Abstract
Objective
We evaluated the interaction of serum folate and vitamin B12 with methylenetetrahydrofolate reductase (MTHFR) C677T genotypes on the risk of first ischemic stroke and on the efficacy of folic acid treatment in prevention of first ischemic stroke.
Methods
A total of 20,702 hypertensive adults were randomized to a double-blind treatment of daily enalapril 10 mg and folic acid 0.8 mg or enalapril 10 mg alone. Participants were followed up every 3 months.
Results
Median values of folate and B12 concentrations at baseline were 8.1 ng/mL and 280.2 pmol/L, respectively. Over a median of 4.5 years, among those not receiving folic acid, participants with baseline serum B12 or serum folate above the median had a significantly lower risk of first ischemic stroke (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.57–0.96), especially in those with MTHFR 677 CC genotype (wild-type) (HR, 0.49; 95% CI, 0.31–0.78). Folic acid treatment significantly reduced the risk of first ischemic stroke in participants with both folate and B12 below the median (2.3% in enalapril-folic acid group vs 3.6% in enalapril-only group; HR, 0.62; 95% CI, 0.46–0.86), particularly in MTHFR 677 CC carriers (1.6% vs 4.9%; HR, 0.24; 95% CI, 0.11–0.55). However, TT homozygotes responded better with both folate and B12 levels above the median (HR, 0.28; 95% CI, 0.10–0.75).
Conclusions
The risk of first ischemic stroke was significantly higher in hypertensive patients with low levels of both folate and B12. Effect of folic acid treatment was greatest in patients with low folate and B12 with the CC genotype, and with high folate and B12 with the TT genotype.
Stroke is a major cause of death and disability worldwide.1,2 There is increasing interest in identifying novel modifiable risk factors to improve primary prevention of stroke and reduce the related severe disease burden.
Numerous prospective studies have shown a graded, independent association between total homocysteine (tHcy) and stroke.3–5 Folate, vitamin B12, and vitamin B6 deficiencies and reduced major enzyme (such as methylenetetrahydrofolate reductase [MTHFR]) activities in folate and homocysteine metabolism may inhibit the metabolism of homocysteine, thus increasing tHcy levels.6 Moreover, vitamin B12 and folate have direct antioxidant, antithrombotic, and endothelium-protective effects, and have a major role in DNA synthesis or repair.7,8 Therefore, as expected, previous randomized trials have shown that folic acid therapy composed of folic acid, vitamin B12, and vitamin B6 reduced the risk of ischemic stroke.9–11 Furthermore, the beneficial effect on stroke was more pronounced in trials conducted in populations without folic acid fortification and with lower vitamin B12 levels.10 This has led some physicians to think that in countries with folate fortification, only vitamin B12 was necessary for homocysteine lowering to prevent stroke. More importantly, it appears that in the early randomized trials of B vitamins for stroke prevention, toxicity from cyanocobalamin among study participants with impaired renal function may have obscured the benefit of B vitamins.9 However, the joint effect of naturally occurring serum B12 and folate, with MTHFR C677T genotypes, on the risk of first ischemic stroke, and on the efficacy of folic acid treatment in prevention of first ischemic stroke has not been fully investigated in previous studies.
Hypertension is one of the most important risk factors for stroke.12,13 As such, our current study aimed to address the above knowledge gap in hypertensive adults, using data from the China Stroke Primary Prevention Trial (CSPPT).14
Methods
Standard protocol approvals, registrations, and patient consents
The CSPPT was registered in clinicaltrials.gov (identifier: NCT00794885). The current study is a post hoc analysis of the CSPPT. The parent study (the CSPPT) and the current study were approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number: FWA00001263). Informed consent was waived for the post hoc analysis.
Participants
Details regarding the study design and major results of the CSPPT have been reported elsewhere.14 Briefly, the CSPPT was a multicommunity, randomized, double-blind, controlled trial conducted from May 19, 2008, to August 24, 2013, in 32 communities in China. Eligible participants were men and women aged 45–75 years who had hypertension, defined as seated, resting systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg at both the screening and recruitment visit, or who were on antihypertensive medication. The major exclusion criteria included history of physician-diagnosed stroke, myocardial infarction (MI), heart failure, post coronary revascularization, congenital heart disease, or current supplementation by folic acid, vitamin B12, or vitamin B6.
Procedures
Eligible participants, stratified by MTHFR C677T genotypes (CC [wild-type], CT, or TT), were randomly assigned, in a 1:1 ratio, to 1 of 2 treatment groups: a daily tablet containing 10 mg enalapril and 0.8 mg folic acid (the enalapril–folic acid group) or a daily tablet containing 10 mg enalapril only (the enalapril-only group).
During the trial period, concomitant use of other antihypertensive drugs (mainly calcium channel blockers or diuretics) was allowed, but not B vitamins. Participants were scheduled for follow-up every 3 months.
Laboratory assays
Serum folate and vitamin B12 at baseline were measured by a commercial laboratory using a chemiluminescent immunoassay (New Industrial, Shenzhen, China). Serum tHcy (at both the baseline and the exit visit) and fasting lipids and glucose at baseline were measured using automatic clinical analyzers (Beckman Coulter, Brea, CA) at the core lab of the National Clinical Research Center for Kidney Disease (Nanfang Hospital, Guangzhou, China). MTHFR C677T (rs1801133) polymorphisms were detected on an ABI PRISM 7900HT sequence detection system (Life Technologies, Carlsbad, CA) using the TaqMan assay.
