Abstract
Maternal immune activation (MIA) by an infection is considered to be an important environmental factor of fetal brain development. Recent animal model on MIA induced by polyinosinic:polycytidylic acid, a mimic of viral infection, demonstrates that maternal IL-17A signaling is required for the development of autism spectrum disorder (ASD)-like behaviors of offspring. However, there is little information on bacterial infection. In this study, we aim to elucidate the influence of MIA induced by lipopolysaccharide (LPS) to mimic a bacterial infection on fetal brain development. We demonstrated that LPS-induced MIA promoted ASD-like behaviors in mouse offspring. We further found that LPS exposure induced acute phase immune response: elevation of serum IL-17A levels in MIA mothers, upregulation of Il17a mRNA expression and increase of IL-17A-producing γδ T cells in the uterus, and upregulation of Il17ra mRNA expression in the fetal brain. Blocking of IL-17A in LPS-induced MIA ameliorated ASD-like behaviors in offspring. Our data suggest that bacterial-induced maternal IL-17A pathway promotes ASD-like behaviors in offspring.
Keywords: autism spectrum disorder (ASD), IL-17A, immunology, lipopolysaccharide (LPS), maternal immune activation (MIA)
Introduction
During pregnancy, maternal immune system including innate and adaptive immune system needs to keep the balance between immunological tolerance against allogeneic fetus and immune response against invading pathogens [22]. Maternal immune activation (MIA) during pregnancy in human has been implicated an environmental risk factor for developing autism spectrum disorder (ASD) of offspring. Several epidemiological reports indicate that one of the risk factors to induce MIA is a maternal infection with pathogens [2, 7, 29].
To investigate the relationships between MIA and ASD-like behaviors of offspring, animal models of MIA induced by polyinosinic:polycytidylic acid (poly(I:C)) to mimic viral infection and lipopolysaccharide (LPS) to mimic bacterial infection have been reported [4, 10,11,12, 18, 30, 32]. MIA model to mimic viral infection has been frequently used by injecting one dose of poly(I:C) on embryonic day 12.5 (E12.5), which corresponds to the first trimester stage in human [5]. Recent MIA model by poly(I:C) injection has shown that pro-inflammatory cytokine IL-17A produced by T helper 17 (Th17) cells plays a critical role in ASD-like phenotypes of offspring [4, 10, 12] and affects fetal brain development by generating cortical patches on cerebral cortex, which is associated with ASD-like behavioral abnormalities [32]. In addition, recent reports have indicated that maternal intestinal bacteria that promote Th17 cell differentiation regulate ASD-like phenotypes of MIA offspring [10, 12]. Although there is an increasing knowledge on poly(I:C)-induced MIA, there is little evidence for the effect of LPS-induced MIA. Some reports have suggested that the resulting phenotypes of LPS-induced MIA offspring exhibit ASD-like abnormal phenotypes [11, 18, 30], but conditions for MIA induction such as dose and injection timing of LPS during gestation were not consistent among those studies. For example, the following are the MIA induction conditions used in the aforementioned studies: single LPS injection (0.1 µg/g-rats) on E9.5 [11], single LPS injection (0.25 µg/g-rats) on E15 [18], and two LPS injections (0.075 µg/g-mice) on both days on E11.5 and E12 [30]. However, the cellular and molecular mechanisms between LPS-induced MIA and abnormal behavioral phenotypes of offspring are unknown.
In this study, we aim to elucidate the influence of MIA induced by the bacterial mimetic LPS on immune responses in mothers and subsequent behavioral abnormalities in offspring. Our data showed that offspring exposed to MIA by LPS exhibited ASD-like behaviors such as communicative irregularity, repetitive behavior, and defect in social interactions. We further found that acute phase innate immune response of IL-17A was a key determinant for LPS-induced MIA and ASD-like behaviors in offspring.
Materials and Methods
Animals
The Animal Care Committee of Fukuoka Dental College approved all animal procedures used in this study. The protocol for these experiments was reviewed and approved by the committee of Ethics of Animal Experiments of Fukuoka Dental College (#18008). All mouse experiments were performed in accordance with the guidelines of the committee of Ethics of Animal Experiments of Fukuoka Dental College. C57BL/6N (B6) mice (CLEA Japan, Shizuoka, Japan) and IL-17A-GFP reporter mice (The Jackson Laboratory, Bar Harbor, ME, USA) were kept under specific pathogen-free conditions in the animal facility of Fukuoka Dental College. All animals were kept in a controlled environment with a 12 h light/dark cycle (lights on at 7:00 am). Mice were group housed (2–5 per cage) and given access to food and water ad libitum.
Maternal immune activation (MIA)
Female mice (age from 7 to 12 weeks) were mated with male mice (age from 7 to 16 weeks) overnight and checked daily for pregnancy. On embryonic day 14 (E14.0), pregnant female mice were weighted and injected intraperitoneally with a single dose of Escherichia coli LPS (Lipopolysaccharide from Escherichia coli O114:B4, Sigma, St. Lois, MO, USA) (0.05 µg/g-mice) or PBS vehicle. For cytokine blockade experiments, monoclonal anti-IL-17A blocking antibody (clone 17F3; Bio X Cell, West Lebanon, NH, USA) or isotype control antibody (IgG1a, clone MOPC-21, Bio X Cell) was administrated 8 h before MIA by LPS injection.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) from the uterus of MIA mother at 2 h post-injection of LPS or the fetal brain at 4 h post-injection of LPS. cDNA was synthesized using oligodT with the SuperScript III Reverse Transcriptase (ThermoFisher, Carlsbad, CA, USA) according to the manufacture’s protocol. qPCR was performed with CFX96 Real-Time System (BIO-RAD, Foster City, CA, USA) using the SSO advanced universal SYBR Green super mix (BIO-RAD). The primers used were as follows: 5’-CTCCAGAAGGCCCTCAGACTAC-3’ and 5’-AGCTTTCCCTCCGCATTGACACAG-3’ for Il17a; 5’-CCACTCTGTAGCACCCCAAT-3’ and 5’-CAGGCTCCGTAGTTCCTCAG-3’ for Il17ra; 5’-GGTACTGTCCCCAGGGGTAT-3’ and 5’-GAGGCCGGTTTTCATCTCCA-3’ for Il17rc; and 5’-AGGTCGGTGTGAACGGATTTG-3’ and 5’-TGTAGACCATGTAGTTGAGGTCA-3’ for gapdh. The relative expressions of Il17a, Il17ra, and Il17rc were normalized to gapdh, which was determined using 2-ΔΔCt method.
