Abstract
Background
To investigate the effect and possible mechanism of obstructive sleep apnea hypopnea syndrome on coronary microcirculation in stable angina pectoris (SAP) patients with a single borderline lesion.
Methods
We retrospectively analyzed 102 SAP patients with a single critical lesion [fractional flow reserve > 0.80] who were divided into an abnormal microcirculatory function group [index of microcirculatory resistance (IMR) ≥ 25, n = 52] and normal microcirculatory function (NMF) group (IMR < 25, n = 50). We compared indexes including biochemical indicators, coronary lesion characteristics, apnea hypopnea index (AHI), lowest oxygen saturation (LSaO2), night average heart rate, endothelin-1 (ET-1), nitric oxide (NO) and high-sensitivity C-reactive protein in serum between the two groups. Furthermore, risk factors affecting coronary microcirculation were analyzed.
Results
There were no significant differences in biochemical indexes and coronary lesion characteristics between the two groups (p > 0.05). Compared to the NMF group, AHI (23.76 ± 8.41 times/h) and ET-1 (1.96 ± 0.43 ng/L) were obviously increased (p < 0.01), and LSaO2 (77.96 ± 7.26%) and NO (23.63 ± 7.09 μmol/L) was significantly lower in the AMF group (p < 0.01). Moreover, AHI and ET-1 were positively associated with IMR (r1 = 0.887, 0.835, respectively). However, LSaO2 and NO had a negative correlation with IMR (r3 = 0.659, 0.691, respectively). Logistic regression analysis showed that AHI was an independent predictor of coronary microcirculatory dysfunction (odds ratio = 1.260, 95% confidence interval 1.083~1.467, p < 0.01). Receiver operating characteristic (ROC) curve analysis indicated an AHI cut-off value of 13.7 times/h to predict microcirculatory dysfunction (sensitivity 0.942, specificity 0.880).
Conclusions
In SAP patients with a single critical lesion, AHI was associated with coronary microcirculatory dysfunction.
Keywords: Angina pectoris, Apnea hypopnea index, Index of microcirculatory resistance
INTRODUCTION
Obstructive sleep apnea hypopnea syndrome (OSAHS) is a common disorder of sleep and breathing which is characterized by repeated apnea and hypopnea during sleep. OSAHS can result in hypoxemia and/or hypercapnia, and finally cause damage to multiple organs. OSAHS is closely related to cardiovascular and cerebrovascular diseases, such as hypertension, coronary heart disease (CHD), arrhythmia, heart failure and stroke.1-6 OSAHS has a high morbidity in CHD patients, which has long been considered to be the cause of coronary artery stenosis and myocardial ischemia.7,8
With the development of medical technology, there are many non-invasive and invasive methods to detect coronary microcirculatory function.9 Recently, index of microcirculatory resistance (IMR) has become the gold standard for the diagnosis of coronary microcirculatory function.10 Increasing evidence has shown that many patients with typical angina symptoms have no significant stenosis (coronary artery lesion < 50%) in the coronary artery during coronary angiography (CAG), and this has been shown to be caused by coronary microcirculatory dysfunction using IMR analysis.11 Crea and college showed that non-ST-segment elevation acute coronary syndrome (NSTE-ACS) without obstructive coronary artery disease exhibited notable coronary dysfunction mainly involving coronary microcirculation, which seemed to involve both an increased constrictor reactivity and a reduced microvascular dilator function.12 Kakuta et al. reported that IMR showed no significant association with the severity of functional stenosis quantified by fractional flow reserve (FFR) or with the severity of anatomical stenosis on quantitative coronary angiography in patients with intermediate coronary lesions, and found that the location of right coronary artery (RCA) lesions and a history of hypertension were interdependent predictors of microcirculatory dysfunction.13 Taken together, these data suggest that patients with coronary artery stenosis < 70% are usually accompanied with coronary microcirculatory dysfunction.
OSAHS patients usually have endothelial dysfunction and systemic inflammation, which can damage microcirculatory function.14 However, no studies have reported about the relationship between OSAHS and microcirculatory function in patients with stable angina pectoris (SAP) with a single borderline lesion. Therefore, this study mainly focused on the effects of OSAHS on coronary microcirculation and explored its possible mechanism in SAP patients with a single borderline lesion.