Study outcomes
In the CSPPT, the primary outcome was first stroke (ischemic or hemorrhagic). However, folic acid reduced only ischemic stroke and did not affect hemorrhagic stroke.14 For that reason, the primary outcome for this study was a first ischemic stroke (fatal or nonfatal). The secondary outcomes included a first stroke (ischemic or hemorrhagic), excluding subarachnoid hemorrhage and silent stroke, and a composite of cardiovascular events consisting of cardiovascular death, MI, and stroke.
All the study outcomes were reviewed and adjudicated by an independent Endpoint Adjudication Committee, whose members were unaware of study group assignments.
Statistical analysis
Means (SD) and proportions were calculated for population characteristics in accordance with the combined baseline folate and B12 levels (group 1: B12 <280.2 pmol/L [median] and folate <8.1 ng/mL [median]; group 2: B12 <280.2 pmol/L and folate ≥ 8.1 ng/mL; group 3: B12 ≥280.2 pmol/L and folate <8.1 ng/mL; group 4: B12 ≥280.2 pmol/L and folate ≥8.1 ng/mL). The hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of first ischemic stroke associated with the combined baseline folate and B12 levels were estimated using Cox proportional hazards models in the enalapril-only group without and with adjustment for major covariates. Interactions of B12 and folate subgroups with MTHFR C677T genotypes (CC [wild-type] vs CT or TT) were examined by including interaction terms into the Cox models. We further assessed the effect of folic acid treatment on the total population (participants from the enalapril-only group and the enalapril–folic acid group) on the prevention of first ischemic stroke according to different B12 and folate strata by means of Cox proportional hazards regression both before and after adjustment for the major covariates.
As a post hoc analysis, correction for multiple hypothesis testing was not applied, and a 2-tailed p < 0.05 was considered statistically significant in all analyses. R software, version 3.4.3 (R-project.org/), was used to perform all statistical analyses.
Data availability
The data, analytic methods, and study materials that support the findings of this study will be available from the corresponding authors on request, after the request is submitted and formally reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China.
Results
Study participants and baseline characteristics
In this study, a total of 20,499 participants (10,256 in the enalapril-only group and 10,243 in the enalapril–folic acid group) with baseline folate and B12 measurements were included in the final analyses (figure e-1, doi.org/10.5061/dryad.s45jd1v).
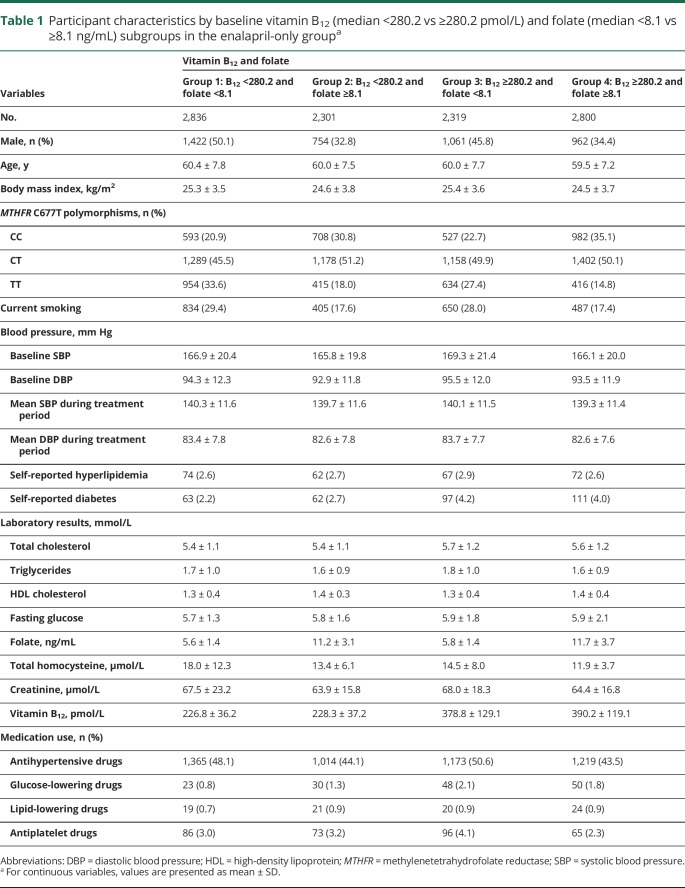
Baseline characteristics of participants in the total population, the enalapril-only group, and the enalapril–folic acid group by baseline folate and B12 level subgroupings (groups 1–4) are summarized in table e-1 (doi.org/10.5061/dryad.s45jd1v) and tables 1 and e-2 (doi.org/10.5061/dryad.s45jd1v), respectively. Median values of folate and B12 concentrations were 8.1 ng/mL (interquartile range [IQR] 5.6–10.5) and 280.2 pmol/L (IQR 232.4–351.7), respectively, in the enalapril group. Compared with participants in groups 2–4 at baseline, participants in group 1 were older and had significantly higher body mass index, creatinine, and tHcy; lower total cholesterol, high-density lipoprotein cholesterol, and fasting glucose levels; a lower prevalence of self-reported diabetes; lower usage of glucose-lowering drugs; and higher usage of antihypertensive drugs at baseline. However, all the differences had small magnitudes. Moreover, participants in group 1 were more likely to be smokers and TT genotype carriers than were the other 3 groups (table e-3, doi.org/10.5061/dryad.s45jd1v).
