Abstract
Background:
The digestive tract malignancies are a series of malignant tumor with high morbidity and mortality. Traditional Chinese medicine (TCM) combined with chemotherapy drugs interventions have been applied for the treatment of malignant tumors in Asian countries for dacades. This study aimed to assess the effectiveness and safety on the combination of Kanglaite injection and fluorouracil-based chemotherapy for treating digestive tract malignancies.
Purpose:
To assess the effectiveness and safety on the combination of Kanglaite injection and fluorouracil-based chemotherapy for digestive tract malignancies.
Methods:
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed when conducting the meta-analysis. Randomized controlled trials (RCTs) of Kanglaite injection combined with fluorouracil-based chemotherapy in the treatment of digestive tract malignant tumors were selected and assessed for inclusion. RevMan 5.3 software (Cochrane Collaboration, Oxford, UK) was used for meta-analysis. The objective response rate (ORR) was defined as the primary endpoint, and the disease control rate (DCR), quality of life (QoL), and toxicities were the secondary outcomes.
Results:
20 RCTs enrolling 1339 patients with advanced digestive tract malignancies were included. The methodological quality of most included trials was low to moderate. Compared with fluorouracil-based chemotherapy alone, Kanglaite injection plus fluorouracil-based chemotherapy can improve DCR (risk ratio (RR) = 1.18, 95% confidence interval (CI) 1.11–1.25, P < .00001), ORR (RR = 1.35, 95% CI 1.18–1.54, P < .00001), QoL (RR = 1.58, 95% CI 1.35–1.85, P < .00001), and can reduce adverse drug reactions (ADRs) such as myelosuppression (RR = 0.33, 95% CI 0.25–0.43, P < .00001), leukopenia (RR = 0.31, 95% CI 0.22–0.43, P < .00001), thrombocytopenia (RR = 0.6, 95% CI 0.38–0.49, P = .03), neutropenia (RR = 0.26, 95% CI 0.12–0.55, P = .0005), anemia (RR = 0.41, 95% CI 0.23–0.75, P = .004), gastrointestinal reaction (RR = 0.35, 95% CI 0.27–0.46, P < .00001), nausea/vomiting (RR = 0.41, 95% CI 0.28–0.61, P < .00001), diarrhea (RR = 0.34, 95% CI 0.18–0.62, P = .0004), hepatotoxicity (RR = 0.28, 95% CI 0.17–0.47, P < .00001), neurotoxicity (RR = 0.58, 95% CI 0.41–0.82, P = .002), mucositis (RR = 0.59, 95% CI 0.29–1.21, P = .15).
Conclusion:
Kanglaite injection combined with fluorouracil-based chemotherapy could remarkably improve the clinical effectiveness and reduce the adverse effects in patients with advanced malignant tumors of the digestive tract which may provide evidence to judge whether TCM is an effective and safe intervention for the digestive tract malignancies.
Keywords: digestive tract malignancy, fluorouracil-based chemotherapy, Kanglaite injection, meta-analysis, randomized controlled trial (RCT)
1. Introduction
Given the intensification of urbanization, industrialization, and aging; ecological environment deterioration; and lifestyle changes, there has been a continuous increase in the malignant tumor incidence. Consequently, it has become a major public health issue that poses a threat to human life and social development. In 2018, the worldwide number of new cancer cases reached 18.1 million with nearly 9.6 million of them resulting in death, which made it the leading cause of death after cardiovascular disease. The leading 3 causes of cancer death were lung cancer (22.0%), liver cancer (10.2%), and stomach cancer (9.5%) in males and breast cancer (15%), lung cancer (13.8%), and colorectal cancer (9.5%) in females.[1] World Health Organization (WHO) reported that there were 2.76 million worldwide cases of digestive tract malignancies in 2012 with an incidence rate of 35.2/100,000. Further, the number of deaths from malignant digestive tract tumors was 1.82 million with a case fatality rate of 22.3/100,000. In China, the annual number of cases and deaths from malignant digestive tract tumors is >2 million and 1.6 million, respectively, which makes it have the highest incidence and mortality among the malignant tumors in China with the 2 showing an annual increase.[2]
Chemotherapy is among the main treatment methods for malignant digestive tract tumors. However, its drug toxicity in tumor treatment often causes many adverse reactions, including bone marrow suppression, immunosuppression, and gastrointestinal reactions, which seriously affect the quality of life (QoL) for individuals with malignant tumors. As a multi-target and multi-effect treatment method with fewer side effects, traditional Chinese medicine confers unique advantages against chemotherapy-induced adverse reactions.[3]
Kanglaite injection (Z10970091, China Food and Drug Administration), which is extracted from Coix seed, is an effective anti-cancer treatment in traditional Chinese medicine. It has been shown to induce apoptosis and inhibit cancer cell proliferation by regulating PI3K/Akt/mTOR signaling pathway.[4] Further, it has been reported to be clinically effective in the treatment of liver cancer, pancreatic cancer, lung cancer, and other malignant tumors.[5–7] Malignant tumor treatment using Kanglaite injection combined with chemotherapy could improve clinical efficacy, reduce toxicity and side effects, and enhance immune function.[8]
Fluorouracil (antineoplastic agent) is a cell-cycle-specific drug that exerts cytotoxic effects through DNA synthesis inhibition and RNA transcription interference.[9] Fluorouracil (5-Fu) remains the first-line treatment for digestive tract tumors.[10,11] Given its broad antineoplastic spectrum, it is also widely used in the treatment of breast cancer, ovarian cancer, bladder cancer, etc.[12–14] Fluorouracil agents include 5-Fu, tegafur, fluorouridine, capecitabine, and tigeo; among them, 5-Fu was the first to be clinically applied. Fluorouracil-based chemotherapy refers to the use of fluorouracil alone or in combination with cisplatin (DDP),[15] oxaliplatin (L-OHP),[16] paclitaxel (Taxol),[17] or docetaxel (Taxotere),[18] which are important therapeutic agents for gastrointestinal malignant tumors. There have been studies comparing the efficacy and safety of Kanglaite injection combined with chemotherapy vs chemotherapy alone in patients with advanced gastric cancer. However, these studies are limited by small sample sizes and low quality, which weakens the validity of their conclusions. A comprehensive evaluation of the efficacy of Kanglaite injection for advanced gastric cancer is difficult. In 2014, a meta-analysis by Wang et al[19] reported that chemotherapy using a combination of Kanglaite and 5-fluorouracil, leucovorin plus oxaliplatin (FOLFOX), a typical fluorouracil-based regimen, significantly improved objective response rate (ORR) and QoL in patients with gastric cancer. Further, Kanglaite injection can significantly reduce the incidence of nausea, vomiting, and leukopenia (III–IV). This meta-analysis, which included 10 Chinese herb injections (CHIs) combined with FOLFOX, focused on horizontally comparing traditional Chinese medicine. A similar analysis[20] reported that Kanglaite injection combined with FOLFOX had a more favorable clinical efficacy than that of the FOLFOX regimen alone. Several studies have compared the efficacy and safety of Kanglaite injection combined with chemotherapy vs chemotherapy alone in advanced digestive tract malignancies. However, they have several limitations including small sample sizes, as well as limited quality and reference values. Moreover, the clinical efficacy and effect of Kanglaite injection combined with fluorouracil-based chemotherapy on the QoL of patients with digestive tract malignancies remains unclear. Therefore, we aimed to conduct a comprehensive meta-analysis of the results of clinical randomized controlled trials (RCTs) on Kanglaite injection plus chemotherapy, as well as systematically evaluate the efficacy and safety of Kanglaite injection plus chemotherapy versus chemotherapy alone for advanced digestive tract malignancies.
2. Materials and methods
This meta-analysis was implemented following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[21] Given that we used data from published studies, there was no requirement for ethical approval. This meta-analysis has been registered at International prospective register of systematic reviews (PROSPERO) under the registration number CRD42019130508.
2.1. Searching strategies
Two investigators (Qi Song and Jie Zhang) independently retrieved related data from the databases using the following screening strategy: (“kang-lai-te” [Supplementary Concept] OR kanglaite OR kanglaite injection OR KLT) AND (Chemotherapy OR Chemotherapies OR “Fluorouracil”[Mesh] OR Fluorouracil OR 5-Fu OR Tegafur OR Capecitabine OR Carmofur OR Fluorouridine) AND (“Esophageal Neoplasms”[Mesh] OR “Stomach Neoplasms”[Mesh] OR “Gastrointestinal Neoplasms”[Mesh] OR “Colorectal Neoplasms”[Mesh] OR “Colonic Neoplasms”[Mesh] OR “Rectal Neoplasms”[Mesh] OR “Intestinal Neoplasms”[Mesh] OR Esophageal Cancer OR Gastric Cancer OR Intestinal Cancer OR Colorectal Cancer OR Colon Cancer OR Rectal Cancer). We searched the following Chinese databases: China Biological Medicine Database, China National Knowledge Infrastructure Database, Chinese Scientific Journals Full-Text Database, Wanfang Database, and Airiti Library. We searched the following English databases: Medline, Embase, Web of Science, and Cochrane Central Register of Controlled Trials. All retrievals were implemented using MeSH and free word. The retrieval period lasted from the established time to March 2019. After the evaluation of similar or related systematic reviews or meta-analysis, we selected the studies that met the inclusion criteria from related references.
2.2. Types of studies
We evaluated RCTs that assessed the beneficial effects and safety outcomes of Kanglaite injection combined with fluorouracil-based chemotherapy versus chemotherapy alone regardless of the language, publication status, or blinding. We excluded cluster randomization trials, cross-over design studies, before–after studies, cohort studies, non-randomized trials, case–control studies, cross-sectional studies, descriptive studies, reviews, case reports, and animal studies.
