not able to fully replicate the anxiety effects that occur following loss of GR throughout brain, and further implies that other brain sites may be important for glucocorticoid regulation of anxiety. One possible candidate is the central nucleus of the amygdala, as surgical implantation of slow-release corticosterone pellets adjacent the central amygdala increases anxiety-related behavior in the elevated plus maze (186, 492). Notably, glucocorticoid implantation also increases the expression of CRH mRNA in the central amygdala (492), suggesting that glucocorticoids may enhance behavioral anxiety, at least in part, by potentiating CRH signaling. In support of this idea, chronic corticosterone administration potentiates CRH-induced increases in the acoustic startle response, despite having no effects on its own (282). Lastly, some evidence suggests that glucocorticoids could also impact anxiety end points via actions at brain MR (55, 506). Acute antagonism of MR in the hippocampus of adult male rats reduced anxiety-related behaviors in multiple tests, including the light-dark box, elevated plus maze, open field, and defensive burying tests, and these effects were lost following adrenalectomy (55, 506). Thus, these results generally support the idea that increased glucocorticoid signaling in brain promotes anxiety-related behaviors.
Summary of anxiety- and trauma-related disorders and anxiety-related behaviors
People with a history of stressful or traumatic life experiences are at increased risk for developing an anxiety- or trauma-related disorder. These disorders are often accompanied by HPA axis disturbances, such that patients with some anxiety disorders show signs of overall HPA over-activity, while patients with PTSD often have HPA hypoactivity. This dichotomy had led to speculation that either insufficient or excessive HPA axis activity could contribute to the development of these disorders. Some preclinical rodent research supports each of these relationships (Table 5). On the one hand, a link between HPA axis hypoactivity and anxiety is supported by studies exploiting individual differences in anxiety end points. Mice identified as displaying inherently high-anxiety have lower plasma glucocorticoids, and supplementation with exogenous glucocorticoids reduces the behavioral anxiety of these high-anxiety mice. On the other hand, a link between HPA axis over-activity and anxiety is supported by chronic stress studies. Chronic stress elevates both HPA axis tone and anxiety-related behaviors in rodents, and manipulations that limit CRH and GR signaling can reduce behavioral anxiety. Clearly, the relationship between anxiety and the HPA axis is complex; current work is directed towards improving our understanding of this relationship, as well as its underlying mechanisms.
HPA axis interaction with appetitively-motivated behaviors
Overview of brain reward circuitry
Appetitively-motivated behaviors are those that are directed towards receiving a positive or pleasurable hedonic outcome. These outcomes are often referred to as ‘rewards’ due to their ability to motivate individuals to perform goal-oriented behavior (i.e., subjects are willing to work to obtain them) (19, 248). Examples of rewards include those that are natural such as tasty foods, sexual activity, and exercise (182, 185, 377, 386, 540), as well as those that are pharmacological, such as drugs of abuse (293, 589).
A common characteristic of rewarding behaviors is that they activate the mesocorticolimbic reward circuit. The pathway starts with the activation of dopamine-containing neurons in the ventral tegmental area (VTA). The main mesolimbic projection of the VTA is to the nucleus accumbens, along with projections to the septum, amygdala, and hippocampus. The mesocortical arm of this pathway projects from the VTA to the PFC (19, 56, 480, 589). In addition, the PFC, nucleus accumbens, and amygdala are anatomically and functionally interconnected, which further modulates activity in this circuitry.
Some have suggested that reward can be considered as two distinct components: “wanting” and “liking” (43, 46). Though they often appear together, they may be independently modulated (46, 544). The wanting of a reward describes its incentive salience, or the motivation to obtain the reward, and is associated with approach and consummatory behaviors (46). An increase in the incentive salience or wanting for a reward will result in a greater willingness to work to acquire that reward. For example, a rodent’s willingness to work for a rewarding food or drug can be measured by the number of times it will press a lever to receive it (21, 217, 437, 548), with more lever presses being considered an indication of greater incentive salience. Neurobiologically, wanting has been linked to activation of VTA dopaminergic projections and dopamine receptor signaling within the mesocorticolimbic pathway (43, 45, 544, 601). For example, experimental manipulations that increase dopamine signaling in the mesocorticolimbic circuit increase wanting for a reward (43, 45, 46, 394, 544), while dopamine receptor antagonists decrease it (43).
The concept of “liking” involves the pleasure or hedonics associated with a reward (44). Pleasure is often thought of as a subjective feeling, but there are objective methods that measure how much a stimulus is liked. One such test is conditioned place preference, which pairs a particular environment with a rewarding stimulus, then tests the extent to which that environment is preferred compared to another that was not associated with the rewarding stimulus (553). Another approach to measure liking is to look at affective facial expressions in response to a stimulus, for example a preferred versus non-preferred food. Across both animals and humans, sweet tastes like sugar will provoke particular “liking” reactions such as tongue protrusions, while bitter tastes like quinine provoke “disliking” reactions such as gapes (46). The neurobiological substrates that are linked to the liking of rewards include the opioid and endocannabinoid systems (307, 395).
In summary, appetitively-motivated behaviors include a diverse group of behaviors that are all directed towards receiving a pleasurable or rewarding outcome. This includes the use of pharmacological drugs of abuse, as well as engaging in naturally-rewarding behaviors like eating highly-palatable foods. In general, engaging in these types of behaviors can impact HPA axis activity, and HPA axis and glucocorticoid tone can in turn influence these behaviors. These interactions are described for each particular type of appetitively-motivated behavior in the following subsections.
Substance use disorders
An estimated 21.6 million people in the United States have a substance use disorder, and this is associated with a total cost of over $700 billion annually (360). A substance use disorder occurs when the repeated use of a rewarding pharmacological substance (e.g., alcohol, tobacco, or other drug of abuse) results in marked clinical and functional impairments that interfere with daily activities (16). The DSM-5 categorizes the most commonly abused substances as alcohol, tobacco, cannabis, stimulants (such as cocaine or amphetamines), opioids (including illegal opioids like heroin and prescription opioids), and hallucinogens (16).
In preclinical research, the development of drug abuse/addiction is often classified into three stages: initiation, maintenance, and withdrawal/reinstatement (500, 575) (although different stage classifications are often used in clinical research (263)). Initiation refers to the initial onset of drug-taking behaviors, while maintenance occurs once drug-taking behaviors are established and escalate. In later stages, withdrawal occurs soon after discontinuing drug use and is accompanied by negative physical and mental symptoms, and reinstatement is the resumption of drug-taking behaviors following a period of abstinence. Importantly, there are critical interactions between drug-taking behavior, stress and the HPA axis. The HPA axis is generally activated by use of drugs of abuse, although the HPA axis effect varies with the particular type of drug, method of administration and stage of abuse. Furthermore, stress is a well-established factor that promotes drug use. This has led to the idea that the increased HPA axis tone that accompanies drug use and/or stress exposure can promote continued drug taking. The bidirectional influences between the HPA axis and drug-taking behavior are described below, and have also been reviewed extensively elsewhere (20, 102, 238, 263, 268, 297, 314, 317, 331, 332, 404, 405, 485, 500, 521, 575).
Effect of drug use on the HPA axis
Acute drug use in humans
Acute (i.e., single-time) use of almost all drugs of abuse including cocaine, amphetamines, alcohol, nicotine and marijuana, acutely activates the HPA axis resulting in increased circulating ACTH and cortisol (20, 34, 122, 204, 242, 332-334, 500, 541, 582). [Note that HPA activation by exogenous cannabinoids like marijuana appears to be distinct from the HPA effects of physiological levels of brain endocannabinoids, which are typically thought to blunt HPA stress responsivity (216, 348).] Although opiates are an exception to this, as this class of drugs typically reduces plasma ACTH and cortisol following a single use in people (14, 170, 441, 573, 613), indicating that the endogenous opioid receptors that reside in stress-regulatory brain regions normally restrain HPA axis activity in humans. Table 6 provides a summary of the effects of acute drug use on the HPA axis in humans.
Table 6.
The impact of acute exposure to a drug of abuse on HPA axis activity. The studies in non-human experimental animals assessed the HPA effects of a single, first-time drug exposure. However, for the studies in human subjects, prior drug exposure varied based on each study’s inclusion criteria.
| Drug of abuse | Species | Effect on HPA axis activity |
Literature References |
|---|---|---|---|
| Amphetamine | Rat | ↑ | (260, 531) |
| Human | ↑ | (242) | |
| Cocaine | Rat | ↑ | (61, 72, 285, 345, 407, 445, 470, 471) |
| Rhesus monkey | ↑ | (473) | |
| Human | ↑ | (34, 204, 333, 541) | |
| Ethanol | Rat | ↑ | (194, 264, 280, 391, 436, 442, 516) |
| Mouse | ↑ | (281) | |
| Nicotine | Rat | ↑ | (138, 326) |
| Human | ↑ | (334, 582) | |
| Opioid | Rat | ↑ | (232, 366, 619) |
| Human | ↓ | (14, 170, 441, 613) | |
| Cannabinoid | Rat | ↑ | (229, 261, 315, 419, 580, 618) |
| Mouse | ↑ | (236, 365) | |
| Human | ↑ | (122) |
Acute drug administration to rodents
In preclinical studies, there is typically a robust activation of the HPA axis when naïve rodents are first given a drug of abuse. This includes HPA axis activation by cocaine (72, 172, 285, 345, 407, 445, 470, 471), amphetamine (72, 260, 531), nicotine (138, 326), morphine (366, 619), ethanol (194, 264, 280, 281, 391, 436, 442, 516), and cannabinoids (236, 261, 315, 365, 419, 580, 618). As expected, the increased HPA axis tone following drug use is generally accompanied by increased CRH tone in the hypothalamus/PVH. For example, cocaine, alcohol, and amphetamines stimulate CRH release from in vitro hypothalamic explants or primary hypothalamic cell cultures (80, 288, 332, 446). After a single acute exposure in vivo, cocaine increases CRH mRNA in the PVH (443). Similarly, alcohol upregulates CRH hnRNA in the PVH (444), as well as CRH-R1 mRNA in the PVH, supraoptic hypothalamic nucleus and amygdala (279). Moreover, as one would predict, interventions that limit CRH signaling in the PVH prevent HPA axis activation by drugs of abuse. Lesions of the PVH attenuate ACTH release after cocaine exposure (332, 443). CRH immunoneutralization and CRH receptor antagonists can also blunt the drug-induced HPA axis response to most drugs (13, 445, 472, 531). Likewise, transgenic mice that lack CRH-R1 have a blunted ACTH response to alcohol (281, 446). Taken together, it is clear that initial use of most pharmacological drugs of abuse robustly activate the HPA axis. Table 6 includes a summary of the effects of acute drug use on the HPA axis in non-human experimental animals.
Mechanisms by which drug use impacts HPA axis tone:
The mechanisms by which drugs of abuse activate the HPA axis depend, at least in part, on the particular drug that is used. Abused drugs can have direct actions in the brain by binding to related neurotransmitter receptors in stress-regulatory brain regions, and/or by activation of the central reward pathways. However, many abused drugs can also have direct actions in the periphery that could induce a secondary activation of the HPA axis. For instance, cocaine activates the sympathetic nervous system (likely through a combination of central and peripheral actions) resulting in robust increases in heart rate and blood pressure in rats (344), and these cardiovascular and sympathetic effects may then contribute indirectly to increased glucocorticoid levels (93, 142-145).
The important role of contingency and “choice” in the HPA effects of drug use:
It is also important to note that the effects of acute drug use on the HPA axis can vary with whether the drug is self-administered or experimenter-administered. For instance, while intragastric ethanol treatment increases plasma corticosterone, HPA axis activation does not occur in rats that voluntary ingest ethanol despite achieving equivalent blood ethanol concentrations (264). This relationship between self- vs. investigator-administered drug and the HPA axis has also been studied by comparing rats that are trained to press a lever in order to self-administer a drug (i.e., response-contingent administration) to a yoked control group that is passively receiving the same dose as the contingent group (i.e., non-contingent or response-independent administration). In this setting, the plasma corticosterone response to cocaine is lower after contingent self-administration relative to non-contingent controls (169). While another study showed that despite equivalent levels of circulating corticosterone, the concentration of corticosterone in medial PFC microdialysates is lower when rats receive self-administered (vs. yoked) cocaine (384). Also, HPA axis activation by contingent nicotine administration is more transient than that produced by non-contingent administration (138). Similarly, electrical stimulation of brain reward areas produces varying levels of HPA axis activation and habituation depending on whether the stimulation is self-administered. Burgess et al. (73) showed that self-administered electrical stimulation of the VTA increased plasma corticosterone while investigator-administered electrical stimulation did not. In contrast, Terry and Martin (542) found that both self- and investigator-administered electrical stimulation of the lateral hypothalamic area increased plasma corticosterone, but the response to self-administration decreased over repeated trials while the response to passive administration did not. Collectively, this suggests that the HPA axis response to drugs is modulated by whether the individual chooses to use the drug and/or can control the amount and schedule of drug dosing.
Chronic drug use in humans and the relationship to the stage of addiction
Given that substance abuse develops with repeated use of the drug, it is also important to characterize the effects of chronic drug use on the HPA axis while considering that HPA axis effects may vary with the stage of addiction (e.g., maintenance vs. withdrawal). Cocaine addicts have higher ACTH and cortisol levels upon entrance to a treatment facility than controls (non-cocaine users), with urine testing indicating recent cocaine use (570). These HPA axis effects normalize after 15 days of cocaine abstinence (570). Moreover, people addicted to heroin have elevated cortisol levels for up to 25 days after stopping drug use (36, 287). Lastly, individuals that are experiencing withdrawal generally have increased HPA axis activation consistent with the aversive emotional and physical consequences of acute withdrawal (8, 83, 102, 209, 225, 268, 440, 500, 570). For example, individuals undergoing cocaine withdrawal show enhanced HPA axis activity and sensitivity to stress (496, 497, 499). Likewise, alcoholics have increased resting plasma cortisol on day 1 of withdrawal/abstinence compared to day 8 of abstinence (8). These effects of withdrawal/abstinence on the HPA axis may depend on the amount of time since cessation of drug-taking behaviors. After 1-3 weeks of abstinence from alcohol, alcoholic patients have lower ACTH and cortisol responses to exogenous CRH administration than non-drinking controls, whereas beyond 3 weeks abstinence there is no difference in HPA response between alcoholics and non-drinking controls (7, 115). Similarly, polysubstance abusers (after an average of 5 days abstinence) have a lower ACTH and cortisol response to CRH stimulation than non-drug controls (110). Moreover, HPA axis responses to stress can also be decreased during abstinence. For instance, recent smokers have a lower cortisol response to a problem-solving task than non-smokers (551), and infants exposed to cocaine in utero have a lower cortisol response to stress than non-cocaine exposed controls (306). Taken together, this suggests that the use of abusive drugs impacts the HPA axis in a manner that varies with stage of addiction, such that HPA axis tone is generally increased during chronic, ongoing drug use and early abstinence (e.g., withdrawal), whereas HPA axis responsivity may be blunted during sustained abstinence from some drugs of abuse. The HPA effects likely also vary with the type of drug that is abused.
However, it should be noted that the design and interpretation of clinical studies that measure HPA axis indices during and after chronic drug use are complicated by several factors. HPA axis effects may vary with the amount and frequency of past use which can be difficult to determine in addicted patients. Also, many addicted individuals use more than one drug, making it difficult to attribute observed HPA axis effects to any particular substance. Given these caveats for studies using human subjects, experimental animals can provide additional opportunities to clarify the relationship between chronic drug use and its HPA axis effects.
Chronic drug administration in rodents
The net effect of chronic drug administration in rodents is generally an increase in HPA axis tone that resembles a chronic stress-like state, and in which responses to subsequent stress exposures are facilitated. For example, while chronic morphine treatment by constant infusion of an implanted pellet does not activate the HPA axis and reduces HPA axis responses to subsequent stressors, intermittent treatment with escalating doses increases basal and stress-evoked levels of plasma ACTH and corticosterone, adrenal weight and CRH mRNA in the PVH during early withdrawal (reviewed and/or shown in (224)). Chronic amphetamine treatment increases HPA axis responses to restraint stress (30). Chronic cocaine administration increases adrenal weight and basal plasma corticosterone (60, 472); stress-evoked plasma corticosterone levels are also facilitated during withdrawal/abstinence (311, 313). Similarly, chronic liquid ethanol diet increases adrenal weight, decreases thymus weight and increases basal and/or post-stress levels of plasma corticosterone (278, 302, 392). Although it is important to note that most studies exploring the effects of chronic drug exposure on the HPA axis have used administration paradigms that lack choice (e.g., using non-contingent drug administration, or not offering a dietary choice). As the choice (or contingency) of acute drug administration impacts HPA activation (as described above), it is possible that choice could similarly modulate the HPA effects of chronic drug use.
Modulation of drug-taking behavior by the HPA axis
Role of stress in human drug-taking behavior at the various stages of addiction
Stress is considered a critical factor that promotes addiction through its actions in all stages of drug abuse. Epidemiological and clinical studies show that stressful life events increase an individual’s likelihood of beginning drug use, and can further contribute to escalation of drug-taking behaviors. For example, self-reports of stressful life events are associated with an increase in both the initiation and escalation of drug-taking behaviors in adolescents and young adults (498, 500, 588), with cumulative stress experiences predicting alcohol and drug dependence (500, 552). Moreover, stressful events that occur early in life, such as childhood physical or sexual abuse or the loss of a parent, increase drug use and abuse later in adulthood (237, 364, 500, 587). Lastly, stress can impact the reinstatement of drug use following a period of abstinence. For example, addicts who have been abstinent report increased drug craving and anxiety during times of stress (24, 163, 500). Collectively this research suggests that life stress is an important factor that promotes drug taking and addiction in many patients.
Role of stress in rodent drug-taking behavior at the various stages of addiction
Similar results are seen in experimental animals in that stress can promote drug-taking behavior during multiple stages of drug use. In the acquisition and maintenance phases, a variety of types of stress are able to increase self-administration of drugs of abuse in rodents. For example, tail pinch stress increases self-administration and locomotor sensitivity to amphetamine (403), social isolation early in life increases oral self-administration of ethanol (475), and immobilization stress increases self-administration of heroin (487). Furthermore, both footshock and social stress increase self-administration of cocaine (173, 202, 340, 476) and opioids (64, 319, 488). Stress can also promote the reinstatement of drug-seeking behavior after a period of abstinence. Stressors such as food restriction (489), forced swim (109), and footshock can reinstate the self-administration of cocaine, heroin and alcohol after a prolonged drug free period ((10, 149, 277, 309, 310) and reviewed in (485)). Thus, there is a clear relationship between stress and increased drug-taking behavior during multiple stages of drug abuse, in both experimental animals and humans.
Contribution of the HPA axis to drug-taking behavior
The clear relationship between stress and increased drug taking has led to a focus on understanding the mechanisms for these stress effects. Much of this work has implicated stress-induced HPA axis activation as an important factor that potentiates drug-taking behavior. This idea is supported by the observation that GR is highly expressed in many of the brain regions that regulate reward processes, including the VTA, nucleus accumbens, PFC, and amygdala (201, 258, 259, 350). Clinical studies have also shown that the amount of ACTH and cortisol released during stress is predictive of the amount of cocaine consumed in a relapse episode (498). As a result, preclinical research has explored whether manipulations that limit HPA axis tone in rodents can inhibit drug-taking during the various stages of drug use.
