Abstract
Calcific aortic valve disease (CAVD) represents a significant threat to cardiovascular health worldwide, and the incidence of this sclerocalcific valve disease has rapidly increased along with a rise in life expectancy. Compelling evidence has suggested that CAVD is an actively and finely regulated pathophysiological process even though it has been referred to as "degenerative" for decades. A striking similarity has been noted in the etiopathogenesis between CAVD and atherosclerosis, a classical proliferative sclerotic vascular disease.1 Nevertheless, pharmaceutical trials that attempted to target inflammation and dyslipidemia have produced disappointing results in CAVD. While senescence is a well-documented risk factor, the sophisticated regulatory networks have not been adequately explored underlying the aberrant calcification and osteogenesis in CAVD. Valvular endothelial cells (VECs), a type of resident effector cells in aortic leaflets, are crucial in maintaining valvular integrity and homeostasis, and dysfunctional VECs are a major contributor to disease initiation and progression. Accumulating evidence suggests that VECs undergo a phenotypic and functional transition to mesenchymal or fibroblast-like cells in CAVD, a process known as the endothelial-to-mesenchymal transition (EndMT) process. The relevance of this transition in CAVD has recently drawn great interest due to its importance in both valve genesis at an embryonic stage and CAVD development at an adult stage. Hence EndMT might be a valuable diagnostic and therapeutic target for disease prevention and treatment. This mini-review summarized the relevant literature that delineates the EndMT process and the underlying regulatory networks involved in CAVD.
Keywords: Calcific aortic valve disease, Endothelial-to-mesenchymal transition, Regulatory network, Valvular endothelial cell
INTRODUCTION
Calcific aortic valve disease (CAVD) affects nearly 3% of people older than 75 years in the United States and the incidence is also increasing in China.2 This entity of aortic valve pathology develops progressively from initial valve thickening to the end-stage formation of calcification nodules.3,4 The affected aortic valve malfunctions to maintain stable hemodynamics between the left ventricle and ascending aorta, presenting as varying degrees of aortic valve stenosis and/or insufficiency, left ventricle pressure overload and hypertrophy, ascending aorta dilatation and aneurysm and even aortic dissection.5 Patients with CAVD commonly have chest tightness and pain, dyspnea and syncope, which can lead to congestive heart failure and sudden cardiac death (SCD).6 At present, the only curable approaches are invasive therapeutic strategies including surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI) which radically replace the diseased cusps with a mechanical or bioprosthetic valve.7,8 Even though a successful SAVR or TAVI improves the long-term prognosis, postprocedural outcomes are still complicated by major adverse cardiovascular and cerebrovascular events (MACCEs).9 Improper postoperative antithrombotic therapy after valve replacement can lead to catastrophic ischemic or hemorrhagic events,10 and the invasive nature of valve replacement surgery inevitably results in more trauma and medical expense.11 In addition, mechanical/bioprosthetic valve and artificial blood vessels can, to a great extent, simulate natural blood flow across the valve. However, the physiological hemodynamics of the aortic root is not entirely restored, and the anatomical structure of aortic sinuses remains incompletely reconstructed.12 In addition, the bioprosthetic valves are still at risk of calcification and osteogenesis.13 Making an early diagnosis and providing effective medical therapy for CAVD remain a huge challenge.14 Hence, early diagnostic markers and pharmacological targets are urgently required in the prevention and treatment of CAVD.15
CAVD is generally believed to be an actively and finely regulated biological process in which specific stimulators activate and perpetuate calcific and osteogenic programs.14 Although intensive investigations have recently focused on this field, the underlying mechanism remains largely elusive.3 Aging is obviously a risk factor for CAVD, and it might partially account for the increasing prevalence of CAVD.16 In particular, atherosclerosis and CAVD share similar pathophysiological events, and accumulating evidence suggests an overlap of mechanisms that include inflammation, dyslipidemia and oxidative stress.17 However, clinical trials testing statins for CAVD have produced disappointing results, even though statins have long been recognized as the cornerstone in the pharmaceutical treatment of atherosclerosis.18 Besides, while the role of intimal calcification is still under debate in promoting plaque instability, it is generally accepted that calcification compromises the functional and structural properties of aortic leaflets.14 This indicates that the currently known mechanisms may account for only a fraction of the susceptibility to CAVD.
Very recently, endothelial-to-mesenchymal transition (EndMT) has been shown to play a role in the pathogenesis of CAVD.19 In this transition, endothelial cells progressively acquire the phenotypic and functional characteristics of mesenchymal cells and express both endothelial and mesenchymal cell markers.20 Numerous reports have demonstrated that the EndMT and epithelial-to-mesenchymal transition (EMT) are key regulators in common human diseases including cancer, kidney fibrosis and cardiovascular diseases.21,22 In particular, the EndMT process has been associated with various cardiovascular disorders including atherosclerosis, pulmonary hypertension, cardiac fibrosis, myocardiopathy and bypass graft restenosis.23 The EndMT process is crucial in valve development at the embryonic stage, and adult valvular endothelial cells (VECs) might also re-experience this cellular reprogramming to contribute to the abnormal stiffening and calcification of aortic valve leaflets.24,25 Several studies have focused on the relationship between EndMT and CAVD and identified the related stimulants, cellular transition features and underlying signaling pathways.19,25,26 In this mini-review, we collected the relevant literature to: 1) summarize the known knowledge of EndMT in CAVD; 2) describe our hypothesis of the role of EndMT in CAVD progression; and 3) suggest several key questions that warrant intensive investigations in the future.
BASIC KNOWLEDGE OF AORTIC VALVE HISTOLOGY
Aortic valve leaflets are usually comprised of three distinct extracellular matrix (ECM) layers including the fibrosa, ventricularis and spongiosa. This highly organized "sandwich" architecture plays a pivotal role in maintaining the normal functions of aortic valves. The fibrosa is rich in collagen and is situated adjacent to the aorta, whereas the elastin-rich ventricularis lies close to the left ventricle. A middle spongiosa layer rich in glycosaminoglycans is located between the fibrosa and ventricularis.3 The fibrosa/aortic aspect and ventricularis/ventricular aspect of aortic leaflets are differentially affected in CAVD, most likely due to distinct models of shear stress. The fibrosa is more prone to calcification, with calcific nodules mainly forming in the base of the aortic aspect.3,27 The whole disease progression evolves from initial thickening, subsequent fibrosis, and then calcific nodules which ultimately influence the opening and closing of leaflets.