ELISA
Blood was collected by venipuncture and then serum was collected after centrifugation. IL-17A and IL-6 cytokine levels in serum were measured according to the manufacture’s protocol (BioLegend, San Diego, CA, USA). The detection limit of ELISA was 16.8 pg/ml.
Cell preparation and flow cytometry
Uterine tissue was dissected and treated enzymatically with 0.28 WU/ml Liberase (Roche, Mannheim, Germany) and 30 µg/ml DNase I (Roche) for 30 min at 37°C with mixing. Digested tissue was washed with PBS containing 5% fetal bovine serum and 5 mM EDTA, and then was incubated with the same buffer for 15 min at 37°C prior to filtration. Mononuclear cells were obtained with discontinuous 40% and 80% Percoll gradient. Mononuclear cells were stained with BV510-conjugated CD4 Ab (clone RM4-5, BD Bioscience, San Jose, CA, USA), V450-conjugated CD45 Ab (clone 30-F11, BD Bioscience), PerCp-Cy5.5-conjugated CD3 Ab (clone TC11-18H10, BD Bioscience), and PE-conjugated TCRγδ Ab (clone GL3, BD Bioscience). Flowcytometric analysis was performed on FACSVerse (BD Bioscience). All mononuclear cells were applied to flow cytometer and the cell number was counted. All data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
Ultrasonic vocalizations (USVs)
On postnatal day 8 (P8), mouse pups were habituated to testing room for 15 min and separated from their mother. Each mouse pup was placed in a clean 500 ml glass beaker (90Φ × 125 mm). Ultrasonic vocalizations (USVs) were detected for 3 min using a Pettersson M500-384 USB Ultra Sound Microphone (NHBS Ltd., Pettersson Elektronik AB, Uppsala, Sweden). USVs were recorded and measured between 33–125 kHz using UltraVox XT software (Noldus Information Technology, Wageningen, Netherlands). All pup USV calls were counted manually. Both sexes were used for the experiments.
Marble burying test
Male mice at 8 weeks of age were used in this test. After habituating on testing cage (arena size: 24 × 17.2 cm, bedding depth: 5 cm) for 20 min, the mice were placed in a testing cage containing 20 glass marbles - four rows of five marbles with equidistant distances apart. At the end of a 15 min exploration period, the mice were removed from the testing cages and the number of marbles buried was counted. A marble burying index was scored as percentage of buried marbles base on following scales: 1 for marbles covered >50% with bedding or 0 for anything less.
Three-chamber social test
12-week-old male mice were tested for social behavior using a three-chamber social test. The mice were habituated to a testing room for 15 min and were then placed in a three-chamber arena without objects for 10 min. The next day, the mice were habituated for 30 min to the testing room and placed in the center chamber without removing barriers to limit access to the left and right test areas. After removing barriers, the mice were allowed to move over to the left and right arenas for 10 min in one session. One chamber contained a social object (live B6 male mouse) and the other chamber contained an inanimate objective (black color toy). Sessions for 10 min were video-recorded, and the total distance of movement and interaction time with the objects in each chamber were measured using SMART 3.0. Video tracking software (Panlab, Harvard Apparatus, USA). Social preference was calculated as the percentage of time investigating the two objectives.
Statistics
Statistical analyses were performed using SPSS. Data were analyzed using a paired two-tailed Student’s t-test, one-way ANOVA or two-way ANOVA followed by Tukey post hoc test. Values of P<0.05 were considered significant. All data are represented as mean ± SEM.
Results
LPS-induced MIA promotes ASD-like behaviors in offspring
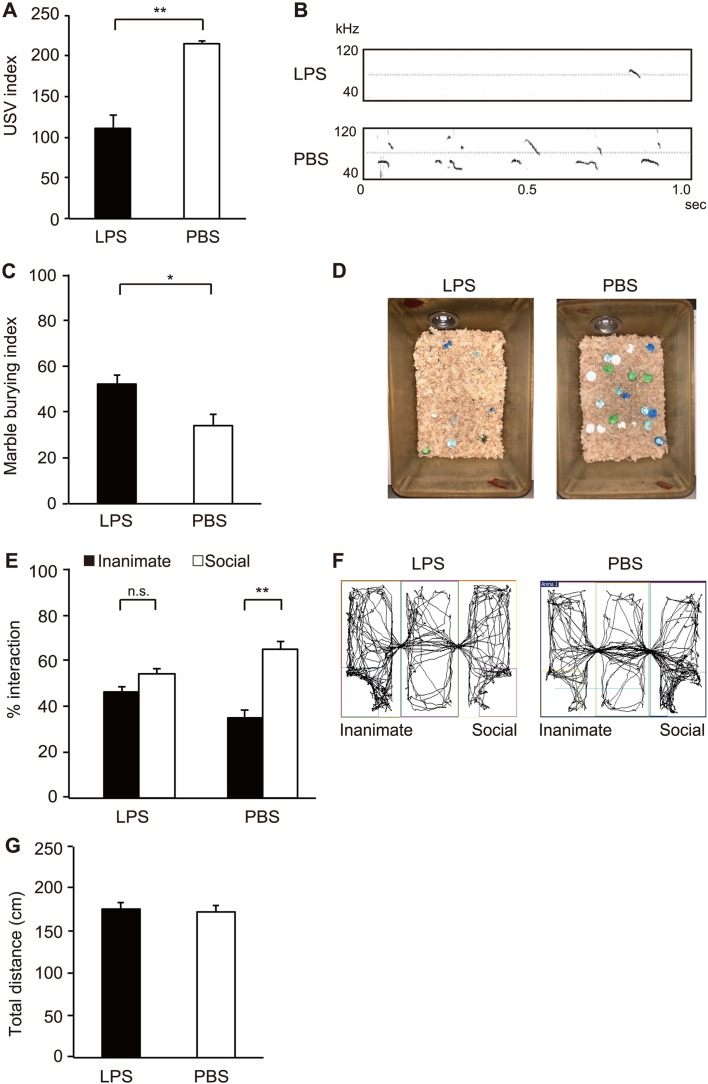
We developed the bacterial-induced MIA model by LPS derived from E. coli (Supplementary Fig. 1A). We first investigated several conditions for inducing MIA such as dosage of LPS and injection timing. High dose of LPS led to a high rate of spontaneous abortion. In addition, it has been suggested that MIA on the window from E12.5 to E14.5 may be critical for fetal brain development. We injected 0.05 µg/g-mice of LPS to pregnant mice on E14.0. After post-injection, some behavioral tests were conducted on the offspring of MIA mice. We first investigated abnormal communication in MIA pups by conducting a USV test (Figs. 1A and B). Pups from LPS-injected mothers showed