Effects of HPA axis manipulations on the acquisition and maintenance of drug taking:
The HPA axis plays a role in the acquisition and/or maintenance of drug-taking behavior in rodents. For example, adrenalectomy blocks the acquisition of cocaine self-administration and this is partially recovered by corticosterone replacement (174). Constitutive deletion of GR in the brain of mice does not affect the initial acquisition of cocaine self-administration, but greatly flattens the dose response curve for cocaine (133) indicating that glucocorticoids may act in brain to maintain cocaine reinforcement. Consistent with this idea, treatment with metyrapone, which blocks the synthesis of corticosterone, decreases ongoing cocaine self-administration in rats that have already acquired the behavior (174). Preclinical research has focused in part on identifying the brain region(s) and/or neuronal phenotype(s) that are responsible for mediating the impact of GR signaling on drug self-administration. GR specifically in reward-related brain regions may be especially important for maintaining cocaine self-administration, as selective deletion of GR in neurons expressing dopamine receptor 1a also flattens the dose response curve for cocaine self-administration and reduces conditioned place preference for cocaine (15, 29). Though the role of GR in dopamine receptor 1a-expressing neurons appears to depend on the type of drug tested, as this specific GR deletion also reduces amphetamine, but not morphine, conditioned place preference (29, 389). Glucocorticoid signaling within the central nucleus of the amygdala is linked with alcohol intake, as administration of the GR antagonist mifepristone either directly into this brain region or systemically reduces alcohol intake in alcohol-dependent rats (568). CRH neurotransmission in brain may also contribute to the maintenance of drug use. Treatment with the CRH receptor antagonists CP-154,526, antalarmin, or MPZP reduces cocaine self-administration in rats during maintenance (175, 515).
Effects of HPA axis manipulations on the reinstatement of drug taking:
Importantly, the HPA axis also plays a critical role in the reinstatement of drug taking following a period of abstinence. CRH may be an important mediator of this phenomenon. The infusion of CRH into the lateral ventricle (148, 309), BNST (151), or VTA (576) reinstates cocaine-seeking behavior, and icv infusions of CRH reinstate both heroin and alcohol self-administration (485, 486). Moreover, stress-induced reinstatement is prevented by interventions that limit HPA axis activation. Cocaine reinstatement can be induced by footshock, and this effect is prevented by adrenalectomy, and restored by replacement of corticosterone to circadian levels (150). Ketoconazole, which can inhibit corticosteroid synthesis and act as a GR antagonist, blocks stress-induced reinstatement of cocaine self-administration (312). Similarly, the CRH receptor antagonists D-PHE CRF12-41 and CP-154,256 can attenuate footshock stress-induced reinstatement for cocaine and heroin (150, 484, 485). The activation of the HPA axis that occurs during cocaine-self administration also appears to be important for stress-induced reinstatement. Graf et al. (183) examined this using adrenalectomy with diurnal replacement of corticosterone to basal levels. Rats that underwent adrenalectomy with basal corticosterone replacement before self-administration (and thus had no cocaine-induced increases in corticosterone) acquired the behavior normally, but footshock- and CRH-induced reinstatement was eliminated. This is compared to rats that underwent adrenalectomy with basal corticosterone replacement after acquisition of self-administration, which had no effect on stress- or CRH-induced reinstatement (183).
Association between HPA axis tone and the locomotor response to drug administration:
Notably, HPA axis activity is not only linked with promoting self-administration of drugs of abuse, it is also associated with the behavioral responses that accompany drug administration. For example, stress can increase the locomotor response to drugs, including cocaine, amphetamine, and morphine (131, 318), suggestive of behavioral sensitivity. Moreover, this stress-induced behavioral sensitivity can be blocked by a GR antagonist or adrenalectomy, and the effects of adrenalectomy can be restored by corticosterone treatment (131, 132, 318). Thus, the HPA axis appears to contribute to multiple aspects of drug-related behaviors.
Summary of substance use disorders and drug-taking behavior
Acute and chronic use of most drugs of abuse increases HPA axis activity in both human and rodent studies. Furthermore, the preclinical research indicates that the extent of HPA axis activation can be modulated by whether the individual self-administers the drug, indicating that some aspect of choice or control of drug schedule/dosing impacts HPA axis responsivity. Stress also increases drug-taking behavior in people and rodents, and is thought to promote the development of substance use disorders. Preclinical studies indicate that the HPA axis promotes the use of drugs of abuse and likely mediates, at least in part, the impact of stress on the initiation, maintenance and reinstatement of drug taking.
Given the clear bi-directional relationship between the HPA axis and use of pharmacological rewards, it is of interest to explore whether a similar bi-directional relationship exists between the HPA axis and natural rewards. As such, the next portion of this review focuses on one of the most studied natural rewards – the consumption of highly-palatable foods.
Consumption of highly-palatable foods
Individuals eat food for a number of reasons, including physiological or homeostatic need, as well as non-homeostatic factors such as pleasure (reviewed in (43, 47, 48, 469)). Highly-palatable foods are those foods that are particularly tasty and are rewarding; these foods are generally calorically-dense and high in sugars/carbohydrates and/or fats. Stress profoundly impacts feeding behavior; it affects the total amount of food eaten, as well as the types of foods that are chosen, with an overall effect of preferentially promoting the intake of highly-palatable foods. At least some of these stress effects are related to increased glucocorticoids. Moreover, the overall amount and type of food an individual eats can have differential effects on the HPA axis and responses to stress, as described in the following sections.
Modulation of palatable food intake by stress in humans and rodents
Effect of stress on total food intake
The effect of stress on eating behavior and total caloric intake varies between individuals, with about 35-60% of people reporting that they eat more food during stress, while 25-40% report eating less (146, 375, 578). Moreover, increased food intake during stress is associated with increased body weight and obesity (284, 447), suggesting that stress is important factor promoting metabolic disease in at least a subset of people. The divergent effect of stress on eating behaviors likely depends, at least in part, on the type of stress. Daily life stressors, such as interpersonal relationships, work and school, and early-life stress generally promote overeating and obesity (147, 328, 410, 457, 577), whereas immediate and direct physical threats, such as imminent combat or surgery, tend to reduce food intake (41, 411). Similar results are observed in experimental animals, in that social hierarchy stress often promotes hyperphagia, and increases body weight and adiposity (33, 339, 509). In contrast, paradigms with physical stressors (e.g., restraint, forced swim) reduce caloric intake, body weight and adiposity (383, 559, 562). Even the anticipation of a physical stressor will reduce food intake, as rats that have learned to associate a predictive cue with the receipt of a subsequent shock, will reduce their food intake when presented with the cue alone (400, 401, 430, 559) (note that this phenomenon is the basis of some rodent models of anorexia nervosa). Individual differences also likely contribute to the divergent effects of stress on caloric intake. For example, people who describe themselves as ‘restrained’ or ‘emotional’ eaters are more prone to overeating in response to stress (375, 457, 577). Stress also disinhibits food intake (193) and interferes with behavioral modifications like “dieting” (reviewed in (81)). Thus, life stress clearly promotes increased food intake in at least a subset of people.
Effect of stress on the types of food eaten
Importantly, stress not only affects the total amount of food eaten – it also affects the types of food that are chosen. In people, stress generally promotes the choice of highly-palatable foods relative to less-tasty, more nutritious alternatives (193, 254, 275, 328, 375, 550), and this can occur even among people who decrease their total caloric intake (375). When rodents with a diet choice experience stress, they generally maintain their intake of the highly palatable food (e.g. sucrose or lard) despite decreasing chow intake (126, 160, 383, 396, 562). As a result, highly-palatable foods represent a greater proportion of total food intake during stress. This dietary shift towards greater palatable food intake may result, at least in part, from increased motivation to obtain these foods. Both humans and rodents are willing to work more to obtain palatable foods during stress (284, 396, 583), and this is associated with greater activation of reward-related brain structures by palatable foods (456, 550). Stress also increases the conditioned place preference for high-fat diet in rodents (98). Notably, the increased motivation to obtain preferred foods may occur despite the presence of stress-induced anhedonia (see Depression section above), in which stress decreases the consumption of weakly-preferred foods (e.g., dilute sucrose or saccharin drink), but not highly-preferred foods (e.g., concentrated sucrose drink) (462, 562, 583). Taken together, this suggests that stress increases the ‘wanting’ and overall intake of palatable foods, even though it may also reduce the ‘liking’ of some palatable foods (584).
Contribution of the HPA axis to palatable food intake in rodents
Many of the effects of stress on food intake and diet choice have been attributed to glucocorticoids. Circulating glucocorticoid levels are positively associated with increased food intake in humans (164, 171, 536). The central administration of corticosterone or GR agonists (RU28362 or dexamethasone) increases total food intake, as well as palatable food intake, and promotes body weight gain in rodents (184, 539, 611). In contrast, adrenalectomy reduces both total food intake in ad libitum-fed rodents, as well as the refeeding response to a 24-hour fast (40, 134, 159, 161, 417, 508). Adrenalectomy also decreases fat and sucrose intake in rodents that are given these foods as a dietary option (3, 40, 134, 159, 161, 508). Moreover, replacing corticosterone or administering the GR agonist RU28362 can reverse the effects of adrenalectomy on total food intake (89, 230, 539), while administering the MR agonist aldosterone can restore fat intake (134, 161). GR is widely-expressed throughout metabolism-related brain regions (201, 258, 259, 350), indicating that GR may alter food intake via changes in the homeostatic regulation of food intake. Consistent with this idea, glucocorticoids contribute to plastic changes in the organization of synaptic inputs in the arcuate nucleus in a manner that is predicted to drive food intake (195). Furthermore, GR is also expressed in reward-related brain regions (201, 258, 259, 350), and circulating glucocorticoid levels are linked with increased consumption of the artificial sweetener saccharin (52), suggesting that glucocorticoids may also act via promoting non-homeostatic, reward-driven food intake.
Effect of food intake on stress and the HPA axis
HPA axis activity is profoundly influenced by an individual’s metabolic state. Prolonged food restriction, fasting or starvation generally increases HPA axis tone (127, 367), which likely promotes the continued availability of energy to supply immediate metabolic needs. However, HPA axis tone is also often increased during marked obesity with its associated metabolic disorders (57, 58, 140, 390, 454, 535). This suggests that the HPA axis has the capacity to respond to gross metabolic deviations from ‘normal’ energy balance regardless of whether they are associated with positive or negative energy balance.
Reduced HPA axis tone following highly-palatable food intake
The HPA axis is also affected by the types of food that an individual eats. As described in the preceding section, individuals often preferentially eat highly palatable food during stress. This has led to speculation that this behavior is a form of ‘self-medication’, and is so-called ‘comfort’ food eating (125), since the consumption of these foods is linked with reduced stress responsivity. In humans, consuming preferred foods (i.e., comfort food, chocolate) improves mood and reduces perceived stress (139, 283, 303, 546) while also decreasing circulating glucocorticoid levels (18, 320). Similarly, when rodents are given the option of consuming highly-palatable foods in addition to chow, they have less behavioral anxiety (75, 342, 416, 555) and blunted HPA axis activation. These HPA axis effects include reduced expression of CRH mRNA in the PVH (94, 162, 274, 396, 562), as well as blunted plasma ACTH and corticosterone responses to stress (97, 103, 125, 159, 160, 162, 379, 416, 555, 562).
Role of the caloric and macronutrient properties of highly-palatable foods:
Ongoing work is determining the mechanisms by which highly-palatable foods elicit this HPA axis stress ‘relief’. Highly palatable foods have many different attributes that can be loosely divided into two categories: metabolic factors including calories and macronutrient composition, and non-metabolic factors including hedonics, pleasure and reward (although it should be noted that metabolic factors can also contribute to food reward (482)). HPA axis-dampening by the metabolic features of palatable foods have been most commonly studied in paradigms in which rodents with free access to chow are given additional, unlimited access to one or more highly-preferred foods (e.g., sucrose drink, lard). In these studies (39, 160, 274, 396, 523, 527), rodents eat large amounts of the preferred food(s), representing ~30-55% of their total caloric intake. Moreover, despite reducing their chow intake by ~30-45%, total caloric intake increases by ~10-20%, resulting in increased adiposity without changes in overall body weight. Under these conditions, free access to sucrose drink prevents the augmented indices of HPA axis tone (e.g., CRH mRNA expression in the PVH and plasma ACTH) that typically accompany adrenalectomy (and its concomitant loss of glucocorticoid negative feedback) (274). In contrast, free access to the artificial sweetener saccharin is ineffective (274), suggesting that the metabolic consequences of sucrose ingestion are critical for regulating HPA-axis tone in the absence of glucocorticoids. This type of unlimited, optional palatable food paradigm also reduces HPA axis tone in adrenal-intact rodents, including attenuated CRH mRNA expression in the PVH and reduced HPA axis hormonal responses to acute and chronic stress (160, 396, 523, 527). Moreover, in these paradigms the degree of HPA axis blunting has been inversely related to the amount of visceral fat, leading to speculation that an abdominal fat-derived signal may contribute to reduced HPA axis tone (124, 126). Recent work suggests that GR expression in the adipocyte may play an important role in this fat-to-brain feedback (255).
Role of the pleasurable and rewarding properties of highly-palatable foods:
Importantly, the pleasurable and rewarding properties of preferred foods can also contribute to stress ‘relief’. For instance, eating highly-palatable chocolate improves negative mood in people, while eating unpalatable chocolate does not (303). Also, free access to saccharin drink blunts HPA axis activation by paradoxical sleep deprivation in rodents as effectively as sucrose drink (527). In order to further explore the mechanisms by which the pleasurable and rewarding properties of preferred foods blunt HPA axis tone, our group has developed and characterized a paradigm of limited sucrose intake (LSI) in rats. In this paradigm, adult male rats are given additional brief (up to 30 min) twice-daily access to a small amount (up to 4 ml) of 30% sucrose solution (or water as a control) in addition to ad libitum chow and water (555, 562). The rats that are offered these limited sucrose ‘snacks’ rapidly begin to drink the sucrose in amounts reaching the maximum permitted (8 ml per day with ~9 calories per day) (562). In contrast, control rats that are offered equivalent amounts of water drink little, consistent with the fact that they maintain free access to their normal water bottle. Rats with this LSI modestly decrease chow intake (~9 calories per day), resulting in no effect on body weight or adiposity (562).
A history of LSI (typically for 2-4 weeks), lessens HPA axis hormonal responses to a subsequent acute stressor (97, 555, 561, 562) and decreases CRH mRNA expression in the PVH (562). Rats given limited saccharin (0.1%) drink showed HPA axis-dampening similar to sucrose (555), whereas twice-daily orogastric gavage of 4 ml of sucrose did not produce HPA axis-dampening (555). Taken together, this suggests that the post-ingestive effects of sucrose (e.g., calories, macronutrients) are not sufficient, and furthermore indicate that the hedonic/rewarding properties of sucrose are likely necessary. Lastly, limited intermittent sexual activity, another type of naturally rewarding behavior, can replicate these HPA axis effects (555). Collectively, this work indicates that the pleasurable and rewarding properties of palatable food, as well as other types of natural rewards, can contribute to lowering the HPA axis response to stress.
The mechanisms by which the non-metabolic properties of palatable foods blunt HPA axis responsivity appear to involve forebrain structures that regulate both reward and stress systems. For example, the BLA is a reward-regulatory site that is activated by palatable food intake (355, 368, 483, 533), and also modulates HPA axis activity (53, 178, 538). Moreover, the induction of c-fos (a marker of neuronal activation) mRNA in the BLA following restraint stress is reduced by a prior history of LSI (562), indicating that the BLA is well-positioned to mediate HPA axis stress ‘relief’. Consistent with this idea, bilateral ibotenate lesions of the BLA prevent HPA axis-dampening by sucrose relative to controls groups that were either vehicle-injected or that had lesions that missed the BLA, despite equivalent sucrose intake (555). In order to identify potential intra-BLA mechanisms for the HPA axis effects of LSI, BLA mRNA expression was assessed after sucrose vs. water exposure. Microarray analysis identified 145 genes whose expression was altered by sucrose (555). Functional clustering analyses indicated that this collection of 145 genes was significantly enriched in members of the intracellular calcium signaling and long-term potentiation (LTP) pathways, suggestive of synaptic plasticity (555). Subsequent neuroanatomical studies showed that LSI increases BLA immunolabeling for multiple markers of synaptic plasticity, including synaptophysin, phospho-cAMP response element-binding protein (pCREB)-positive cells, phospho-calcium/calmodulin-dependent protein kinase II alpha (pCamKII)-positive cells, and FosB/deltaFosB-positive cells (97, 555). Collectively, this work indicates that HPA axis dampening by LSI may result, at least in part, from sucrose-induced plasticity and remodeling in the BLA.
More recently, work has sought to identify brain regions that likely work with the BLA to impart sucrose stress relief. Advanced statistical modeling approaches were applied to a dataset that included measures of multiple plasticity indices in several stress- and reward-regulatory brain regions (556). These procedures identified that sucrose may act via a combination of dampening an excitatory BLA-to-medial amygdala circuit, while also potentiating an inhibitory BNST-mediated circuit, resulting in reduced HPA activation after stress (556). Future empirical research can now be directed towards explicitly testing whether the implicated circuitry underlies stress relief by LSI.
Increased HPA axis tone following highly-palatable food intake
It is also important to note that palatable food intake does not necessarily lead to reduced HPA axis tone. For example, mice that have become obese following prolonged (6-12 weeks) ad libitum access to a high fat diet have increased plasma corticosterone following restraint stress (490, 491). Thus, the consumption of preferred foods can have opposing effects on the HPA axis that likely depend on several additional factors. First, the HPA axis effects of palatable foods may vary with the degree of metabolic dysfunction that accompanies the palatable food intake. For instance, paradigms that involve prolonged, unlimited access to palatable food produce marked obesity and increase the HPA axis response to stress (490, 491), whereas short-term, limited access to palatable food does not alter adiposity and reduces HPA axis tone (555, 562). In support of this idea, increased adiposity is often associated with increased HPA axis tone (164, 276, 296, 563). Second, the HPA axis effects of palatable foods may vary with their particular macronutrient composition. Dietary sugars and carbohydrates may preferentially limit HPA axis tone relative to dietary fat (18, 31, 90, 97, 320, 555, 561, 562, 564). Third, the valence of the HPA axis effects may be influenced by whether paradigms include an aspect of dietary choice. For example, rats that are allowed to choose between two foods (chow and highly-palatable lard) exhibit reduced HPA axis responsivity, whereas eating the same ratio of lard/chow without choice (e.g., the foods are mixed together) does not blunt HPA axis responses (160). Thus, the HPA axis effects of palatable food intake, and perhaps other types of naturally rewarding behaviors, likely depend, at least in part, on several contextual factors (Figure 2).
Figure 2).
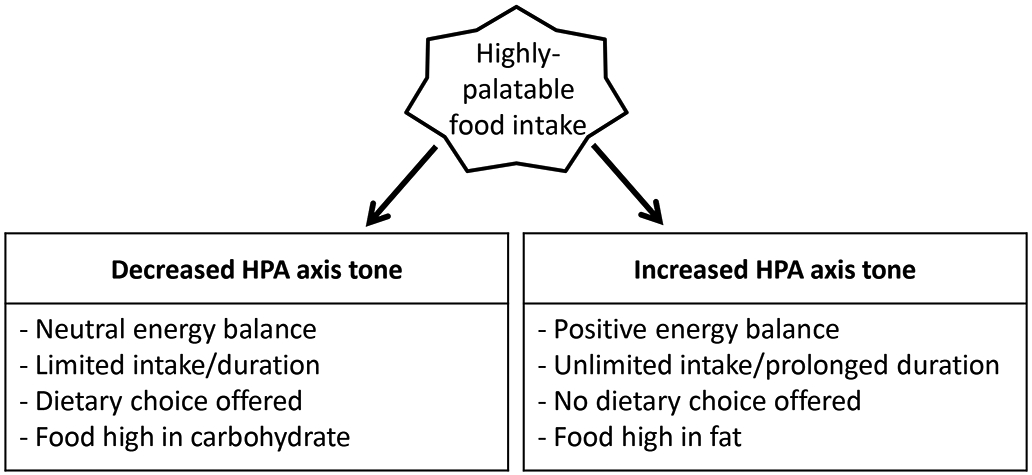
Consumption of highly-palatable foods has opposing effects on hypothalamic-pituitary-adrenocortical (HPA) axis activity depending on the particular conditions surrounding the food intake. Eating small amounts of highly-palatable foods (particularly carbohydrates) in the context of a dietary choice reduces HPA axis tone. In contrast, eating large amounts of highly-palatable foods (particularly lipids) without a dietary choice generally leads to positive energy balance (e.g., obesity) and elevated HPA axis tone.