MATERIALS AND METHODS
Study population
Patients with SAP were enrolled in the study from January 2016 to December 2016 at our hospital. Basic clinical data were recorded during hospitalization. FFR and IMR of target coronary arteries were measured, and polysomnographic breathing was used to record hypopnea index (AHI) and lowest oxygen saturation (LSaO2) values in single critical lesion patients with FFR > 0.80. According to the IMR, the patients were divided into two groups: abnormal microcirculatory function group (AMF) (IMR ≥ 25, n = 52), and normal microcirculatory function group (NMF) (IMR < 25, n = 50).10,11 The inclusion criteria were: (1) stable angina pectoris; (2) age range from 30 to 80 years; (3) single coronary artery critical lesion; (4) target coronary artery lesion (FFR > 0.80). The exclusion criteria were: (1) rheumatic heart disease and patients with cardiomyopathy; (2) patients with major adverse cardiac events during hospitalization; (3) percutaneous coronary intervention (PCI) and coronary artery bypass grafting history; (4) multiple coronary artery lesions; and (5) asthma. Nitric oxide (NO), endothelin-1 (ET-1) and high sensitive-C reactive protein (hs-CRP) levels were detected by indirect colorimetry, radioimmunoassay and immunoprotective turbidimetry, respectively. The study protocol was approved by the hospital’s ethics committees, and all patients gave written informed consent.
OSAHS was diagnosed according to the Guidelines for Diagnosis and Treatment of OSAHS (revised version 2011) as follows: Light OSAHS: apnea AHI 5~15 times/h, LSaO2 between 85%~90%; Moderate OSAHS: AHI 15~30 times/h, LSaO2 between 80% and < 85%; Severe OSAHS: AHI > 30 times/h, LSaO2 < 80%.15
Measurement of FFR and IMR
All of the patients were given 300 mg clopidogrel as a loading dose before CAG. Unfractionated heparin (100-120 u/kg) was administered by bolus injection via a sheath to maintain an activated clotting time > 300 seconds during the whole procedure. Standard selective coronary angiography was performed via a radial approach with a 6 French catheter with no side hole according to routine practice. Coronary angiograms were quantitatively analyzed using a quantitative coronary angiographic analysis (QCA) system (CAAS II 5.0, Netherlands) by an experienced observer.
All patients had a single coronary critical lesion (stenosis 50%~70%) in QCA analysis, and then their FFR and IMR values were measured. We used a pressure wire head (PressureWireTM Certus, St. Jude Medical) and a guide rod temperature sensor to measure data using the temperature dilution method. Briefly, the method involved placing the temperature sensor at the end of the guide wire at least 3 cm away from the target lesion section and keeping the position of the guide wire relatively fixed and stable. We used a syringe to inject 3 ml normal saline at room temperature bullet-wise from the guide tube, and then recorded the time the normal saline ran from the guide tube to the tip of the guide wire, called the transit mean time (Tmn), which was inversely proportional to the flow velocity. We repeated this procedure three times and determined that the differences in values among them were less than 10%, and then took the average value. Finally we injected adenosine triphosphate intravenously to induce maximum hyperemia, and repeated the measurements three times to get the mean time of maximum hyperemia and the distal pressure (Pd) of the lesion. FFR was calculated as [FFR = Pd (distal coronary artery pressure)/Pa (aortic mean pressure of maximum expansion)]. Because there were no significant differences in all of the target lesions in the present study (FFR > 0.80), accurate determination of IMR required coronary wedge pressure (Pw) measured at the time when the target vessel was totally occluded by balloon inflation. When there were no coronary stenosis or severe stenosis (FFR > 0.80) and no collateral circulation, the Pw value was 0. Finally, we calculated IMR as (IMR = Tmn × Pd).16
Polysomnographic breathing monitoring
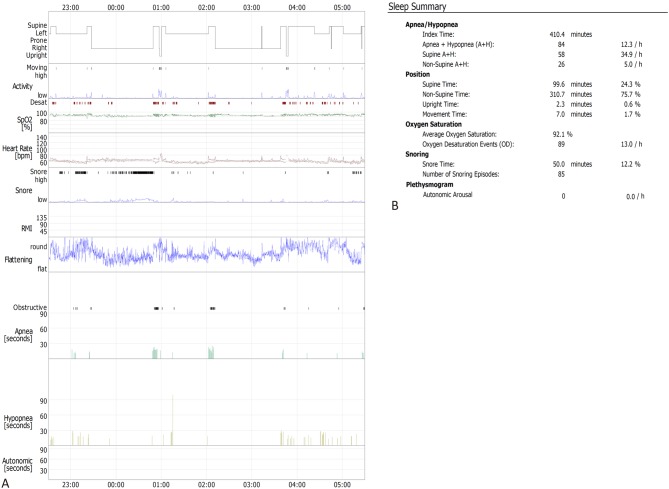