Table 1.
Participant characteristics by baseline vitamin B12 (median <280.2 vs ≥280.2 pmol/L) and folate (median <8.1 vs ≥8.1 ng/mL) subgroups in the enalapril-only groupa
Relationship of baseline serum B12 and folate with baseline tHcy levels in the enalapril-only group
Compared with participants with both lower B12 and lower folate levels (group 1) at baseline, significantly lower tHcy levels were found in those with higher folate alone (group 2; β, −2.7; 95% CI, −3.1 to −2.3 μmol/L), higher B12 alone (group 3; β, −3.1; 95% CI, −3.5 to −2.7 μmol/L), both higher B12 and higher folate (group 4; β, −4.1; 95% CI, −4.6 to −3.7 μmol/L), and higher B12 or higher folate levels (groups 2–4; β, −3.3; 95% CI, −3.6 to −2.9 μmol/L).
Similar results were found in participants with the MTHFR 677 CC, CT, or TT genotype (table e-4, doi.org/10.5061/dryad.s45jd1v).
Interaction of baseline serum B12 and folate levels with MTHFR C677T genotypes on the risk of first ischemic stroke in the enalapril-only group
The median treatment duration was 4.5 years. Overall, there was no significant association of baseline B12 or folate levels alone with the risk of first ischemic stroke (figure e-2 and table e-5, doi.org/10.5061/dryad.s45jd1v) in the enalapril-only group. To make our findings more applicable to clinical practice, folate and B12 levels were both divided at the median to create a low and high category (figure e-5, doi.org/10.5061/dryad.s45jd1v).
Compared with participants in group 1 (both lower B12 and lower folate levels at baseline), a lower risk of first ischemic stroke was found in those with higher folate alone (group 2: HR, 0.65; 95% CI, 0.45–0.93), higher B12 alone (group 3: HR, 0.79; 95% CI, 0.57–1.07), both higher B12 and higher folate (group 4: HR, 0.77; 95% CI, 0.55–1.09), or higher B12 or higher folate levels (groups 2–4: HR, 0.74; 95% CI, 0.57–0.96) (table 2). Due to the similar risk reduction, we combined groups 2–4 together in the following analysis.
Table 2.
Interaction of vitamin B12 (median <280.2 vs ≥ 280.2 pmol/L) and folate (median <8.1 vs ≥8.1 ng/mL) with methylenetetrahydrofolate reductase (MTHFR) C677T genotypes on risk of first ischemic stroke in the enalapril-only group
When participants were stratified by baseline tHcy levels, among those with lower (<12.5 μmol/L [median]) tHcy levels, a significantly lower risk of first ischemic stroke was found in those with higher folate alone (group 2: HR, 0.38; 95% CI, 0.20–0.72), higher B12 alone (group 3: HR, 0.53; 95% CI, 0.31–0.91), both higher B12 and higher folate (group 4: HR, 0.55; 95% CI, 0.33–0.91), and higher B12 or higher folate levels (groups 2–4: HR, 0.50; 95% CI, 0.32–0.77), compared with those in group 1. However, no significant association was found in participants with higher tHcy levels (table e-6, doi.org/10.5061/dryad.s45jd1v).
When participants were stratified by the MTHFR C677T genotypes, a stronger association (groups 2–4 vs group 1) was found in those with CC genotype (wild-type) (HR, 0.49; 95% CI, 0.31–0.78; vs CT/TT genotype: HR, 0.83; 95% CI, 0.61–1.11; p interaction = 0.044) (tables 2 and e-7, doi.org/10.5061/dryad.s45jd1v), especially in those with higher (≥12.5 μmol/L) baseline tHcy levels (p interaction = 0.014) (tables 2 and e-6, doi.org/10.5061/dryad.s45jd1v).
Similar results were found when folate levels were divided at quartile 1 (5.6 ng/mL) (table e-8, doi.org/10.5061/dryad.s45jd1v), as well as for first stroke (table e-9, doi.org/10.5061/dryad.s45jd1v), and the composite of cardiovascular events (table e-10, doi.org/10.5061/dryad.s45jd1v).