Summary of feeding behavior and the consumption of preferred foods
In summary, stress can have a significant effect on feeding behavior by altering the total amount eaten, as well as the types of foods that are chosen. Stress generally promotes the intake of highly-palatable foods relative to less-palatable (and highly-nutritious) foods, and at least some of this effect has been linked with glucocorticoids (Figure 3). Moreover, the overall amount and type of food an individual eats can have differential effects on the HPA axis (Figure 2). Eating small amounts of highly-palatable foods (particularly carbohydrates) in the context of a dietary choice reduces HPA axis tone. Though eating large amounts of highly-palatable foods (particularly lipids) without a dietary choice generally leads to positive energy balance (e.g., obesity) and elevated HPA axis tone.
Figure 3).
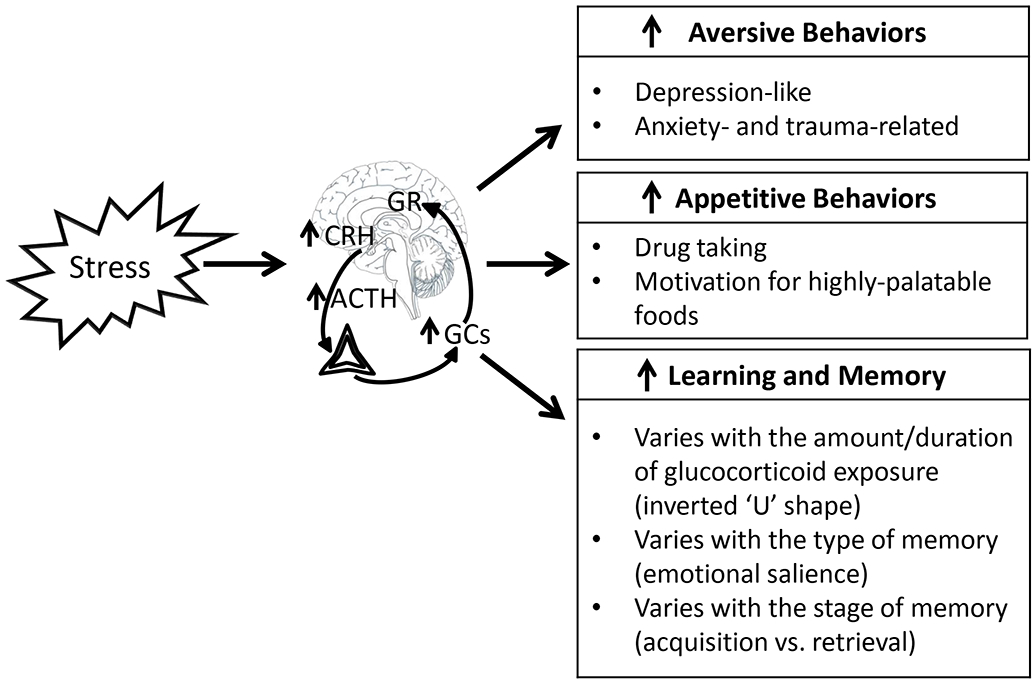
Stress impacts most aspects of behavior, and the hypothalamic-pituitary-adrenocortical (HPA) axis is well-poised to mediate much of these stress effects. For example, HPA axis activation is linked with promoting aversively-motivated behaviors, appetitively-motivated behaviors, and learning and memory processes. In turn, when individuals respond to particular internal or external stimuli by evoking one of these various behaviors, HPA axis activity is also impacted. Other abbreviations shown on figure: ACTH – adrenocorticotropin hormone, CRH – corticotropin releasing hormone, GCs – glucocorticoids, GR – glucocorticoid receptor signaling.
HPA axis interaction with learning and memory
Overview of learning and memory
Learning and memory is a multi-step process that involves: (1) the acquisition of new information, (2) the consolidation or storage of the information, (3) the retention or preservation of the information over time, (4) the retrieval or recall of the information, and/or (5) the extinction or loss of the previously learned information (118, 243, 244, 380, 467). Learning and memory processes play a critical role in shaping behavior, and can be profoundly influenced by stress and the HPA axis. Preclinical research in experimental animals has provided critical insights into the complex relationships between stress, the HPA axis, and learning and memory.
Common assessments of learning and memory in rodents
A number of behavioral tests have been developed to measure various types of memory in rodents, including, associative, spatial, and working memory (Table 7). The most commonly used tests include:
Table 7.
Behavioral tests that are commonly used to assess learning and memory in rodents. CS = conditioned stimulus; US = unconditioned stimulus.
| Behavioral paradigm |
Description | Type of memory tested |
Indicator of learning/memor y |
References |
|---|---|---|---|---|
| Eyeblink conditioning | Rodents or humans learn to associate a predictive cue (CS) with an eyeblink-eliciting stimulus (US) | Associative | Percentage of CS-US trials that elicit a conditioned eyeblink response | (50, 51, 495, 596) |
| Fear conditioning | Rodent learns to associate a predictive cue or context (CS) with an aversive stimulus (US) | Associative | Amount of time spent in a conditioned freezing response | (112, 113, 153, 235) |
| Morris water maze | Rodent learns to find a hidden escape platform that is located in a fixed position in a pool of water | Spatial | Time to escape, path length | (65, 121, 353) |
| Radial arm water maze | Rodent learns to find a hidden escape platform that is located at the end of one of the maze arms. | Spatial working memory | Number of incorrect arm entries | (136, 228, 398, 597) |
| Delayed alternation T-maze | Rodents must remember which arm was entered in the most recent trial and then enter the opposite arm | Working memory | Proportion of correct responses | (32, 453, 609, 610) |
Eyeblink conditioning: This test is a form of classical conditioning that is used to test associative memory. In this test a neutral stimulus (either a visual or auditory cue; the conditioned stimulus (CS)) is paired with an eyeblink-eliciting stimulus (e.g., a puff of air directed at the eye or a periorbital shock; the unconditioned stimulus (US)) (50, 51, 495, 596). Initially the receipt of the US is followed by a reflexive unconditioned eyeblink response (UR). However, after repeated pairings of the CS and US, the subject learns to associate the CS and US, and begins to make a conditioned eyeblink response (CR) prior to, and even in the absence of, the US (50, 51, 495, 596). Performance in this test is measured as the percentage of CS-US trials that elicit a CR (50, 51). Acquisition can be assessed by measuring the CR during initial CS-US pairings. Once the CR is established, retention can assessed by measuring the CR after a prolonged post-training delay, and extinction can be assessed by measuring the CR following CS-alone training (i.e., no US is given). The eyeblink conditioning test can be performed in both rodent and human subjects ((165, 199, 290, 322) and see references above).
Fear conditioning: This test is another form of classical conditioning that assesses associative memory. In this test, a neutral cue (a visual or auditory tone or a particular environmental context; the CS) is paired with the receipt of an aversive event (e.g., a footshock; the US) (112, 113, 153). After pairing of the CS-US, rats and mice begin to make a CR (e.g., freezing behavior in which movement is limited to that needed for respiration) in response to the CS (112, 113, 153, 235). Performance in this test is typically measured as the amount of time spent freezing (the CR) in response to CS, with more time spent immobile being considered as greater fear memory (112, 113, 153, 235). Fear conditioning is often used to study the mechanisms underlying the consolidation, retention and extinction of fear memory (112, 113, 153, 466).
Morris water maze: This behavioral test is used to study spatial memory in rodents (353). In this test, the subject is placed into a large circular pool (that is typically filled with opaque water) and must find a hidden escape platform that is located in a fixed position (65, 121, 353). Through repeated trials, rodents learn to use distal spatial cues to locate the platform when starting from different (random) locations in the pool (65, 353). The simplest and most common measure of performance during testing is the latency to escape, but directionality, path length, and time spent in each quadrant can also be used as indices of spatial learning (65, 121, 353).
Radial arm water maze: This behavioral test is used to measure spatial working memory in rodents. The apparatus consists of a circular pool with internal walls that are placed to form several (typically 4, 6 or 8) arms that radiate from an open central compartment (136, 228, 398, 597). Similar to the Morris water maze, the subject must find a hidden escape platform that is located at the end of one of the arms (i.e., the goal arm). Subjects are given multiple acquisition trials each day (keeping the platform location fixed on a given day; but changing the platform location across days) and retention is tested after a brief delay. The number of incorrect arm entries is used as an index of learning and memory, with fewer incorrect entries being considered as improved memory performance (136, 228, 398, 597).
Delayed alternation T-maze: This test is designed to measure working memory in rodents (32, 453, 609, 610). The testing apparatus consists of a starting arm/runway that has two test arms on either side of its far end (forming a “T” shape). Subjects are typically food-restricted, and are tested over multiple trials. In the first trial, both arms of the maze are baited (with a small food reward like a chocolate morsel) and the subject chooses to enter one of the arms and consumes the food reward. After a variable delay, the subject is returned to the maze and it must remember to enter the other (alternate) arm in order to receive a food reward (32, 453, 609, 610). The proportion of correct responses is used as an index of working memory (32, 453, 609, 610).
Collectively, these various rodent behavioral assays are routinely used in ongoing preclinical learning and memory research. Much of this work has been directed towards understanding the impact of stress and the HPA axis on learning and memory processes.
Modulation of learning and memory by the HPA axis
Evidence that stress and glucocorticoids promote memory processes
There have been extensive studies showing that stress and glucocorticoids have robust effects on learning and memory, with mild stress generally promoting memory processes (12, 111, 461, 463, 464). Moreover, at least some of this memory enhancement appears to be mediated by the elevated glucocorticoids that accompany stress exposure. For instance, performance in the Morris water maze test is enhanced (i.e., faster rate of acquisition and improved retention) by lowering the water temperature from 25°C to 19°C, a manipulation that also elevates plasma corticosterone (463). Moreover, this spatial memory improvement can be replicated by direct administration of corticosterone immediately after training in 25°C water (463). Spatial memory is impaired by the GR antagonist RU-38486, as well as by reducing corticosterone levels via either metyrapone treatment or adrenalectomy (12, 108, 372, 450, 565). Fear conditioning (in which rodents learn to associate an aversive event like footshock with either a cue or a context (112, 113, 153)) shows a linear relationship between the intensity of the shock (the stressor) and the strength of the fear memory (112, 338, 464). This was again shown to be mediated by the HPA axis, as administering corticosterone post-training can dose-dependently enhance fear memory (113, 226, 433), and since both inhibition of corticosterone release and GR antagonism reduce fear memory (111, 158). Eyeblink conditioning is also enhanced by stress, but only if the stressor is of high intensity; glucocorticoids are again necessary for this effect (50, 51, 495, 596). Furthermore, working memory in a T maze task is impaired by adrenalectomy, and this impairment can be rescued by corticosterone replacement (343). These effects are at least in part mediated by signaling at GR, as mice with mutations in GR that render either the entire receptor (371) or the DNA-binding domain of GR (373) dysfunctional have impaired spatial memory. Thus, moderate levels of glucocorticoids, either from an acute stress or from pharmacological interventions, can improve some types of learning and memory in rodents, while loss of glucocorticoid signaling prevents the enhancing effects of stress.
Evidence that stress and glucocorticoids impair memory processes
It must be noted that other research has shown that stress and glucocorticoids can inhibit memory processes in some situations. For example, while lowering water temperature from 25°C to 19°C improves spatial memory performance in the Morris water maze, further lowering it to 16°C impairs performance (461). Several studies have shown that stress or pharmacological manipulations that increase glucocorticoids can impair spatial memory in rodents (32, 135, 136, 422, 452, 453, 465, 597) and humans (349, 370, 378, 448, 478, 479, 537, 590, 591), while other studies have found that stress improves working memory performance (608, 609).
Factors contributing to the diverse effects of stress and glucocorticoids on memory processes
Several important factors contribute to the diverse effects of stress and glucocorticoids on memory (Figure 3). First, the effect may depend on the timing of the memory testing relative to stress/glucocorticoid exposure. Experiments that test memory relatively soon (~30-60 minutes) after stress exposure generally observe memory impairments (135, 136, 422, 452, 465, 597), whereas those that test memory after a longer delay (~4-24 hours after stress) generally see memory improvements (608, 609). Second, a “dose” relationship between glucocorticoids and memory has been described as an “inverted U shaped” function (234, 257, 300, 464). That is, a brief, moderate increase in glucocorticoid levels can improve at least some types of learning and memory, while higher and/or prolonged increases in glucocorticoid concentrations can be detrimental. Third, the effect of stress/glucocorticoids may depend on the type and/or stage of memory. Glucocorticoids may particularly enhance memory of emotionally-salient stimuli relative to some other types of memory (2, 71, 77, 374, 438, 450, 451). Alternatively, glucocorticoids may preferentially enhance learning/acquisition of memories while impairing retrieval ((32, 107, 433, 434, 467, 595) and reviewed in (452)).
Mechanisms by which glucocorticoid signaling alters learning and memory
Importantly, the molecular mechanisms by which glucocorticoid signaling impacts learning and memory processes are the focus of much research. Glucocorticoids alter glutamatergic neurotransmission, which is critically important for memory processes (reviewed in (415)). Glucocorticoid signaling at GR can activate the MAPK pathway leading to increased glutamate release (433, 434), and this signaling cascade is necessary for stress-induced facilitation of memory (433, 434). Moderate levels of glucocorticoids can also facilitate the trafficking of AMPA glutamate receptors to the cell membrane, which may promote memory acquisition and consolidation (107, 190, 270, 467). In contrast, high levels of glucocorticoids are associated with endocytosis of AMPA receptors from the cell membrane, and this may contribute to the impairing effects of high glucocorticoid levels on memory (467, 595). Lastly, glucocorticoids increase the expression of common cell adhesion molecules, such as N-cadherin, NCAM, and L1, in the hippocampus and prefrontal cortex; these cell adhesion molecules can support memory consolidation by increasing dendritic spines and promoting synapse stability (294, 338, 460, 465, 466, 569).
Effects of learning and memory on the HPA axis
Since a primary purpose of the HPA axis response to stress is to evoke appropriate physiological responses to promote survival during stress, there is clear benefit for an individual to be able to learn to predict stressors so that the brain can pre-emptively activate the HPA axis. Thus, during exposure to aversive or stress-inducing stimuli, animals readily learn to associate predictive cues with the subsequent onset of stress exposure (US). During this conditioning process, the predictive (CS) stimulus begins to elicit a conditioned HPA axis response, presumably in anticipation of the forthcoming stressor.
Conditioned activation of the HPA axis by an aversive US
Some of the earliest work showing conditioned activation of the HPA axis employed a conditioned taste aversion paradigm. In this model, rats are exposed to a novel tastant (e.g., saccharin drink) that is followed by administration of a drug that induces visceral illness (e.g., lithium chloride). Later when these same rats are water-restricted (to increase motivation to drink) and given saccharin drink instead of water, there is a conditioned increase in plasma corticosterone relative to rats that are given water, or rats that are given saccharin drink that was not previously paired to lithium chloride administration (e.g., non-conditioned) (6, 211). Conditioned HPA axis activation is also observed with the conditioned fear paradigm, one of the most studied models of classical conditioning to an aversive stimulus. As summarized in the preceding section, in this paradigm rats are either placed into a shock box for subsequent receipt of a footshock (contextual conditioning to shock box), or rats are given a discrete signal (either tone or light cue) paired with a shock. Upon re-exposure to either the context (e.g., shock box) or cue that was previously paired with shock, there is a conditioned increase in plasma corticosterone (104, 130, 198). Similarly, in the conditioned shock probe burying test, re-exposure of rats to a non-electrified shock probe after previously receiving a shock from the probe is accompanied by an increase in plasma corticosterone (267).
Several of the brain regions known to regulate the HPA axis are also heavily implicated in learning and memory processes, including the hippocampus, central amygdala and BNST (505, 528, 592). So it is not surprising that these same brain regions are important for conditioned HPA axis responses. HPA axis activation by conditioned taste aversion is prevented by hippocampal lesion (505), while HPA axis activation by conditioned (contextual or tone) footshock is blocked by lesion of the central amygdala (528). Moreover, lesions of the BNST prevent corticosterone responses to contextual, but not tone, conditioning purportedly due to connections between the BNST and the hippocampus, a structure that is critical for contextual conditioning (528).
Conditioned activation of the HPA axis by an appetitive US
Just as associations are readily formed between predictive cues and aversive stimuli, so too are associations between predictive cues and appetitive or rewarding stimuli. When the appetitive stimuli are drugs of abuse, such as cocaine, heroin or nicotine, predictive cues elicit conditioned activation of the HPA axis (74, 76, 128, 572). When the appetitive stimuli are natural rewards, such as presentation of food or water to deprived rats, the conditioned responses are more complex. For example, food- and/or water-restricted rats can be trained to receive food or drink in a particular context, at particular times of day, or after performing a particular task. In these instances, there is generally an elevation of plasma ACTH and corticosterone prior to receipt of the food/drink (i.e., ‘anticipation’) and a rapid reduction in these hormones upon refeeding or drinking (59, 105, 449). If instead food and drink are not provided as expected, then there is a further increase in HPA axis activation (i.e., ‘frustration’) (59, 105, 247, 449, 477).
Summary of learning and memory processes
In summary, the effects of stress and glucocorticoids on learning and memory are complex, and multiple factors influence this relationship. These factors likely include: the particular type of memory (e.g., working, explicit, emotional, etc.); the stage of memory (e.g., acquisition, consolidation or retrieval); the age and sex of the subject; the timing, intensity, and nature of the stress; and/or the timing and magnitude of glucocorticoid signaling. Readers who would like additional details about the relationship between glucocorticoids and memory are directed to a number of excellent reviews that focus on this complex topic (155, 233, 257, 270, 299, 464, 467, 592). Moreover, it is important to consider that the relationship between glucocorticoids and memory is reciprocal, such that learning and memory processes can correspondingly impact HPA axis function, through the conditioning of HPA axis responses to aversive or appetitive US.
Summary and Perspectives
HPA axis interactions with aversely-motivated behaviors
The HPA axis is generally activated when internal or external (environmental) conditions deviate from the norm or the expected, resulting in increased circulating glucocorticoid levels. Glucocorticoids can then act in brain to shape behavior (as reviewed above). For instance, when a stressor or threatening situation is experienced, animals display increased depression-like (e.g., behavioral despair, allodynia) and anxiety-related (e.g., less exploration and risk-taking) behaviors. These stress-related behaviors are likely adaptive. Consider for example an animal that ceases exploration and instead hides in its burrow when a predator is in the vicinity. The elevated glucocorticoids that accompany the predator/stressor exposure likely contribute to and reinforce such self-protective behaviors. Moreover, excessive or prolonged increases in glucocorticoid levels, as occurs during chronic and/or severe stress, can promote long-term increases in stress-related behaviors. While these long-term behavioral adaptations could be protective if the stressor is ongoing, they may also predispose for the development of a stress-related neuropsychiatric condition (e.g., depression, anxiety- and trauma-related disorders), in which behavioral and mood disturbances interfere with an individual’s ability to live a normal life (101, 231, 256, 327, 387). In addition, insufficient HPA axis responses to stress or trauma have also been linked to the development of some stress-related disorders (176, 231, 602, 604, 606) suggesting that either insufficient or excessive glucocorticoid tone can contribute to the pathophysiology of these diseases. Consistent with this idea, treatments for stress-related disorders often alter HPA axis activity, and these HPA effects may contribute to their therapeutic efficacy. Current research is exploring the mechanisms by which the HPA axis activity influences depressive and anxiety-related behaviors, including exploring the role of CRH and glucocorticoid signaling in brain, as well as the involvement of synaptic plasticity, epigenetic regulation and circadian/clock gene regulation.