An EMBLA Sleep Monitoring System (Embla Systems Inc., Broomfield, CO, USA) was used to record the sleep and breathing of selected patients at night the second day after CAG. Before monitoring, the patients were told to avoid drinking coffee and tea, oral sedatives and hypnotic drugs, going to the toilet before bed, brushing teeth and ensuring adequate power supply to the monitor. The monitoring time ranged from 22:00 to 5:00, and the sleep status of the patients was recorded continuously for 7 hours. AHI, minimum oxygen saturation and average heart rate were recorded during sleep. The operating steps of polysomnographic breathing monitoring were as follows: (1) Connect a nasal airflow tube, thoracoabdominal band, oxygen saturation clip and snoring monitor to the sleep monitor; (2) Measure nasal airflow: place a nasal airflow tube, measure tidal volume and stop breathing according to airflow sensing; (3) Record chest and abdominal breathing movements: a piezoelectric crystal recording device is the most common device used to record respiratory movement, and it was placed on the chest and abdominal band, which could sense an increase in tension of the chest and abdominal band during the expansion of the chest and abdomen and convert it into electrical signals. The thoracic and abdominal bands were tied transversely at the level of the papilla in the chest and the umbilical cord in the abdomen, which was suitable tightening. (4) Measurement of blood oxygen saturation: pulse oximetry is the most simple, reliable and non-invasive method used to evaluate blood flow oxygenation; (5) Install a snoring monitor: snoring was measured by a small microphone, usually fixed near the patient’s trachea to record snoring sounds.17 The monitoring results are shown in Figure 1.
Figure 1.
Polysomnographic breathing monitoring indexes in one representative case. (A) Summary graph of this case. (B) Sleep summary report of this case. Sleep monitoring results of 63-year-old male patient were recorded continuously for 7 hours (22:30~05:30 the next day). The AHI was 12.3/h. The average oxygen saturation was 92.1%. The snoring time was 50.0 min, and the average heart rate was 60.7 bpm. AHI: apnea hypopnea index [Apnea + Hypopnea (A + H)].
Statistical analysis
Data processing and analysis were performed using SPSS 18.0 software. Comparisons of continuous variables were performed using the Student’s or Mann-Whitney test. Analyses of discrete variables were performed using the chi-square test or Fisher’s exact test where appropriate. Logistic regression analysis was used to explore the best predictors. Areas under the receiver operating characteristic (ROC) curves were generated to assess the predictive ability of AHI for coronary microcirculatory dysfunction. The optimum cut-off value was determined as the highest Youden index (Sensitivity + Specificity-1). Scatter plots were used to reflect the relationship between IMR and sleep monitoring results, or with indexes of endothelial function, which were also performed using correlation analysis. A p-value < 0.05 was regarded as being statistically significant.
RESULTS
Basic clinical data between the AMF and NMF groups according to IMR value
A total of 102 patients were enrolled in this study. According to the cut-off value of IMR reported in references,10,11 these patients were divided into AMF and NMF groups. With regards to age, gender, body mass index (BMI), history (hypertension, diabetes mellitus, hyperlipidemia, brain infarction and current smoker), left ventricular ejection fractions (LVEF), total cholesterol (TCH), triglyceride (TRIG), low density lipoprotein (LDL-C), high density lipoprotein cholesterol (HDL-C), serum creatinine (Scr), glycated hemoglobin (GHb), hemoglobin (Hb), hospitalized drug medications [aspirin, clopidogrel, ticagrelor, statin, angiotensin converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARB) and β-blockers], there were no significant differences between the AMF and NMF groups (all p > 0.05) (Table 1).
Table 1. Basic clinical data between AMF and NMF groups according to IMR value.