reduced USV calls compared to those from PBS-injected control mothers (110.8 ± 15.8 pup calls in LPS vs 215.0 ± 3.2 pup calls in PBS, P=0.003). Next we conducted a behavioral test for MIA offspring. We used male offspring in this study because several studies have demonstrated a higher incidence of repetitive and/or restricted interests in male compared to female autism patients [8, 14, 24, 25, 28, 33]. We assessed the marble burying test to investigate repetitive and perseverative behaviors of offspring (Figs. 1C and D). Offspring from LPS-injected mothers buried more marble than those from PBS-injected mothers (52.0% ± 4.1% in LPS vs 34.0% ± 5.0% in PBS, P=0.022). We further examined social behaviors using the three chamber social test (Figs. 1E and F). Offspring from LPS-injected mothers exhibited abnormal behaviors, where they tended to be interested in inanimate compared to novel live mouse (within group: F1,74=32.24, P<0.0001 for inanimate vs social) (Inanimate 46.1% ± 2.6% vs Social 53.9% ± 2.6% in LPS, P=0.21; Inanimate 35.2% ± 3.3% vs Social 64.8% ± 3.3% in PBS, P=0.0010). We further investigated statistical difference between LPS and PBS groups by comparing the ratio of inanimate and social. Significant difference was observed between LPS and PBS groups (LPS 1.4% ± 0.2% vs PBS 2.4% ± 0.3%, P=0.013). Total distance traveled in the three-chamber social test was similar between LPS- or PBS-injected group (Fig. 1G). Moreover, the body weights of offspring from LPS- or PBS-injected group were comparable (176.1 ± 8.2 g in LPS, 172.7 ± 7.9 g in PBS). These results clearly showed that offspring from LPS-injected mothers exhibited ASD-like behaviors, such as communicative irregularities, repetitive and perseverative behaviors, and social interaction defects.
Fig. 1.
Autism spectrum disorder (ASD)-like behaviors of offspring from lipopolysaccharide (LPS)-induced maternal immune activation (MIA). (A and B) Ultrasonic vocalization (USV) assay. At P8, pups from the indicated experimental groups were separated from their mothers to elicit USV calls. The number of pup calls is plotted on the y axis [pups n=16 (LPS), n=16 (PBS); from three or four independent dams]. Statistical significance was assessed using Student’s t-test. (C and D) Marble burying test. The percentage of marbles buried is plotted on the y axis [mice n=50 (LPS), n=16 (PBS); from three to sixteen independent dams]. Statistical significance was assessed using Student’s t-test. (E and F) Three-chamber social test. Graphed as a social preference index (% time spent investigating social or inanimate stimulus out of total object investigation time) [mice n=21 (LPS), n=18 (PBS); from five to eight independent dams]. Statistical significance was assessed using two-way ANOVA with Tukey post hoc tests. (G) Total distance traveled during three-chamber social test. Statistical significance was assessed using Student’s t-test. Error bars represent SEM. *P<0.05; **P<0.01, n.s.: not significant.
LPS-induced MIA elevates serum IL-17A level, and upregulates the expression of Il17a in the uterus and Il17ra in the fetal brain
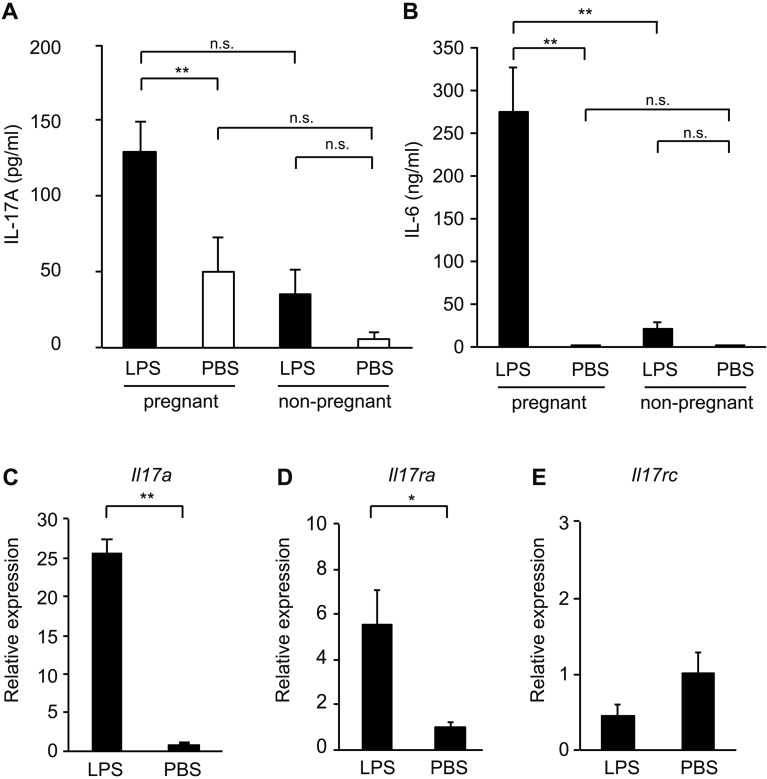
Poly(I:C)-induced MIA model shows that IL-17A signaling by Th17 cells at about E14.5 is an important factor to promote ASD-like behaviors of offspring [4]. To understand major causes of ASD-like behaviors of offspring in our bacterial-induced MIA model, we measured serum cytokines IL-17A and IL-6 by ELISA. LPS-induced MIA resulted in high levels of both IL-17A and IL-6 in pregnant mice serum at 3 h post-injection compared to PBS-injected control mice (IL-17A, F3,34=4.28, P=0.0115; IL-6, F3,26=17.25, P<0.0001) (IL-17A, 146.1 ± 20.9 pg/ml in LPS vs 31.7 ± 31.2 pg/ml in PBS, P=0.045; IL-6, 274.4 ± 51.9 ng/ml in LPS vs 0.003 ± 0.003 ng/ml in PBS, P=0.0010) (Figs. 2A and B). We also compared pregnant and non-pregnant mice to investigate whether the increase of serum cytokine levels by LPS-induced MIA is specifically observed in pregnant mice. Meanwhile, both serum IL-17A and IL-6 levels in non-pregnant mice were not significantly increased in the presence or absence of LPS (Figs. 2A and B). Serum IL-17A level was increased at 3 h post-injection of LPS and expressed low amounts at 12, 24, and 48 h (Supplementary Fig. 1B). These results indicate that LPS injection resulted in an increase of serum IL-17A at about E14.5, similar to the poly(I:C)-induced MIA model.