VALVULAR ENDOTHELIAL CELLS AND VALVULAR INTERSTITIAL CELLS
VECs and valvular interstitial cells (VICs) are two resident effector cells in aortic cusps that are highly heterogeneous cell populations. VICs and VECs, in a physiological state, contribute synergistically to valve homeostasis and structure integrity.28,29 Once aberrantly or excessively activated, these two types of cells can transform from guardians of valve health to culprits of diseased valves. Current evidence has indicated that VICs and VECs might orchestrate a complex interplay in the pathological initiation and progression of CAVD, and that VICs might also fine-tune the EndMT process in CAVD.25
VALVULAR ENDOTHELIAL CELLS
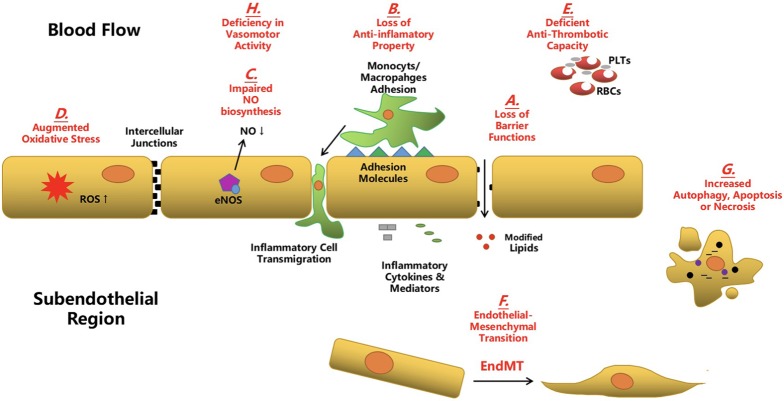
VECs mainly line the outmost surface of leaflets of both the aortic and ventricular aspects. In line with the role of vascular endothelial cells (ECs) in atherosclerosis, the integrity of VECs is crucial in the maintenance of valve physiology and function. Anomalies in VECs, including activation, dysfunction and even transformation, have been demonstrated to be substantially responsible for CAVD pathogenesis.29 From the standpoint of atherosclerosis, once ECs are aberrantly activated, they lose their protective and regulative mechanisms for vascular homeostasis and integrity, which means: 1) the loss of polarity; 2) impairment of barrier function; 3) deficiency of anti-inflammatory, anti-thrombotic and anti-oxidative capacities; and 4) decline in the production of nitric oxide (NO).25,30 As a consequence, perturbations in EC physiology lead to: 1) the leakage of cell junction and infiltration of modified lipids into vascular media; 2) adhesion and transmigration of circulating inflammatory cells into vessel walls; 3) descending regulation of vasodilation and vasoconstriction; and 4) vascular inflammation and thrombus formation.31 In addition, ECs lining the vascular endothelium might further experience decreased cellular proliferation and augmented apoptosis, autophagy and even necrosis if atherogenic stresses persist.32 Very recently, several reports have highlighted the role of the EndMT process in the initiation and progression of atherosclerosis.33 During plaque development, ECs lose their specific markers and acquire a mesenchymal or myofibroblastic phenotype, and this transition has been reported to accelerate vulnerable plaque progression and increase clinical coronary events.14 Similarly for CAVD, VEC activation and dysfunction has been linked with: 1) impaired NO generation; 2) increased oxidative stress; 3) enhanced pro-inflammatory pathways; and 4) the EndMT process.14 Additionally, previous studies have identified several systemic or localized stimulators/risk factors that can cause VEC anomalies in CAVD, including inflammation, oxidative stress, dyslipidemia, diabetes and hyperphosphatemia.3,16,34 However, the detailed mechanisms and similarities/differences between vascular EC and VEC dysfunction in response to environmental stimuli have yet to be elucidated. Compared to VICs, the involvement of VECs in CAVD has been less convincingly verified and extensively studied. Very recently, an inhibitory role of activated VICs in regulating the EndMT process of VECs has been reported, suggesting that further studies on the reciprocal interactions between VECs and VICs in the pathogenesis of CAVD are worthwhile (Figure 1).
Figure 1.
Aberrant endothelial cells in atherosclerosis and calcific aortic valve disease. During the development of atherosclerosis and calcific aortic valve disease (CAVD), aberrant endothelial cells (ECs) disturb markedly the micro-environmental homeostasis of cardiovascular system and contribute greatly to the disease progression. (A) Endothelial dysfunction leads to the loss of barrier function and results in the infiltration of modified lipids to the subendothelial region. (B) Dysfunctional ECs transfer from an anti-inflammatory phenotype to a pro-inflammatory phenotype. Particularly, an increased expression of adhesion molecules and pro-inflammatory cytokines and mediators by these ECs promote the adhesion and subsequent transmigration of circulating immune cells to the subendothelial region. (C) The bio-synthesis and release of nitric oxide (NO) is compromised in the dysfunctional ECs and it impedes the physiological functions of ECs. (D) An array of pathological environmental stimuli induces the augmented oxidative stress in the aberrant ECs. (E) The anti-thrombotic property of ECs is deficient in the dysfunctional ECs. (F) Accumulating evidence suggests that the aberrant ECs undergo the endothelial-to-mesenchymal transition (EndMT) during which these ECs co-express the endothelial and mesenchymal markers and acquire the mesenchymal phenotypic and functional properties. (G) Persistent or potent pathological stimulators cause the increased level of autophagy and apoptosis and even the abnormal necrosis of ECs. (H) Dysfunctional ECs malfunction to regulate properly the vasomotor activity.