| NMF (n = 50) | AMF (n = 52) | p value | |
| Age (years) | 61.90 ± 9.96 | 62.87 ± 9.64 | 0.620 |
| Gender (female/male) | 19/31 | 21/31 | 0.805 |
| BMI (kg/m2) | 22.55 ± 2.80 | 25.23 ± 3.04 | 0.113 |
| History | |||
| Hypertension | 28 (56.00%) | 30 (57.69%) | 0.863 |
| Diabetes mellitus | 17 (34.00%) | 20 (38.46%) | 0.639 |
| Hyperlipidemia | 13 (26.00%) | 16 (30.77%) | 0.593 |
| Brain infarction | 8 (16.00%) | 12 (23.08%) | 0.457 |
| Current smoker | 24 (48.00%) | 27 (51.92%) | 0.692 |
| LVEF (%) | 59.88 ± 4.30 | 61.13 ± 4.18 | 0.138 |
| TCH (mmol/L) | 3.93 ± 0.96 | 4.21 ± 0.93 | 0.132 |
| TRIG (mmol/L) | 2.06 ± 0.89 | 1.99 ± 0.94 | 0.697 |
| LDL-C (mmol/L) | 2.38 ± 0.88 | 2.58 ± 0.74 | 0.208 |
| HDL-C (mmol/L) | 1.17 ± 0.43 | 1.15 ± 0.33 | 0.739 |
| Scr (μmol/L) | 75.84 ± 14.06 | 74.19 ± 11.90 | 0.524 |
| gHb (%) | 6.28 ± 1.10 | 6.56 ± 0.85 | 0.148 |
| Hb (g/L) | 127.96 ± 13.30 | 129.85 ± 13.90 | 0.486 |
| Hospitalized drug medication | |||
| Aspirin | 30 (60.00%) | 33 (63.46%) | 0.719 |
| Clopidogrel | 8 (16.00%) | 7 (13.46%) | 0.717 |
| Ticagrelor | 3 (6.00%) | 4 (7.69%) | 0.675 |
| Statin | 33 (66.00%) | 32 (61.54%) | 0.639 |
| ACEI/ARB | 24 (48.00%) | 27 (51.92%) | 0.692 |
| β-blocker | 29 (58.00%) | 33 (63.46%) | 0.572 |
Data were expressed as n (%), mean ± SD.
ACEI, angiotension converting enzyme inhibitors; AMF, abnormal microcirculatory function; ARB, angiotensin II receptor blockers; BMI, body mass index; gHb, glycosylated hemoglobin; Hb, hemoglobin; HDL-C, high density lipoprotein cholesterol; IMR, index of microcirculatory resistance; LDL-C, low density lipoprotein; LVEF, left ventricular ejection fraction; NMF, normal microcirculatory function; Scr, serum creatinine; SD, standard deviation; TCH, total cholesterol; TRIG, triglyceride.
Angiographic results and coronary physiological indexes between the AMF and NMF groups
Angiographic results of the patients with a single borderline lesion are shown in Table 2. There were no significant differences in target vessels [left anterior descending coronary artery (LAD), left circumflex artery (LCX) and RCA] and quantitative coronary analysis (QCA) results [lesion length (LL), minimum luminal diameter (MLD) and reference vessel diameter (RVD)] before primary PCI (pPCI) between the AMF and NMF groups (all p > 0.05).
Table 2. Angiographic results and coronary physiological indexes between AMF and NMF groups.
| NMF (n = 50) | AMF (n = 52) | p value | |
| Lesion location | 0.827 | ||
| LAD | 21 (42.00%) | 25 (48.08%) | |
| LCX | 13 (26.00%) | 12 (23.08%) | |
| RCA | 16 (32.00%) | 15 (28.85%) | |
| QCA results | |||
| LL (mm) | 27.34 ± 8.43 | 27.80 ± 9.37 | 0.797 |
| MLD (mm) | 1.66 ± 0.36 | 1.65 ± 0.33 | 0.848 |
| RVD (mm) | 3.00 ± 0.42 | 3.04 ± 0.38 | 0.635 |
| physiological indexes | |||
| FFR | 0.89 ± 0.05 | 0.87 ± 0.04 | 0.100 |
| Pd (mmHg) | 71.32 ± 14.57 | 69.50 ± 13.88 | 0.520 |
| Pa (mmHg) | 80.16 ± 14.36 | 79.73 ± 15.47 | 0.885 |
| Tmn (s) | 0.26 ± 0.04 | 0.66 ± 0.26 | 0.000 |
Data were expressed as n (%), mean ± SD.
AMF, abnormal microcirculatory function; FFR, fractional flow reserve; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LL, lesion length; MLD, minimum luminal diameter; NMF, normal microcirculatory function; Pa, mean aortic pressure; Pd, mean distal coronary pressure; QCA, quantitative coronary angiographic analysis; RCA, right coronary artery; RVD, reference vessel diameter; SD, standard deviation; Tmn, mean transit time.
We then measured Pa, Pd and Tmn at hyperemia and calculated FFR and IMR. There were no significant differences about Pa, Pd and FFR between the AMF and NMF groups (p > 0.05). However, Tmn in AMF group was significantly high as compared to that in NMF group (p < 0.01).
Sleep breathing monitoring indicators and indexes of endothelial function and inflammation between the AMF and NMF groups
The AHI value in the AMF group was significantly higher than that in the NMF group, and LSaO2 was markedly lower in the AMF group (both p < 0.01). However, there was no significant difference in HR between the two groups (p > 0.05) (Table 3).