Fig. 2.
Lipopolysaccharide (LPS)-induced maternal immune activation (MIA) leads to elevation of IL-17A in MIA mother and upregulation of Il17ra in offspring. (A) Serum concentration of IL-17A [pregnant; n=11 (LPS), n=17 (PBS), non-pregnant; n=5 for all groups] at 3 h after LPS or PBS injection into pregnant dams at E14.0 or non-pregnant female mice. Statistical significance was assessed using one-way ANOVA with Tukey post hoc tests. (B) Serum concentration of IL-6 [pregnant; n=10 (LPS), n=10 (PBS), non-pregnant; n=5 for all groups] at 3 h after LPS or PBS injection into pregnant or non-pregnant dams at E14.0. Statistical significance was assessed using one-way ANOVA with Tukey post hoc tests. (C) Relative Il17a mRNA expression in the uterus of LPS- or PBS-injected mothers at 2 h post-injection of LPS. The relative mRNA fold change, compared with the LPS- and PBS-injected groups, is plotted on the y axis. Statistical significance was assessed using Student’s t-test. (D and E) Relative Il17ra (D) and Il17rc (E) mRNA levels in the fetal brain derived from LPS- or PBS-injected mothers at 4 h post-injection of LPS. The relative mRNA fold change, compared with the LPS- and PBS- treated groups, is plotted on the y axis. Statistical significance was assessed using Student’s t-test. Graph error bars represent SEM. *P<0.05; **P<0.01, n.s.: not significant.
We next explored the IL-17A-producing tissues in the LPS-induced MIA model. The uterus is an important female reproductive organ that protects fetal health during pregnancy; we therefore targeted the uterus to investigate Il17a mRNA expression in LPS-induced MIA. Il17a mRNA expression was significantly upregulated in the uterus of LPS-injected pregnant mice at 2 h post-injection compared to PBS-injected control mice (25.7 ± 1.6 in LPS vs 1.0 ± 0.1 in PBS, P=0.0005) (Fig. 2C). To investigate the relevant of IL-17A pathway in the LPS-induced MIA model, we examined the expression of IL-17A receptor in the fetal brain at 4 h post-injection of LPS. Increased expression of IL-17A receptor subunit A (Il17ra), but not subunit C (Il17rc), was observed in the fetal brain of LPS-injected mice compared to PBS-injected control mice (Il17ra, 5.6 ± 1.4 in LPS vs 1.0 ± 0.1 in PBS, P=0.016; Il17rc, 0.4 ± 0.1 in LPS vs 1.0 ± 0.2 in PBS, P=0.3) (Figs. 2D and E).