HPA axis interactions with appetitively-motivated behaviors
The HPA axis also plays an important role in the regulation of appetitively-motivated behaviors. The use of drugs of abuse can acutely and chronically activate the HPA axis, while stressful life events that increase HPA axis output can promote drug-taking behavior. This is thought to be an important factor that contributes to the cycle of drug addiction. In contrast, the interaction between stress and the natural reward of highly-palatable food intake appears to be more complicated. For example, the impact of stress on palatable food intake varies with the type, intensity and predictability of the stressor. Moderate intensity and/or socially-related stress are most strongly linked with increased motivation to eat highly palatable foods, whereas anticipation of an imminent, intense stress is more strongly linked with anhedonia. In turn, depending on various factors, including the amount and type of palatable food offered and the availability of dietary choice, the intake of palatable foods can either blunt HPA axis responses to stress (a concept often thought of as stress relief by “comfort” foods) or elevate HPA axis tone (e.g., during marked obesity). Current research is focused on understanding how glucocorticoids impact brain reward processing, as well as understanding how altered brain reward pathways impact HPA axis function.
HPA axis interactions with learning and memory
Another important aspect of behavior is the ability to acquire and remember new knowledge in the process of learning and memory, and these cognitive processes also have important interactions with the HPA axis. First, HPA axis responses can be conditioned to a previously neutral stimulus when paired with an aversive (or appetitive) stimulus that itself can cause an HPA response. Mechanistic studies have implicated the hippocampus, BNST, and amygdala in the conditioning of HPA axis responses (505, 528, 592). Second, the HPA axis can reciprocally influence learning and memory processes in a manner that seems to vary with the extent of HPA axis activation, as well as the type and stage of memory. Investigation of the mechanisms by which the HPA axis exerts cognitive effects has revealed that glucocorticoids modulate synaptic glutamatergic neurotransmission in brain regions that regulate learning and memory processes (107, 190, 270, 433, 467, 595).
Significance of HPA axis interactions with these diverse types of behaviors
At first glance, the fact that the HPA axis can be activated by both aversive and rewarding stimuli, and can act to promote both aversely-motivated (depression-like, anxiety-related, conditioned fear, etc.) and appetitively-motivated (use of drugs of abuse, consumption of highly-palatable foods, conditioned place preference, etc.) behaviors, may seem to be a paradox. What is the adaptive significance of having a common mechanism (the HPA axis) for increasing seemingly opposite types of behavior? One possible explanation is that HPA axis activation does not inherently signal the valence of an internal or external stimulus (i.e., good/rewarding vs. bad/threatening), but rather signals the magnitude of the stimulus (i.e., very good/very bad vs. modestly good/modestly bad). This would imply that the HPA axis is well-positioned to reinforce a variety of behaviors in the face of extreme circumstances. For example, while stress-related behaviors accompany exposure to stress/threats, robust activation of the HPA axis during severe and/or unexpected threats can further promote these behaviors, thereby aiding survival (as described above). By this same reasoning, while appetitively-motivated behaviors accompany rewarding stimuli, activation of the HPA axis by large and/or unexpected rewards (like drugs of abuse) or stress can further stimulate these rewarding behaviors. This arrangement may be particularly helpful during times of scarcity, where glucocorticoids can act in brain to promote the consumption of highly-palatable, calorically-dense foods in a sparse environment (126, 254, 275, 396, 550, 563). However, it may be maladaptive in a modern environment where access to food (and drugs of abuse) is plentiful and daily-life stress is commonplace, and could thus contribute to the development of obesity and substance use disorders.
Conclusion
The HPA axis plays an important role in modulating behavior (Figure 3). More specifically, the HPA axis can promote depressive-like and anxiety-related behaviors in aversive circumstances, increases the motivation to obtain rewards in appetitive circumstances, and improves aspects of learning and memory. Taken together, this suggests that a primary role of the HPA axis may be to strengthen the behavioral outcomes that are most appropriate for a particular situation. Furthermore, engaging in these types of behaviors has reciprocal effects on HPA axis activity that can then feedback to further influence behavior. Lastly, as the HPA axis is strongly impacted by stressful experiences, this scenario provides an important avenue for stress to have broad effects on multiple aspects of behavior. Ongoing research into the neurobiological mechanisms underlying these relationships will greatly improve our understanding of the functional links between stress, the HPA axis and behavior.
Table 8.
Effects of glucocorticoid manipulations on performance in rodent learning and memory tests. GR= glucocorticoid receptor.
| Glucocorticoid Manipulation |
Test | Effect on Performance |
Literature References |
|---|---|---|---|
| Reduced GR signaling (blocking synthesis, GR antagonism, GR genetic inactivation) | Morris water maze Fear conditioning Eye blink conditioning |
↓ ↓ ↓ |
(12, 108, 371–373, 450, 565) (111, 158) (50, 51, 495, 596) |
| Moderate increase during or directly after training (either stress- or pharmacologically-induced) | Morris water maze Fear conditioning Eye blink conditioning |
↑ ↑ ↑ |
(107, 353, 463) (113, 338, 433, 464) (50, 51, 495, 596) |
| Large increase during training or before testing (severe stress- or pharmacologically-induced) | Morris water maze Fear conditioning Radial arm water maze Delayed alternation T-maze Delayed alternation T-maze |
↓ ↓ ↓ ↓ ↑ |
(135, 136, 422, 452, 461, 465, 597) (595) (135, 136, 597) (32, 453) (608, 609) |
Cross-References.
Hypothalamic-pituitary-adrenal axis – the stress response
Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects
Physiological and pathophysiological implications of social stress in mammals
Reward, motivation, cognition: psychobiology of mesotelencephalic dopamine systems
HPA axis – rhythms
Acknowledgements
This work was supported by NIH R01 DK091425 (YMU), F32 DK102334 (AEBP), T32 DK059803 (AEBP), and T32 NS007453 (AEE), as well as an Albert J. Ryan Foundation Fellowship (AEE).
References
- 1.Abe H, Hidaka N, Kawagoe C, Odagiri K, Watanabe Y, Ikeda T, Ishizuka Y, Hashiguchi H, Takeda R, Nishimori T, Ishida Y. Prenatal psychological stress causes higher emotionality, depression-like behavior, and elevated activity in the hypothalamo-pituitary-adrenal axis. Neurosci Res 59: 145–51, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology 31: 187–196, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav 91: 449–458, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Adamec RE, McKay D. Amygdala kindling, anxiety, and corticotrophin releasing factor (CRF). Physiol Behav 54: 423–431, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav 54: 101–109, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Ader R Conditioned adrenocortical steroid elevations in the rat. J Comp Physiol Psychol 90: 1156–1163, 1976. [DOI] [PubMed] [Google Scholar]
- 7.Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry 47: 325–330, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry 148: 1023–1025, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ-F. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry 161: 1488–1490, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 132: 289–295, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Akana SF, Scribner KA, Bradbury MJ, Strack AM, Walker CD, Dallman MF. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology 131: 585–94, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A Facilitative Role for Corticosterone in the Acquisition of a Spatial Task Under Moderate Stress. Learn Mem 11: 188–195, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun 25: 1–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allolio B, Deuss U, Kaulen D, Leonhardt U, Kallabis D, Hamel E, Winkelmann W. FK 33-824, a met-enkephalin analog, blocks corticotropin-releasing hormone-induced adrenocorticotropin secretion in normal subjects but not in patients with Cushing’s disease. J Clin Endocrinol Metab 63: 1427–1431, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schütz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci 12: 247–249, 2009. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of metal disorders (5th Edition). 5th ed. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 17.Amsterdam JD, Marinelli DL, Arger P, Winokur A. Assessment of adrenal gland volume by computed tomography in depressed patients and healthy volunteers: a pilot study. Psychiatry Res 21: 189–97, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Anderson KE, Rosner W, Khan MS, New MI, Pang SY, Wissel PS, Kappas A. Diet-hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci 40: 1761–1768, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, Pöppel E. Dopaminergic reward system: a short integrative review. Int Arch Med 3: 24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armario A Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: Different pathways, common outcome. Trends Pharmacol Sci 31: 318–325, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57: 441–447, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Babb JA, Masini CV, Day HEW, Campeau S. Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress 17: 224–34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. [Online]. Am J Psychiatry 156: 585–8, 1999. http://www.ncbi.nlm.nih.gov/pubmed/10200738. [DOI] [PubMed] [Google Scholar]
- 24.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 111: 33–51, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Bakshi V, Kalin N. Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry 48: 1175–1198, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of Circadian Time in Peripheral Tissues by Glucocorticoid Signaling. Science (80- ) 289: 2344–2347, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Banki CM, Karmacsi L, Bissette G, Nemeroff CB. Cerebrospinal fluid neuropeptides in dementia. Biol Psychiatry 32: 452–456, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Bao A-M, Hestiantoro A, Van Someren EJW, Swaab DF, Zhou J-N. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain 128: 1301–1313, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Golib Dzib JF, Bocquet O, Bailly A, Benecke A, Tronche F. Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biol Psychiatry 68: 231–239, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacology 26: 286–294, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Barr RG, Pantel MS, Young SN, Wright JH, Hendricks LA, Gravel R. The response of crying newborns to sucrose: Is it a “sweetness” effect? Physiol Behav 66: 409–417, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci U S A 107: 16655–16660, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell’Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann MH, Gendron TM, Becketts KM, Henningfield JE, Gorelick DA, Rothman RB. Effects of intravenous cocaine on plasma cortisol and prolactin in human cocaine abusers. Biol Psychiatry 38: 751–755, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Baxter JD. Glucocorticoid Hormone Action. Pharmac 2: 605–659, 1976. [DOI] [PubMed] [Google Scholar]
- 36.Bearn J, Buntwal N, Papadopoulos A, Checkley S. Salivary cortisol during opiate dependence and withdrawal. Addict Biol 6: 157–162, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Belanoff J, Flores B, Kalezhan M, Sund B, Schatzberg AF. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol 21: 516–521, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Belanoff JK, Rothschild AJ, Cassidy F, DeBattista C, Baulieu E-E, Schold C, Schatzberg AF. An open label trial of C-1073 (mifepristone) for psychotic major depression. Biol Psychiatry 52: 386–92, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF, Dallman MF. Sucrose intake and corticosterone interact with cold to modulate ingestive behaviour, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. J Neuroendocrinol 14: 330–342, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF. Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol 12: 461–470, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Bellisle F, Louis-Sylvestre J, Linet N, Rocaboy B, Dalle B, Cheneau F, L’Hinoret D, Guyot L. Anxiety and food intake in men. Psychosom Med 52: 452–457, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Benedek DM, Fullerton C, Ursano RJ. First responders: mental health consequences of natural and human-made disasters for public health and public safety workers. Annu Rev Public Health 28: 55–68, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev 28: 309–369, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20: 1–25, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology (Berl) 191: 391–431, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Berridge KC. “Liking” and “wanting” food rewards: Brain substrates and roles in eating disorders. Physiol Behav 97: 537–550, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berthoud H-R. Neural control of appetite: Cross-talk between homeostatic and non-homeostatic systems. Appetite 43: 315–317, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Berthoud H-R. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity 14 Suppl 5: 197S–200S, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Beylin A V, Shors TJ. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav Neurosci 112: 1327–1338, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Beylin A V, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav 43: 124–131, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF. Corticosterone facilitates saccharin intake in adrenalectomized rats: Does corticosterone increase stimulus salience? J Neuroendocrinol 12: 453–460, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci 1032: 315–319, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299: 1291–1305, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bitran D, Shiekh M, Dowd JA, Dugan MM, Renda P. Corticosterone is permissive to the anxiolytic effect that results from the blockade of hippocampal mineralocorticoid receptors. Pharmacol Biochem Behav 60: 879–887, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci 30: 194–202, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Björntorp P, Rosmond R. Obesity and cortisol. Nutrition 16: 924–936, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Björntorp P Endocrine abnormalities of obesity. Metabolism 44: 21–23, 1995. [DOI] [PubMed] [Google Scholar]
- 59.de Boer SF, de Beun R, Slangen JL, van der Gugten J. Dynamics of plasma catecholamine and corticosterone concentrations during reinforced and extinguished operant behavior in rats. Physiol Behav 47: 691–698, 1990. [DOI] [PubMed] [Google Scholar]
- 60.Borowsky B, Kuhn CM. Chronic cocaine administration sensitizes behavioral but not neuroendocrine responses. Brain Res 543: 301–306, 1991. [DOI] [PubMed] [Google Scholar]
- 61.Borowsky B, Kuhn CM. Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther 256: 204–210, 1991. [PubMed] [Google Scholar]
- 62.Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A 102: 473–8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci 26: 1971–8, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav 33: 903–907, 1989. [DOI] [PubMed] [Google Scholar]
- 65.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci 48: 29–69, 1989. [DOI] [PubMed] [Google Scholar]
- 66.Bravo JA, Díaz-Veliz G, Mora S, Ulloa JL, Berthoud VM, Morales P, Arancibia S, Fiedler JL. Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol 20: 273–85, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 154: 624–629, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci 31: 363–7, 1982. [DOI] [PubMed] [Google Scholar]
- 69.Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role? Biol Psychiatry 55: 1–9, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus: Treatment and preventive therapy. Ann N Y Acad Sci 1179: 41–55, 2009. [DOI] [PubMed] [Google Scholar]
- 71.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 26: 307–317, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Budziszewska B, Jaworska-Feil L, Lasoń W. The effect of repeated amphetamine and cocaine administration on adrenal, gonadal and thyroid hormone levels in the rat plasma. Exp Clin Endocrinol Diabetes 104: 334–338, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Burgess ML, Davis JM, Wilson SP, Borg TK, Burgess WA, Buggy J. Effects of intracranial self-stimulation on selected physiological variables in rats. Am J Physiol 264: R149–R155, 1993. [DOI] [PubMed] [Google Scholar]
- 74.Buske-Kirschbaum A, Grota L, Kirschbaum C, Bienen T, Moynihan J, Ader R, Blair ML, Hellhammer DH, Felten DL. Conditioned increase in peripheral blood mononuclear cell (PBMC) number and corticosterone secretion in the rat. Pharmacol Biochem Behav 55: 27–32, 1996. [DOI] [PubMed] [Google Scholar]
- 75.Buwalda B, Blom WAM, Koolhaas JM, van Dijk G. Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav 73: 371–377, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Caggiula AR, Epstein LH, Antelman SM, Saylor S, Knopf S, Perkins KA, Stiller R. Acute stress or corticosterone administration reduces responsiveness to nicotine: implications for a mechanism of conditioned tolerance. Psychopharmacology (Berl) 111: 499–507, 1993. [DOI] [PubMed] [Google Scholar]
- 77.Cahill L, Gorski L, Le K. Enhanced Human Memory Consolidation With Post-Learning Stress: Interaction With the Degree of Arousal at Encoding. Learn Mem 10: 270–274, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABA(A) and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22: 219–229, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A 95: 5335–5340, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calogero AE, Gallucci WT, Kling MA, Chrousos GP, Gold PW. Cocaine stimulates rat hypothalamic corticotropin-releasing hormone secretion in vitro. Brain Res 505: 7–11, 1989. [DOI] [PubMed] [Google Scholar]
- 81.Calu DJ, Chen YW, Kawa AB, Nair SG, Shaham Y. The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology 76: 395–406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience 183: 81–89, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Camí J, Gilabert M, San L, de la Torre R. Hypercortisolism after opioid discontinuation in rapid detoxification of heroin addicts. Br J Addict 87: 1145–1151, 1992. [DOI] [PubMed] [Google Scholar]
- 84.Cancela LM, Bregonzio C, Molina VA. Anxiolytic-like effect induced by chronic stress is reversed by naloxone pretreatment. Brain Res Bull 36: 209–213, 1995. [DOI] [PubMed] [Google Scholar]
- 85.Carroll B, Greden J, Feinberg M. Neuroendocrine disturbances and the diagnosis and aetiology of endogenous depression. Lancet 1: 321–322, 1980. [DOI] [PubMed] [Google Scholar]
- 86.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine Regulation in Depression. Arch Gen Psychiatry 33: 1039–1044, 1976. [DOI] [PubMed] [Google Scholar]
- 87.Casarotto PC, Andreatini R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. Eur Neuropsychopharmacol 17: 735–42, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Cassella J V, Davis M. The design and calibration of a startle measurement system. Physiol Behav 36: 377–383, 1986. [DOI] [PubMed] [Google Scholar]
- 89.Castonguay TW. Glucocorticoids as modulators in the control of feeding. Brain Res Bull 27: 423–428, 1991. [DOI] [PubMed] [Google Scholar]
- 90.de Castro JM. Macronutrient relationships with meal patterns and mood in the spontaneous feeding behavior of humans. Physiol Behav 39: 561–569, 1987. [DOI] [PubMed] [Google Scholar]
- 91.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 15: 6340–6350, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang CP, Pearse RV., O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin- releasing factor and sauvagine in mammalian brain. Neuron 11: 1187–1195, 1993. [DOI] [PubMed] [Google Scholar]
- 93.Charlton BG. Adrenal cortical innervation and glucocorticoid secretion. J Endocrinol 126: 5–8, 1990. [DOI] [PubMed] [Google Scholar]
- 94.Chavez M, Seeley RJ, Havel PJ, Friedman MI, Matson CA, Woods SC, Schwartz MW. Effect of a high-fat diet on food intake and hypothalamic neuropeptide gene expression in streptozotocin diabetes. J Clin Invest 102: 340–346, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U S A 102: 2620–2625, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A 90: 8967–8971, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christiansen AM, DeKloet AD, Ulrich-Lai YM, Herman JP. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav 103: 111–6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest 121: 2684–2692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Claes S, Villafuerte S, Forsgren T, Sluijs S, Del-Favero J, Adolfsson R, Van Broeckhoven C. The corticotropin-releasing hormone binding protein is associated with major depression in a population from Northern Sweden. Biol Psychiatry 54: 867–872, 2003. [DOI] [PubMed] [Google Scholar]
- 100.Claes S Corticotropin-releasing hormone (CRH) in psychiatry: from stress to psychopathology. Ann Med 36: 50–61, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Claes SJ. CRH, stress, and major depression: a psychobiological interplay. Vitam Horm 69: 117–50, 2004. [DOI] [PubMed] [Google Scholar]
- 102.Cleck JN, Blendy JA. Making a bad thing worse: Adverse effects of stress on drug addiction. J Clin Invest 118: 454–461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coccurello R, D’Amato FR, Moles A. Chronic social stress, hedonism and vulnerability to obesity: Lessons from Rodents. Neurosci Biobehav Rev 33: 537–550, 2009. [DOI] [PubMed] [Google Scholar]
- 104.Coco ML, Kuhn CM, Ely TD, Kilts CD. Selective activation of mesoamygdaloid dopamine neurons by conditioned stress: attenuation by diazepam. Brain Res 590: 39–47, 1992. [DOI] [PubMed] [Google Scholar]
- 105.Coe CL, Stanton ME, Levine S. Adrenal responses to reinforcement and extinction: role of expectancy versus instrumental responding. Behav Neurosci 97: 654–657, 1983. [DOI] [PubMed] [Google Scholar]
- 106.Cole BJ, Hillmann M, Seidelmann D, Klewer M, Jones GH. Effects of benzodiazepine receptor partial inverse agonists in the elevated plus maze test of anxiety in the rat. Psychopharmacology (Berl) 121: 118–126, 1995. [DOI] [PubMed] [Google Scholar]
- 107.Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology 35: 674–685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110: 1321–1334, 1996. [DOI] [PubMed] [Google Scholar]
- 109.Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol Psychiatry 68: 303–5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Contoreggi C, Herning RI, Na P, Gold PW, Chrousos G, Negro PJ, Better W, Cadet JL. Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol Psychiatry 54: 873–878, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Cordero MI, Kruyt ND, Merino JJ, Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress 5: 73–79, 2002. [DOI] [PubMed] [Google Scholar]
- 112.Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci 112: 885–891, 1998. [DOI] [PubMed] [Google Scholar]
- 113.Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: Dependence upon training intensity. Brain Res 786: 11–17, 1998. [DOI] [PubMed] [Google Scholar]
- 114.Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, Wu X, Gentile KA, Huang SP, Emison E, Delmonte T, D’Souza BB, Zimbroff DL, Grebb JA, Goddard AW, Stock EG. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety 27: 417–425, 2010. [DOI] [PubMed] [Google Scholar]
- 115.Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology 21: 263–275, 1996. [DOI] [PubMed] [Google Scholar]
- 116.Coyne J, Downey G. Social factors in psychopathology: Stress, social support, and coping processes. Annu Rev Psycology 42: 401–25, 1991. [DOI] [PubMed] [Google Scholar]
- 117.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13: 167–170, 1980. [DOI] [PubMed] [Google Scholar]
- 118.Crowder RG. Principles of Learning and Memory: Classic Edition. 2014.