Table 3. Sleep breathing monitoring indicators and indexes of endothelial function and inflammation between AMF and NMF groups.
| NMF (n = 50) | AMF (n = 52) | p value | |
| Sleep breathing monitoring indicators | |||
| AHI | 8.69 ± 5.23 | 23.76 ± 8.41 | 0.000 |
| LSaO2 (%) | 87.06 ± 4.64 | 77.96 ± 7.26 | 0.000 |
| HR (bpm) | 61.55 ± 6.12 | 60.31 ± 7.10 | 0.347 |
| Indexes of endothelial function and inflammation | |||
| NO (μmol/L) | 35.13 ± 9.48 | 23.63 ± 7.09 | 0.000 |
| ET-1 (ng/L) | 1.30 ± 0.31 | 1.96 ± 0.43 | 0.000 |
| hs-CRP (mg/L) | 3.44 ± 1.67 | 3.83 ± 1.63 | 0.237 |
Data were expressed as n (%), mean ± SD.
AHI, apnea hypopnea index; AMF, abnormal microcirculatory function; ET-1, endothelin-1; hs-CRP, high sensitive-C reactive protein; HR, heart rate; LSaO2, lowest oxygen saturation; NMF, normal microcirculatory function; NO, nitric oxide; SD, standard deviation.
The AMF group had a higher level of ET-1 than the NMF group, while a lower level of NO was observed in the AMF group (both p < 0.01). However, there was no significant difference in hs-CRP between the two groups (p > 0.05) (Table 3).
Taken together, the results indicated that microcirculatory dysfunction in the SAP patients with a single borderline lesion was usually accompanied with OSAHS and endothelial dysfunction.
Correlations among IMR with AHI, LSaO2, ET-1 and NO in the SAP patients with a single borderline lesion
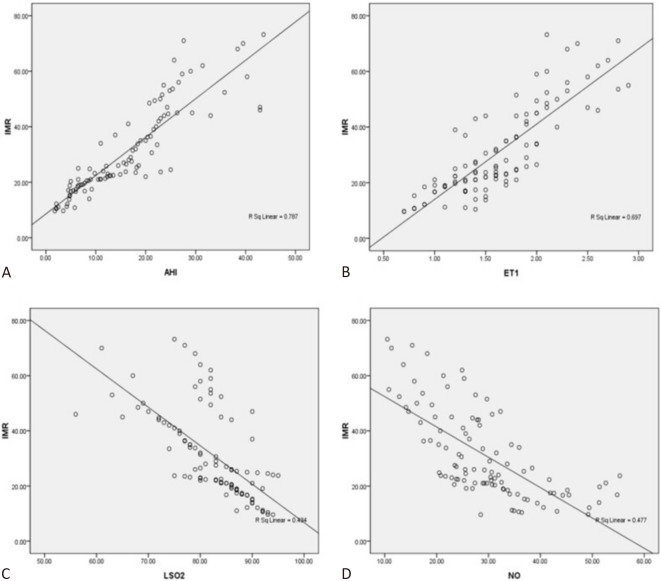
AHI and ET-1 were positively correlated with IMR (r = 0.887, 0.835, respectively) (Figure 2A, 2B), while LSaO2 and NO were negatively correlated with IMR (r = 0.659, 0.691, respectively) (Figure 2C, 2D). These results suggested that microcirculatory dysfunction in the SAP patients with a single borderline lesion was closely related to OSAHS and endothelial dysfunction.
Figure 2.
Correlation of IMR with AHI, ET-1, LSaO2 and NO in SAP patients with single borderline lesion. (A) Correlation of IMR with AHI; (B) Correlation of IMR with ET-1; (C) Correlation of IMR with LSaO2; (D) Correlation of IMR with NO. AHI, apnea hypopnea index; ET-1, endothelin-1; IMR, index of microcirculatory resistance; LSaO2, lowest oxygen saturation; NO, nitric oxide; SAP, stable angina pectoris.
Logistic regression analysis of the risk factors to predict microcirculatory dysfunction in the SAP patients with a single borderline lesion
Logistic regression analysis showed that AHI [odds ratio (OR) = 1.260, 95% CI 1.083-1.467, p < 0.01] was an independent predictor of microcirculatory dysfunction in the SAP patients with a single borderline lesion (Table 4). The cut-off value of AHI for predicting microcirculatory dysfunction was 13.7 times/h (sensitivity: 0.942, specificity: 0.880) (Figure 3).