2.3. Inclusion and exclusion criteria
We applied the following inclusion criteria:
Studies enrolling histopathologically or cytologically confirmed patients with advanced (stage III–IV) digestive tract malignancies without surgical operations.
Studies that employed an RCT study design.
Experiment group with patients treated with the combination of Kanglaite injection and fluorouracil-based chemotherapy and a control group with patients treated with a corresponding routine fluorouracil-based chemotherapy alone.
Patients not receiving any other adjuvant treatments, including other chemotherapies, radiotherapy, and additional traditional Chinese herbs prior to study enrollment.
Main outcomes involving short-term clinical efficacy evaluated as tumor responses, QoL, and adverse drug reactions (ADRs).
Additional outcomes involving the immune function index, including CD3+, CD4+, CD8+, CD4+/CD8+, and NK.
We did not employ restrictions regarding the times and types of follow-up.
We excluded the following studies: case report studies; meeting abstracts; reviews; cohort studies; non-RCT studies; in vitro and animal studies; duplicated studies; studies including patients that received surgery, radiotherapy, or other traditional Chinese medicine treatment during the intervention period; studies that lacked at least 1 outcome among those for clinical efficacy, QoL, and ADRs; studies with a drop out ratio >10%.
2.4. Definition of outcome measures
We assessed the treatment efficacy according to Response Evaluation Criteria in Solid Tumors (RECIST) developed by WHO and the curative effect evaluation criteria after the scheduled treatment and follow-up. We classified the patients into 4 categories; namely, complete response (CR), partial response (PR), stable disease (SD), and progressive disease. The ORR, which is also known as the clinical effective rate, was considered as (CR + PR) and as the primary endpoint. Moreover, we defined the disease control rate (DCR) as (CR + PR + SD) against the total number of patients in each group. Moreover, we employed the standardization of the diagnosis and curative effect from the Guiding Principles for Clinical Research of New Drugs for Syndromes of traditional Chinese medicine (TCM) (National Medical Products Administration. Guiding Principles for Clinical Research of New Drugs for Syndromes of TCM, 2018) as a reference for curative effect evaluation. According to this evaluation system, we considered a decrease of post-treatment scores by >70%, 30% to 70%, <30% as significant, partial, and non-improvement, respectively.
QoL was assessed according to the Karnofsky Performance Status (KPS) scale.[22] We defined QoL improvement as a post-treatment KPS score increase of ≥10 points. A post-treatment KPS score of <10 points was considered as declined QoL. A stable QoL was considered when the post-treatment score increased or decreased by <10 points.
ADRs were assessed and graded as I through IV degrees according to Recommendations for Grading of Acute and Subacute Toxicity.[23] Moreover, we employed the Common Terminology Criteria Adverse Events version 4.0 developed by the National Cancer Institute of U.S. as a reference for evaluating anticancer drug toxicity [http://evs.nci.nih.gov/ftp1/CTCAE/About.html].
We set immune function indexes as the additional outcomes defined as peripheral blood T lymphocyte subsets (CD3+, CD4+, CD8+, and CD4+/CD8+ levels) and serum NK cell count.
2.5. Study selection
Two investigators (Qi Song and Jie Zhang) independently evaluated and screened the identified studies in strict accordance with the inclusion and exclusion criteria. Disagreements were resolved through discussion with a third investigator (Elaine Leung).
2.6. Data extraction
We applied the search strategy to retrieve titles and abstracts of the selected studies. Studies identified from additional sources were independently screened by 2 review authors (Qi Song and Jie Zhang) to identify studies that potentially met the inclusion criteria. The 2 review authors independently assessed full-text articles for eligibility. Disagreements over the eligibility of certain studies were resolved through discussion with the third reviewer. The 2 review authors independently extracted the following information from each trial: name of the first author; published year; demographic characteristics, for example, sample size, age, and gender; study design and methodology; Kanglaite injection and chemotherapy regimen usage; and evaluation criteria and main outcome indicators, including ORR, DCR, QoL, and ADRs. Study details were not only obtained through direct information extraction from the articles but also by contacting the original authors for further information if necessary; further, data in diagram form were extracted through calculation.
2.7. Risk of bias (quality) assessment
We evaluated the methodological quality of the included clinical trials based on the Cochrane evaluation handbook of RCTs (5.1.0) (Higgins J, Green S, editors) and the Cochrane handbook for systematic reviews of interventions (Wiley Online Library; 2008). Two investigators used a unified method to independently evaluate and cross-check the quality of the eligible articles through 6 domains: random sequence generation (selection bias), allocation concealment (selection bias), participant and personnel blinding (performance bias), outcome assessment blinding (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Disagreements were jointly resolved through discussions with the third investigator. We regarded trials with low-bias risk for some domains as high-quality research and were judged with “Yes”. Studies with a high-bias risk in any domain were defined as poor-quality research and were judged with “No”. Finally, the remaining studies were thought to have an unclear risk of bias and were judged with “Unclear”.
2.8. Strategy for data synthesis
Two reviewers performed the statistical analysis using Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK). We determined the risk ratio (RR) with a 95% confidence interval (CI) for dichotomous variables and P < .05 as statistically significant. We determined the mean difference (MD) with 95% CI for continuous variables. We examined the heterogeneity of the studies using the χ2-based Q test. Between-study heterogeneity was described using the I2 index. Significant heterogeneity was confirmed when I2 > 50% while non-significant heterogeneity was considered when P > .05 and I2 ≤ 50%. The fixed-effects model (FEM) was applied to estimate the summary RR (or OR) while the random-effects model was applied for the MD and 95% CI. Subgroup or sensitivity analyses were used to explore heterogeneity when necessary. In the case of an unclear heterogeneity source, the stochastic effect model was employed to analyze the source. The combined effect was tested using the Z test with P < .05 indicating a significant difference. Funnel plots were used to assess publication bias if more than 10 studies were included in 1 outcome index.
Two independent reviewers (Qi Song and Jie Zhang) independently assessed the outcome evidence quality for each study based on the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) approach.[24] Disagreements were resolved through discussion between themselves or with a third investigator (Guoping Li). GRADE classifies the evidence quality in the evaluation of diagnostic test systems by examining 5 downgrading domains, that is, the risk of bias, indirectness, inconsistency, imprecision, and publication bias, as well as 2 upgrading factors, that is, dose–response gradient and plausible confounding. The recommendation strength was graded into 4 levels; namely, high, moderate, low, and very low.
3. Results
3.1. Types of studies
The initial database search identified 184 records based on the established search strategy. Among them, 108 records were excluded after title screening for duplicates. After abstract screening, 41 records were rejected for failing to meet inclusion criteria. The full texts of the remaining 35 records were downloaded with the subsequent exclusion of 15 unqualified studies (single-arm, cohort, non-random, etc). Finally, 20 trials were included in the meta-analysis (Fig. 1).
Figure 1.
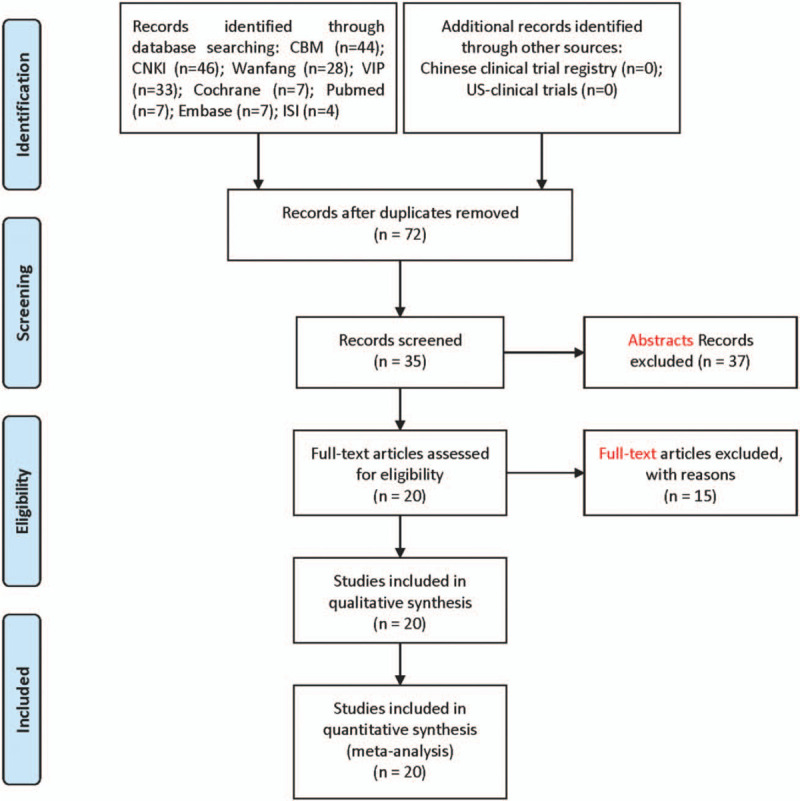
Articles retrieved and assessed for eligibility.