- 119.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4: 775–790, 2005. [DOI] [PubMed] [Google Scholar]
- 120.Cuesta M, Cermakian N, Boivin DB. Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J 29: 1360–1370, 2014. [DOI] [PubMed] [Google Scholar]
- 121.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. 2001. [DOI] [PubMed]
- 122.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu Y-T, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29: 1558–1572, 2004. [DOI] [PubMed] [Google Scholar]
- 123.Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, Al-Shabbout M, Meyer VA, Perel J. 24-Hour Cortisol Measures in Adolescents With Major Depression: a Controlled Study. Biol Psychiatry 30: 25–36, 1991. [DOI] [PubMed] [Google Scholar]
- 124.Dallman M, Akana S, Pecoraro N, Warne J, la Fleur S, Foster M. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr altzheimers Res 4: 199–204, 2007. [DOI] [PubMed] [Google Scholar]
- 125.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A 100: 11696–701, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dallman MF, Pecoraro NC, La Fleur SE. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain Behav Immun 19: 275–280, 2005. [DOI] [PubMed] [Google Scholar]
- 127.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front. Neuroendocrinol. 14: 303–347, 1993. [DOI] [PubMed] [Google Scholar]
- 128.Davis KW, Cepeda-Benito A, Harraid JH, Wellman PJ. Plasma corticosterone in the rat in response to nicotine and saline injections in a context previously paired or unpaired with nicotine. Psychopharmacology (Berl) 180: 466–472, 2005. [DOI] [PubMed] [Google Scholar]
- 129.Davis M, Redmond DE, Baraban JM. Noradrenergic agonists and antagonists: Effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 65: 111–118, 1979. [DOI] [PubMed] [Google Scholar]
- 130.Daviu N, Andero R, Armario A, Nadal R. Sex differences in the behavioural and hypothalamic–pituitary–adrenal response to contextual fear conditioning in rats. Horm Behav 66: 713–723, 2014. [DOI] [PubMed] [Google Scholar]
- 131.Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza P. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci 15: 7181–7188, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deroche V, Piazza P, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res 598: 343–348, 1992. [DOI] [PubMed] [Google Scholar]
- 133.Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, Kellendonk C, Le Moal M, Spanagel R, Schütz G, Tronche F, Piazza PV. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci 23: 4785–4790, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Devenport L, Knehans A, Thomas T, Sundstrom A. Macronutrient intake and utilization by rats: interactions with type I adrenocorticoid receptor stimulation. Am J Physiol 260: R73–R81, 1991. [DOI] [PubMed] [Google Scholar]
- 135.Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci 110: 661–672, 1996. [DOI] [PubMed] [Google Scholar]
- 136.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 9: 542–552, 1999. [DOI] [PubMed] [Google Scholar]
- 137.Donner NC, Montoya CD, Lukkes JL, Lowry C a. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37: 645–61, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Donny EC, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF. Differential effects of response-contingent and response-independent nicotine in rats. Eur J Pharmacol 402: 231–240, 2000. [DOI] [PubMed] [Google Scholar]
- 139.Dubé L, LeBel JL, Lu J. Affect asymmetry and comfort food consumption. Physiol Behav 86: 559–567, 2005. [DOI] [PubMed] [Google Scholar]
- 140.Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res 13: 1157–1166, 2005. [DOI] [PubMed] [Google Scholar]
- 141.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev 15: 71–100, 1990. [DOI] [PubMed] [Google Scholar]
- 142.Edwards A V, Jones CT, Bloom SR. Reduced adrenal cortical sensitivity to ACTH in lambs with cut splanchnic nerves. J Endocrinol 110: 81–85, 1986. [DOI] [PubMed] [Google Scholar]
- 143.Edwards A V, Jones CT. Autonomic control of adrenal function. J Anat 183 ( Pt 2: 291–307, 1993. [PMC free article] [PubMed] [Google Scholar]
- 144.Engeland W, Gann D. Splanchnic nerve stimulation modulates steroid secretion in hypophysectomized dogs. Neuroendocrinology1 50: 124–31, 1989. [DOI] [PubMed] [Google Scholar]
- 145.Engeland W, Lilly M, Gann D. Sympathetic adrenal denervation decreases adrenal blood flow without altering the cortisol response to hemorrhage. Endocrinology 117: 100–10, 1985. [DOI] [PubMed] [Google Scholar]
- 146.Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci 1032: 208–10, 2004. [DOI] [PubMed] [Google Scholar]
- 147.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26: 37–49, 2001. [DOI] [PubMed] [Google Scholar]
- 148.Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 187: 112–20, 2006. [DOI] [PubMed] [Google Scholar]
- 149.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 128: 408–412, 1996. [DOI] [PubMed] [Google Scholar]
- 150.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 18: 5529–5536, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19: 1–6, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Falter U, Gower AJ, Gobert J. Resistance of baseline activity in the elevated plus-maze to exogenous influences. Behav Pharmacol 3: 123–128, 1992. [PubMed] [Google Scholar]
- 153.Fanselow MS. Conditional and unconditional components of post-shock freezing. Pavlov J Biol Sci Off J Pavlov 15: 177–182, 1980. [DOI] [PubMed] [Google Scholar]
- 154.Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav 54: 31–40, 1996. [DOI] [PubMed] [Google Scholar]
- 155.Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol Learn Mem 112: 17–29, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav 104: 228–34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol 517: 156–65, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fleshner M, Pugh CR, Tremblay D, Rudy JW. DHEA-S selectively impairs contextual-fear conditioning: support for the antiglucocorticoid hypothesis. Behav Neurosci 111: 512–517, 1997. [DOI] [PubMed] [Google Scholar]
- 159.La Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: Regulation of lard intake and fat stores. Endocrinology 145: 2174–2185, 2004. [DOI] [PubMed] [Google Scholar]
- 160.La Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology 146: 2193–2199, 2005. [DOI] [PubMed] [Google Scholar]
- 161.la Fleur SE. The effects of glucocorticoids on feeding behavior in rats. Physiol Behav 89: 110–114, 2006. [DOI] [PubMed] [Google Scholar]
- 162.Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropic and corticosterone concentrations after restraint. Endocrinology 150: 2325–2333, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology 30: 880–891, 2005. [DOI] [PubMed] [Google Scholar]
- 164.Francis LA, Granger DA, Susman EJ. Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite 64: 32–38, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn Mem 18: 666–677, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, Dutriez-Casteloot I, Enache M, Maccari S, Barden N, Cohen-Salmon C, Hamon M, Lanfumey L. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci 24: 2787–2796, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 149: 5482–90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci 15: 2007–2015, 2002. [DOI] [PubMed] [Google Scholar]
- 169.Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol 387: 59–62, 2000. [DOI] [PubMed] [Google Scholar]
- 170.Garland EJ, Zis AP. Effect of codeine and oxazepam on afternoon cortisol secretion in men. Psychoneuroendocrinology 14: 397–402, 1989. [DOI] [PubMed] [Google Scholar]
- 171.George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 35: 607–612, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Goeders NE, Bienvenu OJ, De Souza EB. Chronic cocaine administration alters corticotropin-releasing factor receptors in the rat brain. Brain Res 531: 322–328, 1990. [DOI] [PubMed] [Google Scholar]
- 173.Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 114: 63–70, 1994. [DOI] [PubMed] [Google Scholar]
- 174.Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res 722: 145–152, 1996. [DOI] [PubMed] [Google Scholar]
- 175.Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology 23: 577–586, 2000. [DOI] [PubMed] [Google Scholar]
- 176.Gold P, Chrousos G. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7: 254–275, 2002. [DOI] [PubMed] [Google Scholar]
- 177.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress. N Engl J Med 319: 413–20, 1988. [DOI] [PubMed] [Google Scholar]
- 178.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci 16: 4787–4798, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gómez-Abellán P, Díez-Noguera A, Madrid J a, Luján J a, Ordovás JM, Garaulet M. Glucocorticoids affect 24 h clock genes expression in human adipose tissue explant cultures. PLoS One 7: e50435, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goodyer IM, Park RJ, Herbert J. Psychosocial and endocrine features of chronic first-episode major depression in 8-16 year olds. Biol Psychiatry 50: 351–357, 2001. [DOI] [PubMed] [Google Scholar]
- 181.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13: 717–28, 2008. [DOI] [PubMed] [Google Scholar]
- 182.Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. Int J Obes (Lond) 33 Suppl 2: S54–S58, 2009. [DOI] [PubMed] [Google Scholar]
- 183.Graf EN, Hoks M a, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker D a, Mantsch JR. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-Induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology 36: 1444–54, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Green PK, Wilkinson CW, Woods SC. Intraventricular corticosterone increases the rate of body weight gain in underweight adrenalectomized rats. Endocrinology 130: 269–275, 1992. [DOI] [PubMed] [Google Scholar]
- 185.Greenwood BN, Foley TE, Le TV, Strong P V, Loughridge AB, Day HEW, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res 217: 354–362, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 893: 135–142, 2001. [DOI] [PubMed] [Google Scholar]
- 187.Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 114: 1557–1579, 2003. [DOI] [PubMed] [Google Scholar]
- 188.Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. Acute Stress Potentiates Anxiety in Humans. Biol Psychiatry 62: 1183–1186, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol 282: R1333–41, 2002. [DOI] [PubMed] [Google Scholar]
- 190.Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci 11: 868–870, 2008. [DOI] [PubMed] [Google Scholar]
- 191.Groenink L, Dirks A, Verdouw P, Schipholt M, Veening J, van der Gugten J, Olivier B. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry 51: 875–881, 2002. [DOI] [PubMed] [Google Scholar]
- 192.Groenink L, Pattij T, De Jongh R, Van der Gugten J, Oosting RS, Dirks A, Olivier B. 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur J Pharmacol 463: 185–197, 2003. [DOI] [PubMed] [Google Scholar]
- 193.Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Laraia B, Epel E. What is eating you? Stress and the drive to eat. Appetite 58: 717–721, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guaza C, Torrellas A, Borrell S. Adrenocortical response to acute and chronic ethanol administration in rats. Psychopharmacology (Berl) 79: 173–176, 1983. [DOI] [PubMed] [Google Scholar]
- 195.Gyengesi E, Liu ZW, D’Agostino G, Gan G, Horvath TL, Gao XB, Diano S. Corticosterone regulates synaptic input organization of POMC and NPY/AgRP neurons in adult mice. Endocrinology 151: 5395–5402, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci 97: 6079–6084, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Haenisch B, Bilkei-Gorzo A, Caron MG, Bönisch H. Knockout of the norepinephrine transporter and pharmacologically diverse antidepressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J Neurochem 111: 403–16, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JM, Meerlo P. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J Sleep Res 20: 259–266, 2011. [DOI] [PubMed] [Google Scholar]
- 199.Hajcak G, Castille C, Olvet D, Dunning J, Roohl J, Hatchwell E. Genetic variation in brain-derived neurotrophic factor and human fear conditioning. Genes Brain Behav 8: 80–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol 18: 385–403, 1934. [Google Scholar]
- 201.Han F, Ozawa H, Matsuda KI, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res 51: 371–381, 2005. [DOI] [PubMed] [Google Scholar]
- 202.Haney M, Maccari S, Le Moal M, Simon H, Piazza P. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698: 46–52, 1995. [DOI] [PubMed] [Google Scholar]
- 203.Haskett RF. Diagnostic categorization of psychiatric disturbance in Cushing’s syndrome. Am J Psychiatry 142: 911–6, 1985. [DOI] [PubMed] [Google Scholar]
- 204.Heesch CM, Negus BH, Keffer JH, Snyder RW, Risser RC, Eichhorn EJ. Effects of cocaine on cortisol secretion in humans. Am J Med Sci 310: 61–64, 1995. [DOI] [PubMed] [Google Scholar]
- 205.Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol 233: 102–111, 2012. [DOI] [PubMed] [Google Scholar]
- 206.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49: 1023–1039, 2001. [DOI] [PubMed] [Google Scholar]
- 207.Heim C, Newport DJ, Bonsall R, Miller a H, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. [Online]. Am J Psychiatry 158: 575–81, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11282691. [DOI] [PubMed] [Google Scholar]
- 208.Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, Koob GF. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology 11: 179–186, 1994. [DOI] [PubMed] [Google Scholar]
- 209.Hellman L, Fukushima D, Roffwarg H, Fishman J. Changes in estradiol and cortisol production rates in men under the influence of narcotics. J Clin Endocrinol Metab 41: 1014–9, 1975. [DOI] [PubMed] [Google Scholar]
- 210.Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther 284: 651–660, 1998. [PubMed] [Google Scholar]
- 211.Hennessy JW, Smotherman WP, Levine S. Conditioned taste aversion and the pituitary-adrenal system. Behav Biol 16: 413–424, 1976. [DOI] [PubMed] [Google Scholar]
- 212.Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol 6: 341–5, 1994. [DOI] [PubMed] [Google Scholar]
- 213.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61: 180–90, 1995. [DOI] [PubMed] [Google Scholar]
- 214.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res 28: 341–356, 1994. [DOI] [PubMed] [Google Scholar]
- 215.Hill MN, Hellemans KGC, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36: 2085–117, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience 204: 5–16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hodos W Progressive ratio as a measure of reward strength. Science 134: 943–944, 1961. [DOI] [PubMed] [Google Scholar]
- 218.Holmes S, Robins L. The role of parental disciplinary practices in the development of depression and alcoholism. Psychiatry 51: 24–36, 1988. [DOI] [PubMed] [Google Scholar]
- 219.Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol 61: 81–109, C1–C11, 2010. [DOI] [PubMed] [Google Scholar]
- 220.Holsboer F, Liebl R, Hofschuster E. Repeated Dexamethasone Suppression Test during depressive illness. J Affect Disord 4: 93–101, 1982. [DOI] [PubMed] [Google Scholar]
- 221.Holsboer F The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23: 477–501, 2000. [DOI] [PubMed] [Google Scholar]
- 222.Holsboer-Trachsler E, Stohler R, Hatzinger M. Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatry Res 38: 163–71, 1991. [DOI] [PubMed] [Google Scholar]
- 223.Horn CAC, Pietrzak RH, Corsi-Travali S, Neumeister A. Linking plasma cortisol levels to phenotypic heterogeneity of posttraumatic stress symptomatology. Psychoneuroendocrinology 39: 88–93, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Houshyar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: Possible mechanisms involved in facilitated and attenuated stress response. J Neuroendocrinol 13: 875–886, 2001. [DOI] [PubMed] [Google Scholar]
- 225.Hughes JR, Arana G, Amori G, Stewart F, Workman R. Effect of tobacco withdrawal on the dexamethasone suppression test. Biol Psychiatry 23: 96–98, 1988. [DOI] [PubMed] [Google Scholar]
- 226.Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem 81: 67–74, 2004. [DOI] [PubMed] [Google Scholar]
- 227.Huot RL, Thrivikraman K V, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 158: 366–373, 2001. [DOI] [PubMed] [Google Scholar]
- 228.Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: Learning in three inbred strins of mice. Brain Res 785: 236–244, 1998. [DOI] [PubMed] [Google Scholar]
- 229.Jackson A, Murphy L. Role of the hypothalamic-pituitary-adrenal axis in the suppression of luteinizing hormone release by delta-9-tetrahydrocannabinol. Neuroendocrinology 65: 446–52, 1997. [DOI] [PubMed] [Google Scholar]
- 230.Jacobson L Glucocorticoid Replacement, but not Corticotropin- Releasing Hormone Deficiency, Prevents Adrenalectomy- Induced Anorexia in Mice. Endocrinology 140: 310–317, 1999. [DOI] [PubMed] [Google Scholar]
- 231.Jacobson L Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr Physiol 4: 715–738, 2014. [DOI] [PubMed] [Google Scholar]
- 232.Jezová D, Vigas M, Jurcovicová J. ACTH and corticosterone response to naloxone and morphine in normal, hypophysectomized and dexamethasone-treated rats. Life Sci 31: 307–314, 1982. [DOI] [PubMed] [Google Scholar]
- 233.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci 10: 152–8, 2006. [DOI] [PubMed] [Google Scholar]
- 234.Joëls M Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 27: 244–250, 2006. [DOI] [PubMed] [Google Scholar]
- 235.Johansen JP, Cain CK, Ostroff LE, Ledoux JE. Molecular mechanisms of fear learning and memory. Cell 147: 509–524, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Johnson KM, Dewey WL, Ritter KS, Beckner JS. Cannabinoid effects on plasma corticosterone and uptake of H-Corticosterone by mouse brain. Eur J Pharmacol 47: 303–310, 1978. [DOI] [PubMed] [Google Scholar]
- 237.Johnson V, Pandina RJ. Alcohol problems among a community sample: longitudinal influences of stress, coping, and gender. Subst Use Misuse 35: 669–686, 2000. [DOI] [PubMed] [Google Scholar]
- 238.De Jong IEM, De Kloet ER. Glucocorticoids and Vulnerability to Psychostimulant Drugs: Toward Substrate and Mechanism. Ann N Y Acad Sci 1018: 192–198, 2004. [DOI] [PubMed] [Google Scholar]
- 239.Jovanovic T, Phifer JE, Sicking K, Weiss T, Norrholm SD, Bradley B, Ressler KJ. Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology 36: 1540–1552, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci 20: 6983–6988, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kabbaj M, Morley-Fletcher S, Le Moal M, Maccari S. Individual differences in the effects of chronic prazosin hydrochloride treatment on hippocampal mineralocorticoid and glucocorticoid receptors. Eur J Neurosci 25: 3312–8, 2007. [DOI] [PubMed] [Google Scholar]
- 242.van Kammen DP, Schulz SC. d-Amphetamine raises cortisol levels in schizophrenic patients with and without chronic naltrexone pretreatment. J Neural Transm 64: 35–43, 1985. [DOI] [PubMed] [Google Scholar]
- 243.Kandel E, Schwartz J, Jessell T, Siegelbaum S, Hudspeth A. Principles of Neural Science, Fifth Edition. 5th ed. McGraw-Hill Companies. [Google Scholar]
- 244.Kandel ER, Dudai Y, Mayford MR. The Molecular and Systems Biology of Memory. Cell 157: 163–186, 2014. [DOI] [PubMed] [Google Scholar]