Table 4. Logistic regression analysis of risk factors to predict microcirculatory dysfunction in SAP patients with single borderline lesion.
| Variable | OR (95%CI) | p value |
| AHI | 1.260 (1.083~1.467) | 0.003 |
| LSaO2 | 0.908 (0.781~1.056) | 0.211 |
| NO | 0.935 (0.850~1.030) | 0.173 |
| ET-1 | 13.019 (0.689~246.022) | 0.087 |
CI, confidential interval; ET-1, endothelin-1; IMR, index of microcirculatory resistance; LSaO2, lowest oxygen saturation; NO, nitric oxide; OR, odds ratio; SAP, stable angina pectoris.
Figure 3.
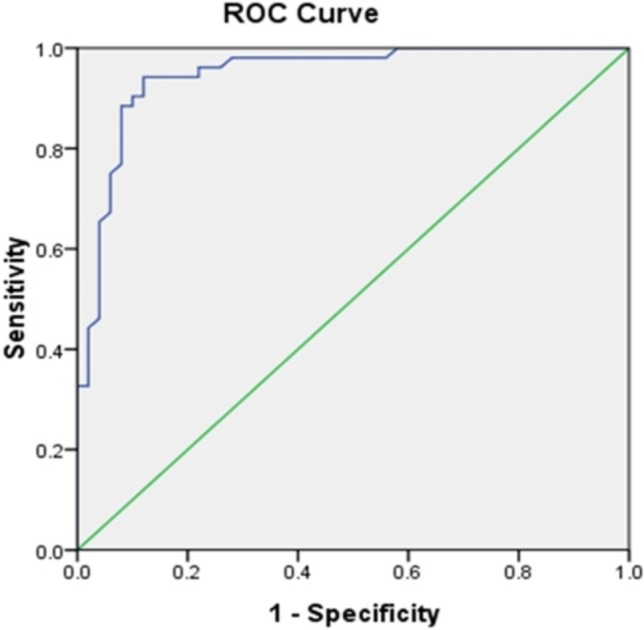
ROC curve analysis of AHI to predict microcirculatory dysfunction in SAP patients with single borderline lesion. The cut-off value of AHI for predicting microcirculatory dysfunction was 13.7 times/h (sensitivity: 0.942, specificity: 0.880). ROC, receiver operating characteristic curve. SAP, stable angina pectoris.
DISCUSSION
OSAHS is a chronic respiratory disease with a high incidence. Epidemiological data have indicated that about 4% of adult males and 2% of adult females suffer from OSAHS.18 The diagnostic criteria for OSAHS are as follow: (1) symptoms: typical nocturnal snoring with apnea, daytime sleepiness, etc. (2) signs: upper airway stenosis or obstruction. (3) laboratory examination: AHI ≥ 5 times/h.19 OSAHS patients are prone to chronic intermittent hypoxia or night arousal due to intermittent airflow interruption, which can lead to cardiovascular and cerebrovascular diseases and increase mortality.20,21 In addition, CHD patients usually also have OSAHS.22 However, the relationship between CHD and OSAHS is still unclear. It has been reported that microcirculatory dysfunction plays an important role in the pathogenesis of CHD.11-13 Endothelial dysfunction and systemic inflammation caused by OSAHS can lead to microcirculatory dysfunction.14 Therefore, the aim of this study was to observe the effect of OSAHS on coronary microcirculation and explore its possible mechanism in SAP patients with a single borderline lesion. Our results showed that OSAHS was an independent predictor of microcirculatory dysfunction in the SAP patients with a single borderline lesion, which has not been reported before.
LSaO2 and AHI are the two most important polysomnographic indicators for estimating the severity of sleep apnea. LSaO2 reflects the degree of nocturnal hypoxia, and AHI reflects the frequency of apnea hypopnea. The results of this study also confirmed the presence of repeated apnea and hypoxia events in patients with OSAHS at night. AHI is defined as the frequency of respiratory disorder events every hour in patients with OSAHS, however it cannot assess the severity of hypoxia in every respiratory disorder event.23,24 Pearson et al. reported that hypoxia may cause damage to coronary microcirculatory function.25 The results of our study also indicated that microcirculatory dysfunction in SAP patients with a single borderline lesion was closely associated with OSAHS.