3.2. Characteristics of the included trials
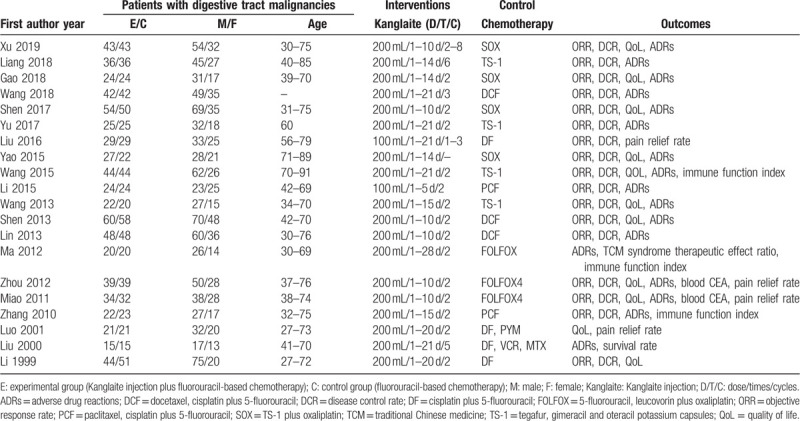
We enrolled 20 trials on 1339 patients with advanced (stage III–IV) digestive tract malignancies (Table 1). All the selected trials had been performed in China and had been published between 1999 and 2019 with no significant baseline differences. A total of 1339 cases were studied with 673 in the Kanglaite injection combined with fluorouracil-based chemotherapy group (treatment group) and 666 in the routine chemotherapy group (control group). The number of males and females was 848 and 500, respectively, and the age ranged from 27 to 89 years. Nineteen trials reported administering intravenous Kanglaite injections at 200 mL/time, 1 to 4 week/cycle, and 2 to 4 cycles. The control groups underwent the following sole fluorouracil-based chemotherapies: SOX (TS-1 plus oxaliplatin), FOLFOX, DCF (docetaxel, cisplatin plus 5-fluorouracil), PCF (paclitaxel, cisplatin plus 5-fluorouracil), DF (cisplatin plus 5-fluorouracil), and TS-1 (tegafur, gimeracil, and oteracil potassium capsules). None of the selected trials reported information regarding placebo in the control groups. Seventeen trials reported the short-term efficacy as tumor responses according to the RECIST. Fourteen trials reported the QoL based on the KPS evaluation. Seventeen trials eligibly reported the ADRs based on the WHO criteria. There was only 1 trial with a follow-up duration of 36 months reported the overall survival (OS).
Table 1.
Characteristics of the included studies.
3.3. The methodological bias of the included studies
Only 4 trials[25–28] clearly described the random sequence generation. None of the studies provided specific information regarding random allocation methods. Selection bias could exist in these included studies. Only 1 trial[29] reported the details of the participant and personnel blinding, which indicates performance bias in the included studies. All the included trials had unclear details regarding the blinding of the outcome assessment. The data were complete in all trials and there was no evidence of selective reporting. Three,[28,30,31] 5,[26,29,31–33] and 2 trials[32,34] did not completely report the DCR, QoL, and ADRs, respectively. Other biases were unclear (Fig. 2).
Figure 2.
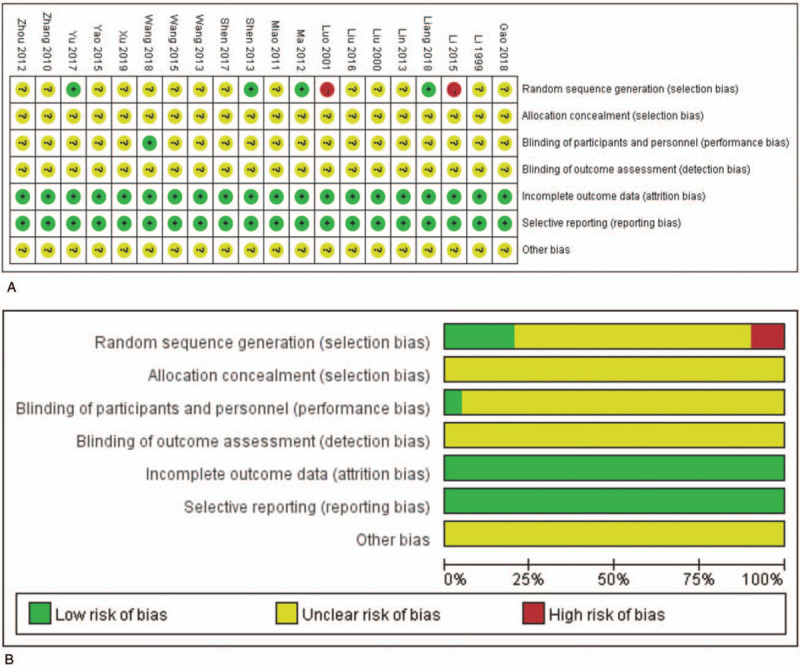
The risk of methodological bias. Risk of bias summary (A): review of authors’ judgments about each risk of bias item for included studies. Risk of bias graph (B): review of authors’ judgments about each risk of bias item presented as percentages across all included studies. Note: Each color represents a different level of bias: red for high-risk, green for low-risk, and yellow for unclear-risk of bias.
We applied the trial sequential analysis (TSA) software, sensitivity analysis, and subgroup analysis to assess the results’ robustness and calculate the required information size in the meta-analysis.[35] TSA indicated that Kanglaite injection combined with fluorouracil-based chemotherapy was significantly superior to fluorouracil-based chemotherapy alone and that the cumulated sample size of all the RCTs reached the required information size required for a conclusive and reliable meta-analysis (Fig. 3). This suggests that the findings for the DCR in this meta-analysis are robust.
Figure 3.
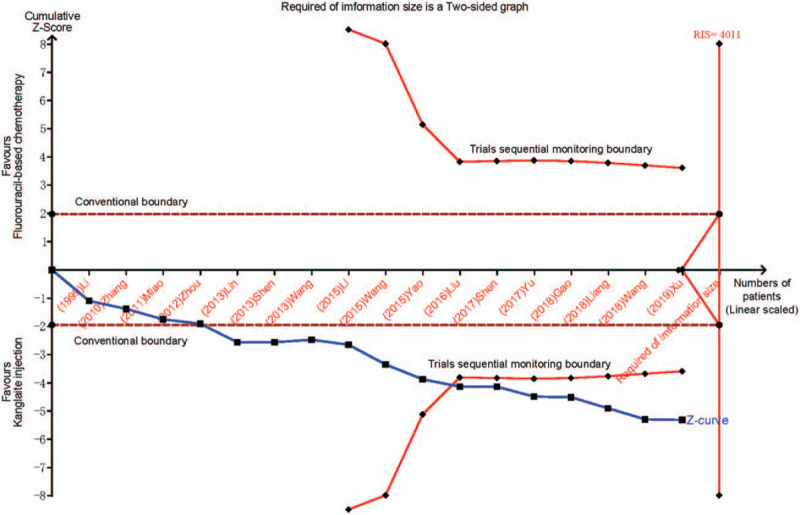
Trial sequential analysis. The Z curve is a measure of treatment effect, and the boundaries are thresholds for statistical significance that is adjusted for heterogeneousness of multiple statistical testing and trial results. The treatment effect outside the statistical significance boundary (red line) indicates that there is a reliable evidence of treatment effect, and the treatment effect within the futility boundary (dotted line) indicates that there is no reliable evidence of treatment effect. The calculated optimum sample size is indicated by optimum size for statistical inference. RIS, required information size.
3.4. Clinical efficacy
Seventeen trials[25–27,29,32–34,36–45] detailedly reported the short-term efficacy using tumor responses (Figs. 4 and 5). A total of 1227 patients were included in subgroup analyses based on different therapeutic evaluation criteria. We used the DCR and ORR to assess the short-term efficacy based on the RECIST criteria. There was no statistical heterogeneity among the trials in the ORR (P = .85, I2 = 0%) and DCR (P = .60, I2 = 0%) after the I2 test and Pearson's chi-square test. Therefore, we used FEM to analyze the results. The short-term efficacy rate was significantly higher in the experiment group than that in the control group (RR = 1.18, 95% CI 1.11–1.25, P < .00001). Compared with fluorouracil-based chemotherapy alone, the FEM indicated that fluorouracil-based chemotherapy combined with Kanglaite injection substantially improved the ORR (RR = 1.35, 95% CI 1.18–1.54, P < .00001).
Figure 4.
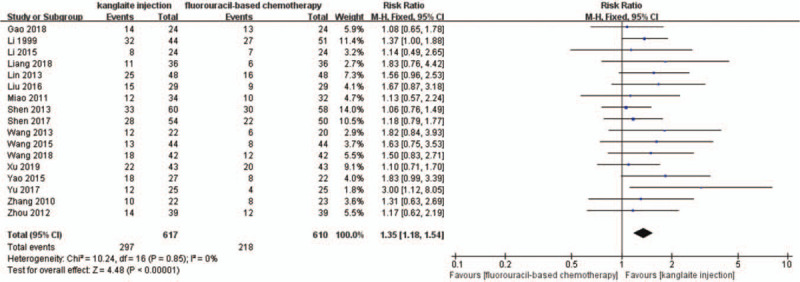
The analysis of objective response rate (ORR) between 2 groups. Forest plot of the comparison of ORR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
Figure 5.