Effect of baseline serum B12 and folate levels with MTHFR C677T genotypes on the efficacy of folic acid treatment in preventing first ischemic stroke among the total population
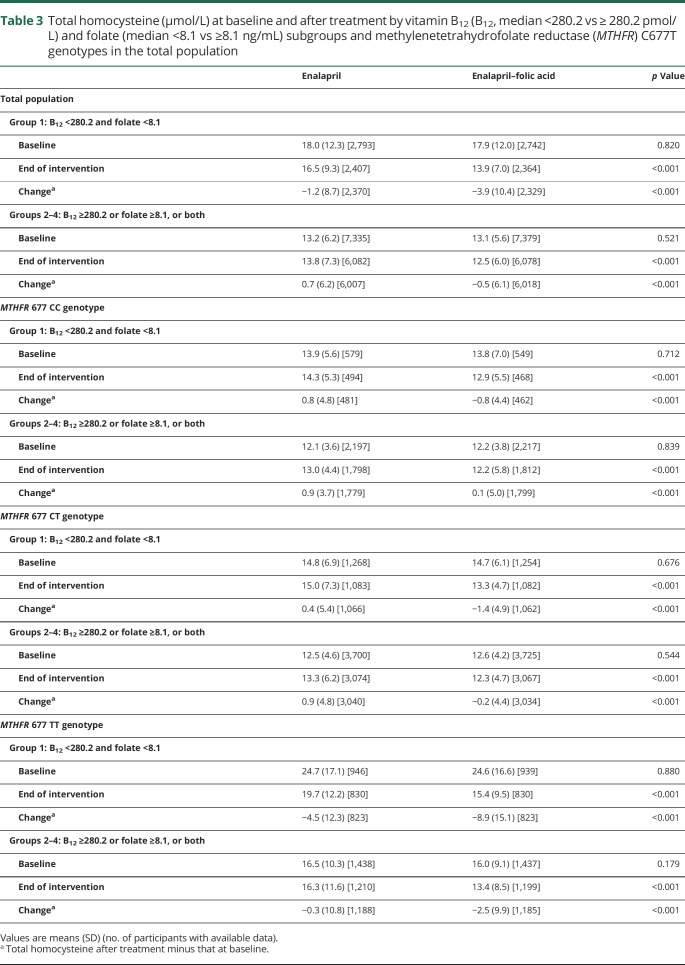
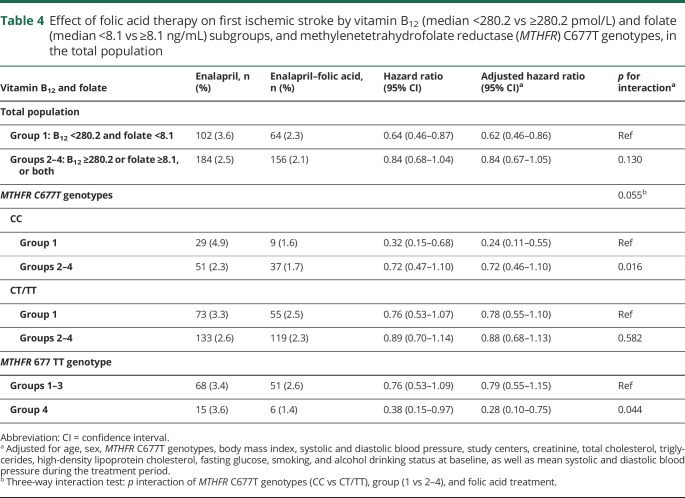
Folic acid treatment significantly reduced tHcy levels. As expected, a greater reduction of tHcy was observed in participants in group 1 (both lower B12 and lower folate levels) compared with those in groups 2–4 (table 3). Consistently, folic acid treatment significantly reduced the risk of first ischemic stroke by 38% in participants in group 1 (HR, 0.62; 95% CI, 0.46–0.86) (figure 1 and table 4).
Table 3.
Total homocysteine (μmol/L) at baseline and after treatment by vitamin B12 (B12, median <280.2 vs ≥ 280.2 pmol/L) and folate (median <8.1 vs ≥8.1 ng/mL) subgroups and methylenetetrahydrofolate reductase (MTHFR) C677T genotypes in the total population
Figure 1. Kaplan-Meier curves for first ischemic stroke by treatment group and B12/folate subgroups.
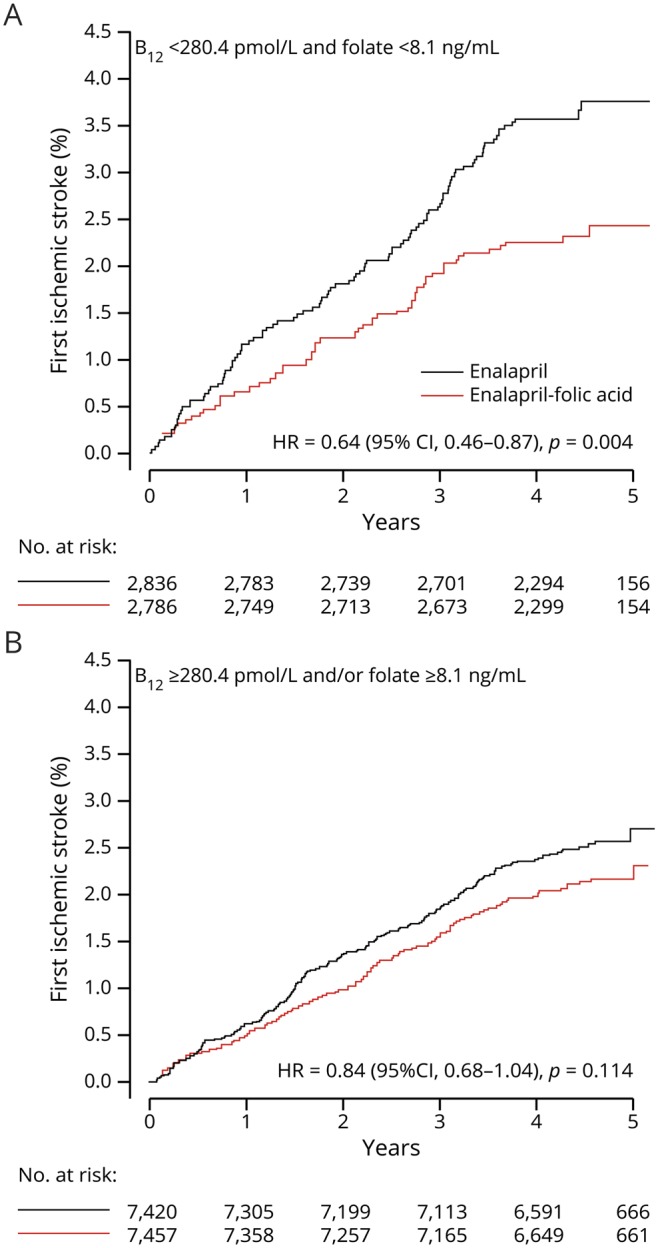
Kaplan-Meier curves of cumulative hazards for first ischemic stroke by treatment group in participants with (A) both lower vitamin B12 (median <280.2 pmol/L) and lower folate (median <8.1 ng/mL) levels or (B) higher vitamin B12 (≥280.2 pmol/L) or higher folate levels (≥8.1 ng/mL) or both. CI = confidence interval; HR = hazard ratio.