IL-17A-producing γδ T cells are enriched in the uterus of MIA mother
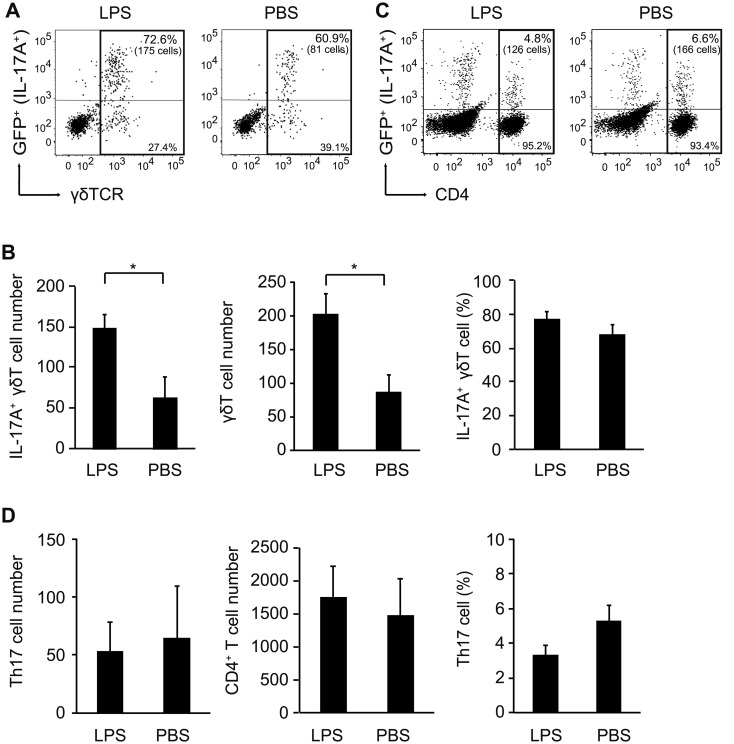
We next analyzed the cellular source of IL-17A in the uterus of MIA mother using IL-17A-GFP reporter mice in which the mice express GFP as a marker of IL-17A expression. We first confirmed serum IL-17A elevation in MIA mother by LPS injection in IL-17A-GFP reporter mice (Supplementary Fig. 2A). It has been known that IL-17A protein is produced by Th17 cells, γδ T cells, or ILC3 cells [9, 13, 15]. Acute phase of IL-17A production at 3 h in LPS-injected MIA model implicated innate immune cells stimulated with LPS rather than adaptive immune cells. Previous reports indicate that γδ T cells as innate immune cells accumulate in mucosal sites for epithelial barrier roles [1, 19] and are enriched in the uterus during pregnancy to prevent intrauterine infection [3, 20, 21]. Therefore, we first focused on γδ T cells in the uterus of LPS-injected pregnant mice. Cell numbers of γδ T cells, particularly IL-17A+ γδ T cells, were increased in the uterus of LPS-injected mice compared to PBS-injected control mice (IL-17A+ γδ T cells, 148.8 ± 17.4 cells in LPS vs 63.8 ± 25.2 cells in PBS, P=0.031; γδ T cells, 203.0 ± 29.8 cells in LPS vs 86.5 ± 25.9 cells in PBS, P=0.038), although the proportions of these cells were comparable between the two mice groups (Figs. 3A and B). In contrast, Th17 cells were comparable between LPS or PBS-injected mice in cell numbers and proportion of the cells (Figs. 3C and D). Meanwhile, almost no GFP+ cells were detected in the population of CD45+, CD3−, CD4− plus γδ TCR− cells, including ILC3 cells (Supplementary Fig. 2B). On the other hand, we could not observe any difference in γδ T cells or Th17 cells between LPS- or PBS-injected pregnant mice in the small intestine lamina propria, which were known as IL-17A-producing cells (Supplementary Figs. 2C and D). These results suggest that IL-17A-producing γδ T cells, but not Th17 cells or ILC3 cells, are significantly recruited in the uterus by LPS injection in MIA mothers.
Fig. 3.
γδ T cells but not T helper 17 (Th17) cells are increased in the uterus of lipopolysaccharide (LPS)-injected maternal immune activation (MIA) mothers. (A) Flow cytometric analysis of GFP+ (IL-17A+) cells among γδT cells (Gated as CD45+, CD3+, CD4−, TCRγδ+). Proportion and absolute cell number (in parenthesis) of IL-17A+ γδ T cells are shown. Representative data from three independent experiments are shown. (B) Total number of IL-17A+ γδ T cells (left) and γδ T cells (middle), and percentage of IL-17A+ γδ T cells among γδ T cells (right) in the uterus at 3 h post-injection of LPS. (C) Flow cytometric analysis of GFP+ (IL-17A+) cells (Th17 cells) among CD4+ T cells (Gated as CD45+, CD4+). Proportion and absolute number (in parenthesis) of Th17 cells are shown. Representative data from three independent experiments are shown. (D) Total number of Th17 cells (left) and CD4+ T cells (middle), and proportion of Th17 cells among CD4+ T cells (right) in the uterus at 3 h post-injection of LPS. Error bars represent SEM. Statistical significance was assessed using Student’s t-test (*P<0.05).
Pretreatment of LPS-induced MIA mother with IL-17A-blocking antibody ameliorates ASD-like behaviors of offspring
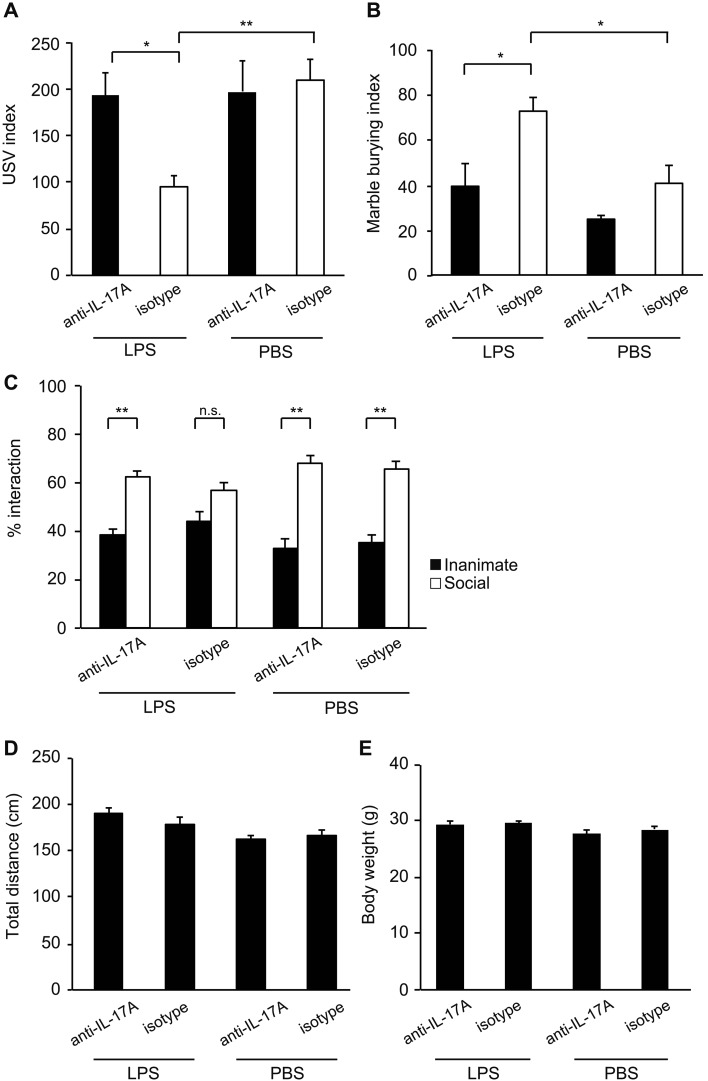
To test the relevance of IL-17A in LPS-induced ASD-like behaviors of offspring, pregnant mother mice were pre-injected with anti-IL-17A-blocking antibody before LPS injection. We evaluated the effect of IL-17A-blocking antibody on ASD-like behaviors of LPS-induced MIA offspring (Fig. 4). As expected, LPS-induced MIA resulted in reduced USV calls of pups under pretreatment with isotype control antibody (F3,76=6.09, P=0.0009) (113.7 ± 17.3 pup calls in LPS vs 191.4 ± 18.5 pup calls in PBS, P=0.002) (Fig. 4A). Pretreatment with anti-IL-17A-blocking antibody resulted in increased numbers of USV calls compared to pretreatment with isotype control antibody (178.9 ± 19.4 pup calls with anti-IL-17A vs 113.7 ± 17.3 pup calls with isotype, P=0.023) (Fig. 4A). We also tested repetitive and perseverative behaviors using the marble burying test. Although MIA offspring from LPS-injected mothers enhanced marble burying under pretreatment with isotype control antibody (72.3% ± 5.7% in LPS vs 40.6% ± 8.0% in PBS, P=0.018), pretreating with anti-IL-17A-blocking antibody rescued marble burying behavior compared