VALVULAR INTERSTITIAL CELLS
VICs are ubiquitously distributed in all of the three "sandwich" ECM layers. This group of mesenchymal cells has recently been shown to dynamically mediate complex valvular alterations, both physiologically and pathologically.28 Their roles are to a certain extent akin to those of vascular smooth muscle cells and fibroblasts resident in the vascular wall or atherosclerotic plaques. Within healthy adult aortic valves, VICs mostly resemble quiescent fibroblasts, called quiescent VICs (qVICs), and are primarily responsible for balancing the production and degradation of the ECM and various types of collagen. Once stimulated, qVICs are reprogrammed to differentiate into activated myofibroblast-like VICs (aVICs) and regulate the functional remodeling of valve ECM and collagen. In addition, aVICs are also thought to transform back to qVICs if physiological stimulation recedes or terminates.35 This dynamic conversion of qVICs and aVICs plays a key role in sustaining normal valve remodeling and function. Nevertheless, if pathological stimulation occurs or physiological stimulation perpetuates, aVICs can further adapt themselves into an osteoblastic phenotype (oVICs), the chief culprit of calcium deposition in CAVD.36 oVICs can enhance the expression of metalloproteinases (MMPs) and proinflammatory cytokines and mediators that promote pathological remodeling of valve tissue.35,37 Thus, a heterogeneity of VIC subpopulations existduring disease progression, and studying how this heterogeneity predisposes to CAVD will become a research hotspot in the near future.25 Recent investigations have suggested the effects of VECs on the proliferative and osteogenic activation of VICs, indicating the relevance of VEC-VIC interactions in determining the fate of VICs.25,38,39
BASIC CONCEPT AND KNOWLEDGE OF EndMT
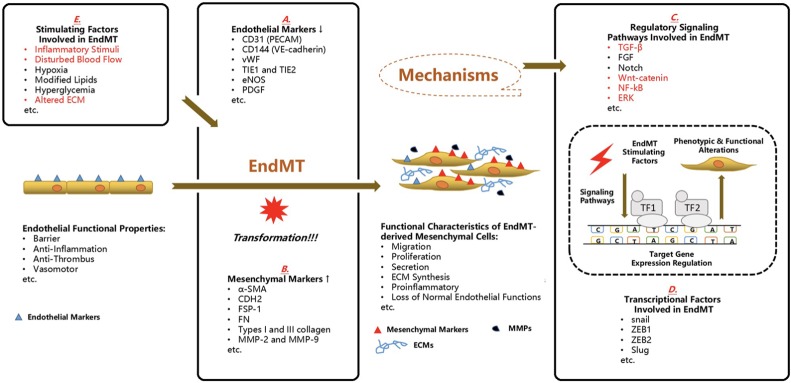
EndMT is generally considered to be a specialized form of EMT, but it has unique characteristics compared to EMT.40 EndMT represents a cellular reprogramming process in which ECs acquire mesenchymal cell markers and features upon certain stimulations. Via EndMT, ECs act like mesenchymal cells by regaining mesenchymal phenotype characteristics of proliferative, migratory, secretory and productive features with a simultaneous loss of specific endothelial markers and functions.41 Positive staining of both mesenchymal markers and endothelial markers in stimulated ECs is a landmark of EMT, as well as a switch of cellular shape from a pyramidal to spindle type (Figure 2).
Figure 2.
Endothelial-to-mesenchymal transition in the cardiovascular diseases. It has gained increasing attention that endothelial cells (ECs) experience the endothelial-to-mesenchymal transition (EndMT) in the evolution of common cardiovascular diseases including calcific aortic valve disease (CAVD) and this cellular reprogramming is potentially utilized as a novel diagnostic signature and therapeutic target. Under physiological conditions, ECs undertake a fundamental role in maintaining the homeostasis and integrity of cardiovascular architecture and their common duties include barrier, anti-inflammation, anti-thrombus and vasomotor. However, once the EndMT process is initiated, the normal functions of ECs are aberrantly interfered and progressively deprived. Furthermore, these transformed ECs gain the mesenchymal phenotypic characteristics such as proliferation, migration, secretion, extracellular matrix (ECM) synthesis and above all, pro-inflammatory capacities. In the process of EndMT, the cells co-expressing endothelial markers and mesenchymal markers is a hallmark. And in these cells, the listed endothelial markers (A) are down-regulated whereas the expression of mesenchymal markers (B) are enhanced. This cellular transition is launched by certain environmental stimuli, after which several well-documented signaling pathways (C) have been linked with the regulation of expression of certain target genes, on the transcriptional level by transcription factors (TF) (D), in most cases. (The red ones have been associated with the EndMT process in CAVD.) Several stimulators (E) have been correlated with the induction of EndMT. (The red ones have been reported in the EndMT process in CAVD). CD31, cluster of differentiation 31; CD144, cluster of differentiation 144; CDH2, N-cadherin; ECM, extracellular matrix; ECMs, extracellular matrixs; EndMT, endothelial-to-mesenchymal transition; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated protein kinases; FGF, fibroblast growth factor; FN, fibronectin; FSP-1, fibroblast-specific protein-1; MMPs, matrix metalloproteinases; MMP-2, matrix metalloproteinase 2; MMP-9, matrix metalloproteinase 9; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; PDGF, platelet derived growth factor; PECAM, platelet endothelial cell adhesion molecule; TF1, transcription factor 1; TF2, transcription factor 2; TIE1, tyrosine kinase with immunoglobulin-like and EGF-like domains 1; TIE2, tyrosine kinase with immunoglobulin-like and EGF-like domains 2; VE-cadherin, vascular endothelial cadherin; vWF, von willebrand factor; ZEB1, zinc finger E-box-binding homeobox 1; ZEB2, zinc finger E-box-binding homeobox 2; α-SMA, alpha-smooth muscle actin; TGF-β, transforming growth factor-β.
ALTERATIONS OF MOLECULAR MARKERS AND TRANSCRIPTION FACTORS IN EndMT
During EndMT, there is a decline in the expression of endothelial markers. These endothelial markers commonly include PECAM (CD31), vWF, VE-cadherin (CD144), tyrosine kinase with immunoglobulin-like and EGF-like domains 1 and 2 (TIE1 and TIE2), eNOS and platelet-derived growth factor (PDGF). The most commonly used mesenchymal markers include alpha-smooth muscle actin (a-SMA), N-cadherin (CDH2), calponin, fibroblast-specific protein-1 (FSP-1), vimentin, fibronectin (FN), collagen types I and III, and matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9 (MMP-9). In particular, a frequently used EMT marker, E-cadherin (CDH1), is not expressed in ECs. In addition, epithelial surface markers such as claudins, occludins and cytokeratin are not usually used in studies of EndMT. Cells co-expressing epithelial/endothelial and mesenchymal markers can be regarded as indirect evidence of EMT/EndMT because it might also be the consequence of a reversed process, mesenchymal-to-epithelial/endothelial transition22,42 (Figure 2A and B).
EndMT is "powered" by the strength of transcription regulation, and a series of transcription factors (TFs) have been noted to modulate EndMT once the process is initiated. The commonly involved TFs include snail, ZEB1, ZEB2 and Slug, which play key roles in the transcriptional control of the expression of endothelial and mesenchymal markers22,23 (Figure 2C and D).