Table 4.
Effect of folic acid therapy on first ischemic stroke by vitamin B12 (median <280.2 vs ≥280.2 pmol/L) and folate (median <8.1 vs ≥8.1 ng/mL) subgroups, and methylenetetrahydrofolate reductase (MTHFR) C677T genotypes, in the total population
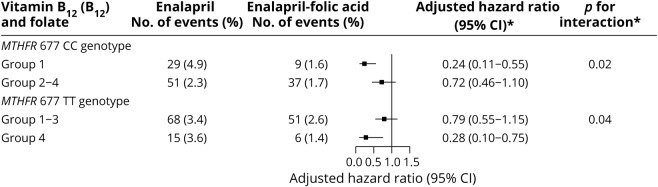
When participants were stratified by the MTHFR C677T genotypes, among participants with CC genotype, the beneficial effect was greater in participants in group 1 (HR, 0.24; 95% CI, 0.11–0.55; vs groups 2–4: HR, 0.72; 95% CI, 0.46–1.10; p interaction = 0.016) (figures 2 and e-3, doi.org/10.5061/dryad.s45jd1v, table 4). Similar trends were found in those with higher (≥12.5 μmol/L [median]) or lower (<12.5 μmol/L) tHcy levels (table e-11, doi.org/10.5061/dryad.s45jd1v).
Figure 2. Folic acid therapy effect on first ischemic stroke by B12/folate subgroups and methylenetetrahydrofolate reductase (MTHFR 677) genotypes (CC/TT).
Effect of folic acid therapy on first ischemic stroke by vitamin B12 (median <280.2 vs ≥280.2 pmol/L) and folate (median <8.1 vs ≥8.1 ng/mL) subgroups and MTHFR C677T genotypes (CC and TT genotype) in the total population. *Group 1: B12 <280.2 pmol/L and folate <8.1 ng/mL; group 2: B12 < 280.2 pmol/L and folate ≥8.1 ng/mL; group 3: B12 ≥280.2 pmol/L and folate <8.1 ng/mL; group 4: B12 ≥280.2 pmol/L and folate ≥8.1 ng/mL; adjusted for age, sex, body mass index, systolic and diastolic blood pressure, study centers, creatinine, total cholesterol, triglycerides, HDL cholesterol, fasting glucose, smoking and alcohol drinking status at baseline, and mean systolic and diastolic blood pressure during the treatment period. CI = confidence interval.
Among participants with the CT or TT genotype, a beneficial effect was observed in participants in group 1 with lower (<12.5 μmol/L) tHcy levels (HR, 0.44; 95% CI, 0.21–0.92). Nevertheless, that benefit is not established in groups 2–4 with higher (≥12.5 μmol/L) tHcy levels (HR, 0.77; 95% CI, 0.54–1.08) or in group 1 with higher (≥12.5 μmol/L) tHcy levels (HR, 0.94; 95% CI, 0.63–1.41) (table e-11, doi.org/10.5061/dryad.s45jd1v).
However, among those with the 677 TT genotype, the greatest benefit of folic acid treatment was found in participants in group 4, those participants with both higher B12 and higher folate levels at baseline (HR, 0.28; 95% CI, 0.10–0.75) (figures 2 and e-3 [doi.org/10.5061/dryad.s45jd1v] and table 4). The trends were similar in those with higher (≥12.5 μmol/L) or lower (<12.5 μmol/L) tHcy levels (table e-11, doi.org/10.5061/dryad.s45jd1v).
Similar results were found for first stroke (table e-12, doi.org/10.5061/dryad.s45jd1v) or composite of cardiovascular events (table e-13, doi.org/10.5061/dryad.s45jd1v).
Discussion
A recent meta-analysis15 reported that folic acid therapy would be beneficial for prevention of stroke, particularly in countries where folic acid fortification is not in place. However, in countries where folic acid fortification exists, the main nutritional determinant of tHcy is B12, and biochemical B12 deficiency and metabolic B12 deficiency (inadequate active B12) are common,16 so it may be important to add vitamin B12 to folic acid therapy, even in countries with folic acid fortification. We found that patients with low levels of both folate and B12 had significantly higher risk of first ischemic stroke, especially in those with the MTHFR 677 CC genotype (wild-type), suggesting that folic acid therapy alone would not be optimal for reduction of stroke. Furthermore, in patients with low levels of both folate and B12, folic acid treatment reduced first ischemic stroke for all such patients by 38%, and by 76% in those with the MTHFR 677 CC genotype.