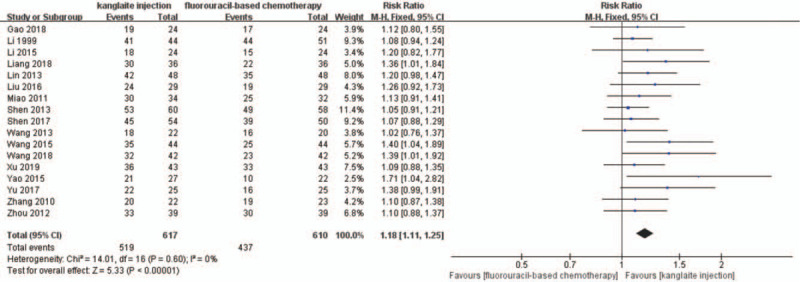
The analysis of disease control rate (DCR) between 2 groups. Forest plot of the comparison of DCR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
We divided the patients based on the different tumor sites into 3 subgroups; namely, gastric, colorectal, and esophageal cancer. The random-effects model showed that Kanglaite injection combined with fluorouracil-based chemotherapy for esophageal cancer (RR = 1.14, 95% CI 0.99–1.31, P = .07), gastric cancer (RR = 1.15, 95% CI 1.06–1.24, P = .0005), and colorectal cancer (RR = 1.26, 95% CI 1.11–1.42, P = .0002) were significantly superior to chemotherapy alone (Fig. 6). The ORR meta-analysis results similarly indicated that Kanglaite injection combined with fluorouracil-based chemotherapy for esophageal cancer (RR = 2.37, 95% CI 1.21–4.65, P = .01), gastric cancer (RR = 1.59, 95% CI 1.18–2.15, P = .002), and colorectal cancer (RR = 1.79, 95% CI 1.11–2.88, P = .02) were significantly superior to chemotherapy alone (Fig. 7).
Figure 6.
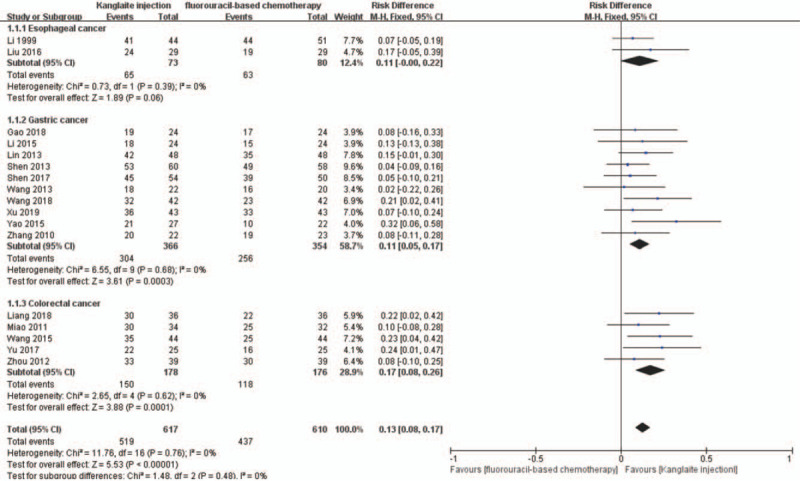
The analysis of disease control rate (DCR) between 2 groups based on tumor sites. Forest plot of the comparison of DCR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
Figure 7.
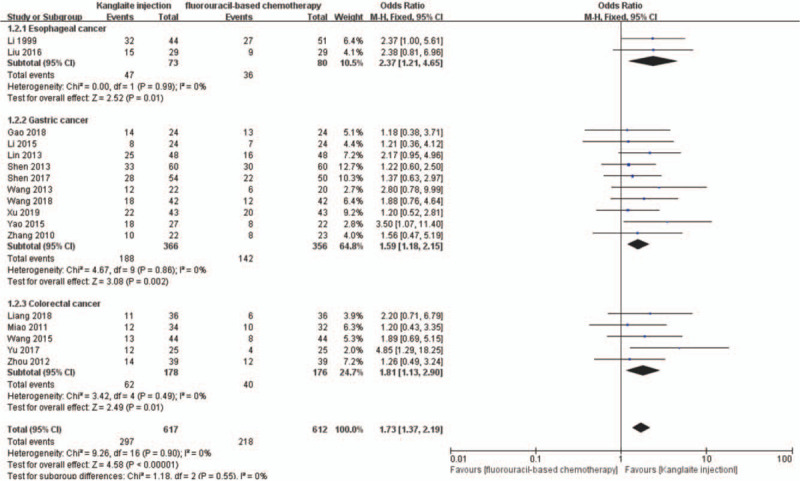
The analysis of objective response rate (ORR) between 2 groups based on tumor sites. Forest plot of the comparison of ORR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
3.5. QoL evaluation
A total of 11 RCTs were included.[27,30,34,36–40,42,44,45]I2 test and Pearson's chi-square test indicated that there was non-significant heterogeneity among the trials (I2 = 18%). FEM meta-analysis showed that the QoL improvement rate after Kanglaite injection combined with chemotherapy was better than that after chemotherapy alone (RR = 1.58, 95% CI 1.35–1.85, P < .00001) (Fig. 8). Four trials[25,28,41,43] reported the QoL as specific KPS scale scores with FEM meta-analysis showing that Kanglaite injection combined with chemotherapy was superior to chemotherapy alone (RR = 4.46, 95% CI 2.66–6.26, P < .00001) (Fig. 9).
Figure 8.
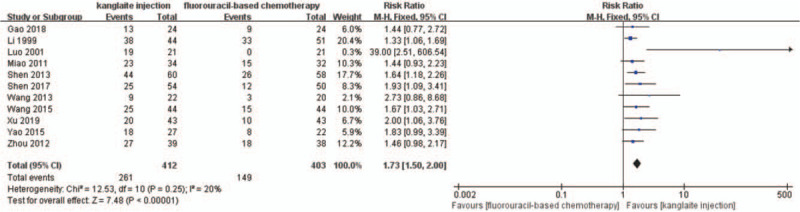
The analysis of quality of life (QoL) between 2 groups. Forest plot of the comparison of QoL between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
Figure 9.
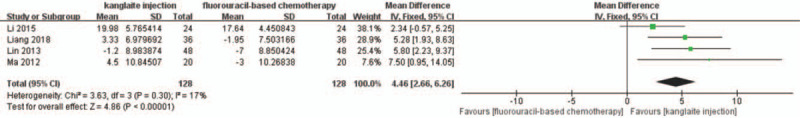
The analysis of Karnofsky Performance Status (KPS) scales between 2 groups. Forest plot of the comparison of ORR between the experimental and control group. Control group, chemotherapy alone group; Experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
3.6. ADRs evaluation
A total of 20 RCTs were included; among them, 3 RCTs[30,32,34] did not report specific cases, and thus could not be used in this analysis. A meta-analysis of the remaining 17 RCTs[25–29,31,33,36–45] showed that Kanglaite injection combined with chemotherapy for treating malignant digestive tract tumors (Table 2), including myelosuppression, leukopenia, gastrointestinal reaction, nausea/vomiting, diarrhea, hepatotoxicity, and neurotoxicity. Compared with the experiment group, the control group had a significantly lower incidence; however, there was no significant between-group difference in the incidence of mucositis. Although there were significant between-group differences in the incidence of thrombocytopenia, anemia, and neutropenia, there could be assessment bias due to sample size limitations.
Table 2.
Meta analysis results of ADRs between 2 groups.
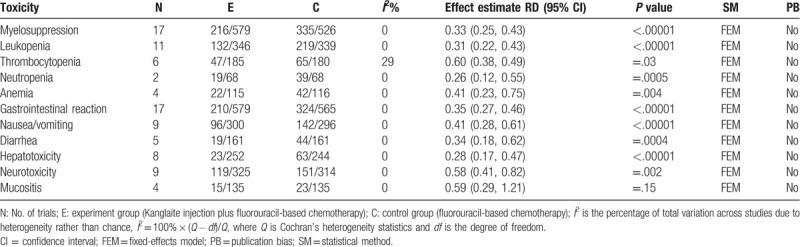
3.7. Evaluation of immune function index
Only 3 RCTs[28,33,40] reported the immune function index according to standards that allowed inclusion. There was no heterogeneity among the 3 studies using the CD3+ cell count as the immune indicator (I2 = 24%, P = .27); therefore, FEM meta-analysis was performed. The results indicated a significantly higher CD3+ cell count in the experiment group than that in the control group (RR = 7.67, 95% CI 5.71–9.63, P < .00001) (Fig. 10). Moreover, we observed significant heterogeneity in the CD4+ cell count in the 3 studies (I2 = 68% and P = .04); therefore, a CD3+ cell count meta-analysis was conducted. The results indicated a significantly higher post-treatment CD4+ cell count in the experiment group than that in the control group (RR = 5.51, 95% CI 1.99–9.02, P = .002) (Fig. 11).
Figure 10.
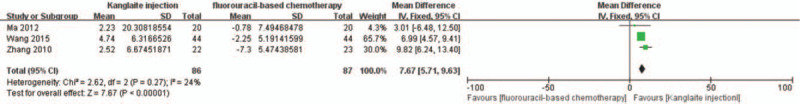
The analysis of CD3+ between 2 groups. Forest plot of the comparison of CD3+ levels between the experimental and control group. Control group, chemotherapy alone group; Experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
Figure 11.
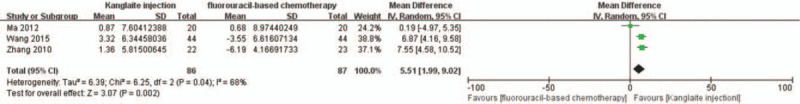
The analysis of CD4+ between 2 groups. Forest plot of the comparison of CD4+ levels between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The random-effects model was used.