- 245.Karolyi IJ, Burrows HL, Ramesh TM, Nakajima M, Lesh JS, Seong E, Camper SA, Seasholtz AF. Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc Natl Acad Sci U S A 96: 11595–600, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Katz RJ. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav 16: 965–968, 1982. [DOI] [PubMed] [Google Scholar]
- 247.Kawasaki K, Iwasaki T. Corticosterone levels during extrinction of runway response in rats. Life Sci 61: 1721–1728, 1997. [DOI] [PubMed] [Google Scholar]
- 248.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22: 3306–3311, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kellner M, Baker DG, Yehuda R. Salivary cortisol in Operation Desert Storm returnees. Biol Psychiatry 42: 849–50, 1997. [DOI] [PubMed] [Google Scholar]
- 250.Kenna HA, Poon AW, De Los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: Review with case report. Psychiatry Clin Neurosci 65: 549–560, 2011. [DOI] [PubMed] [Google Scholar]
- 251.Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustün TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc 18: 23–33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kilfoil T, Michel A, Montgomery D, Whiting RL. Effects of anxiolytic and anxiogenic drugs on exploratory activity in a simple model of anxiety in mice. Neuropharmacology 28: 901–905, 1989. [DOI] [PubMed] [Google Scholar]
- 254.Kim Y, Yang HY, Kim AJ, Lim Y. Academic stress levels were positively associated with sweet food consumption among Korean high-school students. Nutrition 29: 213–218, 2013. [DOI] [PubMed] [Google Scholar]
- 255.de Kloet AD, Krause EG, Solomon MB, Flak JN, Scott KA, Kim D-H, Myers B, Ulrich-Lai YM, Woods SC, Seeley RJ, Herman JP. Adipocyte glucocorticoid receptors mediate fat-to-brain signaling. Psychoneuroendocrinology 56: 110–119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–75, 2005. [DOI] [PubMed] [Google Scholar]
- 257.de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci 22: 422–426, 1999. [DOI] [PubMed] [Google Scholar]
- 258.de Kloet ER, Reul JM, Sutanto W. Corticosteroids and the brain. J Steroid Biochem Mol Biol 37: 387–394, 1990. [DOI] [PubMed] [Google Scholar]
- 259.de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19: 269–301, 1998. [DOI] [PubMed] [Google Scholar]
- 260.Knych ET, Eisenberg RM. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology 29: 110–118, 1979. [DOI] [PubMed] [Google Scholar]
- 261.Kokka N, Garcia JF. Effects of delta 9-THC on growth hormone and ACTH secretion in rats. Life Sci 15: 329–338, 1974. [DOI] [PubMed] [Google Scholar]
- 262.Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp 172: 277–89; discussion 290–5, 1993. [DOI] [PubMed] [Google Scholar]
- 263.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science 278: 52–58, 1997. [DOI] [PubMed] [Google Scholar]
- 264.Koranyi L, Endroczi E, Tal E, Levay G. The effect of acute and chronic ethanol administration on serum corticosterone concentration in rats. Acta Physiol Hung 69: 123–128, 1987. [PubMed] [Google Scholar]
- 265.Korte SM, De Boer SF, Bohus B. Fear-potentiation in the elevated plus-maze test depends on stressor controllability and fear conditioning. Stress 3: 27–40, 1999. [DOI] [PubMed] [Google Scholar]
- 266.Korte SM, De Boer SF. A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol 463: 163–175, 2003. [DOI] [PubMed] [Google Scholar]
- 267.Korte SM, Buwalda B, Bouws GAH, Koolhaas JM, Maes FW, Bohus B. Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiol Behav 51: 815–822, 1992. [DOI] [PubMed] [Google Scholar]
- 268.Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51: 23–47, 1998. [DOI] [PubMed] [Google Scholar]
- 269.Krishnan K, Doraiswamy P. Pituitary size in depression. J Clin Endocrinol Metab 72: 256–259, 1991. [DOI] [PubMed] [Google Scholar]
- 270.Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA plasticity and memory. Nat Publ Gr 11: 675–681, 2010. [DOI] [PubMed] [Google Scholar]
- 271.Kuipers SD, Trentani A, Westenbroek C, Bramham CR, Korf J, Kema IP, Ter Horst GJ, Den Boer JA. Unique patterns of FOS, phospho-CREB and BrdU immunoreactivity in the female rat brain following chronic stress and citalopram treatment. Neuropharmacology 50: 428–40, 2006. [DOI] [PubMed] [Google Scholar]
- 272.Ladd CO, Huot RL, Thrivikraman K V, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res 122: 81–103, 2000. [DOI] [PubMed] [Google Scholar]
- 273.Lamia K a., Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480: 552–556, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: A glucocorticoid-metabolic-brain axis? Endocrinology 142: 2796–2804, 2001. [DOI] [PubMed] [Google Scholar]
- 275.Laugero KD, Falcon LM, Tucker KL. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite 56: 194–204, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lawlor DA, Harbord RM, Tybjaerg-Hansen A, Palmer TM, Zacho J, Benn M, Timpson NJ, Davey Smith G, Nordestgaard BG. Using genetic loci to understand the relationship between adiposity and psychological distress: A Mendelian Randomization study in the Copenhagen General Population Study of 53221 adults. J Intern Med 269: 525–537, 2011. [DOI] [PubMed] [Google Scholar]
- 277.Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 135: 169–174, 1998. [DOI] [PubMed] [Google Scholar]
- 278.Lee S, Rivier C. Effect of Exposure to an Alcohol Diet for 10 Days on the Ability of Interleukin-1β to Release ACTH and Corticosterone in the Adult Ovariectomized Female Rat. Alcohol Clin Exp Res 17: 1009–1013, 1993. [DOI] [PubMed] [Google Scholar]
- 279.Lee S, Rivier C. Alcohol increases the expression of type 1, but not type 2 alpha corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Brain Res Mol Brain Res 52: 78–89, 1997. [DOI] [PubMed] [Google Scholar]
- 280.Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: Comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology 145: 4470–4479, 2004. [DOI] [PubMed] [Google Scholar]
- 281.Lee S, Smith GW, Vale W, Lee K, Rivier C. Mice that lack corticotropin-releasing factor (CRF) receptors type 1 show a blunted ACTH response to acute alcohol despite up-regulated constitutive hypothalamic CRF gene expression. Alcohol Clin Exp Res 25: 427–433, 2001. [PubMed] [Google Scholar]
- 282.Lee Y, Schulkin J, Davis M. Effect of corticosterone on the enhancement of the acoustic startle reflex by corticotropin releasing factor (CRF). Brain Res 666: 93–98, 1994. [DOI] [PubMed] [Google Scholar]
- 283.Leigh Gibson E Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol Behav 89: 53–61, 2006. [DOI] [PubMed] [Google Scholar]
- 284.Lemmens SG, Rutters F, Born JM, Westerterp-Plantenga MS. Stress augments food “wanting” and energy intake in visceral overweight subjects in the absence of hunger. Physiol Behav 103: 157–163, 2011. [DOI] [PubMed] [Google Scholar]
- 285.Levy AD, Li QA, Kerr JE, Rittenhouse PA, Milonas G, Cabrera TM, Battaglia G, Alvarez Sanz MC, Van de Kar LD. Cocaine-induced elevation of plasma adrenocorticotropin hormone and corticosterone is mediated by serotonergic neurons. J Pharmacol Exp Ther 259: 495–500, 1991. [PubMed] [Google Scholar]
- 286.Lewis DA, Smith RE. Steroid-induced psychiatric syndromes. J Affect Disord 5: 319–332, 1983. [DOI] [PubMed] [Google Scholar]
- 287.Li SX, Shi J, Epstein DH, Wang X, Zhang XL, Bao YP, Zhang D, Zhang XY, Kosten TR, Lu L. Circadian Alteration in Neurobiology During 30 Days of Abstinence in Heroin Users. Biol Psychiatry 65: 905–912, 2009. [DOI] [PubMed] [Google Scholar]
- 288.Li Z, Kang SS, Lee S, Rivier C. Effect of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci 29: 345–354, 2005. [DOI] [PubMed] [Google Scholar]
- 289.Licinio J, O’Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, Lake S, Tantisira KG, Weiss ST, Wong M-L. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry 9: 1075–1082, 2004. [DOI] [PubMed] [Google Scholar]
- 290.Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behav Res Ther 46: 678–687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659–1662, 1997. [DOI] [PubMed] [Google Scholar]
- 292.Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, Wang H, Liu H, Wang X, Wu Y, Cao Z, Li W. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett 414: 155–158, 2007. [DOI] [PubMed] [Google Scholar]
- 293.Lobo MK. Lighting up the brain’s reward circuitry. Ann N Y Acad Sci 1260: 24–33, 2012. [DOI] [PubMed] [Google Scholar]
- 294.Lopez-Fernandez MA, Montaron M-F, Varea E, Rougon G, Venero C, Abrous DN, Sandi C. Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J Neurosci 27: 4552–4561, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A 92: 836–840, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lu Q, Tao F, Hou F, Zhang Z, Sun Y, Xu Y, Xu S, Zhao Y. Cortisol reactivity, delay discounting and percent body fat in Chinese urban young adolescents. Appetite 72: 13–20, 2014. [DOI] [PubMed] [Google Scholar]
- 297.Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neuroscience 277: 139–151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lucki I The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8: 523–532, 1997. [DOI] [PubMed] [Google Scholar]
- 299.Luksys G, Sandi C. Neural mechanisms and computations underlying stress effects on learning and memory. Curr Opin Neurobiol 21: 502–508, 2011. [DOI] [PubMed] [Google Scholar]
- 300.Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can't live without it. Behav Brain Res 127: 137–158, 2001. [DOI] [PubMed] [Google Scholar]
- 301.Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology 32 Suppl 1: S10–5, 2007. [DOI] [PubMed] [Google Scholar]
- 302.Macho L, Zorad S, Radikova Z, Patterson-Buckedahl P, Kvetnansky R. Ethanol consumption affects stress response and insulin binding in tissues of rats. Endocr Regul 37: 195–202, 2003. [PubMed] [Google Scholar]
- 303.Macht M, Mueller J. Immediate effects of chocolate on experimentally induced mood states. Appetite 49: 667–674, 2007. [DOI] [PubMed] [Google Scholar]
- 304.Magarinos A, McEwen B. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 69: 83–88, 1995. [DOI] [PubMed] [Google Scholar]
- 305.Magarinos A, McEwen B. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69: 89–98, 1995. [DOI] [PubMed] [Google Scholar]
- 306.Magnano CL, Gardner JM, Karmel BZ. Differences in salivary cortisol levels in cocaine-exposed and noncocaine-exposed NICU infants. Dev Psychobiol 25: 93–103, 1992. [DOI] [PubMed] [Google Scholar]
- 307.Mahler S V, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances “liking” of a sweet reward. Neuropsychopharmacology 32: 2267–2278, 2007. [DOI] [PubMed] [Google Scholar]
- 308.Makino S, Asaba K, Nishiyama M, Hashimoto K. Decreased type 2 corticotropin-releasing hormone receptor mRNA expression in the ventromedial hypothalamus during repeated immobilization stress. Neuroendocrinology 70: 160–167, 1999. [DOI] [PubMed] [Google Scholar]
- 309.Mantsch JR, Baker D a., Francis DM, Katz ES, Hoks M a., Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 195: 591–603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mantsch JR, Baker DA, Serge JP, Hoks MA, Francis DM, Katz ES. Surgical Adrenalectomy with Diurnal Corticosterone Replacement Slows Escalation and Prevents the Augmentation of Cocaine-Induced Reinstatement in Rats Self-Administering Cocaine Under Long-Access Conditions. Neuropsychopharmacology 33: 814–826, 2008. [DOI] [PubMed] [Google Scholar]
- 311.Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res 1167: 101–111, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: Relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl) 142: 399–407, 1999. [DOI] [PubMed] [Google Scholar]
- 313.Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, Cullinan WE, Ziegler DR. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett 415: 269–273, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mantsch JR, Vranjkovic O, Twining RC, Gasser PJ, Mcreynolds JR, Blacktop JM. Neurobiological mechanisms that contribute to stress-related cocaine use. Neuropharmacology 76: 383–394, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Manzanares J, Corchero J, Fuentes JA. Opioid and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of Δ9-tetrahydrocannabinol in rats. Brain Res 839: 173–179, 1999. [DOI] [PubMed] [Google Scholar]
- 316.Marcus M, Yasamy MT, van Ommeren M, Chisholm D. Depression, a global public health concern. WHO Dep. Ment. Heal. Subst. Abus. .
- 317.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs*. Eur J Neurosci 16: 387–394, 2002. [DOI] [PubMed] [Google Scholar]
- 318.Marinelli M, Rougé-Pont F, Deroche V, Barrot M, De Jésus-Oliveira C, Le Moal M, Piazza P. Glucocorticoids and behavioral effects of psychostimulants. I: locomotor response to cocaine depends on basal levels of glucocorticoids. J Pharmacol Exp Ther 281: 1392–1400, 1997. [PubMed] [Google Scholar]
- 319.Marks-Kaufman R, Lewis M. Early housing experience modifies morphine self-administration and physical dependence in adult rats. Addict Behav 9: 235–243, 1984. [DOI] [PubMed] [Google Scholar]
- 320.Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav 70: 333–342, 2000. [DOI] [PubMed] [Google Scholar]
- 321.Martijena ID, Calvo N, Volosin M, Molina VA. Prior exposure to a brief restraint session facilitates the occurrence of fear in response to a conflict situation: Behavioral and neurochemical correlates. Brain Res 752: 136–142, 1997. [DOI] [PubMed] [Google Scholar]
- 322.Maschke M, Drepper J, Kindsvater K, Kolb FP, Diener HC, Timmann D. Fear conditioned potentiation of the acoustic blink reflex in patients with cerebellar lesions. J Neurol Neurosurg Psychiatry 68: 358–364, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis 174: 145–9, 1986. [DOI] [PubMed] [Google Scholar]
- 324.Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philos Trans R Soc Lond B Biol Sci 367: 2485–94, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Mathew SJ, Coplan JD, Goetz RR, Feder A, Greenwald S, Dahl RE, Ryan ND, Mann JJ, Weissman MM. Differentiating depressed adolescent 24 h cortisol secretion in light of their adult clinical outcome. Neuropsychopharmacology 28: 1336–1343, 2003. [DOI] [PubMed] [Google Scholar]
- 326.Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther 243: 217–226, 1987. [PubMed] [Google Scholar]
- 327.Mazure CM. Life Stressors as Risk Factors in Depression. Clin Psychol Sci Pract 5: 291–313, 1998. [Google Scholar]
- 328.McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom Med 52: 97–108, 1990. [DOI] [PubMed] [Google Scholar]
- 329.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62: 3–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mcewen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci 110: 1561–1561, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.McReynolds JR, Peña DF, Blacktop JM, Mantsch JR. Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress 17: 22–38, 2014. [DOI] [PubMed] [Google Scholar]
- 332.Mello NK, Mendelson JH. Cocaine’s effects on neuroendocrine systems: Clinical and preclinical studies. Pharmacol Biochem Behav 57: 571–599, 1997. [DOI] [PubMed] [Google Scholar]
- 333.Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: Behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology 18: 263–271, 1998. [DOI] [PubMed] [Google Scholar]
- 334.Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology 30: 1751–1763, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Mendonça FH, Guimarães FS. Intra-hippocampal administration of cycloheximide attenuates the restraint-induced exploratory deficit of an elevated plus maze. Behav Brain Res 91: 207–211, 1998. [DOI] [PubMed] [Google Scholar]
- 336.Le Menuet D, Lombès M. The neuronal mineralocorticoid receptor: From cell survival to neurogenesis. Steroids 91: 1–9, 2014. [DOI] [PubMed] [Google Scholar]
- 337.Merchenthaler I Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides 5 Suppl 1: 53–69, 1984. [DOI] [PubMed] [Google Scholar]
- 338.Merino JJ, Cordero MI, Sandi C. Regulation of hippocampal cell adhesion molecules NCAM and L1 by contextual fear conditioning is dependent upon time and stressor intensity. Eur J Neurosci 12: 3283–3290, 2000. [DOI] [PubMed] [Google Scholar]
- 339.Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology 37: 1479–1490, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 128: 256–264, 1996. [DOI] [PubMed] [Google Scholar]
- 341.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev 31: 3–17, 2007. [DOI] [PubMed] [Google Scholar]
- 342.Minor TR, Saade S. Poststress glucose mitigates behavioral impairment in rats in the “learned helplessness” model of psychopathology. Biol Psychiatry 42: 324–334, 1997. [DOI] [PubMed] [Google Scholar]
- 343.Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci 24: 5492–5499, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Mo W, Arruda JA, Dunea G, Singh AK. Cocaine-induced hypertension: role of the peripheral sympathetic system. Pharmacol Res 40: 139–145, 1999. [DOI] [PubMed] [Google Scholar]
- 345.Moldow RL, Fischman AJ. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides 8: 819–822, 1987. [DOI] [PubMed] [Google Scholar]
- 346.Montkowski A, Barden N, Wotjak C, Stec I, Ganster J, Meaney M, Engelmann M, Reul JMHM, Landgraf R, Holsboer F. Long-Term Antidepressant Treatment Reduces Behavioural Deficits in Transgenic Mice with Impaired Glucocorticoid Receptor Function. J Neuroendocrinol 7: 841–845, 1995. [DOI] [PubMed] [Google Scholar]
- 347.Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol 2: 43–49, 1992. [DOI] [PubMed] [Google Scholar]
- 348.Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology (2015). doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Morgan C a., Doran A, Steffian G, Hazlett G, Southwick SM. Stress-Induced Deficits in Working Memory and Visuo-Constructive Abilities in Special Operations Soldiers. Biol Psychiatry 60: 722–729, 2006. [DOI] [PubMed] [Google Scholar]
- 350.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistochemical and in situ hybridization study. Neurosci Res 26: 235–269, 1996. [DOI] [PubMed] [Google Scholar]
- 351.Morley-Fletcher S, Darnaudery M, Koehl M, Casolini P, Van Reeth O, Maccari S. Prenatal stress in rats predicts immobility behavior in the forced swim test. Brain Res 989: 246–251, 2003. [DOI] [PubMed] [Google Scholar]
- 352.Morley-Fletcher S, Darnaudéry M, Mocaer E, Froger N, Lanfumey L, Laviola G, Casolini P, Zuena a. R, Marzano L, Hamon M, Maccari S. Chronic treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT1A receptor mRNA in prenatally stressed rats. Neuropharmacology 47: 841–847, 2004. [DOI] [PubMed] [Google Scholar]
- 353.Morris R Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60, 1984. [DOI] [PubMed] [Google Scholar]
- 354.Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MSD, Droste SK, Kühn R, Reul JMHM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 6: 1100–1107, 2003. [DOI] [PubMed] [Google Scholar]
- 355.Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience 52: 621–636, 1993. [DOI] [PubMed] [Google Scholar]
- 356.Murphy BE, Filipini D, Ghadirian AM. Possible use of glucocorticoid receptor antagonists in the treatment of major depression: preliminary results using RU 486. J psychiatry Neurosci 18: 209–13, 1993. [PMC free article] [PubMed] [Google Scholar]
- 357.Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response: The many faces of feedback. Cell Mol Neurobiol 32: 683–694, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Front Neuroendocrinol 35: 180–196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days’coricosteroid treatment. a prospective study. psychoneu 21: 25–31, 1996. [DOI] [PubMed] [Google Scholar]
- 360.National Institute on Drug Abuse. Trends & Statistics [Online]. 2015. http://www.drugabuse.gov/related-topics/trends-statistics.