The prospective relationship of serum B12 or folate with the risk of stroke is controversial. Van Guelpen et al.17 suggested a protective role for folate in hemorrhagic stroke, and found no obvious association between B12 and either hemorrhagic or ischemic stroke in a prospective, nested case-referent study. Giles et al.18 reported an inverse association between serum folate and ischemic stroke in black but not in white patients. The Kuopio Ischaemic Heart Disease Risk Factor Study19 showed that men in the highest third of serum folate concentrations had an adjusted relative risk (RR) for any stroke of 0.35 (95% CI, 0.14–0.87), compared with men in the lowest third. Only one previous case–cohort study20 comprising 779 controls and 188 incident cases of cerebral ischemia (ischemic stroke or TIA) by Weikert et al.20 evaluated the combined effect of folate and B12 on stroke. Consistent with our study, the authors found that the combination of low B12 with low folate levels significantly increased the risk of cerebral ischemia. However, this study had rather limited stroke cases, and did not consider possible confounding factors such as MTHFR C677T genotypes, blood pressure control, and renal function.
A previous meta-analysis that focused on genetic studies and prospective studies concluded that a 3 μmol/L homocysteine decrease could reduce the risk of stroke by 24%.4 Consistent with this prediction, in the current study, among participants in the enalapril-only group, a tHcy reduction of 3.3 μmol/L was seen between group 1 (both lower B12 and lower folate levels) and groups 2–4 (higher B12 or higher folate levels or both), and was associated with a 26% stroke risk reduction. However, the stroke risk reduction between group 1 and groups 2–4 was mainly found in participants with lower tHcy levels. In fact, in addition to its effect on tHcy levels, folate has direct antioxidant and antithrombotic effects, and can ameliorate endothelial dysfunction.7 B12 insufficiency is also associated with a reduction in glutathione and serum total antioxidant capacity.8 More importantly, B12 and folate play a fundamental role in DNA synthesis or repair. These results suggest that the increased stroke risk may be associated with both lower vitamin (folate or B12) and higher tHcy levels. Among those with higher tHcy levels in the enalapril-only group without additional supplementation, the relatively higher folate or B12 levels were still insufficient to fully correct for deficiencies and excesses in vitamin and tHcy levels.
MTHFR is a key enzyme involved in folate metabolism. The common MTHFR gene 677C→T polymorphism is associated with reduced enzyme activity (30% reduction in CT and 70% reduction in TT), resulting in decreased folate and increased tHcy levels.6 Our previous study suggested that baseline tHcy was associated with an increased risk of first stroke among participants with the CC/CT genotype, but not among those with the TT genotype.21 Moreover, folic acid intervention mainly reduced the stroke risk in participants with the TT genotype and lower tHcy levels.21 Accordingly, our current study found that a greater tHcy reduction between group 1 (both lower B12 and lower folate levels) and groups 2–4 (higher B12 or higher folate levels or both) in those with the MTHFR 677 TT genotype did not lead to a greater risk reduction of stroke risk. More importantly, among MTHFR 677 TT carriers, the greatest benefit of folic acid treatment was in those with higher baseline levels of both B12 and folate (group 4) regardless of baseline tHcy levels. We speculate that TT carriers may require a higher dose of folic acid, in addition to B12 supplementation, to further lower the stroke risk.
Our previous study also found that folic acid treatment significantly reduced stroke risk in participants with CC/CT genotypes and higher tHcy levels.21 Our current study further suggested that among those with higher tHcy levels, the greatest benefit of folic acid treatment still occurred in those with the CC genotype and low levels of both folate and B12 (group 1), while a beneficial trend was also observed in participants in groups 2–4 (higher B12 or higher folate levels or both) regardless of genotype. The lack of benefit from folic acid treatment in participants in group 1 with the CT/TT genotype may be due to the inability of folic acid treatment to fully correct the tHcy levels in those groups. However, even among those with lower tHcy levels, folic acid treatment significantly reduced the stroke risk in group 1 (both lower B12 and lower folate levels), especially in those with the CC genotype. 5,10-Methylene-THF is involved in the conversion of deoxyuridylate monophosphate to deoxythymidylate monophosphate, and low levels of 5,10-methylene-THF can lead to an increased ratio of deoxyuridine monophosphate to deoxythymidine monophosphate, inducing uracil misincorporation.22 DNA damage has been associated with increased cardiovascular disease.23,24 The enzyme MTHFR irreversibly reduces 5,10-methylene-THF to 5-methyl-THF. We hypothesize that when B12 and folate are both insufficient, a less active form of MTHFR could possibly lead, all other factors being equal, to an accumulation of 5,10-methylene-THF, which leads to a presumably protective effect against DNA damage.25 As such, MTHFR 677 CC carriers are possibly more sensitive to the increase of tHcy and insufficiency of folate and B12, and thereby benefit more from folic acid treatment. Consistently, among participants in group 1 (low levels of both folate and B12), the higher ischemic stroke risk was found in participants who were MTHFR CC carriers (4.9% vs 3.3% in CT or TT carriers) (table 2) in our current study. These results indicate that the levels of both folate and B12 and tHcy should be considered in the prediction and prevention of stroke.