EndMT-RELATED DISEASES
Decades of research have correlated EMT/EndMT with various types of common human diseases, including malignant cancers, cardiovascular diseases, transplant arteriopathy, fibrodysplasia ossificans progressiva and organ fibrosis. In the field of cardiovascular disorders, EndMT has been confirmed to contribute to atherosclerosis, myocardial fibrosis due to diabetes, infarction or pulmonary hypertension, pulmonary hypertension, graft vessel remodeling after coronary artery bypass grafting, vascular malformation and calcification, as well as CAVD. These cardiovascular diseases can be categorized as fibrotic diseases, suggesting that EndMT might be a new target and a common mechanism for the prevention and treatment of these diseases. EndMT in CAVD has been relatively less intensively studied, and it is considered to be a promising research hotspot in exploring the remodeling of valve structure except for canonical theories such as chronic inflammation and dyslipidemia.43
EndMT-RELATED ENVIRONMENTAL STIMULI IN CARDIOVASCULAR DISEASES
In adults, EndMT and EMT have to be launched and augmented by environmental stimuli that are persistently pathological. Inflammatory stimuli are amongst the strongest inducers of the EndMT process, and include TNF-α, IL-6, IFN-γ and lipopolysaccharide (LPS). These inflammatory mediators can promote EndMT alone or synergistically. TGF-β is also a potent stimulating factor for EndMT, and TGF-β signaling has been reported to be one of the most important signaling pathways in the EndMT process.42,44 In addition, disturbed blood flow has been demonstrated to contribute to the occurrence of EndMT. Several studies have shown that oscillatory flow induces harmful shear stress and facilitates the initiation and progression of EndMT in certain locations of the cardiovascular system.45 Typical locations are arterial bifurcations such as the orifice of supra-aortic arteries, lesser curvature of the aorta, as well as the aortic aspect of aortic valves.19,46 Conversely, laminar flow seems to have a protective role against EndMT. For example, compared to lesser curvature of the aorta, greater curvature of the aorta has been proven to be an atherosclerotic-proof area.46 In addition, hypoxia, modified lipids and hyperglycemia have been shown to drive the process of EndMT individually.43 Furthermore, EndMT might also be induced by altered ECM in aortic valve ECs, as shown in an in vitro model of CAVD26 (Figure 2E).
PIVOTAL SIGNALING PATHWAYS REGULATING EndMT
Among all of the related signaling pathways regulating EndMT, TGF-β is the most frequently mentioned. It is a major regulator of fibroproliferative disease and has been extensively investigated in EndMT/EMT. Three TGF-β members, TGF-β1, -2 and -3 act through type I and II TGF-β receptors to form multimeric complexes and subsequently launch the downstream Smad-dependent and Smad-independent signaling pathways.47 The fibroblast growth factor signaling pathway is crucial in modulating EC physiology, and it has been recently been demonstrated to be a vital negative regulator for EndMT and to be impeded by inflammatory stimuli and shear stress in atherosclerotic plaques.46,48 Another significant pathway is the Notch signaling pathway, which plays key roles in cell differentiation, proliferation and apoptosis. It can mediate EndMT by fine-tuning the expression of EndMT-related transcriptional factors.49 Several other signaling pathways involved in EndMT include Wnt-catenin pathway, Akt/NF-kB and microRNAs19,20,50-52 (Figure 2C and D).
EndMT IN ATHEROSCLEROSIS
In atherosclerosis, vascular ECs undergo the EndMT process upon stimulation by hemodynamic shear stress and pro-inflammatory signals, and EndMT is involved in the whole course of disease from initial stable plaques to end-stage vulnerable plaques.33 In addition, the EndMT process has also been linked with disease severity, with the severity being directly proportional to human coronary remodeling and plaque instability.46 Kim and colleagues first demonstrated in delayed coronary artery disease (CAD) after chest radiotherapy that radiation can induce the EndMT process, which can then be reinforced by oxidized low-density lipoprotein (ox-LDL).53 Recently a group led by Chen showed the pivotal role of the FGF signaling pathway in the inhibition of the TGF-β signaling pathway and regulation of EndMT. In vitro evidence has suggested that inflammatory cytokines promote the EndMT process and stimulate the TGF-β signaling pathway by down-regulating the expression of FGFR1. Additionally, it has been reported that conditional knockout (CKO) of Fgfr1 in Apoe-/- mice can facilitate the development of both early and advanced atherosclerotic plaques and accentuate conversion from the stable stage to the vulnerable stage. More importantly, the severity of human coronary disease has been positively correlated to the expression of markers of the TGF-β signaling pathway and the extent of EndMT.46 Kovacic and colleagues presented their findings using an endothelial-tracking system in a mice model in which EC-derived fibroblast-like cells were a significant source of mesenchymal cells in atherosclerotic plaques. In addition, in human atherosclerotic plaques, the extent of EndMT has been proven to be more serious in vulnerable plaques and ruptured plaques. Moreover, they proposed the role of EndMT in driving plaque evolution by disturbing ECM homeostasis between collagen production and MMP degradation.54 Subsequent to myocardial infarction, the infarcted region undergoes remodeling and fibrosis, and Hatzopoulos et al. reported that ECs in the infarcted region also experienced the EndMT process, which was mediated by Wnt-catenin signaling pathway.50 Coronary artery bypass grafting (CABG) is still the standard of care for CAD, especially in multivessel and complicated coronary lesions. However, the mid- and long-term patency of saphenous vein grafts (SVGs) remains unsatisfactory, with a reported rate of graft failure being more that 50% at 10 years of follow-up. Boehm et al. also reported that the EndMT process contributes to neointimal formation and SVG restenosis mainly via the TGF-β-Smad2/3-Slug signaling pathway.55
EndMT IN CALCIFIC AORTIC VALVE DISEASE
Current evidence suggests that the molecular regulatory mechanisms and TFs involved in heart valve progenitor development might be activated in adult diseased aortic valves. Amongst them, the EndMT process is crucial in generating valve progenitor cells and certain TFs expressed in these progenitor cells that play a key role in valve development and EndMT.24 Intriguingly, these TFs might also become active in CAVD and exert potential pathological functions via mediating EndMT. These modulators of the EndMT process include Msx1/2, RBPJ and Sox9,56 which have been associated with cardiovascular calcification and aortic valve disease. However, to date there is very limited evidence of whether or not these factors quicken the process of CAVD through EndMT (Figure 3).