NO is one of the important substances produced by vascular endothelial cells, which can dilate blood vessels, prevent platelet aggregation, adhesion and release of active substances, and also prevent vasospasm and thrombosis.26 ET-1 is the most powerful vasoconstriction factor secreted by vascular endothelial cells, and it can cause vasoconstriction of various vessels.27 Under physiological conditions, the NO released by vascular endothelial cells is dominant and can counteract the vasoconstriction of ET-1. Repeated hypoxic events in patients with OSAHS can increase ET-1 and decrease the biological activity of NO, which can lead to increased vascular endothelial damage and dysfunction.28 In addition, we found that microcirculatory function impairment in the SAP patients with a single borderline lesion was usually accompanied by night hypoxia and endothelial dysfunction, indicating that endothelial dysfunction caused by hypoxia could contribute to microcirculatory dysfunction in these patients.
Intermittent hypoxia during sleep in patients with OSAHS can further up-regulate the expression of inflammatory mediators.29 Hs-CRP is a biomarker of inflammation and an independent risk factor for myocardial infarction, unstable angina pectoris and multiple peripheral artery diseases.30 However, this study showed no significant difference in hs-CRP level between the AMF and NMF groups, which was probably due to the small sample size.
CHD caused by coronary microvascular dysfunction is called microvascular angina pectoris clinically.31 Coronary microvascular dysfunction can cause the mismatch of myocardial blood supply and oxygen demand, and then lead to myocardial ischemia.32 Increasing evidence has shown high morbidity of adverse cardiovascular events such as heart failure, sudden cardiac death and acute myocardial infarction in patients with coronary microvascular dysfunction.21,33 In this study, we found that AHI was an independent predictor of microcirculatory dysfunction in SAP patients with a single borderline lesion, suggesting that early treatment of OSAHS such as ventilator therapy may be a good method to improve microvascular dysfunction in these patients.
Limitations
The sample size of this study was small and all of the study subjects only had a single coronary lesion. In addition, there were no data of IMR in patients with normal coronary arteries. Moreover, we explored only the effects of hs-CRP and endothelial factors on coronary microcirculation, however there were no data of these effects after ventilator therapy. Furthermore, this study was only a preliminary observational study conducted at one center, so the results need to be further validated in a multicenter study.
CONCLUSIONS
In conclusion, OSAHS could cause or aggravate coronary microcirculatory dysfunction, which was partly due to endothelial dysfunction in SAP patients with a single critical lesion. In addition, AHI was an independent predictor of coronary microcirculatory impairment in these patients.
CONLICTS OF INTEREST
All the authors declare no conflict of interest.
FUNDING
This project was supported by the National Natural Science Foundation of China (grant number 81600296), China Postdoctoral Science Foundation (grant number 2019M651886), Jiangsu Postdoctoral Research Foundation (grant number 2018K242C) and Nanjing Municipal Science and Technology Bureau (grant number: 201803008).
REFERENCES
- 1.Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 2.Bravata DM, McClain V, Austin C, et al. Diagnosing and managing sleep apnea in patients with chronic cerebrovascular disease: a randomized trial of a home-based strategy. Sleep Breath. 2017;21:713–725. doi: 10.1007/s11325-017-1494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the sleep heart health study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz R, Blau A, Borgel J, et al. Sleep apnoea in heart failure. Eur Respir J. 2007;29:1201–1205. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 5.Umut Somuncu M, Bulut U, Karakurt H, et al. The relationship between obstructive sleep apnea and coronary plaque: a coronary computed tomographic angiography study. Acta Cardiol Sin. 2019;35:325–334. doi: 10.6515/ACS.201905_35(3).20181029A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin YS, Liu PH, Chu PH. Obstructive sleep apnea independently increases the incidence of heart failure and major adverse cardiac events: a retrospective population-based follow-up study. Acta Cardiol Sin. 2017;33:656–663. doi: 10.6515/ACS20170825A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200:493–506. doi: 10.1164/rccm.201808-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera-Perez SJ, Martinez D, Araujo GN, et al. Severity of obstructive sleep apnea and extension of coronary artery disease. Sleep Breath. 2018;23:747–752. doi: 10.1007/s11325-018-1769-5. [DOI] [PubMed] [Google Scholar]