3.8. Subgroup analysis
We conducted subgroup analyses to determine the influence of different Kanglaite injection scenarios and fluorouracil-based regimens on ORR and DCR. The dosages of Kanglaite injection were 200 mL/day in 18 trials. The treatment time and cycles were 10 days/2–8 cycles, 14–15 days/2–6 cycles, and 20–28 days/2–5 cycles, respectively. Subgroup analysis showed that Kanglaite injection at 200 mL/day doses could improve the ORR and DCR (Figs. 12 and 13). The fluorouracil-based chemotherapy regimens included SOX, FOLFOX, DCF, PCF, DF, and TS-1 (tegafur, gimeracil, and oteracil potassium capsules). Subgroup analyses indicated the effectiveness of SOX, DCF, and PCF, but not FOLFOX and DF, for the ORR and DCR (Figs. 14 and 15). Tumor responses were evaluated using the WHO criteria or RECIST. Subgroup analysis showed that Kanglaite injection combined with fluorouracil-based chemotherapy could increase the ORR and DCR based on the assessment of tumor responses.
Figure 12.
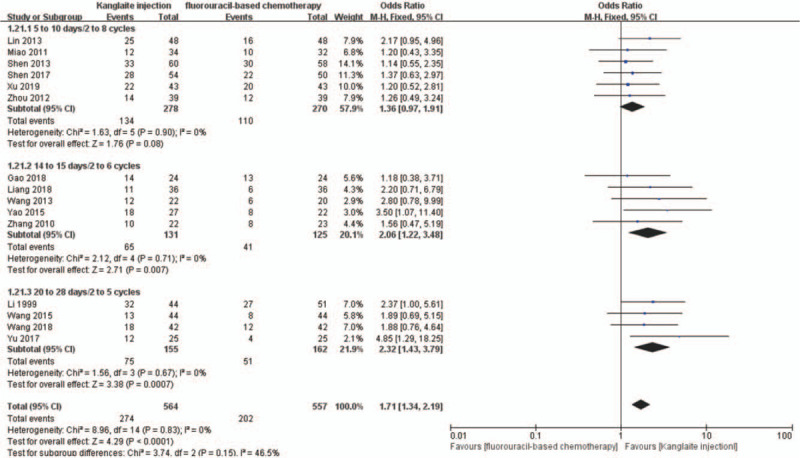
The subgroup analysis of objective response rate (ORR) between 2 groups based on usage of Kanglaite injection. Forest plot of the comparison of ORR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
Figure 13.
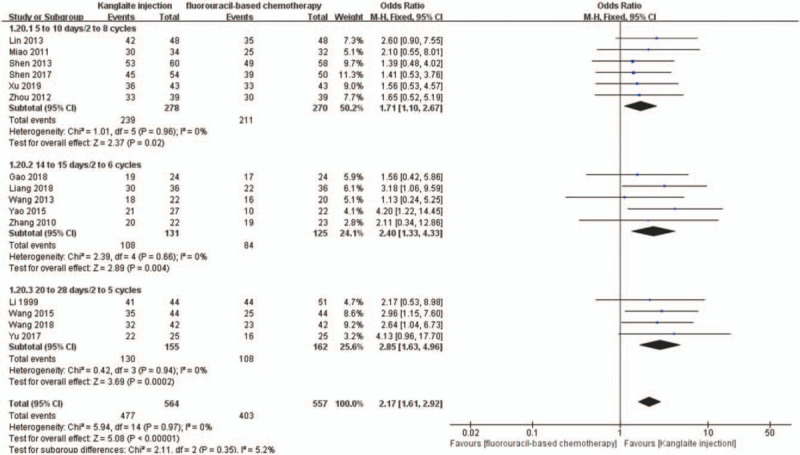
The subgroup analysis of disease control rate (DCR) between 2 groups based on usage of Kanglaite injection. Forest plot of the comparison of DCR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
Figure 14.
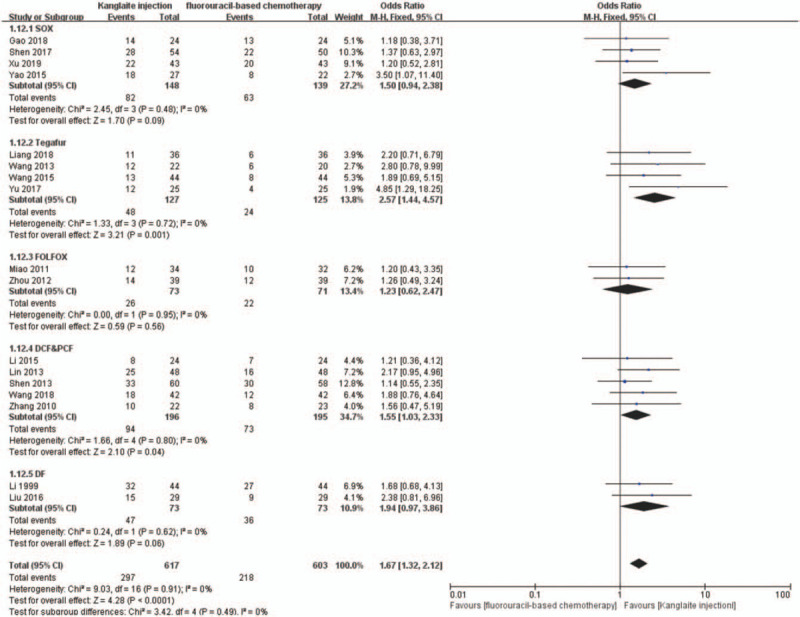
The subgroup analysis of objective response rate (ORR) between 2 groups based on regimen of chemotherapy. Forest plot of the comparison of ORR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
Figure 15.
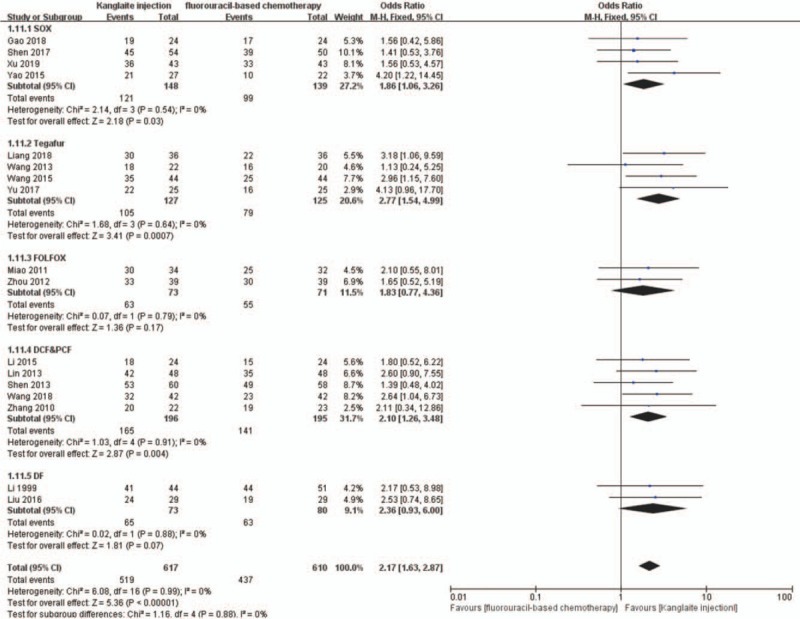
The subgroup analysis of disease control rate (DCR) between 2 groups based on regimen of chemotherapy. Forest plot of the comparison of DCR between the experimental and control group. Control group, chemotherapy alone group; experimental group, Kanglaite injection and fluorouracil-based chemotherapy combined group. The fixed-effects model was used.
3.9. Publication bias analysis
The funnel plots were symmetric for the ORR, DCR, myelosuppression, leukopenia, gastrointestinal reaction, nausea/vomiting, diarrhea, hepatotoxicity, and neurotoxicity (Fig. 16). This indicated no publication bias and objective reporting by the included studies. Funnel plots were significantly asymmetric in QoL (Fig. 16C), which indicated publication bias. The QoL risk was over-estimated in 1 trial[30] and under-estimated in another.[34] Therefore, we implemented meta-analysis by excluding the 2 aforementioned studies. In summary, except for QoL, all the results had good objectivity.
Figure 16.
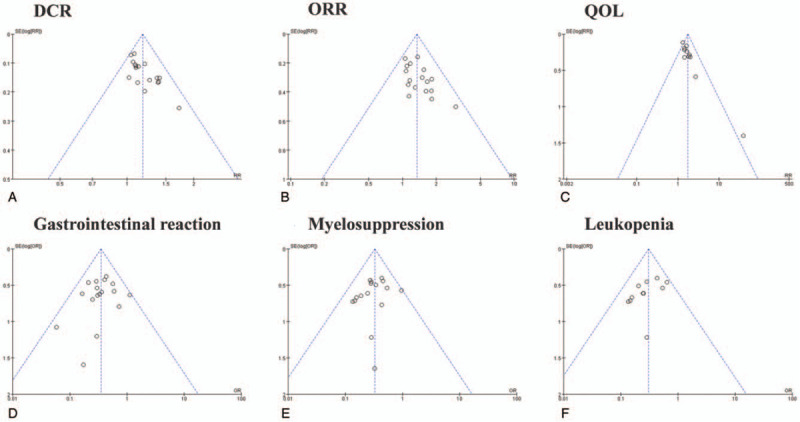
Publication bias analysis. Funnel plot of DCR (A), ORR (B), QoL (C), gastrointestinal reaction (D), myelosuppression (E), leukopenia (F).
3.10. Sensitivity analysis
Two poor-quality trials[30,34] showed a potential effect on the QoL with good consistency being acquired after their exclusion. Therefore, sensitivity assessment was performed after the exclusion of the inferior trials. The results had good stability before and after excluding the poor-quality trials.