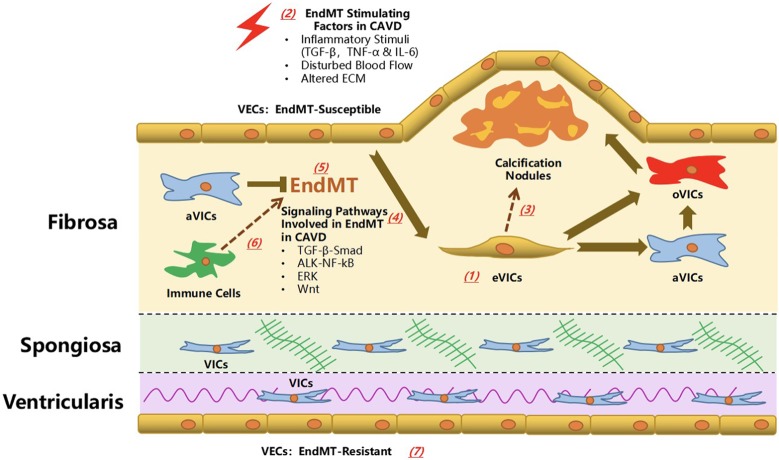
Figure 3.
Endothelial-to-mesenchymal transition in calcific aortic valve disease: the known knowledge and unresolved questions. The investigation on the role of endothelial-to-mesenchymal transition (EndMT) in calcific aortic valve disease has recently emerged. The evidence of EndMT in vivo has been found in the fibrosa layer (aortic aspect) of aortic valve, which was distal to the calcification nodule. In vitro evidence supported that inflammatory cytokines (including TNF-α, IL-6 and TGF-β), disturbed blood flow and altered extracellular matrix (ECM) was responsible for the induction of EndMT process, during which the TGF-β-Smad, ALK-NF-kB, ERK and Wnt signaling pathways might play a regulatory role. The transformed VECs (eVICs) might serve as an important reservoir for activated VICs (aVICs) whereas the EndMT process might be negatively regulated by VICs. eVICs might further transformed into an osteogenic type (oVIC) and cooperate with the VIC-derived oVICs to promote the CAVD progression. Several key unresolved questions are crucial in understanding the role of EndMT process in CAVS. (1) The origin of cells co-expressing endothelial and mesenchymal markers in the diseased aortic valve has to be further validated. (2) What are the effects of other risk factors/stimulating factors of CAVD on the EndMT process? (3) The relationship between EndMT process and calcific and osteogenic process remains to be explored in the future. In other words, it is elusive that how the EndMT process contributes to the different phases of CAVD from valve thickening, stenosis to calcification. (4) Several other well-known signaling pathways involved in the regulation of EndMT/EMT might also exert effects on the development of CAVD via EndMT. (5) Further in-depth investigation is required to verify a clearer correlation between the extent of EndMT, and severity and progression of CAVD based on pathological examination and echocardiography evaluation. (6) It is not clear how the interactions between VECs and other immune cells influence the EndMT process, and vice versa. (7) Last but not least, it is worthwhile of intensive efforts to verify the sub-population of EndMT-resistant VECs and the mechanisms modulating this obvious heterogeneity.
In vitro and in vivo evidence of EndMT in CAVD
EndMT is a relatively novel frontier even in the basic research of atherosclerosis, and related findings are also scarce when it comes to EndMT in CAVD. In 2001, Paranya et al. reported that ECs isolated from adult mature aortic valves could transdifferentiate to a mesenchymal phenotype in medium containing either TGF-β or low levels of serum without basic fibroblast growth factor. These transdifferentiated ECs co-expressed the endothelial marker CD31 and mesenchymal marker α-SMA, and exhibited increased migration upon stimulation with platelet-derived growth factor-BB. In addition, the presence of this transdifferentiation has also been detected in vivo, with positive staining of both markers in a subpopulation of cells in frozen sections of aortic valves. This evidence supports the existence of the EndMT process both in vivo and In vitro, and that it is potentially mediated by TGF-β-dependent and -independent signaling. Moreover, Mahler et al. supported the spatiotemporal nature of the EndMT process in CAVD, and it will be discussed later19 (Figure 3).
Inflammation induces the EndMT process in CAVD
Mahler et al. were amongst the first to demonstrate indirect evidence of the presence of EndMT by showing a population of subendothelial cells co-expressing α-SMA and CD31 in explanted calcific human aortic valves. A majority of these EndMT-derived cells also co-expressed nuclear NF-κB. It appeared to be a side-specific and disease-specific property of the EndMT process in CAVD, as healthy pediatric human valves and the ventricularis layer of aortic cusps did not co-express endothelial or mesenchymal markers. In addition, the EndMT and potential EndMT-derived cells were found distal to the sites of calcified lesions, indicating that EndMT is not likely to be involved in the mineralization process. The same group further found that inflammatory cytokines, both TNF-α and IL-6, promoted the EndMT process in not only adult VECs but also embryonic valve endocardium partially through NF-κB signaling, an effect which resembled that of TGF-β. Mechanistic studies revealed that Akt/MAPK/NF-κB signaling was essential for the EndMT process in both adult VECs and embryonic valve endocardium, while TGF-β-ALK-Smad2/3 signaling downstream of NF-κ B seemed to be unique to the EndMT process at an embryonic stage. To sum up, inflammatory cytokines induced EndMT in VECs in both embryonic and adult stages via Akt/NF-κB signaling, but with distinct utilization of TGF-β signaling19 (Figure 3).
Mechanical stress serves as a stimulating factor for EndMT in CAVD
Oscillatory shear stress is very likely to be a causative factor for EndMT in CAVD. The typical pathological changes are limited to the fibrosa layer, because the aortic aspect of leaflets bears the disturbed blood flow while the ventricular aspect undergoes a laminar flow. Balachandran et al. were amongst the first to demonstrate that EndMT could also be induced by mechanical stress in diseased aortic valves. By using microcontact printing to simulate the regions of isotropy and anisotropy of the leaflet and applying cyclic mechanical strain in the 2D valve endothelium, they found that both low and high strain contributed to the EndMT process, but in distinct regulatory pathways. Moreover, TGF-β signaling was increased with low strain-induced EndMT while high strain strengthened Wnt/β-catenin signaling, suggesting that in healthy or diseased aortic valves, different modes of strain are utilized. The effect of cyclic strain was also directionally dependent, and transformed cells displayed increased capacity for migration in response to endothelin-1 and larger basal mechanical tone.