- 361.Nemeroff C, Krishnan K, Reed D, Leder R, Beam C, Dunnick R. Adrenal gland enlargement in major depression: a computed tomographic study. Arch Gen Psychiatry 49: 384–387, 1992. [DOI] [PubMed] [Google Scholar]
- 362.Nemeroff C The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1: 336–342, 1996. [PubMed] [Google Scholar]
- 363.Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science (80- ) 226: 1342–4, 1984. [DOI] [PubMed] [Google Scholar]
- 364.Newcomb MD, Harlow LL. Life events and substance use among adolescents: Mediating effects of perceived loss of control and meaninglessness in life. J Pers Soc Psychol 51: 564–577, 1986. [DOI] [PubMed] [Google Scholar]
- 365.Newton CA, Lu T, Nazian SJ, Perkins I, Friedman H, Klein TW. The THC-induced suppression of Th1 polarization in response to Legionella pneumophila infection is not mediated by increases in corticosterone and PGE2. J Leukoc Biol 76: 854–861, 2004. [DOI] [PubMed] [Google Scholar]
- 366.Nikolarakis KE, Pfeiffer A, Stalla GK, Herz A. Facilitation of ACTH secretion by morphine is mediated by activation of CRF releasing neurons and sympathetic neuronal pathways. Brain Res 498: 385–388, 1989. [DOI] [PubMed] [Google Scholar]
- 367.Nishiyama M, Makino S, Iwasaki Y, Tanaka Y, Nazarloo HP, Kaneda T, Asaba K, Hashimoto K. CRH mRNA expression in the hypothalamic paraventricular nucleus is inhibited despite the activation of the hypothalamo-pituitary-adrenal axis during starvation. Brain Res 1228: 107–112, 2008. [DOI] [PubMed] [Google Scholar]
- 368.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol 85: 1315–1321, 2001. [DOI] [PubMed] [Google Scholar]
- 369.O’Dwyer A, Lightman S, Marks MN, Checkley S. Treatment of major depression with metyrapone and hydrocortisone. J Affect Disord 33: 123–128, 1995. [DOI] [PubMed] [Google Scholar]
- 370.Oei NYL, Everaerd WTAM, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress 9: 133–141, 2006. [DOI] [PubMed] [Google Scholar]
- 371.Oitzl MS, de Kloet ER, Joëls M, Schmid W, Cole TJ. Spatial learning deficits in mice with a targeted glucocorticoid receptor gene disruption. [Online]. Eur J Neurosci 9: 2284–96, 1997. http://www.ncbi.nlm.nih.gov/pubmed/9464923. [DOI] [PubMed] [Google Scholar]
- 372.Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci 106: 62–71, 1992. [DOI] [PubMed] [Google Scholar]
- 373.Oitzl MS, Reichardt HM, Joëls M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci U S A 98: 12790–12795, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A 101: 853–858, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav 66: 511–5, 1999. [DOI] [PubMed] [Google Scholar]
- 376.Olschowka JA, O’Donohue TL, Mueller GP, Jacobowitz DM. The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain. Peptides 3: 995–1015, 1982. [DOI] [PubMed] [Google Scholar]
- 377.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: When reward outweighs homeostasis. Physiol Behav 91: 506–512, 2007. [DOI] [PubMed] [Google Scholar]
- 378.Olver JS, Pinney M, Maruff P, Norman TR. Impairments of Spatial Working Memory and Attention Following Acute Psychosocial Stress. Stress Heal 31: 115–123, 2015. [DOI] [PubMed] [Google Scholar]
- 379.Ortolani D, Oyama LM, Ferrari EM, Melo LL, Spadari-Bratfisch RC. Effects of comfort food on food intake, anxiety-like behavior and the stress response in rats. Physiol Behav 103: 487–492, 2011. [DOI] [PubMed] [Google Scholar]
- 380.Owen GR, Brenner EA. Mapping molecular memory: Navigating the cellular pathways of learning. Cell Mol Neurobiol 32: 919–941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev 43: 425–73, 1991. [PubMed] [Google Scholar]
- 382.Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp 172: 296–308; discussion 308–16, 1993. [DOI] [PubMed] [Google Scholar]
- 383.Packard AEB, Ghosal S, Herman JP, Woods SC, Ulrich-Lai YM. Chronic variable stress improves glucose tolerance in rats with sucrose-induced prediabetes. Psychoneuroendocrinology 47: 178–188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Palamarchouk V, Smagin G, Goeders NE. Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats. Pharmacol Biochem Behav 94: 163–168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Palkovits M, Brownstein M, Vale W. Distribution of corticotropin-releasing factor in rat brain. Fed Proc 44: 215–219, 1985. [PubMed] [Google Scholar]
- 386.Paredes RG. Evaluating the neurobiology of sexual reward. ILAR J 50: 15–27, 2009. [DOI] [PubMed] [Google Scholar]
- 387.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49: 391–404, 2001. [DOI] [PubMed] [Google Scholar]
- 388.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 43: 60–66, 2003. [DOI] [PubMed] [Google Scholar]
- 389.Parnaudeau S, Dongelmans M-L, Turiault M, Ambroggi F, Delbes A-S, Cansell C, Luquet S, Piazza P-V, Tronche F, Barik J. Glucocorticoid receptor gene inactivation in dopamine-innervated areas selectively decreases behavioral responses to amphetamine. Front Behav Neurosci 8: 35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pasquali R, Vicennati V. The abdominal obesity phenotype and insulin resistance are associated with abnormalities of the hypothalamic-pituitary-adrenal axis in humans. Horm Metab Res 32: 521–525, 2000. [DOI] [PubMed] [Google Scholar]
- 391.Patel VA, Pohorecky LA. Acute and chronic ethanol treatment on beta-endorphin and catecholamine levels. Alcohol 6: 59–63, 1989. [DOI] [PubMed] [Google Scholar]
- 392.Patterson-Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R. Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol 37: 157–166, 2005. [DOI] [PubMed] [Google Scholar]
- 393.Paykel E, Myers J, Dienelt M, Klerman G, Lindenthal J, Pepper M. Life events and depression: A controlled study. Arch Gen Psychiatry 21, 1969. [DOI] [PubMed] [Google Scholar]
- 394.Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci 23: 9395–9402, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist 12: 500–511, 2006. [DOI] [PubMed] [Google Scholar]
- 396.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology 145: 3754–3762, 2004. [DOI] [PubMed] [Google Scholar]
- 397.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167, 1985. [DOI] [PubMed] [Google Scholar]
- 398.Penley SC, Gaudet CM, Threlkeld SW. Use of an eight-arm radial water maze to assess working and reference memory following neonatal brain injury. J Vis Exp : 50940, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Perrin M, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology 133: 3058–61, 1993. [DOI] [PubMed] [Google Scholar]
- 400.Petrovich GD, Lougee MA. Sex differences in fear-induced feeding cessation: Prolonged effect in female rats. Physiol Behav 104: 996–1001, 2011. [DOI] [PubMed] [Google Scholar]
- 401.Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues. J Neurosci 29: 15205–15212, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Pfohl B, Sherman B, Schlechte J, Winokur G. Differences in plasma ACTH and cortisol between depressed patients and normal controls. Biol Psychiatry 20: 1055–1072, 1985. [DOI] [PubMed] [Google Scholar]
- 403.Piazza P, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 514: 22–26, 1990. [DOI] [PubMed] [Google Scholar]
- 404.Piazza P, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci 19: 67–71, 1998. [DOI] [PubMed] [Google Scholar]
- 405.Piazza P, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 36: 359–378, 1996. [DOI] [PubMed] [Google Scholar]
- 406.Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology 18: 485–493, 1993. [DOI] [PubMed] [Google Scholar]
- 407.Pilotte NS, Sharpe LG, Dax EM. Multiple, but not acute, infusions of cocaine alter the release of prolactin in male rats. Brain Res 512: 107–112, 1990. [DOI] [PubMed] [Google Scholar]
- 408.Plotsky PM, Thrivikraman K V, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30: 2192–2204, 2005. [DOI] [PubMed] [Google Scholar]
- 409.Pollak DD, Rey CE, Monje FJ. Rodent models in depression research: classical strategies and new directions. Ann Med 42: 252–64, 2010. [DOI] [PubMed] [Google Scholar]
- 410.Pollard T, Steptoe A, Canaan L, Davies GJ, Wardle J. Effects of Academic Examination Stress on Eating Behavior and Blood Lipid Levels. Int. J. Behav. Med 2: 299–320, 1995. [DOI] [PubMed] [Google Scholar]
- 411.Popper R, Smits G, Meiselman HL, Hirsch E. Eating in combat: a survey of U.S. Marines. Mil Med 154: 619–623, 1989. [PubMed] [Google Scholar]
- 412.Porsolt R, Deniel M, Jalfre M. Forced swimming in rats: hypothermia, immobility and the effects of imipramine. Eur J Pharmacol 57: 431–436, 1979. [DOI] [PubMed] [Google Scholar]
- 413.Porsolt R, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 731–732, 1977. [DOI] [PubMed] [Google Scholar]
- 414.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A 91: 8777–8781, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Prager EM, Johnson LR. Stress at the synapse: signal transduction mechanisms of adrenal steroids at neuronal membranes. Sci Signal 2: re5, 2009. [DOI] [PubMed] [Google Scholar]
- 416.Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav 60: 1039–1042, 1996. [DOI] [PubMed] [Google Scholar]
- 417.Proulx K, Vahl TP, Drazen DL, Woods SC, Seeley RJ. The effect of adrenalectomy on ghrelin secretion and orexigenic action. J Neuroendocrinol 17: 445–451, 2005. [DOI] [PubMed] [Google Scholar]
- 418.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol 463: 3–33, 2003. [DOI] [PubMed] [Google Scholar]
- 419.Puder M, Weidenfeld J, Chowers I, Nir I, Conforti N, Siegel RA. Corticotrophin and corticosterone secretion following delta 1-tetrahydrocannabinol, in intact and in hypothalamic deafferentated male rats. Exp Brain Res 46: 85–88, 1982. [DOI] [PubMed] [Google Scholar]
- 420.de Quervain DJF, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol 30: 358–370, 2009. [DOI] [PubMed] [Google Scholar]
- 421.de Quervain DJ-F, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH. Glucocorticoids enhance extinction-based psychotherapy. Proc Natl Acad Sci U S A 108: 6621–5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.de Quervain DJ-F, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394: 787–790, 1998. [DOI] [PubMed] [Google Scholar]
- 423.Raadsheer F, van Heerikhuize J, Lucassen P, Hoogendijk WJ, Tilders FJH, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry 152: 1372–1376, 1995. [DOI] [PubMed] [Google Scholar]
- 424.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 60: 436–44, 1994. [DOI] [PubMed] [Google Scholar]
- 425.Radley J, Morilak D, Viau V, Campeau S. Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev 58: 79–91, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Radley JJ, Sisti HM, Hao J, Rocher a. B, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125: 1–6, 2004. [DOI] [PubMed] [Google Scholar]
- 427.Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Giles DE, Rao R, Kaufman J, Nelson B. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biol Psychiatry 40: 474–484, 1996. [DOI] [PubMed] [Google Scholar]
- 428.Raskin DE. Steroid-induced panic disorder. Am J Psychiatry 141: 1647, 1984. [DOI] [PubMed] [Google Scholar]
- 429.Raven P, O’Dwyer A, Taylor N, Checkley S. The relationship between the effects of metyrapone treatment on depressed mood and urinary steroid profiles. Psychoneuroendocrinology 21: 277–286, 1996. [DOI] [PubMed] [Google Scholar]
- 430.Reppucci CJ, Kuthyar M, Petrovich GD. Contextual fear cues inhibit eating in food-deprived male and female rats. Appetite 69: 186–195, 2013. [DOI] [PubMed] [Google Scholar]
- 431.Reul J, de Kloet E. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117: 2505–11, 1985. [DOI] [PubMed] [Google Scholar]
- 432.Reul JM, de Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem 24: 269–272, 1986. [DOI] [PubMed] [Google Scholar]
- 433.Revest J-M, Di Blasi F, Kitchener P, Rougé-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci 8: 664–672, 2005. [DOI] [PubMed] [Google Scholar]
- 434.Revest J-M, Kaouane N, Mondin M, Le Roux A, Rougé-Pont F, Vallée M, Barik J, Tronche F, Desmedt A, Piazza PV. The enhancement of stress-related memory by glucocorticoids depends on synapsin-Ia/Ib. Mol Psychiatry 15: 1140–1151, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ribeiro S, Tandon R, Grunhaus L, Greden J. The DST as a predictor of outcome in depression: a meta-analysis. Am J Psychiatry 150: 1618–1629, 1993. [DOI] [PubMed] [Google Scholar]
- 436.Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary- adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci 28: 1641–1653, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11, 1996. [DOI] [PubMed] [Google Scholar]
- 438.Rimmele U, Domes G, Mathiak K, Hautzinger M. Cortisol has different effects on human memory for emotional and neutral stimuli. Neuroreport 14: 2485–8, 2003. [DOI] [PubMed] [Google Scholar]
- 439.Risch SC, Lewine RJ, Kalin NH, Jewart RD, Risby ED, Caudle JM, Stipetic M, Turner J, Eccard MB, Pollard WE. Limbic-hypothalamic-pituitary-adrenal axis activity and ventricular-to-brain ratio studies in affective illness and schizophrenia. Neuropsychopharmacology 6: 95–100, 1992. [PubMed] [Google Scholar]
- 440.Risher-Flowers D, Adinoff B, Ravitz B, Bone G, Martin P, Nutt D, Linnoila M. Circadian rhythms of cortisol during alcohol withdrawal. Adv alcohol Subst Abus 7: 37–41, 1988. [DOI] [PubMed] [Google Scholar]
- 441.Rittmaster RS, Cutler GBJ, Sobel DO, Goldstein DS, Koppelman MC, Loriaux DL, Chrousos GP. Morphine inhibits the pituitary-adrenal response to ovine corticotropin-releasing hormone in normal subjects. J Clin Endocrinol Metab 60: 891–895, 1985. [DOI] [PubMed] [Google Scholar]
- 442.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther 229: 127–131, 1984. [PubMed] [Google Scholar]
- 443.Rivier C, Lee S. Stimulatory effect of cocaine on ACTH secretion: role of the hypothalamus. Mol Cell Neurosci 5: 189–195, 1994. [DOI] [PubMed] [Google Scholar]
- 444.Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res 726: 1–10, 1996. [PubMed] [Google Scholar]
- 445.Rivier C, Vale W. cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res 422: 403–406, 1987. [DOI] [PubMed] [Google Scholar]
- 446.Rivier C Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Front Neuroendocrinol 35: 221–233, 2014. [DOI] [PubMed] [Google Scholar]
- 447.Roberts CJ, Campbell IC, Troop N. Increases in weight during chronic stress are partially associated with a switch in food choice towards increased carbohydrate and saturated fat intake. Eur Eat Disord Rev 22: 77–82, 2014. [DOI] [PubMed] [Google Scholar]
- 448.Robinson SJ, Sünram-Lea SI, Leach J, Owen-Lynch PJ. The effects of exposure to an acute naturalistic stressor on working memory, state anxiety and salivary cortisol concentrations. Stress 11: 115–24, 2008. [DOI] [PubMed] [Google Scholar]
- 449.Romero LM, Levine S, Sapolsky RM. Adrenocorticotropin secretagog release: stimulation by frustration and paradoxically by reward presentation. Brain Res 676: 151–156, 1995. [DOI] [PubMed] [Google Scholar]
- 450.Roozendaal B, Carmi O, McGaugh JL. Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proc Natl Acad Sci U S A 93: 1429–1433, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Roozendaal B, Okuda S, De Quervain DJF, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience 138: 901–910, 2006. [DOI] [PubMed] [Google Scholar]
- 452.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 78: 578–595, 2002. [DOI] [PubMed] [Google Scholar]
- 453.Roozendaal B The Basolateral Amygdala Interacts with the Medial Prefrontal Cortex in Regulating Glucocorticoid Effects on Working Memory Impairment. J Neurosci 24: 1385–1392, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 83: 1853–1859, 1998. [DOI] [PubMed] [Google Scholar]
- 455.Rubin RT, Jeffrey J, Sadow TF, Mccracken JT. Adrenal Gland Volume in Major Depression. Arch Gen Psychiatry 52: 213–218, 1995. [DOI] [PubMed] [Google Scholar]
- 456.Rudenga KJ, Small DM. Ventromedial prefrontal cortex response to concentrated sucrose reflects liking rather than sweet quality coding. Chem Senses 38: 585–594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Rutters F, Nieuwenhuizen AG, Lemmens SGT, Born JM, Westerterp-Plantenga MS. Acute Stress-related Changes in Eating in the Absence of Hunger. Obesity 17: 72–77, 2009. [DOI] [PubMed] [Google Scholar]
- 458.Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher TF. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry 28: 19–24, 1973. [DOI] [PubMed] [Google Scholar]
- 459.Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. Tenth Lippincott Williams & Wilkins, 2011. [Google Scholar]
- 460.Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M, Passafaro M. Extracellular Interactions between GluR2 and N-Cadherin in Spine Regulation. Neuron 54: 461–477, 2007. [DOI] [PubMed] [Google Scholar]
- 461.Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem 17: 522–530, 2010. [DOI] [PubMed] [Google Scholar]
- 462.Sampson D, Muscat R, Phillips G, Willner P. Decreased reactivity to sweetness following chronic exposure to mild unpredictable stress or acute administration of pimozide. Neurosci Biobehav Rev 16: 519–524, 1992. [DOI] [PubMed] [Google Scholar]
- 463.Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci 9: 637–642, 1997. [DOI] [PubMed] [Google Scholar]
- 464.Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast 2007, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, Lopez-Fernandez MA, Venero C, Diamond DM. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry 57: 856–864, 2005. [DOI] [PubMed] [Google Scholar]
- 466.Sandi C The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plast 6: 41–52, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Sandi C Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci 34: 165–176, 2011. [DOI] [PubMed] [Google Scholar]
- 468.Santiago PN, Ursano RJ, Gray CL, Pynoos RS, Spiegel D, Lewis-Fernandez R, Friedman MJ, Fullerton CS. A Systematic Review of PTSD Prevalence and Trajectories in DSM-5 Defined Trauma Exposed Populations: Intentional and Non-Intentional Traumatic Events. PLoS One 8: 1–5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron 36: 199–211, 2002. [DOI] [PubMed] [Google Scholar]
- 470.Saphier D, Welch JE, Farrar GE, Goeders NE. Effects of intracerebroventricular and intrahypothalamic cocaine administration on adrenocortical secretion. Neuroendocrinology 57: 54–62, 1993. [DOI] [PubMed] [Google Scholar]
- 471.Sarnyai Z, Bíró E, Penke B, Telegdy G. The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res 589: 154–156, 1992. [DOI] [PubMed] [Google Scholar]
- 472.Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, “binge” cocaine administration: Diminished individual differences in stress-induced corticosterone response. Neuroendocrinology 68: 334–344, 1998. [DOI] [PubMed] [Google Scholar]
- 473.Sarnyai Z, Mello NK, Mendelson JH, Erös-Sarnyai M, Mercer G. Effects of cocaine on pulsatile activity of hypothalamic-pituitary-adrenal axis in male rhesus monkeys: neuroendocrine and behavioral correlates. J Pharmacol Exp Ther 277: 225–234, 1996. [PubMed] [Google Scholar]
- 474.Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, De Quervain D, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann N Y Acad Sci 1071: 46–53, 2006. [DOI] [PubMed] [Google Scholar]
- 475.Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol 7: 321–326, 1990. [DOI] [PubMed] [Google Scholar]
- 476.Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett 81: 227–231, 1987. [DOI] [PubMed] [Google Scholar]
- 477.Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol 5: 16, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Schoofs D, Preuß D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology 33: 643–653, 2008. [DOI] [PubMed] [Google Scholar]
- 479.Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci 123: 1066–1075, 2009. [DOI] [PubMed] [Google Scholar]
- 480.Schultz W Reward signaling by dopamine neurons. Neuroscientist 7: 293–302, 2001. [DOI] [PubMed] [Google Scholar]
- 481.Sclafani A, Nissenbaum JW. Taste preference thresholds for Polycose, maltose, and sucrose in rats. Neurosci Biobehav Rev 11: 181–185, 1987. [DOI] [PubMed] [Google Scholar]
- 482.Sclafani A. Oralx and postoral determinants of food reward. Physiol Behav 81: 773–779, 2004. [DOI] [PubMed] [Google Scholar]
- 483.Scott TR, Karadi Z, Oomura Y, Nishino H, Plata-Salaman CR, Lenard L, Giza BK, Aou S. Gustatory neural coding in the amygdala of the alert macaque monkey. J Neurophysiol 69: 1810–1820, 1993. [DOI] [PubMed] [Google Scholar]
- 484.Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 137: 184–190, 1998. [DOI] [PubMed] [Google Scholar]
- 485.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: A review. Brain Res Rev 33: 13–33, 2000. [DOI] [PubMed] [Google Scholar]
- 486.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17: 2605–2614, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Shaham Y Immobilization stress-induced oral opioid self-administration and withdrawal in rats: role of conditioning factors and the effect of stress on “relapse” to opioid drugs. Psychopharmacology (Berl) 111: 477–485, 1993. [DOI] [PubMed] [Google Scholar]