There was no significant heterogeneity in the ORR, DCR, gastrointestinal reaction, myelosuppression, and leukopenia; however, they all had publication bias. The ORR, DCR, gastrointestinal reaction, myelosuppression, and leukopenia had good consistency before and after excluding the trials with over- and under-estimated QoL. The results had good stability before and after excluding the over- and under-estimated studies. In conclusion, the results of this meta-analysis had good robustness.
3.11. Quality of evidence
A majority of the 20 included trials showed uncertainty in the risk of methodological bias. Only 4 trials reported using random number tables to generate random sequences. Eight trials had at least 1 domain with a high risk of bias. Sensitivity analysis showed that the results had good robustness before and after excluding the poor-quality trials. Thus, we rated down the outcomes by 1 level due to design limitations. There was heterogeneity in the ORR, DCR, myelosuppression, leukopenia, gastrointestinal reaction, nausea/vomiting, diarrhea, hepatotoxicity, neurotoxicity, and thrombocytopenia. Except for QoL, all the other results showed good robustness. Consequently, only QoL was rated down by 1 level. The number of events for anemia, neutropenia, and mucositis were <300; therefore, we rated down these outcomes by 1 level. The QoL, which showed publication bias, had been over- and under-estimated. The evidence was not rated down since the results showed good robustness. There were no outcomes that met the eligibility for an upgrade. In general, the quality of the evidence was moderate.
4. Discussion
In this meta-analysis, we included 20 trials involving 1339 patients with advanced (stage III–IV) digestive tract malignancies. There were 848 males and 500 females with an age range of 27 to 89 years. The interventions included intravenous Kanglaite injections administered at 200 mL/time for 5–10 days/2–8 cycles, 14–15 days/2–6 cycles, and 20–28 days/2–5 cycles, as well as at 100 mL/time for 21 days/1–3 cycles. This meta-analysis evaluated the tumor response, QoL, ADRs, and immune function indexes.
Kanglaite injection is a Coix seed extract prepared by modern pharmaceutical technology. The active components of Coix seed oil have been shown to inhibit tumor cell proliferation and reduce tumor sizes, including lung cancer, pancreatic cancer, stomach cancer, and breast cancer.[46,47] Kanglaite injection regulates human immune function; further, it promotes cancer cell apoptosis and inhibits cancer cell proliferation. In vivo, in vitro, and clinical trials have suggested that Kanglaite injection could directly promote tumor cell apoptosis,[48] reverse chemotherapy resistance of tumor cells,[49] regulate and enhance the immune system.[50] Further, the prevention and reversal of cancer cachexia contribute to improving chemotherapy effectiveness and reducing side effects in patients with cancer.[51]
Kanglaite injection combined with chemotherapy is widely used in China. It is unclear whether Kanglaite injection combined with chemotherapy could have improved clinical efficacy and survival rate in patients with digestive tract malignancies. Seventeen trials with 1227 patients evaluated the ORR and DCR based on solid tumor response guidelines. A meta-analysis of these trials showed that Kanglaite injection combined with chemotherapy significantly improved ORR and DCR in patients with digestive tract malignancies compared with chemotherapy alone. Further, we performed subgroup analyses based on different tumor sites, chemotherapy regimens, and treatment courses to assess the effects of Kanglaite injection on digestive tract malignancies. Subgroup analysis based on tumor sites showed that Kanglaite injection combined with chemotherapy increased the ORR and DCR in patients with esophageal cancer, gastric cancer, or colorectal cancer. Subgroup analysis based on different chemotherapy regimens showed that Kanglaite injection combined with all the different fluorouracil-based chemotherapy regimens increased the ORR and DCR. Further subgroup analysis based on different Kanglaite injection treatment courses revealed that Kanglaite injection administration at 5–10 days/2–8 cycles, 14–15 days/2–6 cycles, and 21–28 days/2–5 cycles improved the DCR and ORR.
QoL is an indispensable indicator for evaluating chemotherapy effectiveness. We included 11 trials with 815 patients to assess the QoL in patients with advanced (stage III–IV) digestive tract malignancies. A meta-analysis of the QoL showed that Kanglaite injection combined with chemotherapy significantly improved the QoL in the patients. Moreover, a meta-analysis of 4 trials with 256 patients reported the QoL as specific KPS scale scores revealed similar results. The sufficient sample sizes of the included studies ensured accurate evaluation of the ORR, DCR, and QoL. Unfortunately, there was an unclear risk of bias in the majority of the included trials. The studies objectively reported the ORR and DCR; further, the good robustness of the results was confirmed by sensitivity analysis.
A previous network meta-analysis showed that among the CHIs, Kanglaite injection is the best for strengthening the ORR, improve QoL, and reducing nausea, vomiting, and leukopenia incidence in patients with gastric cancer. Further, Kanglaite injection combined with the FOLFOX regimen was better than other CHIs combined with the FOLFOX regimen and FOLFOX alone.[19] A related meta-analysis showed that compared with the FOLFOX regimen alone, Kanglaite injection combined with the FOLFOX regimen could improve the clinical efficacy for colorectal cancer treatment.[61] A similar strategy has been adopted by Chinese meta-analysis reports,[52–55] which indicated that Kanglaite injection might have a synergistic effect on chemotherapy for digestive tract malignancies. They all horizontally compared the efficacy of various traditional Chinese medicine injections combined with a similar chemotherapy regimen. In our meta-analysis, we performed a subgroup analysis of efficacy based on different chemotherapy regimens, including SOX, TS-1, FOLFOX, DCF and PCF, and DF. The subgroup analysis confirmed the effectiveness of SOX, DCF, and PCF, but not FOLFOX and DF, for ORR and DCR. The results regarding the FOLFOX and DF subgroups are inconsistent with those of previous studies, which could be attributed to small sample sizes. This study might be associated with statistical bias. The effect of different treatment courses of Kanglaite injection on patients with advanced digestive tract malignancies remains unclear. Therefore, in an attempt to clarify this, we selected 15 trials with 880 cases for further subgroup meta-analysis. We found significant differences in all the subgroups (10 days/2–8 cycles, 14–15 days/2–6 cycles, and 20–28 days/2–5 cycles). This indicated that Kanglaite injection improved the DCR and ORR in all the 3 subgroups of Kanglaite injection administration.
There have been studies on whether Kanglaite injection combined with chemotherapy decreases the risk of ADRs. A previous meta-analysis[56,57] reported that Kanglaite injection combined with routine chemotherapy had a lower risk of hepatotoxicity, leukopenia, and gastrointestinal reactions compared with chemotherapy alone. Another meta-analysis[58] reported that Kanglaite injection had a low risk of hepatotoxicity and cachexia. Consistent with these previous findings, we found that Kanglaite injection combined with chemotherapy lowered the risk of hematological, gastrointestinal, and other chemotherapy-induced toxicities in patients with digestive tract malignancies. Our meta-analysis had a sufficient number of included RCTs and patients with sensitivity analysis of results showing good robustness.
The immune system function is an important prognosis indicator in patients with advanced malignancies undergoing systemic chemotherapy. The most direct index of immune function is the peripheral blood CD8+ and CD4+ T cell count. We found that Kanglaite injection could affect the immunity level in patients with advanced digestive tract malignancies. Three trials reported changes in immune function after assessing the population of T cell subsets in peripheral blood.[28,33,40] They all reported that Kanglaite injection effectively protected the immune function in patients after chemotherapy. Meta-analysis of CD3+, CD4+, CD8+, and CD4+/CD8+ levels indicated significant difference in CD3+ (RR = 7.67, 95% CI 5.71–9.63, P < .00001) and CD4+ (RR = 5.51, 95% CI 1.99–9.02, P = .002). Unfortunately, an insufficient number of trials and patients might have led to insufficient assessment; therefore, there is a need for new evidence to support these findings.
A majority of the included studies had an unclear risk of bias. The sample size of clinical trials ranged from 30 to 124 patients; further, none of the studies performed a priori calculation of the sample size, which might have resulted in selection bias. Although all the clinical trials randomly recruited patients, only 4 trials[25–28] reported information generated by specific random sequences, which increases the risk of selection bias. None of the studies provided information regarding allocation concealment, which also increases the risk of selection bias. Only 1 clinical study[29] provided details regarding participant and personnel blinding. None of the RCTs reported information regarding outcome assessment blinding, which increases the risk of reporting bias. Only 1 trial[31] reported follow-up details with the remaining studies not reporting details regarding follow-up or withdrawal, which indicates a high withdrawal bias. Generally, 11 trials reported the QoL[27,30,34,36–40,42,44,45] with the remaining studies adopting other evaluation methods, which further increases the risk of reporting bias. Although all the studies reported ADRs, there did not use a common standard in the degree confirmation. Among them, 7,[27,28,33,40,42,43,45] 2,[38,41] 1,[39] and 1 trial[37] used grade I–IV, III–IV, I–II, and II–IV ADRs, respectively, as the evaluation standard with the remaining not explicitly introducing an evaluation standard. Avoiding these biases requires a more in-depth analysis of the studies as well as supplements regarding the essential descriptions in the literature, including direct emails or phone calls to the researchers, to allow a more precise and detailed literature review. Further, subgroup analysis could be performed to rule out heterogeneity in case of different evaluation criteria. Finally, more comprehensive design, implementation, and addressing of statistical irregularities in clinical studies might increase the reliability of the evaluation.