Mahler et al. developed a dynamic, 3D environment to quantify the effects of a shear stress pattern and magnitude on the EndMT process and invasion of VECs, and found that low steady shear stress and oscillatory shear stress up-regulated EndMT-related and inflammation-related gene expressions and matrix invasion in comparison with static controls or high steady shear (Figure 3).
Alterations in ECM contribute to the EndMT process in CAVD
Except for inflammatory conditions, altered ECM might also contribute to the EndMT process in CAVD. A very recent report by Dahal and colleagues used in vitro models of early- and late-stage valve disease by incorporating the glycosaminoglycan chondroitin sulfate (CS) into a 3D collagen gel culture system with or without TGF-β1 stimulation to induce EndMT. They noticed that high levels of CS facilitated the EndMT process to the highest grade, showed by most collagen and GAG production by the EndMT-derived cells. In addition, the EndMT process due to altered ECM depended on cell-ECM bond strength and ERK1/2 signaling26 (Figure 3).
VIC and VEC interactions modulate the EndMT process in CAVD
Hjortnaes et al. recently reported that VECs undergo osteogenic differentiation via the EndMT process, and that this could be reversed by VICs. In addition, they found that TGF-β1 stimulation promoted EndMT in VECs, and that osteogenic alterations were induced in VECs by osteogenic media (OM). Moreover, co-culture with VICs could inhibit both TGF-β1-mediated EndMT and OM-induced osteogenic differentiation. Interestingly, time course analysis demonstrated that EndMT occurred in advance of osteogenesis. Increased expressions of EndMT markers (α-SMA and MMP-2) were first observed at 7 days after stimulation by OM alone or OM plus TGF-β1, which was followed by subsequent increases in osteogenic markers (osteopontin and osteocalcin) at 14 days after stimulation. In addition, VICs suppressed osteogenesis in VECs in CAVD, but not vice versa25 (Figure 3).
Different responsiveness of distinct sets of VECs to inflammation-induced EndMT process in CAVD
A recent report reported the heterogeneous responsiveness of VECs to EndMT transformation stimulated by TNF-α. Two distinct subsets of VECs were present, among which one group converted into the mesenchymal phenotype and the other group remained resistant to mesenchymal transformation. Transformed cells abandoned the endothelial characteristics and up-regulated the mesenchymal marker α-SMA, whereas untransformed cells expressed stabilized levels of the endothelial markers eNOS and VE-cadherin. Both subsets of cells presented with an inflammatory phenotype with an increased expression of ICAM-1, but only the transformed cells up-regulated MMP-9, Notch1, TGF-β and BMP-4. In addition, the transformed cells also exerted distinct effects on collagen fiber alignment at migration. It remains unknown how the different destinies of VECs are determined. Elucidating the molecular signature of transformation-resistant VECs could promote the development of diagnostic and treatment strategies (Figure 3).
VECs might serve as a reservoir for VICs via the EndMT process
Hjortnaes et al. also advanced a theory that VECs might act as a reservoir for VICs via the EndMT process. A subset of VEC-derived VICs (eVICs) arise from the EndMT process, accompanied by a decline in the expression of VE-cadherin and an increased expression of α-SMA. This transition is very likely to be negatively regulated by VICs. eVICs might further differentiate into oVICs and contribute to the calcification in CAVD, a process in which osteoblastic markers such as runx2, osteocalcin and osteopontin are up-regulated.25 The effects of eVICs under physiological or pathological conditions are not clear. For normal aortic valve tissue, this transition might be a functional supplement for resident VICs in order to maintain valve physiology and homeostasis. eVICs might also experience a reversed phenotypic transition back to VECs if the viability of VICs is restored. While in diseased aortic valves, persistent pathological stimuli make this special subset of VECs play a role in valve stiffening and calcification. If there is a continuum from VECs to eVICs to oVICs, then it might be precisely modulated by a negative feedback regulatory loop. However, during the advanced stage of CAVD, this balance might be progressively disrupted due to a loss of well-functioning VICs, and the inhibitory effects of VICs on the EndMT process then become deficient (Figure 3).
QUESTIONS AWAITING ANSWERS FOR THE EndMT PROCESS IN CAVD
Despite the evidence listed above, investigations of the role of the EndMT process in CAVD and the underlying molecular regulatory networks are in the preliminary phase. First, the origin of cells co-expressing endothelial and mesenchymal markers has to be further validated. The endothelial tracking system used in mice models to study atherosclerosis is highly recommended in CAVD to confirm that the population of EndMT-derived mesenchymal cells is one major source of aVICs or oVICs in diseased aortic valves (Figure 3(1)).
Second, based on the current knowledge learned from CAVD and other cardiovascular diseases, this cellular transformation might be initiated in CAVD by inflammatory stimuli, ECM composition alterations, hemodynamic shear stress, oxidative stress, and metabolic disorders. While the former three have recently emerged in this field, investigations of hyperglycemia, dyslipidemia, hyperphosphatemia and oxidative stress are almost completely lacking. Thus, in the future, intensive investigations should focus on the role of these potential risk factors in mediating EndMT in CAVD (Figure 3(2)).
Third, the explicit role of the EndMT process in the early- and end-stage phases of CAVD is still unclear. The current evidence suggests that EndMT precedes osteogenic changes in vitro, and that EndMT-derived cells are located in regions not proximal to calcification nodules in vivo. The EndMT process might mainly influence the initial stage of the CAVD process, but future studies should continue to investigate the association between the EndMT process and calcification (Figure 3(3)).
Fourth, the detailed molecular regulatory mechanisms involved in the EndMT process in CAVD should be investigated in detail. As mechanistic exploration has been intensively performed regarding EMT in malignancy and EndMT in atherosclerosis, relevant work is thought to be relatively easier in CAVD. To date, canonical TGF-β-Smad signaling and Akt-MAPK-NF-κB signaling have been found to play roles in the regulation of EndMT in CAVD, however this cannot explain all of the observed changes. Other well-documented signaling pathways should be taken into consideration for in-depth explorations including Wnt-catenin, non-canonical TGF-β and Notch signaling pathways (Figure 3(4)).57,58
Fifth, clinical investigations are required to verify the correlation between the extent of EndMT and severity and progression of CAVD based on pathological and echocardiography evaluations. However, it remains a challenge to obtain sufficient explanted human aortic valves because surgical aortic valve replacement is not indicated for patients with mild and mild-to-moderate aortic stenosis due to CAVD (Figure 3(5)).