to isotype control antibody (F3,46=8.40, P=0.0001) (39.2% ± 9.7% with anti-IL-17A vs 72.3% ± 5.7% with isotype, P=0.010) (Fig. 4B). Finally, we tested the effect of anti-IL-17A-blocking antibody on MIA-induced social interaction defect using the three-chamber social test. MIA offspring from LPS-injected mothers exhibited defect in social interaction (Figs. 1E and F). We found that pretreatment with anti-IL-17A-blocking antibody in LPS-injected mother rescued social behavior abnormalities of offspring, showing augmentation of interest in novel live animal compared to inanimate (within group: F3,82=115.57, P<0.0001 for inanimate vs social) (between groups: F1,41=4.67, P=0.037 for LPS vs. PBS; F1,41=1.53, P=0.223 for anti-IL-17A vs isotype; F1,41=0.28, P=0.593 for (LPS vs. PBS) × (anti-IL-17A vs. isotype)) (Inanimate 38.2% ± 2.6% vs. Social 61.8% ± 2.6% with anti-IL-17A, P=0.0010; Inanimate 44.0% ± 3.8% vs. Social 56.0% ± 3.8% with isotype, P=0.14) (Fig. 4C). We further investigated statistical difference between anti-IL-17A and isotype groups in LPS-injected mothers by comparing the ratio of inanimate and social. Although social interaction of offspring from LPS-induced mothers was rescued by anti-IL-17A-blocking antibody but not by isotype control as shown above, significant difference was not observed between two groups (Anti-IL-17A 1.7% ± 0.2% vs Isotype 1.5% ± 0.3%, P=0.553). Observed rescue of abnormal behavior by anti-IL-17A-blocking antibody was not due to the difference in arousal or activity because we confirmed offspring in all tested groups traveled a similar amount of distance in total (Fig. 4D). In addition, the body weights of offspring from the tested group were comparable (Fig. 4E). Taken together, these results indicate that IL-17A is necessary for ASD-like behaviors of offspring in LPS-induced MIA model.
Fig. 4.
IL-17A is critical for autism spectrum disorder (ASD)-like behaviors of offspring from lipopolysaccharide (LPS)-induced maternal immune activation (MIA). At E14.0, pregnant mothers were pretreated with isotype or anti-IL-17A-blocking antibody. 8 h after the pretreatment, the mothers were injected with LPS to induce MIA or with PBS. Offspring from LPS- or PBS-injected mothers at P8 were assessed for ultrasonic vocalization (USV) assay, at 8 weeks and 12 weeks were evaluated in the marble burying test and the social approach test, respectively. (A) USV assay. The number of pup calls is plotted on the y axis [pups n=17 (LPS, anti-IL-17A); n=23 (LPS, isotype control); n=18 (PBS, anti-IL-17A); n=17 (PBS, isotype control); from three or four independent dams per treatment]. Statistical significance was assessed using one-way ANOVA with Tukey post hoc tests. (B) Marble burying test. Percentage of the number of buried marbles is plotted on the y axis [mice n=12 (LPS, anti-IL-17A); n=13 (LPS, isotype control); n=14 (PBS, anti-IL-17A); n=11 (PBS, isotype control); from three or four independent dams per treatment]. Statistical significance was assessed using one-way ANOVA with Tukey post hoc tests. (C) Three-chamber social test. Graphed as a social preference index (% time spent investigating social or inanimate stimulus out of total object investigation time)[mice n=10 (LPS, anti-IL-17A); n=12 (LPS, isotype control); n=11 (PBS, anti-IL-17A); n=12 (PBS, isotype control); from three or four independent dams per treatment]. Statistical significance was assessed using two-way ANOVA with Tukey post hoc tests. (D) Total distance traveled during the three-chamber social test. (E) Body weight of offspring used in the three-chamber social test. 12 weeks old male mice were used for measuring weights. Error bars represent SEM. *P<0.05; **P<0.01, n.s.: not significant.
Discussion
There are various MIA models with wide range of protocols that vary in timing of exposure, dose, mode of delivery, and kinds of immune antigens. These differences in MIA are key factors in determining the severity of the outcome of offspring phenotypes [17]. In particular, MIA on the window from E12.5 to E14.5 during gestation may be critical stage for neurodevelopment in fetus. In fact, poly(I:C) injection on E12.5 results in strong induction of IL-17A at E14.5 that promotes ASD-like phenotypes of offspring [4, 12]. In this study, we induced MIA on E14.0 by injecting LPS and demonstrated that bacterial-induced maternal IL-17A pathway by LPS promoted ASD-like behaviors of offspring.