- 9.McAlindon E, Pufulete M, Harris J, et al. Microvascular dysfunction determines infarct characteristics in patients with reperfused ST-segment elevation myocardial infarction: the microcirculation in acute myocardial infarction (Micro-Ami) study. PLoS One. 2018;13:e0203750. doi: 10.1371/journal.pone.0203750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran D, Young R, Adlam D, et al. Coronary microvascular dysfunction in patients with stable coronary artery disease: the Ce-Marc 2 coronary physiology sub-study. Int J Cardiol. 2018;266:7–14. doi: 10.1016/j.ijcard.2018.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vita A, Manfredonia L, Lamendola P, et al. Coronary microvascular dysfunction in patients with acute coronary syndrome and no obstructive coronary artery disease. Clin Res Cardiol. 2019;108:1364–1370. doi: 10.1007/s00392-019-01472-4. [DOI] [PubMed] [Google Scholar]
- 13.Murai T, Lee T, Yonetsu T, et al. Variability of microcirculatory resistance index and its relationship with fractional flow reserve in patients with intermediate coronary artery lesions. Circ J. 2013;77:1769–1776. doi: 10.1253/circj.cj-12-1442. [DOI] [PubMed] [Google Scholar]
- 14.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–685. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 15.Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: The Wisconsin Sleep Cohort Study. Sleep. 2015;38:677–684. doi: 10.5665/sleep.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aarnoudse W, van den Berg P, van de Vosse F, et al. Myocardial resistance assessed by guidewire-based pressure-temperature measurement: in vitro validation. Catheter Cardiovasc Interv. 2004;62:56–63. doi: 10.1002/ccd.10793. [DOI] [PubMed] [Google Scholar]
- 17.Cintra FD, Leite RP, Storti LJ, et al. Sleep apnea and nocturnal cardiac arrhythmia: a populational study. Arq Bras Cardiol. 2014;103:368–374. doi: 10.5935/abc.20140142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 19.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott M, Brown DL, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurother. 2018;18:523–531. doi: 10.1080/14737175.2018.1489239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baratta F, Pastori D, Fabiani M, et al. Severity of Osas, Cpap and cardiovascular events: a follow-up study. Eur J Clin Invest. 2018;48:e12908. doi: 10.1111/eci.12908. [DOI] [PubMed] [Google Scholar]
- 22.Zhao GQ, Wang X, Fan JY, et al. Association between hypothyroidism and sleep breathing disorders in patients with coronary heart disease. Zhonghua Nei Ke Za Zhi. 2018;57:571–575. doi: 10.3760/cma.j.issn.0578-1426.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Shi S, Xia Y, et al. Changes in sleep characteristics and airway obstruction in Osahs patients with multi-level obstruction following simple Uppp, Uppp-Ga, or Uppp-Tba: a prospective, single-center, parallel group study. ORL J Otorhinolaryngol Relat Spec. 2014;76:179–188. doi: 10.1159/000358012. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Li Y. Clinical and polysomnographic characteristics in elderly patients with obstructive sleep apnea hypopnea syndrome. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22:222–225. [PubMed] [Google Scholar]
- 25.Chen YC, Inagaki T, Fujii Y, et al. Chronic intermittent hypoxia accelerates coronary microcirculatory dysfunction in insulin-resistant Goto-Kakizaki rats. Am J Physiol Regul Integr Comp Physiol. 2016;311:R426–R439. doi: 10.1152/ajpregu.00112.2016. [DOI] [PubMed] [Google Scholar]
- 26.Friebe A, Voussen B, Groneberg D. No-Gc in cells ‘off the beaten track’. Nitric Oxide. 2018;77:12–18. doi: 10.1016/j.niox.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Yammine L, Kang DH, Baun MM, et al. Endothelin-1 and psychosocial risk factors for cardiovascular disease: a systematic review. Psychosom Med. 2014;76:109–121. doi: 10.1097/PSY.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 28.Rodella LF, Favero G, Rossini C, et al. Aging and vascular dysfunction: beneficial melatonin effects. Age (Dordr) 2013;35:103–115. doi: 10.1007/s11357-011-9336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Yang F, Guo Y, et al. The contribution of chronic intermittent hypoxia to Osahs: from the perspective of serum extracellular microvesicle proteins. Metabolism. 2018;85:97–108. doi: 10.1016/j.metabol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Kumakura H, Fujita K, Kanai H, et al. High-sensitivity C-reactive protein, lipoprotein(a) and homocysteine are risk factors for coronary artery disease in Japanese patients with peripheral arterial disease. J Atheroscler Thromb. 2015;22:344–354. doi: 10.5551/jat.25478. [DOI] [PubMed] [Google Scholar]
- 31.Jaarsma C, Vink H, van Haare J, et al. Non-invasive assessment of microvascular dysfunction in patients with microvascular angina. Int J Cardiol. 2017;248:433–439. doi: 10.1016/j.ijcard.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Schindler TH, Dilsizian V. Coronary microvascular dysfunction: clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging. 2020;13:140–155. doi: 10.1016/j.jcmg.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Peker Y, Thunstrom E, Glantz H, et al. Outcomes in coronary artery disease patients with sleepy obstructive sleep apnoea on Cpap. Eur Respir J. 2017;50 doi: 10.1183/13993003.00749-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]