Sixth, except for resident VICs and VECs, infiltrating immune cells also contribute to disease progression, and it still remains unclear as to how VECs and these effector cells interactively influence the EndMT process. Similar research, however, has recently been performed in atherosclerosis (Figure 3(6)).
Seventh, as mentioned above, the simultaneous presence of two heterogeneous subsets of VECs that react differently to EndMT stimuli implies that one acts as the guardian of valve integrity with the other serving as the culprit of pathological remodeling. However, the fundamental mechanisms regulating the distinct behaviors and fates of VECs remain undetermined. Whether these two groups can be converted to each other is still an unresolved question (Figure 3(7)).
CONCLUSIONS
Increasing evidence indicates that the EndMT process is an emerging theory driving the development of CAVD, and that it can potentially be used as a novel diagnostic signature and therapeutic target in disease prevention and treatment. Future efforts should focus on investigating the association between EndMT and CAVD with regards to clinical progression, different effects of EndMT at different disease stages, and detailed mechanisms modulating various stimuli, distinct responsiveness of VECs and cell-to-cell interactions in the EndMT process.
Acknowledgments
None.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
FUNDING SOURCES
The study was supported by the Natural Science Foundation of Shandong Province (ZR2018BH002 and ZR2015HQ001), National Natural Science Foundation of China (81500375 and 81800255), National Key R&D Program of China (2017YFC1308000) and Key Research and Development Program of Shandong Province (2016GSF 201039).
REFERENCES
- 1.Huang PH, Chen JW, Lin SJ. Effects of cardiovascular risk factors on endothelial progenitor cell. Acta Cardiol Sin. 2014;30:375–381. [PMC free article] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa E, Libby P. A rock and a hard place: chiseling away at the multiple mechanisms of aortic stenosis. Circulation. 2017;135:1951–1955. doi: 10.1161/CIRCULATIONAHA.117.027776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman H, Bahçeci I, Hamur H, et al. The relationship between serum apelin levels and the severity of calcific aortic stenosis. Acta Cardiol Sin. 2018;34:259–266. doi: 10.6515/ACS.201805_34(3).20180207A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen M, Tastet L, Bergler-Klein J, et al. Blood, tissue and imaging biomarkers in calcific aortic valve stenosis: past, present and future. Curr Opin Cardiol. 2018;33:125–133. doi: 10.1097/HCO.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 6.Perrucci GL, Zanobini M, Gripari P, et al. Pathophysiology of aortic stenosis and mitral regurgitation. Compr Physiol. 2017;7:799–818. doi: 10.1002/cphy.c160020. [DOI] [PubMed] [Google Scholar]
- 7.Partida RA, Elmariah S. Transcatheter mitral valve interventions: current therapies and future directions. Curr Treat Options Cardiovasc Med. 2017;19:32. doi: 10.1007/s11936-017-0538-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen YH, Chang HH, Chen PL, et al. Procedural characteristics and outcomes of transcatheter aortic valve implantation: a single-center experience of the first 100 inoperable or high surgical risk patients with severe aortic stenosis. Acta Cardiol Sin. 2017;33:339–349. doi: 10.6515/ACS20170620A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbanti M, Buccheri S, Capodanno D, et al. Transcatheter or surgical treatment of severe aortic stenosis and coronary artery disease: a comparative analysis from the Italian observant study. Int J Cardiol. 2018;270:102–106. doi: 10.1016/j.ijcard.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Yang FY, Wang Y, et al. Single versus dual antiplatelet therapy after transcatheter aortic valve implantation: a systematic review and meta-analysis. Cardiology. 2018;141:52–65. doi: 10.1159/000490307. [DOI] [PubMed] [Google Scholar]
- 11.Kundi H, Popma JJ, Khabbaz KR, et al. Association of hospital surgical aortic valve replacement quality with 30-day and 1-year mortality after transcatheter aortic valve replacement. JAMA Cardiology. 2018 doi: 10.1001/jamacardio.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramlawi B, Garcia-Morales LJ. Advanced in aortic root surgery. Methodist Debakey Cardiovasc J. 2011;7:48–52. doi: 10.14797/mdcj-7-3-48. [DOI] [PubMed] [Google Scholar]
- 13.Takagi H, Mitta S, Ando T. Meta-analysis of valve-in-valve transcatheter versus redo surgical aortic valve replacement. Thorac Cardiovasc Surg. 2019;67:243–250. doi: 10.1055/s-0038-1668135. [DOI] [PubMed] [Google Scholar]
- 14.Rattazzi M, Pauletto P. Valvular endothelial cells: guardians or destroyers of aortic valve integrity? Atherosclerosis. 2015;242:396–398. doi: 10.1016/j.atherosclerosis.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Peeters F, Meex SJR, Dweck MR, et al. Calcific aortic valve stenosis: hard disease in the heart: a biomolecular approach towards diagnosis and treatment. Eur Heart J. 2018;39:2618–2624. doi: 10.1093/eurheartj/ehx653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulin A, Hego A, Lancellotti P, et al. Advances in pathophysiology of calcific aortic valve disease propose novel molecular therapeutic targets. Front Cardiovasc Med. 2018;5:21. doi: 10.3389/fcvm.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohukainen P, Ruskoaho H, Rysa J. Cellular mechanisms of valvular thickening in early and intermediate calcific aortic valve disease. Curr Cardiol Rev. 2018;14:264–271. doi: 10.2174/1573403X14666180820151325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 19.Mahler GJ, Farrar EJ, Butcher JT. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:121–130. doi: 10.1161/ATVBAHA.112.300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulshoff MS, Xu X, Krenning G, et al. Epigenetic regulation of endothelial-to-mesenchymal transition in chronic heart disease. Arterioscler Thromb Vasc Biol. 2018;38:1986–1996. doi: 10.1161/ATVBAHA.118.311276. [DOI] [PubMed] [Google Scholar]
- 21.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2018 doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Duffhues G, Garcia de Vinuesa A, Ten Dijke P. Endothelial-to-mesenchymal transition in cardiovascular diseases: developmental signaling pathways gone awry. Dev Dyn. 2018;247:492–508. doi: 10.1002/dvdy.24589. [DOI] [PubMed] [Google Scholar]