Our previous meta-analysis of randomized trials lends further support to our current findings. We found that among trials conducted in populations without folic acid fortification, the benefit of folic acid therapy in stroke prevention was mainly found in trials of populations with lower baseline B12 levels (<283.4 pmol/L: RR, 0.78; 95% CI, 0.68–0.89; vs ≥283.4 pmol/L: RR, 1.00; 95% CI, 0.86–1.16; p interaction = 0.049).10 However, due to the apparent toxicity of cyanocobalamin among patients with impaired renal function,9,26 the potential benefits of a combined treatment of methylcobalamin and folic acid should be further investigated in future studies.9
Inadequate folate intake and B12 insufficiency are prevalent in most countries without folic acid fortification. Even in the era of post folic acid fortification and widespread use of vitamin supplementation, there is substantial variability in blood folate levels within the US population and across racial/ethnic groups.27 Although biochemical B12 deficiency is usually defined as a serum B12 <150 pmol/L, metabolic B12 deficiency is common among patients with a serum B12 <248 pmol/L.16,26 Our study suggests that higher doses of folic acid should be used to ensure adequate doses for persons with the MTHFR 677 TT genotype, and B12 (probably as methylcobalamin) should be combined with folic acid to maximize reduction of stroke, even in countries with folic acid fortification.
The prevalence of the 677 CT and TT genotypes of MTHFR was much higher in this Chinese population than previously reported in other populations. We found that 27% had CC, 49% had CT, and 24% had the TT genotype. In contrast, in a study population mainly of European origin, Spence et al.28 reported that 40.4% had CC, 46.6% had CT, and only 13% had TT genotypes. This may account for the much higher prevalence of hyperhomocysteinemia in China than in other populations. Yang et al.29 reported a Chinese population prevalence of tHcy >15 µmol/L of 27.5%. In contrast, in a French population-based study,30 the prevalence of tHcy >15 µmol/L was only 9.6% in men and 3.2% in women. The higher prevalence of the T allele in our population may have made it possible to observe the differences in response to folic acid that we observed among the MTHFR genotypes.
Our study had some limitations. First, this is a post hoc secondary analysis that did not take multiple testing into consideration; therefore, additional research is needed to further investigate and confirm our findings and determine an optimal dosage and strategy for folic acid and B12 therapy that is based on an individual's MTHFR 677 genotype. Second, the CSPPT used a fixed dose of folic acid (0.8 mg daily). While this is believed to be a reasonable dose according to previous studies,10,31 the relatively low dose of folic acid made it possible to recognize that a higher dose folate is probably needed for patients with the TT genotype. Third, our study was conducted in Chinese hypertensive patients, and more studies are needed to confirm our findings in other populations with similar and different characteristics. Another limitation of the CSPPT is the lack of classification of ischemic stroke subtypes, such as large artery disease, small artery disease, or cardioembolic stroke. In addition, we did not have folate or B12 measurements at the cellular level. Therefore, our results are merely hypothesis-generating. Confirmation of our findings in an independent population is essential.
The combination of lower folate and lower B12 levels significantly increased the risk of first ischemic stroke in hypertensive patients. Folic acid treatment reduced the first ischemic stroke risk for such patients by 38%. Effect of folic acid treatment was greatest in patients with low folate and B12 with the CC genotype, and with high folate and B12 with TT genotypes. This finding suggests that folic acid fortification (which delivers ∼400 μg/d of folic acid) may not be adequate for TT homozygous patients, so treatment with B12 alone may not be sufficient for prevention of stroke in countries with folate fortification.
These results are applicable to individuals receiving B vitamin therapy to lower tHcy for preventive purposes, and may also aid health care administrators in developing appropriate strategies that are based on specific population characteristics when implementing or creating guidelines for mandatory folic acid or B12 fortification.
Glossary
- CI
confidence interval
- CSPPT
China Stroke Primary Prevention Trial
- HR
hazard ratio
- IQR
interquartile range
- MI
myocardial infarction
- MTHFR
methylenetetrahydrofolate reductase
- tHcy
total homocysteine
Appendix. Authors


Study funding
The study was supported by funding from the following: the National Key Research and Development Program (2016YFE0205400, 2018ZX09739, 2018ZX09301034003); the National Natural Science Foundation of China (81730019, 81973133); the Science and Technology Planning Project of Guangzhou, China (201707020010); the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040, GJHS20170314114526143); the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390); and the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009). The funders had no role in the design and/or conduct of the study (data collection, management, analysis, and interpretation); the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosure
X. Qin reports grants from the National Natural Science Foundation of China (81730019, 81973133) and the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009). J. Spence is a consultant to Amgen and Orphan Technologies and has received lecture fees from Pfizer and Bristol-Myers-Squibb. J. Li, Y. Zhang, Y. Li, N. Sun, M. Liang, Y. Song, Y. Zhang, B. Wang, X. Cheng, L. Zhao, and X. Wang report no disclosures relevant to the manuscript. X. Xu reports grants from the National Key Research and Development Program (2016YFE0205400, 2018ZX09739, 2018ZX09301034003), the Science and Technology Planning Project of Guangzhou, China (201707020010), the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040, GJHS20170314114526143), and the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390). Y. Huo reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics: 2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Li Y, Li P, et al. Association between percent decline in serum total homocysteine and risk of first stroke. Neurology 2017;89:2101–2107. [DOI] [PubMed] [Google Scholar]