Serum IL-17A in pregnant mother was quickly detected in 3 h post-injection of LPS. The cytokine IL-17A plays an important role in defense against extracellular pathogens, but its dysregulation results in inflammation and tissue damage [27]. We focused on uterine tissue to identify the source of IL-17A-producing cells because uterine tissue is known to be important at the maternal-fetal interface during pregnancy. As expected, the Il17a mRNA level in the uterus of LPS-injected mothers was upregulated at 2 h post-injection. These acute phase responses of IL-17A suggest that pre-existing innate immune cells are the source of IL-17A rather than adaptive immune cells. It has been known that γδ T cells are enriched in the uterus during pregnancy to prevent intrauterine infection [3, 20, 21]. We found the augmentation of cell numbers of IL-17A-producing γδ T cells, but not its Th17 cells, in the uterus at 3 h post-injection of LPS. Our results suggested that IL-17A-producing γδ T cells in the uterus could contribute to acute phase IL-17A responses and also play a pathogenic role in MIA-induced neurodevelopmental disorders. It has been reported that γδ T cells in peritoneal cavity secrete IL-17A in Toll-like receptor (TLR)-4 dependent manner against E. coli intraperitoneal infection [23]. We proposed that IL-17A production of maternal γδ T cells including intraperitoneal cavity is promoted by LPS-induced MIA via TLR4 dependent manner and, in turn, IL-17A-producing γδ T cells are recruited to the uterus via unknown mechanism. Further studies are required to dissect the specific role of uterine γδ T cells in LPS-induced MIA. In viral mimetic poly(I:C)-induced MIA models, maternal gut bacteria with the ability to induce Th17 cells are critical for ASD-like behaviors of offspring [10]. However, we could not observe the difference in proportion of Th17 cells and γδ T cells in the small intestine lamina propria between LPS- or PBS-injected mice group. LPS and poly(I:C) are recognized by TLRs and activate intracellular signaling to secrete a multitude of pro-inflammatory cytokines (e.g., TNF, IL-6, and IL-12) and chemokines that mediate the inflammatory response to infection [16]. LPS is specifically recognized by innate immune receptor TLR4, while poly(I:C) is recognized by TLR3. We suggest that timing of exposure, dose and/or type of immune antigens, and kinds of target immune receptors may cause the difference in immune responses between LPS- and poly(I:C)-induced MIA. IL-17A targets the receptor IL-17R to trigger downstream signaling. IL-17A induces expression of Il17ra mRNA prior to recruiting IL-17Rc subunit to complete IL-17R [6, 26, 31]. Similar to poly(I:C)-induced MIA models, we also found an upregulation of Il17ra but not Il17rc in the fetal brain at 4 h post-injection in the LPS-induced MIA model. The IL-17A-IL-17R signaling axis could also adversely impact the fetal brain development and may be associated with ASD-like behaviors of offspring in LPS-induced MIA. Choi et al. [4] demonstrated that injection of recombinant IL-17A into the ventricles of the developing brain induces MIA-associated phenotypes of offspring. However, there is no direct evidence that maternal IL-17A is transferred into the fetal brain via blood brain barrier. We need further studies to reveal this question. Notably, the relevance of IL-17A pathway in LPS-induced MIA model was further supported by anti-IL-17A-blocking antibody treatments, which reduced the severity of ASD-like behaviors of offspring. Our data clearly showed that maternal IL-17A was a critical factor for ASD-like behaviors of offspring not only in poly(I:C)-induced MIA but also in LPS-induced MIA. An increase of serum IL-17A level by LPS-induced MIA was observed in pregnant mice but not in non-pregnant mice. These results suggested that pregnancy is required for maternal serum IL-17A increase that induces ASD-like behaviors of offspring.
In conclusion, we demonstrated, for the first time to our knowledge, that IL-17A is a key immune mediator in LPS-mediated MIA, which contributes to inducing ASD-like behaviors of offspring. IL-17A-mediated inflammatory responses against an infection of bacteria containing LPS, gram-negative bacteria, during pregnancy may be a risk factor for neurodevelopmental disorders such as ASD in children.
Supplementary Material
Acknowledgments
This work was supported in part by grants from Japan Society for the Promotion of Science KAKENHI Grant Number JP18K09546 (to J.N.), JP17H04371, JP18K19657 and JP19H04819 (to Y.T.); Astellas Research Support (to Y.T.); TERUMO FOUNDATION for LIFE SCIENCES and ARTS (to Y.T.); Private University Research Branding Project (to J.N. and Y.N.). This work was partly supported by the Cooperative Research Project Program of the Medical Institute of Bioregulation, Kyushu University and Joint Usage/Research Center at the Institute of Development, Aging and Cancer, Tohoku University.