A recent meta-analysis[59] on the effectiveness of Kanglaite injection in patients with advanced pancreatic cancer recommended collection of information regarding patients’ smoking history and past medical history for enhanced rationality. Contrastingly, since malignant digestive tract tumors involve a high genetic correlation with family heritability, it is better to evaluate the curative effect with the inclusion of information regarding family history. Another Chinese meta-analysis[60] evaluated the QoL in patients with advanced non-small-cell lung cancer undergoing Kanglaite injection combined with chemotherapy. It included cancer pain in the evaluation system and concluded that Kanglaite injection could effectively alleviate cancer pain. Future studies on chemotherapy for malignant digestive tract tumors should consider digestive tract obstruction and constipation in addition to common gastrointestinal toxicity, including nausea, vomiting, and anorexia. A network meta-analysis[61] attempted to explore the comparative effectiveness and safety of different CHIs combined with the FOLFOX regimen versus FOLFOX alone for colorectal cancer. Similarly to our study, this previous meta-analysis included RCTs on patients of Asian descent; therefore, the conclusions might not be suitable for other populations.
This study has several limitations. First, there were few RCTs on the use of Kanglaite injection in the treatment of advanced digestive tract malignancies, which might contribute to sample size bias. Second, a majority of the included clinical trials lacked detailed descriptions of random sequence generation, allocation concealment, and blinding methods. Further, they did not provide sufficient information to allow determination of the study quality, which might have led to over- or under-estimation of efficacy. Third, the use of different chemotherapy regimens and administration modes might have affected efficacy and safety evaluation. Fourth, the treatment course of the included studies was insufficient for evaluating long-term efficacy, for example, OS and progression-free survival. Therefore, there is a need for well-designed, strictly implemented, high-quality, and double-blinded RCTs with large sample sizes for further evaluation.
5. Conclusion
We found that Kanglaite injection combined with fluorouracil-based chemotherapy, including TS-1, could remarkably improve the clinical effectiveness and QoL and reduce the risk of hematotoxicity, gastrointestinal reactions, neurotoxicity, and hepatotoxicity in patients with advanced digestive tract malignancies. The subgroup analysis indicated that the optimal regime for Kanglaite injection treatment could be 200 mL/day, 20 to 28 days, and 2 to 5 cycles. Kanglaite injection shows synergistic effects on fluorouracil-based chemotherapy.
However, given the intrinsic limitations of the enrolled RCTs, there is a need for well-designed, strictly implemented, and high-quality studies with longer follow-ups and important survival outcomes for further evaluation.
Author contributions
Conceptualization: Qi Song.
Data curation: Qi Song.
Formal analysis: Qi Song, Jie Zhang.
Investigation: Qi Song, Jie Zhang.
Methodology: Qibiao Wu.
Project administration: Qi Song, Qibiao Wu.
Resources: Qi Song.
Software: Qi Song.
Supervision: Guoping Li, Elaine Lai-Han Leung.
Writing – original draft: Qi Song.
Writing – review & editing: Qibiao Wu, Guoping Li, Elaine Lai-Han Leung.
qi song orcid: 0000-0001-5733-9222.
Footnotes
Abbreviations: ADRs = adverse drug reactions, CHIs = Chinese herb injections, CI = confidence interval, CR = complete response, DCF = docetaxel, cisplatin plus 5-fluorouracil, DCR = disease control rate, DDP = cisplatin, DF = cisplatin plus 5-fluorouracil, FEM = fixed-effects model, FOLFOX = 5-fluorouracil, leucovorin plus oxaliplatin, GRADE = Grades of Recommendation Assessment, Development, and Evaluation, KPS = Karnofsky Performance Status, L-OHP = oxaliplatin, MD = mean difference, NMA = network meta-analysis, ORR = objective response rate, OS = overall survival, PCF = paclitaxel, cisplatin plus 5-fluorouracil, PD = progressive disease, PFS = progression-free survival, PR = partial response, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PROSPERO = International prospective register of systematic reviews, QoL = quality of life, RCTs = randomized controlled trials, RECIST = Response Evaluation Criteria In Solid Tumors, REM = random-effects model, RR = risk ratio, SD = stable disease, SOX = TS-1 plus oxaliplatin, Taxol = paclitaxel, Taxotere = docetaxel, TCM = traditional Chinese medicine, TS-1 = tegafur, gimeracil and oteracil potassium capsules, TSA = trial sequential analysis, WHO = World Health Organization.
How to cite this article: Song Q, Zhang J, Wu Q, Li G, Leung EH. Kanglaite injection plus fluorouracil-based chemotherapy on the reduction of adverse effects and improvement of clinical effectiveness in patients with advanced malignant tumors of the digestive tract: A meta-analysis of 20 RCTs following the PRISMA guidelines. Medicine. 2020;99:17(e19480).
QS and JZ contributed equally to this work.
This article does not contain any studies of human participants or animals performed by any of the authors.
For this type of study, formal consent is not required.
This work is supported by the Macao Science and Technology Development Fund (Project code: 0096/2018/A3).
The authors have no conflicts of interest to disclose.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 2018;68:394–424. doi: 10.3322/caac.21492 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- [2].Committee of Experts on Rational Drug Use National Health, Family Planning Commission of the P.R. China. Guide of rational use of drug on malignant tumors in digestive tract. Chin J Rational Drug Use 2017;14:5–4. doi: 10.3969/j.issn.2096-3327.2017.09.002 [published Online First: Epub Date]. [Google Scholar]
- [3].Zhang KL, Si WT, Shao HM. Application of traditional Chinese medicine in adverse reactions of tumor chemotherapy. J Emerg Tradit Chin Med 2015;24:1212-14-3.doi: 10.3969/j.issn.1004-745X.2015.07.031 [published Online First: Epub Date]. [Google Scholar]
- [4].Liu Y, Zhang W, Wang XJ, et al. Antitumor effect of Kanglaite(R) injection in human pancreatic cancer xenografts. BMC Complement Altern Med 2014;14:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fu F, Wan Y, Wu T. Kanglaite injection combined with hepatic arterial intervention for unresectable hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther 2014;10: Suppl 1: 38–41. [DOI] [PubMed] [Google Scholar]
- [6].Zhang D, Wu J, Liu S, et al. Network meta-analysis of Chinese herbal injections combined with the chemotherapy for the treatment of pancreatic cancer. Medicine 2017;96:e7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu X, Chung VC, Lu P, et al. Chinese herbal medicine for improving quality of life among nonsmall cell lung cancer patients: overview of systematic reviews and network meta-analysis. Medicine 2016;95:e2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fanghua Q, Anyuan L, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemoor radio-therapy for cancer. Biosci Trends 2010;4:297. [PubMed] [Google Scholar]
- [9].Thorn CF, Marsh S, Carrillo MW, et al. PharmGKB summary: fluoropyrimidine pathways. Pharmacogenet Genom 2011;21:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Doosti-Irani A, Holakouie-Naieni K, Rahimi-Foroushani A, et al. A network meta-analysis of the treatments for esophageal squamous cell carcinoma in terms of survival. Crit Rev Oncol Hematol 2018;127:80–90. [DOI] [PubMed] [Google Scholar]
- [11].Zhang L, Xing X, Meng F, et al. Oral fluoropyrimidine versus intravenous 5-fluorouracil for the treatment of advanced gastric and colorectal cancer: meta-analysis. J Gastroenterol Hepatol 2018;33:209–25. [DOI] [PubMed] [Google Scholar]
- [12].Tokunaga S, Takashima T, Kashiwagi S, et al. Neoadjuvant chemotherapy with nab-paclitaxel plus trastuzumab followed by 5-fluorouracil/epirubicin/cyclophosphamide for HER2-positive operable breast cancer: a multicenter Phase II trial. Anticancer Res 2019;39:2053–9. [DOI] [PubMed] [Google Scholar]
- [13].Conteduca V, Gurioli G, Rossi L, et al. Oxaliplatin plus leucovorin and 5-fluorouracil (FOLFOX-4) as a salvage chemotherapy in heavily-pretreated platinum-resistant ovarian cancer. BMC Cancer 2018;18:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coen JJ, Zhang P, Saylor PJ, et al. Bladder preservation with twice-a-day radiation plus fluorouracil/cisplatin or once daily radiation plus gemcitabine for muscle-invasive bladder cancer: NRG/RTOG 0712 – a randomized Phase II trial. J Clin Oncol: Off J Am Soc Clin Oncol 2019;37:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 2010;28:1547–53. [DOI] [PubMed] [Google Scholar]
- [16].Schmoll H-J, Twelves C, Sun W, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol 2014;15:1481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hainsworth JD, Meluch AA, Greco FA. Paclitaxel, carboplatin, and long-term continuous 5-fluorouracil infusion in the treatment of upper aerodigestive malignancies: preliminary results of phase II trial. Semin Oncol 1997;24: Suppl 19: S19-38–s19-42. [PubMed] [Google Scholar]
- [18].Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7. [DOI] [PubMed] [Google Scholar]