- 4.Wald D, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a metaanalysis. BMJ 2002;325:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin X, Huo Y. H-type hypertension, stroke and diabetes in China: opportunities for primary prevention. J Diabetes 2016;8:38–40. [DOI] [PubMed] [Google Scholar]
- 6.Stanger O, Herrmann W, Pietrzik K, et al. DACH-LIGA homocysteine (German, Austrian and Swiss homocysteine society): consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: guidelines and recommendations. Clin Chem Lab Med 2003;41:1392–1403. [DOI] [PubMed] [Google Scholar]
- 7.Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol 2002;22:6–13. [DOI] [PubMed] [Google Scholar]
- 8.UK, Kalita J, Singh SK, Rahi SK. Oxidative stress markers in vitamin B12 deficiency. Mol Neurobiol 2017;54:1278–1284. [DOI] [PubMed] [Google Scholar]
- 9.Spence JD, Yi Q, Hankey GJ. B vitamins in stroke prevention: time to reconsider. Lancet Neurol 2017;16:750–760. [DOI] [PubMed] [Google Scholar]
- 10.Zhao M, Wu G, Li Y, et al. Meta-analysis of folic acid efficacy trials in stroke prevention: insight into effect modifiers. Neurology 2017;88:1830–1838. [DOI] [PubMed] [Google Scholar]
- 11.Qin X, Li J, Spence JD, et al. Folic acid therapy reduces the first stroke risk associated with hypercholesterolemia among hypertensive patients. Stroke 2016;47:2805–2812. [DOI] [PubMed] [Google Scholar]
- 12.Fan F, Yuan Z, Qin X, et al. Optimal systolic blood pressure levels for primary prevention of stroke in general hypertensive adults: findings from the CSPPT (China Stroke Primary Prevention Trial). Hypertension 2017;69:697–704. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Xu B, Xu R, et al. Independent and joint effect of brachial-ankle pulse wave velocity and blood pressure control on incident stroke in hypertensive adults. Hypertension 2016;68:46–53. [DOI] [PubMed] [Google Scholar]
- 14.Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins DJA, Spence JD, Giovannucci EL, et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol 2018;71:2570–2584. [DOI] [PubMed] [Google Scholar]
- 16.Spence JD. Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Nutr Res 2016;36:109–116. [DOI] [PubMed] [Google Scholar]
- 17.Van Guelpen B, Hultdin J, Johansson I, et al. Folate, vitamin B12, and risk of ischemic and hemorrhagic stroke: a prospective, nested case-referent study of plasma concentrations and dietary intake. Stroke 2005;36:1426–1431. [DOI] [PubMed] [Google Scholar]
- 18.Giles WH, Kittner SJ, Anda RF, Croft JB, Casper ML. Serum folate and risk for ischemic stroke. First National Health and Nutrition Examination Survey epidemiologic follow-up study. Stroke 1995;26:1166–1170. [DOI] [PubMed] [Google Scholar]
- 19.Virtanen JK, Voutilainen S, Happonen P, et al. Serum homocysteine, folate and risk of stroke: Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Eur J Cardiovasc Prev Rehabil 2005;12:369–375. [DOI] [PubMed] [Google Scholar]
- 20.Weikert C, Dierkes J, Hoffmann K, et al. B vitamin plasma levels and the risk of ischemic stroke and transient ischemic attack in a German cohort. Stroke 2007;38:2912–2918. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Wang X, He M, et al. Homocysteine and stroke risk: modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke 2017;48:1183–1190. [DOI] [PubMed] [Google Scholar]
- 22.Boccia S, Hung R, Ricciardi G, et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol 2008;167:505–516. [DOI] [PubMed] [Google Scholar]
- 23.Izzotti A. DNA damage and alterations of gene expression in chronic-degenerative diseases. Acta Biochim Pol 2003;50:145–154. [PubMed] [Google Scholar]
- 24.Federici C, Botto N, Manfredi S, Rizza A, Del Fiandra M, Andreassi MG. Relation of increased chromosomal damage to future adverse cardiac events in patients with known coronary artery disease. Am J Cardiol 2008;102:1296–1300. [DOI] [PubMed] [Google Scholar]
- 25.Roest M, van der Schouw YT, Grobbee DE, et al. Methylenetetrahydrofolate reductase 677 C/T genotype and cardiovascular disease mortality in postmenopausal women. Am J Epidemiol 2001;153:673–679. [DOI] [PubMed] [Google Scholar]
- 26.Spence JD, Stampfer MJ. Understanding the complexity of homocysteine lowering with vitamins: the potential role of subgroup analyses. JAMA 2011;306:2610–2611. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Cogswell ME, Hamner HC, et al. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: national Health and Nutrition Examination Survey (NHANES) 2003-2006. Am J Clin Nutr 2010;91:64–72. [DOI] [PubMed] [Google Scholar]
- 28.Spence JD, Barnett PA, Hegele RA, Marian AJ, Freeman D, Malinow MR. Plasma homocyst(e)ine, but not MTHFR genotype, is associated with variation in carotid plaque area. Stroke 1999;30:969–973. [DOI] [PubMed] [Google Scholar]
- 29.Yang B, Fan S, Zhi X, et al. Prevalence of hyperhomocysteinemia in China: a systematic review and meta-analysis. Nutrients 2014;7:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabi H, Bochud M, Glaus J, et al. Association of serum homocysteine with major depressive disorder: results from a large population-based study. Psychoneuroendocrinology 2013;38:2309–2318. [DOI] [PubMed] [Google Scholar]
- 31.Wald DS, Bishop L, Wald NJ, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med 2001;161:695–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data, analytic methods, and study materials that support the findings of this study will be available from the corresponding authors on request, after the request is submitted and formally reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China.