References
- 1.Akitsu A., Iwakura Y.2018. Interleukin-17-producing γδ T (γδ17) cells in inflammatory diseases. Immunology 155: 418–426. doi: 10.1111/imm.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atladóttir H.O., Thorsen P., Østergaard L., Schendel D.E., Lemcke S., Abdallah M., Parner E.T.2010. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 40: 1423–1430. doi: 10.1007/s10803-010-1006-y [DOI] [PubMed] [Google Scholar]
- 3.Cai D., Tang Y., Yao X.2019. Changes of γδT cell subtypes during pregnancy and their influences in spontaneous abortion. J. Reprod. Immunol. 131: 57–62. doi: 10.1016/j.jri.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 4.Choi G.B., Yim Y.S., Wong H., Kim S., Kim H., Kim S.V., Hoeffer C.A., Littman D.R., Huh J.R.2016. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351: 933–939. doi: 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy B., Finlay B.L., Darlington R.B., Anand K.J.2007. Extrapolating brain development from experimental species to humans. Neurotoxicology 28: 931–937. doi: 10.1016/j.neuro.2007.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ely L.K., Fischer S., Garcia K.C.2009. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 10: 1245–1251. doi: 10.1038/ni.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estes M.L., McAllister A.K.2016. Maternal immune activation: Implications for neuropsychiatric disorders. Science 353: 772–777. doi: 10.1126/science.aag3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattier M.A., Matson J.L., Tureck K., Horovitz M.2011. The effects of gender and age on repetitive and/or restricted behaviors and interests in adults with autism spectrum disorders and intellectual disability. Res. Dev. Disabil. 32: 2346–2351. doi: 10.1016/j.ridd.2011.07.028 [DOI] [PubMed] [Google Scholar]
- 9.Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R.2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133. doi: 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- 10.Kim S., Kim H., Yim Y.S., Ha S., Atarashi K., Tan T.G., Longman R.S., Honda K., Littman D.R., Choi G.B., Huh J.R.2017. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549: 528–532. doi: 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirsten T.B., Chaves-Kirsten G.P., Chaible L.M., Silva A.C., Martins D.O., Britto L.R., Dagli M.L., Torrão A.S., Palermo-Neto J., Bernardi M.M.2012. Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. J. Neurosci. Res. 90: 1903–1912. doi: 10.1002/jnr.23089 [DOI] [PubMed] [Google Scholar]
- 12.Lammert C.R., Frost E.L., Bolte A.C., Paysour M.J., Shaw M.E., Bellinger C.E., Weigel T.K., Zunder E.R., Lukens J.R.2018. Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. J. Immunol. 201: 845–850. doi: 10.4049/jimmunol.1701755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G.2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J. Exp. Med. 205: 1381–1393. doi: 10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandy W., Chilvers R., Chowdhury U., Salter G., Seigal A., Skuse D.2012. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J. Autism Dev. Disord. 42: 1304–1313. doi: 10.1007/s10803-011-1356-0 [DOI] [PubMed] [Google Scholar]
- 15.Manel N., Unutmaz D., Littman D.R.2008. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 9: 641–649. doi: 10.1038/ni.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov R., Janeway C.A., Jr1998. Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 10: 351–353. doi: 10.1006/smim.1998.0136 [DOI] [PubMed] [Google Scholar]
- 17.Meyer U.2014. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 75: 307–315. doi: 10.1016/j.biopsych.2013.07.011 [DOI] [PubMed] [Google Scholar]
- 18.Oskvig D.B., Elkahloun A.G., Johnson K.R., Phillips T.M., Herkenham M.2012. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav. Immun. 26: 623–634. doi: 10.1016/j.bbi.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papotto P.H., Ribot J.C., Silva-Santos B.2017. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18: 604–611. doi: 10.1038/ni.3726 [DOI] [PubMed] [Google Scholar]
- 20.Pinget G.V., Corpuz T.M., Stolp J., Lousberg E.L., Diener K.R., Robertson S.A., Sprent J., Webster K.E.2016. The majority of murine γδ T cells at the maternal-fetal interface in pregnancy produce IL-17. Immunol. Cell Biol. 94: 623–630. doi: 10.1038/icb.2016.48 [DOI] [PubMed] [Google Scholar]
- 21.Polese B., Gridelet V., Perrier d’Hauterive S., Renard C., Munaut C., Martens H., Vermijlen D., King I.L., Jacobs N., Geenen V.2018. Accumulation of IL-17+ Vγ6+ γδ T cells in pregnant mice is not associated with spontaneous abortion. Clin. Transl. Immunology 7: e1008. doi: 10.1002/cti2.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.PrabhuDas M., Bonney E., Caron K., Dey S., Erlebacher A., Fazleabas A., Fisher S., Golos T., Matzuk M., McCune J.M., Mor G., Schulz L., Soares M., Spencer T., Strominger J., Way S.S., Yoshinaga K.2015. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat. Immunol. 16: 328–334. doi: 10.1038/ni.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata K., Yamada H., Hara H., Kishihara K., Yoshikai Y.2007. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178: 4466–4472. doi: 10.4049/jimmunol.178.7.4466 [DOI] [PubMed] [Google Scholar]
- 24.Sipes M., Matson J.L., Worley J.A., Kozlowski A.M.2011. Gender differences in symptoms of autism spectrum disorders in toddlers. Res. Autism Spectr. Disord. 5: 1465–1470. doi: 10.1016/j.rasd.2011.02.007 [DOI] [Google Scholar]
- 25.Szatmari P., Liu X., Goldberg J., Zwaigenbaum L., Paterson A.D., Woodbury-Smith M., Georgiades S., Duku E., Thompson A.2012. Sex differences in repetitive stereotyped behaviors in autism: Implications for genetic liability. Am. J. Med. Genet Part B. 159: 5–12. doi: 10.1002/ajmg.b.31238 [DOI] [PubMed] [Google Scholar]
- 26.Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., Tocker J., Peschon J.2006. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177: 36–39. doi: 10.4049/jimmunol.177.1.36 [DOI] [PubMed] [Google Scholar]
- 27.Veldhoen M.2017. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 18: 612–621. doi: 10.1038/ni.3742 [DOI] [PubMed] [Google Scholar]
- 28.Werling D.M., Geschwind D.H.2013. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26: 146–153. doi: 10.1097/WCO.0b013e32835ee548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong H., Hoeffer C.2018. Maternal IL-17A in autism. Exp. Neurol. 299:(Pt A): 228–240. doi: 10.1016/j.expneurol.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xuan I.C., Hampson D.R.2014. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS One 9: e104433. doi: 10.1371/journal.pone.0104433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.M., Painter S.L., Comeau M.R., Cohen J.I., Spriggs M.K.1995. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3: 811–821. doi: 10.1016/1074-7613(95)90070-5 [DOI] [PubMed] [Google Scholar]
- 32.Shin Yim Y., Park A., Berrios J., Lafourcade M., Pascual L.M., Soares N., Yeon Kim J., Kim S., Kim H., Waisman A., Littman D.R., Wickersham I.R., Harnett M.T., Huh J.R., Choi G.B.2017. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549: 482–487. doi: 10.1038/nature23909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwaigenbaum L., Bryson S.E., Szatmari P., Brian J., Smith I.M., Roberts W., Vaillancourt T., Roncadin C.2012. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. J. Autism Dev. Disord. 42: 2585–2596. doi: 10.1007/s10803-012-1515-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.