- 23.Gong H, Lyu X, Wang Q, et al. Endothelial to mesenchymal transition in the cardiovascular system. Life Sci. 2017;184:95–102. doi: 10.1016/j.lfs.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Wirrig EE, Yutzey KE. Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler Thromb Vasc Biol. 2014;34:737–741. doi: 10.1161/ATVBAHA.113.302071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjortnaes J, Shapero K, Goettsch C, et al. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis. 2015;242:251–260. doi: 10.1016/j.atherosclerosis.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahal S, Huang P, Murray BT, et al. Endothelial to mesenchymal transformation is induced by altered extracellular matrix in aortic valve endothelial cells. J Biomed Mater Res A. 2017;105:2729–2741. doi: 10.1002/jbm.a.36133. [DOI] [PubMed] [Google Scholar]
- 27.Richards J, El-Hamamsy I, Chen S, et al. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol. 2013;182:1922–1931. doi: 10.1016/j.ajpath.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cirka HA, Kural MH, Billiar KL. Mechanoregulation of aortic valvular interstitial cell life and death. J Long Term Eff Med Implants. 2015;25:3–16. doi: 10.1615/jlongtermeffmedimplants.2015011759. [DOI] [PubMed] [Google Scholar]
- 29.Wu B, Wang Y, Xiao F, et al. Developmental mechanisms of aortic valve malformation and disease. Annu Rev Physiol. 2017;79:21–41. doi: 10.1146/annurev-physiol-022516-034001. [DOI] [PubMed] [Google Scholar]
- 30.Haybar H, Shahrabi S, Rezaeeyan H, et al. Endothelial cells: from dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovasc Toxicol. 2018 doi: 10.1007/s12012-018-9493-8. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Qian M, Kyler K, et al. Endothelial-vascular smooth muscle cells interactions in atherosclerosis. Front Cardiovasc Med. 2018;5:151. doi: 10.3389/fcvm.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pi X, Xie L, Patterson C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ Res. 2018;123:477–494. doi: 10.1161/CIRCRESAHA.118.313237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho JG, Lee A, Chang W, et al. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front Immunol. 2018;9:294. doi: 10.3389/fimmu.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta P, Lincoln J. Calcific aortic valve disease: a developmental biology perspective. Curr Cardiol Rep. 2018;20:21. doi: 10.1007/s11886-018-0968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Rodriguez C, Parra-Izquierdo I, Castanos-Mollor I, et al. Toll-like receptors, inflammation, and calcific aortic valve disease. Front Physiol. 2018;9:201. doi: 10.3389/fphys.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galeone A, Brunetti G, Oranger A, et al. Aortic valvular interstitial cells apoptosis and calcification are mediated by tnf-related apoptosis-inducing ligand. Int J Cardiol. 2013;169:296–304. doi: 10.1016/j.ijcard.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Venardos N, Nadlonek NA, Zhan Q, et al. Aortic valve calcification is mediated by a differential response of aortic valve interstitial cells to inflammation. J Surg Res. 2014;190:1–8. doi: 10.1016/j.jss.2014.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arevalos CA, Berg JM, Nguyen JM, et al. Valve interstitial cells act in a pericyte manner promoting angiogensis and invasion by valve endothelial cells. Ann Biomed Eng. 2016;44:2707–2723. doi: 10.1007/s10439-016-1567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huk DJ, Austin BF, Horne TE, et al. Valve endothelial cell-derived tgfbeta1 signaling promotes nuclear localization of sox9 in interstitial cells associated with attenuated calcification. Arterioscler Thromb Vasc Biol. 2016;36:328–338. doi: 10.1161/ATVBAHA.115.306091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito A. EMT and EndMT: regulated in similar ways? J Biochem. 2013;153:493–495. doi: 10.1093/jb/mvt032. [DOI] [PubMed] [Google Scholar]
- 41.Yu W, Liu Z, An S, et al. The endothelial-mesenchymal transition (EndMT) and tissue regeneration. Curr Stem Cell Res Ther. 2014;9:196–204. doi: 10.2174/1574888x09666140213154144. [DOI] [PubMed] [Google Scholar]
- 42.Perez L, Munoz-Durango N, Riedel CA, et al. Endothelial-to-mesenchymal transition: cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017;33:41–54. doi: 10.1016/j.cytogfr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Jackson AO, Zhang J, Jiang Z, et al. Endothelial-to-mesenchymal transition: a novel therapeutic target for cardiovascular diseases. Trends Cardiovasc Med. 2017;27:383–393. doi: 10.1016/j.tcm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Correia AC, Moonen JR, Brinker MG, et al. Fgf2 inhibits endothelial-mesenchymal transition through microrna-20a-mediated repression of canonical tgf-β signaling. J Cell Sci. 2016;129:569–579. doi: 10.1242/jcs.176248. [DOI] [PubMed] [Google Scholar]
- 45.Krenning G, Barauna VG, Krieger JE, et al. Endothelial plasticity: shifting phenotypes through force feedback. Stem Cells Int. 2016;2016:9762959. doi: 10.1155/2016/9762959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen PY, Qin L, Baeyens N, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–4528. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, et al. Tgf-beta-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen PY, Simons M. Fibroblast growth factor-transforming growth factor beta dialogues, endothelial cell to mesenchymal transition, and atherosclerosis. Curr Opin Lipidol. 2018;29:397–403. doi: 10.1097/MOL.0000000000000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin F, Wang N, Zhang TC. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life. 2012;64:717–723. doi: 10.1002/iub.1059. [DOI] [PubMed] [Google Scholar]
- 50.Aisagbonhi O, Rai M, Ryzhov S, et al. Experimental myocardial infarction triggers canonical wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.S M, Ka C, Fa P, et al. The mir-200 family regulates key pathogenic events in ascending aortas of individuals with bicuspid aortic valves. J Intern Med. 2019;285:102–114. doi: 10.1111/joim.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ak G, V N, Jw C, et al. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): differential expression of micrornas during endmt. Cell Signal. 2012;24:1031–1036. doi: 10.1016/j.cellsig.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim M, Choi SH, Jin YB, et al. The effect of oxidized low-density lipoprotein (ox-ldl) on radiation-induced endothelial-to-mesenchymal transition. Int J Radiat Biol. 2013;89:356–363. doi: 10.3109/09553002.2013.763193. [DOI] [PubMed] [Google Scholar]
- 54.Evrard SM, Lecce L, Michelis KC, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016;7:11853. doi: 10.1038/ncomms11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooley BC, Nevado J, Mellad J, et al. Tgf-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6:227ra234. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Js S, Sl C, Jm P, et al. Msx2 promotes cardiovascular calcification by activating paracrine wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timmerman LA, Grego-Bessa J, Raya A, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liebner S, Cattelino A, Gallini R, et al. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]