- 488.Shaham Y Exposure to mild stress enhance the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berl) 114: 523–527, 1994. [DOI] [PubMed] [Google Scholar]
- 489.Shalev U, Marinelli M, Baumann MH, Piazza P-V, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 168: 170–6, 2003. [DOI] [PubMed] [Google Scholar]
- 490.Sharma S, Fernandes MF, Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes 37: 1183–91, 2013. [DOI] [PubMed] [Google Scholar]
- 491.Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes 37: 382–389, 2013. [DOI] [PubMed] [Google Scholar]
- 492.Shepard J, Barron K, Myers D. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 861: 288–295, 2000. [DOI] [PubMed] [Google Scholar]
- 493.Sherman JE, Kalin NH. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol Biochem Behav 30: 801–7, 1988. [DOI] [PubMed] [Google Scholar]
- 494.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35: 169–191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol Learn Mem 68: 92–96, 1997. [DOI] [PubMed] [Google Scholar]
- 496.Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 142: 343–351, 1999. [DOI] [PubMed] [Google Scholar]
- 497.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 152: 140–148, 2000. [DOI] [PubMed] [Google Scholar]
- 498.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63: 324–331, 2006. [DOI] [PubMed] [Google Scholar]
- 499.Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 170: 62–72, 2003. [DOI] [PubMed] [Google Scholar]
- 500.Sinha R Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141: 105–130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Skutella T, Probst JC, Renner U, Holsboer F, Behl C. Corticotropin-releasing hormone receptor (type I) antisense targeting reduces anxiety. Neuroscience 85: 795–805, 1998. [DOI] [PubMed] [Google Scholar]
- 502.Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20: 1093–1102, 1998. [DOI] [PubMed] [Google Scholar]
- 503.Smith MA, Davidson J, Ritchie JC, Kudler H, Lipper S, Chappell P, Nemeroff CB. The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biol Psychiatry 26: 349–55, 1989. [DOI] [PubMed] [Google Scholar]
- 504.Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone S V., Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry 57: 1485–1492, 2005. [DOI] [PubMed] [Google Scholar]
- 505.Smotherman WP, Kolp LA, Coyle S, Levine S. Hippocampal lesion effects on conditioned taste aversion and pituitary-adrenal activity in rats. Behav Brain Res 2: 33–48, 1981. [DOI] [PubMed] [Google Scholar]
- 506.Smythe JW, Murphy D, Timothy C, Costall B. Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharmacol Biochem Behav 56: 507–513, 1997. [DOI] [PubMed] [Google Scholar]
- 507.So AY-L, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A 106: 17582–17587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Solomon J, Mayer J. The effect of adrenalectomy on the development of the obese-hyperglycemic syndrome in ob-ob mice. Endocrinology 93: 510–512, 1973. [DOI] [PubMed] [Google Scholar]
- 509.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 292: R283–R290, 2007. [DOI] [PubMed] [Google Scholar]
- 510.Solomon MB, Furay AR, Jones K, Packard AEB, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience 203: 135–143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, Flak JN, Ulrich-Lai Y, Herman JP. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm Behav 65: 363–371, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Sonino N, Fava G. Psychiatric disorders associated with Cushing’s syndrome. CNS Drugs 15: 361–373, 2001. [DOI] [PubMed] [Google Scholar]
- 513.Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ-F. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A 103: 5585–5590, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Sotnikov S, Wittmann A, Bunck M, Bauer S, Deussing J, Schmidt M, Touma C, Landgraf R, Czibere L. Blunted HPA axis reactivity reveals glucocorticoid system dysbalance in a mouse model of high anxiety-related behavior. Psychoneuroendocrinology 48: 41–51, 2014. [DOI] [PubMed] [Google Scholar]
- 515.Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 196: 473–482, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology 52: 481–489, 1990. [DOI] [PubMed] [Google Scholar]
- 517.Spiga F, Walker JJ, Terry JR, Lightman SL. HPA axis-rhythms. Compr Physiol 4: 1273–98, 2014. [DOI] [PubMed] [Google Scholar]
- 518.Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry 42: 680–6, 1997. [DOI] [PubMed] [Google Scholar]
- 519.Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology 130: 3378–86, 1992. [DOI] [PubMed] [Google Scholar]
- 520.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci 14: 2579–84, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Stephens MAC, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res Curr Rev 34: 468–83, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85: 367–370, 1985. [DOI] [PubMed] [Google Scholar]
- 523.Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol 272: R840–R848, 1997. [DOI] [PubMed] [Google Scholar]
- 524.Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol 17: 271–87, 2006. [DOI] [PubMed] [Google Scholar]
- 525.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29: 2007–2017, 2004. [DOI] [PubMed] [Google Scholar]
- 526.Ströhle A, Scheel M, Modell S, Holsboer F. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. J Psychiatr Res 42: 1185–1188, 2008. [DOI] [PubMed] [Google Scholar]
- 527.Sucheki D, Antunes J, Tufik S. Palatable solutions during paradoxical sleep deprivation: Reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect of energy imbalance. J Neuroendocrinol 15: 815–821, 2003. [DOI] [PubMed] [Google Scholar]
- 528.Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128: 7–14, 2004. [DOI] [PubMed] [Google Scholar]
- 529.Swanson L, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36: 165–86, 1983. [DOI] [PubMed] [Google Scholar]
- 530.Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9-41). Neuropsychopharmacology 2: 285–92, 1989. [DOI] [PubMed] [Google Scholar]
- 531.Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav 45: 629–637, 1993. [DOI] [PubMed] [Google Scholar]
- 532.Szigethy E, Conwell Y, Forbes NT, Cox C, Caine ED. Adrenal weight and morphology in victims of completed suicide. Biol Psychiatry 36: 374–80, 1994. [DOI] [PubMed] [Google Scholar]
- 533.Tabuchi E, Yokawa T, Mallick H, Inubushi T, Kondoh T, Ono T, Torii K. Spatio-temporal dynamics of brain activated regions during drinking behavior in rats. Brain Res 951: 270–279, 2002. [DOI] [PubMed] [Google Scholar]
- 534.Tagliari B, dos Santos TM, Cunha AA, Lima DD, Delwing D, Sitta A, Vargas CR, Dalmaz C, Wyse ATS. Chronic variable stress induces oxidative stress and decreases butyrylcholinesterase activity in blood of rats. J Neural Transm 117: 1067–76, 2010. [DOI] [PubMed] [Google Scholar]
- 535.Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol 273: E1168–E1177, 1997. [DOI] [PubMed] [Google Scholar]
- 536.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol 271: E317–E325, 1996. [DOI] [PubMed] [Google Scholar]
- 537.Taverniers J, Van Ruysseveldt J, Smeets T, von Grumbkow J. High-intensity stress elicits robust cortisol increases, and impairs working memory and visuo-spatial declarative memory in Special Forces candidates: A field experiment. Stress 13: 323–33, 2010. [DOI] [PubMed] [Google Scholar]
- 538.Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol 95: 197–211, 2008. [DOI] [PubMed] [Google Scholar]
- 539.Tempel DL, McEwen BS, Leibowitz SF. Effects of adrenal steroid agonists on food intake and macronutrient selection. Physiol Behav 52: 1161–1166, 1992. [DOI] [PubMed] [Google Scholar]
- 540.Tenk CM, Wilson H, Zhang Q, Pitchers KK, Lique M. Sexual reward in male rats: Effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav 55: 93–97, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Teoh SK, Sarnyai Z, Mendelson JH, Mello NK, Springer SA, Sholar JW, Wapler M, Kuehnle JC, Gelles H. Cocaine effects on pulsatile secretion of ACTH in men. J Pharmacol Exp Ther 270: 1134–1138, 1994. [PubMed] [Google Scholar]
- 542.Terry LC, Martin JB. Hypothalamic-pituitary responses to intracranial self-stimulation in the rat. Brain Res 157: 89–104, 1978. [DOI] [PubMed] [Google Scholar]
- 543.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor. Nat Genet 19: 162–166, 1998. [DOI] [PubMed] [Google Scholar]
- 544.Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: Amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci 22: 2617–2634, 2005. [DOI] [PubMed] [Google Scholar]
- 545.Tizzano JP, Griffey KI, Schoepp DD. The anxiolytic action of mGlu2/3 receptor agonist, LY354740, in the fear-potentiated startle model in rats is mechanistically distinct from diazepam. Pharmacol Biochem Behav 73: 367–374, 2002. [DOI] [PubMed] [Google Scholar]
- 546.Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 36: 1513–1519, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Treit D, Menard J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav 44: 463–469, 1993. [DOI] [PubMed] [Google Scholar]
- 548.Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci 1: 444–458, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban P, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23: 99–103, 1999. [DOI] [PubMed] [Google Scholar]
- 550.Tryon MS, Carter CS, DeCant R, Laugero KD. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav 120: 233–242, 2013. [DOI] [PubMed] [Google Scholar]
- 551.Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C. Cigarette smoking and psychophysiological stress responsiveness: Effects of recent smoking and temporary abstinence. Psychopharmacology (Berl) 126: 226–233, 1996. [DOI] [PubMed] [Google Scholar]
- 552.Turner RJ, Lloyd DA. Cumulative adversity and drug dependence in young adults : racial / ethnic contrasts. Addiction 98: 305–315, 2003. [DOI] [PubMed] [Google Scholar]
- 553.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict Biol 12: 227–462, 2007. [DOI] [PubMed] [Google Scholar]
- 554.Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290: R1128–R1135, 2006. [DOI] [PubMed] [Google Scholar]
- 555.Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A 107: 20529–34, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Ulrich-Lai YM, Christiansen AM, Wang X, Song S, Herman JP. Statistical modeling implicates neuroanatomical circuit mediating stress relief by “comfort” food. Brain Struct. Funct (2015). doi: 10.1007/s00429-015-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291: E965–E973, 2006. [DOI] [PubMed] [Google Scholar]
- 558.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291: E965–73, 2006. [DOI] [PubMed] [Google Scholar]
- 559.Ulrich-lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure , food intake and emotional state. Stress 00: 1–19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav 103: 104–10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology 148: 1823–34, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Ulrich-Lai YM, Ryan KK. Neuroendocrine Circuits Governing Energy Balance and Stress Regulation: Functional Overlap and Therapeutic Implications. Cell Metab 19: 910–925, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Utter AC, Kang J, Niemann DC, Williams F, Robertson RJ, Henson DA, Davis JM, Butterworth DE. Effect of carbohydrate ingestion and hormonal respones on ratings of perceived exertion during prolonged cycling and running. Eur J Appl Physiol 80: 92–99, 1999. [DOI] [PubMed] [Google Scholar]
- 565.Vaher PR, Luine VN, Gould E, McEwen BS. Effects of adrenalectomy on spatial memory performance and dentate gyrus morphology. Brain Res 656: 71–78, 1994. [DOI] [PubMed] [Google Scholar]
- 566.Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci 17: 2626–2636, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Veenit V, Riccio O, Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J Psychiatr Res 53: 1–7, 2014. [DOI] [PubMed] [Google Scholar]
- 568.Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, Mcginn MA, Zamora-martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 125: 3193–3197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Venero C, Herrero AI, Touyarot K, Cambon K, López-Fernández M a., Berezin V, Bock E, Sandi C. Hippocampal up-regulation of NCAM expression and polysialylation plays a key role on spatial memory. Eur J Neurosci 23: 1585–1595, 2006. [DOI] [PubMed] [Google Scholar]
- 570.Vescovi PP, Coiro V, Volpi R, Passeri M. Diurnal variations in plasma ACTH, cortisol and Beta-endorphin levels in cocaine addicts. Horm Res 37: 221–224, 1992. [DOI] [PubMed] [Google Scholar]
- 571.Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, Le Fur G, Caput D, Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett 335: 1–5, 1993. [DOI] [PubMed] [Google Scholar]
- 572.De Vries AC, Taymans SE, Sundstrom JM, Pert A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res 786: 39–46, 1998. [DOI] [PubMed] [Google Scholar]
- 573.Vuong C, Van Uum SHM, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev 31: 98–132, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Wand G The Influence of Stress on the Transition From Drug Use to Addiction. Alcohol Res Health 31: 119–136, 2008. [PMC free article] [PubMed] [Google Scholar]
- 576.Wang B, You Z-B, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 193: 283–294, 2007. [DOI] [PubMed] [Google Scholar]
- 577.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res 48: 195–202, 2000. [DOI] [PubMed] [Google Scholar]
- 578.Weinstein SE, Shide DJ, Rolls BJ. Changes in food intake in response to stress in men and women: psychological factors. Appetite 28: 7–18, 1997. [DOI] [PubMed] [Google Scholar]
- 579.Weinstock M Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 65: 427–451, 2001. [DOI] [PubMed] [Google Scholar]
- 580.Wenger T, Jamali KA, Juanéda C, Léonardelli J, Tramu G. Arachidonyl ethanolamide (anandamide) activates the parvocellular part of hypothalamic paraventricular nucleus. Biochem Biophys Res Commun 237: 724–728, 1997. [DOI] [PubMed] [Google Scholar]
- 581.van West D, Del-Favero J, Aulchenko Y, Oswald P, Souery D, Forsgren T, Sluijs S, Bel-Kacem S, Adolfsson R, Mendlewicz J, Van Duijn C, Deboutte D, Van Broeckhoven C, Claes S. A major SNP haplotype of the arginine vasopressin 1B receptor protects against recurrent major depression. Mol Psychiatry 9: 287–92, 2004. [DOI] [PubMed] [Google Scholar]
- 582.Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl) 78: 305–308, 1982. [DOI] [PubMed] [Google Scholar]
- 583.Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, Morgan M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology (Berl) 136: 272–283, 1998. [DOI] [PubMed] [Google Scholar]
- 584.Willner P, Healy S. Decreased hedonic responsiveness during a brief depressive mood swing. J Affect Disord 32: 13–20, 1994. [DOI] [PubMed] [Google Scholar]
- 585.Willner P Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134: 319–29, 1997. [DOI] [PubMed] [Google Scholar]
- 586.Willner P Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52: 90–110, 2005. [DOI] [PubMed] [Google Scholar]
- 587.Wills TA, Cleary SD. How are social support effects mediated? A test with parental support and adolescent substance use. J Pers Soc Psychol 71: 937–952, 1996. [DOI] [PubMed] [Google Scholar]
- 588.Wills TA, Sandy JM, Yaeger AM, Cleary SD, Shinar O. Coping Dimensions, Life Stress, and Adolescent Substance Use: A Latent Growth Analysis. J Abnorm Psychol 110: 309–323, 2001. [DOI] [PubMed] [Google Scholar]
- 589.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol 40: 191–225, 1989. [DOI] [PubMed] [Google Scholar]
- 590.Wolf OT, Convit a, McHugh PF, Kandil E, Thorn EL, De Santi S, McEwen BS, de Leon MJ. Cortisol differentially affects memory in young and elderly men. Behav Neurosci 115: 1002–1011, 2001. [DOI] [PubMed] [Google Scholar]
- 591.Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced coritsol levels and memory differs between men and women. Psychoneuroendocrinology 26: 711–720, 2001. [DOI] [PubMed] [Google Scholar]
- 592.Wolf OT. HPA axis and memory. Best Pract Res Clin Endocrinol Metab 17: 287–299, 2003. [DOI] [PubMed] [Google Scholar]
- 593.Wolkowitz O, Reus V, Manfredi F, Ingbar J, Brizendine L, Weingartner H. Ketoconazole administration in hypercortisolemic depression. Am J Psychiatry 150: 810–812, 1993. [DOI] [PubMed] [Google Scholar]
- 594.Wong M, Kling M, Munson P, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon I, Geracioti T, DeBellis M, Rice K, Goldstein D, Veldhuis J, Chrousos G, Oldfield E, McCann S, Gold P. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA 97: 325–330, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, Brebner K, Liu L, Weinberg J, Christie BR, Phillips AG, Wang YT. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci U S A 104: 11471–6, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A 95: 4066–4071, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-Induced Amnesia in Rats: Working Memory-Specific Impairment, Corticosterone-Memory Correlation, and Fear Versus Arousal Effects on Memory. Learn Mem 10: 326–336, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.World Health Organization. WHO: Depression Fact Sheet N369 [Online]. 2012. http://www.who.int/mediacentre/factsheets/fs369/en/ [9 June. 2015].
- 599.Wu L-M, Han H, Wang Q-N, Hou H-L, Tong H, Yan X-B, Zhou J-N. Mifepristone repairs region-dependent alteration of synapsin I in hippocampus in rat model of depression. Neuropsychopharmacology 32: 2500–10, 2007. [DOI] [PubMed] [Google Scholar]
- 600.Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology 35: 1100–1112, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20: 8122–8130, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Yehuda R, LeDoux J. Response Variation following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron 56: 19–32, 2007. [DOI] [PubMed] [Google Scholar]
- 603.Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biol Psychiatry 40: 79–88, 1996. [DOI] [PubMed] [Google Scholar]
- 604.Yehuda R Biology of posttraumatic stress disorder. J Clin Psychiatry 62 Suppl 1: 41–6, 2001. [PubMed] [Google Scholar]
- 605.Yehuda R Risk and resilience in posttraumatic stress disorder. J Clin Psychiatry 65 Suppl 1: 29–36, 2004. [PubMed] [Google Scholar]
- 606.Yehuda R Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci 1179: 56–69, 2009. [DOI] [PubMed] [Google Scholar]
- 607.Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology 29: 1538–45, 2004. [DOI] [PubMed] [Google Scholar]
- 608.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A 106: 14075–14079, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, MCEWEN BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 16: 156–170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D-1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. [Online]. J Neurosci 17: 8528–8535, 1997. <Go to ISI>://WOS:A1997YC94700044\nhttp://www.jneurosci.org/content/17/21/8528.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Zakrzewska KE, Cusin I, Stricker-Krongrad A, Boss O, Ricquier D, Jeanrenaud B, Rohner-Jeanrenaud F. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes 48: 365–370, 1999. [DOI] [PubMed] [Google Scholar]
- 612.Zangrossi H, File SE. Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Res Bull 29: 381–388, 1992. [DOI] [PubMed] [Google Scholar]
- 613.Zis AP, Haskett RF, Ariav Albala A, Carroll BJ. Morphine inhibits cortisol and stimulates prolactin secretion in man. Psychoneuroendocrinology 9: 423–427, 1984. [DOI] [PubMed] [Google Scholar]
- 614.Zobel A, Nickel T, Künzel H, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 34: 171–181, 2000. [DOI] [PubMed] [Google Scholar]
- 615.Zobel AW, Yassouridis A, Frieboes RM, Holsboer F. Prediction of medium-term outcome by cortisol response to the combined dexamethasone-CRH test in patients with remitted depression. Am J Psychiatry 156: 949–51, 1999. [DOI] [PubMed] [Google Scholar]
- 616.Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, Yehuda R, Cohen H. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. Eur Neuropsychopharmacol 21: 796–809, 2011. [DOI] [PubMed] [Google Scholar]
- 617.Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress 11: 259–281, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Zuardi AW, Teixeira NA, Karniol IC. Pharmacological interaction of the effects of delta 9-trans-tetrahydrocannabinol and cannabidiol on serum corticosterone levels in rats. Arch Int Pharmacodyn Ther 269: 12–19, 1984. [PubMed] [Google Scholar]
- 619.Zubelewicz B, Muc-Qierzgon M, Harbuz M, Brodziak A. Central single and chronic administration of morphine stimulates corticosterone and IL-6 in adjuvant induced arthritis.pdf. J. Physiol. Pharmacol. 51: 897–906, 2000. [PubMed] [Google Scholar]