- [19].Wang JC, Tian JH, Ge L, et al. Which is the best Chinese herb injection based on the FOLFOX regimen for gastric cancer? A network metaanalysis of randomized controlled trials. Asian Pac J Cancer Prev 2014;15:4795–800. [DOI] [PubMed] [Google Scholar]
- [20].Zhang D, Wu J, Duan X, et al. Network meta-analysis of Chinese herbal injections plus the FOLFOX regimen for the treatment of colorectal cancer in China. Integr Cancer Ther 2019;18: doi: 10.1177/1534735419827098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.doi: 10.1371/journal.pmed.1000097 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980;45:2220–4. [DOI] [PubMed] [Google Scholar]
- [23].Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207–14. [DOI] [PubMed] [Google Scholar]
- [24].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liang L, Yang QM. The clinical observation of kanglaite injection combined with S-1 in the treatment of recurrent or metastatic colorectal cancer. Oncol Prog 2018;16:1178–80. doi: 10.11877/j.issn.1672-1535.2018.16.09.30 [published Online First: Epub Date]. [Google Scholar]
- [26].Yu T. Efficacy evaluation of Kanglaite combined with tegafur, gimeracil and oteracil potassium in treatment of elderly patients with advanced colorectal cancer. J Clin Med 2017;4:20377–8. [Google Scholar]
- [27].Shen WX, Zhou LN, Wang LQ, et al. A randomized controlled study on Kanglaite combined with docetaxel, cisplatin, 5-fluorouracil in the treatment of patients with advanced gastric cancer. Chin J Hemorheol 2013;23:451–3. doi: 10.3969/j.issn.1009-881X.2013.03.021 [published Online First: Epub Date]. [Google Scholar]
- [28].Nanjing University of Chinese Medicine, Ma J. The Clinical Research of Coix Seed Oil Combined with FOLFOX4 for Treatment of Colorectal Cancer. 2012. [Google Scholar]
- [29].Wang XJ, Liu Y. Clinical application of Kanglaite combined with DCF chemotherapy in patients with gastric cancer. Yin Shi Bao Jian 2018;5:49–50. [Google Scholar]
- [30].Luo ZH, Kong LY. The clinical study of life quality improvement of advanced esophageal carcinoma patient by using kanglaite. Chin J Cancer Prev Treat 2001;8:418–9. [Google Scholar]
- [31].Liu H, Li JL, Wang HP. Coix seed extract combined with chemotherapy for super-long esophageal cancer. Tumor 2000;20: 78-79-2. [Google Scholar]
- [32].Liu YH. Clinical observation of Kanglaite injection combined with chemotherapy in the treatment of pain in advanced esophageal cancer. Chin J Crit Car Med 2016;36:109–10. [Google Scholar]
- [33].Zhang L, Wang YF. Effect of Kanglaite injection combined with chemotherapy on advanced gastric cancer and immune function. J Nanjing Med Univ (Nat Sci) 2010;30:1657–9. [Google Scholar]
- [34].Li XY, Shen SJ, Fan QX, et al. Combmation of Kanglaite injection and DDP-5-Fu chemotherapy in the treatment of esophageal cancer. Chin J Clin Oncol 1999;26:296–7. [Google Scholar]
- [35].Thorlund K, Imberger G, Walsh M, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis – a simulation study. PloS One 2011;6:e25491.doi: 10.1371/journal.pone.0025491 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xu RQ. Efficacy of Kanglaite injection combined with SOX regimen in the treatment of advanced gastric cancer. World Latest Med Inform (Electronic Version) 2019;19:10–1. doi: 10.19613/j.cnki.1671-3141.2019.13.005 [published Online First: Epub Date]. [Google Scholar]
- [37].Gao NN, Chen RX, Li QY. Clinical analysis of Kanglaite injection combined with SOX regimen in the treatment of advanced gastric cancer. Chin Youjiang Med J 2018;46:411–4. doi: 10.3969/j.issn.1003-1383.2018.04.010 [published Online First: Epub Date]. [Google Scholar]
- [38].Shen G, Zhang Y, Chen MB, et al. Efficacy of combined use of SOX chemotherapy regimen and Kanglaite injection in treating advanced gastric cancer. Jiangsu Med J 2017;43:919–21. doi: 10.19460/j.cnki.0253-3685.2017.13.005 [published Online First: Epub Date]. [Google Scholar]
- [39].Yao XJ. Clinical observation of Kanglaite injection combined with chemotherapy in the treatment of elderly patients with gastric cancer. J Basic Clin Oncol 2015;28:160–1. doi: 10.3969/j.issn.1673-5412.2015.02.025 [published Online First: Epub Date]. [Google Scholar]
- [40].Wang Y, Chen X, Gao XL. Clinical study on Kanglaite combined with tegafur, gimeracil and oteracil potassium in treatment of elderly patients with advanced colorectal cancer. Drugs Clinic 2015;30:65–9. doi: 10.7501/j.issn.1674-5515.2015.01.015 [published Online First: Epub Date]. [Google Scholar]
- [41].Li YH, Li B, Huang JB, et al. Clinical study of Kanglaite injection in treating advanced gastric cancer based on the changed-expression of EPOR and HMGA2. Pract Clin J Integr Tradit Chin West Med 2015;15:5–8. doi: 10.13638/j.issn.1671-4040.2015.12.002 [published Online First: Epub Date]. [Google Scholar]
- [42].Wang LR, Duan FF, Zhang Y, et al. The clinical research of Kanglaite injection combined with S-1 in advanced gastric cancer. Guide China Med 2013;11:424–5. [Google Scholar]
- [43].Lin R. The effect of Kanglaite combined with DCF chemotherapy in the treatment of advanced gastric cancer. China Pract Med 2013;8:9–11. [Google Scholar]
- [44].Zhou YZ. Efficacy of KLT combined with chemotherapy for colorectal liver metastases. China Med Pharm 2012;2:37–8+40. [Google Scholar]
- [45].Miao YJ, Wang YF. Clinical observation of Kanglaite injection combined with chemotherapy in the treatment of liver metastasis from colorectal cancer. Chin J Cancer Prev Treat 2011;18:726–7. doi: 10.16073/j.cnki.cjcpt.2011.09.026 [published Online First: Epub Date]. [Google Scholar]
- [46].Liu X, Xu F, Wang G, et al. Kanglaite injection plus chemotherapy versus chemotherapy alone for non-small cell lung cancer patients: a systematic review and meta-analysis. Curr Ther Res 2008;69:381–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schwartzberg LS, Arena FP, Bienvenu BJ, et al. A randomized, open-label, safety and exploratory efficacy study of kanglaite injection (KLTi) plus Gemcitabine versus Gemcitabine in patients with advanced pancreatic cancer. J Cancer 2017;8:1872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lu Y, Li CS, Dong Q. Chinese herb related molecules of cancer-cell-apoptosis: a minireview of progress between Kanglaite injection and related genes. J Exp Clin Cancer Res: CR 2008;27:31.doi: 10.1186/1756-9966-27-31 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang C, Hou A, Yu C, et al. Kanglaite reverses multidrug resistance of HCC by inducing apoptosis and cell cycle arrest via PI3K/AKT pathway. OncoTargets Ther 2018;11:983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [50].Li D, Xu X, Bao D, et al. Effects of kanglaite capsules combined with transcatheter arterial chemoembolization (TACE) on patients with mid or late-stage primary hepatocellular carcinoma (HCC). Chin Ger J Clin Oncol 2009;8:65–8. [Google Scholar]
- [51].Liu H, Li L, Zou J, et al. Coix seed oil ameliorates cancer cachexia by counteracting muscle loss and fat lipolysis. BMC Complement Altern Med 2019;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Beijing University of Chinese Medicine, Zhang D. Clinical Evaluation of Chinese Herbal Injection for Digestive System Tumor Based on Network Meta-analysis. 2018. [Google Scholar]
- [53].Wu ZL, Tan WJ, Pan SS, et al. Network meta-analysis of Chinese medicine injections combined with L-OHP-based chemotherapy regimen in treating colorectal neoplasms. Chin J Exp Tradit Med Formulae 2017;23:203–11. doi: 10.13422/j.cnki.syfjx.2017130203 [published Online First: Epub Date]. [Google Scholar]
- [54].Lou LL, Xie W, Zhang P, et al. Effectiveness of traditional Chinese medicine plus chemotherapy in treatment of esophageal cancer: a network meta-analysis. J Lanzhou Univ (Med Sci) 2016;42:55–60. doi: 10.13885/j.issn.1000-2812.2016.01.010 [published Online First: Epub Date]. [Google Scholar]
- [55].Lanzhou University, Wang JC. Network Meta-analysis of Chinese Herb Injection Combined with Chemotherapy for Gastric Cancer. 2014. [Google Scholar]
- [56].Ma YF, Sun X, Nian JY, et al. Meta-analysis of Kanglaite combined with chemotherapy for advanced gastric cancer. Guiding J Tradit Chin Med Pharm 2017;23:40–4. doi: 10.13862/j.cnki.cn43-1446/r.2017.15.012 [published Online First: Epub Date]. [Google Scholar]
- [57].Liao JF, Lin CX, Li ZF, et al. Meta-analysis of Kanglaite combined with chemotherapy and chemotherapy alone in the treatment of colorectal cancer. Chin J Health Stat 2017;34:620–4. [Google Scholar]
- [58].Wang C, Wang Q, He XR, et al. Meta-analysis on clinical curative effect of KLT plus chemotherapy in treatment of advanced gastric cancer. Modern J Integr Tradit Chin West Med 2011;20:3774–7. [Google Scholar]
- [59].Liu J, Yu L, Ding W. Efficacy and safety of Kanglaite injection combined with radiochemotherapy in the treatment of advanced pancreatic cancer: a PRISMA-compliant meta-analysis. Medicine 2019;98: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen Z, Lin U, Sun L, et al. Kanglaite injection combined with chemotherapy on quality of life for patients with advanced non-small cell lung cancer: a meta-analysis. Chin J Surg Oncol 2019;11: 183-89+94-8. [Google Scholar]
- [61].Zhang D, Wu J, Duan X, et al. Network meta-analysis of Chinese herbal injections plus the FOLFOX regimen for the treatment of colorectal cancer in China. Integr Cancer Ther 2019;18: doi: 10.1177/1534735419827098 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]


